User login
South American Hospitalist Conference Draws Record Attendance
A record number of hospitalists attended the combined biannual conference of the Chilean Society of Hospitalists and the Pan American Society of Hospitalists (PASHA) July 19-20 in Santiago, Chile. The meeting sold out with more than 250 attendees, and the energy of the South American hospitalist movement could be felt inside as hospitalists from across the Americas packed into the conference center.
It was the third such combined meeting, and hospitalists from Argentina, Colombia, the United States, and all parts of Chile were present. The first national meeting of hospitalists in Chile was held in 2008, and meetings have occurred every other year since 2008. PASHA was established in 2010 to unite hospitalists in South America and build collaborations with hospitalists in North America. PASHA’s past meetings were held in Brazil and Argentina, and organizers plan to continue it annually.
Hospitalists and subspecialists gathered to update their clinical knowledge and share ideas on patient safety, quality improvement (QI), and transitions of care.
“All of our countries have different challenges, but hospitalists everywhere are trying to do the same thing—provide quality healthcare as efficiently as possible. This central goal of hospitalists allows us to share ideas and learn from each other,” said Andres Aizman, MD, director of the division of hospital medicine at Pontificia Universidad Católica de Chile in Santiago.
Lectures were translated into multiple languages, including English, to engage not only local physicians, but also those from abroad.
“This has been an incredible experience,” said Dr. Eddie Greene, a nephrologist from the Mayo Clinic in Rochester, Minn., who presented a lecture on hypertensive crises. “Getting so many talented people from different backgrounds in the same place exchanging ideas on diverse topics has been an amazing experience. This is something special, and I hope to see it continue to grow.”
Two internists at Pontificia Universidad Católica de Chile founded its first HM division in 2004. The division has grown to include nine physicians, and there is a second, five-physician group of hospitalists at Universidad de Chile, a neighboring teaching hospital. The actual number of practicing hospitalists in Chile is hard to estimate because there are many hospital-based internists that have yet to be recognized as hospitalists; however, the most active academic hospitalists in the Chilean Society of Hospitalists are at the two universities.
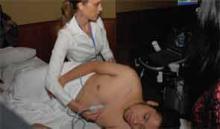
One of the highlights of this year’s conference was a workshop on bedside applications of portable ultrasound for hospitalists. Lectures on procedural and diagnostic applications of portable ultrasound were presented to the general assembly, and a hands-on workshop with limited seating was sold out.
The workshop included brief lectures, simulation-based training, and scanning of live models.
“This meeting has enlightened me on so many topics and tools that I can easily use in my hospital,” said Ofelia Leiva, MD, a hospitalist from Curico, a city of 250,000 in southern Chile. “There are so many ways we can use ultrasound in the hospital. I’m grateful that I was able to attend.”
Nilam Soni, MD, of the University of Texas Health Science Center at San Antonio, spearheaded the ultrasound workshop in collaboration with Dr. Ricardo Franco from John H. Stroger Hospital in Chicago and Dr. Carolina Candotti from Penn State Hershey Medical Center.
Faculty from the Mayo Clinic, including Drs. Jamie Newman, Fernando Rivera, John Park, and Eddie Greene, attended the meeting for a second consecutive time. Their contributions made the event even more attractive to local hospitalists.
—Ofelia Leiva, hospitalist, Curico, Chile
Although HM continues to grow in South America, barriers still exist. Each country has a different healthcare system, and the economic and political forces that drive these systems affect hospitalists and their patients. Certain metrics that are often used to gauge hospitalists’ impact in North America, such as length of stay, are less meaningful in Chile and other countries. Additionally, hospitalists’ ability to participate in conferences and exchange ideas is limited by distance and circumstance; the average hospitalist in Chile earns the equivalent of about $70,000 a year. Lastly, many hospitalists in South America aspire to develop research programs, but obtaining research training is challenging. Such opportunities are limited in South America, and physicians often encounter cost and immigration issues when they seek training abroad.
Despite the challenges, the enthusiasm to advance HM in South America and build international collaborations continues to grow. Hospitalists from different institutions in the U.S. have returned every year to participate in the conference. The opportunity to meet hospitalists from different countries, exchange ideas, and be part of the hospitalist movement abroad draws people back. The energy of the hospitalist movement is still alive.
Dr. Abbott and Dr. Rodriguez are adjunct professors and Dr. Aizman is an assistant professor in the department of internal medicine at Pontificia Universidad Católica de Chile School of Medicine in Santiago; Dr. Newman is an assistant professor in the division of hospital medicine at Mayo Clinic in Rochester, Minn.; and Dr. Soni is an assistant professor of medicine in the division of hospital medicine at the University of Texas Health Science Center at San Antonio.
A record number of hospitalists attended the combined biannual conference of the Chilean Society of Hospitalists and the Pan American Society of Hospitalists (PASHA) July 19-20 in Santiago, Chile. The meeting sold out with more than 250 attendees, and the energy of the South American hospitalist movement could be felt inside as hospitalists from across the Americas packed into the conference center.
It was the third such combined meeting, and hospitalists from Argentina, Colombia, the United States, and all parts of Chile were present. The first national meeting of hospitalists in Chile was held in 2008, and meetings have occurred every other year since 2008. PASHA was established in 2010 to unite hospitalists in South America and build collaborations with hospitalists in North America. PASHA’s past meetings were held in Brazil and Argentina, and organizers plan to continue it annually.
Hospitalists and subspecialists gathered to update their clinical knowledge and share ideas on patient safety, quality improvement (QI), and transitions of care.
“All of our countries have different challenges, but hospitalists everywhere are trying to do the same thing—provide quality healthcare as efficiently as possible. This central goal of hospitalists allows us to share ideas and learn from each other,” said Andres Aizman, MD, director of the division of hospital medicine at Pontificia Universidad Católica de Chile in Santiago.
Lectures were translated into multiple languages, including English, to engage not only local physicians, but also those from abroad.
“This has been an incredible experience,” said Dr. Eddie Greene, a nephrologist from the Mayo Clinic in Rochester, Minn., who presented a lecture on hypertensive crises. “Getting so many talented people from different backgrounds in the same place exchanging ideas on diverse topics has been an amazing experience. This is something special, and I hope to see it continue to grow.”
Two internists at Pontificia Universidad Católica de Chile founded its first HM division in 2004. The division has grown to include nine physicians, and there is a second, five-physician group of hospitalists at Universidad de Chile, a neighboring teaching hospital. The actual number of practicing hospitalists in Chile is hard to estimate because there are many hospital-based internists that have yet to be recognized as hospitalists; however, the most active academic hospitalists in the Chilean Society of Hospitalists are at the two universities.
One of the highlights of this year’s conference was a workshop on bedside applications of portable ultrasound for hospitalists. Lectures on procedural and diagnostic applications of portable ultrasound were presented to the general assembly, and a hands-on workshop with limited seating was sold out.
The workshop included brief lectures, simulation-based training, and scanning of live models.
“This meeting has enlightened me on so many topics and tools that I can easily use in my hospital,” said Ofelia Leiva, MD, a hospitalist from Curico, a city of 250,000 in southern Chile. “There are so many ways we can use ultrasound in the hospital. I’m grateful that I was able to attend.”
Nilam Soni, MD, of the University of Texas Health Science Center at San Antonio, spearheaded the ultrasound workshop in collaboration with Dr. Ricardo Franco from John H. Stroger Hospital in Chicago and Dr. Carolina Candotti from Penn State Hershey Medical Center.
Faculty from the Mayo Clinic, including Drs. Jamie Newman, Fernando Rivera, John Park, and Eddie Greene, attended the meeting for a second consecutive time. Their contributions made the event even more attractive to local hospitalists.
—Ofelia Leiva, hospitalist, Curico, Chile
Although HM continues to grow in South America, barriers still exist. Each country has a different healthcare system, and the economic and political forces that drive these systems affect hospitalists and their patients. Certain metrics that are often used to gauge hospitalists’ impact in North America, such as length of stay, are less meaningful in Chile and other countries. Additionally, hospitalists’ ability to participate in conferences and exchange ideas is limited by distance and circumstance; the average hospitalist in Chile earns the equivalent of about $70,000 a year. Lastly, many hospitalists in South America aspire to develop research programs, but obtaining research training is challenging. Such opportunities are limited in South America, and physicians often encounter cost and immigration issues when they seek training abroad.
Despite the challenges, the enthusiasm to advance HM in South America and build international collaborations continues to grow. Hospitalists from different institutions in the U.S. have returned every year to participate in the conference. The opportunity to meet hospitalists from different countries, exchange ideas, and be part of the hospitalist movement abroad draws people back. The energy of the hospitalist movement is still alive.
Dr. Abbott and Dr. Rodriguez are adjunct professors and Dr. Aizman is an assistant professor in the department of internal medicine at Pontificia Universidad Católica de Chile School of Medicine in Santiago; Dr. Newman is an assistant professor in the division of hospital medicine at Mayo Clinic in Rochester, Minn.; and Dr. Soni is an assistant professor of medicine in the division of hospital medicine at the University of Texas Health Science Center at San Antonio.
A record number of hospitalists attended the combined biannual conference of the Chilean Society of Hospitalists and the Pan American Society of Hospitalists (PASHA) July 19-20 in Santiago, Chile. The meeting sold out with more than 250 attendees, and the energy of the South American hospitalist movement could be felt inside as hospitalists from across the Americas packed into the conference center.
It was the third such combined meeting, and hospitalists from Argentina, Colombia, the United States, and all parts of Chile were present. The first national meeting of hospitalists in Chile was held in 2008, and meetings have occurred every other year since 2008. PASHA was established in 2010 to unite hospitalists in South America and build collaborations with hospitalists in North America. PASHA’s past meetings were held in Brazil and Argentina, and organizers plan to continue it annually.
Hospitalists and subspecialists gathered to update their clinical knowledge and share ideas on patient safety, quality improvement (QI), and transitions of care.
“All of our countries have different challenges, but hospitalists everywhere are trying to do the same thing—provide quality healthcare as efficiently as possible. This central goal of hospitalists allows us to share ideas and learn from each other,” said Andres Aizman, MD, director of the division of hospital medicine at Pontificia Universidad Católica de Chile in Santiago.
Lectures were translated into multiple languages, including English, to engage not only local physicians, but also those from abroad.
“This has been an incredible experience,” said Dr. Eddie Greene, a nephrologist from the Mayo Clinic in Rochester, Minn., who presented a lecture on hypertensive crises. “Getting so many talented people from different backgrounds in the same place exchanging ideas on diverse topics has been an amazing experience. This is something special, and I hope to see it continue to grow.”
Two internists at Pontificia Universidad Católica de Chile founded its first HM division in 2004. The division has grown to include nine physicians, and there is a second, five-physician group of hospitalists at Universidad de Chile, a neighboring teaching hospital. The actual number of practicing hospitalists in Chile is hard to estimate because there are many hospital-based internists that have yet to be recognized as hospitalists; however, the most active academic hospitalists in the Chilean Society of Hospitalists are at the two universities.
One of the highlights of this year’s conference was a workshop on bedside applications of portable ultrasound for hospitalists. Lectures on procedural and diagnostic applications of portable ultrasound were presented to the general assembly, and a hands-on workshop with limited seating was sold out.
