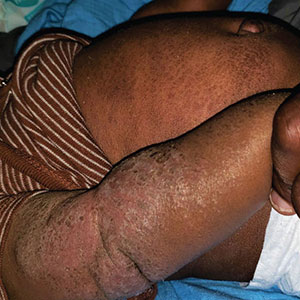
User login
Botulinum Toxin, Dermal Fillers Safe in Skin of Color Patients, Review Finds
TOPLINE:
, and more data on Black and Latinx populations are needed, according to a literature review.
METHODOLOGY:
- Understanding the efficacy and safety of cosmetic injectables in diverse skin types is important because individuals identifying as racial and ethnic minorities accounted for 18% of neuromodulator procedures and 22% of soft tissue augmentation procedures in 2020 in the United States.
- Researchers reviewed available literature on the usability and efficacy of neuromodulators and soft tissue augmentation in individuals with SOC because of the limited data available in these populations, particularly non-Asian, SOC populations.
- Overall, 88 studies in English were included, which were either dedicated to discussing safety and/or efficacy of injectables in SOC populations or enrolled more than 20% of participants from SOC populations.
- High-quality level I and II evidence was found in 50 studies, and 9940 patients were analyzed in this review.
TAKEAWAY:
- Studies considered high quality indicated that botulinum toxin is safe and effective for treating glabellar lines in Asians; tailored guidelines recommended specific strategies; and adverse events, such as eyelid issues, were more common in Asians.
- Hyaluronic acid fillers showed significant improvement in moderate to severe cases of nasolabial folds in Asians, and adverse effects like swelling and pain were mild to moderate — some cases of granuloma formation and vascular compromise have been reported.
- In Black individuals, botulinum toxin was well tolerated; hyaluronic acid fillers showed favorable safety, with mild to moderate adverse events; and measures like slower injections and subdermal techniques minimized risks.
- In Latinx populations, there was a lack of robust study data on safety and efficacy of botulinum toxin, whereas hyaluronic acid and poly-L-lactic acid fillers were well tolerated.
IN PRACTICE:
“Neuromodulators and dermal fillers are useful and safe as cosmetic and antiaging treatments in SOC populations, with the greatest amount of data supporting its use in Asian populations,” although more data on Black and Latinx populations are needed, the authors concluded. “During cosmetic consultations, physicians should consider the impact of different cultural beauty norms on the aesthetic goals of diverse patient populations,” they added.
SOURCE:
This study led by Shanice McKenzie, MD, from the Department of Dermatology, University of Southern California, Los Angeles, California, was published online in the Journal of Cosmetic Dermatology.
LIMITATIONS:
Most of the recent data and formal consensus guidelines on injectables in the review came from Asian countries, and there was “a relative paucity of research on Black and Latinx populations,” the authors noted.
DISCLOSURES:
The study did not receive any funding. Two authors declared serving as a consultant, investigator, and/or speaker for various companies; the rest had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
, and more data on Black and Latinx populations are needed, according to a literature review.
METHODOLOGY:
- Understanding the efficacy and safety of cosmetic injectables in diverse skin types is important because individuals identifying as racial and ethnic minorities accounted for 18% of neuromodulator procedures and 22% of soft tissue augmentation procedures in 2020 in the United States.
- Researchers reviewed available literature on the usability and efficacy of neuromodulators and soft tissue augmentation in individuals with SOC because of the limited data available in these populations, particularly non-Asian, SOC populations.
- Overall, 88 studies in English were included, which were either dedicated to discussing safety and/or efficacy of injectables in SOC populations or enrolled more than 20% of participants from SOC populations.
- High-quality level I and II evidence was found in 50 studies, and 9940 patients were analyzed in this review.
TAKEAWAY:
- Studies considered high quality indicated that botulinum toxin is safe and effective for treating glabellar lines in Asians; tailored guidelines recommended specific strategies; and adverse events, such as eyelid issues, were more common in Asians.
- Hyaluronic acid fillers showed significant improvement in moderate to severe cases of nasolabial folds in Asians, and adverse effects like swelling and pain were mild to moderate — some cases of granuloma formation and vascular compromise have been reported.
- In Black individuals, botulinum toxin was well tolerated; hyaluronic acid fillers showed favorable safety, with mild to moderate adverse events; and measures like slower injections and subdermal techniques minimized risks.
- In Latinx populations, there was a lack of robust study data on safety and efficacy of botulinum toxin, whereas hyaluronic acid and poly-L-lactic acid fillers were well tolerated.
IN PRACTICE:
“Neuromodulators and dermal fillers are useful and safe as cosmetic and antiaging treatments in SOC populations, with the greatest amount of data supporting its use in Asian populations,” although more data on Black and Latinx populations are needed, the authors concluded. “During cosmetic consultations, physicians should consider the impact of different cultural beauty norms on the aesthetic goals of diverse patient populations,” they added.
SOURCE:
This study led by Shanice McKenzie, MD, from the Department of Dermatology, University of Southern California, Los Angeles, California, was published online in the Journal of Cosmetic Dermatology.
LIMITATIONS:
Most of the recent data and formal consensus guidelines on injectables in the review came from Asian countries, and there was “a relative paucity of research on Black and Latinx populations,” the authors noted.
DISCLOSURES:
The study did not receive any funding. Two authors declared serving as a consultant, investigator, and/or speaker for various companies; the rest had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
, and more data on Black and Latinx populations are needed, according to a literature review.
METHODOLOGY:
- Understanding the efficacy and safety of cosmetic injectables in diverse skin types is important because individuals identifying as racial and ethnic minorities accounted for 18% of neuromodulator procedures and 22% of soft tissue augmentation procedures in 2020 in the United States.
- Researchers reviewed available literature on the usability and efficacy of neuromodulators and soft tissue augmentation in individuals with SOC because of the limited data available in these populations, particularly non-Asian, SOC populations.
- Overall, 88 studies in English were included, which were either dedicated to discussing safety and/or efficacy of injectables in SOC populations or enrolled more than 20% of participants from SOC populations.
- High-quality level I and II evidence was found in 50 studies, and 9940 patients were analyzed in this review.
TAKEAWAY:
- Studies considered high quality indicated that botulinum toxin is safe and effective for treating glabellar lines in Asians; tailored guidelines recommended specific strategies; and adverse events, such as eyelid issues, were more common in Asians.
- Hyaluronic acid fillers showed significant improvement in moderate to severe cases of nasolabial folds in Asians, and adverse effects like swelling and pain were mild to moderate — some cases of granuloma formation and vascular compromise have been reported.
- In Black individuals, botulinum toxin was well tolerated; hyaluronic acid fillers showed favorable safety, with mild to moderate adverse events; and measures like slower injections and subdermal techniques minimized risks.
- In Latinx populations, there was a lack of robust study data on safety and efficacy of botulinum toxin, whereas hyaluronic acid and poly-L-lactic acid fillers were well tolerated.
IN PRACTICE:
“Neuromodulators and dermal fillers are useful and safe as cosmetic and antiaging treatments in SOC populations, with the greatest amount of data supporting its use in Asian populations,” although more data on Black and Latinx populations are needed, the authors concluded. “During cosmetic consultations, physicians should consider the impact of different cultural beauty norms on the aesthetic goals of diverse patient populations,” they added.
SOURCE:
This study led by Shanice McKenzie, MD, from the Department of Dermatology, University of Southern California, Los Angeles, California, was published online in the Journal of Cosmetic Dermatology.
LIMITATIONS:
Most of the recent data and formal consensus guidelines on injectables in the review came from Asian countries, and there was “a relative paucity of research on Black and Latinx populations,” the authors noted.
DISCLOSURES:
The study did not receive any funding. Two authors declared serving as a consultant, investigator, and/or speaker for various companies; the rest had no disclosures to report.
A version of this article appeared on Medscape.com.
What’s Driving the Higher Breast Cancer Death Rate in Black Women?
More women today are surviving breast cancer if it’s caught early, largely because of better screening and more effective and targeted treatments.
However, not everyone has benefited equitably from this progress. Critical gaps in breast cancer outcomes and survival remain for women in racial and ethnic minority groups.
Black women for instance, have a 41% higher death rate from breast cancer compared with White patients. They also have a greater incidence of aggressive disease like triple-negative breast cancer. Native American and Hispanic women, meanwhile, are more likely to be diagnosed with breast cancer at an earlier age than White women and experience more aggressive breast cancers.
In 2023, Farhad Islami, MD, PhD, and his team published an updated analysis of racial/ethnic and socioeconomic disparities in cancer trends based on data from 2014 to 2020. The analysis found that Black women in particular, were the least likely to have an early-stage diagnosis of breast cancer. Localized‐stage breast cancer was diagnosed in 57% of Black women versus 68% of White women.
“Despite substantial progress in cancer prevention, early detection, and treatments, the burden of cancer remains greater among populations that have been historically marginalized, including people of color, people with lower socioeconomic status, and people living in nonmetropolitan areas,” said Dr. Islami, who is senior scientific director of cancer disparity research in the Surveillance & Health Equity Science Department at the American Cancer Society.
The reasons behind outcomes disparities in breast cancer are complex, making solutions challenging, say experts researching racial differences in cancer outcomes.
Among the findings of this research is that breast cancer tests may be contributing to the disparities and misguiding care for some patients of color.
SDH and Screening Rates Differences By Race
A range of factors contribute to racial and ethnic disparities in breast cancer outcomes, said Pamela Ganschow, MD, an associate professor in the Department of Internal Medicine at the University of Illinois Cancer Center in Chicago and part of the university’s Cancer Prevention and Control research program. These include socioeconomic status, access to timely and high-quality care across the cancer control continuum, cultural beliefs, differences in genetic makeup and tumor biology, as well as system biases, such as implicit biases and systemic racism, Dr. Ganschow said.
Dr. Islami adds that gaps in access to cancer prevention, early detection, and treatment are largely rooted in fundamental inequities in social determinants of health (SDH), such as whether a patient has safe housing, transportation, education, job opportunities, income, access to nutritious foods, and language and literacy skills, among others.
Dr. Islami’s analysis, for example, shows that people of color are generally more likely to have lower educational attainment and to experience poverty, food insecurity, and housing insecurity compared with White people. Among people aged 18-64 years, the age-adjusted proportion of individuals with no health insurance in 2021 was also higher among Black (13.7%), American Indian/Alaskan Native (18.7%), and Hispanic (28.7%) patients than among White (7.8%) or Asian (5.9%) people, according to the report.
Competing needs can also get in the way of prioritizing cancer screenings, especially for patients in lower socio-economic populations, Dr. Ganschow said.
“You’ve got people who are working a job or three jobs, just to make ends meet for their family and can’t necessarily take time off to get that done,” she said. “Nor is it prioritized in their head because they’ve got to put a meal on the table.”
But the racial disparities between Black and White women, at least, are not clearly explained by differences between the screening rates..
Of patients who received mammograms 76% were White and 79% were Black, according to another recent study coauthored by Dr. Islami. While Black women appear to have the highest breast cancer screening rates, some data suggest such rates are being overreported.
Lower screening rates were seen in American Indian/Alaska Native (59%), Asian (67%), and Hispanic women (74%).
Biological Differences, Bad Testing Recommendations May Contribute to Poor Outcomes
Differences in biology may be one overlooked internal driver of lower breast cancer survival in Black women.
Researchers at Sanford Burnham Prebys in La Jolla, California, recently analyzed the breast cells of White and Black women, finding significant molecular differences that may be contributing to higher breast cancer mortality rates in Black women.
Investigators analyzed both healthy tissue and tumor tissue from 185 Black women and compared the samples to that of White women. They discovered differences among Black and White women in the way their DNA repair genes are expressed, both in healthy breast tissue and in tumors positive for estrogen receptor breast cancer. Molecular differences were also present in the cellular signals that control how fast cells, including cancer cells, grow.
DNA repair is part of normal cellular function and helps cells recover from damage that can occur during DNA replication or in response to external factors, such as stress.
“One of the first lines of defense, to prevent the cell from becoming a tumor are DNA damage repair pathways,” said Svasti Haricharan, PhD, a coauthor of the study and an assistant professor at Sanford Burnham Prebys. “We know there are many different DNA damage repair pathways that respond to different types of DNA damage. What we didn’t know was that, even in our normal cells, based on your race and ethnicity, you have different levels of DNA repair proteins.”
The study found that many of the proteins associated with endocrine resistance and poor outcomes in breast cancer patients are differently regulated in Black women compared with White woman. These differences contribute to resistance to standard endocrine therapy, Dr. Haricharan said.
“Because we never studied the biology in Black woman, it was just assumed that across all demographics, it must be the same,” she said. “We are not even accounting for the possibility there are likely intrinsic differences for how you will respond to an endocrine treatment.”
Testing and treatment may also be playing a role in worse breast cancer outcomes for Black women.
In an analysis of 73,363 women with early-stage, estrogen receptor–positive breast cancer, investigators found that a common test used to decide the treatment course for patients may be leading to bad recommendations for Black women.
The test, known as the 21-gene breast recurrence score, is the most commonly ordered biomarker test used to guide doctor’s recommendations for patients with estrogen receptor–positive breast cancer, the most common form of cancer in Black women, representing about 70%-80% of cases.
The test helps physicians identify which patients are good candidates for chemo, but the test may underestimate the benefit of chemo for Black women. It ranks some Black women as unlikely to benefit from chemo, when they actually would have benefited, according to the January 2024 study, published in the Journal of the National Comprehensive Cancer Network.
The test gives a score of zero to 100, explains Kent Hoskins, MD, oncology service line medical director at the University of Illinois (UI) Health and director of the Familial Breast Cancer Clinic at UI Health, both in Chicago. The higher the score, the higher the risk and the greater the benefit of chemotherapy. A patient is either above the cut-off score and receives chemo, or is below the cut-off score and does not. In the analysis, investigators found that Black women start improving with chemo at a lower score than White women do.
Dr. Hoskins said the results raise questions about whether the biomarker test should be modified to be more applicable to Black women, whether other tests should be used, or if physicians should judge cut-off scores differently, depending on race.
How Neighborhood Impacts Breast Cancer, Death Rates
Living in a disadvantaged neighborhood also lowers breast cancer survival, according to new research. A disadvantaged neighborhood is generally defined as a location associated with higher concentrations of poverty, higher rates of unemployment, and less access to health care, quality housing, food, and community resources, according to the Centers for Disease Control and Prevention.
Authors of a study published in JAMA Network Open on April 18 identified 350,824 patients with breast cancer. Of these, 41,519 (11.8%) were Hispanic, 39,631 (11.3%) were non-Hispanic Black, and 234,698 (66.9%) were non-Hispanic White. Investigators divided the patients into five groups representing the lowest to highest neighborhood socioeconomic indices using the Yost Index. (The Yost Index is used by the National Cancer Institute for cancer surveillance and is based on variables such as household income, home value, median rent, percentage below 150% of the poverty line, education, and unemployment.)
Of the Black and Hispanic patients in the study, the highest proportions of both demographics lived in the most disadvantaged neighborhoods. (16,141 Black patients [30.9%]) and 10,168 Hispanic patients [19.5%]). Although 45% of White patients also fell into that same category, the highest proportion of White patients in the study lived in the most advantaged neighborhoods (66,529 patients [76.2%]).
