User login
Depressed patients respond faster to IV ketamine than intranasal ketamine
NEW ORLEANS – New research reveals that patients with treatment-resistant depression who were treated with repeated intravenous ketamine show no significant differences in achieving response or remission, compared with those receiving the intranasal formulation of the drug, esketamine – although fewer treatments appear necessary with the intravenous formulation.
“ although at the end, the responses are similar,” said first author Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, in Rochester, Minn.
The findings were presented at the annual meeting of the American Psychiatric Association.
Commenting on the study, Roger S. McIntyre, MD, underscored that “this is an important study that addresses the priority questions that everyone wants to know – not only for clinical reasons, but economic reasons.” Dr. McIntyre, a professor of psychiatry and pharmacology at the University of Toronto, and head of the university’s mood disorders psychopharmacology unit, said that “there are implications not only for clinical outcomes and cost, but also implementation because IV is obviously more demanding and complicated.”
As intravenous ketamine increasingly gained interest as a rapid-acting treatment for patients with severe, treatment-resistant depression, the introduction of a more convenient intranasal formulation was seen as a welcome improvement and received approval from the Food and Drug Administration in 2019. However, while the approval ushered in more coverage by insurance companies, the treatment can still be expensive. Intravenous ketamine does not have FDA approval.
With a lack of studies in the real-world setting comparing efficacy of the two formulations, Dr. Singh and his colleagues conducted the observational study, evaluating the responses of 62 adults with treatment-resistant depression who had received either up to six IV ketamine infusions of 0.5 mg/kg, infused over 40 minutes, or up to eight intranasal esketamine treatments of 56/84 mg, as approved by the FDA, at the Mayo Clinic Depression Center.
Of the patients, who had a mean age of 47 years, 59 had major depression and 3 had bipolar depression. Among them, 76% (47) received intravenous ketamine and 24% (15) received esketamine, which Dr. Singh noted reflected the higher number of patients included before esketamine received FDA approval. The patients had similar comorbidity profiles, with the intravenous ketamine group having a higher body mass index at baseline.
Overall, the patients all had significant improvement in their depression at the end of the acute phase of 4 weeks, with a mean change in on the 16-Item Quick Inventory of Depressive Symptomatology (QIDS-SR) scale of –8.6 from baseline (P < .001).
The overall remission rate was 38.7% and overall response rate was 58.1%. Those receiving intravenous ketamine had response and remission rates of 57.4% and 42.6%, versus response and remission rates of 60.0% and 26.7% among the esketamine group, which Dr. Singh said were not significant differences (P > .05).
However, the mean number of treatments necessary to achieve response in the intravenous ketamine group was just 2.3 versus 4.6 with esketamine, and the mean number of treatments to achieve remission were 2.5 versus 6.3, respectively (P = .008).
After a multivariate adjustment, the time to response was determined to be faster with intravenous ketamine versus esketamine (hazard ratio, 2.61; P = .05) and the time to remission was also faster (HR, 5.0; P = .02).
“What this means is you would need fewer treatments to achieve a response or remission with IV ketamine, so there could be an acceleration of patients’ antidepressant response,” Dr. Singh explained.
There were no significant differences between the groups in terms of side effects, and most patients tolerated the treatments well.
Dr. Singh noted the limitation of the study is that it was observational and included a small sample size. Nevertheless, when asked which he would choose if starting treatment when insurance was not an issue, Dr. Singh replied: “I would take patient preference into account, but certainly IV seems to have an advantage.”
Dr. McIntyre noted that, though small, the study’s setting in a real world clinical environment is important.
“Obviously this is observational and not controlled, but the strength is that this involved a real-world cohort of patients and real world applications,” he said. “It’s difficult to have a true comparator head-to-head trial, so that makes this all the more important because it takes into consideration all of the complexities of real world patients.”
Dr. McIntyre emphasized that the study is not “the last word on the story because we need to see a larger sample and replication. But certainly they make an argument that IV ketamine may have an advantage over the speed of onset with intranasal ketamine, which will need to be either replicated or refuted, but it’s a great starting point in the conversation.”
Navigating patient preference
Robert Meisner, MD, founding medical director of the McLean Ketamine Service, Division of Psychiatric Neurotherapeutics, McLean Hospital, Harvard Medical School, in Boston, noted that wide-ranging factors may influence patient as well as clinician decisions about which ketamine treatment approach to use.
“When a patient appears to be equally well-suited for both interventions, I continue to be surprised by why one patient will indicate a preference for intranasal esketamine, while another will lean toward IV racemic ketamine,” he said in an interview.
“Some patients find esketamine’s clear and consistent protocol optimal for scheduling and navigating the logistics of daily life; others value the flexibility offered by certain evidence-based, racemic (IV) protocols,” he said. “Predicting who will prefer each treatment, even with the apparent temporal advantage with IV ketamine, is extremely difficult.”
Likewise, in terms of clinician preference, Dr. Meisner notes that key concerns may sway decisions.
“If I’m concerned with labile pressures or hypertension, for example, or if I have a patient with, say, Erlos Danlos Syndrome without a clear subtype, and hence, some risk of undiscovered aneurysmal vascular disease, I may lean toward racemic IV ketamine.”
On the other hand, “some patients find the simplicity and predictability of the maintenance esketamine protocol comforting and psychologically stabilizing,” he added. “Yet others find that their work or family’s erratic demands on their time make one of the evidence-based racemic regimens preferable – inasmuch as it integrates more flexibility and allows them to remain more fully engaged in the basic activities or work and family.”
Dr. Meisner noted the caveat that efforts to decide which method to use are often complicated by substantial misinformation.
“I can’t emphasize how much misinformation continues to abound regarding appropriate (evidence-based) and safe use of ketamine and esketamine,” he said. “Especially on the IV racemic side, there simply is no substantive evidence base for many of the claims that some providers are preaching.”
The confusion, driven in part by social media, “has diffused into sectors of the field and industry that one might assume are relatively immune (i.e., allied physicians, sophisticated payers, etc),” he added.
“In short, two mantra continue to apply,” Dr. Meisner said. “One – if it sounds too good to be true, it probably is; and two – in pharmacology and interventional psychiatry, we see remarkable progress and potential, but there simply is no such thing as a magic bullet.”
Dr. Singh and Dr. Meisner had no disclosures to report. Dr. McIntyre has received research grant support from Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/National Natural Science Foundation of China, and speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
NEW ORLEANS – New research reveals that patients with treatment-resistant depression who were treated with repeated intravenous ketamine show no significant differences in achieving response or remission, compared with those receiving the intranasal formulation of the drug, esketamine – although fewer treatments appear necessary with the intravenous formulation.
“ although at the end, the responses are similar,” said first author Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, in Rochester, Minn.
The findings were presented at the annual meeting of the American Psychiatric Association.
Commenting on the study, Roger S. McIntyre, MD, underscored that “this is an important study that addresses the priority questions that everyone wants to know – not only for clinical reasons, but economic reasons.” Dr. McIntyre, a professor of psychiatry and pharmacology at the University of Toronto, and head of the university’s mood disorders psychopharmacology unit, said that “there are implications not only for clinical outcomes and cost, but also implementation because IV is obviously more demanding and complicated.”
As intravenous ketamine increasingly gained interest as a rapid-acting treatment for patients with severe, treatment-resistant depression, the introduction of a more convenient intranasal formulation was seen as a welcome improvement and received approval from the Food and Drug Administration in 2019. However, while the approval ushered in more coverage by insurance companies, the treatment can still be expensive. Intravenous ketamine does not have FDA approval.
With a lack of studies in the real-world setting comparing efficacy of the two formulations, Dr. Singh and his colleagues conducted the observational study, evaluating the responses of 62 adults with treatment-resistant depression who had received either up to six IV ketamine infusions of 0.5 mg/kg, infused over 40 minutes, or up to eight intranasal esketamine treatments of 56/84 mg, as approved by the FDA, at the Mayo Clinic Depression Center.
Of the patients, who had a mean age of 47 years, 59 had major depression and 3 had bipolar depression. Among them, 76% (47) received intravenous ketamine and 24% (15) received esketamine, which Dr. Singh noted reflected the higher number of patients included before esketamine received FDA approval. The patients had similar comorbidity profiles, with the intravenous ketamine group having a higher body mass index at baseline.
Overall, the patients all had significant improvement in their depression at the end of the acute phase of 4 weeks, with a mean change in on the 16-Item Quick Inventory of Depressive Symptomatology (QIDS-SR) scale of –8.6 from baseline (P < .001).
The overall remission rate was 38.7% and overall response rate was 58.1%. Those receiving intravenous ketamine had response and remission rates of 57.4% and 42.6%, versus response and remission rates of 60.0% and 26.7% among the esketamine group, which Dr. Singh said were not significant differences (P > .05).
However, the mean number of treatments necessary to achieve response in the intravenous ketamine group was just 2.3 versus 4.6 with esketamine, and the mean number of treatments to achieve remission were 2.5 versus 6.3, respectively (P = .008).
After a multivariate adjustment, the time to response was determined to be faster with intravenous ketamine versus esketamine (hazard ratio, 2.61; P = .05) and the time to remission was also faster (HR, 5.0; P = .02).
“What this means is you would need fewer treatments to achieve a response or remission with IV ketamine, so there could be an acceleration of patients’ antidepressant response,” Dr. Singh explained.
There were no significant differences between the groups in terms of side effects, and most patients tolerated the treatments well.
Dr. Singh noted the limitation of the study is that it was observational and included a small sample size. Nevertheless, when asked which he would choose if starting treatment when insurance was not an issue, Dr. Singh replied: “I would take patient preference into account, but certainly IV seems to have an advantage.”
Dr. McIntyre noted that, though small, the study’s setting in a real world clinical environment is important.
“Obviously this is observational and not controlled, but the strength is that this involved a real-world cohort of patients and real world applications,” he said. “It’s difficult to have a true comparator head-to-head trial, so that makes this all the more important because it takes into consideration all of the complexities of real world patients.”
Dr. McIntyre emphasized that the study is not “the last word on the story because we need to see a larger sample and replication. But certainly they make an argument that IV ketamine may have an advantage over the speed of onset with intranasal ketamine, which will need to be either replicated or refuted, but it’s a great starting point in the conversation.”
Navigating patient preference
Robert Meisner, MD, founding medical director of the McLean Ketamine Service, Division of Psychiatric Neurotherapeutics, McLean Hospital, Harvard Medical School, in Boston, noted that wide-ranging factors may influence patient as well as clinician decisions about which ketamine treatment approach to use.
“When a patient appears to be equally well-suited for both interventions, I continue to be surprised by why one patient will indicate a preference for intranasal esketamine, while another will lean toward IV racemic ketamine,” he said in an interview.
“Some patients find esketamine’s clear and consistent protocol optimal for scheduling and navigating the logistics of daily life; others value the flexibility offered by certain evidence-based, racemic (IV) protocols,” he said. “Predicting who will prefer each treatment, even with the apparent temporal advantage with IV ketamine, is extremely difficult.”
Likewise, in terms of clinician preference, Dr. Meisner notes that key concerns may sway decisions.
“If I’m concerned with labile pressures or hypertension, for example, or if I have a patient with, say, Erlos Danlos Syndrome without a clear subtype, and hence, some risk of undiscovered aneurysmal vascular disease, I may lean toward racemic IV ketamine.”
On the other hand, “some patients find the simplicity and predictability of the maintenance esketamine protocol comforting and psychologically stabilizing,” he added. “Yet others find that their work or family’s erratic demands on their time make one of the evidence-based racemic regimens preferable – inasmuch as it integrates more flexibility and allows them to remain more fully engaged in the basic activities or work and family.”
Dr. Meisner noted the caveat that efforts to decide which method to use are often complicated by substantial misinformation.
“I can’t emphasize how much misinformation continues to abound regarding appropriate (evidence-based) and safe use of ketamine and esketamine,” he said. “Especially on the IV racemic side, there simply is no substantive evidence base for many of the claims that some providers are preaching.”
The confusion, driven in part by social media, “has diffused into sectors of the field and industry that one might assume are relatively immune (i.e., allied physicians, sophisticated payers, etc),” he added.
“In short, two mantra continue to apply,” Dr. Meisner said. “One – if it sounds too good to be true, it probably is; and two – in pharmacology and interventional psychiatry, we see remarkable progress and potential, but there simply is no such thing as a magic bullet.”
Dr. Singh and Dr. Meisner had no disclosures to report. Dr. McIntyre has received research grant support from Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/National Natural Science Foundation of China, and speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
NEW ORLEANS – New research reveals that patients with treatment-resistant depression who were treated with repeated intravenous ketamine show no significant differences in achieving response or remission, compared with those receiving the intranasal formulation of the drug, esketamine – although fewer treatments appear necessary with the intravenous formulation.
“ although at the end, the responses are similar,” said first author Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, in Rochester, Minn.
The findings were presented at the annual meeting of the American Psychiatric Association.
Commenting on the study, Roger S. McIntyre, MD, underscored that “this is an important study that addresses the priority questions that everyone wants to know – not only for clinical reasons, but economic reasons.” Dr. McIntyre, a professor of psychiatry and pharmacology at the University of Toronto, and head of the university’s mood disorders psychopharmacology unit, said that “there are implications not only for clinical outcomes and cost, but also implementation because IV is obviously more demanding and complicated.”
As intravenous ketamine increasingly gained interest as a rapid-acting treatment for patients with severe, treatment-resistant depression, the introduction of a more convenient intranasal formulation was seen as a welcome improvement and received approval from the Food and Drug Administration in 2019. However, while the approval ushered in more coverage by insurance companies, the treatment can still be expensive. Intravenous ketamine does not have FDA approval.