The workshop included brief lectures, simulation-based training, and scanning of live models.
“This meeting has enlightened me on so many topics and tools that I can easily use in my hospital,” said Ofelia Leiva, MD, a hospitalist from Curico, a city of 250,000 in southern Chile. “There are so many ways we can use ultrasound in the hospital. I’m grateful that I was able to attend.”
Nilam Soni, MD, of the University of Texas Health Science Center at San Antonio, spearheaded the ultrasound workshop in collaboration with Dr. Ricardo Franco from John H. Stroger Hospital in Chicago and Dr. Carolina Candotti from Penn State Hershey Medical Center.
Faculty from the Mayo Clinic, including Drs. Jamie Newman, Fernando Rivera, John Park, and Eddie Greene, attended the meeting for a second consecutive time. Their contributions made the event even more attractive to local hospitalists.
—Ofelia Leiva, hospitalist, Curico, Chile
Although HM continues to grow in South America, barriers still exist. Each country has a different healthcare system, and the economic and political forces that drive these systems affect hospitalists and their patients. Certain metrics that are often used to gauge hospitalists’ impact in North America, such as length of stay, are less meaningful in Chile and other countries. Additionally, hospitalists’ ability to participate in conferences and exchange ideas is limited by distance and circumstance; the average hospitalist in Chile earns the equivalent of about $70,000 a year. Lastly, many hospitalists in South America aspire to develop research programs, but obtaining research training is challenging. Such opportunities are limited in South America, and physicians often encounter cost and immigration issues when they seek training abroad.
Despite the challenges, the enthusiasm to advance HM in South America and build international collaborations continues to grow. Hospitalists from different institutions in the U.S. have returned every year to participate in the conference. The opportunity to meet hospitalists from different countries, exchange ideas, and be part of the hospitalist movement abroad draws people back. The energy of the hospitalist movement is still alive.
Dr. Abbott and Dr. Rodriguez are adjunct professors and Dr. Aizman is an assistant professor in the department of internal medicine at Pontificia Universidad Católica de Chile School of Medicine in Santiago; Dr. Newman is an assistant professor in the division of hospital medicine at Mayo Clinic in Rochester, Minn.; and Dr. Soni is an assistant professor of medicine in the division of hospital medicine at the University of Texas Health Science Center at San Antonio.
Debate Rages Over Hospitalists' Role in ICU Physician Shortage
The long-simmering debate over whether and how hospitalists might help solve the worsening shortage of critical-care physicians is beginning to boil over.
In June, SHM and the Society of Critical Care Medicine (SCCM) issued a joint position paper proposing an expedited, one-year, critical-care fellowship for hospitalists with at least three years of clinical job experience, in lieu of the two-year fellowship now required for board certification.1
“Bringing qualified hospitalists into the critical-care workforce through rigorous sanctioned and accredited one-year training programs,” the paper asserted, “will open a new intensivist training pipeline and potentially offer more critically ill patients the benefits of providers who are unequivocally qualified to care for them.”
The backlash was swift and sharp. In a strongly worded editorial response published in July, the American College of Chest Physicians (ACCP) and the American Association of Critical-Care Nurses (AACN) declared that one year of fellowship training is inadequate for HM physicians to achieve competence in critical-care medicine.2 “No, the perfect should not be the enemy of the good in our efforts to craft solutions,” the editorial stated. “But the current imperfect SCCM/SHM proposal is an enemy of the existing good training processes already in place.”
HM leaders counter that the current strategies for bolstering the ranks of board-certified intensivists simply aren’t working, and that creative, outside-the-box thinking is required to solve the dilemma.
“Hospitalists are rapidly becoming a dominant, if not the dominant, block of physicians who are providing critical care in the United States. You can decide, if you want, whether that’s good or bad, but that’s the reality,” says Eric Siegal, MD, SFHM, lead author of the SHM/SCCM position paper, director of critical-care medicine at Aurora St. Luke’s Medical Center in Milwaukee, and an SHM board member. Given the escalating shortage of intensivists, he says, he believes that concerned stakeholders can either try to help develop the skills and knowledge of those hospitalists already in the ICU or “hope that a whole bunch of hospitalists suddenly decide to abandon their practices and complete two-year medical
critical-care fellowships.”
Intensivist leaders say that less training will do nothing to improve patient outcomes. “The reality is that hospitalists are doing it. The question should be, ‘Are they doing it well or at the detriment of the patient?’” asks Michael Baumann, MD, MS, FCCP, professor of medicine in the division of pulmonary, critical-care, and sleep medicine at the University of Mississippi Medical Center in Jackson. “The patient is the one who loses if we have somebody pinch-hitting, which is really what we’re talking about here,” adds Dr. Baumann, lead author of the ACCP/AACN editorial.
Staffing Shortfall
Despite the heated rhetoric, interviews with leaders on both sides suggest an eagerness to move forward in trying to collectively solve a problem that has vexed the entire medical community.
In 2000, the Leapfrog Group, a Washington, D.C.-based consortium of major healthcare purchasers focused on improving the safety, quality, and value of care, recommended that all ICUs should be staffed with physicians certified in critical-care medicine.3 As part of its rationale, the group cited research suggesting that greater intensivist use can yield better patient outcomes.
But a seminal study published the same year hinted at just how difficult meeting Leapfrog’s ambitious goal might be. Based on the trajectory of supply and demand, the authors forecast a 22% shortfall in intensivist hours by 2020, and a 35% shortfall by 2030, mainly due to a surge in demand from an aging U.S. population.4 A follow-up report in 2006 estimated that 53% of the nation’s ICU units had no intensivist coverage at all, and that only 4% of adult ICUs were meeting the full Leapfrog standards of high-intensity ICU staffing, dedicated attending physician coverage during the day, and dedicated coverage by any physician at night.5

—Timothy Buchman, PhD, MD, director, Emory University Center for Critical Care, Atlanta
Given the recent push for more outpatient treatment of less-critically-ill patients, many observers say the increased acuity of hospitalized patients—with more comorbidities—only exacerbates the mismatch between supply and demand. Making matters worse, providers are not evenly distributed throughout the country, with many smaller and rural hospitals already facing an acute shortage of intensivist services.
As a result, many hospitalists have been forced to step into the breach. According to SHM’s 2012 State of Hospital Medicine survey, 83.5% of responding nonacademic adult medicine groups said they routinely provide care for patients in an ICU setting, along with 27.9% of academic HM groups.
“So we have hospitalists who, either by choice or by default, care for patients who they may or may not be fully qualified to manage,” Dr. Siegal says. Practically speaking, he and his coauthors assert, the question of whether hospitalists should be in the ICU is now moot. The real question is how to ensure that those providers can deliver safe and effective care.
Experience vs. Training
Currently, internists who have completed fellowships in such specialties as pulmonary medicine, nephrology, and infectious disease can complete a one-year critical-care fellowship to obtain board certification. Experienced hospitalists have questioned the requirement that they instead complete a two-year fellowship, with no consideration given to the relevant clinical experience and maturity gained after years of hospitalist practice. In addition, they argue, it is logistically and financially unrealistic to expect a large cadre of experienced hospitalists to abandon their practices for two years to pursue critical-care training.
But Dr. Baumann says subpar internal-medicine residency requirements deserve much of the blame for offering inadequate training. “Critical care is a blend of critical thinking skills and procedural skills. Both of those are diminished tremendously in the current programs for internal medicine,” he says. “It’s really an indictment of our current training of internal-medicine residents now.”
SCCM, for its part, is sticking to its guns, albeit more quietly. When asked for comment, a spokesman issued a carefully worded statement that reads, “The paper reflects the society’s concerns regarding workforce shortages and the realities of today’s environment.”
The SHM/SCCM proposal makes sense provided that hospitalists are realistic about the types of patients they’ll see, says Timothy Buchman, PhD, MD, director of Emory University’s Center for Critical Care in Atlanta. “No one in their right mind will say one year is as good as two years. That would be folly,” he says. “On the other hand, that’s not the question. The question is, ‘Can we structure training that is competency-focused, so that the majority of people who enter the training will achieve the necessary levels of competency within a year?’”
Derek Angus, MD, chair of critical-care medicine at the University of Pittsburgh Medical Center and lead author of the 2000 study chronicling the intensivist shortfall, is more ambivalent. “Hospitalists and intensivists have to work hand in hand. In many ways, they are the two groups that run inpatient hospital medicine,” he says. In that respect, sorting out and streamlining training pathways might be a good idea.
“On the other hand, all of intensive-care training in the United States is a little thin in comparison to what goes on in many other countries,” Dr. Angus adds. “If anything, I would like to be seeing more vigorous training. So creating one more pathway that helps reinforce pretty light training feels like accreditation, in general, may be moving slightly in the wrong direction.”
Dr. Buchman and other observers view the debate as a difference in opinion among well-meaning people who are passionate about patient care. And they concede that no one knows yet who may be right.
“We do know that advanced training is required. We do know that it should be competency-focused,” Dr. Buchman says. “But what we don’t know is how long it’s really going to take to get to the competency levels that we believe are necessary to care for the patients.”
That point may provide one important opening for further discussions. Dr. Baumann agrees that the real issues are how to define critical-care competencies, how to measure them, and how to ensure that trainees prove their mettle as competent providers. “It really shouldn’t be time-based; it should be outcome-based,” he says.
The SHM/SCCM proposal, Dr. Siegal says, should be viewed as a conversation-starter. The true test will be whether everyone can reach an agreement on how to evaluate whether an ICU caregiver has attained the necessary knowledge, skills, and attitudes—and how relevant professional experience should factor into discussions over the length of training required for intensivist certification.
A Tiered Solution
The concept of tiered ICU care—already used in neonatal ICUs—might offer another opening for productive debate. “Can patients who are not that critically ill be managed by someone who hasn’t done that much critical-care training?” Dr. Angus asks. He believes it’s possible, provided patients are properly sorted and that hospitalists aren’t put in the uncomfortable position of managing medical conditions that they see only rarely. He has no problem, though, envisioning a tiered system in which fully trained intensivists spend most of their time managing the sickest patients, while other providers—including hospitalists—care for patients at intermediate risk.
Hospitalists have greeted the idea cautiously, noting that a two-tiered model might be difficult to define and standardize, and that it could present logistical challenges around transferring patients. However, Daniel D. Dressler, MD, MSc, SFHM, FACP, associate professor of internal medicine at Emory University School of Medicine and coauthor of the SHM/SCCM position paper, led a recent study that offers at least some support for a risk-based system.6
Overall, the study found no statistically significant difference in the length of stay or inpatient mortality rates for ICU patients cared for by hospitalist-led or intensivist-led teams. Among mechanically ventilated patients with intermediate illness severity, though, the study suggested that intensivist-led care resulted in a lower length of stay in both the hospital and ICU, as well as in a trend toward reduced inpatient mortality. “There may be some value in designing or developing a stratification system,” Dr. Dressler says, “but it definitely needs more study.”
In the meantime, Dr. Dressler says, more rapid solutions are needed. And although he says he understands and respects many of the doubts expressed about the SHM/SCCM proposal, he also believes some of the fear might be based on anecdotes about individual hospitalists who were deemed unlikely to thrive in an ICU environment. “For each person like that, we also know 10 or 20 people who might do really well” with just a year of additional training, says Dr. Dressler, a former SHM board member.
