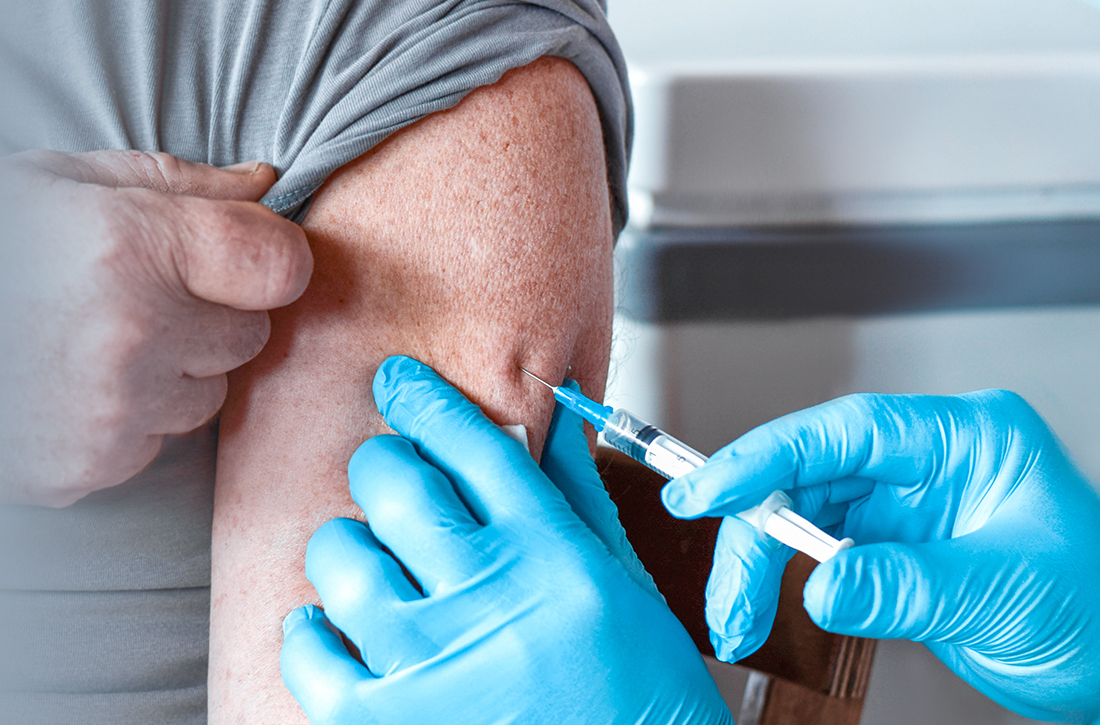
User login
52-year-old man • intermittent fevers • recently received second dose of COVID-19 vaccine • tremors in all 4 extremities • Dx?
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
► Intermittent fevers
► Recently received second dose of COVID-19 vaccine
► Tremors in all 4 extremities
Maternal pertussis vax effective for infants in most vulnerable months
Maternal pertussis vaccinations, given during pregnancy, prevent an estimated 65% of pertussis infections in infants, new research indicates.
The study, led by Annette K. Regan, PhD, MPH, a perinatal and pediatric infectious disease epidemiologist at Curtin University, Perth, Australia, was published online in Pediatrics.
Dr. Regan – who is also with the University of San Francisco and the University of California, Los Angeles – and colleagues reviewed data on 279,418 infants born to 252,444 mothers in Australia.
There, about 52% of the women in this study received the Tdap vaccine through a maternal pertussis vaccination program.
Duration of effectiveness in infants was one of the main questions the study sought to answer.
The authors wrote that they assessed vaccine effectiveness through 18 months of age. “We observed significant protection against disease until at least 8 months of age, 2 months longer than reported in previous studies.” From 70% to 90% of all pertussis-attributable hospitalizations and death occur in infancy.
Answering the ‘blunting’ question
This study also set out to clarify an important clinical question regarding a potential “blunting” effect in infants. Previous work had suggested that maternal antibodies from the vaccination could interfere with the effectiveness of infants’ DtaP (the version of Tdap for infants) and other vaccines.
Dr. Regan and colleagues found that, “although we observed slightly lower VE [vaccine effectiveness] point estimates for the third dose of infant pertussis vaccine among maternally vaccinated compared with unvaccinated infants (76.5% vs. 92.9%, P = .002), we did not observe higher rates of pertussis infection (hazard ratio, 0.70; 95% confidence interval, 0.61-3.39).
Best time to give mothers the vaccine
Another clinical debate has centered on when to give the mother the vaccine during pregnancy. The authors concluded: “Our findings support the infant health benefits of recommendations to administer a booster dose of pertussis vaccine near 28 weeks of gestational age.”
That 28-week mark was associated with lower risk of infection in infants through 8 months of age, they wrote.
Positive results in the United States
In an invited commentary, Kathryn M. Edwards, MD, with the division of infectious diseases, department of pediatrics, at Vanderbilt University Medical Center, Nashville, Tenn., highlighted similar positive findings for maternal pertussis vaccination in the United States.
The Centers for Disease Control and Prevention did an ecologic study of infant pertussis cases reported between Jan. 1, 2000, and Dec. 31, 2019. Rates were compared for the years before maternal Tdap vaccinations were recommended against the 7-year period after they were implemented.
That study found that in the period before maternal Tdap vaccination, annual pertussis incidence did not change among infants younger than 2 months and increased slightly in infants 6-12 months.
However, during the period after maternal Tdap vaccination had started (2012-2019), pertussis incidence significantly decreased in infants younger than 2 months and was unchanged in infants 6-12 months.
“As with the Australian data, the U.S. data support the overall benefit of the maternal Tdap program and, as with the Australian data, do not suggest that blunting has led to an increase in cases within the first year of life,” Dr. Edwards wrote.
The CDC notes that pertussis cases are rising and outbreaks are happening across the United States.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” the CDC states.
Uptake low despite positive data
Dr. Edwards noted that, despite positive data supporting maternal vaccination to reduce pertussis, uptake rates are low – between 50% and 60% in Australia, the United Kingdom, and the United States. “Active engagement to increase these rates should be implemented.”
Maternal vaccination might also be implemented soon to protect against other diseases including respiratory syncytial virus and group B streptococcal disease after promising study data, she said.
As with pertussis, the potential “blunting” effect will need to be carefully monitored, she said, “as was done in the carefully conducted study of pertussis reported in this issue of Pediatrics.”
One coauthor has received institutional honoraria for participation in advisory groups for Merck Sharpe & Dohme and Pfizer unrelated to this work. Another coauthor was supported by scholarships provided by the Wesfarmers Centre of Vaccines and Infectious Disease at the Telethon Kids Institute. Dr. Edwards reported receiving grants from the CDC and consulting for Bionet, Dynavax, and IBM. She is a member of the data safety and monitoring board for Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, Merck, Roche, Novavax, and Brighton Collaboration.
Maternal pertussis vaccinations, given during pregnancy, prevent an estimated 65% of pertussis infections in infants, new research indicates.
The study, led by Annette K. Regan, PhD, MPH, a perinatal and pediatric infectious disease epidemiologist at Curtin University, Perth, Australia, was published online in Pediatrics.
Dr. Regan – who is also with the University of San Francisco and the University of California, Los Angeles – and colleagues reviewed data on 279,418 infants born to 252,444 mothers in Australia.
There, about 52% of the women in this study received the Tdap vaccine through a maternal pertussis vaccination program.
Duration of effectiveness in infants was one of the main questions the study sought to answer.
The authors wrote that they assessed vaccine effectiveness through 18 months of age. “We observed significant protection against disease until at least 8 months of age, 2 months longer than reported in previous studies.” From 70% to 90% of all pertussis-attributable hospitalizations and death occur in infancy.
Answering the ‘blunting’ question
This study also set out to clarify an important clinical question regarding a potential “blunting” effect in infants. Previous work had suggested that maternal antibodies from the vaccination could interfere with the effectiveness of infants’ DtaP (the version of Tdap for infants) and other vaccines.
Dr. Regan and colleagues found that, “although we observed slightly lower VE [vaccine effectiveness] point estimates for the third dose of infant pertussis vaccine among maternally vaccinated compared with unvaccinated infants (76.5% vs. 92.9%, P = .002), we did not observe higher rates of pertussis infection (hazard ratio, 0.70; 95% confidence interval, 0.61-3.39).
Best time to give mothers the vaccine
Another clinical debate has centered on when to give the mother the vaccine during pregnancy. The authors concluded: “Our findings support the infant health benefits of recommendations to administer a booster dose of pertussis vaccine near 28 weeks of gestational age.”
That 28-week mark was associated with lower risk of infection in infants through 8 months of age, they wrote.
Positive results in the United States
In an invited commentary, Kathryn M. Edwards, MD, with the division of infectious diseases, department of pediatrics, at Vanderbilt University Medical Center, Nashville, Tenn., highlighted similar positive findings for maternal pertussis vaccination in the United States.
The Centers for Disease Control and Prevention did an ecologic study of infant pertussis cases reported between Jan. 1, 2000, and Dec. 31, 2019. Rates were compared for the years before maternal Tdap vaccinations were recommended against the 7-year period after they were implemented.
That study found that in the period before maternal Tdap vaccination, annual pertussis incidence did not change among infants younger than 2 months and increased slightly in infants 6-12 months.
However, during the period after maternal Tdap vaccination had started (2012-2019), pertussis incidence significantly decreased in infants younger than 2 months and was unchanged in infants 6-12 months.
“As with the Australian data, the U.S. data support the overall benefit of the maternal Tdap program and, as with the Australian data, do not suggest that blunting has led to an increase in cases within the first year of life,” Dr. Edwards wrote.
The CDC notes that pertussis cases are rising and outbreaks are happening across the United States.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” the CDC states.
Uptake low despite positive data
Dr. Edwards noted that, despite positive data supporting maternal vaccination to reduce pertussis, uptake rates are low – between 50% and 60% in Australia, the United Kingdom, and the United States. “Active engagement to increase these rates should be implemented.”
Maternal vaccination might also be implemented soon to protect against other diseases including respiratory syncytial virus and group B streptococcal disease after promising study data, she said.
As with pertussis, the potential “blunting” effect will need to be carefully monitored, she said, “as was done in the carefully conducted study of pertussis reported in this issue of Pediatrics.”
One coauthor has received institutional honoraria for participation in advisory groups for Merck Sharpe & Dohme and Pfizer unrelated to this work. Another coauthor was supported by scholarships provided by the Wesfarmers Centre of Vaccines and Infectious Disease at the Telethon Kids Institute. Dr. Edwards reported receiving grants from the CDC and consulting for Bionet, Dynavax, and IBM. She is a member of the data safety and monitoring board for Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, Merck, Roche, Novavax, and Brighton Collaboration.
Maternal pertussis vaccinations, given during pregnancy, prevent an estimated 65% of pertussis infections in infants, new research indicates.
The study, led by Annette K. Regan, PhD, MPH, a perinatal and pediatric infectious disease epidemiologist at Curtin University, Perth, Australia, was published online in Pediatrics.
Dr. Regan – who is also with the University of San Francisco and the University of California, Los Angeles – and colleagues reviewed data on 279,418 infants born to 252,444 mothers in Australia.
There, about 52% of the women in this study received the Tdap vaccine through a maternal pertussis vaccination program.
Duration of effectiveness in infants was one of the main questions the study sought to answer.
The authors wrote that they assessed vaccine effectiveness through 18 months of age. “We observed significant protection against disease until at least 8 months of age, 2 months longer than reported in previous studies.” From 70% to 90% of all pertussis-attributable hospitalizations and death occur in infancy.
Answering the ‘blunting’ question
This study also set out to clarify an important clinical question regarding a potential “blunting” effect in infants. Previous work had suggested that maternal antibodies from the vaccination could interfere with the effectiveness of infants’ DtaP (the version of Tdap for infants) and other vaccines.
Dr. Regan and colleagues found that, “although we observed slightly lower VE [vaccine effectiveness] point estimates for the third dose of infant pertussis vaccine among maternally vaccinated compared with unvaccinated infants (76.5% vs. 92.9%, P = .002), we did not observe higher rates of pertussis infection (hazard ratio, 0.70; 95% confidence interval, 0.61-3.39).
Best time to give mothers the vaccine
Another clinical debate has centered on when to give the mother the vaccine during pregnancy. The authors concluded: “Our findings support the infant health benefits of recommendations to administer a booster dose of pertussis vaccine near 28 weeks of gestational age.”
That 28-week mark was associated with lower risk of infection in infants through 8 months of age, they wrote.
Positive results in the United States
In an invited commentary, Kathryn M. Edwards, MD, with the division of infectious diseases, department of pediatrics, at Vanderbilt University Medical Center, Nashville, Tenn., highlighted similar positive findings for maternal pertussis vaccination in the United States.
The Centers for Disease Control and Prevention did an ecologic study of infant pertussis cases reported between Jan. 1, 2000, and Dec. 31, 2019. Rates were compared for the years before maternal Tdap vaccinations were recommended against the 7-year period after they were implemented.
That study found that in the period before maternal Tdap vaccination, annual pertussis incidence did not change among infants younger than 2 months and increased slightly in infants 6-12 months.
However, during the period after maternal Tdap vaccination had started (2012-2019), pertussis incidence significantly decreased in infants younger than 2 months and was unchanged in infants 6-12 months.
“As with the Australian data, the U.S. data support the overall benefit of the maternal Tdap program and, as with the Australian data, do not suggest that blunting has led to an increase in cases within the first year of life,” Dr. Edwards wrote.
The CDC notes that pertussis cases are rising and outbreaks are happening across the United States.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” the CDC states.
Uptake low despite positive data
Dr. Edwards noted that, despite positive data supporting maternal vaccination to reduce pertussis, uptake rates are low – between 50% and 60% in Australia, the United Kingdom, and the United States. “Active engagement to increase these rates should be implemented.”
