User login
Infiltrating Wound Vacuum-Assisted Closure With Topical Amphotericin for Mucormycosis Infection of the Achilles Tendon
Vacuum-assisted closure (VAC) of wounds has become a foundational tool in the armamentarium of wound care specialists. Using a system consisting of a sponge, semi-occlusive barrier, and fluid collection device, VAC systems apply constant negative pressure resulting in macro and micro deformation to a wound, stabilization of the wound environment, and removal of inflammatory factors in wound fluid.1 These conditions allow for the removal of drainage and fluid from a wound bed, reduced edema and inflammation, reduced bacterial load, recruitment of healing factors, approximation of wound edges, and increased blood flow to the wound.2
In complex, infected wounds, a variation of negative pressure wound therapy (NPWT) via the instillation of topical antibiotics (instillation VAC) has been used.3 This variation has been advantageous even in soft tissue fungal infections. Early and aggressive treatment of such infections is critical to prevent dissemination, particularly in aggressive infections, such as mucormycosis.4 We present a case of a patient with a mucormycosis infection of his left Achilles tendon and overlying skin who was successfully treated with surgical debridement and wound care with instillation NPWT with topical amphotericin B.
Case Presentation
A 53-year-old man underwent left Achilles tendon reconstruction with allograft after a complete tear during exercise. He had no relevant medical history and was otherwise healthy, which he attributed to working out daily. About a week after the operation, he began having incisional breakdown, prompting presentation to an emergency department. There, he received IV antibiotics along with multiple debridements. After the wound failed to improve and intra-operative cultures grew mucormycosis, he was transferred to our facility for a higher level of care. On admission, he was immediately given IV amphotericin B and scheduled for repeat debridement.
After 1 prior debridement and 10 total days of IV amphotericin, a repeat debridement was performed. After the debridement, the installation VAC was applied to the patient’s left lower extremity wound with an instilling fluid of amphotericin B and the settings as follows: smart phase instill volume, 110 mL; soak time, 3.5 hours; target pressure, 125 mm Hg; intensity, low; and VAC therapy mode, continuous. After 5 days, the wound bed appeared clean without overt signs of infection. However, due to some toxicity to healthy surrounding soft tissue, the instillation VAC was discontinued and standard NPWT was started. The patient underwent 2 additional rounds of debridement with partial delayed closure. Four weeks after discontinuation of the instillation VAC, the wound appeared healthy and granulated so the patient underwent split-thickness skin grafting to the left posterior ankle. He subsequently completed a course of oral antifungal medication as an outpatient.
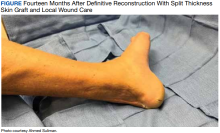
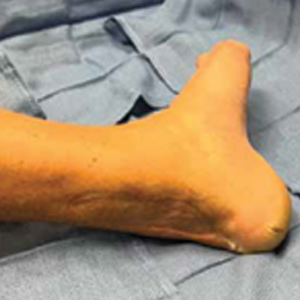
The patient was seen in the outpatient clinic for 14 months from the initial mucormycosis infection (Figure).
Discussion
Mucormycosis is an infection caused by fungi in the class Zygomycetes and of the order Mucorales that typically occurs in immunocompromised patients, especially those with diabetic ketoacidosis and neutropenia. Given that this patient had no relevant medical history and was otherwise healthy, he was at extremely low risk of this type of infection. In this patient’s case, the spores of this nonseptate hyphae wide-branching species were most likely introduced at the time of left Achilles tendon repair. Mucormycosis is progressive and can be fatal unless treated, with a mortality rate approaching 70%.5 The rarity and heterogeneity of mucormycosis make treatment variable.6 No prospective or randomized clinical trials exist in plastic surgery literature.
The use of wound VAC in combination with the instillation of amphotericin B to treat cutaneous mucormycosis is not well documented. Mucormycosis infections are traditionally addressed with surgical debridement and antifungal therapy, specifically IV amphotericin B.7,8 As previously noted, NPWT has become the gold standard in treating complex wounds.3 Additionally, wound VAC therapy with instillation has been noted in the literature as a reliable method to treat bacteria-infected wounds, providing a shorter treatment period and earlier wound closure.9 Instillation VAC therapy has proven particularly useful in complex, infected wounds, such as aggressive fungal infections.
Mucormycosis treatment is challenging particularly in the extremities as management must balance both mortality and limb salvage. In this case, the use of NPWT with wound VAC and intervals of instilling amphotericin B facilitated infection control in this lower extremity mucormycosis infection. The significant adverse effect profile of amphotericin B, particularly the nephrotoxicity, should be seriously considered when deciding the treatment regimen for patients affected by mucormycosis. Locally, topical amphotericin B has been reported to cause blistering, itchiness, redness, peeling, and dryness. However, topical preparations of amphotericin B are nontoxic unlike their IV counterpart, able to cross the physiological barriers of the skin while simultaneously targeting macrophages in the dermis and epidermis.10
Conclusions
Although the mainstay of treatment for systemic mucormycosis is radical debridement and IV amphotericin B, a more localized infection may benefit from an adjunct like an instillation wound VAC with topical amphotericin B, as presented in this case study. Swift treatment with wound VAC was beneficial in the overall recovery and tissue healing of this patient and may be beneficial in similar cases.
1. Normandin S, Safran T, Winocour S, et al. negative pressure wound therapy: mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164-170. doi:10.1055/s-0041-1731792
2. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
3. Gabriel A, Shores J, Bernstein B, et al. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: experience and case series. Int Wound J. 2009;6(suppl 2):1-25. doi:10.1111/j.1742-481X.2009.00628.x
4. Guégan S, Lanternier F, Rouzaud C, Dupin N, Lortholary O. Fungal skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):124-130. doi:10.1097/QCO.0000000000000252
5. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22. doi:10.1093/cid/cir865
6. Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. J Fungi (Basel). 2018;4(3):90. Published 2018 Jul 31. doi:10.3390/jof4030090
7. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569. doi:10.1128/CMR.18.3.556-569.2005
8. Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49(4):385-390. doi:10.1097/00000637-200210000-00009
9. Webb LX. New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg. 2002;10(5):303-311. doi:10.5435/00124635-200209000-00002
10. Varikuti S, Oghumu S, Saljoughian N, et al. Topical treatment with nanoliposomal Amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop. 2017;173:102-108. doi:10.1016/j.actatropica.2017.06.004
Vacuum-assisted closure (VAC) of wounds has become a foundational tool in the armamentarium of wound care specialists. Using a system consisting of a sponge, semi-occlusive barrier, and fluid collection device, VAC systems apply constant negative pressure resulting in macro and micro deformation to a wound, stabilization of the wound environment, and removal of inflammatory factors in wound fluid.1 These conditions allow for the removal of drainage and fluid from a wound bed, reduced edema and inflammation, reduced bacterial load, recruitment of healing factors, approximation of wound edges, and increased blood flow to the wound.2
In complex, infected wounds, a variation of negative pressure wound therapy (NPWT) via the instillation of topical antibiotics (instillation VAC) has been used.3 This variation has been advantageous even in soft tissue fungal infections. Early and aggressive treatment of such infections is critical to prevent dissemination, particularly in aggressive infections, such as mucormycosis.4 We present a case of a patient with a mucormycosis infection of his left Achilles tendon and overlying skin who was successfully treated with surgical debridement and wound care with instillation NPWT with topical amphotericin B.
Case Presentation
A 53-year-old man underwent left Achilles tendon reconstruction with allograft after a complete tear during exercise. He had no relevant medical history and was otherwise healthy, which he attributed to working out daily. About a week after the operation, he began having incisional breakdown, prompting presentation to an emergency department. There, he received IV antibiotics along with multiple debridements. After the wound failed to improve and intra-operative cultures grew mucormycosis, he was transferred to our facility for a higher level of care. On admission, he was immediately given IV amphotericin B and scheduled for repeat debridement.
After 1 prior debridement and 10 total days of IV amphotericin, a repeat debridement was performed. After the debridement, the installation VAC was applied to the patient’s left lower extremity wound with an instilling fluid of amphotericin B and the settings as follows: smart phase instill volume, 110 mL; soak time, 3.5 hours; target pressure, 125 mm Hg; intensity, low; and VAC therapy mode, continuous. After 5 days, the wound bed appeared clean without overt signs of infection. However, due to some toxicity to healthy surrounding soft tissue, the instillation VAC was discontinued and standard NPWT was started. The patient underwent 2 additional rounds of debridement with partial delayed closure. Four weeks after discontinuation of the instillation VAC, the wound appeared healthy and granulated so the patient underwent split-thickness skin grafting to the left posterior ankle. He subsequently completed a course of oral antifungal medication as an outpatient.
The patient was seen in the outpatient clinic for 14 months from the initial mucormycosis infection (Figure).
Discussion
Mucormycosis is an infection caused by fungi in the class Zygomycetes and of the order Mucorales that typically occurs in immunocompromised patients, especially those with diabetic ketoacidosis and neutropenia. Given that this patient had no relevant medical history and was otherwise healthy, he was at extremely low risk of this type of infection. In this patient’s case, the spores of this nonseptate hyphae wide-branching species were most likely introduced at the time of left Achilles tendon repair. Mucormycosis is progressive and can be fatal unless treated, with a mortality rate approaching 70%.5 The rarity and heterogeneity of mucormycosis make treatment variable.6 No prospective or randomized clinical trials exist in plastic surgery literature.
