User login
How to integrate mental health care into primary care
During my training as a child and adolescent psychiatry fellow, I “lived” down the hall from 10 other people just like me who had similar offices and training. Our pace was tailored to pediatric psychiatry. Appointments were 30 minutes or more. Our goal was to provide the most comprehensive mental health care for the families whom we grew to know and love.
The impetus to create an integrated mental health care approach has been well elucidated by the American Academy of Child and Adolescent Psychiatry (AACAP) in its report, Collaborative mental health care in pediatric primary care. It is based on some telling statistics: Fifty percent of all cases of mental illness begin before age 14 years and 75% begin by age 24. Half of all pediatric office visits involve behavioral, psychosocial, or educational concerns. The American Academy of Pediatrics’ Task Force on Mental Health similarly has stated that primary care clinicians can and should be able to provide mental health services to children and adolescents in a primary care setting.
Integrative psychiatry and primary care treatment comes in three forms: classic consultation, in which a specialist sees a patient and refers back to the PCP with recommendations; colocation, in which mental health specialists practice in the same office but essentially are “ships crossing in the night” with PCPs; and the most-lauded form, collaborative/integrative care, in which back-and-forth consultation and discussions of a case occur between mental health specialists and PCPs, with in-person follow-up as needed.
Several institutions offer programs to address the AACAP and AAP imperatives, most prominently the University of Washington, Seattle, and the University of Massachusetts, Worcester. Both offer resources on how to create an integrated care model (University of Washington AIMS Resource Center; The University of Massachusetts Center for Integrated Care).
What can one do in a busy pediatric primary care practice to address mental health imperatives on the individual provider level? Often PCPs can, as I do, offer families some resources by having a set of mental health handouts and resources. I have gathered useful handouts for families throughout my residency to use as shortcuts and visual aids to promote mental health. I use the AACAP Facts for Families for handouts on mental health diagnoses and topics. I use the National Sleep Foundation for its sleep hygiene tips. I also offer some low-cost mindfulness resources to help kids and parents with their anxiety, such as the Calm app and Headspace app. If parents have difficulty with access to parent management training (the first-line treatment to manage aggression in children), I often recommend “The Defiant Child: A Parent’s Guide to Oppositional Defiant Disorder” (Lanham, Md.: Taylor Trade Publishing, 1997), which shows how to create a rewards system in the home to promote positive behavior. “How to Talk So Kids Will Listen & Listen So Kids Will Talk” (New York: Scribner, 2012 ) is a beloved book for parents (and there is a teenager version) that I recommend when parents launch into questions about how to talk to kids and teens about difficult topics so that, ultimately, they can improve their relationship.
Dr. Pawlowski is an adult, adolescent, and child psychiatrist at the University of Vermont Medical Center and an assistant professor of psychiatry at UVM, both in Burlington. Email her at [email protected].
Resources
The AACAP website has materials to help clinicians develop a collaborative mental health care model in the primary care setting: Search for “collaboration with primary care.”
The journal Pediatrics also has a useful resource: Improving mental health services in primary care: Reducing administrative and financial barriers to access and collaboration. (2009;123;1248-51).
During my training as a child and adolescent psychiatry fellow, I “lived” down the hall from 10 other people just like me who had similar offices and training. Our pace was tailored to pediatric psychiatry. Appointments were 30 minutes or more. Our goal was to provide the most comprehensive mental health care for the families whom we grew to know and love.
The impetus to create an integrated mental health care approach has been well elucidated by the American Academy of Child and Adolescent Psychiatry (AACAP) in its report, Collaborative mental health care in pediatric primary care. It is based on some telling statistics: Fifty percent of all cases of mental illness begin before age 14 years and 75% begin by age 24. Half of all pediatric office visits involve behavioral, psychosocial, or educational concerns. The American Academy of Pediatrics’ Task Force on Mental Health similarly has stated that primary care clinicians can and should be able to provide mental health services to children and adolescents in a primary care setting.
Integrative psychiatry and primary care treatment comes in three forms: classic consultation, in which a specialist sees a patient and refers back to the PCP with recommendations; colocation, in which mental health specialists practice in the same office but essentially are “ships crossing in the night” with PCPs; and the most-lauded form, collaborative/integrative care, in which back-and-forth consultation and discussions of a case occur between mental health specialists and PCPs, with in-person follow-up as needed.
Several institutions offer programs to address the AACAP and AAP imperatives, most prominently the University of Washington, Seattle, and the University of Massachusetts, Worcester. Both offer resources on how to create an integrated care model (University of Washington AIMS Resource Center; The University of Massachusetts Center for Integrated Care).
What can one do in a busy pediatric primary care practice to address mental health imperatives on the individual provider level? Often PCPs can, as I do, offer families some resources by having a set of mental health handouts and resources. I have gathered useful handouts for families throughout my residency to use as shortcuts and visual aids to promote mental health. I use the AACAP Facts for Families for handouts on mental health diagnoses and topics. I use the National Sleep Foundation for its sleep hygiene tips. I also offer some low-cost mindfulness resources to help kids and parents with their anxiety, such as the Calm app and Headspace app. If parents have difficulty with access to parent management training (the first-line treatment to manage aggression in children), I often recommend “The Defiant Child: A Parent’s Guide to Oppositional Defiant Disorder” (Lanham, Md.: Taylor Trade Publishing, 1997), which shows how to create a rewards system in the home to promote positive behavior. “How to Talk So Kids Will Listen & Listen So Kids Will Talk” (New York: Scribner, 2012 ) is a beloved book for parents (and there is a teenager version) that I recommend when parents launch into questions about how to talk to kids and teens about difficult topics so that, ultimately, they can improve their relationship.
Dr. Pawlowski is an adult, adolescent, and child psychiatrist at the University of Vermont Medical Center and an assistant professor of psychiatry at UVM, both in Burlington. Email her at [email protected].
Resources
The AACAP website has materials to help clinicians develop a collaborative mental health care model in the primary care setting: Search for “collaboration with primary care.”
The journal Pediatrics also has a useful resource: Improving mental health services in primary care: Reducing administrative and financial barriers to access and collaboration. (2009;123;1248-51).
During my training as a child and adolescent psychiatry fellow, I “lived” down the hall from 10 other people just like me who had similar offices and training. Our pace was tailored to pediatric psychiatry. Appointments were 30 minutes or more. Our goal was to provide the most comprehensive mental health care for the families whom we grew to know and love.
The impetus to create an integrated mental health care approach has been well elucidated by the American Academy of Child and Adolescent Psychiatry (AACAP) in its report, Collaborative mental health care in pediatric primary care. It is based on some telling statistics: Fifty percent of all cases of mental illness begin before age 14 years and 75% begin by age 24. Half of all pediatric office visits involve behavioral, psychosocial, or educational concerns. The American Academy of Pediatrics’ Task Force on Mental Health similarly has stated that primary care clinicians can and should be able to provide mental health services to children and adolescents in a primary care setting.
Integrative psychiatry and primary care treatment comes in three forms: classic consultation, in which a specialist sees a patient and refers back to the PCP with recommendations; colocation, in which mental health specialists practice in the same office but essentially are “ships crossing in the night” with PCPs; and the most-lauded form, collaborative/integrative care, in which back-and-forth consultation and discussions of a case occur between mental health specialists and PCPs, with in-person follow-up as needed.
Several institutions offer programs to address the AACAP and AAP imperatives, most prominently the University of Washington, Seattle, and the University of Massachusetts, Worcester. Both offer resources on how to create an integrated care model (University of Washington AIMS Resource Center; The University of Massachusetts Center for Integrated Care).
What can one do in a busy pediatric primary care practice to address mental health imperatives on the individual provider level? Often PCPs can, as I do, offer families some resources by having a set of mental health handouts and resources. I have gathered useful handouts for families throughout my residency to use as shortcuts and visual aids to promote mental health. I use the AACAP Facts for Families for handouts on mental health diagnoses and topics. I use the National Sleep Foundation for its sleep hygiene tips. I also offer some low-cost mindfulness resources to help kids and parents with their anxiety, such as the Calm app and Headspace app. If parents have difficulty with access to parent management training (the first-line treatment to manage aggression in children), I often recommend “The Defiant Child: A Parent’s Guide to Oppositional Defiant Disorder” (Lanham, Md.: Taylor Trade Publishing, 1997), which shows how to create a rewards system in the home to promote positive behavior. “How to Talk So Kids Will Listen & Listen So Kids Will Talk” (New York: Scribner, 2012 ) is a beloved book for parents (and there is a teenager version) that I recommend when parents launch into questions about how to talk to kids and teens about difficult topics so that, ultimately, they can improve their relationship.
Dr. Pawlowski is an adult, adolescent, and child psychiatrist at the University of Vermont Medical Center and an assistant professor of psychiatry at UVM, both in Burlington. Email her at [email protected].
Resources
The AACAP website has materials to help clinicians develop a collaborative mental health care model in the primary care setting: Search for “collaboration with primary care.”
The journal Pediatrics also has a useful resource: Improving mental health services in primary care: Reducing administrative and financial barriers to access and collaboration. (2009;123;1248-51).
Commentary—Finding Important Nutrients in Unexpected Places
While you might not typically put chopped or blended, unsalted, boiled canned oysters on your usual list of recommended infant and toddler foods, maybe you should.
The American Academy of Pediatrics just published a new policy statement on advocacy to improve child nutrition in the first 1,000 days (from conception to age 2). The statement emphasizes the importance of nutrition to optimal brain development. Pediatricians are encouraged to be familiar with community services to support optimal nutrition such as the Special Supplemental Nutrition Program for Women, Infants, and Children, the Supplemental Nutrition Assistance Program, the Child and Adult Care Food Program, and food pantries and soup kitchens, but also to get beyond recommending a “good diet” to something more specific that is high in key nutrients important for brain development such as protein; zinc; iron; choline; folate; iodine; vitamins A, D, B6, and B12; and polyunsaturated fatty acids. That’s where the boiled oysters, a decent source of the listed nutrients and especially loaded with zinc, iron, and vitamin B12, come in. While not everyone is going to rush out to buy their babies such an unexpected (and for many, unfamiliar) food, the statement reminds pediatricians to recommend foods that are good sources of the nutrients that babies and toddlers need most. Other foods that fit the bill include oatmeal, meat and poultry, fish like salmon and tuna, eggs, tofu and soybeans, and other legumes and beans like chickpeas and lentils.
—Natalie D. Muth, MD
Pediatrician
Children's Primary Care Medical Group
Carlsbad, California
While you might not typically put chopped or blended, unsalted, boiled canned oysters on your usual list of recommended infant and toddler foods, maybe you should.
The American Academy of Pediatrics just published a new policy statement on advocacy to improve child nutrition in the first 1,000 days (from conception to age 2). The statement emphasizes the importance of nutrition to optimal brain development. Pediatricians are encouraged to be familiar with community services to support optimal nutrition such as the Special Supplemental Nutrition Program for Women, Infants, and Children, the Supplemental Nutrition Assistance Program, the Child and Adult Care Food Program, and food pantries and soup kitchens, but also to get beyond recommending a “good diet” to something more specific that is high in key nutrients important for brain development such as protein; zinc; iron; choline; folate; iodine; vitamins A, D, B6, and B12; and polyunsaturated fatty acids. That’s where the boiled oysters, a decent source of the listed nutrients and especially loaded with zinc, iron, and vitamin B12, come in. While not everyone is going to rush out to buy their babies such an unexpected (and for many, unfamiliar) food, the statement reminds pediatricians to recommend foods that are good sources of the nutrients that babies and toddlers need most. Other foods that fit the bill include oatmeal, meat and poultry, fish like salmon and tuna, eggs, tofu and soybeans, and other legumes and beans like chickpeas and lentils.
