User login
AI detects ugly-duckling skin lesions for melanoma follow-up
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
Methotrexate-associated hepatotoxicity risk differs between psoriasis, PsA, and RA patients
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
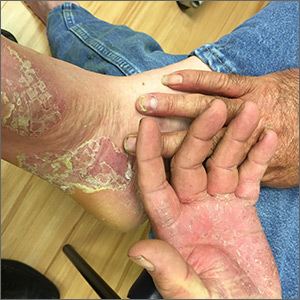
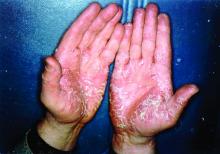
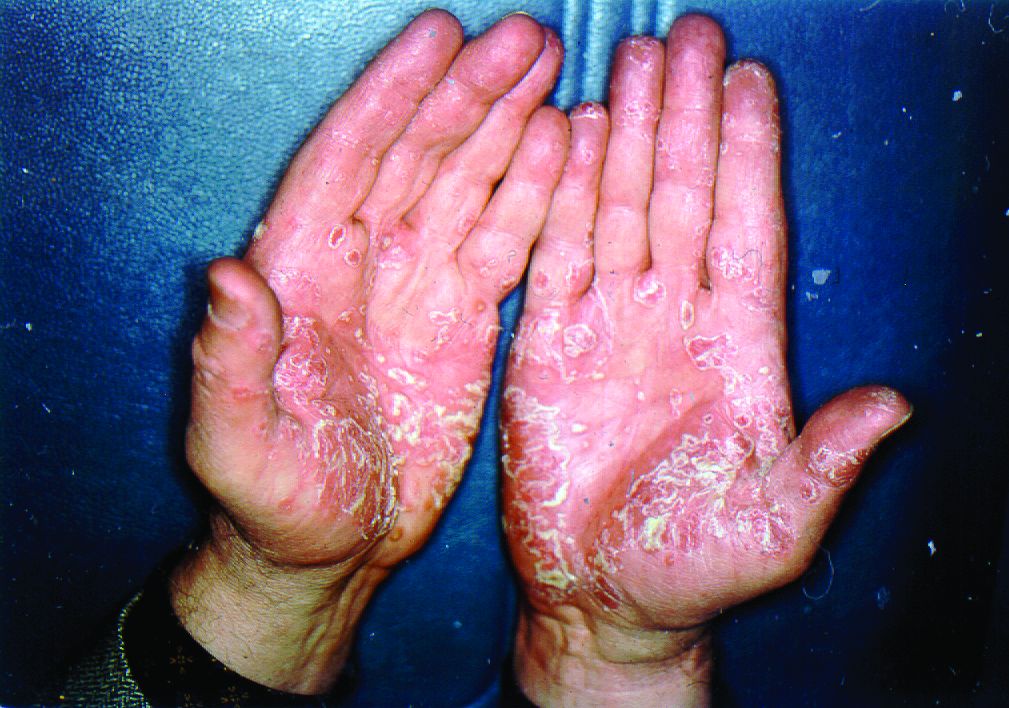
Painful hand and foot plaques
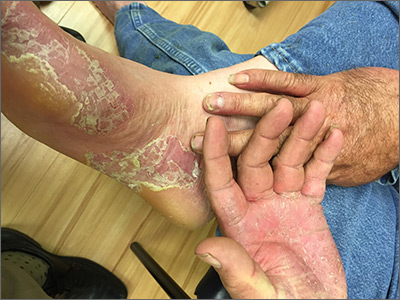
This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.

This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.
Emerging treatments for molluscum contagiosum and acne show promise
, but that could soon change, according to Leon H. Kircik, MD.
“The treatment of molluscum is still an unmet need,” Dr. Kircik, clinical professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said at the Orlando Dermatology Aesthetic and Clinical Conference. However, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been tested in phase 3 studies, is currently under FDA review. The manufacturer, Verrica Pharmaceuticals resubmitted a new drug application for the product, VP-102, in December 2020.
“VP-102 features a visualization agent so the injector can see which lesions have been treated, as well as a bittering agent to mitigate oral ingestion by children. Complete clearance at 12 weeks ranged from 46% to 54% of patients, while lesion count reduction compared with baseline ranged from 69% to 82%.”
Acne
In August, 2020, clascoterone 1% cream was approved for the treatment of acne in patients 12 years and older, a development that Dr. Kircik said “can be a game changer in acne treatment.” Clascoterone cream 1% exhibits strong, selective anti-androgen activity by targeting androgen receptors in the skin, not systemically. “It limits or blocks transcription of androgen responsive genes, but it also has an anti-inflammatory effect and an anti-sebum effect,” he explained.
According to results from two phase 3 trials of the product, a response of clear or almost clear on the IGA scale at week 12 was achieved in 18.4% of those on treatment vs. 9% of those on vehicle in one study (P less than .001) and 20.3% vs. 6.5%, respectively, in the second study (P less than .001). Clascoterone is also being evaluated for treating androgenetic alopecia.
In Dr. Kircik’s clinical experience, retinoids can be helpful for patients with moderate to severe acne. “We always use them for anticomedogenic effects, but we also know that they have anti-inflammatory effects,” he said. “They actually inhibit toll-like receptor activity. They also inhibit the AP-1 pathway by causing a reduction in inflammatory signaling associated with collagen degradation and scarring.”
The most recent retinoid to be approved for the topical treatment of acne was 0.005% trifarotene cream, in 2019, for patients aged 9 years and older. “But when we got the results, it was not that exciting,” a difference of about 3.6 (mean) inflammatory lesion reduction between the active and the vehicle arm, said Dr. Kircik, medical director of Physicians Skin Care in Louisville, Ky. “According to the package insert, treatment side effects included mild to moderate erythema in 59% of patients, scaling in 65%, dryness in 69%, and stinging/burning in 56%, which makes it difficult to use in our clinical practice.”
The drug was also tested for treating truncal acne. However, one comparative study showed that tazarotene 0.045% lotion spread an average of 36.7 square centimeters farther than the trifarotene cream, which makes the tazarotene lotion easier to use on the chest and back, he said.
