User login
A Physician With Thigh Pain
Necrotizing soft-tissue infection (NSTI) often is difficult to distinguish from a superficial soft-tissue infection like cellulitis. Both conditions present with pain, edema, and erythema and can be accompanied by fever and malaise. The diagnosis of NSTI must be made quickly because successful treatment requires early surgical debridement and broad-spectrum antibiotics. The following case demonstrates the challenge of diagnosing NSTI.
Case Presentation
A 50-year-old physician developed a sore throat with subjective fevers, night sweats, and chills. After 2 days, his symptoms resolved. The next day he developed right thigh pain while playing tennis and limped off the court. That night he had fevers, chills, and sweats. For the next 3 days, his right thigh pain persisted with waxing and waning fevers.
The patient’s medical history included gastroesophageal reflux disease, vitamin D deficiency, and a positive purified protein derivative test for which he had completed 1 year of isoniazid therapy. The patient was married and in a monogamous relationship with his wife. He had traveled to the Sierra National Forest and Yosemite Park during the preceding winter. He did not swim in a lake or recall a tick bite. He had not consumed raw food, imported meats, or dairy products. He recently started oral fluconazole for tinea corporis.
The patient’s temperature was 39.5°C, heart rate was 115 beats per minute, blood pressure (BP) was 142/88 mm Hg, and respiratory rate was 18 breaths per minute with an oxygen saturation of 95% while breathing ambient air. He was drenched in sweat yet remained comfortable throughout the interview. The oropharyngeal mucosa was moist without lesions or erythema. There was no rash or lymphadenopathy. The lungs were clear to auscultation. The cardiac exam revealed tachycardia. There was point tenderness to deep palpation of the mid-anterior right thigh without crepitus, erythema, or edema.
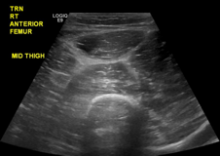
The patient’s sodium level was 129 mmol/L (normal range 135-145 mmol/L), bicarbonate was 20 mmol/L (normal range 22-32 mmol/L), creatinine was 1.1 mg/dL (normal range 0.7-1.2 mg/dL), and glucose was 194 mg/dL. The white blood cell count (WBC) was 12,900 cells/mm3 (normal range 3,400-10,000 cells/mm3) with 96% neutrophils. The hematocrit was 41% (normal range 41-53%), and the platelet count was 347,000 cells/mm3 (normal range 140,000-450,000 cells/mm3). The lactate level was 2.2 mmol/L (normal range 0-2 mmol/L). The creatine kinase level was 347 U/L (normal range 50-388 U/L), and the lactate dehydrogenase level was 254 U/L (normal range 102-199 U/L). A rapid group A streptococcal (GAS) antigen test was negative. A radiograph of the right femur revealed mildly edematous soft tissue. On ultrasound the right quadriceps appeared mildly edematous, but there was no evidence of abscess or discrete fluid collection (eFigure 1).
eFigure 1. Ultrasound of the Right Anterior Thigh Ultrasound revealed heterogeneous, mildly edematous quadriceps muscle. There was no abscess or discrete fluid collection. There was trace fluid along the fascia of the quadriceps muscle.
Four liters of normal saline, acetaminophen, ceftriaxone, and doxycycline were administered to the patient. Overnight he was afebrile, tachycardic, and normotensive. The following morning his BP decreased to 81/53 mm Hg. His WBC count was 33,000 cells/mm3 with 96% neutrophils. A peripheral blood smear showed immature granulocytes. The sodium and creatinine increased to 135 mmol/L and 1.3 mg/dL, respectively. The erythrocyte sedimentation rate was 20 mm/h (normal range 0-10 mm/h), and the C-reactive protein level was 174 mg/L (normal range < 6.3 mg/L).The right thigh became erythematous and edematous.
Given concern for necrotizing fasciitis, antibiotics were changed to vancomycin, piperacillin-tazobactam, and clindamycin. The patient was taken to the operating room (OR). The right quadriceps muscle was markedly edematous with overlying necrotic fibrofatty tissue with easy separation of the fascia from the anterolateral rectus femoris and rectus lateralis muscles. Necrotizing fasciitis was diagnosed.
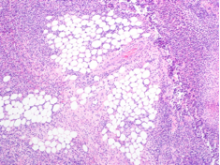
The tissue was debrided, and surgical pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation (eFigure 2). After the anterolateral space between the fascia and underlying thigh muscle was drained, a Penrose drain was placed, and the wound was left open with plans for a second-look operation within 24 hours.
eFigure 2. Surgical Pathology of Debrided Right Thigh
Pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation.
eFigure 3. Right Anterior Thigh
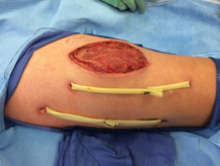
Two Penrose drains inserted after second operation.
In the ensuing hours erythema extended proximal to the operative site. The patient was emergently taken to the OR. The focus of necrotizing fasciitis along the anterolateral aspect of the thigh had extended posteriorly and superiorly. This area was irrigated, all loculations were disrupted, and a second Penrose drain was placed.
The wound was left open for 6 more days. On hospital day 9, operative exploration revealed no necrotizing fasciitis. The fascia and skin wound were then closed (eFigure 3).
Cultures from the fascia grew the GAS bacteria Streptococcus pyogenes (S pyogenes), which was sensitive to penicillin. The blood cultures from admission were sterile. A test for Epstein-Barr virus immunoglobulin M antibody was negative. The patient was discharged after 10 days in the hospital to complete a 2-week course of IV penicillin. Two months later he resumed playing tennis and returned to his clinical duties.
Discussion
In the U.S., there are approximately 3.5 cases of invasive GAS infection per 100,000 persons.1 Type I NSTI is polymicrobial (aerobic and anaerobic organisms). Risk factors include recent surgery, immunocompromised states, drug use, diabetes mellitus, and traumatic wounds.2 Type II NSTI is caused by GAS or other β-hemolytic streptococci either alone or in association with another organism, most commonly Staphylococcus aureus. Type II NSTI is classically found on the extremities and occurs in young, healthy, immunocompetent patients—such as this patient.3
The portal of entry in nearly half of type II NSTI is unknown; minor local trauma is often suspected.4 However, cases have been reported in which the only identifiable source was a preceding sore throat.4 The origin of this patient’s GAS remains unknown, but perhaps his pharyngitis led to transient bacteremia, which then seeded his injured thigh muscle. An in vitro model demonstrated that injured muscles increase surface expression of the cytoskeletal protein vimentin, which binds GAS.5 Exotoxins and endotoxins produced by S pyogenes may lead to microvascular thrombosis, tissue ischemia, liquefactive necrosis, and systemic release of cytokines followed by systemic illness, multiorgan dysfunction, and death.6
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was developed to aid in early diagnosis of NSTI.7 It was derived from a series of 2,555 patients admitted with cellulitis or abscesses at a single institution. Scores > 8 have a positive predictive value of 93% for NSTI. This patient had a LRINEC score of 9. Radiographs or computed tomography scans may demonstrate soft-tissue air collections but lack sensitivity and are often nondiagnostic.8,9 T1-weighted magnetic resonance imaging can delineate the anatomic extent of soft-tissue infections but is time consuming and may delay treatment.10 When the pretest probability is high, proceeding directly to the OR for direct visualization and possible debridement is advisable. Histologic features of necrotizing fasciitis include inflammation with polymorphonuclear cells and necrosis of the subcutaneous fat and fascia with relative sparing of the muscle.11Necrotizing soft-tissue infection requires early surgical debridement and broad-spectrum antibiotic coverage. Without surgical debridement, the mortality rate approaches 100%.2 Antibiotics should include activity against Gram-positive, Gram-negative, and anaerobic organisms. The duration of antibiotic therapy has not been defined and is dependent on the patient’s clinical status. Adjunctive treatment options may include IV immunoglobulin and hyperbaric oxygen therapy, although the data supporting their utility are limited.12,13
Conclusion
Despite the LRINEC scoring systems and advanced imaging, necrotizing fasciitis remains challenging to diagnose in a timely manner. In this case, close monitoring of the patient facilitated timely evaluation and treatment of a fatal disease.
1. O'Loughlin RE, Roberson A, Cieslak PR, et al; Active Bacterial Core Surveillance Team. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853-857.
2. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705-710.
3. Naqvi GA, Malik SA, Jan W. Necrotizing fasciitis of the lower extremity: a case report and current concept of diagnosis and management. Scand J Trauma Resusc Emerg Med. 2009;17:28.
4. Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1195;1(3):69-78.
5. Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685-1692.
6. Cainzos M, Gonzalez-Rodriguez FJ. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13(4):433-439.
7. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
8. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):119-125.
9. Lancerotto L, Tocco I, Salmaso R, Vindigni V, Basetto F. Necrotizing fasciitis: classification, diagnosis and management. J Trauma Acute Care Surg. 2012;72(3):560-566.
10. Brothers TE, Tagge DU, Stutley JE, Conway WF, Del Schutte H Jr, Byrne TK. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg. 1998;187(4):416-421.
11. Bakleh M, Wold LE, Mandrekar JN, Harmsen WS, Dimashkieh HH, Baddour LM. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40(3):410-414.
12. Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267(24):3315-3316.
13. Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg. 2004;139(12):1339-1345.
Necrotizing soft-tissue infection (NSTI) often is difficult to distinguish from a superficial soft-tissue infection like cellulitis. Both conditions present with pain, edema, and erythema and can be accompanied by fever and malaise. The diagnosis of NSTI must be made quickly because successful treatment requires early surgical debridement and broad-spectrum antibiotics. The following case demonstrates the challenge of diagnosing NSTI.
Case Presentation
A 50-year-old physician developed a sore throat with subjective fevers, night sweats, and chills. After 2 days, his symptoms resolved. The next day he developed right thigh pain while playing tennis and limped off the court. That night he had fevers, chills, and sweats. For the next 3 days, his right thigh pain persisted with waxing and waning fevers.
The patient’s medical history included gastroesophageal reflux disease, vitamin D deficiency, and a positive purified protein derivative test for which he had completed 1 year of isoniazid therapy. The patient was married and in a monogamous relationship with his wife. He had traveled to the Sierra National Forest and Yosemite Park during the preceding winter. He did not swim in a lake or recall a tick bite. He had not consumed raw food, imported meats, or dairy products. He recently started oral fluconazole for tinea corporis.
The patient’s temperature was 39.5°C, heart rate was 115 beats per minute, blood pressure (BP) was 142/88 mm Hg, and respiratory rate was 18 breaths per minute with an oxygen saturation of 95% while breathing ambient air. He was drenched in sweat yet remained comfortable throughout the interview. The oropharyngeal mucosa was moist without lesions or erythema. There was no rash or lymphadenopathy. The lungs were clear to auscultation. The cardiac exam revealed tachycardia. There was point tenderness to deep palpation of the mid-anterior right thigh without crepitus, erythema, or edema.
The patient’s sodium level was 129 mmol/L (normal range 135-145 mmol/L), bicarbonate was 20 mmol/L (normal range 22-32 mmol/L), creatinine was 1.1 mg/dL (normal range 0.7-1.2 mg/dL), and glucose was 194 mg/dL. The white blood cell count (WBC) was 12,900 cells/mm3 (normal range 3,400-10,000 cells/mm3) with 96% neutrophils. The hematocrit was 41% (normal range 41-53%), and the platelet count was 347,000 cells/mm3 (normal range 140,000-450,000 cells/mm3). The lactate level was 2.2 mmol/L (normal range 0-2 mmol/L). The creatine kinase level was 347 U/L (normal range 50-388 U/L), and the lactate dehydrogenase level was 254 U/L (normal range 102-199 U/L). A rapid group A streptococcal (GAS) antigen test was negative. A radiograph of the right femur revealed mildly edematous soft tissue. On ultrasound the right quadriceps appeared mildly edematous, but there was no evidence of abscess or discrete fluid collection (eFigure 1).
eFigure 1. Ultrasound of the Right Anterior Thigh Ultrasound revealed heterogeneous, mildly edematous quadriceps muscle. There was no abscess or discrete fluid collection. There was trace fluid along the fascia of the quadriceps muscle.
Four liters of normal saline, acetaminophen, ceftriaxone, and doxycycline were administered to the patient. Overnight he was afebrile, tachycardic, and normotensive. The following morning his BP decreased to 81/53 mm Hg. His WBC count was 33,000 cells/mm3 with 96% neutrophils. A peripheral blood smear showed immature granulocytes. The sodium and creatinine increased to 135 mmol/L and 1.3 mg/dL, respectively. The erythrocyte sedimentation rate was 20 mm/h (normal range 0-10 mm/h), and the C-reactive protein level was 174 mg/L (normal range < 6.3 mg/L).The right thigh became erythematous and edematous.
Given concern for necrotizing fasciitis, antibiotics were changed to vancomycin, piperacillin-tazobactam, and clindamycin. The patient was taken to the operating room (OR). The right quadriceps muscle was markedly edematous with overlying necrotic fibrofatty tissue with easy separation of the fascia from the anterolateral rectus femoris and rectus lateralis muscles. Necrotizing fasciitis was diagnosed.
The tissue was debrided, and surgical pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation (eFigure 2). After the anterolateral space between the fascia and underlying thigh muscle was drained, a Penrose drain was placed, and the wound was left open with plans for a second-look operation within 24 hours.
eFigure 2. Surgical Pathology of Debrided Right Thigh
Pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation.
eFigure 3. Right Anterior Thigh
Two Penrose drains inserted after second operation.
In the ensuing hours erythema extended proximal to the operative site. The patient was emergently taken to the OR. The focus of necrotizing fasciitis along the anterolateral aspect of the thigh had extended posteriorly and superiorly. This area was irrigated, all loculations were disrupted, and a second Penrose drain was placed.
The wound was left open for 6 more days. On hospital day 9, operative exploration revealed no necrotizing fasciitis. The fascia and skin wound were then closed (eFigure 3).
Cultures from the fascia grew the GAS bacteria Streptococcus pyogenes (S pyogenes), which was sensitive to penicillin. The blood cultures from admission were sterile. A test for Epstein-Barr virus immunoglobulin M antibody was negative. The patient was discharged after 10 days in the hospital to complete a 2-week course of IV penicillin. Two months later he resumed playing tennis and returned to his clinical duties.
Discussion
In the U.S., there are approximately 3.5 cases of invasive GAS infection per 100,000 persons.1 Type I NSTI is polymicrobial (aerobic and anaerobic organisms). Risk factors include recent surgery, immunocompromised states, drug use, diabetes mellitus, and traumatic wounds.2 Type II NSTI is caused by GAS or other β-hemolytic streptococci either alone or in association with another organism, most commonly Staphylococcus aureus. Type II NSTI is classically found on the extremities and occurs in young, healthy, immunocompetent patients—such as this patient.3
The portal of entry in nearly half of type II NSTI is unknown; minor local trauma is often suspected.4 However, cases have been reported in which the only identifiable source was a preceding sore throat.4 The origin of this patient’s GAS remains unknown, but perhaps his pharyngitis led to transient bacteremia, which then seeded his injured thigh muscle. An in vitro model demonstrated that injured muscles increase surface expression of the cytoskeletal protein vimentin, which binds GAS.5 Exotoxins and endotoxins produced by S pyogenes may lead to microvascular thrombosis, tissue ischemia, liquefactive necrosis, and systemic release of cytokines followed by systemic illness, multiorgan dysfunction, and death.6
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was developed to aid in early diagnosis of NSTI.7 It was derived from a series of 2,555 patients admitted with cellulitis or abscesses at a single institution. Scores > 8 have a positive predictive value of 93% for NSTI. This patient had a LRINEC score of 9. Radiographs or computed tomography scans may demonstrate soft-tissue air collections but lack sensitivity and are often nondiagnostic.8,9 T1-weighted magnetic resonance imaging can delineate the anatomic extent of soft-tissue infections but is time consuming and may delay treatment.10 When the pretest probability is high, proceeding directly to the OR for direct visualization and possible debridement is advisable. Histologic features of necrotizing fasciitis include inflammation with polymorphonuclear cells and necrosis of the subcutaneous fat and fascia with relative sparing of the muscle.11Necrotizing soft-tissue infection requires early surgical debridement and broad-spectrum antibiotic coverage. Without surgical debridement, the mortality rate approaches 100%.2 Antibiotics should include activity against Gram-positive, Gram-negative, and anaerobic organisms. The duration of antibiotic therapy has not been defined and is dependent on the patient’s clinical status. Adjunctive treatment options may include IV immunoglobulin and hyperbaric oxygen therapy, although the data supporting their utility are limited.12,13
Conclusion
Despite the LRINEC scoring systems and advanced imaging, necrotizing fasciitis remains challenging to diagnose in a timely manner. In this case, close monitoring of the patient facilitated timely evaluation and treatment of a fatal disease.
Necrotizing soft-tissue infection (NSTI) often is difficult to distinguish from a superficial soft-tissue infection like cellulitis. Both conditions present with pain, edema, and erythema and can be accompanied by fever and malaise. The diagnosis of NSTI must be made quickly because successful treatment requires early surgical debridement and broad-spectrum antibiotics. The following case demonstrates the challenge of diagnosing NSTI.
Case Presentation
A 50-year-old physician developed a sore throat with subjective fevers, night sweats, and chills. After 2 days, his symptoms resolved. The next day he developed right thigh pain while playing tennis and limped off the court. That night he had fevers, chills, and sweats. For the next 3 days, his right thigh pain persisted with waxing and waning fevers.
The patient’s medical history included gastroesophageal reflux disease, vitamin D deficiency, and a positive purified protein derivative test for which he had completed 1 year of isoniazid therapy. The patient was married and in a monogamous relationship with his wife. He had traveled to the Sierra National Forest and Yosemite Park during the preceding winter. He did not swim in a lake or recall a tick bite. He had not consumed raw food, imported meats, or dairy products. He recently started oral fluconazole for tinea corporis.
The patient’s temperature was 39.5°C, heart rate was 115 beats per minute, blood pressure (BP) was 142/88 mm Hg, and respiratory rate was 18 breaths per minute with an oxygen saturation of 95% while breathing ambient air. He was drenched in sweat yet remained comfortable throughout the interview. The oropharyngeal mucosa was moist without lesions or erythema. There was no rash or lymphadenopathy. The lungs were clear to auscultation. The cardiac exam revealed tachycardia. There was point tenderness to deep palpation of the mid-anterior right thigh without crepitus, erythema, or edema.
The patient’s sodium level was 129 mmol/L (normal range 135-145 mmol/L), bicarbonate was 20 mmol/L (normal range 22-32 mmol/L), creatinine was 1.1 mg/dL (normal range 0.7-1.2 mg/dL), and glucose was 194 mg/dL. The white blood cell count (WBC) was 12,900 cells/mm3 (normal range 3,400-10,000 cells/mm3) with 96% neutrophils. The hematocrit was 41% (normal range 41-53%), and the platelet count was 347,000 cells/mm3 (normal range 140,000-450,000 cells/mm3). The lactate level was 2.2 mmol/L (normal range 0-2 mmol/L). The creatine kinase level was 347 U/L (normal range 50-388 U/L), and the lactate dehydrogenase level was 254 U/L (normal range 102-199 U/L). A rapid group A streptococcal (GAS) antigen test was negative. A radiograph of the right femur revealed mildly edematous soft tissue. On ultrasound the right quadriceps appeared mildly edematous, but there was no evidence of abscess or discrete fluid collection (eFigure 1).
eFigure 1. Ultrasound of the Right Anterior Thigh Ultrasound revealed heterogeneous, mildly edematous quadriceps muscle. There was no abscess or discrete fluid collection. There was trace fluid along the fascia of the quadriceps muscle.
Four liters of normal saline, acetaminophen, ceftriaxone, and doxycycline were administered to the patient. Overnight he was afebrile, tachycardic, and normotensive. The following morning his BP decreased to 81/53 mm Hg. His WBC count was 33,000 cells/mm3 with 96% neutrophils. A peripheral blood smear showed immature granulocytes. The sodium and creatinine increased to 135 mmol/L and 1.3 mg/dL, respectively. The erythrocyte sedimentation rate was 20 mm/h (normal range 0-10 mm/h), and the C-reactive protein level was 174 mg/L (normal range < 6.3 mg/L).The right thigh became erythematous and edematous.
Given concern for necrotizing fasciitis, antibiotics were changed to vancomycin, piperacillin-tazobactam, and clindamycin. The patient was taken to the operating room (OR). The right quadriceps muscle was markedly edematous with overlying necrotic fibrofatty tissue with easy separation of the fascia from the anterolateral rectus femoris and rectus lateralis muscles. Necrotizing fasciitis was diagnosed.
The tissue was debrided, and surgical pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation (eFigure 2). After the anterolateral space between the fascia and underlying thigh muscle was drained, a Penrose drain was placed, and the wound was left open with plans for a second-look operation within 24 hours.
eFigure 2. Surgical Pathology of Debrided Right Thigh
Pathology revealed fibroadipose tissue with extensive necrosis and dense acute inflammation.
eFigure 3. Right Anterior Thigh
Two Penrose drains inserted after second operation.
In the ensuing hours erythema extended proximal to the operative site. The patient was emergently taken to the OR. The focus of necrotizing fasciitis along the anterolateral aspect of the thigh had extended posteriorly and superiorly. This area was irrigated, all loculations were disrupted, and a second Penrose drain was placed.
The wound was left open for 6 more days. On hospital day 9, operative exploration revealed no necrotizing fasciitis. The fascia and skin wound were then closed (eFigure 3).
Cultures from the fascia grew the GAS bacteria Streptococcus pyogenes (S pyogenes), which was sensitive to penicillin. The blood cultures from admission were sterile. A test for Epstein-Barr virus immunoglobulin M antibody was negative. The patient was discharged after 10 days in the hospital to complete a 2-week course of IV penicillin. Two months later he resumed playing tennis and returned to his clinical duties.
Discussion
In the U.S., there are approximately 3.5 cases of invasive GAS infection per 100,000 persons.1 Type I NSTI is polymicrobial (aerobic and anaerobic organisms). Risk factors include recent surgery, immunocompromised states, drug use, diabetes mellitus, and traumatic wounds.2 Type II NSTI is caused by GAS or other β-hemolytic streptococci either alone or in association with another organism, most commonly Staphylococcus aureus. Type II NSTI is classically found on the extremities and occurs in young, healthy, immunocompetent patients—such as this patient.3
The portal of entry in nearly half of type II NSTI is unknown; minor local trauma is often suspected.4 However, cases have been reported in which the only identifiable source was a preceding sore throat.4 The origin of this patient’s GAS remains unknown, but perhaps his pharyngitis led to transient bacteremia, which then seeded his injured thigh muscle. An in vitro model demonstrated that injured muscles increase surface expression of the cytoskeletal protein vimentin, which binds GAS.5 Exotoxins and endotoxins produced by S pyogenes may lead to microvascular thrombosis, tissue ischemia, liquefactive necrosis, and systemic release of cytokines followed by systemic illness, multiorgan dysfunction, and death.6
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was developed to aid in early diagnosis of NSTI.7 It was derived from a series of 2,555 patients admitted with cellulitis or abscesses at a single institution. Scores > 8 have a positive predictive value of 93% for NSTI. This patient had a LRINEC score of 9. Radiographs or computed tomography scans may demonstrate soft-tissue air collections but lack sensitivity and are often nondiagnostic.8,9 T1-weighted magnetic resonance imaging can delineate the anatomic extent of soft-tissue infections but is time consuming and may delay treatment.10 When the pretest probability is high, proceeding directly to the OR for direct visualization and possible debridement is advisable. Histologic features of necrotizing fasciitis include inflammation with polymorphonuclear cells and necrosis of the subcutaneous fat and fascia with relative sparing of the muscle.11Necrotizing soft-tissue infection requires early surgical debridement and broad-spectrum antibiotic coverage. Without surgical debridement, the mortality rate approaches 100%.2 Antibiotics should include activity against Gram-positive, Gram-negative, and anaerobic organisms. The duration of antibiotic therapy has not been defined and is dependent on the patient’s clinical status. Adjunctive treatment options may include IV immunoglobulin and hyperbaric oxygen therapy, although the data supporting their utility are limited.12,13
Conclusion
Despite the LRINEC scoring systems and advanced imaging, necrotizing fasciitis remains challenging to diagnose in a timely manner. In this case, close monitoring of the patient facilitated timely evaluation and treatment of a fatal disease.
1. O'Loughlin RE, Roberson A, Cieslak PR, et al; Active Bacterial Core Surveillance Team. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853-857.
2. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705-710.
3. Naqvi GA, Malik SA, Jan W. Necrotizing fasciitis of the lower extremity: a case report and current concept of diagnosis and management. Scand J Trauma Resusc Emerg Med. 2009;17:28.
4. Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1195;1(3):69-78.
5. Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685-1692.
6. Cainzos M, Gonzalez-Rodriguez FJ. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13(4):433-439.
7. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
8. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):119-125.
9. Lancerotto L, Tocco I, Salmaso R, Vindigni V, Basetto F. Necrotizing fasciitis: classification, diagnosis and management. J Trauma Acute Care Surg. 2012;72(3):560-566.
10. Brothers TE, Tagge DU, Stutley JE, Conway WF, Del Schutte H Jr, Byrne TK. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg. 1998;187(4):416-421.
11. Bakleh M, Wold LE, Mandrekar JN, Harmsen WS, Dimashkieh HH, Baddour LM. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40(3):410-414.
12. Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267(24):3315-3316.
13. Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg. 2004;139(12):1339-1345.
1. O'Loughlin RE, Roberson A, Cieslak PR, et al; Active Bacterial Core Surveillance Team. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853-857.
2. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705-710.
3. Naqvi GA, Malik SA, Jan W. Necrotizing fasciitis of the lower extremity: a case report and current concept of diagnosis and management. Scand J Trauma Resusc Emerg Med. 2009;17:28.
4. Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1195;1(3):69-78.
5. Bryant AE, Bayer CR, Huntington JD, Stevens DL. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685-1692.
6. Cainzos M, Gonzalez-Rodriguez FJ. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13(4):433-439.
7. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
8. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):119-125.
9. Lancerotto L, Tocco I, Salmaso R, Vindigni V, Basetto F. Necrotizing fasciitis: classification, diagnosis and management. J Trauma Acute Care Surg. 2012;72(3):560-566.
10. Brothers TE, Tagge DU, Stutley JE, Conway WF, Del Schutte H Jr, Byrne TK. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg. 1998;187(4):416-421.
11. Bakleh M, Wold LE, Mandrekar JN, Harmsen WS, Dimashkieh HH, Baddour LM. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40(3):410-414.
12. Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267(24):3315-3316.
13. Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg. 2004;139(12):1339-1345.
The Effect of Humeral Inclination on Range of Motion in Reverse Total Shoulder Arthroplasty: A Systematic Review
Reverse total shoulder arthroplasty (RTSA) has become a reliable treatment option for many pathologic conditions of the shoulder, including rotator cuff arthropathy, proximal humerus fractures, and others.1-4 While the treatment outcomes have generally been reported as good, some concern exists over the postoperative range of motion (ROM) in patients following RTSA, including external rotation.5-7 The original RTSA design was introduced by Neer in the 1970s and has undergone many modifications since that time.1,2 The original Grammont-style prosthesis involved medialization of the glenoid, inferiorizing the center of rotation (with increased deltoid tensioning), and a neck-shaft angle of 155°.1,8 While clinical results of the 155° design were encouraging, concerns arose over the significance of the common finding of scapular notching, or contact between the scapular neck and inferior portion of the humeral polyethylene when the arm is adducted.9,10
To address this concern, a prosthesis design with a 135° neck-shaft angle was introduced.11 This new design did significantly decrease the rate of scapular notching, and although some reported a concern over implant stability with the 135° prosthesis, recent data has shown no difference in dislocation rates between the 135° and 155° prostheses.3 A different variable that has not been evaluated between these prostheses is the active ROM that is achieved postoperatively, and the change in ROM from pre- to post-RTSA.12,13 As active ROM plays a significant role in shoulder function and patient satisfaction, the question of whether a significant difference exists in postoperative ROM between the 135° and 155° prostheses must be addressed.
The purpose of this study was to perform a systematic review investigating active ROM following RTSA to determine if active postoperative ROM following RTSA differs between the 135° and 155° humeral inclination prostheses, and to determine if there is a significant difference between the change in preoperative and postoperative ROM between the 135° and 155° prostheses. The authors hypothesize that there will be no significant difference in active postoperative ROM between the 135° and 155° prostheses, and that the difference between preoperative and postoperative ROM (that is, the amount of motion gained by the surgery) will not significantly differ between the 135° and 155° prostheses.
Methods
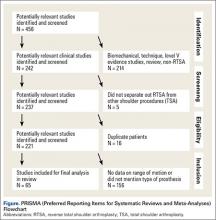
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.15 Systematic review registration was performed using the PROSPERO international prospective register of systematic reviews (registration date 3/9/15, registration number CRD42015017367).16 Two reviewers independently conducted the search on March 7, 2015 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I-IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine17) clinical studies that reported the type of RTSA prosthesis that was used as well as postoperative ROM with at least 12 months follow-up were eligible. All references within included studies were cross-referenced for inclusion if missed by the initial search. If duplicate subject publications were discovered, the study with the longer duration of follow-up or larger number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical studies, arthroscopic shoulder surgery, imaging, surgical technique, and classification studies were excluded. Studies were excluded if both a 135° and 155° prosthesis were utilized and the outcomes were not stratified by the humeral inclination. Studies that did not report ROM were excluded.
A total of 456 studies were located, and, after implementation of the exclusion criteria, 65 studies from 2005-2015 were included in the final analysis (Figure). Subjects of interest in this systematic review underwent a RTSA. Studies were not excluded based on the surgical indications (rotator cuff tear arthropathy, proximal humerus fractures, osteoarthritis) and there was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, journal of publication, country and continent of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, the manufacturer and type of prosthesis used, and the degree of the humeral inclination (135° vs 155° humeral cup). Preoperative ROM, including forward elevation, abduction, external rotation with the arm adducted, and external rotation with the arm at 90° of abduction, were recorded. The same ROM measurements were recorded for the final follow-up visit that was reported. Internal rotation was recorded, but because of the variability with how this measurement was reported, it was not analyzed. Clinical outcome scores and complications were not assessed. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).18
Statistical Analysis
Descriptive statistics were calculated, including mean ± standard deviation for quantitative continuous data and frequencies with percentages for qualitative categorical data. ROM comparisons between 135° and 155° components (pre- vs postoperative for each and postoperative between the 2) were made using 2 proportion z-test calculator (http://in-silico.net/tools/statistics/ztest) using alpha .05 because of the difference in sample sizes between compared groups.
Results
Sixty-five studies with 3302 patients (3434 shoulders) were included in this study. There was a total of 1211 shoulders in the 135° lateralized glenosphere group and 2223 shoulders in the 155° group. The studies had an average MCMS of 40.4 ± 8.2 (poor), 48% of studies reported a conflict of interest, 32% had no conflict of interest, and 20% did not report whether a conflict of interest existed or not. The majority of studies included were level IV evidence (85%). Mean patient age was 71.1 ± 7.6 years; 29% of patients were male and 71% were female. No significant difference existed between patient age at the time of surgery; the average age of patients in the 135° lateralized glenosphere group was 71.67 ± 3.8 years, while the average patient age of patients in the 155° group was 70.97 ± 8.8 years. Mean follow-up for all patients included in this study was 37.2 ± 16.5 months. Of the 65 studies included, 3 were published from Asia, 4 were published from Australia, 24 were from North America, and 34 were from Europe. Of the individual countries whose studies were included, the United States had 23 included studies, France had 13 included studies, and Italy had 4 included studies. All other countries had <4 studies included.
Patients who received either a 135° or a 155° prosthesis showed significant improvements in external rotation with the arm at the side (P < .05), forward elevation (P < .05), and abduction (P < .05) following surgery (Table). When comparing the 135° and 155° groups, patients who received a 135° prosthesis showed significantly greater improvements in external rotation with the arm at the side (P < .001) and had significantly more overall external rotation postoperatively (P < .001) than patients who received a 155° prosthesis. The only preoperative ROM difference between groups was the 155° group started with significantly more forward elevation than the 135°group prior to surgery (P = .002).
Discussion
RTSA is indicated in patients with rotator cuff tear arthropathy, pseudoparalysis, and a functional deltoid.1,2,4 The purpose of this systematic review was to determine if active ROM following RTSA differs between the 135° and 155° humeral inclination prostheses, and to determine if there is a significant difference between the change in preoperative and postoperative ROM between the 135° and 155° prostheses. Forward elevation, abduction, and external rotation all significantly improved following surgery in both groups, with no significant difference between groups in motion or amount of motion improvement, mostly confirming the study hypotheses. However, patients in the 135° group had significantly greater postoperative external rotation and greater amount of external rotation improvement compared to the 155° group.
Two of the frequently debated issues regarding implant geometry is stability and scapular notching between the 135° and 155° humeral inclination designs. Erickson and colleagues3 recently evaluated the rate of scapular notching and dislocations between the 135° and 155° RTSA prostheses. The authors found that the 135° prosthesis had a significantly lower incidence of scapular notching vs the 155° group and that the rate of dislocations was not significantly different between groups.3 In the latter systematic review, the authors attempted to evaluate ROM between the 135° and 155° prostheses, but as the inclusion criteria of the study was reporting on scapular notching and dislocation rates, many studies reporting solely on ROM were excluded, and the influence of humeral inclination on ROM was inconclusive.3 Furthermore, there have been no studies that have directly compared ROM following RTSA between the 135° and 155° prostheses. While studies evaluating each prosthesis on an individual level have shown an improvement in ROM from pre- to postsurgery, there have been no large studies that have compared the postoperative ROM and change in pre- to postoperative ROM between the 135° and 155° prostheses.11,13,19,20
One study by Valenti and colleagues21 evaluated a group of 30 patients with an average age of 69.5 years who underwent RTSA using either a 135° or a 155° prosthesis. Although the study did not directly compare the 2 types of prostheses, it did report the separate outcomes for each prosthesis. At an average follow-up of 36.4 months, the authors found that patients who had the 135° prosthesis implanted had a mean increase in forward elevation and external rotation of 53° and 9°, while patients who had the 155° showed an increase of 56° in forward elevation and a loss of 1° of external rotation. Both prostheses showed a significant increase in forward elevation, but neither had a significant increase in external rotation. Furthermore, scapular notching was seen in 4 patients in the 155° group, while no patients in the 135° group had evidence of notching.
The results of the current study were similar in that both the 135° and 155° prosthesis showed improvements in forward elevation following surgery, and the 135° group showed a significantly greater gain in external rotation than the 155° group. A significant component of shoulder function and patient satisfaction following RTSA is active ROM. However, this variable has not explicitly been evaluated in the literature until now. The clinical significance of this finding is unclear. Patients with adequate external rotation prior to surgery likely would not see a functional difference between prostheses, while those patients who were borderline on a functional amount of external rotation would see a clinically significant benefit with the 135° prosthesis. Studies have shown that the 135° prosthesis is more anatomic than the 155°, and this could explain the difference seen in ROM outcomes between the 2 prostheses.19 Ladermann and colleagues22 recently created and evaluated a 3-dimensional computer model to evaluate possible differences between the 135° and 155° prosthesis. The authors found a significant increase in external rotation of the 135° compared to the 155°, likely related to a difference in acromiohumeral distance as well as inlay vs onlay humeral trays between the 2 prostheses. The results of this study parallel the computer model, thereby validating these experimental results.
It is important to understand what the minimum functional ROM of the shoulder is (in other words, the ROM necessary to complete activities of daily living (ADLs).23 Namdari and colleagues24 used motion analysis software to evaluate the shoulder ROM necessary to complete 10 different ADLs, including combing hair, washing the back of the opposite shoulder, and reaching a shelf above their head without bending their elbow in 20 patients with a mean age of 29.2 years. They found that patients required 121° ± 6.7° of flexion, 46° ± 5.3° of extension, 128° ± 7.9° of abduction, 116° ± 9.1° of cross-body adduction, 59° ± 10° of external rotation with the arm 90° abducted, and 102° ± 7.7° of internal rotation with the arm at the side (external rotation with the arm at the side was not well defined).24 Hence, while abduction and forward elevation seem comparable, the results from the current study do raise concerns about the amount of external rotation obtained following RTSA as it relates to a patients’ ability to perform ADLs, specifically in the 155° prosthesis, as the average postoperative external rotation in this group was 20.5°. Therefore, based on the results of this study, it appears that, while both the 135° and 155° RTSA prostheses provide similar gain in forward elevation and abduction ROM as well as overall forward elevation and abduction, the 135° prosthesis provides significantly more external rotation with the arm at the side than the 155° prosthesis.
Limitations
Although this study attempted to look at all studies that reported active ROM in patients following a RTSA, and 2 authors performed the search, there is a possibility that some studies were missed, introducing study selection bias. Furthermore, the mean follow-up was over 3 years following surgery, but the minimum follow-up requirement for studies to be included was only 12 months. Hence, this transfer bias introduces the possibility that the patient’s ROM would have changed had they been followed for a standard period of time. There are many variables that come into play in evaluating ROM, and although the study attempted to control for these, there are some that could not be controlled for due to lack of reporting by some studies. Glenosphere size and humeral retroversion were not recorded, as they were not reliably reported in all studies, so motion outcomes based on these variables was not evaluated. Complications and clinical outcomes were not assessed in this review and as such, conclusions regarding these variables cannot be drawn from this study. Finally, indications for surgery were not reliably reported in the studies included in this paper, so differences may have existed between surgical indications of the 135° and 155° groups that could have affected outcomes.
Conclusion
Patients who receive a 135° RTSA gain significantly more external rotation from pre- to postsurgery and have an overall greater amount of external rotation than patients who receive a 155° prosthesis. Both groups show improvements in forward elevation, external rotation, and abduction following surgery.
1. Flatow EL, Harrison AK. A history of reverse total shoulder arthroplasty. Clin Orthop Relat Res. 2011;469(9):2432-2439.
2. Hyun YS, Huri G, Garbis NG, McFarland EG. Uncommon indications for reverse total shoulder arthroplasty. Clin Orthop Surg. 2013;5(4):243-255.
3. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993.
4. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures--asystematic review of 92 studies including 4500 patients. J Orthop Trauma. 2015;29(1):54-59.
5. Feeley BT, Zhang AL, Barry JJ, et al. Decreased scapular notching with lateralization and inferior baseplate placement in reverse shoulder arthroplasty with high humeral inclination. Int J Shoulder Surg. 2014;8(3):65-71.
6. Kiet TK, Feeley BT, Naimark M, et al. Outcomes after shoulder replacement: comparison between reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(2):179-185.
7. Alentorn-Geli E, Guirro P, Santana F, Torrens C. Treatment of fracture sequelae of the proximal humerus: comparison of hemiarthroplasty and reverse total shoulder arthroplasty. Arch Orthop Trauma Surg. 2014;134(11):1545-1550.
8. Baulot E, Sirveaux F, Boileau P. Grammont’s idea: The story of Paul Grammont’s functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res. 2011;469(9):2425-2431.
9. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97.
10. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61.
11. Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89(2):292-300.
12. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
13. Atalar AC, Salduz A, Cil H, Sungur M, Celik D, Demirhan M. Reverse shoulder arthroplasty: radiological and clinical short-term results. Acta Orthop Traumatol Turc. 2014;48(1):25-31.
14. Raiss P, Edwards TB, da Silva MR, Bruckner T, Loew M, Walch G. Reverse shoulder arthroplasty for the treatment of nonunions of the surgical neck of the proximal part of the humerus (type-3 fracture sequelae). J Bone Joint Surg Am. 2014;96(24):2070-2076.
15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34.
16. The University of York Centre for Reviews and Dissemination. PROSPERO International prospective register of systematic reviews. Available at: http://www.crd.york.ac.uk/PROSPERO/. Accessed April 11, 2016.
17. The University of Oxford. Oxford Centre for Evidence Based Medicine. Available at: http://www.cebm.net/. Accessed April 11, 2016
18. Cowan J, Lozano-Calderon S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
19. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
20. Sayana MK, Kakarala G, Bandi S, Wynn-Jones C. Medium term results of reverse total shoulder replacement in patients with rotator cuff arthropathy. Ir J Med Sci. 2009;178(2):147-150.
21. Valenti P, Kilinc AS, Sauzieres P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
22. Ladermann A, Denard PJ, Boileau P, et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop. 2015;39(11):2205-2213.
23. Vasen AP, Lacey SH, Keith MW, Shaffer JW. Functional range of motion of the elbow. J Hand Surg Am. 1995;20(2):288-292.
24. Namdari S, Yagnik G, Ebaugh DD, et al. Defining functional shoulder range of motion for activities of daily living. J Shoulder Elbow Surg. 2012;21(9):1177-1183.
Reverse total shoulder arthroplasty (RTSA) has become a reliable treatment option for many pathologic conditions of the shoulder, including rotator cuff arthropathy, proximal humerus fractures, and others.1-4 While the treatment outcomes have generally been reported as good, some concern exists over the postoperative range of motion (ROM) in patients following RTSA, including external rotation.5-7 The original RTSA design was introduced by Neer in the 1970s and has undergone many modifications since that time.1,2 The original Grammont-style prosthesis involved medialization of the glenoid, inferiorizing the center of rotation (with increased deltoid tensioning), and a neck-shaft angle of 155°.1,8 While clinical results of the 155° design were encouraging, concerns arose over the significance of the common finding of scapular notching, or contact between the scapular neck and inferior portion of the humeral polyethylene when the arm is adducted.9,10
To address this concern, a prosthesis design with a 135° neck-shaft angle was introduced.11 This new design did significantly decrease the rate of scapular notching, and although some reported a concern over implant stability with the 135° prosthesis, recent data has shown no difference in dislocation rates between the 135° and 155° prostheses.3 A different variable that has not been evaluated between these prostheses is the active ROM that is achieved postoperatively, and the change in ROM from pre- to post-RTSA.12,13 As active ROM plays a significant role in shoulder function and patient satisfaction, the question of whether a significant difference exists in postoperative ROM between the 135° and 155° prostheses must be addressed.
The purpose of this study was to perform a systematic review investigating active ROM following RTSA to determine if active postoperative ROM following RTSA differs between the 135° and 155° humeral inclination prostheses, and to determine if there is a significant difference between the change in preoperative and postoperative ROM between the 135° and 155° prostheses. The authors hypothesize that there will be no significant difference in active postoperative ROM between the 135° and 155° prostheses, and that the difference between preoperative and postoperative ROM (that is, the amount of motion gained by the surgery) will not significantly differ between the 135° and 155° prostheses.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.15 Systematic review registration was performed using the PROSPERO international prospective register of systematic reviews (registration date 3/9/15, registration number CRD42015017367).16 Two reviewers independently conducted the search on March 7, 2015 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I-IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine17) clinical studies that reported the type of RTSA prosthesis that was used as well as postoperative ROM with at least 12 months follow-up were eligible. All references within included studies were cross-referenced for inclusion if missed by the initial search. If duplicate subject publications were discovered, the study with the longer duration of follow-up or larger number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical studies, arthroscopic shoulder surgery, imaging, surgical technique, and classification studies were excluded. Studies were excluded if both a 135° and 155° prosthesis were utilized and the outcomes were not stratified by the humeral inclination. Studies that did not report ROM were excluded.
A total of 456 studies were located, and, after implementation of the exclusion criteria, 65 studies from 2005-2015 were included in the final analysis (Figure). Subjects of interest in this systematic review underwent a RTSA. Studies were not excluded based on the surgical indications (rotator cuff tear arthropathy, proximal humerus fractures, osteoarthritis) and there was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, journal of publication, country and continent of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, the manufacturer and type of prosthesis used, and the degree of the humeral inclination (135° vs 155° humeral cup). Preoperative ROM, including forward elevation, abduction, external rotation with the arm adducted, and external rotation with the arm at 90° of abduction, were recorded. The same ROM measurements were recorded for the final follow-up visit that was reported. Internal rotation was recorded, but because of the variability with how this measurement was reported, it was not analyzed. Clinical outcome scores and complications were not assessed. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).18
Statistical Analysis
Descriptive statistics were calculated, including mean ± standard deviation for quantitative continuous data and frequencies with percentages for qualitative categorical data. ROM comparisons between 135° and 155° components (pre- vs postoperative for each and postoperative between the 2) were made using 2 proportion z-test calculator (http://in-silico.net/tools/statistics/ztest) using alpha .05 because of the difference in sample sizes between compared groups.
Results
Sixty-five studies with 3302 patients (3434 shoulders) were included in this study. There was a total of 1211 shoulders in the 135° lateralized glenosphere group and 2223 shoulders in the 155° group. The studies had an average MCMS of 40.4 ± 8.2 (poor), 48% of studies reported a conflict of interest, 32% had no conflict of interest, and 20% did not report whether a conflict of interest existed or not. The majority of studies included were level IV evidence (85%). Mean patient age was 71.1 ± 7.6 years; 29% of patients were male and 71% were female. No significant difference existed between patient age at the time of surgery; the average age of patients in the 135° lateralized glenosphere group was 71.67 ± 3.8 years, while the average patient age of patients in the 155° group was 70.97 ± 8.8 years. Mean follow-up for all patients included in this study was 37.2 ± 16.5 months. Of the 65 studies included, 3 were published from Asia, 4 were published from Australia, 24 were from North America, and 34 were from Europe. Of the individual countries whose studies were included, the United States had 23 included studies, France had 13 included studies, and Italy had 4 included studies. All other countries had <4 studies included.
Patients who received either a 135° or a 155° prosthesis showed significant improvements in external rotation with the arm at the side (P < .05), forward elevation (P < .05), and abduction (P < .05) following surgery (Table). When comparing the 135° and 155° groups, patients who received a 135° prosthesis showed significantly greater improvements in external rotation with the arm at the side (P < .001) and had significantly more overall external rotation postoperatively (P < .001) than patients who received a 155° prosthesis. The only preoperative ROM difference between groups was the 155° group started with significantly more forward elevation than the 135°group prior to surgery (P = .002).
Discussion
RTSA is indicated in patients with rotator cuff tear arthropathy, pseudoparalysis, and a functional deltoid.1,2,4 The purpose of this systematic review was to determine if active ROM following RTSA differs between the 135° and 155° humeral inclination prostheses, and to determine if there is a significant difference between the change in preoperative and postoperative ROM between the 135° and 155° prostheses. Forward elevation, abduction, and external rotation all significantly improved following surgery in both groups, with no significant difference between groups in motion or amount of motion improvement, mostly confirming the study hypotheses. However, patients in the 135° group had significantly greater postoperative external rotation and greater amount of external rotation improvement compared to the 155° group.
Two of the frequently debated issues regarding implant geometry is stability and scapular notching between the 135° and 155° humeral inclination designs. Erickson and colleagues3 recently evaluated the rate of scapular notching and dislocations between the 135° and 155° RTSA prostheses. The authors found that the 135° prosthesis had a significantly lower incidence of scapular notching vs the 155° group and that the rate of dislocations was not significantly different between groups.3 In the latter systematic review, the authors attempted to evaluate ROM between the 135° and 155° prostheses, but as the inclusion criteria of the study was reporting on scapular notching and dislocation rates, many studies reporting solely on ROM were excluded, and the influence of humeral inclination on ROM was inconclusive.3 Furthermore, there have been no studies that have directly compared ROM following RTSA between the 135° and 155° prostheses. While studies evaluating each prosthesis on an individual level have shown an improvement in ROM from pre- to postsurgery, there have been no large studies that have compared the postoperative ROM and change in pre- to postoperative ROM between the 135° and 155° prostheses.11,13,19,20
One study by Valenti and colleagues21 evaluated a group of 30 patients with an average age of 69.5 years who underwent RTSA using either a 135° or a 155° prosthesis. Although the study did not directly compare the 2 types of prostheses, it did report the separate outcomes for each prosthesis. At an average follow-up of 36.4 months, the authors found that patients who had the 135° prosthesis implanted had a mean increase in forward elevation and external rotation of 53° and 9°, while patients who had the 155° showed an increase of 56° in forward elevation and a loss of 1° of external rotation. Both prostheses showed a significant increase in forward elevation, but neither had a significant increase in external rotation. Furthermore, scapular notching was seen in 4 patients in the 155° group, while no patients in the 135° group had evidence of notching.
The results of the current study were similar in that both the 135° and 155° prosthesis showed improvements in forward elevation following surgery, and the 135° group showed a significantly greater gain in external rotation than the 155° group. A significant component of shoulder function and patient satisfaction following RTSA is active ROM. However, this variable has not explicitly been evaluated in the literature until now. The clinical significance of this finding is unclear. Patients with adequate external rotation prior to surgery likely would not see a functional difference between prostheses, while those patients who were borderline on a functional amount of external rotation would see a clinically significant benefit with the 135° prosthesis. Studies have shown that the 135° prosthesis is more anatomic than the 155°, and this could explain the difference seen in ROM outcomes between the 2 prostheses.19 Ladermann and colleagues22 recently created and evaluated a 3-dimensional computer model to evaluate possible differences between the 135° and 155° prosthesis. The authors found a significant increase in external rotation of the 135° compared to the 155°, likely related to a difference in acromiohumeral distance as well as inlay vs onlay humeral trays between the 2 prostheses. The results of this study parallel the computer model, thereby validating these experimental results.
