User login
Hospital to Home Transitions
Hospital readmissions, which account for a substantial proportion of healthcare expenditures, have increasingly become a focus for hospitals and health systems. Hospitals now assume greater responsibility for population health, and face financial penalties by federal and state agencies that consider readmissions a key measure of the quality of care provided during hospitalization. Consequently, there is broad interest in identifying approaches to reduce hospital reutilization, including emergency department (ED) revisits and hospital readmissions. In this issue of the Journal of Hospital Medicine, Auger et al.[1] report the results of a systematic review, which evaluates the effect of discharge interventions on hospital reutilization among children.
As Auger et al. note, the transition from hospital to home is a vulnerable time for children and their families, with 1 in 5 parents reporting major challenges with such transitions.[2] Auger and colleagues identified 14 studies spanning 3 pediatric disease processes that addressed this issue. The authors concluded that several interventions were potentially effective, but individual studies frequently used multifactorial interventions, precluding determination of discrete elements essential to success. The larger body of care transitions literature in adult populations provides insights for interventions that may benefit pediatric patients, as well as informs future research and quality improvement priorities.
The authors identified some distinct interventions that may successfully decrease hospital reutilization, which share common themes from the adult literature. The first is the use of a dedicated transition coordinator (eg, nurse) or coordinating center to assist with the patient's transition home after discharge. In adult studies, this bridging strategy[3, 4] (ie, use of a dedicated transition coordinator or provider) is initiated during the hospitalization and continues postdischarge in the form of phone calls or home visits. The second theme illustrated in both this pediatric review[1] and adult reviews[3, 4, 5] focuses on enhanced or individualized patient education. Most studies have used a combination of these strategies. For example, the Care Transitions Intervention (one of the best validated adult discharge approaches) uses a transition coach to aid the patient in medication self‐management, creation of a patient‐centered record, scheduling follow‐up appointments, and understanding signs and symptoms of a worsening condition.[6] In a randomized study, this intervention demonstrated a reduction in readmissions within 90 days to 16.7% in the intervention group, compared with 22.5% in the control group.[6] One of the pediatric studies highlighted in the review by Auger et al. achieved a decrease in 14‐day ED revisits from 8% prior to implementation of the program to 2.7% following implementation of the program.[7] This program was for patients discharged from the neonatal intensive care unit and involved a nurse coordinator (similar to a transition coach) who worked closely with families and ensured adequate resources prior to discharge as well as a home visitation program.[7]
Although Auger et al. identify some effective approaches to reducing hospital reutilization after discharge in children, their review and the complementary adult literature bring to light 4 main unresolved questions for hospitalists seeking to improve care transitions: (1) how to dissect diverse and heterogeneous interventions to determine the key driver of success, (2) how to interpret and generally apply interventions from single centers where they may have been tailored to a specific healthcare environment, (3) how to generalize the findings of many disease‐specific interventions to other populations, and (4) how to evaluate the cost and assess the costbenefit of implementing many of the more resource intensive interventions. An example of a heterogeneous intervention addressed in this pediatric systematic review was described by Ng et al.,[8] in which the intervention group received a combination of an enhanced discharge education session, disease‐specific nurse evaluation, an animated education booklet, and postdischarge telephone follow‐up, whereas the control group received a shorter discharge education session, a disease‐specific nurse evaluation only if referred by a physician, a written education booklet, and no telephone follow‐up. Investigators found that intervention patients were less likely to be readmitted or revisit the ED as compared with controls. A similarly multifaceted intervention introduced by Taggart et al.[9] was unable to detect a difference in readmissions or ED revisits. It is unclear whether or not the differences in outcomes were related to differences in the intervention bundle itself or institutional or local contextual factors, thus limiting application to other hospitals. Generalizability of interventions is similarly complicated in adults.
The studies presented in this pediatric review article are specific to 3 disease processes: cancer, asthma, and neonatal intensive care (ie, premature) populations. Beyond these populations, there were no other pediatric conditions that met inclusion criteria, thus limiting the generalizability of the findings. As described by Rennke et al.,[3] adult systematic reviews that have focused only on disease‐specific interventions to reduce hospital reutilization are also difficult to generalize to broader populations. Two of the 3 recent adult transition intervention systematic reviews excluded disease‐specific interventions in an attempt to find more broadly applicable interventions but struggled with the same heterogeneity discussed in this review by Auger et al.[3, 4] Although disease‐specific interventions were included in the third adult systematic review and the evaluation was restricted to randomized controlled trials, the authors still grappled with finding 1 or 2 common, successful intervention components.[5] The fourth unresolved question involves understanding the financial burden of implementing more resource‐intensive interventions such as postdischarge home nurse visits. For example, it may be difficult to justify the business case for hiring a transition coach or initiating home nurse visits when the cost and financial implications are unclear. Neither the pediatric nor adult literature describes this well.
Some of the challenges in identifying effective interventions differ between adult and pediatric populations. Adults tend to have multiple comorbid conditions, making them more medically complex and at greater risk for adverse outcomes, medication errors, and hospital utilization.[10] Although a small subset of the pediatric population with complex chronic medical conditions accounts for a majority of hospital reutilization and cost,[11] most hospitalized pediatric patients are otherwise healthy with acute illnesses.[12] Additionally, pediatric patients have lower overall hospital reutilization rates when compared with adults. Adult 30‐day readmission rates are approximately 20%[13] compared with pediatric patients whose mean 30‐day readmission rate is 6.5%.[14] With readmission being an outcome upon which studies are basing intervention success or failure, the relatively low readmission rates in the pediatric population make shifting that outcome more challenging.
There is also controversy about whether policymakers should be focusing on decreasing 30‐day readmission rates as a measure of success. We believe that efforts should focus on identifying more meaningful outcomes, especially outcomes important to patients and their families. No single metric is likely to be an adequate measure of the quality of care transitions, but a combination of outcome measures could potentially be more informative both for patients and clinicians. Patient satisfaction with the discharge process is measured as part of standard patient experience surveys, and the 3‐question Care Transitions Measure[15] has been validated and endorsed as a measure of patient perception of discharge safety in adult populations. There is a growing consensus that 30‐day readmission rates are lacking as a measure of discharge quality, and therefore, measuring shorter‐term7‐ or 14‐dayreadmission rates along with short‐term ED utilization after discharge would likely be more helpful for identifying care transitions problems. Attention should also be paid to measuring rates of specific adverse events in the postdischarge period, such as adverse drug events or failure to follow up on pending test results, as these failures are often implicated in reutilization.
In reflecting upon the published data on adult and pediatric transitions of care interventions and the lingering unanswered questions, we propose a few considerations for future direction of the field. First, engagement of the primary care provider may be beneficial. In many interventions describing a care transition coordinator, nursing fulfilled this role; however, there are opportunities for the primary care provider to play a greater role in this arena. Second, the use of factorial design in future studies may help elucidate which specific parts of each intervention may be the most crucial.[16] Finally, readmission rates are a controversial quality measure in adults. Pediatric readmissions are relatively uncommon, making it difficult to track measurements and show improvement. Clinicians, patients, and policymakers should prioritize outcome measures that are most meaningful to patients and their families that occur at a much higher rate than that of readmissions.
- , , , . Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(0):000–000.
- , , , , . Are hospital characteristics associated with parental views of pediatric inpatient care quality? Pediatrics. 2003;111(2):308–314.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157(6):417–428.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , , . Description and evaluation of a program for the early discharge of infants from a neonatal intensive care unit. J Pediatr. 1995;127(2):285–290.
- , , , , , . Effect of a structured asthma education program on hospitalized asthmatic children: a randomized controlled study. Pediatr Int. 2006;48(2):158–162.
- , , , et al. You Can Control Asthma: evaluation of an asthma education‐program for hospitalized inner‐city children. Patient Educ Couns. 1991;17(1):35–47.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372–380.
- , , , . Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46(3):317–322.
- , , . Quality Improvement Through Planned Experimentation. 2nd ed. New York, NY: McGraw‐Hill; 1999.
Hospital readmissions, which account for a substantial proportion of healthcare expenditures, have increasingly become a focus for hospitals and health systems. Hospitals now assume greater responsibility for population health, and face financial penalties by federal and state agencies that consider readmissions a key measure of the quality of care provided during hospitalization. Consequently, there is broad interest in identifying approaches to reduce hospital reutilization, including emergency department (ED) revisits and hospital readmissions. In this issue of the Journal of Hospital Medicine, Auger et al.[1] report the results of a systematic review, which evaluates the effect of discharge interventions on hospital reutilization among children.
As Auger et al. note, the transition from hospital to home is a vulnerable time for children and their families, with 1 in 5 parents reporting major challenges with such transitions.[2] Auger and colleagues identified 14 studies spanning 3 pediatric disease processes that addressed this issue. The authors concluded that several interventions were potentially effective, but individual studies frequently used multifactorial interventions, precluding determination of discrete elements essential to success. The larger body of care transitions literature in adult populations provides insights for interventions that may benefit pediatric patients, as well as informs future research and quality improvement priorities.
The authors identified some distinct interventions that may successfully decrease hospital reutilization, which share common themes from the adult literature. The first is the use of a dedicated transition coordinator (eg, nurse) or coordinating center to assist with the patient's transition home after discharge. In adult studies, this bridging strategy[3, 4] (ie, use of a dedicated transition coordinator or provider) is initiated during the hospitalization and continues postdischarge in the form of phone calls or home visits. The second theme illustrated in both this pediatric review[1] and adult reviews[3, 4, 5] focuses on enhanced or individualized patient education. Most studies have used a combination of these strategies. For example, the Care Transitions Intervention (one of the best validated adult discharge approaches) uses a transition coach to aid the patient in medication self‐management, creation of a patient‐centered record, scheduling follow‐up appointments, and understanding signs and symptoms of a worsening condition.[6] In a randomized study, this intervention demonstrated a reduction in readmissions within 90 days to 16.7% in the intervention group, compared with 22.5% in the control group.[6] One of the pediatric studies highlighted in the review by Auger et al. achieved a decrease in 14‐day ED revisits from 8% prior to implementation of the program to 2.7% following implementation of the program.[7] This program was for patients discharged from the neonatal intensive care unit and involved a nurse coordinator (similar to a transition coach) who worked closely with families and ensured adequate resources prior to discharge as well as a home visitation program.[7]
Although Auger et al. identify some effective approaches to reducing hospital reutilization after discharge in children, their review and the complementary adult literature bring to light 4 main unresolved questions for hospitalists seeking to improve care transitions: (1) how to dissect diverse and heterogeneous interventions to determine the key driver of success, (2) how to interpret and generally apply interventions from single centers where they may have been tailored to a specific healthcare environment, (3) how to generalize the findings of many disease‐specific interventions to other populations, and (4) how to evaluate the cost and assess the costbenefit of implementing many of the more resource intensive interventions. An example of a heterogeneous intervention addressed in this pediatric systematic review was described by Ng et al.,[8] in which the intervention group received a combination of an enhanced discharge education session, disease‐specific nurse evaluation, an animated education booklet, and postdischarge telephone follow‐up, whereas the control group received a shorter discharge education session, a disease‐specific nurse evaluation only if referred by a physician, a written education booklet, and no telephone follow‐up. Investigators found that intervention patients were less likely to be readmitted or revisit the ED as compared with controls. A similarly multifaceted intervention introduced by Taggart et al.[9] was unable to detect a difference in readmissions or ED revisits. It is unclear whether or not the differences in outcomes were related to differences in the intervention bundle itself or institutional or local contextual factors, thus limiting application to other hospitals. Generalizability of interventions is similarly complicated in adults.
The studies presented in this pediatric review article are specific to 3 disease processes: cancer, asthma, and neonatal intensive care (ie, premature) populations. Beyond these populations, there were no other pediatric conditions that met inclusion criteria, thus limiting the generalizability of the findings. As described by Rennke et al.,[3] adult systematic reviews that have focused only on disease‐specific interventions to reduce hospital reutilization are also difficult to generalize to broader populations. Two of the 3 recent adult transition intervention systematic reviews excluded disease‐specific interventions in an attempt to find more broadly applicable interventions but struggled with the same heterogeneity discussed in this review by Auger et al.[3, 4] Although disease‐specific interventions were included in the third adult systematic review and the evaluation was restricted to randomized controlled trials, the authors still grappled with finding 1 or 2 common, successful intervention components.[5] The fourth unresolved question involves understanding the financial burden of implementing more resource‐intensive interventions such as postdischarge home nurse visits. For example, it may be difficult to justify the business case for hiring a transition coach or initiating home nurse visits when the cost and financial implications are unclear. Neither the pediatric nor adult literature describes this well.
Some of the challenges in identifying effective interventions differ between adult and pediatric populations. Adults tend to have multiple comorbid conditions, making them more medically complex and at greater risk for adverse outcomes, medication errors, and hospital utilization.[10] Although a small subset of the pediatric population with complex chronic medical conditions accounts for a majority of hospital reutilization and cost,[11] most hospitalized pediatric patients are otherwise healthy with acute illnesses.[12] Additionally, pediatric patients have lower overall hospital reutilization rates when compared with adults. Adult 30‐day readmission rates are approximately 20%[13] compared with pediatric patients whose mean 30‐day readmission rate is 6.5%.[14] With readmission being an outcome upon which studies are basing intervention success or failure, the relatively low readmission rates in the pediatric population make shifting that outcome more challenging.
There is also controversy about whether policymakers should be focusing on decreasing 30‐day readmission rates as a measure of success. We believe that efforts should focus on identifying more meaningful outcomes, especially outcomes important to patients and their families. No single metric is likely to be an adequate measure of the quality of care transitions, but a combination of outcome measures could potentially be more informative both for patients and clinicians. Patient satisfaction with the discharge process is measured as part of standard patient experience surveys, and the 3‐question Care Transitions Measure[15] has been validated and endorsed as a measure of patient perception of discharge safety in adult populations. There is a growing consensus that 30‐day readmission rates are lacking as a measure of discharge quality, and therefore, measuring shorter‐term7‐ or 14‐dayreadmission rates along with short‐term ED utilization after discharge would likely be more helpful for identifying care transitions problems. Attention should also be paid to measuring rates of specific adverse events in the postdischarge period, such as adverse drug events or failure to follow up on pending test results, as these failures are often implicated in reutilization.
In reflecting upon the published data on adult and pediatric transitions of care interventions and the lingering unanswered questions, we propose a few considerations for future direction of the field. First, engagement of the primary care provider may be beneficial. In many interventions describing a care transition coordinator, nursing fulfilled this role; however, there are opportunities for the primary care provider to play a greater role in this arena. Second, the use of factorial design in future studies may help elucidate which specific parts of each intervention may be the most crucial.[16] Finally, readmission rates are a controversial quality measure in adults. Pediatric readmissions are relatively uncommon, making it difficult to track measurements and show improvement. Clinicians, patients, and policymakers should prioritize outcome measures that are most meaningful to patients and their families that occur at a much higher rate than that of readmissions.
Hospital readmissions, which account for a substantial proportion of healthcare expenditures, have increasingly become a focus for hospitals and health systems. Hospitals now assume greater responsibility for population health, and face financial penalties by federal and state agencies that consider readmissions a key measure of the quality of care provided during hospitalization. Consequently, there is broad interest in identifying approaches to reduce hospital reutilization, including emergency department (ED) revisits and hospital readmissions. In this issue of the Journal of Hospital Medicine, Auger et al.[1] report the results of a systematic review, which evaluates the effect of discharge interventions on hospital reutilization among children.
As Auger et al. note, the transition from hospital to home is a vulnerable time for children and their families, with 1 in 5 parents reporting major challenges with such transitions.[2] Auger and colleagues identified 14 studies spanning 3 pediatric disease processes that addressed this issue. The authors concluded that several interventions were potentially effective, but individual studies frequently used multifactorial interventions, precluding determination of discrete elements essential to success. The larger body of care transitions literature in adult populations provides insights for interventions that may benefit pediatric patients, as well as informs future research and quality improvement priorities.
The authors identified some distinct interventions that may successfully decrease hospital reutilization, which share common themes from the adult literature. The first is the use of a dedicated transition coordinator (eg, nurse) or coordinating center to assist with the patient's transition home after discharge. In adult studies, this bridging strategy[3, 4] (ie, use of a dedicated transition coordinator or provider) is initiated during the hospitalization and continues postdischarge in the form of phone calls or home visits. The second theme illustrated in both this pediatric review[1] and adult reviews[3, 4, 5] focuses on enhanced or individualized patient education. Most studies have used a combination of these strategies. For example, the Care Transitions Intervention (one of the best validated adult discharge approaches) uses a transition coach to aid the patient in medication self‐management, creation of a patient‐centered record, scheduling follow‐up appointments, and understanding signs and symptoms of a worsening condition.[6] In a randomized study, this intervention demonstrated a reduction in readmissions within 90 days to 16.7% in the intervention group, compared with 22.5% in the control group.[6] One of the pediatric studies highlighted in the review by Auger et al. achieved a decrease in 14‐day ED revisits from 8% prior to implementation of the program to 2.7% following implementation of the program.[7] This program was for patients discharged from the neonatal intensive care unit and involved a nurse coordinator (similar to a transition coach) who worked closely with families and ensured adequate resources prior to discharge as well as a home visitation program.[7]
Although Auger et al. identify some effective approaches to reducing hospital reutilization after discharge in children, their review and the complementary adult literature bring to light 4 main unresolved questions for hospitalists seeking to improve care transitions: (1) how to dissect diverse and heterogeneous interventions to determine the key driver of success, (2) how to interpret and generally apply interventions from single centers where they may have been tailored to a specific healthcare environment, (3) how to generalize the findings of many disease‐specific interventions to other populations, and (4) how to evaluate the cost and assess the costbenefit of implementing many of the more resource intensive interventions. An example of a heterogeneous intervention addressed in this pediatric systematic review was described by Ng et al.,[8] in which the intervention group received a combination of an enhanced discharge education session, disease‐specific nurse evaluation, an animated education booklet, and postdischarge telephone follow‐up, whereas the control group received a shorter discharge education session, a disease‐specific nurse evaluation only if referred by a physician, a written education booklet, and no telephone follow‐up. Investigators found that intervention patients were less likely to be readmitted or revisit the ED as compared with controls. A similarly multifaceted intervention introduced by Taggart et al.[9] was unable to detect a difference in readmissions or ED revisits. It is unclear whether or not the differences in outcomes were related to differences in the intervention bundle itself or institutional or local contextual factors, thus limiting application to other hospitals. Generalizability of interventions is similarly complicated in adults.
The studies presented in this pediatric review article are specific to 3 disease processes: cancer, asthma, and neonatal intensive care (ie, premature) populations. Beyond these populations, there were no other pediatric conditions that met inclusion criteria, thus limiting the generalizability of the findings. As described by Rennke et al.,[3] adult systematic reviews that have focused only on disease‐specific interventions to reduce hospital reutilization are also difficult to generalize to broader populations. Two of the 3 recent adult transition intervention systematic reviews excluded disease‐specific interventions in an attempt to find more broadly applicable interventions but struggled with the same heterogeneity discussed in this review by Auger et al.[3, 4] Although disease‐specific interventions were included in the third adult systematic review and the evaluation was restricted to randomized controlled trials, the authors still grappled with finding 1 or 2 common, successful intervention components.[5] The fourth unresolved question involves understanding the financial burden of implementing more resource‐intensive interventions such as postdischarge home nurse visits. For example, it may be difficult to justify the business case for hiring a transition coach or initiating home nurse visits when the cost and financial implications are unclear. Neither the pediatric nor adult literature describes this well.
Some of the challenges in identifying effective interventions differ between adult and pediatric populations. Adults tend to have multiple comorbid conditions, making them more medically complex and at greater risk for adverse outcomes, medication errors, and hospital utilization.[10] Although a small subset of the pediatric population with complex chronic medical conditions accounts for a majority of hospital reutilization and cost,[11] most hospitalized pediatric patients are otherwise healthy with acute illnesses.[12] Additionally, pediatric patients have lower overall hospital reutilization rates when compared with adults. Adult 30‐day readmission rates are approximately 20%[13] compared with pediatric patients whose mean 30‐day readmission rate is 6.5%.[14] With readmission being an outcome upon which studies are basing intervention success or failure, the relatively low readmission rates in the pediatric population make shifting that outcome more challenging.
There is also controversy about whether policymakers should be focusing on decreasing 30‐day readmission rates as a measure of success. We believe that efforts should focus on identifying more meaningful outcomes, especially outcomes important to patients and their families. No single metric is likely to be an adequate measure of the quality of care transitions, but a combination of outcome measures could potentially be more informative both for patients and clinicians. Patient satisfaction with the discharge process is measured as part of standard patient experience surveys, and the 3‐question Care Transitions Measure[15] has been validated and endorsed as a measure of patient perception of discharge safety in adult populations. There is a growing consensus that 30‐day readmission rates are lacking as a measure of discharge quality, and therefore, measuring shorter‐term7‐ or 14‐dayreadmission rates along with short‐term ED utilization after discharge would likely be more helpful for identifying care transitions problems. Attention should also be paid to measuring rates of specific adverse events in the postdischarge period, such as adverse drug events or failure to follow up on pending test results, as these failures are often implicated in reutilization.
In reflecting upon the published data on adult and pediatric transitions of care interventions and the lingering unanswered questions, we propose a few considerations for future direction of the field. First, engagement of the primary care provider may be beneficial. In many interventions describing a care transition coordinator, nursing fulfilled this role; however, there are opportunities for the primary care provider to play a greater role in this arena. Second, the use of factorial design in future studies may help elucidate which specific parts of each intervention may be the most crucial.[16] Finally, readmission rates are a controversial quality measure in adults. Pediatric readmissions are relatively uncommon, making it difficult to track measurements and show improvement. Clinicians, patients, and policymakers should prioritize outcome measures that are most meaningful to patients and their families that occur at a much higher rate than that of readmissions.
- , , , . Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(0):000–000.
- , , , , . Are hospital characteristics associated with parental views of pediatric inpatient care quality? Pediatrics. 2003;111(2):308–314.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157(6):417–428.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , , . Description and evaluation of a program for the early discharge of infants from a neonatal intensive care unit. J Pediatr. 1995;127(2):285–290.
- , , , , , . Effect of a structured asthma education program on hospitalized asthmatic children: a randomized controlled study. Pediatr Int. 2006;48(2):158–162.
- , , , et al. You Can Control Asthma: evaluation of an asthma education‐program for hospitalized inner‐city children. Patient Educ Couns. 1991;17(1):35–47.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372–380.
- , , , . Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46(3):317–322.
- , , . Quality Improvement Through Planned Experimentation. 2nd ed. New York, NY: McGraw‐Hill; 1999.
- , , , . Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(0):000–000.
- , , , , . Are hospital characteristics associated with parental views of pediatric inpatient care quality? Pediatrics. 2003;111(2):308–314.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157(6):417–428.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , , . Description and evaluation of a program for the early discharge of infants from a neonatal intensive care unit. J Pediatr. 1995;127(2):285–290.
- , , , , , . Effect of a structured asthma education program on hospitalized asthmatic children: a randomized controlled study. Pediatr Int. 2006;48(2):158–162.
- , , , et al. You Can Control Asthma: evaluation of an asthma education‐program for hospitalized inner‐city children. Patient Educ Couns. 1991;17(1):35–47.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372–380.
- , , , . Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46(3):317–322.
- , , . Quality Improvement Through Planned Experimentation. 2nd ed. New York, NY: McGraw‐Hill; 1999.
AAN issues nonvalvular atrial fibrillation stroke prevention guideline
A new evidence-based guideline on how to identify and treat patients with nonvalvular atrial fibrillation to prevent cardioembolic stroke from the American Academy of Neurology suggests when to conduct cardiac rhythm monitoring and offer anticoagulation, including newer agents in place of warfarin.
But the guideline might already be outdated in not considering the results of the recent CRYSTAL-AF study, in which long-term cardiac rhythm monitoring of patients with a previous cryptogenic stroke detected asymptomatic patients at a significantly higher rate than did standard monitoring methods.
The guideline also extends the routine use of anticoagulation for patients with nonvalvular atrial fibrillation (NVAF) who are generally undertreated or whose health was thought a possible barrier to their use, such as those aged 75 years or older, those with mild dementia, and those at moderate risk of falls.
"Cognizant of the global reach of the AAN [American Academy of Neurology], the guideline also examines the evidence base for a treatment alternative to warfarin or its analogues for patients in developing countries who may not have access to the new oral anticoagulants," said lead author Dr. Antonio Culebras in an interview.
"The World Health Organization has determined that atrial fibrillation has reached near-epidemic proportions," observed Dr. Culebras of the State University of New York, Syracuse. "Approximately 1 in 20 individuals with AF will have a stroke unless treated appropriately."
The risk for stroke among patients with NVAF is highest in those with a history of transient ischemic attack (TIA) or prior stroke, at an absolute value of around 10% per year. Patients with "lone NVAF," meaning they have no additional risk factors, have less than a 2% increased risk of stroke per year.
The AAN issued a practice parameter on this topic in 1998 (Neurology 1998;51:671-3). At the time, warfarin, adjusted to an international normalized ratio (INR) of 2.0, was, and largely remains, the recommended standard for patients at risk for cardioembolic stroke. Aspirin was the only recommended alterative for those unable to receive the vitamin K antagonist or who were deemed to be at low risk of stroke, although the evidence was scanty.
Since then, several new oral anticoagulant agents have become available, including the direct thrombin inhibitor dabigatran (Pradaxa), and two factor Xa inhibitors – rivaroxaban (Xarelto) and apixaban (Eliquis) – which have been shown to be at least as effective as, if not more effective than, warfarin. Cardiac rhythm monitoring via a variety of methods has also been introduced as a means to try to detect NVAF in asymptomatic patients.
The aim of the AAN guideline (Neurology 2014;82:716-24) was therefore to look at the latest evidence on the detection of AF using new technologies, as well as the use of treatments to reduce the risk of stroke without increasing the risk of hemorrhage versus the long-standing standard of therapy, warfarin. Data published from 1998 to March 2013 were considered in the preparation of the guideline.
Cardiac rhythm monitoring for NVAF
Seventeen studies were found that examined the use of cardiac monitoring technologies to detect new cases of NVAF. The most common methods used were 24-hour Holter monitoring and serial electrocardiograms, but some emerging evidence on newer technologies was included. The proportion of patients identified with NVAF ranged from 0% to 23%, with the average detection rate 10.7% in all of the studies included.
"The guideline addresses the question of long-term monitoring of patients with NVAF," Dr. Culebras said. "It recommends that clinicians ‘might’ [level C evidence] obtain outpatient cardiac rhythm studies in patients with cryptogenic stroke without known NVAF to identify patients with occult NVAF." He added that the guideline also recommends that monitoring might be needed for prolonged periods of 1 or more weeks rather than for shorter periods, such as 24 hours.
However, at the time the guideline was being prepared, recent data from the CRYSTAL-AF study were not available, and this means the guideline is already outdated, Dr. Richard A. Bernstein, professor of neurology at Northwestern University, Chicago, said in an interview. He was not a guideline author.
Dr. Bernstein was on the steering committee for the CRYSTAL-AF trial, which assessed the performance of Medtronic’s Reveal XT Insertable Cardiac Monitor and found that the implanted device could detect NVAF better than serial ECGs or Holter monitoring (8.6% vs. 1.4%; P = .0006); most (74%) cases of NVAF found were asymptomatic.*
"CRYSTAL-AF represents the state of the art for cardiac monitoring in cryptogenic stroke patients and makes the AAN guidelines obsolete," Dr. Berstein said. "[The study] shows that even intermediate-term monitoring (less than 1 month) will miss the majority of AF in this population, and that most of the AF we find with long-term (greater than 1 year) monitoring is likely to be clinically significant."
With regard to the AAN guideline, he added: "There is no discussion of truly long-term monitoring in the guideline, which is unfortunate." That said, "anything that gets neurologists thinking about long-term cardiac monitoring is likely to be beneficial."
Anticoagulation for stroke prevention
The AAN guideline also provides general recommendations on the use of novel oral anticoagulant agents (NOACs) as alternatives to warfarin. Specifically, it notes that in comparison with warfarin, these NOACs are probably at least as effective (rivaroxaban) or more effective (dabigatran and apixaban). Additionally, while apixaban is also likely to be more effective than aspirin, it is associated with a similar risk for bleeding. NOACs have the following advantages over warfarin: an overall lower risk of intracranial hemorrhage and no need for routine anticoagulant monitoring.
From a practical perspective, the AAN guideline suggests that clinicians have the following options available: warfarin to reach an INR of 2.0-3.0, dabigatran 150 mg twice daily, rivaroxaban 15-20 mg/dL, apixaban 2.5-5 mg twice a day, and triflusal 600 mg plus acenocoumarol to reach an INR target of 1.25-2.0. If a patient is already taking warfarin and is well controlled, then they should remain on that therapy and not switch to a newer oral anticoagulant.
The guideline also notes that clopidogrel plus aspirin is probably less effective than warfarin, but the combination is probably better than aspirin alone. However, the risk of hemorrhage is higher.
Where used, triflusal plus acenocoumarol is "likely more effective" than acenocoumarol alone. Triflusal is an antiplatelet drug related to aspirin, used in Europe, Latin America, and Southeast Asia. Acenocoumarol is mostly used in European countries.
Dr. Culebras explained that the guideline was not intended to dictate which treatment to use. "The guideline leaves room on purpose for clinicians to use their judgment," he said. "The overall objective of the guideline is to reduce therapeutic uncertainty and not to issue commandments for treatment."
Although Dr. Bernstein was critical of the guidelines for not advocating the use of anticoagulants strongly enough, he said that the recommendations on anticoagulant choice are "reasonable in that they impute potential clinical profiles of patients who might particularly benefit from one NOAC over another, without making a claim that these recommendations are based on solid data. This reflects how doctors make decisions when we don’t have direct comparative studies, and I think that is helpful."
The guideline was developed with financial support from the American Academy of Neurology. None of the authors received reimbursement, honoraria, or stipends for their participation in the development of the guideline.
Dr. Culebras has received one-time funding for travel from J. Uriach & Co, and he serves on the editorial boards of MedLink, UpToDate.com, and the International Journal of Stroke. He has received royalties from Informa Healthcare and Cambridge University Press, and has held stock in Clinical Stroke Research. Other authors reported current or past ties to companies marketing oral anticoagulants and stroke treatments.
Dr. Bernstein was on the steering committee for the CRYSTAL-AF study and is a paid speaker, researcher, and consultant for Medtronic, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, and Lifewatch.
*Correction, 4/8/2014: The article previously misstated what the implantable device was detecting in the CRYSTAL-AF study.