Dr. Kircik also discussed 4% minocycline, a hydrophobic, topical foam formulation of minocycline that was approved by the FDA in 2019 for the treatment of moderate to severe acne, for patients aged 9 and older. In a 12-week study that involved 1,488 patients (mean age was about 20 years), investigators observed a 56% reduction in inflammatory lesion count among those treated with minocycline 4%, compared with 43% in the vehicle group.
Dr. Kircik, one of the authors of the study, noted that the hydrophobic composition of minocycline 4% allows for stable and efficient delivery of an inherently unstable active pharmaceutical ingredient such as minocycline. “It’s free of primary irritants such as surfactants and short chain alcohols, which makes it much more tolerable,” he said. “The unique physical foam characteristics facilitate ease of application and absorption at target sites.”
Dr. Kircik reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including Galderma, the manufacturer of trifarotene cream.
[email protected]
, but that could soon change, according to Leon H. Kircik, MD.
“The treatment of molluscum is still an unmet need,” Dr. Kircik, clinical professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said at the Orlando Dermatology Aesthetic and Clinical Conference. However, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been tested in phase 3 studies, is currently under FDA review. The manufacturer, Verrica Pharmaceuticals resubmitted a new drug application for the product, VP-102, in December 2020.
“VP-102 features a visualization agent so the injector can see which lesions have been treated, as well as a bittering agent to mitigate oral ingestion by children. Complete clearance at 12 weeks ranged from 46% to 54% of patients, while lesion count reduction compared with baseline ranged from 69% to 82%.”
Acne
In August, 2020, clascoterone 1% cream was approved for the treatment of acne in patients 12 years and older, a development that Dr. Kircik said “can be a game changer in acne treatment.” Clascoterone cream 1% exhibits strong, selective anti-androgen activity by targeting androgen receptors in the skin, not systemically. “It limits or blocks transcription of androgen responsive genes, but it also has an anti-inflammatory effect and an anti-sebum effect,” he explained.
According to results from two phase 3 trials of the product, a response of clear or almost clear on the IGA scale at week 12 was achieved in 18.4% of those on treatment vs. 9% of those on vehicle in one study (P less than .001) and 20.3% vs. 6.5%, respectively, in the second study (P less than .001). Clascoterone is also being evaluated for treating androgenetic alopecia.
In Dr. Kircik’s clinical experience, retinoids can be helpful for patients with moderate to severe acne. “We always use them for anticomedogenic effects, but we also know that they have anti-inflammatory effects,” he said. “They actually inhibit toll-like receptor activity. They also inhibit the AP-1 pathway by causing a reduction in inflammatory signaling associated with collagen degradation and scarring.”
The most recent retinoid to be approved for the topical treatment of acne was 0.005% trifarotene cream, in 2019, for patients aged 9 years and older. “But when we got the results, it was not that exciting,” a difference of about 3.6 (mean) inflammatory lesion reduction between the active and the vehicle arm, said Dr. Kircik, medical director of Physicians Skin Care in Louisville, Ky. “According to the package insert, treatment side effects included mild to moderate erythema in 59% of patients, scaling in 65%, dryness in 69%, and stinging/burning in 56%, which makes it difficult to use in our clinical practice.”
The drug was also tested for treating truncal acne. However, one comparative study showed that tazarotene 0.045% lotion spread an average of 36.7 square centimeters farther than the trifarotene cream, which makes the tazarotene lotion easier to use on the chest and back, he said.
Dr. Kircik also discussed 4% minocycline, a hydrophobic, topical foam formulation of minocycline that was approved by the FDA in 2019 for the treatment of moderate to severe acne, for patients aged 9 and older. In a 12-week study that involved 1,488 patients (mean age was about 20 years), investigators observed a 56% reduction in inflammatory lesion count among those treated with minocycline 4%, compared with 43% in the vehicle group.
Dr. Kircik, one of the authors of the study, noted that the hydrophobic composition of minocycline 4% allows for stable and efficient delivery of an inherently unstable active pharmaceutical ingredient such as minocycline. “It’s free of primary irritants such as surfactants and short chain alcohols, which makes it much more tolerable,” he said. “The unique physical foam characteristics facilitate ease of application and absorption at target sites.”
Dr. Kircik reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including Galderma, the manufacturer of trifarotene cream.
[email protected]
, but that could soon change, according to Leon H. Kircik, MD.
“The treatment of molluscum is still an unmet need,” Dr. Kircik, clinical professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said at the Orlando Dermatology Aesthetic and Clinical Conference. However, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been tested in phase 3 studies, is currently under FDA review. The manufacturer, Verrica Pharmaceuticals resubmitted a new drug application for the product, VP-102, in December 2020.
“VP-102 features a visualization agent so the injector can see which lesions have been treated, as well as a bittering agent to mitigate oral ingestion by children. Complete clearance at 12 weeks ranged from 46% to 54% of patients, while lesion count reduction compared with baseline ranged from 69% to 82%.”
Acne
In August, 2020, clascoterone 1% cream was approved for the treatment of acne in patients 12 years and older, a development that Dr. Kircik said “can be a game changer in acne treatment.” Clascoterone cream 1% exhibits strong, selective anti-androgen activity by targeting androgen receptors in the skin, not systemically. “It limits or blocks transcription of androgen responsive genes, but it also has an anti-inflammatory effect and an anti-sebum effect,” he explained.
According to results from two phase 3 trials of the product, a response of clear or almost clear on the IGA scale at week 12 was achieved in 18.4% of those on treatment vs. 9% of those on vehicle in one study (P less than .001) and 20.3% vs. 6.5%, respectively, in the second study (P less than .001). Clascoterone is also being evaluated for treating androgenetic alopecia.
In Dr. Kircik’s clinical experience, retinoids can be helpful for patients with moderate to severe acne. “We always use them for anticomedogenic effects, but we also know that they have anti-inflammatory effects,” he said. “They actually inhibit toll-like receptor activity. They also inhibit the AP-1 pathway by causing a reduction in inflammatory signaling associated with collagen degradation and scarring.”
