User login
FDA announces approval of fifth adalimumab biosimilar, Abrilada
The Food and Drug Administration has cleared adalimumab-afzb (Abrilada) as the fifth approved Humira biosimilar and the 25th approved biosimilar drug overall, the agency said in a Nov. 15 announcement.
According to a press release from Pfizer, approval for Abrilada was based on review of a comprehensive data package demonstrating biosimilarity of the drug to the reference product. This included data from a clinical comparative study, which found no clinically meaningful difference between Abrilada and the reference in terms of efficacy, safety, and immunogenicity in patients with moderate to severe rheumatoid arthritis (RA). In addition to RA, Abrilada is indicated for juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis.
Common adverse events in adalimumab clinical trials included infection, injection-site reactions, headache, and rash.
Pfizer said that it “is working to make Abrilada available to U.S. patients as soon as feasible based on the terms of our agreement with AbbVie [the manufacturer of Humira]. Our current plans are to launch in 2023.”
The Food and Drug Administration has cleared adalimumab-afzb (Abrilada) as the fifth approved Humira biosimilar and the 25th approved biosimilar drug overall, the agency said in a Nov. 15 announcement.
According to a press release from Pfizer, approval for Abrilada was based on review of a comprehensive data package demonstrating biosimilarity of the drug to the reference product. This included data from a clinical comparative study, which found no clinically meaningful difference between Abrilada and the reference in terms of efficacy, safety, and immunogenicity in patients with moderate to severe rheumatoid arthritis (RA). In addition to RA, Abrilada is indicated for juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis.
Common adverse events in adalimumab clinical trials included infection, injection-site reactions, headache, and rash.
Pfizer said that it “is working to make Abrilada available to U.S. patients as soon as feasible based on the terms of our agreement with AbbVie [the manufacturer of Humira]. Our current plans are to launch in 2023.”
The Food and Drug Administration has cleared adalimumab-afzb (Abrilada) as the fifth approved Humira biosimilar and the 25th approved biosimilar drug overall, the agency said in a Nov. 15 announcement.
According to a press release from Pfizer, approval for Abrilada was based on review of a comprehensive data package demonstrating biosimilarity of the drug to the reference product. This included data from a clinical comparative study, which found no clinically meaningful difference between Abrilada and the reference in terms of efficacy, safety, and immunogenicity in patients with moderate to severe rheumatoid arthritis (RA). In addition to RA, Abrilada is indicated for juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis.
Common adverse events in adalimumab clinical trials included infection, injection-site reactions, headache, and rash.
Pfizer said that it “is working to make Abrilada available to U.S. patients as soon as feasible based on the terms of our agreement with AbbVie [the manufacturer of Humira]. Our current plans are to launch in 2023.”
GALILEO, GALILEO 4D: Mixed results in post-TAVR anticoagulation
PHILADELPHIA – The results of the first randomized prospective trial of an anticoagulation strategy versus standard dual antiplatelet (DAPT) therapy for patients undergoing transcatheter aortic valve replacement (TAVR) show that routine anticoagulation is not suitable for all comers in a high-risk population.
In the main GALILEO trial of elderly patients after TAVR, those who received an investigational anticoagulation strategy with the direct factor Xa inhibitor rivaroxaban (Xarelto; Bayer/Janssen) had worse survival and more thromboembolic and bleeding events than patients who received standard DAPT.
However, in the GALILEO 4D substudy of patients who underwent four-dimensional computed tomography (4DCT) randomized to the two therapies, those in the rivaroxaban arm were less likely to show subclinical leaflet motion abnormalities and leaflet thickening.
Preliminary results from GALILEO were disclosed in an October 3, 2018, “Dear Healthcare Professional” letter from Bayer, and the trial was stopped after a median of 17 months due to safety concerns.
The full data analysis from GALILEO as well as the results from GALILEO 4D were presented at the American Heart Association scientific sessions to coincide with their publication on Nov. 16, 2019, in the New England Journal of Medicine.
The takeaway message is that, despite the positive imaging finding in GALILEO 4D, “there is no reason to give 10 mg rivaroxaban-based treatment routinely after TAVR in patients who don’t need anticoagulation anyhow,” lead author in the main GALILEO trial, George D. Dangas, MD, PhD, Mount Sinai Hospital, New York, said in an interview.
However, because rivaroxaban had an effect in reducing the clots on leaflets, he said, further investigation is required to determine the optimal therapeutic strategy after TAVR.
Similarly, the assigned discussant for GALILEO, Elaine Hylek, MD, of Boston University said in an interview that “we just don’t know right now what the overall added benefit of an oral anticoagulant would be in this high-risk patient population after having a TAVR.”
Ole De Backer, MD, PhD, of Rigshospitalet University Hospital, Copenhagen, lead author of the GALILEO 4D substudy, concluded that, although the rivaroxaban-based strategy was associated with fewer valve abnormalities in this analysis, those positive outcomes need to be taken in context with worse clinical outcomes in the main GALILEO trial.
GALILEO
Guidelines recommend DAPT after TAVR, but this advice is based on expert consensus or small studies, the GALILEO study authors noted. Several years ago, there were random case reports and then case series of patients who had undergone TAVR or surgical aortic valve replacement (SAVR) and developed clots around the valve, Dr. Dangas explained.
These developments coincided with the first available high-quality CT angiography images that captured valve abnormalities that had not been seen before.
In parallel, there were rare reports of stroke and transient ischemic attack (TIA) that may have been associated with TAVR or SAVR. This triggered a series of studies to investigate an anticoagulation strategy after TAVR.
From December 2015 to May 2018, GALILEO enrolled 1,644 patients at 136 sites in 16 countries who had undergone successful TAVR, and had no indication for an anticoagulant (e.g., no atrial fibrillation).
The patients had a mean age of 80.6 years (plus or minus 6.6 years) and 49.5% were female. The median time from TAVR to randomization was 2 days (range, 0-8 days).
Half were randomized to receive an antithrombotic strategy, rivaroxaban 10 mg once daily plus aspirin 75-100 mg once daily for the first 90 days followed by rivaroxaban alone. The other half received an antiplatelet-based strategy, aspirin 75-100 mg once daily plus clopidogrel 75 mg once daily for the first 90 days followed by aspirin alone.
In the intention-to-treat analysis, death or first thromboembolic event, the primary efficacy outcome, occurred in 105 patients in the rivaroxaban group and 78 patients in the antiplatelet group (hazard ratio, 1.35; 95% CI, 1.01-1.81; P = .04).
Major, disabling, or life-threatening bleeding, the primary safety outcome, occurred in 46 and 31 patients, respectively (HR, 1.50; P = .08).
A total of 64 deaths occurred in the rivaroxaban group and 38 in the antiplatelet group (HR, 1.69; 95% CI, 1.13-2.53).
The individuals who were enrolled in this study were 80 and older, Dr. Hylek pointed out. “The age in and of itself is an uncontested risk factor for everything, whether it be bleeding, embolic event, or obviously mortality.”
Although the dose was half that used to prevent stroke in patients with atrial fibrillation, perhaps a “twice-daily lower dose” might be the way to go, moving forward, she said.
Patients who did not have atrial fibrillation may have developed atrial fibrillation in the interim, and “you would have to change the dose of the rivaroxaban.”
Also, patients who may have been taking aspirin for 5 or 10 years and “survived” aspirin, who were then newly exposed to an anticoagulant, would be more likely to experience bleeding.
“I certainly wouldn’t close the door on novel anticoagulants,” she concluded. “There are still other drug trials that are out there with this TAVR population. We’ll wait for that,” and see if the results corroborate these findings.
The high-risk patients may turn out to be a potential niche group for drugs being developed to inhibit factor XIa, she speculated.
GALILEO 4D
However, despite the negative results of the overall GALILEO study, results from the substudy that used 4DCT to evaluate function of the bioprosthetic aortic valves suggested rivaroxaban may have potentially beneficial effects on valve function.
The results showed that patients on the rivaroxaban and aspirin regimen had lower rates of subclinical reduced leaflet motion and leaflet thickening than patients on the antiplatelet strategy, said Dr. De Backer, reporting on behalf of the GALILEO-4D investigators.
The substudy evaluated 205 patients who had 4DCT 90 days after TAVR. The primary substudy endpoint was at least one prosthetic valve leaflet with a grade 3 or higher motion reduction, which 2 of 97 patients in the rivaroxaban group had (2.1%) versus 11 of 101 in the antiplatelet group (10.9%, P = .01).
“This indicated an 80% greater reduction of the primary endpoint in the rivaroxaban arm,” Dr. De Backer said. The chief secondary endpoint, the proportion of patients with at least one thickened leaflet, was met by 12.4% of the rivaroxaban group and 32.4% of the antiplatelet arm, “a 60% significant reduction by rivaroxaban,” Dr. De Backer said.
However, when the 10 patients in each group who didn’t adhere to the study drug regimen were excluded, he said, “then we see no single patient had reduced leaflet motion of grade 3 or more in the rivaroxaban arm.”
Another takeaway from the substudy is the ineffectiveness of transthoracic echocardiography as opposed to 4DCT in TAVR patients. Echocardiography (ECG) failed to show any significant differences in the mean valve gradient between the treatment groups, Dr. De Backer said.
Eleven patients who didn’t have leaflet thickening (7.3%) and 7 patients who did (15.9%) showed an increase of 5 mm Hg or more in the mean valve gradient on echo. ECG also showed a similar increase in the mean valve gradient in 14 patients who had no to moderate reduced leaflet motion (grade 3 or lower, 7.7%) and in four patients (30.8) who had grade 3 or higher reduced leaflet motion.
“This basically confirms results from observational studies that transthoracic echocardiography is often not good enough to detect these phenomena,” Dr. De Backer said.
The percentages of substudy patients who had major clinical events – major bleeding, thromboembolic events, or death at 90 days – were each less than 3%, he said. “There were too few clinical events to permit any assessment of the impact of leaflet thickening or reduced leaflet motion on clinical outcomes,” he said.
That lack of clarity with regard to clinical events is one of the questions the study leaves unanswered, said discussant Victoria Delgado, MD, PhD, of Leiden University Medical Center in the Netherlands.
“With stroke or TIA, there are too few events to draw any conclusions,” she said of the substudy. “We don’t know when we need to use CT, when we need to evaluate these patients, or maybe when we should go for more advanced imaging techniques where we can see the biology of those changes in the leaflets.” Hopefully, she said, future studies provide those insights.
“CT can be more sensitive than ECG to see these subclinical changes,” she said, “but the open questions that we have are to see if there is a correlation between thrombosis rate on imaging versus the stroke rate.”
The substudy’s conclusion on ECG, however, has been borne out by previous retrospective studies, Dr. Delgado added.
Robert A. Harrington, MD, of Stanford Medicine, tried to put the seemingly conflicting findings of the main GALILEO study and the 4D substudy into context.
“There you have the disconnect between the mechanism and the clinical observation and those are sometimes difficult to reconcile because the assumption is that the mechanism leads to the clinical outcome.”
While the main study shows that routine anticoagulation after TAVR is not indicated, the findings raise questions about the risk of clots forming on bioprosthetic valves. “Yes, maybe there are clots forming on these valves, but maybe that’s not causing the bad clinical outcomes,” Dr. Harrington said.
The findings also raise questions about the use of newer anticoagulants to prevent stroke post TAVR, he said. “It appears that warfarin is better than the newer anticoagulants for reasons that aren’t entirely clear.”
Dr. Dangas, lead author of the main GALILEO trial, said the substudy results could help design future trials of even-lower doses of anticoagulation in a more selective group of TAVR patients.
“In order to decrease the clots, first of all you don’t need the full dose of anticoagulation; even a low dose may do the trick,” he said. Further investigations can evaluate the clinical significance of having a blood clot in the valve as an indication for anticoagulation versus antiplatelet therapy.
“Even though this obviously doesn’t mean you’re going to have a stroke in a year or two,” Dr. Dangas said, “could it perhaps mean that the valve is not going to have such a good durability later on?”
Perhaps future studies of anticoagulation in TAVR should concentrate on patients who actually have clotting in the valve, he said.
The trial was supported by Bayer and Janssen. Dr. Dangas reported receiving grants from Bayer during the conduct of the study, personal fees from Bayer and Janssen, grants and personal fees from Daiichi-Sankyo, and “other” funding from Medtronic outside the submitted work. Dr. De Backer reported receiving grants from Bayer during the conduct of the study and personal fees from Abbott and Boston Scientific outside the submitted work.
SOURCE: Dangas GD and De Backer O. AHA 19, Late-Breaking Science 3 session.
This article also appears on Medscape.com.
PHILADELPHIA – The results of the first randomized prospective trial of an anticoagulation strategy versus standard dual antiplatelet (DAPT) therapy for patients undergoing transcatheter aortic valve replacement (TAVR) show that routine anticoagulation is not suitable for all comers in a high-risk population.
In the main GALILEO trial of elderly patients after TAVR, those who received an investigational anticoagulation strategy with the direct factor Xa inhibitor rivaroxaban (Xarelto; Bayer/Janssen) had worse survival and more thromboembolic and bleeding events than patients who received standard DAPT.
However, in the GALILEO 4D substudy of patients who underwent four-dimensional computed tomography (4DCT) randomized to the two therapies, those in the rivaroxaban arm were less likely to show subclinical leaflet motion abnormalities and leaflet thickening.
Preliminary results from GALILEO were disclosed in an October 3, 2018, “Dear Healthcare Professional” letter from Bayer, and the trial was stopped after a median of 17 months due to safety concerns.
The full data analysis from GALILEO as well as the results from GALILEO 4D were presented at the American Heart Association scientific sessions to coincide with their publication on Nov. 16, 2019, in the New England Journal of Medicine.
The takeaway message is that, despite the positive imaging finding in GALILEO 4D, “there is no reason to give 10 mg rivaroxaban-based treatment routinely after TAVR in patients who don’t need anticoagulation anyhow,” lead author in the main GALILEO trial, George D. Dangas, MD, PhD, Mount Sinai Hospital, New York, said in an interview.
However, because rivaroxaban had an effect in reducing the clots on leaflets, he said, further investigation is required to determine the optimal therapeutic strategy after TAVR.
Similarly, the assigned discussant for GALILEO, Elaine Hylek, MD, of Boston University said in an interview that “we just don’t know right now what the overall added benefit of an oral anticoagulant would be in this high-risk patient population after having a TAVR.”
Ole De Backer, MD, PhD, of Rigshospitalet University Hospital, Copenhagen, lead author of the GALILEO 4D substudy, concluded that, although the rivaroxaban-based strategy was associated with fewer valve abnormalities in this analysis, those positive outcomes need to be taken in context with worse clinical outcomes in the main GALILEO trial.
GALILEO
Guidelines recommend DAPT after TAVR, but this advice is based on expert consensus or small studies, the GALILEO study authors noted. Several years ago, there were random case reports and then case series of patients who had undergone TAVR or surgical aortic valve replacement (SAVR) and developed clots around the valve, Dr. Dangas explained.
These developments coincided with the first available high-quality CT angiography images that captured valve abnormalities that had not been seen before.
In parallel, there were rare reports of stroke and transient ischemic attack (TIA) that may have been associated with TAVR or SAVR. This triggered a series of studies to investigate an anticoagulation strategy after TAVR.
From December 2015 to May 2018, GALILEO enrolled 1,644 patients at 136 sites in 16 countries who had undergone successful TAVR, and had no indication for an anticoagulant (e.g., no atrial fibrillation).
The patients had a mean age of 80.6 years (plus or minus 6.6 years) and 49.5% were female. The median time from TAVR to randomization was 2 days (range, 0-8 days).
Half were randomized to receive an antithrombotic strategy, rivaroxaban 10 mg once daily plus aspirin 75-100 mg once daily for the first 90 days followed by rivaroxaban alone. The other half received an antiplatelet-based strategy, aspirin 75-100 mg once daily plus clopidogrel 75 mg once daily for the first 90 days followed by aspirin alone.
In the intention-to-treat analysis, death or first thromboembolic event, the primary efficacy outcome, occurred in 105 patients in the rivaroxaban group and 78 patients in the antiplatelet group (hazard ratio, 1.35; 95% CI, 1.01-1.81; P = .04).
Major, disabling, or life-threatening bleeding, the primary safety outcome, occurred in 46 and 31 patients, respectively (HR, 1.50; P = .08).
A total of 64 deaths occurred in the rivaroxaban group and 38 in the antiplatelet group (HR, 1.69; 95% CI, 1.13-2.53).
The individuals who were enrolled in this study were 80 and older, Dr. Hylek pointed out. “The age in and of itself is an uncontested risk factor for everything, whether it be bleeding, embolic event, or obviously mortality.”