|
|
These guidelines are a missed opportunity to empower neurologists to advocate in favor of anticoagulation to prevent stroke. The biggest public health problem in AF is that only half of patients who need anticoagulation are getting it. This disgraceful state of affairs results in patients having cardioembolic strokes that are fatal or worse and that could have been prevented. We neurologists see these complications of inadequate treatment and should be on the front lines of prevention. These tepid guidelines give as much space to bleeding as they do to ischemic stroke prevention, which is inappropriate, and I fear will make neurologists, who are not terribly assertive under any circumstances, even less willing to push doctors to use anticoagulants.
I would have been happier with a single page that said: "Stop using aspirin. Patients fear major stroke more than they fear bleeding or death, and they are right. Stop undertreating your patients and start preventing strokes."
Dr. Richard A. Bernstein is professor of neurology and director of the stroke program at Northwestern University, Chicago.

|
|
These guidelines are a missed opportunity to empower neurologists to advocate in favor of anticoagulation to prevent stroke. The biggest public health problem in AF is that only half of patients who need anticoagulation are getting it. This disgraceful state of affairs results in patients having cardioembolic strokes that are fatal or worse and that could have been prevented. We neurologists see these complications of inadequate treatment and should be on the front lines of prevention. These tepid guidelines give as much space to bleeding as they do to ischemic stroke prevention, which is inappropriate, and I fear will make neurologists, who are not terribly assertive under any circumstances, even less willing to push doctors to use anticoagulants.
I would have been happier with a single page that said: "Stop using aspirin. Patients fear major stroke more than they fear bleeding or death, and they are right. Stop undertreating your patients and start preventing strokes."
Dr. Richard A. Bernstein is professor of neurology and director of the stroke program at Northwestern University, Chicago.

|
|
These guidelines are a missed opportunity to empower neurologists to advocate in favor of anticoagulation to prevent stroke. The biggest public health problem in AF is that only half of patients who need anticoagulation are getting it. This disgraceful state of affairs results in patients having cardioembolic strokes that are fatal or worse and that could have been prevented. We neurologists see these complications of inadequate treatment and should be on the front lines of prevention. These tepid guidelines give as much space to bleeding as they do to ischemic stroke prevention, which is inappropriate, and I fear will make neurologists, who are not terribly assertive under any circumstances, even less willing to push doctors to use anticoagulants.
I would have been happier with a single page that said: "Stop using aspirin. Patients fear major stroke more than they fear bleeding or death, and they are right. Stop undertreating your patients and start preventing strokes."
Dr. Richard A. Bernstein is professor of neurology and director of the stroke program at Northwestern University, Chicago.
A new evidence-based guideline on how to identify and treat patients with nonvalvular atrial fibrillation to prevent cardioembolic stroke from the American Academy of Neurology suggests when to conduct cardiac rhythm monitoring and offer anticoagulation, including newer agents in place of warfarin.
But the guideline might already be outdated in not considering the results of the recent CRYSTAL-AF study, in which long-term cardiac rhythm monitoring of patients with a previous cryptogenic stroke detected asymptomatic patients at a significantly higher rate than did standard monitoring methods.
The guideline also extends the routine use of anticoagulation for patients with nonvalvular atrial fibrillation (NVAF) who are generally undertreated or whose health was thought a possible barrier to their use, such as those aged 75 years or older, those with mild dementia, and those at moderate risk of falls.
"Cognizant of the global reach of the AAN [American Academy of Neurology], the guideline also examines the evidence base for a treatment alternative to warfarin or its analogues for patients in developing countries who may not have access to the new oral anticoagulants," said lead author Dr. Antonio Culebras in an interview.
"The World Health Organization has determined that atrial fibrillation has reached near-epidemic proportions," observed Dr. Culebras of the State University of New York, Syracuse. "Approximately 1 in 20 individuals with AF will have a stroke unless treated appropriately."
The risk for stroke among patients with NVAF is highest in those with a history of transient ischemic attack (TIA) or prior stroke, at an absolute value of around 10% per year. Patients with "lone NVAF," meaning they have no additional risk factors, have less than a 2% increased risk of stroke per year.
The AAN issued a practice parameter on this topic in 1998 (Neurology 1998;51:671-3). At the time, warfarin, adjusted to an international normalized ratio (INR) of 2.0, was, and largely remains, the recommended standard for patients at risk for cardioembolic stroke. Aspirin was the only recommended alterative for those unable to receive the vitamin K antagonist or who were deemed to be at low risk of stroke, although the evidence was scanty.
Since then, several new oral anticoagulant agents have become available, including the direct thrombin inhibitor dabigatran (Pradaxa), and two factor Xa inhibitors – rivaroxaban (Xarelto) and apixaban (Eliquis) – which have been shown to be at least as effective as, if not more effective than, warfarin. Cardiac rhythm monitoring via a variety of methods has also been introduced as a means to try to detect NVAF in asymptomatic patients.
The aim of the AAN guideline (Neurology 2014;82:716-24) was therefore to look at the latest evidence on the detection of AF using new technologies, as well as the use of treatments to reduce the risk of stroke without increasing the risk of hemorrhage versus the long-standing standard of therapy, warfarin. Data published from 1998 to March 2013 were considered in the preparation of the guideline.
Cardiac rhythm monitoring for NVAF
Seventeen studies were found that examined the use of cardiac monitoring technologies to detect new cases of NVAF. The most common methods used were 24-hour Holter monitoring and serial electrocardiograms, but some emerging evidence on newer technologies was included. The proportion of patients identified with NVAF ranged from 0% to 23%, with the average detection rate 10.7% in all of the studies included.
"The guideline addresses the question of long-term monitoring of patients with NVAF," Dr. Culebras said. "It recommends that clinicians ‘might’ [level C evidence] obtain outpatient cardiac rhythm studies in patients with cryptogenic stroke without known NVAF to identify patients with occult NVAF." He added that the guideline also recommends that monitoring might be needed for prolonged periods of 1 or more weeks rather than for shorter periods, such as 24 hours.
However, at the time the guideline was being prepared, recent data from the CRYSTAL-AF study were not available, and this means the guideline is already outdated, Dr. Richard A. Bernstein, professor of neurology at Northwestern University, Chicago, said in an interview. He was not a guideline author.
Dr. Bernstein was on the steering committee for the CRYSTAL-AF trial, which assessed the performance of Medtronic’s Reveal XT Insertable Cardiac Monitor and found that the implanted device could detect NVAF better than serial ECGs or Holter monitoring (8.6% vs. 1.4%; P = .0006); most (74%) cases of NVAF found were asymptomatic.*
"CRYSTAL-AF represents the state of the art for cardiac monitoring in cryptogenic stroke patients and makes the AAN guidelines obsolete," Dr. Berstein said. "[The study] shows that even intermediate-term monitoring (less than 1 month) will miss the majority of AF in this population, and that most of the AF we find with long-term (greater than 1 year) monitoring is likely to be clinically significant."
With regard to the AAN guideline, he added: "There is no discussion of truly long-term monitoring in the guideline, which is unfortunate." That said, "anything that gets neurologists thinking about long-term cardiac monitoring is likely to be beneficial."
Anticoagulation for stroke prevention
The AAN guideline also provides general recommendations on the use of novel oral anticoagulant agents (NOACs) as alternatives to warfarin. Specifically, it notes that in comparison with warfarin, these NOACs are probably at least as effective (rivaroxaban) or more effective (dabigatran and apixaban). Additionally, while apixaban is also likely to be more effective than aspirin, it is associated with a similar risk for bleeding. NOACs have the following advantages over warfarin: an overall lower risk of intracranial hemorrhage and no need for routine anticoagulant monitoring.
From a practical perspective, the AAN guideline suggests that clinicians have the following options available: warfarin to reach an INR of 2.0-3.0, dabigatran 150 mg twice daily, rivaroxaban 15-20 mg/dL, apixaban 2.5-5 mg twice a day, and triflusal 600 mg plus acenocoumarol to reach an INR target of 1.25-2.0. If a patient is already taking warfarin and is well controlled, then they should remain on that therapy and not switch to a newer oral anticoagulant.
The guideline also notes that clopidogrel plus aspirin is probably less effective than warfarin, but the combination is probably better than aspirin alone. However, the risk of hemorrhage is higher.
Where used, triflusal plus acenocoumarol is "likely more effective" than acenocoumarol alone. Triflusal is an antiplatelet drug related to aspirin, used in Europe, Latin America, and Southeast Asia. Acenocoumarol is mostly used in European countries.
Dr. Culebras explained that the guideline was not intended to dictate which treatment to use. "The guideline leaves room on purpose for clinicians to use their judgment," he said. "The overall objective of the guideline is to reduce therapeutic uncertainty and not to issue commandments for treatment."
Although Dr. Bernstein was critical of the guidelines for not advocating the use of anticoagulants strongly enough, he said that the recommendations on anticoagulant choice are "reasonable in that they impute potential clinical profiles of patients who might particularly benefit from one NOAC over another, without making a claim that these recommendations are based on solid data. This reflects how doctors make decisions when we don’t have direct comparative studies, and I think that is helpful."
The guideline was developed with financial support from the American Academy of Neurology. None of the authors received reimbursement, honoraria, or stipends for their participation in the development of the guideline.
Dr. Culebras has received one-time funding for travel from J. Uriach & Co, and he serves on the editorial boards of MedLink, UpToDate.com, and the International Journal of Stroke. He has received royalties from Informa Healthcare and Cambridge University Press, and has held stock in Clinical Stroke Research. Other authors reported current or past ties to companies marketing oral anticoagulants and stroke treatments.
Dr. Bernstein was on the steering committee for the CRYSTAL-AF study and is a paid speaker, researcher, and consultant for Medtronic, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, and Lifewatch.
*Correction, 4/8/2014: The article previously misstated what the implantable device was detecting in the CRYSTAL-AF study.
A new evidence-based guideline on how to identify and treat patients with nonvalvular atrial fibrillation to prevent cardioembolic stroke from the American Academy of Neurology suggests when to conduct cardiac rhythm monitoring and offer anticoagulation, including newer agents in place of warfarin.
But the guideline might already be outdated in not considering the results of the recent CRYSTAL-AF study, in which long-term cardiac rhythm monitoring of patients with a previous cryptogenic stroke detected asymptomatic patients at a significantly higher rate than did standard monitoring methods.
The guideline also extends the routine use of anticoagulation for patients with nonvalvular atrial fibrillation (NVAF) who are generally undertreated or whose health was thought a possible barrier to their use, such as those aged 75 years or older, those with mild dementia, and those at moderate risk of falls.
"Cognizant of the global reach of the AAN [American Academy of Neurology], the guideline also examines the evidence base for a treatment alternative to warfarin or its analogues for patients in developing countries who may not have access to the new oral anticoagulants," said lead author Dr. Antonio Culebras in an interview.
"The World Health Organization has determined that atrial fibrillation has reached near-epidemic proportions," observed Dr. Culebras of the State University of New York, Syracuse. "Approximately 1 in 20 individuals with AF will have a stroke unless treated appropriately."
The risk for stroke among patients with NVAF is highest in those with a history of transient ischemic attack (TIA) or prior stroke, at an absolute value of around 10% per year. Patients with "lone NVAF," meaning they have no additional risk factors, have less than a 2% increased risk of stroke per year.
The AAN issued a practice parameter on this topic in 1998 (Neurology 1998;51:671-3). At the time, warfarin, adjusted to an international normalized ratio (INR) of 2.0, was, and largely remains, the recommended standard for patients at risk for cardioembolic stroke. Aspirin was the only recommended alterative for those unable to receive the vitamin K antagonist or who were deemed to be at low risk of stroke, although the evidence was scanty.
Since then, several new oral anticoagulant agents have become available, including the direct thrombin inhibitor dabigatran (Pradaxa), and two factor Xa inhibitors – rivaroxaban (Xarelto) and apixaban (Eliquis) – which have been shown to be at least as effective as, if not more effective than, warfarin. Cardiac rhythm monitoring via a variety of methods has also been introduced as a means to try to detect NVAF in asymptomatic patients.
The aim of the AAN guideline (Neurology 2014;82:716-24) was therefore to look at the latest evidence on the detection of AF using new technologies, as well as the use of treatments to reduce the risk of stroke without increasing the risk of hemorrhage versus the long-standing standard of therapy, warfarin. Data published from 1998 to March 2013 were considered in the preparation of the guideline.
Cardiac rhythm monitoring for NVAF
Seventeen studies were found that examined the use of cardiac monitoring technologies to detect new cases of NVAF. The most common methods used were 24-hour Holter monitoring and serial electrocardiograms, but some emerging evidence on newer technologies was included. The proportion of patients identified with NVAF ranged from 0% to 23%, with the average detection rate 10.7% in all of the studies included.
"The guideline addresses the question of long-term monitoring of patients with NVAF," Dr. Culebras said. "It recommends that clinicians ‘might’ [level C evidence] obtain outpatient cardiac rhythm studies in patients with cryptogenic stroke without known NVAF to identify patients with occult NVAF." He added that the guideline also recommends that monitoring might be needed for prolonged periods of 1 or more weeks rather than for shorter periods, such as 24 hours.
However, at the time the guideline was being prepared, recent data from the CRYSTAL-AF study were not available, and this means the guideline is already outdated, Dr. Richard A. Bernstein, professor of neurology at Northwestern University, Chicago, said in an interview. He was not a guideline author.
Dr. Bernstein was on the steering committee for the CRYSTAL-AF trial, which assessed the performance of Medtronic’s Reveal XT Insertable Cardiac Monitor and found that the implanted device could detect NVAF better than serial ECGs or Holter monitoring (8.6% vs. 1.4%; P = .0006); most (74%) cases of NVAF found were asymptomatic.*
"CRYSTAL-AF represents the state of the art for cardiac monitoring in cryptogenic stroke patients and makes the AAN guidelines obsolete," Dr. Berstein said. "[The study] shows that even intermediate-term monitoring (less than 1 month) will miss the majority of AF in this population, and that most of the AF we find with long-term (greater than 1 year) monitoring is likely to be clinically significant."
With regard to the AAN guideline, he added: "There is no discussion of truly long-term monitoring in the guideline, which is unfortunate." That said, "anything that gets neurologists thinking about long-term cardiac monitoring is likely to be beneficial."
Anticoagulation for stroke prevention
The AAN guideline also provides general recommendations on the use of novel oral anticoagulant agents (NOACs) as alternatives to warfarin. Specifically, it notes that in comparison with warfarin, these NOACs are probably at least as effective (rivaroxaban) or more effective (dabigatran and apixaban). Additionally, while apixaban is also likely to be more effective than aspirin, it is associated with a similar risk for bleeding. NOACs have the following advantages over warfarin: an overall lower risk of intracranial hemorrhage and no need for routine anticoagulant monitoring.
From a practical perspective, the AAN guideline suggests that clinicians have the following options available: warfarin to reach an INR of 2.0-3.0, dabigatran 150 mg twice daily, rivaroxaban 15-20 mg/dL, apixaban 2.5-5 mg twice a day, and triflusal 600 mg plus acenocoumarol to reach an INR target of 1.25-2.0. If a patient is already taking warfarin and is well controlled, then they should remain on that therapy and not switch to a newer oral anticoagulant.
The guideline also notes that clopidogrel plus aspirin is probably less effective than warfarin, but the combination is probably better than aspirin alone. However, the risk of hemorrhage is higher.
Where used, triflusal plus acenocoumarol is "likely more effective" than acenocoumarol alone. Triflusal is an antiplatelet drug related to aspirin, used in Europe, Latin America, and Southeast Asia. Acenocoumarol is mostly used in European countries.
Dr. Culebras explained that the guideline was not intended to dictate which treatment to use. "The guideline leaves room on purpose for clinicians to use their judgment," he said. "The overall objective of the guideline is to reduce therapeutic uncertainty and not to issue commandments for treatment."
Although Dr. Bernstein was critical of the guidelines for not advocating the use of anticoagulants strongly enough, he said that the recommendations on anticoagulant choice are "reasonable in that they impute potential clinical profiles of patients who might particularly benefit from one NOAC over another, without making a claim that these recommendations are based on solid data. This reflects how doctors make decisions when we don’t have direct comparative studies, and I think that is helpful."
The guideline was developed with financial support from the American Academy of Neurology. None of the authors received reimbursement, honoraria, or stipends for their participation in the development of the guideline.
Dr. Culebras has received one-time funding for travel from J. Uriach & Co, and he serves on the editorial boards of MedLink, UpToDate.com, and the International Journal of Stroke. He has received royalties from Informa Healthcare and Cambridge University Press, and has held stock in Clinical Stroke Research. Other authors reported current or past ties to companies marketing oral anticoagulants and stroke treatments.
Dr. Bernstein was on the steering committee for the CRYSTAL-AF study and is a paid speaker, researcher, and consultant for Medtronic, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, and Lifewatch.
*Correction, 4/8/2014: The article previously misstated what the implantable device was detecting in the CRYSTAL-AF study.
FROM NEUROLOGY
Mammogram data are not to die for
I remember that day like it was yesterday, though it occurred more than a decade ago. I stood leaning over a black entertainment center in my family room, legs wobbly, heart weary – a surreal and solemn snapshot in time. From a speaker streamed a now-favorite Donnie McClurkin song, called "Stand," with its introspective lyrics: "You’ve prayed and you’ve cried ... . After you’ve done all you can, you just stand."
In the next room I could hear her softly gurgling on her secretions. I needed a moment, no, two or three moments, to collect my thoughts and pull myself together before I returned to face the nightmare I was living. My mother was actively dying in my guestroom. Why? I believed then and, today, many years later, believe just as strongly it was because she had not been getting her mammograms.
As a writer, sometimes I struggle with how personal to get in my blogs, but rest assured. I got her permission to share her story while she was still very lucid and competent. You see, she did not want others’ lives to end as hers was ending. She realized, in her final stages of life, that things would have likely been much different had she had her screening mammograms as recommended.
By the time of her diagnosis in her early 60s, the cancer had already spread. Would a mammogram in her late 50s have saved her life? I believe so, and I’m not alone. So I take issue with a recent article published in BMJ that downplays the significance of mammography (BMJ 2014;348:g366).
In 1980, Canadian researchers randomized 89,835 women, aged 40-59 to receive five annual mammograms or physical breast examinations. They followed these women over a 25-year period, and concluded that yearly mammography in women aged 40-59 did not decrease breast cancer mortality "beyond that of physical examination or usual care when adjuvant therapy for breast cancer is freely available."
Well, how many of us have taken care of women in their 50s, 40s, and even 30s with terminal breast cancer? How many of us would advise a mother, aunt, sister (or self) not to have routine mammography? Not many, I’m sure. There is the art of medicine and the science of medicine. Sometimes these two clash, but I believe the art of medicine is realizing that the science of medicine really doesn’t matter to dying patients and their family members. Sometimes, we have to act in the best interest of individual patients and not rely too heavily on the "data." Data changes, risk factors emerge, or research findings may prove to be skewed or wrong in hindsight. Explains Dr. Poornima Sharma, an oncologist/hematologist at the University of Maryland Baltimore-Washington Medical Center: "While the methodology, mammographic technique, and equipment used in the Canadian study is being assessed and compared to the mammography standards used in the United States, the standard in this country remains annual mammography starting at age 40."
Still, many women consider themselves at low risk for breast cancer if they have no close relatives with the disease. As erroneous as this assumption may be, this subset of women may be particularly vulnerable to the implication that yearly mammography is not needed.
So do this: Discuss screening mammography with your own family and then use those feelings when a teachable moment presents itself at bedside.
Our patients rely on us to look into their eyes and give them our best advice. Even though I am a hospitalist, there are still those women I feel compelled to counsel about screening mammography, and this study will not lessen my fervor.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
I remember that day like it was yesterday, though it occurred more than a decade ago. I stood leaning over a black entertainment center in my family room, legs wobbly, heart weary – a surreal and solemn snapshot in time. From a speaker streamed a now-favorite Donnie McClurkin song, called "Stand," with its introspective lyrics: "You’ve prayed and you’ve cried ... . After you’ve done all you can, you just stand."
In the next room I could hear her softly gurgling on her secretions. I needed a moment, no, two or three moments, to collect my thoughts and pull myself together before I returned to face the nightmare I was living. My mother was actively dying in my guestroom. Why? I believed then and, today, many years later, believe just as strongly it was because she had not been getting her mammograms.
As a writer, sometimes I struggle with how personal to get in my blogs, but rest assured. I got her permission to share her story while she was still very lucid and competent. You see, she did not want others’ lives to end as hers was ending. She realized, in her final stages of life, that things would have likely been much different had she had her screening mammograms as recommended.
By the time of her diagnosis in her early 60s, the cancer had already spread. Would a mammogram in her late 50s have saved her life? I believe so, and I’m not alone. So I take issue with a recent article published in BMJ that downplays the significance of mammography (BMJ 2014;348:g366).
In 1980, Canadian researchers randomized 89,835 women, aged 40-59 to receive five annual mammograms or physical breast examinations. They followed these women over a 25-year period, and concluded that yearly mammography in women aged 40-59 did not decrease breast cancer mortality "beyond that of physical examination or usual care when adjuvant therapy for breast cancer is freely available."
Well, how many of us have taken care of women in their 50s, 40s, and even 30s with terminal breast cancer? How many of us would advise a mother, aunt, sister (or self) not to have routine mammography? Not many, I’m sure. There is the art of medicine and the science of medicine. Sometimes these two clash, but I believe the art of medicine is realizing that the science of medicine really doesn’t matter to dying patients and their family members. Sometimes, we have to act in the best interest of individual patients and not rely too heavily on the "data." Data changes, risk factors emerge, or research findings may prove to be skewed or wrong in hindsight. Explains Dr. Poornima Sharma, an oncologist/hematologist at the University of Maryland Baltimore-Washington Medical Center: "While the methodology, mammographic technique, and equipment used in the Canadian study is being assessed and compared to the mammography standards used in the United States, the standard in this country remains annual mammography starting at age 40."
Still, many women consider themselves at low risk for breast cancer if they have no close relatives with the disease. As erroneous as this assumption may be, this subset of women may be particularly vulnerable to the implication that yearly mammography is not needed.
So do this: Discuss screening mammography with your own family and then use those feelings when a teachable moment presents itself at bedside.
Our patients rely on us to look into their eyes and give them our best advice. Even though I am a hospitalist, there are still those women I feel compelled to counsel about screening mammography, and this study will not lessen my fervor.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
I remember that day like it was yesterday, though it occurred more than a decade ago. I stood leaning over a black entertainment center in my family room, legs wobbly, heart weary – a surreal and solemn snapshot in time. From a speaker streamed a now-favorite Donnie McClurkin song, called "Stand," with its introspective lyrics: "You’ve prayed and you’ve cried ... . After you’ve done all you can, you just stand."
In the next room I could hear her softly gurgling on her secretions. I needed a moment, no, two or three moments, to collect my thoughts and pull myself together before I returned to face the nightmare I was living. My mother was actively dying in my guestroom. Why? I believed then and, today, many years later, believe just as strongly it was because she had not been getting her mammograms.
As a writer, sometimes I struggle with how personal to get in my blogs, but rest assured. I got her permission to share her story while she was still very lucid and competent. You see, she did not want others’ lives to end as hers was ending. She realized, in her final stages of life, that things would have likely been much different had she had her screening mammograms as recommended.
By the time of her diagnosis in her early 60s, the cancer had already spread. Would a mammogram in her late 50s have saved her life? I believe so, and I’m not alone. So I take issue with a recent article published in BMJ that downplays the significance of mammography (BMJ 2014;348:g366).
In 1980, Canadian researchers randomized 89,835 women, aged 40-59 to receive five annual mammograms or physical breast examinations. They followed these women over a 25-year period, and concluded that yearly mammography in women aged 40-59 did not decrease breast cancer mortality "beyond that of physical examination or usual care when adjuvant therapy for breast cancer is freely available."
Well, how many of us have taken care of women in their 50s, 40s, and even 30s with terminal breast cancer? How many of us would advise a mother, aunt, sister (or self) not to have routine mammography? Not many, I’m sure. There is the art of medicine and the science of medicine. Sometimes these two clash, but I believe the art of medicine is realizing that the science of medicine really doesn’t matter to dying patients and their family members. Sometimes, we have to act in the best interest of individual patients and not rely too heavily on the "data." Data changes, risk factors emerge, or research findings may prove to be skewed or wrong in hindsight. Explains Dr. Poornima Sharma, an oncologist/hematologist at the University of Maryland Baltimore-Washington Medical Center: "While the methodology, mammographic technique, and equipment used in the Canadian study is being assessed and compared to the mammography standards used in the United States, the standard in this country remains annual mammography starting at age 40."
Still, many women consider themselves at low risk for breast cancer if they have no close relatives with the disease. As erroneous as this assumption may be, this subset of women may be particularly vulnerable to the implication that yearly mammography is not needed.
So do this: Discuss screening mammography with your own family and then use those feelings when a teachable moment presents itself at bedside.
Our patients rely on us to look into their eyes and give them our best advice. Even though I am a hospitalist, there are still those women I feel compelled to counsel about screening mammography, and this study will not lessen my fervor.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
Protein appears essential to malaria transmission
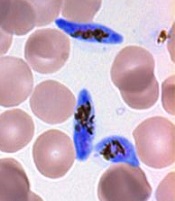
gametocyte stage (blue) and
uninfected red blood cells
Credit: The Llinás lab
Results of 2 new studies suggest that a single regulatory protein acts as a master switch to trigger development of the sexual forms of malaria parasites.
It appears that the protein, AP2-G, is necessary for activating a set of genes that initiate the development of Plasmodium gametocytes, the only forms of the parasite that are infectious to mosquitoes.
This suggests that if researchers can target AP2-G, they can stop sexual parasites from forming.
And if the sexual forms of the parasite never develop in an infected person’s blood, none will enter the mosquito’s gut, and the mosquito will be unable to infect anyone else with malaria.
“Exciting opportunities now lie ahead for finding an effective way to break the chain of malaria transmission by preventing the malaria parasite from completing its full lifecycle,” said Manuel Llinás, PhD, a professor at Pennsylvania State University who was involved in both studies.
The 2 studies, which were published as letters to Nature, had remarkably similar results, despite the fact that the groups worked with 2 different malaria parasites—Plasmodium falciparum and Plasmodium berghei.
In one study, researchers analyzed the whole-genome sequences of 2 P falciparum strains that were unable to produce gametocytes. The only mutated, non-functional gene common to both strains was the AP2-G gene.
In the other study, researchers sequenced P berghei parasites that had lost their ability to make gametocytes. Again, the only common mutated gene in these parasites was AP2-G.
To confirm these observations, both groups of researchers disabled the AP2-G gene in parasites that could generate gametocytes.
As expected, disabling the gene prevented the parasites from producing gametocytes. But the parasites regained their ability to make gametocytes when the mutated gene was repaired.
These results, as well as results of additional experiments, suggest that sexual-stage malaria parasites are produced only when the AP2-G protein is in working order.
“Our research has demonstrated unequivocally that the AP2-G transcription factor protein is essential for flipping the switch that initiates the transformation of malaria parasites in the blood from the asexual stage to the critical sexual stage of their life cycle,” Dr Llinás said.
He and his colleagues believe their discovery is exciting for the future of malaria research. It could spur the development of a sexual-stage vaccine, which would help a person infected with malaria mount an immune response to prevent their parasites from being transmitted to a mosquito, effectively ending the life cycle for that person’s batch of malaria parasites. ![]()

