User login
Neurotransmitter-based diagnosis and treatment: A hypothesis (Part 3)
Optimal diagnosis and treatment of psychiatric illness requires clinicians to be able to connect mental and physical symptoms. Direct brain neurotransmitter testing is presently in its infancy and the science of defining the underlying mechanisms of psychiatric disorders lags behind the obvious clinical needs. We are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. In this article series, we suggest an indirect way of judging neurotransmitter activity by recognizing specific mental and physical symptoms connected by common biology. Here we present hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. The descriptions we present in this series do not reflect the entire set of symptoms caused by the neurotransmitters we discuss; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypothesis we present. We argue that in cases of multiple psychiatric disorders and chronic pain, the development and approval of medications currently is based on an umbrella descriptive diagnoses, and disregards the various underlying causes of such conditions. Similar to how the many types of pneumonias are treated differently depending on the infective agent, we suggested the same possible causative approach to various types of depression and pain.
Part 1 of this series (
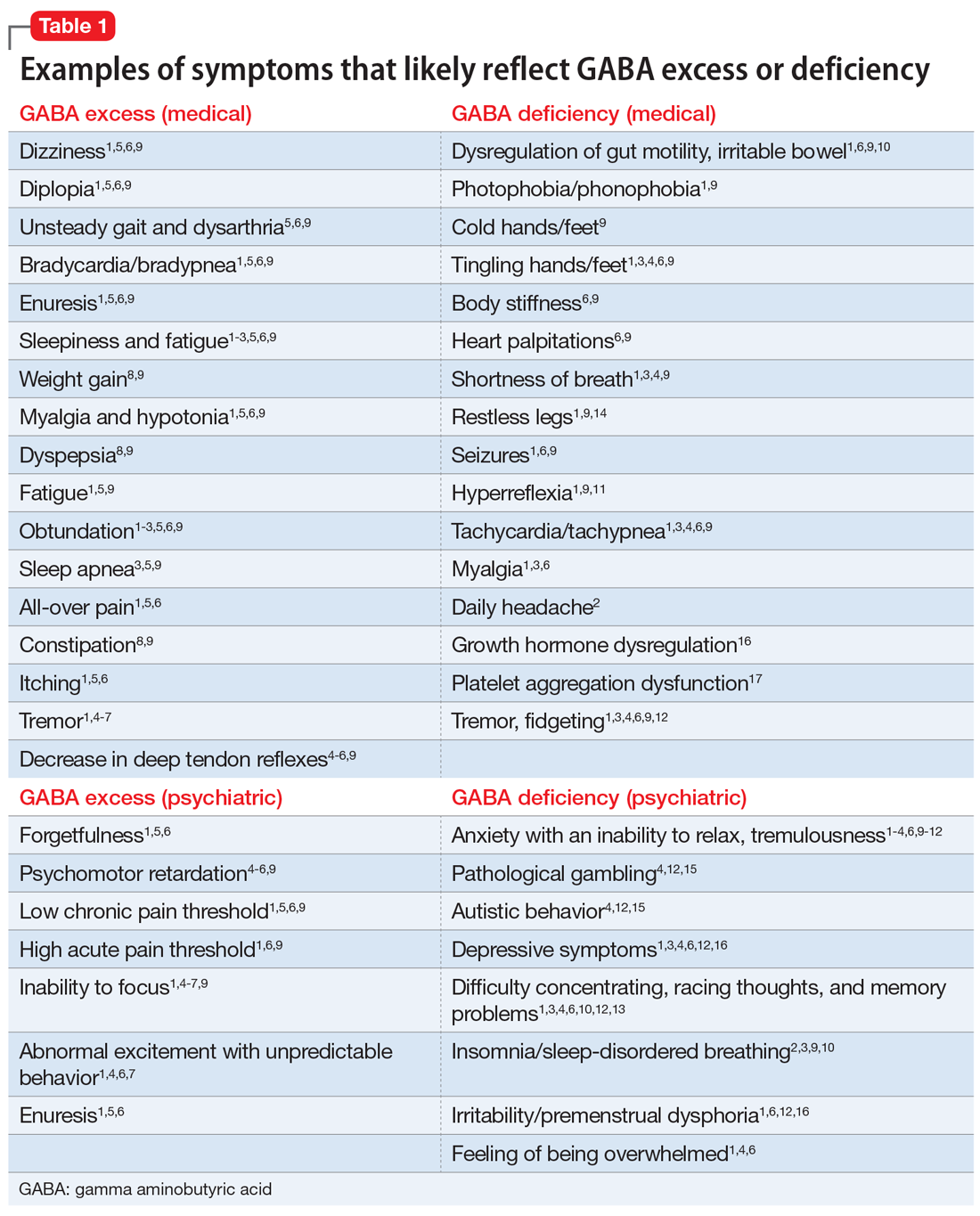
GABA excess (Table 11-9)
Ms. V is brought to your office by a friend. She complains of pain all over her body, itchiness, inability to focus, and dizziness.1,5,6,9 She is puzzled by how little pain she feels when she cuts her finger but by how much pain she is in every day, though her clinicians have not discovered a reason for her pain.1,6,9 She states that her fatigue is so severe that she can sleep 15 hours a day.1-6,9 Her obstructive and central sleep apnea have been treated, but this did not improve her fatigue.3,5,9 She is forgetful and has been diagnosed with depression, though she says she does not feel depressed.1,5,6 Nothing is pleasant to her, but she is prone to abnormal excitement and unpredictable behavior.1,4,6,7
A physical exam shows slow breathing, bradycardia, decreased deep tendon reflexes, and decreased muscle tone.1,5,6,9 Ms. V complains of double vision1,5,6,9 and problems with gait and balance,5,6,9 as well as tremors.1,4-7 She experienced enuresis well into adulthood1,5,6,9 and is prone to weight gain, dyspepsia, and constipation.8,9 She cannot understand people who have anxiety, and is prone to melancholy.4-6,9 Ms. V had been treated with electroconvulsive therapy in the past but states that she “had to have so much electricity, they gave up on me.”
Impression. Ms. V exhibits multiple symptoms associated with GABA excess. Dopaminergic medications such as methylphenidate or amphetamines may be helpful, as they suppress GABA. GABAergic medications and supplements should be avoided in such a patient. Noradrenergic medications including antidepressants with corresponding activity or vasopressors may be beneficial. Suppression of glutamate increases GABA, which is why ketamine in any formulation should be avoided in a patient such as Ms. V.
GABA deficiency (Table 11-4,6,9-17)
Mr. N complains of depression,1,3,4,6,12,16 pain all over his body, tingling in his hands and feet,1,6,9 a constant dull headache,2 and severe insomnia.2,3,9,10 He cannot control his anxiety and, in general, has problems relaxing. In the office, he is jumpy, tremulous, and fidgety during the interview and examination.1,3,4,6,9,12 His muscle tone is high1,9,11 and he feels stiff.6,9 Mr. N’s pupils are narrow1,9; he is hyper-reflexive1,9,11 and reports “Klonopin withdrawal seizures.”1,6,9 He loves alcohol because “it makes me feel good” and helps with his mind, which otherwise “never stops.”1,6,13 Mr. N is frequently anxious and very sensitive to pain, especially when he is upset. He was diagnosed with fibromyalgia by his primary care doctor, who says that irritable bowel is common in patients like him.1,6 His anxiety disables him.1-4,6,9-12 His sister reports that in addition to having difficulty relaxing, Mr. N is easily frustrated and sleeps poorly because he says he has racing thoughts.10 She mentions that her brother’s gambling addiction endangered his finances on several occasions4,12,15 and he was suspected of having autism spectrum disorder.4,12 Mr. N is frequently overwhelmed, including during your interview.1,3,4,6 He is sensitive to light and noise1,9 and complains of palpitations1,3,4,6,9 and frequent shortness of breath.1,3,4,9 He mentions his hands and feet often are cold, especially when he is anxious.1,3,4,6,9 Not eating at regular times makes his symptoms even worse. Mr. N commonly feels depressed, but his anxiety is more bothersome.1,3,4,6,12,16 His ongoing complaints include difficulty concentrating and memory problems,3,4,12,13 as well as a constant feeling of being overwhelmed.1,3,4,6 His restless leg syndrome requires ongoing treatment.1,9,14 Though uncommon, Mr. N has episodes of slowing and weakness, which are associated with growth hormone problems.16 In the past, he experienced gut motility dysregulation9,10 and prolonged bleeding that worried his doctors.17
Impression. Mr. N shows multiple symptoms associated with GABA deficiency. The deficiency of GABA activity ultimately causes an increase in norepinephrine and dopamine firing; therefore, symptoms of GABA deficiency are partially aligned with symptoms of dopamine and norepinephrine excess. GABAergic medications would be most beneficial for such patients. Anticonvulsants (eg, gabapentin and pregabalin) are preferable. Acamprostate may be considered. For long-term use, benzodiapines are as problematic as opioids and should be avoided, if possible. The use of opioids in such patients is especially counterproductive. Some supplements and vitamins may enhance GABA activity. Avoiding bupropion and stimulants would be wise. Ketamine in any formulation would be a good choice in this scenario. Sedating antipsychotic medications have a promise for patients such as Mr. N. The muscle relaxant baclofen frequently helps with these patients’ pain, anxiety, and sleep.
Continue to: Glutamate excess
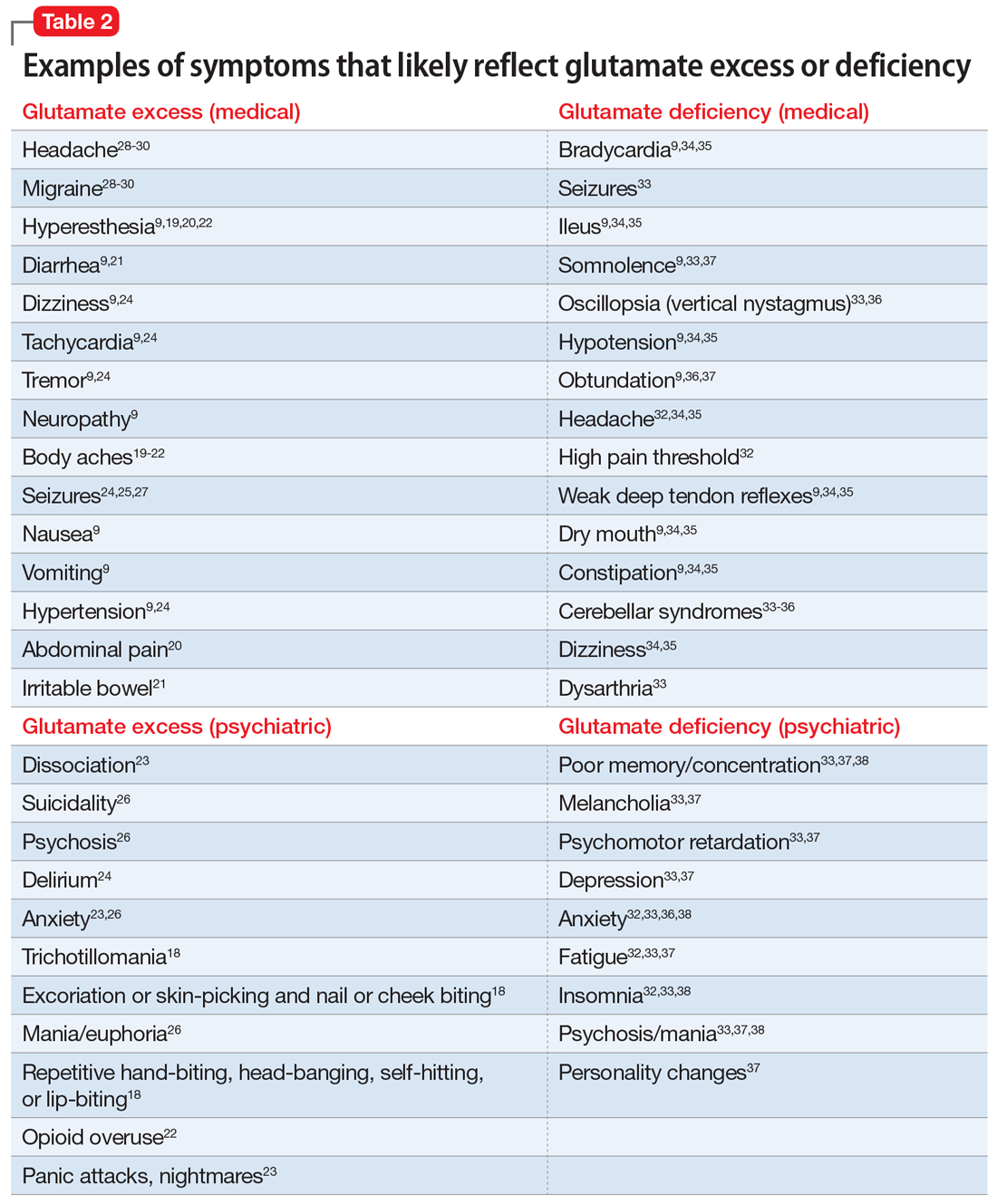
Glutamate excess (Table 29,18-30)
Mr. B is anxious and bites his fingernails and cheek while you interview him.18 He has scars on his lower arms that were caused by years of picking his skin.18 He complains of headache28-30 and deep muscle, whole body,19-23 and abdominal pain.20 Both hyperesthesia (he calls it “fibromyalgia”)9,19,20,22 and irritable bowel syndrome flare up if he eats Chinese food that contains monosodium glutamate.21 This also increases nausea, vomiting, and hypertensive episodes.9,19,20,22,24,26 Mr. B developed and received treatment for opioid use disorder after being prescribed morphine for the treatment of fibromyalgia.22 He is being treated for posttraumatic stress disorder at the VA hospital and is bitter that his flashbacks are not controlled.23 Once, he experienced a frank psychosis.26 He commonly experiences dissociative symptoms and suicidality.23,26 The sensations of crawling skin,18 panic attacks, and nightmares complicate his life.23 Mr. B is angry that his “incompetent” psychiatrist stopped his diazepam and that it “almost killed him” by causing delirium.24 He suffers from severe neuropathic pain in his feet and says that his pain, depression, and anxiety respond especially well to ketamine treatment.9,23,26 He is prone to euphoria and has had several manic episodes.26 In childhood, his parents brought him to a psychiatrist to address episodes of head-banging and self-hitting.18 Mr. B developed seizures; presently, they are controlled, but he remains chronically dizzy.9,24,25,27 He claims that his headaches and migraines respond only to methadone and that sumatriptan makes them worse, especially in prolonged treatment.28-30 He is tachycardic, tremulous, and makes you feel deeply uneasy.9,24
Impression. Mr. B has many symptoms of glutamate hyperactivity. The use of N-methyl-
Glutamate deficiency (Table 29,32-38)
Mr. Z feels dull, fatigued, and unhappy.32,33,37 He is overweight and moves slowly. Sometimes he is so slow and clumsy that he seems obtunded.9,36,37 He states that his peripheral neuropathy does not cause him pain, though his neurodiagnostic results are unfavorable.32 Mr. Z’s overall pain threshold is high, and he is unhappy with people who complain about pain because “who cares?”32 His memory and concentration were never good.33,37,38 He suffers from insomnia and is frequently miserable and disheartened.32,33,38 People view him as melancholic.33,37 Mr. Z is mildly depressed, but he experiences aggressive outbursts37,38 and bouts of anxiety,32,33,36,38 psychosis, and mania.33,37,38 He is visibly confused37 and says it is easy for him to get disoriented and lost.37,38 His medical history includes long-term constipation and several episodes of ileus.9,34,35 His childhood-onset seizures are controlled presently.33 He complains of frequent bouts of dizziness and headache.32,34,35 On physical exam, Mr. Z has dry mouth, hypotension, diminished deep tendon reflexes, and bradycardia.9,34,35 He sought a consultation from an ophthalmologist to evaluate an eye movement problem.33,36 No cause was found, but the ophthalmologist thought this problem might have the same underlying mechanism as his dysarthria.33 Mr. Z’s balance is bothersome, but his podiatrist was unable to help him to correct his abnormal gait.33-36 A friend who came with Mr. Z mentioned she had noticed personality changes in him over the last several months.37
Impression. Mr. Z exhibits multiple signs of low glutamatergic function. Amino acid taurine has been shown in rodents to increase brain levels of both GABA and glutamate. Glutamate is metabolized into GABA, so low glutamate and low GABA symptoms overlap. Glutamine, which is present in meat, fish, eggs, dairy, wheat, and some vegetables, is converted in the body into glutamate and may be considered for a patient with low glutamate function. The medication approach to such a patient would be similar to the treatment of a low GABA patient and includes glutamate-enhancing magnesium and dextromethorphan.
Rarely is just 1 neurotransmitter involved
Most real-world patients have mixed presentations with more than 1 neurotransmitter implicated in the pathology of their symptoms. A clinician’s ability to dissect the clinical picture and select an appropriate treatment must be based on history and observed behavior because no lab results or reliable tests are presently available.
Continue to: The most studied...
The most studied neurotransmitter in depression and anxiety is serotonin, and for many years psychiatrists have paid too much attention to it. Similarly, pain physicians have been overly focused on the opioid system. Excessive attention to these neurochemicals has overshadowed multiple other (no less impactful) neurotransmitters. Dopamine is frequently not attended to by many physicians who treat chronic pain. Psychiatrists also may overlook underlying endorphin or glutamate dysfunction in patients with psychiatric illness.
Nonpharmacologic approaches can affect neurotransmitters
With all the emphasis on pharmacologic treatments, it is important to remember that nonpharmacologic modalities such as exercise, diet, hydrotherapy, acupuncture, and psychotherapy can help normalize neurotransmitter function in the brain and ultimately help patients with chronic conditions. Careful use of nutritional supplements and vitamins may also be beneficial.
A hypothesis for future research
Multiple peripheral and central mechanisms define various chronic pain and psychiatric symptoms and disorders, including depression, anxiety, and fibromyalgia. The variety of mechanisms of pathologic mood and pain perception may be expressed to a different extent and in countless combinations in individual patients. This, in part, explains the variable responses to the same treatment observed in similar patients, or even in the same patient.