Findings showed patients in the most disadvantaged neighborhoods had the highest proportion of triple-negative breast cancer. Patients in this group also had the lowest proportion of patients who completed surgery and radiation, and the highest proportion of patients who received chemotherapy, compared with all other neighborhood groups. The most advantaged neighborhoods group had higher proportions of localized-stage cancer, a higher proportion of patients who underwent surgery and radiation, and the lowest proportion of patients receiving chemotherapy treatment.
Patients in the most disadvantaged neighborhoods also had the highest risk of mortality (hazard ratio [HR,] 1.53; 95% CI, 1.48-1.59; P less than .001) compared with patients living in the most advantaged neighborhoods. Non-Hispanic Black patients in particular, had the highest risk of mortality, compared with non-Hispanic White patients (HR, 1.16; 95% CI, 1.13-1.20; P less than .001).
Authors wrote that the findings suggest neighborhood disadvantage is independently associated with shorter survival in patients with breast cancer, even after controlling for individual-level factors, tumor characteristics, and treatment.
“To address these residual disparities associated with neighborhood disadvantage, research must focus on which components of the built environment influence outcomes,” the authors said.
Another recent study also found correlations among where breast cancer patients lived and how they fared with the disease.
Jasmine M. Miller-Kleinhenz, PhD, an assistant professor at University of Mississippi Medical Center in Jackson, studied how historical redlining impacts breast cancer development and outcomes in her research published in JAMA Network Open, earlier this year. Redlining refers to the practice of denying people access to credit because of where they live. Historically, mortgage lenders widely redlined neighborhoods with predominantly Black residents. The 1968 Fair Housing Act outlawed racially motivated redlining, but consequences from historical redlining still exist.
Dr. Miller-Kleinhenz and her colleagues analyzed a cohort of 1764 women diagnosed with breast cancer between January 2010 and December 2017, who were followed up through December 2019. Investigators accessed the cohort based on three exposures: historic redlining (HRL), contemporary mortgage discrimination (CMD), and persistent mortgage discrimination (PMD). Contemporary mortgage discrimination refers to current-day discriminatory mortgage practices and persistent mortgage discrimination refers to neighborhoods that have experienced both HRL and CMD.
Findings showed that Black women living in historical redlined areas had increased odds of being diagnosed with aggressive forms of breast cancer, while White women in redlined areas had increased odds of late-stage diagnosis.
White women exposed to persistent mortgage discrimination were twice as likely to die of breast cancer, compared with their White counterparts living in areas without historical redlining or contemporary mortgage discrimination, the study found.
That is not to say that Black women did not have an increased risk of breast cancer mortality, Dr. Miller-Kleinhenz explained. Black women had a more than threefold elevated risk of breast cancer mortality compared with White women no matter where they lived, according to the findings.
“These results were surprising because it is showing that while neighborhood conditions might be a major driver of breast cancer mortality in White women, there are factors beyond the neighborhood that are additional drivers that are contributing to poor outcomes in Black women,” she said.
Hope for Improved Outcomes, Higher Survival Rates
Investigators hope the findings of all of this new research lead to better, more targeted treatments and, in turn, improved outcomes.
Dr. Haricharan is optimistic about the improvement of breast cancer outcomes as more is learned about the biology of Black patients and other non-White patients.
There is a growing effort to include more data from minoritized populations in breast cancer research studies, Dr. Haricharan said, and she foresees associated changes to clinical protocols in the future. Her own team is working on creating larger data sets that are more representative of non-White patients to further analyze the differences found in their prior study.
“I think there’s this understanding that, until we have data sets that are more representative, we really are catering to [only one] population in terms of our diagnostic and therapeutic technological advances,” she said.
The American Cancer Society meanwhile, is launching a new initiative in May that aims to collect more health data from Black women to ultimately develop more effective cancer interventions. VOICES of Black Women will focus on collecting and studying health data from Black women through online surveys. The society’s goal is to enroll at least 100,000 Black women in the United States between ages 25 and 55.
Dr. Miller-Kleinhenz called the initiative “an important step to starting to research and answer some of these lingering questions about why there continue to be breast cancer disparities.”
More women today are surviving breast cancer if it’s caught early, largely because of better screening and more effective and targeted treatments.
However, not everyone has benefited equitably from this progress. Critical gaps in breast cancer outcomes and survival remain for women in racial and ethnic minority groups.
Black women for instance, have a 41% higher death rate from breast cancer compared with White patients. They also have a greater incidence of aggressive disease like triple-negative breast cancer. Native American and Hispanic women, meanwhile, are more likely to be diagnosed with breast cancer at an earlier age than White women and experience more aggressive breast cancers.
In 2023, Farhad Islami, MD, PhD, and his team published an updated analysis of racial/ethnic and socioeconomic disparities in cancer trends based on data from 2014 to 2020. The analysis found that Black women in particular, were the least likely to have an early-stage diagnosis of breast cancer. Localized‐stage breast cancer was diagnosed in 57% of Black women versus 68% of White women.
“Despite substantial progress in cancer prevention, early detection, and treatments, the burden of cancer remains greater among populations that have been historically marginalized, including people of color, people with lower socioeconomic status, and people living in nonmetropolitan areas,” said Dr. Islami, who is senior scientific director of cancer disparity research in the Surveillance & Health Equity Science Department at the American Cancer Society.
The reasons behind outcomes disparities in breast cancer are complex, making solutions challenging, say experts researching racial differences in cancer outcomes.
Among the findings of this research is that breast cancer tests may be contributing to the disparities and misguiding care for some patients of color.
SDH and Screening Rates Differences By Race
A range of factors contribute to racial and ethnic disparities in breast cancer outcomes, said Pamela Ganschow, MD, an associate professor in the Department of Internal Medicine at the University of Illinois Cancer Center in Chicago and part of the university’s Cancer Prevention and Control research program. These include socioeconomic status, access to timely and high-quality care across the cancer control continuum, cultural beliefs, differences in genetic makeup and tumor biology, as well as system biases, such as implicit biases and systemic racism, Dr. Ganschow said.
Dr. Islami adds that gaps in access to cancer prevention, early detection, and treatment are largely rooted in fundamental inequities in social determinants of health (SDH), such as whether a patient has safe housing, transportation, education, job opportunities, income, access to nutritious foods, and language and literacy skills, among others.
Dr. Islami’s analysis, for example, shows that people of color are generally more likely to have lower educational attainment and to experience poverty, food insecurity, and housing insecurity compared with White people. Among people aged 18-64 years, the age-adjusted proportion of individuals with no health insurance in 2021 was also higher among Black (13.7%), American Indian/Alaskan Native (18.7%), and Hispanic (28.7%) patients than among White (7.8%) or Asian (5.9%) people, according to the report.
Competing needs can also get in the way of prioritizing cancer screenings, especially for patients in lower socio-economic populations, Dr. Ganschow said.
“You’ve got people who are working a job or three jobs, just to make ends meet for their family and can’t necessarily take time off to get that done,” she said. “Nor is it prioritized in their head because they’ve got to put a meal on the table.”
But the racial disparities between Black and White women, at least, are not clearly explained by differences between the screening rates..
Of patients who received mammograms 76% were White and 79% were Black, according to another recent study coauthored by Dr. Islami. While Black women appear to have the highest breast cancer screening rates, some data suggest such rates are being overreported.
Lower screening rates were seen in American Indian/Alaska Native (59%), Asian (67%), and Hispanic women (74%).
Biological Differences, Bad Testing Recommendations May Contribute to Poor Outcomes
Differences in biology may be one overlooked internal driver of lower breast cancer survival in Black women.
Researchers at Sanford Burnham Prebys in La Jolla, California, recently analyzed the breast cells of White and Black women, finding significant molecular differences that may be contributing to higher breast cancer mortality rates in Black women.
Investigators analyzed both healthy tissue and tumor tissue from 185 Black women and compared the samples to that of White women. They discovered differences among Black and White women in the way their DNA repair genes are expressed, both in healthy breast tissue and in tumors positive for estrogen receptor breast cancer. Molecular differences were also present in the cellular signals that control how fast cells, including cancer cells, grow.
DNA repair is part of normal cellular function and helps cells recover from damage that can occur during DNA replication or in response to external factors, such as stress.
“One of the first lines of defense, to prevent the cell from becoming a tumor are DNA damage repair pathways,” said Svasti Haricharan, PhD, a coauthor of the study and an assistant professor at Sanford Burnham Prebys. “We know there are many different DNA damage repair pathways that respond to different types of DNA damage. What we didn’t know was that, even in our normal cells, based on your race and ethnicity, you have different levels of DNA repair proteins.”
The study found that many of the proteins associated with endocrine resistance and poor outcomes in breast cancer patients are differently regulated in Black women compared with White woman. These differences contribute to resistance to standard endocrine therapy, Dr. Haricharan said.
“Because we never studied the biology in Black woman, it was just assumed that across all demographics, it must be the same,” she said. “We are not even accounting for the possibility there are likely intrinsic differences for how you will respond to an endocrine treatment.”
Testing and treatment may also be playing a role in worse breast cancer outcomes for Black women.
In an analysis of 73,363 women with early-stage, estrogen receptor–positive breast cancer, investigators found that a common test used to decide the treatment course for patients may be leading to bad recommendations for Black women.
The test, known as the 21-gene breast recurrence score, is the most commonly ordered biomarker test used to guide doctor’s recommendations for patients with estrogen receptor–positive breast cancer, the most common form of cancer in Black women, representing about 70%-80% of cases.
The test helps physicians identify which patients are good candidates for chemo, but the test may underestimate the benefit of chemo for Black women. It ranks some Black women as unlikely to benefit from chemo, when they actually would have benefited, according to the January 2024 study, published in the Journal of the National Comprehensive Cancer Network.
The test gives a score of zero to 100, explains Kent Hoskins, MD, oncology service line medical director at the University of Illinois (UI) Health and director of the Familial Breast Cancer Clinic at UI Health, both in Chicago. The higher the score, the higher the risk and the greater the benefit of chemotherapy. A patient is either above the cut-off score and receives chemo, or is below the cut-off score and does not. In the analysis, investigators found that Black women start improving with chemo at a lower score than White women do.
Dr. Hoskins said the results raise questions about whether the biomarker test should be modified to be more applicable to Black women, whether other tests should be used, or if physicians should judge cut-off scores differently, depending on race.
How Neighborhood Impacts Breast Cancer, Death Rates
Living in a disadvantaged neighborhood also lowers breast cancer survival, according to new research. A disadvantaged neighborhood is generally defined as a location associated with higher concentrations of poverty, higher rates of unemployment, and less access to health care, quality housing, food, and community resources, according to the Centers for Disease Control and Prevention.
Authors of a study published in JAMA Network Open on April 18 identified 350,824 patients with breast cancer. Of these, 41,519 (11.8%) were Hispanic, 39,631 (11.3%) were non-Hispanic Black, and 234,698 (66.9%) were non-Hispanic White. Investigators divided the patients into five groups representing the lowest to highest neighborhood socioeconomic indices using the Yost Index. (The Yost Index is used by the National Cancer Institute for cancer surveillance and is based on variables such as household income, home value, median rent, percentage below 150% of the poverty line, education, and unemployment.)
Of the Black and Hispanic patients in the study, the highest proportions of both demographics lived in the most disadvantaged neighborhoods. (16,141 Black patients [30.9%]) and 10,168 Hispanic patients [19.5%]). Although 45% of White patients also fell into that same category, the highest proportion of White patients in the study lived in the most advantaged neighborhoods (66,529 patients [76.2%]).
Findings showed patients in the most disadvantaged neighborhoods had the highest proportion of triple-negative breast cancer. Patients in this group also had the lowest proportion of patients who completed surgery and radiation, and the highest proportion of patients who received chemotherapy, compared with all other neighborhood groups. The most advantaged neighborhoods group had higher proportions of localized-stage cancer, a higher proportion of patients who underwent surgery and radiation, and the lowest proportion of patients receiving chemotherapy treatment.
Patients in the most disadvantaged neighborhoods also had the highest risk of mortality (hazard ratio [HR,] 1.53; 95% CI, 1.48-1.59; P less than .001) compared with patients living in the most advantaged neighborhoods. Non-Hispanic Black patients in particular, had the highest risk of mortality, compared with non-Hispanic White patients (HR, 1.16; 95% CI, 1.13-1.20; P less than .001).
Authors wrote that the findings suggest neighborhood disadvantage is independently associated with shorter survival in patients with breast cancer, even after controlling for individual-level factors, tumor characteristics, and treatment.
“To address these residual disparities associated with neighborhood disadvantage, research must focus on which components of the built environment influence outcomes,” the authors said.
Another recent study also found correlations among where breast cancer patients lived and how they fared with the disease.
Jasmine M. Miller-Kleinhenz, PhD, an assistant professor at University of Mississippi Medical Center in Jackson, studied how historical redlining impacts breast cancer development and outcomes in her research published in JAMA Network Open, earlier this year. Redlining refers to the practice of denying people access to credit because of where they live. Historically, mortgage lenders widely redlined neighborhoods with predominantly Black residents. The 1968 Fair Housing Act outlawed racially motivated redlining, but consequences from historical redlining still exist.
Dr. Miller-Kleinhenz and her colleagues analyzed a cohort of 1764 women diagnosed with breast cancer between January 2010 and December 2017, who were followed up through December 2019. Investigators accessed the cohort based on three exposures: historic redlining (HRL), contemporary mortgage discrimination (CMD), and persistent mortgage discrimination (PMD). Contemporary mortgage discrimination refers to current-day discriminatory mortgage practices and persistent mortgage discrimination refers to neighborhoods that have experienced both HRL and CMD.
Findings showed that Black women living in historical redlined areas had increased odds of being diagnosed with aggressive forms of breast cancer, while White women in redlined areas had increased odds of late-stage diagnosis.
White women exposed to persistent mortgage discrimination were twice as likely to die of breast cancer, compared with their White counterparts living in areas without historical redlining or contemporary mortgage discrimination, the study found.
That is not to say that Black women did not have an increased risk of breast cancer mortality, Dr. Miller-Kleinhenz explained. Black women had a more than threefold elevated risk of breast cancer mortality compared with White women no matter where they lived, according to the findings.
“These results were surprising because it is showing that while neighborhood conditions might be a major driver of breast cancer mortality in White women, there are factors beyond the neighborhood that are additional drivers that are contributing to poor outcomes in Black women,” she said.
Hope for Improved Outcomes, Higher Survival Rates
Investigators hope the findings of all of this new research lead to better, more targeted treatments and, in turn, improved outcomes.
Dr. Haricharan is optimistic about the improvement of breast cancer outcomes as more is learned about the biology of Black patients and other non-White patients.
There is a growing effort to include more data from minoritized populations in breast cancer research studies, Dr. Haricharan said, and she foresees associated changes to clinical protocols in the future. Her own team is working on creating larger data sets that are more representative of non-White patients to further analyze the differences found in their prior study.
“I think there’s this understanding that, until we have data sets that are more representative, we really are catering to [only one] population in terms of our diagnostic and therapeutic technological advances,” she said.
The American Cancer Society meanwhile, is launching a new initiative in May that aims to collect more health data from Black women to ultimately develop more effective cancer interventions. VOICES of Black Women will focus on collecting and studying health data from Black women through online surveys. The society’s goal is to enroll at least 100,000 Black women in the United States between ages 25 and 55.
Dr. Miller-Kleinhenz called the initiative “an important step to starting to research and answer some of these lingering questions about why there continue to be breast cancer disparities.”
More women today are surviving breast cancer if it’s caught early, largely because of better screening and more effective and targeted treatments.
