User login
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
Express Scripts, the contractor that manages the pharmacy benefit for Tricare, the military health insurance program, announced in 2021 that after a 5-year absence, CVS Pharmacy was once more in the network. In 2023, CVS had the largest profits of any pharmacy chain in the United States, about $159 billion, and generated a quarter of the overall revenue of the US pharmacy industry.1 Tricare officials heralded the return of CVS as a move that would offer US Department of Defense (DoD) beneficiaries more competitive prices, convenient access, and overall quality.2
DOJ Files Lawsuit Against CVS
In December 2024, the US Department of Justice (DoJ) filed a lawsuit alleging that CVS violated both the Controlled Substances Act (CSA) and the False Claims Act (FCA).3,4 The United States ex rel. Estright v Health Corporation, et al, filed in Rhode Island, charged that CVS “routinely” and “knowingly” filled invalid prescriptions for controlled substances violating the CSA and then billed federal health care programs for payment for these prescriptions, a breach of the FCA.5 The DoJ alleged that CVS pharmacies and pharmacists filled prescriptions for controlled substances that (1) lacked a legitimate medical purpose; (2) were not legally valid; and/or (3) were not issued in the usual course of medical practice. 6 CVS contests the charges and issued an official response, stating that it disputes the allegations as false, plans to disprove them in litigation, and has nonetheless fully cooperated with the investigation.7
The allegations involved prescriptions for drugs like opioids and benzodiazepines, primary culprits in the American overdose epidemic.8 The complaint notes that the prescriptions were early refills in excessive quantities and included what has been called the “holy trinity” of dangerous medications: opioids, benzodiazepines, and muscle relaxants. 5,8 Even worse (if that is possible), as the complaint outlines, CVS had access to data from both inside and outside the company that these prescriptions came from notorious pill mills and were hence unlawful and yet continued to fill them, leading the DoJ to file the more serious charge that the corporation “knowingly” violated the CSA and “prioritized profits over safety in dispensing controlled substances.”5,6
The Unholy Trinity
The infamous members of what I prefer to call the “unholy trinity” are a benzodiazepine, often alprazolam, an opioid, and the muscle relaxant carisoprodol. The combination amplifies each agent’s independent risk of respiratory depression. The latter is a schedule IV medication with an active metabolite, meprobamate, that also has this adverse effect. All 3 drugs have high abuse potential and, when combined, increase the risk of fatal overdose. The colloquial name holy trinity derives from the synergistic euphoria experienced when taking this triple cocktail of sedative agents.9 This pharmacological recipe for disaster is the house specialty of pill mills: infamous storefront practices that generate high profits and exploit persons with chronic pain and addiction by handing out controlled substances with little clinical assessment and even less oversight.10
When the Means Become the End
The DoJ allegations suggest that the violations resulted from “corporate-mandated performance metrics, incentive compensation, and staffing policies that prioritized corporate profits over patient safety.”6 If the allegations are true, why would a company reinvited by Tricare to serve the nation’s heroes seemingly engage in illegal practices? While CVS has not responded in court, their statement argued that “too often, we have seen government agencies and trial lawyers question the good-faith decisions made by pharmacists while a patient waits at the pharmacy counter, often in pain.”6
The DoJ complaint offers a cautionary warning for the US health care system, which is increasingly being micromanaged in the pursuit of efficiency. Like many practitioners in and out of the federal system, I get a cold chill when I read the word productivity. “CVS pharmacists described working at CVS as ‘soul crushing’ because it was impossible to meet the company’s expectations,” the complaint alleges, because “CVS set staffing levels so low that it was impossible for pharmacists to comply with their legal obligations and meet CVS’s demanding metrics.”5 Did top-down mandates drive the alleged activities by imposing unattainable performance metrics on pharmacists, offering incentives that encouraged and rewarded corner-cutting, and refusing to fund sufficient staffing to ensure patient safety? This may be what happens when the means (efficiency) become the end rather than a mechanism to achieve the goal of more accessible, affordable, high-quality health care.
Ethically, what is most concerning is that leadership intentionally “deprived its pharmacists of crucial information” about specific practitioners known to engage in illegal prescribing practices.6 CVS did not provide pharmacists with “information about prescribers’ prescribing habits that CVS routinely collected and reviewed at the corporate level,” and even removed prescriber blocks that were implemented at Target pharmacies before it was acquired by CVS.5 The first element of informed consent is providing patients with adequate information upon which to decide whether to accept or decline treatment. 11 In this situation, however, CVS allegedly prevented “pharmacists from warning one another about certain prescribers.”6
If true, the company deprived frontline pharmacists of the information they needed to safely and responsibly dispense medications: “The practices alleged contributed to the opioid crisis and opioid-related deaths, and today’s complaint seeks to hold CVS accountable for its misconduct.”6 Though the cost in human life that may have resulted from CSA violations must absolutely and always outweigh financial considerations, the economic damage to Tricare from fraudulent billing and the betrayal of its fiduciary responsinility cannot be underestimated.
A Corporate Morality Play
CVS is not the only company, nor is pharmacy the only industry in health care, that has been the subject of watchdog agency lawsuits or variegated forms of wrongdoing, including violations of the CSA and FCA.10,12 As of this writing, the DoJ case against CVS has not been heard, much less adjudicated in a court of law. It is ironic that both the DoJ claims and the CVS rebuttal describe the manifest conflict of obligation that pharmacists confront between protecting their livelihood and safeguarding patients’ lives as suggested in the epigraph that has been attributed to the 19th-century British physician and medical educator Peter Mere Latham. It is a dilemma that a growing number of health care practitioners face daily in a vocation becoming increasingly commercialized. It is all too easy for an individual physician, nurse, or pharmacist to feel hopeless and helpless before the behemoth might of a large and looming entity. Yet, it was a whistleblower whose moral courage led to the DoJ investigation and subsequent charges.13 We must all never doubt the power of a committed person of conscience to withstand the pressure to mutate medications into poison and stand up for the principles of our professions and inspire a community of colleagues to follow their example.
- Fein AJ. The Top U.S. pharmacy markets of 2023: market shares and revenues at the biggest chains and PBMs. Drug Channels. March 12, 2024. Accessed February 24, 2025. https://www.drugchannels.net/2024/03/the-top-15-us-pharmacies-of-2023-market.html
- Jowers K. CVS returns to the military Tricare network. Walmart’s out. Military Times. October 18, 2021. Accessed February 24, 2025. https://www.militarytimes.com/pay-benefits/mil-money/2021/10/28/cvs-returns-to-the-military-tricare-pharmacy-network-walmarts-out/
- False Claims, 31 USC § 3729 (2009). Accessed February 24, 2025. https://www.govinfo.gov/content/pkg/USCODE-2011-title31/pdf/USCODE-2011-title31-subtitleIII-chap37-subchapIII-sec3729.pdf
- Drug Abuse Prevention and Control, Control and Enforcement, 21 USC 13 § 801 (2022). Accessed February 24, 2025. https://www.govinfo.gov/app/details/USCODE-2021-title21/USCODE-2021-title21-chap13-subchapI-partA-sec801
- United States ex rel. Estright v Health Corporation, et al. Accessed February 26, 2025. https://www.justice.gov/archives/opa/media/1381111/dl
- US Department of Justice. Justice Department files nationwide lawsuit alleging CVS knowingly dispensed controlled substances in violation of the Controlled Substances ACT and the False Claims Act. News release. December 18, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/justice-department-files-nationwide-lawsuit-alleging-cvs-knowingly-dispensed-controlled
- CVS Health. CVS Health statement regarding the U.S. Department of Justice’s lawsuit against CVS pharmacy. News release. December 18, 2024. Accessed February 24, 2025. https://www.cvshealth.com/impact/healthy-community/our-opioid-response.html
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi:10.1136/bmj.h2698
- Wang Y, Delcher C, Li Y, Goldberger BA, Reisfield GM. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019;205:107693. doi:10.1016/j.drugalcdep.2019.107693
- Wolf AA. The perfect storm: opioid risks and ‘The Holy Trinity’. Pharmacy Times. September 24, 2014. Accessed February 24, 2025. https://www.pharmacytimes.com/view/the-perfect-storm-opioid-risks-and-the-holy-trinity
- The meaning and justification of informed consent. In: Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Eighth Edition. Oxford University Press; 2019:118-123.
- US Department of Justice. OptumRX agrees to pay $20M to resolve allegations that it filled certain opioid prescriptions in violation of the Controlled Substances Act. News release. June 27, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/optumrx-agrees-pay-20m-resolve-allegations-it-filled-certain-opioid-prescriptions-violation
- US Department of Justice. False Claims Act settlements and judgments exceed $2.9B in fiscal year 2024. News release. January 15, 2025. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/false-claims-act-settlements-and-judgments-exceed-29b-fiscal-year-2024
Express Scripts, the contractor that manages the pharmacy benefit for Tricare, the military health insurance program, announced in 2021 that after a 5-year absence, CVS Pharmacy was once more in the network. In 2023, CVS had the largest profits of any pharmacy chain in the United States, about $159 billion, and generated a quarter of the overall revenue of the US pharmacy industry.1 Tricare officials heralded the return of CVS as a move that would offer US Department of Defense (DoD) beneficiaries more competitive prices, convenient access, and overall quality.2
DOJ Files Lawsuit Against CVS
In December 2024, the US Department of Justice (DoJ) filed a lawsuit alleging that CVS violated both the Controlled Substances Act (CSA) and the False Claims Act (FCA).3,4 The United States ex rel. Estright v Health Corporation, et al, filed in Rhode Island, charged that CVS “routinely” and “knowingly” filled invalid prescriptions for controlled substances violating the CSA and then billed federal health care programs for payment for these prescriptions, a breach of the FCA.5 The DoJ alleged that CVS pharmacies and pharmacists filled prescriptions for controlled substances that (1) lacked a legitimate medical purpose; (2) were not legally valid; and/or (3) were not issued in the usual course of medical practice. 6 CVS contests the charges and issued an official response, stating that it disputes the allegations as false, plans to disprove them in litigation, and has nonetheless fully cooperated with the investigation.7
The allegations involved prescriptions for drugs like opioids and benzodiazepines, primary culprits in the American overdose epidemic.8 The complaint notes that the prescriptions were early refills in excessive quantities and included what has been called the “holy trinity” of dangerous medications: opioids, benzodiazepines, and muscle relaxants. 5,8 Even worse (if that is possible), as the complaint outlines, CVS had access to data from both inside and outside the company that these prescriptions came from notorious pill mills and were hence unlawful and yet continued to fill them, leading the DoJ to file the more serious charge that the corporation “knowingly” violated the CSA and “prioritized profits over safety in dispensing controlled substances.”5,6
The Unholy Trinity
The infamous members of what I prefer to call the “unholy trinity” are a benzodiazepine, often alprazolam, an opioid, and the muscle relaxant carisoprodol. The combination amplifies each agent’s independent risk of respiratory depression. The latter is a schedule IV medication with an active metabolite, meprobamate, that also has this adverse effect. All 3 drugs have high abuse potential and, when combined, increase the risk of fatal overdose. The colloquial name holy trinity derives from the synergistic euphoria experienced when taking this triple cocktail of sedative agents.9 This pharmacological recipe for disaster is the house specialty of pill mills: infamous storefront practices that generate high profits and exploit persons with chronic pain and addiction by handing out controlled substances with little clinical assessment and even less oversight.10
When the Means Become the End
The DoJ allegations suggest that the violations resulted from “corporate-mandated performance metrics, incentive compensation, and staffing policies that prioritized corporate profits over patient safety.”6 If the allegations are true, why would a company reinvited by Tricare to serve the nation’s heroes seemingly engage in illegal practices? While CVS has not responded in court, their statement argued that “too often, we have seen government agencies and trial lawyers question the good-faith decisions made by pharmacists while a patient waits at the pharmacy counter, often in pain.”6
The DoJ complaint offers a cautionary warning for the US health care system, which is increasingly being micromanaged in the pursuit of efficiency. Like many practitioners in and out of the federal system, I get a cold chill when I read the word productivity. “CVS pharmacists described working at CVS as ‘soul crushing’ because it was impossible to meet the company’s expectations,” the complaint alleges, because “CVS set staffing levels so low that it was impossible for pharmacists to comply with their legal obligations and meet CVS’s demanding metrics.”5 Did top-down mandates drive the alleged activities by imposing unattainable performance metrics on pharmacists, offering incentives that encouraged and rewarded corner-cutting, and refusing to fund sufficient staffing to ensure patient safety? This may be what happens when the means (efficiency) become the end rather than a mechanism to achieve the goal of more accessible, affordable, high-quality health care.
Ethically, what is most concerning is that leadership intentionally “deprived its pharmacists of crucial information” about specific practitioners known to engage in illegal prescribing practices.6 CVS did not provide pharmacists with “information about prescribers’ prescribing habits that CVS routinely collected and reviewed at the corporate level,” and even removed prescriber blocks that were implemented at Target pharmacies before it was acquired by CVS.5 The first element of informed consent is providing patients with adequate information upon which to decide whether to accept or decline treatment. 11 In this situation, however, CVS allegedly prevented “pharmacists from warning one another about certain prescribers.”6
If true, the company deprived frontline pharmacists of the information they needed to safely and responsibly dispense medications: “The practices alleged contributed to the opioid crisis and opioid-related deaths, and today’s complaint seeks to hold CVS accountable for its misconduct.”6 Though the cost in human life that may have resulted from CSA violations must absolutely and always outweigh financial considerations, the economic damage to Tricare from fraudulent billing and the betrayal of its fiduciary responsinility cannot be underestimated.
A Corporate Morality Play
CVS is not the only company, nor is pharmacy the only industry in health care, that has been the subject of watchdog agency lawsuits or variegated forms of wrongdoing, including violations of the CSA and FCA.10,12 As of this writing, the DoJ case against CVS has not been heard, much less adjudicated in a court of law. It is ironic that both the DoJ claims and the CVS rebuttal describe the manifest conflict of obligation that pharmacists confront between protecting their livelihood and safeguarding patients’ lives as suggested in the epigraph that has been attributed to the 19th-century British physician and medical educator Peter Mere Latham. It is a dilemma that a growing number of health care practitioners face daily in a vocation becoming increasingly commercialized. It is all too easy for an individual physician, nurse, or pharmacist to feel hopeless and helpless before the behemoth might of a large and looming entity. Yet, it was a whistleblower whose moral courage led to the DoJ investigation and subsequent charges.13 We must all never doubt the power of a committed person of conscience to withstand the pressure to mutate medications into poison and stand up for the principles of our professions and inspire a community of colleagues to follow their example.
Express Scripts, the contractor that manages the pharmacy benefit for Tricare, the military health insurance program, announced in 2021 that after a 5-year absence, CVS Pharmacy was once more in the network. In 2023, CVS had the largest profits of any pharmacy chain in the United States, about $159 billion, and generated a quarter of the overall revenue of the US pharmacy industry.1 Tricare officials heralded the return of CVS as a move that would offer US Department of Defense (DoD) beneficiaries more competitive prices, convenient access, and overall quality.2
DOJ Files Lawsuit Against CVS
In December 2024, the US Department of Justice (DoJ) filed a lawsuit alleging that CVS violated both the Controlled Substances Act (CSA) and the False Claims Act (FCA).3,4 The United States ex rel. Estright v Health Corporation, et al, filed in Rhode Island, charged that CVS “routinely” and “knowingly” filled invalid prescriptions for controlled substances violating the CSA and then billed federal health care programs for payment for these prescriptions, a breach of the FCA.5 The DoJ alleged that CVS pharmacies and pharmacists filled prescriptions for controlled substances that (1) lacked a legitimate medical purpose; (2) were not legally valid; and/or (3) were not issued in the usual course of medical practice. 6 CVS contests the charges and issued an official response, stating that it disputes the allegations as false, plans to disprove them in litigation, and has nonetheless fully cooperated with the investigation.7
The allegations involved prescriptions for drugs like opioids and benzodiazepines, primary culprits in the American overdose epidemic.8 The complaint notes that the prescriptions were early refills in excessive quantities and included what has been called the “holy trinity” of dangerous medications: opioids, benzodiazepines, and muscle relaxants. 5,8 Even worse (if that is possible), as the complaint outlines, CVS had access to data from both inside and outside the company that these prescriptions came from notorious pill mills and were hence unlawful and yet continued to fill them, leading the DoJ to file the more serious charge that the corporation “knowingly” violated the CSA and “prioritized profits over safety in dispensing controlled substances.”5,6
The Unholy Trinity
The infamous members of what I prefer to call the “unholy trinity” are a benzodiazepine, often alprazolam, an opioid, and the muscle relaxant carisoprodol. The combination amplifies each agent’s independent risk of respiratory depression. The latter is a schedule IV medication with an active metabolite, meprobamate, that also has this adverse effect. All 3 drugs have high abuse potential and, when combined, increase the risk of fatal overdose. The colloquial name holy trinity derives from the synergistic euphoria experienced when taking this triple cocktail of sedative agents.9 This pharmacological recipe for disaster is the house specialty of pill mills: infamous storefront practices that generate high profits and exploit persons with chronic pain and addiction by handing out controlled substances with little clinical assessment and even less oversight.10
When the Means Become the End
The DoJ allegations suggest that the violations resulted from “corporate-mandated performance metrics, incentive compensation, and staffing policies that prioritized corporate profits over patient safety.”6 If the allegations are true, why would a company reinvited by Tricare to serve the nation’s heroes seemingly engage in illegal practices? While CVS has not responded in court, their statement argued that “too often, we have seen government agencies and trial lawyers question the good-faith decisions made by pharmacists while a patient waits at the pharmacy counter, often in pain.”6
The DoJ complaint offers a cautionary warning for the US health care system, which is increasingly being micromanaged in the pursuit of efficiency. Like many practitioners in and out of the federal system, I get a cold chill when I read the word productivity. “CVS pharmacists described working at CVS as ‘soul crushing’ because it was impossible to meet the company’s expectations,” the complaint alleges, because “CVS set staffing levels so low that it was impossible for pharmacists to comply with their legal obligations and meet CVS’s demanding metrics.”5 Did top-down mandates drive the alleged activities by imposing unattainable performance metrics on pharmacists, offering incentives that encouraged and rewarded corner-cutting, and refusing to fund sufficient staffing to ensure patient safety? This may be what happens when the means (efficiency) become the end rather than a mechanism to achieve the goal of more accessible, affordable, high-quality health care.
Ethically, what is most concerning is that leadership intentionally “deprived its pharmacists of crucial information” about specific practitioners known to engage in illegal prescribing practices.6 CVS did not provide pharmacists with “information about prescribers’ prescribing habits that CVS routinely collected and reviewed at the corporate level,” and even removed prescriber blocks that were implemented at Target pharmacies before it was acquired by CVS.5 The first element of informed consent is providing patients with adequate information upon which to decide whether to accept or decline treatment. 11 In this situation, however, CVS allegedly prevented “pharmacists from warning one another about certain prescribers.”6
If true, the company deprived frontline pharmacists of the information they needed to safely and responsibly dispense medications: “The practices alleged contributed to the opioid crisis and opioid-related deaths, and today’s complaint seeks to hold CVS accountable for its misconduct.”6 Though the cost in human life that may have resulted from CSA violations must absolutely and always outweigh financial considerations, the economic damage to Tricare from fraudulent billing and the betrayal of its fiduciary responsinility cannot be underestimated.