Clinicians should always remember that depression and anxiety as well as chronic pain (including fibromyalgia and chronic headache) are not a representation of a single condition but are the result of an assembly of different syndromes; therefore, 1 treatment does not fit all patients. Pain is ultimately recognized and comprehended centrally, making it very much a neuropsychiatric field. The optimal treatment for 2 patients with similar pain or psychiatric symptoms may be drastically different due to different underlying mechanisms that can be distinguished by looking at the symptoms other than “pain” or “depression.”
Remembering that every neurotransmitter deficiency or excess has an identifiable clinical correlation is important. Basing a treatment approach on a specific clinical presentation in a particular depressed or chronic pain patient would assure a more successful and reliable outcome.
Continue to: This 3-part series...
This 3-part series was designed to bring attention to a notion that diagnosis and treatment of diverse conditions such as “depression,” “anxiety,” or “chronic pain” should be based on clinically identifiable symptoms that may suggest specific neurotransmitter(s) involved in a specific type of each of these conditions. However, there are no well-recognized, well-established, reliable, or validated syndromes described in this series. The collection of symptoms associated with the various neurotransmitters described in this series is not complete. We have assembled what is described in the literature as a suggestion for future research.
Bottom Line
Both high and low levels of gamma aminobutyric acid (GABA) and glutamate may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 2). Current Psychiatry. 2022;21(6):28-33. doi:10.12788/cp.0253
Drug Brand Names
Acamprostate • Campral
Amantadine • Gocovri
Bupropion • Wellbutrin
Clonazepam • Klonopin
Clonidine • Catapres
Diazepam • Valium
Gabapentin • Neurontin
Ketamine • Ketalar
Memantine • Namenda
Methylphenidate • Concerta
Morphine • Kadian
Pregabalin • Lyrica
Sumatriptan • Imitrex
Tizanidine • Zanaflex
1. Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562-573.
2. Winkelman JW, Buxton OM, Jensen JE, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008;31(11):1499-1506.
3. Pereira AC, Mao X, Jiang CS, et al. Dorsolateral prefrontal cortex GABA deficit in older adults with sleep-disordered breathing. Proc Natl Acad Sci U S A. 2017;114(38):10250-10255.
4. Schür RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37(9):3337-3352.
5. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
6. Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother. 2016;50(3):229-233.
7. Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445-461.
8. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability, and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
9. Guyton AC, Hall JE. Guyton and Hall Textbook of Medical Physiology. 12th ed. Elsevier; 2011:550-551,692-693.
10. Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13(3):239-244.
11. Vianello M, Tavolato B, Giometto B. Glutamic acid decarboxylase autoantibodies and neurological disorders. Neurol Sci. 2002;23(4):145-151.
12. Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107-120.
13. Huang D, Liu D, Yin J, et al. Glutamate-glutamine and GABA in the brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27(7):2698-2705.
14. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, et al. Neurochemical features of idiopathic restless legs syndrome. Sleep Med Rev. 2019;45:70-87.
15. Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol. 2017;22(6):1601-1609.
16. Brambilla P, Perez J, Barale F, et al. Gabaergic dysfunction in mood disorders. Molecular Psychiatry. 2003;8:721-737.
17. Kaneez FS, Saeed SA. Investigating GABA and its function in platelets as compared to neurons. Platelets. 2009;20(5):328-333.
18. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702.
19. Miranda A, Peles S, Rudolph C, et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082-1089.
20. Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98(1-2):69-78.
21. Holton KF, Taren DL, Thomson CA, et al. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin Exp Rheumatol. 2012;30(6 Suppl 74):10-70.
22. Sekiya Y, Nakagawa T, Ozawa T, et al. Facilitation of morphine withdrawal symptoms and morphine-induced conditioned place preference by a glutamate transporter inhibitor DL-threo-beta-benzyloxy aspartate in rats. Eur J Pharmacol. 2004;485(1-3):201-210.
23. Bestha D, Soliman L, Blankenship K. et al. The walking wounded: emerging treatments for PTSD. Curr Psychiatry Rep. 2018;20(10):94.
24. Tsuda M, Shimizu N, Suzuki T. Contribution of glutamate receptors to benzodiazepine withdrawal signs. Jpn J Pharmacol. 1999;81(1):1-6.
25. Spravato [package insert]. Janssen Pharmaceuticals, Inc; 2019.
26. Mattingly GW, Anderson RH. Intranasal ketamine. Current Psychiatry. 2019;18(5):31-38.
27. Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269-1275.
28. Ferrari A, Spaccapelo L, Pinetti D, et al. Effective prophylactic treatment of migraines lower plasma glutamate levels. Cephalalgia. 2009;29(4):423-429.
29. Vieira DS, Naffah-Mazzacoratti Mda G, Zukerman E, et al. Glutamate levels in cerebrospinal fluid and triptans overuse in chronic migraine. Headache. 2007;47(6):842-847.
30. Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs. 2014;74:1165-1176.
31. Pappa S, Tsouli S, Apostolou G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
32. Kraal AZ, Arvanitis NR, Jaeger AP, et al. Could dietary glutamate play a role in psychiatric distress? Neuro Psych. 2020;79:13-19.
33. Levite M. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, Anti-NMDA-NR1 antibodies, Anti-NMDA-NR2A/B antibodies, Anti-mGluR1 antibodies or Anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren’s syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor’s expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm (Vienna). 2014;121(8):1029-1075.
34. Lancaster E. CNS syndromes associated with antibodies against metabotropic receptors. Curr Opin Neurol. 2017;30:354-360.
35. Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21-27.
36. Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol. 2010;67(5):627-630.
37. Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698-1701.
38. Mat A, Adler H, Merwick A, et al. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology. 2013;80(14):1349-1350.
Optimal diagnosis and treatment of psychiatric illness requires clinicians to be able to connect mental and physical symptoms. Direct brain neurotransmitter testing is presently in its infancy and the science of defining the underlying mechanisms of psychiatric disorders lags behind the obvious clinical needs. We are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. In this article series, we suggest an indirect way of judging neurotransmitter activity by recognizing specific mental and physical symptoms connected by common biology. Here we present hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. The descriptions we present in this series do not reflect the entire set of symptoms caused by the neurotransmitters we discuss; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypothesis we present. We argue that in cases of multiple psychiatric disorders and chronic pain, the development and approval of medications currently is based on an umbrella descriptive diagnoses, and disregards the various underlying causes of such conditions. Similar to how the many types of pneumonias are treated differently depending on the infective agent, we suggested the same possible causative approach to various types of depression and pain.

Part 1 of this series (

GABA excess (Table 11-9)
Ms. V is brought to your office by a friend. She complains of pain all over her body, itchiness, inability to focus, and dizziness.1,5,6,9 She is puzzled by how little pain she feels when she cuts her finger but by how much pain she is in every day, though her clinicians have not discovered a reason for her pain.1,6,9 She states that her fatigue is so severe that she can sleep 15 hours a day.1-6,9 Her obstructive and central sleep apnea have been treated, but this did not improve her fatigue.3,5,9 She is forgetful and has been diagnosed with depression, though she says she does not feel depressed.1,5,6 Nothing is pleasant to her, but she is prone to abnormal excitement and unpredictable behavior.1,4,6,7
A physical exam shows slow breathing, bradycardia, decreased deep tendon reflexes, and decreased muscle tone.1,5,6,9 Ms. V complains of double vision1,5,6,9 and problems with gait and balance,5,6,9 as well as tremors.1,4-7 She experienced enuresis well into adulthood1,5,6,9 and is prone to weight gain, dyspepsia, and constipation.8,9 She cannot understand people who have anxiety, and is prone to melancholy.4-6,9 Ms. V had been treated with electroconvulsive therapy in the past but states that she “had to have so much electricity, they gave up on me.”
Impression. Ms. V exhibits multiple symptoms associated with GABA excess. Dopaminergic medications such as methylphenidate or amphetamines may be helpful, as they suppress GABA. GABAergic medications and supplements should be avoided in such a patient. Noradrenergic medications including antidepressants with corresponding activity or vasopressors may be beneficial. Suppression of glutamate increases GABA, which is why ketamine in any formulation should be avoided in a patient such as Ms. V.
GABA deficiency (Table 11-4,6,9-17)
Mr. N complains of depression,1,3,4,6,12,16 pain all over his body, tingling in his hands and feet,1,6,9 a constant dull headache,2 and severe insomnia.2,3,9,10 He cannot control his anxiety and, in general, has problems relaxing. In the office, he is jumpy, tremulous, and fidgety during the interview and examination.1,3,4,6,9,12 His muscle tone is high1,9,11 and he feels stiff.6,9 Mr. N’s pupils are narrow1,9; he is hyper-reflexive1,9,11 and reports “Klonopin withdrawal seizures.”1,6,9 He loves alcohol because “it makes me feel good” and helps with his mind, which otherwise “never stops.”1,6,13 Mr. N is frequently anxious and very sensitive to pain, especially when he is upset. He was diagnosed with fibromyalgia by his primary care doctor, who says that irritable bowel is common in patients like him.1,6 His anxiety disables him.1-4,6,9-12 His sister reports that in addition to having difficulty relaxing, Mr. N is easily frustrated and sleeps poorly because he says he has racing thoughts.10 She mentions that her brother’s gambling addiction endangered his finances on several occasions4,12,15 and he was suspected of having autism spectrum disorder.4,12 Mr. N is frequently overwhelmed, including during your interview.1,3,4,6 He is sensitive to light and noise1,9 and complains of palpitations1,3,4,6,9 and frequent shortness of breath.1,3,4,9 He mentions his hands and feet often are cold, especially when he is anxious.1,3,4,6,9 Not eating at regular times makes his symptoms even worse. Mr. N commonly feels depressed, but his anxiety is more bothersome.1,3,4,6,12,16 His ongoing complaints include difficulty concentrating and memory problems,3,4,12,13 as well as a constant feeling of being overwhelmed.1,3,4,6 His restless leg syndrome requires ongoing treatment.1,9,14 Though uncommon, Mr. N has episodes of slowing and weakness, which are associated with growth hormone problems.16 In the past, he experienced gut motility dysregulation9,10 and prolonged bleeding that worried his doctors.17
Impression. Mr. N shows multiple symptoms associated with GABA deficiency. The deficiency of GABA activity ultimately causes an increase in norepinephrine and dopamine firing; therefore, symptoms of GABA deficiency are partially aligned with symptoms of dopamine and norepinephrine excess. GABAergic medications would be most beneficial for such patients. Anticonvulsants (eg, gabapentin and pregabalin) are preferable. Acamprostate may be considered. For long-term use, benzodiapines are as problematic as opioids and should be avoided, if possible. The use of opioids in such patients is especially counterproductive. Some supplements and vitamins may enhance GABA activity. Avoiding bupropion and stimulants would be wise. Ketamine in any formulation would be a good choice in this scenario. Sedating antipsychotic medications have a promise for patients such as Mr. N. The muscle relaxant baclofen frequently helps with these patients’ pain, anxiety, and sleep.
Continue to: Glutamate excess
Glutamate excess (Table 29,18-30)
Mr. B is anxious and bites his fingernails and cheek while you interview him.18 He has scars on his lower arms that were caused by years of picking his skin.18 He complains of headache28-30 and deep muscle, whole body,19-23 and abdominal pain.20 Both hyperesthesia (he calls it “fibromyalgia”)9,19,20,22 and irritable bowel syndrome flare up if he eats Chinese food that contains monosodium glutamate.21 This also increases nausea, vomiting, and hypertensive episodes.9,19,20,22,24,26 Mr. B developed and received treatment for opioid use disorder after being prescribed morphine for the treatment of fibromyalgia.22 He is being treated for posttraumatic stress disorder at the VA hospital and is bitter that his flashbacks are not controlled.23 Once, he experienced a frank psychosis.26 He commonly experiences dissociative symptoms and suicidality.23,26 The sensations of crawling skin,18 panic attacks, and nightmares complicate his life.23 Mr. B is angry that his “incompetent” psychiatrist stopped his diazepam and that it “almost killed him” by causing delirium.24 He suffers from severe neuropathic pain in his feet and says that his pain, depression, and anxiety respond especially well to ketamine treatment.9,23,26 He is prone to euphoria and has had several manic episodes.26 In childhood, his parents brought him to a psychiatrist to address episodes of head-banging and self-hitting.18 Mr. B developed seizures; presently, they are controlled, but he remains chronically dizzy.9,24,25,27 He claims that his headaches and migraines respond only to methadone and that sumatriptan makes them worse, especially in prolonged treatment.28-30 He is tachycardic, tremulous, and makes you feel deeply uneasy.9,24
Impression. Mr. B has many symptoms of glutamate hyperactivity. The use of N-methyl-
Glutamate deficiency (Table 29,32-38)
Mr. Z feels dull, fatigued, and unhappy.32,33,37 He is overweight and moves slowly. Sometimes he is so slow and clumsy that he seems obtunded.9,36,37 He states that his peripheral neuropathy does not cause him pain, though his neurodiagnostic results are unfavorable.32 Mr. Z’s overall pain threshold is high, and he is unhappy with people who complain about pain because “who cares?”32 His memory and concentration were never good.33,37,38 He suffers from insomnia and is frequently miserable and disheartened.32,33,38 People view him as melancholic.33,37 Mr. Z is mildly depressed, but he experiences aggressive outbursts37,38 and bouts of anxiety,32,33,36,38 psychosis, and mania.33,37,38 He is visibly confused37 and says it is easy for him to get disoriented and lost.37,38 His medical history includes long-term constipation and several episodes of ileus.9,34,35 His childhood-onset seizures are controlled presently.33 He complains of frequent bouts of dizziness and headache.32,34,35 On physical exam, Mr. Z has dry mouth, hypotension, diminished deep tendon reflexes, and bradycardia.9,34,35 He sought a consultation from an ophthalmologist to evaluate an eye movement problem.33,36 No cause was found, but the ophthalmologist thought this problem might have the same underlying mechanism as his dysarthria.33 Mr. Z’s balance is bothersome, but his podiatrist was unable to help him to correct his abnormal gait.33-36 A friend who came with Mr. Z mentioned she had noticed personality changes in him over the last several months.37
Impression. Mr. Z exhibits multiple signs of low glutamatergic function. Amino acid taurine has been shown in rodents to increase brain levels of both GABA and glutamate. Glutamate is metabolized into GABA, so low glutamate and low GABA symptoms overlap. Glutamine, which is present in meat, fish, eggs, dairy, wheat, and some vegetables, is converted in the body into glutamate and may be considered for a patient with low glutamate function. The medication approach to such a patient would be similar to the treatment of a low GABA patient and includes glutamate-enhancing magnesium and dextromethorphan.
Rarely is just 1 neurotransmitter involved
Most real-world patients have mixed presentations with more than 1 neurotransmitter implicated in the pathology of their symptoms. A clinician’s ability to dissect the clinical picture and select an appropriate treatment must be based on history and observed behavior because no lab results or reliable tests are presently available.
Continue to: The most studied...
The most studied neurotransmitter in depression and anxiety is serotonin, and for many years psychiatrists have paid too much attention to it. Similarly, pain physicians have been overly focused on the opioid system. Excessive attention to these neurochemicals has overshadowed multiple other (no less impactful) neurotransmitters. Dopamine is frequently not attended to by many physicians who treat chronic pain. Psychiatrists also may overlook underlying endorphin or glutamate dysfunction in patients with psychiatric illness.
Nonpharmacologic approaches can affect neurotransmitters
With all the emphasis on pharmacologic treatments, it is important to remember that nonpharmacologic modalities such as exercise, diet, hydrotherapy, acupuncture, and psychotherapy can help normalize neurotransmitter function in the brain and ultimately help patients with chronic conditions. Careful use of nutritional supplements and vitamins may also be beneficial.
A hypothesis for future research
Multiple peripheral and central mechanisms define various chronic pain and psychiatric symptoms and disorders, including depression, anxiety, and fibromyalgia. The variety of mechanisms of pathologic mood and pain perception may be expressed to a different extent and in countless combinations in individual patients. This, in part, explains the variable responses to the same treatment observed in similar patients, or even in the same patient.
Clinicians should always remember that depression and anxiety as well as chronic pain (including fibromyalgia and chronic headache) are not a representation of a single condition but are the result of an assembly of different syndromes; therefore, 1 treatment does not fit all patients. Pain is ultimately recognized and comprehended centrally, making it very much a neuropsychiatric field. The optimal treatment for 2 patients with similar pain or psychiatric symptoms may be drastically different due to different underlying mechanisms that can be distinguished by looking at the symptoms other than “pain” or “depression.”
Remembering that every neurotransmitter deficiency or excess has an identifiable clinical correlation is important. Basing a treatment approach on a specific clinical presentation in a particular depressed or chronic pain patient would assure a more successful and reliable outcome.
Continue to: This 3-part series...
This 3-part series was designed to bring attention to a notion that diagnosis and treatment of diverse conditions such as “depression,” “anxiety,” or “chronic pain” should be based on clinically identifiable symptoms that may suggest specific neurotransmitter(s) involved in a specific type of each of these conditions. However, there are no well-recognized, well-established, reliable, or validated syndromes described in this series. The collection of symptoms associated with the various neurotransmitters described in this series is not complete. We have assembled what is described in the literature as a suggestion for future research.
Bottom Line
Both high and low levels of gamma aminobutyric acid (GABA) and glutamate may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 2). Current Psychiatry. 2022;21(6):28-33. doi:10.12788/cp.0253
Drug Brand Names
Acamprostate • Campral
Amantadine • Gocovri
Bupropion • Wellbutrin
Clonazepam • Klonopin
Clonidine • Catapres
Diazepam • Valium
Gabapentin • Neurontin
Ketamine • Ketalar
Memantine • Namenda
Methylphenidate • Concerta
Morphine • Kadian
Pregabalin • Lyrica
Sumatriptan • Imitrex
Tizanidine • Zanaflex
Optimal diagnosis and treatment of psychiatric illness requires clinicians to be able to connect mental and physical symptoms. Direct brain neurotransmitter testing is presently in its infancy and the science of defining the underlying mechanisms of psychiatric disorders lags behind the obvious clinical needs. We are not yet equipped to clearly recognize which neurotransmitters cause which symptoms. In this article series, we suggest an indirect way of judging neurotransmitter activity by recognizing specific mental and physical symptoms connected by common biology. Here we present hypothetical clinical cases to emphasize a possible way of analyzing symptoms in order to identify underlying pathology and guide more effective treatment. The descriptions we present in this series do not reflect the entire set of symptoms caused by the neurotransmitters we discuss; we created them based on what is presently known (or suspected). Additional research is needed to confirm or disprove the hypothesis we present. We argue that in cases of multiple psychiatric disorders and chronic pain, the development and approval of medications currently is based on an umbrella descriptive diagnoses, and disregards the various underlying causes of such conditions. Similar to how the many types of pneumonias are treated differently depending on the infective agent, we suggested the same possible causative approach to various types of depression and pain.

