User login
Proposed Medicare bill would raise docs’ pay with inflation
Introduced by four physician U.S. House representatives, HR 2474 would link Medicare fee schedule updates to the Medicare Economic Index, a measure of inflation related to physicians’ practice costs and wages.
That’s a long-sought goal of the American Medical Association, which is leading 120 state medical societies and medical specialty groups in championing the bill.
The legislation is essential to enabling physician practices to better absorb payment distributions triggered by budget neutrality rules, performance adjustments, and periods of high inflation, the groups wrote in a joint letter sent to the bill’s sponsors. The sponsors say they hope the legislation will improve access to care, as low reimbursements cause some physicians to limit their number of Medicare patients.
Physicians groups for years have urged federal lawmakers to scrap short-term fixes staving off Medicare pay cuts in favor of permanent reforms. Unlike nearly all other Medicare clinicians including hospitals, physicians’ Medicare payment updates aren’t currently tied to inflation.
Adjusted for inflation, Medicare payments to physicians have declined 26% between 2001 and 2023, including a 2% payment reduction in 2023, according to the AMA. Small and rural physician practices have been disproportionately affected by these reductions, as have doctors treating low-income or uninsured patients, the AMA said.
Last month, an influential federal advisory panel recommended permanently tying Medicare physician pay increases to inflation. Clinicians’ cost of providing services, measured by the Medicare Economic Index, rose by 2.6% in 2021 and are estimated to have risen 4.7% in 2022, significantly more than in recent years, the Medicare Payment Advisory Commission said.
A version of this article originally appeared on Medscape.com.
Introduced by four physician U.S. House representatives, HR 2474 would link Medicare fee schedule updates to the Medicare Economic Index, a measure of inflation related to physicians’ practice costs and wages.
That’s a long-sought goal of the American Medical Association, which is leading 120 state medical societies and medical specialty groups in championing the bill.
The legislation is essential to enabling physician practices to better absorb payment distributions triggered by budget neutrality rules, performance adjustments, and periods of high inflation, the groups wrote in a joint letter sent to the bill’s sponsors. The sponsors say they hope the legislation will improve access to care, as low reimbursements cause some physicians to limit their number of Medicare patients.
Physicians groups for years have urged federal lawmakers to scrap short-term fixes staving off Medicare pay cuts in favor of permanent reforms. Unlike nearly all other Medicare clinicians including hospitals, physicians’ Medicare payment updates aren’t currently tied to inflation.
Adjusted for inflation, Medicare payments to physicians have declined 26% between 2001 and 2023, including a 2% payment reduction in 2023, according to the AMA. Small and rural physician practices have been disproportionately affected by these reductions, as have doctors treating low-income or uninsured patients, the AMA said.
Last month, an influential federal advisory panel recommended permanently tying Medicare physician pay increases to inflation. Clinicians’ cost of providing services, measured by the Medicare Economic Index, rose by 2.6% in 2021 and are estimated to have risen 4.7% in 2022, significantly more than in recent years, the Medicare Payment Advisory Commission said.
A version of this article originally appeared on Medscape.com.
Introduced by four physician U.S. House representatives, HR 2474 would link Medicare fee schedule updates to the Medicare Economic Index, a measure of inflation related to physicians’ practice costs and wages.
That’s a long-sought goal of the American Medical Association, which is leading 120 state medical societies and medical specialty groups in championing the bill.
The legislation is essential to enabling physician practices to better absorb payment distributions triggered by budget neutrality rules, performance adjustments, and periods of high inflation, the groups wrote in a joint letter sent to the bill’s sponsors. The sponsors say they hope the legislation will improve access to care, as low reimbursements cause some physicians to limit their number of Medicare patients.
Physicians groups for years have urged federal lawmakers to scrap short-term fixes staving off Medicare pay cuts in favor of permanent reforms. Unlike nearly all other Medicare clinicians including hospitals, physicians’ Medicare payment updates aren’t currently tied to inflation.
Adjusted for inflation, Medicare payments to physicians have declined 26% between 2001 and 2023, including a 2% payment reduction in 2023, according to the AMA. Small and rural physician practices have been disproportionately affected by these reductions, as have doctors treating low-income or uninsured patients, the AMA said.
Last month, an influential federal advisory panel recommended permanently tying Medicare physician pay increases to inflation. Clinicians’ cost of providing services, measured by the Medicare Economic Index, rose by 2.6% in 2021 and are estimated to have risen 4.7% in 2022, significantly more than in recent years, the Medicare Payment Advisory Commission said.
A version of this article originally appeared on Medscape.com.
Medicare expands CGM coverage to more with type 2 diabetes
Medicare is now covering continuous glucose monitoring (CGM) for all beneficiaries with diabetes who use insulin, as well as those with a “history of problematic hypoglycemia.”
The new policy decision, announced earlier this year by the Centers for Medicare and Medicaid Services, means that coverage is expanded to those who take even just a single dose of basal insulin daily or who don’t take insulin but who for other reasons experience “problematic” hypoglycemia, defined as a history of more than one level 2 event (glucose < 54 mg/dL) or at least one level 3 event (< 54 mg/dL requiring assistance).
Previously, coverage was limited to those taking frequent daily insulin doses.
The additional number of people covered, most with type 2 diabetes, is estimated to be at least 1.5 million. That number could more than double if private insurers follow suit, reported an industry analyst.
Chuck Henderson, chief executive officer of the American Diabetes Association, said in a statement: “We applaud CMS’ decision allowing for all insulin-dependent people as well as others who have a history of problematic hypoglycemia to have access to a continuous glucose monitor, a potentially life-saving tool for diabetes management.”
According to Dexcom, which manufacturers the G6 and the recently approved G7 CGMs, the decision was based in part on their MOBILE study. The trial demonstrated the benefit of CGM in people with type 2 diabetes who use only basal insulin or have a history of problematic hypoglycemic events.
On April 14, Abbott, which manufactures the Freestyle Libre 2 and the recently approved Libre 3, received clearance from the U.S. Food and Drug Administration for the Libre 3’s stand-alone reader device. Previously, the Libre 3 had been approved for use only with a smartphone app. The small handheld reader is considered durable medical equipment, making it eligible for Medicare coverage. Abbott is “working on having the FreeStyle Libre 3 system available to Medicare beneficiaries,” the company said in a statement.
A version of this article first appeared on Medscape.com.
Medicare is now covering continuous glucose monitoring (CGM) for all beneficiaries with diabetes who use insulin, as well as those with a “history of problematic hypoglycemia.”
The new policy decision, announced earlier this year by the Centers for Medicare and Medicaid Services, means that coverage is expanded to those who take even just a single dose of basal insulin daily or who don’t take insulin but who for other reasons experience “problematic” hypoglycemia, defined as a history of more than one level 2 event (glucose < 54 mg/dL) or at least one level 3 event (< 54 mg/dL requiring assistance).
Previously, coverage was limited to those taking frequent daily insulin doses.
The additional number of people covered, most with type 2 diabetes, is estimated to be at least 1.5 million. That number could more than double if private insurers follow suit, reported an industry analyst.
Chuck Henderson, chief executive officer of the American Diabetes Association, said in a statement: “We applaud CMS’ decision allowing for all insulin-dependent people as well as others who have a history of problematic hypoglycemia to have access to a continuous glucose monitor, a potentially life-saving tool for diabetes management.”
According to Dexcom, which manufacturers the G6 and the recently approved G7 CGMs, the decision was based in part on their MOBILE study. The trial demonstrated the benefit of CGM in people with type 2 diabetes who use only basal insulin or have a history of problematic hypoglycemic events.
On April 14, Abbott, which manufactures the Freestyle Libre 2 and the recently approved Libre 3, received clearance from the U.S. Food and Drug Administration for the Libre 3’s stand-alone reader device. Previously, the Libre 3 had been approved for use only with a smartphone app. The small handheld reader is considered durable medical equipment, making it eligible for Medicare coverage. Abbott is “working on having the FreeStyle Libre 3 system available to Medicare beneficiaries,” the company said in a statement.
A version of this article first appeared on Medscape.com.
Medicare is now covering continuous glucose monitoring (CGM) for all beneficiaries with diabetes who use insulin, as well as those with a “history of problematic hypoglycemia.”
The new policy decision, announced earlier this year by the Centers for Medicare and Medicaid Services, means that coverage is expanded to those who take even just a single dose of basal insulin daily or who don’t take insulin but who for other reasons experience “problematic” hypoglycemia, defined as a history of more than one level 2 event (glucose < 54 mg/dL) or at least one level 3 event (< 54 mg/dL requiring assistance).
Previously, coverage was limited to those taking frequent daily insulin doses.
The additional number of people covered, most with type 2 diabetes, is estimated to be at least 1.5 million. That number could more than double if private insurers follow suit, reported an industry analyst.
Chuck Henderson, chief executive officer of the American Diabetes Association, said in a statement: “We applaud CMS’ decision allowing for all insulin-dependent people as well as others who have a history of problematic hypoglycemia to have access to a continuous glucose monitor, a potentially life-saving tool for diabetes management.”
According to Dexcom, which manufacturers the G6 and the recently approved G7 CGMs, the decision was based in part on their MOBILE study. The trial demonstrated the benefit of CGM in people with type 2 diabetes who use only basal insulin or have a history of problematic hypoglycemic events.
On April 14, Abbott, which manufactures the Freestyle Libre 2 and the recently approved Libre 3, received clearance from the U.S. Food and Drug Administration for the Libre 3’s stand-alone reader device. Previously, the Libre 3 had been approved for use only with a smartphone app. The small handheld reader is considered durable medical equipment, making it eligible for Medicare coverage. Abbott is “working on having the FreeStyle Libre 3 system available to Medicare beneficiaries,” the company said in a statement.
A version of this article first appeared on Medscape.com.
Single bivalent COVID booster is enough for now: CDC
“If you have completed your updated booster dose, you are currently up to date. There is not a recommendation to get another updated booster dose,” the CDC website now explains.
In January, the nation’s expert COVID panel recommended that the United States move toward an annual COVID booster shot in the fall, similar to the annual flu shot, that targets the most widely circulating strains of the virus. Recent studies have shown that booster strength wanes after a few months, spurring discussions of whether people at high risk of getting a severe case of COVID may need more than one annual shot.
September was the last time a new booster dose was recommended, when, at the time, the bivalent booster was released, offering new protection against Omicron variants of the virus. Health officials’ focus is now shifting from preventing infections to reducing the likelihood of severe ones, the San Francisco Chronicle reported.
“The bottom line is that there is some waning of protection for those who got boosters more than six months ago and haven’t had an intervening infection,” said Bob Wachter, MD, head of the University of California–San Francisco’s department of medicine, according to the Chronicle. “But the level of protection versus severe infection continues to be fairly high, good enough that people who aren’t at super high risk are probably fine waiting until a new booster comes out in the fall.”
The Wall Street Journal reported recently that many people have been asking their doctors to give them another booster, which is not authorized by the Food and Drug Administration.
About 8 in 10 people in the United States got the initial set of COVID-19 vaccines, which were first approved in August 2021. But just 16.4% of people in the United States have gotten the latest booster that was released in September, CDC data show.
A version of this article originally appeared on WebMD.com.
“If you have completed your updated booster dose, you are currently up to date. There is not a recommendation to get another updated booster dose,” the CDC website now explains.
In January, the nation’s expert COVID panel recommended that the United States move toward an annual COVID booster shot in the fall, similar to the annual flu shot, that targets the most widely circulating strains of the virus. Recent studies have shown that booster strength wanes after a few months, spurring discussions of whether people at high risk of getting a severe case of COVID may need more than one annual shot.
September was the last time a new booster dose was recommended, when, at the time, the bivalent booster was released, offering new protection against Omicron variants of the virus. Health officials’ focus is now shifting from preventing infections to reducing the likelihood of severe ones, the San Francisco Chronicle reported.
“The bottom line is that there is some waning of protection for those who got boosters more than six months ago and haven’t had an intervening infection,” said Bob Wachter, MD, head of the University of California–San Francisco’s department of medicine, according to the Chronicle. “But the level of protection versus severe infection continues to be fairly high, good enough that people who aren’t at super high risk are probably fine waiting until a new booster comes out in the fall.”
The Wall Street Journal reported recently that many people have been asking their doctors to give them another booster, which is not authorized by the Food and Drug Administration.
About 8 in 10 people in the United States got the initial set of COVID-19 vaccines, which were first approved in August 2021. But just 16.4% of people in the United States have gotten the latest booster that was released in September, CDC data show.
A version of this article originally appeared on WebMD.com.
“If you have completed your updated booster dose, you are currently up to date. There is not a recommendation to get another updated booster dose,” the CDC website now explains.
In January, the nation’s expert COVID panel recommended that the United States move toward an annual COVID booster shot in the fall, similar to the annual flu shot, that targets the most widely circulating strains of the virus. Recent studies have shown that booster strength wanes after a few months, spurring discussions of whether people at high risk of getting a severe case of COVID may need more than one annual shot.
September was the last time a new booster dose was recommended, when, at the time, the bivalent booster was released, offering new protection against Omicron variants of the virus. Health officials’ focus is now shifting from preventing infections to reducing the likelihood of severe ones, the San Francisco Chronicle reported.
“The bottom line is that there is some waning of protection for those who got boosters more than six months ago and haven’t had an intervening infection,” said Bob Wachter, MD, head of the University of California–San Francisco’s department of medicine, according to the Chronicle. “But the level of protection versus severe infection continues to be fairly high, good enough that people who aren’t at super high risk are probably fine waiting until a new booster comes out in the fall.”
The Wall Street Journal reported recently that many people have been asking their doctors to give them another booster, which is not authorized by the Food and Drug Administration.
About 8 in 10 people in the United States got the initial set of COVID-19 vaccines, which were first approved in August 2021. But just 16.4% of people in the United States have gotten the latest booster that was released in September, CDC data show.
A version of this article originally appeared on WebMD.com.
Kickback Scheme Nets Prison Time for Philadelphia VAMC Service Chief
A former manager at the Philadelphia Veterans Affairs Medical Center (VAMC) has been sentenced to 6 months in federal prison for his part in a bribery scheme.
Ralph Johnson was convicted of accepting $30,000 in kickbacks and bribes for steering contracts to Earron and Carlicha Starks, who ran Ekno Medical Supply and Collondale Medical Supply from 2009 to 2019. Johnson served as chief of environmental services at the medical center. He admitted to receiving cash in binders and packages mailed to his home between 2018 and 2019.
The Starkses pleaded guilty first to paying kickbacks on $7 million worth of contracts to Florida VA facilities, then participated in a sting that implicated Johnson.
The VA Office of Inspector General began investigating Johnson in 2018 after the Starkses, who were indicted for bribing staff at US Department of Veterans Affairs (VA) hospitals in Miami and West Palm Beach, Florida, said they also paid officials in VA facilities on the East Coast.
According to the Philadelphia Inquirer, the judge credited Johnson’s past military service and his “extensive cooperation” with federal authorities investigating fraud within the VA. Johnson apologized to his former employers: “Throughout these 2 and a half years [since the arrest] there’s not a day I don’t think about the wrongness that I did.”
In addition to the prison sentence, Johnson has been ordered to pay back, at $50 a month, the $440,000-plus he cost the Philadelphia VAMC in fraudulent and bloated contracts.
Johnson is at least the third Philadelphia VAMC employee indicted or sentenced for fraud since 2020.
A former manager at the Philadelphia Veterans Affairs Medical Center (VAMC) has been sentenced to 6 months in federal prison for his part in a bribery scheme.
Ralph Johnson was convicted of accepting $30,000 in kickbacks and bribes for steering contracts to Earron and Carlicha Starks, who ran Ekno Medical Supply and Collondale Medical Supply from 2009 to 2019. Johnson served as chief of environmental services at the medical center. He admitted to receiving cash in binders and packages mailed to his home between 2018 and 2019.
The Starkses pleaded guilty first to paying kickbacks on $7 million worth of contracts to Florida VA facilities, then participated in a sting that implicated Johnson.
The VA Office of Inspector General began investigating Johnson in 2018 after the Starkses, who were indicted for bribing staff at US Department of Veterans Affairs (VA) hospitals in Miami and West Palm Beach, Florida, said they also paid officials in VA facilities on the East Coast.
According to the Philadelphia Inquirer, the judge credited Johnson’s past military service and his “extensive cooperation” with federal authorities investigating fraud within the VA. Johnson apologized to his former employers: “Throughout these 2 and a half years [since the arrest] there’s not a day I don’t think about the wrongness that I did.”
In addition to the prison sentence, Johnson has been ordered to pay back, at $50 a month, the $440,000-plus he cost the Philadelphia VAMC in fraudulent and bloated contracts.
Johnson is at least the third Philadelphia VAMC employee indicted or sentenced for fraud since 2020.
A former manager at the Philadelphia Veterans Affairs Medical Center (VAMC) has been sentenced to 6 months in federal prison for his part in a bribery scheme.