—Natalie D. Muth, MD
Pediatrician
Children's Primary Care Medical Group
Carlsbad, California
While you might not typically put chopped or blended, unsalted, boiled canned oysters on your usual list of recommended infant and toddler foods, maybe you should.
The American Academy of Pediatrics just published a new policy statement on advocacy to improve child nutrition in the first 1,000 days (from conception to age 2). The statement emphasizes the importance of nutrition to optimal brain development. Pediatricians are encouraged to be familiar with community services to support optimal nutrition such as the Special Supplemental Nutrition Program for Women, Infants, and Children, the Supplemental Nutrition Assistance Program, the Child and Adult Care Food Program, and food pantries and soup kitchens, but also to get beyond recommending a “good diet” to something more specific that is high in key nutrients important for brain development such as protein; zinc; iron; choline; folate; iodine; vitamins A, D, B6, and B12; and polyunsaturated fatty acids. That’s where the boiled oysters, a decent source of the listed nutrients and especially loaded with zinc, iron, and vitamin B12, come in. While not everyone is going to rush out to buy their babies such an unexpected (and for many, unfamiliar) food, the statement reminds pediatricians to recommend foods that are good sources of the nutrients that babies and toddlers need most. Other foods that fit the bill include oatmeal, meat and poultry, fish like salmon and tuna, eggs, tofu and soybeans, and other legumes and beans like chickpeas and lentils.
—Natalie D. Muth, MD
Pediatrician
Children's Primary Care Medical Group
Carlsbad, California
Serotonin syndrome warnings magnify its rare probability
Serotonin syndrome.
What do those words make you think of? A rare neurological condition? The differential diagnosis you got at 2:00 a.m. from an overly enthusiastic resident? Or a fax from a pharmacy that you get a few times a week?
I’ll say the last.
Triptans are not as common, but are still out there in large numbers. To date, they’re the most effective migraine treatment we have.
Inevitably, these roads will cross, especially because SNRIs, and their older cousins the tricylic antidepressants, are commonly used for migraine prevention. And that’s where the fun begins. There’s a suspected incidence of serotonin syndrome when the two are combined, which became widespread knowledge following a 2006 Food and Drug Administration alert. This is a fact drilled into us by multiple call-backs and faxes from pharmacies, terrified patients who use Google, and electronic prescribing systems that flag our attempts to combine them to make sure we know THIS IS DANGEROUS!
But a study published Feb. 26 in JAMA Neurology found that it’s rarer than anyone suspected (JAMA Neurol. 2018 Feb 26. doi: 10.1001/jamaneurol.2017.5144). Breaking down 14 years’ worth of patient data with more than 19,000 patients on both triptans and serotonergic drugs, there were only two cases (0.01%) that clearly met criteria for serotonin syndrome.
I also understand where the warnings come from. When things go bad, medicine becomes a blame game as each side points at another. The pharmacy wants to say they warned us. The e-prescribing system company wants to say they warned us. The patients want to know why no one warned them when a Google search makes it sound common. And the malpractice lawyers want to blame everyone.
But there are more serious side effects out there. Dilantin has been linked to lymphoma. Sinemet (possibly) to melanoma. But do you remember the last time you had to click or sign off on a pharmacy warning for those? Me neither.
Using any drug is a balance of risks and benefits. We make our judgments, discuss concerns with the patients, and roll the dice every day. Side effects aren’t uncommon. Most serious side effects are rare. But warnings that magnify issues with a rare probability don’t help the situation and can keep patients from receiving the help they need.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Serotonin syndrome.
What do those words make you think of? A rare neurological condition? The differential diagnosis you got at 2:00 a.m. from an overly enthusiastic resident? Or a fax from a pharmacy that you get a few times a week?
I’ll say the last.
Triptans are not as common, but are still out there in large numbers. To date, they’re the most effective migraine treatment we have.
Inevitably, these roads will cross, especially because SNRIs, and their older cousins the tricylic antidepressants, are commonly used for migraine prevention. And that’s where the fun begins. There’s a suspected incidence of serotonin syndrome when the two are combined, which became widespread knowledge following a 2006 Food and Drug Administration alert. This is a fact drilled into us by multiple call-backs and faxes from pharmacies, terrified patients who use Google, and electronic prescribing systems that flag our attempts to combine them to make sure we know THIS IS DANGEROUS!
But a study published Feb. 26 in JAMA Neurology found that it’s rarer than anyone suspected (JAMA Neurol. 2018 Feb 26. doi: 10.1001/jamaneurol.2017.5144). Breaking down 14 years’ worth of patient data with more than 19,000 patients on both triptans and serotonergic drugs, there were only two cases (0.01%) that clearly met criteria for serotonin syndrome.
I also understand where the warnings come from. When things go bad, medicine becomes a blame game as each side points at another. The pharmacy wants to say they warned us. The e-prescribing system company wants to say they warned us. The patients want to know why no one warned them when a Google search makes it sound common. And the malpractice lawyers want to blame everyone.
But there are more serious side effects out there. Dilantin has been linked to lymphoma. Sinemet (possibly) to melanoma. But do you remember the last time you had to click or sign off on a pharmacy warning for those? Me neither.
Using any drug is a balance of risks and benefits. We make our judgments, discuss concerns with the patients, and roll the dice every day. Side effects aren’t uncommon. Most serious side effects are rare. But warnings that magnify issues with a rare probability don’t help the situation and can keep patients from receiving the help they need.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Serotonin syndrome.
What do those words make you think of? A rare neurological condition? The differential diagnosis you got at 2:00 a.m. from an overly enthusiastic resident? Or a fax from a pharmacy that you get a few times a week?
I’ll say the last.
Triptans are not as common, but are still out there in large numbers. To date, they’re the most effective migraine treatment we have.
Inevitably, these roads will cross, especially because SNRIs, and their older cousins the tricylic antidepressants, are commonly used for migraine prevention. And that’s where the fun begins. There’s a suspected incidence of serotonin syndrome when the two are combined, which became widespread knowledge following a 2006 Food and Drug Administration alert. This is a fact drilled into us by multiple call-backs and faxes from pharmacies, terrified patients who use Google, and electronic prescribing systems that flag our attempts to combine them to make sure we know THIS IS DANGEROUS!
But a study published Feb. 26 in JAMA Neurology found that it’s rarer than anyone suspected (JAMA Neurol. 2018 Feb 26. doi: 10.1001/jamaneurol.2017.5144). Breaking down 14 years’ worth of patient data with more than 19,000 patients on both triptans and serotonergic drugs, there were only two cases (0.01%) that clearly met criteria for serotonin syndrome.
I also understand where the warnings come from. When things go bad, medicine becomes a blame game as each side points at another. The pharmacy wants to say they warned us. The e-prescribing system company wants to say they warned us. The patients want to know why no one warned them when a Google search makes it sound common. And the malpractice lawyers want to blame everyone.
But there are more serious side effects out there. Dilantin has been linked to lymphoma. Sinemet (possibly) to melanoma. But do you remember the last time you had to click or sign off on a pharmacy warning for those? Me neither.
Using any drug is a balance of risks and benefits. We make our judgments, discuss concerns with the patients, and roll the dice every day. Side effects aren’t uncommon. Most serious side effects are rare. But warnings that magnify issues with a rare probability don’t help the situation and can keep patients from receiving the help they need.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Pediatric Dermatology Consult - February 2018
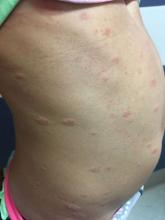
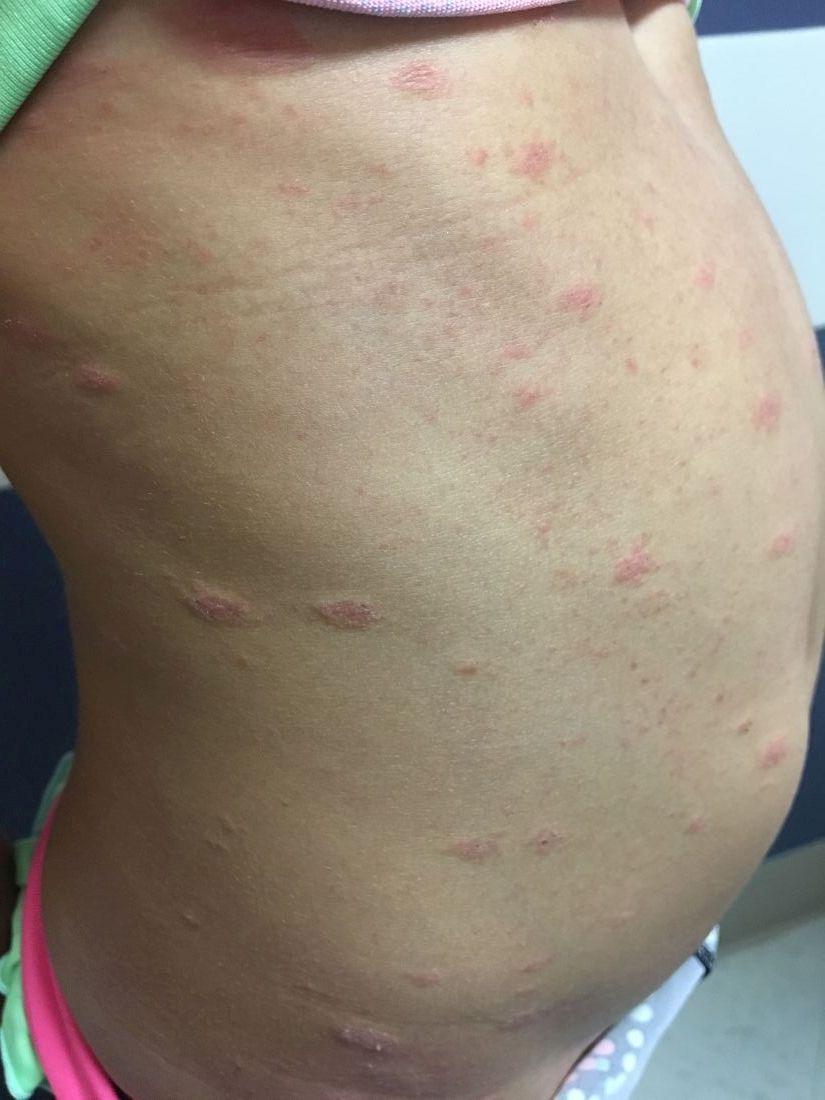
The patient was diagnosed with pityriasis rosea (PR) on the basis of the clinical findings; a biopsy was not performed. The patient’s pruritus was treated with oral hydroxyzine and topical 1% triamcinolone ointment. She experienced itch relief with these treatments. On follow-up at 3 months, the patient’s lesions had mostly resolved with some postinflammatory hyperpigmentation.