Part 1 of this series (

GABA excess (Table 11-9)
Ms. V is brought to your office by a friend. She complains of pain all over her body, itchiness, inability to focus, and dizziness.1,5,6,9 She is puzzled by how little pain she feels when she cuts her finger but by how much pain she is in every day, though her clinicians have not discovered a reason for her pain.1,6,9 She states that her fatigue is so severe that she can sleep 15 hours a day.1-6,9 Her obstructive and central sleep apnea have been treated, but this did not improve her fatigue.3,5,9 She is forgetful and has been diagnosed with depression, though she says she does not feel depressed.1,5,6 Nothing is pleasant to her, but she is prone to abnormal excitement and unpredictable behavior.1,4,6,7
A physical exam shows slow breathing, bradycardia, decreased deep tendon reflexes, and decreased muscle tone.1,5,6,9 Ms. V complains of double vision1,5,6,9 and problems with gait and balance,5,6,9 as well as tremors.1,4-7 She experienced enuresis well into adulthood1,5,6,9 and is prone to weight gain, dyspepsia, and constipation.8,9 She cannot understand people who have anxiety, and is prone to melancholy.4-6,9 Ms. V had been treated with electroconvulsive therapy in the past but states that she “had to have so much electricity, they gave up on me.”
Impression. Ms. V exhibits multiple symptoms associated with GABA excess. Dopaminergic medications such as methylphenidate or amphetamines may be helpful, as they suppress GABA. GABAergic medications and supplements should be avoided in such a patient. Noradrenergic medications including antidepressants with corresponding activity or vasopressors may be beneficial. Suppression of glutamate increases GABA, which is why ketamine in any formulation should be avoided in a patient such as Ms. V.
GABA deficiency (Table 11-4,6,9-17)
Mr. N complains of depression,1,3,4,6,12,16 pain all over his body, tingling in his hands and feet,1,6,9 a constant dull headache,2 and severe insomnia.2,3,9,10 He cannot control his anxiety and, in general, has problems relaxing. In the office, he is jumpy, tremulous, and fidgety during the interview and examination.1,3,4,6,9,12 His muscle tone is high1,9,11 and he feels stiff.6,9 Mr. N’s pupils are narrow1,9; he is hyper-reflexive1,9,11 and reports “Klonopin withdrawal seizures.”1,6,9 He loves alcohol because “it makes me feel good” and helps with his mind, which otherwise “never stops.”1,6,13 Mr. N is frequently anxious and very sensitive to pain, especially when he is upset. He was diagnosed with fibromyalgia by his primary care doctor, who says that irritable bowel is common in patients like him.1,6 His anxiety disables him.1-4,6,9-12 His sister reports that in addition to having difficulty relaxing, Mr. N is easily frustrated and sleeps poorly because he says he has racing thoughts.10 She mentions that her brother’s gambling addiction endangered his finances on several occasions4,12,15 and he was suspected of having autism spectrum disorder.4,12 Mr. N is frequently overwhelmed, including during your interview.1,3,4,6 He is sensitive to light and noise1,9 and complains of palpitations1,3,4,6,9 and frequent shortness of breath.1,3,4,9 He mentions his hands and feet often are cold, especially when he is anxious.1,3,4,6,9 Not eating at regular times makes his symptoms even worse. Mr. N commonly feels depressed, but his anxiety is more bothersome.1,3,4,6,12,16 His ongoing complaints include difficulty concentrating and memory problems,3,4,12,13 as well as a constant feeling of being overwhelmed.1,3,4,6 His restless leg syndrome requires ongoing treatment.1,9,14 Though uncommon, Mr. N has episodes of slowing and weakness, which are associated with growth hormone problems.16 In the past, he experienced gut motility dysregulation9,10 and prolonged bleeding that worried his doctors.17
Impression. Mr. N shows multiple symptoms associated with GABA deficiency. The deficiency of GABA activity ultimately causes an increase in norepinephrine and dopamine firing; therefore, symptoms of GABA deficiency are partially aligned with symptoms of dopamine and norepinephrine excess. GABAergic medications would be most beneficial for such patients. Anticonvulsants (eg, gabapentin and pregabalin) are preferable. Acamprostate may be considered. For long-term use, benzodiapines are as problematic as opioids and should be avoided, if possible. The use of opioids in such patients is especially counterproductive. Some supplements and vitamins may enhance GABA activity. Avoiding bupropion and stimulants would be wise. Ketamine in any formulation would be a good choice in this scenario. Sedating antipsychotic medications have a promise for patients such as Mr. N. The muscle relaxant baclofen frequently helps with these patients’ pain, anxiety, and sleep.
Continue to: Glutamate excess
Glutamate excess (Table 29,18-30)
Mr. B is anxious and bites his fingernails and cheek while you interview him.18 He has scars on his lower arms that were caused by years of picking his skin.18 He complains of headache28-30 and deep muscle, whole body,19-23 and abdominal pain.20 Both hyperesthesia (he calls it “fibromyalgia”)9,19,20,22 and irritable bowel syndrome flare up if he eats Chinese food that contains monosodium glutamate.21 This also increases nausea, vomiting, and hypertensive episodes.9,19,20,22,24,26 Mr. B developed and received treatment for opioid use disorder after being prescribed morphine for the treatment of fibromyalgia.22 He is being treated for posttraumatic stress disorder at the VA hospital and is bitter that his flashbacks are not controlled.23 Once, he experienced a frank psychosis.26 He commonly experiences dissociative symptoms and suicidality.23,26 The sensations of crawling skin,18 panic attacks, and nightmares complicate his life.23 Mr. B is angry that his “incompetent” psychiatrist stopped his diazepam and that it “almost killed him” by causing delirium.24 He suffers from severe neuropathic pain in his feet and says that his pain, depression, and anxiety respond especially well to ketamine treatment.9,23,26 He is prone to euphoria and has had several manic episodes.26 In childhood, his parents brought him to a psychiatrist to address episodes of head-banging and self-hitting.18 Mr. B developed seizures; presently, they are controlled, but he remains chronically dizzy.9,24,25,27 He claims that his headaches and migraines respond only to methadone and that sumatriptan makes them worse, especially in prolonged treatment.28-30 He is tachycardic, tremulous, and makes you feel deeply uneasy.9,24
Impression. Mr. B has many symptoms of glutamate hyperactivity. The use of N-methyl-
Glutamate deficiency (Table 29,32-38)
Mr. Z feels dull, fatigued, and unhappy.32,33,37 He is overweight and moves slowly. Sometimes he is so slow and clumsy that he seems obtunded.9,36,37 He states that his peripheral neuropathy does not cause him pain, though his neurodiagnostic results are unfavorable.32 Mr. Z’s overall pain threshold is high, and he is unhappy with people who complain about pain because “who cares?”32 His memory and concentration were never good.33,37,38 He suffers from insomnia and is frequently miserable and disheartened.32,33,38 People view him as melancholic.33,37 Mr. Z is mildly depressed, but he experiences aggressive outbursts37,38 and bouts of anxiety,32,33,36,38 psychosis, and mania.33,37,38 He is visibly confused37 and says it is easy for him to get disoriented and lost.37,38 His medical history includes long-term constipation and several episodes of ileus.9,34,35 His childhood-onset seizures are controlled presently.33 He complains of frequent bouts of dizziness and headache.32,34,35 On physical exam, Mr. Z has dry mouth, hypotension, diminished deep tendon reflexes, and bradycardia.9,34,35 He sought a consultation from an ophthalmologist to evaluate an eye movement problem.33,36 No cause was found, but the ophthalmologist thought this problem might have the same underlying mechanism as his dysarthria.33 Mr. Z’s balance is bothersome, but his podiatrist was unable to help him to correct his abnormal gait.33-36 A friend who came with Mr. Z mentioned she had noticed personality changes in him over the last several months.37
Impression. Mr. Z exhibits multiple signs of low glutamatergic function. Amino acid taurine has been shown in rodents to increase brain levels of both GABA and glutamate. Glutamate is metabolized into GABA, so low glutamate and low GABA symptoms overlap. Glutamine, which is present in meat, fish, eggs, dairy, wheat, and some vegetables, is converted in the body into glutamate and may be considered for a patient with low glutamate function. The medication approach to such a patient would be similar to the treatment of a low GABA patient and includes glutamate-enhancing magnesium and dextromethorphan.
Rarely is just 1 neurotransmitter involved
Most real-world patients have mixed presentations with more than 1 neurotransmitter implicated in the pathology of their symptoms. A clinician’s ability to dissect the clinical picture and select an appropriate treatment must be based on history and observed behavior because no lab results or reliable tests are presently available.
Continue to: The most studied...
The most studied neurotransmitter in depression and anxiety is serotonin, and for many years psychiatrists have paid too much attention to it. Similarly, pain physicians have been overly focused on the opioid system. Excessive attention to these neurochemicals has overshadowed multiple other (no less impactful) neurotransmitters. Dopamine is frequently not attended to by many physicians who treat chronic pain. Psychiatrists also may overlook underlying endorphin or glutamate dysfunction in patients with psychiatric illness.
Nonpharmacologic approaches can affect neurotransmitters
With all the emphasis on pharmacologic treatments, it is important to remember that nonpharmacologic modalities such as exercise, diet, hydrotherapy, acupuncture, and psychotherapy can help normalize neurotransmitter function in the brain and ultimately help patients with chronic conditions. Careful use of nutritional supplements and vitamins may also be beneficial.
A hypothesis for future research
Multiple peripheral and central mechanisms define various chronic pain and psychiatric symptoms and disorders, including depression, anxiety, and fibromyalgia. The variety of mechanisms of pathologic mood and pain perception may be expressed to a different extent and in countless combinations in individual patients. This, in part, explains the variable responses to the same treatment observed in similar patients, or even in the same patient.
Clinicians should always remember that depression and anxiety as well as chronic pain (including fibromyalgia and chronic headache) are not a representation of a single condition but are the result of an assembly of different syndromes; therefore, 1 treatment does not fit all patients. Pain is ultimately recognized and comprehended centrally, making it very much a neuropsychiatric field. The optimal treatment for 2 patients with similar pain or psychiatric symptoms may be drastically different due to different underlying mechanisms that can be distinguished by looking at the symptoms other than “pain” or “depression.”
Remembering that every neurotransmitter deficiency or excess has an identifiable clinical correlation is important. Basing a treatment approach on a specific clinical presentation in a particular depressed or chronic pain patient would assure a more successful and reliable outcome.
Continue to: This 3-part series...
This 3-part series was designed to bring attention to a notion that diagnosis and treatment of diverse conditions such as “depression,” “anxiety,” or “chronic pain” should be based on clinically identifiable symptoms that may suggest specific neurotransmitter(s) involved in a specific type of each of these conditions. However, there are no well-recognized, well-established, reliable, or validated syndromes described in this series. The collection of symptoms associated with the various neurotransmitters described in this series is not complete. We have assembled what is described in the literature as a suggestion for future research.
Bottom Line
Both high and low levels of gamma aminobutyric acid (GABA) and glutamate may be associated with certain psychiatric and medical symptoms and disorders. An astute clinician may judge which neurotransmitter is dysfunctional based on the patient’s presentation, and tailor treatment accordingly.
Related Resources
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 1). Current Psychiatry. 2022;21(5):30-36. doi:10.12788/cp.0242
- Arbuck DM, Salmerón JM, Mueller R. Neurotransmitter-based diagnosis and treatment: a hypothesis (part 2). Current Psychiatry. 2022;21(6):28-33. doi:10.12788/cp.0253
Drug Brand Names
Acamprostate • Campral
Amantadine • Gocovri
Bupropion • Wellbutrin
Clonazepam • Klonopin
Clonidine • Catapres
Diazepam • Valium
Gabapentin • Neurontin
Ketamine • Ketalar
Memantine • Namenda
Methylphenidate • Concerta
Morphine • Kadian
Pregabalin • Lyrica
Sumatriptan • Imitrex
Tizanidine • Zanaflex
1. Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562-573.
2. Winkelman JW, Buxton OM, Jensen JE, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008;31(11):1499-1506.
3. Pereira AC, Mao X, Jiang CS, et al. Dorsolateral prefrontal cortex GABA deficit in older adults with sleep-disordered breathing. Proc Natl Acad Sci U S A. 2017;114(38):10250-10255.
4. Schür RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37(9):3337-3352.
5. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
6. Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother. 2016;50(3):229-233.
7. Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445-461.
8. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability, and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
9. Guyton AC, Hall JE. Guyton and Hall Textbook of Medical Physiology. 12th ed. Elsevier; 2011:550-551,692-693.
10. Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13(3):239-244.
11. Vianello M, Tavolato B, Giometto B. Glutamic acid decarboxylase autoantibodies and neurological disorders. Neurol Sci. 2002;23(4):145-151.
12. Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107-120.
13. Huang D, Liu D, Yin J, et al. Glutamate-glutamine and GABA in the brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27(7):2698-2705.
14. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, et al. Neurochemical features of idiopathic restless legs syndrome. Sleep Med Rev. 2019;45:70-87.
15. Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol. 2017;22(6):1601-1609.
16. Brambilla P, Perez J, Barale F, et al. Gabaergic dysfunction in mood disorders. Molecular Psychiatry. 2003;8:721-737.
17. Kaneez FS, Saeed SA. Investigating GABA and its function in platelets as compared to neurons. Platelets. 2009;20(5):328-333.
18. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702.
19. Miranda A, Peles S, Rudolph C, et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082-1089.
20. Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98(1-2):69-78.
21. Holton KF, Taren DL, Thomson CA, et al. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin Exp Rheumatol. 2012;30(6 Suppl 74):10-70.
22. Sekiya Y, Nakagawa T, Ozawa T, et al. Facilitation of morphine withdrawal symptoms and morphine-induced conditioned place preference by a glutamate transporter inhibitor DL-threo-beta-benzyloxy aspartate in rats. Eur J Pharmacol. 2004;485(1-3):201-210.
23. Bestha D, Soliman L, Blankenship K. et al. The walking wounded: emerging treatments for PTSD. Curr Psychiatry Rep. 2018;20(10):94.
24. Tsuda M, Shimizu N, Suzuki T. Contribution of glutamate receptors to benzodiazepine withdrawal signs. Jpn J Pharmacol. 1999;81(1):1-6.
25. Spravato [package insert]. Janssen Pharmaceuticals, Inc; 2019.
26. Mattingly GW, Anderson RH. Intranasal ketamine. Current Psychiatry. 2019;18(5):31-38.
27. Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269-1275.
28. Ferrari A, Spaccapelo L, Pinetti D, et al. Effective prophylactic treatment of migraines lower plasma glutamate levels. Cephalalgia. 2009;29(4):423-429.
29. Vieira DS, Naffah-Mazzacoratti Mda G, Zukerman E, et al. Glutamate levels in cerebrospinal fluid and triptans overuse in chronic migraine. Headache. 2007;47(6):842-847.
30. Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs. 2014;74:1165-1176.
31. Pappa S, Tsouli S, Apostolou G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
32. Kraal AZ, Arvanitis NR, Jaeger AP, et al. Could dietary glutamate play a role in psychiatric distress? Neuro Psych. 2020;79:13-19.
33. Levite M. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, Anti-NMDA-NR1 antibodies, Anti-NMDA-NR2A/B antibodies, Anti-mGluR1 antibodies or Anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren’s syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor’s expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm (Vienna). 2014;121(8):1029-1075.
34. Lancaster E. CNS syndromes associated with antibodies against metabotropic receptors. Curr Opin Neurol. 2017;30:354-360.
35. Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21-27.
36. Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol. 2010;67(5):627-630.
37. Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698-1701.
38. Mat A, Adler H, Merwick A, et al. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology. 2013;80(14):1349-1350.
1. Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562-573.
2. Winkelman JW, Buxton OM, Jensen JE, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008;31(11):1499-1506.
3. Pereira AC, Mao X, Jiang CS, et al. Dorsolateral prefrontal cortex GABA deficit in older adults with sleep-disordered breathing. Proc Natl Acad Sci U S A. 2017;114(38):10250-10255.
4. Schür RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37(9):3337-3352.
5. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
6. Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother. 2016;50(3):229-233.
7. Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445-461.
8. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability, and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
9. Guyton AC, Hall JE. Guyton and Hall Textbook of Medical Physiology. 12th ed. Elsevier; 2011:550-551,692-693.
10. Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13(3):239-244.
11. Vianello M, Tavolato B, Giometto B. Glutamic acid decarboxylase autoantibodies and neurological disorders. Neurol Sci. 2002;23(4):145-151.
12. Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107-120.
13. Huang D, Liu D, Yin J, et al. Glutamate-glutamine and GABA in the brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27(7):2698-2705.
14. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, et al. Neurochemical features of idiopathic restless legs syndrome. Sleep Med Rev. 2019;45:70-87.
15. Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol. 2017;22(6):1601-1609.
16. Brambilla P, Perez J, Barale F, et al. Gabaergic dysfunction in mood disorders. Molecular Psychiatry. 2003;8:721-737.
17. Kaneez FS, Saeed SA. Investigating GABA and its function in platelets as compared to neurons. Platelets. 2009;20(5):328-333.
18. Paholpak P, Mendez MF. Trichotillomania as a manifestation of dementia. Case Rep Psychiatry. 2016;2016:9782702.
19. Miranda A, Peles S, Rudolph C, et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082-1089.
20. Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98(1-2):69-78.
21. Holton KF, Taren DL, Thomson CA, et al. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin Exp Rheumatol. 2012;30(6 Suppl 74):10-70.