Maternal vaccination might also be implemented soon to protect against other diseases including respiratory syncytial virus and group B streptococcal disease after promising study data, she said.
As with pertussis, the potential “blunting” effect will need to be carefully monitored, she said, “as was done in the carefully conducted study of pertussis reported in this issue of Pediatrics.”
One coauthor has received institutional honoraria for participation in advisory groups for Merck Sharpe & Dohme and Pfizer unrelated to this work. Another coauthor was supported by scholarships provided by the Wesfarmers Centre of Vaccines and Infectious Disease at the Telethon Kids Institute. Dr. Edwards reported receiving grants from the CDC and consulting for Bionet, Dynavax, and IBM. She is a member of the data safety and monitoring board for Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, Merck, Roche, Novavax, and Brighton Collaboration.
FROM PEDIATRICS
FDA approves first tocilizumab biosimilar
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
FDA issues letter regarding lebrikizumab review for atopic dermatitis
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
Study finds inflammatory bowel disease risk higher in children, adults with atopic dermatitis
The published recently in JAMA Dermatology.
The study also found an increased risk for Crohn’s disease (CD) in adults and children with AD, as well as an increased risk for ulcerative colitis (UC) in adults with AD and in children with severe AD, researchers reported.
“It is imperative for clinicians to understand atopic dermatitis and the trajectory of our patients with it in order to provide the best standard of care,” senior author Joel M. Gelfand, MD, MSCE, professor in clinical investigation with the department of dermatology at the University of Pennsylvania, Philadelphia, said in a news release.
“There are new and better treatments for AD today, and there will likely continue to be more,” continued Dr. Gelfand. “But providers have to understand how those treatments could impact other autoimmune diseases. For patients with AD and another autoimmune disease, some currently available medications can exacerbate symptoms of their other disease or can help treat two immune diseases at the same time.”
The study results support the idea that AD and IBD may have some common underlying causes, said Sheilagh Maguiness, MD, pediatric dermatologist and associate professor in the department of dermatology at the University of Minnesota, Minneapolis, who was asked to comment on the findings.
“As the pathogenesis of AD is becoming better understood, we are recognizing that, rather than simply a cutaneous disease, the underlying inflammation and immune dysregulation that leads to AD best categorizes it as a systemic inflammatory disease with significant comorbidities,” she told this news organization. “I will be more likely to ask patients and families about GI symptoms, and if positive, may plan to refer to GI more readily than in the past,” added Dr. Maguiness, who was not involved in the study.
UK general practice cohort
AD has been associated with an increasing number of comorbidities, including IBD, but studies linking AD with IBD, including UC, have had mixed results, the authors wrote. And few studies have separately examined how AD or AD severity may be linked with UC or CD risk.
To examine the risk for new-onset IBD, UC, and CD in children and adults with atopic dermatitis, the researchers conducted a population-based cohort study using the THIN (The Health Improvement Network) electronic medical record database of patients registered with United Kingdom general practices. They used 21 years of data collected from January 1994 to February 2015.
The researchers matched each patient who had AD with up to five controls based on age, practice, and index date. Because THIN does not capture AD severity, they used treatment exposure assessed by dermatologic referrals and treatments patients received as proxy for severity. The authors used logistic regression to examine the risks for IBD, UC, and CD in children (aged 1-10) with AD, and in adults (aged 30-68) with AD, and they compared their outcomes with the outcomes for controls.
In the pediatric cohort, the team compared 409,431 children who had AD with 1.8 million children without AD. Slightly more than half were boys. In the adult cohort, they compared 625,083 people who had AD with 2.68 million controls, and slightly more than half were women. Data on race or ethnicity were not available, the authors wrote, but the THIN database is considered to be representative of the UK population.
AD severity linked with IBD risk
The risk for new-onset inflammatory bowel disease appears to be higher in children and adults with AD, and the risk varies based on age, AD severity, and subtype of inflammatory bowel disease, the authors reported.
Overall, AD in children was associated with a 44% increased risk for IBD (adjusted hazard ratio (HR), 1.44; 95% confidence interval [CI], 1.31-1.58) compared with controls, the authors reported. They found a 74% increased risk for CD in children with AD compared with controls (HR, 1.74; 95% CI, 1.54-1.97). More severe AD was linked with increased risk for both IBD and CD.
AD did not appear to increase risk for UC in children, except those with severe AD (HR, 1.65; 95% CI, 1.02-2.67).
Overall, adults with AD had a 34% (HR, 1.34; 95% CI, 1.27-1.40) increased risk for IBD, a 36% (HR, 1.36; 95% CI, 1.26-1.47) increased risk for CD, and a 32% (HR, 1.32; 95% CI, 1.24-1.41) increased risk for UC, with risk increasing with increased AD severity.
Robust data with cautionary note
“This study provides the most robust data to date on the association between IBD and AD. It provides clear evidence for an association that most dermatologists or primary care providers are not typically taught in training,” Kelly Scarberry, MD, assistant professor of dermatology at Case Western Reserve University in Cleveland, told this news organization. “I will be much more likely to pursue diagnostic workup in my AD patients who have GI complaints.”
However, AD severity was measured by proxy, added Dr. Scarberry, who was not involved in the study, and the study lacked important racial and ethnic data.
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., also not involved in the study, said in an interview that she found the size of the cohort and the longitudinal data to be strengths of the study.
But, she added, the “lack of family IBD history, race and ethnicity, and comorbidities, are limitations, as is treatment exposure used as a proxy for disease severity, given that physician treatment practices differ.”
For Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest, “the most important conclusion, and it is a definitive finding, [is] that IBD is uncommon, even in patients with AD.
“The findings could be misinterpreted,” cautioned Dr. Feldman, who was not involved in the study. “While there is an increased relative risk, the absolute risk is small.” The study found that “the highest relative risk group is children with severe AD, who have a roughly fivefold increased risk for CD.” However, he added, the incidence rates of CD were 0.68 per 1,000 person-years in children with severe AD and 0.08 per 1,000 person-years in controls.
“Basically, because Crohn’s disease and IBD don’t happen very often, the modest increase in relative risk the investigators found doesn’t amount to much we’d have to worry about,” he said. “The findings do not show any need to screen patients with atopic dermatitis for IBD any more than we’d need to screen patients without atopic dermatitis.”
The increased relative risk “could be a clue to possible genetic connections between diseases,” he added. “But when we’re making clinical decisions, those decisions should be based on the absolute risk that some event may occur.”
Susan Massick, MD, dermatologist and associate professor at The Ohio State University in Columbus, who was not involved with the study, said in an interview, “We are still scratching the surface of the complexity of the immune and inflammatory pathways in AD and IBD.
“It is important to remember that correlation does not mean causation,” Dr. Massick said. “It would be premature to draw direct conclusions based on this study alone.”
The authors recommend future related studies in more diverse populations.
Dr. Gelfand and two coauthors reported ties with Pfizer, which supported the study. Dr. Gelfand and three coauthors reported ties with other pharmaceutical companies. Dr. Maguiness, Dr. Scarberry, Dr. Strowd, and Dr. Massick reported having no relevant disclosures. Dr. Feldman reported ties with Pfizer and other pharmaceutical companies.
A version of this article appeared on Medscape.com.
The published recently in JAMA Dermatology.
The study also found an increased risk for Crohn’s disease (CD) in adults and children with AD, as well as an increased risk for ulcerative colitis (UC) in adults with AD and in children with severe AD, researchers reported.
“It is imperative for clinicians to understand atopic dermatitis and the trajectory of our patients with it in order to provide the best standard of care,” senior author Joel M. Gelfand, MD, MSCE, professor in clinical investigation with the department of dermatology at the University of Pennsylvania, Philadelphia, said in a news release.
“There are new and better treatments for AD today, and there will likely continue to be more,” continued Dr. Gelfand. “But providers have to understand how those treatments could impact other autoimmune diseases. For patients with AD and another autoimmune disease, some currently available medications can exacerbate symptoms of their other disease or can help treat two immune diseases at the same time.”
The study results support the idea that AD and IBD may have some common underlying causes, said Sheilagh Maguiness, MD, pediatric dermatologist and associate professor in the department of dermatology at the University of Minnesota, Minneapolis, who was asked to comment on the findings.
“As the pathogenesis of AD is becoming better understood, we are recognizing that, rather than simply a cutaneous disease, the underlying inflammation and immune dysregulation that leads to AD best categorizes it as a systemic inflammatory disease with significant comorbidities,” she told this news organization. “I will be more likely to ask patients and families about GI symptoms, and if positive, may plan to refer to GI more readily than in the past,” added Dr. Maguiness, who was not involved in the study.
UK general practice cohort
AD has been associated with an increasing number of comorbidities, including IBD, but studies linking AD with IBD, including UC, have had mixed results, the authors wrote. And few studies have separately examined how AD or AD severity may be linked with UC or CD risk.
To examine the risk for new-onset IBD, UC, and CD in children and adults with atopic dermatitis, the researchers conducted a population-based cohort study using the THIN (The Health Improvement Network) electronic medical record database of patients registered with United Kingdom general practices. They used 21 years of data collected from January 1994 to February 2015.
The researchers matched each patient who had AD with up to five controls based on age, practice, and index date. Because THIN does not capture AD severity, they used treatment exposure assessed by dermatologic referrals and treatments patients received as proxy for severity. The authors used logistic regression to examine the risks for IBD, UC, and CD in children (aged 1-10) with AD, and in adults (aged 30-68) with AD, and they compared their outcomes with the outcomes for controls.
In the pediatric cohort, the team compared 409,431 children who had AD with 1.8 million children without AD. Slightly more than half were boys. In the adult cohort, they compared 625,083 people who had AD with 2.68 million controls, and slightly more than half were women. Data on race or ethnicity were not available, the authors wrote, but the THIN database is considered to be representative of the UK population.
AD severity linked with IBD risk
The risk for new-onset inflammatory bowel disease appears to be higher in children and adults with AD, and the risk varies based on age, AD severity, and subtype of inflammatory bowel disease, the authors reported.
Overall, AD in children was associated with a 44% increased risk for IBD (adjusted hazard ratio (HR), 1.44; 95% confidence interval [CI], 1.31-1.58) compared with controls, the authors reported. They found a 74% increased risk for CD in children with AD compared with controls (HR, 1.74; 95% CI, 1.54-1.97). More severe AD was linked with increased risk for both IBD and CD.
AD did not appear to increase risk for UC in children, except those with severe AD (HR, 1.65; 95% CI, 1.02-2.67).
Overall, adults with AD had a 34% (HR, 1.34; 95% CI, 1.27-1.40) increased risk for IBD, a 36% (HR, 1.36; 95% CI, 1.26-1.47) increased risk for CD, and a 32% (HR, 1.32; 95% CI, 1.24-1.41) increased risk for UC, with risk increasing with increased AD severity.
Robust data with cautionary note
“This study provides the most robust data to date on the association between IBD and AD. It provides clear evidence for an association that most dermatologists or primary care providers are not typically taught in training,” Kelly Scarberry, MD, assistant professor of dermatology at Case Western Reserve University in Cleveland, told this news organization. “I will be much more likely to pursue diagnostic workup in my AD patients who have GI complaints.”
However, AD severity was measured by proxy, added Dr. Scarberry, who was not involved in the study, and the study lacked important racial and ethnic data.
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., also not involved in the study, said in an interview that she found the size of the cohort and the longitudinal data to be strengths of the study.
But, she added, the “lack of family IBD history, race and ethnicity, and comorbidities, are limitations, as is treatment exposure used as a proxy for disease severity, given that physician treatment practices differ.”
For Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest, “the most important conclusion, and it is a definitive finding, [is] that IBD is uncommon, even in patients with AD.
“The findings could be misinterpreted,” cautioned Dr. Feldman, who was not involved in the study. “While there is an increased relative risk, the absolute risk is small.” The study found that “the highest relative risk group is children with severe AD, who have a roughly fivefold increased risk for CD.” However, he added, the incidence rates of CD were 0.68 per 1,000 person-years in children with severe AD and 0.08 per 1,000 person-years in controls.
“Basically, because Crohn’s disease and IBD don’t happen very often, the modest increase in relative risk the investigators found doesn’t amount to much we’d have to worry about,” he said. “The findings do not show any need to screen patients with atopic dermatitis for IBD any more than we’d need to screen patients without atopic dermatitis.”
The increased relative risk “could be a clue to possible genetic connections between diseases,” he added. “But when we’re making clinical decisions, those decisions should be based on the absolute risk that some event may occur.”
Susan Massick, MD, dermatologist and associate professor at The Ohio State University in Columbus, who was not involved with the study, said in an interview, “We are still scratching the surface of the complexity of the immune and inflammatory pathways in AD and IBD.
“It is important to remember that correlation does not mean causation,” Dr. Massick said. “It would be premature to draw direct conclusions based on this study alone.”
The authors recommend future related studies in more diverse populations.
Dr. Gelfand and two coauthors reported ties with Pfizer, which supported the study. Dr. Gelfand and three coauthors reported ties with other pharmaceutical companies. Dr. Maguiness, Dr. Scarberry, Dr. Strowd, and Dr. Massick reported having no relevant disclosures. Dr. Feldman reported ties with Pfizer and other pharmaceutical companies.
A version of this article appeared on Medscape.com.
The published recently in JAMA Dermatology.
The study also found an increased risk for Crohn’s disease (CD) in adults and children with AD, as well as an increased risk for ulcerative colitis (UC) in adults with AD and in children with severe AD, researchers reported.
“It is imperative for clinicians to understand atopic dermatitis and the trajectory of our patients with it in order to provide the best standard of care,” senior author Joel M. Gelfand, MD, MSCE, professor in clinical investigation with the department of dermatology at the University of Pennsylvania, Philadelphia, said in a news release.
