User login
Novel Watchman device approved as warfarin alternative in atrial fib
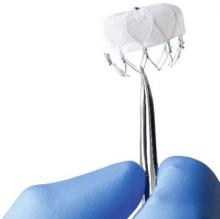
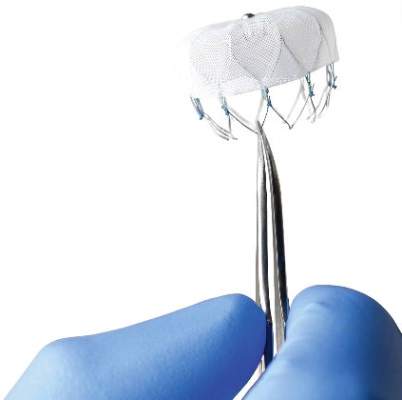
The Watchman left atrial appendage (LAA) closure device has been approved in the United States as an alternative to warfarin for patients with nonvalvular atrial fibrillation, for a narrower indication than the one submitted for approval to the Food and Drug Administration.
The device is a percutaneously delivered permanent cardiac implant placed in the LAA to prevent the embolization of thrombi formed in the LAA, and is manufactured by Boston Scientific. The FDA approved the Watchman for reducing the risk of thromboembolism from the LAA in patients with nonvalvular atrial fibrillation “who are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores, are deemed by their physicians to be suitable for warfarin; and have an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device, compared to warfarin,” according to a statement issued by the company on March 13.
The approved indication is worded differently than the proposed indication that was submitted to the FDA for approval and discussed at an FDA panel meeting in October, to “prevent thromboembolism from the left atrial appendage.” The changes in the indication include the replacement of “prevent” with “reduce the risk” of thromboembolism, and the addition of the following qualifiers: In patients who “are deemed by their physicians to be suitable for warfarin,” and who have “an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.”
“These changes were made to more accurately reflect the appropriate patient population for this device,” according to an FDA spokesperson.
At a meeting in October 2014, the FDA’s Circulatory System Devices Panel voted 6-5 with one abstention that the benefits of the device outweighed its risks for the proposed indication, but several panelists who voted no said they would support approval of a second-line indication. In addition, panelists voting on both sides of this question said that the indication was too broad and should be revised to describe the device as a second-line alternative to warfarin, making clear it is not appropriate for all warfarin-eligible patients. (At the meeting, the panel unanimously agreed that there was “reasonable assurance” that the device was safe for use in this population.)
At the first advisory panel meeting on the device, in December 2013, the panel voted 13-1 to recommend approval, based on data from the PREVAIL and PROTECT-AF studies, which compared the device to chronic warfarin, and information from the Continued Access to PREVAIL (CAP2) registry.PREVAIL compared implantation of the device – with 45 days of warfarin plus 81 mg of aspirin for 45 days, followed by 325 mg of aspirin and 75 mg of clopidogrel through 6 months, followed by 325 mg of aspirin a day indefinitely – to chronic warfarin.
The October meeting was convened by the FDA to review longer follow-up data from PREVAIL, which found additional cases of ischemic strokes in the Watchman group and none in the warfarin-treated group.
The Watchman device has been available outside of the United States since 2009, is registered in 75 countries, and has been used to treat more than 10,000 patients, according to Boston Scientific.
The Watchman left atrial appendage (LAA) closure device has been approved in the United States as an alternative to warfarin for patients with nonvalvular atrial fibrillation, for a narrower indication than the one submitted for approval to the Food and Drug Administration.
The device is a percutaneously delivered permanent cardiac implant placed in the LAA to prevent the embolization of thrombi formed in the LAA, and is manufactured by Boston Scientific. The FDA approved the Watchman for reducing the risk of thromboembolism from the LAA in patients with nonvalvular atrial fibrillation “who are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores, are deemed by their physicians to be suitable for warfarin; and have an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device, compared to warfarin,” according to a statement issued by the company on March 13.
The approved indication is worded differently than the proposed indication that was submitted to the FDA for approval and discussed at an FDA panel meeting in October, to “prevent thromboembolism from the left atrial appendage.” The changes in the indication include the replacement of “prevent” with “reduce the risk” of thromboembolism, and the addition of the following qualifiers: In patients who “are deemed by their physicians to be suitable for warfarin,” and who have “an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.”
“These changes were made to more accurately reflect the appropriate patient population for this device,” according to an FDA spokesperson.
At a meeting in October 2014, the FDA’s Circulatory System Devices Panel voted 6-5 with one abstention that the benefits of the device outweighed its risks for the proposed indication, but several panelists who voted no said they would support approval of a second-line indication. In addition, panelists voting on both sides of this question said that the indication was too broad and should be revised to describe the device as a second-line alternative to warfarin, making clear it is not appropriate for all warfarin-eligible patients. (At the meeting, the panel unanimously agreed that there was “reasonable assurance” that the device was safe for use in this population.)
At the first advisory panel meeting on the device, in December 2013, the panel voted 13-1 to recommend approval, based on data from the PREVAIL and PROTECT-AF studies, which compared the device to chronic warfarin, and information from the Continued Access to PREVAIL (CAP2) registry.PREVAIL compared implantation of the device – with 45 days of warfarin plus 81 mg of aspirin for 45 days, followed by 325 mg of aspirin and 75 mg of clopidogrel through 6 months, followed by 325 mg of aspirin a day indefinitely – to chronic warfarin.
The October meeting was convened by the FDA to review longer follow-up data from PREVAIL, which found additional cases of ischemic strokes in the Watchman group and none in the warfarin-treated group.
The Watchman device has been available outside of the United States since 2009, is registered in 75 countries, and has been used to treat more than 10,000 patients, according to Boston Scientific.
The Watchman left atrial appendage (LAA) closure device has been approved in the United States as an alternative to warfarin for patients with nonvalvular atrial fibrillation, for a narrower indication than the one submitted for approval to the Food and Drug Administration.
The device is a percutaneously delivered permanent cardiac implant placed in the LAA to prevent the embolization of thrombi formed in the LAA, and is manufactured by Boston Scientific. The FDA approved the Watchman for reducing the risk of thromboembolism from the LAA in patients with nonvalvular atrial fibrillation “who are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores, are deemed by their physicians to be suitable for warfarin; and have an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device, compared to warfarin,” according to a statement issued by the company on March 13.
The approved indication is worded differently than the proposed indication that was submitted to the FDA for approval and discussed at an FDA panel meeting in October, to “prevent thromboembolism from the left atrial appendage.” The changes in the indication include the replacement of “prevent” with “reduce the risk” of thromboembolism, and the addition of the following qualifiers: In patients who “are deemed by their physicians to be suitable for warfarin,” and who have “an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.”
“These changes were made to more accurately reflect the appropriate patient population for this device,” according to an FDA spokesperson.
At a meeting in October 2014, the FDA’s Circulatory System Devices Panel voted 6-5 with one abstention that the benefits of the device outweighed its risks for the proposed indication, but several panelists who voted no said they would support approval of a second-line indication. In addition, panelists voting on both sides of this question said that the indication was too broad and should be revised to describe the device as a second-line alternative to warfarin, making clear it is not appropriate for all warfarin-eligible patients. (At the meeting, the panel unanimously agreed that there was “reasonable assurance” that the device was safe for use in this population.)
At the first advisory panel meeting on the device, in December 2013, the panel voted 13-1 to recommend approval, based on data from the PREVAIL and PROTECT-AF studies, which compared the device to chronic warfarin, and information from the Continued Access to PREVAIL (CAP2) registry.PREVAIL compared implantation of the device – with 45 days of warfarin plus 81 mg of aspirin for 45 days, followed by 325 mg of aspirin and 75 mg of clopidogrel through 6 months, followed by 325 mg of aspirin a day indefinitely – to chronic warfarin.
The October meeting was convened by the FDA to review longer follow-up data from PREVAIL, which found additional cases of ischemic strokes in the Watchman group and none in the warfarin-treated group.
The Watchman device has been available outside of the United States since 2009, is registered in 75 countries, and has been used to treat more than 10,000 patients, according to Boston Scientific.
Apple’s ResearchKit
Doctors have been conjecturing about how the new Apple Watch, with its spectacular fitness and wellness tracking features, will transform health care. The real rock star at Apple’s March 9 “Spring Forward” event, however, was the opening band, ResearchKit.
What is it?
ResearchKit is Apple’s (beautiful) solution to one of the great problems of medical research: recruiting subjects. ResearchKit allows researchers to collect data in a way that before today was impossible: with just a click from their smartphones. The open-source software platform allows developers to design studies and to recruit subjects right from the app store. Researchers can leverage high-tech smartphone sensors and can push out surveys, collecting both objective and subjective data from thousands (heck, potentially millions) of participants.
Five apps were developed for the launch: mPower for Parkinson’s disease, from the University of Rochester, N.Y.; GlucoSuccess for diabetes, from Massachusetts General Hospital, Boston; MyHeart Counts for cardiovascular disease, from Stanford (Calif.) University and the University of Oxford, England; Asthma Health from Mount Sinai and Weill Medical College of Cornell University, New York, N.Y.; and Share the Journey for breast cancer, from the Dana-Farber Cancer Institute, Boston; the University of California, Los Angeles Fielding School of Public Health; and Penn Medicine, Philadelphia.
My take
I took a closer look at MyHeart Counts, which evaluates how patients’ activity levels influence their cardiovascular health. According to Stanford University, a mere 4 days after its release, the MyHeart Counts app had been downloaded 52,900 times in the United States and Canada and had more than 22,000 users who had consented to the study. Try getting that kind of response to your research study with a flyer with tear-off phone number posted in your hospital cafeteria.
I was impressed with its beautiful interface and ease of use. Designed to gather sensor and health data from your iPhone and personal devices, this app is designed to help researchers (and you) detect patterns or details about your heart health. To start, you download the app, give your consent, answer questions about your health and lifestyle, and begin recording your activity with your phone or wearable device. You do a walk test to determine your heart health and potential health risk.
What happens to the data you input? It is sent (with your permission) to a secure database, and your name is replaced with a random code. Your coded and encrypted data are then shared with scientists and physicians to use in medical research.
For this particular study, they ask you to participate 10-15 minutes per day for 1 week, then hope that you can contribute further for 1 week every 3 months answering surveys about your health, lifestyle, and physical activity. Apple reassures users that they can withdraw at any time.
Why? Who cares?
The value proposition for researchers is obvious: The platform provides access to many more subjects than even imaginable. The accelerometer, barometer, gyroscope, and GPS send interesting data to researchers friction free. The Parkinson’s app, for example, uses a cool algorithm and the phone’s microphone to detect symptoms by having patients say “ahhhh.” By pushing out questionnaires regularly, you can collect much more data with shorter intervals for longer periods of time.
The advantages for patients are equally compelling. In addition to sending their data to researchers, they also receive information back from the researchers, helping them monitor their cardiovascular health. In fact, just knowing they are participating in the study might be of benefit. As dermatologist Dr. Steve Feldman of Wake Forest Baptist Medical Center, Winston Salem, N.C., has shown, patients are more likely to adhere to therapies when they know they are being watched, a manifestation of the Hawthorne effect.
Shortcomings
Surely there is a catch? And there is. With potentially millions of participants sending self-reported data, there is the potential that ResearchKit studies glean big, beautiful, bad data. How, for example, could you verify that self-reported asthma patients actually have asthma? Maybe they just read about ResearchKit and wanted to be part of the fun.
For patients, privacy concerns are paramount. Apple promised that no one, not even Apple, will see your data without your permission. But with privacy breaches reported in the news weekly, what can Apple’s assurance mean? Didn’t Target and Aetna promise to keep your data safe as well?
The potential for interesting research is enormous. By the time you read this, I wouldn’t be surprised if a psoriasis study had already launched. In fact, a year from now, the problem might be a dozen or more interesting psoriasis studies all competing for the same patients. Ah, maybe we should be glad if we should be so lucky.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
Doctors have been conjecturing about how the new Apple Watch, with its spectacular fitness and wellness tracking features, will transform health care. The real rock star at Apple’s March 9 “Spring Forward” event, however, was the opening band, ResearchKit.
