User login
The Safety and Efficacy of AUC/MIC-Guided vs Trough-Guided Vancomycin Monitoring Among Veterans
Vancomycin is a commonly used glycopeptide antibiotic used to treat infections caused by gram-positive organisms. Vancomycin is most often used as a parenteral agent for empiric or definitive treatment of methicillin-resistant Staphylococcus aureus (MRSA). It can also be used for the treatment of other susceptible Staphylococcus or Enterococcus species. Adverse effects of parenteral vancomycin include infusion-related reactions, ototoxicity, and nephrotoxicity.1 Higher vancomycin trough levels have been associated with an increased risk of nephrotoxicity.1-4 The major safety concern with vancomycin is acute kidney injury (AKI). Even mild AKI can prolong hospitalizations, increase the cost of health care, and increase morbidity.2
In March 2020, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America (IDSA), the Pediatric Infectious Disease Society, and the Society of Infectious Diseases Pharmacists released a consensus statement and guidelines regarding the optimization of vancomycin dosing and monitoring for patients with suspected or definitive serious MRSA infections. Based on these guidelines, it is recommended to target an individualized area under the curve/minimum inhibitory concentration (AUC/MIC) ratio of 400 to 600 mg × h/L to maximize clinical efficacy and minimize the risk of AKI.2
Before March 2020, the vancomycin monitoring recommendation was to target trough levels of 10 to 20 mg/L. A goal trough of 15 to 20 mg/L was recommended for severe infections, including sepsis, endocarditis, hospital-acquired pneumonia, meningitis, and osteomyelitis, caused by MRSA. A goal trough of 10 to 15 mg/L was recommended for noninvasive infections, such as skin and soft tissue infections and urinary tract infections, caused by MRSA. Targeting these trough levels was thought to achieve an AUC/MIC ≥ 400 mg × h/L.5 Evidence has since shown that trough values may not be an optimal marker for AUC/MIC values.2
The updated vancomycin therapeutic drug monitoring (TDM) guidelines recommend that health systems transition to AUC/MIC-guided monitoring for suspected or confirmed infections caused by MRSA. There is not enough evidence to recommend AUC/MIC-guided monitoring in patients with noninvasive infections or infections caused by other microbes.2
AUC/MIC-guided monitoring can be achieved in 2 ways. The first method is collecting Cmax (peak level) and Cmin (trough level) serum concentrations, preferably during the same dosing interval. Ideally, Cmax should be drawn 1 to 2 hours after the vancomycin infusion and Cmin should be drawn at the end of the dosing interval. First-order pharmacokinetic equations are used to estimate the AUC/MIC with this method. Bayesian software pharmacokinetic modeling based on 1 or 2 vancomycin concentrations with 1 trough level also can be used for monitoring. Preferably, 2 levels would be obtained to estimate the AUC/MIC when using Bayesian modeling.2
The bactericidal activity of vancomycin was achieved with AUC/MIC ratios of ≥ 400 mg × h/L. AUC/MIC ratios of < 400 mg × h/L increase the incidence of resistant and intermediate strains of S aureus. AUC/MIC-guided monitoring assumes an MIC of 1 mg/L. When the MIC is > 1 mg/L, it is less likely that an AUC/MIC ≥ 400 mg × h/L is achievable. Regardless of the TDM method used, AUC/MIC ratios ≥ 400 mg × h/L are not achievable with conventional dosing methods if the vancomycin MIC is > 2 mg/L in patients with normal renal function. Alternative therapy is recommended to be used for these patients.2
There are multiple studies investigating the therapeutic dosing of vancomycin and the associated incidence of AKI. Previous studies have correlated vancomycin AUC/MICs of 400 mg to 600 mg × h/L with clinical effectiveness.2,6 In 2017, Neely and colleagues looked at the therapeutic dosing of vancomycin in 252 adults with ≥ 1 vancomycin level.7 During this prospective trial, they evaluated patients for 1 year and targeted trough concentrations of 10 to 20 mg/L with infection-specific goal ranges of 10 to 15 mg/L and 15 to 20 mg/L for noninvasive and invasive infections, respectively. They also targeted AUC/MIC ratios ≥ 400 mg × h/L regardless of trough concentration using Bayesian estimated AUC/MICs for 2 years. They found only 19% of trough concentrations to be therapeutic compared with 70% of AUC/MICs. A secondary outcome assessed by Neely and colleagues was nephrotoxicity, which was identified in 8% of patients with trough targets and 2% of patients with AUC/MIC targets.8
Previous studies evaluating the use of vancomycin in the veteran population have focused on AKI incidence, general nephrotoxicity, and 30-day readmission rates.4,7,9,10 Poston-Blahnik and colleagues investigated the rates of AKI in 200 veterans using AUC/MIC-guided vancomycin TDM.5 They found an AKI incidence of 42% of patients with AUC/MICs ≥ 550 mg × h/L and 2% of patients with AUC/MICs < 550 mg × h/L.5 Gyamlani and colleagues investigated the rates of AKI in 33,527 veterans and found that serum vancomycin trough levels ≥ 20 mg/L were associated with a higher risk of AKI.8 Prabaker and colleagues investigated the association between vancomycin trough levels and nephrotoxicity, defined as 0.5 mg/L or a 50% increase in serum creatinine (sCr) in 348 veterans. They found nephrotoxicity in 8.9% of patients.10 Patel and colleagues investigated the effect of AKI on 30-day readmission rates in 216 veterans.10 AKI occurred in 8.8% of patients and of those 19.4% were readmitted within 30 days.10 Current literature lacks evidence regarding the comparison of the safety and efficacy of vancomycin trough-guided vs AUC/MIC-guided TDM in the veteran population. Therefore, the objective of this study was to investigate the differences in the safety and efficacy of vancomycin TDM in the veteran population based on the different monitoring methods used.
METHODS
This study was a retrospective, single-center, quasi-experimental chart review conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS) in South Dakota. Data were collected from the Computerized Patient Record System (CPRS). The SFVAHCS transitioned from trough-guided to AUC/MIC-guided TDM in November 2020.
Patients included in this study were veterans aged ≥ 18 years with orders for parenteral vancomycin between February 1, 2020, and October 31, 2020, for the trough-guided TDM group and between December 1, 2020, and August 31, 2021, for the AUC/MIC-guided TDM group. Patients with vancomycin courses initiated during November 2020 were excluded as both TDM methods were being used at that time. Patients were excluded if their vancomycin course began before February 1, 2020, for the trough-guided TDM group or began during November 2020 for the AUC/MIC-guided TDM group. Patients were excluded if their vancomycin course extended past October 31, 2020, for the trough group or past August 31, 2021, for the AUC/MIC group. Patients on dialysis or missing Cmax, Cmin, or sCr levels were excluded.
This study evaluated both safety (AKI incidence) and effectiveness (time spent in therapeutic range and time to therapeutic range). The primary endpoint was presence of vancomycin-induced AKI, which was based on the most recent Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition: increased sCr of ≥ 0.3 mg/dL or by 50% from baseline sustained over 48 hours without any other explanation for the change.11 A secondary endpoint was the absence or presence of AKI.
Additional secondary endpoints included the presence of the initial trough or AUC/MIC of each vancomycin course within the therapeutic range and the percentage of all trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges. The therapeutic range for AUC/MIC-guided TDM was 400 to 600 mg × h/L and 10 to 20 mg/L depending on indication for trough-guided TDM (15-20 mg/L for severe infections and 10-15 mg/L for less invasive infections). The percentage of trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges were calculated as a ratio of levels within each range to total levels taken for each patient.
For AUC/MIC-guided TDM the Cmax levels were ideally drawn 1 to 2 hours after vancomycin infusion and Cmin levels were ideally drawn 30 minutes before the next dose. First-order pharmacokinetic equations were used to estimate the AUC/MIC.12 If the timing of a vancomycin level was inappropriate, actual levels were extrapolated based on the timing of the blood draw compared with the ideal Cmin or Cmax time. Extrapolated levels were used for both trough-guided and AUC/MIC-guided TDM groups when appropriate. Vancomycin levels were excluded if they were drawn during the vancomycin infusion.
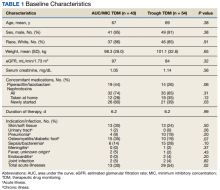
Study participant age, sex, race, weight, baseline estimated glomerular filtration (eGFR) rate, baseline sCr, concomitant nephrotoxic medications, duration of vancomycin course, indication of vancomycin, and acuity of illness based on indication were collected. sCr levels were collected from the initial day vancomycin was ordered through 72 hours following completion of a vancomycin course to evaluate for AKI. Patients’ charts were reviewed for the use of the following nephrotoxic medications: nonsteroidal anti-inflammatories, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aminoglycosides, piperacillin/tazobactam, loop diuretics, amphotericin B, acyclovir, intravenous contrast, and nephrotoxic chemotherapy (cisplatin). The category of concomitant nephrotoxic medications was also collected including the continuation of a home nephrotoxic medication vs the initiation of a new nephrotoxic medication.
Statistical Analysis
The primary endpoint of the incidence of vancomycin-induced AKI was compared using a Fisher exact test. The secondary endpoint of the percentage of trough levels or AUC/MICs in the therapeutic, subtherapeutic, and supratherapeutic range were compared using a student t test. The secondary endpoint of first level or AUC/MIC within goal range was compared using a χ2 test. Continuous baseline characteristics were reported as a mean and compared using a student t test. Nominal baseline characteristics were reported as a percentage and compared using the χ2 test. P values < .05 were considered statistically significant.
RESULTS
This study included 97 patients, 43 in the AUC/MIC group and 54 in the trough group.
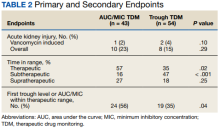
One (2%) patient in the AUC/MIC group and 2 (4%) patients in the trough group experienced vancomycin-induced AKI (P = .10) (Table 2).