A Corporate Morality Play
CVS is not the only company, nor is pharmacy the only industry in health care, that has been the subject of watchdog agency lawsuits or variegated forms of wrongdoing, including violations of the CSA and FCA.10,12 As of this writing, the DoJ case against CVS has not been heard, much less adjudicated in a court of law. It is ironic that both the DoJ claims and the CVS rebuttal describe the manifest conflict of obligation that pharmacists confront between protecting their livelihood and safeguarding patients’ lives as suggested in the epigraph that has been attributed to the 19th-century British physician and medical educator Peter Mere Latham. It is a dilemma that a growing number of health care practitioners face daily in a vocation becoming increasingly commercialized. It is all too easy for an individual physician, nurse, or pharmacist to feel hopeless and helpless before the behemoth might of a large and looming entity. Yet, it was a whistleblower whose moral courage led to the DoJ investigation and subsequent charges.13 We must all never doubt the power of a committed person of conscience to withstand the pressure to mutate medications into poison and stand up for the principles of our professions and inspire a community of colleagues to follow their example.
- Fein AJ. The Top U.S. pharmacy markets of 2023: market shares and revenues at the biggest chains and PBMs. Drug Channels. March 12, 2024. Accessed February 24, 2025. https://www.drugchannels.net/2024/03/the-top-15-us-pharmacies-of-2023-market.html
- Jowers K. CVS returns to the military Tricare network. Walmart’s out. Military Times. October 18, 2021. Accessed February 24, 2025. https://www.militarytimes.com/pay-benefits/mil-money/2021/10/28/cvs-returns-to-the-military-tricare-pharmacy-network-walmarts-out/
- False Claims, 31 USC § 3729 (2009). Accessed February 24, 2025. https://www.govinfo.gov/content/pkg/USCODE-2011-title31/pdf/USCODE-2011-title31-subtitleIII-chap37-subchapIII-sec3729.pdf
- Drug Abuse Prevention and Control, Control and Enforcement, 21 USC 13 § 801 (2022). Accessed February 24, 2025. https://www.govinfo.gov/app/details/USCODE-2021-title21/USCODE-2021-title21-chap13-subchapI-partA-sec801
- United States ex rel. Estright v Health Corporation, et al. Accessed February 26, 2025. https://www.justice.gov/archives/opa/media/1381111/dl
- US Department of Justice. Justice Department files nationwide lawsuit alleging CVS knowingly dispensed controlled substances in violation of the Controlled Substances ACT and the False Claims Act. News release. December 18, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/justice-department-files-nationwide-lawsuit-alleging-cvs-knowingly-dispensed-controlled
- CVS Health. CVS Health statement regarding the U.S. Department of Justice’s lawsuit against CVS pharmacy. News release. December 18, 2024. Accessed February 24, 2025. https://www.cvshealth.com/impact/healthy-community/our-opioid-response.html
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi:10.1136/bmj.h2698
- Wang Y, Delcher C, Li Y, Goldberger BA, Reisfield GM. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019;205:107693. doi:10.1016/j.drugalcdep.2019.107693
- Wolf AA. The perfect storm: opioid risks and ‘The Holy Trinity’. Pharmacy Times. September 24, 2014. Accessed February 24, 2025. https://www.pharmacytimes.com/view/the-perfect-storm-opioid-risks-and-the-holy-trinity
- The meaning and justification of informed consent. In: Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Eighth Edition. Oxford University Press; 2019:118-123.
- US Department of Justice. OptumRX agrees to pay $20M to resolve allegations that it filled certain opioid prescriptions in violation of the Controlled Substances Act. News release. June 27, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/optumrx-agrees-pay-20m-resolve-allegations-it-filled-certain-opioid-prescriptions-violation
- US Department of Justice. False Claims Act settlements and judgments exceed $2.9B in fiscal year 2024. News release. January 15, 2025. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/false-claims-act-settlements-and-judgments-exceed-29b-fiscal-year-2024
- Fein AJ. The Top U.S. pharmacy markets of 2023: market shares and revenues at the biggest chains and PBMs. Drug Channels. March 12, 2024. Accessed February 24, 2025. https://www.drugchannels.net/2024/03/the-top-15-us-pharmacies-of-2023-market.html
- Jowers K. CVS returns to the military Tricare network. Walmart’s out. Military Times. October 18, 2021. Accessed February 24, 2025. https://www.militarytimes.com/pay-benefits/mil-money/2021/10/28/cvs-returns-to-the-military-tricare-pharmacy-network-walmarts-out/
- False Claims, 31 USC § 3729 (2009). Accessed February 24, 2025. https://www.govinfo.gov/content/pkg/USCODE-2011-title31/pdf/USCODE-2011-title31-subtitleIII-chap37-subchapIII-sec3729.pdf
- Drug Abuse Prevention and Control, Control and Enforcement, 21 USC 13 § 801 (2022). Accessed February 24, 2025. https://www.govinfo.gov/app/details/USCODE-2021-title21/USCODE-2021-title21-chap13-subchapI-partA-sec801
- United States ex rel. Estright v Health Corporation, et al. Accessed February 26, 2025. https://www.justice.gov/archives/opa/media/1381111/dl
- US Department of Justice. Justice Department files nationwide lawsuit alleging CVS knowingly dispensed controlled substances in violation of the Controlled Substances ACT and the False Claims Act. News release. December 18, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/justice-department-files-nationwide-lawsuit-alleging-cvs-knowingly-dispensed-controlled
- CVS Health. CVS Health statement regarding the U.S. Department of Justice’s lawsuit against CVS pharmacy. News release. December 18, 2024. Accessed February 24, 2025. https://www.cvshealth.com/impact/healthy-community/our-opioid-response.html
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi:10.1136/bmj.h2698
- Wang Y, Delcher C, Li Y, Goldberger BA, Reisfield GM. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019;205:107693. doi:10.1016/j.drugalcdep.2019.107693
- Wolf AA. The perfect storm: opioid risks and ‘The Holy Trinity’. Pharmacy Times. September 24, 2014. Accessed February 24, 2025. https://www.pharmacytimes.com/view/the-perfect-storm-opioid-risks-and-the-holy-trinity
- The meaning and justification of informed consent. In: Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Eighth Edition. Oxford University Press; 2019:118-123.
- US Department of Justice. OptumRX agrees to pay $20M to resolve allegations that it filled certain opioid prescriptions in violation of the Controlled Substances Act. News release. June 27, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/optumrx-agrees-pay-20m-resolve-allegations-it-filled-certain-opioid-prescriptions-violation
- US Department of Justice. False Claims Act settlements and judgments exceed $2.9B in fiscal year 2024. News release. January 15, 2025. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/false-claims-act-settlements-and-judgments-exceed-29b-fiscal-year-2024
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
The Value of Public Service
Former Secretary of State Condoleezza Rice once said: “There is no greater challenge and there is no greater honor than to be in public service.” It has been a challenging few months for public servants, including the thousands of federal healthcare and public health workers who care for our veterans, provide critical services to underserved communities, work to fund high-impact biomedical research that improves health outcomes, and otherwise further important public health goals.
From the VA to the Department of Health & Human Services and its operating divisions, including the Centers for Disease Control and Prevention, National Institutes of Health, Centers for Medicare & Medicaid Services, and others, dedicated federal civil servants have had their work ethic, commitment, and productivity questioned in late-night emails from anonymous authors. They have been encouraged indiscriminately to resign and “move from [their] lower-productivity jobs in the public sector to higher-productivity jobs in the private sector,” and been subjected to vague threats of future job loss regardless of role, duration of service, performance, or political persuasion. This includes the roughly 30% of federal employees who are themselves US military veterans.
In essence, the message is that their work does not matter, and their service and sacrifice is not valued (which, of course, could not be further from the truth). These actions, along with a plethora of other divisive policies, not only threaten our democratic principles, but also serve to degrade our collective values and norms. We are at a “fork in the road” as a nation. I hope for the greater good that we can work together to uphold the value of public service, of community, of civility — both for the sake of our democracy and to preserve our nation’s health.
In our March issue, This month’s Member Spotlight features Dr. Pooja Singhal (Oklahoma Gastro Health and Wellness), who describes how she integrates wellness principles into her clinical practice, discusses the evolution of her interest in women’s digestive health, and shares how she serves her community outside of medicine.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Former Secretary of State Condoleezza Rice once said: “There is no greater challenge and there is no greater honor than to be in public service.” It has been a challenging few months for public servants, including the thousands of federal healthcare and public health workers who care for our veterans, provide critical services to underserved communities, work to fund high-impact biomedical research that improves health outcomes, and otherwise further important public health goals.
From the VA to the Department of Health & Human Services and its operating divisions, including the Centers for Disease Control and Prevention, National Institutes of Health, Centers for Medicare & Medicaid Services, and others, dedicated federal civil servants have had their work ethic, commitment, and productivity questioned in late-night emails from anonymous authors. They have been encouraged indiscriminately to resign and “move from [their] lower-productivity jobs in the public sector to higher-productivity jobs in the private sector,” and been subjected to vague threats of future job loss regardless of role, duration of service, performance, or political persuasion. This includes the roughly 30% of federal employees who are themselves US military veterans.
In essence, the message is that their work does not matter, and their service and sacrifice is not valued (which, of course, could not be further from the truth). These actions, along with a plethora of other divisive policies, not only threaten our democratic principles, but also serve to degrade our collective values and norms. We are at a “fork in the road” as a nation. I hope for the greater good that we can work together to uphold the value of public service, of community, of civility — both for the sake of our democracy and to preserve our nation’s health.
In our March issue, This month’s Member Spotlight features Dr. Pooja Singhal (Oklahoma Gastro Health and Wellness), who describes how she integrates wellness principles into her clinical practice, discusses the evolution of her interest in women’s digestive health, and shares how she serves her community outside of medicine.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Former Secretary of State Condoleezza Rice once said: “There is no greater challenge and there is no greater honor than to be in public service.” It has been a challenging few months for public servants, including the thousands of federal healthcare and public health workers who care for our veterans, provide critical services to underserved communities, work to fund high-impact biomedical research that improves health outcomes, and otherwise further important public health goals.
From the VA to the Department of Health & Human Services and its operating divisions, including the Centers for Disease Control and Prevention, National Institutes of Health, Centers for Medicare & Medicaid Services, and others, dedicated federal civil servants have had their work ethic, commitment, and productivity questioned in late-night emails from anonymous authors. They have been encouraged indiscriminately to resign and “move from [their] lower-productivity jobs in the public sector to higher-productivity jobs in the private sector,” and been subjected to vague threats of future job loss regardless of role, duration of service, performance, or political persuasion. This includes the roughly 30% of federal employees who are themselves US military veterans.
In essence, the message is that their work does not matter, and their service and sacrifice is not valued (which, of course, could not be further from the truth). These actions, along with a plethora of other divisive policies, not only threaten our democratic principles, but also serve to degrade our collective values and norms. We are at a “fork in the road” as a nation. I hope for the greater good that we can work together to uphold the value of public service, of community, of civility — both for the sake of our democracy and to preserve our nation’s health.
In our March issue, This month’s Member Spotlight features Dr. Pooja Singhal (Oklahoma Gastro Health and Wellness), who describes how she integrates wellness principles into her clinical practice, discusses the evolution of her interest in women’s digestive health, and shares how she serves her community outside of medicine.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Surgical vs Endoscopic Excision of Large Colon Polyps
Dear colleagues,
We now have the ability to remove almost any large colon polyp endoscopically using a variety of techniques — from the widely used endoscopic mucosal resection to the increasingly prevalent endoscopic submucosal dissection. Yet, in this new era,
In this issue of Perspectives, Dr. Jeffrey Mosko and Dr. Moamen Gabr discuss the importance of careful polyp selection and argue that almost all polyps can be safely removed endoscopically, with low recurrence rates. In contrast, Dr. Ira Leeds from colorectal surgery offers a counterpoint, urging caution when managing polyps in the cecum and rectum while highlighting the role of minimally invasive surgical approaches. We hope these discussions provide valuable insights to support your approach to managing large colorectal polyps, especially in an era of increasing colon cancer screening.
We also welcome your thoughts on this topic — join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Advantages of Endoscopic Resection for Large Colon Polyps
BY MOAMEN GABR, MD, MSC, AND JEFFREY D. MOSKO MD, MSC
General Advantages
Endoscopy has revolutionized the management of large colorectal polyps, offering a minimally invasive alternative to surgical resection. The dawn of endoscopic resection in the late 20th century, particularly the evolution of endoscopic mucosal resection (EMR) in Japan, marked a paradigm shift in the treatment of colonic lesions by enabling the removal of lesions that would otherwise necessitate surgery.
Endoscopic resection of colorectal polyps is generally performed in an outpatient setting, allowing patients to recover at home the same day. This not only minimizes disruption to daily life but also significantly enhances patient satisfaction.
Most procedures are performed under moderate or deep sedation eliminating the need for general anesthesia. This represents a critical benefit, particularly for older or medically frail patients who are at higher risk of anesthesia-related complications.
From an economic perspective, endoscopic resection reduces healthcare costs by eliminating prolonged hospital stays and complex perioperative care. Additionally, preserving the colon’s structure and function avoids long-term consequences such as altered bowel habits or ostomy dependence, common with surgical interventions.
The advantages of endoscopic intervention are clear: safety, cost-effectiveness, organ preservation, and convenience for patients.
Lesion Selection
The superiority of endoscopic resection relies on selecting lesions appropriately, specifically those with a low risk of lymph node metastases. This meticulous process should include assessing a lesion’s size, location, morphology, granularity, microvascular and surface pit pattern using a combination of high-definition white light endoscopy, virtual chromoendoscopy and image magnification (when available).
Gross morphologic assessment utilizes the Paris and LST classifications. Combining the Paris classification, lesion granularity and location is both straightforward and revealing. Ulcerated/excavated lesions (0-III) are concerning for deep invasion. Depressed (0-IIc) morphologies are strongly associated with T1 CRC. Nodular lesions (0-Is or IIa + Is) have a higher risk of T1 colorectal cancer (CRC), compared with flat lesions (0-IIa or 0-IIb). Non-granular lesions (0-Is and 0-IIa + Is) have a higher risk of covert cancer. Finally, the rectosigmoid location is associated with an increased risk of T1 CRC (vs. proximal locations).
Endoscopic surface pattern assessment increases one’s diagnostic accuracy. There are three primary endoscopic surface pattern classifications: NBI International Colorectal Endoscopic (NICE), Japanese NBI expert team (JNET), and Kudo pit pattern classifications. Colonic lesions that have a NICE Type 3, JNET 3, or Kudo type Vn pattern should be referred promptly for surgical resection. Lesions with a JNET 2B or Kudo type VI carry a higher risk of superficial T1 CRC but can still be removed endoscopically (see below) in expert centers. All other lesions should undergo endoscopic resection.
Endoscopic Resection Techniques
Endoscopic resection of large colorectal polyps encompasses two primary techniques: EMR and endoscopic submucosal dissection (ESD), each tailored to specific lesion characteristics and operator expertise.
EMR, the technique of choice for the vast majority of lesions, relies on injecting a submucosal cushion to lift the lesion before excision. Recent advances, including enhanced snare designs and underwater EMR, have improved en-bloc resection rates, significantly reducing recurrence and enhancing the efficacy of this technique.
ESD offers unparalleled precision for en-bloc resection of complex lesions, particularly those with fibrosis or high-risk features. Cutting-edge innovations, such as traction devices, have streamlined the procedure, addressing the traditional challenges of ESD. Despite being more time intensive, ESD minimizes recurrence and provides complete histopathological evaluation, critical for the management of malignant or pre-malignant lesions.
For non-lifting polyps, newer techniques such as endoscopic full-thickness resection (eFTR), using tools like the Full-Thickness Resection Device (FTRD), enable resection of up to 2-3 cm of the colonic or rectal wall. This ensures complete removal of any lesion and its underlying tissue, effectively preventing recurrence.
These advancements demonstrate how endoscopy can tackle even the most challenging colorectal polyps, reinforcing its position as the preferred treatment modality.
Perceived Limitations
With ongoing refinement over the last 2 decades, many of the perceived limitations (below) of endoscopic resection have now been overcome.
- Difficult locations/access: Historically lesions at the anorectal junction, ileocecal valve, appendiceal orifice and anastomoses were preferentially sent for surgery. In spite of unique technical challenges at each of these locations, there is now compelling data supporting EMR for these scenarios. We now also have techniques aimed at enabling the resection of lesions with poor access including patient repositioning, distal attachments, variable endoscope diameter/flexibility, traction and overtube devices.
- Recurrence: In the past, recurrence after endoscopic resection of lesions > 20 mm has been reported to be as high as 20%. With our current systematic approach to complete resection, meticulous examination of the post-resection defect for residual polyp tissue, adjunctive techniques to address submucosal fibrosis (hot avulsion, CAST, submucosal release) and thermal ablation to the resection margin (EMR-T), the risk of recurrence for piecemeal resections can be decreased to < 5%. In fact, some groups argue for the en-bloc resection of all large colorectal lesions based on the extremely low (< 1%) recurrence rates and potential for decreased follow-up.
- Post-resection bleeding: Post-resection bleeding is no longer a major limitation of any endoscopic approach because of the combination of improved intra-procedural hemostatic and resection techniques, optimized electrosurgical technology, and enhanced defect closure capabilities and devices (with prophylactic defect closure now supported by randomized control trial level data).
- Perforation: Deep mural injury, once an endoscopists’ worst fear during resection, is no longer a surgical emergency. It can now be predicted, identified (Sydney classification) and successfully managed. In spite of more widespread aggressive resection strategies, the risk of emergency surgery in patients undergoing EMR and even ESD (where the risk of DMI is significantly higher) is extremely low.
Endoscopic resection for large colorectal polyps is effective, available, minimally invasive and organ sparing making it the standard of care for the management of colonic polyps. With ongoing iteration in techniques, more invasive surgical approaches can be avoided in almost all patients with benign and low-risk T1 colorectal cancers.
Dr. Gabr is associate GI division director at the University of Cincinnati, Ohio. Dr. Mosko is based in the division of gastroenterology at St. Michael’s Hospital, Toronto, Ontario, Canada. The authors declare no conflicts of interest.
Blurred Lines: Polyp Needing Surgical versus Endoscopic Excision
BY IRA LEEDS, MD
I am grateful for the invitation to join in discussion with Dr. Gabr and Dr. Mosko on the ever-increasing role of endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). However, as a surgeon, I do carry at least mild trepidation entering one of the literary “safe spaces” of my gastroenterology colleagues.
