User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Breastfeeding and colorectal cancer
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Teens have easy online access to Delta-8 cannabinoid products
WASHINGTON – , researchers reported at the 2023 annual meeting of the American Academy of Pediatrics. Most of the products identified came in bright, colorful, kid-friendly packaging and cost less than $10, the researchers found, and only 2 out of 45 sites had a third-party age verification requirement for purchases.
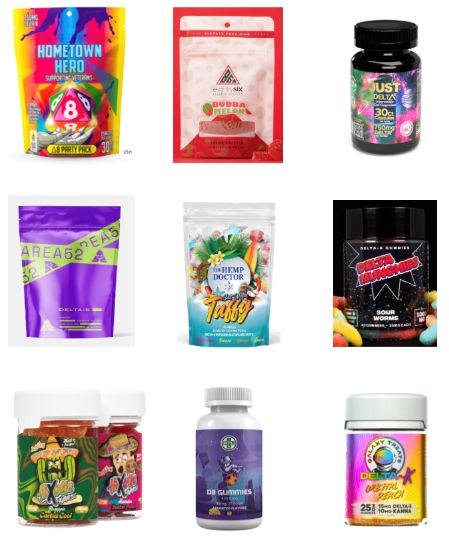
Delta-8 THC, also called D8, is a synthetically produced cannabinoid whose chemical structure and effects are nearly identical to traditional THC, the authors explained, and past research has found that D8 products, such as e-cigarettes, can contain toxic byproducts and contaminants.
”Since D8 is not traditional THC, minors may underestimate its strength and potential danger,” wrote lead author Abhijeet Grewal, BS, a research assistant at Cohen Children’s Medical Center, New York, and senior author Ruth Milanaik, DO, director of the Neonatal Neurodevelopmental Program at Cohen Children’s and a developmental/behavioral pediatrician at Northwell Health, also in New York. “Although traditional THC is a federally banned substance, D8 is legal on a federal level and less restricted on a state by state basis, making it easier for individuals to acquire D8.”
Easily accessible
During the first seven moments of 2021, 77% of reports of accidental exposure occurred in people under age 18, including some children who required ICU admission. The U.S. Food and Drug Administration also received 104 reports of adverse events from products containing D8 between December 2020-February 2022, and more than half of those required medical intervention.
To better understand how easy it is to access D8, the authors collected data on 45 websites they identified that sold D8. The researchers looked for age verification questions for accessing the site, third-party age certification, what kinds of products (edibles, smoke products, or tinctures) were sold, the price and dosage of the cheapest product, and examples of packaging, flavors, marketing claims, and warning statements at each site.
More than a third of the sites (36%) did not ask for customers’ age and almost none of the sites asked for proof: 96% of the sites lacked formal third-party age verification procedures. All but one of the sites sold D8 edibles, and most (82%) sold D8 vaping or smoking products. Only 42% sold tinctures, a mix of concentrated D8 with oil that’s orally consumed.
The cheapest product was priced under $5 on one-third of the sites and under $10 on another third of the sites. The cheapest product was between $10-20 on 16% of the sites while the remaining nine sites’ cheapest product was more than $20. In assessing only the cheapest D8 products on each site, nearly half (47%) contained 51 mg or more of D8, and 20% of the products didn’t report the dosage. Another 22% contained 41-50 mg of D8, and the remaining five products contained 20-40 mg.
Kid-friendly D8
More than half of the D8 products were sold in kid-friendly packaging – packages with bright, colorful designs and fonts that resemble candy or snack food, sometimes cartoon characters or fun items like dice on the packaging. Further, 24% of the websites did not include any warnings or other health information about D8.
“The low prices, high dosages available, and eye-popping packaging make these products extremely attractive to teens who are looking for a high,” the researchers concluded. They advised clinicians to talk with teen patients about the dangers of D8 and advocated for policymakers to more strictly regulate online distributors of D8 products, particularly in requiring age verification procedures and prohibiting kid-friendly packaging.
Megan Moreno, MD, MSEd, MPH, an adolescent medicine physician and researcher at the University of Wisconsin, Madison, School of Medicine and Public Health and UWHealthKids, was particularly struck by how eye-catching the packaging was. “The bright colors and font choices are really designed to attract adolescents,” commented Dr. Moreno, who was not involved in the study. But she was not surprised overall by the findings.
“Other studies have found that the cannabis industry leverages online tools and social media, alongside youth-friendly packaging, to attract youth to their products,” she said. “What is disappointing is that these companies do not use industry standard approaches, such as the alcohol industry, to age-gate their websites.”
It’s important for providers who care for adolescents to ask about substance use but to especially include questions about substances that teens might not think of as “drugs,” such as Delta 8, Dr. Moreno said.
“Prior research on other types of substance such as these has found that teens can think these are less dangerous versions of cannabis, so providing accurate information and asking about these products can prevent harm to kids,” Dr. Moreno said. Although this study focused on websites that sell D8 products, she said that “another important area of influence to consider is social media messaging around these products, which may drive traffic to the purchasing site.” It’s clear this industry is not going to self-regulate without policy changes, Dr. Moreno added, so she noted the importance of advocating for policy that regulates these sites.
Mr. Grewal, Dr. Milanaik and Dr. Moreno had no disclosures. No external funding sources were noted.
WASHINGTON – , researchers reported at the 2023 annual meeting of the American Academy of Pediatrics. Most of the products identified came in bright, colorful, kid-friendly packaging and cost less than $10, the researchers found, and only 2 out of 45 sites had a third-party age verification requirement for purchases.

Delta-8 THC, also called D8, is a synthetically produced cannabinoid whose chemical structure and effects are nearly identical to traditional THC, the authors explained, and past research has found that D8 products, such as e-cigarettes, can contain toxic byproducts and contaminants.
”Since D8 is not traditional THC, minors may underestimate its strength and potential danger,” wrote lead author Abhijeet Grewal, BS, a research assistant at Cohen Children’s Medical Center, New York, and senior author Ruth Milanaik, DO, director of the Neonatal Neurodevelopmental Program at Cohen Children’s and a developmental/behavioral pediatrician at Northwell Health, also in New York. “Although traditional THC is a federally banned substance, D8 is legal on a federal level and less restricted on a state by state basis, making it easier for individuals to acquire D8.”
Easily accessible
During the first seven moments of 2021, 77% of reports of accidental exposure occurred in people under age 18, including some children who required ICU admission. The U.S. Food and Drug Administration also received 104 reports of adverse events from products containing D8 between December 2020-February 2022, and more than half of those required medical intervention.
To better understand how easy it is to access D8, the authors collected data on 45 websites they identified that sold D8. The researchers looked for age verification questions for accessing the site, third-party age certification, what kinds of products (edibles, smoke products, or tinctures) were sold, the price and dosage of the cheapest product, and examples of packaging, flavors, marketing claims, and warning statements at each site.
More than a third of the sites (36%) did not ask for customers’ age and almost none of the sites asked for proof: 96% of the sites lacked formal third-party age verification procedures. All but one of the sites sold D8 edibles, and most (82%) sold D8 vaping or smoking products. Only 42% sold tinctures, a mix of concentrated D8 with oil that’s orally consumed.
The cheapest product was priced under $5 on one-third of the sites and under $10 on another third of the sites. The cheapest product was between $10-20 on 16% of the sites while the remaining nine sites’ cheapest product was more than $20. In assessing only the cheapest D8 products on each site, nearly half (47%) contained 51 mg or more of D8, and 20% of the products didn’t report the dosage. Another 22% contained 41-50 mg of D8, and the remaining five products contained 20-40 mg.
Kid-friendly D8
More than half of the D8 products were sold in kid-friendly packaging – packages with bright, colorful designs and fonts that resemble candy or snack food, sometimes cartoon characters or fun items like dice on the packaging. Further, 24% of the websites did not include any warnings or other health information about D8.
“The low prices, high dosages available, and eye-popping packaging make these products extremely attractive to teens who are looking for a high,” the researchers concluded. They advised clinicians to talk with teen patients about the dangers of D8 and advocated for policymakers to more strictly regulate online distributors of D8 products, particularly in requiring age verification procedures and prohibiting kid-friendly packaging.
Megan Moreno, MD, MSEd, MPH, an adolescent medicine physician and researcher at the University of Wisconsin, Madison, School of Medicine and Public Health and UWHealthKids, was particularly struck by how eye-catching the packaging was. “The bright colors and font choices are really designed to attract adolescents,” commented Dr. Moreno, who was not involved in the study. But she was not surprised overall by the findings.
“Other studies have found that the cannabis industry leverages online tools and social media, alongside youth-friendly packaging, to attract youth to their products,” she said. “What is disappointing is that these companies do not use industry standard approaches, such as the alcohol industry, to age-gate their websites.”
It’s important for providers who care for adolescents to ask about substance use but to especially include questions about substances that teens might not think of as “drugs,” such as Delta 8, Dr. Moreno said.
“Prior research on other types of substance such as these has found that teens can think these are less dangerous versions of cannabis, so providing accurate information and asking about these products can prevent harm to kids,” Dr. Moreno said. Although this study focused on websites that sell D8 products, she said that “another important area of influence to consider is social media messaging around these products, which may drive traffic to the purchasing site.” It’s clear this industry is not going to self-regulate without policy changes, Dr. Moreno added, so she noted the importance of advocating for policy that regulates these sites.
Mr. Grewal, Dr. Milanaik and Dr. Moreno had no disclosures. No external funding sources were noted.
WASHINGTON – , researchers reported at the 2023 annual meeting of the American Academy of Pediatrics. Most of the products identified came in bright, colorful, kid-friendly packaging and cost less than $10, the researchers found, and only 2 out of 45 sites had a third-party age verification requirement for purchases.

Delta-8 THC, also called D8, is a synthetically produced cannabinoid whose chemical structure and effects are nearly identical to traditional THC, the authors explained, and past research has found that D8 products, such as e-cigarettes, can contain toxic byproducts and contaminants.
”Since D8 is not traditional THC, minors may underestimate its strength and potential danger,” wrote lead author Abhijeet Grewal, BS, a research assistant at Cohen Children’s Medical Center, New York, and senior author Ruth Milanaik, DO, director of the Neonatal Neurodevelopmental Program at Cohen Children’s and a developmental/behavioral pediatrician at Northwell Health, also in New York. “Although traditional THC is a federally banned substance, D8 is legal on a federal level and less restricted on a state by state basis, making it easier for individuals to acquire D8.”
Easily accessible
During the first seven moments of 2021, 77% of reports of accidental exposure occurred in people under age 18, including some children who required ICU admission. The U.S. Food and Drug Administration also received 104 reports of adverse events from products containing D8 between December 2020-February 2022, and more than half of those required medical intervention.
To better understand how easy it is to access D8, the authors collected data on 45 websites they identified that sold D8. The researchers looked for age verification questions for accessing the site, third-party age certification, what kinds of products (edibles, smoke products, or tinctures) were sold, the price and dosage of the cheapest product, and examples of packaging, flavors, marketing claims, and warning statements at each site.
More than a third of the sites (36%) did not ask for customers’ age and almost none of the sites asked for proof: 96% of the sites lacked formal third-party age verification procedures. All but one of the sites sold D8 edibles, and most (82%) sold D8 vaping or smoking products. Only 42% sold tinctures, a mix of concentrated D8 with oil that’s orally consumed.