“There are new and better treatments for AD today, and there will likely continue to be more,” continued Dr. Gelfand. “But providers have to understand how those treatments could impact other autoimmune diseases. For patients with AD and another autoimmune disease, some currently available medications can exacerbate symptoms of their other disease or can help treat two immune diseases at the same time.”
The study results support the idea that AD and IBD may have some common underlying causes, said Sheilagh Maguiness, MD, pediatric dermatologist and associate professor in the department of dermatology at the University of Minnesota, Minneapolis, who was asked to comment on the findings.
“As the pathogenesis of AD is becoming better understood, we are recognizing that, rather than simply a cutaneous disease, the underlying inflammation and immune dysregulation that leads to AD best categorizes it as a systemic inflammatory disease with significant comorbidities,” she told this news organization. “I will be more likely to ask patients and families about GI symptoms, and if positive, may plan to refer to GI more readily than in the past,” added Dr. Maguiness, who was not involved in the study.
UK general practice cohort
AD has been associated with an increasing number of comorbidities, including IBD, but studies linking AD with IBD, including UC, have had mixed results, the authors wrote. And few studies have separately examined how AD or AD severity may be linked with UC or CD risk.
To examine the risk for new-onset IBD, UC, and CD in children and adults with atopic dermatitis, the researchers conducted a population-based cohort study using the THIN (The Health Improvement Network) electronic medical record database of patients registered with United Kingdom general practices. They used 21 years of data collected from January 1994 to February 2015.
The researchers matched each patient who had AD with up to five controls based on age, practice, and index date. Because THIN does not capture AD severity, they used treatment exposure assessed by dermatologic referrals and treatments patients received as proxy for severity. The authors used logistic regression to examine the risks for IBD, UC, and CD in children (aged 1-10) with AD, and in adults (aged 30-68) with AD, and they compared their outcomes with the outcomes for controls.
In the pediatric cohort, the team compared 409,431 children who had AD with 1.8 million children without AD. Slightly more than half were boys. In the adult cohort, they compared 625,083 people who had AD with 2.68 million controls, and slightly more than half were women. Data on race or ethnicity were not available, the authors wrote, but the THIN database is considered to be representative of the UK population.
AD severity linked with IBD risk
The risk for new-onset inflammatory bowel disease appears to be higher in children and adults with AD, and the risk varies based on age, AD severity, and subtype of inflammatory bowel disease, the authors reported.
Overall, AD in children was associated with a 44% increased risk for IBD (adjusted hazard ratio (HR), 1.44; 95% confidence interval [CI], 1.31-1.58) compared with controls, the authors reported. They found a 74% increased risk for CD in children with AD compared with controls (HR, 1.74; 95% CI, 1.54-1.97). More severe AD was linked with increased risk for both IBD and CD.
AD did not appear to increase risk for UC in children, except those with severe AD (HR, 1.65; 95% CI, 1.02-2.67).
Overall, adults with AD had a 34% (HR, 1.34; 95% CI, 1.27-1.40) increased risk for IBD, a 36% (HR, 1.36; 95% CI, 1.26-1.47) increased risk for CD, and a 32% (HR, 1.32; 95% CI, 1.24-1.41) increased risk for UC, with risk increasing with increased AD severity.
Robust data with cautionary note
“This study provides the most robust data to date on the association between IBD and AD. It provides clear evidence for an association that most dermatologists or primary care providers are not typically taught in training,” Kelly Scarberry, MD, assistant professor of dermatology at Case Western Reserve University in Cleveland, told this news organization. “I will be much more likely to pursue diagnostic workup in my AD patients who have GI complaints.”
However, AD severity was measured by proxy, added Dr. Scarberry, who was not involved in the study, and the study lacked important racial and ethnic data.
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., also not involved in the study, said in an interview that she found the size of the cohort and the longitudinal data to be strengths of the study.
But, she added, the “lack of family IBD history, race and ethnicity, and comorbidities, are limitations, as is treatment exposure used as a proxy for disease severity, given that physician treatment practices differ.”
For Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest, “the most important conclusion, and it is a definitive finding, [is] that IBD is uncommon, even in patients with AD.
“The findings could be misinterpreted,” cautioned Dr. Feldman, who was not involved in the study. “While there is an increased relative risk, the absolute risk is small.” The study found that “the highest relative risk group is children with severe AD, who have a roughly fivefold increased risk for CD.” However, he added, the incidence rates of CD were 0.68 per 1,000 person-years in children with severe AD and 0.08 per 1,000 person-years in controls.
“Basically, because Crohn’s disease and IBD don’t happen very often, the modest increase in relative risk the investigators found doesn’t amount to much we’d have to worry about,” he said. “The findings do not show any need to screen patients with atopic dermatitis for IBD any more than we’d need to screen patients without atopic dermatitis.”
The increased relative risk “could be a clue to possible genetic connections between diseases,” he added. “But when we’re making clinical decisions, those decisions should be based on the absolute risk that some event may occur.”
Susan Massick, MD, dermatologist and associate professor at The Ohio State University in Columbus, who was not involved with the study, said in an interview, “We are still scratching the surface of the complexity of the immune and inflammatory pathways in AD and IBD.
“It is important to remember that correlation does not mean causation,” Dr. Massick said. “It would be premature to draw direct conclusions based on this study alone.”
The authors recommend future related studies in more diverse populations.
Dr. Gelfand and two coauthors reported ties with Pfizer, which supported the study. Dr. Gelfand and three coauthors reported ties with other pharmaceutical companies. Dr. Maguiness, Dr. Scarberry, Dr. Strowd, and Dr. Massick reported having no relevant disclosures. Dr. Feldman reported ties with Pfizer and other pharmaceutical companies.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Many young people stop ulcerative colitis maintenance treatment, risking relapse
, new research from the United Kingdom indicates.
“This is concerning as they are at risk of their condition returning and further complications. It can also lead to severe complications such as surgery to remove part of the gut,” Sonia Saxena, MBBS, director, Imperial Child Health Unit, School of Public Health, Imperial College London, said in a news release.
The study “highlights the importance of counseling and education of patients at diagnosis as this is a critical window that influences long-term health behavior,” Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, said in an interview.
“It has not been my experience in U.S.-based practice that the rates of discontinuation are that high, but it would be important to examine this in different locations,” added Dr. Ananthakrishnan, who wasn’t involved with the study.
The study was published online in the British Journal of General Practice.
Cases on the rise
Globally, the incidence of UC is increasing fastest in younger populations. It’s estimated that up to 30% of individuals with UC are diagnosed in childhood or young adulthood, and these individuals are more likely to have a severe disease course and years living with disability, compared with peers diagnosed later in life. This makes achieving disease control and maintaining remission “paramount” for those diagnosed in early life, Dr. Saxena and colleagues write.
International UC guidelines recommend starting therapy with 5-ASA, also known as mesalamine, soon after diagnosis and continuing it long term to maintain remission. However, some prior evidence suggests that adherence to UC medication may be less optimal in younger people – findings supported by the U.K. study.
Leveraging data from the UK Clinical Practice Research Datalink, Dr. Saxena and colleagues analyzed data for 607 children and young adults aged 10-24 years starting oral 5-ASA maintenance therapy for UC.
They found that 152 individuals (25%) stopped 5-ASA treatment after 1 month, and 419 (69%) discontinued it within 1 year of starting treatment. The median time to stopping the anti-inflammatory drug was 162 days.
Discontinuation rates were highest in young adults aged 18-24 years (74%). The transition to adult care and loss of support from caregivers who encourage adherence in adolescents and provide financial and practical support could be one explanation for this, the researchers write.
After accounting for other factors, young adults aged 18-24 years starting 5-ASA were 43% more likely to discontinue it in the first year than adolescents aged 10-14 years.
Individuals living in socioeconomically deprived areas were 46% more likely to stop treatment, compared with those living in more affluent areas, a finding that suggests the need to address socioeconomic disparities that could drive discontinuation, the authors say.
They also found that early corticosteroid use for an acute UC flare was associated with a 32% lower likelihood of stopping 5-ASA therapy.
Adherence falls short
In terms of adherence, defined as the proportion of days covered by 5-ASA medication, the mean was 72%, equivalent to just under 9 months. Adherence fell with older age at initiation of therapy. Adherence was 80% among those 10-14 years, 78% among those 15-17 years, and 69% among those 18-24 years. Prior research has shown that nonadherence to UC medication – defined as proportion of days covered less than 80% – has been associated with a five-fold risk of disease, compared with adherence above 80%, the investigators note.
“If clinicians are unaware of suboptimal adherence to first-line medication, they may incorrectly assume therapy has failed, which may lead to unnecessary escalation in treatment and avoidable steroid use that remains high in UC,” the researchers write.
Psychiatric comorbidity (depression, anxiety, or antidepressant use) was not associated with discontinuation or adherence to treatment.
“As doctors, this study shows we need to be keeping a close eye on patients, particularly within that first year of starting medication,” Dr. Saxena said in the release.
“We should check if these patients are getting their medications and whether they have difficulty paying for them. We should also use the opportunity to talk through any recurring symptoms and how to access advice from providers such as a nurse specialist,” Dr. Saxena said.
Effectiveness of therapy in young adults
Reached for comment, Michael Dolinger, MD, assistant professor of pediatric gastroenterology, Icahn School of Medicine and Mount Sinai Kravis Children’s Hospital, both in New York, said he has seen 5-ASA stoppage among his younger patients.
“Generally, what we see is that the majority of patients over time are not able to be sustained on oral mesalamine treatment, and they need a more advanced therapy,” Dr. Dolinger said in an interview.
And while the U.K. study did not delve into the reasons for discontinuation, ineffectiveness of therapy is likely a main cause, Dr. Dolinger said.
“We especially see this in our younger adolescent and young adult patients. In these younger patients, the immune system is potentially driving inflammation a bit more than in older patients, often going beyond the inner lining of the colon to the entire bowel wall, even in ulcerative colitis, and therefore mesalamine may be ineffective over the first year,” Dr. Dolinger explained.
When choosing a more advanced therapy, Dr. Dolinger said, “it’s all about having that conversation in a shared decision-making process about what may be the most effective short- and long-term treatment options with the best safety for that patient. It’s a very individualized discussion.”
“One of the main things we preach and talk about is control of inflammation, getting into early deep remission, because the longer you have inflammation, even if it’s just smoldering, the harder it is to get into deep remission,” Dr. Dolinger added.
The study had no commercial funding. Dr. Saxena, Dr. Ananthakrishnan, and Dr. Dolinger have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research from the United Kingdom indicates.
“This is concerning as they are at risk of their condition returning and further complications. It can also lead to severe complications such as surgery to remove part of the gut,” Sonia Saxena, MBBS, director, Imperial Child Health Unit, School of Public Health, Imperial College London, said in a news release.
The study “highlights the importance of counseling and education of patients at diagnosis as this is a critical window that influences long-term health behavior,” Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, said in an interview.
“It has not been my experience in U.S.-based practice that the rates of discontinuation are that high, but it would be important to examine this in different locations,” added Dr. Ananthakrishnan, who wasn’t involved with the study.
The study was published online in the British Journal of General Practice.
Cases on the rise
Globally, the incidence of UC is increasing fastest in younger populations. It’s estimated that up to 30% of individuals with UC are diagnosed in childhood or young adulthood, and these individuals are more likely to have a severe disease course and years living with disability, compared with peers diagnosed later in life. This makes achieving disease control and maintaining remission “paramount” for those diagnosed in early life, Dr. Saxena and colleagues write.
International UC guidelines recommend starting therapy with 5-ASA, also known as mesalamine, soon after diagnosis and continuing it long term to maintain remission. However, some prior evidence suggests that adherence to UC medication may be less optimal in younger people – findings supported by the U.K. study.
Leveraging data from the UK Clinical Practice Research Datalink, Dr. Saxena and colleagues analyzed data for 607 children and young adults aged 10-24 years starting oral 5-ASA maintenance therapy for UC.
They found that 152 individuals (25%) stopped 5-ASA treatment after 1 month, and 419 (69%) discontinued it within 1 year of starting treatment. The median time to stopping the anti-inflammatory drug was 162 days.
Discontinuation rates were highest in young adults aged 18-24 years (74%). The transition to adult care and loss of support from caregivers who encourage adherence in adolescents and provide financial and practical support could be one explanation for this, the researchers write.
After accounting for other factors, young adults aged 18-24 years starting 5-ASA were 43% more likely to discontinue it in the first year than adolescents aged 10-14 years.
Individuals living in socioeconomically deprived areas were 46% more likely to stop treatment, compared with those living in more affluent areas, a finding that suggests the need to address socioeconomic disparities that could drive discontinuation, the authors say.
They also found that early corticosteroid use for an acute UC flare was associated with a 32% lower likelihood of stopping 5-ASA therapy.
Adherence falls short
In terms of adherence, defined as the proportion of days covered by 5-ASA medication, the mean was 72%, equivalent to just under 9 months. Adherence fell with older age at initiation of therapy. Adherence was 80% among those 10-14 years, 78% among those 15-17 years, and 69% among those 18-24 years. Prior research has shown that nonadherence to UC medication – defined as proportion of days covered less than 80% – has been associated with a five-fold risk of disease, compared with adherence above 80%, the investigators note.
“If clinicians are unaware of suboptimal adherence to first-line medication, they may incorrectly assume therapy has failed, which may lead to unnecessary escalation in treatment and avoidable steroid use that remains high in UC,” the researchers write.
Psychiatric comorbidity (depression, anxiety, or antidepressant use) was not associated with discontinuation or adherence to treatment.
“As doctors, this study shows we need to be keeping a close eye on patients, particularly within that first year of starting medication,” Dr. Saxena said in the release.
“We should check if these patients are getting their medications and whether they have difficulty paying for them. We should also use the opportunity to talk through any recurring symptoms and how to access advice from providers such as a nurse specialist,” Dr. Saxena said.
Effectiveness of therapy in young adults
Reached for comment, Michael Dolinger, MD, assistant professor of pediatric gastroenterology, Icahn School of Medicine and Mount Sinai Kravis Children’s Hospital, both in New York, said he has seen 5-ASA stoppage among his younger patients.