What is it?
ResearchKit is Apple’s (beautiful) solution to one of the great problems of medical research: recruiting subjects. ResearchKit allows researchers to collect data in a way that before today was impossible: with just a click from their smartphones. The open-source software platform allows developers to design studies and to recruit subjects right from the app store. Researchers can leverage high-tech smartphone sensors and can push out surveys, collecting both objective and subjective data from thousands (heck, potentially millions) of participants.
Five apps were developed for the launch: mPower for Parkinson’s disease, from the University of Rochester, N.Y.; GlucoSuccess for diabetes, from Massachusetts General Hospital, Boston; MyHeart Counts for cardiovascular disease, from Stanford (Calif.) University and the University of Oxford, England; Asthma Health from Mount Sinai and Weill Medical College of Cornell University, New York, N.Y.; and Share the Journey for breast cancer, from the Dana-Farber Cancer Institute, Boston; the University of California, Los Angeles Fielding School of Public Health; and Penn Medicine, Philadelphia.
My take
I took a closer look at MyHeart Counts, which evaluates how patients’ activity levels influence their cardiovascular health. According to Stanford University, a mere 4 days after its release, the MyHeart Counts app had been downloaded 52,900 times in the United States and Canada and had more than 22,000 users who had consented to the study. Try getting that kind of response to your research study with a flyer with tear-off phone number posted in your hospital cafeteria.
I was impressed with its beautiful interface and ease of use. Designed to gather sensor and health data from your iPhone and personal devices, this app is designed to help researchers (and you) detect patterns or details about your heart health. To start, you download the app, give your consent, answer questions about your health and lifestyle, and begin recording your activity with your phone or wearable device. You do a walk test to determine your heart health and potential health risk.
What happens to the data you input? It is sent (with your permission) to a secure database, and your name is replaced with a random code. Your coded and encrypted data are then shared with scientists and physicians to use in medical research.
For this particular study, they ask you to participate 10-15 minutes per day for 1 week, then hope that you can contribute further for 1 week every 3 months answering surveys about your health, lifestyle, and physical activity. Apple reassures users that they can withdraw at any time.
Why? Who cares?
The value proposition for researchers is obvious: The platform provides access to many more subjects than even imaginable. The accelerometer, barometer, gyroscope, and GPS send interesting data to researchers friction free. The Parkinson’s app, for example, uses a cool algorithm and the phone’s microphone to detect symptoms by having patients say “ahhhh.” By pushing out questionnaires regularly, you can collect much more data with shorter intervals for longer periods of time.
The advantages for patients are equally compelling. In addition to sending their data to researchers, they also receive information back from the researchers, helping them monitor their cardiovascular health. In fact, just knowing they are participating in the study might be of benefit. As dermatologist Dr. Steve Feldman of Wake Forest Baptist Medical Center, Winston Salem, N.C., has shown, patients are more likely to adhere to therapies when they know they are being watched, a manifestation of the Hawthorne effect.
Shortcomings
Surely there is a catch? And there is. With potentially millions of participants sending self-reported data, there is the potential that ResearchKit studies glean big, beautiful, bad data. How, for example, could you verify that self-reported asthma patients actually have asthma? Maybe they just read about ResearchKit and wanted to be part of the fun.
For patients, privacy concerns are paramount. Apple promised that no one, not even Apple, will see your data without your permission. But with privacy breaches reported in the news weekly, what can Apple’s assurance mean? Didn’t Target and Aetna promise to keep your data safe as well?
The potential for interesting research is enormous. By the time you read this, I wouldn’t be surprised if a psoriasis study had already launched. In fact, a year from now, the problem might be a dozen or more interesting psoriasis studies all competing for the same patients. Ah, maybe we should be glad if we should be so lucky.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
Doctors have been conjecturing about how the new Apple Watch, with its spectacular fitness and wellness tracking features, will transform health care. The real rock star at Apple’s March 9 “Spring Forward” event, however, was the opening band, ResearchKit.
What is it?
ResearchKit is Apple’s (beautiful) solution to one of the great problems of medical research: recruiting subjects. ResearchKit allows researchers to collect data in a way that before today was impossible: with just a click from their smartphones. The open-source software platform allows developers to design studies and to recruit subjects right from the app store. Researchers can leverage high-tech smartphone sensors and can push out surveys, collecting both objective and subjective data from thousands (heck, potentially millions) of participants.
Five apps were developed for the launch: mPower for Parkinson’s disease, from the University of Rochester, N.Y.; GlucoSuccess for diabetes, from Massachusetts General Hospital, Boston; MyHeart Counts for cardiovascular disease, from Stanford (Calif.) University and the University of Oxford, England; Asthma Health from Mount Sinai and Weill Medical College of Cornell University, New York, N.Y.; and Share the Journey for breast cancer, from the Dana-Farber Cancer Institute, Boston; the University of California, Los Angeles Fielding School of Public Health; and Penn Medicine, Philadelphia.
My take
I took a closer look at MyHeart Counts, which evaluates how patients’ activity levels influence their cardiovascular health. According to Stanford University, a mere 4 days after its release, the MyHeart Counts app had been downloaded 52,900 times in the United States and Canada and had more than 22,000 users who had consented to the study. Try getting that kind of response to your research study with a flyer with tear-off phone number posted in your hospital cafeteria.
I was impressed with its beautiful interface and ease of use. Designed to gather sensor and health data from your iPhone and personal devices, this app is designed to help researchers (and you) detect patterns or details about your heart health. To start, you download the app, give your consent, answer questions about your health and lifestyle, and begin recording your activity with your phone or wearable device. You do a walk test to determine your heart health and potential health risk.
What happens to the data you input? It is sent (with your permission) to a secure database, and your name is replaced with a random code. Your coded and encrypted data are then shared with scientists and physicians to use in medical research.
For this particular study, they ask you to participate 10-15 minutes per day for 1 week, then hope that you can contribute further for 1 week every 3 months answering surveys about your health, lifestyle, and physical activity. Apple reassures users that they can withdraw at any time.
Why? Who cares?
The value proposition for researchers is obvious: The platform provides access to many more subjects than even imaginable. The accelerometer, barometer, gyroscope, and GPS send interesting data to researchers friction free. The Parkinson’s app, for example, uses a cool algorithm and the phone’s microphone to detect symptoms by having patients say “ahhhh.” By pushing out questionnaires regularly, you can collect much more data with shorter intervals for longer periods of time.
The advantages for patients are equally compelling. In addition to sending their data to researchers, they also receive information back from the researchers, helping them monitor their cardiovascular health. In fact, just knowing they are participating in the study might be of benefit. As dermatologist Dr. Steve Feldman of Wake Forest Baptist Medical Center, Winston Salem, N.C., has shown, patients are more likely to adhere to therapies when they know they are being watched, a manifestation of the Hawthorne effect.
Shortcomings
Surely there is a catch? And there is. With potentially millions of participants sending self-reported data, there is the potential that ResearchKit studies glean big, beautiful, bad data. How, for example, could you verify that self-reported asthma patients actually have asthma? Maybe they just read about ResearchKit and wanted to be part of the fun.
For patients, privacy concerns are paramount. Apple promised that no one, not even Apple, will see your data without your permission. But with privacy breaches reported in the news weekly, what can Apple’s assurance mean? Didn’t Target and Aetna promise to keep your data safe as well?
The potential for interesting research is enormous. By the time you read this, I wouldn’t be surprised if a psoriasis study had already launched. In fact, a year from now, the problem might be a dozen or more interesting psoriasis studies all competing for the same patients. Ah, maybe we should be glad if we should be so lucky.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
Addressing pain at the end of life
A few months ago, a colleague asked me about treating a patient’s pain that he was managing for months both in and out of the hospital for what was now an incurable condition. This very skilled surgeon believed that the patient should “not require” such high doses of opioids based on the clinical picture of a healed surgical wound but felt at a loss of what else to do. He did not want to abandon his relationship with the patient. He considered referral to the anesthesia pain clinic as escalating pain requirements were exceeding his comfort level.
Alternatively, he considered deferring pain management to the patient’s primary care provider. Instead, we worked together through a rational pain approach and explored external factors that may have been contributing to the patient’s total pain experience. This brief vignette is not atypical and sheds light onto the ongoing need to fill an education gap for surgeons who deal with patients at the end of life.
It has been almost 25 years since the term “pain as the fifth vital sign” was first introduced into the lexicon of clinical practice. The idea was to provide as much zeal to the topic of pain as we do to a patient’s other vital physiological measures. Yet, seriously ill patients with potential life-limiting conditions continue to experience significant pain, especially at the end of life. Among patients with nonmalignant diagnoses, more than 40% experience severe pain within days of their death. For those with malignant conditions, 15%-75% report moderate to severe pain during the final weeks of life. Whether in the ICU, hospital ward, or outpatient setting, our surgical community struggles to provide effective symptomatic pain control in many patients who have transitioned from a curative pathway to one of comfort.
Although we never intend to allow patients to suffer at the end of life, barriers to appropriate pain control persist. In some case cases, patients may feel embarrassed or ashamed to accept escalating opioid doses. In other cases, patients and families may possess misconceptions about addiction to pain medication. It is important to dispel such myths and distinguish tolerance from dependence. Among opioid-naive patients, the risk of dependence (in other words, addiction) is estimated to be 0.1%. Among patients with a history of opioid abuse, the risk of addiction is still only 1%.
Large proportions of physicians continue to report inadequate training in pain control and are reluctant to prescribe high-enough doses of opioids to relieve pain, even at the end of life. One well-described reason has been physician fear of regulatory action and possible litigation for higher than typical opioid dosing.
This was the case for my colleague who was reluctant to escalate pain control.
This in turn leads to undertreating pain which, in fact, has been a source of successful litigation. Because undertreatment of pain may be akin to patient negligence, we should strive to become more comfortable with optimal pain treatment strategies. But pain control is not merely about intravenous opioids or pain tablets. Surgeons should at least have an appreciation for, if not a better understanding, of the modern palliative care approach to “total pain.” This construct consists of four interrelated pain domains: physical, psychological (emotional), spiritual, and social.
Although we tend to focus on physical pain, other domains are influenced by anxiety, depression, and fear. If such an approach seems a bridge too far, optimal care should involve a multidisciplinary team that touches on such areas. This may be most efficiently achieved through consultation and coordination with palliative care services when available. This patient’s surgeon soon discovered that family financial concerns were contributing to the patient’s sleepless nights and worsening somatic pain.
Somewhat outside the scope of typical postoperative care, pain relief at the end of life requires dosing and medication choices for extended periods of time. When establishing a treatment strategy, the surgeon should consider the feasibility and efficacy (half-life, duration, bioavailability, active metabolites) of each modality. In our patient, standard dosing was inadequate; for some, basal doses may increase by 25%-100% for progressive disease. To support the surgeon in learning more about this important area of care, multiple online tools and websites are available to assist with pain management choices. A short while ago, I learned from my colleague that this patient died comfortably and essentially pain free for the last months of his life.
Dr. Zonies is an associate professor of surgery in the trauma/critical care division at Oregon Health & Science University, Portland. He is board certified in hospice and palliative medicine.
A few months ago, a colleague asked me about treating a patient’s pain that he was managing for months both in and out of the hospital for what was now an incurable condition. This very skilled surgeon believed that the patient should “not require” such high doses of opioids based on the clinical picture of a healed surgical wound but felt at a loss of what else to do. He did not want to abandon his relationship with the patient. He considered referral to the anesthesia pain clinic as escalating pain requirements were exceeding his comfort level.
Alternatively, he considered deferring pain management to the patient’s primary care provider. Instead, we worked together through a rational pain approach and explored external factors that may have been contributing to the patient’s total pain experience. This brief vignette is not atypical and sheds light onto the ongoing need to fill an education gap for surgeons who deal with patients at the end of life.
It has been almost 25 years since the term “pain as the fifth vital sign” was first introduced into the lexicon of clinical practice. The idea was to provide as much zeal to the topic of pain as we do to a patient’s other vital physiological measures. Yet, seriously ill patients with potential life-limiting conditions continue to experience significant pain, especially at the end of life. Among patients with nonmalignant diagnoses, more than 40% experience severe pain within days of their death. For those with malignant conditions, 15%-75% report moderate to severe pain during the final weeks of life. Whether in the ICU, hospital ward, or outpatient setting, our surgical community struggles to provide effective symptomatic pain control in many patients who have transitioned from a curative pathway to one of comfort.