However, not everyone has benefited equitably from this progress. Critical gaps in breast cancer outcomes and survival remain for women in racial and ethnic minority groups.
Black women for instance, have a 41% higher death rate from breast cancer compared with White patients. They also have a greater incidence of aggressive disease like triple-negative breast cancer. Native American and Hispanic women, meanwhile, are more likely to be diagnosed with breast cancer at an earlier age than White women and experience more aggressive breast cancers.
In 2023, Farhad Islami, MD, PhD, and his team published an updated analysis of racial/ethnic and socioeconomic disparities in cancer trends based on data from 2014 to 2020. The analysis found that Black women in particular, were the least likely to have an early-stage diagnosis of breast cancer. Localized‐stage breast cancer was diagnosed in 57% of Black women versus 68% of White women.
“Despite substantial progress in cancer prevention, early detection, and treatments, the burden of cancer remains greater among populations that have been historically marginalized, including people of color, people with lower socioeconomic status, and people living in nonmetropolitan areas,” said Dr. Islami, who is senior scientific director of cancer disparity research in the Surveillance & Health Equity Science Department at the American Cancer Society.
The reasons behind outcomes disparities in breast cancer are complex, making solutions challenging, say experts researching racial differences in cancer outcomes.
Among the findings of this research is that breast cancer tests may be contributing to the disparities and misguiding care for some patients of color.
SDH and Screening Rates Differences By Race
A range of factors contribute to racial and ethnic disparities in breast cancer outcomes, said Pamela Ganschow, MD, an associate professor in the Department of Internal Medicine at the University of Illinois Cancer Center in Chicago and part of the university’s Cancer Prevention and Control research program. These include socioeconomic status, access to timely and high-quality care across the cancer control continuum, cultural beliefs, differences in genetic makeup and tumor biology, as well as system biases, such as implicit biases and systemic racism, Dr. Ganschow said.
Dr. Islami adds that gaps in access to cancer prevention, early detection, and treatment are largely rooted in fundamental inequities in social determinants of health (SDH), such as whether a patient has safe housing, transportation, education, job opportunities, income, access to nutritious foods, and language and literacy skills, among others.
Dr. Islami’s analysis, for example, shows that people of color are generally more likely to have lower educational attainment and to experience poverty, food insecurity, and housing insecurity compared with White people. Among people aged 18-64 years, the age-adjusted proportion of individuals with no health insurance in 2021 was also higher among Black (13.7%), American Indian/Alaskan Native (18.7%), and Hispanic (28.7%) patients than among White (7.8%) or Asian (5.9%) people, according to the report.
Competing needs can also get in the way of prioritizing cancer screenings, especially for patients in lower socio-economic populations, Dr. Ganschow said.
“You’ve got people who are working a job or three jobs, just to make ends meet for their family and can’t necessarily take time off to get that done,” she said. “Nor is it prioritized in their head because they’ve got to put a meal on the table.”
But the racial disparities between Black and White women, at least, are not clearly explained by differences between the screening rates..
Of patients who received mammograms 76% were White and 79% were Black, according to another recent study coauthored by Dr. Islami. While Black women appear to have the highest breast cancer screening rates, some data suggest such rates are being overreported.
Lower screening rates were seen in American Indian/Alaska Native (59%), Asian (67%), and Hispanic women (74%).
Biological Differences, Bad Testing Recommendations May Contribute to Poor Outcomes
Differences in biology may be one overlooked internal driver of lower breast cancer survival in Black women.
Researchers at Sanford Burnham Prebys in La Jolla, California, recently analyzed the breast cells of White and Black women, finding significant molecular differences that may be contributing to higher breast cancer mortality rates in Black women.
Investigators analyzed both healthy tissue and tumor tissue from 185 Black women and compared the samples to that of White women. They discovered differences among Black and White women in the way their DNA repair genes are expressed, both in healthy breast tissue and in tumors positive for estrogen receptor breast cancer. Molecular differences were also present in the cellular signals that control how fast cells, including cancer cells, grow.
DNA repair is part of normal cellular function and helps cells recover from damage that can occur during DNA replication or in response to external factors, such as stress.
“One of the first lines of defense, to prevent the cell from becoming a tumor are DNA damage repair pathways,” said Svasti Haricharan, PhD, a coauthor of the study and an assistant professor at Sanford Burnham Prebys. “We know there are many different DNA damage repair pathways that respond to different types of DNA damage. What we didn’t know was that, even in our normal cells, based on your race and ethnicity, you have different levels of DNA repair proteins.”
The study found that many of the proteins associated with endocrine resistance and poor outcomes in breast cancer patients are differently regulated in Black women compared with White woman. These differences contribute to resistance to standard endocrine therapy, Dr. Haricharan said.
“Because we never studied the biology in Black woman, it was just assumed that across all demographics, it must be the same,” she said. “We are not even accounting for the possibility there are likely intrinsic differences for how you will respond to an endocrine treatment.”
Testing and treatment may also be playing a role in worse breast cancer outcomes for Black women.
In an analysis of 73,363 women with early-stage, estrogen receptor–positive breast cancer, investigators found that a common test used to decide the treatment course for patients may be leading to bad recommendations for Black women.
The test, known as the 21-gene breast recurrence score, is the most commonly ordered biomarker test used to guide doctor’s recommendations for patients with estrogen receptor–positive breast cancer, the most common form of cancer in Black women, representing about 70%-80% of cases.
The test helps physicians identify which patients are good candidates for chemo, but the test may underestimate the benefit of chemo for Black women. It ranks some Black women as unlikely to benefit from chemo, when they actually would have benefited, according to the January 2024 study, published in the Journal of the National Comprehensive Cancer Network.
The test gives a score of zero to 100, explains Kent Hoskins, MD, oncology service line medical director at the University of Illinois (UI) Health and director of the Familial Breast Cancer Clinic at UI Health, both in Chicago. The higher the score, the higher the risk and the greater the benefit of chemotherapy. A patient is either above the cut-off score and receives chemo, or is below the cut-off score and does not. In the analysis, investigators found that Black women start improving with chemo at a lower score than White women do.
Dr. Hoskins said the results raise questions about whether the biomarker test should be modified to be more applicable to Black women, whether other tests should be used, or if physicians should judge cut-off scores differently, depending on race.
How Neighborhood Impacts Breast Cancer, Death Rates
Living in a disadvantaged neighborhood also lowers breast cancer survival, according to new research. A disadvantaged neighborhood is generally defined as a location associated with higher concentrations of poverty, higher rates of unemployment, and less access to health care, quality housing, food, and community resources, according to the Centers for Disease Control and Prevention.
Authors of a study published in JAMA Network Open on April 18 identified 350,824 patients with breast cancer. Of these, 41,519 (11.8%) were Hispanic, 39,631 (11.3%) were non-Hispanic Black, and 234,698 (66.9%) were non-Hispanic White. Investigators divided the patients into five groups representing the lowest to highest neighborhood socioeconomic indices using the Yost Index. (The Yost Index is used by the National Cancer Institute for cancer surveillance and is based on variables such as household income, home value, median rent, percentage below 150% of the poverty line, education, and unemployment.)
Of the Black and Hispanic patients in the study, the highest proportions of both demographics lived in the most disadvantaged neighborhoods. (16,141 Black patients [30.9%]) and 10,168 Hispanic patients [19.5%]). Although 45% of White patients also fell into that same category, the highest proportion of White patients in the study lived in the most advantaged neighborhoods (66,529 patients [76.2%]).
Findings showed patients in the most disadvantaged neighborhoods had the highest proportion of triple-negative breast cancer. Patients in this group also had the lowest proportion of patients who completed surgery and radiation, and the highest proportion of patients who received chemotherapy, compared with all other neighborhood groups. The most advantaged neighborhoods group had higher proportions of localized-stage cancer, a higher proportion of patients who underwent surgery and radiation, and the lowest proportion of patients receiving chemotherapy treatment.
Patients in the most disadvantaged neighborhoods also had the highest risk of mortality (hazard ratio [HR,] 1.53; 95% CI, 1.48-1.59; P less than .001) compared with patients living in the most advantaged neighborhoods. Non-Hispanic Black patients in particular, had the highest risk of mortality, compared with non-Hispanic White patients (HR, 1.16; 95% CI, 1.13-1.20; P less than .001).
Authors wrote that the findings suggest neighborhood disadvantage is independently associated with shorter survival in patients with breast cancer, even after controlling for individual-level factors, tumor characteristics, and treatment.
“To address these residual disparities associated with neighborhood disadvantage, research must focus on which components of the built environment influence outcomes,” the authors said.
Another recent study also found correlations among where breast cancer patients lived and how they fared with the disease.
Jasmine M. Miller-Kleinhenz, PhD, an assistant professor at University of Mississippi Medical Center in Jackson, studied how historical redlining impacts breast cancer development and outcomes in her research published in JAMA Network Open, earlier this year. Redlining refers to the practice of denying people access to credit because of where they live. Historically, mortgage lenders widely redlined neighborhoods with predominantly Black residents. The 1968 Fair Housing Act outlawed racially motivated redlining, but consequences from historical redlining still exist.
Dr. Miller-Kleinhenz and her colleagues analyzed a cohort of 1764 women diagnosed with breast cancer between January 2010 and December 2017, who were followed up through December 2019. Investigators accessed the cohort based on three exposures: historic redlining (HRL), contemporary mortgage discrimination (CMD), and persistent mortgage discrimination (PMD). Contemporary mortgage discrimination refers to current-day discriminatory mortgage practices and persistent mortgage discrimination refers to neighborhoods that have experienced both HRL and CMD.
Findings showed that Black women living in historical redlined areas had increased odds of being diagnosed with aggressive forms of breast cancer, while White women in redlined areas had increased odds of late-stage diagnosis.
White women exposed to persistent mortgage discrimination were twice as likely to die of breast cancer, compared with their White counterparts living in areas without historical redlining or contemporary mortgage discrimination, the study found.
That is not to say that Black women did not have an increased risk of breast cancer mortality, Dr. Miller-Kleinhenz explained. Black women had a more than threefold elevated risk of breast cancer mortality compared with White women no matter where they lived, according to the findings.
“These results were surprising because it is showing that while neighborhood conditions might be a major driver of breast cancer mortality in White women, there are factors beyond the neighborhood that are additional drivers that are contributing to poor outcomes in Black women,” she said.
Hope for Improved Outcomes, Higher Survival Rates
Investigators hope the findings of all of this new research lead to better, more targeted treatments and, in turn, improved outcomes.
Dr. Haricharan is optimistic about the improvement of breast cancer outcomes as more is learned about the biology of Black patients and other non-White patients.
There is a growing effort to include more data from minoritized populations in breast cancer research studies, Dr. Haricharan said, and she foresees associated changes to clinical protocols in the future. Her own team is working on creating larger data sets that are more representative of non-White patients to further analyze the differences found in their prior study.
“I think there’s this understanding that, until we have data sets that are more representative, we really are catering to [only one] population in terms of our diagnostic and therapeutic technological advances,” she said.
The American Cancer Society meanwhile, is launching a new initiative in May that aims to collect more health data from Black women to ultimately develop more effective cancer interventions. VOICES of Black Women will focus on collecting and studying health data from Black women through online surveys. The society’s goal is to enroll at least 100,000 Black women in the United States between ages 25 and 55.
Dr. Miller-Kleinhenz called the initiative “an important step to starting to research and answer some of these lingering questions about why there continue to be breast cancer disparities.”
Half-Truths Produce Whole Failures in Health Policy
On May 5, 2023, the director of the Centers for Disease Control and Prevention (CDC), Rochelle Walensky, in announcing her resignation after more than 2 years of dedicated service, wrote that she “took on this role … with the goal of leaving behind the dark days of the pandemic and moving the CDC — and public health — forward into a much better and more trusted place.”
Three times in the past 3 years I have written a Beyond the White Coat column emphasizing the importance of trust. Trust in the expertise of scientists. Trust in the integrity of medical research and public health institutions. Trust in the commitment of providers — doctors, nurses, therapists, and first responders — to shepherd us through the pandemic and other medical crises in our lives. This column is take four.
All human institutions have human imperfections. However, imperfect humans working together in community are more productive and more reliable than nihilism and political polarization. Underlying all of healthcare are compassion and honesty. Honesty means the truth, the whole truth, and nothing but the truth. Honesty is such a simple concept in the moral formation of children, but the concept has evolved aberrantly in the world of woke adults. There appear to be irresistible temptations to shade that truth for political gain. The dominant current mutation is the half-truth. One tells the part of the truth that appears to advance one’s own political aspirations and at the same time one omits or censors other viewpoints.
On April 17, 2023, the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the American Psychiatric Association wrote an open letter to Congressional leaders advocating for transgender female students’ participation in girl’s and women’s sports. The letter was written “On behalf of the more than 165,000” members of those organizations, though public opinion polls show a majority of those members likely oppose the opinion expressed. The letter goes on to extol the benefits that sports might bring to transgender students, but it contains not one word acknowledging the negative impact that participation has on others. That is a half-truth.
The same half-truth methodology distorts dialogue about various therapies for gender dysphoria in children and young adults.
In April 2022, U.S. Assistant Secretary for Health Rachel Levine in an NPR interview declared that, “There is no argument about the value and importance of gender-affirming care.” That might be a half-truth, since I could not locate U.S. specialists who dare to go on record questioning the party line of the World Professional Association for Transgender Health. However, Dr. Levine’s dismissal of any dissent is bizarre since in the prior 2 years multiple countries, including Australia, New Zealand, Sweden, Finland, and the United Kingdom had all issued reports questioning and even rescinding the practices that evolved since the 2012 WPATH guidance. Their main concerns included 1) the marked increase in incidence of gender dysphoria first manifesting in tween and early teenage girls, 2) the inadequate access to mental health screening before considering transitioning, 3) the long-term risks of puberty blockers particularly to bone density, and 4) the low quality of evidence supporting a measurable reduction in suicide rates. There may be reasonable counterarguments to each of those concerns, but a high ranking U.S. government official labeling all those international reports as “no argument” does not produce high quality decision making and does not foster the public’s trust.
Indeed, the public in many cases has decided its elected legislators are more trustworthy on these topics than the medical organizations. As I wrote the first draft of this column, the Missouri state legislators had passed a bill banning gender-affirming health care for transgender minors. They also passed a bill preventing participation of transgender females in women’s sports. Per reckoning by CBS News in the summer of 2023, 16 states had recently enacted laws restricting gender-affirming care and 22 states had restricted transgender participation in sports.
In 2022, I wrote a column claiming that suppressing viewpoints and debate leads to exploding spaceships. I believe the current legislative carnage is just such an explosion. It harms children.
The AAP has experts in advocacy. I am no expert in political advocacy. Perhaps politics has to be played by different rules where half-truths are normalized. Criminal law and advertising use those rules. But this explosion of vitriol and legislative intrusion into medicine should prompt everyone to reassess the use of one-sided advocacy in public and professional circles in healthcare. I want to be associated with a profession that uses evidence-based medicine that is not corrupted with political agendas. I want to be associated with a profession known for telling the whole truth.
In a society that is increasingly polarized, I want to embrace the advice of John Stuart Mill, a 19th century English philosopher best known for utilitarianism, which is often expressed as “the greatest good for the greatest number.” Mr. Mill also wrote on social theory, liberty, and even some early feminist theory. His 1859 work, On Liberty, chapter II, asserts: “He who knows only his own side of the case, knows little of that. His reasons may be good, and no one may have been able to refute them. But if he is equally unable to refute the reasons on the opposite side; if he does not so much as know what they are, he has no ground for preferring either opinion.”