The most recent retinoid to be approved for the topical treatment of acne was 0.005% trifarotene cream, in 2019, for patients aged 9 years and older. “But when we got the results, it was not that exciting,” a difference of about 3.6 (mean) inflammatory lesion reduction between the active and the vehicle arm, said Dr. Kircik, medical director of Physicians Skin Care in Louisville, Ky. “According to the package insert, treatment side effects included mild to moderate erythema in 59% of patients, scaling in 65%, dryness in 69%, and stinging/burning in 56%, which makes it difficult to use in our clinical practice.”
The drug was also tested for treating truncal acne. However, one comparative study showed that tazarotene 0.045% lotion spread an average of 36.7 square centimeters farther than the trifarotene cream, which makes the tazarotene lotion easier to use on the chest and back, he said.
Dr. Kircik also discussed 4% minocycline, a hydrophobic, topical foam formulation of minocycline that was approved by the FDA in 2019 for the treatment of moderate to severe acne, for patients aged 9 and older. In a 12-week study that involved 1,488 patients (mean age was about 20 years), investigators observed a 56% reduction in inflammatory lesion count among those treated with minocycline 4%, compared with 43% in the vehicle group.
Dr. Kircik, one of the authors of the study, noted that the hydrophobic composition of minocycline 4% allows for stable and efficient delivery of an inherently unstable active pharmaceutical ingredient such as minocycline. “It’s free of primary irritants such as surfactants and short chain alcohols, which makes it much more tolerable,” he said. “The unique physical foam characteristics facilitate ease of application and absorption at target sites.”
Dr. Kircik reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including Galderma, the manufacturer of trifarotene cream.
[email protected]
FROM ODAC 2021
Cellulitis treatment recommendations
He noticed discomfort today and saw that his left lower leg had redness and was warm. He does not recall scratches or injury to his leg. He has not had fever or chills. He has no other symptoms. His diabetes has been well controlled with diet and metformin.
On exam, his blood pressure is 120/70, pulse is 80, temperature is 37 degrees Celsius.
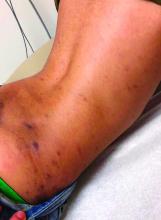
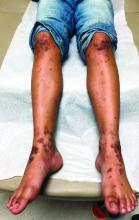
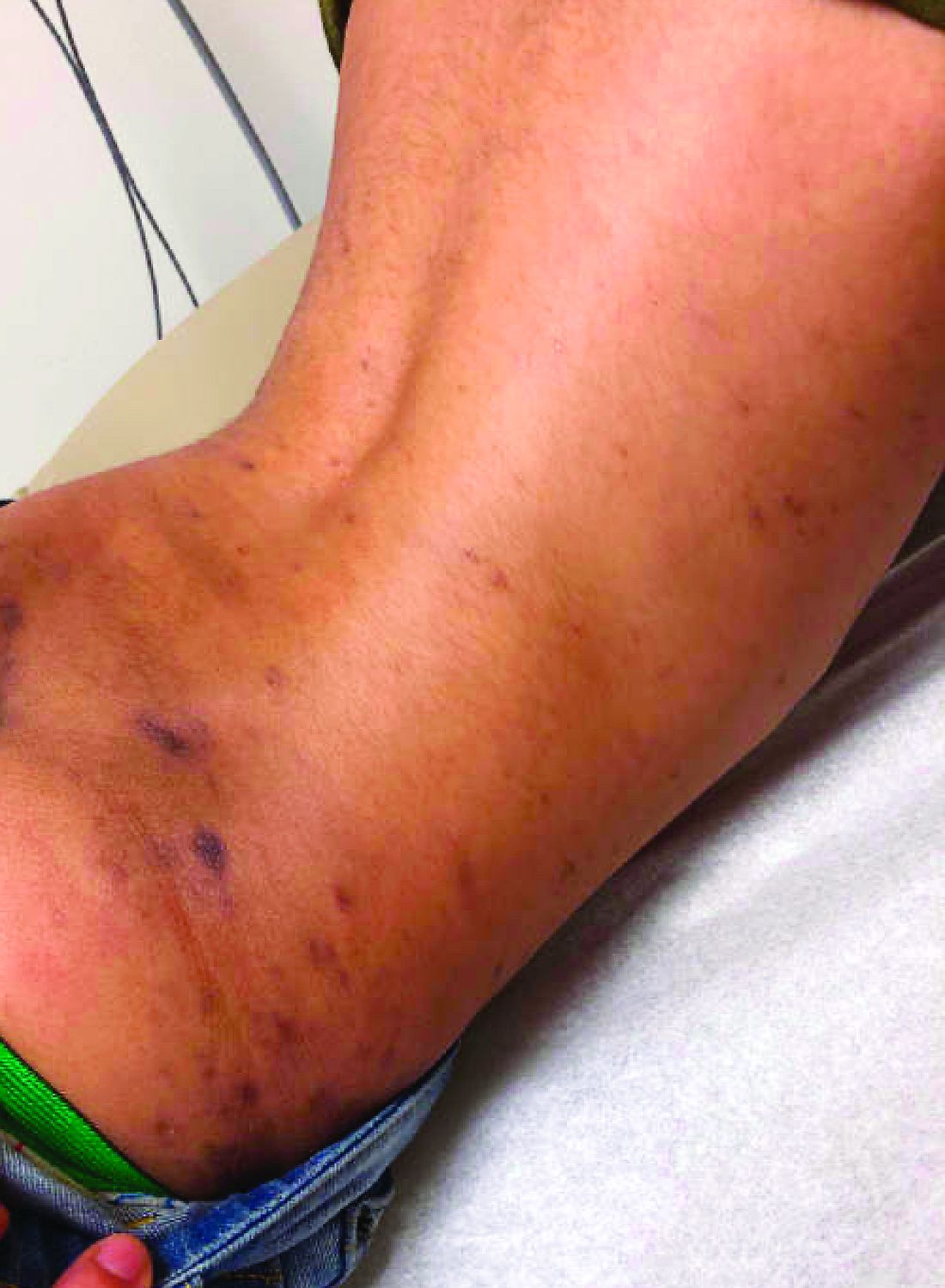
In the left lower extremity, the patient had 1+ edema at the ankle, with a 14-cm x 20-cm warm, erythematous area just above the ankle and extending proximally.
