User login
Biosimilar Business Deals Keep Up ‘Musical Chairs’ Game of Formulary Construction
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).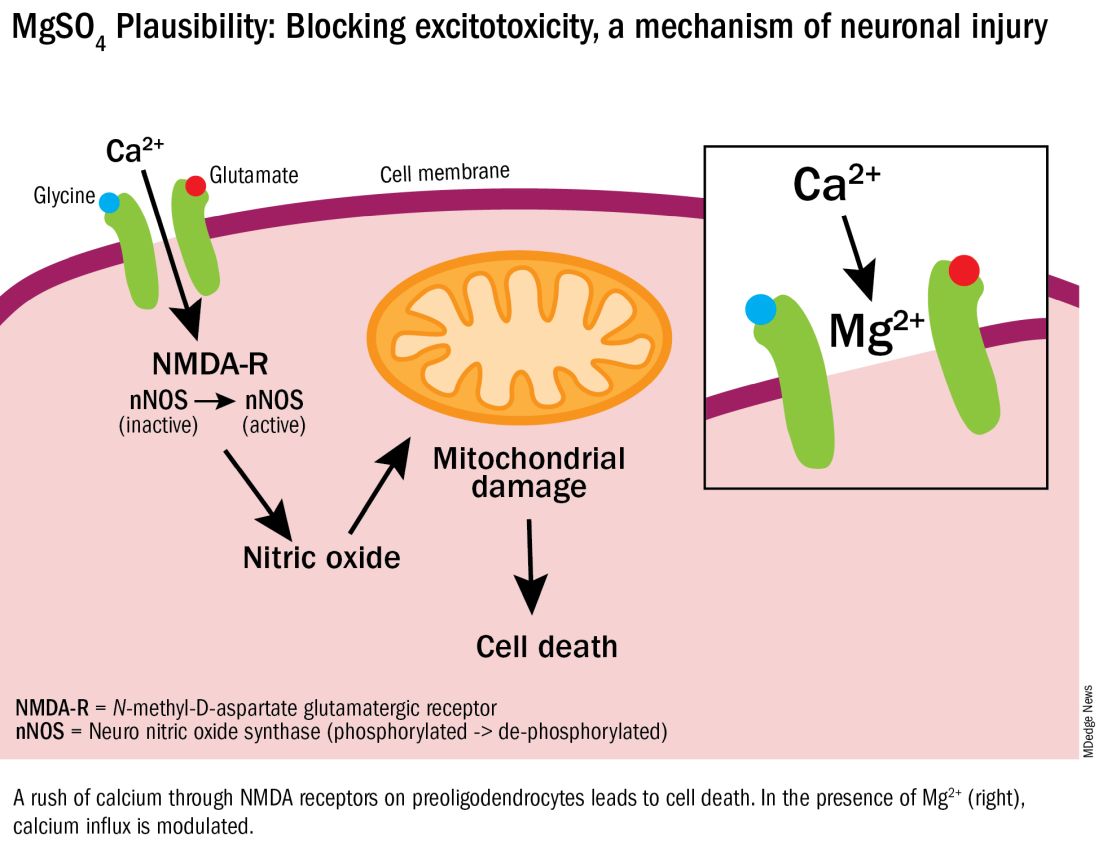
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.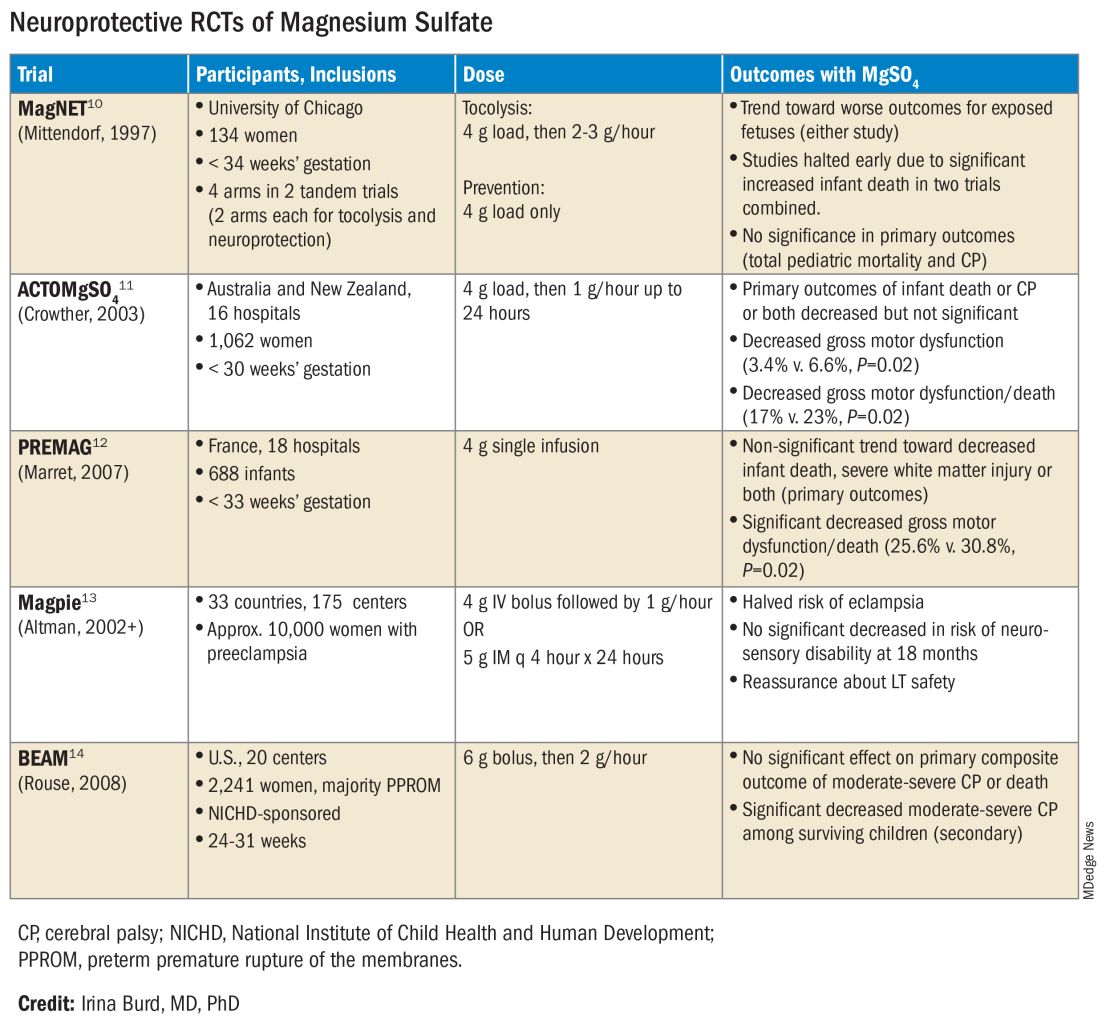
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
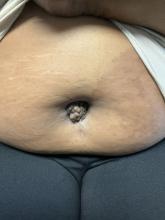
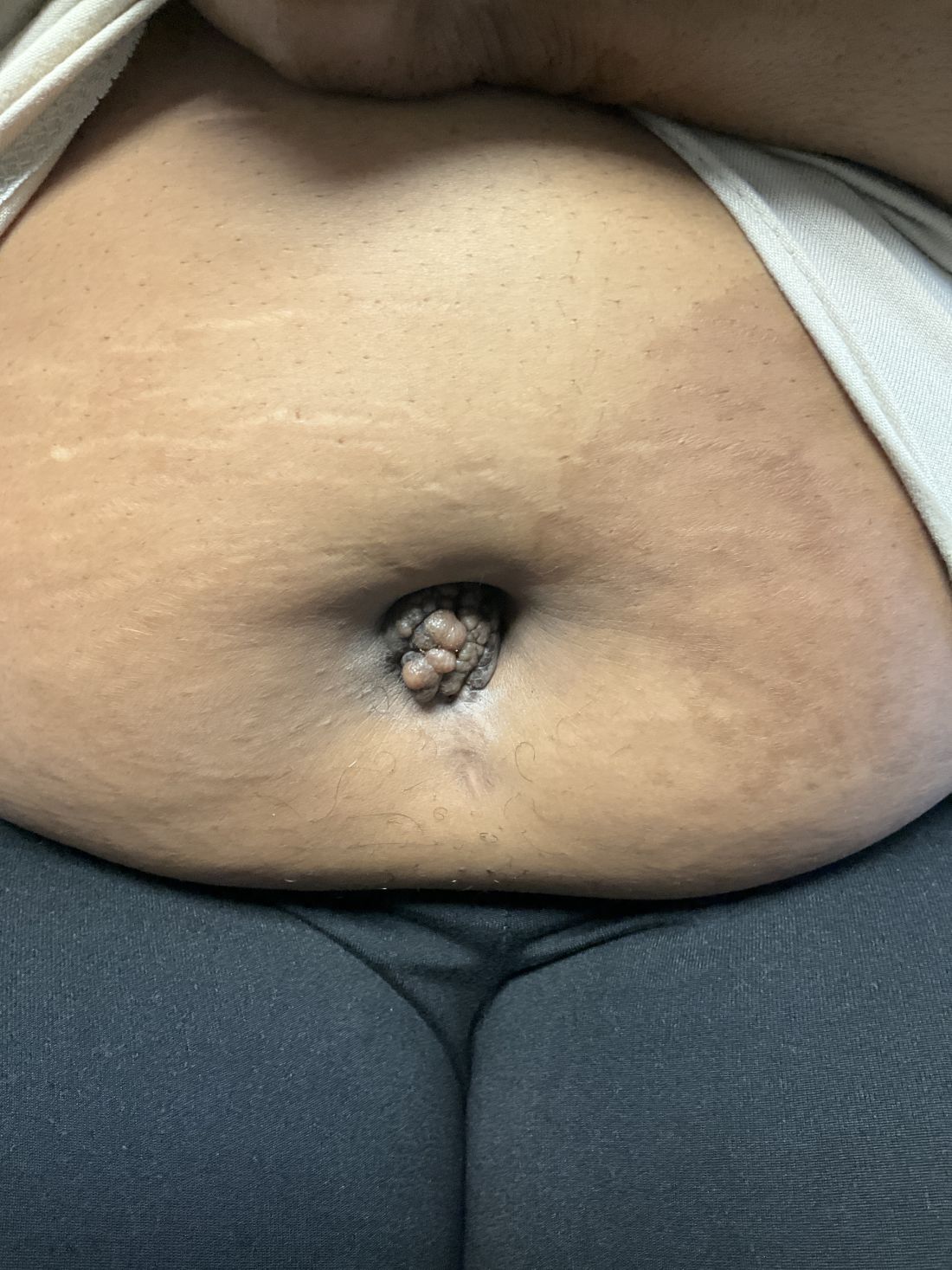
A 27-year-old Haitian woman presented with a painful umbilical mass which had been growing in size for 5 months
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Is This the Cure for Restless Legs?
