User login
Is hemoglobin A1c an accurate measure of glycemic control in all diabetic patients?
No. Hemoglobin A1c has been validated as a predictor of diabetes-related complications and is a standard measure of the adequacy of glucose control. But sometimes we need to regard its values with suspicion, especially when they are not concordant with the patient’s self-monitored blood glucose levels.
UNIVERSALLY USED
Measuring glycated hemoglobin has become an essential tool for detecting impaired glucose tolerance (when levels are between 5.7% and 6.5%), for diagnosing diabetes mellitus (when levels are ≥ 6.5%), and for following the adequacy of control in established disease. The results reflect glycemic control over the preceding 2 to 3 months and possibly indicate the risk of complications, particularly microvascular disease in the long term.
The significance of hemoglobin A1c was further accentuated with the results of the DETECT-2 project,1 which showed that the risk of diabetic retinopathy is insignificant with levels lower than 6% and rises substantially when it is greater than 6.5%.
However, because the biochemical hallmark of diabetes is hyperglycemia (and not the glycation of proteins), concerns have been raised about the universal validity of hemoglobin A1c in all diabetic patients, especially when it is used to monitor glucose control in the long term.2
FACTORS THAT AFFECT THE GLYCATED HEMOGLOBIN LEVEL
Altered glycation
Although the hemoglobin A1c value correlates well with the mean blood glucose level over the previous months, it is affected more by the most recent glucose levels than by earlier levels, and it is especially affected by the most recent peak in blood glucose.3 It is estimated that approximately 50% of the hemoglobin A1c level is determined by the plasma glucose level during the preceding 1-month period.3
Other factors that affect levels of glycated hemoglobin independently of the average glucose level during the previous months include genetic predisposition (some people are “rapid glycators”), labile glycation (ie, transient glycation of hemoglobin when exposed to very high concentrations of glucose), and the 2,3-diphosphoglycerate concentration and pH of the blood.2
Hemoglobin factors
Age of red blood cells. Red blood cells last about 120 days, and the mean age of all red blood cells in circulation ranges from 38 to 60 days (50 on average). Turnover is dictated by a number of factors, including ethnicity, which in turn significantly affect hemoglobin A1c values.
Race and ethnicity. African American, Asian, and Hispanic patients may have higher hemoglobin A1c values than white people who have the same blood glucose levels. In one study of racial and ethnic differences in mean plasma glucose, levels were higher by 0.37% in African American patients, 0.27% in Hispanics, and 0.33% in Asians than in white patients, and the differences were statistically significant.4 However, there is no clear evidence that these differences are associated with differences in the incidence of microvascular disease.5
Effects due to heritable factors could vary among ethnic groups. Racial differences in hemoglobin A1c may be ascribed to the degree of glycation, caused by multiple factors, and to socioeconomic status. Interestingly, many of the interracial differences in conditions that affect erythrocyte turnover would in theory lead to a lower hemoglobin A1c in nonwhites, which is not the case.6
Pregnancy. The mechanisms of hemoglobin A1c discrepancy in pregnancy are not clear. It has been demonstrated that pregnant women may have lower hemoglobin A1c levels than nonpregnant women.7–9 Hemodilution and increased cell turnover have been postulated to account for the decrease, although a mechanism has not been described. Interestingly, conflicting data have been reported regarding hemoglobin A1c in the last trimester of pregnancy (increase, decrease, or no change). Iron deficiency has been presumed to cause the increase of hemoglobin A1c in the last trimester.10
Moreover, hemoglobin A1c may reflect glucose levels during a shorter time because of increased turnover of red blood cells that occurs during this state. Erythropoietin and erythrocyte production are increased during normal pregnancy while hemoglobin and hematocrit continuously dilute into the third trimester. In normal pregnancy, the red blood cell life span is decreased due to “emergency hemopoiesis” in response to these elevated erythropoietin levels.
Anemia. Hemolytic anemia, acute bleeding, and iron-deficiency anemia all influence glycated hemoglobin levels. The formation of reticulocytes whose hemoglobin lacks glycosylation may lead to falsely low hemoglobin A1c values. Interestingly, iron deficiency by itself has been observed to cause elevation of hemoglobin A1c through unclear mechanisms11; however, iron replacement may lead to reticulocytosis. Alternatively, asplenic patients may have deceptively higher hemoglobin A1c values because of the increased life span of their red blood cells.12
Hemoglobinopathy. Hemoglobin F may cause overestimation of hemoglobin A1c levels, whereas hemoglobin S and hemoglobin C may cause underestimation. Of note, these effects are method-specific, and newer immunoassay techniques are relatively robust even in the presence of common hemoglobin variants. Clinicians should be aware of their institution’s laboratory method for measuring glycated hemoglobin.13
Comorbidities
Chronic illnesses can cause fluctuation in hemoglobin A1c and make it unreliable. Uremia, severe hypertriglyceridemia, severe hyperbilirubinemia, chronic alcoholism, chronic salicylate use, chronic opioid use, and lead poisoning all can falsely increase hemoglobin A1c levels.
Vitamin and mineral deficiencies (eg, deficiencies of vitamin B12 and iron) can reduce red blood cell turnover and therefore falsely elevate hemoglobin A1c levels. Conversely, medical replacement of these deficiencies could lead to higher red blood cell turnover and reduced hemoglobin A1c levels.
Blood transfusions. Recent reports suggest that red blood cell transfusions reduce the hemoglobin A1c concentration in diabetic patients. This effect was most pronounced in patients who received large transfusion volumes or who had a high hemoglobin A1c level before the transfusion.14
Renal failure. Patients with renal failure have higher levels of carbamylated hemoglobin, which is reported to interfere with measurement and interpretation of hemoglobin A1c. Moreover, there is concern that hemoglobin A1c values may be falsely low in these patients because of shortened erythrocyte survival. Other factors that influence hemoglobin A1c and cause the measured levels to be misleadingly low in renal failure patients include use of recombinant human erythropoietin, the uremic environment, and blood transfusions.15
It has been suggested that glycated albumin may be a better marker for assessing glycemic control in patients with severe chronic kidney disease.16
Medications and supplements that affect hemoglobin
Drugs that may cause hemolysis could lower hemoglobin A1c levels. Examples are dapsone, ribavirin, and sulfonamides. Other drugs can change the structure of hemoglobin. For example, hydroxyurea alters hemoglobin A into hemoglobin F, thus lowering the hemoglobin A1c level. Chronic opiate use has been reported to increase hemoglobin A1c levels through mechanisms yet unclear.
Aspirin, vitamin C, and vitamin E have been postulated to interfere with hemoglobin A1c measurement assays, although studies have not been consistent in demonstrating these effects.
Labile diabetes
In some patients with diabetes, blood glucose levels are labile and oscillate between states of hypoglycemia and hyperglycemia, despite optimal hemoglobin A1c levels.17 In these patients, the average blood glucose level may very well correlate appropriately with the glycated hemoglobin level, but the degree of control would not be acceptable. Fasting hyperglycemia or postprandial hyperglycemia, or both, especially in the setting of significant glycemic variability over the month before testing, may not be captured by the hemoglobin A1c measurement. These glycemic excursions may be important, as data suggest that this variability may independently worsen microvascular complications in diabetic patients.18
ALTERNATIVES TO MEASURING THE GLYCATED HEMOGLOBIN
When hemoglobin A1c levels are suspected to be inaccurate, other tests of the adequacy of glycemic control can be used.19
Continuous glucose monitoring is the gold standard and precisely shows the degree of glycemic variability, usually over 5 days. It is often used when hypoglycemia and wide fluctuations in within-day and day-to-day glucose levels are suspected. In addition, we believe that continuous monitoring could be used to confirm the validity of hemoglobin A1c testing. In a clinical setting in which the level does not seem to match the fingerstick blood glucose readings, it can be a useful tool to assess the range and variation in glycemic control.
This method, however, is not practical in all diabetic patients, and it certainly does not have the same long-term predictive prognostic value. Yet it may still have a role in validating measures of long-term glycemic control (eg, hemoglobin A1c). There is evidence that using continuous glucose monitoring periodically can improve glycemic control, lower hemoglobin A1c levels, and lead to fewer hypoglycemic events.20 As discussed earlier, patients who have labile glycemic excursions and higher risk of microvascular complications can still have “normal” hemoglobin A1c levels; in this scenario, the use of continuous glucose monitoring can lead to lower risk and better control.
1,5-anhydroglucitol and fructosamine are circulating biomarkers that reflect short-term glucose control, ie, over 2 to 3 weeks. The higher the average blood glucose level, the lower the 1,5-anhydroglucitol level, since higher glucose levels competitively inhibit renal reabsorption of this molecule. However, its utility is limited in renal failure, liver disease, and pregnancy.
Fructosamines are nonenzymatically glycated proteins. As markers, they are reliable in renal disease but are unreliable in hypoproteinemic states such as liver disease, nephrosis, and lipemia. This group of proteins represents all of serum-stable glycated proteins; they are strongly influenced by the concentration of serum proteins, as well as by coexisting low-molecular-weight substances in the plasma.
Glycated albumin is superior to glycated hemoglobin in reflecting glycemic control, as it has a faster metabolic turnover than hemoglobin and is not affected by hemoglobin-opathies. Unlike fructosamines, it is not influenced by the serum albumin concentration. Moreover, it may be superior to the hemoglobin A1c in patients who have postprandial hypoglycemia.21
Interestingly, recent cross-sectional analyses suggest that fructosamines and glycated albumin are at least as strongly associated with microvascular complications as the hemoglobin A1c is.22
BE ALERT TO FACTORS THAT AFFECT GLYCATED HEMOGLOBIN
Hemoglobin A1c reflects exposure of red blood cells to glucose. Multiple factors—pathologic, physiologic, and environmental—can influence the glycation process, red blood cell turnover, and the hemoglobin structure in ways that can decrease the reliability of the hemoglobin A1c measurement.
Clinicians should be vigilant for the various clinical situations in which hemoglobin A1c is hard to interpret, and they should be familiar with alternative tests (eg, continuous glucose monitoring, 1,5-anhydroglucitol, fructosamines) that can be used to monitor adequate glycemic control in these patients.
- Colaguiri S, Lee CM, Wong TY, Balkau B, Shaw JE, Borch-Johnsen K; DETECT-2 Collaboration Writing Group. Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care 2011; 34:145–150.
- Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care 2011; 34(suppl 2):S184–S190.
- Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 2002; 25:275–278.
- Herman WH, Dungan KM, Wolffenbuttel BH, et al. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94:1689–1694.
- Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362:800–811.
- Tahara Y, Shima K. The response of GHb to stepwise plasma glucose change over time in diabetic patients. Diabetes Care 1993; 16:1313–1314.
- Radder JK, van Roosmalen J. HbA1c in healthy, pregnant women. Neth J Med 2005; 63:256–259.
- Mosca A, Paleari R, Dalfra MG, et al. Reference intervals for hemoglobin A1c in pregnant women: data from an Italian multicenter study. Clin Chem 2006; 52:1138–1143.
- Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care 2004; 27:1200–1201.
- Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol 2011; 5:1572–1583.
- Tarim O, Kucukerdogan A, Gunay U, Eralp O, Ercan I. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int 1999; 41:357–362.
- Panzer S, Kronik G, Lechner K, Bettelheim P, Neumann E, Dudczak R. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood 1982; 59:1348–1350.
- National Glycohemoglobin Standardization Program. HbA1c assay interferences. www.ngsp.org/interf.asp. Accessed December 27, 2013.
- Spencer DH, Grossman BJ, Scott MG. Red cell transfusion decreases hemoglobin A1c in patients with diabetes. Clin Chem 2011; 57:344–346.
- Little RR, Rohlfing CL, Tennill AL, et al. Measurement of Hba(1C) in patients with chronic renal failure. Clin Chim Acta 2013; 418:73–76.
- Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. NDT Plus 2011; 4:368–375.
- Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia 2007; 50:2553–2561.
- Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295:1681–1687.
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med 2013; Sep 4 [epub ahead of print]. http://link.springer.com/article/10.1007%2Fs11606-013-2595-x/fulltext.html. Accessed January 29, 2014.
- Leinung M, Nardacci E, Patel N, Bettadahalli S, Paika K, Thompson S. Benefits of short-term professional continuous glucose monitoring in clinical practice. Diabetes Technol Ther 2013; 15:744–747.
- Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J 2010; 57:751–762.
- Selvin E, Francis LM, Ballantyne CM, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care 2011; 34:960–967.
No. Hemoglobin A1c has been validated as a predictor of diabetes-related complications and is a standard measure of the adequacy of glucose control. But sometimes we need to regard its values with suspicion, especially when they are not concordant with the patient’s self-monitored blood glucose levels.
UNIVERSALLY USED
Measuring glycated hemoglobin has become an essential tool for detecting impaired glucose tolerance (when levels are between 5.7% and 6.5%), for diagnosing diabetes mellitus (when levels are ≥ 6.5%), and for following the adequacy of control in established disease. The results reflect glycemic control over the preceding 2 to 3 months and possibly indicate the risk of complications, particularly microvascular disease in the long term.
The significance of hemoglobin A1c was further accentuated with the results of the DETECT-2 project,1 which showed that the risk of diabetic retinopathy is insignificant with levels lower than 6% and rises substantially when it is greater than 6.5%.
However, because the biochemical hallmark of diabetes is hyperglycemia (and not the glycation of proteins), concerns have been raised about the universal validity of hemoglobin A1c in all diabetic patients, especially when it is used to monitor glucose control in the long term.2
FACTORS THAT AFFECT THE GLYCATED HEMOGLOBIN LEVEL
Altered glycation
Although the hemoglobin A1c value correlates well with the mean blood glucose level over the previous months, it is affected more by the most recent glucose levels than by earlier levels, and it is especially affected by the most recent peak in blood glucose.3 It is estimated that approximately 50% of the hemoglobin A1c level is determined by the plasma glucose level during the preceding 1-month period.3
Other factors that affect levels of glycated hemoglobin independently of the average glucose level during the previous months include genetic predisposition (some people are “rapid glycators”), labile glycation (ie, transient glycation of hemoglobin when exposed to very high concentrations of glucose), and the 2,3-diphosphoglycerate concentration and pH of the blood.2
Hemoglobin factors
Age of red blood cells. Red blood cells last about 120 days, and the mean age of all red blood cells in circulation ranges from 38 to 60 days (50 on average). Turnover is dictated by a number of factors, including ethnicity, which in turn significantly affect hemoglobin A1c values.
Race and ethnicity. African American, Asian, and Hispanic patients may have higher hemoglobin A1c values than white people who have the same blood glucose levels. In one study of racial and ethnic differences in mean plasma glucose, levels were higher by 0.37% in African American patients, 0.27% in Hispanics, and 0.33% in Asians than in white patients, and the differences were statistically significant.4 However, there is no clear evidence that these differences are associated with differences in the incidence of microvascular disease.5
Effects due to heritable factors could vary among ethnic groups. Racial differences in hemoglobin A1c may be ascribed to the degree of glycation, caused by multiple factors, and to socioeconomic status. Interestingly, many of the interracial differences in conditions that affect erythrocyte turnover would in theory lead to a lower hemoglobin A1c in nonwhites, which is not the case.6
Pregnancy. The mechanisms of hemoglobin A1c discrepancy in pregnancy are not clear. It has been demonstrated that pregnant women may have lower hemoglobin A1c levels than nonpregnant women.7–9 Hemodilution and increased cell turnover have been postulated to account for the decrease, although a mechanism has not been described. Interestingly, conflicting data have been reported regarding hemoglobin A1c in the last trimester of pregnancy (increase, decrease, or no change). Iron deficiency has been presumed to cause the increase of hemoglobin A1c in the last trimester.10
Moreover, hemoglobin A1c may reflect glucose levels during a shorter time because of increased turnover of red blood cells that occurs during this state. Erythropoietin and erythrocyte production are increased during normal pregnancy while hemoglobin and hematocrit continuously dilute into the third trimester. In normal pregnancy, the red blood cell life span is decreased due to “emergency hemopoiesis” in response to these elevated erythropoietin levels.
Anemia. Hemolytic anemia, acute bleeding, and iron-deficiency anemia all influence glycated hemoglobin levels. The formation of reticulocytes whose hemoglobin lacks glycosylation may lead to falsely low hemoglobin A1c values. Interestingly, iron deficiency by itself has been observed to cause elevation of hemoglobin A1c through unclear mechanisms11; however, iron replacement may lead to reticulocytosis. Alternatively, asplenic patients may have deceptively higher hemoglobin A1c values because of the increased life span of their red blood cells.12
Hemoglobinopathy. Hemoglobin F may cause overestimation of hemoglobin A1c levels, whereas hemoglobin S and hemoglobin C may cause underestimation. Of note, these effects are method-specific, and newer immunoassay techniques are relatively robust even in the presence of common hemoglobin variants. Clinicians should be aware of their institution’s laboratory method for measuring glycated hemoglobin.13
Comorbidities
Chronic illnesses can cause fluctuation in hemoglobin A1c and make it unreliable. Uremia, severe hypertriglyceridemia, severe hyperbilirubinemia, chronic alcoholism, chronic salicylate use, chronic opioid use, and lead poisoning all can falsely increase hemoglobin A1c levels.
Vitamin and mineral deficiencies (eg, deficiencies of vitamin B12 and iron) can reduce red blood cell turnover and therefore falsely elevate hemoglobin A1c levels. Conversely, medical replacement of these deficiencies could lead to higher red blood cell turnover and reduced hemoglobin A1c levels.
Blood transfusions. Recent reports suggest that red blood cell transfusions reduce the hemoglobin A1c concentration in diabetic patients. This effect was most pronounced in patients who received large transfusion volumes or who had a high hemoglobin A1c level before the transfusion.14
Renal failure. Patients with renal failure have higher levels of carbamylated hemoglobin, which is reported to interfere with measurement and interpretation of hemoglobin A1c. Moreover, there is concern that hemoglobin A1c values may be falsely low in these patients because of shortened erythrocyte survival. Other factors that influence hemoglobin A1c and cause the measured levels to be misleadingly low in renal failure patients include use of recombinant human erythropoietin, the uremic environment, and blood transfusions.15
It has been suggested that glycated albumin may be a better marker for assessing glycemic control in patients with severe chronic kidney disease.16
Medications and supplements that affect hemoglobin
Drugs that may cause hemolysis could lower hemoglobin A1c levels. Examples are dapsone, ribavirin, and sulfonamides. Other drugs can change the structure of hemoglobin. For example, hydroxyurea alters hemoglobin A into hemoglobin F, thus lowering the hemoglobin A1c level. Chronic opiate use has been reported to increase hemoglobin A1c levels through mechanisms yet unclear.
Aspirin, vitamin C, and vitamin E have been postulated to interfere with hemoglobin A1c measurement assays, although studies have not been consistent in demonstrating these effects.
Labile diabetes
In some patients with diabetes, blood glucose levels are labile and oscillate between states of hypoglycemia and hyperglycemia, despite optimal hemoglobin A1c levels.17 In these patients, the average blood glucose level may very well correlate appropriately with the glycated hemoglobin level, but the degree of control would not be acceptable. Fasting hyperglycemia or postprandial hyperglycemia, or both, especially in the setting of significant glycemic variability over the month before testing, may not be captured by the hemoglobin A1c measurement. These glycemic excursions may be important, as data suggest that this variability may independently worsen microvascular complications in diabetic patients.18
ALTERNATIVES TO MEASURING THE GLYCATED HEMOGLOBIN
When hemoglobin A1c levels are suspected to be inaccurate, other tests of the adequacy of glycemic control can be used.19
Continuous glucose monitoring is the gold standard and precisely shows the degree of glycemic variability, usually over 5 days. It is often used when hypoglycemia and wide fluctuations in within-day and day-to-day glucose levels are suspected. In addition, we believe that continuous monitoring could be used to confirm the validity of hemoglobin A1c testing. In a clinical setting in which the level does not seem to match the fingerstick blood glucose readings, it can be a useful tool to assess the range and variation in glycemic control.
This method, however, is not practical in all diabetic patients, and it certainly does not have the same long-term predictive prognostic value. Yet it may still have a role in validating measures of long-term glycemic control (eg, hemoglobin A1c). There is evidence that using continuous glucose monitoring periodically can improve glycemic control, lower hemoglobin A1c levels, and lead to fewer hypoglycemic events.20 As discussed earlier, patients who have labile glycemic excursions and higher risk of microvascular complications can still have “normal” hemoglobin A1c levels; in this scenario, the use of continuous glucose monitoring can lead to lower risk and better control.
1,5-anhydroglucitol and fructosamine are circulating biomarkers that reflect short-term glucose control, ie, over 2 to 3 weeks. The higher the average blood glucose level, the lower the 1,5-anhydroglucitol level, since higher glucose levels competitively inhibit renal reabsorption of this molecule. However, its utility is limited in renal failure, liver disease, and pregnancy.
Fructosamines are nonenzymatically glycated proteins. As markers, they are reliable in renal disease but are unreliable in hypoproteinemic states such as liver disease, nephrosis, and lipemia. This group of proteins represents all of serum-stable glycated proteins; they are strongly influenced by the concentration of serum proteins, as well as by coexisting low-molecular-weight substances in the plasma.
Glycated albumin is superior to glycated hemoglobin in reflecting glycemic control, as it has a faster metabolic turnover than hemoglobin and is not affected by hemoglobin-opathies. Unlike fructosamines, it is not influenced by the serum albumin concentration. Moreover, it may be superior to the hemoglobin A1c in patients who have postprandial hypoglycemia.21
Interestingly, recent cross-sectional analyses suggest that fructosamines and glycated albumin are at least as strongly associated with microvascular complications as the hemoglobin A1c is.22
BE ALERT TO FACTORS THAT AFFECT GLYCATED HEMOGLOBIN
Hemoglobin A1c reflects exposure of red blood cells to glucose. Multiple factors—pathologic, physiologic, and environmental—can influence the glycation process, red blood cell turnover, and the hemoglobin structure in ways that can decrease the reliability of the hemoglobin A1c measurement.
Clinicians should be vigilant for the various clinical situations in which hemoglobin A1c is hard to interpret, and they should be familiar with alternative tests (eg, continuous glucose monitoring, 1,5-anhydroglucitol, fructosamines) that can be used to monitor adequate glycemic control in these patients.
No. Hemoglobin A1c has been validated as a predictor of diabetes-related complications and is a standard measure of the adequacy of glucose control. But sometimes we need to regard its values with suspicion, especially when they are not concordant with the patient’s self-monitored blood glucose levels.
UNIVERSALLY USED
Measuring glycated hemoglobin has become an essential tool for detecting impaired glucose tolerance (when levels are between 5.7% and 6.5%), for diagnosing diabetes mellitus (when levels are ≥ 6.5%), and for following the adequacy of control in established disease. The results reflect glycemic control over the preceding 2 to 3 months and possibly indicate the risk of complications, particularly microvascular disease in the long term.
The significance of hemoglobin A1c was further accentuated with the results of the DETECT-2 project,1 which showed that the risk of diabetic retinopathy is insignificant with levels lower than 6% and rises substantially when it is greater than 6.5%.
However, because the biochemical hallmark of diabetes is hyperglycemia (and not the glycation of proteins), concerns have been raised about the universal validity of hemoglobin A1c in all diabetic patients, especially when it is used to monitor glucose control in the long term.2
FACTORS THAT AFFECT THE GLYCATED HEMOGLOBIN LEVEL
Altered glycation
Although the hemoglobin A1c value correlates well with the mean blood glucose level over the previous months, it is affected more by the most recent glucose levels than by earlier levels, and it is especially affected by the most recent peak in blood glucose.3 It is estimated that approximately 50% of the hemoglobin A1c level is determined by the plasma glucose level during the preceding 1-month period.3
Other factors that affect levels of glycated hemoglobin independently of the average glucose level during the previous months include genetic predisposition (some people are “rapid glycators”), labile glycation (ie, transient glycation of hemoglobin when exposed to very high concentrations of glucose), and the 2,3-diphosphoglycerate concentration and pH of the blood.2
Hemoglobin factors
Age of red blood cells. Red blood cells last about 120 days, and the mean age of all red blood cells in circulation ranges from 38 to 60 days (50 on average). Turnover is dictated by a number of factors, including ethnicity, which in turn significantly affect hemoglobin A1c values.
Race and ethnicity. African American, Asian, and Hispanic patients may have higher hemoglobin A1c values than white people who have the same blood glucose levels. In one study of racial and ethnic differences in mean plasma glucose, levels were higher by 0.37% in African American patients, 0.27% in Hispanics, and 0.33% in Asians than in white patients, and the differences were statistically significant.4 However, there is no clear evidence that these differences are associated with differences in the incidence of microvascular disease.5
Effects due to heritable factors could vary among ethnic groups. Racial differences in hemoglobin A1c may be ascribed to the degree of glycation, caused by multiple factors, and to socioeconomic status. Interestingly, many of the interracial differences in conditions that affect erythrocyte turnover would in theory lead to a lower hemoglobin A1c in nonwhites, which is not the case.6
Pregnancy. The mechanisms of hemoglobin A1c discrepancy in pregnancy are not clear. It has been demonstrated that pregnant women may have lower hemoglobin A1c levels than nonpregnant women.7–9 Hemodilution and increased cell turnover have been postulated to account for the decrease, although a mechanism has not been described. Interestingly, conflicting data have been reported regarding hemoglobin A1c in the last trimester of pregnancy (increase, decrease, or no change). Iron deficiency has been presumed to cause the increase of hemoglobin A1c in the last trimester.10
Moreover, hemoglobin A1c may reflect glucose levels during a shorter time because of increased turnover of red blood cells that occurs during this state. Erythropoietin and erythrocyte production are increased during normal pregnancy while hemoglobin and hematocrit continuously dilute into the third trimester. In normal pregnancy, the red blood cell life span is decreased due to “emergency hemopoiesis” in response to these elevated erythropoietin levels.
Anemia. Hemolytic anemia, acute bleeding, and iron-deficiency anemia all influence glycated hemoglobin levels. The formation of reticulocytes whose hemoglobin lacks glycosylation may lead to falsely low hemoglobin A1c values. Interestingly, iron deficiency by itself has been observed to cause elevation of hemoglobin A1c through unclear mechanisms11; however, iron replacement may lead to reticulocytosis. Alternatively, asplenic patients may have deceptively higher hemoglobin A1c values because of the increased life span of their red blood cells.12
Hemoglobinopathy. Hemoglobin F may cause overestimation of hemoglobin A1c levels, whereas hemoglobin S and hemoglobin C may cause underestimation. Of note, these effects are method-specific, and newer immunoassay techniques are relatively robust even in the presence of common hemoglobin variants. Clinicians should be aware of their institution’s laboratory method for measuring glycated hemoglobin.13
Comorbidities
Chronic illnesses can cause fluctuation in hemoglobin A1c and make it unreliable. Uremia, severe hypertriglyceridemia, severe hyperbilirubinemia, chronic alcoholism, chronic salicylate use, chronic opioid use, and lead poisoning all can falsely increase hemoglobin A1c levels.
Vitamin and mineral deficiencies (eg, deficiencies of vitamin B12 and iron) can reduce red blood cell turnover and therefore falsely elevate hemoglobin A1c levels. Conversely, medical replacement of these deficiencies could lead to higher red blood cell turnover and reduced hemoglobin A1c levels.
Blood transfusions. Recent reports suggest that red blood cell transfusions reduce the hemoglobin A1c concentration in diabetic patients. This effect was most pronounced in patients who received large transfusion volumes or who had a high hemoglobin A1c level before the transfusion.14
Renal failure. Patients with renal failure have higher levels of carbamylated hemoglobin, which is reported to interfere with measurement and interpretation of hemoglobin A1c. Moreover, there is concern that hemoglobin A1c values may be falsely low in these patients because of shortened erythrocyte survival. Other factors that influence hemoglobin A1c and cause the measured levels to be misleadingly low in renal failure patients include use of recombinant human erythropoietin, the uremic environment, and blood transfusions.15
It has been suggested that glycated albumin may be a better marker for assessing glycemic control in patients with severe chronic kidney disease.16
Medications and supplements that affect hemoglobin
Drugs that may cause hemolysis could lower hemoglobin A1c levels. Examples are dapsone, ribavirin, and sulfonamides. Other drugs can change the structure of hemoglobin. For example, hydroxyurea alters hemoglobin A into hemoglobin F, thus lowering the hemoglobin A1c level. Chronic opiate use has been reported to increase hemoglobin A1c levels through mechanisms yet unclear.
Aspirin, vitamin C, and vitamin E have been postulated to interfere with hemoglobin A1c measurement assays, although studies have not been consistent in demonstrating these effects.
Labile diabetes
In some patients with diabetes, blood glucose levels are labile and oscillate between states of hypoglycemia and hyperglycemia, despite optimal hemoglobin A1c levels.17 In these patients, the average blood glucose level may very well correlate appropriately with the glycated hemoglobin level, but the degree of control would not be acceptable. Fasting hyperglycemia or postprandial hyperglycemia, or both, especially in the setting of significant glycemic variability over the month before testing, may not be captured by the hemoglobin A1c measurement. These glycemic excursions may be important, as data suggest that this variability may independently worsen microvascular complications in diabetic patients.18
ALTERNATIVES TO MEASURING THE GLYCATED HEMOGLOBIN
When hemoglobin A1c levels are suspected to be inaccurate, other tests of the adequacy of glycemic control can be used.19
Continuous glucose monitoring is the gold standard and precisely shows the degree of glycemic variability, usually over 5 days. It is often used when hypoglycemia and wide fluctuations in within-day and day-to-day glucose levels are suspected. In addition, we believe that continuous monitoring could be used to confirm the validity of hemoglobin A1c testing. In a clinical setting in which the level does not seem to match the fingerstick blood glucose readings, it can be a useful tool to assess the range and variation in glycemic control.
This method, however, is not practical in all diabetic patients, and it certainly does not have the same long-term predictive prognostic value. Yet it may still have a role in validating measures of long-term glycemic control (eg, hemoglobin A1c). There is evidence that using continuous glucose monitoring periodically can improve glycemic control, lower hemoglobin A1c levels, and lead to fewer hypoglycemic events.20 As discussed earlier, patients who have labile glycemic excursions and higher risk of microvascular complications can still have “normal” hemoglobin A1c levels; in this scenario, the use of continuous glucose monitoring can lead to lower risk and better control.
1,5-anhydroglucitol and fructosamine are circulating biomarkers that reflect short-term glucose control, ie, over 2 to 3 weeks. The higher the average blood glucose level, the lower the 1,5-anhydroglucitol level, since higher glucose levels competitively inhibit renal reabsorption of this molecule. However, its utility is limited in renal failure, liver disease, and pregnancy.
Fructosamines are nonenzymatically glycated proteins. As markers, they are reliable in renal disease but are unreliable in hypoproteinemic states such as liver disease, nephrosis, and lipemia. This group of proteins represents all of serum-stable glycated proteins; they are strongly influenced by the concentration of serum proteins, as well as by coexisting low-molecular-weight substances in the plasma.
Glycated albumin is superior to glycated hemoglobin in reflecting glycemic control, as it has a faster metabolic turnover than hemoglobin and is not affected by hemoglobin-opathies. Unlike fructosamines, it is not influenced by the serum albumin concentration. Moreover, it may be superior to the hemoglobin A1c in patients who have postprandial hypoglycemia.21
Interestingly, recent cross-sectional analyses suggest that fructosamines and glycated albumin are at least as strongly associated with microvascular complications as the hemoglobin A1c is.22
BE ALERT TO FACTORS THAT AFFECT GLYCATED HEMOGLOBIN
Hemoglobin A1c reflects exposure of red blood cells to glucose. Multiple factors—pathologic, physiologic, and environmental—can influence the glycation process, red blood cell turnover, and the hemoglobin structure in ways that can decrease the reliability of the hemoglobin A1c measurement.
Clinicians should be vigilant for the various clinical situations in which hemoglobin A1c is hard to interpret, and they should be familiar with alternative tests (eg, continuous glucose monitoring, 1,5-anhydroglucitol, fructosamines) that can be used to monitor adequate glycemic control in these patients.
- Colaguiri S, Lee CM, Wong TY, Balkau B, Shaw JE, Borch-Johnsen K; DETECT-2 Collaboration Writing Group. Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care 2011; 34:145–150.
- Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care 2011; 34(suppl 2):S184–S190.
- Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 2002; 25:275–278.
- Herman WH, Dungan KM, Wolffenbuttel BH, et al. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94:1689–1694.
- Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362:800–811.
- Tahara Y, Shima K. The response of GHb to stepwise plasma glucose change over time in diabetic patients. Diabetes Care 1993; 16:1313–1314.
- Radder JK, van Roosmalen J. HbA1c in healthy, pregnant women. Neth J Med 2005; 63:256–259.
- Mosca A, Paleari R, Dalfra MG, et al. Reference intervals for hemoglobin A1c in pregnant women: data from an Italian multicenter study. Clin Chem 2006; 52:1138–1143.
- Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care 2004; 27:1200–1201.
- Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol 2011; 5:1572–1583.
- Tarim O, Kucukerdogan A, Gunay U, Eralp O, Ercan I. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int 1999; 41:357–362.
- Panzer S, Kronik G, Lechner K, Bettelheim P, Neumann E, Dudczak R. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood 1982; 59:1348–1350.
- National Glycohemoglobin Standardization Program. HbA1c assay interferences. www.ngsp.org/interf.asp. Accessed December 27, 2013.
- Spencer DH, Grossman BJ, Scott MG. Red cell transfusion decreases hemoglobin A1c in patients with diabetes. Clin Chem 2011; 57:344–346.
- Little RR, Rohlfing CL, Tennill AL, et al. Measurement of Hba(1C) in patients with chronic renal failure. Clin Chim Acta 2013; 418:73–76.
- Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. NDT Plus 2011; 4:368–375.
- Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia 2007; 50:2553–2561.
- Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295:1681–1687.
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med 2013; Sep 4 [epub ahead of print]. http://link.springer.com/article/10.1007%2Fs11606-013-2595-x/fulltext.html. Accessed January 29, 2014.
- Leinung M, Nardacci E, Patel N, Bettadahalli S, Paika K, Thompson S. Benefits of short-term professional continuous glucose monitoring in clinical practice. Diabetes Technol Ther 2013; 15:744–747.
- Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J 2010; 57:751–762.
- Selvin E, Francis LM, Ballantyne CM, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care 2011; 34:960–967.
- Colaguiri S, Lee CM, Wong TY, Balkau B, Shaw JE, Borch-Johnsen K; DETECT-2 Collaboration Writing Group. Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care 2011; 34:145–150.
- Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care 2011; 34(suppl 2):S184–S190.
- Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 2002; 25:275–278.
- Herman WH, Dungan KM, Wolffenbuttel BH, et al. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94:1689–1694.
- Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362:800–811.
- Tahara Y, Shima K. The response of GHb to stepwise plasma glucose change over time in diabetic patients. Diabetes Care 1993; 16:1313–1314.
- Radder JK, van Roosmalen J. HbA1c in healthy, pregnant women. Neth J Med 2005; 63:256–259.
- Mosca A, Paleari R, Dalfra MG, et al. Reference intervals for hemoglobin A1c in pregnant women: data from an Italian multicenter study. Clin Chem 2006; 52:1138–1143.
- Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care 2004; 27:1200–1201.
- Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol 2011; 5:1572–1583.
- Tarim O, Kucukerdogan A, Gunay U, Eralp O, Ercan I. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int 1999; 41:357–362.
- Panzer S, Kronik G, Lechner K, Bettelheim P, Neumann E, Dudczak R. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood 1982; 59:1348–1350.
- National Glycohemoglobin Standardization Program. HbA1c assay interferences. www.ngsp.org/interf.asp. Accessed December 27, 2013.
- Spencer DH, Grossman BJ, Scott MG. Red cell transfusion decreases hemoglobin A1c in patients with diabetes. Clin Chem 2011; 57:344–346.
- Little RR, Rohlfing CL, Tennill AL, et al. Measurement of Hba(1C) in patients with chronic renal failure. Clin Chim Acta 2013; 418:73–76.
- Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. NDT Plus 2011; 4:368–375.
- Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia 2007; 50:2553–2561.
- Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295:1681–1687.
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med 2013; Sep 4 [epub ahead of print]. http://link.springer.com/article/10.1007%2Fs11606-013-2595-x/fulltext.html. Accessed January 29, 2014.
- Leinung M, Nardacci E, Patel N, Bettadahalli S, Paika K, Thompson S. Benefits of short-term professional continuous glucose monitoring in clinical practice. Diabetes Technol Ther 2013; 15:744–747.
- Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J 2010; 57:751–762.
- Selvin E, Francis LM, Ballantyne CM, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care 2011; 34:960–967.
New practice guidelines: Constrained or enhanced by the evidence?
Recent guidelines have revisited the management of two major modifiable risk factors for cardiovascular morbidity: hypercholesterolemia and hypertension. The authors of each purposefully emphasized high-grade evidence in generating their recommendations. But, as pointed out by Thomas et al in this issue of the Journal,1 the authors of the hypertension guidelines still resorted to “expert opinion” in five of their 10 recommendations.
The authors of the new hypertension guidelines from the Eighth Joint National Committee (JNC 8),2 as well as the new cholesterol guidelines3 discussed by Raymond et al in the January 2014 issue of the Journal,4 relied on interventional clinical trial evidence for their recommendations. The good news in the context of pay-for-performance metrics is that both of these new guidelines are easier to adhere to than the previous ones. But will the new guidelines really help us achieve better patient outcomes?
Concerns about these guidelines spring directly from their assumed major strength—ie, that they are based on interventional trial data. Well-run, randomized, controlled trials are the brass ring of evidence-based medical practice, presumably providing the cleanest demonstration of therapeutic efficacy. But with “clean” data potentially come sterile, non-real-world conclusions that may advise but should not limit our practice decisions. Most of our patients do not fit neatly into trial inclusion and exclusion criteria, nor do they exactly match the demographics of trial volunteers. Patients who participate in clinical trials are not the same as the patients who populate our clinics. Nor, unfortunately, is the blood pressure measurement technique likely the same in the clinical trial setting as in many of our offices.
In the clinic, it seems obvious not to be overly zealous about blood pressure control in an elderly, frail, hypertensive patient. But at the same time, aiming for a systolic pressure lower than 150 mm Hg (which is looser than in the last set of guidelines) as a target for those over age 60 is incredibly arbitrary, given that physiology and biologic risk rarely act in a step-function manner. Biologic metrics tend to behave as a continuum. If we recognize that the blood pressure can be readily and safely reduced further in a given patient, and if there are observational data to support the concept that risk for cardiovascular events roughly parallels the systolic blood pressure in a continuous manner to lower than 150 mm Hg, why aim to lower it only slightly? Trial-based guidelines are valuable, but they should not replace sound physiologic reasoning and common sense (also known as “expert opinion”). Yet we must temper this logical reasoning with lessons learned from trials such as ACCORD,5 which showed that overly vigorous efforts at reaching theoretical therapeutic targets may be fraught with unexpected adverse outcomes.
Our challenge is to appropriately individualize therapy, usually in the absence of relevant comparative efficacy studies. Trying to apply homogenized clinical trial data to the individual patient in the examination room is not always reasonable. Treating a 59-year-old who has a slowly escalating systolic pressure of 142 mm Hg is not the same as treating a 32-year-old who has a chronic pressure of 138 and an audible S4.
The new hypertension guidelines should be easier to implement than the previous ones in JNC 7. I like some of the specificity of the new recommendations and the disappearance of beta-blockers from the list of recommended early therapies. Yet I think that in the presence of comorbidities and end-organ damage, they may be too lax. And certain groups are left relatively undiscussed, such as patients with cerebrovascular disease, known hypertensive vascular injury, and obstructive sleep apnea, as there were limited trial data to provide guidance (although for some clinical subsets we do have very suggestive observational and experiential data). We can’t always wait for the perfect trial to be done in order to make clinical decisions.
To paraphrase Thomas et al,1 for these guidelines, one size fits many, but we still must do significant custom tailoring in the office. In the months ahead, we will try to provide some guidance on how to effectively deal with those situations where robust trial evidence is insufficient to direct clinical decision-making.
- Thomas G, Shishehbor M, Brill D, Nally JV Jr. New hypertension guidelines: one size fits most? Cleve Clin J Med 2014; 81:178–188.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; doi: 10.1016/jacc.2013.11.002.
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81:11–19.
- Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
Recent guidelines have revisited the management of two major modifiable risk factors for cardiovascular morbidity: hypercholesterolemia and hypertension. The authors of each purposefully emphasized high-grade evidence in generating their recommendations. But, as pointed out by Thomas et al in this issue of the Journal,1 the authors of the hypertension guidelines still resorted to “expert opinion” in five of their 10 recommendations.
The authors of the new hypertension guidelines from the Eighth Joint National Committee (JNC 8),2 as well as the new cholesterol guidelines3 discussed by Raymond et al in the January 2014 issue of the Journal,4 relied on interventional clinical trial evidence for their recommendations. The good news in the context of pay-for-performance metrics is that both of these new guidelines are easier to adhere to than the previous ones. But will the new guidelines really help us achieve better patient outcomes?
Concerns about these guidelines spring directly from their assumed major strength—ie, that they are based on interventional trial data. Well-run, randomized, controlled trials are the brass ring of evidence-based medical practice, presumably providing the cleanest demonstration of therapeutic efficacy. But with “clean” data potentially come sterile, non-real-world conclusions that may advise but should not limit our practice decisions. Most of our patients do not fit neatly into trial inclusion and exclusion criteria, nor do they exactly match the demographics of trial volunteers. Patients who participate in clinical trials are not the same as the patients who populate our clinics. Nor, unfortunately, is the blood pressure measurement technique likely the same in the clinical trial setting as in many of our offices.
In the clinic, it seems obvious not to be overly zealous about blood pressure control in an elderly, frail, hypertensive patient. But at the same time, aiming for a systolic pressure lower than 150 mm Hg (which is looser than in the last set of guidelines) as a target for those over age 60 is incredibly arbitrary, given that physiology and biologic risk rarely act in a step-function manner. Biologic metrics tend to behave as a continuum. If we recognize that the blood pressure can be readily and safely reduced further in a given patient, and if there are observational data to support the concept that risk for cardiovascular events roughly parallels the systolic blood pressure in a continuous manner to lower than 150 mm Hg, why aim to lower it only slightly? Trial-based guidelines are valuable, but they should not replace sound physiologic reasoning and common sense (also known as “expert opinion”). Yet we must temper this logical reasoning with lessons learned from trials such as ACCORD,5 which showed that overly vigorous efforts at reaching theoretical therapeutic targets may be fraught with unexpected adverse outcomes.
Our challenge is to appropriately individualize therapy, usually in the absence of relevant comparative efficacy studies. Trying to apply homogenized clinical trial data to the individual patient in the examination room is not always reasonable. Treating a 59-year-old who has a slowly escalating systolic pressure of 142 mm Hg is not the same as treating a 32-year-old who has a chronic pressure of 138 and an audible S4.
The new hypertension guidelines should be easier to implement than the previous ones in JNC 7. I like some of the specificity of the new recommendations and the disappearance of beta-blockers from the list of recommended early therapies. Yet I think that in the presence of comorbidities and end-organ damage, they may be too lax. And certain groups are left relatively undiscussed, such as patients with cerebrovascular disease, known hypertensive vascular injury, and obstructive sleep apnea, as there were limited trial data to provide guidance (although for some clinical subsets we do have very suggestive observational and experiential data). We can’t always wait for the perfect trial to be done in order to make clinical decisions.
To paraphrase Thomas et al,1 for these guidelines, one size fits many, but we still must do significant custom tailoring in the office. In the months ahead, we will try to provide some guidance on how to effectively deal with those situations where robust trial evidence is insufficient to direct clinical decision-making.
Recent guidelines have revisited the management of two major modifiable risk factors for cardiovascular morbidity: hypercholesterolemia and hypertension. The authors of each purposefully emphasized high-grade evidence in generating their recommendations. But, as pointed out by Thomas et al in this issue of the Journal,1 the authors of the hypertension guidelines still resorted to “expert opinion” in five of their 10 recommendations.
The authors of the new hypertension guidelines from the Eighth Joint National Committee (JNC 8),2 as well as the new cholesterol guidelines3 discussed by Raymond et al in the January 2014 issue of the Journal,4 relied on interventional clinical trial evidence for their recommendations. The good news in the context of pay-for-performance metrics is that both of these new guidelines are easier to adhere to than the previous ones. But will the new guidelines really help us achieve better patient outcomes?
Concerns about these guidelines spring directly from their assumed major strength—ie, that they are based on interventional trial data. Well-run, randomized, controlled trials are the brass ring of evidence-based medical practice, presumably providing the cleanest demonstration of therapeutic efficacy. But with “clean” data potentially come sterile, non-real-world conclusions that may advise but should not limit our practice decisions. Most of our patients do not fit neatly into trial inclusion and exclusion criteria, nor do they exactly match the demographics of trial volunteers. Patients who participate in clinical trials are not the same as the patients who populate our clinics. Nor, unfortunately, is the blood pressure measurement technique likely the same in the clinical trial setting as in many of our offices.
In the clinic, it seems obvious not to be overly zealous about blood pressure control in an elderly, frail, hypertensive patient. But at the same time, aiming for a systolic pressure lower than 150 mm Hg (which is looser than in the last set of guidelines) as a target for those over age 60 is incredibly arbitrary, given that physiology and biologic risk rarely act in a step-function manner. Biologic metrics tend to behave as a continuum. If we recognize that the blood pressure can be readily and safely reduced further in a given patient, and if there are observational data to support the concept that risk for cardiovascular events roughly parallels the systolic blood pressure in a continuous manner to lower than 150 mm Hg, why aim to lower it only slightly? Trial-based guidelines are valuable, but they should not replace sound physiologic reasoning and common sense (also known as “expert opinion”). Yet we must temper this logical reasoning with lessons learned from trials such as ACCORD,5 which showed that overly vigorous efforts at reaching theoretical therapeutic targets may be fraught with unexpected adverse outcomes.
Our challenge is to appropriately individualize therapy, usually in the absence of relevant comparative efficacy studies. Trying to apply homogenized clinical trial data to the individual patient in the examination room is not always reasonable. Treating a 59-year-old who has a slowly escalating systolic pressure of 142 mm Hg is not the same as treating a 32-year-old who has a chronic pressure of 138 and an audible S4.
The new hypertension guidelines should be easier to implement than the previous ones in JNC 7. I like some of the specificity of the new recommendations and the disappearance of beta-blockers from the list of recommended early therapies. Yet I think that in the presence of comorbidities and end-organ damage, they may be too lax. And certain groups are left relatively undiscussed, such as patients with cerebrovascular disease, known hypertensive vascular injury, and obstructive sleep apnea, as there were limited trial data to provide guidance (although for some clinical subsets we do have very suggestive observational and experiential data). We can’t always wait for the perfect trial to be done in order to make clinical decisions.
To paraphrase Thomas et al,1 for these guidelines, one size fits many, but we still must do significant custom tailoring in the office. In the months ahead, we will try to provide some guidance on how to effectively deal with those situations where robust trial evidence is insufficient to direct clinical decision-making.
- Thomas G, Shishehbor M, Brill D, Nally JV Jr. New hypertension guidelines: one size fits most? Cleve Clin J Med 2014; 81:178–188.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; doi: 10.1016/jacc.2013.11.002.
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81:11–19.
- Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Thomas G, Shishehbor M, Brill D, Nally JV Jr. New hypertension guidelines: one size fits most? Cleve Clin J Med 2014; 81:178–188.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; doi: 10.1016/jacc.2013.11.002.
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81:11–19.
- Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
New hypertension guidelines: One size fits most?
The report of the panel appointed to the eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8),1 published in December 2013 after considerable delay, contains some important changes from earlier guidelines from this group.2 For example:
- The blood pressure goal has been changed to less than 150/90 mm Hg in people age 60 and older. Formerly, the goal was less than 140/90 mm Hg.
- The goal has been changed to less than 140/90 mm Hg in all others, including people with diabetes mellitus and chronic kidney disease. Formerly, those two groups had a goal of less than 130/80 mm Hg.
- The initial choice of therapy can be from any of four classes of drugs: thiazide-type diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs). Formerly, the list also contained beta-blockers. Also, thiazide-type diuretics have lost their “preferred” status.
The new guidelines are evidence-based and are intended to simplify the way that hypertension is managed. Below, we summarize them—how they were developed, their strengths and limitations, and the main changes from earlier JNC reports.
WHOSE GUIDELINES ARE THESE?
The JNC has issued guidelines for managing hypertension since 1976, traditionally sanctioned by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. The guidelines have generally been updated every 4 to 5 years, with the last update, JNC 7,2 published in 2003.
The JNC 8 panel, consisting of 17 members, was commissioned by the NHLBI in 2008. However, in June 2013, the NHLBI announced it was withdrawing from guideline development and was delegating it to selected specialty organizations.3,4 In the interest of bringing the already delayed guidelines to the public in a timely manner, the JNC 8 panel decided to pursue publication independently and submitted the report to a medical journal. It is therefore not an official NHLBI-sanctioned report.
Here, we will refer to the new guidelines as “JNC 8,” but they are officially from “panel members appointed to the Eighth Joint National Committee (JNC 8).”
THREE QUESTIONS THAT GUIDED THE GUIDELINES
Epidemiologic studies clearly show a close relationship between blood pressure and the risk of heart disease, stroke, and kidney disease, these risks being lowest at a blood pressure of around 115/75 mm Hg.5 However, clinical trials have failed to show any evidence to justify treatment with antihypertensive medications to such a low level once hypertension has been diagnosed.
Patients and health care providers thus face questions about when to begin treatment, how low to aim for, and which antihypertensive medications to use. The JNC 8 panel focused on these three questions, believing them to be of greatest relevance to primary care providers.
A RIGOROUS PROCESS OF EVIDENCE REVIEW AND GUIDELINE DEVELOPMENT
The JNC 8 panel followed the guideline-development pathway outlined by the Institute of Medicine report, Clinical Practice Guidelines We Can Trust.6
Studies published from January 1966 through December 2009 that met specified criteria were selected for evidence review. Specifically, the studies had to be randomized controlled trials—no observational studies, systematic reviews, or meta-analyses, which were allowed in the JNC 7 report—with sample sizes of more than 100. Follow-up had to be for more than 1 year. Participants had to be age 18 or older and have hypertension—studies with patients with normal blood pressure or prehypertension were excluded. Health outcomes had to be reported, ie, “hard” end points such as rates of death, myocardial infarction, heart failure, hospitalization for heart failure, stroke, revascularization, and end-stage renal disease. Post hoc analyses were not allowed. The studies had to be rated by the NHLBI’s standardized quality rating tool as “good” (which has the least risk of bias, with valid results) or “fair (which is susceptible to some bias, but not enough to invalidate the results).
Subsequently, another search was conducted for relevant studies published from December 2009 through August 2013. In addition to meeting all the other criteria, this bridging search further restricted selection to major multicenter studies with sample sizes of more than 2,000.
An external methodology team performed the initial literature review and summarized the data. The JNC panel then crafted evidence statements and clinical recommendations using the evidence quality rating and grading systems developed by the NHLBI. In January 2013, the NHLBI submitted the guidelines for external review by individual reviewers with expertise in hypertension and to federal agencies, and a revised document was framed based on their comments and suggestions.
The evidence statements are detailed in an online 300-page supplemental review, and the panel members have indicated that reviewer comments and responses from the presubmission review process will be made available on request.
NINE RECOMMENDATIONS AND ONE COROLLARY
The panel made nine recommendations and one corollary recommendation based on a review of the evidence. Of the 10 total recommendations, five are based on expert opinion. Another two were rated as “moderate” in strength, one was “weak,” and only two were rated as “strong” (ie, based on high-quality evidence).
Recommendation 1: < 150/90 for those 60 and older
In the general population age 60 and older, the JNC 8 recommends starting drug treatment if the systolic pressure is 150 mm Hg or higher or if the diastolic pressure is 90 mm Hg or higher, and aiming for a systolic goal of less than 150 mm Hg and a diastolic goal of less than 90 mm Hg.
Strength of recommendation—strong (grade A).
Comments. Of all the recommendations, this one will probably have the greatest impact on clinical practice. Consider a frail 70-year-old patient at risk of falls who is taking two antihypertensive medications and whose blood pressure is 148/85 mm Hg. This level would have been considered too high under JNC 7 but is now acceptable, and the patient’s therapy does not have to be escalated.
The age cutoff of 60 years for this recommendation is debatable. The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)7 included patients ages 60 to 85 (mean age 74) and found no difference in outcomes comparing a goal systolic pressure of less than 140 mm Hg (this group achieved a mean systolic pressure of 135.9 mm Hg) and a goal systolic pressure of 140 to 160 mm Hg (achieved systolic pressure 145.6 mm Hg).
Similarly, the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) trial8 included patients ages 70 to 84 (mean age 76.1) and found no difference in outcomes between a goal systolic pressure of less than 140 mm Hg (achieved systolic pressure 136.6 mm Hg) and a goal of 140 to 150 mm Hg (achieved systolic pressure 142 mm Hg).
The Hypertension in the Very Elderly Trial (HYVET)9 found lower rates of stroke, death, and heart failure in patients age 80 and older when their systolic pressure was less than 150 mm Hg.
While these trials support a goal pressure of less than 150 mm Hg in the elderly, it is unclear whether this goal should be applied beginning at age 60. Other guidelines, including those recently released jointly by the American Society of Hypertension and the International Society of Hypertension, recommend a systolic goal of less than 150 mm Hg in people age 80 and older—not age 60.10
The recommendation for a goal systolic pressure of less than 150 mm Hg in people age 60 and older was not unanimous; some panel members recommended continuing the JNC 7 goal of less than 140 mm Hg based on expert opinion, as they believed that the evidence was insufficient, especially in high-risk subgroups such as black people and those with cerebrovascular disease and other risk factors.
A subsequent minority report from five panel members discusses in more detail why they believe the systolic target should be kept lower than 140 mm Hg in patients age 60 or older until the risks and benefits of a higher target become clearer.11
Corollary recommendation: No need to down-titrate if lower than 140
In the general population age 60 and older, dosages do not have to be adjusted downward if the patient’s systolic pressure is already lower than 140 mm Hg and treatment is well tolerated without adverse effects on health or quality of life.
Strength of recommendation—expert opinion (grade E).
Comments. In the studies that supported a systolic goal lower than 150 mm Hg, many participants actually achieved a systolic pressure lower than 140 mm Hg without any adverse events. Trials that showed no benefit from a systolic goal lower than 140 mm Hg were graded as lower in quality. Thus, the possibility remains that a systolic goal lower than 140 mm Hg could have a clinically important benefit. Therefore, medications do not have to be adjusted so that blood pressure can “ride up.”
For example, therapy does not need to be down-titrated in a 65-year-old patient whose blood pressure is 138/85 mm Hg on two medications that he or she is tolerating well. On the other hand, based on Recommendation 1, therapy can be down-titrated in a 65-year-old whose pressure is 138/85 mm Hg on four medications that are causing side effects.
Recommendation 2: Diastolic < 90 for those younger than 60
In the general population younger than 60 years, JNC 8 recommends starting pharmacologic treatment if the diastolic pressure is 90 mm Hg or higher and aiming for a goal diastolic pressure of less than 90 mm Hg.
Strength of recommendation—strong (grade A) for ages 30 to 59, expert opinion (grade E) for ages 18 to 29.
Comments. The panel found no evidence to support a goal diastolic pressure of 80 mm Hg or less (or 85 mm Hg or less) compared with 90 mm Hg or less in this population.
It is reasonable to aim for the same diastolic goal in younger persons (under age 30), given the higher prevalence of diastolic hypertension in younger people.
Recommendation 3: Systolic < 140 for those younger than 60
In the general population younger than 60 years, we should start drug treatment at a systolic pressure of 140 mm Hg or higher and treat to a systolic goal of less than 140 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Although evidence was insufficient to support this recommendation, the panel decided to keep the same systolic goal for people younger than 60 as in the JNC 7 recommendations, for the following two reasons.
First, there is strong evidence supporting a diastolic goal of less than 90 mm Hg in this population (Recommendation 2), and many study participants who achieved a diastolic pressure lower than 90 mm Hg also achieved a systolic pressure lower than 140. Therefore, it is not possible to tease out whether the outcome benefits were due to lower systolic pressure or to lower diastolic pressure, or to both.
Second, the panel believed the guidelines would be simpler to implement if the systolic goals were the same in the general population as in those with chronic kidney disease or diabetes (see below).
Recommendation 4: < 140/90 in chronic kidney disease
In patients age 18 and older with chronic kidney disease, JNC 8 recommends starting drug treatment at a systolic pressure of 140 mm Hg or higher or a diastolic pressure of 90 mm Hg or higher and treating to a goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Chronic kidney disease is defined as either a glomerular filtration rate (estimated or measured) less than 60 mL/min/1.73 m2 in people up to age 70, or albuminuria, defined as more than 30 mg/g of creatinine at any glomerular filtration rate at any age.
Strength of recommendation—expert opinion (grade E).
Comments. There was insufficient evidence that aiming for a lower goal of 130/80 mm Hg (as in the JNC 7 recommendations) had any beneficial effect on cardiovascular, cerebrovascular, or mortality outcomes compared with 140/90 mm Hg, and there was moderate-quality evidence showing that treatment to lower goal (< 130/80 mm Hg) did not slow the progression of chronic kidney disease any better than a goal of less than 140/90 mm Hg. (One study that did find better renal outcomes with a lower blood pressure goal was a post hoc analysis of the Modification of Diet in Renal Disease study data in patients with proteinuria of more than 3 g per day.12)
We believe that decisions should be individualized regarding goal blood pressures and pharmacologic therapy in patients with chronic kidney disease and proteinuria, who may benefit from lower blood pressure goals (<130/80 mm Hg), based on low-level evidence.13,14 Risks and benefits should also be weighed in considering the blood pressure goal in the elderly with chronic kidney disease, taking into account functional status, comorbidities, and level of proteinuria.
Recommendation 5: < 140/90 for people with diabetes
In patients with diabetes who are age 18 and older, JNC 8 says to start drug treatment at a systolic pressure of 140 mm Hg or higher or diastolic pressure of 90 mm Hg or higher, and treat to goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Moderate-quality evidence showed cardiovascular, cerebrovascular, and mortality outcome benefits with treatment to a systolic goal of less than 150 mm Hg in patients with diabetes and hypertension.
The panel found no randomized controlled trials that compared a treatment goal of less than 140 mm Hg with one of less than 150 mm Hg for outcome benefits, but decided to base its recommendations on the results of the Action to Control Cardiovascular Risk in Diabetes—Blood-pressure-lowering Arm (ACCORD-BP) trial.15 The control group in this trial had a goal systolic pressure of less than 140 mm Hg and had similar outcomes compared with a lower goal.
The panel found no evidence to support a lower blood pressure goal (< 130/80) as in JNC 7. ACCORD-BP showed no differences in outcomes with a systolic goal lower than 140 mm Hg vs lower than 120 mm Hg except for a small reduction in stroke, and the risks of trying to achieve intensive lowering of blood pressure may outweigh the benefit of a small reduction in stroke.12 There was no evidence for a goal diastolic pressure below 80 mm Hg.
Recommendation 6: In nonblack patients, start with a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB
In the general nonblack population, including those with diabetes, initial drug treatment should include a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB.
Strength of recommendation—moderate (grade B).
Comments. All these drug classes had comparable outcome benefits in terms of rates of death, cardiovascular disease, cerebrovascular disease, and kidney disease, but not heart failure. For improving heart failure outcomes, thiazide-type diuretics are better than ACE inhibitors, which in turn are better than calcium channel blockers.
Thiazide-type diuretics (eg, hydrochlorothiazide, chlorthalidone, and indapamide) were recommended as first-line therapy for most patients in JNC 7, but they no longer carry this preferred status in JNC 8. In addition, the panel did not address preferential use of chlorthalidone as opposed to hydrochlorothiazide, or the use of spironolactone in resistant hypertension.
The panel did not recommend beta-blockers as first-line therapy because there were no differences in outcomes (or insufficient evidence) compared with the above medication classes; additionally, the Losartan Intervention for Endpoint Reduction in Hypertension study16 reported a higher incidence of stroke with a beta-blocker than with an ARB. However, JNC 8 did not consider randomized controlled trials in specific nonhypertensive populations such as patients with coronary artery disease or heart failure. We believe decisions should be individualized as to the use of beta-blockers in these two conditions.
The panel recommended the same approach in patients with diabetes, as there were no differences in major cardiovascular or cerebrovascular outcomes compared with the general population.
Recommendation 7: In black patients, start with a thiazide-type diuretic or calcium channel blocker
In the general black population, including those with diabetes, JNC 8 recommends starting drug treatment with a thiazide-type diuretic or a calcium channel blocker.
Strength of recommendation—moderate (grade B) for the general black population; weak (grade C) for blacks with diabetes.
Comments. In the black subgroup in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack trial (ALLHAT),17 a thiazide-type diuretic (chlorthalidone) was better than an ACE inhibitor (lisinopril) in terms of cerebrovascular, heart failure, and composite outcomes, but similar for mortality rates and cardiovascular, and kidney outcomes. Also in this subgroup, a calcium channel blocker (amlodipine) was better than the ACE inhibitor for cerebrovascular outcomes (there was a 51% higher rate of stroke with the ACE inhibitor as initial therapy than with the calcium channel blocker); the ACE inhibitor was also less effective in reducing blood pressure in blacks than the calcium channel blocker.
For improving heart failure outcomes, the thiazide-type diuretic was better than the ACE inhibitor, which in turn was better than the calcium channel blocker.
Evidence for black patients with diabetes (graded as weak) was extrapolated from ALLHAT, in which 46% had diabetes.17 We would consider using an ACE inhibitor or ARB in this population on an individual basis, especially if the patient had proteinuria.
Recommendation 8: ACEs and ARBs for chronic kidney disease
In patients age 18 and older with chronic kidney disease, irrespective of race, diabetes, or proteinuria, initial or add-on drug treatment should include an ACE inhibitor or ARB to improve kidney outcomes.
Strength of recommendation—moderate (grade B).
Comments. Treatment with an ACE inhibitor or ARB improves kidney outcomes in patients with chronic kidney disease. But in this population, these drugs are no more beneficial than calcium channel blockers or beta-blockers in terms of cardiovascular outcomes.
No randomized controlled trial has compared ACE inhibitors and ARBs for cardiovascular outcomes in chronic kidney disease, and these drugs have similar effects on kidney outcomes.
The panel did not make any recommendations about direct renin inhibitors, as there were no eligible studies demonstrating benefits on cardiovascular or kidney outcomes.
In black patients with chronic kidney disease and proteinuria, the panel recommended initial therapy with an ACE inhibitor or ARB to slow progression to end-stage renal disease (contrast with Recommendation 7).
In black patients with chronic kidney disease and no proteinuria, the panel recommended choosing from a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB. If an ACE inhibitor or ARB is not used as initial therapy, then one can be added on as a second-line medication (contrast with Recommendation 7).
The panel found no evidence to support this recommendation in people over age 75 and noted that although an ACE inhibitor or ARB may be beneficial in this group, a thiazide-type diuretic or calcium channel blocker can be considered.
Recommendation 9: If not at goal, step up
The main objective of pharmacologic treatment of hypertension is to attain and maintain the goal blood pressure. Lifestyle interventions should be maintained throughout treatment (Table 1). Medications can be initiated and titrated according to any of three strategies used in the randomized controlled trials selected by the panel (detailed below). Do not use an ACE inhibitor and ARB together in same patient.
If blood pressure is not at goal using all medication classes as in Recommendation 6 (ie, the triple combination of a thiazide-type diuretic, calcium channel blocker, and either an ACE inhibitor or an ARB), if there is a contraindication to any of these medication classes, or if there is need to use more than three medications to reach the goal, drugs from other classes can be used.
Referral to a hypertension specialist may be indicated for patients who are not at goal using the above strategy or for whom additional clinical consultation is needed.
Strength of recommendation—expert opinion (grade E).
Comments. Blood pressure should be monitored and assessed regularly, treatment adjusted as needed, and lifestyle modifications encouraged.
The panel did not recommend any monitoring schedule before or after goal blood pressure is achieved, and this should be individualized.
ADDITIONAL TOPICS IN JNC 8
A supplemental report covered some additional topics for which formal evidence review was not conducted but which the panel considered important.
Measuring and monitoring blood pressure
The panel recommended measuring the blood pressure with an automated oscillometric device that is properly calibrated and validated, or carefully measuring it manually.
Blood pressure should be measured in a quiet and relaxed environment with the patient seated comfortably for at least 5 minutes in a chair (rather than on an examination table) with feet flat on the floor, back supported, and arm supported at heart level. Blood pressure should be taken on the bare upper arm with an appropriate-sized cuff whose bladder encircles at least 80% of the mid-upper arm circumference, and patients should avoid caffeine, smoking, and physical activity for at least 30 minutes before measurement. In addition, patients should be asked about the need to empty the bladder (and encouraged to do so if they have to).
To establish the diagnosis of hypertension and to assess whether blood pressure goals are being met, two or three measurements should be taken at each visit as outlined above, and the average recorded.
At the first visit, blood pressure should be measured in both arms, and the arm with the higher pressure should be used for subsequent measurements.
Appropriate dosing of antihypertensive medications
Dosing should be individualized for each patient, but in general, target doses can be achieved within 2 to 4 weeks, and generally should not take longer than 2 months.
In general, to minimize potential adverse effects, treatment is started at a lower dose than the target dose and is then titrated up. This is especially important in older patients and patients on multiple medications with other comorbidities, and if two antihypertensive medications are being started simultaneously.
The panel reviewed evidence-based dosing of antihypertensive medications that were shown to improve cardiovascular outcomes from the studies that were selected for review. Hydrochlorothiazide gets a special mention: although doses up to 100 mg were used in some studies, the panel recommended an evidence-based dose of 25 or 50 mg daily to balance efficacy and safety.
Three strategies for dosing antihypertensive medications that were used in the selected randomized controlled trials were provided. These strategies were not compared with each other, nor is it known if one is better than the others in terms of health outcomes. In all cases, avoid combining an ACE inhibitor and an ARB.
- Start one drug from the four classes in Recommendation 6, titrate to the maximum dose, then add a second drug and titrate, then add a third drug and titrate to achieve the goal blood pressure.
- Start one drug from the four classes in Recommendation 6 and add a second drug before increasing the initial drug to its maximal dose. Titrate both to maximal doses, and add a third drug if needed and titrate to achieve the goal blood pressure.
- Start with two drugs at the same time from the four classes in Recommendation 6, either as separate pills or in a fixed-dose combination. Add a third drug if needed to achieve the goal blood pressure.
Lifestyle modification
The panel did not extensively review the evidence for lifestyle modification but endorsed the recommendations of the Lifestyle Work Group, which was convened by the NHLBI to focus on the effects of diet and physical activity on cardiovascular disease risk factors.18
Diet. The Lifestyle Work Group recommends combining the Dietary Approaches to Stop Hypertension (DASH) diet with reduced sodium intake, as there is evidence of a greater blood-pressure-lowering effect when the two are combined. The effect on blood pressure is independent of changes in weight.
The Lifestyle Work Group recommends consuming no more than 2,400 mg of sodium per day, noting that limiting intake to 1,500 mg can result in even greater reduction in blood pressure, and that even without achieving these goals, reducing sodium intake by at least 1,000 mg per day lowers blood pressure.
Physical activity. The Lifestyle Work Group recommends moderate to vigorous physical activity for approximately 160 minutes per week (three to four sessions a week, lasting an average of 40 minutes per session).
Weight loss. The Lifestyle Work Group did not review the blood-pressure-lowering effect of weight loss in those who are overweight or obese. The JNC 8 panel endorsed maintaining a healthy weight in controlling blood pressure.
Alcohol intake received no specific recommendations in JNC 8.
JNC 8 IN PERSPECTIVE
JNC 8 takes a rigorous, evidence-based approach and focuses on a few key questions. Thus, it is very different from the earlier reports: it has a narrower focus and does not address the full range of issues related to hypertension.
Strengths of JNC 8
The panel followed a rigorous process of review and evaluation of evidence from randomized controlled trials, adhering closely to standards set by the Institute of Medicine for guideline development. In contrast, JNC 7 relied on consensus and expert opinion.
The JNC 8 guidelines aim to simplify recommendations, with only two goals to remember: treat to lower than 150/90 mm Hg in patients age 60 and older, and lower than 140/90 mm Hg for everybody else. The initial drug regimen was simplified as well, with any of four choices for initial therapy in nonblacks and two in blacks.
Relaxing the blood pressure goals in elderly patients (although a cutoff of age 60 vs age 80 is likely to be debated) will also allay concerns about overtreating hypertension and causing adverse events in this population that is particularly susceptible to orthostatic changes and is at increased risk of falls.
Limitations and concerns
While the evidence-based nature of the recommendations is a strength, information from observational studies, systematic reviews, and meta-analyses was not incorporated into the formulation of these guidelines. This limits the available evidence, reflected in the fact that despite an extensive attempt to provide recommendations based on good evidence, five of the 10 recommendations (including the corollary recommendation) are still based on expert consensus opinion. Comparing and combining studies from different time periods is also problematic because of different methods of conducting clinical trials and analysis, and also because clinical care in a different period may differ from current standard practices.