gametocyte stage (blue) and
uninfected red blood cells
Credit: The Llinás lab
Results of 2 new studies suggest that a single regulatory protein acts as a master switch to trigger development of the sexual forms of malaria parasites.
It appears that the protein, AP2-G, is necessary for activating a set of genes that initiate the development of Plasmodium gametocytes, the only forms of the parasite that are infectious to mosquitoes.
This suggests that if researchers can target AP2-G, they can stop sexual parasites from forming.
And if the sexual forms of the parasite never develop in an infected person’s blood, none will enter the mosquito’s gut, and the mosquito will be unable to infect anyone else with malaria.
“Exciting opportunities now lie ahead for finding an effective way to break the chain of malaria transmission by preventing the malaria parasite from completing its full lifecycle,” said Manuel Llinás, PhD, a professor at Pennsylvania State University who was involved in both studies.
The 2 studies, which were published as letters to Nature, had remarkably similar results, despite the fact that the groups worked with 2 different malaria parasites—Plasmodium falciparum and Plasmodium berghei.
In one study, researchers analyzed the whole-genome sequences of 2 P falciparum strains that were unable to produce gametocytes. The only mutated, non-functional gene common to both strains was the AP2-G gene.
In the other study, researchers sequenced P berghei parasites that had lost their ability to make gametocytes. Again, the only common mutated gene in these parasites was AP2-G.
To confirm these observations, both groups of researchers disabled the AP2-G gene in parasites that could generate gametocytes.
As expected, disabling the gene prevented the parasites from producing gametocytes. But the parasites regained their ability to make gametocytes when the mutated gene was repaired.
These results, as well as results of additional experiments, suggest that sexual-stage malaria parasites are produced only when the AP2-G protein is in working order.
“Our research has demonstrated unequivocally that the AP2-G transcription factor protein is essential for flipping the switch that initiates the transformation of malaria parasites in the blood from the asexual stage to the critical sexual stage of their life cycle,” Dr Llinás said.
He and his colleagues believe their discovery is exciting for the future of malaria research. It could spur the development of a sexual-stage vaccine, which would help a person infected with malaria mount an immune response to prevent their parasites from being transmitted to a mosquito, effectively ending the life cycle for that person’s batch of malaria parasites. ![]()

gametocyte stage (blue) and
uninfected red blood cells
Credit: The Llinás lab
Results of 2 new studies suggest that a single regulatory protein acts as a master switch to trigger development of the sexual forms of malaria parasites.
It appears that the protein, AP2-G, is necessary for activating a set of genes that initiate the development of Plasmodium gametocytes, the only forms of the parasite that are infectious to mosquitoes.
This suggests that if researchers can target AP2-G, they can stop sexual parasites from forming.
And if the sexual forms of the parasite never develop in an infected person’s blood, none will enter the mosquito’s gut, and the mosquito will be unable to infect anyone else with malaria.
“Exciting opportunities now lie ahead for finding an effective way to break the chain of malaria transmission by preventing the malaria parasite from completing its full lifecycle,” said Manuel Llinás, PhD, a professor at Pennsylvania State University who was involved in both studies.
The 2 studies, which were published as letters to Nature, had remarkably similar results, despite the fact that the groups worked with 2 different malaria parasites—Plasmodium falciparum and Plasmodium berghei.
In one study, researchers analyzed the whole-genome sequences of 2 P falciparum strains that were unable to produce gametocytes. The only mutated, non-functional gene common to both strains was the AP2-G gene.
In the other study, researchers sequenced P berghei parasites that had lost their ability to make gametocytes. Again, the only common mutated gene in these parasites was AP2-G.
To confirm these observations, both groups of researchers disabled the AP2-G gene in parasites that could generate gametocytes.
As expected, disabling the gene prevented the parasites from producing gametocytes. But the parasites regained their ability to make gametocytes when the mutated gene was repaired.
These results, as well as results of additional experiments, suggest that sexual-stage malaria parasites are produced only when the AP2-G protein is in working order.
“Our research has demonstrated unequivocally that the AP2-G transcription factor protein is essential for flipping the switch that initiates the transformation of malaria parasites in the blood from the asexual stage to the critical sexual stage of their life cycle,” Dr Llinás said.
He and his colleagues believe their discovery is exciting for the future of malaria research. It could spur the development of a sexual-stage vaccine, which would help a person infected with malaria mount an immune response to prevent their parasites from being transmitted to a mosquito, effectively ending the life cycle for that person’s batch of malaria parasites. ![]()
Drug could enhance effects of chemo

Credit: Rhoda Baer
The drug spironolactone could improve the efficacy of platinum-based chemotherapy by preventing tumor cell repair, according to research published in Chemistry & Biology.
The researchers knew that platinum-based chemotherapy drugs bind to cellular DNA to induce damage.
So they theorized that blocking DNA repair mechanisms would help potentiate chemotherapy by reducing cancer cells’ resistance to treatment.
The team focused their efforts on inhibiting nucleotide excision repair (NER), in which a damaged DNA fragment is replaced with an intact fragment.
Frédéric Coin, PhD, of the Institute of Genetics and Molecular and Cellular Biology in Illkirch, France, and his colleagues screened more than 1200 drugs looking for one that would inhibit NER activity.
And they found that spironolactone—a drug already used to treat fluid retention, high blood pressure, and other conditions—affects NER activity.
Specifically, the team found that, when combined with platinum derivatives, spironolactone significantly increased cytotoxicity in ovarian and colon cancer cells.
As platinum-based chemotherapy is used to treat a range of cancers, similar results might occur in other malignancies as well.
The researchers also noted that, because spironolactone is already in use for other purposes, it doesn’t require a new application for marketing authorization. And its side effects are already known.
The team said this suggests that protocols testing spironolactone in combination with platinum-based chemotherapy could be organized rather quickly. ![]()

Credit: Rhoda Baer
The drug spironolactone could improve the efficacy of platinum-based chemotherapy by preventing tumor cell repair, according to research published in Chemistry & Biology.
The researchers knew that platinum-based chemotherapy drugs bind to cellular DNA to induce damage.
So they theorized that blocking DNA repair mechanisms would help potentiate chemotherapy by reducing cancer cells’ resistance to treatment.
The team focused their efforts on inhibiting nucleotide excision repair (NER), in which a damaged DNA fragment is replaced with an intact fragment.
Frédéric Coin, PhD, of the Institute of Genetics and Molecular and Cellular Biology in Illkirch, France, and his colleagues screened more than 1200 drugs looking for one that would inhibit NER activity.
And they found that spironolactone—a drug already used to treat fluid retention, high blood pressure, and other conditions—affects NER activity.
Specifically, the team found that, when combined with platinum derivatives, spironolactone significantly increased cytotoxicity in ovarian and colon cancer cells.
As platinum-based chemotherapy is used to treat a range of cancers, similar results might occur in other malignancies as well.
The researchers also noted that, because spironolactone is already in use for other purposes, it doesn’t require a new application for marketing authorization. And its side effects are already known.
The team said this suggests that protocols testing spironolactone in combination with platinum-based chemotherapy could be organized rather quickly. ![]()

Credit: Rhoda Baer
The drug spironolactone could improve the efficacy of platinum-based chemotherapy by preventing tumor cell repair, according to research published in Chemistry & Biology.
The researchers knew that platinum-based chemotherapy drugs bind to cellular DNA to induce damage.
So they theorized that blocking DNA repair mechanisms would help potentiate chemotherapy by reducing cancer cells’ resistance to treatment.
The team focused their efforts on inhibiting nucleotide excision repair (NER), in which a damaged DNA fragment is replaced with an intact fragment.
Frédéric Coin, PhD, of the Institute of Genetics and Molecular and Cellular Biology in Illkirch, France, and his colleagues screened more than 1200 drugs looking for one that would inhibit NER activity.
And they found that spironolactone—a drug already used to treat fluid retention, high blood pressure, and other conditions—affects NER activity.
Specifically, the team found that, when combined with platinum derivatives, spironolactone significantly increased cytotoxicity in ovarian and colon cancer cells.
As platinum-based chemotherapy is used to treat a range of cancers, similar results might occur in other malignancies as well.
The researchers also noted that, because spironolactone is already in use for other purposes, it doesn’t require a new application for marketing authorization. And its side effects are already known.
The team said this suggests that protocols testing spironolactone in combination with platinum-based chemotherapy could be organized rather quickly. ![]()
Evolving Role of the PNP Hospitalist
The Accreditation Council for Graduate Medical Education implemented rules limiting work hours for residents in 2003 and 2011, decreasing the availability of residents as providers at teaching hospitals.[1] These restrictions have increased reliance on advance practice providers (APPs) including nurse practitioners (NPs) and physicians' assistants in providing inpatient care. The NP hospitalist role includes inpatient medical management, coordination of care, patient and staff education, and quality improvement activities.[2] The NP hospitalist role has expanded beyond a replacement for reduced resident work hours, adding value through resident teaching, development of clinical care guidelines (CCGs), continuity of care, and familiarity with inpatient management.[3] The NP hospitalist role has been shown to improve the quality, efficiency, and cost effectiveness of inpatient care.[4, 5]
Favorable quality and cost measure results have been documented for adult NP hospitalists compared to housestaff, including improved patient outcomes, increased patient and staff satisfaction, decreased length of stay (LOS) and cost of care, and improved access to care.[6] These findings are supported by NP inpatient program evaluations at several academic medical centers, which also show increased patient and family satisfaction and improved communication between physicians, nurses, and families.[6, 7, 8] One study demonstrated that collaborative care management of adult medical patients by a hospitalist physician and advanced practice nurse led to decreased LOS and improved hospital profit without changing patient readmission or mortality.[9] Although there is a growing body of evidence supporting the quality and cost effectiveness of the NP hospitalist role in adult inpatient care, there are little published data for pediatric programs.
METHODS
The pediatric nurse practitioner (PNP) hospitalist role at Children's Hospital Colorado (CHCO) was initiated in 2006 to meet the need for additional inpatient providers. Inpatient staffing challenges included decreased resident work hours as well as high inpatient volume during the winter respiratory season. The PNP hospitalist providers at CHCO independently manage care throughout hospitalization for patients within their scope of practice, and comanage more complex patients with the attending doctor of medicine (MD). The PNPs complete history and physical exams, order and interpret diagnostic tests, perform procedures, prescribe medications, and assist with discharge coordination. Patient populations within the PNP hospitalist scope of practice include uncomplicated bronchiolitis, pneumonia, and asthma.
The hospitalist section at CHCO's main campus includes 2 resident teams and 1 PNP team. The hospitalist section also provides inpatient care at several network of care (NOC) sites. These NOC sites are CHCO‐staffed facilities that are either freestanding or connected to a community hospital, with an emergency department and 6 to 8 inpatient beds. The PNP hospitalist role includes inpatient management at the CHCO main campus as well as in the NOC. The NOC sites are staffed with a PNP and MD team who work collaboratively to manage inpatient care. The Advanced Practice Hospitalist Program was implemented to improve staffing and maintain quality of patient care in a cost‐effective manner. We undertook a program evaluation with the goal of comparing quality and cost of care between the PNP team, PNP/MD team, and resident teams.
Administrative and electronic medical record data from July 1, 2009 through June 30, 2010 were reviewed retrospectively. Data were obtained from inpatient records at CHCO inpatient medical unit and inpatient satellite sites in the CHCO NOC. The 2008 versions 26 and 27 of the 3M All Patient Refined Diagnosis‐Related Groups (APR‐DRG) were used to categorize patients by diagnosis, severity of illness, and risk of mortality.[10, 11] The top 3 APR‐DRGs at CHCO, based on volume of inpatient admissions, were selected for this analysis, including bronchiolitis and RSV pneumonia (APR‐DRG 138), pneumonia NEC (APR‐DRG 139), and asthma (APR‐DRG 141) (N = 1664). These 3 diagnoses accounted for approximately 60% of all inpatient hospitalist encounters and comprised 78% of the PNP encounters, 52% of the resident encounters, and 76% of the PNP/MD encounters. APR‐DRG severity of illness categories include I, II, III, and IV (minor, moderate, major, and extreme, respectively).[12] Severity of illness levels I and II were used for this analysis. Severity III and IV levels were excluded due to lack of patients in these categories on the PNP team and in the NOC. We also included observation status patients. The PNP team accounted for approximately 20% of the inpatient encounters, with 45% on the resident teams and 35% on the PNP/MD team in the NOC (Table 1).
| Distribution of Patients | Patient Type/Severity of Illness | NP | Resident | PNP/MD |
|---|---|---|---|---|
| ||||
| Bronchiolitis | Observation | 26 (23%) | 32 (28%) | 55 (49%) |
| Severity I | 93 (29%) | 77 (24%) | 151 (47%) | |
| Severity II | 49 (24%) | 95 (47%) | 60 (29%) | |
| Asthma | Observation | 7 (14%) | 23 (45%) | 21 (41%) |
| Severity I | 48 (14%) | 191 (57%) | 97 (29%) | |
| Severity II | 19 (12%) | 106 (66%) | 35 (22%) | |
| Pneumonia | Observation | 6 (22%) | 12 (44%) | 9 (34%) |
| Severity I | 33 (17%) | 68 (35%) | 93 (48%) | |
| Severity II | 37 (14%) | 152 (59%) | 69 (27%) | |
The PNP hospitalist program was evaluated by comparing patient records from the PNP team, the PNP/MD team, and the resident teams. Evaluation measures included compliance with specific components of the bronchiolitis and asthma CCGs, LOS, and cost of care.
Outcomes Measured
Quality measures for this program evaluation included compliance with the bronchiolitis CCG recommendation to diagnose bronchiolitis based on history and exam findings while minimizing the use of chest x‐ray and respiratory viral testing.[13] Current evidence suggests that these tests add cost and exposure to radiation and do not necessarily predict severity of disease or change medical management.[14] This program evaluation also measured compliance with the asthma CCG recommendation to give every asthma patient an asthma action plan (AAP) prior to hospital discharge.[15] Of note, this evaluation was completed prior to more recent evidence that questions the utility of AAP for improving asthma clinical outcomes.[16] There were no related measures for pneumonia available because there was no CCG in place at the time of this evaluation.
Outcomes measures for this evaluation included LOS and cost of care for the top 3 inpatient diagnoses: bronchiolitis, asthma, and pneumonia. LOS for the inpatient hospitalization was measured in hours. Direct cost of care was used for this analysis, which included medical supplies, pharmacy, radiology, laboratory, and bed charges. Nursing charges were also included in the direct cost due to the proximity of nursing cost to the patient, versus more distant costs such as infrastructure or administration. Hospitalist physician and NP salaries were not included in direct cost analysis. Outcomes were compared for the PNP team, the resident teams, and the PN/MD team in the NOC.
Analysis
Patients were summarized by diagnosis‐related groups (APR‐DRG) and severity of illness using counts and percentages across the PNP team, resident teams, and the PNP/MD team in the NOC (Table 1). LOS and direct cost is skewed, therefore natural log transformations were used to meet normal assumption for statistical testing and modeling. Chi squared and t tests were performed to compare outcomes between the PNP and resident physician teams, stratified by APR‐DRG. Analysis of variance was used to analyze LOS and direct cost for the top 3 APR‐DRG admission codes while adjusting for acuity. The outcomes were also compared pairwise among the 3 teams using a linear mixed model to adjust for APR‐DRG and severity of illness, treating severity as a nested effect within the APR‐DRG. Bonferroni corrections were used to adjust for multiple comparisons; a P value 0.017 was considered statistically significant. Post hoc power analysis was completed for the analysis of bronchiolitis chest x‐ray ordering, even though the sample size was relatively large (PNP team 128, resident team 204) (Table 1). There was a 7% difference between the PNP and resident groups, and the power of detecting a significant difference was 40%. A sample size of 482 for each group would be necessary to achieve 80% power of detecting a 7% difference, while controlling for 5% type I error. All statistical analyses were performed with SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
PNP adherence to CCGs was comparable to resident teams for the specific measures used in this evaluation. Based on a hospital‐wide goal of ordering diagnostic tests for less than 25% of inpatients with bronchiolitis, there was no significant difference between the PNP team and resident teams. There was no significant difference in the rate of chest x‐ray ordering between the PNP team and the resident teams (15% vs 22%, P = 0.1079). Similarly, there was no significant difference in viral testing between the PNP and physician teams (24% vs 25%, P = 0.9813) (Table 2). Post hoc power analysis indicated that a larger sample size would be required to increase the power of detecting a statistically significant difference in chest x‐ray ordering between these groups. The PNP and resident teams were also compared using compliance with the asthma CCGs, specifically related to the goal of providing an accurate AAP to every patient admitted for asthma. The PNP and resident teams had a similar rate of compliance, with PNPs achieving 81% compliance and MDs 76% (P = 0.4351) (Table 2).
| Clinical Care Guidelines | Diagnostic Test | PNP Team | Resident Teams | P Value |
|---|---|---|---|---|
| ||||
| Bronchiolitis care | Chest x‐ray | 15% | 22% | 0.1079 |
| Diagnostic testing | Viral test | 24% | 25% | 0.9813 |
| Completed asthma action plans | 81% | 76% | 0.4351 | |
LOS and direct costs were compared for the 3 teams for the top 3 APR‐DRGs and controlling for acuity. Table 3 illustrates that there were no significant differences in LOS between the PNP and resident teams or between the PNP and PNP/MD teams for these 3 APR‐DRGs (P 0.017 considered statistically significant). There was a statistically significant difference in LOS between resident and PNP/MD teams for asthma and pneumonia (P 0.001). The direct cost of care per patient encounter provided by the PNP team was significantly less than the PNP/MD team for all 3 APR‐DRGs (P 0.001). The direct cost of care per patient encounter provided by the PNP team was significantly less than the resident teams for asthma (P = 0.0021) and pneumonia (P = 0.0001), although the difference was not statistically significant for bronchiolitis (P = 0.0228) for level of significance P 0.0017 (Table 3, 4).
| PNP | Resident | PNP/MD | P Value PNP vs Resident | P Value
PNP vs PNP/MD |
P Value Resident vs PNP/MD | |
|---|---|---|---|---|---|---|
| ||||||
| Cost | ||||||
| Bronchiolitis | $2190 | $2513 | $3072 | 0.0228 | 0.0001 | 0.0002 |
| Asthma | $2089 | $2655 | $3220 | 0.0021 | 0.0001 | 0.0190 |
| Pneumonia | $2348 | $3185 | $3185 | 0.0001 | 0.0001 | 0.1142 |
| LOS, h | ||||||
| Bronchiolitis | 52 | 52 | 51 | 0.9112 | 0.1600 | 0.1728 |
| Asthma | 36 | 42 | 48 | 0.0158 | 0.3151 | 0.0001 |
| Pneumonia | 54 | 61 | 68 | 0.1136 | 0.1605 | 0.0001 |
| PNP | Resident | PNP/MD | PHIS Observation | PHIS SeverityIII | |
|---|---|---|---|---|---|
| |||||
| LOS, h | |||||
| Bronchiolitis | 52 | 52 | 51 | 43 | 70 |
| Asthma | 36 | 42 | 48 | 31 | 48 |
| Pneumonia | 54 | 61 | 68 | 46 | 64 |
Figure 1 illustrates the monthly patient census on the PNP and resident teams obtained from daily midnight census. There was a dramatic seasonal fluctuation in PNP team census, with a low census in July 2009 (22 patients) and high census in February 2010 (355 patients). The resident teams maintained a relatively stable census year round compared to the PNP team.
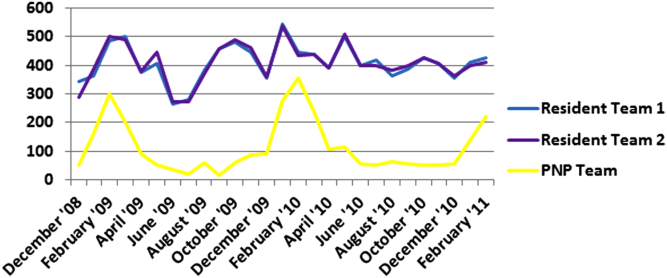
CONCLUSIONS/DISCUSSION
The results of this program evaluation suggest that the PNP team at CHCO provides inpatient care comparable to the resident teams at a lower cost per patient encounter for uncomplicated bronchiolitis, pneumonia, and asthma. The results of this program evaluation are consistent with previously published studies demonstrating that NPs improve outcomes such as decreased LOS and cost of care.[9]
In the setting of increasingly stringent restrictions in residency work hours, PNP hospitalists are a valuable resource for managing inpatient care. PNPs can provide additional benefits not explored in this program evaluation, such as increased access to care, increased patient and family satisfaction, improved documentation, and improved communication between nurses and physicians.[6] NP hospitalist providers can also decrease the patient care burden on housestaff, allowing teaching teams to focus on resident education.[6] This point could be made for the PNP team at CHCO, which contributed to care of inpatients during the peak respiratory season census. This strategy has allowed the resident teaching teams to maintain a more manageable patient census during the winter respiratory season, and presumably has allowed greater focus on resident education year round.[17]
Hospitals have been increasingly using evidence based CCGs as a strategy to improve patient outcomes and decrease LOS and cost.[18] CCGs provide an excellent tool for hospitalist physicians and APPs to deliver consistent inpatient care for common diagnoses such as bronchiolitis, asthma, and pneumonia. Increased reliance on CCGs has provided an opportunity to standardize evidence‐based practices and has allowed PNPs to expand their inpatient role at CHCO. The addition of a PNP inpatient team at CHCO also provided an effective strategy for management of seasonal fluctuations in inpatient census, particularly during the winter respiratory season.
Limitations
This is a single‐site program evaluation at a free standing children's hospital. Colorado law allows NPs to practice independently and obtain full prescriptive authority, although licensing and certification regulations for APPs vary from state to state. Our results may not be generalizable to other hospitals or to states where regulations differ. Patients admitted to the NOC sites and those assigned to the PNP team at the main campus are generally lower acuity and complexity compared to patients assigned to the resident teams at the main campus. Although we controlled for severity using the APR‐DRG severity classification, it is possible that our results were biased due to different patient profiles among the PNP and MD hospitalist teams. There were also potential limitations in the cost analysis, which included nursing in direct costs. Although nurse‐to‐patient ratios are comparable across hospitalist sites, the ratios may have varied due to fluctuations in patient census at each site. The CCG monitoring measures used in this evaluation also presented limitations. These measures were selected due to the availability of these data in the electronic medical record. Future studies may provide more clinically relevant information by including additional patient outcomes measures specifically related to inpatient medical management.
Despite the limitations in this program evaluation, we feel that these data add to the current knowledge in pediatrics by showing equipoise between these 2 groups. The PNP hospitalist role continues to evolve at CHCO, and the utility of this role must continue to be evaluated and reported.
Acknowledgements
Dashka Ranade provided Children's Hospital Colorado CCG comparison data for this program evaluation. David Bertoch provided LOS data from the Children's Hospital Association Pediatric Health Information System database.
Disclosures: Supported by NIH/NCATS Colorado CTSI grant number UL1 TR000154. The contents are the authors' sole responsibility and do not necessarily represent official NIH views.
- Education ACfGM. Common Program Requirements. Accreditation Council for Graduate Medical Education, 2011.
- , , , , , . Hospitalist services: an evolving opportunity. Nurse Pract. 2008;33(5):9–10.
- . APRN hospitalist: just a resident replacement? J Pediatr Health Care. 2004;18(4):208–210.
- , , , . Evaluation of the role of the pediatric nurse practitioner in an inpatient asthma program. J Pediatr Health Care. 2008;22(5):273–281.
- , . Acute care nurse practitioner as hospitalist: role description. AACN Adv Crit Care. 2009;20(2):133–136.
- , . Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service. Am J Crit Care. 2002;11(5):448–458.
- , , , et al. Pediatric trauma nurse practitioners provide excellent care with superior patient satisfaction for injured children. J Pediatr Surg. 2006;41(1):277–281.
- , , , et al. Pediatric trauma nurse practitioners increase bedside nurses' satisfaction with pediatric trauma patient care. J Trauma Nurs. 2006;13(2):66–69.
- , , , et al. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J Nurs Adm. 2006;36(2):79–85.
- , , , , . A closer look at all‐patient refined DRGs. J AHIMA. 2002;73(1):46–50.
- . Structure and performance of different DRG classification systems for neonatal medicine. Pediatrics. 1999;103(1 suppl E):302–318.
- Association CsH. Patient classification system, Children's Hospital Association. Available at: http://www.childrenshospitals.org/. Accessed January 4, 2014.
- . Children's Hospital Colorado bronchiolitis clinical care guideline, Bronchiolitis CCG Task Force 2011. Available at: http://www.childrenscolorado.org/conditions/lung/healthcare_professionals/clinical_care_guidelines.aspx. Accessed January 4, 2014.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793.
- .Children's Hospital Colorado asthma clinical care guideline, Asthma Task Force, 2011. Available at: http://www.childrenscolorado.org/conditions/lung/healthcare_professionals/clinical_care_guidelines.aspx. Accessed January 4, 2014.
- , , . Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;(3):CD005306.
- , . Pediatric nurse practitioners as hospitalists. J Pediatr Health Care. 2010;24(5):347–350.
- , , . Health policy issues and applications for evidence‐based medicine and clinical practice guidelines. Health Policy. 1998;46(1):1–19.
The Accreditation Council for Graduate Medical Education implemented rules limiting work hours for residents in 2003 and 2011, decreasing the availability of residents as providers at teaching hospitals.[1] These restrictions have increased reliance on advance practice providers (APPs) including nurse practitioners (NPs) and physicians' assistants in providing inpatient care. The NP hospitalist role includes inpatient medical management, coordination of care, patient and staff education, and quality improvement activities.[2] The NP hospitalist role has expanded beyond a replacement for reduced resident work hours, adding value through resident teaching, development of clinical care guidelines (CCGs), continuity of care, and familiarity with inpatient management.[3] The NP hospitalist role has been shown to improve the quality, efficiency, and cost effectiveness of inpatient care.[4, 5]
Favorable quality and cost measure results have been documented for adult NP hospitalists compared to housestaff, including improved patient outcomes, increased patient and staff satisfaction, decreased length of stay (LOS) and cost of care, and improved access to care.[6] These findings are supported by NP inpatient program evaluations at several academic medical centers, which also show increased patient and family satisfaction and improved communication between physicians, nurses, and families.[6, 7, 8] One study demonstrated that collaborative care management of adult medical patients by a hospitalist physician and advanced practice nurse led to decreased LOS and improved hospital profit without changing patient readmission or mortality.[9] Although there is a growing body of evidence supporting the quality and cost effectiveness of the NP hospitalist role in adult inpatient care, there are little published data for pediatric programs.
METHODS
The pediatric nurse practitioner (PNP) hospitalist role at Children's Hospital Colorado (CHCO) was initiated in 2006 to meet the need for additional inpatient providers. Inpatient staffing challenges included decreased resident work hours as well as high inpatient volume during the winter respiratory season. The PNP hospitalist providers at CHCO independently manage care throughout hospitalization for patients within their scope of practice, and comanage more complex patients with the attending doctor of medicine (MD). The PNPs complete history and physical exams, order and interpret diagnostic tests, perform procedures, prescribe medications, and assist with discharge coordination. Patient populations within the PNP hospitalist scope of practice include uncomplicated bronchiolitis, pneumonia, and asthma.
The hospitalist section at CHCO's main campus includes 2 resident teams and 1 PNP team. The hospitalist section also provides inpatient care at several network of care (NOC) sites. These NOC sites are CHCO‐staffed facilities that are either freestanding or connected to a community hospital, with an emergency department and 6 to 8 inpatient beds. The PNP hospitalist role includes inpatient management at the CHCO main campus as well as in the NOC. The NOC sites are staffed with a PNP and MD team who work collaboratively to manage inpatient care. The Advanced Practice Hospitalist Program was implemented to improve staffing and maintain quality of patient care in a cost‐effective manner. We undertook a program evaluation with the goal of comparing quality and cost of care between the PNP team, PNP/MD team, and resident teams.
Administrative and electronic medical record data from July 1, 2009 through June 30, 2010 were reviewed retrospectively. Data were obtained from inpatient records at CHCO inpatient medical unit and inpatient satellite sites in the CHCO NOC. The 2008 versions 26 and 27 of the 3M All Patient Refined Diagnosis‐Related Groups (APR‐DRG) were used to categorize patients by diagnosis, severity of illness, and risk of mortality.[10, 11] The top 3 APR‐DRGs at CHCO, based on volume of inpatient admissions, were selected for this analysis, including bronchiolitis and RSV pneumonia (APR‐DRG 138), pneumonia NEC (APR‐DRG 139), and asthma (APR‐DRG 141) (N = 1664). These 3 diagnoses accounted for approximately 60% of all inpatient hospitalist encounters and comprised 78% of the PNP encounters, 52% of the resident encounters, and 76% of the PNP/MD encounters. APR‐DRG severity of illness categories include I, II, III, and IV (minor, moderate, major, and extreme, respectively).[12] Severity of illness levels I and II were used for this analysis. Severity III and IV levels were excluded due to lack of patients in these categories on the PNP team and in the NOC. We also included observation status patients. The PNP team accounted for approximately 20% of the inpatient encounters, with 45% on the resident teams and 35% on the PNP/MD team in the NOC (Table 1).
| Distribution of Patients | Patient Type/Severity of Illness | NP | Resident | PNP/MD |
|---|---|---|---|---|
| ||||
| Bronchiolitis | Observation | 26 (23%) | 32 (28%) | 55 (49%) |
| Severity I | 93 (29%) | 77 (24%) | 151 (47%) | |
| Severity II | 49 (24%) | 95 (47%) | 60 (29%) | |
| Asthma | Observation | 7 (14%) | 23 (45%) | 21 (41%) |
| Severity I | 48 (14%) | 191 (57%) | 97 (29%) | |
| Severity II | 19 (12%) | 106 (66%) | 35 (22%) | |
| Pneumonia | Observation | 6 (22%) | 12 (44%) | 9 (34%) |
| Severity I | 33 (17%) | 68 (35%) | 93 (48%) | |
| Severity II | 37 (14%) | 152 (59%) | 69 (27%) | |
The PNP hospitalist program was evaluated by comparing patient records from the PNP team, the PNP/MD team, and the resident teams. Evaluation measures included compliance with specific components of the bronchiolitis and asthma CCGs, LOS, and cost of care.
Outcomes Measured
Quality measures for this program evaluation included compliance with the bronchiolitis CCG recommendation to diagnose bronchiolitis based on history and exam findings while minimizing the use of chest x‐ray and respiratory viral testing.[13] Current evidence suggests that these tests add cost and exposure to radiation and do not necessarily predict severity of disease or change medical management.[14] This program evaluation also measured compliance with the asthma CCG recommendation to give every asthma patient an asthma action plan (AAP) prior to hospital discharge.[15] Of note, this evaluation was completed prior to more recent evidence that questions the utility of AAP for improving asthma clinical outcomes.[16] There were no related measures for pneumonia available because there was no CCG in place at the time of this evaluation.
Outcomes measures for this evaluation included LOS and cost of care for the top 3 inpatient diagnoses: bronchiolitis, asthma, and pneumonia. LOS for the inpatient hospitalization was measured in hours. Direct cost of care was used for this analysis, which included medical supplies, pharmacy, radiology, laboratory, and bed charges. Nursing charges were also included in the direct cost due to the proximity of nursing cost to the patient, versus more distant costs such as infrastructure or administration. Hospitalist physician and NP salaries were not included in direct cost analysis. Outcomes were compared for the PNP team, the resident teams, and the PN/MD team in the NOC.
Analysis
Patients were summarized by diagnosis‐related groups (APR‐DRG) and severity of illness using counts and percentages across the PNP team, resident teams, and the PNP/MD team in the NOC (Table 1). LOS and direct cost is skewed, therefore natural log transformations were used to meet normal assumption for statistical testing and modeling. Chi squared and t tests were performed to compare outcomes between the PNP and resident physician teams, stratified by APR‐DRG. Analysis of variance was used to analyze LOS and direct cost for the top 3 APR‐DRG admission codes while adjusting for acuity. The outcomes were also compared pairwise among the 3 teams using a linear mixed model to adjust for APR‐DRG and severity of illness, treating severity as a nested effect within the APR‐DRG. Bonferroni corrections were used to adjust for multiple comparisons; a P value 0.017 was considered statistically significant. Post hoc power analysis was completed for the analysis of bronchiolitis chest x‐ray ordering, even though the sample size was relatively large (PNP team 128, resident team 204) (Table 1). There was a 7% difference between the PNP and resident groups, and the power of detecting a significant difference was 40%. A sample size of 482 for each group would be necessary to achieve 80% power of detecting a 7% difference, while controlling for 5% type I error. All statistical analyses were performed with SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
PNP adherence to CCGs was comparable to resident teams for the specific measures used in this evaluation. Based on a hospital‐wide goal of ordering diagnostic tests for less than 25% of inpatients with bronchiolitis, there was no significant difference between the PNP team and resident teams. There was no significant difference in the rate of chest x‐ray ordering between the PNP team and the resident teams (15% vs 22%, P = 0.1079). Similarly, there was no significant difference in viral testing between the PNP and physician teams (24% vs 25%, P = 0.9813) (Table 2). Post hoc power analysis indicated that a larger sample size would be required to increase the power of detecting a statistically significant difference in chest x‐ray ordering between these groups. The PNP and resident teams were also compared using compliance with the asthma CCGs, specifically related to the goal of providing an accurate AAP to every patient admitted for asthma. The PNP and resident teams had a similar rate of compliance, with PNPs achieving 81% compliance and MDs 76% (P = 0.4351) (Table 2).
| Clinical Care Guidelines | Diagnostic Test | PNP Team | Resident Teams | P Value |
|---|---|---|---|---|
| ||||
| Bronchiolitis care | Chest x‐ray | 15% | 22% | 0.1079 |
| Diagnostic testing | Viral test | 24% | 25% | 0.9813 |
| Completed asthma action plans | 81% | 76% | 0.4351 | |
LOS and direct costs were compared for the 3 teams for the top 3 APR‐DRGs and controlling for acuity. Table 3 illustrates that there were no significant differences in LOS between the PNP and resident teams or between the PNP and PNP/MD teams for these 3 APR‐DRGs (P 0.017 considered statistically significant). There was a statistically significant difference in LOS between resident and PNP/MD teams for asthma and pneumonia (P 0.001). The direct cost of care per patient encounter provided by the PNP team was significantly less than the PNP/MD team for all 3 APR‐DRGs (P 0.001). The direct cost of care per patient encounter provided by the PNP team was significantly less than the resident teams for asthma (P = 0.0021) and pneumonia (P = 0.0001), although the difference was not statistically significant for bronchiolitis (P = 0.0228) for level of significance P 0.0017 (Table 3, 4).
| PNP | Resident | PNP/MD | P Value PNP vs Resident | P Value
PNP vs PNP/MD |
P Value Resident vs PNP/MD | |
|---|---|---|---|---|---|---|
| ||||||
| Cost | ||||||
| Bronchiolitis | $2190 | $2513 | $3072 | 0.0228 | 0.0001 | 0.0002 |
| Asthma | $2089 | $2655 | $3220 | 0.0021 | 0.0001 | 0.0190 |
| Pneumonia | $2348 | $3185 | $3185 | 0.0001 | 0.0001 | 0.1142 |
| LOS, h | ||||||
| Bronchiolitis | 52 | 52 | 51 | 0.9112 | 0.1600 | 0.1728 |
| Asthma | 36 | 42 | 48 | 0.0158 | 0.3151 | 0.0001 |
| Pneumonia | 54 | 61 | 68 | 0.1136 | 0.1605 | 0.0001 |
| PNP | Resident | PNP/MD | PHIS Observation | PHIS SeverityIII | |
|---|---|---|---|---|---|
| |||||
| LOS, h | |||||
| Bronchiolitis | 52 | 52 | 51 | 43 | 70 |
| Asthma | 36 | 42 | 48 | 31 | 48 |
| Pneumonia | 54 | 61 | 68 | 46 | 64 |
Figure 1 illustrates the monthly patient census on the PNP and resident teams obtained from daily midnight census. There was a dramatic seasonal fluctuation in PNP team census, with a low census in July 2009 (22 patients) and high census in February 2010 (355 patients). The resident teams maintained a relatively stable census year round compared to the PNP team.