Although the dose was half that used to prevent stroke in patients with atrial fibrillation, perhaps a “twice-daily lower dose” might be the way to go, moving forward, she said.
Patients who did not have atrial fibrillation may have developed atrial fibrillation in the interim, and “you would have to change the dose of the rivaroxaban.”
Also, patients who may have been taking aspirin for 5 or 10 years and “survived” aspirin, who were then newly exposed to an anticoagulant, would be more likely to experience bleeding.
“I certainly wouldn’t close the door on novel anticoagulants,” she concluded. “There are still other drug trials that are out there with this TAVR population. We’ll wait for that,” and see if the results corroborate these findings.
The high-risk patients may turn out to be a potential niche group for drugs being developed to inhibit factor XIa, she speculated.
GALILEO 4D
However, despite the negative results of the overall GALILEO study, results from the substudy that used 4DCT to evaluate function of the bioprosthetic aortic valves suggested rivaroxaban may have potentially beneficial effects on valve function.
The results showed that patients on the rivaroxaban and aspirin regimen had lower rates of subclinical reduced leaflet motion and leaflet thickening than patients on the antiplatelet strategy, said Dr. De Backer, reporting on behalf of the GALILEO-4D investigators.
The substudy evaluated 205 patients who had 4DCT 90 days after TAVR. The primary substudy endpoint was at least one prosthetic valve leaflet with a grade 3 or higher motion reduction, which 2 of 97 patients in the rivaroxaban group had (2.1%) versus 11 of 101 in the antiplatelet group (10.9%, P = .01).
“This indicated an 80% greater reduction of the primary endpoint in the rivaroxaban arm,” Dr. De Backer said. The chief secondary endpoint, the proportion of patients with at least one thickened leaflet, was met by 12.4% of the rivaroxaban group and 32.4% of the antiplatelet arm, “a 60% significant reduction by rivaroxaban,” Dr. De Backer said.
However, when the 10 patients in each group who didn’t adhere to the study drug regimen were excluded, he said, “then we see no single patient had reduced leaflet motion of grade 3 or more in the rivaroxaban arm.”
Another takeaway from the substudy is the ineffectiveness of transthoracic echocardiography as opposed to 4DCT in TAVR patients. Echocardiography (ECG) failed to show any significant differences in the mean valve gradient between the treatment groups, Dr. De Backer said.
Eleven patients who didn’t have leaflet thickening (7.3%) and 7 patients who did (15.9%) showed an increase of 5 mm Hg or more in the mean valve gradient on echo. ECG also showed a similar increase in the mean valve gradient in 14 patients who had no to moderate reduced leaflet motion (grade 3 or lower, 7.7%) and in four patients (30.8) who had grade 3 or higher reduced leaflet motion.
“This basically confirms results from observational studies that transthoracic echocardiography is often not good enough to detect these phenomena,” Dr. De Backer said.
The percentages of substudy patients who had major clinical events – major bleeding, thromboembolic events, or death at 90 days – were each less than 3%, he said. “There were too few clinical events to permit any assessment of the impact of leaflet thickening or reduced leaflet motion on clinical outcomes,” he said.
That lack of clarity with regard to clinical events is one of the questions the study leaves unanswered, said discussant Victoria Delgado, MD, PhD, of Leiden University Medical Center in the Netherlands.
“With stroke or TIA, there are too few events to draw any conclusions,” she said of the substudy. “We don’t know when we need to use CT, when we need to evaluate these patients, or maybe when we should go for more advanced imaging techniques where we can see the biology of those changes in the leaflets.” Hopefully, she said, future studies provide those insights.
“CT can be more sensitive than ECG to see these subclinical changes,” she said, “but the open questions that we have are to see if there is a correlation between thrombosis rate on imaging versus the stroke rate.”
The substudy’s conclusion on ECG, however, has been borne out by previous retrospective studies, Dr. Delgado added.
Robert A. Harrington, MD, of Stanford Medicine, tried to put the seemingly conflicting findings of the main GALILEO study and the 4D substudy into context.
“There you have the disconnect between the mechanism and the clinical observation and those are sometimes difficult to reconcile because the assumption is that the mechanism leads to the clinical outcome.”
While the main study shows that routine anticoagulation after TAVR is not indicated, the findings raise questions about the risk of clots forming on bioprosthetic valves. “Yes, maybe there are clots forming on these valves, but maybe that’s not causing the bad clinical outcomes,” Dr. Harrington said.
The findings also raise questions about the use of newer anticoagulants to prevent stroke post TAVR, he said. “It appears that warfarin is better than the newer anticoagulants for reasons that aren’t entirely clear.”
Dr. Dangas, lead author of the main GALILEO trial, said the substudy results could help design future trials of even-lower doses of anticoagulation in a more selective group of TAVR patients.
“In order to decrease the clots, first of all you don’t need the full dose of anticoagulation; even a low dose may do the trick,” he said. Further investigations can evaluate the clinical significance of having a blood clot in the valve as an indication for anticoagulation versus antiplatelet therapy.
“Even though this obviously doesn’t mean you’re going to have a stroke in a year or two,” Dr. Dangas said, “could it perhaps mean that the valve is not going to have such a good durability later on?”
Perhaps future studies of anticoagulation in TAVR should concentrate on patients who actually have clotting in the valve, he said.
The trial was supported by Bayer and Janssen. Dr. Dangas reported receiving grants from Bayer during the conduct of the study, personal fees from Bayer and Janssen, grants and personal fees from Daiichi-Sankyo, and “other” funding from Medtronic outside the submitted work. Dr. De Backer reported receiving grants from Bayer during the conduct of the study and personal fees from Abbott and Boston Scientific outside the submitted work.
SOURCE: Dangas GD and De Backer O. AHA 19, Late-Breaking Science 3 session.
This article also appears on Medscape.com.
PHILADELPHIA – The results of the first randomized prospective trial of an anticoagulation strategy versus standard dual antiplatelet (DAPT) therapy for patients undergoing transcatheter aortic valve replacement (TAVR) show that routine anticoagulation is not suitable for all comers in a high-risk population.
In the main GALILEO trial of elderly patients after TAVR, those who received an investigational anticoagulation strategy with the direct factor Xa inhibitor rivaroxaban (Xarelto; Bayer/Janssen) had worse survival and more thromboembolic and bleeding events than patients who received standard DAPT.
However, in the GALILEO 4D substudy of patients who underwent four-dimensional computed tomography (4DCT) randomized to the two therapies, those in the rivaroxaban arm were less likely to show subclinical leaflet motion abnormalities and leaflet thickening.
Preliminary results from GALILEO were disclosed in an October 3, 2018, “Dear Healthcare Professional” letter from Bayer, and the trial was stopped after a median of 17 months due to safety concerns.
The full data analysis from GALILEO as well as the results from GALILEO 4D were presented at the American Heart Association scientific sessions to coincide with their publication on Nov. 16, 2019, in the New England Journal of Medicine.
The takeaway message is that, despite the positive imaging finding in GALILEO 4D, “there is no reason to give 10 mg rivaroxaban-based treatment routinely after TAVR in patients who don’t need anticoagulation anyhow,” lead author in the main GALILEO trial, George D. Dangas, MD, PhD, Mount Sinai Hospital, New York, said in an interview.
However, because rivaroxaban had an effect in reducing the clots on leaflets, he said, further investigation is required to determine the optimal therapeutic strategy after TAVR.
Similarly, the assigned discussant for GALILEO, Elaine Hylek, MD, of Boston University said in an interview that “we just don’t know right now what the overall added benefit of an oral anticoagulant would be in this high-risk patient population after having a TAVR.”
Ole De Backer, MD, PhD, of Rigshospitalet University Hospital, Copenhagen, lead author of the GALILEO 4D substudy, concluded that, although the rivaroxaban-based strategy was associated with fewer valve abnormalities in this analysis, those positive outcomes need to be taken in context with worse clinical outcomes in the main GALILEO trial.
GALILEO
Guidelines recommend DAPT after TAVR, but this advice is based on expert consensus or small studies, the GALILEO study authors noted. Several years ago, there were random case reports and then case series of patients who had undergone TAVR or surgical aortic valve replacement (SAVR) and developed clots around the valve, Dr. Dangas explained.
These developments coincided with the first available high-quality CT angiography images that captured valve abnormalities that had not been seen before.
In parallel, there were rare reports of stroke and transient ischemic attack (TIA) that may have been associated with TAVR or SAVR. This triggered a series of studies to investigate an anticoagulation strategy after TAVR.
From December 2015 to May 2018, GALILEO enrolled 1,644 patients at 136 sites in 16 countries who had undergone successful TAVR, and had no indication for an anticoagulant (e.g., no atrial fibrillation).
The patients had a mean age of 80.6 years (plus or minus 6.6 years) and 49.5% were female. The median time from TAVR to randomization was 2 days (range, 0-8 days).
Half were randomized to receive an antithrombotic strategy, rivaroxaban 10 mg once daily plus aspirin 75-100 mg once daily for the first 90 days followed by rivaroxaban alone. The other half received an antiplatelet-based strategy, aspirin 75-100 mg once daily plus clopidogrel 75 mg once daily for the first 90 days followed by aspirin alone.
In the intention-to-treat analysis, death or first thromboembolic event, the primary efficacy outcome, occurred in 105 patients in the rivaroxaban group and 78 patients in the antiplatelet group (hazard ratio, 1.35; 95% CI, 1.01-1.81; P = .04).
Major, disabling, or life-threatening bleeding, the primary safety outcome, occurred in 46 and 31 patients, respectively (HR, 1.50; P = .08).
A total of 64 deaths occurred in the rivaroxaban group and 38 in the antiplatelet group (HR, 1.69; 95% CI, 1.13-2.53).
The individuals who were enrolled in this study were 80 and older, Dr. Hylek pointed out. “The age in and of itself is an uncontested risk factor for everything, whether it be bleeding, embolic event, or obviously mortality.”
Although the dose was half that used to prevent stroke in patients with atrial fibrillation, perhaps a “twice-daily lower dose” might be the way to go, moving forward, she said.
Patients who did not have atrial fibrillation may have developed atrial fibrillation in the interim, and “you would have to change the dose of the rivaroxaban.”
Also, patients who may have been taking aspirin for 5 or 10 years and “survived” aspirin, who were then newly exposed to an anticoagulant, would be more likely to experience bleeding.
“I certainly wouldn’t close the door on novel anticoagulants,” she concluded. “There are still other drug trials that are out there with this TAVR population. We’ll wait for that,” and see if the results corroborate these findings.
The high-risk patients may turn out to be a potential niche group for drugs being developed to inhibit factor XIa, she speculated.
GALILEO 4D
However, despite the negative results of the overall GALILEO study, results from the substudy that used 4DCT to evaluate function of the bioprosthetic aortic valves suggested rivaroxaban may have potentially beneficial effects on valve function.
The results showed that patients on the rivaroxaban and aspirin regimen had lower rates of subclinical reduced leaflet motion and leaflet thickening than patients on the antiplatelet strategy, said Dr. De Backer, reporting on behalf of the GALILEO-4D investigators.
The substudy evaluated 205 patients who had 4DCT 90 days after TAVR. The primary substudy endpoint was at least one prosthetic valve leaflet with a grade 3 or higher motion reduction, which 2 of 97 patients in the rivaroxaban group had (2.1%) versus 11 of 101 in the antiplatelet group (10.9%, P = .01).
“This indicated an 80% greater reduction of the primary endpoint in the rivaroxaban arm,” Dr. De Backer said. The chief secondary endpoint, the proportion of patients with at least one thickened leaflet, was met by 12.4% of the rivaroxaban group and 32.4% of the antiplatelet arm, “a 60% significant reduction by rivaroxaban,” Dr. De Backer said.
However, when the 10 patients in each group who didn’t adhere to the study drug regimen were excluded, he said, “then we see no single patient had reduced leaflet motion of grade 3 or more in the rivaroxaban arm.”
Another takeaway from the substudy is the ineffectiveness of transthoracic echocardiography as opposed to 4DCT in TAVR patients. Echocardiography (ECG) failed to show any significant differences in the mean valve gradient between the treatment groups, Dr. De Backer said.
Eleven patients who didn’t have leaflet thickening (7.3%) and 7 patients who did (15.9%) showed an increase of 5 mm Hg or more in the mean valve gradient on echo. ECG also showed a similar increase in the mean valve gradient in 14 patients who had no to moderate reduced leaflet motion (grade 3 or lower, 7.7%) and in four patients (30.8) who had grade 3 or higher reduced leaflet motion.
“This basically confirms results from observational studies that transthoracic echocardiography is often not good enough to detect these phenomena,” Dr. De Backer said.
The percentages of substudy patients who had major clinical events – major bleeding, thromboembolic events, or death at 90 days – were each less than 3%, he said. “There were too few clinical events to permit any assessment of the impact of leaflet thickening or reduced leaflet motion on clinical outcomes,” he said.
That lack of clarity with regard to clinical events is one of the questions the study leaves unanswered, said discussant Victoria Delgado, MD, PhD, of Leiden University Medical Center in the Netherlands.
“With stroke or TIA, there are too few events to draw any conclusions,” she said of the substudy. “We don’t know when we need to use CT, when we need to evaluate these patients, or maybe when we should go for more advanced imaging techniques where we can see the biology of those changes in the leaflets.” Hopefully, she said, future studies provide those insights.
“CT can be more sensitive than ECG to see these subclinical changes,” she said, “but the open questions that we have are to see if there is a correlation between thrombosis rate on imaging versus the stroke rate.”
The substudy’s conclusion on ECG, however, has been borne out by previous retrospective studies, Dr. Delgado added.
Robert A. Harrington, MD, of Stanford Medicine, tried to put the seemingly conflicting findings of the main GALILEO study and the 4D substudy into context.
“There you have the disconnect between the mechanism and the clinical observation and those are sometimes difficult to reconcile because the assumption is that the mechanism leads to the clinical outcome.”
While the main study shows that routine anticoagulation after TAVR is not indicated, the findings raise questions about the risk of clots forming on bioprosthetic valves. “Yes, maybe there are clots forming on these valves, but maybe that’s not causing the bad clinical outcomes,” Dr. Harrington said.
The findings also raise questions about the use of newer anticoagulants to prevent stroke post TAVR, he said. “It appears that warfarin is better than the newer anticoagulants for reasons that aren’t entirely clear.”
Dr. Dangas, lead author of the main GALILEO trial, said the substudy results could help design future trials of even-lower doses of anticoagulation in a more selective group of TAVR patients.
“In order to decrease the clots, first of all you don’t need the full dose of anticoagulation; even a low dose may do the trick,” he said. Further investigations can evaluate the clinical significance of having a blood clot in the valve as an indication for anticoagulation versus antiplatelet therapy.
“Even though this obviously doesn’t mean you’re going to have a stroke in a year or two,” Dr. Dangas said, “could it perhaps mean that the valve is not going to have such a good durability later on?”
Perhaps future studies of anticoagulation in TAVR should concentrate on patients who actually have clotting in the valve, he said.
The trial was supported by Bayer and Janssen. Dr. Dangas reported receiving grants from Bayer during the conduct of the study, personal fees from Bayer and Janssen, grants and personal fees from Daiichi-Sankyo, and “other” funding from Medtronic outside the submitted work. Dr. De Backer reported receiving grants from Bayer during the conduct of the study and personal fees from Abbott and Boston Scientific outside the submitted work.
SOURCE: Dangas GD and De Backer O. AHA 19, Late-Breaking Science 3 session.
This article also appears on Medscape.com.
REPORTING FROM AHA 2019
DAPA-HF: Dapagliflozin’s HFrEF efficacy confirmed in nondiabetics
PHILADELPHIA – The primary outcome results from the practice-changing DAPA-HF trial gave clinicians strong evidence that the diabetes drug dapagliflozin was equally effective at reducing cardiovascular death and acute exacerbations in patients with heart failure with reduced ejection fraction, whether or not they also had type 2 diabetes. More detailed findings from the 2,605 enrolled patients in DAPA-HF who lacked diabetes (55% of the total study population) have now sealed the deal.
“The relative and absolute reductions in cardiovascular death and hospitalizations or urgent visits for heart failure were substantial, clinically important, and consistent in patients with or without type 2 diabetes,” John McMurray, MD, declared at the American Heart Association scientific sessions as he summarized new trial results that confirmed the initial finding he reported previously.
While the initial report of the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) by the study’s lead investigator, Dr. McMurray, was limited to the finding that the relative risk reduction for the study’s primary endpoint was a highly statistically significant 25% in heart failure patients with diabetes and an equally strongly significant 27% relative cut among patients without diabetes (N Engl J Med. 2019 Sep 19;doi: 10.1056/NEJMoa1911303), the new data showed that same consistency across the range of outcomes studied in the trial as well as across the range of glycosylated hemoglobin levels that patients had at study entry.