Blood pressure targets in some subgroups are not clearly addressed, including those with proteinuria and with a history of stroke. Peterson et al,19 in an editorial accompanying the JNC 8 publication, commented on the need for larger randomized controlled trials to compare different blood pressure thresholds in various patient populations.
Some health care providers will likely be concerned that relaxing blood pressure goals could lead to higher real-world blood pressures, eventually leading to adverse cardiovascular outcomes, particularly on a population level. This is akin to the “speed limit rule”—people are more likely to hover above target, no matter what the target is.
In another editorial, Sox20 raised concerns about the external review process, ie, that the guidelines were not published in draft form to solicit public comment. Additionally, although the recommendations underwent extensive review, they were not endorsed by the specialty societies that the NHLBI designated to develop guidelines. In its defense, however, the JNC 8 panel has offered to share records of the review process on request, and this should serve to increase confidence in the review process.
The original literature search was limited to studies published through December 2009, which is more than 4 years before the publication of the recommendations. Although a bridge search was conducted until August 2013 to identify additional studies, this search used different inclusion criteria than the original criteria.
With its narrow focus, JNC 8 does not address many relevant issues. The American Society of Hypertension/International Society of Hypertension guidelines, published around the same time that the JNC 8 report was released, provide a more comprehensive review that will be of practical use for health care providers in the community.10
Ambulatory blood pressure monitoring is increasingly being used in clinical practice to detect white coat hypertension and, in many cases, to assess hypertension that is resistant to medications. It has also been shown to have better prognostic value in predicting cardiovascular risk and progression of kidney disease than office blood pressures.21,22 The UK National Institute of Health and Care Excellence guideline recommends ambulatory monitoring for the diagnosis of hypertension.23 However, JNC 8 did not provide specific recommendations for the use of this technology. Additionally, the JNC 8 evidence review is based on studies that used office blood pressure readings, and the recommendations are not necessarily applicable to measurements obtained by ambulatory monitoring.
Other topics covered in JNC 7 but not in JNC 8 include:
- Definitions and stages of hypertension (which remain the same)
- Initial treatment of stage 2 hypertension with two medications
- The J-curve phenomenon
- Preferred medications for patients with coronary artery disease or congestive heart failure
- A detailed list of oral antihypertensive agents—JNC 8 confines itself to the drugs and doses used in randomized controlled trials
- Patient evaluation
- Secondary hypertension
- Resistant hypertension
- Adherence issues.
Contrast with other guidelines
While the goal of these recommendations is to make treatment standards more understandable and uniform, contrasting recommendations on blood pressure goals and medications from various groups muddy the waters. Other groups that have issued hypertension guidelines in recent years include:
- The American Diabetes Association24
- The American Society of Hypertension and the International Society of Hypertension10
- The European Society of Hypertension and the European Society of Cardiology25
- The Canadian Hypertension Education Program26
- The Kidney Disease: Improving Global Outcomes initiative14
- The National Institute for Health and Clinical Excellence (UK)23
- The International Society on Hypertension in Blacks27
- The American Heart Association, the American College of Cardiology, and the US Centers for Disease Control and Prevention.28
Future directions
Despite the emphasis on making treatment decisions on an individual basis and using guidelines only as a framework for a safe direction in managing difficult clinical scenarios, guideline recommendations are increasingly being used to assess provider performance and quality of care, and so they assume even more importance in the current health care environment. As specialty organizations review and decide whether to endorse the JNC 8 recommendations, reconciling seemingly disparate recommendations from various groups is needed to send a clear and concise message to practitioners taking care of patients with high blood pressure.
Although a daunting task, integrating guidelines on hypertension management with other cardiovascular risk guidelines (eg, cholesterol, obesity) with assessment of overall cardiovascular risk profile would likely help in developing a more effective cardiovascular prevention strategy.
Despite the panel’s best efforts at providing evidence-based recommendations, many of the recommendations are based on expert opinion, reflecting the need for larger well-conducted studies. It is hoped that ongoing studies such as the Systolic Blood Pressure Intervention Trial29 will provide more clarity about blood pressure goals, especially in the elderly.
Final thoughts
Guidelines are not rules, and while they provide a framework by synthesizing the best available evidence, any treatment plan should be formulated on the basis of individual patient characteristics, including comorbidities, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572. Erratum in JAMA 2003; 290:197.
- Gibbons GH, Harold JG, Jessup M, Robertson RM, Oetgen WJ. The next steps in developing clinical practice guidelines for prevention. J Am Coll Cardiol 2013; 62:1399–1400.
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2013; 62:1396–1398.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. Erratum in: Lancet 2003; 361:1060.
- Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. http://www.iom.edu/Reports/2011/Clinical-Practice-Guide-lines-We-Can-Trust.aspx. Accessed February 4, 2014.
- JATOS Study Group. Principal results of the Japanese Trial To Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56:196–202.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014 Jan 14. [Epub ahead of print]
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 2011; 154:541–548.
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2:337–414.
- Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003.
- Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2003; 42:239–246.
- Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013 Nov 12. [Epub ahead of print]
- Peterson ED, Gaziano JM, Greenland P. Recommendations for treating hypertension: what are the right goals and purposes? JAMA Editorial. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Sox HC. Assessing the trustworthiness of the guideline for management of high blood pressure in adults (editorial). JAMA. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005; 46:156–161.
- Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69:1175–1180.
- National Institute for Health and Clinical Excellence. Hypertension (CG127). http://publications.nice.org.uk/hypertension-cg127. Accessed February 4, 2014.
- American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2013 Dec 20. [Epub ahead of print]
- Hypertension without compelling indications: 2013 CHEP recommendations. Hypertension Canada website. http://www.hypertension.ca/hypertension-without-compelling-indications. Accessed February 4, 2014.
- Flack JM, Sica DA, Bakris G, et al; International Society on Hypertension in Blacks. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800.
- Go AS, Bauman M, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2013 Nov 15.
- Systolic Blood Pressure Intervention Trial (SPRINT). http://clinicaltrials.gov/ct2/show/NCT01206062. Accessed February 4, 2014.
The report of the panel appointed to the eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8),1 published in December 2013 after considerable delay, contains some important changes from earlier guidelines from this group.2 For example:
- The blood pressure goal has been changed to less than 150/90 mm Hg in people age 60 and older. Formerly, the goal was less than 140/90 mm Hg.
- The goal has been changed to less than 140/90 mm Hg in all others, including people with diabetes mellitus and chronic kidney disease. Formerly, those two groups had a goal of less than 130/80 mm Hg.
- The initial choice of therapy can be from any of four classes of drugs: thiazide-type diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs). Formerly, the list also contained beta-blockers. Also, thiazide-type diuretics have lost their “preferred” status.
The new guidelines are evidence-based and are intended to simplify the way that hypertension is managed. Below, we summarize them—how they were developed, their strengths and limitations, and the main changes from earlier JNC reports.
WHOSE GUIDELINES ARE THESE?
The JNC has issued guidelines for managing hypertension since 1976, traditionally sanctioned by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. The guidelines have generally been updated every 4 to 5 years, with the last update, JNC 7,2 published in 2003.
The JNC 8 panel, consisting of 17 members, was commissioned by the NHLBI in 2008. However, in June 2013, the NHLBI announced it was withdrawing from guideline development and was delegating it to selected specialty organizations.3,4 In the interest of bringing the already delayed guidelines to the public in a timely manner, the JNC 8 panel decided to pursue publication independently and submitted the report to a medical journal. It is therefore not an official NHLBI-sanctioned report.
Here, we will refer to the new guidelines as “JNC 8,” but they are officially from “panel members appointed to the Eighth Joint National Committee (JNC 8).”
THREE QUESTIONS THAT GUIDED THE GUIDELINES
Epidemiologic studies clearly show a close relationship between blood pressure and the risk of heart disease, stroke, and kidney disease, these risks being lowest at a blood pressure of around 115/75 mm Hg.5 However, clinical trials have failed to show any evidence to justify treatment with antihypertensive medications to such a low level once hypertension has been diagnosed.
Patients and health care providers thus face questions about when to begin treatment, how low to aim for, and which antihypertensive medications to use. The JNC 8 panel focused on these three questions, believing them to be of greatest relevance to primary care providers.
A RIGOROUS PROCESS OF EVIDENCE REVIEW AND GUIDELINE DEVELOPMENT
The JNC 8 panel followed the guideline-development pathway outlined by the Institute of Medicine report, Clinical Practice Guidelines We Can Trust.6
Studies published from January 1966 through December 2009 that met specified criteria were selected for evidence review. Specifically, the studies had to be randomized controlled trials—no observational studies, systematic reviews, or meta-analyses, which were allowed in the JNC 7 report—with sample sizes of more than 100. Follow-up had to be for more than 1 year. Participants had to be age 18 or older and have hypertension—studies with patients with normal blood pressure or prehypertension were excluded. Health outcomes had to be reported, ie, “hard” end points such as rates of death, myocardial infarction, heart failure, hospitalization for heart failure, stroke, revascularization, and end-stage renal disease. Post hoc analyses were not allowed. The studies had to be rated by the NHLBI’s standardized quality rating tool as “good” (which has the least risk of bias, with valid results) or “fair (which is susceptible to some bias, but not enough to invalidate the results).
Subsequently, another search was conducted for relevant studies published from December 2009 through August 2013. In addition to meeting all the other criteria, this bridging search further restricted selection to major multicenter studies with sample sizes of more than 2,000.
An external methodology team performed the initial literature review and summarized the data. The JNC panel then crafted evidence statements and clinical recommendations using the evidence quality rating and grading systems developed by the NHLBI. In January 2013, the NHLBI submitted the guidelines for external review by individual reviewers with expertise in hypertension and to federal agencies, and a revised document was framed based on their comments and suggestions.
The evidence statements are detailed in an online 300-page supplemental review, and the panel members have indicated that reviewer comments and responses from the presubmission review process will be made available on request.
NINE RECOMMENDATIONS AND ONE COROLLARY
The panel made nine recommendations and one corollary recommendation based on a review of the evidence. Of the 10 total recommendations, five are based on expert opinion. Another two were rated as “moderate” in strength, one was “weak,” and only two were rated as “strong” (ie, based on high-quality evidence).
Recommendation 1: < 150/90 for those 60 and older
In the general population age 60 and older, the JNC 8 recommends starting drug treatment if the systolic pressure is 150 mm Hg or higher or if the diastolic pressure is 90 mm Hg or higher, and aiming for a systolic goal of less than 150 mm Hg and a diastolic goal of less than 90 mm Hg.
Strength of recommendation—strong (grade A).
Comments. Of all the recommendations, this one will probably have the greatest impact on clinical practice. Consider a frail 70-year-old patient at risk of falls who is taking two antihypertensive medications and whose blood pressure is 148/85 mm Hg. This level would have been considered too high under JNC 7 but is now acceptable, and the patient’s therapy does not have to be escalated.
The age cutoff of 60 years for this recommendation is debatable. The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)7 included patients ages 60 to 85 (mean age 74) and found no difference in outcomes comparing a goal systolic pressure of less than 140 mm Hg (this group achieved a mean systolic pressure of 135.9 mm Hg) and a goal systolic pressure of 140 to 160 mm Hg (achieved systolic pressure 145.6 mm Hg).
Similarly, the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) trial8 included patients ages 70 to 84 (mean age 76.1) and found no difference in outcomes between a goal systolic pressure of less than 140 mm Hg (achieved systolic pressure 136.6 mm Hg) and a goal of 140 to 150 mm Hg (achieved systolic pressure 142 mm Hg).
The Hypertension in the Very Elderly Trial (HYVET)9 found lower rates of stroke, death, and heart failure in patients age 80 and older when their systolic pressure was less than 150 mm Hg.
While these trials support a goal pressure of less than 150 mm Hg in the elderly, it is unclear whether this goal should be applied beginning at age 60. Other guidelines, including those recently released jointly by the American Society of Hypertension and the International Society of Hypertension, recommend a systolic goal of less than 150 mm Hg in people age 80 and older—not age 60.10
The recommendation for a goal systolic pressure of less than 150 mm Hg in people age 60 and older was not unanimous; some panel members recommended continuing the JNC 7 goal of less than 140 mm Hg based on expert opinion, as they believed that the evidence was insufficient, especially in high-risk subgroups such as black people and those with cerebrovascular disease and other risk factors.
A subsequent minority report from five panel members discusses in more detail why they believe the systolic target should be kept lower than 140 mm Hg in patients age 60 or older until the risks and benefits of a higher target become clearer.11
Corollary recommendation: No need to down-titrate if lower than 140
In the general population age 60 and older, dosages do not have to be adjusted downward if the patient’s systolic pressure is already lower than 140 mm Hg and treatment is well tolerated without adverse effects on health or quality of life.
Strength of recommendation—expert opinion (grade E).
Comments. In the studies that supported a systolic goal lower than 150 mm Hg, many participants actually achieved a systolic pressure lower than 140 mm Hg without any adverse events. Trials that showed no benefit from a systolic goal lower than 140 mm Hg were graded as lower in quality. Thus, the possibility remains that a systolic goal lower than 140 mm Hg could have a clinically important benefit. Therefore, medications do not have to be adjusted so that blood pressure can “ride up.”
For example, therapy does not need to be down-titrated in a 65-year-old patient whose blood pressure is 138/85 mm Hg on two medications that he or she is tolerating well. On the other hand, based on Recommendation 1, therapy can be down-titrated in a 65-year-old whose pressure is 138/85 mm Hg on four medications that are causing side effects.
Recommendation 2: Diastolic < 90 for those younger than 60
In the general population younger than 60 years, JNC 8 recommends starting pharmacologic treatment if the diastolic pressure is 90 mm Hg or higher and aiming for a goal diastolic pressure of less than 90 mm Hg.
Strength of recommendation—strong (grade A) for ages 30 to 59, expert opinion (grade E) for ages 18 to 29.
Comments. The panel found no evidence to support a goal diastolic pressure of 80 mm Hg or less (or 85 mm Hg or less) compared with 90 mm Hg or less in this population.
It is reasonable to aim for the same diastolic goal in younger persons (under age 30), given the higher prevalence of diastolic hypertension in younger people.
Recommendation 3: Systolic < 140 for those younger than 60
In the general population younger than 60 years, we should start drug treatment at a systolic pressure of 140 mm Hg or higher and treat to a systolic goal of less than 140 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Although evidence was insufficient to support this recommendation, the panel decided to keep the same systolic goal for people younger than 60 as in the JNC 7 recommendations, for the following two reasons.
First, there is strong evidence supporting a diastolic goal of less than 90 mm Hg in this population (Recommendation 2), and many study participants who achieved a diastolic pressure lower than 90 mm Hg also achieved a systolic pressure lower than 140. Therefore, it is not possible to tease out whether the outcome benefits were due to lower systolic pressure or to lower diastolic pressure, or to both.
Second, the panel believed the guidelines would be simpler to implement if the systolic goals were the same in the general population as in those with chronic kidney disease or diabetes (see below).
Recommendation 4: < 140/90 in chronic kidney disease
In patients age 18 and older with chronic kidney disease, JNC 8 recommends starting drug treatment at a systolic pressure of 140 mm Hg or higher or a diastolic pressure of 90 mm Hg or higher and treating to a goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Chronic kidney disease is defined as either a glomerular filtration rate (estimated or measured) less than 60 mL/min/1.73 m2 in people up to age 70, or albuminuria, defined as more than 30 mg/g of creatinine at any glomerular filtration rate at any age.
Strength of recommendation—expert opinion (grade E).
Comments. There was insufficient evidence that aiming for a lower goal of 130/80 mm Hg (as in the JNC 7 recommendations) had any beneficial effect on cardiovascular, cerebrovascular, or mortality outcomes compared with 140/90 mm Hg, and there was moderate-quality evidence showing that treatment to lower goal (< 130/80 mm Hg) did not slow the progression of chronic kidney disease any better than a goal of less than 140/90 mm Hg. (One study that did find better renal outcomes with a lower blood pressure goal was a post hoc analysis of the Modification of Diet in Renal Disease study data in patients with proteinuria of more than 3 g per day.12)
We believe that decisions should be individualized regarding goal blood pressures and pharmacologic therapy in patients with chronic kidney disease and proteinuria, who may benefit from lower blood pressure goals (<130/80 mm Hg), based on low-level evidence.13,14 Risks and benefits should also be weighed in considering the blood pressure goal in the elderly with chronic kidney disease, taking into account functional status, comorbidities, and level of proteinuria.
Recommendation 5: < 140/90 for people with diabetes
In patients with diabetes who are age 18 and older, JNC 8 says to start drug treatment at a systolic pressure of 140 mm Hg or higher or diastolic pressure of 90 mm Hg or higher, and treat to goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Moderate-quality evidence showed cardiovascular, cerebrovascular, and mortality outcome benefits with treatment to a systolic goal of less than 150 mm Hg in patients with diabetes and hypertension.
The panel found no randomized controlled trials that compared a treatment goal of less than 140 mm Hg with one of less than 150 mm Hg for outcome benefits, but decided to base its recommendations on the results of the Action to Control Cardiovascular Risk in Diabetes—Blood-pressure-lowering Arm (ACCORD-BP) trial.15 The control group in this trial had a goal systolic pressure of less than 140 mm Hg and had similar outcomes compared with a lower goal.
The panel found no evidence to support a lower blood pressure goal (< 130/80) as in JNC 7. ACCORD-BP showed no differences in outcomes with a systolic goal lower than 140 mm Hg vs lower than 120 mm Hg except for a small reduction in stroke, and the risks of trying to achieve intensive lowering of blood pressure may outweigh the benefit of a small reduction in stroke.12 There was no evidence for a goal diastolic pressure below 80 mm Hg.
Recommendation 6: In nonblack patients, start with a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB
In the general nonblack population, including those with diabetes, initial drug treatment should include a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB.
Strength of recommendation—moderate (grade B).
Comments. All these drug classes had comparable outcome benefits in terms of rates of death, cardiovascular disease, cerebrovascular disease, and kidney disease, but not heart failure. For improving heart failure outcomes, thiazide-type diuretics are better than ACE inhibitors, which in turn are better than calcium channel blockers.
Thiazide-type diuretics (eg, hydrochlorothiazide, chlorthalidone, and indapamide) were recommended as first-line therapy for most patients in JNC 7, but they no longer carry this preferred status in JNC 8. In addition, the panel did not address preferential use of chlorthalidone as opposed to hydrochlorothiazide, or the use of spironolactone in resistant hypertension.
The panel did not recommend beta-blockers as first-line therapy because there were no differences in outcomes (or insufficient evidence) compared with the above medication classes; additionally, the Losartan Intervention for Endpoint Reduction in Hypertension study16 reported a higher incidence of stroke with a beta-blocker than with an ARB. However, JNC 8 did not consider randomized controlled trials in specific nonhypertensive populations such as patients with coronary artery disease or heart failure. We believe decisions should be individualized as to the use of beta-blockers in these two conditions.
The panel recommended the same approach in patients with diabetes, as there were no differences in major cardiovascular or cerebrovascular outcomes compared with the general population.
Recommendation 7: In black patients, start with a thiazide-type diuretic or calcium channel blocker
In the general black population, including those with diabetes, JNC 8 recommends starting drug treatment with a thiazide-type diuretic or a calcium channel blocker.
Strength of recommendation—moderate (grade B) for the general black population; weak (grade C) for blacks with diabetes.
Comments. In the black subgroup in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack trial (ALLHAT),17 a thiazide-type diuretic (chlorthalidone) was better than an ACE inhibitor (lisinopril) in terms of cerebrovascular, heart failure, and composite outcomes, but similar for mortality rates and cardiovascular, and kidney outcomes. Also in this subgroup, a calcium channel blocker (amlodipine) was better than the ACE inhibitor for cerebrovascular outcomes (there was a 51% higher rate of stroke with the ACE inhibitor as initial therapy than with the calcium channel blocker); the ACE inhibitor was also less effective in reducing blood pressure in blacks than the calcium channel blocker.
For improving heart failure outcomes, the thiazide-type diuretic was better than the ACE inhibitor, which in turn was better than the calcium channel blocker.
Evidence for black patients with diabetes (graded as weak) was extrapolated from ALLHAT, in which 46% had diabetes.17 We would consider using an ACE inhibitor or ARB in this population on an individual basis, especially if the patient had proteinuria.
Recommendation 8: ACEs and ARBs for chronic kidney disease
In patients age 18 and older with chronic kidney disease, irrespective of race, diabetes, or proteinuria, initial or add-on drug treatment should include an ACE inhibitor or ARB to improve kidney outcomes.
Strength of recommendation—moderate (grade B).
Comments. Treatment with an ACE inhibitor or ARB improves kidney outcomes in patients with chronic kidney disease. But in this population, these drugs are no more beneficial than calcium channel blockers or beta-blockers in terms of cardiovascular outcomes.
No randomized controlled trial has compared ACE inhibitors and ARBs for cardiovascular outcomes in chronic kidney disease, and these drugs have similar effects on kidney outcomes.
The panel did not make any recommendations about direct renin inhibitors, as there were no eligible studies demonstrating benefits on cardiovascular or kidney outcomes.
In black patients with chronic kidney disease and proteinuria, the panel recommended initial therapy with an ACE inhibitor or ARB to slow progression to end-stage renal disease (contrast with Recommendation 7).
In black patients with chronic kidney disease and no proteinuria, the panel recommended choosing from a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB. If an ACE inhibitor or ARB is not used as initial therapy, then one can be added on as a second-line medication (contrast with Recommendation 7).
The panel found no evidence to support this recommendation in people over age 75 and noted that although an ACE inhibitor or ARB may be beneficial in this group, a thiazide-type diuretic or calcium channel blocker can be considered.
Recommendation 9: If not at goal, step up
The main objective of pharmacologic treatment of hypertension is to attain and maintain the goal blood pressure. Lifestyle interventions should be maintained throughout treatment (Table 1). Medications can be initiated and titrated according to any of three strategies used in the randomized controlled trials selected by the panel (detailed below). Do not use an ACE inhibitor and ARB together in same patient.
If blood pressure is not at goal using all medication classes as in Recommendation 6 (ie, the triple combination of a thiazide-type diuretic, calcium channel blocker, and either an ACE inhibitor or an ARB), if there is a contraindication to any of these medication classes, or if there is need to use more than three medications to reach the goal, drugs from other classes can be used.
Referral to a hypertension specialist may be indicated for patients who are not at goal using the above strategy or for whom additional clinical consultation is needed.
Strength of recommendation—expert opinion (grade E).
Comments. Blood pressure should be monitored and assessed regularly, treatment adjusted as needed, and lifestyle modifications encouraged.
The panel did not recommend any monitoring schedule before or after goal blood pressure is achieved, and this should be individualized.
ADDITIONAL TOPICS IN JNC 8
A supplemental report covered some additional topics for which formal evidence review was not conducted but which the panel considered important.
Measuring and monitoring blood pressure
The panel recommended measuring the blood pressure with an automated oscillometric device that is properly calibrated and validated, or carefully measuring it manually.
Blood pressure should be measured in a quiet and relaxed environment with the patient seated comfortably for at least 5 minutes in a chair (rather than on an examination table) with feet flat on the floor, back supported, and arm supported at heart level. Blood pressure should be taken on the bare upper arm with an appropriate-sized cuff whose bladder encircles at least 80% of the mid-upper arm circumference, and patients should avoid caffeine, smoking, and physical activity for at least 30 minutes before measurement. In addition, patients should be asked about the need to empty the bladder (and encouraged to do so if they have to).
To establish the diagnosis of hypertension and to assess whether blood pressure goals are being met, two or three measurements should be taken at each visit as outlined above, and the average recorded.
At the first visit, blood pressure should be measured in both arms, and the arm with the higher pressure should be used for subsequent measurements.
Appropriate dosing of antihypertensive medications
Dosing should be individualized for each patient, but in general, target doses can be achieved within 2 to 4 weeks, and generally should not take longer than 2 months.
In general, to minimize potential adverse effects, treatment is started at a lower dose than the target dose and is then titrated up. This is especially important in older patients and patients on multiple medications with other comorbidities, and if two antihypertensive medications are being started simultaneously.
The panel reviewed evidence-based dosing of antihypertensive medications that were shown to improve cardiovascular outcomes from the studies that were selected for review. Hydrochlorothiazide gets a special mention: although doses up to 100 mg were used in some studies, the panel recommended an evidence-based dose of 25 or 50 mg daily to balance efficacy and safety.
Three strategies for dosing antihypertensive medications that were used in the selected randomized controlled trials were provided. These strategies were not compared with each other, nor is it known if one is better than the others in terms of health outcomes. In all cases, avoid combining an ACE inhibitor and an ARB.
- Start one drug from the four classes in Recommendation 6, titrate to the maximum dose, then add a second drug and titrate, then add a third drug and titrate to achieve the goal blood pressure.
- Start one drug from the four classes in Recommendation 6 and add a second drug before increasing the initial drug to its maximal dose. Titrate both to maximal doses, and add a third drug if needed and titrate to achieve the goal blood pressure.
- Start with two drugs at the same time from the four classes in Recommendation 6, either as separate pills or in a fixed-dose combination. Add a third drug if needed to achieve the goal blood pressure.
Lifestyle modification
The panel did not extensively review the evidence for lifestyle modification but endorsed the recommendations of the Lifestyle Work Group, which was convened by the NHLBI to focus on the effects of diet and physical activity on cardiovascular disease risk factors.18
Diet. The Lifestyle Work Group recommends combining the Dietary Approaches to Stop Hypertension (DASH) diet with reduced sodium intake, as there is evidence of a greater blood-pressure-lowering effect when the two are combined. The effect on blood pressure is independent of changes in weight.
The Lifestyle Work Group recommends consuming no more than 2,400 mg of sodium per day, noting that limiting intake to 1,500 mg can result in even greater reduction in blood pressure, and that even without achieving these goals, reducing sodium intake by at least 1,000 mg per day lowers blood pressure.
Physical activity. The Lifestyle Work Group recommends moderate to vigorous physical activity for approximately 160 minutes per week (three to four sessions a week, lasting an average of 40 minutes per session).
Weight loss. The Lifestyle Work Group did not review the blood-pressure-lowering effect of weight loss in those who are overweight or obese. The JNC 8 panel endorsed maintaining a healthy weight in controlling blood pressure.
Alcohol intake received no specific recommendations in JNC 8.
JNC 8 IN PERSPECTIVE
JNC 8 takes a rigorous, evidence-based approach and focuses on a few key questions. Thus, it is very different from the earlier reports: it has a narrower focus and does not address the full range of issues related to hypertension.
Strengths of JNC 8
The panel followed a rigorous process of review and evaluation of evidence from randomized controlled trials, adhering closely to standards set by the Institute of Medicine for guideline development. In contrast, JNC 7 relied on consensus and expert opinion.
The JNC 8 guidelines aim to simplify recommendations, with only two goals to remember: treat to lower than 150/90 mm Hg in patients age 60 and older, and lower than 140/90 mm Hg for everybody else. The initial drug regimen was simplified as well, with any of four choices for initial therapy in nonblacks and two in blacks.
Relaxing the blood pressure goals in elderly patients (although a cutoff of age 60 vs age 80 is likely to be debated) will also allay concerns about overtreating hypertension and causing adverse events in this population that is particularly susceptible to orthostatic changes and is at increased risk of falls.
Limitations and concerns
While the evidence-based nature of the recommendations is a strength, information from observational studies, systematic reviews, and meta-analyses was not incorporated into the formulation of these guidelines. This limits the available evidence, reflected in the fact that despite an extensive attempt to provide recommendations based on good evidence, five of the 10 recommendations (including the corollary recommendation) are still based on expert consensus opinion. Comparing and combining studies from different time periods is also problematic because of different methods of conducting clinical trials and analysis, and also because clinical care in a different period may differ from current standard practices.
Blood pressure targets in some subgroups are not clearly addressed, including those with proteinuria and with a history of stroke. Peterson et al,19 in an editorial accompanying the JNC 8 publication, commented on the need for larger randomized controlled trials to compare different blood pressure thresholds in various patient populations.
Some health care providers will likely be concerned that relaxing blood pressure goals could lead to higher real-world blood pressures, eventually leading to adverse cardiovascular outcomes, particularly on a population level. This is akin to the “speed limit rule”—people are more likely to hover above target, no matter what the target is.
In another editorial, Sox20 raised concerns about the external review process, ie, that the guidelines were not published in draft form to solicit public comment. Additionally, although the recommendations underwent extensive review, they were not endorsed by the specialty societies that the NHLBI designated to develop guidelines. In its defense, however, the JNC 8 panel has offered to share records of the review process on request, and this should serve to increase confidence in the review process.
The original literature search was limited to studies published through December 2009, which is more than 4 years before the publication of the recommendations. Although a bridge search was conducted until August 2013 to identify additional studies, this search used different inclusion criteria than the original criteria.
With its narrow focus, JNC 8 does not address many relevant issues. The American Society of Hypertension/International Society of Hypertension guidelines, published around the same time that the JNC 8 report was released, provide a more comprehensive review that will be of practical use for health care providers in the community.10
Ambulatory blood pressure monitoring is increasingly being used in clinical practice to detect white coat hypertension and, in many cases, to assess hypertension that is resistant to medications. It has also been shown to have better prognostic value in predicting cardiovascular risk and progression of kidney disease than office blood pressures.21,22 The UK National Institute of Health and Care Excellence guideline recommends ambulatory monitoring for the diagnosis of hypertension.23 However, JNC 8 did not provide specific recommendations for the use of this technology. Additionally, the JNC 8 evidence review is based on studies that used office blood pressure readings, and the recommendations are not necessarily applicable to measurements obtained by ambulatory monitoring.
Other topics covered in JNC 7 but not in JNC 8 include:
- Definitions and stages of hypertension (which remain the same)
- Initial treatment of stage 2 hypertension with two medications
- The J-curve phenomenon
- Preferred medications for patients with coronary artery disease or congestive heart failure
- A detailed list of oral antihypertensive agents—JNC 8 confines itself to the drugs and doses used in randomized controlled trials
- Patient evaluation
- Secondary hypertension
- Resistant hypertension
- Adherence issues.
Contrast with other guidelines
While the goal of these recommendations is to make treatment standards more understandable and uniform, contrasting recommendations on blood pressure goals and medications from various groups muddy the waters. Other groups that have issued hypertension guidelines in recent years include:
- The American Diabetes Association24
- The American Society of Hypertension and the International Society of Hypertension10
- The European Society of Hypertension and the European Society of Cardiology25
- The Canadian Hypertension Education Program26
- The Kidney Disease: Improving Global Outcomes initiative14
- The National Institute for Health and Clinical Excellence (UK)23
- The International Society on Hypertension in Blacks27
- The American Heart Association, the American College of Cardiology, and the US Centers for Disease Control and Prevention.28
Future directions
Despite the emphasis on making treatment decisions on an individual basis and using guidelines only as a framework for a safe direction in managing difficult clinical scenarios, guideline recommendations are increasingly being used to assess provider performance and quality of care, and so they assume even more importance in the current health care environment. As specialty organizations review and decide whether to endorse the JNC 8 recommendations, reconciling seemingly disparate recommendations from various groups is needed to send a clear and concise message to practitioners taking care of patients with high blood pressure.
Although a daunting task, integrating guidelines on hypertension management with other cardiovascular risk guidelines (eg, cholesterol, obesity) with assessment of overall cardiovascular risk profile would likely help in developing a more effective cardiovascular prevention strategy.
Despite the panel’s best efforts at providing evidence-based recommendations, many of the recommendations are based on expert opinion, reflecting the need for larger well-conducted studies. It is hoped that ongoing studies such as the Systolic Blood Pressure Intervention Trial29 will provide more clarity about blood pressure goals, especially in the elderly.
Final thoughts
Guidelines are not rules, and while they provide a framework by synthesizing the best available evidence, any treatment plan should be formulated on the basis of individual patient characteristics, including comorbidities, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence.
The report of the panel appointed to the eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8),1 published in December 2013 after considerable delay, contains some important changes from earlier guidelines from this group.2 For example:
- The blood pressure goal has been changed to less than 150/90 mm Hg in people age 60 and older. Formerly, the goal was less than 140/90 mm Hg.
- The goal has been changed to less than 140/90 mm Hg in all others, including people with diabetes mellitus and chronic kidney disease. Formerly, those two groups had a goal of less than 130/80 mm Hg.
- The initial choice of therapy can be from any of four classes of drugs: thiazide-type diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs). Formerly, the list also contained beta-blockers. Also, thiazide-type diuretics have lost their “preferred” status.
The new guidelines are evidence-based and are intended to simplify the way that hypertension is managed. Below, we summarize them—how they were developed, their strengths and limitations, and the main changes from earlier JNC reports.
WHOSE GUIDELINES ARE THESE?
The JNC has issued guidelines for managing hypertension since 1976, traditionally sanctioned by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. The guidelines have generally been updated every 4 to 5 years, with the last update, JNC 7,2 published in 2003.
The JNC 8 panel, consisting of 17 members, was commissioned by the NHLBI in 2008. However, in June 2013, the NHLBI announced it was withdrawing from guideline development and was delegating it to selected specialty organizations.3,4 In the interest of bringing the already delayed guidelines to the public in a timely manner, the JNC 8 panel decided to pursue publication independently and submitted the report to a medical journal. It is therefore not an official NHLBI-sanctioned report.
Here, we will refer to the new guidelines as “JNC 8,” but they are officially from “panel members appointed to the Eighth Joint National Committee (JNC 8).”
THREE QUESTIONS THAT GUIDED THE GUIDELINES
Epidemiologic studies clearly show a close relationship between blood pressure and the risk of heart disease, stroke, and kidney disease, these risks being lowest at a blood pressure of around 115/75 mm Hg.5 However, clinical trials have failed to show any evidence to justify treatment with antihypertensive medications to such a low level once hypertension has been diagnosed.
Patients and health care providers thus face questions about when to begin treatment, how low to aim for, and which antihypertensive medications to use. The JNC 8 panel focused on these three questions, believing them to be of greatest relevance to primary care providers.
A RIGOROUS PROCESS OF EVIDENCE REVIEW AND GUIDELINE DEVELOPMENT
The JNC 8 panel followed the guideline-development pathway outlined by the Institute of Medicine report, Clinical Practice Guidelines We Can Trust.6
Studies published from January 1966 through December 2009 that met specified criteria were selected for evidence review. Specifically, the studies had to be randomized controlled trials—no observational studies, systematic reviews, or meta-analyses, which were allowed in the JNC 7 report—with sample sizes of more than 100. Follow-up had to be for more than 1 year. Participants had to be age 18 or older and have hypertension—studies with patients with normal blood pressure or prehypertension were excluded. Health outcomes had to be reported, ie, “hard” end points such as rates of death, myocardial infarction, heart failure, hospitalization for heart failure, stroke, revascularization, and end-stage renal disease. Post hoc analyses were not allowed. The studies had to be rated by the NHLBI’s standardized quality rating tool as “good” (which has the least risk of bias, with valid results) or “fair (which is susceptible to some bias, but not enough to invalidate the results).