The use of wound VAC in combination with the instillation of amphotericin B to treat cutaneous mucormycosis is not well documented. Mucormycosis infections are traditionally addressed with surgical debridement and antifungal therapy, specifically IV amphotericin B.7,8 As previously noted, NPWT has become the gold standard in treating complex wounds.3 Additionally, wound VAC therapy with instillation has been noted in the literature as a reliable method to treat bacteria-infected wounds, providing a shorter treatment period and earlier wound closure.9 Instillation VAC therapy has proven particularly useful in complex, infected wounds, such as aggressive fungal infections.
Mucormycosis treatment is challenging particularly in the extremities as management must balance both mortality and limb salvage. In this case, the use of NPWT with wound VAC and intervals of instilling amphotericin B facilitated infection control in this lower extremity mucormycosis infection. The significant adverse effect profile of amphotericin B, particularly the nephrotoxicity, should be seriously considered when deciding the treatment regimen for patients affected by mucormycosis. Locally, topical amphotericin B has been reported to cause blistering, itchiness, redness, peeling, and dryness. However, topical preparations of amphotericin B are nontoxic unlike their IV counterpart, able to cross the physiological barriers of the skin while simultaneously targeting macrophages in the dermis and epidermis.10
Conclusions
Although the mainstay of treatment for systemic mucormycosis is radical debridement and IV amphotericin B, a more localized infection may benefit from an adjunct like an instillation wound VAC with topical amphotericin B, as presented in this case study. Swift treatment with wound VAC was beneficial in the overall recovery and tissue healing of this patient and may be beneficial in similar cases.
Vacuum-assisted closure (VAC) of wounds has become a foundational tool in the armamentarium of wound care specialists. Using a system consisting of a sponge, semi-occlusive barrier, and fluid collection device, VAC systems apply constant negative pressure resulting in macro and micro deformation to a wound, stabilization of the wound environment, and removal of inflammatory factors in wound fluid.1 These conditions allow for the removal of drainage and fluid from a wound bed, reduced edema and inflammation, reduced bacterial load, recruitment of healing factors, approximation of wound edges, and increased blood flow to the wound.2
In complex, infected wounds, a variation of negative pressure wound therapy (NPWT) via the instillation of topical antibiotics (instillation VAC) has been used.3 This variation has been advantageous even in soft tissue fungal infections. Early and aggressive treatment of such infections is critical to prevent dissemination, particularly in aggressive infections, such as mucormycosis.4 We present a case of a patient with a mucormycosis infection of his left Achilles tendon and overlying skin who was successfully treated with surgical debridement and wound care with instillation NPWT with topical amphotericin B.
Case Presentation
A 53-year-old man underwent left Achilles tendon reconstruction with allograft after a complete tear during exercise. He had no relevant medical history and was otherwise healthy, which he attributed to working out daily. About a week after the operation, he began having incisional breakdown, prompting presentation to an emergency department. There, he received IV antibiotics along with multiple debridements. After the wound failed to improve and intra-operative cultures grew mucormycosis, he was transferred to our facility for a higher level of care. On admission, he was immediately given IV amphotericin B and scheduled for repeat debridement.
After 1 prior debridement and 10 total days of IV amphotericin, a repeat debridement was performed. After the debridement, the installation VAC was applied to the patient’s left lower extremity wound with an instilling fluid of amphotericin B and the settings as follows: smart phase instill volume, 110 mL; soak time, 3.5 hours; target pressure, 125 mm Hg; intensity, low; and VAC therapy mode, continuous. After 5 days, the wound bed appeared clean without overt signs of infection. However, due to some toxicity to healthy surrounding soft tissue, the instillation VAC was discontinued and standard NPWT was started. The patient underwent 2 additional rounds of debridement with partial delayed closure. Four weeks after discontinuation of the instillation VAC, the wound appeared healthy and granulated so the patient underwent split-thickness skin grafting to the left posterior ankle. He subsequently completed a course of oral antifungal medication as an outpatient.
The patient was seen in the outpatient clinic for 14 months from the initial mucormycosis infection (Figure).
Discussion
Mucormycosis is an infection caused by fungi in the class Zygomycetes and of the order Mucorales that typically occurs in immunocompromised patients, especially those with diabetic ketoacidosis and neutropenia. Given that this patient had no relevant medical history and was otherwise healthy, he was at extremely low risk of this type of infection. In this patient’s case, the spores of this nonseptate hyphae wide-branching species were most likely introduced at the time of left Achilles tendon repair. Mucormycosis is progressive and can be fatal unless treated, with a mortality rate approaching 70%.5 The rarity and heterogeneity of mucormycosis make treatment variable.6 No prospective or randomized clinical trials exist in plastic surgery literature.
The use of wound VAC in combination with the instillation of amphotericin B to treat cutaneous mucormycosis is not well documented. Mucormycosis infections are traditionally addressed with surgical debridement and antifungal therapy, specifically IV amphotericin B.7,8 As previously noted, NPWT has become the gold standard in treating complex wounds.3 Additionally, wound VAC therapy with instillation has been noted in the literature as a reliable method to treat bacteria-infected wounds, providing a shorter treatment period and earlier wound closure.9 Instillation VAC therapy has proven particularly useful in complex, infected wounds, such as aggressive fungal infections.
Mucormycosis treatment is challenging particularly in the extremities as management must balance both mortality and limb salvage. In this case, the use of NPWT with wound VAC and intervals of instilling amphotericin B facilitated infection control in this lower extremity mucormycosis infection. The significant adverse effect profile of amphotericin B, particularly the nephrotoxicity, should be seriously considered when deciding the treatment regimen for patients affected by mucormycosis. Locally, topical amphotericin B has been reported to cause blistering, itchiness, redness, peeling, and dryness. However, topical preparations of amphotericin B are nontoxic unlike their IV counterpart, able to cross the physiological barriers of the skin while simultaneously targeting macrophages in the dermis and epidermis.10
Conclusions