With the increasing evidentiary support of EMR approaches and the increasing experience of those performing ESD, these two techniques are quickly becoming the options of choice. As these practices become ubiquitous, it is important to recognize both their advantages and limitations, compared with available surgical options. The decision to proceed with EMR and ESD is essentially a turning point away from early surgical referral for a complex lesion. In this discussion, I intend to highlight when EMR and ESD have a clear advantage to early surgical referral, why I believe that early surgical referral is still superior to advanced endoscopic techniques in the rectum, and why the approach for right-sided lesions should hinge on careful shared decision-making.
Endoscopic approaches nearly always beat surgical approaches when considering short-term risks. Even in the best surgical series, colorectal surgery typically leads to complications in 10%-15% of patients, 1%-5% being serious. Moreover, transabdominal surgical interventions (ie, colectomy) require considerable recovery involving at least a few days in the inpatient setting and over a month of activity restrictions. Finally, there is a minority of chronically unwell patients who cannot tolerate surgical intervention but may be fortunate enough to have a lesion that with enhanced attention can be endoscopically resectioned. While EMR and ESD also contribute a disproportionate burden of complications to endoscopy practice, overall complication rates are still favorable when compared with surgical resection.
Moreover, the most feared short-term complication of EMR and ESD, perforation, has the added benefit of a “controlled failure” to colectomy. Advanced endoscopic approaches already require a prepared colon, and patients are given strict return instructions. Hence, the yearly handful of postprocedural perforations that I get called upon to assist with typically tolerate a routine surgical exploration, repair or resection, and recover at rates equal to or better than elective colon resections. For these reasons, lesions that can be endoscopically removed within appropriate risk tolerances, can and should be considered for EMR or ESD at time of diagnosis.
There are two clinical scenarios where this consideration for up-front EMR or ESD requires further caution. First, any rectal lesion considered for advanced endoscopic techniques really needs to be done in multidisciplinary conference with a colorectal surgeon. In the modern era of colorectal surgery, surgeons now have numerous approaches to reach the rectum that bridge the gap between traditional endoscopy and transabdominal resection. For many rectal lesions, transanal laparoscopic and robotic approaches offer the opportunity for local excision. The most commonly practiced approach, transanal minimally invasive microsurgery (TAMIS), provides many of the benefits of endoscopy (eg, same-day discharge, no activity restrictions, limited periprocedural physiologic stress, low complication rates) while providing the surgical precision, repair strategies, and specimen orientation of conventional surgery. Anecdotally, the time it takes to do a high-quality TAMIS excision in the rectum can be substantially less than that required for a comparable ESD.
For rectal lesions in particular, specimen quality is paramount for oncologic prognosis. Regardless of any intrinsic favorable histopathology or deft hand of the endoscopist, a TAMIS approach will typically provide for a deeper partial thickness or even full thickness excision. More times each year than I would like, I find myself at a multidisciplinary tumor board discussing an endoscopically removed rectal lesion done in a piecemeal fashion or insufficient deep ESD where appropriate risk stratification is impossible and we end up offering patients a likely overly aggressive proctectomy or a potentially oncologically unsound re-excision. Consideration of EMR/ESD vs TAMIS up front would allow better sorting of which technique is most suited to which lesion and avoid these diagnostic dilemmas that only seem to be more common as EMR and ESD practices proliferate.
For a different set of reasons, an advanced cecal adenoma may also be more suited to upfront surgical considerations. Right colon lesions can be more challenging for surveillance for a host of reasons. Procedurally, right colon lesions are undeniably more difficult. The thin-walled cecum can be unforgiving for repeated polypectomies. Despite it being an uncomfortable subject for colonoscopists, the evidence suggests that getting to the cecum is not consistent or 100% expected. Finally, patients can be unwilling to undergo serial bowel preparation and endoscopic examination. In contrast, a laparoscopic right colectomy avoids these issues while also attributing little additional risk. Laparoscopic right-colon operations have overall complication rates of less than 10% and major complications of less than 1%. Hospital stays for laparoscopic right colectomy are typically 3 days or less. Finally, surgery reduces both the frequency of surveillance, and a shortened colon makes surveillance easier.
Advanced polypectomy techniques broaden our ability to address even difficult lesions under the ideally aligned degree of invasive procedure. However, like any procedure, these techniques have their own advantages and limitations. There will always be a minority of premalignant colon lesions that are best suited to surgery-first approaches to treatment. In my practice, maintaining open lines of communication and regular interaction with my endoscopy colleagues naturally leads to polyps being addressed in their most suitable fashion.
Dr. Leeds is assistant professor of surgery at the Yale School of Medicine and a staff surgeon at the VA Connecticut Healthcare System. He declares no conflicts of interest.
Dear colleagues,
We now have the ability to remove almost any large colon polyp endoscopically using a variety of techniques — from the widely used endoscopic mucosal resection to the increasingly prevalent endoscopic submucosal dissection. Yet, in this new era,
In this issue of Perspectives, Dr. Jeffrey Mosko and Dr. Moamen Gabr discuss the importance of careful polyp selection and argue that almost all polyps can be safely removed endoscopically, with low recurrence rates. In contrast, Dr. Ira Leeds from colorectal surgery offers a counterpoint, urging caution when managing polyps in the cecum and rectum while highlighting the role of minimally invasive surgical approaches. We hope these discussions provide valuable insights to support your approach to managing large colorectal polyps, especially in an era of increasing colon cancer screening.
We also welcome your thoughts on this topic — join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Advantages of Endoscopic Resection for Large Colon Polyps
BY MOAMEN GABR, MD, MSC, AND JEFFREY D. MOSKO MD, MSC
General Advantages
Endoscopy has revolutionized the management of large colorectal polyps, offering a minimally invasive alternative to surgical resection. The dawn of endoscopic resection in the late 20th century, particularly the evolution of endoscopic mucosal resection (EMR) in Japan, marked a paradigm shift in the treatment of colonic lesions by enabling the removal of lesions that would otherwise necessitate surgery.
Endoscopic resection of colorectal polyps is generally performed in an outpatient setting, allowing patients to recover at home the same day. This not only minimizes disruption to daily life but also significantly enhances patient satisfaction.
Most procedures are performed under moderate or deep sedation eliminating the need for general anesthesia. This represents a critical benefit, particularly for older or medically frail patients who are at higher risk of anesthesia-related complications.
From an economic perspective, endoscopic resection reduces healthcare costs by eliminating prolonged hospital stays and complex perioperative care. Additionally, preserving the colon’s structure and function avoids long-term consequences such as altered bowel habits or ostomy dependence, common with surgical interventions.
The advantages of endoscopic intervention are clear: safety, cost-effectiveness, organ preservation, and convenience for patients.
Lesion Selection
The superiority of endoscopic resection relies on selecting lesions appropriately, specifically those with a low risk of lymph node metastases. This meticulous process should include assessing a lesion’s size, location, morphology, granularity, microvascular and surface pit pattern using a combination of high-definition white light endoscopy, virtual chromoendoscopy and image magnification (when available).
Gross morphologic assessment utilizes the Paris and LST classifications. Combining the Paris classification, lesion granularity and location is both straightforward and revealing. Ulcerated/excavated lesions (0-III) are concerning for deep invasion. Depressed (0-IIc) morphologies are strongly associated with T1 CRC. Nodular lesions (0-Is or IIa + Is) have a higher risk of T1 colorectal cancer (CRC), compared with flat lesions (0-IIa or 0-IIb). Non-granular lesions (0-Is and 0-IIa + Is) have a higher risk of covert cancer. Finally, the rectosigmoid location is associated with an increased risk of T1 CRC (vs. proximal locations).
Endoscopic surface pattern assessment increases one’s diagnostic accuracy. There are three primary endoscopic surface pattern classifications: NBI International Colorectal Endoscopic (NICE), Japanese NBI expert team (JNET), and Kudo pit pattern classifications. Colonic lesions that have a NICE Type 3, JNET 3, or Kudo type Vn pattern should be referred promptly for surgical resection. Lesions with a JNET 2B or Kudo type VI carry a higher risk of superficial T1 CRC but can still be removed endoscopically (see below) in expert centers. All other lesions should undergo endoscopic resection.
Endoscopic Resection Techniques
Endoscopic resection of large colorectal polyps encompasses two primary techniques: EMR and endoscopic submucosal dissection (ESD), each tailored to specific lesion characteristics and operator expertise.
EMR, the technique of choice for the vast majority of lesions, relies on injecting a submucosal cushion to lift the lesion before excision. Recent advances, including enhanced snare designs and underwater EMR, have improved en-bloc resection rates, significantly reducing recurrence and enhancing the efficacy of this technique.
ESD offers unparalleled precision for en-bloc resection of complex lesions, particularly those with fibrosis or high-risk features. Cutting-edge innovations, such as traction devices, have streamlined the procedure, addressing the traditional challenges of ESD. Despite being more time intensive, ESD minimizes recurrence and provides complete histopathological evaluation, critical for the management of malignant or pre-malignant lesions.
For non-lifting polyps, newer techniques such as endoscopic full-thickness resection (eFTR), using tools like the Full-Thickness Resection Device (FTRD), enable resection of up to 2-3 cm of the colonic or rectal wall. This ensures complete removal of any lesion and its underlying tissue, effectively preventing recurrence.
These advancements demonstrate how endoscopy can tackle even the most challenging colorectal polyps, reinforcing its position as the preferred treatment modality.
Perceived Limitations
With ongoing refinement over the last 2 decades, many of the perceived limitations (below) of endoscopic resection have now been overcome.
- Difficult locations/access: Historically lesions at the anorectal junction, ileocecal valve, appendiceal orifice and anastomoses were preferentially sent for surgery. In spite of unique technical challenges at each of these locations, there is now compelling data supporting EMR for these scenarios. We now also have techniques aimed at enabling the resection of lesions with poor access including patient repositioning, distal attachments, variable endoscope diameter/flexibility, traction and overtube devices.
- Recurrence: In the past, recurrence after endoscopic resection of lesions > 20 mm has been reported to be as high as 20%. With our current systematic approach to complete resection, meticulous examination of the post-resection defect for residual polyp tissue, adjunctive techniques to address submucosal fibrosis (hot avulsion, CAST, submucosal release) and thermal ablation to the resection margin (EMR-T), the risk of recurrence for piecemeal resections can be decreased to < 5%. In fact, some groups argue for the en-bloc resection of all large colorectal lesions based on the extremely low (< 1%) recurrence rates and potential for decreased follow-up.
- Post-resection bleeding: Post-resection bleeding is no longer a major limitation of any endoscopic approach because of the combination of improved intra-procedural hemostatic and resection techniques, optimized electrosurgical technology, and enhanced defect closure capabilities and devices (with prophylactic defect closure now supported by randomized control trial level data).
- Perforation: Deep mural injury, once an endoscopists’ worst fear during resection, is no longer a surgical emergency. It can now be predicted, identified (Sydney classification) and successfully managed. In spite of more widespread aggressive resection strategies, the risk of emergency surgery in patients undergoing EMR and even ESD (where the risk of DMI is significantly higher) is extremely low.
Endoscopic resection for large colorectal polyps is effective, available, minimally invasive and organ sparing making it the standard of care for the management of colonic polyps. With ongoing iteration in techniques, more invasive surgical approaches can be avoided in almost all patients with benign and low-risk T1 colorectal cancers.
Dr. Gabr is associate GI division director at the University of Cincinnati, Ohio. Dr. Mosko is based in the division of gastroenterology at St. Michael’s Hospital, Toronto, Ontario, Canada. The authors declare no conflicts of interest.
Blurred Lines: Polyp Needing Surgical versus Endoscopic Excision
BY IRA LEEDS, MD
I am grateful for the invitation to join in discussion with Dr. Gabr and Dr. Mosko on the ever-increasing role of endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). However, as a surgeon, I do carry at least mild trepidation entering one of the literary “safe spaces” of my gastroenterology colleagues.
With the increasing evidentiary support of EMR approaches and the increasing experience of those performing ESD, these two techniques are quickly becoming the options of choice. As these practices become ubiquitous, it is important to recognize both their advantages and limitations, compared with available surgical options. The decision to proceed with EMR and ESD is essentially a turning point away from early surgical referral for a complex lesion. In this discussion, I intend to highlight when EMR and ESD have a clear advantage to early surgical referral, why I believe that early surgical referral is still superior to advanced endoscopic techniques in the rectum, and why the approach for right-sided lesions should hinge on careful shared decision-making.
Endoscopic approaches nearly always beat surgical approaches when considering short-term risks. Even in the best surgical series, colorectal surgery typically leads to complications in 10%-15% of patients, 1%-5% being serious. Moreover, transabdominal surgical interventions (ie, colectomy) require considerable recovery involving at least a few days in the inpatient setting and over a month of activity restrictions. Finally, there is a minority of chronically unwell patients who cannot tolerate surgical intervention but may be fortunate enough to have a lesion that with enhanced attention can be endoscopically resectioned. While EMR and ESD also contribute a disproportionate burden of complications to endoscopy practice, overall complication rates are still favorable when compared with surgical resection.
Moreover, the most feared short-term complication of EMR and ESD, perforation, has the added benefit of a “controlled failure” to colectomy. Advanced endoscopic approaches already require a prepared colon, and patients are given strict return instructions. Hence, the yearly handful of postprocedural perforations that I get called upon to assist with typically tolerate a routine surgical exploration, repair or resection, and recover at rates equal to or better than elective colon resections. For these reasons, lesions that can be endoscopically removed within appropriate risk tolerances, can and should be considered for EMR or ESD at time of diagnosis.
There are two clinical scenarios where this consideration for up-front EMR or ESD requires further caution. First, any rectal lesion considered for advanced endoscopic techniques really needs to be done in multidisciplinary conference with a colorectal surgeon. In the modern era of colorectal surgery, surgeons now have numerous approaches to reach the rectum that bridge the gap between traditional endoscopy and transabdominal resection. For many rectal lesions, transanal laparoscopic and robotic approaches offer the opportunity for local excision. The most commonly practiced approach, transanal minimally invasive microsurgery (TAMIS), provides many of the benefits of endoscopy (eg, same-day discharge, no activity restrictions, limited periprocedural physiologic stress, low complication rates) while providing the surgical precision, repair strategies, and specimen orientation of conventional surgery. Anecdotally, the time it takes to do a high-quality TAMIS excision in the rectum can be substantially less than that required for a comparable ESD.
For rectal lesions in particular, specimen quality is paramount for oncologic prognosis. Regardless of any intrinsic favorable histopathology or deft hand of the endoscopist, a TAMIS approach will typically provide for a deeper partial thickness or even full thickness excision. More times each year than I would like, I find myself at a multidisciplinary tumor board discussing an endoscopically removed rectal lesion done in a piecemeal fashion or insufficient deep ESD where appropriate risk stratification is impossible and we end up offering patients a likely overly aggressive proctectomy or a potentially oncologically unsound re-excision. Consideration of EMR/ESD vs TAMIS up front would allow better sorting of which technique is most suited to which lesion and avoid these diagnostic dilemmas that only seem to be more common as EMR and ESD practices proliferate.
For a different set of reasons, an advanced cecal adenoma may also be more suited to upfront surgical considerations. Right colon lesions can be more challenging for surveillance for a host of reasons. Procedurally, right colon lesions are undeniably more difficult. The thin-walled cecum can be unforgiving for repeated polypectomies. Despite it being an uncomfortable subject for colonoscopists, the evidence suggests that getting to the cecum is not consistent or 100% expected. Finally, patients can be unwilling to undergo serial bowel preparation and endoscopic examination. In contrast, a laparoscopic right colectomy avoids these issues while also attributing little additional risk. Laparoscopic right-colon operations have overall complication rates of less than 10% and major complications of less than 1%. Hospital stays for laparoscopic right colectomy are typically 3 days or less. Finally, surgery reduces both the frequency of surveillance, and a shortened colon makes surveillance easier.
Advanced polypectomy techniques broaden our ability to address even difficult lesions under the ideally aligned degree of invasive procedure. However, like any procedure, these techniques have their own advantages and limitations. There will always be a minority of premalignant colon lesions that are best suited to surgery-first approaches to treatment. In my practice, maintaining open lines of communication and regular interaction with my endoscopy colleagues naturally leads to polyps being addressed in their most suitable fashion.
Dr. Leeds is assistant professor of surgery at the Yale School of Medicine and a staff surgeon at the VA Connecticut Healthcare System. He declares no conflicts of interest.
Dear colleagues,
We now have the ability to remove almost any large colon polyp endoscopically using a variety of techniques — from the widely used endoscopic mucosal resection to the increasingly prevalent endoscopic submucosal dissection. Yet, in this new era,
In this issue of Perspectives, Dr. Jeffrey Mosko and Dr. Moamen Gabr discuss the importance of careful polyp selection and argue that almost all polyps can be safely removed endoscopically, with low recurrence rates. In contrast, Dr. Ira Leeds from colorectal surgery offers a counterpoint, urging caution when managing polyps in the cecum and rectum while highlighting the role of minimally invasive surgical approaches. We hope these discussions provide valuable insights to support your approach to managing large colorectal polyps, especially in an era of increasing colon cancer screening.
We also welcome your thoughts on this topic — join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Advantages of Endoscopic Resection for Large Colon Polyps
BY MOAMEN GABR, MD, MSC, AND JEFFREY D. MOSKO MD, MSC
General Advantages
Endoscopy has revolutionized the management of large colorectal polyps, offering a minimally invasive alternative to surgical resection. The dawn of endoscopic resection in the late 20th century, particularly the evolution of endoscopic mucosal resection (EMR) in Japan, marked a paradigm shift in the treatment of colonic lesions by enabling the removal of lesions that would otherwise necessitate surgery.
Endoscopic resection of colorectal polyps is generally performed in an outpatient setting, allowing patients to recover at home the same day. This not only minimizes disruption to daily life but also significantly enhances patient satisfaction.
Most procedures are performed under moderate or deep sedation eliminating the need for general anesthesia. This represents a critical benefit, particularly for older or medically frail patients who are at higher risk of anesthesia-related complications.
From an economic perspective, endoscopic resection reduces healthcare costs by eliminating prolonged hospital stays and complex perioperative care. Additionally, preserving the colon’s structure and function avoids long-term consequences such as altered bowel habits or ostomy dependence, common with surgical interventions.
The advantages of endoscopic intervention are clear: safety, cost-effectiveness, organ preservation, and convenience for patients.
Lesion Selection
The superiority of endoscopic resection relies on selecting lesions appropriately, specifically those with a low risk of lymph node metastases. This meticulous process should include assessing a lesion’s size, location, morphology, granularity, microvascular and surface pit pattern using a combination of high-definition white light endoscopy, virtual chromoendoscopy and image magnification (when available).
Gross morphologic assessment utilizes the Paris and LST classifications. Combining the Paris classification, lesion granularity and location is both straightforward and revealing. Ulcerated/excavated lesions (0-III) are concerning for deep invasion. Depressed (0-IIc) morphologies are strongly associated with T1 CRC. Nodular lesions (0-Is or IIa + Is) have a higher risk of T1 colorectal cancer (CRC), compared with flat lesions (0-IIa or 0-IIb). Non-granular lesions (0-Is and 0-IIa + Is) have a higher risk of covert cancer. Finally, the rectosigmoid location is associated with an increased risk of T1 CRC (vs. proximal locations).