Subsequently, another search was conducted for relevant studies published from December 2009 through August 2013. In addition to meeting all the other criteria, this bridging search further restricted selection to major multicenter studies with sample sizes of more than 2,000.
An external methodology team performed the initial literature review and summarized the data. The JNC panel then crafted evidence statements and clinical recommendations using the evidence quality rating and grading systems developed by the NHLBI. In January 2013, the NHLBI submitted the guidelines for external review by individual reviewers with expertise in hypertension and to federal agencies, and a revised document was framed based on their comments and suggestions.
The evidence statements are detailed in an online 300-page supplemental review, and the panel members have indicated that reviewer comments and responses from the presubmission review process will be made available on request.
NINE RECOMMENDATIONS AND ONE COROLLARY
The panel made nine recommendations and one corollary recommendation based on a review of the evidence. Of the 10 total recommendations, five are based on expert opinion. Another two were rated as “moderate” in strength, one was “weak,” and only two were rated as “strong” (ie, based on high-quality evidence).
Recommendation 1: < 150/90 for those 60 and older
In the general population age 60 and older, the JNC 8 recommends starting drug treatment if the systolic pressure is 150 mm Hg or higher or if the diastolic pressure is 90 mm Hg or higher, and aiming for a systolic goal of less than 150 mm Hg and a diastolic goal of less than 90 mm Hg.
Strength of recommendation—strong (grade A).
Comments. Of all the recommendations, this one will probably have the greatest impact on clinical practice. Consider a frail 70-year-old patient at risk of falls who is taking two antihypertensive medications and whose blood pressure is 148/85 mm Hg. This level would have been considered too high under JNC 7 but is now acceptable, and the patient’s therapy does not have to be escalated.
The age cutoff of 60 years for this recommendation is debatable. The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)7 included patients ages 60 to 85 (mean age 74) and found no difference in outcomes comparing a goal systolic pressure of less than 140 mm Hg (this group achieved a mean systolic pressure of 135.9 mm Hg) and a goal systolic pressure of 140 to 160 mm Hg (achieved systolic pressure 145.6 mm Hg).
Similarly, the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) trial8 included patients ages 70 to 84 (mean age 76.1) and found no difference in outcomes between a goal systolic pressure of less than 140 mm Hg (achieved systolic pressure 136.6 mm Hg) and a goal of 140 to 150 mm Hg (achieved systolic pressure 142 mm Hg).
The Hypertension in the Very Elderly Trial (HYVET)9 found lower rates of stroke, death, and heart failure in patients age 80 and older when their systolic pressure was less than 150 mm Hg.
While these trials support a goal pressure of less than 150 mm Hg in the elderly, it is unclear whether this goal should be applied beginning at age 60. Other guidelines, including those recently released jointly by the American Society of Hypertension and the International Society of Hypertension, recommend a systolic goal of less than 150 mm Hg in people age 80 and older—not age 60.10
The recommendation for a goal systolic pressure of less than 150 mm Hg in people age 60 and older was not unanimous; some panel members recommended continuing the JNC 7 goal of less than 140 mm Hg based on expert opinion, as they believed that the evidence was insufficient, especially in high-risk subgroups such as black people and those with cerebrovascular disease and other risk factors.
A subsequent minority report from five panel members discusses in more detail why they believe the systolic target should be kept lower than 140 mm Hg in patients age 60 or older until the risks and benefits of a higher target become clearer.11
Corollary recommendation: No need to down-titrate if lower than 140
In the general population age 60 and older, dosages do not have to be adjusted downward if the patient’s systolic pressure is already lower than 140 mm Hg and treatment is well tolerated without adverse effects on health or quality of life.
Strength of recommendation—expert opinion (grade E).
Comments. In the studies that supported a systolic goal lower than 150 mm Hg, many participants actually achieved a systolic pressure lower than 140 mm Hg without any adverse events. Trials that showed no benefit from a systolic goal lower than 140 mm Hg were graded as lower in quality. Thus, the possibility remains that a systolic goal lower than 140 mm Hg could have a clinically important benefit. Therefore, medications do not have to be adjusted so that blood pressure can “ride up.”
For example, therapy does not need to be down-titrated in a 65-year-old patient whose blood pressure is 138/85 mm Hg on two medications that he or she is tolerating well. On the other hand, based on Recommendation 1, therapy can be down-titrated in a 65-year-old whose pressure is 138/85 mm Hg on four medications that are causing side effects.
Recommendation 2: Diastolic < 90 for those younger than 60
In the general population younger than 60 years, JNC 8 recommends starting pharmacologic treatment if the diastolic pressure is 90 mm Hg or higher and aiming for a goal diastolic pressure of less than 90 mm Hg.
Strength of recommendation—strong (grade A) for ages 30 to 59, expert opinion (grade E) for ages 18 to 29.
Comments. The panel found no evidence to support a goal diastolic pressure of 80 mm Hg or less (or 85 mm Hg or less) compared with 90 mm Hg or less in this population.
It is reasonable to aim for the same diastolic goal in younger persons (under age 30), given the higher prevalence of diastolic hypertension in younger people.
Recommendation 3: Systolic < 140 for those younger than 60
In the general population younger than 60 years, we should start drug treatment at a systolic pressure of 140 mm Hg or higher and treat to a systolic goal of less than 140 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Although evidence was insufficient to support this recommendation, the panel decided to keep the same systolic goal for people younger than 60 as in the JNC 7 recommendations, for the following two reasons.
First, there is strong evidence supporting a diastolic goal of less than 90 mm Hg in this population (Recommendation 2), and many study participants who achieved a diastolic pressure lower than 90 mm Hg also achieved a systolic pressure lower than 140. Therefore, it is not possible to tease out whether the outcome benefits were due to lower systolic pressure or to lower diastolic pressure, or to both.
Second, the panel believed the guidelines would be simpler to implement if the systolic goals were the same in the general population as in those with chronic kidney disease or diabetes (see below).
Recommendation 4: < 140/90 in chronic kidney disease
In patients age 18 and older with chronic kidney disease, JNC 8 recommends starting drug treatment at a systolic pressure of 140 mm Hg or higher or a diastolic pressure of 90 mm Hg or higher and treating to a goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Chronic kidney disease is defined as either a glomerular filtration rate (estimated or measured) less than 60 mL/min/1.73 m2 in people up to age 70, or albuminuria, defined as more than 30 mg/g of creatinine at any glomerular filtration rate at any age.
Strength of recommendation—expert opinion (grade E).
Comments. There was insufficient evidence that aiming for a lower goal of 130/80 mm Hg (as in the JNC 7 recommendations) had any beneficial effect on cardiovascular, cerebrovascular, or mortality outcomes compared with 140/90 mm Hg, and there was moderate-quality evidence showing that treatment to lower goal (< 130/80 mm Hg) did not slow the progression of chronic kidney disease any better than a goal of less than 140/90 mm Hg. (One study that did find better renal outcomes with a lower blood pressure goal was a post hoc analysis of the Modification of Diet in Renal Disease study data in patients with proteinuria of more than 3 g per day.12)
We believe that decisions should be individualized regarding goal blood pressures and pharmacologic therapy in patients with chronic kidney disease and proteinuria, who may benefit from lower blood pressure goals (<130/80 mm Hg), based on low-level evidence.13,14 Risks and benefits should also be weighed in considering the blood pressure goal in the elderly with chronic kidney disease, taking into account functional status, comorbidities, and level of proteinuria.
Recommendation 5: < 140/90 for people with diabetes
In patients with diabetes who are age 18 and older, JNC 8 says to start drug treatment at a systolic pressure of 140 mm Hg or higher or diastolic pressure of 90 mm Hg or higher, and treat to goal systolic pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg.
Strength of recommendation—expert opinion (grade E).
Comments. Moderate-quality evidence showed cardiovascular, cerebrovascular, and mortality outcome benefits with treatment to a systolic goal of less than 150 mm Hg in patients with diabetes and hypertension.
The panel found no randomized controlled trials that compared a treatment goal of less than 140 mm Hg with one of less than 150 mm Hg for outcome benefits, but decided to base its recommendations on the results of the Action to Control Cardiovascular Risk in Diabetes—Blood-pressure-lowering Arm (ACCORD-BP) trial.15 The control group in this trial had a goal systolic pressure of less than 140 mm Hg and had similar outcomes compared with a lower goal.
The panel found no evidence to support a lower blood pressure goal (< 130/80) as in JNC 7. ACCORD-BP showed no differences in outcomes with a systolic goal lower than 140 mm Hg vs lower than 120 mm Hg except for a small reduction in stroke, and the risks of trying to achieve intensive lowering of blood pressure may outweigh the benefit of a small reduction in stroke.12 There was no evidence for a goal diastolic pressure below 80 mm Hg.
Recommendation 6: In nonblack patients, start with a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB
In the general nonblack population, including those with diabetes, initial drug treatment should include a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB.
Strength of recommendation—moderate (grade B).
Comments. All these drug classes had comparable outcome benefits in terms of rates of death, cardiovascular disease, cerebrovascular disease, and kidney disease, but not heart failure. For improving heart failure outcomes, thiazide-type diuretics are better than ACE inhibitors, which in turn are better than calcium channel blockers.
Thiazide-type diuretics (eg, hydrochlorothiazide, chlorthalidone, and indapamide) were recommended as first-line therapy for most patients in JNC 7, but they no longer carry this preferred status in JNC 8. In addition, the panel did not address preferential use of chlorthalidone as opposed to hydrochlorothiazide, or the use of spironolactone in resistant hypertension.
The panel did not recommend beta-blockers as first-line therapy because there were no differences in outcomes (or insufficient evidence) compared with the above medication classes; additionally, the Losartan Intervention for Endpoint Reduction in Hypertension study16 reported a higher incidence of stroke with a beta-blocker than with an ARB. However, JNC 8 did not consider randomized controlled trials in specific nonhypertensive populations such as patients with coronary artery disease or heart failure. We believe decisions should be individualized as to the use of beta-blockers in these two conditions.
The panel recommended the same approach in patients with diabetes, as there were no differences in major cardiovascular or cerebrovascular outcomes compared with the general population.
Recommendation 7: In black patients, start with a thiazide-type diuretic or calcium channel blocker
In the general black population, including those with diabetes, JNC 8 recommends starting drug treatment with a thiazide-type diuretic or a calcium channel blocker.
Strength of recommendation—moderate (grade B) for the general black population; weak (grade C) for blacks with diabetes.
Comments. In the black subgroup in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack trial (ALLHAT),17 a thiazide-type diuretic (chlorthalidone) was better than an ACE inhibitor (lisinopril) in terms of cerebrovascular, heart failure, and composite outcomes, but similar for mortality rates and cardiovascular, and kidney outcomes. Also in this subgroup, a calcium channel blocker (amlodipine) was better than the ACE inhibitor for cerebrovascular outcomes (there was a 51% higher rate of stroke with the ACE inhibitor as initial therapy than with the calcium channel blocker); the ACE inhibitor was also less effective in reducing blood pressure in blacks than the calcium channel blocker.
For improving heart failure outcomes, the thiazide-type diuretic was better than the ACE inhibitor, which in turn was better than the calcium channel blocker.
Evidence for black patients with diabetes (graded as weak) was extrapolated from ALLHAT, in which 46% had diabetes.17 We would consider using an ACE inhibitor or ARB in this population on an individual basis, especially if the patient had proteinuria.
Recommendation 8: ACEs and ARBs for chronic kidney disease
In patients age 18 and older with chronic kidney disease, irrespective of race, diabetes, or proteinuria, initial or add-on drug treatment should include an ACE inhibitor or ARB to improve kidney outcomes.
Strength of recommendation—moderate (grade B).
Comments. Treatment with an ACE inhibitor or ARB improves kidney outcomes in patients with chronic kidney disease. But in this population, these drugs are no more beneficial than calcium channel blockers or beta-blockers in terms of cardiovascular outcomes.
No randomized controlled trial has compared ACE inhibitors and ARBs for cardiovascular outcomes in chronic kidney disease, and these drugs have similar effects on kidney outcomes.
The panel did not make any recommendations about direct renin inhibitors, as there were no eligible studies demonstrating benefits on cardiovascular or kidney outcomes.
In black patients with chronic kidney disease and proteinuria, the panel recommended initial therapy with an ACE inhibitor or ARB to slow progression to end-stage renal disease (contrast with Recommendation 7).
In black patients with chronic kidney disease and no proteinuria, the panel recommended choosing from a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or ARB. If an ACE inhibitor or ARB is not used as initial therapy, then one can be added on as a second-line medication (contrast with Recommendation 7).
The panel found no evidence to support this recommendation in people over age 75 and noted that although an ACE inhibitor or ARB may be beneficial in this group, a thiazide-type diuretic or calcium channel blocker can be considered.
Recommendation 9: If not at goal, step up
The main objective of pharmacologic treatment of hypertension is to attain and maintain the goal blood pressure. Lifestyle interventions should be maintained throughout treatment (Table 1). Medications can be initiated and titrated according to any of three strategies used in the randomized controlled trials selected by the panel (detailed below). Do not use an ACE inhibitor and ARB together in same patient.
If blood pressure is not at goal using all medication classes as in Recommendation 6 (ie, the triple combination of a thiazide-type diuretic, calcium channel blocker, and either an ACE inhibitor or an ARB), if there is a contraindication to any of these medication classes, or if there is need to use more than three medications to reach the goal, drugs from other classes can be used.
Referral to a hypertension specialist may be indicated for patients who are not at goal using the above strategy or for whom additional clinical consultation is needed.
Strength of recommendation—expert opinion (grade E).
Comments. Blood pressure should be monitored and assessed regularly, treatment adjusted as needed, and lifestyle modifications encouraged.
The panel did not recommend any monitoring schedule before or after goal blood pressure is achieved, and this should be individualized.
ADDITIONAL TOPICS IN JNC 8
A supplemental report covered some additional topics for which formal evidence review was not conducted but which the panel considered important.
Measuring and monitoring blood pressure
The panel recommended measuring the blood pressure with an automated oscillometric device that is properly calibrated and validated, or carefully measuring it manually.
Blood pressure should be measured in a quiet and relaxed environment with the patient seated comfortably for at least 5 minutes in a chair (rather than on an examination table) with feet flat on the floor, back supported, and arm supported at heart level. Blood pressure should be taken on the bare upper arm with an appropriate-sized cuff whose bladder encircles at least 80% of the mid-upper arm circumference, and patients should avoid caffeine, smoking, and physical activity for at least 30 minutes before measurement. In addition, patients should be asked about the need to empty the bladder (and encouraged to do so if they have to).
To establish the diagnosis of hypertension and to assess whether blood pressure goals are being met, two or three measurements should be taken at each visit as outlined above, and the average recorded.
At the first visit, blood pressure should be measured in both arms, and the arm with the higher pressure should be used for subsequent measurements.
Appropriate dosing of antihypertensive medications
Dosing should be individualized for each patient, but in general, target doses can be achieved within 2 to 4 weeks, and generally should not take longer than 2 months.
In general, to minimize potential adverse effects, treatment is started at a lower dose than the target dose and is then titrated up. This is especially important in older patients and patients on multiple medications with other comorbidities, and if two antihypertensive medications are being started simultaneously.
The panel reviewed evidence-based dosing of antihypertensive medications that were shown to improve cardiovascular outcomes from the studies that were selected for review. Hydrochlorothiazide gets a special mention: although doses up to 100 mg were used in some studies, the panel recommended an evidence-based dose of 25 or 50 mg daily to balance efficacy and safety.
Three strategies for dosing antihypertensive medications that were used in the selected randomized controlled trials were provided. These strategies were not compared with each other, nor is it known if one is better than the others in terms of health outcomes. In all cases, avoid combining an ACE inhibitor and an ARB.
- Start one drug from the four classes in Recommendation 6, titrate to the maximum dose, then add a second drug and titrate, then add a third drug and titrate to achieve the goal blood pressure.
- Start one drug from the four classes in Recommendation 6 and add a second drug before increasing the initial drug to its maximal dose. Titrate both to maximal doses, and add a third drug if needed and titrate to achieve the goal blood pressure.
- Start with two drugs at the same time from the four classes in Recommendation 6, either as separate pills or in a fixed-dose combination. Add a third drug if needed to achieve the goal blood pressure.
Lifestyle modification
The panel did not extensively review the evidence for lifestyle modification but endorsed the recommendations of the Lifestyle Work Group, which was convened by the NHLBI to focus on the effects of diet and physical activity on cardiovascular disease risk factors.18
Diet. The Lifestyle Work Group recommends combining the Dietary Approaches to Stop Hypertension (DASH) diet with reduced sodium intake, as there is evidence of a greater blood-pressure-lowering effect when the two are combined. The effect on blood pressure is independent of changes in weight.
The Lifestyle Work Group recommends consuming no more than 2,400 mg of sodium per day, noting that limiting intake to 1,500 mg can result in even greater reduction in blood pressure, and that even without achieving these goals, reducing sodium intake by at least 1,000 mg per day lowers blood pressure.
Physical activity. The Lifestyle Work Group recommends moderate to vigorous physical activity for approximately 160 minutes per week (three to four sessions a week, lasting an average of 40 minutes per session).
Weight loss. The Lifestyle Work Group did not review the blood-pressure-lowering effect of weight loss in those who are overweight or obese. The JNC 8 panel endorsed maintaining a healthy weight in controlling blood pressure.
Alcohol intake received no specific recommendations in JNC 8.
JNC 8 IN PERSPECTIVE
JNC 8 takes a rigorous, evidence-based approach and focuses on a few key questions. Thus, it is very different from the earlier reports: it has a narrower focus and does not address the full range of issues related to hypertension.
Strengths of JNC 8
The panel followed a rigorous process of review and evaluation of evidence from randomized controlled trials, adhering closely to standards set by the Institute of Medicine for guideline development. In contrast, JNC 7 relied on consensus and expert opinion.
The JNC 8 guidelines aim to simplify recommendations, with only two goals to remember: treat to lower than 150/90 mm Hg in patients age 60 and older, and lower than 140/90 mm Hg for everybody else. The initial drug regimen was simplified as well, with any of four choices for initial therapy in nonblacks and two in blacks.
Relaxing the blood pressure goals in elderly patients (although a cutoff of age 60 vs age 80 is likely to be debated) will also allay concerns about overtreating hypertension and causing adverse events in this population that is particularly susceptible to orthostatic changes and is at increased risk of falls.
Limitations and concerns
While the evidence-based nature of the recommendations is a strength, information from observational studies, systematic reviews, and meta-analyses was not incorporated into the formulation of these guidelines. This limits the available evidence, reflected in the fact that despite an extensive attempt to provide recommendations based on good evidence, five of the 10 recommendations (including the corollary recommendation) are still based on expert consensus opinion. Comparing and combining studies from different time periods is also problematic because of different methods of conducting clinical trials and analysis, and also because clinical care in a different period may differ from current standard practices.
Blood pressure targets in some subgroups are not clearly addressed, including those with proteinuria and with a history of stroke. Peterson et al,19 in an editorial accompanying the JNC 8 publication, commented on the need for larger randomized controlled trials to compare different blood pressure thresholds in various patient populations.
Some health care providers will likely be concerned that relaxing blood pressure goals could lead to higher real-world blood pressures, eventually leading to adverse cardiovascular outcomes, particularly on a population level. This is akin to the “speed limit rule”—people are more likely to hover above target, no matter what the target is.
In another editorial, Sox20 raised concerns about the external review process, ie, that the guidelines were not published in draft form to solicit public comment. Additionally, although the recommendations underwent extensive review, they were not endorsed by the specialty societies that the NHLBI designated to develop guidelines. In its defense, however, the JNC 8 panel has offered to share records of the review process on request, and this should serve to increase confidence in the review process.
The original literature search was limited to studies published through December 2009, which is more than 4 years before the publication of the recommendations. Although a bridge search was conducted until August 2013 to identify additional studies, this search used different inclusion criteria than the original criteria.
With its narrow focus, JNC 8 does not address many relevant issues. The American Society of Hypertension/International Society of Hypertension guidelines, published around the same time that the JNC 8 report was released, provide a more comprehensive review that will be of practical use for health care providers in the community.10
Ambulatory blood pressure monitoring is increasingly being used in clinical practice to detect white coat hypertension and, in many cases, to assess hypertension that is resistant to medications. It has also been shown to have better prognostic value in predicting cardiovascular risk and progression of kidney disease than office blood pressures.21,22 The UK National Institute of Health and Care Excellence guideline recommends ambulatory monitoring for the diagnosis of hypertension.23 However, JNC 8 did not provide specific recommendations for the use of this technology. Additionally, the JNC 8 evidence review is based on studies that used office blood pressure readings, and the recommendations are not necessarily applicable to measurements obtained by ambulatory monitoring.
Other topics covered in JNC 7 but not in JNC 8 include:
- Definitions and stages of hypertension (which remain the same)
- Initial treatment of stage 2 hypertension with two medications
- The J-curve phenomenon
- Preferred medications for patients with coronary artery disease or congestive heart failure
- A detailed list of oral antihypertensive agents—JNC 8 confines itself to the drugs and doses used in randomized controlled trials
- Patient evaluation
- Secondary hypertension
- Resistant hypertension
- Adherence issues.
Contrast with other guidelines
While the goal of these recommendations is to make treatment standards more understandable and uniform, contrasting recommendations on blood pressure goals and medications from various groups muddy the waters. Other groups that have issued hypertension guidelines in recent years include:
- The American Diabetes Association24
- The American Society of Hypertension and the International Society of Hypertension10
- The European Society of Hypertension and the European Society of Cardiology25
- The Canadian Hypertension Education Program26
- The Kidney Disease: Improving Global Outcomes initiative14
- The National Institute for Health and Clinical Excellence (UK)23
- The International Society on Hypertension in Blacks27
- The American Heart Association, the American College of Cardiology, and the US Centers for Disease Control and Prevention.28
Future directions
Despite the emphasis on making treatment decisions on an individual basis and using guidelines only as a framework for a safe direction in managing difficult clinical scenarios, guideline recommendations are increasingly being used to assess provider performance and quality of care, and so they assume even more importance in the current health care environment. As specialty organizations review and decide whether to endorse the JNC 8 recommendations, reconciling seemingly disparate recommendations from various groups is needed to send a clear and concise message to practitioners taking care of patients with high blood pressure.
Although a daunting task, integrating guidelines on hypertension management with other cardiovascular risk guidelines (eg, cholesterol, obesity) with assessment of overall cardiovascular risk profile would likely help in developing a more effective cardiovascular prevention strategy.
Despite the panel’s best efforts at providing evidence-based recommendations, many of the recommendations are based on expert opinion, reflecting the need for larger well-conducted studies. It is hoped that ongoing studies such as the Systolic Blood Pressure Intervention Trial29 will provide more clarity about blood pressure goals, especially in the elderly.
Final thoughts
Guidelines are not rules, and while they provide a framework by synthesizing the best available evidence, any treatment plan should be formulated on the basis of individual patient characteristics, including comorbidities, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572. Erratum in JAMA 2003; 290:197.
- Gibbons GH, Harold JG, Jessup M, Robertson RM, Oetgen WJ. The next steps in developing clinical practice guidelines for prevention. J Am Coll Cardiol 2013; 62:1399–1400.
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2013; 62:1396–1398.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. Erratum in: Lancet 2003; 361:1060.
- Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. http://www.iom.edu/Reports/2011/Clinical-Practice-Guide-lines-We-Can-Trust.aspx. Accessed February 4, 2014.
- JATOS Study Group. Principal results of the Japanese Trial To Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56:196–202.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014 Jan 14. [Epub ahead of print]
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 2011; 154:541–548.
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2:337–414.
- Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003.
- Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2003; 42:239–246.
- Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013 Nov 12. [Epub ahead of print]
- Peterson ED, Gaziano JM, Greenland P. Recommendations for treating hypertension: what are the right goals and purposes? JAMA Editorial. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Sox HC. Assessing the trustworthiness of the guideline for management of high blood pressure in adults (editorial). JAMA. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005; 46:156–161.
- Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69:1175–1180.
- National Institute for Health and Clinical Excellence. Hypertension (CG127). http://publications.nice.org.uk/hypertension-cg127. Accessed February 4, 2014.
- American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2013 Dec 20. [Epub ahead of print]
- Hypertension without compelling indications: 2013 CHEP recommendations. Hypertension Canada website. http://www.hypertension.ca/hypertension-without-compelling-indications. Accessed February 4, 2014.
- Flack JM, Sica DA, Bakris G, et al; International Society on Hypertension in Blacks. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800.
- Go AS, Bauman M, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2013 Nov 15.
- Systolic Blood Pressure Intervention Trial (SPRINT). http://clinicaltrials.gov/ct2/show/NCT01206062. Accessed February 4, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572. Erratum in JAMA 2003; 290:197.
- Gibbons GH, Harold JG, Jessup M, Robertson RM, Oetgen WJ. The next steps in developing clinical practice guidelines for prevention. J Am Coll Cardiol 2013; 62:1399–1400.
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2013; 62:1396–1398.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. Erratum in: Lancet 2003; 361:1060.
- Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. http://www.iom.edu/Reports/2011/Clinical-Practice-Guide-lines-We-Can-Trust.aspx. Accessed February 4, 2014.
- JATOS Study Group. Principal results of the Japanese Trial To Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56:196–202.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014 Jan 14. [Epub ahead of print]
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330:877–884.
- Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 2011; 154:541–548.
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2:337–414.
- Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003.
- Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2003; 42:239–246.
- Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013 Nov 12. [Epub ahead of print]
- Peterson ED, Gaziano JM, Greenland P. Recommendations for treating hypertension: what are the right goals and purposes? JAMA Editorial. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Sox HC. Assessing the trustworthiness of the guideline for management of high blood pressure in adults (editorial). JAMA. Published online December 18, 2013. doi: 10.1001/jama.2013.284430.
- Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005; 46:156–161.
- Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69:1175–1180.
- National Institute for Health and Clinical Excellence. Hypertension (CG127). http://publications.nice.org.uk/hypertension-cg127. Accessed February 4, 2014.
- American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2013 Dec 20. [Epub ahead of print]
- Hypertension without compelling indications: 2013 CHEP recommendations. Hypertension Canada website. http://www.hypertension.ca/hypertension-without-compelling-indications. Accessed February 4, 2014.
- Flack JM, Sica DA, Bakris G, et al; International Society on Hypertension in Blacks. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800.
- Go AS, Bauman M, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2013 Nov 15.
- Systolic Blood Pressure Intervention Trial (SPRINT). http://clinicaltrials.gov/ct2/show/NCT01206062. Accessed February 4, 2014.
KEY POINTS
- JNC 8 focuses on three main questions: when to begin treatment, how low to aim for, and which antihypertensive medications to use. It does not cover many topics that were included in JNC 7.
- In patients age 60 or older, JNC 8 recommends starting antihypertensive treatment if the blood pressure is 150/90 mm Hg or higher, with a goal of less than 150/90.
- For everyone else, including people with diabetes or chronic kidney disease, the threshold is 140/90 mm Hg, and the goal is less than 140/90.
- The recommended classes of drugs for initial therapy in nonblack patients without chronic kidney disease are thiazide-type diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs), although the last two classes should not be used in combination.
- For black patients, the initial classes of drugs are diuretics and calcium channel blockers; patients with chronic kidney disease should receive an ACE inhibitor or ARB.
Prevention and treatment of influenza in the primary care office
Every year, 5% to 20% of US residents contract the flu, 200,000 are hospitalized for it, and 36,000 die of influenza-related complications. The economic impact, including direct medical costs and lost earnings, exceeds $87 billion.1 Despite this, less than half of eligible US residents were vaccinated in the 2012–2013 season, with uninsured people more than twice as likely to forgo vaccination.2,3
Several studies have shown that influenza vaccination reduces the need for outpatient encounters and hospitalizations and lowers the incidence of death from acute myocardial infarction, the rate of all-cause mortality, and even the incidence of therapies administered by implantable defibrillators.4–6 In the 2012–2013 influenza season, vaccination prevented an estimated 3.2 million medically attended illnesses and almost 80,000 hospitalizations; 70% of hospitalizations prevented were in children age 6 months to 4 years and in adults over age 65.7
After the 2009 H1N1 pandemic, which disproportionately killed previously healthy adults, the US Centers for Disease Control and Prevention (CDC) expanded its vaccination recommendations to include everyone above the age of 6 months, with few contraindications.8
In addition, recent years have seen a great expansion in vaccine options, changes in the at-risk demographics, and continued widespread resistance to certain antiviral agents, with implications for practice in primary care.
Here, we review the barriers and the new options for treatment and prevention of influenza.
HEMAGGLUTININ AND NEURAMINIDASE
Influenza infection is caused by one of the circulating strains of influenza virus A or B.
The major viral surface glycoproteins are hemagglutinin and neuraminidase. Hemagglutinin plays an important role in viral attachment to host cells and is the major immunogen in the influenza vaccine. Neuraminidase contains an active enzymatic site that cleaves the newly formed budding influenza viruses from host-cell sialic acid residues and allows them to be released from the cell membrane to infect other respiratory epithelial cells. It is the target of currently recommended antiviral drugs.
VACCINE PRODUCTION
Throughout the year, 130 influenza centers around the world sample circulating strains and share their data with five World Health Organization (WHO) Collaborating Centers for Reference and Research on Influenza. The WHO analyzes the circulation patterns, predicts the strains most likely to be circulating in the next influenza season, and shares these strains with manufacturers of the vaccine.
Pharmaceutical companies then begin an elaborate process of producing and distributing hundreds of millions of doses of vaccine worldwide. The production traditionally uses millions of fertilized chicken eggs to produce strain-specific influenza hemagglutinin. Individual vaccine strains are combined into the final product after being inactivated by chemical or physical splitting of the viral envelope with or without subsequent purification of the hemagglutinin particles.
Before 2013, the WHO’s yearly recommendations included two strains of influenza A and a single strain of influenza B. In 2013, new quadrivalent vaccines that include protection against a second strain of influenza B were approved.
The WHO strain-selection process allows manufacturers about 6 months to produce the vaccine. In a typical year, the worldwide demand is about 400 million doses. The theoretical maximal annual worldwide capacity, given current techniques, is fewer than 1 billion doses, which is well short of the 10 billion doses necessary to allow for the double vaccination needed in a pandemic.9 Newly approved recombinant manufacturing techniques offer greater production efficiency, while novel methods of intradermal administration increase vaccine immunogenicity, decreasing the amount of viral antigens used per dose.
INACTIVATED VS LIVE-ATTENUATED
In addition to intramuscular inactivated influenza vaccine, a live-attenuated vaccine in the form of an intranasal spray (FluMist) became available in 2003. This form is generally favored in children, as it avoids the discomfort of an injection. It contains live, weakened, cold-adapted influenza strains that reproduce in the relatively colder temperatures of the exterior nares but cannot survive in the warmer temperatures of the lung and proximal airways. It is approved for healthy people 2 to 49 years of age, and some evidence suggests that it may be more effective than inactivated influenza vaccine in children,10 although its utility is limited by multiple contraindications (see below).
INFLUENZA VACCINE INDICATIONS AND CONTRAINDICATIONS
Vaccination for influenza is recommended for all persons 6 months of age and older, an expansion from pre-2009 guidelines that did not recommend vaccination for healthy adults age 19 to 49 who were not in contact with people at high risk of influenza-related complications.8 Many new vaccine formulations have become available in recent years, each with specific benefits, risks, and target populations (Table 1).
Contraindications to inactivated vaccine
The only firm contraindication to inactivated influenza vaccine is previous severe allergic reaction to influenza vaccine or any of its components. Those with moderate to severe acute illness are advised to wait until their condition improves before being vaccinated. People who have had Guillain-Barré syndrome and those with egg allergy are discussed in MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION, below. There is no risk of influenza infection from inactivated influenza vaccine.
Contraindications to live-attenuated influenza vaccine
Unlike inactivated influenza vaccine, the live-attenuated vaccine does result in shedding of vaccine-strain virus from the vaccinated host, with the theoretical potential for transmission of the virus from the vaccine recipient to other people, as well as the potential for influenza-like illness in vaccine recipients.11,12 Based on reported events, the former is estimated to occur in 10 to 20 per 1 million vaccinations, although these cases have never been proven to be caused by a cold-adapted vaccine-strain rather than by coincidental transmission of circulating wild-type viral strains.13
Despite this exceedingly small risk of viral transmission, live-attenuated influenza vaccine has multiple contraindications, including age less than 2 years and more than 49 years, disease- or drug-related compromised immune status, pregnancy, egg allergy, and history of allergic reaction to the formulation. These limit its use and are important to review in detail before prescribing.14
Use of neuraminidase inhibitors within 2 days before or 2 weeks after receiving live-attenuated influenza vaccine may interfere with replication of the cold-adapted strain and decrease the vaccine’s effectiveness.14
EFFECTIVENESS OF INFLUENZA VACCINATION IN OLDER ADULTS
The effectiveness of influenza vaccination depends on the age and health status of the person being vaccinated, as well as on the quality of the match between the vaccine and the circulating influenza viruses.
In the 2012–2013 season, the adjusted vaccine effectiveness was 56% overall, 47% for influenza A H3N2, and 67% for influenza B. However, in people age 65 and older, the overall adjusted vaccine effectiveness was 27%, and only 9% for influenza A H3N2.15 Thus, even though the vaccine-virus match was considered good, the vaccine was suboptimally effective in the older group. This may be an argument for using the recently approved high-dose vaccine in that age group. Although the high-dose vaccine has been shown to be significantly more immunogenic in older adults, it is too early to know if it is clinically more effective in preventing influenza in this age group.
Despite the lower-than-expected effectiveness in preventing influenza in the 2012–2013 season in people age 65 and older, several well-designed studies found that influenza vaccination prevented severe disease, including one study that found vaccination to be 89% effective in reducing influenza-associated hospitalizations in the 2010–2011 flu season.4,16
The limited effectiveness of vaccination in the older age group reminds us of the importance of early recognition and treatment of patients at high risk of influenza-related complications (see Table 2). It is also a call for greater compliance with vaccination in younger people, with a goal of achieving the 80% vaccination rate that has been calculated as adequate to achieve herd immunity.17
MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION
Concern about potential adverse effects is the most common reason for refusing influenza vaccination, even among health care workers.18 However, the only commonly encountered adverse effect of the intramuscular inactivated influenza vaccine is injection-site pain.