With a lack of studies in the real-world setting comparing efficacy of the two formulations, Dr. Singh and his colleagues conducted the observational study, evaluating the responses of 62 adults with treatment-resistant depression who had received either up to six IV ketamine infusions of 0.5 mg/kg, infused over 40 minutes, or up to eight intranasal esketamine treatments of 56/84 mg, as approved by the FDA, at the Mayo Clinic Depression Center.
Of the patients, who had a mean age of 47 years, 59 had major depression and 3 had bipolar depression. Among them, 76% (47) received intravenous ketamine and 24% (15) received esketamine, which Dr. Singh noted reflected the higher number of patients included before esketamine received FDA approval. The patients had similar comorbidity profiles, with the intravenous ketamine group having a higher body mass index at baseline.
Overall, the patients all had significant improvement in their depression at the end of the acute phase of 4 weeks, with a mean change in on the 16-Item Quick Inventory of Depressive Symptomatology (QIDS-SR) scale of –8.6 from baseline (P < .001).
The overall remission rate was 38.7% and overall response rate was 58.1%. Those receiving intravenous ketamine had response and remission rates of 57.4% and 42.6%, versus response and remission rates of 60.0% and 26.7% among the esketamine group, which Dr. Singh said were not significant differences (P > .05).
However, the mean number of treatments necessary to achieve response in the intravenous ketamine group was just 2.3 versus 4.6 with esketamine, and the mean number of treatments to achieve remission were 2.5 versus 6.3, respectively (P = .008).
After a multivariate adjustment, the time to response was determined to be faster with intravenous ketamine versus esketamine (hazard ratio, 2.61; P = .05) and the time to remission was also faster (HR, 5.0; P = .02).
“What this means is you would need fewer treatments to achieve a response or remission with IV ketamine, so there could be an acceleration of patients’ antidepressant response,” Dr. Singh explained.
There were no significant differences between the groups in terms of side effects, and most patients tolerated the treatments well.
Dr. Singh noted the limitation of the study is that it was observational and included a small sample size. Nevertheless, when asked which he would choose if starting treatment when insurance was not an issue, Dr. Singh replied: “I would take patient preference into account, but certainly IV seems to have an advantage.”
Dr. McIntyre noted that, though small, the study’s setting in a real world clinical environment is important.
“Obviously this is observational and not controlled, but the strength is that this involved a real-world cohort of patients and real world applications,” he said. “It’s difficult to have a true comparator head-to-head trial, so that makes this all the more important because it takes into consideration all of the complexities of real world patients.”
Dr. McIntyre emphasized that the study is not “the last word on the story because we need to see a larger sample and replication. But certainly they make an argument that IV ketamine may have an advantage over the speed of onset with intranasal ketamine, which will need to be either replicated or refuted, but it’s a great starting point in the conversation.”
Navigating patient preference
Robert Meisner, MD, founding medical director of the McLean Ketamine Service, Division of Psychiatric Neurotherapeutics, McLean Hospital, Harvard Medical School, in Boston, noted that wide-ranging factors may influence patient as well as clinician decisions about which ketamine treatment approach to use.
“When a patient appears to be equally well-suited for both interventions, I continue to be surprised by why one patient will indicate a preference for intranasal esketamine, while another will lean toward IV racemic ketamine,” he said in an interview.
“Some patients find esketamine’s clear and consistent protocol optimal for scheduling and navigating the logistics of daily life; others value the flexibility offered by certain evidence-based, racemic (IV) protocols,” he said. “Predicting who will prefer each treatment, even with the apparent temporal advantage with IV ketamine, is extremely difficult.”
Likewise, in terms of clinician preference, Dr. Meisner notes that key concerns may sway decisions.
“If I’m concerned with labile pressures or hypertension, for example, or if I have a patient with, say, Erlos Danlos Syndrome without a clear subtype, and hence, some risk of undiscovered aneurysmal vascular disease, I may lean toward racemic IV ketamine.”
On the other hand, “some patients find the simplicity and predictability of the maintenance esketamine protocol comforting and psychologically stabilizing,” he added. “Yet others find that their work or family’s erratic demands on their time make one of the evidence-based racemic regimens preferable – inasmuch as it integrates more flexibility and allows them to remain more fully engaged in the basic activities or work and family.”
Dr. Meisner noted the caveat that efforts to decide which method to use are often complicated by substantial misinformation.
“I can’t emphasize how much misinformation continues to abound regarding appropriate (evidence-based) and safe use of ketamine and esketamine,” he said. “Especially on the IV racemic side, there simply is no substantive evidence base for many of the claims that some providers are preaching.”
The confusion, driven in part by social media, “has diffused into sectors of the field and industry that one might assume are relatively immune (i.e., allied physicians, sophisticated payers, etc),” he added.
“In short, two mantra continue to apply,” Dr. Meisner said. “One – if it sounds too good to be true, it probably is; and two – in pharmacology and interventional psychiatry, we see remarkable progress and potential, but there simply is no such thing as a magic bullet.”
Dr. Singh and Dr. Meisner had no disclosures to report. Dr. McIntyre has received research grant support from Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/National Natural Science Foundation of China, and speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
AT APA 2022
More evidence dementia not linked to PPI use in older people
Controversy regarding the purported link between the use of proton pump inhibitors (PPIs) or histamine H2 receptor antagonists (H2RAs) and risk for dementia continues.
Adding to the “no link” column comes new evidence from a study presented at the annual Digestive Disease Week® (DDW) .
Among almost 19,000 people, no association was found between the use of these agents and a greater likelihood of incident dementia, Alzheimer’s disease, or cognitive decline in people older than 65 years.
“We found that baseline PPI or H2RA use in older adults was not associated with dementia, with mild cognitive impairment, or declines in cognitive scores over time,” said lead author Raaj Shishir Mehta, MD, a gastroenterology fellow at Massachusetts General Hospital in Boston.
“While deprescribing efforts are important, especially when medications are not indicated, these data provide reassurance about the cognitive impacts of long-term use of PPIs in older adults,” he added.
Growing use, growing concern
As PPI use has increased worldwide, so too have concerns over the adverse effects from their long-term use, Dr. Mehta said.
“One particular area of concern, especially among older adults, is the link between long-term PPI use and risk for dementia,” he said.
Igniting the controversy was a February 2016 study published in JAMA Neurology that showed a positive association between PPI use and dementia in residents of Germany aged 75 years and older. Researchers linked PPI use to a 44% increased risk of dementia over 5 years.
The 2016 study was based on claims data, which can introduce “inaccuracy or bias in defining dementia cases,” Dr. Mehta said. He noted that it and other previous studies also were limited by an inability to account for concomitant medications or comorbidities.
To overcome these limitations in their study, Dr. Mehta and colleagues analyzed medication data collected during in-person visits and asked experts to confirm dementia outcomes. The research data come from ASPREE, a large aspirin study of 18,846 people older than 65 years in the United States and Australia. Participants were enrolled from 2010 to 2014. A total of 566 people developed incident dementia during follow-up.
The researchers had data on alcohol consumption and other lifestyle factors, as well as information on comorbidities, hospitalizations, and overall well-being.
“Perhaps the biggest strength of our study is our rigorous neurocognitive assessments,” Dr. Mehta said.
They assessed cognition at baseline and at years 1, 3, 5, and 7 using a battery of tests. An expert panel of neurologists, neuropsychologists, and geriatricians adjudicated cases of dementia, in accordance with DSM-IV criteria. If the diagnosis was unclear, they referred people for additional workup, including neuroimaging.
Cox proportional hazards, regression, and/or mixed effects modeling were used to relate medication use with cognitive scores.
All analyses were adjusted for age, sex, body mass index, alcohol use, family history of dementia, medications, and other medical comorbidities.
At baseline, PPI users were more likely to be White, have fewer years of education, and have higher rates of hypertension, diabetes, and kidney disease. This group also was more likely to be taking five or more medications.
Key points
During 80,976 person-years of follow-up, there were 566 incident cases of dementia, including 235 probable cases of Alzheimer’s disease and 331 other dementias.
Baseline PPI use, in comparison with nonuse, was not associated with incident dementia (hazard ratio, 0.86; 95% confidence interval, 0.70-1.05).
“Similarly, when we look specifically at Alzheimer’s disease or mixed types of dementia, we find no association between baseline PPI use and dementia,” Dr. Mehta said.
When they excluded people already taking PPIs at baseline, they found no association between starting PPIs and developing dementia over time.
Secondary aims of the study included looking for a link between PPI use and mild cognitive impairment or significant changes in cognition over time. In both cases, no association emerged. PPI use at baseline also was not associated with cognitive impairment/no dementia (also known as mild cognitive impairment) or with changes in overall cognitive test scores over time.
To determine whether any association could be a class effect of acid suppression medication, they assessed use of H2RA medications and development of incident dementia. Again, the researchers found no link.
A diverse multinational population from urban and rural areas was a strength of the study, as was the “very rigorous cognitive testing with expert adjudication of our endpoints,” Dr. Mehta said. In addition, fewer than 5% of patients were lost to follow-up.
In terms of limitations, this was an observational study “so residual confounding is always possible,” he added. “But I’ll emphasize that we are among the largest studies to date with wealth of covariates.”
Why the different findings?
The study was “really well done,” session moderator Paul Moayyedi, MD, said during the Q&A session at DDW 2022.
Dr. Moayyedi, a professor of medicine at McMaster University, Hamilton, Ont., asked Dr. Mehta why he “found absolutely no signal, whereas the German study did.”
“It’s a good question,” Dr. Mehta responded. “If you look across the board, there have been conflicting results.”
The disparity could be related to how researchers conducting claims data studies classify dementia, he noted.
“If you look at the nitty-gritty details over 5 years, almost 40% of participants [in those studies] end up with a diagnosis of dementia, which is quite high,” Dr. Mehta said. “That raises questions about whether the diagnosis of dementia is truly accurate.”
Dr. Mehta and Dr. Moayyedi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Controversy regarding the purported link between the use of proton pump inhibitors (PPIs) or histamine H2 receptor antagonists (H2RAs) and risk for dementia continues.
Adding to the “no link” column comes new evidence from a study presented at the annual Digestive Disease Week® (DDW) .
Among almost 19,000 people, no association was found between the use of these agents and a greater likelihood of incident dementia, Alzheimer’s disease, or cognitive decline in people older than 65 years.
“We found that baseline PPI or H2RA use in older adults was not associated with dementia, with mild cognitive impairment, or declines in cognitive scores over time,” said lead author Raaj Shishir Mehta, MD, a gastroenterology fellow at Massachusetts General Hospital in Boston.
“While deprescribing efforts are important, especially when medications are not indicated, these data provide reassurance about the cognitive impacts of long-term use of PPIs in older adults,” he added.
Growing use, growing concern
As PPI use has increased worldwide, so too have concerns over the adverse effects from their long-term use, Dr. Mehta said.
“One particular area of concern, especially among older adults, is the link between long-term PPI use and risk for dementia,” he said.
Igniting the controversy was a February 2016 study published in JAMA Neurology that showed a positive association between PPI use and dementia in residents of Germany aged 75 years and older. Researchers linked PPI use to a 44% increased risk of dementia over 5 years.
The 2016 study was based on claims data, which can introduce “inaccuracy or bias in defining dementia cases,” Dr. Mehta said. He noted that it and other previous studies also were limited by an inability to account for concomitant medications or comorbidities.
To overcome these limitations in their study, Dr. Mehta and colleagues analyzed medication data collected during in-person visits and asked experts to confirm dementia outcomes. The research data come from ASPREE, a large aspirin study of 18,846 people older than 65 years in the United States and Australia. Participants were enrolled from 2010 to 2014. A total of 566 people developed incident dementia during follow-up.
The researchers had data on alcohol consumption and other lifestyle factors, as well as information on comorbidities, hospitalizations, and overall well-being.
“Perhaps the biggest strength of our study is our rigorous neurocognitive assessments,” Dr. Mehta said.
They assessed cognition at baseline and at years 1, 3, 5, and 7 using a battery of tests. An expert panel of neurologists, neuropsychologists, and geriatricians adjudicated cases of dementia, in accordance with DSM-IV criteria. If the diagnosis was unclear, they referred people for additional workup, including neuroimaging.
Cox proportional hazards, regression, and/or mixed effects modeling were used to relate medication use with cognitive scores.
All analyses were adjusted for age, sex, body mass index, alcohol use, family history of dementia, medications, and other medical comorbidities.
At baseline, PPI users were more likely to be White, have fewer years of education, and have higher rates of hypertension, diabetes, and kidney disease. This group also was more likely to be taking five or more medications.
Key points
During 80,976 person-years of follow-up, there were 566 incident cases of dementia, including 235 probable cases of Alzheimer’s disease and 331 other dementias.
Baseline PPI use, in comparison with nonuse, was not associated with incident dementia (hazard ratio, 0.86; 95% confidence interval, 0.70-1.05).
“Similarly, when we look specifically at Alzheimer’s disease or mixed types of dementia, we find no association between baseline PPI use and dementia,” Dr. Mehta said.
When they excluded people already taking PPIs at baseline, they found no association between starting PPIs and developing dementia over time.
Secondary aims of the study included looking for a link between PPI use and mild cognitive impairment or significant changes in cognition over time. In both cases, no association emerged. PPI use at baseline also was not associated with cognitive impairment/no dementia (also known as mild cognitive impairment) or with changes in overall cognitive test scores over time.
To determine whether any association could be a class effect of acid suppression medication, they assessed use of H2RA medications and development of incident dementia. Again, the researchers found no link.
A diverse multinational population from urban and rural areas was a strength of the study, as was the “very rigorous cognitive testing with expert adjudication of our endpoints,” Dr. Mehta said. In addition, fewer than 5% of patients were lost to follow-up.