His labs found an HCT of 44 and a WBC of 12,000. What do you recommend?
A) Vascular duplex exam
B) 1st generation cephalosporin
C) 1st generation cephalosporin + TMP/Sulfa
D) Oral clindamycin
E) IV vancomycin
This patient has cellulitis and should receive a beta lactam antibiotic, which will have the best coverage and lowest minimal inhibitory concentration for the likely organism, beta hemolytic streptococci. Clindamycin would likely work, but it has greater side effects. This patient does not need coverage for methicillin-resistant staphylococcus aureus (MRSA). I know many of you, if not most, know this, but I want to go through relevant data and formal recommendations, because of a recent call I received from a patient.
My patient had a full body rash after receiving cephalexin + TMP/sulfa [trimethoprim-sulfamethoxazole] treatment for cellulitis. In recent years the addition of TMP/sulfa to strep treatment to also cover MRSA has become popular, especially in emergency department and urgent care settings.
Moran and colleagues studied cephalexin + TMP/sulfa vs. cephalexin and placebo in patients with uncomplicated cellulitis.1 The outcome measured was clinical cure, and there was no difference between groups; clinical cure occurred in 182 (83.5%) of 218 participants in the cephalexin plus TMP/sulfa group vs. 165 (85.5%) of 193 in the cephalexin group (difference, −2.0%; 95% confidence interval, −9.7% to 5.7%; P = .50).
Jeng and colleagues studied patients admitted for a cellulitis, and evaluated the patients’ response to beta-lactam antibiotics.2 Patients had acute and convalescent serologies for beta hemolytic strep. Almost all evaluable patients with positive strep studies (97%) responded to beta-lactams, and 21 of 23 (91%) with negative studies responded to beta-lactams (overall response rate 95%). This study was done during a time of high MRSA prevalence.
The most recent Infectious Diseases Society of America guidelines for skin and soft tissue infections, recommend oral penicillin, cephalexin, dicloxacillin, or clindamycin for mild cellulitis, and IV equivalent if patients have moderate cellulitis.3 If abscesses are present, then drainage is recommended and MRSA coverage. Kamath and colleagues reported on how closely guidelines for skin and soft tissue infections were followed.4 In patients with mild cellulitis, only 36% received guideline-suggested antibiotics. The most common antibiotic prescribed that was outside the guidelines was trimethoprim-sulfamethoxazole.
Myth: Cellulitis treatment should include MRSA coverage.
My advice: Stick with beta-lactam antibiotics, unless an abscess is present. There is no need to add MRSA coverage for initial treatment of mild to moderate cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Moran GJ et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis: A randomized clinical trial. JAMA 2017 May 23;317(20):2088-96.
2. Jeng Arthur et al. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;July;89(4):217-26.
3. Stevens DL et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52.
4. Kamath RS et al. Guidelines vs. actual management of skin and soft tissue infections in the emergency department. Open Forum Infect Dis. 2018 Jan 12;5(1):ofx188.
He noticed discomfort today and saw that his left lower leg had redness and was warm. He does not recall scratches or injury to his leg. He has not had fever or chills. He has no other symptoms. His diabetes has been well controlled with diet and metformin.
On exam, his blood pressure is 120/70, pulse is 80, temperature is 37 degrees Celsius.
In the left lower extremity, the patient had 1+ edema at the ankle, with a 14-cm x 20-cm warm, erythematous area just above the ankle and extending proximally.
His labs found an HCT of 44 and a WBC of 12,000. What do you recommend?
A) Vascular duplex exam
B) 1st generation cephalosporin
C) 1st generation cephalosporin + TMP/Sulfa
D) Oral clindamycin
E) IV vancomycin
This patient has cellulitis and should receive a beta lactam antibiotic, which will have the best coverage and lowest minimal inhibitory concentration for the likely organism, beta hemolytic streptococci. Clindamycin would likely work, but it has greater side effects. This patient does not need coverage for methicillin-resistant staphylococcus aureus (MRSA). I know many of you, if not most, know this, but I want to go through relevant data and formal recommendations, because of a recent call I received from a patient.
My patient had a full body rash after receiving cephalexin + TMP/sulfa [trimethoprim-sulfamethoxazole] treatment for cellulitis. In recent years the addition of TMP/sulfa to strep treatment to also cover MRSA has become popular, especially in emergency department and urgent care settings.
Moran and colleagues studied cephalexin + TMP/sulfa vs. cephalexin and placebo in patients with uncomplicated cellulitis.1 The outcome measured was clinical cure, and there was no difference between groups; clinical cure occurred in 182 (83.5%) of 218 participants in the cephalexin plus TMP/sulfa group vs. 165 (85.5%) of 193 in the cephalexin group (difference, −2.0%; 95% confidence interval, −9.7% to 5.7%; P = .50).
Jeng and colleagues studied patients admitted for a cellulitis, and evaluated the patients’ response to beta-lactam antibiotics.2 Patients had acute and convalescent serologies for beta hemolytic strep. Almost all evaluable patients with positive strep studies (97%) responded to beta-lactams, and 21 of 23 (91%) with negative studies responded to beta-lactams (overall response rate 95%). This study was done during a time of high MRSA prevalence.
The most recent Infectious Diseases Society of America guidelines for skin and soft tissue infections, recommend oral penicillin, cephalexin, dicloxacillin, or clindamycin for mild cellulitis, and IV equivalent if patients have moderate cellulitis.3 If abscesses are present, then drainage is recommended and MRSA coverage. Kamath and colleagues reported on how closely guidelines for skin and soft tissue infections were followed.4 In patients with mild cellulitis, only 36% received guideline-suggested antibiotics. The most common antibiotic prescribed that was outside the guidelines was trimethoprim-sulfamethoxazole.
Myth: Cellulitis treatment should include MRSA coverage.
