User login
Potential new treatment for REM sleep behavior disorder
Dual orexin receptor antagonists (DORAs), a class of drugs approved to treat insomnia, may also be effective for rapid eye movement sleep behavior disorder (RBD), a study suggests.
About 3 million people in the United States have RBD, which is often a precursor to Parkinson’s disease. People with the disorder act out their dreams by talking, flailing their arms and legs, punching, kicking, and exhibiting other behaviors while asleep.
Researchers used an animal model for the study, which they say is the first to identify a new form of treatment for RBD.
“REM behavior disorder is difficult to treat, and the treatments are mostly limited to clonazepam and melatonin,” which may have side effects, senior investigator Andrew Varga, MD, PhD, associate professor of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai, New York, told this news organization. “We’re using something completely different, which raises the possibility this might be something useful for REM behavior disorders.”
The findings, with Mount Sinai assistant professor Korey Kam, PhD, as lead author, were published online in the Journal of Neuroscience.
A new model for RBD?
RBD can signal risk for synucleinopathies, a group of neurological conditions such as Parkinson’s disease that involve the formation of clumps of alpha-synuclein protein in the brain.
Prior research on RBD was done in synucleinopathy mouse models. For this study, however, researchers used a tauopathy mouse model to investigate how the abnormal accumulation of tau protein might affect RBD.
Researchers collected data on biophysical properties when the mice were awake and in REM and non-REM sleep. They examined length of sleep, transitions from waking to sleep, and how some factors are related to age.
Nearly a third of the older animals showed behaviors similar to REM sleep behavior disorder in humans, including chewing and limb extension.
But after researchers administered a DORA medication twice during a 24-hour period, they noted that the medication not only helped the animals fall asleep faster and for longer, it also reduced levels of dream enactment that are a hallmark of RBD.
The ‘bigger highlight’
Finding RBD behaviors in a tauopathy animal model was surprising, Dr. Varga said, because RBD has been previously linked to synucleinopathies. There was no known correlation between RBD and abnormal accumulation of tau.
Another unexpected finding was the detection of RBD in some of the younger animals, who had not yet shown evidence of tau accumulation.
“It appears to be a biomarker or a signature of something that’s going on that predicts the impending tauopathy at a time where there is very little, or no, tau pathology going on in the brain,” Dr. Varga said.
If RBD is an early predictor of future tau accumulation, the model could guide future prevention and treatment. However, the more important finding is the potential new treatment for the condition.
“The bigger highlight here is less about what’s causing the RBD [than about] what you can do to make it better,” he said.
The next step in the work is to study whether the effect of DORAs on RBD seen in this tauopathy mouse model is evidenced in other animals and whether it is effective in humans with RBD, Dr. Varga said.
The study was funded by the Alzheimer’s Association and Merck Investigator Studies Program. Dr. Kam, Dr. Varga, and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dual orexin receptor antagonists (DORAs), a class of drugs approved to treat insomnia, may also be effective for rapid eye movement sleep behavior disorder (RBD), a study suggests.
About 3 million people in the United States have RBD, which is often a precursor to Parkinson’s disease. People with the disorder act out their dreams by talking, flailing their arms and legs, punching, kicking, and exhibiting other behaviors while asleep.
Researchers used an animal model for the study, which they say is the first to identify a new form of treatment for RBD.
“REM behavior disorder is difficult to treat, and the treatments are mostly limited to clonazepam and melatonin,” which may have side effects, senior investigator Andrew Varga, MD, PhD, associate professor of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai, New York, told this news organization. “We’re using something completely different, which raises the possibility this might be something useful for REM behavior disorders.”
The findings, with Mount Sinai assistant professor Korey Kam, PhD, as lead author, were published online in the Journal of Neuroscience.
A new model for RBD?
RBD can signal risk for synucleinopathies, a group of neurological conditions such as Parkinson’s disease that involve the formation of clumps of alpha-synuclein protein in the brain.
Prior research on RBD was done in synucleinopathy mouse models. For this study, however, researchers used a tauopathy mouse model to investigate how the abnormal accumulation of tau protein might affect RBD.
Researchers collected data on biophysical properties when the mice were awake and in REM and non-REM sleep. They examined length of sleep, transitions from waking to sleep, and how some factors are related to age.
Nearly a third of the older animals showed behaviors similar to REM sleep behavior disorder in humans, including chewing and limb extension.
But after researchers administered a DORA medication twice during a 24-hour period, they noted that the medication not only helped the animals fall asleep faster and for longer, it also reduced levels of dream enactment that are a hallmark of RBD.
The ‘bigger highlight’
Finding RBD behaviors in a tauopathy animal model was surprising, Dr. Varga said, because RBD has been previously linked to synucleinopathies. There was no known correlation between RBD and abnormal accumulation of tau.
Another unexpected finding was the detection of RBD in some of the younger animals, who had not yet shown evidence of tau accumulation.
“It appears to be a biomarker or a signature of something that’s going on that predicts the impending tauopathy at a time where there is very little, or no, tau pathology going on in the brain,” Dr. Varga said.
If RBD is an early predictor of future tau accumulation, the model could guide future prevention and treatment. However, the more important finding is the potential new treatment for the condition.
“The bigger highlight here is less about what’s causing the RBD [than about] what you can do to make it better,” he said.
The next step in the work is to study whether the effect of DORAs on RBD seen in this tauopathy mouse model is evidenced in other animals and whether it is effective in humans with RBD, Dr. Varga said.
The study was funded by the Alzheimer’s Association and Merck Investigator Studies Program. Dr. Kam, Dr. Varga, and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dual orexin receptor antagonists (DORAs), a class of drugs approved to treat insomnia, may also be effective for rapid eye movement sleep behavior disorder (RBD), a study suggests.
About 3 million people in the United States have RBD, which is often a precursor to Parkinson’s disease. People with the disorder act out their dreams by talking, flailing their arms and legs, punching, kicking, and exhibiting other behaviors while asleep.
Researchers used an animal model for the study, which they say is the first to identify a new form of treatment for RBD.
“REM behavior disorder is difficult to treat, and the treatments are mostly limited to clonazepam and melatonin,” which may have side effects, senior investigator Andrew Varga, MD, PhD, associate professor of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai, New York, told this news organization. “We’re using something completely different, which raises the possibility this might be something useful for REM behavior disorders.”
The findings, with Mount Sinai assistant professor Korey Kam, PhD, as lead author, were published online in the Journal of Neuroscience.
A new model for RBD?
RBD can signal risk for synucleinopathies, a group of neurological conditions such as Parkinson’s disease that involve the formation of clumps of alpha-synuclein protein in the brain.
Prior research on RBD was done in synucleinopathy mouse models. For this study, however, researchers used a tauopathy mouse model to investigate how the abnormal accumulation of tau protein might affect RBD.
Researchers collected data on biophysical properties when the mice were awake and in REM and non-REM sleep. They examined length of sleep, transitions from waking to sleep, and how some factors are related to age.
Nearly a third of the older animals showed behaviors similar to REM sleep behavior disorder in humans, including chewing and limb extension.
But after researchers administered a DORA medication twice during a 24-hour period, they noted that the medication not only helped the animals fall asleep faster and for longer, it also reduced levels of dream enactment that are a hallmark of RBD.
The ‘bigger highlight’
Finding RBD behaviors in a tauopathy animal model was surprising, Dr. Varga said, because RBD has been previously linked to synucleinopathies. There was no known correlation between RBD and abnormal accumulation of tau.
Another unexpected finding was the detection of RBD in some of the younger animals, who had not yet shown evidence of tau accumulation.
“It appears to be a biomarker or a signature of something that’s going on that predicts the impending tauopathy at a time where there is very little, or no, tau pathology going on in the brain,” Dr. Varga said.
If RBD is an early predictor of future tau accumulation, the model could guide future prevention and treatment. However, the more important finding is the potential new treatment for the condition.
“The bigger highlight here is less about what’s causing the RBD [than about] what you can do to make it better,” he said.
The next step in the work is to study whether the effect of DORAs on RBD seen in this tauopathy mouse model is evidenced in other animals and whether it is effective in humans with RBD, Dr. Varga said.
The study was funded by the Alzheimer’s Association and Merck Investigator Studies Program. Dr. Kam, Dr. Varga, and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROSCIENCE
Meet the JCOM Author with Dr. Barkoudah: EHR Interventions to Improve Glucagon Prescription Rates for Individuals With T1DM
Glucagon Prescription Rates for Individuals With Type 1 Diabetes Mellitus Following Implementation of an Electronic Health Records Intervention
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.
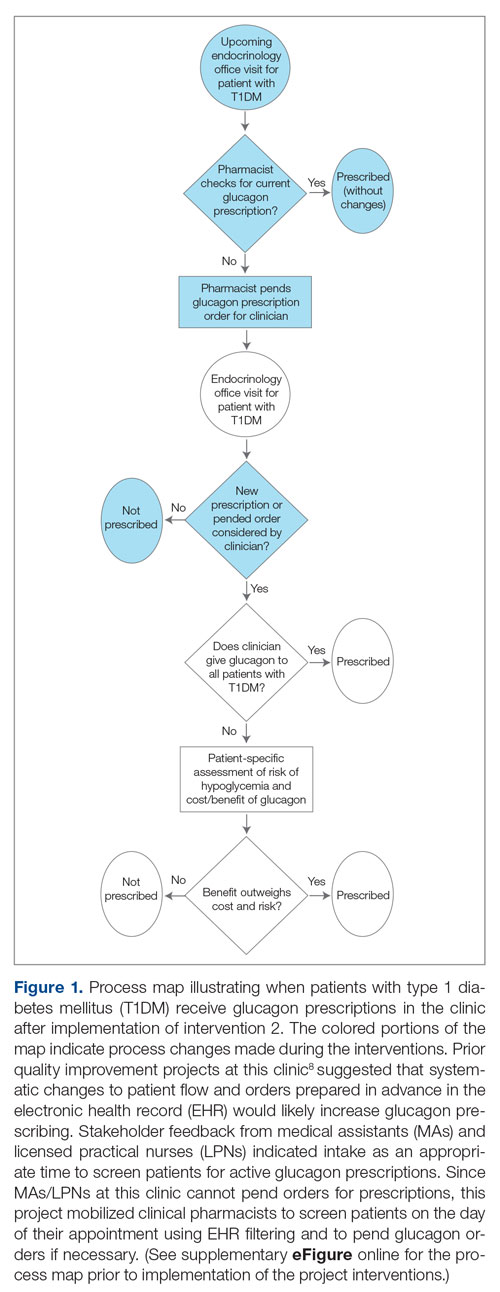
In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).