22. Sekiya Y, Nakagawa T, Ozawa T, et al. Facilitation of morphine withdrawal symptoms and morphine-induced conditioned place preference by a glutamate transporter inhibitor DL-threo-beta-benzyloxy aspartate in rats. Eur J Pharmacol. 2004;485(1-3):201-210.
23. Bestha D, Soliman L, Blankenship K. et al. The walking wounded: emerging treatments for PTSD. Curr Psychiatry Rep. 2018;20(10):94.
24. Tsuda M, Shimizu N, Suzuki T. Contribution of glutamate receptors to benzodiazepine withdrawal signs. Jpn J Pharmacol. 1999;81(1):1-6.
25. Spravato [package insert]. Janssen Pharmaceuticals, Inc; 2019.
26. Mattingly GW, Anderson RH. Intranasal ketamine. Current Psychiatry. 2019;18(5):31-38.
27. Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269-1275.
28. Ferrari A, Spaccapelo L, Pinetti D, et al. Effective prophylactic treatment of migraines lower plasma glutamate levels. Cephalalgia. 2009;29(4):423-429.
29. Vieira DS, Naffah-Mazzacoratti Mda G, Zukerman E, et al. Glutamate levels in cerebrospinal fluid and triptans overuse in chronic migraine. Headache. 2007;47(6):842-847.
30. Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs. 2014;74:1165-1176.
31. Pappa S, Tsouli S, Apostolou G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
32. Kraal AZ, Arvanitis NR, Jaeger AP, et al. Could dietary glutamate play a role in psychiatric distress? Neuro Psych. 2020;79:13-19.
33. Levite M. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, Anti-NMDA-NR1 antibodies, Anti-NMDA-NR2A/B antibodies, Anti-mGluR1 antibodies or Anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren’s syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor’s expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm (Vienna). 2014;121(8):1029-1075.
34. Lancaster E. CNS syndromes associated with antibodies against metabotropic receptors. Curr Opin Neurol. 2017;30:354-360.
35. Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342(1):21-27.
36. Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol. 2010;67(5):627-630.
37. Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698-1701.
38. Mat A, Adler H, Merwick A, et al. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology. 2013;80(14):1349-1350.
3 steps to bend the curve of schizophrenia
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24

Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24

Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
Schizophrenia is arguably the most serious psychiatric brain syndrome. It disables teens and young adults and robs them of their potential and life dreams. It is widely regarded as a hopeless illness.
But it does not have to be. The reason most patients with schizophrenia do not return to their baseline is because obsolete clinical management approaches, a carryover from the last century, continue to be used.
Approximately 20 years ago, psychiatric researchers made a major discovery: psychosis is a neurotoxic state, and each psychotic episode is associated with significant brain damage in both gray and white matter.1 Based on that discovery, a more rational management of schizophrenia has emerged, focused on protecting patients from experiencing psychotic recurrence after the first-episode psychosis (FEP). In the past century, this strategy did not exist because psychiatrists were in a state of scientific ignorance, completely unaware that the malignant component of schizophrenia that leads to disability is psychotic relapses, the primary cause of which is very poor medication adherence after hospital discharge following the FEP.
Based on the emerging scientific evidence, here are 3 essential principles to halt the deterioration and bend the curve of outcomes in schizophrenia:
1. Minimize the duration of untreated psychosis (DUP)
Numerous studies have shown that the longer the DUP, the worse the outcome in schizophrenia.2,3 It is therefore vital to shorten the DUP spanning the emergence of psychotic symptoms at home, prior to the first hospital admission.4 The DUP is often prolonged from weeks to months by a combination of anosognosia by the patient, who fails to recognize how pathological their hallucinations and delusions are, plus the stigma of mental illness, which leads parents to delay bringing their son or daughter for psychiatric evaluation and treatment.
Another reason for a prolonged DUP is the legal system’s governing of the initiation of antipsychotic medications for an acutely psychotic patient who does not believe he/she is sick, and who adamantly refuses to receive medications. Laws passed decades ago have not kept up with scientific advances about brain damage during the DUP. Instead of delegating the rapid administration of an antipsychotic medication to the psychiatric physician who evaluated and diagnosed a patient with acute psychosis, the legal system further prolongs the DUP by requiring the psychiatrist to go to court and have a judge order the administration of antipsychotic medications. Such a legal requirement that delays urgently needed treatment has never been imposed on neurologists when administering medication to an obtunded stroke patient. Yet psychosis damages brain tissue and must be treated as urgently as stroke.5
Perhaps the most common reason for a long DUP is the recurrent relapses of psychosis, almost always caused by the high nonadherence rate among patients with schizophrenia due to multiple factors related to the illness itself.6 Ensuring uninterrupted delivery of an antipsychotic to a patient’s brain is as important to maintaining remission in schizophrenia as uninterrupted insulin treatment is for an individual with diabetes. The only way to guarantee ongoing daily pharmacotherapy in schizophrenia and avoid a longer DUP and more brain damage is to use long-acting injectable (LAI) formulations of antipsychotic medications, which are infrequently used despite making eminent sense to protect patients from the tragic consequences of psychotic relapse.7
Continue to: Start very early use of LAIs
2. Start very early use of LAIs
There is no doubt that switching from an oral to an LAI antipsychotic immediately after hospital discharge for the FEP is the single most important medical decision psychiatrists can make for patients with schizophrenia.8 This is because disability in schizophrenia begins after the second episode, not the first.9-11 Therefore, psychiatrists must behave like cardiologists,12 who strive to prevent a second destructive myocardial infarction. Regrettably, 99.9% of psychiatric practitioners never start an LAI after the FEP, and usually wait until the patient experiences multiple relapses, after extensive gray matter atrophy and white matter disintegration have occurred due to the neuroinflammation and oxidative stress (free radicals) that occur with every psychotic episode.13,14 This clearly does not make clinical sense, but remains the standard current practice.
In oncology, chemotherapy is far more effective in Stage 1 cancer, immediately after the diagnosis is made, rather than in Stage 4, when the prognosis is very poor. Similarly, LAIs are best used in Stage 1 schizophrenia, which is the first episode (schizophrenia researchers now regard the illness as having stages).15 Unfortunately, it is now rare for patients with schizophrenia to be switched to LAI pharmacotherapy right after recovery from the FEP. Instead, LAIs are more commonly used in Stage 3 or Stage 4, when the brains of patients with chronic schizophrenia have been already structurally damaged, and functional disability had set in. Bending the cure of outcome in schizophrenia is only possible when LAIs are used very early to prevent the second episode.
The prevention of relapse by using LAIs in FEP is truly remarkable. Subotnik et al16 reported that only 5% of FEP patients who received an LAI antipsychotic relapsed, compared to 33% of those who received an oral formulation of the same antipsychotic (a 650% difference). It is frankly inexplicable why psychiatrists do not exploit the relapse-preventing properties of LAIs at the time of discharge after the FEP, and instead continue to perpetuate the use of prescribing oral tablets to patients who are incapable of full adherence and doomed to “self-destruct.” This was the practice model in the previous century, when there was total ignorance about the brain-damaging effects of psychosis, and no sense of urgency about preventing psychotic relapses and DUP. Psychiatrists regarded LAIs as a last resort instead of a life-saving first resort.
In addition to relapse prevention,17 the benefits of second-generation LAIs include neuroprotection18 and lower all-cause mortality,19 a remarkable triad of benefits for patients with schizophrenia.20
3. Implement comprehensive psychosocial treatment
Most patients with schizophrenia do not have access to the array of psychosocial treatments that have been shown to be vital for rehabilitation following the FEP, just as physical rehabilitation is indispensable after the first stroke. Studies such as RAISE,21 which was funded by the National Institute of Mental Health, have demonstrated the value of psychosocial therapies (Table21-23). Collaborative care with primary care physicians is also essential due to the high prevalence of metabolic disorders (obesity, diabetics, dyslipidemia, hypertension), which tend to be undertreated in patients with schizophrenia.24

Finally, when patients continue to experience delusions and hallucinations despite full adherence (with LAIs), clozapine must be used. Like LAIs, clozapine is woefully underutilized25 despite having been shown to restore mental health and full recovery to many (but not all) patients written off as hopeless due to persistent and refractory psychotic symptoms.26
If clinicians who treat schizophrenia implement these 3 steps in their FEP patients, they will be gratified to witness a more benign trajectory of schizophrenia, which I have personally seen. The curve can indeed be bent in favor of better outcomes. By using the 3 evidence-based steps described here, clinicians will realize that schizophrenia does not have to carry the label of “the worst disease affecting mankind,” as an editorial in a top-tier journal pessimistically stated over 3 decades ago.27
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
1. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
2. Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75-95.
3. Oliver D, Davies C, Crossland G, et al. Can we reduce the duration of untreated psychosis? A systematic review and meta-analysis of controlled interventional studies. Schizophr Bull. 2018;44(6):1362-1372.
4. Srihari VH, Ferrara M, Li F, et al. Reducing the duration of untreated psychosis (DUP) in a US community: a quasi-experimental trial. Schizophr Bull Open. 2022;3(1):sgab057. doi:10.1093/schizbullopen/sgab057
5. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
6. Lieslehto J, Tiihonen J, Lähteenvuo M, et al. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48(3):665-663.
7. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
8. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
9. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
10. Taipale H, Tanskanen A, Correll CU, et al. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9(4):271-279.
11. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):38-45,e3.
12. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
13. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93.
14. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400-409.
15. Lavoie S, Polari AR, Goldstone S, et al. Staging model in psychiatry: review of the evolution of electroencephalography abnormalities in major psychiatric disorders. Early Interv Psychiatry. 2019;13(6):1319-1328.
16. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
17. Lin YH, Wu CS, Liu CC, et al. Comparative effectiveness of antipsychotics in preventing readmission for first-admission schizophrenia patients in national cohorts from 2001 to 2017 in Taiwan. Schizophr Bull. 2022;sbac046. doi:10.1093/schbul/sbac046
18. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
21. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
22. Keshavan MS, Ongur D, Srihari VH. Toward an expanded and personalized approach to coordinated specialty care in early course psychoses. Schizophr Res. 2022;241:119-121.
23. Srihari VH, Keshavan MS. Early intervention services for schizophrenia: looking back and looking ahead. Schizophr Bull. 2022;48(3):544-550.
24. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
25. Nasrallah HA. Clozapine is a vastly underutilized, unique agent with multiple applications. Current Psychiatry. 2014;13(10):21,24-25.
26. CureSZ Foundation. Clozapine success stories. Accessed June 1, 2022. https://curesz.org/clozapine-success-stories/
27. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
Why we should be scrutinizing the rising prevalence of adult ADHD
In patients with attention-deficit/hyperactivity disorder (ADHD), stimulants reduce impulsivity and improve attention and focus. In individuals who do not have this disorder, stimulants are believed to enhance cognition, attention, and physical performance. In this article, I describe how a patient whose intermittent use of stimulants for motivation and cognitive enhancement shaped my approach to the diagnosis of ADHD.
Instant gratification and quick solutions
I asked him questions to confirm the diagnosis, but he rushed to reassure me that he had already been diagnosed with ADHD and had been doing well on dextroamphetamine and amphetamine for many years. I was inclined to question his diagnosis of ADHD after learning of his “as-needed” use of stimulants as brain enhancers. His medical record reflecting the diagnosis of ADHD dated back to when he was a first-year dental student. The diagnosis was based on the patient’s report of procrastination for as long as he could remember. It also hinged on difficulties learning a second language and math being a challenging subject for him. Despite this, he managed to do well in school and earn an undergraduate degree, well enough to later pursue dentistry at a reputable university.
I thought, “Isn’t it normal to lose motivation and have doubts when preparing for a high-stakes exam like the boards? Aren’t these negative thoughts distracting enough to render sustained focus impossible? Doesn’t everyone struggle with procrastination, especially when they need to study? If learning a new language requires devotion, consistency, and sacrifice, isn’t it inherently challenging? Doesn’t good performance in math depend on multiple factors (ie, a strong foundation, cumulative learning, frequent practice), and thus leaves many students struggling?”
This interaction and many similar ones made me scrutinize the diagnosis of ADHD in patients I encounter in clinical settings. We live in a society where instant gratification is cherished, and quick fixes are pursued with little contemplation of pitfalls. Students use stimulants to cram for exams, high-functioning professionals use them to meet deadlines, and athletes use them to enhance performance and improve reaction times. Psychiatry seems to be drawn into the demands of society and may be fueling the “quick-fix” mentality by prescribing stimulants to healthy individuals who want to improve their focus, and then diagnosing them with ADHD to align the prescription with an appropriate diagnosis. Research on the adverse effects of stimulant use in adults is not convincing nor conclusive enough to sway prescribers from denying the average adult patient a stimulant to enhance cognitive function before a high-stakes exam or a critical, career-shaping project if they present with some ADHD traits, which the patient might even hyperbolize to secure the desired prescription. All of this may contribute to the perceived rising prevalence of ADHD among adults.
As for my 30-year-old dental student, I reasoned that continuing his medication, for now, would help me establish rapport and trust. This would allow me to counsel him on the long-term adverse effects of stimulants, and develop a plan to optimize his sleep, focus, and time management skills, eventually improving his cognition and attention naturally. Unfortunately, he did not show up to future appointments after I sent him the refill.
In patients with attention-deficit/hyperactivity disorder (ADHD), stimulants reduce impulsivity and improve attention and focus. In individuals who do not have this disorder, stimulants are believed to enhance cognition, attention, and physical performance. In this article, I describe how a patient whose intermittent use of stimulants for motivation and cognitive enhancement shaped my approach to the diagnosis of ADHD.
Instant gratification and quick solutions
I asked him questions to confirm the diagnosis, but he rushed to reassure me that he had already been diagnosed with ADHD and had been doing well on dextroamphetamine and amphetamine for many years. I was inclined to question his diagnosis of ADHD after learning of his “as-needed” use of stimulants as brain enhancers. His medical record reflecting the diagnosis of ADHD dated back to when he was a first-year dental student. The diagnosis was based on the patient’s report of procrastination for as long as he could remember. It also hinged on difficulties learning a second language and math being a challenging subject for him. Despite this, he managed to do well in school and earn an undergraduate degree, well enough to later pursue dentistry at a reputable university.
I thought, “Isn’t it normal to lose motivation and have doubts when preparing for a high-stakes exam like the boards? Aren’t these negative thoughts distracting enough to render sustained focus impossible? Doesn’t everyone struggle with procrastination, especially when they need to study? If learning a new language requires devotion, consistency, and sacrifice, isn’t it inherently challenging? Doesn’t good performance in math depend on multiple factors (ie, a strong foundation, cumulative learning, frequent practice), and thus leaves many students struggling?”
This interaction and many similar ones made me scrutinize the diagnosis of ADHD in patients I encounter in clinical settings. We live in a society where instant gratification is cherished, and quick fixes are pursued with little contemplation of pitfalls. Students use stimulants to cram for exams, high-functioning professionals use them to meet deadlines, and athletes use them to enhance performance and improve reaction times. Psychiatry seems to be drawn into the demands of society and may be fueling the “quick-fix” mentality by prescribing stimulants to healthy individuals who want to improve their focus, and then diagnosing them with ADHD to align the prescription with an appropriate diagnosis. Research on the adverse effects of stimulant use in adults is not convincing nor conclusive enough to sway prescribers from denying the average adult patient a stimulant to enhance cognitive function before a high-stakes exam or a critical, career-shaping project if they present with some ADHD traits, which the patient might even hyperbolize to secure the desired prescription. All of this may contribute to the perceived rising prevalence of ADHD among adults.
As for my 30-year-old dental student, I reasoned that continuing his medication, for now, would help me establish rapport and trust. This would allow me to counsel him on the long-term adverse effects of stimulants, and develop a plan to optimize his sleep, focus, and time management skills, eventually improving his cognition and attention naturally. Unfortunately, he did not show up to future appointments after I sent him the refill.
In patients with attention-deficit/hyperactivity disorder (ADHD), stimulants reduce impulsivity and improve attention and focus. In individuals who do not have this disorder, stimulants are believed to enhance cognition, attention, and physical performance. In this article, I describe how a patient whose intermittent use of stimulants for motivation and cognitive enhancement shaped my approach to the diagnosis of ADHD.
Instant gratification and quick solutions
I asked him questions to confirm the diagnosis, but he rushed to reassure me that he had already been diagnosed with ADHD and had been doing well on dextroamphetamine and amphetamine for many years. I was inclined to question his diagnosis of ADHD after learning of his “as-needed” use of stimulants as brain enhancers. His medical record reflecting the diagnosis of ADHD dated back to when he was a first-year dental student. The diagnosis was based on the patient’s report of procrastination for as long as he could remember. It also hinged on difficulties learning a second language and math being a challenging subject for him. Despite this, he managed to do well in school and earn an undergraduate degree, well enough to later pursue dentistry at a reputable university.
I thought, “Isn’t it normal to lose motivation and have doubts when preparing for a high-stakes exam like the boards? Aren’t these negative thoughts distracting enough to render sustained focus impossible? Doesn’t everyone struggle with procrastination, especially when they need to study? If learning a new language requires devotion, consistency, and sacrifice, isn’t it inherently challenging? Doesn’t good performance in math depend on multiple factors (ie, a strong foundation, cumulative learning, frequent practice), and thus leaves many students struggling?”
This interaction and many similar ones made me scrutinize the diagnosis of ADHD in patients I encounter in clinical settings. We live in a society where instant gratification is cherished, and quick fixes are pursued with little contemplation of pitfalls. Students use stimulants to cram for exams, high-functioning professionals use them to meet deadlines, and athletes use them to enhance performance and improve reaction times. Psychiatry seems to be drawn into the demands of society and may be fueling the “quick-fix” mentality by prescribing stimulants to healthy individuals who want to improve their focus, and then diagnosing them with ADHD to align the prescription with an appropriate diagnosis. Research on the adverse effects of stimulant use in adults is not convincing nor conclusive enough to sway prescribers from denying the average adult patient a stimulant to enhance cognitive function before a high-stakes exam or a critical, career-shaping project if they present with some ADHD traits, which the patient might even hyperbolize to secure the desired prescription. All of this may contribute to the perceived rising prevalence of ADHD among adults.