Although we never intend to allow patients to suffer at the end of life, barriers to appropriate pain control persist. In some case cases, patients may feel embarrassed or ashamed to accept escalating opioid doses. In other cases, patients and families may possess misconceptions about addiction to pain medication. It is important to dispel such myths and distinguish tolerance from dependence. Among opioid-naive patients, the risk of dependence (in other words, addiction) is estimated to be 0.1%. Among patients with a history of opioid abuse, the risk of addiction is still only 1%.
Large proportions of physicians continue to report inadequate training in pain control and are reluctant to prescribe high-enough doses of opioids to relieve pain, even at the end of life. One well-described reason has been physician fear of regulatory action and possible litigation for higher than typical opioid dosing.
This was the case for my colleague who was reluctant to escalate pain control.
This in turn leads to undertreating pain which, in fact, has been a source of successful litigation. Because undertreatment of pain may be akin to patient negligence, we should strive to become more comfortable with optimal pain treatment strategies. But pain control is not merely about intravenous opioids or pain tablets. Surgeons should at least have an appreciation for, if not a better understanding, of the modern palliative care approach to “total pain.” This construct consists of four interrelated pain domains: physical, psychological (emotional), spiritual, and social.
Although we tend to focus on physical pain, other domains are influenced by anxiety, depression, and fear. If such an approach seems a bridge too far, optimal care should involve a multidisciplinary team that touches on such areas. This may be most efficiently achieved through consultation and coordination with palliative care services when available. This patient’s surgeon soon discovered that family financial concerns were contributing to the patient’s sleepless nights and worsening somatic pain.
Somewhat outside the scope of typical postoperative care, pain relief at the end of life requires dosing and medication choices for extended periods of time. When establishing a treatment strategy, the surgeon should consider the feasibility and efficacy (half-life, duration, bioavailability, active metabolites) of each modality. In our patient, standard dosing was inadequate; for some, basal doses may increase by 25%-100% for progressive disease. To support the surgeon in learning more about this important area of care, multiple online tools and websites are available to assist with pain management choices. A short while ago, I learned from my colleague that this patient died comfortably and essentially pain free for the last months of his life.
Dr. Zonies is an associate professor of surgery in the trauma/critical care division at Oregon Health & Science University, Portland. He is board certified in hospice and palliative medicine.
A few months ago, a colleague asked me about treating a patient’s pain that he was managing for months both in and out of the hospital for what was now an incurable condition. This very skilled surgeon believed that the patient should “not require” such high doses of opioids based on the clinical picture of a healed surgical wound but felt at a loss of what else to do. He did not want to abandon his relationship with the patient. He considered referral to the anesthesia pain clinic as escalating pain requirements were exceeding his comfort level.
Alternatively, he considered deferring pain management to the patient’s primary care provider. Instead, we worked together through a rational pain approach and explored external factors that may have been contributing to the patient’s total pain experience. This brief vignette is not atypical and sheds light onto the ongoing need to fill an education gap for surgeons who deal with patients at the end of life.
It has been almost 25 years since the term “pain as the fifth vital sign” was first introduced into the lexicon of clinical practice. The idea was to provide as much zeal to the topic of pain as we do to a patient’s other vital physiological measures. Yet, seriously ill patients with potential life-limiting conditions continue to experience significant pain, especially at the end of life. Among patients with nonmalignant diagnoses, more than 40% experience severe pain within days of their death. For those with malignant conditions, 15%-75% report moderate to severe pain during the final weeks of life. Whether in the ICU, hospital ward, or outpatient setting, our surgical community struggles to provide effective symptomatic pain control in many patients who have transitioned from a curative pathway to one of comfort.
Although we never intend to allow patients to suffer at the end of life, barriers to appropriate pain control persist. In some case cases, patients may feel embarrassed or ashamed to accept escalating opioid doses. In other cases, patients and families may possess misconceptions about addiction to pain medication. It is important to dispel such myths and distinguish tolerance from dependence. Among opioid-naive patients, the risk of dependence (in other words, addiction) is estimated to be 0.1%. Among patients with a history of opioid abuse, the risk of addiction is still only 1%.
Large proportions of physicians continue to report inadequate training in pain control and are reluctant to prescribe high-enough doses of opioids to relieve pain, even at the end of life. One well-described reason has been physician fear of regulatory action and possible litigation for higher than typical opioid dosing.
This was the case for my colleague who was reluctant to escalate pain control.
This in turn leads to undertreating pain which, in fact, has been a source of successful litigation. Because undertreatment of pain may be akin to patient negligence, we should strive to become more comfortable with optimal pain treatment strategies. But pain control is not merely about intravenous opioids or pain tablets. Surgeons should at least have an appreciation for, if not a better understanding, of the modern palliative care approach to “total pain.” This construct consists of four interrelated pain domains: physical, psychological (emotional), spiritual, and social.
Although we tend to focus on physical pain, other domains are influenced by anxiety, depression, and fear. If such an approach seems a bridge too far, optimal care should involve a multidisciplinary team that touches on such areas. This may be most efficiently achieved through consultation and coordination with palliative care services when available. This patient’s surgeon soon discovered that family financial concerns were contributing to the patient’s sleepless nights and worsening somatic pain.
Somewhat outside the scope of typical postoperative care, pain relief at the end of life requires dosing and medication choices for extended periods of time. When establishing a treatment strategy, the surgeon should consider the feasibility and efficacy (half-life, duration, bioavailability, active metabolites) of each modality. In our patient, standard dosing was inadequate; for some, basal doses may increase by 25%-100% for progressive disease. To support the surgeon in learning more about this important area of care, multiple online tools and websites are available to assist with pain management choices. A short while ago, I learned from my colleague that this patient died comfortably and essentially pain free for the last months of his life.
Dr. Zonies is an associate professor of surgery in the trauma/critical care division at Oregon Health & Science University, Portland. He is board certified in hospice and palliative medicine.
Sharpening the saw
Recently, I wrote that springtime is an excellent time to spruce up your office, to check your equipment for malfunctions, to resharpen your curettes and scissors, and to back up your computer files and upgrade software. More important than any of that, though, is reevaluating your most important asset: yourself.
I write this reminder every couple of years because it’s so easy to lose sight of the big picture among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a piano or sailing lesson; or a long weekend away with my wife. And I take no less than 4 weeks vacation per year.
I know how some of you feel about “wasting” a workday. Vacations are even worse, because patients might go elsewhere while we’re gone, and every day the office is idle we “lose money.”
That whole paradigm is wrong. Stop thinking day to day; think year to year instead. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacation days; it all averages out in the end.
Besides, this is much more important than money. This is breaking the routine, clearing the cobwebs, living your life. Trust me – your practice will still be there when you return.
Last month, my wife and I hiked up a mountain in the Himalayas to the fabled Tiger’s Nest Monastery in Bhutan. As I huffed and puffed up the trail, I didn’t have the time – or the slightest inclination – to worry about the office. When the trek was over, I returned ready to take on the world, and my practice, anew.
And I jotted down some great ideas – practical, medical, and literary. Original thoughts are hard to come by during the daily grind, but they often appear, unannounced, in a new and refreshing environment.
Creative people have long recognized the value of “sharpening the saw.” A classic example is the oft-told story of Swiss physicist K. Alex Müller and German physicist J. Georg Bednorz. In 1986, they reached a major impasse in their superconductivity research; it appeared 2 decades of work might be for naught. The harder they pressed, the more elusive the answer became. So Müller decided to take some time off, put aside his troubles, and research a subject that had always interested him: ceramics.
Nothing could have been further from his research field, of course, since ceramics are among the poorest conductors known. Yet, as he relaxed, it occurred to Müller that a unique property of ceramics might apply to their project. Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor.
The rest, as they say, is history; Müller and Bednorz won a Nobel Prize and triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically levitated trains, and many other applications that are still being realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at the same old problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Recently, I wrote that springtime is an excellent time to spruce up your office, to check your equipment for malfunctions, to resharpen your curettes and scissors, and to back up your computer files and upgrade software. More important than any of that, though, is reevaluating your most important asset: yourself.
I write this reminder every couple of years because it’s so easy to lose sight of the big picture among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a piano or sailing lesson; or a long weekend away with my wife. And I take no less than 4 weeks vacation per year.
I know how some of you feel about “wasting” a workday. Vacations are even worse, because patients might go elsewhere while we’re gone, and every day the office is idle we “lose money.”
That whole paradigm is wrong. Stop thinking day to day; think year to year instead. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacation days; it all averages out in the end.
Besides, this is much more important than money. This is breaking the routine, clearing the cobwebs, living your life. Trust me – your practice will still be there when you return.
Last month, my wife and I hiked up a mountain in the Himalayas to the fabled Tiger’s Nest Monastery in Bhutan. As I huffed and puffed up the trail, I didn’t have the time – or the slightest inclination – to worry about the office. When the trek was over, I returned ready to take on the world, and my practice, anew.
And I jotted down some great ideas – practical, medical, and literary. Original thoughts are hard to come by during the daily grind, but they often appear, unannounced, in a new and refreshing environment.
Creative people have long recognized the value of “sharpening the saw.” A classic example is the oft-told story of Swiss physicist K. Alex Müller and German physicist J. Georg Bednorz. In 1986, they reached a major impasse in their superconductivity research; it appeared 2 decades of work might be for naught. The harder they pressed, the more elusive the answer became. So Müller decided to take some time off, put aside his troubles, and research a subject that had always interested him: ceramics.
Nothing could have been further from his research field, of course, since ceramics are among the poorest conductors known. Yet, as he relaxed, it occurred to Müller that a unique property of ceramics might apply to their project. Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor.
The rest, as they say, is history; Müller and Bednorz won a Nobel Prize and triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically levitated trains, and many other applications that are still being realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at the same old problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Recently, I wrote that springtime is an excellent time to spruce up your office, to check your equipment for malfunctions, to resharpen your curettes and scissors, and to back up your computer files and upgrade software. More important than any of that, though, is reevaluating your most important asset: yourself.
I write this reminder every couple of years because it’s so easy to lose sight of the big picture among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a piano or sailing lesson; or a long weekend away with my wife. And I take no less than 4 weeks vacation per year.
I know how some of you feel about “wasting” a workday. Vacations are even worse, because patients might go elsewhere while we’re gone, and every day the office is idle we “lose money.”
That whole paradigm is wrong. Stop thinking day to day; think year to year instead. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacation days; it all averages out in the end.
Besides, this is much more important than money. This is breaking the routine, clearing the cobwebs, living your life. Trust me – your practice will still be there when you return.
Last month, my wife and I hiked up a mountain in the Himalayas to the fabled Tiger’s Nest Monastery in Bhutan. As I huffed and puffed up the trail, I didn’t have the time – or the slightest inclination – to worry about the office. When the trek was over, I returned ready to take on the world, and my practice, anew.
And I jotted down some great ideas – practical, medical, and literary. Original thoughts are hard to come by during the daily grind, but they often appear, unannounced, in a new and refreshing environment.
Creative people have long recognized the value of “sharpening the saw.” A classic example is the oft-told story of Swiss physicist K. Alex Müller and German physicist J. Georg Bednorz. In 1986, they reached a major impasse in their superconductivity research; it appeared 2 decades of work might be for naught. The harder they pressed, the more elusive the answer became. So Müller decided to take some time off, put aside his troubles, and research a subject that had always interested him: ceramics.
Nothing could have been further from his research field, of course, since ceramics are among the poorest conductors known. Yet, as he relaxed, it occurred to Müller that a unique property of ceramics might apply to their project. Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor.
The rest, as they say, is history; Müller and Bednorz won a Nobel Prize and triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically levitated trains, and many other applications that are still being realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at the same old problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
From the Washington Office
In November 2014, the Centers for Medicare & Medicaid Services finalized a policy that will transition all 10-day and 90-day global codes to 0-day global codes in 2017 and 2018, respectively.