This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
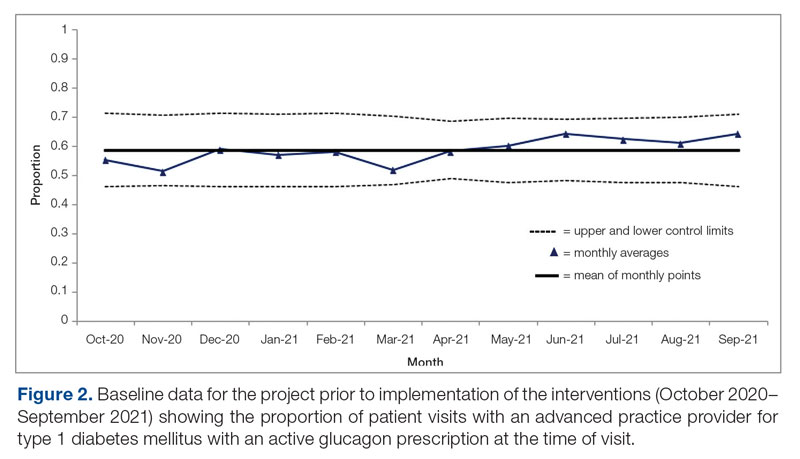
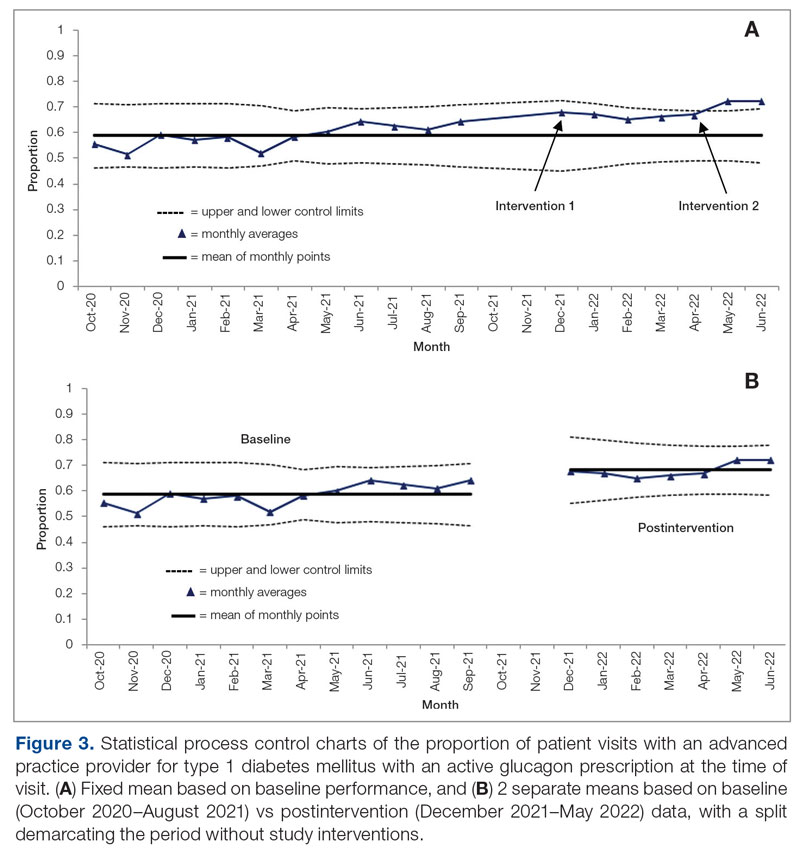
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).
Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.
Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.

In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).

This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).

Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.

Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.

In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).

This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).

Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.

Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
Differences in 30-Day Readmission Rates in Older Adults With Dementia
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
Patient Safety in Transitions of Care: Addressing Discharge Communication Gaps and the Potential of the Teach-Back Method
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
Study 1 Overview (Trivedi et al)
Objective: This observational quality improvement study aimed to evaluate the discharge communication practices in internal medicine services at 2 urban academic teaching hospitals, specifically focusing on patient education and counseling in 6 key discharge communication domains.
Design: Observations were conducted over a 13-month period from September 2018 through October 2019, following the Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines.
Setting and participants: The study involved a total of 33 English- and Spanish-speaking patients purposefully selected from the “discharge before noon” list at 2 urban tertiary-care teaching hospitals. A total of 155 observation hours were accumulated, with an average observation time of 4.7 hours per patient on the day of discharge.
Main outcome measures: The study assessed 6 discharge communication domains: (1) the name and function of medication changes, (2) the purpose of postdischarge appointments, (3) disease self-management, (4) red flags or warning signs for complications, (5) teach-back techniques to confirm patient understanding, and (6) staff solicitation of patient questions or concerns.
Main results: The study found several gaps in discharge communication practices. Among the 29 patients with medication changes, 28% were not informed about the name and basic function of the changes, while 59% did not receive counseling on the purpose for the medication change. In terms of postdischarge appointments, 48% of patients were not told the purpose of these appointments. Moreover, 54% of patients did not receive counseling on self-management of their primary discharge diagnosis or other diagnoses, and 73% were not informed about symptom expectations or the expected course of their illness after leaving the hospital. Most patients (82%) were not counseled on red-flag signs and symptoms that should prompt immediate return to care.
Teach-back techniques, which are critical for ensuring patient understanding, were used in only 3% of cases, and 85% of patients were not asked by health care providers if there might be barriers to following the care plan. Less than half (42%) of the patients were asked if they had any questions, with most questions being logistical and often deferred to another team member or met with uncertainty. Of note, among the 33 patients, only 2 patients received extensive information that covered 5 or 6 out of 6 discharge communication domains.
The study found variable roles in who communicated what aspects of discharge education, with most domains being communicated in an ad hoc manner and no clear pattern of responsibility. However, 2 exceptions were observed: nurses were more likely to provide information about new or changed medications and follow-up appointments, and the only example of teach-back was conducted by an attending physician.
Conclusion: The study highlights a significant need for improved discharge techniques to enhance patient safety and quality of care upon leaving the hospital. Interventions should focus on increasing transparency in patient education and understanding, clarifying assumptions of roles among the interprofessional team, and implementing effective communication strategies and system redesigns that foster patient-centered discharge education. Also, the study revealed that some patients received more robust discharge education than others, indicating systemic inequality in the patient experience. Further studies are needed to explore the development and assessment of such interventions to ensure optimal patient outcomes and equal care following hospital discharge.
Study 2 Overview (Marks et al)
Objective: This study aimed to investigate the impact of a nurse-led discharge medication education program, Teaching Important Medication Effects (TIME), on patients’ new medication knowledge at discharge and 48 to 72 hours post discharge. The specific objectives were to identify patients’ priority learning needs, evaluate the influence of TIME on patients’ new medication knowledge before and after discharge, and assess the effect of TIME on patients’ experience and satisfaction with medication education.
Design: The study employed a longitudinal pretest/post-test, 2-group design involving 107 randomly selected medical-surgical patients from an academic hospital. Participants were interviewed before and within 72 hours after discharge following administration of medication instructions. Bivariate analyses were performed to assess demographic and outcome variable differences between groups.
Setting and participants: Conducted on a 24-bed medical-surgical unit at a large Magnet® hospital over 18 months (2018-2019), the study included patients with at least 1 new medication, aged 18 years or older, able to read and speak English or Spanish, admitted from home with a minimum 1 overnight stay, and planning to return home post discharge. Excluded were cognitively impaired patients, those assigned to a resource pool nurse without TIME training, and those having a research team member assigned. Participants were randomly selected from a computerized list of patients scheduled for discharge.
Main outcome measures: Primary outcome measures included patients’ new medication knowledge before and after discharge and patients’ experience and satisfaction with medication education.
Main results: The usual care (n = 52) and TIME (n = 55) patients had similar baseline demographic characteristics. The study revealed that almost all patients in both usual care and TIME groups were aware of their new medication and its purpose at discharge. However, differences were observed in medication side effect responses, with 72.5% of the usual-care group knowing side effects compared to 94.3% of the TIME group (P = .003). Additionally, 81.5% of the usual-care group understood the medication purpose compared to 100% of the TIME group (P = .02). During the 48- to 72-hour postdischarge calls, consistent responses were found from both groups regarding knowledge of new medication, medication name, and medication purpose. Similar to discharge results, differences in medication side effect responses were observed, with 75.8% of the usual care group correctly identifying at least 1 medication side effect compared to 93.9% of the TIME group (P = .04). TIME was associated with higher satisfaction with medication education compared to usual care (97% vs. 46.9%, P < .001).
Conclusion: The nurse-led discharge medication education program TIME effectively enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge. The program also significantly improved patients’ experience and satisfaction with medication education. These findings indicate that TIME is a valuable tool for augmenting patient education and medication adherence in a hospital setting. By incorporating the teach-back method, TIME offers a structured approach to educating patients about their medications at hospital discharge, leading to improved care transitions.
Commentary
Suboptimal communication between patients, caregivers, and providers upon hospital discharge is a major contributor to patients’ inadequate understanding of postdischarge care plans. This inadequate understanding leads to preventable harms, such as medication errors, adverse events, emergency room visits, and costly hospital readmissions.1 The issue is further exacerbated by a lack of clarity among health care team members’ respective roles in providing information that optimizes care transitions during the discharge communication process. Moreover, low health literacy, particularly prevalent among seniors, those from disadvantaged backgrouds, and those with lower education attainment or chronic illnesses, create additional barriers to effective discharge communication. A potential solution to this problem is the adoption of effective teaching strategies, specifically the teach-back method. This method employs techniques that ensure patients’ understanding and recall of new information regardless of health literacy, and places accountability on clinicians rather than patients. By closing communication gaps between clinicians and patients, the teach-back method can reduce hospital readmissions, hospital-acquired conditions, and mortality rates, while improving patient satisfaction with health care instructions and the overall hospital experience.2
Study 1, by Trivedi et al, and study 2, by Marks et al, aimed to identify and address problems related to poor communication between patients and health care team members at hospital discharge. Specifically, study 1 examined routine discharge communication practices to determine communication gaps, while study 2 evaluated a nurse-led teach-back intervention program designed to improve patients’ medication knowledge and satisfaction. These distinct objectives and designs reflected the unique ways each study approached the challenges associated with care transitions at the time of hospital discharge.
