User login
Black-box warnings: How they can improve your clinical practice
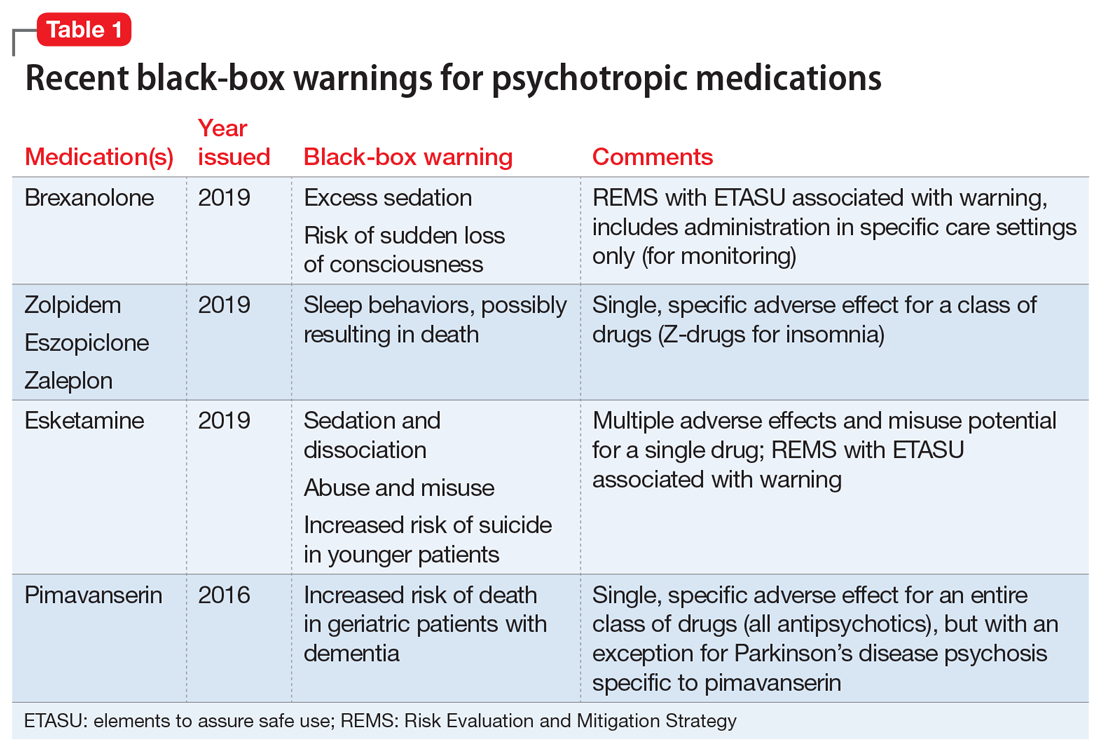
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).
In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
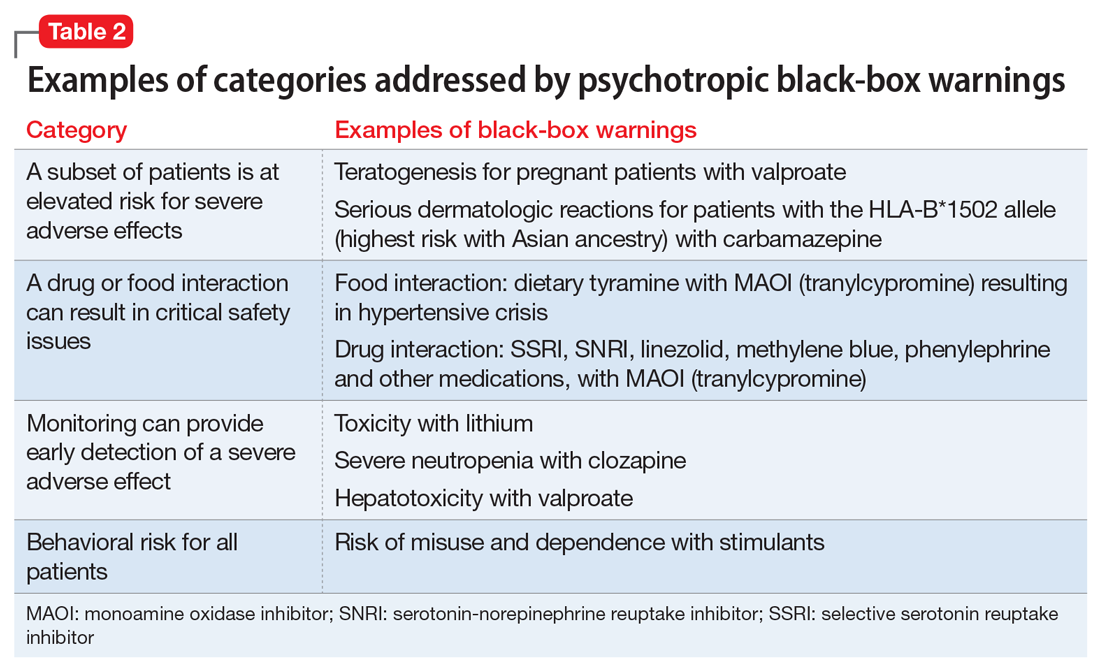
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5
Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
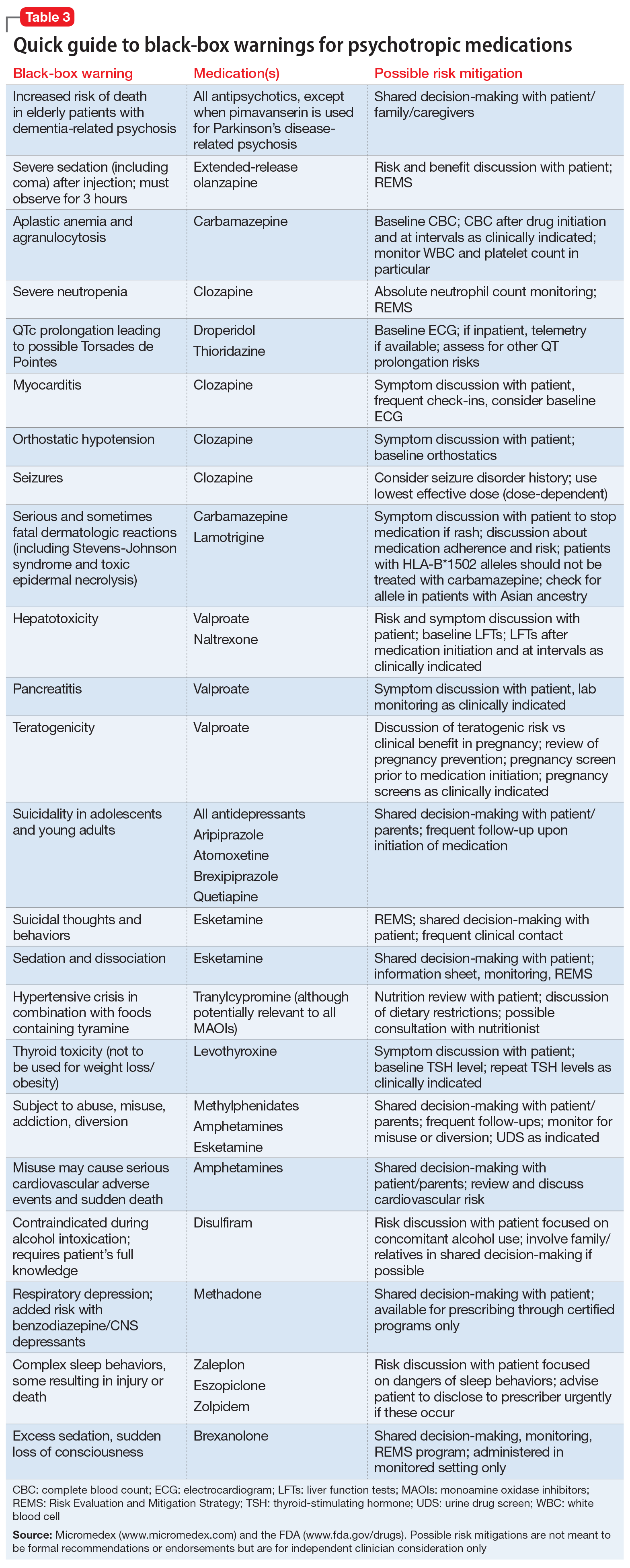
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.
The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).

In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5

Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.

The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Recently, the FDA issued “black-box” warnings, its most prominent drug safety statements, for esketamine,1 which is indicated for treatment-resistant depression, and the Z-drugs, which are indicated for insomnia2 (Table 1). A black-box warning also comes with brexanolone, which was recently approved for postpartum depression.3 While these newly issued warnings serve as a timely reminder of the importance of black-box warnings, older black-box warnings also cover large areas of psychiatric prescribing, including all medications indicated for treating psychosis or schizophrenia (increased mortality in patients with dementia), and all psychotropic medications with a depression indication (suicidality in younger people).

In this article, we help busy prescribers navigate the landscape of black-box warnings by providing a concise review of how to use them in clinical practice, and where to find information to keep up-to-date.
What are black-box warnings?
A black-box warning is a summary of the potential serious or life-threatening risks of a specific prescription medication. The black-box warning is formatted within a black border found at the top of the manufacturer’s prescribing information document (also known as the package insert or product label). Below the black-box warning, potential risks appear in descending order in sections titled “Contraindications,” “Warnings and Precautions,” and “Adverse Reactions.”4 The FDA issues black-box warnings either during drug development, to take effect upon approval of a new agent, or (more commonly) based on post-marketing safety information,5 which the FDA continuously gathers from reports by patients, clinicians, and industry.6 Federal law mandates the existence of black-box warnings, stating in part that, “special problems, particularly those that may lead to death or serious injury, may be required by the [FDA] to be placed in a prominently displayed box” (21 CFR 201.57(e)).
When is a black-box warning necessary?
The FDA issues a black-box warning based upon its judgment of the seriousness of the adverse effect. However, by definition, these risks do not inherently outweigh the benefits a medication may offer to certain patients. According to the FDA,7 black-box warnings are placed when:
- an adverse reaction so significant exists that this potential negative effect must be considered in risks and benefits when prescribing the medication
- a serious adverse reaction exists that can be prevented, or the risk reduced, by appropriate use of the medication
- the FDA has approved the medication with restrictions to ensure safe use.
Table 2 shows examples of scenarios where black-box warnings have been issued.8 Black-box warnings may be placed on an individual agent or on an entire class of medications. For example, both antipsychotics and antidepressants have class-wide warnings. Finally, black-box warnings are not static, and their content may change; in a study of black-box warnings issued from 2007 to 2015, 29% were entirely new, 32% were considered major updates to existing black-box warnings, and 40% were minor updates.5

Critiques of black-box warnings focus on the absence of published, formal criteria for instituting such warnings, the lack of a consistent approach in their content, and the infrequent inclusion of any information on the relative size of the risk.9 Suggestions for improvement include offering guidance on how to implement the black-box warnings in a patient-centered, shared decision-making model by adding evidence profiles and implementation guides.10 Less frequently considered, black-box warnings may be discontinued if new evidence demonstrates that the risk is lower than previously appreciated; however, similarly to their placement, no explicit criteria for the removal of black-box warnings have been made public.11
When a medication poses an especially high safety risk, the FDA may require the manufacturer to implement a Risk Evaluation and Mitigation Strategy (REMS) program. These programs can describe specific steps to improve medication safety, known as elements to assure safe use (ETASU).4 A familiar example is the clozapine REMS. In order to reduce the risk of severe neutropenia, the clozapine REMS requires prescribers (and pharmacists) to complete specialized training (making up the ETASU). Surprisingly, not every medication with a REMS has a corresponding black-box warning12; more understandably, many medications with black-box warnings do not have an associated REMS, because their risks are evaluated to be manageable by an individual prescriber’s clinical judgment. Most recently, esketamine carries both a black-box warning and a REMS. The black-box warning focuses on adverse effects (Table 1), while the REMS focuses on specific steps used to lessen these risks, including requiring use of a patient enrollment and monitoring form, a fact sheet for patients, and health care setting and pharmacy enrollment forms.13
Continue to: Psychotropic medications and black-box warnings
Psychotropic medications and black-box warnings
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.

The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...
This class warning has had widespread effects. For example, it has prompted less use of antipsychotics in nursing home facilities, as a result of stricter Centers for Medicare and Medicaid Services regulations20; overall, there is some evidence that there has been reduced prescribing of antipsychotics in general.21 Additionally, this black-box warning is unusual in that it warns about a specific off-label indication, which is itself poorly supported by evidence.21 Concomitantly, few other treatment options are available for this clinical situation. These medications are often seen as the only option for patients with dementia complicated by severe behavioral disturbance, and thus this black-box warning reflects real-world practices.14
Varenicline and neuropsychiatric complications. The withdrawal of the black-box warning on potential neuropsychiatric complications of using varenicline for smoking cessation shows that black-box warnings are not static and can, though infrequently, be removed as more safety data accumulates.11 As additional post-marketing information emerged on this risk, this black-box warning was reconsidered and withdrawn in 2016.22 Its withdrawal could potentially make clinicians more comfortable prescribing varenicline and in turn, help to reduce smoking rates.
How to use black-box warnings
To enhance their clinical practice, prescribers can use black-box warnings to inform safe prescribing practices, to guide shared decision-making, and to improve documentation of their treatment decisions.
Informing safe prescribing practices. A prescriber should be aware of the main safety concerns contained in a medication’s black-box warning; at the same time, these warnings are not meant to unduly limit use when crucial treatment is needed.14 In issuing a black-box warning, the FDA has clearly stated the priority and seriousness of its concern. These safety issues must be balanced against the medication’s utility for a given patient, at the prescriber’s clinical judgment.
Guiding shared decision-making. Clinicians are not required to disclose black-box warnings to patients, and there are no criteria that clearly define the role of these warnings in patient care. As is often noted, the FDA does not regulate the practice of medicine.6 However, given the seriousness of the potential adverse effects delineated by black-box warnings, it is reasonable for clinicians to have a solid grasp of black-box warnings for all medications they prescribe, and to be able to relate these warnings to patients, in appropriate language. This patient-centered discussion should include weighing the risks and benefits with the patient and educating the patient about the risks and strategies to mitigate those risks. This discussion can be augmented by patient handouts, which are often offered by pharmaceutical manufacturers, and by shared decision-making tools. A proactive discussion with patients and families about black-box warnings and other risks discussed in product labels can help reduce fears associated with taking medications and may improve adherence.