It is important to understand what the minimum functional ROM of the shoulder is (in other words, the ROM necessary to complete activities of daily living (ADLs).23 Namdari and colleagues24 used motion analysis software to evaluate the shoulder ROM necessary to complete 10 different ADLs, including combing hair, washing the back of the opposite shoulder, and reaching a shelf above their head without bending their elbow in 20 patients with a mean age of 29.2 years. They found that patients required 121° ± 6.7° of flexion, 46° ± 5.3° of extension, 128° ± 7.9° of abduction, 116° ± 9.1° of cross-body adduction, 59° ± 10° of external rotation with the arm 90° abducted, and 102° ± 7.7° of internal rotation with the arm at the side (external rotation with the arm at the side was not well defined).24 Hence, while abduction and forward elevation seem comparable, the results from the current study do raise concerns about the amount of external rotation obtained following RTSA as it relates to a patients’ ability to perform ADLs, specifically in the 155° prosthesis, as the average postoperative external rotation in this group was 20.5°. Therefore, based on the results of this study, it appears that, while both the 135° and 155° RTSA prostheses provide similar gain in forward elevation and abduction ROM as well as overall forward elevation and abduction, the 135° prosthesis provides significantly more external rotation with the arm at the side than the 155° prosthesis.
Limitations
Although this study attempted to look at all studies that reported active ROM in patients following a RTSA, and 2 authors performed the search, there is a possibility that some studies were missed, introducing study selection bias. Furthermore, the mean follow-up was over 3 years following surgery, but the minimum follow-up requirement for studies to be included was only 12 months. Hence, this transfer bias introduces the possibility that the patient’s ROM would have changed had they been followed for a standard period of time. There are many variables that come into play in evaluating ROM, and although the study attempted to control for these, there are some that could not be controlled for due to lack of reporting by some studies. Glenosphere size and humeral retroversion were not recorded, as they were not reliably reported in all studies, so motion outcomes based on these variables was not evaluated. Complications and clinical outcomes were not assessed in this review and as such, conclusions regarding these variables cannot be drawn from this study. Finally, indications for surgery were not reliably reported in the studies included in this paper, so differences may have existed between surgical indications of the 135° and 155° groups that could have affected outcomes.
Conclusion
Patients who receive a 135° RTSA gain significantly more external rotation from pre- to postsurgery and have an overall greater amount of external rotation than patients who receive a 155° prosthesis. Both groups show improvements in forward elevation, external rotation, and abduction following surgery.
Reverse total shoulder arthroplasty (RTSA) has become a reliable treatment option for many pathologic conditions of the shoulder, including rotator cuff arthropathy, proximal humerus fractures, and others.1-4 While the treatment outcomes have generally been reported as good, some concern exists over the postoperative range of motion (ROM) in patients following RTSA, including external rotation.5-7 The original RTSA design was introduced by Neer in the 1970s and has undergone many modifications since that time.1,2 The original Grammont-style prosthesis involved medialization of the glenoid, inferiorizing the center of rotation (with increased deltoid tensioning), and a neck-shaft angle of 155°.1,8 While clinical results of the 155° design were encouraging, concerns arose over the significance of the common finding of scapular notching, or contact between the scapular neck and inferior portion of the humeral polyethylene when the arm is adducted.9,10
To address this concern, a prosthesis design with a 135° neck-shaft angle was introduced.11 This new design did significantly decrease the rate of scapular notching, and although some reported a concern over implant stability with the 135° prosthesis, recent data has shown no difference in dislocation rates between the 135° and 155° prostheses.3 A different variable that has not been evaluated between these prostheses is the active ROM that is achieved postoperatively, and the change in ROM from pre- to post-RTSA.12,13 As active ROM plays a significant role in shoulder function and patient satisfaction, the question of whether a significant difference exists in postoperative ROM between the 135° and 155° prostheses must be addressed.
The purpose of this study was to perform a systematic review investigating active ROM following RTSA to determine if active postoperative ROM following RTSA differs between the 135° and 155° humeral inclination prostheses, and to determine if there is a significant difference between the change in preoperative and postoperative ROM between the 135° and 155° prostheses. The authors hypothesize that there will be no significant difference in active postoperative ROM between the 135° and 155° prostheses, and that the difference between preoperative and postoperative ROM (that is, the amount of motion gained by the surgery) will not significantly differ between the 135° and 155° prostheses.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.15 Systematic review registration was performed using the PROSPERO international prospective register of systematic reviews (registration date 3/9/15, registration number CRD42015017367).16 Two reviewers independently conducted the search on March 7, 2015 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm utilized was: (((((reverse[Title/Abstract]) AND shoulder[Title/Abstract]) AND arthroplasty[Title/Abstract]) NOT arthroscopic[Title/Abstract]) NOT cadaver[Title/Abstract]) NOT biomechanical[Title/Abstract]. English language Level I-IV evidence (2011 update by the Oxford Centre for Evidence-Based Medicine17) clinical studies that reported the type of RTSA prosthesis that was used as well as postoperative ROM with at least 12 months follow-up were eligible. All references within included studies were cross-referenced for inclusion if missed by the initial search. If duplicate subject publications were discovered, the study with the longer duration of follow-up or larger number of patients was included. Level V evidence reviews, letters to the editor, basic science, biomechanical studies, arthroscopic shoulder surgery, imaging, surgical technique, and classification studies were excluded. Studies were excluded if both a 135° and 155° prosthesis were utilized and the outcomes were not stratified by the humeral inclination. Studies that did not report ROM were excluded.
A total of 456 studies were located, and, after implementation of the exclusion criteria, 65 studies from 2005-2015 were included in the final analysis (Figure). Subjects of interest in this systematic review underwent a RTSA. Studies were not excluded based on the surgical indications (rotator cuff tear arthropathy, proximal humerus fractures, osteoarthritis) and there was no minimum follow-up or rehabilitation requirement. Study and subject demographic parameters analyzed included year of publication, journal of publication, country and continent of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and shoulders, gender, age, the manufacturer and type of prosthesis used, and the degree of the humeral inclination (135° vs 155° humeral cup). Preoperative ROM, including forward elevation, abduction, external rotation with the arm adducted, and external rotation with the arm at 90° of abduction, were recorded. The same ROM measurements were recorded for the final follow-up visit that was reported. Internal rotation was recorded, but because of the variability with how this measurement was reported, it was not analyzed. Clinical outcome scores and complications were not assessed. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).18
Statistical Analysis
Descriptive statistics were calculated, including mean ± standard deviation for quantitative continuous data and frequencies with percentages for qualitative categorical data. ROM comparisons between 135° and 155° components (pre- vs postoperative for each and postoperative between the 2) were made using 2 proportion z-test calculator (http://in-silico.net/tools/statistics/ztest) using alpha .05 because of the difference in sample sizes between compared groups.
Results
Sixty-five studies with 3302 patients (3434 shoulders) were included in this study. There was a total of 1211 shoulders in the 135° lateralized glenosphere group and 2223 shoulders in the 155° group. The studies had an average MCMS of 40.4 ± 8.2 (poor), 48% of studies reported a conflict of interest, 32% had no conflict of interest, and 20% did not report whether a conflict of interest existed or not. The majority of studies included were level IV evidence (85%). Mean patient age was 71.1 ± 7.6 years; 29% of patients were male and 71% were female. No significant difference existed between patient age at the time of surgery; the average age of patients in the 135° lateralized glenosphere group was 71.67 ± 3.8 years, while the average patient age of patients in the 155° group was 70.97 ± 8.8 years. Mean follow-up for all patients included in this study was 37.2 ± 16.5 months. Of the 65 studies included, 3 were published from Asia, 4 were published from Australia, 24 were from North America, and 34 were from Europe. Of the individual countries whose studies were included, the United States had 23 included studies, France had 13 included studies, and Italy had 4 included studies. All other countries had <4 studies included.
Patients who received either a 135° or a 155° prosthesis showed significant improvements in external rotation with the arm at the side (P < .05), forward elevation (P < .05), and abduction (P < .05) following surgery (Table). When comparing the 135° and 155° groups, patients who received a 135° prosthesis showed significantly greater improvements in external rotation with the arm at the side (P < .001) and had significantly more overall external rotation postoperatively (P < .001) than patients who received a 155° prosthesis. The only preoperative ROM difference between groups was the 155° group started with significantly more forward elevation than the 135°group prior to surgery (P = .002).
Discussion
RTSA is indicated in patients with rotator cuff tear arthropathy, pseudoparalysis, and a functional deltoid.1,2,4 The purpose of this systematic review was to determine if active ROM following RTSA differs between the 135° and 155° humeral inclination prostheses, and to determine if there is a significant difference between the change in preoperative and postoperative ROM between the 135° and 155° prostheses. Forward elevation, abduction, and external rotation all significantly improved following surgery in both groups, with no significant difference between groups in motion or amount of motion improvement, mostly confirming the study hypotheses. However, patients in the 135° group had significantly greater postoperative external rotation and greater amount of external rotation improvement compared to the 155° group.
Two of the frequently debated issues regarding implant geometry is stability and scapular notching between the 135° and 155° humeral inclination designs. Erickson and colleagues3 recently evaluated the rate of scapular notching and dislocations between the 135° and 155° RTSA prostheses. The authors found that the 135° prosthesis had a significantly lower incidence of scapular notching vs the 155° group and that the rate of dislocations was not significantly different between groups.3 In the latter systematic review, the authors attempted to evaluate ROM between the 135° and 155° prostheses, but as the inclusion criteria of the study was reporting on scapular notching and dislocation rates, many studies reporting solely on ROM were excluded, and the influence of humeral inclination on ROM was inconclusive.3 Furthermore, there have been no studies that have directly compared ROM following RTSA between the 135° and 155° prostheses. While studies evaluating each prosthesis on an individual level have shown an improvement in ROM from pre- to postsurgery, there have been no large studies that have compared the postoperative ROM and change in pre- to postoperative ROM between the 135° and 155° prostheses.11,13,19,20
One study by Valenti and colleagues21 evaluated a group of 30 patients with an average age of 69.5 years who underwent RTSA using either a 135° or a 155° prosthesis. Although the study did not directly compare the 2 types of prostheses, it did report the separate outcomes for each prosthesis. At an average follow-up of 36.4 months, the authors found that patients who had the 135° prosthesis implanted had a mean increase in forward elevation and external rotation of 53° and 9°, while patients who had the 155° showed an increase of 56° in forward elevation and a loss of 1° of external rotation. Both prostheses showed a significant increase in forward elevation, but neither had a significant increase in external rotation. Furthermore, scapular notching was seen in 4 patients in the 155° group, while no patients in the 135° group had evidence of notching.
The results of the current study were similar in that both the 135° and 155° prosthesis showed improvements in forward elevation following surgery, and the 135° group showed a significantly greater gain in external rotation than the 155° group. A significant component of shoulder function and patient satisfaction following RTSA is active ROM. However, this variable has not explicitly been evaluated in the literature until now. The clinical significance of this finding is unclear. Patients with adequate external rotation prior to surgery likely would not see a functional difference between prostheses, while those patients who were borderline on a functional amount of external rotation would see a clinically significant benefit with the 135° prosthesis. Studies have shown that the 135° prosthesis is more anatomic than the 155°, and this could explain the difference seen in ROM outcomes between the 2 prostheses.19 Ladermann and colleagues22 recently created and evaluated a 3-dimensional computer model to evaluate possible differences between the 135° and 155° prosthesis. The authors found a significant increase in external rotation of the 135° compared to the 155°, likely related to a difference in acromiohumeral distance as well as inlay vs onlay humeral trays between the 2 prostheses. The results of this study parallel the computer model, thereby validating these experimental results.
It is important to understand what the minimum functional ROM of the shoulder is (in other words, the ROM necessary to complete activities of daily living (ADLs).23 Namdari and colleagues24 used motion analysis software to evaluate the shoulder ROM necessary to complete 10 different ADLs, including combing hair, washing the back of the opposite shoulder, and reaching a shelf above their head without bending their elbow in 20 patients with a mean age of 29.2 years. They found that patients required 121° ± 6.7° of flexion, 46° ± 5.3° of extension, 128° ± 7.9° of abduction, 116° ± 9.1° of cross-body adduction, 59° ± 10° of external rotation with the arm 90° abducted, and 102° ± 7.7° of internal rotation with the arm at the side (external rotation with the arm at the side was not well defined).24 Hence, while abduction and forward elevation seem comparable, the results from the current study do raise concerns about the amount of external rotation obtained following RTSA as it relates to a patients’ ability to perform ADLs, specifically in the 155° prosthesis, as the average postoperative external rotation in this group was 20.5°. Therefore, based on the results of this study, it appears that, while both the 135° and 155° RTSA prostheses provide similar gain in forward elevation and abduction ROM as well as overall forward elevation and abduction, the 135° prosthesis provides significantly more external rotation with the arm at the side than the 155° prosthesis.
Limitations
Although this study attempted to look at all studies that reported active ROM in patients following a RTSA, and 2 authors performed the search, there is a possibility that some studies were missed, introducing study selection bias. Furthermore, the mean follow-up was over 3 years following surgery, but the minimum follow-up requirement for studies to be included was only 12 months. Hence, this transfer bias introduces the possibility that the patient’s ROM would have changed had they been followed for a standard period of time. There are many variables that come into play in evaluating ROM, and although the study attempted to control for these, there are some that could not be controlled for due to lack of reporting by some studies. Glenosphere size and humeral retroversion were not recorded, as they were not reliably reported in all studies, so motion outcomes based on these variables was not evaluated. Complications and clinical outcomes were not assessed in this review and as such, conclusions regarding these variables cannot be drawn from this study. Finally, indications for surgery were not reliably reported in the studies included in this paper, so differences may have existed between surgical indications of the 135° and 155° groups that could have affected outcomes.
Conclusion
Patients who receive a 135° RTSA gain significantly more external rotation from pre- to postsurgery and have an overall greater amount of external rotation than patients who receive a 155° prosthesis. Both groups show improvements in forward elevation, external rotation, and abduction following surgery.
1. Flatow EL, Harrison AK. A history of reverse total shoulder arthroplasty. Clin Orthop Relat Res. 2011;469(9):2432-2439.
2. Hyun YS, Huri G, Garbis NG, McFarland EG. Uncommon indications for reverse total shoulder arthroplasty. Clin Orthop Surg. 2013;5(4):243-255.
3. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993.
4. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures--asystematic review of 92 studies including 4500 patients. J Orthop Trauma. 2015;29(1):54-59.
5. Feeley BT, Zhang AL, Barry JJ, et al. Decreased scapular notching with lateralization and inferior baseplate placement in reverse shoulder arthroplasty with high humeral inclination. Int J Shoulder Surg. 2014;8(3):65-71.
6. Kiet TK, Feeley BT, Naimark M, et al. Outcomes after shoulder replacement: comparison between reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(2):179-185.
7. Alentorn-Geli E, Guirro P, Santana F, Torrens C. Treatment of fracture sequelae of the proximal humerus: comparison of hemiarthroplasty and reverse total shoulder arthroplasty. Arch Orthop Trauma Surg. 2014;134(11):1545-1550.
8. Baulot E, Sirveaux F, Boileau P. Grammont’s idea: The story of Paul Grammont’s functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res. 2011;469(9):2425-2431.
9. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97.
10. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61.
11. Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89(2):292-300.
12. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
13. Atalar AC, Salduz A, Cil H, Sungur M, Celik D, Demirhan M. Reverse shoulder arthroplasty: radiological and clinical short-term results. Acta Orthop Traumatol Turc. 2014;48(1):25-31.
14. Raiss P, Edwards TB, da Silva MR, Bruckner T, Loew M, Walch G. Reverse shoulder arthroplasty for the treatment of nonunions of the surgical neck of the proximal part of the humerus (type-3 fracture sequelae). J Bone Joint Surg Am. 2014;96(24):2070-2076.
15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34.
16. The University of York Centre for Reviews and Dissemination. PROSPERO International prospective register of systematic reviews. Available at: http://www.crd.york.ac.uk/PROSPERO/. Accessed April 11, 2016.
17. The University of Oxford. Oxford Centre for Evidence Based Medicine. Available at: http://www.cebm.net/. Accessed April 11, 2016
18. Cowan J, Lozano-Calderon S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
19. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
20. Sayana MK, Kakarala G, Bandi S, Wynn-Jones C. Medium term results of reverse total shoulder replacement in patients with rotator cuff arthropathy. Ir J Med Sci. 2009;178(2):147-150.
21. Valenti P, Kilinc AS, Sauzieres P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
22. Ladermann A, Denard PJ, Boileau P, et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop. 2015;39(11):2205-2213.
23. Vasen AP, Lacey SH, Keith MW, Shaffer JW. Functional range of motion of the elbow. J Hand Surg Am. 1995;20(2):288-292.
24. Namdari S, Yagnik G, Ebaugh DD, et al. Defining functional shoulder range of motion for activities of daily living. J Shoulder Elbow Surg. 2012;21(9):1177-1183.
1. Flatow EL, Harrison AK. A history of reverse total shoulder arthroplasty. Clin Orthop Relat Res. 2011;469(9):2432-2439.
2. Hyun YS, Huri G, Garbis NG, McFarland EG. Uncommon indications for reverse total shoulder arthroplasty. Clin Orthop Surg. 2013;5(4):243-255.
3. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988-993.
4. Gupta AK, Harris JD, Erickson BJ, et al. Surgical management of complex proximal humerus fractures--asystematic review of 92 studies including 4500 patients. J Orthop Trauma. 2015;29(1):54-59.
5. Feeley BT, Zhang AL, Barry JJ, et al. Decreased scapular notching with lateralization and inferior baseplate placement in reverse shoulder arthroplasty with high humeral inclination. Int J Shoulder Surg. 2014;8(3):65-71.
6. Kiet TK, Feeley BT, Naimark M, et al. Outcomes after shoulder replacement: comparison between reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(2):179-185.
7. Alentorn-Geli E, Guirro P, Santana F, Torrens C. Treatment of fracture sequelae of the proximal humerus: comparison of hemiarthroplasty and reverse total shoulder arthroplasty. Arch Orthop Trauma Surg. 2014;134(11):1545-1550.
8. Baulot E, Sirveaux F, Boileau P. Grammont’s idea: The story of Paul Grammont’s functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res. 2011;469(9):2425-2431.
9. Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop Traumatol Surg Res. 2014;100(1):93-97.
10. Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57-61.
11. Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89(2):292-300.
12. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
13. Atalar AC, Salduz A, Cil H, Sungur M, Celik D, Demirhan M. Reverse shoulder arthroplasty: radiological and clinical short-term results. Acta Orthop Traumatol Turc. 2014;48(1):25-31.
14. Raiss P, Edwards TB, da Silva MR, Bruckner T, Loew M, Walch G. Reverse shoulder arthroplasty for the treatment of nonunions of the surgical neck of the proximal part of the humerus (type-3 fracture sequelae). J Bone Joint Surg Am. 2014;96(24):2070-2076.
15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34.
16. The University of York Centre for Reviews and Dissemination. PROSPERO International prospective register of systematic reviews. Available at: http://www.crd.york.ac.uk/PROSPERO/. Accessed April 11, 2016.
17. The University of Oxford. Oxford Centre for Evidence Based Medicine. Available at: http://www.cebm.net/. Accessed April 11, 2016
18. Cowan J, Lozano-Calderon S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
19. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
20. Sayana MK, Kakarala G, Bandi S, Wynn-Jones C. Medium term results of reverse total shoulder replacement in patients with rotator cuff arthropathy. Ir J Med Sci. 2009;178(2):147-150.
21. Valenti P, Kilinc AS, Sauzieres P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
22. Ladermann A, Denard PJ, Boileau P, et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop. 2015;39(11):2205-2213.
23. Vasen AP, Lacey SH, Keith MW, Shaffer JW. Functional range of motion of the elbow. J Hand Surg Am. 1995;20(2):288-292.
24. Namdari S, Yagnik G, Ebaugh DD, et al. Defining functional shoulder range of motion for activities of daily living. J Shoulder Elbow Surg. 2012;21(9):1177-1183.
Promoting Early Literacy in the Pediatrician’s Office: What Have We Learned?
From the Hasbro Children’s Hospital/Warren Alpert School of Medicine at Brown University, Providence, RI.
Abstract
- Objective: To describe current knowledge about the effects of promoting literacy and early language development in young children.
- Methods: Review of the literature.
- Results: Children who are exposed to literacy-promoting interventions in their pediatricians’ offices are more likely to be read to frequently by their caregivers and have improved language skills when compared to children who are not. Language disparities can have life-long consequences that are particularly important in children from disadvantaged socioeconomic backgrounds. The power of the intervention may lie in the fact that it begins in a parent's lap and helps build strong and nurturing parent-child relationships as well as language skills.
- Conclusion: Pediatric providers are in a unique position to positively influence a child’s life course by promoting literacy starting at birth.
Over the past few decades, pediatric providers and parents have been inundated with information about the importance of reading to children, starting at a young age. In fact, a national organization, Reach Out and Read (ROR), has been promoting this idea for the past 25 years. ROR began in 1989 at Boston City Hospital when it was noticed that the books brought in by staff for the pediatric waiting room area were disappearing. Pediatricians and staff members realized that this was likely the result of a lack of children’s books in homes of disadvantaged children, and they decided to provide quality children’s books and guidance about reading with young children as a component of their primary care [1,2]. Since then, ROR has proliferated, with now over 5000 sites throughout the nation. Millions of children between the ages of 6 months and 5 years are given books by their pediatricians at every well child visit. Their parents receive anticipatory guidance about the benefits and joys of reading aloud to their children.
Most pediatricians trained in the past 10 to 15 years cannot imagine a visit that will not include giving a book to a child and talking to his or her parents about the benefits of sharing books together. This practice was reinforced when in 2014 the American Academy of Pediatrics (AAP) released a policy statement making literacy promotion in pediatric practice the standard of care [3]. In this paper, we review the data supporting early literacy promoting interventions and the role that pediatricians have in improving children’s literacy environments. We also discuss the ROR model as well as the impact of electronic media on children’s language skills.
Early Brain Development and Literacy Interventions
About 90% of brain growth occurs before the age of 5. In the first year of life, the brain triples in volume and there is a dramatic increase in the number of synapses. As many as 700 new neural connections are formed every second, and the number grows exponentially from 50 trillion at birth to 1000 trillion by the time of the child’s first birthday. This period of rapid proliferation is followed by a phase of synaptic retraction or “pruning,” so that brain circuits become more efficient. The time course for synaptic “blooming and pruning” varies by brain region. Overproduction in the sensory pathways like those for basic vision and hearing peaks at about the 4th postnatal month and is followed by a gradual retraction that occurs until the middle-end of the preschool period. A similar pattern is observed in areas of the brain that govern development of early language skills but with a somewhat later time course observed, peaking at about 9 months, followed by decline and stabilization in the preschool years. The prefrontal cortex, involved in higher cognitive functions, is the last to develop, reaching a peak overproduction in synapses by age 1, and it is not until late adolescence to early adulthood that a more streamlined density of synapses is obtained [4,5].
Both genetic guidance and experiential exposure are important and play a crucial role in brain development. In fact, the purpose of synaptic overproduction is in part to capture and incorporate experience into the developing synaptic architecture of the brain. Exposure is particularly important during “critical” and “sensitive” periods of development. Critical periods are times during which a set of signals must be present for neural systems to differentiate normally. For example, exposure to patterned visual information in the first few years of life is crucial for stereoscopic vision to develop. Sensitive developmental periods are times when opportunity exists for experience to define patterns of synaptic connectivity, optimizing a child’s ability to adapt to specific environmental factors. Brain plasticity however decreases with age, and as the maturing brain becomes more specialized it is less capable of adapting to new or unexpected challenges. This makes early childhood an important sensitive period in a child’s life, during which experiences directly mold neuronal circuits, offering a critical window for learning [6–9].
Pediatric providers have the unique opportunity to intervene at a time in which the brain is absorbing information at an incredible pace. When children miss the chance to acquire foundational language skills at a very young age, they in turn are at risk for immediate struggles with literacy when they begin attending school. Therefore, for an intervention to have a significant impact on the development of early literacy skills, it has to start early. In the ROR model, pediatric providers start providing anticipatory guidance about the benefits of shared reading, talking, singing, and rhyming starting soon after birth.
Impact of the “Word Gap”
The term “word gap” was first coined by psychologists Betty Hart and Todd Risley in their 1995 book, Meaningful Differences in the Everyday Lives of Young American Children [10]. Their study included 42 healthy and intact young families: 13 high-income families (professional families), 23 families of middle/low socioeconomic status (working-class families), and 6 families who received welfare benefits. Monthly hour-long recordings of parent-child conversations and observations of each family were conducted from the time their index child was about 12 months old until they turned 3 years of age. Gender and race were balanced within the sample.
This study identified remarkable differences in the early vocabulary experiences of young children. The average child raised in a family receiving welfare was hearing half as many words per hour (616 words per hour) as was the average child in working-class family (1251 words per hour) and less than one-third as many than the average child raised in a professional family (2153 words per hour). By extrapolating these numbers in a linear fashion, their study found that the average child growing up in a family living in poverty would listen to about 13 million fewer words than the average child being raised by working class parents and 30 million fewer words than children living in higher income/professional families by the time they reached the age of 3.
To investigate if these findings had longer-term implications, 29 of the 42 families included in their initial study were recruited for follow-up when the children were in third grade. Researchers found that measures of accomplishment at age 3 were highly predictive of performance at the ages of 9 and 10 on several standardized vocabulary, language development, and reading comprehension measures. Thus, the foundation built at age 3 had a great bearing on their progress many years later [11]. This is important because it confirmed that vocabulary development during the toddler and preschool years is directly related to later reading skills and school success in general.
Outcomes of Poor Literacy
Poor early literacy skills are associated with lifelong academic, social, and income disparities. Studies have repeatedly shown that high school graduation rates are directly correlated to reading abilities by the end of 3rd grade. Poor early readers are at a much higher risk of dropping out of school later on. In turn, dropping out of high school is associated with higher risks of delinquency, substance abuse, and incarceration [12,13].
To break the cycle of poverty, we need to help our children—particularly children coming from low-income, disadvantaged homes—become better readers. One of the ways in which we can achieve this is by giving them the tools they need starting in infancy. By giving them books at every well child visit and by encouraging parents to read aloud with their children every day, we can strengthen their early literacy skills, providing a foundation for later success in school and ultimately impacting the quality of their lives.
As Nobel laureate economist James Heckman stated [14]:
Investment in early education for disadvantaged children from birth to age 5 helps reduce the achievement gap, reduce the need for special education, increase the likelihood of healthier lifestyles, lower the crime rate, and reduce overall social costs. In fact, every dollar invested in high-quality early childhood education produces a 7 to 10 percent per annum return on investment.
Why Books? What About Electronics and TV?
In an era of electronics par excellence, we have to look at what the data say about the effects of electronics on children’s brains and language development. To date, studies looking at the effects of electronic media on infant and toddler development have failed to show any benefits. In fact, heavy exposure to electronic devices has been linked to language delays [15]. The data is so strong that in 2011, the AAP released an update of the 1999 policy statement on media use in children. The revised policy stated once again that “pediatricians should urge parents to avoid television viewing in children less than 2 years of age.” The updated statement addresses (1) the lack of evidence supporting educational or developmental benefits for media use by children younger than 2 years, (2) the potential adverse health and developmental effects of media use by children younger than 2 years, and (3) adverse effects of parental media use (background media) on children younger than 2 years [16].
The existing literature suggests that media use does not promote language skills in infants and toddlers and that vocabulary growth is directly related to the amount of time parents spend speaking to and interacting with their children [17–19]. For example, a study comparing the quantity and quality of language interactions of 25 parent-infant dyads during a total of six 15-minute play sessions with electronic toys, traditional toys, and books showed that during play with electronic toys, there were fewer adult words, fewer conversational turns, fewer parental responses, and fewer productions of content-specific words than during play with traditional toys or books. Children vocalized less during play with electronic toys than during play with books. Parents produced fewer words during play with traditional toys than during play with books and use of content-specific words was lower during play with traditional toys than during play with books. This study included primarily college-educated white non-Hispanic parents [20].
Heavy television use in a household can interfere with a child’s language development likely because parents spend less time talking to their child. In turn, children who live in households with heavy media use spend less time being read to. In the short-term, children younger than 2 years who spend a significant amount of time watching television or videos have higher chances of having a language delay [21–23]. Children who are exposed to infant videos also develop fewer language skills than children who are read to [24,25]. What is clear from all of this work is that young children learn best by interacting with the caring people in their lives, not with screens.
Given these facts, the AAP continues to discourage media use among children younger than 2, encourages parents to spend time reading and playing with their children, and discourages parents from having the TV or other electronics on as “background noise” when their children are present, since it decreases the amount of talking and interacting between parents and their children [16].
Benefits of the Reach Out and Read Model
For the past 25 years, pediatricians have been promoting early literacy in their practices following the ROR model, which consists of the following components:
- Giving a new, colorful, age-appropriate book to babies, toddlers, and preschoolers at every well child visit starting at 6 months of age
- Providing anticipatory guidance to parents on the benefits of reading aloud to children starting at birth
- Having a literacy-rich waiting room area (which at times includes volunteers reading to the children)
The data supporting this very simple, inexpensive intervention is robust. Multiple studies have shown that children exposed to the ROR model have improved language skills when compared to children who are not. Parents also report a much higher frequency of reading with their children when exposed to ROR than parents who are not [26–28].
In a randomized controlled study of literacy promotion in Hispanic families, when parents were asked open-endedly “What are your 3 most favorite things to do with your child?,” parents who had received literacy-promoting anticipatory guidance and books reported “reading with my toddler” significantly more often than parents who had not (43% intervention vs. 13% controls). When asked about the frequency of reading to their toddlers, intervention parents were significantly more likely to report reading books with their children at least 3 days/week than controls (66% intervention vs. 24% controls). Applying a multiple logistic regression model controlling for child and parent age, parent reading habits, and English proficiency, we found that the odds of parents reading to their child at least 3 days/week were 10 times greater in intervention families (odds ratio [OR] 10.1, 95% confidence interval 4.0–25.6) than in controls [29].
In a parallel study with English-speaking low income families, when parents were asked open-endedly, “What are your child’s 3 most favorite activities?,” parents who had been exposed to the intervention, were significantly more likely to report “reading books” as one of their toddler’s 3 favorite activities than parents who were not exposed (27% intervention vs. 12% controls). Toddler expressive and receptive vocabulary scores were higher in intervention families and were associated with more frequent shared reading [30].
A multicenter study (19 clinical sites in 10 different states) that compared 730 children aged 6 to 72 months exposed to the ROR model with a comparison group of 917 matched children who did not participate in this literacy promoting model found significant associations between exposure to ROR and reading aloud as a favorite parent activity (adjusted OR 1.6, P < 0.001); reading aloud at bedtime (adjusted OR 1.5, P < 0.001); reading aloud 3 or more days per week (adjusted OR 1.8, P < 0.001); and ownership of 10 or more picture books (adjusted OR 1.6, P < 0.001) [31].
Across the world, others have been replicating and testing the ROR model. Interestingly, studies conducted in Taiwan and with immigrants from Latin America and Asia have all shown similar effects on parental literacy behaviors and on the development of children’s early oral language skills [32–35].
Parent-Child Bonding from Sharing Books
According to the 2014 AAP policy statement, literacy promotion is an essential component of pediatric primary care [3]. The statement emphasizes that parent-child shared reading is a “very personal and nurturing experience that promotes parent-child interaction, social-emotional development, and language and literacy skills during this critical period of early brain and child development.” It recognizes the importance of shared reading as a bonding experience that could start in early infancy. These early nurturing relationships are critical to promoting healthy child development [36].
Most studies of practice-based literacy promotion have asked parents what their favorite things are to do with their child. All of these studies have shown that parents who have received guidance around the importance of reading together and high-quality books to share with their infants, toddlers, and preschoolers include reading aloud as one of their 3 most favorite activities, compared to control families who did not receive this intervention [28–31]. When activities are favorites, they are enriched by this shared enjoyment and are far more likely to occur often and perhaps become treasured family routines. Children’s books and early play and discussions around the themes in these books stimulate increased interaction between caregivers and children [37]. These interactions build secure relationships that are key to children’s healthy cognitive, language, and social-emotional development [38–40].
The Effects on the Brain From Listening to Stories
In a recent study, 48 children aged 6 to 11 years were classified as early talkers (16), on-time talkers (16), or late talkers (16) by parental report [41]. Group assignments were based on whether the parent recalled their child making 2- to 3-word sentences early, on-time, or late. None of the “early talkers” had spoken their first sentences after 24 months, and none of the “late talkers” had spoken sentences before age 2. Utilizing functional MRI, researchers analyzed talker group differences in processing of speech and print and functional activation differences on auditory stimuli and when visualizing print. The groups were matched by age, gender, and performance IQ. This study showed strong group differences in the activation of several regions of the brain, including the left superior temporal gyrus, left putamen, globus pallidus, right putamen, left insula, and thalamus. In each of these areas, late talkers demonstrated significantly less activation that early talkers in both speech and print conditions (P < 0.001). Talker group status was strongly related to neural activation patterns during simple linguistic tasks. These cortical differences in activation are consistent with other studies that demonstrate the role of these regions in understanding speech [42] and processing print [43,44]. These findings highlight the importance of early language development on the formation of critical language and reading circuits and how these neural pathways are affected many years later [41].
In another study of nineteen 3- to 5-year-olds, researchers used functional MRI to examine the relationship between home reading environment and brain activity during a story listening task. The study showed that while listening to stories, children with greater home reading exposure exhibited higher activation of left-sided brain regions involved with processing of meaning. Higher reading exposure at home as measured by the StimQ-P Reading subscale score, was positively correlated with neural activation in the left-sided parietal-temporal-occipital association cortex, a region of the brain supporting semantic language processing, when controlling for household income (P < 0.05) [45].
Conclusion
Pediatric providers are in a unique position to impact a child’s life by promoting literacy starting at birth. The effects of shared reading and parent-child interactions on early language development, on the formation of brain circuitry, and on children’s ability to become better readers and arrive to school ready to learn is now known.
We have an obligation to not only make literacy promotion in pediatric encounters the standard of care, but to continue to expand these types of interventions to other settings to reach as many young children as possible. Children from disadvantaged socioeconomic backgrounds and those from immigrant families are at highest risk and should be the primary focus of our intervention efforts. However, data from the 2011–2012 National Survey of Children’s Health found that only 60% of US children raised in households with income > 400% of the federal poverty level were read to daily [46]. These data suggest that more affluent, professional families should also be counseled by their pediatricians about the benefits of shared reading and about the detrimental effects of “electronics” at this critical time in their child’s development.
More research is needed to fully understand the long-term impacts of literacy promotion interventions in primary care settings. Longitudinal studies directly measuring the potential effects of the ROR model on reading skills in 3rd grade, on high school graduation rates, and on other measures of social and academic success are lacking. However, the existing evidence suggests that this kind of program can fulfill the promise of child health supervision visits. While providing guidance and the tools aimed at improving the home environment, pediatric providers can shape the course of young children’s lives.
Corresponding author: Natalia Golova, MD, Hasbro Children’s Hospital, 593 Eddy St., Hasbro Lower Level, Providence, RI 02903, [email protected].
Financial disclosures: None.
1. Needlman R, Fried L, Morley D, et al. Clinic-based intervention to promote literacy. Am J Dis Child 1991;145:881–4.
2. Reach Out and Read: a national pediatric literacy program. Available at http://reachoutandread.org.
3. High PC, Klass P. Literacy promotion: an essential component of primary care pediatric practice. Council on Early Childhood. Pediatrics 2014;134:404–9.
4. Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 1997; 387:167–78.
5. Center on the Developing Child. The science of early childhood development (in brief); 2007. Accessed 6 May 2016 at www.developingchild.harvard.edu.
6. Connecting Science, Policy, and Practice: Zero to Three’s National Training Institute, 2015. Zero Three 2016;36(3).
7. Fox NA, Zeanah CH, Nelson CA. A matter of timing: enhancing positive change for the developing brain. Zero Three 2014;34(3):4–9.
8. Halfon N, Shulman E, Hochstein M. Brain development in early childhood. Technical report. UCLA Center for Healthier Children, Families and Communities. Aug 2001.
9. National Scientific Council on the Developing Child. The science of early childhood development: closing the gap between what we know and what we do. Center on the Developing Child. Harvard University; 2007.
10. Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Baltimore: Brookes; 1995.
11. Hart B, Risley TR. The early catastrophe: the 30 million word gap by age 3. Am Educator 2003;27:4–9.
12. The Annie E. Casey Foundation. Double jeopardy: how third grade reading skills and poverty influence high school graduation. 2012. Accessed 21 Feb 2016 at www.aecf.org/resources/double-jeopardy.
13. The Annie E. Casey Foundation. Early warning confirmed: a research update on third grade reading. 2013 Nov. Accessed 23 Feb 2016 at www.aecf.org/m/resourcedoc/AECF-EarlyWarningConfirmed-2013.pdf
14. Heckman J. The economics of inequality: the value of early childhood education. Am Educator 2011;47:31–5.
15. Christakis DA. The effects of infant media usage: what do we know and what should we learn? Acta Paediatr 2009;98: 8–16.
16. American Academy of Pediatrics, Council on Communications and Media. Policy statement. Media use by children younger than 2 years. Pediatrics 2011;128:1040–5.
17. Linebarger DL, Walker D. Infants’ and toddlers’ television viewing and language outcomes. Am Behav Sci 2005;48:624–45.
18. Masako T, Okuma K, Kyoshima K. Television viewing and reduced parental utterance, and delayed speech development in infants and young children. Arch Pediatr Adolesc Med 2007;161:618–9.
19. Rideout VJ, Hamel E. The media family: electronic media in the lives of infants, toddlers, preschoolers, and their parents. Menlo Park, CA: Kaiser Family Foundation; 2006.
20. Sosa AV. Association of the type of toy used during play with the quantity and quality of parent-infant communication. JAMA Pediatr 2016;170:132–7.
21. Vandewater EA, Bickham DS, Lee JH et al. When the television is always on: heavy television exposure and young children’s development. Am Behav Sci 2005;48:562–77.
22. Zimmerman FJ, Christakis DA, Meltzoff AN. Associations between media viewing and language development in children under age two years. J Pediatr 2007;151:364–8.
23. Chonchaiya W, Pruksananonda C. Television viewing associates with delayed language development. Acta Paediatr 2008;97:977–82.
24. Robb MB, Richert RA, Wartella EA. Just a talking book? Word learning from watching baby videos. Br J Dev Psychol 2009;27(Pt 1):27–45.
25. DeLoache JS, Chiong C, Sherman K, et al. Do babies learn from baby media? Psychol Sci 2010;21:1570–4.
26. Mendelsohn A, Mogliner L, Dreyer B, et al. The impact of a clinic-based literacy intervention on language development in inner-city preschool children. Pediatrics 2001;107:130–4.
27. Mendelsohn AL. Promoting language and literacy through reading aloud: the role of the pediatrician. Curr Probl Pediatr Adolesc Health Care 2002;32:183–210.
28. High P, Hopman M, LaGasse L, et al. Evaluation of a clinic-based program to promote book sharing and bedtime routines among low-income urban families with young children. Arch Pediatr Adolesc Med 1998;152:459–65.
29. Golova N, Alario A, Vivier P, et al. Literacy promotion for Hispanic families in a primary care setting: a randomized controlled trial. Pediatrics 1999;103:993–7.
30. High PC, LaGasse L, Becker S, et al. Literacy promotion in primary care pediatrics: can we make a difference? Pediatrics 2000;105:927–34.
31. Needlman R, Toker KH, Dreyer BP, et al. Effectiveness of a primary care intervention to support reading aloud: a multicenter evaluation. Ambul Pediatr 2005;5:209–15.
32. Wu SC, Lue HC, Tseng LL. A pediatric clinic-based approach to early literacy promotion--experience in a well-baby clinic in Taiwan. J Formos Med Assoc 2012;111:258–64.
33. Sanders LM, Gershon TD, Huffman LC, et al. Prescribing books for immigrant children: a pilot study to promote emergent literacy among the children of Hispanic immigrants. Arch Pediatr Adolesc Med 2000;154:771–7.
34. Kitabayashi KM, Huang GY, Linskey KR, et al. Parent-child reading interactions among English and English as a second language speakers in an underserved pediatric clinic in Hawai’i. Hawaii Med J 2008;67:260–3.
35. Festa N, Loftus PD, Cullen MR, Mendoza FS. Disparities in early exposure to book sharing within immigrant families. Pediatrics. 2014;134:e162–8.
36. Shonkoff JP, Phillips DA, editors. From neurons to neighborhoods: the science of early childhood development. National Research Council (US) and Institute of Medicine (US) Committee on Integrating the Science of Early Childhood Development. Washington, DC: National Academies Press; 2000.
37. Neuman SB. Guiding young children’s participation in early literacy development: a family literacy program for adolescent mothers. Early Child Dev Care 1997;127:119–29.
38. Tomopoulos S, Dreyer BP, Tamis-LeMonda C, et al. Books, toys, parent-child interaction, and development in young Latino children. Ambul Pediatr 2006;6:72–8.
39. Mendelsohn AL, Huberman HS, Berkule SB, et al. Primary care strategies for promoting parent-child interactions and school readiness in at-risk families: the Bellevue Project for Early Language, Literacy, and Education Success. Arch Pediatr Adolesc Med 2011;165:33–41.
40. Ginsburg K; American Academy of Pediatrics, Committee on Communications, Committee on Psychosocial Aspects of Child and Family Health. The importance of play in promoting healthy child development and maintaining strong parent-child bonds. Pediatrics 2007;119:182–91.
41. Preston JL, Frost SJ, Mencl WE, et al. Early and late talkers: school-age language, literacy and neurolinguistic differences. Brain 2010;133:2185–95.
42. Hugdahl K, Gundersen H, Brekke C, et al. fMRI Brain activation in a Finnish family with specific language impairment compared with a normal control group. J Speech Lang Hear Res 2004;47:162–72.
43. Pugh KR, Mencl WE, Jenner AR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 2000;6:207–13.
44. Pugh KR, Mencl WE, Jenner AR, et al. Neurobiological studies of reading and reading disability. J Commun Disord 2001;34:479–92.
45. Hutton JS, Horowitz-Kraus T, Mendelsohn AL, et al. Home reading environment and brain activation in preschool children listening to stories. Pediatrics 2015;136:466–78.
46. Data Resource Center for Child and Adolescent Health. 2011/12 National Survey of Children’s Health. Accessed 28 Feb 2016 at www.nschdata.org.
From the Hasbro Children’s Hospital/Warren Alpert School of Medicine at Brown University, Providence, RI.
Abstract
- Objective: To describe current knowledge about the effects of promoting literacy and early language development in young children.
- Methods: Review of the literature.
- Results: Children who are exposed to literacy-promoting interventions in their pediatricians’ offices are more likely to be read to frequently by their caregivers and have improved language skills when compared to children who are not. Language disparities can have life-long consequences that are particularly important in children from disadvantaged socioeconomic backgrounds. The power of the intervention may lie in the fact that it begins in a parent's lap and helps build strong and nurturing parent-child relationships as well as language skills.
- Conclusion: Pediatric providers are in a unique position to positively influence a child’s life course by promoting literacy starting at birth.
Over the past few decades, pediatric providers and parents have been inundated with information about the importance of reading to children, starting at a young age. In fact, a national organization, Reach Out and Read (ROR), has been promoting this idea for the past 25 years. ROR began in 1989 at Boston City Hospital when it was noticed that the books brought in by staff for the pediatric waiting room area were disappearing. Pediatricians and staff members realized that this was likely the result of a lack of children’s books in homes of disadvantaged children, and they decided to provide quality children’s books and guidance about reading with young children as a component of their primary care [1,2]. Since then, ROR has proliferated, with now over 5000 sites throughout the nation. Millions of children between the ages of 6 months and 5 years are given books by their pediatricians at every well child visit. Their parents receive anticipatory guidance about the benefits and joys of reading aloud to their children.
Most pediatricians trained in the past 10 to 15 years cannot imagine a visit that will not include giving a book to a child and talking to his or her parents about the benefits of sharing books together. This practice was reinforced when in 2014 the American Academy of Pediatrics (AAP) released a policy statement making literacy promotion in pediatric practice the standard of care [3]. In this paper, we review the data supporting early literacy promoting interventions and the role that pediatricians have in improving children’s literacy environments. We also discuss the ROR model as well as the impact of electronic media on children’s language skills.
Early Brain Development and Literacy Interventions
About 90% of brain growth occurs before the age of 5. In the first year of life, the brain triples in volume and there is a dramatic increase in the number of synapses. As many as 700 new neural connections are formed every second, and the number grows exponentially from 50 trillion at birth to 1000 trillion by the time of the child’s first birthday. This period of rapid proliferation is followed by a phase of synaptic retraction or “pruning,” so that brain circuits become more efficient. The time course for synaptic “blooming and pruning” varies by brain region. Overproduction in the sensory pathways like those for basic vision and hearing peaks at about the 4th postnatal month and is followed by a gradual retraction that occurs until the middle-end of the preschool period. A similar pattern is observed in areas of the brain that govern development of early language skills but with a somewhat later time course observed, peaking at about 9 months, followed by decline and stabilization in the preschool years. The prefrontal cortex, involved in higher cognitive functions, is the last to develop, reaching a peak overproduction in synapses by age 1, and it is not until late adolescence to early adulthood that a more streamlined density of synapses is obtained [4,5].
Both genetic guidance and experiential exposure are important and play a crucial role in brain development. In fact, the purpose of synaptic overproduction is in part to capture and incorporate experience into the developing synaptic architecture of the brain. Exposure is particularly important during “critical” and “sensitive” periods of development. Critical periods are times during which a set of signals must be present for neural systems to differentiate normally. For example, exposure to patterned visual information in the first few years of life is crucial for stereoscopic vision to develop. Sensitive developmental periods are times when opportunity exists for experience to define patterns of synaptic connectivity, optimizing a child’s ability to adapt to specific environmental factors. Brain plasticity however decreases with age, and as the maturing brain becomes more specialized it is less capable of adapting to new or unexpected challenges. This makes early childhood an important sensitive period in a child’s life, during which experiences directly mold neuronal circuits, offering a critical window for learning [6–9].
Pediatric providers have the unique opportunity to intervene at a time in which the brain is absorbing information at an incredible pace. When children miss the chance to acquire foundational language skills at a very young age, they in turn are at risk for immediate struggles with literacy when they begin attending school. Therefore, for an intervention to have a significant impact on the development of early literacy skills, it has to start early. In the ROR model, pediatric providers start providing anticipatory guidance about the benefits of shared reading, talking, singing, and rhyming starting soon after birth.
Impact of the “Word Gap”
The term “word gap” was first coined by psychologists Betty Hart and Todd Risley in their 1995 book, Meaningful Differences in the Everyday Lives of Young American Children [10]. Their study included 42 healthy and intact young families: 13 high-income families (professional families), 23 families of middle/low socioeconomic status (working-class families), and 6 families who received welfare benefits. Monthly hour-long recordings of parent-child conversations and observations of each family were conducted from the time their index child was about 12 months old until they turned 3 years of age. Gender and race were balanced within the sample.
This study identified remarkable differences in the early vocabulary experiences of young children. The average child raised in a family receiving welfare was hearing half as many words per hour (616 words per hour) as was the average child in working-class family (1251 words per hour) and less than one-third as many than the average child raised in a professional family (2153 words per hour). By extrapolating these numbers in a linear fashion, their study found that the average child growing up in a family living in poverty would listen to about 13 million fewer words than the average child being raised by working class parents and 30 million fewer words than children living in higher income/professional families by the time they reached the age of 3.
To investigate if these findings had longer-term implications, 29 of the 42 families included in their initial study were recruited for follow-up when the children were in third grade. Researchers found that measures of accomplishment at age 3 were highly predictive of performance at the ages of 9 and 10 on several standardized vocabulary, language development, and reading comprehension measures. Thus, the foundation built at age 3 had a great bearing on their progress many years later [11]. This is important because it confirmed that vocabulary development during the toddler and preschool years is directly related to later reading skills and school success in general.
Outcomes of Poor Literacy
Poor early literacy skills are associated with lifelong academic, social, and income disparities. Studies have repeatedly shown that high school graduation rates are directly correlated to reading abilities by the end of 3rd grade. Poor early readers are at a much higher risk of dropping out of school later on. In turn, dropping out of high school is associated with higher risks of delinquency, substance abuse, and incarceration [12,13].
To break the cycle of poverty, we need to help our children—particularly children coming from low-income, disadvantaged homes—become better readers. One of the ways in which we can achieve this is by giving them the tools they need starting in infancy. By giving them books at every well child visit and by encouraging parents to read aloud with their children every day, we can strengthen their early literacy skills, providing a foundation for later success in school and ultimately impacting the quality of their lives.
As Nobel laureate economist James Heckman stated [14]:
Investment in early education for disadvantaged children from birth to age 5 helps reduce the achievement gap, reduce the need for special education, increase the likelihood of healthier lifestyles, lower the crime rate, and reduce overall social costs. In fact, every dollar invested in high-quality early childhood education produces a 7 to 10 percent per annum return on investment.
Why Books? What About Electronics and TV?