My advice: Stick with beta-lactam antibiotics, unless an abscess is present. There is no need to add MRSA coverage for initial treatment of mild to moderate cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Moran GJ et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis: A randomized clinical trial. JAMA 2017 May 23;317(20):2088-96.
2. Jeng Arthur et al. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;July;89(4):217-26.
3. Stevens DL et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52.
4. Kamath RS et al. Guidelines vs. actual management of skin and soft tissue infections in the emergency department. Open Forum Infect Dis. 2018 Jan 12;5(1):ofx188.
He noticed discomfort today and saw that his left lower leg had redness and was warm. He does not recall scratches or injury to his leg. He has not had fever or chills. He has no other symptoms. His diabetes has been well controlled with diet and metformin.
On exam, his blood pressure is 120/70, pulse is 80, temperature is 37 degrees Celsius.
In the left lower extremity, the patient had 1+ edema at the ankle, with a 14-cm x 20-cm warm, erythematous area just above the ankle and extending proximally.
His labs found an HCT of 44 and a WBC of 12,000. What do you recommend?
A) Vascular duplex exam
B) 1st generation cephalosporin
C) 1st generation cephalosporin + TMP/Sulfa
D) Oral clindamycin
E) IV vancomycin
This patient has cellulitis and should receive a beta lactam antibiotic, which will have the best coverage and lowest minimal inhibitory concentration for the likely organism, beta hemolytic streptococci. Clindamycin would likely work, but it has greater side effects. This patient does not need coverage for methicillin-resistant staphylococcus aureus (MRSA). I know many of you, if not most, know this, but I want to go through relevant data and formal recommendations, because of a recent call I received from a patient.
My patient had a full body rash after receiving cephalexin + TMP/sulfa [trimethoprim-sulfamethoxazole] treatment for cellulitis. In recent years the addition of TMP/sulfa to strep treatment to also cover MRSA has become popular, especially in emergency department and urgent care settings.
Moran and colleagues studied cephalexin + TMP/sulfa vs. cephalexin and placebo in patients with uncomplicated cellulitis.1 The outcome measured was clinical cure, and there was no difference between groups; clinical cure occurred in 182 (83.5%) of 218 participants in the cephalexin plus TMP/sulfa group vs. 165 (85.5%) of 193 in the cephalexin group (difference, −2.0%; 95% confidence interval, −9.7% to 5.7%; P = .50).
Jeng and colleagues studied patients admitted for a cellulitis, and evaluated the patients’ response to beta-lactam antibiotics.2 Patients had acute and convalescent serologies for beta hemolytic strep. Almost all evaluable patients with positive strep studies (97%) responded to beta-lactams, and 21 of 23 (91%) with negative studies responded to beta-lactams (overall response rate 95%). This study was done during a time of high MRSA prevalence.
The most recent Infectious Diseases Society of America guidelines for skin and soft tissue infections, recommend oral penicillin, cephalexin, dicloxacillin, or clindamycin for mild cellulitis, and IV equivalent if patients have moderate cellulitis.3 If abscesses are present, then drainage is recommended and MRSA coverage. Kamath and colleagues reported on how closely guidelines for skin and soft tissue infections were followed.4 In patients with mild cellulitis, only 36% received guideline-suggested antibiotics. The most common antibiotic prescribed that was outside the guidelines was trimethoprim-sulfamethoxazole.
Myth: Cellulitis treatment should include MRSA coverage.
My advice: Stick with beta-lactam antibiotics, unless an abscess is present. There is no need to add MRSA coverage for initial treatment of mild to moderate cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Moran GJ et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis: A randomized clinical trial. JAMA 2017 May 23;317(20):2088-96.
2. Jeng Arthur et al. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;July;89(4):217-26.
3. Stevens DL et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52.
4. Kamath RS et al. Guidelines vs. actual management of skin and soft tissue infections in the emergency department. Open Forum Infect Dis. 2018 Jan 12;5(1):ofx188.
Pandemic puts patients with psoriatic disease off seeking medical help
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
FROM CARC 2021
Less pain, same gain with tirbanibulin for actinic keratosis
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
ASDSA warns of rogue insulin pen use for DIY fillers
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
Make the Diagnosis - March 2021
Because of the lack of improvement with topical corticosteroids, a skin biopsy was performed from a lesion on the lower back which showed an epidermis with compact hyperkeratosis and a thickened granular layer. Within the dermis, there was a lichenoid infiltrate of lymphocytes with a prominent interface change and rare dyskeratotic keratinocytes consistent with lichen planus.
Lichen planus is an inflammatory condition of the skin seen mainly in the adult population and is rare in children. This condition affects 0.5%-1% of the population, with maybe a higher prevalence in woman with no racial predilection in the adult or pediatric population. Most patients diagnosed are described to be over 40 years of age, but in children, the mean age for presentation is reported between the ages of 7 and 11.8 years.1 Interestingly, most of the published larger studies of lichen planus in children originate from India. In a U.K. study, about 80% of the cases reported were from children of Indian descent, as is our patient; so it is possible that lichen planus may be more prevalent in India.1 In a study based in the United States, cases were more prevalent in African American children.2
The exact cause of this condition is not known but studies have suggested that activated T cells, particularly CD8+, attack and cause apoptosis of the basal keratinocytes.3 There appears to be an up-regulation of Th1 cytokines such as interferon‐gamma, tumor necrosis factor–alpha, interleukin‐1 alpha, IL‐6, and IL‐8, as well as other apoptosis-related molecules.3
Lichen planus has been associated with other systemic conditions especially liver disease (chronic active hepatitis C and primary biliary cirrhosis). Children and adults may also have coexistence of other autoimmune diseases such as autoimmune polyendocrinopathy, myasthenia gravis, autoimmune thyroid disease, vitiligo, and thymoma. Some reports have also found a higher prevalence of atopic dermatitis in children with lichen planus.4
The lesions are typically described as the four “Ps” for pruritic, polygonal, purpuric flat-topped papules, and plaques. The papules of lichen planus have characteristically dry fine white streaks known as Wickham’s striae. The lesions can occur anywhere on the body, but they tend to occur more commonly on the flexures of the forearms, the wrists, ankles, shins, knees, and the torso. The face is rarely affected. In some patients oral, scalp (lichen planopilaris), nails, and rarely conjunctival, genital, and esophageal involvement can occur.2
In histopathology, the lesions are characterized by a wedge-shaped hypergranulosis, marked hyperkeratosis, and irregular sawtooth-like acanthosis of rete ridges on the epidermis. The dermal-epidermal junction typically shows an interstitial dermatitis. Civatte bodies may also be seen. On direct immunofluorescence, IgM-staining of the cytoid bodies in the dermal papilla or peribasilar areas are suggestive of lichen planus.1
The differential diagnosis of lichen planus includes severe lichenified atopic dermatitis, drug-induced lichen planus, graft-versus-host disease, psoriasis, pityriasis rosea, subacute cutaneous lupus, discoid lupus, secondary syphilis, and lichen simplex chronicus. Interestingly, our patient presented with lesions that were not pruritic and more generalized. Compared with eczema, were flexures are commonly affected, our patient’s lesions were localized to the ankles, wrists, extensor knees, and elbows, and no pruritus was reported. Lichenification of skin lesions occurs as a response to chronic scratching as it occurs in atopic dermatitis and lichen simplex chronicus, was considered in our patient, but the lack of pruritus and the more acute presentation made it unlikely.