In terms of limitations, this was an observational study “so residual confounding is always possible,” he added. “But I’ll emphasize that we are among the largest studies to date with wealth of covariates.”
Why the different findings?
The study was “really well done,” session moderator Paul Moayyedi, MD, said during the Q&A session at DDW 2022.
Dr. Moayyedi, a professor of medicine at McMaster University, Hamilton, Ont., asked Dr. Mehta why he “found absolutely no signal, whereas the German study did.”
“It’s a good question,” Dr. Mehta responded. “If you look across the board, there have been conflicting results.”
The disparity could be related to how researchers conducting claims data studies classify dementia, he noted.
“If you look at the nitty-gritty details over 5 years, almost 40% of participants [in those studies] end up with a diagnosis of dementia, which is quite high,” Dr. Mehta said. “That raises questions about whether the diagnosis of dementia is truly accurate.”
Dr. Mehta and Dr. Moayyedi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Controversy regarding the purported link between the use of proton pump inhibitors (PPIs) or histamine H2 receptor antagonists (H2RAs) and risk for dementia continues.
Adding to the “no link” column comes new evidence from a study presented at the annual Digestive Disease Week® (DDW) .
Among almost 19,000 people, no association was found between the use of these agents and a greater likelihood of incident dementia, Alzheimer’s disease, or cognitive decline in people older than 65 years.
“We found that baseline PPI or H2RA use in older adults was not associated with dementia, with mild cognitive impairment, or declines in cognitive scores over time,” said lead author Raaj Shishir Mehta, MD, a gastroenterology fellow at Massachusetts General Hospital in Boston.
“While deprescribing efforts are important, especially when medications are not indicated, these data provide reassurance about the cognitive impacts of long-term use of PPIs in older adults,” he added.
Growing use, growing concern
As PPI use has increased worldwide, so too have concerns over the adverse effects from their long-term use, Dr. Mehta said.
“One particular area of concern, especially among older adults, is the link between long-term PPI use and risk for dementia,” he said.
Igniting the controversy was a February 2016 study published in JAMA Neurology that showed a positive association between PPI use and dementia in residents of Germany aged 75 years and older. Researchers linked PPI use to a 44% increased risk of dementia over 5 years.
The 2016 study was based on claims data, which can introduce “inaccuracy or bias in defining dementia cases,” Dr. Mehta said. He noted that it and other previous studies also were limited by an inability to account for concomitant medications or comorbidities.
To overcome these limitations in their study, Dr. Mehta and colleagues analyzed medication data collected during in-person visits and asked experts to confirm dementia outcomes. The research data come from ASPREE, a large aspirin study of 18,846 people older than 65 years in the United States and Australia. Participants were enrolled from 2010 to 2014. A total of 566 people developed incident dementia during follow-up.
The researchers had data on alcohol consumption and other lifestyle factors, as well as information on comorbidities, hospitalizations, and overall well-being.
“Perhaps the biggest strength of our study is our rigorous neurocognitive assessments,” Dr. Mehta said.
They assessed cognition at baseline and at years 1, 3, 5, and 7 using a battery of tests. An expert panel of neurologists, neuropsychologists, and geriatricians adjudicated cases of dementia, in accordance with DSM-IV criteria. If the diagnosis was unclear, they referred people for additional workup, including neuroimaging.
Cox proportional hazards, regression, and/or mixed effects modeling were used to relate medication use with cognitive scores.
All analyses were adjusted for age, sex, body mass index, alcohol use, family history of dementia, medications, and other medical comorbidities.
At baseline, PPI users were more likely to be White, have fewer years of education, and have higher rates of hypertension, diabetes, and kidney disease. This group also was more likely to be taking five or more medications.
Key points
During 80,976 person-years of follow-up, there were 566 incident cases of dementia, including 235 probable cases of Alzheimer’s disease and 331 other dementias.
Baseline PPI use, in comparison with nonuse, was not associated with incident dementia (hazard ratio, 0.86; 95% confidence interval, 0.70-1.05).
“Similarly, when we look specifically at Alzheimer’s disease or mixed types of dementia, we find no association between baseline PPI use and dementia,” Dr. Mehta said.
When they excluded people already taking PPIs at baseline, they found no association between starting PPIs and developing dementia over time.
Secondary aims of the study included looking for a link between PPI use and mild cognitive impairment or significant changes in cognition over time. In both cases, no association emerged. PPI use at baseline also was not associated with cognitive impairment/no dementia (also known as mild cognitive impairment) or with changes in overall cognitive test scores over time.
To determine whether any association could be a class effect of acid suppression medication, they assessed use of H2RA medications and development of incident dementia. Again, the researchers found no link.
A diverse multinational population from urban and rural areas was a strength of the study, as was the “very rigorous cognitive testing with expert adjudication of our endpoints,” Dr. Mehta said. In addition, fewer than 5% of patients were lost to follow-up.
In terms of limitations, this was an observational study “so residual confounding is always possible,” he added. “But I’ll emphasize that we are among the largest studies to date with wealth of covariates.”
Why the different findings?
The study was “really well done,” session moderator Paul Moayyedi, MD, said during the Q&A session at DDW 2022.
Dr. Moayyedi, a professor of medicine at McMaster University, Hamilton, Ont., asked Dr. Mehta why he “found absolutely no signal, whereas the German study did.”
“It’s a good question,” Dr. Mehta responded. “If you look across the board, there have been conflicting results.”
The disparity could be related to how researchers conducting claims data studies classify dementia, he noted.
“If you look at the nitty-gritty details over 5 years, almost 40% of participants [in those studies] end up with a diagnosis of dementia, which is quite high,” Dr. Mehta said. “That raises questions about whether the diagnosis of dementia is truly accurate.”
Dr. Mehta and Dr. Moayyedi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DDW 2022
Metformin bombs in breast cancer in landmark trial
Metformin, a common option for patients with type 2 diabetes, had previously been shown in observational studies to be associated with improved survival of cancer patients. Those studies mostly involved older patients with cancer who also had diabetes.
These findings have led to trials of the use of metformin for patients with cancer who do not have diabetes, but two lung cancer trials found no effect on survival.
Now this latest trial in breast cancer, which included 3,649 patients with hormone receptor–positive or –negative disease – who did not have diabetes – also found that metformin had no effect on survival.
These results “tell us that metformin is not effective against the most common types of breast cancer and any off-label use [of] this drug for the treatment of these common types of breast cancer should be stopped,” lead investigator and medical oncologist Pamela Goodwin, MD, a breast cancer researcher at the Lunenfeld-Tanenbaum Research Institute in Toronto, said in a press release.
The negative results “underscore the need for well-conducted randomized trials” before observational studies are put into practice, Dr. Goodwin and her team said.
However, the investigators cautioned against extrapolating their results to patients with diabetes, noting that “because metformin is effective in type 2 diabetes, the results ... should not affect the use of metformin” in breast cancer patients who have diabetes.
The study was published online in JAMA.
Patients were enrolled from 2010 to 2013 while undergoing adjuvant treatment – chemotherapy, radiotherapy, hormone therapy, and/or others – following complete resection of T1-3, N0-3 tumors. They were almost exclusively women (mean age, 52.4 years), and almost 90% were non-Hispanic White. They were primarily from the United States and Canada, with some patients from the United Kingdom and Switzerland.
Patients were randomly assigned equally to receive either metformin 850 mg twice daily or placebo for 5 years. Median follow-up was about 8 years.
Among 2,533 patients with estrogen receptor– and/or progesterone receptor–positive disease, the incidence of invasive disease–free survival events was 2.78 per 100 patient-years in the metformin group, vs. 2.74 per 100 patient-years in the placebo arm (hazard ratio [HR], 1.01, P = .93). There were 1.46 deaths per 100 patient-years with metformin, vs. 1.32 with placebo (HR, 1.10, P = .47).
Metformin was stopped early at about 3 years for the 1,116 hormone receptor–negative patients after futility was declared on interim analysis. The incidence of invasive disease–free survival events was 3.58 with metformin, vs. 3.60 with placebo per 100 patient-years (HR, 1.01, P = .92). There were 1.91 deaths per 100 patient-years in the metformin arm, vs. 2.15 in the group that received placebo (HR, 0.89, P = .46).
However, the findings were different and suggested a signal among the small subset of patients (17% of the total) who had HER2-positive disease. There were 1.93 disease-free survival events with metformin per 100 patient-years, vs. 3.05 events with placebo (HR, 0.64, P = .03), and 0.78 deaths in the metformin arm, vs. 1.43 deaths per 100 patient-years in the placebo arm (HR, 0.54, P = .04).
The benefit seen in this HER2-postive subgroup was limited to patients with any C allele of the rs11212617 single-nucleotide variant.
This was an exploratory analysis, so the results need to be confirmed in a randomized trial, but it’s possible that metformin “could provide an additional treatment option for HER2-positive breast cancer,” Dr. Goodwin said.
Grade 3 or higher adverse events were more common with metformin (21.5% vs. 17.5%). The most common such events were hypertension (2.4% vs. 1.9%), irregular menses (1.5% vs. 1.4%), and diarrhea (1.9% vs. 0.8%).
The study was conducted by the Canadian Cancer Trials Group and was funded by the Canadian Cancer Society, the National Cancer Institute, and others. Dr. Goodwin has disclosed no relevant financial relationships. Several coauthors reported ties to Pfizer, Eli Lilly, Roche, and a number of other companies. One coauthor is an AstraZeneca employee.
A version of this article first appeared on Medscape.com.
Metformin, a common option for patients with type 2 diabetes, had previously been shown in observational studies to be associated with improved survival of cancer patients. Those studies mostly involved older patients with cancer who also had diabetes.
These findings have led to trials of the use of metformin for patients with cancer who do not have diabetes, but two lung cancer trials found no effect on survival.
Now this latest trial in breast cancer, which included 3,649 patients with hormone receptor–positive or –negative disease – who did not have diabetes – also found that metformin had no effect on survival.
These results “tell us that metformin is not effective against the most common types of breast cancer and any off-label use [of] this drug for the treatment of these common types of breast cancer should be stopped,” lead investigator and medical oncologist Pamela Goodwin, MD, a breast cancer researcher at the Lunenfeld-Tanenbaum Research Institute in Toronto, said in a press release.
The negative results “underscore the need for well-conducted randomized trials” before observational studies are put into practice, Dr. Goodwin and her team said.
However, the investigators cautioned against extrapolating their results to patients with diabetes, noting that “because metformin is effective in type 2 diabetes, the results ... should not affect the use of metformin” in breast cancer patients who have diabetes.
The study was published online in JAMA.
Patients were enrolled from 2010 to 2013 while undergoing adjuvant treatment – chemotherapy, radiotherapy, hormone therapy, and/or others – following complete resection of T1-3, N0-3 tumors. They were almost exclusively women (mean age, 52.4 years), and almost 90% were non-Hispanic White. They were primarily from the United States and Canada, with some patients from the United Kingdom and Switzerland.
Patients were randomly assigned equally to receive either metformin 850 mg twice daily or placebo for 5 years. Median follow-up was about 8 years.
Among 2,533 patients with estrogen receptor– and/or progesterone receptor–positive disease, the incidence of invasive disease–free survival events was 2.78 per 100 patient-years in the metformin group, vs. 2.74 per 100 patient-years in the placebo arm (hazard ratio [HR], 1.01, P = .93). There were 1.46 deaths per 100 patient-years with metformin, vs. 1.32 with placebo (HR, 1.10, P = .47).
Metformin was stopped early at about 3 years for the 1,116 hormone receptor–negative patients after futility was declared on interim analysis. The incidence of invasive disease–free survival events was 3.58 with metformin, vs. 3.60 with placebo per 100 patient-years (HR, 1.01, P = .92). There were 1.91 deaths per 100 patient-years in the metformin arm, vs. 2.15 in the group that received placebo (HR, 0.89, P = .46).
However, the findings were different and suggested a signal among the small subset of patients (17% of the total) who had HER2-positive disease. There were 1.93 disease-free survival events with metformin per 100 patient-years, vs. 3.05 events with placebo (HR, 0.64, P = .03), and 0.78 deaths in the metformin arm, vs. 1.43 deaths per 100 patient-years in the placebo arm (HR, 0.54, P = .04).
The benefit seen in this HER2-postive subgroup was limited to patients with any C allele of the rs11212617 single-nucleotide variant.
This was an exploratory analysis, so the results need to be confirmed in a randomized trial, but it’s possible that metformin “could provide an additional treatment option for HER2-positive breast cancer,” Dr. Goodwin said.
Grade 3 or higher adverse events were more common with metformin (21.5% vs. 17.5%). The most common such events were hypertension (2.4% vs. 1.9%), irregular menses (1.5% vs. 1.4%), and diarrhea (1.9% vs. 0.8%).
The study was conducted by the Canadian Cancer Trials Group and was funded by the Canadian Cancer Society, the National Cancer Institute, and others. Dr. Goodwin has disclosed no relevant financial relationships. Several coauthors reported ties to Pfizer, Eli Lilly, Roche, and a number of other companies. One coauthor is an AstraZeneca employee.
A version of this article first appeared on Medscape.com.
Metformin, a common option for patients with type 2 diabetes, had previously been shown in observational studies to be associated with improved survival of cancer patients. Those studies mostly involved older patients with cancer who also had diabetes.
These findings have led to trials of the use of metformin for patients with cancer who do not have diabetes, but two lung cancer trials found no effect on survival.
Now this latest trial in breast cancer, which included 3,649 patients with hormone receptor–positive or –negative disease – who did not have diabetes – also found that metformin had no effect on survival.