The cheapest product was priced under $5 on one-third of the sites and under $10 on another third of the sites. The cheapest product was between $10-20 on 16% of the sites while the remaining nine sites’ cheapest product was more than $20. In assessing only the cheapest D8 products on each site, nearly half (47%) contained 51 mg or more of D8, and 20% of the products didn’t report the dosage. Another 22% contained 41-50 mg of D8, and the remaining five products contained 20-40 mg.
Kid-friendly D8
More than half of the D8 products were sold in kid-friendly packaging – packages with bright, colorful designs and fonts that resemble candy or snack food, sometimes cartoon characters or fun items like dice on the packaging. Further, 24% of the websites did not include any warnings or other health information about D8.
“The low prices, high dosages available, and eye-popping packaging make these products extremely attractive to teens who are looking for a high,” the researchers concluded. They advised clinicians to talk with teen patients about the dangers of D8 and advocated for policymakers to more strictly regulate online distributors of D8 products, particularly in requiring age verification procedures and prohibiting kid-friendly packaging.
Megan Moreno, MD, MSEd, MPH, an adolescent medicine physician and researcher at the University of Wisconsin, Madison, School of Medicine and Public Health and UWHealthKids, was particularly struck by how eye-catching the packaging was. “The bright colors and font choices are really designed to attract adolescents,” commented Dr. Moreno, who was not involved in the study. But she was not surprised overall by the findings.
“Other studies have found that the cannabis industry leverages online tools and social media, alongside youth-friendly packaging, to attract youth to their products,” she said. “What is disappointing is that these companies do not use industry standard approaches, such as the alcohol industry, to age-gate their websites.”
It’s important for providers who care for adolescents to ask about substance use but to especially include questions about substances that teens might not think of as “drugs,” such as Delta 8, Dr. Moreno said.
“Prior research on other types of substance such as these has found that teens can think these are less dangerous versions of cannabis, so providing accurate information and asking about these products can prevent harm to kids,” Dr. Moreno said. Although this study focused on websites that sell D8 products, she said that “another important area of influence to consider is social media messaging around these products, which may drive traffic to the purchasing site.” It’s clear this industry is not going to self-regulate without policy changes, Dr. Moreno added, so she noted the importance of advocating for policy that regulates these sites.
Mr. Grewal, Dr. Milanaik and Dr. Moreno had no disclosures. No external funding sources were noted.
At AAP 2023
FDA okays drug for Duchenne muscular dystrophy
Duchenne muscular dystrophy (DMD) in patients as young as age 2 years, the company has announced Vamorolone is a structurally unique steroidal anti-inflammatory drug that potently inhibits proinflammatory NFkB pathways via high-affinity binding to the glucocorticoid receptor.
“Corticosteroids have been a first line treatment for DMD for many years, but their utility has always been limited by the side effect profile, which includes weight gain, short stature, and decreased bone density, among others,” Sharon Hesterlee, PhD, chief research officer for the Muscular Dystrophy Association, said in a statement.
The approval of vamorolone “provides people living with Duchenne, and their families, a powerful tool to treat the disease, while limiting some negative side effects associated with corticosteroids,” Dr. Hesterlee added.
The approval was based on data from the phase 2b VISION-DMD study, supplemented with safety information collected from three open-label studies.
Vamorolone was administered at doses ranging from 2-6 mg/kg/d for a period of up to 48 months.
Vamorolone demonstrated efficacy similar to that of traditional corticosteroids, with data suggesting a reduction in adverse events – notably related to bone health, growth trajectory, and behavior.
Vamorolone had received orphan drug status for DMD, as well as fast track and rare pediatric disease designations. It will be made available in the United States by Catalyst Pharmaceuticals.
A version of this article first appeared on Medscape.com .
Duchenne muscular dystrophy (DMD) in patients as young as age 2 years, the company has announced Vamorolone is a structurally unique steroidal anti-inflammatory drug that potently inhibits proinflammatory NFkB pathways via high-affinity binding to the glucocorticoid receptor.
“Corticosteroids have been a first line treatment for DMD for many years, but their utility has always been limited by the side effect profile, which includes weight gain, short stature, and decreased bone density, among others,” Sharon Hesterlee, PhD, chief research officer for the Muscular Dystrophy Association, said in a statement.
The approval of vamorolone “provides people living with Duchenne, and their families, a powerful tool to treat the disease, while limiting some negative side effects associated with corticosteroids,” Dr. Hesterlee added.
The approval was based on data from the phase 2b VISION-DMD study, supplemented with safety information collected from three open-label studies.
Vamorolone was administered at doses ranging from 2-6 mg/kg/d for a period of up to 48 months.
Vamorolone demonstrated efficacy similar to that of traditional corticosteroids, with data suggesting a reduction in adverse events – notably related to bone health, growth trajectory, and behavior.
Vamorolone had received orphan drug status for DMD, as well as fast track and rare pediatric disease designations. It will be made available in the United States by Catalyst Pharmaceuticals.
A version of this article first appeared on Medscape.com .
Duchenne muscular dystrophy (DMD) in patients as young as age 2 years, the company has announced Vamorolone is a structurally unique steroidal anti-inflammatory drug that potently inhibits proinflammatory NFkB pathways via high-affinity binding to the glucocorticoid receptor.
“Corticosteroids have been a first line treatment for DMD for many years, but their utility has always been limited by the side effect profile, which includes weight gain, short stature, and decreased bone density, among others,” Sharon Hesterlee, PhD, chief research officer for the Muscular Dystrophy Association, said in a statement.
The approval of vamorolone “provides people living with Duchenne, and their families, a powerful tool to treat the disease, while limiting some negative side effects associated with corticosteroids,” Dr. Hesterlee added.
The approval was based on data from the phase 2b VISION-DMD study, supplemented with safety information collected from three open-label studies.
Vamorolone was administered at doses ranging from 2-6 mg/kg/d for a period of up to 48 months.
Vamorolone demonstrated efficacy similar to that of traditional corticosteroids, with data suggesting a reduction in adverse events – notably related to bone health, growth trajectory, and behavior.
Vamorolone had received orphan drug status for DMD, as well as fast track and rare pediatric disease designations. It will be made available in the United States by Catalyst Pharmaceuticals.
A version of this article first appeared on Medscape.com .
Online nicotine toothpick vendors ignore age restrictions
WASHINGTON – according to a study of 77 stores and 16 online sites.
Online nicotine toothpick sales are “the Wild West” in terms of regulation, said Abhijeet Grewal, a research assistant at Cohen Children’s Medical Center, in New Hyde Park, N.Y., who presented the findings at the annual meeting of the American Academy of Pediatrics.
Nicotine toothpicks have become popular among teenagers as a relatively inconspicuous way to access the drug, Mr. Grewal said. The nicotine content of the toothpicks varies, but many contain as much as 2-3 mg per pick compared with the 1.1-1.8–mg amount inhaled per the average cigarette, he said. The cheap price and teen-friendly flavors like cherry and mocha add to the appeal of the picks. However, data on the marketplace and accessibility of these products are lacking, Mr. Grewal said.
To find out how easily youth can buy nicotine toothpicks through in-person and online channels, Mr. Grewal and colleagues identified and called 404 brick-and-mortar retailers across the United States by phone and asked whether they required ID for purchase of nicotine toothpicks; of the 77 locations that responded, only 1 said that they would sell nicotine toothpicks without asking for proof of age.
The researchers also collected data on 16 vendor websites that sold nicotine toothpicks with shipment to the United States (identified from pixotine.com).
Overall, 11 sites (69%) prompted users to confirm that they were aged 21 years or older to either view the site or place orders, but 12 sites (75%) required no formal method of verification.
Warnings or disclaimers, such as “nicotine is an addictive chemical,” appeared on 69% of sites. Marketing statements including terms such as “discreet” and “cost-effective” to describe the toothpicks, Mr. Grewal said, and online reviews endorsed the products as “convenient” and “rich in flavor.”
The sites in the study offered a total of 32 different flavors, Mr. Grewal said, and 44% of the sites offered some type of discount on prices, which land in the range of approximately $5 for a tube of 20 toothpicks.
Nicotine toothpicks and flavored toothpicks without nicotine were originally marketed as smoking cessation aids, said Mr. Grewal, but their low price point and ability to be consumed discreetly makes them appealing to teens for nicotine use in many environments.
More research is needed to characterize youth use of nicotine toothpick products, as well as purchasing patterns, he said. However, the results highlight the need for regulation of nicotine toothpick vendors to protect youth from accessing nicotine in this form, he said.
Ask adolescents about toothpicks
“While nicotine replacement therapy [NRT] products may be an effective way for people to quit smoking, these products have the potential to introduce minors to nicotine in a seemingly innocent way resulting in dependence,” senior author Ruth Milanaik, DO, also of Cohen Children’s Medical Center, said in an interview. “Many children are intrigued by these fun flavored products, and our team was interested in examining the availability of these products to minors.”
Overall, “our team was quite pleased with brick-and-mortar stores’ spoken requirements of age verification for purchase, and quite worried about the availability of nic picks through online vendors,” she continued.
Clinicians, educators, and parents should be aware of the existence of nicotine toothpicks and the ease with which minors can attain them through online vendors, Dr. Milanaik said. “While NRT is a part of smoking cessation programs, nicotine toothpicks should not be used by minors without clinical reasons,” she said. “The innocuous and innocent nature of these toothpicks may entice minors to try and regularly use these without regard to future dependence.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
WASHINGTON – according to a study of 77 stores and 16 online sites.
Online nicotine toothpick sales are “the Wild West” in terms of regulation, said Abhijeet Grewal, a research assistant at Cohen Children’s Medical Center, in New Hyde Park, N.Y., who presented the findings at the annual meeting of the American Academy of Pediatrics.
Nicotine toothpicks have become popular among teenagers as a relatively inconspicuous way to access the drug, Mr. Grewal said. The nicotine content of the toothpicks varies, but many contain as much as 2-3 mg per pick compared with the 1.1-1.8–mg amount inhaled per the average cigarette, he said. The cheap price and teen-friendly flavors like cherry and mocha add to the appeal of the picks. However, data on the marketplace and accessibility of these products are lacking, Mr. Grewal said.
To find out how easily youth can buy nicotine toothpicks through in-person and online channels, Mr. Grewal and colleagues identified and called 404 brick-and-mortar retailers across the United States by phone and asked whether they required ID for purchase of nicotine toothpicks; of the 77 locations that responded, only 1 said that they would sell nicotine toothpicks without asking for proof of age.
The researchers also collected data on 16 vendor websites that sold nicotine toothpicks with shipment to the United States (identified from pixotine.com).
Overall, 11 sites (69%) prompted users to confirm that they were aged 21 years or older to either view the site or place orders, but 12 sites (75%) required no formal method of verification.
Warnings or disclaimers, such as “nicotine is an addictive chemical,” appeared on 69% of sites. Marketing statements including terms such as “discreet” and “cost-effective” to describe the toothpicks, Mr. Grewal said, and online reviews endorsed the products as “convenient” and “rich in flavor.”
The sites in the study offered a total of 32 different flavors, Mr. Grewal said, and 44% of the sites offered some type of discount on prices, which land in the range of approximately $5 for a tube of 20 toothpicks.