Mr. Mill did not like half-truths.
It’s About Trust
My column is not the instrument to debate the use of hormones as puberty blockers or the fairness of transgender women participating in women’s sports. Those judgments will be rendered by others. I may report on those deliberations, but my column’s emphasis is on how professionals, and their organizations, go about making those determinations
For instance, the National Health Service in the United Kingdom spent 2 years reassessing transgender care for children and in October 2022 released a draft proposal to reduce and limit the aggressive therapies. On June 9, 2023, the NHS fully enacted those changes. Puberty blockers for gender dysphoria would be used only in experimental trials. In April 2024 the NHS began implementing those changes, joining other European countries that have imposed similar restrictions.
Similarly, the debate about transgender participation in women’s sports has continued to rage for years. On April 8, 2024, the National Association of Intercollegiate Athletics passed a resolution that bans almost all transgender participation in NAIA-regulated intercollegiate women’s sports. Dance and cheerleading are exceptions. Participation is still permissible at the intramural level. The NCAA has different rules.
Go to those sources to learn more substance for those debates. This column is about trust.
A major problem currently facing medicine is the public’s trust in expertise. That trust had been seriously weakened before the pandemic and was repeatedly wounded during the pandemic with arguments over masks, vaccines, and shutdowns. It needs repair.
This is especially true for pediatric hospitalists that do not have the opportunity that office-based pediatricians have to build rapport with a family over years. At a recent university conference on diversity, equity, and inclusivity, one female rabbi stated, “I cannot be rabbi to everybody.” I agreed, but as a medical professional, sometimes I must be.
Telling half-truths harms the public’s trust in their personal physicians and in the medical establishment. Once people suspect an organization is making decisions based on ideology rather than science, credibility is lost and difficult to recover.
Let us stop telling half-truths. Let us stop suppressing dialogue. Truth can never be completely captured by humans, but if one side of an issue is suppressed by cancel culture, censorship, accusations of homophobia, or threat of cultural war, the search for truth is severely impaired.
Let us, as medical professionals, adopt Stephen Covey’s habit number 5, “Seek first to understand, then to be understood.” Empower voices. Listen to all stakeholders. And when we finally do speak, remember John Stuart Mill and tell the whole truth.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
On May 5, 2023, the director of the Centers for Disease Control and Prevention (CDC), Rochelle Walensky, in announcing her resignation after more than 2 years of dedicated service, wrote that she “took on this role … with the goal of leaving behind the dark days of the pandemic and moving the CDC — and public health — forward into a much better and more trusted place.”
Three times in the past 3 years I have written a Beyond the White Coat column emphasizing the importance of trust. Trust in the expertise of scientists. Trust in the integrity of medical research and public health institutions. Trust in the commitment of providers — doctors, nurses, therapists, and first responders — to shepherd us through the pandemic and other medical crises in our lives. This column is take four.
All human institutions have human imperfections. However, imperfect humans working together in community are more productive and more reliable than nihilism and political polarization. Underlying all of healthcare are compassion and honesty. Honesty means the truth, the whole truth, and nothing but the truth. Honesty is such a simple concept in the moral formation of children, but the concept has evolved aberrantly in the world of woke adults. There appear to be irresistible temptations to shade that truth for political gain. The dominant current mutation is the half-truth. One tells the part of the truth that appears to advance one’s own political aspirations and at the same time one omits or censors other viewpoints.
On April 17, 2023, the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the American Psychiatric Association wrote an open letter to Congressional leaders advocating for transgender female students’ participation in girl’s and women’s sports. The letter was written “On behalf of the more than 165,000” members of those organizations, though public opinion polls show a majority of those members likely oppose the opinion expressed. The letter goes on to extol the benefits that sports might bring to transgender students, but it contains not one word acknowledging the negative impact that participation has on others. That is a half-truth.
The same half-truth methodology distorts dialogue about various therapies for gender dysphoria in children and young adults.
In April 2022, U.S. Assistant Secretary for Health Rachel Levine in an NPR interview declared that, “There is no argument about the value and importance of gender-affirming care.” That might be a half-truth, since I could not locate U.S. specialists who dare to go on record questioning the party line of the World Professional Association for Transgender Health. However, Dr. Levine’s dismissal of any dissent is bizarre since in the prior 2 years multiple countries, including Australia, New Zealand, Sweden, Finland, and the United Kingdom had all issued reports questioning and even rescinding the practices that evolved since the 2012 WPATH guidance. Their main concerns included 1) the marked increase in incidence of gender dysphoria first manifesting in tween and early teenage girls, 2) the inadequate access to mental health screening before considering transitioning, 3) the long-term risks of puberty blockers particularly to bone density, and 4) the low quality of evidence supporting a measurable reduction in suicide rates. There may be reasonable counterarguments to each of those concerns, but a high ranking U.S. government official labeling all those international reports as “no argument” does not produce high quality decision making and does not foster the public’s trust.
Indeed, the public in many cases has decided its elected legislators are more trustworthy on these topics than the medical organizations. As I wrote the first draft of this column, the Missouri state legislators had passed a bill banning gender-affirming health care for transgender minors. They also passed a bill preventing participation of transgender females in women’s sports. Per reckoning by CBS News in the summer of 2023, 16 states had recently enacted laws restricting gender-affirming care and 22 states had restricted transgender participation in sports.
In 2022, I wrote a column claiming that suppressing viewpoints and debate leads to exploding spaceships. I believe the current legislative carnage is just such an explosion. It harms children.
The AAP has experts in advocacy. I am no expert in political advocacy. Perhaps politics has to be played by different rules where half-truths are normalized. Criminal law and advertising use those rules. But this explosion of vitriol and legislative intrusion into medicine should prompt everyone to reassess the use of one-sided advocacy in public and professional circles in healthcare. I want to be associated with a profession that uses evidence-based medicine that is not corrupted with political agendas. I want to be associated with a profession known for telling the whole truth.
In a society that is increasingly polarized, I want to embrace the advice of John Stuart Mill, a 19th century English philosopher best known for utilitarianism, which is often expressed as “the greatest good for the greatest number.” Mr. Mill also wrote on social theory, liberty, and even some early feminist theory. His 1859 work, On Liberty, chapter II, asserts: “He who knows only his own side of the case, knows little of that. His reasons may be good, and no one may have been able to refute them. But if he is equally unable to refute the reasons on the opposite side; if he does not so much as know what they are, he has no ground for preferring either opinion.”
Mr. Mill did not like half-truths.
It’s About Trust
My column is not the instrument to debate the use of hormones as puberty blockers or the fairness of transgender women participating in women’s sports. Those judgments will be rendered by others. I may report on those deliberations, but my column’s emphasis is on how professionals, and their organizations, go about making those determinations
For instance, the National Health Service in the United Kingdom spent 2 years reassessing transgender care for children and in October 2022 released a draft proposal to reduce and limit the aggressive therapies. On June 9, 2023, the NHS fully enacted those changes. Puberty blockers for gender dysphoria would be used only in experimental trials. In April 2024 the NHS began implementing those changes, joining other European countries that have imposed similar restrictions.
Similarly, the debate about transgender participation in women’s sports has continued to rage for years. On April 8, 2024, the National Association of Intercollegiate Athletics passed a resolution that bans almost all transgender participation in NAIA-regulated intercollegiate women’s sports. Dance and cheerleading are exceptions. Participation is still permissible at the intramural level. The NCAA has different rules.
Go to those sources to learn more substance for those debates. This column is about trust.
A major problem currently facing medicine is the public’s trust in expertise. That trust had been seriously weakened before the pandemic and was repeatedly wounded during the pandemic with arguments over masks, vaccines, and shutdowns. It needs repair.
This is especially true for pediatric hospitalists that do not have the opportunity that office-based pediatricians have to build rapport with a family over years. At a recent university conference on diversity, equity, and inclusivity, one female rabbi stated, “I cannot be rabbi to everybody.” I agreed, but as a medical professional, sometimes I must be.
Telling half-truths harms the public’s trust in their personal physicians and in the medical establishment. Once people suspect an organization is making decisions based on ideology rather than science, credibility is lost and difficult to recover.
Let us stop telling half-truths. Let us stop suppressing dialogue. Truth can never be completely captured by humans, but if one side of an issue is suppressed by cancel culture, censorship, accusations of homophobia, or threat of cultural war, the search for truth is severely impaired.
Let us, as medical professionals, adopt Stephen Covey’s habit number 5, “Seek first to understand, then to be understood.” Empower voices. Listen to all stakeholders. And when we finally do speak, remember John Stuart Mill and tell the whole truth.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
On May 5, 2023, the director of the Centers for Disease Control and Prevention (CDC), Rochelle Walensky, in announcing her resignation after more than 2 years of dedicated service, wrote that she “took on this role … with the goal of leaving behind the dark days of the pandemic and moving the CDC — and public health — forward into a much better and more trusted place.”
Three times in the past 3 years I have written a Beyond the White Coat column emphasizing the importance of trust. Trust in the expertise of scientists. Trust in the integrity of medical research and public health institutions. Trust in the commitment of providers — doctors, nurses, therapists, and first responders — to shepherd us through the pandemic and other medical crises in our lives. This column is take four.
All human institutions have human imperfections. However, imperfect humans working together in community are more productive and more reliable than nihilism and political polarization. Underlying all of healthcare are compassion and honesty. Honesty means the truth, the whole truth, and nothing but the truth. Honesty is such a simple concept in the moral formation of children, but the concept has evolved aberrantly in the world of woke adults. There appear to be irresistible temptations to shade that truth for political gain. The dominant current mutation is the half-truth. One tells the part of the truth that appears to advance one’s own political aspirations and at the same time one omits or censors other viewpoints.
On April 17, 2023, the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the American Psychiatric Association wrote an open letter to Congressional leaders advocating for transgender female students’ participation in girl’s and women’s sports. The letter was written “On behalf of the more than 165,000” members of those organizations, though public opinion polls show a majority of those members likely oppose the opinion expressed. The letter goes on to extol the benefits that sports might bring to transgender students, but it contains not one word acknowledging the negative impact that participation has on others. That is a half-truth.
The same half-truth methodology distorts dialogue about various therapies for gender dysphoria in children and young adults.
In April 2022, U.S. Assistant Secretary for Health Rachel Levine in an NPR interview declared that, “There is no argument about the value and importance of gender-affirming care.” That might be a half-truth, since I could not locate U.S. specialists who dare to go on record questioning the party line of the World Professional Association for Transgender Health. However, Dr. Levine’s dismissal of any dissent is bizarre since in the prior 2 years multiple countries, including Australia, New Zealand, Sweden, Finland, and the United Kingdom had all issued reports questioning and even rescinding the practices that evolved since the 2012 WPATH guidance. Their main concerns included 1) the marked increase in incidence of gender dysphoria first manifesting in tween and early teenage girls, 2) the inadequate access to mental health screening before considering transitioning, 3) the long-term risks of puberty blockers particularly to bone density, and 4) the low quality of evidence supporting a measurable reduction in suicide rates. There may be reasonable counterarguments to each of those concerns, but a high ranking U.S. government official labeling all those international reports as “no argument” does not produce high quality decision making and does not foster the public’s trust.
Indeed, the public in many cases has decided its elected legislators are more trustworthy on these topics than the medical organizations. As I wrote the first draft of this column, the Missouri state legislators had passed a bill banning gender-affirming health care for transgender minors. They also passed a bill preventing participation of transgender females in women’s sports. Per reckoning by CBS News in the summer of 2023, 16 states had recently enacted laws restricting gender-affirming care and 22 states had restricted transgender participation in sports.
In 2022, I wrote a column claiming that suppressing viewpoints and debate leads to exploding spaceships. I believe the current legislative carnage is just such an explosion. It harms children.
The AAP has experts in advocacy. I am no expert in political advocacy. Perhaps politics has to be played by different rules where half-truths are normalized. Criminal law and advertising use those rules. But this explosion of vitriol and legislative intrusion into medicine should prompt everyone to reassess the use of one-sided advocacy in public and professional circles in healthcare. I want to be associated with a profession that uses evidence-based medicine that is not corrupted with political agendas. I want to be associated with a profession known for telling the whole truth.
In a society that is increasingly polarized, I want to embrace the advice of John Stuart Mill, a 19th century English philosopher best known for utilitarianism, which is often expressed as “the greatest good for the greatest number.” Mr. Mill also wrote on social theory, liberty, and even some early feminist theory. His 1859 work, On Liberty, chapter II, asserts: “He who knows only his own side of the case, knows little of that. His reasons may be good, and no one may have been able to refute them. But if he is equally unable to refute the reasons on the opposite side; if he does not so much as know what they are, he has no ground for preferring either opinion.”
Mr. Mill did not like half-truths.
It’s About Trust
My column is not the instrument to debate the use of hormones as puberty blockers or the fairness of transgender women participating in women’s sports. Those judgments will be rendered by others. I may report on those deliberations, but my column’s emphasis is on how professionals, and their organizations, go about making those determinations
For instance, the National Health Service in the United Kingdom spent 2 years reassessing transgender care for children and in October 2022 released a draft proposal to reduce and limit the aggressive therapies. On June 9, 2023, the NHS fully enacted those changes. Puberty blockers for gender dysphoria would be used only in experimental trials. In April 2024 the NHS began implementing those changes, joining other European countries that have imposed similar restrictions.
Similarly, the debate about transgender participation in women’s sports has continued to rage for years. On April 8, 2024, the National Association of Intercollegiate Athletics passed a resolution that bans almost all transgender participation in NAIA-regulated intercollegiate women’s sports. Dance and cheerleading are exceptions. Participation is still permissible at the intramural level. The NCAA has different rules.
Go to those sources to learn more substance for those debates. This column is about trust.
A major problem currently facing medicine is the public’s trust in expertise. That trust had been seriously weakened before the pandemic and was repeatedly wounded during the pandemic with arguments over masks, vaccines, and shutdowns. It needs repair.
This is especially true for pediatric hospitalists that do not have the opportunity that office-based pediatricians have to build rapport with a family over years. At a recent university conference on diversity, equity, and inclusivity, one female rabbi stated, “I cannot be rabbi to everybody.” I agreed, but as a medical professional, sometimes I must be.
Telling half-truths harms the public’s trust in their personal physicians and in the medical establishment. Once people suspect an organization is making decisions based on ideology rather than science, credibility is lost and difficult to recover.
Let us stop telling half-truths. Let us stop suppressing dialogue. Truth can never be completely captured by humans, but if one side of an issue is suppressed by cancel culture, censorship, accusations of homophobia, or threat of cultural war, the search for truth is severely impaired.
Let us, as medical professionals, adopt Stephen Covey’s habit number 5, “Seek first to understand, then to be understood.” Empower voices. Listen to all stakeholders. And when we finally do speak, remember John Stuart Mill and tell the whole truth.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Internists blame bureaucracy as top cause of burnout
Reported burnout among internal medicine physicians decreased over the past year based on data from Medscape’s annual survey of burnout and depression among physicians in the United States.
Approximately 80% of male internists and 85% of female internists said that their feelings of burnout and/or depression were driven by their jobs all or most of the time. The job-related stress and burnout come home with them — 76% of respondents overall said that burnout had negatively affected their personal relationships.
Too many bureaucratic tasks such as charting and paperwork were by far the top contributor to burnout, reported by 70% of respondents, with insufficient compensation and lack of respect from employers, colleagues, and staff as relatively distant second and third contributors (40% and 37%, respectively).