In an era of electronics par excellence, we have to look at what the data say about the effects of electronics on children’s brains and language development. To date, studies looking at the effects of electronic media on infant and toddler development have failed to show any benefits. In fact, heavy exposure to electronic devices has been linked to language delays [15]. The data is so strong that in 2011, the AAP released an update of the 1999 policy statement on media use in children. The revised policy stated once again that “pediatricians should urge parents to avoid television viewing in children less than 2 years of age.” The updated statement addresses (1) the lack of evidence supporting educational or developmental benefits for media use by children younger than 2 years, (2) the potential adverse health and developmental effects of media use by children younger than 2 years, and (3) adverse effects of parental media use (background media) on children younger than 2 years [16].
The existing literature suggests that media use does not promote language skills in infants and toddlers and that vocabulary growth is directly related to the amount of time parents spend speaking to and interacting with their children [17–19]. For example, a study comparing the quantity and quality of language interactions of 25 parent-infant dyads during a total of six 15-minute play sessions with electronic toys, traditional toys, and books showed that during play with electronic toys, there were fewer adult words, fewer conversational turns, fewer parental responses, and fewer productions of content-specific words than during play with traditional toys or books. Children vocalized less during play with electronic toys than during play with books. Parents produced fewer words during play with traditional toys than during play with books and use of content-specific words was lower during play with traditional toys than during play with books. This study included primarily college-educated white non-Hispanic parents [20].
Heavy television use in a household can interfere with a child’s language development likely because parents spend less time talking to their child. In turn, children who live in households with heavy media use spend less time being read to. In the short-term, children younger than 2 years who spend a significant amount of time watching television or videos have higher chances of having a language delay [21–23]. Children who are exposed to infant videos also develop fewer language skills than children who are read to [24,25]. What is clear from all of this work is that young children learn best by interacting with the caring people in their lives, not with screens.
Given these facts, the AAP continues to discourage media use among children younger than 2, encourages parents to spend time reading and playing with their children, and discourages parents from having the TV or other electronics on as “background noise” when their children are present, since it decreases the amount of talking and interacting between parents and their children [16].
Benefits of the Reach Out and Read Model
For the past 25 years, pediatricians have been promoting early literacy in their practices following the ROR model, which consists of the following components:
- Giving a new, colorful, age-appropriate book to babies, toddlers, and preschoolers at every well child visit starting at 6 months of age
- Providing anticipatory guidance to parents on the benefits of reading aloud to children starting at birth
- Having a literacy-rich waiting room area (which at times includes volunteers reading to the children)
The data supporting this very simple, inexpensive intervention is robust. Multiple studies have shown that children exposed to the ROR model have improved language skills when compared to children who are not. Parents also report a much higher frequency of reading with their children when exposed to ROR than parents who are not [26–28].
In a randomized controlled study of literacy promotion in Hispanic families, when parents were asked open-endedly “What are your 3 most favorite things to do with your child?,” parents who had received literacy-promoting anticipatory guidance and books reported “reading with my toddler” significantly more often than parents who had not (43% intervention vs. 13% controls). When asked about the frequency of reading to their toddlers, intervention parents were significantly more likely to report reading books with their children at least 3 days/week than controls (66% intervention vs. 24% controls). Applying a multiple logistic regression model controlling for child and parent age, parent reading habits, and English proficiency, we found that the odds of parents reading to their child at least 3 days/week were 10 times greater in intervention families (odds ratio [OR] 10.1, 95% confidence interval 4.0–25.6) than in controls [29].
In a parallel study with English-speaking low income families, when parents were asked open-endedly, “What are your child’s 3 most favorite activities?,” parents who had been exposed to the intervention, were significantly more likely to report “reading books” as one of their toddler’s 3 favorite activities than parents who were not exposed (27% intervention vs. 12% controls). Toddler expressive and receptive vocabulary scores were higher in intervention families and were associated with more frequent shared reading [30].
A multicenter study (19 clinical sites in 10 different states) that compared 730 children aged 6 to 72 months exposed to the ROR model with a comparison group of 917 matched children who did not participate in this literacy promoting model found significant associations between exposure to ROR and reading aloud as a favorite parent activity (adjusted OR 1.6, P < 0.001); reading aloud at bedtime (adjusted OR 1.5, P < 0.001); reading aloud 3 or more days per week (adjusted OR 1.8, P < 0.001); and ownership of 10 or more picture books (adjusted OR 1.6, P < 0.001) [31].
Across the world, others have been replicating and testing the ROR model. Interestingly, studies conducted in Taiwan and with immigrants from Latin America and Asia have all shown similar effects on parental literacy behaviors and on the development of children’s early oral language skills [32–35].
Parent-Child Bonding from Sharing Books
According to the 2014 AAP policy statement, literacy promotion is an essential component of pediatric primary care [3]. The statement emphasizes that parent-child shared reading is a “very personal and nurturing experience that promotes parent-child interaction, social-emotional development, and language and literacy skills during this critical period of early brain and child development.” It recognizes the importance of shared reading as a bonding experience that could start in early infancy. These early nurturing relationships are critical to promoting healthy child development [36].
Most studies of practice-based literacy promotion have asked parents what their favorite things are to do with their child. All of these studies have shown that parents who have received guidance around the importance of reading together and high-quality books to share with their infants, toddlers, and preschoolers include reading aloud as one of their 3 most favorite activities, compared to control families who did not receive this intervention [28–31]. When activities are favorites, they are enriched by this shared enjoyment and are far more likely to occur often and perhaps become treasured family routines. Children’s books and early play and discussions around the themes in these books stimulate increased interaction between caregivers and children [37]. These interactions build secure relationships that are key to children’s healthy cognitive, language, and social-emotional development [38–40].
The Effects on the Brain From Listening to Stories
In a recent study, 48 children aged 6 to 11 years were classified as early talkers (16), on-time talkers (16), or late talkers (16) by parental report [41]. Group assignments were based on whether the parent recalled their child making 2- to 3-word sentences early, on-time, or late. None of the “early talkers” had spoken their first sentences after 24 months, and none of the “late talkers” had spoken sentences before age 2. Utilizing functional MRI, researchers analyzed talker group differences in processing of speech and print and functional activation differences on auditory stimuli and when visualizing print. The groups were matched by age, gender, and performance IQ. This study showed strong group differences in the activation of several regions of the brain, including the left superior temporal gyrus, left putamen, globus pallidus, right putamen, left insula, and thalamus. In each of these areas, late talkers demonstrated significantly less activation that early talkers in both speech and print conditions (P < 0.001). Talker group status was strongly related to neural activation patterns during simple linguistic tasks. These cortical differences in activation are consistent with other studies that demonstrate the role of these regions in understanding speech [42] and processing print [43,44]. These findings highlight the importance of early language development on the formation of critical language and reading circuits and how these neural pathways are affected many years later [41].
In another study of nineteen 3- to 5-year-olds, researchers used functional MRI to examine the relationship between home reading environment and brain activity during a story listening task. The study showed that while listening to stories, children with greater home reading exposure exhibited higher activation of left-sided brain regions involved with processing of meaning. Higher reading exposure at home as measured by the StimQ-P Reading subscale score, was positively correlated with neural activation in the left-sided parietal-temporal-occipital association cortex, a region of the brain supporting semantic language processing, when controlling for household income (P < 0.05) [45].
Conclusion
Pediatric providers are in a unique position to impact a child’s life by promoting literacy starting at birth. The effects of shared reading and parent-child interactions on early language development, on the formation of brain circuitry, and on children’s ability to become better readers and arrive to school ready to learn is now known.
We have an obligation to not only make literacy promotion in pediatric encounters the standard of care, but to continue to expand these types of interventions to other settings to reach as many young children as possible. Children from disadvantaged socioeconomic backgrounds and those from immigrant families are at highest risk and should be the primary focus of our intervention efforts. However, data from the 2011–2012 National Survey of Children’s Health found that only 60% of US children raised in households with income > 400% of the federal poverty level were read to daily [46]. These data suggest that more affluent, professional families should also be counseled by their pediatricians about the benefits of shared reading and about the detrimental effects of “electronics” at this critical time in their child’s development.
More research is needed to fully understand the long-term impacts of literacy promotion interventions in primary care settings. Longitudinal studies directly measuring the potential effects of the ROR model on reading skills in 3rd grade, on high school graduation rates, and on other measures of social and academic success are lacking. However, the existing evidence suggests that this kind of program can fulfill the promise of child health supervision visits. While providing guidance and the tools aimed at improving the home environment, pediatric providers can shape the course of young children’s lives.
Corresponding author: Natalia Golova, MD, Hasbro Children’s Hospital, 593 Eddy St., Hasbro Lower Level, Providence, RI 02903, [email protected].
Financial disclosures: None.
From the Hasbro Children’s Hospital/Warren Alpert School of Medicine at Brown University, Providence, RI.
Abstract
- Objective: To describe current knowledge about the effects of promoting literacy and early language development in young children.
- Methods: Review of the literature.
- Results: Children who are exposed to literacy-promoting interventions in their pediatricians’ offices are more likely to be read to frequently by their caregivers and have improved language skills when compared to children who are not. Language disparities can have life-long consequences that are particularly important in children from disadvantaged socioeconomic backgrounds. The power of the intervention may lie in the fact that it begins in a parent's lap and helps build strong and nurturing parent-child relationships as well as language skills.
- Conclusion: Pediatric providers are in a unique position to positively influence a child’s life course by promoting literacy starting at birth.
Over the past few decades, pediatric providers and parents have been inundated with information about the importance of reading to children, starting at a young age. In fact, a national organization, Reach Out and Read (ROR), has been promoting this idea for the past 25 years. ROR began in 1989 at Boston City Hospital when it was noticed that the books brought in by staff for the pediatric waiting room area were disappearing. Pediatricians and staff members realized that this was likely the result of a lack of children’s books in homes of disadvantaged children, and they decided to provide quality children’s books and guidance about reading with young children as a component of their primary care [1,2]. Since then, ROR has proliferated, with now over 5000 sites throughout the nation. Millions of children between the ages of 6 months and 5 years are given books by their pediatricians at every well child visit. Their parents receive anticipatory guidance about the benefits and joys of reading aloud to their children.
Most pediatricians trained in the past 10 to 15 years cannot imagine a visit that will not include giving a book to a child and talking to his or her parents about the benefits of sharing books together. This practice was reinforced when in 2014 the American Academy of Pediatrics (AAP) released a policy statement making literacy promotion in pediatric practice the standard of care [3]. In this paper, we review the data supporting early literacy promoting interventions and the role that pediatricians have in improving children’s literacy environments. We also discuss the ROR model as well as the impact of electronic media on children’s language skills.
Early Brain Development and Literacy Interventions
About 90% of brain growth occurs before the age of 5. In the first year of life, the brain triples in volume and there is a dramatic increase in the number of synapses. As many as 700 new neural connections are formed every second, and the number grows exponentially from 50 trillion at birth to 1000 trillion by the time of the child’s first birthday. This period of rapid proliferation is followed by a phase of synaptic retraction or “pruning,” so that brain circuits become more efficient. The time course for synaptic “blooming and pruning” varies by brain region. Overproduction in the sensory pathways like those for basic vision and hearing peaks at about the 4th postnatal month and is followed by a gradual retraction that occurs until the middle-end of the preschool period. A similar pattern is observed in areas of the brain that govern development of early language skills but with a somewhat later time course observed, peaking at about 9 months, followed by decline and stabilization in the preschool years. The prefrontal cortex, involved in higher cognitive functions, is the last to develop, reaching a peak overproduction in synapses by age 1, and it is not until late adolescence to early adulthood that a more streamlined density of synapses is obtained [4,5].
Both genetic guidance and experiential exposure are important and play a crucial role in brain development. In fact, the purpose of synaptic overproduction is in part to capture and incorporate experience into the developing synaptic architecture of the brain. Exposure is particularly important during “critical” and “sensitive” periods of development. Critical periods are times during which a set of signals must be present for neural systems to differentiate normally. For example, exposure to patterned visual information in the first few years of life is crucial for stereoscopic vision to develop. Sensitive developmental periods are times when opportunity exists for experience to define patterns of synaptic connectivity, optimizing a child’s ability to adapt to specific environmental factors. Brain plasticity however decreases with age, and as the maturing brain becomes more specialized it is less capable of adapting to new or unexpected challenges. This makes early childhood an important sensitive period in a child’s life, during which experiences directly mold neuronal circuits, offering a critical window for learning [6–9].
Pediatric providers have the unique opportunity to intervene at a time in which the brain is absorbing information at an incredible pace. When children miss the chance to acquire foundational language skills at a very young age, they in turn are at risk for immediate struggles with literacy when they begin attending school. Therefore, for an intervention to have a significant impact on the development of early literacy skills, it has to start early. In the ROR model, pediatric providers start providing anticipatory guidance about the benefits of shared reading, talking, singing, and rhyming starting soon after birth.
Impact of the “Word Gap”
The term “word gap” was first coined by psychologists Betty Hart and Todd Risley in their 1995 book, Meaningful Differences in the Everyday Lives of Young American Children [10]. Their study included 42 healthy and intact young families: 13 high-income families (professional families), 23 families of middle/low socioeconomic status (working-class families), and 6 families who received welfare benefits. Monthly hour-long recordings of parent-child conversations and observations of each family were conducted from the time their index child was about 12 months old until they turned 3 years of age. Gender and race were balanced within the sample.
This study identified remarkable differences in the early vocabulary experiences of young children. The average child raised in a family receiving welfare was hearing half as many words per hour (616 words per hour) as was the average child in working-class family (1251 words per hour) and less than one-third as many than the average child raised in a professional family (2153 words per hour). By extrapolating these numbers in a linear fashion, their study found that the average child growing up in a family living in poverty would listen to about 13 million fewer words than the average child being raised by working class parents and 30 million fewer words than children living in higher income/professional families by the time they reached the age of 3.
To investigate if these findings had longer-term implications, 29 of the 42 families included in their initial study were recruited for follow-up when the children were in third grade. Researchers found that measures of accomplishment at age 3 were highly predictive of performance at the ages of 9 and 10 on several standardized vocabulary, language development, and reading comprehension measures. Thus, the foundation built at age 3 had a great bearing on their progress many years later [11]. This is important because it confirmed that vocabulary development during the toddler and preschool years is directly related to later reading skills and school success in general.
Outcomes of Poor Literacy
Poor early literacy skills are associated with lifelong academic, social, and income disparities. Studies have repeatedly shown that high school graduation rates are directly correlated to reading abilities by the end of 3rd grade. Poor early readers are at a much higher risk of dropping out of school later on. In turn, dropping out of high school is associated with higher risks of delinquency, substance abuse, and incarceration [12,13].
To break the cycle of poverty, we need to help our children—particularly children coming from low-income, disadvantaged homes—become better readers. One of the ways in which we can achieve this is by giving them the tools they need starting in infancy. By giving them books at every well child visit and by encouraging parents to read aloud with their children every day, we can strengthen their early literacy skills, providing a foundation for later success in school and ultimately impacting the quality of their lives.
As Nobel laureate economist James Heckman stated [14]:
Investment in early education for disadvantaged children from birth to age 5 helps reduce the achievement gap, reduce the need for special education, increase the likelihood of healthier lifestyles, lower the crime rate, and reduce overall social costs. In fact, every dollar invested in high-quality early childhood education produces a 7 to 10 percent per annum return on investment.
Why Books? What About Electronics and TV?
In an era of electronics par excellence, we have to look at what the data say about the effects of electronics on children’s brains and language development. To date, studies looking at the effects of electronic media on infant and toddler development have failed to show any benefits. In fact, heavy exposure to electronic devices has been linked to language delays [15]. The data is so strong that in 2011, the AAP released an update of the 1999 policy statement on media use in children. The revised policy stated once again that “pediatricians should urge parents to avoid television viewing in children less than 2 years of age.” The updated statement addresses (1) the lack of evidence supporting educational or developmental benefits for media use by children younger than 2 years, (2) the potential adverse health and developmental effects of media use by children younger than 2 years, and (3) adverse effects of parental media use (background media) on children younger than 2 years [16].
The existing literature suggests that media use does not promote language skills in infants and toddlers and that vocabulary growth is directly related to the amount of time parents spend speaking to and interacting with their children [17–19]. For example, a study comparing the quantity and quality of language interactions of 25 parent-infant dyads during a total of six 15-minute play sessions with electronic toys, traditional toys, and books showed that during play with electronic toys, there were fewer adult words, fewer conversational turns, fewer parental responses, and fewer productions of content-specific words than during play with traditional toys or books. Children vocalized less during play with electronic toys than during play with books. Parents produced fewer words during play with traditional toys than during play with books and use of content-specific words was lower during play with traditional toys than during play with books. This study included primarily college-educated white non-Hispanic parents [20].
Heavy television use in a household can interfere with a child’s language development likely because parents spend less time talking to their child. In turn, children who live in households with heavy media use spend less time being read to. In the short-term, children younger than 2 years who spend a significant amount of time watching television or videos have higher chances of having a language delay [21–23]. Children who are exposed to infant videos also develop fewer language skills than children who are read to [24,25]. What is clear from all of this work is that young children learn best by interacting with the caring people in their lives, not with screens.
Given these facts, the AAP continues to discourage media use among children younger than 2, encourages parents to spend time reading and playing with their children, and discourages parents from having the TV or other electronics on as “background noise” when their children are present, since it decreases the amount of talking and interacting between parents and their children [16].
Benefits of the Reach Out and Read Model
For the past 25 years, pediatricians have been promoting early literacy in their practices following the ROR model, which consists of the following components:
- Giving a new, colorful, age-appropriate book to babies, toddlers, and preschoolers at every well child visit starting at 6 months of age
- Providing anticipatory guidance to parents on the benefits of reading aloud to children starting at birth
- Having a literacy-rich waiting room area (which at times includes volunteers reading to the children)
The data supporting this very simple, inexpensive intervention is robust. Multiple studies have shown that children exposed to the ROR model have improved language skills when compared to children who are not. Parents also report a much higher frequency of reading with their children when exposed to ROR than parents who are not [26–28].
In a randomized controlled study of literacy promotion in Hispanic families, when parents were asked open-endedly “What are your 3 most favorite things to do with your child?,” parents who had received literacy-promoting anticipatory guidance and books reported “reading with my toddler” significantly more often than parents who had not (43% intervention vs. 13% controls). When asked about the frequency of reading to their toddlers, intervention parents were significantly more likely to report reading books with their children at least 3 days/week than controls (66% intervention vs. 24% controls). Applying a multiple logistic regression model controlling for child and parent age, parent reading habits, and English proficiency, we found that the odds of parents reading to their child at least 3 days/week were 10 times greater in intervention families (odds ratio [OR] 10.1, 95% confidence interval 4.0–25.6) than in controls [29].
In a parallel study with English-speaking low income families, when parents were asked open-endedly, “What are your child’s 3 most favorite activities?,” parents who had been exposed to the intervention, were significantly more likely to report “reading books” as one of their toddler’s 3 favorite activities than parents who were not exposed (27% intervention vs. 12% controls). Toddler expressive and receptive vocabulary scores were higher in intervention families and were associated with more frequent shared reading [30].
A multicenter study (19 clinical sites in 10 different states) that compared 730 children aged 6 to 72 months exposed to the ROR model with a comparison group of 917 matched children who did not participate in this literacy promoting model found significant associations between exposure to ROR and reading aloud as a favorite parent activity (adjusted OR 1.6, P < 0.001); reading aloud at bedtime (adjusted OR 1.5, P < 0.001); reading aloud 3 or more days per week (adjusted OR 1.8, P < 0.001); and ownership of 10 or more picture books (adjusted OR 1.6, P < 0.001) [31].
Across the world, others have been replicating and testing the ROR model. Interestingly, studies conducted in Taiwan and with immigrants from Latin America and Asia have all shown similar effects on parental literacy behaviors and on the development of children’s early oral language skills [32–35].
Parent-Child Bonding from Sharing Books
According to the 2014 AAP policy statement, literacy promotion is an essential component of pediatric primary care [3]. The statement emphasizes that parent-child shared reading is a “very personal and nurturing experience that promotes parent-child interaction, social-emotional development, and language and literacy skills during this critical period of early brain and child development.” It recognizes the importance of shared reading as a bonding experience that could start in early infancy. These early nurturing relationships are critical to promoting healthy child development [36].
Most studies of practice-based literacy promotion have asked parents what their favorite things are to do with their child. All of these studies have shown that parents who have received guidance around the importance of reading together and high-quality books to share with their infants, toddlers, and preschoolers include reading aloud as one of their 3 most favorite activities, compared to control families who did not receive this intervention [28–31]. When activities are favorites, they are enriched by this shared enjoyment and are far more likely to occur often and perhaps become treasured family routines. Children’s books and early play and discussions around the themes in these books stimulate increased interaction between caregivers and children [37]. These interactions build secure relationships that are key to children’s healthy cognitive, language, and social-emotional development [38–40].
The Effects on the Brain From Listening to Stories
In a recent study, 48 children aged 6 to 11 years were classified as early talkers (16), on-time talkers (16), or late talkers (16) by parental report [41]. Group assignments were based on whether the parent recalled their child making 2- to 3-word sentences early, on-time, or late. None of the “early talkers” had spoken their first sentences after 24 months, and none of the “late talkers” had spoken sentences before age 2. Utilizing functional MRI, researchers analyzed talker group differences in processing of speech and print and functional activation differences on auditory stimuli and when visualizing print. The groups were matched by age, gender, and performance IQ. This study showed strong group differences in the activation of several regions of the brain, including the left superior temporal gyrus, left putamen, globus pallidus, right putamen, left insula, and thalamus. In each of these areas, late talkers demonstrated significantly less activation that early talkers in both speech and print conditions (P < 0.001). Talker group status was strongly related to neural activation patterns during simple linguistic tasks. These cortical differences in activation are consistent with other studies that demonstrate the role of these regions in understanding speech [42] and processing print [43,44]. These findings highlight the importance of early language development on the formation of critical language and reading circuits and how these neural pathways are affected many years later [41].
In another study of nineteen 3- to 5-year-olds, researchers used functional MRI to examine the relationship between home reading environment and brain activity during a story listening task. The study showed that while listening to stories, children with greater home reading exposure exhibited higher activation of left-sided brain regions involved with processing of meaning. Higher reading exposure at home as measured by the StimQ-P Reading subscale score, was positively correlated with neural activation in the left-sided parietal-temporal-occipital association cortex, a region of the brain supporting semantic language processing, when controlling for household income (P < 0.05) [45].
Conclusion
Pediatric providers are in a unique position to impact a child’s life by promoting literacy starting at birth. The effects of shared reading and parent-child interactions on early language development, on the formation of brain circuitry, and on children’s ability to become better readers and arrive to school ready to learn is now known.
We have an obligation to not only make literacy promotion in pediatric encounters the standard of care, but to continue to expand these types of interventions to other settings to reach as many young children as possible. Children from disadvantaged socioeconomic backgrounds and those from immigrant families are at highest risk and should be the primary focus of our intervention efforts. However, data from the 2011–2012 National Survey of Children’s Health found that only 60% of US children raised in households with income > 400% of the federal poverty level were read to daily [46]. These data suggest that more affluent, professional families should also be counseled by their pediatricians about the benefits of shared reading and about the detrimental effects of “electronics” at this critical time in their child’s development.
More research is needed to fully understand the long-term impacts of literacy promotion interventions in primary care settings. Longitudinal studies directly measuring the potential effects of the ROR model on reading skills in 3rd grade, on high school graduation rates, and on other measures of social and academic success are lacking. However, the existing evidence suggests that this kind of program can fulfill the promise of child health supervision visits. While providing guidance and the tools aimed at improving the home environment, pediatric providers can shape the course of young children’s lives.
Corresponding author: Natalia Golova, MD, Hasbro Children’s Hospital, 593 Eddy St., Hasbro Lower Level, Providence, RI 02903, [email protected].
Financial disclosures: None.
1. Needlman R, Fried L, Morley D, et al. Clinic-based intervention to promote literacy. Am J Dis Child 1991;145:881–4.
2. Reach Out and Read: a national pediatric literacy program. Available at http://reachoutandread.org.
3. High PC, Klass P. Literacy promotion: an essential component of primary care pediatric practice. Council on Early Childhood. Pediatrics 2014;134:404–9.
4. Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 1997; 387:167–78.
5. Center on the Developing Child. The science of early childhood development (in brief); 2007. Accessed 6 May 2016 at www.developingchild.harvard.edu.
6. Connecting Science, Policy, and Practice: Zero to Three’s National Training Institute, 2015. Zero Three 2016;36(3).
7. Fox NA, Zeanah CH, Nelson CA. A matter of timing: enhancing positive change for the developing brain. Zero Three 2014;34(3):4–9.
8. Halfon N, Shulman E, Hochstein M. Brain development in early childhood. Technical report. UCLA Center for Healthier Children, Families and Communities. Aug 2001.
9. National Scientific Council on the Developing Child. The science of early childhood development: closing the gap between what we know and what we do. Center on the Developing Child. Harvard University; 2007.
10. Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Baltimore: Brookes; 1995.
11. Hart B, Risley TR. The early catastrophe: the 30 million word gap by age 3. Am Educator 2003;27:4–9.
12. The Annie E. Casey Foundation. Double jeopardy: how third grade reading skills and poverty influence high school graduation. 2012. Accessed 21 Feb 2016 at www.aecf.org/resources/double-jeopardy.
13. The Annie E. Casey Foundation. Early warning confirmed: a research update on third grade reading. 2013 Nov. Accessed 23 Feb 2016 at www.aecf.org/m/resourcedoc/AECF-EarlyWarningConfirmed-2013.pdf
14. Heckman J. The economics of inequality: the value of early childhood education. Am Educator 2011;47:31–5.
15. Christakis DA. The effects of infant media usage: what do we know and what should we learn? Acta Paediatr 2009;98: 8–16.
16. American Academy of Pediatrics, Council on Communications and Media. Policy statement. Media use by children younger than 2 years. Pediatrics 2011;128:1040–5.
17. Linebarger DL, Walker D. Infants’ and toddlers’ television viewing and language outcomes. Am Behav Sci 2005;48:624–45.
18. Masako T, Okuma K, Kyoshima K. Television viewing and reduced parental utterance, and delayed speech development in infants and young children. Arch Pediatr Adolesc Med 2007;161:618–9.
19. Rideout VJ, Hamel E. The media family: electronic media in the lives of infants, toddlers, preschoolers, and their parents. Menlo Park, CA: Kaiser Family Foundation; 2006.
20. Sosa AV. Association of the type of toy used during play with the quantity and quality of parent-infant communication. JAMA Pediatr 2016;170:132–7.
21. Vandewater EA, Bickham DS, Lee JH et al. When the television is always on: heavy television exposure and young children’s development. Am Behav Sci 2005;48:562–77.
22. Zimmerman FJ, Christakis DA, Meltzoff AN. Associations between media viewing and language development in children under age two years. J Pediatr 2007;151:364–8.
23. Chonchaiya W, Pruksananonda C. Television viewing associates with delayed language development. Acta Paediatr 2008;97:977–82.
24. Robb MB, Richert RA, Wartella EA. Just a talking book? Word learning from watching baby videos. Br J Dev Psychol 2009;27(Pt 1):27–45.
25. DeLoache JS, Chiong C, Sherman K, et al. Do babies learn from baby media? Psychol Sci 2010;21:1570–4.
26. Mendelsohn A, Mogliner L, Dreyer B, et al. The impact of a clinic-based literacy intervention on language development in inner-city preschool children. Pediatrics 2001;107:130–4.
27. Mendelsohn AL. Promoting language and literacy through reading aloud: the role of the pediatrician. Curr Probl Pediatr Adolesc Health Care 2002;32:183–210.
28. High P, Hopman M, LaGasse L, et al. Evaluation of a clinic-based program to promote book sharing and bedtime routines among low-income urban families with young children. Arch Pediatr Adolesc Med 1998;152:459–65.
29. Golova N, Alario A, Vivier P, et al. Literacy promotion for Hispanic families in a primary care setting: a randomized controlled trial. Pediatrics 1999;103:993–7.
30. High PC, LaGasse L, Becker S, et al. Literacy promotion in primary care pediatrics: can we make a difference? Pediatrics 2000;105:927–34.
31. Needlman R, Toker KH, Dreyer BP, et al. Effectiveness of a primary care intervention to support reading aloud: a multicenter evaluation. Ambul Pediatr 2005;5:209–15.
32. Wu SC, Lue HC, Tseng LL. A pediatric clinic-based approach to early literacy promotion--experience in a well-baby clinic in Taiwan. J Formos Med Assoc 2012;111:258–64.
33. Sanders LM, Gershon TD, Huffman LC, et al. Prescribing books for immigrant children: a pilot study to promote emergent literacy among the children of Hispanic immigrants. Arch Pediatr Adolesc Med 2000;154:771–7.
34. Kitabayashi KM, Huang GY, Linskey KR, et al. Parent-child reading interactions among English and English as a second language speakers in an underserved pediatric clinic in Hawai’i. Hawaii Med J 2008;67:260–3.
35. Festa N, Loftus PD, Cullen MR, Mendoza FS. Disparities in early exposure to book sharing within immigrant families. Pediatrics. 2014;134:e162–8.
36. Shonkoff JP, Phillips DA, editors. From neurons to neighborhoods: the science of early childhood development. National Research Council (US) and Institute of Medicine (US) Committee on Integrating the Science of Early Childhood Development. Washington, DC: National Academies Press; 2000.
37. Neuman SB. Guiding young children’s participation in early literacy development: a family literacy program for adolescent mothers. Early Child Dev Care 1997;127:119–29.
38. Tomopoulos S, Dreyer BP, Tamis-LeMonda C, et al. Books, toys, parent-child interaction, and development in young Latino children. Ambul Pediatr 2006;6:72–8.
39. Mendelsohn AL, Huberman HS, Berkule SB, et al. Primary care strategies for promoting parent-child interactions and school readiness in at-risk families: the Bellevue Project for Early Language, Literacy, and Education Success. Arch Pediatr Adolesc Med 2011;165:33–41.
40. Ginsburg K; American Academy of Pediatrics, Committee on Communications, Committee on Psychosocial Aspects of Child and Family Health. The importance of play in promoting healthy child development and maintaining strong parent-child bonds. Pediatrics 2007;119:182–91.
41. Preston JL, Frost SJ, Mencl WE, et al. Early and late talkers: school-age language, literacy and neurolinguistic differences. Brain 2010;133:2185–95.
42. Hugdahl K, Gundersen H, Brekke C, et al. fMRI Brain activation in a Finnish family with specific language impairment compared with a normal control group. J Speech Lang Hear Res 2004;47:162–72.
43. Pugh KR, Mencl WE, Jenner AR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 2000;6:207–13.
44. Pugh KR, Mencl WE, Jenner AR, et al. Neurobiological studies of reading and reading disability. J Commun Disord 2001;34:479–92.
45. Hutton JS, Horowitz-Kraus T, Mendelsohn AL, et al. Home reading environment and brain activation in preschool children listening to stories. Pediatrics 2015;136:466–78.
46. Data Resource Center for Child and Adolescent Health. 2011/12 National Survey of Children’s Health. Accessed 28 Feb 2016 at www.nschdata.org.
1. Needlman R, Fried L, Morley D, et al. Clinic-based intervention to promote literacy. Am J Dis Child 1991;145:881–4.
2. Reach Out and Read: a national pediatric literacy program. Available at http://reachoutandread.org.
3. High PC, Klass P. Literacy promotion: an essential component of primary care pediatric practice. Council on Early Childhood. Pediatrics 2014;134:404–9.
4. Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 1997; 387:167–78.
5. Center on the Developing Child. The science of early childhood development (in brief); 2007. Accessed 6 May 2016 at www.developingchild.harvard.edu.
6. Connecting Science, Policy, and Practice: Zero to Three’s National Training Institute, 2015. Zero Three 2016;36(3).
7. Fox NA, Zeanah CH, Nelson CA. A matter of timing: enhancing positive change for the developing brain. Zero Three 2014;34(3):4–9.
8. Halfon N, Shulman E, Hochstein M. Brain development in early childhood. Technical report. UCLA Center for Healthier Children, Families and Communities. Aug 2001.
9. National Scientific Council on the Developing Child. The science of early childhood development: closing the gap between what we know and what we do. Center on the Developing Child. Harvard University; 2007.
10. Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Baltimore: Brookes; 1995.
11. Hart B, Risley TR. The early catastrophe: the 30 million word gap by age 3. Am Educator 2003;27:4–9.
12. The Annie E. Casey Foundation. Double jeopardy: how third grade reading skills and poverty influence high school graduation. 2012. Accessed 21 Feb 2016 at www.aecf.org/resources/double-jeopardy.
13. The Annie E. Casey Foundation. Early warning confirmed: a research update on third grade reading. 2013 Nov. Accessed 23 Feb 2016 at www.aecf.org/m/resourcedoc/AECF-EarlyWarningConfirmed-2013.pdf
14. Heckman J. The economics of inequality: the value of early childhood education. Am Educator 2011;47:31–5.
15. Christakis DA. The effects of infant media usage: what do we know and what should we learn? Acta Paediatr 2009;98: 8–16.
16. American Academy of Pediatrics, Council on Communications and Media. Policy statement. Media use by children younger than 2 years. Pediatrics 2011;128:1040–5.
17. Linebarger DL, Walker D. Infants’ and toddlers’ television viewing and language outcomes. Am Behav Sci 2005;48:624–45.
18. Masako T, Okuma K, Kyoshima K. Television viewing and reduced parental utterance, and delayed speech development in infants and young children. Arch Pediatr Adolesc Med 2007;161:618–9.
19. Rideout VJ, Hamel E. The media family: electronic media in the lives of infants, toddlers, preschoolers, and their parents. Menlo Park, CA: Kaiser Family Foundation; 2006.
20. Sosa AV. Association of the type of toy used during play with the quantity and quality of parent-infant communication. JAMA Pediatr 2016;170:132–7.
21. Vandewater EA, Bickham DS, Lee JH et al. When the television is always on: heavy television exposure and young children’s development. Am Behav Sci 2005;48:562–77.
22. Zimmerman FJ, Christakis DA, Meltzoff AN. Associations between media viewing and language development in children under age two years. J Pediatr 2007;151:364–8.
23. Chonchaiya W, Pruksananonda C. Television viewing associates with delayed language development. Acta Paediatr 2008;97:977–82.
24. Robb MB, Richert RA, Wartella EA. Just a talking book? Word learning from watching baby videos. Br J Dev Psychol 2009;27(Pt 1):27–45.
25. DeLoache JS, Chiong C, Sherman K, et al. Do babies learn from baby media? Psychol Sci 2010;21:1570–4.
26. Mendelsohn A, Mogliner L, Dreyer B, et al. The impact of a clinic-based literacy intervention on language development in inner-city preschool children. Pediatrics 2001;107:130–4.
27. Mendelsohn AL. Promoting language and literacy through reading aloud: the role of the pediatrician. Curr Probl Pediatr Adolesc Health Care 2002;32:183–210.
28. High P, Hopman M, LaGasse L, et al. Evaluation of a clinic-based program to promote book sharing and bedtime routines among low-income urban families with young children. Arch Pediatr Adolesc Med 1998;152:459–65.
29. Golova N, Alario A, Vivier P, et al. Literacy promotion for Hispanic families in a primary care setting: a randomized controlled trial. Pediatrics 1999;103:993–7.
30. High PC, LaGasse L, Becker S, et al. Literacy promotion in primary care pediatrics: can we make a difference? Pediatrics 2000;105:927–34.
31. Needlman R, Toker KH, Dreyer BP, et al. Effectiveness of a primary care intervention to support reading aloud: a multicenter evaluation. Ambul Pediatr 2005;5:209–15.
32. Wu SC, Lue HC, Tseng LL. A pediatric clinic-based approach to early literacy promotion--experience in a well-baby clinic in Taiwan. J Formos Med Assoc 2012;111:258–64.
33. Sanders LM, Gershon TD, Huffman LC, et al. Prescribing books for immigrant children: a pilot study to promote emergent literacy among the children of Hispanic immigrants. Arch Pediatr Adolesc Med 2000;154:771–7.
34. Kitabayashi KM, Huang GY, Linskey KR, et al. Parent-child reading interactions among English and English as a second language speakers in an underserved pediatric clinic in Hawai’i. Hawaii Med J 2008;67:260–3.
35. Festa N, Loftus PD, Cullen MR, Mendoza FS. Disparities in early exposure to book sharing within immigrant families. Pediatrics. 2014;134:e162–8.
36. Shonkoff JP, Phillips DA, editors. From neurons to neighborhoods: the science of early childhood development. National Research Council (US) and Institute of Medicine (US) Committee on Integrating the Science of Early Childhood Development. Washington, DC: National Academies Press; 2000.
37. Neuman SB. Guiding young children’s participation in early literacy development: a family literacy program for adolescent mothers. Early Child Dev Care 1997;127:119–29.
38. Tomopoulos S, Dreyer BP, Tamis-LeMonda C, et al. Books, toys, parent-child interaction, and development in young Latino children. Ambul Pediatr 2006;6:72–8.
39. Mendelsohn AL, Huberman HS, Berkule SB, et al. Primary care strategies for promoting parent-child interactions and school readiness in at-risk families: the Bellevue Project for Early Language, Literacy, and Education Success. Arch Pediatr Adolesc Med 2011;165:33–41.
40. Ginsburg K; American Academy of Pediatrics, Committee on Communications, Committee on Psychosocial Aspects of Child and Family Health. The importance of play in promoting healthy child development and maintaining strong parent-child bonds. Pediatrics 2007;119:182–91.
41. Preston JL, Frost SJ, Mencl WE, et al. Early and late talkers: school-age language, literacy and neurolinguistic differences. Brain 2010;133:2185–95.
42. Hugdahl K, Gundersen H, Brekke C, et al. fMRI Brain activation in a Finnish family with specific language impairment compared with a normal control group. J Speech Lang Hear Res 2004;47:162–72.
43. Pugh KR, Mencl WE, Jenner AR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 2000;6:207–13.
44. Pugh KR, Mencl WE, Jenner AR, et al. Neurobiological studies of reading and reading disability. J Commun Disord 2001;34:479–92.
45. Hutton JS, Horowitz-Kraus T, Mendelsohn AL, et al. Home reading environment and brain activation in preschool children listening to stories. Pediatrics 2015;136:466–78.
46. Data Resource Center for Child and Adolescent Health. 2011/12 National Survey of Children’s Health. Accessed 28 Feb 2016 at www.nschdata.org.
2016 Update on infectious disease
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.
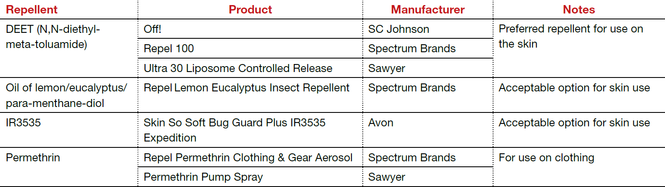
Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.

Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.

Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
In this article
• Azithromycin vs doxycycline for chlamydia
• Insect repellents to prevent Zika virus
Medical Mimics of Psychiatric Conditions, Part 2
Although the emergency physician (EP) typically encounters common conditions such as chest pain, urinary tract infection, and gastroenteritis, many other clinical presentations can confound diagnosis of the true underlying condition. This may be the case with a patient who presents with apparent psychiatric symptoms that are actually masking an acute medical condition. For example, a patient who appears to be depressed may actually be exhibiting early signs of dementia. Likewise, a manic patient may not have a true underlying psychiatric disorder but rather rhabdomyolysis and hyperthermia from ingesting an illicit substance such as synthetic cathinones (“bath salts”).
Part 1 of this series reviewed psychiatric presentations caused by underlying infectious, pharmacological withdrawal, metabolic, autoimmune, traumatic, and central nervous system etiologies (Emerg Med. 2016;48[5]:202-211). Part 2 covers psychiatric presentations related to dementia, cancer, cardiac disease, nutritional deficiencies, endocrine disorders, or toxins (Table 1).
Case Scenarios
Case 1
A 62-year-old man with a history of hypertension, hyperlipidemia, and past alcohol abuse presented to the ED with reported mental status changes after he was pulled over by police for driving the wrong way down the highway. On presentation, the patient’s vital signs were normal. When questioned, the patient was alert and fully oriented and believed the officers were mistaken about what was reported. He denied any recent illness and had a normal physical examination, including neurological examination.
A brief work-up was ordered and the patient passed the time by politely flirting with the nurses. When his wife arrived at the ED, she was relieved that her husband seemed to be all right. She confirmed that the patient had not consumed any alcohol in years. The patient, meanwhile, playfully minimized his wife’s concern at his presence in the ED. A full toxicology screen, laboratory evaluation, and head computed tomography (CT) scan were ordered.
Case 2
A 48-year-old woman with a history of anxiety disorder, depression, and diabetes mellitus presented to the ED with a 2-hour history of chest pain. She stated that the pain had started toward the end of a heated argument with her son. The patient was escorted into the examination room by hospital security because she was still agitated and kept yelling at her son. On examination the patient was tachycardic (110 beats/minute), diaphoretic, and crying. During the examination, she asked the EP for a “Xanax”; her son further noted that this would help his mother’s condition.
The patient repeatedly claimed she could not breathe and could not lie flat on the stretcher. After verbal de-escalation, she cooperated with the electrocardiography (ECG) technician and phlebotomist. Her ECG showed nonspecific ST changes with no prior study for comparison. While glaring at her son, she maintained that she had constant chest pain.
Dementia
Alzheimer’s Disease
Alzheimer’s disease (AD), the most common cause of dementia, is a chronic neurodegenerative disease characterized by an insidiously progressive cognitive decline and loss of function. There is considerable apparent variability in the early signs of the disease, and recent literature has suggested that the manifestation of initial symptoms may be age-dependent. Younger patients tend to present with non-memory cognitive changes such as problem-solving difficulties, as well as personality changes and behavioral symptoms of depression, apathy, and withdrawal.1
Lewy Body Dementia
Lewy body dementia (LBD) is a chronic neurodegenerative disease with a presentation that overlaps substantially with AD. However, LBD is associated with a significantly more rapid course than AD and presents more frequently with visual hallucinations or illusions due to specific visuospatial dysfunction.2
Frontotemporal Dementia
Frontotemporal dementia is a comparatively rare chronic neurodegenerative disease characterized by early-onset memory impairment with cognitive decline, as well as behavioral changes such as disinhibition, emotional blunting, and language difficulty. Initial presentations can also include atypical features such as paranoia or delusion, and misdiagnosis as a primary psychiatric problem is common.3
Cancer
Brain Tumor
Primary and metastatic brain tumors classically present with either focal neurological signs or less specific symptoms such as headaches, seizures, or syncope. Additionally, central nervous system (CNS) tumors can also initially present with primary psychiatric complaints (eg, personality changes, depression, mania, panic attacks, auditory or visual hallucinations). Patients with a brain neoplasm who are initially misdiagnosed with a primary psychiatric disorder face significant delays in proper diagnosis and treatment, leading to increased morbidity. To correctly diagnose the true cause as soon as possible, early imaging is recommended for patients who present with psychiatric symptoms that are abrupt in onset, atypical in presentation, resistant to conventional treatments, or associated with a change in headache pattern.4
Paraneoplastic Limbic Encephalitis
Paraneoplastic limbic encephalitis (PLE) is a rare neurological consequence of certain cancers. Although PLE most commonly occurs in patients with small cell lung cancer, the condition has also been reported (though less frequently) in cases of esophageal adenocarcinoma, ovarian teratoma, metastatic breast cancer, and germ cell testicular cancer.5 This disease overlaps substantially with anti-N-methyl-D-aspartate (anti-NMDA) receptor encephalitis. Moreover, PLE can present initially with prominent neuropsychiatric symptoms such as confusion, cognitive problems, behavioral changes, irritability, depression, or frank psychosis with hallucinations. Paraneoplastic limbic encephalitis can occur early in the course of cancer—often before other systemic signs appear—and its significance is often only recognized in retrospect or postmortem. A higher index of suspicion for the disorder may lead to earlier detection of treatable cancers.
Malignant Meningitis
Malignant meningitis is the metastatic spread of a primary solid tumor to the leptomeninges. It can present as a wide variety of neuropsychiatric complaints, including depression, anxiety, disorientation, and paranoia. Diagnosis can often be made through lumbar puncture. Malignant meningitis should be considered in the differential diagnosis of new psychiatric symptoms in a patient with a history of cancer—even in the absence of focal neurological deficits or meningeal signs.6
Pancreatic Insulinoma
Pancreatic insulinoma is a rare, potentially curable endocrine tumor that can present initially with vague psychiatric complaints such as irrational behavior, confusion, depression, or anxiety. In up to 64% of patients, insulinomas are misdiagnosed as primary neurological or psychiatric disease, which can delay potentially curative surgery—sometimes for years.7 The EP should suspect pancreatic insulinoma in any patient who presents with psychiatric symptoms and unexplained episodes of hypoglycemia.7
Cardiac Disease
Transient Left Ventricular Apical Ballooning Syndrome
Transient left ventricular apical ballooning syndrome (TLVABS), first identified in Japan as Takotsubo syndrome, has more recently been recognized worldwide as overlapping with the classic broken heart syndrome. In postmenopausal women, TLVABS appears to follow a catecholamine surge triggered by extreme emotional stress, resulting in an acute coronary artery spasm. Researchers have hypothesized that there may be a link between TLVABS and dissociative amnesia, which is also thought to result from a catecholamine surge in response to emotional stress.8
Nutritional Deficiencies
Wernicke/Korsakoff Syndrome and Thiamine Deficiency
Wernicke encephalopathy and Korsakoff syndrome (WKS) represent a spectrum of neurodegenerative disorders caused by thiamine deficiency. The condition typically occurs in malnourished alcoholic patients, manifesting as a triad of mental status changes, ophthalmoplegia, and ataxia. Recent research has suggested that WKS is more common than previously thought, is not confined exclusively to alcoholic patients, is unlikely to present with the full classic triad, and is typically only diagnosed postmortem.9
Nonalcoholic WKS tends to occur in younger female patients with a wide array of conditions that affect nutrition (eg, gastrointestinal malignancy, bariatric surgery, hyperemesis gravidarum, anorexia nervosa).9 In a patient with chronic alcoholism, application of the Caine criteria (any two of the following findings: ophthalmoplegia, ataxia, even mild memory impairment or confusion without another cause, evidence of malnutrition) has been shown to be more sensitive and specific than the classic triad.10
Subacute Combined Degeneration
Patients with subacute combined degeneration and extrapyramidal symptoms due to B12 (cobalamin) deficiency are well documented. However, patients with B12 deficiency can also present with mood disorders, acute psychosis, psychotic depression, or paranoid hallucinations. The EP should always consider vitamin B12 deficiency as an important, reversible cause of altered mental status—even in the absence of megaloblastic anemia—especially in patients with celiac disease or anorexia nervosa, and in teenagers and those who are vegans/vegetarians.11
Zinc/Vitamin D Deficiency
Zinc and vitamin D deficiency are both highly prevalent in geriatric patients and have been associated with a range of psychiatric complaints, including depressive disorders, bipolar disorder, and psychotic episodes. Though the neurodevelopmental effects of long-term deficiency of these nutrients are well documented in pediatric patients, the role and relationship to acute psychiatric complaints in elderly patients remain unclear.12,13
Endocrine Disorders
Hypothyroidism
Hypothyroidism is a commonly encountered endocrine disruption that classically presents with fatigue, cold insensitivity, weight gain, and thinning hair. Thyroid dysfunction can result in various neuropsychiatric presentations, including mood disorders, cognitive impairment, and exacerbation of underlying psychiatric disorders. Though rare, primary hypothyroidism can present as mania, psychosis, and auditory or visual hallucinations, a phenomenon termed “myxedema madness.” Myxedema madness typically occurs in older women, but has also been described in adolescents and as a postoperative complication of thyroidectomy.14
Hyperthyroidism
Hyperthyroidism classically presents with tachycardia, nervousness or anxiety, heat insensitivity, and weight loss despite increased appetite. Involvement of the CNS in thyrotoxicosis is rare, but when present, it is a significant predictor of mortality. Neuropsychiatric presentations of hyperthyroidism or thyroid storm vary widely, and have been reported to include psychosis, catatonia, auditory hallucinations, delusional parasitosis, new-onset sleepwalking, dissociative disorder, and suicide attempts.15
Steroid Dysregulation
Steroid dysregulation, either endogenous or iatrogenic in nature, has been reported to cause neuropsychiatric symptoms. Major depression with psychotic features can be an initial presentation of Cushing disease, especially in the presence of other systemic signs.16 Adrenal insufficiency has also been shown to cause severe psychotic disorder.17
Chronic treatment with exogenous corticosteroids can cause a recurrent steroid psychosis, primarily manifesting as subacute mania with psychotic features. Treatment of acute adrenal crisis can also cause an acute steroid psychosis with hallucinations, delusions, and dangerous behavior.17
Parathyroid Dysregulation
Elevated calcium levels caused by primary hyperparathyroidism can present as cognitive slowing, reductions in psychomotor speed, memory impairment, and depression. While the disorder is most prevalent in older women, it has been reported in adolescents, and often remains undiagnosed in younger patients until end-organ damage occurs.18 Hypoparathyroidism has also been reported to cause mood disorders, which can occur with or without the classic symptoms of hypocalcemia (eg, tetany, seizures, dementia, and parkinsonism).18
Pheochromocytoma
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that causes sympathetic hyperactivity by the release of large amounts of catecholamines. Pheochromocytoma is well-reported to present with nervousness, anxiety, panic attacks, or depression.19
Gonadal Hormone Dysregulation
Gonadal hormone dysregulation can be either congenital or acquired and is typically caused by a pituitary tumor or traumatic brain injury. Thought to be a result of dopaminergic hyperactivity, acute psychosis can develop in cases of hypogonadotropic hypogonadism, hypopituitarism, and/or hyperprolactinemia.20 There is a high incidence of psychotic manifestations in hypogonadal disorders such as Klinefelter syndrome and Prader-Willi syndrome.