I don’t rightly remember when I first learned of restless legs syndrome (RLS). It was many decades ago, and I recognized that once in a while, I would be restless during sleep, tossing and turning, seeking a favorable sleeping position. I felt like I just needed to move my legs around; my gastrocnemii and hamstrings might cramp; and my torso skin might strangely “crawl” a bit, but then normal sleep would return. I never sought medical care for it and used no treatment, except moving my legs when indicated.
My trusty LLM (large language model), Bard, tells me that there are about 53,000 articles about RLS in English, of which, some 20,000 are in the primary source, peer reviewed literature. Count this as one more article. Will it make a difference? Read on and see.
For many centuries (since Sir Thomas Willis in 1672), the symptoms now grouped and categorized as RLS have been recognized and reported but were often dismissed as bizarre and unexplained. The name was applied in 1948 by Dr Karl-Axel Ekborn.
In the 1960s, in sleep labs, RLS became better studied and characterized.
Mayo Clinic describes RLS as “… compelling, unpleasant sensations in the legs or feet ... both sides of the body ... within the limb rather than on the skin ... crawling, creeping, pulling, throbbing, aching, itching, electric ... difficult to explain …” Not numbness, but a consistent desire to move the legs.
When I read about it many decades ago, I realized that I may have RLS. But then many months would pass with no recurrence, so I dismissed it as just another of those “symptoms of unknown origin” that my late friend Clifton Meador has written about so eloquently.
I am sure that a lot of people experience this, don’t understand it, and don’t consider it important enough to do anything about. The cause is unknown, but it seems to run in families. It may be autosomal dominant, but no causative genes have been confirmed.
Treatment of RLS
Many pharmacologic and physical treatments have been tried with some success for some patients, but over time, these treatments have mostly failed.
We know how Big Pharma often operates. A company owns a drug, preferably under patent protection, but without an apparent profitable indication. They need to find a medical condition, ideally one with troublesome symptoms, that the drug might ameliorate to some degree. Armed with a plausible candidate symptom, the company embarks upon a campaign to find people who might want to take the drug. Mass communications, such as direct-to-consumer advertising, can identify large numbers of people who match to pretty much any symptoms, although many of these people never suspected they had a disease, much less a treatable one.
I figured long ago that RLS was just another of those nonspecific entities experienced by many people, making them good candidates for disease mongering.
In 2005, the marketing of GlaxoSmithKline’s (GSK’s) dopamine agonist drug Requip (ropinirole) was approved by the FDA. GSK had already undertaken an intensive promotional campaign for Requip, issuing press releases, advertising to doctors in medical journals, and advertising directly to consumers. To increase general awareness of RLS, GSK’s campaign told consumers that a “new survey reveals that a common yet underrecognized disorder-restless legs syndrome—is keeping Americans awake at night.” GSK was accused of “disease mongering,” trying to turn ordinary people into patients who needed specific drugs.
Within a year, sales of the drug had doubled, climbing from $165 million in 2005 to nearly $330 million in 2006. Soon, 4.4 million prescriptions were written annually for the drug, with sales reported to be nearly $491 million. However, the focus on RLS faded rapidly as the Requip television commercials were pulled from the airwaves following approval of generic ropinirole.
And Requip had competition. Boehringer Ingelheim manufactures pramipexole (brand name Mirapex) another dopamine agonist. Gabapentin enacarbil (marketed as Horizant by UCB Pharma) is also approved for RLS, and Pfizer’s pregabalin (brand name Lyrica) is used off-label to manage symptoms of RLS. Janssen Pharmaceuticals manufactures rotigotine, (brand name Neupro), a dopamine agonist delivered via a transdermal patch.
It is safe to say that RLS is a real clinical entity composed of clearly recognizable symptoms, with no cure and no ending, unless it is associated with iron-deficiency anemia. However, as a disease, it seems to lack etiology, pathology, pathogenesis, pathophysiology, diagnostic findings on physical examination, laboratory tests, or imaging, and any clear strategy for prevention.
Pharmacologic treatments include dopaminergic agents, benzodiazepines, opioids, anticonvulsants, alpha 2–adrenergic agonists and iron salts. Yes, you read that right; RLS is treated with a broad array of different drugs, which is usually a sign that nothing works very well. Some agents work for a while, but none seem to be the definitive solution.
Same for the physical interventions: sleep hygiene, exercise, hot or cold bathing, limb massage, vibratory or electrical stimulation of the feet, stopping caffeine before bedtime. Try everything and see if something works.
Taking the Sugar Challenge
Could the culprit be sugar?
Lacking clarity of scientific understanding of RLS or its treatment from an extensive clinical literature, after ascertaining that RLS is real, one might look for real-world evidence, including well-performed N-of-1 trials.
I am an antisugar guy. Read my prior Medscape columns. I practice what I preach, but sugar does taste good.
Early in November 2023, after a healthy, conservative dinner at home with some wine, I enjoyed a mini Dove bar for dessert. But I didn’t stop there.
Mini Dove bars contain 11 grams sugar. It was also just a few days after Halloween. Having had fewer trick-or-treaters than expected, we had leftovers. Snickers, Milky Ways, Twix mini bars, each with at least 20 grams of sugar.
I ate several of these not long before bedtime. Lo and behold, in the dark of that night, and continuing off and on for a few fitful hours, I had bad RLS. Shifting, tossing, turning, compulsively seeking a new sleeping position only to have to soon move again. Plus, I had repetitive leg cramps and that creepy-crawly skin sensation. An altogether unpleasant experience. Sound sleep eventually arrived, and there were no recurrences over subsequent weeks.
The classic way to determine whether a drug is causing a reaction, condition, or disease is to apply the challenge-dechallenge-rechallenge testing method.
Give the drug, the patient demonstrates the disease finding. Remove the drug, the problem disappears. Rinse and repeat three times. We pathologists first worked this out for drug-induced liver disease, such as steatosis, in the late 1960s. Blinding or double blinding in these N-of-1 situations would be nice but often not practical.
Siwert de Groot, in the Netherlands, published a very convincing use of this technique in 2023: Big-time sugar consumption for a week, then low intake of sugar for the following week, repeated three times on one patient.
Very elaborate RLS symptom reporting. I’m pretty convinced from my unintentional challenge and single dechallenge that my unusually high sugar intake resulted in RLS. I will not undergo a rechallenge, although it might be fun to binge on sucrose and see what happens.
If you are serious about identifying or treating RLS, I suggest that you incorporate the International Restless Legs Study Group Severity Rating Scale into your practice, and begin the systematic use of the dechallenge-rechallenge exclusion process for your patients with RLS. Start with sugar and see what happens. Keep records and let the world know what you discover. Be your own clinical investigator. Social media offers you abundant opportunity to share your results, whatever they may be.
How many millions of dollars would Big Pharma lose if patients with RLS just said no to sugar and it worked? Of course, humans being humans, many would probably prefer to continue to gorge on sugar, gain weight, develop diabetes, and then take medications to control their RLS symptoms. But patients ought to at least be given an informed choice.
I will be watching for your reports.
Dr. Lundberg had no disclosures.
A version of this article appeared on Medscape.com.
I don’t rightly remember when I first learned of restless legs syndrome (RLS). It was many decades ago, and I recognized that once in a while, I would be restless during sleep, tossing and turning, seeking a favorable sleeping position. I felt like I just needed to move my legs around; my gastrocnemii and hamstrings might cramp; and my torso skin might strangely “crawl” a bit, but then normal sleep would return. I never sought medical care for it and used no treatment, except moving my legs when indicated.
My trusty LLM (large language model), Bard, tells me that there are about 53,000 articles about RLS in English, of which, some 20,000 are in the primary source, peer reviewed literature. Count this as one more article. Will it make a difference? Read on and see.
For many centuries (since Sir Thomas Willis in 1672), the symptoms now grouped and categorized as RLS have been recognized and reported but were often dismissed as bizarre and unexplained. The name was applied in 1948 by Dr Karl-Axel Ekborn.
In the 1960s, in sleep labs, RLS became better studied and characterized.
Mayo Clinic describes RLS as “… compelling, unpleasant sensations in the legs or feet ... both sides of the body ... within the limb rather than on the skin ... crawling, creeping, pulling, throbbing, aching, itching, electric ... difficult to explain …” Not numbness, but a consistent desire to move the legs.
When I read about it many decades ago, I realized that I may have RLS. But then many months would pass with no recurrence, so I dismissed it as just another of those “symptoms of unknown origin” that my late friend Clifton Meador has written about so eloquently.
I am sure that a lot of people experience this, don’t understand it, and don’t consider it important enough to do anything about. The cause is unknown, but it seems to run in families. It may be autosomal dominant, but no causative genes have been confirmed.
Treatment of RLS
Many pharmacologic and physical treatments have been tried with some success for some patients, but over time, these treatments have mostly failed.
We know how Big Pharma often operates. A company owns a drug, preferably under patent protection, but without an apparent profitable indication. They need to find a medical condition, ideally one with troublesome symptoms, that the drug might ameliorate to some degree. Armed with a plausible candidate symptom, the company embarks upon a campaign to find people who might want to take the drug. Mass communications, such as direct-to-consumer advertising, can identify large numbers of people who match to pretty much any symptoms, although many of these people never suspected they had a disease, much less a treatable one.
I figured long ago that RLS was just another of those nonspecific entities experienced by many people, making them good candidates for disease mongering.
In 2005, the marketing of GlaxoSmithKline’s (GSK’s) dopamine agonist drug Requip (ropinirole) was approved by the FDA. GSK had already undertaken an intensive promotional campaign for Requip, issuing press releases, advertising to doctors in medical journals, and advertising directly to consumers. To increase general awareness of RLS, GSK’s campaign told consumers that a “new survey reveals that a common yet underrecognized disorder-restless legs syndrome—is keeping Americans awake at night.” GSK was accused of “disease mongering,” trying to turn ordinary people into patients who needed specific drugs.
Within a year, sales of the drug had doubled, climbing from $165 million in 2005 to nearly $330 million in 2006. Soon, 4.4 million prescriptions were written annually for the drug, with sales reported to be nearly $491 million. However, the focus on RLS faded rapidly as the Requip television commercials were pulled from the airwaves following approval of generic ropinirole.
And Requip had competition. Boehringer Ingelheim manufactures pramipexole (brand name Mirapex) another dopamine agonist. Gabapentin enacarbil (marketed as Horizant by UCB Pharma) is also approved for RLS, and Pfizer’s pregabalin (brand name Lyrica) is used off-label to manage symptoms of RLS. Janssen Pharmaceuticals manufactures rotigotine, (brand name Neupro), a dopamine agonist delivered via a transdermal patch.