‘Catching the flu from a flu shot’
Many people think that they can “catch the flu from a flu shot” (or think that they actually did), but vaccine-acquired influenza is not possible with the inactivated influenza vaccine,19 and it is only a theoretical, undocumented consideration with the live-attenuated vaccine.
Various respiratory viruses other than influenza also cause viral upper-respiratory infections during the influenza season. These infections may coincide with influenza vaccination and are frequently misconstrued as a side effect of the influenza vaccine or as evidence of vaccine ineffectiveness.
Unnecessary concerns about simultaneous vaccinations
Patients and doctors are often concerned about simultaneous administration of multiple vaccines and choose to spread out indicated vaccinations over multiple visits. This practice increases patients’ risk of illness from vaccine-preventable diseases. Research shows that simultaneous administration does not alter the safety or effectiveness of vaccination.20–22 The CDC recommends simultaneous administration of all indicated live and inactivated vaccinations in order to reduce barriers to vaccination.20
Fear of Guillain-Barré syndrome
Guillain-Barré syndrome, an acute ascending polyneuropathy, has been blamed on influenza vaccination in cases that developed after the 1976 influenza A (H1N1) epidemic.
Most cases are self-limiting but require intensive treatment and supportive care. Full recovery occurs in 60% of cases, though some people experience persistent symptoms. The mortality rate is less than 5%.23
After the 1976 influenza pandemic, approximately 400 cases of Guillain-Barré syndrome arose in 45 million vaccine recipients, or about 1 case per 100,000 people.24 Multiple subsequent population analyses concluded that the actual incidence of Guillain-Barré syndrome attributable to influenza vaccination is negligible, at less than 1 case in 1 million vaccinations. Against this, we should compare the real risk of illness and death from influenza infection, which itself is a risk factor for Guillain-Barré syndrome.25
Should a person with a history of Guillain-Barré syndrome be revaccinated against influenza? The risk was evaluated in a large retrospective analysis of cases identified in the Kaiser Permanente Northern California Database from 1995 to 2006.26 Five hundred fifty cases of Guillain-Barré syndrome were identified, of which 18 had arisen within 6 weeks of the patient receiving a flu shot. Four hundred five doses of inactivated influenza vaccine were subsequently given to 105 patients who had a history of Guillain-Barré syndrome, two of whom had developed the syndrome within 6 weeks of receiving the shot. There were no documented episodes of recurrent Guillain-Barré syndrome in any of these patients. Only 6 of 550 patients with a history of the disease developed it again; none of these 6 had received the influenza vaccine in the preceding 2 months, and only 1 had been exposed to the measles-mumps-rubella vaccine in the 4 months before vaccination.
Nevertheless, expert opinion recommends lifelong avoidance of any immunization that had been given within 6 weeks before the onset of symptoms of Guillain-Barré syndrome.27
Overstated concern about egg allergy
Anaphylactic reactions can occur after influenza vaccination in people who have severe egg allergy, and concern about these reactions unfortunately prevents many otherwise eligible people with mild allergy from being vaccinated.
These reactions are much less common than feared. In a well-designed prospective cohort study of 367 patients with a history of egg allergy and positive skin-prick tests, including 132 with a history of severe allergy and 4 with a history of mild allergic symptoms arising in response to previous influenza vaccinations, none developed anaphylaxis.28
The same authors reviewed 26 studies in more than 4,000 egg-allergic patients, of whom more than 500 had a history of severe egg-associated reactions, and likewise found no cases of influenza vaccine-associated anaphylaxis. They concluded that the inactivated influenza vaccine is safer than the egg-derived mumps-measles-rubella vaccine, for which precautions for egg allergy no longer exist.28
People with a history of more serious reactions, ranging from stomach upset to anaphylaxis, can be safely vaccinated with a recombinant vaccine or referred to an allergist for further testing. People who experience hives as their only reaction to egg exposure should receive full-dose vaccination but then be observed for a half hour afterward.
The recombinant trivalent influenza vaccine Flublok was approved in 2013 for people age 18 to 49. It is the first commercially available influenza vaccine produced in a continuous insect cell line using a baculovirus vector. No eggs are used in its production, and it is approved for use in patients with egg allergy of any severity.
People who have a history of more serious reactions, including abdominal pain, nausea, vomiting, dizziness, or wheezing can be vaccinated with the recombinant vaccine or referred to an allergy specialist.
Despite this new option, understanding of alternative immunization guidelines for people with egg allergies, available on the CDC website29 remains important, as the availability of the recombinant trivalent influenza vaccine remains limited in the 2013–2014 influenza season.
Misconception about mercury toxicity
Thimerosal is an ethylmercury-containing preservative used in multidose antiviral vaccines, including some influenza vaccines.30 It is designed to prevent bacterial and fungal colonization of the vaccine vial while not reducing vaccine effectiveness or causing toxicity.
Contemporary understanding of mercury neurotoxicity is based largely on studies of methylmercury, including long-term, low-dose exposure in remote communities in the Faroe Islands and the Seychelles through regular consumption of fish and whale meat.31,32 These exposure studies had conflicting results: those in the Faroe Islands demonstrated toxicity, but the Seychelles studies actually showed better neurologic test scores at higher mercury levels, a trend the authors attributed to the beneficial effects of maternal fish consumption.
The results of the methylmercury studies have been extrapolated to ethylmercury (contained in thimerosal), although the two chemicals have vastly different pharmacologic properties. For example, methylmercury has a longer half-life and greater transport across the blood-brain barrier.33 A direct comparison found that ethylmercury is less toxic than methylmercury, although an increase in ethylmercury concentration of only 20% resulted in similar toxicity profiles.34 These studies were performed at concentrations of mercury thousands of times higher than those resulting from vaccination: nearly 150,000 times greater than those in an average adult or 15,000 times greater than those in a 1-year-old child from the typical 25-μg thimerosal dose allowed in contemporary influenza vaccines.
Despite much negative publicity, no link has been shown between thimerosal and autism.30 Multiple regulatory, scientific, and medical organizations including the US Food and Drug Administration (FDA), the WHO, the National Institutes of Health, the CDC, the American Academy of Pediatrics, and the American Congress of Obstetricians and Gynecologists (ACOG) have evaluated the data on the safety of thimerosal in vaccines and have agreed that it is safe. However, most of them urged vaccine manufacturers to eliminate mercury from vaccines as a precaution.30,35 Thimerosal has subsequently been eliminated from all childhood vaccines except for influenza vaccine, with no resulting decrease in childhood autism diagnoses.36
Considering that no harm from thimerosal at FDA-approved doses has been documented, and considering the real risk of influenza-related complications, particularly in young children and pregnant women, we recommend vaccination using whatever vaccine formulation is locally available for all patients, including children age 6 months and older and pregnant women. Nevertheless, given that mercury is being eliminated from childhood vaccines and that preservative-free single-dose vials are increasingly available in the United States, it seems reasonable to use thimerosal-free formulations for children, expectant mothers, and patients concerned about exposure if these formulations are readily available. Influenza vaccination should not be delayed if a thimerosal-free formulation is not readily available.
NEW VACCINE FORMULATIONS
Recent years have seen a dramatic expansion in influenza vaccine options (Table 1).
Quadrivalent vaccines
Quadrivalent vaccines protect against two strains of influenza A and two strains of influenza B, whereas earlier formulations included only one influenza B strain. Vaccination against either influenza B strain offers only limited cross-protection against the other B strain, and previous formulations involved assumptions about which strain would predominate in any given year. The CDC estimates that switching to quadrivalent vaccines will prevent up to 970,000 cases of influenza, 8,200 hospitalizations, and 485 deaths per year.37
Intradermal vaccine
The newly available Fluzone Intradermal vaccine contains smaller doses of hemagglutinin but is still effective because antigen-presenting dendritic cells in the skin reduce the required amount of vaccine antigen necessary for inducing protection.38 This may provide an advantage in the event of vaccine shortage. Also, since it is given in needles only 1.5 mm long, it may appeal to people who are afraid of needles.
The stronger immune reaction with intradermal administration causes more redness, induration, and tenderness at the injection site than with intramuscular administration.39 Patients should not be surprised by this reaction and can be advised to apply ice packs for symptomatic relief.
High-dose vaccine
A high-dose vaccine was approved in 2009 for use in adults age 65 and older. It contains 60 μg of hemagglutinin, compared with 15 μg in standard-dose vaccines, and has been shown to improve seroconversion rates. It remains to be seen if this translates into better clinical outcomes in older adults.40 Further studies will be necessary before we can recommend high-dose vaccines to other people with weakened immune response, such as those undergoing chemotherapy or those infected with human immunodeficiency virus (HIV).
Cell-based vaccines
Flucelvax was the first cell-based influenza vaccine. However, unlike the recombinant trivalent influenza vaccine, which uses no eggs in its manufacturing process, Flucelvax production starts with egg-derived influenza strains that are subsequently propagated in liquid culture of animal cells. It may therefore contain traces of egg protein, and it has not been studied in people with egg allergy.41
An advantage of the cell-based production technique is the use of fewer or no eggs at all, which may result in greater manufacturing efficiency. Also, it is a closed process that reduces the risk of bacterial contamination as well as reliance on antibiotics or preservatives, such as thimerosal, in the manufacturing process.42
CHEMOPROPHYLAXIS WITH NEURAMINIDASE INHIBITORS
The mainstays of influenza prevention are seasonal vaccination and appropriate infection-prevention practices. In addition, in patients at high risk of influenza-related complications (Table 2),43 postexposure chemoprophylaxis with a neuraminidase inhibitor, ie, oseltamivir (Tamiflu) or zanamivir (Relenza), is an effective preventive strategy, especially in years when the match between vaccine and circulating virus strains is suboptimal.44,45
Neuraminidase inhibitors are competitive inhibitors of the active site of the influenza glycoprotein neuraminidase, responsible for viral release from infected respiratory epithelial cells. Rates of resistance to neuraminidase inhibitors have been less than 1% in the United States in recent years, while resistance to the adamantanes amantadine (Symmetrel) and rimantadine (Flumadine) can be as high as 92%, depending on the virus isolate. Thus, their use for treatment or prophylaxis of influenza is not currently recommended by the CDC.46
Chemoprophylaxis with any agent may promote emergence of resistant strains, can cause adverse reactions, and should never be considered a substitute for vaccination.
ANTI-INFLUENZA AGENTS
Two neuraminidase inhibitors, oseltamivir and zanamivir, are approved by the FDA for preventing and treating uncomplicated influenza. Treatment must be instituted within 2 days of onset of symptoms to be effective.
Oseltamivir is available as an oral capsule or powder for liquid suspension. Its most common adverse effects are gastrointestinal upset including diarrhea, nausea, and vomiting.44
Zanamivir is only available in the form of a dry powder inhaler because of the drug’s poor oral bioavailability, and only 4% to 17% of the inhaled dose is systemically absorbed.45 There is a theoretical benefit in targeted delivery of zanamivir to the primary organ affected by influenza, and gastrointestinal side effects are less common with this drug.44,45 Unfortunately, the zanamivir inhaler requires complicated assembly and dexterity for administration (see the video on YouTube47), which may make it unreliable in certain patient groups, especially handicapped and elderly patients. Administration has been associated with bronchospasm, resulting in a more than 20% reduction in the forced expiratory volume in 1 second, and it is contraindicated in patients with underlying reactive airway disease such as chronic obstructive pulmonary disease or asthma.45
Table 3 lists the doses and duration of therapy for oseltamivir and zanamivir in adults with normal renal function, as well as approximate costs. No generic formulations of neuraminidase inhibitors are currently available, and outpatient use may not be covered by medical insurance. Several other neuraminidase inhibitors are either under development or at various stages in the FDA approval process.
EFFECTIVENESS OF ANTI-INFLUENZA DRUGS
Treatment with oseltamivir has been shown to reduce the duration of symptoms by approximately 1 day if initiated within 36 hours of onset of illness and 1.5 to 2 days if initiated within 24 hours.48,49 Trials and meta-analyses of zanamivir show similar effectiveness, though some suggest that symptoms were alleviated as much as 3 days sooner than in controls in a subgroup of patients who were febrile at presentation.50,51 Dual neuraminidase inhibitor therapy in an attempt to prevent emergence of resistance seems logical but was actually found to be less effective than monotherapy, according to a 2010 study.52
The effectiveness of neuraminidase inhibitors in reducing influenza-related complications and mortality rates has been controversial in recent years, as these outcomes were not addressed in initial studies that secured FDA approval. Several meta-analyses differ in their assessments of available data quality and conclusions. A 2009 Cochrane review questioned the completeness and the veracity of the data from manufacturer-funded trial data, much of which was unpublished and not made available to reviewers, and it concluded that a reduction of complications could not be supported by the available data.53 Hernán and Lipsitch,54 in a 2011 review, calculated that oseltamivir reduces the risk of lower respiratory tract complications by 28% in patients with influenza-like symptoms and by 37% in patients with confirmed influenza infection.
Additional trials and better access to available data are needed to settle the question of the effectiveness of neuraminidase inhibitors in reducing complications of influenza. Meanwhile, they remain strongly recommended by major health organizations, including the CDC and the WHO, which lists oseltamivir on its “model list of essential medicines.”
VIRAL RESISTANCE TO NEURAMINIDASE INHIBITORS
Viral resistance to neuraminidase inhibitors occurs through multiple mechanisms and may arise without selective pressure from exposure to these drugs.55
Oseltamivir possesses a hydrophobic moiety that requires viral neuraminidase to undergo a complex reconfiguration to expose the active site prior to binding. Any mutation affecting its ability to undergo this structural rearrangement can promote resistance by decreased oseltamivir access to the active site.
Zanamivir has a structural homology to the neuraminidase active site and requires no such reconfiguration. Additionally, mutations promoting resistance to zanamivir may actually decrease viral fitness; thus, resistance to zanamivir is significantly less common than to oseltamivir.55
About 2,000 influenza virus isolates currently circulating in the United States were tested for resistance; only 1% of the 2009 influenza A H1N1 isolates demonstrated resistance to oseltamivir, and none to zanamivir.56
The CDC regularly updates the resistance patterns of circulating influenza strains at www.cdc.gov/flu/weekly/index.htm.
SPECIAL CONSIDERATIONS
Pregnancy
Pregnant women may be at higher risk of severe influenza complications. This was especially true during the 2009 H1N1 pandemic, when pregnant women had a five times higher risk of death from influenza-related complications. Additionally, fever during pregnancy is an independent risk factor for adverse outcomes in the offspring.57 Maternal vaccination against influenza effectively protects the infant for the first 6 months of life, when vaccination is not recommended because of a poor immune response.58
Live-attenuated influenza vaccine is contraindicated during pregnancy. Given the documented risks to the mother from influenza and no documented harm from preservatives in multiuse vaccine vials, the Advisory Committee on Immunization Practices (ACIP) and ACOG do not state a preference for thimerosal-containing or thimerosal-free vaccine for any group, including pregnant women. Pregnant women should be vaccinated with whatever inactivated influenza vaccine formulation is available at the earliest opportunity in the beginning of the influenza season, regardless of the trimester of pregnancy.
Pregnant women are at high risk of influenza-related complications and should be considered for postexposure antiviral prophylaxis or early treatment with a neuraminidase inhibitor. However, both of the approved neuraminidase inhibitors are in pregnancy safety category C, indicating possible adverse effects in animal studies and a lack of safety data in pregnant humans. As with all category C medications, the risks and benefits must be considered, taking into account maternal comorbidities, vaccination status, effectiveness of the season’s influenza vaccine, and the virulence of circulating influenza strains.
As oseltamivir is associated with nausea and gastrointestinal side effects and as zanamivir has less systemic absorption, it may be reasonable to prescribe zanamivir for women already experiencing severe pregnancy-related nausea.
Immunocompromised people
Inactivated influenza vaccine is recommended and live-attenuated influenza vaccine is contraindicated for all immunocompromised people. Generally speaking, any form of immune compromise will decrease the immunogenicity of the vaccine. Additional considerations vary depending on the cause and severity of the immunocompromised status.
HIV-infected patients have higher seroconversion rates when vaccinated with the high-dose vaccine than with the standard-dose vaccine; however, as in adults over age 65, the clinical benefit has yet to be evaluated.59 The efficacy of vaccination is predictably related to the CD4 cell count, as T cells are necessary to mount a response.60 No documented benefit is gained from booster influenza vaccination in this group of patients.
Cancer patients should receive inactivated influenza vaccine every year. Postexposure chemoprophylaxis should be considered, and early treatment with a neuraminidase inhibitor is recommended in patients undergoing chemotherapy.
Solid-organ transplant recipients face a risk of organ rejection if they contract influenza infection, in addition to a higher risk of influenza-related complications.61 Transplant recipients should receive inactivated influenza vaccine as soon as it becomes available at the beginning of every influenza season. Additional research is necessary to evaluate the safety and effectiveness of the high-dose influenza vaccine in this patient group.
MORE OPTIONS, GREAT BENEFIT
Influenza remains a significant source of morbidity and mortality in the United States, and emerging pandemic strains as well as the aging population pose the risk of increased disease burden. New vaccine options offer hope of greater safety, improved efficacy, and higher vaccination rates though broader appeal to individuals. The actual differences in protection between various vaccine options are insignificant relative to the overall benefit of vaccination.
Health care providers should inquire about patients’ understanding and address their concerns about vaccination. Giving an available influenza vaccine within approved indications should not be delayed if alternative vaccine options are not readily available.
In addition to vaccination, patients at high risk of complications should be advised early in the influenza season to inform their doctors about potential exposure to influenza or the development of flu-like symptoms for consideration of early treatment or postexposure prophylaxis with a neuraminidase inhibitor.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–5096.
- Soni A. Influenza Immunization Rates for Selected at Risk Populations among the US Adult Civilian Noninstitutionalized Population, 2006. Statistical Brief #226. December 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://meps.ahrq.gov/data_files/publications/st226/stat226.pdf. Accessed January 31, 2014.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2012–13 Influenza Season. http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm - age-group-adults. Accessed January 31, 2014.
- Castilla J, Godoy P, Domínguez A, et al; CIBERESP Cases and Controls in Influenza Working Group Spain. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013; 57:167–175.
- Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011–2012 influenza season. Clin Infect Dis 2013; 56:1774–1777.
- Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013; 310:1711–1720.
- Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by influenza vaccination—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:997–1000.
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013; 62:1–43.
- Friede M. Snapshot of influenza vaccine manufacturing capacity worldwide and summary of WHO-HHS activities to promote technology transfer. World Health Organization Global Action Plan for Influenza II Meeting 2011. www.who.int/phi/Session1B_Current_Manufacturing_Capacity_Worldwide_Friede.pdf. Accessed February 5, 2014.
- Ashkenazi S, Vertruyen A, Arístegui J, et al., CAIV-T Study Group. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879.
- Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA 2005; 294:2720–2725.
- Vesikari T, Karvonen A, Korhonen T, et al; CAIV-T Transmission Study Group. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 2006; 25:590–595.
- Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control Hosp Epidemiol 2007; 28:702–707.
- Centers for Disease Control and Prevention (CDC). Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). http://www.cdc.gov/flu/about/qa/nasalspray.htm. Accessed February 3, 2014.
- Centers for Disease Control and Prevention (CDC). Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep 2013; 62:119–123.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med 2012; 55:72–77.
- Aziz NA, Muhamad S, Manaf MR, Hamid MZ. Factors Influencing H1N1 vaccination among primary health care workers: a cross-sectional study. Int J Prev Med 2013; 4:664–670.
- Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med 1996; 156:1546–1550.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60( 2):1–64.
- Tseng HF, Smith N, Sy LS, Jacobsen LJ. Evaluation of the incidence of herpes zoster after concomitant administration of zoster vaccine and polysaccharide pneumococcal vaccine. Vaccine 2011; 29:3628–3632.
- Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 2002; 109:124–129.
- Rajabally YA, Uncini A. Outcome and its predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 2012; 83:711–718.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976—1977. Am J Epidemiol 1979; 110:105–123.
- Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis 2010; 10:643–651.
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP, Network C. Recurrent Guillain-Barré syndrome following vaccination. Clin Infect Dis 2012; 54:800–804.
- Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol 2005; 62:1194–1198.
- Des Roches A, Paradis L, Gagnon R, et al. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol 2012; 130:1213–1216.e1.
- US Centers for Disease Control and Prevention. Influenza vaccination of people with a history of egg allergy. www.immunize.org/catg.d/p3094.pdf. Accessed February 3, 2014.
- US Food Drug Administration. Thimerosal in vaccines. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed February 3, 2014.
- Davidson PW, Kost J, Myers GJ, Cox C, Clarkson TW, Shamlaye CF. Methylmercury and neurodevelopment: reanalysis of the Seychelles Child Development Study outcomes at 66 months of age. JAMA 2001; 285:1291–1293.
- Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997; 19:417–428.
- Nelson KB, Bauman ML. Thimerosal and autism? Pediatrics 2003; 111:674–679.
- Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR. The comparative toxicology of ethyl- and methylmercury. Arch Toxicol 1985; 57:260–267.
- American Congress of Obstetricians and Gynecologists. Influenza vaccination during pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Influenza_Vaccination_During_Pregnancy. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. Understanding thimerosal, mercury, and vaccine safety. www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-thimerosal-color-office.pdf. Accessed February 3, 2014.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine 2013. doi: 10.1016/j.vaccine.2013.09.074. [Epub ahead of print]
- Sanofi Pasteur. Fluzone package insert. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305080.pdf. Accessed February 3, 2014.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food Drug Administration. Flucelvax FDA application. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM332069.pdf. Accessed February 3, 2014.
- Novartis. Flucelvax (influenza virus vaccine) fact sheet. www.novartis-vaccines.com/downloads/flucelvax/Flucelvax_Fact_Sheet.pdf. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. People at high risk for developing flu-related complications. www.cdc.gov/flu/about/disease/high_risk.htm. Accessed February 3, 2014.
- Roche Pharmaceuticals. Tamiflu package insert. http://www.gene.com/download/pdf/tamiflu_prescribing.pdf. Accessed February 3, 2014.
- GlaxoSmithKline. Relenza package insert. http://us.gsk.com/products/assets/us_relenza.pdf. Accessed February 3, 2014.
- Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60:1–24.
- Administration technique for zanamivir (Relenza) Diskhaler. YouTube. 2009. www.youtube.com/watch?v=sQI0a0ToSPo. Accessed February 6, 2014.
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355:1845–1850.
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283:1016–1624.
- Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326:1235.
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 1997; 337:874–880.
- Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med 2010; 7:e1000362.
- Jefferson T, Jones M, Doshi P, Del Mar C. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 2009; 339:b5106.
- Hernán MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011; 53:277–279.
- Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 2013; 98:174–185.
- US Centers for Disease Control and Prevention. FluView. www.cdc.gov/flu/weekly. Accessed February 3, 2014.
- Acs N, Bánhidy F, Puhó E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 2005; 73:989–996.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med 2013; 158:19–26.
- Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994; 8:469–476.
- Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2:287–291.
Every year, 5% to 20% of US residents contract the flu, 200,000 are hospitalized for it, and 36,000 die of influenza-related complications. The economic impact, including direct medical costs and lost earnings, exceeds $87 billion.1 Despite this, less than half of eligible US residents were vaccinated in the 2012–2013 season, with uninsured people more than twice as likely to forgo vaccination.2,3
Several studies have shown that influenza vaccination reduces the need for outpatient encounters and hospitalizations and lowers the incidence of death from acute myocardial infarction, the rate of all-cause mortality, and even the incidence of therapies administered by implantable defibrillators.4–6 In the 2012–2013 influenza season, vaccination prevented an estimated 3.2 million medically attended illnesses and almost 80,000 hospitalizations; 70% of hospitalizations prevented were in children age 6 months to 4 years and in adults over age 65.7
After the 2009 H1N1 pandemic, which disproportionately killed previously healthy adults, the US Centers for Disease Control and Prevention (CDC) expanded its vaccination recommendations to include everyone above the age of 6 months, with few contraindications.8
In addition, recent years have seen a great expansion in vaccine options, changes in the at-risk demographics, and continued widespread resistance to certain antiviral agents, with implications for practice in primary care.
Here, we review the barriers and the new options for treatment and prevention of influenza.
HEMAGGLUTININ AND NEURAMINIDASE
Influenza infection is caused by one of the circulating strains of influenza virus A or B.
The major viral surface glycoproteins are hemagglutinin and neuraminidase. Hemagglutinin plays an important role in viral attachment to host cells and is the major immunogen in the influenza vaccine. Neuraminidase contains an active enzymatic site that cleaves the newly formed budding influenza viruses from host-cell sialic acid residues and allows them to be released from the cell membrane to infect other respiratory epithelial cells. It is the target of currently recommended antiviral drugs.
VACCINE PRODUCTION
Throughout the year, 130 influenza centers around the world sample circulating strains and share their data with five World Health Organization (WHO) Collaborating Centers for Reference and Research on Influenza. The WHO analyzes the circulation patterns, predicts the strains most likely to be circulating in the next influenza season, and shares these strains with manufacturers of the vaccine.
Pharmaceutical companies then begin an elaborate process of producing and distributing hundreds of millions of doses of vaccine worldwide. The production traditionally uses millions of fertilized chicken eggs to produce strain-specific influenza hemagglutinin. Individual vaccine strains are combined into the final product after being inactivated by chemical or physical splitting of the viral envelope with or without subsequent purification of the hemagglutinin particles.
Before 2013, the WHO’s yearly recommendations included two strains of influenza A and a single strain of influenza B. In 2013, new quadrivalent vaccines that include protection against a second strain of influenza B were approved.
The WHO strain-selection process allows manufacturers about 6 months to produce the vaccine. In a typical year, the worldwide demand is about 400 million doses. The theoretical maximal annual worldwide capacity, given current techniques, is fewer than 1 billion doses, which is well short of the 10 billion doses necessary to allow for the double vaccination needed in a pandemic.9 Newly approved recombinant manufacturing techniques offer greater production efficiency, while novel methods of intradermal administration increase vaccine immunogenicity, decreasing the amount of viral antigens used per dose.
INACTIVATED VS LIVE-ATTENUATED
In addition to intramuscular inactivated influenza vaccine, a live-attenuated vaccine in the form of an intranasal spray (FluMist) became available in 2003. This form is generally favored in children, as it avoids the discomfort of an injection. It contains live, weakened, cold-adapted influenza strains that reproduce in the relatively colder temperatures of the exterior nares but cannot survive in the warmer temperatures of the lung and proximal airways. It is approved for healthy people 2 to 49 years of age, and some evidence suggests that it may be more effective than inactivated influenza vaccine in children,10 although its utility is limited by multiple contraindications (see below).
INFLUENZA VACCINE INDICATIONS AND CONTRAINDICATIONS
Vaccination for influenza is recommended for all persons 6 months of age and older, an expansion from pre-2009 guidelines that did not recommend vaccination for healthy adults age 19 to 49 who were not in contact with people at high risk of influenza-related complications.8 Many new vaccine formulations have become available in recent years, each with specific benefits, risks, and target populations (Table 1).
Contraindications to inactivated vaccine
The only firm contraindication to inactivated influenza vaccine is previous severe allergic reaction to influenza vaccine or any of its components. Those with moderate to severe acute illness are advised to wait until their condition improves before being vaccinated. People who have had Guillain-Barré syndrome and those with egg allergy are discussed in MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION, below. There is no risk of influenza infection from inactivated influenza vaccine.
Contraindications to live-attenuated influenza vaccine
Unlike inactivated influenza vaccine, the live-attenuated vaccine does result in shedding of vaccine-strain virus from the vaccinated host, with the theoretical potential for transmission of the virus from the vaccine recipient to other people, as well as the potential for influenza-like illness in vaccine recipients.11,12 Based on reported events, the former is estimated to occur in 10 to 20 per 1 million vaccinations, although these cases have never been proven to be caused by a cold-adapted vaccine-strain rather than by coincidental transmission of circulating wild-type viral strains.13
Despite this exceedingly small risk of viral transmission, live-attenuated influenza vaccine has multiple contraindications, including age less than 2 years and more than 49 years, disease- or drug-related compromised immune status, pregnancy, egg allergy, and history of allergic reaction to the formulation. These limit its use and are important to review in detail before prescribing.14
Use of neuraminidase inhibitors within 2 days before or 2 weeks after receiving live-attenuated influenza vaccine may interfere with replication of the cold-adapted strain and decrease the vaccine’s effectiveness.14
EFFECTIVENESS OF INFLUENZA VACCINATION IN OLDER ADULTS
The effectiveness of influenza vaccination depends on the age and health status of the person being vaccinated, as well as on the quality of the match between the vaccine and the circulating influenza viruses.
In the 2012–2013 season, the adjusted vaccine effectiveness was 56% overall, 47% for influenza A H3N2, and 67% for influenza B. However, in people age 65 and older, the overall adjusted vaccine effectiveness was 27%, and only 9% for influenza A H3N2.15 Thus, even though the vaccine-virus match was considered good, the vaccine was suboptimally effective in the older group. This may be an argument for using the recently approved high-dose vaccine in that age group. Although the high-dose vaccine has been shown to be significantly more immunogenic in older adults, it is too early to know if it is clinically more effective in preventing influenza in this age group.
Despite the lower-than-expected effectiveness in preventing influenza in the 2012–2013 season in people age 65 and older, several well-designed studies found that influenza vaccination prevented severe disease, including one study that found vaccination to be 89% effective in reducing influenza-associated hospitalizations in the 2010–2011 flu season.4,16
The limited effectiveness of vaccination in the older age group reminds us of the importance of early recognition and treatment of patients at high risk of influenza-related complications (see Table 2). It is also a call for greater compliance with vaccination in younger people, with a goal of achieving the 80% vaccination rate that has been calculated as adequate to achieve herd immunity.17
MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION
Concern about potential adverse effects is the most common reason for refusing influenza vaccination, even among health care workers.18 However, the only commonly encountered adverse effect of the intramuscular inactivated influenza vaccine is injection-site pain.
‘Catching the flu from a flu shot’
Many people think that they can “catch the flu from a flu shot” (or think that they actually did), but vaccine-acquired influenza is not possible with the inactivated influenza vaccine,19 and it is only a theoretical, undocumented consideration with the live-attenuated vaccine.
Various respiratory viruses other than influenza also cause viral upper-respiratory infections during the influenza season. These infections may coincide with influenza vaccination and are frequently misconstrued as a side effect of the influenza vaccine or as evidence of vaccine ineffectiveness.
Unnecessary concerns about simultaneous vaccinations
Patients and doctors are often concerned about simultaneous administration of multiple vaccines and choose to spread out indicated vaccinations over multiple visits. This practice increases patients’ risk of illness from vaccine-preventable diseases. Research shows that simultaneous administration does not alter the safety or effectiveness of vaccination.20–22 The CDC recommends simultaneous administration of all indicated live and inactivated vaccinations in order to reduce barriers to vaccination.20
Fear of Guillain-Barré syndrome
Guillain-Barré syndrome, an acute ascending polyneuropathy, has been blamed on influenza vaccination in cases that developed after the 1976 influenza A (H1N1) epidemic.
Most cases are self-limiting but require intensive treatment and supportive care. Full recovery occurs in 60% of cases, though some people experience persistent symptoms. The mortality rate is less than 5%.23
After the 1976 influenza pandemic, approximately 400 cases of Guillain-Barré syndrome arose in 45 million vaccine recipients, or about 1 case per 100,000 people.24 Multiple subsequent population analyses concluded that the actual incidence of Guillain-Barré syndrome attributable to influenza vaccination is negligible, at less than 1 case in 1 million vaccinations. Against this, we should compare the real risk of illness and death from influenza infection, which itself is a risk factor for Guillain-Barré syndrome.25
Should a person with a history of Guillain-Barré syndrome be revaccinated against influenza? The risk was evaluated in a large retrospective analysis of cases identified in the Kaiser Permanente Northern California Database from 1995 to 2006.26 Five hundred fifty cases of Guillain-Barré syndrome were identified, of which 18 had arisen within 6 weeks of the patient receiving a flu shot. Four hundred five doses of inactivated influenza vaccine were subsequently given to 105 patients who had a history of Guillain-Barré syndrome, two of whom had developed the syndrome within 6 weeks of receiving the shot. There were no documented episodes of recurrent Guillain-Barré syndrome in any of these patients. Only 6 of 550 patients with a history of the disease developed it again; none of these 6 had received the influenza vaccine in the preceding 2 months, and only 1 had been exposed to the measles-mumps-rubella vaccine in the 4 months before vaccination.
Nevertheless, expert opinion recommends lifelong avoidance of any immunization that had been given within 6 weeks before the onset of symptoms of Guillain-Barré syndrome.27
Overstated concern about egg allergy
Anaphylactic reactions can occur after influenza vaccination in people who have severe egg allergy, and concern about these reactions unfortunately prevents many otherwise eligible people with mild allergy from being vaccinated.
These reactions are much less common than feared. In a well-designed prospective cohort study of 367 patients with a history of egg allergy and positive skin-prick tests, including 132 with a history of severe allergy and 4 with a history of mild allergic symptoms arising in response to previous influenza vaccinations, none developed anaphylaxis.28
The same authors reviewed 26 studies in more than 4,000 egg-allergic patients, of whom more than 500 had a history of severe egg-associated reactions, and likewise found no cases of influenza vaccine-associated anaphylaxis. They concluded that the inactivated influenza vaccine is safer than the egg-derived mumps-measles-rubella vaccine, for which precautions for egg allergy no longer exist.28
People with a history of more serious reactions, ranging from stomach upset to anaphylaxis, can be safely vaccinated with a recombinant vaccine or referred to an allergist for further testing. People who experience hives as their only reaction to egg exposure should receive full-dose vaccination but then be observed for a half hour afterward.
The recombinant trivalent influenza vaccine Flublok was approved in 2013 for people age 18 to 49. It is the first commercially available influenza vaccine produced in a continuous insect cell line using a baculovirus vector. No eggs are used in its production, and it is approved for use in patients with egg allergy of any severity.
People who have a history of more serious reactions, including abdominal pain, nausea, vomiting, dizziness, or wheezing can be vaccinated with the recombinant vaccine or referred to an allergy specialist.
Despite this new option, understanding of alternative immunization guidelines for people with egg allergies, available on the CDC website29 remains important, as the availability of the recombinant trivalent influenza vaccine remains limited in the 2013–2014 influenza season.
Misconception about mercury toxicity
Thimerosal is an ethylmercury-containing preservative used in multidose antiviral vaccines, including some influenza vaccines.30 It is designed to prevent bacterial and fungal colonization of the vaccine vial while not reducing vaccine effectiveness or causing toxicity.