Continue to: Improving documentation of treatment decisions
Improving documentation of treatment decisions. Fluent knowledge of black-box warnings may help clinicians improve documentation of their treatment decisions, particularly the risks and benefits of their medication choices. Fluency with black-box warnings will help clinicians accurately document both their awareness of these risks, and how these risks informed their risk-benefit analysis in specific clinical situations.
Despite the clear importance the FDA places on black-box warnings, they are not often a topic of study in training or in postgraduate continuing education, and as a result, not all clinicians may be equally conversant with black-box warnings. While black-box warnings do change over time, many psychotropic medication black-box warnings are long-standing and well-established, and they evolve slowly enough to make mastering these warnings worthwhile in order to make the most informed clinical decisions for patient care.
Keeping up-to-date
There are practical and useful ways for busy clinicians to stay up-to-date with black-box warnings. Although these resources exist in multiple locations, together they provide convenient ways to keep current.
The FDA provides access to black-box warnings via its comprehensive database, DRUGS@FDA (https://www.accessdata.fda.gov/scripts/cder/daf/). Detailed information about REMS (and corresponding ETASU and other information related to REMS programs) is available at REMS@FDA (https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm). Clinicians can make safety reports that may contribute to FDA decision-making on black-box warnings by contacting MedWatch (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program), the FDA’s adverse events reporting system. MedWatch releases safety information reports, which can be followed on Twitter @FDAMedWatch. Note that FDA information generally is organized by specific drug, and not into categories, such as psychotropic medications.
BlackBoxRx (www.blackboxrx.com) is a subscription-based web service that some clinicians may have access to via facility or academic resources as part of a larger FormWeb software package. Individuals also can subscribe (currently, $89/year).
Continue to: Micromedex
Micromedex (www.micromedex.com), which is widely available through medical libraries, is a subscription-based web service that provides black-box warning information from a separate tab that is easily accessed in each drug’s information front page. There is also an alphabetical list of black-box warnings under a separate tab on the Micromedex landing page.
ePocrates (www.epocrates.com) is a subscription-based service that provides extensive drug information, including black-box warnings, in a convenient mobile app.
Bottom Line
Black-box warnings are the most prominent drug safety warnings issued by the FDA. Many psychotropic medications carry black-box warnings that are crucial to everyday psychiatric prescribing. A better understanding of blackbox warnings can enhance your clinical practice by informing safe prescribing practices, guiding shared decision-making, and improving documentation of your treatment decisions.
Related Resources
- US Food and Drug Administration. DRUGS@FDA: FDAapproved drug products. www.accessdata.fda.gov/scripts/cder/daf/.
- US Food and Drug Administration. Drug safety and availability. www.fda.gov/drugs/drug-safety-and-availability. Updated October 10, 2019.
- BlackBoxRx. www.blackboxrx.com. (Subscription required.)
- Mircromedex. www.micromedex.com. (Subscription required.)
- ePocrates. www.epocrates.com. (Subscription required.)
Drug Brand Names
Amitriptyline • Elavil, Vanatrip
Amoxatine • Strattera
Amoxapine • Asendin
Aripiprazole • Abilify
Asenapine • Saphris
Brexanolone • Zulresso
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dexmethylphenidate • Focalin
Dextroamphetamine/amphetamine • Adderall
Disulfiram • Antabuse
Doxepin • Prudoxin, Silenor
Droperidol • Inapsine
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imipramine • Tofranil
Isocarboxazid • Marplan
Lamotrigine • Lamictal
Levomilnacipran • Fetzima
Levothyroxine • Synthroid
Linezolid • Zyvox
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Lurasidone • Latuda
Maprotiline • Ludiomil
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin, Concerta
Midazolam • Versed
Milnacipran • Savella
Mirtazapine • Remeron
Naltrexone • Revia, Vivitrol
Nefazodone • Serzone
Nortriptyline • Aventyl, Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenelzine • Nardil
Pimavanserin • Nuplazid
Prochlorperazine • Compro
Protriptyline • Vivactil
Quetiapine • Seroquel
Risperidone • Risperdal
Selegiline • Emsam
Sertraline • Zoloft
Thioridazine • Mellaril
Thiothixene • Navane
Tranylcypromine • Parnate
Trazodone • Desyrel, Oleptro
Trifluoperazine • Stelazine
Trimipramine • Surmontil
Valproate • Depakote
Varenicline • Chantix, Wellbutrin
Vilazodone • Viibryd
Venlafaxine • Effexor
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
1. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
2. U.S. Food and Drug Administration. FDA drug safety announcement: FDA adds boxed warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Published April 30, 2019. Accessed October 28, 2019.
3. Zulresso [package insert]. Cambridge, Mass.: Sage Therapeutics Inc.; 2019.
4. Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682.
5. Solotke MT, Dhruva SS, Downing NS, et al. New and incremental FDA black box warnings from 2008 to 2015. Expert Opin Drug Saf. 2018;17(2):117-123.
6. Murphy S, Roberts R. “Black box” 101: how the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006;117(1):34-39.
7. U.S. Food and Drug Administration. Guidance document: Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products – content and format. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/warnings-and-precautions-contraindications-and-boxed-warning-sections-labeling-human-prescription. Published October 2011. Accessed October 28, 2019.
8. Beach JE, Faich GA, Bormel FG, et al. Black box warnings in prescription drug labeling: results of a survey of 206 drugs. Food Drug Law J. 1998;53(3):403-411.
9. Matlock A, Allan N, Wills B, et al. A continuing black hole? The FDA boxed warning: an appeal to improve its clinical utility. Clinical Toxicol (Phila). 2011;49(6):443-447.
10. Elraiyah T, Gionfriddo MR, Montori VM, et al. Content, consistency, and quality of black box warnings: time for a change. Ann Intern Med. 2015;163(11):875-876.
11. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf. 2016;39(8):709-714.
12. Boudes PF. Risk Evaluation and Mitigation Strategies (REMSs): are they improving drug safety? A critical review of REMSs requiring Elements to Assure Safe Use (ETASU). Drugs R D. 2017;17(2):245-254.
13. U.S. Food and Drug Administration. Approved risk evaluation mitigation strategies (REMS): Spravato (esketamine) REMS program. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386. Updated June 25, 2019. Accessed October 28, 2018.
14. Stevens JR, Jarrahzadeh T, Brendel RW, et al. Strategies for the prescription of psychotropic drugs with black box warnings. Psychosomatics. 2014;55(2):123-133.
15. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668.
16. Stone MB. The FDA warning on antidepressants and suicidality--why the controversy? N Engl J Med. 2014;371(18):1668-1671.
17. Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668-e673. doi: 10.4088/JCP.16r11119.
18. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals Inc.; May 2019.
19. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17(3):213-222.
20. Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640-647.
21. Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170(1):96-103.
22. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. Published December 16, 2016. Accessed October 28, 2019.
Trial finds three drugs equally effective for established status epilepticus
according to a study published Nov. 27 in the New England Journal of Medicine. The effectiveness and safety of the intravenous medications do not differ significantly, the researchers wrote.
“Having three equally effective second-line intravenous medications means that the clinician may choose a drug that takes into account individual situations,” wrote Phil E.M. Smith, MD, in an accompanying editorial (doi: 10.1056/NEJMe1913775). Clinicians may consider “factors such as the presumed underlying cause of status epilepticus; coexisting conditions, including allergy, liver and renal disease, hypotension, propensity to cardiac arrhythmia, and alcohol and drug dependence; the currently prescribed antiepileptic treatment; the cost of the medication; and governmental agency drug approval,” said Dr. Smith, who is affiliated with University Hospital of Wales in Cardiff.
A gap in guidance
Evidence supports benzodiazepines as the initial treatment for status epilepticus, but these drugs do not work in up to a third of patients, said first study author Jaideep Kapur, MBBS, PhD, and colleagues. “Clinical guidelines emphasize the need for rapid control of benzodiazepine-refractory status epilepticus but do not provide guidance regarding the choice of medication on the basis of either efficacy or safety,” they wrote. Dr. Kapur is a professor of neurology and the director of UVA Brain Institute at University of Virginia in Charlottesville.
Levetiracetam, fosphenytoin, and valproate are the three most commonly used medications for benzodiazepine-refractory status epilepticus. The Food and Drug Administration has labeled fosphenytoin for this indication in adults, and none of the drugs is approved for children. To determine the superiority or inferiority of these medications, the researchers conducted the Established Status Epilepticus Treatment Trial (ESETT). The blinded, comparative-effectiveness trial enrolled 384 patients at 57 hospital EDs in the United States. Patients were aged 2 years or older, had received a generally accepted cumulative dose of benzodiazepines for generalized convulsive seizures lasting more than 5 minutes and continued to have persistent or recurrent convulsions between 5-30 minutes after the last dose of benzodiazepine.
Patients randomly received one of the three trial drugs, which “were identical in appearance, formulation, packaging, and administration,” the authors said. The primary outcome was absence of clinically apparent seizures and improving responsiveness at 60 minutes after the start of the infusion without administration of additional anticonvulsant medication. ED physicians determined the presence of seizure and improvement in responsiveness.
Trial was stopped for futility
The trial included 400 enrollments of 384 unique patients during 2015-2017. Sixteen patients were enrolled twice, and their second enrollments were not included in the intention-to-treat analysis. A planned interim analysis after 400 enrollments to assess the likelihood of success or futility found that the trial had met the futility criterion. “There was a 1% chance of showing a most effective or least effective treatment if the trial were to continue to the maximum sample size” of 795 patients, Dr. Kapur and coauthors wrote. The researchers continued enrollment in a pediatric subcohort for a planned subgroup analysis by age.
In all, 55% of the patients were male, 43% were black, and 16% were Hispanic. The population was 39% children and adolescents, 48% adults aged 18-65 years, and 13% older than 65 years. Most patients had a final diagnosis of status epilepticus (87%). Other final diagnoses included psychogenic nonepileptic seizures (10%).
At 60 minutes after treatment administration, absence of seizures and improved responsiveness occurred in 47% of patients who received levetiracetam, 45% who received fosphenytoin, and 46% who received valproate.
In 39 patients for whom the researchers had reliable information about time to seizure cessation, median time to seizure cessation numerically favored valproate (7 minutes for valproate vs. 10.5 minutes for levetiracetam vs. 11.7 minutes for fosphenytoin), but the number of patients was limited, the authors noted.
“Hypotension and endotracheal intubation were more frequent with fosphenytoin than with the other two drugs, and deaths were more frequent with levetiracetam, but these differences were not significant,” wrote Dr. Kapur and colleagues. Seven patients who received levetiracetam died, compared with three who received fosphenytoin and two who received valproate. Life-threatening hypotension occurred in 3.2% of patients who received fosphenytoin, compared with 1.6% who received valproate and 0.7% who received levetiracetam. Endotracheal intubation occurred in 26.4% or patients who received fosphenytoin, compared with 20% of patients in the levetiracetam group and 16.8% in the valproate group.
The trial’s limitations include the enrollment of patients with psychogenic nonepileptic seizures and the use of clinical instead of electroencephalographic criteria for the primary outcome measure, the investigators wrote.
Dr. Smith noted that third- and fourth-line management of status epilepticus is not supported by high-quality evidence, and further studies are needed. Given the evidence from ESETT, “the practical challenge for the management of status epilepticus remains the same as in the past: ensuring that clinicians are familiar with, and follow, a treatment protocol,” he said.
The trial was funded by the National Institute of Neurological Disorders and Stroke. Dr. Kapur had no financial disclosures. A coauthor holds a patent on intravenous carbamazepine and intellectual property on intravenous topiramate. Other coauthors have ties to pharmaceutical and medical device companies.
Dr. Smith is coeditor of Practical Neurology and a member of the U.K. National Institute for Health and Clinical Excellence (NICE) guidelines committee for epilepsy.
SOURCE: Kapur J et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1905795.
according to a study published Nov. 27 in the New England Journal of Medicine. The effectiveness and safety of the intravenous medications do not differ significantly, the researchers wrote.
“Having three equally effective second-line intravenous medications means that the clinician may choose a drug that takes into account individual situations,” wrote Phil E.M. Smith, MD, in an accompanying editorial (doi: 10.1056/NEJMe1913775). Clinicians may consider “factors such as the presumed underlying cause of status epilepticus; coexisting conditions, including allergy, liver and renal disease, hypotension, propensity to cardiac arrhythmia, and alcohol and drug dependence; the currently prescribed antiepileptic treatment; the cost of the medication; and governmental agency drug approval,” said Dr. Smith, who is affiliated with University Hospital of Wales in Cardiff.
A gap in guidance
Evidence supports benzodiazepines as the initial treatment for status epilepticus, but these drugs do not work in up to a third of patients, said first study author Jaideep Kapur, MBBS, PhD, and colleagues. “Clinical guidelines emphasize the need for rapid control of benzodiazepine-refractory status epilepticus but do not provide guidance regarding the choice of medication on the basis of either efficacy or safety,” they wrote. Dr. Kapur is a professor of neurology and the director of UVA Brain Institute at University of Virginia in Charlottesville.
Levetiracetam, fosphenytoin, and valproate are the three most commonly used medications for benzodiazepine-refractory status epilepticus. The Food and Drug Administration has labeled fosphenytoin for this indication in adults, and none of the drugs is approved for children. To determine the superiority or inferiority of these medications, the researchers conducted the Established Status Epilepticus Treatment Trial (ESETT). The blinded, comparative-effectiveness trial enrolled 384 patients at 57 hospital EDs in the United States. Patients were aged 2 years or older, had received a generally accepted cumulative dose of benzodiazepines for generalized convulsive seizures lasting more than 5 minutes and continued to have persistent or recurrent convulsions between 5-30 minutes after the last dose of benzodiazepine.
Patients randomly received one of the three trial drugs, which “were identical in appearance, formulation, packaging, and administration,” the authors said. The primary outcome was absence of clinically apparent seizures and improving responsiveness at 60 minutes after the start of the infusion without administration of additional anticonvulsant medication. ED physicians determined the presence of seizure and improvement in responsiveness.