Lichen planus is considered a self-limiting disease, so treatment is focused on the control of pruritus and to accelerate resolution. The first-line therapy for classic cutaneous lichen planus is the use of potent or superpotent topical corticosteroids for localized disease on the body and extremities and mild to mid-potency for intertriginous areas and the face. Clinical response should be assessed after 2-3 weeks of treatment. For patients with more generalized or recalcitrant disease like our patient, other treatment modalities like phototherapy (narrow-band UVB), a 4- to 6-week course of oral glucocorticoids, or acitretin may be considered. Our patient recently started narrow-band UVB. Other medications that have been reported beneficial for more severe cases include methotrexate, cyclosporine, griseofulvin, hydroxychloroquine, metronidazole, dapsone, and mycophenolate. Recent studies in the adult population have shown apremilast, a phosphodiesterase inhibitor, to be a promising medication for patients with cutaneous lichen planus, though this medication has not been approved yet for use in the pediatric population.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Payette MJ et al. Clin Dermatol. 2015 Nov-Dec;33(6):631-43.
2. Walton KE et al. Pediatr Dermatol. 2010;27:34-8.
3. Lehman JS et al. Int J Dermatol. 2009 Jul;48(7):682-94.
4. Laughter D et al. J Am Acad Dermatol. 2000;43:649-55.
5. Paul J et al. J Am Acad Dermatol. 2013 Feb;68(2):255-61.
Because of the lack of improvement with topical corticosteroids, a skin biopsy was performed from a lesion on the lower back which showed an epidermis with compact hyperkeratosis and a thickened granular layer. Within the dermis, there was a lichenoid infiltrate of lymphocytes with a prominent interface change and rare dyskeratotic keratinocytes consistent with lichen planus.
Lichen planus is an inflammatory condition of the skin seen mainly in the adult population and is rare in children. This condition affects 0.5%-1% of the population, with maybe a higher prevalence in woman with no racial predilection in the adult or pediatric population. Most patients diagnosed are described to be over 40 years of age, but in children, the mean age for presentation is reported between the ages of 7 and 11.8 years.1 Interestingly, most of the published larger studies of lichen planus in children originate from India. In a U.K. study, about 80% of the cases reported were from children of Indian descent, as is our patient; so it is possible that lichen planus may be more prevalent in India.1 In a study based in the United States, cases were more prevalent in African American children.2
The exact cause of this condition is not known but studies have suggested that activated T cells, particularly CD8+, attack and cause apoptosis of the basal keratinocytes.3 There appears to be an up-regulation of Th1 cytokines such as interferon‐gamma, tumor necrosis factor–alpha, interleukin‐1 alpha, IL‐6, and IL‐8, as well as other apoptosis-related molecules.3
Lichen planus has been associated with other systemic conditions especially liver disease (chronic active hepatitis C and primary biliary cirrhosis). Children and adults may also have coexistence of other autoimmune diseases such as autoimmune polyendocrinopathy, myasthenia gravis, autoimmune thyroid disease, vitiligo, and thymoma. Some reports have also found a higher prevalence of atopic dermatitis in children with lichen planus.4
The lesions are typically described as the four “Ps” for pruritic, polygonal, purpuric flat-topped papules, and plaques. The papules of lichen planus have characteristically dry fine white streaks known as Wickham’s striae. The lesions can occur anywhere on the body, but they tend to occur more commonly on the flexures of the forearms, the wrists, ankles, shins, knees, and the torso. The face is rarely affected. In some patients oral, scalp (lichen planopilaris), nails, and rarely conjunctival, genital, and esophageal involvement can occur.2
In histopathology, the lesions are characterized by a wedge-shaped hypergranulosis, marked hyperkeratosis, and irregular sawtooth-like acanthosis of rete ridges on the epidermis. The dermal-epidermal junction typically shows an interstitial dermatitis. Civatte bodies may also be seen. On direct immunofluorescence, IgM-staining of the cytoid bodies in the dermal papilla or peribasilar areas are suggestive of lichen planus.1
The differential diagnosis of lichen planus includes severe lichenified atopic dermatitis, drug-induced lichen planus, graft-versus-host disease, psoriasis, pityriasis rosea, subacute cutaneous lupus, discoid lupus, secondary syphilis, and lichen simplex chronicus. Interestingly, our patient presented with lesions that were not pruritic and more generalized. Compared with eczema, were flexures are commonly affected, our patient’s lesions were localized to the ankles, wrists, extensor knees, and elbows, and no pruritus was reported. Lichenification of skin lesions occurs as a response to chronic scratching as it occurs in atopic dermatitis and lichen simplex chronicus, was considered in our patient, but the lack of pruritus and the more acute presentation made it unlikely.