In an analysis that divided the entire study population of 4,744 patients with heart failure with reduced ejection fraction (HFrEF) into tertiles based on their entry blood level of hemoglobin A1c, patients with a normal level at or below 5.6% had a 26% relative reduction in the study’s primary endpoint, essentially the same response as the 29% relative cut in adverse events in the tertile of patients with a glycosylated hemoglobin level of 5.7%-5.9% and the relative 28% relative reduction in events in patients diagnosed with type 2 diabetes and having a hemoglobin A1c of 6.0% or greater, reported Dr. McMurray, professor of cardiology at the University of Glasgow. The results also showed a very benign safety profile in the patients without diabetes, similar to patients with diabetes and to placebo, and with no episodes of major hypoglycemia or diabetic ketoacidosis.
“It’s quite impressive that the result was consistent regardless of the level of hemoglobin A1c,” commented Larry A. Allen, MD, professor of medicine at the University of Colorado in Aurora and designated discussant for the report. Even though the patients without diabetes constituted just over half of the full DAPA-HF enrollment, the comparison of the effect of dapagliflozin in patients with or without diabetes was prespecified in a trial that enrolled a relatively large number of patients into each of the two subgroups by diabetes status. “I think there a good chance dapagliflozin will get an indication” for treating HFrEF patients without diabetes, Dr. Allen suggested in a video interview.
If the DAPA-HF results persuade the U.S. Food and Drug Administration to grant a supplemental indication to dapagliflozin for use in cutting cardiovascular deaths and acute heart failure exacerbations in patients without diabetes, it would pave the way for health insurers to pay for the drug. Right now, even though Dr. Allen and other heart failure physicians have been impressed by the DAPA-HF findings and are eager to add the drug to the list of agents that HFrEF patients routinely receive, he’s been stymied so far by patients’ out-of pocket cost for using dapagliflozin off-label, roughly $500 a month.
“The DAPA-HF results suggest there is strong reason to consider dapagliflozin for patients without diabetes, and for payers to pay for it. I’m not prescribing dapagliflozin to HFrEF patients without diabetes right now; not because of the data, but because of noncoverage. Payers have not yet caught up with the data,” he said, and they likely will continue to not pay for the drug when used by patients without diabetes until a new labeled indication appears for those patients.
The immediate availability of dapagliflozin (Farxiga) and the two other approved members of the sodium-glucose co-transporter 2 inhibitor class of drugs, empagliflozin (Jardiance) and canagliflozin (Invokana), to treat patients with HFrEF, and the prospect of soon having dapagliflozin and possibly the other drugs in this class to treat patients with HFrEF but without diabetes also raises issues of drug sequencing in these patients and the overall number of drugs that HFrEF patients must now take to be on optimized medical therapy, Dr. Allen noted.
The already-existing lineup of medications for HFrEF patients includes starting on an ACE inhibitor or angiotensin receptor blocker and adding a beta-blocker, a mineralocorticoid receptor antagonist, then swapping out the initial renin-angiotensin system inhibitor for sacubitril/valsartan, and then, on top of all this, adding dapagliflozin or another drug in the same class. It raises questions of what is objectively the best way to introduce all these drugs into patients, and how to do it without subjecting patients to “financial toxicity,” Dr. Allen said during his discussion of the trial’s results.
DAPA-HF was sponsored by AstraZeneca, which markets dapagliflozin (Farxiga). The University of Glasgow received payment from AstraZeneca to compensate for the time Dr. McMurray spent running the study. Dr. Allen has been a consultant to ACI Clinical, Boston Scientific, and Janssen.
SOURCE: McMurray JJV. AHA 19, Late-Breaking Science 1.
A labeling change for dapagliflozin that says the drug is approved for use in patients with heart failure with reduced ejection fraction (HFrEF) and without diabetes is critical so that payers will get on board with this new and important treatment. The evidence for efficacy and safety in patients without diabetes was so strong in the DAPA-HF trial that I don’t think a second trial will be needed for the Food and Drug Administration to add this indication to dapagliflozin’s label.
For patients with type 2 diabetes as well as HFrEF, it’s already full steam ahead to use dapagliflozin or another drug from the class of sodium glucose co-transporter 2 (SGLT2) inhibitors, empagliflozin and canagliflozin. However, so far these drugs are not being widely prescribed by clinicians to patients with HFrEF but without diabetes. We need to build up the familiarity of clinicians with the SGLT2 inhibitor drugs so that primary care physicians will feel comfortable starting HFrEF patients on them. It’s relatively easy to start patients on the drugs in this class because of their good safety and no signal of problems when using them with other HFrEF medications.
The growing list of key drugs to use on patients with HFrEF means that we need to become smarter on how we start patients on these agents. Currently it’s done without evidence for which order of introduction works best. We also need to confirm that all five types of drugs that now appear indicated for HFrEF patients are all truly additive: an angiotensin receptor blocker coupled with the angiotensin receptor neprilysin inhibitor sacubitril, a beta-blocker, a mineralocorticoid receptor antagonist, and now an SGLT2 inhibitor. I propose that researchers run studies that systematically stop one of these drugs to see whether the overall benefit to HFrEF patients remains unchanged, thereby identifying an agent that could be dropped from what is a growing list of drug classes, with possibly more classes to follow depending on results from studies now underway.
Christopher M. O’Connor, MD, is a heart failure physician and president of the Inova Heart and Vascular Institute in Falls Church, Va. He has been a consultant to Arena, Bayer, Bristol-Meyers Squibb, Merck, and Windtree Therapeutics. He made these comments in an interview.
A labeling change for dapagliflozin that says the drug is approved for use in patients with heart failure with reduced ejection fraction (HFrEF) and without diabetes is critical so that payers will get on board with this new and important treatment. The evidence for efficacy and safety in patients without diabetes was so strong in the DAPA-HF trial that I don’t think a second trial will be needed for the Food and Drug Administration to add this indication to dapagliflozin’s label.
For patients with type 2 diabetes as well as HFrEF, it’s already full steam ahead to use dapagliflozin or another drug from the class of sodium glucose co-transporter 2 (SGLT2) inhibitors, empagliflozin and canagliflozin. However, so far these drugs are not being widely prescribed by clinicians to patients with HFrEF but without diabetes. We need to build up the familiarity of clinicians with the SGLT2 inhibitor drugs so that primary care physicians will feel comfortable starting HFrEF patients on them. It’s relatively easy to start patients on the drugs in this class because of their good safety and no signal of problems when using them with other HFrEF medications.
The growing list of key drugs to use on patients with HFrEF means that we need to become smarter on how we start patients on these agents. Currently it’s done without evidence for which order of introduction works best. We also need to confirm that all five types of drugs that now appear indicated for HFrEF patients are all truly additive: an angiotensin receptor blocker coupled with the angiotensin receptor neprilysin inhibitor sacubitril, a beta-blocker, a mineralocorticoid receptor antagonist, and now an SGLT2 inhibitor. I propose that researchers run studies that systematically stop one of these drugs to see whether the overall benefit to HFrEF patients remains unchanged, thereby identifying an agent that could be dropped from what is a growing list of drug classes, with possibly more classes to follow depending on results from studies now underway.
Christopher M. O’Connor, MD, is a heart failure physician and president of the Inova Heart and Vascular Institute in Falls Church, Va. He has been a consultant to Arena, Bayer, Bristol-Meyers Squibb, Merck, and Windtree Therapeutics. He made these comments in an interview.
A labeling change for dapagliflozin that says the drug is approved for use in patients with heart failure with reduced ejection fraction (HFrEF) and without diabetes is critical so that payers will get on board with this new and important treatment. The evidence for efficacy and safety in patients without diabetes was so strong in the DAPA-HF trial that I don’t think a second trial will be needed for the Food and Drug Administration to add this indication to dapagliflozin’s label.
For patients with type 2 diabetes as well as HFrEF, it’s already full steam ahead to use dapagliflozin or another drug from the class of sodium glucose co-transporter 2 (SGLT2) inhibitors, empagliflozin and canagliflozin. However, so far these drugs are not being widely prescribed by clinicians to patients with HFrEF but without diabetes. We need to build up the familiarity of clinicians with the SGLT2 inhibitor drugs so that primary care physicians will feel comfortable starting HFrEF patients on them. It’s relatively easy to start patients on the drugs in this class because of their good safety and no signal of problems when using them with other HFrEF medications.
The growing list of key drugs to use on patients with HFrEF means that we need to become smarter on how we start patients on these agents. Currently it’s done without evidence for which order of introduction works best. We also need to confirm that all five types of drugs that now appear indicated for HFrEF patients are all truly additive: an angiotensin receptor blocker coupled with the angiotensin receptor neprilysin inhibitor sacubitril, a beta-blocker, a mineralocorticoid receptor antagonist, and now an SGLT2 inhibitor. I propose that researchers run studies that systematically stop one of these drugs to see whether the overall benefit to HFrEF patients remains unchanged, thereby identifying an agent that could be dropped from what is a growing list of drug classes, with possibly more classes to follow depending on results from studies now underway.
Christopher M. O’Connor, MD, is a heart failure physician and president of the Inova Heart and Vascular Institute in Falls Church, Va. He has been a consultant to Arena, Bayer, Bristol-Meyers Squibb, Merck, and Windtree Therapeutics. He made these comments in an interview.
PHILADELPHIA – The primary outcome results from the practice-changing DAPA-HF trial gave clinicians strong evidence that the diabetes drug dapagliflozin was equally effective at reducing cardiovascular death and acute exacerbations in patients with heart failure with reduced ejection fraction, whether or not they also had type 2 diabetes. More detailed findings from the 2,605 enrolled patients in DAPA-HF who lacked diabetes (55% of the total study population) have now sealed the deal.
“The relative and absolute reductions in cardiovascular death and hospitalizations or urgent visits for heart failure were substantial, clinically important, and consistent in patients with or without type 2 diabetes,” John McMurray, MD, declared at the American Heart Association scientific sessions as he summarized new trial results that confirmed the initial finding he reported previously.
While the initial report of the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) by the study’s lead investigator, Dr. McMurray, was limited to the finding that the relative risk reduction for the study’s primary endpoint was a highly statistically significant 25% in heart failure patients with diabetes and an equally strongly significant 27% relative cut among patients without diabetes (N Engl J Med. 2019 Sep 19;doi: 10.1056/NEJMoa1911303), the new data showed that same consistency across the range of outcomes studied in the trial as well as across the range of glycosylated hemoglobin levels that patients had at study entry.
In an analysis that divided the entire study population of 4,744 patients with heart failure with reduced ejection fraction (HFrEF) into tertiles based on their entry blood level of hemoglobin A1c, patients with a normal level at or below 5.6% had a 26% relative reduction in the study’s primary endpoint, essentially the same response as the 29% relative cut in adverse events in the tertile of patients with a glycosylated hemoglobin level of 5.7%-5.9% and the relative 28% relative reduction in events in patients diagnosed with type 2 diabetes and having a hemoglobin A1c of 6.0% or greater, reported Dr. McMurray, professor of cardiology at the University of Glasgow. The results also showed a very benign safety profile in the patients without diabetes, similar to patients with diabetes and to placebo, and with no episodes of major hypoglycemia or diabetic ketoacidosis.
“It’s quite impressive that the result was consistent regardless of the level of hemoglobin A1c,” commented Larry A. Allen, MD, professor of medicine at the University of Colorado in Aurora and designated discussant for the report. Even though the patients without diabetes constituted just over half of the full DAPA-HF enrollment, the comparison of the effect of dapagliflozin in patients with or without diabetes was prespecified in a trial that enrolled a relatively large number of patients into each of the two subgroups by diabetes status. “I think there a good chance dapagliflozin will get an indication” for treating HFrEF patients without diabetes, Dr. Allen suggested in a video interview.
If the DAPA-HF results persuade the U.S. Food and Drug Administration to grant a supplemental indication to dapagliflozin for use in cutting cardiovascular deaths and acute heart failure exacerbations in patients without diabetes, it would pave the way for health insurers to pay for the drug. Right now, even though Dr. Allen and other heart failure physicians have been impressed by the DAPA-HF findings and are eager to add the drug to the list of agents that HFrEF patients routinely receive, he’s been stymied so far by patients’ out-of pocket cost for using dapagliflozin off-label, roughly $500 a month.
“The DAPA-HF results suggest there is strong reason to consider dapagliflozin for patients without diabetes, and for payers to pay for it. I’m not prescribing dapagliflozin to HFrEF patients without diabetes right now; not because of the data, but because of noncoverage. Payers have not yet caught up with the data,” he said, and they likely will continue to not pay for the drug when used by patients without diabetes until a new labeled indication appears for those patients.
The immediate availability of dapagliflozin (Farxiga) and the two other approved members of the sodium-glucose co-transporter 2 inhibitor class of drugs, empagliflozin (Jardiance) and canagliflozin (Invokana), to treat patients with HFrEF, and the prospect of soon having dapagliflozin and possibly the other drugs in this class to treat patients with HFrEF but without diabetes also raises issues of drug sequencing in these patients and the overall number of drugs that HFrEF patients must now take to be on optimized medical therapy, Dr. Allen noted.
The already-existing lineup of medications for HFrEF patients includes starting on an ACE inhibitor or angiotensin receptor blocker and adding a beta-blocker, a mineralocorticoid receptor antagonist, then swapping out the initial renin-angiotensin system inhibitor for sacubitril/valsartan, and then, on top of all this, adding dapagliflozin or another drug in the same class. It raises questions of what is objectively the best way to introduce all these drugs into patients, and how to do it without subjecting patients to “financial toxicity,” Dr. Allen said during his discussion of the trial’s results.
DAPA-HF was sponsored by AstraZeneca, which markets dapagliflozin (Farxiga). The University of Glasgow received payment from AstraZeneca to compensate for the time Dr. McMurray spent running the study. Dr. Allen has been a consultant to ACI Clinical, Boston Scientific, and Janssen.
SOURCE: McMurray JJV. AHA 19, Late-Breaking Science 1.
PHILADELPHIA – The primary outcome results from the practice-changing DAPA-HF trial gave clinicians strong evidence that the diabetes drug dapagliflozin was equally effective at reducing cardiovascular death and acute exacerbations in patients with heart failure with reduced ejection fraction, whether or not they also had type 2 diabetes. More detailed findings from the 2,605 enrolled patients in DAPA-HF who lacked diabetes (55% of the total study population) have now sealed the deal.
“The relative and absolute reductions in cardiovascular death and hospitalizations or urgent visits for heart failure were substantial, clinically important, and consistent in patients with or without type 2 diabetes,” John McMurray, MD, declared at the American Heart Association scientific sessions as he summarized new trial results that confirmed the initial finding he reported previously.
While the initial report of the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) by the study’s lead investigator, Dr. McMurray, was limited to the finding that the relative risk reduction for the study’s primary endpoint was a highly statistically significant 25% in heart failure patients with diabetes and an equally strongly significant 27% relative cut among patients without diabetes (N Engl J Med. 2019 Sep 19;doi: 10.1056/NEJMoa1911303), the new data showed that same consistency across the range of outcomes studied in the trial as well as across the range of glycosylated hemoglobin levels that patients had at study entry.
In an analysis that divided the entire study population of 4,744 patients with heart failure with reduced ejection fraction (HFrEF) into tertiles based on their entry blood level of hemoglobin A1c, patients with a normal level at or below 5.6% had a 26% relative reduction in the study’s primary endpoint, essentially the same response as the 29% relative cut in adverse events in the tertile of patients with a glycosylated hemoglobin level of 5.7%-5.9% and the relative 28% relative reduction in events in patients diagnosed with type 2 diabetes and having a hemoglobin A1c of 6.0% or greater, reported Dr. McMurray, professor of cardiology at the University of Glasgow. The results also showed a very benign safety profile in the patients without diabetes, similar to patients with diabetes and to placebo, and with no episodes of major hypoglycemia or diabetic ketoacidosis.
“It’s quite impressive that the result was consistent regardless of the level of hemoglobin A1c,” commented Larry A. Allen, MD, professor of medicine at the University of Colorado in Aurora and designated discussant for the report. Even though the patients without diabetes constituted just over half of the full DAPA-HF enrollment, the comparison of the effect of dapagliflozin in patients with or without diabetes was prespecified in a trial that enrolled a relatively large number of patients into each of the two subgroups by diabetes status. “I think there a good chance dapagliflozin will get an indication” for treating HFrEF patients without diabetes, Dr. Allen suggested in a video interview.