Trial was stopped for futility
The trial included 400 enrollments of 384 unique patients during 2015-2017. Sixteen patients were enrolled twice, and their second enrollments were not included in the intention-to-treat analysis. A planned interim analysis after 400 enrollments to assess the likelihood of success or futility found that the trial had met the futility criterion. “There was a 1% chance of showing a most effective or least effective treatment if the trial were to continue to the maximum sample size” of 795 patients, Dr. Kapur and coauthors wrote. The researchers continued enrollment in a pediatric subcohort for a planned subgroup analysis by age.
In all, 55% of the patients were male, 43% were black, and 16% were Hispanic. The population was 39% children and adolescents, 48% adults aged 18-65 years, and 13% older than 65 years. Most patients had a final diagnosis of status epilepticus (87%). Other final diagnoses included psychogenic nonepileptic seizures (10%).
At 60 minutes after treatment administration, absence of seizures and improved responsiveness occurred in 47% of patients who received levetiracetam, 45% who received fosphenytoin, and 46% who received valproate.
In 39 patients for whom the researchers had reliable information about time to seizure cessation, median time to seizure cessation numerically favored valproate (7 minutes for valproate vs. 10.5 minutes for levetiracetam vs. 11.7 minutes for fosphenytoin), but the number of patients was limited, the authors noted.
“Hypotension and endotracheal intubation were more frequent with fosphenytoin than with the other two drugs, and deaths were more frequent with levetiracetam, but these differences were not significant,” wrote Dr. Kapur and colleagues. Seven patients who received levetiracetam died, compared with three who received fosphenytoin and two who received valproate. Life-threatening hypotension occurred in 3.2% of patients who received fosphenytoin, compared with 1.6% who received valproate and 0.7% who received levetiracetam. Endotracheal intubation occurred in 26.4% or patients who received fosphenytoin, compared with 20% of patients in the levetiracetam group and 16.8% in the valproate group.
The trial’s limitations include the enrollment of patients with psychogenic nonepileptic seizures and the use of clinical instead of electroencephalographic criteria for the primary outcome measure, the investigators wrote.
Dr. Smith noted that third- and fourth-line management of status epilepticus is not supported by high-quality evidence, and further studies are needed. Given the evidence from ESETT, “the practical challenge for the management of status epilepticus remains the same as in the past: ensuring that clinicians are familiar with, and follow, a treatment protocol,” he said.
The trial was funded by the National Institute of Neurological Disorders and Stroke. Dr. Kapur had no financial disclosures. A coauthor holds a patent on intravenous carbamazepine and intellectual property on intravenous topiramate. Other coauthors have ties to pharmaceutical and medical device companies.
Dr. Smith is coeditor of Practical Neurology and a member of the U.K. National Institute for Health and Clinical Excellence (NICE) guidelines committee for epilepsy.
SOURCE: Kapur J et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1905795.
according to a study published Nov. 27 in the New England Journal of Medicine. The effectiveness and safety of the intravenous medications do not differ significantly, the researchers wrote.
“Having three equally effective second-line intravenous medications means that the clinician may choose a drug that takes into account individual situations,” wrote Phil E.M. Smith, MD, in an accompanying editorial (doi: 10.1056/NEJMe1913775). Clinicians may consider “factors such as the presumed underlying cause of status epilepticus; coexisting conditions, including allergy, liver and renal disease, hypotension, propensity to cardiac arrhythmia, and alcohol and drug dependence; the currently prescribed antiepileptic treatment; the cost of the medication; and governmental agency drug approval,” said Dr. Smith, who is affiliated with University Hospital of Wales in Cardiff.
A gap in guidance
Evidence supports benzodiazepines as the initial treatment for status epilepticus, but these drugs do not work in up to a third of patients, said first study author Jaideep Kapur, MBBS, PhD, and colleagues. “Clinical guidelines emphasize the need for rapid control of benzodiazepine-refractory status epilepticus but do not provide guidance regarding the choice of medication on the basis of either efficacy or safety,” they wrote. Dr. Kapur is a professor of neurology and the director of UVA Brain Institute at University of Virginia in Charlottesville.
Levetiracetam, fosphenytoin, and valproate are the three most commonly used medications for benzodiazepine-refractory status epilepticus. The Food and Drug Administration has labeled fosphenytoin for this indication in adults, and none of the drugs is approved for children. To determine the superiority or inferiority of these medications, the researchers conducted the Established Status Epilepticus Treatment Trial (ESETT). The blinded, comparative-effectiveness trial enrolled 384 patients at 57 hospital EDs in the United States. Patients were aged 2 years or older, had received a generally accepted cumulative dose of benzodiazepines for generalized convulsive seizures lasting more than 5 minutes and continued to have persistent or recurrent convulsions between 5-30 minutes after the last dose of benzodiazepine.
Patients randomly received one of the three trial drugs, which “were identical in appearance, formulation, packaging, and administration,” the authors said. The primary outcome was absence of clinically apparent seizures and improving responsiveness at 60 minutes after the start of the infusion without administration of additional anticonvulsant medication. ED physicians determined the presence of seizure and improvement in responsiveness.
Trial was stopped for futility
The trial included 400 enrollments of 384 unique patients during 2015-2017. Sixteen patients were enrolled twice, and their second enrollments were not included in the intention-to-treat analysis. A planned interim analysis after 400 enrollments to assess the likelihood of success or futility found that the trial had met the futility criterion. “There was a 1% chance of showing a most effective or least effective treatment if the trial were to continue to the maximum sample size” of 795 patients, Dr. Kapur and coauthors wrote. The researchers continued enrollment in a pediatric subcohort for a planned subgroup analysis by age.
In all, 55% of the patients were male, 43% were black, and 16% were Hispanic. The population was 39% children and adolescents, 48% adults aged 18-65 years, and 13% older than 65 years. Most patients had a final diagnosis of status epilepticus (87%). Other final diagnoses included psychogenic nonepileptic seizures (10%).
At 60 minutes after treatment administration, absence of seizures and improved responsiveness occurred in 47% of patients who received levetiracetam, 45% who received fosphenytoin, and 46% who received valproate.
In 39 patients for whom the researchers had reliable information about time to seizure cessation, median time to seizure cessation numerically favored valproate (7 minutes for valproate vs. 10.5 minutes for levetiracetam vs. 11.7 minutes for fosphenytoin), but the number of patients was limited, the authors noted.
“Hypotension and endotracheal intubation were more frequent with fosphenytoin than with the other two drugs, and deaths were more frequent with levetiracetam, but these differences were not significant,” wrote Dr. Kapur and colleagues. Seven patients who received levetiracetam died, compared with three who received fosphenytoin and two who received valproate. Life-threatening hypotension occurred in 3.2% of patients who received fosphenytoin, compared with 1.6% who received valproate and 0.7% who received levetiracetam. Endotracheal intubation occurred in 26.4% or patients who received fosphenytoin, compared with 20% of patients in the levetiracetam group and 16.8% in the valproate group.
The trial’s limitations include the enrollment of patients with psychogenic nonepileptic seizures and the use of clinical instead of electroencephalographic criteria for the primary outcome measure, the investigators wrote.
Dr. Smith noted that third- and fourth-line management of status epilepticus is not supported by high-quality evidence, and further studies are needed. Given the evidence from ESETT, “the practical challenge for the management of status epilepticus remains the same as in the past: ensuring that clinicians are familiar with, and follow, a treatment protocol,” he said.
The trial was funded by the National Institute of Neurological Disorders and Stroke. Dr. Kapur had no financial disclosures. A coauthor holds a patent on intravenous carbamazepine and intellectual property on intravenous topiramate. Other coauthors have ties to pharmaceutical and medical device companies.
Dr. Smith is coeditor of Practical Neurology and a member of the U.K. National Institute for Health and Clinical Excellence (NICE) guidelines committee for epilepsy.
SOURCE: Kapur J et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1905795.
FROM NEJM
Key clinical point: Among children and adults with benzodiazepine-refractory status epilepticus, fosphenytoin, valproate, and levetiracetam each stop seizures by 60 minutes in approximately half of patients.
Major finding: Absence of seizures and improved responsiveness occurred in 47% of patients who received levetiracetam, 45% who received fosphenytoin, and 46% who received valproate.
Study details: The Established Status Epilepticus Treatment Trial (ESETT) was a blinded, comparative-effectiveness trial that enrolled 384 patients at 57 hospital EDs in the United States.
Disclosures: The trial was funded by the National Institute of Neurological Disorders and Stroke. Dr. Kapur had no financial disclosures. A coauthor holds a patent on intravenous carbamazepine and intellectual property on intravenous topiramate. Other coauthors have ties to pharmaceutical and medical device companies.
Source: Kapur J et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1905795.
ASH preview: Key themes include tackling CAR T obstacles, sickle cell advances, VTE
Chimeric antigen receptor (CAR) T-cell therapies have garnered a great deal of attention given their “incredible efficacy” in treating B-cell malignancies, and new findings are taking aim at the drawbacks of therapy, such as the time, expense, and toxicity involved, according to Robert A. Brodsky, MD.
One example, from a study slated for presentation during a plenary session at the upcoming annual meeting of the American Society of Hematology involves the investigational T-cell bispecific antibody mosunetuzumab, which targets both CD20 on the surface of malignant B cells, and CD3 on cytotoxic T cells, engaging the T cells and directing their cytotoxicity against B cells.
In a study (Abstract 6) of 218 non-Hodgkin lymphoma patients, including 23 who had already received CAR T-cell therapy and had relapsed or were refractory to the treatment, 64% responded, 42% had a complete response, and the median duration of response is now out to 9 months, Dr. Brodsky, ASH secretary and director of the division of hematology at Johns Hopkins University, Baltimore, said during a premeeting press conference.
“It’s basically an antibody using the patient’s own T cell to do what a CAR-T cell would do – [a] very exciting study and large study,” he said. “It is an off-the-shelf product, it completely gets around the problem of the time to generate the CAR T-cell product, and because it’s going to be much simpler and faster to produce, it’s likely going to be much cheaper than CAR T cells.”
The preliminary results also suggest it is less toxic than CAR T-cell therapy, he added.
Two other CAR T-cell therapy–related studies highlighted during the press conference address its use for multiple myeloma. One, the phase 1b/2 CARTITUDE study (Abstract 577) uses CAR T cells against the B-cell maturation antigen (BCMA) in the relapsed/refractory setting.
Of 25 patients treated with chemotherapy followed by CAR T-cell infusion and followed for a median of 3 months, 91% responded, two achieved a complete remission, and “many other responses were very deep responses,” Dr. Brodsky said, noting that the second featured multiple myeloma trial (Abstract 930) looked at bispecific CAR T-cell therapy targeting BCMA and CD38 in an effort to reduce resistance to the therapy.
“Again, very interesting preliminary results,” he said, noting that of 16 patients followed for a median of 36 weeks, 87.5% responded, the treatment was well tolerated, and progression-free survival at 9 months was 75%.
In addition to the “key theme” of overcoming CAR T-cell therapy obstacles, three other themes have emerged from among the thousands of abstracts submitted for presentation at ASH. These, as presented during the press conference, include new venous thromboembolism (VTE) therapies and approaches to research; inclusive medicine, with abstracts focused on age- and race-related issues in clinical trials; and new advances in the treatment of sickle cell disease. All of these have potentially practice-changing implications, as do the six late-breaking abstracts selected from 93 abstracts submitted for consideration for oral presentation at ASH, Dr. Brodsky said.
One of the “truly practice-changing” late-breakers is a randomized phase 3 trial (Abstract LBA-1) comparing the bispecific antibody blinatumomab to chemotherapy for post-re-induction therapy in high- and intermediate-risk acute lymphoblastic leukemia (ALL) at first relapse in children, adolescents and young adults.
The study demonstrated the superiority of blinatumomab for efficacy and tolerability, which is particularly encouraging given the challenges in getting relapsed ALL patients back into remission so they can undergo bone marrow transplant, Dr. Brodsky said.
Of 208 patients randomized, 73% vs. 45% in the blinatumomab vs. chemotherapy arms were able to get to transplant – and therefore to potential cure, he said.
“Of note, the blinatumomab arm was less toxic and there was marked improvement in disease-free and overall survival, so this is clearly going to become a new standard of care for relapsed and refractory ALL,” he added.
Chimeric antigen receptor (CAR) T-cell therapies have garnered a great deal of attention given their “incredible efficacy” in treating B-cell malignancies, and new findings are taking aim at the drawbacks of therapy, such as the time, expense, and toxicity involved, according to Robert A. Brodsky, MD.
One example, from a study slated for presentation during a plenary session at the upcoming annual meeting of the American Society of Hematology involves the investigational T-cell bispecific antibody mosunetuzumab, which targets both CD20 on the surface of malignant B cells, and CD3 on cytotoxic T cells, engaging the T cells and directing their cytotoxicity against B cells.
In a study (Abstract 6) of 218 non-Hodgkin lymphoma patients, including 23 who had already received CAR T-cell therapy and had relapsed or were refractory to the treatment, 64% responded, 42% had a complete response, and the median duration of response is now out to 9 months, Dr. Brodsky, ASH secretary and director of the division of hematology at Johns Hopkins University, Baltimore, said during a premeeting press conference.
“It’s basically an antibody using the patient’s own T cell to do what a CAR-T cell would do – [a] very exciting study and large study,” he said. “It is an off-the-shelf product, it completely gets around the problem of the time to generate the CAR T-cell product, and because it’s going to be much simpler and faster to produce, it’s likely going to be much cheaper than CAR T cells.”
The preliminary results also suggest it is less toxic than CAR T-cell therapy, he added.
Two other CAR T-cell therapy–related studies highlighted during the press conference address its use for multiple myeloma. One, the phase 1b/2 CARTITUDE study (Abstract 577) uses CAR T cells against the B-cell maturation antigen (BCMA) in the relapsed/refractory setting.
Of 25 patients treated with chemotherapy followed by CAR T-cell infusion and followed for a median of 3 months, 91% responded, two achieved a complete remission, and “many other responses were very deep responses,” Dr. Brodsky said, noting that the second featured multiple myeloma trial (Abstract 930) looked at bispecific CAR T-cell therapy targeting BCMA and CD38 in an effort to reduce resistance to the therapy.