It is safe to say that RLS is a real clinical entity composed of clearly recognizable symptoms, with no cure and no ending, unless it is associated with iron-deficiency anemia. However, as a disease, it seems to lack etiology, pathology, pathogenesis, pathophysiology, diagnostic findings on physical examination, laboratory tests, or imaging, and any clear strategy for prevention.
Pharmacologic treatments include dopaminergic agents, benzodiazepines, opioids, anticonvulsants, alpha 2–adrenergic agonists and iron salts. Yes, you read that right; RLS is treated with a broad array of different drugs, which is usually a sign that nothing works very well. Some agents work for a while, but none seem to be the definitive solution.
Same for the physical interventions: sleep hygiene, exercise, hot or cold bathing, limb massage, vibratory or electrical stimulation of the feet, stopping caffeine before bedtime. Try everything and see if something works.
Taking the Sugar Challenge
Could the culprit be sugar?
Lacking clarity of scientific understanding of RLS or its treatment from an extensive clinical literature, after ascertaining that RLS is real, one might look for real-world evidence, including well-performed N-of-1 trials.
I am an antisugar guy. Read my prior Medscape columns. I practice what I preach, but sugar does taste good.
Early in November 2023, after a healthy, conservative dinner at home with some wine, I enjoyed a mini Dove bar for dessert. But I didn’t stop there.
Mini Dove bars contain 11 grams sugar. It was also just a few days after Halloween. Having had fewer trick-or-treaters than expected, we had leftovers. Snickers, Milky Ways, Twix mini bars, each with at least 20 grams of sugar.
I ate several of these not long before bedtime. Lo and behold, in the dark of that night, and continuing off and on for a few fitful hours, I had bad RLS. Shifting, tossing, turning, compulsively seeking a new sleeping position only to have to soon move again. Plus, I had repetitive leg cramps and that creepy-crawly skin sensation. An altogether unpleasant experience. Sound sleep eventually arrived, and there were no recurrences over subsequent weeks.
The classic way to determine whether a drug is causing a reaction, condition, or disease is to apply the challenge-dechallenge-rechallenge testing method.
Give the drug, the patient demonstrates the disease finding. Remove the drug, the problem disappears. Rinse and repeat three times. We pathologists first worked this out for drug-induced liver disease, such as steatosis, in the late 1960s. Blinding or double blinding in these N-of-1 situations would be nice but often not practical.
Siwert de Groot, in the Netherlands, published a very convincing use of this technique in 2023: Big-time sugar consumption for a week, then low intake of sugar for the following week, repeated three times on one patient.
Very elaborate RLS symptom reporting. I’m pretty convinced from my unintentional challenge and single dechallenge that my unusually high sugar intake resulted in RLS. I will not undergo a rechallenge, although it might be fun to binge on sucrose and see what happens.
If you are serious about identifying or treating RLS, I suggest that you incorporate the International Restless Legs Study Group Severity Rating Scale into your practice, and begin the systematic use of the dechallenge-rechallenge exclusion process for your patients with RLS. Start with sugar and see what happens. Keep records and let the world know what you discover. Be your own clinical investigator. Social media offers you abundant opportunity to share your results, whatever they may be.
How many millions of dollars would Big Pharma lose if patients with RLS just said no to sugar and it worked? Of course, humans being humans, many would probably prefer to continue to gorge on sugar, gain weight, develop diabetes, and then take medications to control their RLS symptoms. But patients ought to at least be given an informed choice.
I will be watching for your reports.
Dr. Lundberg had no disclosures.
A version of this article appeared on Medscape.com.
I don’t rightly remember when I first learned of restless legs syndrome (RLS). It was many decades ago, and I recognized that once in a while, I would be restless during sleep, tossing and turning, seeking a favorable sleeping position. I felt like I just needed to move my legs around; my gastrocnemii and hamstrings might cramp; and my torso skin might strangely “crawl” a bit, but then normal sleep would return. I never sought medical care for it and used no treatment, except moving my legs when indicated.
My trusty LLM (large language model), Bard, tells me that there are about 53,000 articles about RLS in English, of which, some 20,000 are in the primary source, peer reviewed literature. Count this as one more article. Will it make a difference? Read on and see.
For many centuries (since Sir Thomas Willis in 1672), the symptoms now grouped and categorized as RLS have been recognized and reported but were often dismissed as bizarre and unexplained. The name was applied in 1948 by Dr Karl-Axel Ekborn.
In the 1960s, in sleep labs, RLS became better studied and characterized.
Mayo Clinic describes RLS as “… compelling, unpleasant sensations in the legs or feet ... both sides of the body ... within the limb rather than on the skin ... crawling, creeping, pulling, throbbing, aching, itching, electric ... difficult to explain …” Not numbness, but a consistent desire to move the legs.
When I read about it many decades ago, I realized that I may have RLS. But then many months would pass with no recurrence, so I dismissed it as just another of those “symptoms of unknown origin” that my late friend Clifton Meador has written about so eloquently.
I am sure that a lot of people experience this, don’t understand it, and don’t consider it important enough to do anything about. The cause is unknown, but it seems to run in families. It may be autosomal dominant, but no causative genes have been confirmed.
Treatment of RLS
Many pharmacologic and physical treatments have been tried with some success for some patients, but over time, these treatments have mostly failed.
We know how Big Pharma often operates. A company owns a drug, preferably under patent protection, but without an apparent profitable indication. They need to find a medical condition, ideally one with troublesome symptoms, that the drug might ameliorate to some degree. Armed with a plausible candidate symptom, the company embarks upon a campaign to find people who might want to take the drug. Mass communications, such as direct-to-consumer advertising, can identify large numbers of people who match to pretty much any symptoms, although many of these people never suspected they had a disease, much less a treatable one.
I figured long ago that RLS was just another of those nonspecific entities experienced by many people, making them good candidates for disease mongering.
In 2005, the marketing of GlaxoSmithKline’s (GSK’s) dopamine agonist drug Requip (ropinirole) was approved by the FDA. GSK had already undertaken an intensive promotional campaign for Requip, issuing press releases, advertising to doctors in medical journals, and advertising directly to consumers. To increase general awareness of RLS, GSK’s campaign told consumers that a “new survey reveals that a common yet underrecognized disorder-restless legs syndrome—is keeping Americans awake at night.” GSK was accused of “disease mongering,” trying to turn ordinary people into patients who needed specific drugs.
Within a year, sales of the drug had doubled, climbing from $165 million in 2005 to nearly $330 million in 2006. Soon, 4.4 million prescriptions were written annually for the drug, with sales reported to be nearly $491 million. However, the focus on RLS faded rapidly as the Requip television commercials were pulled from the airwaves following approval of generic ropinirole.
And Requip had competition. Boehringer Ingelheim manufactures pramipexole (brand name Mirapex) another dopamine agonist. Gabapentin enacarbil (marketed as Horizant by UCB Pharma) is also approved for RLS, and Pfizer’s pregabalin (brand name Lyrica) is used off-label to manage symptoms of RLS. Janssen Pharmaceuticals manufactures rotigotine, (brand name Neupro), a dopamine agonist delivered via a transdermal patch.
It is safe to say that RLS is a real clinical entity composed of clearly recognizable symptoms, with no cure and no ending, unless it is associated with iron-deficiency anemia. However, as a disease, it seems to lack etiology, pathology, pathogenesis, pathophysiology, diagnostic findings on physical examination, laboratory tests, or imaging, and any clear strategy for prevention.
Pharmacologic treatments include dopaminergic agents, benzodiazepines, opioids, anticonvulsants, alpha 2–adrenergic agonists and iron salts. Yes, you read that right; RLS is treated with a broad array of different drugs, which is usually a sign that nothing works very well. Some agents work for a while, but none seem to be the definitive solution.
Same for the physical interventions: sleep hygiene, exercise, hot or cold bathing, limb massage, vibratory or electrical stimulation of the feet, stopping caffeine before bedtime. Try everything and see if something works.
Taking the Sugar Challenge
Could the culprit be sugar?
Lacking clarity of scientific understanding of RLS or its treatment from an extensive clinical literature, after ascertaining that RLS is real, one might look for real-world evidence, including well-performed N-of-1 trials.
I am an antisugar guy. Read my prior Medscape columns. I practice what I preach, but sugar does taste good.
Early in November 2023, after a healthy, conservative dinner at home with some wine, I enjoyed a mini Dove bar for dessert. But I didn’t stop there.
Mini Dove bars contain 11 grams sugar. It was also just a few days after Halloween. Having had fewer trick-or-treaters than expected, we had leftovers. Snickers, Milky Ways, Twix mini bars, each with at least 20 grams of sugar.
I ate several of these not long before bedtime. Lo and behold, in the dark of that night, and continuing off and on for a few fitful hours, I had bad RLS. Shifting, tossing, turning, compulsively seeking a new sleeping position only to have to soon move again. Plus, I had repetitive leg cramps and that creepy-crawly skin sensation. An altogether unpleasant experience. Sound sleep eventually arrived, and there were no recurrences over subsequent weeks.
The classic way to determine whether a drug is causing a reaction, condition, or disease is to apply the challenge-dechallenge-rechallenge testing method.
Give the drug, the patient demonstrates the disease finding. Remove the drug, the problem disappears. Rinse and repeat three times. We pathologists first worked this out for drug-induced liver disease, such as steatosis, in the late 1960s. Blinding or double blinding in these N-of-1 situations would be nice but often not practical.
Siwert de Groot, in the Netherlands, published a very convincing use of this technique in 2023: Big-time sugar consumption for a week, then low intake of sugar for the following week, repeated three times on one patient.
Very elaborate RLS symptom reporting. I’m pretty convinced from my unintentional challenge and single dechallenge that my unusually high sugar intake resulted in RLS. I will not undergo a rechallenge, although it might be fun to binge on sucrose and see what happens.
If you are serious about identifying or treating RLS, I suggest that you incorporate the International Restless Legs Study Group Severity Rating Scale into your practice, and begin the systematic use of the dechallenge-rechallenge exclusion process for your patients with RLS. Start with sugar and see what happens. Keep records and let the world know what you discover. Be your own clinical investigator. Social media offers you abundant opportunity to share your results, whatever they may be.
How many millions of dollars would Big Pharma lose if patients with RLS just said no to sugar and it worked? Of course, humans being humans, many would probably prefer to continue to gorge on sugar, gain weight, develop diabetes, and then take medications to control their RLS symptoms. But patients ought to at least be given an informed choice.
I will be watching for your reports.
Dr. Lundberg had no disclosures.
A version of this article appeared on Medscape.com.
Cannabis for Psychiatric Disorders? ‘Not Today,’ Experts Say
This transcript has been edited for clarity.
Stephen M. Strakowski, MD: Hello. Thank you all for joining us today. I’m very excited to have some great guests to talk about what I consider an active controversy. I’m Stephen M. Strakowski. I’m a professor and vice chair of psychiatry at Indiana University, and professor and associate vice president at University of Texas in Austin.