Nicotine toothpicks and flavored toothpicks without nicotine were originally marketed as smoking cessation aids, said Mr. Grewal, but their low price point and ability to be consumed discreetly makes them appealing to teens for nicotine use in many environments.
More research is needed to characterize youth use of nicotine toothpick products, as well as purchasing patterns, he said. However, the results highlight the need for regulation of nicotine toothpick vendors to protect youth from accessing nicotine in this form, he said.
Ask adolescents about toothpicks
“While nicotine replacement therapy [NRT] products may be an effective way for people to quit smoking, these products have the potential to introduce minors to nicotine in a seemingly innocent way resulting in dependence,” senior author Ruth Milanaik, DO, also of Cohen Children’s Medical Center, said in an interview. “Many children are intrigued by these fun flavored products, and our team was interested in examining the availability of these products to minors.”
Overall, “our team was quite pleased with brick-and-mortar stores’ spoken requirements of age verification for purchase, and quite worried about the availability of nic picks through online vendors,” she continued.
Clinicians, educators, and parents should be aware of the existence of nicotine toothpicks and the ease with which minors can attain them through online vendors, Dr. Milanaik said. “While NRT is a part of smoking cessation programs, nicotine toothpicks should not be used by minors without clinical reasons,” she said. “The innocuous and innocent nature of these toothpicks may entice minors to try and regularly use these without regard to future dependence.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
WASHINGTON – according to a study of 77 stores and 16 online sites.
Online nicotine toothpick sales are “the Wild West” in terms of regulation, said Abhijeet Grewal, a research assistant at Cohen Children’s Medical Center, in New Hyde Park, N.Y., who presented the findings at the annual meeting of the American Academy of Pediatrics.
Nicotine toothpicks have become popular among teenagers as a relatively inconspicuous way to access the drug, Mr. Grewal said. The nicotine content of the toothpicks varies, but many contain as much as 2-3 mg per pick compared with the 1.1-1.8–mg amount inhaled per the average cigarette, he said. The cheap price and teen-friendly flavors like cherry and mocha add to the appeal of the picks. However, data on the marketplace and accessibility of these products are lacking, Mr. Grewal said.
To find out how easily youth can buy nicotine toothpicks through in-person and online channels, Mr. Grewal and colleagues identified and called 404 brick-and-mortar retailers across the United States by phone and asked whether they required ID for purchase of nicotine toothpicks; of the 77 locations that responded, only 1 said that they would sell nicotine toothpicks without asking for proof of age.
The researchers also collected data on 16 vendor websites that sold nicotine toothpicks with shipment to the United States (identified from pixotine.com).
Overall, 11 sites (69%) prompted users to confirm that they were aged 21 years or older to either view the site or place orders, but 12 sites (75%) required no formal method of verification.
Warnings or disclaimers, such as “nicotine is an addictive chemical,” appeared on 69% of sites. Marketing statements including terms such as “discreet” and “cost-effective” to describe the toothpicks, Mr. Grewal said, and online reviews endorsed the products as “convenient” and “rich in flavor.”
The sites in the study offered a total of 32 different flavors, Mr. Grewal said, and 44% of the sites offered some type of discount on prices, which land in the range of approximately $5 for a tube of 20 toothpicks.
Nicotine toothpicks and flavored toothpicks without nicotine were originally marketed as smoking cessation aids, said Mr. Grewal, but their low price point and ability to be consumed discreetly makes them appealing to teens for nicotine use in many environments.
More research is needed to characterize youth use of nicotine toothpick products, as well as purchasing patterns, he said. However, the results highlight the need for regulation of nicotine toothpick vendors to protect youth from accessing nicotine in this form, he said.
Ask adolescents about toothpicks
“While nicotine replacement therapy [NRT] products may be an effective way for people to quit smoking, these products have the potential to introduce minors to nicotine in a seemingly innocent way resulting in dependence,” senior author Ruth Milanaik, DO, also of Cohen Children’s Medical Center, said in an interview. “Many children are intrigued by these fun flavored products, and our team was interested in examining the availability of these products to minors.”
Overall, “our team was quite pleased with brick-and-mortar stores’ spoken requirements of age verification for purchase, and quite worried about the availability of nic picks through online vendors,” she continued.
Clinicians, educators, and parents should be aware of the existence of nicotine toothpicks and the ease with which minors can attain them through online vendors, Dr. Milanaik said. “While NRT is a part of smoking cessation programs, nicotine toothpicks should not be used by minors without clinical reasons,” she said. “The innocuous and innocent nature of these toothpicks may entice minors to try and regularly use these without regard to future dependence.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
AT AAP 2023
Dupilumab promising for children aged 1-11 with EoE
VANCOUVER –
High exposure to dupilumab was associated with significantly improved histologic, endoscopic, and transcriptomic improvements, compared with placebo at week 16. Sustained response or improvements continued to week 52 with continued treatment in the high-exposure dupilumab group. Children in the high-exposure dupilumab group also gained more weight during the study than those initially assigned to placebo.
“Eosinophilic esophagitis is a chronic, aggressive, type 2 inflammatory disease that has a substantial impact on quality of life,” said Mirna Chehade, MD, MPH, of the Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai in New York. And the incidence and prevalence of the disease is increasing.
Dupilumab is already indicated for treating EoE in adolescents aged 12 or older as well as adults, but “there are no approved treatments for EoE in children under 12,” said Dr. Chehade, who presented the results of the late-breaking abstract at the ACG: American College of Gastroenterology 2023 annual scientific meeting.
She and her colleagues randomly assigned 102 children aged 1-11 years with active EoE to three groups for the first 16 weeks of the study: 37 to high-exposure dupilumab; 31 to low-exposure dupilumab; and 34 others to placebo, followed by either high- or low-dose dupilumab. Baseline demographics and disease characteristics were comparable between groups.
During an active 36-week extension period, the 37 participants who were initially assigned to receive high-exposure dupilumab continued the same treatment up to week 52. A total of 29 participants initially assigned to receive low-exposure dupilumab continues their regimen as well. Those initially assigned to receive placebo switched to a preassigned active treatment group; 18 children started to take high-exposure dupilumab, and 14 began to take low-exposure dupilumab.
The children in the study had a high burden of disease, as reflected by the duration of EoE as well as histologic, endoscopic, and clinical scores. The mean age was 7.2 years in the placebo group and 6.8 years in the dupilumab group. They were mostly White boys, Dr. Chehade said.
Key outcomes
At week 16, the high-exposure dupilumab group met the primary study endpoint with a peak esophageal intraepithelial eosinophil count ≤ 6 on high-power field assessment. This was significantly different from the placebo group (least squares mean difference, 64.5; 95% confidence interval, 48.19-80.85; P < .0001).
At week 52, 63% of children who remained on high-exposure dupilumab and 53% of those who switched from placebo to high-exposure dupilumab achieved a peak eosinophil count ≤ 6.
The study included multiple secondary outcomes. For example, at week 16, the following measures improved from baseline with high-exposure dupilumab, compared with placebo:
- EoE-Histologic Scoring System grade and stage scores (–0.88 and –0.84 vs. +0.02 and +0.05; both P < .0001).
- EoE-Endoscopic Reference Score (–3.5 vs. +0.3; P < .0001).
- Change in body weight for age percentile (+3.09 vs. +0.29).
- Numeric improvement in caregiver-reported proportion of days experiencing one or more EoE sign (–0.28 vs. –0.17).
At week 52, these outcomes were sustained or improved with continued high-exposure dupilumab. The researchers also saw improvements among the placebo recipients who switched to high-exposure dupilumab.
The reason the children were randomly assigned to high-exposure or low-exposure groups instead of high-dose and low-dose cohorts is because the children grew during the study, Dr. Chehade explained. “As you can see, there was a nice change in weight, and at specific time periods the doses were adjusted to match.”
‘Good safety profile’
Dupilumab was well tolerated. “The safety profile is very similar to what has been so far described and published for dupilumab in adults,” said Dr. Chehade. At week 16, adverse events that were more frequent with dupilumab vs. placebo included COVID-19, rash, headache, and injection-site erythema, for example. Similar safety results were seen up to week 52.
“I think it’s promising as we wait for the actual study to be published,” said Asmeen Bhatt, MD, PhD, co-moderator of the session and assistant professor of medicine at University of Texas Health Science Center, Houston. “The drug was recently approved for adult EOE use, just last year, and it has been shown to be effective.”
“There are a lot of adult drugs that are now being tested in the pediatric population, and this is one of them,” Dr. Bhatt added. “It has a very good safety profile. I’m not a pediatric gastroenterologist but I expect that it will have a lot of utility.”
The study was funded by Regeneron and Sanofi. Dr. Chehade is a consultant for Sanofi and Regeneron and receives research funding from Regeneron. Dr. Bhatt had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
VANCOUVER –
High exposure to dupilumab was associated with significantly improved histologic, endoscopic, and transcriptomic improvements, compared with placebo at week 16. Sustained response or improvements continued to week 52 with continued treatment in the high-exposure dupilumab group. Children in the high-exposure dupilumab group also gained more weight during the study than those initially assigned to placebo.
“Eosinophilic esophagitis is a chronic, aggressive, type 2 inflammatory disease that has a substantial impact on quality of life,” said Mirna Chehade, MD, MPH, of the Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai in New York. And the incidence and prevalence of the disease is increasing.
Dupilumab is already indicated for treating EoE in adolescents aged 12 or older as well as adults, but “there are no approved treatments for EoE in children under 12,” said Dr. Chehade, who presented the results of the late-breaking abstract at the ACG: American College of Gastroenterology 2023 annual scientific meeting.
She and her colleagues randomly assigned 102 children aged 1-11 years with active EoE to three groups for the first 16 weeks of the study: 37 to high-exposure dupilumab; 31 to low-exposure dupilumab; and 34 others to placebo, followed by either high- or low-dose dupilumab. Baseline demographics and disease characteristics were comparable between groups.
During an active 36-week extension period, the 37 participants who were initially assigned to receive high-exposure dupilumab continued the same treatment up to week 52. A total of 29 participants initially assigned to receive low-exposure dupilumab continues their regimen as well. Those initially assigned to receive placebo switched to a preassigned active treatment group; 18 children started to take high-exposure dupilumab, and 14 began to take low-exposure dupilumab.
The children in the study had a high burden of disease, as reflected by the duration of EoE as well as histologic, endoscopic, and clinical scores. The mean age was 7.2 years in the placebo group and 6.8 years in the dupilumab group. They were mostly White boys, Dr. Chehade said.
Key outcomes
At week 16, the high-exposure dupilumab group met the primary study endpoint with a peak esophageal intraepithelial eosinophil count ≤ 6 on high-power field assessment. This was significantly different from the placebo group (least squares mean difference, 64.5; 95% confidence interval, 48.19-80.85; P < .0001).
At week 52, 63% of children who remained on high-exposure dupilumab and 53% of those who switched from placebo to high-exposure dupilumab achieved a peak eosinophil count ≤ 6.
The study included multiple secondary outcomes. For example, at week 16, the following measures improved from baseline with high-exposure dupilumab, compared with placebo:
- EoE-Histologic Scoring System grade and stage scores (–0.88 and –0.84 vs. +0.02 and +0.05; both P < .0001).