If the DAPA-HF results persuade the U.S. Food and Drug Administration to grant a supplemental indication to dapagliflozin for use in cutting cardiovascular deaths and acute heart failure exacerbations in patients without diabetes, it would pave the way for health insurers to pay for the drug. Right now, even though Dr. Allen and other heart failure physicians have been impressed by the DAPA-HF findings and are eager to add the drug to the list of agents that HFrEF patients routinely receive, he’s been stymied so far by patients’ out-of pocket cost for using dapagliflozin off-label, roughly $500 a month.
“The DAPA-HF results suggest there is strong reason to consider dapagliflozin for patients without diabetes, and for payers to pay for it. I’m not prescribing dapagliflozin to HFrEF patients without diabetes right now; not because of the data, but because of noncoverage. Payers have not yet caught up with the data,” he said, and they likely will continue to not pay for the drug when used by patients without diabetes until a new labeled indication appears for those patients.
The immediate availability of dapagliflozin (Farxiga) and the two other approved members of the sodium-glucose co-transporter 2 inhibitor class of drugs, empagliflozin (Jardiance) and canagliflozin (Invokana), to treat patients with HFrEF, and the prospect of soon having dapagliflozin and possibly the other drugs in this class to treat patients with HFrEF but without diabetes also raises issues of drug sequencing in these patients and the overall number of drugs that HFrEF patients must now take to be on optimized medical therapy, Dr. Allen noted.
The already-existing lineup of medications for HFrEF patients includes starting on an ACE inhibitor or angiotensin receptor blocker and adding a beta-blocker, a mineralocorticoid receptor antagonist, then swapping out the initial renin-angiotensin system inhibitor for sacubitril/valsartan, and then, on top of all this, adding dapagliflozin or another drug in the same class. It raises questions of what is objectively the best way to introduce all these drugs into patients, and how to do it without subjecting patients to “financial toxicity,” Dr. Allen said during his discussion of the trial’s results.
DAPA-HF was sponsored by AstraZeneca, which markets dapagliflozin (Farxiga). The University of Glasgow received payment from AstraZeneca to compensate for the time Dr. McMurray spent running the study. Dr. Allen has been a consultant to ACI Clinical, Boston Scientific, and Janssen.
SOURCE: McMurray JJV. AHA 19, Late-Breaking Science 1.
REPORTING FROM AHA 2019
Key clinical point: Dapaglifozin produced as much benefit in HFrEF patients without diabetes as it did in those with type 2 diabetes.
Major finding: The relative risk reduction with dapagliflozin was 26% in patients with a hemoglobin A1c of 5.6% or less.
Study details: DAPA-HF is a multicenter, randomized trial involving 4,744 patients with heart failure with reduced ejection fraction.
Disclosures: DAPA-HF was sponsored by AstraZeneca, which markets dapagliflozin (Farxiga). The University of Glasgow received payment from AstraZeneca to compensate for the time Dr. McMurray spent running the study. Dr. Allen has been a consultant to ACI Clinical, Boston Scientific, and Janssen.
Source: McMurray JJV et al. AHA 19, Late-Breaking Science 1.
FDA panel supports Vascepa expanded indication for CVD reduction
Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid, received unanimous backing from a Food and Drug Administration advisory panel for a new indication for reducing cardiovascular event risk.
Icosapent ethyl (Vascepa) received initial agency approval in 2012 for the indication of cutting triglyceride levels once they reached at least 500 mg/dL.
The target patient population for this new, cardiovascular-event protection role will reflect some or all of the types of patients enrolled in REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial), which tested icosapent ethyl in 8,179 patients with either established cardiovascular disease or diabetes and at least one additional cardiovascular disease risk factor. This study provided the bulk of the data considered by the FDA panel.
REDUCE-IT showed that, during a median of 4.9 years, patients who received icosapent ethyl had a statistically significant 25% relative risk reduction in the trial’s primary, composite endpoint (New Engl J Med. 2019 Jan 3;380[1]:11-22).
Icosapent ethyl “appeared effective and safe,” and would be a “useful, new, added agent for treating patients” like those enrolled in the trial, said Kenneth D. Burman, MD, professor and chief of endocrinology at Medstar Washington (D.C.) Hospital Center and chair of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee.
The advisory panel members appeared uniformly comfortable with recommending that the FDA add a cardiovascular disease indication based on the REDUCE-IT findings.
But while they agreed that icosapent ethyl should receive some type of indication for cardiovascular event reduction, the committee split over which patients the indication should include. Specifically, they diverged on the issue of primary prevention.
Some said that the patient enrollment that produced a positive result in REDUCE-IT should not be retrospectively subdivided, while others said that combining secondary- and primary-prevention patients in a single large trial inappropriately lumped together patients who would be better considered separately.
Committee members also expressed uncertainty over the appropriate triglyceride level to warrant treatment. The REDUCE-IT trial was designed to enroll patients with triglycerides of 135 mg/dL or greater, but several panel members suggested that, for labeling, the threshold should be at least 150 mg/dL, or even 200 mg/dL.
Safety was another aspect that generated a lot of panel discussion throughout their day-long discussion, with particular focus on a signal of a small but concerning increased rate of incident atrial fibrillation among patients who received icosapent ethyl, as well as a small but nearly significant increase in the rate of serious bleeds.
Further analyses presented during the meeting showed that an increased bleeding rate linked with icosapent ethyl was focused in patients who concurrently received one or more antiplatelet drugs or an anticoagulant.
However, panel members rejected the notion that these safety concerns warranted a boxed warning, agreeing that it could be managed with appropriate labeling information.
Clinician reaction
Clinicians who manage these types of patients viewed the prospect of an expanded indication for icosapent ethyl as an important advance.
The REDUCE-IT results by themselves “were convincing” for patients with established cardiovascular disease without need for a confirmatory trial, Robert H. Eckel, MD, an endocrinologist and professor of medicine at the University of Colorado at Denver, Aurora, said in an interview. But he remained unconvinced about efficacy for primary-prevention patients, or even for secondary-prevention patients with a triglyceride level below 150 mg/dL.
“Icosapent ethyl will clearly be a mainstay for managing high-risk patients. It gives us another treatment option,” Yehuda Handelsman, MD, an endocrinologist and medical director and principal investigator of the Metabolic Institute of America in Tarzana, Calif., said in an interview. “I do not see the atrial fibrillation or bleeding effects as reasons not to approve this drug. It should be a precaution. Overall, icosapent ethyl is one of the easier drugs for patients to take.”
Dr. Handelsman said it would be unethical to run a confirmatory trial and randomize patients to placebo. “Another trial makes no sense,” he said.
But the data from REDUCE-IT were “not as convincing” for primary-prevention patients, suggesting a need for caution about using icosapent ethyl for patients without established cardiovascular disease, Paul S. Jellinger, MD, an endocrinologist in Fort Lauderdale, Fla., said in an interview.
Cost-effectiveness
An analysis of the cost-effectiveness of icosapent ethyl as used in REDUCE-IT showed that the drug fell into the rare category of being a “dominant” treatment, meaning that it both improved patient outcomes and reduced medical costs. William S. Weintraub, MD, will report findings from this analysis on Nov. 16, 2019, at the annual scientific sessions of the American Heart Association.
The analysis used a wholesale acquisition cost for a 1-day dosage of icosapent ethyl of $4.16, derived from a commercial source for prescription-drug pricing and actual hospitalization costs for the patients in the trial.
Based on the REDUCE-IT outcomes, treatment with icosapent ethyl was linked with a boost in quality-adjusted life-years that extrapolated to an average 0.26 increase during the full lifetime of REDUCE-IT participants, at a cost that averaged $1,284 less per treated patient over their lifetime, according to Dr. Weintraub, director of Outcomes Research at Medstar Washington Hospital Center, Washington.
Although the 0.26 lifetime increase in quality-adjusted life-years may sound modest, “in the cost-effectiveness world, 0.26 is actually significant,” Dr. Weintraub said. He also highlighted how unusual it is to find a patented drug that improves quality of life and longevity while also saving money.
“I know of no other on-patent, branded pharmaceutical that is dominant,” he said.
Off-patent pharmaceuticals, like statins, can be quite inexpensive and may also be dominant, he noted. Being dominant for cost-effectiveness means that icosapent ethyl “provides good value and is worth what we pay for it, well within social thresholds of willingness to pay,” Dr. Weintraub said.
REDUCE-IT was sponsored by Amarin, the company that markets icosapent ethyl (Vascepa). Dr. Burman has received research funding from AstraZeneca, Eisai, and IBSA. Dr. Eckel has received personal fees from Kowa Pharmaceuticals, Merck, Novartis, and Sanofi/Regeneron, as well as research funding from Endece, Ionis Pharmaceuticals, and UniQure. Dr. Handelsman has been a consultant to and received research funding from Amarin and several other companies. Dr. Jellinger has been a speaker on behalf of Amarin, Amgen, and Regeneron. Dr. Weintraub has received honoraria and research support from Amarin, and honoraria from AstraZeneca.
Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid, received unanimous backing from a Food and Drug Administration advisory panel for a new indication for reducing cardiovascular event risk.
Icosapent ethyl (Vascepa) received initial agency approval in 2012 for the indication of cutting triglyceride levels once they reached at least 500 mg/dL.
The target patient population for this new, cardiovascular-event protection role will reflect some or all of the types of patients enrolled in REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial), which tested icosapent ethyl in 8,179 patients with either established cardiovascular disease or diabetes and at least one additional cardiovascular disease risk factor. This study provided the bulk of the data considered by the FDA panel.
REDUCE-IT showed that, during a median of 4.9 years, patients who received icosapent ethyl had a statistically significant 25% relative risk reduction in the trial’s primary, composite endpoint (New Engl J Med. 2019 Jan 3;380[1]:11-22).
Icosapent ethyl “appeared effective and safe,” and would be a “useful, new, added agent for treating patients” like those enrolled in the trial, said Kenneth D. Burman, MD, professor and chief of endocrinology at Medstar Washington (D.C.) Hospital Center and chair of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee.
The advisory panel members appeared uniformly comfortable with recommending that the FDA add a cardiovascular disease indication based on the REDUCE-IT findings.
But while they agreed that icosapent ethyl should receive some type of indication for cardiovascular event reduction, the committee split over which patients the indication should include. Specifically, they diverged on the issue of primary prevention.
Some said that the patient enrollment that produced a positive result in REDUCE-IT should not be retrospectively subdivided, while others said that combining secondary- and primary-prevention patients in a single large trial inappropriately lumped together patients who would be better considered separately.
Committee members also expressed uncertainty over the appropriate triglyceride level to warrant treatment. The REDUCE-IT trial was designed to enroll patients with triglycerides of 135 mg/dL or greater, but several panel members suggested that, for labeling, the threshold should be at least 150 mg/dL, or even 200 mg/dL.
Safety was another aspect that generated a lot of panel discussion throughout their day-long discussion, with particular focus on a signal of a small but concerning increased rate of incident atrial fibrillation among patients who received icosapent ethyl, as well as a small but nearly significant increase in the rate of serious bleeds.
Further analyses presented during the meeting showed that an increased bleeding rate linked with icosapent ethyl was focused in patients who concurrently received one or more antiplatelet drugs or an anticoagulant.
However, panel members rejected the notion that these safety concerns warranted a boxed warning, agreeing that it could be managed with appropriate labeling information.
Clinician reaction
Clinicians who manage these types of patients viewed the prospect of an expanded indication for icosapent ethyl as an important advance.
The REDUCE-IT results by themselves “were convincing” for patients with established cardiovascular disease without need for a confirmatory trial, Robert H. Eckel, MD, an endocrinologist and professor of medicine at the University of Colorado at Denver, Aurora, said in an interview. But he remained unconvinced about efficacy for primary-prevention patients, or even for secondary-prevention patients with a triglyceride level below 150 mg/dL.
“Icosapent ethyl will clearly be a mainstay for managing high-risk patients. It gives us another treatment option,” Yehuda Handelsman, MD, an endocrinologist and medical director and principal investigator of the Metabolic Institute of America in Tarzana, Calif., said in an interview. “I do not see the atrial fibrillation or bleeding effects as reasons not to approve this drug. It should be a precaution. Overall, icosapent ethyl is one of the easier drugs for patients to take.”
Dr. Handelsman said it would be unethical to run a confirmatory trial and randomize patients to placebo. “Another trial makes no sense,” he said.
But the data from REDUCE-IT were “not as convincing” for primary-prevention patients, suggesting a need for caution about using icosapent ethyl for patients without established cardiovascular disease, Paul S. Jellinger, MD, an endocrinologist in Fort Lauderdale, Fla., said in an interview.
Cost-effectiveness
An analysis of the cost-effectiveness of icosapent ethyl as used in REDUCE-IT showed that the drug fell into the rare category of being a “dominant” treatment, meaning that it both improved patient outcomes and reduced medical costs. William S. Weintraub, MD, will report findings from this analysis on Nov. 16, 2019, at the annual scientific sessions of the American Heart Association.
The analysis used a wholesale acquisition cost for a 1-day dosage of icosapent ethyl of $4.16, derived from a commercial source for prescription-drug pricing and actual hospitalization costs for the patients in the trial.
Based on the REDUCE-IT outcomes, treatment with icosapent ethyl was linked with a boost in quality-adjusted life-years that extrapolated to an average 0.26 increase during the full lifetime of REDUCE-IT participants, at a cost that averaged $1,284 less per treated patient over their lifetime, according to Dr. Weintraub, director of Outcomes Research at Medstar Washington Hospital Center, Washington.
Although the 0.26 lifetime increase in quality-adjusted life-years may sound modest, “in the cost-effectiveness world, 0.26 is actually significant,” Dr. Weintraub said. He also highlighted how unusual it is to find a patented drug that improves quality of life and longevity while also saving money.
“I know of no other on-patent, branded pharmaceutical that is dominant,” he said.
Off-patent pharmaceuticals, like statins, can be quite inexpensive and may also be dominant, he noted. Being dominant for cost-effectiveness means that icosapent ethyl “provides good value and is worth what we pay for it, well within social thresholds of willingness to pay,” Dr. Weintraub said.
REDUCE-IT was sponsored by Amarin, the company that markets icosapent ethyl (Vascepa). Dr. Burman has received research funding from AstraZeneca, Eisai, and IBSA. Dr. Eckel has received personal fees from Kowa Pharmaceuticals, Merck, Novartis, and Sanofi/Regeneron, as well as research funding from Endece, Ionis Pharmaceuticals, and UniQure. Dr. Handelsman has been a consultant to and received research funding from Amarin and several other companies. Dr. Jellinger has been a speaker on behalf of Amarin, Amgen, and Regeneron. Dr. Weintraub has received honoraria and research support from Amarin, and honoraria from AstraZeneca.
Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid, received unanimous backing from a Food and Drug Administration advisory panel for a new indication for reducing cardiovascular event risk.
Icosapent ethyl (Vascepa) received initial agency approval in 2012 for the indication of cutting triglyceride levels once they reached at least 500 mg/dL.
The target patient population for this new, cardiovascular-event protection role will reflect some or all of the types of patients enrolled in REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial), which tested icosapent ethyl in 8,179 patients with either established cardiovascular disease or diabetes and at least one additional cardiovascular disease risk factor. This study provided the bulk of the data considered by the FDA panel.
REDUCE-IT showed that, during a median of 4.9 years, patients who received icosapent ethyl had a statistically significant 25% relative risk reduction in the trial’s primary, composite endpoint (New Engl J Med. 2019 Jan 3;380[1]:11-22).
Icosapent ethyl “appeared effective and safe,” and would be a “useful, new, added agent for treating patients” like those enrolled in the trial, said Kenneth D. Burman, MD, professor and chief of endocrinology at Medstar Washington (D.C.) Hospital Center and chair of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee.
The advisory panel members appeared uniformly comfortable with recommending that the FDA add a cardiovascular disease indication based on the REDUCE-IT findings.
But while they agreed that icosapent ethyl should receive some type of indication for cardiovascular event reduction, the committee split over which patients the indication should include. Specifically, they diverged on the issue of primary prevention.
Some said that the patient enrollment that produced a positive result in REDUCE-IT should not be retrospectively subdivided, while others said that combining secondary- and primary-prevention patients in a single large trial inappropriately lumped together patients who would be better considered separately.
Committee members also expressed uncertainty over the appropriate triglyceride level to warrant treatment. The REDUCE-IT trial was designed to enroll patients with triglycerides of 135 mg/dL or greater, but several panel members suggested that, for labeling, the threshold should be at least 150 mg/dL, or even 200 mg/dL.
Safety was another aspect that generated a lot of panel discussion throughout their day-long discussion, with particular focus on a signal of a small but concerning increased rate of incident atrial fibrillation among patients who received icosapent ethyl, as well as a small but nearly significant increase in the rate of serious bleeds.