“Again, very interesting preliminary results,” he said, noting that of 16 patients followed for a median of 36 weeks, 87.5% responded, the treatment was well tolerated, and progression-free survival at 9 months was 75%.
In addition to the “key theme” of overcoming CAR T-cell therapy obstacles, three other themes have emerged from among the thousands of abstracts submitted for presentation at ASH. These, as presented during the press conference, include new venous thromboembolism (VTE) therapies and approaches to research; inclusive medicine, with abstracts focused on age- and race-related issues in clinical trials; and new advances in the treatment of sickle cell disease. All of these have potentially practice-changing implications, as do the six late-breaking abstracts selected from 93 abstracts submitted for consideration for oral presentation at ASH, Dr. Brodsky said.
One of the “truly practice-changing” late-breakers is a randomized phase 3 trial (Abstract LBA-1) comparing the bispecific antibody blinatumomab to chemotherapy for post-re-induction therapy in high- and intermediate-risk acute lymphoblastic leukemia (ALL) at first relapse in children, adolescents and young adults.
The study demonstrated the superiority of blinatumomab for efficacy and tolerability, which is particularly encouraging given the challenges in getting relapsed ALL patients back into remission so they can undergo bone marrow transplant, Dr. Brodsky said.
Of 208 patients randomized, 73% vs. 45% in the blinatumomab vs. chemotherapy arms were able to get to transplant – and therefore to potential cure, he said.
“Of note, the blinatumomab arm was less toxic and there was marked improvement in disease-free and overall survival, so this is clearly going to become a new standard of care for relapsed and refractory ALL,” he added.
Chimeric antigen receptor (CAR) T-cell therapies have garnered a great deal of attention given their “incredible efficacy” in treating B-cell malignancies, and new findings are taking aim at the drawbacks of therapy, such as the time, expense, and toxicity involved, according to Robert A. Brodsky, MD.
One example, from a study slated for presentation during a plenary session at the upcoming annual meeting of the American Society of Hematology involves the investigational T-cell bispecific antibody mosunetuzumab, which targets both CD20 on the surface of malignant B cells, and CD3 on cytotoxic T cells, engaging the T cells and directing their cytotoxicity against B cells.
In a study (Abstract 6) of 218 non-Hodgkin lymphoma patients, including 23 who had already received CAR T-cell therapy and had relapsed or were refractory to the treatment, 64% responded, 42% had a complete response, and the median duration of response is now out to 9 months, Dr. Brodsky, ASH secretary and director of the division of hematology at Johns Hopkins University, Baltimore, said during a premeeting press conference.
“It’s basically an antibody using the patient’s own T cell to do what a CAR-T cell would do – [a] very exciting study and large study,” he said. “It is an off-the-shelf product, it completely gets around the problem of the time to generate the CAR T-cell product, and because it’s going to be much simpler and faster to produce, it’s likely going to be much cheaper than CAR T cells.”
The preliminary results also suggest it is less toxic than CAR T-cell therapy, he added.
Two other CAR T-cell therapy–related studies highlighted during the press conference address its use for multiple myeloma. One, the phase 1b/2 CARTITUDE study (Abstract 577) uses CAR T cells against the B-cell maturation antigen (BCMA) in the relapsed/refractory setting.
Of 25 patients treated with chemotherapy followed by CAR T-cell infusion and followed for a median of 3 months, 91% responded, two achieved a complete remission, and “many other responses were very deep responses,” Dr. Brodsky said, noting that the second featured multiple myeloma trial (Abstract 930) looked at bispecific CAR T-cell therapy targeting BCMA and CD38 in an effort to reduce resistance to the therapy.
“Again, very interesting preliminary results,” he said, noting that of 16 patients followed for a median of 36 weeks, 87.5% responded, the treatment was well tolerated, and progression-free survival at 9 months was 75%.
In addition to the “key theme” of overcoming CAR T-cell therapy obstacles, three other themes have emerged from among the thousands of abstracts submitted for presentation at ASH. These, as presented during the press conference, include new venous thromboembolism (VTE) therapies and approaches to research; inclusive medicine, with abstracts focused on age- and race-related issues in clinical trials; and new advances in the treatment of sickle cell disease. All of these have potentially practice-changing implications, as do the six late-breaking abstracts selected from 93 abstracts submitted for consideration for oral presentation at ASH, Dr. Brodsky said.
One of the “truly practice-changing” late-breakers is a randomized phase 3 trial (Abstract LBA-1) comparing the bispecific antibody blinatumomab to chemotherapy for post-re-induction therapy in high- and intermediate-risk acute lymphoblastic leukemia (ALL) at first relapse in children, adolescents and young adults.
The study demonstrated the superiority of blinatumomab for efficacy and tolerability, which is particularly encouraging given the challenges in getting relapsed ALL patients back into remission so they can undergo bone marrow transplant, Dr. Brodsky said.
Of 208 patients randomized, 73% vs. 45% in the blinatumomab vs. chemotherapy arms were able to get to transplant – and therefore to potential cure, he said.
“Of note, the blinatumomab arm was less toxic and there was marked improvement in disease-free and overall survival, so this is clearly going to become a new standard of care for relapsed and refractory ALL,” he added.
DAPA-HF: Dapagliflozin benefits regardless of age, HF severity
PHILADELPHIA – The substantial benefits from adding dapagliflozin to guideline-directed medical therapy for patients with heart failure with reduced ejection fraction enrolled in the DAPA-HF trial applied to patients regardless of their age or baseline health status, a pair of new post hoc analyses suggest.
These findings emerged a day after a report that more fully delineated dapagliflozin’s consistent safety and efficacy in patients with heart failure with reduced ejection fraction (HFrEF) regardless of whether they also had type 2 diabetes. One of the new, post hoc analyses reported at the American Heart Association scientific sessions suggested that even the most elderly enrolled patients, 75 years and older, had a similar cut in mortality and acute heart failure exacerbations, compared with younger patients. A second post hoc analysis indicated that patients with severe heart failure symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with mild baseline symptoms, measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ).
The primary results from the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial, first reported in August 2019, showed that among more than 4,700 patients with HFrEF randomized to receive the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) on top of standard HFrEF medications or placebo, those who received dapagliflozin had a statistically significant, 26% decrease in their incidence of the primary study endpoint over a median 18 months, regardless of diabetes status (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“These benefits were entirely consistent across the range of ages studied,” extending from patients younger than 55 years to those older than 75 years, John McMurray, MD, said at the meeting. “In many parts of the world, particularly North America and Western Europe, we have an increasingly elderly population. Many patients with heart failure are much older than in clinical trials,” he said.
“The thing of concern is whether elderly patients get as much benefit and tolerate treatment as well as younger patients,” said Dr. McMurray, professor of medical cardiology at the University of Glasgow.
“Dapagliflozin worked across all ages, including some very elderly patients enrolled in the trial,” said Mary Norine Walsh, MD, medical director of the heart failure and transplant program at St. Vincent Heart Center of Indiana in Indianapolis. “Many trials have not looked at age like this. I hope this is a new way to analyze trials to produce more information that can help patients,” she said in an interview.
Quality-of-life outcomes
The other new, post hoc analysis showed that patients with severe HF symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with milder baseline symptoms and less impaired function, measured by the KCCQ. Dapagliflozin treatment “improved cardiovascular death and worsening heart failure to a similar extent across the entire range of KCCQ at baseline,” Mikhail N. Kosiborod, MD, said in a separate talk at the meeting. In addition, dapagliflozin treatment increased the rate of small, moderate, and large clinically meaningful improvements in patients’ KCCQ scores across all key domains of the metric, which scores symptom frequency and severity, physical and social limitations, and quality of life, said Dr. Kosiborod, a cardiologist and professor of medicine at the University of Missouri–Kansas City.
After the first 8 months of treatment in the DAPA-HF trial, 58% of the 2,373 patients who received dapagliflozin had a clinically meaningful improvement in their total KCCQ symptom score of at least 5 points, compared with a 51% rate in the 2,371 patients in the control arm, a statistically significant difference. This meant that the number needed to treat with dapagliflozin was 14 patients to produce one additional patient with at least a 5-point KCCQ improvement compared with controls, a “very small” number needed to treat, Dr. Kosiborod said in an interview.
Addition of the KCCQ to the panel of assessments that patients underwent during DAPA-HF reflected an evolved approach to measuring efficacy outcomes in clinical trials by including patient-reported outcomes. Earlier in 2019, the Food and Drug Administration released draft guidance for heart failure drug development that explicitly called for efficacy endpoints in pivotal studies that measure how patients feel and function, and stating that these endpoints can be the basis for new drug approvals.
“To many patients, how they feel matters as much if not more than how long they live,” Dr. Kosiborod noted. The goals of heart failure treatments are not only to extend survival and reduced hospitalizations, but also to improve symptoms, function, and quality of life, he said.
“There is a lot of interest now in having outcomes in heart failure trials that are more meaningful to patients, like feeling better and being able to do more,” noted Dr. Walsh.
The DAPA-HF results also showed that patients had similar rates of reduction in death, heart failure hospitalization, or urgent clinical visits, regardless of how severely they were affected by their heart failure when they began dapagliflozin treatment. The researchers ran an analysis that divided the entire trial population into tertiles based on their KCCQ score on entering the study. Patients in the most severely-affected tertile had a 30% cut in their rate of death or acute heart failure exacerbation on dapagliflozin compared with placebo, while patients in the tertile with the mildest symptoms at baseline had a 38% reduction in their primary outcome incidence compared with controls who received placebo. Concurrently with Dr. Kosiborod’s report, the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044138).
Outcomes by age
Not surprisingly in DAPA-HF, the older patients were, the sicker, Dr. McMurray observed. Of the study’s 1,149 patients (24% of the study cohort) who were at least 75 years old, 62% had chronic kidney disease, compared with a 14% prevalence among the 636 patients younger than age 55. The 75-and-older group showed a steeper, 32% decline in incidence of the primary endpoint – a composite of cardiovascular (CV) death, HF hospitalization, or urgent HF visit requiring intravenous therapy – than in the other studied age groups: a 24% decline in those 65-74 years old, a 29% cut in those 55-64 years old, and a 13% drop in patients younger than 55 years old.
In addition, patients aged 75 years or greater were just as likely as the overall group to show at least a 5-point improvement in their KCCQ Total Symptom Score on dapagliflozin, as well as about the same reduced rate of deterioration compared with placebo as tracked with the KCCQ.
Patients “got as much benefit in terms of symptoms as well as morbidity and mortality,” Dr. McMurray concluded. Concurrently with the meeting report the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044133).
“These data are of critical importance, as improving patient-reported outcomes in heart failure, especially in highly symptomatic patients, is an important goal in drug development,” G. Michael Felker, MD, wrote in an editorial accompanying the two published analyses (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044578). These new analyses also highlight another attractive feature of dapagliflozin and, apparently, the entire class of SGLT2 inhibitors: They “ ‘play well with others’ when it comes to overlapping intolerances that often limit (either in reality or in perception) optimization of GDMT [guideline-directed medical therapy]. Although SGLT2 inhibitor therapy may lead to volume depletion and require adjustment of diuretics, the SGLT2 inhibitors generally lack some of the other dose-limiting adverse effects (such as renal dysfunction, hyperkalemia, and hypotension) that can make aggressive up-titration of GDMT problematic, particularly in older patients or those with more advanced disease,“ wrote Dr. Felker, professor of medicine at Duke University in Durham, N.C. “We stand at the beginning of a new era of ‘quadruple therapy’ for HFrEF with beta-blockers, an angiotensin receptor neprilysin inhibitor, mineralocorticoid receptor antagonists, and SGLT2 inhibitors,” he concluded.
A version of this article also appears on Medscape.com
In DAPA-HF, treatment with dapagliflozin met the three critical goals of heart failure management. When used on top of current guideline-directed medical therapy, the treatment reduced mortality, cut hospitalizations, and improved heart failure–related health status – all to a similar extent regardless of patients’ age or symptom severity at entry. These new, post hoc findings provide important, additional data supporting inhibition of sodium-glucose cotransporter (SGLT) 2 with dapagliflozin as the newest foundational pillar of treatment for heart failure with reduced ejection fraction (HFrEF).
The results of the analysis by baseline symptoms severity as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) showed similar treatment effects from dapagliflozin regardless of a patient’s baseline KCCQ score, suggesting that the prior report of a blunted effect of dapagliflozin in patients classified at baseline as being in New York Heart Association functional class III or IV compared with class I and II patients was likely a chance finding.
Both the analyses by age and by KCCQ scores were limited by their post hoc status using data collected in a single study. No evidence addresses whether these are class effects for all drugs in the SGLT2-inhibitor class, whether these findings from DAPA-HF are generalizable to real world practice, or whether treatment with dapagliflozin would have similar effects on outcomes if it had been used more often in combination with sacubitril/valsartan. In DAPA-HF, 11% of patients also received sacubitril/valsartan even though existing management guidelines recommend sacubitril/valsartan as the preferred agent for inhibiting the renin-angiotensin system.
It’s also unclear whether patient-reported outcomes such as those measured by the KCCQ will help in sequencing the introduction of drugs for HFrEF patients, or drug selection by patients, providers, payers, and in guidelines.
Carolyn S.P. Lam, MD, is professor of medicine at Duke-National University of Singapore. She has been a consultant to and has received research funding from AstraZeneca and several other companies. She made these comments as designated discussant for the two reports.
In DAPA-HF, treatment with dapagliflozin met the three critical goals of heart failure management. When used on top of current guideline-directed medical therapy, the treatment reduced mortality, cut hospitalizations, and improved heart failure–related health status – all to a similar extent regardless of patients’ age or symptom severity at entry. These new, post hoc findings provide important, additional data supporting inhibition of sodium-glucose cotransporter (SGLT) 2 with dapagliflozin as the newest foundational pillar of treatment for heart failure with reduced ejection fraction (HFrEF).
The results of the analysis by baseline symptoms severity as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) showed similar treatment effects from dapagliflozin regardless of a patient’s baseline KCCQ score, suggesting that the prior report of a blunted effect of dapagliflozin in patients classified at baseline as being in New York Heart Association functional class III or IV compared with class I and II patients was likely a chance finding.