Ralph Johnson was convicted of accepting $30,000 in kickbacks and bribes for steering contracts to Earron and Carlicha Starks, who ran Ekno Medical Supply and Collondale Medical Supply from 2009 to 2019. Johnson served as chief of environmental services at the medical center. He admitted to receiving cash in binders and packages mailed to his home between 2018 and 2019.
The Starkses pleaded guilty first to paying kickbacks on $7 million worth of contracts to Florida VA facilities, then participated in a sting that implicated Johnson.
The VA Office of Inspector General began investigating Johnson in 2018 after the Starkses, who were indicted for bribing staff at US Department of Veterans Affairs (VA) hospitals in Miami and West Palm Beach, Florida, said they also paid officials in VA facilities on the East Coast.
According to the Philadelphia Inquirer, the judge credited Johnson’s past military service and his “extensive cooperation” with federal authorities investigating fraud within the VA. Johnson apologized to his former employers: “Throughout these 2 and a half years [since the arrest] there’s not a day I don’t think about the wrongness that I did.”
In addition to the prison sentence, Johnson has been ordered to pay back, at $50 a month, the $440,000-plus he cost the Philadelphia VAMC in fraudulent and bloated contracts.
Johnson is at least the third Philadelphia VAMC employee indicted or sentenced for fraud since 2020.
Frustration over iPLEDGE evident at FDA meeting
During 2 days of after the chaotic rollout of the new REMS platform at the end of 2021.
On March 29, at the end of the FDA’s joint meeting of two advisory committees that addressed ways to improve the iPLEDGE program, most panelists voted to change the 19-day lockout period for patients who can become pregnant, and the requirement that every month, providers must document counseling of those who cannot get pregnant and are taking the drug for acne.
However, there was no consensus on whether there should be a lockout at all or for how long, and what an appropriate interval for counseling those who cannot get pregnant would be, if not monthly. Those voting on the questions repeatedly cited a lack of data to make well-informed decisions.
The meeting of the two panels, the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee, was held March 28-29, to discuss proposed changes to iPLEDGE requirements, to minimize the program’s burden on patients, prescribers, and pharmacies – while maintaining safe use of the highly teratogenic drug.
Lockout based on outdated reasoning
John S. Barbieri, MD, a dermatologist and epidemiologist, and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital in Boston, speaking as deputy chair of the American Academy of Dermatology Association (AADA) iPLEDGE work group, described the burden of getting the drug to patients. He was not on the panel, but spoke during the open public hearing.
“Compared to other acne medications, the time it takes to successfully go from prescribed (isotretinoin) to when the patient actually has it in their hands is 5- to 10-fold higher,” he said.
Among the barriers is the 19-day lockout period for people who can get pregnant and miss the 7-day window for picking up their prescriptions. They must then wait 19 days to get a pregnancy test to clear them for receiving the medication.
Gregory Wedin, PharmD, pharmacovigilance and risk management director of Upsher-Smith Laboratories, who spoke on behalf of the Isotretinoin Products Manufacturer Group (IPMG), which manages iPLEDGE, said, “The rationale for the 19-day wait is to ensure the next confirmatory pregnancy test is completed after the most fertile period of the menstrual cycle is passed.”
Many don’t have a monthly cycle
But Dr. Barbieri said that reasoning is outdated.
“The current program’s focus on the menstrual cycle is really an antiquated approach,” he said. “Many patients do not have a monthly cycle due to medical conditions like polycystic ovarian syndrome, or due to [certain kinds of] contraception.”
He added, “By removing this 19-day lockout and, really, the archaic timing around the menstrual cycle in general in this program, we can simplify the program, improve it, and better align it with the real-world biology of our patients.” He added that patients are often missing the 7-day window for picking up their prescriptions through no fault of their own. Speakers at the hearing also mentioned insurance hassles and ordering delays.
Communication with IPMG
Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and outgoing chair of the AADA iPLEDGE work group, cited difficulty in working with IPMG on modifications as another barrier. She also spoke during the open public hearing.
“Despite many, many attempts to work with the IPMG, we are not aware of any organizational structure or key leaders to communicate with. Instead we have been given repeatedly a generic email address for trying to establish a working relationship and we believe this may explain the inaction of the IPMG since our proposals 4 years ago in 2019.”
Among those proposals, she said, were allowing telemedicine visits as part of the iPLEDGE REMS program and reducing counseling attestation to every 6 months instead of monthly for those who cannot become pregnant.
She pointed to the chaotic rollout of modifications to the iPLEDGE program on a new website at the end of 2021.
In 2021, she said, “despite 6 months of notification, no prescriber input was solicited before revamping the website. This lack of transparency and accountability has been a major hurdle in improving iPLEDGE.”
Dr. Barbieri called the rollout “a debacle” that could have been mitigated with communication with IPMG. “We warned about every issue that happened and talked about ways to mitigate it and were largely ignored,” he said.
“By including dermatologists and key stakeholders in these discussions, as we move forward with changes to improve this program, we can make sure that it’s patient-centered.”
IPMG did not address the specific complaints about the working relationship with the AADA workgroup at the meeting.
Monthly attestation for counseling patients who cannot get pregnant
Dr. Barbieri said the monthly requirement to counsel patients who cannot get pregnant and document that counseling unfairly burdens clinicians and patients. “We’re essentially asking patients to come in monthly just to tell them not to share their drugs [or] donate blood,” he said.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among the panel members voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
IPMG representative Dr. Wedin, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while an extension to 120 days would reduce burden on prescribers, it comes with the risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Dr. Wedin said.
Home pregnancy testing
The advisory groups were also tasked with discussing whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most committee members and those in the public hearing who spoke on the issue agreed that home tests should continue in an effort to increase access and decrease burden.
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory.
Lindsey Crist, PharmD, a risk management analyst at the FDA, who presented the FDA review committee’s analysis, said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” Dr. Crist said.
Dr. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Workaround to avoid falsification
Advisory committee member Brian P. Green, DO, associate professor of dermatology at Penn State University, Hershey, Pa., spoke in support of home pregnancy tests.
“What we have people do for telemedicine is take the stick, write their name, write the date on it, and send a picture of that the same day as their visit,” he said. “That way we have the pregnancy test the same day. Allowing this to continue to happen at home is important. Bringing people in is burdensome and costly.”
Emmy Graber, MD, a dermatologist who practices in Boston, and a director of the American Acne and Rosacea Society (AARS), relayed an example of the burden for a patient using isotretinoin who lives 1.5 hours away from the dermatology office. She is able to meet the requirements of iPLEDGE only through telehealth.
“Home pregnancy tests are highly sensitive, equal to the ones done in CLIA-certified labs, and highly accurate when interpreted by a dermatology provider,” said Dr. Graber, who spoke on behalf of the AARS during the open public hearing.
“Notably, CLIA [Clinical Laboratory Improvement Amendments] certification is not required by other REMS programs” for teratogenic drugs, she added.
Dr. Graber said it’s important to note that in the time the pandemic exceptions have been made for isotretinoin patients, “there has been no reported spike in pregnancy in the past three years.
“We do have some data to show that it is not imposing additional harms,” she said.
Suggestions for improvement
At the end of the hearing, advisory committee members were asked to propose improvements to the iPLEDGE REMS program.
Dr. Green advocated for the addition of an iPLEDGE mobile app.
“Most people go to their phones rather than their computers, particularly teenagers and younger people,” he noted.
Advisory committee member Megha M. Tollefson, MD, professor of dermatology and pediatric and adolescent medicine at Mayo Clinic in Rochester, Minn., echoed the need for an iPLEDGE app.
The young patients getting isotretinoin “don’t respond to email, they don’t necessarily go onto web pages. If we’re going to be as effective as possible, it’s going to have to be through an app-based system.”
Dr. Tollefson said she would like to see patient counseling standardized through the app. “I think there’s a lot of variability in what counseling is given when it’s left to the individual prescriber or practice,” she said.
Exceptions for long-acting contraceptives?
Advisory committee member Abbey B. Berenson, MD, PhD, professor of obstetrics and gynecology at University of Texas Medical Branch in Galveston, said that patients taking long-acting reversible contraceptives (LARCs) may need to be considered differently when deciding the intervals for attestation or whether to have a lockout period.
“LARC methods’ rate of failure is extremely low,” she said. “While it is true, as it has been pointed out, that all methods can fail, when they’re over 99% effective, I think that we can treat those methods differently than we treat methods such as birth control pills or abstinence that fail far more often. That is one way we could minimize burden on the providers and the patients.”
She also suggested using members of the health care team other than physicians to complete counseling, such as a nurse or pharmacist.
Prescriptions for emergency contraception
Advisory committee member Sascha Dublin, MD, PhD, senior scientific investigator for Kaiser Permanente Washington Health Research Institute in Seattle, said most patients taking the drug who can get pregnant should get a prescription for emergency contraception at the time of the first isotretinoin prescription.
“They don’t have to buy it, but to make it available at the very beginning sets the expectation that it would be good to have in your medicine cabinet, particularly if the [contraception] choice is abstinence or birth control pills.”
Dr. Dublin also called for better transparency surrounding the role of IPMG.
She said IPMG should be expected to collect data in a way that allows examination of health disparities, including by race and ethnicity and insurance status. Dr. Dublin added that she was concerned about the poor communication between dermatological societies and IPMG.
“The FDA should really require that IPMG hold periodic, regularly scheduled stakeholder forums,” she said. “There has to be a mechanism in place for IPMG to listen to those concerns in real time and respond.”
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
During 2 days of after the chaotic rollout of the new REMS platform at the end of 2021.
On March 29, at the end of the FDA’s joint meeting of two advisory committees that addressed ways to improve the iPLEDGE program, most panelists voted to change the 19-day lockout period for patients who can become pregnant, and the requirement that every month, providers must document counseling of those who cannot get pregnant and are taking the drug for acne.
However, there was no consensus on whether there should be a lockout at all or for how long, and what an appropriate interval for counseling those who cannot get pregnant would be, if not monthly. Those voting on the questions repeatedly cited a lack of data to make well-informed decisions.
The meeting of the two panels, the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee, was held March 28-29, to discuss proposed changes to iPLEDGE requirements, to minimize the program’s burden on patients, prescribers, and pharmacies – while maintaining safe use of the highly teratogenic drug.
Lockout based on outdated reasoning
John S. Barbieri, MD, a dermatologist and epidemiologist, and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital in Boston, speaking as deputy chair of the American Academy of Dermatology Association (AADA) iPLEDGE work group, described the burden of getting the drug to patients. He was not on the panel, but spoke during the open public hearing.
“Compared to other acne medications, the time it takes to successfully go from prescribed (isotretinoin) to when the patient actually has it in their hands is 5- to 10-fold higher,” he said.
Among the barriers is the 19-day lockout period for people who can get pregnant and miss the 7-day window for picking up their prescriptions. They must then wait 19 days to get a pregnancy test to clear them for receiving the medication.
Gregory Wedin, PharmD, pharmacovigilance and risk management director of Upsher-Smith Laboratories, who spoke on behalf of the Isotretinoin Products Manufacturer Group (IPMG), which manages iPLEDGE, said, “The rationale for the 19-day wait is to ensure the next confirmatory pregnancy test is completed after the most fertile period of the menstrual cycle is passed.”
Many don’t have a monthly cycle
But Dr. Barbieri said that reasoning is outdated.
“The current program’s focus on the menstrual cycle is really an antiquated approach,” he said. “Many patients do not have a monthly cycle due to medical conditions like polycystic ovarian syndrome, or due to [certain kinds of] contraception.”
He added, “By removing this 19-day lockout and, really, the archaic timing around the menstrual cycle in general in this program, we can simplify the program, improve it, and better align it with the real-world biology of our patients.” He added that patients are often missing the 7-day window for picking up their prescriptions through no fault of their own. Speakers at the hearing also mentioned insurance hassles and ordering delays.
Communication with IPMG
Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and outgoing chair of the AADA iPLEDGE work group, cited difficulty in working with IPMG on modifications as another barrier. She also spoke during the open public hearing.
“Despite many, many attempts to work with the IPMG, we are not aware of any organizational structure or key leaders to communicate with. Instead we have been given repeatedly a generic email address for trying to establish a working relationship and we believe this may explain the inaction of the IPMG since our proposals 4 years ago in 2019.”
Among those proposals, she said, were allowing telemedicine visits as part of the iPLEDGE REMS program and reducing counseling attestation to every 6 months instead of monthly for those who cannot become pregnant.
She pointed to the chaotic rollout of modifications to the iPLEDGE program on a new website at the end of 2021.
In 2021, she said, “despite 6 months of notification, no prescriber input was solicited before revamping the website. This lack of transparency and accountability has been a major hurdle in improving iPLEDGE.”
Dr. Barbieri called the rollout “a debacle” that could have been mitigated with communication with IPMG. “We warned about every issue that happened and talked about ways to mitigate it and were largely ignored,” he said.
“By including dermatologists and key stakeholders in these discussions, as we move forward with changes to improve this program, we can make sure that it’s patient-centered.”
IPMG did not address the specific complaints about the working relationship with the AADA workgroup at the meeting.
Monthly attestation for counseling patients who cannot get pregnant
Dr. Barbieri said the monthly requirement to counsel patients who cannot get pregnant and document that counseling unfairly burdens clinicians and patients. “We’re essentially asking patients to come in monthly just to tell them not to share their drugs [or] donate blood,” he said.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among the panel members voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
IPMG representative Dr. Wedin, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while an extension to 120 days would reduce burden on prescribers, it comes with the risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Dr. Wedin said.
Home pregnancy testing
The advisory groups were also tasked with discussing whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most committee members and those in the public hearing who spoke on the issue agreed that home tests should continue in an effort to increase access and decrease burden.
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory.
Lindsey Crist, PharmD, a risk management analyst at the FDA, who presented the FDA review committee’s analysis, said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” Dr. Crist said.
Dr. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Workaround to avoid falsification
Advisory committee member Brian P. Green, DO, associate professor of dermatology at Penn State University, Hershey, Pa., spoke in support of home pregnancy tests.
“What we have people do for telemedicine is take the stick, write their name, write the date on it, and send a picture of that the same day as their visit,” he said. “That way we have the pregnancy test the same day. Allowing this to continue to happen at home is important. Bringing people in is burdensome and costly.”
Emmy Graber, MD, a dermatologist who practices in Boston, and a director of the American Acne and Rosacea Society (AARS), relayed an example of the burden for a patient using isotretinoin who lives 1.5 hours away from the dermatology office. She is able to meet the requirements of iPLEDGE only through telehealth.
“Home pregnancy tests are highly sensitive, equal to the ones done in CLIA-certified labs, and highly accurate when interpreted by a dermatology provider,” said Dr. Graber, who spoke on behalf of the AARS during the open public hearing.
“Notably, CLIA [Clinical Laboratory Improvement Amendments] certification is not required by other REMS programs” for teratogenic drugs, she added.
Dr. Graber said it’s important to note that in the time the pandemic exceptions have been made for isotretinoin patients, “there has been no reported spike in pregnancy in the past three years.
“We do have some data to show that it is not imposing additional harms,” she said.
Suggestions for improvement
At the end of the hearing, advisory committee members were asked to propose improvements to the iPLEDGE REMS program.
Dr. Green advocated for the addition of an iPLEDGE mobile app.
“Most people go to their phones rather than their computers, particularly teenagers and younger people,” he noted.
Advisory committee member Megha M. Tollefson, MD, professor of dermatology and pediatric and adolescent medicine at Mayo Clinic in Rochester, Minn., echoed the need for an iPLEDGE app.
The young patients getting isotretinoin “don’t respond to email, they don’t necessarily go onto web pages. If we’re going to be as effective as possible, it’s going to have to be through an app-based system.”
Dr. Tollefson said she would like to see patient counseling standardized through the app. “I think there’s a lot of variability in what counseling is given when it’s left to the individual prescriber or practice,” she said.
Exceptions for long-acting contraceptives?
Advisory committee member Abbey B. Berenson, MD, PhD, professor of obstetrics and gynecology at University of Texas Medical Branch in Galveston, said that patients taking long-acting reversible contraceptives (LARCs) may need to be considered differently when deciding the intervals for attestation or whether to have a lockout period.
“LARC methods’ rate of failure is extremely low,” she said. “While it is true, as it has been pointed out, that all methods can fail, when they’re over 99% effective, I think that we can treat those methods differently than we treat methods such as birth control pills or abstinence that fail far more often. That is one way we could minimize burden on the providers and the patients.”
She also suggested using members of the health care team other than physicians to complete counseling, such as a nurse or pharmacist.
Prescriptions for emergency contraception
Advisory committee member Sascha Dublin, MD, PhD, senior scientific investigator for Kaiser Permanente Washington Health Research Institute in Seattle, said most patients taking the drug who can get pregnant should get a prescription for emergency contraception at the time of the first isotretinoin prescription.