As for my 30-year-old dental student, I reasoned that continuing his medication, for now, would help me establish rapport and trust. This would allow me to counsel him on the long-term adverse effects of stimulants, and develop a plan to optimize his sleep, focus, and time management skills, eventually improving his cognition and attention naturally. Unfortunately, he did not show up to future appointments after I sent him the refill.
Murder of physician raises the stress level for all clinicians
As if it weren’t enough that doctors work in a profession where it’s almost more a question of when they’ll be sued than if they’ll be sued – where COVID, staff shortages, long hours, and patients frustrated over canceled procedures have caused unrelenting fatigue and stress – they now have to worry that an unhappy patient is going to buy a gun, walk into their office, and kill them.
That’s exactly what happened in Tulsa, Okla., where a patient complaining of pain after back surgery murdered his doctor and several others who happened to be in the wrong place at the wrong time.
The temptation in the aftermath of such tragedies is to think about preventive measures: Make medical facilities “hardened” targets, like schools have become, with armed guards, metal detectors, automatically locking doors, physical barriers within, security cameras, and buzzers for entry – although hardening a large medical center where members of the community routinely come and go would be challenging.
What about the enormous stress on doctors, nurses, and others in the medical workplace? Physicians who have been sued for malpractice often describe how it changes the way they interact with patients: They now size patients up and make judgments about their potential litigiousness. Will the physicians now look over their patients’ shoulders at the video feed from a security camera when they’re taking a history? Will medical professionals be forced to make snap judgments about patients’ psychological state before deciding whether to treat them?
Remember, there was a time when school shootings were unimaginable. Once one person crosses that line, others inevitably follow.
It could be a drug-seeking patient complaining of ongoing pain, angry because he can’t get a new prescription. It could be a patient whose unpaid bill was turned over to a collection agency, angry because he’s now getting calls from collectors. It could be someone who blames a physician for the loss of a loved one. It could be someone who would otherwise have filed a lawsuit, who now thinks he has a more effective option for exacting retribution.
Most of us would find it unbearable to live and work under the kind of stress faced by medical professionals today. And unfortunately, there is no short-term, systemic relief on the horizon. But there are methods of relieving at least some of the psychological burden being carried by these dedicated individuals.
For starters, the government should provide funds to improve safety and security at medical facilities. It’s sad but it’s a fact of life. The physical structure of schools, along with emergency procedures, have been changed since Columbine and Sandy Hook, and our children and their teachers undergo active shooter drills. Health care facilities will need to adopt similar strategies.
But if we don’t also support the individuals who work in health care, we’ll no longer have even partially staffed health care facilities. Hospitals and medical groups need to be conscious of the effects stress may have on them. Medical staff and administrators need to recognize changes in their colleagues’ behavior and refer those cohorts to professional stress coaches who can get them back on track.
Medical personnel should be picking up on warning signs, like irritability, depression, sudden weight gain or loss, lack of motivation and job satisfaction, obsessiveness, unusual levels of fatigue, alcohol or drug use, and, of course, avoidable medical errors.
In addition, colleagues in the medical workplace need to know each other well. They are usually the first ones to notice if something is off and may be in the best position to refer coworkers for help. Also, medical malpractice insurance carriers should consider encouraging and covering coaching sessions, because helping physicians cope with this heightened stress will prevent medical errors and the lawsuits that inevitably accompany mistakes.
This needn’t be a long-term process like ongoing psychotherapy; a few sessions with a well-trained coach may help psychologically challenged peers restore their focus and perspective. It won’t eliminate the threat any more than litigation stress coaching eliminates the threat of being sued, but it can prevent that stress from leading to avoidable errors. It also can prevent physicians’ personal lives and relationships from going off the rails and driving them out of the medical profession.
None of us can afford to ignore the impacts that these new stressors are having and simply act as if it’s business as usual. The people in the trenches need our help.
Ms. Fiore is President of Winning Focus in Murrysville, Pa. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As if it weren’t enough that doctors work in a profession where it’s almost more a question of when they’ll be sued than if they’ll be sued – where COVID, staff shortages, long hours, and patients frustrated over canceled procedures have caused unrelenting fatigue and stress – they now have to worry that an unhappy patient is going to buy a gun, walk into their office, and kill them.
That’s exactly what happened in Tulsa, Okla., where a patient complaining of pain after back surgery murdered his doctor and several others who happened to be in the wrong place at the wrong time.
The temptation in the aftermath of such tragedies is to think about preventive measures: Make medical facilities “hardened” targets, like schools have become, with armed guards, metal detectors, automatically locking doors, physical barriers within, security cameras, and buzzers for entry – although hardening a large medical center where members of the community routinely come and go would be challenging.
What about the enormous stress on doctors, nurses, and others in the medical workplace? Physicians who have been sued for malpractice often describe how it changes the way they interact with patients: They now size patients up and make judgments about their potential litigiousness. Will the physicians now look over their patients’ shoulders at the video feed from a security camera when they’re taking a history? Will medical professionals be forced to make snap judgments about patients’ psychological state before deciding whether to treat them?
Remember, there was a time when school shootings were unimaginable. Once one person crosses that line, others inevitably follow.
It could be a drug-seeking patient complaining of ongoing pain, angry because he can’t get a new prescription. It could be a patient whose unpaid bill was turned over to a collection agency, angry because he’s now getting calls from collectors. It could be someone who blames a physician for the loss of a loved one. It could be someone who would otherwise have filed a lawsuit, who now thinks he has a more effective option for exacting retribution.
Most of us would find it unbearable to live and work under the kind of stress faced by medical professionals today. And unfortunately, there is no short-term, systemic relief on the horizon. But there are methods of relieving at least some of the psychological burden being carried by these dedicated individuals.
For starters, the government should provide funds to improve safety and security at medical facilities. It’s sad but it’s a fact of life. The physical structure of schools, along with emergency procedures, have been changed since Columbine and Sandy Hook, and our children and their teachers undergo active shooter drills. Health care facilities will need to adopt similar strategies.
But if we don’t also support the individuals who work in health care, we’ll no longer have even partially staffed health care facilities. Hospitals and medical groups need to be conscious of the effects stress may have on them. Medical staff and administrators need to recognize changes in their colleagues’ behavior and refer those cohorts to professional stress coaches who can get them back on track.
Medical personnel should be picking up on warning signs, like irritability, depression, sudden weight gain or loss, lack of motivation and job satisfaction, obsessiveness, unusual levels of fatigue, alcohol or drug use, and, of course, avoidable medical errors.
In addition, colleagues in the medical workplace need to know each other well. They are usually the first ones to notice if something is off and may be in the best position to refer coworkers for help. Also, medical malpractice insurance carriers should consider encouraging and covering coaching sessions, because helping physicians cope with this heightened stress will prevent medical errors and the lawsuits that inevitably accompany mistakes.
This needn’t be a long-term process like ongoing psychotherapy; a few sessions with a well-trained coach may help psychologically challenged peers restore their focus and perspective. It won’t eliminate the threat any more than litigation stress coaching eliminates the threat of being sued, but it can prevent that stress from leading to avoidable errors. It also can prevent physicians’ personal lives and relationships from going off the rails and driving them out of the medical profession.
None of us can afford to ignore the impacts that these new stressors are having and simply act as if it’s business as usual. The people in the trenches need our help.
Ms. Fiore is President of Winning Focus in Murrysville, Pa. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As if it weren’t enough that doctors work in a profession where it’s almost more a question of when they’ll be sued than if they’ll be sued – where COVID, staff shortages, long hours, and patients frustrated over canceled procedures have caused unrelenting fatigue and stress – they now have to worry that an unhappy patient is going to buy a gun, walk into their office, and kill them.
That’s exactly what happened in Tulsa, Okla., where a patient complaining of pain after back surgery murdered his doctor and several others who happened to be in the wrong place at the wrong time.
The temptation in the aftermath of such tragedies is to think about preventive measures: Make medical facilities “hardened” targets, like schools have become, with armed guards, metal detectors, automatically locking doors, physical barriers within, security cameras, and buzzers for entry – although hardening a large medical center where members of the community routinely come and go would be challenging.
What about the enormous stress on doctors, nurses, and others in the medical workplace? Physicians who have been sued for malpractice often describe how it changes the way they interact with patients: They now size patients up and make judgments about their potential litigiousness. Will the physicians now look over their patients’ shoulders at the video feed from a security camera when they’re taking a history? Will medical professionals be forced to make snap judgments about patients’ psychological state before deciding whether to treat them?
Remember, there was a time when school shootings were unimaginable. Once one person crosses that line, others inevitably follow.
It could be a drug-seeking patient complaining of ongoing pain, angry because he can’t get a new prescription. It could be a patient whose unpaid bill was turned over to a collection agency, angry because he’s now getting calls from collectors. It could be someone who blames a physician for the loss of a loved one. It could be someone who would otherwise have filed a lawsuit, who now thinks he has a more effective option for exacting retribution.
Most of us would find it unbearable to live and work under the kind of stress faced by medical professionals today. And unfortunately, there is no short-term, systemic relief on the horizon. But there are methods of relieving at least some of the psychological burden being carried by these dedicated individuals.
For starters, the government should provide funds to improve safety and security at medical facilities. It’s sad but it’s a fact of life. The physical structure of schools, along with emergency procedures, have been changed since Columbine and Sandy Hook, and our children and their teachers undergo active shooter drills. Health care facilities will need to adopt similar strategies.
But if we don’t also support the individuals who work in health care, we’ll no longer have even partially staffed health care facilities. Hospitals and medical groups need to be conscious of the effects stress may have on them. Medical staff and administrators need to recognize changes in their colleagues’ behavior and refer those cohorts to professional stress coaches who can get them back on track.
Medical personnel should be picking up on warning signs, like irritability, depression, sudden weight gain or loss, lack of motivation and job satisfaction, obsessiveness, unusual levels of fatigue, alcohol or drug use, and, of course, avoidable medical errors.
In addition, colleagues in the medical workplace need to know each other well. They are usually the first ones to notice if something is off and may be in the best position to refer coworkers for help. Also, medical malpractice insurance carriers should consider encouraging and covering coaching sessions, because helping physicians cope with this heightened stress will prevent medical errors and the lawsuits that inevitably accompany mistakes.
This needn’t be a long-term process like ongoing psychotherapy; a few sessions with a well-trained coach may help psychologically challenged peers restore their focus and perspective. It won’t eliminate the threat any more than litigation stress coaching eliminates the threat of being sued, but it can prevent that stress from leading to avoidable errors. It also can prevent physicians’ personal lives and relationships from going off the rails and driving them out of the medical profession.
None of us can afford to ignore the impacts that these new stressors are having and simply act as if it’s business as usual. The people in the trenches need our help.
Ms. Fiore is President of Winning Focus in Murrysville, Pa. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comments & Controversies
More on T. gondii
We reviewed the article by Dr. Torrey on Toxoplasma gondii (T. gondii) and schizophrenia (“Cats, toxoplasmosis, and psychosis: Understanding the risks,”
The natural history of toxoplasmosis is an extraordinary example of nature’s complexity. The life cycle of this parasite uses the nervous system of the mouse to increase its transmission. Behavior changes ranging from reduced cat urine avoidance and increased risk-taking are observed in mice infected with T. gondii.2 Chronic toxoplasmosis may also affect human behavior.3
Cats are fascinating, complex creatures. Of note, they produce a protein structurally like the secretion of the slow loris.4 The loris uses this brachial gland protein secretion as part of a defense strategy.5 Consideration of a possible toxic, neuroimmune role of these small mammal proteins in psychiatric disorders may open other avenues to explore.6
Our relationship to domesticated animals has been connected to serious diseases throughout human history.7 Severe acute respiratory syndrome and COVID-19 appear to be linked to animal reservoirs, mammals of the small animal trade, and the fur industry.8,9 The rapid development of vaccines for COVID-19 is commendable. In conditions with multifactorial causation, managing an infectious component is worthy of consideration.
With mounting evidence suggesting a link between T. gondii and schizophrenia, ASD, and other diseases, further epidemiological studies and pilot interventions offer value. Interventions, including encouraging keeping cats indoors only, cat immunization, and human treatment, could be implemented in high-risk families. Efficacy requires data collection. While not easy, collaborative work by psychiatrists, developmental pediatricians, veterinarians, and epidemiologists is encouraged.
Mark C. Chandler, MD
Triangle Neuropsychiatry
Durham, North Carolina
Michelle Douglass, PA-S2
Duke University Physician Assistant Program
Durham, North Carolina
References
1. Nayeri T, Sarvi S, Moosazadeh M, et al. Relationship between toxoplasmosis and autism: a systematic review and meta-analysis. Microb Pathog. 2020;147:104434. doi:10.1016/j.micpath.2020.104434
2. Kochanowsky JA, Koshy AA. Toxoplasma gondii. Curr Biol. 2018;28(14):R770-R771. doi:10.1016/j.cub.2018.05.035
3. Letcher S. Parasite mind control: how a single celled parasite carried in the cat intestine may be quietly tweaking our behavior. Scientific Kenyon: The Neuroscience Edition. 2018;22(1):4-11.
4. Scheib H, Nekaris KA, Rode-Margono J, et al. The toxicological intersection between allergen and toxin: a structural comparison of the cat dander allergenic protein Fel d1 and the slow loris brachial gland secretion protein. Toxins (Basel). 2020;12(2):86. doi:10.3390/toxins12020086
5. Nekaris KA, Moore RS, Rode EJ, et al. Mad, bad and dangerous to know: the biochemistry, ecology and evolution of slow loris venom. J Venom Anim Toxins Incl Trop Dis. 2013;19(1):21. doi:10.1186/1678-9199-19-21
6. Ligabue-Braun R. Hello, kitty: could cat allergy be a form of intoxication? J Venom Anim Toxins Incl Trop Dis. 2020;26:e20200051. doi:10.1590/1678-9199-JVATITD-2020-0051
7. Pearce-Duvet JM. The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease. Biol Rev Camb Philos Soc. 2006;81(3):369-382. doi:10.1017/S1464793106007020
8. Jo WK, de Oliveira-Filho EF, Rasche A, et al. Potential zoonotic sources of SARS-CoV-2 infections. Transbound Emerg Dis. 2021;68(4):1824-1834. doi:10.1111/tbed.13872
9. Bell D, Roberton S, Hunter PR. Animal origins of SARS coronavirus: possible links with the international trade in small carnivores. Philos Trans R Soc Lond B Biol Sci. 2004;359(1447):1107-1114. doi:10.1098/rstb.2004.1492
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Continue to: Pramipexole for MDD
Pramipexole for MDD
I appreciate Dr. Espejo’s recommendations for treating patients who experience limited response from initial antidepressant therapy (“Treating major depressive disorder after limited response to an initial agent,”
Jonathan R. Scarff, MD
Lexington VA Health Care System
Lexington, Kentucky
References
1. Tundo A, de Filippis R, De Crescenzo F. Pramipexole in the treatment of unipolar and bipolar depression. A systematic review and meta-analysis. Acta Psychiatr Scand. 2019;140(2):116-125.
2. Tundo A, Betrò S, Iommi M, et al. Efficacy and safety of 24-week pramipexole augmentation in patients with treatment resistant depression. A retrospective cohort study. Prog Neuropsychopharmacol Biol Psychiatry. 2022;112:110425. doi:10.1016/j.pnpbp.2021.110425
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
More on T. gondii
We reviewed the article by Dr. Torrey on Toxoplasma gondii (T. gondii) and schizophrenia (“Cats, toxoplasmosis, and psychosis: Understanding the risks,”
The natural history of toxoplasmosis is an extraordinary example of nature’s complexity. The life cycle of this parasite uses the nervous system of the mouse to increase its transmission. Behavior changes ranging from reduced cat urine avoidance and increased risk-taking are observed in mice infected with T. gondii.2 Chronic toxoplasmosis may also affect human behavior.3
Cats are fascinating, complex creatures. Of note, they produce a protein structurally like the secretion of the slow loris.4 The loris uses this brachial gland protein secretion as part of a defense strategy.5 Consideration of a possible toxic, neuroimmune role of these small mammal proteins in psychiatric disorders may open other avenues to explore.6
Our relationship to domesticated animals has been connected to serious diseases throughout human history.7 Severe acute respiratory syndrome and COVID-19 appear to be linked to animal reservoirs, mammals of the small animal trade, and the fur industry.8,9 The rapid development of vaccines for COVID-19 is commendable. In conditions with multifactorial causation, managing an infectious component is worthy of consideration.
With mounting evidence suggesting a link between T. gondii and schizophrenia, ASD, and other diseases, further epidemiological studies and pilot interventions offer value. Interventions, including encouraging keeping cats indoors only, cat immunization, and human treatment, could be implemented in high-risk families. Efficacy requires data collection. While not easy, collaborative work by psychiatrists, developmental pediatricians, veterinarians, and epidemiologists is encouraged.
Mark C. Chandler, MD
Triangle Neuropsychiatry
Durham, North Carolina
Michelle Douglass, PA-S2
Duke University Physician Assistant Program
Durham, North Carolina
References
1. Nayeri T, Sarvi S, Moosazadeh M, et al. Relationship between toxoplasmosis and autism: a systematic review and meta-analysis. Microb Pathog. 2020;147:104434. doi:10.1016/j.micpath.2020.104434
2. Kochanowsky JA, Koshy AA. Toxoplasma gondii. Curr Biol. 2018;28(14):R770-R771. doi:10.1016/j.cub.2018.05.035
3. Letcher S. Parasite mind control: how a single celled parasite carried in the cat intestine may be quietly tweaking our behavior. Scientific Kenyon: The Neuroscience Edition. 2018;22(1):4-11.