Now that both sides clearly have the attention of the other, leaders say they hope the opening salvos give way to more temperate discussions about how to move more skilled providers to the front lines.
“Health professionals are a smart and clever lot,” says Mary Stahl, RN, MSN, ACNS-BC, CCNS-CMC, CCRN, immediate past president of AACN and a clinical nurse specialist at the Mid America Heart Institute at Saint Luke’s Hospital in Kansas City, Mo. “I’m confident we’ll develop an effective solution—maybe several—by focusing on the fundamental belief that patients’ needs must drive caregivers’ knowledge and skills.”
Bryn Nelson is a freelance medical writer in Seattle.
References
- Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7:359-364.
- Baumann MH, Simpson SQ, Stahl M, et al. First, do no harm: less training ≠ quality care. Chest. 2012;142:5-7.
- Milstein A, Galvin RS, Delbanco SF, et al. Improving the safety of health care: the Leapfrog initiative. Eff Clin Pract. 2000;3:313-316.
- Angus DC, Kelley MA, Schmitz RJ, et al. Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284:2762-2770.
- Angus DC, Shorr AF, White A, et al. Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34:1016-1024.
- Wise KR, Akopov VA, Williams BR Jr., Ido MS, Leeper KV, Dressler DD. Hospitalists and intensivists in the medical ICU: a prospective observational study comparing mortality and length of stay between two staffing models. J Hosp Med. 2012;7:183-189.
The long-simmering debate over whether and how hospitalists might help solve the worsening shortage of critical-care physicians is beginning to boil over.
In June, SHM and the Society of Critical Care Medicine (SCCM) issued a joint position paper proposing an expedited, one-year, critical-care fellowship for hospitalists with at least three years of clinical job experience, in lieu of the two-year fellowship now required for board certification.1
“Bringing qualified hospitalists into the critical-care workforce through rigorous sanctioned and accredited one-year training programs,” the paper asserted, “will open a new intensivist training pipeline and potentially offer more critically ill patients the benefits of providers who are unequivocally qualified to care for them.”
The backlash was swift and sharp. In a strongly worded editorial response published in July, the American College of Chest Physicians (ACCP) and the American Association of Critical-Care Nurses (AACN) declared that one year of fellowship training is inadequate for HM physicians to achieve competence in critical-care medicine.2 “No, the perfect should not be the enemy of the good in our efforts to craft solutions,” the editorial stated. “But the current imperfect SCCM/SHM proposal is an enemy of the existing good training processes already in place.”
HM leaders counter that the current strategies for bolstering the ranks of board-certified intensivists simply aren’t working, and that creative, outside-the-box thinking is required to solve the dilemma.
“Hospitalists are rapidly becoming a dominant, if not the dominant, block of physicians who are providing critical care in the United States. You can decide, if you want, whether that’s good or bad, but that’s the reality,” says Eric Siegal, MD, SFHM, lead author of the SHM/SCCM position paper, director of critical-care medicine at Aurora St. Luke’s Medical Center in Milwaukee, and an SHM board member. Given the escalating shortage of intensivists, he says, he believes that concerned stakeholders can either try to help develop the skills and knowledge of those hospitalists already in the ICU or “hope that a whole bunch of hospitalists suddenly decide to abandon their practices and complete two-year medical
critical-care fellowships.”
Intensivist leaders say that less training will do nothing to improve patient outcomes. “The reality is that hospitalists are doing it. The question should be, ‘Are they doing it well or at the detriment of the patient?’” asks Michael Baumann, MD, MS, FCCP, professor of medicine in the division of pulmonary, critical-care, and sleep medicine at the University of Mississippi Medical Center in Jackson. “The patient is the one who loses if we have somebody pinch-hitting, which is really what we’re talking about here,” adds Dr. Baumann, lead author of the ACCP/AACN editorial.
Staffing Shortfall
Despite the heated rhetoric, interviews with leaders on both sides suggest an eagerness to move forward in trying to collectively solve a problem that has vexed the entire medical community.
In 2000, the Leapfrog Group, a Washington, D.C.-based consortium of major healthcare purchasers focused on improving the safety, quality, and value of care, recommended that all ICUs should be staffed with physicians certified in critical-care medicine.3 As part of its rationale, the group cited research suggesting that greater intensivist use can yield better patient outcomes.
But a seminal study published the same year hinted at just how difficult meeting Leapfrog’s ambitious goal might be. Based on the trajectory of supply and demand, the authors forecast a 22% shortfall in intensivist hours by 2020, and a 35% shortfall by 2030, mainly due to a surge in demand from an aging U.S. population.4 A follow-up report in 2006 estimated that 53% of the nation’s ICU units had no intensivist coverage at all, and that only 4% of adult ICUs were meeting the full Leapfrog standards of high-intensity ICU staffing, dedicated attending physician coverage during the day, and dedicated coverage by any physician at night.5

—Timothy Buchman, PhD, MD, director, Emory University Center for Critical Care, Atlanta
Given the recent push for more outpatient treatment of less-critically-ill patients, many observers say the increased acuity of hospitalized patients—with more comorbidities—only exacerbates the mismatch between supply and demand. Making matters worse, providers are not evenly distributed throughout the country, with many smaller and rural hospitals already facing an acute shortage of intensivist services.
As a result, many hospitalists have been forced to step into the breach. According to SHM’s 2012 State of Hospital Medicine survey, 83.5% of responding nonacademic adult medicine groups said they routinely provide care for patients in an ICU setting, along with 27.9% of academic HM groups.
“So we have hospitalists who, either by choice or by default, care for patients who they may or may not be fully qualified to manage,” Dr. Siegal says. Practically speaking, he and his coauthors assert, the question of whether hospitalists should be in the ICU is now moot. The real question is how to ensure that those providers can deliver safe and effective care.
Experience vs. Training
Currently, internists who have completed fellowships in such specialties as pulmonary medicine, nephrology, and infectious disease can complete a one-year critical-care fellowship to obtain board certification. Experienced hospitalists have questioned the requirement that they instead complete a two-year fellowship, with no consideration given to the relevant clinical experience and maturity gained after years of hospitalist practice. In addition, they argue, it is logistically and financially unrealistic to expect a large cadre of experienced hospitalists to abandon their practices for two years to pursue critical-care training.
But Dr. Baumann says subpar internal-medicine residency requirements deserve much of the blame for offering inadequate training. “Critical care is a blend of critical thinking skills and procedural skills. Both of those are diminished tremendously in the current programs for internal medicine,” he says. “It’s really an indictment of our current training of internal-medicine residents now.”
SCCM, for its part, is sticking to its guns, albeit more quietly. When asked for comment, a spokesman issued a carefully worded statement that reads, “The paper reflects the society’s concerns regarding workforce shortages and the realities of today’s environment.”
The SHM/SCCM proposal makes sense provided that hospitalists are realistic about the types of patients they’ll see, says Timothy Buchman, PhD, MD, director of Emory University’s Center for Critical Care in Atlanta. “No one in their right mind will say one year is as good as two years. That would be folly,” he says. “On the other hand, that’s not the question. The question is, ‘Can we structure training that is competency-focused, so that the majority of people who enter the training will achieve the necessary levels of competency within a year?’”
Derek Angus, MD, chair of critical-care medicine at the University of Pittsburgh Medical Center and lead author of the 2000 study chronicling the intensivist shortfall, is more ambivalent. “Hospitalists and intensivists have to work hand in hand. In many ways, they are the two groups that run inpatient hospital medicine,” he says. In that respect, sorting out and streamlining training pathways might be a good idea.
“On the other hand, all of intensive-care training in the United States is a little thin in comparison to what goes on in many other countries,” Dr. Angus adds. “If anything, I would like to be seeing more vigorous training. So creating one more pathway that helps reinforce pretty light training feels like accreditation, in general, may be moving slightly in the wrong direction.”
Dr. Buchman and other observers view the debate as a difference in opinion among well-meaning people who are passionate about patient care. And they concede that no one knows yet who may be right.
“We do know that advanced training is required. We do know that it should be competency-focused,” Dr. Buchman says. “But what we don’t know is how long it’s really going to take to get to the competency levels that we believe are necessary to care for the patients.”
That point may provide one important opening for further discussions. Dr. Baumann agrees that the real issues are how to define critical-care competencies, how to measure them, and how to ensure that trainees prove their mettle as competent providers. “It really shouldn’t be time-based; it should be outcome-based,” he says.
The SHM/SCCM proposal, Dr. Siegal says, should be viewed as a conversation-starter. The true test will be whether everyone can reach an agreement on how to evaluate whether an ICU caregiver has attained the necessary knowledge, skills, and attitudes—and how relevant professional experience should factor into discussions over the length of training required for intensivist certification.
A Tiered Solution
The concept of tiered ICU care—already used in neonatal ICUs—might offer another opening for productive debate. “Can patients who are not that critically ill be managed by someone who hasn’t done that much critical-care training?” Dr. Angus asks. He believes it’s possible, provided patients are properly sorted and that hospitalists aren’t put in the uncomfortable position of managing medical conditions that they see only rarely. He has no problem, though, envisioning a tiered system in which fully trained intensivists spend most of their time managing the sickest patients, while other providers—including hospitalists—care for patients at intermediate risk.
Hospitalists have greeted the idea cautiously, noting that a two-tiered model might be difficult to define and standardize, and that it could present logistical challenges around transferring patients. However, Daniel D. Dressler, MD, MSc, SFHM, FACP, associate professor of internal medicine at Emory University School of Medicine and coauthor of the SHM/SCCM position paper, led a recent study that offers at least some support for a risk-based system.6
Overall, the study found no statistically significant difference in the length of stay or inpatient mortality rates for ICU patients cared for by hospitalist-led or intensivist-led teams. Among mechanically ventilated patients with intermediate illness severity, though, the study suggested that intensivist-led care resulted in a lower length of stay in both the hospital and ICU, as well as in a trend toward reduced inpatient mortality. “There may be some value in designing or developing a stratification system,” Dr. Dressler says, “but it definitely needs more study.”
In the meantime, Dr. Dressler says, more rapid solutions are needed. And although he says he understands and respects many of the doubts expressed about the SHM/SCCM proposal, he also believes some of the fear might be based on anecdotes about individual hospitalists who were deemed unlikely to thrive in an ICU environment. “For each person like that, we also know 10 or 20 people who might do really well” with just a year of additional training, says Dr. Dressler, a former SHM board member.
Now that both sides clearly have the attention of the other, leaders say they hope the opening salvos give way to more temperate discussions about how to move more skilled providers to the front lines.
“Health professionals are a smart and clever lot,” says Mary Stahl, RN, MSN, ACNS-BC, CCNS-CMC, CCRN, immediate past president of AACN and a clinical nurse specialist at the Mid America Heart Institute at Saint Luke’s Hospital in Kansas City, Mo. “I’m confident we’ll develop an effective solution—maybe several—by focusing on the fundamental belief that patients’ needs must drive caregivers’ knowledge and skills.”
Bryn Nelson is a freelance medical writer in Seattle.
References
- Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7:359-364.