These results “tell us that metformin is not effective against the most common types of breast cancer and any off-label use [of] this drug for the treatment of these common types of breast cancer should be stopped,” lead investigator and medical oncologist Pamela Goodwin, MD, a breast cancer researcher at the Lunenfeld-Tanenbaum Research Institute in Toronto, said in a press release.
The negative results “underscore the need for well-conducted randomized trials” before observational studies are put into practice, Dr. Goodwin and her team said.
However, the investigators cautioned against extrapolating their results to patients with diabetes, noting that “because metformin is effective in type 2 diabetes, the results ... should not affect the use of metformin” in breast cancer patients who have diabetes.
The study was published online in JAMA.
Patients were enrolled from 2010 to 2013 while undergoing adjuvant treatment – chemotherapy, radiotherapy, hormone therapy, and/or others – following complete resection of T1-3, N0-3 tumors. They were almost exclusively women (mean age, 52.4 years), and almost 90% were non-Hispanic White. They were primarily from the United States and Canada, with some patients from the United Kingdom and Switzerland.
Patients were randomly assigned equally to receive either metformin 850 mg twice daily or placebo for 5 years. Median follow-up was about 8 years.
Among 2,533 patients with estrogen receptor– and/or progesterone receptor–positive disease, the incidence of invasive disease–free survival events was 2.78 per 100 patient-years in the metformin group, vs. 2.74 per 100 patient-years in the placebo arm (hazard ratio [HR], 1.01, P = .93). There were 1.46 deaths per 100 patient-years with metformin, vs. 1.32 with placebo (HR, 1.10, P = .47).
Metformin was stopped early at about 3 years for the 1,116 hormone receptor–negative patients after futility was declared on interim analysis. The incidence of invasive disease–free survival events was 3.58 with metformin, vs. 3.60 with placebo per 100 patient-years (HR, 1.01, P = .92). There were 1.91 deaths per 100 patient-years in the metformin arm, vs. 2.15 in the group that received placebo (HR, 0.89, P = .46).
However, the findings were different and suggested a signal among the small subset of patients (17% of the total) who had HER2-positive disease. There were 1.93 disease-free survival events with metformin per 100 patient-years, vs. 3.05 events with placebo (HR, 0.64, P = .03), and 0.78 deaths in the metformin arm, vs. 1.43 deaths per 100 patient-years in the placebo arm (HR, 0.54, P = .04).
The benefit seen in this HER2-postive subgroup was limited to patients with any C allele of the rs11212617 single-nucleotide variant.
This was an exploratory analysis, so the results need to be confirmed in a randomized trial, but it’s possible that metformin “could provide an additional treatment option for HER2-positive breast cancer,” Dr. Goodwin said.
Grade 3 or higher adverse events were more common with metformin (21.5% vs. 17.5%). The most common such events were hypertension (2.4% vs. 1.9%), irregular menses (1.5% vs. 1.4%), and diarrhea (1.9% vs. 0.8%).
The study was conducted by the Canadian Cancer Trials Group and was funded by the Canadian Cancer Society, the National Cancer Institute, and others. Dr. Goodwin has disclosed no relevant financial relationships. Several coauthors reported ties to Pfizer, Eli Lilly, Roche, and a number of other companies. One coauthor is an AstraZeneca employee.
A version of this article first appeared on Medscape.com.
How to manage drug interactions with Paxlovid for COVID-19
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
OTC meds, supplements, and other drugs may interact with HIV antiretrovirals
Over-the-counter medications, food supplements, and other drugs may interact with antiretroviral therapy (ART) in people living with HIV and be harmful, an industry-sponsored clinical survey from Denmark reports.
“Our study confirms that polypharmacy and being on a protease inhibitor–based regimen increase the risk of potential drug-drug interactions [PDDIs] considerably and highlights the importance of questioning people living with HIV [PLWH] about dietary supplement intake,” the authors, led by Michaela Tinggaard, MD, Copenhagen University Hospital, wrote in HIV Medicine.
“Potential drug-drug interactions were common among our study population. Although the clinical significance of the majority of the identified PDDIs may be low, most of them were avoidable through a change or discontinuation of the comedication, a change in ART or by spacing drugs,” they added.
Senior author Thomas Benfield, MD, DTMH, DMSc, a professor of infectious diseases at the University of Copenhagen, and colleagues collected information on prescription medication, over-the-counter medication, and dietary supplements from adults living with HIV who received ART from two outpatient clinics.
The researchers estimated the prevalence of non-HIV comedications, and they used the University of Liverpool HIV Drug Interactions database to identify potential drug-drug interactions. They evaluated PDDIs and used logistic regression models to investigate links between PDDIs and relevant variables.
The study included 337 people living with HIV receiving ART. The median age was 53 years, 77% of them were male, and 96% were virally suppressed, with HIV-RNA viral load less than 50 copies/mL.
Overall, 26% of participants received five or more comedications, and 56% took dietary supplements.
In the medication lists of 52% of patients, the authors identified coadministration of drugs that required dose adjustment or monitoring; 4.5% of patients were taking drugs that should not be coadministered.
The researchers detected several factors that independently predicted PDDIs:
- Male sex (odds ratio, 1.9; 95% confidence interval, 1.0-3.4)
- Being on a protease inhibitor (OR, 4.3; 95% CI, 1.9-9.7)
- Receiving five or more comedications (OR, 3.3; 95% CI, 1.5-7.2)
- Taking over-the-counter medications (OR, 1.9; 95% CI, 1.1-3.3)
- Taking dietary supplements (OR, 2.0; 95% CI, 1.2-3.3)
Comorbidities and OTC medications increase in aging people with HIV
Indira Brar, MD, an infectious diseases senior staff physician and the medical director of HIV services at Henry Ford Health in Detroit, called the study and important resource for educating providers and patients about over-the-counter drugs.
“The main strength of the study is that it includes a decent number of aging patients living with HIV, the age group in which we worry about drug interactions,” she said in an interview.
“As patients get older, they have increased comorbidities. As comorbidities increase, the number of medications increases. As the number of medications increases, the drug interactions increase,” said Dr. Brar, who was not involved in the study. “Also, as patients get older, they tend to take more over-the-counter drugs.”
Dr. Brar explained how drug-drug interactions can harm patients.
“Drugs added to a patient who is already on ART could decrease the level of the ART and cause the patient to develop a drug-resistant HIV infection,” she said. “Or the ART the patient is on can increase the levels of the new drugs that have been added, and that could have potential toxicity and side effects.
“Food supplements, including multivitamins, calcium, and magnesium, are often overlooked because we think they’re benign. But these drugs can bind our new antiretrovirals, the integrase inhibitors. They can decrease their levels in the patient and cause drug-resistant HIV infection.
“In our clinic, we always tell our patients to please call us before they take any medication, so we can make sure there is no drug interaction,” Dr. Brar said.
Nan Wang, PharmD, a clinical pharmacy specialist at University Hospitals Cleveland Medical Center, noted in an email that drug-drug interactions with ARTs are common.
“Understanding the prevalence of antiretroviral drug interactions in a patient population can help identify certain medications that require enhanced vigilance and can guide our clinical interventions,” said Dr. Wang, who was not associated with the research.
Joseph Alvarnas, MD, a hematologist and oncologist at City of Hope Comprehensive Cancer Center in Duarte, Calif., said that this is “a methodologically sound and well-designed study that’s a timely, important reminder that providers need to think carefully and comprehensively when caring for their patients living with HIV.”
Dr. Alvarnas, who was not involved in the study, said that, with the widespread availability of ART, HIV has become a chronic, manageable condition in an aging population.
“ART agents, particularly the ritonavir-boosted protease inhibitors, increase the likelihood of patients having a potentially significant drug-drug interaction with one of their chronic care medications,” he added. “Even seemingly low-risk supplements such as multivitamins may result in a negative impact upon effective ART treatment of PLWH.”
“The essential next step is that these findings are integrated carefully into decision-support systems, electronic health record prescribing systems, and pharmacy safety-check systems to ensure that we reduce the risk of patient harm,” Dr. Alvarnas advised.
Dr. Benfield and several study coauthors reported financial relationships with GlaxoSmithKline and other pharmaceutical companies. Other coauthors, as well as Dr. Alvarnas, Dr. Brar, and Dr. Wang, reported no relevant financial relationships. The study was supported by GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
Over-the-counter medications, food supplements, and other drugs may interact with antiretroviral therapy (ART) in people living with HIV and be harmful, an industry-sponsored clinical survey from Denmark reports.
“Our study confirms that polypharmacy and being on a protease inhibitor–based regimen increase the risk of potential drug-drug interactions [PDDIs] considerably and highlights the importance of questioning people living with HIV [PLWH] about dietary supplement intake,” the authors, led by Michaela Tinggaard, MD, Copenhagen University Hospital, wrote in HIV Medicine.
“Potential drug-drug interactions were common among our study population. Although the clinical significance of the majority of the identified PDDIs may be low, most of them were avoidable through a change or discontinuation of the comedication, a change in ART or by spacing drugs,” they added.
Senior author Thomas Benfield, MD, DTMH, DMSc, a professor of infectious diseases at the University of Copenhagen, and colleagues collected information on prescription medication, over-the-counter medication, and dietary supplements from adults living with HIV who received ART from two outpatient clinics.
The researchers estimated the prevalence of non-HIV comedications, and they used the University of Liverpool HIV Drug Interactions database to identify potential drug-drug interactions. They evaluated PDDIs and used logistic regression models to investigate links between PDDIs and relevant variables.
The study included 337 people living with HIV receiving ART. The median age was 53 years, 77% of them were male, and 96% were virally suppressed, with HIV-RNA viral load less than 50 copies/mL.
Overall, 26% of participants received five or more comedications, and 56% took dietary supplements.
In the medication lists of 52% of patients, the authors identified coadministration of drugs that required dose adjustment or monitoring; 4.5% of patients were taking drugs that should not be coadministered.
The researchers detected several factors that independently predicted PDDIs:
- Male sex (odds ratio, 1.9; 95% confidence interval, 1.0-3.4)
- Being on a protease inhibitor (OR, 4.3; 95% CI, 1.9-9.7)
- Receiving five or more comedications (OR, 3.3; 95% CI, 1.5-7.2)
- Taking over-the-counter medications (OR, 1.9; 95% CI, 1.1-3.3)
- Taking dietary supplements (OR, 2.0; 95% CI, 1.2-3.3)
Comorbidities and OTC medications increase in aging people with HIV
Indira Brar, MD, an infectious diseases senior staff physician and the medical director of HIV services at Henry Ford Health in Detroit, called the study and important resource for educating providers and patients about over-the-counter drugs.
“The main strength of the study is that it includes a decent number of aging patients living with HIV, the age group in which we worry about drug interactions,” she said in an interview.
“As patients get older, they have increased comorbidities. As comorbidities increase, the number of medications increases. As the number of medications increases, the drug interactions increase,” said Dr. Brar, who was not involved in the study. “Also, as patients get older, they tend to take more over-the-counter drugs.”
Dr. Brar explained how drug-drug interactions can harm patients.
“Drugs added to a patient who is already on ART could decrease the level of the ART and cause the patient to develop a drug-resistant HIV infection,” she said. “Or the ART the patient is on can increase the levels of the new drugs that have been added, and that could have potential toxicity and side effects.
“Food supplements, including multivitamins, calcium, and magnesium, are often overlooked because we think they’re benign. But these drugs can bind our new antiretrovirals, the integrase inhibitors. They can decrease their levels in the patient and cause drug-resistant HIV infection.
“In our clinic, we always tell our patients to please call us before they take any medication, so we can make sure there is no drug interaction,” Dr. Brar said.
Nan Wang, PharmD, a clinical pharmacy specialist at University Hospitals Cleveland Medical Center, noted in an email that drug-drug interactions with ARTs are common.
“Understanding the prevalence of antiretroviral drug interactions in a patient population can help identify certain medications that require enhanced vigilance and can guide our clinical interventions,” said Dr. Wang, who was not associated with the research.
Joseph Alvarnas, MD, a hematologist and oncologist at City of Hope Comprehensive Cancer Center in Duarte, Calif., said that this is “a methodologically sound and well-designed study that’s a timely, important reminder that providers need to think carefully and comprehensively when caring for their patients living with HIV.”
Dr. Alvarnas, who was not involved in the study, said that, with the widespread availability of ART, HIV has become a chronic, manageable condition in an aging population.
“ART agents, particularly the ritonavir-boosted protease inhibitors, increase the likelihood of patients having a potentially significant drug-drug interaction with one of their chronic care medications,” he added. “Even seemingly low-risk supplements such as multivitamins may result in a negative impact upon effective ART treatment of PLWH.”
“The essential next step is that these findings are integrated carefully into decision-support systems, electronic health record prescribing systems, and pharmacy safety-check systems to ensure that we reduce the risk of patient harm,” Dr. Alvarnas advised.
Dr. Benfield and several study coauthors reported financial relationships with GlaxoSmithKline and other pharmaceutical companies. Other coauthors, as well as Dr. Alvarnas, Dr. Brar, and Dr. Wang, reported no relevant financial relationships. The study was supported by GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
Over-the-counter medications, food supplements, and other drugs may interact with antiretroviral therapy (ART) in people living with HIV and be harmful, an industry-sponsored clinical survey from Denmark reports.
“Our study confirms that polypharmacy and being on a protease inhibitor–based regimen increase the risk of potential drug-drug interactions [PDDIs] considerably and highlights the importance of questioning people living with HIV [PLWH] about dietary supplement intake,” the authors, led by Michaela Tinggaard, MD, Copenhagen University Hospital, wrote in HIV Medicine.
“Potential drug-drug interactions were common among our study population. Although the clinical significance of the majority of the identified PDDIs may be low, most of them were avoidable through a change or discontinuation of the comedication, a change in ART or by spacing drugs,” they added.