“Generally, what we see is that the majority of patients over time are not able to be sustained on oral mesalamine treatment, and they need a more advanced therapy,” Dr. Dolinger said in an interview.
And while the U.K. study did not delve into the reasons for discontinuation, ineffectiveness of therapy is likely a main cause, Dr. Dolinger said.
“We especially see this in our younger adolescent and young adult patients. In these younger patients, the immune system is potentially driving inflammation a bit more than in older patients, often going beyond the inner lining of the colon to the entire bowel wall, even in ulcerative colitis, and therefore mesalamine may be ineffective over the first year,” Dr. Dolinger explained.
When choosing a more advanced therapy, Dr. Dolinger said, “it’s all about having that conversation in a shared decision-making process about what may be the most effective short- and long-term treatment options with the best safety for that patient. It’s a very individualized discussion.”
“One of the main things we preach and talk about is control of inflammation, getting into early deep remission, because the longer you have inflammation, even if it’s just smoldering, the harder it is to get into deep remission,” Dr. Dolinger added.
The study had no commercial funding. Dr. Saxena, Dr. Ananthakrishnan, and Dr. Dolinger have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research from the United Kingdom indicates.
“This is concerning as they are at risk of their condition returning and further complications. It can also lead to severe complications such as surgery to remove part of the gut,” Sonia Saxena, MBBS, director, Imperial Child Health Unit, School of Public Health, Imperial College London, said in a news release.
The study “highlights the importance of counseling and education of patients at diagnosis as this is a critical window that influences long-term health behavior,” Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, said in an interview.
“It has not been my experience in U.S.-based practice that the rates of discontinuation are that high, but it would be important to examine this in different locations,” added Dr. Ananthakrishnan, who wasn’t involved with the study.
The study was published online in the British Journal of General Practice.
Cases on the rise
Globally, the incidence of UC is increasing fastest in younger populations. It’s estimated that up to 30% of individuals with UC are diagnosed in childhood or young adulthood, and these individuals are more likely to have a severe disease course and years living with disability, compared with peers diagnosed later in life. This makes achieving disease control and maintaining remission “paramount” for those diagnosed in early life, Dr. Saxena and colleagues write.
International UC guidelines recommend starting therapy with 5-ASA, also known as mesalamine, soon after diagnosis and continuing it long term to maintain remission. However, some prior evidence suggests that adherence to UC medication may be less optimal in younger people – findings supported by the U.K. study.
Leveraging data from the UK Clinical Practice Research Datalink, Dr. Saxena and colleagues analyzed data for 607 children and young adults aged 10-24 years starting oral 5-ASA maintenance therapy for UC.
They found that 152 individuals (25%) stopped 5-ASA treatment after 1 month, and 419 (69%) discontinued it within 1 year of starting treatment. The median time to stopping the anti-inflammatory drug was 162 days.
Discontinuation rates were highest in young adults aged 18-24 years (74%). The transition to adult care and loss of support from caregivers who encourage adherence in adolescents and provide financial and practical support could be one explanation for this, the researchers write.
After accounting for other factors, young adults aged 18-24 years starting 5-ASA were 43% more likely to discontinue it in the first year than adolescents aged 10-14 years.
Individuals living in socioeconomically deprived areas were 46% more likely to stop treatment, compared with those living in more affluent areas, a finding that suggests the need to address socioeconomic disparities that could drive discontinuation, the authors say.
They also found that early corticosteroid use for an acute UC flare was associated with a 32% lower likelihood of stopping 5-ASA therapy.
Adherence falls short
In terms of adherence, defined as the proportion of days covered by 5-ASA medication, the mean was 72%, equivalent to just under 9 months. Adherence fell with older age at initiation of therapy. Adherence was 80% among those 10-14 years, 78% among those 15-17 years, and 69% among those 18-24 years. Prior research has shown that nonadherence to UC medication – defined as proportion of days covered less than 80% – has been associated with a five-fold risk of disease, compared with adherence above 80%, the investigators note.
“If clinicians are unaware of suboptimal adherence to first-line medication, they may incorrectly assume therapy has failed, which may lead to unnecessary escalation in treatment and avoidable steroid use that remains high in UC,” the researchers write.
Psychiatric comorbidity (depression, anxiety, or antidepressant use) was not associated with discontinuation or adherence to treatment.
“As doctors, this study shows we need to be keeping a close eye on patients, particularly within that first year of starting medication,” Dr. Saxena said in the release.
“We should check if these patients are getting their medications and whether they have difficulty paying for them. We should also use the opportunity to talk through any recurring symptoms and how to access advice from providers such as a nurse specialist,” Dr. Saxena said.
Effectiveness of therapy in young adults
Reached for comment, Michael Dolinger, MD, assistant professor of pediatric gastroenterology, Icahn School of Medicine and Mount Sinai Kravis Children’s Hospital, both in New York, said he has seen 5-ASA stoppage among his younger patients.
“Generally, what we see is that the majority of patients over time are not able to be sustained on oral mesalamine treatment, and they need a more advanced therapy,” Dr. Dolinger said in an interview.
And while the U.K. study did not delve into the reasons for discontinuation, ineffectiveness of therapy is likely a main cause, Dr. Dolinger said.
“We especially see this in our younger adolescent and young adult patients. In these younger patients, the immune system is potentially driving inflammation a bit more than in older patients, often going beyond the inner lining of the colon to the entire bowel wall, even in ulcerative colitis, and therefore mesalamine may be ineffective over the first year,” Dr. Dolinger explained.
When choosing a more advanced therapy, Dr. Dolinger said, “it’s all about having that conversation in a shared decision-making process about what may be the most effective short- and long-term treatment options with the best safety for that patient. It’s a very individualized discussion.”
“One of the main things we preach and talk about is control of inflammation, getting into early deep remission, because the longer you have inflammation, even if it’s just smoldering, the harder it is to get into deep remission,” Dr. Dolinger added.
The study had no commercial funding. Dr. Saxena, Dr. Ananthakrishnan, and Dr. Dolinger have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF GENERAL PRACTICE
Are ketogenic supplements the key to healthy aging?
A century ago, pediatricians began prescribing for children with intractable seizures the “keto diet,” which they also used to treat diabetes in children and adults. The low-carbohydrate, high-fat meals were designed to induce a near hypoglycemic state, forcing the body to use ketones for fuel instead of glucose.
The strategy fell out of favor after the discovery of insulin in the 1920s and the development of better antiseizure medications. The global market for the ketogenic diet topped $11 billion in 2022.
Is it just a fad, or has the public – and science – caught up with the 100-year-old approach?
Although scientists still don’t know why the ketogenic diet was effective for controlling seizures, they have documented the effectiveness of ketogenic diets for the treatment of diabetes and metabolic syndrome. An extensive body of literature has documented their use in athletes, but less is known regarding conditions such as heart disease and dementia.
Although the data are promising, much of the research has been conducted with mice or has come from trials of short-term use in humans. But recently, the National Institutes of Health awarded a $3.5 million federal grant for a double-blind, randomized, placebo-controlled clinical trial to understand the effects of the long-term use of ketone ester supplementation on frailty. Developed 20 years ago, ketone esters are precursor molecules that the body quickly breaks down into ketone bodies when carbohydrates aren’t available.
“We’ve learned so much recently about how ketone bodies interact with aging biology,” John Newman, MD, PhD, of the Buck Institute for Research on Aging in Novato, Calif., and the study’s principal investigator, said in an interview. “And we’re only just starting to translate that out of the laboratory and into human studies to see how we can take advantage of ketone bodies to improve people’s health.”
Researchers from the Ohio State University and the University of Connecticut will also participate in the TAKEOFF (Targeting Aging With Ketone Ester in Older Adults for Function in Frailty) trial, which seeks to recruit a total of 180 people across the three sites.
Dr. Newman, assistant professor at the Buck Institute and associate professor in the division of geriatrics at the University of California, San Francisco, said
One of the common things that happen during aging is that tissues – such as of the heart, brain, and muscle – lose the ability to metabolize glucose effectively. Over time, resistance to insulin can develop.
Researchers can map out areas of the brain affected by Alzheimer’s disease, for example, by assessing where patients’ glucose uptake drops. In heart failure, the heart has difficulty obtaining enough energy from glucose and instead burns fats and ketone bodies.
How might ketones affect frailty in the elderly?
As a practicing geriatrician, Dr. Newman measures frailty by evaluating patients’ strength, endurance, and how they react to stresses. He and his colleagues believe certain molecular and cellular changes may make patients more likely to fall, to recover more slowly from surgery, or to lose mobility.
The main hypothesis of the TAKEOFF study is “that if you target these fundamental mechanisms of aging, you would be able to impact many different diseases of aging across different organ systems.”
Dr. Newman and Brianna Stubbs, DPhil, lead translational scientist at the Buck Institute, are still finishing up the BIKE (Buck Institute Ketone Ester) pilot study, which was the first double-blind, randomized, placebo-controlled study to evaluate the use of ketone ester supplements in adults older than 65 years. “The BIKE study is 12 weeks long. That’s actually the longest that anyone has studied ketone ester supplements in humans,” Dr. Stubbs said. The results will help them firm up the protocol for the TAKEOFF trial, which will likely treat patients for up to 24 weeks.
The primary outcome measure at all three study sites will be leg press strength. Researchers will also assess a variety of secondary outcomes that cover geriatric and cognitive function – measures such as gait speed and walking endurance, cognitive tests, and quality of life. And at the Buck, Dr. Newman and Dr. Stubbs will be evaluating the use of biomarkers that are often available in clinical labs – insulin, C-reactive protein, cystatin, and natriuretic peptide tests – for use as outcome measures that are responsive to treatment interventions and that can be used to track outcomes in future research on aging.
To achieve the goal of looking broadly at different organ systems likely to be affected by ketogenic supplements, they have assembled a team of coinvestigators with wide-ranging expertise in ketone and aging research.
Jeff Volek, PhD, professor in the department of human sciences at the Ohio State University, in Columbus, has contributed extensively to the literature on the use of ketogenic diets and supplements in a variety of populations, such as endurance athletes and patients with insulin resistance or diabetes.
Dr. Volek has demonstrated that ketones can have an anticatabolic effect on muscle tissue. “They could help offset some of the muscle loss with aging, which would in turn improve their physical functioning and ability to do daily activities,” he said.
The anti-inflammatory property of ketones may provide another benefit to older people. They can reduce oxidative stress, which is considered one of the chief pathologic mechanisms responsible for conditions such as heart disease, Alzheimer’s disease, asthma, and arthritis.
In addition to the main study outcomes, Dr. Volek’s lab will study muscle physiology by performing biopsies at baseline and after consumption of ketogenic supplements to assess metabolic changes in muscle cells as they consume energy. Study participants will also undergo MRIs to detect subtle changes in muscle size before and after treatment.
From elite athletes to everyday agers
As a graduate student in Dr. Volek’s lab, Jenna Bartley, PhD, studied the effects of a ketogenic diet on elite athletes. But her work has taken a turn. Now an assistant professor in the department of immunology and the center on aging at the University of Connecticut in Farmington, she focuses on how immune responses and physical function decline with age.
“Ketogenic diets and the main ketone bodies – mainly beta-hydroxybutyrate – have been shown to have really powerful influences on a lot of things that go wrong with aging,” Dr. Bartley said. The decline in immune function in the elderly is not isolated to one cell type or even one arm of the immune system. There is reason to believe ketone supplementation could improve immune function.
“T cells really love ketones for energy,” Dr. Bartley said. Some data show that production of ketone bodies is impaired in individuals with severe SARS-CoV-2 infection. Mouse models of SARS-CoV-2 infection have found that ketogenic diets led to improvement in the response to antiviral therapy.
In her lab, she’ll assess serum markers of inflammation in patients, as well as cytokine secretion following stimulation of T cells. T cells in culture from older people produce more inflammatory cytokines than those from younger people, leading to a dysfunctional immune response. Dr. Bartley is curious to see whether ketones can fix that. Additional work will include single-cell RNA sequencing of different classes of immune cells to investigate how ketones might change metabolic pathways.
Why use ketogenic supplements instead of having people consume ketogenic diets? “There are no cheat days in the keto diet,” Dr. Bartley said. Administering the diet requires intense supervision of research participants to enforce adherence. Use of supplements will improve compliance and likely make any findings translatable to more of the population, she said.
Drawbacks of the initial formulations of ketone esters, first developed 20 years ago, included high cost and terrible taste. Dr, Stubbs, a former world class rowing champion who competed in the Ironman World Championship last year, has firsthand experience with them as a research participant.
“It tasted like drinking nail polish,” she said. Recent advances in manufacturing have made them cheaper – roughly $5 per day – and more palatable, enabling research studies such as TAKEOFF.
For Dr. Newman, the studies are early building blocks in the emerging field of geroscience, which aims to translate fundamental mechanisms of aging into therapies to treat disease.
“We’re hoping that this will be an example of a proof-of-concept geroscience study that will really help to translate ketone body biology out of the laboratory and hopefully into a diversity of clinical applications,” he said. “There’s a lot we don’t understand still about the molecular mechanisms of frailty.”
Dr. Newman and Dr. Stubbs own stock in BHB Therapeutics Ltd, the company providing the product being studied, and are inventors on patents that relate to the product being studied. The Buck Institute has an ownership interest in BHB Therapeutics. Dr. Bartley and Dr. Volek report no relevant financial relationships.
A version of this article appeared on Medscape.com .
A century ago, pediatricians began prescribing for children with intractable seizures the “keto diet,” which they also used to treat diabetes in children and adults. The low-carbohydrate, high-fat meals were designed to induce a near hypoglycemic state, forcing the body to use ketones for fuel instead of glucose.
The strategy fell out of favor after the discovery of insulin in the 1920s and the development of better antiseizure medications. The global market for the ketogenic diet topped $11 billion in 2022.