Toxins
Many toxins can cause altered mental status and psychiatric manifestations. The administration of these toxins can be iatrogenic, related to prescribed use, or overdose—whether accidental, recreational, or intentional (eg, suicide attempt). Table 2 lists common drugs and toxins associated with psychiatric symptoms.21
Synthetic Drugs
The use of numerous unregulated, synthetic analogues of popular recreational drugs has greatly increased over the last several years. Synthetic cannabinoids are available under a variety of names (eg, “Spice,” “K2”) and can cause prominent psychiatric symptoms, including new-onset psychosis, paranoid delusions, hallucinations, and suicide ideation or attempt. While most clinical symptoms are self-limited and require only supportive care, more serious complications have been reported, including myocardial infarction, ischemic stroke, and acute kidney injury.22 Synthetic cathinones (bath salts) can also cause autonomic instability and prominent acute psychosis, sometimes creating a clinical picture indistinguishable from excited delirium syndrome.23
Heavy Metals
Chronic toxicity of many heavy metals is implicated in abnormal neurodevelopment, behavioral disturbances, and progression of neurodegenerative diseases. Recent literature has also implicated acute metal overload in new-onset impaired emotional behavior, though the mechanism is not currently well understood.24
Case Scenarios Continued
Case 1
[The 62-year-old man with altered mental status.]
The patient’s laboratory evaluation and toxicology screen were negative, including a screen for alcohol. He remained jovial but otherwise in no distress. Since the noncontrast head CT scan showed a subtle asymmetry in the frontal lobes, a magnetic resonance imaging (MRI) study was recommended. The brain MRI showed a 5-cm mass in the right frontal lobe with surrounding edema, findings consistent with glioblastoma multiforme. A neurosurgeon was consulted, and the patient was admitted to the intensive care unit.
Case Scenarios Continued
Case 2
[The 48-year-old woman with chest pain.]
The patient received a dose of oral lorazepam, after which she began to feel less anxious, and her chest pain and shortness of breath also improved slightly. The repeat ECG showed worsening of the ST segment changes. The laboratory evaluation was negative. The patient’s son asked if he could take his mother home for what he felt was much needed rest. The EP, however, ordered a stat two-dimensional echocardiogram (ECHO) and repeat troponin level test. The repeat troponin test was positive, and the ECHO was remarkable for a decreased left ventricular ejection fraction of 15%, with apical ballooning. These findings were consistent with stress cardiomyopathy (Takotsubo syndrome). The patient was admitted to the cardiology service and given a beta blocker and an angiotensin-converting enzyme inhibitor.
After a normal coronary angiogram, the patient developed cardiogenic shock and was intubated. Seven days later, she was extubated and transferred to inpatient rehabilitation services where she also received an assessment and treatment for her underlying depression. Eight weeks postdiagnosis, the patient’s ejection fraction had returned to 50%, and she was close to her baseline exercise tolerance.
1. Barnes J, Dickerson BC, Frost C, Jiskoot LC, Wolk D, van der Flier WM. Alzheimer’s disease first symptoms are age dependent: Evidence from the NACC dataset. Alzheimers Dement. 2015;11(11):1349-1357.
2. Yoshizawa H, Vonsattel JP, Honig LS. Early neuropsychological discriminants for Lewy body disease: an autopsy series. J Neurol Neurosurg Psychiatry. 2013;84(12):1326-1330.
3. Iroka N, Jehangir W, Ii JL, Pattan V, Yousif A, Mishra AK. Paranoid personality masking an atypical case of frontotemporal dementia. J Clin Med Res. 2015;7(5):364-366.
4. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
5. Said S, Cooper CJ, Reyna E, Alkhateeb H, Diaz J, Nahleh Z. Paraneoplastic limbic encephalitis, an uncommon presentation of a common cancer: Case report and discussion. Am J Case Rep. 2013;14:391-394.
6. Weitzner MA, Olofsson SM, Forman AD. Patients with malignant meningitis presenting with neuropsychiatric manifestations. Cancer. 1995;76(10):1804-1808.
7. Ding Y, Wang S, Liu J. Neuropsychiatric profiles of patients with insulinomas. Eur Neurol. 2010;63(1):48-51.
8. Toussi A, Bryk J, Alam A. Forgetting heart break: a fascinating case of transient left ventricular apical ballooning syndrome associated with dissociative amnesia. Gen Hosp Psychiatry. 2014;36(2):225-227.
9. Scalzo SJ, Bowden SC, Ambrose ML, Whelan G, Cook MJ. Wernicke-Korsakoff syndrome not related to alcohol use: a systematic review. J Neurol Neurosurg Psychiatry. 2015;86(12):1362-1368.
10. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
11. Issac TG, Soundarya S, Christopher R, Chandra SR. Vitamin B12 deficiency: an important reversible co-morbidity in neuropsychiatric manifestations. Indian J Psychol Med. 2015;37(1):26-29.
12. Grønli O, Kvamme JM, Friborg O, Wynn R. Zinc deficiency is common in several psychiatric disorders. PLoS One. 2013;8(12):e82793.
13. Grønli O, Kvamme JM, Jorde R, Wynn R. Vitamin D deficiency is common in psychogeriatric patients, independent of diagnosis. BMC Psychiatry. 2014;14:134.
14. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry. 2003;5(6):260-266.
15. Swee du S, Chng CL, Lim A. Clinical characteristics and outcome of thyroid storm: a case series and review of neuropsychiatric derangements in thyrotoxicosis. Endocr Pract. 2015;21(2):182-189.
16. Tang A, O’Sullivan AJ, Diamond T, Gerard A, Campbell P. Psychiatric symptoms as a clinical presentation of Cushing’s syndrome. Ann Gen Psychiatry. 2013;12(1):23.
17. Farah Jde L, Lauand CV, Chequi L, et al. Severe psychotic disorder as the main manifestation of adrenal insufficiency. Case Rep Psychiatry. 2015;2015:512430.
18. Rice T, Azova S, Coffey BJ. Negative symptoms in a depressed teen? Primary hyperparathyroidism and its psychiatric manifestations. J Child Adolesc Psychopharmacol. 2015;25(8):653-655.
19. Zardawi IM. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
20. Kate S, Dhanwal DK, Kumar S, Bharti P. Acute psychosis as a presentation of hypopituitarism. BMJ Case Rep. 2013;2013.
21. Abramowicz M. Drugs that may cause psychiatric symptoms. Med Lett Drugs Ther. 2008;50(1301-1302):100-103.
22. Tait RJ, Caldicott D, Mountain D, Hill SL, Lenton S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila). 2016;54(1):1-13.
23. Karch SB. Cathinone neurotoxicity (“The “3Ms”). Curr Neuropharmacol. 2015;13(1): 21-25.
24. Menon AV, Chang J, Kim J. Mechanisms of divalent metal toxicity in affective disorders. Toxicology. 2016;339:58-72.
Although the emergency physician (EP) typically encounters common conditions such as chest pain, urinary tract infection, and gastroenteritis, many other clinical presentations can confound diagnosis of the true underlying condition. This may be the case with a patient who presents with apparent psychiatric symptoms that are actually masking an acute medical condition. For example, a patient who appears to be depressed may actually be exhibiting early signs of dementia. Likewise, a manic patient may not have a true underlying psychiatric disorder but rather rhabdomyolysis and hyperthermia from ingesting an illicit substance such as synthetic cathinones (“bath salts”).
Part 1 of this series reviewed psychiatric presentations caused by underlying infectious, pharmacological withdrawal, metabolic, autoimmune, traumatic, and central nervous system etiologies (Emerg Med. 2016;48[5]:202-211). Part 2 covers psychiatric presentations related to dementia, cancer, cardiac disease, nutritional deficiencies, endocrine disorders, or toxins (Table 1).
Case Scenarios
Case 1
A 62-year-old man with a history of hypertension, hyperlipidemia, and past alcohol abuse presented to the ED with reported mental status changes after he was pulled over by police for driving the wrong way down the highway. On presentation, the patient’s vital signs were normal. When questioned, the patient was alert and fully oriented and believed the officers were mistaken about what was reported. He denied any recent illness and had a normal physical examination, including neurological examination.
A brief work-up was ordered and the patient passed the time by politely flirting with the nurses. When his wife arrived at the ED, she was relieved that her husband seemed to be all right. She confirmed that the patient had not consumed any alcohol in years. The patient, meanwhile, playfully minimized his wife’s concern at his presence in the ED. A full toxicology screen, laboratory evaluation, and head computed tomography (CT) scan were ordered.
Case 2
A 48-year-old woman with a history of anxiety disorder, depression, and diabetes mellitus presented to the ED with a 2-hour history of chest pain. She stated that the pain had started toward the end of a heated argument with her son. The patient was escorted into the examination room by hospital security because she was still agitated and kept yelling at her son. On examination the patient was tachycardic (110 beats/minute), diaphoretic, and crying. During the examination, she asked the EP for a “Xanax”; her son further noted that this would help his mother’s condition.
The patient repeatedly claimed she could not breathe and could not lie flat on the stretcher. After verbal de-escalation, she cooperated with the electrocardiography (ECG) technician and phlebotomist. Her ECG showed nonspecific ST changes with no prior study for comparison. While glaring at her son, she maintained that she had constant chest pain.
Dementia
Alzheimer’s Disease
Alzheimer’s disease (AD), the most common cause of dementia, is a chronic neurodegenerative disease characterized by an insidiously progressive cognitive decline and loss of function. There is considerable apparent variability in the early signs of the disease, and recent literature has suggested that the manifestation of initial symptoms may be age-dependent. Younger patients tend to present with non-memory cognitive changes such as problem-solving difficulties, as well as personality changes and behavioral symptoms of depression, apathy, and withdrawal.1
Lewy Body Dementia
Lewy body dementia (LBD) is a chronic neurodegenerative disease with a presentation that overlaps substantially with AD. However, LBD is associated with a significantly more rapid course than AD and presents more frequently with visual hallucinations or illusions due to specific visuospatial dysfunction.2
Frontotemporal Dementia
Frontotemporal dementia is a comparatively rare chronic neurodegenerative disease characterized by early-onset memory impairment with cognitive decline, as well as behavioral changes such as disinhibition, emotional blunting, and language difficulty. Initial presentations can also include atypical features such as paranoia or delusion, and misdiagnosis as a primary psychiatric problem is common.3
Cancer
Brain Tumor
Primary and metastatic brain tumors classically present with either focal neurological signs or less specific symptoms such as headaches, seizures, or syncope. Additionally, central nervous system (CNS) tumors can also initially present with primary psychiatric complaints (eg, personality changes, depression, mania, panic attacks, auditory or visual hallucinations). Patients with a brain neoplasm who are initially misdiagnosed with a primary psychiatric disorder face significant delays in proper diagnosis and treatment, leading to increased morbidity. To correctly diagnose the true cause as soon as possible, early imaging is recommended for patients who present with psychiatric symptoms that are abrupt in onset, atypical in presentation, resistant to conventional treatments, or associated with a change in headache pattern.4
Paraneoplastic Limbic Encephalitis
Paraneoplastic limbic encephalitis (PLE) is a rare neurological consequence of certain cancers. Although PLE most commonly occurs in patients with small cell lung cancer, the condition has also been reported (though less frequently) in cases of esophageal adenocarcinoma, ovarian teratoma, metastatic breast cancer, and germ cell testicular cancer.5 This disease overlaps substantially with anti-N-methyl-D-aspartate (anti-NMDA) receptor encephalitis. Moreover, PLE can present initially with prominent neuropsychiatric symptoms such as confusion, cognitive problems, behavioral changes, irritability, depression, or frank psychosis with hallucinations. Paraneoplastic limbic encephalitis can occur early in the course of cancer—often before other systemic signs appear—and its significance is often only recognized in retrospect or postmortem. A higher index of suspicion for the disorder may lead to earlier detection of treatable cancers.
Malignant Meningitis
Malignant meningitis is the metastatic spread of a primary solid tumor to the leptomeninges. It can present as a wide variety of neuropsychiatric complaints, including depression, anxiety, disorientation, and paranoia. Diagnosis can often be made through lumbar puncture. Malignant meningitis should be considered in the differential diagnosis of new psychiatric symptoms in a patient with a history of cancer—even in the absence of focal neurological deficits or meningeal signs.6
Pancreatic Insulinoma
Pancreatic insulinoma is a rare, potentially curable endocrine tumor that can present initially with vague psychiatric complaints such as irrational behavior, confusion, depression, or anxiety. In up to 64% of patients, insulinomas are misdiagnosed as primary neurological or psychiatric disease, which can delay potentially curative surgery—sometimes for years.7 The EP should suspect pancreatic insulinoma in any patient who presents with psychiatric symptoms and unexplained episodes of hypoglycemia.7
Cardiac Disease
Transient Left Ventricular Apical Ballooning Syndrome
Transient left ventricular apical ballooning syndrome (TLVABS), first identified in Japan as Takotsubo syndrome, has more recently been recognized worldwide as overlapping with the classic broken heart syndrome. In postmenopausal women, TLVABS appears to follow a catecholamine surge triggered by extreme emotional stress, resulting in an acute coronary artery spasm. Researchers have hypothesized that there may be a link between TLVABS and dissociative amnesia, which is also thought to result from a catecholamine surge in response to emotional stress.8
Nutritional Deficiencies
Wernicke/Korsakoff Syndrome and Thiamine Deficiency
Wernicke encephalopathy and Korsakoff syndrome (WKS) represent a spectrum of neurodegenerative disorders caused by thiamine deficiency. The condition typically occurs in malnourished alcoholic patients, manifesting as a triad of mental status changes, ophthalmoplegia, and ataxia. Recent research has suggested that WKS is more common than previously thought, is not confined exclusively to alcoholic patients, is unlikely to present with the full classic triad, and is typically only diagnosed postmortem.9
Nonalcoholic WKS tends to occur in younger female patients with a wide array of conditions that affect nutrition (eg, gastrointestinal malignancy, bariatric surgery, hyperemesis gravidarum, anorexia nervosa).9 In a patient with chronic alcoholism, application of the Caine criteria (any two of the following findings: ophthalmoplegia, ataxia, even mild memory impairment or confusion without another cause, evidence of malnutrition) has been shown to be more sensitive and specific than the classic triad.10
Subacute Combined Degeneration
Patients with subacute combined degeneration and extrapyramidal symptoms due to B12 (cobalamin) deficiency are well documented. However, patients with B12 deficiency can also present with mood disorders, acute psychosis, psychotic depression, or paranoid hallucinations. The EP should always consider vitamin B12 deficiency as an important, reversible cause of altered mental status—even in the absence of megaloblastic anemia—especially in patients with celiac disease or anorexia nervosa, and in teenagers and those who are vegans/vegetarians.11
Zinc/Vitamin D Deficiency
Zinc and vitamin D deficiency are both highly prevalent in geriatric patients and have been associated with a range of psychiatric complaints, including depressive disorders, bipolar disorder, and psychotic episodes. Though the neurodevelopmental effects of long-term deficiency of these nutrients are well documented in pediatric patients, the role and relationship to acute psychiatric complaints in elderly patients remain unclear.12,13
Endocrine Disorders
Hypothyroidism
Hypothyroidism is a commonly encountered endocrine disruption that classically presents with fatigue, cold insensitivity, weight gain, and thinning hair. Thyroid dysfunction can result in various neuropsychiatric presentations, including mood disorders, cognitive impairment, and exacerbation of underlying psychiatric disorders. Though rare, primary hypothyroidism can present as mania, psychosis, and auditory or visual hallucinations, a phenomenon termed “myxedema madness.” Myxedema madness typically occurs in older women, but has also been described in adolescents and as a postoperative complication of thyroidectomy.14
Hyperthyroidism
Hyperthyroidism classically presents with tachycardia, nervousness or anxiety, heat insensitivity, and weight loss despite increased appetite. Involvement of the CNS in thyrotoxicosis is rare, but when present, it is a significant predictor of mortality. Neuropsychiatric presentations of hyperthyroidism or thyroid storm vary widely, and have been reported to include psychosis, catatonia, auditory hallucinations, delusional parasitosis, new-onset sleepwalking, dissociative disorder, and suicide attempts.15
Steroid Dysregulation
Steroid dysregulation, either endogenous or iatrogenic in nature, has been reported to cause neuropsychiatric symptoms. Major depression with psychotic features can be an initial presentation of Cushing disease, especially in the presence of other systemic signs.16 Adrenal insufficiency has also been shown to cause severe psychotic disorder.17
Chronic treatment with exogenous corticosteroids can cause a recurrent steroid psychosis, primarily manifesting as subacute mania with psychotic features. Treatment of acute adrenal crisis can also cause an acute steroid psychosis with hallucinations, delusions, and dangerous behavior.17
Parathyroid Dysregulation
Elevated calcium levels caused by primary hyperparathyroidism can present as cognitive slowing, reductions in psychomotor speed, memory impairment, and depression. While the disorder is most prevalent in older women, it has been reported in adolescents, and often remains undiagnosed in younger patients until end-organ damage occurs.18 Hypoparathyroidism has also been reported to cause mood disorders, which can occur with or without the classic symptoms of hypocalcemia (eg, tetany, seizures, dementia, and parkinsonism).18
Pheochromocytoma
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that causes sympathetic hyperactivity by the release of large amounts of catecholamines. Pheochromocytoma is well-reported to present with nervousness, anxiety, panic attacks, or depression.19
Gonadal Hormone Dysregulation
Gonadal hormone dysregulation can be either congenital or acquired and is typically caused by a pituitary tumor or traumatic brain injury. Thought to be a result of dopaminergic hyperactivity, acute psychosis can develop in cases of hypogonadotropic hypogonadism, hypopituitarism, and/or hyperprolactinemia.20 There is a high incidence of psychotic manifestations in hypogonadal disorders such as Klinefelter syndrome and Prader-Willi syndrome.
Toxins
Many toxins can cause altered mental status and psychiatric manifestations. The administration of these toxins can be iatrogenic, related to prescribed use, or overdose—whether accidental, recreational, or intentional (eg, suicide attempt). Table 2 lists common drugs and toxins associated with psychiatric symptoms.21
Synthetic Drugs
The use of numerous unregulated, synthetic analogues of popular recreational drugs has greatly increased over the last several years. Synthetic cannabinoids are available under a variety of names (eg, “Spice,” “K2”) and can cause prominent psychiatric symptoms, including new-onset psychosis, paranoid delusions, hallucinations, and suicide ideation or attempt. While most clinical symptoms are self-limited and require only supportive care, more serious complications have been reported, including myocardial infarction, ischemic stroke, and acute kidney injury.22 Synthetic cathinones (bath salts) can also cause autonomic instability and prominent acute psychosis, sometimes creating a clinical picture indistinguishable from excited delirium syndrome.23
Heavy Metals
Chronic toxicity of many heavy metals is implicated in abnormal neurodevelopment, behavioral disturbances, and progression of neurodegenerative diseases. Recent literature has also implicated acute metal overload in new-onset impaired emotional behavior, though the mechanism is not currently well understood.24
Case Scenarios Continued
Case 1
[The 62-year-old man with altered mental status.]
The patient’s laboratory evaluation and toxicology screen were negative, including a screen for alcohol. He remained jovial but otherwise in no distress. Since the noncontrast head CT scan showed a subtle asymmetry in the frontal lobes, a magnetic resonance imaging (MRI) study was recommended. The brain MRI showed a 5-cm mass in the right frontal lobe with surrounding edema, findings consistent with glioblastoma multiforme. A neurosurgeon was consulted, and the patient was admitted to the intensive care unit.
Case Scenarios Continued
Case 2
[The 48-year-old woman with chest pain.]
The patient received a dose of oral lorazepam, after which she began to feel less anxious, and her chest pain and shortness of breath also improved slightly. The repeat ECG showed worsening of the ST segment changes. The laboratory evaluation was negative. The patient’s son asked if he could take his mother home for what he felt was much needed rest. The EP, however, ordered a stat two-dimensional echocardiogram (ECHO) and repeat troponin level test. The repeat troponin test was positive, and the ECHO was remarkable for a decreased left ventricular ejection fraction of 15%, with apical ballooning. These findings were consistent with stress cardiomyopathy (Takotsubo syndrome). The patient was admitted to the cardiology service and given a beta blocker and an angiotensin-converting enzyme inhibitor.
After a normal coronary angiogram, the patient developed cardiogenic shock and was intubated. Seven days later, she was extubated and transferred to inpatient rehabilitation services where she also received an assessment and treatment for her underlying depression. Eight weeks postdiagnosis, the patient’s ejection fraction had returned to 50%, and she was close to her baseline exercise tolerance.
Although the emergency physician (EP) typically encounters common conditions such as chest pain, urinary tract infection, and gastroenteritis, many other clinical presentations can confound diagnosis of the true underlying condition. This may be the case with a patient who presents with apparent psychiatric symptoms that are actually masking an acute medical condition. For example, a patient who appears to be depressed may actually be exhibiting early signs of dementia. Likewise, a manic patient may not have a true underlying psychiatric disorder but rather rhabdomyolysis and hyperthermia from ingesting an illicit substance such as synthetic cathinones (“bath salts”).
Part 1 of this series reviewed psychiatric presentations caused by underlying infectious, pharmacological withdrawal, metabolic, autoimmune, traumatic, and central nervous system etiologies (Emerg Med. 2016;48[5]:202-211). Part 2 covers psychiatric presentations related to dementia, cancer, cardiac disease, nutritional deficiencies, endocrine disorders, or toxins (Table 1).
Case Scenarios
Case 1
A 62-year-old man with a history of hypertension, hyperlipidemia, and past alcohol abuse presented to the ED with reported mental status changes after he was pulled over by police for driving the wrong way down the highway. On presentation, the patient’s vital signs were normal. When questioned, the patient was alert and fully oriented and believed the officers were mistaken about what was reported. He denied any recent illness and had a normal physical examination, including neurological examination.
A brief work-up was ordered and the patient passed the time by politely flirting with the nurses. When his wife arrived at the ED, she was relieved that her husband seemed to be all right. She confirmed that the patient had not consumed any alcohol in years. The patient, meanwhile, playfully minimized his wife’s concern at his presence in the ED. A full toxicology screen, laboratory evaluation, and head computed tomography (CT) scan were ordered.
Case 2
A 48-year-old woman with a history of anxiety disorder, depression, and diabetes mellitus presented to the ED with a 2-hour history of chest pain. She stated that the pain had started toward the end of a heated argument with her son. The patient was escorted into the examination room by hospital security because she was still agitated and kept yelling at her son. On examination the patient was tachycardic (110 beats/minute), diaphoretic, and crying. During the examination, she asked the EP for a “Xanax”; her son further noted that this would help his mother’s condition.
The patient repeatedly claimed she could not breathe and could not lie flat on the stretcher. After verbal de-escalation, she cooperated with the electrocardiography (ECG) technician and phlebotomist. Her ECG showed nonspecific ST changes with no prior study for comparison. While glaring at her son, she maintained that she had constant chest pain.
Dementia
Alzheimer’s Disease
Alzheimer’s disease (AD), the most common cause of dementia, is a chronic neurodegenerative disease characterized by an insidiously progressive cognitive decline and loss of function. There is considerable apparent variability in the early signs of the disease, and recent literature has suggested that the manifestation of initial symptoms may be age-dependent. Younger patients tend to present with non-memory cognitive changes such as problem-solving difficulties, as well as personality changes and behavioral symptoms of depression, apathy, and withdrawal.1
Lewy Body Dementia
Lewy body dementia (LBD) is a chronic neurodegenerative disease with a presentation that overlaps substantially with AD. However, LBD is associated with a significantly more rapid course than AD and presents more frequently with visual hallucinations or illusions due to specific visuospatial dysfunction.2
Frontotemporal Dementia
Frontotemporal dementia is a comparatively rare chronic neurodegenerative disease characterized by early-onset memory impairment with cognitive decline, as well as behavioral changes such as disinhibition, emotional blunting, and language difficulty. Initial presentations can also include atypical features such as paranoia or delusion, and misdiagnosis as a primary psychiatric problem is common.3
Cancer
Brain Tumor
Primary and metastatic brain tumors classically present with either focal neurological signs or less specific symptoms such as headaches, seizures, or syncope. Additionally, central nervous system (CNS) tumors can also initially present with primary psychiatric complaints (eg, personality changes, depression, mania, panic attacks, auditory or visual hallucinations). Patients with a brain neoplasm who are initially misdiagnosed with a primary psychiatric disorder face significant delays in proper diagnosis and treatment, leading to increased morbidity. To correctly diagnose the true cause as soon as possible, early imaging is recommended for patients who present with psychiatric symptoms that are abrupt in onset, atypical in presentation, resistant to conventional treatments, or associated with a change in headache pattern.4
Paraneoplastic Limbic Encephalitis
Paraneoplastic limbic encephalitis (PLE) is a rare neurological consequence of certain cancers. Although PLE most commonly occurs in patients with small cell lung cancer, the condition has also been reported (though less frequently) in cases of esophageal adenocarcinoma, ovarian teratoma, metastatic breast cancer, and germ cell testicular cancer.5 This disease overlaps substantially with anti-N-methyl-D-aspartate (anti-NMDA) receptor encephalitis. Moreover, PLE can present initially with prominent neuropsychiatric symptoms such as confusion, cognitive problems, behavioral changes, irritability, depression, or frank psychosis with hallucinations. Paraneoplastic limbic encephalitis can occur early in the course of cancer—often before other systemic signs appear—and its significance is often only recognized in retrospect or postmortem. A higher index of suspicion for the disorder may lead to earlier detection of treatable cancers.
Malignant Meningitis
Malignant meningitis is the metastatic spread of a primary solid tumor to the leptomeninges. It can present as a wide variety of neuropsychiatric complaints, including depression, anxiety, disorientation, and paranoia. Diagnosis can often be made through lumbar puncture. Malignant meningitis should be considered in the differential diagnosis of new psychiatric symptoms in a patient with a history of cancer—even in the absence of focal neurological deficits or meningeal signs.6
Pancreatic Insulinoma
Pancreatic insulinoma is a rare, potentially curable endocrine tumor that can present initially with vague psychiatric complaints such as irrational behavior, confusion, depression, or anxiety. In up to 64% of patients, insulinomas are misdiagnosed as primary neurological or psychiatric disease, which can delay potentially curative surgery—sometimes for years.7 The EP should suspect pancreatic insulinoma in any patient who presents with psychiatric symptoms and unexplained episodes of hypoglycemia.7
Cardiac Disease
Transient Left Ventricular Apical Ballooning Syndrome
Transient left ventricular apical ballooning syndrome (TLVABS), first identified in Japan as Takotsubo syndrome, has more recently been recognized worldwide as overlapping with the classic broken heart syndrome. In postmenopausal women, TLVABS appears to follow a catecholamine surge triggered by extreme emotional stress, resulting in an acute coronary artery spasm. Researchers have hypothesized that there may be a link between TLVABS and dissociative amnesia, which is also thought to result from a catecholamine surge in response to emotional stress.8
Nutritional Deficiencies
Wernicke/Korsakoff Syndrome and Thiamine Deficiency
Wernicke encephalopathy and Korsakoff syndrome (WKS) represent a spectrum of neurodegenerative disorders caused by thiamine deficiency. The condition typically occurs in malnourished alcoholic patients, manifesting as a triad of mental status changes, ophthalmoplegia, and ataxia. Recent research has suggested that WKS is more common than previously thought, is not confined exclusively to alcoholic patients, is unlikely to present with the full classic triad, and is typically only diagnosed postmortem.9
Nonalcoholic WKS tends to occur in younger female patients with a wide array of conditions that affect nutrition (eg, gastrointestinal malignancy, bariatric surgery, hyperemesis gravidarum, anorexia nervosa).9 In a patient with chronic alcoholism, application of the Caine criteria (any two of the following findings: ophthalmoplegia, ataxia, even mild memory impairment or confusion without another cause, evidence of malnutrition) has been shown to be more sensitive and specific than the classic triad.10
Subacute Combined Degeneration
Patients with subacute combined degeneration and extrapyramidal symptoms due to B12 (cobalamin) deficiency are well documented. However, patients with B12 deficiency can also present with mood disorders, acute psychosis, psychotic depression, or paranoid hallucinations. The EP should always consider vitamin B12 deficiency as an important, reversible cause of altered mental status—even in the absence of megaloblastic anemia—especially in patients with celiac disease or anorexia nervosa, and in teenagers and those who are vegans/vegetarians.11
Zinc/Vitamin D Deficiency
Zinc and vitamin D deficiency are both highly prevalent in geriatric patients and have been associated with a range of psychiatric complaints, including depressive disorders, bipolar disorder, and psychotic episodes. Though the neurodevelopmental effects of long-term deficiency of these nutrients are well documented in pediatric patients, the role and relationship to acute psychiatric complaints in elderly patients remain unclear.12,13
Endocrine Disorders
Hypothyroidism
Hypothyroidism is a commonly encountered endocrine disruption that classically presents with fatigue, cold insensitivity, weight gain, and thinning hair. Thyroid dysfunction can result in various neuropsychiatric presentations, including mood disorders, cognitive impairment, and exacerbation of underlying psychiatric disorders. Though rare, primary hypothyroidism can present as mania, psychosis, and auditory or visual hallucinations, a phenomenon termed “myxedema madness.” Myxedema madness typically occurs in older women, but has also been described in adolescents and as a postoperative complication of thyroidectomy.14
Hyperthyroidism
Hyperthyroidism classically presents with tachycardia, nervousness or anxiety, heat insensitivity, and weight loss despite increased appetite. Involvement of the CNS in thyrotoxicosis is rare, but when present, it is a significant predictor of mortality. Neuropsychiatric presentations of hyperthyroidism or thyroid storm vary widely, and have been reported to include psychosis, catatonia, auditory hallucinations, delusional parasitosis, new-onset sleepwalking, dissociative disorder, and suicide attempts.15
Steroid Dysregulation
Steroid dysregulation, either endogenous or iatrogenic in nature, has been reported to cause neuropsychiatric symptoms. Major depression with psychotic features can be an initial presentation of Cushing disease, especially in the presence of other systemic signs.16 Adrenal insufficiency has also been shown to cause severe psychotic disorder.17
Chronic treatment with exogenous corticosteroids can cause a recurrent steroid psychosis, primarily manifesting as subacute mania with psychotic features. Treatment of acute adrenal crisis can also cause an acute steroid psychosis with hallucinations, delusions, and dangerous behavior.17
Parathyroid Dysregulation
Elevated calcium levels caused by primary hyperparathyroidism can present as cognitive slowing, reductions in psychomotor speed, memory impairment, and depression. While the disorder is most prevalent in older women, it has been reported in adolescents, and often remains undiagnosed in younger patients until end-organ damage occurs.18 Hypoparathyroidism has also been reported to cause mood disorders, which can occur with or without the classic symptoms of hypocalcemia (eg, tetany, seizures, dementia, and parkinsonism).18
Pheochromocytoma
Pheochromocytoma is a neuroendocrine tumor of the adrenal medulla that causes sympathetic hyperactivity by the release of large amounts of catecholamines. Pheochromocytoma is well-reported to present with nervousness, anxiety, panic attacks, or depression.19
Gonadal Hormone Dysregulation
Gonadal hormone dysregulation can be either congenital or acquired and is typically caused by a pituitary tumor or traumatic brain injury. Thought to be a result of dopaminergic hyperactivity, acute psychosis can develop in cases of hypogonadotropic hypogonadism, hypopituitarism, and/or hyperprolactinemia.20 There is a high incidence of psychotic manifestations in hypogonadal disorders such as Klinefelter syndrome and Prader-Willi syndrome.
Toxins
Many toxins can cause altered mental status and psychiatric manifestations. The administration of these toxins can be iatrogenic, related to prescribed use, or overdose—whether accidental, recreational, or intentional (eg, suicide attempt). Table 2 lists common drugs and toxins associated with psychiatric symptoms.21
Synthetic Drugs
The use of numerous unregulated, synthetic analogues of popular recreational drugs has greatly increased over the last several years. Synthetic cannabinoids are available under a variety of names (eg, “Spice,” “K2”) and can cause prominent psychiatric symptoms, including new-onset psychosis, paranoid delusions, hallucinations, and suicide ideation or attempt. While most clinical symptoms are self-limited and require only supportive care, more serious complications have been reported, including myocardial infarction, ischemic stroke, and acute kidney injury.22 Synthetic cathinones (bath salts) can also cause autonomic instability and prominent acute psychosis, sometimes creating a clinical picture indistinguishable from excited delirium syndrome.23
Heavy Metals
Chronic toxicity of many heavy metals is implicated in abnormal neurodevelopment, behavioral disturbances, and progression of neurodegenerative diseases. Recent literature has also implicated acute metal overload in new-onset impaired emotional behavior, though the mechanism is not currently well understood.24
Case Scenarios Continued
Case 1
[The 62-year-old man with altered mental status.]
The patient’s laboratory evaluation and toxicology screen were negative, including a screen for alcohol. He remained jovial but otherwise in no distress. Since the noncontrast head CT scan showed a subtle asymmetry in the frontal lobes, a magnetic resonance imaging (MRI) study was recommended. The brain MRI showed a 5-cm mass in the right frontal lobe with surrounding edema, findings consistent with glioblastoma multiforme. A neurosurgeon was consulted, and the patient was admitted to the intensive care unit.
Case Scenarios Continued
Case 2
[The 48-year-old woman with chest pain.]
The patient received a dose of oral lorazepam, after which she began to feel less anxious, and her chest pain and shortness of breath also improved slightly. The repeat ECG showed worsening of the ST segment changes. The laboratory evaluation was negative. The patient’s son asked if he could take his mother home for what he felt was much needed rest. The EP, however, ordered a stat two-dimensional echocardiogram (ECHO) and repeat troponin level test. The repeat troponin test was positive, and the ECHO was remarkable for a decreased left ventricular ejection fraction of 15%, with apical ballooning. These findings were consistent with stress cardiomyopathy (Takotsubo syndrome). The patient was admitted to the cardiology service and given a beta blocker and an angiotensin-converting enzyme inhibitor.
After a normal coronary angiogram, the patient developed cardiogenic shock and was intubated. Seven days later, she was extubated and transferred to inpatient rehabilitation services where she also received an assessment and treatment for her underlying depression. Eight weeks postdiagnosis, the patient’s ejection fraction had returned to 50%, and she was close to her baseline exercise tolerance.
1. Barnes J, Dickerson BC, Frost C, Jiskoot LC, Wolk D, van der Flier WM. Alzheimer’s disease first symptoms are age dependent: Evidence from the NACC dataset. Alzheimers Dement. 2015;11(11):1349-1357.
2. Yoshizawa H, Vonsattel JP, Honig LS. Early neuropsychological discriminants for Lewy body disease: an autopsy series. J Neurol Neurosurg Psychiatry. 2013;84(12):1326-1330.
3. Iroka N, Jehangir W, Ii JL, Pattan V, Yousif A, Mishra AK. Paranoid personality masking an atypical case of frontotemporal dementia. J Clin Med Res. 2015;7(5):364-366.
4. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
5. Said S, Cooper CJ, Reyna E, Alkhateeb H, Diaz J, Nahleh Z. Paraneoplastic limbic encephalitis, an uncommon presentation of a common cancer: Case report and discussion. Am J Case Rep. 2013;14:391-394.
6. Weitzner MA, Olofsson SM, Forman AD. Patients with malignant meningitis presenting with neuropsychiatric manifestations. Cancer. 1995;76(10):1804-1808.
7. Ding Y, Wang S, Liu J. Neuropsychiatric profiles of patients with insulinomas. Eur Neurol. 2010;63(1):48-51.
8. Toussi A, Bryk J, Alam A. Forgetting heart break: a fascinating case of transient left ventricular apical ballooning syndrome associated with dissociative amnesia. Gen Hosp Psychiatry. 2014;36(2):225-227.
9. Scalzo SJ, Bowden SC, Ambrose ML, Whelan G, Cook MJ. Wernicke-Korsakoff syndrome not related to alcohol use: a systematic review. J Neurol Neurosurg Psychiatry. 2015;86(12):1362-1368.
10. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
11. Issac TG, Soundarya S, Christopher R, Chandra SR. Vitamin B12 deficiency: an important reversible co-morbidity in neuropsychiatric manifestations. Indian J Psychol Med. 2015;37(1):26-29.
12. Grønli O, Kvamme JM, Friborg O, Wynn R. Zinc deficiency is common in several psychiatric disorders. PLoS One. 2013;8(12):e82793.
13. Grønli O, Kvamme JM, Jorde R, Wynn R. Vitamin D deficiency is common in psychogeriatric patients, independent of diagnosis. BMC Psychiatry. 2014;14:134.
14. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry. 2003;5(6):260-266.
15. Swee du S, Chng CL, Lim A. Clinical characteristics and outcome of thyroid storm: a case series and review of neuropsychiatric derangements in thyrotoxicosis. Endocr Pract. 2015;21(2):182-189.
16. Tang A, O’Sullivan AJ, Diamond T, Gerard A, Campbell P. Psychiatric symptoms as a clinical presentation of Cushing’s syndrome. Ann Gen Psychiatry. 2013;12(1):23.
17. Farah Jde L, Lauand CV, Chequi L, et al. Severe psychotic disorder as the main manifestation of adrenal insufficiency. Case Rep Psychiatry. 2015;2015:512430.
18. Rice T, Azova S, Coffey BJ. Negative symptoms in a depressed teen? Primary hyperparathyroidism and its psychiatric manifestations. J Child Adolesc Psychopharmacol. 2015;25(8):653-655.
19. Zardawi IM. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
20. Kate S, Dhanwal DK, Kumar S, Bharti P. Acute psychosis as a presentation of hypopituitarism. BMJ Case Rep. 2013;2013.
21. Abramowicz M. Drugs that may cause psychiatric symptoms. Med Lett Drugs Ther. 2008;50(1301-1302):100-103.
22. Tait RJ, Caldicott D, Mountain D, Hill SL, Lenton S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila). 2016;54(1):1-13.
23. Karch SB. Cathinone neurotoxicity (“The “3Ms”). Curr Neuropharmacol. 2015;13(1): 21-25.
24. Menon AV, Chang J, Kim J. Mechanisms of divalent metal toxicity in affective disorders. Toxicology. 2016;339:58-72.
1. Barnes J, Dickerson BC, Frost C, Jiskoot LC, Wolk D, van der Flier WM. Alzheimer’s disease first symptoms are age dependent: Evidence from the NACC dataset. Alzheimers Dement. 2015;11(11):1349-1357.
2. Yoshizawa H, Vonsattel JP, Honig LS. Early neuropsychological discriminants for Lewy body disease: an autopsy series. J Neurol Neurosurg Psychiatry. 2013;84(12):1326-1330.
3. Iroka N, Jehangir W, Ii JL, Pattan V, Yousif A, Mishra AK. Paranoid personality masking an atypical case of frontotemporal dementia. J Clin Med Res. 2015;7(5):364-366.
4. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
5. Said S, Cooper CJ, Reyna E, Alkhateeb H, Diaz J, Nahleh Z. Paraneoplastic limbic encephalitis, an uncommon presentation of a common cancer: Case report and discussion. Am J Case Rep. 2013;14:391-394.
6. Weitzner MA, Olofsson SM, Forman AD. Patients with malignant meningitis presenting with neuropsychiatric manifestations. Cancer. 1995;76(10):1804-1808.
7. Ding Y, Wang S, Liu J. Neuropsychiatric profiles of patients with insulinomas. Eur Neurol. 2010;63(1):48-51.
8. Toussi A, Bryk J, Alam A. Forgetting heart break: a fascinating case of transient left ventricular apical ballooning syndrome associated with dissociative amnesia. Gen Hosp Psychiatry. 2014;36(2):225-227.
9. Scalzo SJ, Bowden SC, Ambrose ML, Whelan G, Cook MJ. Wernicke-Korsakoff syndrome not related to alcohol use: a systematic review. J Neurol Neurosurg Psychiatry. 2015;86(12):1362-1368.
10. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
11. Issac TG, Soundarya S, Christopher R, Chandra SR. Vitamin B12 deficiency: an important reversible co-morbidity in neuropsychiatric manifestations. Indian J Psychol Med. 2015;37(1):26-29.
12. Grønli O, Kvamme JM, Friborg O, Wynn R. Zinc deficiency is common in several psychiatric disorders. PLoS One. 2013;8(12):e82793.
13. Grønli O, Kvamme JM, Jorde R, Wynn R. Vitamin D deficiency is common in psychogeriatric patients, independent of diagnosis. BMC Psychiatry. 2014;14:134.
14. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry. 2003;5(6):260-266.
15. Swee du S, Chng CL, Lim A. Clinical characteristics and outcome of thyroid storm: a case series and review of neuropsychiatric derangements in thyrotoxicosis. Endocr Pract. 2015;21(2):182-189.
16. Tang A, O’Sullivan AJ, Diamond T, Gerard A, Campbell P. Psychiatric symptoms as a clinical presentation of Cushing’s syndrome. Ann Gen Psychiatry. 2013;12(1):23.
17. Farah Jde L, Lauand CV, Chequi L, et al. Severe psychotic disorder as the main manifestation of adrenal insufficiency. Case Rep Psychiatry. 2015;2015:512430.
18. Rice T, Azova S, Coffey BJ. Negative symptoms in a depressed teen? Primary hyperparathyroidism and its psychiatric manifestations. J Child Adolesc Psychopharmacol. 2015;25(8):653-655.
19. Zardawi IM. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
20. Kate S, Dhanwal DK, Kumar S, Bharti P. Acute psychosis as a presentation of hypopituitarism. BMJ Case Rep. 2013;2013.
21. Abramowicz M. Drugs that may cause psychiatric symptoms. Med Lett Drugs Ther. 2008;50(1301-1302):100-103.
22. Tait RJ, Caldicott D, Mountain D, Hill SL, Lenton S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila). 2016;54(1):1-13.
23. Karch SB. Cathinone neurotoxicity (“The “3Ms”). Curr Neuropharmacol. 2015;13(1): 21-25.
24. Menon AV, Chang J, Kim J. Mechanisms of divalent metal toxicity in affective disorders. Toxicology. 2016;339:58-72.
Pediatric Heat-Related Illnesses
Heat-related illnesses in children encompass a wide range of disease processes—from minor conditions such as heat rash to life-threatening thermoregulatory emergencies such as heatstroke. Physiological differences in children compared to adults make them particularly susceptible to illnesses caused by heat exposure.
Pediatric heat-related illnesses can usually be prevented if appropriate precautions are taken (see “Taking Steps to Prevent Heat-Related Illnesses” box). In lieu of prevention, early recognition and treatment of heatstroke in children may drastically reduce life-threatening complications related to multisystem organ dysfunction. Management of heatstroke rests primarily on prompt initiation of rapid cooling measures and evaluation for organ dysfunction.
Case Scenarios
Case 1
An obese 10-year-old boy was brought to the ED by emergency medical services (EMS) during the first week of youth football tryouts. It was a hot day in late August, with 100% humidity and temperatures over 95°F. The patient, who weighed approximately 240 lb, was trying out for football but had no previous athletic-conditioning experience. Despite his obesity, he had been generally healthy and only took a stimulant medication for attention-deficit/hyperactivity disorder (ADHD).
At approximately noon, the boy collapsed on the field and had a seizure. When the EMS technicians arrived, they administered a dose of intramuscular (IM) midazolam. Although his seizure ceased, he remained obtunded and was intubated. A rectal temperature revealed a temperature of 105.8°F and paramedics noted that while the patient felt hot, he was no longer sweating. While en route to the ED, EMS technicians removed the patient’s football uniform; placed a fan in front of him; and sprayed cool water on him in an effort to lower his body temperature. At the time of arrival to the ED, his rectal temperature was 104.9°F.
Case 2
A previously healthy 3-month-old female infant was brought to the ED by EMS after she was accidentally left in a car on a summer day with a temperature of 90°F and 100% humidity. The infant’s father said that while running errands, he had forgotten his daughter was in the car and had left her in the rear facing backseat car carrier for approximately 10 minutes. When he returned to the car, he found his daughter awake but crying inconsolably. She had sweated through her clothes, vomited, and felt very hot, so he called 911. Her initial rectal temperature was 102.2°F, and her clothes were removed as she was being transported in an air-conditioned ambulance to the ED for further evaluation. Once undressed, she was noted to have an erythematous rash with multiple papules and pustules on her trunk.
Epidemiology
From 2006 to 2010, an average of 668 heat-related deaths per year occurred among people of all ages in the United States. Of these deaths, approximately 7% occurred in children younger than age 4 years (2.5% in those younger than age 1 year and 4.5% in those age 1-4 years). These figures have remained relatively stable over the last 10 years.1,2 Adolescents are particularly at risk for overexertion, and heatstroke is the third leading cause of death in young athletes, after traumatic and cardiac causes.3 As may be expected, most heat-related deaths (76%) occur in the southern and western regions of the United States.
Pathophysiology of Heat-Related Illnesses
The hypothalamus is the main control center for temperature homeostasis. As the core temperature rises due to either metabolic or environmental causes of heat, the hypothalamus primarily acts on the autonomic nervous system to engage mechanisms of heat dissipation.4 Evaporation of sweat is believed to be the most important mechanism of heat dissipation in humans; however, this method becomes less effective when humidity levels are above 75%.5 Radiation allows heat to transfer from the skin to the air, but is reliant on a temperature gradient. Conduction can allow heat to transfer to a cooler object through physical contact (as seen with cold-water immersion), while convection utilizes air movement to transfer heat (as illustrated by fanning).6
Thermoregulation is disrupted when the body is unable to balance metabolic heat production and heat dissipation. Heat dissipation mechanisms are easily overwhelmed when a person is exposed to excessive heat from the environment. The resulting stress from hyperthermia can directly injure cells, leading to a cytokine storm and endothelial injury. Heat can cause proteins to denature and cells to undergo apoptosis, which, if severe, can result in multisystem organ dysfunction.7
Physiological Differences in Children
Several physiological differences in children compared to adults compromise their ability to manage heat exposure. Thermoregulation in infants is less developed secondary to an immature hypothalamus; therefore, they are less able to utilize compensatory mechanisms to dissipate heat.8 In addition, infants and young children have a decreased sweating capacity, which makes evaporative cooling less effective.9 Children also produce more endogenous heat per kilogram than adults, which is believed to be secondary to a higher basal metabolic rate. They have less blood volume than adults, which decreases their ability to transfer warm blood into the periphery in order to cool the central core. Lastly, children have a higher surface area-to-body mass ratio, which causes increased heat absorption. All of these factors ultimately result in a slower rate of acclimatization in children compared to adults.10
Environmental Factors
Several environmental risk factors predispose children to heat-related illnesses. Infants are completely dependent on their caregivers for hydration and environmental protection from the heat. Infants who are over-bundled or left in a hot car are particularly at risk for heat-related illnesses.11 Older children are at risk for sports-related overexertion and typically must depend on permission from a coach or supervising adult to hydrate or take a break from exercise. Lastly, medications such as stimulants frequently prescribed for ADHD or medications with anticholinergic properties (secondary to decreased sweating) can predispose children to heat intolerance.12
Minor Heat-Related Illnesses
Heat-related illnesses range from benign conditions (eg, heat rash) to life-threatening processes (eg, heatstroke).
Miliaria Rubra
There are several forms of miliaria. Miliaria rubra, also known as heat rash or prickly heat, is a common, benign manifestation of heat exposure in infants and young children. A combination of heat exposure and obstructed sweat glands results in a pruritic, erythematous rash with papules and pustules (Figure). This is often seen in areas of friction from skin rubbing against skin or clothing.13
Heat Edema/Heat Cramps
Heat edema is another benign process related to heat exposure that generally occurs in older adults but can also occur in children. It is the result of peripheral vasodilation as the body attempts to shunt warm blood to the periphery.14 Heat cramps are a common manifestation in young athletes exercising in hot, summer conditions. Although benign, the cramps are very painful spasms that often affect large muscle groups, particularly in the legs, such as the calves, quadriceps, and hamstrings. There is conflicting data regarding the underlying cause of heat cramps. Many believe there is a significant component related to dehydration, while others attribute the cramps to fatigue or a combination
of the two.15
Heat Syncope
Heat syncope secondary to peripheral vasodilation, and venous pooling occurs as the body attempts to dissipate heat by transferring warm blood to the periphery. Relative dehydration plays a role in heat syncope, which is often precipitated by a rapid change in positioning during exercise, such as moving from a sitting to standing position. Heat syncope usually improves after the patient is supine, and children with heat syncope do not have an elevation in core body temperature.14 Some patients who experience heat syncope, however, may also have heat exhaustion.