As most surgeons will know, global codes include all necessary services normally furnished before, during, and after a surgical procedure. Approximately 4,200 of the more than 9,900 Current Procedural Terminology codes are 10-day or 90-day global codes. The CMS claims the transition is necessary, in part, to increase the accuracy of payment for these codes. Despite the fact that the policy for the 10-day codes will be put into effect in 2017, the CMS has yet to develop a methodology for making this transition.
Prior to the release of the final rule, the ACS sent a detailed letter to the CMS asserting that the agency should postpone moving forward with this proposal until a comprehensive analysis of the effect on surgical patients and access to surgical care was completed. The ACS included recommendations on a number of issues that the CMS must resolve before moving forward with the proposed policy and stressed, above all, that the CMS should not make policy changes that infringe on surgeons’ ability to provide high-quality care to surgical patients. Despite the ACS regulatory advocacy efforts and those similar from other surgical and medical specialty groups, the CMS finalized the rule and continues to indicate that it plans to move forward.
During the lame duck session of Congress following the November election, a coalition of surgical groups led by the ACS drafted and provided to Congress legislative language the effect of which would be to preclude the CMS from moving forward with its plan to transition the 10-day and 90-day global codes to 0-day global codes.
The groups involved mounted an aggressive campaign, strongly advocating for the inclusion of the legislative language in the “CRomnibus” bill. Despite strong support from the Congressional “Doc Caucus” and other members of Congress, no language addressing transitioning of the global codes was included in the “CRomnibus” bill, which passed both chambers at the conclusion of the 113th Congress.
Now that the 114th Congress has begun, the ACS, working again in concert with the aforementioned coalition of other surgical and medical specialty groups, is taking a variety of strategic actions on both the legislative and regulatory fronts. Working with key members of Congress including Rep. Larry Bucshon, MD, FACS, Rep. Tom Price, MD, FACS, and Rep. Dan Benishek, MD, FACS, the ACS will continue to oppose implementation of the policy change by seeking congressional intervention to rescind the rule until such time as the CMS can ensure that the transition will not have a negative impact on patients and can be implemented in a way that accurately accounts for the care that surgeons provide.
Revised legislative language has been provided to Congress. Members of the ACS DAHP legislative affairs staff are engaged in daily advocacy efforts for inclusion of that language in legislation. ACS leaders and DAHP regulatory affairs staff have met with the CMS in an attempt to provide education concerning what is believed will be significant, negative impact of the policy on both patients and surgeons.
Fellows are encouraged to augment the efforts of the DAHP by personally contacting their senators and representatives to educate them about the following negative potential consequences the implementation of this policy would be expected to have:
1. Reduces patient access and quality of care. If 10-day and 90-day global codes are transitioned to 0-day global codes, patients will have a copay for the procedure and additional, separate copays for other services including each of the follow-up visits. Patients may also be responsible for separate payment of supplies and drugs necessary during postop visits currently bundled into the global payment, but not bundled into visit codes. This could considerably increase the financial burden on patients, or worse, discourage them from returning for follow-up care.
2. Undermines the current SGR legislation and other Medicare reform initiatives. The CMS initiatives for payment are all moving toward larger bundled payments. Deconstruction of the current payment structure for physicians is counterintuitive to the end goal of providing more comprehensive and coordinated care for patients.
3. Increases administrative burden. The administrative burden on surgical practices, the CMS, and its contractors will be significant. The American Medical Association estimates that eliminating the global package will result in 63 million additional claims per year to account for postsurgical evaluation and management services. This will add unnecessary costs to the claims processing system.
4. Obstructs clinical registry data collection and quality improvement. If patients forgo follow-up treatment or seek it from other providers, the policy would have a deleterious effect on surgeons’ ability to collect information on patient outcomes in clinical registries and undermine many meaningful quality improvement initiatives.
Staff members of the DC office are available to assist surgeons interested in contacting their individual senators and representatives to assist in the advocacy efforts relative to this policy. I can be reached by phone at 202-337-2701 or by e-mail at [email protected].
Until next month …
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
In November 2014, the Centers for Medicare & Medicaid Services finalized a policy that will transition all 10-day and 90-day global codes to 0-day global codes in 2017 and 2018, respectively.
As most surgeons will know, global codes include all necessary services normally furnished before, during, and after a surgical procedure. Approximately 4,200 of the more than 9,900 Current Procedural Terminology codes are 10-day or 90-day global codes. The CMS claims the transition is necessary, in part, to increase the accuracy of payment for these codes. Despite the fact that the policy for the 10-day codes will be put into effect in 2017, the CMS has yet to develop a methodology for making this transition.
Prior to the release of the final rule, the ACS sent a detailed letter to the CMS asserting that the agency should postpone moving forward with this proposal until a comprehensive analysis of the effect on surgical patients and access to surgical care was completed. The ACS included recommendations on a number of issues that the CMS must resolve before moving forward with the proposed policy and stressed, above all, that the CMS should not make policy changes that infringe on surgeons’ ability to provide high-quality care to surgical patients. Despite the ACS regulatory advocacy efforts and those similar from other surgical and medical specialty groups, the CMS finalized the rule and continues to indicate that it plans to move forward.
During the lame duck session of Congress following the November election, a coalition of surgical groups led by the ACS drafted and provided to Congress legislative language the effect of which would be to preclude the CMS from moving forward with its plan to transition the 10-day and 90-day global codes to 0-day global codes.
The groups involved mounted an aggressive campaign, strongly advocating for the inclusion of the legislative language in the “CRomnibus” bill. Despite strong support from the Congressional “Doc Caucus” and other members of Congress, no language addressing transitioning of the global codes was included in the “CRomnibus” bill, which passed both chambers at the conclusion of the 113th Congress.
Now that the 114th Congress has begun, the ACS, working again in concert with the aforementioned coalition of other surgical and medical specialty groups, is taking a variety of strategic actions on both the legislative and regulatory fronts. Working with key members of Congress including Rep. Larry Bucshon, MD, FACS, Rep. Tom Price, MD, FACS, and Rep. Dan Benishek, MD, FACS, the ACS will continue to oppose implementation of the policy change by seeking congressional intervention to rescind the rule until such time as the CMS can ensure that the transition will not have a negative impact on patients and can be implemented in a way that accurately accounts for the care that surgeons provide.
Revised legislative language has been provided to Congress. Members of the ACS DAHP legislative affairs staff are engaged in daily advocacy efforts for inclusion of that language in legislation. ACS leaders and DAHP regulatory affairs staff have met with the CMS in an attempt to provide education concerning what is believed will be significant, negative impact of the policy on both patients and surgeons.
Fellows are encouraged to augment the efforts of the DAHP by personally contacting their senators and representatives to educate them about the following negative potential consequences the implementation of this policy would be expected to have:
1. Reduces patient access and quality of care. If 10-day and 90-day global codes are transitioned to 0-day global codes, patients will have a copay for the procedure and additional, separate copays for other services including each of the follow-up visits. Patients may also be responsible for separate payment of supplies and drugs necessary during postop visits currently bundled into the global payment, but not bundled into visit codes. This could considerably increase the financial burden on patients, or worse, discourage them from returning for follow-up care.
2. Undermines the current SGR legislation and other Medicare reform initiatives. The CMS initiatives for payment are all moving toward larger bundled payments. Deconstruction of the current payment structure for physicians is counterintuitive to the end goal of providing more comprehensive and coordinated care for patients.
3. Increases administrative burden. The administrative burden on surgical practices, the CMS, and its contractors will be significant. The American Medical Association estimates that eliminating the global package will result in 63 million additional claims per year to account for postsurgical evaluation and management services. This will add unnecessary costs to the claims processing system.
4. Obstructs clinical registry data collection and quality improvement. If patients forgo follow-up treatment or seek it from other providers, the policy would have a deleterious effect on surgeons’ ability to collect information on patient outcomes in clinical registries and undermine many meaningful quality improvement initiatives.
Staff members of the DC office are available to assist surgeons interested in contacting their individual senators and representatives to assist in the advocacy efforts relative to this policy. I can be reached by phone at 202-337-2701 or by e-mail at [email protected].
Until next month …
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
In November 2014, the Centers for Medicare & Medicaid Services finalized a policy that will transition all 10-day and 90-day global codes to 0-day global codes in 2017 and 2018, respectively.
As most surgeons will know, global codes include all necessary services normally furnished before, during, and after a surgical procedure. Approximately 4,200 of the more than 9,900 Current Procedural Terminology codes are 10-day or 90-day global codes. The CMS claims the transition is necessary, in part, to increase the accuracy of payment for these codes. Despite the fact that the policy for the 10-day codes will be put into effect in 2017, the CMS has yet to develop a methodology for making this transition.
Prior to the release of the final rule, the ACS sent a detailed letter to the CMS asserting that the agency should postpone moving forward with this proposal until a comprehensive analysis of the effect on surgical patients and access to surgical care was completed. The ACS included recommendations on a number of issues that the CMS must resolve before moving forward with the proposed policy and stressed, above all, that the CMS should not make policy changes that infringe on surgeons’ ability to provide high-quality care to surgical patients. Despite the ACS regulatory advocacy efforts and those similar from other surgical and medical specialty groups, the CMS finalized the rule and continues to indicate that it plans to move forward.
During the lame duck session of Congress following the November election, a coalition of surgical groups led by the ACS drafted and provided to Congress legislative language the effect of which would be to preclude the CMS from moving forward with its plan to transition the 10-day and 90-day global codes to 0-day global codes.
The groups involved mounted an aggressive campaign, strongly advocating for the inclusion of the legislative language in the “CRomnibus” bill. Despite strong support from the Congressional “Doc Caucus” and other members of Congress, no language addressing transitioning of the global codes was included in the “CRomnibus” bill, which passed both chambers at the conclusion of the 113th Congress.
Now that the 114th Congress has begun, the ACS, working again in concert with the aforementioned coalition of other surgical and medical specialty groups, is taking a variety of strategic actions on both the legislative and regulatory fronts. Working with key members of Congress including Rep. Larry Bucshon, MD, FACS, Rep. Tom Price, MD, FACS, and Rep. Dan Benishek, MD, FACS, the ACS will continue to oppose implementation of the policy change by seeking congressional intervention to rescind the rule until such time as the CMS can ensure that the transition will not have a negative impact on patients and can be implemented in a way that accurately accounts for the care that surgeons provide.
Revised legislative language has been provided to Congress. Members of the ACS DAHP legislative affairs staff are engaged in daily advocacy efforts for inclusion of that language in legislation. ACS leaders and DAHP regulatory affairs staff have met with the CMS in an attempt to provide education concerning what is believed will be significant, negative impact of the policy on both patients and surgeons.
Fellows are encouraged to augment the efforts of the DAHP by personally contacting their senators and representatives to educate them about the following negative potential consequences the implementation of this policy would be expected to have:
1. Reduces patient access and quality of care. If 10-day and 90-day global codes are transitioned to 0-day global codes, patients will have a copay for the procedure and additional, separate copays for other services including each of the follow-up visits. Patients may also be responsible for separate payment of supplies and drugs necessary during postop visits currently bundled into the global payment, but not bundled into visit codes. This could considerably increase the financial burden on patients, or worse, discourage them from returning for follow-up care.
2. Undermines the current SGR legislation and other Medicare reform initiatives. The CMS initiatives for payment are all moving toward larger bundled payments. Deconstruction of the current payment structure for physicians is counterintuitive to the end goal of providing more comprehensive and coordinated care for patients.
3. Increases administrative burden. The administrative burden on surgical practices, the CMS, and its contractors will be significant. The American Medical Association estimates that eliminating the global package will result in 63 million additional claims per year to account for postsurgical evaluation and management services. This will add unnecessary costs to the claims processing system.
4. Obstructs clinical registry data collection and quality improvement. If patients forgo follow-up treatment or seek it from other providers, the policy would have a deleterious effect on surgeons’ ability to collect information on patient outcomes in clinical registries and undermine many meaningful quality improvement initiatives.
Staff members of the DC office are available to assist surgeons interested in contacting their individual senators and representatives to assist in the advocacy efforts relative to this policy. I can be reached by phone at 202-337-2701 or by e-mail at [email protected].