In addition, nearly half of the physicians said that their burnout was severe enough that they might leave medicine.
To help manage burnout, more internists reported positive coping strategies such as exercise (51%), talking with friends and family (47%), spending time alone (41%), and sleeping (40%), compared to less healthy strategies such as eating junk food, drinking alcohol, and using nicotine or cannabis products.
When asked what workplace measures would help with burnout, no one strategy rose to the top, but the top three were increased compensation (49%), additional support staff (48%), and more flexible work schedules (45%).
Notably, 62% of internists reported depression they defined as colloquial (feeling down or sad) and 27% described their depression as clinical. However, only 9% said they had sought professional help for depression, and 15% said they had sought help for burnout.
Staying in Practice Despite Burnout
The percentage of physicians across specialties who report depression and burnout worsened during the COVID-19 pandemic, said Noel Deep, MD, an internal medicine physician in group practice in Antigo, Wisconsin, in an interview.
Since the pandemic, newer stressors have replaced the pandemic-related stressors, and increasing bureaucratic burdens and paperwork continue to cause more physicians to report burnout, he said.
“If not assessed and addressed, this will lead to attrition in the physician workforce leading to increased burden on other physicians and impact patient access to healthcare,” he added.
The survey findings reflect Dr. Deep’s observations. “When talking to physicians across specialties, I have heard universally from many physicians about their experiences and ongoing struggles with potential burnout and mood-related issues,” he said. “While many of them feel that they are getting to the point of burnout, most of them also stoically continue to provide care to patients because they feel an obligation to them,” he said.
This feeling of obligation to patients is why less than one third of the physicians who consider retiring or leaving medicine because of burnout actually do, he said.
As for measures to reduce burnout, “I personally feel that increasing the compensation will not lead to decreased burnout,” Dr. Deep said. Although more money may provide temporary satisfaction, it will not yield long-term improvement in burnout, he said. “Based on personal experiences and my interactions with physicians, providing them more autonomy and control over their practices ... would contribute to decreasing the burnout,” Dr. Deep emphasized.
What Is to Be Done?
“I would favor having physician leaders in healthcare organizations take the time to talk to physicians [and] provide mentoring programs when new physicians are recruited, with ongoing discussions at operations and governance meetings about physician health and wellness,” Dr. Deep said. Providing frequent updates to physicians about wellness resources and encouraging them to seek out help anonymously through Employee Assistance Programs and other counseling services would be beneficial, he added.
“I would also consider peer mentoring when possible. Employers, healthcare organizations, and other key stakeholders should continue to work toward decreasing the stigma of depression and burnout,” Dr. Deep said.
Employers can help physicians manage and reduce burnout and depression by engaging with them, listening to their concerns, and trying to address them, said Dr. Deep. These actions will increase physicians’ trust in their administrations and promote a positive and healthy work environment, he said. “This will lead to reduced attrition in the workforce, retention of experienced physicians and support staff, and lead to increased patient satisfaction as well.”
The data come from Medscape’s annual report on Physician Burnout & Depression, which included 9226 practicing physicians in the United States across more than 29 specialties.
Dr. Deep had no financial conflicts to disclose; he serves on the Editorial Advisory Board of Internal Medicine News.
Reported burnout among internal medicine physicians decreased over the past year based on data from Medscape’s annual survey of burnout and depression among physicians in the United States.
Approximately 80% of male internists and 85% of female internists said that their feelings of burnout and/or depression were driven by their jobs all or most of the time. The job-related stress and burnout come home with them — 76% of respondents overall said that burnout had negatively affected their personal relationships.
Too many bureaucratic tasks such as charting and paperwork were by far the top contributor to burnout, reported by 70% of respondents, with insufficient compensation and lack of respect from employers, colleagues, and staff as relatively distant second and third contributors (40% and 37%, respectively).
In addition, nearly half of the physicians said that their burnout was severe enough that they might leave medicine.
To help manage burnout, more internists reported positive coping strategies such as exercise (51%), talking with friends and family (47%), spending time alone (41%), and sleeping (40%), compared to less healthy strategies such as eating junk food, drinking alcohol, and using nicotine or cannabis products.
When asked what workplace measures would help with burnout, no one strategy rose to the top, but the top three were increased compensation (49%), additional support staff (48%), and more flexible work schedules (45%).
Notably, 62% of internists reported depression they defined as colloquial (feeling down or sad) and 27% described their depression as clinical. However, only 9% said they had sought professional help for depression, and 15% said they had sought help for burnout.
Staying in Practice Despite Burnout
The percentage of physicians across specialties who report depression and burnout worsened during the COVID-19 pandemic, said Noel Deep, MD, an internal medicine physician in group practice in Antigo, Wisconsin, in an interview.
Since the pandemic, newer stressors have replaced the pandemic-related stressors, and increasing bureaucratic burdens and paperwork continue to cause more physicians to report burnout, he said.
“If not assessed and addressed, this will lead to attrition in the physician workforce leading to increased burden on other physicians and impact patient access to healthcare,” he added.
The survey findings reflect Dr. Deep’s observations. “When talking to physicians across specialties, I have heard universally from many physicians about their experiences and ongoing struggles with potential burnout and mood-related issues,” he said. “While many of them feel that they are getting to the point of burnout, most of them also stoically continue to provide care to patients because they feel an obligation to them,” he said.
This feeling of obligation to patients is why less than one third of the physicians who consider retiring or leaving medicine because of burnout actually do, he said.
As for measures to reduce burnout, “I personally feel that increasing the compensation will not lead to decreased burnout,” Dr. Deep said. Although more money may provide temporary satisfaction, it will not yield long-term improvement in burnout, he said. “Based on personal experiences and my interactions with physicians, providing them more autonomy and control over their practices ... would contribute to decreasing the burnout,” Dr. Deep emphasized.
What Is to Be Done?
“I would favor having physician leaders in healthcare organizations take the time to talk to physicians [and] provide mentoring programs when new physicians are recruited, with ongoing discussions at operations and governance meetings about physician health and wellness,” Dr. Deep said. Providing frequent updates to physicians about wellness resources and encouraging them to seek out help anonymously through Employee Assistance Programs and other counseling services would be beneficial, he added.
“I would also consider peer mentoring when possible. Employers, healthcare organizations, and other key stakeholders should continue to work toward decreasing the stigma of depression and burnout,” Dr. Deep said.
Employers can help physicians manage and reduce burnout and depression by engaging with them, listening to their concerns, and trying to address them, said Dr. Deep. These actions will increase physicians’ trust in their administrations and promote a positive and healthy work environment, he said. “This will lead to reduced attrition in the workforce, retention of experienced physicians and support staff, and lead to increased patient satisfaction as well.”
The data come from Medscape’s annual report on Physician Burnout & Depression, which included 9226 practicing physicians in the United States across more than 29 specialties.
Dr. Deep had no financial conflicts to disclose; he serves on the Editorial Advisory Board of Internal Medicine News.
Reported burnout among internal medicine physicians decreased over the past year based on data from Medscape’s annual survey of burnout and depression among physicians in the United States.
Approximately 80% of male internists and 85% of female internists said that their feelings of burnout and/or depression were driven by their jobs all or most of the time. The job-related stress and burnout come home with them — 76% of respondents overall said that burnout had negatively affected their personal relationships.
Too many bureaucratic tasks such as charting and paperwork were by far the top contributor to burnout, reported by 70% of respondents, with insufficient compensation and lack of respect from employers, colleagues, and staff as relatively distant second and third contributors (40% and 37%, respectively).
In addition, nearly half of the physicians said that their burnout was severe enough that they might leave medicine.
To help manage burnout, more internists reported positive coping strategies such as exercise (51%), talking with friends and family (47%), spending time alone (41%), and sleeping (40%), compared to less healthy strategies such as eating junk food, drinking alcohol, and using nicotine or cannabis products.
When asked what workplace measures would help with burnout, no one strategy rose to the top, but the top three were increased compensation (49%), additional support staff (48%), and more flexible work schedules (45%).
Notably, 62% of internists reported depression they defined as colloquial (feeling down or sad) and 27% described their depression as clinical. However, only 9% said they had sought professional help for depression, and 15% said they had sought help for burnout.
Staying in Practice Despite Burnout
The percentage of physicians across specialties who report depression and burnout worsened during the COVID-19 pandemic, said Noel Deep, MD, an internal medicine physician in group practice in Antigo, Wisconsin, in an interview.
Since the pandemic, newer stressors have replaced the pandemic-related stressors, and increasing bureaucratic burdens and paperwork continue to cause more physicians to report burnout, he said.
“If not assessed and addressed, this will lead to attrition in the physician workforce leading to increased burden on other physicians and impact patient access to healthcare,” he added.
The survey findings reflect Dr. Deep’s observations. “When talking to physicians across specialties, I have heard universally from many physicians about their experiences and ongoing struggles with potential burnout and mood-related issues,” he said. “While many of them feel that they are getting to the point of burnout, most of them also stoically continue to provide care to patients because they feel an obligation to them,” he said.
This feeling of obligation to patients is why less than one third of the physicians who consider retiring or leaving medicine because of burnout actually do, he said.
As for measures to reduce burnout, “I personally feel that increasing the compensation will not lead to decreased burnout,” Dr. Deep said. Although more money may provide temporary satisfaction, it will not yield long-term improvement in burnout, he said. “Based on personal experiences and my interactions with physicians, providing them more autonomy and control over their practices ... would contribute to decreasing the burnout,” Dr. Deep emphasized.
What Is to Be Done?
“I would favor having physician leaders in healthcare organizations take the time to talk to physicians [and] provide mentoring programs when new physicians are recruited, with ongoing discussions at operations and governance meetings about physician health and wellness,” Dr. Deep said. Providing frequent updates to physicians about wellness resources and encouraging them to seek out help anonymously through Employee Assistance Programs and other counseling services would be beneficial, he added.
“I would also consider peer mentoring when possible. Employers, healthcare organizations, and other key stakeholders should continue to work toward decreasing the stigma of depression and burnout,” Dr. Deep said.
Employers can help physicians manage and reduce burnout and depression by engaging with them, listening to their concerns, and trying to address them, said Dr. Deep. These actions will increase physicians’ trust in their administrations and promote a positive and healthy work environment, he said. “This will lead to reduced attrition in the workforce, retention of experienced physicians and support staff, and lead to increased patient satisfaction as well.”
The data come from Medscape’s annual report on Physician Burnout & Depression, which included 9226 practicing physicians in the United States across more than 29 specialties.
Dr. Deep had no financial conflicts to disclose; he serves on the Editorial Advisory Board of Internal Medicine News.
Progressively Worsening Scaly Patches and Plaques in an Infant
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
A 5-month-old male with moderately brown skin that rarely burns and tans profusely presented to the emergency department with a worsening red rash of more than 4 months’ duration. The patient had diffuse erythroderma and eczematous patches and plaques covering 95% of the total body surface area, including lichenified plaques on the arms and elbows, with no signs of infection. He initially presented for his 1-month appointment at the pediatric clinic with scaly patches and plaques on the face and trunk as well as diffuse xerosis. He was prescribed daily oatmeal baths and topical Minerin (Major Pharmaceuticals)—containing water, petrolatum, mineral oil, mineral wax, lanolin alcohol, methylchloroisothiazolinone, and methylisothiazolinone—to be applied to the whole body twice daily. At the patient’s 2-month well visit, symptoms persisted. The patient’s pediatrician increased application of Minerin to 2 to 3 times daily, and hydrocortisone cream 2.5% application 2 to 3 times daily was added.
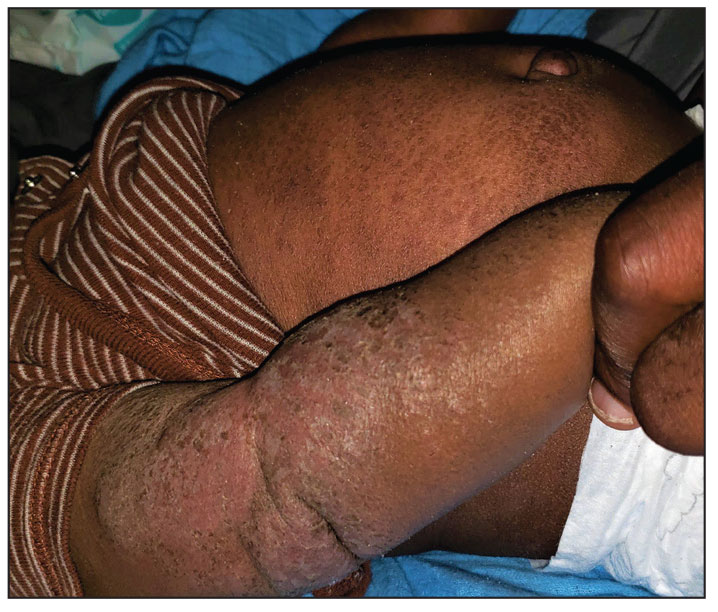
Lichenoid Dermatosis on the Feet
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).
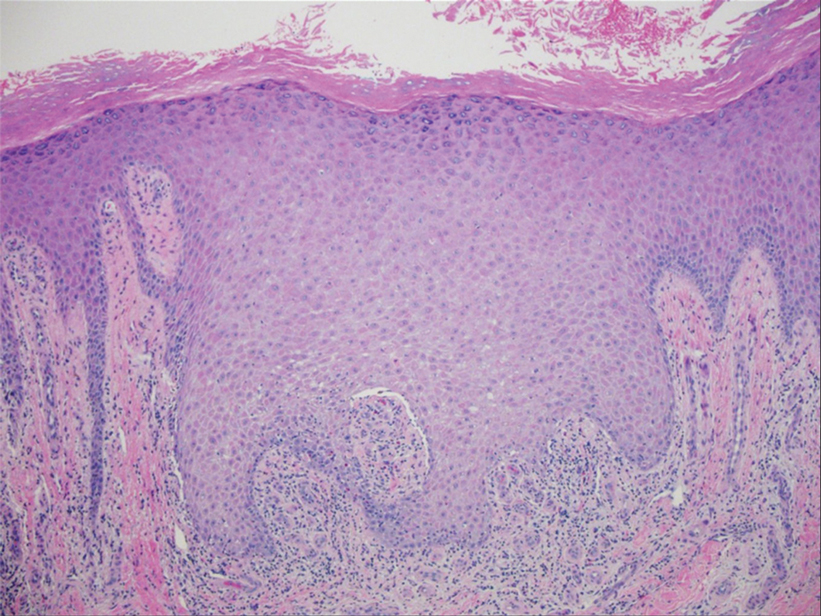
Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.
The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).

Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.

The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).

Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.

The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
An 83-year-old woman presented for evaluation of hyperkeratotic plaques on the medial and lateral aspects of the left heel (top). Physical examination also revealed onychodystrophy of the toenails on the halluces (bottom). A crusted friable plaque on the lower lip and white plaques with peripheral reticulation and erosions on the buccal mucosa also were present. The patient had a history of nummular eczema, stasis dermatitis, and hand dermatitis. She denied a history of cold sores.