Study 1 used direct observation of patient-practitioner interactions to evaluate routine discharge communication practices in internal medicine services at 2 urban academic teaching hospitals. In the 33 patients observed, significant gaps in discharge communication practices were identified in the domains of medication changes, postdischarge appointments, disease self-management, and red flags or warning signs. Unsurprisingly, most of these domains were communicated in an ad hoc manner by members of the health care team without a clear pattern of responsibility in reference to patient discharge education, and teach-back was seldom used. These findings underscore the need for improved discharge techniques, effective communication strategies, and clarification of roles among the interprofessional team to enhance the safety, quality of care, and overall patient experience during hospital discharge.
Study 2 aimed to augment the hospital discharge communication process by implementing a nurse-led discharge medication education program (TIME), which targeted patients’ priority learning needs, new medication knowledge, and satisfaction with medication education. In the 107 patients assessed, this teach-back method enhanced patients’ new medication knowledge at discharge and 48 to 72 hours after discharge, as well as improved patients’ experience and satisfaction with medication education. These results suggest that a teach-back method such as the TIME program could be a solution to care transition problems identified in the Trivedi et al study by providing a structured approach to patient education and enhancing communication practices during the hospital discharge process. Thus, by implementing the TIME program, hospitals may improve patient outcomes, safety, and overall quality of care upon leaving the hospital.
Applications for Clinical Practice and System Implementation
Care transition at the time of hospital discharge is a particularly pivotal period in the care of vulnerable individuals. There is growing literature, including studies discussed in this review, to indicate that by focusing on improving patient-practitioner communication during the discharge process and using strategies such as the teach-back method, health care professionals can better prepare patients for self-management in the post-acute period and help them make informed decisions about their care. This emphasis on care-transition communication strategies may lead to a reduction in medication errors, adverse events, and hospital readmissions, ultimately improving patient outcomes and satisfaction. Barriers to system implementation of such strategies may include competing demands and responsibilities of busy practitioners as well as the inherent complexities associated with hospital discharge. Creative solutions, such as the utilization of telehealth and early transition-of-care visits, represent some potential approaches to counter these barriers.
While both studies illustrated barriers and facilitators of hospital discharge communication, each study had limitations that impacted their generalizability to real-world clinical practice. Limitations in study 1 included a small sample size, purposive sampling method, and a focus on planned discharges in a teaching hospital, which may introduce selection bias. The study’s findings may not be generalizable to unplanned discharges, patients who do not speak English or Spanish, or nonteaching hospitals. Additionally, the data were collected before the COVID-19 pandemic, which could have further impacted discharge education practices. The study also revealed that some patients received more robust discharge education than others, which indicated systemic inequality in the patient experience. Further research is required to address this discrepancy. Limitations in study 2 included a relatively small and homogeneous sample, with most participants being younger, non-Hispanic White, English-speaking, and well-educated. This lack of diversity may limit the generalizability of the findings. Furthermore, the study did not evaluate the patients’ knowledge of medication dosage and focused only on new medications. Future studies should examine the effect of teach-back on a broader range of self-management topics in preparation for discharge, while also including a more diverse population to account for factors related to social determinants of health. Taken together, further research is needed to address these limitations and ensure more generalizable results that can more broadly improve discharge education and care transitions that bridge acute and post-acute care.
Practice Points
- There is a significant need for improved discharge strategies to enhance patient safety and quality of care upon leaving the hospital.
- Teach-back method may offer a structured approach to educating patients about their medications at hospital discharge and improve care transitions.
–Yuka Shichijo, MD, and Fred Ko, MD, Mount Sinai Beth Israel Hospital, New York, NY
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
1. Snow V, Beck D, Budnitz T, Miller DC, Potter J, Wears RL, Weiss KB, Williams MV; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of care consensus policy statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971-976. doi:10.1007/s11606-009-0969-x
2. Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed. Pract. 2019;36(6):284-289.
No expiration date for sex
For health professionals, the thought that our parents and grandparents don’t have sex – or didn’t – might be comforting.
The reality is that, for a significant proportion of our older patients, sex has no use-by date. Humans are sexual beings throughout their lives, yet the culture has concealed that fact.
According to Rome, the purpose of sex is to make children. According to Hollywood, sex is only for the young, the healthy, and the beautiful. For the medical profession, sex consists mainly of risks or dysfunctions.
The results of these biases? Sexuality and intimacy are essential elements for quality of life, with clear physical, emotional, and relational benefits.
Let’s look at the data when researchers dared to ask seniors about their sexuality.
We start with the 2015 U.K. national research on sexuality. The study found a link between age and a decline in various aspects of sexual activity – but not a zeroing-out. For example, among men aged 70-79, 59% reported having had sex in the past year, with 19% having intercourse at least twice a month and 18% masturbating at least that often. Above age 80, those numbers dropped to 39%, 6%, and 5%, respectively. The reason behind the declines? A combination of taboo, fear of disease, use of medications or other interventions that disrupt sexual function or cause disfigurement, and a little bit of age itself.
What about women? Among women ages 70-79, 39% said they’d had sex in the past year, with 6% having intercourse at least twice per month and 5% masturbating two times or more monthly. Above age 80, those numbers were 10%, 4.5%, and 1%, respectively. Driving the falloff in women were the same factors as for men, plus the sad reality that many heterosexual women become widowed because their older male partners die earlier.
The male-female difference also reflects lower levels of testosterone in women. And, because women say they value intimacy more than performance, we have two explanations for their lower frequency of masturbation. After all, a lot of intimacy occurs without either intercourse or masturbation.
Surprising and relevant is the amount of distress – or rather, their relative lack thereof – older patients report because of sexual problems. At age 18-44, 11% of U.S. women indicated sexual distress; at age 45-64, the figure was 15%; and at age 65 and up, 9%.
For clinicians, those figures should prompt us to look more closely at alternative forms of sexual expression – those not involving intercourse or masturbation – in the aged, a field physicians typically do not consider.
Although dyspareunia or erectile problems affect many in long-standing relationships, neither is a reason to abstain from sexual pleasure. Indeed, in many couples, oral sex will replace vaginal intercourse, and if urinary, fecal, or flatal incontinence intrude, couples often waive oral sex in favor of more cuddling, kissing, digital stimulation, and other forms of sexual pleasure.
What about the expiry date for sex?
Fascinating research from Nils Beckman, PhD, and colleagues found that the sex drive persists even as people (and men in particular) reach their 100th year. Dr. Beckman’s group interviewed 269 Swedish seniors, all without dementia, at age 97. Sexual desire was affirmed by 27% of men and 5% of women in the survey. Among the men, 32% said they still had sexual thoughts, compared with 18% of women. Meanwhile, 26% of the men and 15% of the women said they missed sexual activity.
What should clinicians do with this information? First, we could start talking about sex with our older patients. According to the 97-year-old Swedes, most want us to! More than 8 in 10 of both women and men in the survey expressed positive views about questions on sexuality. And please don’t be scared to address the subject in the single senior. They, too, can have a sexual or relationship issue and are happy when we raise the subject. They’re not scared to talk about masturbation, either.
When caring for those with chronic diseases, cancer, in the course of physical rehabilitation, and even in the last phase of life, the clinical experience indicates that our patients are happy when we address sexuality and intimacy. Doing so can open the door to the admission of a problem and a corresponding solution, a lubricant or a PDE5 inhibitor.
But sometimes the solution is the conversation itself: Roughly 25% of patients are sufficiently helped simply by talking about sex. Addressing the importance of sexual pleasure is nearly always valuable.
Here are a few ice-breakers I find helpful:
- Did taking this medication change aspects of sexuality? If so, does that bother you?
- Knowing that continuing intimacy is healthy, do you mind if I address that subject?
- We know that aspects of sexuality and intimacy are healthy. Without a partner, some people become sexually isolated. Would you like to talk about that?’
If addressing sexuality has benefits, what about sex itself?
We are gradually learning more about the many short-, intermediate-, and long-term health benefits of solo and joint sexual activity. Short-term benefits include muscle relaxation, pain relief (even, perhaps ironically, for headaches), and better sleep – all pretty valuable for older adults. Examples of intermediate-term benefits include stress relief and less depression. Research from the United States has found that hugging can reduce the concentrations of proinflammatory cytokines, and kissing positively influences cholesterol levels.
Finally, while the long-term benefits of sex might be less relevant for seniors, they do exist.
Among them are delayed onset of dementia and a substantial reduction in cardiovascular and cerebrovascular problems in men. More sex has been linked to longevity, with men benefiting a bit more than women from going through the entire process, including an orgasm, whereas women appear to gain from having a “satisfying” sex life, which does not always require an orgasm.
Let us not forget that these benefits apply to both patients and clinicians alike. Addressing intimacy and sexuality can ease eventual sexual concerns and potentially create a stronger clinician-patient relationship.
Dr. Gianotten, MD is emeritus senior lecturer in medical sexology, Erasmus University Medical Centre, Rotterdam, the Netherlands. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
For health professionals, the thought that our parents and grandparents don’t have sex – or didn’t – might be comforting.
The reality is that, for a significant proportion of our older patients, sex has no use-by date. Humans are sexual beings throughout their lives, yet the culture has concealed that fact.
According to Rome, the purpose of sex is to make children. According to Hollywood, sex is only for the young, the healthy, and the beautiful. For the medical profession, sex consists mainly of risks or dysfunctions.
The results of these biases? Sexuality and intimacy are essential elements for quality of life, with clear physical, emotional, and relational benefits.
Let’s look at the data when researchers dared to ask seniors about their sexuality.
We start with the 2015 U.K. national research on sexuality. The study found a link between age and a decline in various aspects of sexual activity – but not a zeroing-out. For example, among men aged 70-79, 59% reported having had sex in the past year, with 19% having intercourse at least twice a month and 18% masturbating at least that often. Above age 80, those numbers dropped to 39%, 6%, and 5%, respectively. The reason behind the declines? A combination of taboo, fear of disease, use of medications or other interventions that disrupt sexual function or cause disfigurement, and a little bit of age itself.