In some patients, flu-like symptoms precede the onset of skin lesions; this has led to speculation regarding a viral etiology for PR. This prodrome, which is present in as many as half of all cases,can include mild headache, low-grade fever, joint aches, or malaise.2 Pityriasis rosea is thought to occur secondary to a systemic activation of human herpesviruses (HHV) 6 and/or HHV-7. Three cases of PR have been reported in the setting of H1N1 influenza virus infection.3 In one small study, HHV-8 was detected by polymerase chain reaction in approximately 20% of biopsy samples of lesional skin in patients with PR.4 However, most research on a viral etiology for pityriasis rosea has focused on HHV-6 and to a lesser extent HHV-7. DNA from both viruses has been isolated from PR lesions, but at varying detection rates.5,6 Furthermore, HHV-7 DNA has been isolated in as many as 14% of normal individuals without pityriasis rosea, suggesting that the presence of this virus on the skin is fairly common.7
Pityriasis rosea occurs in males and females of all ethnicities, with a slight female predominance. It is rare in young children and older adults. Most cases occur in adolescents and in adults in their twenties and early thirties. Cases occur most frequently in fall and spring.8
The herald patch of pityriasis rosea is typically solitary, but cases with multiple herald patches have been described. The herald patch can range in size from 1-10 cm and usually contains the best example of trailing scale – scale seen on the inside edge of the annular lesion. The satellite lesions of pityriasis rosea are typically papules or plaques with a collarette of scale. These lesions usually are oriented along the Langer cleavage lines, giving them a “Christmas tree” configuration when they appear on the posterior trunk.
Mimics
The herald patch of pityriasis rosea can resemble tinea corporis, and if there is any doubt as to the diagnosis, potassium hydroxide examination (also known as a KOH test) and/or fungal culture should be done to rule out a fungal etiology. However, certain features of this case, particularly the subsequent development of satellite lesions, are more consistent with pityriasis rosea.
Secondary syphilis should be considered in patients who are sexually active. The lesions of secondary syphilis are not typically pruritic, and involvement of the palms and soles is common (whereas such involvement is rare in pityriasis rosea).
Like pityriasis rosea, pityriasis lichenoides et varioliformis acuta (PLEVA) is characterized by papular lesions that resolve spontaneously; the lesions of PLEVA usually evolve to vesicular, necrotic, and purpuric papules that take longer to resolve than PR lesions. The lesions of PLEVA are more erythematous, pustular, and crusting than the lesions of pityriasis rosea.
Guttate psoriasis, which occurs following streptococcal pharyngitis in over 50% of patients, does not present with a herald lesion or distribution along Langer’s lines.10 If guttate psoriasis is suspected, rapid streptococcal testing of the throat or perianal area may be considered.
Nummular eczema presents as papules that enlarge to form erythematous, lichenified plaques that measure 1-2 cm in diameter. A relatively sudden eruption, such as this patient’s, would be unusual for nummular eczema. Also, nummular eczema typically occurs on xerotic skin, more often on the extremities than the trunk.
Diagnostic tests, treatment
Most patients do not require specific therapy for pityriasis rosea. Patients should be reassured that PR is typically a self-limited disease without long-term sequelae. Pregnant patients who develop pityriasis rosea in the first trimester may be at higher risk for spontaneous abortion,although data on the subject are sorely lacking.11 Oral antihistamines are useful in reducing pruritus associated with PR, and some patients experience relief by applying a low-potency topical corticosteroid.
In more severe cases, or in cases in which the patient is greatly distressed by the lesions, both broadband and narrowband UVB phototherapy effectively improve severity of lesions and reduces symptoms.12 These observations suggest that moderate sun exposure can help to reduce severity of PR lesions and hasten their resolution, but no studies assessing the effect of sun exposure on pityriasis rosea symptoms have been performed.
Furthermore, the possible role of the HHV-6 in PR has led some investigators to explore the utility of acyclovir in managing pityriasis rosea.13 One group recently found that 400 mg of acyclovir three times per day for 7 days decreased the number of lesions and pruritus associated with pityriasis rosea, compared those seen in controls, at 1-month follow-up.13
Timely recognition of the diagnosis, consideration of mimics, and ample reassurance are appropriate when approaching this disease.
Mr. Kusari is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, and the departments of dermatology and pediatrics, University of California, San Diego. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. They have no relevant financial disclosures. Email them at [email protected].
References
1. Dermatology. 2015;231(1):9-14.
2. World J Clin Cases. 2017 Jun 16;5(6):203-11.
3. Pediatr Dermatol. 2011 May-Jun;28(3):341-2.
4. J Eur Acad Dermatol Venereol. 2006 Jul;20(6):667-71.
5. Dermatology. 1997;195(4):374-8.
6. J Invest Dermatol. 2005 Jun;124(6):1234-40.
7. Arch Dermatol. 1999 Sep;135(9):1070-2.
8. J Am Acad Dermatol. 1982 Jul;7(1):80-9.
9. Iran J Pediatr. 2010 Jun;20(2):237-41.
10. J Pediatr. 1988 Dec;113(6):1037-9.
11. J Am Acad Dermatol. 2008 May;58(5 Suppl 1):S78-83.
12. J Am Acad Dermatol. 1995 Dec;33(6):996-9.
13. Indian Dermatol Online J. 2015 May-Jun;6(3):181-4.
The patient was diagnosed with pityriasis rosea (PR) on the basis of the clinical findings; a biopsy was not performed. The patient’s pruritus was treated with oral hydroxyzine and topical 1% triamcinolone ointment. She experienced itch relief with these treatments. On follow-up at 3 months, the patient’s lesions had mostly resolved with some postinflammatory hyperpigmentation.
In some patients, flu-like symptoms precede the onset of skin lesions; this has led to speculation regarding a viral etiology for PR. This prodrome, which is present in as many as half of all cases,can include mild headache, low-grade fever, joint aches, or malaise.2 Pityriasis rosea is thought to occur secondary to a systemic activation of human herpesviruses (HHV) 6 and/or HHV-7. Three cases of PR have been reported in the setting of H1N1 influenza virus infection.3 In one small study, HHV-8 was detected by polymerase chain reaction in approximately 20% of biopsy samples of lesional skin in patients with PR.4 However, most research on a viral etiology for pityriasis rosea has focused on HHV-6 and to a lesser extent HHV-7. DNA from both viruses has been isolated from PR lesions, but at varying detection rates.5,6 Furthermore, HHV-7 DNA has been isolated in as many as 14% of normal individuals without pityriasis rosea, suggesting that the presence of this virus on the skin is fairly common.7
Pityriasis rosea occurs in males and females of all ethnicities, with a slight female predominance. It is rare in young children and older adults. Most cases occur in adolescents and in adults in their twenties and early thirties. Cases occur most frequently in fall and spring.8
The herald patch of pityriasis rosea is typically solitary, but cases with multiple herald patches have been described. The herald patch can range in size from 1-10 cm and usually contains the best example of trailing scale – scale seen on the inside edge of the annular lesion. The satellite lesions of pityriasis rosea are typically papules or plaques with a collarette of scale. These lesions usually are oriented along the Langer cleavage lines, giving them a “Christmas tree” configuration when they appear on the posterior trunk.
Mimics
The herald patch of pityriasis rosea can resemble tinea corporis, and if there is any doubt as to the diagnosis, potassium hydroxide examination (also known as a KOH test) and/or fungal culture should be done to rule out a fungal etiology. However, certain features of this case, particularly the subsequent development of satellite lesions, are more consistent with pityriasis rosea.
Secondary syphilis should be considered in patients who are sexually active. The lesions of secondary syphilis are not typically pruritic, and involvement of the palms and soles is common (whereas such involvement is rare in pityriasis rosea).
Like pityriasis rosea, pityriasis lichenoides et varioliformis acuta (PLEVA) is characterized by papular lesions that resolve spontaneously; the lesions of PLEVA usually evolve to vesicular, necrotic, and purpuric papules that take longer to resolve than PR lesions. The lesions of PLEVA are more erythematous, pustular, and crusting than the lesions of pityriasis rosea.
Guttate psoriasis, which occurs following streptococcal pharyngitis in over 50% of patients, does not present with a herald lesion or distribution along Langer’s lines.10 If guttate psoriasis is suspected, rapid streptococcal testing of the throat or perianal area may be considered.
Nummular eczema presents as papules that enlarge to form erythematous, lichenified plaques that measure 1-2 cm in diameter. A relatively sudden eruption, such as this patient’s, would be unusual for nummular eczema. Also, nummular eczema typically occurs on xerotic skin, more often on the extremities than the trunk.
Diagnostic tests, treatment
Most patients do not require specific therapy for pityriasis rosea. Patients should be reassured that PR is typically a self-limited disease without long-term sequelae. Pregnant patients who develop pityriasis rosea in the first trimester may be at higher risk for spontaneous abortion,although data on the subject are sorely lacking.11 Oral antihistamines are useful in reducing pruritus associated with PR, and some patients experience relief by applying a low-potency topical corticosteroid.
In more severe cases, or in cases in which the patient is greatly distressed by the lesions, both broadband and narrowband UVB phototherapy effectively improve severity of lesions and reduces symptoms.12 These observations suggest that moderate sun exposure can help to reduce severity of PR lesions and hasten their resolution, but no studies assessing the effect of sun exposure on pityriasis rosea symptoms have been performed.
Furthermore, the possible role of the HHV-6 in PR has led some investigators to explore the utility of acyclovir in managing pityriasis rosea.13 One group recently found that 400 mg of acyclovir three times per day for 7 days decreased the number of lesions and pruritus associated with pityriasis rosea, compared those seen in controls, at 1-month follow-up.13
Timely recognition of the diagnosis, consideration of mimics, and ample reassurance are appropriate when approaching this disease.
Mr. Kusari is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, and the departments of dermatology and pediatrics, University of California, San Diego. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. They have no relevant financial disclosures. Email them at [email protected].
References
1. Dermatology. 2015;231(1):9-14.
2. World J Clin Cases. 2017 Jun 16;5(6):203-11.
3. Pediatr Dermatol. 2011 May-Jun;28(3):341-2.
4. J Eur Acad Dermatol Venereol. 2006 Jul;20(6):667-71.
5. Dermatology. 1997;195(4):374-8.
6. J Invest Dermatol. 2005 Jun;124(6):1234-40.
7. Arch Dermatol. 1999 Sep;135(9):1070-2.
8. J Am Acad Dermatol. 1982 Jul;7(1):80-9.
9. Iran J Pediatr. 2010 Jun;20(2):237-41.
10. J Pediatr. 1988 Dec;113(6):1037-9.
11. J Am Acad Dermatol. 2008 May;58(5 Suppl 1):S78-83.
12. J Am Acad Dermatol. 1995 Dec;33(6):996-9.
13. Indian Dermatol Online J. 2015 May-Jun;6(3):181-4.
The patient was diagnosed with pityriasis rosea (PR) on the basis of the clinical findings; a biopsy was not performed. The patient’s pruritus was treated with oral hydroxyzine and topical 1% triamcinolone ointment. She experienced itch relief with these treatments. On follow-up at 3 months, the patient’s lesions had mostly resolved with some postinflammatory hyperpigmentation.