Endoscopic surface pattern assessment increases one’s diagnostic accuracy. There are three primary endoscopic surface pattern classifications: NBI International Colorectal Endoscopic (NICE), Japanese NBI expert team (JNET), and Kudo pit pattern classifications. Colonic lesions that have a NICE Type 3, JNET 3, or Kudo type Vn pattern should be referred promptly for surgical resection. Lesions with a JNET 2B or Kudo type VI carry a higher risk of superficial T1 CRC but can still be removed endoscopically (see below) in expert centers. All other lesions should undergo endoscopic resection.
Endoscopic Resection Techniques
Endoscopic resection of large colorectal polyps encompasses two primary techniques: EMR and endoscopic submucosal dissection (ESD), each tailored to specific lesion characteristics and operator expertise.
EMR, the technique of choice for the vast majority of lesions, relies on injecting a submucosal cushion to lift the lesion before excision. Recent advances, including enhanced snare designs and underwater EMR, have improved en-bloc resection rates, significantly reducing recurrence and enhancing the efficacy of this technique.
ESD offers unparalleled precision for en-bloc resection of complex lesions, particularly those with fibrosis or high-risk features. Cutting-edge innovations, such as traction devices, have streamlined the procedure, addressing the traditional challenges of ESD. Despite being more time intensive, ESD minimizes recurrence and provides complete histopathological evaluation, critical for the management of malignant or pre-malignant lesions.
For non-lifting polyps, newer techniques such as endoscopic full-thickness resection (eFTR), using tools like the Full-Thickness Resection Device (FTRD), enable resection of up to 2-3 cm of the colonic or rectal wall. This ensures complete removal of any lesion and its underlying tissue, effectively preventing recurrence.
These advancements demonstrate how endoscopy can tackle even the most challenging colorectal polyps, reinforcing its position as the preferred treatment modality.
Perceived Limitations
With ongoing refinement over the last 2 decades, many of the perceived limitations (below) of endoscopic resection have now been overcome.
- Difficult locations/access: Historically lesions at the anorectal junction, ileocecal valve, appendiceal orifice and anastomoses were preferentially sent for surgery. In spite of unique technical challenges at each of these locations, there is now compelling data supporting EMR for these scenarios. We now also have techniques aimed at enabling the resection of lesions with poor access including patient repositioning, distal attachments, variable endoscope diameter/flexibility, traction and overtube devices.
- Recurrence: In the past, recurrence after endoscopic resection of lesions > 20 mm has been reported to be as high as 20%. With our current systematic approach to complete resection, meticulous examination of the post-resection defect for residual polyp tissue, adjunctive techniques to address submucosal fibrosis (hot avulsion, CAST, submucosal release) and thermal ablation to the resection margin (EMR-T), the risk of recurrence for piecemeal resections can be decreased to < 5%. In fact, some groups argue for the en-bloc resection of all large colorectal lesions based on the extremely low (< 1%) recurrence rates and potential for decreased follow-up.
- Post-resection bleeding: Post-resection bleeding is no longer a major limitation of any endoscopic approach because of the combination of improved intra-procedural hemostatic and resection techniques, optimized electrosurgical technology, and enhanced defect closure capabilities and devices (with prophylactic defect closure now supported by randomized control trial level data).
- Perforation: Deep mural injury, once an endoscopists’ worst fear during resection, is no longer a surgical emergency. It can now be predicted, identified (Sydney classification) and successfully managed. In spite of more widespread aggressive resection strategies, the risk of emergency surgery in patients undergoing EMR and even ESD (where the risk of DMI is significantly higher) is extremely low.
Endoscopic resection for large colorectal polyps is effective, available, minimally invasive and organ sparing making it the standard of care for the management of colonic polyps. With ongoing iteration in techniques, more invasive surgical approaches can be avoided in almost all patients with benign and low-risk T1 colorectal cancers.
Dr. Gabr is associate GI division director at the University of Cincinnati, Ohio. Dr. Mosko is based in the division of gastroenterology at St. Michael’s Hospital, Toronto, Ontario, Canada. The authors declare no conflicts of interest.
Blurred Lines: Polyp Needing Surgical versus Endoscopic Excision
BY IRA LEEDS, MD
I am grateful for the invitation to join in discussion with Dr. Gabr and Dr. Mosko on the ever-increasing role of endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). However, as a surgeon, I do carry at least mild trepidation entering one of the literary “safe spaces” of my gastroenterology colleagues.
With the increasing evidentiary support of EMR approaches and the increasing experience of those performing ESD, these two techniques are quickly becoming the options of choice. As these practices become ubiquitous, it is important to recognize both their advantages and limitations, compared with available surgical options. The decision to proceed with EMR and ESD is essentially a turning point away from early surgical referral for a complex lesion. In this discussion, I intend to highlight when EMR and ESD have a clear advantage to early surgical referral, why I believe that early surgical referral is still superior to advanced endoscopic techniques in the rectum, and why the approach for right-sided lesions should hinge on careful shared decision-making.
Endoscopic approaches nearly always beat surgical approaches when considering short-term risks. Even in the best surgical series, colorectal surgery typically leads to complications in 10%-15% of patients, 1%-5% being serious. Moreover, transabdominal surgical interventions (ie, colectomy) require considerable recovery involving at least a few days in the inpatient setting and over a month of activity restrictions. Finally, there is a minority of chronically unwell patients who cannot tolerate surgical intervention but may be fortunate enough to have a lesion that with enhanced attention can be endoscopically resectioned. While EMR and ESD also contribute a disproportionate burden of complications to endoscopy practice, overall complication rates are still favorable when compared with surgical resection.
Moreover, the most feared short-term complication of EMR and ESD, perforation, has the added benefit of a “controlled failure” to colectomy. Advanced endoscopic approaches already require a prepared colon, and patients are given strict return instructions. Hence, the yearly handful of postprocedural perforations that I get called upon to assist with typically tolerate a routine surgical exploration, repair or resection, and recover at rates equal to or better than elective colon resections. For these reasons, lesions that can be endoscopically removed within appropriate risk tolerances, can and should be considered for EMR or ESD at time of diagnosis.
There are two clinical scenarios where this consideration for up-front EMR or ESD requires further caution. First, any rectal lesion considered for advanced endoscopic techniques really needs to be done in multidisciplinary conference with a colorectal surgeon. In the modern era of colorectal surgery, surgeons now have numerous approaches to reach the rectum that bridge the gap between traditional endoscopy and transabdominal resection. For many rectal lesions, transanal laparoscopic and robotic approaches offer the opportunity for local excision. The most commonly practiced approach, transanal minimally invasive microsurgery (TAMIS), provides many of the benefits of endoscopy (eg, same-day discharge, no activity restrictions, limited periprocedural physiologic stress, low complication rates) while providing the surgical precision, repair strategies, and specimen orientation of conventional surgery. Anecdotally, the time it takes to do a high-quality TAMIS excision in the rectum can be substantially less than that required for a comparable ESD.
For rectal lesions in particular, specimen quality is paramount for oncologic prognosis. Regardless of any intrinsic favorable histopathology or deft hand of the endoscopist, a TAMIS approach will typically provide for a deeper partial thickness or even full thickness excision. More times each year than I would like, I find myself at a multidisciplinary tumor board discussing an endoscopically removed rectal lesion done in a piecemeal fashion or insufficient deep ESD where appropriate risk stratification is impossible and we end up offering patients a likely overly aggressive proctectomy or a potentially oncologically unsound re-excision. Consideration of EMR/ESD vs TAMIS up front would allow better sorting of which technique is most suited to which lesion and avoid these diagnostic dilemmas that only seem to be more common as EMR and ESD practices proliferate.
For a different set of reasons, an advanced cecal adenoma may also be more suited to upfront surgical considerations. Right colon lesions can be more challenging for surveillance for a host of reasons. Procedurally, right colon lesions are undeniably more difficult. The thin-walled cecum can be unforgiving for repeated polypectomies. Despite it being an uncomfortable subject for colonoscopists, the evidence suggests that getting to the cecum is not consistent or 100% expected. Finally, patients can be unwilling to undergo serial bowel preparation and endoscopic examination. In contrast, a laparoscopic right colectomy avoids these issues while also attributing little additional risk. Laparoscopic right-colon operations have overall complication rates of less than 10% and major complications of less than 1%. Hospital stays for laparoscopic right colectomy are typically 3 days or less. Finally, surgery reduces both the frequency of surveillance, and a shortened colon makes surveillance easier.
Advanced polypectomy techniques broaden our ability to address even difficult lesions under the ideally aligned degree of invasive procedure. However, like any procedure, these techniques have their own advantages and limitations. There will always be a minority of premalignant colon lesions that are best suited to surgery-first approaches to treatment. In my practice, maintaining open lines of communication and regular interaction with my endoscopy colleagues naturally leads to polyps being addressed in their most suitable fashion.
Dr. Leeds is assistant professor of surgery at the Yale School of Medicine and a staff surgeon at the VA Connecticut Healthcare System. He declares no conflicts of interest.
Clinical Research in Early Career Academic Medicine
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
Conducting clinical research as an early career gastroenterologist can take on many forms and has varying definitions of success. This article focuses on key factors to consider and should be supplemented with mentorship tailored to personal interests, goals, and institutional criteria for success. In this article, we will discuss selected high-yield topics that assist in early-career research. We will briefly discuss 1. Defining your niche, 2. Collaboration, 3. Visibility, 4. Time management, 5. Funding, 6. Receiving mentorship, and 7. Providing mentorship. We will conclude with discussing several authors’ experience in the research lab of the first author (FELD Lab – Fostering Equity in Liver and Digestive disease).
Defining Your Niche
Defining your niche is an essential component of an early career, as when academicians must transition from a trainee, who is supporting the research of an established mentor, to defining their own subspeciality area of investigation. Early-career academics should build on their prior work, but should also explore their own passions and skill set to define what will be unique about their research program and contributions to the field. Of course, positioning oneself at the intersection of two or more seemingly unrelated fields opens much opportunity for large impact but comes at a cost of identifying mentorship and justifying the niche to funders.
Collaboration
Fostering a collaborative environment is essential for early-career physician-researchers. One effective approach is to establish collaboration circles with other early career academics. Expanding research endeavors beyond a single institution to a multi-center framework enriches both scope and impact. This collaborative approach not only amplifies the depth of research but also facilitates peer mentorship and sponsorship. Participation in such networks can significantly enhance scholarly output and broaden professional reach during this critical phase of academic progression. Furthermore, prioritizing the promotion of colleagues within these networks is crucial. Proactive sponsorship opportunities, such as inviting peers to present at institutional seminars, strengthen both individual and collective academic visibility.
Collaboration is also essential to foster between trainees involved in early-career investigators’ work. An interconnected lab environment ensures that trainees remain informed about concurrent projects, thereby fostering a culture of shared knowledge and optimized productivity. Encouraging trainees to spearhead research aligned with their interests, under mentor guidance, nurtures independent inquiry and leadership. By establishing explicit roles, responsibilities, and authorship agreements at the outset of collaborative projects, early career mentors can avoid future conflicts and preserve a collaborative culture within the lab. This structured approach cultivates a supportive ecosystem, advancing both individual and collective research achievements.
Visibility
Establishing visibility and developing name recognition are crucial components of career advancement for early-career academic physicians. By clearly defining their areas of expertise, faculty can position themselves as leaders within their discipline. Active participation in professional societies, both at the local and national level, engagement with interest groups, and frequent contributions to educational events can be effective strategies for gaining recognition. Leveraging social media platforms can be helpful in enhancing visibility by facilitating connections and promoting research to a broader audience.
Moreover, research visibility plays a vital role in academic promotion. A strong publication record, reflected by an increasing h-index, demonstrates the impact and relevance of one’s research. Self-citation, when appropriate, can reinforce the continuity and progression of scholarly contributions. While publishing in high-impact journals is desirable, adaptability in resubmitting to other journals following rejections ensures that research remains visible and accessible. It also clearly establishes by whom the work was first done, before someone else investigates the line of inquiry. Through a combination of strategic engagement and publication efforts, early-career physicians can effectively build their professional reputation and advance their academic careers.
Time Management
Time management is essential for any research, and particularly in early career when efficiency in clinical care duties is still being gained. Securing protected time for research is essential to develop a niche, build connections (both institutionally and beyond their institutions), and demonstrate productivity that can be utilized to support future grant efforts.
Similarly, using protected time efficiently is required. Without organization and planning, research time can be spent with scattered meetings and responding to various tasks that do not directly support your research. It is helpful to be introspective about the time of the day you are most productive in your research efforts and blocking off that time to focus on research tasks and minimizing distractions. Blocking monthly time for larger scale thinking and planning is also important. Weekly lab and individual one-on-one meetings also support time management for trainees and lab members, to ensure efficiency and progress. Additionally, robust clinical support is essential to ensure that research time remains protected and patient care moves forward. When negotiating for positions, and in regular meetings thereafter, it is important to advocate for sufficient clinical staffing such that non-physician tasks can be appropriately delegated to another member of the care team.
Funding
Securing adequate funding poses a significant challenge for all early-career physician-scientists, particularly because of the discrepancy between National Institutes of Health salary caps and the higher average salaries in academic gastroenterology. This financial gap can deter physicians from pursuing research-intensive careers altogether and can derail early investigators who do not obtain funding rapidly. To overcome this, early-career investigators may need to adopt flexible strategies, such as accepting a lower salary that aligns with grant funding limits or funneling incentive or bonus pay to research accounts. Alternatively, they can advocate for institutional support to bridge the salary gap, ensuring their research efforts remain financially viable.
Institutions committed to fostering research excellence may offer supplemental funding or bridge programs to retain talented physician-scientists, thereby mitigating the financial strain and encouraging long-term engagement in research. Regular meetings to review salary and support sources, including philanthropy, foundation grants, and other streams, should be undertaken with leadership to align the researcher’s timeline and available funding. If career development funding appears untenable, consideration of multi–principal investigator R01s or equivalent with senior established investigators can be a promising path.
Receiving Mentorship
Effective mentorship for early-career physician-scientists should be approached through a team-based model that leverages both internal and external mentors. Internal mentors, familiar with the specific culture, expectations, and advancement pathways of the institution, can provide invaluable guidance on navigating institutional metrics for success, such as promotion criteria, grant acquisition, and clinical-research balance. External mentors, on the other hand, bring a broader perspective by offering innovative career development strategies and solutions derived from experiences at their home institutions. This multimodal mentorship model ensures a well-rounded approach to professional growth.
All national gastroenterology societies, including the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, offer structured early-career mentorship programs designed to connect emerging researchers with experienced leaders in the field (see below). These programs typically require a formal application process and are highly regarded for their exceptional quality and impact. Participation in such initiatives can significantly enhance career development by expanding networks, fostering interdisciplinary collaboration, and providing tailored guidance that complements institutional support. Integrating both internal and external mentorship opportunities ensures a robust and dynamic foundation for long-term success in academic medicine.
- AGA Career Compass (https://gastro.org/fellows-and-early-career/mentoring/)
- ACG Early Career Leadership Program (https://gi.org/acg-institute/early-career-leadership-program/)
- AGA-AASLD Academic Skills Workshop (https://gastro.org/news/applications-now-being-accepted-for-the-2024-aga-aasld-academic-skills-workshop/)
- AASLD Women’s Initiative Committee Leadership Program (https://www.aasld.org/promoting-leadership-potential-women-hepatology)
- Scrubs n Heels Mentorship Matrix (https://scrubsandheels.com/matrix/)
Providing Mentorship
The trainee authors on this manuscript describe in this section what has been helpful for them as mentees in the FELD research lab.
Student doctor Nguyen describes her experience as a lab member and things she finds most helpful as a medical student in the lab:
- Upon joining the team, a one-to-one meeting to discuss trainee’s personal and professional goals, and availability, was crucial to building the mentor-mentee relationship. Establishing this meaningful mentorship early on clarified expectations on both sides, built trust, and increased motivation. As a trainee, it is essential for me to see how my work aligns with a long-term goal and to receive ample guidance throughout the process.
- One of the most impactful experiences has been joining informal lunch sessions where trainees discussed data collection protocols and exchanged insights. In doing so, Dr. Feld has cultivated a lab culture that encourages curiosity, constructive feedback, and collaborative learning.
- To increase productivity, our team of trainees created a useful group message thread where we coordinated more sessions to collaborate. This coordination formed stronger relationships between team members and fostered a sense of shared purpose.
Dr. Cooper, a third year internal medicine resident, describes her experience as both a research mentee and a mentor to the junior trainees: “As a resident pursuing a career in academic gastroenterology and hepatology, I have found three key elements to be most helpful: intentional mentorship, structured meetings, and leadership development.”
- Intentional mentorship: Prior to joining the lab, I met with Dr. Feld to discuss my research experience and my goals. She took the time to understand these within the context of my training timeline and tailored project opportunities that aligned with my interests and were both feasible and impactful for my next steps. This intentional approach not only fostered a productive research experience but also established a mentor-mentee relationship built on genuine care for my growth and development.
- Regular meetings: Frequent lab meetings promote accountability, teamwork, and shared problem-solving skills. The open exchange of ideas fosters collaboration and joint problem solving to elevate the quality of our research. They are also an opportunity to observe key decision-making points during the research process and have been a great way to learn more about solid methodology.
- Supervised leadership: I have had ample time to lead discussions and coordinate projects among the junior trainees. These monitored leadership experiences promote project management skills, mentorship, and team dynamic awareness while maintaining the safety net of senior guidance. This model helped me transition from a trainee supporting others’ research to a more independent role, contributing to multi-disciplinary projects while mentoring junior members.
Conclusion
In conclusion, many exciting opportunities and notable barriers exist to establishing a clinical research laboratory in the early career. While excellence in each of the areas outlined may evolve, some aspects will come easier than others and with time, persistence, and a bit of luck, the research world will be a better place because of your contributions!
Dr. Feld is assistant professor of gastroenterology and hepatology and physician executive of Diversity, Equity, Inclusion and Belonging for the department of medicine at the University of Massachusetts (UMass) Chan Medical School, Worcester. Ms. Nguyen is a medical student at UMass Chan Medical School. Dr. Cooper is a resident physician at UMass Chan Medical School. Dr. Rabinowitz is an attending physician in the Inflammatory Bowel Disease Center at Beth Israel Deaconess Medical Center, Boston, Mass. Dr. Uchida is codirector of the Multidisciplinary Eosinophilic Gastrointestinal Disease Clinic at the University of Utah School of Medicine, Salt Lake City.
The Federal Trade Commission’s Non-Compete Ban
Non-compete agreements (NCAs) in physician contracts, also termed “restrictive covenants” or “covenants not to compete,” have become a hot topic recently because of the Federal Trade Commission’s (FTC’s) April 2024 ruling invalidating almost all NCAs. But in fact, NCAs have long been controversial, and no more so than in the realm of physician NCAs, which involve substantial policy concerns.
how it relates to a physician employment contract currently, and its possible evolution.
What is It?