Is it just a fad, or has the public – and science – caught up with the 100-year-old approach?
Although scientists still don’t know why the ketogenic diet was effective for controlling seizures, they have documented the effectiveness of ketogenic diets for the treatment of diabetes and metabolic syndrome. An extensive body of literature has documented their use in athletes, but less is known regarding conditions such as heart disease and dementia.
Although the data are promising, much of the research has been conducted with mice or has come from trials of short-term use in humans. But recently, the National Institutes of Health awarded a $3.5 million federal grant for a double-blind, randomized, placebo-controlled clinical trial to understand the effects of the long-term use of ketone ester supplementation on frailty. Developed 20 years ago, ketone esters are precursor molecules that the body quickly breaks down into ketone bodies when carbohydrates aren’t available.
“We’ve learned so much recently about how ketone bodies interact with aging biology,” John Newman, MD, PhD, of the Buck Institute for Research on Aging in Novato, Calif., and the study’s principal investigator, said in an interview. “And we’re only just starting to translate that out of the laboratory and into human studies to see how we can take advantage of ketone bodies to improve people’s health.”
Researchers from the Ohio State University and the University of Connecticut will also participate in the TAKEOFF (Targeting Aging With Ketone Ester in Older Adults for Function in Frailty) trial, which seeks to recruit a total of 180 people across the three sites.
Dr. Newman, assistant professor at the Buck Institute and associate professor in the division of geriatrics at the University of California, San Francisco, said
One of the common things that happen during aging is that tissues – such as of the heart, brain, and muscle – lose the ability to metabolize glucose effectively. Over time, resistance to insulin can develop.
Researchers can map out areas of the brain affected by Alzheimer’s disease, for example, by assessing where patients’ glucose uptake drops. In heart failure, the heart has difficulty obtaining enough energy from glucose and instead burns fats and ketone bodies.
How might ketones affect frailty in the elderly?
As a practicing geriatrician, Dr. Newman measures frailty by evaluating patients’ strength, endurance, and how they react to stresses. He and his colleagues believe certain molecular and cellular changes may make patients more likely to fall, to recover more slowly from surgery, or to lose mobility.
The main hypothesis of the TAKEOFF study is “that if you target these fundamental mechanisms of aging, you would be able to impact many different diseases of aging across different organ systems.”
Dr. Newman and Brianna Stubbs, DPhil, lead translational scientist at the Buck Institute, are still finishing up the BIKE (Buck Institute Ketone Ester) pilot study, which was the first double-blind, randomized, placebo-controlled study to evaluate the use of ketone ester supplements in adults older than 65 years. “The BIKE study is 12 weeks long. That’s actually the longest that anyone has studied ketone ester supplements in humans,” Dr. Stubbs said. The results will help them firm up the protocol for the TAKEOFF trial, which will likely treat patients for up to 24 weeks.
The primary outcome measure at all three study sites will be leg press strength. Researchers will also assess a variety of secondary outcomes that cover geriatric and cognitive function – measures such as gait speed and walking endurance, cognitive tests, and quality of life. And at the Buck, Dr. Newman and Dr. Stubbs will be evaluating the use of biomarkers that are often available in clinical labs – insulin, C-reactive protein, cystatin, and natriuretic peptide tests – for use as outcome measures that are responsive to treatment interventions and that can be used to track outcomes in future research on aging.
To achieve the goal of looking broadly at different organ systems likely to be affected by ketogenic supplements, they have assembled a team of coinvestigators with wide-ranging expertise in ketone and aging research.
Jeff Volek, PhD, professor in the department of human sciences at the Ohio State University, in Columbus, has contributed extensively to the literature on the use of ketogenic diets and supplements in a variety of populations, such as endurance athletes and patients with insulin resistance or diabetes.
Dr. Volek has demonstrated that ketones can have an anticatabolic effect on muscle tissue. “They could help offset some of the muscle loss with aging, which would in turn improve their physical functioning and ability to do daily activities,” he said.
The anti-inflammatory property of ketones may provide another benefit to older people. They can reduce oxidative stress, which is considered one of the chief pathologic mechanisms responsible for conditions such as heart disease, Alzheimer’s disease, asthma, and arthritis.
In addition to the main study outcomes, Dr. Volek’s lab will study muscle physiology by performing biopsies at baseline and after consumption of ketogenic supplements to assess metabolic changes in muscle cells as they consume energy. Study participants will also undergo MRIs to detect subtle changes in muscle size before and after treatment.
From elite athletes to everyday agers
As a graduate student in Dr. Volek’s lab, Jenna Bartley, PhD, studied the effects of a ketogenic diet on elite athletes. But her work has taken a turn. Now an assistant professor in the department of immunology and the center on aging at the University of Connecticut in Farmington, she focuses on how immune responses and physical function decline with age.
“Ketogenic diets and the main ketone bodies – mainly beta-hydroxybutyrate – have been shown to have really powerful influences on a lot of things that go wrong with aging,” Dr. Bartley said. The decline in immune function in the elderly is not isolated to one cell type or even one arm of the immune system. There is reason to believe ketone supplementation could improve immune function.
“T cells really love ketones for energy,” Dr. Bartley said. Some data show that production of ketone bodies is impaired in individuals with severe SARS-CoV-2 infection. Mouse models of SARS-CoV-2 infection have found that ketogenic diets led to improvement in the response to antiviral therapy.
In her lab, she’ll assess serum markers of inflammation in patients, as well as cytokine secretion following stimulation of T cells. T cells in culture from older people produce more inflammatory cytokines than those from younger people, leading to a dysfunctional immune response. Dr. Bartley is curious to see whether ketones can fix that. Additional work will include single-cell RNA sequencing of different classes of immune cells to investigate how ketones might change metabolic pathways.
Why use ketogenic supplements instead of having people consume ketogenic diets? “There are no cheat days in the keto diet,” Dr. Bartley said. Administering the diet requires intense supervision of research participants to enforce adherence. Use of supplements will improve compliance and likely make any findings translatable to more of the population, she said.
Drawbacks of the initial formulations of ketone esters, first developed 20 years ago, included high cost and terrible taste. Dr, Stubbs, a former world class rowing champion who competed in the Ironman World Championship last year, has firsthand experience with them as a research participant.
“It tasted like drinking nail polish,” she said. Recent advances in manufacturing have made them cheaper – roughly $5 per day – and more palatable, enabling research studies such as TAKEOFF.
For Dr. Newman, the studies are early building blocks in the emerging field of geroscience, which aims to translate fundamental mechanisms of aging into therapies to treat disease.
“We’re hoping that this will be an example of a proof-of-concept geroscience study that will really help to translate ketone body biology out of the laboratory and hopefully into a diversity of clinical applications,” he said. “There’s a lot we don’t understand still about the molecular mechanisms of frailty.”
Dr. Newman and Dr. Stubbs own stock in BHB Therapeutics Ltd, the company providing the product being studied, and are inventors on patents that relate to the product being studied. The Buck Institute has an ownership interest in BHB Therapeutics. Dr. Bartley and Dr. Volek report no relevant financial relationships.
A version of this article appeared on Medscape.com .
A century ago, pediatricians began prescribing for children with intractable seizures the “keto diet,” which they also used to treat diabetes in children and adults. The low-carbohydrate, high-fat meals were designed to induce a near hypoglycemic state, forcing the body to use ketones for fuel instead of glucose.
The strategy fell out of favor after the discovery of insulin in the 1920s and the development of better antiseizure medications. The global market for the ketogenic diet topped $11 billion in 2022.
Is it just a fad, or has the public – and science – caught up with the 100-year-old approach?
Although scientists still don’t know why the ketogenic diet was effective for controlling seizures, they have documented the effectiveness of ketogenic diets for the treatment of diabetes and metabolic syndrome. An extensive body of literature has documented their use in athletes, but less is known regarding conditions such as heart disease and dementia.
Although the data are promising, much of the research has been conducted with mice or has come from trials of short-term use in humans. But recently, the National Institutes of Health awarded a $3.5 million federal grant for a double-blind, randomized, placebo-controlled clinical trial to understand the effects of the long-term use of ketone ester supplementation on frailty. Developed 20 years ago, ketone esters are precursor molecules that the body quickly breaks down into ketone bodies when carbohydrates aren’t available.
“We’ve learned so much recently about how ketone bodies interact with aging biology,” John Newman, MD, PhD, of the Buck Institute for Research on Aging in Novato, Calif., and the study’s principal investigator, said in an interview. “And we’re only just starting to translate that out of the laboratory and into human studies to see how we can take advantage of ketone bodies to improve people’s health.”
Researchers from the Ohio State University and the University of Connecticut will also participate in the TAKEOFF (Targeting Aging With Ketone Ester in Older Adults for Function in Frailty) trial, which seeks to recruit a total of 180 people across the three sites.
Dr. Newman, assistant professor at the Buck Institute and associate professor in the division of geriatrics at the University of California, San Francisco, said
One of the common things that happen during aging is that tissues – such as of the heart, brain, and muscle – lose the ability to metabolize glucose effectively. Over time, resistance to insulin can develop.
Researchers can map out areas of the brain affected by Alzheimer’s disease, for example, by assessing where patients’ glucose uptake drops. In heart failure, the heart has difficulty obtaining enough energy from glucose and instead burns fats and ketone bodies.
How might ketones affect frailty in the elderly?
As a practicing geriatrician, Dr. Newman measures frailty by evaluating patients’ strength, endurance, and how they react to stresses. He and his colleagues believe certain molecular and cellular changes may make patients more likely to fall, to recover more slowly from surgery, or to lose mobility.
The main hypothesis of the TAKEOFF study is “that if you target these fundamental mechanisms of aging, you would be able to impact many different diseases of aging across different organ systems.”
Dr. Newman and Brianna Stubbs, DPhil, lead translational scientist at the Buck Institute, are still finishing up the BIKE (Buck Institute Ketone Ester) pilot study, which was the first double-blind, randomized, placebo-controlled study to evaluate the use of ketone ester supplements in adults older than 65 years. “The BIKE study is 12 weeks long. That’s actually the longest that anyone has studied ketone ester supplements in humans,” Dr. Stubbs said. The results will help them firm up the protocol for the TAKEOFF trial, which will likely treat patients for up to 24 weeks.
The primary outcome measure at all three study sites will be leg press strength. Researchers will also assess a variety of secondary outcomes that cover geriatric and cognitive function – measures such as gait speed and walking endurance, cognitive tests, and quality of life. And at the Buck, Dr. Newman and Dr. Stubbs will be evaluating the use of biomarkers that are often available in clinical labs – insulin, C-reactive protein, cystatin, and natriuretic peptide tests – for use as outcome measures that are responsive to treatment interventions and that can be used to track outcomes in future research on aging.
To achieve the goal of looking broadly at different organ systems likely to be affected by ketogenic supplements, they have assembled a team of coinvestigators with wide-ranging expertise in ketone and aging research.
Jeff Volek, PhD, professor in the department of human sciences at the Ohio State University, in Columbus, has contributed extensively to the literature on the use of ketogenic diets and supplements in a variety of populations, such as endurance athletes and patients with insulin resistance or diabetes.
Dr. Volek has demonstrated that ketones can have an anticatabolic effect on muscle tissue. “They could help offset some of the muscle loss with aging, which would in turn improve their physical functioning and ability to do daily activities,” he said.
The anti-inflammatory property of ketones may provide another benefit to older people. They can reduce oxidative stress, which is considered one of the chief pathologic mechanisms responsible for conditions such as heart disease, Alzheimer’s disease, asthma, and arthritis.
In addition to the main study outcomes, Dr. Volek’s lab will study muscle physiology by performing biopsies at baseline and after consumption of ketogenic supplements to assess metabolic changes in muscle cells as they consume energy. Study participants will also undergo MRIs to detect subtle changes in muscle size before and after treatment.
From elite athletes to everyday agers
As a graduate student in Dr. Volek’s lab, Jenna Bartley, PhD, studied the effects of a ketogenic diet on elite athletes. But her work has taken a turn. Now an assistant professor in the department of immunology and the center on aging at the University of Connecticut in Farmington, she focuses on how immune responses and physical function decline with age.
“Ketogenic diets and the main ketone bodies – mainly beta-hydroxybutyrate – have been shown to have really powerful influences on a lot of things that go wrong with aging,” Dr. Bartley said. The decline in immune function in the elderly is not isolated to one cell type or even one arm of the immune system. There is reason to believe ketone supplementation could improve immune function.
“T cells really love ketones for energy,” Dr. Bartley said. Some data show that production of ketone bodies is impaired in individuals with severe SARS-CoV-2 infection. Mouse models of SARS-CoV-2 infection have found that ketogenic diets led to improvement in the response to antiviral therapy.
In her lab, she’ll assess serum markers of inflammation in patients, as well as cytokine secretion following stimulation of T cells. T cells in culture from older people produce more inflammatory cytokines than those from younger people, leading to a dysfunctional immune response. Dr. Bartley is curious to see whether ketones can fix that. Additional work will include single-cell RNA sequencing of different classes of immune cells to investigate how ketones might change metabolic pathways.
Why use ketogenic supplements instead of having people consume ketogenic diets? “There are no cheat days in the keto diet,” Dr. Bartley said. Administering the diet requires intense supervision of research participants to enforce adherence. Use of supplements will improve compliance and likely make any findings translatable to more of the population, she said.
Drawbacks of the initial formulations of ketone esters, first developed 20 years ago, included high cost and terrible taste. Dr, Stubbs, a former world class rowing champion who competed in the Ironman World Championship last year, has firsthand experience with them as a research participant.
“It tasted like drinking nail polish,” she said. Recent advances in manufacturing have made them cheaper – roughly $5 per day – and more palatable, enabling research studies such as TAKEOFF.
For Dr. Newman, the studies are early building blocks in the emerging field of geroscience, which aims to translate fundamental mechanisms of aging into therapies to treat disease.