Further analyses presented during the meeting showed that an increased bleeding rate linked with icosapent ethyl was focused in patients who concurrently received one or more antiplatelet drugs or an anticoagulant.
However, panel members rejected the notion that these safety concerns warranted a boxed warning, agreeing that it could be managed with appropriate labeling information.
Clinician reaction
Clinicians who manage these types of patients viewed the prospect of an expanded indication for icosapent ethyl as an important advance.
The REDUCE-IT results by themselves “were convincing” for patients with established cardiovascular disease without need for a confirmatory trial, Robert H. Eckel, MD, an endocrinologist and professor of medicine at the University of Colorado at Denver, Aurora, said in an interview. But he remained unconvinced about efficacy for primary-prevention patients, or even for secondary-prevention patients with a triglyceride level below 150 mg/dL.
“Icosapent ethyl will clearly be a mainstay for managing high-risk patients. It gives us another treatment option,” Yehuda Handelsman, MD, an endocrinologist and medical director and principal investigator of the Metabolic Institute of America in Tarzana, Calif., said in an interview. “I do not see the atrial fibrillation or bleeding effects as reasons not to approve this drug. It should be a precaution. Overall, icosapent ethyl is one of the easier drugs for patients to take.”
Dr. Handelsman said it would be unethical to run a confirmatory trial and randomize patients to placebo. “Another trial makes no sense,” he said.
But the data from REDUCE-IT were “not as convincing” for primary-prevention patients, suggesting a need for caution about using icosapent ethyl for patients without established cardiovascular disease, Paul S. Jellinger, MD, an endocrinologist in Fort Lauderdale, Fla., said in an interview.
Cost-effectiveness
An analysis of the cost-effectiveness of icosapent ethyl as used in REDUCE-IT showed that the drug fell into the rare category of being a “dominant” treatment, meaning that it both improved patient outcomes and reduced medical costs. William S. Weintraub, MD, will report findings from this analysis on Nov. 16, 2019, at the annual scientific sessions of the American Heart Association.
The analysis used a wholesale acquisition cost for a 1-day dosage of icosapent ethyl of $4.16, derived from a commercial source for prescription-drug pricing and actual hospitalization costs for the patients in the trial.
Based on the REDUCE-IT outcomes, treatment with icosapent ethyl was linked with a boost in quality-adjusted life-years that extrapolated to an average 0.26 increase during the full lifetime of REDUCE-IT participants, at a cost that averaged $1,284 less per treated patient over their lifetime, according to Dr. Weintraub, director of Outcomes Research at Medstar Washington Hospital Center, Washington.
Although the 0.26 lifetime increase in quality-adjusted life-years may sound modest, “in the cost-effectiveness world, 0.26 is actually significant,” Dr. Weintraub said. He also highlighted how unusual it is to find a patented drug that improves quality of life and longevity while also saving money.
“I know of no other on-patent, branded pharmaceutical that is dominant,” he said.
Off-patent pharmaceuticals, like statins, can be quite inexpensive and may also be dominant, he noted. Being dominant for cost-effectiveness means that icosapent ethyl “provides good value and is worth what we pay for it, well within social thresholds of willingness to pay,” Dr. Weintraub said.
REDUCE-IT was sponsored by Amarin, the company that markets icosapent ethyl (Vascepa). Dr. Burman has received research funding from AstraZeneca, Eisai, and IBSA. Dr. Eckel has received personal fees from Kowa Pharmaceuticals, Merck, Novartis, and Sanofi/Regeneron, as well as research funding from Endece, Ionis Pharmaceuticals, and UniQure. Dr. Handelsman has been a consultant to and received research funding from Amarin and several other companies. Dr. Jellinger has been a speaker on behalf of Amarin, Amgen, and Regeneron. Dr. Weintraub has received honoraria and research support from Amarin, and honoraria from AstraZeneca.
CDC releases update of 2013 Antibiotic Resistance Threats Report
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
Opioid reduction works after minimally invasive gynecologic surgery
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
REPORTING FROM THE AAGL GLOBAL CONGRESS
ACP recommends ways to address rising drug prices
such as promoting the use of lower-cost generics and introducing annual out-of-pocket spending caps.
Two position papers, published in Annals of Internal Medicine, outlined the College’s concerns about the increasing price of prescription drugs for Medicare and Medicaid, pointing out that the United States has an average annual per capita spend of $1,443 on pharmaceutical drugs and $1,026 on retail prescription drugs.
“The primary differences between health care expenditures in the United States versus other high-income nations are pricing of medical goods and services and the lack of direct price controls or negotiating power by centralized government health care systems,” wrote Hilary Daniel and Sue S. Bornstein, MD, of the Health Public Policy Committee of the American College of Physicians, in one of the papers.
They cited the example of new drugs for hepatitis C which, at more than $80,000 for a treatment course, accounted for 40% of the net growth in prescription drug spending in 2014.
Their first recommendation was to modify the Medicare Part D low-income subsidy (LIS) program, which currently supports approximately 12 million beneficiaries, to encourage the use of lower-cost generic or biosimilar drugs.
The rate of generic drug dispensing among LIS enrollees has been consistently 4%-5% lower than among non-LIS enrollees, they wrote. The Centers for Medicare & Medicaid Services estimated that Medicare could have saved nearly $9 billion, and passed on $3 billion in savings to the Part D program and its beneficiaries, if available equivalent generics were prescribed instead of brand-name drugs.
The authors wrote that zero-copay generics have had the strongest effect on generic drug use, both for LIS and non-LIS enrollees.
“Reducing or eliminating cost sharing for LIS enrollees would not require legislative action, because it would not increase cost sharing, would reduce overall out-of pocket costs for LIS enrollees, and would encourage use of generics among them,” they wrote. They authors of the paper also argued that this move could reduce Medicare spending on reinsurance payments, because most enrollees who reach the ‘catastrophic’ phase of coverage were in the LIS program.
The second recommendation was for annual out-of-pocket spending caps for Medicare Part D beneficiaries who reach the catastrophic phase of coverage. During 2007-2015, the number of seniors in Medicare Part D who reached this catastrophic limit of coverage doubled to more than 1 million, with those enrollees paying an average of more than $3,000 out of pocket in 2015 alone, the authors noted.
“Caps have been proposed in other areas of Medicare; a 2016 resolution from the House Committee on the Budget included a Medicare proposal with a catastrophic coverage cap on annual out-of-pocket expenses, which it called, ‘an important aspect of the private insurance market currently absent from Medicare that would safeguard the sickest and poorest beneficiaries,’ ” they wrote.
In an accompanying editorial, Shelley A. Jazowski of the University of North Carolina at Chapel Hill and coauthor Stacie B. Dusetzina, PhD, of Vanderbilt University, in Nashville, Tenn., said that, while a cap would improve financial protection for beneficiaries, it could result in trade-offs such as increased premiums to accommodate lower spending by some beneficiaries.
The American College of Physicians (ACP) supports a full repeal of the noninterference clause in the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, wrote the position paper’s authors. This Act prohibits Medicare from negotiating directly with pharmaceutical manufacturers over the price of drugs.
The position paper also advocated for interim approaches, such as allowing the Secretary of Health and Human Services to negotiate on the price of a limited set of high-cost or sole-source drugs.
“Although negotiation alone may not be enough to rein in drug prices, this approach would allow the government to leverage its purchasing power to reduce Medicare program costs while also allowing plan sponsors to maintain the power to negotiate for the vast majority of drugs covered in the program,” they wrote.
The editorial’s authors pointed out that the success of these negotiations would rely on the ability of Medicare to walk away from a bad deal, which could delay or limit the availability of some drugs with limited competitors.
The ACP also called for efforts to minimize the financial impact of misclassifications of prescription drugs in the Medicaid Drug Rebate Program on the federal government. One study found that these misclassifications occurred in 885 of the 30,000 drugs in the program. The EpiPen and EpiPen Jr autoinjectors, for example, were improperly classified as generic drugs, the paper states.
The authors’ final recommendation in the position paper was for further study of payment models that could reduce incentives to prescribe higher-priced drugs instead of lower-cost and similarly effective options.
In the second position paper, Ms. Daniel and Dr. Bornstein made policy recommendations targeted at pharmacy benefit managers. The first was to improve transparency for pharmacy benefit managers, such as by banning gag clauses that might prevent pharmacies from sharing pricing information with consumers.
“The continued lack of transparency from [pharmacy benefit managers] and insurers can hinder how patients, physicians, and others view the drug supply chain and can make it difficult to identify whether a particular entity is inappropriately driving up drug prices,” the authors wrote in the second position paper.
This was accompanied by a recommendation that accurate, understandable and actionable information on the price of prescription medication should be made available to physicians and patients at the point of prescription. They also called for health plans, pharmacy benefit managers and pharmaceutical manufacturers to share information on the amount paid for prescription drugs, aggregate amount of rebates, and pricing decisions to the Department of Health & Human Services and make that information publicly available, with exceptions for confidential data.
The editorial’s authors commented that many of the policy recommendations raised in the position paper were currently being debated in Congress, and there was clear support from physician groups to address drug pricing, out-of-pocket spending, and access.
“Although trade-offs will need to be considered before selection or implementation of policy solutions, policymakers must act to ensure that patients have access to the prescription drugs they need at a price that reflects the benefits to patients and society,” they wrote.
One author declared book royalties but no other conflicts of interest were declared.
SOURCES: Daniel H et al. Ann Intern Med. 2019 Nov 12. doi. 10.7326/M19-0013; doi. 10.7326/M19-0035.
such as promoting the use of lower-cost generics and introducing annual out-of-pocket spending caps.
Two position papers, published in Annals of Internal Medicine, outlined the College’s concerns about the increasing price of prescription drugs for Medicare and Medicaid, pointing out that the United States has an average annual per capita spend of $1,443 on pharmaceutical drugs and $1,026 on retail prescription drugs.
“The primary differences between health care expenditures in the United States versus other high-income nations are pricing of medical goods and services and the lack of direct price controls or negotiating power by centralized government health care systems,” wrote Hilary Daniel and Sue S. Bornstein, MD, of the Health Public Policy Committee of the American College of Physicians, in one of the papers.
They cited the example of new drugs for hepatitis C which, at more than $80,000 for a treatment course, accounted for 40% of the net growth in prescription drug spending in 2014.
Their first recommendation was to modify the Medicare Part D low-income subsidy (LIS) program, which currently supports approximately 12 million beneficiaries, to encourage the use of lower-cost generic or biosimilar drugs.
The rate of generic drug dispensing among LIS enrollees has been consistently 4%-5% lower than among non-LIS enrollees, they wrote. The Centers for Medicare & Medicaid Services estimated that Medicare could have saved nearly $9 billion, and passed on $3 billion in savings to the Part D program and its beneficiaries, if available equivalent generics were prescribed instead of brand-name drugs.
The authors wrote that zero-copay generics have had the strongest effect on generic drug use, both for LIS and non-LIS enrollees.
“Reducing or eliminating cost sharing for LIS enrollees would not require legislative action, because it would not increase cost sharing, would reduce overall out-of pocket costs for LIS enrollees, and would encourage use of generics among them,” they wrote. They authors of the paper also argued that this move could reduce Medicare spending on reinsurance payments, because most enrollees who reach the ‘catastrophic’ phase of coverage were in the LIS program.
The second recommendation was for annual out-of-pocket spending caps for Medicare Part D beneficiaries who reach the catastrophic phase of coverage. During 2007-2015, the number of seniors in Medicare Part D who reached this catastrophic limit of coverage doubled to more than 1 million, with those enrollees paying an average of more than $3,000 out of pocket in 2015 alone, the authors noted.
“Caps have been proposed in other areas of Medicare; a 2016 resolution from the House Committee on the Budget included a Medicare proposal with a catastrophic coverage cap on annual out-of-pocket expenses, which it called, ‘an important aspect of the private insurance market currently absent from Medicare that would safeguard the sickest and poorest beneficiaries,’ ” they wrote.
In an accompanying editorial, Shelley A. Jazowski of the University of North Carolina at Chapel Hill and coauthor Stacie B. Dusetzina, PhD, of Vanderbilt University, in Nashville, Tenn., said that, while a cap would improve financial protection for beneficiaries, it could result in trade-offs such as increased premiums to accommodate lower spending by some beneficiaries.
The American College of Physicians (ACP) supports a full repeal of the noninterference clause in the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, wrote the position paper’s authors. This Act prohibits Medicare from negotiating directly with pharmaceutical manufacturers over the price of drugs.
The position paper also advocated for interim approaches, such as allowing the Secretary of Health and Human Services to negotiate on the price of a limited set of high-cost or sole-source drugs.
“Although negotiation alone may not be enough to rein in drug prices, this approach would allow the government to leverage its purchasing power to reduce Medicare program costs while also allowing plan sponsors to maintain the power to negotiate for the vast majority of drugs covered in the program,” they wrote.
The editorial’s authors pointed out that the success of these negotiations would rely on the ability of Medicare to walk away from a bad deal, which could delay or limit the availability of some drugs with limited competitors.
The ACP also called for efforts to minimize the financial impact of misclassifications of prescription drugs in the Medicaid Drug Rebate Program on the federal government. One study found that these misclassifications occurred in 885 of the 30,000 drugs in the program. The EpiPen and EpiPen Jr autoinjectors, for example, were improperly classified as generic drugs, the paper states.
The authors’ final recommendation in the position paper was for further study of payment models that could reduce incentives to prescribe higher-priced drugs instead of lower-cost and similarly effective options.
In the second position paper, Ms. Daniel and Dr. Bornstein made policy recommendations targeted at pharmacy benefit managers. The first was to improve transparency for pharmacy benefit managers, such as by banning gag clauses that might prevent pharmacies from sharing pricing information with consumers.
“The continued lack of transparency from [pharmacy benefit managers] and insurers can hinder how patients, physicians, and others view the drug supply chain and can make it difficult to identify whether a particular entity is inappropriately driving up drug prices,” the authors wrote in the second position paper.
This was accompanied by a recommendation that accurate, understandable and actionable information on the price of prescription medication should be made available to physicians and patients at the point of prescription. They also called for health plans, pharmacy benefit managers and pharmaceutical manufacturers to share information on the amount paid for prescription drugs, aggregate amount of rebates, and pricing decisions to the Department of Health & Human Services and make that information publicly available, with exceptions for confidential data.
The editorial’s authors commented that many of the policy recommendations raised in the position paper were currently being debated in Congress, and there was clear support from physician groups to address drug pricing, out-of-pocket spending, and access.
“Although trade-offs will need to be considered before selection or implementation of policy solutions, policymakers must act to ensure that patients have access to the prescription drugs they need at a price that reflects the benefits to patients and society,” they wrote.
One author declared book royalties but no other conflicts of interest were declared.
SOURCES: Daniel H et al. Ann Intern Med. 2019 Nov 12. doi. 10.7326/M19-0013; doi. 10.7326/M19-0035.
such as promoting the use of lower-cost generics and introducing annual out-of-pocket spending caps.
Two position papers, published in Annals of Internal Medicine, outlined the College’s concerns about the increasing price of prescription drugs for Medicare and Medicaid, pointing out that the United States has an average annual per capita spend of $1,443 on pharmaceutical drugs and $1,026 on retail prescription drugs.
“The primary differences between health care expenditures in the United States versus other high-income nations are pricing of medical goods and services and the lack of direct price controls or negotiating power by centralized government health care systems,” wrote Hilary Daniel and Sue S. Bornstein, MD, of the Health Public Policy Committee of the American College of Physicians, in one of the papers.
They cited the example of new drugs for hepatitis C which, at more than $80,000 for a treatment course, accounted for 40% of the net growth in prescription drug spending in 2014.
Their first recommendation was to modify the Medicare Part D low-income subsidy (LIS) program, which currently supports approximately 12 million beneficiaries, to encourage the use of lower-cost generic or biosimilar drugs.
The rate of generic drug dispensing among LIS enrollees has been consistently 4%-5% lower than among non-LIS enrollees, they wrote. The Centers for Medicare & Medicaid Services estimated that Medicare could have saved nearly $9 billion, and passed on $3 billion in savings to the Part D program and its beneficiaries, if available equivalent generics were prescribed instead of brand-name drugs.
The authors wrote that zero-copay generics have had the strongest effect on generic drug use, both for LIS and non-LIS enrollees.
“Reducing or eliminating cost sharing for LIS enrollees would not require legislative action, because it would not increase cost sharing, would reduce overall out-of pocket costs for LIS enrollees, and would encourage use of generics among them,” they wrote. They authors of the paper also argued that this move could reduce Medicare spending on reinsurance payments, because most enrollees who reach the ‘catastrophic’ phase of coverage were in the LIS program.