Generally speaking, an NCA, usually in the form of an employment contract clause, is an agreement between the employer and the employee that the employee will not enter into post-contract competition with that employer within the limitations of a specific duration, scope of practice, and/or geography. NCAs have traditionally been regulated under state statutory law and common law and have been permitted based on policy considerations that attempt to balance competing employee and employer interests. Physicians should understand their states’ statutory treatment of an NCA.
NCAs protect important employer business interests, including the protection of proprietary information, safeguarding trade secrets, reducing employee turnover, and protecting patient lists. Employees, though, have limited mobility in changing professional positions, have less bargaining power with the employer, and may find themselves with limited options for comparable professional positions.
The NCA ostensibly appears to greatly benefit the employer’s interests over the employee’s; however, NCA protection of employer interests may also substantially benefit employees by encouraging substantial employer investment in employees whom the employer recognizes as a stable and likely long-term human resource, ultimately fostering increased employee satisfaction and innovation. Indeed, one concern with the FTC’s non-compete ban is the potential for significant underinvestment in information sharing and employee training, because employers would, without a NCA, be less likely to recoup those employee investments and would have limited ability to keep competitors from free-riding on investments in employees who leave and join competitors. Ultimately, this would lead to decreased market efficiency.
What is Its Status Today?
Regulation of NCAs, including physician NCAs, has traditionally been based on state statutory law and by common law. Perhaps because of the increasing use of the NCA in professional settings, the NCA has been increasingly scrutinized by courts and state legislatures in the last few decades, with an overall increasing focus on NCA reasonableness and appropriate fit in individual employment settings, and with an emphasis on employer demonstration of legitimate and significant business interests for using a NCA.
States have evolved differently in their treatment of NCAs; some states ban NCAs altogether while others allow them with varying interpretation and enforceability, frequently focused upon the NCA’s duration, scope, and geography. Similarly, in common law, courts will frequently invalidate NCAs that are found to be unreasonably overbroad, either geographically, temporally, and/or in regard to scope.
On April 23, 2024, however, the FTC altered this existing state of affairs by issuing a rule banning new NCAs in all employment situations after September 3, 2024. The rule also holds that existing NCAs are not enforceable, with a small carve-out for some senior executives. It applies to for-profit businesses, and some, but not all, non-profit organizations. The FTC’s stated intent is to reduce healthcare spending by increasing employee compensation and mobility. The FTC’s ban is likely meant to reduce transaction costs by increasing physician mobility.
There have been several lawsuits regarding the FTC ruling, challenging it on different grounds. The US District Court for the Northern District of Texas in Ryan LLC v. FTC issued first a preliminary injunction, then a final decision overturning the FTC’s rule. The Court held that the FTC had exceeded its statutory authority, and further, that the rule was arbitrary and capricious. It noted that the rule’s “categorical ban” has no equivalent in state law, is “unreasonably overbroad without a reasonable explanation,” “provides no evidence or reasoned basis,” does not “consider the positive benefits of non-compete agreements,” and does not “address alternatives to the Rule.” The Ryan Court reasoned that as an administrative agency, the FTC can only act as Congress authorizes by statute. On Oct. 18, 2024, the FTC appealed the Court’s decision to the Fifth Circuit Court of Appeals, seeking to reverse the holding setting aside its NCA ban.
The United States District Court for the Eastern District of Pennsylvania in ATS Tree Services LLC v. FTC denied the plaintiff’s motion to stay enforcement of the rule, refusing to issue a preliminary injunction preventing its implementation. As in Ryan, the ATS Tree Services LLC v. FTC plaintiffs argued that the FTC had exceeded its statutory authority in issuing the rule. However, the Plaintiff did not appeal the holding.
The US District Court for the Middle District of Florida in Properties of the Villages, Inc. v. FTCheld, like Ryan, that the rule exceeds the FTC’s statutory authority, noting the FTC’s prior lack of any NCA enforcement actions; however, its reasoning differed from Ryan. The Florida Court held that the FTC in fact has statutory authority to issue such rules; however, the Court held that the FTC could not enforce its rule because it violates the “major questions doctrine.” The “major questions doctrine” requires an agency such as the FTC to “point to clear congressional authorization” for any rule it issues that has “extraordinary ... economic and political significance,” as the NCA ban rule certainly does.
What is Its Future?
The FTC’s NCA ban remains unsettled. State legislatures, in response to the recent court holdings, are reassessing their statutory law regarding NCAs. The Ryan Court’s holding prevented the FTC’s rule from going into effect on September 4, 2024. The Texas and Florida court decisions are awaiting 5th and 11th Circuit Court of Appeals review, respectively. Assuming affirmation of either of the cases on appeal, a circuit split regarding the NCA ban may occur. The US Supreme Court may be called upon to determine the validity of the FTC rule banning NCAs. The Circuit Court decisions are likely to occur in 2025, and any Supreme Court decision would not likely occur until 2026. Meanwhile, state statutory law and common law still apply to NCAs, and the FTC may challenge the validity of NCAs on a case-by-case basis.
US antitrust law remains a potential remedy to scrutinize and restrain inappropriate business practices, including NCA-related abuses. The Sherman Act allows federal and state actors and private citizens, to sue for redress. Antitrust cases are typically considered using the “rule of reason” formulated by the Supreme Court in 1911, which requires plaintiffs show that defendant businesses possessing market power did in fact undertake anticompetitive conduct that had or likely had anticompetitive effects. In other words, the court in an antitrust case will require that the plaintiff show that the business actually had a significant controlling market presence in the geographic area; and further, that the plaintiff show that the business’ actions in fact had an anticompetitive effect, or likely had one. The latter can be found by showing an anticompetitive effect such as abusive pricing
The FTC’s ruling is legally and academically controversial and in fact may not withstand court scrutiny. The rule was put forth by the FTC as an ambitious rule to reduce healthcare spending. But businesses survive only if their revenue surpasses their costs, including personnel costs. Further, maximization of capitalization is attained when businesses require NCAs. Businesses invest heavily in recruiting, hiring, and training personnel, and increased personnel turnover increases these expenditures. NCAs arguably provide a collective benefit by ensuring force continuity, mitigating the risk of the loss of highly trained personnel with proprietary knowledge. NCAs also help a business maintain a skilled workforce, helping maximize business valuation. If FTC’s NCA ban rule were ultimately upheld, businesses would likely respond by instituting longer-term employee contracts, extended termination notice periods, and disincentives for employees who do not fully serve their contract length, including substantial financial disincentives. Business valuation might decrease, reducing investment incentives.
NCAs have long been a method of balancing the interests of employees and employers. They protect businesses’ confidential information, trade secrets, and patient lists, at some cost to employees pursuing new opportunities. The employee, though, is also provided with some benefit from the NCA, albeit indirect. State statutory law and courts have traditionally worked to ensure an appropriate delicate balance between interests, with courts generally finding unbalanced NCAs unenforceable.
For now, physicians should understand the policy considerations of and recognize the uncertainty surrounding NCAs, become familiar with their state’s statutory NCA law, review employment contracts carefully for NCA reasonableness, and seek legal advice if necessary.
Perhaps the FTC’s approach is the correct one for our future. Or perhaps the appropriate future of NCA interpretation and enforcement should continue to rest on state statutory law and common law, where antitrust enforcement is on a case-by-case basis, rather than FTC rulemaking. The results of high court decisions, state statutory law changes in response to the FTC rule, and perhaps US congressional action will provide the final answer.
Dr. Allen is based at the University of Oklahoma Health Sciences Center in Oklahoma City. He has declared no conflicts of interest in relation to this article.
Non-compete agreements (NCAs) in physician contracts, also termed “restrictive covenants” or “covenants not to compete,” have become a hot topic recently because of the Federal Trade Commission’s (FTC’s) April 2024 ruling invalidating almost all NCAs. But in fact, NCAs have long been controversial, and no more so than in the realm of physician NCAs, which involve substantial policy concerns.
how it relates to a physician employment contract currently, and its possible evolution.
What is It?
Generally speaking, an NCA, usually in the form of an employment contract clause, is an agreement between the employer and the employee that the employee will not enter into post-contract competition with that employer within the limitations of a specific duration, scope of practice, and/or geography. NCAs have traditionally been regulated under state statutory law and common law and have been permitted based on policy considerations that attempt to balance competing employee and employer interests. Physicians should understand their states’ statutory treatment of an NCA.
NCAs protect important employer business interests, including the protection of proprietary information, safeguarding trade secrets, reducing employee turnover, and protecting patient lists. Employees, though, have limited mobility in changing professional positions, have less bargaining power with the employer, and may find themselves with limited options for comparable professional positions.
The NCA ostensibly appears to greatly benefit the employer’s interests over the employee’s; however, NCA protection of employer interests may also substantially benefit employees by encouraging substantial employer investment in employees whom the employer recognizes as a stable and likely long-term human resource, ultimately fostering increased employee satisfaction and innovation. Indeed, one concern with the FTC’s non-compete ban is the potential for significant underinvestment in information sharing and employee training, because employers would, without a NCA, be less likely to recoup those employee investments and would have limited ability to keep competitors from free-riding on investments in employees who leave and join competitors. Ultimately, this would lead to decreased market efficiency.
What is Its Status Today?
Regulation of NCAs, including physician NCAs, has traditionally been based on state statutory law and by common law. Perhaps because of the increasing use of the NCA in professional settings, the NCA has been increasingly scrutinized by courts and state legislatures in the last few decades, with an overall increasing focus on NCA reasonableness and appropriate fit in individual employment settings, and with an emphasis on employer demonstration of legitimate and significant business interests for using a NCA.
States have evolved differently in their treatment of NCAs; some states ban NCAs altogether while others allow them with varying interpretation and enforceability, frequently focused upon the NCA’s duration, scope, and geography. Similarly, in common law, courts will frequently invalidate NCAs that are found to be unreasonably overbroad, either geographically, temporally, and/or in regard to scope.
On April 23, 2024, however, the FTC altered this existing state of affairs by issuing a rule banning new NCAs in all employment situations after September 3, 2024. The rule also holds that existing NCAs are not enforceable, with a small carve-out for some senior executives. It applies to for-profit businesses, and some, but not all, non-profit organizations. The FTC’s stated intent is to reduce healthcare spending by increasing employee compensation and mobility. The FTC’s ban is likely meant to reduce transaction costs by increasing physician mobility.
There have been several lawsuits regarding the FTC ruling, challenging it on different grounds. The US District Court for the Northern District of Texas in Ryan LLC v. FTC issued first a preliminary injunction, then a final decision overturning the FTC’s rule. The Court held that the FTC had exceeded its statutory authority, and further, that the rule was arbitrary and capricious. It noted that the rule’s “categorical ban” has no equivalent in state law, is “unreasonably overbroad without a reasonable explanation,” “provides no evidence or reasoned basis,” does not “consider the positive benefits of non-compete agreements,” and does not “address alternatives to the Rule.” The Ryan Court reasoned that as an administrative agency, the FTC can only act as Congress authorizes by statute. On Oct. 18, 2024, the FTC appealed the Court’s decision to the Fifth Circuit Court of Appeals, seeking to reverse the holding setting aside its NCA ban.
The United States District Court for the Eastern District of Pennsylvania in ATS Tree Services LLC v. FTC denied the plaintiff’s motion to stay enforcement of the rule, refusing to issue a preliminary injunction preventing its implementation. As in Ryan, the ATS Tree Services LLC v. FTC plaintiffs argued that the FTC had exceeded its statutory authority in issuing the rule. However, the Plaintiff did not appeal the holding.
The US District Court for the Middle District of Florida in Properties of the Villages, Inc. v. FTCheld, like Ryan, that the rule exceeds the FTC’s statutory authority, noting the FTC’s prior lack of any NCA enforcement actions; however, its reasoning differed from Ryan. The Florida Court held that the FTC in fact has statutory authority to issue such rules; however, the Court held that the FTC could not enforce its rule because it violates the “major questions doctrine.” The “major questions doctrine” requires an agency such as the FTC to “point to clear congressional authorization” for any rule it issues that has “extraordinary ... economic and political significance,” as the NCA ban rule certainly does.
What is Its Future?
The FTC’s NCA ban remains unsettled. State legislatures, in response to the recent court holdings, are reassessing their statutory law regarding NCAs. The Ryan Court’s holding prevented the FTC’s rule from going into effect on September 4, 2024. The Texas and Florida court decisions are awaiting 5th and 11th Circuit Court of Appeals review, respectively. Assuming affirmation of either of the cases on appeal, a circuit split regarding the NCA ban may occur. The US Supreme Court may be called upon to determine the validity of the FTC rule banning NCAs. The Circuit Court decisions are likely to occur in 2025, and any Supreme Court decision would not likely occur until 2026. Meanwhile, state statutory law and common law still apply to NCAs, and the FTC may challenge the validity of NCAs on a case-by-case basis.
US antitrust law remains a potential remedy to scrutinize and restrain inappropriate business practices, including NCA-related abuses. The Sherman Act allows federal and state actors and private citizens, to sue for redress. Antitrust cases are typically considered using the “rule of reason” formulated by the Supreme Court in 1911, which requires plaintiffs show that defendant businesses possessing market power did in fact undertake anticompetitive conduct that had or likely had anticompetitive effects. In other words, the court in an antitrust case will require that the plaintiff show that the business actually had a significant controlling market presence in the geographic area; and further, that the plaintiff show that the business’ actions in fact had an anticompetitive effect, or likely had one. The latter can be found by showing an anticompetitive effect such as abusive pricing
The FTC’s ruling is legally and academically controversial and in fact may not withstand court scrutiny. The rule was put forth by the FTC as an ambitious rule to reduce healthcare spending. But businesses survive only if their revenue surpasses their costs, including personnel costs. Further, maximization of capitalization is attained when businesses require NCAs. Businesses invest heavily in recruiting, hiring, and training personnel, and increased personnel turnover increases these expenditures. NCAs arguably provide a collective benefit by ensuring force continuity, mitigating the risk of the loss of highly trained personnel with proprietary knowledge. NCAs also help a business maintain a skilled workforce, helping maximize business valuation. If FTC’s NCA ban rule were ultimately upheld, businesses would likely respond by instituting longer-term employee contracts, extended termination notice periods, and disincentives for employees who do not fully serve their contract length, including substantial financial disincentives. Business valuation might decrease, reducing investment incentives.
NCAs have long been a method of balancing the interests of employees and employers. They protect businesses’ confidential information, trade secrets, and patient lists, at some cost to employees pursuing new opportunities. The employee, though, is also provided with some benefit from the NCA, albeit indirect. State statutory law and courts have traditionally worked to ensure an appropriate delicate balance between interests, with courts generally finding unbalanced NCAs unenforceable.
For now, physicians should understand the policy considerations of and recognize the uncertainty surrounding NCAs, become familiar with their state’s statutory NCA law, review employment contracts carefully for NCA reasonableness, and seek legal advice if necessary.
Perhaps the FTC’s approach is the correct one for our future. Or perhaps the appropriate future of NCA interpretation and enforcement should continue to rest on state statutory law and common law, where antitrust enforcement is on a case-by-case basis, rather than FTC rulemaking. The results of high court decisions, state statutory law changes in response to the FTC rule, and perhaps US congressional action will provide the final answer.
Dr. Allen is based at the University of Oklahoma Health Sciences Center in Oklahoma City. He has declared no conflicts of interest in relation to this article.
Non-compete agreements (NCAs) in physician contracts, also termed “restrictive covenants” or “covenants not to compete,” have become a hot topic recently because of the Federal Trade Commission’s (FTC’s) April 2024 ruling invalidating almost all NCAs. But in fact, NCAs have long been controversial, and no more so than in the realm of physician NCAs, which involve substantial policy concerns.
how it relates to a physician employment contract currently, and its possible evolution.
What is It?
Generally speaking, an NCA, usually in the form of an employment contract clause, is an agreement between the employer and the employee that the employee will not enter into post-contract competition with that employer within the limitations of a specific duration, scope of practice, and/or geography. NCAs have traditionally been regulated under state statutory law and common law and have been permitted based on policy considerations that attempt to balance competing employee and employer interests. Physicians should understand their states’ statutory treatment of an NCA.
NCAs protect important employer business interests, including the protection of proprietary information, safeguarding trade secrets, reducing employee turnover, and protecting patient lists. Employees, though, have limited mobility in changing professional positions, have less bargaining power with the employer, and may find themselves with limited options for comparable professional positions.
The NCA ostensibly appears to greatly benefit the employer’s interests over the employee’s; however, NCA protection of employer interests may also substantially benefit employees by encouraging substantial employer investment in employees whom the employer recognizes as a stable and likely long-term human resource, ultimately fostering increased employee satisfaction and innovation. Indeed, one concern with the FTC’s non-compete ban is the potential for significant underinvestment in information sharing and employee training, because employers would, without a NCA, be less likely to recoup those employee investments and would have limited ability to keep competitors from free-riding on investments in employees who leave and join competitors. Ultimately, this would lead to decreased market efficiency.
What is Its Status Today?
Regulation of NCAs, including physician NCAs, has traditionally been based on state statutory law and by common law. Perhaps because of the increasing use of the NCA in professional settings, the NCA has been increasingly scrutinized by courts and state legislatures in the last few decades, with an overall increasing focus on NCA reasonableness and appropriate fit in individual employment settings, and with an emphasis on employer demonstration of legitimate and significant business interests for using a NCA.
States have evolved differently in their treatment of NCAs; some states ban NCAs altogether while others allow them with varying interpretation and enforceability, frequently focused upon the NCA’s duration, scope, and geography. Similarly, in common law, courts will frequently invalidate NCAs that are found to be unreasonably overbroad, either geographically, temporally, and/or in regard to scope.
On April 23, 2024, however, the FTC altered this existing state of affairs by issuing a rule banning new NCAs in all employment situations after September 3, 2024. The rule also holds that existing NCAs are not enforceable, with a small carve-out for some senior executives. It applies to for-profit businesses, and some, but not all, non-profit organizations. The FTC’s stated intent is to reduce healthcare spending by increasing employee compensation and mobility. The FTC’s ban is likely meant to reduce transaction costs by increasing physician mobility.
There have been several lawsuits regarding the FTC ruling, challenging it on different grounds. The US District Court for the Northern District of Texas in Ryan LLC v. FTC issued first a preliminary injunction, then a final decision overturning the FTC’s rule. The Court held that the FTC had exceeded its statutory authority, and further, that the rule was arbitrary and capricious. It noted that the rule’s “categorical ban” has no equivalent in state law, is “unreasonably overbroad without a reasonable explanation,” “provides no evidence or reasoned basis,” does not “consider the positive benefits of non-compete agreements,” and does not “address alternatives to the Rule.” The Ryan Court reasoned that as an administrative agency, the FTC can only act as Congress authorizes by statute. On Oct. 18, 2024, the FTC appealed the Court’s decision to the Fifth Circuit Court of Appeals, seeking to reverse the holding setting aside its NCA ban.
