User login
Meaningful improvement for patients like Tante Ilse
Last year, after a long delay due to COVID, my father’s ashes were finally laid to rest at Arlington National Cemetery. Among the loved ones who came was my favorite aunt, Tante Ilse, who was suffering from dementia. While she wasn’t “following” everything that was going on, she did perk up when she heard my father’s name and would comment on how she liked him and how wonderful he had been to her.
After the ceremony, our family of about 30 gathered at a restaurant where we shared stories and old pictures. Tante Ilse seemed to relish the photos and the time with family. She was doing so well that when we went back to my mom’s home after the reception, my cousins decided to bring Tante Ilse there, too. She had a great time, as evidenced by her famous total-body laugh. In the months before her death, we all commented about that day and how happy she seemed.
My aunt’s decline comes to mind as I reflect on media reports of 2 Alzheimer drugs— aducanumab and lecanemab—that have been billed by some as “gamechangers.” These new drugs are monoclonal antibodies directed at amyloid, one of several agents thought to cause Alzheimer disease. The details of aducanumab’s approval by the US Food and Drug Administration (FDA) generated a great deal of criticism—with good reason.
Two manufacturer-sponsored studies of aducanumab were halted due to futility of finding a benefit.1 The FDA’s scientific advisory panel recommended against approval due to a lack of evidence that it did anything more than remove amyloid plaque from the brain. And yet aducanumab received accelerated approval from the FDA. (This author collaborated on an additional analysis using data presented to the FDA, after its approval, which also reported no clinically meaningful effects.2) The other agent, lecanemab, also reduces markers of amyloid and was shown to be only moderately better than placebo in decreasing the rate of decline on various measures of cognition.3 Quite notably, both aducanumab and lecanemab, which are administered parenterally, cost more than $25,000 per year4,5 and cause amyloid-related imaging abnormalities (brain edema or hemorrhage).
Expensive agents without meaningful benefit. So far, neither of these agents has shown a reduction in things that are truly important to our patients and their families/caregivers: a reduction in caregiver burden and a reduction in the need for placement in long-term care facilities.
This is in contrast to cholinesterase inhibitors, which also slow the rate of cognitive decline.6 Among the differences that exist between these agents: Cholinesterase inhibitors are taken orally and are available as generics, which cost less than a thousand dollars per year.7 Limited data also suggest that they are associated with a lower risk for nursing home placement.8,9 (A February 2023 search of clinicaltrials.gov did not reveal any completed or planned head-to-head comparisons of monoclonal antibodies and anticholinergic agents.)
Our patients, their families, and caregivers hold out hope for something that will improve the patient’s cognition and extend the meaningful time they have with their loved ones. So far, the best we have to offer falls far short of these goals. I certainly would have hoped for something better than merely clearing amyloid for my aunt.
It’s time that the FDA adopt more rigorous standards requiring new drugs to, among other things, demonstrate meaningful clinical benefits, provide real cost savings, and be safer than currently available therapies. Other nations seem to be able to do this.10,11 It is bad enough to provide “hope in a bottle”; it is worse when what is offered is false hope.
1. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:197-210. doi: 10.14283/jpad.2022.30
2. Ebell MH, Barry HC. Why physicians should not prescribe aducanumab for Alzheimer disease. Am Fam Physician. 2022;105:353-354.
3. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9-21. doi: 10.1056/NEJMoa2212948
4. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023; 613:227-228. doi: 10.1038/d41586-023-00030-3
5. Biogen announces reduced price for Aduhelm to improve access for patients with early Alzheimer’s disease. December 20, 2021. Accessed February 20, 2023. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-reduced-price-aduhelmr-improve-access-patients
6. Takramah WK, Asem L. The efficacy of pharmacological interventions to improve cognitive and behavior symptoms in people with dementia: A systematic review and meta-analysis. Health Sci Rep. 2022;5:e913. doi: 10.1002/hsr2.913
7. GoodRx. Donepezil generic Aricept. Accessed February 20, 2023. www.goodrx.com/donepezil
8. Howard R, McShane R, Lindesay J, et al. Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer’s disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14:1171-1181. doi: 10.1016/S1474-4422(15)00258-6
9. Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51:937-944. doi: 10.1046/j.1365-2389.2003.51306.x
10. Pham C, Le K, Draves M, et al. Assessment of FDA-approved drugs not recommended for use or reimbursement in other countries, 2017-2020. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6787
11. Johnston JL, Ross JS, Ramachandran R. US Food and Drug Administration approval of drugs not meeting pivotal trial primary end points, 2018-2021. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6444
Last year, after a long delay due to COVID, my father’s ashes were finally laid to rest at Arlington National Cemetery. Among the loved ones who came was my favorite aunt, Tante Ilse, who was suffering from dementia. While she wasn’t “following” everything that was going on, she did perk up when she heard my father’s name and would comment on how she liked him and how wonderful he had been to her.
After the ceremony, our family of about 30 gathered at a restaurant where we shared stories and old pictures. Tante Ilse seemed to relish the photos and the time with family. She was doing so well that when we went back to my mom’s home after the reception, my cousins decided to bring Tante Ilse there, too. She had a great time, as evidenced by her famous total-body laugh. In the months before her death, we all commented about that day and how happy she seemed.
My aunt’s decline comes to mind as I reflect on media reports of 2 Alzheimer drugs— aducanumab and lecanemab—that have been billed by some as “gamechangers.” These new drugs are monoclonal antibodies directed at amyloid, one of several agents thought to cause Alzheimer disease. The details of aducanumab’s approval by the US Food and Drug Administration (FDA) generated a great deal of criticism—with good reason.
Two manufacturer-sponsored studies of aducanumab were halted due to futility of finding a benefit.1 The FDA’s scientific advisory panel recommended against approval due to a lack of evidence that it did anything more than remove amyloid plaque from the brain. And yet aducanumab received accelerated approval from the FDA. (This author collaborated on an additional analysis using data presented to the FDA, after its approval, which also reported no clinically meaningful effects.2) The other agent, lecanemab, also reduces markers of amyloid and was shown to be only moderately better than placebo in decreasing the rate of decline on various measures of cognition.3 Quite notably, both aducanumab and lecanemab, which are administered parenterally, cost more than $25,000 per year4,5 and cause amyloid-related imaging abnormalities (brain edema or hemorrhage).
Expensive agents without meaningful benefit. So far, neither of these agents has shown a reduction in things that are truly important to our patients and their families/caregivers: a reduction in caregiver burden and a reduction in the need for placement in long-term care facilities.
This is in contrast to cholinesterase inhibitors, which also slow the rate of cognitive decline.6 Among the differences that exist between these agents: Cholinesterase inhibitors are taken orally and are available as generics, which cost less than a thousand dollars per year.7 Limited data also suggest that they are associated with a lower risk for nursing home placement.8,9 (A February 2023 search of clinicaltrials.gov did not reveal any completed or planned head-to-head comparisons of monoclonal antibodies and anticholinergic agents.)
Our patients, their families, and caregivers hold out hope for something that will improve the patient’s cognition and extend the meaningful time they have with their loved ones. So far, the best we have to offer falls far short of these goals. I certainly would have hoped for something better than merely clearing amyloid for my aunt.
It’s time that the FDA adopt more rigorous standards requiring new drugs to, among other things, demonstrate meaningful clinical benefits, provide real cost savings, and be safer than currently available therapies. Other nations seem to be able to do this.10,11 It is bad enough to provide “hope in a bottle”; it is worse when what is offered is false hope.
Last year, after a long delay due to COVID, my father’s ashes were finally laid to rest at Arlington National Cemetery. Among the loved ones who came was my favorite aunt, Tante Ilse, who was suffering from dementia. While she wasn’t “following” everything that was going on, she did perk up when she heard my father’s name and would comment on how she liked him and how wonderful he had been to her.
After the ceremony, our family of about 30 gathered at a restaurant where we shared stories and old pictures. Tante Ilse seemed to relish the photos and the time with family. She was doing so well that when we went back to my mom’s home after the reception, my cousins decided to bring Tante Ilse there, too. She had a great time, as evidenced by her famous total-body laugh. In the months before her death, we all commented about that day and how happy she seemed.
My aunt’s decline comes to mind as I reflect on media reports of 2 Alzheimer drugs— aducanumab and lecanemab—that have been billed by some as “gamechangers.” These new drugs are monoclonal antibodies directed at amyloid, one of several agents thought to cause Alzheimer disease. The details of aducanumab’s approval by the US Food and Drug Administration (FDA) generated a great deal of criticism—with good reason.
Two manufacturer-sponsored studies of aducanumab were halted due to futility of finding a benefit.1 The FDA’s scientific advisory panel recommended against approval due to a lack of evidence that it did anything more than remove amyloid plaque from the brain. And yet aducanumab received accelerated approval from the FDA. (This author collaborated on an additional analysis using data presented to the FDA, after its approval, which also reported no clinically meaningful effects.2) The other agent, lecanemab, also reduces markers of amyloid and was shown to be only moderately better than placebo in decreasing the rate of decline on various measures of cognition.3 Quite notably, both aducanumab and lecanemab, which are administered parenterally, cost more than $25,000 per year4,5 and cause amyloid-related imaging abnormalities (brain edema or hemorrhage).
Expensive agents without meaningful benefit. So far, neither of these agents has shown a reduction in things that are truly important to our patients and their families/caregivers: a reduction in caregiver burden and a reduction in the need for placement in long-term care facilities.
This is in contrast to cholinesterase inhibitors, which also slow the rate of cognitive decline.6 Among the differences that exist between these agents: Cholinesterase inhibitors are taken orally and are available as generics, which cost less than a thousand dollars per year.7 Limited data also suggest that they are associated with a lower risk for nursing home placement.8,9 (A February 2023 search of clinicaltrials.gov did not reveal any completed or planned head-to-head comparisons of monoclonal antibodies and anticholinergic agents.)
Our patients, their families, and caregivers hold out hope for something that will improve the patient’s cognition and extend the meaningful time they have with their loved ones. So far, the best we have to offer falls far short of these goals. I certainly would have hoped for something better than merely clearing amyloid for my aunt.
It’s time that the FDA adopt more rigorous standards requiring new drugs to, among other things, demonstrate meaningful clinical benefits, provide real cost savings, and be safer than currently available therapies. Other nations seem to be able to do this.10,11 It is bad enough to provide “hope in a bottle”; it is worse when what is offered is false hope.
1. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:197-210. doi: 10.14283/jpad.2022.30
2. Ebell MH, Barry HC. Why physicians should not prescribe aducanumab for Alzheimer disease. Am Fam Physician. 2022;105:353-354.
3. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9-21. doi: 10.1056/NEJMoa2212948
4. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023; 613:227-228. doi: 10.1038/d41586-023-00030-3
5. Biogen announces reduced price for Aduhelm to improve access for patients with early Alzheimer’s disease. December 20, 2021. Accessed February 20, 2023. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-reduced-price-aduhelmr-improve-access-patients
6. Takramah WK, Asem L. The efficacy of pharmacological interventions to improve cognitive and behavior symptoms in people with dementia: A systematic review and meta-analysis. Health Sci Rep. 2022;5:e913. doi: 10.1002/hsr2.913
7. GoodRx. Donepezil generic Aricept. Accessed February 20, 2023. www.goodrx.com/donepezil
8. Howard R, McShane R, Lindesay J, et al. Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer’s disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14:1171-1181. doi: 10.1016/S1474-4422(15)00258-6
9. Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51:937-944. doi: 10.1046/j.1365-2389.2003.51306.x
10. Pham C, Le K, Draves M, et al. Assessment of FDA-approved drugs not recommended for use or reimbursement in other countries, 2017-2020. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6787
11. Johnston JL, Ross JS, Ramachandran R. US Food and Drug Administration approval of drugs not meeting pivotal trial primary end points, 2018-2021. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6444
1. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:197-210. doi: 10.14283/jpad.2022.30
2. Ebell MH, Barry HC. Why physicians should not prescribe aducanumab for Alzheimer disease. Am Fam Physician. 2022;105:353-354.
3. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9-21. doi: 10.1056/NEJMoa2212948
4. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023; 613:227-228. doi: 10.1038/d41586-023-00030-3
5. Biogen announces reduced price for Aduhelm to improve access for patients with early Alzheimer’s disease. December 20, 2021. Accessed February 20, 2023. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-reduced-price-aduhelmr-improve-access-patients
6. Takramah WK, Asem L. The efficacy of pharmacological interventions to improve cognitive and behavior symptoms in people with dementia: A systematic review and meta-analysis. Health Sci Rep. 2022;5:e913. doi: 10.1002/hsr2.913
7. GoodRx. Donepezil generic Aricept. Accessed February 20, 2023. www.goodrx.com/donepezil
8. Howard R, McShane R, Lindesay J, et al. Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer’s disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14:1171-1181. doi: 10.1016/S1474-4422(15)00258-6
9. Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51:937-944. doi: 10.1046/j.1365-2389.2003.51306.x
10. Pham C, Le K, Draves M, et al. Assessment of FDA-approved drugs not recommended for use or reimbursement in other countries, 2017-2020. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6787
11. Johnston JL, Ross JS, Ramachandran R. US Food and Drug Administration approval of drugs not meeting pivotal trial primary end points, 2018-2021. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6444
5 non-COVID vaccine recommendations from ACIP
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
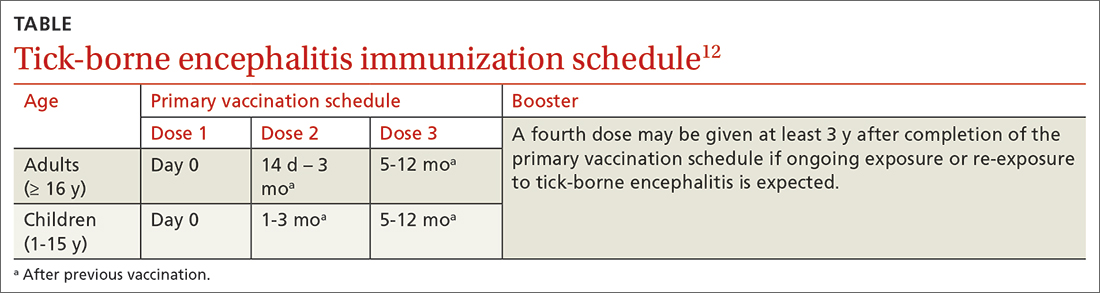
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13
Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13

Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13

Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1
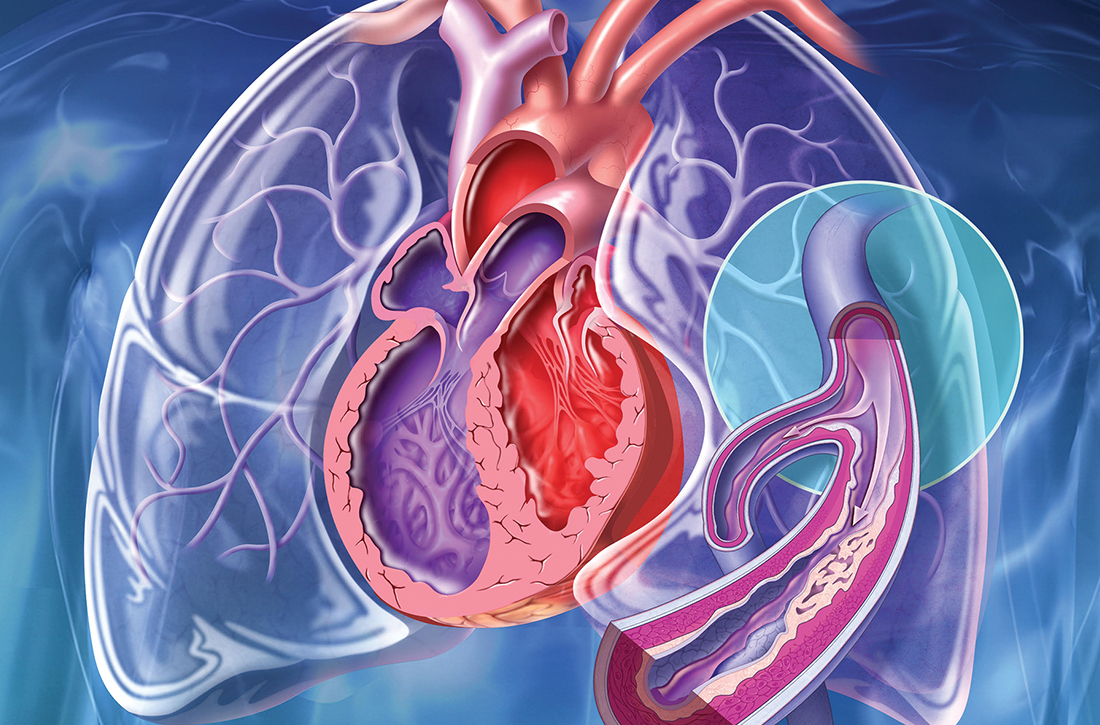
Pulmonary hypertension: An update of Dx and Tx guidelines
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.
Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
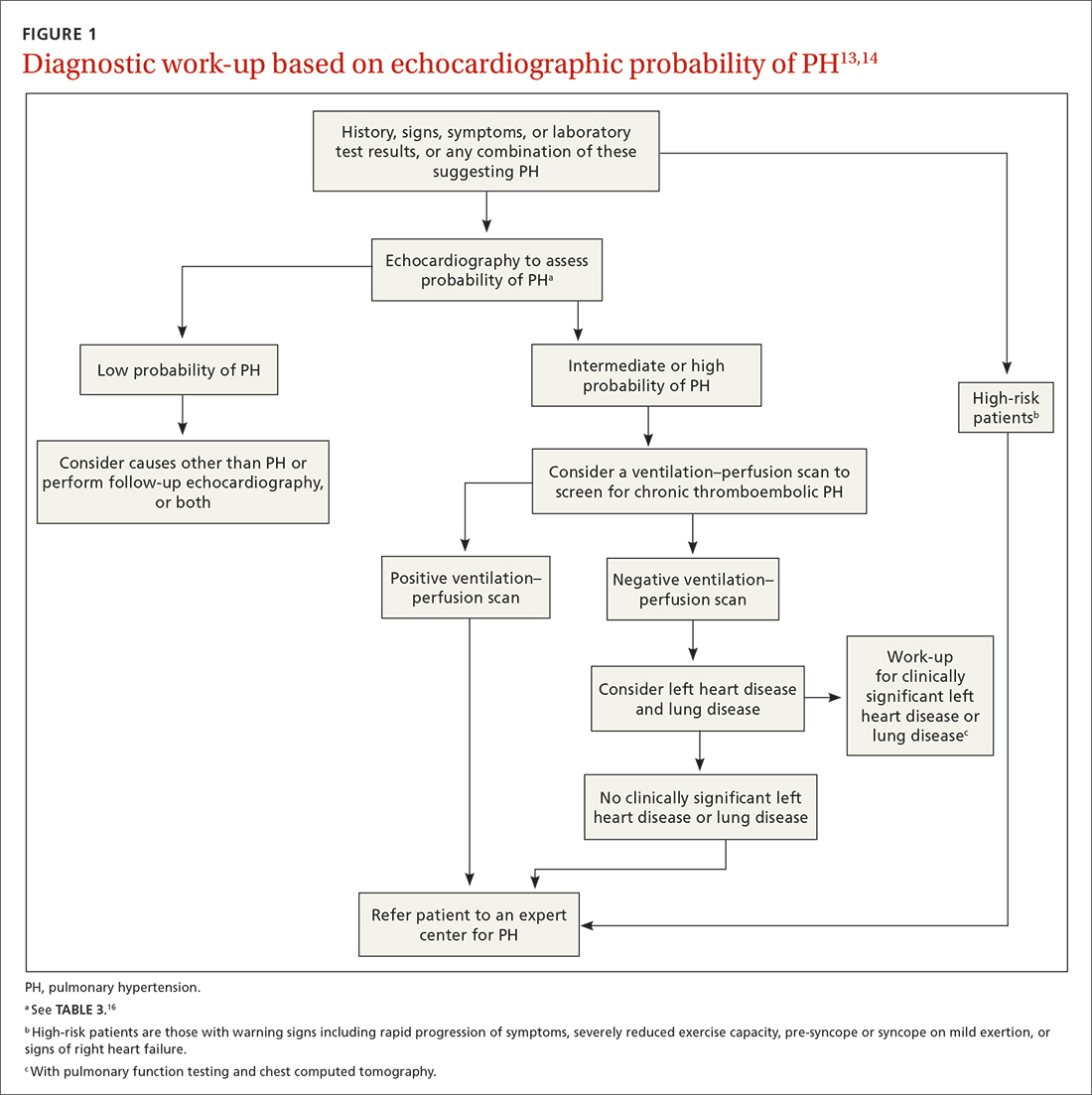
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.
Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7
Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6
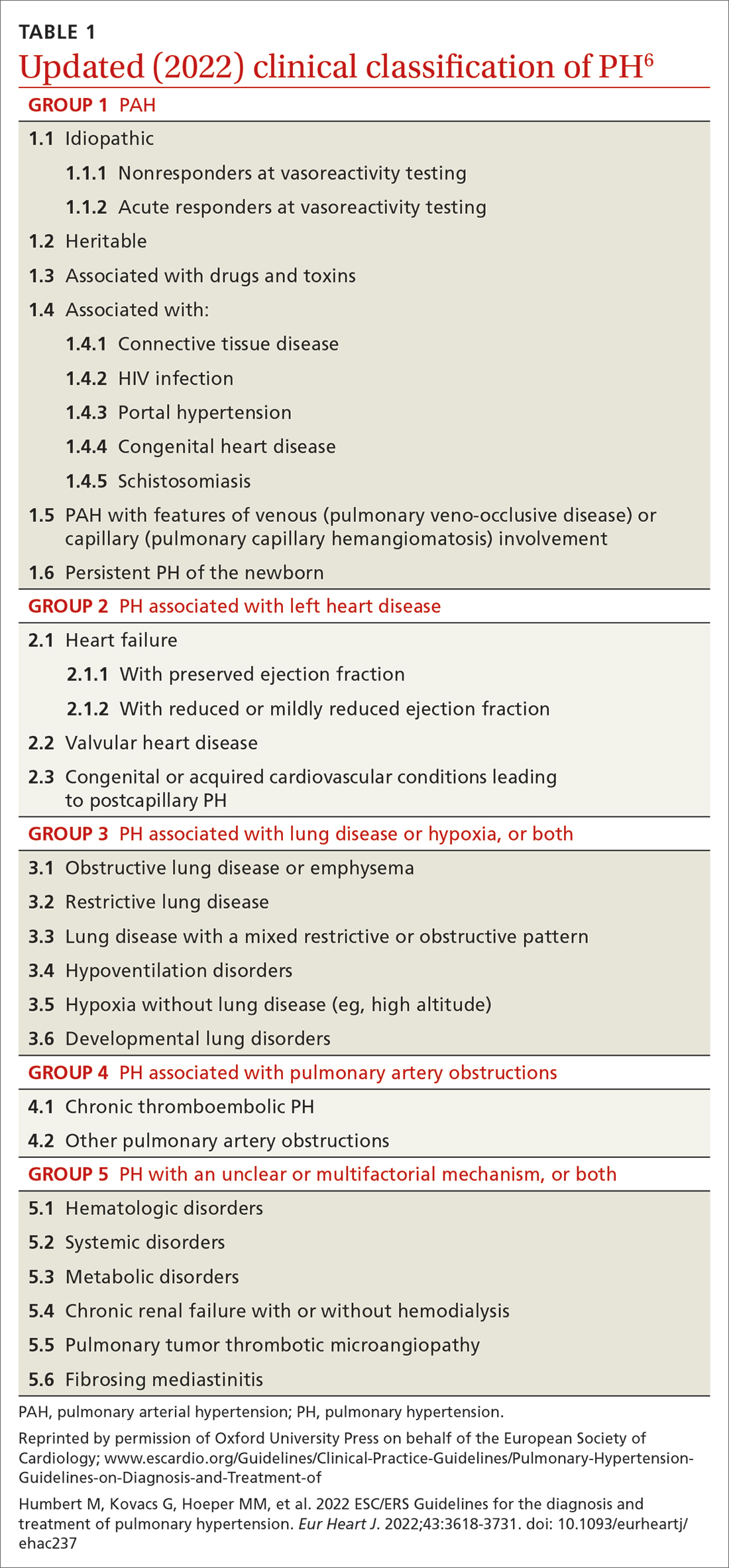
Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).
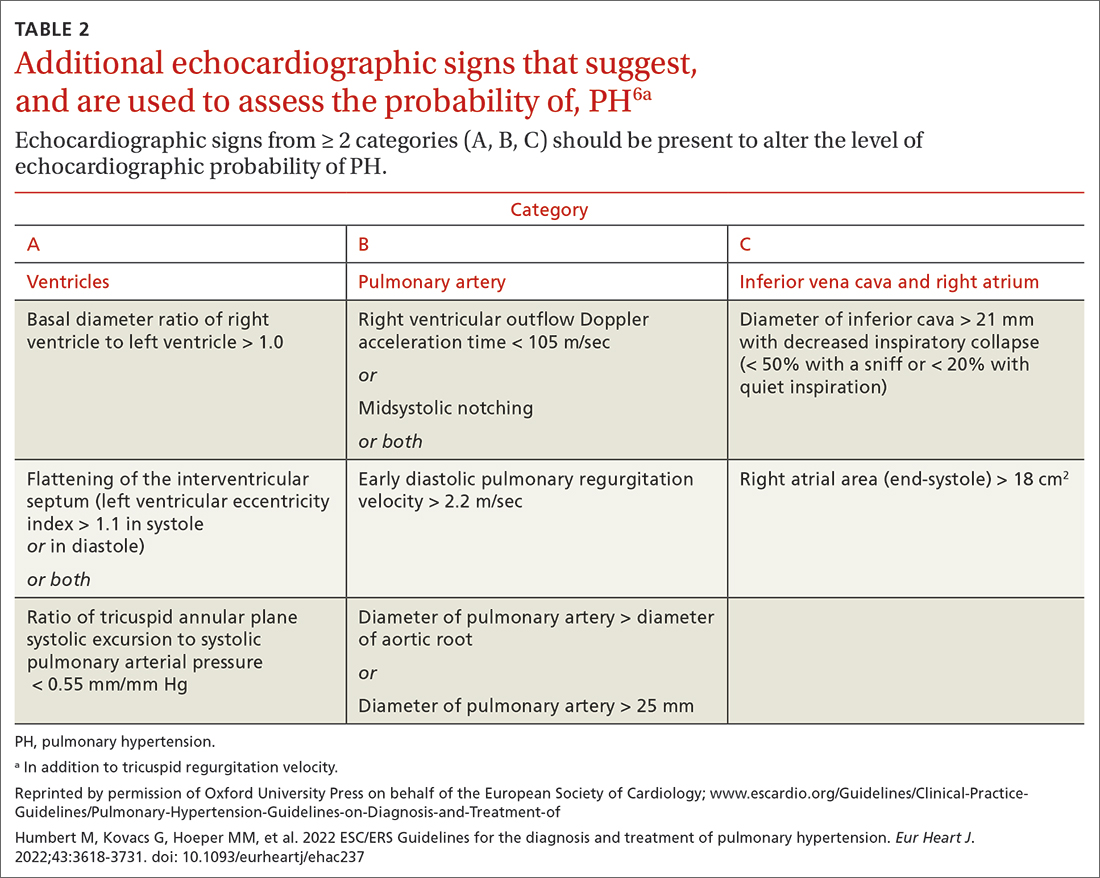
Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18
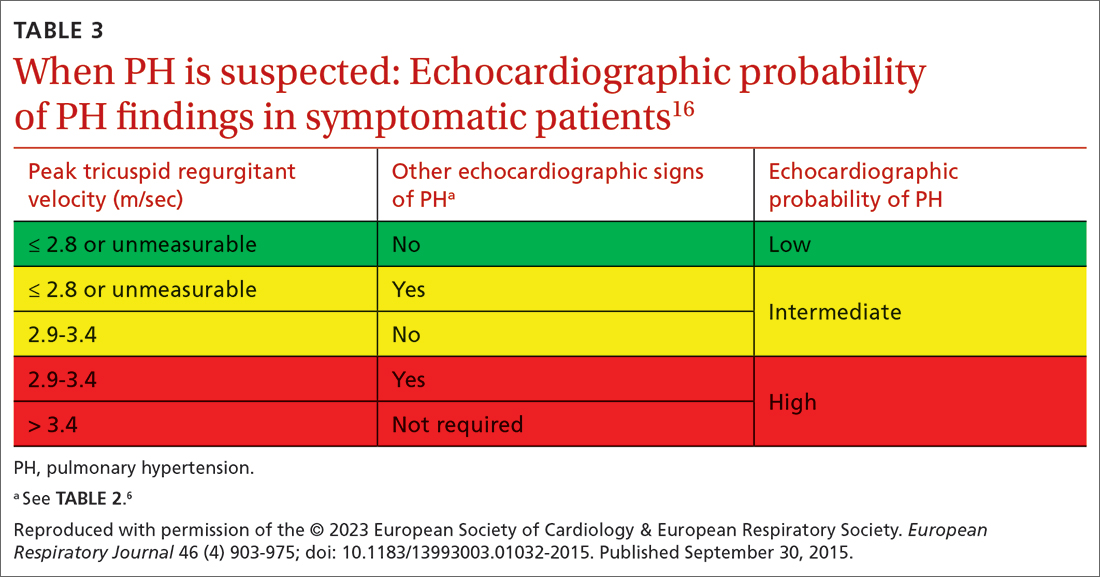
TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.

Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.

Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7

Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6

Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).

Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18

TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.

Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.

Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7

Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6

Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).

Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18

TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
PRACTICE RECOMMENDATIONS
› Employ echocardiography as the first-line diagnostic test when pulmonary hypertension (PH) is suspected. C
› Order a ventilation– perfusion scan in patients with unexplained PH to exclude chronic thromboembolic PH. C
› Order lung function testing with diffusion capacity for carbon monoxide as part of the initial evaluation of PH. C
› Use right heart catheterization to confirm the diagnosis of pulmonary arterial hypertension. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Long-term BP reductions with renal denervation not race specific
WASHINGTON – On the heels the recently published final report from the SYMPLICITY HTN-3 renal denervation trial, a new analysis showed that Black patients, like non-Blacks, had sustained blood pressure control.
Contrary to a signal from earlier results, “there is nothing race specific about renal denervation,” said presenter Deepak L. Bhatt, MD, at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute.
Black patients are well represented among patients with treatment-resistant hypertension and considered an important subgroup to target, according to Dr. Bhatt, director of Mount Sinai Heart, New York. This is the reason that they were not only a prespecified subgroup in SYMPLICITY HTN-3, but race was one of two stratification factors at enrollment. At the time of the study design, there was an expectation that Black patients would benefit more than non-Blacks.
This did not prove to be the case during the 6-month controlled phase of the trial. When patients randomized to renal denervation or the sham procedure were stratified by race, the primary endpoint of reduction in office systolic blood pressure (SBP) reached significance in the experimental arm among non-Black patients (–6.63 mm Hg; P = .01), but not among Black patients (–2.25 mm Hg; P = .09).
Blacks comprised 26% of SYMPLICITY HTN-3 trial
In the initial controlled analysis, published in the New England Journal of Medicine, the lack of benefit in the substantial Black enrollment – representing 26% of the study total – weighed against the ability of the trial to demonstrate a benefit, but Dr. Bhatt pointed out that BP reductions were unexpectedly high in the sham group regardless of race. Patients randomized to the sham group were encouraged to adhere to antihypertensive therapy, and based on response, this was particularly effective in the Black sham subgroup.
In SYMPLICITY HTN-3, patients with treatment-resistant hypertension were randomized to renal denervation or a sham procedure in a 2:1 ratio. While the controlled phase lasted just 6 months, the follow-up after the study was unblinded has continued out to 3 years. Safety and efficacy were assessed at 12, 24, and 36 months.
Unlike the disappointing results at 6 months, renal denervation has been consistently associated with significantly lower BP over long-term follow-up, even though those randomized to the sham procedure were permitted to cross over. About two-thirds of the sham group did so.
In the recently published final report of SYMPLICITY, the overall median change in office SBP at 3 years regardless of race was –26.4 mm Hg in the group initially randomized to renal denervation versus –5.7 mm Hg (P < .0001) among those randomized to the sham procedure.
In the subgroup analysis presented by Dr. Bhatt, the relative control of office SBP, as well as other measures of blood pressure, were similarly and significantly reduced in both Black and non-Black patients. In general, the relative control offered by being randomized initially to renal denervation increased over time in both groups.
For example, the relative reduction in office SBP favoring renal denervation climbed from –12.0 mm Hg at 12 months (P = .0066) to –21.0 at 18 months (P = .0002) and then to –24.9 mm Hg (P < .0001) at 36 months in the Black subgroup. In non-Blacks, the same type of relative reductions were seen at each time point, climbing from –13.5 (P < .0001) to –20.5 (P < .0001) and then to –21.0 (P < .0001).
The comparisons for other measures of BP control, including office diastolic BP, 24-hour SBP, and BP control during morning, day, and night periods were also statistically and similarly improved for those initially randomized to renal denervation rather than a sham procedure among both Blacks and non-Blacks.
Renal denervation safe in Black and non-Black patients
Renal denervation was well tolerated in both Black and non-Black participants with no signal of long-term risks over 36 months in either group. Among Blacks, rates of death at 36 months (3% vs. 11%) and stroke (7% vs. 11%) were lower among those randomized to renal denervation relative to sham patients who never crossed over, but Dr. Bhatt said the numbers are too small to draw any conclusions about outcomes.
While this subgroup analysis, along with the final SYMPLICITY report, supports the efficacy of renal denervation over the long term, these data are also consistent with the recently published analysis of SPYRAL ON-MED . Together, these data have led many experts, including Dr. Bhatt, to conclude that renal denervation is effective and deserves regulatory approval.
“In out-of-control blood pressure, when patients have maxed out on medications and lifestyle, I think renal denervation is efficacious, and it is equally efficacious in Blacks and non-Blacks,” Dr. Bhatt said.
This subgroup analysis is important because of the need for options in treatment-resistant hypertension among Black as well as non-Black patients, pointed out Sripal Bangalore, MBBS, director of complex coronary intervention at New York University.
“I am glad that we did not conclude too soon that it does not work in Blacks,” Dr. Bangalore said. If renal denervation is approved, he expects this procedure to be a valuable tool in this racial group.
Dr. Bhatt reported financial relationship with more than 20 pharmaceutical and device companies, including Medtronic, which provided funding for the SYMPLICITY HTN-3 trial. Dr. Bangalore has financial relationships with Abbott Vascular, Amgen, Biotronik, Inari, Pfizer, Reata, and Truvic.
WASHINGTON – On the heels the recently published final report from the SYMPLICITY HTN-3 renal denervation trial, a new analysis showed that Black patients, like non-Blacks, had sustained blood pressure control.
Contrary to a signal from earlier results, “there is nothing race specific about renal denervation,” said presenter Deepak L. Bhatt, MD, at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute.
Black patients are well represented among patients with treatment-resistant hypertension and considered an important subgroup to target, according to Dr. Bhatt, director of Mount Sinai Heart, New York. This is the reason that they were not only a prespecified subgroup in SYMPLICITY HTN-3, but race was one of two stratification factors at enrollment. At the time of the study design, there was an expectation that Black patients would benefit more than non-Blacks.
This did not prove to be the case during the 6-month controlled phase of the trial. When patients randomized to renal denervation or the sham procedure were stratified by race, the primary endpoint of reduction in office systolic blood pressure (SBP) reached significance in the experimental arm among non-Black patients (–6.63 mm Hg; P = .01), but not among Black patients (–2.25 mm Hg; P = .09).
Blacks comprised 26% of SYMPLICITY HTN-3 trial
In the initial controlled analysis, published in the New England Journal of Medicine, the lack of benefit in the substantial Black enrollment – representing 26% of the study total – weighed against the ability of the trial to demonstrate a benefit, but Dr. Bhatt pointed out that BP reductions were unexpectedly high in the sham group regardless of race. Patients randomized to the sham group were encouraged to adhere to antihypertensive therapy, and based on response, this was particularly effective in the Black sham subgroup.
In SYMPLICITY HTN-3, patients with treatment-resistant hypertension were randomized to renal denervation or a sham procedure in a 2:1 ratio. While the controlled phase lasted just 6 months, the follow-up after the study was unblinded has continued out to 3 years. Safety and efficacy were assessed at 12, 24, and 36 months.
Unlike the disappointing results at 6 months, renal denervation has been consistently associated with significantly lower BP over long-term follow-up, even though those randomized to the sham procedure were permitted to cross over. About two-thirds of the sham group did so.
In the recently published final report of SYMPLICITY, the overall median change in office SBP at 3 years regardless of race was –26.4 mm Hg in the group initially randomized to renal denervation versus –5.7 mm Hg (P < .0001) among those randomized to the sham procedure.
In the subgroup analysis presented by Dr. Bhatt, the relative control of office SBP, as well as other measures of blood pressure, were similarly and significantly reduced in both Black and non-Black patients. In general, the relative control offered by being randomized initially to renal denervation increased over time in both groups.
For example, the relative reduction in office SBP favoring renal denervation climbed from –12.0 mm Hg at 12 months (P = .0066) to –21.0 at 18 months (P = .0002) and then to –24.9 mm Hg (P < .0001) at 36 months in the Black subgroup. In non-Blacks, the same type of relative reductions were seen at each time point, climbing from –13.5 (P < .0001) to –20.5 (P < .0001) and then to –21.0 (P < .0001).
The comparisons for other measures of BP control, including office diastolic BP, 24-hour SBP, and BP control during morning, day, and night periods were also statistically and similarly improved for those initially randomized to renal denervation rather than a sham procedure among both Blacks and non-Blacks.
Renal denervation safe in Black and non-Black patients
Renal denervation was well tolerated in both Black and non-Black participants with no signal of long-term risks over 36 months in either group. Among Blacks, rates of death at 36 months (3% vs. 11%) and stroke (7% vs. 11%) were lower among those randomized to renal denervation relative to sham patients who never crossed over, but Dr. Bhatt said the numbers are too small to draw any conclusions about outcomes.
While this subgroup analysis, along with the final SYMPLICITY report, supports the efficacy of renal denervation over the long term, these data are also consistent with the recently published analysis of SPYRAL ON-MED . Together, these data have led many experts, including Dr. Bhatt, to conclude that renal denervation is effective and deserves regulatory approval.
“In out-of-control blood pressure, when patients have maxed out on medications and lifestyle, I think renal denervation is efficacious, and it is equally efficacious in Blacks and non-Blacks,” Dr. Bhatt said.
This subgroup analysis is important because of the need for options in treatment-resistant hypertension among Black as well as non-Black patients, pointed out Sripal Bangalore, MBBS, director of complex coronary intervention at New York University.
“I am glad that we did not conclude too soon that it does not work in Blacks,” Dr. Bangalore said. If renal denervation is approved, he expects this procedure to be a valuable tool in this racial group.
Dr. Bhatt reported financial relationship with more than 20 pharmaceutical and device companies, including Medtronic, which provided funding for the SYMPLICITY HTN-3 trial. Dr. Bangalore has financial relationships with Abbott Vascular, Amgen, Biotronik, Inari, Pfizer, Reata, and Truvic.
WASHINGTON – On the heels the recently published final report from the SYMPLICITY HTN-3 renal denervation trial, a new analysis showed that Black patients, like non-Blacks, had sustained blood pressure control.
Contrary to a signal from earlier results, “there is nothing race specific about renal denervation,” said presenter Deepak L. Bhatt, MD, at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute.
Black patients are well represented among patients with treatment-resistant hypertension and considered an important subgroup to target, according to Dr. Bhatt, director of Mount Sinai Heart, New York. This is the reason that they were not only a prespecified subgroup in SYMPLICITY HTN-3, but race was one of two stratification factors at enrollment. At the time of the study design, there was an expectation that Black patients would benefit more than non-Blacks.
This did not prove to be the case during the 6-month controlled phase of the trial. When patients randomized to renal denervation or the sham procedure were stratified by race, the primary endpoint of reduction in office systolic blood pressure (SBP) reached significance in the experimental arm among non-Black patients (–6.63 mm Hg; P = .01), but not among Black patients (–2.25 mm Hg; P = .09).
Blacks comprised 26% of SYMPLICITY HTN-3 trial
In the initial controlled analysis, published in the New England Journal of Medicine, the lack of benefit in the substantial Black enrollment – representing 26% of the study total – weighed against the ability of the trial to demonstrate a benefit, but Dr. Bhatt pointed out that BP reductions were unexpectedly high in the sham group regardless of race. Patients randomized to the sham group were encouraged to adhere to antihypertensive therapy, and based on response, this was particularly effective in the Black sham subgroup.
In SYMPLICITY HTN-3, patients with treatment-resistant hypertension were randomized to renal denervation or a sham procedure in a 2:1 ratio. While the controlled phase lasted just 6 months, the follow-up after the study was unblinded has continued out to 3 years. Safety and efficacy were assessed at 12, 24, and 36 months.
Unlike the disappointing results at 6 months, renal denervation has been consistently associated with significantly lower BP over long-term follow-up, even though those randomized to the sham procedure were permitted to cross over. About two-thirds of the sham group did so.
In the recently published final report of SYMPLICITY, the overall median change in office SBP at 3 years regardless of race was –26.4 mm Hg in the group initially randomized to renal denervation versus –5.7 mm Hg (P < .0001) among those randomized to the sham procedure.
In the subgroup analysis presented by Dr. Bhatt, the relative control of office SBP, as well as other measures of blood pressure, were similarly and significantly reduced in both Black and non-Black patients. In general, the relative control offered by being randomized initially to renal denervation increased over time in both groups.
For example, the relative reduction in office SBP favoring renal denervation climbed from –12.0 mm Hg at 12 months (P = .0066) to –21.0 at 18 months (P = .0002) and then to –24.9 mm Hg (P < .0001) at 36 months in the Black subgroup. In non-Blacks, the same type of relative reductions were seen at each time point, climbing from –13.5 (P < .0001) to –20.5 (P < .0001) and then to –21.0 (P < .0001).
The comparisons for other measures of BP control, including office diastolic BP, 24-hour SBP, and BP control during morning, day, and night periods were also statistically and similarly improved for those initially randomized to renal denervation rather than a sham procedure among both Blacks and non-Blacks.
Renal denervation safe in Black and non-Black patients
Renal denervation was well tolerated in both Black and non-Black participants with no signal of long-term risks over 36 months in either group. Among Blacks, rates of death at 36 months (3% vs. 11%) and stroke (7% vs. 11%) were lower among those randomized to renal denervation relative to sham patients who never crossed over, but Dr. Bhatt said the numbers are too small to draw any conclusions about outcomes.
While this subgroup analysis, along with the final SYMPLICITY report, supports the efficacy of renal denervation over the long term, these data are also consistent with the recently published analysis of SPYRAL ON-MED . Together, these data have led many experts, including Dr. Bhatt, to conclude that renal denervation is effective and deserves regulatory approval.
“In out-of-control blood pressure, when patients have maxed out on medications and lifestyle, I think renal denervation is efficacious, and it is equally efficacious in Blacks and non-Blacks,” Dr. Bhatt said.
This subgroup analysis is important because of the need for options in treatment-resistant hypertension among Black as well as non-Black patients, pointed out Sripal Bangalore, MBBS, director of complex coronary intervention at New York University.
“I am glad that we did not conclude too soon that it does not work in Blacks,” Dr. Bangalore said. If renal denervation is approved, he expects this procedure to be a valuable tool in this racial group.
Dr. Bhatt reported financial relationship with more than 20 pharmaceutical and device companies, including Medtronic, which provided funding for the SYMPLICITY HTN-3 trial. Dr. Bangalore has financial relationships with Abbott Vascular, Amgen, Biotronik, Inari, Pfizer, Reata, and Truvic.
AT CRT 2023
EoE: One-food elimination works as well as six-food elimination
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
FROM THE LANCET GASTROENTEROLOGY AND HEPATOLOGY
Widespread flaky red skin
This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345