- Baumann MH, Simpson SQ, Stahl M, et al. First, do no harm: less training ≠ quality care. Chest. 2012;142:5-7.
- Milstein A, Galvin RS, Delbanco SF, et al. Improving the safety of health care: the Leapfrog initiative. Eff Clin Pract. 2000;3:313-316.
- Angus DC, Kelley MA, Schmitz RJ, et al. Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284:2762-2770.
- Angus DC, Shorr AF, White A, et al. Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34:1016-1024.
- Wise KR, Akopov VA, Williams BR Jr., Ido MS, Leeper KV, Dressler DD. Hospitalists and intensivists in the medical ICU: a prospective observational study comparing mortality and length of stay between two staffing models. J Hosp Med. 2012;7:183-189.
The long-simmering debate over whether and how hospitalists might help solve the worsening shortage of critical-care physicians is beginning to boil over.
In June, SHM and the Society of Critical Care Medicine (SCCM) issued a joint position paper proposing an expedited, one-year, critical-care fellowship for hospitalists with at least three years of clinical job experience, in lieu of the two-year fellowship now required for board certification.1
“Bringing qualified hospitalists into the critical-care workforce through rigorous sanctioned and accredited one-year training programs,” the paper asserted, “will open a new intensivist training pipeline and potentially offer more critically ill patients the benefits of providers who are unequivocally qualified to care for them.”
The backlash was swift and sharp. In a strongly worded editorial response published in July, the American College of Chest Physicians (ACCP) and the American Association of Critical-Care Nurses (AACN) declared that one year of fellowship training is inadequate for HM physicians to achieve competence in critical-care medicine.2 “No, the perfect should not be the enemy of the good in our efforts to craft solutions,” the editorial stated. “But the current imperfect SCCM/SHM proposal is an enemy of the existing good training processes already in place.”
HM leaders counter that the current strategies for bolstering the ranks of board-certified intensivists simply aren’t working, and that creative, outside-the-box thinking is required to solve the dilemma.
“Hospitalists are rapidly becoming a dominant, if not the dominant, block of physicians who are providing critical care in the United States. You can decide, if you want, whether that’s good or bad, but that’s the reality,” says Eric Siegal, MD, SFHM, lead author of the SHM/SCCM position paper, director of critical-care medicine at Aurora St. Luke’s Medical Center in Milwaukee, and an SHM board member. Given the escalating shortage of intensivists, he says, he believes that concerned stakeholders can either try to help develop the skills and knowledge of those hospitalists already in the ICU or “hope that a whole bunch of hospitalists suddenly decide to abandon their practices and complete two-year medical
critical-care fellowships.”
Intensivist leaders say that less training will do nothing to improve patient outcomes. “The reality is that hospitalists are doing it. The question should be, ‘Are they doing it well or at the detriment of the patient?’” asks Michael Baumann, MD, MS, FCCP, professor of medicine in the division of pulmonary, critical-care, and sleep medicine at the University of Mississippi Medical Center in Jackson. “The patient is the one who loses if we have somebody pinch-hitting, which is really what we’re talking about here,” adds Dr. Baumann, lead author of the ACCP/AACN editorial.
Staffing Shortfall
Despite the heated rhetoric, interviews with leaders on both sides suggest an eagerness to move forward in trying to collectively solve a problem that has vexed the entire medical community.
In 2000, the Leapfrog Group, a Washington, D.C.-based consortium of major healthcare purchasers focused on improving the safety, quality, and value of care, recommended that all ICUs should be staffed with physicians certified in critical-care medicine.3 As part of its rationale, the group cited research suggesting that greater intensivist use can yield better patient outcomes.
But a seminal study published the same year hinted at just how difficult meeting Leapfrog’s ambitious goal might be. Based on the trajectory of supply and demand, the authors forecast a 22% shortfall in intensivist hours by 2020, and a 35% shortfall by 2030, mainly due to a surge in demand from an aging U.S. population.4 A follow-up report in 2006 estimated that 53% of the nation’s ICU units had no intensivist coverage at all, and that only 4% of adult ICUs were meeting the full Leapfrog standards of high-intensity ICU staffing, dedicated attending physician coverage during the day, and dedicated coverage by any physician at night.5

—Timothy Buchman, PhD, MD, director, Emory University Center for Critical Care, Atlanta
Given the recent push for more outpatient treatment of less-critically-ill patients, many observers say the increased acuity of hospitalized patients—with more comorbidities—only exacerbates the mismatch between supply and demand. Making matters worse, providers are not evenly distributed throughout the country, with many smaller and rural hospitals already facing an acute shortage of intensivist services.
As a result, many hospitalists have been forced to step into the breach. According to SHM’s 2012 State of Hospital Medicine survey, 83.5% of responding nonacademic adult medicine groups said they routinely provide care for patients in an ICU setting, along with 27.9% of academic HM groups.
“So we have hospitalists who, either by choice or by default, care for patients who they may or may not be fully qualified to manage,” Dr. Siegal says. Practically speaking, he and his coauthors assert, the question of whether hospitalists should be in the ICU is now moot. The real question is how to ensure that those providers can deliver safe and effective care.
Experience vs. Training
Currently, internists who have completed fellowships in such specialties as pulmonary medicine, nephrology, and infectious disease can complete a one-year critical-care fellowship to obtain board certification. Experienced hospitalists have questioned the requirement that they instead complete a two-year fellowship, with no consideration given to the relevant clinical experience and maturity gained after years of hospitalist practice. In addition, they argue, it is logistically and financially unrealistic to expect a large cadre of experienced hospitalists to abandon their practices for two years to pursue critical-care training.
But Dr. Baumann says subpar internal-medicine residency requirements deserve much of the blame for offering inadequate training. “Critical care is a blend of critical thinking skills and procedural skills. Both of those are diminished tremendously in the current programs for internal medicine,” he says. “It’s really an indictment of our current training of internal-medicine residents now.”
SCCM, for its part, is sticking to its guns, albeit more quietly. When asked for comment, a spokesman issued a carefully worded statement that reads, “The paper reflects the society’s concerns regarding workforce shortages and the realities of today’s environment.”
The SHM/SCCM proposal makes sense provided that hospitalists are realistic about the types of patients they’ll see, says Timothy Buchman, PhD, MD, director of Emory University’s Center for Critical Care in Atlanta. “No one in their right mind will say one year is as good as two years. That would be folly,” he says. “On the other hand, that’s not the question. The question is, ‘Can we structure training that is competency-focused, so that the majority of people who enter the training will achieve the necessary levels of competency within a year?’”
Derek Angus, MD, chair of critical-care medicine at the University of Pittsburgh Medical Center and lead author of the 2000 study chronicling the intensivist shortfall, is more ambivalent. “Hospitalists and intensivists have to work hand in hand. In many ways, they are the two groups that run inpatient hospital medicine,” he says. In that respect, sorting out and streamlining training pathways might be a good idea.
“On the other hand, all of intensive-care training in the United States is a little thin in comparison to what goes on in many other countries,” Dr. Angus adds. “If anything, I would like to be seeing more vigorous training. So creating one more pathway that helps reinforce pretty light training feels like accreditation, in general, may be moving slightly in the wrong direction.”
Dr. Buchman and other observers view the debate as a difference in opinion among well-meaning people who are passionate about patient care. And they concede that no one knows yet who may be right.
“We do know that advanced training is required. We do know that it should be competency-focused,” Dr. Buchman says. “But what we don’t know is how long it’s really going to take to get to the competency levels that we believe are necessary to care for the patients.”
That point may provide one important opening for further discussions. Dr. Baumann agrees that the real issues are how to define critical-care competencies, how to measure them, and how to ensure that trainees prove their mettle as competent providers. “It really shouldn’t be time-based; it should be outcome-based,” he says.
The SHM/SCCM proposal, Dr. Siegal says, should be viewed as a conversation-starter. The true test will be whether everyone can reach an agreement on how to evaluate whether an ICU caregiver has attained the necessary knowledge, skills, and attitudes—and how relevant professional experience should factor into discussions over the length of training required for intensivist certification.
A Tiered Solution
The concept of tiered ICU care—already used in neonatal ICUs—might offer another opening for productive debate. “Can patients who are not that critically ill be managed by someone who hasn’t done that much critical-care training?” Dr. Angus asks. He believes it’s possible, provided patients are properly sorted and that hospitalists aren’t put in the uncomfortable position of managing medical conditions that they see only rarely. He has no problem, though, envisioning a tiered system in which fully trained intensivists spend most of their time managing the sickest patients, while other providers—including hospitalists—care for patients at intermediate risk.
Hospitalists have greeted the idea cautiously, noting that a two-tiered model might be difficult to define and standardize, and that it could present logistical challenges around transferring patients. However, Daniel D. Dressler, MD, MSc, SFHM, FACP, associate professor of internal medicine at Emory University School of Medicine and coauthor of the SHM/SCCM position paper, led a recent study that offers at least some support for a risk-based system.6
Overall, the study found no statistically significant difference in the length of stay or inpatient mortality rates for ICU patients cared for by hospitalist-led or intensivist-led teams. Among mechanically ventilated patients with intermediate illness severity, though, the study suggested that intensivist-led care resulted in a lower length of stay in both the hospital and ICU, as well as in a trend toward reduced inpatient mortality. “There may be some value in designing or developing a stratification system,” Dr. Dressler says, “but it definitely needs more study.”
In the meantime, Dr. Dressler says, more rapid solutions are needed. And although he says he understands and respects many of the doubts expressed about the SHM/SCCM proposal, he also believes some of the fear might be based on anecdotes about individual hospitalists who were deemed unlikely to thrive in an ICU environment. “For each person like that, we also know 10 or 20 people who might do really well” with just a year of additional training, says Dr. Dressler, a former SHM board member.
Now that both sides clearly have the attention of the other, leaders say they hope the opening salvos give way to more temperate discussions about how to move more skilled providers to the front lines.
“Health professionals are a smart and clever lot,” says Mary Stahl, RN, MSN, ACNS-BC, CCNS-CMC, CCRN, immediate past president of AACN and a clinical nurse specialist at the Mid America Heart Institute at Saint Luke’s Hospital in Kansas City, Mo. “I’m confident we’ll develop an effective solution—maybe several—by focusing on the fundamental belief that patients’ needs must drive caregivers’ knowledge and skills.”
Bryn Nelson is a freelance medical writer in Seattle.
References
- Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7:359-364.
- Baumann MH, Simpson SQ, Stahl M, et al. First, do no harm: less training ≠ quality care. Chest. 2012;142:5-7.
- Milstein A, Galvin RS, Delbanco SF, et al. Improving the safety of health care: the Leapfrog initiative. Eff Clin Pract. 2000;3:313-316.
- Angus DC, Kelley MA, Schmitz RJ, et al. Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284:2762-2770.
- Angus DC, Shorr AF, White A, et al. Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34:1016-1024.
- Wise KR, Akopov VA, Williams BR Jr., Ido MS, Leeper KV, Dressler DD. Hospitalists and intensivists in the medical ICU: a prospective observational study comparing mortality and length of stay between two staffing models. J Hosp Med. 2012;7:183-189.