Heat Exhaustion
Heat exhaustion occurs in patients with a known heat exposure. As opposed to the previously described processes, heat exhaustion is characterized by a body temperature elevated up to 104°F. Heat exhaustion is often accompanied by diffuse, nonspecific symptoms such as tachycardia, sweating, nausea, vomiting, weakness, fatigue, headache, and mild confusion. Dehydration often plays a significant role in heat exhaustion, but in contrast to heatstroke (described in the following section), mentation is normal, or there is a transient, mild confusion.16
Heatstroke
Heatstroke is observed in patients with a known heat exposure who have a temperature greater than 104°F accompanied by central nervous system (CNS) dysfunction.14 The CNS dysfunction involves an alteration in mental status manifested by slurred speech, ataxia, delirium, hallucinations, or seizure activity. In severe cases, obtundation or coma may result in airway compromise.17 Vital signs are unstable, and tachycardia and hypotension are often present. Patients with heatstroke may stop sweating, although the absence of sweating is not required for the diagnosis. Other nonspecific findings such as vomiting and diarrhea are common.6
The hallmark of heatstroke is multisystem organ dysfunction, which is caused by heat-induced tissue damage resulting in a systemic inflammatory response.18 Since the pediatric brain is particularly sensitive to temperature extremes, cerebral edema and herniation are potential complications of heatstroke.17 Damage to myocardial tissue, coupled with dehydration and systemic vasodilation, results in hypotension and poor systemic perfusion.19 Muscle breakdown causes rhabdomyolysis that can lead to kidney failure and hepatic injury. Degradation of clotting factors disrupts the clotting system and can cause disseminated intravascular coagulation (DIC).20 Damage to the mucosal lining of the intestines may result in ischemia and massive hematochezia.21
Heatstroke is classified as either nonexertional or exertional. Nonexertional heatstroke occurs most frequently in younger children who are exposed to a hot environment, such as an infant left in a car on a warm day. Exertional heatstroke occurs primarily in children exercising on a hot day, such as young athletes.6
Due to its significant morbidity and mortality, heatstroke is the most concerning manifestation of excessive heat exposure. The mortality rate for children with heatstroke is significantly lower than for adults; however, approximately 10% of children with heatstroke will not survive,22 and 20% will have long-term neurological disabilities, including permanent impairment in vision, speech, memory, behavior, and coordination.23
Management of Minor Heat-Related Illnesses
For most minor heat-related illnesses, supportive care is the mainstay of treatment (Table).
Miliaria Rubra
Infants with miliaria rubra typically improve once they are placed in a cool environment and their clothing is removed. In infants, lotions may contribute to sweat gland obstruction and should be used sparingly.13
Heat Edema/Heat Cramps
Similarly, heat edema generally improves once the child is removed from the hot environment and the extremities are elevated.14 Heat cramps are likely the result of fatigue and dehydration; therefore, these painful contractions often improve with rest, stretching, oral hydration, and removal from the hot environment. If cramps persist despite these measures, parenteral rehydration (20 mL/kg of normal saline) may be beneficial.15
Heat Syncope
Patients with orthostatic hypotension from heat syncope usually improve once they are resting in a cool environment and have been rehydrated. Pediatric oral rehydration with salt-containing fluids, such as commercial sports drinks, is safe; nonetheless, these patients may require intravenous (IV) rehydration with normal saline if orthostatic hypotension does not improve with oral rehydration alone.14
Heat Exhaustion
Differentiating heat exhaustion from heatstroke is of upmost importance because the treatment courses vary greatly. The difference in neurological status is the most effective way of differentiating the two diseases. All patients with slurred speech, ataxia, delirium, hallucinations, or seizure activity should be treated for presumptive heatstroke until proven otherwise (see “Management of Heatstroke” section).
Although children with heat exhaustion may have mild confusion, this tends to be transient and resolves with supportive care. Patients with heat exhaustion should stop exercising and be placed in a cool environment without excess clothing. Oral rehydration with salt-containing fluids is important, and most patients improve with these measures alone.
Children with apparent heat exhaustion who do not improve should be evaluated in the hospital setting, and laboratory studies should be obtained to evaluate for electrolyte abnormalities. Such patients typically warrant a 20 mL/kg IV bolus of normal saline. A complete neurological examination and a rectal temperature should be obtained on initial presentation.16
The evaluation of an overbundled infant with hyperthermia may be particularly challenging. Studies have demonstrated that it is possible for an infant to develop core temperature elevation if overbundled and placed in a warm environment.24 Nonetheless, it is important to address these patients with a broad differential diagnosis in mind, and always consider the possibility of sepsis. If the history and examination are consistent with hyperthermia secondary to heat exposure, a period of observation with supportive care may be a reasonable option. Infants should have a rectal temperature assessed every 15 to 30 minutes to monitor for improvement; if they improve with supportive care alone, a septic evaluation can be potentially avoided. Antipyretics will confuse the clinical picture and should be avoided in this situation.24
Management of Heatstroke
Significant morbidity and mortality are associated with heatstroke, and prompt recognition and initiation of therapy are required to prevent or minimize serious complications.22 As in any other life-threatening condition, the initial treatment of heatstroke requires support of the airway, breathing, and circulation. Patients are often neurologically unstable and cannot protect their airway, which should prompt endotracheal intubation. Children who are tachycardic and hypotensive should be resuscitated with normal saline prior to intubation if oxygenation and ventilation are maintained with supplemental oxygen alone. Most patients require at least 20 mL/kg of IV normal saline but many ultimately need up to 60 mL/kg.14 If blood pressure (BP) does not respond adequately to fluid resuscitation alone, vasopressors may be necessary. Seizure activity can be managed with IV benzodiazepines, such as lorazepam (0.1 mg/kg with maximum 4 mg per dose).14
Rapid cooling therapy is the mainstay of treatment for heatstroke and should be initiated as soon as the diagnosis is suspected, since morbidity and mortality correlates directly with the duration of hyperthermia. These measures are ideally started prior to arrival at the hospital. Evaporative cooling can be achieved in the field or ambulance with a cool water spray and air conditioning. Additionally, ice packs can be placed along the neck and axilla to augment rapid cooling measures and can be continued in the ED until the patient’s core temperature decreases to 101.4°F.25
Medications have a limited role in the treatment of heatstroke. Antipyretics such as acetaminophen and ibuprofen have no proven benefit and may exacerbate hepatic, gastrointestinal, clotting, and renal dysfunction.26 Benzodiazepines are helpful for seizure activity and may have a role in seizure prophylaxis. Dantrolene is not recommended for treating heatstroke as studies have not demonstrated a statistical improvement in cooling time, complications, or mortality.14 The use of chilled IV fluids instead of room-temperature fluids is not definitively supported in the literature.27
Further diagnostic evaluation is directed at determining the degree of multisystem organ dysfunction that results from heatstroke. A head computed tomography (CT) scan can evaluate for cerebral edema, whereas a comprehensive metabolic profile (CMP) will screen for electrolyte abnormalities such as hyponatremia (salt loss), hypernatremia (volume depletion), and possible transaminase elevation, which may indicate hepatic injury. Prolonged coagulation studies may reveal DIC and an arterial blood gas (ABG) analysis often may reveal metabolic acidosis. A serum creatine phosphokinase (CPK) and urinalysis (UA) can help to identify rhabdomyolysis or the presence of an acute kidney injury (AKI).16
After their condition is stabilized, children with heatstroke should be monitored in the pediatric intensive care unit (PICU) to effectively address complications of multisystem organ dysfunction.
Case Scenarios Continued
Case 1
[The 10-year-old boy who collapsed during football tryouts.]
The initial evaluation revealed an obese child who was intubated and obtunded. His vital signs included the following: rectal temperature, 104.9°F; heart rate (HR), 149 beats/minute; and BP, 82/36 mm Hg. Heatstroke was diagnosed and rapid cooling measures were initiated.
Evaporative heat loss was maintained with a fan and water spray, and ice packs were placed along the patient’s groin and axillae. Laboratory evaluation included a complete blood count (CBC), CMP, CPK, UA, coagulation panel, and ABG. A normal saline IV bolus at room temperature was given and a postintubation chest X-ray confirmed appropriate position of the endotracheal tube, without any evidence of acute respiratory distress syndrome (ARDS). A head CT scan did not reveal cerebral edema. Since the child’s BP and HR did not improve after the first normal saline bolus, he was given a total of 40 mL/kg of IV normal saline in the ED. The patient’s laboratory results were concerning for an AKI, with elevated CPK, hepatic injury, coagulopathy, and severe metabolic acidosis. He was subsequently admitted to the PICU for further care.
The child’s PICU course was complicated by multisystem organ failure, which ultimately included DIC, ARDS, acute renal failure requiring hemodialysis, and hypotension requiring vasopressors. A repeat head CT scan 3 days after admission revealed marked cerebral edema. The patient subsequently died within a week of presentation.
Case 2
[The 3-month-old girl who was left in a hot vehicle.]
The initial evaluation revealed a fussy infant with dry mucous membranes, elevated HR, and sunken fontanelle. Her rectal temperature on arrival to the ED was 100.7°F after conservative measures were taken (ie, removing her from the hot environment and removing her clothing). A peripheral IV was placed due to her clinical dehydration and she received a 20 mL/kg bolus of normal saline at room temperature. A glucose level was obtained and was normal. The patient’s rectal temperature was monitored every 30 minutes over the next 4 hours, and her temperature and HR gradually normalized.
The patient’s rash appeared consistent with miliaria rubra and improved as her temperature decreased. The infant underwent a brief period of observation in the ED where she continued to look well and tolerated oral fluids without vomiting. Neither a septic work-up nor empiric antibiotics were initiated, since heat exposure was felt to be the likely source of her core temperature elevation. Child Protective Services (CPS) was notified and opened a case for further evaluation of possible child neglect. The patient ultimately returned to her baseline in the ED and was discharged home with a family member, according to the safety plan outlined by CPS, and close follow-up with her pediatrician.
1. Berko J, Ingram DD, Saha S, Parker JD. Deaths attributed to heat, cold, and other weather events in the United States, 2006-2010. National health statistics reports; no 76. Hyattsville, MD: National Center for Health Statistics; 2014. http://www.cdc.gov/nchs/data/nhsr/nhsr076.pdf. Accessed May 22, 2016.
2. Centers for Disease Control and Prevention(CDC). Heat-related deaths--United States, 1999-2003. MMWR Morb Mortal Wkly Rep. 2006;55(29):796-798.
3. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119(8):1085-1092.
4. Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R37-R46.
5. Smith CJ, Johnson, JM. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: Neural control of skin blood flow and sweating in humans. Auton Neurosci. 2016;196:25-36.
6. Becker JA, Stewart LK. Heat-related illness. Am Fam Physician. 2011;83(11):1325-1330.
7. Aggarwal Y, Karan BM, Das BN, Sinha RK. Prediction of heat-illness symptoms with the prediction of human vascular response in hot environment under resting condition. J Med Syst. 2008;32(2):167-176.
8. Charkoudian N. Human hermoregulation from the autonomic perspective. Auton Neurosci. 2016;196:1-2.
9. Wendt D, van Loon LJ, Lichtenbelt WD. Thermoregulation during exercise in the heat: strategies for maintaining health and performance. Sports Med. 2007;37(8):669-682.
10. Falk B, Dotan R. Children’s thermoregulation during exercise in the heat: a revisit. Appl Physiol Nutr Metab. 2008;33(2):420-427.
11. Booth JN 3rd, Davis GG, Waterbor J, McGwin G Jr. Hyperthermia deaths among children in parked vehicles: an analysis of 231 fatalities in the United States, 1999-2007. Forensic Sci Med Pathol. 2010;6(2):99-105.
12. Levine M, LoVecchio F, Ruha AM, Chu G, Roque P. Influence of drug use on morbidity and mortality in heatstroke. J Med Toxicol. 2012;8(3):252-257.
13. O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. Common rashes. Am Fam Physician. 2008;77(1):47-52.
14. Howe AS, Boden BP. Heat-related illness in athletes. Am J Sports Med. 2007;35(8):1384-1395.
15. Bergeron MF. Muscle cramps during exercise – Is it fatigue or electrolyte deficit? Curr Sports Med Rep. 2008;7(4):S50-S55.
16. Glazer JL. Management of heatstroke and heat exhaustion. Am Fam Physician. 2005;71(11):2133-2140.
17. Sharma HS. Methods to produce hyperthermia-induced brain dysfunction. Prog Brain Res. 2007;162:173-199.
18. Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J Appl Physiol. 2010;109(6):1980-1988.
19. Wilson TE, Crandall CG. Effect of thermal stress on cardiac function. Exerc Sport Sci Rev. 2011;39(1):12-17.
20. Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29(1):17-24.
21. Lambert GP. Intestinal barrier dysfunction, endotoxemia, and gastrointestinal symptoms: the ‘canary in the coal mine’ during exercise-heat stress? Med Sport Sci. 2008;53:61-73.
22. Jardine DS. Heat illness and heat stroke. Pediatr Rev. 2007;28(7):249-258
23. Argaud L, Ferry T, Le QH, et al. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167(20):2177-2183.
24. Cheng TL, Partridge JC. Effect of bundling and high environmental temperature on neonatal body temperature. Pediatrics. 1993;92(2):238-240.
25. Bouchama A, Dehbi M, Chaves-Carballo E. Cooling and hemodynamic management in heatstroke: practical recommendations. Crit Care. 2007;11(3):R54.
26. Walker JS, Hogan DE. Heat emergencies. In: Tintinalli JE, Kelen GD, Stapczynski S. The American College of Emergency Physicians, eds. Emergency Medicine: A Comprehensive Study Guide, Section 15. China: The McGraw-Hill Companies, Inc; 2004:1183-1189.
27. Smith JE. Cooling methods used in the treatment of exertional heat illness. Br J Sports Med. 2005;39(8):503-507.
28. Rowland T. Fluid replacement requirements for child athletes. Sports Med. 2011;41(4):279-288.
29. National Weather Service, National Oceanic and Atmospheric Administration: NWS Heat Index. http://www.nws.noaa.gov/om/heat/heat_index.shtml. Accessed May 19, 2016.
30. Council on Sports Medicine and Fitness and Council on School Health; Bergeron MF, Devore C, Rice SG; American Academy of Pediatrics. Policy statement—Climatic heat stress and exercising children and adolescents. Pediatrics. 2011;128(3):e741-e777.
Heat-related illnesses in children encompass a wide range of disease processes—from minor conditions such as heat rash to life-threatening thermoregulatory emergencies such as heatstroke. Physiological differences in children compared to adults make them particularly susceptible to illnesses caused by heat exposure.
Pediatric heat-related illnesses can usually be prevented if appropriate precautions are taken (see “Taking Steps to Prevent Heat-Related Illnesses” box). In lieu of prevention, early recognition and treatment of heatstroke in children may drastically reduce life-threatening complications related to multisystem organ dysfunction. Management of heatstroke rests primarily on prompt initiation of rapid cooling measures and evaluation for organ dysfunction.
Case Scenarios
Case 1
An obese 10-year-old boy was brought to the ED by emergency medical services (EMS) during the first week of youth football tryouts. It was a hot day in late August, with 100% humidity and temperatures over 95°F. The patient, who weighed approximately 240 lb, was trying out for football but had no previous athletic-conditioning experience. Despite his obesity, he had been generally healthy and only took a stimulant medication for attention-deficit/hyperactivity disorder (ADHD).
At approximately noon, the boy collapsed on the field and had a seizure. When the EMS technicians arrived, they administered a dose of intramuscular (IM) midazolam. Although his seizure ceased, he remained obtunded and was intubated. A rectal temperature revealed a temperature of 105.8°F and paramedics noted that while the patient felt hot, he was no longer sweating. While en route to the ED, EMS technicians removed the patient’s football uniform; placed a fan in front of him; and sprayed cool water on him in an effort to lower his body temperature. At the time of arrival to the ED, his rectal temperature was 104.9°F.
Case 2
A previously healthy 3-month-old female infant was brought to the ED by EMS after she was accidentally left in a car on a summer day with a temperature of 90°F and 100% humidity. The infant’s father said that while running errands, he had forgotten his daughter was in the car and had left her in the rear facing backseat car carrier for approximately 10 minutes. When he returned to the car, he found his daughter awake but crying inconsolably. She had sweated through her clothes, vomited, and felt very hot, so he called 911. Her initial rectal temperature was 102.2°F, and her clothes were removed as she was being transported in an air-conditioned ambulance to the ED for further evaluation. Once undressed, she was noted to have an erythematous rash with multiple papules and pustules on her trunk.
Epidemiology
From 2006 to 2010, an average of 668 heat-related deaths per year occurred among people of all ages in the United States. Of these deaths, approximately 7% occurred in children younger than age 4 years (2.5% in those younger than age 1 year and 4.5% in those age 1-4 years). These figures have remained relatively stable over the last 10 years.1,2 Adolescents are particularly at risk for overexertion, and heatstroke is the third leading cause of death in young athletes, after traumatic and cardiac causes.3 As may be expected, most heat-related deaths (76%) occur in the southern and western regions of the United States.
Pathophysiology of Heat-Related Illnesses
The hypothalamus is the main control center for temperature homeostasis. As the core temperature rises due to either metabolic or environmental causes of heat, the hypothalamus primarily acts on the autonomic nervous system to engage mechanisms of heat dissipation.4 Evaporation of sweat is believed to be the most important mechanism of heat dissipation in humans; however, this method becomes less effective when humidity levels are above 75%.5 Radiation allows heat to transfer from the skin to the air, but is reliant on a temperature gradient. Conduction can allow heat to transfer to a cooler object through physical contact (as seen with cold-water immersion), while convection utilizes air movement to transfer heat (as illustrated by fanning).6
Thermoregulation is disrupted when the body is unable to balance metabolic heat production and heat dissipation. Heat dissipation mechanisms are easily overwhelmed when a person is exposed to excessive heat from the environment. The resulting stress from hyperthermia can directly injure cells, leading to a cytokine storm and endothelial injury. Heat can cause proteins to denature and cells to undergo apoptosis, which, if severe, can result in multisystem organ dysfunction.7
Physiological Differences in Children
Several physiological differences in children compared to adults compromise their ability to manage heat exposure. Thermoregulation in infants is less developed secondary to an immature hypothalamus; therefore, they are less able to utilize compensatory mechanisms to dissipate heat.8 In addition, infants and young children have a decreased sweating capacity, which makes evaporative cooling less effective.9 Children also produce more endogenous heat per kilogram than adults, which is believed to be secondary to a higher basal metabolic rate. They have less blood volume than adults, which decreases their ability to transfer warm blood into the periphery in order to cool the central core. Lastly, children have a higher surface area-to-body mass ratio, which causes increased heat absorption. All of these factors ultimately result in a slower rate of acclimatization in children compared to adults.10
Environmental Factors
Several environmental risk factors predispose children to heat-related illnesses. Infants are completely dependent on their caregivers for hydration and environmental protection from the heat. Infants who are over-bundled or left in a hot car are particularly at risk for heat-related illnesses.11 Older children are at risk for sports-related overexertion and typically must depend on permission from a coach or supervising adult to hydrate or take a break from exercise. Lastly, medications such as stimulants frequently prescribed for ADHD or medications with anticholinergic properties (secondary to decreased sweating) can predispose children to heat intolerance.12
Minor Heat-Related Illnesses
Heat-related illnesses range from benign conditions (eg, heat rash) to life-threatening processes (eg, heatstroke).
Miliaria Rubra
There are several forms of miliaria. Miliaria rubra, also known as heat rash or prickly heat, is a common, benign manifestation of heat exposure in infants and young children. A combination of heat exposure and obstructed sweat glands results in a pruritic, erythematous rash with papules and pustules (Figure). This is often seen in areas of friction from skin rubbing against skin or clothing.13
Heat Edema/Heat Cramps
Heat edema is another benign process related to heat exposure that generally occurs in older adults but can also occur in children. It is the result of peripheral vasodilation as the body attempts to shunt warm blood to the periphery.14 Heat cramps are a common manifestation in young athletes exercising in hot, summer conditions. Although benign, the cramps are very painful spasms that often affect large muscle groups, particularly in the legs, such as the calves, quadriceps, and hamstrings. There is conflicting data regarding the underlying cause of heat cramps. Many believe there is a significant component related to dehydration, while others attribute the cramps to fatigue or a combination
of the two.15
Heat Syncope
Heat syncope secondary to peripheral vasodilation, and venous pooling occurs as the body attempts to dissipate heat by transferring warm blood to the periphery. Relative dehydration plays a role in heat syncope, which is often precipitated by a rapid change in positioning during exercise, such as moving from a sitting to standing position. Heat syncope usually improves after the patient is supine, and children with heat syncope do not have an elevation in core body temperature.14 Some patients who experience heat syncope, however, may also have heat exhaustion.
Heat Exhaustion
Heat exhaustion occurs in patients with a known heat exposure. As opposed to the previously described processes, heat exhaustion is characterized by a body temperature elevated up to 104°F. Heat exhaustion is often accompanied by diffuse, nonspecific symptoms such as tachycardia, sweating, nausea, vomiting, weakness, fatigue, headache, and mild confusion. Dehydration often plays a significant role in heat exhaustion, but in contrast to heatstroke (described in the following section), mentation is normal, or there is a transient, mild confusion.16
Heatstroke
Heatstroke is observed in patients with a known heat exposure who have a temperature greater than 104°F accompanied by central nervous system (CNS) dysfunction.14 The CNS dysfunction involves an alteration in mental status manifested by slurred speech, ataxia, delirium, hallucinations, or seizure activity. In severe cases, obtundation or coma may result in airway compromise.17 Vital signs are unstable, and tachycardia and hypotension are often present. Patients with heatstroke may stop sweating, although the absence of sweating is not required for the diagnosis. Other nonspecific findings such as vomiting and diarrhea are common.6
The hallmark of heatstroke is multisystem organ dysfunction, which is caused by heat-induced tissue damage resulting in a systemic inflammatory response.18 Since the pediatric brain is particularly sensitive to temperature extremes, cerebral edema and herniation are potential complications of heatstroke.17 Damage to myocardial tissue, coupled with dehydration and systemic vasodilation, results in hypotension and poor systemic perfusion.19 Muscle breakdown causes rhabdomyolysis that can lead to kidney failure and hepatic injury. Degradation of clotting factors disrupts the clotting system and can cause disseminated intravascular coagulation (DIC).20 Damage to the mucosal lining of the intestines may result in ischemia and massive hematochezia.21
Heatstroke is classified as either nonexertional or exertional. Nonexertional heatstroke occurs most frequently in younger children who are exposed to a hot environment, such as an infant left in a car on a warm day. Exertional heatstroke occurs primarily in children exercising on a hot day, such as young athletes.6
Due to its significant morbidity and mortality, heatstroke is the most concerning manifestation of excessive heat exposure. The mortality rate for children with heatstroke is significantly lower than for adults; however, approximately 10% of children with heatstroke will not survive,22 and 20% will have long-term neurological disabilities, including permanent impairment in vision, speech, memory, behavior, and coordination.23
Management of Minor Heat-Related Illnesses
For most minor heat-related illnesses, supportive care is the mainstay of treatment (Table).
Miliaria Rubra
Infants with miliaria rubra typically improve once they are placed in a cool environment and their clothing is removed. In infants, lotions may contribute to sweat gland obstruction and should be used sparingly.13
Heat Edema/Heat Cramps
Similarly, heat edema generally improves once the child is removed from the hot environment and the extremities are elevated.14 Heat cramps are likely the result of fatigue and dehydration; therefore, these painful contractions often improve with rest, stretching, oral hydration, and removal from the hot environment. If cramps persist despite these measures, parenteral rehydration (20 mL/kg of normal saline) may be beneficial.15
Heat Syncope
Patients with orthostatic hypotension from heat syncope usually improve once they are resting in a cool environment and have been rehydrated. Pediatric oral rehydration with salt-containing fluids, such as commercial sports drinks, is safe; nonetheless, these patients may require intravenous (IV) rehydration with normal saline if orthostatic hypotension does not improve with oral rehydration alone.14
Heat Exhaustion
Differentiating heat exhaustion from heatstroke is of upmost importance because the treatment courses vary greatly. The difference in neurological status is the most effective way of differentiating the two diseases. All patients with slurred speech, ataxia, delirium, hallucinations, or seizure activity should be treated for presumptive heatstroke until proven otherwise (see “Management of Heatstroke” section).
Although children with heat exhaustion may have mild confusion, this tends to be transient and resolves with supportive care. Patients with heat exhaustion should stop exercising and be placed in a cool environment without excess clothing. Oral rehydration with salt-containing fluids is important, and most patients improve with these measures alone.
Children with apparent heat exhaustion who do not improve should be evaluated in the hospital setting, and laboratory studies should be obtained to evaluate for electrolyte abnormalities. Such patients typically warrant a 20 mL/kg IV bolus of normal saline. A complete neurological examination and a rectal temperature should be obtained on initial presentation.16
The evaluation of an overbundled infant with hyperthermia may be particularly challenging. Studies have demonstrated that it is possible for an infant to develop core temperature elevation if overbundled and placed in a warm environment.24 Nonetheless, it is important to address these patients with a broad differential diagnosis in mind, and always consider the possibility of sepsis. If the history and examination are consistent with hyperthermia secondary to heat exposure, a period of observation with supportive care may be a reasonable option. Infants should have a rectal temperature assessed every 15 to 30 minutes to monitor for improvement; if they improve with supportive care alone, a septic evaluation can be potentially avoided. Antipyretics will confuse the clinical picture and should be avoided in this situation.24
Management of Heatstroke
Significant morbidity and mortality are associated with heatstroke, and prompt recognition and initiation of therapy are required to prevent or minimize serious complications.22 As in any other life-threatening condition, the initial treatment of heatstroke requires support of the airway, breathing, and circulation. Patients are often neurologically unstable and cannot protect their airway, which should prompt endotracheal intubation. Children who are tachycardic and hypotensive should be resuscitated with normal saline prior to intubation if oxygenation and ventilation are maintained with supplemental oxygen alone. Most patients require at least 20 mL/kg of IV normal saline but many ultimately need up to 60 mL/kg.14 If blood pressure (BP) does not respond adequately to fluid resuscitation alone, vasopressors may be necessary. Seizure activity can be managed with IV benzodiazepines, such as lorazepam (0.1 mg/kg with maximum 4 mg per dose).14
Rapid cooling therapy is the mainstay of treatment for heatstroke and should be initiated as soon as the diagnosis is suspected, since morbidity and mortality correlates directly with the duration of hyperthermia. These measures are ideally started prior to arrival at the hospital. Evaporative cooling can be achieved in the field or ambulance with a cool water spray and air conditioning. Additionally, ice packs can be placed along the neck and axilla to augment rapid cooling measures and can be continued in the ED until the patient’s core temperature decreases to 101.4°F.25
Medications have a limited role in the treatment of heatstroke. Antipyretics such as acetaminophen and ibuprofen have no proven benefit and may exacerbate hepatic, gastrointestinal, clotting, and renal dysfunction.26 Benzodiazepines are helpful for seizure activity and may have a role in seizure prophylaxis. Dantrolene is not recommended for treating heatstroke as studies have not demonstrated a statistical improvement in cooling time, complications, or mortality.14 The use of chilled IV fluids instead of room-temperature fluids is not definitively supported in the literature.27
Further diagnostic evaluation is directed at determining the degree of multisystem organ dysfunction that results from heatstroke. A head computed tomography (CT) scan can evaluate for cerebral edema, whereas a comprehensive metabolic profile (CMP) will screen for electrolyte abnormalities such as hyponatremia (salt loss), hypernatremia (volume depletion), and possible transaminase elevation, which may indicate hepatic injury. Prolonged coagulation studies may reveal DIC and an arterial blood gas (ABG) analysis often may reveal metabolic acidosis. A serum creatine phosphokinase (CPK) and urinalysis (UA) can help to identify rhabdomyolysis or the presence of an acute kidney injury (AKI).16
After their condition is stabilized, children with heatstroke should be monitored in the pediatric intensive care unit (PICU) to effectively address complications of multisystem organ dysfunction.
Case Scenarios Continued
Case 1
[The 10-year-old boy who collapsed during football tryouts.]
The initial evaluation revealed an obese child who was intubated and obtunded. His vital signs included the following: rectal temperature, 104.9°F; heart rate (HR), 149 beats/minute; and BP, 82/36 mm Hg. Heatstroke was diagnosed and rapid cooling measures were initiated.
Evaporative heat loss was maintained with a fan and water spray, and ice packs were placed along the patient’s groin and axillae. Laboratory evaluation included a complete blood count (CBC), CMP, CPK, UA, coagulation panel, and ABG. A normal saline IV bolus at room temperature was given and a postintubation chest X-ray confirmed appropriate position of the endotracheal tube, without any evidence of acute respiratory distress syndrome (ARDS). A head CT scan did not reveal cerebral edema. Since the child’s BP and HR did not improve after the first normal saline bolus, he was given a total of 40 mL/kg of IV normal saline in the ED. The patient’s laboratory results were concerning for an AKI, with elevated CPK, hepatic injury, coagulopathy, and severe metabolic acidosis. He was subsequently admitted to the PICU for further care.
The child’s PICU course was complicated by multisystem organ failure, which ultimately included DIC, ARDS, acute renal failure requiring hemodialysis, and hypotension requiring vasopressors. A repeat head CT scan 3 days after admission revealed marked cerebral edema. The patient subsequently died within a week of presentation.
Case 2
[The 3-month-old girl who was left in a hot vehicle.]
The initial evaluation revealed a fussy infant with dry mucous membranes, elevated HR, and sunken fontanelle. Her rectal temperature on arrival to the ED was 100.7°F after conservative measures were taken (ie, removing her from the hot environment and removing her clothing). A peripheral IV was placed due to her clinical dehydration and she received a 20 mL/kg bolus of normal saline at room temperature. A glucose level was obtained and was normal. The patient’s rectal temperature was monitored every 30 minutes over the next 4 hours, and her temperature and HR gradually normalized.
The patient’s rash appeared consistent with miliaria rubra and improved as her temperature decreased. The infant underwent a brief period of observation in the ED where she continued to look well and tolerated oral fluids without vomiting. Neither a septic work-up nor empiric antibiotics were initiated, since heat exposure was felt to be the likely source of her core temperature elevation. Child Protective Services (CPS) was notified and opened a case for further evaluation of possible child neglect. The patient ultimately returned to her baseline in the ED and was discharged home with a family member, according to the safety plan outlined by CPS, and close follow-up with her pediatrician.
Heat-related illnesses in children encompass a wide range of disease processes—from minor conditions such as heat rash to life-threatening thermoregulatory emergencies such as heatstroke. Physiological differences in children compared to adults make them particularly susceptible to illnesses caused by heat exposure.
Pediatric heat-related illnesses can usually be prevented if appropriate precautions are taken (see “Taking Steps to Prevent Heat-Related Illnesses” box). In lieu of prevention, early recognition and treatment of heatstroke in children may drastically reduce life-threatening complications related to multisystem organ dysfunction. Management of heatstroke rests primarily on prompt initiation of rapid cooling measures and evaluation for organ dysfunction.
Case Scenarios
Case 1
An obese 10-year-old boy was brought to the ED by emergency medical services (EMS) during the first week of youth football tryouts. It was a hot day in late August, with 100% humidity and temperatures over 95°F. The patient, who weighed approximately 240 lb, was trying out for football but had no previous athletic-conditioning experience. Despite his obesity, he had been generally healthy and only took a stimulant medication for attention-deficit/hyperactivity disorder (ADHD).
At approximately noon, the boy collapsed on the field and had a seizure. When the EMS technicians arrived, they administered a dose of intramuscular (IM) midazolam. Although his seizure ceased, he remained obtunded and was intubated. A rectal temperature revealed a temperature of 105.8°F and paramedics noted that while the patient felt hot, he was no longer sweating. While en route to the ED, EMS technicians removed the patient’s football uniform; placed a fan in front of him; and sprayed cool water on him in an effort to lower his body temperature. At the time of arrival to the ED, his rectal temperature was 104.9°F.
Case 2
A previously healthy 3-month-old female infant was brought to the ED by EMS after she was accidentally left in a car on a summer day with a temperature of 90°F and 100% humidity. The infant’s father said that while running errands, he had forgotten his daughter was in the car and had left her in the rear facing backseat car carrier for approximately 10 minutes. When he returned to the car, he found his daughter awake but crying inconsolably. She had sweated through her clothes, vomited, and felt very hot, so he called 911. Her initial rectal temperature was 102.2°F, and her clothes were removed as she was being transported in an air-conditioned ambulance to the ED for further evaluation. Once undressed, she was noted to have an erythematous rash with multiple papules and pustules on her trunk.
Epidemiology
From 2006 to 2010, an average of 668 heat-related deaths per year occurred among people of all ages in the United States. Of these deaths, approximately 7% occurred in children younger than age 4 years (2.5% in those younger than age 1 year and 4.5% in those age 1-4 years). These figures have remained relatively stable over the last 10 years.1,2 Adolescents are particularly at risk for overexertion, and heatstroke is the third leading cause of death in young athletes, after traumatic and cardiac causes.3 As may be expected, most heat-related deaths (76%) occur in the southern and western regions of the United States.
Pathophysiology of Heat-Related Illnesses
The hypothalamus is the main control center for temperature homeostasis. As the core temperature rises due to either metabolic or environmental causes of heat, the hypothalamus primarily acts on the autonomic nervous system to engage mechanisms of heat dissipation.4 Evaporation of sweat is believed to be the most important mechanism of heat dissipation in humans; however, this method becomes less effective when humidity levels are above 75%.5 Radiation allows heat to transfer from the skin to the air, but is reliant on a temperature gradient. Conduction can allow heat to transfer to a cooler object through physical contact (as seen with cold-water immersion), while convection utilizes air movement to transfer heat (as illustrated by fanning).6
Thermoregulation is disrupted when the body is unable to balance metabolic heat production and heat dissipation. Heat dissipation mechanisms are easily overwhelmed when a person is exposed to excessive heat from the environment. The resulting stress from hyperthermia can directly injure cells, leading to a cytokine storm and endothelial injury. Heat can cause proteins to denature and cells to undergo apoptosis, which, if severe, can result in multisystem organ dysfunction.7
Physiological Differences in Children
Several physiological differences in children compared to adults compromise their ability to manage heat exposure. Thermoregulation in infants is less developed secondary to an immature hypothalamus; therefore, they are less able to utilize compensatory mechanisms to dissipate heat.8 In addition, infants and young children have a decreased sweating capacity, which makes evaporative cooling less effective.9 Children also produce more endogenous heat per kilogram than adults, which is believed to be secondary to a higher basal metabolic rate. They have less blood volume than adults, which decreases their ability to transfer warm blood into the periphery in order to cool the central core. Lastly, children have a higher surface area-to-body mass ratio, which causes increased heat absorption. All of these factors ultimately result in a slower rate of acclimatization in children compared to adults.10
Environmental Factors
Several environmental risk factors predispose children to heat-related illnesses. Infants are completely dependent on their caregivers for hydration and environmental protection from the heat. Infants who are over-bundled or left in a hot car are particularly at risk for heat-related illnesses.11 Older children are at risk for sports-related overexertion and typically must depend on permission from a coach or supervising adult to hydrate or take a break from exercise. Lastly, medications such as stimulants frequently prescribed for ADHD or medications with anticholinergic properties (secondary to decreased sweating) can predispose children to heat intolerance.12
Minor Heat-Related Illnesses
Heat-related illnesses range from benign conditions (eg, heat rash) to life-threatening processes (eg, heatstroke).
Miliaria Rubra
There are several forms of miliaria. Miliaria rubra, also known as heat rash or prickly heat, is a common, benign manifestation of heat exposure in infants and young children. A combination of heat exposure and obstructed sweat glands results in a pruritic, erythematous rash with papules and pustules (Figure). This is often seen in areas of friction from skin rubbing against skin or clothing.13
Heat Edema/Heat Cramps
Heat edema is another benign process related to heat exposure that generally occurs in older adults but can also occur in children. It is the result of peripheral vasodilation as the body attempts to shunt warm blood to the periphery.14 Heat cramps are a common manifestation in young athletes exercising in hot, summer conditions. Although benign, the cramps are very painful spasms that often affect large muscle groups, particularly in the legs, such as the calves, quadriceps, and hamstrings. There is conflicting data regarding the underlying cause of heat cramps. Many believe there is a significant component related to dehydration, while others attribute the cramps to fatigue or a combination
of the two.15
Heat Syncope
Heat syncope secondary to peripheral vasodilation, and venous pooling occurs as the body attempts to dissipate heat by transferring warm blood to the periphery. Relative dehydration plays a role in heat syncope, which is often precipitated by a rapid change in positioning during exercise, such as moving from a sitting to standing position. Heat syncope usually improves after the patient is supine, and children with heat syncope do not have an elevation in core body temperature.14 Some patients who experience heat syncope, however, may also have heat exhaustion.
Heat Exhaustion
Heat exhaustion occurs in patients with a known heat exposure. As opposed to the previously described processes, heat exhaustion is characterized by a body temperature elevated up to 104°F. Heat exhaustion is often accompanied by diffuse, nonspecific symptoms such as tachycardia, sweating, nausea, vomiting, weakness, fatigue, headache, and mild confusion. Dehydration often plays a significant role in heat exhaustion, but in contrast to heatstroke (described in the following section), mentation is normal, or there is a transient, mild confusion.16
Heatstroke
Heatstroke is observed in patients with a known heat exposure who have a temperature greater than 104°F accompanied by central nervous system (CNS) dysfunction.14 The CNS dysfunction involves an alteration in mental status manifested by slurred speech, ataxia, delirium, hallucinations, or seizure activity. In severe cases, obtundation or coma may result in airway compromise.17 Vital signs are unstable, and tachycardia and hypotension are often present. Patients with heatstroke may stop sweating, although the absence of sweating is not required for the diagnosis. Other nonspecific findings such as vomiting and diarrhea are common.6
The hallmark of heatstroke is multisystem organ dysfunction, which is caused by heat-induced tissue damage resulting in a systemic inflammatory response.18 Since the pediatric brain is particularly sensitive to temperature extremes, cerebral edema and herniation are potential complications of heatstroke.17 Damage to myocardial tissue, coupled with dehydration and systemic vasodilation, results in hypotension and poor systemic perfusion.19 Muscle breakdown causes rhabdomyolysis that can lead to kidney failure and hepatic injury. Degradation of clotting factors disrupts the clotting system and can cause disseminated intravascular coagulation (DIC).20 Damage to the mucosal lining of the intestines may result in ischemia and massive hematochezia.21
Heatstroke is classified as either nonexertional or exertional. Nonexertional heatstroke occurs most frequently in younger children who are exposed to a hot environment, such as an infant left in a car on a warm day. Exertional heatstroke occurs primarily in children exercising on a hot day, such as young athletes.6
Due to its significant morbidity and mortality, heatstroke is the most concerning manifestation of excessive heat exposure. The mortality rate for children with heatstroke is significantly lower than for adults; however, approximately 10% of children with heatstroke will not survive,22 and 20% will have long-term neurological disabilities, including permanent impairment in vision, speech, memory, behavior, and coordination.23
Management of Minor Heat-Related Illnesses
For most minor heat-related illnesses, supportive care is the mainstay of treatment (Table).
Miliaria Rubra
Infants with miliaria rubra typically improve once they are placed in a cool environment and their clothing is removed. In infants, lotions may contribute to sweat gland obstruction and should be used sparingly.13
Heat Edema/Heat Cramps
Similarly, heat edema generally improves once the child is removed from the hot environment and the extremities are elevated.14 Heat cramps are likely the result of fatigue and dehydration; therefore, these painful contractions often improve with rest, stretching, oral hydration, and removal from the hot environment. If cramps persist despite these measures, parenteral rehydration (20 mL/kg of normal saline) may be beneficial.15
Heat Syncope
Patients with orthostatic hypotension from heat syncope usually improve once they are resting in a cool environment and have been rehydrated. Pediatric oral rehydration with salt-containing fluids, such as commercial sports drinks, is safe; nonetheless, these patients may require intravenous (IV) rehydration with normal saline if orthostatic hypotension does not improve with oral rehydration alone.14
Heat Exhaustion
Differentiating heat exhaustion from heatstroke is of upmost importance because the treatment courses vary greatly. The difference in neurological status is the most effective way of differentiating the two diseases. All patients with slurred speech, ataxia, delirium, hallucinations, or seizure activity should be treated for presumptive heatstroke until proven otherwise (see “Management of Heatstroke” section).
Although children with heat exhaustion may have mild confusion, this tends to be transient and resolves with supportive care. Patients with heat exhaustion should stop exercising and be placed in a cool environment without excess clothing. Oral rehydration with salt-containing fluids is important, and most patients improve with these measures alone.
Children with apparent heat exhaustion who do not improve should be evaluated in the hospital setting, and laboratory studies should be obtained to evaluate for electrolyte abnormalities. Such patients typically warrant a 20 mL/kg IV bolus of normal saline. A complete neurological examination and a rectal temperature should be obtained on initial presentation.16
The evaluation of an overbundled infant with hyperthermia may be particularly challenging. Studies have demonstrated that it is possible for an infant to develop core temperature elevation if overbundled and placed in a warm environment.24 Nonetheless, it is important to address these patients with a broad differential diagnosis in mind, and always consider the possibility of sepsis. If the history and examination are consistent with hyperthermia secondary to heat exposure, a period of observation with supportive care may be a reasonable option. Infants should have a rectal temperature assessed every 15 to 30 minutes to monitor for improvement; if they improve with supportive care alone, a septic evaluation can be potentially avoided. Antipyretics will confuse the clinical picture and should be avoided in this situation.24
Management of Heatstroke
Significant morbidity and mortality are associated with heatstroke, and prompt recognition and initiation of therapy are required to prevent or minimize serious complications.22 As in any other life-threatening condition, the initial treatment of heatstroke requires support of the airway, breathing, and circulation. Patients are often neurologically unstable and cannot protect their airway, which should prompt endotracheal intubation. Children who are tachycardic and hypotensive should be resuscitated with normal saline prior to intubation if oxygenation and ventilation are maintained with supplemental oxygen alone. Most patients require at least 20 mL/kg of IV normal saline but many ultimately need up to 60 mL/kg.14 If blood pressure (BP) does not respond adequately to fluid resuscitation alone, vasopressors may be necessary. Seizure activity can be managed with IV benzodiazepines, such as lorazepam (0.1 mg/kg with maximum 4 mg per dose).14
Rapid cooling therapy is the mainstay of treatment for heatstroke and should be initiated as soon as the diagnosis is suspected, since morbidity and mortality correlates directly with the duration of hyperthermia. These measures are ideally started prior to arrival at the hospital. Evaporative cooling can be achieved in the field or ambulance with a cool water spray and air conditioning. Additionally, ice packs can be placed along the neck and axilla to augment rapid cooling measures and can be continued in the ED until the patient’s core temperature decreases to 101.4°F.25
Medications have a limited role in the treatment of heatstroke. Antipyretics such as acetaminophen and ibuprofen have no proven benefit and may exacerbate hepatic, gastrointestinal, clotting, and renal dysfunction.26 Benzodiazepines are helpful for seizure activity and may have a role in seizure prophylaxis. Dantrolene is not recommended for treating heatstroke as studies have not demonstrated a statistical improvement in cooling time, complications, or mortality.14 The use of chilled IV fluids instead of room-temperature fluids is not definitively supported in the literature.27
Further diagnostic evaluation is directed at determining the degree of multisystem organ dysfunction that results from heatstroke. A head computed tomography (CT) scan can evaluate for cerebral edema, whereas a comprehensive metabolic profile (CMP) will screen for electrolyte abnormalities such as hyponatremia (salt loss), hypernatremia (volume depletion), and possible transaminase elevation, which may indicate hepatic injury. Prolonged coagulation studies may reveal DIC and an arterial blood gas (ABG) analysis often may reveal metabolic acidosis. A serum creatine phosphokinase (CPK) and urinalysis (UA) can help to identify rhabdomyolysis or the presence of an acute kidney injury (AKI).16
After their condition is stabilized, children with heatstroke should be monitored in the pediatric intensive care unit (PICU) to effectively address complications of multisystem organ dysfunction.
Case Scenarios Continued
Case 1
[The 10-year-old boy who collapsed during football tryouts.]
The initial evaluation revealed an obese child who was intubated and obtunded. His vital signs included the following: rectal temperature, 104.9°F; heart rate (HR), 149 beats/minute; and BP, 82/36 mm Hg. Heatstroke was diagnosed and rapid cooling measures were initiated.
Evaporative heat loss was maintained with a fan and water spray, and ice packs were placed along the patient’s groin and axillae. Laboratory evaluation included a complete blood count (CBC), CMP, CPK, UA, coagulation panel, and ABG. A normal saline IV bolus at room temperature was given and a postintubation chest X-ray confirmed appropriate position of the endotracheal tube, without any evidence of acute respiratory distress syndrome (ARDS). A head CT scan did not reveal cerebral edema. Since the child’s BP and HR did not improve after the first normal saline bolus, he was given a total of 40 mL/kg of IV normal saline in the ED. The patient’s laboratory results were concerning for an AKI, with elevated CPK, hepatic injury, coagulopathy, and severe metabolic acidosis. He was subsequently admitted to the PICU for further care.
The child’s PICU course was complicated by multisystem organ failure, which ultimately included DIC, ARDS, acute renal failure requiring hemodialysis, and hypotension requiring vasopressors. A repeat head CT scan 3 days after admission revealed marked cerebral edema. The patient subsequently died within a week of presentation.
Case 2
[The 3-month-old girl who was left in a hot vehicle.]
The initial evaluation revealed a fussy infant with dry mucous membranes, elevated HR, and sunken fontanelle. Her rectal temperature on arrival to the ED was 100.7°F after conservative measures were taken (ie, removing her from the hot environment and removing her clothing). A peripheral IV was placed due to her clinical dehydration and she received a 20 mL/kg bolus of normal saline at room temperature. A glucose level was obtained and was normal. The patient’s rectal temperature was monitored every 30 minutes over the next 4 hours, and her temperature and HR gradually normalized.
The patient’s rash appeared consistent with miliaria rubra and improved as her temperature decreased. The infant underwent a brief period of observation in the ED where she continued to look well and tolerated oral fluids without vomiting. Neither a septic work-up nor empiric antibiotics were initiated, since heat exposure was felt to be the likely source of her core temperature elevation. Child Protective Services (CPS) was notified and opened a case for further evaluation of possible child neglect. The patient ultimately returned to her baseline in the ED and was discharged home with a family member, according to the safety plan outlined by CPS, and close follow-up with her pediatrician.
1. Berko J, Ingram DD, Saha S, Parker JD. Deaths attributed to heat, cold, and other weather events in the United States, 2006-2010. National health statistics reports; no 76. Hyattsville, MD: National Center for Health Statistics; 2014. http://www.cdc.gov/nchs/data/nhsr/nhsr076.pdf. Accessed May 22, 2016.
2. Centers for Disease Control and Prevention(CDC). Heat-related deaths--United States, 1999-2003. MMWR Morb Mortal Wkly Rep. 2006;55(29):796-798.
3. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119(8):1085-1092.
4. Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R37-R46.
5. Smith CJ, Johnson, JM. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: Neural control of skin blood flow and sweating in humans. Auton Neurosci. 2016;196:25-36.
6. Becker JA, Stewart LK. Heat-related illness. Am Fam Physician. 2011;83(11):1325-1330.
7. Aggarwal Y, Karan BM, Das BN, Sinha RK. Prediction of heat-illness symptoms with the prediction of human vascular response in hot environment under resting condition. J Med Syst. 2008;32(2):167-176.
8. Charkoudian N. Human hermoregulation from the autonomic perspective. Auton Neurosci. 2016;196:1-2.
9. Wendt D, van Loon LJ, Lichtenbelt WD. Thermoregulation during exercise in the heat: strategies for maintaining health and performance. Sports Med. 2007;37(8):669-682.
10. Falk B, Dotan R. Children’s thermoregulation during exercise in the heat: a revisit. Appl Physiol Nutr Metab. 2008;33(2):420-427.
11. Booth JN 3rd, Davis GG, Waterbor J, McGwin G Jr. Hyperthermia deaths among children in parked vehicles: an analysis of 231 fatalities in the United States, 1999-2007. Forensic Sci Med Pathol. 2010;6(2):99-105.
12. Levine M, LoVecchio F, Ruha AM, Chu G, Roque P. Influence of drug use on morbidity and mortality in heatstroke. J Med Toxicol. 2012;8(3):252-257.
13. O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. Common rashes. Am Fam Physician. 2008;77(1):47-52.
14. Howe AS, Boden BP. Heat-related illness in athletes. Am J Sports Med. 2007;35(8):1384-1395.
15. Bergeron MF. Muscle cramps during exercise – Is it fatigue or electrolyte deficit? Curr Sports Med Rep. 2008;7(4):S50-S55.
16. Glazer JL. Management of heatstroke and heat exhaustion. Am Fam Physician. 2005;71(11):2133-2140.
17. Sharma HS. Methods to produce hyperthermia-induced brain dysfunction. Prog Brain Res. 2007;162:173-199.
18. Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J Appl Physiol. 2010;109(6):1980-1988.
19. Wilson TE, Crandall CG. Effect of thermal stress on cardiac function. Exerc Sport Sci Rev. 2011;39(1):12-17.
20. Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29(1):17-24.
21. Lambert GP. Intestinal barrier dysfunction, endotoxemia, and gastrointestinal symptoms: the ‘canary in the coal mine’ during exercise-heat stress? Med Sport Sci. 2008;53:61-73.