4. Scheib H, Nekaris KA, Rode-Margono J, et al. The toxicological intersection between allergen and toxin: a structural comparison of the cat dander allergenic protein Fel d1 and the slow loris brachial gland secretion protein. Toxins (Basel). 2020;12(2):86. doi:10.3390/toxins12020086
5. Nekaris KA, Moore RS, Rode EJ, et al. Mad, bad and dangerous to know: the biochemistry, ecology and evolution of slow loris venom. J Venom Anim Toxins Incl Trop Dis. 2013;19(1):21. doi:10.1186/1678-9199-19-21
6. Ligabue-Braun R. Hello, kitty: could cat allergy be a form of intoxication? J Venom Anim Toxins Incl Trop Dis. 2020;26:e20200051. doi:10.1590/1678-9199-JVATITD-2020-0051
7. Pearce-Duvet JM. The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease. Biol Rev Camb Philos Soc. 2006;81(3):369-382. doi:10.1017/S1464793106007020
8. Jo WK, de Oliveira-Filho EF, Rasche A, et al. Potential zoonotic sources of SARS-CoV-2 infections. Transbound Emerg Dis. 2021;68(4):1824-1834. doi:10.1111/tbed.13872
9. Bell D, Roberton S, Hunter PR. Animal origins of SARS coronavirus: possible links with the international trade in small carnivores. Philos Trans R Soc Lond B Biol Sci. 2004;359(1447):1107-1114. doi:10.1098/rstb.2004.1492
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Continue to: Pramipexole for MDD
Pramipexole for MDD
I appreciate Dr. Espejo’s recommendations for treating patients who experience limited response from initial antidepressant therapy (“Treating major depressive disorder after limited response to an initial agent,”
Jonathan R. Scarff, MD
Lexington VA Health Care System
Lexington, Kentucky
References
1. Tundo A, de Filippis R, De Crescenzo F. Pramipexole in the treatment of unipolar and bipolar depression. A systematic review and meta-analysis. Acta Psychiatr Scand. 2019;140(2):116-125.
2. Tundo A, Betrò S, Iommi M, et al. Efficacy and safety of 24-week pramipexole augmentation in patients with treatment resistant depression. A retrospective cohort study. Prog Neuropsychopharmacol Biol Psychiatry. 2022;112:110425. doi:10.1016/j.pnpbp.2021.110425
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
More on T. gondii
We reviewed the article by Dr. Torrey on Toxoplasma gondii (T. gondii) and schizophrenia (“Cats, toxoplasmosis, and psychosis: Understanding the risks,”
The natural history of toxoplasmosis is an extraordinary example of nature’s complexity. The life cycle of this parasite uses the nervous system of the mouse to increase its transmission. Behavior changes ranging from reduced cat urine avoidance and increased risk-taking are observed in mice infected with T. gondii.2 Chronic toxoplasmosis may also affect human behavior.3
Cats are fascinating, complex creatures. Of note, they produce a protein structurally like the secretion of the slow loris.4 The loris uses this brachial gland protein secretion as part of a defense strategy.5 Consideration of a possible toxic, neuroimmune role of these small mammal proteins in psychiatric disorders may open other avenues to explore.6
Our relationship to domesticated animals has been connected to serious diseases throughout human history.7 Severe acute respiratory syndrome and COVID-19 appear to be linked to animal reservoirs, mammals of the small animal trade, and the fur industry.8,9 The rapid development of vaccines for COVID-19 is commendable. In conditions with multifactorial causation, managing an infectious component is worthy of consideration.
With mounting evidence suggesting a link between T. gondii and schizophrenia, ASD, and other diseases, further epidemiological studies and pilot interventions offer value. Interventions, including encouraging keeping cats indoors only, cat immunization, and human treatment, could be implemented in high-risk families. Efficacy requires data collection. While not easy, collaborative work by psychiatrists, developmental pediatricians, veterinarians, and epidemiologists is encouraged.
Mark C. Chandler, MD
Triangle Neuropsychiatry
Durham, North Carolina
Michelle Douglass, PA-S2
Duke University Physician Assistant Program
Durham, North Carolina
References
1. Nayeri T, Sarvi S, Moosazadeh M, et al. Relationship between toxoplasmosis and autism: a systematic review and meta-analysis. Microb Pathog. 2020;147:104434. doi:10.1016/j.micpath.2020.104434
2. Kochanowsky JA, Koshy AA. Toxoplasma gondii. Curr Biol. 2018;28(14):R770-R771. doi:10.1016/j.cub.2018.05.035
3. Letcher S. Parasite mind control: how a single celled parasite carried in the cat intestine may be quietly tweaking our behavior. Scientific Kenyon: The Neuroscience Edition. 2018;22(1):4-11.
4. Scheib H, Nekaris KA, Rode-Margono J, et al. The toxicological intersection between allergen and toxin: a structural comparison of the cat dander allergenic protein Fel d1 and the slow loris brachial gland secretion protein. Toxins (Basel). 2020;12(2):86. doi:10.3390/toxins12020086
5. Nekaris KA, Moore RS, Rode EJ, et al. Mad, bad and dangerous to know: the biochemistry, ecology and evolution of slow loris venom. J Venom Anim Toxins Incl Trop Dis. 2013;19(1):21. doi:10.1186/1678-9199-19-21
6. Ligabue-Braun R. Hello, kitty: could cat allergy be a form of intoxication? J Venom Anim Toxins Incl Trop Dis. 2020;26:e20200051. doi:10.1590/1678-9199-JVATITD-2020-0051
7. Pearce-Duvet JM. The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease. Biol Rev Camb Philos Soc. 2006;81(3):369-382. doi:10.1017/S1464793106007020
8. Jo WK, de Oliveira-Filho EF, Rasche A, et al. Potential zoonotic sources of SARS-CoV-2 infections. Transbound Emerg Dis. 2021;68(4):1824-1834. doi:10.1111/tbed.13872
9. Bell D, Roberton S, Hunter PR. Animal origins of SARS coronavirus: possible links with the international trade in small carnivores. Philos Trans R Soc Lond B Biol Sci. 2004;359(1447):1107-1114. doi:10.1098/rstb.2004.1492
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Continue to: Pramipexole for MDD
Pramipexole for MDD
I appreciate Dr. Espejo’s recommendations for treating patients who experience limited response from initial antidepressant therapy (“Treating major depressive disorder after limited response to an initial agent,”
Jonathan R. Scarff, MD
Lexington VA Health Care System
Lexington, Kentucky
References
1. Tundo A, de Filippis R, De Crescenzo F. Pramipexole in the treatment of unipolar and bipolar depression. A systematic review and meta-analysis. Acta Psychiatr Scand. 2019;140(2):116-125.
2. Tundo A, Betrò S, Iommi M, et al. Efficacy and safety of 24-week pramipexole augmentation in patients with treatment resistant depression. A retrospective cohort study. Prog Neuropsychopharmacol Biol Psychiatry. 2022;112:110425. doi:10.1016/j.pnpbp.2021.110425
Disclosures
The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Sugar highs and royal meltdowns
I can dimly recall watching Queen Elizabeth’s coronation on a very small black and white television screen. Even in monochrome it was a riveting event. Recently, the Queen celebrated her Platinum Jubilee, marking her 70-year reign. Apparently it was a multiday event with all the trappings, floating above an undercurrent of scandal and intrigue. I had better things to do than I did as a 7-year-old entranced by the novelty of a neighbor’s television set.
But, it turns out that I had missed the opportunity to see live and in color a royal meltdown starring the Queen’s great-grandson, 4-year-old Prince Louis. Not to worry. It remains on video archives for our education and pleasure ad infinitum. His performance was no more dramatic than what you have seen numerous times in the checkout line of the grocery store. However, this meltdown was on the world stage in the front row of the royal box and performed in various venues on each day of a 4-day event.
As long as you weren’t his parents, Kate Middleton and Prince William, the meltdown had its moments of hilarity. Louis made full use of his youthful and plastic face, creating a wide variety of taunts and responses to his mother’s praiseworthy and understated attempts at regaining control. Of course, the British press and every armchair parent with a Twitter account had a field day contributing their explanations and advice.
For example, here’s the headline on an international news website that caught my eye: “Royal reveals why Prince Louis was so ‘mischievous’ during the Jubilee”. In the article, a fellow royal and former rugby star who was sitting directly behind the little Prince during one of his performances chalked up the 4-year-old’s behavior to a “sugar high” resulting from the ample supply of sweets available behind the royal box.
Nowhere in the article is there a question of whether the “sugar high” is a science-based phenomenon. In fact, the reporter assumes we all know it exists and writes that “parents across the globe can probably [read: definitely] relate.”
I’m curious: How do you respond when a parent in the office explains the child’s behavior as the result of a “sugar high”? Or when you’re at a cookout and someone makes a comment that makes it obvious that they believe that “sugar highs” are real? Do you immediately pause the conversation and launch into a short but tasteful observation that you know of no scientific studies that sugar can cause a high? Or, figuring that in the face of an overwhelming burden of old wives’ tales it’s not worth mounting a rebuttal, do you pretend you didn’t hear the comment?
Or am I completely off base because your experience has left you convinced that despite the lack of supporting studies the “sugar high” phenomenon exists? Maybe you even include it on your list of explanations and remedies for pediatric misbehaviors. I am ready to listen, but it will take some heavy lifting to convince me.
I suspect your response to offhand comments about “sugar highs” is similar to mine. It depends on the situation. If I think there are obvious and correctable causes for the child’s misbehavior such as sleep deprivation or a mismatch between parental expectation and the child’s tolerance for a stimulating environment I will include in my parenting advice the comment, “Sugar highs probably don’t exist.”
On the other hand, if I’m tired and think my observation will fall on deaf ears I let the conversation drift. I worry that my silence will be interpreted as a confirmation of an old wives’ tale. What I really don’t want to do is perpetuate a myth that may prevent some children from getting the care they need.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I can dimly recall watching Queen Elizabeth’s coronation on a very small black and white television screen. Even in monochrome it was a riveting event. Recently, the Queen celebrated her Platinum Jubilee, marking her 70-year reign. Apparently it was a multiday event with all the trappings, floating above an undercurrent of scandal and intrigue. I had better things to do than I did as a 7-year-old entranced by the novelty of a neighbor’s television set.
But, it turns out that I had missed the opportunity to see live and in color a royal meltdown starring the Queen’s great-grandson, 4-year-old Prince Louis. Not to worry. It remains on video archives for our education and pleasure ad infinitum. His performance was no more dramatic than what you have seen numerous times in the checkout line of the grocery store. However, this meltdown was on the world stage in the front row of the royal box and performed in various venues on each day of a 4-day event.
As long as you weren’t his parents, Kate Middleton and Prince William, the meltdown had its moments of hilarity. Louis made full use of his youthful and plastic face, creating a wide variety of taunts and responses to his mother’s praiseworthy and understated attempts at regaining control. Of course, the British press and every armchair parent with a Twitter account had a field day contributing their explanations and advice.
For example, here’s the headline on an international news website that caught my eye: “Royal reveals why Prince Louis was so ‘mischievous’ during the Jubilee”. In the article, a fellow royal and former rugby star who was sitting directly behind the little Prince during one of his performances chalked up the 4-year-old’s behavior to a “sugar high” resulting from the ample supply of sweets available behind the royal box.
Nowhere in the article is there a question of whether the “sugar high” is a science-based phenomenon. In fact, the reporter assumes we all know it exists and writes that “parents across the globe can probably [read: definitely] relate.”
I’m curious: How do you respond when a parent in the office explains the child’s behavior as the result of a “sugar high”? Or when you’re at a cookout and someone makes a comment that makes it obvious that they believe that “sugar highs” are real? Do you immediately pause the conversation and launch into a short but tasteful observation that you know of no scientific studies that sugar can cause a high? Or, figuring that in the face of an overwhelming burden of old wives’ tales it’s not worth mounting a rebuttal, do you pretend you didn’t hear the comment?
Or am I completely off base because your experience has left you convinced that despite the lack of supporting studies the “sugar high” phenomenon exists? Maybe you even include it on your list of explanations and remedies for pediatric misbehaviors. I am ready to listen, but it will take some heavy lifting to convince me.
I suspect your response to offhand comments about “sugar highs” is similar to mine. It depends on the situation. If I think there are obvious and correctable causes for the child’s misbehavior such as sleep deprivation or a mismatch between parental expectation and the child’s tolerance for a stimulating environment I will include in my parenting advice the comment, “Sugar highs probably don’t exist.”
On the other hand, if I’m tired and think my observation will fall on deaf ears I let the conversation drift. I worry that my silence will be interpreted as a confirmation of an old wives’ tale. What I really don’t want to do is perpetuate a myth that may prevent some children from getting the care they need.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I can dimly recall watching Queen Elizabeth’s coronation on a very small black and white television screen. Even in monochrome it was a riveting event. Recently, the Queen celebrated her Platinum Jubilee, marking her 70-year reign. Apparently it was a multiday event with all the trappings, floating above an undercurrent of scandal and intrigue. I had better things to do than I did as a 7-year-old entranced by the novelty of a neighbor’s television set.
But, it turns out that I had missed the opportunity to see live and in color a royal meltdown starring the Queen’s great-grandson, 4-year-old Prince Louis. Not to worry. It remains on video archives for our education and pleasure ad infinitum. His performance was no more dramatic than what you have seen numerous times in the checkout line of the grocery store. However, this meltdown was on the world stage in the front row of the royal box and performed in various venues on each day of a 4-day event.
As long as you weren’t his parents, Kate Middleton and Prince William, the meltdown had its moments of hilarity. Louis made full use of his youthful and plastic face, creating a wide variety of taunts and responses to his mother’s praiseworthy and understated attempts at regaining control. Of course, the British press and every armchair parent with a Twitter account had a field day contributing their explanations and advice.
For example, here’s the headline on an international news website that caught my eye: “Royal reveals why Prince Louis was so ‘mischievous’ during the Jubilee”. In the article, a fellow royal and former rugby star who was sitting directly behind the little Prince during one of his performances chalked up the 4-year-old’s behavior to a “sugar high” resulting from the ample supply of sweets available behind the royal box.
Nowhere in the article is there a question of whether the “sugar high” is a science-based phenomenon. In fact, the reporter assumes we all know it exists and writes that “parents across the globe can probably [read: definitely] relate.”
I’m curious: How do you respond when a parent in the office explains the child’s behavior as the result of a “sugar high”? Or when you’re at a cookout and someone makes a comment that makes it obvious that they believe that “sugar highs” are real? Do you immediately pause the conversation and launch into a short but tasteful observation that you know of no scientific studies that sugar can cause a high? Or, figuring that in the face of an overwhelming burden of old wives’ tales it’s not worth mounting a rebuttal, do you pretend you didn’t hear the comment?
Or am I completely off base because your experience has left you convinced that despite the lack of supporting studies the “sugar high” phenomenon exists? Maybe you even include it on your list of explanations and remedies for pediatric misbehaviors. I am ready to listen, but it will take some heavy lifting to convince me.
I suspect your response to offhand comments about “sugar highs” is similar to mine. It depends on the situation. If I think there are obvious and correctable causes for the child’s misbehavior such as sleep deprivation or a mismatch between parental expectation and the child’s tolerance for a stimulating environment I will include in my parenting advice the comment, “Sugar highs probably don’t exist.”
On the other hand, if I’m tired and think my observation will fall on deaf ears I let the conversation drift. I worry that my silence will be interpreted as a confirmation of an old wives’ tale. What I really don’t want to do is perpetuate a myth that may prevent some children from getting the care they need.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Ob.gyns. on the day that Roe v. Wade was overturned
“I’m happy to contribute, but can you keep it anonymous? It’s a safety concern for me.”
On the day that the Supreme Court of the United States voted to strike down Roe v. Wade, I reached out to ob.gyn.s across the country, wanting to hear their reactions. My own response, like that of many doctors and women, was a visceral mix of anger, fear, and grief. I could only begin to imagine what the real experts on reproductive health care were going through.
When the first ob.gyn. responded to my request by expressing concerns around anonymity and personal safety, I was shocked – but I shouldn’t have been. For starters, there is already a storied history in this country of deadly attacks on abortion providers. David Gunn, MD; Barnett Slepian, MD; and George Tiller, MD, were all tragically murdered by antiabortion extremists. Then, there’s the existence of websites that keep logs of abortion providers and sometimes include photos, office contact information, or even home addresses.
The idea that any reproductive health care provider should have to think twice before offering their uniquely qualified opinion is profoundly disturbing, nearly as disturbing as the Supreme Court’s decision itself. But it’s more critical than ever for ob.gyn. voices to be amplified. This is the time for the healthcare community to rally around women’s health providers, to learn from them, to support them.
I asked ob.gyns. around the country to tell me what they were thinking and feeling on the day that Roe v. Wade was overturned. We agreed to keep the responses anonymous, given that several people expressed very understandable safety concerns.
Here’s what they had to say.
Tennessee ob.gyn.
“Today is an emotionally charged day for many people in this country, yet as I type this, with my ob.gyn. practice continuing around me, with my own almost 10-week pregnancy growing inside me, I feel quite blunted. I feel powerless to answer questions that are variations on ‘what next?’ or ‘how do we fight back?’ All I can think of is, I am so glad I do not have anyone on my schedule right now who does not want to be pregnant. But what will happen when that eventually changes? What about my colleagues who do have these patients on their schedules today? On a personal level, what if my prenatal genetic testing comes back abnormal? How can we so blatantly disregard a separation of church and state in this country? What ways will our government interfere with my practice next? My head is spinning, but I have to go see my next patient. She is a 25-year-old who is here to have an IUD placed, and that seems like the most important thing I can do today.”
South Carolina ob.gyn.
“I’m really scared. For my patients and for myself. I don’t know how to be a good ob.gyn. if my ability to offer safe and accessible abortion care is being threatened.”
Massachusetts ob.gyn.
“Livid and devastated and sad and terrified.”
California family planning specialist
“The fact is that about one in four people with uteruses have had an abortion. I can’t tell you how many abortions I’ve provided for people who say that they don’t ‘believe’ in them or that they thought they’d never be in this situation. ... The fact is that pregnancy is a life-threatening condition in and of itself. I am an ob.gyn., a medical doctor, and an abortion provider. I will not stop providing abortions or helping people access them. I will dedicate my life to ensuring this right to bodily autonomy. Today I am devastated by the Supreme Court’s decision to force parenthood that will result in increased maternal mortality. I am broken, but I have never been more proud to be an abortion provider.”
New York ob.gyn.
“Grateful to live in a state and work for a hospital where I can provide abortions but feel terrible for so many people less fortunate and underserved.”