In some patients, flu-like symptoms precede the onset of skin lesions; this has led to speculation regarding a viral etiology for PR. This prodrome, which is present in as many as half of all cases,can include mild headache, low-grade fever, joint aches, or malaise.2 Pityriasis rosea is thought to occur secondary to a systemic activation of human herpesviruses (HHV) 6 and/or HHV-7. Three cases of PR have been reported in the setting of H1N1 influenza virus infection.3 In one small study, HHV-8 was detected by polymerase chain reaction in approximately 20% of biopsy samples of lesional skin in patients with PR.4 However, most research on a viral etiology for pityriasis rosea has focused on HHV-6 and to a lesser extent HHV-7. DNA from both viruses has been isolated from PR lesions, but at varying detection rates.5,6 Furthermore, HHV-7 DNA has been isolated in as many as 14% of normal individuals without pityriasis rosea, suggesting that the presence of this virus on the skin is fairly common.7
Pityriasis rosea occurs in males and females of all ethnicities, with a slight female predominance. It is rare in young children and older adults. Most cases occur in adolescents and in adults in their twenties and early thirties. Cases occur most frequently in fall and spring.8
The herald patch of pityriasis rosea is typically solitary, but cases with multiple herald patches have been described. The herald patch can range in size from 1-10 cm and usually contains the best example of trailing scale – scale seen on the inside edge of the annular lesion. The satellite lesions of pityriasis rosea are typically papules or plaques with a collarette of scale. These lesions usually are oriented along the Langer cleavage lines, giving them a “Christmas tree” configuration when they appear on the posterior trunk.
Mimics
The herald patch of pityriasis rosea can resemble tinea corporis, and if there is any doubt as to the diagnosis, potassium hydroxide examination (also known as a KOH test) and/or fungal culture should be done to rule out a fungal etiology. However, certain features of this case, particularly the subsequent development of satellite lesions, are more consistent with pityriasis rosea.
Secondary syphilis should be considered in patients who are sexually active. The lesions of secondary syphilis are not typically pruritic, and involvement of the palms and soles is common (whereas such involvement is rare in pityriasis rosea).
Like pityriasis rosea, pityriasis lichenoides et varioliformis acuta (PLEVA) is characterized by papular lesions that resolve spontaneously; the lesions of PLEVA usually evolve to vesicular, necrotic, and purpuric papules that take longer to resolve than PR lesions. The lesions of PLEVA are more erythematous, pustular, and crusting than the lesions of pityriasis rosea.
Guttate psoriasis, which occurs following streptococcal pharyngitis in over 50% of patients, does not present with a herald lesion or distribution along Langer’s lines.10 If guttate psoriasis is suspected, rapid streptococcal testing of the throat or perianal area may be considered.
Nummular eczema presents as papules that enlarge to form erythematous, lichenified plaques that measure 1-2 cm in diameter. A relatively sudden eruption, such as this patient’s, would be unusual for nummular eczema. Also, nummular eczema typically occurs on xerotic skin, more often on the extremities than the trunk.
Diagnostic tests, treatment
Most patients do not require specific therapy for pityriasis rosea. Patients should be reassured that PR is typically a self-limited disease without long-term sequelae. Pregnant patients who develop pityriasis rosea in the first trimester may be at higher risk for spontaneous abortion,although data on the subject are sorely lacking.11 Oral antihistamines are useful in reducing pruritus associated with PR, and some patients experience relief by applying a low-potency topical corticosteroid.
In more severe cases, or in cases in which the patient is greatly distressed by the lesions, both broadband and narrowband UVB phototherapy effectively improve severity of lesions and reduces symptoms.12 These observations suggest that moderate sun exposure can help to reduce severity of PR lesions and hasten their resolution, but no studies assessing the effect of sun exposure on pityriasis rosea symptoms have been performed.
Furthermore, the possible role of the HHV-6 in PR has led some investigators to explore the utility of acyclovir in managing pityriasis rosea.13 One group recently found that 400 mg of acyclovir three times per day for 7 days decreased the number of lesions and pruritus associated with pityriasis rosea, compared those seen in controls, at 1-month follow-up.13
Timely recognition of the diagnosis, consideration of mimics, and ample reassurance are appropriate when approaching this disease.
Mr. Kusari is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, and the departments of dermatology and pediatrics, University of California, San Diego. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. They have no relevant financial disclosures. Email them at [email protected].
References
1. Dermatology. 2015;231(1):9-14.
2. World J Clin Cases. 2017 Jun 16;5(6):203-11.
3. Pediatr Dermatol. 2011 May-Jun;28(3):341-2.
4. J Eur Acad Dermatol Venereol. 2006 Jul;20(6):667-71.
5. Dermatology. 1997;195(4):374-8.
6. J Invest Dermatol. 2005 Jun;124(6):1234-40.
7. Arch Dermatol. 1999 Sep;135(9):1070-2.
8. J Am Acad Dermatol. 1982 Jul;7(1):80-9.
9. Iran J Pediatr. 2010 Jun;20(2):237-41.
10. J Pediatr. 1988 Dec;113(6):1037-9.
11. J Am Acad Dermatol. 2008 May;58(5 Suppl 1):S78-83.
12. J Am Acad Dermatol. 1995 Dec;33(6):996-9.
13. Indian Dermatol Online J. 2015 May-Jun;6(3):181-4.
A 6-year-old female presents to the pediatric dermatology office with a 2-day history of a slightly itchy skin lesion on her back. Her birthday was a week prior, and her mother gave her a new kitten, and since then she has been playing with the kitten daily. She has tried some over-the-counter antifungal cream since the lesion first appeared, but there hasn’t been much improvement. The night prior to presenting to the office, the mother noticed more lesions developing on the child’s torso, and because of this, she became worried.
On physical exam, the patient is well appearing, and vital signs are normal. She has multiple scaly, pink, oval plaques and papules on her torso. There are no oral lesions, and her palms and soles are spared.
25 Years of Movement Disorders
Peter A. LeWitt, MD
Dr. LeWitt is the Director of the Parkinson Disease and Movement Disorder Program at Henry Ford Hospital, West Bloomfield, Michigan.
For most neurology subspecialties, the past quarter-century was only the latest chapter of a productive history extending through the mid-19th century, but for movement disorders, these years were formative. Before then, movement disorder specialists were few in number, and their medical literature was far more scanty than it is now, with multiple journals and texts now devoted to this topic. Twenty-five years ago, the international society devoted to Parkinson disease and other movement disorders was only a recent arrival. Fortunately, two charismatic and brilliant leaders—the American neurologist Stanley Fahn, MD, and the British neurologist C. David Marsden, MBBS—came forward to inspire a generation of trainees with their broad interests in bridging clinical neurology, experimental neuroscience, and patient advocacy. Today, the impact of these pioneers is still felt by a growing community of movement disorder clinicians and researchers who have transformed movement disorders from an earlier realm of descriptive neurology into a vibrant field exploring disease mechanisms and therapeutics, as well as an expanding array of clinical insights.
When Neurology Reviews was founded in 1993, the majority of significant developments in movement disorders concerned Parkinson’s disease. Strategies to improve upon dopaminergic therapy were being tested in clinical trials, along with ventures into pallidotomy as a neurosurgical treatment. Animal models of parkinsonism and neurochemical analysis of brain and putative biomarkers provided scientific guidance for trials of disease modification. Another milestone from the 1990s was discovery of the first gene leading to autosomal-dominant parkinsonism. This rare mutation in the structural protein alpha-synuclein had a much broader impact on Parkinson’s disease research in that it provided important information about the sporadic disorder’s final common pathway of pathogenesis. Today, alpha-synuclein is the focus of therapeutic intervention utilizing immunologic therapies to halt disease progression. Over the past quarter-century, more than two dozen additional gene mutations associated with parkinsonism have been discovered. These mutations provide additional insight into disease mechanisms. Beyond the science and clinical insights that have propelled advances in understanding Parkinson’s disease and its related disorders, the growth of the patient advocacy and caregiver movement has also been remarkable in expanding what is needed to improve daily life with this disorder.
Since 1993, other movement disorders have also profited from the tremendous growth of molecular neuroscience and its applications. Novel neuroimaging techniques like functional MRI, computation of neural pathways, and scanning using PET and SPECT have helped work out the brain mechanisms of several disorders. Genetic probing of familial disorders like dystonia and myoclonus have helped to unravel the neurochemistry of what has gone wrong to cause certain movement disorders. In turn, transgenic animal models using these human genetic mutations have allowed exploration of what might be done to fix the problems. These developments could have huge implications for making progress in the therapies of the future, since the most common categories of movement disorders—dystonia, myoclonus, ataxia, tremor, restless legs syndrome, Tourette syndrome, choreic disorders, and paroxysmal movement disorders—all can have hereditary patterns. Genetic testing can be valuable for choosing at-risk individuals for participation in trials of disease-modifying drugs for Huntington’s disease, for example, and this strategy may be the way of the future for other late-life emergent disorders. In the past decade, several gene therapy studies have been carried out in patients with Parkinson’s disease. The promise of this approach and the recent gene editing and RNA interference approaches, all part of the molecular biology revolution of the past 25 years, may further change the landscape of movement disorder therapeutics ahead.
Today, movement disorder specialists routinely use therapeutic approaches that were in their infancy in 1993. Botulinum toxin selective denervation has had a major impact by improving quality of life for patients affected with a wide range of movement disorders: dystonic necks, hands, and feet; involuntary facial movements and voice aberrations; tics; spasticity; and drooling. Deep brain stimulation is a routine option for many patients with tremor, Parkinson’s disease, and dystonia. Developments in this field have refined the choice of targets for stimulation and the possibility of treating other disorders with these techniques, like Tourette syndrome. A nonsurgical approach to abolishing tremor, MRI-guided focused ultrasound, recently was approved. In the background of these advances is the increasing knowledge of movement disorders that practitioners have to offer many patients with disorders that in the past were merely neurologic curiosities. Through clinical trial research and serendipity (the major treatment options for essential tremor arose this way), there is an expanding search for repurposing older medications and harnessing new biotechnology to target the mechanisms leading to various movement disorders.
It is impressive how much the neurologic discipline of movement disorders has changed in response to the new era of electronic communications and other technologies. In the past quarter-century, medical students, residents, and practicing neurologists have had instant access to a curriculum that includes resources such as video-recorded movement disorders. Clinical trials are being revolutionized by electronic technologies that record information in ways that improve upon human ratings. A patient with Parkinson’s disease, essential tremor, or restless legs syndrome can now have symptoms monitored remotely by wearable devices that offer a real-world view on how a new therapy is performing. But perhaps the greatest revolution in the recent past has been increasing confidence that progressive disabilities caused by movement disorders might be halted by emerging insights into disease mechanisms. There has been tremendous growth of interest among the basic neuroscience community in human diseases affecting motor control. More than half of research presentations at recent international Parkinson’s disease and movement disorder conferences reflect this trend. These efforts support an optimistic view that, over the nex
Peter A. LeWitt, MD
Dr. LeWitt is the Director of the Parkinson Disease and Movement Disorder Program at Henry Ford Hospital, West Bloomfield, Michigan.