22. Jardine DS. Heat illness and heat stroke. Pediatr Rev. 2007;28(7):249-258
23. Argaud L, Ferry T, Le QH, et al. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167(20):2177-2183.
24. Cheng TL, Partridge JC. Effect of bundling and high environmental temperature on neonatal body temperature. Pediatrics. 1993;92(2):238-240.
25. Bouchama A, Dehbi M, Chaves-Carballo E. Cooling and hemodynamic management in heatstroke: practical recommendations. Crit Care. 2007;11(3):R54.
26. Walker JS, Hogan DE. Heat emergencies. In: Tintinalli JE, Kelen GD, Stapczynski S. The American College of Emergency Physicians, eds. Emergency Medicine: A Comprehensive Study Guide, Section 15. China: The McGraw-Hill Companies, Inc; 2004:1183-1189.
27. Smith JE. Cooling methods used in the treatment of exertional heat illness. Br J Sports Med. 2005;39(8):503-507.
28. Rowland T. Fluid replacement requirements for child athletes. Sports Med. 2011;41(4):279-288.
29. National Weather Service, National Oceanic and Atmospheric Administration: NWS Heat Index. http://www.nws.noaa.gov/om/heat/heat_index.shtml. Accessed May 19, 2016.
30. Council on Sports Medicine and Fitness and Council on School Health; Bergeron MF, Devore C, Rice SG; American Academy of Pediatrics. Policy statement—Climatic heat stress and exercising children and adolescents. Pediatrics. 2011;128(3):e741-e777.
1. Berko J, Ingram DD, Saha S, Parker JD. Deaths attributed to heat, cold, and other weather events in the United States, 2006-2010. National health statistics reports; no 76. Hyattsville, MD: National Center for Health Statistics; 2014. http://www.cdc.gov/nchs/data/nhsr/nhsr076.pdf. Accessed May 22, 2016.
2. Centers for Disease Control and Prevention(CDC). Heat-related deaths--United States, 1999-2003. MMWR Morb Mortal Wkly Rep. 2006;55(29):796-798.
3. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119(8):1085-1092.
4. Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R37-R46.
5. Smith CJ, Johnson, JM. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: Neural control of skin blood flow and sweating in humans. Auton Neurosci. 2016;196:25-36.
6. Becker JA, Stewart LK. Heat-related illness. Am Fam Physician. 2011;83(11):1325-1330.
7. Aggarwal Y, Karan BM, Das BN, Sinha RK. Prediction of heat-illness symptoms with the prediction of human vascular response in hot environment under resting condition. J Med Syst. 2008;32(2):167-176.
8. Charkoudian N. Human hermoregulation from the autonomic perspective. Auton Neurosci. 2016;196:1-2.
9. Wendt D, van Loon LJ, Lichtenbelt WD. Thermoregulation during exercise in the heat: strategies for maintaining health and performance. Sports Med. 2007;37(8):669-682.
10. Falk B, Dotan R. Children’s thermoregulation during exercise in the heat: a revisit. Appl Physiol Nutr Metab. 2008;33(2):420-427.
11. Booth JN 3rd, Davis GG, Waterbor J, McGwin G Jr. Hyperthermia deaths among children in parked vehicles: an analysis of 231 fatalities in the United States, 1999-2007. Forensic Sci Med Pathol. 2010;6(2):99-105.
12. Levine M, LoVecchio F, Ruha AM, Chu G, Roque P. Influence of drug use on morbidity and mortality in heatstroke. J Med Toxicol. 2012;8(3):252-257.
13. O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. Common rashes. Am Fam Physician. 2008;77(1):47-52.
14. Howe AS, Boden BP. Heat-related illness in athletes. Am J Sports Med. 2007;35(8):1384-1395.
15. Bergeron MF. Muscle cramps during exercise – Is it fatigue or electrolyte deficit? Curr Sports Med Rep. 2008;7(4):S50-S55.
16. Glazer JL. Management of heatstroke and heat exhaustion. Am Fam Physician. 2005;71(11):2133-2140.
17. Sharma HS. Methods to produce hyperthermia-induced brain dysfunction. Prog Brain Res. 2007;162:173-199.
18. Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J Appl Physiol. 2010;109(6):1980-1988.
19. Wilson TE, Crandall CG. Effect of thermal stress on cardiac function. Exerc Sport Sci Rev. 2011;39(1):12-17.
20. Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29(1):17-24.
21. Lambert GP. Intestinal barrier dysfunction, endotoxemia, and gastrointestinal symptoms: the ‘canary in the coal mine’ during exercise-heat stress? Med Sport Sci. 2008;53:61-73.
22. Jardine DS. Heat illness and heat stroke. Pediatr Rev. 2007;28(7):249-258
23. Argaud L, Ferry T, Le QH, et al. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167(20):2177-2183.
24. Cheng TL, Partridge JC. Effect of bundling and high environmental temperature on neonatal body temperature. Pediatrics. 1993;92(2):238-240.
25. Bouchama A, Dehbi M, Chaves-Carballo E. Cooling and hemodynamic management in heatstroke: practical recommendations. Crit Care. 2007;11(3):R54.
26. Walker JS, Hogan DE. Heat emergencies. In: Tintinalli JE, Kelen GD, Stapczynski S. The American College of Emergency Physicians, eds. Emergency Medicine: A Comprehensive Study Guide, Section 15. China: The McGraw-Hill Companies, Inc; 2004:1183-1189.
27. Smith JE. Cooling methods used in the treatment of exertional heat illness. Br J Sports Med. 2005;39(8):503-507.
28. Rowland T. Fluid replacement requirements for child athletes. Sports Med. 2011;41(4):279-288.
29. National Weather Service, National Oceanic and Atmospheric Administration: NWS Heat Index. http://www.nws.noaa.gov/om/heat/heat_index.shtml. Accessed May 19, 2016.
30. Council on Sports Medicine and Fitness and Council on School Health; Bergeron MF, Devore C, Rice SG; American Academy of Pediatrics. Policy statement—Climatic heat stress and exercising children and adolescents. Pediatrics. 2011;128(3):e741-e777.
Novel Melanoma Therapies and Their Side Effects
In the last few years, melanoma treatment has been revolutionized by the development of immune checkpoint–blocking antibodies or immune checkpoint inhibitors. These drugs act through receptor or ligand blockades at certain points along the immunologic cascade to enhance the immune system’s ability to fight malignancies.1 In 2011, the US Food and Drug Administration approved ipilimumab, an inhibitor of cytotoxic T-lymphocyte antigen 4 (CTLA-4), for treatment of patients with unresectable or metastatic melanoma. Other immune-modulating agents followed thereafter. Vemurafenib and dabrafenib, 2 selective BRAF inhibitors, were approved in 2011 and 2013, respectively, and trametinib, a mitogen-activated extracellular signal-regulated kinase 1 (MEK1) and MEK2 blocker, was approved in 2013. These agents are being used to treat patients with activating BRAF or NRAS mutations.2,3 Nivolumab and pembrolizumab, which target programmed death receptor-1 (PD-1) and programmed death ligand 1 (PD-L1), respectively, were approved in 2014. Furthermore, phase 2 and 3 trials are ongoing for patients with unresectable stage III or IV melanomas harboring activating c-KIT mutations, which are rare and usually are found in acral or mucosal melanomas. The multikinase inhibitors imatinib, sunitinib, dasatinib, and nilotinib are being used in clinical trials for this purpose and are not yet approved.4
Although immune checkpoint inhibitors have shown promising results, they lack direct activity against malignant cells. The nonspecific enhanced immune system response promoted by these drugs has been shown to cause multiple adverse events (AEs). A subset of these side effects has been termed immune-related AEs (irAEs), which occur secondary to reduced tolerance to antigens previously recognized as self-antigens, leading to immune-related side effects.5 The majority of these AEs involve the skin and are mild to moderate in severity; however, other organ systems (eg, gastrointestinal, hepatic, endocrine, and neurologic systems) also may be affected. Most of the toxicities have been successfully treated with immunosuppressive agents such as corticosteroids, tumor necrosis factor α antagonists, and mycophenolate mofetil.6
Dermatologic Side Effects
The most common AEs associated with immune checkpoint inhibitors are cutaneous reactions, which commonly present after 2 to 3 weeks of treatment.7 Approximately 50% of patients receiving ipilimumab (CTLA-4 inhibitor) will experience cutaneous reactions, including erythematous, reticulated, or maculopapular rashes.8 Vitiligo and Sweet syndrome also have been observed.9,10
Antibodies against PD-1 and PD-L1 have been associated with oral mucositis and dry mouth.11 Most patients treated with BRAF, MEK, and KIT inhibitors also experience dermatologic AEs. Rashes caused by BRAF inhibitors commonly are maculopapular to verrucous and hyperkeratotic. Keratoacanthomas, squamous cell carcinomas, and other hyperkeratotic lesions such as verruca vulgaris, actinic keratoses, and milia have been reported, usually in sun-exposed areas.4,12,13 Other types of keratotic lesions have been observed, such as areolar hyperkeratosis with vemurafenib (BRAF inhibitor).14 Photosensitivity, panniculitis (eg, erythema nodosum), and mild alopecia also have been reported.15 Radiosensitization and radiation recall also have been reported in patients treated with BRAF inhibitors.16-19 Cutaneous reactions observed with MEK inhibitors are acneiform to papulopustular and appear in seborrheic areas such as the face and chest.4 In contrast to BRAF inhibitors, increased rates of squamous cell carcinomas and keratoacanthomas have not been reported with MEK inhibitors. Severe cutaneous effects such as toxic epidermal necrolysis and Stevens-Johnson syndrome may occur, and although rare, treatment should be discontinued in these cases.
Gastrointestinal Tract Side Effects
Gastrointestinal (GI) tract side effects commonly result from treatment with immunomodulators, usually occurring after 6 to 7 weeks.7 Most patients will experience mild to moderate GI adverse effects (eg, diarrhea), but a few patients have had episodes of colitis, some of which have been fatal.20 Diarrhea and other GI effects are more common in patients treated with ipilimumab, occurring in approximately 30% of patients,20 in comparison to 1% to 2% of those treated with PD-1 and PD-L1 inhibitors.11,21
Liver abnormalities and asymptomatic elevations in liver enzymes can occur with KIT, BRAF, CTLA-4, and PD-L1 inhibitors.11,20-23 More serious abnormalities such as symptomatic hepatitis and fever are mostly seen with CTLA-4 inhibitors.
Endocrinologic Side Effects
Immune-related AEs also can affect the pituitary, adrenal, and thyroid glands. These events occur after an average of 9 weeks and usually consist of nausea, headache, and/or fatigue.7 Hypophysitis and hypothyroidism are the most common endocrinopathies reported based on characteristic laboratory or radiographic findings and are observed most often with CTLA-4 inhibitors, though they also have been reported with PD-1/PD-L1 blockers.24,25 Ipilimumab-induced thyrotoxicosis also has been reported, though it is far less common than hypothyroidism.26
Other Side Effects
Other irAEs that are less common include neurologic side effects ranging from Bell palsy27 and Guillain-Barré syndrome20 to paresthesia, as well as pancreatitis,28 ophthalmologic reactions,29-33 nephritis,34,35 and hematologic side effects.36-38 One distinctive AE is lung toxicity, which has been reported with PD-1 inhibitors and presents as cough, dyspnea, or pneumonitis early in treatment.21
It is unclear whether immunomodulating agents exacerbate autoimmune diseases. Patients with autoimmune diseases were not included in the clinical trials but reportedly have been treated with ipilimumab without exacerbations. Nevertheless, there has been a report of worsening multiple sclerosis in a melanoma patient treated with ipilimumab.39
Conclusion
Immunomodulators have dramatically improved the survival and care of patients with unresectable melanomas. Because of their mechanism of action, they have the capability to produce substantial toxicity. Although most AEs are mild, lethal side effects can ensue. Therefore, all specialists treating patients with melanoma should be familiar with these side effects and their treatment options, as survival rates and survival times will be increasing over the next few years. Rapid AE identification and treatment can improve patient outcomes and optimize the therapeutic potential of these medications. Because immune checkpoint inhibitors are fairly new, further studies are needed to assess irAEs and the long-term impact in patients treated with immunomodulators.
- Ito A, Kondo S, Tada K, et al. Clinical development of immune checkpoint inhibitors. Biomed Res Int. 2015;2015:605478.
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694-1703.
- Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14:249-256.
- Livingstone E, Zimmer L, Vaubel J, et al. BRAF, MEK and KIT inhibitors for melanoma: adverse events and their management. Chin Clin Oncol. 2014;3:29.
- Schmerling RA. Toxicity of checkpoint inhibitors. Chin Clin Oncol. 2014;3:31.
- Yervoy [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2011.
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
- Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-169.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study [published online December 8, 2009]. Lancet Oncol. 2010;11:155-164.
- Pintova S, Sidhu H, Friedlander PA, et al. Sweet’s syndrome in a patient with metastatic melanoma after ipilimumab therapy. Melanoma Res. 2013;23:498-501.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Lacouture ME, Duvic M, Hauschild A, et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist. 2013;18:314-322.
- Martinez-Garcia E, Taibjee S, Koch D, et al. Vemurafenib-induced hyperkeratosis of the areola treated with topical adapalene [published online February 22, 2015]. Clin Exp Dermatol. 2016;41:148-151.
- Sanlorenzo M, Choudhry A, Vujic I, et al. Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma. J Am Acad Dermatol. 2014;71:1102-1109.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Ducassou A, David I, Delannes M, et al. Radiosensitization induced by vemurafenib. Cancer Radiother. 2013;17:304-307.
- Peuvrel L, Ruellan AL, Thillays F, et al. Severe radiotherapy-induced extracutaneous toxicity under vemurafenib. Eur J Dermatol. 2013;23:879-881.
- Satzger I, Degen A, Asper H, et al. Serious skin toxicity with the combination of BRAF inhibitors and radiotherapy. J Clin Oncol. 2013;31:e220-e222.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
- Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616-622.
- Corsello SM, Barnabei A, Marchetti P, et al. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98:1361-1375.
- Ryder M, Callahan M, Postow MA, et al. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371-381.
- Yu C, Chopra IJ, Ha E. A novel melanoma therapy stirs up a storm: ipilimumab-induced thyrotoxicosis. Endocrinol Diabetes Metab Case Rep. 2015;2015:140092.
- Klein O, Ribas A, Chmielowski B, et al. Facial palsy as a side effect of vemurafenib treatment in patients with metastatic melanoma. J Clin Oncol. 2013;31:e215-e217.
- Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: case report. Pharmacotherapy. 2013;33:e43-e44.
- Flaherty L, Hamid O, Linette G, et al. A single-arm, open-label, expanded access study of vemurafenib in patients with metastatic melanoma in the United States. Cancer J. 2014;20:18-24.
- Wolf SE, Meenken C, Moll AC, et al. Severe pan-uveitis in a patient treated with vemurafenib for metastatic melanoma. BMC Cancer. 2013;13:561.
- Sandhu SS, Ling C, Lim L, et al. Vemurafenib (B-RAF inhibitor) associated uveitis in patients with metastatic cutaneous melanoma. Clin Exp Ophthalmol. 2012;40:118.
- Joshi L, Karydis A, Gemenetzi M, et al. Uveitis as a result of MAP kinase pathway inhibition. Case Rep Ophthalmol. 2013;4:279-282.
- Robinson MR, Chan CC, Yang JC, et al. Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: a new cause of uveitis. J Immunother. 2004;27:478-479.
- Regnier-Rosencher E, Lazareth H, Gressier L, et al. Acute kidney injury in patients with severe rash on vemurafenib treatment for metastatic melanomas. Br J Dermatol. 2013;169:934-938.
- Izzedine H, Gueutin V, Gharbi C, et al. Kidney injuries related to ipilimumab [published online April 1, 2014]. Invest New Drugs. 2014;32:769-773.
- Akhtari M, Waller EK, Jaye DL, et al. Neutropenia in a patient treated with ipilimumab (anti-CTLA-4 antibody). J Immunother. 2009;32:322-324.
- Gordon IO, Wade T, Chin K, et al. Immune mediated red cell aplasia after anti-CTLA-4 immunotherapy for metastatic melanoma. Cancer Immunol Immunother. 2009;58:1351-1353.
- Kopecký J, Trojanová P, Kubeček O, et al. Treatment possibilities of ipilimumab-induced thrombocytopenia—case study and literature review. Jpn J Clin Oncol. 2015;45:381-384.
- Gettings EJ, Hackett CT, Scott TF. Severe relapse in a multiple sclerosis patient associated with ipilimumab treatment of melanoma. Mult Scler. 2015;21:670.
In the last few years, melanoma treatment has been revolutionized by the development of immune checkpoint–blocking antibodies or immune checkpoint inhibitors. These drugs act through receptor or ligand blockades at certain points along the immunologic cascade to enhance the immune system’s ability to fight malignancies.1 In 2011, the US Food and Drug Administration approved ipilimumab, an inhibitor of cytotoxic T-lymphocyte antigen 4 (CTLA-4), for treatment of patients with unresectable or metastatic melanoma. Other immune-modulating agents followed thereafter. Vemurafenib and dabrafenib, 2 selective BRAF inhibitors, were approved in 2011 and 2013, respectively, and trametinib, a mitogen-activated extracellular signal-regulated kinase 1 (MEK1) and MEK2 blocker, was approved in 2013. These agents are being used to treat patients with activating BRAF or NRAS mutations.2,3 Nivolumab and pembrolizumab, which target programmed death receptor-1 (PD-1) and programmed death ligand 1 (PD-L1), respectively, were approved in 2014. Furthermore, phase 2 and 3 trials are ongoing for patients with unresectable stage III or IV melanomas harboring activating c-KIT mutations, which are rare and usually are found in acral or mucosal melanomas. The multikinase inhibitors imatinib, sunitinib, dasatinib, and nilotinib are being used in clinical trials for this purpose and are not yet approved.4
Although immune checkpoint inhibitors have shown promising results, they lack direct activity against malignant cells. The nonspecific enhanced immune system response promoted by these drugs has been shown to cause multiple adverse events (AEs). A subset of these side effects has been termed immune-related AEs (irAEs), which occur secondary to reduced tolerance to antigens previously recognized as self-antigens, leading to immune-related side effects.5 The majority of these AEs involve the skin and are mild to moderate in severity; however, other organ systems (eg, gastrointestinal, hepatic, endocrine, and neurologic systems) also may be affected. Most of the toxicities have been successfully treated with immunosuppressive agents such as corticosteroids, tumor necrosis factor α antagonists, and mycophenolate mofetil.6
Dermatologic Side Effects
The most common AEs associated with immune checkpoint inhibitors are cutaneous reactions, which commonly present after 2 to 3 weeks of treatment.7 Approximately 50% of patients receiving ipilimumab (CTLA-4 inhibitor) will experience cutaneous reactions, including erythematous, reticulated, or maculopapular rashes.8 Vitiligo and Sweet syndrome also have been observed.9,10
Antibodies against PD-1 and PD-L1 have been associated with oral mucositis and dry mouth.11 Most patients treated with BRAF, MEK, and KIT inhibitors also experience dermatologic AEs. Rashes caused by BRAF inhibitors commonly are maculopapular to verrucous and hyperkeratotic. Keratoacanthomas, squamous cell carcinomas, and other hyperkeratotic lesions such as verruca vulgaris, actinic keratoses, and milia have been reported, usually in sun-exposed areas.4,12,13 Other types of keratotic lesions have been observed, such as areolar hyperkeratosis with vemurafenib (BRAF inhibitor).14 Photosensitivity, panniculitis (eg, erythema nodosum), and mild alopecia also have been reported.15 Radiosensitization and radiation recall also have been reported in patients treated with BRAF inhibitors.16-19 Cutaneous reactions observed with MEK inhibitors are acneiform to papulopustular and appear in seborrheic areas such as the face and chest.4 In contrast to BRAF inhibitors, increased rates of squamous cell carcinomas and keratoacanthomas have not been reported with MEK inhibitors. Severe cutaneous effects such as toxic epidermal necrolysis and Stevens-Johnson syndrome may occur, and although rare, treatment should be discontinued in these cases.
Gastrointestinal Tract Side Effects
Gastrointestinal (GI) tract side effects commonly result from treatment with immunomodulators, usually occurring after 6 to 7 weeks.7 Most patients will experience mild to moderate GI adverse effects (eg, diarrhea), but a few patients have had episodes of colitis, some of which have been fatal.20 Diarrhea and other GI effects are more common in patients treated with ipilimumab, occurring in approximately 30% of patients,20 in comparison to 1% to 2% of those treated with PD-1 and PD-L1 inhibitors.11,21
Liver abnormalities and asymptomatic elevations in liver enzymes can occur with KIT, BRAF, CTLA-4, and PD-L1 inhibitors.11,20-23 More serious abnormalities such as symptomatic hepatitis and fever are mostly seen with CTLA-4 inhibitors.
Endocrinologic Side Effects
Immune-related AEs also can affect the pituitary, adrenal, and thyroid glands. These events occur after an average of 9 weeks and usually consist of nausea, headache, and/or fatigue.7 Hypophysitis and hypothyroidism are the most common endocrinopathies reported based on characteristic laboratory or radiographic findings and are observed most often with CTLA-4 inhibitors, though they also have been reported with PD-1/PD-L1 blockers.24,25 Ipilimumab-induced thyrotoxicosis also has been reported, though it is far less common than hypothyroidism.26
Other Side Effects
Other irAEs that are less common include neurologic side effects ranging from Bell palsy27 and Guillain-Barré syndrome20 to paresthesia, as well as pancreatitis,28 ophthalmologic reactions,29-33 nephritis,34,35 and hematologic side effects.36-38 One distinctive AE is lung toxicity, which has been reported with PD-1 inhibitors and presents as cough, dyspnea, or pneumonitis early in treatment.21
It is unclear whether immunomodulating agents exacerbate autoimmune diseases. Patients with autoimmune diseases were not included in the clinical trials but reportedly have been treated with ipilimumab without exacerbations. Nevertheless, there has been a report of worsening multiple sclerosis in a melanoma patient treated with ipilimumab.39
Conclusion
Immunomodulators have dramatically improved the survival and care of patients with unresectable melanomas. Because of their mechanism of action, they have the capability to produce substantial toxicity. Although most AEs are mild, lethal side effects can ensue. Therefore, all specialists treating patients with melanoma should be familiar with these side effects and their treatment options, as survival rates and survival times will be increasing over the next few years. Rapid AE identification and treatment can improve patient outcomes and optimize the therapeutic potential of these medications. Because immune checkpoint inhibitors are fairly new, further studies are needed to assess irAEs and the long-term impact in patients treated with immunomodulators.
In the last few years, melanoma treatment has been revolutionized by the development of immune checkpoint–blocking antibodies or immune checkpoint inhibitors. These drugs act through receptor or ligand blockades at certain points along the immunologic cascade to enhance the immune system’s ability to fight malignancies.1 In 2011, the US Food and Drug Administration approved ipilimumab, an inhibitor of cytotoxic T-lymphocyte antigen 4 (CTLA-4), for treatment of patients with unresectable or metastatic melanoma. Other immune-modulating agents followed thereafter. Vemurafenib and dabrafenib, 2 selective BRAF inhibitors, were approved in 2011 and 2013, respectively, and trametinib, a mitogen-activated extracellular signal-regulated kinase 1 (MEK1) and MEK2 blocker, was approved in 2013. These agents are being used to treat patients with activating BRAF or NRAS mutations.2,3 Nivolumab and pembrolizumab, which target programmed death receptor-1 (PD-1) and programmed death ligand 1 (PD-L1), respectively, were approved in 2014. Furthermore, phase 2 and 3 trials are ongoing for patients with unresectable stage III or IV melanomas harboring activating c-KIT mutations, which are rare and usually are found in acral or mucosal melanomas. The multikinase inhibitors imatinib, sunitinib, dasatinib, and nilotinib are being used in clinical trials for this purpose and are not yet approved.4
Although immune checkpoint inhibitors have shown promising results, they lack direct activity against malignant cells. The nonspecific enhanced immune system response promoted by these drugs has been shown to cause multiple adverse events (AEs). A subset of these side effects has been termed immune-related AEs (irAEs), which occur secondary to reduced tolerance to antigens previously recognized as self-antigens, leading to immune-related side effects.5 The majority of these AEs involve the skin and are mild to moderate in severity; however, other organ systems (eg, gastrointestinal, hepatic, endocrine, and neurologic systems) also may be affected. Most of the toxicities have been successfully treated with immunosuppressive agents such as corticosteroids, tumor necrosis factor α antagonists, and mycophenolate mofetil.6
Dermatologic Side Effects
The most common AEs associated with immune checkpoint inhibitors are cutaneous reactions, which commonly present after 2 to 3 weeks of treatment.7 Approximately 50% of patients receiving ipilimumab (CTLA-4 inhibitor) will experience cutaneous reactions, including erythematous, reticulated, or maculopapular rashes.8 Vitiligo and Sweet syndrome also have been observed.9,10
Antibodies against PD-1 and PD-L1 have been associated with oral mucositis and dry mouth.11 Most patients treated with BRAF, MEK, and KIT inhibitors also experience dermatologic AEs. Rashes caused by BRAF inhibitors commonly are maculopapular to verrucous and hyperkeratotic. Keratoacanthomas, squamous cell carcinomas, and other hyperkeratotic lesions such as verruca vulgaris, actinic keratoses, and milia have been reported, usually in sun-exposed areas.4,12,13 Other types of keratotic lesions have been observed, such as areolar hyperkeratosis with vemurafenib (BRAF inhibitor).14 Photosensitivity, panniculitis (eg, erythema nodosum), and mild alopecia also have been reported.15 Radiosensitization and radiation recall also have been reported in patients treated with BRAF inhibitors.16-19 Cutaneous reactions observed with MEK inhibitors are acneiform to papulopustular and appear in seborrheic areas such as the face and chest.4 In contrast to BRAF inhibitors, increased rates of squamous cell carcinomas and keratoacanthomas have not been reported with MEK inhibitors. Severe cutaneous effects such as toxic epidermal necrolysis and Stevens-Johnson syndrome may occur, and although rare, treatment should be discontinued in these cases.
Gastrointestinal Tract Side Effects
Gastrointestinal (GI) tract side effects commonly result from treatment with immunomodulators, usually occurring after 6 to 7 weeks.7 Most patients will experience mild to moderate GI adverse effects (eg, diarrhea), but a few patients have had episodes of colitis, some of which have been fatal.20 Diarrhea and other GI effects are more common in patients treated with ipilimumab, occurring in approximately 30% of patients,20 in comparison to 1% to 2% of those treated with PD-1 and PD-L1 inhibitors.11,21
Liver abnormalities and asymptomatic elevations in liver enzymes can occur with KIT, BRAF, CTLA-4, and PD-L1 inhibitors.11,20-23 More serious abnormalities such as symptomatic hepatitis and fever are mostly seen with CTLA-4 inhibitors.
Endocrinologic Side Effects
Immune-related AEs also can affect the pituitary, adrenal, and thyroid glands. These events occur after an average of 9 weeks and usually consist of nausea, headache, and/or fatigue.7 Hypophysitis and hypothyroidism are the most common endocrinopathies reported based on characteristic laboratory or radiographic findings and are observed most often with CTLA-4 inhibitors, though they also have been reported with PD-1/PD-L1 blockers.24,25 Ipilimumab-induced thyrotoxicosis also has been reported, though it is far less common than hypothyroidism.26
Other Side Effects
Other irAEs that are less common include neurologic side effects ranging from Bell palsy27 and Guillain-Barré syndrome20 to paresthesia, as well as pancreatitis,28 ophthalmologic reactions,29-33 nephritis,34,35 and hematologic side effects.36-38 One distinctive AE is lung toxicity, which has been reported with PD-1 inhibitors and presents as cough, dyspnea, or pneumonitis early in treatment.21
It is unclear whether immunomodulating agents exacerbate autoimmune diseases. Patients with autoimmune diseases were not included in the clinical trials but reportedly have been treated with ipilimumab without exacerbations. Nevertheless, there has been a report of worsening multiple sclerosis in a melanoma patient treated with ipilimumab.39
Conclusion
Immunomodulators have dramatically improved the survival and care of patients with unresectable melanomas. Because of their mechanism of action, they have the capability to produce substantial toxicity. Although most AEs are mild, lethal side effects can ensue. Therefore, all specialists treating patients with melanoma should be familiar with these side effects and their treatment options, as survival rates and survival times will be increasing over the next few years. Rapid AE identification and treatment can improve patient outcomes and optimize the therapeutic potential of these medications. Because immune checkpoint inhibitors are fairly new, further studies are needed to assess irAEs and the long-term impact in patients treated with immunomodulators.
- Ito A, Kondo S, Tada K, et al. Clinical development of immune checkpoint inhibitors. Biomed Res Int. 2015;2015:605478.
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694-1703.
- Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14:249-256.
- Livingstone E, Zimmer L, Vaubel J, et al. BRAF, MEK and KIT inhibitors for melanoma: adverse events and their management. Chin Clin Oncol. 2014;3:29.
- Schmerling RA. Toxicity of checkpoint inhibitors. Chin Clin Oncol. 2014;3:31.
- Yervoy [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2011.
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
- Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-169.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study [published online December 8, 2009]. Lancet Oncol. 2010;11:155-164.
- Pintova S, Sidhu H, Friedlander PA, et al. Sweet’s syndrome in a patient with metastatic melanoma after ipilimumab therapy. Melanoma Res. 2013;23:498-501.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Lacouture ME, Duvic M, Hauschild A, et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist. 2013;18:314-322.
- Martinez-Garcia E, Taibjee S, Koch D, et al. Vemurafenib-induced hyperkeratosis of the areola treated with topical adapalene [published online February 22, 2015]. Clin Exp Dermatol. 2016;41:148-151.
- Sanlorenzo M, Choudhry A, Vujic I, et al. Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma. J Am Acad Dermatol. 2014;71:1102-1109.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Ducassou A, David I, Delannes M, et al. Radiosensitization induced by vemurafenib. Cancer Radiother. 2013;17:304-307.
- Peuvrel L, Ruellan AL, Thillays F, et al. Severe radiotherapy-induced extracutaneous toxicity under vemurafenib. Eur J Dermatol. 2013;23:879-881.
- Satzger I, Degen A, Asper H, et al. Serious skin toxicity with the combination of BRAF inhibitors and radiotherapy. J Clin Oncol. 2013;31:e220-e222.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
- Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616-622.
- Corsello SM, Barnabei A, Marchetti P, et al. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98:1361-1375.
- Ryder M, Callahan M, Postow MA, et al. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371-381.
- Yu C, Chopra IJ, Ha E. A novel melanoma therapy stirs up a storm: ipilimumab-induced thyrotoxicosis. Endocrinol Diabetes Metab Case Rep. 2015;2015:140092.
- Klein O, Ribas A, Chmielowski B, et al. Facial palsy as a side effect of vemurafenib treatment in patients with metastatic melanoma. J Clin Oncol. 2013;31:e215-e217.
- Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: case report. Pharmacotherapy. 2013;33:e43-e44.
- Flaherty L, Hamid O, Linette G, et al. A single-arm, open-label, expanded access study of vemurafenib in patients with metastatic melanoma in the United States. Cancer J. 2014;20:18-24.
- Wolf SE, Meenken C, Moll AC, et al. Severe pan-uveitis in a patient treated with vemurafenib for metastatic melanoma. BMC Cancer. 2013;13:561.
- Sandhu SS, Ling C, Lim L, et al. Vemurafenib (B-RAF inhibitor) associated uveitis in patients with metastatic cutaneous melanoma. Clin Exp Ophthalmol. 2012;40:118.
- Joshi L, Karydis A, Gemenetzi M, et al. Uveitis as a result of MAP kinase pathway inhibition. Case Rep Ophthalmol. 2013;4:279-282.
- Robinson MR, Chan CC, Yang JC, et al. Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: a new cause of uveitis. J Immunother. 2004;27:478-479.
- Regnier-Rosencher E, Lazareth H, Gressier L, et al. Acute kidney injury in patients with severe rash on vemurafenib treatment for metastatic melanomas. Br J Dermatol. 2013;169:934-938.
- Izzedine H, Gueutin V, Gharbi C, et al. Kidney injuries related to ipilimumab [published online April 1, 2014]. Invest New Drugs. 2014;32:769-773.
- Akhtari M, Waller EK, Jaye DL, et al. Neutropenia in a patient treated with ipilimumab (anti-CTLA-4 antibody). J Immunother. 2009;32:322-324.
- Gordon IO, Wade T, Chin K, et al. Immune mediated red cell aplasia after anti-CTLA-4 immunotherapy for metastatic melanoma. Cancer Immunol Immunother. 2009;58:1351-1353.
- Kopecký J, Trojanová P, Kubeček O, et al. Treatment possibilities of ipilimumab-induced thrombocytopenia—case study and literature review. Jpn J Clin Oncol. 2015;45:381-384.
- Gettings EJ, Hackett CT, Scott TF. Severe relapse in a multiple sclerosis patient associated with ipilimumab treatment of melanoma. Mult Scler. 2015;21:670.
- Ito A, Kondo S, Tada K, et al. Clinical development of immune checkpoint inhibitors. Biomed Res Int. 2015;2015:605478.
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694-1703.
- Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14:249-256.
- Livingstone E, Zimmer L, Vaubel J, et al. BRAF, MEK and KIT inhibitors for melanoma: adverse events and their management. Chin Clin Oncol. 2014;3:29.
- Schmerling RA. Toxicity of checkpoint inhibitors. Chin Clin Oncol. 2014;3:31.
- Yervoy [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2011.
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
- Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161-169.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study [published online December 8, 2009]. Lancet Oncol. 2010;11:155-164.
- Pintova S, Sidhu H, Friedlander PA, et al. Sweet’s syndrome in a patient with metastatic melanoma after ipilimumab therapy. Melanoma Res. 2013;23:498-501.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Lacouture ME, Duvic M, Hauschild A, et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist. 2013;18:314-322.
- Martinez-Garcia E, Taibjee S, Koch D, et al. Vemurafenib-induced hyperkeratosis of the areola treated with topical adapalene [published online February 22, 2015]. Clin Exp Dermatol. 2016;41:148-151.
- Sanlorenzo M, Choudhry A, Vujic I, et al. Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma. J Am Acad Dermatol. 2014;71:1102-1109.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Ducassou A, David I, Delannes M, et al. Radiosensitization induced by vemurafenib. Cancer Radiother. 2013;17:304-307.
- Peuvrel L, Ruellan AL, Thillays F, et al. Severe radiotherapy-induced extracutaneous toxicity under vemurafenib. Eur J Dermatol. 2013;23:879-881.
- Satzger I, Degen A, Asper H, et al. Serious skin toxicity with the combination of BRAF inhibitors and radiotherapy. J Clin Oncol. 2013;31:e220-e222.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
- Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616-622.
- Corsello SM, Barnabei A, Marchetti P, et al. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98:1361-1375.
- Ryder M, Callahan M, Postow MA, et al. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371-381.
- Yu C, Chopra IJ, Ha E. A novel melanoma therapy stirs up a storm: ipilimumab-induced thyrotoxicosis. Endocrinol Diabetes Metab Case Rep. 2015;2015:140092.
- Klein O, Ribas A, Chmielowski B, et al. Facial palsy as a side effect of vemurafenib treatment in patients with metastatic melanoma. J Clin Oncol. 2013;31:e215-e217.
- Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: case report. Pharmacotherapy. 2013;33:e43-e44.
- Flaherty L, Hamid O, Linette G, et al. A single-arm, open-label, expanded access study of vemurafenib in patients with metastatic melanoma in the United States. Cancer J. 2014;20:18-24.
- Wolf SE, Meenken C, Moll AC, et al. Severe pan-uveitis in a patient treated with vemurafenib for metastatic melanoma. BMC Cancer. 2013;13:561.
- Sandhu SS, Ling C, Lim L, et al. Vemurafenib (B-RAF inhibitor) associated uveitis in patients with metastatic cutaneous melanoma. Clin Exp Ophthalmol. 2012;40:118.
- Joshi L, Karydis A, Gemenetzi M, et al. Uveitis as a result of MAP kinase pathway inhibition. Case Rep Ophthalmol. 2013;4:279-282.
- Robinson MR, Chan CC, Yang JC, et al. Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: a new cause of uveitis. J Immunother. 2004;27:478-479.
- Regnier-Rosencher E, Lazareth H, Gressier L, et al. Acute kidney injury in patients with severe rash on vemurafenib treatment for metastatic melanomas. Br J Dermatol. 2013;169:934-938.
- Izzedine H, Gueutin V, Gharbi C, et al. Kidney injuries related to ipilimumab [published online April 1, 2014]. Invest New Drugs. 2014;32:769-773.
- Akhtari M, Waller EK, Jaye DL, et al. Neutropenia in a patient treated with ipilimumab (anti-CTLA-4 antibody). J Immunother. 2009;32:322-324.
- Gordon IO, Wade T, Chin K, et al. Immune mediated red cell aplasia after anti-CTLA-4 immunotherapy for metastatic melanoma. Cancer Immunol Immunother. 2009;58:1351-1353.
- Kopecký J, Trojanová P, Kubeček O, et al. Treatment possibilities of ipilimumab-induced thrombocytopenia—case study and literature review. Jpn J Clin Oncol. 2015;45:381-384.
- Gettings EJ, Hackett CT, Scott TF. Severe relapse in a multiple sclerosis patient associated with ipilimumab treatment of melanoma. Mult Scler. 2015;21:670.
Practice Points
- Immune checkpoint inhibitors can cause immune-related adverse events (irAEs), which most commonly involve the skin but also involve the gastrointestinal, hepatic, endocrine, and neurologic systems.
- These irAEs can be treated with corticosteroids, tumor necrosis factor α antagonists, and mycopheno-late mofetil.
Beyond the bull's eye: Recognizing Lyme disease
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.
Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17
Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18
Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.
Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17
Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18
Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.
Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17
Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18
Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
From The Journal of Family Practice | 2016;65(6):373-379.
Woman, 35, With Jaundice and Altered Mental Status
IN THIS ARTICLE
- Results of case patient's initial laboratory work-up
- Top 10 prescription medications associated with idiosyncratic disease
- Outcome for the case patient
A 35-year-old African-American woman presented to the emergency department (ED) after being found disoriented and lethargic in her apartment by her friends. Given her altered mental status, the history of present illness was limited and informed mainly by her mother and friends. She had been unreachable by telephone for three days, and friends grew concerned when she was absent from work on two consecutive days. After obtaining access to her apartment, they found her in the bathroom jaundiced, incoherent, and surrounded by nonbloody, nonbilious vomit. She had no prior significant medical history, no documented daily medication, and no recent travel. Of note, previous medical contact was limited, and she did not have an established primary care provider. Additionally, there was no contributory family history, including autoimmune illness or liver disease.
ED presentation was marked by indications of grade 4 encephalopathy, including unresponsiveness to noxious stimuli. Initial laboratory work-up was notable for significantly elevated liver function test results (see Table 1). Based on her international normalized ratio (INR), total bilirubin, and creatinine, her initial Model for End-Stage Liver Disease score was 39, correlating to an 83% three-month mortality rate.1 Autoimmune marker testing revealed a positive antinuclear antibody (ANA), elevated immunoglobulin G (IgG), elevated smooth muscle antibody (IgG), normal antimitochondrial antibody, and normal anti-liver/kidney microsome antibody (IgG). Viral hepatitis serologies, including A, B, C, and E, were unremarkable. Ceruloplasmin and iron saturation were within normal limits. Acetaminophen, salicylate, and ethanol levels were negligible. Pregnancy testing and urine toxin testing were negative. Thyroid function tests were normal. Infectious work-up, including pan-culture, remained negative. Syphilis, herpes simplex virus (HSV), HIV, and varicella zoster testing were unremarkable.
CT of the head was not consistent with cerebral edema. CT of the abdomen and pelvis showed evidence of chronic pancreatitis and trace perihepatic ascites. She was intubated for airway protection and transferred to the medical ICU.
On liver biopsy, the patient was found to have acute hepatitis with centrilobular necrosis, approximately 30% to 40%, and prominent cholestasis. Histologically, these findings were reported as most consistent with drug-induced liver injury. Given her comatose state, coagulopathy, and extremely limited life expectancy without liver transplantation, the patient was listed for transplant as a status 1A candidate with fulminant hepatic failure.
She was placed on propofol and N-acetylcysteine infusions in addition to supportive IV resuscitation. The patient’s synthetic and neurocognitive function improved gradually over several weeks, and she was able to provide collateral history. She denied taking any prescription medications or having any ongoing medical issues. She did report that for two months prior to admission she had been taking an oral beauty supplement designed to enhance hair, skin, and nails. She obtained the supplement online. She could not recall the week leading up to admission, but she did note increasing malaise and fatigue beginning two weeks prior to admission. She denied any recreational drug or alcohol use.
Continue for discussion >>
DISCUSSION
Drug-induced liver injury (DILI) is a relatively uncommon occurrence in the United States.2 It is estimated to occur in approximately 20 individuals per 100,000 persons per year.2 However, DILI incidence secondary to herbal and dietary supplement use appears to be on the rise in the US. In a prospective study conducted by the Drug-Induced Liver Injury Network (DILIN) that included patients with liver injury referred to eight DILIN centers between 2004 and 2013, the proportion of DILI cases caused by herbal and dietary supplements increased from 7% to 20% over the study period.3
DILI can be subclassified into intrinsic and idiosyncratic. Intrinsic DILI results from substances causing a predictable time course and natural history. Substances causing a varied, unpredictable occurrence of DILI in susceptible individuals are idiosyncratic.4 Overall, acetaminophen overdose is the most common cause of DILI.2 However, the most common idiosyncratic offending agents, taken at FDA-approved dosages, are antimicrobials (see Table 2).5 The second most common offending agents are herbal and dietary supplements.5
In a retrospective cohort study evaluating all cases of acute liver failure (ALF) over a six-year period in an integrated health care system, the leading cause of ALF was DILI.6 Of the 32 patients with confirmed drug-induced ALF in this study, the majority of cases (18) were associated with acetaminophen. Herbal and dietary supplements were implicated in six cases, with miscellaneous medications accounting for the remaining eight cases.6 In terms of outcomes, 18.8% of patients with ALF due to DILI underwent liver transplantation, 68.8% were discharged, and 12.5% died during hospitalization.6
DILI disproportionately affects women and minorities7;although the etiology is unclear, it is hypothesized that increased use of antibiotics may play a role among women.2 Providers should be aware of the increased risk for DILI in these populations and consider this diagnosis in the appropriate setting.
Teasing out the diagnosis
DILI is a diagnosis of exclusion, aided in large part by the history and physical exam.4 An extensive history may alert the health care provider to a potential offending substance as well as provide information on timing of exposure.4 DILI should be suspected in patients with persistently elevated liver enzymes, unremarkable work-up for all other underlying liver disease (including autoimmune and viral serologies), and negative abdominal imaging.4 In particular, acute hepatitis C virus (HCV) and hepatitis E virus (HEV) infection mimic the clinical presentation of DILI and should be excluded with HCV RNA and IgM anti-HEV testing, with reflex HEV RNA testing to confirm positive IgM anti-HEV results.8,9 Liver biopsy is rarely indicated for the diagnosis of DILI.2
The presentation of DILI ranges from asymptomatic, with mildly abnormal results on liver function testing, to fulminant hepatic failure. Acetaminophen is the most frequently reported cause of intrinsic DILI in the US, playing a role in approximately half of all ALF cases.10 DILI can be further subdivided according to the pattern of liver test abnormalities as hepatocellular, mixed, or cholestatic based on the ratio of ALT to alkaline phosphatase (R value).2 Utilizing the formula serum ALT/upper limit of normal (ULN) divided by the serum alkaline phosphatase/ULN to determine R value, liver test abnormalities are defined as hepatocellular (R > 5), mixed (R = 2-5), and cholestatic (R < 2).4 These liver test patterns can be used to predict prognosis (see “Prognosis: Hy’s law”). In a prospective, longitudinal study, DILIN found that chronic DILI was present in 18% of the study population at 6 months following onset.5 Patients with the cholestatic presentation were more likely to develop chronic DILI than were those with the hepatocellular or mixed pattern. Furthermore, the hepatocellular pattern on presentation was associated with greater mortality.5 Patients with the mixed pattern had the most favorable outcomes. Another prospective cohort study found that persistently elevated liver enzymes in DILI patients at 12 months is associated with older age and the cholestatic pattern of liver test abnormalities at presentation, in particular, alkaline phosphatase elevation.11 However, neither length of therapy nor type of offending medication was associated with long-term liver test abnormalities.11
Managing DILI and ALF
In all DILI cases, immediate discontinuation of the offending agent is the initial treatment recommendation.2 Patients presenting with DILI who have an accompanying bilirubin level > 2 mg/dL should be referred to a hepatology specialist due to an increased risk for ALF.2 ALF is defined as coagulopathy to INR ≥ 1.5 and hepatic encephalopathy within 26 weeks of initial symptom onset in individuals without known underlying liver disease, with the exception of autoimmune hepatitis, Wilson disease, and reactivation of hepatitis B.12-15 Fulminant hepatic failure is further specified as encephalopathy occurring within 8 weeks of jaundice onset.12
Patients presenting with ALF should be transferred to an intensive care setting, preferably within a liver transplant center, for supportive care and potential liver transplant evaluation.12 CT of the head should be used to rule out other etiologies for altered mental status.16N-Acetylcysteine is the treatment of choice for acetaminophen-induced ALF, and it has also been shown to improve transplant-free survival outcomes in patients with non-acetaminophen–related early ALF.17 Infectious work-up and continuous monitoring are essential in ALF care, since up to 80% of patients with ALF will develop a bacterial infection.18 A comprehensive infectious work-up should include pan-culture of blood, urine, and sputum in addition to assessment for Epstein-Barr virus, cytomegalovirus, and HSV.4,18 For irreversible ALF, liver transplantation remains the only validated treatment option.12,19
Prognosis: Hy’s law
Hy’s law refers to a method used in clinical trials to assess a drug’s likelihood of causing severe hepatotoxicity; it is also used to predict which patients with DILI will develop ALF.12,20 According to Hy’s law, patients with AST or ALT elevations three times ULN and total bilirubin elevations two times ULN are at increased risk for ALF.In a retrospective cohort study of more than 15,000 patients with DILI, the Hy’s law criteria were found to have high specificity but low sensitivity for detecting individuals at risk for ALF.15 An alternative model, the Drug-Induced Liver Toxicity ALF Score, uses platelet count and bilirubin level to identify patients at risk for ALF with high sensitivity.15
Patient education
Effective patient education is essential to decreasing DILI incidence at a time when herbal and dietary supplement consumption is increasing. Patients will often bring herbal and dietary supplements to their providers to obtain a safety profile prior to initiation. In these cases, it is essential to reinforce with patients the absence of federal regulation of these products. It should be stressed to patients that, due to the lack of government oversight, it is impossible to confidently identify the entirety and quantity of ingredients in these supplements. Furthermore, there is no existing protocol for surveillance or adverse event reporting for these products.21 Because these products are not routinely or systematically studied, even health care providers have no evidence on which to base monitoring or usage recommendations. Providers may direct patients to the National Institutes of Health’s LiverTox website (livertox.NIH.gov) to review prior case reports of hepatotoxicity for specific dietary and herbal supplements.
Level of education is associated with knowledge of the potential for overdose when taking OTC medications that contain acetaminophen.22 As a result, health care providers should strongly reinforce with patients the importance of reading all medication labels and abiding by the listed administration directions. In particular, providers should emphasize that the maximum daily dosage of acetaminophen is 4 g.23 For patients with chronic liver disease, a more conservative recommendation is warranted. Generally, patients with cirrhosis may be advised to consume up to 2 g/d of acetaminophen as a firstline treatment for pain. However, providers should ensure acetaminophen ingestion is limited to a brief period.24
Additionally, it is important to educate patients that many combination OTC medications contain acetaminophen. Of note, chronic opioid users are more likely to accurately identify OTC medications containing acetaminophen, compared with acute opioid users.22 These findings should compel health care providers to deliver in-depth education for all patients, particularly those with less education or experience with medications. Education on avoidance of offending medications, including medications within the same class, when appropriate, is essential for quality patient care.2
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Following discharge, the patient was monitored closely with regular clinic visits and blood work. Her liver test results improved gradually, with consideration of a repeat biopsy to evaluate for overlap or missed autoimmune disease. Her repeat ANA was negative and IgG was within normal limits. Within three months of admission, her liver tests normalized and repeat biopsy was deferred.
Upon review of the herbal beauty supplement the patient reported taking, shark cartilage was noted as a primary ingredient. In a case report, shark cartilage was identified as a hepatotoxin.25 The patient was advised never to ingest the offending supplement, or any other substances not regulated by the FDA, again. Furthermore, the offending medication was listed as a medication allergy in her electronic health record.
CONCLUSION
It is crucial to emphasize to patients the potential hepatotoxicity of medications and herbal and dietary supplements, especially OTC medications that pose an overdose risk. Patients should review all new supplements with their providers prior to therapy initiation. With known hepatotoxins, providers should closely monitor patients for liver injury while treatment is ongoing. In suspected cases of DILI, a thorough history and physical exam will greatly inform the diagnosis. In the majority of cases, the suspect medication should be discontinued immediately, with subsequent assessment of liver response. Identification of DILI early in the course increases the likelihood of full hepatic recovery and improves patient outcomes.
References
1. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-470.
2. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89(1):95-106.
3. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the US Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408.
4. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966.