Senior author Thomas Benfield, MD, DTMH, DMSc, a professor of infectious diseases at the University of Copenhagen, and colleagues collected information on prescription medication, over-the-counter medication, and dietary supplements from adults living with HIV who received ART from two outpatient clinics.
The researchers estimated the prevalence of non-HIV comedications, and they used the University of Liverpool HIV Drug Interactions database to identify potential drug-drug interactions. They evaluated PDDIs and used logistic regression models to investigate links between PDDIs and relevant variables.
The study included 337 people living with HIV receiving ART. The median age was 53 years, 77% of them were male, and 96% were virally suppressed, with HIV-RNA viral load less than 50 copies/mL.
Overall, 26% of participants received five or more comedications, and 56% took dietary supplements.
In the medication lists of 52% of patients, the authors identified coadministration of drugs that required dose adjustment or monitoring; 4.5% of patients were taking drugs that should not be coadministered.
The researchers detected several factors that independently predicted PDDIs:
- Male sex (odds ratio, 1.9; 95% confidence interval, 1.0-3.4)
- Being on a protease inhibitor (OR, 4.3; 95% CI, 1.9-9.7)
- Receiving five or more comedications (OR, 3.3; 95% CI, 1.5-7.2)
- Taking over-the-counter medications (OR, 1.9; 95% CI, 1.1-3.3)
- Taking dietary supplements (OR, 2.0; 95% CI, 1.2-3.3)
Comorbidities and OTC medications increase in aging people with HIV
Indira Brar, MD, an infectious diseases senior staff physician and the medical director of HIV services at Henry Ford Health in Detroit, called the study and important resource for educating providers and patients about over-the-counter drugs.
“The main strength of the study is that it includes a decent number of aging patients living with HIV, the age group in which we worry about drug interactions,” she said in an interview.
“As patients get older, they have increased comorbidities. As comorbidities increase, the number of medications increases. As the number of medications increases, the drug interactions increase,” said Dr. Brar, who was not involved in the study. “Also, as patients get older, they tend to take more over-the-counter drugs.”
Dr. Brar explained how drug-drug interactions can harm patients.
“Drugs added to a patient who is already on ART could decrease the level of the ART and cause the patient to develop a drug-resistant HIV infection,” she said. “Or the ART the patient is on can increase the levels of the new drugs that have been added, and that could have potential toxicity and side effects.
“Food supplements, including multivitamins, calcium, and magnesium, are often overlooked because we think they’re benign. But these drugs can bind our new antiretrovirals, the integrase inhibitors. They can decrease their levels in the patient and cause drug-resistant HIV infection.
“In our clinic, we always tell our patients to please call us before they take any medication, so we can make sure there is no drug interaction,” Dr. Brar said.
Nan Wang, PharmD, a clinical pharmacy specialist at University Hospitals Cleveland Medical Center, noted in an email that drug-drug interactions with ARTs are common.
“Understanding the prevalence of antiretroviral drug interactions in a patient population can help identify certain medications that require enhanced vigilance and can guide our clinical interventions,” said Dr. Wang, who was not associated with the research.
Joseph Alvarnas, MD, a hematologist and oncologist at City of Hope Comprehensive Cancer Center in Duarte, Calif., said that this is “a methodologically sound and well-designed study that’s a timely, important reminder that providers need to think carefully and comprehensively when caring for their patients living with HIV.”
Dr. Alvarnas, who was not involved in the study, said that, with the widespread availability of ART, HIV has become a chronic, manageable condition in an aging population.
“ART agents, particularly the ritonavir-boosted protease inhibitors, increase the likelihood of patients having a potentially significant drug-drug interaction with one of their chronic care medications,” he added. “Even seemingly low-risk supplements such as multivitamins may result in a negative impact upon effective ART treatment of PLWH.”
“The essential next step is that these findings are integrated carefully into decision-support systems, electronic health record prescribing systems, and pharmacy safety-check systems to ensure that we reduce the risk of patient harm,” Dr. Alvarnas advised.
Dr. Benfield and several study coauthors reported financial relationships with GlaxoSmithKline and other pharmaceutical companies. Other coauthors, as well as Dr. Alvarnas, Dr. Brar, and Dr. Wang, reported no relevant financial relationships. The study was supported by GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
FROM HIV MEDICINE
NAVIGATOR steers uncontrolled asthma toward calmer seas
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.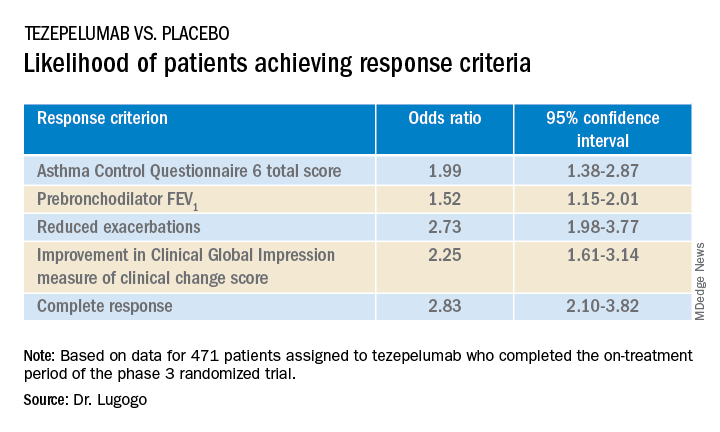
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
AT ATS 2022
Bupivacaine following Mohs surgery reduces opioid use, study finds
An injection of a randomized trial shows.
“Single-dose, in-office bupivacaine administration immediately following reconstructions known to be high risk for pain reduces postoperative narcotic use and acute pain during the time period when our patients have the highest levels of pain,” said first author Vanessa B. Voss, MD, of the University of Missouri–Columbia, who presented the findings at the annual meeting of the American College of Mohs Surgery.
“It was well tolerated, there were no adverse effects, and we recommend the consideration of using this in Mohs micrographic surgery reconstructions that are at the highest risk for pain,” she said.
Recent research has shown that Mohs micrographic surgeons have the highest rates of opioid prescribing of all dermatologists, with about 11% of patients undergoing a Mohs procedure prescribed the drugs for postoperative use, Dr. Voss explained.
Yet, with the ongoing opioid epidemic and even short courses of postoperative opioids placing patients at risk for addiction, the pressure is on to find alternative, nonaddictive strategies for the treatment of acute postoperative pain.
Bupivacaine is commonly used intraoperatively with other types of surgeries to reduce postoperative pain, with a favorable duration of action lasting up to 7 hours, compared with just 2-3 hours with lidocaine. And while its use in Mohs surgery is typically also intraoperative, along with lidocaine, the unique postoperative treatment approach in Mohs surgery has not been well studied, Dr. Voss noted.
To investigate, Dr. Voss and colleagues conducted the prospective, multicenter randomized trial, enrolling 174 patients undergoing Mohs micrographic surgery for skin cancer.
Patients were receiving complex flap reconstructions that have been specifically designated in an American Academy of Dermatology position statement to be high risk for pain following Mohs surgeries, and hence, more likely to involve prescriptions for opioids. These include reconstruction flaps of the scalp, ear, nose or lip, a wedge repair of the ear or lip, or a Mustarde cheek rotation flap.
The mean age of the patients was about 69 years, and about 65% were male. The two groups had no significant differences in demographics, tumor types, or repairs. They were randomized to receive either local injections of bupivacaine 0.5% (with no epinephrine) or placebo with sterile saline injection immediately following the procedure, with the total amount of injection standardized and dependent upon the flap surface area, ranging from 2.5 to 5 cm3.
For postoperative pain, all patients were prescribed acetaminophen 1,000 mg alternating with ibuprofen 400 mg, and tramadol, with instructions to only use tramadol as needed for breakthrough pain.
The reported use of narcotic analgesics by participants was significantly higher among those receiving placebo versus bupivacaine in the first 24 hours following surgery (odds ratio, 2.18; P = .03), as well as in the second 24 hours (OR, 2.18; P = .08) and at 48 hours combined (OR, 2.58; P < .01).
Those in the bupivacaine group also reported lower average pain scores, on a scale of 0-10, during the first 8-hour interval (mean difference, 1.6; P < .001). Importantly, overall, reports of pain medication use and the percentage of patients reporting pain under control were similar between groups, despite lower opioid use in the bupivacaine group.
“The percentage of patients reporting their pain to be under control was similar at all time intervals in both groups, so this means the bupivacaine group had their pain well-controlled despite fewer narcotics, with significant reductions in opioid use,” Dr. Voss noted.
Bupivacaine, though generally regarded as safe, has a reputation for being the most cardiotoxic of the local anesthetic agents; however, there were no such side effects reported in the study. Dr. Voss said the likely explanation is the use of low doses.
“In our study, we had no cardiotoxic effects when using up to 5 cc of 0.5%, which equates to 25 mg per patient,” she explained. This is considered a “very low dose,” since the maximum in the Food and Drug Administration pamphlet for local infiltration is 175 mg per patient every 3 hours, “yet is sufficient for reducing pain/narcotic use.”
She added that “surgeons must be careful to avoid accidental intravascular injection, which could increase risks of systemic toxicity, but this is very rare in the reconstruction settings described.”
Overall, the study suggests a potentially beneficial and unique nonopioid approach that is currently lacking for Mohs procedures associated with a high level of pain. “These findings offer a very effective intervention to reduce postoperative opioid use in this subset of patients,” Dr. Voss told this news organization. “There is not any other intervention that I am aware of to address this, although further study into other long-acting anesthetics may demonstrate similar effects.”
Commenting on the study, Justin J. Leitenberger, MD, session moderator, said that these “data could be impactful for reducing pain as well as the need for opioid medication after dermatologic surgery, both of which would be significant for our patients and public health outcomes.”
Among the challenges in treating pain following Mohs surgeries is that “every patient has a different pain threshold and expectation after surgery,” said Dr. Leitenberger, assistant professor of medicine and dermatology and codirector of dermatologic surgery, Mohs micrographic surgery, and laser and cosmetic surgery at Oregon Health & Science University, Portland.
“Patients undergoing larger repairs in tense areas of skin can experience increased pain and require prescription pain medication,” he said. “Bupivacaine, in this study, shows promise to provide longer lasting pain control from the surgical appointment and easier bridging to nonopioid pain control.”
Regarding the potential cardiotoxicities associated with the drug, Dr. Leitenberger agreed that the risks are low, and added that many surgeons have, in fact, switched to full use of bupivacaine, as opposed to combination with lidocaine, apparently without problems. “This is a small dose locally to the area after a procedure and I agree that the risks are minuscule,” he said.
“Of note, during national lidocaine shortages over the past few years, many practices transitioned to exclusive use of bupivacaine for the entire Mohs procedure, and [anecdotally], this transition did not result in toxicities that were reported,” Dr. Leitenberger said.
Commenting further, Vishal Patel, MD, assistant professor of dermatology and hematology/oncology at George Washington University and director of cutaneous oncology at the GW Cancer Center, both in Washington, also agreed that the benefits appear important. “The benefit from using bupivacaine is encouraging on multiple levels,” he said in an interview.
“Given all that we know about opioids and their negative side effect profile as well as their limited help in cutaneous surgery pain control, the use of long-acting anesthetics is an innovative and reasonable approach to provide pain control in the immediate postoperative window when patients tend to have the most pain,” said Dr. Patel, who is also director of dermatologic surgery at George Washington University.
“After this window, acetaminophen and ibuprofen, which have been shown when used in tandem in an alternating schedule to be superior to opioids, provides an effective pain regimen,” he said. “For larger and more pain-sensitive patients, this appears to be a promising combination.”
Dr. Voss, Dr. Leitenberger, and Dr. Patel have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An injection of a randomized trial shows.
“Single-dose, in-office bupivacaine administration immediately following reconstructions known to be high risk for pain reduces postoperative narcotic use and acute pain during the time period when our patients have the highest levels of pain,” said first author Vanessa B. Voss, MD, of the University of Missouri–Columbia, who presented the findings at the annual meeting of the American College of Mohs Surgery.
“It was well tolerated, there were no adverse effects, and we recommend the consideration of using this in Mohs micrographic surgery reconstructions that are at the highest risk for pain,” she said.
Recent research has shown that Mohs micrographic surgeons have the highest rates of opioid prescribing of all dermatologists, with about 11% of patients undergoing a Mohs procedure prescribed the drugs for postoperative use, Dr. Voss explained.
Yet, with the ongoing opioid epidemic and even short courses of postoperative opioids placing patients at risk for addiction, the pressure is on to find alternative, nonaddictive strategies for the treatment of acute postoperative pain.
Bupivacaine is commonly used intraoperatively with other types of surgeries to reduce postoperative pain, with a favorable duration of action lasting up to 7 hours, compared with just 2-3 hours with lidocaine. And while its use in Mohs surgery is typically also intraoperative, along with lidocaine, the unique postoperative treatment approach in Mohs surgery has not been well studied, Dr. Voss noted.
To investigate, Dr. Voss and colleagues conducted the prospective, multicenter randomized trial, enrolling 174 patients undergoing Mohs micrographic surgery for skin cancer.
Patients were receiving complex flap reconstructions that have been specifically designated in an American Academy of Dermatology position statement to be high risk for pain following Mohs surgeries, and hence, more likely to involve prescriptions for opioids. These include reconstruction flaps of the scalp, ear, nose or lip, a wedge repair of the ear or lip, or a Mustarde cheek rotation flap.
The mean age of the patients was about 69 years, and about 65% were male. The two groups had no significant differences in demographics, tumor types, or repairs. They were randomized to receive either local injections of bupivacaine 0.5% (with no epinephrine) or placebo with sterile saline injection immediately following the procedure, with the total amount of injection standardized and dependent upon the flap surface area, ranging from 2.5 to 5 cm3.