Dx Across the Skin Color Spectrum: Longitudinal Melanonychia
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P =.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥ 18 years) with subungual melanoma, with no reported cases in childhood (aged < 18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
• In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
• Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
• Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
• Dermoscopic findings of LM in patients with skin of color include wider bands (P = .0125), lower band brightness (P < .032), and higher frequency of changing appearance of bands (P = .0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
1. Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
2. Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
4. Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
5. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017;31:732-736. doi:10.1111/jdv.13991
6. Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
7. Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019;9:38-43. doi:10.5826/dpc.0901a10
8. Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
9. Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
10. Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
11. Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016/j.jaad.2016.11.053
12. LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390/medsci9030057
13. Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
14. Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.13652133.2005.06668.x
15. Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
16. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Trauma, Racism Linked to Increased Suicide Risk in Black Men
One in three Black men in rural America experienced suicidal or death ideation (SDI) in the past week, new research showed.
A developmental model used in the study showed a direct association between experiences pertaining to threat, deprivation, and racial discrimination during childhood and suicide risk in adulthood, suggesting that a broad range of adverse experiences in early life may affect SDI risk among Black men.
“During the past 20-30 years, young Black men have evinced increasing levels of suicidal behavior and related cognitions,” lead author Steven Kogan, PhD, professor of family and consumer sciences at the University of Georgia, Athens, Georgia, and colleagues wrote.
“By controlling for depressive symptoms in assessing increases in SDI over time, our study’s design directly informed the extent to which social adversities affect SDI independent of other depressive problems,” they added.
The findings were published online in Cultural Diversity and Ethnic Minority Psychology.
Second Leading Cause of Death
Suicide is the second leading cause of death for Black Americans ages 15-24, according to the Centers for Disease Control and Prevention. The outlook is worse for Black men, whose death rate from suicide is about four times greater than for Black women.
Previous research suggests Black men are disproportionately exposed to social adversity, including poverty and discrimination, which may increase the risk for SDI. In addition, racial discrimination has been shown to increase the risks for depression, anxiety, and psychological distress among Black youth and adults.
But little research exists to better understand how these negative experiences affect vulnerability to SDI. The new study tested a model linking adversity during childhood and emerging exposure to racial discrimination to increases in suicidal thoughts.
Researchers analyzed data from 504 participants in the African American Men’s Project, which included a series of surveys completed by young men in rural Georgia at three different time points over a period of about 3 years.
Composite scores for childhood threat and deprivation were developed using the Adverse Childhood Experiences Scale and Childhood Trauma Questionnaire. Everyday discrimination was measured on the Schedule of Racist Events response scale.
To assess their experience with childhood threats, the men in the study, who were about 21 years old on average when they enrolled, were asked if they experienced a series of adverse childhood experiences and deprivation through age 16. Questions explored issues such as directly experiencing physical violence or witnessing abuse in the home and whether the men felt loved and “important or special” as children.
The investigators also asked the men about their experiences of racial discrimination, the quality of their relationships, their belief that aggression is a means of gaining respect, and their cynicism regarding romantic relationships.
Targeted Prevention
Overall, 33.6% of participants reported SDI in the previous week. A history of childhood threats and deprivation was associated with an increased likelihood of SDI (P < .001).
Researchers also found that a history of racial discrimination was significantly associated with the development of negative relational schemas, which are characterized by beliefs that other people are untrustworthy, uncaring, and/or hostile. Negative schemas were in turn associated with an increased risk for suicidal thoughts (P = .03).
“Clinical and preventive interventions for suicidality should target the influence of racism and adverse experiences and the negative relational schemas they induce,” the investigators noted.
“Policy efforts designed to dismantle systemic racism are critically needed. Interventions that address SDI, including programming designed to support Black men through their experiences with racial discrimination and processing of childhood experiences of adversity, may help young Black men resist the psychological impacts of racism, expand their positive support networks, and decrease their risk of SDI,” they added.
The study authors reported no funding sources or relevant financial relationships.
A version of this article appeared on Medscape.com.
One in three Black men in rural America experienced suicidal or death ideation (SDI) in the past week, new research showed.
A developmental model used in the study showed a direct association between experiences pertaining to threat, deprivation, and racial discrimination during childhood and suicide risk in adulthood, suggesting that a broad range of adverse experiences in early life may affect SDI risk among Black men.
“During the past 20-30 years, young Black men have evinced increasing levels of suicidal behavior and related cognitions,” lead author Steven Kogan, PhD, professor of family and consumer sciences at the University of Georgia, Athens, Georgia, and colleagues wrote.
“By controlling for depressive symptoms in assessing increases in SDI over time, our study’s design directly informed the extent to which social adversities affect SDI independent of other depressive problems,” they added.
The findings were published online in Cultural Diversity and Ethnic Minority Psychology.
Second Leading Cause of Death
Suicide is the second leading cause of death for Black Americans ages 15-24, according to the Centers for Disease Control and Prevention. The outlook is worse for Black men, whose death rate from suicide is about four times greater than for Black women.
Previous research suggests Black men are disproportionately exposed to social adversity, including poverty and discrimination, which may increase the risk for SDI. In addition, racial discrimination has been shown to increase the risks for depression, anxiety, and psychological distress among Black youth and adults.
But little research exists to better understand how these negative experiences affect vulnerability to SDI. The new study tested a model linking adversity during childhood and emerging exposure to racial discrimination to increases in suicidal thoughts.
Researchers analyzed data from 504 participants in the African American Men’s Project, which included a series of surveys completed by young men in rural Georgia at three different time points over a period of about 3 years.
Composite scores for childhood threat and deprivation were developed using the Adverse Childhood Experiences Scale and Childhood Trauma Questionnaire. Everyday discrimination was measured on the Schedule of Racist Events response scale.
To assess their experience with childhood threats, the men in the study, who were about 21 years old on average when they enrolled, were asked if they experienced a series of adverse childhood experiences and deprivation through age 16. Questions explored issues such as directly experiencing physical violence or witnessing abuse in the home and whether the men felt loved and “important or special” as children.
The investigators also asked the men about their experiences of racial discrimination, the quality of their relationships, their belief that aggression is a means of gaining respect, and their cynicism regarding romantic relationships.
Targeted Prevention
Overall, 33.6% of participants reported SDI in the previous week. A history of childhood threats and deprivation was associated with an increased likelihood of SDI (P < .001).
Researchers also found that a history of racial discrimination was significantly associated with the development of negative relational schemas, which are characterized by beliefs that other people are untrustworthy, uncaring, and/or hostile. Negative schemas were in turn associated with an increased risk for suicidal thoughts (P = .03).
“Clinical and preventive interventions for suicidality should target the influence of racism and adverse experiences and the negative relational schemas they induce,” the investigators noted.
“Policy efforts designed to dismantle systemic racism are critically needed. Interventions that address SDI, including programming designed to support Black men through their experiences with racial discrimination and processing of childhood experiences of adversity, may help young Black men resist the psychological impacts of racism, expand their positive support networks, and decrease their risk of SDI,” they added.
The study authors reported no funding sources or relevant financial relationships.
A version of this article appeared on Medscape.com.
One in three Black men in rural America experienced suicidal or death ideation (SDI) in the past week, new research showed.
A developmental model used in the study showed a direct association between experiences pertaining to threat, deprivation, and racial discrimination during childhood and suicide risk in adulthood, suggesting that a broad range of adverse experiences in early life may affect SDI risk among Black men.
“During the past 20-30 years, young Black men have evinced increasing levels of suicidal behavior and related cognitions,” lead author Steven Kogan, PhD, professor of family and consumer sciences at the University of Georgia, Athens, Georgia, and colleagues wrote.
“By controlling for depressive symptoms in assessing increases in SDI over time, our study’s design directly informed the extent to which social adversities affect SDI independent of other depressive problems,” they added.
The findings were published online in Cultural Diversity and Ethnic Minority Psychology.
Second Leading Cause of Death
Suicide is the second leading cause of death for Black Americans ages 15-24, according to the Centers for Disease Control and Prevention. The outlook is worse for Black men, whose death rate from suicide is about four times greater than for Black women.
Previous research suggests Black men are disproportionately exposed to social adversity, including poverty and discrimination, which may increase the risk for SDI. In addition, racial discrimination has been shown to increase the risks for depression, anxiety, and psychological distress among Black youth and adults.
But little research exists to better understand how these negative experiences affect vulnerability to SDI. The new study tested a model linking adversity during childhood and emerging exposure to racial discrimination to increases in suicidal thoughts.
Researchers analyzed data from 504 participants in the African American Men’s Project, which included a series of surveys completed by young men in rural Georgia at three different time points over a period of about 3 years.
Composite scores for childhood threat and deprivation were developed using the Adverse Childhood Experiences Scale and Childhood Trauma Questionnaire. Everyday discrimination was measured on the Schedule of Racist Events response scale.
To assess their experience with childhood threats, the men in the study, who were about 21 years old on average when they enrolled, were asked if they experienced a series of adverse childhood experiences and deprivation through age 16. Questions explored issues such as directly experiencing physical violence or witnessing abuse in the home and whether the men felt loved and “important or special” as children.
The investigators also asked the men about their experiences of racial discrimination, the quality of their relationships, their belief that aggression is a means of gaining respect, and their cynicism regarding romantic relationships.
Targeted Prevention
Overall, 33.6% of participants reported SDI in the previous week. A history of childhood threats and deprivation was associated with an increased likelihood of SDI (P < .001).
Researchers also found that a history of racial discrimination was significantly associated with the development of negative relational schemas, which are characterized by beliefs that other people are untrustworthy, uncaring, and/or hostile. Negative schemas were in turn associated with an increased risk for suicidal thoughts (P = .03).
“Clinical and preventive interventions for suicidality should target the influence of racism and adverse experiences and the negative relational schemas they induce,” the investigators noted.
“Policy efforts designed to dismantle systemic racism are critically needed. Interventions that address SDI, including programming designed to support Black men through their experiences with racial discrimination and processing of childhood experiences of adversity, may help young Black men resist the psychological impacts of racism, expand their positive support networks, and decrease their risk of SDI,” they added.
The study authors reported no funding sources or relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CULTURAL DIVERSITY AND ETHNIC MINORITY PSYCHOLOGY
Best Practices for Clinical Image Collection and Utilization in Patients With Skin of Color
Clinical images are integral to dermatologic care, research, and education. Studies have highlighted the underrepresentation of images of skin of color (SOC) in educational materials,1 clinical trials,2 and research publications.3 Recognition of this disparity has ignited a call to action by dermatologists and dermatologic organizations to address the gap by improving the collection and use of SOC images.4 It is critical to remind dermatologists of the importance of properly obtaining informed consent and ensuring images are not used without a patient’s permission, as images in journal articles, conference presentations, and educational materials can be widely distributed and shared. Herein, we summarize current practices of clinical image storage and make general recommendations on how dermatologists can better protect patient privacy. Certain cultural and social factors in patients with SOC should be considered when obtaining informed consent and collecting images.
Clinical Image Acquisition
Consenting procedures are crucial components of proper image usage. However, current consenting practices are inconsistent across various platforms, including academic journals, websites, printed text, social media, and educational presentations.5
Current regulations for use of patient health information in the United States are governed by the Health Insurance Portability and Accountability Act (HIPAA)of 1996. Although this act explicitly prohibits use of “full face photographic images and any comparable images” without consent from the patient or the patient’s representative, there is less restriction regarding the use of deidentified images.6 Some clinicians or researchers may consider using a black bar or a masking technique over the eyes or face, but this is not always a sufficient method of anonymizing an image.
One study investigating the different requirements listed by the top 20 dermatology journals (as determined by the Google Scholar h5-index) found that while 95% (19/20) of journals stated that written or signed consent or permission was a requirement for use of patient images, only 20% (4/20) instructed authors to inform the patient or the patient’s representative that images may become available on the internet.5 Once an article is accepted for publication by a medical journal, it eventually may be accessible online; however, patients may not be aware of this factor, which is particularly concerning for those with SOC due to the increased demand for diverse dermatologic resources and images as well as the highly digitalized manner in which we access and share media.
Furthermore, cultural and social factors exist that present challenges to informed decision-making during the consenting process for certain SOC populations such as a lack of trust in the medical and scientific research community, inadequate comprehension of the consent material, health illiteracy, language barriers, or use of complex terminology in consent documentation.7,8 Studies also have shown that patients in ethnic minority groups have greater barriers to health literacy compared to other patient groups, and patients with limited health literacy are less likely to ask questions during their medical visits.9,10 Therefore, when obtaining informed consent for images, it is important that measures are taken to ensure that the patient has full knowledge and understanding of what the consent covers, including the extent to which the images will be used and/or shared and whether the patient’s confidentiality and/or anonymity are at risk.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Encourage influential dermatology organizations such as the American Academy of Dermatology to establish standardized consenting procedures for image acquisition and use, including requirements to provide (a) written consent for all patient images and (b) specific details as to where and how the image may be used and/or shared.
2. Ensure that consent terminology is presented at a sixth-grade reading level or below, minimize the use of medical jargon and complex terms, and provide consent documentation in the patient’s preferred language.
3. Allow patients to take the consent document home so they can have additional time to comprehensively review the material or have it reviewed by family or friends.
4. Employ strategies such as teach-back methods and encourage questions to maximize the level of understanding during the consent process.
Clinical Image Storage
Clinical image storage procedures can have an impact on a patient’s health information remaining anonymous and confidential. In a survey evaluating medical photography use among 153 US board-certified dermatologists, 69.1% of respondents reported emailing or texting images between patients and colleagues. Additionally, 30.3% (46/152) reported having patient photographs stored on their personal phone at the time of the survey, and 39.1% (18/46) of those individuals had images that showed identifiable features, such as the patient’s face or a tattoo.11
Although most providers state that their devices are password protected, it cannot be guaranteed that the device and consequently the images remain secure and inaccessible to unauthorized individuals. As sharing and viewing images continue to play an essential role in assessing disease state, progression, treatment response, and inclusion in research, we must establish and encourage clear guidelines for the storage and retention of such images.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Store clinical images exclusively on password-protected devices and in password-protected files.
2. Use work-related cameras or electronic devices rather than personal devices, unless the personal device is being used to upload directly into the patient’s medical record. In such cases, use a HIPAA-compliant electronic medical record mobile application that does not store images on the application or the device itself.
3. Avoid using text-messaging systems or unencrypted email to share identifying images without clear patient consent.
Clinical Image Use
Once a thorough consenting process has been completed, it is crucial that the use and distribution of the clinical image are in accordance with the terms specified in the original consent. With the current state of technologic advancement, widespread social media usage, and constant sharing of information, adherence to these terms can be challenging. For example, an image initially intended for use in an educational presentation at a professional conference can be shared on social media if an audience member captures a photo of it. In another example, a patient may consent to their image being shown on a dermatologic website but that image can be duplicated and shared on other unauthorized sites and locations. This situation can be particularly distressing to patients whose image may include all or most of their face, an intimate area, or other physical features that they did not wish to share widely.
Individuals identifying as Black/African American, Latino/Hispanic, or Asian have been shown to express less comfort with providing permission for images of a nonidentifiable sensitive area to be taken (or obtained) or for use for teaching irrespective of identifiability compared to their White counterparts,12 which may be due to the aforementioned lack of trust in medical providers and the health care system in general, both of which may contribute to concerns with how a clinical image is used and/or shared. Although consent from a patient or the patient’s representative can be granted, we must ensure that the use of these images adheres to the patient’s initial agreement. Ultimately, medical providers, researchers, and other parties involved in acquiring or sharing patient images have both an ethical and legal responsibility to ensure that anonymity, privacy, and confidentiality are preserved to the greatest extent possible.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Display a message on websites containing patient images stating that the sharing of the images outside the established guidelines and intended use is prohibited.