For most neurology subspecialties, the past quarter-century was only the latest chapter of a productive history extending through the mid-19th century, but for movement disorders, these years were formative. Before then, movement disorder specialists were few in number, and their medical literature was far more scanty than it is now, with multiple journals and texts now devoted to this topic. Twenty-five years ago, the international society devoted to Parkinson disease and other movement disorders was only a recent arrival. Fortunately, two charismatic and brilliant leaders—the American neurologist Stanley Fahn, MD, and the British neurologist C. David Marsden, MBBS—came forward to inspire a generation of trainees with their broad interests in bridging clinical neurology, experimental neuroscience, and patient advocacy. Today, the impact of these pioneers is still felt by a growing community of movement disorder clinicians and researchers who have transformed movement disorders from an earlier realm of descriptive neurology into a vibrant field exploring disease mechanisms and therapeutics, as well as an expanding array of clinical insights.
When Neurology Reviews was founded in 1993, the majority of significant developments in movement disorders concerned Parkinson’s disease. Strategies to improve upon dopaminergic therapy were being tested in clinical trials, along with ventures into pallidotomy as a neurosurgical treatment. Animal models of parkinsonism and neurochemical analysis of brain and putative biomarkers provided scientific guidance for trials of disease modification. Another milestone from the 1990s was discovery of the first gene leading to autosomal-dominant parkinsonism. This rare mutation in the structural protein alpha-synuclein had a much broader impact on Parkinson’s disease research in that it provided important information about the sporadic disorder’s final common pathway of pathogenesis. Today, alpha-synuclein is the focus of therapeutic intervention utilizing immunologic therapies to halt disease progression. Over the past quarter-century, more than two dozen additional gene mutations associated with parkinsonism have been discovered. These mutations provide additional insight into disease mechanisms. Beyond the science and clinical insights that have propelled advances in understanding Parkinson’s disease and its related disorders, the growth of the patient advocacy and caregiver movement has also been remarkable in expanding what is needed to improve daily life with this disorder.
Since 1993, other movement disorders have also profited from the tremendous growth of molecular neuroscience and its applications. Novel neuroimaging techniques like functional MRI, computation of neural pathways, and scanning using PET and SPECT have helped work out the brain mechanisms of several disorders. Genetic probing of familial disorders like dystonia and myoclonus have helped to unravel the neurochemistry of what has gone wrong to cause certain movement disorders. In turn, transgenic animal models using these human genetic mutations have allowed exploration of what might be done to fix the problems. These developments could have huge implications for making progress in the therapies of the future, since the most common categories of movement disorders—dystonia, myoclonus, ataxia, tremor, restless legs syndrome, Tourette syndrome, choreic disorders, and paroxysmal movement disorders—all can have hereditary patterns. Genetic testing can be valuable for choosing at-risk individuals for participation in trials of disease-modifying drugs for Huntington’s disease, for example, and this strategy may be the way of the future for other late-life emergent disorders. In the past decade, several gene therapy studies have been carried out in patients with Parkinson’s disease. The promise of this approach and the recent gene editing and RNA interference approaches, all part of the molecular biology revolution of the past 25 years, may further change the landscape of movement disorder therapeutics ahead.
Today, movement disorder specialists routinely use therapeutic approaches that were in their infancy in 1993. Botulinum toxin selective denervation has had a major impact by improving quality of life for patients affected with a wide range of movement disorders: dystonic necks, hands, and feet; involuntary facial movements and voice aberrations; tics; spasticity; and drooling. Deep brain stimulation is a routine option for many patients with tremor, Parkinson’s disease, and dystonia. Developments in this field have refined the choice of targets for stimulation and the possibility of treating other disorders with these techniques, like Tourette syndrome. A nonsurgical approach to abolishing tremor, MRI-guided focused ultrasound, recently was approved. In the background of these advances is the increasing knowledge of movement disorders that practitioners have to offer many patients with disorders that in the past were merely neurologic curiosities. Through clinical trial research and serendipity (the major treatment options for essential tremor arose this way), there is an expanding search for repurposing older medications and harnessing new biotechnology to target the mechanisms leading to various movement disorders.
It is impressive how much the neurologic discipline of movement disorders has changed in response to the new era of electronic communications and other technologies. In the past quarter-century, medical students, residents, and practicing neurologists have had instant access to a curriculum that includes resources such as video-recorded movement disorders. Clinical trials are being revolutionized by electronic technologies that record information in ways that improve upon human ratings. A patient with Parkinson’s disease, essential tremor, or restless legs syndrome can now have symptoms monitored remotely by wearable devices that offer a real-world view on how a new therapy is performing. But perhaps the greatest revolution in the recent past has been increasing confidence that progressive disabilities caused by movement disorders might be halted by emerging insights into disease mechanisms. There has been tremendous growth of interest among the basic neuroscience community in human diseases affecting motor control. More than half of research presentations at recent international Parkinson’s disease and movement disorder conferences reflect this trend. These efforts support an optimistic view that, over the nex
Peter A. LeWitt, MD
Dr. LeWitt is the Director of the Parkinson Disease and Movement Disorder Program at Henry Ford Hospital, West Bloomfield, Michigan.
For most neurology subspecialties, the past quarter-century was only the latest chapter of a productive history extending through the mid-19th century, but for movement disorders, these years were formative. Before then, movement disorder specialists were few in number, and their medical literature was far more scanty than it is now, with multiple journals and texts now devoted to this topic. Twenty-five years ago, the international society devoted to Parkinson disease and other movement disorders was only a recent arrival. Fortunately, two charismatic and brilliant leaders—the American neurologist Stanley Fahn, MD, and the British neurologist C. David Marsden, MBBS—came forward to inspire a generation of trainees with their broad interests in bridging clinical neurology, experimental neuroscience, and patient advocacy. Today, the impact of these pioneers is still felt by a growing community of movement disorder clinicians and researchers who have transformed movement disorders from an earlier realm of descriptive neurology into a vibrant field exploring disease mechanisms and therapeutics, as well as an expanding array of clinical insights.
When Neurology Reviews was founded in 1993, the majority of significant developments in movement disorders concerned Parkinson’s disease. Strategies to improve upon dopaminergic therapy were being tested in clinical trials, along with ventures into pallidotomy as a neurosurgical treatment. Animal models of parkinsonism and neurochemical analysis of brain and putative biomarkers provided scientific guidance for trials of disease modification. Another milestone from the 1990s was discovery of the first gene leading to autosomal-dominant parkinsonism. This rare mutation in the structural protein alpha-synuclein had a much broader impact on Parkinson’s disease research in that it provided important information about the sporadic disorder’s final common pathway of pathogenesis. Today, alpha-synuclein is the focus of therapeutic intervention utilizing immunologic therapies to halt disease progression. Over the past quarter-century, more than two dozen additional gene mutations associated with parkinsonism have been discovered. These mutations provide additional insight into disease mechanisms. Beyond the science and clinical insights that have propelled advances in understanding Parkinson’s disease and its related disorders, the growth of the patient advocacy and caregiver movement has also been remarkable in expanding what is needed to improve daily life with this disorder.
Since 1993, other movement disorders have also profited from the tremendous growth of molecular neuroscience and its applications. Novel neuroimaging techniques like functional MRI, computation of neural pathways, and scanning using PET and SPECT have helped work out the brain mechanisms of several disorders. Genetic probing of familial disorders like dystonia and myoclonus have helped to unravel the neurochemistry of what has gone wrong to cause certain movement disorders. In turn, transgenic animal models using these human genetic mutations have allowed exploration of what might be done to fix the problems. These developments could have huge implications for making progress in the therapies of the future, since the most common categories of movement disorders—dystonia, myoclonus, ataxia, tremor, restless legs syndrome, Tourette syndrome, choreic disorders, and paroxysmal movement disorders—all can have hereditary patterns. Genetic testing can be valuable for choosing at-risk individuals for participation in trials of disease-modifying drugs for Huntington’s disease, for example, and this strategy may be the way of the future for other late-life emergent disorders. In the past decade, several gene therapy studies have been carried out in patients with Parkinson’s disease. The promise of this approach and the recent gene editing and RNA interference approaches, all part of the molecular biology revolution of the past 25 years, may further change the landscape of movement disorder therapeutics ahead.
Today, movement disorder specialists routinely use therapeutic approaches that were in their infancy in 1993. Botulinum toxin selective denervation has had a major impact by improving quality of life for patients affected with a wide range of movement disorders: dystonic necks, hands, and feet; involuntary facial movements and voice aberrations; tics; spasticity; and drooling. Deep brain stimulation is a routine option for many patients with tremor, Parkinson’s disease, and dystonia. Developments in this field have refined the choice of targets for stimulation and the possibility of treating other disorders with these techniques, like Tourette syndrome. A nonsurgical approach to abolishing tremor, MRI-guided focused ultrasound, recently was approved. In the background of these advances is the increasing knowledge of movement disorders that practitioners have to offer many patients with disorders that in the past were merely neurologic curiosities. Through clinical trial research and serendipity (the major treatment options for essential tremor arose this way), there is an expanding search for repurposing older medications and harnessing new biotechnology to target the mechanisms leading to various movement disorders.
It is impressive how much the neurologic discipline of movement disorders has changed in response to the new era of electronic communications and other technologies. In the past quarter-century, medical students, residents, and practicing neurologists have had instant access to a curriculum that includes resources such as video-recorded movement disorders. Clinical trials are being revolutionized by electronic technologies that record information in ways that improve upon human ratings. A patient with Parkinson’s disease, essential tremor, or restless legs syndrome can now have symptoms monitored remotely by wearable devices that offer a real-world view on how a new therapy is performing. But perhaps the greatest revolution in the recent past has been increasing confidence that progressive disabilities caused by movement disorders might be halted by emerging insights into disease mechanisms. There has been tremendous growth of interest among the basic neuroscience community in human diseases affecting motor control. More than half of research presentations at recent international Parkinson’s disease and movement disorder conferences reflect this trend. These efforts support an optimistic view that, over the nex
Learning from the 2017 Oscar fiasco
It was a “never event.” At the very end of the 2017 Academy Awards presentation, the winner for Best Picture was announced. It was wrong. Two and a half minutes later it was corrected. The true winner was “Moonlight,” not “La La Land.” But by then much damage had been done.
I watched it happen live on TV and reviewed it again on YouTube. Several news agencies investigated and reported on what happened. I don’t have any inside information beyond that, but my engineering perspective can illuminate how to reduce mistakes.
The first lesson is how quickly people seek to assign blame after something goes wrong. I saw various online news agencies say Warren Beatty had announced the wrong winner. While he opened the envelope, it was Faye Dunaway who actually made the announcement of “La La Land.” Furthermore, Warren and Faye were merely reading the card. Warren had been given the wrong envelope, as high resolution photographs prove. The envelope was a duplicate for the prize announced just before them for the Best Actress award. The card said Emma Stone and in a smaller font “La La Land,” the film in which she starred. Warren hesitated because of how this was written on the card. Faye thought he was trying to pause as a shtick to increase suspense so she glanced at the card and blurted out “La La Land.”
Experts in quality improvement have learned that the best way to reduce errors is to resist this tendency to assign blame. A better approach is to assume, absent evidence to the contrary, that everyone is acting responsibly and sincerely to help the patient. Hear both sides of the story before jumping to any conclusions. Find systemic factors that contributed to a human error. Then focus on ameliorating systemic weaknesses.
One contributing factor for the error at the Oscars was that there were two copies of the set of award envelopes, with one set available on each side of the stage. This way the presenters can enter from either side of the stage. They are handed an envelope by one of the two auditors from PricewaterhouseCoopers, who are the only ones who know the contents.