Although the mainstay of treatment for systemic mucormycosis is radical debridement and IV amphotericin B, a more localized infection may benefit from an adjunct like an instillation wound VAC with topical amphotericin B, as presented in this case study. Swift treatment with wound VAC was beneficial in the overall recovery and tissue healing of this patient and may be beneficial in similar cases.
1. Normandin S, Safran T, Winocour S, et al. negative pressure wound therapy: mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164-170. doi:10.1055/s-0041-1731792
2. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
3. Gabriel A, Shores J, Bernstein B, et al. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: experience and case series. Int Wound J. 2009;6(suppl 2):1-25. doi:10.1111/j.1742-481X.2009.00628.x
4. Guégan S, Lanternier F, Rouzaud C, Dupin N, Lortholary O. Fungal skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):124-130. doi:10.1097/QCO.0000000000000252
5. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22. doi:10.1093/cid/cir865
6. Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. J Fungi (Basel). 2018;4(3):90. Published 2018 Jul 31. doi:10.3390/jof4030090
7. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569. doi:10.1128/CMR.18.3.556-569.2005
8. Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49(4):385-390. doi:10.1097/00000637-200210000-00009
9. Webb LX. New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg. 2002;10(5):303-311. doi:10.5435/00124635-200209000-00002
10. Varikuti S, Oghumu S, Saljoughian N, et al. Topical treatment with nanoliposomal Amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop. 2017;173:102-108. doi:10.1016/j.actatropica.2017.06.004
1. Normandin S, Safran T, Winocour S, et al. negative pressure wound therapy: mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164-170. doi:10.1055/s-0041-1731792
2. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
3. Gabriel A, Shores J, Bernstein B, et al. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: experience and case series. Int Wound J. 2009;6(suppl 2):1-25. doi:10.1111/j.1742-481X.2009.00628.x
4. Guégan S, Lanternier F, Rouzaud C, Dupin N, Lortholary O. Fungal skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):124-130. doi:10.1097/QCO.0000000000000252
5. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22. doi:10.1093/cid/cir865
6. Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. J Fungi (Basel). 2018;4(3):90. Published 2018 Jul 31. doi:10.3390/jof4030090
7. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569. doi:10.1128/CMR.18.3.556-569.2005
8. Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49(4):385-390. doi:10.1097/00000637-200210000-00009
9. Webb LX. New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg. 2002;10(5):303-311. doi:10.5435/00124635-200209000-00002
10. Varikuti S, Oghumu S, Saljoughian N, et al. Topical treatment with nanoliposomal Amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop. 2017;173:102-108. doi:10.1016/j.actatropica.2017.06.004
A technicality could keep RSV shots from kids in need
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
A White male presented with a 1½-year history of a progressive hypoesthetic annular, hyperpigmented plaque on the upper arm
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
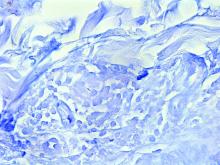
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
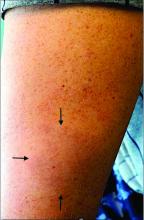
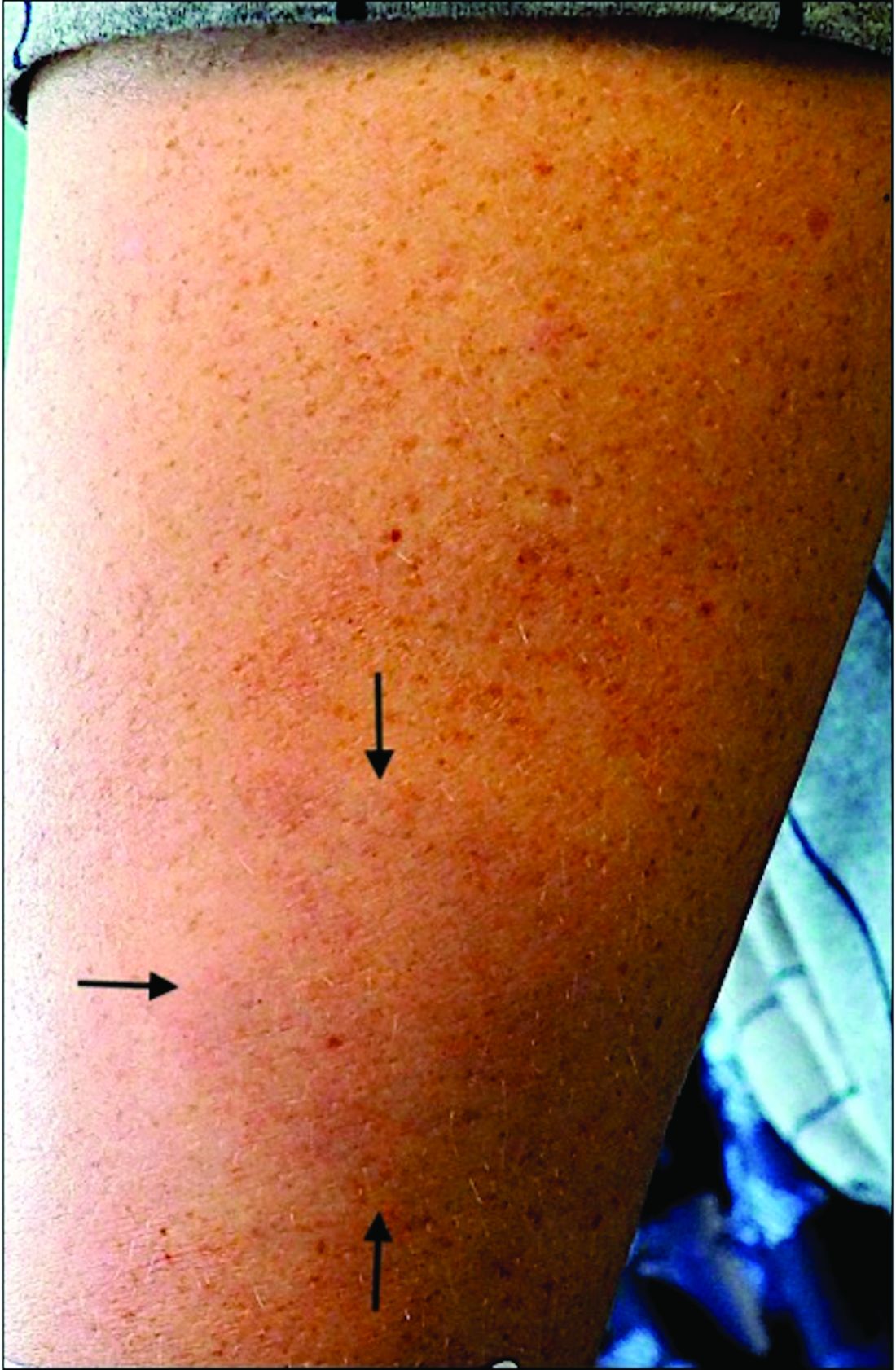
A 44-year-old White male presented with a 1½-year history of a progressive hypoesthetic annular, mildly hyperpigmented plaque on the left posterior upper arm.
He denied pruritus, pain, or systemic symptoms including weight loss, visual changes, cough, dyspnea, and abdominal pain. He also denied any paresthesia or weakness. On physical examination, there is a subtle, solitary 4-cm annular skin-colored thin plaque on the patient's left posterior upper arm (Figure 1).
Punch biopsy of the lesion was performed, and the histopathological findings are illustrated in Figure 2.
Advice on antibiotics for kids during shortages
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
Inflammation and immunity troubles top long-COVID suspect list
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
COVID emergency orders ending: What’s next?
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
Children and COVID: Weekly cases may have doubled in early January
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”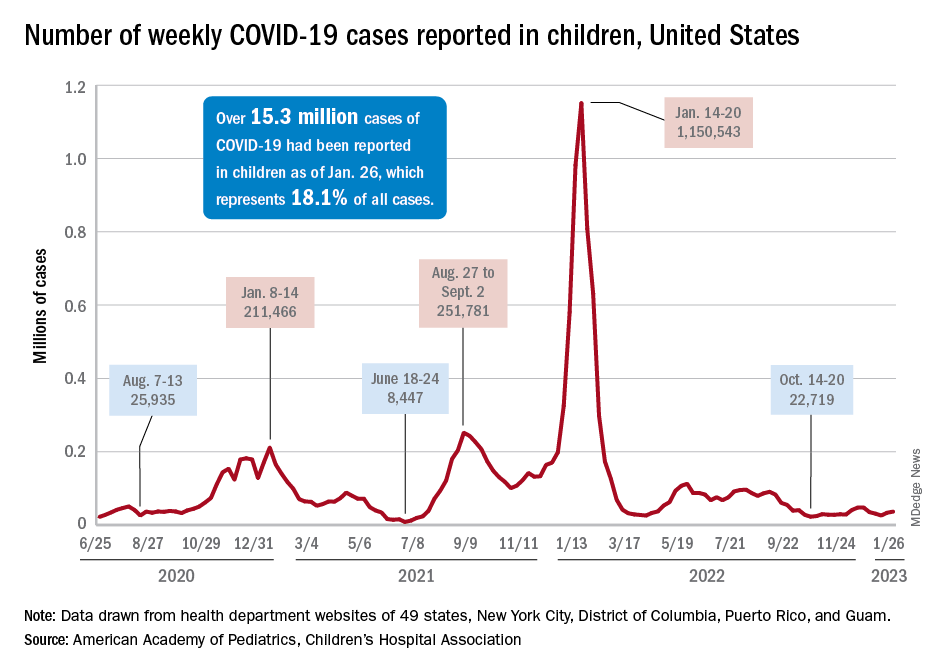
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Fungal Osler Nodes Indicate Candidal Infective Endocarditis
To the Editor:
A 44-year-old woman presented with a low-grade fever (temperature, 38.0 °C) and painful acral lesions of 1 week’s duration. She had a history of hepatitis C viral infection and intravenous (IV) drug use, as well as polymicrobial infective endocarditis that involved the tricuspid and aortic valves; pathogenic organisms were identified via blood culture as Enterococcus faecalis, Serratia species, Streptococcus viridans, and Candida albicans. The patient had received a mechanical aortic valve and bioprosthetic tricuspid valve replacement 5 months prior with warfarin therapy and had completed a postsurgical 6-week course of high-dose micafungin. She reported that she had developed painful, violaceous, thin papules on the plantar surface of the left foot 2 weeks prior to presentation. Her symptoms improved with a short systemic steroid taper; however, within a week she developed new tender, erythematous, thin papules on the plantar surface of the right foot and the palmar surface of the left hand with associated lower extremity swelling. She denied other symptoms, including fever, chills, neurologic symptoms, shortness of breath, chest pain, nausea, vomiting, hematuria, and hematochezia. Due to worsening cutaneous findings, the patient presented to the emergency department, prompting hospital admission for empiric antibacterial therapy with vancomycin and piperacillin-tazobactam for suspected infectious endocarditis. Dermatology was consulted after 1 day of antibacterial therapy without improvement to determine the etiology of the patient’s skin findings.
Physical examination revealed the patient was afebrile with partially blanching violaceous to purpuric, tender, edematous papules on the left fourth and fifth finger pads, as well as scattered, painful, purpuric patches with stellate borders on the right plantar foot (Figure 1). Laboratory test results revealed mild anemia (hemoglobin, 11.9 g/dL [reference range, 12.0–15.0 g/dL], mild neutrophilia (neutrophils, 8.4×109/L [reference range, 1.9–7.9×109/L], elevated acute-phase reactants (erythrocyte sedimentation rate, 71 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 5.7 mg/dL [reference range, 0.0–0.5 mg/dL]), and positive hepatitis C virus antibody with an undetectable viral load. At the time of dermatologic evaluation, admission blood cultures and transthoracic echocardiogram were negative. Additionally, a transesophageal echocardiogram, limited by artifact from the mechanical aortic valve, was equivocal for valvular pathology. Subsequent ophthalmologic evaluation was negative for lesions associated with endocarditis, such as retinal hemorrhages.