2. Place a watermark on images to discourage unauthorized duplication.
3. Issue explicit instructions to audiences prohibiting the copying or reproducing of any patient images during teaching events or presentations.
Final Thoughts
The use of clinical images is an essential component of dermatologic care, education, and research. Due to the higher demand for diverse and representative images and the dearth of images in the medical literature, many SOC images have been widely disseminated and utilized by dermatologists, raising concerns of the adequacy of informed consent for the storage and use of such material. Therefore, dermatologists should implement streamlined guidelines and consent procedures to ensure a patient’s informed consent is provided with full knowledge of how and where their images might be used and shared. Additional efforts should be made to protect patients’ privacy and unauthorized use of their images. Furthermore, we encourage our leading dermatology organizations to develop expert consensus on best practices for appropriate clinical image consent, storage, and use.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198. doi:10.1001/jamadermatol.2016.4129
- Marroquin NA, Carboni A, Zueger M, et al. Skin of color representation trends in JAAD case reports 2015-2021: content analysis. JMIR Dermatol. 2023;6:e40816. doi:10.2196/40816
- Kim Y, Miller JJ, Hollins LC. Skin of color matters: a call to action. J Am Acad Dermatol. 2021;84:E273-E274. doi:10.1016/j.jaad.2020.11.026
- Nanda JK, Marchetti MA. Consent and deidentification of patient images in dermatology journals: observational study. JMIR Dermatol. 2022;5:E37398. doi:10.2196/37398
- US Department of Health and Human Services. Summary of the HIPAA privacy rule. Updated October 19, 2022. Accessed March 15, 2024. https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html
- Quinn SC, Garza MA, Butler J, et al. Improving informed consent with minority participants: results from researcher and community surveys. J Empir Res Hum Res Ethics. 2012;7:44-55. doi:10.1525/jer.2012.7.5.44
- Hadden KB, Prince LY, Moore TD, et al. Improving readability of informed consents for research at an academic medical institution. J Clin Transl Sci. 2017;1:361-365. doi:10.1017/cts.2017.312
- Muvuka B, Combs RM, Ayangeakaa SD, et al. Health literacy in African-American communities: barriers and strategies. Health Lit Res Pract. 2020;4:E138-E143. doi:10.3928/24748307-20200617-01
- Menendez ME, van Hoorn BT, Mackert M, et al. Patients with limited health literacy ask fewer questions during office visits with hand surgeons. Clin Orthop Relat Res. 2017;475:1291-1297. doi:10.1007/s11999-016-5140-5
- Milam EC, Leger MC. Use of medical photography among dermatologists: a nationwide online survey study. J Eur Acad Dermatol Venereol. 2018;32:1804-1809. doi:10.1111/jdv.14839
- Leger MC, Wu T, Haimovic A, et al. Patient perspectives on medical photography in dermatology. Dermatol Surg. 2014;40:1028-1037. doi:10.1097/01.DSS.0000452632.22081.79
Clinical images are integral to dermatologic care, research, and education. Studies have highlighted the underrepresentation of images of skin of color (SOC) in educational materials,1 clinical trials,2 and research publications.3 Recognition of this disparity has ignited a call to action by dermatologists and dermatologic organizations to address the gap by improving the collection and use of SOC images.4 It is critical to remind dermatologists of the importance of properly obtaining informed consent and ensuring images are not used without a patient’s permission, as images in journal articles, conference presentations, and educational materials can be widely distributed and shared. Herein, we summarize current practices of clinical image storage and make general recommendations on how dermatologists can better protect patient privacy. Certain cultural and social factors in patients with SOC should be considered when obtaining informed consent and collecting images.
Clinical Image Acquisition
Consenting procedures are crucial components of proper image usage. However, current consenting practices are inconsistent across various platforms, including academic journals, websites, printed text, social media, and educational presentations.5
Current regulations for use of patient health information in the United States are governed by the Health Insurance Portability and Accountability Act (HIPAA)of 1996. Although this act explicitly prohibits use of “full face photographic images and any comparable images” without consent from the patient or the patient’s representative, there is less restriction regarding the use of deidentified images.6 Some clinicians or researchers may consider using a black bar or a masking technique over the eyes or face, but this is not always a sufficient method of anonymizing an image.
One study investigating the different requirements listed by the top 20 dermatology journals (as determined by the Google Scholar h5-index) found that while 95% (19/20) of journals stated that written or signed consent or permission was a requirement for use of patient images, only 20% (4/20) instructed authors to inform the patient or the patient’s representative that images may become available on the internet.5 Once an article is accepted for publication by a medical journal, it eventually may be accessible online; however, patients may not be aware of this factor, which is particularly concerning for those with SOC due to the increased demand for diverse dermatologic resources and images as well as the highly digitalized manner in which we access and share media.
Furthermore, cultural and social factors exist that present challenges to informed decision-making during the consenting process for certain SOC populations such as a lack of trust in the medical and scientific research community, inadequate comprehension of the consent material, health illiteracy, language barriers, or use of complex terminology in consent documentation.7,8 Studies also have shown that patients in ethnic minority groups have greater barriers to health literacy compared to other patient groups, and patients with limited health literacy are less likely to ask questions during their medical visits.9,10 Therefore, when obtaining informed consent for images, it is important that measures are taken to ensure that the patient has full knowledge and understanding of what the consent covers, including the extent to which the images will be used and/or shared and whether the patient’s confidentiality and/or anonymity are at risk.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Encourage influential dermatology organizations such as the American Academy of Dermatology to establish standardized consenting procedures for image acquisition and use, including requirements to provide (a) written consent for all patient images and (b) specific details as to where and how the image may be used and/or shared.
2. Ensure that consent terminology is presented at a sixth-grade reading level or below, minimize the use of medical jargon and complex terms, and provide consent documentation in the patient’s preferred language.
3. Allow patients to take the consent document home so they can have additional time to comprehensively review the material or have it reviewed by family or friends.
4. Employ strategies such as teach-back methods and encourage questions to maximize the level of understanding during the consent process.
Clinical Image Storage
Clinical image storage procedures can have an impact on a patient’s health information remaining anonymous and confidential. In a survey evaluating medical photography use among 153 US board-certified dermatologists, 69.1% of respondents reported emailing or texting images between patients and colleagues. Additionally, 30.3% (46/152) reported having patient photographs stored on their personal phone at the time of the survey, and 39.1% (18/46) of those individuals had images that showed identifiable features, such as the patient’s face or a tattoo.11
Although most providers state that their devices are password protected, it cannot be guaranteed that the device and consequently the images remain secure and inaccessible to unauthorized individuals. As sharing and viewing images continue to play an essential role in assessing disease state, progression, treatment response, and inclusion in research, we must establish and encourage clear guidelines for the storage and retention of such images.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Store clinical images exclusively on password-protected devices and in password-protected files.
2. Use work-related cameras or electronic devices rather than personal devices, unless the personal device is being used to upload directly into the patient’s medical record. In such cases, use a HIPAA-compliant electronic medical record mobile application that does not store images on the application or the device itself.
3. Avoid using text-messaging systems or unencrypted email to share identifying images without clear patient consent.
Clinical Image Use
Once a thorough consenting process has been completed, it is crucial that the use and distribution of the clinical image are in accordance with the terms specified in the original consent. With the current state of technologic advancement, widespread social media usage, and constant sharing of information, adherence to these terms can be challenging. For example, an image initially intended for use in an educational presentation at a professional conference can be shared on social media if an audience member captures a photo of it. In another example, a patient may consent to their image being shown on a dermatologic website but that image can be duplicated and shared on other unauthorized sites and locations. This situation can be particularly distressing to patients whose image may include all or most of their face, an intimate area, or other physical features that they did not wish to share widely.
Individuals identifying as Black/African American, Latino/Hispanic, or Asian have been shown to express less comfort with providing permission for images of a nonidentifiable sensitive area to be taken (or obtained) or for use for teaching irrespective of identifiability compared to their White counterparts,12 which may be due to the aforementioned lack of trust in medical providers and the health care system in general, both of which may contribute to concerns with how a clinical image is used and/or shared. Although consent from a patient or the patient’s representative can be granted, we must ensure that the use of these images adheres to the patient’s initial agreement. Ultimately, medical providers, researchers, and other parties involved in acquiring or sharing patient images have both an ethical and legal responsibility to ensure that anonymity, privacy, and confidentiality are preserved to the greatest extent possible.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Display a message on websites containing patient images stating that the sharing of the images outside the established guidelines and intended use is prohibited.
2. Place a watermark on images to discourage unauthorized duplication.
3. Issue explicit instructions to audiences prohibiting the copying or reproducing of any patient images during teaching events or presentations.
Final Thoughts
The use of clinical images is an essential component of dermatologic care, education, and research. Due to the higher demand for diverse and representative images and the dearth of images in the medical literature, many SOC images have been widely disseminated and utilized by dermatologists, raising concerns of the adequacy of informed consent for the storage and use of such material. Therefore, dermatologists should implement streamlined guidelines and consent procedures to ensure a patient’s informed consent is provided with full knowledge of how and where their images might be used and shared. Additional efforts should be made to protect patients’ privacy and unauthorized use of their images. Furthermore, we encourage our leading dermatology organizations to develop expert consensus on best practices for appropriate clinical image consent, storage, and use.
Clinical images are integral to dermatologic care, research, and education. Studies have highlighted the underrepresentation of images of skin of color (SOC) in educational materials,1 clinical trials,2 and research publications.3 Recognition of this disparity has ignited a call to action by dermatologists and dermatologic organizations to address the gap by improving the collection and use of SOC images.4 It is critical to remind dermatologists of the importance of properly obtaining informed consent and ensuring images are not used without a patient’s permission, as images in journal articles, conference presentations, and educational materials can be widely distributed and shared. Herein, we summarize current practices of clinical image storage and make general recommendations on how dermatologists can better protect patient privacy. Certain cultural and social factors in patients with SOC should be considered when obtaining informed consent and collecting images.
Clinical Image Acquisition
Consenting procedures are crucial components of proper image usage. However, current consenting practices are inconsistent across various platforms, including academic journals, websites, printed text, social media, and educational presentations.5
Current regulations for use of patient health information in the United States are governed by the Health Insurance Portability and Accountability Act (HIPAA)of 1996. Although this act explicitly prohibits use of “full face photographic images and any comparable images” without consent from the patient or the patient’s representative, there is less restriction regarding the use of deidentified images.6 Some clinicians or researchers may consider using a black bar or a masking technique over the eyes or face, but this is not always a sufficient method of anonymizing an image.
One study investigating the different requirements listed by the top 20 dermatology journals (as determined by the Google Scholar h5-index) found that while 95% (19/20) of journals stated that written or signed consent or permission was a requirement for use of patient images, only 20% (4/20) instructed authors to inform the patient or the patient’s representative that images may become available on the internet.5 Once an article is accepted for publication by a medical journal, it eventually may be accessible online; however, patients may not be aware of this factor, which is particularly concerning for those with SOC due to the increased demand for diverse dermatologic resources and images as well as the highly digitalized manner in which we access and share media.
Furthermore, cultural and social factors exist that present challenges to informed decision-making during the consenting process for certain SOC populations such as a lack of trust in the medical and scientific research community, inadequate comprehension of the consent material, health illiteracy, language barriers, or use of complex terminology in consent documentation.7,8 Studies also have shown that patients in ethnic minority groups have greater barriers to health literacy compared to other patient groups, and patients with limited health literacy are less likely to ask questions during their medical visits.9,10 Therefore, when obtaining informed consent for images, it is important that measures are taken to ensure that the patient has full knowledge and understanding of what the consent covers, including the extent to which the images will be used and/or shared and whether the patient’s confidentiality and/or anonymity are at risk.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Encourage influential dermatology organizations such as the American Academy of Dermatology to establish standardized consenting procedures for image acquisition and use, including requirements to provide (a) written consent for all patient images and (b) specific details as to where and how the image may be used and/or shared.
2. Ensure that consent terminology is presented at a sixth-grade reading level or below, minimize the use of medical jargon and complex terms, and provide consent documentation in the patient’s preferred language.
3. Allow patients to take the consent document home so they can have additional time to comprehensively review the material or have it reviewed by family or friends.
4. Employ strategies such as teach-back methods and encourage questions to maximize the level of understanding during the consent process.
Clinical Image Storage
Clinical image storage procedures can have an impact on a patient’s health information remaining anonymous and confidential. In a survey evaluating medical photography use among 153 US board-certified dermatologists, 69.1% of respondents reported emailing or texting images between patients and colleagues. Additionally, 30.3% (46/152) reported having patient photographs stored on their personal phone at the time of the survey, and 39.1% (18/46) of those individuals had images that showed identifiable features, such as the patient’s face or a tattoo.11
Although most providers state that their devices are password protected, it cannot be guaranteed that the device and consequently the images remain secure and inaccessible to unauthorized individuals. As sharing and viewing images continue to play an essential role in assessing disease state, progression, treatment response, and inclusion in research, we must establish and encourage clear guidelines for the storage and retention of such images.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Store clinical images exclusively on password-protected devices and in password-protected files.
2. Use work-related cameras or electronic devices rather than personal devices, unless the personal device is being used to upload directly into the patient’s medical record. In such cases, use a HIPAA-compliant electronic medical record mobile application that does not store images on the application or the device itself.
3. Avoid using text-messaging systems or unencrypted email to share identifying images without clear patient consent.
Clinical Image Use
Once a thorough consenting process has been completed, it is crucial that the use and distribution of the clinical image are in accordance with the terms specified in the original consent. With the current state of technologic advancement, widespread social media usage, and constant sharing of information, adherence to these terms can be challenging. For example, an image initially intended for use in an educational presentation at a professional conference can be shared on social media if an audience member captures a photo of it. In another example, a patient may consent to their image being shown on a dermatologic website but that image can be duplicated and shared on other unauthorized sites and locations. This situation can be particularly distressing to patients whose image may include all or most of their face, an intimate area, or other physical features that they did not wish to share widely.
Individuals identifying as Black/African American, Latino/Hispanic, or Asian have been shown to express less comfort with providing permission for images of a nonidentifiable sensitive area to be taken (or obtained) or for use for teaching irrespective of identifiability compared to their White counterparts,12 which may be due to the aforementioned lack of trust in medical providers and the health care system in general, both of which may contribute to concerns with how a clinical image is used and/or shared. Although consent from a patient or the patient’s representative can be granted, we must ensure that the use of these images adheres to the patient’s initial agreement. Ultimately, medical providers, researchers, and other parties involved in acquiring or sharing patient images have both an ethical and legal responsibility to ensure that anonymity, privacy, and confidentiality are preserved to the greatest extent possible.
Recommendations—We propose that dermatologists should follow these recommendations:
1. Display a message on websites containing patient images stating that the sharing of the images outside the established guidelines and intended use is prohibited.
2. Place a watermark on images to discourage unauthorized duplication.
3. Issue explicit instructions to audiences prohibiting the copying or reproducing of any patient images during teaching events or presentations.