Today we’re going to talk about cannabis. As all of you are aware, everyone’s talking about cannabis. We hear constantly on social media and in interviews, particularly with relevance to psychiatric disorders, that everyone should be thinking about using cannabis. That seems to be the common conversation.
Last week, I had a patient who said, “All my friends tell me I need to be on cannabis.” That was their solution to her problems. With that in mind, let me introduce our guests, who are both experts on this, to talk about the role of cannabis in psychiatric disorders today.
First, I want to welcome Dr. Leslie Hulvershorn. Dr. Hulvershorn is an associate professor and chair at Indiana University in Indianapolis. Dr. Christopher Hammond is an assistant professor and the director of the co-occurring disorders program at Johns Hopkins. Welcome!
Leslie A. Hulvershorn, MD, MSc: Thank you.
Christopher J. Hammond, MD, PhD: Thank you.
Dr. Strakowski: Leslie, as I mentioned, many people are talking about how cannabis could be a good treatment for psychiatric disorders. Is that true?
Dr. Hulvershorn: If you look at what defines a good treatment, what you’re looking for is clinical trials, ideally randomized, placebo-controlled clinical trials.
When we look at research related to cannabis, we see very few of those trials, and we see that the cannabis plant is actually quite complicated and there are many different compounds that come from it. So we need to look at all the different compounds.
If you think about THC, delta 9 or delta 8, depending on the version, that’s the active ingredient that we most often think about when we say “cannabis.” If you look at THC studies, there really is no evidence that I could find that it helps psychiatric disorders.
What we do find is an enormous literature, many hunDr.eds of studies, actually, that show that THC actually worsens or even brings on psychiatric disorders. There’s a separate conversation about other compounds within the cannabis plant, like CBD, cannabidiol, where there’s maybe a signal that certain anxiety disorders might be improved by a compound like that.
Certainly, rare forms of epilepsy have been found to be improved with that compound. It really depends on what you’re looking at within the cannabis plant, but if we’re thinking about THC, the answer really is no, this is not a helpful thing. In fact, it’s probably a harmful thing to be ingesting in terms of psychiatric disorders.
Dr. Strakowski: Thank you, Leslie. Chris, what would you add to that? Do we know anything about the use of cannabis in any psychiatric condition?
Dr. Hammond: I definitely would echo what Leslie said. The popular opinion, that the media and the state legislatures have really, in many ways, put the cart before the horse — they speak about cannabis as a medication for the treatment of psychiatric conditions before we have sufficient evidence to say that it’s safe or effective for these conditions. Most of the evidence that we have, particularly in regard to the cannabinoid compound, delta 9, tetrahyDr.ocannabinol, or THC, suggests that that cannabinoid is associated with adverse mental health outcomes across different categories.
Dr. Strakowski: Our group, a long time ago, conducted a study looking at first episode of mania, and found that regular cannabis use increases the risk for subsequent manic episodes. I’m not aware of many other studies like that.
You referred, Chris, to the safety aspect. This is something anybody can use. There are no negative consequences. Is that true? I mean, is it really risk free?
Dr. Hammond: Research shows that that’s an inaccurate framing of the safety profile of cannabis. Again, as Leslie put it very well, cannabis is many different compounds. Using this catchall phrase of «cannabis» is not very helpful.
In regard to the main bioactive compounds of the cannabis plant, THC and cannabidiol, or CBD, what we know from studies of THC administration and from medications that have been designed to mimic THC and act on receptors that THC acts on is that those medications have clear side effects and adverse events in a percentage of patients who take them, particularly in regard to precipitating panic attacks, dysphoric episodes, and psychosis in some individuals.
Dr. Hulvershorn: I would add that it really depends on the age of the person that you’re talking about and when they’re first exposed to cannabis. If you’re talking about a person, say, under the age of 14 who uses cannabis, there’s a large amount of concern about the worsening of psychosis and mental health symptoms, but also cognitive features like memory.
There’s a very good study that was conducted in New Zealand that followed a large number of kids over time and showed significant decreases in working memory capacity for kids who used quite heavily.
Then you think about pregnant women. That’s very interesting literature, where people are finding that cannabis not only affects brain development but also a host of other systems in the body. For example, I think the risk for asthma is increased. If you look at the genes in the placenta that are affected, it has much to do with the immune system.
Women who are using cannabis during pregnancy are really exposing their fetus to a range of potential risks that we certainly don’t understand well enough, but there’s enough science that suggests this is really concerning.
If you take a step back and look at animal models, even with things like CBD products, which, again, everybody seems to be buying and they’re viewed as very safe — it’s almost hard to find things without CBD these days.
There we find, for example, in developing rats that testicular development seems to be affected with high doses of CBD. There’s just a huge array of effects, even outside of the psychiatric world, that make me very nervous about anyone using, especially a pregnant woman or a young person.
Then there’s a whole separate literature on adults. It’s hard to find studies that suggest this is a great idea. You’re going to find on the mental health side of things, and the cognitive side of things, many effects as well.
I, personally, am agnostic one way or the other. If cannabis turns out to be helpful, great. We love things that are helpful in medicine. We don’t really care where they come from. I’m not biased politically one way or the other. It’s just when you look at the totality of the literature, it’s hard to feel excited about people using cannabis at any age.
Dr. Hammond: It’s difficult to interpret the literature because of some biases there. It speaks to the importance of thoughtful research being done in this space that takes a neutral approach to assessing cannabis and looking for evidence of both potential benefit and potential harm.
The other piece that I think is of value that builds off what Leslie mentioned is the effects of cannabis and THC. The risk for harm appears to be greater in pregnant women and in young people. For adults, I think, we’re also still trying to understand what the effects are.
The other way of parsing out effects and thinking about them is in terms of the acute effects and the acute response in the moment right after one ingests cannabis vs the long-term effects.
After acute ingestion of cannabis, it can precipitate a psychotic episode, dysphoria or severe depressive symptoms, or severe anxiety, and can cause one to be disoriented, have delayed response time, and affect the ability to Dr.ive. In that capacity, it is related to a higher risk for motor vehicle crashes.
Dr. Strakowski: That’s very interesting. In my practice, and maybe it’s atypical, but half to two thirds of my patients, particularly the younger ones, are using cannabis in some form or another. In my experience, if they’re under 21, they’re more likely to use cannabis than alcohol.
What do we tell our patients? Is there a safe level of use? Do we say to never touch it? How do we manage the social pressure and environment that our patients have to live in?
Dr. Hulvershorn: I think about what we call motivational interviewing and the substance use disorder field, which is a style of interacting with someone that’s very neutral to discuss the pros and the cons. In my practice, people are usually coming to us because of problems related to their substance use.
Not everyone is experiencing those, but for those people, it’s a pretty easy discussion. It sounds like you’re getting into trouble. Your athletic performance is suffering. Your scholastic performance is suffering.
You walk them toward understanding that, wait a minute, if I smoked less weed or no weed, I would probably be doing better in this or that domain of my life. That seems to be the most helpful thing, by allowing them to come to that conclusion.
I think it is a more difficult conversation for people who don’t identify any problems related to their use. What is the right answer? Again, I just go back to saying, “Is this good for you? It’s hard to find the literature that suggests that. Is it neutral for you? Maybe, for some people. Is it harmful for some people? Absolutely.”
I think, for me, the most impactful studies have been those that showed for certain people with certain genetic makeup, cannabis is an absolutely terrible idea. Their risk for psychosis development and things like that are so high. For other people, they could smoke weed all day and never have a problem, based on their genetics — maybe. We don’t know. It’s not like we’re doing blood tests to figure out who you are.
The safest advice, I think, is no use. That’s never going to be bad advice.
Dr. Hammond: I mostly agree with Leslie on this point but feel very, very strongly that — in this era, where in the context of popular media, celebrities and other people are stating that cannabis is good and should be put in everything — clinical providers, especially pediatric providers, need to be extremely grounded in the science, and not let popular media sway our approach and strategy for working with these young people.
There’s two decades worth of data from longitudinal studies that have followed individuals from birth or from preadolescence into their thirties and forties, that show us that, for this association between cannabis use and later adverse mental health outcomes, there is a dose effect there.
The earlier an individual starts using, the more frequent they use, and more persistent their use is over time, those individuals have poorer mental health outcomes compared with individuals who choose to abstain or individuals who use just a few times and stop.
There’s also a signal for higher-THC-potency products being associated with poorer mental health outcomes, particularly when used during adolescence.
I apply a motivational interviewing approach as well to disseminate this information to both the young people and their parents about the risks, and to communicate what the data clearly show in regard to using THC-based cannabinoid products, which is that we don’t have evidence that shows that any use is healthy to the developing brain.
There’s a large amount of evidence that suggests it’s harmful to the developing brain, so the recommendation is not to use, to delay the onset of use, if you want to use, until adulthood. Many youth choose to use. For those young people, we meet them where they’re at and try to work with them on cutting down.
Dr. Strakowski: Thank you both. There’s an interesting effort in different states, with lobbying by celebrities and legislators pushing insurance companies to fund cannabis use broadly, including in a number of psychiatric indications, with no FDA approval at this point. Do you support that? Is that a good idea?
Dr. Hammond: Absolutely not.
Dr. Strakowski: Thank you.
Dr. Hammond: I think that’s a very important statement to make. For the medical and healthcare profession to stand strong related to states requiring insurance companies to cover medical cannabis really opens the door to lawsuits that would force insurance companies to cover other undertested bioactive chemicals and health supplements.
There are insufficient safety data for medical cannabis for FDA approval for any condition right now. The FDA has approved cannabinoid-based medications. Those cannabinoid-based medications have really undergone rigorous safety and efficacy testing, and have been approved for very narrow indications, none of which are psychiatric conditions.
They’ve been approved for chemotherapy-associated nausea and vomiting, treatment-resistant seizures related to two rare seizure disorders that emerge during childhood, and related to tuberous sclerosis, and one related to treating multiple sclerosis–associated spasticity and central neuropathic pain.
Dr. Hulvershorn: Steve, I think it’s important for listeners to be aware that there is a process in place for any therapeutic to become tested and reviewed. We see an industry that stands to make an enormous amount of money, and that is really the motivation for this industry.
These are not folks who are, out of the kindness of their heart, just hoping for better treatments for people. There are many ways you could channel that desire that does not include cannabis making money.
It’s really a profit-motivated industry. They’re very effective at lobbying. The public, unfortunately, has been sort of manipulated by this industry to believe that these are healthy, safe, and natural just because they grow in the ground.
Unfortunately, that’s really the issue. I think people just need to keep that in mind. Someone stands to make a large amount of money off of this. This is a very calculated, strategic approach that goes state by state but is nationally organized, and is potentially, like Chris says, for many reasons, really harmful.