The United States District Court for the Eastern District of Pennsylvania in ATS Tree Services LLC v. FTC denied the plaintiff’s motion to stay enforcement of the rule, refusing to issue a preliminary injunction preventing its implementation. As in Ryan, the ATS Tree Services LLC v. FTC plaintiffs argued that the FTC had exceeded its statutory authority in issuing the rule. However, the Plaintiff did not appeal the holding.
The US District Court for the Middle District of Florida in Properties of the Villages, Inc. v. FTCheld, like Ryan, that the rule exceeds the FTC’s statutory authority, noting the FTC’s prior lack of any NCA enforcement actions; however, its reasoning differed from Ryan. The Florida Court held that the FTC in fact has statutory authority to issue such rules; however, the Court held that the FTC could not enforce its rule because it violates the “major questions doctrine.” The “major questions doctrine” requires an agency such as the FTC to “point to clear congressional authorization” for any rule it issues that has “extraordinary ... economic and political significance,” as the NCA ban rule certainly does.
What is Its Future?
The FTC’s NCA ban remains unsettled. State legislatures, in response to the recent court holdings, are reassessing their statutory law regarding NCAs. The Ryan Court’s holding prevented the FTC’s rule from going into effect on September 4, 2024. The Texas and Florida court decisions are awaiting 5th and 11th Circuit Court of Appeals review, respectively. Assuming affirmation of either of the cases on appeal, a circuit split regarding the NCA ban may occur. The US Supreme Court may be called upon to determine the validity of the FTC rule banning NCAs. The Circuit Court decisions are likely to occur in 2025, and any Supreme Court decision would not likely occur until 2026. Meanwhile, state statutory law and common law still apply to NCAs, and the FTC may challenge the validity of NCAs on a case-by-case basis.
US antitrust law remains a potential remedy to scrutinize and restrain inappropriate business practices, including NCA-related abuses. The Sherman Act allows federal and state actors and private citizens, to sue for redress. Antitrust cases are typically considered using the “rule of reason” formulated by the Supreme Court in 1911, which requires plaintiffs show that defendant businesses possessing market power did in fact undertake anticompetitive conduct that had or likely had anticompetitive effects. In other words, the court in an antitrust case will require that the plaintiff show that the business actually had a significant controlling market presence in the geographic area; and further, that the plaintiff show that the business’ actions in fact had an anticompetitive effect, or likely had one. The latter can be found by showing an anticompetitive effect such as abusive pricing
The FTC’s ruling is legally and academically controversial and in fact may not withstand court scrutiny. The rule was put forth by the FTC as an ambitious rule to reduce healthcare spending. But businesses survive only if their revenue surpasses their costs, including personnel costs. Further, maximization of capitalization is attained when businesses require NCAs. Businesses invest heavily in recruiting, hiring, and training personnel, and increased personnel turnover increases these expenditures. NCAs arguably provide a collective benefit by ensuring force continuity, mitigating the risk of the loss of highly trained personnel with proprietary knowledge. NCAs also help a business maintain a skilled workforce, helping maximize business valuation. If FTC’s NCA ban rule were ultimately upheld, businesses would likely respond by instituting longer-term employee contracts, extended termination notice periods, and disincentives for employees who do not fully serve their contract length, including substantial financial disincentives. Business valuation might decrease, reducing investment incentives.
NCAs have long been a method of balancing the interests of employees and employers. They protect businesses’ confidential information, trade secrets, and patient lists, at some cost to employees pursuing new opportunities. The employee, though, is also provided with some benefit from the NCA, albeit indirect. State statutory law and courts have traditionally worked to ensure an appropriate delicate balance between interests, with courts generally finding unbalanced NCAs unenforceable.
For now, physicians should understand the policy considerations of and recognize the uncertainty surrounding NCAs, become familiar with their state’s statutory NCA law, review employment contracts carefully for NCA reasonableness, and seek legal advice if necessary.
Perhaps the FTC’s approach is the correct one for our future. Or perhaps the appropriate future of NCA interpretation and enforcement should continue to rest on state statutory law and common law, where antitrust enforcement is on a case-by-case basis, rather than FTC rulemaking. The results of high court decisions, state statutory law changes in response to the FTC rule, and perhaps US congressional action will provide the final answer.
Dr. Allen is based at the University of Oklahoma Health Sciences Center in Oklahoma City. He has declared no conflicts of interest in relation to this article.
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
Oral Biologics: The New Wave for Treating Psoriasis
Oral Biologics: The New Wave for Treating Psoriasis
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.
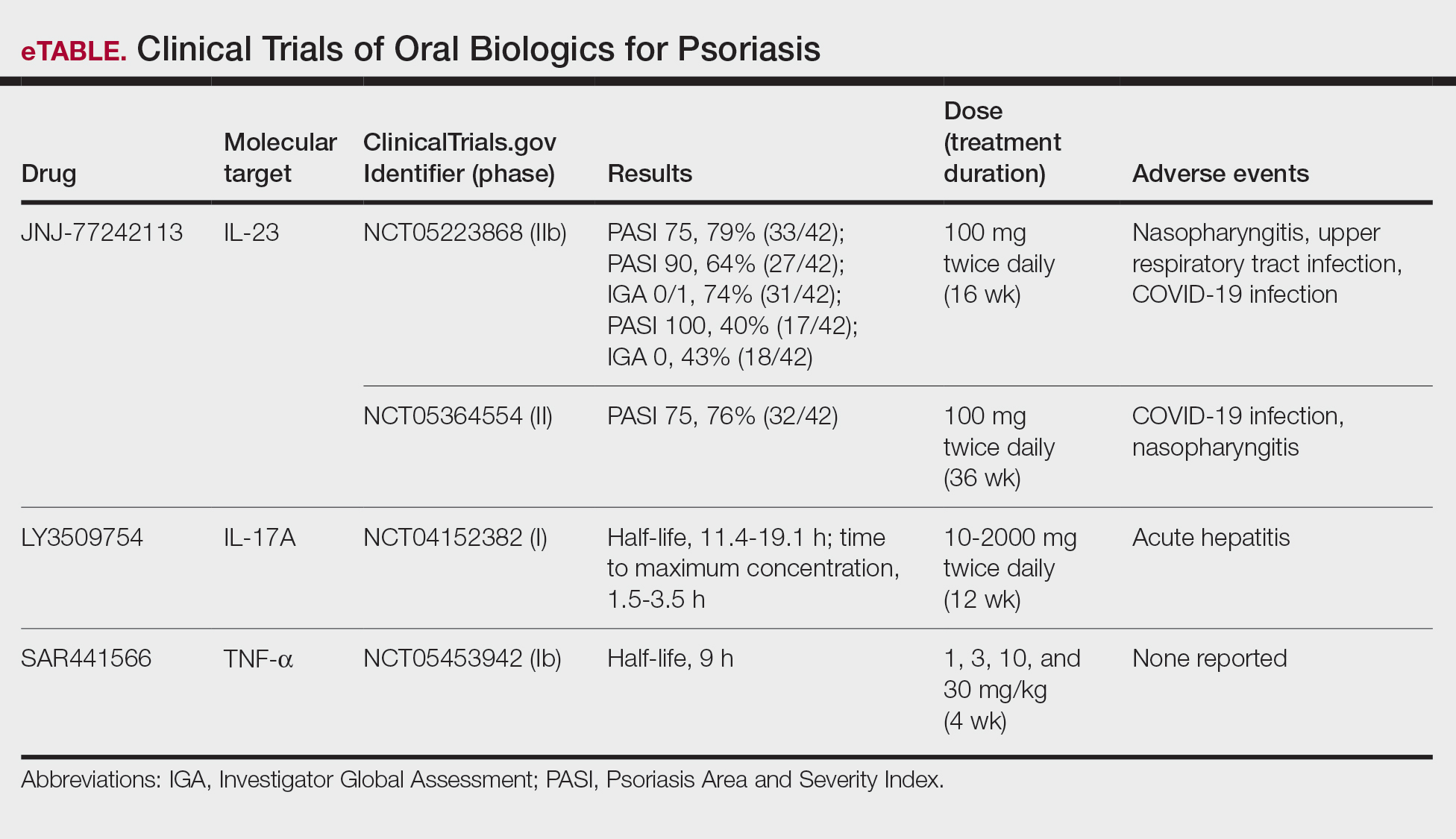
A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.

A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.

A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
Oral Biologics: The New Wave for Treating Psoriasis
Oral Biologics: The New Wave for Treating Psoriasis
PRACTICE POINTS
- The biologics that currently are approved for psoriasis are expensive and must be administered via injection due to their large molecule size.
- Emerging small-molecule oral therapies for psoriasis are effective and affordable and may represent the future for psoriasis patients.
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
Psoriasis treatments have come a long way in the past 20 years. We now have more than a dozen systemic targeted treatments for psoriatic disease, with more on the way; however, with each successive class of medications introduced, the gap has narrowed in terms of increasing efficacy. In an era of medications reporting complete clearance rates in the 70% range, the average improvement in Psoriasis Area and Severity Index (PASI) for most biologics has remained at 90% to 95% in the past half-decade. While this is a far cry from the mean PASI improvements of 70% seen with the first biologics,1 it is becoming more challenging to base our treatment decisions solely on PASI outcome measures.
How, then, do we approach rational selection of a systemic psoriasis treatment? We could try to delineate based on mechanism of action, but it may be disingenuous to dissect minor differences in pathways (eg, IL-17 vs IL-23) that are fundamentally related and on the same continuum in psoriasis pathophysiology. Therefore, the most meaningful way to select an appropriate therapeutic may be to adopt a patient-centered approach that accounts for both individual preferences and specific medical needs by evaluating for other comorbidities2 to exclude or select certain medicines or types of treatments. We have long known to avoid tumor necrosis factor (TNF) α inhibitors in patients with congestive heart failure or a history of demyelinating disorders while regularly considering the presence of psoriatic arthritis and family planning when making treatment decisions. Now, we can be more nuanced in our approaches to psoriasis biologics. Specifically, the most important comorbidities to consider broadly encompass cardiometabolic disorders, gastrointestinal conditions, and psychiatric conditions.
Cardiometabolic Disorders
Possibly the hottest topic in psoriasis for some years now, the relationship between cardiometabolic disorders and psoriasis is of great interest to clinicians, scientists, and patients alike. There is a clear link between development of atherosclerosis and Th17-related immune mechanisms that also are implicated in the pathogenesis of psoriasis.3 Furthermore, the incidence of cardiovascular disease is markedly increased in patients with psoriasis, which is an independent risk factor for myocardial infarction, particularly among younger patients.4,5 Although several retrospective studies6-8 have shown that TNF-α inhibitors are associated with a reduction in cardiovascular outcomes, it is yet to be seen whether biologic treatment actually has a direct impact on cardiovascular outcomes, multiple studies investigating the effect of biologics on arterial inflammation markers notwithstanding.9
There are some direct factors to keep in mind when considering cardiometabolic comorbidities in patients with psoriasis. Obesity is common in the psoriasis population and can have a direct negative effect on cardiovascular health.10 However, the data on obesity and psoriasis are somewhat mixed with regard to treatment outcomes. In general, with increased volumes of distribution for biologics in patients with obesity, it has been shown that treatment success is more difficult to achieve in those with a body mass index greater than 30.11 Rather surprisingly, a separate nationwide study in South Korea found that patients on biologics for psoriasis were more likely to experience weight gain, even after controlling for factors such as exercise, smoking, and drinking,12 but it is unclear whether this is driven mostly by a known connection between weight gain and TNF-α inhibitors.13 These contrasting results point to the need for further studies in this area, as our intuitive approach would involve promoting weight loss while starting on a systemic treatment for psoriasis—but perhaps it is important not to assume that one will come with the other in tow, reinforcing the need to discuss a healthy diet with our patients with psoriasis regardless of treatment decisions.
The data that we have do not directly answer the big questions about biologic treatment and cardiovascular health, but we are starting to see interesting signals. For example, in a report of tildrakizumab treatment in patients with and without metabolic syndrome, the rates of major adverse cardiovascular events as well as cardiac disorders were essentially the same in both groups after receiving treatment for up to 244 weeks.14 This is interesting, more because of the lack of an increase in cardiovascular adverse events in the metabolic syndrome group, who entered the trial on average 25 kg to 30 kg heavier than those without metabolic syndrome. There is an increased risk for adverse cardiovascular events among patients with metabolic syndrome, a roughly 2-fold relative risk in as few as 5 to 6 years of follow-up.15 While the cohorts in the tildrakizumab study14 were too small to draw firm conclusions, the data are interesting and a step in the right direction; we need much larger data sets for analysis. Among other agents, similar efficacy and safety have been reported for guselkumab in a long-term psoriasis study; as a class, IL-23 inhibitors also tend to perform well from an efficacy standpoint in patients with obesity.16
Overall, when assessing the evidence for cardiometabolic disorders, it is reasonable to consider starting a biologic from the IL-17 or IL-23 inhibitor classes— thus avoiding both the potential downside of weight gain and contraindication in patients with congestive heart failure associated with TNF-α inhibitors. It is important to counsel patients about weight loss in conjunction with these treatments, both to improve efficacy and reduce cardiovascular risk factors. There may be a preference for IL-23 inhibitors in patients with obesity, as this class of medications maintains efficacy particularly well in these patients. Patients with psoriasis should be counseled to follow up with a primary care physician given their higher risk for metabolic syndrome and adverse cardiovascular outcomes.
Gastrointestinal Conditions
Psoriasis and inflammatory bowel disease (IBD) have a bidirectional association, and patients with psoriasis are about 1.7 times more likely to have either Crohn disease or ulcerative colitis.17,18 This association may be related to a shared pathogenesis with regard to immune dysregulation and overactivated inflammatory pathways, but there are some important differences to consider from a therapeutic standpoint. Given the increased expression of IL-17 in patients with IBD,19 a phase II trial of secukinumab yielded surprising results—not only was secukinumab ineffective in treating Crohn disease, but there also were higher rates of adverse events20 (as noted on the product label for all IL-17 inhibitors). We have come to understand that there are regulatory subsets of IL-17 cells that are important in mucosal homeostasis and also regulate IL-10, which generally is considered an anti-inflammatory cytokine.21 Thus, while IL-17 inhibition can reduce some component of inflammatory signaling, it also can increase inflammatory signaling through indirect pathways while increasing intestinal permeability to microbes. Importantly, this process seems to occur via IL-23–independent pathways; as such, while direct inhibition of IL-17 can be deleterious, IL-23 inhibitors have become important therapeutics for IBD.22
IL-17 family, IL-17A clearly is the culprit for worsening colitis as evidenced by both human and animal models. On the contrary, IL-17F blockade has been shown to ameliorate colitis in a murine model, whereas IL-17A inhibition worsens it.23 Furthermore, dual blockade of IL-17A and IL-17F has a protective effect against colitis, suggesting that the IL-17F inhibition is dominant. This interesting finding has some mechanistic backing, since blockade of IL-17F induces Treg cells that serve to maintain gut epithelium homeostasis and integrity.24
Overall, IL-17A inhibitors should be avoided in patients with a history of IBD—namely, secukinumab and ixekizumab. While there may be theoretical reasons that brodalumab or bimekizumab may confer a somewhat different risk for IBD exacerbation, there may be better choices that would be expected to effectively treat both the psoriasis and IBD manifestations. Given the US Food and Drug Administration approval of IL-23 inhibitors for IBD and their high levels of efficacy in treating psoriasis, these likely will be the best choice for most patients. Another mainstay of IBD treatment is TNF-α inhibitors, but they come with other risks such as increased immunosuppression and increased risk for nonmelanoma skin cancer.
An important question remains: What about patients who do not have known IBD? Do we proactively change our treatment choice due to fear of IBD development given the higher incidence of both Crohn disease and ulcerative colitis in patients with psoriasis? What about patients with a family history of IBD? First-degree relatives of patients with Crohn disease and ulcerative colitis have an 8- and 4-fold higher risk for those same conditions, respectively.25 Postmarketing surveillance and database findings of low rates of IBD development with IL-17 inhibitors gives only modest reassurance, as dermatologists generally know to avoid these medications for patients with even questionable IBD symptoms. It is important to emphasize to our patients that in no case do we believe that a psoriasis medication actually will cause IBD—rather, someone with subclinical IBD could experience a flare and a first manifestation of colitis. The drug is not the culprit in inducing IBD but rather may serve to unmask existing disease.
One study suggested that for patients who move on to the IL-17 inhibitor secukinumab after being treated with TNF-α inhibitors for psoriasis, the rates of IBD development are higher (4.8%) than in those who start IL-17A inhibition without prior treatment (1%)(OR, 8.38; P=.018).26 This begs the question of whether subclinical IBD in many patients with psoriasis who are treated with TNF-α inhibitors can be unmasked later when they are transitioned to a treatment that either does not treat the IBD or could worsen it. There may be a mechanistic drive behind this sequencing of treatments that predisposes patients to colitis, which would suggest selecting an IL-23 inhibitor after failing/trying a TNF-α inhibitor. However, the data are very preliminary, and in real practice, other concerns such as severe psoriatic arthritis may outweigh these considerations, as the IL-17 inhibitor class still is considered to be more effective than IL-23 inhibition at treating psoriatic arthritis overall. For most patients with no personal history of IBD and no strong family history of IBD (ie, first-degree relatives), the choice of biologic should not be affected by concern over gastrointestinal issues.
Psychiatric Conditions
It has been well established that psoriasis is linked to higher rates of depression, anxiety, and suicidality.27 How do we take this into account when treating patients with psoriasis, especially when we have biologics with a warning label for suicidality and a Risk Evaluation and Mitigation Strategies program (brodalumab) and language around suicidal ideation in the label (bimekizumab)? While it is challenging to discuss mental health, it is not a conversation that we as dermatologists should shy away from. Appropriate treatment of psoriasis is an important tool to get our patients on the path to better mental health. A recent database study of more than 4000 patients showed that patients with psoriasis treated with biologics had a 17% lower risk for depression than those treated with conventional disease-modifying drugs such as methotrexate.28 The comparator of the conventional disease-modifying drug class is important as it serves as a control for disease severity. Too often, a higher rate of depression, anxiety, or suicidality can be attributed to a medication when we in fact may just be capturing the background of higher incidence of all 3 in patients with severe psoriasis.
Indeed, even with the medication that many worry about on this front (brodalumab), multiple studies have confirmed that the effect on mental health generally is a positive one, with decreases in depressive symptoms.29 In a cohort switched from TNF-α inhibitors to brodalumab, symptoms of depression actually improved,30 so attributing a direct treatment effect to negative mental health outcomes does not seem to be justified, especially in light of the low number of suicide events in global postmarketing surveillance for brodalumab, comparable to or lower than other biologics for psoriasis.31 Similarly, bimekizumab has language in the label about discussing suicidality with patients, although the rates of suicidal ideation and behavior are no different from other biologics and rates of depression improved with its use.32
Heightened awareness of our patients’ mental health is something that we as providers should embrace, even when it seems that we do not have much time to see each patient. The priority when a patient comes in with mental health symptoms should be to treat what is within our scope (ie, psoriasis) as quickly and effectively as possible— with a newer-generation biologic such as an IL-17 or IL-23 inhibitor—while encouraging the patient to seek care from a mental health professional. In these cases, one might even argue that the rapidity of action of IL-17 inhibitors may be of additional benefit.