Until next month …
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
Practicing surgery and having a life
For those who have chosen the surgeon’s path, finding a sustainable work/life balance is challenging. For surgeons with young families and spousal responsibilities, achieving that balance may seem like an unattainable goal. As a rural surgeon with a spouse and children, I am here to say that rural practice has many benefits to those who love their work and also want a balanced life. I recommend that young surgeons, women in particular, consider this path for both the professional and personal advantages it offers.
I always tell the medical students that rotate with me, “When it is 4 o’clock in the morning and you forgot to go home ... be that.” That is to say, whatever rotation they are on when they have that feeling, that is the specialty they should choose. That was us when it came to surgery. When we were students going through surgical rotations we could not get enough. We wanted to see everything, do everything, and we didn’t want to miss anything. Many of our colleagues recommended against choosing surgery. We were told, “You’ll never have a life or a family;” “Your life will be horrible,” etc.
Now, many years later, I still love what I do as a surgeon. But I also love the other half of my life. I chose to practice rural surgery because I sensed that a balanced life would be possible in the rural setting. So I offer the following 10 tips for young surgeons who might be considering a rural practice:
1. Pick a good small town
Sit down and write out a list of all the things you want in a small town. My list, of course, will be different from your list but most importantly, make the list! It is no use taking a job in rural Colorado if you hate to hunt and fish. Consider the town and the job equally. For women surgeons, you may want to look for a place where there are women in upper administrative roles and other female physicians on staff. All small towns are definitely not alike and if you pick one for the hospital or the job without considering your surroundings, you will not likely be happy.
2. Don’t commute
Some people take a job in a small town or a rural setting but choose to live 30 miles away in the next larger town. I suggest living in the town you practice in. If at all possible, live close to the hospital. Every minute that you spend on the road is a minute away from your family. Living far away makes that 2 a.m. call from the ER that much more painful. You also miss out on the opportunity to become a real part of your community.
3. Choose the right partner
Small town physicians are tough to keep. Rural practice turnover is high. One of the most common reasons I hear for why a physician has left the area is because his or her spouse wasn’t happy. No matter how appealing the job seems, no matter how much bonus money they offer, no matter how great the hospital appears, if your spouse doesn’t like it there, you’ll be leaving. You may love your job and back country camping every weekend, but if your spouse pines for Whole Foods and the opera, you will be moving on eventually.
A large part of rural life revolves around events in the school system, and even if you have no young children of your own, it is helpful to make an effort to attend some of these events, if only to support your neighbor’s kids. It goes a long way to establish you as someone who is involved in the community and who cares. Remember, it is the mothers young and old who make many of the health care decisions for their families. Volunteer for events or get involved in the science program at the high school. You may find that you are a unique role model for teenagers in your community.
4. Choose the right partners
Rural general surgery is challenging enough without having to compete with those around you. Surgery in the small town setting becomes infinitely more pleasant when you have good partners. Is the practice set up to help and support each partner, or are you pitted against one another, competing for RVUs? Would your partners tolerate covering your call for 3 months of maternity leave or would this cause resentment? Look for a practice where the surgeons work together and cover for each other, and your small town life will be greatly enhanced.
5. Live within your means
A small town surgical practice can make for a very comfortable life. The cost of living is less, which means that your money goes further, with more left over for expenses such as saving for your kids’ college education. In addition, when you live in rural America, you won’t likely feel pressured to join the super expensive country club, send your kids to an expensive school, or drive a six-figure car. Although we may not make quite as much as the big city folk, when you account for the cost of living, rural surgeons do quite well.
6. Hire help
My husband is a firefighter, and at one point early in our marriage he was working night shift. With me on call and small children at home, that presented somewhat of a problem. But we solved it with a live-in nanny. Some people say, “I don’t want someone else raising my children,” and then they spend all their free time washing baby’s laundry and cleaning house instead of playing with the baby and having family meals and other family time together. How you utilize a nanny or any other help you hire is determined by you. But the idea is to hire help to do the mundane things so that you can do the fun things.
7. Learn to charm a rock
Small towns can be tough. Make one mistake, make one person angry, and before you eat your breakfast the next morning, the whole town knows about it. A very wise mentor of mine once said, “You catch more flies with honey.” Of course, he was absolutely right. When we work in bigger places, we tend to become numb to our own behavior because outbursts and conflicts are so common that our own little outbursts get lost in the mix. Such is not the case in a small town.
In rural America, people stop their cars to let you pull out. This can be very shocking when you come from New York and your first thought is …am I being carjacked? In a rural town, if you walk around with a Band-Aid on your face, every single person you pass on the street will ask if you are ok. It teaches you to treat other people well, even if you are having an absolutely terrible day. You can’t get away with taking your mood out on other people in a small town.
One very nice thing about working in a rural community is that people are grateful and have a very long memory for whatever you’ve done for them. Whether you’ve cured their colon cancer or removed a lipoma, they will stop you on the street to thank you or tell their friends for years afterward about how wonderful you were.
8. Get a hobby
Although life as a small town general surgeon can seem like a 24/7 occupation, careful time management and household support can create space for a hobby. And you need one. No matter how much we all love surgery, it is essential that we cultivate the ability to leave it be for a time here and there. A hobby (preferably one that helps alleviate stress) can help stave off burnout.
9. Don’t forget to sleep
This much-neglected survival tip is so important. We all have to be on call, some more than others. But most of us also have nights where we are not on call. These should not be the nights where we stay up until 3 a.m. watching every episode of the latest Netflix series. You must resist that temptation. The older you get, the harder it is to recover from a night out operating.
10. Never forget the 4 a.m. feeling
So you may learn to adjust to the small town and then to love it fiercely and protectively. You may even buy your own cow once a year or join a shooting range. You may learn to discuss the fall elk hunt, ice fishing, ranching, and the best place to buy ammunition with your patients. You walk through the hospital and you know every single person you pass in the hallway as well as their kids.
Let us never forget why we chose to be surgeons. Most of us would weather any challenge to continue to do what we love. And choosing a rural practice is one way to practice surgery and also achieve a satisfying work/life balance.
Dr. Long is an ACS Fellow and a general surgeon in rural West Virginia. She is the mother of five and an ironman triathlete. She is currently preparing for her 18th surgical mission trip to Central America in April. Dr. Justine Gavagan and Dr. Catherine O’Connor contributed to this article.
For those who have chosen the surgeon’s path, finding a sustainable work/life balance is challenging. For surgeons with young families and spousal responsibilities, achieving that balance may seem like an unattainable goal. As a rural surgeon with a spouse and children, I am here to say that rural practice has many benefits to those who love their work and also want a balanced life. I recommend that young surgeons, women in particular, consider this path for both the professional and personal advantages it offers.
I always tell the medical students that rotate with me, “When it is 4 o’clock in the morning and you forgot to go home ... be that.” That is to say, whatever rotation they are on when they have that feeling, that is the specialty they should choose. That was us when it came to surgery. When we were students going through surgical rotations we could not get enough. We wanted to see everything, do everything, and we didn’t want to miss anything. Many of our colleagues recommended against choosing surgery. We were told, “You’ll never have a life or a family;” “Your life will be horrible,” etc.
Now, many years later, I still love what I do as a surgeon. But I also love the other half of my life. I chose to practice rural surgery because I sensed that a balanced life would be possible in the rural setting. So I offer the following 10 tips for young surgeons who might be considering a rural practice:
1. Pick a good small town
Sit down and write out a list of all the things you want in a small town. My list, of course, will be different from your list but most importantly, make the list! It is no use taking a job in rural Colorado if you hate to hunt and fish. Consider the town and the job equally. For women surgeons, you may want to look for a place where there are women in upper administrative roles and other female physicians on staff. All small towns are definitely not alike and if you pick one for the hospital or the job without considering your surroundings, you will not likely be happy.
2. Don’t commute
Some people take a job in a small town or a rural setting but choose to live 30 miles away in the next larger town. I suggest living in the town you practice in. If at all possible, live close to the hospital. Every minute that you spend on the road is a minute away from your family. Living far away makes that 2 a.m. call from the ER that much more painful. You also miss out on the opportunity to become a real part of your community.
3. Choose the right partner
Small town physicians are tough to keep. Rural practice turnover is high. One of the most common reasons I hear for why a physician has left the area is because his or her spouse wasn’t happy. No matter how appealing the job seems, no matter how much bonus money they offer, no matter how great the hospital appears, if your spouse doesn’t like it there, you’ll be leaving. You may love your job and back country camping every weekend, but if your spouse pines for Whole Foods and the opera, you will be moving on eventually.
A large part of rural life revolves around events in the school system, and even if you have no young children of your own, it is helpful to make an effort to attend some of these events, if only to support your neighbor’s kids. It goes a long way to establish you as someone who is involved in the community and who cares. Remember, it is the mothers young and old who make many of the health care decisions for their families. Volunteer for events or get involved in the science program at the high school. You may find that you are a unique role model for teenagers in your community.
4. Choose the right partners
Rural general surgery is challenging enough without having to compete with those around you. Surgery in the small town setting becomes infinitely more pleasant when you have good partners. Is the practice set up to help and support each partner, or are you pitted against one another, competing for RVUs? Would your partners tolerate covering your call for 3 months of maternity leave or would this cause resentment? Look for a practice where the surgeons work together and cover for each other, and your small town life will be greatly enhanced.
5. Live within your means
A small town surgical practice can make for a very comfortable life. The cost of living is less, which means that your money goes further, with more left over for expenses such as saving for your kids’ college education. In addition, when you live in rural America, you won’t likely feel pressured to join the super expensive country club, send your kids to an expensive school, or drive a six-figure car. Although we may not make quite as much as the big city folk, when you account for the cost of living, rural surgeons do quite well.
6. Hire help
My husband is a firefighter, and at one point early in our marriage he was working night shift. With me on call and small children at home, that presented somewhat of a problem. But we solved it with a live-in nanny. Some people say, “I don’t want someone else raising my children,” and then they spend all their free time washing baby’s laundry and cleaning house instead of playing with the baby and having family meals and other family time together. How you utilize a nanny or any other help you hire is determined by you. But the idea is to hire help to do the mundane things so that you can do the fun things.
7. Learn to charm a rock
Small towns can be tough. Make one mistake, make one person angry, and before you eat your breakfast the next morning, the whole town knows about it. A very wise mentor of mine once said, “You catch more flies with honey.” Of course, he was absolutely right. When we work in bigger places, we tend to become numb to our own behavior because outbursts and conflicts are so common that our own little outbursts get lost in the mix. Such is not the case in a small town.
In rural America, people stop their cars to let you pull out. This can be very shocking when you come from New York and your first thought is …am I being carjacked? In a rural town, if you walk around with a Band-Aid on your face, every single person you pass on the street will ask if you are ok. It teaches you to treat other people well, even if you are having an absolutely terrible day. You can’t get away with taking your mood out on other people in a small town.
One very nice thing about working in a rural community is that people are grateful and have a very long memory for whatever you’ve done for them. Whether you’ve cured their colon cancer or removed a lipoma, they will stop you on the street to thank you or tell their friends for years afterward about how wonderful you were.
8. Get a hobby
Although life as a small town general surgeon can seem like a 24/7 occupation, careful time management and household support can create space for a hobby. And you need one. No matter how much we all love surgery, it is essential that we cultivate the ability to leave it be for a time here and there. A hobby (preferably one that helps alleviate stress) can help stave off burnout.
9. Don’t forget to sleep
This much-neglected survival tip is so important. We all have to be on call, some more than others. But most of us also have nights where we are not on call. These should not be the nights where we stay up until 3 a.m. watching every episode of the latest Netflix series. You must resist that temptation. The older you get, the harder it is to recover from a night out operating.
10. Never forget the 4 a.m. feeling
So you may learn to adjust to the small town and then to love it fiercely and protectively. You may even buy your own cow once a year or join a shooting range. You may learn to discuss the fall elk hunt, ice fishing, ranching, and the best place to buy ammunition with your patients. You walk through the hospital and you know every single person you pass in the hallway as well as their kids.