“We’re hoping that this will be an example of a proof-of-concept geroscience study that will really help to translate ketone body biology out of the laboratory and hopefully into a diversity of clinical applications,” he said. “There’s a lot we don’t understand still about the molecular mechanisms of frailty.”
Dr. Newman and Dr. Stubbs own stock in BHB Therapeutics Ltd, the company providing the product being studied, and are inventors on patents that relate to the product being studied. The Buck Institute has an ownership interest in BHB Therapeutics. Dr. Bartley and Dr. Volek report no relevant financial relationships.
A version of this article appeared on Medscape.com .
People with long COVID have specific blood biomarkers, study says
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
Patients with rheumatism have premature immune system aging
LEIPZIG, GERMANY – With age comes illness: Cancer, cardiovascular and neurodegenerative diseases, increased infections, and autoimmune diseases such as rheumatism become more common. This is because the immune system also ages. In the case of autoimmune diseases, this aging happens particularly quickly.
“There is this phenomenon of premature aging of the immune system,” said Cornelia Weyand, PhD, director of Stanford (Calif.) University’s Center for Translational Medicine at the German Rheumatology Congress 2023. In healthy people, the immune system begins to age at age 20. From that point on, the thymus gland, which reaches peak function at 14-15 years old, plays an increasingly minor role. “At age 50 years, the aging process of the immune system gains momentum.”
“What’s good about this is that the T and B cells age together, but all a little differently, each system by itself,” said Thomas Dörner, MD, PhD, head of consultation hours for clinical hemostaseology at the Charité University Hospitals in Berlin.
While the reduced formation of naive T cells can be attributed to the regression of the thymus gland, the naive B cells are a consequence of age-related, fatty bone marrow degeneration. The influence of adipocyte-derived tumor necrosis factor (TNF)–alpha also causes the bone marrow to develop B cells more and more weakly and slowly. “Through this [process], the preimmune range of B-cells decreases and becomes less healthy than in a young person.”
‘Inflamm-aging’
“In the periphery, we have identified a process we call “inflamm-aging,” where the cytokines interferon-gamma, interleukin (IL)–10, and IL-17 play a predominant role. This also alters the primary and secondary immune response,” said Dr. Dörner. Here, decreasing stimulation via the B-cell receptor by aging T-lymphocytes makes a difference.
As we age, the immune system restructures itself completely. “Protective immunity regresses and the inferior immunity emerges,” explained Dr. Weyand. Wounds heal more poorly, the protective action against infections and above all malignancies, as well as the immune response to vaccinations, decreases.
The increased occurrence of neurodegenerative, cardiovascular, and autoimmune diseases is not because of a loss of function, but rather to newly gained, undesired functions. These are associated with inflammatory changes. Hence, the term inflamm-aging.
With the B cells, functional germinal centers in the lymphoid organs and protective antibodies become rarer, and age-associated B cells accumulate. As Dr. Dörner emphasized, these cells are not under the command of the B-cell receptor and are independent of the cytokine BAFF (B-cell activating factor). Instead, they react to signals that are sent from the toll-like receptors 7 and 9.
This potentially also explains the increased development of autoantibodies in older people and the association of viral and autoimmune diseases. This means that age-associated B cells develop more frequently, such as with rheumatoid arthritis, scleroderma, and systemic lupus erythematosus. “There are good data that show that they are triggered by infections and that they are specialized to form autoantibodies,” Dr. Weyand said about the age-associated B cells.
‘Bad old T cells’
Under the influence of genetic stop-and-go signals, the composition of the T-cell population also changes over the course of our lives. It becomes less diverse. T-helper cells become less common, and as a result, terminally differentiated effector memory T cells become more common. According to Dr. Weyand, herein lies the problem. “These cells are not just lazy, old cells that sit around. Unfortunately, they are also malicious. What we see in both the T- and B-cell systems is that they become increasingly innate with age,” he said. “They are not quite so precise or good.”
In turn, myeloid cells are less active in old age because of phagocytosis and antigen presentation, and they get more mutations. They are released more often from the bone marrow, produce more cytokines, and essentially contribute to inflamm-aging.
Power sources fail
In her cellular and microbiological investigations, Dr. Weyand has devoted a lot of time to studying why T cells age prematurely in patients with RA. The key was in the cellular microbiology. “We learned how the T-cell aging process translates into metabolic reprogramming of the T cells – how a good, strong, and protective T cell transforms into a disease-inducing T cell.”
At the center of premature T-cell aging in RA are disrupted mitochondrial function and insufficient communication of the mitochondria with the lysosomes and the endoplasmic reticulum.
T cells of RA patients (RA T cells) contain less MRE11A, compared with those in healthy people. This is a nuclease that allows the repair of breaks in DNA. If MRE11A is inhibited, then senescent T cells accumulate and form proinflammatory cytokines such as IL-B, IL-6, and TNF. “This is the trio that we rheumatologists are always concerned with.”
Since mitochondrial DNA repair is essential for maintaining mitochondrial fitness, the cellular power sources in patients with RA cannot provide as much energy in the form of adenosine triphosphate as in healthy people. Metabolically, they are not so fit, Dr. Weyand said.
Inflammatory cell death
In fact, all metabolic pathways in the T cells are reduced. The bioenergetic failure has consequences. “Unfortunately, as the mitochondrion ages, its DNA leaks into the cytosol,” explained Dr. Weyand. “Cells do not like this.” This is because DNA activates inflammasomes in the cytosol via caspase-1. This process results in a highly inflammatory cell death: pyroptosis. Subsequently, there is no trace of the cells in the tissue. “RA patients’ synovial tissue is a graveyard of dying T cells.”
In the lysosomes, the cells’ “intestine,” problems arise because patients with RA can no longer activate the adenosine monophosphate–activated protein kinase enzyme. It does not receive the lipid tail it needs to take its position as energy sensor on the lysosomal membrane. As a result, its antagonist, mTOR – both usually keep each other in check – gains the upper hand. According to Dr. Weyand, “mTOR has a party.” It activates and stresses the cells.
Additional changes affect the endoplasmic reticulum (ER). “This is where all of your proteins are synthesized and packaged to migrate from within to outside the cell, or to the cell membrane.” Compared with healthy T cells, RA T cells have around 50% more ER. “The less that mitochondria work, the larger the ER. It gets really fat.”
Mitochondria communicate with the ER via aspartate, oxaloacetate, and malate. In so doing, they control their size. RA T cells appear to be aspartate deficient. In animal models, amino acids had an anti-inflammatory effect.
When sequencing the mRNA bound to the ER, Dr. Weyand and her colleagues encountered the building blocks for TNF. “There is more than three times as much mRNA as TNF. It transforms these T cells into TNF superproducers,” said the rheumatologist. “No wonder this kind of cell is proinflammatory – it forms precisely that cytokine on which you focus every day.”
This article was translated from Medscape’s German edition. A version of this article first appeared on Medscape.com.
LEIPZIG, GERMANY – With age comes illness: Cancer, cardiovascular and neurodegenerative diseases, increased infections, and autoimmune diseases such as rheumatism become more common. This is because the immune system also ages. In the case of autoimmune diseases, this aging happens particularly quickly.
“There is this phenomenon of premature aging of the immune system,” said Cornelia Weyand, PhD, director of Stanford (Calif.) University’s Center for Translational Medicine at the German Rheumatology Congress 2023. In healthy people, the immune system begins to age at age 20. From that point on, the thymus gland, which reaches peak function at 14-15 years old, plays an increasingly minor role. “At age 50 years, the aging process of the immune system gains momentum.”
“What’s good about this is that the T and B cells age together, but all a little differently, each system by itself,” said Thomas Dörner, MD, PhD, head of consultation hours for clinical hemostaseology at the Charité University Hospitals in Berlin.
While the reduced formation of naive T cells can be attributed to the regression of the thymus gland, the naive B cells are a consequence of age-related, fatty bone marrow degeneration. The influence of adipocyte-derived tumor necrosis factor (TNF)–alpha also causes the bone marrow to develop B cells more and more weakly and slowly. “Through this [process], the preimmune range of B-cells decreases and becomes less healthy than in a young person.”
‘Inflamm-aging’
“In the periphery, we have identified a process we call “inflamm-aging,” where the cytokines interferon-gamma, interleukin (IL)–10, and IL-17 play a predominant role. This also alters the primary and secondary immune response,” said Dr. Dörner. Here, decreasing stimulation via the B-cell receptor by aging T-lymphocytes makes a difference.
As we age, the immune system restructures itself completely. “Protective immunity regresses and the inferior immunity emerges,” explained Dr. Weyand. Wounds heal more poorly, the protective action against infections and above all malignancies, as well as the immune response to vaccinations, decreases.
The increased occurrence of neurodegenerative, cardiovascular, and autoimmune diseases is not because of a loss of function, but rather to newly gained, undesired functions. These are associated with inflammatory changes. Hence, the term inflamm-aging.
With the B cells, functional germinal centers in the lymphoid organs and protective antibodies become rarer, and age-associated B cells accumulate. As Dr. Dörner emphasized, these cells are not under the command of the B-cell receptor and are independent of the cytokine BAFF (B-cell activating factor). Instead, they react to signals that are sent from the toll-like receptors 7 and 9.
This potentially also explains the increased development of autoantibodies in older people and the association of viral and autoimmune diseases. This means that age-associated B cells develop more frequently, such as with rheumatoid arthritis, scleroderma, and systemic lupus erythematosus. “There are good data that show that they are triggered by infections and that they are specialized to form autoantibodies,” Dr. Weyand said about the age-associated B cells.
‘Bad old T cells’
Under the influence of genetic stop-and-go signals, the composition of the T-cell population also changes over the course of our lives. It becomes less diverse. T-helper cells become less common, and as a result, terminally differentiated effector memory T cells become more common. According to Dr. Weyand, herein lies the problem. “These cells are not just lazy, old cells that sit around. Unfortunately, they are also malicious. What we see in both the T- and B-cell systems is that they become increasingly innate with age,” he said. “They are not quite so precise or good.”
In turn, myeloid cells are less active in old age because of phagocytosis and antigen presentation, and they get more mutations. They are released more often from the bone marrow, produce more cytokines, and essentially contribute to inflamm-aging.
Power sources fail
In her cellular and microbiological investigations, Dr. Weyand has devoted a lot of time to studying why T cells age prematurely in patients with RA. The key was in the cellular microbiology. “We learned how the T-cell aging process translates into metabolic reprogramming of the T cells – how a good, strong, and protective T cell transforms into a disease-inducing T cell.”
At the center of premature T-cell aging in RA are disrupted mitochondrial function and insufficient communication of the mitochondria with the lysosomes and the endoplasmic reticulum.
T cells of RA patients (RA T cells) contain less MRE11A, compared with those in healthy people. This is a nuclease that allows the repair of breaks in DNA. If MRE11A is inhibited, then senescent T cells accumulate and form proinflammatory cytokines such as IL-B, IL-6, and TNF. “This is the trio that we rheumatologists are always concerned with.”
Since mitochondrial DNA repair is essential for maintaining mitochondrial fitness, the cellular power sources in patients with RA cannot provide as much energy in the form of adenosine triphosphate as in healthy people. Metabolically, they are not so fit, Dr. Weyand said.
Inflammatory cell death
In fact, all metabolic pathways in the T cells are reduced. The bioenergetic failure has consequences. “Unfortunately, as the mitochondrion ages, its DNA leaks into the cytosol,” explained Dr. Weyand. “Cells do not like this.” This is because DNA activates inflammasomes in the cytosol via caspase-1. This process results in a highly inflammatory cell death: pyroptosis. Subsequently, there is no trace of the cells in the tissue. “RA patients’ synovial tissue is a graveyard of dying T cells.”
In the lysosomes, the cells’ “intestine,” problems arise because patients with RA can no longer activate the adenosine monophosphate–activated protein kinase enzyme. It does not receive the lipid tail it needs to take its position as energy sensor on the lysosomal membrane. As a result, its antagonist, mTOR – both usually keep each other in check – gains the upper hand. According to Dr. Weyand, “mTOR has a party.” It activates and stresses the cells.
Additional changes affect the endoplasmic reticulum (ER). “This is where all of your proteins are synthesized and packaged to migrate from within to outside the cell, or to the cell membrane.” Compared with healthy T cells, RA T cells have around 50% more ER. “The less that mitochondria work, the larger the ER. It gets really fat.”
Mitochondria communicate with the ER via aspartate, oxaloacetate, and malate. In so doing, they control their size. RA T cells appear to be aspartate deficient. In animal models, amino acids had an anti-inflammatory effect.
When sequencing the mRNA bound to the ER, Dr. Weyand and her colleagues encountered the building blocks for TNF. “There is more than three times as much mRNA as TNF. It transforms these T cells into TNF superproducers,” said the rheumatologist. “No wonder this kind of cell is proinflammatory – it forms precisely that cytokine on which you focus every day.”
This article was translated from Medscape’s German edition. A version of this article first appeared on Medscape.com.
LEIPZIG, GERMANY – With age comes illness: Cancer, cardiovascular and neurodegenerative diseases, increased infections, and autoimmune diseases such as rheumatism become more common. This is because the immune system also ages. In the case of autoimmune diseases, this aging happens particularly quickly.
“There is this phenomenon of premature aging of the immune system,” said Cornelia Weyand, PhD, director of Stanford (Calif.) University’s Center for Translational Medicine at the German Rheumatology Congress 2023. In healthy people, the immune system begins to age at age 20. From that point on, the thymus gland, which reaches peak function at 14-15 years old, plays an increasingly minor role. “At age 50 years, the aging process of the immune system gains momentum.”
“What’s good about this is that the T and B cells age together, but all a little differently, each system by itself,” said Thomas Dörner, MD, PhD, head of consultation hours for clinical hemostaseology at the Charité University Hospitals in Berlin.