The second recommendation was for annual out-of-pocket spending caps for Medicare Part D beneficiaries who reach the catastrophic phase of coverage. During 2007-2015, the number of seniors in Medicare Part D who reached this catastrophic limit of coverage doubled to more than 1 million, with those enrollees paying an average of more than $3,000 out of pocket in 2015 alone, the authors noted.
“Caps have been proposed in other areas of Medicare; a 2016 resolution from the House Committee on the Budget included a Medicare proposal with a catastrophic coverage cap on annual out-of-pocket expenses, which it called, ‘an important aspect of the private insurance market currently absent from Medicare that would safeguard the sickest and poorest beneficiaries,’ ” they wrote.
In an accompanying editorial, Shelley A. Jazowski of the University of North Carolina at Chapel Hill and coauthor Stacie B. Dusetzina, PhD, of Vanderbilt University, in Nashville, Tenn., said that, while a cap would improve financial protection for beneficiaries, it could result in trade-offs such as increased premiums to accommodate lower spending by some beneficiaries.
The American College of Physicians (ACP) supports a full repeal of the noninterference clause in the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, wrote the position paper’s authors. This Act prohibits Medicare from negotiating directly with pharmaceutical manufacturers over the price of drugs.
The position paper also advocated for interim approaches, such as allowing the Secretary of Health and Human Services to negotiate on the price of a limited set of high-cost or sole-source drugs.
“Although negotiation alone may not be enough to rein in drug prices, this approach would allow the government to leverage its purchasing power to reduce Medicare program costs while also allowing plan sponsors to maintain the power to negotiate for the vast majority of drugs covered in the program,” they wrote.
The editorial’s authors pointed out that the success of these negotiations would rely on the ability of Medicare to walk away from a bad deal, which could delay or limit the availability of some drugs with limited competitors.
The ACP also called for efforts to minimize the financial impact of misclassifications of prescription drugs in the Medicaid Drug Rebate Program on the federal government. One study found that these misclassifications occurred in 885 of the 30,000 drugs in the program. The EpiPen and EpiPen Jr autoinjectors, for example, were improperly classified as generic drugs, the paper states.
The authors’ final recommendation in the position paper was for further study of payment models that could reduce incentives to prescribe higher-priced drugs instead of lower-cost and similarly effective options.
In the second position paper, Ms. Daniel and Dr. Bornstein made policy recommendations targeted at pharmacy benefit managers. The first was to improve transparency for pharmacy benefit managers, such as by banning gag clauses that might prevent pharmacies from sharing pricing information with consumers.
“The continued lack of transparency from [pharmacy benefit managers] and insurers can hinder how patients, physicians, and others view the drug supply chain and can make it difficult to identify whether a particular entity is inappropriately driving up drug prices,” the authors wrote in the second position paper.
This was accompanied by a recommendation that accurate, understandable and actionable information on the price of prescription medication should be made available to physicians and patients at the point of prescription. They also called for health plans, pharmacy benefit managers and pharmaceutical manufacturers to share information on the amount paid for prescription drugs, aggregate amount of rebates, and pricing decisions to the Department of Health & Human Services and make that information publicly available, with exceptions for confidential data.
The editorial’s authors commented that many of the policy recommendations raised in the position paper were currently being debated in Congress, and there was clear support from physician groups to address drug pricing, out-of-pocket spending, and access.
“Although trade-offs will need to be considered before selection or implementation of policy solutions, policymakers must act to ensure that patients have access to the prescription drugs they need at a price that reflects the benefits to patients and society,” they wrote.
One author declared book royalties but no other conflicts of interest were declared.
SOURCES: Daniel H et al. Ann Intern Med. 2019 Nov 12. doi. 10.7326/M19-0013; doi. 10.7326/M19-0035.
FROM ANNALS OF INTERNAL MEDICINE
MS-related disability may be decreasing
STOCKHOLM – , according to an overview provided at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Data consistently indicate that the time that elapses before a patient requires a cane for ambulation has increased, and survival has likewise improved. “Some of the improvement can be attributed confidently to treatment effect,” said Ilya Kister, MD, associate professor of neurology at NYU Langone Health in New York. “We hope to see an even greater change with newer therapies.”
At the same time, neurologists appear to be diagnosing more cases of MS than they previously did, said Dr. Kister, which suggests that neurologists probably are diagnosing milder cases. The overall societal burden of MS remains high.
The relative prevalence of mild disability has increased
About 25 years have elapsed since the first disease-modifying treatment (DMT) for MS became available, and treatment has become widespread during that time. Dr. Kister and colleagues sought to determine whether the current clinical population of patients with MS, who for the most part receive DMTs, has less disability than do untreated patients or patients from natural history studies do. They identified the MS Severity Score (MSSS) as a measure with which to compare populations. The MSSS assigns a patient a ranking according to his or her level of disability, using a reference population of patients with the same disease duration for comparison. “MSSS can be conceptualized as rate of disability accumulation,” said Dr. Kister. “Lower MSSS corresponds to relatively slower disability accumulation, and higher MSSS to higher disability accumulation.”
The MSSS was developed using the Expanded Disability Status Scale (EDSS) score as a measure of disability. Because many neurologists do not routinely obtain EDSS scores for their patients, Dr. Kister and colleagues used the Patient-Determined Disease Steps (PDDS) to measure disability. As its name implies, the PDDS is a patient-reported outcome measure that mainly measures ambulation. It correlates strongly with EDSS, said Dr. Kister. He and colleagues used the PDDS to develop a reference table of MS disability, which they called the Patient-Derived MSSS.
The investigators examined a large sample of patients at NYU MS Center and Barnabas MS Center in Livingston, N.J. They grouped patients into sextiles according to their Patient-Derived MSSS. Dr. Kister and colleagues found that, rather than arriving at sextiles that contained equal numbers of patients, as would be expected if disability were distributed as in the reference population, they had significantly more patients in the two lowest sextiles and significantly fewer patients in the two highest sextiles. “This [result] suggests that the disability curve has indeed shifted toward the more benign end of the spectrum in the contemporary clinic population,” said Dr. Kister.
Other researchers have observed a similar phenomenon. George et al. published the results of a large, international collaboration in Neurology Genetics in 2016. After examining more than 7,000 patients, the investigators noted a similar overrepresentation of patients with milder severity scores and underrepresentation of patients with higher severity scores. These results support the hypothesis of a shift toward milder disability, said Dr. Kister.
Trend toward milder disability
The investigators next examined whether the rate of accumulation of disability among patients with MS had changed from year to year since DMTs were introduced. They conducted a univariate analysis of MSSS for 6,238 patients who were enrolled in the N.Y. State MS Consortium during 1996-2007. They found that patients who were enrolled in more recent years had significantly lower MSSS than patients who were enrolled in earlier years, regardless of disease duration. When Dr. Kister and colleagues replicated their analysis using EDSS, they found significantly lower levels of disability for patients enrolled in more recent years, except for patients with disease duration of 26-30 years. A multivariate analysis showed that the median MSSS of enrollees into the N.Y. State MS Consortium decreased from 5.04 in 1996 to 3.78 in 2006.
In a subsequent study, Dr. Kister and colleagues examined the age at which patients in the MSBase registry reached various disability milestones (e.g., EDSS of 6, which indicates the need of a cane to walk outdoors), according to their year of enrollment in the registry. They found a significant increase in age at milestone achievement with each subsequent calendar year. For example, for every consecutive year of enrollment, the age at which patients attained an EDSS of 6 increased by 0.38 years. These analyses were confirmed for the subgroups of patients diagnosed according to the Poser and McDonald criteria. The increase in age “is probably not just related to the shift in diagnostic criteria,” said Dr. Kister. When the researchers calculated the net average gains in years over the 13-year follow-up period, they found that patients who entered at the end of the enrollment period were 4.9 years older when they reached an EDSS of 6, compared with patients with an EDSS of 6 who entered at the beginning of the enrollment period.
International data show similar trends
Research conducted around the world shows similar trends, said Dr. Kister. In 2009, Veugelers et al. published the results of a study that included 1,752 patients with MS in Nova Scotia. Before the 1998 introduction of a drug insurance program that provides DMTs, the time to an EDSS of 6 was 14.4 years. After the introduction of this program, the time to EDSS of 6 was 18.6 years.
More recently, Capra et al. examined 1,324 patients with MS who attended an MS center in Brescia, Italy, during 1980-2010. They found that the age at which 50% of patients reached an EDSS of 6 was approximately 55 years in 1990. By 2010, the age at achieving this milestone had increased to approximately 63 years.
In a prospective study, Cree et al. examined the evolution of disability in 448 actively treated patients with relapsing-remitting MS and 69 patients with progressive MS. Approximately 45% of patients had no disability worsening during a 10-year follow-up period. Furthermore, a comparatively low 11% of patients had reached an EDSS of 6 at 10 years. The average disease duration of the cohort at that time was 17 years, said Dr. Kister. The results indicated that about 50% of patients would be expected to reach an EDSS of 6 after a disease duration of approximately 38 years, “which is much longer than in the natural history studies,” he added.
In 2019, Beiki et al. found that among patients with relapsing-remitting MS, the risk of reaching an EDSS of 6 decreased by 7% with each subsequent calendar year of diagnosis. The researchers did not observe a similar trend among patients with progressive MS. Their population-based, retrospective study included 7,331 patients in Sweden.
Two additional studies in Scandinavian populations add to the evidence of decreasing disability. In their examination of Swedish patients with MS who received a diagnosis of MS during 1968-2012, Burkill et al. found that the risk of death decreased over time. The hazard ratio of mortality for patients with MS, compared with a non-MS comparator group, decreased from 6.52 among those diagnosed during 1968-1980 to 2.08 for patients diagnosed during 2001-2012. The decrease in the risk of mortality was greater among patients with MS than in a matched comparator population. Similarly, in a nationwide, population-based study, Koch-Henriksen et al. found that all-cause excess mortality in Danish patients with MS decreased from 1950 through 1999.
The role of DMTs
The evidence suggests that DMTs are affecting the long-term progression of MS, said Dr. Kister. Palace et al. compared patients with MS in the UK who received treatment with interferon-beta with a modeled untreated cohort of patients in British Columbia. They found that treated patients reached an EDSS of 6 4 years later than did untreated patients.
Furthermore, an analysis by Brown et al. showed that the time to conversion to secondary progressive MS was longer among treated patients, compared with untreated patients. The risk of conversion was lower for patients treated with newer, more effective therapies (i.e., fingolimod, alemtuzumab, or natalizumab) than for those treated with glatiramer acetate or interferon beta.
Finally, Kingwell and colleagues examined the effect of treatment with interferon-beta on survival using an international cohort of approximately 6,000 patients with relapsing-remitting MS. They found that exposure to interferon-beta for more than 3 years was associated with a 32% reduction in the risk of mortality. They observed no similar risk reduction among patients exposed to interferon-beta for 6 months to 3 years.
Although these data are encouraging, other evidence indicates that the prevalence of MS in the United States has increased considerably in the past 40 years. Researchers estimate that 1 million Americans have MS, which “suggests that we are diagnosing many more mild cases,” said Dr. Kister. The burden of the disease remains high, he concluded.
Dr. Kister reported receiving consulting fees or research grants from Biogen, Roche, Genzyme and Genentech.
SOURCE: Kister I et al. ECTRIMS 2019. Abstract 281754.
STOCKHOLM – , according to an overview provided at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Data consistently indicate that the time that elapses before a patient requires a cane for ambulation has increased, and survival has likewise improved. “Some of the improvement can be attributed confidently to treatment effect,” said Ilya Kister, MD, associate professor of neurology at NYU Langone Health in New York. “We hope to see an even greater change with newer therapies.”
At the same time, neurologists appear to be diagnosing more cases of MS than they previously did, said Dr. Kister, which suggests that neurologists probably are diagnosing milder cases. The overall societal burden of MS remains high.
The relative prevalence of mild disability has increased
About 25 years have elapsed since the first disease-modifying treatment (DMT) for MS became available, and treatment has become widespread during that time. Dr. Kister and colleagues sought to determine whether the current clinical population of patients with MS, who for the most part receive DMTs, has less disability than do untreated patients or patients from natural history studies do. They identified the MS Severity Score (MSSS) as a measure with which to compare populations. The MSSS assigns a patient a ranking according to his or her level of disability, using a reference population of patients with the same disease duration for comparison. “MSSS can be conceptualized as rate of disability accumulation,” said Dr. Kister. “Lower MSSS corresponds to relatively slower disability accumulation, and higher MSSS to higher disability accumulation.”
The MSSS was developed using the Expanded Disability Status Scale (EDSS) score as a measure of disability. Because many neurologists do not routinely obtain EDSS scores for their patients, Dr. Kister and colleagues used the Patient-Determined Disease Steps (PDDS) to measure disability. As its name implies, the PDDS is a patient-reported outcome measure that mainly measures ambulation. It correlates strongly with EDSS, said Dr. Kister. He and colleagues used the PDDS to develop a reference table of MS disability, which they called the Patient-Derived MSSS.
The investigators examined a large sample of patients at NYU MS Center and Barnabas MS Center in Livingston, N.J. They grouped patients into sextiles according to their Patient-Derived MSSS. Dr. Kister and colleagues found that, rather than arriving at sextiles that contained equal numbers of patients, as would be expected if disability were distributed as in the reference population, they had significantly more patients in the two lowest sextiles and significantly fewer patients in the two highest sextiles. “This [result] suggests that the disability curve has indeed shifted toward the more benign end of the spectrum in the contemporary clinic population,” said Dr. Kister.
Other researchers have observed a similar phenomenon. George et al. published the results of a large, international collaboration in Neurology Genetics in 2016. After examining more than 7,000 patients, the investigators noted a similar overrepresentation of patients with milder severity scores and underrepresentation of patients with higher severity scores. These results support the hypothesis of a shift toward milder disability, said Dr. Kister.
Trend toward milder disability
The investigators next examined whether the rate of accumulation of disability among patients with MS had changed from year to year since DMTs were introduced. They conducted a univariate analysis of MSSS for 6,238 patients who were enrolled in the N.Y. State MS Consortium during 1996-2007. They found that patients who were enrolled in more recent years had significantly lower MSSS than patients who were enrolled in earlier years, regardless of disease duration. When Dr. Kister and colleagues replicated their analysis using EDSS, they found significantly lower levels of disability for patients enrolled in more recent years, except for patients with disease duration of 26-30 years. A multivariate analysis showed that the median MSSS of enrollees into the N.Y. State MS Consortium decreased from 5.04 in 1996 to 3.78 in 2006.
In a subsequent study, Dr. Kister and colleagues examined the age at which patients in the MSBase registry reached various disability milestones (e.g., EDSS of 6, which indicates the need of a cane to walk outdoors), according to their year of enrollment in the registry. They found a significant increase in age at milestone achievement with each subsequent calendar year. For example, for every consecutive year of enrollment, the age at which patients attained an EDSS of 6 increased by 0.38 years. These analyses were confirmed for the subgroups of patients diagnosed according to the Poser and McDonald criteria. The increase in age “is probably not just related to the shift in diagnostic criteria,” said Dr. Kister. When the researchers calculated the net average gains in years over the 13-year follow-up period, they found that patients who entered at the end of the enrollment period were 4.9 years older when they reached an EDSS of 6, compared with patients with an EDSS of 6 who entered at the beginning of the enrollment period.
International data show similar trends
Research conducted around the world shows similar trends, said Dr. Kister. In 2009, Veugelers et al. published the results of a study that included 1,752 patients with MS in Nova Scotia. Before the 1998 introduction of a drug insurance program that provides DMTs, the time to an EDSS of 6 was 14.4 years. After the introduction of this program, the time to EDSS of 6 was 18.6 years.
More recently, Capra et al. examined 1,324 patients with MS who attended an MS center in Brescia, Italy, during 1980-2010. They found that the age at which 50% of patients reached an EDSS of 6 was approximately 55 years in 1990. By 2010, the age at achieving this milestone had increased to approximately 63 years.
In a prospective study, Cree et al. examined the evolution of disability in 448 actively treated patients with relapsing-remitting MS and 69 patients with progressive MS. Approximately 45% of patients had no disability worsening during a 10-year follow-up period. Furthermore, a comparatively low 11% of patients had reached an EDSS of 6 at 10 years. The average disease duration of the cohort at that time was 17 years, said Dr. Kister. The results indicated that about 50% of patients would be expected to reach an EDSS of 6 after a disease duration of approximately 38 years, “which is much longer than in the natural history studies,” he added.
In 2019, Beiki et al. found that among patients with relapsing-remitting MS, the risk of reaching an EDSS of 6 decreased by 7% with each subsequent calendar year of diagnosis. The researchers did not observe a similar trend among patients with progressive MS. Their population-based, retrospective study included 7,331 patients in Sweden.