Contemporary understanding of mercury neurotoxicity is based largely on studies of methylmercury, including long-term, low-dose exposure in remote communities in the Faroe Islands and the Seychelles through regular consumption of fish and whale meat.31,32 These exposure studies had conflicting results: those in the Faroe Islands demonstrated toxicity, but the Seychelles studies actually showed better neurologic test scores at higher mercury levels, a trend the authors attributed to the beneficial effects of maternal fish consumption.
The results of the methylmercury studies have been extrapolated to ethylmercury (contained in thimerosal), although the two chemicals have vastly different pharmacologic properties. For example, methylmercury has a longer half-life and greater transport across the blood-brain barrier.33 A direct comparison found that ethylmercury is less toxic than methylmercury, although an increase in ethylmercury concentration of only 20% resulted in similar toxicity profiles.34 These studies were performed at concentrations of mercury thousands of times higher than those resulting from vaccination: nearly 150,000 times greater than those in an average adult or 15,000 times greater than those in a 1-year-old child from the typical 25-μg thimerosal dose allowed in contemporary influenza vaccines.
Despite much negative publicity, no link has been shown between thimerosal and autism.30 Multiple regulatory, scientific, and medical organizations including the US Food and Drug Administration (FDA), the WHO, the National Institutes of Health, the CDC, the American Academy of Pediatrics, and the American Congress of Obstetricians and Gynecologists (ACOG) have evaluated the data on the safety of thimerosal in vaccines and have agreed that it is safe. However, most of them urged vaccine manufacturers to eliminate mercury from vaccines as a precaution.30,35 Thimerosal has subsequently been eliminated from all childhood vaccines except for influenza vaccine, with no resulting decrease in childhood autism diagnoses.36
Considering that no harm from thimerosal at FDA-approved doses has been documented, and considering the real risk of influenza-related complications, particularly in young children and pregnant women, we recommend vaccination using whatever vaccine formulation is locally available for all patients, including children age 6 months and older and pregnant women. Nevertheless, given that mercury is being eliminated from childhood vaccines and that preservative-free single-dose vials are increasingly available in the United States, it seems reasonable to use thimerosal-free formulations for children, expectant mothers, and patients concerned about exposure if these formulations are readily available. Influenza vaccination should not be delayed if a thimerosal-free formulation is not readily available.
NEW VACCINE FORMULATIONS
Recent years have seen a dramatic expansion in influenza vaccine options (Table 1).
Quadrivalent vaccines
Quadrivalent vaccines protect against two strains of influenza A and two strains of influenza B, whereas earlier formulations included only one influenza B strain. Vaccination against either influenza B strain offers only limited cross-protection against the other B strain, and previous formulations involved assumptions about which strain would predominate in any given year. The CDC estimates that switching to quadrivalent vaccines will prevent up to 970,000 cases of influenza, 8,200 hospitalizations, and 485 deaths per year.37
Intradermal vaccine
The newly available Fluzone Intradermal vaccine contains smaller doses of hemagglutinin but is still effective because antigen-presenting dendritic cells in the skin reduce the required amount of vaccine antigen necessary for inducing protection.38 This may provide an advantage in the event of vaccine shortage. Also, since it is given in needles only 1.5 mm long, it may appeal to people who are afraid of needles.
The stronger immune reaction with intradermal administration causes more redness, induration, and tenderness at the injection site than with intramuscular administration.39 Patients should not be surprised by this reaction and can be advised to apply ice packs for symptomatic relief.
High-dose vaccine
A high-dose vaccine was approved in 2009 for use in adults age 65 and older. It contains 60 μg of hemagglutinin, compared with 15 μg in standard-dose vaccines, and has been shown to improve seroconversion rates. It remains to be seen if this translates into better clinical outcomes in older adults.40 Further studies will be necessary before we can recommend high-dose vaccines to other people with weakened immune response, such as those undergoing chemotherapy or those infected with human immunodeficiency virus (HIV).
Cell-based vaccines
Flucelvax was the first cell-based influenza vaccine. However, unlike the recombinant trivalent influenza vaccine, which uses no eggs in its manufacturing process, Flucelvax production starts with egg-derived influenza strains that are subsequently propagated in liquid culture of animal cells. It may therefore contain traces of egg protein, and it has not been studied in people with egg allergy.41
An advantage of the cell-based production technique is the use of fewer or no eggs at all, which may result in greater manufacturing efficiency. Also, it is a closed process that reduces the risk of bacterial contamination as well as reliance on antibiotics or preservatives, such as thimerosal, in the manufacturing process.42
CHEMOPROPHYLAXIS WITH NEURAMINIDASE INHIBITORS
The mainstays of influenza prevention are seasonal vaccination and appropriate infection-prevention practices. In addition, in patients at high risk of influenza-related complications (Table 2),43 postexposure chemoprophylaxis with a neuraminidase inhibitor, ie, oseltamivir (Tamiflu) or zanamivir (Relenza), is an effective preventive strategy, especially in years when the match between vaccine and circulating virus strains is suboptimal.44,45
Neuraminidase inhibitors are competitive inhibitors of the active site of the influenza glycoprotein neuraminidase, responsible for viral release from infected respiratory epithelial cells. Rates of resistance to neuraminidase inhibitors have been less than 1% in the United States in recent years, while resistance to the adamantanes amantadine (Symmetrel) and rimantadine (Flumadine) can be as high as 92%, depending on the virus isolate. Thus, their use for treatment or prophylaxis of influenza is not currently recommended by the CDC.46
Chemoprophylaxis with any agent may promote emergence of resistant strains, can cause adverse reactions, and should never be considered a substitute for vaccination.
ANTI-INFLUENZA AGENTS
Two neuraminidase inhibitors, oseltamivir and zanamivir, are approved by the FDA for preventing and treating uncomplicated influenza. Treatment must be instituted within 2 days of onset of symptoms to be effective.
Oseltamivir is available as an oral capsule or powder for liquid suspension. Its most common adverse effects are gastrointestinal upset including diarrhea, nausea, and vomiting.44
Zanamivir is only available in the form of a dry powder inhaler because of the drug’s poor oral bioavailability, and only 4% to 17% of the inhaled dose is systemically absorbed.45 There is a theoretical benefit in targeted delivery of zanamivir to the primary organ affected by influenza, and gastrointestinal side effects are less common with this drug.44,45 Unfortunately, the zanamivir inhaler requires complicated assembly and dexterity for administration (see the video on YouTube47), which may make it unreliable in certain patient groups, especially handicapped and elderly patients. Administration has been associated with bronchospasm, resulting in a more than 20% reduction in the forced expiratory volume in 1 second, and it is contraindicated in patients with underlying reactive airway disease such as chronic obstructive pulmonary disease or asthma.45
Table 3 lists the doses and duration of therapy for oseltamivir and zanamivir in adults with normal renal function, as well as approximate costs. No generic formulations of neuraminidase inhibitors are currently available, and outpatient use may not be covered by medical insurance. Several other neuraminidase inhibitors are either under development or at various stages in the FDA approval process.
EFFECTIVENESS OF ANTI-INFLUENZA DRUGS
Treatment with oseltamivir has been shown to reduce the duration of symptoms by approximately 1 day if initiated within 36 hours of onset of illness and 1.5 to 2 days if initiated within 24 hours.48,49 Trials and meta-analyses of zanamivir show similar effectiveness, though some suggest that symptoms were alleviated as much as 3 days sooner than in controls in a subgroup of patients who were febrile at presentation.50,51 Dual neuraminidase inhibitor therapy in an attempt to prevent emergence of resistance seems logical but was actually found to be less effective than monotherapy, according to a 2010 study.52
The effectiveness of neuraminidase inhibitors in reducing influenza-related complications and mortality rates has been controversial in recent years, as these outcomes were not addressed in initial studies that secured FDA approval. Several meta-analyses differ in their assessments of available data quality and conclusions. A 2009 Cochrane review questioned the completeness and the veracity of the data from manufacturer-funded trial data, much of which was unpublished and not made available to reviewers, and it concluded that a reduction of complications could not be supported by the available data.53 Hernán and Lipsitch,54 in a 2011 review, calculated that oseltamivir reduces the risk of lower respiratory tract complications by 28% in patients with influenza-like symptoms and by 37% in patients with confirmed influenza infection.
Additional trials and better access to available data are needed to settle the question of the effectiveness of neuraminidase inhibitors in reducing complications of influenza. Meanwhile, they remain strongly recommended by major health organizations, including the CDC and the WHO, which lists oseltamivir on its “model list of essential medicines.”
VIRAL RESISTANCE TO NEURAMINIDASE INHIBITORS
Viral resistance to neuraminidase inhibitors occurs through multiple mechanisms and may arise without selective pressure from exposure to these drugs.55
Oseltamivir possesses a hydrophobic moiety that requires viral neuraminidase to undergo a complex reconfiguration to expose the active site prior to binding. Any mutation affecting its ability to undergo this structural rearrangement can promote resistance by decreased oseltamivir access to the active site.
Zanamivir has a structural homology to the neuraminidase active site and requires no such reconfiguration. Additionally, mutations promoting resistance to zanamivir may actually decrease viral fitness; thus, resistance to zanamivir is significantly less common than to oseltamivir.55
About 2,000 influenza virus isolates currently circulating in the United States were tested for resistance; only 1% of the 2009 influenza A H1N1 isolates demonstrated resistance to oseltamivir, and none to zanamivir.56
The CDC regularly updates the resistance patterns of circulating influenza strains at www.cdc.gov/flu/weekly/index.htm.
SPECIAL CONSIDERATIONS
Pregnancy
Pregnant women may be at higher risk of severe influenza complications. This was especially true during the 2009 H1N1 pandemic, when pregnant women had a five times higher risk of death from influenza-related complications. Additionally, fever during pregnancy is an independent risk factor for adverse outcomes in the offspring.57 Maternal vaccination against influenza effectively protects the infant for the first 6 months of life, when vaccination is not recommended because of a poor immune response.58
Live-attenuated influenza vaccine is contraindicated during pregnancy. Given the documented risks to the mother from influenza and no documented harm from preservatives in multiuse vaccine vials, the Advisory Committee on Immunization Practices (ACIP) and ACOG do not state a preference for thimerosal-containing or thimerosal-free vaccine for any group, including pregnant women. Pregnant women should be vaccinated with whatever inactivated influenza vaccine formulation is available at the earliest opportunity in the beginning of the influenza season, regardless of the trimester of pregnancy.
Pregnant women are at high risk of influenza-related complications and should be considered for postexposure antiviral prophylaxis or early treatment with a neuraminidase inhibitor. However, both of the approved neuraminidase inhibitors are in pregnancy safety category C, indicating possible adverse effects in animal studies and a lack of safety data in pregnant humans. As with all category C medications, the risks and benefits must be considered, taking into account maternal comorbidities, vaccination status, effectiveness of the season’s influenza vaccine, and the virulence of circulating influenza strains.
As oseltamivir is associated with nausea and gastrointestinal side effects and as zanamivir has less systemic absorption, it may be reasonable to prescribe zanamivir for women already experiencing severe pregnancy-related nausea.
Immunocompromised people
Inactivated influenza vaccine is recommended and live-attenuated influenza vaccine is contraindicated for all immunocompromised people. Generally speaking, any form of immune compromise will decrease the immunogenicity of the vaccine. Additional considerations vary depending on the cause and severity of the immunocompromised status.
HIV-infected patients have higher seroconversion rates when vaccinated with the high-dose vaccine than with the standard-dose vaccine; however, as in adults over age 65, the clinical benefit has yet to be evaluated.59 The efficacy of vaccination is predictably related to the CD4 cell count, as T cells are necessary to mount a response.60 No documented benefit is gained from booster influenza vaccination in this group of patients.
Cancer patients should receive inactivated influenza vaccine every year. Postexposure chemoprophylaxis should be considered, and early treatment with a neuraminidase inhibitor is recommended in patients undergoing chemotherapy.
Solid-organ transplant recipients face a risk of organ rejection if they contract influenza infection, in addition to a higher risk of influenza-related complications.61 Transplant recipients should receive inactivated influenza vaccine as soon as it becomes available at the beginning of every influenza season. Additional research is necessary to evaluate the safety and effectiveness of the high-dose influenza vaccine in this patient group.
MORE OPTIONS, GREAT BENEFIT
Influenza remains a significant source of morbidity and mortality in the United States, and emerging pandemic strains as well as the aging population pose the risk of increased disease burden. New vaccine options offer hope of greater safety, improved efficacy, and higher vaccination rates though broader appeal to individuals. The actual differences in protection between various vaccine options are insignificant relative to the overall benefit of vaccination.
Health care providers should inquire about patients’ understanding and address their concerns about vaccination. Giving an available influenza vaccine within approved indications should not be delayed if alternative vaccine options are not readily available.
In addition to vaccination, patients at high risk of complications should be advised early in the influenza season to inform their doctors about potential exposure to influenza or the development of flu-like symptoms for consideration of early treatment or postexposure prophylaxis with a neuraminidase inhibitor.
Every year, 5% to 20% of US residents contract the flu, 200,000 are hospitalized for it, and 36,000 die of influenza-related complications. The economic impact, including direct medical costs and lost earnings, exceeds $87 billion.1 Despite this, less than half of eligible US residents were vaccinated in the 2012–2013 season, with uninsured people more than twice as likely to forgo vaccination.2,3
Several studies have shown that influenza vaccination reduces the need for outpatient encounters and hospitalizations and lowers the incidence of death from acute myocardial infarction, the rate of all-cause mortality, and even the incidence of therapies administered by implantable defibrillators.4–6 In the 2012–2013 influenza season, vaccination prevented an estimated 3.2 million medically attended illnesses and almost 80,000 hospitalizations; 70% of hospitalizations prevented were in children age 6 months to 4 years and in adults over age 65.7
After the 2009 H1N1 pandemic, which disproportionately killed previously healthy adults, the US Centers for Disease Control and Prevention (CDC) expanded its vaccination recommendations to include everyone above the age of 6 months, with few contraindications.8
In addition, recent years have seen a great expansion in vaccine options, changes in the at-risk demographics, and continued widespread resistance to certain antiviral agents, with implications for practice in primary care.
Here, we review the barriers and the new options for treatment and prevention of influenza.
HEMAGGLUTININ AND NEURAMINIDASE
Influenza infection is caused by one of the circulating strains of influenza virus A or B.
The major viral surface glycoproteins are hemagglutinin and neuraminidase. Hemagglutinin plays an important role in viral attachment to host cells and is the major immunogen in the influenza vaccine. Neuraminidase contains an active enzymatic site that cleaves the newly formed budding influenza viruses from host-cell sialic acid residues and allows them to be released from the cell membrane to infect other respiratory epithelial cells. It is the target of currently recommended antiviral drugs.
VACCINE PRODUCTION
Throughout the year, 130 influenza centers around the world sample circulating strains and share their data with five World Health Organization (WHO) Collaborating Centers for Reference and Research on Influenza. The WHO analyzes the circulation patterns, predicts the strains most likely to be circulating in the next influenza season, and shares these strains with manufacturers of the vaccine.
Pharmaceutical companies then begin an elaborate process of producing and distributing hundreds of millions of doses of vaccine worldwide. The production traditionally uses millions of fertilized chicken eggs to produce strain-specific influenza hemagglutinin. Individual vaccine strains are combined into the final product after being inactivated by chemical or physical splitting of the viral envelope with or without subsequent purification of the hemagglutinin particles.
Before 2013, the WHO’s yearly recommendations included two strains of influenza A and a single strain of influenza B. In 2013, new quadrivalent vaccines that include protection against a second strain of influenza B were approved.
The WHO strain-selection process allows manufacturers about 6 months to produce the vaccine. In a typical year, the worldwide demand is about 400 million doses. The theoretical maximal annual worldwide capacity, given current techniques, is fewer than 1 billion doses, which is well short of the 10 billion doses necessary to allow for the double vaccination needed in a pandemic.9 Newly approved recombinant manufacturing techniques offer greater production efficiency, while novel methods of intradermal administration increase vaccine immunogenicity, decreasing the amount of viral antigens used per dose.
INACTIVATED VS LIVE-ATTENUATED
In addition to intramuscular inactivated influenza vaccine, a live-attenuated vaccine in the form of an intranasal spray (FluMist) became available in 2003. This form is generally favored in children, as it avoids the discomfort of an injection. It contains live, weakened, cold-adapted influenza strains that reproduce in the relatively colder temperatures of the exterior nares but cannot survive in the warmer temperatures of the lung and proximal airways. It is approved for healthy people 2 to 49 years of age, and some evidence suggests that it may be more effective than inactivated influenza vaccine in children,10 although its utility is limited by multiple contraindications (see below).
INFLUENZA VACCINE INDICATIONS AND CONTRAINDICATIONS
Vaccination for influenza is recommended for all persons 6 months of age and older, an expansion from pre-2009 guidelines that did not recommend vaccination for healthy adults age 19 to 49 who were not in contact with people at high risk of influenza-related complications.8 Many new vaccine formulations have become available in recent years, each with specific benefits, risks, and target populations (Table 1).
Contraindications to inactivated vaccine
The only firm contraindication to inactivated influenza vaccine is previous severe allergic reaction to influenza vaccine or any of its components. Those with moderate to severe acute illness are advised to wait until their condition improves before being vaccinated. People who have had Guillain-Barré syndrome and those with egg allergy are discussed in MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION, below. There is no risk of influenza infection from inactivated influenza vaccine.
Contraindications to live-attenuated influenza vaccine
Unlike inactivated influenza vaccine, the live-attenuated vaccine does result in shedding of vaccine-strain virus from the vaccinated host, with the theoretical potential for transmission of the virus from the vaccine recipient to other people, as well as the potential for influenza-like illness in vaccine recipients.11,12 Based on reported events, the former is estimated to occur in 10 to 20 per 1 million vaccinations, although these cases have never been proven to be caused by a cold-adapted vaccine-strain rather than by coincidental transmission of circulating wild-type viral strains.13
Despite this exceedingly small risk of viral transmission, live-attenuated influenza vaccine has multiple contraindications, including age less than 2 years and more than 49 years, disease- or drug-related compromised immune status, pregnancy, egg allergy, and history of allergic reaction to the formulation. These limit its use and are important to review in detail before prescribing.14
Use of neuraminidase inhibitors within 2 days before or 2 weeks after receiving live-attenuated influenza vaccine may interfere with replication of the cold-adapted strain and decrease the vaccine’s effectiveness.14
EFFECTIVENESS OF INFLUENZA VACCINATION IN OLDER ADULTS
The effectiveness of influenza vaccination depends on the age and health status of the person being vaccinated, as well as on the quality of the match between the vaccine and the circulating influenza viruses.
In the 2012–2013 season, the adjusted vaccine effectiveness was 56% overall, 47% for influenza A H3N2, and 67% for influenza B. However, in people age 65 and older, the overall adjusted vaccine effectiveness was 27%, and only 9% for influenza A H3N2.15 Thus, even though the vaccine-virus match was considered good, the vaccine was suboptimally effective in the older group. This may be an argument for using the recently approved high-dose vaccine in that age group. Although the high-dose vaccine has been shown to be significantly more immunogenic in older adults, it is too early to know if it is clinically more effective in preventing influenza in this age group.
Despite the lower-than-expected effectiveness in preventing influenza in the 2012–2013 season in people age 65 and older, several well-designed studies found that influenza vaccination prevented severe disease, including one study that found vaccination to be 89% effective in reducing influenza-associated hospitalizations in the 2010–2011 flu season.4,16
The limited effectiveness of vaccination in the older age group reminds us of the importance of early recognition and treatment of patients at high risk of influenza-related complications (see Table 2). It is also a call for greater compliance with vaccination in younger people, with a goal of achieving the 80% vaccination rate that has been calculated as adequate to achieve herd immunity.17
MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION
Concern about potential adverse effects is the most common reason for refusing influenza vaccination, even among health care workers.18 However, the only commonly encountered adverse effect of the intramuscular inactivated influenza vaccine is injection-site pain.
‘Catching the flu from a flu shot’
Many people think that they can “catch the flu from a flu shot” (or think that they actually did), but vaccine-acquired influenza is not possible with the inactivated influenza vaccine,19 and it is only a theoretical, undocumented consideration with the live-attenuated vaccine.
Various respiratory viruses other than influenza also cause viral upper-respiratory infections during the influenza season. These infections may coincide with influenza vaccination and are frequently misconstrued as a side effect of the influenza vaccine or as evidence of vaccine ineffectiveness.
Unnecessary concerns about simultaneous vaccinations
Patients and doctors are often concerned about simultaneous administration of multiple vaccines and choose to spread out indicated vaccinations over multiple visits. This practice increases patients’ risk of illness from vaccine-preventable diseases. Research shows that simultaneous administration does not alter the safety or effectiveness of vaccination.20–22 The CDC recommends simultaneous administration of all indicated live and inactivated vaccinations in order to reduce barriers to vaccination.20
Fear of Guillain-Barré syndrome
Guillain-Barré syndrome, an acute ascending polyneuropathy, has been blamed on influenza vaccination in cases that developed after the 1976 influenza A (H1N1) epidemic.
Most cases are self-limiting but require intensive treatment and supportive care. Full recovery occurs in 60% of cases, though some people experience persistent symptoms. The mortality rate is less than 5%.23
After the 1976 influenza pandemic, approximately 400 cases of Guillain-Barré syndrome arose in 45 million vaccine recipients, or about 1 case per 100,000 people.24 Multiple subsequent population analyses concluded that the actual incidence of Guillain-Barré syndrome attributable to influenza vaccination is negligible, at less than 1 case in 1 million vaccinations. Against this, we should compare the real risk of illness and death from influenza infection, which itself is a risk factor for Guillain-Barré syndrome.25
Should a person with a history of Guillain-Barré syndrome be revaccinated against influenza? The risk was evaluated in a large retrospective analysis of cases identified in the Kaiser Permanente Northern California Database from 1995 to 2006.26 Five hundred fifty cases of Guillain-Barré syndrome were identified, of which 18 had arisen within 6 weeks of the patient receiving a flu shot. Four hundred five doses of inactivated influenza vaccine were subsequently given to 105 patients who had a history of Guillain-Barré syndrome, two of whom had developed the syndrome within 6 weeks of receiving the shot. There were no documented episodes of recurrent Guillain-Barré syndrome in any of these patients. Only 6 of 550 patients with a history of the disease developed it again; none of these 6 had received the influenza vaccine in the preceding 2 months, and only 1 had been exposed to the measles-mumps-rubella vaccine in the 4 months before vaccination.
Nevertheless, expert opinion recommends lifelong avoidance of any immunization that had been given within 6 weeks before the onset of symptoms of Guillain-Barré syndrome.27
Overstated concern about egg allergy
Anaphylactic reactions can occur after influenza vaccination in people who have severe egg allergy, and concern about these reactions unfortunately prevents many otherwise eligible people with mild allergy from being vaccinated.
These reactions are much less common than feared. In a well-designed prospective cohort study of 367 patients with a history of egg allergy and positive skin-prick tests, including 132 with a history of severe allergy and 4 with a history of mild allergic symptoms arising in response to previous influenza vaccinations, none developed anaphylaxis.28
The same authors reviewed 26 studies in more than 4,000 egg-allergic patients, of whom more than 500 had a history of severe egg-associated reactions, and likewise found no cases of influenza vaccine-associated anaphylaxis. They concluded that the inactivated influenza vaccine is safer than the egg-derived mumps-measles-rubella vaccine, for which precautions for egg allergy no longer exist.28
People with a history of more serious reactions, ranging from stomach upset to anaphylaxis, can be safely vaccinated with a recombinant vaccine or referred to an allergist for further testing. People who experience hives as their only reaction to egg exposure should receive full-dose vaccination but then be observed for a half hour afterward.
The recombinant trivalent influenza vaccine Flublok was approved in 2013 for people age 18 to 49. It is the first commercially available influenza vaccine produced in a continuous insect cell line using a baculovirus vector. No eggs are used in its production, and it is approved for use in patients with egg allergy of any severity.
People who have a history of more serious reactions, including abdominal pain, nausea, vomiting, dizziness, or wheezing can be vaccinated with the recombinant vaccine or referred to an allergy specialist.
Despite this new option, understanding of alternative immunization guidelines for people with egg allergies, available on the CDC website29 remains important, as the availability of the recombinant trivalent influenza vaccine remains limited in the 2013–2014 influenza season.
Misconception about mercury toxicity
Thimerosal is an ethylmercury-containing preservative used in multidose antiviral vaccines, including some influenza vaccines.30 It is designed to prevent bacterial and fungal colonization of the vaccine vial while not reducing vaccine effectiveness or causing toxicity.
Contemporary understanding of mercury neurotoxicity is based largely on studies of methylmercury, including long-term, low-dose exposure in remote communities in the Faroe Islands and the Seychelles through regular consumption of fish and whale meat.31,32 These exposure studies had conflicting results: those in the Faroe Islands demonstrated toxicity, but the Seychelles studies actually showed better neurologic test scores at higher mercury levels, a trend the authors attributed to the beneficial effects of maternal fish consumption.
The results of the methylmercury studies have been extrapolated to ethylmercury (contained in thimerosal), although the two chemicals have vastly different pharmacologic properties. For example, methylmercury has a longer half-life and greater transport across the blood-brain barrier.33 A direct comparison found that ethylmercury is less toxic than methylmercury, although an increase in ethylmercury concentration of only 20% resulted in similar toxicity profiles.34 These studies were performed at concentrations of mercury thousands of times higher than those resulting from vaccination: nearly 150,000 times greater than those in an average adult or 15,000 times greater than those in a 1-year-old child from the typical 25-μg thimerosal dose allowed in contemporary influenza vaccines.
Despite much negative publicity, no link has been shown between thimerosal and autism.30 Multiple regulatory, scientific, and medical organizations including the US Food and Drug Administration (FDA), the WHO, the National Institutes of Health, the CDC, the American Academy of Pediatrics, and the American Congress of Obstetricians and Gynecologists (ACOG) have evaluated the data on the safety of thimerosal in vaccines and have agreed that it is safe. However, most of them urged vaccine manufacturers to eliminate mercury from vaccines as a precaution.30,35 Thimerosal has subsequently been eliminated from all childhood vaccines except for influenza vaccine, with no resulting decrease in childhood autism diagnoses.36
Considering that no harm from thimerosal at FDA-approved doses has been documented, and considering the real risk of influenza-related complications, particularly in young children and pregnant women, we recommend vaccination using whatever vaccine formulation is locally available for all patients, including children age 6 months and older and pregnant women. Nevertheless, given that mercury is being eliminated from childhood vaccines and that preservative-free single-dose vials are increasingly available in the United States, it seems reasonable to use thimerosal-free formulations for children, expectant mothers, and patients concerned about exposure if these formulations are readily available. Influenza vaccination should not be delayed if a thimerosal-free formulation is not readily available.
NEW VACCINE FORMULATIONS
Recent years have seen a dramatic expansion in influenza vaccine options (Table 1).
Quadrivalent vaccines
Quadrivalent vaccines protect against two strains of influenza A and two strains of influenza B, whereas earlier formulations included only one influenza B strain. Vaccination against either influenza B strain offers only limited cross-protection against the other B strain, and previous formulations involved assumptions about which strain would predominate in any given year. The CDC estimates that switching to quadrivalent vaccines will prevent up to 970,000 cases of influenza, 8,200 hospitalizations, and 485 deaths per year.37
Intradermal vaccine
The newly available Fluzone Intradermal vaccine contains smaller doses of hemagglutinin but is still effective because antigen-presenting dendritic cells in the skin reduce the required amount of vaccine antigen necessary for inducing protection.38 This may provide an advantage in the event of vaccine shortage. Also, since it is given in needles only 1.5 mm long, it may appeal to people who are afraid of needles.
The stronger immune reaction with intradermal administration causes more redness, induration, and tenderness at the injection site than with intramuscular administration.39 Patients should not be surprised by this reaction and can be advised to apply ice packs for symptomatic relief.
High-dose vaccine
A high-dose vaccine was approved in 2009 for use in adults age 65 and older. It contains 60 μg of hemagglutinin, compared with 15 μg in standard-dose vaccines, and has been shown to improve seroconversion rates. It remains to be seen if this translates into better clinical outcomes in older adults.40 Further studies will be necessary before we can recommend high-dose vaccines to other people with weakened immune response, such as those undergoing chemotherapy or those infected with human immunodeficiency virus (HIV).
Cell-based vaccines
Flucelvax was the first cell-based influenza vaccine. However, unlike the recombinant trivalent influenza vaccine, which uses no eggs in its manufacturing process, Flucelvax production starts with egg-derived influenza strains that are subsequently propagated in liquid culture of animal cells. It may therefore contain traces of egg protein, and it has not been studied in people with egg allergy.41
An advantage of the cell-based production technique is the use of fewer or no eggs at all, which may result in greater manufacturing efficiency. Also, it is a closed process that reduces the risk of bacterial contamination as well as reliance on antibiotics or preservatives, such as thimerosal, in the manufacturing process.42
CHEMOPROPHYLAXIS WITH NEURAMINIDASE INHIBITORS
The mainstays of influenza prevention are seasonal vaccination and appropriate infection-prevention practices. In addition, in patients at high risk of influenza-related complications (Table 2),43 postexposure chemoprophylaxis with a neuraminidase inhibitor, ie, oseltamivir (Tamiflu) or zanamivir (Relenza), is an effective preventive strategy, especially in years when the match between vaccine and circulating virus strains is suboptimal.44,45
Neuraminidase inhibitors are competitive inhibitors of the active site of the influenza glycoprotein neuraminidase, responsible for viral release from infected respiratory epithelial cells. Rates of resistance to neuraminidase inhibitors have been less than 1% in the United States in recent years, while resistance to the adamantanes amantadine (Symmetrel) and rimantadine (Flumadine) can be as high as 92%, depending on the virus isolate. Thus, their use for treatment or prophylaxis of influenza is not currently recommended by the CDC.46
Chemoprophylaxis with any agent may promote emergence of resistant strains, can cause adverse reactions, and should never be considered a substitute for vaccination.
ANTI-INFLUENZA AGENTS
Two neuraminidase inhibitors, oseltamivir and zanamivir, are approved by the FDA for preventing and treating uncomplicated influenza. Treatment must be instituted within 2 days of onset of symptoms to be effective.
Oseltamivir is available as an oral capsule or powder for liquid suspension. Its most common adverse effects are gastrointestinal upset including diarrhea, nausea, and vomiting.44
Zanamivir is only available in the form of a dry powder inhaler because of the drug’s poor oral bioavailability, and only 4% to 17% of the inhaled dose is systemically absorbed.45 There is a theoretical benefit in targeted delivery of zanamivir to the primary organ affected by influenza, and gastrointestinal side effects are less common with this drug.44,45 Unfortunately, the zanamivir inhaler requires complicated assembly and dexterity for administration (see the video on YouTube47), which may make it unreliable in certain patient groups, especially handicapped and elderly patients. Administration has been associated with bronchospasm, resulting in a more than 20% reduction in the forced expiratory volume in 1 second, and it is contraindicated in patients with underlying reactive airway disease such as chronic obstructive pulmonary disease or asthma.45
Table 3 lists the doses and duration of therapy for oseltamivir and zanamivir in adults with normal renal function, as well as approximate costs. No generic formulations of neuraminidase inhibitors are currently available, and outpatient use may not be covered by medical insurance. Several other neuraminidase inhibitors are either under development or at various stages in the FDA approval process.
EFFECTIVENESS OF ANTI-INFLUENZA DRUGS
Treatment with oseltamivir has been shown to reduce the duration of symptoms by approximately 1 day if initiated within 36 hours of onset of illness and 1.5 to 2 days if initiated within 24 hours.48,49 Trials and meta-analyses of zanamivir show similar effectiveness, though some suggest that symptoms were alleviated as much as 3 days sooner than in controls in a subgroup of patients who were febrile at presentation.50,51 Dual neuraminidase inhibitor therapy in an attempt to prevent emergence of resistance seems logical but was actually found to be less effective than monotherapy, according to a 2010 study.52
The effectiveness of neuraminidase inhibitors in reducing influenza-related complications and mortality rates has been controversial in recent years, as these outcomes were not addressed in initial studies that secured FDA approval. Several meta-analyses differ in their assessments of available data quality and conclusions. A 2009 Cochrane review questioned the completeness and the veracity of the data from manufacturer-funded trial data, much of which was unpublished and not made available to reviewers, and it concluded that a reduction of complications could not be supported by the available data.53 Hernán and Lipsitch,54 in a 2011 review, calculated that oseltamivir reduces the risk of lower respiratory tract complications by 28% in patients with influenza-like symptoms and by 37% in patients with confirmed influenza infection.
Additional trials and better access to available data are needed to settle the question of the effectiveness of neuraminidase inhibitors in reducing complications of influenza. Meanwhile, they remain strongly recommended by major health organizations, including the CDC and the WHO, which lists oseltamivir on its “model list of essential medicines.”
VIRAL RESISTANCE TO NEURAMINIDASE INHIBITORS
Viral resistance to neuraminidase inhibitors occurs through multiple mechanisms and may arise without selective pressure from exposure to these drugs.55
Oseltamivir possesses a hydrophobic moiety that requires viral neuraminidase to undergo a complex reconfiguration to expose the active site prior to binding. Any mutation affecting its ability to undergo this structural rearrangement can promote resistance by decreased oseltamivir access to the active site.
Zanamivir has a structural homology to the neuraminidase active site and requires no such reconfiguration. Additionally, mutations promoting resistance to zanamivir may actually decrease viral fitness; thus, resistance to zanamivir is significantly less common than to oseltamivir.55
About 2,000 influenza virus isolates currently circulating in the United States were tested for resistance; only 1% of the 2009 influenza A H1N1 isolates demonstrated resistance to oseltamivir, and none to zanamivir.56
The CDC regularly updates the resistance patterns of circulating influenza strains at www.cdc.gov/flu/weekly/index.htm.
SPECIAL CONSIDERATIONS
Pregnancy
Pregnant women may be at higher risk of severe influenza complications. This was especially true during the 2009 H1N1 pandemic, when pregnant women had a five times higher risk of death from influenza-related complications. Additionally, fever during pregnancy is an independent risk factor for adverse outcomes in the offspring.57 Maternal vaccination against influenza effectively protects the infant for the first 6 months of life, when vaccination is not recommended because of a poor immune response.58
Live-attenuated influenza vaccine is contraindicated during pregnancy. Given the documented risks to the mother from influenza and no documented harm from preservatives in multiuse vaccine vials, the Advisory Committee on Immunization Practices (ACIP) and ACOG do not state a preference for thimerosal-containing or thimerosal-free vaccine for any group, including pregnant women. Pregnant women should be vaccinated with whatever inactivated influenza vaccine formulation is available at the earliest opportunity in the beginning of the influenza season, regardless of the trimester of pregnancy.