While the reduced formation of naive T cells can be attributed to the regression of the thymus gland, the naive B cells are a consequence of age-related, fatty bone marrow degeneration. The influence of adipocyte-derived tumor necrosis factor (TNF)–alpha also causes the bone marrow to develop B cells more and more weakly and slowly. “Through this [process], the preimmune range of B-cells decreases and becomes less healthy than in a young person.”
‘Inflamm-aging’
“In the periphery, we have identified a process we call “inflamm-aging,” where the cytokines interferon-gamma, interleukin (IL)–10, and IL-17 play a predominant role. This also alters the primary and secondary immune response,” said Dr. Dörner. Here, decreasing stimulation via the B-cell receptor by aging T-lymphocytes makes a difference.
As we age, the immune system restructures itself completely. “Protective immunity regresses and the inferior immunity emerges,” explained Dr. Weyand. Wounds heal more poorly, the protective action against infections and above all malignancies, as well as the immune response to vaccinations, decreases.
The increased occurrence of neurodegenerative, cardiovascular, and autoimmune diseases is not because of a loss of function, but rather to newly gained, undesired functions. These are associated with inflammatory changes. Hence, the term inflamm-aging.
With the B cells, functional germinal centers in the lymphoid organs and protective antibodies become rarer, and age-associated B cells accumulate. As Dr. Dörner emphasized, these cells are not under the command of the B-cell receptor and are independent of the cytokine BAFF (B-cell activating factor). Instead, they react to signals that are sent from the toll-like receptors 7 and 9.
This potentially also explains the increased development of autoantibodies in older people and the association of viral and autoimmune diseases. This means that age-associated B cells develop more frequently, such as with rheumatoid arthritis, scleroderma, and systemic lupus erythematosus. “There are good data that show that they are triggered by infections and that they are specialized to form autoantibodies,” Dr. Weyand said about the age-associated B cells.
‘Bad old T cells’
Under the influence of genetic stop-and-go signals, the composition of the T-cell population also changes over the course of our lives. It becomes less diverse. T-helper cells become less common, and as a result, terminally differentiated effector memory T cells become more common. According to Dr. Weyand, herein lies the problem. “These cells are not just lazy, old cells that sit around. Unfortunately, they are also malicious. What we see in both the T- and B-cell systems is that they become increasingly innate with age,” he said. “They are not quite so precise or good.”
In turn, myeloid cells are less active in old age because of phagocytosis and antigen presentation, and they get more mutations. They are released more often from the bone marrow, produce more cytokines, and essentially contribute to inflamm-aging.
Power sources fail
In her cellular and microbiological investigations, Dr. Weyand has devoted a lot of time to studying why T cells age prematurely in patients with RA. The key was in the cellular microbiology. “We learned how the T-cell aging process translates into metabolic reprogramming of the T cells – how a good, strong, and protective T cell transforms into a disease-inducing T cell.”
At the center of premature T-cell aging in RA are disrupted mitochondrial function and insufficient communication of the mitochondria with the lysosomes and the endoplasmic reticulum.
T cells of RA patients (RA T cells) contain less MRE11A, compared with those in healthy people. This is a nuclease that allows the repair of breaks in DNA. If MRE11A is inhibited, then senescent T cells accumulate and form proinflammatory cytokines such as IL-B, IL-6, and TNF. “This is the trio that we rheumatologists are always concerned with.”
Since mitochondrial DNA repair is essential for maintaining mitochondrial fitness, the cellular power sources in patients with RA cannot provide as much energy in the form of adenosine triphosphate as in healthy people. Metabolically, they are not so fit, Dr. Weyand said.
Inflammatory cell death
In fact, all metabolic pathways in the T cells are reduced. The bioenergetic failure has consequences. “Unfortunately, as the mitochondrion ages, its DNA leaks into the cytosol,” explained Dr. Weyand. “Cells do not like this.” This is because DNA activates inflammasomes in the cytosol via caspase-1. This process results in a highly inflammatory cell death: pyroptosis. Subsequently, there is no trace of the cells in the tissue. “RA patients’ synovial tissue is a graveyard of dying T cells.”
In the lysosomes, the cells’ “intestine,” problems arise because patients with RA can no longer activate the adenosine monophosphate–activated protein kinase enzyme. It does not receive the lipid tail it needs to take its position as energy sensor on the lysosomal membrane. As a result, its antagonist, mTOR – both usually keep each other in check – gains the upper hand. According to Dr. Weyand, “mTOR has a party.” It activates and stresses the cells.
Additional changes affect the endoplasmic reticulum (ER). “This is where all of your proteins are synthesized and packaged to migrate from within to outside the cell, or to the cell membrane.” Compared with healthy T cells, RA T cells have around 50% more ER. “The less that mitochondria work, the larger the ER. It gets really fat.”
Mitochondria communicate with the ER via aspartate, oxaloacetate, and malate. In so doing, they control their size. RA T cells appear to be aspartate deficient. In animal models, amino acids had an anti-inflammatory effect.
When sequencing the mRNA bound to the ER, Dr. Weyand and her colleagues encountered the building blocks for TNF. “There is more than three times as much mRNA as TNF. It transforms these T cells into TNF superproducers,” said the rheumatologist. “No wonder this kind of cell is proinflammatory – it forms precisely that cytokine on which you focus every day.”
This article was translated from Medscape’s German edition. A version of this article first appeared on Medscape.com.
Primary care clinicians should spearhead HIV prevention
HIV continues to be a significant public health concern in the United States, with an estimated 1.2 million people currently living with the virus and more than 30,000 new diagnoses in 2020 alone.
Primary care clinicians can help decrease rates of HIV infection by prescribing pre-exposure prophylaxis to people who are sexually active.
But many do not.
“In medical school, we don’t spend much time discussing sexuality, sexual behavior, sexually transmitted infections, and such, so providers may feel uncomfortable asking what kind of sex their patient is having and with whom, whether they use a condom, and other basics,” said Matthew M. Hamill, MBChB, PhD, MPH, a specialist in sexually transmitted diseases at Johns Hopkins Medicine, Baltimore.
PrEP (pre-exposure prophylaxis) is an antiviral medication that cuts the risk of contracting HIV through sex by around 99% when taken as prescribed, according to the Centers for Disease Control and Prevention.
“Many people who would benefit from PrEP are not receiving this highly effective medication,” said John B. Wong, MD, a primary care internist and professor of medicine at Tufts University, Boston. The gap is particularly acute among Black, Hispanic, and Latino people, who are significantly more likely to be diagnosed with HIV but are much less likely than Whites to receive PrEP, he said.
Dr. Wong, a member of the U.S. Preventive Services Task Force, helped write the group’s new PrEP recommendations. Published in August, the guidelines call for clinicians to prescribe the drugs to adolescents and adults who do not have HIV but are at an increased risk for infection.
“Primary care physicians are ideally positioned to prescribe PrEP for their patients because they have longitudinal relationships: They get to know their patients, and hopefully their patients feel comfortable talking with them about their sexual health,” said Brandon Pollak, MD, a primary care physician and HIV specialist at the Ohio State University College of Medicine, Columbus.
Dr. Pollak, who was not involved with the USPSTF recommendations, cares for patients who are heterosexual and living with HIV.
Clinicians should consider PrEP for all patients who have sex with someone who has HIV, do not use condoms, or have had a sexually transmitted infection within the previous 6 months. Men who have sex with men, transgender women who have sex with men, people who inject illicit drugs or engage in transactional sex, and Black, Hispanic, and Latino individuals also are at increased risk for the infection.
“The vast majority of patients on PrEP in any form sail through with no problems; they have regular lab work and can follow up in person or by telemedicine,” Dr. Hamill said. “They tend to be young, fit people without complicated medical histories, and the medications are very well-tolerated, particularly if people expect some short-term side effects.”
What you need to know when prescribing PrEP
Prescribing PrEP is similar in complexity to prescribing hypertension or diabetes medications, Dr. Hamill said.
Because taking the medications while already infected with the virus can lead to the emergence of drug-resistant HIV, patients must have a negative HIV test before starting PrEP. In addition, the USPSTF recommends testing for other sexually transmitted infections and for pregnancy, if appropriate. The task force also recommends conducting kidney function and hepatitis B tests, and a lipid profile before starting specific types of PrEP.
HIV screening is also recommended at 3-month intervals.
“Providers may order labs done at 3- to 4-month intervals but only see patients in clinic once or twice per year, depending on patient needs and risk behaviors,” said Jill S. Blumenthal, MD, associate professor of medicine at UC San Diego Health.
Clinicians should consider medication adherence and whether a patient is likely to take a pill once a day or could benefit from receiving an injection every 2 months. Patients may experience side effects such as diarrhea or headache with oral PrEP or soreness at the injection site. In rare cases, some of the drugs may cause kidney toxicity or bone mineral loss, according to Dr. Hamill.
Three similarly effective forms of PrEP approved by the U.S. Food and Drug Administration enable clinicians to tailor the medications to the specific needs and preferences of each patient. Truvada (emtricitabine and tenofovir disoproxil fumarate) and Descovy (emtricitabine and tenofovir alafenamide) are both daily tablets, although the latter is not advised for people assigned female sex at birth who have receptive vaginal sex. Apretude (cabotegravir), an injectable agent, is not recommended for people who inject illegal drugs.
Patients with renal or bone disease are not good candidates for Truvada.
“Truvada can decrease bone density, so for someone with osteoporosis, you might choose Descovy or Apretude,” Dr. Pollak said. “For someone with chronic kidney disease, consider Descovy or Apretude. “If a patient has hepatitis B, Truvada or Descovy are appropriate, because hepatitis B is treatable.”
Patients taking an injectable PrEP may need more attention, because the concentration of the medication in the body decreases slowly and may linger for many months at low levels that don’t prevent HIV, according to Dr. Hamill. Someone who acquires HIV during that “tail” period might develop resistance to PrEP.
New research also showed that Descovy users were at elevated risk of developing hypertension and statin initiation, especially among those over age 40 years.
Primary care physicians may want to consult with renal specialists about medication safety in patients with severe kidney disease or with rheumatologists or endocrinologists about metabolic bone disease concerns, Dr. Hamill said.
Meanwhile, if a person begins a monogamous relationship and their risk for HIV drops, “it’s fine to stop taking PrEP tablets,” Dr. Pollak said. “I would still recommend routine HIV screening every 6 or 12 months or however often, depending on other risk factors.”
Caring for these patients entails ensuring labs are completed, monitoring adherence, ordering refills, and scheduling regular follow-up visits.
“For the vast majority of patients, the primary care physician is perfectly equipped for their care through the entire PrEP journey, from discussion and initiation to provision of PrEP,” and most cases do not require specialist care, Dr. Hamill said.
However, “if PrEP fails, which is exceedingly rare, primary care physicians should refer patients immediately, preferably with a warm handoff, for linkage to HIV care,” Dr. Blumenthal said.
Talking about PrEP opens the door to conversations with patients about sexual health and broader health issues, Dr. Hamill said. Although these may not come naturally to primary care clinicians, training is available. The National Network of STD Clinical Prevention Training Centers, funded by the CDC, trains providers on how to overcome their anxiety and have open, inclusive conversations about sexuality and sexual behaviors with transgender and gender-diverse, nonbinary people.
“People worry about saying the wrong thing, about causing offense,” Dr. Hamill said. “But once you get comfortable discussing sexuality, you may open conversations around other health issues.”
Barriers for patients
The task force identified several barriers to PrEP access for patients because of lack of trusting relationships with health care, the effects of structural racism on health disparities, and persistent biases within the health care system.
Racial and ethnic disparities in HIV incidence persist, with 42% of new diagnoses occurring among Black people, 27% among Hispanic or Latino people, and 26% among White people in 2020.
Rates of PrEP usage for a year or longer are also low. Sometimes the patient no longer needs PrEP, but barriers often involve the costs of taking time off from work and arranging transportation to clinic visits.
Although nearly all insurance plans and state Medicaid programs cover PrEP, if a patient does not have coverage, the drugs and required tests and office visits can be expensive.
“One of the biggest barriers for all providers is navigating our complicated health system and drug assistance programs,” said Mehri S. McKellar, MD, associate professor of medicine at Duke University School of Medicine, Durham, N.C.
But lower-cost FDA-approved generic emtricitabine/tenofovir disoproxil fumarate is now available, and clinicians can direct patients to programs that help provide the medications at low or no cost.
“Providing PrEP care is straightforward, beneficial, and satisfying,” Dr. Hamill said. “You help people protect themselves from a life-changing diagnosis, and the health system doesn’t need to pay the cost of treating HIV. Everyone wins.”
Dr. Hamill, Dr. McKellar, Dr. Pollak, and Dr. Wong have reported no relevant financial relationships. Dr. Blumenthal has reported a financial relationship with Gilead Sciences.
A version of this article appeared on Medscape.com.
HIV continues to be a significant public health concern in the United States, with an estimated 1.2 million people currently living with the virus and more than 30,000 new diagnoses in 2020 alone.
Primary care clinicians can help decrease rates of HIV infection by prescribing pre-exposure prophylaxis to people who are sexually active.
But many do not.
“In medical school, we don’t spend much time discussing sexuality, sexual behavior, sexually transmitted infections, and such, so providers may feel uncomfortable asking what kind of sex their patient is having and with whom, whether they use a condom, and other basics,” said Matthew M. Hamill, MBChB, PhD, MPH, a specialist in sexually transmitted diseases at Johns Hopkins Medicine, Baltimore.
PrEP (pre-exposure prophylaxis) is an antiviral medication that cuts the risk of contracting HIV through sex by around 99% when taken as prescribed, according to the Centers for Disease Control and Prevention.
“Many people who would benefit from PrEP are not receiving this highly effective medication,” said John B. Wong, MD, a primary care internist and professor of medicine at Tufts University, Boston. The gap is particularly acute among Black, Hispanic, and Latino people, who are significantly more likely to be diagnosed with HIV but are much less likely than Whites to receive PrEP, he said.