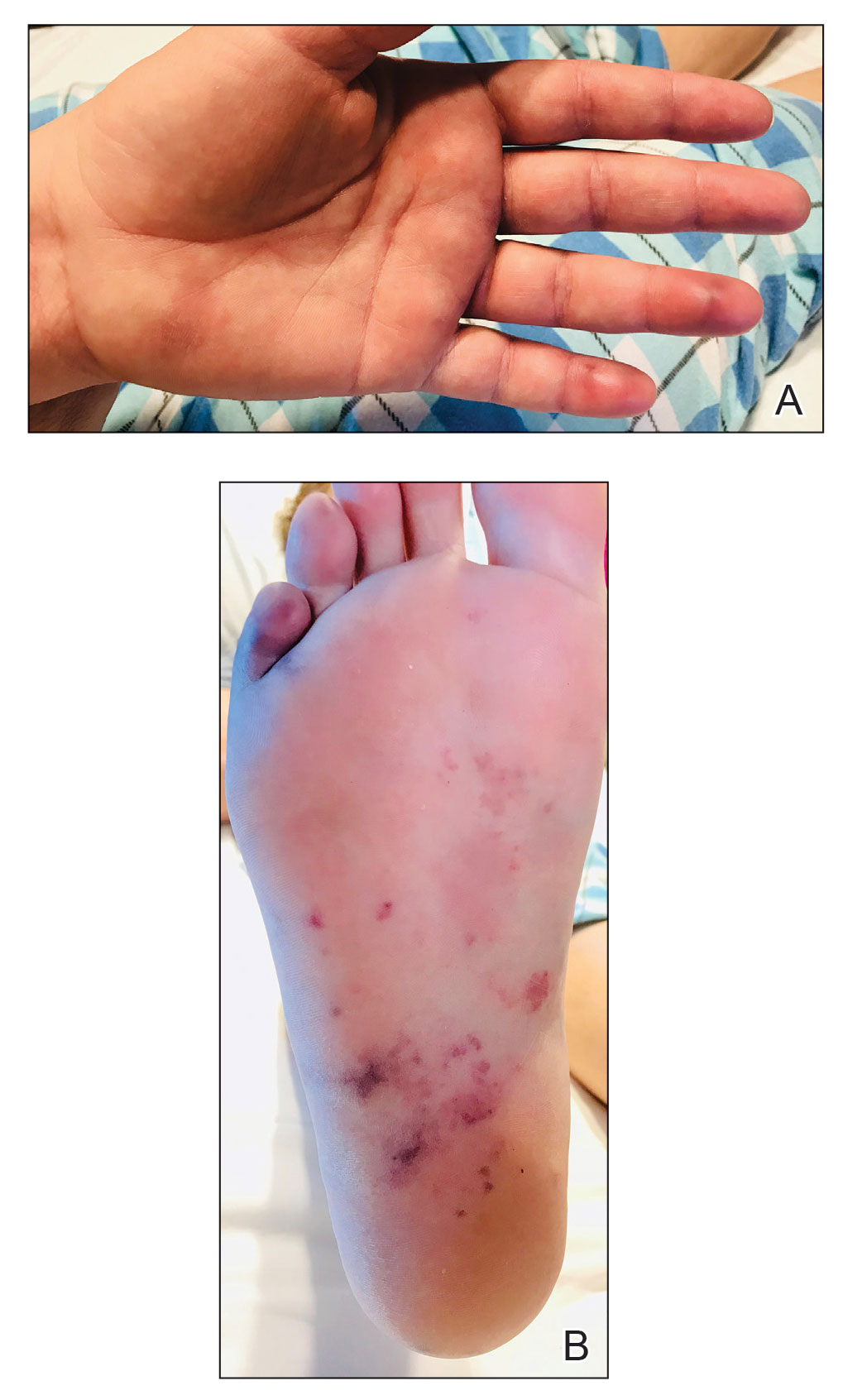
Punch biopsies of the left fourth finger pad were submitted for histopathologic analysis and tissue cultures. Histopathology demonstrated deep dermal perivascular neutrophilic inflammation with multiple intravascular thrombi, perivascular fibrin, and karyorrhectic debris (Figure 2). Periodic acid–Schiff and Grocott-Gomori methenamine-silver stains revealed fungal spores with rare pseudohyphae within the thrombosed vascular spaces and the perivascular dermis, consistent with fungal septic emboli (Figure 3).
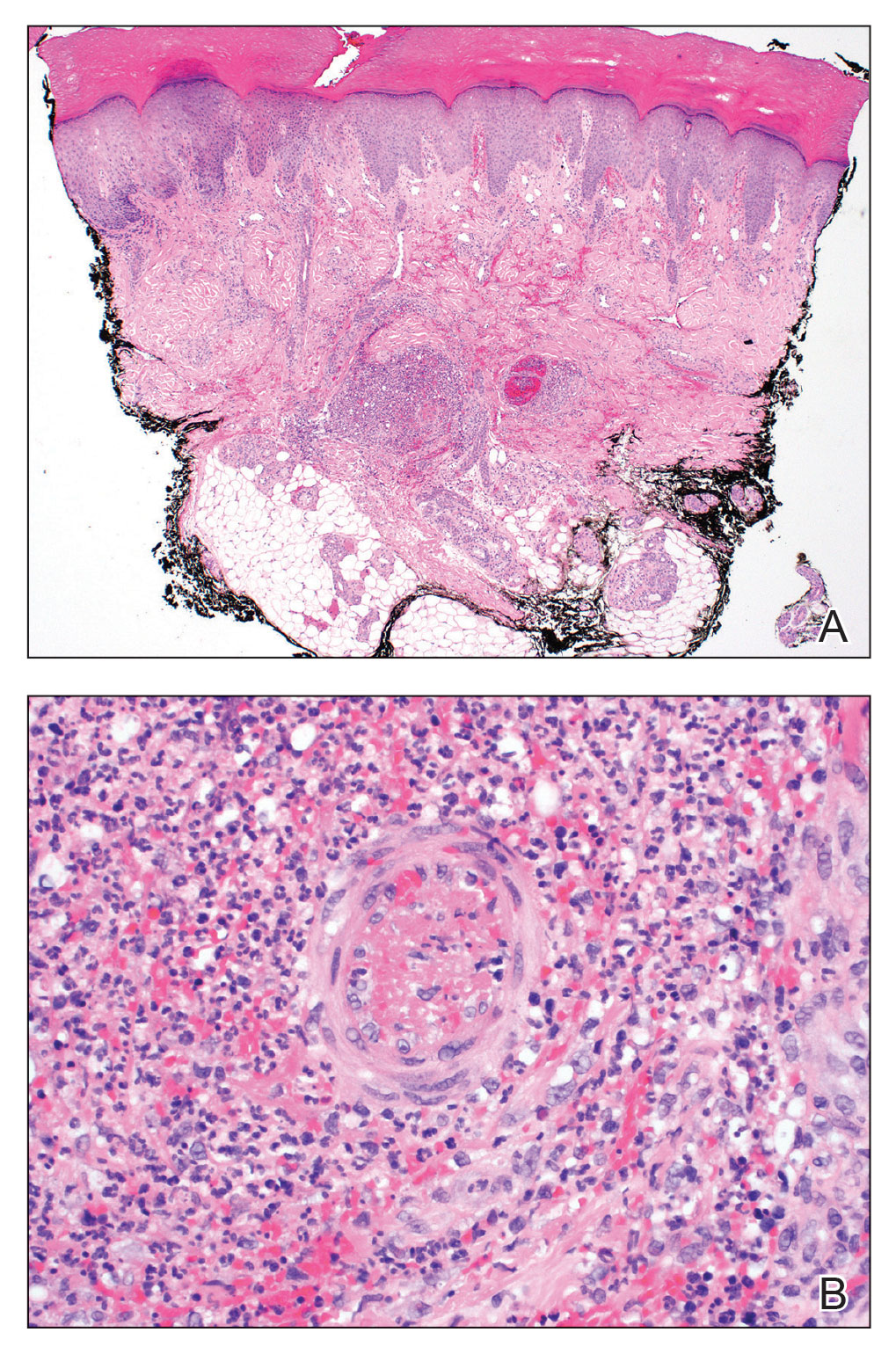
Empiric systemic antifungal coverage composed of IV liposomal amphotericin B and oral flucytosine was initiated, and the patient’s tender acral papules rapidly improved. Within 48 hours of biopsy, skin tissue culture confirmed the presence of C albicans. Four days after the preliminary dermatopathology report, confirmatory blood cultures resulted with pansensitive C albicans. Final tissue and blood cultures were negative for bacteria including mycobacteria. In addition to a 6-week course of IV amphotericin B and flucytosine, repeat surgical intervention was considered, and lifelong suppressive antifungal oral therapy was recommended. Unfortunately, the patient did not present for follow-up. Three months later, she presented to the emergency department with peritonitis; in the operating room, she was found to have ischemia of the entirety of the small and large intestines and died shortly thereafter.