“They don’t have to buy it, but to make it available at the very beginning sets the expectation that it would be good to have in your medicine cabinet, particularly if the [contraception] choice is abstinence or birth control pills.”
Dr. Dublin also called for better transparency surrounding the role of IPMG.
She said IPMG should be expected to collect data in a way that allows examination of health disparities, including by race and ethnicity and insurance status. Dr. Dublin added that she was concerned about the poor communication between dermatological societies and IPMG.
“The FDA should really require that IPMG hold periodic, regularly scheduled stakeholder forums,” she said. “There has to be a mechanism in place for IPMG to listen to those concerns in real time and respond.”
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
During 2 days of after the chaotic rollout of the new REMS platform at the end of 2021.
On March 29, at the end of the FDA’s joint meeting of two advisory committees that addressed ways to improve the iPLEDGE program, most panelists voted to change the 19-day lockout period for patients who can become pregnant, and the requirement that every month, providers must document counseling of those who cannot get pregnant and are taking the drug for acne.
However, there was no consensus on whether there should be a lockout at all or for how long, and what an appropriate interval for counseling those who cannot get pregnant would be, if not monthly. Those voting on the questions repeatedly cited a lack of data to make well-informed decisions.
The meeting of the two panels, the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee, was held March 28-29, to discuss proposed changes to iPLEDGE requirements, to minimize the program’s burden on patients, prescribers, and pharmacies – while maintaining safe use of the highly teratogenic drug.
Lockout based on outdated reasoning
John S. Barbieri, MD, a dermatologist and epidemiologist, and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital in Boston, speaking as deputy chair of the American Academy of Dermatology Association (AADA) iPLEDGE work group, described the burden of getting the drug to patients. He was not on the panel, but spoke during the open public hearing.
“Compared to other acne medications, the time it takes to successfully go from prescribed (isotretinoin) to when the patient actually has it in their hands is 5- to 10-fold higher,” he said.
Among the barriers is the 19-day lockout period for people who can get pregnant and miss the 7-day window for picking up their prescriptions. They must then wait 19 days to get a pregnancy test to clear them for receiving the medication.
Gregory Wedin, PharmD, pharmacovigilance and risk management director of Upsher-Smith Laboratories, who spoke on behalf of the Isotretinoin Products Manufacturer Group (IPMG), which manages iPLEDGE, said, “The rationale for the 19-day wait is to ensure the next confirmatory pregnancy test is completed after the most fertile period of the menstrual cycle is passed.”
Many don’t have a monthly cycle
But Dr. Barbieri said that reasoning is outdated.
“The current program’s focus on the menstrual cycle is really an antiquated approach,” he said. “Many patients do not have a monthly cycle due to medical conditions like polycystic ovarian syndrome, or due to [certain kinds of] contraception.”
He added, “By removing this 19-day lockout and, really, the archaic timing around the menstrual cycle in general in this program, we can simplify the program, improve it, and better align it with the real-world biology of our patients.” He added that patients are often missing the 7-day window for picking up their prescriptions through no fault of their own. Speakers at the hearing also mentioned insurance hassles and ordering delays.
Communication with IPMG
Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and outgoing chair of the AADA iPLEDGE work group, cited difficulty in working with IPMG on modifications as another barrier. She also spoke during the open public hearing.
“Despite many, many attempts to work with the IPMG, we are not aware of any organizational structure or key leaders to communicate with. Instead we have been given repeatedly a generic email address for trying to establish a working relationship and we believe this may explain the inaction of the IPMG since our proposals 4 years ago in 2019.”
Among those proposals, she said, were allowing telemedicine visits as part of the iPLEDGE REMS program and reducing counseling attestation to every 6 months instead of monthly for those who cannot become pregnant.
She pointed to the chaotic rollout of modifications to the iPLEDGE program on a new website at the end of 2021.
In 2021, she said, “despite 6 months of notification, no prescriber input was solicited before revamping the website. This lack of transparency and accountability has been a major hurdle in improving iPLEDGE.”
Dr. Barbieri called the rollout “a debacle” that could have been mitigated with communication with IPMG. “We warned about every issue that happened and talked about ways to mitigate it and were largely ignored,” he said.
“By including dermatologists and key stakeholders in these discussions, as we move forward with changes to improve this program, we can make sure that it’s patient-centered.”
IPMG did not address the specific complaints about the working relationship with the AADA workgroup at the meeting.
Monthly attestation for counseling patients who cannot get pregnant
Dr. Barbieri said the monthly requirement to counsel patients who cannot get pregnant and document that counseling unfairly burdens clinicians and patients. “We’re essentially asking patients to come in monthly just to tell them not to share their drugs [or] donate blood,” he said.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among the panel members voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
IPMG representative Dr. Wedin, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while an extension to 120 days would reduce burden on prescribers, it comes with the risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Dr. Wedin said.
Home pregnancy testing
The advisory groups were also tasked with discussing whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most committee members and those in the public hearing who spoke on the issue agreed that home tests should continue in an effort to increase access and decrease burden.
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory.
Lindsey Crist, PharmD, a risk management analyst at the FDA, who presented the FDA review committee’s analysis, said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” Dr. Crist said.
Dr. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Workaround to avoid falsification
Advisory committee member Brian P. Green, DO, associate professor of dermatology at Penn State University, Hershey, Pa., spoke in support of home pregnancy tests.
“What we have people do for telemedicine is take the stick, write their name, write the date on it, and send a picture of that the same day as their visit,” he said. “That way we have the pregnancy test the same day. Allowing this to continue to happen at home is important. Bringing people in is burdensome and costly.”
Emmy Graber, MD, a dermatologist who practices in Boston, and a director of the American Acne and Rosacea Society (AARS), relayed an example of the burden for a patient using isotretinoin who lives 1.5 hours away from the dermatology office. She is able to meet the requirements of iPLEDGE only through telehealth.
“Home pregnancy tests are highly sensitive, equal to the ones done in CLIA-certified labs, and highly accurate when interpreted by a dermatology provider,” said Dr. Graber, who spoke on behalf of the AARS during the open public hearing.
“Notably, CLIA [Clinical Laboratory Improvement Amendments] certification is not required by other REMS programs” for teratogenic drugs, she added.
Dr. Graber said it’s important to note that in the time the pandemic exceptions have been made for isotretinoin patients, “there has been no reported spike in pregnancy in the past three years.
“We do have some data to show that it is not imposing additional harms,” she said.
Suggestions for improvement
At the end of the hearing, advisory committee members were asked to propose improvements to the iPLEDGE REMS program.
Dr. Green advocated for the addition of an iPLEDGE mobile app.
“Most people go to their phones rather than their computers, particularly teenagers and younger people,” he noted.
Advisory committee member Megha M. Tollefson, MD, professor of dermatology and pediatric and adolescent medicine at Mayo Clinic in Rochester, Minn., echoed the need for an iPLEDGE app.
The young patients getting isotretinoin “don’t respond to email, they don’t necessarily go onto web pages. If we’re going to be as effective as possible, it’s going to have to be through an app-based system.”
Dr. Tollefson said she would like to see patient counseling standardized through the app. “I think there’s a lot of variability in what counseling is given when it’s left to the individual prescriber or practice,” she said.
Exceptions for long-acting contraceptives?
Advisory committee member Abbey B. Berenson, MD, PhD, professor of obstetrics and gynecology at University of Texas Medical Branch in Galveston, said that patients taking long-acting reversible contraceptives (LARCs) may need to be considered differently when deciding the intervals for attestation or whether to have a lockout period.
“LARC methods’ rate of failure is extremely low,” she said. “While it is true, as it has been pointed out, that all methods can fail, when they’re over 99% effective, I think that we can treat those methods differently than we treat methods such as birth control pills or abstinence that fail far more often. That is one way we could minimize burden on the providers and the patients.”
She also suggested using members of the health care team other than physicians to complete counseling, such as a nurse or pharmacist.
Prescriptions for emergency contraception
Advisory committee member Sascha Dublin, MD, PhD, senior scientific investigator for Kaiser Permanente Washington Health Research Institute in Seattle, said most patients taking the drug who can get pregnant should get a prescription for emergency contraception at the time of the first isotretinoin prescription.
“They don’t have to buy it, but to make it available at the very beginning sets the expectation that it would be good to have in your medicine cabinet, particularly if the [contraception] choice is abstinence or birth control pills.”
Dr. Dublin also called for better transparency surrounding the role of IPMG.
She said IPMG should be expected to collect data in a way that allows examination of health disparities, including by race and ethnicity and insurance status. Dr. Dublin added that she was concerned about the poor communication between dermatological societies and IPMG.
“The FDA should really require that IPMG hold periodic, regularly scheduled stakeholder forums,” she said. “There has to be a mechanism in place for IPMG to listen to those concerns in real time and respond.”
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
New state bill could protect docs prescribing abortion pills to out-of-state patients
California lawmakers are considering legislation to protect California physicians and pharmacists who prescribe abortion pills to out-of-state patients. The proposed law would shield health care providers who are legally performing their jobs in California from facing prosecution in another state or being extradited.
State Sen. Nancy Skinner, who introduced the bill, said the legislation is necessary in a fractured, post-Roe legal landscape where doctors in some states can face felony charges or civil penalties for providing reproductive health care. It’s part of a package of 17 new bills aiming to “strengthen California’s standing as a safe haven for abortion, contraception, and pregnancy care,” according to a press release.
“I’m trying to protect our healthcare practitioners so they can do their jobs, without fear,” Ms. Skinner said in a statement on March 24.
Most abortions are banned in 14 states after the Supreme Court overturned Roe v. Wade. Lawmakers in those states have established a variety of penalties for doctors, pharmacists, and other clinicians to provide abortion care or assist patients in obtaining abortions, including jail time, fines, and loss of professional licenses.
As a result, doctors in restrictive states have anguished over having to delay treatment for patients experiencing miscarriages, ectopic pregnancies, and other conditions until their lives are enough at risk to satisfy exceptions to state abortion laws.
“As a physician, I believe everyone deserves the care they need, regardless of where they live,” said Daniel Grossman, MD, a University of California, San Francisco, ob.gyn. professor who directs the university’s Advancing New Standards in Reproductive Health program.
“Since the fall of Roe v. Wade, patients are being forced to travel long distances – often over 500 miles – to access abortion care in a clinic. People should be able to access this essential care closer to home, including by telemedicine, which has been shown to be safe and effective. I am hopeful that SB 345 will provide additional legal protections that would allow California clinicians to help patients in other states,” he stated.
Other states, including New York, Vermont, New Jersey, Massachusetts, and Connecticut, have passed or are considering similar legislation to protect doctors using telemedicine to prescribe abortion medication to out-of-state patients. These laws come amid a growing push by some states and anti-abortion groups to severely restrict access to abortion pills.
Wyoming is the first state to explicitly ban the pills, although a judge on March 22 blocked that ban. And, in a closely watched case, a conservative federal judge could soon rule to ban sales of mifepristone, one of the medications in a two-pill regimen approved for abortions early in pregnancy.
California’s legislation protects clinicians from losing their California professional licenses if an out-of-state medical board takes action against them. It also allows clinicians to sue anyone who tries to legally interfere with the care they are providing.
It also covers California physicians prescribing contraceptives or gender-affirming care to out-of-state patients. At least 21 states are considering restrictions on gender-affirming care for minors and another 9 states have passed them, according to the advocacy group Human Rights Campaign. Courts have blocked the restrictions in some states.
“It’s understandable that states like California want to reassure their doctors ... that, if one of their patients is caught in one of those states and can’t get help locally, they can step up to help and feel safe in doing so,” said Matthew Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora.
“This is also a crazy development in terms of the law. It’s just one part of the legal mayhem that was predicted when the Supreme Court overturned Roe,” Dr. Wynia said of the growing number of bills protecting in-state doctors. These bills “will almost certainly end up being litigated over issues of interstate commerce, cross-state licensure and practice compacts, FDA regulations and authorities, and maybe more. It’s a huge mess, in which both doctors and patients are being hurt.”
A version of this article first appeared on Medscape.com.
California lawmakers are considering legislation to protect California physicians and pharmacists who prescribe abortion pills to out-of-state patients. The proposed law would shield health care providers who are legally performing their jobs in California from facing prosecution in another state or being extradited.
State Sen. Nancy Skinner, who introduced the bill, said the legislation is necessary in a fractured, post-Roe legal landscape where doctors in some states can face felony charges or civil penalties for providing reproductive health care. It’s part of a package of 17 new bills aiming to “strengthen California’s standing as a safe haven for abortion, contraception, and pregnancy care,” according to a press release.
“I’m trying to protect our healthcare practitioners so they can do their jobs, without fear,” Ms. Skinner said in a statement on March 24.
Most abortions are banned in 14 states after the Supreme Court overturned Roe v. Wade. Lawmakers in those states have established a variety of penalties for doctors, pharmacists, and other clinicians to provide abortion care or assist patients in obtaining abortions, including jail time, fines, and loss of professional licenses.
As a result, doctors in restrictive states have anguished over having to delay treatment for patients experiencing miscarriages, ectopic pregnancies, and other conditions until their lives are enough at risk to satisfy exceptions to state abortion laws.
“As a physician, I believe everyone deserves the care they need, regardless of where they live,” said Daniel Grossman, MD, a University of California, San Francisco, ob.gyn. professor who directs the university’s Advancing New Standards in Reproductive Health program.
“Since the fall of Roe v. Wade, patients are being forced to travel long distances – often over 500 miles – to access abortion care in a clinic. People should be able to access this essential care closer to home, including by telemedicine, which has been shown to be safe and effective. I am hopeful that SB 345 will provide additional legal protections that would allow California clinicians to help patients in other states,” he stated.
Other states, including New York, Vermont, New Jersey, Massachusetts, and Connecticut, have passed or are considering similar legislation to protect doctors using telemedicine to prescribe abortion medication to out-of-state patients. These laws come amid a growing push by some states and anti-abortion groups to severely restrict access to abortion pills.
Wyoming is the first state to explicitly ban the pills, although a judge on March 22 blocked that ban. And, in a closely watched case, a conservative federal judge could soon rule to ban sales of mifepristone, one of the medications in a two-pill regimen approved for abortions early in pregnancy.
California’s legislation protects clinicians from losing their California professional licenses if an out-of-state medical board takes action against them. It also allows clinicians to sue anyone who tries to legally interfere with the care they are providing.
It also covers California physicians prescribing contraceptives or gender-affirming care to out-of-state patients. At least 21 states are considering restrictions on gender-affirming care for minors and another 9 states have passed them, according to the advocacy group Human Rights Campaign. Courts have blocked the restrictions in some states.
“It’s understandable that states like California want to reassure their doctors ... that, if one of their patients is caught in one of those states and can’t get help locally, they can step up to help and feel safe in doing so,” said Matthew Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora.
“This is also a crazy development in terms of the law. It’s just one part of the legal mayhem that was predicted when the Supreme Court overturned Roe,” Dr. Wynia said of the growing number of bills protecting in-state doctors. These bills “will almost certainly end up being litigated over issues of interstate commerce, cross-state licensure and practice compacts, FDA regulations and authorities, and maybe more. It’s a huge mess, in which both doctors and patients are being hurt.”
A version of this article first appeared on Medscape.com.
California lawmakers are considering legislation to protect California physicians and pharmacists who prescribe abortion pills to out-of-state patients. The proposed law would shield health care providers who are legally performing their jobs in California from facing prosecution in another state or being extradited.
State Sen. Nancy Skinner, who introduced the bill, said the legislation is necessary in a fractured, post-Roe legal landscape where doctors in some states can face felony charges or civil penalties for providing reproductive health care. It’s part of a package of 17 new bills aiming to “strengthen California’s standing as a safe haven for abortion, contraception, and pregnancy care,” according to a press release.
“I’m trying to protect our healthcare practitioners so they can do their jobs, without fear,” Ms. Skinner said in a statement on March 24.
Most abortions are banned in 14 states after the Supreme Court overturned Roe v. Wade. Lawmakers in those states have established a variety of penalties for doctors, pharmacists, and other clinicians to provide abortion care or assist patients in obtaining abortions, including jail time, fines, and loss of professional licenses.
As a result, doctors in restrictive states have anguished over having to delay treatment for patients experiencing miscarriages, ectopic pregnancies, and other conditions until their lives are enough at risk to satisfy exceptions to state abortion laws.
“As a physician, I believe everyone deserves the care they need, regardless of where they live,” said Daniel Grossman, MD, a University of California, San Francisco, ob.gyn. professor who directs the university’s Advancing New Standards in Reproductive Health program.
“Since the fall of Roe v. Wade, patients are being forced to travel long distances – often over 500 miles – to access abortion care in a clinic. People should be able to access this essential care closer to home, including by telemedicine, which has been shown to be safe and effective. I am hopeful that SB 345 will provide additional legal protections that would allow California clinicians to help patients in other states,” he stated.