Final Thoughts
The use of clinical images is an essential component of dermatologic care, education, and research. Due to the higher demand for diverse and representative images and the dearth of images in the medical literature, many SOC images have been widely disseminated and utilized by dermatologists, raising concerns of the adequacy of informed consent for the storage and use of such material. Therefore, dermatologists should implement streamlined guidelines and consent procedures to ensure a patient’s informed consent is provided with full knowledge of how and where their images might be used and shared. Additional efforts should be made to protect patients’ privacy and unauthorized use of their images. Furthermore, we encourage our leading dermatology organizations to develop expert consensus on best practices for appropriate clinical image consent, storage, and use.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198. doi:10.1001/jamadermatol.2016.4129
- Marroquin NA, Carboni A, Zueger M, et al. Skin of color representation trends in JAAD case reports 2015-2021: content analysis. JMIR Dermatol. 2023;6:e40816. doi:10.2196/40816
- Kim Y, Miller JJ, Hollins LC. Skin of color matters: a call to action. J Am Acad Dermatol. 2021;84:E273-E274. doi:10.1016/j.jaad.2020.11.026
- Nanda JK, Marchetti MA. Consent and deidentification of patient images in dermatology journals: observational study. JMIR Dermatol. 2022;5:E37398. doi:10.2196/37398
- US Department of Health and Human Services. Summary of the HIPAA privacy rule. Updated October 19, 2022. Accessed March 15, 2024. https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html
- Quinn SC, Garza MA, Butler J, et al. Improving informed consent with minority participants: results from researcher and community surveys. J Empir Res Hum Res Ethics. 2012;7:44-55. doi:10.1525/jer.2012.7.5.44
- Hadden KB, Prince LY, Moore TD, et al. Improving readability of informed consents for research at an academic medical institution. J Clin Transl Sci. 2017;1:361-365. doi:10.1017/cts.2017.312
- Muvuka B, Combs RM, Ayangeakaa SD, et al. Health literacy in African-American communities: barriers and strategies. Health Lit Res Pract. 2020;4:E138-E143. doi:10.3928/24748307-20200617-01
- Menendez ME, van Hoorn BT, Mackert M, et al. Patients with limited health literacy ask fewer questions during office visits with hand surgeons. Clin Orthop Relat Res. 2017;475:1291-1297. doi:10.1007/s11999-016-5140-5
- Milam EC, Leger MC. Use of medical photography among dermatologists: a nationwide online survey study. J Eur Acad Dermatol Venereol. 2018;32:1804-1809. doi:10.1111/jdv.14839
- Leger MC, Wu T, Haimovic A, et al. Patient perspectives on medical photography in dermatology. Dermatol Surg. 2014;40:1028-1037. doi:10.1097/01.DSS.0000452632.22081.79
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198. doi:10.1001/jamadermatol.2016.4129
- Marroquin NA, Carboni A, Zueger M, et al. Skin of color representation trends in JAAD case reports 2015-2021: content analysis. JMIR Dermatol. 2023;6:e40816. doi:10.2196/40816
- Kim Y, Miller JJ, Hollins LC. Skin of color matters: a call to action. J Am Acad Dermatol. 2021;84:E273-E274. doi:10.1016/j.jaad.2020.11.026
- Nanda JK, Marchetti MA. Consent and deidentification of patient images in dermatology journals: observational study. JMIR Dermatol. 2022;5:E37398. doi:10.2196/37398
- US Department of Health and Human Services. Summary of the HIPAA privacy rule. Updated October 19, 2022. Accessed March 15, 2024. https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html
- Quinn SC, Garza MA, Butler J, et al. Improving informed consent with minority participants: results from researcher and community surveys. J Empir Res Hum Res Ethics. 2012;7:44-55. doi:10.1525/jer.2012.7.5.44
- Hadden KB, Prince LY, Moore TD, et al. Improving readability of informed consents for research at an academic medical institution. J Clin Transl Sci. 2017;1:361-365. doi:10.1017/cts.2017.312
- Muvuka B, Combs RM, Ayangeakaa SD, et al. Health literacy in African-American communities: barriers and strategies. Health Lit Res Pract. 2020;4:E138-E143. doi:10.3928/24748307-20200617-01
- Menendez ME, van Hoorn BT, Mackert M, et al. Patients with limited health literacy ask fewer questions during office visits with hand surgeons. Clin Orthop Relat Res. 2017;475:1291-1297. doi:10.1007/s11999-016-5140-5
- Milam EC, Leger MC. Use of medical photography among dermatologists: a nationwide online survey study. J Eur Acad Dermatol Venereol. 2018;32:1804-1809. doi:10.1111/jdv.14839
- Leger MC, Wu T, Haimovic A, et al. Patient perspectives on medical photography in dermatology. Dermatol Surg. 2014;40:1028-1037. doi:10.1097/01.DSS.0000452632.22081.79
Prostate Cancer Tsunami Coming, Experts Caution
An “inevitable” global surge in prostate cancer is coming, with a worldwide doubling of cases to 2.9 million and an 85% increase in deaths to nearly 700,000 by the year 2040, the Lancet Commission on Prostate Cancer warned this week.
At a meeting of urologists in Paris, the commission said that the acceleration is already underway in high-income countries such as the United States and the United Kingdom but will gain momentum in low- and medium-income countries.
Nick James, MD, lead author of The Lancet report and professor of prostate and bladder cancer research at The Institute of Cancer Research in London, said that the surge, in part, is a medical success story.
Dr. James told this news organization.
“There is a big rise in the high-income countries. But we’re going to see a big rise in the number of 50-, 60-, 70-year-olds in the coming decades in the poorer countries, and with that comes more prostate cancer. High-income countries such as the UK and USA will also see smaller increases for the same reason.”
According to the report, to be presented April 6 at the 2024 European Association of Urology Congress in Paris, “The case for prostate cancer screening for all men aged 50-70 years (and all men of African origin aged 45–70 years) in high-income countries is strengthening with improved use of technologies such as MRI and growing evidence for the safety of active surveillance.”
Andrew Vickers, PhD, a biostatistician at Memorial Sloan Kettering Cancer Center in New York City, said that the Lancet Commission came to similar conclusions as he and an international group of researchers did in a 2023 policy paper in The BMJ. A major gap, Dr. Vickers said, is misuse of prostate-specific antigen (PSA) screening.
“We found that the ubiquitous policy compromise of letting patients decide for themselves about PSA has led to the worst possible outcomes of overuse in men unlikely to benefit, high rates of overdiagnosis and overtreatment, and economic and racial inequity,” Dr. Vickers said. “Our view is that PSA screening should be done well — by implementing straightforward harm-reduction strategies like restricting screening in older men and use of secondary tests before biopsy — or not at all.”
Dr. James said that undertreatment of advanced disease is widespread; only about 30%-40% of men in the United States receive combination hormone therapy for metastatic disease, for example. “Simply doing what we know works would improve outcomes,” he said.
Dr. James said that men of African ancestry are twice as likely to develop prostate cancer, but whether treatment should follow a different approach in these men is unclear. The new report stressed the need to include more men of African ancestry in research.
Brandon Mahal, MD, vice chair of research in radiation oncology the University of Miami Sylvester Comprehensive Cancer Center and a coauthor of the report, said that new approaches are needed to enable earlier diagnosis of prostate cancer in men in low- to middle-income countries, where most patients present with metastatic disease and are less likely to survive for long periods.
Dr. James recommended pop-up clinics and mobile testing to encourage men who are at high risk for prostate cancer but feel well to detect lethal cancers early.
In England, for example, Dr. James helped introduce an outreach program called The Man Van which provided free health checks, including PSA tests, to high-risk men in London.
“By bringing a van with quick and easy testing straight to men at work and in the community, and targeting those who have a higher risk of prostate cancer, we provided thousands of health checks which resulted in almost 100 cancer diagnoses in men who might otherwise have only seen a doctor once their cancer has progressed to a more advanced stage,” he said.
He noted that the medical community worldwide is ill-prepared for the onslaught of prostate cancer cases.
“The solution cannot be training more urologists, radiation oncologists, pathologists, and radiologists because it simply takes too long,” Dr. James said. However, increased use of nurses and artificial intelligence may help. “In my own hospital, biopsies are a nurse-led and -delivered service. AI is extraordinarily good at diagnosis already and will only get better,” he said.
In poorer countries, smartphones could fill gaps too. “The same technology that does face recognition already can say that’s a Gleason 7 prostate cancer,” Dr. James said. “It’s not being rolled out in countries like America of course because pathologists’ income is at risk.”
Dr. James, Dr. Vickers, and Dr. Mahal reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
An “inevitable” global surge in prostate cancer is coming, with a worldwide doubling of cases to 2.9 million and an 85% increase in deaths to nearly 700,000 by the year 2040, the Lancet Commission on Prostate Cancer warned this week.
At a meeting of urologists in Paris, the commission said that the acceleration is already underway in high-income countries such as the United States and the United Kingdom but will gain momentum in low- and medium-income countries.
Nick James, MD, lead author of The Lancet report and professor of prostate and bladder cancer research at The Institute of Cancer Research in London, said that the surge, in part, is a medical success story.
Dr. James told this news organization.
“There is a big rise in the high-income countries. But we’re going to see a big rise in the number of 50-, 60-, 70-year-olds in the coming decades in the poorer countries, and with that comes more prostate cancer. High-income countries such as the UK and USA will also see smaller increases for the same reason.”
According to the report, to be presented April 6 at the 2024 European Association of Urology Congress in Paris, “The case for prostate cancer screening for all men aged 50-70 years (and all men of African origin aged 45–70 years) in high-income countries is strengthening with improved use of technologies such as MRI and growing evidence for the safety of active surveillance.”
Andrew Vickers, PhD, a biostatistician at Memorial Sloan Kettering Cancer Center in New York City, said that the Lancet Commission came to similar conclusions as he and an international group of researchers did in a 2023 policy paper in The BMJ. A major gap, Dr. Vickers said, is misuse of prostate-specific antigen (PSA) screening.
“We found that the ubiquitous policy compromise of letting patients decide for themselves about PSA has led to the worst possible outcomes of overuse in men unlikely to benefit, high rates of overdiagnosis and overtreatment, and economic and racial inequity,” Dr. Vickers said. “Our view is that PSA screening should be done well — by implementing straightforward harm-reduction strategies like restricting screening in older men and use of secondary tests before biopsy — or not at all.”
Dr. James said that undertreatment of advanced disease is widespread; only about 30%-40% of men in the United States receive combination hormone therapy for metastatic disease, for example. “Simply doing what we know works would improve outcomes,” he said.
Dr. James said that men of African ancestry are twice as likely to develop prostate cancer, but whether treatment should follow a different approach in these men is unclear. The new report stressed the need to include more men of African ancestry in research.
Brandon Mahal, MD, vice chair of research in radiation oncology the University of Miami Sylvester Comprehensive Cancer Center and a coauthor of the report, said that new approaches are needed to enable earlier diagnosis of prostate cancer in men in low- to middle-income countries, where most patients present with metastatic disease and are less likely to survive for long periods.
Dr. James recommended pop-up clinics and mobile testing to encourage men who are at high risk for prostate cancer but feel well to detect lethal cancers early.
In England, for example, Dr. James helped introduce an outreach program called The Man Van which provided free health checks, including PSA tests, to high-risk men in London.
“By bringing a van with quick and easy testing straight to men at work and in the community, and targeting those who have a higher risk of prostate cancer, we provided thousands of health checks which resulted in almost 100 cancer diagnoses in men who might otherwise have only seen a doctor once their cancer has progressed to a more advanced stage,” he said.
He noted that the medical community worldwide is ill-prepared for the onslaught of prostate cancer cases.
“The solution cannot be training more urologists, radiation oncologists, pathologists, and radiologists because it simply takes too long,” Dr. James said. However, increased use of nurses and artificial intelligence may help. “In my own hospital, biopsies are a nurse-led and -delivered service. AI is extraordinarily good at diagnosis already and will only get better,” he said.
In poorer countries, smartphones could fill gaps too. “The same technology that does face recognition already can say that’s a Gleason 7 prostate cancer,” Dr. James said. “It’s not being rolled out in countries like America of course because pathologists’ income is at risk.”
Dr. James, Dr. Vickers, and Dr. Mahal reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
An “inevitable” global surge in prostate cancer is coming, with a worldwide doubling of cases to 2.9 million and an 85% increase in deaths to nearly 700,000 by the year 2040, the Lancet Commission on Prostate Cancer warned this week.
At a meeting of urologists in Paris, the commission said that the acceleration is already underway in high-income countries such as the United States and the United Kingdom but will gain momentum in low- and medium-income countries.
Nick James, MD, lead author of The Lancet report and professor of prostate and bladder cancer research at The Institute of Cancer Research in London, said that the surge, in part, is a medical success story.
Dr. James told this news organization.
“There is a big rise in the high-income countries. But we’re going to see a big rise in the number of 50-, 60-, 70-year-olds in the coming decades in the poorer countries, and with that comes more prostate cancer. High-income countries such as the UK and USA will also see smaller increases for the same reason.”
According to the report, to be presented April 6 at the 2024 European Association of Urology Congress in Paris, “The case for prostate cancer screening for all men aged 50-70 years (and all men of African origin aged 45–70 years) in high-income countries is strengthening with improved use of technologies such as MRI and growing evidence for the safety of active surveillance.”
Andrew Vickers, PhD, a biostatistician at Memorial Sloan Kettering Cancer Center in New York City, said that the Lancet Commission came to similar conclusions as he and an international group of researchers did in a 2023 policy paper in The BMJ. A major gap, Dr. Vickers said, is misuse of prostate-specific antigen (PSA) screening.
“We found that the ubiquitous policy compromise of letting patients decide for themselves about PSA has led to the worst possible outcomes of overuse in men unlikely to benefit, high rates of overdiagnosis and overtreatment, and economic and racial inequity,” Dr. Vickers said. “Our view is that PSA screening should be done well — by implementing straightforward harm-reduction strategies like restricting screening in older men and use of secondary tests before biopsy — or not at all.”
Dr. James said that undertreatment of advanced disease is widespread; only about 30%-40% of men in the United States receive combination hormone therapy for metastatic disease, for example. “Simply doing what we know works would improve outcomes,” he said.
Dr. James said that men of African ancestry are twice as likely to develop prostate cancer, but whether treatment should follow a different approach in these men is unclear. The new report stressed the need to include more men of African ancestry in research.
Brandon Mahal, MD, vice chair of research in radiation oncology the University of Miami Sylvester Comprehensive Cancer Center and a coauthor of the report, said that new approaches are needed to enable earlier diagnosis of prostate cancer in men in low- to middle-income countries, where most patients present with metastatic disease and are less likely to survive for long periods.
Dr. James recommended pop-up clinics and mobile testing to encourage men who are at high risk for prostate cancer but feel well to detect lethal cancers early.
In England, for example, Dr. James helped introduce an outreach program called The Man Van which provided free health checks, including PSA tests, to high-risk men in London.
“By bringing a van with quick and easy testing straight to men at work and in the community, and targeting those who have a higher risk of prostate cancer, we provided thousands of health checks which resulted in almost 100 cancer diagnoses in men who might otherwise have only seen a doctor once their cancer has progressed to a more advanced stage,” he said.
He noted that the medical community worldwide is ill-prepared for the onslaught of prostate cancer cases.
“The solution cannot be training more urologists, radiation oncologists, pathologists, and radiologists because it simply takes too long,” Dr. James said. However, increased use of nurses and artificial intelligence may help. “In my own hospital, biopsies are a nurse-led and -delivered service. AI is extraordinarily good at diagnosis already and will only get better,” he said.
In poorer countries, smartphones could fill gaps too. “The same technology that does face recognition already can say that’s a Gleason 7 prostate cancer,” Dr. James said. “It’s not being rolled out in countries like America of course because pathologists’ income is at risk.”
Dr. James, Dr. Vickers, and Dr. Mahal reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.