Both the analyses by age and by KCCQ scores were limited by their post hoc status using data collected in a single study. No evidence addresses whether these are class effects for all drugs in the SGLT2-inhibitor class, whether these findings from DAPA-HF are generalizable to real world practice, or whether treatment with dapagliflozin would have similar effects on outcomes if it had been used more often in combination with sacubitril/valsartan. In DAPA-HF, 11% of patients also received sacubitril/valsartan even though existing management guidelines recommend sacubitril/valsartan as the preferred agent for inhibiting the renin-angiotensin system.
It’s also unclear whether patient-reported outcomes such as those measured by the KCCQ will help in sequencing the introduction of drugs for HFrEF patients, or drug selection by patients, providers, payers, and in guidelines.
Carolyn S.P. Lam, MD, is professor of medicine at Duke-National University of Singapore. She has been a consultant to and has received research funding from AstraZeneca and several other companies. She made these comments as designated discussant for the two reports.
In DAPA-HF, treatment with dapagliflozin met the three critical goals of heart failure management. When used on top of current guideline-directed medical therapy, the treatment reduced mortality, cut hospitalizations, and improved heart failure–related health status – all to a similar extent regardless of patients’ age or symptom severity at entry. These new, post hoc findings provide important, additional data supporting inhibition of sodium-glucose cotransporter (SGLT) 2 with dapagliflozin as the newest foundational pillar of treatment for heart failure with reduced ejection fraction (HFrEF).
The results of the analysis by baseline symptoms severity as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) showed similar treatment effects from dapagliflozin regardless of a patient’s baseline KCCQ score, suggesting that the prior report of a blunted effect of dapagliflozin in patients classified at baseline as being in New York Heart Association functional class III or IV compared with class I and II patients was likely a chance finding.
Both the analyses by age and by KCCQ scores were limited by their post hoc status using data collected in a single study. No evidence addresses whether these are class effects for all drugs in the SGLT2-inhibitor class, whether these findings from DAPA-HF are generalizable to real world practice, or whether treatment with dapagliflozin would have similar effects on outcomes if it had been used more often in combination with sacubitril/valsartan. In DAPA-HF, 11% of patients also received sacubitril/valsartan even though existing management guidelines recommend sacubitril/valsartan as the preferred agent for inhibiting the renin-angiotensin system.
It’s also unclear whether patient-reported outcomes such as those measured by the KCCQ will help in sequencing the introduction of drugs for HFrEF patients, or drug selection by patients, providers, payers, and in guidelines.
Carolyn S.P. Lam, MD, is professor of medicine at Duke-National University of Singapore. She has been a consultant to and has received research funding from AstraZeneca and several other companies. She made these comments as designated discussant for the two reports.
PHILADELPHIA – The substantial benefits from adding dapagliflozin to guideline-directed medical therapy for patients with heart failure with reduced ejection fraction enrolled in the DAPA-HF trial applied to patients regardless of their age or baseline health status, a pair of new post hoc analyses suggest.
These findings emerged a day after a report that more fully delineated dapagliflozin’s consistent safety and efficacy in patients with heart failure with reduced ejection fraction (HFrEF) regardless of whether they also had type 2 diabetes. One of the new, post hoc analyses reported at the American Heart Association scientific sessions suggested that even the most elderly enrolled patients, 75 years and older, had a similar cut in mortality and acute heart failure exacerbations, compared with younger patients. A second post hoc analysis indicated that patients with severe heart failure symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with mild baseline symptoms, measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ).
The primary results from the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial, first reported in August 2019, showed that among more than 4,700 patients with HFrEF randomized to receive the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) on top of standard HFrEF medications or placebo, those who received dapagliflozin had a statistically significant, 26% decrease in their incidence of the primary study endpoint over a median 18 months, regardless of diabetes status (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“These benefits were entirely consistent across the range of ages studied,” extending from patients younger than 55 years to those older than 75 years, John McMurray, MD, said at the meeting. “In many parts of the world, particularly North America and Western Europe, we have an increasingly elderly population. Many patients with heart failure are much older than in clinical trials,” he said.
“The thing of concern is whether elderly patients get as much benefit and tolerate treatment as well as younger patients,” said Dr. McMurray, professor of medical cardiology at the University of Glasgow.
“Dapagliflozin worked across all ages, including some very elderly patients enrolled in the trial,” said Mary Norine Walsh, MD, medical director of the heart failure and transplant program at St. Vincent Heart Center of Indiana in Indianapolis. “Many trials have not looked at age like this. I hope this is a new way to analyze trials to produce more information that can help patients,” she said in an interview.
Quality-of-life outcomes
The other new, post hoc analysis showed that patients with severe HF symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with milder baseline symptoms and less impaired function, measured by the KCCQ. Dapagliflozin treatment “improved cardiovascular death and worsening heart failure to a similar extent across the entire range of KCCQ at baseline,” Mikhail N. Kosiborod, MD, said in a separate talk at the meeting. In addition, dapagliflozin treatment increased the rate of small, moderate, and large clinically meaningful improvements in patients’ KCCQ scores across all key domains of the metric, which scores symptom frequency and severity, physical and social limitations, and quality of life, said Dr. Kosiborod, a cardiologist and professor of medicine at the University of Missouri–Kansas City.
After the first 8 months of treatment in the DAPA-HF trial, 58% of the 2,373 patients who received dapagliflozin had a clinically meaningful improvement in their total KCCQ symptom score of at least 5 points, compared with a 51% rate in the 2,371 patients in the control arm, a statistically significant difference. This meant that the number needed to treat with dapagliflozin was 14 patients to produce one additional patient with at least a 5-point KCCQ improvement compared with controls, a “very small” number needed to treat, Dr. Kosiborod said in an interview.
Addition of the KCCQ to the panel of assessments that patients underwent during DAPA-HF reflected an evolved approach to measuring efficacy outcomes in clinical trials by including patient-reported outcomes. Earlier in 2019, the Food and Drug Administration released draft guidance for heart failure drug development that explicitly called for efficacy endpoints in pivotal studies that measure how patients feel and function, and stating that these endpoints can be the basis for new drug approvals.
“To many patients, how they feel matters as much if not more than how long they live,” Dr. Kosiborod noted. The goals of heart failure treatments are not only to extend survival and reduced hospitalizations, but also to improve symptoms, function, and quality of life, he said.
“There is a lot of interest now in having outcomes in heart failure trials that are more meaningful to patients, like feeling better and being able to do more,” noted Dr. Walsh.
The DAPA-HF results also showed that patients had similar rates of reduction in death, heart failure hospitalization, or urgent clinical visits, regardless of how severely they were affected by their heart failure when they began dapagliflozin treatment. The researchers ran an analysis that divided the entire trial population into tertiles based on their KCCQ score on entering the study. Patients in the most severely-affected tertile had a 30% cut in their rate of death or acute heart failure exacerbation on dapagliflozin compared with placebo, while patients in the tertile with the mildest symptoms at baseline had a 38% reduction in their primary outcome incidence compared with controls who received placebo. Concurrently with Dr. Kosiborod’s report, the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044138).
Outcomes by age
Not surprisingly in DAPA-HF, the older patients were, the sicker, Dr. McMurray observed. Of the study’s 1,149 patients (24% of the study cohort) who were at least 75 years old, 62% had chronic kidney disease, compared with a 14% prevalence among the 636 patients younger than age 55. The 75-and-older group showed a steeper, 32% decline in incidence of the primary endpoint – a composite of cardiovascular (CV) death, HF hospitalization, or urgent HF visit requiring intravenous therapy – than in the other studied age groups: a 24% decline in those 65-74 years old, a 29% cut in those 55-64 years old, and a 13% drop in patients younger than 55 years old.
In addition, patients aged 75 years or greater were just as likely as the overall group to show at least a 5-point improvement in their KCCQ Total Symptom Score on dapagliflozin, as well as about the same reduced rate of deterioration compared with placebo as tracked with the KCCQ.
Patients “got as much benefit in terms of symptoms as well as morbidity and mortality,” Dr. McMurray concluded. Concurrently with the meeting report the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044133).
“These data are of critical importance, as improving patient-reported outcomes in heart failure, especially in highly symptomatic patients, is an important goal in drug development,” G. Michael Felker, MD, wrote in an editorial accompanying the two published analyses (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044578). These new analyses also highlight another attractive feature of dapagliflozin and, apparently, the entire class of SGLT2 inhibitors: They “ ‘play well with others’ when it comes to overlapping intolerances that often limit (either in reality or in perception) optimization of GDMT [guideline-directed medical therapy]. Although SGLT2 inhibitor therapy may lead to volume depletion and require adjustment of diuretics, the SGLT2 inhibitors generally lack some of the other dose-limiting adverse effects (such as renal dysfunction, hyperkalemia, and hypotension) that can make aggressive up-titration of GDMT problematic, particularly in older patients or those with more advanced disease,“ wrote Dr. Felker, professor of medicine at Duke University in Durham, N.C. “We stand at the beginning of a new era of ‘quadruple therapy’ for HFrEF with beta-blockers, an angiotensin receptor neprilysin inhibitor, mineralocorticoid receptor antagonists, and SGLT2 inhibitors,” he concluded.
A version of this article also appears on Medscape.com
PHILADELPHIA – The substantial benefits from adding dapagliflozin to guideline-directed medical therapy for patients with heart failure with reduced ejection fraction enrolled in the DAPA-HF trial applied to patients regardless of their age or baseline health status, a pair of new post hoc analyses suggest.
These findings emerged a day after a report that more fully delineated dapagliflozin’s consistent safety and efficacy in patients with heart failure with reduced ejection fraction (HFrEF) regardless of whether they also had type 2 diabetes. One of the new, post hoc analyses reported at the American Heart Association scientific sessions suggested that even the most elderly enrolled patients, 75 years and older, had a similar cut in mortality and acute heart failure exacerbations, compared with younger patients. A second post hoc analysis indicated that patients with severe heart failure symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with mild baseline symptoms, measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ).
The primary results from the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial, first reported in August 2019, showed that among more than 4,700 patients with HFrEF randomized to receive the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) on top of standard HFrEF medications or placebo, those who received dapagliflozin had a statistically significant, 26% decrease in their incidence of the primary study endpoint over a median 18 months, regardless of diabetes status (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“These benefits were entirely consistent across the range of ages studied,” extending from patients younger than 55 years to those older than 75 years, John McMurray, MD, said at the meeting. “In many parts of the world, particularly North America and Western Europe, we have an increasingly elderly population. Many patients with heart failure are much older than in clinical trials,” he said.
“The thing of concern is whether elderly patients get as much benefit and tolerate treatment as well as younger patients,” said Dr. McMurray, professor of medical cardiology at the University of Glasgow.
“Dapagliflozin worked across all ages, including some very elderly patients enrolled in the trial,” said Mary Norine Walsh, MD, medical director of the heart failure and transplant program at St. Vincent Heart Center of Indiana in Indianapolis. “Many trials have not looked at age like this. I hope this is a new way to analyze trials to produce more information that can help patients,” she said in an interview.
Quality-of-life outcomes
The other new, post hoc analysis showed that patients with severe HF symptoms at entry into the trial received about as much benefit from the addition of dapagliflozin as did patients with milder baseline symptoms and less impaired function, measured by the KCCQ. Dapagliflozin treatment “improved cardiovascular death and worsening heart failure to a similar extent across the entire range of KCCQ at baseline,” Mikhail N. Kosiborod, MD, said in a separate talk at the meeting. In addition, dapagliflozin treatment increased the rate of small, moderate, and large clinically meaningful improvements in patients’ KCCQ scores across all key domains of the metric, which scores symptom frequency and severity, physical and social limitations, and quality of life, said Dr. Kosiborod, a cardiologist and professor of medicine at the University of Missouri–Kansas City.
After the first 8 months of treatment in the DAPA-HF trial, 58% of the 2,373 patients who received dapagliflozin had a clinically meaningful improvement in their total KCCQ symptom score of at least 5 points, compared with a 51% rate in the 2,371 patients in the control arm, a statistically significant difference. This meant that the number needed to treat with dapagliflozin was 14 patients to produce one additional patient with at least a 5-point KCCQ improvement compared with controls, a “very small” number needed to treat, Dr. Kosiborod said in an interview.
Addition of the KCCQ to the panel of assessments that patients underwent during DAPA-HF reflected an evolved approach to measuring efficacy outcomes in clinical trials by including patient-reported outcomes. Earlier in 2019, the Food and Drug Administration released draft guidance for heart failure drug development that explicitly called for efficacy endpoints in pivotal studies that measure how patients feel and function, and stating that these endpoints can be the basis for new drug approvals.
“To many patients, how they feel matters as much if not more than how long they live,” Dr. Kosiborod noted. The goals of heart failure treatments are not only to extend survival and reduced hospitalizations, but also to improve symptoms, function, and quality of life, he said.
“There is a lot of interest now in having outcomes in heart failure trials that are more meaningful to patients, like feeling better and being able to do more,” noted Dr. Walsh.
The DAPA-HF results also showed that patients had similar rates of reduction in death, heart failure hospitalization, or urgent clinical visits, regardless of how severely they were affected by their heart failure when they began dapagliflozin treatment. The researchers ran an analysis that divided the entire trial population into tertiles based on their KCCQ score on entering the study. Patients in the most severely-affected tertile had a 30% cut in their rate of death or acute heart failure exacerbation on dapagliflozin compared with placebo, while patients in the tertile with the mildest symptoms at baseline had a 38% reduction in their primary outcome incidence compared with controls who received placebo. Concurrently with Dr. Kosiborod’s report, the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044138).
Outcomes by age
Not surprisingly in DAPA-HF, the older patients were, the sicker, Dr. McMurray observed. Of the study’s 1,149 patients (24% of the study cohort) who were at least 75 years old, 62% had chronic kidney disease, compared with a 14% prevalence among the 636 patients younger than age 55. The 75-and-older group showed a steeper, 32% decline in incidence of the primary endpoint – a composite of cardiovascular (CV) death, HF hospitalization, or urgent HF visit requiring intravenous therapy – than in the other studied age groups: a 24% decline in those 65-74 years old, a 29% cut in those 55-64 years old, and a 13% drop in patients younger than 55 years old.
In addition, patients aged 75 years or greater were just as likely as the overall group to show at least a 5-point improvement in their KCCQ Total Symptom Score on dapagliflozin, as well as about the same reduced rate of deterioration compared with placebo as tracked with the KCCQ.