Illinois maternal-fetal medicine specialist
“As a maternal-fetal medicine specialist, I fear for my patients who are at the highest risk of pregnancy complications having their freedom taken away. For the tragic ultrasound findings that make a pregnant person carry a baby who will never live. For the patients who cannot use most forms of contraception because of their medical comorbidities. For the patients who are victims of intimate partner violence or under the influence of their culture, to continue having children regardless of their desires or their health. ... The freedom to prevent or end a pregnancy has enabled women to become independent and productive members of society on their own terms, with or without children. My heart breaks for the children and adolescents and adults who are being told they are second-class citizens, not worthy of making their own decisions. Politicians and Supreme Court justices are not in the clinic room, ultrasound suite, operating room, or delivery room when we have these intense conversations and pregnancy outcomes. They have no idea that of which they speak, and it’s unconscionable that they can determine what healthcare decisions my patients can make for their own lives. Nobody knows a body better than the patient themselves.”
Texas ob.gyn.
“In the area where I live and practice, it feels like guns and the people who use them have more legal rights than people with uteruses in desperate or life-threatening situations. I’m afraid for my personal safety as a women’s health practitioner in this political climate. I feel helpless, but I’m supposed to be able to help my patients.”
Missouri family planning specialist
“Abortion is an essential part of healthcare, and the only people that should get a say in it are the patient and their doctor. Period. The fact that some far-off court without any medical expertise can insert itself into individual medical decisions is oppressive and unethical.”
Georgia ob.gyn.
“I can’t even think straight right now. I feel sick. Honestly, I’ve been thinking about moving for a long time now. Somewhere where I would actually be able to offer good, comprehensive care.”
New York ob.gyn.
“I graduated from my ob.gyn. residency hours after the Roe v. Wade news broke. It was so emotional for me. I’ve dedicated my life to caring for people with uteruses and I will not let this heartbreaking news change that. I feel more committed than ever to women’s health. I fully plan to continue delivering babies, providing contraception, and performing abortions. I will be there to help women with desired pregnancies who received unspeakably bad news about fetal anomalies. I will be there to help women with life-threatening pregnancy complications before fetal viability. I will be there to help women with ectopic pregnancies. I will be there to help women who were raped or otherwise forced into pregnancy. I will always be there to help women.”
Dr. Croll is a neurovascular fellow at New York University Langone Health. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
“I’m happy to contribute, but can you keep it anonymous? It’s a safety concern for me.”
On the day that the Supreme Court of the United States voted to strike down Roe v. Wade, I reached out to ob.gyn.s across the country, wanting to hear their reactions. My own response, like that of many doctors and women, was a visceral mix of anger, fear, and grief. I could only begin to imagine what the real experts on reproductive health care were going through.
When the first ob.gyn. responded to my request by expressing concerns around anonymity and personal safety, I was shocked – but I shouldn’t have been. For starters, there is already a storied history in this country of deadly attacks on abortion providers. David Gunn, MD; Barnett Slepian, MD; and George Tiller, MD, were all tragically murdered by antiabortion extremists. Then, there’s the existence of websites that keep logs of abortion providers and sometimes include photos, office contact information, or even home addresses.
The idea that any reproductive health care provider should have to think twice before offering their uniquely qualified opinion is profoundly disturbing, nearly as disturbing as the Supreme Court’s decision itself. But it’s more critical than ever for ob.gyn. voices to be amplified. This is the time for the healthcare community to rally around women’s health providers, to learn from them, to support them.
I asked ob.gyns. around the country to tell me what they were thinking and feeling on the day that Roe v. Wade was overturned. We agreed to keep the responses anonymous, given that several people expressed very understandable safety concerns.
Here’s what they had to say.
Tennessee ob.gyn.
“Today is an emotionally charged day for many people in this country, yet as I type this, with my ob.gyn. practice continuing around me, with my own almost 10-week pregnancy growing inside me, I feel quite blunted. I feel powerless to answer questions that are variations on ‘what next?’ or ‘how do we fight back?’ All I can think of is, I am so glad I do not have anyone on my schedule right now who does not want to be pregnant. But what will happen when that eventually changes? What about my colleagues who do have these patients on their schedules today? On a personal level, what if my prenatal genetic testing comes back abnormal? How can we so blatantly disregard a separation of church and state in this country? What ways will our government interfere with my practice next? My head is spinning, but I have to go see my next patient. She is a 25-year-old who is here to have an IUD placed, and that seems like the most important thing I can do today.”
South Carolina ob.gyn.
“I’m really scared. For my patients and for myself. I don’t know how to be a good ob.gyn. if my ability to offer safe and accessible abortion care is being threatened.”
Massachusetts ob.gyn.
“Livid and devastated and sad and terrified.”
California family planning specialist
“The fact is that about one in four people with uteruses have had an abortion. I can’t tell you how many abortions I’ve provided for people who say that they don’t ‘believe’ in them or that they thought they’d never be in this situation. ... The fact is that pregnancy is a life-threatening condition in and of itself. I am an ob.gyn., a medical doctor, and an abortion provider. I will not stop providing abortions or helping people access them. I will dedicate my life to ensuring this right to bodily autonomy. Today I am devastated by the Supreme Court’s decision to force parenthood that will result in increased maternal mortality. I am broken, but I have never been more proud to be an abortion provider.”
New York ob.gyn.
“Grateful to live in a state and work for a hospital where I can provide abortions but feel terrible for so many people less fortunate and underserved.”
Illinois maternal-fetal medicine specialist
“As a maternal-fetal medicine specialist, I fear for my patients who are at the highest risk of pregnancy complications having their freedom taken away. For the tragic ultrasound findings that make a pregnant person carry a baby who will never live. For the patients who cannot use most forms of contraception because of their medical comorbidities. For the patients who are victims of intimate partner violence or under the influence of their culture, to continue having children regardless of their desires or their health. ... The freedom to prevent or end a pregnancy has enabled women to become independent and productive members of society on their own terms, with or without children. My heart breaks for the children and adolescents and adults who are being told they are second-class citizens, not worthy of making their own decisions. Politicians and Supreme Court justices are not in the clinic room, ultrasound suite, operating room, or delivery room when we have these intense conversations and pregnancy outcomes. They have no idea that of which they speak, and it’s unconscionable that they can determine what healthcare decisions my patients can make for their own lives. Nobody knows a body better than the patient themselves.”
Texas ob.gyn.
“In the area where I live and practice, it feels like guns and the people who use them have more legal rights than people with uteruses in desperate or life-threatening situations. I’m afraid for my personal safety as a women’s health practitioner in this political climate. I feel helpless, but I’m supposed to be able to help my patients.”
Missouri family planning specialist
“Abortion is an essential part of healthcare, and the only people that should get a say in it are the patient and their doctor. Period. The fact that some far-off court without any medical expertise can insert itself into individual medical decisions is oppressive and unethical.”
Georgia ob.gyn.
“I can’t even think straight right now. I feel sick. Honestly, I’ve been thinking about moving for a long time now. Somewhere where I would actually be able to offer good, comprehensive care.”
New York ob.gyn.
“I graduated from my ob.gyn. residency hours after the Roe v. Wade news broke. It was so emotional for me. I’ve dedicated my life to caring for people with uteruses and I will not let this heartbreaking news change that. I feel more committed than ever to women’s health. I fully plan to continue delivering babies, providing contraception, and performing abortions. I will be there to help women with desired pregnancies who received unspeakably bad news about fetal anomalies. I will be there to help women with life-threatening pregnancy complications before fetal viability. I will be there to help women with ectopic pregnancies. I will be there to help women who were raped or otherwise forced into pregnancy. I will always be there to help women.”
Dr. Croll is a neurovascular fellow at New York University Langone Health. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
“I’m happy to contribute, but can you keep it anonymous? It’s a safety concern for me.”
On the day that the Supreme Court of the United States voted to strike down Roe v. Wade, I reached out to ob.gyn.s across the country, wanting to hear their reactions. My own response, like that of many doctors and women, was a visceral mix of anger, fear, and grief. I could only begin to imagine what the real experts on reproductive health care were going through.
When the first ob.gyn. responded to my request by expressing concerns around anonymity and personal safety, I was shocked – but I shouldn’t have been. For starters, there is already a storied history in this country of deadly attacks on abortion providers. David Gunn, MD; Barnett Slepian, MD; and George Tiller, MD, were all tragically murdered by antiabortion extremists. Then, there’s the existence of websites that keep logs of abortion providers and sometimes include photos, office contact information, or even home addresses.
The idea that any reproductive health care provider should have to think twice before offering their uniquely qualified opinion is profoundly disturbing, nearly as disturbing as the Supreme Court’s decision itself. But it’s more critical than ever for ob.gyn. voices to be amplified. This is the time for the healthcare community to rally around women’s health providers, to learn from them, to support them.
I asked ob.gyns. around the country to tell me what they were thinking and feeling on the day that Roe v. Wade was overturned. We agreed to keep the responses anonymous, given that several people expressed very understandable safety concerns.
Here’s what they had to say.
Tennessee ob.gyn.
“Today is an emotionally charged day for many people in this country, yet as I type this, with my ob.gyn. practice continuing around me, with my own almost 10-week pregnancy growing inside me, I feel quite blunted. I feel powerless to answer questions that are variations on ‘what next?’ or ‘how do we fight back?’ All I can think of is, I am so glad I do not have anyone on my schedule right now who does not want to be pregnant. But what will happen when that eventually changes? What about my colleagues who do have these patients on their schedules today? On a personal level, what if my prenatal genetic testing comes back abnormal? How can we so blatantly disregard a separation of church and state in this country? What ways will our government interfere with my practice next? My head is spinning, but I have to go see my next patient. She is a 25-year-old who is here to have an IUD placed, and that seems like the most important thing I can do today.”
South Carolina ob.gyn.
“I’m really scared. For my patients and for myself. I don’t know how to be a good ob.gyn. if my ability to offer safe and accessible abortion care is being threatened.”
Massachusetts ob.gyn.
“Livid and devastated and sad and terrified.”
California family planning specialist
“The fact is that about one in four people with uteruses have had an abortion. I can’t tell you how many abortions I’ve provided for people who say that they don’t ‘believe’ in them or that they thought they’d never be in this situation. ... The fact is that pregnancy is a life-threatening condition in and of itself. I am an ob.gyn., a medical doctor, and an abortion provider. I will not stop providing abortions or helping people access them. I will dedicate my life to ensuring this right to bodily autonomy. Today I am devastated by the Supreme Court’s decision to force parenthood that will result in increased maternal mortality. I am broken, but I have never been more proud to be an abortion provider.”
New York ob.gyn.
“Grateful to live in a state and work for a hospital where I can provide abortions but feel terrible for so many people less fortunate and underserved.”
Illinois maternal-fetal medicine specialist
“As a maternal-fetal medicine specialist, I fear for my patients who are at the highest risk of pregnancy complications having their freedom taken away. For the tragic ultrasound findings that make a pregnant person carry a baby who will never live. For the patients who cannot use most forms of contraception because of their medical comorbidities. For the patients who are victims of intimate partner violence or under the influence of their culture, to continue having children regardless of their desires or their health. ... The freedom to prevent or end a pregnancy has enabled women to become independent and productive members of society on their own terms, with or without children. My heart breaks for the children and adolescents and adults who are being told they are second-class citizens, not worthy of making their own decisions. Politicians and Supreme Court justices are not in the clinic room, ultrasound suite, operating room, or delivery room when we have these intense conversations and pregnancy outcomes. They have no idea that of which they speak, and it’s unconscionable that they can determine what healthcare decisions my patients can make for their own lives. Nobody knows a body better than the patient themselves.”
Texas ob.gyn.
“In the area where I live and practice, it feels like guns and the people who use them have more legal rights than people with uteruses in desperate or life-threatening situations. I’m afraid for my personal safety as a women’s health practitioner in this political climate. I feel helpless, but I’m supposed to be able to help my patients.”
Missouri family planning specialist
“Abortion is an essential part of healthcare, and the only people that should get a say in it are the patient and their doctor. Period. The fact that some far-off court without any medical expertise can insert itself into individual medical decisions is oppressive and unethical.”
Georgia ob.gyn.
“I can’t even think straight right now. I feel sick. Honestly, I’ve been thinking about moving for a long time now. Somewhere where I would actually be able to offer good, comprehensive care.”
New York ob.gyn.
“I graduated from my ob.gyn. residency hours after the Roe v. Wade news broke. It was so emotional for me. I’ve dedicated my life to caring for people with uteruses and I will not let this heartbreaking news change that. I feel more committed than ever to women’s health. I fully plan to continue delivering babies, providing contraception, and performing abortions. I will be there to help women with desired pregnancies who received unspeakably bad news about fetal anomalies. I will be there to help women with life-threatening pregnancy complications before fetal viability. I will be there to help women with ectopic pregnancies. I will be there to help women who were raped or otherwise forced into pregnancy. I will always be there to help women.”
Dr. Croll is a neurovascular fellow at New York University Langone Health. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
My picks for best of ASCO 2022
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
AT ASCO 2022
Adjuvant vs. neoadjuvant? What has ASCO 2022 taught us regarding resectable NSCLC?
for non–small cell lung cancer (NSCLC). While there has been some notable progress in this area, we need phase 3 trials that compare the two therapeutic approaches.
Investigators reporting at the 2022 annual meeting of American Society of Clinical Oncology focused primarily on neoadjuvant treatment, which I’ll address here.
In the randomized, phase 2 NADIM II clinical trial reported at the meeting, researchers expanded on the results of NADIM published in 2020 in the Lancet Oncology and in May 2022 in the Journal of Clinical Oncology along with CheckMate 816 results published in the New England Journal of Medicine.
In each of these three studies, researchers compared nivolumab plus chemotherapy versus chemotherapy alone (abstract 8501) as a neoadjuvant treatment for resectable stage IIIA NSCLC. In the study reported at ASCO 2022, patients with resectable clinical stage IIIA-B (per American Joint Committee on Cancer 8th edition) NSCLC and no known EGFR/ALK alterations, were randomized to receive preoperative nivolumab plus chemotherapy (paclitaxel and carboplatin; n = 57) or chemotherapy (n = 29) alone followed by surgery.
The primary endpoint was pathological complete response (pCR); secondary endpoints included major pathological response, safety and tolerability, impact on surgical issues such as delayed or canceled surgeries or length of hospital stay, overall survival and progression free survival. The pCR rate was 36.8% in the neoadjuvant nivolumab plus chemotherapy arm and 6.9% in the chemotherapy alone arm. (P = .0068). 25% of patients on the nivolumab plus chemo arm had grade 3-4 adverse events, compared with 10.3% in the control arm. 93% of patients on the nivolumab plus chemo arm underwent definitive surgery whereas 69.0% of the patients on the chemo alone arm had definitive surgery. (P = .008)
What else did we learn about neoadjuvant treatment at the meeting?
Investigators looking at the optimal number of neoadjuvant cycles (abstract 8500) found that three cycles of sintilimab (an investigational PD-1 inhibitor) produced a numerically higher major pathological response rate, compared with two cycles (when given in concert with platinum-doublet chemotherapy). And, neoadjuvant chemoradiotherapy does not result in significant survival benefits when compared with neoadjuvant chemotherapy alone (abstract 8503).
Of course, when it comes to resectable NSCLC, the goal of treatment is to increase the cure rate and improve survival. No randomized studies have reported yet on overall survival, probably because they are too immature. Instead, disease-free survival (DFS) or event-free survival (EFS) are often used as surrogate endpoints. Since none of the studies reported at ASCO reported on DFS or EFS, we need to look elsewhere. CheckMate 816 was a phase 3 study which randomized patients with stages IB-IIIA NSCLC to receive neoadjuvant nivolumab plus platinum-based chemotherapy or neoadjuvant platinum-based chemotherapy alone, followed by resection. The median EFS was 31.6 months with nivolumab plus chemotherapy and 20.8 months with chemotherapy alone (P = .005). The percentage of patients with a pCR was 24.0% and 2.2%, respectively (P < .001).
We all know one has to be careful when doing cross-trial comparisons as these studies differ by the percentage of patients with various stages of disease, the type of immunotherapy and chemotherapy used, etc. However, I think we can agree that neoadjuvant chemoimmunotherapy results in better outcomes than chemotherapy alone.
Of course, resectable NSCLC is, by definition, resectable. And traditionally, resection is followed by adjuvant chemotherapy to eradicate micrometastases. Unfortunately, the current standard of care for completely resected early-stage NSCLC (stage I [tumor ≥ 4 cm] to IIIA) involves adjuvant platinum-based combination chemotherapy which results in only a modest 4%-5% improvement in survival versus observation.
Given these modest results, as in the neoadjuvant space, investigators have looked at the benefit of adding immunotherapy to adjuvant chemotherapy. One such study has been reported. IMpower 010 randomly assigned patients with completely resected stage IB (tumors ≥ 4 cm) to IIIA NSCLC, whose tumor cells expressed at least 1% PD-L1, to receive adjuvant atezolizumab or best supportive care after adjuvant platinum-based chemotherapy. In the stage II-IIIA population whose tumors expressed PD-L1 on 1% or more of tumor cells, 3-year DFS rates were 60% and 48% in the atezolizumab and best supportive care arms, respectively (hazard ratio, 0·66 P =·.0039). In all patients in the stage II-IIIA population, the 3-year DFS rates were 56% in the atezolizumab group and 49% in the best supportive care group, (HR, 0.79; P = .020).
KEYNOTE-091, reported at the 2021 annual meeting of the European Society for Medical Oncology, randomized early-stage NSCLC patients following complete resection and adjuvant chemotherapy to pembrolizumab or placebo. Median DFS for the overall population was 53.6 months for patients in the pembro arm versus 42 months in the placebo arm (HR, 0.76; P = .0014). Interestingly, the benefit was not seen in patients with PD-L1 with at least 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm (HR, 0.82; P = .14). Although the contradictory results of PD-L1 as a biomarker is puzzling, I think we can agree that the addition of immunotherapy following adjuvant chemotherapy improves outcomes compared to adjuvant chemotherapy alone.
What to do when a patient presents with resectable disease?