Let us never forget why we chose to be surgeons. Most of us would weather any challenge to continue to do what we love. And choosing a rural practice is one way to practice surgery and also achieve a satisfying work/life balance.
Dr. Long is an ACS Fellow and a general surgeon in rural West Virginia. She is the mother of five and an ironman triathlete. She is currently preparing for her 18th surgical mission trip to Central America in April. Dr. Justine Gavagan and Dr. Catherine O’Connor contributed to this article.
For those who have chosen the surgeon’s path, finding a sustainable work/life balance is challenging. For surgeons with young families and spousal responsibilities, achieving that balance may seem like an unattainable goal. As a rural surgeon with a spouse and children, I am here to say that rural practice has many benefits to those who love their work and also want a balanced life. I recommend that young surgeons, women in particular, consider this path for both the professional and personal advantages it offers.
I always tell the medical students that rotate with me, “When it is 4 o’clock in the morning and you forgot to go home ... be that.” That is to say, whatever rotation they are on when they have that feeling, that is the specialty they should choose. That was us when it came to surgery. When we were students going through surgical rotations we could not get enough. We wanted to see everything, do everything, and we didn’t want to miss anything. Many of our colleagues recommended against choosing surgery. We were told, “You’ll never have a life or a family;” “Your life will be horrible,” etc.
Now, many years later, I still love what I do as a surgeon. But I also love the other half of my life. I chose to practice rural surgery because I sensed that a balanced life would be possible in the rural setting. So I offer the following 10 tips for young surgeons who might be considering a rural practice:
1. Pick a good small town
Sit down and write out a list of all the things you want in a small town. My list, of course, will be different from your list but most importantly, make the list! It is no use taking a job in rural Colorado if you hate to hunt and fish. Consider the town and the job equally. For women surgeons, you may want to look for a place where there are women in upper administrative roles and other female physicians on staff. All small towns are definitely not alike and if you pick one for the hospital or the job without considering your surroundings, you will not likely be happy.
2. Don’t commute
Some people take a job in a small town or a rural setting but choose to live 30 miles away in the next larger town. I suggest living in the town you practice in. If at all possible, live close to the hospital. Every minute that you spend on the road is a minute away from your family. Living far away makes that 2 a.m. call from the ER that much more painful. You also miss out on the opportunity to become a real part of your community.
3. Choose the right partner
Small town physicians are tough to keep. Rural practice turnover is high. One of the most common reasons I hear for why a physician has left the area is because his or her spouse wasn’t happy. No matter how appealing the job seems, no matter how much bonus money they offer, no matter how great the hospital appears, if your spouse doesn’t like it there, you’ll be leaving. You may love your job and back country camping every weekend, but if your spouse pines for Whole Foods and the opera, you will be moving on eventually.
A large part of rural life revolves around events in the school system, and even if you have no young children of your own, it is helpful to make an effort to attend some of these events, if only to support your neighbor’s kids. It goes a long way to establish you as someone who is involved in the community and who cares. Remember, it is the mothers young and old who make many of the health care decisions for their families. Volunteer for events or get involved in the science program at the high school. You may find that you are a unique role model for teenagers in your community.
4. Choose the right partners
Rural general surgery is challenging enough without having to compete with those around you. Surgery in the small town setting becomes infinitely more pleasant when you have good partners. Is the practice set up to help and support each partner, or are you pitted against one another, competing for RVUs? Would your partners tolerate covering your call for 3 months of maternity leave or would this cause resentment? Look for a practice where the surgeons work together and cover for each other, and your small town life will be greatly enhanced.
5. Live within your means
A small town surgical practice can make for a very comfortable life. The cost of living is less, which means that your money goes further, with more left over for expenses such as saving for your kids’ college education. In addition, when you live in rural America, you won’t likely feel pressured to join the super expensive country club, send your kids to an expensive school, or drive a six-figure car. Although we may not make quite as much as the big city folk, when you account for the cost of living, rural surgeons do quite well.
6. Hire help
My husband is a firefighter, and at one point early in our marriage he was working night shift. With me on call and small children at home, that presented somewhat of a problem. But we solved it with a live-in nanny. Some people say, “I don’t want someone else raising my children,” and then they spend all their free time washing baby’s laundry and cleaning house instead of playing with the baby and having family meals and other family time together. How you utilize a nanny or any other help you hire is determined by you. But the idea is to hire help to do the mundane things so that you can do the fun things.
7. Learn to charm a rock
Small towns can be tough. Make one mistake, make one person angry, and before you eat your breakfast the next morning, the whole town knows about it. A very wise mentor of mine once said, “You catch more flies with honey.” Of course, he was absolutely right. When we work in bigger places, we tend to become numb to our own behavior because outbursts and conflicts are so common that our own little outbursts get lost in the mix. Such is not the case in a small town.
In rural America, people stop their cars to let you pull out. This can be very shocking when you come from New York and your first thought is …am I being carjacked? In a rural town, if you walk around with a Band-Aid on your face, every single person you pass on the street will ask if you are ok. It teaches you to treat other people well, even if you are having an absolutely terrible day. You can’t get away with taking your mood out on other people in a small town.
One very nice thing about working in a rural community is that people are grateful and have a very long memory for whatever you’ve done for them. Whether you’ve cured their colon cancer or removed a lipoma, they will stop you on the street to thank you or tell their friends for years afterward about how wonderful you were.
8. Get a hobby
Although life as a small town general surgeon can seem like a 24/7 occupation, careful time management and household support can create space for a hobby. And you need one. No matter how much we all love surgery, it is essential that we cultivate the ability to leave it be for a time here and there. A hobby (preferably one that helps alleviate stress) can help stave off burnout.
9. Don’t forget to sleep
This much-neglected survival tip is so important. We all have to be on call, some more than others. But most of us also have nights where we are not on call. These should not be the nights where we stay up until 3 a.m. watching every episode of the latest Netflix series. You must resist that temptation. The older you get, the harder it is to recover from a night out operating.
10. Never forget the 4 a.m. feeling
So you may learn to adjust to the small town and then to love it fiercely and protectively. You may even buy your own cow once a year or join a shooting range. You may learn to discuss the fall elk hunt, ice fishing, ranching, and the best place to buy ammunition with your patients. You walk through the hospital and you know every single person you pass in the hallway as well as their kids.
Let us never forget why we chose to be surgeons. Most of us would weather any challenge to continue to do what we love. And choosing a rural practice is one way to practice surgery and also achieve a satisfying work/life balance.
Dr. Long is an ACS Fellow and a general surgeon in rural West Virginia. She is the mother of five and an ironman triathlete. She is currently preparing for her 18th surgical mission trip to Central America in April. Dr. Justine Gavagan and Dr. Catherine O’Connor contributed to this article.
Only as good as the prep
In the realm of screening routinely offered to our patients in primary care, colon cancer screening stands with cervical cancer screening with a grade A recommendation from the U.S. Preventive Services Task Force. As such, our systems have been set up to gently remind us of colon cancer screening when it is due. If your practice is like mine, it has been almost exclusively colonoscopy. As such, I hear a lot of patient complaints about the colonoscopy prep.
Recently, one of our patients had uncontrolled vomiting with one of the commonly used polyethylene glycol (PEG) 3350 preparations. Many of us may have been aware of the Miralax and Gatorade (M-G) colon prep, and I considered recommending it to my patient. But is it just as good?
Dr. Sameer Siddique of the University of Missouri, Columbia, and colleagues published a systematic review evaluating the comparability of the M-G prep (238-255 g in 1.9 L) to PEG (3.8-4 L) (Am. J. Gastroenterol. 2014;109:1566-74).
The investigators identified five articles and observed that the M-G prep was associated with significantly fewer satisfactory bowel preparations, compared with PEG (odds ratio, 0.65; 95% confidence interval, 0.43-0.98; P = .04). In a subgroup analysis, split-dose M-G was inferior to split-dose PEG in the number of satisfactory preparations.
Patients, however, had a greater willingness to repeat the preparation (OR, 7.32; 95% CI, 4.88-10.98; P <.01). No significant differences were observed with polyp detection or in side effects such as nausea, cramping, or bloating.
The study authors point out that the dose of Miralax in the M-G prep is not FDA approved (because it is 15 times higher than the dose for constipation), and that the solution is hypotonic and can potentially cause hyponatremia. Furthermore, the cost of a colonoscopy ranges from $600 to more than $5,400. At those prices, patient agreeableness to repeat the test does not mean the health care system can bear a deluge of re-do’s.
Remember that split-dose PEG (one half the day before and one half the day of) has been shown to be superior to the night-before preparation, and it can increase tolerability. If a patient has time to get the job done the day of the colonoscopy, maybe this is the way to go.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
In the realm of screening routinely offered to our patients in primary care, colon cancer screening stands with cervical cancer screening with a grade A recommendation from the U.S. Preventive Services Task Force. As such, our systems have been set up to gently remind us of colon cancer screening when it is due. If your practice is like mine, it has been almost exclusively colonoscopy. As such, I hear a lot of patient complaints about the colonoscopy prep.
Recently, one of our patients had uncontrolled vomiting with one of the commonly used polyethylene glycol (PEG) 3350 preparations. Many of us may have been aware of the Miralax and Gatorade (M-G) colon prep, and I considered recommending it to my patient. But is it just as good?
Dr. Sameer Siddique of the University of Missouri, Columbia, and colleagues published a systematic review evaluating the comparability of the M-G prep (238-255 g in 1.9 L) to PEG (3.8-4 L) (Am. J. Gastroenterol. 2014;109:1566-74).
The investigators identified five articles and observed that the M-G prep was associated with significantly fewer satisfactory bowel preparations, compared with PEG (odds ratio, 0.65; 95% confidence interval, 0.43-0.98; P = .04). In a subgroup analysis, split-dose M-G was inferior to split-dose PEG in the number of satisfactory preparations.
Patients, however, had a greater willingness to repeat the preparation (OR, 7.32; 95% CI, 4.88-10.98; P <.01). No significant differences were observed with polyp detection or in side effects such as nausea, cramping, or bloating.
The study authors point out that the dose of Miralax in the M-G prep is not FDA approved (because it is 15 times higher than the dose for constipation), and that the solution is hypotonic and can potentially cause hyponatremia. Furthermore, the cost of a colonoscopy ranges from $600 to more than $5,400. At those prices, patient agreeableness to repeat the test does not mean the health care system can bear a deluge of re-do’s.
Remember that split-dose PEG (one half the day before and one half the day of) has been shown to be superior to the night-before preparation, and it can increase tolerability. If a patient has time to get the job done the day of the colonoscopy, maybe this is the way to go.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
In the realm of screening routinely offered to our patients in primary care, colon cancer screening stands with cervical cancer screening with a grade A recommendation from the U.S. Preventive Services Task Force. As such, our systems have been set up to gently remind us of colon cancer screening when it is due. If your practice is like mine, it has been almost exclusively colonoscopy. As such, I hear a lot of patient complaints about the colonoscopy prep.
Recently, one of our patients had uncontrolled vomiting with one of the commonly used polyethylene glycol (PEG) 3350 preparations. Many of us may have been aware of the Miralax and Gatorade (M-G) colon prep, and I considered recommending it to my patient. But is it just as good?
Dr. Sameer Siddique of the University of Missouri, Columbia, and colleagues published a systematic review evaluating the comparability of the M-G prep (238-255 g in 1.9 L) to PEG (3.8-4 L) (Am. J. Gastroenterol. 2014;109:1566-74).
The investigators identified five articles and observed that the M-G prep was associated with significantly fewer satisfactory bowel preparations, compared with PEG (odds ratio, 0.65; 95% confidence interval, 0.43-0.98; P = .04). In a subgroup analysis, split-dose M-G was inferior to split-dose PEG in the number of satisfactory preparations.
Patients, however, had a greater willingness to repeat the preparation (OR, 7.32; 95% CI, 4.88-10.98; P <.01). No significant differences were observed with polyp detection or in side effects such as nausea, cramping, or bloating.
The study authors point out that the dose of Miralax in the M-G prep is not FDA approved (because it is 15 times higher than the dose for constipation), and that the solution is hypotonic and can potentially cause hyponatremia. Furthermore, the cost of a colonoscopy ranges from $600 to more than $5,400. At those prices, patient agreeableness to repeat the test does not mean the health care system can bear a deluge of re-do’s.