What about women? Among women ages 70-79, 39% said they’d had sex in the past year, with 6% having intercourse at least twice per month and 5% masturbating two times or more monthly. Above age 80, those numbers were 10%, 4.5%, and 1%, respectively. Driving the falloff in women were the same factors as for men, plus the sad reality that many heterosexual women become widowed because their older male partners die earlier.
The male-female difference also reflects lower levels of testosterone in women. And, because women say they value intimacy more than performance, we have two explanations for their lower frequency of masturbation. After all, a lot of intimacy occurs without either intercourse or masturbation.
Surprising and relevant is the amount of distress – or rather, their relative lack thereof – older patients report because of sexual problems. At age 18-44, 11% of U.S. women indicated sexual distress; at age 45-64, the figure was 15%; and at age 65 and up, 9%.
For clinicians, those figures should prompt us to look more closely at alternative forms of sexual expression – those not involving intercourse or masturbation – in the aged, a field physicians typically do not consider.
Although dyspareunia or erectile problems affect many in long-standing relationships, neither is a reason to abstain from sexual pleasure. Indeed, in many couples, oral sex will replace vaginal intercourse, and if urinary, fecal, or flatal incontinence intrude, couples often waive oral sex in favor of more cuddling, kissing, digital stimulation, and other forms of sexual pleasure.
What about the expiry date for sex?
Fascinating research from Nils Beckman, PhD, and colleagues found that the sex drive persists even as people (and men in particular) reach their 100th year. Dr. Beckman’s group interviewed 269 Swedish seniors, all without dementia, at age 97. Sexual desire was affirmed by 27% of men and 5% of women in the survey. Among the men, 32% said they still had sexual thoughts, compared with 18% of women. Meanwhile, 26% of the men and 15% of the women said they missed sexual activity.
What should clinicians do with this information? First, we could start talking about sex with our older patients. According to the 97-year-old Swedes, most want us to! More than 8 in 10 of both women and men in the survey expressed positive views about questions on sexuality. And please don’t be scared to address the subject in the single senior. They, too, can have a sexual or relationship issue and are happy when we raise the subject. They’re not scared to talk about masturbation, either.
When caring for those with chronic diseases, cancer, in the course of physical rehabilitation, and even in the last phase of life, the clinical experience indicates that our patients are happy when we address sexuality and intimacy. Doing so can open the door to the admission of a problem and a corresponding solution, a lubricant or a PDE5 inhibitor.
But sometimes the solution is the conversation itself: Roughly 25% of patients are sufficiently helped simply by talking about sex. Addressing the importance of sexual pleasure is nearly always valuable.
Here are a few ice-breakers I find helpful:
- Did taking this medication change aspects of sexuality? If so, does that bother you?
- Knowing that continuing intimacy is healthy, do you mind if I address that subject?
- We know that aspects of sexuality and intimacy are healthy. Without a partner, some people become sexually isolated. Would you like to talk about that?’
If addressing sexuality has benefits, what about sex itself?
We are gradually learning more about the many short-, intermediate-, and long-term health benefits of solo and joint sexual activity. Short-term benefits include muscle relaxation, pain relief (even, perhaps ironically, for headaches), and better sleep – all pretty valuable for older adults. Examples of intermediate-term benefits include stress relief and less depression. Research from the United States has found that hugging can reduce the concentrations of proinflammatory cytokines, and kissing positively influences cholesterol levels.
Finally, while the long-term benefits of sex might be less relevant for seniors, they do exist.
Among them are delayed onset of dementia and a substantial reduction in cardiovascular and cerebrovascular problems in men. More sex has been linked to longevity, with men benefiting a bit more than women from going through the entire process, including an orgasm, whereas women appear to gain from having a “satisfying” sex life, which does not always require an orgasm.
Let us not forget that these benefits apply to both patients and clinicians alike. Addressing intimacy and sexuality can ease eventual sexual concerns and potentially create a stronger clinician-patient relationship.
Dr. Gianotten, MD is emeritus senior lecturer in medical sexology, Erasmus University Medical Centre, Rotterdam, the Netherlands. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
For health professionals, the thought that our parents and grandparents don’t have sex – or didn’t – might be comforting.
The reality is that, for a significant proportion of our older patients, sex has no use-by date. Humans are sexual beings throughout their lives, yet the culture has concealed that fact.
According to Rome, the purpose of sex is to make children. According to Hollywood, sex is only for the young, the healthy, and the beautiful. For the medical profession, sex consists mainly of risks or dysfunctions.
The results of these biases? Sexuality and intimacy are essential elements for quality of life, with clear physical, emotional, and relational benefits.
Let’s look at the data when researchers dared to ask seniors about their sexuality.
We start with the 2015 U.K. national research on sexuality. The study found a link between age and a decline in various aspects of sexual activity – but not a zeroing-out. For example, among men aged 70-79, 59% reported having had sex in the past year, with 19% having intercourse at least twice a month and 18% masturbating at least that often. Above age 80, those numbers dropped to 39%, 6%, and 5%, respectively. The reason behind the declines? A combination of taboo, fear of disease, use of medications or other interventions that disrupt sexual function or cause disfigurement, and a little bit of age itself.
What about women? Among women ages 70-79, 39% said they’d had sex in the past year, with 6% having intercourse at least twice per month and 5% masturbating two times or more monthly. Above age 80, those numbers were 10%, 4.5%, and 1%, respectively. Driving the falloff in women were the same factors as for men, plus the sad reality that many heterosexual women become widowed because their older male partners die earlier.
The male-female difference also reflects lower levels of testosterone in women. And, because women say they value intimacy more than performance, we have two explanations for their lower frequency of masturbation. After all, a lot of intimacy occurs without either intercourse or masturbation.
Surprising and relevant is the amount of distress – or rather, their relative lack thereof – older patients report because of sexual problems. At age 18-44, 11% of U.S. women indicated sexual distress; at age 45-64, the figure was 15%; and at age 65 and up, 9%.
For clinicians, those figures should prompt us to look more closely at alternative forms of sexual expression – those not involving intercourse or masturbation – in the aged, a field physicians typically do not consider.
Although dyspareunia or erectile problems affect many in long-standing relationships, neither is a reason to abstain from sexual pleasure. Indeed, in many couples, oral sex will replace vaginal intercourse, and if urinary, fecal, or flatal incontinence intrude, couples often waive oral sex in favor of more cuddling, kissing, digital stimulation, and other forms of sexual pleasure.
What about the expiry date for sex?
Fascinating research from Nils Beckman, PhD, and colleagues found that the sex drive persists even as people (and men in particular) reach their 100th year. Dr. Beckman’s group interviewed 269 Swedish seniors, all without dementia, at age 97. Sexual desire was affirmed by 27% of men and 5% of women in the survey. Among the men, 32% said they still had sexual thoughts, compared with 18% of women. Meanwhile, 26% of the men and 15% of the women said they missed sexual activity.
What should clinicians do with this information? First, we could start talking about sex with our older patients. According to the 97-year-old Swedes, most want us to! More than 8 in 10 of both women and men in the survey expressed positive views about questions on sexuality. And please don’t be scared to address the subject in the single senior. They, too, can have a sexual or relationship issue and are happy when we raise the subject. They’re not scared to talk about masturbation, either.
When caring for those with chronic diseases, cancer, in the course of physical rehabilitation, and even in the last phase of life, the clinical experience indicates that our patients are happy when we address sexuality and intimacy. Doing so can open the door to the admission of a problem and a corresponding solution, a lubricant or a PDE5 inhibitor.
But sometimes the solution is the conversation itself: Roughly 25% of patients are sufficiently helped simply by talking about sex. Addressing the importance of sexual pleasure is nearly always valuable.
Here are a few ice-breakers I find helpful:
- Did taking this medication change aspects of sexuality? If so, does that bother you?
- Knowing that continuing intimacy is healthy, do you mind if I address that subject?
- We know that aspects of sexuality and intimacy are healthy. Without a partner, some people become sexually isolated. Would you like to talk about that?’
If addressing sexuality has benefits, what about sex itself?
We are gradually learning more about the many short-, intermediate-, and long-term health benefits of solo and joint sexual activity. Short-term benefits include muscle relaxation, pain relief (even, perhaps ironically, for headaches), and better sleep – all pretty valuable for older adults. Examples of intermediate-term benefits include stress relief and less depression. Research from the United States has found that hugging can reduce the concentrations of proinflammatory cytokines, and kissing positively influences cholesterol levels.
Finally, while the long-term benefits of sex might be less relevant for seniors, they do exist.
Among them are delayed onset of dementia and a substantial reduction in cardiovascular and cerebrovascular problems in men. More sex has been linked to longevity, with men benefiting a bit more than women from going through the entire process, including an orgasm, whereas women appear to gain from having a “satisfying” sex life, which does not always require an orgasm.
Let us not forget that these benefits apply to both patients and clinicians alike. Addressing intimacy and sexuality can ease eventual sexual concerns and potentially create a stronger clinician-patient relationship.
Dr. Gianotten, MD is emeritus senior lecturer in medical sexology, Erasmus University Medical Centre, Rotterdam, the Netherlands. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Internet use a modifiable dementia risk factor in older adults?
Investigators followed more than 18,000 older individuals and found that regular Internet use was associated with about a 50% reduction in dementia risk, compared with their counterparts who did not use the Internet regularly.
They also found that longer duration of regular Internet use was associated with a reduced risk of dementia, although excessive daily Internet usage appeared to adversely affect dementia risk.
“Online engagement can develop and maintain cognitive reserve – resiliency against physiological damage to the brain – and increased cognitive reserve can, in turn, compensate for brain aging and reduce the risk of dementia,” study investigator Gawon Cho, a doctoral candidate at New York University School of Global Public Health, said in an interview.
The study was published online in the Journal of the American Geriatrics Society.
Unexamined benefits
Prior research has shown that older adult Internet users have “better overall cognitive performance, verbal reasoning, and memory,” compared with nonusers, the authors note.
However, because this body of research consists of cross-sectional analyses and longitudinal studies with brief follow-up periods, the long-term cognitive benefits of Internet usage remain “unexamined.”