Patients “got as much benefit in terms of symptoms as well as morbidity and mortality,” Dr. McMurray concluded. Concurrently with the meeting report the results appeared in an article online (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044133).
“These data are of critical importance, as improving patient-reported outcomes in heart failure, especially in highly symptomatic patients, is an important goal in drug development,” G. Michael Felker, MD, wrote in an editorial accompanying the two published analyses (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044578). These new analyses also highlight another attractive feature of dapagliflozin and, apparently, the entire class of SGLT2 inhibitors: They “ ‘play well with others’ when it comes to overlapping intolerances that often limit (either in reality or in perception) optimization of GDMT [guideline-directed medical therapy]. Although SGLT2 inhibitor therapy may lead to volume depletion and require adjustment of diuretics, the SGLT2 inhibitors generally lack some of the other dose-limiting adverse effects (such as renal dysfunction, hyperkalemia, and hypotension) that can make aggressive up-titration of GDMT problematic, particularly in older patients or those with more advanced disease,“ wrote Dr. Felker, professor of medicine at Duke University in Durham, N.C. “We stand at the beginning of a new era of ‘quadruple therapy’ for HFrEF with beta-blockers, an angiotensin receptor neprilysin inhibitor, mineralocorticoid receptor antagonists, and SGLT2 inhibitors,” he concluded.
A version of this article also appears on Medscape.com
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Combo elicits lasting responses in metastatic melanoma
NATIONAL HARBOR, MD. – The combination of bempegaldesleukin and nivolumab produced durable responses in a phase 1/2 trial of patients with previously untreated metastatic melanoma.
The overall response rate was 53%, and most responders were still in response at a median follow-up of about 19 months. The median progression-free survival was not reached, and the combination was considered well tolerated.
Adi Diab, MD, of the University of Texas MD Anderson Cancer Center, Houston, presented these results from the PIVOT-02 study at the annual meeting of the Society for Immunotherapy of Cancer.
Dr. Diab explained that bempegaldesleukin (bempeg) is a CD122-preferential interleukin-2 pathway agonist, and earlier results from the PIVOT-02 trial showed that adding bempeg to nivolumab can convert baseline tumors from programmed death–ligand 1 (PD-L1) negative to PD-L1 positive (SITC 2018, Abstract O4).
Dr. Diab presented updated results from PIVOT-02 (NCT02983045) in 41 patients with metastatic melanoma who received bempeg plus nivolumab as first-line treatment. The patients had a median age of 63 years (range, 22-80 years) at baseline, and 58.5% were male. Most patients (58.5%) were PD-L1 positive, although PD-L1 status was unknown in 7.3% of patients.
Patients received bempeg at 0.006 mg/kg and nivolumab at 360 mg every 3 weeks. They received a median of nine cycles (range, 1-34), and the median follow-up was 18.6 months.
Efficacy
In the 38 patients who were evaluable for efficacy, the overall response rate was 53% (n = 20), and the complete response rate was 34% (n = 13). The median time to response was 2.0 months, and the median time to complete response was 7.9 months.
Dr. Diab noted that responses were seen regardless of PD-L1 expression at baseline. The response rate was 39% among PD-L1-negative patients, 64% among PD-L1-positive patients, and 33% among patients whose PD-L1 status was unknown.
Dr. Diab also pointed out that responses were durable and deepened over time. The median duration of response was not reached, and 17 of the 20 responders had ongoing responses at last follow-up. The median progression-free survival has not been reached.
Safety
“This combination is safe and tolerable, there’s no overlapping immune-related adverse events, and the most common side effects are grade 1/2 flu-like symptoms,” Dr. Diab said.
The most common grade 1/2 treatment-related adverse events (AEs) were flu-like symptoms (80.5%), rash (70.7%), fatigue (65.9%), pruritus (48.8%), nausea (46.3%), arthralgia (43.9%), decreased appetite (36.6%), and myalgia (36.6%).
Dr. Diab noted that cytokine-related AEs (flu-like symptoms, rash, and pruritus) were easily managed with NSAIDs; decreased with subsequent cycles of treatment; and did not necessitate dose delays, reductions, or discontinuations.
Grade 3/4 treatment-related AEs included two cases of acute kidney injury, two cases of atrial fibrillation, one case of dizziness, one case of dyspnea, one case of hypoxia, one case of hyperglycemia, and one case of hypernatremia.
Five patients discontinued treatment because of related AEs, including cerebrovascular accident, peripheral edema, blood creatinine increase, malaise, and pharyngitis. There were no treatment-related deaths.
Dr. Diab said these results were used to support the recent breakthrough therapy designation granted to bempeg in combination with nivolumab. The results have also prompted a phase 3 trial in which researchers are comparing the combination with nivolumab alone (NCT03635983).
The phase 1/2 trial is sponsored by Nektar Therapeutics in collaboration with Bristol-Myers Squibb. Dr. Diab reported relationships with Nektar, Celgene, CureVac, Idera, and Pfizer.
SOURCE: Diab A et al. SITC 2019, Abstract O35.
NATIONAL HARBOR, MD. – The combination of bempegaldesleukin and nivolumab produced durable responses in a phase 1/2 trial of patients with previously untreated metastatic melanoma.
The overall response rate was 53%, and most responders were still in response at a median follow-up of about 19 months. The median progression-free survival was not reached, and the combination was considered well tolerated.
Adi Diab, MD, of the University of Texas MD Anderson Cancer Center, Houston, presented these results from the PIVOT-02 study at the annual meeting of the Society for Immunotherapy of Cancer.
Dr. Diab explained that bempegaldesleukin (bempeg) is a CD122-preferential interleukin-2 pathway agonist, and earlier results from the PIVOT-02 trial showed that adding bempeg to nivolumab can convert baseline tumors from programmed death–ligand 1 (PD-L1) negative to PD-L1 positive (SITC 2018, Abstract O4).
Dr. Diab presented updated results from PIVOT-02 (NCT02983045) in 41 patients with metastatic melanoma who received bempeg plus nivolumab as first-line treatment. The patients had a median age of 63 years (range, 22-80 years) at baseline, and 58.5% were male. Most patients (58.5%) were PD-L1 positive, although PD-L1 status was unknown in 7.3% of patients.
Patients received bempeg at 0.006 mg/kg and nivolumab at 360 mg every 3 weeks. They received a median of nine cycles (range, 1-34), and the median follow-up was 18.6 months.
Efficacy
In the 38 patients who were evaluable for efficacy, the overall response rate was 53% (n = 20), and the complete response rate was 34% (n = 13). The median time to response was 2.0 months, and the median time to complete response was 7.9 months.
Dr. Diab noted that responses were seen regardless of PD-L1 expression at baseline. The response rate was 39% among PD-L1-negative patients, 64% among PD-L1-positive patients, and 33% among patients whose PD-L1 status was unknown.
Dr. Diab also pointed out that responses were durable and deepened over time. The median duration of response was not reached, and 17 of the 20 responders had ongoing responses at last follow-up. The median progression-free survival has not been reached.
Safety
“This combination is safe and tolerable, there’s no overlapping immune-related adverse events, and the most common side effects are grade 1/2 flu-like symptoms,” Dr. Diab said.
The most common grade 1/2 treatment-related adverse events (AEs) were flu-like symptoms (80.5%), rash (70.7%), fatigue (65.9%), pruritus (48.8%), nausea (46.3%), arthralgia (43.9%), decreased appetite (36.6%), and myalgia (36.6%).
Dr. Diab noted that cytokine-related AEs (flu-like symptoms, rash, and pruritus) were easily managed with NSAIDs; decreased with subsequent cycles of treatment; and did not necessitate dose delays, reductions, or discontinuations.
Grade 3/4 treatment-related AEs included two cases of acute kidney injury, two cases of atrial fibrillation, one case of dizziness, one case of dyspnea, one case of hypoxia, one case of hyperglycemia, and one case of hypernatremia.
Five patients discontinued treatment because of related AEs, including cerebrovascular accident, peripheral edema, blood creatinine increase, malaise, and pharyngitis. There were no treatment-related deaths.
Dr. Diab said these results were used to support the recent breakthrough therapy designation granted to bempeg in combination with nivolumab. The results have also prompted a phase 3 trial in which researchers are comparing the combination with nivolumab alone (NCT03635983).
The phase 1/2 trial is sponsored by Nektar Therapeutics in collaboration with Bristol-Myers Squibb. Dr. Diab reported relationships with Nektar, Celgene, CureVac, Idera, and Pfizer.
SOURCE: Diab A et al. SITC 2019, Abstract O35.
NATIONAL HARBOR, MD. – The combination of bempegaldesleukin and nivolumab produced durable responses in a phase 1/2 trial of patients with previously untreated metastatic melanoma.
The overall response rate was 53%, and most responders were still in response at a median follow-up of about 19 months. The median progression-free survival was not reached, and the combination was considered well tolerated.
Adi Diab, MD, of the University of Texas MD Anderson Cancer Center, Houston, presented these results from the PIVOT-02 study at the annual meeting of the Society for Immunotherapy of Cancer.
Dr. Diab explained that bempegaldesleukin (bempeg) is a CD122-preferential interleukin-2 pathway agonist, and earlier results from the PIVOT-02 trial showed that adding bempeg to nivolumab can convert baseline tumors from programmed death–ligand 1 (PD-L1) negative to PD-L1 positive (SITC 2018, Abstract O4).
Dr. Diab presented updated results from PIVOT-02 (NCT02983045) in 41 patients with metastatic melanoma who received bempeg plus nivolumab as first-line treatment. The patients had a median age of 63 years (range, 22-80 years) at baseline, and 58.5% were male. Most patients (58.5%) were PD-L1 positive, although PD-L1 status was unknown in 7.3% of patients.
Patients received bempeg at 0.006 mg/kg and nivolumab at 360 mg every 3 weeks. They received a median of nine cycles (range, 1-34), and the median follow-up was 18.6 months.
Efficacy
In the 38 patients who were evaluable for efficacy, the overall response rate was 53% (n = 20), and the complete response rate was 34% (n = 13). The median time to response was 2.0 months, and the median time to complete response was 7.9 months.
Dr. Diab noted that responses were seen regardless of PD-L1 expression at baseline. The response rate was 39% among PD-L1-negative patients, 64% among PD-L1-positive patients, and 33% among patients whose PD-L1 status was unknown.
Dr. Diab also pointed out that responses were durable and deepened over time. The median duration of response was not reached, and 17 of the 20 responders had ongoing responses at last follow-up. The median progression-free survival has not been reached.
Safety
“This combination is safe and tolerable, there’s no overlapping immune-related adverse events, and the most common side effects are grade 1/2 flu-like symptoms,” Dr. Diab said.
The most common grade 1/2 treatment-related adverse events (AEs) were flu-like symptoms (80.5%), rash (70.7%), fatigue (65.9%), pruritus (48.8%), nausea (46.3%), arthralgia (43.9%), decreased appetite (36.6%), and myalgia (36.6%).
Dr. Diab noted that cytokine-related AEs (flu-like symptoms, rash, and pruritus) were easily managed with NSAIDs; decreased with subsequent cycles of treatment; and did not necessitate dose delays, reductions, or discontinuations.
Grade 3/4 treatment-related AEs included two cases of acute kidney injury, two cases of atrial fibrillation, one case of dizziness, one case of dyspnea, one case of hypoxia, one case of hyperglycemia, and one case of hypernatremia.
Five patients discontinued treatment because of related AEs, including cerebrovascular accident, peripheral edema, blood creatinine increase, malaise, and pharyngitis. There were no treatment-related deaths.
Dr. Diab said these results were used to support the recent breakthrough therapy designation granted to bempeg in combination with nivolumab. The results have also prompted a phase 3 trial in which researchers are comparing the combination with nivolumab alone (NCT03635983).
The phase 1/2 trial is sponsored by Nektar Therapeutics in collaboration with Bristol-Myers Squibb. Dr. Diab reported relationships with Nektar, Celgene, CureVac, Idera, and Pfizer.
SOURCE: Diab A et al. SITC 2019, Abstract O35.
REPORTING FROM SITC 2019
Survey: Cancer-related pain, opioid use up since 2018
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
OA management guidelines forgo treatment hierarchy or order but emphasize severity, patient risk factors
ATLANTA – New guidelines for management of osteoarthritis of the hand, knee, and hip from the American College of Rheumatology and the Arthritis Foundation lay out a wide range of treatment options without an algorithm or hierarchy, making strong recommendations for nondrug interventions and for tailoring plans to individual patient-level factors.
Since the ACR last released OA management guidelines in 2012, a number of recommendations have been added, changed, and removed, and the structure of the guidelines has also changed. For instance, the new OA guidelines include a broad list of management options, Sharon L. Kolasinski, MD, chair of the ACR guidelines panel and professor of clinical medicine in the division of rheumatology at the University of Pennsylvania, Philadelphia, said in a presentation at the annual meeting of the American College of Rheumatology.
“The new guideline emphasizes comprehensive management of patients with OA, rather than a stepwise algorithm in a linear manner,” she said.
There is also no hierarchy to the recommendations, apart from the strength of the recommendation. “For any individual patient, a single option may be chosen at a particular time point, perhaps with or without other options, and may be reused in the future. For a given intervention, there might be a period of time over which it’s useful, and then the option might be changed,” Dr. Kolasinski noted.
Dr. Kolasinski advised making treatment decisions based on a patient’s disease severity, whether the patient uses medical devices, and in consideration of patient risk factors. “A history of injuries, surgical history, access to care, personal beliefs and preferences should all be brought to bear on decision making for osteoarthritis management,” she said.
The guidelines also advise considering a patient’s overall well-being and factors related to a patient’s perception of pain and function, such as mood disorders, altered sleep, chronic pain, impaired coping measures, and stress level. “Comprehensive management requires a broad assessment of how pain and function are affecting the patient with OA as a whole and recognizing that multiple options are available. They might be used in combination or change over time,” Dr. Kolasinski said.