- EoE-Endoscopic Reference Score (–3.5 vs. +0.3; P < .0001).
- Change in body weight for age percentile (+3.09 vs. +0.29).
- Numeric improvement in caregiver-reported proportion of days experiencing one or more EoE sign (–0.28 vs. –0.17).
At week 52, these outcomes were sustained or improved with continued high-exposure dupilumab. The researchers also saw improvements among the placebo recipients who switched to high-exposure dupilumab.
The reason the children were randomly assigned to high-exposure or low-exposure groups instead of high-dose and low-dose cohorts is because the children grew during the study, Dr. Chehade explained. “As you can see, there was a nice change in weight, and at specific time periods the doses were adjusted to match.”
‘Good safety profile’
Dupilumab was well tolerated. “The safety profile is very similar to what has been so far described and published for dupilumab in adults,” said Dr. Chehade. At week 16, adverse events that were more frequent with dupilumab vs. placebo included COVID-19, rash, headache, and injection-site erythema, for example. Similar safety results were seen up to week 52.
“I think it’s promising as we wait for the actual study to be published,” said Asmeen Bhatt, MD, PhD, co-moderator of the session and assistant professor of medicine at University of Texas Health Science Center, Houston. “The drug was recently approved for adult EOE use, just last year, and it has been shown to be effective.”
“There are a lot of adult drugs that are now being tested in the pediatric population, and this is one of them,” Dr. Bhatt added. “It has a very good safety profile. I’m not a pediatric gastroenterologist but I expect that it will have a lot of utility.”
The study was funded by Regeneron and Sanofi. Dr. Chehade is a consultant for Sanofi and Regeneron and receives research funding from Regeneron. Dr. Bhatt had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
VANCOUVER –
High exposure to dupilumab was associated with significantly improved histologic, endoscopic, and transcriptomic improvements, compared with placebo at week 16. Sustained response or improvements continued to week 52 with continued treatment in the high-exposure dupilumab group. Children in the high-exposure dupilumab group also gained more weight during the study than those initially assigned to placebo.
“Eosinophilic esophagitis is a chronic, aggressive, type 2 inflammatory disease that has a substantial impact on quality of life,” said Mirna Chehade, MD, MPH, of the Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai in New York. And the incidence and prevalence of the disease is increasing.
Dupilumab is already indicated for treating EoE in adolescents aged 12 or older as well as adults, but “there are no approved treatments for EoE in children under 12,” said Dr. Chehade, who presented the results of the late-breaking abstract at the ACG: American College of Gastroenterology 2023 annual scientific meeting.
She and her colleagues randomly assigned 102 children aged 1-11 years with active EoE to three groups for the first 16 weeks of the study: 37 to high-exposure dupilumab; 31 to low-exposure dupilumab; and 34 others to placebo, followed by either high- or low-dose dupilumab. Baseline demographics and disease characteristics were comparable between groups.
During an active 36-week extension period, the 37 participants who were initially assigned to receive high-exposure dupilumab continued the same treatment up to week 52. A total of 29 participants initially assigned to receive low-exposure dupilumab continues their regimen as well. Those initially assigned to receive placebo switched to a preassigned active treatment group; 18 children started to take high-exposure dupilumab, and 14 began to take low-exposure dupilumab.
The children in the study had a high burden of disease, as reflected by the duration of EoE as well as histologic, endoscopic, and clinical scores. The mean age was 7.2 years in the placebo group and 6.8 years in the dupilumab group. They were mostly White boys, Dr. Chehade said.
Key outcomes
At week 16, the high-exposure dupilumab group met the primary study endpoint with a peak esophageal intraepithelial eosinophil count ≤ 6 on high-power field assessment. This was significantly different from the placebo group (least squares mean difference, 64.5; 95% confidence interval, 48.19-80.85; P < .0001).
At week 52, 63% of children who remained on high-exposure dupilumab and 53% of those who switched from placebo to high-exposure dupilumab achieved a peak eosinophil count ≤ 6.
The study included multiple secondary outcomes. For example, at week 16, the following measures improved from baseline with high-exposure dupilumab, compared with placebo:
- EoE-Histologic Scoring System grade and stage scores (–0.88 and –0.84 vs. +0.02 and +0.05; both P < .0001).
- EoE-Endoscopic Reference Score (–3.5 vs. +0.3; P < .0001).
- Change in body weight for age percentile (+3.09 vs. +0.29).
- Numeric improvement in caregiver-reported proportion of days experiencing one or more EoE sign (–0.28 vs. –0.17).
At week 52, these outcomes were sustained or improved with continued high-exposure dupilumab. The researchers also saw improvements among the placebo recipients who switched to high-exposure dupilumab.
The reason the children were randomly assigned to high-exposure or low-exposure groups instead of high-dose and low-dose cohorts is because the children grew during the study, Dr. Chehade explained. “As you can see, there was a nice change in weight, and at specific time periods the doses were adjusted to match.”
‘Good safety profile’
Dupilumab was well tolerated. “The safety profile is very similar to what has been so far described and published for dupilumab in adults,” said Dr. Chehade. At week 16, adverse events that were more frequent with dupilumab vs. placebo included COVID-19, rash, headache, and injection-site erythema, for example. Similar safety results were seen up to week 52.
“I think it’s promising as we wait for the actual study to be published,” said Asmeen Bhatt, MD, PhD, co-moderator of the session and assistant professor of medicine at University of Texas Health Science Center, Houston. “The drug was recently approved for adult EOE use, just last year, and it has been shown to be effective.”
“There are a lot of adult drugs that are now being tested in the pediatric population, and this is one of them,” Dr. Bhatt added. “It has a very good safety profile. I’m not a pediatric gastroenterologist but I expect that it will have a lot of utility.”
The study was funded by Regeneron and Sanofi. Dr. Chehade is a consultant for Sanofi and Regeneron and receives research funding from Regeneron. Dr. Bhatt had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
AT ACG 2023
Clustered Vesicles on the Neck
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.
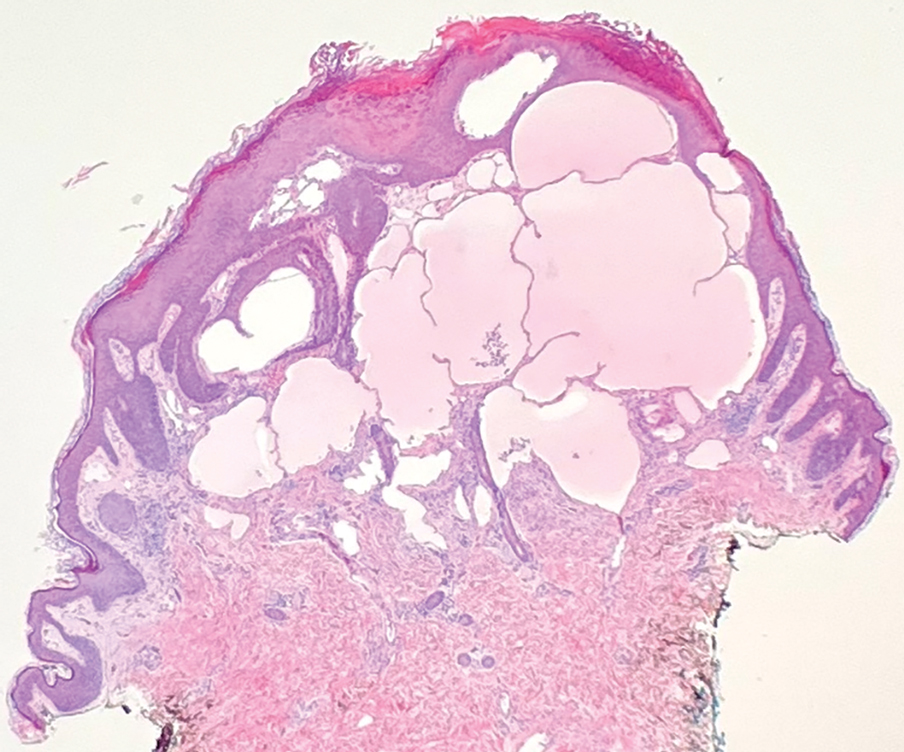
The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.

The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.

The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
A 6-year-old girl presented to the dermatology clinic with a rash on the right side of the neck that was noted at birth as a small raised lesion but slowly increased over time in size and number of lesions. She reported pruritus and irritation, particularly when rubbed or scratched. There was no family history of similar skin abnormalities. Her medical history was notable for a left-sided cholesteatoma on tympanomastoidectomy. Physical examination revealed clustered vesicles on the right side of the neck with underlying erythema. The vesicles contained mostly clear fluid with a few focal areas of hemorrhagic fluid. Ultrasonography was unremarkable, and magnetic resonance imaging revealed superficial T2 hyperintense nonenhancing cutaneous and subcutaneous lesions overlying the right lateral neck with minimal extension into the superficial right supraclavicular soft tissues.
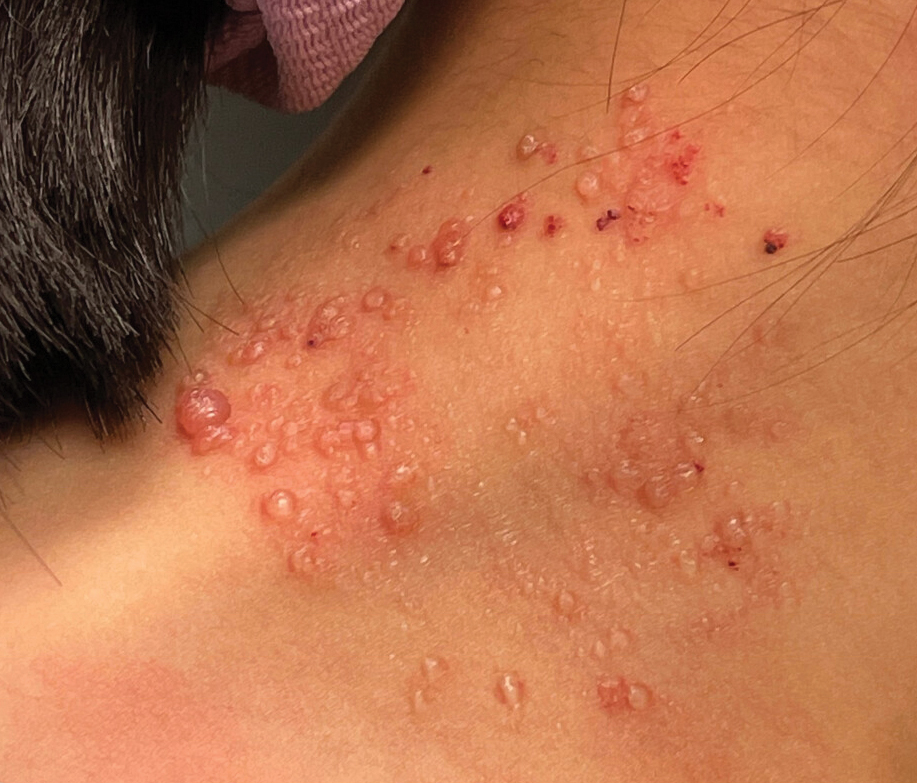
Neurologic nuggets of wisdom for pediatric practice
WASHINGTON – Get the back story before rushing to diagnose a seizure disorder in a child, Michael Strunc, MD, said in a presentation at the annual meeting of the American Academy of Pediatrics.