A key component of safety is having check backs. The envelopes have the name of the award on the outside. One might hope the presenter would double check that they are being given the correct envelope by the auditor. But backstage is a very nervous and hectic place for the presenters. Actors are not professionals dedicated to safety.
Medical care is different. Before giving a transfusion, one nurse reads the number on the bag of blood to another nurse, who confirms that it matches a paper form. That simple act can prevent mistakes. Perhaps the auditor handing the envelope to the Oscar presenter should ask the presenter, who knows which award s/he is scheduled to announce, to read out loud the award title on the front of the envelope.
Clearly, Warren Beatty was confused by the contents of the envelope. He was expecting a card to have the name of a film, not the name of an actress with the film’s name in small print below it. He didn’t know what action to take and hesitated. Faye Dunaway plunged forward and misinterpreted the card. A key component of quality is making it safe for anyone, if they are not confident in what is happening, to stop the proceeding, ask questions, and challenge plans. For example, there are time-outs prior to surgery. A second component is presenting information in a form less likely to be misinterpreted. Medicine has a problem with many sound-alike and look-alike drug names, so sometimes these words are spelled with particular letters capitalized, to distinguish them. I wish EHRs would present lab results in large, bold font.
Another contributing factor here was that Faye misinterpreted Warren’s behaviors as a joke. Major airlines utilize the “sterile cockpit.” During the few minutes that they are running through the preflight checklist, the pilot and copilot do not discuss last night’s football game, crack jokes, or engage in any other extraneous conversations. They avoid interruptions and distractions, focusing solely on the task. Sign outs in medicine need to adopt this habit.
There is a concern that one of the auditors tweeted a picture of Emma Stone backstage holding her Oscar at the same time the fiasco was happening on stage. In the modern world, cell phones and selfies are a key source of distraction, errors, and car accidents.
Per the Army, “Prior planning prevents poor performance.” A couple days before the Oscar fiasco, the auditors were interviewed and they revealed that they didn’t have an action plan to deal with the situation of a mistaken announcement. They figured it was extremely unlikely and that the circumstances would determine the best response.
Experience has shown that in the hours leading up to a pediatric code, there may be several opportunities to recognize the risk and intervene so that blame cannot be assigned to a single person or action. Mock codes prepare people to think on their feet. And it is important to have a clearly designated person in charge of a code. Leadership matters.
In the Oscar fiasco, the damage was quickly limited by the gracious words of a “La La Land” producer He assessed the situation, announced the mistake, beckoned the “Moonlight” cast and crew to the stage, graciously complimented them, showed the correct award envelope and card to the camera, and offered the statue to the correct producer. Then he hastened his team off the stage. These actions of responsibility, truthfulness, transparency, and grace staunched the bleeding, minimized the damage, and as best as possible, remediated the error. Movie producers are experts at dealing with crises and catastrophes. Medical staff, when revealing errors to patients, can learn from this role model.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
It was a “never event.” At the very end of the 2017 Academy Awards presentation, the winner for Best Picture was announced. It was wrong. Two and a half minutes later it was corrected. The true winner was “Moonlight,” not “La La Land.” But by then much damage had been done.
I watched it happen live on TV and reviewed it again on YouTube. Several news agencies investigated and reported on what happened. I don’t have any inside information beyond that, but my engineering perspective can illuminate how to reduce mistakes.
The first lesson is how quickly people seek to assign blame after something goes wrong. I saw various online news agencies say Warren Beatty had announced the wrong winner. While he opened the envelope, it was Faye Dunaway who actually made the announcement of “La La Land.” Furthermore, Warren and Faye were merely reading the card. Warren had been given the wrong envelope, as high resolution photographs prove. The envelope was a duplicate for the prize announced just before them for the Best Actress award. The card said Emma Stone and in a smaller font “La La Land,” the film in which she starred. Warren hesitated because of how this was written on the card. Faye thought he was trying to pause as a shtick to increase suspense so she glanced at the card and blurted out “La La Land.”
Experts in quality improvement have learned that the best way to reduce errors is to resist this tendency to assign blame. A better approach is to assume, absent evidence to the contrary, that everyone is acting responsibly and sincerely to help the patient. Hear both sides of the story before jumping to any conclusions. Find systemic factors that contributed to a human error. Then focus on ameliorating systemic weaknesses.
One contributing factor for the error at the Oscars was that there were two copies of the set of award envelopes, with one set available on each side of the stage. This way the presenters can enter from either side of the stage. They are handed an envelope by one of the two auditors from PricewaterhouseCoopers, who are the only ones who know the contents.
A key component of safety is having check backs. The envelopes have the name of the award on the outside. One might hope the presenter would double check that they are being given the correct envelope by the auditor. But backstage is a very nervous and hectic place for the presenters. Actors are not professionals dedicated to safety.
Medical care is different. Before giving a transfusion, one nurse reads the number on the bag of blood to another nurse, who confirms that it matches a paper form. That simple act can prevent mistakes. Perhaps the auditor handing the envelope to the Oscar presenter should ask the presenter, who knows which award s/he is scheduled to announce, to read out loud the award title on the front of the envelope.
Clearly, Warren Beatty was confused by the contents of the envelope. He was expecting a card to have the name of a film, not the name of an actress with the film’s name in small print below it. He didn’t know what action to take and hesitated. Faye Dunaway plunged forward and misinterpreted the card. A key component of quality is making it safe for anyone, if they are not confident in what is happening, to stop the proceeding, ask questions, and challenge plans. For example, there are time-outs prior to surgery. A second component is presenting information in a form less likely to be misinterpreted. Medicine has a problem with many sound-alike and look-alike drug names, so sometimes these words are spelled with particular letters capitalized, to distinguish them. I wish EHRs would present lab results in large, bold font.
Another contributing factor here was that Faye misinterpreted Warren’s behaviors as a joke. Major airlines utilize the “sterile cockpit.” During the few minutes that they are running through the preflight checklist, the pilot and copilot do not discuss last night’s football game, crack jokes, or engage in any other extraneous conversations. They avoid interruptions and distractions, focusing solely on the task. Sign outs in medicine need to adopt this habit.
There is a concern that one of the auditors tweeted a picture of Emma Stone backstage holding her Oscar at the same time the fiasco was happening on stage. In the modern world, cell phones and selfies are a key source of distraction, errors, and car accidents.
Per the Army, “Prior planning prevents poor performance.” A couple days before the Oscar fiasco, the auditors were interviewed and they revealed that they didn’t have an action plan to deal with the situation of a mistaken announcement. They figured it was extremely unlikely and that the circumstances would determine the best response.
Experience has shown that in the hours leading up to a pediatric code, there may be several opportunities to recognize the risk and intervene so that blame cannot be assigned to a single person or action. Mock codes prepare people to think on their feet. And it is important to have a clearly designated person in charge of a code. Leadership matters.
In the Oscar fiasco, the damage was quickly limited by the gracious words of a “La La Land” producer He assessed the situation, announced the mistake, beckoned the “Moonlight” cast and crew to the stage, graciously complimented them, showed the correct award envelope and card to the camera, and offered the statue to the correct producer. Then he hastened his team off the stage. These actions of responsibility, truthfulness, transparency, and grace staunched the bleeding, minimized the damage, and as best as possible, remediated the error. Movie producers are experts at dealing with crises and catastrophes. Medical staff, when revealing errors to patients, can learn from this role model.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
It was a “never event.” At the very end of the 2017 Academy Awards presentation, the winner for Best Picture was announced. It was wrong. Two and a half minutes later it was corrected. The true winner was “Moonlight,” not “La La Land.” But by then much damage had been done.
I watched it happen live on TV and reviewed it again on YouTube. Several news agencies investigated and reported on what happened. I don’t have any inside information beyond that, but my engineering perspective can illuminate how to reduce mistakes.
The first lesson is how quickly people seek to assign blame after something goes wrong. I saw various online news agencies say Warren Beatty had announced the wrong winner. While he opened the envelope, it was Faye Dunaway who actually made the announcement of “La La Land.” Furthermore, Warren and Faye were merely reading the card. Warren had been given the wrong envelope, as high resolution photographs prove. The envelope was a duplicate for the prize announced just before them for the Best Actress award. The card said Emma Stone and in a smaller font “La La Land,” the film in which she starred. Warren hesitated because of how this was written on the card. Faye thought he was trying to pause as a shtick to increase suspense so she glanced at the card and blurted out “La La Land.”
Experts in quality improvement have learned that the best way to reduce errors is to resist this tendency to assign blame. A better approach is to assume, absent evidence to the contrary, that everyone is acting responsibly and sincerely to help the patient. Hear both sides of the story before jumping to any conclusions. Find systemic factors that contributed to a human error. Then focus on ameliorating systemic weaknesses.
One contributing factor for the error at the Oscars was that there were two copies of the set of award envelopes, with one set available on each side of the stage. This way the presenters can enter from either side of the stage. They are handed an envelope by one of the two auditors from PricewaterhouseCoopers, who are the only ones who know the contents.
A key component of safety is having check backs. The envelopes have the name of the award on the outside. One might hope the presenter would double check that they are being given the correct envelope by the auditor. But backstage is a very nervous and hectic place for the presenters. Actors are not professionals dedicated to safety.
Medical care is different. Before giving a transfusion, one nurse reads the number on the bag of blood to another nurse, who confirms that it matches a paper form. That simple act can prevent mistakes. Perhaps the auditor handing the envelope to the Oscar presenter should ask the presenter, who knows which award s/he is scheduled to announce, to read out loud the award title on the front of the envelope.
Clearly, Warren Beatty was confused by the contents of the envelope. He was expecting a card to have the name of a film, not the name of an actress with the film’s name in small print below it. He didn’t know what action to take and hesitated. Faye Dunaway plunged forward and misinterpreted the card. A key component of quality is making it safe for anyone, if they are not confident in what is happening, to stop the proceeding, ask questions, and challenge plans. For example, there are time-outs prior to surgery. A second component is presenting information in a form less likely to be misinterpreted. Medicine has a problem with many sound-alike and look-alike drug names, so sometimes these words are spelled with particular letters capitalized, to distinguish them. I wish EHRs would present lab results in large, bold font.
Another contributing factor here was that Faye misinterpreted Warren’s behaviors as a joke. Major airlines utilize the “sterile cockpit.” During the few minutes that they are running through the preflight checklist, the pilot and copilot do not discuss last night’s football game, crack jokes, or engage in any other extraneous conversations. They avoid interruptions and distractions, focusing solely on the task. Sign outs in medicine need to adopt this habit.
There is a concern that one of the auditors tweeted a picture of Emma Stone backstage holding her Oscar at the same time the fiasco was happening on stage. In the modern world, cell phones and selfies are a key source of distraction, errors, and car accidents.
Per the Army, “Prior planning prevents poor performance.” A couple days before the Oscar fiasco, the auditors were interviewed and they revealed that they didn’t have an action plan to deal with the situation of a mistaken announcement. They figured it was extremely unlikely and that the circumstances would determine the best response.
Experience has shown that in the hours leading up to a pediatric code, there may be several opportunities to recognize the risk and intervene so that blame cannot be assigned to a single person or action. Mock codes prepare people to think on their feet. And it is important to have a clearly designated person in charge of a code. Leadership matters.