I see it as sort of a bullying approach. Like if your Dr.ug works, Medicaid will pay for it. Medicaid in each state will review the studies. The FDA obviously leads the way. To cut the line without the research is really not helpful — circumventing the process that’s been in place for a long time and works well.
Dr. Hammond: Yes, it sets a dangerous precedent.
Dr. Strakowski: I was going to add the same, that it’s potentially dangerous. Thank you both, Dr.s Hulvershorn and Hammond, for a really good, lively discussion. I know we could talk for a very long time about this situation.
I do think it’s clear for listeners, most of whom are practitioners, that at this point in time, there just really does not seem to be strong evidence for the use of cannabis-based products for any psychiatric condition.
I do think we have to approach the people we’re working with around their psychiatric conditions to manage use and abuse wisely, like we would with any other substance. I appreciate everyone who’s tuned in today to watch us. I hope this is useful for your practice. Thank you.
Stephen M. Strakowski, MD, has disclosed the following relevant financial relationships:
- Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Roche; Procter & Gamble; Novartis; Sunovion
- Received income in an amount equal to or greater than $250 from: Roche; Procter & Gamble; Novartis; Sunovion; Oxford University Press
Leslie A. Hulvershorn, MD, MSc, has disclosed the following relevant financial relationships:
- Received income in an amount equal to or greater than $250 from: Greenwich Biosciences, educational grant for Summit
Christopher J. Hammond, MD, PhD, has disclosed the following relevant financial relationships:
- Received research grant from National Institutes of Health Grants; Bench to Bench Award; Substance Abuse and Mental Health Services Administration; Doris Duke.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Stephen M. Strakowski, MD: Hello. Thank you all for joining us today. I’m very excited to have some great guests to talk about what I consider an active controversy. I’m Stephen M. Strakowski. I’m a professor and vice chair of psychiatry at Indiana University, and professor and associate vice president at University of Texas in Austin.
Today we’re going to talk about cannabis. As all of you are aware, everyone’s talking about cannabis. We hear constantly on social media and in interviews, particularly with relevance to psychiatric disorders, that everyone should be thinking about using cannabis. That seems to be the common conversation.
Last week, I had a patient who said, “All my friends tell me I need to be on cannabis.” That was their solution to her problems. With that in mind, let me introduce our guests, who are both experts on this, to talk about the role of cannabis in psychiatric disorders today.
First, I want to welcome Dr. Leslie Hulvershorn. Dr. Hulvershorn is an associate professor and chair at Indiana University in Indianapolis. Dr. Christopher Hammond is an assistant professor and the director of the co-occurring disorders program at Johns Hopkins. Welcome!
Leslie A. Hulvershorn, MD, MSc: Thank you.
Christopher J. Hammond, MD, PhD: Thank you.
Dr. Strakowski: Leslie, as I mentioned, many people are talking about how cannabis could be a good treatment for psychiatric disorders. Is that true?
Dr. Hulvershorn: If you look at what defines a good treatment, what you’re looking for is clinical trials, ideally randomized, placebo-controlled clinical trials.
When we look at research related to cannabis, we see very few of those trials, and we see that the cannabis plant is actually quite complicated and there are many different compounds that come from it. So we need to look at all the different compounds.
If you think about THC, delta 9 or delta 8, depending on the version, that’s the active ingredient that we most often think about when we say “cannabis.” If you look at THC studies, there really is no evidence that I could find that it helps psychiatric disorders.
What we do find is an enormous literature, many hunDr.eds of studies, actually, that show that THC actually worsens or even brings on psychiatric disorders. There’s a separate conversation about other compounds within the cannabis plant, like CBD, cannabidiol, where there’s maybe a signal that certain anxiety disorders might be improved by a compound like that.
Certainly, rare forms of epilepsy have been found to be improved with that compound. It really depends on what you’re looking at within the cannabis plant, but if we’re thinking about THC, the answer really is no, this is not a helpful thing. In fact, it’s probably a harmful thing to be ingesting in terms of psychiatric disorders.
Dr. Strakowski: Thank you, Leslie. Chris, what would you add to that? Do we know anything about the use of cannabis in any psychiatric condition?
Dr. Hammond: I definitely would echo what Leslie said. The popular opinion, that the media and the state legislatures have really, in many ways, put the cart before the horse — they speak about cannabis as a medication for the treatment of psychiatric conditions before we have sufficient evidence to say that it’s safe or effective for these conditions. Most of the evidence that we have, particularly in regard to the cannabinoid compound, delta 9, tetrahyDr.ocannabinol, or THC, suggests that that cannabinoid is associated with adverse mental health outcomes across different categories.
Dr. Strakowski: Our group, a long time ago, conducted a study looking at first episode of mania, and found that regular cannabis use increases the risk for subsequent manic episodes. I’m not aware of many other studies like that.
You referred, Chris, to the safety aspect. This is something anybody can use. There are no negative consequences. Is that true? I mean, is it really risk free?
Dr. Hammond: Research shows that that’s an inaccurate framing of the safety profile of cannabis. Again, as Leslie put it very well, cannabis is many different compounds. Using this catchall phrase of «cannabis» is not very helpful.
In regard to the main bioactive compounds of the cannabis plant, THC and cannabidiol, or CBD, what we know from studies of THC administration and from medications that have been designed to mimic THC and act on receptors that THC acts on is that those medications have clear side effects and adverse events in a percentage of patients who take them, particularly in regard to precipitating panic attacks, dysphoric episodes, and psychosis in some individuals.
Dr. Hulvershorn: I would add that it really depends on the age of the person that you’re talking about and when they’re first exposed to cannabis. If you’re talking about a person, say, under the age of 14 who uses cannabis, there’s a large amount of concern about the worsening of psychosis and mental health symptoms, but also cognitive features like memory.
There’s a very good study that was conducted in New Zealand that followed a large number of kids over time and showed significant decreases in working memory capacity for kids who used quite heavily.
Then you think about pregnant women. That’s very interesting literature, where people are finding that cannabis not only affects brain development but also a host of other systems in the body. For example, I think the risk for asthma is increased. If you look at the genes in the placenta that are affected, it has much to do with the immune system.
Women who are using cannabis during pregnancy are really exposing their fetus to a range of potential risks that we certainly don’t understand well enough, but there’s enough science that suggests this is really concerning.
If you take a step back and look at animal models, even with things like CBD products, which, again, everybody seems to be buying and they’re viewed as very safe — it’s almost hard to find things without CBD these days.
There we find, for example, in developing rats that testicular development seems to be affected with high doses of CBD. There’s just a huge array of effects, even outside of the psychiatric world, that make me very nervous about anyone using, especially a pregnant woman or a young person.
Then there’s a whole separate literature on adults. It’s hard to find studies that suggest this is a great idea. You’re going to find on the mental health side of things, and the cognitive side of things, many effects as well.
I, personally, am agnostic one way or the other. If cannabis turns out to be helpful, great. We love things that are helpful in medicine. We don’t really care where they come from. I’m not biased politically one way or the other. It’s just when you look at the totality of the literature, it’s hard to feel excited about people using cannabis at any age.
Dr. Hammond: It’s difficult to interpret the literature because of some biases there. It speaks to the importance of thoughtful research being done in this space that takes a neutral approach to assessing cannabis and looking for evidence of both potential benefit and potential harm.
The other piece that I think is of value that builds off what Leslie mentioned is the effects of cannabis and THC. The risk for harm appears to be greater in pregnant women and in young people. For adults, I think, we’re also still trying to understand what the effects are.
The other way of parsing out effects and thinking about them is in terms of the acute effects and the acute response in the moment right after one ingests cannabis vs the long-term effects.
After acute ingestion of cannabis, it can precipitate a psychotic episode, dysphoria or severe depressive symptoms, or severe anxiety, and can cause one to be disoriented, have delayed response time, and affect the ability to Dr.ive. In that capacity, it is related to a higher risk for motor vehicle crashes.
Dr. Strakowski: That’s very interesting. In my practice, and maybe it’s atypical, but half to two thirds of my patients, particularly the younger ones, are using cannabis in some form or another. In my experience, if they’re under 21, they’re more likely to use cannabis than alcohol.
What do we tell our patients? Is there a safe level of use? Do we say to never touch it? How do we manage the social pressure and environment that our patients have to live in?
Dr. Hulvershorn: I think about what we call motivational interviewing and the substance use disorder field, which is a style of interacting with someone that’s very neutral to discuss the pros and the cons. In my practice, people are usually coming to us because of problems related to their substance use.
Not everyone is experiencing those, but for those people, it’s a pretty easy discussion. It sounds like you’re getting into trouble. Your athletic performance is suffering. Your scholastic performance is suffering.
You walk them toward understanding that, wait a minute, if I smoked less weed or no weed, I would probably be doing better in this or that domain of my life. That seems to be the most helpful thing, by allowing them to come to that conclusion.
I think it is a more difficult conversation for people who don’t identify any problems related to their use. What is the right answer? Again, I just go back to saying, “Is this good for you? It’s hard to find the literature that suggests that. Is it neutral for you? Maybe, for some people. Is it harmful for some people? Absolutely.”
I think, for me, the most impactful studies have been those that showed for certain people with certain genetic makeup, cannabis is an absolutely terrible idea. Their risk for psychosis development and things like that are so high. For other people, they could smoke weed all day and never have a problem, based on their genetics — maybe. We don’t know. It’s not like we’re doing blood tests to figure out who you are.
The safest advice, I think, is no use. That’s never going to be bad advice.
Dr. Hammond: I mostly agree with Leslie on this point but feel very, very strongly that — in this era, where in the context of popular media, celebrities and other people are stating that cannabis is good and should be put in everything — clinical providers, especially pediatric providers, need to be extremely grounded in the science, and not let popular media sway our approach and strategy for working with these young people.
There’s two decades worth of data from longitudinal studies that have followed individuals from birth or from preadolescence into their thirties and forties, that show us that, for this association between cannabis use and later adverse mental health outcomes, there is a dose effect there.
The earlier an individual starts using, the more frequent they use, and more persistent their use is over time, those individuals have poorer mental health outcomes compared with individuals who choose to abstain or individuals who use just a few times and stop.
There’s also a signal for higher-THC-potency products being associated with poorer mental health outcomes, particularly when used during adolescence.
I apply a motivational interviewing approach as well to disseminate this information to both the young people and their parents about the risks, and to communicate what the data clearly show in regard to using THC-based cannabinoid products, which is that we don’t have evidence that shows that any use is healthy to the developing brain.
There’s a large amount of evidence that suggests it’s harmful to the developing brain, so the recommendation is not to use, to delay the onset of use, if you want to use, until adulthood. Many youth choose to use. For those young people, we meet them where they’re at and try to work with them on cutting down.