This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345
The SHOW UP Act Threatens VA Telehealth
In February, the US House of Representatives hurriedly passed the Stopping Home Office Work’s Unproductive Problems (SHOW UP) Act, H.R. 139, a bill that calls into question the contributions of federal employees allowed to work from home and resets telework policies to those in place in 2019. Its author, House Oversight Committee Chairman James Comer (R, Kentucky) claimed that this change was necessary because the expansion of federal telework during the COVID-19 pandemic “has crippled the ability of agencies to get their jobs done and created backlogs.” His targets included the US Department of Veterans Affairs (VA), where, he charged, “veterans have been unable…to obtain care they have earned.” He added, “it’s hard to argue that teleworking has helped the VA.”
While oversight of government programs is an authority of Congress, the SHOW UP Act is based on unsubstantiated assumptions of dereliction. It also disregards the devastating impact the proposed changes will have on veterans’ ability to receive care and inaccurately implies improving it. As the Senate considers the bill, they should take heed of these and other facts involving this often misunderstood form of labor.
COVID-19 irrevocably transformed the use of virtual care within the VA and across the world. Even as the pandemic subsides, public and private health care systems have continued to use telework-centered telehealth far above prepandemic levels, especially for mental health and primary care. Employers, including the VA, capitalize on telework for its benefits to both consumers and the workforce. For consumers, research supports the clinical effectiveness of telemental health service, as well as its cost-effectiveness and consumer satisfaction. On the workforce side, research has documented heightened productivity, lower distractibility, and higher job satisfaction among counselors who shifted to remote work.
Remote work also serves as a key tool in attracting and retaining a qualified workforce. As one VA service chief explained, “I am having enough trouble competing with the private sector, where extensive telework is now the norm. If telework options were rolled back, the private sector will have a field day picking off my best staff.” These comments are consistent with the data. McKinsey’s American Opportunity Survey shows that Americans have embraced remote work and want more of it. Recent data from Gallup show that 6 of 10 currently exclusively remote employees would be extremely likely to change companies if they lost their remote flexibility. Further, Gallup data show that when an employee’s location preference does not match their current work location, burnout rises, and engagement drops.
Between 2019 and 2023, the VA’s telework expansion is what has enabled it to meet the growing demand for mental health services. VA is keeping pace by having 2 or more clinicians rotate between home and a shared VA office. Forcing these hybrid practitioners to work full time at VA facilities would drastically reduce the number of patients they can care for. There simply are not enough offices on crammed VA grounds to house staff who telework today. The net result would be that fewer appointments would be available, creating longer wait times. And that is just for existing patients. It does not factor in the expected influx due to new veteran eligibility made possible by the toxic exposures PACT Act.
Here is another good example of crucial VA telework: With the advent of the 988 Suicide & Crisis Lifeline, VA is adding more than 1000 new Veterans Crisis Line responders. All these new positions are remote. The SHOW UP Act would inhibit this expansion of lifesaving programs.
Veterans want more, not fewer, telehealth options. At a House Committee on Veterans’ Affairs hearing this past September, the VA reported that most veterans would prefer to receive mental health services virtually than to have to commute to a VA medical center or clinic. Telehealth benefits veterans in meaningful ways, including that it reduces their travel time, travel expense, depletion of sick leave, and need for childcare. Veterans with posttraumatic stress disorder, military sexual trauma, those with mobility issues, or those who struggle with the stigma of mental health treatment may prefer the familiarity of their own homes for care. Virtual options also relieve a patient’s need to enter a hospital and be unnecessarily exposed to contagious viruses. That’s safer not only for veterans but also for VA staff.
Finally, virtual care improves treatment. Research has revealed that the likelihood of missing telehealth appointments is lower than for in-person appointments. When patients miss appointments, continuity of care is disrupted, and health care outcomes are diminished.
The pandemic is receding, but the advantages of telework-centered virtual care are greater than ever. Political representatives who want to show up for veterans should do everything in their power to expand—not cut—VA’s ability to authorize working from home.
In February, the US House of Representatives hurriedly passed the Stopping Home Office Work’s Unproductive Problems (SHOW UP) Act, H.R. 139, a bill that calls into question the contributions of federal employees allowed to work from home and resets telework policies to those in place in 2019. Its author, House Oversight Committee Chairman James Comer (R, Kentucky) claimed that this change was necessary because the expansion of federal telework during the COVID-19 pandemic “has crippled the ability of agencies to get their jobs done and created backlogs.” His targets included the US Department of Veterans Affairs (VA), where, he charged, “veterans have been unable…to obtain care they have earned.” He added, “it’s hard to argue that teleworking has helped the VA.”
While oversight of government programs is an authority of Congress, the SHOW UP Act is based on unsubstantiated assumptions of dereliction. It also disregards the devastating impact the proposed changes will have on veterans’ ability to receive care and inaccurately implies improving it. As the Senate considers the bill, they should take heed of these and other facts involving this often misunderstood form of labor.
COVID-19 irrevocably transformed the use of virtual care within the VA and across the world. Even as the pandemic subsides, public and private health care systems have continued to use telework-centered telehealth far above prepandemic levels, especially for mental health and primary care. Employers, including the VA, capitalize on telework for its benefits to both consumers and the workforce. For consumers, research supports the clinical effectiveness of telemental health service, as well as its cost-effectiveness and consumer satisfaction. On the workforce side, research has documented heightened productivity, lower distractibility, and higher job satisfaction among counselors who shifted to remote work.
Remote work also serves as a key tool in attracting and retaining a qualified workforce. As one VA service chief explained, “I am having enough trouble competing with the private sector, where extensive telework is now the norm. If telework options were rolled back, the private sector will have a field day picking off my best staff.” These comments are consistent with the data. McKinsey’s American Opportunity Survey shows that Americans have embraced remote work and want more of it. Recent data from Gallup show that 6 of 10 currently exclusively remote employees would be extremely likely to change companies if they lost their remote flexibility. Further, Gallup data show that when an employee’s location preference does not match their current work location, burnout rises, and engagement drops.
Between 2019 and 2023, the VA’s telework expansion is what has enabled it to meet the growing demand for mental health services. VA is keeping pace by having 2 or more clinicians rotate between home and a shared VA office. Forcing these hybrid practitioners to work full time at VA facilities would drastically reduce the number of patients they can care for. There simply are not enough offices on crammed VA grounds to house staff who telework today. The net result would be that fewer appointments would be available, creating longer wait times. And that is just for existing patients. It does not factor in the expected influx due to new veteran eligibility made possible by the toxic exposures PACT Act.
Here is another good example of crucial VA telework: With the advent of the 988 Suicide & Crisis Lifeline, VA is adding more than 1000 new Veterans Crisis Line responders. All these new positions are remote. The SHOW UP Act would inhibit this expansion of lifesaving programs.
Veterans want more, not fewer, telehealth options. At a House Committee on Veterans’ Affairs hearing this past September, the VA reported that most veterans would prefer to receive mental health services virtually than to have to commute to a VA medical center or clinic. Telehealth benefits veterans in meaningful ways, including that it reduces their travel time, travel expense, depletion of sick leave, and need for childcare. Veterans with posttraumatic stress disorder, military sexual trauma, those with mobility issues, or those who struggle with the stigma of mental health treatment may prefer the familiarity of their own homes for care. Virtual options also relieve a patient’s need to enter a hospital and be unnecessarily exposed to contagious viruses. That’s safer not only for veterans but also for VA staff.
Finally, virtual care improves treatment. Research has revealed that the likelihood of missing telehealth appointments is lower than for in-person appointments. When patients miss appointments, continuity of care is disrupted, and health care outcomes are diminished.
The pandemic is receding, but the advantages of telework-centered virtual care are greater than ever. Political representatives who want to show up for veterans should do everything in their power to expand—not cut—VA’s ability to authorize working from home.
In February, the US House of Representatives hurriedly passed the Stopping Home Office Work’s Unproductive Problems (SHOW UP) Act, H.R. 139, a bill that calls into question the contributions of federal employees allowed to work from home and resets telework policies to those in place in 2019. Its author, House Oversight Committee Chairman James Comer (R, Kentucky) claimed that this change was necessary because the expansion of federal telework during the COVID-19 pandemic “has crippled the ability of agencies to get their jobs done and created backlogs.” His targets included the US Department of Veterans Affairs (VA), where, he charged, “veterans have been unable…to obtain care they have earned.” He added, “it’s hard to argue that teleworking has helped the VA.”
While oversight of government programs is an authority of Congress, the SHOW UP Act is based on unsubstantiated assumptions of dereliction. It also disregards the devastating impact the proposed changes will have on veterans’ ability to receive care and inaccurately implies improving it. As the Senate considers the bill, they should take heed of these and other facts involving this often misunderstood form of labor.
COVID-19 irrevocably transformed the use of virtual care within the VA and across the world. Even as the pandemic subsides, public and private health care systems have continued to use telework-centered telehealth far above prepandemic levels, especially for mental health and primary care. Employers, including the VA, capitalize on telework for its benefits to both consumers and the workforce. For consumers, research supports the clinical effectiveness of telemental health service, as well as its cost-effectiveness and consumer satisfaction. On the workforce side, research has documented heightened productivity, lower distractibility, and higher job satisfaction among counselors who shifted to remote work.
Remote work also serves as a key tool in attracting and retaining a qualified workforce. As one VA service chief explained, “I am having enough trouble competing with the private sector, where extensive telework is now the norm. If telework options were rolled back, the private sector will have a field day picking off my best staff.” These comments are consistent with the data. McKinsey’s American Opportunity Survey shows that Americans have embraced remote work and want more of it. Recent data from Gallup show that 6 of 10 currently exclusively remote employees would be extremely likely to change companies if they lost their remote flexibility. Further, Gallup data show that when an employee’s location preference does not match their current work location, burnout rises, and engagement drops.
Between 2019 and 2023, the VA’s telework expansion is what has enabled it to meet the growing demand for mental health services. VA is keeping pace by having 2 or more clinicians rotate between home and a shared VA office. Forcing these hybrid practitioners to work full time at VA facilities would drastically reduce the number of patients they can care for. There simply are not enough offices on crammed VA grounds to house staff who telework today. The net result would be that fewer appointments would be available, creating longer wait times. And that is just for existing patients. It does not factor in the expected influx due to new veteran eligibility made possible by the toxic exposures PACT Act.
Here is another good example of crucial VA telework: With the advent of the 988 Suicide & Crisis Lifeline, VA is adding more than 1000 new Veterans Crisis Line responders. All these new positions are remote. The SHOW UP Act would inhibit this expansion of lifesaving programs.
Veterans want more, not fewer, telehealth options. At a House Committee on Veterans’ Affairs hearing this past September, the VA reported that most veterans would prefer to receive mental health services virtually than to have to commute to a VA medical center or clinic. Telehealth benefits veterans in meaningful ways, including that it reduces their travel time, travel expense, depletion of sick leave, and need for childcare. Veterans with posttraumatic stress disorder, military sexual trauma, those with mobility issues, or those who struggle with the stigma of mental health treatment may prefer the familiarity of their own homes for care. Virtual options also relieve a patient’s need to enter a hospital and be unnecessarily exposed to contagious viruses. That’s safer not only for veterans but also for VA staff.
Finally, virtual care improves treatment. Research has revealed that the likelihood of missing telehealth appointments is lower than for in-person appointments. When patients miss appointments, continuity of care is disrupted, and health care outcomes are diminished.
The pandemic is receding, but the advantages of telework-centered virtual care are greater than ever. Political representatives who want to show up for veterans should do everything in their power to expand—not cut—VA’s ability to authorize working from home.
COORDINATEd effort boosts optimal therapy in patients with T2D and ASCVD
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
AT ACC 2023
Shaved costs, high risk, maximum profits: Regulators worry about Florida’s butt lift boom
The office in Miami where she scheduled what’s known as a Brazilian butt lift had closed and transferred her records to a different facility, she said. The price she was quoted – and paid upfront – increased the day of the procedure, and she said she did not meet her surgeon until she was about to be placed under general anesthesia.
“I was ready to walk out,” said Ms. Ruston, 44, of Lake Alfred in Central Florida. “But I had paid everything.”
A few days after the July procedure, Ms. Ruston was hospitalized because of infection, blood loss, and nausea, her medical records show.
“I went cheap. That’s what I did,” Ms. Ruston recalled recently. “I looked for the lowest price, and I found him on Instagram.”