In the Oscar fiasco, the damage was quickly limited by the gracious words of a “La La Land” producer He assessed the situation, announced the mistake, beckoned the “Moonlight” cast and crew to the stage, graciously complimented them, showed the correct award envelope and card to the camera, and offered the statue to the correct producer. Then he hastened his team off the stage. These actions of responsibility, truthfulness, transparency, and grace staunched the bleeding, minimized the damage, and as best as possible, remediated the error. Movie producers are experts at dealing with crises and catastrophes. Medical staff, when revealing errors to patients, can learn from this role model.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
RNS May Reduce the Risk of SUDEP
Responsive neurostimulation (RNS) appears to reduce the risk of sudden unexpected death in epilepsy (SUDEP), according to research published online ahead of print January 16 in Epilepsia. The results provide evidence that treatments that reduce seizures decrease the risk of SUDEP, according to the authors. The data also indicate that not every SUDEP follows a seizure.
Orrin Devinsky, MD, Director of the Comprehensive Epilepsy Center at New York University Langone Medical Center, and colleagues examined data for all deaths in patients with epilepsy who received RNS in clinical trials or following FDA approval through May 5, 2016, and adjudicated them for SUDEP. In all, 256 patients received treatment during trials, and 451 received treatment after FDA approval.
The investigators observed 14 deaths, including two possible, one probable, and four definite cases of SUDEP. The rate of probable or definite SUDEP was 2.0/1,000 over 2,036 patient stimulation years and 2.3/1,000 over 2,208 patient implant years. The age-adjusted standardized mortality ratio for definite and probable SUDEP was 0.75.
“The SUDEP rate of 2.0/1,000 patient stimulation years among patients undergoing treatment with RNS is favorable relative to patients [with treatment-resistant epilepsy] randomized to the placebo arm of add-on drug studies (SUDEP rate of 6.1/1,000 patient years), patients who were referred for epilepsy surgery but did not receive epilepsy surgery (SUDEP rate of 6.3/1,000 patient years), and patients with recurrent seizures after epilepsy surgery (SUDEP rate 6.3/1,000 patient years),” said the authors. “Future explorations can examine whether certain seizure patterns are particularly associated with SUDEP.”
—Erik Greb
Suggested Reading
Devinsky O, Friedman D, Duckrow RB, et al. Sudden unexpected death in epilepsy in patients treated with brain-responsive neurostimulation. Epilepsia. 2018 Jan 16 [Epub ahead of print].
Responsive neurostimulation (RNS) appears to reduce the risk of sudden unexpected death in epilepsy (SUDEP), according to research published online ahead of print January 16 in Epilepsia. The results provide evidence that treatments that reduce seizures decrease the risk of SUDEP, according to the authors. The data also indicate that not every SUDEP follows a seizure.
Orrin Devinsky, MD, Director of the Comprehensive Epilepsy Center at New York University Langone Medical Center, and colleagues examined data for all deaths in patients with epilepsy who received RNS in clinical trials or following FDA approval through May 5, 2016, and adjudicated them for SUDEP. In all, 256 patients received treatment during trials, and 451 received treatment after FDA approval.
The investigators observed 14 deaths, including two possible, one probable, and four definite cases of SUDEP. The rate of probable or definite SUDEP was 2.0/1,000 over 2,036 patient stimulation years and 2.3/1,000 over 2,208 patient implant years. The age-adjusted standardized mortality ratio for definite and probable SUDEP was 0.75.
“The SUDEP rate of 2.0/1,000 patient stimulation years among patients undergoing treatment with RNS is favorable relative to patients [with treatment-resistant epilepsy] randomized to the placebo arm of add-on drug studies (SUDEP rate of 6.1/1,000 patient years), patients who were referred for epilepsy surgery but did not receive epilepsy surgery (SUDEP rate of 6.3/1,000 patient years), and patients with recurrent seizures after epilepsy surgery (SUDEP rate 6.3/1,000 patient years),” said the authors. “Future explorations can examine whether certain seizure patterns are particularly associated with SUDEP.”
—Erik Greb
Suggested Reading
Devinsky O, Friedman D, Duckrow RB, et al. Sudden unexpected death in epilepsy in patients treated with brain-responsive neurostimulation. Epilepsia. 2018 Jan 16 [Epub ahead of print].
Responsive neurostimulation (RNS) appears to reduce the risk of sudden unexpected death in epilepsy (SUDEP), according to research published online ahead of print January 16 in Epilepsia. The results provide evidence that treatments that reduce seizures decrease the risk of SUDEP, according to the authors. The data also indicate that not every SUDEP follows a seizure.
Orrin Devinsky, MD, Director of the Comprehensive Epilepsy Center at New York University Langone Medical Center, and colleagues examined data for all deaths in patients with epilepsy who received RNS in clinical trials or following FDA approval through May 5, 2016, and adjudicated them for SUDEP. In all, 256 patients received treatment during trials, and 451 received treatment after FDA approval.
The investigators observed 14 deaths, including two possible, one probable, and four definite cases of SUDEP. The rate of probable or definite SUDEP was 2.0/1,000 over 2,036 patient stimulation years and 2.3/1,000 over 2,208 patient implant years. The age-adjusted standardized mortality ratio for definite and probable SUDEP was 0.75.
“The SUDEP rate of 2.0/1,000 patient stimulation years among patients undergoing treatment with RNS is favorable relative to patients [with treatment-resistant epilepsy] randomized to the placebo arm of add-on drug studies (SUDEP rate of 6.1/1,000 patient years), patients who were referred for epilepsy surgery but did not receive epilepsy surgery (SUDEP rate of 6.3/1,000 patient years), and patients with recurrent seizures after epilepsy surgery (SUDEP rate 6.3/1,000 patient years),” said the authors. “Future explorations can examine whether certain seizure patterns are particularly associated with SUDEP.”
—Erik Greb
Suggested Reading
Devinsky O, Friedman D, Duckrow RB, et al. Sudden unexpected death in epilepsy in patients treated with brain-responsive neurostimulation. Epilepsia. 2018 Jan 16 [Epub ahead of print].
How to advise adolescents ISO drugs on the ‘dark web’
There was a time, not so long ago, when in the popular imagination, a drug deal involved an aging hippie in a tie-dyed shirt and love beads, copping a joint at a Dead concert. Today, however, in the age of the Internet and smartphones, a teenager in his bedroom can select, order, and have delivered to his door illicit drugs via the marketplaces on the “dark web.”
As the name implies, the dark web is a subterranean layer of the Internet that is mysterious, ominous, and sometimes lawless. It lies “beneath” the surface web – the layer where grandmothers post on Facebook and purchase on Amazon.
The dark web largely was the brainchild of three mathematicians at the Naval Research Laboratory as a means of encrypting messages exchanged by the intelligence community. They dubbed their project “Tor,” for “The Onion Router,” as the system consists of layer after layer of random relays, permitting anonymity on the Internet with little risk of tracking or surveillance.
This online underworld first came to my attention several years ago. The father of a teen who was being treated for disruptive mood dysregulation, attention-deficit disorder, and alcohol and cannabis use disorder called to inform me that his son had been arrested at Lollapalooza with hundreds of Adderall tablets and Xanax bars. Several weeks later, in session, the young man disclosed to me that he had found simple instructions online about installing Tor, creating a VPN (a virtual private network), accessing the dark web, transacting with bitcoin, and identifying drug marketplaces. He also demonstrated a detailed knowledge of chemical manufacturing in China, pill pressing in Canada, and money laundering in Switzerland.
With the air of an insider sharing “trade secrets,” this young man described how dealers on the dark web avoid detection and ensure secure delivery of the goods: latex gloves, vacuum sealing, and bleach dipping to obviate fingerprints – human and chemical. He said that dealers will send “dummy” packages to throw off the authorities and that buyers often will use the address of a clueless or absent neighbor. In his case, however, the parcels were delivered to his doorstep.
. For one thing, acquiring drugs in this way can seem less risky, as there is no chance of being robbed at gunpoint in a sketchy neighborhood or being busted for possession during a routine traffic stop.
Crossing over to the world of the dark web also can give an adolescent a sense of being clever and rebellious and of pulling a “fast one” on the parents – and on us, the clinicians who are treating them. And a teen who is interested in using illicit substances and plays “Call of Duty” from the comfort of his family’s basement without actual injury or death might assume that he can attain illicit drugs that are safe and inexpensive.
There are counterarguments to misinformation about the dark web. For example, contrary to the notion that buying drugs on the dark web minimizes interdiction or arrest, clinicians should point out that since international law enforcement shut down Silk Road and incarcerated its founder, Ross Ulbricht (also known as “Dread Pirate Roberts”) in 2013, hundreds of other dark web marketplaces such as AlphaBay and Hansa have been silenced and their operators prosecuted.
Moreover, the U.S. Department of Justice recently launched the Joint Criminal Opioid Darknet Enforcement (J-CODE) group, and the U.S. Postal Service Inspection Service reportedly has been hiring cybercrime and dark web specialists to combat drug trafficking. It might come as a surprise to a teen that, in a state where recreational marijuana is legal, transport and delivery of cannabis by the postal service elevates purchase and possession to the level of a violation of federal law. Informing even the most oblivious or oppositional adolescent that a drug felony can disqualify him for college grants and loans, impede his search for gainful employment, or prohibit him from obtaining a professional license, might give him a moment’s pause.
Adolescents seeking to buy drugs on the dark web should brace themselves for another shock. Whether lulled by custom after years of shopping on Amazon or using PayPal, or simply dulled by addiction, they might have a blind trust that bitcoin tumblers and “dark” escrow accounts will secure their payments. It will be rude awakening when they learn that the transfer and holding of currency on the dark web is vulnerable to hackers and to operators of the marketplaces – who are known to simply abscond with funds.
Currently, the drug marketplaces on the dark web represent a thin slice of the total illicit drug trade. These marketplaces, however, are growing quickly and offer buyers a virtual smorgasbord: the leading prescription drugs bought are Xanax and OxyContin, whereas 3,4-methylenedioxymethamphetamine (MDMA)/ecstasy and cannabis are the most commonly purchased controlled Schedule I substances, according to an article in The Economist. The estimated annual sales for 2016 ranged from $100 million to $200 million, but even more alarming is the percentage of substance abusers who have purchased drugs on the dark web: A recent article in The Independent reported that 13.2% of U.S. respondents self-reported making at least one purchase online, whereas in the United Kingdom, the self-reported percentage was 25.3 %, and in Finland, it was 41.4%.
Evidence abounds that drugs purchased on the dark web often are counterfeit and sometimes “dirty.” There is a report of “Viagra” containing cement dust, of “Ambien” containing haloperidol, and “Xanax” laced with fentanyl, the latter having been linked to several deaths and hospital admissions in the Bay Area in 2016. (Big Pharma, in fact, is engaged in surveillance and investigation of the sale of knockoffs online, The Telegraph reported in article about the proliferation of fake drugs available on the surface web).Unfortunately, attempting to reduce teen substance abuse using law enforcement measures directed at dark web marketplaces might be a game of whack-a-mole: As soon as one supply source is staunched, another surfaces. Indeed, addiction should be viewed not simply in terms of the biopsychosocial model, but also as an economic activity. Thus, it might be more beneficial for clinicians to concentrate our resources and efforts on curtailing the demand through education and treatment.