Remember that split-dose PEG (one half the day before and one half the day of) has been shown to be superior to the night-before preparation, and it can increase tolerability. If a patient has time to get the job done the day of the colonoscopy, maybe this is the way to go.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
Novel anticoagulant system no better than existing drug

SAN DIEGO—In a now-terminated phase 3 trial, a novel anticoagulant system proved about as effective as an established drug in patients undergoing percutaneous coronary intervention (PCI).
The system also conferred higher rates of bleeding and prompted more allergic reactions.
The trial, known as REGULATE-PCI, was designed to compare the Revolixys Kit (also known as the REG-1 Anticoagulation System) to bivalirudin (Angiomax).
The study was officially halted in August due to an excess of severe allergic reactions associated with the Revolixys Kit. Given the early termination, investigators said the data should be considered exploratory.
Roxana Mehran, MD, of Mount Sinai Hospital in New York, New York, presented the study’s results at the American College of Cardiology’s 64th Annual Scientific Session (abstract 402-12).
The goal of the REGULATE-PCI trial was to compare the reversible thrombin inhibitor bivalirudin to the Revolixys Kit—a 2-component system consisting of pegnivacogin, an anticoagulant aptamer targeting coagulation factor IXa, and its complementary oligonucleotide active control agent, anivamersen—in patients undergoing PCI.
Before the trial was stopped, 3232 patients were enrolled. They underwent PCI at 225 hospitals in 17 countries. Patients were equally randomized to the bivalirudin or Revolixys arms, and investigators collected data at 3 days and 30 days.
Efficacy and safety results
There were no differences between the treatment arms in terms of the study’s primary efficacy endpoint—a composite of all-cause death, heart attack, stroke, or urgent revascularization.
The endpoint occurred in 6.7% of patients in the Revolixys arm and 6.4% of patients receiving bivalirudin 3 days after PCI (P=0.72). Efficacy was still comparable at 30 days.
In addition, the Revolixys system failed to show a benefit over bivalirudin with regard to the primary safety endpoint of bleeding.
The rate of BARC 3 or 5 bleeding was 0.4% in the Revolixys arm and 0.1% in the bivalirudin arm (P=0.09). And the rates of BARC 2, 3, or 5 bleeding were 6.5% and 4.1%, respectively (P=0.002).
Serious allergic reactions occurred in 10 of 1605 patients in the Revolixys arm. One of these reactions was fatal, and the others were anaphylactic reactions. Only one patient in the bivalirudin group had a serious allergic event.
“This anticoagulant system is associated with infrequent but an unacceptably high rate of severe allergic reactions,” Dr Mehran said.
Research is ongoing to determine the exact cause of the allergic reactions, and Dr Mehran said she hopes this does not deter the search for novel anticoagulants for use in this patient population.
More about REGULATE-PCI
Investigators started recruiting patients to the trial in September 2013. In April, because there were a handful of allergic reactions seen in the earlier phase 2 trial, a data safety and monitoring board reviewed all of the safety endpoints for the first 1000 patients enrolled in REGULATE-PCI.
In June, both the executive committee and the sponsor decided to suspend the trial, and the US Food and Drug Administration announced a clinical hold in July. The trial was permanently halted in August.
This study was funded by Regado Biosciences Inc., the company developing the Revolixys kit. Dr Mehran has served on the scientific advisory board for Regado Biosciences. ![]()

SAN DIEGO—In a now-terminated phase 3 trial, a novel anticoagulant system proved about as effective as an established drug in patients undergoing percutaneous coronary intervention (PCI).
The system also conferred higher rates of bleeding and prompted more allergic reactions.
The trial, known as REGULATE-PCI, was designed to compare the Revolixys Kit (also known as the REG-1 Anticoagulation System) to bivalirudin (Angiomax).
The study was officially halted in August due to an excess of severe allergic reactions associated with the Revolixys Kit. Given the early termination, investigators said the data should be considered exploratory.
Roxana Mehran, MD, of Mount Sinai Hospital in New York, New York, presented the study’s results at the American College of Cardiology’s 64th Annual Scientific Session (abstract 402-12).
The goal of the REGULATE-PCI trial was to compare the reversible thrombin inhibitor bivalirudin to the Revolixys Kit—a 2-component system consisting of pegnivacogin, an anticoagulant aptamer targeting coagulation factor IXa, and its complementary oligonucleotide active control agent, anivamersen—in patients undergoing PCI.
Before the trial was stopped, 3232 patients were enrolled. They underwent PCI at 225 hospitals in 17 countries. Patients were equally randomized to the bivalirudin or Revolixys arms, and investigators collected data at 3 days and 30 days.
Efficacy and safety results
There were no differences between the treatment arms in terms of the study’s primary efficacy endpoint—a composite of all-cause death, heart attack, stroke, or urgent revascularization.
The endpoint occurred in 6.7% of patients in the Revolixys arm and 6.4% of patients receiving bivalirudin 3 days after PCI (P=0.72). Efficacy was still comparable at 30 days.
In addition, the Revolixys system failed to show a benefit over bivalirudin with regard to the primary safety endpoint of bleeding.
The rate of BARC 3 or 5 bleeding was 0.4% in the Revolixys arm and 0.1% in the bivalirudin arm (P=0.09). And the rates of BARC 2, 3, or 5 bleeding were 6.5% and 4.1%, respectively (P=0.002).
Serious allergic reactions occurred in 10 of 1605 patients in the Revolixys arm. One of these reactions was fatal, and the others were anaphylactic reactions. Only one patient in the bivalirudin group had a serious allergic event.
“This anticoagulant system is associated with infrequent but an unacceptably high rate of severe allergic reactions,” Dr Mehran said.
Research is ongoing to determine the exact cause of the allergic reactions, and Dr Mehran said she hopes this does not deter the search for novel anticoagulants for use in this patient population.
More about REGULATE-PCI
Investigators started recruiting patients to the trial in September 2013. In April, because there were a handful of allergic reactions seen in the earlier phase 2 trial, a data safety and monitoring board reviewed all of the safety endpoints for the first 1000 patients enrolled in REGULATE-PCI.
In June, both the executive committee and the sponsor decided to suspend the trial, and the US Food and Drug Administration announced a clinical hold in July. The trial was permanently halted in August.
This study was funded by Regado Biosciences Inc., the company developing the Revolixys kit. Dr Mehran has served on the scientific advisory board for Regado Biosciences. ![]()

SAN DIEGO—In a now-terminated phase 3 trial, a novel anticoagulant system proved about as effective as an established drug in patients undergoing percutaneous coronary intervention (PCI).
The system also conferred higher rates of bleeding and prompted more allergic reactions.
The trial, known as REGULATE-PCI, was designed to compare the Revolixys Kit (also known as the REG-1 Anticoagulation System) to bivalirudin (Angiomax).
The study was officially halted in August due to an excess of severe allergic reactions associated with the Revolixys Kit. Given the early termination, investigators said the data should be considered exploratory.
Roxana Mehran, MD, of Mount Sinai Hospital in New York, New York, presented the study’s results at the American College of Cardiology’s 64th Annual Scientific Session (abstract 402-12).
The goal of the REGULATE-PCI trial was to compare the reversible thrombin inhibitor bivalirudin to the Revolixys Kit—a 2-component system consisting of pegnivacogin, an anticoagulant aptamer targeting coagulation factor IXa, and its complementary oligonucleotide active control agent, anivamersen—in patients undergoing PCI.
Before the trial was stopped, 3232 patients were enrolled. They underwent PCI at 225 hospitals in 17 countries. Patients were equally randomized to the bivalirudin or Revolixys arms, and investigators collected data at 3 days and 30 days.
Efficacy and safety results
There were no differences between the treatment arms in terms of the study’s primary efficacy endpoint—a composite of all-cause death, heart attack, stroke, or urgent revascularization.
The endpoint occurred in 6.7% of patients in the Revolixys arm and 6.4% of patients receiving bivalirudin 3 days after PCI (P=0.72). Efficacy was still comparable at 30 days.
In addition, the Revolixys system failed to show a benefit over bivalirudin with regard to the primary safety endpoint of bleeding.
The rate of BARC 3 or 5 bleeding was 0.4% in the Revolixys arm and 0.1% in the bivalirudin arm (P=0.09). And the rates of BARC 2, 3, or 5 bleeding were 6.5% and 4.1%, respectively (P=0.002).
Serious allergic reactions occurred in 10 of 1605 patients in the Revolixys arm. One of these reactions was fatal, and the others were anaphylactic reactions. Only one patient in the bivalirudin group had a serious allergic event.
“This anticoagulant system is associated with infrequent but an unacceptably high rate of severe allergic reactions,” Dr Mehran said.
Research is ongoing to determine the exact cause of the allergic reactions, and Dr Mehran said she hopes this does not deter the search for novel anticoagulants for use in this patient population.
More about REGULATE-PCI
Investigators started recruiting patients to the trial in September 2013. In April, because there were a handful of allergic reactions seen in the earlier phase 2 trial, a data safety and monitoring board reviewed all of the safety endpoints for the first 1000 patients enrolled in REGULATE-PCI.
In June, both the executive committee and the sponsor decided to suspend the trial, and the US Food and Drug Administration announced a clinical hold in July. The trial was permanently halted in August.
This study was funded by Regado Biosciences Inc., the company developing the Revolixys kit. Dr Mehran has served on the scientific advisory board for Regado Biosciences. ![]()
Group reprograms B-ALL cells into macrophage-like cells

pseudopodia to engulf particles
Investigators have reported methods for reprogramming leukemia cells into non-leukemic, macrophage-like cells.
The team reprogrammed cells derived from patients with BCR-ABL1+ precursor B-cell acute lymphoblastic leukemia (B-ALL) by exposing the cells to myeloid differentiation-promoting cytokines or by transient expression of certain transcription factors.
The group described this work in Proceedings of the National Academy of Sciences.
The research began when the investigators were trying to keep patient-derived leukemia cells alive in culture.
“We were throwing everything at them to help them survive,” said Ravindra Majeti, MD, PhD, of Stanford University School of Medicine in California.
Then, James Scott McClellan, MD, PhD, also of Stanford University School of Medicine, mentioned that some of the cells were changing shape and size, morphing into what looked like macrophages.
Dr Majeti concurred with that observation, but the reasons for the changed cells were a mystery. That is, until he remembered an old research paper, which showed that early B-cell mouse progenitor cells could be forced to become macrophages when exposed to certain transcription factors.
So he and his colleagues set out to confirm that they could transform leukemic cells into macrophage-like cells.
The team isolated CD19+CD34+ blasts from 12 adults with BCR-ABL1+ B-ALL and cultured the blasts in the presence of myeloid-differentiation-promoting cytokines. This resulted in CD14high/CD19low cells that expressed the surface markers and had the functional properties typical of normal macrophages.
The investigators also cultured B-ALL cells with the myeloid transcription factor C/EBPα or the myeloid/lymphoid transcription factor PU.1. Both factors were able to reprogram blasts into macrophage-like cells.
Experiments in mice revealed that reprogramming the blasts into macrophage-like cells eliminated their leukemogenicity.
And the investigators’ final experiments suggested that myeloid reprogramming occurs, to some degree, in humans. In samples from patients with BCR-ABL1+ B-ALL, the team found primary CD14+ monocytes/macrophages that had the BCR-ABL1+ translocation and clonally recombined VDJ regions.
Dr Majeti and his colleagues said they have reason to hope that, when the leukemic cells become macrophage-like cells, they are not only neutralized but may actually assist in fighting the leukemia.
“Because the macrophage cells came from the cancer cells, they will already carry with them the chemical signals that will identify the cancer cells, making an immune attack against the cancer more likely,” Dr Majeti said. ![]()

pseudopodia to engulf particles
Investigators have reported methods for reprogramming leukemia cells into non-leukemic, macrophage-like cells.
The team reprogrammed cells derived from patients with BCR-ABL1+ precursor B-cell acute lymphoblastic leukemia (B-ALL) by exposing the cells to myeloid differentiation-promoting cytokines or by transient expression of certain transcription factors.