Pregnant women are at high risk of influenza-related complications and should be considered for postexposure antiviral prophylaxis or early treatment with a neuraminidase inhibitor. However, both of the approved neuraminidase inhibitors are in pregnancy safety category C, indicating possible adverse effects in animal studies and a lack of safety data in pregnant humans. As with all category C medications, the risks and benefits must be considered, taking into account maternal comorbidities, vaccination status, effectiveness of the season’s influenza vaccine, and the virulence of circulating influenza strains.
As oseltamivir is associated with nausea and gastrointestinal side effects and as zanamivir has less systemic absorption, it may be reasonable to prescribe zanamivir for women already experiencing severe pregnancy-related nausea.
Immunocompromised people
Inactivated influenza vaccine is recommended and live-attenuated influenza vaccine is contraindicated for all immunocompromised people. Generally speaking, any form of immune compromise will decrease the immunogenicity of the vaccine. Additional considerations vary depending on the cause and severity of the immunocompromised status.
HIV-infected patients have higher seroconversion rates when vaccinated with the high-dose vaccine than with the standard-dose vaccine; however, as in adults over age 65, the clinical benefit has yet to be evaluated.59 The efficacy of vaccination is predictably related to the CD4 cell count, as T cells are necessary to mount a response.60 No documented benefit is gained from booster influenza vaccination in this group of patients.
Cancer patients should receive inactivated influenza vaccine every year. Postexposure chemoprophylaxis should be considered, and early treatment with a neuraminidase inhibitor is recommended in patients undergoing chemotherapy.
Solid-organ transplant recipients face a risk of organ rejection if they contract influenza infection, in addition to a higher risk of influenza-related complications.61 Transplant recipients should receive inactivated influenza vaccine as soon as it becomes available at the beginning of every influenza season. Additional research is necessary to evaluate the safety and effectiveness of the high-dose influenza vaccine in this patient group.
MORE OPTIONS, GREAT BENEFIT
Influenza remains a significant source of morbidity and mortality in the United States, and emerging pandemic strains as well as the aging population pose the risk of increased disease burden. New vaccine options offer hope of greater safety, improved efficacy, and higher vaccination rates though broader appeal to individuals. The actual differences in protection between various vaccine options are insignificant relative to the overall benefit of vaccination.
Health care providers should inquire about patients’ understanding and address their concerns about vaccination. Giving an available influenza vaccine within approved indications should not be delayed if alternative vaccine options are not readily available.
In addition to vaccination, patients at high risk of complications should be advised early in the influenza season to inform their doctors about potential exposure to influenza or the development of flu-like symptoms for consideration of early treatment or postexposure prophylaxis with a neuraminidase inhibitor.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–5096.
- Soni A. Influenza Immunization Rates for Selected at Risk Populations among the US Adult Civilian Noninstitutionalized Population, 2006. Statistical Brief #226. December 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://meps.ahrq.gov/data_files/publications/st226/stat226.pdf. Accessed January 31, 2014.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2012–13 Influenza Season. http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm - age-group-adults. Accessed January 31, 2014.
- Castilla J, Godoy P, Domínguez A, et al; CIBERESP Cases and Controls in Influenza Working Group Spain. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013; 57:167–175.
- Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011–2012 influenza season. Clin Infect Dis 2013; 56:1774–1777.
- Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013; 310:1711–1720.
- Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by influenza vaccination—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:997–1000.
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013; 62:1–43.
- Friede M. Snapshot of influenza vaccine manufacturing capacity worldwide and summary of WHO-HHS activities to promote technology transfer. World Health Organization Global Action Plan for Influenza II Meeting 2011. www.who.int/phi/Session1B_Current_Manufacturing_Capacity_Worldwide_Friede.pdf. Accessed February 5, 2014.
- Ashkenazi S, Vertruyen A, Arístegui J, et al., CAIV-T Study Group. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879.
- Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA 2005; 294:2720–2725.
- Vesikari T, Karvonen A, Korhonen T, et al; CAIV-T Transmission Study Group. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 2006; 25:590–595.
- Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control Hosp Epidemiol 2007; 28:702–707.
- Centers for Disease Control and Prevention (CDC). Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). http://www.cdc.gov/flu/about/qa/nasalspray.htm. Accessed February 3, 2014.
- Centers for Disease Control and Prevention (CDC). Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep 2013; 62:119–123.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med 2012; 55:72–77.
- Aziz NA, Muhamad S, Manaf MR, Hamid MZ. Factors Influencing H1N1 vaccination among primary health care workers: a cross-sectional study. Int J Prev Med 2013; 4:664–670.
- Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med 1996; 156:1546–1550.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60( 2):1–64.
- Tseng HF, Smith N, Sy LS, Jacobsen LJ. Evaluation of the incidence of herpes zoster after concomitant administration of zoster vaccine and polysaccharide pneumococcal vaccine. Vaccine 2011; 29:3628–3632.
- Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 2002; 109:124–129.
- Rajabally YA, Uncini A. Outcome and its predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 2012; 83:711–718.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976—1977. Am J Epidemiol 1979; 110:105–123.
- Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis 2010; 10:643–651.
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP, Network C. Recurrent Guillain-Barré syndrome following vaccination. Clin Infect Dis 2012; 54:800–804.
- Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol 2005; 62:1194–1198.
- Des Roches A, Paradis L, Gagnon R, et al. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol 2012; 130:1213–1216.e1.
- US Centers for Disease Control and Prevention. Influenza vaccination of people with a history of egg allergy. www.immunize.org/catg.d/p3094.pdf. Accessed February 3, 2014.
- US Food Drug Administration. Thimerosal in vaccines. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed February 3, 2014.
- Davidson PW, Kost J, Myers GJ, Cox C, Clarkson TW, Shamlaye CF. Methylmercury and neurodevelopment: reanalysis of the Seychelles Child Development Study outcomes at 66 months of age. JAMA 2001; 285:1291–1293.
- Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997; 19:417–428.
- Nelson KB, Bauman ML. Thimerosal and autism? Pediatrics 2003; 111:674–679.
- Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR. The comparative toxicology of ethyl- and methylmercury. Arch Toxicol 1985; 57:260–267.
- American Congress of Obstetricians and Gynecologists. Influenza vaccination during pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Influenza_Vaccination_During_Pregnancy. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. Understanding thimerosal, mercury, and vaccine safety. www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-thimerosal-color-office.pdf. Accessed February 3, 2014.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine 2013. doi: 10.1016/j.vaccine.2013.09.074. [Epub ahead of print]
- Sanofi Pasteur. Fluzone package insert. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305080.pdf. Accessed February 3, 2014.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food Drug Administration. Flucelvax FDA application. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM332069.pdf. Accessed February 3, 2014.
- Novartis. Flucelvax (influenza virus vaccine) fact sheet. www.novartis-vaccines.com/downloads/flucelvax/Flucelvax_Fact_Sheet.pdf. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. People at high risk for developing flu-related complications. www.cdc.gov/flu/about/disease/high_risk.htm. Accessed February 3, 2014.
- Roche Pharmaceuticals. Tamiflu package insert. http://www.gene.com/download/pdf/tamiflu_prescribing.pdf. Accessed February 3, 2014.
- GlaxoSmithKline. Relenza package insert. http://us.gsk.com/products/assets/us_relenza.pdf. Accessed February 3, 2014.
- Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60:1–24.
- Administration technique for zanamivir (Relenza) Diskhaler. YouTube. 2009. www.youtube.com/watch?v=sQI0a0ToSPo. Accessed February 6, 2014.
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355:1845–1850.
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283:1016–1624.
- Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326:1235.
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 1997; 337:874–880.
- Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med 2010; 7:e1000362.
- Jefferson T, Jones M, Doshi P, Del Mar C. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 2009; 339:b5106.
- Hernán MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011; 53:277–279.
- Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 2013; 98:174–185.
- US Centers for Disease Control and Prevention. FluView. www.cdc.gov/flu/weekly. Accessed February 3, 2014.
- Acs N, Bánhidy F, Puhó E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 2005; 73:989–996.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med 2013; 158:19–26.
- Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994; 8:469–476.
- Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2:287–291.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–5096.
- Soni A. Influenza Immunization Rates for Selected at Risk Populations among the US Adult Civilian Noninstitutionalized Population, 2006. Statistical Brief #226. December 2008. Agency for Healthcare Research and Quality, Rockville, MD. http://meps.ahrq.gov/data_files/publications/st226/stat226.pdf. Accessed January 31, 2014.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2012–13 Influenza Season. http://www.cdc.gov/flu/fluvaxview/coverage-1213estimates.htm - age-group-adults. Accessed January 31, 2014.
- Castilla J, Godoy P, Domínguez A, et al; CIBERESP Cases and Controls in Influenza Working Group Spain. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013; 57:167–175.
- Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011–2012 influenza season. Clin Infect Dis 2013; 56:1774–1777.
- Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013; 310:1711–1720.
- Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by influenza vaccination—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep 2013; 62:997–1000.
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013; 62:1–43.
- Friede M. Snapshot of influenza vaccine manufacturing capacity worldwide and summary of WHO-HHS activities to promote technology transfer. World Health Organization Global Action Plan for Influenza II Meeting 2011. www.who.int/phi/Session1B_Current_Manufacturing_Capacity_Worldwide_Friede.pdf. Accessed February 5, 2014.
- Ashkenazi S, Vertruyen A, Arístegui J, et al., CAIV-T Study Group. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879.
- Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA 2005; 294:2720–2725.
- Vesikari T, Karvonen A, Korhonen T, et al; CAIV-T Transmission Study Group. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 2006; 25:590–595.
- Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control Hosp Epidemiol 2007; 28:702–707.
- Centers for Disease Control and Prevention (CDC). Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). http://www.cdc.gov/flu/about/qa/nasalspray.htm. Accessed February 3, 2014.
- Centers for Disease Control and Prevention (CDC). Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb Mortal Wkly Rep 2013; 62:119–123.
- Voordouw AC, Sturkenboom MC, Dieleman JP, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004; 292:2089–2095.
- Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med 2012; 55:72–77.
- Aziz NA, Muhamad S, Manaf MR, Hamid MZ. Factors Influencing H1N1 vaccination among primary health care workers: a cross-sectional study. Int J Prev Med 2013; 4:664–670.
- Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med 1996; 156:1546–1550.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60( 2):1–64.
- Tseng HF, Smith N, Sy LS, Jacobsen LJ. Evaluation of the incidence of herpes zoster after concomitant administration of zoster vaccine and polysaccharide pneumococcal vaccine. Vaccine 2011; 29:3628–3632.
- Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 2002; 109:124–129.
- Rajabally YA, Uncini A. Outcome and its predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 2012; 83:711–718.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976—1977. Am J Epidemiol 1979; 110:105–123.
- Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis 2010; 10:643–651.
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP, Network C. Recurrent Guillain-Barré syndrome following vaccination. Clin Infect Dis 2012; 54:800–804.
- Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol 2005; 62:1194–1198.
- Des Roches A, Paradis L, Gagnon R, et al. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol 2012; 130:1213–1216.e1.
- US Centers for Disease Control and Prevention. Influenza vaccination of people with a history of egg allergy. www.immunize.org/catg.d/p3094.pdf. Accessed February 3, 2014.
- US Food Drug Administration. Thimerosal in vaccines. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed February 3, 2014.
- Davidson PW, Kost J, Myers GJ, Cox C, Clarkson TW, Shamlaye CF. Methylmercury and neurodevelopment: reanalysis of the Seychelles Child Development Study outcomes at 66 months of age. JAMA 2001; 285:1291–1293.
- Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997; 19:417–428.
- Nelson KB, Bauman ML. Thimerosal and autism? Pediatrics 2003; 111:674–679.
- Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR. The comparative toxicology of ethyl- and methylmercury. Arch Toxicol 1985; 57:260–267.
- American Congress of Obstetricians and Gynecologists. Influenza vaccination during pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Influenza_Vaccination_During_Pregnancy. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. Understanding thimerosal, mercury, and vaccine safety. www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-thimerosal-color-office.pdf. Accessed February 3, 2014.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Tsang P, Gorse GJ, Strout CB, et al. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine 2013. doi: 10.1016/j.vaccine.2013.09.074. [Epub ahead of print]
- Sanofi Pasteur. Fluzone package insert. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM305080.pdf. Accessed February 3, 2014.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food Drug Administration. Flucelvax FDA application. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM332069.pdf. Accessed February 3, 2014.
- Novartis. Flucelvax (influenza virus vaccine) fact sheet. www.novartis-vaccines.com/downloads/flucelvax/Flucelvax_Fact_Sheet.pdf. Accessed February 3, 2014.
- US Centers for Disease Control and Prevention. People at high risk for developing flu-related complications. www.cdc.gov/flu/about/disease/high_risk.htm. Accessed February 3, 2014.
- Roche Pharmaceuticals. Tamiflu package insert. http://www.gene.com/download/pdf/tamiflu_prescribing.pdf. Accessed February 3, 2014.
- GlaxoSmithKline. Relenza package insert. http://us.gsk.com/products/assets/us_relenza.pdf. Accessed February 3, 2014.
- Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60:1–24.
- Administration technique for zanamivir (Relenza) Diskhaler. YouTube. 2009. www.youtube.com/watch?v=sQI0a0ToSPo. Accessed February 6, 2014.
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355:1845–1850.
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283:1016–1624.
- Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326:1235.
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 1997; 337:874–880.
- Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med 2010; 7:e1000362.
- Jefferson T, Jones M, Doshi P, Del Mar C. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 2009; 339:b5106.
- Hernán MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011; 53:277–279.
- Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 2013; 98:174–185.
- US Centers for Disease Control and Prevention. FluView. www.cdc.gov/flu/weekly. Accessed February 3, 2014.
- Acs N, Bánhidy F, Puhó E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 2005; 73:989–996.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564.
- McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med 2013; 158:19–26.
- Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994; 8:469–476.
- Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2:287–291.
KEY POINTS
- Influenza vaccination is effective at preventing influenza-associated disease.
- Influenza vaccine is safe in people with a history of mild egg allergy.
- Many new vaccine formulations exist and may offer benefits to different patient groups.
- Neuraminidase inhibitors are recommended for treatment and postexposure prophylaxis in patients at high risk of influenza-related complications; however, they are not a substitute for vaccination.
Hand Pain Following an Altercation
ANSWER
The radiograph shows moderate soft-tissue swelling with dislocation of the proximal interphalangeal joint. No definite fracture is seen. In addition, there are some metallic-appearing foreign bodies.
The patient was treated with closed reduction and splinting. He also received a referral to outpatient orthopedics for follow-up.
ANSWER
The radiograph shows moderate soft-tissue swelling with dislocation of the proximal interphalangeal joint. No definite fracture is seen. In addition, there are some metallic-appearing foreign bodies.
The patient was treated with closed reduction and splinting. He also received a referral to outpatient orthopedics for follow-up.
ANSWER
The radiograph shows moderate soft-tissue swelling with dislocation of the proximal interphalangeal joint. No definite fracture is seen. In addition, there are some metallic-appearing foreign bodies.
The patient was treated with closed reduction and splinting. He also received a referral to outpatient orthopedics for follow-up.
A 60-year-old man presents with a complaint of pain in his right fifth finger following an altercation. He is not sure exactly how the injury occurred, but he does recall that at one point his hand was twisted awkwardly. He denies any significant medical history. His vital signs are normal. Primary survey appears normal as well. On examination, you notice moderate swelling around the fifth finger of his right hand, which does appear to be slightly deformed. There are no obvious wounds or lacerations. He has moderate tenderness at the base of his finger. Range of motion is limited due to the swelling. Good capillary refill time is noted. The triage nurse already sent the patient for a radiograph of his finger (shown). What is your impression?
For Lethargic Patient, Trouble Is Brewing
ANSWER
The correct interpretation is an atrial tachycardia with 2:1 ventricular conduction. The ventricular rate is 87 beats/min (690 ms), and the atrial rate is 174 beats/min (345 ms). Two P waves are present for each QRS, which excludes a first-degree atrioventricular block. The less obvious P wave is found in the terminal portion of the QRS complex. (You may convince yourself of this by using calipers to measure the R-R interval, dividing that measurement in half, and then applying it to the ECG. You will see the P waves march through without changing the ventricular response.)
A nonspecific intraventricular conduction delay is also present. The QRS duration is > 100 ms; however, the criteria for right or left bundle branch block are absent.
A thorough investigation revealed that the clerk formulating the herbs for the tea was using, among other things, dried foxglove. Foxglove has been used as a remedy for lethargy in the elderly, presumably because it inadvertently treats symptoms of congestive heart failure. It was the tea consumption that accounted for the presence of digoxin in the patient’s blood. (Recall that there is a substantial overlap between therapeutic and toxic serum concentrations of digoxin.) When the patient stopped consuming the tea, his atrial tachycardia resolved, as did his symptoms.
ANSWER
The correct interpretation is an atrial tachycardia with 2:1 ventricular conduction. The ventricular rate is 87 beats/min (690 ms), and the atrial rate is 174 beats/min (345 ms). Two P waves are present for each QRS, which excludes a first-degree atrioventricular block. The less obvious P wave is found in the terminal portion of the QRS complex. (You may convince yourself of this by using calipers to measure the R-R interval, dividing that measurement in half, and then applying it to the ECG. You will see the P waves march through without changing the ventricular response.)
A nonspecific intraventricular conduction delay is also present. The QRS duration is > 100 ms; however, the criteria for right or left bundle branch block are absent.
A thorough investigation revealed that the clerk formulating the herbs for the tea was using, among other things, dried foxglove. Foxglove has been used as a remedy for lethargy in the elderly, presumably because it inadvertently treats symptoms of congestive heart failure. It was the tea consumption that accounted for the presence of digoxin in the patient’s blood. (Recall that there is a substantial overlap between therapeutic and toxic serum concentrations of digoxin.) When the patient stopped consuming the tea, his atrial tachycardia resolved, as did his symptoms.
ANSWER
The correct interpretation is an atrial tachycardia with 2:1 ventricular conduction. The ventricular rate is 87 beats/min (690 ms), and the atrial rate is 174 beats/min (345 ms). Two P waves are present for each QRS, which excludes a first-degree atrioventricular block. The less obvious P wave is found in the terminal portion of the QRS complex. (You may convince yourself of this by using calipers to measure the R-R interval, dividing that measurement in half, and then applying it to the ECG. You will see the P waves march through without changing the ventricular response.)
A nonspecific intraventricular conduction delay is also present. The QRS duration is > 100 ms; however, the criteria for right or left bundle branch block are absent.
A thorough investigation revealed that the clerk formulating the herbs for the tea was using, among other things, dried foxglove. Foxglove has been used as a remedy for lethargy in the elderly, presumably because it inadvertently treats symptoms of congestive heart failure. It was the tea consumption that accounted for the presence of digoxin in the patient’s blood. (Recall that there is a substantial overlap between therapeutic and toxic serum concentrations of digoxin.) When the patient stopped consuming the tea, his atrial tachycardia resolved, as did his symptoms.
A 72-year-old man presents with a primary complaint of lethargy. He emigrated from Southeast Asia to the United States about a year ago and neither speaks nor understands English. His grandson, who is fluent, accompanies him to his appointment. Through his grandson, the patient explains that he has become increasingly tired in the past four months—to the extent that exercise and activities of daily living have become difficult. The patient’s libido also has been affected. In an effort to correct this, he visited a local Asian goods store, where he was given a mixture of herbs from which to brew tea to treat his symptoms. For three weeks, he consumed the tea twice daily. Initially, his energy, stamina, and libido improved. However, his symptoms eventually returned, so he doubled his tea consumption with the idea that this would improve his condition. Unfortunately, in addition to his lethargy, he is now experiencing palpitations, a fluttering sensation in his chest, and occasional dizziness. He denies chest pain, shortness of breath, nocturnal dyspnea, syncope, or near syncope. Medical history is difficult to elicit. He denies prior history of hypertension, myocardial infarction, congestive heart failure, or diabetes. Neither he nor his grandson understands the concept of arrhythmias (eg, atrial fibrillation). He was treated for tuberculosis as a child and has had no recurrence. He has had no surgeries. The patient takes no prescribed medications. He does, however, use herbal products including ginseng, horny goat weed, and fenugreek (in addition to his herbal tea). He has no known drug allergies. Social history reveals that the patient lives with his son’s family, having moved to the US from Thailand after his wife died of old age. He worked as a farmer his entire life. He drinks one ounce of whiskey daily and smokes 1 to 1.5 packs of cigarettes a day. The review of systems is noncontributory. His grandson is reluctant to ask the patient many questions regarding his health, once he notices his grandfather’s agitation at answering questions. The physical exam reveals a thin, elderly male with weathered skin who is in no acute distress. Vital signs include a blood pressure of 118/62 mm Hg; pulse, 80 beats/min and regular; respiratory rate, 16 breaths/min; and temperature, 97.8°F. His height is 62 in and his weight, 117 lb. The HEENT exam is remarkable for arcus senilis and multiple missing teeth. There is no jugular distention, and the thyroid is not enlarged. The lungs reveal coarse breath sounds that clear with coughing in all lung fields. (The patient has an occasional harsh cough.) The cardiac exam is positive for a grade II/VI systolic murmur best heard at the left upper sternal border, which radiates to the carotid arteries. The rhythm is regular at a rate of 80 beats/min, and there are no clicks or rubs. The abdomen is scaphoid, soft, and nontender, with no palpable masses. The peripheral pulses are strong and equal bilaterally. Extremities demonstrate full range of motion, and the neurologic exam is grossly intact. Routine laboratory tests including a complete blood count and electrolyte panel are obtained. Because you are unsure of his medication regimen, you order a toxicology screen. You are surprised to see a serum digoxin level of 0.7 ng/mL. Finally, given the patient’s symptoms of palpitations and dizziness, you order an ECG. It shows the following: a ventricular rate of 87 beats/min; PR interval, 218 ms; QRS duration, 130 ms; QT/QTc interval, 416/500 ms; P axis, 24°; R axis, 49°; and T axis, 45°. What is your interpretation of this ECG?
These Old Lesions? She’s Had Them for Years …
ANSWER
The correct answer is disseminated superficial actinic porokeratosis (DSAP; choice “a”). This condition, caused by an inherited defect of the SART3 gene, is seen mostly on the sun-exposed skin of middle-aged women.
Stasis dermatitis (choice “b”) can cause a number of skin changes, but not the discrete annular lesions seen with DSAP.
Seborrheic keratoses (choice “c”) are common on the legs. However, they don’t display this same morphology.
Nummular eczema (choice “d”) presents with annular papulosquamous lesions (as opposed to the fixed lesions seen with DSAP), often on the legs and lower trunk, but without the thready circumferential scaly border.
Continue reading for Joe Monroe's discussion...
DISCUSSION
Leg skin is prey to an astonishing array of problems; many have to do with increased hydrostatic pressure (eg, venous stasis disease), with the almost complete lack of sebaceous glands (eg, nummular eczema), or with the simple fact of being “in harm’s way.” And there is no law that says a given patient can’t have more than one problem at a time, co-existing and serving to confuse the examiner. Such is the case with this patient.
Her concern about possible blood clots is misplaced but understandable. Deep vein thromboses would not present in multiples, would not be on the surface or scaly, and would almost certainly be painful.
The fixed nature of this patient’s scaly lesions is extremely significant—but only if you know about DSAP, which typically manifests in the third decade of life and slowly worsens. The lesions’ highly palpable and unique scaly border makes them hard to leave alone. This might not be a problem except for the warfarin, which makes otherwise minor trauma visible as purpuric macules. Chronic sun damage tends to accentuate them as well. The positive family history is nicely corroborative and quite common.
The brown macules on the patient’s legs are solar lentigines (sun-caused freckles), which many patients (and even younger providers) erroneously call “age spots.” When these individuals become “aged,” they’ll understand that there is no such thing as an age spot.
This patient could easily have had nummular eczema, but not for 30 years! Those lesions, treated or not, will come and go. But not DSAP, about which many questions remain: If they’re caused by sun exposure, why don’t we see them more often on the face and arms? And why don’t we see them on the sun-damaged skin of older men?
If needed, a biopsy could have been performed. It would have been confirmatory of the diagnosis and effectively would have ruled out the other items in the differential, including wart, squamous cell carcinoma, and actinic or seborrheic keratosis.
ANSWER
The correct answer is disseminated superficial actinic porokeratosis (DSAP; choice “a”). This condition, caused by an inherited defect of the SART3 gene, is seen mostly on the sun-exposed skin of middle-aged women.
Stasis dermatitis (choice “b”) can cause a number of skin changes, but not the discrete annular lesions seen with DSAP.
Seborrheic keratoses (choice “c”) are common on the legs. However, they don’t display this same morphology.
Nummular eczema (choice “d”) presents with annular papulosquamous lesions (as opposed to the fixed lesions seen with DSAP), often on the legs and lower trunk, but without the thready circumferential scaly border.
Continue reading for Joe Monroe's discussion...
DISCUSSION
Leg skin is prey to an astonishing array of problems; many have to do with increased hydrostatic pressure (eg, venous stasis disease), with the almost complete lack of sebaceous glands (eg, nummular eczema), or with the simple fact of being “in harm’s way.” And there is no law that says a given patient can’t have more than one problem at a time, co-existing and serving to confuse the examiner. Such is the case with this patient.
Her concern about possible blood clots is misplaced but understandable. Deep vein thromboses would not present in multiples, would not be on the surface or scaly, and would almost certainly be painful.
The fixed nature of this patient’s scaly lesions is extremely significant—but only if you know about DSAP, which typically manifests in the third decade of life and slowly worsens. The lesions’ highly palpable and unique scaly border makes them hard to leave alone. This might not be a problem except for the warfarin, which makes otherwise minor trauma visible as purpuric macules. Chronic sun damage tends to accentuate them as well. The positive family history is nicely corroborative and quite common.
The brown macules on the patient’s legs are solar lentigines (sun-caused freckles), which many patients (and even younger providers) erroneously call “age spots.” When these individuals become “aged,” they’ll understand that there is no such thing as an age spot.
This patient could easily have had nummular eczema, but not for 30 years! Those lesions, treated or not, will come and go. But not DSAP, about which many questions remain: If they’re caused by sun exposure, why don’t we see them more often on the face and arms? And why don’t we see them on the sun-damaged skin of older men?
If needed, a biopsy could have been performed. It would have been confirmatory of the diagnosis and effectively would have ruled out the other items in the differential, including wart, squamous cell carcinoma, and actinic or seborrheic keratosis.
ANSWER
The correct answer is disseminated superficial actinic porokeratosis (DSAP; choice “a”). This condition, caused by an inherited defect of the SART3 gene, is seen mostly on the sun-exposed skin of middle-aged women.
Stasis dermatitis (choice “b”) can cause a number of skin changes, but not the discrete annular lesions seen with DSAP.
Seborrheic keratoses (choice “c”) are common on the legs. However, they don’t display this same morphology.
Nummular eczema (choice “d”) presents with annular papulosquamous lesions (as opposed to the fixed lesions seen with DSAP), often on the legs and lower trunk, but without the thready circumferential scaly border.
Continue reading for Joe Monroe's discussion...
DISCUSSION
Leg skin is prey to an astonishing array of problems; many have to do with increased hydrostatic pressure (eg, venous stasis disease), with the almost complete lack of sebaceous glands (eg, nummular eczema), or with the simple fact of being “in harm’s way.” And there is no law that says a given patient can’t have more than one problem at a time, co-existing and serving to confuse the examiner. Such is the case with this patient.
Her concern about possible blood clots is misplaced but understandable. Deep vein thromboses would not present in multiples, would not be on the surface or scaly, and would almost certainly be painful.
The fixed nature of this patient’s scaly lesions is extremely significant—but only if you know about DSAP, which typically manifests in the third decade of life and slowly worsens. The lesions’ highly palpable and unique scaly border makes them hard to leave alone. This might not be a problem except for the warfarin, which makes otherwise minor trauma visible as purpuric macules. Chronic sun damage tends to accentuate them as well. The positive family history is nicely corroborative and quite common.
The brown macules on the patient’s legs are solar lentigines (sun-caused freckles), which many patients (and even younger providers) erroneously call “age spots.” When these individuals become “aged,” they’ll understand that there is no such thing as an age spot.
This patient could easily have had nummular eczema, but not for 30 years! Those lesions, treated or not, will come and go. But not DSAP, about which many questions remain: If they’re caused by sun exposure, why don’t we see them more often on the face and arms? And why don’t we see them on the sun-damaged skin of older men?
If needed, a biopsy could have been performed. It would have been confirmatory of the diagnosis and effectively would have ruled out the other items in the differential, including wart, squamous cell carcinoma, and actinic or seborrheic keratosis.
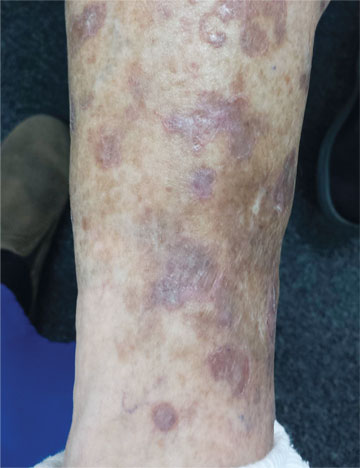
A 65-year-old woman is referred to dermatology with discoloration of her legs that started several weeks ago. Her family suggested it might be “blood clots,” although she has been taking warfarin since she was diagnosed with atrial fibrillation several months ago. Her dermatologic condition is basically asymptomatic, but the patient admits to scratching her legs, saying it’s “hard to leave them alone.” On further questioning, she reveals that she has had “rough places” on her legs for at least 20 years and volunteers that her sister had the same problem, which was diagnosed years ago as “fungal infection.” Both she and her sister spent a great deal of time in the sun as children, long before sunscreen was invented. The patient is otherwise fairly healthy. She takes medication for her lipids, as well as daily vitamins. Her atrial fibrillation is under control and requires no medications other than the warfarin. A great deal of focal discoloration is seen on both legs, circumferentially distributed from well below the knees to just above the ankles. Many of the lesions are brown macules, but more are purplish-red, annular, and scaly. On closer examination, these lesions—the ones the patient says she has had for decades—have a very fine, thready, scaly border that palpation reveals to be tough and adherent. They average about 2 cm in diameter. There are no such lesions noted elsewhere on the patient’s skin. There is, however, abundant evidence of excessive sun exposure, characterized by a multitude of solar lentigines, many fine wrinkles, and extremely thin arm skin.
7 questions to ask when evaluating a noninferiority trial
The traditional clinical trial, designed to test whether a new treatment is better than a placebo or another active treatment, is known as a “superiority” trial—although rarely labeled as such. In contrast, the goal of a noninferiority trial is simply to demonstrate that a new treatment is not substantially less effective than the standard therapy.
Such trials are useful when a new therapy is thought to be safer, easier to administer, or less costly than the existing treatment, but not necessarily more effective. And, because it would be unethical to randomize patients with a serious condition for which there already is an effective treatment to placebo, a noninferiority trial is another means of determining if the new treatment is effective.
Noninferiority trials have unique design features and methodology and require a different analysis than traditional superiority trials. Yet many physicians know far less about them; many investigators appear to be less than proficient, as well. A review of 116 noninferiority trials and 46 equivalence trials found that only 20% fulfilled generally accepted quality criteria.1 To improve the quality of noninferiority trials, the CONSORT (Consolidated Standards of Reporting Trials) Group has published a checklist for trial design and reporting standards.2,3 Based on this checklist, we came up with 7 key questions to consider when evaluating a noninferiority trial. In the pages that follow, you’ll also find an at-a-glance guide (TABLE) and a methodology review using a hypothetical case (page E7).
1. Is a noninferiority trial appropriate?
The introduction to a noninferiority trial should provide the rationale for this design and the absence of a placebo control group. Look for a review of the evidence of the efficacy of the reference treatment that placebo-controlled trials have revealed, along with the effect size. The advantages of the new treatment over the standard treatment—eg, fewer adverse effects, easier administration, or lower cost—should be discussed, as well.
In the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY)—a prominent noninferiority trial—investigators compared the standard anticoagulant (warfarin) for patients with atrial fibrillation (AF) at risk of stroke with a new agent, dabigatran.4 In the methods section of the abstract and the statistical analysis section of the main body, the authors clearly indicated that this was a noninferiority trial. They began by referring to the existing evidence of warfarin’s effectiveness, then detailed the qualities that make warfarin cumbersome to use, including the need for frequent laboratory monitoring. This was followed by evidence that many patients stop taking warfarin and that even for those who persist with treatment, adequate anticoagulation is difficult to maintain.
The authors went on to state that because dabigatran requires no long-term monitoring, it is easier to use. Therefore, if dabigatran could be shown to be no worse than warfarin in preventing strokes, it would be a reasonable alternative, leaving no doubt that this was an appropriate noninferiority trial.
2. Is the noninferiority margin based on clinical judgment and statistical reasoning?
The noninferiority margin should be based on clinical judgment as to how effective a new treatment must be in order to be declared not clinically inferior to the standard treatment. This can be based on several factors, including the severity of the outcome and the expected advantages of the new treatment. The margin should also take into account the size of the standard treatment’s effect vs placebo. In RELY, for example, the authors noted that the noninferiority margin was based on the desire to preserve at least 50% of the lower limit of the confidence interval (CI) of warfarin’s estimated effect; this was done using data from a previously published meta-analysis of 6 trials comparing warfarin with placebo for stroke prevention in patients with AF.4-6
3. Are the hypothesis and statistical analysis formulated correctly?
The clinical hypothesis in a noninferiority trial is that the new treatment is not worse than the standard treatment by a prespecified margin; therefore, the statistical null hypothesis to be tested is that the new treatment is worse than the reference treatment by more than that margin. Rejecting a true null hypothesis (for example, because the P value is <.05) is known as a type l error. In this setting, making a type I error would mean accepting a new treatment that is truly worse than the standard by at least the specified margin. Failure to reject a false null hypothesis is known as a type II error, which in this case would mean failing to identify a new treatment that is truly noninferior to the standard.7
In RE-LY, the authors stated that the upper limit of the one-sided 97.5% CI for the relative risk of a stroke with dabigatran vs warfarin had to fall below 1.46.4 (This is the same as testing the null hypothesis that the hazard ratio is ≥1.46.) Thus, the hypothesis was formulated correctly.
4. Is the sample size appropriate and justified?
The sample size in a noninferiority trial should provide high power to reject the null hypothesis that the difference (or relative risk) between groups is equal to or greater than the noninferiority margin under some clinically meaningful assumption about the true difference (or absolute risk reduction) between groups. A true difference of 0 (or a relative risk of 1) is typically assumed for sample size calculation. However, assuming that the new treatment is truly slightly better or slightly worse than the standard may be clinically appropriate in some cases. This would indicate a need for a smaller or larger sample size, respectively, than that required under the usual assumption of no difference.