Dr. Strakowski: Thank you both. There’s an interesting effort in different states, with lobbying by celebrities and legislators pushing insurance companies to fund cannabis use broadly, including in a number of psychiatric indications, with no FDA approval at this point. Do you support that? Is that a good idea?
Dr. Hammond: Absolutely not.
Dr. Strakowski: Thank you.
Dr. Hammond: I think that’s a very important statement to make. For the medical and healthcare profession to stand strong related to states requiring insurance companies to cover medical cannabis really opens the door to lawsuits that would force insurance companies to cover other undertested bioactive chemicals and health supplements.
There are insufficient safety data for medical cannabis for FDA approval for any condition right now. The FDA has approved cannabinoid-based medications. Those cannabinoid-based medications have really undergone rigorous safety and efficacy testing, and have been approved for very narrow indications, none of which are psychiatric conditions.
They’ve been approved for chemotherapy-associated nausea and vomiting, treatment-resistant seizures related to two rare seizure disorders that emerge during childhood, and related to tuberous sclerosis, and one related to treating multiple sclerosis–associated spasticity and central neuropathic pain.
Dr. Hulvershorn: Steve, I think it’s important for listeners to be aware that there is a process in place for any therapeutic to become tested and reviewed. We see an industry that stands to make an enormous amount of money, and that is really the motivation for this industry.
These are not folks who are, out of the kindness of their heart, just hoping for better treatments for people. There are many ways you could channel that desire that does not include cannabis making money.
It’s really a profit-motivated industry. They’re very effective at lobbying. The public, unfortunately, has been sort of manipulated by this industry to believe that these are healthy, safe, and natural just because they grow in the ground.
Unfortunately, that’s really the issue. I think people just need to keep that in mind. Someone stands to make a large amount of money off of this. This is a very calculated, strategic approach that goes state by state but is nationally organized, and is potentially, like Chris says, for many reasons, really harmful.
I see it as sort of a bullying approach. Like if your Dr.ug works, Medicaid will pay for it. Medicaid in each state will review the studies. The FDA obviously leads the way. To cut the line without the research is really not helpful — circumventing the process that’s been in place for a long time and works well.
Dr. Hammond: Yes, it sets a dangerous precedent.
Dr. Strakowski: I was going to add the same, that it’s potentially dangerous. Thank you both, Dr.s Hulvershorn and Hammond, for a really good, lively discussion. I know we could talk for a very long time about this situation.
I do think it’s clear for listeners, most of whom are practitioners, that at this point in time, there just really does not seem to be strong evidence for the use of cannabis-based products for any psychiatric condition.
I do think we have to approach the people we’re working with around their psychiatric conditions to manage use and abuse wisely, like we would with any other substance. I appreciate everyone who’s tuned in today to watch us. I hope this is useful for your practice. Thank you.
Stephen M. Strakowski, MD, has disclosed the following relevant financial relationships:
- Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Roche; Procter & Gamble; Novartis; Sunovion
- Received income in an amount equal to or greater than $250 from: Roche; Procter & Gamble; Novartis; Sunovion; Oxford University Press
Leslie A. Hulvershorn, MD, MSc, has disclosed the following relevant financial relationships:
- Received income in an amount equal to or greater than $250 from: Greenwich Biosciences, educational grant for Summit
Christopher J. Hammond, MD, PhD, has disclosed the following relevant financial relationships:
- Received research grant from National Institutes of Health Grants; Bench to Bench Award; Substance Abuse and Mental Health Services Administration; Doris Duke.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Stephen M. Strakowski, MD: Hello. Thank you all for joining us today. I’m very excited to have some great guests to talk about what I consider an active controversy. I’m Stephen M. Strakowski. I’m a professor and vice chair of psychiatry at Indiana University, and professor and associate vice president at University of Texas in Austin.
Today we’re going to talk about cannabis. As all of you are aware, everyone’s talking about cannabis. We hear constantly on social media and in interviews, particularly with relevance to psychiatric disorders, that everyone should be thinking about using cannabis. That seems to be the common conversation.
Last week, I had a patient who said, “All my friends tell me I need to be on cannabis.” That was their solution to her problems. With that in mind, let me introduce our guests, who are both experts on this, to talk about the role of cannabis in psychiatric disorders today.
First, I want to welcome Dr. Leslie Hulvershorn. Dr. Hulvershorn is an associate professor and chair at Indiana University in Indianapolis. Dr. Christopher Hammond is an assistant professor and the director of the co-occurring disorders program at Johns Hopkins. Welcome!
Leslie A. Hulvershorn, MD, MSc: Thank you.
Christopher J. Hammond, MD, PhD: Thank you.
Dr. Strakowski: Leslie, as I mentioned, many people are talking about how cannabis could be a good treatment for psychiatric disorders. Is that true?
Dr. Hulvershorn: If you look at what defines a good treatment, what you’re looking for is clinical trials, ideally randomized, placebo-controlled clinical trials.
When we look at research related to cannabis, we see very few of those trials, and we see that the cannabis plant is actually quite complicated and there are many different compounds that come from it. So we need to look at all the different compounds.
If you think about THC, delta 9 or delta 8, depending on the version, that’s the active ingredient that we most often think about when we say “cannabis.” If you look at THC studies, there really is no evidence that I could find that it helps psychiatric disorders.
What we do find is an enormous literature, many hunDr.eds of studies, actually, that show that THC actually worsens or even brings on psychiatric disorders. There’s a separate conversation about other compounds within the cannabis plant, like CBD, cannabidiol, where there’s maybe a signal that certain anxiety disorders might be improved by a compound like that.
Certainly, rare forms of epilepsy have been found to be improved with that compound. It really depends on what you’re looking at within the cannabis plant, but if we’re thinking about THC, the answer really is no, this is not a helpful thing. In fact, it’s probably a harmful thing to be ingesting in terms of psychiatric disorders.
Dr. Strakowski: Thank you, Leslie. Chris, what would you add to that? Do we know anything about the use of cannabis in any psychiatric condition?
Dr. Hammond: I definitely would echo what Leslie said. The popular opinion, that the media and the state legislatures have really, in many ways, put the cart before the horse — they speak about cannabis as a medication for the treatment of psychiatric conditions before we have sufficient evidence to say that it’s safe or effective for these conditions. Most of the evidence that we have, particularly in regard to the cannabinoid compound, delta 9, tetrahyDr.ocannabinol, or THC, suggests that that cannabinoid is associated with adverse mental health outcomes across different categories.
Dr. Strakowski: Our group, a long time ago, conducted a study looking at first episode of mania, and found that regular cannabis use increases the risk for subsequent manic episodes. I’m not aware of many other studies like that.
You referred, Chris, to the safety aspect. This is something anybody can use. There are no negative consequences. Is that true? I mean, is it really risk free?
Dr. Hammond: Research shows that that’s an inaccurate framing of the safety profile of cannabis. Again, as Leslie put it very well, cannabis is many different compounds. Using this catchall phrase of «cannabis» is not very helpful.
In regard to the main bioactive compounds of the cannabis plant, THC and cannabidiol, or CBD, what we know from studies of THC administration and from medications that have been designed to mimic THC and act on receptors that THC acts on is that those medications have clear side effects and adverse events in a percentage of patients who take them, particularly in regard to precipitating panic attacks, dysphoric episodes, and psychosis in some individuals.
Dr. Hulvershorn: I would add that it really depends on the age of the person that you’re talking about and when they’re first exposed to cannabis. If you’re talking about a person, say, under the age of 14 who uses cannabis, there’s a large amount of concern about the worsening of psychosis and mental health symptoms, but also cognitive features like memory.
There’s a very good study that was conducted in New Zealand that followed a large number of kids over time and showed significant decreases in working memory capacity for kids who used quite heavily.
Then you think about pregnant women. That’s very interesting literature, where people are finding that cannabis not only affects brain development but also a host of other systems in the body. For example, I think the risk for asthma is increased. If you look at the genes in the placenta that are affected, it has much to do with the immune system.
Women who are using cannabis during pregnancy are really exposing their fetus to a range of potential risks that we certainly don’t understand well enough, but there’s enough science that suggests this is really concerning.
If you take a step back and look at animal models, even with things like CBD products, which, again, everybody seems to be buying and they’re viewed as very safe — it’s almost hard to find things without CBD these days.
There we find, for example, in developing rats that testicular development seems to be affected with high doses of CBD. There’s just a huge array of effects, even outside of the psychiatric world, that make me very nervous about anyone using, especially a pregnant woman or a young person.
Then there’s a whole separate literature on adults. It’s hard to find studies that suggest this is a great idea. You’re going to find on the mental health side of things, and the cognitive side of things, many effects as well.
I, personally, am agnostic one way or the other. If cannabis turns out to be helpful, great. We love things that are helpful in medicine. We don’t really care where they come from. I’m not biased politically one way or the other. It’s just when you look at the totality of the literature, it’s hard to feel excited about people using cannabis at any age.
Dr. Hammond: It’s difficult to interpret the literature because of some biases there. It speaks to the importance of thoughtful research being done in this space that takes a neutral approach to assessing cannabis and looking for evidence of both potential benefit and potential harm.
The other piece that I think is of value that builds off what Leslie mentioned is the effects of cannabis and THC. The risk for harm appears to be greater in pregnant women and in young people. For adults, I think, we’re also still trying to understand what the effects are.
The other way of parsing out effects and thinking about them is in terms of the acute effects and the acute response in the moment right after one ingests cannabis vs the long-term effects.
After acute ingestion of cannabis, it can precipitate a psychotic episode, dysphoria or severe depressive symptoms, or severe anxiety, and can cause one to be disoriented, have delayed response time, and affect the ability to Dr.ive. In that capacity, it is related to a higher risk for motor vehicle crashes.
Dr. Strakowski: That’s very interesting. In my practice, and maybe it’s atypical, but half to two thirds of my patients, particularly the younger ones, are using cannabis in some form or another. In my experience, if they’re under 21, they’re more likely to use cannabis than alcohol.
What do we tell our patients? Is there a safe level of use? Do we say to never touch it? How do we manage the social pressure and environment that our patients have to live in?
Dr. Hulvershorn: I think about what we call motivational interviewing and the substance use disorder field, which is a style of interacting with someone that’s very neutral to discuss the pros and the cons. In my practice, people are usually coming to us because of problems related to their substance use.
Not everyone is experiencing those, but for those people, it’s a pretty easy discussion. It sounds like you’re getting into trouble. Your athletic performance is suffering. Your scholastic performance is suffering.
You walk them toward understanding that, wait a minute, if I smoked less weed or no weed, I would probably be doing better in this or that domain of my life. That seems to be the most helpful thing, by allowing them to come to that conclusion.
I think it is a more difficult conversation for people who don’t identify any problems related to their use. What is the right answer? Again, I just go back to saying, “Is this good for you? It’s hard to find the literature that suggests that. Is it neutral for you? Maybe, for some people. Is it harmful for some people? Absolutely.”
I think, for me, the most impactful studies have been those that showed for certain people with certain genetic makeup, cannabis is an absolutely terrible idea. Their risk for psychosis development and things like that are so high. For other people, they could smoke weed all day and never have a problem, based on their genetics — maybe. We don’t know. It’s not like we’re doing blood tests to figure out who you are.
The safest advice, I think, is no use. That’s never going to be bad advice.
Dr. Hammond: I mostly agree with Leslie on this point but feel very, very strongly that — in this era, where in the context of popular media, celebrities and other people are stating that cannabis is good and should be put in everything — clinical providers, especially pediatric providers, need to be extremely grounded in the science, and not let popular media sway our approach and strategy for working with these young people.
There’s two decades worth of data from longitudinal studies that have followed individuals from birth or from preadolescence into their thirties and forties, that show us that, for this association between cannabis use and later adverse mental health outcomes, there is a dose effect there.
The earlier an individual starts using, the more frequent they use, and more persistent their use is over time, those individuals have poorer mental health outcomes compared with individuals who choose to abstain or individuals who use just a few times and stop.
There’s also a signal for higher-THC-potency products being associated with poorer mental health outcomes, particularly when used during adolescence.
I apply a motivational interviewing approach as well to disseminate this information to both the young people and their parents about the risks, and to communicate what the data clearly show in regard to using THC-based cannabinoid products, which is that we don’t have evidence that shows that any use is healthy to the developing brain.
There’s a large amount of evidence that suggests it’s harmful to the developing brain, so the recommendation is not to use, to delay the onset of use, if you want to use, until adulthood. Many youth choose to use. For those young people, we meet them where they’re at and try to work with them on cutting down.
Dr. Strakowski: Thank you both. There’s an interesting effort in different states, with lobbying by celebrities and legislators pushing insurance companies to fund cannabis use broadly, including in a number of psychiatric indications, with no FDA approval at this point. Do you support that? Is that a good idea?
Dr. Hammond: Absolutely not.
Dr. Strakowski: Thank you.
Dr. Hammond: I think that’s a very important statement to make. For the medical and healthcare profession to stand strong related to states requiring insurance companies to cover medical cannabis really opens the door to lawsuits that would force insurance companies to cover other undertested bioactive chemicals and health supplements.
There are insufficient safety data for medical cannabis for FDA approval for any condition right now. The FDA has approved cannabinoid-based medications. Those cannabinoid-based medications have really undergone rigorous safety and efficacy testing, and have been approved for very narrow indications, none of which are psychiatric conditions.
They’ve been approved for chemotherapy-associated nausea and vomiting, treatment-resistant seizures related to two rare seizure disorders that emerge during childhood, and related to tuberous sclerosis, and one related to treating multiple sclerosis–associated spasticity and central neuropathic pain.
Dr. Hulvershorn: Steve, I think it’s important for listeners to be aware that there is a process in place for any therapeutic to become tested and reviewed. We see an industry that stands to make an enormous amount of money, and that is really the motivation for this industry.
These are not folks who are, out of the kindness of their heart, just hoping for better treatments for people. There are many ways you could channel that desire that does not include cannabis making money.
It’s really a profit-motivated industry. They’re very effective at lobbying. The public, unfortunately, has been sort of manipulated by this industry to believe that these are healthy, safe, and natural just because they grow in the ground.
Unfortunately, that’s really the issue. I think people just need to keep that in mind. Someone stands to make a large amount of money off of this. This is a very calculated, strategic approach that goes state by state but is nationally organized, and is potentially, like Chris says, for many reasons, really harmful.
I see it as sort of a bullying approach. Like if your Dr.ug works, Medicaid will pay for it. Medicaid in each state will review the studies. The FDA obviously leads the way. To cut the line without the research is really not helpful — circumventing the process that’s been in place for a long time and works well.
Dr. Hammond: Yes, it sets a dangerous precedent.
Dr. Strakowski: I was going to add the same, that it’s potentially dangerous. Thank you both, Dr.s Hulvershorn and Hammond, for a really good, lively discussion. I know we could talk for a very long time about this situation.
I do think it’s clear for listeners, most of whom are practitioners, that at this point in time, there just really does not seem to be strong evidence for the use of cannabis-based products for any psychiatric condition.
I do think we have to approach the people we’re working with around their psychiatric conditions to manage use and abuse wisely, like we would with any other substance. I appreciate everyone who’s tuned in today to watch us. I hope this is useful for your practice. Thank you.
Stephen M. Strakowski, MD, has disclosed the following relevant financial relationships:
- Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Roche; Procter & Gamble; Novartis; Sunovion
- Received income in an amount equal to or greater than $250 from: Roche; Procter & Gamble; Novartis; Sunovion; Oxford University Press
Leslie A. Hulvershorn, MD, MSc, has disclosed the following relevant financial relationships:
- Received income in an amount equal to or greater than $250 from: Greenwich Biosciences, educational grant for Summit
Christopher J. Hammond, MD, PhD, has disclosed the following relevant financial relationships:
- Received research grant from National Institutes of Health Grants; Bench to Bench Award; Substance Abuse and Mental Health Services Administration; Doris Duke.
A version of this article appeared on Medscape.com.
Yes, Patients Are Getting More Complicated
This transcript has been edited for clarity.
The first time I saw a patient in the hospital was in 2004, twenty years ago, when I was a third-year med student. I mean, look at that guy. The things I could tell him.
Since that time, I have spent countless hours in the hospital as a resident, a renal fellow, and finally as an attending. And I’m sure many of you in the medical community feel the same thing I do, which is that patients are much more complicated now than they used to be. I’ll listen to an intern present a new case on rounds and she’ll have an assessment and plan that encompasses a dozen individual medical problems. Sometimes I have to literally be like, “Wait, why is this patient here again?”
But until now, I had no data to convince myself that this feeling was real — that hospitalized patients are getting more and more complicated, or that they only seem more complicated because I’m getting older. Maybe I was better able to keep track of things when I was an intern rather than now as an attending, spending just a couple months of the year in the hospital. I mean, after all, if patients were getting more complicated, surely hospitals would know this and allocate more resources to patient care, right?
Right?
It’s not an illusion. At least not according to this paper, Population-Based Trends in Complexity of Hospital Inpatients, appearing in JAMA Internal Medicine, which examines about 15 years of inpatient hospital admissions in British Columbia.
I like Canada for this study for two reasons: First, their electronic health record system is province-wide, so they don’t have issues of getting data from hospital A vs hospital B. All the data are there — in this case, more than 3 million nonelective hospital admissions from British Columbia. Second, there is universal healthcare. We don’t have to worry about insurance companies changing, or the start of a new program like the Affordable Care Act. It’s just a cleaner set-up.
Of course, complexity is hard to define, and the authors here decide to look at a variety of metrics I think we can agree are tied into complexity. These include things like patient age, comorbidities, medications, frequency of hospitalization, and so on. They also looked at outcomes associated with hospitalization: Did the patient require the ICU? Did they survive? Were they readmitted?
And the tale of the tape is as clear as that British Columbian air: Over the past 15 years, your average hospitalized patient is about 3 years older, is twice as likely to have kidney disease, 70% more likely to have diabetes, is on more medications (particularly anticoagulants), and is much more likely to be admitted through the emergency room. They’ve also spent more time in the hospital in the past year.
Given the increased complexity, you might expect that the outcomes for these patients are worse than years ago, but the data do not bear that out. In fact, inpatient mortality is lower now than it was 15 years ago, although 30-day postdischarge mortality is higher. Put those together and it turns out that death rates are pretty stable: 9% of people admitted for nonelective reasons to the hospital will die within 30 days. It’s just that nowadays, we tend to discharge them before that happens.
Why are our patients getting more complex? Some of it is demographics; the population is aging, after all. Some of it relates to the increasing burden of comorbidities like diabetes and kidney disease, which are associated with the obesity epidemic. But in some ways, we’re a victim of our own success.
Given all that, does it make any sense that many of our hospitals are at skeleton-crew staffing levels? That hospitalists report taking care of more patients than they ever have before?
There’s been so much talk about burnout in the health professions lately. Maybe something people need to start acknowledging — particularly those who haven’t practiced on the front lines for a decade or two — is that the job is, quite simply, harder now. As patients become more complex, we need more resources, human and otherwise, to care for them.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
The first time I saw a patient in the hospital was in 2004, twenty years ago, when I was a third-year med student. I mean, look at that guy. The things I could tell him.
Since that time, I have spent countless hours in the hospital as a resident, a renal fellow, and finally as an attending. And I’m sure many of you in the medical community feel the same thing I do, which is that patients are much more complicated now than they used to be. I’ll listen to an intern present a new case on rounds and she’ll have an assessment and plan that encompasses a dozen individual medical problems. Sometimes I have to literally be like, “Wait, why is this patient here again?”
But until now, I had no data to convince myself that this feeling was real — that hospitalized patients are getting more and more complicated, or that they only seem more complicated because I’m getting older. Maybe I was better able to keep track of things when I was an intern rather than now as an attending, spending just a couple months of the year in the hospital. I mean, after all, if patients were getting more complicated, surely hospitals would know this and allocate more resources to patient care, right?
Right?
It’s not an illusion. At least not according to this paper, Population-Based Trends in Complexity of Hospital Inpatients, appearing in JAMA Internal Medicine, which examines about 15 years of inpatient hospital admissions in British Columbia.
I like Canada for this study for two reasons: First, their electronic health record system is province-wide, so they don’t have issues of getting data from hospital A vs hospital B. All the data are there — in this case, more than 3 million nonelective hospital admissions from British Columbia. Second, there is universal healthcare. We don’t have to worry about insurance companies changing, or the start of a new program like the Affordable Care Act. It’s just a cleaner set-up.
Of course, complexity is hard to define, and the authors here decide to look at a variety of metrics I think we can agree are tied into complexity. These include things like patient age, comorbidities, medications, frequency of hospitalization, and so on. They also looked at outcomes associated with hospitalization: Did the patient require the ICU? Did they survive? Were they readmitted?
And the tale of the tape is as clear as that British Columbian air: Over the past 15 years, your average hospitalized patient is about 3 years older, is twice as likely to have kidney disease, 70% more likely to have diabetes, is on more medications (particularly anticoagulants), and is much more likely to be admitted through the emergency room. They’ve also spent more time in the hospital in the past year.
Given the increased complexity, you might expect that the outcomes for these patients are worse than years ago, but the data do not bear that out. In fact, inpatient mortality is lower now than it was 15 years ago, although 30-day postdischarge mortality is higher. Put those together and it turns out that death rates are pretty stable: 9% of people admitted for nonelective reasons to the hospital will die within 30 days. It’s just that nowadays, we tend to discharge them before that happens.