Cross-trial comparisons are fraught with danger. Until we have a phase 3 study comparing concurrent neoadjuvant chemo/immunotherapy with concurrent adjuvant chemo/immunotherapy, I do not think we can answer the question “which is better?” However, there are some caveats to keep in mind when deciding on which approach to recommend to our patients: First, neoadjuvant treatment requires biomarker testing to ensure the patient does not have EGFR or ALK mutations. This will necessitate a delay in the operation. Will patients be willing to wait? Will the surgeon? Or, would patients prefer to proceed with surgery while the results are pending? Yes, neoadjuvant therapy gives you information regarding the pCR rate, but does that help you in subsequent management of the patient? We do not know.
Secondly, the two adjuvant studies used adjuvant chemotherapy followed by adjuvant immunotherapy, as contrasted to the neoadjuvant study which used concurrent chemo/immunotherapy. Given the longer duration of treatment in postoperative sequential adjuvant studies, there tends to be more drop off because of patients being unwilling or unfit postoperatively to receive long courses of therapy. In IMpower 010, 1,269 patients completed adjuvant chemotherapy; 1,005 were randomized, and of the 507 assigned to the atezolizumab/chemo group, only 323 completed treatment.
Finally, we must beware of using neoadjuvant chemo/immunotherapy to “down-stage” a patient. KEYNOTE-091 included patients with IIIA disease and no benefit to adjuvant chemotherapy followed by immunotherapy was found in this subgroup of patients, which leads me to wonder if these patients were appropriately selected as surgical candidates. In the NADIM II trials, 9 of 29 patients on the neoadjuvant chemotherapy were not resected.
So, many questions remain. In addition to the ones we’ve raised, there is a clear and immediate need for predictive and prognostic biomarkers. In the NADIM II trial, PD-L1 expression was a predictive biomarker of response. The pCR rate for patients with a PD-L1 tumor expression of less than 1%, 1%-49%, and 50% or higher was 15%, 41.7%, and 61.1%, respectively. However, in KEYNOTE-091, the benefit was not seen in patients with PD-L1 of at least than 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm.
Another possible biomarker: circulating tumor DNA. In the first NADIM study, three low pretreatment levels of ctDNA were significantly associated with improved progression-free survival and overall survival (HR, 0.20 and HR, 0.07, respectively). Although clinical response did not predict survival outcomes, undetectable ctDNA levels after neoadjuvant treatment were significantly associated with progression-free survival and overall survival (HR, 0.26 and HR0.04, respectively). Similarly, in CheckMate 816, clearance of ctDNA was associated with longer EFS in patients with ctDNA clearance than in those without ctDNA clearance in both the nivolumab/chemotherapy group (HR, 0.60) and the chemotherapy-alone group (HR, 0.63).
Hopefully, ASCO 2023 will provide more answers.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
for non–small cell lung cancer (NSCLC). While there has been some notable progress in this area, we need phase 3 trials that compare the two therapeutic approaches.
Investigators reporting at the 2022 annual meeting of American Society of Clinical Oncology focused primarily on neoadjuvant treatment, which I’ll address here.
In the randomized, phase 2 NADIM II clinical trial reported at the meeting, researchers expanded on the results of NADIM published in 2020 in the Lancet Oncology and in May 2022 in the Journal of Clinical Oncology along with CheckMate 816 results published in the New England Journal of Medicine.
In each of these three studies, researchers compared nivolumab plus chemotherapy versus chemotherapy alone (abstract 8501) as a neoadjuvant treatment for resectable stage IIIA NSCLC. In the study reported at ASCO 2022, patients with resectable clinical stage IIIA-B (per American Joint Committee on Cancer 8th edition) NSCLC and no known EGFR/ALK alterations, were randomized to receive preoperative nivolumab plus chemotherapy (paclitaxel and carboplatin; n = 57) or chemotherapy (n = 29) alone followed by surgery.
The primary endpoint was pathological complete response (pCR); secondary endpoints included major pathological response, safety and tolerability, impact on surgical issues such as delayed or canceled surgeries or length of hospital stay, overall survival and progression free survival. The pCR rate was 36.8% in the neoadjuvant nivolumab plus chemotherapy arm and 6.9% in the chemotherapy alone arm. (P = .0068). 25% of patients on the nivolumab plus chemo arm had grade 3-4 adverse events, compared with 10.3% in the control arm. 93% of patients on the nivolumab plus chemo arm underwent definitive surgery whereas 69.0% of the patients on the chemo alone arm had definitive surgery. (P = .008)
What else did we learn about neoadjuvant treatment at the meeting?
Investigators looking at the optimal number of neoadjuvant cycles (abstract 8500) found that three cycles of sintilimab (an investigational PD-1 inhibitor) produced a numerically higher major pathological response rate, compared with two cycles (when given in concert with platinum-doublet chemotherapy). And, neoadjuvant chemoradiotherapy does not result in significant survival benefits when compared with neoadjuvant chemotherapy alone (abstract 8503).
Of course, when it comes to resectable NSCLC, the goal of treatment is to increase the cure rate and improve survival. No randomized studies have reported yet on overall survival, probably because they are too immature. Instead, disease-free survival (DFS) or event-free survival (EFS) are often used as surrogate endpoints. Since none of the studies reported at ASCO reported on DFS or EFS, we need to look elsewhere. CheckMate 816 was a phase 3 study which randomized patients with stages IB-IIIA NSCLC to receive neoadjuvant nivolumab plus platinum-based chemotherapy or neoadjuvant platinum-based chemotherapy alone, followed by resection. The median EFS was 31.6 months with nivolumab plus chemotherapy and 20.8 months with chemotherapy alone (P = .005). The percentage of patients with a pCR was 24.0% and 2.2%, respectively (P < .001).
We all know one has to be careful when doing cross-trial comparisons as these studies differ by the percentage of patients with various stages of disease, the type of immunotherapy and chemotherapy used, etc. However, I think we can agree that neoadjuvant chemoimmunotherapy results in better outcomes than chemotherapy alone.
Of course, resectable NSCLC is, by definition, resectable. And traditionally, resection is followed by adjuvant chemotherapy to eradicate micrometastases. Unfortunately, the current standard of care for completely resected early-stage NSCLC (stage I [tumor ≥ 4 cm] to IIIA) involves adjuvant platinum-based combination chemotherapy which results in only a modest 4%-5% improvement in survival versus observation.
Given these modest results, as in the neoadjuvant space, investigators have looked at the benefit of adding immunotherapy to adjuvant chemotherapy. One such study has been reported. IMpower 010 randomly assigned patients with completely resected stage IB (tumors ≥ 4 cm) to IIIA NSCLC, whose tumor cells expressed at least 1% PD-L1, to receive adjuvant atezolizumab or best supportive care after adjuvant platinum-based chemotherapy. In the stage II-IIIA population whose tumors expressed PD-L1 on 1% or more of tumor cells, 3-year DFS rates were 60% and 48% in the atezolizumab and best supportive care arms, respectively (hazard ratio, 0·66 P =·.0039). In all patients in the stage II-IIIA population, the 3-year DFS rates were 56% in the atezolizumab group and 49% in the best supportive care group, (HR, 0.79; P = .020).
KEYNOTE-091, reported at the 2021 annual meeting of the European Society for Medical Oncology, randomized early-stage NSCLC patients following complete resection and adjuvant chemotherapy to pembrolizumab or placebo. Median DFS for the overall population was 53.6 months for patients in the pembro arm versus 42 months in the placebo arm (HR, 0.76; P = .0014). Interestingly, the benefit was not seen in patients with PD-L1 with at least 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm (HR, 0.82; P = .14). Although the contradictory results of PD-L1 as a biomarker is puzzling, I think we can agree that the addition of immunotherapy following adjuvant chemotherapy improves outcomes compared to adjuvant chemotherapy alone.
What to do when a patient presents with resectable disease?
Cross-trial comparisons are fraught with danger. Until we have a phase 3 study comparing concurrent neoadjuvant chemo/immunotherapy with concurrent adjuvant chemo/immunotherapy, I do not think we can answer the question “which is better?” However, there are some caveats to keep in mind when deciding on which approach to recommend to our patients: First, neoadjuvant treatment requires biomarker testing to ensure the patient does not have EGFR or ALK mutations. This will necessitate a delay in the operation. Will patients be willing to wait? Will the surgeon? Or, would patients prefer to proceed with surgery while the results are pending? Yes, neoadjuvant therapy gives you information regarding the pCR rate, but does that help you in subsequent management of the patient? We do not know.
Secondly, the two adjuvant studies used adjuvant chemotherapy followed by adjuvant immunotherapy, as contrasted to the neoadjuvant study which used concurrent chemo/immunotherapy. Given the longer duration of treatment in postoperative sequential adjuvant studies, there tends to be more drop off because of patients being unwilling or unfit postoperatively to receive long courses of therapy. In IMpower 010, 1,269 patients completed adjuvant chemotherapy; 1,005 were randomized, and of the 507 assigned to the atezolizumab/chemo group, only 323 completed treatment.
Finally, we must beware of using neoadjuvant chemo/immunotherapy to “down-stage” a patient. KEYNOTE-091 included patients with IIIA disease and no benefit to adjuvant chemotherapy followed by immunotherapy was found in this subgroup of patients, which leads me to wonder if these patients were appropriately selected as surgical candidates. In the NADIM II trials, 9 of 29 patients on the neoadjuvant chemotherapy were not resected.
So, many questions remain. In addition to the ones we’ve raised, there is a clear and immediate need for predictive and prognostic biomarkers. In the NADIM II trial, PD-L1 expression was a predictive biomarker of response. The pCR rate for patients with a PD-L1 tumor expression of less than 1%, 1%-49%, and 50% or higher was 15%, 41.7%, and 61.1%, respectively. However, in KEYNOTE-091, the benefit was not seen in patients with PD-L1 of at least than 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm.
Another possible biomarker: circulating tumor DNA. In the first NADIM study, three low pretreatment levels of ctDNA were significantly associated with improved progression-free survival and overall survival (HR, 0.20 and HR, 0.07, respectively). Although clinical response did not predict survival outcomes, undetectable ctDNA levels after neoadjuvant treatment were significantly associated with progression-free survival and overall survival (HR, 0.26 and HR0.04, respectively). Similarly, in CheckMate 816, clearance of ctDNA was associated with longer EFS in patients with ctDNA clearance than in those without ctDNA clearance in both the nivolumab/chemotherapy group (HR, 0.60) and the chemotherapy-alone group (HR, 0.63).
Hopefully, ASCO 2023 will provide more answers.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
for non–small cell lung cancer (NSCLC). While there has been some notable progress in this area, we need phase 3 trials that compare the two therapeutic approaches.
Investigators reporting at the 2022 annual meeting of American Society of Clinical Oncology focused primarily on neoadjuvant treatment, which I’ll address here.
In the randomized, phase 2 NADIM II clinical trial reported at the meeting, researchers expanded on the results of NADIM published in 2020 in the Lancet Oncology and in May 2022 in the Journal of Clinical Oncology along with CheckMate 816 results published in the New England Journal of Medicine.
In each of these three studies, researchers compared nivolumab plus chemotherapy versus chemotherapy alone (abstract 8501) as a neoadjuvant treatment for resectable stage IIIA NSCLC. In the study reported at ASCO 2022, patients with resectable clinical stage IIIA-B (per American Joint Committee on Cancer 8th edition) NSCLC and no known EGFR/ALK alterations, were randomized to receive preoperative nivolumab plus chemotherapy (paclitaxel and carboplatin; n = 57) or chemotherapy (n = 29) alone followed by surgery.
The primary endpoint was pathological complete response (pCR); secondary endpoints included major pathological response, safety and tolerability, impact on surgical issues such as delayed or canceled surgeries or length of hospital stay, overall survival and progression free survival. The pCR rate was 36.8% in the neoadjuvant nivolumab plus chemotherapy arm and 6.9% in the chemotherapy alone arm. (P = .0068). 25% of patients on the nivolumab plus chemo arm had grade 3-4 adverse events, compared with 10.3% in the control arm. 93% of patients on the nivolumab plus chemo arm underwent definitive surgery whereas 69.0% of the patients on the chemo alone arm had definitive surgery. (P = .008)
What else did we learn about neoadjuvant treatment at the meeting?
Investigators looking at the optimal number of neoadjuvant cycles (abstract 8500) found that three cycles of sintilimab (an investigational PD-1 inhibitor) produced a numerically higher major pathological response rate, compared with two cycles (when given in concert with platinum-doublet chemotherapy). And, neoadjuvant chemoradiotherapy does not result in significant survival benefits when compared with neoadjuvant chemotherapy alone (abstract 8503).
Of course, when it comes to resectable NSCLC, the goal of treatment is to increase the cure rate and improve survival. No randomized studies have reported yet on overall survival, probably because they are too immature. Instead, disease-free survival (DFS) or event-free survival (EFS) are often used as surrogate endpoints. Since none of the studies reported at ASCO reported on DFS or EFS, we need to look elsewhere. CheckMate 816 was a phase 3 study which randomized patients with stages IB-IIIA NSCLC to receive neoadjuvant nivolumab plus platinum-based chemotherapy or neoadjuvant platinum-based chemotherapy alone, followed by resection. The median EFS was 31.6 months with nivolumab plus chemotherapy and 20.8 months with chemotherapy alone (P = .005). The percentage of patients with a pCR was 24.0% and 2.2%, respectively (P < .001).
We all know one has to be careful when doing cross-trial comparisons as these studies differ by the percentage of patients with various stages of disease, the type of immunotherapy and chemotherapy used, etc. However, I think we can agree that neoadjuvant chemoimmunotherapy results in better outcomes than chemotherapy alone.
Of course, resectable NSCLC is, by definition, resectable. And traditionally, resection is followed by adjuvant chemotherapy to eradicate micrometastases. Unfortunately, the current standard of care for completely resected early-stage NSCLC (stage I [tumor ≥ 4 cm] to IIIA) involves adjuvant platinum-based combination chemotherapy which results in only a modest 4%-5% improvement in survival versus observation.
Given these modest results, as in the neoadjuvant space, investigators have looked at the benefit of adding immunotherapy to adjuvant chemotherapy. One such study has been reported. IMpower 010 randomly assigned patients with completely resected stage IB (tumors ≥ 4 cm) to IIIA NSCLC, whose tumor cells expressed at least 1% PD-L1, to receive adjuvant atezolizumab or best supportive care after adjuvant platinum-based chemotherapy. In the stage II-IIIA population whose tumors expressed PD-L1 on 1% or more of tumor cells, 3-year DFS rates were 60% and 48% in the atezolizumab and best supportive care arms, respectively (hazard ratio, 0·66 P =·.0039). In all patients in the stage II-IIIA population, the 3-year DFS rates were 56% in the atezolizumab group and 49% in the best supportive care group, (HR, 0.79; P = .020).
KEYNOTE-091, reported at the 2021 annual meeting of the European Society for Medical Oncology, randomized early-stage NSCLC patients following complete resection and adjuvant chemotherapy to pembrolizumab or placebo. Median DFS for the overall population was 53.6 months for patients in the pembro arm versus 42 months in the placebo arm (HR, 0.76; P = .0014). Interestingly, the benefit was not seen in patients with PD-L1 with at least 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm (HR, 0.82; P = .14). Although the contradictory results of PD-L1 as a biomarker is puzzling, I think we can agree that the addition of immunotherapy following adjuvant chemotherapy improves outcomes compared to adjuvant chemotherapy alone.
What to do when a patient presents with resectable disease?
Cross-trial comparisons are fraught with danger. Until we have a phase 3 study comparing concurrent neoadjuvant chemo/immunotherapy with concurrent adjuvant chemo/immunotherapy, I do not think we can answer the question “which is better?” However, there are some caveats to keep in mind when deciding on which approach to recommend to our patients: First, neoadjuvant treatment requires biomarker testing to ensure the patient does not have EGFR or ALK mutations. This will necessitate a delay in the operation. Will patients be willing to wait? Will the surgeon? Or, would patients prefer to proceed with surgery while the results are pending? Yes, neoadjuvant therapy gives you information regarding the pCR rate, but does that help you in subsequent management of the patient? We do not know.
Secondly, the two adjuvant studies used adjuvant chemotherapy followed by adjuvant immunotherapy, as contrasted to the neoadjuvant study which used concurrent chemo/immunotherapy. Given the longer duration of treatment in postoperative sequential adjuvant studies, there tends to be more drop off because of patients being unwilling or unfit postoperatively to receive long courses of therapy. In IMpower 010, 1,269 patients completed adjuvant chemotherapy; 1,005 were randomized, and of the 507 assigned to the atezolizumab/chemo group, only 323 completed treatment.
Finally, we must beware of using neoadjuvant chemo/immunotherapy to “down-stage” a patient. KEYNOTE-091 included patients with IIIA disease and no benefit to adjuvant chemotherapy followed by immunotherapy was found in this subgroup of patients, which leads me to wonder if these patients were appropriately selected as surgical candidates. In the NADIM II trials, 9 of 29 patients on the neoadjuvant chemotherapy were not resected.
So, many questions remain. In addition to the ones we’ve raised, there is a clear and immediate need for predictive and prognostic biomarkers. In the NADIM II trial, PD-L1 expression was a predictive biomarker of response. The pCR rate for patients with a PD-L1 tumor expression of less than 1%, 1%-49%, and 50% or higher was 15%, 41.7%, and 61.1%, respectively. However, in KEYNOTE-091, the benefit was not seen in patients with PD-L1 of at least than 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm.
Another possible biomarker: circulating tumor DNA. In the first NADIM study, three low pretreatment levels of ctDNA were significantly associated with improved progression-free survival and overall survival (HR, 0.20 and HR, 0.07, respectively). Although clinical response did not predict survival outcomes, undetectable ctDNA levels after neoadjuvant treatment were significantly associated with progression-free survival and overall survival (HR, 0.26 and HR0.04, respectively). Similarly, in CheckMate 816, clearance of ctDNA was associated with longer EFS in patients with ctDNA clearance than in those without ctDNA clearance in both the nivolumab/chemotherapy group (HR, 0.60) and the chemotherapy-alone group (HR, 0.63).
Hopefully, ASCO 2023 will provide more answers.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.