CORRECTION
A dosage recommendation in the August 2012 article “What is the Optimal Therapy for Acute DVT” (p. 17) should have read: The 2012 American College of Chest Physicians (ACCP) guidelines on antithrombotic therapy for VTE recommends initial therapy with LMWH or fondaparinux (rather than IV or SC UFH). The guidelines suggest that LMWH once-daily dosing is favored over twice-daily dosing, based mainly on patient convenience, although this is a weak recommendation (2C) based on the overall quality of the data. The recommendation applies only if the daily dosing of the LMWH, including tinzaparin, dalteparin, and nadroparin, is equivalent to the twice-daily dosing (i.e. dalteparin may be dosed at 100 units/kg BID vs. 200 units/kg daily). Of importance, enoxaparin has not been studied at a once-daily dose (2 mg/kg), which is equivalent to the twice-daily dosing regimen (1 mg/kg twice daily). Additionally, one study suggests that once-daily dosing of enoxaparin 1.5 mg/kg might be inferior to 1 mg/kg twice daily dosing; therefore, caution must be exercised in applying this recommendation to the LMWH enoxaparin.3,27,28
A dosage recommendation in the August 2012 article “What is the Optimal Therapy for Acute DVT” (p. 17) should have read: The 2012 American College of Chest Physicians (ACCP) guidelines on antithrombotic therapy for VTE recommends initial therapy with LMWH or fondaparinux (rather than IV or SC UFH). The guidelines suggest that LMWH once-daily dosing is favored over twice-daily dosing, based mainly on patient convenience, although this is a weak recommendation (2C) based on the overall quality of the data. The recommendation applies only if the daily dosing of the LMWH, including tinzaparin, dalteparin, and nadroparin, is equivalent to the twice-daily dosing (i.e. dalteparin may be dosed at 100 units/kg BID vs. 200 units/kg daily). Of importance, enoxaparin has not been studied at a once-daily dose (2 mg/kg), which is equivalent to the twice-daily dosing regimen (1 mg/kg twice daily). Additionally, one study suggests that once-daily dosing of enoxaparin 1.5 mg/kg might be inferior to 1 mg/kg twice daily dosing; therefore, caution must be exercised in applying this recommendation to the LMWH enoxaparin.3,27,28
A dosage recommendation in the August 2012 article “What is the Optimal Therapy for Acute DVT” (p. 17) should have read: The 2012 American College of Chest Physicians (ACCP) guidelines on antithrombotic therapy for VTE recommends initial therapy with LMWH or fondaparinux (rather than IV or SC UFH). The guidelines suggest that LMWH once-daily dosing is favored over twice-daily dosing, based mainly on patient convenience, although this is a weak recommendation (2C) based on the overall quality of the data. The recommendation applies only if the daily dosing of the LMWH, including tinzaparin, dalteparin, and nadroparin, is equivalent to the twice-daily dosing (i.e. dalteparin may be dosed at 100 units/kg BID vs. 200 units/kg daily). Of importance, enoxaparin has not been studied at a once-daily dose (2 mg/kg), which is equivalent to the twice-daily dosing regimen (1 mg/kg twice daily). Additionally, one study suggests that once-daily dosing of enoxaparin 1.5 mg/kg might be inferior to 1 mg/kg twice daily dosing; therefore, caution must be exercised in applying this recommendation to the LMWH enoxaparin.3,27,28
Rules of Engagement Necessary for Comanagement of Orthopedic Patients
One of our providers wants to use adult hospitalists for coverage of inpatient orthopedic surgery patients. Is this acceptable practice? Are there qualifiers?
–Libby Gardner
Dr. Hospitalist responds:
Let’s see how far we can tackle this open-ended question. There has been lots of discussion on the topic of comanagement in the past by people eminently more qualified than I am. Still, it never hurts to take a fresh look at things.
For one, on the subject of admissions, I am a firm believer that hospitalists should admit all adult hip fractures. The overwhelming majority of the time, these patients are elderly with comorbid conditions. Sure, they are going to get their hip fixed, because the alternative is usually unacceptable, but some thought needs to go into the process. The orthopedic surgeon sees a hip that needs fixing and not much else. When issues like renal failure, afib, CHF, prior DVT, or dementia are present, hospitalists should take charge of the case. It is the best way to ensure that the patient receives optimal medical care and the documentation that goes along with it. I love our orthopedic surgeons, but I don’t want them primarily admitting, managing, and discharging my elderly patients. Let the surgeon do what they do best, which is operate, and leave the rest to us.
On the subject of orthopedic trauma, I take the exact opposite tack—this is not something for which I or most of my colleagues have expertise. A young, healthy patient with trauma should be admitted by the orthopedic service; that patient population’s complications are much more likely to be directly related to their trauma.
When it comes to elective surgery, when the admitting surgeon (orthopedic or otherwise) wants the help of a hospitalist, then I think it is of paramount importance to have clear “rules of engagement.” I think with good expectations, you can have a fantastic working relationship with your surgeons. Without them, it becomes a nightmare.
Here are my HM group’s rules for elective orthopedic surgery:
- Orthopedics handles all pain medications and VTE prophylaxis, including discharge prescriptions.
- Medicine handles all admit and discharge medication reconciliation (“med rec”).
- There is shared discussion on:
- Need for transfusion; and
- The VTE prophylaxis when a patient already is on chronic anticoagulation.
We do not vary from this protocol. I never adjust a patient’s pain medications. Even the floor nurses know this. Because I’m doing the admit med rec, it also means that the patient doesn’t have their HCTZ continued after 600cc of EBL and spinal anesthesia.
The system works because the rules are clear and the communication is consistent. This does not mean that we cover the orthopedic service at night. They are equally responsible for their patients under the items outlined above. In my view—and this might sound simplistic—the surgeon caused the post-op pain, so they should be responsible for managing it. On VTE prophylaxis, I might take a more nuanced view, but for our surgeons, they own the wound and the post-op follow-up, so they get the choice on what agent to use.
Would I accept an arrangement in which I covered all the orthopedic issues out of regular hours? Nope—not when they have primary responsibility for the case; they should always be directly available to the nurse. I think that anything else would be a system ripe for abuse.
Our exact rules will not work for every situation, but I would strongly encourage the two basic tenets from above: No. 1, the hospitalist should primarily admit and manage elderly hip fractures, and No. 2, clear rules of engagement should be established with your orthopedic or surgery group. It’s a discussion worth having during daylight hours, because trying to figure out the rules at 3 in the morning rarely ends well.
One of our providers wants to use adult hospitalists for coverage of inpatient orthopedic surgery patients. Is this acceptable practice? Are there qualifiers?
–Libby Gardner
Dr. Hospitalist responds:
Let’s see how far we can tackle this open-ended question. There has been lots of discussion on the topic of comanagement in the past by people eminently more qualified than I am. Still, it never hurts to take a fresh look at things.
For one, on the subject of admissions, I am a firm believer that hospitalists should admit all adult hip fractures. The overwhelming majority of the time, these patients are elderly with comorbid conditions. Sure, they are going to get their hip fixed, because the alternative is usually unacceptable, but some thought needs to go into the process. The orthopedic surgeon sees a hip that needs fixing and not much else. When issues like renal failure, afib, CHF, prior DVT, or dementia are present, hospitalists should take charge of the case. It is the best way to ensure that the patient receives optimal medical care and the documentation that goes along with it. I love our orthopedic surgeons, but I don’t want them primarily admitting, managing, and discharging my elderly patients. Let the surgeon do what they do best, which is operate, and leave the rest to us.
On the subject of orthopedic trauma, I take the exact opposite tack—this is not something for which I or most of my colleagues have expertise. A young, healthy patient with trauma should be admitted by the orthopedic service; that patient population’s complications are much more likely to be directly related to their trauma.
When it comes to elective surgery, when the admitting surgeon (orthopedic or otherwise) wants the help of a hospitalist, then I think it is of paramount importance to have clear “rules of engagement.” I think with good expectations, you can have a fantastic working relationship with your surgeons. Without them, it becomes a nightmare.
Here are my HM group’s rules for elective orthopedic surgery:
- Orthopedics handles all pain medications and VTE prophylaxis, including discharge prescriptions.
- Medicine handles all admit and discharge medication reconciliation (“med rec”).
- There is shared discussion on:
- Need for transfusion; and
- The VTE prophylaxis when a patient already is on chronic anticoagulation.
We do not vary from this protocol. I never adjust a patient’s pain medications. Even the floor nurses know this. Because I’m doing the admit med rec, it also means that the patient doesn’t have their HCTZ continued after 600cc of EBL and spinal anesthesia.
The system works because the rules are clear and the communication is consistent. This does not mean that we cover the orthopedic service at night. They are equally responsible for their patients under the items outlined above. In my view—and this might sound simplistic—the surgeon caused the post-op pain, so they should be responsible for managing it. On VTE prophylaxis, I might take a more nuanced view, but for our surgeons, they own the wound and the post-op follow-up, so they get the choice on what agent to use.
Would I accept an arrangement in which I covered all the orthopedic issues out of regular hours? Nope—not when they have primary responsibility for the case; they should always be directly available to the nurse. I think that anything else would be a system ripe for abuse.
Our exact rules will not work for every situation, but I would strongly encourage the two basic tenets from above: No. 1, the hospitalist should primarily admit and manage elderly hip fractures, and No. 2, clear rules of engagement should be established with your orthopedic or surgery group. It’s a discussion worth having during daylight hours, because trying to figure out the rules at 3 in the morning rarely ends well.
One of our providers wants to use adult hospitalists for coverage of inpatient orthopedic surgery patients. Is this acceptable practice? Are there qualifiers?
–Libby Gardner
Dr. Hospitalist responds:
Let’s see how far we can tackle this open-ended question. There has been lots of discussion on the topic of comanagement in the past by people eminently more qualified than I am. Still, it never hurts to take a fresh look at things.
For one, on the subject of admissions, I am a firm believer that hospitalists should admit all adult hip fractures. The overwhelming majority of the time, these patients are elderly with comorbid conditions. Sure, they are going to get their hip fixed, because the alternative is usually unacceptable, but some thought needs to go into the process. The orthopedic surgeon sees a hip that needs fixing and not much else. When issues like renal failure, afib, CHF, prior DVT, or dementia are present, hospitalists should take charge of the case. It is the best way to ensure that the patient receives optimal medical care and the documentation that goes along with it. I love our orthopedic surgeons, but I don’t want them primarily admitting, managing, and discharging my elderly patients. Let the surgeon do what they do best, which is operate, and leave the rest to us.
On the subject of orthopedic trauma, I take the exact opposite tack—this is not something for which I or most of my colleagues have expertise. A young, healthy patient with trauma should be admitted by the orthopedic service; that patient population’s complications are much more likely to be directly related to their trauma.
When it comes to elective surgery, when the admitting surgeon (orthopedic or otherwise) wants the help of a hospitalist, then I think it is of paramount importance to have clear “rules of engagement.” I think with good expectations, you can have a fantastic working relationship with your surgeons. Without them, it becomes a nightmare.
Here are my HM group’s rules for elective orthopedic surgery:
- Orthopedics handles all pain medications and VTE prophylaxis, including discharge prescriptions.
- Medicine handles all admit and discharge medication reconciliation (“med rec”).
- There is shared discussion on:
- Need for transfusion; and
- The VTE prophylaxis when a patient already is on chronic anticoagulation.