Other states, including New York, Vermont, New Jersey, Massachusetts, and Connecticut, have passed or are considering similar legislation to protect doctors using telemedicine to prescribe abortion medication to out-of-state patients. These laws come amid a growing push by some states and anti-abortion groups to severely restrict access to abortion pills.
Wyoming is the first state to explicitly ban the pills, although a judge on March 22 blocked that ban. And, in a closely watched case, a conservative federal judge could soon rule to ban sales of mifepristone, one of the medications in a two-pill regimen approved for abortions early in pregnancy.
California’s legislation protects clinicians from losing their California professional licenses if an out-of-state medical board takes action against them. It also allows clinicians to sue anyone who tries to legally interfere with the care they are providing.
It also covers California physicians prescribing contraceptives or gender-affirming care to out-of-state patients. At least 21 states are considering restrictions on gender-affirming care for minors and another 9 states have passed them, according to the advocacy group Human Rights Campaign. Courts have blocked the restrictions in some states.
“It’s understandable that states like California want to reassure their doctors ... that, if one of their patients is caught in one of those states and can’t get help locally, they can step up to help and feel safe in doing so,” said Matthew Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora.
“This is also a crazy development in terms of the law. It’s just one part of the legal mayhem that was predicted when the Supreme Court overturned Roe,” Dr. Wynia said of the growing number of bills protecting in-state doctors. These bills “will almost certainly end up being litigated over issues of interstate commerce, cross-state licensure and practice compacts, FDA regulations and authorities, and maybe more. It’s a huge mess, in which both doctors and patients are being hurt.”
A version of this article first appeared on Medscape.com.
Meet the JCOM Author with Dr. Barkoudah: Leading for High Reliability During the COVID-19 Pandemic
Meet the JCOM Author with Dr. Barkoudah: Residence Characteristics and Nursing Home Compare Quality Measures
Relationships Between Residence Characteristics and Nursing Home Compare Database Quality Measures
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18
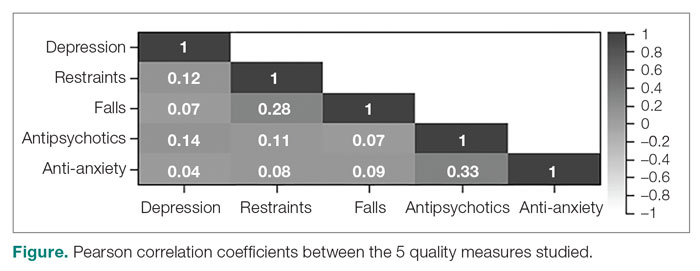
Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).
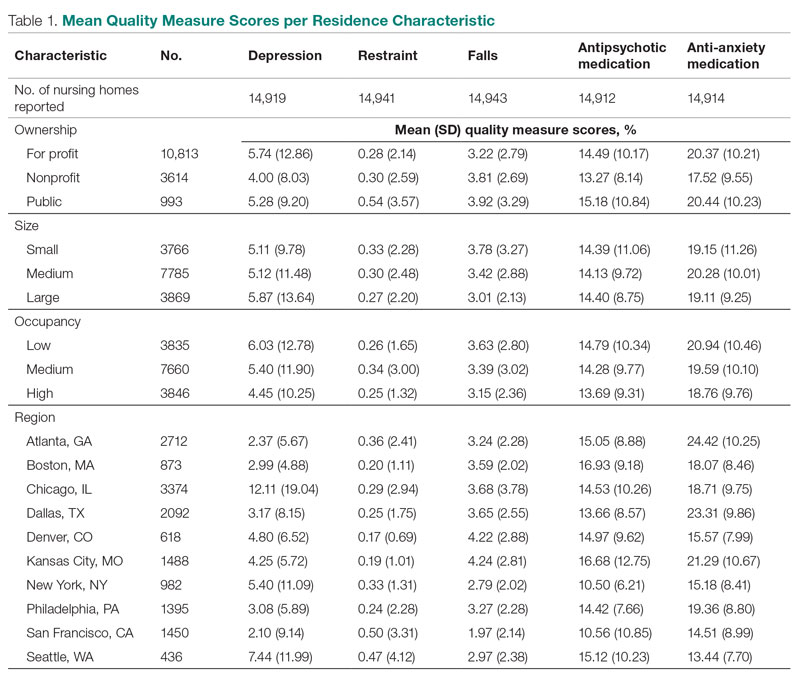
Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.
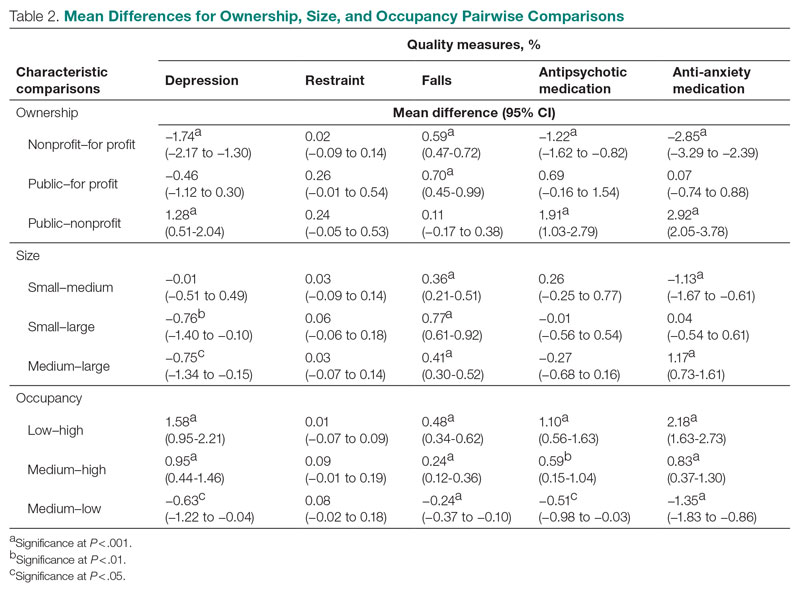
Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.
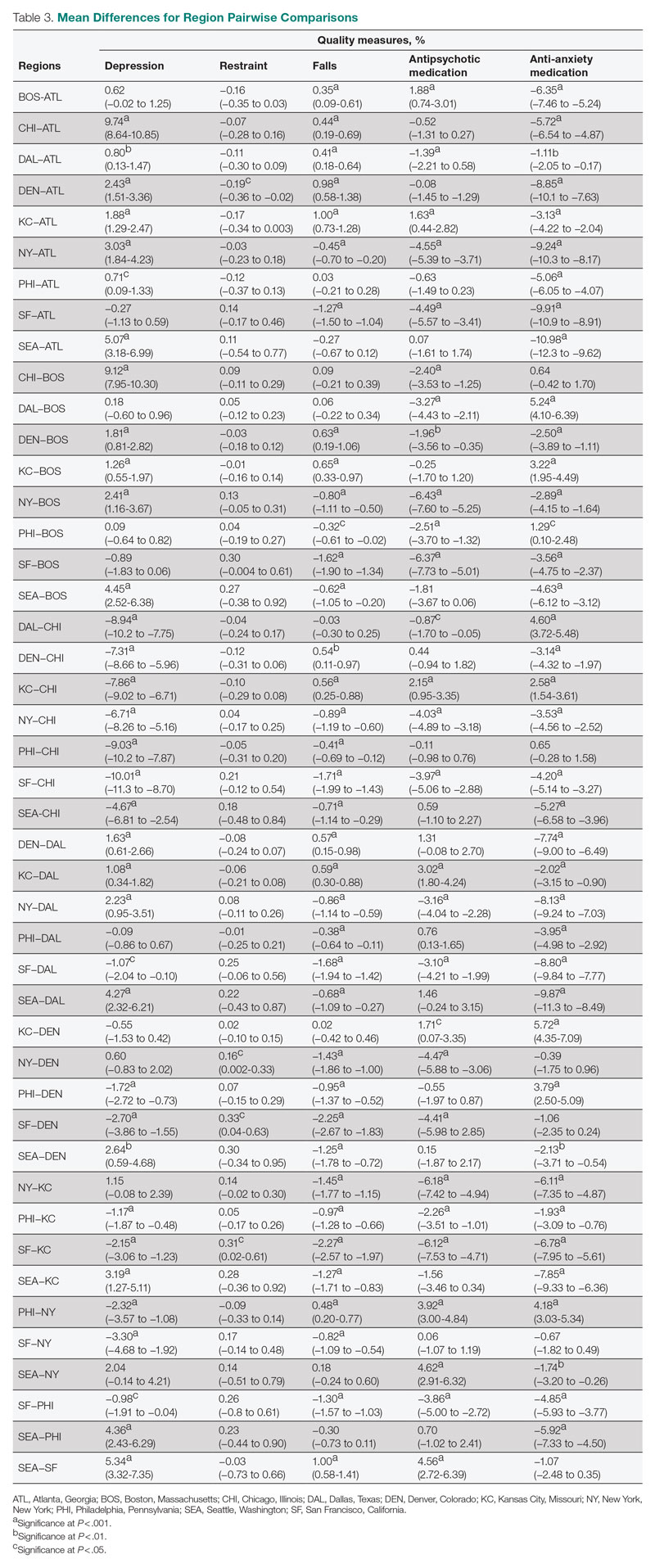
Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
Leading for High Reliability During the COVID-19 Pandemic: A Pilot Quality Improvement Initiative to Identify Challenges Faced and Lessons Learned
From the U.S. Department of Veterans Affairs (all authors), and Cognosante, LLC, Falls Church, VA (Dr. Murray, Dr. Sawyer, and Jessica Fankhauser).
Abstract
Objective: The COVID-19 pandemic posed unprecedented leadership challenges to health care organizations worldwide, especially those on the journey to high reliability. The objective of this pilot quality improvement initiative was to describe the experiences of medical center leaders continuing along the journey to high reliability during the pandemic.
Methods: A convenience sample of Veterans Health Administration medical center directors at facilities that had initiated the journey to high reliability prior to or during the COVID-19 pandemic were asked to complete a confidential survey to explore the challenges experienced and lessons learned.
Results: Of the 35 potential participants, 15 completed the confidential web-based survey. Five major themes emerged from participants’ responses: (1) managing competing priorities, (2) staying committed, (3) adapting and overcoming, (4) prioritizing competing demands, and (5) maintaining momentum.
Conclusion: This pilot quality improvement initiative provides some insight into the challenges experienced and lessons learned during the COVID-19 pandemic to help inform health care leaders’ responses during crises they may encounter along the journey to becoming a high reliability organization.
Keywords: HRO, leadership, patient safety.
Health care leaders worldwide agree that the
Maintaining continuous progress toward advancing high reliability organization (HRO) principles and practices can be especially challenging during crises of unprecedented scale such as the pandemic. HROs must be continually focused on achieving safety, quality, and efficiency goals by attending to the 3 pillars of HRO: culture, leadership, and continuous process improvement. HROs promote a culture where all staff across the organization watch for and report any unsafe conditions before these conditions pose a greater risk in the workplace. Hospital leaders, from executives to frontline managers, must be cognizant of all systems and processes that have the potential to affect patient care.12 All of the principles of HROs must continue without fail to ensure patient safety; these principles include preoccupation with failure, anticipating unexpected risks, sensitivity to dynamic and ever-changing operations, avoiding oversimplifications of identified problems, fostering resilience across the organization, and deferring to those with the expertise to make the best decisions regardless of position, rank, or title.12,13 Given the demands faced by leaders during crises with unprecedented disruption to normal operating procedures, it can be especially difficult to identify systemic challenges and apply lessons learned in a timely manner. However, it is critical to identify such lessons in order to continuously improve and to increase preparedness for subsequent crises.13,14
Because of the COVID-19 pandemic’s unprecedented nature in recent history, a review of the literature produced little evidence exploring the challenges experienced and lessons learned by health care leaders, especially as it relates to implementing or sustaining HRO journeys during the COVID-19 pandemic. Related literature published to date consists of editorials on reliability, uncertainty, and the management of errors15; patient safety and high reliability preventive strategies16; and authentic leadership.17 Five viewpoints were published on HROs and maladaptive stress behaviors,18 mindful organizing and organizational reliability,19 the practical essence of HROs,20 embracing principles of HROs in crisis,8 and using observation and high reliability strategies when facing an unprecedented safety threat.21 Finally, the authors identified 2 studies that used a qualitative research approach to explore leadership functions within an HRO when managing crises22 and organizational change in response to the COVID-19 pandemic.23 Due to the paucity of available information, the authors undertook a pilot quality improvement (QI) initiative to address this knowledge gap.
The aim of this initiative was to gain a better understanding of the challenges experienced, lessons learned, and recommendations to be shared by VHA medical center directors (MCDs) of health care facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The authors hope that this information will help health care leaders across both governmental and nongovernmental organizations, nationally and globally, to prepare for future pandemics, other unanticipated crises (eg, natural disasters, terrorist attacks), and major change initiatives (eg, electronic health record modernization) that may affect the delivery of safe, high-quality, and effective patient care. The initiative is described using the SQUIRE 2.0 guidelines.24,25
Methods
Survey
We used a qualitative approach and administered a confidential web-based survey, developed by the project team, to VHA MCDs at facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The survey consisted of 8 participant characteristic questions (Table 1) and 4 open-ended questions. The open-ended questions were designed to encourage MCD participants to freely provide detailed descriptions of the challenges experienced, lessons learned, recommendations for other health care leaders, and any additional information they believed was relevant.26,27 Participants were asked to respond to the following items:
- Please describe any challenges you experienced while in the role of MCD at a facility that initiated implementation of HRO principles and practices prior to (February 2020) or during (March 2020–September 2021) the initial onset of the COVID-19 pandemic.
- What are some lessons that you learned when responding to the COVID-19 pandemic while on the journey to high reliability?
- What recommendations would you like to make to other health care leaders to enable them to respond effectively to crises while on the journey to high reliability?
- Please provide any additional information that would be of value.
An invitation to participate in this pilot QI initiative was sent via e-mail to 35 potential participants, who were all MCDs at Cohort 1 and Cohort 2 facilities. The invitation was sent on June 17, 2022, by a VHA senior High Reliability Enterprise Support government team member not directly involved with the initiative.
The invitation included the objective of the initiative, estimated time to complete the confidential web-based survey, time allotted for responses to be submitted, and a link to the survey should potential participants agree to participate. Potential participants were informed that their involvement was voluntary, based on their willingness to participate and available time to complete the survey. Finally, the invitation noted that any comments provided would remain confidential and nonattributional for the purpose of publishing and presenting. The inclusion criteria for participation were: (1) serving
Data Gathering and Analysis
To minimize bias and maintain neutrality at the organizational level, only non-VHA individuals working on the project were directly involved with participants’ data review and analysis. Participant characteristics were analyzed using descriptive statistics. Responses to the 4 open-ended questions were coded and analyzed by an experienced researcher and coauthor using NVivo 11 qualitative data analysis software.28 To ensure trustworthiness (credibility, transferability, dependability, and confirmability) in the data analysis procedure,29 inductive thematic analysis was also performed manually using the methodologies of Braun and Clarke (Table 2)30 and Erlingsson and Brysiewicz.31 The goal of inductive analysis is to allow themes to emerge from the data while minimizing preconceptions.32,33 Regular team meetings were held to discuss and review the progress of data collection and analysis. The authors agreed that the themes were representative of the participants’ responses.
Institutional review board (IRB) review and approval were not required, as this project was a pilot QI initiative. The intent of the initiative was to explore ways to improve the quality of care delivered in the participants’ local care settings and not to generalize the findings. Under these circumstances, formal IRB review and approval of a QI initiative are not required.34 Participation in this pilot QI initiative was voluntary, and participants could withdraw at any time without consequences. Completion of the survey indicated consent. Confidentiality was ensured at all times by avoiding both the use of facility names and the collection of participant identifiers. Unique numbers were assigned to each participant. All comments provided by survey participants remained confidential and nonattributional for the purpose of publishing and presenting.
Results
Of the 35 potential participants, 15 VHA MCDs (43%) completed the confidential web-based survey. Out of the 17 potential participants in Cohort 1, 6 (35%) completed the survey. With Cohort 2, 9 (50%) of the potential 18 participants responded. Although saturation was reached at 10 responses, the additional completed surveys were included in the analysis. Saturation can be achieved with a small number of participants (n = 9–17), particularly when the potential participants are relatively homogenous and project aims are narrowly defined.35 Most participants had more than 10 years of executive-level experience and most medical centers had been on the journey to high reliability for more than 12 months at the time of the pandemic (Table 3).