For postoperative pain, all patients were prescribed acetaminophen 1,000 mg alternating with ibuprofen 400 mg, and tramadol, with instructions to only use tramadol as needed for breakthrough pain.
The reported use of narcotic analgesics by participants was significantly higher among those receiving placebo versus bupivacaine in the first 24 hours following surgery (odds ratio, 2.18; P = .03), as well as in the second 24 hours (OR, 2.18; P = .08) and at 48 hours combined (OR, 2.58; P < .01).
Those in the bupivacaine group also reported lower average pain scores, on a scale of 0-10, during the first 8-hour interval (mean difference, 1.6; P < .001). Importantly, overall, reports of pain medication use and the percentage of patients reporting pain under control were similar between groups, despite lower opioid use in the bupivacaine group.
“The percentage of patients reporting their pain to be under control was similar at all time intervals in both groups, so this means the bupivacaine group had their pain well-controlled despite fewer narcotics, with significant reductions in opioid use,” Dr. Voss noted.
Bupivacaine, though generally regarded as safe, has a reputation for being the most cardiotoxic of the local anesthetic agents; however, there were no such side effects reported in the study. Dr. Voss said the likely explanation is the use of low doses.
“In our study, we had no cardiotoxic effects when using up to 5 cc of 0.5%, which equates to 25 mg per patient,” she explained. This is considered a “very low dose,” since the maximum in the Food and Drug Administration pamphlet for local infiltration is 175 mg per patient every 3 hours, “yet is sufficient for reducing pain/narcotic use.”
She added that “surgeons must be careful to avoid accidental intravascular injection, which could increase risks of systemic toxicity, but this is very rare in the reconstruction settings described.”
Overall, the study suggests a potentially beneficial and unique nonopioid approach that is currently lacking for Mohs procedures associated with a high level of pain. “These findings offer a very effective intervention to reduce postoperative opioid use in this subset of patients,” Dr. Voss told this news organization. “There is not any other intervention that I am aware of to address this, although further study into other long-acting anesthetics may demonstrate similar effects.”
Commenting on the study, Justin J. Leitenberger, MD, session moderator, said that these “data could be impactful for reducing pain as well as the need for opioid medication after dermatologic surgery, both of which would be significant for our patients and public health outcomes.”
Among the challenges in treating pain following Mohs surgeries is that “every patient has a different pain threshold and expectation after surgery,” said Dr. Leitenberger, assistant professor of medicine and dermatology and codirector of dermatologic surgery, Mohs micrographic surgery, and laser and cosmetic surgery at Oregon Health & Science University, Portland.
“Patients undergoing larger repairs in tense areas of skin can experience increased pain and require prescription pain medication,” he said. “Bupivacaine, in this study, shows promise to provide longer lasting pain control from the surgical appointment and easier bridging to nonopioid pain control.”
Regarding the potential cardiotoxicities associated with the drug, Dr. Leitenberger agreed that the risks are low, and added that many surgeons have, in fact, switched to full use of bupivacaine, as opposed to combination with lidocaine, apparently without problems. “This is a small dose locally to the area after a procedure and I agree that the risks are minuscule,” he said.
“Of note, during national lidocaine shortages over the past few years, many practices transitioned to exclusive use of bupivacaine for the entire Mohs procedure, and [anecdotally], this transition did not result in toxicities that were reported,” Dr. Leitenberger said.
Commenting further, Vishal Patel, MD, assistant professor of dermatology and hematology/oncology at George Washington University and director of cutaneous oncology at the GW Cancer Center, both in Washington, also agreed that the benefits appear important. “The benefit from using bupivacaine is encouraging on multiple levels,” he said in an interview.
“Given all that we know about opioids and their negative side effect profile as well as their limited help in cutaneous surgery pain control, the use of long-acting anesthetics is an innovative and reasonable approach to provide pain control in the immediate postoperative window when patients tend to have the most pain,” said Dr. Patel, who is also director of dermatologic surgery at George Washington University.
“After this window, acetaminophen and ibuprofen, which have been shown when used in tandem in an alternating schedule to be superior to opioids, provides an effective pain regimen,” he said. “For larger and more pain-sensitive patients, this appears to be a promising combination.”
Dr. Voss, Dr. Leitenberger, and Dr. Patel have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An injection of a randomized trial shows.
“Single-dose, in-office bupivacaine administration immediately following reconstructions known to be high risk for pain reduces postoperative narcotic use and acute pain during the time period when our patients have the highest levels of pain,” said first author Vanessa B. Voss, MD, of the University of Missouri–Columbia, who presented the findings at the annual meeting of the American College of Mohs Surgery.
“It was well tolerated, there were no adverse effects, and we recommend the consideration of using this in Mohs micrographic surgery reconstructions that are at the highest risk for pain,” she said.
Recent research has shown that Mohs micrographic surgeons have the highest rates of opioid prescribing of all dermatologists, with about 11% of patients undergoing a Mohs procedure prescribed the drugs for postoperative use, Dr. Voss explained.
Yet, with the ongoing opioid epidemic and even short courses of postoperative opioids placing patients at risk for addiction, the pressure is on to find alternative, nonaddictive strategies for the treatment of acute postoperative pain.
Bupivacaine is commonly used intraoperatively with other types of surgeries to reduce postoperative pain, with a favorable duration of action lasting up to 7 hours, compared with just 2-3 hours with lidocaine. And while its use in Mohs surgery is typically also intraoperative, along with lidocaine, the unique postoperative treatment approach in Mohs surgery has not been well studied, Dr. Voss noted.
To investigate, Dr. Voss and colleagues conducted the prospective, multicenter randomized trial, enrolling 174 patients undergoing Mohs micrographic surgery for skin cancer.
Patients were receiving complex flap reconstructions that have been specifically designated in an American Academy of Dermatology position statement to be high risk for pain following Mohs surgeries, and hence, more likely to involve prescriptions for opioids. These include reconstruction flaps of the scalp, ear, nose or lip, a wedge repair of the ear or lip, or a Mustarde cheek rotation flap.
The mean age of the patients was about 69 years, and about 65% were male. The two groups had no significant differences in demographics, tumor types, or repairs. They were randomized to receive either local injections of bupivacaine 0.5% (with no epinephrine) or placebo with sterile saline injection immediately following the procedure, with the total amount of injection standardized and dependent upon the flap surface area, ranging from 2.5 to 5 cm3.
For postoperative pain, all patients were prescribed acetaminophen 1,000 mg alternating with ibuprofen 400 mg, and tramadol, with instructions to only use tramadol as needed for breakthrough pain.
The reported use of narcotic analgesics by participants was significantly higher among those receiving placebo versus bupivacaine in the first 24 hours following surgery (odds ratio, 2.18; P = .03), as well as in the second 24 hours (OR, 2.18; P = .08) and at 48 hours combined (OR, 2.58; P < .01).
Those in the bupivacaine group also reported lower average pain scores, on a scale of 0-10, during the first 8-hour interval (mean difference, 1.6; P < .001). Importantly, overall, reports of pain medication use and the percentage of patients reporting pain under control were similar between groups, despite lower opioid use in the bupivacaine group.
“The percentage of patients reporting their pain to be under control was similar at all time intervals in both groups, so this means the bupivacaine group had their pain well-controlled despite fewer narcotics, with significant reductions in opioid use,” Dr. Voss noted.
Bupivacaine, though generally regarded as safe, has a reputation for being the most cardiotoxic of the local anesthetic agents; however, there were no such side effects reported in the study. Dr. Voss said the likely explanation is the use of low doses.
“In our study, we had no cardiotoxic effects when using up to 5 cc of 0.5%, which equates to 25 mg per patient,” she explained. This is considered a “very low dose,” since the maximum in the Food and Drug Administration pamphlet for local infiltration is 175 mg per patient every 3 hours, “yet is sufficient for reducing pain/narcotic use.”
She added that “surgeons must be careful to avoid accidental intravascular injection, which could increase risks of systemic toxicity, but this is very rare in the reconstruction settings described.”
Overall, the study suggests a potentially beneficial and unique nonopioid approach that is currently lacking for Mohs procedures associated with a high level of pain. “These findings offer a very effective intervention to reduce postoperative opioid use in this subset of patients,” Dr. Voss told this news organization. “There is not any other intervention that I am aware of to address this, although further study into other long-acting anesthetics may demonstrate similar effects.”
Commenting on the study, Justin J. Leitenberger, MD, session moderator, said that these “data could be impactful for reducing pain as well as the need for opioid medication after dermatologic surgery, both of which would be significant for our patients and public health outcomes.”
Among the challenges in treating pain following Mohs surgeries is that “every patient has a different pain threshold and expectation after surgery,” said Dr. Leitenberger, assistant professor of medicine and dermatology and codirector of dermatologic surgery, Mohs micrographic surgery, and laser and cosmetic surgery at Oregon Health & Science University, Portland.
“Patients undergoing larger repairs in tense areas of skin can experience increased pain and require prescription pain medication,” he said. “Bupivacaine, in this study, shows promise to provide longer lasting pain control from the surgical appointment and easier bridging to nonopioid pain control.”
Regarding the potential cardiotoxicities associated with the drug, Dr. Leitenberger agreed that the risks are low, and added that many surgeons have, in fact, switched to full use of bupivacaine, as opposed to combination with lidocaine, apparently without problems. “This is a small dose locally to the area after a procedure and I agree that the risks are minuscule,” he said.
“Of note, during national lidocaine shortages over the past few years, many practices transitioned to exclusive use of bupivacaine for the entire Mohs procedure, and [anecdotally], this transition did not result in toxicities that were reported,” Dr. Leitenberger said.
Commenting further, Vishal Patel, MD, assistant professor of dermatology and hematology/oncology at George Washington University and director of cutaneous oncology at the GW Cancer Center, both in Washington, also agreed that the benefits appear important. “The benefit from using bupivacaine is encouraging on multiple levels,” he said in an interview.
“Given all that we know about opioids and their negative side effect profile as well as their limited help in cutaneous surgery pain control, the use of long-acting anesthetics is an innovative and reasonable approach to provide pain control in the immediate postoperative window when patients tend to have the most pain,” said Dr. Patel, who is also director of dermatologic surgery at George Washington University.
“After this window, acetaminophen and ibuprofen, which have been shown when used in tandem in an alternating schedule to be superior to opioids, provides an effective pain regimen,” he said. “For larger and more pain-sensitive patients, this appears to be a promising combination.”
Dr. Voss, Dr. Leitenberger, and Dr. Patel have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACMS 2022
Keeping thyroid hormone treatment on target is key for the heart
A new study highlights the importance of avoiding both exogenous hyperthyroidism and exogenous hypothyroidism to decrease cardiovascular risk and death among patients receiving thyroid hormone treatment.
“Our findings suggest that clinicians should make every effort to maintain euthyroidism in patients on thyroid hormone treatment, regardless of underlying cardiovascular risk, particularly in vulnerable populations, such as older adults,” senior author Maria Papaleontiou, MD, said in an interview.
Commenting on the study, David S. Cooper, MD, of Johns Hopkins University School of Medicine, Baltimore, agreed that the findings are significant.
“Both undertreatment and overtreatment were associated with adverse cardiovascular outcomes, meaning that patients’ thyroid function needs to be monitored, and levothyroxine adjusted if need be, on an ongoing basis,” he told this news organization.
Getting the balance right: a tricky task
Variations in thyroid hormone levels falling above or below target ranges are common with thyroid hormone therapy, as a wide array of factors can prompt the need to regularly adjust dosing to maintain “index” levels. And while guidelines from the American Thyroid Association (ATA) recommend maintaining serum thyroid stimulating hormone (TSH) levels in the normal ranges during treatment, the task is tricky.
“Despite these [ATA] guidelines, prior studies in adults with hypothyroidism have shown that up to 30% are undertreated and up to 48% are overtreated,” said Dr. Papaleontiou, an assistant professor in the Division of Metabolism, Endocrinology at the University of Michigan, Ann Arbor.
In a previous study, Dr. Papaleontiou and colleagues showed that the intensity of thyroid hormone treatment is a modifiable risk factor for incident atrial fibrillation and stroke, however, less is understood about the association with cardiovascular mortality.
For the new study, published in JAMA Network Open, Josh M. Evron, MD, of the University of North Carolina, Chapel Hill, and colleagues further investigated the issue in a large, retrospective cohort of 705,307 adults in the Veterans Health Administration Corporate Data Warehouse treated with thyroid hormone during 2004-2017 who had a median follow-up of 4 years.
They investigated the roles of TSH as well as free thyroxine (FT4) levels among 701,929 adults in the group with data on TSH and 373,981 patients with FT4 measurements.
The mean age of participants was 67 years and 88.7% were male.
Over the course of the study, 10.8% of patients (75,963) died of cardiovascular causes.
Compared with patients with normal thyroid levels, those with exogenous hyperthyroidism related to thyroid hormone treatment had an increased risk of cardiovascular mortality, specifically including when TSH levels were below 0.1 mIU/L (adjusted hazard ratio, 1.39) and when FT4 levels were above 1.9 ng/dL (AHR, 1.29), independent of factors including age, sex, and traditional cardiovascular risk factors, including hypertension, smoking, and previous cardiovascular disease or arrhythmia.
In addition, the increased risk of cardiovascular mortality was observed with exogenous hypothyroidism, specifically among those with TSH levels above 20 mIU/L (AHR, 2.67) and FT4 levels below 0.7 ng/dL (AHR, 1.56), after multivariate adjustment.