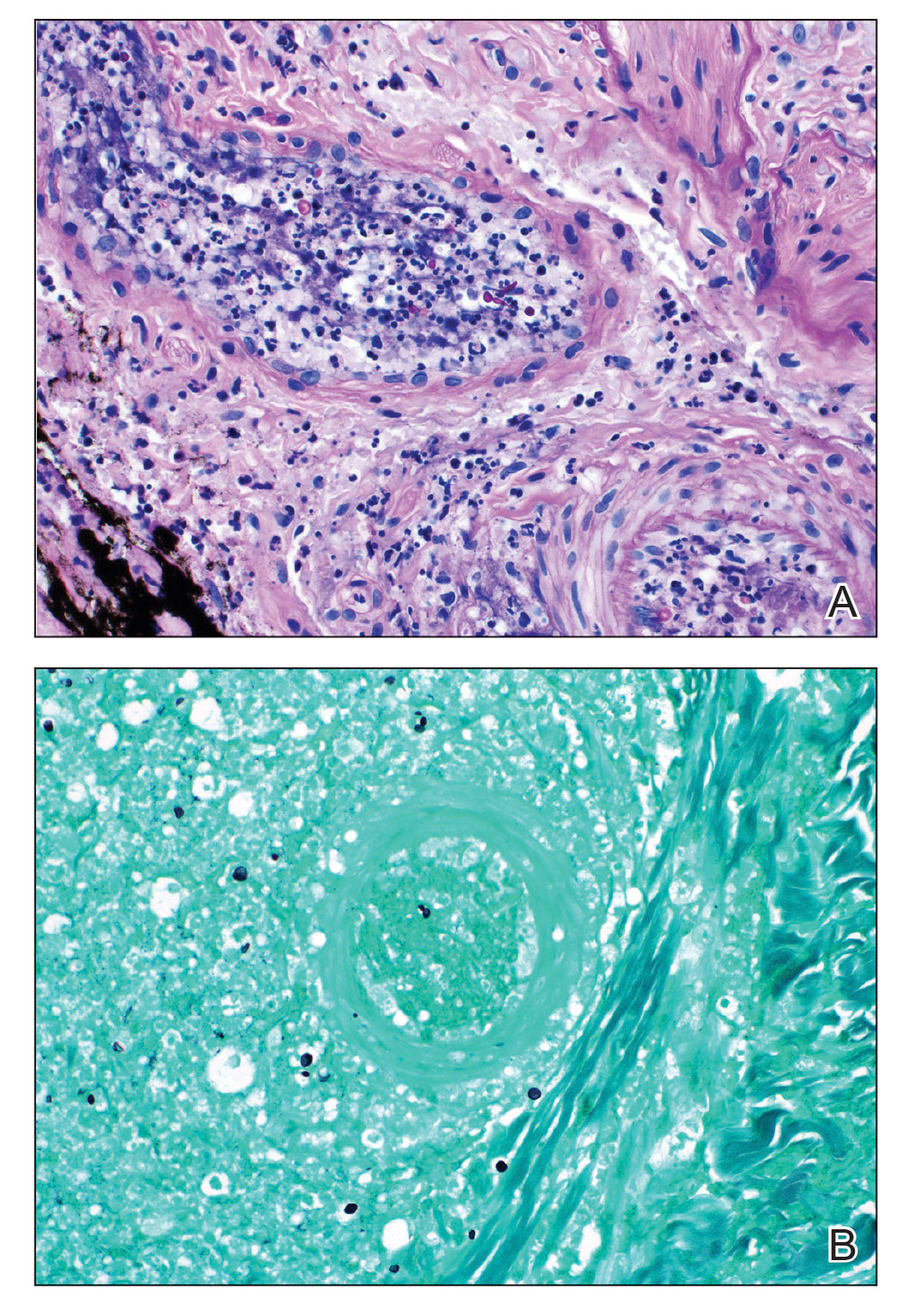
Fungal endocarditis is rare, tending to develop in patient populations with particular risk factors such as immune compromise, structural heart defects or prosthetic valves, and IV drug use. Candida infective endocarditis (CIE) represents less than 2% of infective endocarditis cases and carries a high mortality rate (30%–80%).1-3 Diagnosis may be challenging, as the clinical presentation varies widely. Although some patients may present with classic features of infective endocarditis, including fever, cardiac murmurs, and positive blood cultures, many cases of infective endocarditis present with nonspecific symptoms, raising a broad clinical differential diagnosis. Delay in diagnosis, which is seen in 82% of patients with fungal endocarditis, may be attributed to the slow progression of symptoms, inconclusive cardiac imaging, or negative blood cultures seen in almost one-third of cases.2,3 The feared complication of systemic embolization via infective endocarditis may occur in up to one-half of cases, with the highest rates associated with staphylococcal or fungal pathogens.2 The risk for embolization in fungal endocarditis is independent of the size of the cardiac valve vegetations; accordingly, sequelae of embolic complications may arise despite negative cardiac imaging.4 Embolic complications, which typically are seen within the first 2 to 4 weeks of treatment, may serve as the presenting feature of endocarditis and may even occur after completion of antimicrobial therapy.
Detection of cutaneous manifestations of infective endocarditis, including Janeway lesions, Osler nodes, and splinter hemorrhages, may allow for earlier diagnosis. Despite eponymous recognition, Janeway lesions and Osler nodes are relatively uncommon manifestations of infective endocarditis and may be found in only 5% to 15% of cases.5 Biopsies of suspected Janeway lesions and Osler nodes may allow for recognition of relevant vascular pathology, identification of the causative pathogen, and strong support for the diagnosis of infective endocarditis.4-7
The initial photomicrograph of corresponding Janeway lesion histopathology was published by Kerr in 1955 and revealed dermal microabscesses posited to be secondary to bacterial emboli.8,9 Additional cases through the years have reported overlapping histopathologic features of Janeway lesions and Osler nodes, with the latter often defined by the presence of vasculitis.4 Although there appears to be ongoing debate and overlap between the 2 integumentary findings, a general consensus on differentiation takes into account both the clinical signs and symptoms as well as the histopathologic findings.10,11
Osler nodes present as tender, violaceous, subcutaneous nodules on the acral surfaces, usually on the pads of the fingers and toes.5 The pathogenesis involves the deposition of immune complexes as a sequela of vascular occlusion by microthrombi classically seen in the late phase of subacute endocarditis. Janeway lesions present as nontender erythematous macules on the acral surfaces and are thought to represent microthrombi with dermal microabscesses, more common in acute endocarditis. Our patient demonstrated features of both Osler nodes and Janeway lesions. Despite the presence of fungal thrombi—a pathophysiology closer to that of Janeway lesions—the clinical presentation of painful acral nodules affecting finger pads and histologic features of vasculitis may be better characterized as Osler nodes. Regardless of pathogenesis, these cutaneous findings serve as a minor clinical criterion in the Duke criteria for the diagnosis of infective endocarditis when present.12
Candida infective endocarditis should be suspected in a patient with a history of valvular disease or prior infective endocarditis with fungemia, unexplained neurologic signs, or manifestations of peripheral embolization despite negative blood cultures.3 Particularly in the setting of negative cardiac imaging, recognition of CIE requires heightened diagnostic acumen and clinicopathologic correlation. Although culture and pathologic examination of valvular vegetations represents the gold standard for diagnosis of CIE, aspiration and culture of easily accessible septic emboli may provide rapid identification of the etiologic pathogen. In 1976, Alpert et al13 identified C albicans from an aspirated Osler node. Postmortem examination revealed extensive involvement of the homograft valve and aortic root with C albicans.13 Many other examples exist in the literature demonstrating matching pathogenic isolates from microbiologic cultures of skin and blood.4,9,14,15 Thadepalli and Francis7 investigated 26 cases of endocarditis in heroin users in which the admitting diagnosis was endocarditis in only 4 cases. The etiologic pathogen was aspirated from secondary sites of localized infections secondary to emboli, including cutaneous lesions in 10 of the cases. Gram stain and culture revealed the causative organism leading to the ultimate diagnosis and management in 17 of 26 patients with endocarditis.7
The incidence of fungal endocarditis is increasing, with a reported 67% of cases caused by nosocomial infection.1 Given the rising incidence of fungal endocarditis and its accompanying diagnostic difficulties, including frequently negative blood cultures and cardiac imaging, clinicians must perform careful skin examinations, employ judicious use of skin biopsy, and carefully correlate clinical and pathologic findings to improve recognition of this disease and guide patient care.
- Arnold CJ, Johnson M, Bayer AS, et al. Infective endocarditis: an observational cohort study with a focus on therapy. Antimicrob Agents Chemother. 2015;59:2365. doi:10.1128/AAC.04867-14
- Chaudhary SC, Sawlani KK, Arora R, et al. Native aortic valve fungal endocarditis. BMJ Case Rep. 2013;2013:bcr2012007144. doi:10.1136/bcr-2012-007144
- Ellis ME, Al-Abdely H, Sandridge A, et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis. 2001;32:50-62. doi:10.1086/317550
- Gil MP, Velasco M, Botella R, et al. Janeway lesions: differential diagnosis with Osler’s nodes. Int J Dermatol. 1993;32:673-674. doi:10.1111/j.1365-4362.1993.tb04025.x
- Gomes RT, Tiberto LR, Bello VNM, et al. Dermatologic manifestations of infective endocarditis. An Bras Dermatol. 2016;91:92-94.
- Yee JM. Osler’s nodes and the recognition of infective endocarditis: a lesion of diagnostic importance. South Med J. 1987;80:753-757.
- Thadepalli H, Francis C. Diagnostic clues in metastatic lesions of endocarditia in addicts. West J Med. 1978;128:1-5.
- Kerr A Jr. Subacute Bacterial Endocarditis. Charles C. Thomas; 1955.
- Kerr A Jr, Tan JS. Biopsies of the Janeway lesion of infective endocarditis. J Cutan Pathol. 1979;6:124-129. doi:10.1111/j.1600-0560.1979.tb01113.x
- Marrie TJ. Osler’s nodes and Janeway lesions. Am J Med. 2008;121:105-106. doi:10.1016/j.amjmed.2007.07.035
- Gunson TH, Oliver GF. Osler’s nodes and Janeway lesions. Australas J Dermatol. 2007;48:251-255. doi:10.1111/j.1440-0960.2007.00397.x
- Durack DT, Lukes AS, Bright DK, et al. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-209.
- Alpert JS, Krous HF, Dalen JE, et al. Pathogenesis of Osler’s nodes. Ann Intern Med. 1976;85:471-473. doi:10.7326/0003-4819-85-4-471
- Cardullo AC, Silvers DN, Grossman ME. Janeway lesions and Osler’s nodes: a review of histopathologic findings. J Am Acad Dermatol. 1990;22:1088-1090. doi:10.1016/0190-9622(90)70157-D
- Vinson RP, Chung A, Elston DM, et al. Septic microemboli in a Janeway lesion of bacterial endocarditis. J Am Acad Dermatol. 1996;35:984-985. doi:10.1016/S0190-9622(96)90125-5
To the Editor:
A 44-year-old woman presented with a low-grade fever (temperature, 38.0 °C) and painful acral lesions of 1 week’s duration. She had a history of hepatitis C viral infection and intravenous (IV) drug use, as well as polymicrobial infective endocarditis that involved the tricuspid and aortic valves; pathogenic organisms were identified via blood culture as Enterococcus faecalis, Serratia species, Streptococcus viridans, and Candida albicans. The patient had received a mechanical aortic valve and bioprosthetic tricuspid valve replacement 5 months prior with warfarin therapy and had completed a postsurgical 6-week course of high-dose micafungin. She reported that she had developed painful, violaceous, thin papules on the plantar surface of the left foot 2 weeks prior to presentation. Her symptoms improved with a short systemic steroid taper; however, within a week she developed new tender, erythematous, thin papules on the plantar surface of the right foot and the palmar surface of the left hand with associated lower extremity swelling. She denied other symptoms, including fever, chills, neurologic symptoms, shortness of breath, chest pain, nausea, vomiting, hematuria, and hematochezia. Due to worsening cutaneous findings, the patient presented to the emergency department, prompting hospital admission for empiric antibacterial therapy with vancomycin and piperacillin-tazobactam for suspected infectious endocarditis. Dermatology was consulted after 1 day of antibacterial therapy without improvement to determine the etiology of the patient’s skin findings.