In addition, despite “extensive evidence of a disproportionately high burden of dementia in people of color, individuals without higher education, and adults who experienced other socioeconomic hardships, little is known about whether the Internet has exacerbated population-level disparities in cognitive health,” the investigators add.
Another question concerns whether excessive Internet usage may actually be detrimental to neurocognitive outcomes. However, “existing evidence on the adverse effects of Internet usage is concentrated in younger populations whose brains are still undergoing maturation.”
Ms. Cho said the motivation for the study was the lack of longitudinal studies on this topic, especially those with sufficient follow-up periods. In addition, she said, there is insufficient evidence about how changes in Internet usage in older age are associated with prospective dementia risk.
For the study, investigators turned to participants in the Health and Retirement Study, an ongoing longitudinal survey of a nationally representative sample of U.S.-based older adults (aged ≥ 50 years).
All participants (n = 18,154; 47.36% male; median age, 55.17 years) were dementia-free, community-dwelling older adults who completed a 2002 baseline cognitive assessment and were asked about Internet usage every 2 years thereafter.
Participants were followed from 2002 to 2018 for a maximum of 17.1 years (median, 7.9 years), which is the longest follow-up period to date. Of the total sample, 64.76% were regular Internet users.
The study’s primary outcome was incident dementia, based on performance on the Modified Telephone Interview for Cognitive Status (TICS-M), which was administered every 2 years.
The exposure examined in the study was cumulative Internet usage in late adulthood, defined as “the number of biennial waves where participants used the Internet regularly during the first three waves.”
In addition, participants were asked how many hours they spent using the Internet during the past week for activities other than viewing television shows or movies.
The researchers also investigated whether the link between Internet usage and dementia risk varied by educational attainment, race-ethnicity, sex, and generational cohort.
Covariates included baseline TICS-M score, health, age, household income, marital status, and region of residence.
U-shaped curve
More than half of the sample (52.96%) showed no changes in Internet use from baseline during the study period, while one-fifth (20.54%) did show changes in use.
Investigators found a robust link between Internet usage and lower dementia risk (cause-specific hazard ratio, 0.57 [95% CI, 0.46-0.71]) – a finding that remained even after adjusting for self-selection into baseline usage (csHR, 0.54 [0.41-0.72]) and signs of cognitive decline at baseline (csHR, 0.62 [0.46-0.85]).
Each additional wave of regular Internet usage was associated with a 21% decrease in the risk of dementia (95% CI, 13%-29%), wherein additional regular periods were associated with reduced dementia risk (csHR, 0.80 [95% CI, 0.68-0.95]).
“The difference in risk between regular and nonregular users did not vary by educational attainment, race-ethnicity, sex, and generation,” the investigators note.
A U-shaped association was found between daily hours of online engagement, wherein the lowest risk was observed in those with 0.1-2 hours of usage (compared with 0 hours of usage). The risk increased in a “monotonic fashion” after 2 hours, with 6.1-8 hours of usage showing the highest risk.
This finding was not considered statistically significant, but the “consistent U-shaped trend offers a preliminary suggestion that excessive online engagement may have adverse cognitive effects on older adults,” the investigators note.
“Among older adults, regular Internet users may experience a lower risk of dementia compared to nonregular users, and longer periods of regular Internet usage in late adulthood may help reduce the risks of subsequent dementia incidence,” said Ms. Cho. “Nonetheless, using the Internet excessively daily may negatively affect the risk of dementia in older adults.”
Bidirectional relationship?
Commenting for this article, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that some risk factors for Alzheimer’s or other dementias can’t be changed, while others are modifiable, “either at a personal or a population level.”
She called the current research “important” because it “identifies a potentially modifiable factor that may influence dementia risk.”
However, cautioned Dr. Sexton, who was not involved with the study, the findings cannot establish cause and effect. In fact, the relationship may be bidirectional.
“It may be that regular Internet usage is associated with increased cognitive stimulation, and in turn reduced risk of dementia; or it may be that individuals with lower risk of dementia are more likely to engage in regular Internet usage,” she said. Thus, “interventional studies are able to shed more light on causation.”
The Health and Retirement Study is sponsored by the National Institute on Aging and is conducted by the University of Michigan, Ann Arbor. Ms. Cho, her coauthors, and Dr. Sexton have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators followed more than 18,000 older individuals and found that regular Internet use was associated with about a 50% reduction in dementia risk, compared with their counterparts who did not use the Internet regularly.
They also found that longer duration of regular Internet use was associated with a reduced risk of dementia, although excessive daily Internet usage appeared to adversely affect dementia risk.
“Online engagement can develop and maintain cognitive reserve – resiliency against physiological damage to the brain – and increased cognitive reserve can, in turn, compensate for brain aging and reduce the risk of dementia,” study investigator Gawon Cho, a doctoral candidate at New York University School of Global Public Health, said in an interview.
The study was published online in the Journal of the American Geriatrics Society.
Unexamined benefits
Prior research has shown that older adult Internet users have “better overall cognitive performance, verbal reasoning, and memory,” compared with nonusers, the authors note.
However, because this body of research consists of cross-sectional analyses and longitudinal studies with brief follow-up periods, the long-term cognitive benefits of Internet usage remain “unexamined.”
In addition, despite “extensive evidence of a disproportionately high burden of dementia in people of color, individuals without higher education, and adults who experienced other socioeconomic hardships, little is known about whether the Internet has exacerbated population-level disparities in cognitive health,” the investigators add.
Another question concerns whether excessive Internet usage may actually be detrimental to neurocognitive outcomes. However, “existing evidence on the adverse effects of Internet usage is concentrated in younger populations whose brains are still undergoing maturation.”
Ms. Cho said the motivation for the study was the lack of longitudinal studies on this topic, especially those with sufficient follow-up periods. In addition, she said, there is insufficient evidence about how changes in Internet usage in older age are associated with prospective dementia risk.
For the study, investigators turned to participants in the Health and Retirement Study, an ongoing longitudinal survey of a nationally representative sample of U.S.-based older adults (aged ≥ 50 years).
All participants (n = 18,154; 47.36% male; median age, 55.17 years) were dementia-free, community-dwelling older adults who completed a 2002 baseline cognitive assessment and were asked about Internet usage every 2 years thereafter.
Participants were followed from 2002 to 2018 for a maximum of 17.1 years (median, 7.9 years), which is the longest follow-up period to date. Of the total sample, 64.76% were regular Internet users.
The study’s primary outcome was incident dementia, based on performance on the Modified Telephone Interview for Cognitive Status (TICS-M), which was administered every 2 years.
The exposure examined in the study was cumulative Internet usage in late adulthood, defined as “the number of biennial waves where participants used the Internet regularly during the first three waves.”
In addition, participants were asked how many hours they spent using the Internet during the past week for activities other than viewing television shows or movies.
The researchers also investigated whether the link between Internet usage and dementia risk varied by educational attainment, race-ethnicity, sex, and generational cohort.
Covariates included baseline TICS-M score, health, age, household income, marital status, and region of residence.
U-shaped curve
More than half of the sample (52.96%) showed no changes in Internet use from baseline during the study period, while one-fifth (20.54%) did show changes in use.
Investigators found a robust link between Internet usage and lower dementia risk (cause-specific hazard ratio, 0.57 [95% CI, 0.46-0.71]) – a finding that remained even after adjusting for self-selection into baseline usage (csHR, 0.54 [0.41-0.72]) and signs of cognitive decline at baseline (csHR, 0.62 [0.46-0.85]).
Each additional wave of regular Internet usage was associated with a 21% decrease in the risk of dementia (95% CI, 13%-29%), wherein additional regular periods were associated with reduced dementia risk (csHR, 0.80 [95% CI, 0.68-0.95]).
“The difference in risk between regular and nonregular users did not vary by educational attainment, race-ethnicity, sex, and generation,” the investigators note.
A U-shaped association was found between daily hours of online engagement, wherein the lowest risk was observed in those with 0.1-2 hours of usage (compared with 0 hours of usage). The risk increased in a “monotonic fashion” after 2 hours, with 6.1-8 hours of usage showing the highest risk.
This finding was not considered statistically significant, but the “consistent U-shaped trend offers a preliminary suggestion that excessive online engagement may have adverse cognitive effects on older adults,” the investigators note.
“Among older adults, regular Internet users may experience a lower risk of dementia compared to nonregular users, and longer periods of regular Internet usage in late adulthood may help reduce the risks of subsequent dementia incidence,” said Ms. Cho. “Nonetheless, using the Internet excessively daily may negatively affect the risk of dementia in older adults.”
Bidirectional relationship?
Commenting for this article, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that some risk factors for Alzheimer’s or other dementias can’t be changed, while others are modifiable, “either at a personal or a population level.”
She called the current research “important” because it “identifies a potentially modifiable factor that may influence dementia risk.”
However, cautioned Dr. Sexton, who was not involved with the study, the findings cannot establish cause and effect. In fact, the relationship may be bidirectional.
“It may be that regular Internet usage is associated with increased cognitive stimulation, and in turn reduced risk of dementia; or it may be that individuals with lower risk of dementia are more likely to engage in regular Internet usage,” she said. Thus, “interventional studies are able to shed more light on causation.”
The Health and Retirement Study is sponsored by the National Institute on Aging and is conducted by the University of Michigan, Ann Arbor. Ms. Cho, her coauthors, and Dr. Sexton have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators followed more than 18,000 older individuals and found that regular Internet use was associated with about a 50% reduction in dementia risk, compared with their counterparts who did not use the Internet regularly.
They also found that longer duration of regular Internet use was associated with a reduced risk of dementia, although excessive daily Internet usage appeared to adversely affect dementia risk.
“Online engagement can develop and maintain cognitive reserve – resiliency against physiological damage to the brain – and increased cognitive reserve can, in turn, compensate for brain aging and reduce the risk of dementia,” study investigator Gawon Cho, a doctoral candidate at New York University School of Global Public Health, said in an interview.
The study was published online in the Journal of the American Geriatrics Society.
Unexamined benefits
Prior research has shown that older adult Internet users have “better overall cognitive performance, verbal reasoning, and memory,” compared with nonusers, the authors note.