Lichen planus is considered a self-limiting disease, so treatment is focused on the control of pruritus and to accelerate resolution. The first-line therapy for classic cutaneous lichen planus is the use of potent or superpotent topical corticosteroids for localized disease on the body and extremities and mild to mid-potency for intertriginous areas and the face. Clinical response should be assessed after 2-3 weeks of treatment. For patients with more generalized or recalcitrant disease like our patient, other treatment modalities like phototherapy (narrow-band UVB), a 4- to 6-week course of oral glucocorticoids, or acitretin may be considered. Our patient recently started narrow-band UVB. Other medications that have been reported beneficial for more severe cases include methotrexate, cyclosporine, griseofulvin, hydroxychloroquine, metronidazole, dapsone, and mycophenolate. Recent studies in the adult population have shown apremilast, a phosphodiesterase inhibitor, to be a promising medication for patients with cutaneous lichen planus, though this medication has not been approved yet for use in the pediatric population.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Payette MJ et al. Clin Dermatol. 2015 Nov-Dec;33(6):631-43.
2. Walton KE et al. Pediatr Dermatol. 2010;27:34-8.
3. Lehman JS et al. Int J Dermatol. 2009 Jul;48(7):682-94.
4. Laughter D et al. J Am Acad Dermatol. 2000;43:649-55.
5. Paul J et al. J Am Acad Dermatol. 2013 Feb;68(2):255-61.
Because of the lack of improvement with topical corticosteroids, a skin biopsy was performed from a lesion on the lower back which showed an epidermis with compact hyperkeratosis and a thickened granular layer. Within the dermis, there was a lichenoid infiltrate of lymphocytes with a prominent interface change and rare dyskeratotic keratinocytes consistent with lichen planus.
Lichen planus is an inflammatory condition of the skin seen mainly in the adult population and is rare in children. This condition affects 0.5%-1% of the population, with maybe a higher prevalence in woman with no racial predilection in the adult or pediatric population. Most patients diagnosed are described to be over 40 years of age, but in children, the mean age for presentation is reported between the ages of 7 and 11.8 years.1 Interestingly, most of the published larger studies of lichen planus in children originate from India. In a U.K. study, about 80% of the cases reported were from children of Indian descent, as is our patient; so it is possible that lichen planus may be more prevalent in India.1 In a study based in the United States, cases were more prevalent in African American children.2
The exact cause of this condition is not known but studies have suggested that activated T cells, particularly CD8+, attack and cause apoptosis of the basal keratinocytes.3 There appears to be an up-regulation of Th1 cytokines such as interferon‐gamma, tumor necrosis factor–alpha, interleukin‐1 alpha, IL‐6, and IL‐8, as well as other apoptosis-related molecules.3
Lichen planus has been associated with other systemic conditions especially liver disease (chronic active hepatitis C and primary biliary cirrhosis). Children and adults may also have coexistence of other autoimmune diseases such as autoimmune polyendocrinopathy, myasthenia gravis, autoimmune thyroid disease, vitiligo, and thymoma. Some reports have also found a higher prevalence of atopic dermatitis in children with lichen planus.4
The lesions are typically described as the four “Ps” for pruritic, polygonal, purpuric flat-topped papules, and plaques. The papules of lichen planus have characteristically dry fine white streaks known as Wickham’s striae. The lesions can occur anywhere on the body, but they tend to occur more commonly on the flexures of the forearms, the wrists, ankles, shins, knees, and the torso. The face is rarely affected. In some patients oral, scalp (lichen planopilaris), nails, and rarely conjunctival, genital, and esophageal involvement can occur.2
In histopathology, the lesions are characterized by a wedge-shaped hypergranulosis, marked hyperkeratosis, and irregular sawtooth-like acanthosis of rete ridges on the epidermis. The dermal-epidermal junction typically shows an interstitial dermatitis. Civatte bodies may also be seen. On direct immunofluorescence, IgM-staining of the cytoid bodies in the dermal papilla or peribasilar areas are suggestive of lichen planus.1
The differential diagnosis of lichen planus includes severe lichenified atopic dermatitis, drug-induced lichen planus, graft-versus-host disease, psoriasis, pityriasis rosea, subacute cutaneous lupus, discoid lupus, secondary syphilis, and lichen simplex chronicus. Interestingly, our patient presented with lesions that were not pruritic and more generalized. Compared with eczema, were flexures are commonly affected, our patient’s lesions were localized to the ankles, wrists, extensor knees, and elbows, and no pruritus was reported. Lichenification of skin lesions occurs as a response to chronic scratching as it occurs in atopic dermatitis and lichen simplex chronicus, was considered in our patient, but the lack of pruritus and the more acute presentation made it unlikely.
Lichen planus is considered a self-limiting disease, so treatment is focused on the control of pruritus and to accelerate resolution. The first-line therapy for classic cutaneous lichen planus is the use of potent or superpotent topical corticosteroids for localized disease on the body and extremities and mild to mid-potency for intertriginous areas and the face. Clinical response should be assessed after 2-3 weeks of treatment. For patients with more generalized or recalcitrant disease like our patient, other treatment modalities like phototherapy (narrow-band UVB), a 4- to 6-week course of oral glucocorticoids, or acitretin may be considered. Our patient recently started narrow-band UVB. Other medications that have been reported beneficial for more severe cases include methotrexate, cyclosporine, griseofulvin, hydroxychloroquine, metronidazole, dapsone, and mycophenolate. Recent studies in the adult population have shown apremilast, a phosphodiesterase inhibitor, to be a promising medication for patients with cutaneous lichen planus, though this medication has not been approved yet for use in the pediatric population.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Payette MJ et al. Clin Dermatol. 2015 Nov-Dec;33(6):631-43.
2. Walton KE et al. Pediatr Dermatol. 2010;27:34-8.
3. Lehman JS et al. Int J Dermatol. 2009 Jul;48(7):682-94.
4. Laughter D et al. J Am Acad Dermatol. 2000;43:649-55.
5. Paul J et al. J Am Acad Dermatol. 2013 Feb;68(2):255-61.
There was no prior personal or family history of atopic dermatitis or psoriasis. He has no other medical conditions and is not taking any medications.