Physical examination revealed the patient was afebrile with partially blanching violaceous to purpuric, tender, edematous papules on the left fourth and fifth finger pads, as well as scattered, painful, purpuric patches with stellate borders on the right plantar foot (Figure 1). Laboratory test results revealed mild anemia (hemoglobin, 11.9 g/dL [reference range, 12.0–15.0 g/dL], mild neutrophilia (neutrophils, 8.4×109/L [reference range, 1.9–7.9×109/L], elevated acute-phase reactants (erythrocyte sedimentation rate, 71 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 5.7 mg/dL [reference range, 0.0–0.5 mg/dL]), and positive hepatitis C virus antibody with an undetectable viral load. At the time of dermatologic evaluation, admission blood cultures and transthoracic echocardiogram were negative. Additionally, a transesophageal echocardiogram, limited by artifact from the mechanical aortic valve, was equivocal for valvular pathology. Subsequent ophthalmologic evaluation was negative for lesions associated with endocarditis, such as retinal hemorrhages.

Punch biopsies of the left fourth finger pad were submitted for histopathologic analysis and tissue cultures. Histopathology demonstrated deep dermal perivascular neutrophilic inflammation with multiple intravascular thrombi, perivascular fibrin, and karyorrhectic debris (Figure 2). Periodic acid–Schiff and Grocott-Gomori methenamine-silver stains revealed fungal spores with rare pseudohyphae within the thrombosed vascular spaces and the perivascular dermis, consistent with fungal septic emboli (Figure 3).

Empiric systemic antifungal coverage composed of IV liposomal amphotericin B and oral flucytosine was initiated, and the patient’s tender acral papules rapidly improved. Within 48 hours of biopsy, skin tissue culture confirmed the presence of C albicans. Four days after the preliminary dermatopathology report, confirmatory blood cultures resulted with pansensitive C albicans. Final tissue and blood cultures were negative for bacteria including mycobacteria. In addition to a 6-week course of IV amphotericin B and flucytosine, repeat surgical intervention was considered, and lifelong suppressive antifungal oral therapy was recommended. Unfortunately, the patient did not present for follow-up. Three months later, she presented to the emergency department with peritonitis; in the operating room, she was found to have ischemia of the entirety of the small and large intestines and died shortly thereafter.

Fungal endocarditis is rare, tending to develop in patient populations with particular risk factors such as immune compromise, structural heart defects or prosthetic valves, and IV drug use. Candida infective endocarditis (CIE) represents less than 2% of infective endocarditis cases and carries a high mortality rate (30%–80%).1-3 Diagnosis may be challenging, as the clinical presentation varies widely. Although some patients may present with classic features of infective endocarditis, including fever, cardiac murmurs, and positive blood cultures, many cases of infective endocarditis present with nonspecific symptoms, raising a broad clinical differential diagnosis. Delay in diagnosis, which is seen in 82% of patients with fungal endocarditis, may be attributed to the slow progression of symptoms, inconclusive cardiac imaging, or negative blood cultures seen in almost one-third of cases.2,3 The feared complication of systemic embolization via infective endocarditis may occur in up to one-half of cases, with the highest rates associated with staphylococcal or fungal pathogens.2 The risk for embolization in fungal endocarditis is independent of the size of the cardiac valve vegetations; accordingly, sequelae of embolic complications may arise despite negative cardiac imaging.4 Embolic complications, which typically are seen within the first 2 to 4 weeks of treatment, may serve as the presenting feature of endocarditis and may even occur after completion of antimicrobial therapy.
Detection of cutaneous manifestations of infective endocarditis, including Janeway lesions, Osler nodes, and splinter hemorrhages, may allow for earlier diagnosis. Despite eponymous recognition, Janeway lesions and Osler nodes are relatively uncommon manifestations of infective endocarditis and may be found in only 5% to 15% of cases.5 Biopsies of suspected Janeway lesions and Osler nodes may allow for recognition of relevant vascular pathology, identification of the causative pathogen, and strong support for the diagnosis of infective endocarditis.4-7
The initial photomicrograph of corresponding Janeway lesion histopathology was published by Kerr in 1955 and revealed dermal microabscesses posited to be secondary to bacterial emboli.8,9 Additional cases through the years have reported overlapping histopathologic features of Janeway lesions and Osler nodes, with the latter often defined by the presence of vasculitis.4 Although there appears to be ongoing debate and overlap between the 2 integumentary findings, a general consensus on differentiation takes into account both the clinical signs and symptoms as well as the histopathologic findings.10,11
Osler nodes present as tender, violaceous, subcutaneous nodules on the acral surfaces, usually on the pads of the fingers and toes.5 The pathogenesis involves the deposition of immune complexes as a sequela of vascular occlusion by microthrombi classically seen in the late phase of subacute endocarditis. Janeway lesions present as nontender erythematous macules on the acral surfaces and are thought to represent microthrombi with dermal microabscesses, more common in acute endocarditis. Our patient demonstrated features of both Osler nodes and Janeway lesions. Despite the presence of fungal thrombi—a pathophysiology closer to that of Janeway lesions—the clinical presentation of painful acral nodules affecting finger pads and histologic features of vasculitis may be better characterized as Osler nodes. Regardless of pathogenesis, these cutaneous findings serve as a minor clinical criterion in the Duke criteria for the diagnosis of infective endocarditis when present.12
Candida infective endocarditis should be suspected in a patient with a history of valvular disease or prior infective endocarditis with fungemia, unexplained neurologic signs, or manifestations of peripheral embolization despite negative blood cultures.3 Particularly in the setting of negative cardiac imaging, recognition of CIE requires heightened diagnostic acumen and clinicopathologic correlation. Although culture and pathologic examination of valvular vegetations represents the gold standard for diagnosis of CIE, aspiration and culture of easily accessible septic emboli may provide rapid identification of the etiologic pathogen. In 1976, Alpert et al13 identified C albicans from an aspirated Osler node. Postmortem examination revealed extensive involvement of the homograft valve and aortic root with C albicans.13 Many other examples exist in the literature demonstrating matching pathogenic isolates from microbiologic cultures of skin and blood.4,9,14,15 Thadepalli and Francis7 investigated 26 cases of endocarditis in heroin users in which the admitting diagnosis was endocarditis in only 4 cases. The etiologic pathogen was aspirated from secondary sites of localized infections secondary to emboli, including cutaneous lesions in 10 of the cases. Gram stain and culture revealed the causative organism leading to the ultimate diagnosis and management in 17 of 26 patients with endocarditis.7
The incidence of fungal endocarditis is increasing, with a reported 67% of cases caused by nosocomial infection.1 Given the rising incidence of fungal endocarditis and its accompanying diagnostic difficulties, including frequently negative blood cultures and cardiac imaging, clinicians must perform careful skin examinations, employ judicious use of skin biopsy, and carefully correlate clinical and pathologic findings to improve recognition of this disease and guide patient care.
To the Editor:
A 44-year-old woman presented with a low-grade fever (temperature, 38.0 °C) and painful acral lesions of 1 week’s duration. She had a history of hepatitis C viral infection and intravenous (IV) drug use, as well as polymicrobial infective endocarditis that involved the tricuspid and aortic valves; pathogenic organisms were identified via blood culture as Enterococcus faecalis, Serratia species, Streptococcus viridans, and Candida albicans. The patient had received a mechanical aortic valve and bioprosthetic tricuspid valve replacement 5 months prior with warfarin therapy and had completed a postsurgical 6-week course of high-dose micafungin. She reported that she had developed painful, violaceous, thin papules on the plantar surface of the left foot 2 weeks prior to presentation. Her symptoms improved with a short systemic steroid taper; however, within a week she developed new tender, erythematous, thin papules on the plantar surface of the right foot and the palmar surface of the left hand with associated lower extremity swelling. She denied other symptoms, including fever, chills, neurologic symptoms, shortness of breath, chest pain, nausea, vomiting, hematuria, and hematochezia. Due to worsening cutaneous findings, the patient presented to the emergency department, prompting hospital admission for empiric antibacterial therapy with vancomycin and piperacillin-tazobactam for suspected infectious endocarditis. Dermatology was consulted after 1 day of antibacterial therapy without improvement to determine the etiology of the patient’s skin findings.
Physical examination revealed the patient was afebrile with partially blanching violaceous to purpuric, tender, edematous papules on the left fourth and fifth finger pads, as well as scattered, painful, purpuric patches with stellate borders on the right plantar foot (Figure 1). Laboratory test results revealed mild anemia (hemoglobin, 11.9 g/dL [reference range, 12.0–15.0 g/dL], mild neutrophilia (neutrophils, 8.4×109/L [reference range, 1.9–7.9×109/L], elevated acute-phase reactants (erythrocyte sedimentation rate, 71 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 5.7 mg/dL [reference range, 0.0–0.5 mg/dL]), and positive hepatitis C virus antibody with an undetectable viral load. At the time of dermatologic evaluation, admission blood cultures and transthoracic echocardiogram were negative. Additionally, a transesophageal echocardiogram, limited by artifact from the mechanical aortic valve, was equivocal for valvular pathology. Subsequent ophthalmologic evaluation was negative for lesions associated with endocarditis, such as retinal hemorrhages.