DISCUSSION
There was no statistically significant difference between the 2 groups for the vancomycin-induced AKI (P = .10), the primary endpoint, or overall AKI (P = .29), the secondary endpoint. It should be noted that there was more overall AKI in the AUC/MIC group. Veterans in the AUC/MIC group were found to have their first AUC/MIC within the therapeutic range statistically significantly more often than the first trough level in the trough group (P = .04). The percentage of time spent within therapeutic range was statistically significantly higher in the AUC/MIC-guided TDM group (P = .02). The percentage of time spent subtherapeutic of goal range was statistically significantly higher in the trough-guided TDM group (P < .001). There was no statistically significant difference found in the percent of time spent supratherapeutic of goal range (P = .25). However, the observed percentage of time spent supratherapeutic of goal range was higher in the AUC/MIC group. These results indicate that AUC/MIC-guided TDM may be more efficacious with regard to time in therapeutic range and time to therapeutic range.
The finding of increased AKI with AUC/MIC-guided TDM does not align with previous studies.8 The prospective study by Neely and colleagues found that AUC/MIC-guided TDM resulted in more time in the therapeutic range as well as less nephrotoxicity compared with trough-guided TDM, although it was limited by its lack of randomization and did not account for other causes of nephrotoxicity.8 They found that only 19% of trough concentrations were therapeutic compared with 70% of AUC/MICs and found nephrotoxicity in 8% of trough-guided TDM patients compared with 2% of AUC/MIC-guided TDM patients.8
Unlike Nealy and colleagues, our study did not find lower nephrotoxicity associated with AUC/MIC-guided TDM. Multiple factors may have influenced our results. Our AUC/MIC group had significantly more newly started concomitant nephrotoxins and other nephrotoxic medications used during the vancomycin courses compared with the trough-guided group, which may have influenced AKI outcomes. It also should be noted that there was significantly more time spent subtherapeutic of the goal range and significantly less time in the goal range in the trough group compared with the AUC/MIC group. In our study, the trough-guided group had significantly more patients with acute illness compared with the AUC/MIC group (skin, soft tissue, and joint infections were similar between the groups). The group with more acutely ill patients would have been expected to have more nephrotoxicity. However, despite the acute illnesses, patients in the trough-guided group spent more time in the subtherapeutic range. This may explain the increased nephrotoxicity in the AUC/MIC group since those patients spent more time in the therapeutic range.
This study used the most recent KDIGO AKI definition: either an increase in sCr of ≥ 0.3 mg/dL or a 50% increase in sCr from baseline sustained over 48 hours without any other explanation for the change in renal function.11 This AKI definition is stricter than the previous definition, which was used by earlier studies, including Neely and colleagues, to evaluate rates of vancomycin-induced AKI.2,3 Therefore, the rates of overall AKI found in this study may be higher than in previous studies due to the definition of AKI used.
Limitations
This study was limited by its retrospective nature, lack of randomization, and small sample size. To decrease the potential for error in this study, analysis of power and a larger study sample would have been beneficial. During the COVID-19 pandemic, increased pneumonia cases may have hidden bacterial causes and caused an undercount. Nephrotoxicity may also be related to volume depletion, severe systemic illness, dehydration, or hypotension. Screening was completed via chart review for these alternative causes of nephrotoxicity in this study but may not be completely accounted for due to lack of documentation and the retrospective nature of this study.
CONCLUSIONS
This study did not find a significant difference in the rates of vancomycin-induced or overall AKI between AUC/MIC-guided and trough-guided TDM. However, this study may not have been powered to detect a significant difference in the primary endpoint. This study indicated that AUC/MIC-guided TDM of vancomycin resulted in a quicker time to the therapeutic range and a higher percentage of overall time in the therapeutic range as compared with trough-guided TDM. The results of this study indicated that trough-guided monitoring resulted in a higher percentage of time in a subtherapeutic range. This study also found that the first AUC/MIC calculated was within therapeutic range more often than the first trough level collected.
These results indicate that AUC/MIC-guided TDM may be more effective than trough-guided TDM in the veteran population. However, while AUC/MIC-guided TDM may be more effective with regards to time in therapeutic range and time to therapeutic range, this study did not indicate any safety benefit of AUC/MIC-guided over trough-guided TDM with regards to AKI incidence. Our data indicate that AUC/MIC-guided TDM increases the amount of time in the therapeutic range compared with trough-guided TDM and is not more nephrotoxic. The findings of this study support the recommendation to transition to the use of AUC/MIC-guided TDM of vancomycin in the veteran population.
Acknowledgments
This material is the result of work supported with the use of facilities and resources from the Sioux Falls Veterans Affairs Health Care System.
1. Gallagher J, MacDougall C. Glycopeptides and short-acting lipoglycopeptides In: Antibiotics Simplified. Jones & Bartlett Learning; 2018.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9-14. doi:10.1517/14740330903413514
4. Poston-Blahnik A, Moenster R. Association between vancomycin area under the curve and nephrotoxicity: a single center, retrospective cohort study in a veteran population. Open Forum Infect Dis. 2021;8(5):ofab094. Published 2021 Mar 12. doi:10.1093/ofid/ofab094
5. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. doi:10.2146/ajhp080434
6. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925-942. doi:10.2165/00003088-200443130-00005
7. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-Associated Acute Kidney Injury in a Large Veteran Population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
8. Neely MN, Kato L, Youn G, et al. Prospective Trial on the Use of Trough Concentration versus Area under the Curve To Determine Therapeutic Vancomycin Dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. Published 2018 Jan 25. doi:10.1128/AAC.02042-17
9. Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J Hosp Med. 2012;7(2):91-97. doi:10.1002/jhm.946
10. Patel N, Stornelli N, Sangiovanni RJ, Huang DB, Lodise TP. Effect of vancomycin-associated acute kidney injury on incidence of 30-day readmissions among hospitalized Veterans Affairs patients with skin and skin structure infections. Antimicrob Agents Chemother. 2020;64(10):e01268-20. Published 2020 Sep 21. doi:10.1128/AAC.01268-20
11. Acute Kidney Injury Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(suppl 1):1-138.
12. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50-57. doi:10.1016/j.addr.2014.05.016
Vancomycin is a commonly used glycopeptide antibiotic used to treat infections caused by gram-positive organisms. Vancomycin is most often used as a parenteral agent for empiric or definitive treatment of methicillin-resistant Staphylococcus aureus (MRSA). It can also be used for the treatment of other susceptible Staphylococcus or Enterococcus species. Adverse effects of parenteral vancomycin include infusion-related reactions, ototoxicity, and nephrotoxicity.1 Higher vancomycin trough levels have been associated with an increased risk of nephrotoxicity.1-4 The major safety concern with vancomycin is acute kidney injury (AKI). Even mild AKI can prolong hospitalizations, increase the cost of health care, and increase morbidity.2
In March 2020, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America (IDSA), the Pediatric Infectious Disease Society, and the Society of Infectious Diseases Pharmacists released a consensus statement and guidelines regarding the optimization of vancomycin dosing and monitoring for patients with suspected or definitive serious MRSA infections. Based on these guidelines, it is recommended to target an individualized area under the curve/minimum inhibitory concentration (AUC/MIC) ratio of 400 to 600 mg × h/L to maximize clinical efficacy and minimize the risk of AKI.2
Before March 2020, the vancomycin monitoring recommendation was to target trough levels of 10 to 20 mg/L. A goal trough of 15 to 20 mg/L was recommended for severe infections, including sepsis, endocarditis, hospital-acquired pneumonia, meningitis, and osteomyelitis, caused by MRSA. A goal trough of 10 to 15 mg/L was recommended for noninvasive infections, such as skin and soft tissue infections and urinary tract infections, caused by MRSA. Targeting these trough levels was thought to achieve an AUC/MIC ≥ 400 mg × h/L.5 Evidence has since shown that trough values may not be an optimal marker for AUC/MIC values.2
The updated vancomycin therapeutic drug monitoring (TDM) guidelines recommend that health systems transition to AUC/MIC-guided monitoring for suspected or confirmed infections caused by MRSA. There is not enough evidence to recommend AUC/MIC-guided monitoring in patients with noninvasive infections or infections caused by other microbes.2
AUC/MIC-guided monitoring can be achieved in 2 ways. The first method is collecting Cmax (peak level) and Cmin (trough level) serum concentrations, preferably during the same dosing interval. Ideally, Cmax should be drawn 1 to 2 hours after the vancomycin infusion and Cmin should be drawn at the end of the dosing interval. First-order pharmacokinetic equations are used to estimate the AUC/MIC with this method. Bayesian software pharmacokinetic modeling based on 1 or 2 vancomycin concentrations with 1 trough level also can be used for monitoring. Preferably, 2 levels would be obtained to estimate the AUC/MIC when using Bayesian modeling.2
The bactericidal activity of vancomycin was achieved with AUC/MIC ratios of ≥ 400 mg × h/L. AUC/MIC ratios of < 400 mg × h/L increase the incidence of resistant and intermediate strains of S aureus. AUC/MIC-guided monitoring assumes an MIC of 1 mg/L. When the MIC is > 1 mg/L, it is less likely that an AUC/MIC ≥ 400 mg × h/L is achievable. Regardless of the TDM method used, AUC/MIC ratios ≥ 400 mg × h/L are not achievable with conventional dosing methods if the vancomycin MIC is > 2 mg/L in patients with normal renal function. Alternative therapy is recommended to be used for these patients.2
There are multiple studies investigating the therapeutic dosing of vancomycin and the associated incidence of AKI. Previous studies have correlated vancomycin AUC/MICs of 400 mg to 600 mg × h/L with clinical effectiveness.2,6 In 2017, Neely and colleagues looked at the therapeutic dosing of vancomycin in 252 adults with ≥ 1 vancomycin level.7 During this prospective trial, they evaluated patients for 1 year and targeted trough concentrations of 10 to 20 mg/L with infection-specific goal ranges of 10 to 15 mg/L and 15 to 20 mg/L for noninvasive and invasive infections, respectively. They also targeted AUC/MIC ratios ≥ 400 mg × h/L regardless of trough concentration using Bayesian estimated AUC/MICs for 2 years. They found only 19% of trough concentrations to be therapeutic compared with 70% of AUC/MICs. A secondary outcome assessed by Neely and colleagues was nephrotoxicity, which was identified in 8% of patients with trough targets and 2% of patients with AUC/MIC targets.8
Previous studies evaluating the use of vancomycin in the veteran population have focused on AKI incidence, general nephrotoxicity, and 30-day readmission rates.4,7,9,10 Poston-Blahnik and colleagues investigated the rates of AKI in 200 veterans using AUC/MIC-guided vancomycin TDM.5 They found an AKI incidence of 42% of patients with AUC/MICs ≥ 550 mg × h/L and 2% of patients with AUC/MICs < 550 mg × h/L.5 Gyamlani and colleagues investigated the rates of AKI in 33,527 veterans and found that serum vancomycin trough levels ≥ 20 mg/L were associated with a higher risk of AKI.8 Prabaker and colleagues investigated the association between vancomycin trough levels and nephrotoxicity, defined as 0.5 mg/L or a 50% increase in serum creatinine (sCr) in 348 veterans. They found nephrotoxicity in 8.9% of patients.10 Patel and colleagues investigated the effect of AKI on 30-day readmission rates in 216 veterans.10 AKI occurred in 8.8% of patients and of those 19.4% were readmitted within 30 days.10 Current literature lacks evidence regarding the comparison of the safety and efficacy of vancomycin trough-guided vs AUC/MIC-guided TDM in the veteran population. Therefore, the objective of this study was to investigate the differences in the safety and efficacy of vancomycin TDM in the veteran population based on the different monitoring methods used.