Final Thoughts
We as dermatologists generally are tasked with seeing high volumes of patients, and an initial psoriasis consultation can be a lengthy visit; however, it is rewarding to establish this relationship with patients and a reminder of why we practice medicine to begin with. Psoriasis can be satisfying to treat, and we have so many highly effective medicines that can completely transform our patients’ lives. Applying an understanding of the interplay between psoriasis, its related comorbidities, and treatment choices can be a fulfilling exercise that captures the essence of shared decision-making, which can lead to better outcomes and satisfaction for both providers and patients.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022. doi:10.1056/NEJMoa030409
- Thatiparthi A, Martin A, Liu J, et al. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22:425-442. doi:10.1007/s40257-021-00603-w
- Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5-22. doi:10.1007 /s00281-009-0153-8
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. doi:10.1001/jama.296.14.1735
- Miller IM, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014-1024. doi:10.1016/j.jaad.2013.06.053
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90. doi:10.1016/j.jaad.2016.07.042
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250. doi:10.1001 /archdermatol.2012.2502
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68. doi:10.1016/j.jaad.2018.02.050
- Cai J, Cui L, Wang Y, et al. Cardiometabolic comorbidities in patients with psoriasis: focusing on risk, biological therapy, and pathogenesis. Front Pharmacol. 2021;12:774808. doi:10.3389/fphar.2021.774808
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:E984-E1010. doi:10.1161/CIR.0000000000000973
- Pirro F, Caldarola G, Chiricozzi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig. 2021;41:917-925. doi:10.1007 /s40261-021-01080-z
- Kim H, Hong JY, Cheong S, et al. Impact of biologic agents on body weight and obesity-related disorders in patients with psoriasis: a nationwide population-based cohort study. Obes Res Clin Pract. 2023;17:210-217. doi:10.1016/j.orcp.2023.05.004
- Saraceno R, Schipani C, Mazzotta A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57:290-295. doi:10.1016/j.phrs.2008.02.006
- Fernandez AP, Dauden E, Gerdes S, et al. Tildrakizumab efficacy and safety in patients with psoriasis and concomitant metabolic syndrome: post hoc analysis of 5-year data from reSURFACE 1 and reSURFACE 2. J Eur Acad Dermatol Venereol. 2022;36:1774-1783. doi:10.1111/jdv.18167
- Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. doi:10.1016/j.jacc.2010.05.034
- Ricceri F, Chiricozzi A, Peris K, et al. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: a multicentric retrospective study. Dermatol Ther. 2022;35:E15793. doi:10.1111/dth.15793
- Alinaghi F, Tekin HG, Burisch J, et al. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease— a systematic review and meta-analysis. J Crohns Colitis. 2020;14:351-360. doi:10.1093/ecco-jcc/jjz152
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. doi:10.1136/gut.52.1.65
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebocontrolled trial. Gut. 2012;61:1693-1700. doi:10.1136 /gutjnl-2011-301668
- Brockmann L, Tran A, Huang Y, et al. Intestinal microbiotaspecific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. 2023;56:2719-2735 e7. doi:10.1016/j.immuni.2023.11.003
- Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727-738. doi:10.1016/j.immuni.2015.09.003
- Wedebye Schmidt EG, Larsen HL, Kristensen NN, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19:1567-1576. doi:10.1097 /MIB.0b013e318286fa1c
- Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing T(reg) cells through modification of the intestinal microbiota. Nat Immunol. 2018;19:755-765. doi:10.1038/s41590-018-0134-y
- El Hadad J, Schreiner P, Vavricka SR, Greuter T. The genetics of inflammatory bowel disease. Mol Diagn Ther. 2024;28:27-35. doi:10.1007 /s40291-023-00678-7
- Albayrak F, Gür M, Karatas¸ A, et al. Is the use of secukinumab after anti-TNF therapy greater than expected for the risk of developing inflammatory bowel disease? Reumatol Clin (Engl Ed). 2024;20:123-127. doi:10.1016/j.reumae.2023.11.002
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Strober B, Soliman AM, Truong B, et al. Association between biologic exposure and the risk of depression in patients with psoriasis: a retrospective analysis of large US administrative claims data. Am J Clin Dermatol. 2024;25:853-856. doi:10.1007/s40257 -024-00877-w
- Koo J, Ho RS, Thibodeaux Q. Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review. Cutis. 2019;104:361-365.
- Andersch-Bjorkman Y, Micu E, Seifert O, et al. Effects of brodalumab on psoriasis and depressive symptoms in patients with insufficient response to TNF-alpha inhibitors. J Dermatol. 2023;50:1401-1414. doi:10.1111/1346-8138.16917
- Yeroushalmi S, Chung M, Bartholomew E, et al. Examining worldwide postmarketing suicides from biologics used for psoriasis with a focus on brodalumab: a cross-sectional analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS). JAAD Int. 2022;9:119-121. doi:10.1016/j.jdin.2022.08.010
- Blauvelt A, Armstrong A, Merola JF, et al. Mental health outcomes in patients with moderate to severe psoriasis treated with bimekizumab: analysis of phase 2/3 randomized trials. J Am Acad Dermatol. 2024;91:72-81. doi:10.1016/j.jaad.2024.02.039
Psoriasis treatments have come a long way in the past 20 years. We now have more than a dozen systemic targeted treatments for psoriatic disease, with more on the way; however, with each successive class of medications introduced, the gap has narrowed in terms of increasing efficacy. In an era of medications reporting complete clearance rates in the 70% range, the average improvement in Psoriasis Area and Severity Index (PASI) for most biologics has remained at 90% to 95% in the past half-decade. While this is a far cry from the mean PASI improvements of 70% seen with the first biologics,1 it is becoming more challenging to base our treatment decisions solely on PASI outcome measures.
How, then, do we approach rational selection of a systemic psoriasis treatment? We could try to delineate based on mechanism of action, but it may be disingenuous to dissect minor differences in pathways (eg, IL-17 vs IL-23) that are fundamentally related and on the same continuum in psoriasis pathophysiology. Therefore, the most meaningful way to select an appropriate therapeutic may be to adopt a patient-centered approach that accounts for both individual preferences and specific medical needs by evaluating for other comorbidities2 to exclude or select certain medicines or types of treatments. We have long known to avoid tumor necrosis factor (TNF) α inhibitors in patients with congestive heart failure or a history of demyelinating disorders while regularly considering the presence of psoriatic arthritis and family planning when making treatment decisions. Now, we can be more nuanced in our approaches to psoriasis biologics. Specifically, the most important comorbidities to consider broadly encompass cardiometabolic disorders, gastrointestinal conditions, and psychiatric conditions.
Cardiometabolic Disorders
Possibly the hottest topic in psoriasis for some years now, the relationship between cardiometabolic disorders and psoriasis is of great interest to clinicians, scientists, and patients alike. There is a clear link between development of atherosclerosis and Th17-related immune mechanisms that also are implicated in the pathogenesis of psoriasis.3 Furthermore, the incidence of cardiovascular disease is markedly increased in patients with psoriasis, which is an independent risk factor for myocardial infarction, particularly among younger patients.4,5 Although several retrospective studies6-8 have shown that TNF-α inhibitors are associated with a reduction in cardiovascular outcomes, it is yet to be seen whether biologic treatment actually has a direct impact on cardiovascular outcomes, multiple studies investigating the effect of biologics on arterial inflammation markers notwithstanding.9
There are some direct factors to keep in mind when considering cardiometabolic comorbidities in patients with psoriasis. Obesity is common in the psoriasis population and can have a direct negative effect on cardiovascular health.10 However, the data on obesity and psoriasis are somewhat mixed with regard to treatment outcomes. In general, with increased volumes of distribution for biologics in patients with obesity, it has been shown that treatment success is more difficult to achieve in those with a body mass index greater than 30.11 Rather surprisingly, a separate nationwide study in South Korea found that patients on biologics for psoriasis were more likely to experience weight gain, even after controlling for factors such as exercise, smoking, and drinking,12 but it is unclear whether this is driven mostly by a known connection between weight gain and TNF-α inhibitors.13 These contrasting results point to the need for further studies in this area, as our intuitive approach would involve promoting weight loss while starting on a systemic treatment for psoriasis—but perhaps it is important not to assume that one will come with the other in tow, reinforcing the need to discuss a healthy diet with our patients with psoriasis regardless of treatment decisions.
The data that we have do not directly answer the big questions about biologic treatment and cardiovascular health, but we are starting to see interesting signals. For example, in a report of tildrakizumab treatment in patients with and without metabolic syndrome, the rates of major adverse cardiovascular events as well as cardiac disorders were essentially the same in both groups after receiving treatment for up to 244 weeks.14 This is interesting, more because of the lack of an increase in cardiovascular adverse events in the metabolic syndrome group, who entered the trial on average 25 kg to 30 kg heavier than those without metabolic syndrome. There is an increased risk for adverse cardiovascular events among patients with metabolic syndrome, a roughly 2-fold relative risk in as few as 5 to 6 years of follow-up.15 While the cohorts in the tildrakizumab study14 were too small to draw firm conclusions, the data are interesting and a step in the right direction; we need much larger data sets for analysis. Among other agents, similar efficacy and safety have been reported for guselkumab in a long-term psoriasis study; as a class, IL-23 inhibitors also tend to perform well from an efficacy standpoint in patients with obesity.16
Overall, when assessing the evidence for cardiometabolic disorders, it is reasonable to consider starting a biologic from the IL-17 or IL-23 inhibitor classes— thus avoiding both the potential downside of weight gain and contraindication in patients with congestive heart failure associated with TNF-α inhibitors. It is important to counsel patients about weight loss in conjunction with these treatments, both to improve efficacy and reduce cardiovascular risk factors. There may be a preference for IL-23 inhibitors in patients with obesity, as this class of medications maintains efficacy particularly well in these patients. Patients with psoriasis should be counseled to follow up with a primary care physician given their higher risk for metabolic syndrome and adverse cardiovascular outcomes.
Gastrointestinal Conditions
Psoriasis and inflammatory bowel disease (IBD) have a bidirectional association, and patients with psoriasis are about 1.7 times more likely to have either Crohn disease or ulcerative colitis.17,18 This association may be related to a shared pathogenesis with regard to immune dysregulation and overactivated inflammatory pathways, but there are some important differences to consider from a therapeutic standpoint. Given the increased expression of IL-17 in patients with IBD,19 a phase II trial of secukinumab yielded surprising results—not only was secukinumab ineffective in treating Crohn disease, but there also were higher rates of adverse events20 (as noted on the product label for all IL-17 inhibitors). We have come to understand that there are regulatory subsets of IL-17 cells that are important in mucosal homeostasis and also regulate IL-10, which generally is considered an anti-inflammatory cytokine.21 Thus, while IL-17 inhibition can reduce some component of inflammatory signaling, it also can increase inflammatory signaling through indirect pathways while increasing intestinal permeability to microbes. Importantly, this process seems to occur via IL-23–independent pathways; as such, while direct inhibition of IL-17 can be deleterious, IL-23 inhibitors have become important therapeutics for IBD.22
IL-17 family, IL-17A clearly is the culprit for worsening colitis as evidenced by both human and animal models. On the contrary, IL-17F blockade has been shown to ameliorate colitis in a murine model, whereas IL-17A inhibition worsens it.23 Furthermore, dual blockade of IL-17A and IL-17F has a protective effect against colitis, suggesting that the IL-17F inhibition is dominant. This interesting finding has some mechanistic backing, since blockade of IL-17F induces Treg cells that serve to maintain gut epithelium homeostasis and integrity.24
Overall, IL-17A inhibitors should be avoided in patients with a history of IBD—namely, secukinumab and ixekizumab. While there may be theoretical reasons that brodalumab or bimekizumab may confer a somewhat different risk for IBD exacerbation, there may be better choices that would be expected to effectively treat both the psoriasis and IBD manifestations. Given the US Food and Drug Administration approval of IL-23 inhibitors for IBD and their high levels of efficacy in treating psoriasis, these likely will be the best choice for most patients. Another mainstay of IBD treatment is TNF-α inhibitors, but they come with other risks such as increased immunosuppression and increased risk for nonmelanoma skin cancer.
An important question remains: What about patients who do not have known IBD? Do we proactively change our treatment choice due to fear of IBD development given the higher incidence of both Crohn disease and ulcerative colitis in patients with psoriasis? What about patients with a family history of IBD? First-degree relatives of patients with Crohn disease and ulcerative colitis have an 8- and 4-fold higher risk for those same conditions, respectively.25 Postmarketing surveillance and database findings of low rates of IBD development with IL-17 inhibitors gives only modest reassurance, as dermatologists generally know to avoid these medications for patients with even questionable IBD symptoms. It is important to emphasize to our patients that in no case do we believe that a psoriasis medication actually will cause IBD—rather, someone with subclinical IBD could experience a flare and a first manifestation of colitis. The drug is not the culprit in inducing IBD but rather may serve to unmask existing disease.
One study suggested that for patients who move on to the IL-17 inhibitor secukinumab after being treated with TNF-α inhibitors for psoriasis, the rates of IBD development are higher (4.8%) than in those who start IL-17A inhibition without prior treatment (1%)(OR, 8.38; P=.018).26 This begs the question of whether subclinical IBD in many patients with psoriasis who are treated with TNF-α inhibitors can be unmasked later when they are transitioned to a treatment that either does not treat the IBD or could worsen it. There may be a mechanistic drive behind this sequencing of treatments that predisposes patients to colitis, which would suggest selecting an IL-23 inhibitor after failing/trying a TNF-α inhibitor. However, the data are very preliminary, and in real practice, other concerns such as severe psoriatic arthritis may outweigh these considerations, as the IL-17 inhibitor class still is considered to be more effective than IL-23 inhibition at treating psoriatic arthritis overall. For most patients with no personal history of IBD and no strong family history of IBD (ie, first-degree relatives), the choice of biologic should not be affected by concern over gastrointestinal issues.
Psychiatric Conditions
It has been well established that psoriasis is linked to higher rates of depression, anxiety, and suicidality.27 How do we take this into account when treating patients with psoriasis, especially when we have biologics with a warning label for suicidality and a Risk Evaluation and Mitigation Strategies program (brodalumab) and language around suicidal ideation in the label (bimekizumab)? While it is challenging to discuss mental health, it is not a conversation that we as dermatologists should shy away from. Appropriate treatment of psoriasis is an important tool to get our patients on the path to better mental health. A recent database study of more than 4000 patients showed that patients with psoriasis treated with biologics had a 17% lower risk for depression than those treated with conventional disease-modifying drugs such as methotrexate.28 The comparator of the conventional disease-modifying drug class is important as it serves as a control for disease severity. Too often, a higher rate of depression, anxiety, or suicidality can be attributed to a medication when we in fact may just be capturing the background of higher incidence of all 3 in patients with severe psoriasis.
Indeed, even with the medication that many worry about on this front (brodalumab), multiple studies have confirmed that the effect on mental health generally is a positive one, with decreases in depressive symptoms.29 In a cohort switched from TNF-α inhibitors to brodalumab, symptoms of depression actually improved,30 so attributing a direct treatment effect to negative mental health outcomes does not seem to be justified, especially in light of the low number of suicide events in global postmarketing surveillance for brodalumab, comparable to or lower than other biologics for psoriasis.31 Similarly, bimekizumab has language in the label about discussing suicidality with patients, although the rates of suicidal ideation and behavior are no different from other biologics and rates of depression improved with its use.32
Heightened awareness of our patients’ mental health is something that we as providers should embrace, even when it seems that we do not have much time to see each patient. The priority when a patient comes in with mental health symptoms should be to treat what is within our scope (ie, psoriasis) as quickly and effectively as possible— with a newer-generation biologic such as an IL-17 or IL-23 inhibitor—while encouraging the patient to seek care from a mental health professional. In these cases, one might even argue that the rapidity of action of IL-17 inhibitors may be of additional benefit.
Final Thoughts
We as dermatologists generally are tasked with seeing high volumes of patients, and an initial psoriasis consultation can be a lengthy visit; however, it is rewarding to establish this relationship with patients and a reminder of why we practice medicine to begin with. Psoriasis can be satisfying to treat, and we have so many highly effective medicines that can completely transform our patients’ lives. Applying an understanding of the interplay between psoriasis, its related comorbidities, and treatment choices can be a fulfilling exercise that captures the essence of shared decision-making, which can lead to better outcomes and satisfaction for both providers and patients.
Psoriasis treatments have come a long way in the past 20 years. We now have more than a dozen systemic targeted treatments for psoriatic disease, with more on the way; however, with each successive class of medications introduced, the gap has narrowed in terms of increasing efficacy. In an era of medications reporting complete clearance rates in the 70% range, the average improvement in Psoriasis Area and Severity Index (PASI) for most biologics has remained at 90% to 95% in the past half-decade. While this is a far cry from the mean PASI improvements of 70% seen with the first biologics,1 it is becoming more challenging to base our treatment decisions solely on PASI outcome measures.
How, then, do we approach rational selection of a systemic psoriasis treatment? We could try to delineate based on mechanism of action, but it may be disingenuous to dissect minor differences in pathways (eg, IL-17 vs IL-23) that are fundamentally related and on the same continuum in psoriasis pathophysiology. Therefore, the most meaningful way to select an appropriate therapeutic may be to adopt a patient-centered approach that accounts for both individual preferences and specific medical needs by evaluating for other comorbidities2 to exclude or select certain medicines or types of treatments. We have long known to avoid tumor necrosis factor (TNF) α inhibitors in patients with congestive heart failure or a history of demyelinating disorders while regularly considering the presence of psoriatic arthritis and family planning when making treatment decisions. Now, we can be more nuanced in our approaches to psoriasis biologics. Specifically, the most important comorbidities to consider broadly encompass cardiometabolic disorders, gastrointestinal conditions, and psychiatric conditions.
Cardiometabolic Disorders
Possibly the hottest topic in psoriasis for some years now, the relationship between cardiometabolic disorders and psoriasis is of great interest to clinicians, scientists, and patients alike. There is a clear link between development of atherosclerosis and Th17-related immune mechanisms that also are implicated in the pathogenesis of psoriasis.3 Furthermore, the incidence of cardiovascular disease is markedly increased in patients with psoriasis, which is an independent risk factor for myocardial infarction, particularly among younger patients.4,5 Although several retrospective studies6-8 have shown that TNF-α inhibitors are associated with a reduction in cardiovascular outcomes, it is yet to be seen whether biologic treatment actually has a direct impact on cardiovascular outcomes, multiple studies investigating the effect of biologics on arterial inflammation markers notwithstanding.9
There are some direct factors to keep in mind when considering cardiometabolic comorbidities in patients with psoriasis. Obesity is common in the psoriasis population and can have a direct negative effect on cardiovascular health.10 However, the data on obesity and psoriasis are somewhat mixed with regard to treatment outcomes. In general, with increased volumes of distribution for biologics in patients with obesity, it has been shown that treatment success is more difficult to achieve in those with a body mass index greater than 30.11 Rather surprisingly, a separate nationwide study in South Korea found that patients on biologics for psoriasis were more likely to experience weight gain, even after controlling for factors such as exercise, smoking, and drinking,12 but it is unclear whether this is driven mostly by a known connection between weight gain and TNF-α inhibitors.13 These contrasting results point to the need for further studies in this area, as our intuitive approach would involve promoting weight loss while starting on a systemic treatment for psoriasis—but perhaps it is important not to assume that one will come with the other in tow, reinforcing the need to discuss a healthy diet with our patients with psoriasis regardless of treatment decisions.