People like Ms. Ruston are commonly lured to office-based surgery centers in South Florida through social media marketing that makes Brazilian butt lifts and other cosmetic surgery look deceptively painless, safe, and affordable, say researchers, patient advocates, and surgeon groups.
Unlike ambulatory surgery centers and hospitals, where a patient might stay overnight for observation after treatment, office-based surgery centers offer procedures that don’t typically require an inpatient stay and are regulated as an extension of a doctor’s private practice.

But such surgical offices are often owned by corporations that can offer discount prices by contracting with surgeons who are incentivized to work on as many patients per day as possible, in as little time as possible, according to state regulators and physicians critical of the facilities.
After a rash of deaths, and in the absence of national standards, Florida regulators were the first in the nation to enact rules in 2019 meant to make the procedures safer. More than 3 years later, data shows deaths still occur.

Patient advocates and some surgeons – including those who perform the procedure themselves – anticipate the problem will only get worse. Emergency restrictions imposed by the state’s medical board in June expired in September, and the corporate business model popularized in Miami is spreading to other cities.
“We’re seeing entities that have a strong footprint in low-cost, high-volume cosmetic surgery, based in South Florida, manifesting in other parts of the country,” said Bob Basu, MD, MPH, a vice president of the American Society of Plastic Surgeons and a practicing physician in Houston.
During a Brazilian butt lift, fat is taken via liposuction from other areas of the body – such as the torso, back, or thighs – and injected into the buttocks. More than 61,000 buttock augmentation procedures, both butt lifts and implants, were performed nationwide in 2021, a 37% increase from the previous year, according to data from the Aesthetic Society, a trade group of plastic surgeons.
As with all surgery, complications can occur. Miami-Dade County’s medical examiner has documented nearly three dozen cosmetic surgery patient deaths since 2009, of which 26 resulted from a Brazilian butt lift. In each case, the person died from a pulmonary fat embolism, when fat entered the bloodstream through veins in the gluteal muscles and stopped blood from flowing to the lungs.
No national reporting system or insurance code tracks outcomes and patient demographics for a Brazilian butt lift. About 3% of surgeons worldwide had a patient die as a result of the procedure, according to a 2017 report from an Aesthetic Surgery Education and Research Foundation task force.
Medical experts said the problem is driven, in part, by having medical professionals like physician assistants and nurse practitioners perform key parts of the butt lift instead of doctors. It’s also driven by a business model that is motivated by profit, not safety, and incentivizes surgeons to exceed the number of surgeries outlined in their contracts.
In May, after a fifth patient in as many months died of complications in Miami-Dade County, Kevin Cairns, MD, proposed the state’s emergency rule to limit the number of butt lifts a surgeon could perform each day.
“I was getting sick of reading about women dying and seeing cases come before the board,” said Dr. Cairns, a physician and former member of the Florida Board of Medicine.
Some doctors performed as many as seven, according to disciplinary cases against surgeons prosecuted by the Florida Department of Health. The emergency rule limited them to no more than three, and required the use of an ultrasound to help surgeons lower the risk of a pulmonary fat clot.
But a group of physicians who perform Brazilian butt lifts in South Florida clapped back and formed Surgeons for Safety. They argued the new requirements would make the situation worse. Qualified doctors would have to do fewer procedures, they said, thus driving patients to dangerous medical professionals who don’t follow rules.
The group has since donated more than $350,000 to the state’s Republican Party, Republican candidates, and Republican political action committees, according to campaign contribution data from the Florida Department of State.
Surgeons for Safety declined KHN’s repeated interview requests. Although the group’s president, Constantino Mendieta, MD, wrote in an August editorial that he agreed not all surgeons have followed the standard of care, he called the limits put on surgeons “arbitrary.” The rule sets “a historic precedent of controlling surgeons,” he said during a meeting with Florida’s medical board.
In January, Florida state Sen. Ileana Garcia, a Republican, filed a draft bill with the state legislature that proposes no limit on the number of Brazilian butt lifts a surgeon can perform in a day. Instead, it requires office surgery centers where the procedures are performed to staff one physician per patient and prohibits surgeons from working on more than one person at a time.
The bill would also allow surgeons to delegate some parts of the procedure to other clinicians under their direct supervision.
Florida’s legislature convenes on March 7.
Consumers considering cosmetic procedures are urged to be cautious. Like Ms. Ruston, many people base their expectations on before-and-after photos and marketing videos posted on social media platforms such as Facebook, Snapchat, and Instagram.
“That’s very dangerous,” said Dr. Basu, of the American Society of Plastic Surgeons. “They’re excited about a low price and they forget about doing their homework,” he said.
The average price of a buttocks augmentation in 2021 was $4,000, according to data from the Aesthetic Society. But that’s only for the physician’s fee and does not cover anesthesia, operating room fees, prescriptions, or other expenses. A “safe” Brazilian butt lift, performed in an accredited facility and with proper aftercare, costs between $12,000 and $18,000, according to a recent article on the American Society of Plastic Surgeons’ website.
Although Florida requires a physician’s license to perform liposuction on patients who are under general anesthesia, it’s common in the medical field for midlevel medical practitioners, such as physician assistants and nurse practitioners, to do the procedure in office settings, according to Mark Mofid, MD, who coauthored the 2017 Aesthetic Surgery Education and Research Foundation task force study.
By relying on staffers who don’t have the same specialty training and get paid less, office-based surgeons can complete more butt lifts per day and charge a lower price.
“They’re doing all of them simultaneously in three or four different rooms, and it’s being staffed by one surgeon,” said Dr. Mofid, a plastic surgeon in San Diego, who added that he does not perform more than one Brazilian butt lift in a day. “The surgeon isn’t doing the actual case. It’s assistants.”
Dr. Basu said patients should ask whether their doctor holds privileges to perform the same procedure at a hospital or ambulatory surgery center, which have stricter rules than office surgery centers in terms of who can perform butt lifts and how they should be done.
People in search of bargains are reminded that cosmetic surgery can have other serious risks beyond the deadly fat clots, such as infection and organ puncture, plus problems with the kidneys, heart, and lungs.
Ms. Ruston’s surgery was performed by a board-certified plastic surgeon she said she found on Instagram. She was originally quoted $4,995, which she said she paid in full before surgery. But when she arrived in Miami, she said, the clinic tacked on fees for liposuction and for postsurgical garments and devices.
“I ended up having to pay, like, $8,000,” Ms. Ruston said. A few days after Ms. Ruston returned home to Lake Alfred, she said, she started to feel dizzy and weak and called 911.
Paramedics took her to an emergency room, where doctors diagnosed her with anemia due to blood loss, and blood and abdominal infections, her medical records show.
“If I could go back in time,” she said, “I wouldn’t have had it done.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The office in Miami where she scheduled what’s known as a Brazilian butt lift had closed and transferred her records to a different facility, she said. The price she was quoted – and paid upfront – increased the day of the procedure, and she said she did not meet her surgeon until she was about to be placed under general anesthesia.
“I was ready to walk out,” said Ms. Ruston, 44, of Lake Alfred in Central Florida. “But I had paid everything.”
A few days after the July procedure, Ms. Ruston was hospitalized because of infection, blood loss, and nausea, her medical records show.
“I went cheap. That’s what I did,” Ms. Ruston recalled recently. “I looked for the lowest price, and I found him on Instagram.”

People like Ms. Ruston are commonly lured to office-based surgery centers in South Florida through social media marketing that makes Brazilian butt lifts and other cosmetic surgery look deceptively painless, safe, and affordable, say researchers, patient advocates, and surgeon groups.
Unlike ambulatory surgery centers and hospitals, where a patient might stay overnight for observation after treatment, office-based surgery centers offer procedures that don’t typically require an inpatient stay and are regulated as an extension of a doctor’s private practice.

But such surgical offices are often owned by corporations that can offer discount prices by contracting with surgeons who are incentivized to work on as many patients per day as possible, in as little time as possible, according to state regulators and physicians critical of the facilities.
After a rash of deaths, and in the absence of national standards, Florida regulators were the first in the nation to enact rules in 2019 meant to make the procedures safer. More than 3 years later, data shows deaths still occur.