METHODS
This study was a retrospective, single-center, quasi-experimental chart review conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS) in South Dakota. Data were collected from the Computerized Patient Record System (CPRS). The SFVAHCS transitioned from trough-guided to AUC/MIC-guided TDM in November 2020.
Patients included in this study were veterans aged ≥ 18 years with orders for parenteral vancomycin between February 1, 2020, and October 31, 2020, for the trough-guided TDM group and between December 1, 2020, and August 31, 2021, for the AUC/MIC-guided TDM group. Patients with vancomycin courses initiated during November 2020 were excluded as both TDM methods were being used at that time. Patients were excluded if their vancomycin course began before February 1, 2020, for the trough-guided TDM group or began during November 2020 for the AUC/MIC-guided TDM group. Patients were excluded if their vancomycin course extended past October 31, 2020, for the trough group or past August 31, 2021, for the AUC/MIC group. Patients on dialysis or missing Cmax, Cmin, or sCr levels were excluded.
This study evaluated both safety (AKI incidence) and effectiveness (time spent in therapeutic range and time to therapeutic range). The primary endpoint was presence of vancomycin-induced AKI, which was based on the most recent Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition: increased sCr of ≥ 0.3 mg/dL or by 50% from baseline sustained over 48 hours without any other explanation for the change.11 A secondary endpoint was the absence or presence of AKI.
Additional secondary endpoints included the presence of the initial trough or AUC/MIC of each vancomycin course within the therapeutic range and the percentage of all trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges. The therapeutic range for AUC/MIC-guided TDM was 400 to 600 mg × h/L and 10 to 20 mg/L depending on indication for trough-guided TDM (15-20 mg/L for severe infections and 10-15 mg/L for less invasive infections). The percentage of trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges were calculated as a ratio of levels within each range to total levels taken for each patient.
For AUC/MIC-guided TDM the Cmax levels were ideally drawn 1 to 2 hours after vancomycin infusion and Cmin levels were ideally drawn 30 minutes before the next dose. First-order pharmacokinetic equations were used to estimate the AUC/MIC.12 If the timing of a vancomycin level was inappropriate, actual levels were extrapolated based on the timing of the blood draw compared with the ideal Cmin or Cmax time. Extrapolated levels were used for both trough-guided and AUC/MIC-guided TDM groups when appropriate. Vancomycin levels were excluded if they were drawn during the vancomycin infusion.
Study participant age, sex, race, weight, baseline estimated glomerular filtration (eGFR) rate, baseline sCr, concomitant nephrotoxic medications, duration of vancomycin course, indication of vancomycin, and acuity of illness based on indication were collected. sCr levels were collected from the initial day vancomycin was ordered through 72 hours following completion of a vancomycin course to evaluate for AKI. Patients’ charts were reviewed for the use of the following nephrotoxic medications: nonsteroidal anti-inflammatories, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aminoglycosides, piperacillin/tazobactam, loop diuretics, amphotericin B, acyclovir, intravenous contrast, and nephrotoxic chemotherapy (cisplatin). The category of concomitant nephrotoxic medications was also collected including the continuation of a home nephrotoxic medication vs the initiation of a new nephrotoxic medication.
Statistical Analysis
The primary endpoint of the incidence of vancomycin-induced AKI was compared using a Fisher exact test. The secondary endpoint of the percentage of trough levels or AUC/MICs in the therapeutic, subtherapeutic, and supratherapeutic range were compared using a student t test. The secondary endpoint of first level or AUC/MIC within goal range was compared using a χ2 test. Continuous baseline characteristics were reported as a mean and compared using a student t test. Nominal baseline characteristics were reported as a percentage and compared using the χ2 test. P values < .05 were considered statistically significant.
RESULTS
This study included 97 patients, 43 in the AUC/MIC group and 54 in the trough group.
One (2%) patient in the AUC/MIC group and 2 (4%) patients in the trough group experienced vancomycin-induced AKI (P = .10) (Table 2).
DISCUSSION
There was no statistically significant difference between the 2 groups for the vancomycin-induced AKI (P = .10), the primary endpoint, or overall AKI (P = .29), the secondary endpoint. It should be noted that there was more overall AKI in the AUC/MIC group. Veterans in the AUC/MIC group were found to have their first AUC/MIC within the therapeutic range statistically significantly more often than the first trough level in the trough group (P = .04). The percentage of time spent within therapeutic range was statistically significantly higher in the AUC/MIC-guided TDM group (P = .02). The percentage of time spent subtherapeutic of goal range was statistically significantly higher in the trough-guided TDM group (P < .001). There was no statistically significant difference found in the percent of time spent supratherapeutic of goal range (P = .25). However, the observed percentage of time spent supratherapeutic of goal range was higher in the AUC/MIC group. These results indicate that AUC/MIC-guided TDM may be more efficacious with regard to time in therapeutic range and time to therapeutic range.
The finding of increased AKI with AUC/MIC-guided TDM does not align with previous studies.8 The prospective study by Neely and colleagues found that AUC/MIC-guided TDM resulted in more time in the therapeutic range as well as less nephrotoxicity compared with trough-guided TDM, although it was limited by its lack of randomization and did not account for other causes of nephrotoxicity.8 They found that only 19% of trough concentrations were therapeutic compared with 70% of AUC/MICs and found nephrotoxicity in 8% of trough-guided TDM patients compared with 2% of AUC/MIC-guided TDM patients.8
Unlike Nealy and colleagues, our study did not find lower nephrotoxicity associated with AUC/MIC-guided TDM. Multiple factors may have influenced our results. Our AUC/MIC group had significantly more newly started concomitant nephrotoxins and other nephrotoxic medications used during the vancomycin courses compared with the trough-guided group, which may have influenced AKI outcomes. It also should be noted that there was significantly more time spent subtherapeutic of the goal range and significantly less time in the goal range in the trough group compared with the AUC/MIC group. In our study, the trough-guided group had significantly more patients with acute illness compared with the AUC/MIC group (skin, soft tissue, and joint infections were similar between the groups). The group with more acutely ill patients would have been expected to have more nephrotoxicity. However, despite the acute illnesses, patients in the trough-guided group spent more time in the subtherapeutic range. This may explain the increased nephrotoxicity in the AUC/MIC group since those patients spent more time in the therapeutic range.
This study used the most recent KDIGO AKI definition: either an increase in sCr of ≥ 0.3 mg/dL or a 50% increase in sCr from baseline sustained over 48 hours without any other explanation for the change in renal function.11 This AKI definition is stricter than the previous definition, which was used by earlier studies, including Neely and colleagues, to evaluate rates of vancomycin-induced AKI.2,3 Therefore, the rates of overall AKI found in this study may be higher than in previous studies due to the definition of AKI used.
Limitations
This study was limited by its retrospective nature, lack of randomization, and small sample size. To decrease the potential for error in this study, analysis of power and a larger study sample would have been beneficial. During the COVID-19 pandemic, increased pneumonia cases may have hidden bacterial causes and caused an undercount. Nephrotoxicity may also be related to volume depletion, severe systemic illness, dehydration, or hypotension. Screening was completed via chart review for these alternative causes of nephrotoxicity in this study but may not be completely accounted for due to lack of documentation and the retrospective nature of this study.
CONCLUSIONS
This study did not find a significant difference in the rates of vancomycin-induced or overall AKI between AUC/MIC-guided and trough-guided TDM. However, this study may not have been powered to detect a significant difference in the primary endpoint. This study indicated that AUC/MIC-guided TDM of vancomycin resulted in a quicker time to the therapeutic range and a higher percentage of overall time in the therapeutic range as compared with trough-guided TDM. The results of this study indicated that trough-guided monitoring resulted in a higher percentage of time in a subtherapeutic range. This study also found that the first AUC/MIC calculated was within therapeutic range more often than the first trough level collected.
These results indicate that AUC/MIC-guided TDM may be more effective than trough-guided TDM in the veteran population. However, while AUC/MIC-guided TDM may be more effective with regards to time in therapeutic range and time to therapeutic range, this study did not indicate any safety benefit of AUC/MIC-guided over trough-guided TDM with regards to AKI incidence. Our data indicate that AUC/MIC-guided TDM increases the amount of time in the therapeutic range compared with trough-guided TDM and is not more nephrotoxic. The findings of this study support the recommendation to transition to the use of AUC/MIC-guided TDM of vancomycin in the veteran population.
Acknowledgments
This material is the result of work supported with the use of facilities and resources from the Sioux Falls Veterans Affairs Health Care System.
Vancomycin is a commonly used glycopeptide antibiotic used to treat infections caused by gram-positive organisms. Vancomycin is most often used as a parenteral agent for empiric or definitive treatment of methicillin-resistant Staphylococcus aureus (MRSA). It can also be used for the treatment of other susceptible Staphylococcus or Enterococcus species. Adverse effects of parenteral vancomycin include infusion-related reactions, ototoxicity, and nephrotoxicity.1 Higher vancomycin trough levels have been associated with an increased risk of nephrotoxicity.1-4 The major safety concern with vancomycin is acute kidney injury (AKI). Even mild AKI can prolong hospitalizations, increase the cost of health care, and increase morbidity.2
In March 2020, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America (IDSA), the Pediatric Infectious Disease Society, and the Society of Infectious Diseases Pharmacists released a consensus statement and guidelines regarding the optimization of vancomycin dosing and monitoring for patients with suspected or definitive serious MRSA infections. Based on these guidelines, it is recommended to target an individualized area under the curve/minimum inhibitory concentration (AUC/MIC) ratio of 400 to 600 mg × h/L to maximize clinical efficacy and minimize the risk of AKI.2
Before March 2020, the vancomycin monitoring recommendation was to target trough levels of 10 to 20 mg/L. A goal trough of 15 to 20 mg/L was recommended for severe infections, including sepsis, endocarditis, hospital-acquired pneumonia, meningitis, and osteomyelitis, caused by MRSA. A goal trough of 10 to 15 mg/L was recommended for noninvasive infections, such as skin and soft tissue infections and urinary tract infections, caused by MRSA. Targeting these trough levels was thought to achieve an AUC/MIC ≥ 400 mg × h/L.5 Evidence has since shown that trough values may not be an optimal marker for AUC/MIC values.2
The updated vancomycin therapeutic drug monitoring (TDM) guidelines recommend that health systems transition to AUC/MIC-guided monitoring for suspected or confirmed infections caused by MRSA. There is not enough evidence to recommend AUC/MIC-guided monitoring in patients with noninvasive infections or infections caused by other microbes.2
AUC/MIC-guided monitoring can be achieved in 2 ways. The first method is collecting Cmax (peak level) and Cmin (trough level) serum concentrations, preferably during the same dosing interval. Ideally, Cmax should be drawn 1 to 2 hours after the vancomycin infusion and Cmin should be drawn at the end of the dosing interval. First-order pharmacokinetic equations are used to estimate the AUC/MIC with this method. Bayesian software pharmacokinetic modeling based on 1 or 2 vancomycin concentrations with 1 trough level also can be used for monitoring. Preferably, 2 levels would be obtained to estimate the AUC/MIC when using Bayesian modeling.2
The bactericidal activity of vancomycin was achieved with AUC/MIC ratios of ≥ 400 mg × h/L. AUC/MIC ratios of < 400 mg × h/L increase the incidence of resistant and intermediate strains of S aureus. AUC/MIC-guided monitoring assumes an MIC of 1 mg/L. When the MIC is > 1 mg/L, it is less likely that an AUC/MIC ≥ 400 mg × h/L is achievable. Regardless of the TDM method used, AUC/MIC ratios ≥ 400 mg × h/L are not achievable with conventional dosing methods if the vancomycin MIC is > 2 mg/L in patients with normal renal function. Alternative therapy is recommended to be used for these patients.2
There are multiple studies investigating the therapeutic dosing of vancomycin and the associated incidence of AKI. Previous studies have correlated vancomycin AUC/MICs of 400 mg to 600 mg × h/L with clinical effectiveness.2,6 In 2017, Neely and colleagues looked at the therapeutic dosing of vancomycin in 252 adults with ≥ 1 vancomycin level.7 During this prospective trial, they evaluated patients for 1 year and targeted trough concentrations of 10 to 20 mg/L with infection-specific goal ranges of 10 to 15 mg/L and 15 to 20 mg/L for noninvasive and invasive infections, respectively. They also targeted AUC/MIC ratios ≥ 400 mg × h/L regardless of trough concentration using Bayesian estimated AUC/MICs for 2 years. They found only 19% of trough concentrations to be therapeutic compared with 70% of AUC/MICs. A secondary outcome assessed by Neely and colleagues was nephrotoxicity, which was identified in 8% of patients with trough targets and 2% of patients with AUC/MIC targets.8
Previous studies evaluating the use of vancomycin in the veteran population have focused on AKI incidence, general nephrotoxicity, and 30-day readmission rates.4,7,9,10 Poston-Blahnik and colleagues investigated the rates of AKI in 200 veterans using AUC/MIC-guided vancomycin TDM.5 They found an AKI incidence of 42% of patients with AUC/MICs ≥ 550 mg × h/L and 2% of patients with AUC/MICs < 550 mg × h/L.5 Gyamlani and colleagues investigated the rates of AKI in 33,527 veterans and found that serum vancomycin trough levels ≥ 20 mg/L were associated with a higher risk of AKI.8 Prabaker and colleagues investigated the association between vancomycin trough levels and nephrotoxicity, defined as 0.5 mg/L or a 50% increase in serum creatinine (sCr) in 348 veterans. They found nephrotoxicity in 8.9% of patients.10 Patel and colleagues investigated the effect of AKI on 30-day readmission rates in 216 veterans.10 AKI occurred in 8.8% of patients and of those 19.4% were readmitted within 30 days.10 Current literature lacks evidence regarding the comparison of the safety and efficacy of vancomycin trough-guided vs AUC/MIC-guided TDM in the veteran population. Therefore, the objective of this study was to investigate the differences in the safety and efficacy of vancomycin TDM in the veteran population based on the different monitoring methods used.
METHODS
This study was a retrospective, single-center, quasi-experimental chart review conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS) in South Dakota. Data were collected from the Computerized Patient Record System (CPRS). The SFVAHCS transitioned from trough-guided to AUC/MIC-guided TDM in November 2020.
Patients included in this study were veterans aged ≥ 18 years with orders for parenteral vancomycin between February 1, 2020, and October 31, 2020, for the trough-guided TDM group and between December 1, 2020, and August 31, 2021, for the AUC/MIC-guided TDM group. Patients with vancomycin courses initiated during November 2020 were excluded as both TDM methods were being used at that time. Patients were excluded if their vancomycin course began before February 1, 2020, for the trough-guided TDM group or began during November 2020 for the AUC/MIC-guided TDM group. Patients were excluded if their vancomycin course extended past October 31, 2020, for the trough group or past August 31, 2021, for the AUC/MIC group. Patients on dialysis or missing Cmax, Cmin, or sCr levels were excluded.
This study evaluated both safety (AKI incidence) and effectiveness (time spent in therapeutic range and time to therapeutic range). The primary endpoint was presence of vancomycin-induced AKI, which was based on the most recent Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition: increased sCr of ≥ 0.3 mg/dL or by 50% from baseline sustained over 48 hours without any other explanation for the change.11 A secondary endpoint was the absence or presence of AKI.
Additional secondary endpoints included the presence of the initial trough or AUC/MIC of each vancomycin course within the therapeutic range and the percentage of all trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges. The therapeutic range for AUC/MIC-guided TDM was 400 to 600 mg × h/L and 10 to 20 mg/L depending on indication for trough-guided TDM (15-20 mg/L for severe infections and 10-15 mg/L for less invasive infections). The percentage of trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges were calculated as a ratio of levels within each range to total levels taken for each patient.
For AUC/MIC-guided TDM the Cmax levels were ideally drawn 1 to 2 hours after vancomycin infusion and Cmin levels were ideally drawn 30 minutes before the next dose. First-order pharmacokinetic equations were used to estimate the AUC/MIC.12 If the timing of a vancomycin level was inappropriate, actual levels were extrapolated based on the timing of the blood draw compared with the ideal Cmin or Cmax time. Extrapolated levels were used for both trough-guided and AUC/MIC-guided TDM groups when appropriate. Vancomycin levels were excluded if they were drawn during the vancomycin infusion.
Study participant age, sex, race, weight, baseline estimated glomerular filtration (eGFR) rate, baseline sCr, concomitant nephrotoxic medications, duration of vancomycin course, indication of vancomycin, and acuity of illness based on indication were collected. sCr levels were collected from the initial day vancomycin was ordered through 72 hours following completion of a vancomycin course to evaluate for AKI. Patients’ charts were reviewed for the use of the following nephrotoxic medications: nonsteroidal anti-inflammatories, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aminoglycosides, piperacillin/tazobactam, loop diuretics, amphotericin B, acyclovir, intravenous contrast, and nephrotoxic chemotherapy (cisplatin). The category of concomitant nephrotoxic medications was also collected including the continuation of a home nephrotoxic medication vs the initiation of a new nephrotoxic medication.
Statistical Analysis
The primary endpoint of the incidence of vancomycin-induced AKI was compared using a Fisher exact test. The secondary endpoint of the percentage of trough levels or AUC/MICs in the therapeutic, subtherapeutic, and supratherapeutic range were compared using a student t test. The secondary endpoint of first level or AUC/MIC within goal range was compared using a χ2 test. Continuous baseline characteristics were reported as a mean and compared using a student t test. Nominal baseline characteristics were reported as a percentage and compared using the χ2 test. P values < .05 were considered statistically significant.
RESULTS
This study included 97 patients, 43 in the AUC/MIC group and 54 in the trough group.
One (2%) patient in the AUC/MIC group and 2 (4%) patients in the trough group experienced vancomycin-induced AKI (P = .10) (Table 2).
DISCUSSION
There was no statistically significant difference between the 2 groups for the vancomycin-induced AKI (P = .10), the primary endpoint, or overall AKI (P = .29), the secondary endpoint. It should be noted that there was more overall AKI in the AUC/MIC group. Veterans in the AUC/MIC group were found to have their first AUC/MIC within the therapeutic range statistically significantly more often than the first trough level in the trough group (P = .04). The percentage of time spent within therapeutic range was statistically significantly higher in the AUC/MIC-guided TDM group (P = .02). The percentage of time spent subtherapeutic of goal range was statistically significantly higher in the trough-guided TDM group (P < .001). There was no statistically significant difference found in the percent of time spent supratherapeutic of goal range (P = .25). However, the observed percentage of time spent supratherapeutic of goal range was higher in the AUC/MIC group. These results indicate that AUC/MIC-guided TDM may be more efficacious with regard to time in therapeutic range and time to therapeutic range.