Punch biopsies of the left fourth finger pad were submitted for histopathologic analysis and tissue cultures. Histopathology demonstrated deep dermal perivascular neutrophilic inflammation with multiple intravascular thrombi, perivascular fibrin, and karyorrhectic debris (Figure 2). Periodic acid–Schiff and Grocott-Gomori methenamine-silver stains revealed fungal spores with rare pseudohyphae within the thrombosed vascular spaces and the perivascular dermis, consistent with fungal septic emboli (Figure 3).

Empiric systemic antifungal coverage composed of IV liposomal amphotericin B and oral flucytosine was initiated, and the patient’s tender acral papules rapidly improved. Within 48 hours of biopsy, skin tissue culture confirmed the presence of C albicans. Four days after the preliminary dermatopathology report, confirmatory blood cultures resulted with pansensitive C albicans. Final tissue and blood cultures were negative for bacteria including mycobacteria. In addition to a 6-week course of IV amphotericin B and flucytosine, repeat surgical intervention was considered, and lifelong suppressive antifungal oral therapy was recommended. Unfortunately, the patient did not present for follow-up. Three months later, she presented to the emergency department with peritonitis; in the operating room, she was found to have ischemia of the entirety of the small and large intestines and died shortly thereafter.

Fungal endocarditis is rare, tending to develop in patient populations with particular risk factors such as immune compromise, structural heart defects or prosthetic valves, and IV drug use. Candida infective endocarditis (CIE) represents less than 2% of infective endocarditis cases and carries a high mortality rate (30%–80%).1-3 Diagnosis may be challenging, as the clinical presentation varies widely. Although some patients may present with classic features of infective endocarditis, including fever, cardiac murmurs, and positive blood cultures, many cases of infective endocarditis present with nonspecific symptoms, raising a broad clinical differential diagnosis. Delay in diagnosis, which is seen in 82% of patients with fungal endocarditis, may be attributed to the slow progression of symptoms, inconclusive cardiac imaging, or negative blood cultures seen in almost one-third of cases.2,3 The feared complication of systemic embolization via infective endocarditis may occur in up to one-half of cases, with the highest rates associated with staphylococcal or fungal pathogens.2 The risk for embolization in fungal endocarditis is independent of the size of the cardiac valve vegetations; accordingly, sequelae of embolic complications may arise despite negative cardiac imaging.4 Embolic complications, which typically are seen within the first 2 to 4 weeks of treatment, may serve as the presenting feature of endocarditis and may even occur after completion of antimicrobial therapy.
Detection of cutaneous manifestations of infective endocarditis, including Janeway lesions, Osler nodes, and splinter hemorrhages, may allow for earlier diagnosis. Despite eponymous recognition, Janeway lesions and Osler nodes are relatively uncommon manifestations of infective endocarditis and may be found in only 5% to 15% of cases.5 Biopsies of suspected Janeway lesions and Osler nodes may allow for recognition of relevant vascular pathology, identification of the causative pathogen, and strong support for the diagnosis of infective endocarditis.4-7
The initial photomicrograph of corresponding Janeway lesion histopathology was published by Kerr in 1955 and revealed dermal microabscesses posited to be secondary to bacterial emboli.8,9 Additional cases through the years have reported overlapping histopathologic features of Janeway lesions and Osler nodes, with the latter often defined by the presence of vasculitis.4 Although there appears to be ongoing debate and overlap between the 2 integumentary findings, a general consensus on differentiation takes into account both the clinical signs and symptoms as well as the histopathologic findings.10,11
Osler nodes present as tender, violaceous, subcutaneous nodules on the acral surfaces, usually on the pads of the fingers and toes.5 The pathogenesis involves the deposition of immune complexes as a sequela of vascular occlusion by microthrombi classically seen in the late phase of subacute endocarditis. Janeway lesions present as nontender erythematous macules on the acral surfaces and are thought to represent microthrombi with dermal microabscesses, more common in acute endocarditis. Our patient demonstrated features of both Osler nodes and Janeway lesions. Despite the presence of fungal thrombi—a pathophysiology closer to that of Janeway lesions—the clinical presentation of painful acral nodules affecting finger pads and histologic features of vasculitis may be better characterized as Osler nodes. Regardless of pathogenesis, these cutaneous findings serve as a minor clinical criterion in the Duke criteria for the diagnosis of infective endocarditis when present.12
Candida infective endocarditis should be suspected in a patient with a history of valvular disease or prior infective endocarditis with fungemia, unexplained neurologic signs, or manifestations of peripheral embolization despite negative blood cultures.3 Particularly in the setting of negative cardiac imaging, recognition of CIE requires heightened diagnostic acumen and clinicopathologic correlation. Although culture and pathologic examination of valvular vegetations represents the gold standard for diagnosis of CIE, aspiration and culture of easily accessible septic emboli may provide rapid identification of the etiologic pathogen. In 1976, Alpert et al13 identified C albicans from an aspirated Osler node. Postmortem examination revealed extensive involvement of the homograft valve and aortic root with C albicans.13 Many other examples exist in the literature demonstrating matching pathogenic isolates from microbiologic cultures of skin and blood.4,9,14,15 Thadepalli and Francis7 investigated 26 cases of endocarditis in heroin users in which the admitting diagnosis was endocarditis in only 4 cases. The etiologic pathogen was aspirated from secondary sites of localized infections secondary to emboli, including cutaneous lesions in 10 of the cases. Gram stain and culture revealed the causative organism leading to the ultimate diagnosis and management in 17 of 26 patients with endocarditis.7
The incidence of fungal endocarditis is increasing, with a reported 67% of cases caused by nosocomial infection.1 Given the rising incidence of fungal endocarditis and its accompanying diagnostic difficulties, including frequently negative blood cultures and cardiac imaging, clinicians must perform careful skin examinations, employ judicious use of skin biopsy, and carefully correlate clinical and pathologic findings to improve recognition of this disease and guide patient care.
- Arnold CJ, Johnson M, Bayer AS, et al. Infective endocarditis: an observational cohort study with a focus on therapy. Antimicrob Agents Chemother. 2015;59:2365. doi:10.1128/AAC.04867-14
- Chaudhary SC, Sawlani KK, Arora R, et al. Native aortic valve fungal endocarditis. BMJ Case Rep. 2013;2013:bcr2012007144. doi:10.1136/bcr-2012-007144
- Ellis ME, Al-Abdely H, Sandridge A, et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis. 2001;32:50-62. doi:10.1086/317550
- Gil MP, Velasco M, Botella R, et al. Janeway lesions: differential diagnosis with Osler’s nodes. Int J Dermatol. 1993;32:673-674. doi:10.1111/j.1365-4362.1993.tb04025.x
- Gomes RT, Tiberto LR, Bello VNM, et al. Dermatologic manifestations of infective endocarditis. An Bras Dermatol. 2016;91:92-94.
- Yee JM. Osler’s nodes and the recognition of infective endocarditis: a lesion of diagnostic importance. South Med J. 1987;80:753-757.
- Thadepalli H, Francis C. Diagnostic clues in metastatic lesions of endocarditia in addicts. West J Med. 1978;128:1-5.
- Kerr A Jr. Subacute Bacterial Endocarditis. Charles C. Thomas; 1955.
- Kerr A Jr, Tan JS. Biopsies of the Janeway lesion of infective endocarditis. J Cutan Pathol. 1979;6:124-129. doi:10.1111/j.1600-0560.1979.tb01113.x
- Marrie TJ. Osler’s nodes and Janeway lesions. Am J Med. 2008;121:105-106. doi:10.1016/j.amjmed.2007.07.035
- Gunson TH, Oliver GF. Osler’s nodes and Janeway lesions. Australas J Dermatol. 2007;48:251-255. doi:10.1111/j.1440-0960.2007.00397.x
- Durack DT, Lukes AS, Bright DK, et al. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-209.
- Alpert JS, Krous HF, Dalen JE, et al. Pathogenesis of Osler’s nodes. Ann Intern Med. 1976;85:471-473. doi:10.7326/0003-4819-85-4-471
- Cardullo AC, Silvers DN, Grossman ME. Janeway lesions and Osler’s nodes: a review of histopathologic findings. J Am Acad Dermatol. 1990;22:1088-1090. doi:10.1016/0190-9622(90)70157-D
- Vinson RP, Chung A, Elston DM, et al. Septic microemboli in a Janeway lesion of bacterial endocarditis. J Am Acad Dermatol. 1996;35:984-985. doi:10.1016/S0190-9622(96)90125-5
- Arnold CJ, Johnson M, Bayer AS, et al. Infective endocarditis: an observational cohort study with a focus on therapy. Antimicrob Agents Chemother. 2015;59:2365. doi:10.1128/AAC.04867-14
- Chaudhary SC, Sawlani KK, Arora R, et al. Native aortic valve fungal endocarditis. BMJ Case Rep. 2013;2013:bcr2012007144. doi:10.1136/bcr-2012-007144
- Ellis ME, Al-Abdely H, Sandridge A, et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis. 2001;32:50-62. doi:10.1086/317550
- Gil MP, Velasco M, Botella R, et al. Janeway lesions: differential diagnosis with Osler’s nodes. Int J Dermatol. 1993;32:673-674. doi:10.1111/j.1365-4362.1993.tb04025.x
- Gomes RT, Tiberto LR, Bello VNM, et al. Dermatologic manifestations of infective endocarditis. An Bras Dermatol. 2016;91:92-94.
- Yee JM. Osler’s nodes and the recognition of infective endocarditis: a lesion of diagnostic importance. South Med J. 1987;80:753-757.