Patient advocates and some surgeons – including those who perform the procedure themselves – anticipate the problem will only get worse. Emergency restrictions imposed by the state’s medical board in June expired in September, and the corporate business model popularized in Miami is spreading to other cities.
“We’re seeing entities that have a strong footprint in low-cost, high-volume cosmetic surgery, based in South Florida, manifesting in other parts of the country,” said Bob Basu, MD, MPH, a vice president of the American Society of Plastic Surgeons and a practicing physician in Houston.
During a Brazilian butt lift, fat is taken via liposuction from other areas of the body – such as the torso, back, or thighs – and injected into the buttocks. More than 61,000 buttock augmentation procedures, both butt lifts and implants, were performed nationwide in 2021, a 37% increase from the previous year, according to data from the Aesthetic Society, a trade group of plastic surgeons.
As with all surgery, complications can occur. Miami-Dade County’s medical examiner has documented nearly three dozen cosmetic surgery patient deaths since 2009, of which 26 resulted from a Brazilian butt lift. In each case, the person died from a pulmonary fat embolism, when fat entered the bloodstream through veins in the gluteal muscles and stopped blood from flowing to the lungs.
No national reporting system or insurance code tracks outcomes and patient demographics for a Brazilian butt lift. About 3% of surgeons worldwide had a patient die as a result of the procedure, according to a 2017 report from an Aesthetic Surgery Education and Research Foundation task force.
Medical experts said the problem is driven, in part, by having medical professionals like physician assistants and nurse practitioners perform key parts of the butt lift instead of doctors. It’s also driven by a business model that is motivated by profit, not safety, and incentivizes surgeons to exceed the number of surgeries outlined in their contracts.
In May, after a fifth patient in as many months died of complications in Miami-Dade County, Kevin Cairns, MD, proposed the state’s emergency rule to limit the number of butt lifts a surgeon could perform each day.
“I was getting sick of reading about women dying and seeing cases come before the board,” said Dr. Cairns, a physician and former member of the Florida Board of Medicine.
Some doctors performed as many as seven, according to disciplinary cases against surgeons prosecuted by the Florida Department of Health. The emergency rule limited them to no more than three, and required the use of an ultrasound to help surgeons lower the risk of a pulmonary fat clot.
But a group of physicians who perform Brazilian butt lifts in South Florida clapped back and formed Surgeons for Safety. They argued the new requirements would make the situation worse. Qualified doctors would have to do fewer procedures, they said, thus driving patients to dangerous medical professionals who don’t follow rules.
The group has since donated more than $350,000 to the state’s Republican Party, Republican candidates, and Republican political action committees, according to campaign contribution data from the Florida Department of State.
Surgeons for Safety declined KHN’s repeated interview requests. Although the group’s president, Constantino Mendieta, MD, wrote in an August editorial that he agreed not all surgeons have followed the standard of care, he called the limits put on surgeons “arbitrary.” The rule sets “a historic precedent of controlling surgeons,” he said during a meeting with Florida’s medical board.
In January, Florida state Sen. Ileana Garcia, a Republican, filed a draft bill with the state legislature that proposes no limit on the number of Brazilian butt lifts a surgeon can perform in a day. Instead, it requires office surgery centers where the procedures are performed to staff one physician per patient and prohibits surgeons from working on more than one person at a time.
The bill would also allow surgeons to delegate some parts of the procedure to other clinicians under their direct supervision.
Florida’s legislature convenes on March 7.
Consumers considering cosmetic procedures are urged to be cautious. Like Ms. Ruston, many people base their expectations on before-and-after photos and marketing videos posted on social media platforms such as Facebook, Snapchat, and Instagram.
“That’s very dangerous,” said Dr. Basu, of the American Society of Plastic Surgeons. “They’re excited about a low price and they forget about doing their homework,” he said.
The average price of a buttocks augmentation in 2021 was $4,000, according to data from the Aesthetic Society. But that’s only for the physician’s fee and does not cover anesthesia, operating room fees, prescriptions, or other expenses. A “safe” Brazilian butt lift, performed in an accredited facility and with proper aftercare, costs between $12,000 and $18,000, according to a recent article on the American Society of Plastic Surgeons’ website.
Although Florida requires a physician’s license to perform liposuction on patients who are under general anesthesia, it’s common in the medical field for midlevel medical practitioners, such as physician assistants and nurse practitioners, to do the procedure in office settings, according to Mark Mofid, MD, who coauthored the 2017 Aesthetic Surgery Education and Research Foundation task force study.
By relying on staffers who don’t have the same specialty training and get paid less, office-based surgeons can complete more butt lifts per day and charge a lower price.
“They’re doing all of them simultaneously in three or four different rooms, and it’s being staffed by one surgeon,” said Dr. Mofid, a plastic surgeon in San Diego, who added that he does not perform more than one Brazilian butt lift in a day. “The surgeon isn’t doing the actual case. It’s assistants.”
Dr. Basu said patients should ask whether their doctor holds privileges to perform the same procedure at a hospital or ambulatory surgery center, which have stricter rules than office surgery centers in terms of who can perform butt lifts and how they should be done.
People in search of bargains are reminded that cosmetic surgery can have other serious risks beyond the deadly fat clots, such as infection and organ puncture, plus problems with the kidneys, heart, and lungs.
Ms. Ruston’s surgery was performed by a board-certified plastic surgeon she said she found on Instagram. She was originally quoted $4,995, which she said she paid in full before surgery. But when she arrived in Miami, she said, the clinic tacked on fees for liposuction and for postsurgical garments and devices.
“I ended up having to pay, like, $8,000,” Ms. Ruston said. A few days after Ms. Ruston returned home to Lake Alfred, she said, she started to feel dizzy and weak and called 911.
Paramedics took her to an emergency room, where doctors diagnosed her with anemia due to blood loss, and blood and abdominal infections, her medical records show.
“If I could go back in time,” she said, “I wouldn’t have had it done.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The office in Miami where she scheduled what’s known as a Brazilian butt lift had closed and transferred her records to a different facility, she said. The price she was quoted – and paid upfront – increased the day of the procedure, and she said she did not meet her surgeon until she was about to be placed under general anesthesia.
“I was ready to walk out,” said Ms. Ruston, 44, of Lake Alfred in Central Florida. “But I had paid everything.”
A few days after the July procedure, Ms. Ruston was hospitalized because of infection, blood loss, and nausea, her medical records show.
“I went cheap. That’s what I did,” Ms. Ruston recalled recently. “I looked for the lowest price, and I found him on Instagram.”

People like Ms. Ruston are commonly lured to office-based surgery centers in South Florida through social media marketing that makes Brazilian butt lifts and other cosmetic surgery look deceptively painless, safe, and affordable, say researchers, patient advocates, and surgeon groups.
Unlike ambulatory surgery centers and hospitals, where a patient might stay overnight for observation after treatment, office-based surgery centers offer procedures that don’t typically require an inpatient stay and are regulated as an extension of a doctor’s private practice.

But such surgical offices are often owned by corporations that can offer discount prices by contracting with surgeons who are incentivized to work on as many patients per day as possible, in as little time as possible, according to state regulators and physicians critical of the facilities.
After a rash of deaths, and in the absence of national standards, Florida regulators were the first in the nation to enact rules in 2019 meant to make the procedures safer. More than 3 years later, data shows deaths still occur.