However, because this body of research consists of cross-sectional analyses and longitudinal studies with brief follow-up periods, the long-term cognitive benefits of Internet usage remain “unexamined.”
In addition, despite “extensive evidence of a disproportionately high burden of dementia in people of color, individuals without higher education, and adults who experienced other socioeconomic hardships, little is known about whether the Internet has exacerbated population-level disparities in cognitive health,” the investigators add.
Another question concerns whether excessive Internet usage may actually be detrimental to neurocognitive outcomes. However, “existing evidence on the adverse effects of Internet usage is concentrated in younger populations whose brains are still undergoing maturation.”
Ms. Cho said the motivation for the study was the lack of longitudinal studies on this topic, especially those with sufficient follow-up periods. In addition, she said, there is insufficient evidence about how changes in Internet usage in older age are associated with prospective dementia risk.
For the study, investigators turned to participants in the Health and Retirement Study, an ongoing longitudinal survey of a nationally representative sample of U.S.-based older adults (aged ≥ 50 years).
All participants (n = 18,154; 47.36% male; median age, 55.17 years) were dementia-free, community-dwelling older adults who completed a 2002 baseline cognitive assessment and were asked about Internet usage every 2 years thereafter.
Participants were followed from 2002 to 2018 for a maximum of 17.1 years (median, 7.9 years), which is the longest follow-up period to date. Of the total sample, 64.76% were regular Internet users.
The study’s primary outcome was incident dementia, based on performance on the Modified Telephone Interview for Cognitive Status (TICS-M), which was administered every 2 years.
The exposure examined in the study was cumulative Internet usage in late adulthood, defined as “the number of biennial waves where participants used the Internet regularly during the first three waves.”
In addition, participants were asked how many hours they spent using the Internet during the past week for activities other than viewing television shows or movies.
The researchers also investigated whether the link between Internet usage and dementia risk varied by educational attainment, race-ethnicity, sex, and generational cohort.
Covariates included baseline TICS-M score, health, age, household income, marital status, and region of residence.
U-shaped curve
More than half of the sample (52.96%) showed no changes in Internet use from baseline during the study period, while one-fifth (20.54%) did show changes in use.
Investigators found a robust link between Internet usage and lower dementia risk (cause-specific hazard ratio, 0.57 [95% CI, 0.46-0.71]) – a finding that remained even after adjusting for self-selection into baseline usage (csHR, 0.54 [0.41-0.72]) and signs of cognitive decline at baseline (csHR, 0.62 [0.46-0.85]).
Each additional wave of regular Internet usage was associated with a 21% decrease in the risk of dementia (95% CI, 13%-29%), wherein additional regular periods were associated with reduced dementia risk (csHR, 0.80 [95% CI, 0.68-0.95]).
“The difference in risk between regular and nonregular users did not vary by educational attainment, race-ethnicity, sex, and generation,” the investigators note.
A U-shaped association was found between daily hours of online engagement, wherein the lowest risk was observed in those with 0.1-2 hours of usage (compared with 0 hours of usage). The risk increased in a “monotonic fashion” after 2 hours, with 6.1-8 hours of usage showing the highest risk.
This finding was not considered statistically significant, but the “consistent U-shaped trend offers a preliminary suggestion that excessive online engagement may have adverse cognitive effects on older adults,” the investigators note.
“Among older adults, regular Internet users may experience a lower risk of dementia compared to nonregular users, and longer periods of regular Internet usage in late adulthood may help reduce the risks of subsequent dementia incidence,” said Ms. Cho. “Nonetheless, using the Internet excessively daily may negatively affect the risk of dementia in older adults.”
Bidirectional relationship?
Commenting for this article, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that some risk factors for Alzheimer’s or other dementias can’t be changed, while others are modifiable, “either at a personal or a population level.”
She called the current research “important” because it “identifies a potentially modifiable factor that may influence dementia risk.”
However, cautioned Dr. Sexton, who was not involved with the study, the findings cannot establish cause and effect. In fact, the relationship may be bidirectional.
“It may be that regular Internet usage is associated with increased cognitive stimulation, and in turn reduced risk of dementia; or it may be that individuals with lower risk of dementia are more likely to engage in regular Internet usage,” she said. Thus, “interventional studies are able to shed more light on causation.”
The Health and Retirement Study is sponsored by the National Institute on Aging and is conducted by the University of Michigan, Ann Arbor. Ms. Cho, her coauthors, and Dr. Sexton have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN GERIATRICS SOCIETY
Preventing breaks and falls in older adults
LONG BEACH, CALIF. – Ms. S had recently arrived home after a stay at a skilled nursing facility to recover from a hip fracture resulting from osteoporosis. For many patients, follow-up care would have included a DEXA scan or a prescription for a bisphosphonate from a primary care clinician not trained in geriatrics.
But the 85-year-old received care that went further and that is considered best practice for the management of geriatric fractures: A physical therapist visited her after discharge and provided education on the importance of maintaining mobility. Ms. S also underwent assessment for fall risk and gait balance, and a team of multidisciplinary clinicians managed other factors, from postural hypotension to footwear and foot problems.
Sonja Rosen, MD, professor of medicine and chief of geriatric medicine at Cedars-Sinai Medical Center, Los Angeles, talked about Ms. S as part of a panel discussion on applying the “Geriatric 5Ms” for patients with osteoporosis at the annual meeting of the American Geriatrics Society.
“You have to figure out why they are falling and help them not fall again,” Dr. Rosen said.
Approximately 10 million Americans have osteoporosis, and another 44 million have low bone density. One in two women and up to one in four men will experience a bone fracture as a result of osteoporosis, according to the Bone Health and Osteoporosis Foundation.
, which involves considering the care preferences and goals for health care outcomes of individuals.
Ms. S eventually visited a geriatrician through the Cedars-Sinai Geriatric Fracture Program, which has been shown to lower costs and shorten hospital stays. In the program, she was advised to use a walker. Initially, she saw the aid as a hindrance – she felt she should be able to walk without it, like before. But with education, she learned that it is impossible to predict falls and that the walking aid could reduce her risk of a stumble.
Dr. Rosen said clinicians should address any vision problems, prescriptions for psychotropic drugs,which can affect balance, and heart rate and rhythm abnormalities, and they should suggest modifications to the home environment, such as installing grab bars in showers and removing rugs that can easily be tripped over.
The program at Cedars-Sinai, like similar initiatives, offers a team with resources that some clinicians may not have access to, such as a care coordinator and bone-health coach. But health care providers can utilize aspects, such as making referrals to community exercise classes.
Dr. Rosen and her colleagues studied the effects of such exercise programs and found that the programs lessen loneliness and social isolation. Fear of falling decreased in 75% of participants, “which is so key to these postfracture patients in getting back out into the world and engaging in their prior level of functional status,” Dr. Rosen said.
The second ‘M’: Medication management
The second “M,” medications, can help clinicians sequence osteoporosis drugs, depending on patient characteristics and scenarios.
Cathleen Colon-Emeric, MD, MHS, chief of geriatrics at Duke University, in Durham, N.C., dived into the case history of Ms. S, who had hypertension and insomnia in addition to osteoporosis.
First-line treatment for Ms. S – and for most patients – was an oral bisphosphonate, Dr. Colon-Emeric said. Compared with placebo, the drugs decrease the risk of overall osteoporotic fractures by nearly 40% (odds ratio, 0.62). But the medications are linked to injury of the esophageal mucosa. This risk is decreased when a patient stays upright for 30 minutes after taking oral bisphosphonates. Dr. Colon-Emeric displayed a slide of a woman receiving a pedicure at a nail salon.
“The picture of the pedicure is to share the wonderful idea I got from one skilled nursing facility I was working with, who makes sure they do safe administration to prevent esophagitis in their patients by having them all go to a spa day, where they all sit up and get their nails done while they wait their 30 minutes [after taking the pill] sitting up safely,” Dr. Colon-Emeric said.
This strategy drew applause from the audience.
Dr. Colon-Emeric advised that clinicians use judgment in the interpretation of results from the Fracture Risk Assessment Tool (FRAX). Incorporating race into estimates of fracture risk has pros and cons. While there are racial and ethnic differences in average bone density, the data for race calibrations to estimate risk are dated, she said. Clinicians should compare FRAX estimates with and without race input to help patients understand a range of risks.
Some patients may be reluctant to begin taking osteoporosis drugs because of misinformation originating from inaccurate news reports or anecdotes from friends. Dr. Colon-Emeric advised clinicians to remind patients that one in five who experience a fracture will have another injury in the following 2 years.
“A major osteoporotic fracture is akin to a heart attack; it has a very similar 1-year mortality rate and a very similar rate of a subsequent secondary event,” Dr. Colon-Emeric said. “We have a class of medications that decrease both those risks by nearly a third.”
Shared decision-making can help patients understand the risks and benefits of treatment, she said.
“People are really scared about the side effects,” Michelle Keller, PhD, MPH, a research scientist at Cedars-Sinai who attended the session, said. “The idea that a “bone attack” is like a heart attack gets the message across.”
Mind and multicomplexity
Medical complexity of a patient must be considered when making decisions on treatment, according to Joshua Niznik, PharmD, PhD, assistant professor of medicine in the Center for Aging and Health at the University of North Carolina at Chapel Hill.
“Medical complexity is an acknowledgment of the entire person, the burden of their multiple chronic conditions, advanced illnesses, and also their biopsychosocial needs and how those together might augment treatment selection and decision-making,” Dr. Niznik said.
Studies by Dr. Niznik and others have shown that swallowing difficulties, severe dementia, and being older than 90 are linked with a lower likelihood of receiving treatment for osteoporosis.
But therapies for fracture prevention, especially bisphosphonates, appear to be at least as effective for adults with medical complexity as they are for people without such conditions, Dr. Niznik said. Physicians must consider the potential treatment burden and the likelihood of benefit, he said.
Dr. Niznik’s research has shown a lack of strong evidence on how clinicians can manage patients in nursing homes. In some cases, deprescribing is reasonable, such as for patients who have undergone treatment for several years and whose life expectancy is less than 2 years.
“In the absence of any of those, if they are not already treated for osteoporosis, it makes sense to initiate treatment at that time,” Dr. Niznik said.