CONCLUSIONS/DISCUSSION
The results of this program evaluation suggest that the PNP team at CHCO provides inpatient care comparable to the resident teams at a lower cost per patient encounter for uncomplicated bronchiolitis, pneumonia, and asthma. The results of this program evaluation are consistent with previously published studies demonstrating that NPs improve outcomes such as decreased LOS and cost of care.[9]
In the setting of increasingly stringent restrictions in residency work hours, PNP hospitalists are a valuable resource for managing inpatient care. PNPs can provide additional benefits not explored in this program evaluation, such as increased access to care, increased patient and family satisfaction, improved documentation, and improved communication between nurses and physicians.[6] NP hospitalist providers can also decrease the patient care burden on housestaff, allowing teaching teams to focus on resident education.[6] This point could be made for the PNP team at CHCO, which contributed to care of inpatients during the peak respiratory season census. This strategy has allowed the resident teaching teams to maintain a more manageable patient census during the winter respiratory season, and presumably has allowed greater focus on resident education year round.[17]
Hospitals have been increasingly using evidence based CCGs as a strategy to improve patient outcomes and decrease LOS and cost.[18] CCGs provide an excellent tool for hospitalist physicians and APPs to deliver consistent inpatient care for common diagnoses such as bronchiolitis, asthma, and pneumonia. Increased reliance on CCGs has provided an opportunity to standardize evidence‐based practices and has allowed PNPs to expand their inpatient role at CHCO. The addition of a PNP inpatient team at CHCO also provided an effective strategy for management of seasonal fluctuations in inpatient census, particularly during the winter respiratory season.
Limitations
This is a single‐site program evaluation at a free standing children's hospital. Colorado law allows NPs to practice independently and obtain full prescriptive authority, although licensing and certification regulations for APPs vary from state to state. Our results may not be generalizable to other hospitals or to states where regulations differ. Patients admitted to the NOC sites and those assigned to the PNP team at the main campus are generally lower acuity and complexity compared to patients assigned to the resident teams at the main campus. Although we controlled for severity using the APR‐DRG severity classification, it is possible that our results were biased due to different patient profiles among the PNP and MD hospitalist teams. There were also potential limitations in the cost analysis, which included nursing in direct costs. Although nurse‐to‐patient ratios are comparable across hospitalist sites, the ratios may have varied due to fluctuations in patient census at each site. The CCG monitoring measures used in this evaluation also presented limitations. These measures were selected due to the availability of these data in the electronic medical record. Future studies may provide more clinically relevant information by including additional patient outcomes measures specifically related to inpatient medical management.
Despite the limitations in this program evaluation, we feel that these data add to the current knowledge in pediatrics by showing equipoise between these 2 groups. The PNP hospitalist role continues to evolve at CHCO, and the utility of this role must continue to be evaluated and reported.
Acknowledgements
Dashka Ranade provided Children's Hospital Colorado CCG comparison data for this program evaluation. David Bertoch provided LOS data from the Children's Hospital Association Pediatric Health Information System database.
Disclosures: Supported by NIH/NCATS Colorado CTSI grant number UL1 TR000154. The contents are the authors' sole responsibility and do not necessarily represent official NIH views.
The Accreditation Council for Graduate Medical Education implemented rules limiting work hours for residents in 2003 and 2011, decreasing the availability of residents as providers at teaching hospitals.[1] These restrictions have increased reliance on advance practice providers (APPs) including nurse practitioners (NPs) and physicians' assistants in providing inpatient care. The NP hospitalist role includes inpatient medical management, coordination of care, patient and staff education, and quality improvement activities.[2] The NP hospitalist role has expanded beyond a replacement for reduced resident work hours, adding value through resident teaching, development of clinical care guidelines (CCGs), continuity of care, and familiarity with inpatient management.[3] The NP hospitalist role has been shown to improve the quality, efficiency, and cost effectiveness of inpatient care.[4, 5]
Favorable quality and cost measure results have been documented for adult NP hospitalists compared to housestaff, including improved patient outcomes, increased patient and staff satisfaction, decreased length of stay (LOS) and cost of care, and improved access to care.[6] These findings are supported by NP inpatient program evaluations at several academic medical centers, which also show increased patient and family satisfaction and improved communication between physicians, nurses, and families.[6, 7, 8] One study demonstrated that collaborative care management of adult medical patients by a hospitalist physician and advanced practice nurse led to decreased LOS and improved hospital profit without changing patient readmission or mortality.[9] Although there is a growing body of evidence supporting the quality and cost effectiveness of the NP hospitalist role in adult inpatient care, there are little published data for pediatric programs.
METHODS
The pediatric nurse practitioner (PNP) hospitalist role at Children's Hospital Colorado (CHCO) was initiated in 2006 to meet the need for additional inpatient providers. Inpatient staffing challenges included decreased resident work hours as well as high inpatient volume during the winter respiratory season. The PNP hospitalist providers at CHCO independently manage care throughout hospitalization for patients within their scope of practice, and comanage more complex patients with the attending doctor of medicine (MD). The PNPs complete history and physical exams, order and interpret diagnostic tests, perform procedures, prescribe medications, and assist with discharge coordination. Patient populations within the PNP hospitalist scope of practice include uncomplicated bronchiolitis, pneumonia, and asthma.
The hospitalist section at CHCO's main campus includes 2 resident teams and 1 PNP team. The hospitalist section also provides inpatient care at several network of care (NOC) sites. These NOC sites are CHCO‐staffed facilities that are either freestanding or connected to a community hospital, with an emergency department and 6 to 8 inpatient beds. The PNP hospitalist role includes inpatient management at the CHCO main campus as well as in the NOC. The NOC sites are staffed with a PNP and MD team who work collaboratively to manage inpatient care. The Advanced Practice Hospitalist Program was implemented to improve staffing and maintain quality of patient care in a cost‐effective manner. We undertook a program evaluation with the goal of comparing quality and cost of care between the PNP team, PNP/MD team, and resident teams.
Administrative and electronic medical record data from July 1, 2009 through June 30, 2010 were reviewed retrospectively. Data were obtained from inpatient records at CHCO inpatient medical unit and inpatient satellite sites in the CHCO NOC. The 2008 versions 26 and 27 of the 3M All Patient Refined Diagnosis‐Related Groups (APR‐DRG) were used to categorize patients by diagnosis, severity of illness, and risk of mortality.[10, 11] The top 3 APR‐DRGs at CHCO, based on volume of inpatient admissions, were selected for this analysis, including bronchiolitis and RSV pneumonia (APR‐DRG 138), pneumonia NEC (APR‐DRG 139), and asthma (APR‐DRG 141) (N = 1664). These 3 diagnoses accounted for approximately 60% of all inpatient hospitalist encounters and comprised 78% of the PNP encounters, 52% of the resident encounters, and 76% of the PNP/MD encounters. APR‐DRG severity of illness categories include I, II, III, and IV (minor, moderate, major, and extreme, respectively).[12] Severity of illness levels I and II were used for this analysis. Severity III and IV levels were excluded due to lack of patients in these categories on the PNP team and in the NOC. We also included observation status patients. The PNP team accounted for approximately 20% of the inpatient encounters, with 45% on the resident teams and 35% on the PNP/MD team in the NOC (Table 1).
| Distribution of Patients | Patient Type/Severity of Illness | NP | Resident | PNP/MD |
|---|---|---|---|---|
| ||||
| Bronchiolitis | Observation | 26 (23%) | 32 (28%) | 55 (49%) |
| Severity I | 93 (29%) | 77 (24%) | 151 (47%) | |
| Severity II | 49 (24%) | 95 (47%) | 60 (29%) | |
| Asthma | Observation | 7 (14%) | 23 (45%) | 21 (41%) |
| Severity I | 48 (14%) | 191 (57%) | 97 (29%) | |
| Severity II | 19 (12%) | 106 (66%) | 35 (22%) | |
| Pneumonia | Observation | 6 (22%) | 12 (44%) | 9 (34%) |
| Severity I | 33 (17%) | 68 (35%) | 93 (48%) | |
| Severity II | 37 (14%) | 152 (59%) | 69 (27%) | |
The PNP hospitalist program was evaluated by comparing patient records from the PNP team, the PNP/MD team, and the resident teams. Evaluation measures included compliance with specific components of the bronchiolitis and asthma CCGs, LOS, and cost of care.
Outcomes Measured
Quality measures for this program evaluation included compliance with the bronchiolitis CCG recommendation to diagnose bronchiolitis based on history and exam findings while minimizing the use of chest x‐ray and respiratory viral testing.[13] Current evidence suggests that these tests add cost and exposure to radiation and do not necessarily predict severity of disease or change medical management.[14] This program evaluation also measured compliance with the asthma CCG recommendation to give every asthma patient an asthma action plan (AAP) prior to hospital discharge.[15] Of note, this evaluation was completed prior to more recent evidence that questions the utility of AAP for improving asthma clinical outcomes.[16] There were no related measures for pneumonia available because there was no CCG in place at the time of this evaluation.
Outcomes measures for this evaluation included LOS and cost of care for the top 3 inpatient diagnoses: bronchiolitis, asthma, and pneumonia. LOS for the inpatient hospitalization was measured in hours. Direct cost of care was used for this analysis, which included medical supplies, pharmacy, radiology, laboratory, and bed charges. Nursing charges were also included in the direct cost due to the proximity of nursing cost to the patient, versus more distant costs such as infrastructure or administration. Hospitalist physician and NP salaries were not included in direct cost analysis. Outcomes were compared for the PNP team, the resident teams, and the PN/MD team in the NOC.
Analysis
Patients were summarized by diagnosis‐related groups (APR‐DRG) and severity of illness using counts and percentages across the PNP team, resident teams, and the PNP/MD team in the NOC (Table 1). LOS and direct cost is skewed, therefore natural log transformations were used to meet normal assumption for statistical testing and modeling. Chi squared and t tests were performed to compare outcomes between the PNP and resident physician teams, stratified by APR‐DRG. Analysis of variance was used to analyze LOS and direct cost for the top 3 APR‐DRG admission codes while adjusting for acuity. The outcomes were also compared pairwise among the 3 teams using a linear mixed model to adjust for APR‐DRG and severity of illness, treating severity as a nested effect within the APR‐DRG. Bonferroni corrections were used to adjust for multiple comparisons; a P value 0.017 was considered statistically significant. Post hoc power analysis was completed for the analysis of bronchiolitis chest x‐ray ordering, even though the sample size was relatively large (PNP team 128, resident team 204) (Table 1). There was a 7% difference between the PNP and resident groups, and the power of detecting a significant difference was 40%. A sample size of 482 for each group would be necessary to achieve 80% power of detecting a 7% difference, while controlling for 5% type I error. All statistical analyses were performed with SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
PNP adherence to CCGs was comparable to resident teams for the specific measures used in this evaluation. Based on a hospital‐wide goal of ordering diagnostic tests for less than 25% of inpatients with bronchiolitis, there was no significant difference between the PNP team and resident teams. There was no significant difference in the rate of chest x‐ray ordering between the PNP team and the resident teams (15% vs 22%, P = 0.1079). Similarly, there was no significant difference in viral testing between the PNP and physician teams (24% vs 25%, P = 0.9813) (Table 2). Post hoc power analysis indicated that a larger sample size would be required to increase the power of detecting a statistically significant difference in chest x‐ray ordering between these groups. The PNP and resident teams were also compared using compliance with the asthma CCGs, specifically related to the goal of providing an accurate AAP to every patient admitted for asthma. The PNP and resident teams had a similar rate of compliance, with PNPs achieving 81% compliance and MDs 76% (P = 0.4351) (Table 2).
| Clinical Care Guidelines | Diagnostic Test | PNP Team | Resident Teams | P Value |
|---|---|---|---|---|
| ||||
| Bronchiolitis care | Chest x‐ray | 15% | 22% | 0.1079 |
| Diagnostic testing | Viral test | 24% | 25% | 0.9813 |
| Completed asthma action plans | 81% | 76% | 0.4351 | |
LOS and direct costs were compared for the 3 teams for the top 3 APR‐DRGs and controlling for acuity. Table 3 illustrates that there were no significant differences in LOS between the PNP and resident teams or between the PNP and PNP/MD teams for these 3 APR‐DRGs (P 0.017 considered statistically significant). There was a statistically significant difference in LOS between resident and PNP/MD teams for asthma and pneumonia (P 0.001). The direct cost of care per patient encounter provided by the PNP team was significantly less than the PNP/MD team for all 3 APR‐DRGs (P 0.001). The direct cost of care per patient encounter provided by the PNP team was significantly less than the resident teams for asthma (P = 0.0021) and pneumonia (P = 0.0001), although the difference was not statistically significant for bronchiolitis (P = 0.0228) for level of significance P 0.0017 (Table 3, 4).
| PNP | Resident | PNP/MD | P Value PNP vs Resident | P Value
PNP vs PNP/MD |
P Value Resident vs PNP/MD | |
|---|---|---|---|---|---|---|
| ||||||
| Cost | ||||||
| Bronchiolitis | $2190 | $2513 | $3072 | 0.0228 | 0.0001 | 0.0002 |
| Asthma | $2089 | $2655 | $3220 | 0.0021 | 0.0001 | 0.0190 |
| Pneumonia | $2348 | $3185 | $3185 | 0.0001 | 0.0001 | 0.1142 |
| LOS, h | ||||||
| Bronchiolitis | 52 | 52 | 51 | 0.9112 | 0.1600 | 0.1728 |
| Asthma | 36 | 42 | 48 | 0.0158 | 0.3151 | 0.0001 |
| Pneumonia | 54 | 61 | 68 | 0.1136 | 0.1605 | 0.0001 |
| PNP | Resident | PNP/MD | PHIS Observation | PHIS SeverityIII | |
|---|---|---|---|---|---|
| |||||
| LOS, h | |||||
| Bronchiolitis | 52 | 52 | 51 | 43 | 70 |
| Asthma | 36 | 42 | 48 | 31 | 48 |
| Pneumonia | 54 | 61 | 68 | 46 | 64 |
Figure 1 illustrates the monthly patient census on the PNP and resident teams obtained from daily midnight census. There was a dramatic seasonal fluctuation in PNP team census, with a low census in July 2009 (22 patients) and high census in February 2010 (355 patients). The resident teams maintained a relatively stable census year round compared to the PNP team.