Two additional studies in Scandinavian populations add to the evidence of decreasing disability. In their examination of Swedish patients with MS who received a diagnosis of MS during 1968-2012, Burkill et al. found that the risk of death decreased over time. The hazard ratio of mortality for patients with MS, compared with a non-MS comparator group, decreased from 6.52 among those diagnosed during 1968-1980 to 2.08 for patients diagnosed during 2001-2012. The decrease in the risk of mortality was greater among patients with MS than in a matched comparator population. Similarly, in a nationwide, population-based study, Koch-Henriksen et al. found that all-cause excess mortality in Danish patients with MS decreased from 1950 through 1999.
The role of DMTs
The evidence suggests that DMTs are affecting the long-term progression of MS, said Dr. Kister. Palace et al. compared patients with MS in the UK who received treatment with interferon-beta with a modeled untreated cohort of patients in British Columbia. They found that treated patients reached an EDSS of 6 4 years later than did untreated patients.
Furthermore, an analysis by Brown et al. showed that the time to conversion to secondary progressive MS was longer among treated patients, compared with untreated patients. The risk of conversion was lower for patients treated with newer, more effective therapies (i.e., fingolimod, alemtuzumab, or natalizumab) than for those treated with glatiramer acetate or interferon beta.
Finally, Kingwell and colleagues examined the effect of treatment with interferon-beta on survival using an international cohort of approximately 6,000 patients with relapsing-remitting MS. They found that exposure to interferon-beta for more than 3 years was associated with a 32% reduction in the risk of mortality. They observed no similar risk reduction among patients exposed to interferon-beta for 6 months to 3 years.
Although these data are encouraging, other evidence indicates that the prevalence of MS in the United States has increased considerably in the past 40 years. Researchers estimate that 1 million Americans have MS, which “suggests that we are diagnosing many more mild cases,” said Dr. Kister. The burden of the disease remains high, he concluded.
Dr. Kister reported receiving consulting fees or research grants from Biogen, Roche, Genzyme and Genentech.
SOURCE: Kister I et al. ECTRIMS 2019. Abstract 281754.
STOCKHOLM – , according to an overview provided at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Data consistently indicate that the time that elapses before a patient requires a cane for ambulation has increased, and survival has likewise improved. “Some of the improvement can be attributed confidently to treatment effect,” said Ilya Kister, MD, associate professor of neurology at NYU Langone Health in New York. “We hope to see an even greater change with newer therapies.”
At the same time, neurologists appear to be diagnosing more cases of MS than they previously did, said Dr. Kister, which suggests that neurologists probably are diagnosing milder cases. The overall societal burden of MS remains high.
The relative prevalence of mild disability has increased
About 25 years have elapsed since the first disease-modifying treatment (DMT) for MS became available, and treatment has become widespread during that time. Dr. Kister and colleagues sought to determine whether the current clinical population of patients with MS, who for the most part receive DMTs, has less disability than do untreated patients or patients from natural history studies do. They identified the MS Severity Score (MSSS) as a measure with which to compare populations. The MSSS assigns a patient a ranking according to his or her level of disability, using a reference population of patients with the same disease duration for comparison. “MSSS can be conceptualized as rate of disability accumulation,” said Dr. Kister. “Lower MSSS corresponds to relatively slower disability accumulation, and higher MSSS to higher disability accumulation.”
The MSSS was developed using the Expanded Disability Status Scale (EDSS) score as a measure of disability. Because many neurologists do not routinely obtain EDSS scores for their patients, Dr. Kister and colleagues used the Patient-Determined Disease Steps (PDDS) to measure disability. As its name implies, the PDDS is a patient-reported outcome measure that mainly measures ambulation. It correlates strongly with EDSS, said Dr. Kister. He and colleagues used the PDDS to develop a reference table of MS disability, which they called the Patient-Derived MSSS.
The investigators examined a large sample of patients at NYU MS Center and Barnabas MS Center in Livingston, N.J. They grouped patients into sextiles according to their Patient-Derived MSSS. Dr. Kister and colleagues found that, rather than arriving at sextiles that contained equal numbers of patients, as would be expected if disability were distributed as in the reference population, they had significantly more patients in the two lowest sextiles and significantly fewer patients in the two highest sextiles. “This [result] suggests that the disability curve has indeed shifted toward the more benign end of the spectrum in the contemporary clinic population,” said Dr. Kister.
Other researchers have observed a similar phenomenon. George et al. published the results of a large, international collaboration in Neurology Genetics in 2016. After examining more than 7,000 patients, the investigators noted a similar overrepresentation of patients with milder severity scores and underrepresentation of patients with higher severity scores. These results support the hypothesis of a shift toward milder disability, said Dr. Kister.
Trend toward milder disability
The investigators next examined whether the rate of accumulation of disability among patients with MS had changed from year to year since DMTs were introduced. They conducted a univariate analysis of MSSS for 6,238 patients who were enrolled in the N.Y. State MS Consortium during 1996-2007. They found that patients who were enrolled in more recent years had significantly lower MSSS than patients who were enrolled in earlier years, regardless of disease duration. When Dr. Kister and colleagues replicated their analysis using EDSS, they found significantly lower levels of disability for patients enrolled in more recent years, except for patients with disease duration of 26-30 years. A multivariate analysis showed that the median MSSS of enrollees into the N.Y. State MS Consortium decreased from 5.04 in 1996 to 3.78 in 2006.
In a subsequent study, Dr. Kister and colleagues examined the age at which patients in the MSBase registry reached various disability milestones (e.g., EDSS of 6, which indicates the need of a cane to walk outdoors), according to their year of enrollment in the registry. They found a significant increase in age at milestone achievement with each subsequent calendar year. For example, for every consecutive year of enrollment, the age at which patients attained an EDSS of 6 increased by 0.38 years. These analyses were confirmed for the subgroups of patients diagnosed according to the Poser and McDonald criteria. The increase in age “is probably not just related to the shift in diagnostic criteria,” said Dr. Kister. When the researchers calculated the net average gains in years over the 13-year follow-up period, they found that patients who entered at the end of the enrollment period were 4.9 years older when they reached an EDSS of 6, compared with patients with an EDSS of 6 who entered at the beginning of the enrollment period.
International data show similar trends
Research conducted around the world shows similar trends, said Dr. Kister. In 2009, Veugelers et al. published the results of a study that included 1,752 patients with MS in Nova Scotia. Before the 1998 introduction of a drug insurance program that provides DMTs, the time to an EDSS of 6 was 14.4 years. After the introduction of this program, the time to EDSS of 6 was 18.6 years.
More recently, Capra et al. examined 1,324 patients with MS who attended an MS center in Brescia, Italy, during 1980-2010. They found that the age at which 50% of patients reached an EDSS of 6 was approximately 55 years in 1990. By 2010, the age at achieving this milestone had increased to approximately 63 years.
In a prospective study, Cree et al. examined the evolution of disability in 448 actively treated patients with relapsing-remitting MS and 69 patients with progressive MS. Approximately 45% of patients had no disability worsening during a 10-year follow-up period. Furthermore, a comparatively low 11% of patients had reached an EDSS of 6 at 10 years. The average disease duration of the cohort at that time was 17 years, said Dr. Kister. The results indicated that about 50% of patients would be expected to reach an EDSS of 6 after a disease duration of approximately 38 years, “which is much longer than in the natural history studies,” he added.
In 2019, Beiki et al. found that among patients with relapsing-remitting MS, the risk of reaching an EDSS of 6 decreased by 7% with each subsequent calendar year of diagnosis. The researchers did not observe a similar trend among patients with progressive MS. Their population-based, retrospective study included 7,331 patients in Sweden.
Two additional studies in Scandinavian populations add to the evidence of decreasing disability. In their examination of Swedish patients with MS who received a diagnosis of MS during 1968-2012, Burkill et al. found that the risk of death decreased over time. The hazard ratio of mortality for patients with MS, compared with a non-MS comparator group, decreased from 6.52 among those diagnosed during 1968-1980 to 2.08 for patients diagnosed during 2001-2012. The decrease in the risk of mortality was greater among patients with MS than in a matched comparator population. Similarly, in a nationwide, population-based study, Koch-Henriksen et al. found that all-cause excess mortality in Danish patients with MS decreased from 1950 through 1999.
The role of DMTs
The evidence suggests that DMTs are affecting the long-term progression of MS, said Dr. Kister. Palace et al. compared patients with MS in the UK who received treatment with interferon-beta with a modeled untreated cohort of patients in British Columbia. They found that treated patients reached an EDSS of 6 4 years later than did untreated patients.
Furthermore, an analysis by Brown et al. showed that the time to conversion to secondary progressive MS was longer among treated patients, compared with untreated patients. The risk of conversion was lower for patients treated with newer, more effective therapies (i.e., fingolimod, alemtuzumab, or natalizumab) than for those treated with glatiramer acetate or interferon beta.
Finally, Kingwell and colleagues examined the effect of treatment with interferon-beta on survival using an international cohort of approximately 6,000 patients with relapsing-remitting MS. They found that exposure to interferon-beta for more than 3 years was associated with a 32% reduction in the risk of mortality. They observed no similar risk reduction among patients exposed to interferon-beta for 6 months to 3 years.
Although these data are encouraging, other evidence indicates that the prevalence of MS in the United States has increased considerably in the past 40 years. Researchers estimate that 1 million Americans have MS, which “suggests that we are diagnosing many more mild cases,” said Dr. Kister. The burden of the disease remains high, he concluded.
Dr. Kister reported receiving consulting fees or research grants from Biogen, Roche, Genzyme and Genentech.
SOURCE: Kister I et al. ECTRIMS 2019. Abstract 281754.
EXPERT ANALYSIS FROM ECTRIMS 2019
Benefits of BGF triple fixed-dose therapy consistent in COPD patients without exacerbation history
NEW ORLEANS – When looking specifically at the subgroup patients with no recent exacerbations, in patients with moderate to very severe chronic obstructive pulmonary disorder (COPD), an analysis of the phase 3 KRONOS study shows.
For that subgroup of patients with no moderate to severe exacerbations in the past year, BGF MDI was associated with a nominally significant reduction in the rate of subsequent COPD exacerbations, compared with glycopyrrolate/formoterol fumarate (GFF) MDI, according to investigator Fernando J. Martinez MD, of Weill Cornell Medical College, New York.
Results for the COPD patients with no recent history of exacerbation were consistent with the overall results of KRONOS, suggesting that the benefits of BGF MDI shown in that study were not driven by patients with a recent exacerbation history, Dr. Martinez said at the annual meeting of the American College of Chest Physicians.
“It was clear that the effect of this particular triple did not really appear to be dramatically different by the prior history or not in the comparison of the triple versus dual bronchodilator,” he said in a late-breaking clinical trial session.
About three-quarters of the patients in KRONOS (1,411 out of 1,896) lacked a recent exacerbation history. These patients had a mean age of 65.5 years, 72.8% were male, and 69.9% used inhaled corticosteroids at screening, which are demographics were similar to the overall KRONOS patient population, Dr. Martinez said.
In this subset of patients with no recent exacerbation history, treatment with BGF MDI significantly reduced the rate of moderate or severe exacerbations, compared with GFF MDI, with a treatment incidence rate ratio of 0.52 (95% confidence interval, 0.37-0.72; P = .0001).
“The results were remarkably similar,” Dr. Martinez said. “I was surprised, to be honest with you, when I saw these results. I did not anticipate that would be the case.”
By contrast, BGF MDI did not significantly reduce the rate of moderate or severe exacerbations, compared with budesonide/formoterol fumarate (BFF) MDI or open-label budesonide/formoterol fumarate dihydrate dry-powder inhaler (BUD/FORM DPI) as studied in KRONOS, Dr. Martinez said.
The change from baseline in morning predose trough forced expiratory volume in 1 second (FEV1) at week 24 was significantly improved for BGF MDI, compared with BFF MDI and BUD/FORM DPI, but not compared with GFF MDI, according to the investigator. Similarly, the FEV1 area under the curve from 0 to 4 hours was significantly improved for BGF MDI, compared with BFF MDI and BUD/FORM DPI, but not compared with GFF MDI.
Dr. Martinez reported disclosures related to AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Chiesi, Sunovion, and Teva.
SOURCE: Martinez FJ et al. CHEST 2019, Abstract.
NEW ORLEANS – When looking specifically at the subgroup patients with no recent exacerbations, in patients with moderate to very severe chronic obstructive pulmonary disorder (COPD), an analysis of the phase 3 KRONOS study shows.
For that subgroup of patients with no moderate to severe exacerbations in the past year, BGF MDI was associated with a nominally significant reduction in the rate of subsequent COPD exacerbations, compared with glycopyrrolate/formoterol fumarate (GFF) MDI, according to investigator Fernando J. Martinez MD, of Weill Cornell Medical College, New York.
Results for the COPD patients with no recent history of exacerbation were consistent with the overall results of KRONOS, suggesting that the benefits of BGF MDI shown in that study were not driven by patients with a recent exacerbation history, Dr. Martinez said at the annual meeting of the American College of Chest Physicians.
“It was clear that the effect of this particular triple did not really appear to be dramatically different by the prior history or not in the comparison of the triple versus dual bronchodilator,” he said in a late-breaking clinical trial session.
About three-quarters of the patients in KRONOS (1,411 out of 1,896) lacked a recent exacerbation history. These patients had a mean age of 65.5 years, 72.8% were male, and 69.9% used inhaled corticosteroids at screening, which are demographics were similar to the overall KRONOS patient population, Dr. Martinez said.
In this subset of patients with no recent exacerbation history, treatment with BGF MDI significantly reduced the rate of moderate or severe exacerbations, compared with GFF MDI, with a treatment incidence rate ratio of 0.52 (95% confidence interval, 0.37-0.72; P = .0001).
“The results were remarkably similar,” Dr. Martinez said. “I was surprised, to be honest with you, when I saw these results. I did not anticipate that would be the case.”
By contrast, BGF MDI did not significantly reduce the rate of moderate or severe exacerbations, compared with budesonide/formoterol fumarate (BFF) MDI or open-label budesonide/formoterol fumarate dihydrate dry-powder inhaler (BUD/FORM DPI) as studied in KRONOS, Dr. Martinez said.
The change from baseline in morning predose trough forced expiratory volume in 1 second (FEV1) at week 24 was significantly improved for BGF MDI, compared with BFF MDI and BUD/FORM DPI, but not compared with GFF MDI, according to the investigator. Similarly, the FEV1 area under the curve from 0 to 4 hours was significantly improved for BGF MDI, compared with BFF MDI and BUD/FORM DPI, but not compared with GFF MDI.
Dr. Martinez reported disclosures related to AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Chiesi, Sunovion, and Teva.
SOURCE: Martinez FJ et al. CHEST 2019, Abstract.
NEW ORLEANS – When looking specifically at the subgroup patients with no recent exacerbations, in patients with moderate to very severe chronic obstructive pulmonary disorder (COPD), an analysis of the phase 3 KRONOS study shows.
For that subgroup of patients with no moderate to severe exacerbations in the past year, BGF MDI was associated with a nominally significant reduction in the rate of subsequent COPD exacerbations, compared with glycopyrrolate/formoterol fumarate (GFF) MDI, according to investigator Fernando J. Martinez MD, of Weill Cornell Medical College, New York.
Results for the COPD patients with no recent history of exacerbation were consistent with the overall results of KRONOS, suggesting that the benefits of BGF MDI shown in that study were not driven by patients with a recent exacerbation history, Dr. Martinez said at the annual meeting of the American College of Chest Physicians.
“It was clear that the effect of this particular triple did not really appear to be dramatically different by the prior history or not in the comparison of the triple versus dual bronchodilator,” he said in a late-breaking clinical trial session.
About three-quarters of the patients in KRONOS (1,411 out of 1,896) lacked a recent exacerbation history. These patients had a mean age of 65.5 years, 72.8% were male, and 69.9% used inhaled corticosteroids at screening, which are demographics were similar to the overall KRONOS patient population, Dr. Martinez said.
In this subset of patients with no recent exacerbation history, treatment with BGF MDI significantly reduced the rate of moderate or severe exacerbations, compared with GFF MDI, with a treatment incidence rate ratio of 0.52 (95% confidence interval, 0.37-0.72; P = .0001).
“The results were remarkably similar,” Dr. Martinez said. “I was surprised, to be honest with you, when I saw these results. I did not anticipate that would be the case.”
By contrast, BGF MDI did not significantly reduce the rate of moderate or severe exacerbations, compared with budesonide/formoterol fumarate (BFF) MDI or open-label budesonide/formoterol fumarate dihydrate dry-powder inhaler (BUD/FORM DPI) as studied in KRONOS, Dr. Martinez said.