5. Chalasani N, Bonkovsky HL, Fontana R, et al; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148(7):1340-1352.
6. Goldberg DS, Forde KA, Carbonari DM, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology. 2015;148(7):1353-1361.
7. Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology. 2010;52(6):2065-2076.
8. Davern TJ, Chalasani N, Fontana RJ, et al; Drug-Induced Liver Injury Network (DILIN). Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141(5):1665-1672.e1-9.
9. Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924-1934.
10. Fisher K, Vuppalanchi R, Saxena R. Drug-induced liver injury. Arch Pathol Lab Med. 2015;139(7):876-887.
11. Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110(10):1450-1459.
12. Punzalan CS, Barry CT. Acute liver failure: diagnosis and management. J Intensive Care Med. 2015 Oct 6. [Epub ahead of print]
13. Bower WA, Johns M, Margolis HS, et al. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102(11):2459-2463.
14. O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342(8866):273-275.
15. Lo Re V III, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of Hy’s law and a new prognostic model. Clin Gastroenterol Hepatol. 2015;13(13):2360-2368.
16. Polson J, Lee WM; American Association for the Study of Liver Diseases. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197.
17. Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864.
18. Rolando N, Harvey F, Brahm J. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11(1):49-53.
19. Panackel C, Thomas R, Sebastian B, Mathai SK. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19(1):27-33.
20. Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15(4):241-243.
21. Bunchorntavakul C, Reddy K. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2012;37(1):3-17.
22. Boudreau DM, Wirtz H, Von Korff M, et al. A survey of adult awareness and use of medicine containing acetaminophen. Pharmacoepidemiol Drug Saf. 2013;22(3):229-240.
23. Burns MJ, Friedman SL, Larson AM. Acetaminophen (paracetamol) poisoning in adults: pathophysiology, presentation, and diagnosis. UpToDate. www.uptodate.com/contents/acetaminophen-paracetamol-poisoning-in-adults-pathophysiology-presentation-and-diagnosis. Accessed May 20, 2016.
24. Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132-1156.
25. Ashar B, Vargo E. Shark cartilage-induced hepatitis. Ann Intern Med. 1996;125(9):780-781.
IN THIS ARTICLE
- Results of case patient's initial laboratory work-up
- Top 10 prescription medications associated with idiosyncratic disease
- Outcome for the case patient
A 35-year-old African-American woman presented to the emergency department (ED) after being found disoriented and lethargic in her apartment by her friends. Given her altered mental status, the history of present illness was limited and informed mainly by her mother and friends. She had been unreachable by telephone for three days, and friends grew concerned when she was absent from work on two consecutive days. After obtaining access to her apartment, they found her in the bathroom jaundiced, incoherent, and surrounded by nonbloody, nonbilious vomit. She had no prior significant medical history, no documented daily medication, and no recent travel. Of note, previous medical contact was limited, and she did not have an established primary care provider. Additionally, there was no contributory family history, including autoimmune illness or liver disease.
ED presentation was marked by indications of grade 4 encephalopathy, including unresponsiveness to noxious stimuli. Initial laboratory work-up was notable for significantly elevated liver function test results (see Table 1). Based on her international normalized ratio (INR), total bilirubin, and creatinine, her initial Model for End-Stage Liver Disease score was 39, correlating to an 83% three-month mortality rate.1 Autoimmune marker testing revealed a positive antinuclear antibody (ANA), elevated immunoglobulin G (IgG), elevated smooth muscle antibody (IgG), normal antimitochondrial antibody, and normal anti-liver/kidney microsome antibody (IgG). Viral hepatitis serologies, including A, B, C, and E, were unremarkable. Ceruloplasmin and iron saturation were within normal limits. Acetaminophen, salicylate, and ethanol levels were negligible. Pregnancy testing and urine toxin testing were negative. Thyroid function tests were normal. Infectious work-up, including pan-culture, remained negative. Syphilis, herpes simplex virus (HSV), HIV, and varicella zoster testing were unremarkable.
CT of the head was not consistent with cerebral edema. CT of the abdomen and pelvis showed evidence of chronic pancreatitis and trace perihepatic ascites. She was intubated for airway protection and transferred to the medical ICU.
On liver biopsy, the patient was found to have acute hepatitis with centrilobular necrosis, approximately 30% to 40%, and prominent cholestasis. Histologically, these findings were reported as most consistent with drug-induced liver injury. Given her comatose state, coagulopathy, and extremely limited life expectancy without liver transplantation, the patient was listed for transplant as a status 1A candidate with fulminant hepatic failure.
She was placed on propofol and N-acetylcysteine infusions in addition to supportive IV resuscitation. The patient’s synthetic and neurocognitive function improved gradually over several weeks, and she was able to provide collateral history. She denied taking any prescription medications or having any ongoing medical issues. She did report that for two months prior to admission she had been taking an oral beauty supplement designed to enhance hair, skin, and nails. She obtained the supplement online. She could not recall the week leading up to admission, but she did note increasing malaise and fatigue beginning two weeks prior to admission. She denied any recreational drug or alcohol use.
Continue for discussion >>
DISCUSSION
Drug-induced liver injury (DILI) is a relatively uncommon occurrence in the United States.2 It is estimated to occur in approximately 20 individuals per 100,000 persons per year.2 However, DILI incidence secondary to herbal and dietary supplement use appears to be on the rise in the US. In a prospective study conducted by the Drug-Induced Liver Injury Network (DILIN) that included patients with liver injury referred to eight DILIN centers between 2004 and 2013, the proportion of DILI cases caused by herbal and dietary supplements increased from 7% to 20% over the study period.3
DILI can be subclassified into intrinsic and idiosyncratic. Intrinsic DILI results from substances causing a predictable time course and natural history. Substances causing a varied, unpredictable occurrence of DILI in susceptible individuals are idiosyncratic.4 Overall, acetaminophen overdose is the most common cause of DILI.2 However, the most common idiosyncratic offending agents, taken at FDA-approved dosages, are antimicrobials (see Table 2).5 The second most common offending agents are herbal and dietary supplements.5
In a retrospective cohort study evaluating all cases of acute liver failure (ALF) over a six-year period in an integrated health care system, the leading cause of ALF was DILI.6 Of the 32 patients with confirmed drug-induced ALF in this study, the majority of cases (18) were associated with acetaminophen. Herbal and dietary supplements were implicated in six cases, with miscellaneous medications accounting for the remaining eight cases.6 In terms of outcomes, 18.8% of patients with ALF due to DILI underwent liver transplantation, 68.8% were discharged, and 12.5% died during hospitalization.6
DILI disproportionately affects women and minorities7;although the etiology is unclear, it is hypothesized that increased use of antibiotics may play a role among women.2 Providers should be aware of the increased risk for DILI in these populations and consider this diagnosis in the appropriate setting.
Teasing out the diagnosis
DILI is a diagnosis of exclusion, aided in large part by the history and physical exam.4 An extensive history may alert the health care provider to a potential offending substance as well as provide information on timing of exposure.4 DILI should be suspected in patients with persistently elevated liver enzymes, unremarkable work-up for all other underlying liver disease (including autoimmune and viral serologies), and negative abdominal imaging.4 In particular, acute hepatitis C virus (HCV) and hepatitis E virus (HEV) infection mimic the clinical presentation of DILI and should be excluded with HCV RNA and IgM anti-HEV testing, with reflex HEV RNA testing to confirm positive IgM anti-HEV results.8,9 Liver biopsy is rarely indicated for the diagnosis of DILI.2
The presentation of DILI ranges from asymptomatic, with mildly abnormal results on liver function testing, to fulminant hepatic failure. Acetaminophen is the most frequently reported cause of intrinsic DILI in the US, playing a role in approximately half of all ALF cases.10 DILI can be further subdivided according to the pattern of liver test abnormalities as hepatocellular, mixed, or cholestatic based on the ratio of ALT to alkaline phosphatase (R value).2 Utilizing the formula serum ALT/upper limit of normal (ULN) divided by the serum alkaline phosphatase/ULN to determine R value, liver test abnormalities are defined as hepatocellular (R > 5), mixed (R = 2-5), and cholestatic (R < 2).4 These liver test patterns can be used to predict prognosis (see “Prognosis: Hy’s law”). In a prospective, longitudinal study, DILIN found that chronic DILI was present in 18% of the study population at 6 months following onset.5 Patients with the cholestatic presentation were more likely to develop chronic DILI than were those with the hepatocellular or mixed pattern. Furthermore, the hepatocellular pattern on presentation was associated with greater mortality.5 Patients with the mixed pattern had the most favorable outcomes. Another prospective cohort study found that persistently elevated liver enzymes in DILI patients at 12 months is associated with older age and the cholestatic pattern of liver test abnormalities at presentation, in particular, alkaline phosphatase elevation.11 However, neither length of therapy nor type of offending medication was associated with long-term liver test abnormalities.11
Managing DILI and ALF
In all DILI cases, immediate discontinuation of the offending agent is the initial treatment recommendation.2 Patients presenting with DILI who have an accompanying bilirubin level > 2 mg/dL should be referred to a hepatology specialist due to an increased risk for ALF.2 ALF is defined as coagulopathy to INR ≥ 1.5 and hepatic encephalopathy within 26 weeks of initial symptom onset in individuals without known underlying liver disease, with the exception of autoimmune hepatitis, Wilson disease, and reactivation of hepatitis B.12-15 Fulminant hepatic failure is further specified as encephalopathy occurring within 8 weeks of jaundice onset.12
Patients presenting with ALF should be transferred to an intensive care setting, preferably within a liver transplant center, for supportive care and potential liver transplant evaluation.12 CT of the head should be used to rule out other etiologies for altered mental status.16N-Acetylcysteine is the treatment of choice for acetaminophen-induced ALF, and it has also been shown to improve transplant-free survival outcomes in patients with non-acetaminophen–related early ALF.17 Infectious work-up and continuous monitoring are essential in ALF care, since up to 80% of patients with ALF will develop a bacterial infection.18 A comprehensive infectious work-up should include pan-culture of blood, urine, and sputum in addition to assessment for Epstein-Barr virus, cytomegalovirus, and HSV.4,18 For irreversible ALF, liver transplantation remains the only validated treatment option.12,19
Prognosis: Hy’s law
Hy’s law refers to a method used in clinical trials to assess a drug’s likelihood of causing severe hepatotoxicity; it is also used to predict which patients with DILI will develop ALF.12,20 According to Hy’s law, patients with AST or ALT elevations three times ULN and total bilirubin elevations two times ULN are at increased risk for ALF.In a retrospective cohort study of more than 15,000 patients with DILI, the Hy’s law criteria were found to have high specificity but low sensitivity for detecting individuals at risk for ALF.15 An alternative model, the Drug-Induced Liver Toxicity ALF Score, uses platelet count and bilirubin level to identify patients at risk for ALF with high sensitivity.15
Patient education
Effective patient education is essential to decreasing DILI incidence at a time when herbal and dietary supplement consumption is increasing. Patients will often bring herbal and dietary supplements to their providers to obtain a safety profile prior to initiation. In these cases, it is essential to reinforce with patients the absence of federal regulation of these products. It should be stressed to patients that, due to the lack of government oversight, it is impossible to confidently identify the entirety and quantity of ingredients in these supplements. Furthermore, there is no existing protocol for surveillance or adverse event reporting for these products.21 Because these products are not routinely or systematically studied, even health care providers have no evidence on which to base monitoring or usage recommendations. Providers may direct patients to the National Institutes of Health’s LiverTox website (livertox.NIH.gov) to review prior case reports of hepatotoxicity for specific dietary and herbal supplements.
Level of education is associated with knowledge of the potential for overdose when taking OTC medications that contain acetaminophen.22 As a result, health care providers should strongly reinforce with patients the importance of reading all medication labels and abiding by the listed administration directions. In particular, providers should emphasize that the maximum daily dosage of acetaminophen is 4 g.23 For patients with chronic liver disease, a more conservative recommendation is warranted. Generally, patients with cirrhosis may be advised to consume up to 2 g/d of acetaminophen as a firstline treatment for pain. However, providers should ensure acetaminophen ingestion is limited to a brief period.24
Additionally, it is important to educate patients that many combination OTC medications contain acetaminophen. Of note, chronic opioid users are more likely to accurately identify OTC medications containing acetaminophen, compared with acute opioid users.22 These findings should compel health care providers to deliver in-depth education for all patients, particularly those with less education or experience with medications. Education on avoidance of offending medications, including medications within the same class, when appropriate, is essential for quality patient care.2
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Following discharge, the patient was monitored closely with regular clinic visits and blood work. Her liver test results improved gradually, with consideration of a repeat biopsy to evaluate for overlap or missed autoimmune disease. Her repeat ANA was negative and IgG was within normal limits. Within three months of admission, her liver tests normalized and repeat biopsy was deferred.
Upon review of the herbal beauty supplement the patient reported taking, shark cartilage was noted as a primary ingredient. In a case report, shark cartilage was identified as a hepatotoxin.25 The patient was advised never to ingest the offending supplement, or any other substances not regulated by the FDA, again. Furthermore, the offending medication was listed as a medication allergy in her electronic health record.
CONCLUSION
It is crucial to emphasize to patients the potential hepatotoxicity of medications and herbal and dietary supplements, especially OTC medications that pose an overdose risk. Patients should review all new supplements with their providers prior to therapy initiation. With known hepatotoxins, providers should closely monitor patients for liver injury while treatment is ongoing. In suspected cases of DILI, a thorough history and physical exam will greatly inform the diagnosis. In the majority of cases, the suspect medication should be discontinued immediately, with subsequent assessment of liver response. Identification of DILI early in the course increases the likelihood of full hepatic recovery and improves patient outcomes.
References
1. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-470.
2. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89(1):95-106.
3. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the US Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408.
4. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966.
5. Chalasani N, Bonkovsky HL, Fontana R, et al; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148(7):1340-1352.
6. Goldberg DS, Forde KA, Carbonari DM, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology. 2015;148(7):1353-1361.
7. Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology. 2010;52(6):2065-2076.
8. Davern TJ, Chalasani N, Fontana RJ, et al; Drug-Induced Liver Injury Network (DILIN). Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141(5):1665-1672.e1-9.
9. Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924-1934.
10. Fisher K, Vuppalanchi R, Saxena R. Drug-induced liver injury. Arch Pathol Lab Med. 2015;139(7):876-887.
11. Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110(10):1450-1459.
12. Punzalan CS, Barry CT. Acute liver failure: diagnosis and management. J Intensive Care Med. 2015 Oct 6. [Epub ahead of print]
13. Bower WA, Johns M, Margolis HS, et al. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102(11):2459-2463.
14. O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342(8866):273-275.
15. Lo Re V III, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of Hy’s law and a new prognostic model. Clin Gastroenterol Hepatol. 2015;13(13):2360-2368.
16. Polson J, Lee WM; American Association for the Study of Liver Diseases. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197.
17. Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864.
18. Rolando N, Harvey F, Brahm J. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11(1):49-53.
19. Panackel C, Thomas R, Sebastian B, Mathai SK. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19(1):27-33.
20. Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15(4):241-243.
21. Bunchorntavakul C, Reddy K. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2012;37(1):3-17.
22. Boudreau DM, Wirtz H, Von Korff M, et al. A survey of adult awareness and use of medicine containing acetaminophen. Pharmacoepidemiol Drug Saf. 2013;22(3):229-240.
23. Burns MJ, Friedman SL, Larson AM. Acetaminophen (paracetamol) poisoning in adults: pathophysiology, presentation, and diagnosis. UpToDate. www.uptodate.com/contents/acetaminophen-paracetamol-poisoning-in-adults-pathophysiology-presentation-and-diagnosis. Accessed May 20, 2016.
24. Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132-1156.
25. Ashar B, Vargo E. Shark cartilage-induced hepatitis. Ann Intern Med. 1996;125(9):780-781.
IN THIS ARTICLE
- Results of case patient's initial laboratory work-up
- Top 10 prescription medications associated with idiosyncratic disease
- Outcome for the case patient
A 35-year-old African-American woman presented to the emergency department (ED) after being found disoriented and lethargic in her apartment by her friends. Given her altered mental status, the history of present illness was limited and informed mainly by her mother and friends. She had been unreachable by telephone for three days, and friends grew concerned when she was absent from work on two consecutive days. After obtaining access to her apartment, they found her in the bathroom jaundiced, incoherent, and surrounded by nonbloody, nonbilious vomit. She had no prior significant medical history, no documented daily medication, and no recent travel. Of note, previous medical contact was limited, and she did not have an established primary care provider. Additionally, there was no contributory family history, including autoimmune illness or liver disease.
ED presentation was marked by indications of grade 4 encephalopathy, including unresponsiveness to noxious stimuli. Initial laboratory work-up was notable for significantly elevated liver function test results (see Table 1). Based on her international normalized ratio (INR), total bilirubin, and creatinine, her initial Model for End-Stage Liver Disease score was 39, correlating to an 83% three-month mortality rate.1 Autoimmune marker testing revealed a positive antinuclear antibody (ANA), elevated immunoglobulin G (IgG), elevated smooth muscle antibody (IgG), normal antimitochondrial antibody, and normal anti-liver/kidney microsome antibody (IgG). Viral hepatitis serologies, including A, B, C, and E, were unremarkable. Ceruloplasmin and iron saturation were within normal limits. Acetaminophen, salicylate, and ethanol levels were negligible. Pregnancy testing and urine toxin testing were negative. Thyroid function tests were normal. Infectious work-up, including pan-culture, remained negative. Syphilis, herpes simplex virus (HSV), HIV, and varicella zoster testing were unremarkable.
CT of the head was not consistent with cerebral edema. CT of the abdomen and pelvis showed evidence of chronic pancreatitis and trace perihepatic ascites. She was intubated for airway protection and transferred to the medical ICU.
On liver biopsy, the patient was found to have acute hepatitis with centrilobular necrosis, approximately 30% to 40%, and prominent cholestasis. Histologically, these findings were reported as most consistent with drug-induced liver injury. Given her comatose state, coagulopathy, and extremely limited life expectancy without liver transplantation, the patient was listed for transplant as a status 1A candidate with fulminant hepatic failure.
She was placed on propofol and N-acetylcysteine infusions in addition to supportive IV resuscitation. The patient’s synthetic and neurocognitive function improved gradually over several weeks, and she was able to provide collateral history. She denied taking any prescription medications or having any ongoing medical issues. She did report that for two months prior to admission she had been taking an oral beauty supplement designed to enhance hair, skin, and nails. She obtained the supplement online. She could not recall the week leading up to admission, but she did note increasing malaise and fatigue beginning two weeks prior to admission. She denied any recreational drug or alcohol use.
Continue for discussion >>
DISCUSSION
Drug-induced liver injury (DILI) is a relatively uncommon occurrence in the United States.2 It is estimated to occur in approximately 20 individuals per 100,000 persons per year.2 However, DILI incidence secondary to herbal and dietary supplement use appears to be on the rise in the US. In a prospective study conducted by the Drug-Induced Liver Injury Network (DILIN) that included patients with liver injury referred to eight DILIN centers between 2004 and 2013, the proportion of DILI cases caused by herbal and dietary supplements increased from 7% to 20% over the study period.3
DILI can be subclassified into intrinsic and idiosyncratic. Intrinsic DILI results from substances causing a predictable time course and natural history. Substances causing a varied, unpredictable occurrence of DILI in susceptible individuals are idiosyncratic.4 Overall, acetaminophen overdose is the most common cause of DILI.2 However, the most common idiosyncratic offending agents, taken at FDA-approved dosages, are antimicrobials (see Table 2).5 The second most common offending agents are herbal and dietary supplements.5
In a retrospective cohort study evaluating all cases of acute liver failure (ALF) over a six-year period in an integrated health care system, the leading cause of ALF was DILI.6 Of the 32 patients with confirmed drug-induced ALF in this study, the majority of cases (18) were associated with acetaminophen. Herbal and dietary supplements were implicated in six cases, with miscellaneous medications accounting for the remaining eight cases.6 In terms of outcomes, 18.8% of patients with ALF due to DILI underwent liver transplantation, 68.8% were discharged, and 12.5% died during hospitalization.6
DILI disproportionately affects women and minorities7;although the etiology is unclear, it is hypothesized that increased use of antibiotics may play a role among women.2 Providers should be aware of the increased risk for DILI in these populations and consider this diagnosis in the appropriate setting.
Teasing out the diagnosis
DILI is a diagnosis of exclusion, aided in large part by the history and physical exam.4 An extensive history may alert the health care provider to a potential offending substance as well as provide information on timing of exposure.4 DILI should be suspected in patients with persistently elevated liver enzymes, unremarkable work-up for all other underlying liver disease (including autoimmune and viral serologies), and negative abdominal imaging.4 In particular, acute hepatitis C virus (HCV) and hepatitis E virus (HEV) infection mimic the clinical presentation of DILI and should be excluded with HCV RNA and IgM anti-HEV testing, with reflex HEV RNA testing to confirm positive IgM anti-HEV results.8,9 Liver biopsy is rarely indicated for the diagnosis of DILI.2
The presentation of DILI ranges from asymptomatic, with mildly abnormal results on liver function testing, to fulminant hepatic failure. Acetaminophen is the most frequently reported cause of intrinsic DILI in the US, playing a role in approximately half of all ALF cases.10 DILI can be further subdivided according to the pattern of liver test abnormalities as hepatocellular, mixed, or cholestatic based on the ratio of ALT to alkaline phosphatase (R value).2 Utilizing the formula serum ALT/upper limit of normal (ULN) divided by the serum alkaline phosphatase/ULN to determine R value, liver test abnormalities are defined as hepatocellular (R > 5), mixed (R = 2-5), and cholestatic (R < 2).4 These liver test patterns can be used to predict prognosis (see “Prognosis: Hy’s law”). In a prospective, longitudinal study, DILIN found that chronic DILI was present in 18% of the study population at 6 months following onset.5 Patients with the cholestatic presentation were more likely to develop chronic DILI than were those with the hepatocellular or mixed pattern. Furthermore, the hepatocellular pattern on presentation was associated with greater mortality.5 Patients with the mixed pattern had the most favorable outcomes. Another prospective cohort study found that persistently elevated liver enzymes in DILI patients at 12 months is associated with older age and the cholestatic pattern of liver test abnormalities at presentation, in particular, alkaline phosphatase elevation.11 However, neither length of therapy nor type of offending medication was associated with long-term liver test abnormalities.11
Managing DILI and ALF
In all DILI cases, immediate discontinuation of the offending agent is the initial treatment recommendation.2 Patients presenting with DILI who have an accompanying bilirubin level > 2 mg/dL should be referred to a hepatology specialist due to an increased risk for ALF.2 ALF is defined as coagulopathy to INR ≥ 1.5 and hepatic encephalopathy within 26 weeks of initial symptom onset in individuals without known underlying liver disease, with the exception of autoimmune hepatitis, Wilson disease, and reactivation of hepatitis B.12-15 Fulminant hepatic failure is further specified as encephalopathy occurring within 8 weeks of jaundice onset.12
Patients presenting with ALF should be transferred to an intensive care setting, preferably within a liver transplant center, for supportive care and potential liver transplant evaluation.12 CT of the head should be used to rule out other etiologies for altered mental status.16N-Acetylcysteine is the treatment of choice for acetaminophen-induced ALF, and it has also been shown to improve transplant-free survival outcomes in patients with non-acetaminophen–related early ALF.17 Infectious work-up and continuous monitoring are essential in ALF care, since up to 80% of patients with ALF will develop a bacterial infection.18 A comprehensive infectious work-up should include pan-culture of blood, urine, and sputum in addition to assessment for Epstein-Barr virus, cytomegalovirus, and HSV.4,18 For irreversible ALF, liver transplantation remains the only validated treatment option.12,19
Prognosis: Hy’s law
Hy’s law refers to a method used in clinical trials to assess a drug’s likelihood of causing severe hepatotoxicity; it is also used to predict which patients with DILI will develop ALF.12,20 According to Hy’s law, patients with AST or ALT elevations three times ULN and total bilirubin elevations two times ULN are at increased risk for ALF.In a retrospective cohort study of more than 15,000 patients with DILI, the Hy’s law criteria were found to have high specificity but low sensitivity for detecting individuals at risk for ALF.15 An alternative model, the Drug-Induced Liver Toxicity ALF Score, uses platelet count and bilirubin level to identify patients at risk for ALF with high sensitivity.15
Patient education
Effective patient education is essential to decreasing DILI incidence at a time when herbal and dietary supplement consumption is increasing. Patients will often bring herbal and dietary supplements to their providers to obtain a safety profile prior to initiation. In these cases, it is essential to reinforce with patients the absence of federal regulation of these products. It should be stressed to patients that, due to the lack of government oversight, it is impossible to confidently identify the entirety and quantity of ingredients in these supplements. Furthermore, there is no existing protocol for surveillance or adverse event reporting for these products.21 Because these products are not routinely or systematically studied, even health care providers have no evidence on which to base monitoring or usage recommendations. Providers may direct patients to the National Institutes of Health’s LiverTox website (livertox.NIH.gov) to review prior case reports of hepatotoxicity for specific dietary and herbal supplements.
Level of education is associated with knowledge of the potential for overdose when taking OTC medications that contain acetaminophen.22 As a result, health care providers should strongly reinforce with patients the importance of reading all medication labels and abiding by the listed administration directions. In particular, providers should emphasize that the maximum daily dosage of acetaminophen is 4 g.23 For patients with chronic liver disease, a more conservative recommendation is warranted. Generally, patients with cirrhosis may be advised to consume up to 2 g/d of acetaminophen as a firstline treatment for pain. However, providers should ensure acetaminophen ingestion is limited to a brief period.24
Additionally, it is important to educate patients that many combination OTC medications contain acetaminophen. Of note, chronic opioid users are more likely to accurately identify OTC medications containing acetaminophen, compared with acute opioid users.22 These findings should compel health care providers to deliver in-depth education for all patients, particularly those with less education or experience with medications. Education on avoidance of offending medications, including medications within the same class, when appropriate, is essential for quality patient care.2
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Following discharge, the patient was monitored closely with regular clinic visits and blood work. Her liver test results improved gradually, with consideration of a repeat biopsy to evaluate for overlap or missed autoimmune disease. Her repeat ANA was negative and IgG was within normal limits. Within three months of admission, her liver tests normalized and repeat biopsy was deferred.
Upon review of the herbal beauty supplement the patient reported taking, shark cartilage was noted as a primary ingredient. In a case report, shark cartilage was identified as a hepatotoxin.25 The patient was advised never to ingest the offending supplement, or any other substances not regulated by the FDA, again. Furthermore, the offending medication was listed as a medication allergy in her electronic health record.
CONCLUSION
It is crucial to emphasize to patients the potential hepatotoxicity of medications and herbal and dietary supplements, especially OTC medications that pose an overdose risk. Patients should review all new supplements with their providers prior to therapy initiation. With known hepatotoxins, providers should closely monitor patients for liver injury while treatment is ongoing. In suspected cases of DILI, a thorough history and physical exam will greatly inform the diagnosis. In the majority of cases, the suspect medication should be discontinued immediately, with subsequent assessment of liver response. Identification of DILI early in the course increases the likelihood of full hepatic recovery and improves patient outcomes.
References
1. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-470.
2. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89(1):95-106.
3. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the US Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408.
4. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966.
5. Chalasani N, Bonkovsky HL, Fontana R, et al; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148(7):1340-1352.
6. Goldberg DS, Forde KA, Carbonari DM, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology. 2015;148(7):1353-1361.
7. Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology. 2010;52(6):2065-2076.
8. Davern TJ, Chalasani N, Fontana RJ, et al; Drug-Induced Liver Injury Network (DILIN). Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141(5):1665-1672.e1-9.
9. Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924-1934.
10. Fisher K, Vuppalanchi R, Saxena R. Drug-induced liver injury. Arch Pathol Lab Med. 2015;139(7):876-887.
11. Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110(10):1450-1459.
12. Punzalan CS, Barry CT. Acute liver failure: diagnosis and management. J Intensive Care Med. 2015 Oct 6. [Epub ahead of print]
13. Bower WA, Johns M, Margolis HS, et al. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102(11):2459-2463.
14. O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342(8866):273-275.
15. Lo Re V III, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of Hy’s law and a new prognostic model. Clin Gastroenterol Hepatol. 2015;13(13):2360-2368.
16. Polson J, Lee WM; American Association for the Study of Liver Diseases. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197.
17. Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864.
18. Rolando N, Harvey F, Brahm J. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11(1):49-53.
19. Panackel C, Thomas R, Sebastian B, Mathai SK. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19(1):27-33.
20. Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15(4):241-243.
21. Bunchorntavakul C, Reddy K. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2012;37(1):3-17.
22. Boudreau DM, Wirtz H, Von Korff M, et al. A survey of adult awareness and use of medicine containing acetaminophen. Pharmacoepidemiol Drug Saf. 2013;22(3):229-240.
23. Burns MJ, Friedman SL, Larson AM. Acetaminophen (paracetamol) poisoning in adults: pathophysiology, presentation, and diagnosis. UpToDate. www.uptodate.com/contents/acetaminophen-paracetamol-poisoning-in-adults-pathophysiology-presentation-and-diagnosis. Accessed May 20, 2016.
24. Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132-1156.
25. Ashar B, Vargo E. Shark cartilage-induced hepatitis. Ann Intern Med. 1996;125(9):780-781.
Anterior Cervical Interbody Fusion Using a Polyetheretherketone (PEEK) Cage Device and Local Autograft Bone
Anterior cervical discectomy and fusion (ACDF) has been performed with various techniques and devices for many years. Autologous iliac crest grafts were initially used for the Cloward1,2 and Robinson and Smith3 techniques, but because of iliac crest graft site complications (eg, pain, infection, fracture, dystrophic scarring4,5), the procedure was generally superseded by allograft implants. These implants were then supplemented with anterior locking plate devices. More recently, unitary devices combining a polyetheretherketone (PEEK) spacer with screw or blade fixation have been developed, such as the Zero P (Synthes, Inc.) and the ROI-C cervical cage (LDR). Bone graft is required to fill the cavity of these devices and to promote osseous union. Demineralized bone matrix,6 tricalcium phosphate,7,8 and bone morphogenetic protein (BMP) have been used for these purposes, but they add expense to the procedure and have been associated with several complications (eg, neck swelling, dysphagia associated with BMP).9
Although multiple studies have demonstrated effective fusion rates and good outcomes for both iliac crest autograft and grafting/spacer constructs, the debate over cost and “added value” remains unresolved. One institution, which has published articles reviewing the spine literature and its own data, concluded that iliac crest autograft was the most cost-effective and consistently successful ACDF procedure.5,10
The VA Portland Health Care System (VAPORHCS) has analyzed the use of local autograft sources at the surgical site to circumvent the need to make a second incision at the iliac crest and, theoretically, to decrease risks and expenses associated with iliac crest autograft, allograft bone, and artificial constructs. Given the paucity of data on this method, the case series presented here represents one of a few studies that analyze local autograft for promotion of arthrodesis in a PEEK spacer device.
This article will report on the prospectively collected results of consecutive cases performed by Dr. Ross using a ROI-C cervical cage for 1-level anterior cervical discectomy between August 2011 and November 2014. This study received institutional review board approval.
Methods
Neck disability index (NDI) forms were used to assess the impact of neck pain on patients’ ability to manage in everyday life. The NDI form was completed before surgery and 3 and 9 months after surgery.
Dr. Ross preferred to perform minimally invasive posterior cervical foraminotomy for unilateral radiculopathy. Therefore, all patients with radiculopathy had bilateral symptoms or a symptomatic midline disc protrusion not accessible from a posterior approach. Standard techniques were used to make a left-side approach to the anterior cervical spine except in cases in which a previous right-side approach could be reused. Under the microscope, the anterior longitudinal ligament and annulus were incised, and the anterior contents of the disc space were removed with curettes and pituitary rongeurs. Care was taken to remove all cartilage from beneath the anterior inferior lip of the rostral vertebral body and to remove a few millimeters of the anterior longitudinal ligament from the rostral vertebral body without use of monopolar cautery (Figure 1). A 2 mm Kerrison punch then was used to remove the anterior inferior lip of the rostral vertebral body, and this bone was saved for grafting. No bone wax was used within the disc space.
After all disc space cartilage was removed from the endplates, additional bone was obtained from the uncovertebral joints and posterior vertebral bodies as the decompression proceeded posteriorly. Occasionally, distraction posts were used if the disc space was too narrow for optimal visualization posteriorly. After decompression was achieved, a lordotic ROI-C cervical cage was packed in its lumen with the bone chips and impacted into the disc space under fluoroscopic guidance. The blades were impacted under fluoroscopic guidance as well. The wound was closed with absorbable suture.
Antibiotics were given for no more than 24 hours after surgery. Ketorolac was used for analgesia the night of the surgery, and patients were asked to not use nonsteroidal anti-inflammatory drugs for 3 months after surgery. Lateral radiographs were obtained 3 and 9 months after surgery and every 6 months thereafter until arthrodesis was detected.
Results
Seventy-seven consecutive patients underwent 1-level anterior cervical discectomy (Table 1). Twenty-four procedures were performed for radiculopathy, 52 for myelopathy, and 1 for central cord injury sustained in a fall by a patient with preexisting spinal stenosis. Surgery was performed at C3-C4 (25 cases), C4-C5 (11 cases), C5-C6 (15 cases), and C6-C7 (1 case) for patients with myelopathy. Surgery was performed at C3-C4 (2 cases), C4-C5 (3 cases), C5-C6 (9 cases), and C6-C7 (10 cases) for patients with radiculopathy.
Twenty-eight patients reported presurgery tobacco use. Although all tobacco-using patients agreed to cease use in the perioperative period, at least 9 admitted to resuming tobacco use immediately after surgery. Eighteen patients had diabetes mellitus. In 2 patients, a diagnosis of osteoporosis was made with dual-energy X-ray absorptiometry. One patient was a chronic user of steroids before and after surgery. Mean body mass index (BMI) was 30.6, and 13 patients were morbidly obese (BMI > 34).
In 2 cases, only a single blade was placed. The second blade could not be placed because of broken adjacent screws (1 case) or undetermined reason (1 case).
The mean time for follow-up was 17 months (range 3-34). Four patients were lost to follow-up: 3 after the 1-month postoperative visit and 1 with severe psychiatric problems after hospital discharge.
There were no new neurologic deficits, no wound infections, and no recurrent laryngeal nerve palsies in the 77 patients. Eight months after surgery, 1 patient with radiculopathy underwent foraminotomy at the index level for persisting foraminal stenosis. Two patients whose myelopathic symptoms persisted after surgery returned for minimally invasive posterior laminotomy to remove infolded ligamentum flavum. The presurgery and 3- and 9-month postsurgery NDI scores were available for 52 patients (Table 2). Before surgery the mean NDI score was 24 (range 8-40). Three months postsurgery the mean NDI score was 15 (range 2-27) for patients with myelopathy and 13 (range 2-28) for patients with radiculopathy. The patient with the highest NDI score (28) stated that though all his symptoms were relieved, he had gauged his responses to protect his disability claim. Nine months after surgery, the mean NDI scores were 9.5 (range 5-17) for patients with myelopathy and 6 (range 2-13) for patients with radiculopathy. No NDI score was higher postsurgery than presurgery.
Arthrodesis was defined as bony bridging between the adjacent vertebral bodies and the bone graft within the lumen of the device, anterior to the device, or posterior to the device. In Dr. Ross’ protocol, computed tomography (CT) scans or flexion-extension radiographs were obtained only if pseudarthrosis was suspected to avoid unnecessary radiation exposure. Sixty-six patients had at least the 3-month radiography follow-up available. All 52 patients with 9-month follow-up data achieved complete arthrodesis, as determined by plain film radiography. Bridging ossification was found anterior to the device in all but 9 patients. Trabeculated bone was growing through the lumen of the device in all cases (Figure 2). A broken blade without clinical correlation was noted on imaging for 1 patient.
The total cost of the ROI-C cervical cage (LDR) for VAPORHCS was $3,498, or $1,749 for the PEEK spacer plus $1,749 for 2 metal blades. In comparison, the total cost of a typical anterior locking plate would have been $6,700, or $3,200 for the plate plus $2,000 for 4 screws and $1,500 for an allograft fibular spacer. Demineralized bone matrix (1 mL) as used in cervical arthrodesis by other surgeons at VAPORHCS cost $279, or about $500 including shipping.
DISCUSSION
Anterior cervical discectomy with fusion is a very common and successful surgical procedure for cervical myelopathy, radiculopathy, and degenerative disease that has failed to be corrected with conservative therapy.10 Medicare data documented a 206% increase in 1-level fusion procedures for degenerative spine pathology performed between 1992 and 2005.11 When a procedure is performed so often, it is appropriate to review methods and analyze efficacy, cost, and cost-effectiveness.
According to a 2007 meta-analysis, the fusion rates of 1-level ACDF arthrodesis at 1-year follow-up are 97.1% in patients treated with anterior plates and 92.1% in patients treated with noninstrumented fusion.12 The rate disparity was larger for multiple-level fusion: 50% to 82.5% for instrumented cases12,13 vs 3% to 42% for noninstrumented cases.14-16 Given the higher fusion rates achieved with instrumentation, surgeons have favored its use in ACDF.
Computed Tomography Use
Computed tomography has long been considered the gold standard for assessing arthrodesis outcomes (eg, Siambanes and Mather).17 However, recent data on potential harm caused by CT-related ionizing radiation suggest a need for caution with routine CT use.18,19 For cervical spine CT, Schonfeld and colleagues found that the risk for excess thyroid cancers ranged from 1 to 33 cases per 10,000 CT scans.20 According to another report, “limiting neck CT scanning to a higher risk group would increase the gap between benefit and harm, whereas performing CT routinely on low-risk cases approaches a point where its harm equals or exceeds its benefit.”19 As some have questioned even routinepostoperative use of radiation in patients with unremarkable clinical courses—patients should be spared unnecessary exposure—CT scans or flexion-extensionradiographs were obtained at VAPORHCS only if clinical symptoms or radiographs were suggestive of pseudarthrosis.21 As none of the VAPORHCS patients had those symptoms, none underwent postoperative CT.
For anterior cervical arthrodesis, surgeon preference determines which of many different bone substrates can be used with instrumentation, which impacts the costs. Fusion substrates include structural autografts, structural allografts, morselized autografts, morselized allografts, demineralized allografts, porous ceramics and metals, and BMP. Given these many options, studies comparing the constructs are lacking, especially with regard to the cost of alternative fusion constructs that produce similar outcomes. The Centers for Disease Control and Prevention defines cost-benefit analysis as a “type of economic evaluation that measures both costs and benefits (ie, negative and positive consequences) associated with an intervention in dollar terms.”22 It has been reported that using iliac crest autografts with anterior plate instrumentation is the most cost-effective method, yet alternatives remain in use.5,10
For ACDF, iliac crest bone is an ideal and widely used construct substrate. Structural grafts harvested from the crest provide significant stability due to their bicortical or tricortical configuration with interposed osteoinductive and osteogenic cancellous bone. Few graft complications (eg, graft resorption) and no immunogenic or infectious complications have been reported for iliac crest bone. However, autologous iliac crest increases operative time, and donor-site morbidity has been reported.23,24 A retrospective questionnaire-based investigation by Silber and colleagues, who evaluated iliac crest bone graft site morbidity in 1-level ACDF, found that 26.1% of patients had pain at the iliac crest harvest site, and 15.7% had numbness.24 Other complications, which occurred at lower rates, were bruising, hematoma, pelvic fracture, and poor cosmesis.23,25 In addition, osteoporosis and comorbid conditions have made it a challenge to acquire iliac crest autograft, contributing to the popularity of alternative substrates.25
Allografts
An alternative to autografts, allografts have the advantages of reduced operative time and reduced donor-site morbidity.26 Major historical concerns with allografts have included risk for disease transmission, costs associated with sterilization and serologic screening of grafts, and lack of oversight, leading to human allografts being acquired from dubious sources and ending up in the operating room.27,28 Two major types of allografts are available: mineralized and demineralized.
Arthrodesis rates are inferior for mineralized (structural) allografts with instrumentation than for autografts with instrumentation.29 In addition, smoking and other comorbidities have influenced fusion rates more in allograft than autograft fusions.30-33 However, allografts are being widely used because they avoid the donor-site morbidity associated with autografts and because they are load bearing, can provide structural stability and an osteoconductive matrix, and can be used off the shelf without adding much time to surgery.
Demineralized matrix substrates are commercial osteoconductive and osteoinductive biomaterials approved for filling bone gaps and extending graft when combined with autograft.7,8 Despite their osteoinductive properties, these substrates have had a high degree of product inconsistency, in some cases leading to poor outcomes.34 The lack of randomized studies with these constructs has made the determination of clear indications a challenge.
The initial enthusiasm over use of BMP, another bone-graft substitute for cervical fusion, was curtailed by reports of adverse events (AEs). Effective in anterior lumbar spine fusions, BMP was adapted to off-label use in the cervical spine a few years ago.35 Initial studies by Baskin and colleagues and Bishop and colleagues showed its fusion rates superior to those of allograft.31,32 Both studies reported no significant AEs. However, studies by Dickerman and colleagues and Smucker and colleagues demonstrated increased soft-tissue swelling leading to dysphagia and prolonged hospitalization, which were attributed to higher dosage (no study has identified a precise dose for individual patients).36,37 In addition, the cost of BMP is higher than that of any other bone-graft option for ACDF.3 Osteolysis has also been reported with BMP use.38-40 Carragee and colleagues highlighted the potential carcinogenicity of BMP, but this finding was not corroborated by Lad and colleagues.41,42
Cost Considerations
In addition to surgical effectiveness, spine surgical device costs have come under increased scrutiny.43-45 In 2012, plates were reported to cost (without overhead or profit margin to hospitals) between $1,015 and $3,601, and allograft spacers were estimated to cost between $1,220 and $3,640, cage costs ranged from $1,942 to $4,347, and PEEK spacers cost from $4,930 to $5,246.5 Individual surgeon instrumentation costs varied 10-fold based on the fusion constructs used.5
In a cost-effectiveness review of anterior cervical techniques, cage alone was the least expensive technique, disc arthroplasty or cage/plate/bone substitute groups were the next most expensive, and autograft alone was the most expensive option due to hip graft site morbidity.43 In another study, operative time associated with harvesting an iliac crest graft was equivalent in cost to that of an interbody cage.44 Other studies have compared the costs of various anterior cervical fusion constructs.9,10,45,46 A limitation of these studies is that autologous bone often refers to iliac crest grafts rather than local autograft. Epstein reviewed data from these studies and concluded, “ACDF using dynamic plates and autografts are the most cost effective treatment for anterior cervical discectomy,” citing a cost of $1,015 for this construct.5 Although Epstein demonstrated the cost-effectiveness of autograft in an individual surgeon’s hands, the results also are significant in that the studies identified areas in which improvements can be made at other institutions. The ROI-C cervical cage and local autograft bone cost that the authors report is at the lower end of the range reported by Epstein.5
Device explant rates also can be a concern. Operative waste was well described in a retrospective analysis of 87 ACDF procedures.47 The study found that the cost of explanting devices implanted during the same intraoperative period was equivalent to 9.2% of the cost of permanently implanted constructs. Epstein addressed operative waste by using educational modules to evaluate spine surgeons’ decision making before and after education. After the intervention, the institution noted a marked decline in costs related to explanted devices—from 20% in 2010 (before education) to 5.8% of the total cost of implanted devices in 2010 (after education).5
In the present study, the authors demonstrated that use of local morselized autograft with a PEEK spacer for 1-level ACDF had excellent arthrodesis rates and minimal complications. Of the 52 patients with 9 month postoperative data, all achieved arthrodesis regardless of tobacco use. This method compares favorably with other fusion options in terms of radiographic arthrodesis rates. In addition, it avoids the donor-site morbidity associated with autografts from an iliac site but maintains the benefits of the osteogenic, osteoconductive, and osteoinductive properties of autograft bone. Use of local autograft avoids the costs associated with iliac crest autograft, including increased operating and anesthesia time, additional operating room supplies (drapes, sutures, etc) needed for operating at a second site, and prolonged hospital stay due to pain at the donor site. Use of local autograft also obviates complications at a second surgical site; purchase, storage, and sterilization of allograft; and the neck swelling, possible carcinogenicity, and cost of purchase of BMP. Other than the occasional reuse of distraction posts, this method involves no other expensive explant supplies.
Autografts have osteogenic, osteoconductive, and osteoinductive properties, and autograft fusion rates are generally superior to allograft fusion rates. Bone morphogenetic protein fusion rates may be comparable to autograft fusion rates.9,26,32 Shortcomings of iliac crest autografts include increased operative time, blood loss, and donor-site morbidity. Allografts are osteoconductive and osteoinductive, but their fusion rates are inferior to those of iliac crest autografts. Other shortcomings are infection transmission and immunogenicity risks, higher graft resorption and collapse rates, cost, and previous issues relating to provenance. Bone morphogenetic protein is the most osteoinductive material with fusion rates similar to those of autograft, but its use is associated with neck swelling, dysphagia, osteolysis, potential carcinogenicity, and high cost.9
Conclusion
Overall, use of local autograft with a PEEK spacer has all the advantages of iliac crest autograft along with the benefit of working within the same operative window as the ACDF, thus reducing the infection, bleeding, and pain risks that may be encountered with a second incision. This procedure is effective, inexpensive, and cost-effective compared with alternatives and may be preferable for 1-level ACDF. In a population of patients with high rates of tobacco use, diabetes mellitus, obesity, and other factors that negatively affect fusion rates, local autograft may be a good choice for efficacy and cost savings.
Acknowledgments
The authors thank Shirley McCartney, PhD, for editorial assistance and Andy Rekito, MS, for illustrative assistance.
1. Cloward RB. The anterior approach for removal of ruptured cervical disks. 1958. J Neurosurg Spine. 2007;6(5):496-511.
2. Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15(6):602-617.
3. Robinson RA, Smith GW. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. SAS J. 2010;4(1):34-35.
4. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
5. Epstein NE. Iliac crest autograft versus alternative constructs for anterior cervical spine surgery: pros, cons, and costs. Surg Neurol Int. 2012;3(suppl 3):S143-S156.
6. Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev. 2012;64(12):1063-1077.
7. Becker S, Maissen O, Ponomarev I, Stoll T, Rahn B, Wilke I. Osteopromotion by a beta-tricalcium phosphate/bone marrow hybrid implant for use in spine surgery. Spine (Phila Pa 1976). 2006;31(1):11-17.
8. Muschik M, Ludwig R, Halbhübner S, Bursche K, Stoll T. Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J. 2001;10(suppl 2):S178-S184.
9. Buttermann GR. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8(3):426-435.
10. Epstein NE. Efficacy and outcomes of dynamic-plated single-level anterior diskectomy/fusion with additional analysis of comparative costs. Surg Neurol Int. 2011;2:9.
11. Wang MC, Kreuter W, Wolfla CE, Maiman DJ, Deyo RA. Trends and variations in cervical spine surgery in the United States: Medicare beneficiaries, 1992 to 2005. Spine (Phila Pa 1976). 2009;34(9):955-961.
12. Fraser JF, Härtl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine. 2007;6(4):298-303.
13. Nirala AP, Husain M, Vatsal DK. A retrospective study of multiple interbody grafting and long segment strut grafting following multilevel anterior cervical decompression. Br J Neurosurg. 2004;18(3):227-232.
14. Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75(9):1298-1307.
15. Cauthen JC, Kinard RE, Vogler JB, et al. Outcome analysis of noninstrumented anterior cervical discectomy and interbody fusion in 348 patients. Spine (Phila Pa 1976). 1998;23(2):188-192.
16. Emery SE, Fisher JR, Bohlman HH. Three-level anterior cervical discectomy and fusion: radiographic and clinical results. Spine (Phila Pa 1976). 1997;22(22):2622-2624.
17. Siambanes D, Mather S. Comparison of plain radiographs and CT scans in instrumented posterior lumbar interbody fusion. Orthopedics. 1998;21(2):165-167.
18. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
19. Hikino K, Yamamoto LG. The benefit of neck computed tomography compared with its harm (risk of cancer). J Trauma Acute Care Surg. 2015;78(1):126-131.
20. Schonfeld SJ, Lee C, Berrington de González A. Medical exposure to radiation and thyroid cancer. Clin Oncol (R Coll Radiol). 2011;23(4):244-250.
21. Bartels RH, Beems T, Schutte PJ, Verbeek AL. The rationale of postoperative radiographs after cervical anterior discectomy with stand-alone cage for radicular pain. J Neurosurg Spine. 2010;12(3):275-279.
22. Centers for Disease Control and Prevention. The different types of health assessments. Centers for Disease Control and Prevention website. http://www.cdc.gov/healthyplaces/types_health_assessments.htm. Updated July 25, 2012. Accessed April 8, 2016.
23. Schnee CL, Freese A, Weil RJ, Marcotte PJ. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine (Phila Pa 1976). 1997;22(19):2222-2227.
24. Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2003;28(2):134-139.
25. Seiler JG 3rd, Johnson J. Iliac crest autogenous bone grafting: donor site complications. J South Orthop Assoc. 2000;9(2):91-97.
26. Floyd T, Ohnmeiss D. A meta-analysis of autograft versus allograft in anterior cervical fusion. Eur Spine J. 2000;9(5):398-403.
27. Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: what they can offer and what they cannot. J Bone Joint Surg Br. 2007;89(5):574-579.