CONCLUSIONS/DISCUSSION
The results of this program evaluation suggest that the PNP team at CHCO provides inpatient care comparable to the resident teams at a lower cost per patient encounter for uncomplicated bronchiolitis, pneumonia, and asthma. The results of this program evaluation are consistent with previously published studies demonstrating that NPs improve outcomes such as decreased LOS and cost of care.[9]
In the setting of increasingly stringent restrictions in residency work hours, PNP hospitalists are a valuable resource for managing inpatient care. PNPs can provide additional benefits not explored in this program evaluation, such as increased access to care, increased patient and family satisfaction, improved documentation, and improved communication between nurses and physicians.[6] NP hospitalist providers can also decrease the patient care burden on housestaff, allowing teaching teams to focus on resident education.[6] This point could be made for the PNP team at CHCO, which contributed to care of inpatients during the peak respiratory season census. This strategy has allowed the resident teaching teams to maintain a more manageable patient census during the winter respiratory season, and presumably has allowed greater focus on resident education year round.[17]
Hospitals have been increasingly using evidence based CCGs as a strategy to improve patient outcomes and decrease LOS and cost.[18] CCGs provide an excellent tool for hospitalist physicians and APPs to deliver consistent inpatient care for common diagnoses such as bronchiolitis, asthma, and pneumonia. Increased reliance on CCGs has provided an opportunity to standardize evidence‐based practices and has allowed PNPs to expand their inpatient role at CHCO. The addition of a PNP inpatient team at CHCO also provided an effective strategy for management of seasonal fluctuations in inpatient census, particularly during the winter respiratory season.
Limitations
This is a single‐site program evaluation at a free standing children's hospital. Colorado law allows NPs to practice independently and obtain full prescriptive authority, although licensing and certification regulations for APPs vary from state to state. Our results may not be generalizable to other hospitals or to states where regulations differ. Patients admitted to the NOC sites and those assigned to the PNP team at the main campus are generally lower acuity and complexity compared to patients assigned to the resident teams at the main campus. Although we controlled for severity using the APR‐DRG severity classification, it is possible that our results were biased due to different patient profiles among the PNP and MD hospitalist teams. There were also potential limitations in the cost analysis, which included nursing in direct costs. Although nurse‐to‐patient ratios are comparable across hospitalist sites, the ratios may have varied due to fluctuations in patient census at each site. The CCG monitoring measures used in this evaluation also presented limitations. These measures were selected due to the availability of these data in the electronic medical record. Future studies may provide more clinically relevant information by including additional patient outcomes measures specifically related to inpatient medical management.
Despite the limitations in this program evaluation, we feel that these data add to the current knowledge in pediatrics by showing equipoise between these 2 groups. The PNP hospitalist role continues to evolve at CHCO, and the utility of this role must continue to be evaluated and reported.
Acknowledgements
Dashka Ranade provided Children's Hospital Colorado CCG comparison data for this program evaluation. David Bertoch provided LOS data from the Children's Hospital Association Pediatric Health Information System database.
Disclosures: Supported by NIH/NCATS Colorado CTSI grant number UL1 TR000154. The contents are the authors' sole responsibility and do not necessarily represent official NIH views.
- Education ACfGM. Common Program Requirements. Accreditation Council for Graduate Medical Education, 2011.
- , , , , , . Hospitalist services: an evolving opportunity. Nurse Pract. 2008;33(5):9–10.
- . APRN hospitalist: just a resident replacement? J Pediatr Health Care. 2004;18(4):208–210.
- , , , . Evaluation of the role of the pediatric nurse practitioner in an inpatient asthma program. J Pediatr Health Care. 2008;22(5):273–281.
- , . Acute care nurse practitioner as hospitalist: role description. AACN Adv Crit Care. 2009;20(2):133–136.
- , . Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service. Am J Crit Care. 2002;11(5):448–458.
- , , , et al. Pediatric trauma nurse practitioners provide excellent care with superior patient satisfaction for injured children. J Pediatr Surg. 2006;41(1):277–281.
- , , , et al. Pediatric trauma nurse practitioners increase bedside nurses' satisfaction with pediatric trauma patient care. J Trauma Nurs. 2006;13(2):66–69.
- , , , et al. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J Nurs Adm. 2006;36(2):79–85.
- , , , , . A closer look at all‐patient refined DRGs. J AHIMA. 2002;73(1):46–50.
- . Structure and performance of different DRG classification systems for neonatal medicine. Pediatrics. 1999;103(1 suppl E):302–318.
- Association CsH. Patient classification system, Children's Hospital Association. Available at: http://www.childrenshospitals.org/. Accessed January 4, 2014.
- . Children's Hospital Colorado bronchiolitis clinical care guideline, Bronchiolitis CCG Task Force 2011. Available at: http://www.childrenscolorado.org/conditions/lung/healthcare_professionals/clinical_care_guidelines.aspx. Accessed January 4, 2014.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793.
- .Children's Hospital Colorado asthma clinical care guideline, Asthma Task Force, 2011. Available at: http://www.childrenscolorado.org/conditions/lung/healthcare_professionals/clinical_care_guidelines.aspx. Accessed January 4, 2014.
- , , . Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;(3):CD005306.
- , . Pediatric nurse practitioners as hospitalists. J Pediatr Health Care. 2010;24(5):347–350.
- , , . Health policy issues and applications for evidence‐based medicine and clinical practice guidelines. Health Policy. 1998;46(1):1–19.
- Education ACfGM. Common Program Requirements. Accreditation Council for Graduate Medical Education, 2011.
- , , , , , . Hospitalist services: an evolving opportunity. Nurse Pract. 2008;33(5):9–10.
- . APRN hospitalist: just a resident replacement? J Pediatr Health Care. 2004;18(4):208–210.
- , , , . Evaluation of the role of the pediatric nurse practitioner in an inpatient asthma program. J Pediatr Health Care. 2008;22(5):273–281.
- , . Acute care nurse practitioner as hospitalist: role description. AACN Adv Crit Care. 2009;20(2):133–136.
- , . Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service. Am J Crit Care. 2002;11(5):448–458.
- , , , et al. Pediatric trauma nurse practitioners provide excellent care with superior patient satisfaction for injured children. J Pediatr Surg. 2006;41(1):277–281.
- , , , et al. Pediatric trauma nurse practitioners increase bedside nurses' satisfaction with pediatric trauma patient care. J Trauma Nurs. 2006;13(2):66–69.
- , , , et al. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J Nurs Adm. 2006;36(2):79–85.
- , , , , . A closer look at all‐patient refined DRGs. J AHIMA. 2002;73(1):46–50.
- . Structure and performance of different DRG classification systems for neonatal medicine. Pediatrics. 1999;103(1 suppl E):302–318.
- Association CsH. Patient classification system, Children's Hospital Association. Available at: http://www.childrenshospitals.org/. Accessed January 4, 2014.
- . Children's Hospital Colorado bronchiolitis clinical care guideline, Bronchiolitis CCG Task Force 2011. Available at: http://www.childrenscolorado.org/conditions/lung/healthcare_professionals/clinical_care_guidelines.aspx. Accessed January 4, 2014.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793.
- .Children's Hospital Colorado asthma clinical care guideline, Asthma Task Force, 2011. Available at: http://www.childrenscolorado.org/conditions/lung/healthcare_professionals/clinical_care_guidelines.aspx. Accessed January 4, 2014.
- , , . Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;(3):CD005306.
- , . Pediatric nurse practitioners as hospitalists. J Pediatr Health Care. 2010;24(5):347–350.
- , , . Health policy issues and applications for evidence‐based medicine and clinical practice guidelines. Health Policy. 1998;46(1):1–19.
Bridging the Inpatient–Outpatient Divide
There is consensus that the hospital is an appropriate place to start chronic medications for conditions that caused the hospitalization (e.g., aspirin for a patient admitted with acute myocardial infarction). However, little is known about physician attitudes toward starting chronic medications for conditions unrelated to the reason for hospitalization (e.g., aspirin in a patient with a history of myocardial infarction admitted for cellulitis). Although hospitalists can identify and remedy potential gaps in the management of chronic conditions, changes in such medications during the hospital stay can create a number of problems. Contextual factors, such as prior medication trials, patient preferences, and longstanding patterns of disease management, may be unknown to the inpatient clinician, and medication confusion, nonadherence, and adverse effects can result from multiple medication changes.[1, 2] The lack of consensus about changing chronic medications for conditions unrelated to the reason for admission reflects a lack of clarity regarding the risk‐benefit equation in this area.
The study by Breu and colleagues[3] in this issue provides one of the first studies of hospitalist and primary care physician (PCP) attitudes about changing chronic medications during hospitalization for conditions unrelated to the reason for admission. The authors had hospitalists and PCPs consider six cases, half involving a medication change related to the reason for admission and half involving a medication change unrelated to the reason for admission. They found that PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate when unrelated to the reason for admission. However, the majority of both hospitalists and PCPs did not feel interventions in these cases were appropriate.
Although this study provides useful insight into the attitudes of physicians toward these issues, it is likely that even more physicians would be skeptical of initiating chronic medications in the hospital if the scenarios reflected the messy reality that often faces clinicians when patients are hospitalized. The study asked physician respondents to assume full outpatient electronic medical record (EMR) access and communication at discharge. However, in practice, inpatient physicians often do not have full outpatient EMR access. If they do have full access to records, they typically do not have the time to thoroughly review the chart, leading to over half of internal medicine patients having at least one medication discrepancy at admission.[4] In addition, communication between hospitalists and PCPs occurs infrequently, and discharge summaries are often not available by the time of the first postdischarge clinic visit and lack important information, such as diagnostic test results and discharge medications.[2]
We believe that in most clinical settings, the serious problems that accompany changing medications in hospitalized patients argue for a judicious approach to modifying medications for chronic conditions not related to the reason for hospitalization. However, the more important question is how the prescribing process in hospitalized patients can be re‐envisioned in a manner that allows individualization of these decisions to serve both the short‐ and long‐term needs of patients. Because the success and appropriateness of long‐term treatment decisions often depends on contextual factors, PCP follow‐up, and patient medication compliance, in most cases decisions about initiating long‐term therapy for conditions not central to the hospital admission should involve each of these circumstances. Shared decision making models involve clinicians and patients sharing information, expressing treatment preferences, deliberating the options, and coming to an agreement on a treatment plan,[5] and these models have been associated with improved adherence and disease‐specific outcomes.[6] Shared decision making in many cases could be done quickly and efficiently through a quick check‐in with the PCP and a brief discussion with the patient. When consensus cannot be reached with these methods, then raising the issue with the PCP and patient but deferring the final decision until after discharge would be appropriate.
In hospitalized patients, less is often more, and minimizing the number of nonessential medication changes may ultimately yield better outcomes. Although inpatient clinicians can identify important gaps in care, the best solutions come from discussions that can bridge the inpatient‐outpatient divide and ultimately serve the long‐term needs of patients.
Disclosures
The authors are supported by the National Institutes of Health and the American Federation for Aging Research (1K23‐AG030999) and the Department of Veterans Affairs Quality Scholars Program.
- , , , , . Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20:317–323.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hospital Med. 2007;2:314–323.
- , , , , . Hospitalist and primary care physician perspectives on medication management of chronic conditions for hospitalized patients. J Hosp Med. 2014;9(5):303–309.
- , , , , , . Inpatient medication reconciliation at admission and discharge: a retrospective cohort study of age and other risk factors for medication discrepancies. Am J Geriatr Pharmacother. 2010;8:115–126.
- , , , , . Doctor‐patient communication about drugs: the evidence for shared decision making. Soc Sci Med. 2000;50:829–840.
- , , , et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181:566–577.
There is consensus that the hospital is an appropriate place to start chronic medications for conditions that caused the hospitalization (e.g., aspirin for a patient admitted with acute myocardial infarction). However, little is known about physician attitudes toward starting chronic medications for conditions unrelated to the reason for hospitalization (e.g., aspirin in a patient with a history of myocardial infarction admitted for cellulitis). Although hospitalists can identify and remedy potential gaps in the management of chronic conditions, changes in such medications during the hospital stay can create a number of problems. Contextual factors, such as prior medication trials, patient preferences, and longstanding patterns of disease management, may be unknown to the inpatient clinician, and medication confusion, nonadherence, and adverse effects can result from multiple medication changes.[1, 2] The lack of consensus about changing chronic medications for conditions unrelated to the reason for admission reflects a lack of clarity regarding the risk‐benefit equation in this area.
The study by Breu and colleagues[3] in this issue provides one of the first studies of hospitalist and primary care physician (PCP) attitudes about changing chronic medications during hospitalization for conditions unrelated to the reason for admission. The authors had hospitalists and PCPs consider six cases, half involving a medication change related to the reason for admission and half involving a medication change unrelated to the reason for admission. They found that PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate when unrelated to the reason for admission. However, the majority of both hospitalists and PCPs did not feel interventions in these cases were appropriate.
Although this study provides useful insight into the attitudes of physicians toward these issues, it is likely that even more physicians would be skeptical of initiating chronic medications in the hospital if the scenarios reflected the messy reality that often faces clinicians when patients are hospitalized. The study asked physician respondents to assume full outpatient electronic medical record (EMR) access and communication at discharge. However, in practice, inpatient physicians often do not have full outpatient EMR access. If they do have full access to records, they typically do not have the time to thoroughly review the chart, leading to over half of internal medicine patients having at least one medication discrepancy at admission.[4] In addition, communication between hospitalists and PCPs occurs infrequently, and discharge summaries are often not available by the time of the first postdischarge clinic visit and lack important information, such as diagnostic test results and discharge medications.[2]
We believe that in most clinical settings, the serious problems that accompany changing medications in hospitalized patients argue for a judicious approach to modifying medications for chronic conditions not related to the reason for hospitalization. However, the more important question is how the prescribing process in hospitalized patients can be re‐envisioned in a manner that allows individualization of these decisions to serve both the short‐ and long‐term needs of patients. Because the success and appropriateness of long‐term treatment decisions often depends on contextual factors, PCP follow‐up, and patient medication compliance, in most cases decisions about initiating long‐term therapy for conditions not central to the hospital admission should involve each of these circumstances. Shared decision making models involve clinicians and patients sharing information, expressing treatment preferences, deliberating the options, and coming to an agreement on a treatment plan,[5] and these models have been associated with improved adherence and disease‐specific outcomes.[6] Shared decision making in many cases could be done quickly and efficiently through a quick check‐in with the PCP and a brief discussion with the patient. When consensus cannot be reached with these methods, then raising the issue with the PCP and patient but deferring the final decision until after discharge would be appropriate.
In hospitalized patients, less is often more, and minimizing the number of nonessential medication changes may ultimately yield better outcomes. Although inpatient clinicians can identify important gaps in care, the best solutions come from discussions that can bridge the inpatient‐outpatient divide and ultimately serve the long‐term needs of patients.
Disclosures
The authors are supported by the National Institutes of Health and the American Federation for Aging Research (1K23‐AG030999) and the Department of Veterans Affairs Quality Scholars Program.
There is consensus that the hospital is an appropriate place to start chronic medications for conditions that caused the hospitalization (e.g., aspirin for a patient admitted with acute myocardial infarction). However, little is known about physician attitudes toward starting chronic medications for conditions unrelated to the reason for hospitalization (e.g., aspirin in a patient with a history of myocardial infarction admitted for cellulitis). Although hospitalists can identify and remedy potential gaps in the management of chronic conditions, changes in such medications during the hospital stay can create a number of problems. Contextual factors, such as prior medication trials, patient preferences, and longstanding patterns of disease management, may be unknown to the inpatient clinician, and medication confusion, nonadherence, and adverse effects can result from multiple medication changes.[1, 2] The lack of consensus about changing chronic medications for conditions unrelated to the reason for admission reflects a lack of clarity regarding the risk‐benefit equation in this area.
The study by Breu and colleagues[3] in this issue provides one of the first studies of hospitalist and primary care physician (PCP) attitudes about changing chronic medications during hospitalization for conditions unrelated to the reason for admission. The authors had hospitalists and PCPs consider six cases, half involving a medication change related to the reason for admission and half involving a medication change unrelated to the reason for admission. They found that PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate when unrelated to the reason for admission. However, the majority of both hospitalists and PCPs did not feel interventions in these cases were appropriate.
Although this study provides useful insight into the attitudes of physicians toward these issues, it is likely that even more physicians would be skeptical of initiating chronic medications in the hospital if the scenarios reflected the messy reality that often faces clinicians when patients are hospitalized. The study asked physician respondents to assume full outpatient electronic medical record (EMR) access and communication at discharge. However, in practice, inpatient physicians often do not have full outpatient EMR access. If they do have full access to records, they typically do not have the time to thoroughly review the chart, leading to over half of internal medicine patients having at least one medication discrepancy at admission.[4] In addition, communication between hospitalists and PCPs occurs infrequently, and discharge summaries are often not available by the time of the first postdischarge clinic visit and lack important information, such as diagnostic test results and discharge medications.[2]
We believe that in most clinical settings, the serious problems that accompany changing medications in hospitalized patients argue for a judicious approach to modifying medications for chronic conditions not related to the reason for hospitalization. However, the more important question is how the prescribing process in hospitalized patients can be re‐envisioned in a manner that allows individualization of these decisions to serve both the short‐ and long‐term needs of patients. Because the success and appropriateness of long‐term treatment decisions often depends on contextual factors, PCP follow‐up, and patient medication compliance, in most cases decisions about initiating long‐term therapy for conditions not central to the hospital admission should involve each of these circumstances. Shared decision making models involve clinicians and patients sharing information, expressing treatment preferences, deliberating the options, and coming to an agreement on a treatment plan,[5] and these models have been associated with improved adherence and disease‐specific outcomes.[6] Shared decision making in many cases could be done quickly and efficiently through a quick check‐in with the PCP and a brief discussion with the patient. When consensus cannot be reached with these methods, then raising the issue with the PCP and patient but deferring the final decision until after discharge would be appropriate.
In hospitalized patients, less is often more, and minimizing the number of nonessential medication changes may ultimately yield better outcomes. Although inpatient clinicians can identify important gaps in care, the best solutions come from discussions that can bridge the inpatient‐outpatient divide and ultimately serve the long‐term needs of patients.
Disclosures
The authors are supported by the National Institutes of Health and the American Federation for Aging Research (1K23‐AG030999) and the Department of Veterans Affairs Quality Scholars Program.
- , , , , . Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20:317–323.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hospital Med. 2007;2:314–323.
- , , , , . Hospitalist and primary care physician perspectives on medication management of chronic conditions for hospitalized patients. J Hosp Med. 2014;9(5):303–309.
- , , , , , . Inpatient medication reconciliation at admission and discharge: a retrospective cohort study of age and other risk factors for medication discrepancies. Am J Geriatr Pharmacother. 2010;8:115–126.
- , , , , . Doctor‐patient communication about drugs: the evidence for shared decision making. Soc Sci Med. 2000;50:829–840.
- , , , et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181:566–577.
- , , , , . Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20:317–323.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hospital Med. 2007;2:314–323.
- , , , , . Hospitalist and primary care physician perspectives on medication management of chronic conditions for hospitalized patients. J Hosp Med. 2014;9(5):303–309.
- , , , , , . Inpatient medication reconciliation at admission and discharge: a retrospective cohort study of age and other risk factors for medication discrepancies. Am J Geriatr Pharmacother. 2010;8:115–126.
- , , , , . Doctor‐patient communication about drugs: the evidence for shared decision making. Soc Sci Med. 2000;50:829–840.
- , , , et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181:566–577.
Management of Chronic Conditions
Over the past 2 decades, the care of the hospitalized patient has changed dramatically. Hospitalists now account for the care of more than one‐third of general medicine inpatients, and this number is likely to grow.[1] The emergence of hospital medicine has resulted in a partnership between primary care physicians (PCPs) and hospitalists wherein hospitalists focus on acute medical issues requiring hospitalization, whereas more chronic issues unrelated to the reason for hospitalization remain largely the domain of the PCP.[2, 3]
However, several evolving financial and quality incentives have already begun to blur the distinction between inpatient and outpatient care. First, as private and public payers increasingly scrutinize readmission rates, it has become clear that the responsibility for patient outcomes extends beyond the day of discharge.[4] The birth of Accountable Care Organizations and patient‐centered medical homes may further blur distinctions between what has traditionally constituted inpatient and outpatient care.[5] Bundled payments may force providers to ensure that each visit, whether hospital‐ or clinic‐based, is taken as an opportunity to enact meaningful change.[6] The Centers for Medicare and Medicaid Services (CMS) are already tracking hospital performance on institution of medical therapy for certain conditions regardless of their relatedness to the reason for hospitalization.[7]
No published literature has yet examined the attitudes of inpatient and outpatient providers regarding this issue. Through a case‐based survey conducted at 3 large urban academic medical centers, we aimed to assess opinions among hospitalists and PCPs regarding the role of hospitalists in the management of conditions unrelated to the reason for admission. Our study had 2 main objectives: (1) to determine whether surveyed physicians were more likely to rate an inpatient intervention as appropriate when it related to the reason for admission as compared to interventions unrelated to the reason for admission; and (2) to determine whether these attitudes differed between PCPs and hospitalists.
METHODS
Setting and Subjects
We surveyed hospitalists and hospital‐based PCPs at Beth Israel Deaconess Medical Center (BIDMC), Brigham and Women's Hospital, and Massachusetts General Hospital, 3 large academic medical centers in Boston, Massachusetts. Each hospitalist group includes both teaching and nonteaching services and admits patients from both the surveyed hospital‐based PCP groups and other nonhospital‐based PCP groups. All 3 study sites use electronic medical records with patient information for each hospital‐based PCP available to treating hospitalists.
Survey Design
Using a commercially available online product (SurveyMonkey, Palo Alto, CA), we created a 3‐part case‐based survey instrument. The first section included demographic questions regarding age, sex, primary clinical role (hospitalist or PCP), prior experience as a PCP (for hospitalists only) or a hospitalist (for PCPs only; defined as a position with >30% of clinical time as the attending of record in the inpatient setting), years of clinical experience, and hospital affiliation.
The second section aimed to indirectly assess physician opinions on the appropriateness of inpatient management of conditions unrelated to the reason for admission. It consisted of 6 paired case scenarios, each with an inpatient management decision for a hypothetical hospitalist (Table 1). For each pair, 1 case dealt with management of the condition prompting admission (eg, starting aspirin in a patient admitted with acute nonST‐elevation myocardial infarction). The partner case involved the same intervention (eg, starting aspirin) but for a patient with a chronic condition (eg, history of prior myocardial infarction) and an alternate admitting diagnosis (eg, cellulitis). In an attempt to mitigate concerns regarding the flow of information and communication between providers, the survey asked respondents to assume that the hospitalist has access to the patient's outpatient electronic medical record, and that the hospitalist communicates the details of any hospitalizations at the time of discharge. For each case, the physician was asked to rate the appropriateness of enacting the intervention without discussing it with the PCP on a 5‐point scale from very inappropriate to very appropriate. When a physician answered that an intervention was inappropriate or very inappropriate, an additional question soliciting reasons for inappropriateness was included, with multiple predefined answer choices, as well as the option of a free‐text reply under the other designation.
| |
| Starting aspirin (related to the reason for admission) | A 60‐year‐old patient is admitted with a nonST‐elevation MI, medically managed without cardiac catheterization or percutaneous coronary intervention. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting aspirin (unrelated to the reason for admission) | A 60‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed is admitted to the hospital for treatment of cellulitis. The hospitalist notes the patient is not on aspirin at home. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting spironolactone (related to the reason for admission) | A 70‐year‐old patient with a past medical history significant for NYHA class II congestive heart failure (LVEF of 20%) is admitted for acute on chronic, left‐sided systolic congestive heart failure. The patient has been maintained on furosemide, metoprolol, and lisinopril. Admission serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting spironolactone (unrelated to the reason for admission) | A 70‐year‐old patient with a past history of NYHA class II congestive heart failure (LVEF of 20%) on furosemide, metoprolol, and lisinopril is admitted with pneumonia. Serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting warfarin (related to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension and diabetes is admitted with new atrial fibrillation. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Starting warfarin (unrelated to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension, diabetes, and atrial fibrillation is admitted with pneumonia. The patient is not anticoagulation therapy. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Stopping proton pump inhibitor (related to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of Clostridium difficile colitis. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping proton pump inhibitor (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of a urinary tract infection. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping statin or fibrate (related to the reason for admission) | A 60‐year‐old patient with a history of hyperlipidemia is admitted with an elevated creatine kinase to 5000. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent serum LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Stopping statin or fibrate (unrelated to the reason for admission) | A 60‐year‐old patient is admitted with an acute diarrheal illness. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Changing statin (related to the reason for admission) | A 65‐year‐old patient with a past medical history of hyperlipidemia on maximum‐dose simvastatin is admitted with a nonST‐elevation MI. The patient's cholesterol is noted to be above goal. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
| Changing statin (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed and hyperlipidemia on maximum‐dose simvastatin is admitted with pneumonia. Incidentally, the hospitalist notes that the patient's cholesterol has been above goal for the last 2 years. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
The third section aimed to directly assess physicians' opinions. It consisted of questions regarding the appropriateness of inpatient management of conditions related to and unrelated to a patient's reason for admission.
Prior to administration, we conducted focus groups of hospitalists and PCPs to help hypothesize current physician perceptions on inpatient management, assess physician understanding of survey cases and questions, and to evaluate survey length.
Survey Administration
Between October 23, 2012 and November 10, 2012, 3 emails containing a link to the online survey were sent to all hospitalist and hospital‐based PCPs at the 3 study institutions. The BIDMC Committee on Clinical Investigations, to whom authority was ceded by the remaining 2 study institutions, certified this research protocol as exempt.
Statistical Analysis
We hypothesized that respondents as a whole would be more likely to rate an intervention as appropriate or very appropriate if it was related to the reason for admission, compared to unrelated, and that there would be no difference between PCPs and hospitalists.
We used 2 and Fisher exact tests (where applicable) to compare categorical variables, and a nonparametric median test for continuous variables. We used the Fisher exact test to compare the percent of respondents rating each intervention as appropriate or very appropriate by relatedness or unrelatedness to the reason for admission, and by PCP vs hospitalist. To derive the relative risk (RR) of rating each intervention as appropriate or very appropriate by PCPs compared to hospitalists, adjusting for potential confounders including years out of residency and sex, we used multivariable generalized estimating equation models, each with a Poisson distribution error term, a log link, and an exchangeable working correlation structure to account for dependency of observations arising from clustering at either the hospital or participant level, depending on the comparison: for comparisons within a given case, we controlled for clustering at the hospital level; for comparisons of cases in aggregate, owing to multiple responses from each participant, we controlled for clustering at the individual level.
Assuming a 50% response rate from both PCPs and hospitalists, and that 50% of PCPs would rate a given intervention as appropriate, we calculated that we would have 90% power to detect a 50% increase in the proportion of hospitalists rating an intervention as appropriate as compared to PCPs, using an of .05.
RESULTS
Demographics
One hundred sixty‐two out of 295 providers (55%) responded to the survey (Table 2). The response rate did not differ between hospitalists (70 out of 128; 55%) and PCPs (92 out of 167; 55%). Female respondents made up 58.7% of the PCP and 50.0% of the hospitalist groups (P=0.34). On average, PCPs were older (P<0.001) with a greater median number of years since graduation from residency (P<0.001). A greater percentage of hospitalists spent more than three‐quarters of their time clinically (42.9% vs 19.6%, P=0.009).
| Total, n=162 (100.0%) | PCP, n=92 (6.8%) | Hospitalist, n=70 (43.2%) | P Valuea | |
|---|---|---|---|---|
| ||||
| Hospital, n (%) | ||||
| BIDMC | 79 (48.8) | 48 (60.8) | 31 (39.2) | 0.115 |
| BWH | 36 (22.2) | 15 (41.7) | 21 (58.3) | |
| MGH | 47 (29.0) | 29 (61.7) | 18 (38.3) | |
| Sex, n (%) | ||||
| Male | 73 (45.1) | 38 (41.3) | 35 (50.0) | 0.339 |
| Female | 89 (54.9) | 54 (58.7) | 35 (50.0) | |
| Age interval, y, n (%) | ||||
| 2534 | 36 (22.2) | 9 (9.8) | 27 (38.6) | <0.001 |
| 3544 | 67 (41.4) | 34 (37.0) | 33 (47.1) | |
| 4554 | 35 (21.6) | 29 (31.5) | 6 (8.6) | |
| 5564 | 19 (11.7) | 16 (17.4) | 3 (4.3) | |
| 6574 | 5 (3.1) | 4 (4.4) | 1 (1.4) | |
| Years out of residency, median (IQR) | 10 (417) | 15 (74) | 5 (211) | <0.001 |
| Clinical FTE, n (%) | ||||
| 0.25 | 30 (18.6) | 22 (23.9) | 8 (11.4) | 0.009 |
| 0.260.50 | 41 (25.3) | 25 (27.2) | 16 (22.9) | |
| 0.510.75 | 43 (26.5) | 27 (29.4) | 16 (22.9) | |
| >0.75 | 48 (29.6) | 18 (19.6) | 30 (42.9) | |
| Worked as PCP?b | ||||
| Yes | 6 (8.6) | |||
| No | 64 (91.4) | |||
| Worked as hospitalist? | ||||
| Yes | 11 (12.0) | |||
| No | 81 (88.0) | |||
| AOR for admitted patients | ||||
| Always | 16 (17.4) | |||
| Mostly | 8 (8.7) | |||
| Rarely | 7 (7.6) | |||
| Never | 60 (65.2) | |||
Appropriateness of Inpatient Management Based on Admitting Diagnosis
For each of the 6 case pairings individually and in aggregate, respondents were significantly more likely to deem the intervention appropriate or very appropriate if it was related to the reason for admission, compared to those interventions unrelated to the reason for admission (in aggregate, 78.9% vs 38.8% respectively, P<0.001). For example, whereas 96.9% felt that the addition of aspirin in a patient admitted with acute myocardial infarction (MI) was appropriate, only 54.3% felt it appropriate to start aspirin in a patient with a prior history of MI admitted with cellulitis (P<0.001). Significant differences (all P values <0.001) were seen for all case pairs: starting spironolactone (68.1% when related to the reason for reason for admission vs 43.1% when unrelated to reason for admission); starting warfarin (62.3% vs 23.3%), stopping proton pump inhibitor (72.3% vs 42.8%), stopping statin or fibrate (90.6% vs 28.3%), and changing statin (83.0% vs 40.5%).
Appropriateness of Inpatient Management based on Primary Role
Table 3 compares the percent of PCPs and hospitalists rating each intervention as appropriate or very appropriate, by relatedness of the intervention to the reason for admission. In both unadjusted and adjusted comparisons for all cases in aggregate, PCPs were significantly more likely than hospitalists to rate the inpatient interventions as appropriate or very appropriate when the intervention was related to the reason for admission (83.4% of PCP responses vs 73.0% of hospitalist responses, P<0.001; RR: 1.2, 95% confidence interval [CI]: 1.11.3), unrelated to the reason for admission (44.7% vs 31.1%, P<0.001; RR: 1.5, 95% CI: 1.11.9), and overall (64.1% vs 52.1%, P<0.001; RR: 1.3, 95% CI: 1.11.4).
| Relationship to Admission Diagnosis | PCP, n (%) | Hospitalist, n (%) | P Value | Adjusted RR | 95% CI |
|---|---|---|---|---|---|
| |||||
| Related | 453 (83.4) | 303 (73.0) | <0.001 | 1.2a | 1.11.3 |
| Unrelated | 242 (44.7) | 129 (31.1) | <0.001 | 1.5a | 1.11.9 |
| Overall | 695 (64.1) | 432 (52.1) | <0.001 | 1.3b | 1.11.4 |
Reasons for Inappropriate Designation
Among those respondents rating an intervention as inappropriate or very inappropriate, the 3 most common reasons selected as explanation for perceived inappropriateness from our predefined answer choices were: This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible (chosen by physicians in 49.4% of instances); I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (35.7%); and Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications (34.6%). The least common explanation chosen was I do not believe this is an appropriate pharmacologic intervention for this particular medical problem (6.5%). See Table 4 for a complete list of explanations, overall and stratified by PCP/hospitalist.
| Predefined Reason for Inappropriateness | Total, n=583 (%) | PCP, n=318 (%) | Hospitalist, n=265 (%) | P Value |
|---|---|---|---|---|
| ||||
| This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible. | 288 (49.4) | 151 (47.5) | 137 (51.7) | 0.32 |
| I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision. | 208 (35.7) | 98 (30.8) | 110 (41.5) | 0.009 |
| Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications. | 201 (34.5) | 125 (39.3) | 76 (28.7) | 0.009 |
| I am not confident that the hospitalist will adequately review the medical history necessary to make this decision. | 184 (31.6) | 130 (40.9) | 54 (20.4) | <0.001 |
| Even if the hospitalist has all of the medical history, I do not believe hospitalization is the right time to start this new medication | 106 (21.4) | 69 (21.7) | 56 (21.1) | 0.92 |
| I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient. | 106 (18.2) | 85 (26.7) | 21 (7.9) | <0.001 |
| The benefit of this medication will be too remote to justify starting it in the acute setting. | 66 (11.3) | 40 (12.6) | 26 (9.8) | 0.36 |
| I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. | 38 (6.5) | 27 (8.5) | 11 (4.2) | 0.04 |
There were significant differences in the proportion of PCPs and hospitalists choosing several of the prespecified reasons for inappropriateness. Although hospitalists were more likely than PCPs to select I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (chosen by 41.5% of hospitalists vs 30.8% of PCPs, P=0.009), PCPs were more likely than hospitalists to select, I am not confident that the hospitalist will adequately review the medical history necessary to make this decision (chosen by 40.9% of PCPs vs 20.4% of hospitalists, P<0.001) and I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient (26.7% of PCPs vs 9.8% of hospitalists, P<0.001).
Opinions on Current Management of Conditions Related and Unrelated to Admission
A minority of PCPs and hospitalists agreed or strongly agreed that hospitalists should play a larger role in the management of medical conditions unrelated to the reason for admission (28.1% of PCPs vs 34.8% of hospitalists; P=0.39).
DISCUSSION
In this survey‐based study of PCPs and hospitalists across 3 Boston‐area academic medical centers, we found that: (1) physicians were more likely to see inpatient interventions as appropriate when those interventions dealt with the reason for admission as compared to interventions unrelated to the reason for admission; and (2) PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when they targeted chronic conditions unrelated to the reason for admission. To our knowledge, this study represents the first investigation into the attitudes of PCPs and hospitalists regarding the inpatient management of conditions unrelated to the reason for admission.
That surveyed physicians, regardless of role, were less likely to report an intervention unrelated to the reason for hospitalization as appropriateeven those with likely mortality benefitsuggests that opportunities to affect meaningful change may be missed in a healthcare system that adheres to strict inpatient and outpatient roles. For several of the cases, a change in therapy could lead to benefit soon after implementation. For example, aldosterone antagonists reduce mortality as early as 1 month after initiation in select patients.[8] If a major goal of inpatient care is to reduce 30‐day mortality, it could be argued that hospitalists should more actively adjust congestive heart failure therapy in appropriate inpatients, even when this is not their admitting diagnosis.
For some conditions, CMS is already tracking hospital performance. Since 2003, hospitals have been required to document whether a patient with congestive heart failure (either acute or chronic and regardless of the relationship to admission) was prescribed an angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at the time of discharge.[7] CMS has determined that the proven benefits of ACE inhibitors and ARBs confer hospital accountability for their inclusion in appropriate patients, independent of the acuity of heart failure. There are many potential therapeutic maneuvers on which health systems (and their physicians) may be graded, and accepting the view that a hospitalization provides a window of opportunity for medical optimization may allow for more fruitful interventions and more patient‐centered care.
Despite the potential benefits of addressing chronic medical issues during hospitalization, there are important limitations on what can and/or should be done in the hospital setting. Hospitalizations are a time of fluctuating clinical status, which continues beyond discharge and is often accompanied by several medication changes.[9] In our study, more than 20% of those who believed that a medication intervention was inappropriate selected I do not believe hospitalization is the right time to start this new medication as one of their explanations. Although some medication interventions have been shown in randomized controlled trials to reduce short‐term mortality, the ability to generalize these findings to the average hospitalized patient with multiple comorbidities, concurrent medication changes, and rapidly fluctuating clinical status is limited. Furthermore, there are interventions most would agree should not be dealt with in the hospital (eg, screening colonoscopy) and encounters that may be too short to allow for change (eg, 24‐hour observation). These issues notwithstanding, the average 4‐day hospitalization likely provides an opportunity for monitored change that may currently be underutilized.
Our study suggests several additional explanations for physicians' current practice and opinions. Only 6.5% of respondents who answered that an intervention was inappropriate indicated as a justification that I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. This suggests that the hesitancy has little to do with a lack of benefit but instead relates to systems issues (eg, access to all pertinent records and concerns regarding follow‐up testing) and perceived limitations to what a hospitalist should and should not do without actively involving the PCP. There are likely additional concerns that the medical record and/or patient histories do not fully outline the rationale for exclusion or inclusion of particular medications. Advances in information technology that enhance information exchange and enable streamlined communication may help to address these perceived barriers. However, an additional barrier may be trust, as PCPs appear more concerned that hospitalists will not review all the pertinent records or discuss risks and benefits before enacting important medication changes. Increased attempts at communication between hospitalists and outpatient providers may help to build trust and alleviate concerns regarding the loss of information that often occurs both on admission and at discharge.
We also noted that PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when targeting chronic conditions unrelated to the reason for admission. It may be that PCPs, with an increasing number of problems to address per outpatient visit,[10, 11] are more open to hospitalists managing any medical problems during their patients' admissions. At the same time, with increased acuity[12, 13, 14] and shortened length of stays,[15, 16] hospitalists have only a finite amount of time to ensure acute issues are managed, leaving potentially modifiable chronic conditions to the outpatient setting. These differences aside, a minority of both PCPs and hospitalists in our study were ready to embrace the idea of hospitalists playing a larger role in the management of conditions unrelated to the reason for hospitalization.
Even though our study benefits from its multisite design, there are a number of limitations. First, although we crafted our survey with input from general medicine focus groups, our survey instrument has not been validated. In addition, the cases are necessarily contrived and do not take into account the complexities of inpatient medicine. Furthermore, though our goal was to create paired cases that isolate a management decision as being simply based on whether it was related or unrelated to the reason for admission, it is possible that other factors, not captured by our survey, influenced the responses. For example, the benefits of aspirin as part of secondary prevention are not equal to the benefits in an acute MI.[17]
In an attempt to isolate the hospitalists' role in these management decisions, respondents were instructed to assume that the decisions were being made without discussing it with the primary care physician, but that the hospitalist would communicate the details of any hospitalization at the time of discharge. They were also instructed to assume that the hospitalist has access to the patient's outpatient electronic medical record. These assumptions were made to address concerns regarding the flow of information and communication, and to simulate the ideal system from a communication and information accessibility standpoint. Had these assumptions not been placed, the responses may have differed. It is likely that PCPs and hospitalists practicing in systems without shared, accessible inpatient/outpatient medical records would be even more reluctant to enact medication changes unrelated to the reason for admission.
Along the same lines, our physician cohort consisted of several metropolitan academic physician groups, in which hospitalists have had a presence for almost 20 years. As a result, our findings may not be generalizable to other academic hospitals, community‐based hospitalist programs, or nonhospital‐based PCP practices. Finally, we do not know whether survey nonresponders differed from responders in ways that could have meaningfully affected our results.
In conclusion, our findings suggest that both PCPs and hospitalists see the management of conditions unrelated to the reason for admission as less appropriate than the management of conditions related to the reason for admission. Our findings also suggest that PCPs may be more open to this practice when compared to hospitalists. Failure to capitalize on opportunities for meaningful medical interventions, independent of patient location, suggests a possible lack of patient centeredness in the current partnership between PCPs and hospitalists. Further studies should examine existing barriers and investigate interventions designed to address those barriers, in an effort to improve both quality of care and the degree of patient‐centeredness in our current healthcare system.
Disclosures: Dr. Herzig is supported by a grant from the National Institute on Aging (K23 AG042459). Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Author contributions: study concept and design, Breu, Allen‐Dicker, Mueller, Herzig; acquisition of data, Breu, Allen‐Dicker, Mueller, Palamara, Herzig; analysis and interpretation of data, Breu, Allen‐Dicker, Hinami, Herzig; drafting of the manuscript, Breu; critical revision of the manuscript for important intellectual content, Breu, Allen‐Dicker, Mueller, Palamara, Hinami, Herzig; statistical analysis, Allen‐Dicker, Hinami, Herzig; study supervision, Breu, Herzig. This study was presented as a poster at the Society of Hospital Medicine National Meeting, Washington, DC, May 17, 2013.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- . An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 pt 2):338–342.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , . A national strategy to put accountable care into practice. Health Aff (Millwood). 2010;29(5):982–990.
- . Keeping score under a global payment system. N Engl J Med. 2012;366(5):393–395.
- Reporting Hospital Quality Data for Annual Payment Update. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/Downloads/HospitalRHQDAPU200808. Accessed December 18, 2013.
- , , , et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
- , , , , , . How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open. 2012;2(6):e001461.
- , , . Primary care visit duration and quality: does good care take longer? Arch Intern Med. 2009;169(20):1866–1872.
- , , , The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med. 2008;23(12):2058–2065.
- , , . Multiple chronic conditions among adults aged 45 and over: trends over the past 10 years. NCHS Data Brief. 2012;(100):1–8.
- , , . Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7(1):82.
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(suppl 3):391–395.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
Over the past 2 decades, the care of the hospitalized patient has changed dramatically. Hospitalists now account for the care of more than one‐third of general medicine inpatients, and this number is likely to grow.[1] The emergence of hospital medicine has resulted in a partnership between primary care physicians (PCPs) and hospitalists wherein hospitalists focus on acute medical issues requiring hospitalization, whereas more chronic issues unrelated to the reason for hospitalization remain largely the domain of the PCP.[2, 3]
However, several evolving financial and quality incentives have already begun to blur the distinction between inpatient and outpatient care. First, as private and public payers increasingly scrutinize readmission rates, it has become clear that the responsibility for patient outcomes extends beyond the day of discharge.[4] The birth of Accountable Care Organizations and patient‐centered medical homes may further blur distinctions between what has traditionally constituted inpatient and outpatient care.[5] Bundled payments may force providers to ensure that each visit, whether hospital‐ or clinic‐based, is taken as an opportunity to enact meaningful change.[6] The Centers for Medicare and Medicaid Services (CMS) are already tracking hospital performance on institution of medical therapy for certain conditions regardless of their relatedness to the reason for hospitalization.[7]
No published literature has yet examined the attitudes of inpatient and outpatient providers regarding this issue. Through a case‐based survey conducted at 3 large urban academic medical centers, we aimed to assess opinions among hospitalists and PCPs regarding the role of hospitalists in the management of conditions unrelated to the reason for admission. Our study had 2 main objectives: (1) to determine whether surveyed physicians were more likely to rate an inpatient intervention as appropriate when it related to the reason for admission as compared to interventions unrelated to the reason for admission; and (2) to determine whether these attitudes differed between PCPs and hospitalists.
METHODS
Setting and Subjects
We surveyed hospitalists and hospital‐based PCPs at Beth Israel Deaconess Medical Center (BIDMC), Brigham and Women's Hospital, and Massachusetts General Hospital, 3 large academic medical centers in Boston, Massachusetts. Each hospitalist group includes both teaching and nonteaching services and admits patients from both the surveyed hospital‐based PCP groups and other nonhospital‐based PCP groups. All 3 study sites use electronic medical records with patient information for each hospital‐based PCP available to treating hospitalists.
Survey Design
Using a commercially available online product (SurveyMonkey, Palo Alto, CA), we created a 3‐part case‐based survey instrument. The first section included demographic questions regarding age, sex, primary clinical role (hospitalist or PCP), prior experience as a PCP (for hospitalists only) or a hospitalist (for PCPs only; defined as a position with >30% of clinical time as the attending of record in the inpatient setting), years of clinical experience, and hospital affiliation.
The second section aimed to indirectly assess physician opinions on the appropriateness of inpatient management of conditions unrelated to the reason for admission. It consisted of 6 paired case scenarios, each with an inpatient management decision for a hypothetical hospitalist (Table 1). For each pair, 1 case dealt with management of the condition prompting admission (eg, starting aspirin in a patient admitted with acute nonST‐elevation myocardial infarction). The partner case involved the same intervention (eg, starting aspirin) but for a patient with a chronic condition (eg, history of prior myocardial infarction) and an alternate admitting diagnosis (eg, cellulitis). In an attempt to mitigate concerns regarding the flow of information and communication between providers, the survey asked respondents to assume that the hospitalist has access to the patient's outpatient electronic medical record, and that the hospitalist communicates the details of any hospitalizations at the time of discharge. For each case, the physician was asked to rate the appropriateness of enacting the intervention without discussing it with the PCP on a 5‐point scale from very inappropriate to very appropriate. When a physician answered that an intervention was inappropriate or very inappropriate, an additional question soliciting reasons for inappropriateness was included, with multiple predefined answer choices, as well as the option of a free‐text reply under the other designation.
| |
| Starting aspirin (related to the reason for admission) | A 60‐year‐old patient is admitted with a nonST‐elevation MI, medically managed without cardiac catheterization or percutaneous coronary intervention. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting aspirin (unrelated to the reason for admission) | A 60‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed is admitted to the hospital for treatment of cellulitis. The hospitalist notes the patient is not on aspirin at home. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting spironolactone (related to the reason for admission) | A 70‐year‐old patient with a past medical history significant for NYHA class II congestive heart failure (LVEF of 20%) is admitted for acute on chronic, left‐sided systolic congestive heart failure. The patient has been maintained on furosemide, metoprolol, and lisinopril. Admission serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting spironolactone (unrelated to the reason for admission) | A 70‐year‐old patient with a past history of NYHA class II congestive heart failure (LVEF of 20%) on furosemide, metoprolol, and lisinopril is admitted with pneumonia. Serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting warfarin (related to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension and diabetes is admitted with new atrial fibrillation. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Starting warfarin (unrelated to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension, diabetes, and atrial fibrillation is admitted with pneumonia. The patient is not anticoagulation therapy. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Stopping proton pump inhibitor (related to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of Clostridium difficile colitis. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping proton pump inhibitor (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of a urinary tract infection. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping statin or fibrate (related to the reason for admission) | A 60‐year‐old patient with a history of hyperlipidemia is admitted with an elevated creatine kinase to 5000. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent serum LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Stopping statin or fibrate (unrelated to the reason for admission) | A 60‐year‐old patient is admitted with an acute diarrheal illness. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Changing statin (related to the reason for admission) | A 65‐year‐old patient with a past medical history of hyperlipidemia on maximum‐dose simvastatin is admitted with a nonST‐elevation MI. The patient's cholesterol is noted to be above goal. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
| Changing statin (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed and hyperlipidemia on maximum‐dose simvastatin is admitted with pneumonia. Incidentally, the hospitalist notes that the patient's cholesterol has been above goal for the last 2 years. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
The third section aimed to directly assess physicians' opinions. It consisted of questions regarding the appropriateness of inpatient management of conditions related to and unrelated to a patient's reason for admission.
Prior to administration, we conducted focus groups of hospitalists and PCPs to help hypothesize current physician perceptions on inpatient management, assess physician understanding of survey cases and questions, and to evaluate survey length.
Survey Administration
Between October 23, 2012 and November 10, 2012, 3 emails containing a link to the online survey were sent to all hospitalist and hospital‐based PCPs at the 3 study institutions. The BIDMC Committee on Clinical Investigations, to whom authority was ceded by the remaining 2 study institutions, certified this research protocol as exempt.
Statistical Analysis
We hypothesized that respondents as a whole would be more likely to rate an intervention as appropriate or very appropriate if it was related to the reason for admission, compared to unrelated, and that there would be no difference between PCPs and hospitalists.
We used 2 and Fisher exact tests (where applicable) to compare categorical variables, and a nonparametric median test for continuous variables. We used the Fisher exact test to compare the percent of respondents rating each intervention as appropriate or very appropriate by relatedness or unrelatedness to the reason for admission, and by PCP vs hospitalist. To derive the relative risk (RR) of rating each intervention as appropriate or very appropriate by PCPs compared to hospitalists, adjusting for potential confounders including years out of residency and sex, we used multivariable generalized estimating equation models, each with a Poisson distribution error term, a log link, and an exchangeable working correlation structure to account for dependency of observations arising from clustering at either the hospital or participant level, depending on the comparison: for comparisons within a given case, we controlled for clustering at the hospital level; for comparisons of cases in aggregate, owing to multiple responses from each participant, we controlled for clustering at the individual level.
Assuming a 50% response rate from both PCPs and hospitalists, and that 50% of PCPs would rate a given intervention as appropriate, we calculated that we would have 90% power to detect a 50% increase in the proportion of hospitalists rating an intervention as appropriate as compared to PCPs, using an of .05.
RESULTS
Demographics
One hundred sixty‐two out of 295 providers (55%) responded to the survey (Table 2). The response rate did not differ between hospitalists (70 out of 128; 55%) and PCPs (92 out of 167; 55%). Female respondents made up 58.7% of the PCP and 50.0% of the hospitalist groups (P=0.34). On average, PCPs were older (P<0.001) with a greater median number of years since graduation from residency (P<0.001). A greater percentage of hospitalists spent more than three‐quarters of their time clinically (42.9% vs 19.6%, P=0.009).
| Total, n=162 (100.0%) | PCP, n=92 (6.8%) | Hospitalist, n=70 (43.2%) | P Valuea | |
|---|---|---|---|---|
| ||||
| Hospital, n (%) | ||||
| BIDMC | 79 (48.8) | 48 (60.8) | 31 (39.2) | 0.115 |
| BWH | 36 (22.2) | 15 (41.7) | 21 (58.3) | |
| MGH | 47 (29.0) | 29 (61.7) | 18 (38.3) | |
| Sex, n (%) | ||||
| Male | 73 (45.1) | 38 (41.3) | 35 (50.0) | 0.339 |
| Female | 89 (54.9) | 54 (58.7) | 35 (50.0) | |
| Age interval, y, n (%) | ||||
| 2534 | 36 (22.2) | 9 (9.8) | 27 (38.6) | <0.001 |
| 3544 | 67 (41.4) | 34 (37.0) | 33 (47.1) | |
| 4554 | 35 (21.6) | 29 (31.5) | 6 (8.6) | |
| 5564 | 19 (11.7) | 16 (17.4) | 3 (4.3) | |
| 6574 | 5 (3.1) | 4 (4.4) | 1 (1.4) | |
| Years out of residency, median (IQR) | 10 (417) | 15 (74) | 5 (211) | <0.001 |
| Clinical FTE, n (%) | ||||
| 0.25 | 30 (18.6) | 22 (23.9) | 8 (11.4) | 0.009 |
| 0.260.50 | 41 (25.3) | 25 (27.2) | 16 (22.9) | |
| 0.510.75 | 43 (26.5) | 27 (29.4) | 16 (22.9) | |
| >0.75 | 48 (29.6) | 18 (19.6) | 30 (42.9) | |
| Worked as PCP?b | ||||
| Yes | 6 (8.6) | |||
| No | 64 (91.4) | |||
| Worked as hospitalist? | ||||
| Yes | 11 (12.0) | |||
| No | 81 (88.0) | |||
| AOR for admitted patients | ||||
| Always | 16 (17.4) | |||
| Mostly | 8 (8.7) | |||
| Rarely | 7 (7.6) | |||
| Never | 60 (65.2) | |||
Appropriateness of Inpatient Management Based on Admitting Diagnosis
For each of the 6 case pairings individually and in aggregate, respondents were significantly more likely to deem the intervention appropriate or very appropriate if it was related to the reason for admission, compared to those interventions unrelated to the reason for admission (in aggregate, 78.9% vs 38.8% respectively, P<0.001). For example, whereas 96.9% felt that the addition of aspirin in a patient admitted with acute myocardial infarction (MI) was appropriate, only 54.3% felt it appropriate to start aspirin in a patient with a prior history of MI admitted with cellulitis (P<0.001). Significant differences (all P values <0.001) were seen for all case pairs: starting spironolactone (68.1% when related to the reason for reason for admission vs 43.1% when unrelated to reason for admission); starting warfarin (62.3% vs 23.3%), stopping proton pump inhibitor (72.3% vs 42.8%), stopping statin or fibrate (90.6% vs 28.3%), and changing statin (83.0% vs 40.5%).
Appropriateness of Inpatient Management based on Primary Role
Table 3 compares the percent of PCPs and hospitalists rating each intervention as appropriate or very appropriate, by relatedness of the intervention to the reason for admission. In both unadjusted and adjusted comparisons for all cases in aggregate, PCPs were significantly more likely than hospitalists to rate the inpatient interventions as appropriate or very appropriate when the intervention was related to the reason for admission (83.4% of PCP responses vs 73.0% of hospitalist responses, P<0.001; RR: 1.2, 95% confidence interval [CI]: 1.11.3), unrelated to the reason for admission (44.7% vs 31.1%, P<0.001; RR: 1.5, 95% CI: 1.11.9), and overall (64.1% vs 52.1%, P<0.001; RR: 1.3, 95% CI: 1.11.4).
| Relationship to Admission Diagnosis | PCP, n (%) | Hospitalist, n (%) | P Value | Adjusted RR | 95% CI |
|---|---|---|---|---|---|
| |||||
| Related | 453 (83.4) | 303 (73.0) | <0.001 | 1.2a | 1.11.3 |
| Unrelated | 242 (44.7) | 129 (31.1) | <0.001 | 1.5a | 1.11.9 |
| Overall | 695 (64.1) | 432 (52.1) | <0.001 | 1.3b | 1.11.4 |
Reasons for Inappropriate Designation
Among those respondents rating an intervention as inappropriate or very inappropriate, the 3 most common reasons selected as explanation for perceived inappropriateness from our predefined answer choices were: This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible (chosen by physicians in 49.4% of instances); I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (35.7%); and Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications (34.6%). The least common explanation chosen was I do not believe this is an appropriate pharmacologic intervention for this particular medical problem (6.5%). See Table 4 for a complete list of explanations, overall and stratified by PCP/hospitalist.
| Predefined Reason for Inappropriateness | Total, n=583 (%) | PCP, n=318 (%) | Hospitalist, n=265 (%) | P Value |
|---|---|---|---|---|
| ||||
| This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible. | 288 (49.4) | 151 (47.5) | 137 (51.7) | 0.32 |
| I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision. | 208 (35.7) | 98 (30.8) | 110 (41.5) | 0.009 |
| Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications. | 201 (34.5) | 125 (39.3) | 76 (28.7) | 0.009 |
| I am not confident that the hospitalist will adequately review the medical history necessary to make this decision. | 184 (31.6) | 130 (40.9) | 54 (20.4) | <0.001 |
| Even if the hospitalist has all of the medical history, I do not believe hospitalization is the right time to start this new medication | 106 (21.4) | 69 (21.7) | 56 (21.1) | 0.92 |
| I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient. | 106 (18.2) | 85 (26.7) | 21 (7.9) | <0.001 |
| The benefit of this medication will be too remote to justify starting it in the acute setting. | 66 (11.3) | 40 (12.6) | 26 (9.8) | 0.36 |
| I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. | 38 (6.5) | 27 (8.5) | 11 (4.2) | 0.04 |
There were significant differences in the proportion of PCPs and hospitalists choosing several of the prespecified reasons for inappropriateness. Although hospitalists were more likely than PCPs to select I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (chosen by 41.5% of hospitalists vs 30.8% of PCPs, P=0.009), PCPs were more likely than hospitalists to select, I am not confident that the hospitalist will adequately review the medical history necessary to make this decision (chosen by 40.9% of PCPs vs 20.4% of hospitalists, P<0.001) and I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient (26.7% of PCPs vs 9.8% of hospitalists, P<0.001).
Opinions on Current Management of Conditions Related and Unrelated to Admission
A minority of PCPs and hospitalists agreed or strongly agreed that hospitalists should play a larger role in the management of medical conditions unrelated to the reason for admission (28.1% of PCPs vs 34.8% of hospitalists; P=0.39).
DISCUSSION
In this survey‐based study of PCPs and hospitalists across 3 Boston‐area academic medical centers, we found that: (1) physicians were more likely to see inpatient interventions as appropriate when those interventions dealt with the reason for admission as compared to interventions unrelated to the reason for admission; and (2) PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when they targeted chronic conditions unrelated to the reason for admission. To our knowledge, this study represents the first investigation into the attitudes of PCPs and hospitalists regarding the inpatient management of conditions unrelated to the reason for admission.
That surveyed physicians, regardless of role, were less likely to report an intervention unrelated to the reason for hospitalization as appropriateeven those with likely mortality benefitsuggests that opportunities to affect meaningful change may be missed in a healthcare system that adheres to strict inpatient and outpatient roles. For several of the cases, a change in therapy could lead to benefit soon after implementation. For example, aldosterone antagonists reduce mortality as early as 1 month after initiation in select patients.[8] If a major goal of inpatient care is to reduce 30‐day mortality, it could be argued that hospitalists should more actively adjust congestive heart failure therapy in appropriate inpatients, even when this is not their admitting diagnosis.
For some conditions, CMS is already tracking hospital performance. Since 2003, hospitals have been required to document whether a patient with congestive heart failure (either acute or chronic and regardless of the relationship to admission) was prescribed an angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at the time of discharge.[7] CMS has determined that the proven benefits of ACE inhibitors and ARBs confer hospital accountability for their inclusion in appropriate patients, independent of the acuity of heart failure. There are many potential therapeutic maneuvers on which health systems (and their physicians) may be graded, and accepting the view that a hospitalization provides a window of opportunity for medical optimization may allow for more fruitful interventions and more patient‐centered care.
Despite the potential benefits of addressing chronic medical issues during hospitalization, there are important limitations on what can and/or should be done in the hospital setting. Hospitalizations are a time of fluctuating clinical status, which continues beyond discharge and is often accompanied by several medication changes.[9] In our study, more than 20% of those who believed that a medication intervention was inappropriate selected I do not believe hospitalization is the right time to start this new medication as one of their explanations. Although some medication interventions have been shown in randomized controlled trials to reduce short‐term mortality, the ability to generalize these findings to the average hospitalized patient with multiple comorbidities, concurrent medication changes, and rapidly fluctuating clinical status is limited. Furthermore, there are interventions most would agree should not be dealt with in the hospital (eg, screening colonoscopy) and encounters that may be too short to allow for change (eg, 24‐hour observation). These issues notwithstanding, the average 4‐day hospitalization likely provides an opportunity for monitored change that may currently be underutilized.
Our study suggests several additional explanations for physicians' current practice and opinions. Only 6.5% of respondents who answered that an intervention was inappropriate indicated as a justification that I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. This suggests that the hesitancy has little to do with a lack of benefit but instead relates to systems issues (eg, access to all pertinent records and concerns regarding follow‐up testing) and perceived limitations to what a hospitalist should and should not do without actively involving the PCP. There are likely additional concerns that the medical record and/or patient histories do not fully outline the rationale for exclusion or inclusion of particular medications. Advances in information technology that enhance information exchange and enable streamlined communication may help to address these perceived barriers. However, an additional barrier may be trust, as PCPs appear more concerned that hospitalists will not review all the pertinent records or discuss risks and benefits before enacting important medication changes. Increased attempts at communication between hospitalists and outpatient providers may help to build trust and alleviate concerns regarding the loss of information that often occurs both on admission and at discharge.
We also noted that PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when targeting chronic conditions unrelated to the reason for admission. It may be that PCPs, with an increasing number of problems to address per outpatient visit,[10, 11] are more open to hospitalists managing any medical problems during their patients' admissions. At the same time, with increased acuity[12, 13, 14] and shortened length of stays,[15, 16] hospitalists have only a finite amount of time to ensure acute issues are managed, leaving potentially modifiable chronic conditions to the outpatient setting. These differences aside, a minority of both PCPs and hospitalists in our study were ready to embrace the idea of hospitalists playing a larger role in the management of conditions unrelated to the reason for hospitalization.
Even though our study benefits from its multisite design, there are a number of limitations. First, although we crafted our survey with input from general medicine focus groups, our survey instrument has not been validated. In addition, the cases are necessarily contrived and do not take into account the complexities of inpatient medicine. Furthermore, though our goal was to create paired cases that isolate a management decision as being simply based on whether it was related or unrelated to the reason for admission, it is possible that other factors, not captured by our survey, influenced the responses. For example, the benefits of aspirin as part of secondary prevention are not equal to the benefits in an acute MI.[17]
In an attempt to isolate the hospitalists' role in these management decisions, respondents were instructed to assume that the decisions were being made without discussing it with the primary care physician, but that the hospitalist would communicate the details of any hospitalization at the time of discharge. They were also instructed to assume that the hospitalist has access to the patient's outpatient electronic medical record. These assumptions were made to address concerns regarding the flow of information and communication, and to simulate the ideal system from a communication and information accessibility standpoint. Had these assumptions not been placed, the responses may have differed. It is likely that PCPs and hospitalists practicing in systems without shared, accessible inpatient/outpatient medical records would be even more reluctant to enact medication changes unrelated to the reason for admission.
Along the same lines, our physician cohort consisted of several metropolitan academic physician groups, in which hospitalists have had a presence for almost 20 years. As a result, our findings may not be generalizable to other academic hospitals, community‐based hospitalist programs, or nonhospital‐based PCP practices. Finally, we do not know whether survey nonresponders differed from responders in ways that could have meaningfully affected our results.
In conclusion, our findings suggest that both PCPs and hospitalists see the management of conditions unrelated to the reason for admission as less appropriate than the management of conditions related to the reason for admission. Our findings also suggest that PCPs may be more open to this practice when compared to hospitalists. Failure to capitalize on opportunities for meaningful medical interventions, independent of patient location, suggests a possible lack of patient centeredness in the current partnership between PCPs and hospitalists. Further studies should examine existing barriers and investigate interventions designed to address those barriers, in an effort to improve both quality of care and the degree of patient‐centeredness in our current healthcare system.
Disclosures: Dr. Herzig is supported by a grant from the National Institute on Aging (K23 AG042459). Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Author contributions: study concept and design, Breu, Allen‐Dicker, Mueller, Herzig; acquisition of data, Breu, Allen‐Dicker, Mueller, Palamara, Herzig; analysis and interpretation of data, Breu, Allen‐Dicker, Hinami, Herzig; drafting of the manuscript, Breu; critical revision of the manuscript for important intellectual content, Breu, Allen‐Dicker, Mueller, Palamara, Hinami, Herzig; statistical analysis, Allen‐Dicker, Hinami, Herzig; study supervision, Breu, Herzig. This study was presented as a poster at the Society of Hospital Medicine National Meeting, Washington, DC, May 17, 2013.
Over the past 2 decades, the care of the hospitalized patient has changed dramatically. Hospitalists now account for the care of more than one‐third of general medicine inpatients, and this number is likely to grow.[1] The emergence of hospital medicine has resulted in a partnership between primary care physicians (PCPs) and hospitalists wherein hospitalists focus on acute medical issues requiring hospitalization, whereas more chronic issues unrelated to the reason for hospitalization remain largely the domain of the PCP.[2, 3]
However, several evolving financial and quality incentives have already begun to blur the distinction between inpatient and outpatient care. First, as private and public payers increasingly scrutinize readmission rates, it has become clear that the responsibility for patient outcomes extends beyond the day of discharge.[4] The birth of Accountable Care Organizations and patient‐centered medical homes may further blur distinctions between what has traditionally constituted inpatient and outpatient care.[5] Bundled payments may force providers to ensure that each visit, whether hospital‐ or clinic‐based, is taken as an opportunity to enact meaningful change.[6] The Centers for Medicare and Medicaid Services (CMS) are already tracking hospital performance on institution of medical therapy for certain conditions regardless of their relatedness to the reason for hospitalization.[7]
No published literature has yet examined the attitudes of inpatient and outpatient providers regarding this issue. Through a case‐based survey conducted at 3 large urban academic medical centers, we aimed to assess opinions among hospitalists and PCPs regarding the role of hospitalists in the management of conditions unrelated to the reason for admission. Our study had 2 main objectives: (1) to determine whether surveyed physicians were more likely to rate an inpatient intervention as appropriate when it related to the reason for admission as compared to interventions unrelated to the reason for admission; and (2) to determine whether these attitudes differed between PCPs and hospitalists.
METHODS
Setting and Subjects
We surveyed hospitalists and hospital‐based PCPs at Beth Israel Deaconess Medical Center (BIDMC), Brigham and Women's Hospital, and Massachusetts General Hospital, 3 large academic medical centers in Boston, Massachusetts. Each hospitalist group includes both teaching and nonteaching services and admits patients from both the surveyed hospital‐based PCP groups and other nonhospital‐based PCP groups. All 3 study sites use electronic medical records with patient information for each hospital‐based PCP available to treating hospitalists.
Survey Design
Using a commercially available online product (SurveyMonkey, Palo Alto, CA), we created a 3‐part case‐based survey instrument. The first section included demographic questions regarding age, sex, primary clinical role (hospitalist or PCP), prior experience as a PCP (for hospitalists only) or a hospitalist (for PCPs only; defined as a position with >30% of clinical time as the attending of record in the inpatient setting), years of clinical experience, and hospital affiliation.
The second section aimed to indirectly assess physician opinions on the appropriateness of inpatient management of conditions unrelated to the reason for admission. It consisted of 6 paired case scenarios, each with an inpatient management decision for a hypothetical hospitalist (Table 1). For each pair, 1 case dealt with management of the condition prompting admission (eg, starting aspirin in a patient admitted with acute nonST‐elevation myocardial infarction). The partner case involved the same intervention (eg, starting aspirin) but for a patient with a chronic condition (eg, history of prior myocardial infarction) and an alternate admitting diagnosis (eg, cellulitis). In an attempt to mitigate concerns regarding the flow of information and communication between providers, the survey asked respondents to assume that the hospitalist has access to the patient's outpatient electronic medical record, and that the hospitalist communicates the details of any hospitalizations at the time of discharge. For each case, the physician was asked to rate the appropriateness of enacting the intervention without discussing it with the PCP on a 5‐point scale from very inappropriate to very appropriate. When a physician answered that an intervention was inappropriate or very inappropriate, an additional question soliciting reasons for inappropriateness was included, with multiple predefined answer choices, as well as the option of a free‐text reply under the other designation.
| |
| Starting aspirin (related to the reason for admission) | A 60‐year‐old patient is admitted with a nonST‐elevation MI, medically managed without cardiac catheterization or percutaneous coronary intervention. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting aspirin (unrelated to the reason for admission) | A 60‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed is admitted to the hospital for treatment of cellulitis. The hospitalist notes the patient is not on aspirin at home. Knowing that aspirin reduces mortality as part of secondary prevention in cardiovascular disease, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician? |
| Starting spironolactone (related to the reason for admission) | A 70‐year‐old patient with a past medical history significant for NYHA class II congestive heart failure (LVEF of 20%) is admitted for acute on chronic, left‐sided systolic congestive heart failure. The patient has been maintained on furosemide, metoprolol, and lisinopril. Admission serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting spironolactone (unrelated to the reason for admission) | A 70‐year‐old patient with a past history of NYHA class II congestive heart failure (LVEF of 20%) on furosemide, metoprolol, and lisinopril is admitted with pneumonia. Serum potassium and creatinine are both normal. Knowing that spironolactone decreases mortality in heart failure, how appropriate is it for the hospitalist to start this medication without discussing it with the primary care physician? |
| Starting warfarin (related to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension and diabetes is admitted with new atrial fibrillation. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Starting warfarin (unrelated to the reason for admission) | A 75‐year‐old patient with a past medical history of hypertension, diabetes, and atrial fibrillation is admitted with pneumonia. The patient is not anticoagulation therapy. Given the patient's CHADS2 score of 3, the hospitalist calculates that the patient has a significant risk of thromboembolic stroke. Knowing that warfarin will decrease the risk of thromboembolic stroke, how appropriate is it for the hospitalist to start the patient on this medication without discussing it with the primary care physician (assume that an outpatient anticoagulation clinic is able to see the patient within 3 days of discharge)? |
| Stopping proton pump inhibitor (related to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of Clostridium difficile colitis. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping proton pump inhibitor (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of GERD maintained on a proton pump inhibitor is admitted for treatment of a urinary tract infection. The patient denies having any GERD‐like symptoms for several years. Knowing that proton pump inhibitors can increase the risk of C difficile colitis and recurrence (as well as pneumonia and osteoporosis), how appropriate is it for the hospitalist to initiate a taper of this medication without discussing it with the primary care physician? |
| Stopping statin or fibrate (related to the reason for admission) | A 60‐year‐old patient with a history of hyperlipidemia is admitted with an elevated creatine kinase to 5000. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent serum LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Stopping statin or fibrate (unrelated to the reason for admission) | A 60‐year‐old patient is admitted with an acute diarrheal illness. The hospitalist notes that the patient is on both simvastatin and gemfibrozil. The patient's most recent LDL was at goal. Knowing that coadministration of simvastatin and gemfibrozil can increase the risk of rhabdomyolysis, how appropriate is it for the hospitalist to stop one of these medications without discussing it with the primary care physician? |
| Changing statin (related to the reason for admission) | A 65‐year‐old patient with a past medical history of hyperlipidemia on maximum‐dose simvastatin is admitted with a nonST‐elevation MI. The patient's cholesterol is noted to be above goal. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
| Changing statin (unrelated to the reason for admission) | A 65‐year‐old patient with a past medical history of a prior nonST‐elevation MI that was medically managed and hyperlipidemia on maximum‐dose simvastatin is admitted with pneumonia. Incidentally, the hospitalist notes that the patient's cholesterol has been above goal for the last 2 years. Knowing that improving lipid management reduces mortality in cardiovascular disease, how appropriate is it for the hospitalist to replace simvastatin with atorvastatin without discussing it with the primary care physician? |
The third section aimed to directly assess physicians' opinions. It consisted of questions regarding the appropriateness of inpatient management of conditions related to and unrelated to a patient's reason for admission.
Prior to administration, we conducted focus groups of hospitalists and PCPs to help hypothesize current physician perceptions on inpatient management, assess physician understanding of survey cases and questions, and to evaluate survey length.
Survey Administration
Between October 23, 2012 and November 10, 2012, 3 emails containing a link to the online survey were sent to all hospitalist and hospital‐based PCPs at the 3 study institutions. The BIDMC Committee on Clinical Investigations, to whom authority was ceded by the remaining 2 study institutions, certified this research protocol as exempt.
Statistical Analysis
We hypothesized that respondents as a whole would be more likely to rate an intervention as appropriate or very appropriate if it was related to the reason for admission, compared to unrelated, and that there would be no difference between PCPs and hospitalists.
We used 2 and Fisher exact tests (where applicable) to compare categorical variables, and a nonparametric median test for continuous variables. We used the Fisher exact test to compare the percent of respondents rating each intervention as appropriate or very appropriate by relatedness or unrelatedness to the reason for admission, and by PCP vs hospitalist. To derive the relative risk (RR) of rating each intervention as appropriate or very appropriate by PCPs compared to hospitalists, adjusting for potential confounders including years out of residency and sex, we used multivariable generalized estimating equation models, each with a Poisson distribution error term, a log link, and an exchangeable working correlation structure to account for dependency of observations arising from clustering at either the hospital or participant level, depending on the comparison: for comparisons within a given case, we controlled for clustering at the hospital level; for comparisons of cases in aggregate, owing to multiple responses from each participant, we controlled for clustering at the individual level.
Assuming a 50% response rate from both PCPs and hospitalists, and that 50% of PCPs would rate a given intervention as appropriate, we calculated that we would have 90% power to detect a 50% increase in the proportion of hospitalists rating an intervention as appropriate as compared to PCPs, using an of .05.
RESULTS
Demographics
One hundred sixty‐two out of 295 providers (55%) responded to the survey (Table 2). The response rate did not differ between hospitalists (70 out of 128; 55%) and PCPs (92 out of 167; 55%). Female respondents made up 58.7% of the PCP and 50.0% of the hospitalist groups (P=0.34). On average, PCPs were older (P<0.001) with a greater median number of years since graduation from residency (P<0.001). A greater percentage of hospitalists spent more than three‐quarters of their time clinically (42.9% vs 19.6%, P=0.009).
| Total, n=162 (100.0%) | PCP, n=92 (6.8%) | Hospitalist, n=70 (43.2%) | P Valuea | |
|---|---|---|---|---|
| ||||
| Hospital, n (%) | ||||
| BIDMC | 79 (48.8) | 48 (60.8) | 31 (39.2) | 0.115 |
| BWH | 36 (22.2) | 15 (41.7) | 21 (58.3) | |
| MGH | 47 (29.0) | 29 (61.7) | 18 (38.3) | |
| Sex, n (%) | ||||
| Male | 73 (45.1) | 38 (41.3) | 35 (50.0) | 0.339 |
| Female | 89 (54.9) | 54 (58.7) | 35 (50.0) | |
| Age interval, y, n (%) | ||||
| 2534 | 36 (22.2) | 9 (9.8) | 27 (38.6) | <0.001 |
| 3544 | 67 (41.4) | 34 (37.0) | 33 (47.1) | |
| 4554 | 35 (21.6) | 29 (31.5) | 6 (8.6) | |
| 5564 | 19 (11.7) | 16 (17.4) | 3 (4.3) | |
| 6574 | 5 (3.1) | 4 (4.4) | 1 (1.4) | |
| Years out of residency, median (IQR) | 10 (417) | 15 (74) | 5 (211) | <0.001 |
| Clinical FTE, n (%) | ||||
| 0.25 | 30 (18.6) | 22 (23.9) | 8 (11.4) | 0.009 |
| 0.260.50 | 41 (25.3) | 25 (27.2) | 16 (22.9) | |
| 0.510.75 | 43 (26.5) | 27 (29.4) | 16 (22.9) | |
| >0.75 | 48 (29.6) | 18 (19.6) | 30 (42.9) | |
| Worked as PCP?b | ||||
| Yes | 6 (8.6) | |||
| No | 64 (91.4) | |||
| Worked as hospitalist? | ||||
| Yes | 11 (12.0) | |||
| No | 81 (88.0) | |||
| AOR for admitted patients | ||||
| Always | 16 (17.4) | |||
| Mostly | 8 (8.7) | |||
| Rarely | 7 (7.6) | |||
| Never | 60 (65.2) | |||
Appropriateness of Inpatient Management Based on Admitting Diagnosis
For each of the 6 case pairings individually and in aggregate, respondents were significantly more likely to deem the intervention appropriate or very appropriate if it was related to the reason for admission, compared to those interventions unrelated to the reason for admission (in aggregate, 78.9% vs 38.8% respectively, P<0.001). For example, whereas 96.9% felt that the addition of aspirin in a patient admitted with acute myocardial infarction (MI) was appropriate, only 54.3% felt it appropriate to start aspirin in a patient with a prior history of MI admitted with cellulitis (P<0.001). Significant differences (all P values <0.001) were seen for all case pairs: starting spironolactone (68.1% when related to the reason for reason for admission vs 43.1% when unrelated to reason for admission); starting warfarin (62.3% vs 23.3%), stopping proton pump inhibitor (72.3% vs 42.8%), stopping statin or fibrate (90.6% vs 28.3%), and changing statin (83.0% vs 40.5%).
Appropriateness of Inpatient Management based on Primary Role
Table 3 compares the percent of PCPs and hospitalists rating each intervention as appropriate or very appropriate, by relatedness of the intervention to the reason for admission. In both unadjusted and adjusted comparisons for all cases in aggregate, PCPs were significantly more likely than hospitalists to rate the inpatient interventions as appropriate or very appropriate when the intervention was related to the reason for admission (83.4% of PCP responses vs 73.0% of hospitalist responses, P<0.001; RR: 1.2, 95% confidence interval [CI]: 1.11.3), unrelated to the reason for admission (44.7% vs 31.1%, P<0.001; RR: 1.5, 95% CI: 1.11.9), and overall (64.1% vs 52.1%, P<0.001; RR: 1.3, 95% CI: 1.11.4).
| Relationship to Admission Diagnosis | PCP, n (%) | Hospitalist, n (%) | P Value | Adjusted RR | 95% CI |
|---|---|---|---|---|---|
| |||||
| Related | 453 (83.4) | 303 (73.0) | <0.001 | 1.2a | 1.11.3 |
| Unrelated | 242 (44.7) | 129 (31.1) | <0.001 | 1.5a | 1.11.9 |
| Overall | 695 (64.1) | 432 (52.1) | <0.001 | 1.3b | 1.11.4 |
Reasons for Inappropriate Designation
Among those respondents rating an intervention as inappropriate or very inappropriate, the 3 most common reasons selected as explanation for perceived inappropriateness from our predefined answer choices were: This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible (chosen by physicians in 49.4% of instances); I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (35.7%); and Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications (34.6%). The least common explanation chosen was I do not believe this is an appropriate pharmacologic intervention for this particular medical problem (6.5%). See Table 4 for a complete list of explanations, overall and stratified by PCP/hospitalist.
| Predefined Reason for Inappropriateness | Total, n=583 (%) | PCP, n=318 (%) | Hospitalist, n=265 (%) | P Value |
|---|---|---|---|---|
| ||||
| This medication will necessitate follow‐up testing/monitoring, for which the PCP will be responsible. | 288 (49.4) | 151 (47.5) | 137 (51.7) | 0.32 |
| I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision. | 208 (35.7) | 98 (30.8) | 110 (41.5) | 0.009 |
| Even if the hospitalist has all of the medical history and reviews it, the PCP should be involved in all decisions surrounding new medications. | 201 (34.5) | 125 (39.3) | 76 (28.7) | 0.009 |
| I am not confident that the hospitalist will adequately review the medical history necessary to make this decision. | 184 (31.6) | 130 (40.9) | 54 (20.4) | <0.001 |
| Even if the hospitalist has all of the medical history, I do not believe hospitalization is the right time to start this new medication | 106 (21.4) | 69 (21.7) | 56 (21.1) | 0.92 |
| I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient. | 106 (18.2) | 85 (26.7) | 21 (7.9) | <0.001 |
| The benefit of this medication will be too remote to justify starting it in the acute setting. | 66 (11.3) | 40 (12.6) | 26 (9.8) | 0.36 |
| I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. | 38 (6.5) | 27 (8.5) | 11 (4.2) | 0.04 |
There were significant differences in the proportion of PCPs and hospitalists choosing several of the prespecified reasons for inappropriateness. Although hospitalists were more likely than PCPs to select I am not confident that the hospitalist will have access to all of the medical history necessary to make this decision (chosen by 41.5% of hospitalists vs 30.8% of PCPs, P=0.009), PCPs were more likely than hospitalists to select, I am not confident that the hospitalist will adequately review the medical history necessary to make this decision (chosen by 40.9% of PCPs vs 20.4% of hospitalists, P<0.001) and I am not confident that the hospitalist will appropriately discuss the risks and benefits of this new medication with the patient (26.7% of PCPs vs 9.8% of hospitalists, P<0.001).
Opinions on Current Management of Conditions Related and Unrelated to Admission
A minority of PCPs and hospitalists agreed or strongly agreed that hospitalists should play a larger role in the management of medical conditions unrelated to the reason for admission (28.1% of PCPs vs 34.8% of hospitalists; P=0.39).
DISCUSSION
In this survey‐based study of PCPs and hospitalists across 3 Boston‐area academic medical centers, we found that: (1) physicians were more likely to see inpatient interventions as appropriate when those interventions dealt with the reason for admission as compared to interventions unrelated to the reason for admission; and (2) PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when they targeted chronic conditions unrelated to the reason for admission. To our knowledge, this study represents the first investigation into the attitudes of PCPs and hospitalists regarding the inpatient management of conditions unrelated to the reason for admission.
That surveyed physicians, regardless of role, were less likely to report an intervention unrelated to the reason for hospitalization as appropriateeven those with likely mortality benefitsuggests that opportunities to affect meaningful change may be missed in a healthcare system that adheres to strict inpatient and outpatient roles. For several of the cases, a change in therapy could lead to benefit soon after implementation. For example, aldosterone antagonists reduce mortality as early as 1 month after initiation in select patients.[8] If a major goal of inpatient care is to reduce 30‐day mortality, it could be argued that hospitalists should more actively adjust congestive heart failure therapy in appropriate inpatients, even when this is not their admitting diagnosis.
For some conditions, CMS is already tracking hospital performance. Since 2003, hospitals have been required to document whether a patient with congestive heart failure (either acute or chronic and regardless of the relationship to admission) was prescribed an angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at the time of discharge.[7] CMS has determined that the proven benefits of ACE inhibitors and ARBs confer hospital accountability for their inclusion in appropriate patients, independent of the acuity of heart failure. There are many potential therapeutic maneuvers on which health systems (and their physicians) may be graded, and accepting the view that a hospitalization provides a window of opportunity for medical optimization may allow for more fruitful interventions and more patient‐centered care.
Despite the potential benefits of addressing chronic medical issues during hospitalization, there are important limitations on what can and/or should be done in the hospital setting. Hospitalizations are a time of fluctuating clinical status, which continues beyond discharge and is often accompanied by several medication changes.[9] In our study, more than 20% of those who believed that a medication intervention was inappropriate selected I do not believe hospitalization is the right time to start this new medication as one of their explanations. Although some medication interventions have been shown in randomized controlled trials to reduce short‐term mortality, the ability to generalize these findings to the average hospitalized patient with multiple comorbidities, concurrent medication changes, and rapidly fluctuating clinical status is limited. Furthermore, there are interventions most would agree should not be dealt with in the hospital (eg, screening colonoscopy) and encounters that may be too short to allow for change (eg, 24‐hour observation). These issues notwithstanding, the average 4‐day hospitalization likely provides an opportunity for monitored change that may currently be underutilized.
Our study suggests several additional explanations for physicians' current practice and opinions. Only 6.5% of respondents who answered that an intervention was inappropriate indicated as a justification that I do not believe this is an appropriate pharmacologic intervention for this particular medical problem. This suggests that the hesitancy has little to do with a lack of benefit but instead relates to systems issues (eg, access to all pertinent records and concerns regarding follow‐up testing) and perceived limitations to what a hospitalist should and should not do without actively involving the PCP. There are likely additional concerns that the medical record and/or patient histories do not fully outline the rationale for exclusion or inclusion of particular medications. Advances in information technology that enhance information exchange and enable streamlined communication may help to address these perceived barriers. However, an additional barrier may be trust, as PCPs appear more concerned that hospitalists will not review all the pertinent records or discuss risks and benefits before enacting important medication changes. Increased attempts at communication between hospitalists and outpatient providers may help to build trust and alleviate concerns regarding the loss of information that often occurs both on admission and at discharge.
We also noted that PCPs were more likely than hospitalists to feel that inpatient interventions were appropriate, even when targeting chronic conditions unrelated to the reason for admission. It may be that PCPs, with an increasing number of problems to address per outpatient visit,[10, 11] are more open to hospitalists managing any medical problems during their patients' admissions. At the same time, with increased acuity[12, 13, 14] and shortened length of stays,[15, 16] hospitalists have only a finite amount of time to ensure acute issues are managed, leaving potentially modifiable chronic conditions to the outpatient setting. These differences aside, a minority of both PCPs and hospitalists in our study were ready to embrace the idea of hospitalists playing a larger role in the management of conditions unrelated to the reason for hospitalization.
Even though our study benefits from its multisite design, there are a number of limitations. First, although we crafted our survey with input from general medicine focus groups, our survey instrument has not been validated. In addition, the cases are necessarily contrived and do not take into account the complexities of inpatient medicine. Furthermore, though our goal was to create paired cases that isolate a management decision as being simply based on whether it was related or unrelated to the reason for admission, it is possible that other factors, not captured by our survey, influenced the responses. For example, the benefits of aspirin as part of secondary prevention are not equal to the benefits in an acute MI.[17]
In an attempt to isolate the hospitalists' role in these management decisions, respondents were instructed to assume that the decisions were being made without discussing it with the primary care physician, but that the hospitalist would communicate the details of any hospitalization at the time of discharge. They were also instructed to assume that the hospitalist has access to the patient's outpatient electronic medical record. These assumptions were made to address concerns regarding the flow of information and communication, and to simulate the ideal system from a communication and information accessibility standpoint. Had these assumptions not been placed, the responses may have differed. It is likely that PCPs and hospitalists practicing in systems without shared, accessible inpatient/outpatient medical records would be even more reluctant to enact medication changes unrelated to the reason for admission.
Along the same lines, our physician cohort consisted of several metropolitan academic physician groups, in which hospitalists have had a presence for almost 20 years. As a result, our findings may not be generalizable to other academic hospitals, community‐based hospitalist programs, or nonhospital‐based PCP practices. Finally, we do not know whether survey nonresponders differed from responders in ways that could have meaningfully affected our results.
In conclusion, our findings suggest that both PCPs and hospitalists see the management of conditions unrelated to the reason for admission as less appropriate than the management of conditions related to the reason for admission. Our findings also suggest that PCPs may be more open to this practice when compared to hospitalists. Failure to capitalize on opportunities for meaningful medical interventions, independent of patient location, suggests a possible lack of patient centeredness in the current partnership between PCPs and hospitalists. Further studies should examine existing barriers and investigate interventions designed to address those barriers, in an effort to improve both quality of care and the degree of patient‐centeredness in our current healthcare system.
Disclosures: Dr. Herzig is supported by a grant from the National Institute on Aging (K23 AG042459). Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Author contributions: study concept and design, Breu, Allen‐Dicker, Mueller, Herzig; acquisition of data, Breu, Allen‐Dicker, Mueller, Palamara, Herzig; analysis and interpretation of data, Breu, Allen‐Dicker, Hinami, Herzig; drafting of the manuscript, Breu; critical revision of the manuscript for important intellectual content, Breu, Allen‐Dicker, Mueller, Palamara, Hinami, Herzig; statistical analysis, Allen‐Dicker, Hinami, Herzig; study supervision, Breu, Herzig. This study was presented as a poster at the Society of Hospital Medicine National Meeting, Washington, DC, May 17, 2013.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- . An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 pt 2):338–342.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , . A national strategy to put accountable care into practice. Health Aff (Millwood). 2010;29(5):982–990.
- . Keeping score under a global payment system. N Engl J Med. 2012;366(5):393–395.
- Reporting Hospital Quality Data for Annual Payment Update. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/Downloads/HospitalRHQDAPU200808. Accessed December 18, 2013.
- , , , et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
- , , , , , . How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open. 2012;2(6):e001461.
- , , . Primary care visit duration and quality: does good care take longer? Arch Intern Med. 2009;169(20):1866–1872.
- , , , The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med. 2008;23(12):2058–2065.
- , , . Multiple chronic conditions among adults aged 45 and over: trends over the past 10 years. NCHS Data Brief. 2012;(100):1–8.
- , , . Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7(1):82.
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(suppl 3):391–395.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- . An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 pt 2):338–342.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , . A national strategy to put accountable care into practice. Health Aff (Millwood). 2010;29(5):982–990.
- . Keeping score under a global payment system. N Engl J Med. 2012;366(5):393–395.
- Reporting Hospital Quality Data for Annual Payment Update. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/Downloads/HospitalRHQDAPU200808. Accessed December 18, 2013.
- , , , et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
- , , , , , . How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open. 2012;2(6):e001461.
- , , . Primary care visit duration and quality: does good care take longer? Arch Intern Med. 2009;169(20):1866–1872.
- , , , The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med. 2008;23(12):2058–2065.
- , , . Multiple chronic conditions among adults aged 45 and over: trends over the past 10 years. NCHS Data Brief. 2012;(100):1–8.
- , , . Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7(1):82.
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(suppl 3):391–395.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
© 2014 Society of Hospital Medicine
Supercomputer accelerates whole-genome analysis