We do not vary from this protocol. I never adjust a patient’s pain medications. Even the floor nurses know this. Because I’m doing the admit med rec, it also means that the patient doesn’t have their HCTZ continued after 600cc of EBL and spinal anesthesia.
The system works because the rules are clear and the communication is consistent. This does not mean that we cover the orthopedic service at night. They are equally responsible for their patients under the items outlined above. In my view—and this might sound simplistic—the surgeon caused the post-op pain, so they should be responsible for managing it. On VTE prophylaxis, I might take a more nuanced view, but for our surgeons, they own the wound and the post-op follow-up, so they get the choice on what agent to use.
Would I accept an arrangement in which I covered all the orthopedic issues out of regular hours? Nope—not when they have primary responsibility for the case; they should always be directly available to the nurse. I think that anything else would be a system ripe for abuse.
Our exact rules will not work for every situation, but I would strongly encourage the two basic tenets from above: No. 1, the hospitalist should primarily admit and manage elderly hip fractures, and No. 2, clear rules of engagement should be established with your orthopedic or surgery group. It’s a discussion worth having during daylight hours, because trying to figure out the rules at 3 in the morning rarely ends well.
Society of Hospital Medicine Seeks to Connect Hospitalists Far and Wide
Hospitalists join SHM for lots of reasons, but the ability to network with hospitalists across the country is among the top motivators. In an emerging medical specialty, being able to collaborate and connect with peers is critical to career development and improving care.
Now, SHM has made connecting and collaborating easier than ever with Hospital Medicine Exchange (HMX), the first online community exclusively for hospitalists and hot topics in HM. Using HMX, hospitalists can start public discussions, post responses, and share files in one location.
For fast-moving issues, such as healthcare reform and hospitalist program management, HMX enables hospitalists to go straight to the source of some of the most important innovations: other hospitalists.
Using HMX is easy. Hospitalists log in to HMX using their SHM member username and password at HMXchange.org (automated assistance is available for those who don’t know their usernames or passwords). Once logged into HMX, hospitalists can browse communities, check out recent discussion threads, and update their profiles. Members can browse contacts to connect with thousands of other HMX users.
In addition to being the new home for conversations in hospital medicine, communities within HMX are replacing an array of legacy programs (e.g. the email listservs for practice management and SHM’s Leadership Academy alumni). HMX provides new flexibility not available in older systems like the listservs. Users can now opt into communities easily and decide how often they receive updates via email.
Conversation-Starters
Some hospitalists began collaborating with the HMX platform nearly a year ago. At the time, the platform was known as Higher Logic and supported SHM’s CODE-H, an educational program for hospital coding, along with SHM’s Hospital Value-Based Purchasing Toolkit (HVBP), a set of online resources and a community for hospitalists preparing their hospitals for value-based purchasing.
Patrick Torcson, MD, MMM, FACP, SFHM, led the HVBP community and sees the promise that HMX offers hospitalists. “It’s a nice synthesis of both content and resources because of the networking element,” he says. “It has a very real personal element as well.”
The HVBP community used HMX as equal parts educational session, networking, and library. Dr. Torcson and community members presented webinars, then followed up with discussions. Community members also shared resources about value-based purchasing through the discussion threads and the online library.
The immediate online interaction proved to be especially valuable when discussing a topic that was anything but static, he says.
“Because hospital value-based purchasing was unfolding over a timeline from [the Centers for Medicare & Medicaid Services (CMS)], we were able to add content as the program unfolded, including policy papers from Washington, webinars, and other relevant information,” Dr. Torcson explains. “For something like value-based purchasing, there’s no definitive source, so the collaboration was helpful. It’s true for a lot of topics in hospital medicine.
“To have a community tool like this that can accommodate for all the different inputs is really very valuable,” he adds.

—Patrick Torcson, MD, MMM, FACP, SFHM, chair, SHM’s Performance Measuring and Reporting Committee
Something for Every Member
In addition to a community for general issues affecting hospitalists, HMX also features specific communities designed to facilitate conversations on particular issues. As the communities evolve and conversations develop, SHM will add new communities.
Because so many hospitalists access the Internet from mobile devices, HMX is available for iPhones, iPads, and Android platforms through a third-party mobile application. Instructions for downloading and using the app are available at the HMX website.
SHM notified certain listserv users and others about the HMX introduction in August and September. Users quickly took to the new platform. Within a week of introducing HMX, nearly 100 hospitalists logged in for the first time.
The early interest in HMX isn’t surprising to Dr. Torcson, who says he “definitely” will use HMX in the future.
“It’s a great way to share best practices and case studies,” he says. “The personal dimension was really nice to connect so easily with the hospital medicine community. It’s nice to get the perspectives from other colleagues around the country and in different settings.”
Brendon Shank is SHM’s associate vice president of communications.
Hospitalists join SHM for lots of reasons, but the ability to network with hospitalists across the country is among the top motivators. In an emerging medical specialty, being able to collaborate and connect with peers is critical to career development and improving care.
Now, SHM has made connecting and collaborating easier than ever with Hospital Medicine Exchange (HMX), the first online community exclusively for hospitalists and hot topics in HM. Using HMX, hospitalists can start public discussions, post responses, and share files in one location.
For fast-moving issues, such as healthcare reform and hospitalist program management, HMX enables hospitalists to go straight to the source of some of the most important innovations: other hospitalists.
Using HMX is easy. Hospitalists log in to HMX using their SHM member username and password at HMXchange.org (automated assistance is available for those who don’t know their usernames or passwords). Once logged into HMX, hospitalists can browse communities, check out recent discussion threads, and update their profiles. Members can browse contacts to connect with thousands of other HMX users.
In addition to being the new home for conversations in hospital medicine, communities within HMX are replacing an array of legacy programs (e.g. the email listservs for practice management and SHM’s Leadership Academy alumni). HMX provides new flexibility not available in older systems like the listservs. Users can now opt into communities easily and decide how often they receive updates via email.
Conversation-Starters
Some hospitalists began collaborating with the HMX platform nearly a year ago. At the time, the platform was known as Higher Logic and supported SHM’s CODE-H, an educational program for hospital coding, along with SHM’s Hospital Value-Based Purchasing Toolkit (HVBP), a set of online resources and a community for hospitalists preparing their hospitals for value-based purchasing.
Patrick Torcson, MD, MMM, FACP, SFHM, led the HVBP community and sees the promise that HMX offers hospitalists. “It’s a nice synthesis of both content and resources because of the networking element,” he says. “It has a very real personal element as well.”
The HVBP community used HMX as equal parts educational session, networking, and library. Dr. Torcson and community members presented webinars, then followed up with discussions. Community members also shared resources about value-based purchasing through the discussion threads and the online library.
The immediate online interaction proved to be especially valuable when discussing a topic that was anything but static, he says.
“Because hospital value-based purchasing was unfolding over a timeline from [the Centers for Medicare & Medicaid Services (CMS)], we were able to add content as the program unfolded, including policy papers from Washington, webinars, and other relevant information,” Dr. Torcson explains. “For something like value-based purchasing, there’s no definitive source, so the collaboration was helpful. It’s true for a lot of topics in hospital medicine.
“To have a community tool like this that can accommodate for all the different inputs is really very valuable,” he adds.

—Patrick Torcson, MD, MMM, FACP, SFHM, chair, SHM’s Performance Measuring and Reporting Committee
Something for Every Member
In addition to a community for general issues affecting hospitalists, HMX also features specific communities designed to facilitate conversations on particular issues. As the communities evolve and conversations develop, SHM will add new communities.
Because so many hospitalists access the Internet from mobile devices, HMX is available for iPhones, iPads, and Android platforms through a third-party mobile application. Instructions for downloading and using the app are available at the HMX website.
SHM notified certain listserv users and others about the HMX introduction in August and September. Users quickly took to the new platform. Within a week of introducing HMX, nearly 100 hospitalists logged in for the first time.
The early interest in HMX isn’t surprising to Dr. Torcson, who says he “definitely” will use HMX in the future.
“It’s a great way to share best practices and case studies,” he says. “The personal dimension was really nice to connect so easily with the hospital medicine community. It’s nice to get the perspectives from other colleagues around the country and in different settings.”
Brendon Shank is SHM’s associate vice president of communications.
Hospitalists join SHM for lots of reasons, but the ability to network with hospitalists across the country is among the top motivators. In an emerging medical specialty, being able to collaborate and connect with peers is critical to career development and improving care.
Now, SHM has made connecting and collaborating easier than ever with Hospital Medicine Exchange (HMX), the first online community exclusively for hospitalists and hot topics in HM. Using HMX, hospitalists can start public discussions, post responses, and share files in one location.
For fast-moving issues, such as healthcare reform and hospitalist program management, HMX enables hospitalists to go straight to the source of some of the most important innovations: other hospitalists.
Using HMX is easy. Hospitalists log in to HMX using their SHM member username and password at HMXchange.org (automated assistance is available for those who don’t know their usernames or passwords). Once logged into HMX, hospitalists can browse communities, check out recent discussion threads, and update their profiles. Members can browse contacts to connect with thousands of other HMX users.
In addition to being the new home for conversations in hospital medicine, communities within HMX are replacing an array of legacy programs (e.g. the email listservs for practice management and SHM’s Leadership Academy alumni). HMX provides new flexibility not available in older systems like the listservs. Users can now opt into communities easily and decide how often they receive updates via email.
Conversation-Starters
Some hospitalists began collaborating with the HMX platform nearly a year ago. At the time, the platform was known as Higher Logic and supported SHM’s CODE-H, an educational program for hospital coding, along with SHM’s Hospital Value-Based Purchasing Toolkit (HVBP), a set of online resources and a community for hospitalists preparing their hospitals for value-based purchasing.
Patrick Torcson, MD, MMM, FACP, SFHM, led the HVBP community and sees the promise that HMX offers hospitalists. “It’s a nice synthesis of both content and resources because of the networking element,” he says. “It has a very real personal element as well.”
The HVBP community used HMX as equal parts educational session, networking, and library. Dr. Torcson and community members presented webinars, then followed up with discussions. Community members also shared resources about value-based purchasing through the discussion threads and the online library.
The immediate online interaction proved to be especially valuable when discussing a topic that was anything but static, he says.
“Because hospital value-based purchasing was unfolding over a timeline from [the Centers for Medicare & Medicaid Services (CMS)], we were able to add content as the program unfolded, including policy papers from Washington, webinars, and other relevant information,” Dr. Torcson explains. “For something like value-based purchasing, there’s no definitive source, so the collaboration was helpful. It’s true for a lot of topics in hospital medicine.
“To have a community tool like this that can accommodate for all the different inputs is really very valuable,” he adds.

—Patrick Torcson, MD, MMM, FACP, SFHM, chair, SHM’s Performance Measuring and Reporting Committee
Something for Every Member
In addition to a community for general issues affecting hospitalists, HMX also features specific communities designed to facilitate conversations on particular issues. As the communities evolve and conversations develop, SHM will add new communities.
Because so many hospitalists access the Internet from mobile devices, HMX is available for iPhones, iPads, and Android platforms through a third-party mobile application. Instructions for downloading and using the app are available at the HMX website.