Of note, the risk of cardiovascular mortality was dose-dependent, with the risk increasing progressively with the lower and higher TSH levels, compared with normal levels.
The increased mortality risk in relation to TSH levels was more pronounced among older patients, compared with FT4 associations, the authors note.
“From a clinical perspective, older adults, and particularly the oldest old (aged 85 years), appear to be the most vulnerable, with increased risk of cardiovascular mortality with both exogenous hyperthyroidism and hypothyroidism,” they report.
Among key limitations is that women, who make up the majority of patients with thyroid disease, are under-represented in the predominantly male population of the Veterans Health Administration.
Nevertheless, “because the risk of cardiovascular disease is higher for men than for women, and because more than 70,000 women were included in this cohort, the results of this study are highly clinically relevant,” the authors note.
Addressing over- and under-treatment will avoid harm
The results are also important considering the status of levothyroxine (for hypothyroidism) as consistently ranking among the top three prescription medications in the United States.
And with the common occurrence of exogenous hyperthyroidism or hypothyroidism, the findings have important implications.
“Addressing over- and under-treatment of hypothyroidism promptly will help reduce patient harm, particularly in vulnerable populations such as older adults who are at higher risk for adverse effects,” Dr. Papaleontiou said.
Dr. Cooper further commented that the findings underscore the need to be aware of treatment adjustments and targets that may vary according to patient age.
“In older persons, over 65-70, the target TSH may be higher [for example, 2-4 mIU/L] than in younger persons, and in patients above ages 70 or 80, serum TSH levels may be allowed to rise even further into the 4-6 mIU/L range,” he explained.
“The older the patient, the higher the chance for an adverse cardiovascular outcome if the TSH is subnormal due to iatrogenic thyrotoxicosis,” Dr. Cooper explained.
“In contrast, in younger individuals, an elevated TSH, indicating mild [subclinical] hypothyroidism may be associated with increased cardiovascular risk, especially with serum TSH levels greater than 7 mIU/L.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study highlights the importance of avoiding both exogenous hyperthyroidism and exogenous hypothyroidism to decrease cardiovascular risk and death among patients receiving thyroid hormone treatment.
“Our findings suggest that clinicians should make every effort to maintain euthyroidism in patients on thyroid hormone treatment, regardless of underlying cardiovascular risk, particularly in vulnerable populations, such as older adults,” senior author Maria Papaleontiou, MD, said in an interview.
Commenting on the study, David S. Cooper, MD, of Johns Hopkins University School of Medicine, Baltimore, agreed that the findings are significant.
“Both undertreatment and overtreatment were associated with adverse cardiovascular outcomes, meaning that patients’ thyroid function needs to be monitored, and levothyroxine adjusted if need be, on an ongoing basis,” he told this news organization.
Getting the balance right: a tricky task
Variations in thyroid hormone levels falling above or below target ranges are common with thyroid hormone therapy, as a wide array of factors can prompt the need to regularly adjust dosing to maintain “index” levels. And while guidelines from the American Thyroid Association (ATA) recommend maintaining serum thyroid stimulating hormone (TSH) levels in the normal ranges during treatment, the task is tricky.
“Despite these [ATA] guidelines, prior studies in adults with hypothyroidism have shown that up to 30% are undertreated and up to 48% are overtreated,” said Dr. Papaleontiou, an assistant professor in the Division of Metabolism, Endocrinology at the University of Michigan, Ann Arbor.
In a previous study, Dr. Papaleontiou and colleagues showed that the intensity of thyroid hormone treatment is a modifiable risk factor for incident atrial fibrillation and stroke, however, less is understood about the association with cardiovascular mortality.
For the new study, published in JAMA Network Open, Josh M. Evron, MD, of the University of North Carolina, Chapel Hill, and colleagues further investigated the issue in a large, retrospective cohort of 705,307 adults in the Veterans Health Administration Corporate Data Warehouse treated with thyroid hormone during 2004-2017 who had a median follow-up of 4 years.
They investigated the roles of TSH as well as free thyroxine (FT4) levels among 701,929 adults in the group with data on TSH and 373,981 patients with FT4 measurements.
The mean age of participants was 67 years and 88.7% were male.
Over the course of the study, 10.8% of patients (75,963) died of cardiovascular causes.
Compared with patients with normal thyroid levels, those with exogenous hyperthyroidism related to thyroid hormone treatment had an increased risk of cardiovascular mortality, specifically including when TSH levels were below 0.1 mIU/L (adjusted hazard ratio, 1.39) and when FT4 levels were above 1.9 ng/dL (AHR, 1.29), independent of factors including age, sex, and traditional cardiovascular risk factors, including hypertension, smoking, and previous cardiovascular disease or arrhythmia.
In addition, the increased risk of cardiovascular mortality was observed with exogenous hypothyroidism, specifically among those with TSH levels above 20 mIU/L (AHR, 2.67) and FT4 levels below 0.7 ng/dL (AHR, 1.56), after multivariate adjustment.
Of note, the risk of cardiovascular mortality was dose-dependent, with the risk increasing progressively with the lower and higher TSH levels, compared with normal levels.
The increased mortality risk in relation to TSH levels was more pronounced among older patients, compared with FT4 associations, the authors note.
“From a clinical perspective, older adults, and particularly the oldest old (aged 85 years), appear to be the most vulnerable, with increased risk of cardiovascular mortality with both exogenous hyperthyroidism and hypothyroidism,” they report.
Among key limitations is that women, who make up the majority of patients with thyroid disease, are under-represented in the predominantly male population of the Veterans Health Administration.
Nevertheless, “because the risk of cardiovascular disease is higher for men than for women, and because more than 70,000 women were included in this cohort, the results of this study are highly clinically relevant,” the authors note.
Addressing over- and under-treatment will avoid harm
The results are also important considering the status of levothyroxine (for hypothyroidism) as consistently ranking among the top three prescription medications in the United States.
And with the common occurrence of exogenous hyperthyroidism or hypothyroidism, the findings have important implications.
“Addressing over- and under-treatment of hypothyroidism promptly will help reduce patient harm, particularly in vulnerable populations such as older adults who are at higher risk for adverse effects,” Dr. Papaleontiou said.
Dr. Cooper further commented that the findings underscore the need to be aware of treatment adjustments and targets that may vary according to patient age.
“In older persons, over 65-70, the target TSH may be higher [for example, 2-4 mIU/L] than in younger persons, and in patients above ages 70 or 80, serum TSH levels may be allowed to rise even further into the 4-6 mIU/L range,” he explained.
“The older the patient, the higher the chance for an adverse cardiovascular outcome if the TSH is subnormal due to iatrogenic thyrotoxicosis,” Dr. Cooper explained.
“In contrast, in younger individuals, an elevated TSH, indicating mild [subclinical] hypothyroidism may be associated with increased cardiovascular risk, especially with serum TSH levels greater than 7 mIU/L.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study highlights the importance of avoiding both exogenous hyperthyroidism and exogenous hypothyroidism to decrease cardiovascular risk and death among patients receiving thyroid hormone treatment.
“Our findings suggest that clinicians should make every effort to maintain euthyroidism in patients on thyroid hormone treatment, regardless of underlying cardiovascular risk, particularly in vulnerable populations, such as older adults,” senior author Maria Papaleontiou, MD, said in an interview.
Commenting on the study, David S. Cooper, MD, of Johns Hopkins University School of Medicine, Baltimore, agreed that the findings are significant.
“Both undertreatment and overtreatment were associated with adverse cardiovascular outcomes, meaning that patients’ thyroid function needs to be monitored, and levothyroxine adjusted if need be, on an ongoing basis,” he told this news organization.
Getting the balance right: a tricky task
Variations in thyroid hormone levels falling above or below target ranges are common with thyroid hormone therapy, as a wide array of factors can prompt the need to regularly adjust dosing to maintain “index” levels. And while guidelines from the American Thyroid Association (ATA) recommend maintaining serum thyroid stimulating hormone (TSH) levels in the normal ranges during treatment, the task is tricky.
“Despite these [ATA] guidelines, prior studies in adults with hypothyroidism have shown that up to 30% are undertreated and up to 48% are overtreated,” said Dr. Papaleontiou, an assistant professor in the Division of Metabolism, Endocrinology at the University of Michigan, Ann Arbor.
In a previous study, Dr. Papaleontiou and colleagues showed that the intensity of thyroid hormone treatment is a modifiable risk factor for incident atrial fibrillation and stroke, however, less is understood about the association with cardiovascular mortality.
For the new study, published in JAMA Network Open, Josh M. Evron, MD, of the University of North Carolina, Chapel Hill, and colleagues further investigated the issue in a large, retrospective cohort of 705,307 adults in the Veterans Health Administration Corporate Data Warehouse treated with thyroid hormone during 2004-2017 who had a median follow-up of 4 years.
They investigated the roles of TSH as well as free thyroxine (FT4) levels among 701,929 adults in the group with data on TSH and 373,981 patients with FT4 measurements.
The mean age of participants was 67 years and 88.7% were male.
Over the course of the study, 10.8% of patients (75,963) died of cardiovascular causes.
Compared with patients with normal thyroid levels, those with exogenous hyperthyroidism related to thyroid hormone treatment had an increased risk of cardiovascular mortality, specifically including when TSH levels were below 0.1 mIU/L (adjusted hazard ratio, 1.39) and when FT4 levels were above 1.9 ng/dL (AHR, 1.29), independent of factors including age, sex, and traditional cardiovascular risk factors, including hypertension, smoking, and previous cardiovascular disease or arrhythmia.
In addition, the increased risk of cardiovascular mortality was observed with exogenous hypothyroidism, specifically among those with TSH levels above 20 mIU/L (AHR, 2.67) and FT4 levels below 0.7 ng/dL (AHR, 1.56), after multivariate adjustment.
Of note, the risk of cardiovascular mortality was dose-dependent, with the risk increasing progressively with the lower and higher TSH levels, compared with normal levels.
The increased mortality risk in relation to TSH levels was more pronounced among older patients, compared with FT4 associations, the authors note.
“From a clinical perspective, older adults, and particularly the oldest old (aged 85 years), appear to be the most vulnerable, with increased risk of cardiovascular mortality with both exogenous hyperthyroidism and hypothyroidism,” they report.
Among key limitations is that women, who make up the majority of patients with thyroid disease, are under-represented in the predominantly male population of the Veterans Health Administration.
Nevertheless, “because the risk of cardiovascular disease is higher for men than for women, and because more than 70,000 women were included in this cohort, the results of this study are highly clinically relevant,” the authors note.
Addressing over- and under-treatment will avoid harm
The results are also important considering the status of levothyroxine (for hypothyroidism) as consistently ranking among the top three prescription medications in the United States.
And with the common occurrence of exogenous hyperthyroidism or hypothyroidism, the findings have important implications.
“Addressing over- and under-treatment of hypothyroidism promptly will help reduce patient harm, particularly in vulnerable populations such as older adults who are at higher risk for adverse effects,” Dr. Papaleontiou said.
Dr. Cooper further commented that the findings underscore the need to be aware of treatment adjustments and targets that may vary according to patient age.
“In older persons, over 65-70, the target TSH may be higher [for example, 2-4 mIU/L] than in younger persons, and in patients above ages 70 or 80, serum TSH levels may be allowed to rise even further into the 4-6 mIU/L range,” he explained.
“The older the patient, the higher the chance for an adverse cardiovascular outcome if the TSH is subnormal due to iatrogenic thyrotoxicosis,” Dr. Cooper explained.
“In contrast, in younger individuals, an elevated TSH, indicating mild [subclinical] hypothyroidism may be associated with increased cardiovascular risk, especially with serum TSH levels greater than 7 mIU/L.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA authorizes Pfizer’s COVID booster for kids ages 5 to 11
emergency use authorization (EUA), allowing the Pfizer-BioNTech COVID-19 booster shot for children ages 5 to 11 who are at least 5 months out from their first vaccine series.
According to the most recent data from the Centers for Disease Control and Prevention, 28.6% of children in this age group have received both initial doses of Pfizer’s COVID-19 vaccine, and 35.3% have received their first dose.
Pfizer’s vaccine trial involving 4,500 children showed few side effects among children younger than 12 who received a booster, or third dose, according to a company statement.
Pfizer asked the FDA for an amended authorization in April, after submitting data showing that a third dose in children between 5 and 11 raised antibodies targeting the Omicron variant by 36 times.
“While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer-term effects, even following initially mild disease,” FDA Commissioner Robert M. Califf, MD, said in a news release.
A study done by the New York State Department of Health showed the effectiveness of Pfizer’s two-dose vaccine series fell from 68% to 12% 4-5 months after the second dose was given to children 5 to 11 during the Omicron surge. A CDC study published in March also showed that the Pfizer shot reduced the risk of Omicron by 31% in children 5 to 11, a significantly lower rate than for kids 12 to 15, who had a 59% risk reduction after receiving two doses.
To some experts, this data suggest an even greater need for children under 12 to be eligible for a third dose.
“Since authorizing the vaccine for children down to 5 years of age in October 2021, emerging data suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine in all authorized populations,” says Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research.
The CDC still needs to sign off on the shots before they can be allowed. The agency’s Advisory Committee on Immunization Practices is set to meet on May 19 to discuss boosters in this age group.
FDA advisory panels plan to meet next month to discuss allowing Pfizer’s and Moderna’s COVID-19 vaccines for children under 6 years old.
A version of this article first appeared on WebMD.com.
emergency use authorization (EUA), allowing the Pfizer-BioNTech COVID-19 booster shot for children ages 5 to 11 who are at least 5 months out from their first vaccine series.
According to the most recent data from the Centers for Disease Control and Prevention, 28.6% of children in this age group have received both initial doses of Pfizer’s COVID-19 vaccine, and 35.3% have received their first dose.
Pfizer’s vaccine trial involving 4,500 children showed few side effects among children younger than 12 who received a booster, or third dose, according to a company statement.
Pfizer asked the FDA for an amended authorization in April, after submitting data showing that a third dose in children between 5 and 11 raised antibodies targeting the Omicron variant by 36 times.
“While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer-term effects, even following initially mild disease,” FDA Commissioner Robert M. Califf, MD, said in a news release.
A study done by the New York State Department of Health showed the effectiveness of Pfizer’s two-dose vaccine series fell from 68% to 12% 4-5 months after the second dose was given to children 5 to 11 during the Omicron surge. A CDC study published in March also showed that the Pfizer shot reduced the risk of Omicron by 31% in children 5 to 11, a significantly lower rate than for kids 12 to 15, who had a 59% risk reduction after receiving two doses.
To some experts, this data suggest an even greater need for children under 12 to be eligible for a third dose.
“Since authorizing the vaccine for children down to 5 years of age in October 2021, emerging data suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine in all authorized populations,” says Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research.
The CDC still needs to sign off on the shots before they can be allowed. The agency’s Advisory Committee on Immunization Practices is set to meet on May 19 to discuss boosters in this age group.
FDA advisory panels plan to meet next month to discuss allowing Pfizer’s and Moderna’s COVID-19 vaccines for children under 6 years old.
A version of this article first appeared on WebMD.com.
emergency use authorization (EUA), allowing the Pfizer-BioNTech COVID-19 booster shot for children ages 5 to 11 who are at least 5 months out from their first vaccine series.
According to the most recent data from the Centers for Disease Control and Prevention, 28.6% of children in this age group have received both initial doses of Pfizer’s COVID-19 vaccine, and 35.3% have received their first dose.
Pfizer’s vaccine trial involving 4,500 children showed few side effects among children younger than 12 who received a booster, or third dose, according to a company statement.
Pfizer asked the FDA for an amended authorization in April, after submitting data showing that a third dose in children between 5 and 11 raised antibodies targeting the Omicron variant by 36 times.
“While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer-term effects, even following initially mild disease,” FDA Commissioner Robert M. Califf, MD, said in a news release.
A study done by the New York State Department of Health showed the effectiveness of Pfizer’s two-dose vaccine series fell from 68% to 12% 4-5 months after the second dose was given to children 5 to 11 during the Omicron surge. A CDC study published in March also showed that the Pfizer shot reduced the risk of Omicron by 31% in children 5 to 11, a significantly lower rate than for kids 12 to 15, who had a 59% risk reduction after receiving two doses.
To some experts, this data suggest an even greater need for children under 12 to be eligible for a third dose.
“Since authorizing the vaccine for children down to 5 years of age in October 2021, emerging data suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine in all authorized populations,” says Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research.
The CDC still needs to sign off on the shots before they can be allowed. The agency’s Advisory Committee on Immunization Practices is set to meet on May 19 to discuss boosters in this age group.
FDA advisory panels plan to meet next month to discuss allowing Pfizer’s and Moderna’s COVID-19 vaccines for children under 6 years old.
A version of this article first appeared on WebMD.com.
NeoChemo preserves rectum in half of patients with rectal cancer
Among patients with stage II or stage III rectal adenocarcinoma, organ preservation is achievable in up to half of patients who undergo total neoadjuvant chemotherapy (TNT), according to the results from a new randomized phase 2 trial.
The study included 324 patients from 18 centers who were randomized into one of two groups: induction chemotherapy followed by chemoradiotherapy (INCT-CRT) or chemoradiotherapy followed by consolidation chemotherapy (CRT-CNCT). Patients in both groups then underwent either total mesorectal excision (TME) or a watch-and-wait strategy, depending on tumor response.
“What the study shows is that the order of the chemo and the radiation dose doesn’t affect survival, but it seems to affect the probability of preserving the rectum. That data is consistent with other studies that have compared head-to-head chemotherapy followed by radiation versus radiation followed by chemotherapy. In addition, the survival rate for this study is no different from other prospective studies that included patients with similar-stage tumors selected by MRI. So the data suggest that you can probably avoid surgery in half of the patients with locally advanced rectal cancer and still achieve similar survival compared to patients treated with more conventional neoadjuvant treatments and mandatory surgery,” said lead author Julio Garcia-Aguilar, MD, PhD, in an interview.
“It is a significant shift in the treatment paradigm, that can potentially benefit half of the 50,000 rectal cancer patients diagnosed every year in the United States,” said Dr. Garcia-Aguilar, chief of colorectal surgery at Memorial Sloan Kettering Cancer Center, New York.
The study was published online in the Journal of Clinical Oncology.
Neoadjuvant CRT, TME, and adjuvant chemotherapy is an effective treatment strategy for locally advanced rectal adenocarcinoma, but the regimen can cause bowel, urinary, and sexual dysfunction. The majority of adverse effects from the therapy can be traced to surgery. In addition, some patients with distal rectal cancer often require a permanent colostomy.
TNT is a newer approach that delivers chemotherapy plus radiotherapy before surgery. It is designed to improve treatment compliance and eradicate micrometastases in advance of surgery.
After a median follow-up of 3 years, disease-free survival (76% in both groups) was similar to historical controls (75%). Both groups had similar rates of local recurrence-free survival (94% each) and distant metastasis–free survival (84% for INCT-CRT and 82% for CRT-CNCT).
Following TNT, 26% of patients were recommended for TME, including 28% in the INCT-CRT group and 24% in the CRT-CNCT group, and the rest offered watchful-waiting. Forty percent of those in the INCT-CRT group and 27% in the CRT-CNCT group who went on to watchful waiting had tumor regrowth. Of these combined 75 patients, 67 underwent successful salvage surgery.
In the intention-to-treat analysis, 53% of patients had a preserved rectum at 3 years (95% confidence interval, 45%-62%) in the CRT-CNCT group versus 41% in the INCT-CRT group (95% CI, 33%-50%; P = .01).
The new results reinforce other results and should contribute to shifting clinical practice, according to Dr. Garcia-Aguilar. “I think what we have learned is that rectal cancers respond to chemotherapy and radiation at a higher rate that we thought previously, but that the response takes time. That’s something that we use currently in an adaptive way to modify the treatment as we observe the tumor response,” he said.
The slow regrowth means that patients can be closely monitored without undue risk, but such an approach demands buy-in from the patient. “The patient needs to be compliant with a close surveillance protocol, because otherwise it can be a disaster. I think that’s really part of the message,” Dr. Garcia-Aguilar said.
Dr. Garcia-Aguilar has an ownership interest in Intuitive Surgical and has advised or consulted for Medtronic, Intuitive Surgical, and Johnson & Johnson.
Among patients with stage II or stage III rectal adenocarcinoma, organ preservation is achievable in up to half of patients who undergo total neoadjuvant chemotherapy (TNT), according to the results from a new randomized phase 2 trial.
The study included 324 patients from 18 centers who were randomized into one of two groups: induction chemotherapy followed by chemoradiotherapy (INCT-CRT) or chemoradiotherapy followed by consolidation chemotherapy (CRT-CNCT). Patients in both groups then underwent either total mesorectal excision (TME) or a watch-and-wait strategy, depending on tumor response.
“What the study shows is that the order of the chemo and the radiation dose doesn’t affect survival, but it seems to affect the probability of preserving the rectum. That data is consistent with other studies that have compared head-to-head chemotherapy followed by radiation versus radiation followed by chemotherapy. In addition, the survival rate for this study is no different from other prospective studies that included patients with similar-stage tumors selected by MRI. So the data suggest that you can probably avoid surgery in half of the patients with locally advanced rectal cancer and still achieve similar survival compared to patients treated with more conventional neoadjuvant treatments and mandatory surgery,” said lead author Julio Garcia-Aguilar, MD, PhD, in an interview.
“It is a significant shift in the treatment paradigm, that can potentially benefit half of the 50,000 rectal cancer patients diagnosed every year in the United States,” said Dr. Garcia-Aguilar, chief of colorectal surgery at Memorial Sloan Kettering Cancer Center, New York.
The study was published online in the Journal of Clinical Oncology.
Neoadjuvant CRT, TME, and adjuvant chemotherapy is an effective treatment strategy for locally advanced rectal adenocarcinoma, but the regimen can cause bowel, urinary, and sexual dysfunction. The majority of adverse effects from the therapy can be traced to surgery. In addition, some patients with distal rectal cancer often require a permanent colostomy.
TNT is a newer approach that delivers chemotherapy plus radiotherapy before surgery. It is designed to improve treatment compliance and eradicate micrometastases in advance of surgery.
After a median follow-up of 3 years, disease-free survival (76% in both groups) was similar to historical controls (75%). Both groups had similar rates of local recurrence-free survival (94% each) and distant metastasis–free survival (84% for INCT-CRT and 82% for CRT-CNCT).
Following TNT, 26% of patients were recommended for TME, including 28% in the INCT-CRT group and 24% in the CRT-CNCT group, and the rest offered watchful-waiting. Forty percent of those in the INCT-CRT group and 27% in the CRT-CNCT group who went on to watchful waiting had tumor regrowth. Of these combined 75 patients, 67 underwent successful salvage surgery.
In the intention-to-treat analysis, 53% of patients had a preserved rectum at 3 years (95% confidence interval, 45%-62%) in the CRT-CNCT group versus 41% in the INCT-CRT group (95% CI, 33%-50%; P = .01).
The new results reinforce other results and should contribute to shifting clinical practice, according to Dr. Garcia-Aguilar. “I think what we have learned is that rectal cancers respond to chemotherapy and radiation at a higher rate that we thought previously, but that the response takes time. That’s something that we use currently in an adaptive way to modify the treatment as we observe the tumor response,” he said.
The slow regrowth means that patients can be closely monitored without undue risk, but such an approach demands buy-in from the patient. “The patient needs to be compliant with a close surveillance protocol, because otherwise it can be a disaster. I think that’s really part of the message,” Dr. Garcia-Aguilar said.
Dr. Garcia-Aguilar has an ownership interest in Intuitive Surgical and has advised or consulted for Medtronic, Intuitive Surgical, and Johnson & Johnson.
Among patients with stage II or stage III rectal adenocarcinoma, organ preservation is achievable in up to half of patients who undergo total neoadjuvant chemotherapy (TNT), according to the results from a new randomized phase 2 trial.
The study included 324 patients from 18 centers who were randomized into one of two groups: induction chemotherapy followed by chemoradiotherapy (INCT-CRT) or chemoradiotherapy followed by consolidation chemotherapy (CRT-CNCT). Patients in both groups then underwent either total mesorectal excision (TME) or a watch-and-wait strategy, depending on tumor response.
“What the study shows is that the order of the chemo and the radiation dose doesn’t affect survival, but it seems to affect the probability of preserving the rectum. That data is consistent with other studies that have compared head-to-head chemotherapy followed by radiation versus radiation followed by chemotherapy. In addition, the survival rate for this study is no different from other prospective studies that included patients with similar-stage tumors selected by MRI. So the data suggest that you can probably avoid surgery in half of the patients with locally advanced rectal cancer and still achieve similar survival compared to patients treated with more conventional neoadjuvant treatments and mandatory surgery,” said lead author Julio Garcia-Aguilar, MD, PhD, in an interview.
“It is a significant shift in the treatment paradigm, that can potentially benefit half of the 50,000 rectal cancer patients diagnosed every year in the United States,” said Dr. Garcia-Aguilar, chief of colorectal surgery at Memorial Sloan Kettering Cancer Center, New York.
The study was published online in the Journal of Clinical Oncology.
Neoadjuvant CRT, TME, and adjuvant chemotherapy is an effective treatment strategy for locally advanced rectal adenocarcinoma, but the regimen can cause bowel, urinary, and sexual dysfunction. The majority of adverse effects from the therapy can be traced to surgery. In addition, some patients with distal rectal cancer often require a permanent colostomy.
TNT is a newer approach that delivers chemotherapy plus radiotherapy before surgery. It is designed to improve treatment compliance and eradicate micrometastases in advance of surgery.
After a median follow-up of 3 years, disease-free survival (76% in both groups) was similar to historical controls (75%). Both groups had similar rates of local recurrence-free survival (94% each) and distant metastasis–free survival (84% for INCT-CRT and 82% for CRT-CNCT).
Following TNT, 26% of patients were recommended for TME, including 28% in the INCT-CRT group and 24% in the CRT-CNCT group, and the rest offered watchful-waiting. Forty percent of those in the INCT-CRT group and 27% in the CRT-CNCT group who went on to watchful waiting had tumor regrowth. Of these combined 75 patients, 67 underwent successful salvage surgery.
In the intention-to-treat analysis, 53% of patients had a preserved rectum at 3 years (95% confidence interval, 45%-62%) in the CRT-CNCT group versus 41% in the INCT-CRT group (95% CI, 33%-50%; P = .01).
The new results reinforce other results and should contribute to shifting clinical practice, according to Dr. Garcia-Aguilar. “I think what we have learned is that rectal cancers respond to chemotherapy and radiation at a higher rate that we thought previously, but that the response takes time. That’s something that we use currently in an adaptive way to modify the treatment as we observe the tumor response,” he said.
The slow regrowth means that patients can be closely monitored without undue risk, but such an approach demands buy-in from the patient. “The patient needs to be compliant with a close surveillance protocol, because otherwise it can be a disaster. I think that’s really part of the message,” Dr. Garcia-Aguilar said.
Dr. Garcia-Aguilar has an ownership interest in Intuitive Surgical and has advised or consulted for Medtronic, Intuitive Surgical, and Johnson & Johnson.
FROM JOURNAL OF CLINICAL ONCOLOGY