Matters most: Patient input
Clinicians need to educate patients on how long they must undergo a treatment before they experience benefits, according to Sarah D. Berry, MD, MPH, associate professor of medicine at Harvard Medical School, in Boston.
A meta-analysis of studies that included more than 20,000 women who were randomly assigned to receive bisphosphonate or placebo found that one nonvertebral fracture was avoided during a 12-month period for every 100 persons treated. One hip fracture was avoided during a 20-month period for every 200 patients treated.
“In general, in persons with a 2-year life expectancy, time to benefit favors bisphosphonate use,” Dr. Berry said. “Anabolics may have an even quicker time to benefit.”
Dr. Berry said a shared a decision-making model can help clinicians facilitate discussions that help patients prioritize goals and compare options while considering results, benefits, and harms. And she offered a final tip: Use tools with absolute risk reduction to convey risks and benefits, as the relative risk calculations overestimate how effective treatment will be.
Dr. Rosen has disclosed no relevant financial relationships. Dr. Colon-Emeric has received grants from the National Institutes of Health and VA Health Services Research and Development Funding; has served as endpoint adjudication chair for UCB Pharma; and has received royalties from Wolters Kluwer. Dr. Niznik has received funding from the National Institute of Aging and the Centers for Disease Control and Prevention. Dr. Berry has received funding from the NIH and royalties from Wolters Kluwer.
A version of this article originally appeared on Medscape.com.
LONG BEACH, CALIF. – Ms. S had recently arrived home after a stay at a skilled nursing facility to recover from a hip fracture resulting from osteoporosis. For many patients, follow-up care would have included a DEXA scan or a prescription for a bisphosphonate from a primary care clinician not trained in geriatrics.
But the 85-year-old received care that went further and that is considered best practice for the management of geriatric fractures: A physical therapist visited her after discharge and provided education on the importance of maintaining mobility. Ms. S also underwent assessment for fall risk and gait balance, and a team of multidisciplinary clinicians managed other factors, from postural hypotension to footwear and foot problems.
Sonja Rosen, MD, professor of medicine and chief of geriatric medicine at Cedars-Sinai Medical Center, Los Angeles, talked about Ms. S as part of a panel discussion on applying the “Geriatric 5Ms” for patients with osteoporosis at the annual meeting of the American Geriatrics Society.
“You have to figure out why they are falling and help them not fall again,” Dr. Rosen said.
Approximately 10 million Americans have osteoporosis, and another 44 million have low bone density. One in two women and up to one in four men will experience a bone fracture as a result of osteoporosis, according to the Bone Health and Osteoporosis Foundation.
, which involves considering the care preferences and goals for health care outcomes of individuals.
Ms. S eventually visited a geriatrician through the Cedars-Sinai Geriatric Fracture Program, which has been shown to lower costs and shorten hospital stays. In the program, she was advised to use a walker. Initially, she saw the aid as a hindrance – she felt she should be able to walk without it, like before. But with education, she learned that it is impossible to predict falls and that the walking aid could reduce her risk of a stumble.
Dr. Rosen said clinicians should address any vision problems, prescriptions for psychotropic drugs,which can affect balance, and heart rate and rhythm abnormalities, and they should suggest modifications to the home environment, such as installing grab bars in showers and removing rugs that can easily be tripped over.
The program at Cedars-Sinai, like similar initiatives, offers a team with resources that some clinicians may not have access to, such as a care coordinator and bone-health coach. But health care providers can utilize aspects, such as making referrals to community exercise classes.
Dr. Rosen and her colleagues studied the effects of such exercise programs and found that the programs lessen loneliness and social isolation. Fear of falling decreased in 75% of participants, “which is so key to these postfracture patients in getting back out into the world and engaging in their prior level of functional status,” Dr. Rosen said.
The second ‘M’: Medication management
The second “M,” medications, can help clinicians sequence osteoporosis drugs, depending on patient characteristics and scenarios.
Cathleen Colon-Emeric, MD, MHS, chief of geriatrics at Duke University, in Durham, N.C., dived into the case history of Ms. S, who had hypertension and insomnia in addition to osteoporosis.
First-line treatment for Ms. S – and for most patients – was an oral bisphosphonate, Dr. Colon-Emeric said. Compared with placebo, the drugs decrease the risk of overall osteoporotic fractures by nearly 40% (odds ratio, 0.62). But the medications are linked to injury of the esophageal mucosa. This risk is decreased when a patient stays upright for 30 minutes after taking oral bisphosphonates. Dr. Colon-Emeric displayed a slide of a woman receiving a pedicure at a nail salon.
“The picture of the pedicure is to share the wonderful idea I got from one skilled nursing facility I was working with, who makes sure they do safe administration to prevent esophagitis in their patients by having them all go to a spa day, where they all sit up and get their nails done while they wait their 30 minutes [after taking the pill] sitting up safely,” Dr. Colon-Emeric said.
This strategy drew applause from the audience.
Dr. Colon-Emeric advised that clinicians use judgment in the interpretation of results from the Fracture Risk Assessment Tool (FRAX). Incorporating race into estimates of fracture risk has pros and cons. While there are racial and ethnic differences in average bone density, the data for race calibrations to estimate risk are dated, she said. Clinicians should compare FRAX estimates with and without race input to help patients understand a range of risks.
Some patients may be reluctant to begin taking osteoporosis drugs because of misinformation originating from inaccurate news reports or anecdotes from friends. Dr. Colon-Emeric advised clinicians to remind patients that one in five who experience a fracture will have another injury in the following 2 years.
“A major osteoporotic fracture is akin to a heart attack; it has a very similar 1-year mortality rate and a very similar rate of a subsequent secondary event,” Dr. Colon-Emeric said. “We have a class of medications that decrease both those risks by nearly a third.”
Shared decision-making can help patients understand the risks and benefits of treatment, she said.
“People are really scared about the side effects,” Michelle Keller, PhD, MPH, a research scientist at Cedars-Sinai who attended the session, said. “The idea that a “bone attack” is like a heart attack gets the message across.”
Mind and multicomplexity
Medical complexity of a patient must be considered when making decisions on treatment, according to Joshua Niznik, PharmD, PhD, assistant professor of medicine in the Center for Aging and Health at the University of North Carolina at Chapel Hill.
“Medical complexity is an acknowledgment of the entire person, the burden of their multiple chronic conditions, advanced illnesses, and also their biopsychosocial needs and how those together might augment treatment selection and decision-making,” Dr. Niznik said.
Studies by Dr. Niznik and others have shown that swallowing difficulties, severe dementia, and being older than 90 are linked with a lower likelihood of receiving treatment for osteoporosis.
But therapies for fracture prevention, especially bisphosphonates, appear to be at least as effective for adults with medical complexity as they are for people without such conditions, Dr. Niznik said. Physicians must consider the potential treatment burden and the likelihood of benefit, he said.
Dr. Niznik’s research has shown a lack of strong evidence on how clinicians can manage patients in nursing homes. In some cases, deprescribing is reasonable, such as for patients who have undergone treatment for several years and whose life expectancy is less than 2 years.
“In the absence of any of those, if they are not already treated for osteoporosis, it makes sense to initiate treatment at that time,” Dr. Niznik said.
Matters most: Patient input
Clinicians need to educate patients on how long they must undergo a treatment before they experience benefits, according to Sarah D. Berry, MD, MPH, associate professor of medicine at Harvard Medical School, in Boston.
A meta-analysis of studies that included more than 20,000 women who were randomly assigned to receive bisphosphonate or placebo found that one nonvertebral fracture was avoided during a 12-month period for every 100 persons treated. One hip fracture was avoided during a 20-month period for every 200 patients treated.
“In general, in persons with a 2-year life expectancy, time to benefit favors bisphosphonate use,” Dr. Berry said. “Anabolics may have an even quicker time to benefit.”
Dr. Berry said a shared a decision-making model can help clinicians facilitate discussions that help patients prioritize goals and compare options while considering results, benefits, and harms. And she offered a final tip: Use tools with absolute risk reduction to convey risks and benefits, as the relative risk calculations overestimate how effective treatment will be.
Dr. Rosen has disclosed no relevant financial relationships. Dr. Colon-Emeric has received grants from the National Institutes of Health and VA Health Services Research and Development Funding; has served as endpoint adjudication chair for UCB Pharma; and has received royalties from Wolters Kluwer. Dr. Niznik has received funding from the National Institute of Aging and the Centers for Disease Control and Prevention. Dr. Berry has received funding from the NIH and royalties from Wolters Kluwer.
A version of this article originally appeared on Medscape.com.
LONG BEACH, CALIF. – Ms. S had recently arrived home after a stay at a skilled nursing facility to recover from a hip fracture resulting from osteoporosis. For many patients, follow-up care would have included a DEXA scan or a prescription for a bisphosphonate from a primary care clinician not trained in geriatrics.
But the 85-year-old received care that went further and that is considered best practice for the management of geriatric fractures: A physical therapist visited her after discharge and provided education on the importance of maintaining mobility. Ms. S also underwent assessment for fall risk and gait balance, and a team of multidisciplinary clinicians managed other factors, from postural hypotension to footwear and foot problems.
Sonja Rosen, MD, professor of medicine and chief of geriatric medicine at Cedars-Sinai Medical Center, Los Angeles, talked about Ms. S as part of a panel discussion on applying the “Geriatric 5Ms” for patients with osteoporosis at the annual meeting of the American Geriatrics Society.
“You have to figure out why they are falling and help them not fall again,” Dr. Rosen said.
Approximately 10 million Americans have osteoporosis, and another 44 million have low bone density. One in two women and up to one in four men will experience a bone fracture as a result of osteoporosis, according to the Bone Health and Osteoporosis Foundation.
, which involves considering the care preferences and goals for health care outcomes of individuals.
Ms. S eventually visited a geriatrician through the Cedars-Sinai Geriatric Fracture Program, which has been shown to lower costs and shorten hospital stays. In the program, she was advised to use a walker. Initially, she saw the aid as a hindrance – she felt she should be able to walk without it, like before. But with education, she learned that it is impossible to predict falls and that the walking aid could reduce her risk of a stumble.
Dr. Rosen said clinicians should address any vision problems, prescriptions for psychotropic drugs,which can affect balance, and heart rate and rhythm abnormalities, and they should suggest modifications to the home environment, such as installing grab bars in showers and removing rugs that can easily be tripped over.