Clinicians should ask parents or caregivers about the child’s behavior before the suspected seizure, whether there were any triggers, and if so, what might they have been, according to Dr. Strunc, a child neurologist and sleep medicine specialist at Children’s Hospital of the King’s Daughters, Norfolk, Va.
“Most seizures don’t have triggers,” he said. Rather, patients often become stiff, experience a motor event that builds in intensity then slows and stops, and finally, the patient is sleepy and tired. Clinicians should also find out whether the event had a beginning, middle, and end.
Approximately 0.6% of children younger than 17 years in the United States have active epilepsy, according to the Centers for Disease Control and Prevention.
Dr. Strunc offered a few more tips for diagnosing a child:
- Ask whether the patient’s eyes were open during the event. If the eyes were closed or squished closed, “it is almost never a seizure,” he said.
- Find out whether the patient was awake or asleep, and how, if at all, caregivers attempted to stop the event.
- Ask if the child’s experiences were repeating and predictable, and inquire about a family history of seizures or other events.
- Inquire about any developmental changes and other changes in the child, such as irritability, regression, or ataxia.
The differential diagnosis for a seizure includes nonepileptic events that occur with and without changes in consciousness or sleep. These events range from breath-holding and hyperventilation to night terrors, narcolepsy, migraine, and attention-deficit/hyperactivity disorder, he said.
Is it epilepsy?
Dr. Strunc shared several cases of neurologic “events” ranging from simple to severe.
In one case, a 10-month-old infant girl with a potential tonic/staring seizure presented with a history of events that involved getting stuck in a stiff position, usually while sitting in a car seat or highchair, with adducting of legs, redness of face, and “zoned-out” expression. The infant was healthy, smart, and precocious, with no illness, fever, or trauma, but the mother was very concerned, Dr. Strunc said.
The diagnosis: Self-gratification, which is benign and usually outgrown, although it can become extreme, he said.
By contrast, “absence,” also known as idiopathic generalized epilepsy, presents as brief events of 4-10 seconds that may occur up to hundreds of times a day. This type of epilepsy is associated with the sudden onset of impaired consciousness and unresponsiveness. These events end abruptly, and the child may be unaware. Absence is more common in girls. It usually occurs after age 4 and usually remits by about age 12, Dr. Strunc said.
However, the onset of absence in patients younger than age 3 is associated with increased odds of neurodevelopmental abnormalities “and probably represents another epilepsy syndrome,” he said.
Absence symptoms may mirror those of children who are simply daydreamers, Dr. Strunc noted. One way to confirm absence is by provoking hyperventilation, which will bring on an episode of absence if present, he said. EEGs provide evidence as well.
Acute ataxia in children has a wide differential that sends kids and families to the pediatrician or emergency department, Dr. Strunc said. Acute cerebellar ataxia is characterized by abrupt and symmetric symptoms, with no mental status changes, no fever, no meningitis, and no headache. A wide, unstable gait is a distinguishing feature, Dr. Strunc said.
However, other causes of acute ataxia should be ruled out, including toxic ingestion, tick paralysis, central nervous system infections, vascular conditions, and genetic conditions.
Don’t miss those ticks
Especially during periods when kids are outdoors, clinicians should consider a tick bite as a source of ataxia and neurologic symptoms in children, Dr. Strunc emphasized. Tick paralysis notably resembles many symptoms of Guillain-Barré syndrome (acute inflammatory demyelinating polyneuropathy).
Dr. Strunc described a case involving a 5-year-old girl who developed sudden problems with gait. The problems worsened quickly and prompted an emergency department visit.
The girl had an unremarkable history, she had not experienced mental status changes, her strength was normal, and she had just returned from a Girl Scouts trip. The patient was presumed to have Guillain-Barré. IVIG was initiated when an emergency nurse found a tick on her scalp. The tick was removed, and the patient left the hospital within 24 hours.
Children with tick paralysis are usually symptomatic after 5-7 days with the tick attached, Dr. Strunc said. They recover within a day after tick removal.
Overall, actual seizures are less common than other neurologic events in children, according to Dr. Strunc. Details on history, lack or presence of neurologic feature, and normal child development can help guide evaluation.
Take advantage of videos, he emphasized, as many parents and caregivers record a child’s neurologic events.
“Ataxia is scary, but exam and associated findings will help you with etiology,” he said.
Dr. Strunc has received research support from Jazz and Harmony and has served on the speakers’ bureau for Jazz Pharmaceuticals, Harmony Biosciences, and Avadel, unrelated to his presentation.
A version of this article first appeared on Medscape.com.
WASHINGTON – Get the back story before rushing to diagnose a seizure disorder in a child, Michael Strunc, MD, said in a presentation at the annual meeting of the American Academy of Pediatrics.
Clinicians should ask parents or caregivers about the child’s behavior before the suspected seizure, whether there were any triggers, and if so, what might they have been, according to Dr. Strunc, a child neurologist and sleep medicine specialist at Children’s Hospital of the King’s Daughters, Norfolk, Va.
“Most seizures don’t have triggers,” he said. Rather, patients often become stiff, experience a motor event that builds in intensity then slows and stops, and finally, the patient is sleepy and tired. Clinicians should also find out whether the event had a beginning, middle, and end.
Approximately 0.6% of children younger than 17 years in the United States have active epilepsy, according to the Centers for Disease Control and Prevention.
Dr. Strunc offered a few more tips for diagnosing a child:
- Ask whether the patient’s eyes were open during the event. If the eyes were closed or squished closed, “it is almost never a seizure,” he said.
- Find out whether the patient was awake or asleep, and how, if at all, caregivers attempted to stop the event.
- Ask if the child’s experiences were repeating and predictable, and inquire about a family history of seizures or other events.
- Inquire about any developmental changes and other changes in the child, such as irritability, regression, or ataxia.
The differential diagnosis for a seizure includes nonepileptic events that occur with and without changes in consciousness or sleep. These events range from breath-holding and hyperventilation to night terrors, narcolepsy, migraine, and attention-deficit/hyperactivity disorder, he said.
Is it epilepsy?
Dr. Strunc shared several cases of neurologic “events” ranging from simple to severe.
In one case, a 10-month-old infant girl with a potential tonic/staring seizure presented with a history of events that involved getting stuck in a stiff position, usually while sitting in a car seat or highchair, with adducting of legs, redness of face, and “zoned-out” expression. The infant was healthy, smart, and precocious, with no illness, fever, or trauma, but the mother was very concerned, Dr. Strunc said.
The diagnosis: Self-gratification, which is benign and usually outgrown, although it can become extreme, he said.
By contrast, “absence,” also known as idiopathic generalized epilepsy, presents as brief events of 4-10 seconds that may occur up to hundreds of times a day. This type of epilepsy is associated with the sudden onset of impaired consciousness and unresponsiveness. These events end abruptly, and the child may be unaware. Absence is more common in girls. It usually occurs after age 4 and usually remits by about age 12, Dr. Strunc said.
However, the onset of absence in patients younger than age 3 is associated with increased odds of neurodevelopmental abnormalities “and probably represents another epilepsy syndrome,” he said.
Absence symptoms may mirror those of children who are simply daydreamers, Dr. Strunc noted. One way to confirm absence is by provoking hyperventilation, which will bring on an episode of absence if present, he said. EEGs provide evidence as well.
Acute ataxia in children has a wide differential that sends kids and families to the pediatrician or emergency department, Dr. Strunc said. Acute cerebellar ataxia is characterized by abrupt and symmetric symptoms, with no mental status changes, no fever, no meningitis, and no headache. A wide, unstable gait is a distinguishing feature, Dr. Strunc said.
However, other causes of acute ataxia should be ruled out, including toxic ingestion, tick paralysis, central nervous system infections, vascular conditions, and genetic conditions.
Don’t miss those ticks
Especially during periods when kids are outdoors, clinicians should consider a tick bite as a source of ataxia and neurologic symptoms in children, Dr. Strunc emphasized. Tick paralysis notably resembles many symptoms of Guillain-Barré syndrome (acute inflammatory demyelinating polyneuropathy).
Dr. Strunc described a case involving a 5-year-old girl who developed sudden problems with gait. The problems worsened quickly and prompted an emergency department visit.
The girl had an unremarkable history, she had not experienced mental status changes, her strength was normal, and she had just returned from a Girl Scouts trip. The patient was presumed to have Guillain-Barré. IVIG was initiated when an emergency nurse found a tick on her scalp. The tick was removed, and the patient left the hospital within 24 hours.
Children with tick paralysis are usually symptomatic after 5-7 days with the tick attached, Dr. Strunc said. They recover within a day after tick removal.
Overall, actual seizures are less common than other neurologic events in children, according to Dr. Strunc. Details on history, lack or presence of neurologic feature, and normal child development can help guide evaluation.
Take advantage of videos, he emphasized, as many parents and caregivers record a child’s neurologic events.
“Ataxia is scary, but exam and associated findings will help you with etiology,” he said.
Dr. Strunc has received research support from Jazz and Harmony and has served on the speakers’ bureau for Jazz Pharmaceuticals, Harmony Biosciences, and Avadel, unrelated to his presentation.
A version of this article first appeared on Medscape.com.
WASHINGTON – Get the back story before rushing to diagnose a seizure disorder in a child, Michael Strunc, MD, said in a presentation at the annual meeting of the American Academy of Pediatrics.
Clinicians should ask parents or caregivers about the child’s behavior before the suspected seizure, whether there were any triggers, and if so, what might they have been, according to Dr. Strunc, a child neurologist and sleep medicine specialist at Children’s Hospital of the King’s Daughters, Norfolk, Va.
“Most seizures don’t have triggers,” he said. Rather, patients often become stiff, experience a motor event that builds in intensity then slows and stops, and finally, the patient is sleepy and tired. Clinicians should also find out whether the event had a beginning, middle, and end.
Approximately 0.6% of children younger than 17 years in the United States have active epilepsy, according to the Centers for Disease Control and Prevention.
Dr. Strunc offered a few more tips for diagnosing a child:
- Ask whether the patient’s eyes were open during the event. If the eyes were closed or squished closed, “it is almost never a seizure,” he said.
- Find out whether the patient was awake or asleep, and how, if at all, caregivers attempted to stop the event.
- Ask if the child’s experiences were repeating and predictable, and inquire about a family history of seizures or other events.
- Inquire about any developmental changes and other changes in the child, such as irritability, regression, or ataxia.
The differential diagnosis for a seizure includes nonepileptic events that occur with and without changes in consciousness or sleep. These events range from breath-holding and hyperventilation to night terrors, narcolepsy, migraine, and attention-deficit/hyperactivity disorder, he said.
Is it epilepsy?
Dr. Strunc shared several cases of neurologic “events” ranging from simple to severe.
In one case, a 10-month-old infant girl with a potential tonic/staring seizure presented with a history of events that involved getting stuck in a stiff position, usually while sitting in a car seat or highchair, with adducting of legs, redness of face, and “zoned-out” expression. The infant was healthy, smart, and precocious, with no illness, fever, or trauma, but the mother was very concerned, Dr. Strunc said.
The diagnosis: Self-gratification, which is benign and usually outgrown, although it can become extreme, he said.
By contrast, “absence,” also known as idiopathic generalized epilepsy, presents as brief events of 4-10 seconds that may occur up to hundreds of times a day. This type of epilepsy is associated with the sudden onset of impaired consciousness and unresponsiveness. These events end abruptly, and the child may be unaware. Absence is more common in girls. It usually occurs after age 4 and usually remits by about age 12, Dr. Strunc said.
However, the onset of absence in patients younger than age 3 is associated with increased odds of neurodevelopmental abnormalities “and probably represents another epilepsy syndrome,” he said.
Absence symptoms may mirror those of children who are simply daydreamers, Dr. Strunc noted. One way to confirm absence is by provoking hyperventilation, which will bring on an episode of absence if present, he said. EEGs provide evidence as well.
Acute ataxia in children has a wide differential that sends kids and families to the pediatrician or emergency department, Dr. Strunc said. Acute cerebellar ataxia is characterized by abrupt and symmetric symptoms, with no mental status changes, no fever, no meningitis, and no headache. A wide, unstable gait is a distinguishing feature, Dr. Strunc said.
However, other causes of acute ataxia should be ruled out, including toxic ingestion, tick paralysis, central nervous system infections, vascular conditions, and genetic conditions.
Don’t miss those ticks
Especially during periods when kids are outdoors, clinicians should consider a tick bite as a source of ataxia and neurologic symptoms in children, Dr. Strunc emphasized. Tick paralysis notably resembles many symptoms of Guillain-Barré syndrome (acute inflammatory demyelinating polyneuropathy).
Dr. Strunc described a case involving a 5-year-old girl who developed sudden problems with gait. The problems worsened quickly and prompted an emergency department visit.
The girl had an unremarkable history, she had not experienced mental status changes, her strength was normal, and she had just returned from a Girl Scouts trip. The patient was presumed to have Guillain-Barré. IVIG was initiated when an emergency nurse found a tick on her scalp. The tick was removed, and the patient left the hospital within 24 hours.
Children with tick paralysis are usually symptomatic after 5-7 days with the tick attached, Dr. Strunc said. They recover within a day after tick removal.
Overall, actual seizures are less common than other neurologic events in children, according to Dr. Strunc. Details on history, lack or presence of neurologic feature, and normal child development can help guide evaluation.
Take advantage of videos, he emphasized, as many parents and caregivers record a child’s neurologic events.
“Ataxia is scary, but exam and associated findings will help you with etiology,” he said.
Dr. Strunc has received research support from Jazz and Harmony and has served on the speakers’ bureau for Jazz Pharmaceuticals, Harmony Biosciences, and Avadel, unrelated to his presentation.
A version of this article first appeared on Medscape.com.
FROM AAP 2023
Multicenter study aims to find new treatments for hidradenitis suppurativa
When Haley Naik, MD, joined the University of California, San Francisco, as a dermatologist in 2015, she was struck by the dearth of data in the medical literature about hidradenitis suppurativa (HS).
“For decades there were no datasets to begin to understand HS – its clinical course, how patients respond to medications, and how quality of life improves for patients with therapy,” Dr. Naik, who directs the HS program at UCSF, said in an interview. Inspired to improve the bleak HS knowledge landscape, she began to systematically collect information from HS patient visits, “to try to better understand how treatments were helping them or not and also to better understand their quality-of-life impact,” she said. “This also facilitated research in HS, but over time it became clear that there was a growing need for a larger effort.”
But in 2020, Dr. Naik teamed up with investigative dermatologist Michelle Lowes, MBBS, PhD, to . To date, more than 500 patients are enrolled at 12 participating sites, and 4 more sites plan to join the consortium by the end of 2023. The goal is to enroll a total of 8,000 patients, which will make it the largest dataset of its kind.
“Each site investigator is a physician who specializes in taking care of HS patients,” said Dr. Naik, who is the study’s principal investigator. “These are people who are conducting active research in various aspects of HS, and they’re trusted members of the medical community.”
She highlighted the three main objectives of HS PROGRESS. The first objective is to develop a longitudinal cohort of HS patients so that investigators can understand the clinical course of HS and effectiveness of treatments. The second is to collect biospecimens from patients with HS for translational studies “that can help to drive drug development, help us identify biomarkers that can help us predict disease course and predict patient response to therapies, so we know exactly what to give them,” she explained. The third objective is to provide patients with HS with the opportunity to be recruited for clinical trials, “so they have access to cutting-edge therapies and know what’s happening in this space.”
Collecting biospecimens
The goal of collecting biospecimens is to provide them to multiple investigators to improve the understanding of HS biology and treatment. “Our thought is to apply next generation techniques to these biospecimens to get metagenomic, transcriptomic, and genomic data to better understand HS biology so that we can identify targets for novel therapy,” Dr. Naik said.
Although HS is estimated to affect 1% of Western populations, the tumor necrosis alpha (TNF)-inhibitor adalimumab remains the only Food and Drug Administration-approved therapy for the condition.
However, Dr. Naik said that there are many promising drugs on the horizon for HS, especially interleukin (IL)-17 inhibitors. “One of the most exciting things about these drugs is that they set the bar higher for what we can expect out of therapies for HS, such as reporting a HiSCR (HS Clinical Response) score 75, which is the equivalent of 75% improvement in inflammatory HS lesions without an increase in draining tunnels,” she said. “This is well beyond what adalimumab had demonstrated in landmark trials in 2015. The safety profile on IL-17 inhibitors looks great, too.”
JAK inhibitors also hold promise for HS. “It’s going to be key to see how these drugs perform in the real-world setting in our average HS patients who may have comorbidities,” Dr. Naik said. “This is where an effort like HS PROGRESS will carry weight, because in a dataset like this, we’re going to be able to ask questions like, is there a class of drugs that works better for one specific phenotype of HS, or for patients who have a younger age of onset, or who are earlier in their disease course? These are questions we can’t ask in the context of a clinical trial, but we can ask in the context of real-world data from many practices.”
In addition to USCF, the 11 study locations participating in HS PROGRESS are the University of North Carolina at Chapel Hill; Mayo Clinic; Penn State University, Hershey; University of Virginia, Charlottesville; Washington University in St. Louis; University of Southern California, Los Angeles; Henry Ford Health, Detroit; University of Minnesota; University of Pennsylvania, Philadelphia; Duke University, Durham, N.C.; and University of Miami.
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, UCB, and Novartis; and investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
When Haley Naik, MD, joined the University of California, San Francisco, as a dermatologist in 2015, she was struck by the dearth of data in the medical literature about hidradenitis suppurativa (HS).
“For decades there were no datasets to begin to understand HS – its clinical course, how patients respond to medications, and how quality of life improves for patients with therapy,” Dr. Naik, who directs the HS program at UCSF, said in an interview. Inspired to improve the bleak HS knowledge landscape, she began to systematically collect information from HS patient visits, “to try to better understand how treatments were helping them or not and also to better understand their quality-of-life impact,” she said. “This also facilitated research in HS, but over time it became clear that there was a growing need for a larger effort.”
But in 2020, Dr. Naik teamed up with investigative dermatologist Michelle Lowes, MBBS, PhD, to . To date, more than 500 patients are enrolled at 12 participating sites, and 4 more sites plan to join the consortium by the end of 2023. The goal is to enroll a total of 8,000 patients, which will make it the largest dataset of its kind.
“Each site investigator is a physician who specializes in taking care of HS patients,” said Dr. Naik, who is the study’s principal investigator. “These are people who are conducting active research in various aspects of HS, and they’re trusted members of the medical community.”
She highlighted the three main objectives of HS PROGRESS. The first objective is to develop a longitudinal cohort of HS patients so that investigators can understand the clinical course of HS and effectiveness of treatments. The second is to collect biospecimens from patients with HS for translational studies “that can help to drive drug development, help us identify biomarkers that can help us predict disease course and predict patient response to therapies, so we know exactly what to give them,” she explained. The third objective is to provide patients with HS with the opportunity to be recruited for clinical trials, “so they have access to cutting-edge therapies and know what’s happening in this space.”
Collecting biospecimens
The goal of collecting biospecimens is to provide them to multiple investigators to improve the understanding of HS biology and treatment. “Our thought is to apply next generation techniques to these biospecimens to get metagenomic, transcriptomic, and genomic data to better understand HS biology so that we can identify targets for novel therapy,” Dr. Naik said.
Although HS is estimated to affect 1% of Western populations, the tumor necrosis alpha (TNF)-inhibitor adalimumab remains the only Food and Drug Administration-approved therapy for the condition.
However, Dr. Naik said that there are many promising drugs on the horizon for HS, especially interleukin (IL)-17 inhibitors. “One of the most exciting things about these drugs is that they set the bar higher for what we can expect out of therapies for HS, such as reporting a HiSCR (HS Clinical Response) score 75, which is the equivalent of 75% improvement in inflammatory HS lesions without an increase in draining tunnels,” she said. “This is well beyond what adalimumab had demonstrated in landmark trials in 2015. The safety profile on IL-17 inhibitors looks great, too.”
JAK inhibitors also hold promise for HS. “It’s going to be key to see how these drugs perform in the real-world setting in our average HS patients who may have comorbidities,” Dr. Naik said. “This is where an effort like HS PROGRESS will carry weight, because in a dataset like this, we’re going to be able to ask questions like, is there a class of drugs that works better for one specific phenotype of HS, or for patients who have a younger age of onset, or who are earlier in their disease course? These are questions we can’t ask in the context of a clinical trial, but we can ask in the context of real-world data from many practices.”
In addition to USCF, the 11 study locations participating in HS PROGRESS are the University of North Carolina at Chapel Hill; Mayo Clinic; Penn State University, Hershey; University of Virginia, Charlottesville; Washington University in St. Louis; University of Southern California, Los Angeles; Henry Ford Health, Detroit; University of Minnesota; University of Pennsylvania, Philadelphia; Duke University, Durham, N.C.; and University of Miami.
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, UCB, and Novartis; and investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
When Haley Naik, MD, joined the University of California, San Francisco, as a dermatologist in 2015, she was struck by the dearth of data in the medical literature about hidradenitis suppurativa (HS).
“For decades there were no datasets to begin to understand HS – its clinical course, how patients respond to medications, and how quality of life improves for patients with therapy,” Dr. Naik, who directs the HS program at UCSF, said in an interview. Inspired to improve the bleak HS knowledge landscape, she began to systematically collect information from HS patient visits, “to try to better understand how treatments were helping them or not and also to better understand their quality-of-life impact,” she said. “This also facilitated research in HS, but over time it became clear that there was a growing need for a larger effort.”
But in 2020, Dr. Naik teamed up with investigative dermatologist Michelle Lowes, MBBS, PhD, to . To date, more than 500 patients are enrolled at 12 participating sites, and 4 more sites plan to join the consortium by the end of 2023. The goal is to enroll a total of 8,000 patients, which will make it the largest dataset of its kind.
“Each site investigator is a physician who specializes in taking care of HS patients,” said Dr. Naik, who is the study’s principal investigator. “These are people who are conducting active research in various aspects of HS, and they’re trusted members of the medical community.”