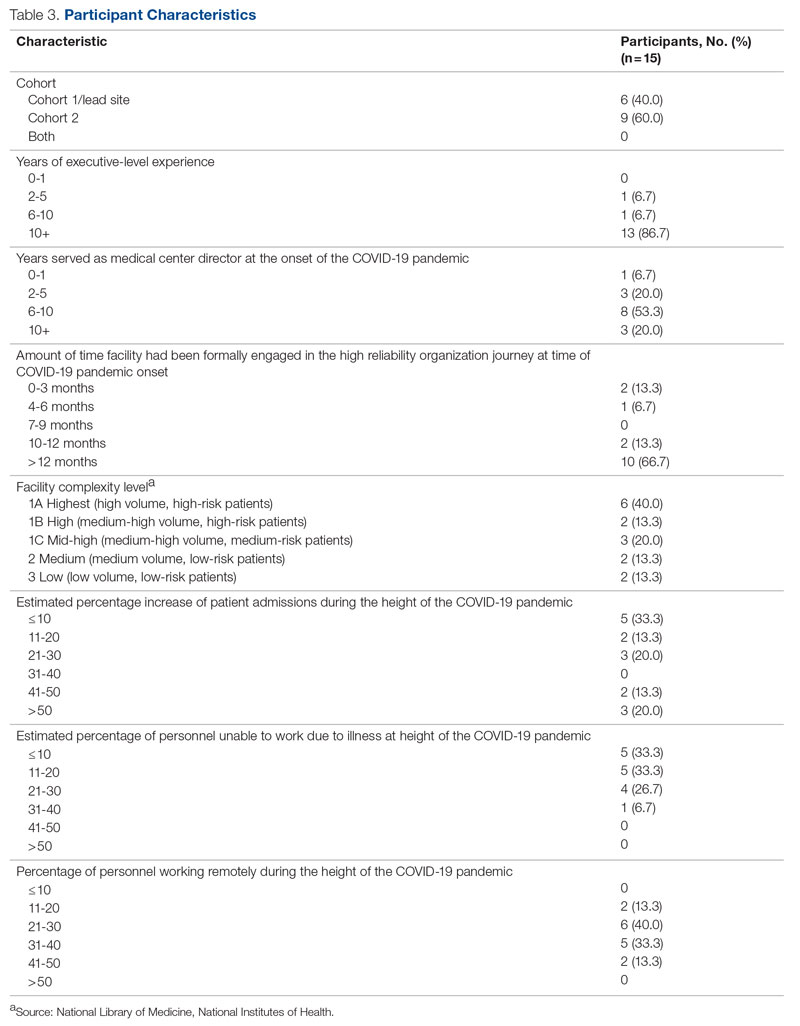
“There were too many competing priorities dealing with the pandemic and staffing crisis.” (Participant 8)
Other participants shared:
“We had our HRO mentor designated just as our first peak was descending on us. It was initially challenging to determine the proper pace of implementation when we clearly had other things going on. There was a real risk that people would say, ‘What, are you kidding?’ as we tried to roll this out.” (Participant 4)
“Prior to COVID, our main challenges were getting organized and operational rollout. During the pandemic, we had to shift our focus to COVID and the training aspects suffered. Also, many other priorities pulled us away from an HRO rollout focus.” (Participant 6)
“If you don’t need a highly reliable organization during a crisis, when do you need it? That was the message that we kicked off with. It was also VERY important to take things slowly. Education had to be done in bits, and we had a much more modest timeline than what would have been the norm for any initiative pre-COVID. The emphasis was on this being a long-term commitment, that we would be doing it the right way rather than rushing it, etc.” (Participant 4)
“Keeping HRO principles and a Just Culture on the forefront of our minds, we looked for opportunities to progress on our HRO journey, despite the challenges of the pandemic. Our monthly Town Halls became weekly events to share COVID updates and information with staff. We used the Town Halls to promote our HRO mission and to open communication lines with staff, designating 1 week each month as a ‘Safety Forum.’ The pandemic provided the springboard and backdrop for staff Safety Stories submissions, many of which were shared at our Town Halls and Safety Forums.” (Participant 7)
“We were able to utilize HRO principles in response to the COVID pandemic. Specifically standardized communication from the facility to VISN [Veterans Integrated Services Network] was initiated on a daily basis. This practice provided daily communication on key operational items and clinical items at the medical center, allowed timely feedback on actions being taken, as was instrumental in daily checks on staffing, COVID testing supplies, overall supply chain issues.” (Participant 9)
The recommendations provided by 10 participants (Cohort 1, n = 6; Cohort 2, n = 4) for other health care leaders experiencing a crisis during the journey to high reliability were insightful. The themes that frequently emerged from the responses to the survey were to adapt and overcome. Participants shared:
“Utilize the many tools you’re given, specifically your team. Try even the craziest ideas from frontline staff.” (Participant 1)
“Use your mentors for younger directors and, even if you think you know the answer, involve your staff. It makes them feel they have a voice and gives them ownership of the issues.” (Participant 5)
“Make sure that you have key leaders in place who are committed to HRO and can help the organization adjust.” (Participant 6)
“Take advantage of HRO Leader Coaching, which pairs MCDs with coaches who act as consultants for HRO leadership practices to ensure progress in reaching the next level in the journey to High Reliability.” (Participant 7)
“Meet regularly with the HRO Lead and team (more frequently during early stages of implementation) to provide support, eliminate barriers, and champion the HRO mission. It is important to include other members of the ELT [Executive Leadership Team] to ensure their involvement with the facility HRO strategic plan.” (Participant 7)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
The theme of prioritizing competing demands emerged again from 5 participants (Cohort 1, n = 3; Cohort 2, n = 2) with question 3 describing recommendations for other leaders:
“Your first priority is to the crisis. Don’t get distracted by this or any other initiative. That was not a very popular message for the people pushing HRO, but it is the reality and the necessity. However, it IS possible to move forward with HRO (or other important initiatives) during crisis times, as long as you carefully consider what you are asking of people and don’t overload/overwhelm them. It is not your ego (or that of Central Office) that needs to be stoked. If the initiative truly has value, you need to be patient to see it done properly, rather than rushed/pushed/forced. Don’t kill it by being overeager and overwhelming your already overtaxed people. That said, keep moving forward. The key is pacing—and remember that your Type A hard-driving leader types (you know who you are) will certainly fail if they push it. Or even if they go at a normal pace that would be appropriate for noncrisis times.” (Participant 4)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
“It was critical for us to always focus on the immediate workplace safety of staff (especially those on the frontlines of the pandemic response) when in the process of rolling out HRO initiatives.” (Participant 14)
Maintaining Momentum
“It seemed as though communication and education from VHA on HRO slowed down at the same time, which further slowed our progress. We are now trying to ramp our engagement up again.” (Participant 3)
“There can be synergy between crisis response and HRO implementation. As an example, one of the first steps we took was leadership rounding. That was necessary anyways for crisis management (raising the spirits on the front lines, so to speak). What we did was include scheduled time instead of (in addition to) ad hoc. And we got credit for taking an HRO step. I resisted whiteboards/visual management systems for a long time because (in my opinion) that would have been much too distracting during the crisis. Having waited for better times, I was able to move forward with that several months later and with good success.” (Participant 4)
Discussion
Health care leaders worldwide experienced an immense set of challenges because of the COVID-19 pandemic, which is a crisis of a magnitude with no parallel in modern times. Strong, adaptive leadership at all levels of health care systems was needed to effectively address the immense crisis at hand.36,37 Findings from this pilot QI initiative suggest that MCDs faced many new challenges, requiring them to perform unfamiliar tasks and manage numerous overlapping challenges (eg, staffing shortages and reassignments, safety concerns, changes to patient appointments, backlogs in essential services), all while also trying to continue with the journey to high reliability. Despite the challenges leaders faced, they recognized the need to manage competing priorities early and effectively. At times, the priority was to address the wide-ranging, urgent issues related to the pandemic. When the conditions improved, there was time to refocus efforts on important but longer-term activities related to the HRO journey. Other participants recognized that their commitment to HRO needed to remain a priority even during the periods of intense focus on COVID-19.
Some participants felt compelled to stay committed to the HRO journey despite numerous competing demands. They stayed committed to looking for opportunities to progress by implementing HRO principles and practices to achieve safety, quality, and efficiency goals. This dedication is noteworthy, especially in light of recently published research that demonstrates the vast number of patient safety issues that presented during the COVID-19 pandemic (eg, ineffective communication, poor teamwork, the absence of coordination)1 as well as perceptions that patient safety and quality of care had significantly declined as a result of the crisis.36,37
Participants also highlighted the need to be adaptive when responding to the complexity and unpredictability of the pandemic. Participants regularly sought ways to increase their knowledge, skills, and abilities by using the resources (eg, tools, experts) available to them. Research shows that in increasingly complex and ever-changing situation such as the COVID-19 pandemic, leaders must be adaptive with all levels of performance, especially when limited information is available.38,39
This is the first initiative of its kind to specifically explore the challenges experienced and lessons learned from health care leaders continuing along the journey to high reliability during the COVID-19 pandemic. Findings from this pilot QI initiative revealed that many participants recommended that leaders adapt and overcome challenges as much as possible when continuing with HRO during a crisis. These findings are echoed in the current literature suggesting that adaptive performance is a highly effective form of leadership during crises.38,40 Being able to effectively adapt during a crisis is essential for reducing further vulnerabilities across health care systems. In fact, this lesson is shared by many countries in response to the unprecedented global crisis.41A limitation of this pilot QI initiative is that the authors did not directly solicit responses from all VHA MCDs or from other health care executives (eg, Chief of Staff, Associate Director for Operations, Associate Director for Patient Care, and Nurse Executive). As such, our findings represent only a small segment of senior leadership perspectives from a large, integrated health care system. Individuals who did not respond to the survey may have had different experiences than those who did, and the authors excluded many MCDs who formally began their HRO journeys in 2022, well after the pandemic was underway. Similarly, the experiences of Veterans Affairs leaders may or may not be similar to that of other health care organizations. Although the goal of this initiative was to explore the participants’ experiences during the period of crisis, time and distance from the events at the height of the COVID-19 pandemic may have resulted in difficulty recalling information as well as making sense of the occurrence. This potential recall bias is a common occurrence in trying to explore past experiences, especially as they relate to crises. Finally, this pilot QI initiative did not explore personal challenges participants may have faced during this period of time (eg, burnout, personal or family illness), which may have also shaped their responses.
Conclusion
This initiative suggests that VHA MCDs often relied on HRO principles to guide and assist with their response to the COVID-19 pandemic, including managing periods of unprecedented crisis. The ability to adapt and prioritize was seen as an especially important lesson. Many MCDs continued their personal and organizational efforts toward high reliability even in periods of intense challenge because of the pandemic. These findings can help with future crises that may occur during an organization’s journey to high reliability. This pilot QI initiative’s findings warrant further investigation to explore the experiences of the broader range of health care leaders while responding to unplanned crises or even planned large-scale cultural change or technology modernization initiatives (eg, electronic health record modernization) to expand the state of the science of high reliability as well as inform policy and decision-making. Finally, another area for future study is examining how leadership responses vary across facilities, depending on factors such as leader roles, facility complexity level, resource availability, patient population characteristics, and organizational culture.
Acknowledgment: The authors express their sincere gratitude to the medical center directors who participated in this pilot study.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel St., Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
1. Editors: Dying in a leadership vacuum. 9.4N Engl J Med. 2020;383(15):1479-1480. doi:10.1056/NEJMe2029812
2. Geerts JM, Kinnair D, Taheri P, et al. Guidance for health care leaders during the recovery stage of the COVID-19 pandemic: a consensus statement. JAMA Netw Open. 2021;4(7):1-16. doi:10.1001/jamanetworkopen.2021.20295
3. Boiral O, Brotherton M-C, Rivaud L, et al. Organizations’ management of the COVID-19 pandemic: a scoping review of business articles. Sustainability. 2021;13:1-20. doi:10.3390/su13073993
4. Razu SR, Yasmin T, Arif TB, et al. Challenges faced by healthcare professionals during the COVID-19 pandemic: a qualitative inquiry from Bangladesh. Front Public Health. 2021;9:1-13. doi:10.3389/fpubh.2021.647315
5. Lyng HB, Ree E, Wibe T, et al. Healthcare leaders’ use of innovative solutions to ensure resilience in healthcare during the Covid-19 pandemic: a qualitative study in Norwegian nursing homes and home care services. BMC Health Serv Res. 2021;21(1):1-11. doi:1186/s12913-021-06923-1
6. Freitas J. Queiroz A, Bortotti I, et al. Nurse leaders’ challenges fighting the COVID-19 pandemic: a qualitative study. Open J Nurs. 2021;11:267-280. doi:10.4236/ojn.2021.115024
7. McGuire AL, Aulisio MP, Davis FD, et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the Association of Bioethics Program Directors (ABPD) Task Force. 9.4Am J Bioeth. 2020;20(7):15-27. doi:10.1080/15265161.2020.1764138
8. Turbow RM, Scibilia JP. Embracing principles of high reliability organizations can improve patient safety during pandemic. AAP News. January 19, 2021. Accessed March 1, 2023. https://publications.aap.org/aapnews/news/8975
9. Roberts BH, Damiano LA, Graham S, et al. A case study in fostering a learning culture in the context of Covid-19. American Association for Physician Leadership. June 24, 2021. Accessed March 1, 2023. https://www.physicianleaders.org/news/a-case-study-in-fostering-a-learning-culture-in-the-context-of-covid-19
10. U.S. Department of Veterans Affairs. Department of Veterans AffairsCOVID-19 National Summary. Veterans Affairs. Accessed December 4, 2022. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary
11. U.S. Department of Veterans Affairs. VA fourth mission summary. Veterans Affairs. Accessed December 4, 2022. https://www.va.gov/health/coronavirus/statesupport.asp#:~:text=As%20part%20of%20the%20Fourth,the%20facilities%20we%20are%20supporting
12. Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18(1):e320-e328. doi:10.1097/PTS.0000000000000768
13. Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. 9.4Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
14. Maison D, Jaworska D, Adamczyk D, et al. The challenges arising from the COVID-19 pandemic and the way people deal with them: a qualitative longitudinal study. PLoS One. 2021;16(10):1-17. doi:10.1371/journal.pone.0258133
15. Schulman PR. Reliability, uncertainty and the management of error: new perspectives in the COVID-19 era. J Contingencies Crisis Manag. 2022;30:92-101. doi:10.1111/1468-5973.12356
16. Adelman JS, Gandhi TK. COVID-19 and patient safety: time to tap into our investment in high reliability. J Patient Saf. 2021;17(4): 331-333. doi:10.1097/PTS.0000000000000843
17. Shingler-Nace A. COVID-19: when leadership calls. Nurs Lead. 2020;18(3):202-203. doi:10.1016/j.mnl.2020.03.017
18. Van Stralen D, Mercer TA. During pandemic COVID 19, the high reliability organization (HRO) identifies maladaptive stress behaviors: the stress-fear-threat cascade. Neonatol Tod. 2020;15(11):113-124. doi: 10.51362/neonatology.today/2020111511113124
19. Vogus TJ, Wilson AD, Randall K, et al. We’re all in this together: how COVID-19 revealed the coconstruction of mindful organising and organisational reliability. BMJ Qual Saf. 2022;31(3):230-233. doi:10.1136/bmjqs-2021-014068
20. Van Stralen D. Pragmatic high-reliability organization (HRO) during pandemic COVID-19. Neonatol Tod. 2020(4);15:109-117. doi:10.51362/neonatology.today/20208158109117
21. Thull-Freedman J, Mondoux S, Stang A, et al. Going to the COVID-19 Gemba: using observation and high reliability strategies to achieve safety in a time of crisis. CJEM. 2020;22(6):738-741. doi:10.1017/cem.2020.380
22. Sarihasan I, Dajnoki K, Oláh J, et al. The importance of the leadership functions of a high-reliability health care organization in managing the COVID-19 pandemic in Turkey. Econ Sociol. 2022;15:78-93. doi:10.14254/2071-789x.2022/15-1/5
23. Crain MA, Bush AL, Hayanga H, et al. Healthcare leadership in the COVID-19 pandemic: from innovative preparation to evolutionary transformation. J Health Leadersh. 2021;13:199-207. doi:10.2147/JHL.S319829
24. SQUIRE. Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) SQUIRE; 2020. Accessed March 1, 2023. http://www.squire-statement.org/index.cfm?fuseaction=Page.ViewPage&pageId=471
25. Lounsbury O. How to write a quality improvement project. Patient Safety J. 2022;4(1):65-67. doi:10.33940/culture/2022.3.6
26. Bengtsson M. How to plan and perform a qualitative study using content analysis. Nurs Plus Open. 2016;2:8-14. doi:10.1016/j.npls.2016.01.001
27. Allen M. The Sage Encyclopedia of Communication Research Methods. (Vols. 1-4). SAGE Publications, Inc; 2017
28. Unlock insights with qualitative data analysis software. Lumivero. Accessed March 2, 2023. https://lumivero.com/products/nvivo/
29. Maher C, Hadfield M, Hutchings M, et al. Ensuring rigor in qualitative data analysis: a design research approach to coding combining NVivo with traditional material methods. Int J Qual Methods. 2018;17:1-13. doi:10.1177/1609406918786362
30. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77-101. doi:10.1191/1478088706qp063oa
31. Erlingsson C, Brysiewicz P. A hands-on guide to doing content analysis. Afr J Emerg Med. 2017;7:93-99. doi:10.1016/j.afjem.2017.08.001
32. Vears DF, Gillam L. Inductive content analysis: a guide for beginning qualitative researchers. FoHPE. 2022;23:111-127. doi:10.11157/fohpe.v23i1.544
33. Nowell LS, Norris JM, White DE, et al. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. 2017;16:1-13. doi:10.1177/1609406917733847
34. Gautham KS, Pearlman S. Do quality improvement projects require IRB approval? J Perinatol. 2021;41:1209-1212. doi:10.1038/s41372-021-01038-1
35. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2022;292:1-10. doi:10.1016/j.socscimed.2021.114523
36. Balogun M, Dada FO, Oladimeji A, et al. Leading in a time of crisis: a qualitative study capturing experiences of health facility leaders during the early phases of the COVID-19 pandemic in Nigeria’s epicentre. Leadersh Health Serv (Bradf Engl). Published online May 12, 2022. doi:10.1108/lhs-02-2022-0017
37. Guttormson J, Calkins K, McAndrew N, et al. Critical care nurses’ experiences during the COVID-19 pandemic: a US national survey. Am J Crit Care. 2022;31:96-103. doi:10.4037/ajcc2022312
38. Bajaba A, Bajaba S, Algarni M, et al. Adaptive managers as emerging leaders during the COVID-19 crisis. Front Psychol. 2021;12:1-11. doi:10.3389/fpsyg.2021.661628
39. Ahern S, Loh E. Leadership during the COVID-19 pandemic: building and sustaining trust in times of uncertainty. BMJ Lead. 2021;59(4):266-269. doi.org/10.1136/leader-2020-000271
40. Cote R. Adaptive leadership approach with COVID 19 adaptive challenges. J Leadersh Account Ethics. 2022;19:34-44. doi:10.33423/jlae.v19i1.4992
41. Juvet TM, Corbaz-Kurth S, Roos P, et al. Adapting to the unexpected: problematic work situations and resilience strategies in healthcare institutions during the COVID-19 pandemic’s first wave. Saf Sci. 2021;139:1-9. doi:10.1016/j.ssci.2021.105277
From the U.S. Department of Veterans Affairs (all authors), and Cognosante, LLC, Falls Church, VA (Dr. Murray, Dr. Sawyer, and Jessica Fankhauser).