The data that we have do not directly answer the big questions about biologic treatment and cardiovascular health, but we are starting to see interesting signals. For example, in a report of tildrakizumab treatment in patients with and without metabolic syndrome, the rates of major adverse cardiovascular events as well as cardiac disorders were essentially the same in both groups after receiving treatment for up to 244 weeks.14 This is interesting, more because of the lack of an increase in cardiovascular adverse events in the metabolic syndrome group, who entered the trial on average 25 kg to 30 kg heavier than those without metabolic syndrome. There is an increased risk for adverse cardiovascular events among patients with metabolic syndrome, a roughly 2-fold relative risk in as few as 5 to 6 years of follow-up.15 While the cohorts in the tildrakizumab study14 were too small to draw firm conclusions, the data are interesting and a step in the right direction; we need much larger data sets for analysis. Among other agents, similar efficacy and safety have been reported for guselkumab in a long-term psoriasis study; as a class, IL-23 inhibitors also tend to perform well from an efficacy standpoint in patients with obesity.16
Overall, when assessing the evidence for cardiometabolic disorders, it is reasonable to consider starting a biologic from the IL-17 or IL-23 inhibitor classes— thus avoiding both the potential downside of weight gain and contraindication in patients with congestive heart failure associated with TNF-α inhibitors. It is important to counsel patients about weight loss in conjunction with these treatments, both to improve efficacy and reduce cardiovascular risk factors. There may be a preference for IL-23 inhibitors in patients with obesity, as this class of medications maintains efficacy particularly well in these patients. Patients with psoriasis should be counseled to follow up with a primary care physician given their higher risk for metabolic syndrome and adverse cardiovascular outcomes.
Gastrointestinal Conditions
Psoriasis and inflammatory bowel disease (IBD) have a bidirectional association, and patients with psoriasis are about 1.7 times more likely to have either Crohn disease or ulcerative colitis.17,18 This association may be related to a shared pathogenesis with regard to immune dysregulation and overactivated inflammatory pathways, but there are some important differences to consider from a therapeutic standpoint. Given the increased expression of IL-17 in patients with IBD,19 a phase II trial of secukinumab yielded surprising results—not only was secukinumab ineffective in treating Crohn disease, but there also were higher rates of adverse events20 (as noted on the product label for all IL-17 inhibitors). We have come to understand that there are regulatory subsets of IL-17 cells that are important in mucosal homeostasis and also regulate IL-10, which generally is considered an anti-inflammatory cytokine.21 Thus, while IL-17 inhibition can reduce some component of inflammatory signaling, it also can increase inflammatory signaling through indirect pathways while increasing intestinal permeability to microbes. Importantly, this process seems to occur via IL-23–independent pathways; as such, while direct inhibition of IL-17 can be deleterious, IL-23 inhibitors have become important therapeutics for IBD.22
IL-17 family, IL-17A clearly is the culprit for worsening colitis as evidenced by both human and animal models. On the contrary, IL-17F blockade has been shown to ameliorate colitis in a murine model, whereas IL-17A inhibition worsens it.23 Furthermore, dual blockade of IL-17A and IL-17F has a protective effect against colitis, suggesting that the IL-17F inhibition is dominant. This interesting finding has some mechanistic backing, since blockade of IL-17F induces Treg cells that serve to maintain gut epithelium homeostasis and integrity.24
Overall, IL-17A inhibitors should be avoided in patients with a history of IBD—namely, secukinumab and ixekizumab. While there may be theoretical reasons that brodalumab or bimekizumab may confer a somewhat different risk for IBD exacerbation, there may be better choices that would be expected to effectively treat both the psoriasis and IBD manifestations. Given the US Food and Drug Administration approval of IL-23 inhibitors for IBD and their high levels of efficacy in treating psoriasis, these likely will be the best choice for most patients. Another mainstay of IBD treatment is TNF-α inhibitors, but they come with other risks such as increased immunosuppression and increased risk for nonmelanoma skin cancer.
An important question remains: What about patients who do not have known IBD? Do we proactively change our treatment choice due to fear of IBD development given the higher incidence of both Crohn disease and ulcerative colitis in patients with psoriasis? What about patients with a family history of IBD? First-degree relatives of patients with Crohn disease and ulcerative colitis have an 8- and 4-fold higher risk for those same conditions, respectively.25 Postmarketing surveillance and database findings of low rates of IBD development with IL-17 inhibitors gives only modest reassurance, as dermatologists generally know to avoid these medications for patients with even questionable IBD symptoms. It is important to emphasize to our patients that in no case do we believe that a psoriasis medication actually will cause IBD—rather, someone with subclinical IBD could experience a flare and a first manifestation of colitis. The drug is not the culprit in inducing IBD but rather may serve to unmask existing disease.
One study suggested that for patients who move on to the IL-17 inhibitor secukinumab after being treated with TNF-α inhibitors for psoriasis, the rates of IBD development are higher (4.8%) than in those who start IL-17A inhibition without prior treatment (1%)(OR, 8.38; P=.018).26 This begs the question of whether subclinical IBD in many patients with psoriasis who are treated with TNF-α inhibitors can be unmasked later when they are transitioned to a treatment that either does not treat the IBD or could worsen it. There may be a mechanistic drive behind this sequencing of treatments that predisposes patients to colitis, which would suggest selecting an IL-23 inhibitor after failing/trying a TNF-α inhibitor. However, the data are very preliminary, and in real practice, other concerns such as severe psoriatic arthritis may outweigh these considerations, as the IL-17 inhibitor class still is considered to be more effective than IL-23 inhibition at treating psoriatic arthritis overall. For most patients with no personal history of IBD and no strong family history of IBD (ie, first-degree relatives), the choice of biologic should not be affected by concern over gastrointestinal issues.
Psychiatric Conditions
It has been well established that psoriasis is linked to higher rates of depression, anxiety, and suicidality.27 How do we take this into account when treating patients with psoriasis, especially when we have biologics with a warning label for suicidality and a Risk Evaluation and Mitigation Strategies program (brodalumab) and language around suicidal ideation in the label (bimekizumab)? While it is challenging to discuss mental health, it is not a conversation that we as dermatologists should shy away from. Appropriate treatment of psoriasis is an important tool to get our patients on the path to better mental health. A recent database study of more than 4000 patients showed that patients with psoriasis treated with biologics had a 17% lower risk for depression than those treated with conventional disease-modifying drugs such as methotrexate.28 The comparator of the conventional disease-modifying drug class is important as it serves as a control for disease severity. Too often, a higher rate of depression, anxiety, or suicidality can be attributed to a medication when we in fact may just be capturing the background of higher incidence of all 3 in patients with severe psoriasis.
Indeed, even with the medication that many worry about on this front (brodalumab), multiple studies have confirmed that the effect on mental health generally is a positive one, with decreases in depressive symptoms.29 In a cohort switched from TNF-α inhibitors to brodalumab, symptoms of depression actually improved,30 so attributing a direct treatment effect to negative mental health outcomes does not seem to be justified, especially in light of the low number of suicide events in global postmarketing surveillance for brodalumab, comparable to or lower than other biologics for psoriasis.31 Similarly, bimekizumab has language in the label about discussing suicidality with patients, although the rates of suicidal ideation and behavior are no different from other biologics and rates of depression improved with its use.32
Heightened awareness of our patients’ mental health is something that we as providers should embrace, even when it seems that we do not have much time to see each patient. The priority when a patient comes in with mental health symptoms should be to treat what is within our scope (ie, psoriasis) as quickly and effectively as possible— with a newer-generation biologic such as an IL-17 or IL-23 inhibitor—while encouraging the patient to seek care from a mental health professional. In these cases, one might even argue that the rapidity of action of IL-17 inhibitors may be of additional benefit.
Final Thoughts
We as dermatologists generally are tasked with seeing high volumes of patients, and an initial psoriasis consultation can be a lengthy visit; however, it is rewarding to establish this relationship with patients and a reminder of why we practice medicine to begin with. Psoriasis can be satisfying to treat, and we have so many highly effective medicines that can completely transform our patients’ lives. Applying an understanding of the interplay between psoriasis, its related comorbidities, and treatment choices can be a fulfilling exercise that captures the essence of shared decision-making, which can lead to better outcomes and satisfaction for both providers and patients.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022. doi:10.1056/NEJMoa030409
- Thatiparthi A, Martin A, Liu J, et al. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22:425-442. doi:10.1007/s40257-021-00603-w
- Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5-22. doi:10.1007 /s00281-009-0153-8
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. doi:10.1001/jama.296.14.1735
- Miller IM, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014-1024. doi:10.1016/j.jaad.2013.06.053
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90. doi:10.1016/j.jaad.2016.07.042
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250. doi:10.1001 /archdermatol.2012.2502
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68. doi:10.1016/j.jaad.2018.02.050
- Cai J, Cui L, Wang Y, et al. Cardiometabolic comorbidities in patients with psoriasis: focusing on risk, biological therapy, and pathogenesis. Front Pharmacol. 2021;12:774808. doi:10.3389/fphar.2021.774808
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:E984-E1010. doi:10.1161/CIR.0000000000000973
- Pirro F, Caldarola G, Chiricozzi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig. 2021;41:917-925. doi:10.1007 /s40261-021-01080-z
- Kim H, Hong JY, Cheong S, et al. Impact of biologic agents on body weight and obesity-related disorders in patients with psoriasis: a nationwide population-based cohort study. Obes Res Clin Pract. 2023;17:210-217. doi:10.1016/j.orcp.2023.05.004
- Saraceno R, Schipani C, Mazzotta A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57:290-295. doi:10.1016/j.phrs.2008.02.006
- Fernandez AP, Dauden E, Gerdes S, et al. Tildrakizumab efficacy and safety in patients with psoriasis and concomitant metabolic syndrome: post hoc analysis of 5-year data from reSURFACE 1 and reSURFACE 2. J Eur Acad Dermatol Venereol. 2022;36:1774-1783. doi:10.1111/jdv.18167
- Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. doi:10.1016/j.jacc.2010.05.034
- Ricceri F, Chiricozzi A, Peris K, et al. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: a multicentric retrospective study. Dermatol Ther. 2022;35:E15793. doi:10.1111/dth.15793
- Alinaghi F, Tekin HG, Burisch J, et al. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease— a systematic review and meta-analysis. J Crohns Colitis. 2020;14:351-360. doi:10.1093/ecco-jcc/jjz152
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. doi:10.1136/gut.52.1.65
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebocontrolled trial. Gut. 2012;61:1693-1700. doi:10.1136 /gutjnl-2011-301668
- Brockmann L, Tran A, Huang Y, et al. Intestinal microbiotaspecific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. 2023;56:2719-2735 e7. doi:10.1016/j.immuni.2023.11.003
- Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727-738. doi:10.1016/j.immuni.2015.09.003
- Wedebye Schmidt EG, Larsen HL, Kristensen NN, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19:1567-1576. doi:10.1097 /MIB.0b013e318286fa1c
- Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing T(reg) cells through modification of the intestinal microbiota. Nat Immunol. 2018;19:755-765. doi:10.1038/s41590-018-0134-y
- El Hadad J, Schreiner P, Vavricka SR, Greuter T. The genetics of inflammatory bowel disease. Mol Diagn Ther. 2024;28:27-35. doi:10.1007 /s40291-023-00678-7
- Albayrak F, Gür M, Karatas¸ A, et al. Is the use of secukinumab after anti-TNF therapy greater than expected for the risk of developing inflammatory bowel disease? Reumatol Clin (Engl Ed). 2024;20:123-127. doi:10.1016/j.reumae.2023.11.002
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Strober B, Soliman AM, Truong B, et al. Association between biologic exposure and the risk of depression in patients with psoriasis: a retrospective analysis of large US administrative claims data. Am J Clin Dermatol. 2024;25:853-856. doi:10.1007/s40257 -024-00877-w
- Koo J, Ho RS, Thibodeaux Q. Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review. Cutis. 2019;104:361-365.
- Andersch-Bjorkman Y, Micu E, Seifert O, et al. Effects of brodalumab on psoriasis and depressive symptoms in patients with insufficient response to TNF-alpha inhibitors. J Dermatol. 2023;50:1401-1414. doi:10.1111/1346-8138.16917
- Yeroushalmi S, Chung M, Bartholomew E, et al. Examining worldwide postmarketing suicides from biologics used for psoriasis with a focus on brodalumab: a cross-sectional analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS). JAAD Int. 2022;9:119-121. doi:10.1016/j.jdin.2022.08.010
- Blauvelt A, Armstrong A, Merola JF, et al. Mental health outcomes in patients with moderate to severe psoriasis treated with bimekizumab: analysis of phase 2/3 randomized trials. J Am Acad Dermatol. 2024;91:72-81. doi:10.1016/j.jaad.2024.02.039
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022. doi:10.1056/NEJMoa030409
- Thatiparthi A, Martin A, Liu J, et al. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22:425-442. doi:10.1007/s40257-021-00603-w
- Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5-22. doi:10.1007 /s00281-009-0153-8
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. doi:10.1001/jama.296.14.1735
- Miller IM, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014-1024. doi:10.1016/j.jaad.2013.06.053
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90. doi:10.1016/j.jaad.2016.07.042
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250. doi:10.1001 /archdermatol.2012.2502
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68. doi:10.1016/j.jaad.2018.02.050
- Cai J, Cui L, Wang Y, et al. Cardiometabolic comorbidities in patients with psoriasis: focusing on risk, biological therapy, and pathogenesis. Front Pharmacol. 2021;12:774808. doi:10.3389/fphar.2021.774808
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:E984-E1010. doi:10.1161/CIR.0000000000000973
- Pirro F, Caldarola G, Chiricozzi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig. 2021;41:917-925. doi:10.1007 /s40261-021-01080-z
- Kim H, Hong JY, Cheong S, et al. Impact of biologic agents on body weight and obesity-related disorders in patients with psoriasis: a nationwide population-based cohort study. Obes Res Clin Pract. 2023;17:210-217. doi:10.1016/j.orcp.2023.05.004
- Saraceno R, Schipani C, Mazzotta A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57:290-295. doi:10.1016/j.phrs.2008.02.006
- Fernandez AP, Dauden E, Gerdes S, et al. Tildrakizumab efficacy and safety in patients with psoriasis and concomitant metabolic syndrome: post hoc analysis of 5-year data from reSURFACE 1 and reSURFACE 2. J Eur Acad Dermatol Venereol. 2022;36:1774-1783. doi:10.1111/jdv.18167
- Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. doi:10.1016/j.jacc.2010.05.034
- Ricceri F, Chiricozzi A, Peris K, et al. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: a multicentric retrospective study. Dermatol Ther. 2022;35:E15793. doi:10.1111/dth.15793
- Alinaghi F, Tekin HG, Burisch J, et al. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease— a systematic review and meta-analysis. J Crohns Colitis. 2020;14:351-360. doi:10.1093/ecco-jcc/jjz152
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. doi:10.1136/gut.52.1.65
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebocontrolled trial. Gut. 2012;61:1693-1700. doi:10.1136 /gutjnl-2011-301668
- Brockmann L, Tran A, Huang Y, et al. Intestinal microbiotaspecific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. 2023;56:2719-2735 e7. doi:10.1016/j.immuni.2023.11.003
- Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727-738. doi:10.1016/j.immuni.2015.09.003
- Wedebye Schmidt EG, Larsen HL, Kristensen NN, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19:1567-1576. doi:10.1097 /MIB.0b013e318286fa1c
- Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing T(reg) cells through modification of the intestinal microbiota. Nat Immunol. 2018;19:755-765. doi:10.1038/s41590-018-0134-y
- El Hadad J, Schreiner P, Vavricka SR, Greuter T. The genetics of inflammatory bowel disease. Mol Diagn Ther. 2024;28:27-35. doi:10.1007 /s40291-023-00678-7
- Albayrak F, Gür M, Karatas¸ A, et al. Is the use of secukinumab after anti-TNF therapy greater than expected for the risk of developing inflammatory bowel disease? Reumatol Clin (Engl Ed). 2024;20:123-127. doi:10.1016/j.reumae.2023.11.002
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Strober B, Soliman AM, Truong B, et al. Association between biologic exposure and the risk of depression in patients with psoriasis: a retrospective analysis of large US administrative claims data. Am J Clin Dermatol. 2024;25:853-856. doi:10.1007/s40257 -024-00877-w
- Koo J, Ho RS, Thibodeaux Q. Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review. Cutis. 2019;104:361-365.
- Andersch-Bjorkman Y, Micu E, Seifert O, et al. Effects of brodalumab on psoriasis and depressive symptoms in patients with insufficient response to TNF-alpha inhibitors. J Dermatol. 2023;50:1401-1414. doi:10.1111/1346-8138.16917
- Yeroushalmi S, Chung M, Bartholomew E, et al. Examining worldwide postmarketing suicides from biologics used for psoriasis with a focus on brodalumab: a cross-sectional analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS). JAAD Int. 2022;9:119-121. doi:10.1016/j.jdin.2022.08.010
- Blauvelt A, Armstrong A, Merola JF, et al. Mental health outcomes in patients with moderate to severe psoriasis treated with bimekizumab: analysis of phase 2/3 randomized trials. J Am Acad Dermatol. 2024;91:72-81. doi:10.1016/j.jaad.2024.02.039
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
Integrating Artificial Intelligence Into Private Practice
In this video, Vasu Appalaneni, MD, a gastroenterologist at Dayton Gastroenterology in Beavercreek, Ohio,
In addition to her work at Dayton Gastroenterology, Dr. Appalaneni is executive vice president of clinical innovation at One GI, a gastroenterology management services organization that partners with gastroenterologists to help them manage and grow their independent gastroenterology practices. One GI is Dayton Gastroenterology’s parent company.

In this video, Vasu Appalaneni, MD, a gastroenterologist at Dayton Gastroenterology in Beavercreek, Ohio,
In addition to her work at Dayton Gastroenterology, Dr. Appalaneni is executive vice president of clinical innovation at One GI, a gastroenterology management services organization that partners with gastroenterologists to help them manage and grow their independent gastroenterology practices. One GI is Dayton Gastroenterology’s parent company.

In this video, Vasu Appalaneni, MD, a gastroenterologist at Dayton Gastroenterology in Beavercreek, Ohio,
In addition to her work at Dayton Gastroenterology, Dr. Appalaneni is executive vice president of clinical innovation at One GI, a gastroenterology management services organization that partners with gastroenterologists to help them manage and grow their independent gastroenterology practices. One GI is Dayton Gastroenterology’s parent company.