Dr. Wong, a member of the U.S. Preventive Services Task Force, helped write the group’s new PrEP recommendations. Published in August, the guidelines call for clinicians to prescribe the drugs to adolescents and adults who do not have HIV but are at an increased risk for infection.
“Primary care physicians are ideally positioned to prescribe PrEP for their patients because they have longitudinal relationships: They get to know their patients, and hopefully their patients feel comfortable talking with them about their sexual health,” said Brandon Pollak, MD, a primary care physician and HIV specialist at the Ohio State University College of Medicine, Columbus.
Dr. Pollak, who was not involved with the USPSTF recommendations, cares for patients who are heterosexual and living with HIV.
Clinicians should consider PrEP for all patients who have sex with someone who has HIV, do not use condoms, or have had a sexually transmitted infection within the previous 6 months. Men who have sex with men, transgender women who have sex with men, people who inject illicit drugs or engage in transactional sex, and Black, Hispanic, and Latino individuals also are at increased risk for the infection.
“The vast majority of patients on PrEP in any form sail through with no problems; they have regular lab work and can follow up in person or by telemedicine,” Dr. Hamill said. “They tend to be young, fit people without complicated medical histories, and the medications are very well-tolerated, particularly if people expect some short-term side effects.”
What you need to know when prescribing PrEP
Prescribing PrEP is similar in complexity to prescribing hypertension or diabetes medications, Dr. Hamill said.
Because taking the medications while already infected with the virus can lead to the emergence of drug-resistant HIV, patients must have a negative HIV test before starting PrEP. In addition, the USPSTF recommends testing for other sexually transmitted infections and for pregnancy, if appropriate. The task force also recommends conducting kidney function and hepatitis B tests, and a lipid profile before starting specific types of PrEP.
HIV screening is also recommended at 3-month intervals.
“Providers may order labs done at 3- to 4-month intervals but only see patients in clinic once or twice per year, depending on patient needs and risk behaviors,” said Jill S. Blumenthal, MD, associate professor of medicine at UC San Diego Health.
Clinicians should consider medication adherence and whether a patient is likely to take a pill once a day or could benefit from receiving an injection every 2 months. Patients may experience side effects such as diarrhea or headache with oral PrEP or soreness at the injection site. In rare cases, some of the drugs may cause kidney toxicity or bone mineral loss, according to Dr. Hamill.
Three similarly effective forms of PrEP approved by the U.S. Food and Drug Administration enable clinicians to tailor the medications to the specific needs and preferences of each patient. Truvada (emtricitabine and tenofovir disoproxil fumarate) and Descovy (emtricitabine and tenofovir alafenamide) are both daily tablets, although the latter is not advised for people assigned female sex at birth who have receptive vaginal sex. Apretude (cabotegravir), an injectable agent, is not recommended for people who inject illegal drugs.
Patients with renal or bone disease are not good candidates for Truvada.
“Truvada can decrease bone density, so for someone with osteoporosis, you might choose Descovy or Apretude,” Dr. Pollak said. “For someone with chronic kidney disease, consider Descovy or Apretude. “If a patient has hepatitis B, Truvada or Descovy are appropriate, because hepatitis B is treatable.”
Patients taking an injectable PrEP may need more attention, because the concentration of the medication in the body decreases slowly and may linger for many months at low levels that don’t prevent HIV, according to Dr. Hamill. Someone who acquires HIV during that “tail” period might develop resistance to PrEP.
New research also showed that Descovy users were at elevated risk of developing hypertension and statin initiation, especially among those over age 40 years.
Primary care physicians may want to consult with renal specialists about medication safety in patients with severe kidney disease or with rheumatologists or endocrinologists about metabolic bone disease concerns, Dr. Hamill said.
Meanwhile, if a person begins a monogamous relationship and their risk for HIV drops, “it’s fine to stop taking PrEP tablets,” Dr. Pollak said. “I would still recommend routine HIV screening every 6 or 12 months or however often, depending on other risk factors.”
Caring for these patients entails ensuring labs are completed, monitoring adherence, ordering refills, and scheduling regular follow-up visits.
“For the vast majority of patients, the primary care physician is perfectly equipped for their care through the entire PrEP journey, from discussion and initiation to provision of PrEP,” and most cases do not require specialist care, Dr. Hamill said.
However, “if PrEP fails, which is exceedingly rare, primary care physicians should refer patients immediately, preferably with a warm handoff, for linkage to HIV care,” Dr. Blumenthal said.
Talking about PrEP opens the door to conversations with patients about sexual health and broader health issues, Dr. Hamill said. Although these may not come naturally to primary care clinicians, training is available. The National Network of STD Clinical Prevention Training Centers, funded by the CDC, trains providers on how to overcome their anxiety and have open, inclusive conversations about sexuality and sexual behaviors with transgender and gender-diverse, nonbinary people.
“People worry about saying the wrong thing, about causing offense,” Dr. Hamill said. “But once you get comfortable discussing sexuality, you may open conversations around other health issues.”
Barriers for patients
The task force identified several barriers to PrEP access for patients because of lack of trusting relationships with health care, the effects of structural racism on health disparities, and persistent biases within the health care system.
Racial and ethnic disparities in HIV incidence persist, with 42% of new diagnoses occurring among Black people, 27% among Hispanic or Latino people, and 26% among White people in 2020.
Rates of PrEP usage for a year or longer are also low. Sometimes the patient no longer needs PrEP, but barriers often involve the costs of taking time off from work and arranging transportation to clinic visits.
Although nearly all insurance plans and state Medicaid programs cover PrEP, if a patient does not have coverage, the drugs and required tests and office visits can be expensive.
“One of the biggest barriers for all providers is navigating our complicated health system and drug assistance programs,” said Mehri S. McKellar, MD, associate professor of medicine at Duke University School of Medicine, Durham, N.C.
But lower-cost FDA-approved generic emtricitabine/tenofovir disoproxil fumarate is now available, and clinicians can direct patients to programs that help provide the medications at low or no cost.
“Providing PrEP care is straightforward, beneficial, and satisfying,” Dr. Hamill said. “You help people protect themselves from a life-changing diagnosis, and the health system doesn’t need to pay the cost of treating HIV. Everyone wins.”
Dr. Hamill, Dr. McKellar, Dr. Pollak, and Dr. Wong have reported no relevant financial relationships. Dr. Blumenthal has reported a financial relationship with Gilead Sciences.
A version of this article appeared on Medscape.com.
HIV continues to be a significant public health concern in the United States, with an estimated 1.2 million people currently living with the virus and more than 30,000 new diagnoses in 2020 alone.
Primary care clinicians can help decrease rates of HIV infection by prescribing pre-exposure prophylaxis to people who are sexually active.
But many do not.
“In medical school, we don’t spend much time discussing sexuality, sexual behavior, sexually transmitted infections, and such, so providers may feel uncomfortable asking what kind of sex their patient is having and with whom, whether they use a condom, and other basics,” said Matthew M. Hamill, MBChB, PhD, MPH, a specialist in sexually transmitted diseases at Johns Hopkins Medicine, Baltimore.
PrEP (pre-exposure prophylaxis) is an antiviral medication that cuts the risk of contracting HIV through sex by around 99% when taken as prescribed, according to the Centers for Disease Control and Prevention.
“Many people who would benefit from PrEP are not receiving this highly effective medication,” said John B. Wong, MD, a primary care internist and professor of medicine at Tufts University, Boston. The gap is particularly acute among Black, Hispanic, and Latino people, who are significantly more likely to be diagnosed with HIV but are much less likely than Whites to receive PrEP, he said.
Dr. Wong, a member of the U.S. Preventive Services Task Force, helped write the group’s new PrEP recommendations. Published in August, the guidelines call for clinicians to prescribe the drugs to adolescents and adults who do not have HIV but are at an increased risk for infection.
“Primary care physicians are ideally positioned to prescribe PrEP for their patients because they have longitudinal relationships: They get to know their patients, and hopefully their patients feel comfortable talking with them about their sexual health,” said Brandon Pollak, MD, a primary care physician and HIV specialist at the Ohio State University College of Medicine, Columbus.
Dr. Pollak, who was not involved with the USPSTF recommendations, cares for patients who are heterosexual and living with HIV.
Clinicians should consider PrEP for all patients who have sex with someone who has HIV, do not use condoms, or have had a sexually transmitted infection within the previous 6 months. Men who have sex with men, transgender women who have sex with men, people who inject illicit drugs or engage in transactional sex, and Black, Hispanic, and Latino individuals also are at increased risk for the infection.
“The vast majority of patients on PrEP in any form sail through with no problems; they have regular lab work and can follow up in person or by telemedicine,” Dr. Hamill said. “They tend to be young, fit people without complicated medical histories, and the medications are very well-tolerated, particularly if people expect some short-term side effects.”
What you need to know when prescribing PrEP
Prescribing PrEP is similar in complexity to prescribing hypertension or diabetes medications, Dr. Hamill said.
Because taking the medications while already infected with the virus can lead to the emergence of drug-resistant HIV, patients must have a negative HIV test before starting PrEP. In addition, the USPSTF recommends testing for other sexually transmitted infections and for pregnancy, if appropriate. The task force also recommends conducting kidney function and hepatitis B tests, and a lipid profile before starting specific types of PrEP.
HIV screening is also recommended at 3-month intervals.
“Providers may order labs done at 3- to 4-month intervals but only see patients in clinic once or twice per year, depending on patient needs and risk behaviors,” said Jill S. Blumenthal, MD, associate professor of medicine at UC San Diego Health.
Clinicians should consider medication adherence and whether a patient is likely to take a pill once a day or could benefit from receiving an injection every 2 months. Patients may experience side effects such as diarrhea or headache with oral PrEP or soreness at the injection site. In rare cases, some of the drugs may cause kidney toxicity or bone mineral loss, according to Dr. Hamill.
Three similarly effective forms of PrEP approved by the U.S. Food and Drug Administration enable clinicians to tailor the medications to the specific needs and preferences of each patient. Truvada (emtricitabine and tenofovir disoproxil fumarate) and Descovy (emtricitabine and tenofovir alafenamide) are both daily tablets, although the latter is not advised for people assigned female sex at birth who have receptive vaginal sex. Apretude (cabotegravir), an injectable agent, is not recommended for people who inject illegal drugs.
Patients with renal or bone disease are not good candidates for Truvada.
“Truvada can decrease bone density, so for someone with osteoporosis, you might choose Descovy or Apretude,” Dr. Pollak said. “For someone with chronic kidney disease, consider Descovy or Apretude. “If a patient has hepatitis B, Truvada or Descovy are appropriate, because hepatitis B is treatable.”
Patients taking an injectable PrEP may need more attention, because the concentration of the medication in the body decreases slowly and may linger for many months at low levels that don’t prevent HIV, according to Dr. Hamill. Someone who acquires HIV during that “tail” period might develop resistance to PrEP.
New research also showed that Descovy users were at elevated risk of developing hypertension and statin initiation, especially among those over age 40 years.
Primary care physicians may want to consult with renal specialists about medication safety in patients with severe kidney disease or with rheumatologists or endocrinologists about metabolic bone disease concerns, Dr. Hamill said.
Meanwhile, if a person begins a monogamous relationship and their risk for HIV drops, “it’s fine to stop taking PrEP tablets,” Dr. Pollak said. “I would still recommend routine HIV screening every 6 or 12 months or however often, depending on other risk factors.”
Caring for these patients entails ensuring labs are completed, monitoring adherence, ordering refills, and scheduling regular follow-up visits.
“For the vast majority of patients, the primary care physician is perfectly equipped for their care through the entire PrEP journey, from discussion and initiation to provision of PrEP,” and most cases do not require specialist care, Dr. Hamill said.
However, “if PrEP fails, which is exceedingly rare, primary care physicians should refer patients immediately, preferably with a warm handoff, for linkage to HIV care,” Dr. Blumenthal said.
Talking about PrEP opens the door to conversations with patients about sexual health and broader health issues, Dr. Hamill said. Although these may not come naturally to primary care clinicians, training is available. The National Network of STD Clinical Prevention Training Centers, funded by the CDC, trains providers on how to overcome their anxiety and have open, inclusive conversations about sexuality and sexual behaviors with transgender and gender-diverse, nonbinary people.
“People worry about saying the wrong thing, about causing offense,” Dr. Hamill said. “But once you get comfortable discussing sexuality, you may open conversations around other health issues.”
Barriers for patients
The task force identified several barriers to PrEP access for patients because of lack of trusting relationships with health care, the effects of structural racism on health disparities, and persistent biases within the health care system.
Racial and ethnic disparities in HIV incidence persist, with 42% of new diagnoses occurring among Black people, 27% among Hispanic or Latino people, and 26% among White people in 2020.
Rates of PrEP usage for a year or longer are also low. Sometimes the patient no longer needs PrEP, but barriers often involve the costs of taking time off from work and arranging transportation to clinic visits.
Although nearly all insurance plans and state Medicaid programs cover PrEP, if a patient does not have coverage, the drugs and required tests and office visits can be expensive.
“One of the biggest barriers for all providers is navigating our complicated health system and drug assistance programs,” said Mehri S. McKellar, MD, associate professor of medicine at Duke University School of Medicine, Durham, N.C.
But lower-cost FDA-approved generic emtricitabine/tenofovir disoproxil fumarate is now available, and clinicians can direct patients to programs that help provide the medications at low or no cost.
“Providing PrEP care is straightforward, beneficial, and satisfying,” Dr. Hamill said. “You help people protect themselves from a life-changing diagnosis, and the health system doesn’t need to pay the cost of treating HIV. Everyone wins.”
Dr. Hamill, Dr. McKellar, Dr. Pollak, and Dr. Wong have reported no relevant financial relationships. Dr. Blumenthal has reported a financial relationship with Gilead Sciences.
A version of this article appeared on Medscape.com.