28. Armour S. Illegal trade in bodies shakes loved ones. USA Today. http://usatoday30.usatoday.com/money/2006-04-26-body-parts-cover-usat_x.htm. Updated April 28, 2006. Accessed April 6, 2016.
29. Wigfield CC, Nelson RJ. Nonautologous interbody fusion materials in cervical spine surgery: how strong is the evidence to justify their use? Spine (Phila Pa 1976). 2001;26(6):687-694.
30. Bärlocher CB, Barth A, Krauss JK, Binggeli R, Seiler RW. Comparative evaluation of microdiscectomy only, autograft fusion, polymethylmethacrylate interposition, and threaded titanium cage fusion for treatment of single-level cervical disc disease: a prospective randomized study in 125 patients. Neurosurg Focus. 2002;12(1):E4.
31. Baskin DS, Ryan P, Sonntag V, Westmark R, Widmayer MA. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine (Phila Pa 1976). 2003;28(12):1219-1224.
32. Bishop RC, Moore KA, Hadley MN. Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg. 1996;85(2):206-210.
33. Martin GJ Jr, Haid RW Jr, MacMillan M, Rodts GE Jr, Berkman R. Anterior cervical discectomy with freeze-dried fibula allograft. Overview of 317 cases and literature review. Spine (Phila Pa 1976). 1999;24(9):852-858.
34. Bae HW, Zhao L, Kanim LE, Wong P, Delamarter RB, Dawson EG. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976). 2006;31(12):1299-1306.
35. Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15(5):337-349.
36. Dickerman RD, Reynolds AS, Morgan BC, Tompkins J, Cattorini J, Bennett M. rh-BMP-2 can be used safely in the cervical spine: dose and containment are the keys! Spine J. 2007;7(4):508-509.
37. Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Phila Pa 1976). 2006;31(24):2813-2819.
38. Vaidya R, Carp J, Sethi A, Bartol S, Craig J, Les CM. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16(8):1257-1265.
39. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
40. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine (Phila Pa 1976). 2011;36(8):672-676.
41. Carragee EJ, Chu G, Rohatgi R, et al. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am. 2013;95(17):1537-1545.
42. Lad SP, Bagley JH, Karikari IO, et al. Cancer after spinal fusion: the role of bone morphogenetic protein. Neurosurgery. 2013;73(3):440-449.
43. Bhadra AK, Raman AS, Casey AT, Crawford RJ. Single-level cervical radiculopathy: clinical outcome and cost-effectiveness of four techniques of anterior cervical discectomy and fusion and disc arthroplasty. Eur Spine J. 2009;18(2):232-237.
44. Castro FP Jr, Holt RT, Majd M, Whitecloud TS 3rd. A cost analysis of two anterior cervical fusion procedures. J Spinal Disord. 2000;13(6):511-514.
45. Kandziora F, Pflugmacher R, Scholz M, et al. Treatment of traumatic cervical spine instability with interbody fusion cages: a prospective controlled study with a 2-year follow-up. Injury. 2005;36(suppl 2):B27-B35.
46. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
47. Epstein NE, Schwall GS, Hood DC. The incidence and cost of devices explanted during single-level anterior diskectomy/fusions. Surg Neurol Int. 2011;2:23.
Anterior cervical discectomy and fusion (ACDF) has been performed with various techniques and devices for many years. Autologous iliac crest grafts were initially used for the Cloward1,2 and Robinson and Smith3 techniques, but because of iliac crest graft site complications (eg, pain, infection, fracture, dystrophic scarring4,5), the procedure was generally superseded by allograft implants. These implants were then supplemented with anterior locking plate devices. More recently, unitary devices combining a polyetheretherketone (PEEK) spacer with screw or blade fixation have been developed, such as the Zero P (Synthes, Inc.) and the ROI-C cervical cage (LDR). Bone graft is required to fill the cavity of these devices and to promote osseous union. Demineralized bone matrix,6 tricalcium phosphate,7,8 and bone morphogenetic protein (BMP) have been used for these purposes, but they add expense to the procedure and have been associated with several complications (eg, neck swelling, dysphagia associated with BMP).9
Although multiple studies have demonstrated effective fusion rates and good outcomes for both iliac crest autograft and grafting/spacer constructs, the debate over cost and “added value” remains unresolved. One institution, which has published articles reviewing the spine literature and its own data, concluded that iliac crest autograft was the most cost-effective and consistently successful ACDF procedure.5,10
The VA Portland Health Care System (VAPORHCS) has analyzed the use of local autograft sources at the surgical site to circumvent the need to make a second incision at the iliac crest and, theoretically, to decrease risks and expenses associated with iliac crest autograft, allograft bone, and artificial constructs. Given the paucity of data on this method, the case series presented here represents one of a few studies that analyze local autograft for promotion of arthrodesis in a PEEK spacer device.
This article will report on the prospectively collected results of consecutive cases performed by Dr. Ross using a ROI-C cervical cage for 1-level anterior cervical discectomy between August 2011 and November 2014. This study received institutional review board approval.
Methods
Neck disability index (NDI) forms were used to assess the impact of neck pain on patients’ ability to manage in everyday life. The NDI form was completed before surgery and 3 and 9 months after surgery.
Dr. Ross preferred to perform minimally invasive posterior cervical foraminotomy for unilateral radiculopathy. Therefore, all patients with radiculopathy had bilateral symptoms or a symptomatic midline disc protrusion not accessible from a posterior approach. Standard techniques were used to make a left-side approach to the anterior cervical spine except in cases in which a previous right-side approach could be reused. Under the microscope, the anterior longitudinal ligament and annulus were incised, and the anterior contents of the disc space were removed with curettes and pituitary rongeurs. Care was taken to remove all cartilage from beneath the anterior inferior lip of the rostral vertebral body and to remove a few millimeters of the anterior longitudinal ligament from the rostral vertebral body without use of monopolar cautery (Figure 1). A 2 mm Kerrison punch then was used to remove the anterior inferior lip of the rostral vertebral body, and this bone was saved for grafting. No bone wax was used within the disc space.
After all disc space cartilage was removed from the endplates, additional bone was obtained from the uncovertebral joints and posterior vertebral bodies as the decompression proceeded posteriorly. Occasionally, distraction posts were used if the disc space was too narrow for optimal visualization posteriorly. After decompression was achieved, a lordotic ROI-C cervical cage was packed in its lumen with the bone chips and impacted into the disc space under fluoroscopic guidance. The blades were impacted under fluoroscopic guidance as well. The wound was closed with absorbable suture.
Antibiotics were given for no more than 24 hours after surgery. Ketorolac was used for analgesia the night of the surgery, and patients were asked to not use nonsteroidal anti-inflammatory drugs for 3 months after surgery. Lateral radiographs were obtained 3 and 9 months after surgery and every 6 months thereafter until arthrodesis was detected.
Results
Seventy-seven consecutive patients underwent 1-level anterior cervical discectomy (Table 1). Twenty-four procedures were performed for radiculopathy, 52 for myelopathy, and 1 for central cord injury sustained in a fall by a patient with preexisting spinal stenosis. Surgery was performed at C3-C4 (25 cases), C4-C5 (11 cases), C5-C6 (15 cases), and C6-C7 (1 case) for patients with myelopathy. Surgery was performed at C3-C4 (2 cases), C4-C5 (3 cases), C5-C6 (9 cases), and C6-C7 (10 cases) for patients with radiculopathy.
Twenty-eight patients reported presurgery tobacco use. Although all tobacco-using patients agreed to cease use in the perioperative period, at least 9 admitted to resuming tobacco use immediately after surgery. Eighteen patients had diabetes mellitus. In 2 patients, a diagnosis of osteoporosis was made with dual-energy X-ray absorptiometry. One patient was a chronic user of steroids before and after surgery. Mean body mass index (BMI) was 30.6, and 13 patients were morbidly obese (BMI > 34).
In 2 cases, only a single blade was placed. The second blade could not be placed because of broken adjacent screws (1 case) or undetermined reason (1 case).
The mean time for follow-up was 17 months (range 3-34). Four patients were lost to follow-up: 3 after the 1-month postoperative visit and 1 with severe psychiatric problems after hospital discharge.
There were no new neurologic deficits, no wound infections, and no recurrent laryngeal nerve palsies in the 77 patients. Eight months after surgery, 1 patient with radiculopathy underwent foraminotomy at the index level for persisting foraminal stenosis. Two patients whose myelopathic symptoms persisted after surgery returned for minimally invasive posterior laminotomy to remove infolded ligamentum flavum. The presurgery and 3- and 9-month postsurgery NDI scores were available for 52 patients (Table 2). Before surgery the mean NDI score was 24 (range 8-40). Three months postsurgery the mean NDI score was 15 (range 2-27) for patients with myelopathy and 13 (range 2-28) for patients with radiculopathy. The patient with the highest NDI score (28) stated that though all his symptoms were relieved, he had gauged his responses to protect his disability claim. Nine months after surgery, the mean NDI scores were 9.5 (range 5-17) for patients with myelopathy and 6 (range 2-13) for patients with radiculopathy. No NDI score was higher postsurgery than presurgery.
Arthrodesis was defined as bony bridging between the adjacent vertebral bodies and the bone graft within the lumen of the device, anterior to the device, or posterior to the device. In Dr. Ross’ protocol, computed tomography (CT) scans or flexion-extension radiographs were obtained only if pseudarthrosis was suspected to avoid unnecessary radiation exposure. Sixty-six patients had at least the 3-month radiography follow-up available. All 52 patients with 9-month follow-up data achieved complete arthrodesis, as determined by plain film radiography. Bridging ossification was found anterior to the device in all but 9 patients. Trabeculated bone was growing through the lumen of the device in all cases (Figure 2). A broken blade without clinical correlation was noted on imaging for 1 patient.
The total cost of the ROI-C cervical cage (LDR) for VAPORHCS was $3,498, or $1,749 for the PEEK spacer plus $1,749 for 2 metal blades. In comparison, the total cost of a typical anterior locking plate would have been $6,700, or $3,200 for the plate plus $2,000 for 4 screws and $1,500 for an allograft fibular spacer. Demineralized bone matrix (1 mL) as used in cervical arthrodesis by other surgeons at VAPORHCS cost $279, or about $500 including shipping.
DISCUSSION
Anterior cervical discectomy with fusion is a very common and successful surgical procedure for cervical myelopathy, radiculopathy, and degenerative disease that has failed to be corrected with conservative therapy.10 Medicare data documented a 206% increase in 1-level fusion procedures for degenerative spine pathology performed between 1992 and 2005.11 When a procedure is performed so often, it is appropriate to review methods and analyze efficacy, cost, and cost-effectiveness.
According to a 2007 meta-analysis, the fusion rates of 1-level ACDF arthrodesis at 1-year follow-up are 97.1% in patients treated with anterior plates and 92.1% in patients treated with noninstrumented fusion.12 The rate disparity was larger for multiple-level fusion: 50% to 82.5% for instrumented cases12,13 vs 3% to 42% for noninstrumented cases.14-16 Given the higher fusion rates achieved with instrumentation, surgeons have favored its use in ACDF.
Computed Tomography Use
Computed tomography has long been considered the gold standard for assessing arthrodesis outcomes (eg, Siambanes and Mather).17 However, recent data on potential harm caused by CT-related ionizing radiation suggest a need for caution with routine CT use.18,19 For cervical spine CT, Schonfeld and colleagues found that the risk for excess thyroid cancers ranged from 1 to 33 cases per 10,000 CT scans.20 According to another report, “limiting neck CT scanning to a higher risk group would increase the gap between benefit and harm, whereas performing CT routinely on low-risk cases approaches a point where its harm equals or exceeds its benefit.”19 As some have questioned even routinepostoperative use of radiation in patients with unremarkable clinical courses—patients should be spared unnecessary exposure—CT scans or flexion-extensionradiographs were obtained at VAPORHCS only if clinical symptoms or radiographs were suggestive of pseudarthrosis.21 As none of the VAPORHCS patients had those symptoms, none underwent postoperative CT.
For anterior cervical arthrodesis, surgeon preference determines which of many different bone substrates can be used with instrumentation, which impacts the costs. Fusion substrates include structural autografts, structural allografts, morselized autografts, morselized allografts, demineralized allografts, porous ceramics and metals, and BMP. Given these many options, studies comparing the constructs are lacking, especially with regard to the cost of alternative fusion constructs that produce similar outcomes. The Centers for Disease Control and Prevention defines cost-benefit analysis as a “type of economic evaluation that measures both costs and benefits (ie, negative and positive consequences) associated with an intervention in dollar terms.”22 It has been reported that using iliac crest autografts with anterior plate instrumentation is the most cost-effective method, yet alternatives remain in use.5,10
For ACDF, iliac crest bone is an ideal and widely used construct substrate. Structural grafts harvested from the crest provide significant stability due to their bicortical or tricortical configuration with interposed osteoinductive and osteogenic cancellous bone. Few graft complications (eg, graft resorption) and no immunogenic or infectious complications have been reported for iliac crest bone. However, autologous iliac crest increases operative time, and donor-site morbidity has been reported.23,24 A retrospective questionnaire-based investigation by Silber and colleagues, who evaluated iliac crest bone graft site morbidity in 1-level ACDF, found that 26.1% of patients had pain at the iliac crest harvest site, and 15.7% had numbness.24 Other complications, which occurred at lower rates, were bruising, hematoma, pelvic fracture, and poor cosmesis.23,25 In addition, osteoporosis and comorbid conditions have made it a challenge to acquire iliac crest autograft, contributing to the popularity of alternative substrates.25
Allografts
An alternative to autografts, allografts have the advantages of reduced operative time and reduced donor-site morbidity.26 Major historical concerns with allografts have included risk for disease transmission, costs associated with sterilization and serologic screening of grafts, and lack of oversight, leading to human allografts being acquired from dubious sources and ending up in the operating room.27,28 Two major types of allografts are available: mineralized and demineralized.
Arthrodesis rates are inferior for mineralized (structural) allografts with instrumentation than for autografts with instrumentation.29 In addition, smoking and other comorbidities have influenced fusion rates more in allograft than autograft fusions.30-33 However, allografts are being widely used because they avoid the donor-site morbidity associated with autografts and because they are load bearing, can provide structural stability and an osteoconductive matrix, and can be used off the shelf without adding much time to surgery.
Demineralized matrix substrates are commercial osteoconductive and osteoinductive biomaterials approved for filling bone gaps and extending graft when combined with autograft.7,8 Despite their osteoinductive properties, these substrates have had a high degree of product inconsistency, in some cases leading to poor outcomes.34 The lack of randomized studies with these constructs has made the determination of clear indications a challenge.
The initial enthusiasm over use of BMP, another bone-graft substitute for cervical fusion, was curtailed by reports of adverse events (AEs). Effective in anterior lumbar spine fusions, BMP was adapted to off-label use in the cervical spine a few years ago.35 Initial studies by Baskin and colleagues and Bishop and colleagues showed its fusion rates superior to those of allograft.31,32 Both studies reported no significant AEs. However, studies by Dickerman and colleagues and Smucker and colleagues demonstrated increased soft-tissue swelling leading to dysphagia and prolonged hospitalization, which were attributed to higher dosage (no study has identified a precise dose for individual patients).36,37 In addition, the cost of BMP is higher than that of any other bone-graft option for ACDF.3 Osteolysis has also been reported with BMP use.38-40 Carragee and colleagues highlighted the potential carcinogenicity of BMP, but this finding was not corroborated by Lad and colleagues.41,42
Cost Considerations
In addition to surgical effectiveness, spine surgical device costs have come under increased scrutiny.43-45 In 2012, plates were reported to cost (without overhead or profit margin to hospitals) between $1,015 and $3,601, and allograft spacers were estimated to cost between $1,220 and $3,640, cage costs ranged from $1,942 to $4,347, and PEEK spacers cost from $4,930 to $5,246.5 Individual surgeon instrumentation costs varied 10-fold based on the fusion constructs used.5
In a cost-effectiveness review of anterior cervical techniques, cage alone was the least expensive technique, disc arthroplasty or cage/plate/bone substitute groups were the next most expensive, and autograft alone was the most expensive option due to hip graft site morbidity.43 In another study, operative time associated with harvesting an iliac crest graft was equivalent in cost to that of an interbody cage.44 Other studies have compared the costs of various anterior cervical fusion constructs.9,10,45,46 A limitation of these studies is that autologous bone often refers to iliac crest grafts rather than local autograft. Epstein reviewed data from these studies and concluded, “ACDF using dynamic plates and autografts are the most cost effective treatment for anterior cervical discectomy,” citing a cost of $1,015 for this construct.5 Although Epstein demonstrated the cost-effectiveness of autograft in an individual surgeon’s hands, the results also are significant in that the studies identified areas in which improvements can be made at other institutions. The ROI-C cervical cage and local autograft bone cost that the authors report is at the lower end of the range reported by Epstein.5
Device explant rates also can be a concern. Operative waste was well described in a retrospective analysis of 87 ACDF procedures.47 The study found that the cost of explanting devices implanted during the same intraoperative period was equivalent to 9.2% of the cost of permanently implanted constructs. Epstein addressed operative waste by using educational modules to evaluate spine surgeons’ decision making before and after education. After the intervention, the institution noted a marked decline in costs related to explanted devices—from 20% in 2010 (before education) to 5.8% of the total cost of implanted devices in 2010 (after education).5
In the present study, the authors demonstrated that use of local morselized autograft with a PEEK spacer for 1-level ACDF had excellent arthrodesis rates and minimal complications. Of the 52 patients with 9 month postoperative data, all achieved arthrodesis regardless of tobacco use. This method compares favorably with other fusion options in terms of radiographic arthrodesis rates. In addition, it avoids the donor-site morbidity associated with autografts from an iliac site but maintains the benefits of the osteogenic, osteoconductive, and osteoinductive properties of autograft bone. Use of local autograft avoids the costs associated with iliac crest autograft, including increased operating and anesthesia time, additional operating room supplies (drapes, sutures, etc) needed for operating at a second site, and prolonged hospital stay due to pain at the donor site. Use of local autograft also obviates complications at a second surgical site; purchase, storage, and sterilization of allograft; and the neck swelling, possible carcinogenicity, and cost of purchase of BMP. Other than the occasional reuse of distraction posts, this method involves no other expensive explant supplies.
Autografts have osteogenic, osteoconductive, and osteoinductive properties, and autograft fusion rates are generally superior to allograft fusion rates. Bone morphogenetic protein fusion rates may be comparable to autograft fusion rates.9,26,32 Shortcomings of iliac crest autografts include increased operative time, blood loss, and donor-site morbidity. Allografts are osteoconductive and osteoinductive, but their fusion rates are inferior to those of iliac crest autografts. Other shortcomings are infection transmission and immunogenicity risks, higher graft resorption and collapse rates, cost, and previous issues relating to provenance. Bone morphogenetic protein is the most osteoinductive material with fusion rates similar to those of autograft, but its use is associated with neck swelling, dysphagia, osteolysis, potential carcinogenicity, and high cost.9
Conclusion
Overall, use of local autograft with a PEEK spacer has all the advantages of iliac crest autograft along with the benefit of working within the same operative window as the ACDF, thus reducing the infection, bleeding, and pain risks that may be encountered with a second incision. This procedure is effective, inexpensive, and cost-effective compared with alternatives and may be preferable for 1-level ACDF. In a population of patients with high rates of tobacco use, diabetes mellitus, obesity, and other factors that negatively affect fusion rates, local autograft may be a good choice for efficacy and cost savings.
Acknowledgments
The authors thank Shirley McCartney, PhD, for editorial assistance and Andy Rekito, MS, for illustrative assistance.
Anterior cervical discectomy and fusion (ACDF) has been performed with various techniques and devices for many years. Autologous iliac crest grafts were initially used for the Cloward1,2 and Robinson and Smith3 techniques, but because of iliac crest graft site complications (eg, pain, infection, fracture, dystrophic scarring4,5), the procedure was generally superseded by allograft implants. These implants were then supplemented with anterior locking plate devices. More recently, unitary devices combining a polyetheretherketone (PEEK) spacer with screw or blade fixation have been developed, such as the Zero P (Synthes, Inc.) and the ROI-C cervical cage (LDR). Bone graft is required to fill the cavity of these devices and to promote osseous union. Demineralized bone matrix,6 tricalcium phosphate,7,8 and bone morphogenetic protein (BMP) have been used for these purposes, but they add expense to the procedure and have been associated with several complications (eg, neck swelling, dysphagia associated with BMP).9
Although multiple studies have demonstrated effective fusion rates and good outcomes for both iliac crest autograft and grafting/spacer constructs, the debate over cost and “added value” remains unresolved. One institution, which has published articles reviewing the spine literature and its own data, concluded that iliac crest autograft was the most cost-effective and consistently successful ACDF procedure.5,10
The VA Portland Health Care System (VAPORHCS) has analyzed the use of local autograft sources at the surgical site to circumvent the need to make a second incision at the iliac crest and, theoretically, to decrease risks and expenses associated with iliac crest autograft, allograft bone, and artificial constructs. Given the paucity of data on this method, the case series presented here represents one of a few studies that analyze local autograft for promotion of arthrodesis in a PEEK spacer device.
This article will report on the prospectively collected results of consecutive cases performed by Dr. Ross using a ROI-C cervical cage for 1-level anterior cervical discectomy between August 2011 and November 2014. This study received institutional review board approval.
Methods
Neck disability index (NDI) forms were used to assess the impact of neck pain on patients’ ability to manage in everyday life. The NDI form was completed before surgery and 3 and 9 months after surgery.
Dr. Ross preferred to perform minimally invasive posterior cervical foraminotomy for unilateral radiculopathy. Therefore, all patients with radiculopathy had bilateral symptoms or a symptomatic midline disc protrusion not accessible from a posterior approach. Standard techniques were used to make a left-side approach to the anterior cervical spine except in cases in which a previous right-side approach could be reused. Under the microscope, the anterior longitudinal ligament and annulus were incised, and the anterior contents of the disc space were removed with curettes and pituitary rongeurs. Care was taken to remove all cartilage from beneath the anterior inferior lip of the rostral vertebral body and to remove a few millimeters of the anterior longitudinal ligament from the rostral vertebral body without use of monopolar cautery (Figure 1). A 2 mm Kerrison punch then was used to remove the anterior inferior lip of the rostral vertebral body, and this bone was saved for grafting. No bone wax was used within the disc space.
After all disc space cartilage was removed from the endplates, additional bone was obtained from the uncovertebral joints and posterior vertebral bodies as the decompression proceeded posteriorly. Occasionally, distraction posts were used if the disc space was too narrow for optimal visualization posteriorly. After decompression was achieved, a lordotic ROI-C cervical cage was packed in its lumen with the bone chips and impacted into the disc space under fluoroscopic guidance. The blades were impacted under fluoroscopic guidance as well. The wound was closed with absorbable suture.
Antibiotics were given for no more than 24 hours after surgery. Ketorolac was used for analgesia the night of the surgery, and patients were asked to not use nonsteroidal anti-inflammatory drugs for 3 months after surgery. Lateral radiographs were obtained 3 and 9 months after surgery and every 6 months thereafter until arthrodesis was detected.
Results
Seventy-seven consecutive patients underwent 1-level anterior cervical discectomy (Table 1). Twenty-four procedures were performed for radiculopathy, 52 for myelopathy, and 1 for central cord injury sustained in a fall by a patient with preexisting spinal stenosis. Surgery was performed at C3-C4 (25 cases), C4-C5 (11 cases), C5-C6 (15 cases), and C6-C7 (1 case) for patients with myelopathy. Surgery was performed at C3-C4 (2 cases), C4-C5 (3 cases), C5-C6 (9 cases), and C6-C7 (10 cases) for patients with radiculopathy.
Twenty-eight patients reported presurgery tobacco use. Although all tobacco-using patients agreed to cease use in the perioperative period, at least 9 admitted to resuming tobacco use immediately after surgery. Eighteen patients had diabetes mellitus. In 2 patients, a diagnosis of osteoporosis was made with dual-energy X-ray absorptiometry. One patient was a chronic user of steroids before and after surgery. Mean body mass index (BMI) was 30.6, and 13 patients were morbidly obese (BMI > 34).
In 2 cases, only a single blade was placed. The second blade could not be placed because of broken adjacent screws (1 case) or undetermined reason (1 case).
The mean time for follow-up was 17 months (range 3-34). Four patients were lost to follow-up: 3 after the 1-month postoperative visit and 1 with severe psychiatric problems after hospital discharge.
There were no new neurologic deficits, no wound infections, and no recurrent laryngeal nerve palsies in the 77 patients. Eight months after surgery, 1 patient with radiculopathy underwent foraminotomy at the index level for persisting foraminal stenosis. Two patients whose myelopathic symptoms persisted after surgery returned for minimally invasive posterior laminotomy to remove infolded ligamentum flavum. The presurgery and 3- and 9-month postsurgery NDI scores were available for 52 patients (Table 2). Before surgery the mean NDI score was 24 (range 8-40). Three months postsurgery the mean NDI score was 15 (range 2-27) for patients with myelopathy and 13 (range 2-28) for patients with radiculopathy. The patient with the highest NDI score (28) stated that though all his symptoms were relieved, he had gauged his responses to protect his disability claim. Nine months after surgery, the mean NDI scores were 9.5 (range 5-17) for patients with myelopathy and 6 (range 2-13) for patients with radiculopathy. No NDI score was higher postsurgery than presurgery.
Arthrodesis was defined as bony bridging between the adjacent vertebral bodies and the bone graft within the lumen of the device, anterior to the device, or posterior to the device. In Dr. Ross’ protocol, computed tomography (CT) scans or flexion-extension radiographs were obtained only if pseudarthrosis was suspected to avoid unnecessary radiation exposure. Sixty-six patients had at least the 3-month radiography follow-up available. All 52 patients with 9-month follow-up data achieved complete arthrodesis, as determined by plain film radiography. Bridging ossification was found anterior to the device in all but 9 patients. Trabeculated bone was growing through the lumen of the device in all cases (Figure 2). A broken blade without clinical correlation was noted on imaging for 1 patient.
The total cost of the ROI-C cervical cage (LDR) for VAPORHCS was $3,498, or $1,749 for the PEEK spacer plus $1,749 for 2 metal blades. In comparison, the total cost of a typical anterior locking plate would have been $6,700, or $3,200 for the plate plus $2,000 for 4 screws and $1,500 for an allograft fibular spacer. Demineralized bone matrix (1 mL) as used in cervical arthrodesis by other surgeons at VAPORHCS cost $279, or about $500 including shipping.
DISCUSSION
Anterior cervical discectomy with fusion is a very common and successful surgical procedure for cervical myelopathy, radiculopathy, and degenerative disease that has failed to be corrected with conservative therapy.10 Medicare data documented a 206% increase in 1-level fusion procedures for degenerative spine pathology performed between 1992 and 2005.11 When a procedure is performed so often, it is appropriate to review methods and analyze efficacy, cost, and cost-effectiveness.
According to a 2007 meta-analysis, the fusion rates of 1-level ACDF arthrodesis at 1-year follow-up are 97.1% in patients treated with anterior plates and 92.1% in patients treated with noninstrumented fusion.12 The rate disparity was larger for multiple-level fusion: 50% to 82.5% for instrumented cases12,13 vs 3% to 42% for noninstrumented cases.14-16 Given the higher fusion rates achieved with instrumentation, surgeons have favored its use in ACDF.
Computed Tomography Use
Computed tomography has long been considered the gold standard for assessing arthrodesis outcomes (eg, Siambanes and Mather).17 However, recent data on potential harm caused by CT-related ionizing radiation suggest a need for caution with routine CT use.18,19 For cervical spine CT, Schonfeld and colleagues found that the risk for excess thyroid cancers ranged from 1 to 33 cases per 10,000 CT scans.20 According to another report, “limiting neck CT scanning to a higher risk group would increase the gap between benefit and harm, whereas performing CT routinely on low-risk cases approaches a point where its harm equals or exceeds its benefit.”19 As some have questioned even routinepostoperative use of radiation in patients with unremarkable clinical courses—patients should be spared unnecessary exposure—CT scans or flexion-extensionradiographs were obtained at VAPORHCS only if clinical symptoms or radiographs were suggestive of pseudarthrosis.21 As none of the VAPORHCS patients had those symptoms, none underwent postoperative CT.
For anterior cervical arthrodesis, surgeon preference determines which of many different bone substrates can be used with instrumentation, which impacts the costs. Fusion substrates include structural autografts, structural allografts, morselized autografts, morselized allografts, demineralized allografts, porous ceramics and metals, and BMP. Given these many options, studies comparing the constructs are lacking, especially with regard to the cost of alternative fusion constructs that produce similar outcomes. The Centers for Disease Control and Prevention defines cost-benefit analysis as a “type of economic evaluation that measures both costs and benefits (ie, negative and positive consequences) associated with an intervention in dollar terms.”22 It has been reported that using iliac crest autografts with anterior plate instrumentation is the most cost-effective method, yet alternatives remain in use.5,10
For ACDF, iliac crest bone is an ideal and widely used construct substrate. Structural grafts harvested from the crest provide significant stability due to their bicortical or tricortical configuration with interposed osteoinductive and osteogenic cancellous bone. Few graft complications (eg, graft resorption) and no immunogenic or infectious complications have been reported for iliac crest bone. However, autologous iliac crest increases operative time, and donor-site morbidity has been reported.23,24 A retrospective questionnaire-based investigation by Silber and colleagues, who evaluated iliac crest bone graft site morbidity in 1-level ACDF, found that 26.1% of patients had pain at the iliac crest harvest site, and 15.7% had numbness.24 Other complications, which occurred at lower rates, were bruising, hematoma, pelvic fracture, and poor cosmesis.23,25 In addition, osteoporosis and comorbid conditions have made it a challenge to acquire iliac crest autograft, contributing to the popularity of alternative substrates.25
Allografts
An alternative to autografts, allografts have the advantages of reduced operative time and reduced donor-site morbidity.26 Major historical concerns with allografts have included risk for disease transmission, costs associated with sterilization and serologic screening of grafts, and lack of oversight, leading to human allografts being acquired from dubious sources and ending up in the operating room.27,28 Two major types of allografts are available: mineralized and demineralized.
Arthrodesis rates are inferior for mineralized (structural) allografts with instrumentation than for autografts with instrumentation.29 In addition, smoking and other comorbidities have influenced fusion rates more in allograft than autograft fusions.30-33 However, allografts are being widely used because they avoid the donor-site morbidity associated with autografts and because they are load bearing, can provide structural stability and an osteoconductive matrix, and can be used off the shelf without adding much time to surgery.
Demineralized matrix substrates are commercial osteoconductive and osteoinductive biomaterials approved for filling bone gaps and extending graft when combined with autograft.7,8 Despite their osteoinductive properties, these substrates have had a high degree of product inconsistency, in some cases leading to poor outcomes.34 The lack of randomized studies with these constructs has made the determination of clear indications a challenge.
The initial enthusiasm over use of BMP, another bone-graft substitute for cervical fusion, was curtailed by reports of adverse events (AEs). Effective in anterior lumbar spine fusions, BMP was adapted to off-label use in the cervical spine a few years ago.35 Initial studies by Baskin and colleagues and Bishop and colleagues showed its fusion rates superior to those of allograft.31,32 Both studies reported no significant AEs. However, studies by Dickerman and colleagues and Smucker and colleagues demonstrated increased soft-tissue swelling leading to dysphagia and prolonged hospitalization, which were attributed to higher dosage (no study has identified a precise dose for individual patients).36,37 In addition, the cost of BMP is higher than that of any other bone-graft option for ACDF.3 Osteolysis has also been reported with BMP use.38-40 Carragee and colleagues highlighted the potential carcinogenicity of BMP, but this finding was not corroborated by Lad and colleagues.41,42
Cost Considerations
In addition to surgical effectiveness, spine surgical device costs have come under increased scrutiny.43-45 In 2012, plates were reported to cost (without overhead or profit margin to hospitals) between $1,015 and $3,601, and allograft spacers were estimated to cost between $1,220 and $3,640, cage costs ranged from $1,942 to $4,347, and PEEK spacers cost from $4,930 to $5,246.5 Individual surgeon instrumentation costs varied 10-fold based on the fusion constructs used.5
In a cost-effectiveness review of anterior cervical techniques, cage alone was the least expensive technique, disc arthroplasty or cage/plate/bone substitute groups were the next most expensive, and autograft alone was the most expensive option due to hip graft site morbidity.43 In another study, operative time associated with harvesting an iliac crest graft was equivalent in cost to that of an interbody cage.44 Other studies have compared the costs of various anterior cervical fusion constructs.9,10,45,46 A limitation of these studies is that autologous bone often refers to iliac crest grafts rather than local autograft. Epstein reviewed data from these studies and concluded, “ACDF using dynamic plates and autografts are the most cost effective treatment for anterior cervical discectomy,” citing a cost of $1,015 for this construct.5 Although Epstein demonstrated the cost-effectiveness of autograft in an individual surgeon’s hands, the results also are significant in that the studies identified areas in which improvements can be made at other institutions. The ROI-C cervical cage and local autograft bone cost that the authors report is at the lower end of the range reported by Epstein.5
Device explant rates also can be a concern. Operative waste was well described in a retrospective analysis of 87 ACDF procedures.47 The study found that the cost of explanting devices implanted during the same intraoperative period was equivalent to 9.2% of the cost of permanently implanted constructs. Epstein addressed operative waste by using educational modules to evaluate spine surgeons’ decision making before and after education. After the intervention, the institution noted a marked decline in costs related to explanted devices—from 20% in 2010 (before education) to 5.8% of the total cost of implanted devices in 2010 (after education).5
In the present study, the authors demonstrated that use of local morselized autograft with a PEEK spacer for 1-level ACDF had excellent arthrodesis rates and minimal complications. Of the 52 patients with 9 month postoperative data, all achieved arthrodesis regardless of tobacco use. This method compares favorably with other fusion options in terms of radiographic arthrodesis rates. In addition, it avoids the donor-site morbidity associated with autografts from an iliac site but maintains the benefits of the osteogenic, osteoconductive, and osteoinductive properties of autograft bone. Use of local autograft avoids the costs associated with iliac crest autograft, including increased operating and anesthesia time, additional operating room supplies (drapes, sutures, etc) needed for operating at a second site, and prolonged hospital stay due to pain at the donor site. Use of local autograft also obviates complications at a second surgical site; purchase, storage, and sterilization of allograft; and the neck swelling, possible carcinogenicity, and cost of purchase of BMP. Other than the occasional reuse of distraction posts, this method involves no other expensive explant supplies.
Autografts have osteogenic, osteoconductive, and osteoinductive properties, and autograft fusion rates are generally superior to allograft fusion rates. Bone morphogenetic protein fusion rates may be comparable to autograft fusion rates.9,26,32 Shortcomings of iliac crest autografts include increased operative time, blood loss, and donor-site morbidity. Allografts are osteoconductive and osteoinductive, but their fusion rates are inferior to those of iliac crest autografts. Other shortcomings are infection transmission and immunogenicity risks, higher graft resorption and collapse rates, cost, and previous issues relating to provenance. Bone morphogenetic protein is the most osteoinductive material with fusion rates similar to those of autograft, but its use is associated with neck swelling, dysphagia, osteolysis, potential carcinogenicity, and high cost.9
Conclusion
Overall, use of local autograft with a PEEK spacer has all the advantages of iliac crest autograft along with the benefit of working within the same operative window as the ACDF, thus reducing the infection, bleeding, and pain risks that may be encountered with a second incision. This procedure is effective, inexpensive, and cost-effective compared with alternatives and may be preferable for 1-level ACDF. In a population of patients with high rates of tobacco use, diabetes mellitus, obesity, and other factors that negatively affect fusion rates, local autograft may be a good choice for efficacy and cost savings.
Acknowledgments
The authors thank Shirley McCartney, PhD, for editorial assistance and Andy Rekito, MS, for illustrative assistance.
1. Cloward RB. The anterior approach for removal of ruptured cervical disks. 1958. J Neurosurg Spine. 2007;6(5):496-511.
2. Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15(6):602-617.
3. Robinson RA, Smith GW. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. SAS J. 2010;4(1):34-35.
4. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
5. Epstein NE. Iliac crest autograft versus alternative constructs for anterior cervical spine surgery: pros, cons, and costs. Surg Neurol Int. 2012;3(suppl 3):S143-S156.
6. Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev. 2012;64(12):1063-1077.
7. Becker S, Maissen O, Ponomarev I, Stoll T, Rahn B, Wilke I. Osteopromotion by a beta-tricalcium phosphate/bone marrow hybrid implant for use in spine surgery. Spine (Phila Pa 1976). 2006;31(1):11-17.
8. Muschik M, Ludwig R, Halbhübner S, Bursche K, Stoll T. Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J. 2001;10(suppl 2):S178-S184.
9. Buttermann GR. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8(3):426-435.
10. Epstein NE. Efficacy and outcomes of dynamic-plated single-level anterior diskectomy/fusion with additional analysis of comparative costs. Surg Neurol Int. 2011;2:9.
11. Wang MC, Kreuter W, Wolfla CE, Maiman DJ, Deyo RA. Trends and variations in cervical spine surgery in the United States: Medicare beneficiaries, 1992 to 2005. Spine (Phila Pa 1976). 2009;34(9):955-961.
12. Fraser JF, Härtl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine. 2007;6(4):298-303.
13. Nirala AP, Husain M, Vatsal DK. A retrospective study of multiple interbody grafting and long segment strut grafting following multilevel anterior cervical decompression. Br J Neurosurg. 2004;18(3):227-232.
14. Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75(9):1298-1307.
15. Cauthen JC, Kinard RE, Vogler JB, et al. Outcome analysis of noninstrumented anterior cervical discectomy and interbody fusion in 348 patients. Spine (Phila Pa 1976). 1998;23(2):188-192.
16. Emery SE, Fisher JR, Bohlman HH. Three-level anterior cervical discectomy and fusion: radiographic and clinical results. Spine (Phila Pa 1976). 1997;22(22):2622-2624.
17. Siambanes D, Mather S. Comparison of plain radiographs and CT scans in instrumented posterior lumbar interbody fusion. Orthopedics. 1998;21(2):165-167.
18. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
19. Hikino K, Yamamoto LG. The benefit of neck computed tomography compared with its harm (risk of cancer). J Trauma Acute Care Surg. 2015;78(1):126-131.
20. Schonfeld SJ, Lee C, Berrington de González A. Medical exposure to radiation and thyroid cancer. Clin Oncol (R Coll Radiol). 2011;23(4):244-250.
21. Bartels RH, Beems T, Schutte PJ, Verbeek AL. The rationale of postoperative radiographs after cervical anterior discectomy with stand-alone cage for radicular pain. J Neurosurg Spine. 2010;12(3):275-279.
22. Centers for Disease Control and Prevention. The different types of health assessments. Centers for Disease Control and Prevention website. http://www.cdc.gov/healthyplaces/types_health_assessments.htm. Updated July 25, 2012. Accessed April 8, 2016.
23. Schnee CL, Freese A, Weil RJ, Marcotte PJ. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine (Phila Pa 1976). 1997;22(19):2222-2227.
24. Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2003;28(2):134-139.
25. Seiler JG 3rd, Johnson J. Iliac crest autogenous bone grafting: donor site complications. J South Orthop Assoc. 2000;9(2):91-97.
26. Floyd T, Ohnmeiss D. A meta-analysis of autograft versus allograft in anterior cervical fusion. Eur Spine J. 2000;9(5):398-403.
27. Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: what they can offer and what they cannot. J Bone Joint Surg Br. 2007;89(5):574-579.
28. Armour S. Illegal trade in bodies shakes loved ones. USA Today. http://usatoday30.usatoday.com/money/2006-04-26-body-parts-cover-usat_x.htm. Updated April 28, 2006. Accessed April 6, 2016.
29. Wigfield CC, Nelson RJ. Nonautologous interbody fusion materials in cervical spine surgery: how strong is the evidence to justify their use? Spine (Phila Pa 1976). 2001;26(6):687-694.
30. Bärlocher CB, Barth A, Krauss JK, Binggeli R, Seiler RW. Comparative evaluation of microdiscectomy only, autograft fusion, polymethylmethacrylate interposition, and threaded titanium cage fusion for treatment of single-level cervical disc disease: a prospective randomized study in 125 patients. Neurosurg Focus. 2002;12(1):E4.
31. Baskin DS, Ryan P, Sonntag V, Westmark R, Widmayer MA. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine (Phila Pa 1976). 2003;28(12):1219-1224.
32. Bishop RC, Moore KA, Hadley MN. Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg. 1996;85(2):206-210.
33. Martin GJ Jr, Haid RW Jr, MacMillan M, Rodts GE Jr, Berkman R. Anterior cervical discectomy with freeze-dried fibula allograft. Overview of 317 cases and literature review. Spine (Phila Pa 1976). 1999;24(9):852-858.
34. Bae HW, Zhao L, Kanim LE, Wong P, Delamarter RB, Dawson EG. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976). 2006;31(12):1299-1306.
35. Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15(5):337-349.
36. Dickerman RD, Reynolds AS, Morgan BC, Tompkins J, Cattorini J, Bennett M. rh-BMP-2 can be used safely in the cervical spine: dose and containment are the keys! Spine J. 2007;7(4):508-509.
37. Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Phila Pa 1976). 2006;31(24):2813-2819.
38. Vaidya R, Carp J, Sethi A, Bartol S, Craig J, Les CM. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16(8):1257-1265.
39. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
40. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine (Phila Pa 1976). 2011;36(8):672-676.
41. Carragee EJ, Chu G, Rohatgi R, et al. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am. 2013;95(17):1537-1545.
42. Lad SP, Bagley JH, Karikari IO, et al. Cancer after spinal fusion: the role of bone morphogenetic protein. Neurosurgery. 2013;73(3):440-449.
43. Bhadra AK, Raman AS, Casey AT, Crawford RJ. Single-level cervical radiculopathy: clinical outcome and cost-effectiveness of four techniques of anterior cervical discectomy and fusion and disc arthroplasty. Eur Spine J. 2009;18(2):232-237.
44. Castro FP Jr, Holt RT, Majd M, Whitecloud TS 3rd. A cost analysis of two anterior cervical fusion procedures. J Spinal Disord. 2000;13(6):511-514.
45. Kandziora F, Pflugmacher R, Scholz M, et al. Treatment of traumatic cervical spine instability with interbody fusion cages: a prospective controlled study with a 2-year follow-up. Injury. 2005;36(suppl 2):B27-B35.
46. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
47. Epstein NE, Schwall GS, Hood DC. The incidence and cost of devices explanted during single-level anterior diskectomy/fusions. Surg Neurol Int. 2011;2:23.
1. Cloward RB. The anterior approach for removal of ruptured cervical disks. 1958. J Neurosurg Spine. 2007;6(5):496-511.
2. Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15(6):602-617.
3. Robinson RA, Smith GW. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. SAS J. 2010;4(1):34-35.
4. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
5. Epstein NE. Iliac crest autograft versus alternative constructs for anterior cervical spine surgery: pros, cons, and costs. Surg Neurol Int. 2012;3(suppl 3):S143-S156.
6. Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev. 2012;64(12):1063-1077.
7. Becker S, Maissen O, Ponomarev I, Stoll T, Rahn B, Wilke I. Osteopromotion by a beta-tricalcium phosphate/bone marrow hybrid implant for use in spine surgery. Spine (Phila Pa 1976). 2006;31(1):11-17.
8. Muschik M, Ludwig R, Halbhübner S, Bursche K, Stoll T. Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J. 2001;10(suppl 2):S178-S184.
9. Buttermann GR. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8(3):426-435.
10. Epstein NE. Efficacy and outcomes of dynamic-plated single-level anterior diskectomy/fusion with additional analysis of comparative costs. Surg Neurol Int. 2011;2:9.
11. Wang MC, Kreuter W, Wolfla CE, Maiman DJ, Deyo RA. Trends and variations in cervical spine surgery in the United States: Medicare beneficiaries, 1992 to 2005. Spine (Phila Pa 1976). 2009;34(9):955-961.
12. Fraser JF, Härtl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine. 2007;6(4):298-303.
13. Nirala AP, Husain M, Vatsal DK. A retrospective study of multiple interbody grafting and long segment strut grafting following multilevel anterior cervical decompression. Br J Neurosurg. 2004;18(3):227-232.
14. Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75(9):1298-1307.
15. Cauthen JC, Kinard RE, Vogler JB, et al. Outcome analysis of noninstrumented anterior cervical discectomy and interbody fusion in 348 patients. Spine (Phila Pa 1976). 1998;23(2):188-192.
16. Emery SE, Fisher JR, Bohlman HH. Three-level anterior cervical discectomy and fusion: radiographic and clinical results. Spine (Phila Pa 1976). 1997;22(22):2622-2624.
17. Siambanes D, Mather S. Comparison of plain radiographs and CT scans in instrumented posterior lumbar interbody fusion. Orthopedics. 1998;21(2):165-167.
18. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
19. Hikino K, Yamamoto LG. The benefit of neck computed tomography compared with its harm (risk of cancer). J Trauma Acute Care Surg. 2015;78(1):126-131.
20. Schonfeld SJ, Lee C, Berrington de González A. Medical exposure to radiation and thyroid cancer. Clin Oncol (R Coll Radiol). 2011;23(4):244-250.
21. Bartels RH, Beems T, Schutte PJ, Verbeek AL. The rationale of postoperative radiographs after cervical anterior discectomy with stand-alone cage for radicular pain. J Neurosurg Spine. 2010;12(3):275-279.
22. Centers for Disease Control and Prevention. The different types of health assessments. Centers for Disease Control and Prevention website. http://www.cdc.gov/healthyplaces/types_health_assessments.htm. Updated July 25, 2012. Accessed April 8, 2016.
23. Schnee CL, Freese A, Weil RJ, Marcotte PJ. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine (Phila Pa 1976). 1997;22(19):2222-2227.
24. Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2003;28(2):134-139.
25. Seiler JG 3rd, Johnson J. Iliac crest autogenous bone grafting: donor site complications. J South Orthop Assoc. 2000;9(2):91-97.
26. Floyd T, Ohnmeiss D. A meta-analysis of autograft versus allograft in anterior cervical fusion. Eur Spine J. 2000;9(5):398-403.
27. Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: what they can offer and what they cannot. J Bone Joint Surg Br. 2007;89(5):574-579.
28. Armour S. Illegal trade in bodies shakes loved ones. USA Today. http://usatoday30.usatoday.com/money/2006-04-26-body-parts-cover-usat_x.htm. Updated April 28, 2006. Accessed April 6, 2016.
29. Wigfield CC, Nelson RJ. Nonautologous interbody fusion materials in cervical spine surgery: how strong is the evidence to justify their use? Spine (Phila Pa 1976). 2001;26(6):687-694.
30. Bärlocher CB, Barth A, Krauss JK, Binggeli R, Seiler RW. Comparative evaluation of microdiscectomy only, autograft fusion, polymethylmethacrylate interposition, and threaded titanium cage fusion for treatment of single-level cervical disc disease: a prospective randomized study in 125 patients. Neurosurg Focus. 2002;12(1):E4.
31. Baskin DS, Ryan P, Sonntag V, Westmark R, Widmayer MA. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine (Phila Pa 1976). 2003;28(12):1219-1224.
32. Bishop RC, Moore KA, Hadley MN. Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg. 1996;85(2):206-210.
33. Martin GJ Jr, Haid RW Jr, MacMillan M, Rodts GE Jr, Berkman R. Anterior cervical discectomy with freeze-dried fibula allograft. Overview of 317 cases and literature review. Spine (Phila Pa 1976). 1999;24(9):852-858.
34. Bae HW, Zhao L, Kanim LE, Wong P, Delamarter RB, Dawson EG. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976). 2006;31(12):1299-1306.
35. Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15(5):337-349.
36. Dickerman RD, Reynolds AS, Morgan BC, Tompkins J, Cattorini J, Bennett M. rh-BMP-2 can be used safely in the cervical spine: dose and containment are the keys! Spine J. 2007;7(4):508-509.
37. Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Phila Pa 1976). 2006;31(24):2813-2819.
38. Vaidya R, Carp J, Sethi A, Bartol S, Craig J, Les CM. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16(8):1257-1265.
39. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
40. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine (Phila Pa 1976). 2011;36(8):672-676.
41. Carragee EJ, Chu G, Rohatgi R, et al. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am. 2013;95(17):1537-1545.
42. Lad SP, Bagley JH, Karikari IO, et al. Cancer after spinal fusion: the role of bone morphogenetic protein. Neurosurgery. 2013;73(3):440-449.
43. Bhadra AK, Raman AS, Casey AT, Crawford RJ. Single-level cervical radiculopathy: clinical outcome and cost-effectiveness of four techniques of anterior cervical discectomy and fusion and disc arthroplasty. Eur Spine J. 2009;18(2):232-237.
44. Castro FP Jr, Holt RT, Majd M, Whitecloud TS 3rd. A cost analysis of two anterior cervical fusion procedures. J Spinal Disord. 2000;13(6):511-514.
45. Kandziora F, Pflugmacher R, Scholz M, et al. Treatment of traumatic cervical spine instability with interbody fusion cages: a prospective controlled study with a 2-year follow-up. Injury. 2005;36(suppl 2):B27-B35.
46. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
47. Epstein NE, Schwall GS, Hood DC. The incidence and cost of devices explanted during single-level anterior diskectomy/fusions. Surg Neurol Int. 2011;2:23.