Patient advocates and some surgeons – including those who perform the procedure themselves – anticipate the problem will only get worse. Emergency restrictions imposed by the state’s medical board in June expired in September, and the corporate business model popularized in Miami is spreading to other cities.
“We’re seeing entities that have a strong footprint in low-cost, high-volume cosmetic surgery, based in South Florida, manifesting in other parts of the country,” said Bob Basu, MD, MPH, a vice president of the American Society of Plastic Surgeons and a practicing physician in Houston.
During a Brazilian butt lift, fat is taken via liposuction from other areas of the body – such as the torso, back, or thighs – and injected into the buttocks. More than 61,000 buttock augmentation procedures, both butt lifts and implants, were performed nationwide in 2021, a 37% increase from the previous year, according to data from the Aesthetic Society, a trade group of plastic surgeons.
As with all surgery, complications can occur. Miami-Dade County’s medical examiner has documented nearly three dozen cosmetic surgery patient deaths since 2009, of which 26 resulted from a Brazilian butt lift. In each case, the person died from a pulmonary fat embolism, when fat entered the bloodstream through veins in the gluteal muscles and stopped blood from flowing to the lungs.
No national reporting system or insurance code tracks outcomes and patient demographics for a Brazilian butt lift. About 3% of surgeons worldwide had a patient die as a result of the procedure, according to a 2017 report from an Aesthetic Surgery Education and Research Foundation task force.
Medical experts said the problem is driven, in part, by having medical professionals like physician assistants and nurse practitioners perform key parts of the butt lift instead of doctors. It’s also driven by a business model that is motivated by profit, not safety, and incentivizes surgeons to exceed the number of surgeries outlined in their contracts.
In May, after a fifth patient in as many months died of complications in Miami-Dade County, Kevin Cairns, MD, proposed the state’s emergency rule to limit the number of butt lifts a surgeon could perform each day.
“I was getting sick of reading about women dying and seeing cases come before the board,” said Dr. Cairns, a physician and former member of the Florida Board of Medicine.
Some doctors performed as many as seven, according to disciplinary cases against surgeons prosecuted by the Florida Department of Health. The emergency rule limited them to no more than three, and required the use of an ultrasound to help surgeons lower the risk of a pulmonary fat clot.
But a group of physicians who perform Brazilian butt lifts in South Florida clapped back and formed Surgeons for Safety. They argued the new requirements would make the situation worse. Qualified doctors would have to do fewer procedures, they said, thus driving patients to dangerous medical professionals who don’t follow rules.
The group has since donated more than $350,000 to the state’s Republican Party, Republican candidates, and Republican political action committees, according to campaign contribution data from the Florida Department of State.
Surgeons for Safety declined KHN’s repeated interview requests. Although the group’s president, Constantino Mendieta, MD, wrote in an August editorial that he agreed not all surgeons have followed the standard of care, he called the limits put on surgeons “arbitrary.” The rule sets “a historic precedent of controlling surgeons,” he said during a meeting with Florida’s medical board.
In January, Florida state Sen. Ileana Garcia, a Republican, filed a draft bill with the state legislature that proposes no limit on the number of Brazilian butt lifts a surgeon can perform in a day. Instead, it requires office surgery centers where the procedures are performed to staff one physician per patient and prohibits surgeons from working on more than one person at a time.
The bill would also allow surgeons to delegate some parts of the procedure to other clinicians under their direct supervision.
Florida’s legislature convenes on March 7.
Consumers considering cosmetic procedures are urged to be cautious. Like Ms. Ruston, many people base their expectations on before-and-after photos and marketing videos posted on social media platforms such as Facebook, Snapchat, and Instagram.
“That’s very dangerous,” said Dr. Basu, of the American Society of Plastic Surgeons. “They’re excited about a low price and they forget about doing their homework,” he said.
The average price of a buttocks augmentation in 2021 was $4,000, according to data from the Aesthetic Society. But that’s only for the physician’s fee and does not cover anesthesia, operating room fees, prescriptions, or other expenses. A “safe” Brazilian butt lift, performed in an accredited facility and with proper aftercare, costs between $12,000 and $18,000, according to a recent article on the American Society of Plastic Surgeons’ website.
Although Florida requires a physician’s license to perform liposuction on patients who are under general anesthesia, it’s common in the medical field for midlevel medical practitioners, such as physician assistants and nurse practitioners, to do the procedure in office settings, according to Mark Mofid, MD, who coauthored the 2017 Aesthetic Surgery Education and Research Foundation task force study.
By relying on staffers who don’t have the same specialty training and get paid less, office-based surgeons can complete more butt lifts per day and charge a lower price.
“They’re doing all of them simultaneously in three or four different rooms, and it’s being staffed by one surgeon,” said Dr. Mofid, a plastic surgeon in San Diego, who added that he does not perform more than one Brazilian butt lift in a day. “The surgeon isn’t doing the actual case. It’s assistants.”
Dr. Basu said patients should ask whether their doctor holds privileges to perform the same procedure at a hospital or ambulatory surgery center, which have stricter rules than office surgery centers in terms of who can perform butt lifts and how they should be done.
People in search of bargains are reminded that cosmetic surgery can have other serious risks beyond the deadly fat clots, such as infection and organ puncture, plus problems with the kidneys, heart, and lungs.
Ms. Ruston’s surgery was performed by a board-certified plastic surgeon she said she found on Instagram. She was originally quoted $4,995, which she said she paid in full before surgery. But when she arrived in Miami, she said, the clinic tacked on fees for liposuction and for postsurgical garments and devices.
“I ended up having to pay, like, $8,000,” Ms. Ruston said. A few days after Ms. Ruston returned home to Lake Alfred, she said, she started to feel dizzy and weak and called 911.
Paramedics took her to an emergency room, where doctors diagnosed her with anemia due to blood loss, and blood and abdominal infections, her medical records show.
“If I could go back in time,” she said, “I wouldn’t have had it done.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nicotinamide does not prevent skin cancer after organ transplant
published in the New England Journal of Medicine.
“No signal of efficacy was observed,” said investigators led by Nicholas Allen, MPH, of the University of Sydney department of dermatology.
These results fill an “important gap in our understanding” and “will probably change the practice of many skin-cancer physicians,” two experts on the topic commented in a related editorial.
The editorialists are David Miller, MD, PhD, a dermatologist and medical oncologist at Massachusetts General Hospital, and Kevin Emerick, MD, a head and neck surgeon as Massachusetts Eye and Ear, both in Boston.
Transplant patients have 50 times the risk of nonmelanoma skin cancers – also known as keratinocyte cancers – than the general public, owing to immunosuppression, and their lesions are more aggressive and are more likely to metastasize, they explain.
Nicotinamide (vitamin B3) has been shown to prevent nonmelanoma skin cancers in healthy, immunocompetent people, so physicians routinely prescribe it to transplant patients on the assumption that it will do the same for them, they comment.
The Australian investigators decided to put the assumption to the test.
The team randomly assigned 79 patients who had undergone solid-organ transplant to receive nicotinamide 500 mg twice a day and 79 other patients to receive twice-daily placebo for a year. Participants underwent dermatology exams every 3 months to check for new lesions.
The participants were at high risk for new lesions; some had had more than 40 in the previous 5 years. The two groups were well balanced; kidney transplants were the most common.
At 12 months, there was virtually no difference in the incidence of new nonmelanoma skin cancers: 207 in the nicotinamide group and 210 in the placebo group (P = .96).
There was also no significant difference in squamous cell and basal cell carcinoma counts or actinic keratosis counts.
“The interpretation of the results is straightforward: nicotinamide lacks clinical usefulness in preventing the development of keratinocyte carcinomas in solid-organ transplant recipients,” the team concludes.
As for why nicotinamide didn’t work in the trial, the investigators say it could be because it is not potent enough to overcome the stifling of antitumor immunity and DNA-repair enzymes with immunosuppression.
Fewer than half of participants in the trial reported using sunscreen at any point during the study, which is in line with past reports that transplant patients don’t routinely use sunscreen.
Two other strategies for preventing squamous cell carcinoma after transplant – use of oral retinoids and mTOR inhibitors – are problematic for various reasons, and use was low in both study arms.
Editorialists Dr. Miller and Dr. Emerick suggest a possible new approach: immune checkpoint inhibitors before transplant to reduce the risk of nonmelanoma skin cancer afterward. They say the strategy should be explored and that ongoing efforts to minimize or eliminate the need for immunosuppression after transplant are promising.
The investigators originally planned to enroll 254 persons, but the trial was stopped early because of poor recruitment. Potential participants may already have been taking nicotinamide, which is commonly used, and that may have affected recruitment, the investigators say.
The work was funded by Australia’s National Health and Medical Research Council. Dr. Allen has disclosed no relevant financial relationships. One investigator has received speaker’s fees from BMS. Another is a consultant for many companies, including Amgen, BMS, GlaxoSmithKline, and Merck. Dr. Emerick is an advisor for Regeneron, Sanofi, and Castle Biosciences. Dr. Miller is a researcher or consultant for those companies as well as Pfizer and others and has stock options in Avstera.
A version of this article first appeared on Medscape.com.
published in the New England Journal of Medicine.
“No signal of efficacy was observed,” said investigators led by Nicholas Allen, MPH, of the University of Sydney department of dermatology.
These results fill an “important gap in our understanding” and “will probably change the practice of many skin-cancer physicians,” two experts on the topic commented in a related editorial.
The editorialists are David Miller, MD, PhD, a dermatologist and medical oncologist at Massachusetts General Hospital, and Kevin Emerick, MD, a head and neck surgeon as Massachusetts Eye and Ear, both in Boston.
Transplant patients have 50 times the risk of nonmelanoma skin cancers – also known as keratinocyte cancers – than the general public, owing to immunosuppression, and their lesions are more aggressive and are more likely to metastasize, they explain.
Nicotinamide (vitamin B3) has been shown to prevent nonmelanoma skin cancers in healthy, immunocompetent people, so physicians routinely prescribe it to transplant patients on the assumption that it will do the same for them, they comment.
The Australian investigators decided to put the assumption to the test.
The team randomly assigned 79 patients who had undergone solid-organ transplant to receive nicotinamide 500 mg twice a day and 79 other patients to receive twice-daily placebo for a year. Participants underwent dermatology exams every 3 months to check for new lesions.
The participants were at high risk for new lesions; some had had more than 40 in the previous 5 years. The two groups were well balanced; kidney transplants were the most common.
At 12 months, there was virtually no difference in the incidence of new nonmelanoma skin cancers: 207 in the nicotinamide group and 210 in the placebo group (P = .96).
There was also no significant difference in squamous cell and basal cell carcinoma counts or actinic keratosis counts.
“The interpretation of the results is straightforward: nicotinamide lacks clinical usefulness in preventing the development of keratinocyte carcinomas in solid-organ transplant recipients,” the team concludes.
As for why nicotinamide didn’t work in the trial, the investigators say it could be because it is not potent enough to overcome the stifling of antitumor immunity and DNA-repair enzymes with immunosuppression.
Fewer than half of participants in the trial reported using sunscreen at any point during the study, which is in line with past reports that transplant patients don’t routinely use sunscreen.
Two other strategies for preventing squamous cell carcinoma after transplant – use of oral retinoids and mTOR inhibitors – are problematic for various reasons, and use was low in both study arms.
Editorialists Dr. Miller and Dr. Emerick suggest a possible new approach: immune checkpoint inhibitors before transplant to reduce the risk of nonmelanoma skin cancer afterward. They say the strategy should be explored and that ongoing efforts to minimize or eliminate the need for immunosuppression after transplant are promising.
The investigators originally planned to enroll 254 persons, but the trial was stopped early because of poor recruitment. Potential participants may already have been taking nicotinamide, which is commonly used, and that may have affected recruitment, the investigators say.
The work was funded by Australia’s National Health and Medical Research Council. Dr. Allen has disclosed no relevant financial relationships. One investigator has received speaker’s fees from BMS. Another is a consultant for many companies, including Amgen, BMS, GlaxoSmithKline, and Merck. Dr. Emerick is an advisor for Regeneron, Sanofi, and Castle Biosciences. Dr. Miller is a researcher or consultant for those companies as well as Pfizer and others and has stock options in Avstera.
A version of this article first appeared on Medscape.com.
published in the New England Journal of Medicine.
“No signal of efficacy was observed,” said investigators led by Nicholas Allen, MPH, of the University of Sydney department of dermatology.
These results fill an “important gap in our understanding” and “will probably change the practice of many skin-cancer physicians,” two experts on the topic commented in a related editorial.
The editorialists are David Miller, MD, PhD, a dermatologist and medical oncologist at Massachusetts General Hospital, and Kevin Emerick, MD, a head and neck surgeon as Massachusetts Eye and Ear, both in Boston.
Transplant patients have 50 times the risk of nonmelanoma skin cancers – also known as keratinocyte cancers – than the general public, owing to immunosuppression, and their lesions are more aggressive and are more likely to metastasize, they explain.
Nicotinamide (vitamin B3) has been shown to prevent nonmelanoma skin cancers in healthy, immunocompetent people, so physicians routinely prescribe it to transplant patients on the assumption that it will do the same for them, they comment.
The Australian investigators decided to put the assumption to the test.
The team randomly assigned 79 patients who had undergone solid-organ transplant to receive nicotinamide 500 mg twice a day and 79 other patients to receive twice-daily placebo for a year. Participants underwent dermatology exams every 3 months to check for new lesions.
The participants were at high risk for new lesions; some had had more than 40 in the previous 5 years. The two groups were well balanced; kidney transplants were the most common.
At 12 months, there was virtually no difference in the incidence of new nonmelanoma skin cancers: 207 in the nicotinamide group and 210 in the placebo group (P = .96).
There was also no significant difference in squamous cell and basal cell carcinoma counts or actinic keratosis counts.
“The interpretation of the results is straightforward: nicotinamide lacks clinical usefulness in preventing the development of keratinocyte carcinomas in solid-organ transplant recipients,” the team concludes.
As for why nicotinamide didn’t work in the trial, the investigators say it could be because it is not potent enough to overcome the stifling of antitumor immunity and DNA-repair enzymes with immunosuppression.
Fewer than half of participants in the trial reported using sunscreen at any point during the study, which is in line with past reports that transplant patients don’t routinely use sunscreen.
Two other strategies for preventing squamous cell carcinoma after transplant – use of oral retinoids and mTOR inhibitors – are problematic for various reasons, and use was low in both study arms.
Editorialists Dr. Miller and Dr. Emerick suggest a possible new approach: immune checkpoint inhibitors before transplant to reduce the risk of nonmelanoma skin cancer afterward. They say the strategy should be explored and that ongoing efforts to minimize or eliminate the need for immunosuppression after transplant are promising.
The investigators originally planned to enroll 254 persons, but the trial was stopped early because of poor recruitment. Potential participants may already have been taking nicotinamide, which is commonly used, and that may have affected recruitment, the investigators say.
The work was funded by Australia’s National Health and Medical Research Council. Dr. Allen has disclosed no relevant financial relationships. One investigator has received speaker’s fees from BMS. Another is a consultant for many companies, including Amgen, BMS, GlaxoSmithKline, and Merck. Dr. Emerick is an advisor for Regeneron, Sanofi, and Castle Biosciences. Dr. Miller is a researcher or consultant for those companies as well as Pfizer and others and has stock options in Avstera.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE