User login
Advanced Primary Care program boosts COVID-19 results
The better outcomes were seen in higher vaccination rates and fewer infections, hospitalizations, and deaths from the disease, according to study authors, led by Emily Gruber, MBA, MPH, with the Maryland Primary Care Program, Maryland Department of Health in Baltimore.
The results were published online in JAMA Network Open.
The study population was divided into MDPCP participants (n = 208,146) and a matched cohort (n = 37,203) of beneficiaries not attributed to MDPCP practices but who met eligibility criteria for study participation from Jan. 1, 2020, through Dec. 31, 2021.
More vaccinations, more antibody treatments
Researchers broke down the comparisons of better outcomes: 84.47% of MDPCP beneficiaries were fully vaccinated vs. 77.93% of nonparticipating beneficiaries (P less than .001). COVID-19–positive program beneficiaries also received monoclonal antibody treatment more often (8.45% vs. 6.11%; P less than .001).
Plus, program participants received more care via telehealth (62.95% vs. 54.53%; P less than .001) compared with those not participating.
Regarding secondary outcomes, MDPCP beneficiaries had lower rates of COVID cases (6.55% vs. 7.09%; P less than .001), lower rates of COVID-19 hospitalizations (1.81% vs. 2.06%; P = .001), and lower rates of death due to COVID-19 (0.56% vs. 0.77%; P less than .001).
Program components
Enrollment in the MDPCP is voluntary, and primary care practices can apply each year to be part of the program.
The model integrates primary care and public health in the pandemic response. It was created by the Maryland Department of Health (MDH) and the Centers for Medicare & Medicaid Services (CMS).
It expands the role of primary care to include services such as expanded care management, integrated behavioral health, data-driven care, and screenings and referrals to address social needs.
Coauthor Howard Haft, MD, MMM, with the Maryland Department of Public Health, said in an interview that among the most important factors in the program’s success were giving providers vaccines to distribute and then giving providers data on how many patients are vaccinated, and who’s not vaccinated but at high risk, and how those rates compare to other practices.
As to whether this could be a widespread model, Dr. Haft said, “It’s highly replicable.”
“Every state in the nation overall has all of these resources. It’s a matter of having the operational and political will to put those resources together. Almost every state has the technological ability to use their health information exchange to help tie pieces together.”
Vaccines and testing made available to providers
Making ample vaccines and testing available to providers in their offices helped patients get those services in a place they trust, Dr. Haft said.
The model also included a payment system for providers that included a significant amount of non–visit-based payments when many locations were closed in the height of the pandemic.
“That helped financially,” as did providing free telehealth platforms to practices with training on how to use them, Dr. Haft said.
‘Innovative and important’
Renu Tipirneni, MD, an assistant professor of internal medicine at the University of Michigan and at the Institute for Healthcare Policy and Innovation in Ann Arbor, said Maryland is out front putting into practice what practices nationwide aspire to do – coordinating physical and mental health and social needs and integrating primary and public health. Dr. Tipirneni, who was not involved with the study, said she was impressed the researchers were able to show statistically significant improvement with COVID-19 outcomes in the first 2 years.
“In terms of health outcomes, we often have to wait longer to see good outcomes,” she said. “It’s a really innovative and important model.”
She said states can learn from each other and this model is an example.
Integrating primary care and public health and addressing social needs may be the biggest challenges for states, she said, as those realms typically have been siloed.
“But they may be the key components to achieving these outcomes,” she said.
Take-home message
The most important benefit of the program is that data suggest it saves lives, according to Dr. Haft. While the actual difference between COVID deaths in the program and nonprogram groups was small, multiplying that savings across the nation shows substantial potential benefit, he explained.
“At a time when we were losing lives at an unconscionable rate, we were able to make a difference in saving lives,” Dr. Haft said.
Authors report no relevant financial disclosures.
The study received financial support from the Maryland Department of Health.
Dr. Tiperneni is helping evaluate Michigan’s Medicaid contract.
The better outcomes were seen in higher vaccination rates and fewer infections, hospitalizations, and deaths from the disease, according to study authors, led by Emily Gruber, MBA, MPH, with the Maryland Primary Care Program, Maryland Department of Health in Baltimore.
The results were published online in JAMA Network Open.
The study population was divided into MDPCP participants (n = 208,146) and a matched cohort (n = 37,203) of beneficiaries not attributed to MDPCP practices but who met eligibility criteria for study participation from Jan. 1, 2020, through Dec. 31, 2021.
More vaccinations, more antibody treatments
Researchers broke down the comparisons of better outcomes: 84.47% of MDPCP beneficiaries were fully vaccinated vs. 77.93% of nonparticipating beneficiaries (P less than .001). COVID-19–positive program beneficiaries also received monoclonal antibody treatment more often (8.45% vs. 6.11%; P less than .001).
Plus, program participants received more care via telehealth (62.95% vs. 54.53%; P less than .001) compared with those not participating.
Regarding secondary outcomes, MDPCP beneficiaries had lower rates of COVID cases (6.55% vs. 7.09%; P less than .001), lower rates of COVID-19 hospitalizations (1.81% vs. 2.06%; P = .001), and lower rates of death due to COVID-19 (0.56% vs. 0.77%; P less than .001).
Program components
Enrollment in the MDPCP is voluntary, and primary care practices can apply each year to be part of the program.
The model integrates primary care and public health in the pandemic response. It was created by the Maryland Department of Health (MDH) and the Centers for Medicare & Medicaid Services (CMS).
It expands the role of primary care to include services such as expanded care management, integrated behavioral health, data-driven care, and screenings and referrals to address social needs.
Coauthor Howard Haft, MD, MMM, with the Maryland Department of Public Health, said in an interview that among the most important factors in the program’s success were giving providers vaccines to distribute and then giving providers data on how many patients are vaccinated, and who’s not vaccinated but at high risk, and how those rates compare to other practices.
As to whether this could be a widespread model, Dr. Haft said, “It’s highly replicable.”
“Every state in the nation overall has all of these resources. It’s a matter of having the operational and political will to put those resources together. Almost every state has the technological ability to use their health information exchange to help tie pieces together.”
Vaccines and testing made available to providers
Making ample vaccines and testing available to providers in their offices helped patients get those services in a place they trust, Dr. Haft said.
The model also included a payment system for providers that included a significant amount of non–visit-based payments when many locations were closed in the height of the pandemic.
“That helped financially,” as did providing free telehealth platforms to practices with training on how to use them, Dr. Haft said.
‘Innovative and important’
Renu Tipirneni, MD, an assistant professor of internal medicine at the University of Michigan and at the Institute for Healthcare Policy and Innovation in Ann Arbor, said Maryland is out front putting into practice what practices nationwide aspire to do – coordinating physical and mental health and social needs and integrating primary and public health. Dr. Tipirneni, who was not involved with the study, said she was impressed the researchers were able to show statistically significant improvement with COVID-19 outcomes in the first 2 years.
“In terms of health outcomes, we often have to wait longer to see good outcomes,” she said. “It’s a really innovative and important model.”
She said states can learn from each other and this model is an example.
Integrating primary care and public health and addressing social needs may be the biggest challenges for states, she said, as those realms typically have been siloed.
“But they may be the key components to achieving these outcomes,” she said.
Take-home message
The most important benefit of the program is that data suggest it saves lives, according to Dr. Haft. While the actual difference between COVID deaths in the program and nonprogram groups was small, multiplying that savings across the nation shows substantial potential benefit, he explained.
“At a time when we were losing lives at an unconscionable rate, we were able to make a difference in saving lives,” Dr. Haft said.
Authors report no relevant financial disclosures.
The study received financial support from the Maryland Department of Health.
Dr. Tiperneni is helping evaluate Michigan’s Medicaid contract.
The better outcomes were seen in higher vaccination rates and fewer infections, hospitalizations, and deaths from the disease, according to study authors, led by Emily Gruber, MBA, MPH, with the Maryland Primary Care Program, Maryland Department of Health in Baltimore.
The results were published online in JAMA Network Open.
The study population was divided into MDPCP participants (n = 208,146) and a matched cohort (n = 37,203) of beneficiaries not attributed to MDPCP practices but who met eligibility criteria for study participation from Jan. 1, 2020, through Dec. 31, 2021.
More vaccinations, more antibody treatments
Researchers broke down the comparisons of better outcomes: 84.47% of MDPCP beneficiaries were fully vaccinated vs. 77.93% of nonparticipating beneficiaries (P less than .001). COVID-19–positive program beneficiaries also received monoclonal antibody treatment more often (8.45% vs. 6.11%; P less than .001).
Plus, program participants received more care via telehealth (62.95% vs. 54.53%; P less than .001) compared with those not participating.
Regarding secondary outcomes, MDPCP beneficiaries had lower rates of COVID cases (6.55% vs. 7.09%; P less than .001), lower rates of COVID-19 hospitalizations (1.81% vs. 2.06%; P = .001), and lower rates of death due to COVID-19 (0.56% vs. 0.77%; P less than .001).
Program components
Enrollment in the MDPCP is voluntary, and primary care practices can apply each year to be part of the program.
The model integrates primary care and public health in the pandemic response. It was created by the Maryland Department of Health (MDH) and the Centers for Medicare & Medicaid Services (CMS).
It expands the role of primary care to include services such as expanded care management, integrated behavioral health, data-driven care, and screenings and referrals to address social needs.
Coauthor Howard Haft, MD, MMM, with the Maryland Department of Public Health, said in an interview that among the most important factors in the program’s success were giving providers vaccines to distribute and then giving providers data on how many patients are vaccinated, and who’s not vaccinated but at high risk, and how those rates compare to other practices.
As to whether this could be a widespread model, Dr. Haft said, “It’s highly replicable.”
“Every state in the nation overall has all of these resources. It’s a matter of having the operational and political will to put those resources together. Almost every state has the technological ability to use their health information exchange to help tie pieces together.”
Vaccines and testing made available to providers
Making ample vaccines and testing available to providers in their offices helped patients get those services in a place they trust, Dr. Haft said.
The model also included a payment system for providers that included a significant amount of non–visit-based payments when many locations were closed in the height of the pandemic.
“That helped financially,” as did providing free telehealth platforms to practices with training on how to use them, Dr. Haft said.
‘Innovative and important’
Renu Tipirneni, MD, an assistant professor of internal medicine at the University of Michigan and at the Institute for Healthcare Policy and Innovation in Ann Arbor, said Maryland is out front putting into practice what practices nationwide aspire to do – coordinating physical and mental health and social needs and integrating primary and public health. Dr. Tipirneni, who was not involved with the study, said she was impressed the researchers were able to show statistically significant improvement with COVID-19 outcomes in the first 2 years.
“In terms of health outcomes, we often have to wait longer to see good outcomes,” she said. “It’s a really innovative and important model.”
She said states can learn from each other and this model is an example.
Integrating primary care and public health and addressing social needs may be the biggest challenges for states, she said, as those realms typically have been siloed.
“But they may be the key components to achieving these outcomes,” she said.
Take-home message
The most important benefit of the program is that data suggest it saves lives, according to Dr. Haft. While the actual difference between COVID deaths in the program and nonprogram groups was small, multiplying that savings across the nation shows substantial potential benefit, he explained.
“At a time when we were losing lives at an unconscionable rate, we were able to make a difference in saving lives,” Dr. Haft said.
Authors report no relevant financial disclosures.
The study received financial support from the Maryland Department of Health.
Dr. Tiperneni is helping evaluate Michigan’s Medicaid contract.
FROM JAMA NETWORK OPEN
Pediatric vaccination rates have failed to recover
I guess we shouldn’t be surprised that vaccination rates in this country fell during the frenzy created by the COVID pandemic. We had a lot on our plates. Schools closed and many of us retreated into what seemed to be the safety of our homes. Parents were reluctant to take their children anywhere, let alone a pediatrician’s office. State health agencies wisely focused on collecting case figures and then shepherding the efforts to immunize against SARS-CoV-2 once vaccines were available. Tracking and promoting the existing children’s vaccinations fell off the priority list, even in places with exemplary vaccination rates.
Whether or not the pandemic is over continues to be a topic for debate, but there is clearly a general shift toward a new normalcy. However, vaccination rates of our children have not rebounded to prepandemic levels. In fact, in some areas they are continuing to fall.
In a recent guest essay in the New York Times, Ezekiel J. Emmanuel, MD, PhD, a physician and professor of medical ethics and health policy at the University of Pennsylvania, and Matthew Guido, his research assistant, explore the reasons for this lack of a significant rebound. The authors cite recent outbreaks of measles in Ohio and polio in New York City as examples of the peril we are facing if we fail to reverse the trend. In some areas measles vaccine rates alarmingly have dipped below the threshold for herd immunity.
While Dr. Emmanuel and Mr. Guido acknowledge that the pandemic was a major driver of the falling vaccination rates they lay blame on the persistent decline on three factors that they view as correctable: nonmedical exemptions, our failure to vigorously enforce existing vaccine requirements, and inadequate public health campaigns.
The authors underestimate the lingering effect of the pandemic on parents’ vaccine hesitancy. As a septuagenarian who often hangs out with other septuagenarians I view the rapid development and effectiveness of the COVID vaccine as astounding and a boost for vaccines in general. However, were I much younger I might treat the vaccine’s success with a shrug. After some initial concern, the younger half of the population didn’t seem to see the illness as much of a threat to themselves or their peers. This attitude was reinforced by the fact that few of their peers, including those who were unvaccinated, were getting seriously ill. Despite all the hype, most parents and their children never ended up getting seriously ill.
You can understand why many parents might be quick to toss what you and I consider a successful COVID vaccine onto what they view as a growing pile of vaccines for diseases that in their experience have never sickened or killed anyone they have known.
Let’s be honest: Over the last half century we have produced several generations of parents who have little knowledge and certainly no personal experience with a childhood disease on the order or magnitude of polio. The vaccines that we have developed during their lifetimes have been targeted at diseases such as haemophilus influenzae meningitis that, while serious and anxiety provoking for pediatricians, occur so sporadically that most parents have no personal experience to motivate them to vaccinate their children.
Dr. Emmanuel and Mr. Guido are correct in advocating for the broader elimination of nonmedical exemptions and urging us to find the political will to vigorously enforce the vaccine requirements we have already enacted. I agree that our promotional campaigns need to be more robust. But, this will be a difficult challenge unless we can impress our audience with our straight talk and honesty. We must acknowledge and then explain why all vaccines are not created equal and that some are of more critical importance than others.
We are slowly learning that education isn’t the cure-all for vaccine hesitancy we once thought it was. And using scare tactics can backfire and create dysfunctional anxiety. We must choosing our words and target audience carefully. And ... having the political will to force parents into doing the right thing will be critical if we wish to restore our vaccination rates.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I guess we shouldn’t be surprised that vaccination rates in this country fell during the frenzy created by the COVID pandemic. We had a lot on our plates. Schools closed and many of us retreated into what seemed to be the safety of our homes. Parents were reluctant to take their children anywhere, let alone a pediatrician’s office. State health agencies wisely focused on collecting case figures and then shepherding the efforts to immunize against SARS-CoV-2 once vaccines were available. Tracking and promoting the existing children’s vaccinations fell off the priority list, even in places with exemplary vaccination rates.
Whether or not the pandemic is over continues to be a topic for debate, but there is clearly a general shift toward a new normalcy. However, vaccination rates of our children have not rebounded to prepandemic levels. In fact, in some areas they are continuing to fall.
In a recent guest essay in the New York Times, Ezekiel J. Emmanuel, MD, PhD, a physician and professor of medical ethics and health policy at the University of Pennsylvania, and Matthew Guido, his research assistant, explore the reasons for this lack of a significant rebound. The authors cite recent outbreaks of measles in Ohio and polio in New York City as examples of the peril we are facing if we fail to reverse the trend. In some areas measles vaccine rates alarmingly have dipped below the threshold for herd immunity.
While Dr. Emmanuel and Mr. Guido acknowledge that the pandemic was a major driver of the falling vaccination rates they lay blame on the persistent decline on three factors that they view as correctable: nonmedical exemptions, our failure to vigorously enforce existing vaccine requirements, and inadequate public health campaigns.
The authors underestimate the lingering effect of the pandemic on parents’ vaccine hesitancy. As a septuagenarian who often hangs out with other septuagenarians I view the rapid development and effectiveness of the COVID vaccine as astounding and a boost for vaccines in general. However, were I much younger I might treat the vaccine’s success with a shrug. After some initial concern, the younger half of the population didn’t seem to see the illness as much of a threat to themselves or their peers. This attitude was reinforced by the fact that few of their peers, including those who were unvaccinated, were getting seriously ill. Despite all the hype, most parents and their children never ended up getting seriously ill.
You can understand why many parents might be quick to toss what you and I consider a successful COVID vaccine onto what they view as a growing pile of vaccines for diseases that in their experience have never sickened or killed anyone they have known.
Let’s be honest: Over the last half century we have produced several generations of parents who have little knowledge and certainly no personal experience with a childhood disease on the order or magnitude of polio. The vaccines that we have developed during their lifetimes have been targeted at diseases such as haemophilus influenzae meningitis that, while serious and anxiety provoking for pediatricians, occur so sporadically that most parents have no personal experience to motivate them to vaccinate their children.
Dr. Emmanuel and Mr. Guido are correct in advocating for the broader elimination of nonmedical exemptions and urging us to find the political will to vigorously enforce the vaccine requirements we have already enacted. I agree that our promotional campaigns need to be more robust. But, this will be a difficult challenge unless we can impress our audience with our straight talk and honesty. We must acknowledge and then explain why all vaccines are not created equal and that some are of more critical importance than others.
We are slowly learning that education isn’t the cure-all for vaccine hesitancy we once thought it was. And using scare tactics can backfire and create dysfunctional anxiety. We must choosing our words and target audience carefully. And ... having the political will to force parents into doing the right thing will be critical if we wish to restore our vaccination rates.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I guess we shouldn’t be surprised that vaccination rates in this country fell during the frenzy created by the COVID pandemic. We had a lot on our plates. Schools closed and many of us retreated into what seemed to be the safety of our homes. Parents were reluctant to take their children anywhere, let alone a pediatrician’s office. State health agencies wisely focused on collecting case figures and then shepherding the efforts to immunize against SARS-CoV-2 once vaccines were available. Tracking and promoting the existing children’s vaccinations fell off the priority list, even in places with exemplary vaccination rates.
Whether or not the pandemic is over continues to be a topic for debate, but there is clearly a general shift toward a new normalcy. However, vaccination rates of our children have not rebounded to prepandemic levels. In fact, in some areas they are continuing to fall.
In a recent guest essay in the New York Times, Ezekiel J. Emmanuel, MD, PhD, a physician and professor of medical ethics and health policy at the University of Pennsylvania, and Matthew Guido, his research assistant, explore the reasons for this lack of a significant rebound. The authors cite recent outbreaks of measles in Ohio and polio in New York City as examples of the peril we are facing if we fail to reverse the trend. In some areas measles vaccine rates alarmingly have dipped below the threshold for herd immunity.
While Dr. Emmanuel and Mr. Guido acknowledge that the pandemic was a major driver of the falling vaccination rates they lay blame on the persistent decline on three factors that they view as correctable: nonmedical exemptions, our failure to vigorously enforce existing vaccine requirements, and inadequate public health campaigns.
The authors underestimate the lingering effect of the pandemic on parents’ vaccine hesitancy. As a septuagenarian who often hangs out with other septuagenarians I view the rapid development and effectiveness of the COVID vaccine as astounding and a boost for vaccines in general. However, were I much younger I might treat the vaccine’s success with a shrug. After some initial concern, the younger half of the population didn’t seem to see the illness as much of a threat to themselves or their peers. This attitude was reinforced by the fact that few of their peers, including those who were unvaccinated, were getting seriously ill. Despite all the hype, most parents and their children never ended up getting seriously ill.
You can understand why many parents might be quick to toss what you and I consider a successful COVID vaccine onto what they view as a growing pile of vaccines for diseases that in their experience have never sickened or killed anyone they have known.
Let’s be honest: Over the last half century we have produced several generations of parents who have little knowledge and certainly no personal experience with a childhood disease on the order or magnitude of polio. The vaccines that we have developed during their lifetimes have been targeted at diseases such as haemophilus influenzae meningitis that, while serious and anxiety provoking for pediatricians, occur so sporadically that most parents have no personal experience to motivate them to vaccinate their children.
Dr. Emmanuel and Mr. Guido are correct in advocating for the broader elimination of nonmedical exemptions and urging us to find the political will to vigorously enforce the vaccine requirements we have already enacted. I agree that our promotional campaigns need to be more robust. But, this will be a difficult challenge unless we can impress our audience with our straight talk and honesty. We must acknowledge and then explain why all vaccines are not created equal and that some are of more critical importance than others.
We are slowly learning that education isn’t the cure-all for vaccine hesitancy we once thought it was. And using scare tactics can backfire and create dysfunctional anxiety. We must choosing our words and target audience carefully. And ... having the political will to force parents into doing the right thing will be critical if we wish to restore our vaccination rates.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Berdazimer gel under review at FDA for treating molluscum contagiosum
, the manufacturer announced.
If the submission is accepted by the FDA, the topical product could be approved in the first quarter of 2024, according to a press release from Novan, the manufacturer. If approved, it would be the first-in-class topical treatment for MC, the common, contagious viral skin infection that affects approximately six million individuals in the United States each year, most of them children aged 1-14 years, the statement noted. No FDA-approved therapies currently exist for the condition, which causes unsightly lesions on the face, trunk, limbs, and axillae that may persist untreated for a period of years.
The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a nitric oxide–releasing agent. A 3.4% formulation is in development for the topical treatment of acne, according to the company.
The submission for FDA approval is based on data from the B-SIMPLE4 study, a phase 3 randomized trial of nearly 900 individuals with MC aged 6 months and older (mean age, 6.6 years), with 3-70 raised lesions. Participants were randomized to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily for 12 weeks. The results were published in JAMA Dermatology.
The primary outcome was complete clearance of all lesions. At 12 weeks, 32.4% of patients in the berdazimer group achieved this outcome vs. 19.7% of those in the vehicle group (P < .001). Overall adverse event rates were low in both groups; 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events across both groups were application-site pain and erythema, and most of these were mild or moderate.
, the manufacturer announced.
If the submission is accepted by the FDA, the topical product could be approved in the first quarter of 2024, according to a press release from Novan, the manufacturer. If approved, it would be the first-in-class topical treatment for MC, the common, contagious viral skin infection that affects approximately six million individuals in the United States each year, most of them children aged 1-14 years, the statement noted. No FDA-approved therapies currently exist for the condition, which causes unsightly lesions on the face, trunk, limbs, and axillae that may persist untreated for a period of years.
The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a nitric oxide–releasing agent. A 3.4% formulation is in development for the topical treatment of acne, according to the company.
The submission for FDA approval is based on data from the B-SIMPLE4 study, a phase 3 randomized trial of nearly 900 individuals with MC aged 6 months and older (mean age, 6.6 years), with 3-70 raised lesions. Participants were randomized to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily for 12 weeks. The results were published in JAMA Dermatology.
The primary outcome was complete clearance of all lesions. At 12 weeks, 32.4% of patients in the berdazimer group achieved this outcome vs. 19.7% of those in the vehicle group (P < .001). Overall adverse event rates were low in both groups; 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events across both groups were application-site pain and erythema, and most of these were mild or moderate.
, the manufacturer announced.
If the submission is accepted by the FDA, the topical product could be approved in the first quarter of 2024, according to a press release from Novan, the manufacturer. If approved, it would be the first-in-class topical treatment for MC, the common, contagious viral skin infection that affects approximately six million individuals in the United States each year, most of them children aged 1-14 years, the statement noted. No FDA-approved therapies currently exist for the condition, which causes unsightly lesions on the face, trunk, limbs, and axillae that may persist untreated for a period of years.
The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a nitric oxide–releasing agent. A 3.4% formulation is in development for the topical treatment of acne, according to the company.
The submission for FDA approval is based on data from the B-SIMPLE4 study, a phase 3 randomized trial of nearly 900 individuals with MC aged 6 months and older (mean age, 6.6 years), with 3-70 raised lesions. Participants were randomized to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily for 12 weeks. The results were published in JAMA Dermatology.
The primary outcome was complete clearance of all lesions. At 12 weeks, 32.4% of patients in the berdazimer group achieved this outcome vs. 19.7% of those in the vehicle group (P < .001). Overall adverse event rates were low in both groups; 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events across both groups were application-site pain and erythema, and most of these were mild or moderate.
Children and COVID: New cases fell as the old year ended
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.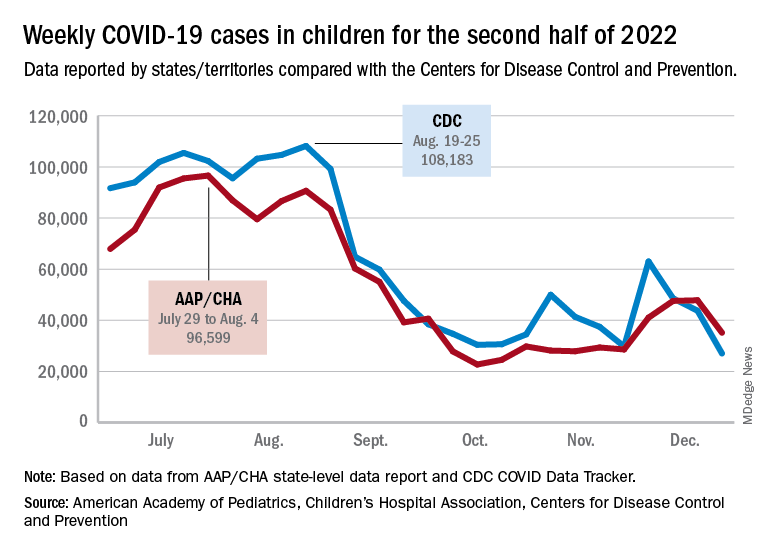
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
Latent TB: The case for vigilance
The US Preventive Services Task Force (USPSTF) recently released draft recommendations on screening for tuberculosis (TB).1 The USPSTF continues to recommend screening for latent TB infection (LTBI) in those at high risk.
Why is this important? Up to one-quarter of the world’s population has been infected with TB, according to World Health Organization (WHO) estimates. In 2021, active TB was diagnosed in 10.6 million people, and it caused 1.6 million deaths.2 Worldwide, TB is still a major cause of mortality: It is the 13th leading cause of death and is the leading cause of infectious disease mortality in non-COVID years.
Although the rate of active TB in the United States has been declining for decades (from 30.7/100,000 in 1960 to 2.4/100,000 in 2021), 7882 cases were reported in 2021, and an estimated 13 million people in the United States have LTBI.3 If not treated, 5% to 10% of LTBI cases will progress to active TB. This risk is higher in those with certain medical conditions.3 People born outside the United States currently account for 71.4% of reported TB cases in the United States.3
To reduce the morbidity and mortality of TB, the Centers for Disease Control and Prevention (CDC), WHO, and USPSTF all recommend screening for and treating LTBI. An effective approach to TB control also includes early detection and completion of treatment for active TB, as well as testing contacts of active TB cases.
Who should be screened? Those at high risk for LTBI include those who were born in, or who have resided in, countries with high rates of TB (eg, Latin America, the Caribbean, Africa, Asia, Eastern Europe, and Russia); those who have lived in a correctional facility or homeless shelter; household and other close contacts of active TB cases; and health care workers who provide care to patients with TB.
Some chronic medical conditions can increase risk for progression to active TB in those with LTBI. Patients who should be tested for LTBI as part of their routine care include those who are HIV positive; are receiving immunosuppressive therapy (chemotherapy, biological immune suppressants); have received an organ transplant; have silicosis; use illicit injected drugs; and/or have had a gastrectomy or jejunoileal bypass.
In addition, local communities may have populations or geographic regions in which TB rates are high. Family physicians can obtain this information from their state or local health departments.
There are 2 screening tests for LTBI: TB blood tests (interferon-gamma release assays [IGRAs]) and the Mantoux tuberculin skin test (TST). Two TB blood tests are available in the United States: QuantiFERON-TB Gold Plus (QFT-Plus) and T-SPOT.TB test (T-Spot).
There are advantages and disadvantages to both types of tests. A TST requires accurate administration and interpretation and 2 clinic visits, 48 to 72 hours apart. The cutoff on a positive test (5, 10, or 15 mm) depends on the patient’s age and risk.4 An IGRA should be processed within 8 to 32 hours and is more expensive. However, a major advantage is that it is more specific, because it is unaffected by previous vaccination with bacille Calmette-Guérin or by most nontuberculous mycobacteria infections.
To rule out active TB ... If a TB screening test is positive, the recommended work-up is to ask about TB symptoms and perform a chest x-ray to rule out active pulmonary TB. Sputum collection for acid-fast smear and culture should be ordered for anyone with a suspicious chest x-ray, respiratory symptoms consistent with TB, or HIV infection.
Treatment for LTBI markedly reduces the risk for active TB. There are 4 options:
- Isoniazid (INH) plus rifapentine (RPT) once per week for 3 months.
- Rifampin (RIF) daily for 4 months.
- INH plus RIF daily for 3 months.
- INH daily for 6 or 9 months.
Details about the variables to consider in choosing a regimen are described on the CDC website.4,5
Know your resources. Local and state public health departments should have TB control programs and are sources of information on TB diagnosis and treatment; they also can assist with follow-up of TB contacts.6 Although LTBI is a reportable condition only in young children, any suspicion of community spread of active TB should be reported to the public health department.
1. USPSTF. Latent tuberculosis infection in adults: screening. Draft recommendation statement. Published November 22, 2022. Accessed December 14, 2022. www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/latent-tuberculosis-infection-adults
2. WHO. Tuberculosis: key facts. Updated October 27, 2022. Accessed December 14, 2022. www.who.int/news-room/fact-sheets/detail/tuberculosis
3. CDC. Tuberculosis: data and statistics. Updated November 29, 2022. Accessed December 14, 2022. www.cdc.gov/tb/statistics/default.htm
4. CDC. Latent TB infection: a guide for primary health care providers. Updated February 3, 2021. Accessed December 14, 2022. www.cdc.gov/tb/publications/ltbi/pdf/LTBIbooklet508.pdf
5. CDC. Treatment regimens for latent TB infection. Updated February 13, 2020. Accessed December 14, 2022. www.cdc.gov/tb/topic/treatment/ltbi.htm
6. CDC. TB control offices. Updated March 28, 2022. Accessed December 14, 2022. www.cdc.gov/tb/links/tboffices.htm
The US Preventive Services Task Force (USPSTF) recently released draft recommendations on screening for tuberculosis (TB).1 The USPSTF continues to recommend screening for latent TB infection (LTBI) in those at high risk.
Why is this important? Up to one-quarter of the world’s population has been infected with TB, according to World Health Organization (WHO) estimates. In 2021, active TB was diagnosed in 10.6 million people, and it caused 1.6 million deaths.2 Worldwide, TB is still a major cause of mortality: It is the 13th leading cause of death and is the leading cause of infectious disease mortality in non-COVID years.
Although the rate of active TB in the United States has been declining for decades (from 30.7/100,000 in 1960 to 2.4/100,000 in 2021), 7882 cases were reported in 2021, and an estimated 13 million people in the United States have LTBI.3 If not treated, 5% to 10% of LTBI cases will progress to active TB. This risk is higher in those with certain medical conditions.3 People born outside the United States currently account for 71.4% of reported TB cases in the United States.3
To reduce the morbidity and mortality of TB, the Centers for Disease Control and Prevention (CDC), WHO, and USPSTF all recommend screening for and treating LTBI. An effective approach to TB control also includes early detection and completion of treatment for active TB, as well as testing contacts of active TB cases.
Who should be screened? Those at high risk for LTBI include those who were born in, or who have resided in, countries with high rates of TB (eg, Latin America, the Caribbean, Africa, Asia, Eastern Europe, and Russia); those who have lived in a correctional facility or homeless shelter; household and other close contacts of active TB cases; and health care workers who provide care to patients with TB.
Some chronic medical conditions can increase risk for progression to active TB in those with LTBI. Patients who should be tested for LTBI as part of their routine care include those who are HIV positive; are receiving immunosuppressive therapy (chemotherapy, biological immune suppressants); have received an organ transplant; have silicosis; use illicit injected drugs; and/or have had a gastrectomy or jejunoileal bypass.
In addition, local communities may have populations or geographic regions in which TB rates are high. Family physicians can obtain this information from their state or local health departments.
There are 2 screening tests for LTBI: TB blood tests (interferon-gamma release assays [IGRAs]) and the Mantoux tuberculin skin test (TST). Two TB blood tests are available in the United States: QuantiFERON-TB Gold Plus (QFT-Plus) and T-SPOT.TB test (T-Spot).
There are advantages and disadvantages to both types of tests. A TST requires accurate administration and interpretation and 2 clinic visits, 48 to 72 hours apart. The cutoff on a positive test (5, 10, or 15 mm) depends on the patient’s age and risk.4 An IGRA should be processed within 8 to 32 hours and is more expensive. However, a major advantage is that it is more specific, because it is unaffected by previous vaccination with bacille Calmette-Guérin or by most nontuberculous mycobacteria infections.
To rule out active TB ... If a TB screening test is positive, the recommended work-up is to ask about TB symptoms and perform a chest x-ray to rule out active pulmonary TB. Sputum collection for acid-fast smear and culture should be ordered for anyone with a suspicious chest x-ray, respiratory symptoms consistent with TB, or HIV infection.
Treatment for LTBI markedly reduces the risk for active TB. There are 4 options:
- Isoniazid (INH) plus rifapentine (RPT) once per week for 3 months.
- Rifampin (RIF) daily for 4 months.
- INH plus RIF daily for 3 months.
- INH daily for 6 or 9 months.
Details about the variables to consider in choosing a regimen are described on the CDC website.4,5
Know your resources. Local and state public health departments should have TB control programs and are sources of information on TB diagnosis and treatment; they also can assist with follow-up of TB contacts.6 Although LTBI is a reportable condition only in young children, any suspicion of community spread of active TB should be reported to the public health department.
The US Preventive Services Task Force (USPSTF) recently released draft recommendations on screening for tuberculosis (TB).1 The USPSTF continues to recommend screening for latent TB infection (LTBI) in those at high risk.
Why is this important? Up to one-quarter of the world’s population has been infected with TB, according to World Health Organization (WHO) estimates. In 2021, active TB was diagnosed in 10.6 million people, and it caused 1.6 million deaths.2 Worldwide, TB is still a major cause of mortality: It is the 13th leading cause of death and is the leading cause of infectious disease mortality in non-COVID years.
Although the rate of active TB in the United States has been declining for decades (from 30.7/100,000 in 1960 to 2.4/100,000 in 2021), 7882 cases were reported in 2021, and an estimated 13 million people in the United States have LTBI.3 If not treated, 5% to 10% of LTBI cases will progress to active TB. This risk is higher in those with certain medical conditions.3 People born outside the United States currently account for 71.4% of reported TB cases in the United States.3
To reduce the morbidity and mortality of TB, the Centers for Disease Control and Prevention (CDC), WHO, and USPSTF all recommend screening for and treating LTBI. An effective approach to TB control also includes early detection and completion of treatment for active TB, as well as testing contacts of active TB cases.
Who should be screened? Those at high risk for LTBI include those who were born in, or who have resided in, countries with high rates of TB (eg, Latin America, the Caribbean, Africa, Asia, Eastern Europe, and Russia); those who have lived in a correctional facility or homeless shelter; household and other close contacts of active TB cases; and health care workers who provide care to patients with TB.
Some chronic medical conditions can increase risk for progression to active TB in those with LTBI. Patients who should be tested for LTBI as part of their routine care include those who are HIV positive; are receiving immunosuppressive therapy (chemotherapy, biological immune suppressants); have received an organ transplant; have silicosis; use illicit injected drugs; and/or have had a gastrectomy or jejunoileal bypass.
In addition, local communities may have populations or geographic regions in which TB rates are high. Family physicians can obtain this information from their state or local health departments.
There are 2 screening tests for LTBI: TB blood tests (interferon-gamma release assays [IGRAs]) and the Mantoux tuberculin skin test (TST). Two TB blood tests are available in the United States: QuantiFERON-TB Gold Plus (QFT-Plus) and T-SPOT.TB test (T-Spot).
There are advantages and disadvantages to both types of tests. A TST requires accurate administration and interpretation and 2 clinic visits, 48 to 72 hours apart. The cutoff on a positive test (5, 10, or 15 mm) depends on the patient’s age and risk.4 An IGRA should be processed within 8 to 32 hours and is more expensive. However, a major advantage is that it is more specific, because it is unaffected by previous vaccination with bacille Calmette-Guérin or by most nontuberculous mycobacteria infections.
To rule out active TB ... If a TB screening test is positive, the recommended work-up is to ask about TB symptoms and perform a chest x-ray to rule out active pulmonary TB. Sputum collection for acid-fast smear and culture should be ordered for anyone with a suspicious chest x-ray, respiratory symptoms consistent with TB, or HIV infection.
Treatment for LTBI markedly reduces the risk for active TB. There are 4 options:
- Isoniazid (INH) plus rifapentine (RPT) once per week for 3 months.
- Rifampin (RIF) daily for 4 months.
- INH plus RIF daily for 3 months.
- INH daily for 6 or 9 months.
Details about the variables to consider in choosing a regimen are described on the CDC website.4,5
Know your resources. Local and state public health departments should have TB control programs and are sources of information on TB diagnosis and treatment; they also can assist with follow-up of TB contacts.6 Although LTBI is a reportable condition only in young children, any suspicion of community spread of active TB should be reported to the public health department.
1. USPSTF. Latent tuberculosis infection in adults: screening. Draft recommendation statement. Published November 22, 2022. Accessed December 14, 2022. www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/latent-tuberculosis-infection-adults
2. WHO. Tuberculosis: key facts. Updated October 27, 2022. Accessed December 14, 2022. www.who.int/news-room/fact-sheets/detail/tuberculosis
3. CDC. Tuberculosis: data and statistics. Updated November 29, 2022. Accessed December 14, 2022. www.cdc.gov/tb/statistics/default.htm
4. CDC. Latent TB infection: a guide for primary health care providers. Updated February 3, 2021. Accessed December 14, 2022. www.cdc.gov/tb/publications/ltbi/pdf/LTBIbooklet508.pdf
5. CDC. Treatment regimens for latent TB infection. Updated February 13, 2020. Accessed December 14, 2022. www.cdc.gov/tb/topic/treatment/ltbi.htm
6. CDC. TB control offices. Updated March 28, 2022. Accessed December 14, 2022. www.cdc.gov/tb/links/tboffices.htm
1. USPSTF. Latent tuberculosis infection in adults: screening. Draft recommendation statement. Published November 22, 2022. Accessed December 14, 2022. www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/latent-tuberculosis-infection-adults
2. WHO. Tuberculosis: key facts. Updated October 27, 2022. Accessed December 14, 2022. www.who.int/news-room/fact-sheets/detail/tuberculosis
3. CDC. Tuberculosis: data and statistics. Updated November 29, 2022. Accessed December 14, 2022. www.cdc.gov/tb/statistics/default.htm
4. CDC. Latent TB infection: a guide for primary health care providers. Updated February 3, 2021. Accessed December 14, 2022. www.cdc.gov/tb/publications/ltbi/pdf/LTBIbooklet508.pdf
5. CDC. Treatment regimens for latent TB infection. Updated February 13, 2020. Accessed December 14, 2022. www.cdc.gov/tb/topic/treatment/ltbi.htm
6. CDC. TB control offices. Updated March 28, 2022. Accessed December 14, 2022. www.cdc.gov/tb/links/tboffices.htm
Atypical Keratotic Nodule on the Knuckle
The Diagnosis: Atypical Mycobacterial Infection
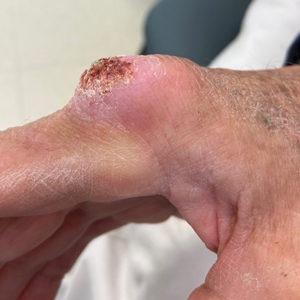
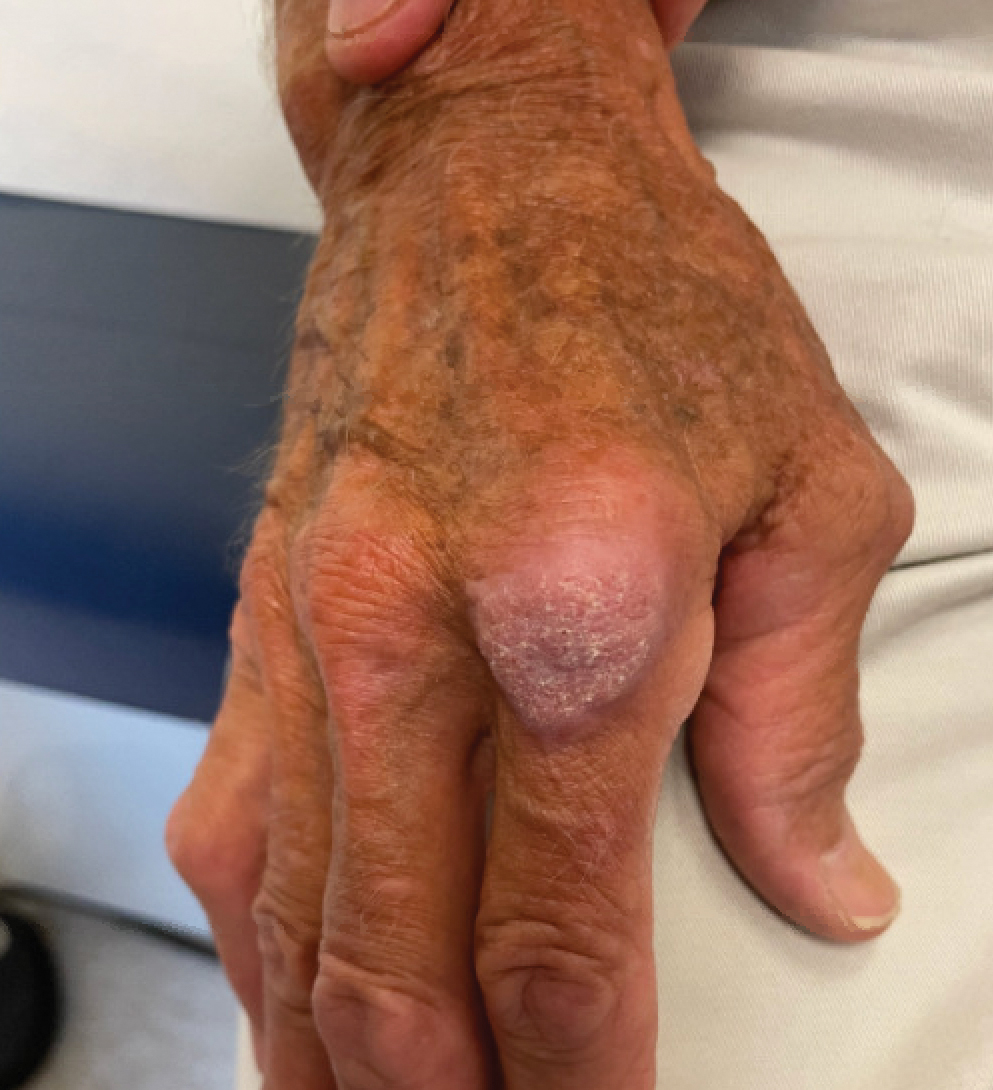
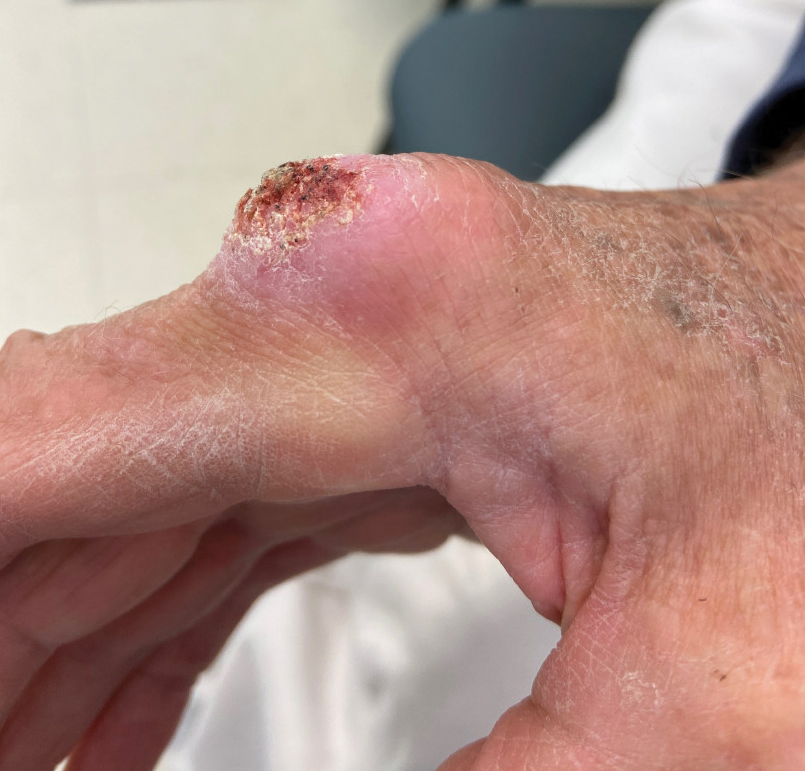
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.
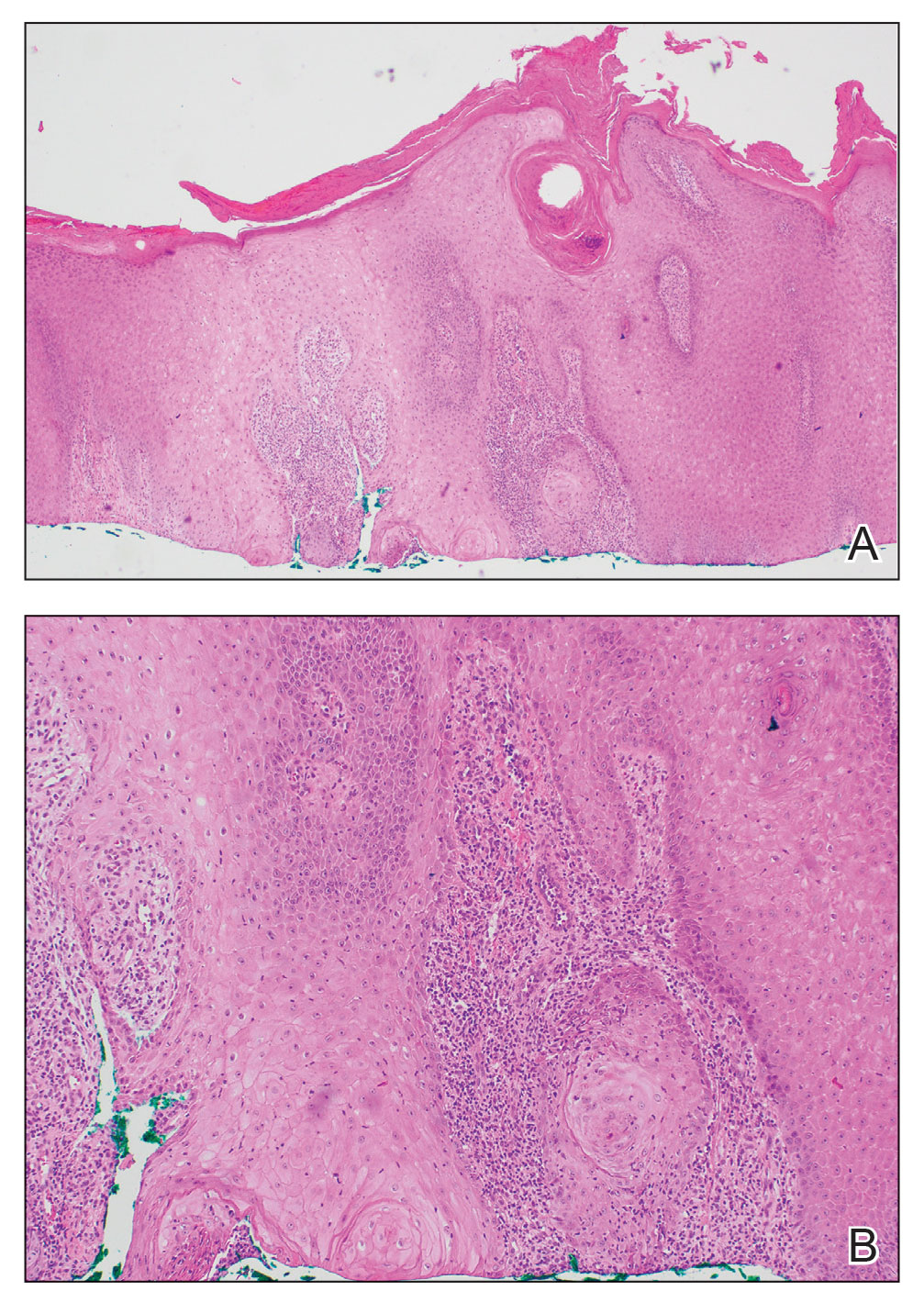
The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2
Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
The Diagnosis: Atypical Mycobacterial Infection
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.

The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2

Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
The Diagnosis: Atypical Mycobacterial Infection
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.

The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2

Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
A 75-year-old man presented with a lesion on the knuckle of 5 months’ duration. He reported that the lesion initially grew very quickly before shrinking down to its current size. He denied any bleeding or pain but thought he may have had a splinter in the area around the time the lesion appeared. He reported spending a lot of time outdoors and noted several recent insect and tick bites. He also owned a boat and frequently went fishing. He previously had been treated for actinic keratoses but had no history of skin cancer and no family history of melanoma. Physical examination revealed a 2-cm erythematous nodule with central hyperkeratosis overlying the metacarpophalangeal joint of the right index finger. A shave biopsy was performed.
Meningococcal B vaccine protects against gonorrhea
PARIS – All the way back in 1907, The Lancet published an article on a gonorrhea vaccine trial. Today, after continuous research throughout the intervening 110-plus years, scientists may finally have achieved success. Sébastien Fouéré, MD, discussed the details at a press conference that focused on the highlights of the Dermatology Days of Paris conference. Dr. Fouéré is the head of the genital dermatology and sexually transmitted infections unit at Saint-Louis Hospital, Paris.
Twin bacteria
Although the gonorrhea vaccine has long been the subject of research, Dr. Fouéré views 2017 as a turning point. This was when the results of a study led by Helen Petousis-Harris, PhD, were published.
“She tried to formalize the not completely indisputable results published by Cuba, where it seemed there were fewer gonococci in individuals vaccinated against meningococcal group B,” he noted.
Dr. Petousis-Harris, an immunologist, conducted a retrospective case-control study involving 11 clinics in New Zealand. The participants were aged 15-30 years, were eligible to receive the meningococcal B vaccine, and had been diagnosed with gonorrhea, chlamydia, or both. The researchers found that receiving the meningococcal B vaccine in childhood provides around 30% protection against Neisseria gonorrhoeae infections.
“It’s not perhaps a coincidence that a meningococcal B vaccine would be protective against gonorrhea,” Dr. Fouéré pointed out. He considers this protection logical, even expected, insofar as “meningococcus and gonococcus are almost twins.” There is 90% and 100% homology between membrane proteins of the two bacteria.
Vaccine is effective
Two retrospective case-control studies confirm that the vaccine is protective. One of the studies, carried out by an Australian team, found that the effectiveness was 32%, quite close to that reported by Petousis-Harris. In the other study, a U.S. team brought to light a dose-response relationship. while a complete vaccination series (two MenB-4C doses) was 40% effective.
Prospective studies are in progress, which will provide a higher level of evidence. The ANRS DOXYVAC trial has been underway since January 2021. The participants are men who have sex with men, who are highly exposed to the risk of sexually transmitted infections, and who presented with at least one STI in the year before their participation in the study. “The study is being conducted by Jean-Michel Molina of Saint-Louis Hospital. What they’re trying to do is protect our cohort of pre-exposure prophylaxis patients with meningococcal vaccine,” explained Dr. Fouéré.
Initial findings demonstrated the efficacy of a meningococcal B vaccine in reducing the risk of gonorrhea and the efficacy of doxycycline as preventive intervention for STIs when taken within 72 hours after sexual intercourse. In light of these results, a decision was made at the end of October to discontinue the trial and to recommend providing both interventions to all ANRS DOXYVAC participants. The follow-up of the participants will continue until the end of 2023. The results that led to stopping the study in its current form will be presented in early 2023.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
PARIS – All the way back in 1907, The Lancet published an article on a gonorrhea vaccine trial. Today, after continuous research throughout the intervening 110-plus years, scientists may finally have achieved success. Sébastien Fouéré, MD, discussed the details at a press conference that focused on the highlights of the Dermatology Days of Paris conference. Dr. Fouéré is the head of the genital dermatology and sexually transmitted infections unit at Saint-Louis Hospital, Paris.
Twin bacteria
Although the gonorrhea vaccine has long been the subject of research, Dr. Fouéré views 2017 as a turning point. This was when the results of a study led by Helen Petousis-Harris, PhD, were published.
“She tried to formalize the not completely indisputable results published by Cuba, where it seemed there were fewer gonococci in individuals vaccinated against meningococcal group B,” he noted.
Dr. Petousis-Harris, an immunologist, conducted a retrospective case-control study involving 11 clinics in New Zealand. The participants were aged 15-30 years, were eligible to receive the meningococcal B vaccine, and had been diagnosed with gonorrhea, chlamydia, or both. The researchers found that receiving the meningococcal B vaccine in childhood provides around 30% protection against Neisseria gonorrhoeae infections.
“It’s not perhaps a coincidence that a meningococcal B vaccine would be protective against gonorrhea,” Dr. Fouéré pointed out. He considers this protection logical, even expected, insofar as “meningococcus and gonococcus are almost twins.” There is 90% and 100% homology between membrane proteins of the two bacteria.
Vaccine is effective
Two retrospective case-control studies confirm that the vaccine is protective. One of the studies, carried out by an Australian team, found that the effectiveness was 32%, quite close to that reported by Petousis-Harris. In the other study, a U.S. team brought to light a dose-response relationship. while a complete vaccination series (two MenB-4C doses) was 40% effective.
Prospective studies are in progress, which will provide a higher level of evidence. The ANRS DOXYVAC trial has been underway since January 2021. The participants are men who have sex with men, who are highly exposed to the risk of sexually transmitted infections, and who presented with at least one STI in the year before their participation in the study. “The study is being conducted by Jean-Michel Molina of Saint-Louis Hospital. What they’re trying to do is protect our cohort of pre-exposure prophylaxis patients with meningococcal vaccine,” explained Dr. Fouéré.
Initial findings demonstrated the efficacy of a meningococcal B vaccine in reducing the risk of gonorrhea and the efficacy of doxycycline as preventive intervention for STIs when taken within 72 hours after sexual intercourse. In light of these results, a decision was made at the end of October to discontinue the trial and to recommend providing both interventions to all ANRS DOXYVAC participants. The follow-up of the participants will continue until the end of 2023. The results that led to stopping the study in its current form will be presented in early 2023.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
PARIS – All the way back in 1907, The Lancet published an article on a gonorrhea vaccine trial. Today, after continuous research throughout the intervening 110-plus years, scientists may finally have achieved success. Sébastien Fouéré, MD, discussed the details at a press conference that focused on the highlights of the Dermatology Days of Paris conference. Dr. Fouéré is the head of the genital dermatology and sexually transmitted infections unit at Saint-Louis Hospital, Paris.
Twin bacteria
Although the gonorrhea vaccine has long been the subject of research, Dr. Fouéré views 2017 as a turning point. This was when the results of a study led by Helen Petousis-Harris, PhD, were published.
“She tried to formalize the not completely indisputable results published by Cuba, where it seemed there were fewer gonococci in individuals vaccinated against meningococcal group B,” he noted.
Dr. Petousis-Harris, an immunologist, conducted a retrospective case-control study involving 11 clinics in New Zealand. The participants were aged 15-30 years, were eligible to receive the meningococcal B vaccine, and had been diagnosed with gonorrhea, chlamydia, or both. The researchers found that receiving the meningococcal B vaccine in childhood provides around 30% protection against Neisseria gonorrhoeae infections.
“It’s not perhaps a coincidence that a meningococcal B vaccine would be protective against gonorrhea,” Dr. Fouéré pointed out. He considers this protection logical, even expected, insofar as “meningococcus and gonococcus are almost twins.” There is 90% and 100% homology between membrane proteins of the two bacteria.
Vaccine is effective
Two retrospective case-control studies confirm that the vaccine is protective. One of the studies, carried out by an Australian team, found that the effectiveness was 32%, quite close to that reported by Petousis-Harris. In the other study, a U.S. team brought to light a dose-response relationship. while a complete vaccination series (two MenB-4C doses) was 40% effective.
Prospective studies are in progress, which will provide a higher level of evidence. The ANRS DOXYVAC trial has been underway since January 2021. The participants are men who have sex with men, who are highly exposed to the risk of sexually transmitted infections, and who presented with at least one STI in the year before their participation in the study. “The study is being conducted by Jean-Michel Molina of Saint-Louis Hospital. What they’re trying to do is protect our cohort of pre-exposure prophylaxis patients with meningococcal vaccine,” explained Dr. Fouéré.
Initial findings demonstrated the efficacy of a meningococcal B vaccine in reducing the risk of gonorrhea and the efficacy of doxycycline as preventive intervention for STIs when taken within 72 hours after sexual intercourse. In light of these results, a decision was made at the end of October to discontinue the trial and to recommend providing both interventions to all ANRS DOXYVAC participants. The follow-up of the participants will continue until the end of 2023. The results that led to stopping the study in its current form will be presented in early 2023.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
CDC reports uptick in invasive Strep A infections
Clinicians in the United States are reporting more cases of invasive group A streptococcal infection (iGAS) in children, according to an alert from the Centers for Disease Control and Prevention. These infections are rare but can be deadly, and they can affect adults as well as children.
including those with recent or co-occurring viral respiratory infections, the agency advised in a Dec. 22 alert.
In some cases, iGAS manifests as persistent or worsening symptoms after a patient with a known viral infection initially starts to show signs of improvement, according to the agency.
In November, the CDC was notified about a possible increase in cases of pediatric iGAS at a hospital in Colorado. Since then, two surveillance systems – the Infectious Diseases Society of America’s Emerging Infections Network and the CDC’s Active Bacterial Core Surveillance System – have detected potential increases in pediatric iGAS cases in other states.
The uptick has coincided with “increased circulation of respiratory syncytial virus (RSV), influenza viruses, SARS-CoV-2, and other respiratory viruses,” the advisory stated. “While the overall number of cases has remained relatively low and iGAS infections remain rare in children, [the] CDC is investigating these reports.”
Not just strep throat
Group A Streptococcus bacteria can cause strep throat and infections in skin and soft tissue. The pathogens also can lead to uncommon but severe diseases, such as sepsis, streptococcal toxic shock syndrome, and necrotizing fasciitis, according to the CDC. The severe illnesses “are associated with high mortality rates and require immediate treatment, including appropriate antibiotic therapy,” the agency said.
Groups at higher risk for iGAS include people aged 65 years or older, American Indian and Alaska Native populations, residents of long-term care facilities, those with wounds or skin disease, people who inject drugs, and people experiencing homelessness.
People with medical conditions such as diabetes, cancer, immunosuppression, and chronic kidney, heart, or respiratory disease also are at increased risk.
Invasive strep A infections initially decreased during the COVID-19 pandemic amid measures to reduce the spread of disease, such as masking and social distancing. But since September, monthly cases have exceeded those in 2020 and 2021. “It is too early to determine whether this rise is beyond what would be expected for pre-COVID” seasonal patterns, the CDC said.
Recommendations
Because iGAS can occur after the flu or chickenpox, health care providers should offer influenza and varicella vaccinations to all eligible people who are not up to date with their vaccines.
In addition, clinicians should educate patients about symptoms of iGAS that require urgent medical attention, including necrotizing fasciitis, cellulitis, and toxic shock syndrome.
They also should obtain cultures for suspected cases of iGAS as clinically indicated, follow guidelines for the diagnosis and treatment of strep throat, and be aware of alternative ways to treat strep throat in children amid a shortage of amoxicillin suspension.
Researchers have reported more cases of iGAS in the United Kingdom this year, as well. According to the UK Health Security Agency, 74 deaths, including 16 children, in England have been attributed to iGAS since September.
“We know that this is concerning for parents, but I want to stress that while we are seeing an increase in cases in children, this remains very uncommon,” UKHSA Deputy Director Colin Brown said in a news release. “There are lots of winter bugs circulating that can make your child feel unwell that mostly aren’t cause for alarm. However, make sure you talk to a health professional if your child is getting worse after a bout of scarlet fever, a sore throat, or respiratory infection.”
A fever that doesn’t resolve, dehydration, extreme tiredness, and difficulty breathing are signs to watch out for, Dr. Brown said.
A version of this article first appeared on Medscape.com.
Clinicians in the United States are reporting more cases of invasive group A streptococcal infection (iGAS) in children, according to an alert from the Centers for Disease Control and Prevention. These infections are rare but can be deadly, and they can affect adults as well as children.
including those with recent or co-occurring viral respiratory infections, the agency advised in a Dec. 22 alert.
In some cases, iGAS manifests as persistent or worsening symptoms after a patient with a known viral infection initially starts to show signs of improvement, according to the agency.
In November, the CDC was notified about a possible increase in cases of pediatric iGAS at a hospital in Colorado. Since then, two surveillance systems – the Infectious Diseases Society of America’s Emerging Infections Network and the CDC’s Active Bacterial Core Surveillance System – have detected potential increases in pediatric iGAS cases in other states.
The uptick has coincided with “increased circulation of respiratory syncytial virus (RSV), influenza viruses, SARS-CoV-2, and other respiratory viruses,” the advisory stated. “While the overall number of cases has remained relatively low and iGAS infections remain rare in children, [the] CDC is investigating these reports.”
Not just strep throat
Group A Streptococcus bacteria can cause strep throat and infections in skin and soft tissue. The pathogens also can lead to uncommon but severe diseases, such as sepsis, streptococcal toxic shock syndrome, and necrotizing fasciitis, according to the CDC. The severe illnesses “are associated with high mortality rates and require immediate treatment, including appropriate antibiotic therapy,” the agency said.
Groups at higher risk for iGAS include people aged 65 years or older, American Indian and Alaska Native populations, residents of long-term care facilities, those with wounds or skin disease, people who inject drugs, and people experiencing homelessness.
People with medical conditions such as diabetes, cancer, immunosuppression, and chronic kidney, heart, or respiratory disease also are at increased risk.
Invasive strep A infections initially decreased during the COVID-19 pandemic amid measures to reduce the spread of disease, such as masking and social distancing. But since September, monthly cases have exceeded those in 2020 and 2021. “It is too early to determine whether this rise is beyond what would be expected for pre-COVID” seasonal patterns, the CDC said.
Recommendations
Because iGAS can occur after the flu or chickenpox, health care providers should offer influenza and varicella vaccinations to all eligible people who are not up to date with their vaccines.
In addition, clinicians should educate patients about symptoms of iGAS that require urgent medical attention, including necrotizing fasciitis, cellulitis, and toxic shock syndrome.
They also should obtain cultures for suspected cases of iGAS as clinically indicated, follow guidelines for the diagnosis and treatment of strep throat, and be aware of alternative ways to treat strep throat in children amid a shortage of amoxicillin suspension.
Researchers have reported more cases of iGAS in the United Kingdom this year, as well. According to the UK Health Security Agency, 74 deaths, including 16 children, in England have been attributed to iGAS since September.
“We know that this is concerning for parents, but I want to stress that while we are seeing an increase in cases in children, this remains very uncommon,” UKHSA Deputy Director Colin Brown said in a news release. “There are lots of winter bugs circulating that can make your child feel unwell that mostly aren’t cause for alarm. However, make sure you talk to a health professional if your child is getting worse after a bout of scarlet fever, a sore throat, or respiratory infection.”
A fever that doesn’t resolve, dehydration, extreme tiredness, and difficulty breathing are signs to watch out for, Dr. Brown said.
A version of this article first appeared on Medscape.com.
Clinicians in the United States are reporting more cases of invasive group A streptococcal infection (iGAS) in children, according to an alert from the Centers for Disease Control and Prevention. These infections are rare but can be deadly, and they can affect adults as well as children.
including those with recent or co-occurring viral respiratory infections, the agency advised in a Dec. 22 alert.
In some cases, iGAS manifests as persistent or worsening symptoms after a patient with a known viral infection initially starts to show signs of improvement, according to the agency.
In November, the CDC was notified about a possible increase in cases of pediatric iGAS at a hospital in Colorado. Since then, two surveillance systems – the Infectious Diseases Society of America’s Emerging Infections Network and the CDC’s Active Bacterial Core Surveillance System – have detected potential increases in pediatric iGAS cases in other states.
The uptick has coincided with “increased circulation of respiratory syncytial virus (RSV), influenza viruses, SARS-CoV-2, and other respiratory viruses,” the advisory stated. “While the overall number of cases has remained relatively low and iGAS infections remain rare in children, [the] CDC is investigating these reports.”
Not just strep throat
Group A Streptococcus bacteria can cause strep throat and infections in skin and soft tissue. The pathogens also can lead to uncommon but severe diseases, such as sepsis, streptococcal toxic shock syndrome, and necrotizing fasciitis, according to the CDC. The severe illnesses “are associated with high mortality rates and require immediate treatment, including appropriate antibiotic therapy,” the agency said.
Groups at higher risk for iGAS include people aged 65 years or older, American Indian and Alaska Native populations, residents of long-term care facilities, those with wounds or skin disease, people who inject drugs, and people experiencing homelessness.
People with medical conditions such as diabetes, cancer, immunosuppression, and chronic kidney, heart, or respiratory disease also are at increased risk.
Invasive strep A infections initially decreased during the COVID-19 pandemic amid measures to reduce the spread of disease, such as masking and social distancing. But since September, monthly cases have exceeded those in 2020 and 2021. “It is too early to determine whether this rise is beyond what would be expected for pre-COVID” seasonal patterns, the CDC said.
Recommendations
Because iGAS can occur after the flu or chickenpox, health care providers should offer influenza and varicella vaccinations to all eligible people who are not up to date with their vaccines.
In addition, clinicians should educate patients about symptoms of iGAS that require urgent medical attention, including necrotizing fasciitis, cellulitis, and toxic shock syndrome.
They also should obtain cultures for suspected cases of iGAS as clinically indicated, follow guidelines for the diagnosis and treatment of strep throat, and be aware of alternative ways to treat strep throat in children amid a shortage of amoxicillin suspension.
Researchers have reported more cases of iGAS in the United Kingdom this year, as well. According to the UK Health Security Agency, 74 deaths, including 16 children, in England have been attributed to iGAS since September.
“We know that this is concerning for parents, but I want to stress that while we are seeing an increase in cases in children, this remains very uncommon,” UKHSA Deputy Director Colin Brown said in a news release. “There are lots of winter bugs circulating that can make your child feel unwell that mostly aren’t cause for alarm. However, make sure you talk to a health professional if your child is getting worse after a bout of scarlet fever, a sore throat, or respiratory infection.”
A fever that doesn’t resolve, dehydration, extreme tiredness, and difficulty breathing are signs to watch out for, Dr. Brown said.
A version of this article first appeared on Medscape.com.
FDA approves first-in-class drug for HIV
The U.S. Food and Drug Administration has approved the medication lenacapavir (Sunlenca) for adults living with multidrug resistant HIV-1 infection. .
“Following today’s decision from the FDA, lenacapavir helps to fill a critical unmet need for people with complex prior treatment histories and offers physicians a long-awaited twice-yearly option for these patients who otherwise have limited therapy choices,” said site principal investigator Sorana Segal-Maurer, MD, a professor of clinical medicine at Weill Cornell Medicine, New York, in a statement.
HIV drug regimens generally consist of two or three HIV medicines combined in a daily pill. In 2021, the FDA approved the first injectable complete drug regimen for HIV-1, Cabenuva, which can be administered monthly or every other month. Lenacapavir is administered only twice annually, but it is also combined with other antiretrovirals. The injections and oral tablets of lenacapavir are estimated to cost $42,250 in the first year of treatment and then $39,000 annually in the subsequent years, Reuters reported.
Lenacapavir is the first of a new class of drug called capsid inhibitors to be FDA-approved for treating HIV-1. The drug blocks the HIV-1 virus’s protein shell and interferes with essential steps of the virus’s evolution. The approval, announced today, was based on a multicenter clinical trial of 72 patients with multidrug resistant HIV-1 infection. After a year of the medication, 30 (83%) of the 36 patients randomly assigned to take lenacapavir, in combination with other HIV medications, had undetectable viral loads.
“Today’s approval ushers in a new class of antiretroviral drugs that may help patients with HIV who have run out of treatment options,” said Debra Birnkrant, MD, director of the division of antivirals in the FDA’s Center for Drug Evaluation and Research, in a press release. “The availability of new classes of antiretroviral medications may possibly help these patients live longer, healthier lives.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the medication lenacapavir (Sunlenca) for adults living with multidrug resistant HIV-1 infection. .
“Following today’s decision from the FDA, lenacapavir helps to fill a critical unmet need for people with complex prior treatment histories and offers physicians a long-awaited twice-yearly option for these patients who otherwise have limited therapy choices,” said site principal investigator Sorana Segal-Maurer, MD, a professor of clinical medicine at Weill Cornell Medicine, New York, in a statement.
HIV drug regimens generally consist of two or three HIV medicines combined in a daily pill. In 2021, the FDA approved the first injectable complete drug regimen for HIV-1, Cabenuva, which can be administered monthly or every other month. Lenacapavir is administered only twice annually, but it is also combined with other antiretrovirals. The injections and oral tablets of lenacapavir are estimated to cost $42,250 in the first year of treatment and then $39,000 annually in the subsequent years, Reuters reported.
Lenacapavir is the first of a new class of drug called capsid inhibitors to be FDA-approved for treating HIV-1. The drug blocks the HIV-1 virus’s protein shell and interferes with essential steps of the virus’s evolution. The approval, announced today, was based on a multicenter clinical trial of 72 patients with multidrug resistant HIV-1 infection. After a year of the medication, 30 (83%) of the 36 patients randomly assigned to take lenacapavir, in combination with other HIV medications, had undetectable viral loads.
“Today’s approval ushers in a new class of antiretroviral drugs that may help patients with HIV who have run out of treatment options,” said Debra Birnkrant, MD, director of the division of antivirals in the FDA’s Center for Drug Evaluation and Research, in a press release. “The availability of new classes of antiretroviral medications may possibly help these patients live longer, healthier lives.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the medication lenacapavir (Sunlenca) for adults living with multidrug resistant HIV-1 infection. .
“Following today’s decision from the FDA, lenacapavir helps to fill a critical unmet need for people with complex prior treatment histories and offers physicians a long-awaited twice-yearly option for these patients who otherwise have limited therapy choices,” said site principal investigator Sorana Segal-Maurer, MD, a professor of clinical medicine at Weill Cornell Medicine, New York, in a statement.
HIV drug regimens generally consist of two or three HIV medicines combined in a daily pill. In 2021, the FDA approved the first injectable complete drug regimen for HIV-1, Cabenuva, which can be administered monthly or every other month. Lenacapavir is administered only twice annually, but it is also combined with other antiretrovirals. The injections and oral tablets of lenacapavir are estimated to cost $42,250 in the first year of treatment and then $39,000 annually in the subsequent years, Reuters reported.
Lenacapavir is the first of a new class of drug called capsid inhibitors to be FDA-approved for treating HIV-1. The drug blocks the HIV-1 virus’s protein shell and interferes with essential steps of the virus’s evolution. The approval, announced today, was based on a multicenter clinical trial of 72 patients with multidrug resistant HIV-1 infection. After a year of the medication, 30 (83%) of the 36 patients randomly assigned to take lenacapavir, in combination with other HIV medications, had undetectable viral loads.
“Today’s approval ushers in a new class of antiretroviral drugs that may help patients with HIV who have run out of treatment options,” said Debra Birnkrant, MD, director of the division of antivirals in the FDA’s Center for Drug Evaluation and Research, in a press release. “The availability of new classes of antiretroviral medications may possibly help these patients live longer, healthier lives.”
A version of this article first appeared on Medscape.com.
AAP offers new guidance on child exploitation and sex trafficking
In a new updated report, the American Academy of Pediatrics urges pediatricians to understand signs of exploitation and labor/sex trafficking and learn how to support children and adolescents who are targeted.
“It’s incredibly scary when you encounter someone you worry is a victim, and you don’t know how to help them, and they’re not saying what’s going on,” pediatrician and report coauthor Dana Kaplan, MD, of Staten Island (N.Y.) University Hospital, said in an interview. “Every case is so unique and different: There’s no algorithm of ‘If A, then B, then C.’ You have to approach each person as an individual, and it takes time to make sure you’re thinking things through about how to provide what’s needed.”
The AAP published the clinical report, which is intended to provide guidance to pediatricians, in the January 2023 issue of Pediatrics. The organization previously tackled this topic in a 2017 clinical report, and Dr. Kaplan said the new report includes updated recommendations.
As the new report notes, there aren’t reliable estimates of exploited children in the United States, although millions are thought to be trafficked and subjected to forced labor around the world. “By virtue of their young age, children and adolescents are vulnerable to manipulation and exploitation, because they have limited life experiences, a need for attachment and acceptance, an immature prefrontal cortex ... and limited options for action,” the report says.
Dr. Kaplan puts it this way: “By the nature of being a child, you’re vulnerable.”
Still, health care professionals often aren’t trained in regard to human trafficking, the report says, even though it’s clear that they “must remain alert for the possibility.”
Dr. Kaplan, who has special training in child abuse and often sees children at risk, cautioned that children usually don’t directly say that they need help. “That’s generally not the case. They don’t articulate what’s going on around them as unsafe, or concerning, or dangerous. If you go and see a doctor for 10 minutes, are you going to tell them everything?
Instead, clinicians must often rely on their own observations. The report lists multiple possible signs of exploitation.
- The patient is accompanied by a domineering adult who does not allow the child to answer questions or accompanied by an unrelated adult. Inconsistent information is provided by the patient or companion. There’s a delay in seeking medical care.
- The patient has multiple sexually transmitted infections, previous pregnancy or termination, and/or frequent visits for emergency contraception. There are signs of prior sexual abuse, assault, or other maltreatment.
- The patient is withdrawn, fearful, hostile, or has a suspicious demeanor. The patient is constantly checking his or her phone and appears anxious or afraid.
What should clinicians do if they suspect exploitation? The report recommends that health care organizations develop guidelines for workers to follow. For her part, Dr. Kaplan advises colleagues to let patients lead conversations and not dig too deeply into their lives.
“Don’t turn into an investigator. This is not [Law & Order] SVU,” she said. “Stay focused on what you’re trained to do – provide health care.”
That doesn’t mean clinicians should ignore signs of trouble. It’s crucial to develop trust with the patient over time, she said, and turn to a specialist in your community or institution if you have suspicions.
And be careful to not portray victims as perpetrators. The new report emphasizes that “it’s important for health care providers to emphasize to authorities that the patient is a victim of exploitation who needs services rather than a juvenile offender.”
The report also highlights the importance of creating an environment that supports clinicians themselves: “Self-care for the clinician is critical in preventing and addressing secondary traumatic stress. A work environment that fosters peer support, encourages open discussion of work-related stress, and implements reasonable work-life balance policies can help protect providers from secondary stress and its consequences.”
Resources for clinicians include the National Human Trafficking Hotline, the federal Office of Trafficking in Persons, and the Centers for Disease Control and Prevention’s domestic refugee screening guidelines.
The study has no external funding. The authors report no disclosures.
In a new updated report, the American Academy of Pediatrics urges pediatricians to understand signs of exploitation and labor/sex trafficking and learn how to support children and adolescents who are targeted.
“It’s incredibly scary when you encounter someone you worry is a victim, and you don’t know how to help them, and they’re not saying what’s going on,” pediatrician and report coauthor Dana Kaplan, MD, of Staten Island (N.Y.) University Hospital, said in an interview. “Every case is so unique and different: There’s no algorithm of ‘If A, then B, then C.’ You have to approach each person as an individual, and it takes time to make sure you’re thinking things through about how to provide what’s needed.”
The AAP published the clinical report, which is intended to provide guidance to pediatricians, in the January 2023 issue of Pediatrics. The organization previously tackled this topic in a 2017 clinical report, and Dr. Kaplan said the new report includes updated recommendations.
As the new report notes, there aren’t reliable estimates of exploited children in the United States, although millions are thought to be trafficked and subjected to forced labor around the world. “By virtue of their young age, children and adolescents are vulnerable to manipulation and exploitation, because they have limited life experiences, a need for attachment and acceptance, an immature prefrontal cortex ... and limited options for action,” the report says.
Dr. Kaplan puts it this way: “By the nature of being a child, you’re vulnerable.”
Still, health care professionals often aren’t trained in regard to human trafficking, the report says, even though it’s clear that they “must remain alert for the possibility.”
Dr. Kaplan, who has special training in child abuse and often sees children at risk, cautioned that children usually don’t directly say that they need help. “That’s generally not the case. They don’t articulate what’s going on around them as unsafe, or concerning, or dangerous. If you go and see a doctor for 10 minutes, are you going to tell them everything?
Instead, clinicians must often rely on their own observations. The report lists multiple possible signs of exploitation.
- The patient is accompanied by a domineering adult who does not allow the child to answer questions or accompanied by an unrelated adult. Inconsistent information is provided by the patient or companion. There’s a delay in seeking medical care.
- The patient has multiple sexually transmitted infections, previous pregnancy or termination, and/or frequent visits for emergency contraception. There are signs of prior sexual abuse, assault, or other maltreatment.
- The patient is withdrawn, fearful, hostile, or has a suspicious demeanor. The patient is constantly checking his or her phone and appears anxious or afraid.
What should clinicians do if they suspect exploitation? The report recommends that health care organizations develop guidelines for workers to follow. For her part, Dr. Kaplan advises colleagues to let patients lead conversations and not dig too deeply into their lives.
“Don’t turn into an investigator. This is not [Law & Order] SVU,” she said. “Stay focused on what you’re trained to do – provide health care.”
That doesn’t mean clinicians should ignore signs of trouble. It’s crucial to develop trust with the patient over time, she said, and turn to a specialist in your community or institution if you have suspicions.
And be careful to not portray victims as perpetrators. The new report emphasizes that “it’s important for health care providers to emphasize to authorities that the patient is a victim of exploitation who needs services rather than a juvenile offender.”
The report also highlights the importance of creating an environment that supports clinicians themselves: “Self-care for the clinician is critical in preventing and addressing secondary traumatic stress. A work environment that fosters peer support, encourages open discussion of work-related stress, and implements reasonable work-life balance policies can help protect providers from secondary stress and its consequences.”
Resources for clinicians include the National Human Trafficking Hotline, the federal Office of Trafficking in Persons, and the Centers for Disease Control and Prevention’s domestic refugee screening guidelines.
The study has no external funding. The authors report no disclosures.
In a new updated report, the American Academy of Pediatrics urges pediatricians to understand signs of exploitation and labor/sex trafficking and learn how to support children and adolescents who are targeted.
“It’s incredibly scary when you encounter someone you worry is a victim, and you don’t know how to help them, and they’re not saying what’s going on,” pediatrician and report coauthor Dana Kaplan, MD, of Staten Island (N.Y.) University Hospital, said in an interview. “Every case is so unique and different: There’s no algorithm of ‘If A, then B, then C.’ You have to approach each person as an individual, and it takes time to make sure you’re thinking things through about how to provide what’s needed.”
The AAP published the clinical report, which is intended to provide guidance to pediatricians, in the January 2023 issue of Pediatrics. The organization previously tackled this topic in a 2017 clinical report, and Dr. Kaplan said the new report includes updated recommendations.
As the new report notes, there aren’t reliable estimates of exploited children in the United States, although millions are thought to be trafficked and subjected to forced labor around the world. “By virtue of their young age, children and adolescents are vulnerable to manipulation and exploitation, because they have limited life experiences, a need for attachment and acceptance, an immature prefrontal cortex ... and limited options for action,” the report says.
Dr. Kaplan puts it this way: “By the nature of being a child, you’re vulnerable.”
Still, health care professionals often aren’t trained in regard to human trafficking, the report says, even though it’s clear that they “must remain alert for the possibility.”
Dr. Kaplan, who has special training in child abuse and often sees children at risk, cautioned that children usually don’t directly say that they need help. “That’s generally not the case. They don’t articulate what’s going on around them as unsafe, or concerning, or dangerous. If you go and see a doctor for 10 minutes, are you going to tell them everything?
Instead, clinicians must often rely on their own observations. The report lists multiple possible signs of exploitation.
- The patient is accompanied by a domineering adult who does not allow the child to answer questions or accompanied by an unrelated adult. Inconsistent information is provided by the patient or companion. There’s a delay in seeking medical care.
- The patient has multiple sexually transmitted infections, previous pregnancy or termination, and/or frequent visits for emergency contraception. There are signs of prior sexual abuse, assault, or other maltreatment.
- The patient is withdrawn, fearful, hostile, or has a suspicious demeanor. The patient is constantly checking his or her phone and appears anxious or afraid.
What should clinicians do if they suspect exploitation? The report recommends that health care organizations develop guidelines for workers to follow. For her part, Dr. Kaplan advises colleagues to let patients lead conversations and not dig too deeply into their lives.
“Don’t turn into an investigator. This is not [Law & Order] SVU,” she said. “Stay focused on what you’re trained to do – provide health care.”
That doesn’t mean clinicians should ignore signs of trouble. It’s crucial to develop trust with the patient over time, she said, and turn to a specialist in your community or institution if you have suspicions.
And be careful to not portray victims as perpetrators. The new report emphasizes that “it’s important for health care providers to emphasize to authorities that the patient is a victim of exploitation who needs services rather than a juvenile offender.”
The report also highlights the importance of creating an environment that supports clinicians themselves: “Self-care for the clinician is critical in preventing and addressing secondary traumatic stress. A work environment that fosters peer support, encourages open discussion of work-related stress, and implements reasonable work-life balance policies can help protect providers from secondary stress and its consequences.”
Resources for clinicians include the National Human Trafficking Hotline, the federal Office of Trafficking in Persons, and the Centers for Disease Control and Prevention’s domestic refugee screening guidelines.
The study has no external funding. The authors report no disclosures.
FROM PEDIATRICS