Dr. Marseille is a psychiatrist who works on the staff at a clinic in Winfield, Ill. His special interests include adolescent and addiction medicine, eating disorders, trauma, bipolar disorder, and the psychiatric manifestations of acute and chronic medical conditions.
There was a time, not so long ago, when in the popular imagination, a drug deal involved an aging hippie in a tie-dyed shirt and love beads, copping a joint at a Dead concert. Today, however, in the age of the Internet and smartphones, a teenager in his bedroom can select, order, and have delivered to his door illicit drugs via the marketplaces on the “dark web.”
As the name implies, the dark web is a subterranean layer of the Internet that is mysterious, ominous, and sometimes lawless. It lies “beneath” the surface web – the layer where grandmothers post on Facebook and purchase on Amazon.
The dark web largely was the brainchild of three mathematicians at the Naval Research Laboratory as a means of encrypting messages exchanged by the intelligence community. They dubbed their project “Tor,” for “The Onion Router,” as the system consists of layer after layer of random relays, permitting anonymity on the Internet with little risk of tracking or surveillance.
This online underworld first came to my attention several years ago. The father of a teen who was being treated for disruptive mood dysregulation, attention-deficit disorder, and alcohol and cannabis use disorder called to inform me that his son had been arrested at Lollapalooza with hundreds of Adderall tablets and Xanax bars. Several weeks later, in session, the young man disclosed to me that he had found simple instructions online about installing Tor, creating a VPN (a virtual private network), accessing the dark web, transacting with bitcoin, and identifying drug marketplaces. He also demonstrated a detailed knowledge of chemical manufacturing in China, pill pressing in Canada, and money laundering in Switzerland.
With the air of an insider sharing “trade secrets,” this young man described how dealers on the dark web avoid detection and ensure secure delivery of the goods: latex gloves, vacuum sealing, and bleach dipping to obviate fingerprints – human and chemical. He said that dealers will send “dummy” packages to throw off the authorities and that buyers often will use the address of a clueless or absent neighbor. In his case, however, the parcels were delivered to his doorstep.
. For one thing, acquiring drugs in this way can seem less risky, as there is no chance of being robbed at gunpoint in a sketchy neighborhood or being busted for possession during a routine traffic stop.
Crossing over to the world of the dark web also can give an adolescent a sense of being clever and rebellious and of pulling a “fast one” on the parents – and on us, the clinicians who are treating them. And a teen who is interested in using illicit substances and plays “Call of Duty” from the comfort of his family’s basement without actual injury or death might assume that he can attain illicit drugs that are safe and inexpensive.
There are counterarguments to misinformation about the dark web. For example, contrary to the notion that buying drugs on the dark web minimizes interdiction or arrest, clinicians should point out that since international law enforcement shut down Silk Road and incarcerated its founder, Ross Ulbricht (also known as “Dread Pirate Roberts”) in 2013, hundreds of other dark web marketplaces such as AlphaBay and Hansa have been silenced and their operators prosecuted.
Moreover, the U.S. Department of Justice recently launched the Joint Criminal Opioid Darknet Enforcement (J-CODE) group, and the U.S. Postal Service Inspection Service reportedly has been hiring cybercrime and dark web specialists to combat drug trafficking. It might come as a surprise to a teen that, in a state where recreational marijuana is legal, transport and delivery of cannabis by the postal service elevates purchase and possession to the level of a violation of federal law. Informing even the most oblivious or oppositional adolescent that a drug felony can disqualify him for college grants and loans, impede his search for gainful employment, or prohibit him from obtaining a professional license, might give him a moment’s pause.
Adolescents seeking to buy drugs on the dark web should brace themselves for another shock. Whether lulled by custom after years of shopping on Amazon or using PayPal, or simply dulled by addiction, they might have a blind trust that bitcoin tumblers and “dark” escrow accounts will secure their payments. It will be rude awakening when they learn that the transfer and holding of currency on the dark web is vulnerable to hackers and to operators of the marketplaces – who are known to simply abscond with funds.
Currently, the drug marketplaces on the dark web represent a thin slice of the total illicit drug trade. These marketplaces, however, are growing quickly and offer buyers a virtual smorgasbord: the leading prescription drugs bought are Xanax and OxyContin, whereas 3,4-methylenedioxymethamphetamine (MDMA)/ecstasy and cannabis are the most commonly purchased controlled Schedule I substances, according to an article in The Economist. The estimated annual sales for 2016 ranged from $100 million to $200 million, but even more alarming is the percentage of substance abusers who have purchased drugs on the dark web: A recent article in The Independent reported that 13.2% of U.S. respondents self-reported making at least one purchase online, whereas in the United Kingdom, the self-reported percentage was 25.3 %, and in Finland, it was 41.4%.
Evidence abounds that drugs purchased on the dark web often are counterfeit and sometimes “dirty.” There is a report of “Viagra” containing cement dust, of “Ambien” containing haloperidol, and “Xanax” laced with fentanyl, the latter having been linked to several deaths and hospital admissions in the Bay Area in 2016. (Big Pharma, in fact, is engaged in surveillance and investigation of the sale of knockoffs online, The Telegraph reported in article about the proliferation of fake drugs available on the surface web).Unfortunately, attempting to reduce teen substance abuse using law enforcement measures directed at dark web marketplaces might be a game of whack-a-mole: As soon as one supply source is staunched, another surfaces. Indeed, addiction should be viewed not simply in terms of the biopsychosocial model, but also as an economic activity. Thus, it might be more beneficial for clinicians to concentrate our resources and efforts on curtailing the demand through education and treatment.
Dr. Marseille is a psychiatrist who works on the staff at a clinic in Winfield, Ill. His special interests include adolescent and addiction medicine, eating disorders, trauma, bipolar disorder, and the psychiatric manifestations of acute and chronic medical conditions.
There was a time, not so long ago, when in the popular imagination, a drug deal involved an aging hippie in a tie-dyed shirt and love beads, copping a joint at a Dead concert. Today, however, in the age of the Internet and smartphones, a teenager in his bedroom can select, order, and have delivered to his door illicit drugs via the marketplaces on the “dark web.”
As the name implies, the dark web is a subterranean layer of the Internet that is mysterious, ominous, and sometimes lawless. It lies “beneath” the surface web – the layer where grandmothers post on Facebook and purchase on Amazon.
The dark web largely was the brainchild of three mathematicians at the Naval Research Laboratory as a means of encrypting messages exchanged by the intelligence community. They dubbed their project “Tor,” for “The Onion Router,” as the system consists of layer after layer of random relays, permitting anonymity on the Internet with little risk of tracking or surveillance.
This online underworld first came to my attention several years ago. The father of a teen who was being treated for disruptive mood dysregulation, attention-deficit disorder, and alcohol and cannabis use disorder called to inform me that his son had been arrested at Lollapalooza with hundreds of Adderall tablets and Xanax bars. Several weeks later, in session, the young man disclosed to me that he had found simple instructions online about installing Tor, creating a VPN (a virtual private network), accessing the dark web, transacting with bitcoin, and identifying drug marketplaces. He also demonstrated a detailed knowledge of chemical manufacturing in China, pill pressing in Canada, and money laundering in Switzerland.
With the air of an insider sharing “trade secrets,” this young man described how dealers on the dark web avoid detection and ensure secure delivery of the goods: latex gloves, vacuum sealing, and bleach dipping to obviate fingerprints – human and chemical. He said that dealers will send “dummy” packages to throw off the authorities and that buyers often will use the address of a clueless or absent neighbor. In his case, however, the parcels were delivered to his doorstep.
. For one thing, acquiring drugs in this way can seem less risky, as there is no chance of being robbed at gunpoint in a sketchy neighborhood or being busted for possession during a routine traffic stop.
Crossing over to the world of the dark web also can give an adolescent a sense of being clever and rebellious and of pulling a “fast one” on the parents – and on us, the clinicians who are treating them. And a teen who is interested in using illicit substances and plays “Call of Duty” from the comfort of his family’s basement without actual injury or death might assume that he can attain illicit drugs that are safe and inexpensive.
There are counterarguments to misinformation about the dark web. For example, contrary to the notion that buying drugs on the dark web minimizes interdiction or arrest, clinicians should point out that since international law enforcement shut down Silk Road and incarcerated its founder, Ross Ulbricht (also known as “Dread Pirate Roberts”) in 2013, hundreds of other dark web marketplaces such as AlphaBay and Hansa have been silenced and their operators prosecuted.
Moreover, the U.S. Department of Justice recently launched the Joint Criminal Opioid Darknet Enforcement (J-CODE) group, and the U.S. Postal Service Inspection Service reportedly has been hiring cybercrime and dark web specialists to combat drug trafficking. It might come as a surprise to a teen that, in a state where recreational marijuana is legal, transport and delivery of cannabis by the postal service elevates purchase and possession to the level of a violation of federal law. Informing even the most oblivious or oppositional adolescent that a drug felony can disqualify him for college grants and loans, impede his search for gainful employment, or prohibit him from obtaining a professional license, might give him a moment’s pause.
Adolescents seeking to buy drugs on the dark web should brace themselves for another shock. Whether lulled by custom after years of shopping on Amazon or using PayPal, or simply dulled by addiction, they might have a blind trust that bitcoin tumblers and “dark” escrow accounts will secure their payments. It will be rude awakening when they learn that the transfer and holding of currency on the dark web is vulnerable to hackers and to operators of the marketplaces – who are known to simply abscond with funds.
Currently, the drug marketplaces on the dark web represent a thin slice of the total illicit drug trade. These marketplaces, however, are growing quickly and offer buyers a virtual smorgasbord: the leading prescription drugs bought are Xanax and OxyContin, whereas 3,4-methylenedioxymethamphetamine (MDMA)/ecstasy and cannabis are the most commonly purchased controlled Schedule I substances, according to an article in The Economist. The estimated annual sales for 2016 ranged from $100 million to $200 million, but even more alarming is the percentage of substance abusers who have purchased drugs on the dark web: A recent article in The Independent reported that 13.2% of U.S. respondents self-reported making at least one purchase online, whereas in the United Kingdom, the self-reported percentage was 25.3 %, and in Finland, it was 41.4%.
Evidence abounds that drugs purchased on the dark web often are counterfeit and sometimes “dirty.” There is a report of “Viagra” containing cement dust, of “Ambien” containing haloperidol, and “Xanax” laced with fentanyl, the latter having been linked to several deaths and hospital admissions in the Bay Area in 2016. (Big Pharma, in fact, is engaged in surveillance and investigation of the sale of knockoffs online, The Telegraph reported in article about the proliferation of fake drugs available on the surface web).Unfortunately, attempting to reduce teen substance abuse using law enforcement measures directed at dark web marketplaces might be a game of whack-a-mole: As soon as one supply source is staunched, another surfaces. Indeed, addiction should be viewed not simply in terms of the biopsychosocial model, but also as an economic activity. Thus, it might be more beneficial for clinicians to concentrate our resources and efforts on curtailing the demand through education and treatment.
Dr. Marseille is a psychiatrist who works on the staff at a clinic in Winfield, Ill. His special interests include adolescent and addiction medicine, eating disorders, trauma, bipolar disorder, and the psychiatric manifestations of acute and chronic medical conditions.