The finding of increased AKI with AUC/MIC-guided TDM does not align with previous studies.8 The prospective study by Neely and colleagues found that AUC/MIC-guided TDM resulted in more time in the therapeutic range as well as less nephrotoxicity compared with trough-guided TDM, although it was limited by its lack of randomization and did not account for other causes of nephrotoxicity.8 They found that only 19% of trough concentrations were therapeutic compared with 70% of AUC/MICs and found nephrotoxicity in 8% of trough-guided TDM patients compared with 2% of AUC/MIC-guided TDM patients.8
Unlike Nealy and colleagues, our study did not find lower nephrotoxicity associated with AUC/MIC-guided TDM. Multiple factors may have influenced our results. Our AUC/MIC group had significantly more newly started concomitant nephrotoxins and other nephrotoxic medications used during the vancomycin courses compared with the trough-guided group, which may have influenced AKI outcomes. It also should be noted that there was significantly more time spent subtherapeutic of the goal range and significantly less time in the goal range in the trough group compared with the AUC/MIC group. In our study, the trough-guided group had significantly more patients with acute illness compared with the AUC/MIC group (skin, soft tissue, and joint infections were similar between the groups). The group with more acutely ill patients would have been expected to have more nephrotoxicity. However, despite the acute illnesses, patients in the trough-guided group spent more time in the subtherapeutic range. This may explain the increased nephrotoxicity in the AUC/MIC group since those patients spent more time in the therapeutic range.
This study used the most recent KDIGO AKI definition: either an increase in sCr of ≥ 0.3 mg/dL or a 50% increase in sCr from baseline sustained over 48 hours without any other explanation for the change in renal function.11 This AKI definition is stricter than the previous definition, which was used by earlier studies, including Neely and colleagues, to evaluate rates of vancomycin-induced AKI.2,3 Therefore, the rates of overall AKI found in this study may be higher than in previous studies due to the definition of AKI used.
Limitations
This study was limited by its retrospective nature, lack of randomization, and small sample size. To decrease the potential for error in this study, analysis of power and a larger study sample would have been beneficial. During the COVID-19 pandemic, increased pneumonia cases may have hidden bacterial causes and caused an undercount. Nephrotoxicity may also be related to volume depletion, severe systemic illness, dehydration, or hypotension. Screening was completed via chart review for these alternative causes of nephrotoxicity in this study but may not be completely accounted for due to lack of documentation and the retrospective nature of this study.
CONCLUSIONS
This study did not find a significant difference in the rates of vancomycin-induced or overall AKI between AUC/MIC-guided and trough-guided TDM. However, this study may not have been powered to detect a significant difference in the primary endpoint. This study indicated that AUC/MIC-guided TDM of vancomycin resulted in a quicker time to the therapeutic range and a higher percentage of overall time in the therapeutic range as compared with trough-guided TDM. The results of this study indicated that trough-guided monitoring resulted in a higher percentage of time in a subtherapeutic range. This study also found that the first AUC/MIC calculated was within therapeutic range more often than the first trough level collected.
These results indicate that AUC/MIC-guided TDM may be more effective than trough-guided TDM in the veteran population. However, while AUC/MIC-guided TDM may be more effective with regards to time in therapeutic range and time to therapeutic range, this study did not indicate any safety benefit of AUC/MIC-guided over trough-guided TDM with regards to AKI incidence. Our data indicate that AUC/MIC-guided TDM increases the amount of time in the therapeutic range compared with trough-guided TDM and is not more nephrotoxic. The findings of this study support the recommendation to transition to the use of AUC/MIC-guided TDM of vancomycin in the veteran population.
Acknowledgments
This material is the result of work supported with the use of facilities and resources from the Sioux Falls Veterans Affairs Health Care System.
1. Gallagher J, MacDougall C. Glycopeptides and short-acting lipoglycopeptides In: Antibiotics Simplified. Jones & Bartlett Learning; 2018.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9-14. doi:10.1517/14740330903413514
4. Poston-Blahnik A, Moenster R. Association between vancomycin area under the curve and nephrotoxicity: a single center, retrospective cohort study in a veteran population. Open Forum Infect Dis. 2021;8(5):ofab094. Published 2021 Mar 12. doi:10.1093/ofid/ofab094
5. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. doi:10.2146/ajhp080434
6. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925-942. doi:10.2165/00003088-200443130-00005
7. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-Associated Acute Kidney Injury in a Large Veteran Population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
8. Neely MN, Kato L, Youn G, et al. Prospective Trial on the Use of Trough Concentration versus Area under the Curve To Determine Therapeutic Vancomycin Dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. Published 2018 Jan 25. doi:10.1128/AAC.02042-17
9. Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J Hosp Med. 2012;7(2):91-97. doi:10.1002/jhm.946
10. Patel N, Stornelli N, Sangiovanni RJ, Huang DB, Lodise TP. Effect of vancomycin-associated acute kidney injury on incidence of 30-day readmissions among hospitalized Veterans Affairs patients with skin and skin structure infections. Antimicrob Agents Chemother. 2020;64(10):e01268-20. Published 2020 Sep 21. doi:10.1128/AAC.01268-20
11. Acute Kidney Injury Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(suppl 1):1-138.
12. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50-57. doi:10.1016/j.addr.2014.05.016
1. Gallagher J, MacDougall C. Glycopeptides and short-acting lipoglycopeptides In: Antibiotics Simplified. Jones & Bartlett Learning; 2018.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9-14. doi:10.1517/14740330903413514
4. Poston-Blahnik A, Moenster R. Association between vancomycin area under the curve and nephrotoxicity: a single center, retrospective cohort study in a veteran population. Open Forum Infect Dis. 2021;8(5):ofab094. Published 2021 Mar 12. doi:10.1093/ofid/ofab094
5. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. doi:10.2146/ajhp080434
6. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925-942. doi:10.2165/00003088-200443130-00005
7. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-Associated Acute Kidney Injury in a Large Veteran Population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
8. Neely MN, Kato L, Youn G, et al. Prospective Trial on the Use of Trough Concentration versus Area under the Curve To Determine Therapeutic Vancomycin Dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. Published 2018 Jan 25. doi:10.1128/AAC.02042-17
9. Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J Hosp Med. 2012;7(2):91-97. doi:10.1002/jhm.946
10. Patel N, Stornelli N, Sangiovanni RJ, Huang DB, Lodise TP. Effect of vancomycin-associated acute kidney injury on incidence of 30-day readmissions among hospitalized Veterans Affairs patients with skin and skin structure infections. Antimicrob Agents Chemother. 2020;64(10):e01268-20. Published 2020 Sep 21. doi:10.1128/AAC.01268-20
11. Acute Kidney Injury Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(suppl 1):1-138.
12. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50-57. doi:10.1016/j.addr.2014.05.016
COVID leading cause of death among law enforcement for third year
A new report says 70 officers died of COVID-related causes after getting the virus while on the job. The number is down dramatically from 2021, when 405 officer deaths were attributed to COVID.
The annual count was published Wednesday by the National Law Enforcement Officers Memorial Fund.
In total, 226 officers died in the line of duty in 2022, which is a decrease of 61% from 2021.
The decrease “is almost entirely related to the significant reduction in COVID-19 deaths,” the report stated. The authors said the decline was likely due to “reduced infection rates and the broad availability and use of vaccinations.”
Reported deaths included federal, state, tribal, and local law enforcement officers.
Firearms-related fatalities were the second-leading cause of death among officers, with 64 in 2022. That count sustains a 21% increase seen in 2021, up from the decade-long average of 53 firearms-related deaths annually from 2010 to 2020.
Traffic-related causes ranked third for cause of death in 2022, accounting for 56 deaths.
“While overall line-of-duty deaths are trending down, the continuing trend of greater-than-average firearms-related deaths continues to be a serious concern,” Marcia Ferranto, the organization’s chief executive officer, said in a news release. “Using and reporting on this data allows us to highlight the continuing cost of maintaining our democracy, regrettably measured in the lives of the many law enforcement professionals who sacrifice everything fulfilling their promise to serve and protect.”
A version of this article first appeared on WebMD.com.
A new report says 70 officers died of COVID-related causes after getting the virus while on the job. The number is down dramatically from 2021, when 405 officer deaths were attributed to COVID.
The annual count was published Wednesday by the National Law Enforcement Officers Memorial Fund.
In total, 226 officers died in the line of duty in 2022, which is a decrease of 61% from 2021.
The decrease “is almost entirely related to the significant reduction in COVID-19 deaths,” the report stated. The authors said the decline was likely due to “reduced infection rates and the broad availability and use of vaccinations.”
Reported deaths included federal, state, tribal, and local law enforcement officers.
Firearms-related fatalities were the second-leading cause of death among officers, with 64 in 2022. That count sustains a 21% increase seen in 2021, up from the decade-long average of 53 firearms-related deaths annually from 2010 to 2020.
Traffic-related causes ranked third for cause of death in 2022, accounting for 56 deaths.
“While overall line-of-duty deaths are trending down, the continuing trend of greater-than-average firearms-related deaths continues to be a serious concern,” Marcia Ferranto, the organization’s chief executive officer, said in a news release. “Using and reporting on this data allows us to highlight the continuing cost of maintaining our democracy, regrettably measured in the lives of the many law enforcement professionals who sacrifice everything fulfilling their promise to serve and protect.”
A version of this article first appeared on WebMD.com.
A new report says 70 officers died of COVID-related causes after getting the virus while on the job. The number is down dramatically from 2021, when 405 officer deaths were attributed to COVID.
The annual count was published Wednesday by the National Law Enforcement Officers Memorial Fund.
In total, 226 officers died in the line of duty in 2022, which is a decrease of 61% from 2021.
The decrease “is almost entirely related to the significant reduction in COVID-19 deaths,” the report stated. The authors said the decline was likely due to “reduced infection rates and the broad availability and use of vaccinations.”
Reported deaths included federal, state, tribal, and local law enforcement officers.
Firearms-related fatalities were the second-leading cause of death among officers, with 64 in 2022. That count sustains a 21% increase seen in 2021, up from the decade-long average of 53 firearms-related deaths annually from 2010 to 2020.
Traffic-related causes ranked third for cause of death in 2022, accounting for 56 deaths.
“While overall line-of-duty deaths are trending down, the continuing trend of greater-than-average firearms-related deaths continues to be a serious concern,” Marcia Ferranto, the organization’s chief executive officer, said in a news release. “Using and reporting on this data allows us to highlight the continuing cost of maintaining our democracy, regrettably measured in the lives of the many law enforcement professionals who sacrifice everything fulfilling their promise to serve and protect.”
A version of this article first appeared on WebMD.com.
Long COVID comes into focus, showing older patients fare worse
These findings help define long COVID, guiding providers and patients through the recovery process, Barak Mizrahi, MSc, of KI Research Institute, Kfar Malal, Israel, and colleagues reported.
“To provide efficient continuous treatment and prevent adverse events related to potential long term effects and delayed symptoms of COVID-19, determining the magnitude and severity of this phenomenon and distinguishing it from similar clinical manifestations that occur normally or following infections with other pathogens is essential,” the investigators wrote in The BMJ.
To this end, they conducted a retrospective, nationwide cohort study involving 1,913,234 people who took a polymerase chain reaction test for SARS-CoV-2 between March 1, 2020, and Oct. 1, 2021. They compared a range of long-term outcomes at different intervals post infection, and compared these trends across subgroups sorted by age, sex, and variant. Outcomes ranged broadly, including respiratory disorders, cough, arthralgia, weakness, hair loss, and others.
The investigators compared hazard ratios for each of these outcomes among patients who tested positive versus those who tested negative at three intervals after testing: 30-90 days, 30-180 days, and 180-360 days. Statistically significant differences in the risks of these outcomes between infected versus uninfected groups suggested that COVID was playing a role.
“The health outcomes that represent long COVID showed a significant increase in both early and late phases,” the investigators wrote. These outcomes included anosmia and dysgeusia, cognitive impairment, dyspnea, weakness, and palpitations. In contrast, chest pain, myalgia, arthralgia, cough, and dizziness were associated with patients who were in the early phase, but not the late phase of long COVID.
“Vaccinated patients with a breakthrough SARS-CoV-2 infection had a lower risk for dyspnea and similar risk for other outcomes compared with unvaccinated infected patients,” the investigators noted.
For the long COVID outcomes, plots of risk differences over time showed that symptoms tended to get milder or resolve within a few months to a year. Patients 41-60 years were most likely to be impacted by long COVID outcomes, and show least improvement at 1 year, compared with other age groups.
“We believe that these findings will shed light on what is ‘long COVID’, support patients and doctors, and facilitate better and more efficient care,” Mr. Mizrahi and coauthor Maytal Bivas-Benita, PhD said in a joint written comment. “Primary care physicians (and patients) will now more clearly understand what are the symptoms that might be related to COVID and for how long they might linger. This would help physicians monitor the patients efficiently, ease their patients’ concerns and navigate a more efficient disease management.”
They suggested that the findings should hold consistent for future variants, although they could not “rule out the possibility of the emergence of new and more severe variants which will be more virulent and cause a more severe illness.”
One “major limitation” of the study, according to Monica Verduzco-Gutierrez, MD, a physiatrist and professor and chair of rehabilitation medicine at the University of Texas Health Science Center, San Antonio, is the lack of data for fatigue and dysautonomia, which are “the major presentations” that she sees in her long COVID clinic.
“The authors of the article focus on the primary damage being related to the lungs, though we know this is a systemic disease beyond the respiratory system, with endothelial dysfunction and immune dysregulation,” Dr. Verduzco-Gutierrez, who is also director of COVID recovery at the University of Texas Health Science Center, said in an interview.
Although it was reassuring to see that younger adults with long COVID trended toward improvement, she noted that patients 41-60 years “still had pretty significant symptoms” after 12 months.
“That [age group comprises] probably the majority of my patients that I’m seeing in the long COVID clinic,” Dr. Verduzco-Gutierrez said. “If you look at the whole thing, it looks better, but then when you drill down to that age group where you’re seeing patients, then it’s not.”
Dr. Verduzco-Gutierrez is so busy managing patients with long COVID that new appointments in her clinic are now delayed until May 31, so most patients will remain under the care of their primary care providers. She recommended that these physicians follow guidance from the American Academy of Physical Medicine and Rehabilitation, who offer consensus statements based on clinical characteristics, with separate recommendations for pediatric patients.
Our understanding of long COVID will continue to improve, and with it, available recommendations, she predicted, but further advances will require persistent effort.
“I think no matter what this [study] shows us, more research is needed,” Dr. Verduzco-Gutierrez said. “We can’t just forget about it, just because there is a population of people who get better. What about the ones who don’t?”
The investigators and Dr. Verduzco-Gutierrez disclosed no conflicts of interest.
These findings help define long COVID, guiding providers and patients through the recovery process, Barak Mizrahi, MSc, of KI Research Institute, Kfar Malal, Israel, and colleagues reported.
“To provide efficient continuous treatment and prevent adverse events related to potential long term effects and delayed symptoms of COVID-19, determining the magnitude and severity of this phenomenon and distinguishing it from similar clinical manifestations that occur normally or following infections with other pathogens is essential,” the investigators wrote in The BMJ.
To this end, they conducted a retrospective, nationwide cohort study involving 1,913,234 people who took a polymerase chain reaction test for SARS-CoV-2 between March 1, 2020, and Oct. 1, 2021. They compared a range of long-term outcomes at different intervals post infection, and compared these trends across subgroups sorted by age, sex, and variant. Outcomes ranged broadly, including respiratory disorders, cough, arthralgia, weakness, hair loss, and others.
The investigators compared hazard ratios for each of these outcomes among patients who tested positive versus those who tested negative at three intervals after testing: 30-90 days, 30-180 days, and 180-360 days. Statistically significant differences in the risks of these outcomes between infected versus uninfected groups suggested that COVID was playing a role.
“The health outcomes that represent long COVID showed a significant increase in both early and late phases,” the investigators wrote. These outcomes included anosmia and dysgeusia, cognitive impairment, dyspnea, weakness, and palpitations. In contrast, chest pain, myalgia, arthralgia, cough, and dizziness were associated with patients who were in the early phase, but not the late phase of long COVID.
“Vaccinated patients with a breakthrough SARS-CoV-2 infection had a lower risk for dyspnea and similar risk for other outcomes compared with unvaccinated infected patients,” the investigators noted.
For the long COVID outcomes, plots of risk differences over time showed that symptoms tended to get milder or resolve within a few months to a year. Patients 41-60 years were most likely to be impacted by long COVID outcomes, and show least improvement at 1 year, compared with other age groups.
“We believe that these findings will shed light on what is ‘long COVID’, support patients and doctors, and facilitate better and more efficient care,” Mr. Mizrahi and coauthor Maytal Bivas-Benita, PhD said in a joint written comment. “Primary care physicians (and patients) will now more clearly understand what are the symptoms that might be related to COVID and for how long they might linger. This would help physicians monitor the patients efficiently, ease their patients’ concerns and navigate a more efficient disease management.”
They suggested that the findings should hold consistent for future variants, although they could not “rule out the possibility of the emergence of new and more severe variants which will be more virulent and cause a more severe illness.”
One “major limitation” of the study, according to Monica Verduzco-Gutierrez, MD, a physiatrist and professor and chair of rehabilitation medicine at the University of Texas Health Science Center, San Antonio, is the lack of data for fatigue and dysautonomia, which are “the major presentations” that she sees in her long COVID clinic.
“The authors of the article focus on the primary damage being related to the lungs, though we know this is a systemic disease beyond the respiratory system, with endothelial dysfunction and immune dysregulation,” Dr. Verduzco-Gutierrez, who is also director of COVID recovery at the University of Texas Health Science Center, said in an interview.
Although it was reassuring to see that younger adults with long COVID trended toward improvement, she noted that patients 41-60 years “still had pretty significant symptoms” after 12 months.
“That [age group comprises] probably the majority of my patients that I’m seeing in the long COVID clinic,” Dr. Verduzco-Gutierrez said. “If you look at the whole thing, it looks better, but then when you drill down to that age group where you’re seeing patients, then it’s not.”
Dr. Verduzco-Gutierrez is so busy managing patients with long COVID that new appointments in her clinic are now delayed until May 31, so most patients will remain under the care of their primary care providers. She recommended that these physicians follow guidance from the American Academy of Physical Medicine and Rehabilitation, who offer consensus statements based on clinical characteristics, with separate recommendations for pediatric patients.
Our understanding of long COVID will continue to improve, and with it, available recommendations, she predicted, but further advances will require persistent effort.
“I think no matter what this [study] shows us, more research is needed,” Dr. Verduzco-Gutierrez said. “We can’t just forget about it, just because there is a population of people who get better. What about the ones who don’t?”
The investigators and Dr. Verduzco-Gutierrez disclosed no conflicts of interest.
These findings help define long COVID, guiding providers and patients through the recovery process, Barak Mizrahi, MSc, of KI Research Institute, Kfar Malal, Israel, and colleagues reported.
“To provide efficient continuous treatment and prevent adverse events related to potential long term effects and delayed symptoms of COVID-19, determining the magnitude and severity of this phenomenon and distinguishing it from similar clinical manifestations that occur normally or following infections with other pathogens is essential,” the investigators wrote in The BMJ.
To this end, they conducted a retrospective, nationwide cohort study involving 1,913,234 people who took a polymerase chain reaction test for SARS-CoV-2 between March 1, 2020, and Oct. 1, 2021. They compared a range of long-term outcomes at different intervals post infection, and compared these trends across subgroups sorted by age, sex, and variant. Outcomes ranged broadly, including respiratory disorders, cough, arthralgia, weakness, hair loss, and others.
The investigators compared hazard ratios for each of these outcomes among patients who tested positive versus those who tested negative at three intervals after testing: 30-90 days, 30-180 days, and 180-360 days. Statistically significant differences in the risks of these outcomes between infected versus uninfected groups suggested that COVID was playing a role.
“The health outcomes that represent long COVID showed a significant increase in both early and late phases,” the investigators wrote. These outcomes included anosmia and dysgeusia, cognitive impairment, dyspnea, weakness, and palpitations. In contrast, chest pain, myalgia, arthralgia, cough, and dizziness were associated with patients who were in the early phase, but not the late phase of long COVID.
“Vaccinated patients with a breakthrough SARS-CoV-2 infection had a lower risk for dyspnea and similar risk for other outcomes compared with unvaccinated infected patients,” the investigators noted.
For the long COVID outcomes, plots of risk differences over time showed that symptoms tended to get milder or resolve within a few months to a year. Patients 41-60 years were most likely to be impacted by long COVID outcomes, and show least improvement at 1 year, compared with other age groups.
“We believe that these findings will shed light on what is ‘long COVID’, support patients and doctors, and facilitate better and more efficient care,” Mr. Mizrahi and coauthor Maytal Bivas-Benita, PhD said in a joint written comment. “Primary care physicians (and patients) will now more clearly understand what are the symptoms that might be related to COVID and for how long they might linger. This would help physicians monitor the patients efficiently, ease their patients’ concerns and navigate a more efficient disease management.”
They suggested that the findings should hold consistent for future variants, although they could not “rule out the possibility of the emergence of new and more severe variants which will be more virulent and cause a more severe illness.”
One “major limitation” of the study, according to Monica Verduzco-Gutierrez, MD, a physiatrist and professor and chair of rehabilitation medicine at the University of Texas Health Science Center, San Antonio, is the lack of data for fatigue and dysautonomia, which are “the major presentations” that she sees in her long COVID clinic.
“The authors of the article focus on the primary damage being related to the lungs, though we know this is a systemic disease beyond the respiratory system, with endothelial dysfunction and immune dysregulation,” Dr. Verduzco-Gutierrez, who is also director of COVID recovery at the University of Texas Health Science Center, said in an interview.
Although it was reassuring to see that younger adults with long COVID trended toward improvement, she noted that patients 41-60 years “still had pretty significant symptoms” after 12 months.
“That [age group comprises] probably the majority of my patients that I’m seeing in the long COVID clinic,” Dr. Verduzco-Gutierrez said. “If you look at the whole thing, it looks better, but then when you drill down to that age group where you’re seeing patients, then it’s not.”
Dr. Verduzco-Gutierrez is so busy managing patients with long COVID that new appointments in her clinic are now delayed until May 31, so most patients will remain under the care of their primary care providers. She recommended that these physicians follow guidance from the American Academy of Physical Medicine and Rehabilitation, who offer consensus statements based on clinical characteristics, with separate recommendations for pediatric patients.
Our understanding of long COVID will continue to improve, and with it, available recommendations, she predicted, but further advances will require persistent effort.
“I think no matter what this [study] shows us, more research is needed,” Dr. Verduzco-Gutierrez said. “We can’t just forget about it, just because there is a population of people who get better. What about the ones who don’t?”
The investigators and Dr. Verduzco-Gutierrez disclosed no conflicts of interest.
FROM THE BMJ
Strong support to provide DAA therapy to all patients with HCV
, a large, real-world analysis finds.
Improved outcomes were seen among patients without cirrhosis, those with compensated cirrhosis, and those with existing liver decompensation, the authors noted.
The findings highlight a “substantial need to provide DAA therapy to all patients with HCV, regardless of disease stage or financial status,” wrote Mindie Nguyen, MD, of Stanford University Medical Center, Palo Alto, Calif., and coinvestigators.
“Additional national efforts are needed to reach and treat U.S. population groups that are underinsured or not insured, incarcerated and otherwise marginalized, such as users of illicit drugs, who are also at higher risk of disease complication and reinfection,” they said.
The study was published online in JAMA Internal Medicine.
CHC and its complications are associated with high rates of illness and death. However, large-scale data on long-term liver and nonliver effects of DAA treatment are limited.
For their study, Dr. Nguyen and colleagues analyzed administrative claims data from 2010 to 2021 for 245,596 adults with CHC, of whom 40,654 had received one or more DAA therapies (without interferon) and 204,942 had not received treatment.
DAA-treated patients were slightly older than their untreated peers (mean age, 59.9 years, vs. 58.5 years) and were more likely to be male (62% vs. 58%) and White (59% vs. 57%), and to have diabetes (26% vs. 25%) and cirrhosis (44% vs. 29%).
For liver outcomes, DAA therapy was associated with a lower incidence of decompensation (28.2 vs. 40.8 per 1,000 person-years; P < .001) and hepatocellular carcinoma (HCC) in compensated cirrhosis (20.1 vs. 41.8; P < .001).
For nonliver outcomes, DAA treatment was associated with a lower incidence of diabetes (30.2 vs. 37.2 per 1,000 person-years; P < .001) and chronic kidney disease (31.1 vs. 34.1; P < .001).
The all-cause mortality rate per 1,000 person-years was 36.5 in the DAA-treated group, vs. 64.7 in the untreated group (P < .001).
In multivariable regression analysis, DAA treatment was independently associated with a significant decrease in the risk for HCC (adjusted hazard ratio [aHR], 0.73), decompensation (aHR, 0.36), diabetes (aHR, 0.74), chronic kidney disease (aHR, 0.81), cardiovascular disease (aHR, 0.90), nonliver cancer (aHR, 0.89), and mortality (aHR, 0.43).
The 57% lower mortality rate observed among DAA-treated vs. untreated patients aligns with a large French study of adults with CHC.
“Because HCV treatment with a DAA regimen is well tolerated for nearly all patients, we believe these findings provide further support for universal HCV treatment coverage for all patients affected by HCV,” Dr. Nguyen and colleagues wrote.
The strengths of this study are its large sample of DAA-treated and untreated patients from diverse racial and ethnic groups from across the United States and from diverse practice settings (not just tertiary centers).
One limitation is that the study cohort included only patients covered by private insurance; therefore, the findings may not be generalizable to individuals who are underinsured or not insured. Miscoding and misclassification are also possible with large claims databases.
Support for the study was provided by Stanford University and the Stanford Center for Population Health Sciences. Dr. Nguyen has received institutional grants and advisory board fees from Gilead Sciences outside the submitted work.
A version of this article first appeared on Medscape.com.
, a large, real-world analysis finds.
Improved outcomes were seen among patients without cirrhosis, those with compensated cirrhosis, and those with existing liver decompensation, the authors noted.
The findings highlight a “substantial need to provide DAA therapy to all patients with HCV, regardless of disease stage or financial status,” wrote Mindie Nguyen, MD, of Stanford University Medical Center, Palo Alto, Calif., and coinvestigators.
“Additional national efforts are needed to reach and treat U.S. population groups that are underinsured or not insured, incarcerated and otherwise marginalized, such as users of illicit drugs, who are also at higher risk of disease complication and reinfection,” they said.
The study was published online in JAMA Internal Medicine.
CHC and its complications are associated with high rates of illness and death. However, large-scale data on long-term liver and nonliver effects of DAA treatment are limited.
For their study, Dr. Nguyen and colleagues analyzed administrative claims data from 2010 to 2021 for 245,596 adults with CHC, of whom 40,654 had received one or more DAA therapies (without interferon) and 204,942 had not received treatment.
DAA-treated patients were slightly older than their untreated peers (mean age, 59.9 years, vs. 58.5 years) and were more likely to be male (62% vs. 58%) and White (59% vs. 57%), and to have diabetes (26% vs. 25%) and cirrhosis (44% vs. 29%).
For liver outcomes, DAA therapy was associated with a lower incidence of decompensation (28.2 vs. 40.8 per 1,000 person-years; P < .001) and hepatocellular carcinoma (HCC) in compensated cirrhosis (20.1 vs. 41.8; P < .001).
For nonliver outcomes, DAA treatment was associated with a lower incidence of diabetes (30.2 vs. 37.2 per 1,000 person-years; P < .001) and chronic kidney disease (31.1 vs. 34.1; P < .001).
The all-cause mortality rate per 1,000 person-years was 36.5 in the DAA-treated group, vs. 64.7 in the untreated group (P < .001).
In multivariable regression analysis, DAA treatment was independently associated with a significant decrease in the risk for HCC (adjusted hazard ratio [aHR], 0.73), decompensation (aHR, 0.36), diabetes (aHR, 0.74), chronic kidney disease (aHR, 0.81), cardiovascular disease (aHR, 0.90), nonliver cancer (aHR, 0.89), and mortality (aHR, 0.43).
The 57% lower mortality rate observed among DAA-treated vs. untreated patients aligns with a large French study of adults with CHC.
“Because HCV treatment with a DAA regimen is well tolerated for nearly all patients, we believe these findings provide further support for universal HCV treatment coverage for all patients affected by HCV,” Dr. Nguyen and colleagues wrote.
The strengths of this study are its large sample of DAA-treated and untreated patients from diverse racial and ethnic groups from across the United States and from diverse practice settings (not just tertiary centers).
One limitation is that the study cohort included only patients covered by private insurance; therefore, the findings may not be generalizable to individuals who are underinsured or not insured. Miscoding and misclassification are also possible with large claims databases.
Support for the study was provided by Stanford University and the Stanford Center for Population Health Sciences. Dr. Nguyen has received institutional grants and advisory board fees from Gilead Sciences outside the submitted work.
A version of this article first appeared on Medscape.com.
, a large, real-world analysis finds.
Improved outcomes were seen among patients without cirrhosis, those with compensated cirrhosis, and those with existing liver decompensation, the authors noted.
The findings highlight a “substantial need to provide DAA therapy to all patients with HCV, regardless of disease stage or financial status,” wrote Mindie Nguyen, MD, of Stanford University Medical Center, Palo Alto, Calif., and coinvestigators.
“Additional national efforts are needed to reach and treat U.S. population groups that are underinsured or not insured, incarcerated and otherwise marginalized, such as users of illicit drugs, who are also at higher risk of disease complication and reinfection,” they said.
The study was published online in JAMA Internal Medicine.
CHC and its complications are associated with high rates of illness and death. However, large-scale data on long-term liver and nonliver effects of DAA treatment are limited.
For their study, Dr. Nguyen and colleagues analyzed administrative claims data from 2010 to 2021 for 245,596 adults with CHC, of whom 40,654 had received one or more DAA therapies (without interferon) and 204,942 had not received treatment.
DAA-treated patients were slightly older than their untreated peers (mean age, 59.9 years, vs. 58.5 years) and were more likely to be male (62% vs. 58%) and White (59% vs. 57%), and to have diabetes (26% vs. 25%) and cirrhosis (44% vs. 29%).
For liver outcomes, DAA therapy was associated with a lower incidence of decompensation (28.2 vs. 40.8 per 1,000 person-years; P < .001) and hepatocellular carcinoma (HCC) in compensated cirrhosis (20.1 vs. 41.8; P < .001).
For nonliver outcomes, DAA treatment was associated with a lower incidence of diabetes (30.2 vs. 37.2 per 1,000 person-years; P < .001) and chronic kidney disease (31.1 vs. 34.1; P < .001).