The new guidelines place a strong emphasis on educational, behavioral, psychosocial, mind-body, and physical approaches. There are strong recommendations for the use of exercise, including aerobic, strengthening, neuromuscular, and aquatic exercise. Weight loss also carries a strong recommendation for patients with hip and knee OA, with a focus on group-based exercise, education, fitness and exercise goals, and a multidisciplinary approach using self-efficacy and self-management programs. The panels made a strong recommendation for tai chi to improve hip and knee OA. There are also strong recommendations for orthoses; aids and assistive devices such as canes, first carpometacarpal (CMC) orthoses, and tibiofemoral knee braces. Other interventions, such as Kinesio tape for first CMC joint and knee OA, hand orthoses, and patellofemoral knee braces, carried a conditional recommendation. Other conditional recommendations made by the panel were for acupuncture, thermal interventions, and radiofrequency ablation for patients with knee OA. Balance training for hip and knee OA, yoga for knee OA, and cognitive-behavioral therapy all were conditionally recommended by the panel.
The panel strongly recommended against the use of transcutaneous nerve stimulation for hip and knee OA, Dr. Kolasinski noted. The panel also conditionally recommended against use of modified shoes and pulsed vibration therapy in knee OA; lateral or medial wedged insoles, massage, and manual therapy with exercise in hip or knee OA; and iontophoresis in first CMC OA.
Tuhina Neogi, MD, PhD, chief of rheumatology at Boston University and member of the core team that developed the guidelines, said in her presentation the panel chose not to use the term “nonpharmacologic” in the guidelines because it may give patients a false impression that they are not receiving a treatment. “We really need to change our language and change the way in which we approach these conversations with our patients so that they don’t feel that they are not getting a treatment when we’re giving these recommendations,” she said.
Recommendations for, against pharmacologic approaches
The ACR has changed conditional recommendations for topical NSAIDs for knee and hand OA, oral NSAIDs, and intra-articular steroids for knee and hip OA into strong recommendations for the 2019 guidelines, Dr. Kolasinski said. While the 2012 guidelines conditionally recommended against topical capsaicin for knee OA, the new guidelines conditionally recommend it.
Other pharmacologic conditional recommendations included topical NSAIDs, chondroitin sulfate, and intra-articular corticosteroid injections for hand OA, acetaminophen, and duloxetine for knee OA.
With the new recommendations come changes that some rheumatologists and health care providers may find controversial. “I think that the practicing rheumatologist may be surprised that we have a recommendation against the use of hyaluronic acid in the knee as a conditional recommendation,” Dr. Kolasinski said. “The assessment of the literature at this point really reveals that there is equivalence between intra-articular hyaluronic acid injection and intra-articular saline injection, and so it was the feeling of the panel that, really, this was worth changing the recommendation from the 2012 guideline.”
The panel made strong recommendations against use of the following pharmacologic interventions:
- Bisphosphonates.
- Glucosamine sulfate.
- Combination glucosamine sulfate-chondroitin sulfate products.
- Hydroxychloroquine.
- Methotrexate.
- Intra-articular hyaluronic acid injections in hip OA.
- Chondroitin sulfate, platelet-rich plasma injections, and stem cell injections in hip and knee OA.
- Tumor necrosis factor (TNF) inhibitors.
- Interleukin-1–receptor antagonists.
Additionally, the panel made a conditional recommendation against topical capsaicin on the hand, colchicine, fish oil, vitamin D, intra-articular hyaluronic acid injections in the first CMC, and intra-articular botulinum toxin and prolotherapy in hip and knee OA.
The panel did not recommend for or against use of yoga for hip and hand OA, topical lidocaine, pregabalin, gabapentin, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors apart from duloxetine, tricyclic antidepressants, and anti-nerve growth factor agents.
While the panel conditionally recommended against use of opioids, they made a conditional recommendation for use of tramadol opioids, and there was “a heated discussion about that distinction,” Dr. Neogi noted in a discussion session at the meeting. “There was a recent observational study that indicated that tramadol may have an increased risk of [all-cause] mortality, but there are lots of issues of confounding by indication in that study.”
The patient panel also raised strong concerns about the ACR and the Arthritis Foundation coming out against opioids for OA management in their guidelines. “They don’t want to damn opioids, but they’re also concerned about a specialty society coming out strongly against opioids in the concern that their physicians may limit their access to opioids if they’re in a situation where nothing else is helping them,” Dr. Neogi said.
Dr. Kolasinski noted the guidelines will be published online in Arthritis & Rheumatology in December, and will appear in print in February of next year.
Dr. Kolasinski reported no relevant financial disclosures. Dr. Neogi reported relationships with EMD Serono, Merck, and Pfizer.
ATLANTA – New guidelines for management of osteoarthritis of the hand, knee, and hip from the American College of Rheumatology and the Arthritis Foundation lay out a wide range of treatment options without an algorithm or hierarchy, making strong recommendations for nondrug interventions and for tailoring plans to individual patient-level factors.
Since the ACR last released OA management guidelines in 2012, a number of recommendations have been added, changed, and removed, and the structure of the guidelines has also changed. For instance, the new OA guidelines include a broad list of management options, Sharon L. Kolasinski, MD, chair of the ACR guidelines panel and professor of clinical medicine in the division of rheumatology at the University of Pennsylvania, Philadelphia, said in a presentation at the annual meeting of the American College of Rheumatology.
“The new guideline emphasizes comprehensive management of patients with OA, rather than a stepwise algorithm in a linear manner,” she said.
There is also no hierarchy to the recommendations, apart from the strength of the recommendation. “For any individual patient, a single option may be chosen at a particular time point, perhaps with or without other options, and may be reused in the future. For a given intervention, there might be a period of time over which it’s useful, and then the option might be changed,” Dr. Kolasinski noted.
Dr. Kolasinski advised making treatment decisions based on a patient’s disease severity, whether the patient uses medical devices, and in consideration of patient risk factors. “A history of injuries, surgical history, access to care, personal beliefs and preferences should all be brought to bear on decision making for osteoarthritis management,” she said.
The guidelines also advise considering a patient’s overall well-being and factors related to a patient’s perception of pain and function, such as mood disorders, altered sleep, chronic pain, impaired coping measures, and stress level. “Comprehensive management requires a broad assessment of how pain and function are affecting the patient with OA as a whole and recognizing that multiple options are available. They might be used in combination or change over time,” Dr. Kolasinski said.
The new guidelines place a strong emphasis on educational, behavioral, psychosocial, mind-body, and physical approaches. There are strong recommendations for the use of exercise, including aerobic, strengthening, neuromuscular, and aquatic exercise. Weight loss also carries a strong recommendation for patients with hip and knee OA, with a focus on group-based exercise, education, fitness and exercise goals, and a multidisciplinary approach using self-efficacy and self-management programs. The panels made a strong recommendation for tai chi to improve hip and knee OA. There are also strong recommendations for orthoses; aids and assistive devices such as canes, first carpometacarpal (CMC) orthoses, and tibiofemoral knee braces. Other interventions, such as Kinesio tape for first CMC joint and knee OA, hand orthoses, and patellofemoral knee braces, carried a conditional recommendation. Other conditional recommendations made by the panel were for acupuncture, thermal interventions, and radiofrequency ablation for patients with knee OA. Balance training for hip and knee OA, yoga for knee OA, and cognitive-behavioral therapy all were conditionally recommended by the panel.
The panel strongly recommended against the use of transcutaneous nerve stimulation for hip and knee OA, Dr. Kolasinski noted. The panel also conditionally recommended against use of modified shoes and pulsed vibration therapy in knee OA; lateral or medial wedged insoles, massage, and manual therapy with exercise in hip or knee OA; and iontophoresis in first CMC OA.
Tuhina Neogi, MD, PhD, chief of rheumatology at Boston University and member of the core team that developed the guidelines, said in her presentation the panel chose not to use the term “nonpharmacologic” in the guidelines because it may give patients a false impression that they are not receiving a treatment. “We really need to change our language and change the way in which we approach these conversations with our patients so that they don’t feel that they are not getting a treatment when we’re giving these recommendations,” she said.
Recommendations for, against pharmacologic approaches
The ACR has changed conditional recommendations for topical NSAIDs for knee and hand OA, oral NSAIDs, and intra-articular steroids for knee and hip OA into strong recommendations for the 2019 guidelines, Dr. Kolasinski said. While the 2012 guidelines conditionally recommended against topical capsaicin for knee OA, the new guidelines conditionally recommend it.
Other pharmacologic conditional recommendations included topical NSAIDs, chondroitin sulfate, and intra-articular corticosteroid injections for hand OA, acetaminophen, and duloxetine for knee OA.
With the new recommendations come changes that some rheumatologists and health care providers may find controversial. “I think that the practicing rheumatologist may be surprised that we have a recommendation against the use of hyaluronic acid in the knee as a conditional recommendation,” Dr. Kolasinski said. “The assessment of the literature at this point really reveals that there is equivalence between intra-articular hyaluronic acid injection and intra-articular saline injection, and so it was the feeling of the panel that, really, this was worth changing the recommendation from the 2012 guideline.”
The panel made strong recommendations against use of the following pharmacologic interventions:
- Bisphosphonates.
- Glucosamine sulfate.
- Combination glucosamine sulfate-chondroitin sulfate products.
- Hydroxychloroquine.
- Methotrexate.
- Intra-articular hyaluronic acid injections in hip OA.
- Chondroitin sulfate, platelet-rich plasma injections, and stem cell injections in hip and knee OA.
- Tumor necrosis factor (TNF) inhibitors.
- Interleukin-1–receptor antagonists.
Additionally, the panel made a conditional recommendation against topical capsaicin on the hand, colchicine, fish oil, vitamin D, intra-articular hyaluronic acid injections in the first CMC, and intra-articular botulinum toxin and prolotherapy in hip and knee OA.
The panel did not recommend for or against use of yoga for hip and hand OA, topical lidocaine, pregabalin, gabapentin, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors apart from duloxetine, tricyclic antidepressants, and anti-nerve growth factor agents.
While the panel conditionally recommended against use of opioids, they made a conditional recommendation for use of tramadol opioids, and there was “a heated discussion about that distinction,” Dr. Neogi noted in a discussion session at the meeting. “There was a recent observational study that indicated that tramadol may have an increased risk of [all-cause] mortality, but there are lots of issues of confounding by indication in that study.”
The patient panel also raised strong concerns about the ACR and the Arthritis Foundation coming out against opioids for OA management in their guidelines. “They don’t want to damn opioids, but they’re also concerned about a specialty society coming out strongly against opioids in the concern that their physicians may limit their access to opioids if they’re in a situation where nothing else is helping them,” Dr. Neogi said.
Dr. Kolasinski noted the guidelines will be published online in Arthritis & Rheumatology in December, and will appear in print in February of next year.
Dr. Kolasinski reported no relevant financial disclosures. Dr. Neogi reported relationships with EMD Serono, Merck, and Pfizer.
ATLANTA – New guidelines for management of osteoarthritis of the hand, knee, and hip from the American College of Rheumatology and the Arthritis Foundation lay out a wide range of treatment options without an algorithm or hierarchy, making strong recommendations for nondrug interventions and for tailoring plans to individual patient-level factors.
Since the ACR last released OA management guidelines in 2012, a number of recommendations have been added, changed, and removed, and the structure of the guidelines has also changed. For instance, the new OA guidelines include a broad list of management options, Sharon L. Kolasinski, MD, chair of the ACR guidelines panel and professor of clinical medicine in the division of rheumatology at the University of Pennsylvania, Philadelphia, said in a presentation at the annual meeting of the American College of Rheumatology.
“The new guideline emphasizes comprehensive management of patients with OA, rather than a stepwise algorithm in a linear manner,” she said.
There is also no hierarchy to the recommendations, apart from the strength of the recommendation. “For any individual patient, a single option may be chosen at a particular time point, perhaps with or without other options, and may be reused in the future. For a given intervention, there might be a period of time over which it’s useful, and then the option might be changed,” Dr. Kolasinski noted.
Dr. Kolasinski advised making treatment decisions based on a patient’s disease severity, whether the patient uses medical devices, and in consideration of patient risk factors. “A history of injuries, surgical history, access to care, personal beliefs and preferences should all be brought to bear on decision making for osteoarthritis management,” she said.
The guidelines also advise considering a patient’s overall well-being and factors related to a patient’s perception of pain and function, such as mood disorders, altered sleep, chronic pain, impaired coping measures, and stress level. “Comprehensive management requires a broad assessment of how pain and function are affecting the patient with OA as a whole and recognizing that multiple options are available. They might be used in combination or change over time,” Dr. Kolasinski said.
The new guidelines place a strong emphasis on educational, behavioral, psychosocial, mind-body, and physical approaches. There are strong recommendations for the use of exercise, including aerobic, strengthening, neuromuscular, and aquatic exercise. Weight loss also carries a strong recommendation for patients with hip and knee OA, with a focus on group-based exercise, education, fitness and exercise goals, and a multidisciplinary approach using self-efficacy and self-management programs. The panels made a strong recommendation for tai chi to improve hip and knee OA. There are also strong recommendations for orthoses; aids and assistive devices such as canes, first carpometacarpal (CMC) orthoses, and tibiofemoral knee braces. Other interventions, such as Kinesio tape for first CMC joint and knee OA, hand orthoses, and patellofemoral knee braces, carried a conditional recommendation. Other conditional recommendations made by the panel were for acupuncture, thermal interventions, and radiofrequency ablation for patients with knee OA. Balance training for hip and knee OA, yoga for knee OA, and cognitive-behavioral therapy all were conditionally recommended by the panel.
The panel strongly recommended against the use of transcutaneous nerve stimulation for hip and knee OA, Dr. Kolasinski noted. The panel also conditionally recommended against use of modified shoes and pulsed vibration therapy in knee OA; lateral or medial wedged insoles, massage, and manual therapy with exercise in hip or knee OA; and iontophoresis in first CMC OA.
Tuhina Neogi, MD, PhD, chief of rheumatology at Boston University and member of the core team that developed the guidelines, said in her presentation the panel chose not to use the term “nonpharmacologic” in the guidelines because it may give patients a false impression that they are not receiving a treatment. “We really need to change our language and change the way in which we approach these conversations with our patients so that they don’t feel that they are not getting a treatment when we’re giving these recommendations,” she said.
Recommendations for, against pharmacologic approaches
The ACR has changed conditional recommendations for topical NSAIDs for knee and hand OA, oral NSAIDs, and intra-articular steroids for knee and hip OA into strong recommendations for the 2019 guidelines, Dr. Kolasinski said. While the 2012 guidelines conditionally recommended against topical capsaicin for knee OA, the new guidelines conditionally recommend it.