Abstract
Objective: The COVID-19 pandemic posed unprecedented leadership challenges to health care organizations worldwide, especially those on the journey to high reliability. The objective of this pilot quality improvement initiative was to describe the experiences of medical center leaders continuing along the journey to high reliability during the pandemic.
Methods: A convenience sample of Veterans Health Administration medical center directors at facilities that had initiated the journey to high reliability prior to or during the COVID-19 pandemic were asked to complete a confidential survey to explore the challenges experienced and lessons learned.
Results: Of the 35 potential participants, 15 completed the confidential web-based survey. Five major themes emerged from participants’ responses: (1) managing competing priorities, (2) staying committed, (3) adapting and overcoming, (4) prioritizing competing demands, and (5) maintaining momentum.
Conclusion: This pilot quality improvement initiative provides some insight into the challenges experienced and lessons learned during the COVID-19 pandemic to help inform health care leaders’ responses during crises they may encounter along the journey to becoming a high reliability organization.
Keywords: HRO, leadership, patient safety.
Health care leaders worldwide agree that the
Maintaining continuous progress toward advancing high reliability organization (HRO) principles and practices can be especially challenging during crises of unprecedented scale such as the pandemic. HROs must be continually focused on achieving safety, quality, and efficiency goals by attending to the 3 pillars of HRO: culture, leadership, and continuous process improvement. HROs promote a culture where all staff across the organization watch for and report any unsafe conditions before these conditions pose a greater risk in the workplace. Hospital leaders, from executives to frontline managers, must be cognizant of all systems and processes that have the potential to affect patient care.12 All of the principles of HROs must continue without fail to ensure patient safety; these principles include preoccupation with failure, anticipating unexpected risks, sensitivity to dynamic and ever-changing operations, avoiding oversimplifications of identified problems, fostering resilience across the organization, and deferring to those with the expertise to make the best decisions regardless of position, rank, or title.12,13 Given the demands faced by leaders during crises with unprecedented disruption to normal operating procedures, it can be especially difficult to identify systemic challenges and apply lessons learned in a timely manner. However, it is critical to identify such lessons in order to continuously improve and to increase preparedness for subsequent crises.13,14
Because of the COVID-19 pandemic’s unprecedented nature in recent history, a review of the literature produced little evidence exploring the challenges experienced and lessons learned by health care leaders, especially as it relates to implementing or sustaining HRO journeys during the COVID-19 pandemic. Related literature published to date consists of editorials on reliability, uncertainty, and the management of errors15; patient safety and high reliability preventive strategies16; and authentic leadership.17 Five viewpoints were published on HROs and maladaptive stress behaviors,18 mindful organizing and organizational reliability,19 the practical essence of HROs,20 embracing principles of HROs in crisis,8 and using observation and high reliability strategies when facing an unprecedented safety threat.21 Finally, the authors identified 2 studies that used a qualitative research approach to explore leadership functions within an HRO when managing crises22 and organizational change in response to the COVID-19 pandemic.23 Due to the paucity of available information, the authors undertook a pilot quality improvement (QI) initiative to address this knowledge gap.
The aim of this initiative was to gain a better understanding of the challenges experienced, lessons learned, and recommendations to be shared by VHA medical center directors (MCDs) of health care facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The authors hope that this information will help health care leaders across both governmental and nongovernmental organizations, nationally and globally, to prepare for future pandemics, other unanticipated crises (eg, natural disasters, terrorist attacks), and major change initiatives (eg, electronic health record modernization) that may affect the delivery of safe, high-quality, and effective patient care. The initiative is described using the SQUIRE 2.0 guidelines.24,25
Methods
Survey
We used a qualitative approach and administered a confidential web-based survey, developed by the project team, to VHA MCDs at facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The survey consisted of 8 participant characteristic questions (Table 1) and 4 open-ended questions. The open-ended questions were designed to encourage MCD participants to freely provide detailed descriptions of the challenges experienced, lessons learned, recommendations for other health care leaders, and any additional information they believed was relevant.26,27 Participants were asked to respond to the following items:
- Please describe any challenges you experienced while in the role of MCD at a facility that initiated implementation of HRO principles and practices prior to (February 2020) or during (March 2020–September 2021) the initial onset of the COVID-19 pandemic.
- What are some lessons that you learned when responding to the COVID-19 pandemic while on the journey to high reliability?
- What recommendations would you like to make to other health care leaders to enable them to respond effectively to crises while on the journey to high reliability?
- Please provide any additional information that would be of value.
An invitation to participate in this pilot QI initiative was sent via e-mail to 35 potential participants, who were all MCDs at Cohort 1 and Cohort 2 facilities. The invitation was sent on June 17, 2022, by a VHA senior High Reliability Enterprise Support government team member not directly involved with the initiative.
The invitation included the objective of the initiative, estimated time to complete the confidential web-based survey, time allotted for responses to be submitted, and a link to the survey should potential participants agree to participate. Potential participants were informed that their involvement was voluntary, based on their willingness to participate and available time to complete the survey. Finally, the invitation noted that any comments provided would remain confidential and nonattributional for the purpose of publishing and presenting. The inclusion criteria for participation were: (1) serving
Data Gathering and Analysis
To minimize bias and maintain neutrality at the organizational level, only non-VHA individuals working on the project were directly involved with participants’ data review and analysis. Participant characteristics were analyzed using descriptive statistics. Responses to the 4 open-ended questions were coded and analyzed by an experienced researcher and coauthor using NVivo 11 qualitative data analysis software.28 To ensure trustworthiness (credibility, transferability, dependability, and confirmability) in the data analysis procedure,29 inductive thematic analysis was also performed manually using the methodologies of Braun and Clarke (Table 2)30 and Erlingsson and Brysiewicz.31 The goal of inductive analysis is to allow themes to emerge from the data while minimizing preconceptions.32,33 Regular team meetings were held to discuss and review the progress of data collection and analysis. The authors agreed that the themes were representative of the participants’ responses.

Institutional review board (IRB) review and approval were not required, as this project was a pilot QI initiative. The intent of the initiative was to explore ways to improve the quality of care delivered in the participants’ local care settings and not to generalize the findings. Under these circumstances, formal IRB review and approval of a QI initiative are not required.34 Participation in this pilot QI initiative was voluntary, and participants could withdraw at any time without consequences. Completion of the survey indicated consent. Confidentiality was ensured at all times by avoiding both the use of facility names and the collection of participant identifiers. Unique numbers were assigned to each participant. All comments provided by survey participants remained confidential and nonattributional for the purpose of publishing and presenting.
Results
Of the 35 potential participants, 15 VHA MCDs (43%) completed the confidential web-based survey. Out of the 17 potential participants in Cohort 1, 6 (35%) completed the survey. With Cohort 2, 9 (50%) of the potential 18 participants responded. Although saturation was reached at 10 responses, the additional completed surveys were included in the analysis. Saturation can be achieved with a small number of participants (n = 9–17), particularly when the potential participants are relatively homogenous and project aims are narrowly defined.35 Most participants had more than 10 years of executive-level experience and most medical centers had been on the journey to high reliability for more than 12 months at the time of the pandemic (Table 3).

“There were too many competing priorities dealing with the pandemic and staffing crisis.” (Participant 8)
Other participants shared:
“We had our HRO mentor designated just as our first peak was descending on us. It was initially challenging to determine the proper pace of implementation when we clearly had other things going on. There was a real risk that people would say, ‘What, are you kidding?’ as we tried to roll this out.” (Participant 4)
“Prior to COVID, our main challenges were getting organized and operational rollout. During the pandemic, we had to shift our focus to COVID and the training aspects suffered. Also, many other priorities pulled us away from an HRO rollout focus.” (Participant 6)
“If you don’t need a highly reliable organization during a crisis, when do you need it? That was the message that we kicked off with. It was also VERY important to take things slowly. Education had to be done in bits, and we had a much more modest timeline than what would have been the norm for any initiative pre-COVID. The emphasis was on this being a long-term commitment, that we would be doing it the right way rather than rushing it, etc.” (Participant 4)
“Keeping HRO principles and a Just Culture on the forefront of our minds, we looked for opportunities to progress on our HRO journey, despite the challenges of the pandemic. Our monthly Town Halls became weekly events to share COVID updates and information with staff. We used the Town Halls to promote our HRO mission and to open communication lines with staff, designating 1 week each month as a ‘Safety Forum.’ The pandemic provided the springboard and backdrop for staff Safety Stories submissions, many of which were shared at our Town Halls and Safety Forums.” (Participant 7)
“We were able to utilize HRO principles in response to the COVID pandemic. Specifically standardized communication from the facility to VISN [Veterans Integrated Services Network] was initiated on a daily basis. This practice provided daily communication on key operational items and clinical items at the medical center, allowed timely feedback on actions being taken, as was instrumental in daily checks on staffing, COVID testing supplies, overall supply chain issues.” (Participant 9)
The recommendations provided by 10 participants (Cohort 1, n = 6; Cohort 2, n = 4) for other health care leaders experiencing a crisis during the journey to high reliability were insightful. The themes that frequently emerged from the responses to the survey were to adapt and overcome. Participants shared:
“Utilize the many tools you’re given, specifically your team. Try even the craziest ideas from frontline staff.” (Participant 1)
“Use your mentors for younger directors and, even if you think you know the answer, involve your staff. It makes them feel they have a voice and gives them ownership of the issues.” (Participant 5)
“Make sure that you have key leaders in place who are committed to HRO and can help the organization adjust.” (Participant 6)
“Take advantage of HRO Leader Coaching, which pairs MCDs with coaches who act as consultants for HRO leadership practices to ensure progress in reaching the next level in the journey to High Reliability.” (Participant 7)
“Meet regularly with the HRO Lead and team (more frequently during early stages of implementation) to provide support, eliminate barriers, and champion the HRO mission. It is important to include other members of the ELT [Executive Leadership Team] to ensure their involvement with the facility HRO strategic plan.” (Participant 7)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
The theme of prioritizing competing demands emerged again from 5 participants (Cohort 1, n = 3; Cohort 2, n = 2) with question 3 describing recommendations for other leaders:
“Your first priority is to the crisis. Don’t get distracted by this or any other initiative. That was not a very popular message for the people pushing HRO, but it is the reality and the necessity. However, it IS possible to move forward with HRO (or other important initiatives) during crisis times, as long as you carefully consider what you are asking of people and don’t overload/overwhelm them. It is not your ego (or that of Central Office) that needs to be stoked. If the initiative truly has value, you need to be patient to see it done properly, rather than rushed/pushed/forced. Don’t kill it by being overeager and overwhelming your already overtaxed people. That said, keep moving forward. The key is pacing—and remember that your Type A hard-driving leader types (you know who you are) will certainly fail if they push it. Or even if they go at a normal pace that would be appropriate for noncrisis times.” (Participant 4)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
“It was critical for us to always focus on the immediate workplace safety of staff (especially those on the frontlines of the pandemic response) when in the process of rolling out HRO initiatives.” (Participant 14)
Maintaining Momentum
“It seemed as though communication and education from VHA on HRO slowed down at the same time, which further slowed our progress. We are now trying to ramp our engagement up again.” (Participant 3)
“There can be synergy between crisis response and HRO implementation. As an example, one of the first steps we took was leadership rounding. That was necessary anyways for crisis management (raising the spirits on the front lines, so to speak). What we did was include scheduled time instead of (in addition to) ad hoc. And we got credit for taking an HRO step. I resisted whiteboards/visual management systems for a long time because (in my opinion) that would have been much too distracting during the crisis. Having waited for better times, I was able to move forward with that several months later and with good success.” (Participant 4)
Discussion
Health care leaders worldwide experienced an immense set of challenges because of the COVID-19 pandemic, which is a crisis of a magnitude with no parallel in modern times. Strong, adaptive leadership at all levels of health care systems was needed to effectively address the immense crisis at hand.36,37 Findings from this pilot QI initiative suggest that MCDs faced many new challenges, requiring them to perform unfamiliar tasks and manage numerous overlapping challenges (eg, staffing shortages and reassignments, safety concerns, changes to patient appointments, backlogs in essential services), all while also trying to continue with the journey to high reliability. Despite the challenges leaders faced, they recognized the need to manage competing priorities early and effectively. At times, the priority was to address the wide-ranging, urgent issues related to the pandemic. When the conditions improved, there was time to refocus efforts on important but longer-term activities related to the HRO journey. Other participants recognized that their commitment to HRO needed to remain a priority even during the periods of intense focus on COVID-19.
Some participants felt compelled to stay committed to the HRO journey despite numerous competing demands. They stayed committed to looking for opportunities to progress by implementing HRO principles and practices to achieve safety, quality, and efficiency goals. This dedication is noteworthy, especially in light of recently published research that demonstrates the vast number of patient safety issues that presented during the COVID-19 pandemic (eg, ineffective communication, poor teamwork, the absence of coordination)1 as well as perceptions that patient safety and quality of care had significantly declined as a result of the crisis.36,37
Participants also highlighted the need to be adaptive when responding to the complexity and unpredictability of the pandemic. Participants regularly sought ways to increase their knowledge, skills, and abilities by using the resources (eg, tools, experts) available to them. Research shows that in increasingly complex and ever-changing situation such as the COVID-19 pandemic, leaders must be adaptive with all levels of performance, especially when limited information is available.38,39
This is the first initiative of its kind to specifically explore the challenges experienced and lessons learned from health care leaders continuing along the journey to high reliability during the COVID-19 pandemic. Findings from this pilot QI initiative revealed that many participants recommended that leaders adapt and overcome challenges as much as possible when continuing with HRO during a crisis. These findings are echoed in the current literature suggesting that adaptive performance is a highly effective form of leadership during crises.38,40 Being able to effectively adapt during a crisis is essential for reducing further vulnerabilities across health care systems. In fact, this lesson is shared by many countries in response to the unprecedented global crisis.41A limitation of this pilot QI initiative is that the authors did not directly solicit responses from all VHA MCDs or from other health care executives (eg, Chief of Staff, Associate Director for Operations, Associate Director for Patient Care, and Nurse Executive). As such, our findings represent only a small segment of senior leadership perspectives from a large, integrated health care system. Individuals who did not respond to the survey may have had different experiences than those who did, and the authors excluded many MCDs who formally began their HRO journeys in 2022, well after the pandemic was underway. Similarly, the experiences of Veterans Affairs leaders may or may not be similar to that of other health care organizations. Although the goal of this initiative was to explore the participants’ experiences during the period of crisis, time and distance from the events at the height of the COVID-19 pandemic may have resulted in difficulty recalling information as well as making sense of the occurrence. This potential recall bias is a common occurrence in trying to explore past experiences, especially as they relate to crises. Finally, this pilot QI initiative did not explore personal challenges participants may have faced during this period of time (eg, burnout, personal or family illness), which may have also shaped their responses.
Conclusion
This initiative suggests that VHA MCDs often relied on HRO principles to guide and assist with their response to the COVID-19 pandemic, including managing periods of unprecedented crisis. The ability to adapt and prioritize was seen as an especially important lesson. Many MCDs continued their personal and organizational efforts toward high reliability even in periods of intense challenge because of the pandemic. These findings can help with future crises that may occur during an organization’s journey to high reliability. This pilot QI initiative’s findings warrant further investigation to explore the experiences of the broader range of health care leaders while responding to unplanned crises or even planned large-scale cultural change or technology modernization initiatives (eg, electronic health record modernization) to expand the state of the science of high reliability as well as inform policy and decision-making. Finally, another area for future study is examining how leadership responses vary across facilities, depending on factors such as leader roles, facility complexity level, resource availability, patient population characteristics, and organizational culture.
Acknowledgment: The authors express their sincere gratitude to the medical center directors who participated in this pilot study.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel St., Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
From the U.S. Department of Veterans Affairs (all authors), and Cognosante, LLC, Falls Church, VA (Dr. Murray, Dr. Sawyer, and Jessica Fankhauser).
Abstract
Objective: The COVID-19 pandemic posed unprecedented leadership challenges to health care organizations worldwide, especially those on the journey to high reliability. The objective of this pilot quality improvement initiative was to describe the experiences of medical center leaders continuing along the journey to high reliability during the pandemic.
Methods: A convenience sample of Veterans Health Administration medical center directors at facilities that had initiated the journey to high reliability prior to or during the COVID-19 pandemic were asked to complete a confidential survey to explore the challenges experienced and lessons learned.
Results: Of the 35 potential participants, 15 completed the confidential web-based survey. Five major themes emerged from participants’ responses: (1) managing competing priorities, (2) staying committed, (3) adapting and overcoming, (4) prioritizing competing demands, and (5) maintaining momentum.
Conclusion: This pilot quality improvement initiative provides some insight into the challenges experienced and lessons learned during the COVID-19 pandemic to help inform health care leaders’ responses during crises they may encounter along the journey to becoming a high reliability organization.
Keywords: HRO, leadership, patient safety.
Health care leaders worldwide agree that the
Maintaining continuous progress toward advancing high reliability organization (HRO) principles and practices can be especially challenging during crises of unprecedented scale such as the pandemic. HROs must be continually focused on achieving safety, quality, and efficiency goals by attending to the 3 pillars of HRO: culture, leadership, and continuous process improvement. HROs promote a culture where all staff across the organization watch for and report any unsafe conditions before these conditions pose a greater risk in the workplace. Hospital leaders, from executives to frontline managers, must be cognizant of all systems and processes that have the potential to affect patient care.12 All of the principles of HROs must continue without fail to ensure patient safety; these principles include preoccupation with failure, anticipating unexpected risks, sensitivity to dynamic and ever-changing operations, avoiding oversimplifications of identified problems, fostering resilience across the organization, and deferring to those with the expertise to make the best decisions regardless of position, rank, or title.12,13 Given the demands faced by leaders during crises with unprecedented disruption to normal operating procedures, it can be especially difficult to identify systemic challenges and apply lessons learned in a timely manner. However, it is critical to identify such lessons in order to continuously improve and to increase preparedness for subsequent crises.13,14
Because of the COVID-19 pandemic’s unprecedented nature in recent history, a review of the literature produced little evidence exploring the challenges experienced and lessons learned by health care leaders, especially as it relates to implementing or sustaining HRO journeys during the COVID-19 pandemic. Related literature published to date consists of editorials on reliability, uncertainty, and the management of errors15; patient safety and high reliability preventive strategies16; and authentic leadership.17 Five viewpoints were published on HROs and maladaptive stress behaviors,18 mindful organizing and organizational reliability,19 the practical essence of HROs,20 embracing principles of HROs in crisis,8 and using observation and high reliability strategies when facing an unprecedented safety threat.21 Finally, the authors identified 2 studies that used a qualitative research approach to explore leadership functions within an HRO when managing crises22 and organizational change in response to the COVID-19 pandemic.23 Due to the paucity of available information, the authors undertook a pilot quality improvement (QI) initiative to address this knowledge gap.
The aim of this initiative was to gain a better understanding of the challenges experienced, lessons learned, and recommendations to be shared by VHA medical center directors (MCDs) of health care facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The authors hope that this information will help health care leaders across both governmental and nongovernmental organizations, nationally and globally, to prepare for future pandemics, other unanticipated crises (eg, natural disasters, terrorist attacks), and major change initiatives (eg, electronic health record modernization) that may affect the delivery of safe, high-quality, and effective patient care. The initiative is described using the SQUIRE 2.0 guidelines.24,25
Methods
Survey
We used a qualitative approach and administered a confidential web-based survey, developed by the project team, to VHA MCDs at facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The survey consisted of 8 participant characteristic questions (Table 1) and 4 open-ended questions. The open-ended questions were designed to encourage MCD participants to freely provide detailed descriptions of the challenges experienced, lessons learned, recommendations for other health care leaders, and any additional information they believed was relevant.26,27 Participants were asked to respond to the following items:
- Please describe any challenges you experienced while in the role of MCD at a facility that initiated implementation of HRO principles and practices prior to (February 2020) or during (March 2020–September 2021) the initial onset of the COVID-19 pandemic.
- What are some lessons that you learned when responding to the COVID-19 pandemic while on the journey to high reliability?
- What recommendations would you like to make to other health care leaders to enable them to respond effectively to crises while on the journey to high reliability?
- Please provide any additional information that would be of value.
An invitation to participate in this pilot QI initiative was sent via e-mail to 35 potential participants, who were all MCDs at Cohort 1 and Cohort 2 facilities. The invitation was sent on June 17, 2022, by a VHA senior High Reliability Enterprise Support government team member not directly involved with the initiative.
The invitation included the objective of the initiative, estimated time to complete the confidential web-based survey, time allotted for responses to be submitted, and a link to the survey should potential participants agree to participate. Potential participants were informed that their involvement was voluntary, based on their willingness to participate and available time to complete the survey. Finally, the invitation noted that any comments provided would remain confidential and nonattributional for the purpose of publishing and presenting. The inclusion criteria for participation were: (1) serving
Data Gathering and Analysis
To minimize bias and maintain neutrality at the organizational level, only non-VHA individuals working on the project were directly involved with participants’ data review and analysis. Participant characteristics were analyzed using descriptive statistics. Responses to the 4 open-ended questions were coded and analyzed by an experienced researcher and coauthor using NVivo 11 qualitative data analysis software.28 To ensure trustworthiness (credibility, transferability, dependability, and confirmability) in the data analysis procedure,29 inductive thematic analysis was also performed manually using the methodologies of Braun and Clarke (Table 2)30 and Erlingsson and Brysiewicz.31 The goal of inductive analysis is to allow themes to emerge from the data while minimizing preconceptions.32,33 Regular team meetings were held to discuss and review the progress of data collection and analysis. The authors agreed that the themes were representative of the participants’ responses.

Institutional review board (IRB) review and approval were not required, as this project was a pilot QI initiative. The intent of the initiative was to explore ways to improve the quality of care delivered in the participants’ local care settings and not to generalize the findings. Under these circumstances, formal IRB review and approval of a QI initiative are not required.34 Participation in this pilot QI initiative was voluntary, and participants could withdraw at any time without consequences. Completion of the survey indicated consent. Confidentiality was ensured at all times by avoiding both the use of facility names and the collection of participant identifiers. Unique numbers were assigned to each participant. All comments provided by survey participants remained confidential and nonattributional for the purpose of publishing and presenting.
Results
Of the 35 potential participants, 15 VHA MCDs (43%) completed the confidential web-based survey. Out of the 17 potential participants in Cohort 1, 6 (35%) completed the survey. With Cohort 2, 9 (50%) of the potential 18 participants responded. Although saturation was reached at 10 responses, the additional completed surveys were included in the analysis. Saturation can be achieved with a small number of participants (n = 9–17), particularly when the potential participants are relatively homogenous and project aims are narrowly defined.35 Most participants had more than 10 years of executive-level experience and most medical centers had been on the journey to high reliability for more than 12 months at the time of the pandemic (Table 3).

“There were too many competing priorities dealing with the pandemic and staffing crisis.” (Participant 8)
Other participants shared:
“We had our HRO mentor designated just as our first peak was descending on us. It was initially challenging to determine the proper pace of implementation when we clearly had other things going on. There was a real risk that people would say, ‘What, are you kidding?’ as we tried to roll this out.” (Participant 4)
“Prior to COVID, our main challenges were getting organized and operational rollout. During the pandemic, we had to shift our focus to COVID and the training aspects suffered. Also, many other priorities pulled us away from an HRO rollout focus.” (Participant 6)
“If you don’t need a highly reliable organization during a crisis, when do you need it? That was the message that we kicked off with. It was also VERY important to take things slowly. Education had to be done in bits, and we had a much more modest timeline than what would have been the norm for any initiative pre-COVID. The emphasis was on this being a long-term commitment, that we would be doing it the right way rather than rushing it, etc.” (Participant 4)
“Keeping HRO principles and a Just Culture on the forefront of our minds, we looked for opportunities to progress on our HRO journey, despite the challenges of the pandemic. Our monthly Town Halls became weekly events to share COVID updates and information with staff. We used the Town Halls to promote our HRO mission and to open communication lines with staff, designating 1 week each month as a ‘Safety Forum.’ The pandemic provided the springboard and backdrop for staff Safety Stories submissions, many of which were shared at our Town Halls and Safety Forums.” (Participant 7)
“We were able to utilize HRO principles in response to the COVID pandemic. Specifically standardized communication from the facility to VISN [Veterans Integrated Services Network] was initiated on a daily basis. This practice provided daily communication on key operational items and clinical items at the medical center, allowed timely feedback on actions being taken, as was instrumental in daily checks on staffing, COVID testing supplies, overall supply chain issues.” (Participant 9)
The recommendations provided by 10 participants (Cohort 1, n = 6; Cohort 2, n = 4) for other health care leaders experiencing a crisis during the journey to high reliability were insightful. The themes that frequently emerged from the responses to the survey were to adapt and overcome. Participants shared:
“Utilize the many tools you’re given, specifically your team. Try even the craziest ideas from frontline staff.” (Participant 1)
“Use your mentors for younger directors and, even if you think you know the answer, involve your staff. It makes them feel they have a voice and gives them ownership of the issues.” (Participant 5)
“Make sure that you have key leaders in place who are committed to HRO and can help the organization adjust.” (Participant 6)
“Take advantage of HRO Leader Coaching, which pairs MCDs with coaches who act as consultants for HRO leadership practices to ensure progress in reaching the next level in the journey to High Reliability.” (Participant 7)
“Meet regularly with the HRO Lead and team (more frequently during early stages of implementation) to provide support, eliminate barriers, and champion the HRO mission. It is important to include other members of the ELT [Executive Leadership Team] to ensure their involvement with the facility HRO strategic plan.” (Participant 7)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
The theme of prioritizing competing demands emerged again from 5 participants (Cohort 1, n = 3; Cohort 2, n = 2) with question 3 describing recommendations for other leaders:
“Your first priority is to the crisis. Don’t get distracted by this or any other initiative. That was not a very popular message for the people pushing HRO, but it is the reality and the necessity. However, it IS possible to move forward with HRO (or other important initiatives) during crisis times, as long as you carefully consider what you are asking of people and don’t overload/overwhelm them. It is not your ego (or that of Central Office) that needs to be stoked. If the initiative truly has value, you need to be patient to see it done properly, rather than rushed/pushed/forced. Don’t kill it by being overeager and overwhelming your already overtaxed people. That said, keep moving forward. The key is pacing—and remember that your Type A hard-driving leader types (you know who you are) will certainly fail if they push it. Or even if they go at a normal pace that would be appropriate for noncrisis times.” (Participant 4)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
“It was critical for us to always focus on the immediate workplace safety of staff (especially those on the frontlines of the pandemic response) when in the process of rolling out HRO initiatives.” (Participant 14)
Maintaining Momentum
“It seemed as though communication and education from VHA on HRO slowed down at the same time, which further slowed our progress. We are now trying to ramp our engagement up again.” (Participant 3)
“There can be synergy between crisis response and HRO implementation. As an example, one of the first steps we took was leadership rounding. That was necessary anyways for crisis management (raising the spirits on the front lines, so to speak). What we did was include scheduled time instead of (in addition to) ad hoc. And we got credit for taking an HRO step. I resisted whiteboards/visual management systems for a long time because (in my opinion) that would have been much too distracting during the crisis. Having waited for better times, I was able to move forward with that several months later and with good success.” (Participant 4)
Discussion
Health care leaders worldwide experienced an immense set of challenges because of the COVID-19 pandemic, which is a crisis of a magnitude with no parallel in modern times. Strong, adaptive leadership at all levels of health care systems was needed to effectively address the immense crisis at hand.36,37 Findings from this pilot QI initiative suggest that MCDs faced many new challenges, requiring them to perform unfamiliar tasks and manage numerous overlapping challenges (eg, staffing shortages and reassignments, safety concerns, changes to patient appointments, backlogs in essential services), all while also trying to continue with the journey to high reliability. Despite the challenges leaders faced, they recognized the need to manage competing priorities early and effectively. At times, the priority was to address the wide-ranging, urgent issues related to the pandemic. When the conditions improved, there was time to refocus efforts on important but longer-term activities related to the HRO journey. Other participants recognized that their commitment to HRO needed to remain a priority even during the periods of intense focus on COVID-19.
Some participants felt compelled to stay committed to the HRO journey despite numerous competing demands. They stayed committed to looking for opportunities to progress by implementing HRO principles and practices to achieve safety, quality, and efficiency goals. This dedication is noteworthy, especially in light of recently published research that demonstrates the vast number of patient safety issues that presented during the COVID-19 pandemic (eg, ineffective communication, poor teamwork, the absence of coordination)1 as well as perceptions that patient safety and quality of care had significantly declined as a result of the crisis.36,37
Participants also highlighted the need to be adaptive when responding to the complexity and unpredictability of the pandemic. Participants regularly sought ways to increase their knowledge, skills, and abilities by using the resources (eg, tools, experts) available to them. Research shows that in increasingly complex and ever-changing situation such as the COVID-19 pandemic, leaders must be adaptive with all levels of performance, especially when limited information is available.38,39
This is the first initiative of its kind to specifically explore the challenges experienced and lessons learned from health care leaders continuing along the journey to high reliability during the COVID-19 pandemic. Findings from this pilot QI initiative revealed that many participants recommended that leaders adapt and overcome challenges as much as possible when continuing with HRO during a crisis. These findings are echoed in the current literature suggesting that adaptive performance is a highly effective form of leadership during crises.38,40 Being able to effectively adapt during a crisis is essential for reducing further vulnerabilities across health care systems. In fact, this lesson is shared by many countries in response to the unprecedented global crisis.41A limitation of this pilot QI initiative is that the authors did not directly solicit responses from all VHA MCDs or from other health care executives (eg, Chief of Staff, Associate Director for Operations, Associate Director for Patient Care, and Nurse Executive). As such, our findings represent only a small segment of senior leadership perspectives from a large, integrated health care system. Individuals who did not respond to the survey may have had different experiences than those who did, and the authors excluded many MCDs who formally began their HRO journeys in 2022, well after the pandemic was underway. Similarly, the experiences of Veterans Affairs leaders may or may not be similar to that of other health care organizations. Although the goal of this initiative was to explore the participants’ experiences during the period of crisis, time and distance from the events at the height of the COVID-19 pandemic may have resulted in difficulty recalling information as well as making sense of the occurrence. This potential recall bias is a common occurrence in trying to explore past experiences, especially as they relate to crises. Finally, this pilot QI initiative did not explore personal challenges participants may have faced during this period of time (eg, burnout, personal or family illness), which may have also shaped their responses.
Conclusion
This initiative suggests that VHA MCDs often relied on HRO principles to guide and assist with their response to the COVID-19 pandemic, including managing periods of unprecedented crisis. The ability to adapt and prioritize was seen as an especially important lesson. Many MCDs continued their personal and organizational efforts toward high reliability even in periods of intense challenge because of the pandemic. These findings can help with future crises that may occur during an organization’s journey to high reliability. This pilot QI initiative’s findings warrant further investigation to explore the experiences of the broader range of health care leaders while responding to unplanned crises or even planned large-scale cultural change or technology modernization initiatives (eg, electronic health record modernization) to expand the state of the science of high reliability as well as inform policy and decision-making. Finally, another area for future study is examining how leadership responses vary across facilities, depending on factors such as leader roles, facility complexity level, resource availability, patient population characteristics, and organizational culture.