She highlighted the three main objectives of HS PROGRESS. The first objective is to develop a longitudinal cohort of HS patients so that investigators can understand the clinical course of HS and effectiveness of treatments. The second is to collect biospecimens from patients with HS for translational studies “that can help to drive drug development, help us identify biomarkers that can help us predict disease course and predict patient response to therapies, so we know exactly what to give them,” she explained. The third objective is to provide patients with HS with the opportunity to be recruited for clinical trials, “so they have access to cutting-edge therapies and know what’s happening in this space.”
Collecting biospecimens
The goal of collecting biospecimens is to provide them to multiple investigators to improve the understanding of HS biology and treatment. “Our thought is to apply next generation techniques to these biospecimens to get metagenomic, transcriptomic, and genomic data to better understand HS biology so that we can identify targets for novel therapy,” Dr. Naik said.
Although HS is estimated to affect 1% of Western populations, the tumor necrosis alpha (TNF)-inhibitor adalimumab remains the only Food and Drug Administration-approved therapy for the condition.
However, Dr. Naik said that there are many promising drugs on the horizon for HS, especially interleukin (IL)-17 inhibitors. “One of the most exciting things about these drugs is that they set the bar higher for what we can expect out of therapies for HS, such as reporting a HiSCR (HS Clinical Response) score 75, which is the equivalent of 75% improvement in inflammatory HS lesions without an increase in draining tunnels,” she said. “This is well beyond what adalimumab had demonstrated in landmark trials in 2015. The safety profile on IL-17 inhibitors looks great, too.”
JAK inhibitors also hold promise for HS. “It’s going to be key to see how these drugs perform in the real-world setting in our average HS patients who may have comorbidities,” Dr. Naik said. “This is where an effort like HS PROGRESS will carry weight, because in a dataset like this, we’re going to be able to ask questions like, is there a class of drugs that works better for one specific phenotype of HS, or for patients who have a younger age of onset, or who are earlier in their disease course? These are questions we can’t ask in the context of a clinical trial, but we can ask in the context of real-world data from many practices.”
In addition to USCF, the 11 study locations participating in HS PROGRESS are the University of North Carolina at Chapel Hill; Mayo Clinic; Penn State University, Hershey; University of Virginia, Charlottesville; Washington University in St. Louis; University of Southern California, Los Angeles; Henry Ford Health, Detroit; University of Minnesota; University of Pennsylvania, Philadelphia; Duke University, Durham, N.C.; and University of Miami.
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, UCB, and Novartis; and investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
Systematic review spotlights the use of nutraceuticals for acne
.
“While many topical and systemic prescription options are available for the treatment of acne, some patients may be interested in natural and complementary therapies as either an adjunctive or an alternative to prescription medications,” researchers led by John S. Barbieri, MD, MBA, of the department of dermatology at Brigham and Women’s Hospital, Boston, wrote in their study, which was published online in JAMA Dermatology. The researchers defined nutraceuticals as products derived from food sources that provide both nutritional and medicinal benefits, such as vitamins, dietary supplements, and herbal products. “Although patients may be interested in nutraceuticals as a potential treatment option for acne, there is uncertainty regarding the efficacy and safety of these products,” they wrote.
For the systematic review, they searched the PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science databases from inception through January 30, 2023, to identify randomized clinical trials that evaluated oral nutraceutical interventions such as vitamins and minerals, botanical extracts, prebiotics, and probiotics in individuals with acne. They extracted clinician-reported outcomes, patient-reported outcomes, and adverse events from the included studies, and used the Cochrane Risk of Bias checklist tool to assess the quality of evidence in randomized clinical trials. Based on this tool, they used Agency for Healthcare Research and Quality standards to categorize the articles as good, fair, or poor quality.
The search yielded 42 unique studies with 3,346 participants. Of these 42 studies, 27 were considered poor quality, 11 were considered fair quality, and 4 were considered good quality. The good-quality studies separately evaluated four interventions: vitamin D, green tea extract, probiotics, and cheongsangbangpoong-tang, an herbal formula approved for use in acne by the Korea Food and Drug Administration.
The 11 fair-quality studies suggested potential effectiveness for pantothenic acid (vitamin B5), the fatty acids omega-3 (eicosapentaenoic acid [EPA] and/or docosahexaenoic acid [DHA]) and omega-6 (gamma-linoleic acid), and probiotics.
Zinc was the most studied nutraceutical identified in the review, but “there was substantial heterogeneity in the results, with only slightly greater than one-half of studies finding zinc to be efficacious,” the authors noted. “Studies using higher doses more often found zinc to be efficacious,” they said, adding that zinc “had the highest rate of adverse effect reporting of any nutraceuticals assessed in this review.”
Dr. Barbieri and colleagues acknowledged limitations of their analysis, including the fact that few of the nutraceuticals considered to have good or fair evidence for their use were evaluated in more than one study. “In addition, some studies had inconsistent results depending on the outcome measure assessed,” they wrote. “For instance, although green tea extract led to statistically significant improvements in lesion counts, it did not result in statistically significant improvements in quality of life, suggesting the observed lesion count differences may not be clinically meaningful to patients.”
And while probiotics had the most studies supporting their efficacy, they were generally of very small sample size.
Asked to comment on the study, Jonette Keri, MD, PhD, a dermatologist who directs the Acne and Rosacea Treatment Center at the University of Miami, who was not involved with the study, said that while the review was exhaustive, more research is needed to better determine the efficacy and side effects of the products studied. “The real strength of this wonderful review is now we have all of this information in one place, and this will serve as a great patient care resource,” she told this news organization.
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Keri disclosed that she is a consultant for L’Oréal.
.
“While many topical and systemic prescription options are available for the treatment of acne, some patients may be interested in natural and complementary therapies as either an adjunctive or an alternative to prescription medications,” researchers led by John S. Barbieri, MD, MBA, of the department of dermatology at Brigham and Women’s Hospital, Boston, wrote in their study, which was published online in JAMA Dermatology. The researchers defined nutraceuticals as products derived from food sources that provide both nutritional and medicinal benefits, such as vitamins, dietary supplements, and herbal products. “Although patients may be interested in nutraceuticals as a potential treatment option for acne, there is uncertainty regarding the efficacy and safety of these products,” they wrote.
For the systematic review, they searched the PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science databases from inception through January 30, 2023, to identify randomized clinical trials that evaluated oral nutraceutical interventions such as vitamins and minerals, botanical extracts, prebiotics, and probiotics in individuals with acne. They extracted clinician-reported outcomes, patient-reported outcomes, and adverse events from the included studies, and used the Cochrane Risk of Bias checklist tool to assess the quality of evidence in randomized clinical trials. Based on this tool, they used Agency for Healthcare Research and Quality standards to categorize the articles as good, fair, or poor quality.
The search yielded 42 unique studies with 3,346 participants. Of these 42 studies, 27 were considered poor quality, 11 were considered fair quality, and 4 were considered good quality. The good-quality studies separately evaluated four interventions: vitamin D, green tea extract, probiotics, and cheongsangbangpoong-tang, an herbal formula approved for use in acne by the Korea Food and Drug Administration.
The 11 fair-quality studies suggested potential effectiveness for pantothenic acid (vitamin B5), the fatty acids omega-3 (eicosapentaenoic acid [EPA] and/or docosahexaenoic acid [DHA]) and omega-6 (gamma-linoleic acid), and probiotics.
Zinc was the most studied nutraceutical identified in the review, but “there was substantial heterogeneity in the results, with only slightly greater than one-half of studies finding zinc to be efficacious,” the authors noted. “Studies using higher doses more often found zinc to be efficacious,” they said, adding that zinc “had the highest rate of adverse effect reporting of any nutraceuticals assessed in this review.”
Dr. Barbieri and colleagues acknowledged limitations of their analysis, including the fact that few of the nutraceuticals considered to have good or fair evidence for their use were evaluated in more than one study. “In addition, some studies had inconsistent results depending on the outcome measure assessed,” they wrote. “For instance, although green tea extract led to statistically significant improvements in lesion counts, it did not result in statistically significant improvements in quality of life, suggesting the observed lesion count differences may not be clinically meaningful to patients.”
And while probiotics had the most studies supporting their efficacy, they were generally of very small sample size.
Asked to comment on the study, Jonette Keri, MD, PhD, a dermatologist who directs the Acne and Rosacea Treatment Center at the University of Miami, who was not involved with the study, said that while the review was exhaustive, more research is needed to better determine the efficacy and side effects of the products studied. “The real strength of this wonderful review is now we have all of this information in one place, and this will serve as a great patient care resource,” she told this news organization.
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Keri disclosed that she is a consultant for L’Oréal.
.
“While many topical and systemic prescription options are available for the treatment of acne, some patients may be interested in natural and complementary therapies as either an adjunctive or an alternative to prescription medications,” researchers led by John S. Barbieri, MD, MBA, of the department of dermatology at Brigham and Women’s Hospital, Boston, wrote in their study, which was published online in JAMA Dermatology. The researchers defined nutraceuticals as products derived from food sources that provide both nutritional and medicinal benefits, such as vitamins, dietary supplements, and herbal products. “Although patients may be interested in nutraceuticals as a potential treatment option for acne, there is uncertainty regarding the efficacy and safety of these products,” they wrote.
For the systematic review, they searched the PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science databases from inception through January 30, 2023, to identify randomized clinical trials that evaluated oral nutraceutical interventions such as vitamins and minerals, botanical extracts, prebiotics, and probiotics in individuals with acne. They extracted clinician-reported outcomes, patient-reported outcomes, and adverse events from the included studies, and used the Cochrane Risk of Bias checklist tool to assess the quality of evidence in randomized clinical trials. Based on this tool, they used Agency for Healthcare Research and Quality standards to categorize the articles as good, fair, or poor quality.
The search yielded 42 unique studies with 3,346 participants. Of these 42 studies, 27 were considered poor quality, 11 were considered fair quality, and 4 were considered good quality. The good-quality studies separately evaluated four interventions: vitamin D, green tea extract, probiotics, and cheongsangbangpoong-tang, an herbal formula approved for use in acne by the Korea Food and Drug Administration.
The 11 fair-quality studies suggested potential effectiveness for pantothenic acid (vitamin B5), the fatty acids omega-3 (eicosapentaenoic acid [EPA] and/or docosahexaenoic acid [DHA]) and omega-6 (gamma-linoleic acid), and probiotics.
Zinc was the most studied nutraceutical identified in the review, but “there was substantial heterogeneity in the results, with only slightly greater than one-half of studies finding zinc to be efficacious,” the authors noted. “Studies using higher doses more often found zinc to be efficacious,” they said, adding that zinc “had the highest rate of adverse effect reporting of any nutraceuticals assessed in this review.”
Dr. Barbieri and colleagues acknowledged limitations of their analysis, including the fact that few of the nutraceuticals considered to have good or fair evidence for their use were evaluated in more than one study. “In addition, some studies had inconsistent results depending on the outcome measure assessed,” they wrote. “For instance, although green tea extract led to statistically significant improvements in lesion counts, it did not result in statistically significant improvements in quality of life, suggesting the observed lesion count differences may not be clinically meaningful to patients.”
And while probiotics had the most studies supporting their efficacy, they were generally of very small sample size.
Asked to comment on the study, Jonette Keri, MD, PhD, a dermatologist who directs the Acne and Rosacea Treatment Center at the University of Miami, who was not involved with the study, said that while the review was exhaustive, more research is needed to better determine the efficacy and side effects of the products studied. “The real strength of this wonderful review is now we have all of this information in one place, and this will serve as a great patient care resource,” she told this news organization.
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Keri disclosed that she is a consultant for L’Oréal.
FROM JAMA DERMATOLOGY
Promising new therapies for managing Tourette syndrome
, according to an overview of new therapies presented at the XXVI World Congress of Neurology.
One recent study by University of Nottingham researchers showed a wrist-worn stimulating device significantly reduces the frequency and severity of tics – repetitive movements or vocalizations that can occur several times a day.
“Wearable nerve stimulation holds great promise because it will be good across the age spectrum; adults can wear it to work, and children can go to school with it to help them concentrate on their schoolwork, and then they take it off at night,” Eileen Joyce, PhD, MB BChir, professor of neuropsychiatry at the Institute of Neurology, University College London, told this news organization.
Dr. Joyce, who was not part of the study, discussed this and other new advances in tic therapy at the meeting.
About 24% of children will suffer from tics at some point. The prevalence of Tourette syndrome among males is about four times that of females, Dr. Joyce told delegates. She added the typical age of onset is about 7 years with peak severity at about 12 years.
Predictors of tics persisting into adulthood include comorbid attention deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder, and autism spectrum disorder, said Dr. Joyce, who also discussed the “highly heritable” nature of the syndrome and the numerous related genes identified to date.
Current and emerging treatments
Current treatments include psychological therapy, Botox for focal tics, and medications such as antipsychotics. Emerging therapies included deep brain stimulation and the new median nerve stimulation approach.
A study published earlier this year included 135 patients with moderate to severe tic disorder who were randomly assigned to receive the investigational neuromodulation treatment, a sham treatment, or a wait-list treatment group.
The intervention involves rhythmic pulse trains of median nerve stimulation delivered via a device worn at the wrist. The device was programmed to deliver rhythmic (10 Hz) trains of low-intensity (1-19 mA) electrical stimulation to the median nerve at home once daily, 5 days a week for 4 weeks.
At 4 weeks, tic severity, as measured by the Yale Global Tic Severity Scale-Total Tic Severity Score (YGTSS-TTSS), was reduced by 7.1 points (35% reduction) in the active stimulation group compared to 2.13 points in the sham and 2.11 points in wait-list control groups.
The reduction for active stimulation was substantially larger, clinically meaningful (effect size, 0.5), and statistically significant (P = .02) compared to both the sham stimulation and wait-list control groups, which did not differ from one another.
Tic frequency (tics per minute or TPM) was reduced more in the active than sham stimulation groups (−15.6 TPM vs. −7.7 TPM; P < .03) and the reduction in tic frequency was clinically meaningful (>25% reduction; effect-size, 0.3).
When the active stimulator was turned off, the tics worsened, noted Dr. Joyce.
“The study showed that if you stimulate the median nerve at the wrist, you can train brain oscillations that are linked to the suppression of movement,” Dr. Joyce said. “So based on physiological knowledge, they have developed a median nerve stimulator to entrain cortical rhythms.”
Simple and exciting
The new device is “really exciting”, she added. “It’s not invasive and is quite simple to use and could help a lot of people with Tourette syndrome.”
Asked to comment, Alan Carson, MD, consultant neuropsychiatrist and honorary professor of neuropsychiatry, University of Edinburgh, who co-chaired the neuropsychiatry session featuring this presentation, called the device “promising.”
“Deep brain stimulation appears to be very effective but it’s a major procedure, so a simple wearable device seems highly desirable,” Dr. Carson said.
Dr. Joyce also discussed a study on the efficacy of cannabis (nabiximols; Sativex) as an intervention for tic management in males, those with severe tics, and those with comorbid ADHD.
And a new oral medication, ecopipam, a highly selective D1 receptor antagonist, is also raising hopes, said Dr. Joyce, with results from a randomized controlled trial showing the drug significantly improved tics and had few adverse effects.
Dr. Joyce and Dr. Carson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to an overview of new therapies presented at the XXVI World Congress of Neurology.
One recent study by University of Nottingham researchers showed a wrist-worn stimulating device significantly reduces the frequency and severity of tics – repetitive movements or vocalizations that can occur several times a day.
“Wearable nerve stimulation holds great promise because it will be good across the age spectrum; adults can wear it to work, and children can go to school with it to help them concentrate on their schoolwork, and then they take it off at night,” Eileen Joyce, PhD, MB BChir, professor of neuropsychiatry at the Institute of Neurology, University College London, told this news organization.
Dr. Joyce, who was not part of the study, discussed this and other new advances in tic therapy at the meeting.
About 24% of children will suffer from tics at some point. The prevalence of Tourette syndrome among males is about four times that of females, Dr. Joyce told delegates. She added the typical age of onset is about 7 years with peak severity at about 12 years.
Predictors of tics persisting into adulthood include comorbid attention deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder, and autism spectrum disorder, said Dr. Joyce, who also discussed the “highly heritable” nature of the syndrome and the numerous related genes identified to date.
Current and emerging treatments
Current treatments include psychological therapy, Botox for focal tics, and medications such as antipsychotics. Emerging therapies included deep brain stimulation and the new median nerve stimulation approach.
A study published earlier this year included 135 patients with moderate to severe tic disorder who were randomly assigned to receive the investigational neuromodulation treatment, a sham treatment, or a wait-list treatment group.
The intervention involves rhythmic pulse trains of median nerve stimulation delivered via a device worn at the wrist. The device was programmed to deliver rhythmic (10 Hz) trains of low-intensity (1-19 mA) electrical stimulation to the median nerve at home once daily, 5 days a week for 4 weeks.
At 4 weeks, tic severity, as measured by the Yale Global Tic Severity Scale-Total Tic Severity Score (YGTSS-TTSS), was reduced by 7.1 points (35% reduction) in the active stimulation group compared to 2.13 points in the sham and 2.11 points in wait-list control groups.
The reduction for active stimulation was substantially larger, clinically meaningful (effect size, 0.5), and statistically significant (P = .02) compared to both the sham stimulation and wait-list control groups, which did not differ from one another.
Tic frequency (tics per minute or TPM) was reduced more in the active than sham stimulation groups (−15.6 TPM vs. −7.7 TPM; P < .03) and the reduction in tic frequency was clinically meaningful (>25% reduction; effect-size, 0.3).
When the active stimulator was turned off, the tics worsened, noted Dr. Joyce.
“The study showed that if you stimulate the median nerve at the wrist, you can train brain oscillations that are linked to the suppression of movement,” Dr. Joyce said. “So based on physiological knowledge, they have developed a median nerve stimulator to entrain cortical rhythms.”
Simple and exciting
The new device is “really exciting”, she added. “It’s not invasive and is quite simple to use and could help a lot of people with Tourette syndrome.”
Asked to comment, Alan Carson, MD, consultant neuropsychiatrist and honorary professor of neuropsychiatry, University of Edinburgh, who co-chaired the neuropsychiatry session featuring this presentation, called the device “promising.”
“Deep brain stimulation appears to be very effective but it’s a major procedure, so a simple wearable device seems highly desirable,” Dr. Carson said.
Dr. Joyce also discussed a study on the efficacy of cannabis (nabiximols; Sativex) as an intervention for tic management in males, those with severe tics, and those with comorbid ADHD.
And a new oral medication, ecopipam, a highly selective D1 receptor antagonist, is also raising hopes, said Dr. Joyce, with results from a randomized controlled trial showing the drug significantly improved tics and had few adverse effects.
Dr. Joyce and Dr. Carson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to an overview of new therapies presented at the XXVI World Congress of Neurology.
One recent study by University of Nottingham researchers showed a wrist-worn stimulating device significantly reduces the frequency and severity of tics – repetitive movements or vocalizations that can occur several times a day.
“Wearable nerve stimulation holds great promise because it will be good across the age spectrum; adults can wear it to work, and children can go to school with it to help them concentrate on their schoolwork, and then they take it off at night,” Eileen Joyce, PhD, MB BChir, professor of neuropsychiatry at the Institute of Neurology, University College London, told this news organization.
Dr. Joyce, who was not part of the study, discussed this and other new advances in tic therapy at the meeting.
About 24% of children will suffer from tics at some point. The prevalence of Tourette syndrome among males is about four times that of females, Dr. Joyce told delegates. She added the typical age of onset is about 7 years with peak severity at about 12 years.
Predictors of tics persisting into adulthood include comorbid attention deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder, and autism spectrum disorder, said Dr. Joyce, who also discussed the “highly heritable” nature of the syndrome and the numerous related genes identified to date.
Current and emerging treatments
Current treatments include psychological therapy, Botox for focal tics, and medications such as antipsychotics. Emerging therapies included deep brain stimulation and the new median nerve stimulation approach.
A study published earlier this year included 135 patients with moderate to severe tic disorder who were randomly assigned to receive the investigational neuromodulation treatment, a sham treatment, or a wait-list treatment group.
The intervention involves rhythmic pulse trains of median nerve stimulation delivered via a device worn at the wrist. The device was programmed to deliver rhythmic (10 Hz) trains of low-intensity (1-19 mA) electrical stimulation to the median nerve at home once daily, 5 days a week for 4 weeks.
At 4 weeks, tic severity, as measured by the Yale Global Tic Severity Scale-Total Tic Severity Score (YGTSS-TTSS), was reduced by 7.1 points (35% reduction) in the active stimulation group compared to 2.13 points in the sham and 2.11 points in wait-list control groups.
The reduction for active stimulation was substantially larger, clinically meaningful (effect size, 0.5), and statistically significant (P = .02) compared to both the sham stimulation and wait-list control groups, which did not differ from one another.
Tic frequency (tics per minute or TPM) was reduced more in the active than sham stimulation groups (−15.6 TPM vs. −7.7 TPM; P < .03) and the reduction in tic frequency was clinically meaningful (>25% reduction; effect-size, 0.3).
When the active stimulator was turned off, the tics worsened, noted Dr. Joyce.
“The study showed that if you stimulate the median nerve at the wrist, you can train brain oscillations that are linked to the suppression of movement,” Dr. Joyce said. “So based on physiological knowledge, they have developed a median nerve stimulator to entrain cortical rhythms.”
Simple and exciting
The new device is “really exciting”, she added. “It’s not invasive and is quite simple to use and could help a lot of people with Tourette syndrome.”
Asked to comment, Alan Carson, MD, consultant neuropsychiatrist and honorary professor of neuropsychiatry, University of Edinburgh, who co-chaired the neuropsychiatry session featuring this presentation, called the device “promising.”
“Deep brain stimulation appears to be very effective but it’s a major procedure, so a simple wearable device seems highly desirable,” Dr. Carson said.
Dr. Joyce also discussed a study on the efficacy of cannabis (nabiximols; Sativex) as an intervention for tic management in males, those with severe tics, and those with comorbid ADHD.
And a new oral medication, ecopipam, a highly selective D1 receptor antagonist, is also raising hopes, said Dr. Joyce, with results from a randomized controlled trial showing the drug significantly improved tics and had few adverse effects.
Dr. Joyce and Dr. Carson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM WCN 2023