He denied any joint pain, sun sensitivity, mouth sores, or other symptoms. After the initial consultation he was treated with fluocinonide 0.05% ointment for 2 weeks with slight improvement on the lesions.
On physical exam he presented with hyperpigmented and violaceous lichenified papules and plaques on the extremities and the torso. (photos 1 and 2). He also had hyperpigmented violaceous macules on the eyelids and around the mouth (photos 1 and 2).
Cluster of hyperpigmented spots
A large hyperpigmented patch with overlying darker macules and papules is characteristic of a speckled lentiginous nevus (SLN), also called a nevus spilus.
SLN is a cafe-au-lait˗like nevus that initially appears with a hyperpigmented background, usually at or around birth. Later, a speckled or polka-dot pattern of dark macules and papules appears over time. SLN is believed to be a form of congenital melanocytic nevus. There are believed to be 2 subtypes of SLN: nevus spilus maculosus and nevus spilus papulosus.
The maculosus subtype is characterized by flat and evenly distributed macules, that look like polka-dots. Histopathology reveals elongated interpapillary ridges containing increased numbers of melanocytes that form nests at the dermo-epidermal junction.
The papulosus subtype (which this patient had) is differentiated by superimposed speckles and papules whose size and distribution vary; this subype looks similar to a starry sky. Histopathology of the papulosus subtype shows melanocytic nevi of either the dermal or compound type—hence the papular appearance.
Given that SLN is a congenital melanocytic nevus, there is a small risk of transformation to malignant melanoma. The papulosus subtype is believed to have a more dynamic course with more lesions appearing over time. The maculosus subtype is considered to have a slightly higher risk of transformation into malignant melanoma compared to the papulosus subtype.
It is important to recognize that SLN is a distinct clinical entity, rather than a large irregular nevus. Mistaking it for a large suspicious nevus would require multiple biopsies of the most suspicious areas or excision of the entire lesion. Treatment for SLN includes serial surveillance with biopsy or excision of any suspicious areas that arise. In this case, the patient did not have any areas warranting biopsy, so the plan was to have him followed with annual clinical surveillance.
Photo and text courtesy of Erik Unruh, MD, MPH, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Happl, R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin Exp Dermatol. 2009;34:133-135. doi: 10.1111/j.1365-2230.2008.02966.x.

A large hyperpigmented patch with overlying darker macules and papules is characteristic of a speckled lentiginous nevus (SLN), also called a nevus spilus.
SLN is a cafe-au-lait˗like nevus that initially appears with a hyperpigmented background, usually at or around birth. Later, a speckled or polka-dot pattern of dark macules and papules appears over time. SLN is believed to be a form of congenital melanocytic nevus. There are believed to be 2 subtypes of SLN: nevus spilus maculosus and nevus spilus papulosus.
The maculosus subtype is characterized by flat and evenly distributed macules, that look like polka-dots. Histopathology reveals elongated interpapillary ridges containing increased numbers of melanocytes that form nests at the dermo-epidermal junction.
The papulosus subtype (which this patient had) is differentiated by superimposed speckles and papules whose size and distribution vary; this subype looks similar to a starry sky. Histopathology of the papulosus subtype shows melanocytic nevi of either the dermal or compound type—hence the papular appearance.
Given that SLN is a congenital melanocytic nevus, there is a small risk of transformation to malignant melanoma. The papulosus subtype is believed to have a more dynamic course with more lesions appearing over time. The maculosus subtype is considered to have a slightly higher risk of transformation into malignant melanoma compared to the papulosus subtype.
It is important to recognize that SLN is a distinct clinical entity, rather than a large irregular nevus. Mistaking it for a large suspicious nevus would require multiple biopsies of the most suspicious areas or excision of the entire lesion. Treatment for SLN includes serial surveillance with biopsy or excision of any suspicious areas that arise. In this case, the patient did not have any areas warranting biopsy, so the plan was to have him followed with annual clinical surveillance.
Photo and text courtesy of Erik Unruh, MD, MPH, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

A large hyperpigmented patch with overlying darker macules and papules is characteristic of a speckled lentiginous nevus (SLN), also called a nevus spilus.
SLN is a cafe-au-lait˗like nevus that initially appears with a hyperpigmented background, usually at or around birth. Later, a speckled or polka-dot pattern of dark macules and papules appears over time. SLN is believed to be a form of congenital melanocytic nevus. There are believed to be 2 subtypes of SLN: nevus spilus maculosus and nevus spilus papulosus.
The maculosus subtype is characterized by flat and evenly distributed macules, that look like polka-dots. Histopathology reveals elongated interpapillary ridges containing increased numbers of melanocytes that form nests at the dermo-epidermal junction.
The papulosus subtype (which this patient had) is differentiated by superimposed speckles and papules whose size and distribution vary; this subype looks similar to a starry sky. Histopathology of the papulosus subtype shows melanocytic nevi of either the dermal or compound type—hence the papular appearance.
Given that SLN is a congenital melanocytic nevus, there is a small risk of transformation to malignant melanoma. The papulosus subtype is believed to have a more dynamic course with more lesions appearing over time. The maculosus subtype is considered to have a slightly higher risk of transformation into malignant melanoma compared to the papulosus subtype.
It is important to recognize that SLN is a distinct clinical entity, rather than a large irregular nevus. Mistaking it for a large suspicious nevus would require multiple biopsies of the most suspicious areas or excision of the entire lesion. Treatment for SLN includes serial surveillance with biopsy or excision of any suspicious areas that arise. In this case, the patient did not have any areas warranting biopsy, so the plan was to have him followed with annual clinical surveillance.
Photo and text courtesy of Erik Unruh, MD, MPH, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Happl, R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin Exp Dermatol. 2009;34:133-135. doi: 10.1111/j.1365-2230.2008.02966.x.
Happl, R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin Exp Dermatol. 2009;34:133-135. doi: 10.1111/j.1365-2230.2008.02966.x.