The all-cause mortality rate per 1,000 person-years was 36.5 in the DAA-treated group, vs. 64.7 in the untreated group (P < .001).
In multivariable regression analysis, DAA treatment was independently associated with a significant decrease in the risk for HCC (adjusted hazard ratio [aHR], 0.73), decompensation (aHR, 0.36), diabetes (aHR, 0.74), chronic kidney disease (aHR, 0.81), cardiovascular disease (aHR, 0.90), nonliver cancer (aHR, 0.89), and mortality (aHR, 0.43).
The 57% lower mortality rate observed among DAA-treated vs. untreated patients aligns with a large French study of adults with CHC.
“Because HCV treatment with a DAA regimen is well tolerated for nearly all patients, we believe these findings provide further support for universal HCV treatment coverage for all patients affected by HCV,” Dr. Nguyen and colleagues wrote.
The strengths of this study are its large sample of DAA-treated and untreated patients from diverse racial and ethnic groups from across the United States and from diverse practice settings (not just tertiary centers).
One limitation is that the study cohort included only patients covered by private insurance; therefore, the findings may not be generalizable to individuals who are underinsured or not insured. Miscoding and misclassification are also possible with large claims databases.
Support for the study was provided by Stanford University and the Stanford Center for Population Health Sciences. Dr. Nguyen has received institutional grants and advisory board fees from Gilead Sciences outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE
FDA OKs Tdap shot in pregnancy to protect newborns from pertussis
The Food and Drug Administration has approved another Tdap vaccine option for use during pregnancy to protect newborns from whooping cough.
The agency on Jan. 9 licensed Adacel (Sanofi Pasteur) for immunization during the third trimester to prevent pertussis in infants younger than 2 months old.
The FDA in October approved a different Tdap vaccine, Boostrix (GlaxoSmithKline), for this indication. Boostrix was the first vaccine specifically approved to prevent a disease in newborns whose mothers receive the vaccine while pregnant.
The Centers for Disease Control and Prevention recommend that women receive a dose of Tdap vaccine during each pregnancy, preferably during gestational weeks 27-36 – and ideally toward the earlier end of that window – to help protect babies from whooping cough, the respiratory tract infection caused by Bordetella pertussis.
Providing a Tdap vaccine – tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed – in the third trimester confers passive immunity to the baby, according to the CDC. It also reduces the likelihood that the mother will get pertussis and pass it on to the infant.
One study found that providing Tdap vaccination during gestational weeks 27-36 was 85% more effective at preventing pertussis in infants younger than 2 months old, compared with providing Tdap vaccination to mothers in the hospital postpartum.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” according to a CDC reference page. “Most of these deaths are among infants who are too young to be protected by the childhood pertussis vaccine series that starts when infants are 2 months old.”
The Food and Drug Administration has approved another Tdap vaccine option for use during pregnancy to protect newborns from whooping cough.
The agency on Jan. 9 licensed Adacel (Sanofi Pasteur) for immunization during the third trimester to prevent pertussis in infants younger than 2 months old.
The FDA in October approved a different Tdap vaccine, Boostrix (GlaxoSmithKline), for this indication. Boostrix was the first vaccine specifically approved to prevent a disease in newborns whose mothers receive the vaccine while pregnant.
The Centers for Disease Control and Prevention recommend that women receive a dose of Tdap vaccine during each pregnancy, preferably during gestational weeks 27-36 – and ideally toward the earlier end of that window – to help protect babies from whooping cough, the respiratory tract infection caused by Bordetella pertussis.
Providing a Tdap vaccine – tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed – in the third trimester confers passive immunity to the baby, according to the CDC. It also reduces the likelihood that the mother will get pertussis and pass it on to the infant.
One study found that providing Tdap vaccination during gestational weeks 27-36 was 85% more effective at preventing pertussis in infants younger than 2 months old, compared with providing Tdap vaccination to mothers in the hospital postpartum.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” according to a CDC reference page. “Most of these deaths are among infants who are too young to be protected by the childhood pertussis vaccine series that starts when infants are 2 months old.”
The Food and Drug Administration has approved another Tdap vaccine option for use during pregnancy to protect newborns from whooping cough.
The agency on Jan. 9 licensed Adacel (Sanofi Pasteur) for immunization during the third trimester to prevent pertussis in infants younger than 2 months old.
The FDA in October approved a different Tdap vaccine, Boostrix (GlaxoSmithKline), for this indication. Boostrix was the first vaccine specifically approved to prevent a disease in newborns whose mothers receive the vaccine while pregnant.
The Centers for Disease Control and Prevention recommend that women receive a dose of Tdap vaccine during each pregnancy, preferably during gestational weeks 27-36 – and ideally toward the earlier end of that window – to help protect babies from whooping cough, the respiratory tract infection caused by Bordetella pertussis.
Providing a Tdap vaccine – tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed – in the third trimester confers passive immunity to the baby, according to the CDC. It also reduces the likelihood that the mother will get pertussis and pass it on to the infant.
One study found that providing Tdap vaccination during gestational weeks 27-36 was 85% more effective at preventing pertussis in infants younger than 2 months old, compared with providing Tdap vaccination to mothers in the hospital postpartum.
“On average, about 1,000 infants are hospitalized and typically between 5 and 15 infants die each year in the United States due to pertussis,” according to a CDC reference page. “Most of these deaths are among infants who are too young to be protected by the childhood pertussis vaccine series that starts when infants are 2 months old.”
Early retirement and the terrible, horrible, no good, very bad cognitive decline
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
Kaposi’s sarcoma: Antiretroviral-related improvements in survival measured
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.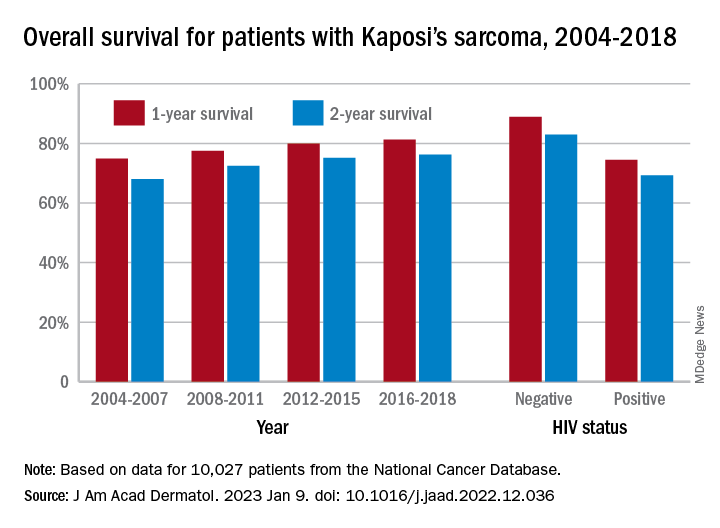
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Measles
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
New Omicron subvariant is ‘crazy infectious,’ COVID expert warns
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.
Autopsies show COVID virus invades entire body
A study on the subject was published in the journal Nature. The researchers completed autopsies from April 2020 to March 2021 of 44 unvaccinated people who had severe COVID-19. The median age was 62.5 years old, and 30% were female. Extensive brain sampling was done for 11 cases.
Because of its nature as a respiratory illness, SARS-CoV-2 was most widespread in the respiratory system such as in the lungs. But it was also found in 79 other body locations, including the heart, kidneys, liver, muscles, nerves, reproductive tract, and eyes.
The researchers said their work shows the SARS-CoV-2 “is capable of infecting and replicating within the human brain.” They also said their results indicate the virus spreads via the blood early during infection, which “seeds the virus throughout the body following infection of the respiratory tract.”
The authors noted that, while the virus was found outside the respiratory tract, they did not find signs of inflammation beyond the respiratory system.
The results will help narrow down treatments for long COVID, and particularly support the idea of using the antiviral drug Paxlovid to treat long COVID, according to a blog post from the National Institute of Allergy and Infectious Diseases. A clinical trial is already underway examining the treatment, and results are expected in January 2024.
A version of this article first appeared on WebMD.com.
A study on the subject was published in the journal Nature. The researchers completed autopsies from April 2020 to March 2021 of 44 unvaccinated people who had severe COVID-19. The median age was 62.5 years old, and 30% were female. Extensive brain sampling was done for 11 cases.
Because of its nature as a respiratory illness, SARS-CoV-2 was most widespread in the respiratory system such as in the lungs. But it was also found in 79 other body locations, including the heart, kidneys, liver, muscles, nerves, reproductive tract, and eyes.
The researchers said their work shows the SARS-CoV-2 “is capable of infecting and replicating within the human brain.” They also said their results indicate the virus spreads via the blood early during infection, which “seeds the virus throughout the body following infection of the respiratory tract.”
The authors noted that, while the virus was found outside the respiratory tract, they did not find signs of inflammation beyond the respiratory system.
The results will help narrow down treatments for long COVID, and particularly support the idea of using the antiviral drug Paxlovid to treat long COVID, according to a blog post from the National Institute of Allergy and Infectious Diseases. A clinical trial is already underway examining the treatment, and results are expected in January 2024.
A version of this article first appeared on WebMD.com.
A study on the subject was published in the journal Nature. The researchers completed autopsies from April 2020 to March 2021 of 44 unvaccinated people who had severe COVID-19. The median age was 62.5 years old, and 30% were female. Extensive brain sampling was done for 11 cases.
Because of its nature as a respiratory illness, SARS-CoV-2 was most widespread in the respiratory system such as in the lungs. But it was also found in 79 other body locations, including the heart, kidneys, liver, muscles, nerves, reproductive tract, and eyes.
The researchers said their work shows the SARS-CoV-2 “is capable of infecting and replicating within the human brain.” They also said their results indicate the virus spreads via the blood early during infection, which “seeds the virus throughout the body following infection of the respiratory tract.”
The authors noted that, while the virus was found outside the respiratory tract, they did not find signs of inflammation beyond the respiratory system.
The results will help narrow down treatments for long COVID, and particularly support the idea of using the antiviral drug Paxlovid to treat long COVID, according to a blog post from the National Institute of Allergy and Infectious Diseases. A clinical trial is already underway examining the treatment, and results are expected in January 2024.
A version of this article first appeared on WebMD.com.
FROM NATURE