The group described this work in Proceedings of the National Academy of Sciences.
The research began when the investigators were trying to keep patient-derived leukemia cells alive in culture.
“We were throwing everything at them to help them survive,” said Ravindra Majeti, MD, PhD, of Stanford University School of Medicine in California.
Then, James Scott McClellan, MD, PhD, also of Stanford University School of Medicine, mentioned that some of the cells were changing shape and size, morphing into what looked like macrophages.
Dr Majeti concurred with that observation, but the reasons for the changed cells were a mystery. That is, until he remembered an old research paper, which showed that early B-cell mouse progenitor cells could be forced to become macrophages when exposed to certain transcription factors.
So he and his colleagues set out to confirm that they could transform leukemic cells into macrophage-like cells.
The team isolated CD19+CD34+ blasts from 12 adults with BCR-ABL1+ B-ALL and cultured the blasts in the presence of myeloid-differentiation-promoting cytokines. This resulted in CD14high/CD19low cells that expressed the surface markers and had the functional properties typical of normal macrophages.
The investigators also cultured B-ALL cells with the myeloid transcription factor C/EBPα or the myeloid/lymphoid transcription factor PU.1. Both factors were able to reprogram blasts into macrophage-like cells.
Experiments in mice revealed that reprogramming the blasts into macrophage-like cells eliminated their leukemogenicity.
And the investigators’ final experiments suggested that myeloid reprogramming occurs, to some degree, in humans. In samples from patients with BCR-ABL1+ B-ALL, the team found primary CD14+ monocytes/macrophages that had the BCR-ABL1+ translocation and clonally recombined VDJ regions.
Dr Majeti and his colleagues said they have reason to hope that, when the leukemic cells become macrophage-like cells, they are not only neutralized but may actually assist in fighting the leukemia.
“Because the macrophage cells came from the cancer cells, they will already carry with them the chemical signals that will identify the cancer cells, making an immune attack against the cancer more likely,” Dr Majeti said. ![]()

pseudopodia to engulf particles
Investigators have reported methods for reprogramming leukemia cells into non-leukemic, macrophage-like cells.
The team reprogrammed cells derived from patients with BCR-ABL1+ precursor B-cell acute lymphoblastic leukemia (B-ALL) by exposing the cells to myeloid differentiation-promoting cytokines or by transient expression of certain transcription factors.
The group described this work in Proceedings of the National Academy of Sciences.
The research began when the investigators were trying to keep patient-derived leukemia cells alive in culture.
“We were throwing everything at them to help them survive,” said Ravindra Majeti, MD, PhD, of Stanford University School of Medicine in California.
Then, James Scott McClellan, MD, PhD, also of Stanford University School of Medicine, mentioned that some of the cells were changing shape and size, morphing into what looked like macrophages.
Dr Majeti concurred with that observation, but the reasons for the changed cells were a mystery. That is, until he remembered an old research paper, which showed that early B-cell mouse progenitor cells could be forced to become macrophages when exposed to certain transcription factors.
So he and his colleagues set out to confirm that they could transform leukemic cells into macrophage-like cells.
The team isolated CD19+CD34+ blasts from 12 adults with BCR-ABL1+ B-ALL and cultured the blasts in the presence of myeloid-differentiation-promoting cytokines. This resulted in CD14high/CD19low cells that expressed the surface markers and had the functional properties typical of normal macrophages.
The investigators also cultured B-ALL cells with the myeloid transcription factor C/EBPα or the myeloid/lymphoid transcription factor PU.1. Both factors were able to reprogram blasts into macrophage-like cells.
Experiments in mice revealed that reprogramming the blasts into macrophage-like cells eliminated their leukemogenicity.
And the investigators’ final experiments suggested that myeloid reprogramming occurs, to some degree, in humans. In samples from patients with BCR-ABL1+ B-ALL, the team found primary CD14+ monocytes/macrophages that had the BCR-ABL1+ translocation and clonally recombined VDJ regions.
Dr Majeti and his colleagues said they have reason to hope that, when the leukemic cells become macrophage-like cells, they are not only neutralized but may actually assist in fighting the leukemia.
“Because the macrophage cells came from the cancer cells, they will already carry with them the chemical signals that will identify the cancer cells, making an immune attack against the cancer more likely,” Dr Majeti said. ![]()
Lowering the cost of cancer drugs in the US

Photo by Petr Kratochvil
Increasingly high prices for cancer drugs are affecting patient care and the overall healthcare system in the US, according to authors of an article in Mayo Clinic Proceedings.
The authors noted that the average price of cancer drugs for about a year of therapy increased from $5000 to $10,000 before 2000, and to more than $100,000 by 2012.
Over nearly the same period, the average household income in the US decreased by about 8%.
“Americans with cancer pay 50% to 100% more for the same patented drug than patients in other countries,” said author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“As oncologists, we have a moral obligation to advocate for affordable cancer drugs for our patients.”
Dr Rajkumar and co-author Hagop Kantarjian, MD, of MD Anderson Cancer Center in Houston, Texas, rebutted the major arguments the pharmaceutical industry uses to justify the high price of cancer drugs; namely, the expense of conducting research and drug development, the comparative benefits to patients, that market forces will settle prices to reasonable levels, and that price controls on cancer drugs will stifle innovation.
“One of the facts that people do not realize is that cancer drugs, for the most part, are not operating under a free market economy,” Dr Rajkumar said. “The fact that there are 5 approved drugs to treat an incurable cancer does not mean there is competition.”
“Typically, the standard of care is that each drug is used sequentially or in combination, so that each new drug represents a monopoly with exclusivity granted by patent protection for many years.”
Drs Rajkumar and Kantarjian said other reasons for the high cost of cancer drugs include legislation that prevents Medicare from being able to negotiate drug prices and a lack of value-based pricing, which ties the cost of a drug to its relative effectiveness compared to other drugs.
The authors recommended a set of potential solutions to help control and reduce the high cost of cancer drugs in the US. Some of their recommendations are already in practice in other developed countries. Their recommendations include:
- Allow Medicare to negotiate drug prices
- Develop cancer treatment pathways/guidelines that incorporate the cost and benefit of cancer drugs
- Allow the Food and Drug Administration or physician panels to recommend target prices based on a drug’s magnitude of benefit (value-based pricing)
- Eliminate “pay-for-delay” strategies in which a pharmaceutical company with a brand name drug shares profits on that drug with a generic drug manufacturer for the remainder of a patent period, effectively eliminating a patent challenge and competition
- Allow the importation of drugs from abroad for personal use
- Allow the Patient-Centered Outcomes Research Institute and other cancer advocacy groups to consider cost in their recommendations
- Create patient-driven grassroots movements and organizations to advocate effectively for the interests of patients with cancer to balance advocacy efforts of pharmaceutical companies, insurance companies, pharmacy outlets, and hospitals.
Dr Kantarjian has organized a petition, which is available on change.org, asking the federal government to implement these changes. ![]()

Photo by Petr Kratochvil
Increasingly high prices for cancer drugs are affecting patient care and the overall healthcare system in the US, according to authors of an article in Mayo Clinic Proceedings.
The authors noted that the average price of cancer drugs for about a year of therapy increased from $5000 to $10,000 before 2000, and to more than $100,000 by 2012.
Over nearly the same period, the average household income in the US decreased by about 8%.
“Americans with cancer pay 50% to 100% more for the same patented drug than patients in other countries,” said author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“As oncologists, we have a moral obligation to advocate for affordable cancer drugs for our patients.”
Dr Rajkumar and co-author Hagop Kantarjian, MD, of MD Anderson Cancer Center in Houston, Texas, rebutted the major arguments the pharmaceutical industry uses to justify the high price of cancer drugs; namely, the expense of conducting research and drug development, the comparative benefits to patients, that market forces will settle prices to reasonable levels, and that price controls on cancer drugs will stifle innovation.
“One of the facts that people do not realize is that cancer drugs, for the most part, are not operating under a free market economy,” Dr Rajkumar said. “The fact that there are 5 approved drugs to treat an incurable cancer does not mean there is competition.”
“Typically, the standard of care is that each drug is used sequentially or in combination, so that each new drug represents a monopoly with exclusivity granted by patent protection for many years.”
Drs Rajkumar and Kantarjian said other reasons for the high cost of cancer drugs include legislation that prevents Medicare from being able to negotiate drug prices and a lack of value-based pricing, which ties the cost of a drug to its relative effectiveness compared to other drugs.
The authors recommended a set of potential solutions to help control and reduce the high cost of cancer drugs in the US. Some of their recommendations are already in practice in other developed countries. Their recommendations include:
- Allow Medicare to negotiate drug prices
- Develop cancer treatment pathways/guidelines that incorporate the cost and benefit of cancer drugs
- Allow the Food and Drug Administration or physician panels to recommend target prices based on a drug’s magnitude of benefit (value-based pricing)
- Eliminate “pay-for-delay” strategies in which a pharmaceutical company with a brand name drug shares profits on that drug with a generic drug manufacturer for the remainder of a patent period, effectively eliminating a patent challenge and competition
- Allow the importation of drugs from abroad for personal use
- Allow the Patient-Centered Outcomes Research Institute and other cancer advocacy groups to consider cost in their recommendations
- Create patient-driven grassroots movements and organizations to advocate effectively for the interests of patients with cancer to balance advocacy efforts of pharmaceutical companies, insurance companies, pharmacy outlets, and hospitals.
Dr Kantarjian has organized a petition, which is available on change.org, asking the federal government to implement these changes. ![]()

Photo by Petr Kratochvil
Increasingly high prices for cancer drugs are affecting patient care and the overall healthcare system in the US, according to authors of an article in Mayo Clinic Proceedings.
The authors noted that the average price of cancer drugs for about a year of therapy increased from $5000 to $10,000 before 2000, and to more than $100,000 by 2012.
Over nearly the same period, the average household income in the US decreased by about 8%.
“Americans with cancer pay 50% to 100% more for the same patented drug than patients in other countries,” said author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“As oncologists, we have a moral obligation to advocate for affordable cancer drugs for our patients.”
Dr Rajkumar and co-author Hagop Kantarjian, MD, of MD Anderson Cancer Center in Houston, Texas, rebutted the major arguments the pharmaceutical industry uses to justify the high price of cancer drugs; namely, the expense of conducting research and drug development, the comparative benefits to patients, that market forces will settle prices to reasonable levels, and that price controls on cancer drugs will stifle innovation.
“One of the facts that people do not realize is that cancer drugs, for the most part, are not operating under a free market economy,” Dr Rajkumar said. “The fact that there are 5 approved drugs to treat an incurable cancer does not mean there is competition.”
“Typically, the standard of care is that each drug is used sequentially or in combination, so that each new drug represents a monopoly with exclusivity granted by patent protection for many years.”
Drs Rajkumar and Kantarjian said other reasons for the high cost of cancer drugs include legislation that prevents Medicare from being able to negotiate drug prices and a lack of value-based pricing, which ties the cost of a drug to its relative effectiveness compared to other drugs.
The authors recommended a set of potential solutions to help control and reduce the high cost of cancer drugs in the US. Some of their recommendations are already in practice in other developed countries. Their recommendations include:
- Allow Medicare to negotiate drug prices
- Develop cancer treatment pathways/guidelines that incorporate the cost and benefit of cancer drugs
- Allow the Food and Drug Administration or physician panels to recommend target prices based on a drug’s magnitude of benefit (value-based pricing)
- Eliminate “pay-for-delay” strategies in which a pharmaceutical company with a brand name drug shares profits on that drug with a generic drug manufacturer for the remainder of a patent period, effectively eliminating a patent challenge and competition
- Allow the importation of drugs from abroad for personal use
- Allow the Patient-Centered Outcomes Research Institute and other cancer advocacy groups to consider cost in their recommendations
- Create patient-driven grassroots movements and organizations to advocate effectively for the interests of patients with cancer to balance advocacy efforts of pharmaceutical companies, insurance companies, pharmacy outlets, and hospitals.
Dr Kantarjian has organized a petition, which is available on change.org, asking the federal government to implement these changes. ![]()