Other pharmacologic conditional recommendations included topical NSAIDs, chondroitin sulfate, and intra-articular corticosteroid injections for hand OA, acetaminophen, and duloxetine for knee OA.
With the new recommendations come changes that some rheumatologists and health care providers may find controversial. “I think that the practicing rheumatologist may be surprised that we have a recommendation against the use of hyaluronic acid in the knee as a conditional recommendation,” Dr. Kolasinski said. “The assessment of the literature at this point really reveals that there is equivalence between intra-articular hyaluronic acid injection and intra-articular saline injection, and so it was the feeling of the panel that, really, this was worth changing the recommendation from the 2012 guideline.”
The panel made strong recommendations against use of the following pharmacologic interventions:
- Bisphosphonates.
- Glucosamine sulfate.
- Combination glucosamine sulfate-chondroitin sulfate products.
- Hydroxychloroquine.
- Methotrexate.
- Intra-articular hyaluronic acid injections in hip OA.
- Chondroitin sulfate, platelet-rich plasma injections, and stem cell injections in hip and knee OA.
- Tumor necrosis factor (TNF) inhibitors.
- Interleukin-1–receptor antagonists.
Additionally, the panel made a conditional recommendation against topical capsaicin on the hand, colchicine, fish oil, vitamin D, intra-articular hyaluronic acid injections in the first CMC, and intra-articular botulinum toxin and prolotherapy in hip and knee OA.
The panel did not recommend for or against use of yoga for hip and hand OA, topical lidocaine, pregabalin, gabapentin, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors apart from duloxetine, tricyclic antidepressants, and anti-nerve growth factor agents.
While the panel conditionally recommended against use of opioids, they made a conditional recommendation for use of tramadol opioids, and there was “a heated discussion about that distinction,” Dr. Neogi noted in a discussion session at the meeting. “There was a recent observational study that indicated that tramadol may have an increased risk of [all-cause] mortality, but there are lots of issues of confounding by indication in that study.”
The patient panel also raised strong concerns about the ACR and the Arthritis Foundation coming out against opioids for OA management in their guidelines. “They don’t want to damn opioids, but they’re also concerned about a specialty society coming out strongly against opioids in the concern that their physicians may limit their access to opioids if they’re in a situation where nothing else is helping them,” Dr. Neogi said.
Dr. Kolasinski noted the guidelines will be published online in Arthritis & Rheumatology in December, and will appear in print in February of next year.
Dr. Kolasinski reported no relevant financial disclosures. Dr. Neogi reported relationships with EMD Serono, Merck, and Pfizer.
REPORTING FROM ACR 2019
What’s new in hepatitis C: Four themes that dominated at the Liver Meeting
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
REPORTING FROM THE LIVER MEETING 2019
Depression linked to persistent opioid use after hysterectomy
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Hepatitis B debrief: Key themes that emerged at AASLD
BOSTON – Some of the most notable abstracts presented here at the annual meeting of the American Association for the Study of Liver Diseases dealt with key topics including the natural history of hepatitis B virus, novel treatment approaches, and prevention, according to Marc Ghany, MD.
During a special hepatitis debriefing session held on the last day of the conference, Dr. Ghany reviewed his key selections in hepatitis B virus (HBV) research, including the following:
Natural history
Steatohepatitis may worsen HBV-related liver injury, according to results of an analysis of liver biopsies from adult patients enrolled in a North American cohort study (Abstract 162). Investigators found that steatohepatitis was associated with a 1.6-fold increased risk of advanced fibrosis.
“For all patients with hepatitis B, I think it’s important to screen and manage metabolic abnormalities to prevent liver disease progression,” said Dr. Ghany, who is with the Liver Diseases Branch of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md.
Risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B was evaluated in two notable studies, Dr. Ghany said, including one that found no difference in risk of HCC development among white patients who received long-term entecavir versus those who received long-term tenofovir (Abstract 454). This stands in contrast to a previous and controversial finding, according to investigators, that HCC incidence was lower in Asian patients treated with tenofovir versus those treated with entecavir.
In the other study, investigators found that dual-antiplatelet therapy (DAPT) was associated with a lower risk of HCC versus aspirin monotherapy in patients with chronic hepatitis B (Abstract 934). Antiplatelet therapy was associated with a near 20% reduction in HCC risk, while DAPT as compared with aspirin monotherapy was linked to a near 30% reduction. “These are very provocative findings,” Dr. Ghany said. “Of course they need to be confirmed, but if so, may open new avenues of HCC chemoprevention.”
Novel therapies
Several new and promising drugs are undergoing clinical trials, including JNJ-64530440 (JNJ-0440), a novel class N capsid assembly modulator. In phase 1a data presented here at The Liver Meeting, the treatment was safe, well tolerated, and resulted in potent inhibition of viral replication (Abstract 0089). “We await further studies on its effect on functional cure,” Dr. Ghany told attendees.
Another treatment to watch is GSK3389404 (GSK404), a liver-targeted antisense oligonucleotide; in a phase 2 placebo-controlled study in patients with chronic hepatitis B on stable nucleos(t)ide therapy, this treatment had acceptable safety and produced dose-dependent declines in hepatitis B surface antigen (HBsAg), according to investigators (Abstract 0695). Dr. Ghany said this constitutes “proof of principle” that antisense oligonucleotides can decrease HBsAg levels.
In a phase 2 randomized, placebo-controlled study in virally suppressed adults with chronic hepatitis B, the toll-like receptor 8 (TLR8) agonist GS-9688 was safe and well tolerated, and resulted in dose-dependent pharmacodynamic changes, with 5% of patients experiencing a 1 log10 IU/mL or greater decline in HBsAg levels or an HBsAg loss by week 24 (Abstract 0697). This is a “promising approach” that merits further study, according to Dr. Ghany.
Prevention: Vaccination and screening
A trivalent HBV vaccine is superior to monovalent vaccine, according to results from the double-blind, randomized, controlled, phase 3 PROTECT study presented here at the meeting (Abstract LP13). Known as Sci-B-Vac, the mammalian cell-derived trivalent vaccine had higher response rates versus the recombinant monovalent vaccine Engerix-B in difficult-to-vaccinate populations, according to Dr. Ghany.
A separate report based on a national health insurance cohort study in Korea demonstrated that regular follow-up, that is to say, every 3-6 months, significantly reduced liver cancer–related mortality (Abstract 0159). Patients compliant with screening in the study not only had a 44% reduction in risk of death from HCC, but also were more likely to receive curative treatments (23.1% versus 15.1%). “Notwithstanding the limitations or cohort studies, I think these data reinforce the need to screen patients with chronic hepatitis B,” Dr. Ghany said.
He provided no disclosures in his presentation.
BOSTON – Some of the most notable abstracts presented here at the annual meeting of the American Association for the Study of Liver Diseases dealt with key topics including the natural history of hepatitis B virus, novel treatment approaches, and prevention, according to Marc Ghany, MD.
During a special hepatitis debriefing session held on the last day of the conference, Dr. Ghany reviewed his key selections in hepatitis B virus (HBV) research, including the following:
Natural history
Steatohepatitis may worsen HBV-related liver injury, according to results of an analysis of liver biopsies from adult patients enrolled in a North American cohort study (Abstract 162). Investigators found that steatohepatitis was associated with a 1.6-fold increased risk of advanced fibrosis.
“For all patients with hepatitis B, I think it’s important to screen and manage metabolic abnormalities to prevent liver disease progression,” said Dr. Ghany, who is with the Liver Diseases Branch of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md.
Risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B was evaluated in two notable studies, Dr. Ghany said, including one that found no difference in risk of HCC development among white patients who received long-term entecavir versus those who received long-term tenofovir (Abstract 454). This stands in contrast to a previous and controversial finding, according to investigators, that HCC incidence was lower in Asian patients treated with tenofovir versus those treated with entecavir.
In the other study, investigators found that dual-antiplatelet therapy (DAPT) was associated with a lower risk of HCC versus aspirin monotherapy in patients with chronic hepatitis B (Abstract 934). Antiplatelet therapy was associated with a near 20% reduction in HCC risk, while DAPT as compared with aspirin monotherapy was linked to a near 30% reduction. “These are very provocative findings,” Dr. Ghany said. “Of course they need to be confirmed, but if so, may open new avenues of HCC chemoprevention.”
Novel therapies
Several new and promising drugs are undergoing clinical trials, including JNJ-64530440 (JNJ-0440), a novel class N capsid assembly modulator. In phase 1a data presented here at The Liver Meeting, the treatment was safe, well tolerated, and resulted in potent inhibition of viral replication (Abstract 0089). “We await further studies on its effect on functional cure,” Dr. Ghany told attendees.
Another treatment to watch is GSK3389404 (GSK404), a liver-targeted antisense oligonucleotide; in a phase 2 placebo-controlled study in patients with chronic hepatitis B on stable nucleos(t)ide therapy, this treatment had acceptable safety and produced dose-dependent declines in hepatitis B surface antigen (HBsAg), according to investigators (Abstract 0695). Dr. Ghany said this constitutes “proof of principle” that antisense oligonucleotides can decrease HBsAg levels.
In a phase 2 randomized, placebo-controlled study in virally suppressed adults with chronic hepatitis B, the toll-like receptor 8 (TLR8) agonist GS-9688 was safe and well tolerated, and resulted in dose-dependent pharmacodynamic changes, with 5% of patients experiencing a 1 log10 IU/mL or greater decline in HBsAg levels or an HBsAg loss by week 24 (Abstract 0697). This is a “promising approach” that merits further study, according to Dr. Ghany.
Prevention: Vaccination and screening
A trivalent HBV vaccine is superior to monovalent vaccine, according to results from the double-blind, randomized, controlled, phase 3 PROTECT study presented here at the meeting (Abstract LP13). Known as Sci-B-Vac, the mammalian cell-derived trivalent vaccine had higher response rates versus the recombinant monovalent vaccine Engerix-B in difficult-to-vaccinate populations, according to Dr. Ghany.
A separate report based on a national health insurance cohort study in Korea demonstrated that regular follow-up, that is to say, every 3-6 months, significantly reduced liver cancer–related mortality (Abstract 0159). Patients compliant with screening in the study not only had a 44% reduction in risk of death from HCC, but also were more likely to receive curative treatments (23.1% versus 15.1%). “Notwithstanding the limitations or cohort studies, I think these data reinforce the need to screen patients with chronic hepatitis B,” Dr. Ghany said.
He provided no disclosures in his presentation.
BOSTON – Some of the most notable abstracts presented here at the annual meeting of the American Association for the Study of Liver Diseases dealt with key topics including the natural history of hepatitis B virus, novel treatment approaches, and prevention, according to Marc Ghany, MD.
During a special hepatitis debriefing session held on the last day of the conference, Dr. Ghany reviewed his key selections in hepatitis B virus (HBV) research, including the following:
Natural history
Steatohepatitis may worsen HBV-related liver injury, according to results of an analysis of liver biopsies from adult patients enrolled in a North American cohort study (Abstract 162). Investigators found that steatohepatitis was associated with a 1.6-fold increased risk of advanced fibrosis.
“For all patients with hepatitis B, I think it’s important to screen and manage metabolic abnormalities to prevent liver disease progression,” said Dr. Ghany, who is with the Liver Diseases Branch of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md.
Risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B was evaluated in two notable studies, Dr. Ghany said, including one that found no difference in risk of HCC development among white patients who received long-term entecavir versus those who received long-term tenofovir (Abstract 454). This stands in contrast to a previous and controversial finding, according to investigators, that HCC incidence was lower in Asian patients treated with tenofovir versus those treated with entecavir.
In the other study, investigators found that dual-antiplatelet therapy (DAPT) was associated with a lower risk of HCC versus aspirin monotherapy in patients with chronic hepatitis B (Abstract 934). Antiplatelet therapy was associated with a near 20% reduction in HCC risk, while DAPT as compared with aspirin monotherapy was linked to a near 30% reduction. “These are very provocative findings,” Dr. Ghany said. “Of course they need to be confirmed, but if so, may open new avenues of HCC chemoprevention.”
Novel therapies
Several new and promising drugs are undergoing clinical trials, including JNJ-64530440 (JNJ-0440), a novel class N capsid assembly modulator. In phase 1a data presented here at The Liver Meeting, the treatment was safe, well tolerated, and resulted in potent inhibition of viral replication (Abstract 0089). “We await further studies on its effect on functional cure,” Dr. Ghany told attendees.
Another treatment to watch is GSK3389404 (GSK404), a liver-targeted antisense oligonucleotide; in a phase 2 placebo-controlled study in patients with chronic hepatitis B on stable nucleos(t)ide therapy, this treatment had acceptable safety and produced dose-dependent declines in hepatitis B surface antigen (HBsAg), according to investigators (Abstract 0695). Dr. Ghany said this constitutes “proof of principle” that antisense oligonucleotides can decrease HBsAg levels.
In a phase 2 randomized, placebo-controlled study in virally suppressed adults with chronic hepatitis B, the toll-like receptor 8 (TLR8) agonist GS-9688 was safe and well tolerated, and resulted in dose-dependent pharmacodynamic changes, with 5% of patients experiencing a 1 log10 IU/mL or greater decline in HBsAg levels or an HBsAg loss by week 24 (Abstract 0697). This is a “promising approach” that merits further study, according to Dr. Ghany.
Prevention: Vaccination and screening
A trivalent HBV vaccine is superior to monovalent vaccine, according to results from the double-blind, randomized, controlled, phase 3 PROTECT study presented here at the meeting (Abstract LP13). Known as Sci-B-Vac, the mammalian cell-derived trivalent vaccine had higher response rates versus the recombinant monovalent vaccine Engerix-B in difficult-to-vaccinate populations, according to Dr. Ghany.
A separate report based on a national health insurance cohort study in Korea demonstrated that regular follow-up, that is to say, every 3-6 months, significantly reduced liver cancer–related mortality (Abstract 0159). Patients compliant with screening in the study not only had a 44% reduction in risk of death from HCC, but also were more likely to receive curative treatments (23.1% versus 15.1%). “Notwithstanding the limitations or cohort studies, I think these data reinforce the need to screen patients with chronic hepatitis B,” Dr. Ghany said.
He provided no disclosures in his presentation.
REPORTING FROM THE LIVER MEETING 2019
