The program at Cedars-Sinai, like similar initiatives, offers a team with resources that some clinicians may not have access to, such as a care coordinator and bone-health coach. But health care providers can utilize aspects, such as making referrals to community exercise classes.
Dr. Rosen and her colleagues studied the effects of such exercise programs and found that the programs lessen loneliness and social isolation. Fear of falling decreased in 75% of participants, “which is so key to these postfracture patients in getting back out into the world and engaging in their prior level of functional status,” Dr. Rosen said.
The second ‘M’: Medication management
The second “M,” medications, can help clinicians sequence osteoporosis drugs, depending on patient characteristics and scenarios.
Cathleen Colon-Emeric, MD, MHS, chief of geriatrics at Duke University, in Durham, N.C., dived into the case history of Ms. S, who had hypertension and insomnia in addition to osteoporosis.
First-line treatment for Ms. S – and for most patients – was an oral bisphosphonate, Dr. Colon-Emeric said. Compared with placebo, the drugs decrease the risk of overall osteoporotic fractures by nearly 40% (odds ratio, 0.62). But the medications are linked to injury of the esophageal mucosa. This risk is decreased when a patient stays upright for 30 minutes after taking oral bisphosphonates. Dr. Colon-Emeric displayed a slide of a woman receiving a pedicure at a nail salon.
“The picture of the pedicure is to share the wonderful idea I got from one skilled nursing facility I was working with, who makes sure they do safe administration to prevent esophagitis in their patients by having them all go to a spa day, where they all sit up and get their nails done while they wait their 30 minutes [after taking the pill] sitting up safely,” Dr. Colon-Emeric said.
This strategy drew applause from the audience.
Dr. Colon-Emeric advised that clinicians use judgment in the interpretation of results from the Fracture Risk Assessment Tool (FRAX). Incorporating race into estimates of fracture risk has pros and cons. While there are racial and ethnic differences in average bone density, the data for race calibrations to estimate risk are dated, she said. Clinicians should compare FRAX estimates with and without race input to help patients understand a range of risks.
Some patients may be reluctant to begin taking osteoporosis drugs because of misinformation originating from inaccurate news reports or anecdotes from friends. Dr. Colon-Emeric advised clinicians to remind patients that one in five who experience a fracture will have another injury in the following 2 years.
“A major osteoporotic fracture is akin to a heart attack; it has a very similar 1-year mortality rate and a very similar rate of a subsequent secondary event,” Dr. Colon-Emeric said. “We have a class of medications that decrease both those risks by nearly a third.”
Shared decision-making can help patients understand the risks and benefits of treatment, she said.
“People are really scared about the side effects,” Michelle Keller, PhD, MPH, a research scientist at Cedars-Sinai who attended the session, said. “The idea that a “bone attack” is like a heart attack gets the message across.”
Mind and multicomplexity
Medical complexity of a patient must be considered when making decisions on treatment, according to Joshua Niznik, PharmD, PhD, assistant professor of medicine in the Center for Aging and Health at the University of North Carolina at Chapel Hill.
“Medical complexity is an acknowledgment of the entire person, the burden of their multiple chronic conditions, advanced illnesses, and also their biopsychosocial needs and how those together might augment treatment selection and decision-making,” Dr. Niznik said.
Studies by Dr. Niznik and others have shown that swallowing difficulties, severe dementia, and being older than 90 are linked with a lower likelihood of receiving treatment for osteoporosis.
But therapies for fracture prevention, especially bisphosphonates, appear to be at least as effective for adults with medical complexity as they are for people without such conditions, Dr. Niznik said. Physicians must consider the potential treatment burden and the likelihood of benefit, he said.
Dr. Niznik’s research has shown a lack of strong evidence on how clinicians can manage patients in nursing homes. In some cases, deprescribing is reasonable, such as for patients who have undergone treatment for several years and whose life expectancy is less than 2 years.
“In the absence of any of those, if they are not already treated for osteoporosis, it makes sense to initiate treatment at that time,” Dr. Niznik said.
Matters most: Patient input
Clinicians need to educate patients on how long they must undergo a treatment before they experience benefits, according to Sarah D. Berry, MD, MPH, associate professor of medicine at Harvard Medical School, in Boston.
A meta-analysis of studies that included more than 20,000 women who were randomly assigned to receive bisphosphonate or placebo found that one nonvertebral fracture was avoided during a 12-month period for every 100 persons treated. One hip fracture was avoided during a 20-month period for every 200 patients treated.
“In general, in persons with a 2-year life expectancy, time to benefit favors bisphosphonate use,” Dr. Berry said. “Anabolics may have an even quicker time to benefit.”
Dr. Berry said a shared a decision-making model can help clinicians facilitate discussions that help patients prioritize goals and compare options while considering results, benefits, and harms. And she offered a final tip: Use tools with absolute risk reduction to convey risks and benefits, as the relative risk calculations overestimate how effective treatment will be.
Dr. Rosen has disclosed no relevant financial relationships. Dr. Colon-Emeric has received grants from the National Institutes of Health and VA Health Services Research and Development Funding; has served as endpoint adjudication chair for UCB Pharma; and has received royalties from Wolters Kluwer. Dr. Niznik has received funding from the National Institute of Aging and the Centers for Disease Control and Prevention. Dr. Berry has received funding from the NIH and royalties from Wolters Kluwer.
A version of this article originally appeared on Medscape.com.
AT AGS 2023
Number of cancer survivors with functional limitations doubled in 20 years
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
FROM JAMA ONCOLOGY
FDA approves first drug to treat Alzheimer’s agitation
(AD), making it the first FDA-approved drug for this indication.
“Agitation is one of the most common and challenging aspects of care among patients with dementia due to Alzheimer’s disease,” Tiffany Farchione, MD, director of the division of psychiatry in the FDA’s Center for Drug Evaluation and Research, said in a news release.
Agitation can include symptoms that range from pacing or restlessness to verbal and physical aggression. “These symptoms are leading causes of assisted living or nursing home placement and have been associated with accelerated disease progression,” Dr. Farchione said.
Brexpiprazole was approved by the FDA in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
Approval of the supplemental application for brexpiprazole for agitation associated with AD dementia was based on results of two randomized, double-blind, placebo-controlled studies.
In both studies, patients who received 2 mg or 3 mg of brexpiprazole showed statistically significant and clinically meaningful improvements in agitation symptoms, as shown by total Cohen-Mansfield Agitation Inventory (CMAI) score, compared with patients who received placebo.
The recommended starting dosage for the treatment of agitation associated with AD dementia is 0.5 mg once daily on days 1-7; it was increased to 1 mg once daily on days 8-14 and then to the recommended target dose of 2 mg once daily.
The dosage can be increased to the maximum recommended daily dosage of 3 mg once daily after at least 14 days, depending on clinical response and tolerability.
The most common side effects of brexpiprazole in patients with agitation associated with AD dementia include headache, dizziness, urinary tract infection, nasopharyngitis, and sleep disturbances.
The drug includes a boxed warning for medications in this class that elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
The supplemental application for brexpiprazole for agitation had fast-track designation.
A version of this article first appeared on Medscape.com.
(AD), making it the first FDA-approved drug for this indication.
“Agitation is one of the most common and challenging aspects of care among patients with dementia due to Alzheimer’s disease,” Tiffany Farchione, MD, director of the division of psychiatry in the FDA’s Center for Drug Evaluation and Research, said in a news release.
Agitation can include symptoms that range from pacing or restlessness to verbal and physical aggression. “These symptoms are leading causes of assisted living or nursing home placement and have been associated with accelerated disease progression,” Dr. Farchione said.
Brexpiprazole was approved by the FDA in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
Approval of the supplemental application for brexpiprazole for agitation associated with AD dementia was based on results of two randomized, double-blind, placebo-controlled studies.
In both studies, patients who received 2 mg or 3 mg of brexpiprazole showed statistically significant and clinically meaningful improvements in agitation symptoms, as shown by total Cohen-Mansfield Agitation Inventory (CMAI) score, compared with patients who received placebo.
The recommended starting dosage for the treatment of agitation associated with AD dementia is 0.5 mg once daily on days 1-7; it was increased to 1 mg once daily on days 8-14 and then to the recommended target dose of 2 mg once daily.
The dosage can be increased to the maximum recommended daily dosage of 3 mg once daily after at least 14 days, depending on clinical response and tolerability.
The most common side effects of brexpiprazole in patients with agitation associated with AD dementia include headache, dizziness, urinary tract infection, nasopharyngitis, and sleep disturbances.
The drug includes a boxed warning for medications in this class that elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
The supplemental application for brexpiprazole for agitation had fast-track designation.
A version of this article first appeared on Medscape.com.
(AD), making it the first FDA-approved drug for this indication.
“Agitation is one of the most common and challenging aspects of care among patients with dementia due to Alzheimer’s disease,” Tiffany Farchione, MD, director of the division of psychiatry in the FDA’s Center for Drug Evaluation and Research, said in a news release.
Agitation can include symptoms that range from pacing or restlessness to verbal and physical aggression. “These symptoms are leading causes of assisted living or nursing home placement and have been associated with accelerated disease progression,” Dr. Farchione said.
Brexpiprazole was approved by the FDA in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
Approval of the supplemental application for brexpiprazole for agitation associated with AD dementia was based on results of two randomized, double-blind, placebo-controlled studies.
In both studies, patients who received 2 mg or 3 mg of brexpiprazole showed statistically significant and clinically meaningful improvements in agitation symptoms, as shown by total Cohen-Mansfield Agitation Inventory (CMAI) score, compared with patients who received placebo.
The recommended starting dosage for the treatment of agitation associated with AD dementia is 0.5 mg once daily on days 1-7; it was increased to 1 mg once daily on days 8-14 and then to the recommended target dose of 2 mg once daily.
The dosage can be increased to the maximum recommended daily dosage of 3 mg once daily after at least 14 days, depending on clinical response and tolerability.
The most common side effects of brexpiprazole in patients with agitation associated with AD dementia include headache, dizziness, urinary tract infection, nasopharyngitis, and sleep disturbances.
The drug includes a boxed warning for medications in this class that elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
The supplemental application for brexpiprazole for agitation had fast-track designation.
A version of this article first appeared on Medscape.com.