The change from baseline in morning predose trough forced expiratory volume in 1 second (FEV1) at week 24 was significantly improved for BGF MDI, compared with BFF MDI and BUD/FORM DPI, but not compared with GFF MDI, according to the investigator. Similarly, the FEV1 area under the curve from 0 to 4 hours was significantly improved for BGF MDI, compared with BFF MDI and BUD/FORM DPI, but not compared with GFF MDI.
Dr. Martinez reported disclosures related to AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Chiesi, Sunovion, and Teva.
SOURCE: Martinez FJ et al. CHEST 2019, Abstract.
REPORTING FROM CHEST 2019
How to use type 2 diabetes meds to lower CV disease risk
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4
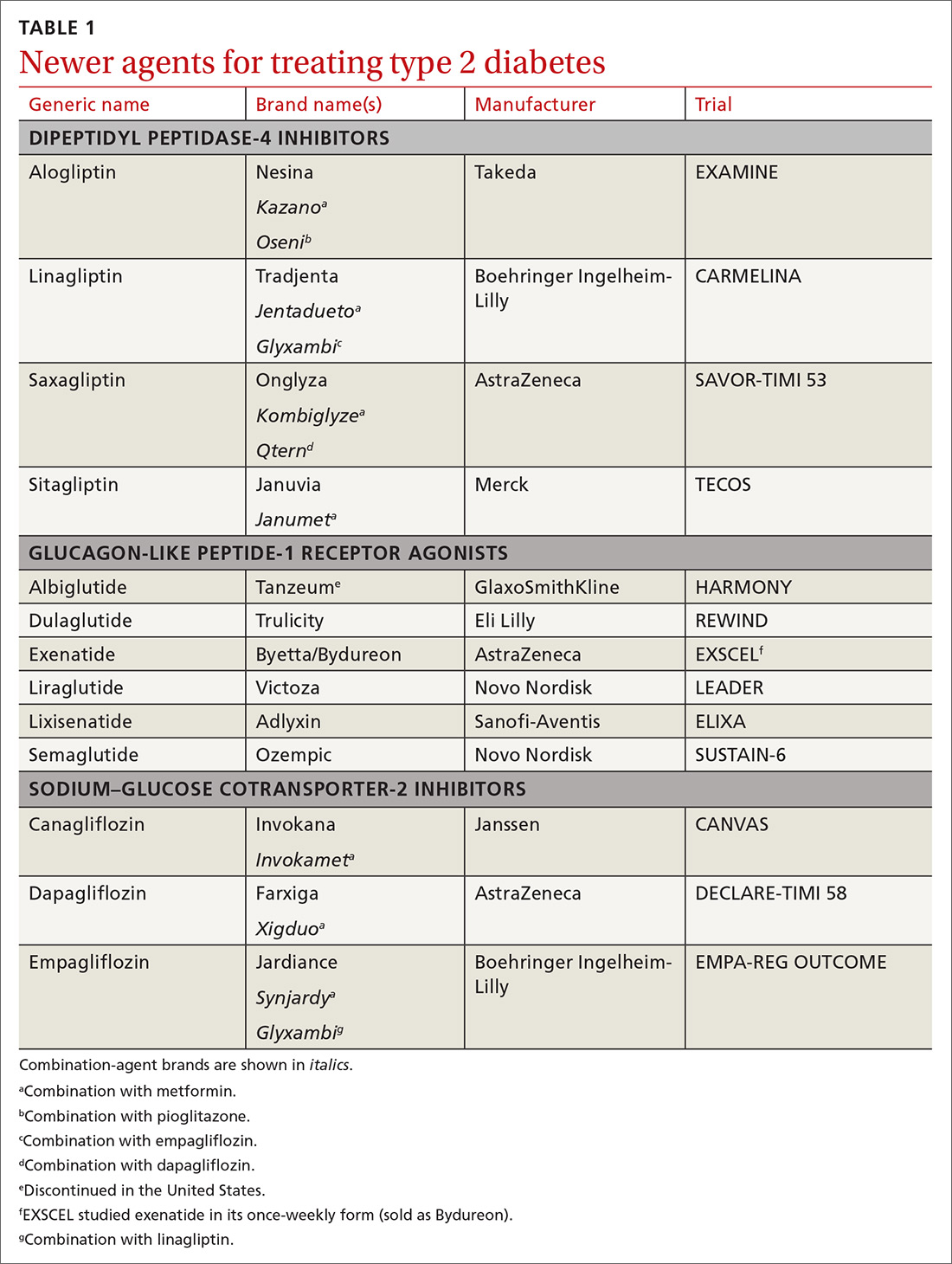
Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).
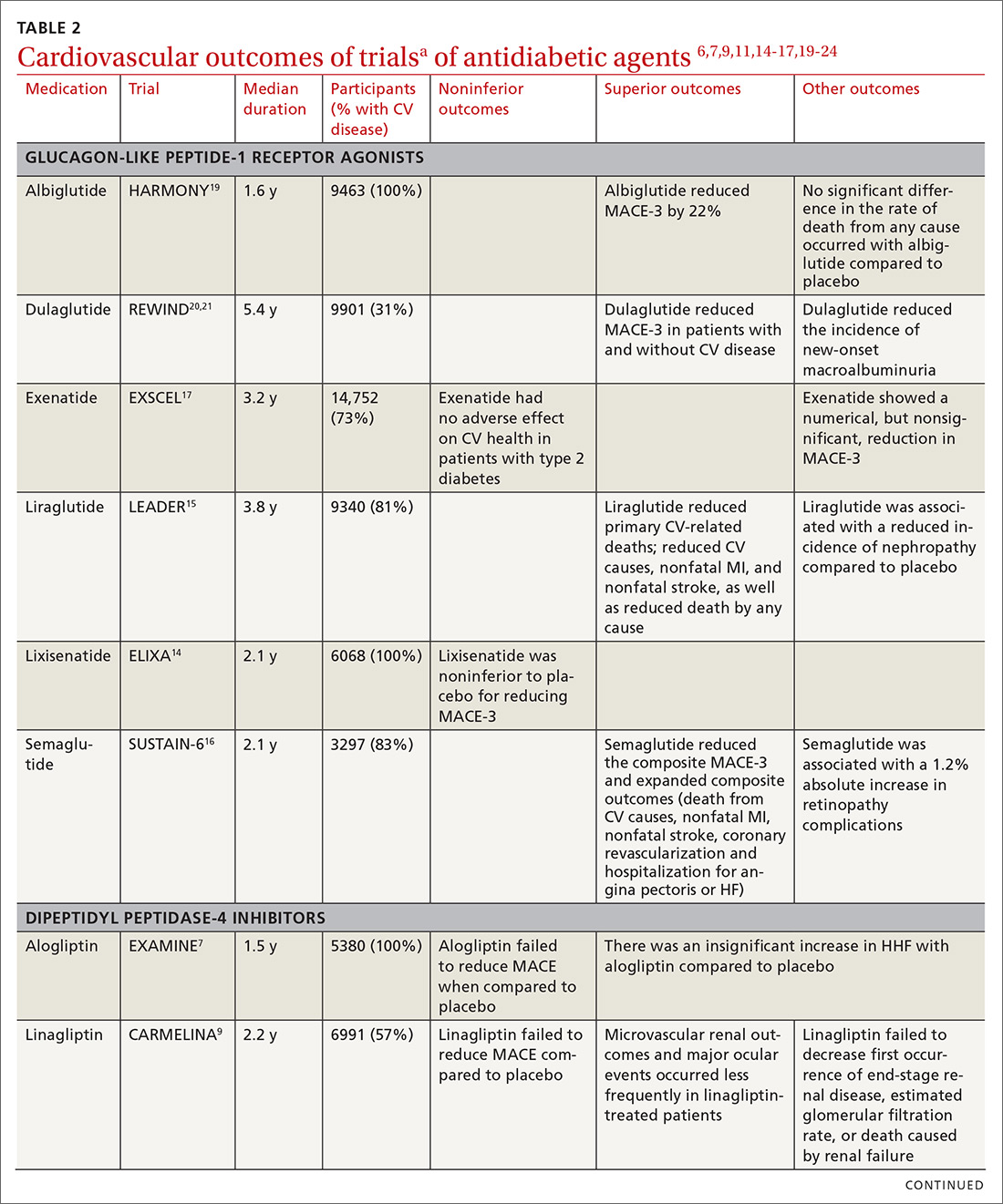
Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32
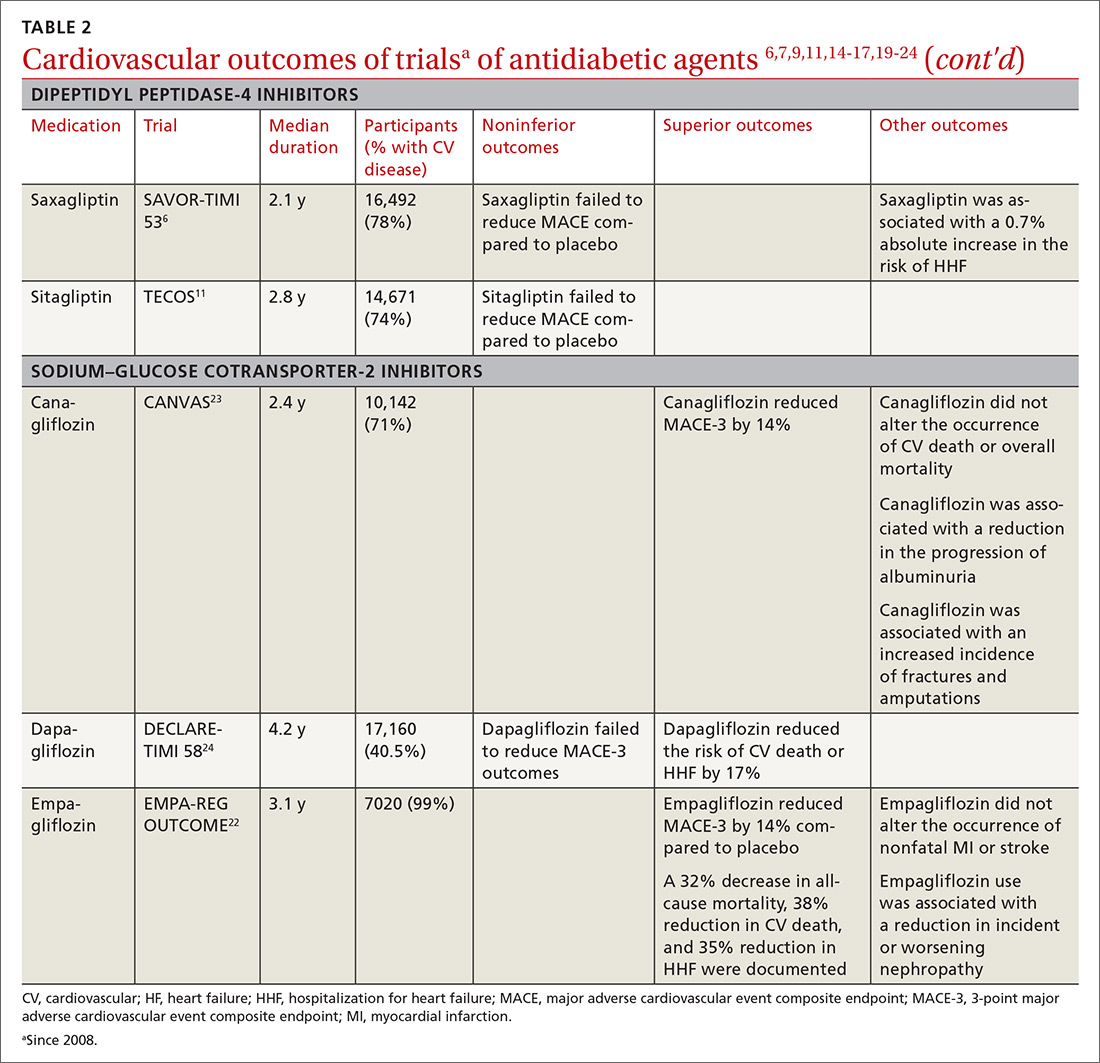
See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.
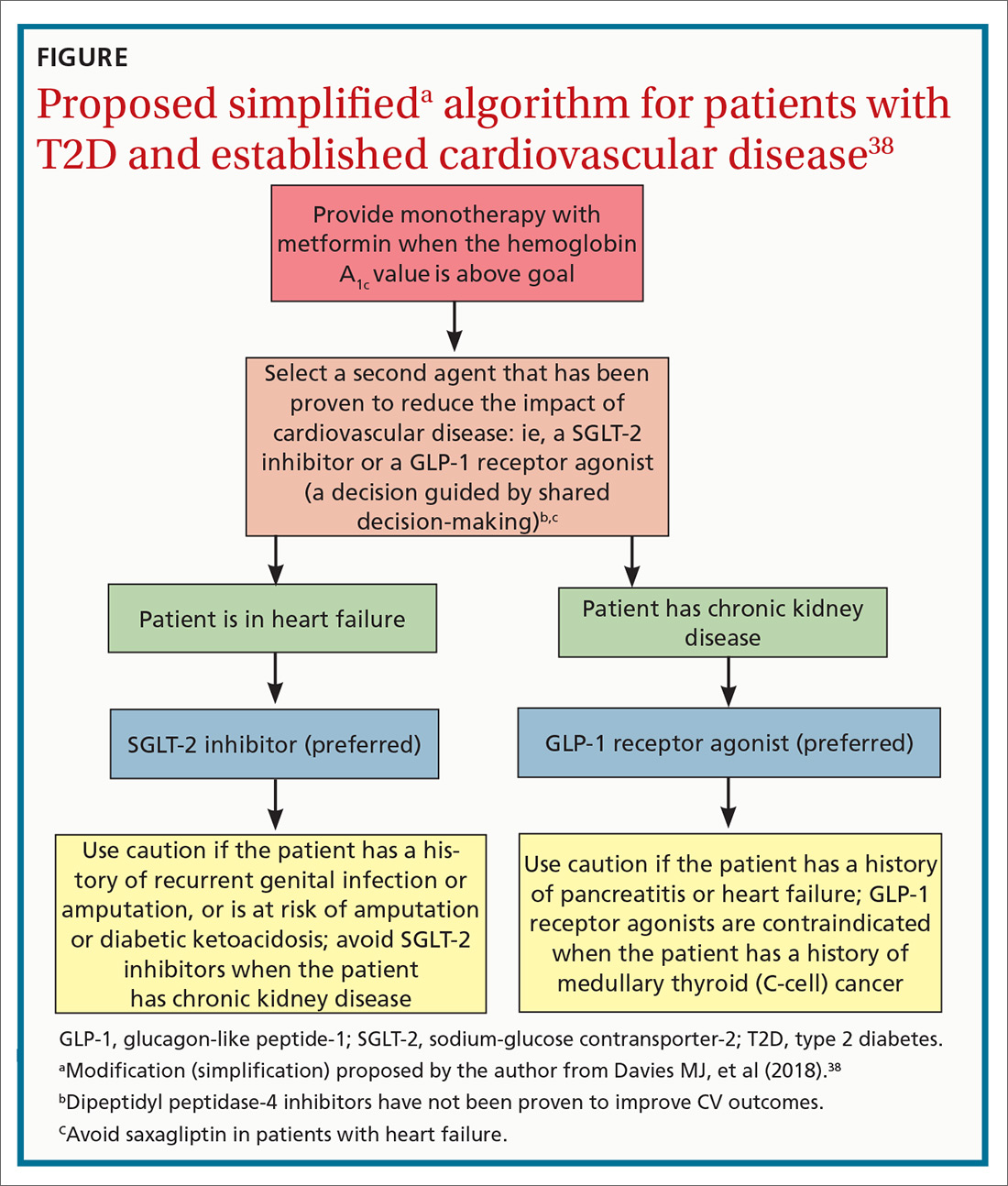
ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; [email protected].
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4

Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).

Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32

See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.

ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; [email protected].
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4

Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).

Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32

See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.

ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; [email protected].
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
PRACTICE RECOMMENDATIONS
› Consider American Diabetes Association (ADA) guidance and prescribe a sodium–glucose cotransporter-2 (SGLT-2) inhibitor or glucagon-like peptide- 1 (GLP-1) receptor agonist that has demonstrated cardiovascular (CV) disease benefit for your patients who have type 2 diabetes (T2D) and established atherosclerotic CV disease. A
› Consider ADA’s recommendation for preferred therapy and prescribe an SGLT-2 inhibitor for your patients with T2D who have atherosclerotic CV disease and are at high risk of heart failure or in whom heart failure coexists. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series