SHM notified certain listserv users and others about the HMX introduction in August and September. Users quickly took to the new platform. Within a week of introducing HMX, nearly 100 hospitalists logged in for the first time.
The early interest in HMX isn’t surprising to Dr. Torcson, who says he “definitely” will use HMX in the future.
“It’s a great way to share best practices and case studies,” he says. “The personal dimension was really nice to connect so easily with the hospital medicine community. It’s nice to get the perspectives from other colleagues around the country and in different settings.”
Brendon Shank is SHM’s associate vice president of communications.
Hospitalists On the Move
Susan D. Hutchins, MD, has been named medical director of hospitalist services at Memorial Hermann The Woodlands Hospital in The Woodlands, Texas. Dr. Hutchins’ new responsibilities include managing nine hospitalists, two nurse practitioners, and one registered nurse as part of Memorial Hermann’s inpatient hospitalist program.
Lewis L. Low, MD, FCCM, FACP, has been promoted to senior vice president and chief medical officer of Legacy Health System in the Portland, Ore., and Vancouver, Wash., areas. Dr. Low has been commended by his colleagues for his supervision of several of Legacy’s hospitalist programs within the Portland metropolitan area.
Business Moves
Helena Regional Medical Center in Helena, Ark., began offering hospitalist services in September. Hospitalists will staff the 155-bed facility 24 hours a day in order to further the hospital’s mission of “Quality Care, Right Here.”
Inpatient Physicians of Southwest Florida (ISSF), a newly formed hospitalist group, has begun offering HM services in the Lee Memorial Health System’s Fort Myers, Fla.-area hospitals. ISSF is a collaborative between Brentwood, Tenn.-based Cogent HMG and the Hospitalist Group of Southwest Florida.
The Mauldin, S.C.-based OB Hospitalist Group has expanded its services to include the Owensboro Medical Health System’s 477-bed flagship facility in Owensboro, Ky., which serves northwestern Kentucky and southwestern Indiana.
—Michael O’Neal
Susan D. Hutchins, MD, has been named medical director of hospitalist services at Memorial Hermann The Woodlands Hospital in The Woodlands, Texas. Dr. Hutchins’ new responsibilities include managing nine hospitalists, two nurse practitioners, and one registered nurse as part of Memorial Hermann’s inpatient hospitalist program.
Lewis L. Low, MD, FCCM, FACP, has been promoted to senior vice president and chief medical officer of Legacy Health System in the Portland, Ore., and Vancouver, Wash., areas. Dr. Low has been commended by his colleagues for his supervision of several of Legacy’s hospitalist programs within the Portland metropolitan area.
Business Moves
Helena Regional Medical Center in Helena, Ark., began offering hospitalist services in September. Hospitalists will staff the 155-bed facility 24 hours a day in order to further the hospital’s mission of “Quality Care, Right Here.”
Inpatient Physicians of Southwest Florida (ISSF), a newly formed hospitalist group, has begun offering HM services in the Lee Memorial Health System’s Fort Myers, Fla.-area hospitals. ISSF is a collaborative between Brentwood, Tenn.-based Cogent HMG and the Hospitalist Group of Southwest Florida.
The Mauldin, S.C.-based OB Hospitalist Group has expanded its services to include the Owensboro Medical Health System’s 477-bed flagship facility in Owensboro, Ky., which serves northwestern Kentucky and southwestern Indiana.
—Michael O’Neal
Susan D. Hutchins, MD, has been named medical director of hospitalist services at Memorial Hermann The Woodlands Hospital in The Woodlands, Texas. Dr. Hutchins’ new responsibilities include managing nine hospitalists, two nurse practitioners, and one registered nurse as part of Memorial Hermann’s inpatient hospitalist program.
Lewis L. Low, MD, FCCM, FACP, has been promoted to senior vice president and chief medical officer of Legacy Health System in the Portland, Ore., and Vancouver, Wash., areas. Dr. Low has been commended by his colleagues for his supervision of several of Legacy’s hospitalist programs within the Portland metropolitan area.
Business Moves
Helena Regional Medical Center in Helena, Ark., began offering hospitalist services in September. Hospitalists will staff the 155-bed facility 24 hours a day in order to further the hospital’s mission of “Quality Care, Right Here.”
Inpatient Physicians of Southwest Florida (ISSF), a newly formed hospitalist group, has begun offering HM services in the Lee Memorial Health System’s Fort Myers, Fla.-area hospitals. ISSF is a collaborative between Brentwood, Tenn.-based Cogent HMG and the Hospitalist Group of Southwest Florida.
The Mauldin, S.C.-based OB Hospitalist Group has expanded its services to include the Owensboro Medical Health System’s 477-bed flagship facility in Owensboro, Ky., which serves northwestern Kentucky and southwestern Indiana.
—Michael O’Neal
Fellow in Hospital Medicine Spotlight: Kenric Maynor, MD, MPH, FHM
CAREER: Prior to joining Geisinger, Dr. Maynor served as a Robert Wood Johnson Clinical Scholar in Internal Medicine at Johns Hopkins Medical Center, Baltimore.
Education: University of North Carolina School of Medicine, internal medicine residency at Yale-New Haven Hospital; and internal medicine fellowship at Johns Hopkins Medical Center.
Notable: Dr. Maynor’s responsibilities at Geisinger will include oversight of eight medical centers, as well as leading the implementation of a new hospitalist program at Geisinger Community Medical Center in Scranton, Pa., which is planned to be operational by fall.
FYI: A Native American member of the Lumbee Tribe in southeastern North Carolina, Dr. Maynor is an avid Baltimore Orioles fan.
Quotable: “Being designated as a Fellow in the Society of Hospital Medicine has confirmed to my institution my commitment to the growth and the development of hospitalists’ principles of quality improvement, innovation, and patient safety. It has given me instant access to other Fellows with similar interests to allow for networking and collaboration.”
CAREER: Prior to joining Geisinger, Dr. Maynor served as a Robert Wood Johnson Clinical Scholar in Internal Medicine at Johns Hopkins Medical Center, Baltimore.
Education: University of North Carolina School of Medicine, internal medicine residency at Yale-New Haven Hospital; and internal medicine fellowship at Johns Hopkins Medical Center.
Notable: Dr. Maynor’s responsibilities at Geisinger will include oversight of eight medical centers, as well as leading the implementation of a new hospitalist program at Geisinger Community Medical Center in Scranton, Pa., which is planned to be operational by fall.
FYI: A Native American member of the Lumbee Tribe in southeastern North Carolina, Dr. Maynor is an avid Baltimore Orioles fan.
Quotable: “Being designated as a Fellow in the Society of Hospital Medicine has confirmed to my institution my commitment to the growth and the development of hospitalists’ principles of quality improvement, innovation, and patient safety. It has given me instant access to other Fellows with similar interests to allow for networking and collaboration.”
CAREER: Prior to joining Geisinger, Dr. Maynor served as a Robert Wood Johnson Clinical Scholar in Internal Medicine at Johns Hopkins Medical Center, Baltimore.
Education: University of North Carolina School of Medicine, internal medicine residency at Yale-New Haven Hospital; and internal medicine fellowship at Johns Hopkins Medical Center.
Notable: Dr. Maynor’s responsibilities at Geisinger will include oversight of eight medical centers, as well as leading the implementation of a new hospitalist program at Geisinger Community Medical Center in Scranton, Pa., which is planned to be operational by fall.
FYI: A Native American member of the Lumbee Tribe in southeastern North Carolina, Dr. Maynor is an avid Baltimore Orioles fan.
Quotable: “Being designated as a Fellow in the Society of Hospital Medicine has confirmed to my institution my commitment to the growth and the development of hospitalists’ principles of quality improvement, innovation, and patient safety. It has given me instant access to other Fellows with similar interests to allow for networking and collaboration.”
We Welcome the Newest SHM Members
Enter text here
Enter text here
Enter text here
Physician Value-Based Payment Initiative Would Change Medicare Reimbursement
The healthcare market is saturated with fee-for-service reimbursement schemes. The Bureau of Labor Statistics estimates that 78% of employer-sponsored health insurance plans are some type of fee-for-service plan.1 In Medicare, about 75% of beneficiaries use the traditional fee-for-service program.2 Fee-for-service denotes that payments are made on individual services, billed separately, irrespective of outcome and, in some cases, necessity.
The physician value-based payment modifier (VBPM) is an initiative that will begin shifting Medicare reimbursement for physicians away from fee-for-service schemes and toward some type of pay-for-performance model.
For hospitalists, this will have a marked impact on HM practice and might have reverberating effects in the field itself.
Established under the 2008 Medicare Improvements for Patients and Providers Act (MIPPA) and expanded under the 2010 Affordable Care Act (ACA), the VBPM will be applied to all physicians by 2017.
The VBPM program is the physician version of hospital value-based purchasing; both are designed to move the basis of payment toward the quality of care delivered, not simply for the quantity of services rendered. By linking quality measurement with payment, the Centers for Medicare & Medicaid Services (CMS) hopes to start paying for value.
While legislation required the creation of pay-for-performance programs for physicians and hospitals, the design and implementation details have been delegated to CMS, part of the U.S. Department of Health and Human Services. Thus, CMS has oversight on the specifics of the program. These specifics are promulgated through the federal rulemaking process, which requires such agencies as CMS to seek input from the general public—as well as medical societies, including SHM—as rules are proposed and finalized. Generally, there is a 30- to 90-day period after a rule is proposed for public comment, after which a rule will be finalized.
For the VBPM and its performance period starting next year, the guidelines were published for public comment in a proposed rule for the fiscal-year 2013 Physician Fee Schedule. The final rule, which will provide more definitive guidance for hospitalists, is slated to come out in November.
Hospitalists should be cognizant of how quality measurements apply to their practice and find ways to participate in such quality measurement programs as the Physician Quality Reporting System (PQRS). PQRS will become the evaluative backbone of the VBPM. It is imperative that hospitalists stay abreast of these transformative changes in the healthcare system and work to ensure that their practice patterns, which fill critical gaps in patient care, are adequately represented in these changes.
Although legislation and legislative advocacy are undoubtedly important features of policymaking, participating in the federal rulemaking process is a vital tool for helping to shape healthcare. SHM actively pursues regulatory issues in order to advocate for hospitalists and their patients. The experiences and expertise of members are critical for SHM to be able to accurately represent the specialty.
By staying informed on health policy and being engaged with SHM, members can provide invaluable perspectives to help transform the field and revolutionize the healthcare system.
Josh Lapps is SHM's government relations specialist.
References
- U.S. Bureau of Labor Statistics. Program Perspectives: fee-for-service plans. U.S. Bureau of Labor Statistics website. Available at: http://www.bls.gov/opub/perspectives/program_perspectives_vol2_issue5.pdf. Accessed Aug. 15, 2012.
- The Henry J. Kaiser Family Foundation. Medicare at a glance. The Henry J. Kaiser Family Foundation website. Available at: http://www.kff.org/medicare/upload/1066_11.pdf. Accessed Aug. 29, 2012.
The healthcare market is saturated with fee-for-service reimbursement schemes. The Bureau of Labor Statistics estimates that 78% of employer-sponsored health insurance plans are some type of fee-for-service plan.1 In Medicare, about 75% of beneficiaries use the traditional fee-for-service program.2 Fee-for-service denotes that payments are made on individual services, billed separately, irrespective of outcome and, in some cases, necessity.