Acknowledgment: The authors express their sincere gratitude to the medical center directors who participated in this pilot study.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel St., Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
1. Editors: Dying in a leadership vacuum. 9.4N Engl J Med. 2020;383(15):1479-1480. doi:10.1056/NEJMe2029812
2. Geerts JM, Kinnair D, Taheri P, et al. Guidance for health care leaders during the recovery stage of the COVID-19 pandemic: a consensus statement. JAMA Netw Open. 2021;4(7):1-16. doi:10.1001/jamanetworkopen.2021.20295
3. Boiral O, Brotherton M-C, Rivaud L, et al. Organizations’ management of the COVID-19 pandemic: a scoping review of business articles. Sustainability. 2021;13:1-20. doi:10.3390/su13073993
4. Razu SR, Yasmin T, Arif TB, et al. Challenges faced by healthcare professionals during the COVID-19 pandemic: a qualitative inquiry from Bangladesh. Front Public Health. 2021;9:1-13. doi:10.3389/fpubh.2021.647315
5. Lyng HB, Ree E, Wibe T, et al. Healthcare leaders’ use of innovative solutions to ensure resilience in healthcare during the Covid-19 pandemic: a qualitative study in Norwegian nursing homes and home care services. BMC Health Serv Res. 2021;21(1):1-11. doi:1186/s12913-021-06923-1
6. Freitas J. Queiroz A, Bortotti I, et al. Nurse leaders’ challenges fighting the COVID-19 pandemic: a qualitative study. Open J Nurs. 2021;11:267-280. doi:10.4236/ojn.2021.115024
7. McGuire AL, Aulisio MP, Davis FD, et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the Association of Bioethics Program Directors (ABPD) Task Force. 9.4Am J Bioeth. 2020;20(7):15-27. doi:10.1080/15265161.2020.1764138
8. Turbow RM, Scibilia JP. Embracing principles of high reliability organizations can improve patient safety during pandemic. AAP News. January 19, 2021. Accessed March 1, 2023. https://publications.aap.org/aapnews/news/8975
9. Roberts BH, Damiano LA, Graham S, et al. A case study in fostering a learning culture in the context of Covid-19. American Association for Physician Leadership. June 24, 2021. Accessed March 1, 2023. https://www.physicianleaders.org/news/a-case-study-in-fostering-a-learning-culture-in-the-context-of-covid-19
10. U.S. Department of Veterans Affairs. Department of Veterans AffairsCOVID-19 National Summary. Veterans Affairs. Accessed December 4, 2022. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary
11. U.S. Department of Veterans Affairs. VA fourth mission summary. Veterans Affairs. Accessed December 4, 2022. https://www.va.gov/health/coronavirus/statesupport.asp#:~:text=As%20part%20of%20the%20Fourth,the%20facilities%20we%20are%20supporting
12. Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18(1):e320-e328. doi:10.1097/PTS.0000000000000768
13. Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. 9.4Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
14. Maison D, Jaworska D, Adamczyk D, et al. The challenges arising from the COVID-19 pandemic and the way people deal with them: a qualitative longitudinal study. PLoS One. 2021;16(10):1-17. doi:10.1371/journal.pone.0258133
15. Schulman PR. Reliability, uncertainty and the management of error: new perspectives in the COVID-19 era. J Contingencies Crisis Manag. 2022;30:92-101. doi:10.1111/1468-5973.12356
16. Adelman JS, Gandhi TK. COVID-19 and patient safety: time to tap into our investment in high reliability. J Patient Saf. 2021;17(4): 331-333. doi:10.1097/PTS.0000000000000843
17. Shingler-Nace A. COVID-19: when leadership calls. Nurs Lead. 2020;18(3):202-203. doi:10.1016/j.mnl.2020.03.017
18. Van Stralen D, Mercer TA. During pandemic COVID 19, the high reliability organization (HRO) identifies maladaptive stress behaviors: the stress-fear-threat cascade. Neonatol Tod. 2020;15(11):113-124. doi: 10.51362/neonatology.today/2020111511113124
19. Vogus TJ, Wilson AD, Randall K, et al. We’re all in this together: how COVID-19 revealed the coconstruction of mindful organising and organisational reliability. BMJ Qual Saf. 2022;31(3):230-233. doi:10.1136/bmjqs-2021-014068
20. Van Stralen D. Pragmatic high-reliability organization (HRO) during pandemic COVID-19. Neonatol Tod. 2020(4);15:109-117. doi:10.51362/neonatology.today/20208158109117
21. Thull-Freedman J, Mondoux S, Stang A, et al. Going to the COVID-19 Gemba: using observation and high reliability strategies to achieve safety in a time of crisis. CJEM. 2020;22(6):738-741. doi:10.1017/cem.2020.380
22. Sarihasan I, Dajnoki K, Oláh J, et al. The importance of the leadership functions of a high-reliability health care organization in managing the COVID-19 pandemic in Turkey. Econ Sociol. 2022;15:78-93. doi:10.14254/2071-789x.2022/15-1/5
23. Crain MA, Bush AL, Hayanga H, et al. Healthcare leadership in the COVID-19 pandemic: from innovative preparation to evolutionary transformation. J Health Leadersh. 2021;13:199-207. doi:10.2147/JHL.S319829
24. SQUIRE. Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) SQUIRE; 2020. Accessed March 1, 2023. http://www.squire-statement.org/index.cfm?fuseaction=Page.ViewPage&pageId=471
25. Lounsbury O. How to write a quality improvement project. Patient Safety J. 2022;4(1):65-67. doi:10.33940/culture/2022.3.6
26. Bengtsson M. How to plan and perform a qualitative study using content analysis. Nurs Plus Open. 2016;2:8-14. doi:10.1016/j.npls.2016.01.001
27. Allen M. The Sage Encyclopedia of Communication Research Methods. (Vols. 1-4). SAGE Publications, Inc; 2017
28. Unlock insights with qualitative data analysis software. Lumivero. Accessed March 2, 2023. https://lumivero.com/products/nvivo/
29. Maher C, Hadfield M, Hutchings M, et al. Ensuring rigor in qualitative data analysis: a design research approach to coding combining NVivo with traditional material methods. Int J Qual Methods. 2018;17:1-13. doi:10.1177/1609406918786362
30. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77-101. doi:10.1191/1478088706qp063oa
31. Erlingsson C, Brysiewicz P. A hands-on guide to doing content analysis. Afr J Emerg Med. 2017;7:93-99. doi:10.1016/j.afjem.2017.08.001
32. Vears DF, Gillam L. Inductive content analysis: a guide for beginning qualitative researchers. FoHPE. 2022;23:111-127. doi:10.11157/fohpe.v23i1.544
33. Nowell LS, Norris JM, White DE, et al. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. 2017;16:1-13. doi:10.1177/1609406917733847
34. Gautham KS, Pearlman S. Do quality improvement projects require IRB approval? J Perinatol. 2021;41:1209-1212. doi:10.1038/s41372-021-01038-1
35. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2022;292:1-10. doi:10.1016/j.socscimed.2021.114523
36. Balogun M, Dada FO, Oladimeji A, et al. Leading in a time of crisis: a qualitative study capturing experiences of health facility leaders during the early phases of the COVID-19 pandemic in Nigeria’s epicentre. Leadersh Health Serv (Bradf Engl). Published online May 12, 2022. doi:10.1108/lhs-02-2022-0017
37. Guttormson J, Calkins K, McAndrew N, et al. Critical care nurses’ experiences during the COVID-19 pandemic: a US national survey. Am J Crit Care. 2022;31:96-103. doi:10.4037/ajcc2022312
38. Bajaba A, Bajaba S, Algarni M, et al. Adaptive managers as emerging leaders during the COVID-19 crisis. Front Psychol. 2021;12:1-11. doi:10.3389/fpsyg.2021.661628
39. Ahern S, Loh E. Leadership during the COVID-19 pandemic: building and sustaining trust in times of uncertainty. BMJ Lead. 2021;59(4):266-269. doi.org/10.1136/leader-2020-000271
40. Cote R. Adaptive leadership approach with COVID 19 adaptive challenges. J Leadersh Account Ethics. 2022;19:34-44. doi:10.33423/jlae.v19i1.4992
41. Juvet TM, Corbaz-Kurth S, Roos P, et al. Adapting to the unexpected: problematic work situations and resilience strategies in healthcare institutions during the COVID-19 pandemic’s first wave. Saf Sci. 2021;139:1-9. doi:10.1016/j.ssci.2021.105277
1. Editors: Dying in a leadership vacuum. 9.4N Engl J Med. 2020;383(15):1479-1480. doi:10.1056/NEJMe2029812
2. Geerts JM, Kinnair D, Taheri P, et al. Guidance for health care leaders during the recovery stage of the COVID-19 pandemic: a consensus statement. JAMA Netw Open. 2021;4(7):1-16. doi:10.1001/jamanetworkopen.2021.20295
3. Boiral O, Brotherton M-C, Rivaud L, et al. Organizations’ management of the COVID-19 pandemic: a scoping review of business articles. Sustainability. 2021;13:1-20. doi:10.3390/su13073993
4. Razu SR, Yasmin T, Arif TB, et al. Challenges faced by healthcare professionals during the COVID-19 pandemic: a qualitative inquiry from Bangladesh. Front Public Health. 2021;9:1-13. doi:10.3389/fpubh.2021.647315
5. Lyng HB, Ree E, Wibe T, et al. Healthcare leaders’ use of innovative solutions to ensure resilience in healthcare during the Covid-19 pandemic: a qualitative study in Norwegian nursing homes and home care services. BMC Health Serv Res. 2021;21(1):1-11. doi:1186/s12913-021-06923-1
6. Freitas J. Queiroz A, Bortotti I, et al. Nurse leaders’ challenges fighting the COVID-19 pandemic: a qualitative study. Open J Nurs. 2021;11:267-280. doi:10.4236/ojn.2021.115024
7. McGuire AL, Aulisio MP, Davis FD, et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the Association of Bioethics Program Directors (ABPD) Task Force. 9.4Am J Bioeth. 2020;20(7):15-27. doi:10.1080/15265161.2020.1764138
8. Turbow RM, Scibilia JP. Embracing principles of high reliability organizations can improve patient safety during pandemic. AAP News. January 19, 2021. Accessed March 1, 2023. https://publications.aap.org/aapnews/news/8975
9. Roberts BH, Damiano LA, Graham S, et al. A case study in fostering a learning culture in the context of Covid-19. American Association for Physician Leadership. June 24, 2021. Accessed March 1, 2023. https://www.physicianleaders.org/news/a-case-study-in-fostering-a-learning-culture-in-the-context-of-covid-19
10. U.S. Department of Veterans Affairs. Department of Veterans AffairsCOVID-19 National Summary. Veterans Affairs. Accessed December 4, 2022. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary
11. U.S. Department of Veterans Affairs. VA fourth mission summary. Veterans Affairs. Accessed December 4, 2022. https://www.va.gov/health/coronavirus/statesupport.asp#:~:text=As%20part%20of%20the%20Fourth,the%20facilities%20we%20are%20supporting
12. Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18(1):e320-e328. doi:10.1097/PTS.0000000000000768
13. Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. 9.4Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
14. Maison D, Jaworska D, Adamczyk D, et al. The challenges arising from the COVID-19 pandemic and the way people deal with them: a qualitative longitudinal study. PLoS One. 2021;16(10):1-17. doi:10.1371/journal.pone.0258133
15. Schulman PR. Reliability, uncertainty and the management of error: new perspectives in the COVID-19 era. J Contingencies Crisis Manag. 2022;30:92-101. doi:10.1111/1468-5973.12356
16. Adelman JS, Gandhi TK. COVID-19 and patient safety: time to tap into our investment in high reliability. J Patient Saf. 2021;17(4): 331-333. doi:10.1097/PTS.0000000000000843
17. Shingler-Nace A. COVID-19: when leadership calls. Nurs Lead. 2020;18(3):202-203. doi:10.1016/j.mnl.2020.03.017
18. Van Stralen D, Mercer TA. During pandemic COVID 19, the high reliability organization (HRO) identifies maladaptive stress behaviors: the stress-fear-threat cascade. Neonatol Tod. 2020;15(11):113-124. doi: 10.51362/neonatology.today/2020111511113124
19. Vogus TJ, Wilson AD, Randall K, et al. We’re all in this together: how COVID-19 revealed the coconstruction of mindful organising and organisational reliability. BMJ Qual Saf. 2022;31(3):230-233. doi:10.1136/bmjqs-2021-014068
20. Van Stralen D. Pragmatic high-reliability organization (HRO) during pandemic COVID-19. Neonatol Tod. 2020(4);15:109-117. doi:10.51362/neonatology.today/20208158109117
21. Thull-Freedman J, Mondoux S, Stang A, et al. Going to the COVID-19 Gemba: using observation and high reliability strategies to achieve safety in a time of crisis. CJEM. 2020;22(6):738-741. doi:10.1017/cem.2020.380
22. Sarihasan I, Dajnoki K, Oláh J, et al. The importance of the leadership functions of a high-reliability health care organization in managing the COVID-19 pandemic in Turkey. Econ Sociol. 2022;15:78-93. doi:10.14254/2071-789x.2022/15-1/5
23. Crain MA, Bush AL, Hayanga H, et al. Healthcare leadership in the COVID-19 pandemic: from innovative preparation to evolutionary transformation. J Health Leadersh. 2021;13:199-207. doi:10.2147/JHL.S319829
24. SQUIRE. Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) SQUIRE; 2020. Accessed March 1, 2023. http://www.squire-statement.org/index.cfm?fuseaction=Page.ViewPage&pageId=471
25. Lounsbury O. How to write a quality improvement project. Patient Safety J. 2022;4(1):65-67. doi:10.33940/culture/2022.3.6
26. Bengtsson M. How to plan and perform a qualitative study using content analysis. Nurs Plus Open. 2016;2:8-14. doi:10.1016/j.npls.2016.01.001
27. Allen M. The Sage Encyclopedia of Communication Research Methods. (Vols. 1-4). SAGE Publications, Inc; 2017
28. Unlock insights with qualitative data analysis software. Lumivero. Accessed March 2, 2023. https://lumivero.com/products/nvivo/
29. Maher C, Hadfield M, Hutchings M, et al. Ensuring rigor in qualitative data analysis: a design research approach to coding combining NVivo with traditional material methods. Int J Qual Methods. 2018;17:1-13. doi:10.1177/1609406918786362
30. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77-101. doi:10.1191/1478088706qp063oa
31. Erlingsson C, Brysiewicz P. A hands-on guide to doing content analysis. Afr J Emerg Med. 2017;7:93-99. doi:10.1016/j.afjem.2017.08.001
32. Vears DF, Gillam L. Inductive content analysis: a guide for beginning qualitative researchers. FoHPE. 2022;23:111-127. doi:10.11157/fohpe.v23i1.544
33. Nowell LS, Norris JM, White DE, et al. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. 2017;16:1-13. doi:10.1177/1609406917733847
34. Gautham KS, Pearlman S. Do quality improvement projects require IRB approval? J Perinatol. 2021;41:1209-1212. doi:10.1038/s41372-021-01038-1
35. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2022;292:1-10. doi:10.1016/j.socscimed.2021.114523
36. Balogun M, Dada FO, Oladimeji A, et al. Leading in a time of crisis: a qualitative study capturing experiences of health facility leaders during the early phases of the COVID-19 pandemic in Nigeria’s epicentre. Leadersh Health Serv (Bradf Engl). Published online May 12, 2022. doi:10.1108/lhs-02-2022-0017
37. Guttormson J, Calkins K, McAndrew N, et al. Critical care nurses’ experiences during the COVID-19 pandemic: a US national survey. Am J Crit Care. 2022;31:96-103. doi:10.4037/ajcc2022312
38. Bajaba A, Bajaba S, Algarni M, et al. Adaptive managers as emerging leaders during the COVID-19 crisis. Front Psychol. 2021;12:1-11. doi:10.3389/fpsyg.2021.661628
39. Ahern S, Loh E. Leadership during the COVID-19 pandemic: building and sustaining trust in times of uncertainty. BMJ Lead. 2021;59(4):266-269. doi.org/10.1136/leader-2020-000271
40. Cote R. Adaptive leadership approach with COVID 19 adaptive challenges. J Leadersh Account Ethics. 2022;19:34-44. doi:10.33423/jlae.v19i1.4992
41. Juvet TM, Corbaz-Kurth S, Roos P, et al. Adapting to the unexpected: problematic work situations and resilience strategies in healthcare institutions during the COVID-19 pandemic’s first wave. Saf Sci. 2021;139:1-9. doi:10.1016/j.ssci.2021.105277