Why are our patients getting more complex? Some of it is demographics; the population is aging, after all. Some of it relates to the increasing burden of comorbidities like diabetes and kidney disease, which are associated with the obesity epidemic. But in some ways, we’re a victim of our own success.
Given all that, does it make any sense that many of our hospitals are at skeleton-crew staffing levels? That hospitalists report taking care of more patients than they ever have before?
There’s been so much talk about burnout in the health professions lately. Maybe something people need to start acknowledging — particularly those who haven’t practiced on the front lines for a decade or two — is that the job is, quite simply, harder now. As patients become more complex, we need more resources, human and otherwise, to care for them.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
The first time I saw a patient in the hospital was in 2004, twenty years ago, when I was a third-year med student. I mean, look at that guy. The things I could tell him.
Since that time, I have spent countless hours in the hospital as a resident, a renal fellow, and finally as an attending. And I’m sure many of you in the medical community feel the same thing I do, which is that patients are much more complicated now than they used to be. I’ll listen to an intern present a new case on rounds and she’ll have an assessment and plan that encompasses a dozen individual medical problems. Sometimes I have to literally be like, “Wait, why is this patient here again?”
But until now, I had no data to convince myself that this feeling was real — that hospitalized patients are getting more and more complicated, or that they only seem more complicated because I’m getting older. Maybe I was better able to keep track of things when I was an intern rather than now as an attending, spending just a couple months of the year in the hospital. I mean, after all, if patients were getting more complicated, surely hospitals would know this and allocate more resources to patient care, right?
Right?
It’s not an illusion. At least not according to this paper, Population-Based Trends in Complexity of Hospital Inpatients, appearing in JAMA Internal Medicine, which examines about 15 years of inpatient hospital admissions in British Columbia.
I like Canada for this study for two reasons: First, their electronic health record system is province-wide, so they don’t have issues of getting data from hospital A vs hospital B. All the data are there — in this case, more than 3 million nonelective hospital admissions from British Columbia. Second, there is universal healthcare. We don’t have to worry about insurance companies changing, or the start of a new program like the Affordable Care Act. It’s just a cleaner set-up.
Of course, complexity is hard to define, and the authors here decide to look at a variety of metrics I think we can agree are tied into complexity. These include things like patient age, comorbidities, medications, frequency of hospitalization, and so on. They also looked at outcomes associated with hospitalization: Did the patient require the ICU? Did they survive? Were they readmitted?
And the tale of the tape is as clear as that British Columbian air: Over the past 15 years, your average hospitalized patient is about 3 years older, is twice as likely to have kidney disease, 70% more likely to have diabetes, is on more medications (particularly anticoagulants), and is much more likely to be admitted through the emergency room. They’ve also spent more time in the hospital in the past year.
Given the increased complexity, you might expect that the outcomes for these patients are worse than years ago, but the data do not bear that out. In fact, inpatient mortality is lower now than it was 15 years ago, although 30-day postdischarge mortality is higher. Put those together and it turns out that death rates are pretty stable: 9% of people admitted for nonelective reasons to the hospital will die within 30 days. It’s just that nowadays, we tend to discharge them before that happens.
Why are our patients getting more complex? Some of it is demographics; the population is aging, after all. Some of it relates to the increasing burden of comorbidities like diabetes and kidney disease, which are associated with the obesity epidemic. But in some ways, we’re a victim of our own success.
Given all that, does it make any sense that many of our hospitals are at skeleton-crew staffing levels? That hospitalists report taking care of more patients than they ever have before?
There’s been so much talk about burnout in the health professions lately. Maybe something people need to start acknowledging — particularly those who haven’t practiced on the front lines for a decade or two — is that the job is, quite simply, harder now. As patients become more complex, we need more resources, human and otherwise, to care for them.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Do Statins Offset Venous Thrombosis Risk With Hormone Therapy?
This transcript has been edited for clarity.
This is Dr JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. I’d like to talk with you about a recent report in JAMA Network Open on the subject of whether statin therapy may be able to offset some of the excess risk for venous thromboembolism (VTE) among women taking menopausal hormone therapy.
It’s an important issue because we know that menopausal hormone therapy, especially oral therapy, is linked to an excess risk for VTE, approximately doubling of risk in the randomized clinical trials. There is also emerging evidence from some randomized trials, such as the Jupiter trial, that step therapy may be linked to a reduction in risk. This may be related to anti-inflammatory or antithrombotic effects of statin therapy.
The authors made use of a very large administrative claims database, Optum Health, to look at more than 15 million annual members. They were able to identify 2000 women with a diagnostic code for VTE treatment. The women were between ages 50 and 64 years, and they were compared with 200,000 controls without VTE, matched in 10-to-1 fashion.
About 50% of the women were taking oral hormone therapy, and about 50% took non-oral transdermal or other non-oral formulations of hormone therapy. The odds ratio for VTE was 1.53 among the women who did not also have prescription records for statin therapy. They were able to look at prescribed prescriptions for both the hormone therapy and the statins. Among the women prescribed hormone therapy and also low- to intermediate-dose statins, the odds ratio was 1.29. So that was quite a mitigation of the elevated risk. Among the women taking high-intensity statins, the odds ratio was 1.06, and there was no significant elevation.
We do need more data and more research on this question. One approach would be a meta-analysis of all of the existing randomized trials of hormone therapy in recent years wherein there was increased uptake of statin therapy to look at this question not only for VTE but also for coronary heart disease, stroke, and other CVD outcomes to see whether statin therapy is associated with some attenuation of the excess risk. We also need a targeted randomized trial of statins vs placebo among women who have clear indications for hormone therapy but may be at some increased risk for VTE. That type of trial would be extremely helpful.
These include choosing a transdermal rather than an oral formulation of hormone therapy and using lower doses of hormone therapy. Also, women who are clear candidates for hormone therapy and also for statins, it’s obvious that statins could be co-prescribed. Even among women who are clear candidates for hormone therapy but only intermediate borderline candidates for statin therapy, the prescription of statins might be considered in that clinical scenario to try to mitigate that excess risk for VTE.
JoAnn E. Manson, MD, DrPH, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from: Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
This is Dr JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. I’d like to talk with you about a recent report in JAMA Network Open on the subject of whether statin therapy may be able to offset some of the excess risk for venous thromboembolism (VTE) among women taking menopausal hormone therapy.
It’s an important issue because we know that menopausal hormone therapy, especially oral therapy, is linked to an excess risk for VTE, approximately doubling of risk in the randomized clinical trials. There is also emerging evidence from some randomized trials, such as the Jupiter trial, that step therapy may be linked to a reduction in risk. This may be related to anti-inflammatory or antithrombotic effects of statin therapy.
The authors made use of a very large administrative claims database, Optum Health, to look at more than 15 million annual members. They were able to identify 2000 women with a diagnostic code for VTE treatment. The women were between ages 50 and 64 years, and they were compared with 200,000 controls without VTE, matched in 10-to-1 fashion.
About 50% of the women were taking oral hormone therapy, and about 50% took non-oral transdermal or other non-oral formulations of hormone therapy. The odds ratio for VTE was 1.53 among the women who did not also have prescription records for statin therapy. They were able to look at prescribed prescriptions for both the hormone therapy and the statins. Among the women prescribed hormone therapy and also low- to intermediate-dose statins, the odds ratio was 1.29. So that was quite a mitigation of the elevated risk. Among the women taking high-intensity statins, the odds ratio was 1.06, and there was no significant elevation.
We do need more data and more research on this question. One approach would be a meta-analysis of all of the existing randomized trials of hormone therapy in recent years wherein there was increased uptake of statin therapy to look at this question not only for VTE but also for coronary heart disease, stroke, and other CVD outcomes to see whether statin therapy is associated with some attenuation of the excess risk. We also need a targeted randomized trial of statins vs placebo among women who have clear indications for hormone therapy but may be at some increased risk for VTE. That type of trial would be extremely helpful.
These include choosing a transdermal rather than an oral formulation of hormone therapy and using lower doses of hormone therapy. Also, women who are clear candidates for hormone therapy and also for statins, it’s obvious that statins could be co-prescribed. Even among women who are clear candidates for hormone therapy but only intermediate borderline candidates for statin therapy, the prescription of statins might be considered in that clinical scenario to try to mitigate that excess risk for VTE.
JoAnn E. Manson, MD, DrPH, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from: Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
This is Dr JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. I’d like to talk with you about a recent report in JAMA Network Open on the subject of whether statin therapy may be able to offset some of the excess risk for venous thromboembolism (VTE) among women taking menopausal hormone therapy.
It’s an important issue because we know that menopausal hormone therapy, especially oral therapy, is linked to an excess risk for VTE, approximately doubling of risk in the randomized clinical trials. There is also emerging evidence from some randomized trials, such as the Jupiter trial, that step therapy may be linked to a reduction in risk. This may be related to anti-inflammatory or antithrombotic effects of statin therapy.
The authors made use of a very large administrative claims database, Optum Health, to look at more than 15 million annual members. They were able to identify 2000 women with a diagnostic code for VTE treatment. The women were between ages 50 and 64 years, and they were compared with 200,000 controls without VTE, matched in 10-to-1 fashion.
About 50% of the women were taking oral hormone therapy, and about 50% took non-oral transdermal or other non-oral formulations of hormone therapy. The odds ratio for VTE was 1.53 among the women who did not also have prescription records for statin therapy. They were able to look at prescribed prescriptions for both the hormone therapy and the statins. Among the women prescribed hormone therapy and also low- to intermediate-dose statins, the odds ratio was 1.29. So that was quite a mitigation of the elevated risk. Among the women taking high-intensity statins, the odds ratio was 1.06, and there was no significant elevation.
We do need more data and more research on this question. One approach would be a meta-analysis of all of the existing randomized trials of hormone therapy in recent years wherein there was increased uptake of statin therapy to look at this question not only for VTE but also for coronary heart disease, stroke, and other CVD outcomes to see whether statin therapy is associated with some attenuation of the excess risk. We also need a targeted randomized trial of statins vs placebo among women who have clear indications for hormone therapy but may be at some increased risk for VTE. That type of trial would be extremely helpful.
These include choosing a transdermal rather than an oral formulation of hormone therapy and using lower doses of hormone therapy. Also, women who are clear candidates for hormone therapy and also for statins, it’s obvious that statins could be co-prescribed. Even among women who are clear candidates for hormone therapy but only intermediate borderline candidates for statin therapy, the prescription of statins might be considered in that clinical scenario to try to mitigate that excess risk for VTE.
JoAnn E. Manson, MD, DrPH, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from: Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
Androgenetic Alopecia: What Works?
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.
When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.

When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.

When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
Age-Friendly Health Systems and Meeting the Principles of High Reliability Organizations in the VHA
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDEprogram for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDEprogram for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDEprogram for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
Perinatal Psychiatry in 2024: Helping More Patients Access Care
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].