The physician value-based payment modifier (VBPM) is an initiative that will begin shifting Medicare reimbursement for physicians away from fee-for-service schemes and toward some type of pay-for-performance model.
For hospitalists, this will have a marked impact on HM practice and might have reverberating effects in the field itself.
Established under the 2008 Medicare Improvements for Patients and Providers Act (MIPPA) and expanded under the 2010 Affordable Care Act (ACA), the VBPM will be applied to all physicians by 2017.
The VBPM program is the physician version of hospital value-based purchasing; both are designed to move the basis of payment toward the quality of care delivered, not simply for the quantity of services rendered. By linking quality measurement with payment, the Centers for Medicare & Medicaid Services (CMS) hopes to start paying for value.
While legislation required the creation of pay-for-performance programs for physicians and hospitals, the design and implementation details have been delegated to CMS, part of the U.S. Department of Health and Human Services. Thus, CMS has oversight on the specifics of the program. These specifics are promulgated through the federal rulemaking process, which requires such agencies as CMS to seek input from the general public—as well as medical societies, including SHM—as rules are proposed and finalized. Generally, there is a 30- to 90-day period after a rule is proposed for public comment, after which a rule will be finalized.
For the VBPM and its performance period starting next year, the guidelines were published for public comment in a proposed rule for the fiscal-year 2013 Physician Fee Schedule. The final rule, which will provide more definitive guidance for hospitalists, is slated to come out in November.
Hospitalists should be cognizant of how quality measurements apply to their practice and find ways to participate in such quality measurement programs as the Physician Quality Reporting System (PQRS). PQRS will become the evaluative backbone of the VBPM. It is imperative that hospitalists stay abreast of these transformative changes in the healthcare system and work to ensure that their practice patterns, which fill critical gaps in patient care, are adequately represented in these changes.
Although legislation and legislative advocacy are undoubtedly important features of policymaking, participating in the federal rulemaking process is a vital tool for helping to shape healthcare. SHM actively pursues regulatory issues in order to advocate for hospitalists and their patients. The experiences and expertise of members are critical for SHM to be able to accurately represent the specialty.
By staying informed on health policy and being engaged with SHM, members can provide invaluable perspectives to help transform the field and revolutionize the healthcare system.
Josh Lapps is SHM's government relations specialist.
References
- U.S. Bureau of Labor Statistics. Program Perspectives: fee-for-service plans. U.S. Bureau of Labor Statistics website. Available at: http://www.bls.gov/opub/perspectives/program_perspectives_vol2_issue5.pdf. Accessed Aug. 15, 2012.
- The Henry J. Kaiser Family Foundation. Medicare at a glance. The Henry J. Kaiser Family Foundation website. Available at: http://www.kff.org/medicare/upload/1066_11.pdf. Accessed Aug. 29, 2012.
The healthcare market is saturated with fee-for-service reimbursement schemes. The Bureau of Labor Statistics estimates that 78% of employer-sponsored health insurance plans are some type of fee-for-service plan.1 In Medicare, about 75% of beneficiaries use the traditional fee-for-service program.2 Fee-for-service denotes that payments are made on individual services, billed separately, irrespective of outcome and, in some cases, necessity.
The physician value-based payment modifier (VBPM) is an initiative that will begin shifting Medicare reimbursement for physicians away from fee-for-service schemes and toward some type of pay-for-performance model.
For hospitalists, this will have a marked impact on HM practice and might have reverberating effects in the field itself.
Established under the 2008 Medicare Improvements for Patients and Providers Act (MIPPA) and expanded under the 2010 Affordable Care Act (ACA), the VBPM will be applied to all physicians by 2017.
The VBPM program is the physician version of hospital value-based purchasing; both are designed to move the basis of payment toward the quality of care delivered, not simply for the quantity of services rendered. By linking quality measurement with payment, the Centers for Medicare & Medicaid Services (CMS) hopes to start paying for value.
While legislation required the creation of pay-for-performance programs for physicians and hospitals, the design and implementation details have been delegated to CMS, part of the U.S. Department of Health and Human Services. Thus, CMS has oversight on the specifics of the program. These specifics are promulgated through the federal rulemaking process, which requires such agencies as CMS to seek input from the general public—as well as medical societies, including SHM—as rules are proposed and finalized. Generally, there is a 30- to 90-day period after a rule is proposed for public comment, after which a rule will be finalized.
For the VBPM and its performance period starting next year, the guidelines were published for public comment in a proposed rule for the fiscal-year 2013 Physician Fee Schedule. The final rule, which will provide more definitive guidance for hospitalists, is slated to come out in November.
Hospitalists should be cognizant of how quality measurements apply to their practice and find ways to participate in such quality measurement programs as the Physician Quality Reporting System (PQRS). PQRS will become the evaluative backbone of the VBPM. It is imperative that hospitalists stay abreast of these transformative changes in the healthcare system and work to ensure that their practice patterns, which fill critical gaps in patient care, are adequately represented in these changes.
Although legislation and legislative advocacy are undoubtedly important features of policymaking, participating in the federal rulemaking process is a vital tool for helping to shape healthcare. SHM actively pursues regulatory issues in order to advocate for hospitalists and their patients. The experiences and expertise of members are critical for SHM to be able to accurately represent the specialty.
By staying informed on health policy and being engaged with SHM, members can provide invaluable perspectives to help transform the field and revolutionize the healthcare system.
Josh Lapps is SHM's government relations specialist.
References
- U.S. Bureau of Labor Statistics. Program Perspectives: fee-for-service plans. U.S. Bureau of Labor Statistics website. Available at: http://www.bls.gov/opub/perspectives/program_perspectives_vol2_issue5.pdf. Accessed Aug. 15, 2012.
- The Henry J. Kaiser Family Foundation. Medicare at a glance. The Henry J. Kaiser Family Foundation website. Available at: http://www.kff.org/medicare/upload/1066_11.pdf. Accessed Aug. 29, 2012.
SHM's Quality and Safety Educators Academy: Preparing Successful Residents and Students
Tomorrow’s hospital will be increasingly oriented around quality and safety; today’s students must prepare to thrive in that environment.
That’s the philosophy behind SHM’s Quality and Safety Educators Academy (QSEA). Now in its second year, the two-and-a-half-day academy trains hospitalist educators to teach medical students and residents about quality and safety.
QSEA, co-hosted by SHM and the Alliance for Academic Internal Medicine, is March 7-9 at Tempe Mission Palms in Tempe, Ariz. Registration is now open at www.hospitalmedicine.org/qsea.
“In order to be successful, we must teach medical students and residents about these goals so that they incorporate them into their practice from day one,” says Jennifer S. Myers, MD, associate professor of clinical medicine, patient safety officer, and director of quality and safety education at the University of Pennsylvania’s Perelman School of Medicine in Philadelphia.
Progress in quality improvement (QI) and patient safety has been slow because many current physicians aren’t familiar with the materials, creating what Dr. Myers refers to as a “faculty development” gap. QSEA is the first and only academy designed to close that gap for hospitalist faculty by giving them specific knowledge, skills, a take-home toolkit, and a brand-new peer network of other quality-minded educators.
A major part of the academy is dedicated to the career trajectory of educators and, in Dr. Myers’ words, “how a hospitalist can be successful in making quality and safety education a career path.”
Despite the serious topics, she also is quick to point out that the academy is anything but dry.
“You have to experience it,” she says. “We have a ton of fun. You will leave with a new family.”
At the end of the inaugural QSEA, the faculty and course directors were so energized by the attendees that they formed a human pyramid. “It was a great moment,” she says.
Dr. Myers says she still enjoys receiving email from QSEA attendees about their new adventures in quality and safety education. “This makes it all worth it and why the QSEA team does this work,” she says.
Tomorrow’s hospital will be increasingly oriented around quality and safety; today’s students must prepare to thrive in that environment.
That’s the philosophy behind SHM’s Quality and Safety Educators Academy (QSEA). Now in its second year, the two-and-a-half-day academy trains hospitalist educators to teach medical students and residents about quality and safety.
QSEA, co-hosted by SHM and the Alliance for Academic Internal Medicine, is March 7-9 at Tempe Mission Palms in Tempe, Ariz. Registration is now open at www.hospitalmedicine.org/qsea.
“In order to be successful, we must teach medical students and residents about these goals so that they incorporate them into their practice from day one,” says Jennifer S. Myers, MD, associate professor of clinical medicine, patient safety officer, and director of quality and safety education at the University of Pennsylvania’s Perelman School of Medicine in Philadelphia.
Progress in quality improvement (QI) and patient safety has been slow because many current physicians aren’t familiar with the materials, creating what Dr. Myers refers to as a “faculty development” gap. QSEA is the first and only academy designed to close that gap for hospitalist faculty by giving them specific knowledge, skills, a take-home toolkit, and a brand-new peer network of other quality-minded educators.
A major part of the academy is dedicated to the career trajectory of educators and, in Dr. Myers’ words, “how a hospitalist can be successful in making quality and safety education a career path.”
Despite the serious topics, she also is quick to point out that the academy is anything but dry.
“You have to experience it,” she says. “We have a ton of fun. You will leave with a new family.”
At the end of the inaugural QSEA, the faculty and course directors were so energized by the attendees that they formed a human pyramid. “It was a great moment,” she says.
Dr. Myers says she still enjoys receiving email from QSEA attendees about their new adventures in quality and safety education. “This makes it all worth it and why the QSEA team does this work,” she says.
Tomorrow’s hospital will be increasingly oriented around quality and safety; today’s students must prepare to thrive in that environment.
That’s the philosophy behind SHM’s Quality and Safety Educators Academy (QSEA). Now in its second year, the two-and-a-half-day academy trains hospitalist educators to teach medical students and residents about quality and safety.
QSEA, co-hosted by SHM and the Alliance for Academic Internal Medicine, is March 7-9 at Tempe Mission Palms in Tempe, Ariz. Registration is now open at www.hospitalmedicine.org/qsea.
“In order to be successful, we must teach medical students and residents about these goals so that they incorporate them into their practice from day one,” says Jennifer S. Myers, MD, associate professor of clinical medicine, patient safety officer, and director of quality and safety education at the University of Pennsylvania’s Perelman School of Medicine in Philadelphia.
Progress in quality improvement (QI) and patient safety has been slow because many current physicians aren’t familiar with the materials, creating what Dr. Myers refers to as a “faculty development” gap. QSEA is the first and only academy designed to close that gap for hospitalist faculty by giving them specific knowledge, skills, a take-home toolkit, and a brand-new peer network of other quality-minded educators.
A major part of the academy is dedicated to the career trajectory of educators and, in Dr. Myers’ words, “how a hospitalist can be successful in making quality and safety education a career path.”
Despite the serious topics, she also is quick to point out that the academy is anything but dry.
“You have to experience it,” she says. “We have a ton of fun. You will leave with a new family.”
At the end of the inaugural QSEA, the faculty and course directors were so energized by the attendees that they formed a human pyramid. “It was a great moment,” she says.
Dr. Myers says she still enjoys receiving email from QSEA attendees about their new adventures in quality and safety education. “This makes it all worth it and why the QSEA team does this work,” she says.