When the justification for the sample size in a noninferiority trial is not provided or the number of participants is based on an inappropriate approach (eg, using superiority trial calculations for a noninferiority trial), questions about the quality of the trial arise. The primary concern is whether the noninferiority margin was actually selected before the trial began, as it should have been. And if the researchers used overly optimistic assumptions about the efficacy of the new treatment relative to the standard therapy, the failure to rule out the margin could be misleading. (As with superiority trials that fail to reject the null hypothesis, post hoc power calculations should be avoided.) After the study has ended, the resulting CIs should be used to evaluate whether the study was large enough to adequately assess the relative effectiveness of the treatments.
The RE-LY trial calculated the sample size that was expected to provide 84% power to rule out the prespecified hazard ratio of 1.46, assuming a true event rate of 1.6% per year (presumably for both groups), a recruitment period of 2 years, and at least one year of follow-up. The sample size was subsequently increased from 15,000 to 18,000 to maintain power in case of a low event rate.4,5
5. Is the noninferiority trial as similar as possible to the trial(s) comparing the standard treatment with placebo?
Characteristics of participants, setting, reference treatment, and outcomes used in a noninferiority trial should be as close as possible to those in the trial(s) comparing the treatment with placebo. This is known as the constancy assumption, and it is key to researchers’ ability to draw a conclusion about noninferiority.
The trials used to calculate the noninferiority margin and the RE-LY trial itself involved similar populations of patients with AF, and the outcome (stroke) was similar.
6. Is a per protocol analysis reported in the results?
In randomized controlled superiority trials, the participants should be analyzed in the groups to which they were originally allocated, regardless of whether they adhered to treatment during the entire follow-up period. Such intention-to-treat (ITT) analysis is important because it provides a more conservative estimate of treatment effect—taking into account that some people who are offered treatment will not accept it and others will discontinue treatment. An ITT analysis therefore tends to minimize treatment effects compared with a “per protocol” analysis, in which participants are analyzed according to the treatment they actually received and are often removed from the analysis if they discontinue or do not adhere to treatment.
In noninferiority trials, if patients in the intervention group cross over to the standard treatment group or those in the standard treatment group have poor adherence, an ITT analysis can increase the risk of wrongly claiming noninferiority.7 Therefore, a per protocol analysis should be included—and indeed may be preferable.
In RE-LY, ITT analyses were reported, and complete follow-up data were available for 99.9% of patients. However, the rates of treatment discontinuation at one year were about 15% for those on dabigatran and 10% for the warfarin group, and 21% and 17%, respectively, at 2 years.4,5 If the new treatment were truly less efficacious than the standard treatment, these moderate discontinuation rates could lead to more similar rates of stroke in the 2 groups than would be expected with higher continuation rates, biasing results towards the alternative of noninferiority. Although the original publication of trial results did not include a per protocol analysis, the RE-LY authors later reported that a per protocol analysis yielded similar results to the ITT analysis.
7. Are the overall design and execution of the trial high quality?
Because a poor quality noninferiority trial can appear to demonstrate noninferiority, looking at such studies critically is crucial. Appropriate randomization, concealed allocation, masking, and careful attention to participant flow must all be assessed.2,3
To continue with our example, the RE-LY trial was well conducted. Randomization was performed centrally via an automated telephone system and 2 doses of dabigatran were administered in a masked fashion, while warfarin was open-label. Remarkably, follow-up was achieved for 99.9% of participants over a median of 2 years, and independent adjudicators masked to treatment group assessed outcomes.4,5
CORRESPONDENCE
Anne Mounsey, MD, UNC Chapel Hill Department of Family Medicine, 590 Manning Drive, CB 7595, Chapel Hill, NC 27590; [email protected]
1. Le Henanff A, Giraudeau B, Baron G, et al. Quality of reporting of noninferiority and equivalence randomized trials. JAMA. 2006;295:1147-1151.
2. Piaggio G, Elbourne DR, Pocock SJ, et al; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594-2604.
3. Moher D, Schulz KF, Altman D; CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987-1991.
4. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
5. Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805-810, 810.e1-2.
6. Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492-501.
7. US Department of Health and Human Services. Guidance for industry non-inferiority clinical trials. US Food and Drug Administration Web site. March 2010. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf. Accessed February 4, 2014.
The traditional clinical trial, designed to test whether a new treatment is better than a placebo or another active treatment, is known as a “superiority” trial—although rarely labeled as such. In contrast, the goal of a noninferiority trial is simply to demonstrate that a new treatment is not substantially less effective than the standard therapy.
Such trials are useful when a new therapy is thought to be safer, easier to administer, or less costly than the existing treatment, but not necessarily more effective. And, because it would be unethical to randomize patients with a serious condition for which there already is an effective treatment to placebo, a noninferiority trial is another means of determining if the new treatment is effective.
Noninferiority trials have unique design features and methodology and require a different analysis than traditional superiority trials. Yet many physicians know far less about them; many investigators appear to be less than proficient, as well. A review of 116 noninferiority trials and 46 equivalence trials found that only 20% fulfilled generally accepted quality criteria.1 To improve the quality of noninferiority trials, the CONSORT (Consolidated Standards of Reporting Trials) Group has published a checklist for trial design and reporting standards.2,3 Based on this checklist, we came up with 7 key questions to consider when evaluating a noninferiority trial. In the pages that follow, you’ll also find an at-a-glance guide (TABLE) and a methodology review using a hypothetical case (page E7).
1. Is a noninferiority trial appropriate?
The introduction to a noninferiority trial should provide the rationale for this design and the absence of a placebo control group. Look for a review of the evidence of the efficacy of the reference treatment that placebo-controlled trials have revealed, along with the effect size. The advantages of the new treatment over the standard treatment—eg, fewer adverse effects, easier administration, or lower cost—should be discussed, as well.
In the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY)—a prominent noninferiority trial—investigators compared the standard anticoagulant (warfarin) for patients with atrial fibrillation (AF) at risk of stroke with a new agent, dabigatran.4 In the methods section of the abstract and the statistical analysis section of the main body, the authors clearly indicated that this was a noninferiority trial. They began by referring to the existing evidence of warfarin’s effectiveness, then detailed the qualities that make warfarin cumbersome to use, including the need for frequent laboratory monitoring. This was followed by evidence that many patients stop taking warfarin and that even for those who persist with treatment, adequate anticoagulation is difficult to maintain.
The authors went on to state that because dabigatran requires no long-term monitoring, it is easier to use. Therefore, if dabigatran could be shown to be no worse than warfarin in preventing strokes, it would be a reasonable alternative, leaving no doubt that this was an appropriate noninferiority trial.
2. Is the noninferiority margin based on clinical judgment and statistical reasoning?
The noninferiority margin should be based on clinical judgment as to how effective a new treatment must be in order to be declared not clinically inferior to the standard treatment. This can be based on several factors, including the severity of the outcome and the expected advantages of the new treatment. The margin should also take into account the size of the standard treatment’s effect vs placebo. In RELY, for example, the authors noted that the noninferiority margin was based on the desire to preserve at least 50% of the lower limit of the confidence interval (CI) of warfarin’s estimated effect; this was done using data from a previously published meta-analysis of 6 trials comparing warfarin with placebo for stroke prevention in patients with AF.4-6
3. Are the hypothesis and statistical analysis formulated correctly?
The clinical hypothesis in a noninferiority trial is that the new treatment is not worse than the standard treatment by a prespecified margin; therefore, the statistical null hypothesis to be tested is that the new treatment is worse than the reference treatment by more than that margin. Rejecting a true null hypothesis (for example, because the P value is <.05) is known as a type l error. In this setting, making a type I error would mean accepting a new treatment that is truly worse than the standard by at least the specified margin. Failure to reject a false null hypothesis is known as a type II error, which in this case would mean failing to identify a new treatment that is truly noninferior to the standard.7
In RE-LY, the authors stated that the upper limit of the one-sided 97.5% CI for the relative risk of a stroke with dabigatran vs warfarin had to fall below 1.46.4 (This is the same as testing the null hypothesis that the hazard ratio is ≥1.46.) Thus, the hypothesis was formulated correctly.
4. Is the sample size appropriate and justified?
The sample size in a noninferiority trial should provide high power to reject the null hypothesis that the difference (or relative risk) between groups is equal to or greater than the noninferiority margin under some clinically meaningful assumption about the true difference (or absolute risk reduction) between groups. A true difference of 0 (or a relative risk of 1) is typically assumed for sample size calculation. However, assuming that the new treatment is truly slightly better or slightly worse than the standard may be clinically appropriate in some cases. This would indicate a need for a smaller or larger sample size, respectively, than that required under the usual assumption of no difference.
When the justification for the sample size in a noninferiority trial is not provided or the number of participants is based on an inappropriate approach (eg, using superiority trial calculations for a noninferiority trial), questions about the quality of the trial arise. The primary concern is whether the noninferiority margin was actually selected before the trial began, as it should have been. And if the researchers used overly optimistic assumptions about the efficacy of the new treatment relative to the standard therapy, the failure to rule out the margin could be misleading. (As with superiority trials that fail to reject the null hypothesis, post hoc power calculations should be avoided.) After the study has ended, the resulting CIs should be used to evaluate whether the study was large enough to adequately assess the relative effectiveness of the treatments.
The RE-LY trial calculated the sample size that was expected to provide 84% power to rule out the prespecified hazard ratio of 1.46, assuming a true event rate of 1.6% per year (presumably for both groups), a recruitment period of 2 years, and at least one year of follow-up. The sample size was subsequently increased from 15,000 to 18,000 to maintain power in case of a low event rate.4,5
5. Is the noninferiority trial as similar as possible to the trial(s) comparing the standard treatment with placebo?
Characteristics of participants, setting, reference treatment, and outcomes used in a noninferiority trial should be as close as possible to those in the trial(s) comparing the treatment with placebo. This is known as the constancy assumption, and it is key to researchers’ ability to draw a conclusion about noninferiority.
The trials used to calculate the noninferiority margin and the RE-LY trial itself involved similar populations of patients with AF, and the outcome (stroke) was similar.
6. Is a per protocol analysis reported in the results?
In randomized controlled superiority trials, the participants should be analyzed in the groups to which they were originally allocated, regardless of whether they adhered to treatment during the entire follow-up period. Such intention-to-treat (ITT) analysis is important because it provides a more conservative estimate of treatment effect—taking into account that some people who are offered treatment will not accept it and others will discontinue treatment. An ITT analysis therefore tends to minimize treatment effects compared with a “per protocol” analysis, in which participants are analyzed according to the treatment they actually received and are often removed from the analysis if they discontinue or do not adhere to treatment.
In noninferiority trials, if patients in the intervention group cross over to the standard treatment group or those in the standard treatment group have poor adherence, an ITT analysis can increase the risk of wrongly claiming noninferiority.7 Therefore, a per protocol analysis should be included—and indeed may be preferable.
In RE-LY, ITT analyses were reported, and complete follow-up data were available for 99.9% of patients. However, the rates of treatment discontinuation at one year were about 15% for those on dabigatran and 10% for the warfarin group, and 21% and 17%, respectively, at 2 years.4,5 If the new treatment were truly less efficacious than the standard treatment, these moderate discontinuation rates could lead to more similar rates of stroke in the 2 groups than would be expected with higher continuation rates, biasing results towards the alternative of noninferiority. Although the original publication of trial results did not include a per protocol analysis, the RE-LY authors later reported that a per protocol analysis yielded similar results to the ITT analysis.
7. Are the overall design and execution of the trial high quality?
Because a poor quality noninferiority trial can appear to demonstrate noninferiority, looking at such studies critically is crucial. Appropriate randomization, concealed allocation, masking, and careful attention to participant flow must all be assessed.2,3
To continue with our example, the RE-LY trial was well conducted. Randomization was performed centrally via an automated telephone system and 2 doses of dabigatran were administered in a masked fashion, while warfarin was open-label. Remarkably, follow-up was achieved for 99.9% of participants over a median of 2 years, and independent adjudicators masked to treatment group assessed outcomes.4,5
CORRESPONDENCE
Anne Mounsey, MD, UNC Chapel Hill Department of Family Medicine, 590 Manning Drive, CB 7595, Chapel Hill, NC 27590; [email protected]
The traditional clinical trial, designed to test whether a new treatment is better than a placebo or another active treatment, is known as a “superiority” trial—although rarely labeled as such. In contrast, the goal of a noninferiority trial is simply to demonstrate that a new treatment is not substantially less effective than the standard therapy.
Such trials are useful when a new therapy is thought to be safer, easier to administer, or less costly than the existing treatment, but not necessarily more effective. And, because it would be unethical to randomize patients with a serious condition for which there already is an effective treatment to placebo, a noninferiority trial is another means of determining if the new treatment is effective.
Noninferiority trials have unique design features and methodology and require a different analysis than traditional superiority trials. Yet many physicians know far less about them; many investigators appear to be less than proficient, as well. A review of 116 noninferiority trials and 46 equivalence trials found that only 20% fulfilled generally accepted quality criteria.1 To improve the quality of noninferiority trials, the CONSORT (Consolidated Standards of Reporting Trials) Group has published a checklist for trial design and reporting standards.2,3 Based on this checklist, we came up with 7 key questions to consider when evaluating a noninferiority trial. In the pages that follow, you’ll also find an at-a-glance guide (TABLE) and a methodology review using a hypothetical case (page E7).
1. Is a noninferiority trial appropriate?
The introduction to a noninferiority trial should provide the rationale for this design and the absence of a placebo control group. Look for a review of the evidence of the efficacy of the reference treatment that placebo-controlled trials have revealed, along with the effect size. The advantages of the new treatment over the standard treatment—eg, fewer adverse effects, easier administration, or lower cost—should be discussed, as well.
In the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY)—a prominent noninferiority trial—investigators compared the standard anticoagulant (warfarin) for patients with atrial fibrillation (AF) at risk of stroke with a new agent, dabigatran.4 In the methods section of the abstract and the statistical analysis section of the main body, the authors clearly indicated that this was a noninferiority trial. They began by referring to the existing evidence of warfarin’s effectiveness, then detailed the qualities that make warfarin cumbersome to use, including the need for frequent laboratory monitoring. This was followed by evidence that many patients stop taking warfarin and that even for those who persist with treatment, adequate anticoagulation is difficult to maintain.
The authors went on to state that because dabigatran requires no long-term monitoring, it is easier to use. Therefore, if dabigatran could be shown to be no worse than warfarin in preventing strokes, it would be a reasonable alternative, leaving no doubt that this was an appropriate noninferiority trial.
2. Is the noninferiority margin based on clinical judgment and statistical reasoning?
The noninferiority margin should be based on clinical judgment as to how effective a new treatment must be in order to be declared not clinically inferior to the standard treatment. This can be based on several factors, including the severity of the outcome and the expected advantages of the new treatment. The margin should also take into account the size of the standard treatment’s effect vs placebo. In RELY, for example, the authors noted that the noninferiority margin was based on the desire to preserve at least 50% of the lower limit of the confidence interval (CI) of warfarin’s estimated effect; this was done using data from a previously published meta-analysis of 6 trials comparing warfarin with placebo for stroke prevention in patients with AF.4-6
3. Are the hypothesis and statistical analysis formulated correctly?
The clinical hypothesis in a noninferiority trial is that the new treatment is not worse than the standard treatment by a prespecified margin; therefore, the statistical null hypothesis to be tested is that the new treatment is worse than the reference treatment by more than that margin. Rejecting a true null hypothesis (for example, because the P value is <.05) is known as a type l error. In this setting, making a type I error would mean accepting a new treatment that is truly worse than the standard by at least the specified margin. Failure to reject a false null hypothesis is known as a type II error, which in this case would mean failing to identify a new treatment that is truly noninferior to the standard.7
In RE-LY, the authors stated that the upper limit of the one-sided 97.5% CI for the relative risk of a stroke with dabigatran vs warfarin had to fall below 1.46.4 (This is the same as testing the null hypothesis that the hazard ratio is ≥1.46.) Thus, the hypothesis was formulated correctly.
4. Is the sample size appropriate and justified?
The sample size in a noninferiority trial should provide high power to reject the null hypothesis that the difference (or relative risk) between groups is equal to or greater than the noninferiority margin under some clinically meaningful assumption about the true difference (or absolute risk reduction) between groups. A true difference of 0 (or a relative risk of 1) is typically assumed for sample size calculation. However, assuming that the new treatment is truly slightly better or slightly worse than the standard may be clinically appropriate in some cases. This would indicate a need for a smaller or larger sample size, respectively, than that required under the usual assumption of no difference.
When the justification for the sample size in a noninferiority trial is not provided or the number of participants is based on an inappropriate approach (eg, using superiority trial calculations for a noninferiority trial), questions about the quality of the trial arise. The primary concern is whether the noninferiority margin was actually selected before the trial began, as it should have been. And if the researchers used overly optimistic assumptions about the efficacy of the new treatment relative to the standard therapy, the failure to rule out the margin could be misleading. (As with superiority trials that fail to reject the null hypothesis, post hoc power calculations should be avoided.) After the study has ended, the resulting CIs should be used to evaluate whether the study was large enough to adequately assess the relative effectiveness of the treatments.
The RE-LY trial calculated the sample size that was expected to provide 84% power to rule out the prespecified hazard ratio of 1.46, assuming a true event rate of 1.6% per year (presumably for both groups), a recruitment period of 2 years, and at least one year of follow-up. The sample size was subsequently increased from 15,000 to 18,000 to maintain power in case of a low event rate.4,5
5. Is the noninferiority trial as similar as possible to the trial(s) comparing the standard treatment with placebo?
Characteristics of participants, setting, reference treatment, and outcomes used in a noninferiority trial should be as close as possible to those in the trial(s) comparing the treatment with placebo. This is known as the constancy assumption, and it is key to researchers’ ability to draw a conclusion about noninferiority.
The trials used to calculate the noninferiority margin and the RE-LY trial itself involved similar populations of patients with AF, and the outcome (stroke) was similar.
6. Is a per protocol analysis reported in the results?
In randomized controlled superiority trials, the participants should be analyzed in the groups to which they were originally allocated, regardless of whether they adhered to treatment during the entire follow-up period. Such intention-to-treat (ITT) analysis is important because it provides a more conservative estimate of treatment effect—taking into account that some people who are offered treatment will not accept it and others will discontinue treatment. An ITT analysis therefore tends to minimize treatment effects compared with a “per protocol” analysis, in which participants are analyzed according to the treatment they actually received and are often removed from the analysis if they discontinue or do not adhere to treatment.
In noninferiority trials, if patients in the intervention group cross over to the standard treatment group or those in the standard treatment group have poor adherence, an ITT analysis can increase the risk of wrongly claiming noninferiority.7 Therefore, a per protocol analysis should be included—and indeed may be preferable.
In RE-LY, ITT analyses were reported, and complete follow-up data were available for 99.9% of patients. However, the rates of treatment discontinuation at one year were about 15% for those on dabigatran and 10% for the warfarin group, and 21% and 17%, respectively, at 2 years.4,5 If the new treatment were truly less efficacious than the standard treatment, these moderate discontinuation rates could lead to more similar rates of stroke in the 2 groups than would be expected with higher continuation rates, biasing results towards the alternative of noninferiority. Although the original publication of trial results did not include a per protocol analysis, the RE-LY authors later reported that a per protocol analysis yielded similar results to the ITT analysis.
7. Are the overall design and execution of the trial high quality?
Because a poor quality noninferiority trial can appear to demonstrate noninferiority, looking at such studies critically is crucial. Appropriate randomization, concealed allocation, masking, and careful attention to participant flow must all be assessed.2,3
To continue with our example, the RE-LY trial was well conducted. Randomization was performed centrally via an automated telephone system and 2 doses of dabigatran were administered in a masked fashion, while warfarin was open-label. Remarkably, follow-up was achieved for 99.9% of participants over a median of 2 years, and independent adjudicators masked to treatment group assessed outcomes.4,5
CORRESPONDENCE
Anne Mounsey, MD, UNC Chapel Hill Department of Family Medicine, 590 Manning Drive, CB 7595, Chapel Hill, NC 27590; [email protected]
1. Le Henanff A, Giraudeau B, Baron G, et al. Quality of reporting of noninferiority and equivalence randomized trials. JAMA. 2006;295:1147-1151.
2. Piaggio G, Elbourne DR, Pocock SJ, et al; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594-2604.
3. Moher D, Schulz KF, Altman D; CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987-1991.
4. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
5. Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805-810, 810.e1-2.
6. Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492-501.
7. US Department of Health and Human Services. Guidance for industry non-inferiority clinical trials. US Food and Drug Administration Web site. March 2010. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf. Accessed February 4, 2014.
1. Le Henanff A, Giraudeau B, Baron G, et al. Quality of reporting of noninferiority and equivalence randomized trials. JAMA. 2006;295:1147-1151.
2. Piaggio G, Elbourne DR, Pocock SJ, et al; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594-2604.
3. Moher D, Schulz KF, Altman D; CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987-1991.
4. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
5. Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805-810, 810.e1-2.
6. Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492-501.
7. US Department of Health and Human Services. Guidance for industry non-inferiority clinical trials. US Food and Drug Administration Web site. March 2010. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf. Accessed February 4, 2014.
Team uses light to measure coagulation
Credit: Максим Кукушкин
Researchers have developed an optical device that requires only a few drops of blood and a few minutes to measure coagulation parameters that can guide blood transfusions and anticoagulant therapy.
The team described their device in Biomedical Optics Express.
“Currently, the most comprehensive measures of coagulation are a battery of lab tests that are expensive and can take hours to perform,” said study author Seemantini Nadkarni, PhD, of Massachusetts General Hospital in Boston.
She noted that other systems have been developed that provide clotting measurements at the point of care, but the systems can be big and expensive or have other limitations, such as requiring significant amounts of blood or only measuring clotting time.
“Our goal is to provide as much information as a lab test, but to provide it quickly and cheaply at a patient’s bedside,” Dr Nadkarni said.
To reach this goal, she and her colleagues turned to an optical technique they pioneered called laser speckle rheology. The technique involves shining a laser into a sample and monitoring the patterns of light that bounce back.
The researchers had previously used the technique to measure the mechanical properties of a range of different tissue types and found that it was extremely sensitive to the coagulation of blood.
When light hits a blood sample, blood cells and platelets scatter the light. In unclotted blood, these light-scattering particles move easily about, making the pattern of scattered light, a speckle pattern, fluctuate rapidly.
“It’s almost like looking at a starry night sky, with twinkling stars,” Dr Nadkarni said. “But as the blood starts to coagulate, blood cells and platelets come together within a fibrin network to form a clot. The motion is restricted as the sample gets stiffer, and the ‘twinkling’ of the speckle pattern is reduced significantly.”
Dr Nadkarni and her colleagues used a miniature high-speed camera to record the fluctuating speckle pattern and then correlated the intensity of changes in the pattern with 2 blood sample measurements: clotting time and fibrinogen concentration.
The team noted that physicians could use the measurements to make decisions about how much blood to give a bleeding patient and what type of blood product is needed most.
“The timely detection of clotting defects followed by the appropriate blood product transfusion is critical in managing bleeding patients,” Dr Nadkarni said. “If you transfuse too much, there could be further coagulation defects that occur, but if you don’t transfuse enough, bleeding continues.”
On the other end of the spectrum, the device could help patients on anticoagulant therapy. Having a small device that can analyze their blood in a doctor’s office or at home could reduce the cost and inconvenience of blood tests, while increasing the safety of anticoagulation treatment, Dr Nadkarni said.
At present, her team’s device is about the size of a tissue box and is connected to a computer. The researchers are working to further miniaturize the system and aim to perform clinical studies with a version smaller than a cell phone within the next year. ![]()

Credit: Максим Кукушкин
Researchers have developed an optical device that requires only a few drops of blood and a few minutes to measure coagulation parameters that can guide blood transfusions and anticoagulant therapy.
The team described their device in Biomedical Optics Express.
“Currently, the most comprehensive measures of coagulation are a battery of lab tests that are expensive and can take hours to perform,” said study author Seemantini Nadkarni, PhD, of Massachusetts General Hospital in Boston.
She noted that other systems have been developed that provide clotting measurements at the point of care, but the systems can be big and expensive or have other limitations, such as requiring significant amounts of blood or only measuring clotting time.
“Our goal is to provide as much information as a lab test, but to provide it quickly and cheaply at a patient’s bedside,” Dr Nadkarni said.
To reach this goal, she and her colleagues turned to an optical technique they pioneered called laser speckle rheology. The technique involves shining a laser into a sample and monitoring the patterns of light that bounce back.
The researchers had previously used the technique to measure the mechanical properties of a range of different tissue types and found that it was extremely sensitive to the coagulation of blood.
When light hits a blood sample, blood cells and platelets scatter the light. In unclotted blood, these light-scattering particles move easily about, making the pattern of scattered light, a speckle pattern, fluctuate rapidly.
“It’s almost like looking at a starry night sky, with twinkling stars,” Dr Nadkarni said. “But as the blood starts to coagulate, blood cells and platelets come together within a fibrin network to form a clot. The motion is restricted as the sample gets stiffer, and the ‘twinkling’ of the speckle pattern is reduced significantly.”
Dr Nadkarni and her colleagues used a miniature high-speed camera to record the fluctuating speckle pattern and then correlated the intensity of changes in the pattern with 2 blood sample measurements: clotting time and fibrinogen concentration.
The team noted that physicians could use the measurements to make decisions about how much blood to give a bleeding patient and what type of blood product is needed most.
“The timely detection of clotting defects followed by the appropriate blood product transfusion is critical in managing bleeding patients,” Dr Nadkarni said. “If you transfuse too much, there could be further coagulation defects that occur, but if you don’t transfuse enough, bleeding continues.”
On the other end of the spectrum, the device could help patients on anticoagulant therapy. Having a small device that can analyze their blood in a doctor’s office or at home could reduce the cost and inconvenience of blood tests, while increasing the safety of anticoagulation treatment, Dr Nadkarni said.
At present, her team’s device is about the size of a tissue box and is connected to a computer. The researchers are working to further miniaturize the system and aim to perform clinical studies with a version smaller than a cell phone within the next year. ![]()

Credit: Максим Кукушкин
Researchers have developed an optical device that requires only a few drops of blood and a few minutes to measure coagulation parameters that can guide blood transfusions and anticoagulant therapy.
The team described their device in Biomedical Optics Express.
“Currently, the most comprehensive measures of coagulation are a battery of lab tests that are expensive and can take hours to perform,” said study author Seemantini Nadkarni, PhD, of Massachusetts General Hospital in Boston.
She noted that other systems have been developed that provide clotting measurements at the point of care, but the systems can be big and expensive or have other limitations, such as requiring significant amounts of blood or only measuring clotting time.
“Our goal is to provide as much information as a lab test, but to provide it quickly and cheaply at a patient’s bedside,” Dr Nadkarni said.
To reach this goal, she and her colleagues turned to an optical technique they pioneered called laser speckle rheology. The technique involves shining a laser into a sample and monitoring the patterns of light that bounce back.
The researchers had previously used the technique to measure the mechanical properties of a range of different tissue types and found that it was extremely sensitive to the coagulation of blood.
When light hits a blood sample, blood cells and platelets scatter the light. In unclotted blood, these light-scattering particles move easily about, making the pattern of scattered light, a speckle pattern, fluctuate rapidly.
“It’s almost like looking at a starry night sky, with twinkling stars,” Dr Nadkarni said. “But as the blood starts to coagulate, blood cells and platelets come together within a fibrin network to form a clot. The motion is restricted as the sample gets stiffer, and the ‘twinkling’ of the speckle pattern is reduced significantly.”
Dr Nadkarni and her colleagues used a miniature high-speed camera to record the fluctuating speckle pattern and then correlated the intensity of changes in the pattern with 2 blood sample measurements: clotting time and fibrinogen concentration.
The team noted that physicians could use the measurements to make decisions about how much blood to give a bleeding patient and what type of blood product is needed most.
“The timely detection of clotting defects followed by the appropriate blood product transfusion is critical in managing bleeding patients,” Dr Nadkarni said. “If you transfuse too much, there could be further coagulation defects that occur, but if you don’t transfuse enough, bleeding continues.”
On the other end of the spectrum, the device could help patients on anticoagulant therapy. Having a small device that can analyze their blood in a doctor’s office or at home could reduce the cost and inconvenience of blood tests, while increasing the safety of anticoagulation treatment, Dr Nadkarni said.
At present, her team’s device is about the size of a tissue box and is connected to a computer. The researchers are working to further miniaturize the system and aim to perform clinical studies with a version smaller than a cell phone within the next year. ![]()
Mutation responsible for insecticide resistance

spray insecticide
Credit: Morgana Wingard
A single genetic mutation can cause resistance to the main insecticides used to combat malaria, according to a study published in Genome Biology.
Researchers identified a mutation in the gene GSTe2 that allows mosquitoes to break down the insecticide DDT into non-toxic substances.
The mutation also makes mosquitoes resistant to pyrethroids, an insecticide class used in mosquito nets.
“We found a population of mosquitoes fully resistant to DDT but also to pyrethroids,” said study author Charles Wondji, PhD, of the Liverpool School of Tropical Medicine in the UK.
“So we wanted to elucidate the molecular basis of that resistance in the population and design a field-applicable diagnostic assay for its monitoring.”
To that end, the researchers did a genome-wide comparison on mosquitoes that were fully susceptible to insecticides and Anopheles funestus mosquitoes from the Republic of Benin in Africa, which were resistant to DDT and the pyrethroid permethrin.
The team found the GSTe2 gene was upregulated in the resistant mosquitoes. And a single mutation (L119F) changed a non-resistant version of the gene to an insecticide-resistant version.
The researchers then designed a DNA-based diagnostic test for this metabolic resistance and confirmed that this mutation was found in mosquitoes from other areas of the world with DDT resistance, but it was completely absent in regions without resistance.
X-ray crystallography of the protein coded by the gene illustrated exactly how the mutation conferred resistance—by opening up the active site where DDT molecules bind to the protein so that more can be broken down. In other words, the mosquito can survive by breaking down the poison into non-toxic substances.
The researchers also introduced the gene into Drosophila melanogaster and found the flies became resistant to DDT and pyrethroids, whereas control flies did not. The team said this confirms that a single mutation is enough to make insects resistant to both DDT and pyrethroids.
“For the first time, we have been able to identify a molecular marker for metabolic resistance in a mosquito population and to design a DNA-based diagnostic assay,” Dr Wondji said.
“Such tools will allow control programs to detect and track resistance at an early stage in the field, which is an essential requirement to successfully tackle the growing problem of insecticide resistance in vector control. This significant progress opens the door for us to do this with other forms of resistance as well and in other vector species.” ![]()

spray insecticide
Credit: Morgana Wingard
A single genetic mutation can cause resistance to the main insecticides used to combat malaria, according to a study published in Genome Biology.
Researchers identified a mutation in the gene GSTe2 that allows mosquitoes to break down the insecticide DDT into non-toxic substances.
The mutation also makes mosquitoes resistant to pyrethroids, an insecticide class used in mosquito nets.
“We found a population of mosquitoes fully resistant to DDT but also to pyrethroids,” said study author Charles Wondji, PhD, of the Liverpool School of Tropical Medicine in the UK.
“So we wanted to elucidate the molecular basis of that resistance in the population and design a field-applicable diagnostic assay for its monitoring.”
To that end, the researchers did a genome-wide comparison on mosquitoes that were fully susceptible to insecticides and Anopheles funestus mosquitoes from the Republic of Benin in Africa, which were resistant to DDT and the pyrethroid permethrin.
The team found the GSTe2 gene was upregulated in the resistant mosquitoes. And a single mutation (L119F) changed a non-resistant version of the gene to an insecticide-resistant version.
The researchers then designed a DNA-based diagnostic test for this metabolic resistance and confirmed that this mutation was found in mosquitoes from other areas of the world with DDT resistance, but it was completely absent in regions without resistance.
X-ray crystallography of the protein coded by the gene illustrated exactly how the mutation conferred resistance—by opening up the active site where DDT molecules bind to the protein so that more can be broken down. In other words, the mosquito can survive by breaking down the poison into non-toxic substances.
The researchers also introduced the gene into Drosophila melanogaster and found the flies became resistant to DDT and pyrethroids, whereas control flies did not. The team said this confirms that a single mutation is enough to make insects resistant to both DDT and pyrethroids.
“For the first time, we have been able to identify a molecular marker for metabolic resistance in a mosquito population and to design a DNA-based diagnostic assay,” Dr Wondji said.
“Such tools will allow control programs to detect and track resistance at an early stage in the field, which is an essential requirement to successfully tackle the growing problem of insecticide resistance in vector control. This significant progress opens the door for us to do this with other forms of resistance as well and in other vector species.” ![]()

spray insecticide
Credit: Morgana Wingard
A single genetic mutation can cause resistance to the main insecticides used to combat malaria, according to a study published in Genome Biology.
Researchers identified a mutation in the gene GSTe2 that allows mosquitoes to break down the insecticide DDT into non-toxic substances.
The mutation also makes mosquitoes resistant to pyrethroids, an insecticide class used in mosquito nets.
“We found a population of mosquitoes fully resistant to DDT but also to pyrethroids,” said study author Charles Wondji, PhD, of the Liverpool School of Tropical Medicine in the UK.
“So we wanted to elucidate the molecular basis of that resistance in the population and design a field-applicable diagnostic assay for its monitoring.”
To that end, the researchers did a genome-wide comparison on mosquitoes that were fully susceptible to insecticides and Anopheles funestus mosquitoes from the Republic of Benin in Africa, which were resistant to DDT and the pyrethroid permethrin.
The team found the GSTe2 gene was upregulated in the resistant mosquitoes. And a single mutation (L119F) changed a non-resistant version of the gene to an insecticide-resistant version.
The researchers then designed a DNA-based diagnostic test for this metabolic resistance and confirmed that this mutation was found in mosquitoes from other areas of the world with DDT resistance, but it was completely absent in regions without resistance.
X-ray crystallography of the protein coded by the gene illustrated exactly how the mutation conferred resistance—by opening up the active site where DDT molecules bind to the protein so that more can be broken down. In other words, the mosquito can survive by breaking down the poison into non-toxic substances.
The researchers also introduced the gene into Drosophila melanogaster and found the flies became resistant to DDT and pyrethroids, whereas control flies did not. The team said this confirms that a single mutation is enough to make insects resistant to both DDT and pyrethroids.
“For the first time, we have been able to identify a molecular marker for metabolic resistance in a mosquito population and to design a DNA-based diagnostic assay,” Dr Wondji said.
“Such tools will allow control programs to detect and track resistance at an early stage in the field, which is an essential requirement to successfully tackle the growing problem of insecticide resistance in vector control. This significant progress opens the door for us to do this with other forms of resistance as well and in other vector species.” ![]()