Credit: NIGMS
The time needed to sequence an entire human genome has decreased greatly in recent years, but analyzing the resulting 3 billion base pairs of genetic information from a single genome can take many months.
Now, researchers have found they can accelerate whole-genome analysis using a Cray XE6 supercomputer.
The team found this computer could process many genomes at once and was able to analyze 240 full genomes in a little over 2 days.
The researchers reported these results in Bioinformatics.
The team used Beagle, a Cray XE6 supercomputer located at Argonne National Laboratory in Illinois, in an attempt to analyze multiple genomes concurrently.
Using publicly available software packages and one quarter of its total capacity, the computer was able to align and call variants on 240 whole genomes in approximately 50 hours.
But the computer did not only speed up whole-genome analysis. It also increased the usable sequences per genome.
“Improving analysis through both speed and accuracy reduces the price per genome,” said study author Elizabeth McNally, MD, PhD, of the University of Chicago.
“With this approach, the price for analyzing an entire genome is less than the cost of looking at just a fraction of the genome. New technology promises to bring the costs of sequencing down to around $1000 per genome. Our goal is get the cost of analysis down into that range.”
The findings of this research have immediate medical applications, according to Dr McNally. She noted that she and her colleagues must often sequence genes from an initial patient as well as multiple family members in order to better understand and either treat or prevent a disease.
“We start genetic testing with the patient,” she said. “But when we find a significant mutation, we have to think about testing the whole family to identify individuals at risk.”
Furthermore, the range of testable mutations has greatly increased in recent years.
“In the early days, we would test 1 to 3 genes,” Dr McNally said. “In 2007, we did our first 5-gene panel. Now, we order 50 to 70 genes at a time, which usually gets us an answer. At that point, it can be more useful and less expensive to sequence the whole genome.”
The information from these genomes combined with careful attention to patient and family histories adds to our knowledge about inherited disorders, according to Dr McNally.
“It can refine the classification of these disorders,” she said. “By paying close attention to family members with genes that place them at increased risk, but who do not yet show signs of disease, we can investigate early phases of a disorder.” ![]()

Credit: NIGMS
The time needed to sequence an entire human genome has decreased greatly in recent years, but analyzing the resulting 3 billion base pairs of genetic information from a single genome can take many months.
Now, researchers have found they can accelerate whole-genome analysis using a Cray XE6 supercomputer.
The team found this computer could process many genomes at once and was able to analyze 240 full genomes in a little over 2 days.
The researchers reported these results in Bioinformatics.
The team used Beagle, a Cray XE6 supercomputer located at Argonne National Laboratory in Illinois, in an attempt to analyze multiple genomes concurrently.
Using publicly available software packages and one quarter of its total capacity, the computer was able to align and call variants on 240 whole genomes in approximately 50 hours.
But the computer did not only speed up whole-genome analysis. It also increased the usable sequences per genome.
“Improving analysis through both speed and accuracy reduces the price per genome,” said study author Elizabeth McNally, MD, PhD, of the University of Chicago.
“With this approach, the price for analyzing an entire genome is less than the cost of looking at just a fraction of the genome. New technology promises to bring the costs of sequencing down to around $1000 per genome. Our goal is get the cost of analysis down into that range.”
The findings of this research have immediate medical applications, according to Dr McNally. She noted that she and her colleagues must often sequence genes from an initial patient as well as multiple family members in order to better understand and either treat or prevent a disease.
“We start genetic testing with the patient,” she said. “But when we find a significant mutation, we have to think about testing the whole family to identify individuals at risk.”
Furthermore, the range of testable mutations has greatly increased in recent years.
“In the early days, we would test 1 to 3 genes,” Dr McNally said. “In 2007, we did our first 5-gene panel. Now, we order 50 to 70 genes at a time, which usually gets us an answer. At that point, it can be more useful and less expensive to sequence the whole genome.”
The information from these genomes combined with careful attention to patient and family histories adds to our knowledge about inherited disorders, according to Dr McNally.
“It can refine the classification of these disorders,” she said. “By paying close attention to family members with genes that place them at increased risk, but who do not yet show signs of disease, we can investigate early phases of a disorder.” ![]()

Credit: NIGMS
The time needed to sequence an entire human genome has decreased greatly in recent years, but analyzing the resulting 3 billion base pairs of genetic information from a single genome can take many months.
Now, researchers have found they can accelerate whole-genome analysis using a Cray XE6 supercomputer.
The team found this computer could process many genomes at once and was able to analyze 240 full genomes in a little over 2 days.
The researchers reported these results in Bioinformatics.
The team used Beagle, a Cray XE6 supercomputer located at Argonne National Laboratory in Illinois, in an attempt to analyze multiple genomes concurrently.
Using publicly available software packages and one quarter of its total capacity, the computer was able to align and call variants on 240 whole genomes in approximately 50 hours.
But the computer did not only speed up whole-genome analysis. It also increased the usable sequences per genome.
“Improving analysis through both speed and accuracy reduces the price per genome,” said study author Elizabeth McNally, MD, PhD, of the University of Chicago.
“With this approach, the price for analyzing an entire genome is less than the cost of looking at just a fraction of the genome. New technology promises to bring the costs of sequencing down to around $1000 per genome. Our goal is get the cost of analysis down into that range.”
The findings of this research have immediate medical applications, according to Dr McNally. She noted that she and her colleagues must often sequence genes from an initial patient as well as multiple family members in order to better understand and either treat or prevent a disease.
“We start genetic testing with the patient,” she said. “But when we find a significant mutation, we have to think about testing the whole family to identify individuals at risk.”
Furthermore, the range of testable mutations has greatly increased in recent years.
“In the early days, we would test 1 to 3 genes,” Dr McNally said. “In 2007, we did our first 5-gene panel. Now, we order 50 to 70 genes at a time, which usually gets us an answer. At that point, it can be more useful and less expensive to sequence the whole genome.”
The information from these genomes combined with careful attention to patient and family histories adds to our knowledge about inherited disorders, according to Dr McNally.
“It can refine the classification of these disorders,” she said. “By paying close attention to family members with genes that place them at increased risk, but who do not yet show signs of disease, we can investigate early phases of a disorder.” ![]()
Mutations linked to blood vessel disorders
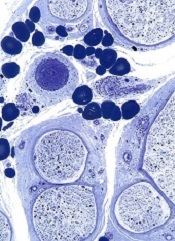
Two groups of researchers have shown that variants in the gene CECR1, which encodes for ADA2, are associated with blood vessel disorders.
One group found that mutations in CECR1 are associated with an ADA2 deficiency syndrome that is characterized by early onset, recurrent strokes and systemic vasculopathy.
The other group discovered that variants in CECR1 can cause polyarteritis nodosa vasculopathy.
Results of both studies appear in NEJM.
ADA2 deficiency syndrome
In the first study, Qing Zhou, PhD, of the National Human Genome Research Institute (NHGRI), and his colleagues used whole-exome sequencing to gain insight into the aforementioned syndrome, which was characterized by early onset lacunar strokes, systemic vasculopathy, and other symptoms.
The researchers performed whole-exome sequencing in 3 patients with the syndrome and their unaffected parents. In comparing 2 of the patients’ exomes to the exomes of their parents, the researchers located 2 variants in CECR1.
Sequencing a third patient revealed another harmful variant of CECR1, in addition to a small genomic deletion that shuts down the second copy of the gene.
The researchers needed only a sequence reading of that single gene to confirm that 3 other patients were affected by variants in CECR1. In all, these 6 patients were compound heterozygous for 8 mutations in CECR1.
The team discovered that these CECR1 mutations lead to the elimination of ADA2, which produces abnormalities and inflammation in blood vessel walls that ultimately result in the syndrome. So the team called the syndrome deficiency of ADA2 (DADA2).
To gain more insight into DADA2, the researchers induced ADA2 deficiency in a zebrafish model. They found that zebrafish embryos that produce less ADA2 than normal embryos have cerebral bleeds similar to those seen in some of the children with DADA2.
“Our study raises the possibility that the ADA2 pathway may contribute to susceptibility to stroke in the more general population,” said Daniel Kastner, MD, PhD, of NHGRI.
“This genome sequencing study expands what has previously been known about vascular biology. The role of ADA2 in such serious human disease is important and suggests that ADA2 variants may contribute to other, more common illnesses.”
Dr Kastner and his colleagues also sequenced the CECR1 gene in 3 patients from Turkey who had some of the symptoms of DADA2. These patients, who had polyarteritis nodosa (n=2) or small-vessel vasculitis (n=1), were homozygous for the p.Gly47Arg mutation.
ADA2 in polyarteritis nodosa
In the second study, researchers found the same mutation (p.Gly47Arg) in patients that emigrated to Israel from the country of Georgia.
“We now know that this mutation exists in the Middle East and in Pakistani populations and that it is not that uncommon,” said Ivona Aksentijevich, MD, of NHGRI. “This is the first time a single gene has been discovered that is involved in causing a system-wide form of vasculitis.”
To uncover this mutation, Paulina Navon-Elkan, MD, of the Shaare Zedek Medical Center in Jerusalem, and her colleagues sequenced 37 patients who had features of polyarteritis nodosa.
Nineteen patients were of Georgian Jewish ancestry. Sixteen of these patients came from 5 families, and 3 patients were unrelated. An additional 14 unrelated patients were of Turkish ancestry. And 4 German patients came from the same family.
The researchers found that, in all the families, vasculitis was a result of recessive mutations in CECR1.
All of the Georgian Jewish patients were homozygous for the p.Gly47Arg mutation. The German patients were compound heterozygous for Arg169Gln and Pro251Leu mutations. And 1 Turkish patient was compound heterozygous for Gly47Val and Trp264Ser mutations.
The researchers also analyzed serum specimens from the patients and found their ADA2 activity was significantly reduced.
Taking these results together, the team concluded that mutations in CECR1 cause loss of ADA2 function that can result in polyarteritis nodosa vasculopathy with a highly varied clinical expression. ![]()

Two groups of researchers have shown that variants in the gene CECR1, which encodes for ADA2, are associated with blood vessel disorders.
One group found that mutations in CECR1 are associated with an ADA2 deficiency syndrome that is characterized by early onset, recurrent strokes and systemic vasculopathy.
The other group discovered that variants in CECR1 can cause polyarteritis nodosa vasculopathy.
Results of both studies appear in NEJM.
ADA2 deficiency syndrome
In the first study, Qing Zhou, PhD, of the National Human Genome Research Institute (NHGRI), and his colleagues used whole-exome sequencing to gain insight into the aforementioned syndrome, which was characterized by early onset lacunar strokes, systemic vasculopathy, and other symptoms.
The researchers performed whole-exome sequencing in 3 patients with the syndrome and their unaffected parents. In comparing 2 of the patients’ exomes to the exomes of their parents, the researchers located 2 variants in CECR1.
Sequencing a third patient revealed another harmful variant of CECR1, in addition to a small genomic deletion that shuts down the second copy of the gene.
The researchers needed only a sequence reading of that single gene to confirm that 3 other patients were affected by variants in CECR1. In all, these 6 patients were compound heterozygous for 8 mutations in CECR1.
The team discovered that these CECR1 mutations lead to the elimination of ADA2, which produces abnormalities and inflammation in blood vessel walls that ultimately result in the syndrome. So the team called the syndrome deficiency of ADA2 (DADA2).
To gain more insight into DADA2, the researchers induced ADA2 deficiency in a zebrafish model. They found that zebrafish embryos that produce less ADA2 than normal embryos have cerebral bleeds similar to those seen in some of the children with DADA2.
“Our study raises the possibility that the ADA2 pathway may contribute to susceptibility to stroke in the more general population,” said Daniel Kastner, MD, PhD, of NHGRI.
“This genome sequencing study expands what has previously been known about vascular biology. The role of ADA2 in such serious human disease is important and suggests that ADA2 variants may contribute to other, more common illnesses.”
Dr Kastner and his colleagues also sequenced the CECR1 gene in 3 patients from Turkey who had some of the symptoms of DADA2. These patients, who had polyarteritis nodosa (n=2) or small-vessel vasculitis (n=1), were homozygous for the p.Gly47Arg mutation.
ADA2 in polyarteritis nodosa
In the second study, researchers found the same mutation (p.Gly47Arg) in patients that emigrated to Israel from the country of Georgia.
“We now know that this mutation exists in the Middle East and in Pakistani populations and that it is not that uncommon,” said Ivona Aksentijevich, MD, of NHGRI. “This is the first time a single gene has been discovered that is involved in causing a system-wide form of vasculitis.”
To uncover this mutation, Paulina Navon-Elkan, MD, of the Shaare Zedek Medical Center in Jerusalem, and her colleagues sequenced 37 patients who had features of polyarteritis nodosa.
Nineteen patients were of Georgian Jewish ancestry. Sixteen of these patients came from 5 families, and 3 patients were unrelated. An additional 14 unrelated patients were of Turkish ancestry. And 4 German patients came from the same family.
The researchers found that, in all the families, vasculitis was a result of recessive mutations in CECR1.
All of the Georgian Jewish patients were homozygous for the p.Gly47Arg mutation. The German patients were compound heterozygous for Arg169Gln and Pro251Leu mutations. And 1 Turkish patient was compound heterozygous for Gly47Val and Trp264Ser mutations.
The researchers also analyzed serum specimens from the patients and found their ADA2 activity was significantly reduced.
Taking these results together, the team concluded that mutations in CECR1 cause loss of ADA2 function that can result in polyarteritis nodosa vasculopathy with a highly varied clinical expression. ![]()

Two groups of researchers have shown that variants in the gene CECR1, which encodes for ADA2, are associated with blood vessel disorders.
One group found that mutations in CECR1 are associated with an ADA2 deficiency syndrome that is characterized by early onset, recurrent strokes and systemic vasculopathy.
The other group discovered that variants in CECR1 can cause polyarteritis nodosa vasculopathy.
Results of both studies appear in NEJM.
ADA2 deficiency syndrome
In the first study, Qing Zhou, PhD, of the National Human Genome Research Institute (NHGRI), and his colleagues used whole-exome sequencing to gain insight into the aforementioned syndrome, which was characterized by early onset lacunar strokes, systemic vasculopathy, and other symptoms.
The researchers performed whole-exome sequencing in 3 patients with the syndrome and their unaffected parents. In comparing 2 of the patients’ exomes to the exomes of their parents, the researchers located 2 variants in CECR1.
Sequencing a third patient revealed another harmful variant of CECR1, in addition to a small genomic deletion that shuts down the second copy of the gene.
The researchers needed only a sequence reading of that single gene to confirm that 3 other patients were affected by variants in CECR1. In all, these 6 patients were compound heterozygous for 8 mutations in CECR1.
The team discovered that these CECR1 mutations lead to the elimination of ADA2, which produces abnormalities and inflammation in blood vessel walls that ultimately result in the syndrome. So the team called the syndrome deficiency of ADA2 (DADA2).
To gain more insight into DADA2, the researchers induced ADA2 deficiency in a zebrafish model. They found that zebrafish embryos that produce less ADA2 than normal embryos have cerebral bleeds similar to those seen in some of the children with DADA2.
“Our study raises the possibility that the ADA2 pathway may contribute to susceptibility to stroke in the more general population,” said Daniel Kastner, MD, PhD, of NHGRI.
“This genome sequencing study expands what has previously been known about vascular biology. The role of ADA2 in such serious human disease is important and suggests that ADA2 variants may contribute to other, more common illnesses.”
Dr Kastner and his colleagues also sequenced the CECR1 gene in 3 patients from Turkey who had some of the symptoms of DADA2. These patients, who had polyarteritis nodosa (n=2) or small-vessel vasculitis (n=1), were homozygous for the p.Gly47Arg mutation.
ADA2 in polyarteritis nodosa
In the second study, researchers found the same mutation (p.Gly47Arg) in patients that emigrated to Israel from the country of Georgia.
“We now know that this mutation exists in the Middle East and in Pakistani populations and that it is not that uncommon,” said Ivona Aksentijevich, MD, of NHGRI. “This is the first time a single gene has been discovered that is involved in causing a system-wide form of vasculitis.”
To uncover this mutation, Paulina Navon-Elkan, MD, of the Shaare Zedek Medical Center in Jerusalem, and her colleagues sequenced 37 patients who had features of polyarteritis nodosa.
Nineteen patients were of Georgian Jewish ancestry. Sixteen of these patients came from 5 families, and 3 patients were unrelated. An additional 14 unrelated patients were of Turkish ancestry. And 4 German patients came from the same family.
The researchers found that, in all the families, vasculitis was a result of recessive mutations in CECR1.
All of the Georgian Jewish patients were homozygous for the p.Gly47Arg mutation. The German patients were compound heterozygous for Arg169Gln and Pro251Leu mutations. And 1 Turkish patient was compound heterozygous for Gly47Val and Trp264Ser mutations.
The researchers also analyzed serum specimens from the patients and found their ADA2 activity was significantly reduced.
Taking these results together, the team concluded that mutations in CECR1 cause loss of ADA2 function that can result in polyarteritis nodosa vasculopathy with a highly varied clinical expression. ![]()