- Thadepalli H, Francis C. Diagnostic clues in metastatic lesions of endocarditia in addicts. West J Med. 1978;128:1-5.
- Kerr A Jr. Subacute Bacterial Endocarditis. Charles C. Thomas; 1955.
- Kerr A Jr, Tan JS. Biopsies of the Janeway lesion of infective endocarditis. J Cutan Pathol. 1979;6:124-129. doi:10.1111/j.1600-0560.1979.tb01113.x
- Marrie TJ. Osler’s nodes and Janeway lesions. Am J Med. 2008;121:105-106. doi:10.1016/j.amjmed.2007.07.035
- Gunson TH, Oliver GF. Osler’s nodes and Janeway lesions. Australas J Dermatol. 2007;48:251-255. doi:10.1111/j.1440-0960.2007.00397.x
- Durack DT, Lukes AS, Bright DK, et al. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-209.
- Alpert JS, Krous HF, Dalen JE, et al. Pathogenesis of Osler’s nodes. Ann Intern Med. 1976;85:471-473. doi:10.7326/0003-4819-85-4-471
- Cardullo AC, Silvers DN, Grossman ME. Janeway lesions and Osler’s nodes: a review of histopathologic findings. J Am Acad Dermatol. 1990;22:1088-1090. doi:10.1016/0190-9622(90)70157-D
- Vinson RP, Chung A, Elston DM, et al. Septic microemboli in a Janeway lesion of bacterial endocarditis. J Am Acad Dermatol. 1996;35:984-985. doi:10.1016/S0190-9622(96)90125-5
PRACTICE POINTS
- Fungal infective endocarditis is rare, and diagnostic tests such as blood cultures and echocardiography may not detect the disease.
- The mortality rate of fungal endocarditis is high, with improved clinical outcomes if diagnosed and treated early.
- Clinicopathologic correlation between integumentary examination and skin biopsy findings may provide timely diagnosis, thereby guiding appropriate therapy.
Managing respiratory symptoms in the ‘tripledemic’ era
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Novel nomogram distinguishes pneumonias
A model incorporating factors such as lymphocytes and lung lesions differentiated adenovirus pneumonias from Chlamydia psittaci (CPP) in a multicenter study of nearly 200 individuals.
Symptoms of pneumonia caused by CPP are often confused with other respiratory infections, particularly adenovirus pneumonia (AVP), which can delay correct diagnosis and impact treatment, Yi Li, MD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues wrote. Detailed comparisons of the two conditions are lacking.
In a retrospective study published in the International Journal of Infectious Diseases, the researchers examined laboratory, clinical, and radiological differences and created a nomogram to distinguish CPP from AVP. The study population included 78 adults with CPP and 102 with AVP who were seen at a single center in China. The mean ages of the CPP and AVP patients were 61.0 years and 38.5 years, and 57.7% men and 91.2% men, respectively. Patients with CPP were significantly more likely to have hypertension and diabetes at baseline, compared with the AVP group.
The primary outcome was 30-day mortality after hospital admission, which was 10.3% and 14.7% for the CPP and AVP patients, respectively (P = 0.376). However, the incidence of cardiac injury was significantly higher in AVP patients versus those with CPP (48.0% vs. 11.5%; P < 0.001).
In a multivariate analysis, age, sex, nervous system symptoms, lymphocyte count, C-reactive protein level (CRP), and bilateral lung lesions were risk factors for CPP. The researchers combined these factors into a nomogram that showed a concordance value of 0.949 for differentiating between the CPP and AVP groups.
Overall, CPP patients were older, had more nervous system symptoms, and had higher CRP levels, compared with patients with AVP, who were more likely to be men and to have higher lymphocyte percentages and more bilateral lung lesions on chest imaging.
The current study is the first known to provide a way to distinguish CPP and AVP, the researchers wrote. “The antibiotic treatments, prognoses, and life support measures of CPP and AVP are considerably different. Therefore, differentiating the two diseases through early identification of specific clinical characteristics is vital.”
The findings were limited by several factors including the small sample size, retrospective design, and the use of mNGS to diagnose CPP in the absence of standard clinical diagnostic kits, which may have resulted in underestimated CPP incidence, the researchers noted.
However, “the nomogram we established combines patient data on age, sex, and readily available laboratory results to reasonably predict CPP, thus making rapid and direct diagnosis possible,” they said.
The study was supported by the Key R&D Program of Hunan Province, Project Program of National Clinical Research Center for Geriatric Disorders, National Natural Science Foundation of China, Hunan Natural Science Youth Foundation, and the national key clinical specialist construction programs of China. The researchers had no financial conflicts to disclose.
A model incorporating factors such as lymphocytes and lung lesions differentiated adenovirus pneumonias from Chlamydia psittaci (CPP) in a multicenter study of nearly 200 individuals.
Symptoms of pneumonia caused by CPP are often confused with other respiratory infections, particularly adenovirus pneumonia (AVP), which can delay correct diagnosis and impact treatment, Yi Li, MD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues wrote. Detailed comparisons of the two conditions are lacking.
In a retrospective study published in the International Journal of Infectious Diseases, the researchers examined laboratory, clinical, and radiological differences and created a nomogram to distinguish CPP from AVP. The study population included 78 adults with CPP and 102 with AVP who were seen at a single center in China. The mean ages of the CPP and AVP patients were 61.0 years and 38.5 years, and 57.7% men and 91.2% men, respectively. Patients with CPP were significantly more likely to have hypertension and diabetes at baseline, compared with the AVP group.
The primary outcome was 30-day mortality after hospital admission, which was 10.3% and 14.7% for the CPP and AVP patients, respectively (P = 0.376). However, the incidence of cardiac injury was significantly higher in AVP patients versus those with CPP (48.0% vs. 11.5%; P < 0.001).
In a multivariate analysis, age, sex, nervous system symptoms, lymphocyte count, C-reactive protein level (CRP), and bilateral lung lesions were risk factors for CPP. The researchers combined these factors into a nomogram that showed a concordance value of 0.949 for differentiating between the CPP and AVP groups.
Overall, CPP patients were older, had more nervous system symptoms, and had higher CRP levels, compared with patients with AVP, who were more likely to be men and to have higher lymphocyte percentages and more bilateral lung lesions on chest imaging.
The current study is the first known to provide a way to distinguish CPP and AVP, the researchers wrote. “The antibiotic treatments, prognoses, and life support measures of CPP and AVP are considerably different. Therefore, differentiating the two diseases through early identification of specific clinical characteristics is vital.”
The findings were limited by several factors including the small sample size, retrospective design, and the use of mNGS to diagnose CPP in the absence of standard clinical diagnostic kits, which may have resulted in underestimated CPP incidence, the researchers noted.
However, “the nomogram we established combines patient data on age, sex, and readily available laboratory results to reasonably predict CPP, thus making rapid and direct diagnosis possible,” they said.
The study was supported by the Key R&D Program of Hunan Province, Project Program of National Clinical Research Center for Geriatric Disorders, National Natural Science Foundation of China, Hunan Natural Science Youth Foundation, and the national key clinical specialist construction programs of China. The researchers had no financial conflicts to disclose.
A model incorporating factors such as lymphocytes and lung lesions differentiated adenovirus pneumonias from Chlamydia psittaci (CPP) in a multicenter study of nearly 200 individuals.
Symptoms of pneumonia caused by CPP are often confused with other respiratory infections, particularly adenovirus pneumonia (AVP), which can delay correct diagnosis and impact treatment, Yi Li, MD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues wrote. Detailed comparisons of the two conditions are lacking.
In a retrospective study published in the International Journal of Infectious Diseases, the researchers examined laboratory, clinical, and radiological differences and created a nomogram to distinguish CPP from AVP. The study population included 78 adults with CPP and 102 with AVP who were seen at a single center in China. The mean ages of the CPP and AVP patients were 61.0 years and 38.5 years, and 57.7% men and 91.2% men, respectively. Patients with CPP were significantly more likely to have hypertension and diabetes at baseline, compared with the AVP group.
The primary outcome was 30-day mortality after hospital admission, which was 10.3% and 14.7% for the CPP and AVP patients, respectively (P = 0.376). However, the incidence of cardiac injury was significantly higher in AVP patients versus those with CPP (48.0% vs. 11.5%; P < 0.001).
In a multivariate analysis, age, sex, nervous system symptoms, lymphocyte count, C-reactive protein level (CRP), and bilateral lung lesions were risk factors for CPP. The researchers combined these factors into a nomogram that showed a concordance value of 0.949 for differentiating between the CPP and AVP groups.
Overall, CPP patients were older, had more nervous system symptoms, and had higher CRP levels, compared with patients with AVP, who were more likely to be men and to have higher lymphocyte percentages and more bilateral lung lesions on chest imaging.
The current study is the first known to provide a way to distinguish CPP and AVP, the researchers wrote. “The antibiotic treatments, prognoses, and life support measures of CPP and AVP are considerably different. Therefore, differentiating the two diseases through early identification of specific clinical characteristics is vital.”
The findings were limited by several factors including the small sample size, retrospective design, and the use of mNGS to diagnose CPP in the absence of standard clinical diagnostic kits, which may have resulted in underestimated CPP incidence, the researchers noted.
However, “the nomogram we established combines patient data on age, sex, and readily available laboratory results to reasonably predict CPP, thus making rapid and direct diagnosis possible,” they said.
The study was supported by the Key R&D Program of Hunan Province, Project Program of National Clinical Research Center for Geriatric Disorders, National Natural Science Foundation of China, Hunan Natural Science Youth Foundation, and the national key clinical specialist construction programs of China. The researchers had no financial conflicts to disclose.
FROM THE INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES