User login
Guidelines for assessing cancer risk may need updating
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
FROM AACR 2023
‘Exciting’ results for cancer vaccine plus pembro in melanoma
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
FROM AACR 2023
Acute Onset of Vitiligolike Depigmentation After Nivolumab Therapy for Systemic Melanoma
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.
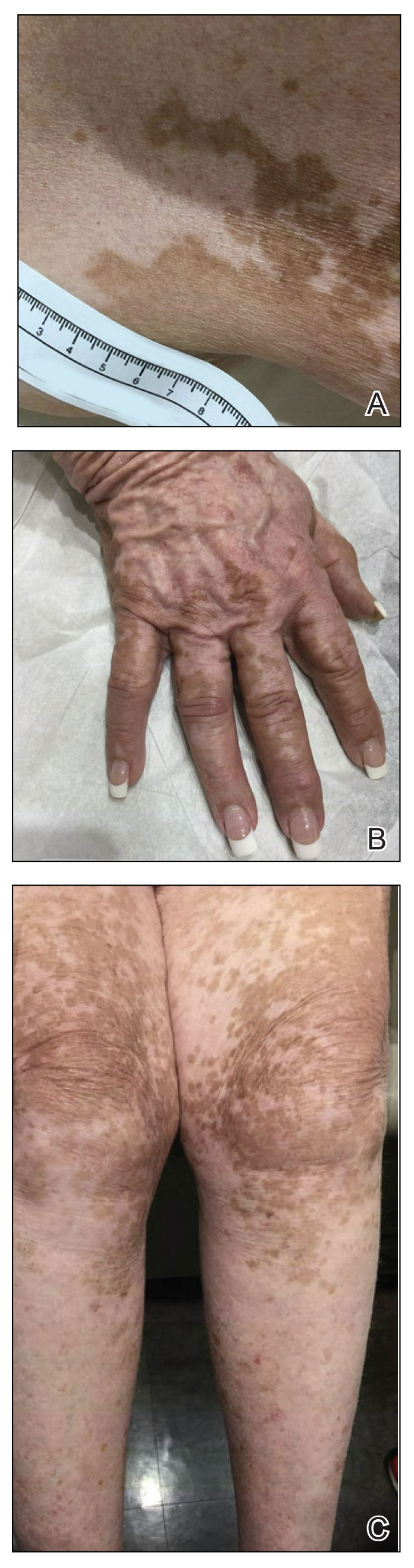
At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.

At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.

At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
Practice Points
- New-onset vitiligo coinciding with malignant melanoma should be considered a good prognostic indicator.
- Daily use of hydroquinone cream 4% in conjunction with diligent photoprotection was shown to even overall skin tone in a patient experiencing leukoderma from nivolumab therapy.
USPSTF releases updated recommendations on skin cancer screening
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
Pembrolizumab monotherapy effective for rare melanoma
The findings could represent a new standard of treatment for this extremely rare tumor.
The study was inspired by a previous retrospective analysis which found an overall response rate of 77% and a complete response of 32% to anti–PD-1 monotherapy.
The ORR is about double what is seen in melanoma more generally, according to Kari Kendra, MD, PhD, who presented the study at the annual meeting of the American Association for Cancer Research.
“Our study was a positive study. Of note, in the retrospective study, they saw a complete response rate of 32%, which was amazingly similar to what we found. [The findings support] the use of single agent anti–PD-1 immunotherapy as first line treatment for most patients with unresectable desmoplastic melanoma. [There was 89% overall response and we saw] dramatic responses across the board,” said Dr. Kendra, who is a medical oncologist at Ohio State University Wexner Medical Center, Columbus.
The findings drew a strong reaction. “In a rare tumor session, to see response curves like that, it’s just outstanding,” said the session’s cochair Brian Van Tine, MD, PhD, who is a professor of medical oncology at Washington University in St. Louis.
“This really is one of the highest tumor response rates to immunotherapy that we are seeing in any cancer. And I think may also highlight the fact that we shouldn’t think of all cutaneous melanomas as one disease, given the heterogeneity in tumor responses based on some of the pathologic and molecular characteristics,” said Zeynep Aroglu, MD, who served as a discussant but was also one of the investigators who enrolled patients for the trial.
Desmoplastic melanoma represents about 4% of all cutaneous melanoma diagnoses, and its unique pathology can make it difficult to diagnose. That often leads to a late diagnosis, according to Dr. Aroglu. They typically occur in elderly patients, in the head and neck area, and are associated with sun exposure. DM also tends to have a high mutation burden, Dr. Aroglu said during the session.
It remains to be seen why there is such a high response rate in this tumor type, even among tumor types with mutation burdens that are nearly as high. DM tumors are often driven by neurofibromatosis type 1, but other tumors driven by NF-1 don’t have as high of a response rate to immunotherapy. The tumor environment could also play a role, she said.
“Is it a combination of all these factors? I think some of the ongoing analysis of tumor samples that Dr. Kendra mentioned may help to answer some of these questions,” Dr. Aroglu continued.
She also noted that the melanoma field is increasingly turning to combination of anti–PD-1 therapy with agents like that target LAG3 or CTLA4. Such combinations can achieve higher response rates, but at a cost of higher rates of grade 3-4 adverse events than anti–PD-1 inhibitors alone. “I wonder if for desmoplastic melanomas in light of this data, do we consider de-escalating therapy, given these very high response rates to PD-1 alone, given also the elderly age of many of these patients, because even the PD-1–LAG3 combo still has a higher rate of toxicity than PD-1 monotherapy. Perhaps the immunotherapy combinations can be reserved for those rare desmoplastic patients who are resistant to PD-1 alone,” said Dr. Aroglu.
Study details and adverse events
Twenty-seven patients were enrolled in the study; 93% were male, all were White, and 22% had elevated baseline lactate dehydrogenase. About 63% had disease located in the head and neck area, 33% experienced a complete response (P < .001), and 56% had a partial response for an ORR of 89%. The result surpassed the primary endpoint target of at least a 20% complete response rate.
The 2-year progression-free survival was 74%, and 2-year overall survival was 89%. The most common toxicities were fatigue (56%), diarrhea (33%), maculopapular rash (30%), pruritus (22%), anemia (19%), arthralgia (19%), and decreased lymphocyte count (19%). There were two grade 4 adverse events: a lipase increase and a lung infection accompanied by sepsis.
The researchers also carried out whole exome sequencing of biopsies and found that 67% had NF-1 loss of function mutations.
Dr. Aroglu has served on advisory boards for Pfizer, Array, Eisai, Genentech, Natera, Novartis, OncoSec, and Regeneron. She has received research support from Boehringer Ingelheim, Pfizer, and Novartis. Dr. Kendra has received institutional support from Bristol Myers-Squibb and trial support from CheckMate Pharmaceuticals, GlaxoSmithKline, Immunocore, Medspace, Merck, Novartis, and Varian Medical Systems. Dr. Van Tine has financial relationships with a wide range of pharmaceutical companies.
The findings could represent a new standard of treatment for this extremely rare tumor.
The study was inspired by a previous retrospective analysis which found an overall response rate of 77% and a complete response of 32% to anti–PD-1 monotherapy.
The ORR is about double what is seen in melanoma more generally, according to Kari Kendra, MD, PhD, who presented the study at the annual meeting of the American Association for Cancer Research.
“Our study was a positive study. Of note, in the retrospective study, they saw a complete response rate of 32%, which was amazingly similar to what we found. [The findings support] the use of single agent anti–PD-1 immunotherapy as first line treatment for most patients with unresectable desmoplastic melanoma. [There was 89% overall response and we saw] dramatic responses across the board,” said Dr. Kendra, who is a medical oncologist at Ohio State University Wexner Medical Center, Columbus.
The findings drew a strong reaction. “In a rare tumor session, to see response curves like that, it’s just outstanding,” said the session’s cochair Brian Van Tine, MD, PhD, who is a professor of medical oncology at Washington University in St. Louis.
“This really is one of the highest tumor response rates to immunotherapy that we are seeing in any cancer. And I think may also highlight the fact that we shouldn’t think of all cutaneous melanomas as one disease, given the heterogeneity in tumor responses based on some of the pathologic and molecular characteristics,” said Zeynep Aroglu, MD, who served as a discussant but was also one of the investigators who enrolled patients for the trial.
Desmoplastic melanoma represents about 4% of all cutaneous melanoma diagnoses, and its unique pathology can make it difficult to diagnose. That often leads to a late diagnosis, according to Dr. Aroglu. They typically occur in elderly patients, in the head and neck area, and are associated with sun exposure. DM also tends to have a high mutation burden, Dr. Aroglu said during the session.
It remains to be seen why there is such a high response rate in this tumor type, even among tumor types with mutation burdens that are nearly as high. DM tumors are often driven by neurofibromatosis type 1, but other tumors driven by NF-1 don’t have as high of a response rate to immunotherapy. The tumor environment could also play a role, she said.
“Is it a combination of all these factors? I think some of the ongoing analysis of tumor samples that Dr. Kendra mentioned may help to answer some of these questions,” Dr. Aroglu continued.
She also noted that the melanoma field is increasingly turning to combination of anti–PD-1 therapy with agents like that target LAG3 or CTLA4. Such combinations can achieve higher response rates, but at a cost of higher rates of grade 3-4 adverse events than anti–PD-1 inhibitors alone. “I wonder if for desmoplastic melanomas in light of this data, do we consider de-escalating therapy, given these very high response rates to PD-1 alone, given also the elderly age of many of these patients, because even the PD-1–LAG3 combo still has a higher rate of toxicity than PD-1 monotherapy. Perhaps the immunotherapy combinations can be reserved for those rare desmoplastic patients who are resistant to PD-1 alone,” said Dr. Aroglu.
Study details and adverse events
Twenty-seven patients were enrolled in the study; 93% were male, all were White, and 22% had elevated baseline lactate dehydrogenase. About 63% had disease located in the head and neck area, 33% experienced a complete response (P < .001), and 56% had a partial response for an ORR of 89%. The result surpassed the primary endpoint target of at least a 20% complete response rate.
The 2-year progression-free survival was 74%, and 2-year overall survival was 89%. The most common toxicities were fatigue (56%), diarrhea (33%), maculopapular rash (30%), pruritus (22%), anemia (19%), arthralgia (19%), and decreased lymphocyte count (19%). There were two grade 4 adverse events: a lipase increase and a lung infection accompanied by sepsis.
The researchers also carried out whole exome sequencing of biopsies and found that 67% had NF-1 loss of function mutations.
Dr. Aroglu has served on advisory boards for Pfizer, Array, Eisai, Genentech, Natera, Novartis, OncoSec, and Regeneron. She has received research support from Boehringer Ingelheim, Pfizer, and Novartis. Dr. Kendra has received institutional support from Bristol Myers-Squibb and trial support from CheckMate Pharmaceuticals, GlaxoSmithKline, Immunocore, Medspace, Merck, Novartis, and Varian Medical Systems. Dr. Van Tine has financial relationships with a wide range of pharmaceutical companies.
The findings could represent a new standard of treatment for this extremely rare tumor.
The study was inspired by a previous retrospective analysis which found an overall response rate of 77% and a complete response of 32% to anti–PD-1 monotherapy.
The ORR is about double what is seen in melanoma more generally, according to Kari Kendra, MD, PhD, who presented the study at the annual meeting of the American Association for Cancer Research.
“Our study was a positive study. Of note, in the retrospective study, they saw a complete response rate of 32%, which was amazingly similar to what we found. [The findings support] the use of single agent anti–PD-1 immunotherapy as first line treatment for most patients with unresectable desmoplastic melanoma. [There was 89% overall response and we saw] dramatic responses across the board,” said Dr. Kendra, who is a medical oncologist at Ohio State University Wexner Medical Center, Columbus.
The findings drew a strong reaction. “In a rare tumor session, to see response curves like that, it’s just outstanding,” said the session’s cochair Brian Van Tine, MD, PhD, who is a professor of medical oncology at Washington University in St. Louis.
“This really is one of the highest tumor response rates to immunotherapy that we are seeing in any cancer. And I think may also highlight the fact that we shouldn’t think of all cutaneous melanomas as one disease, given the heterogeneity in tumor responses based on some of the pathologic and molecular characteristics,” said Zeynep Aroglu, MD, who served as a discussant but was also one of the investigators who enrolled patients for the trial.
Desmoplastic melanoma represents about 4% of all cutaneous melanoma diagnoses, and its unique pathology can make it difficult to diagnose. That often leads to a late diagnosis, according to Dr. Aroglu. They typically occur in elderly patients, in the head and neck area, and are associated with sun exposure. DM also tends to have a high mutation burden, Dr. Aroglu said during the session.
It remains to be seen why there is such a high response rate in this tumor type, even among tumor types with mutation burdens that are nearly as high. DM tumors are often driven by neurofibromatosis type 1, but other tumors driven by NF-1 don’t have as high of a response rate to immunotherapy. The tumor environment could also play a role, she said.
“Is it a combination of all these factors? I think some of the ongoing analysis of tumor samples that Dr. Kendra mentioned may help to answer some of these questions,” Dr. Aroglu continued.
She also noted that the melanoma field is increasingly turning to combination of anti–PD-1 therapy with agents like that target LAG3 or CTLA4. Such combinations can achieve higher response rates, but at a cost of higher rates of grade 3-4 adverse events than anti–PD-1 inhibitors alone. “I wonder if for desmoplastic melanomas in light of this data, do we consider de-escalating therapy, given these very high response rates to PD-1 alone, given also the elderly age of many of these patients, because even the PD-1–LAG3 combo still has a higher rate of toxicity than PD-1 monotherapy. Perhaps the immunotherapy combinations can be reserved for those rare desmoplastic patients who are resistant to PD-1 alone,” said Dr. Aroglu.
Study details and adverse events
Twenty-seven patients were enrolled in the study; 93% were male, all were White, and 22% had elevated baseline lactate dehydrogenase. About 63% had disease located in the head and neck area, 33% experienced a complete response (P < .001), and 56% had a partial response for an ORR of 89%. The result surpassed the primary endpoint target of at least a 20% complete response rate.
The 2-year progression-free survival was 74%, and 2-year overall survival was 89%. The most common toxicities were fatigue (56%), diarrhea (33%), maculopapular rash (30%), pruritus (22%), anemia (19%), arthralgia (19%), and decreased lymphocyte count (19%). There were two grade 4 adverse events: a lipase increase and a lung infection accompanied by sepsis.
The researchers also carried out whole exome sequencing of biopsies and found that 67% had NF-1 loss of function mutations.
Dr. Aroglu has served on advisory boards for Pfizer, Array, Eisai, Genentech, Natera, Novartis, OncoSec, and Regeneron. She has received research support from Boehringer Ingelheim, Pfizer, and Novartis. Dr. Kendra has received institutional support from Bristol Myers-Squibb and trial support from CheckMate Pharmaceuticals, GlaxoSmithKline, Immunocore, Medspace, Merck, Novartis, and Varian Medical Systems. Dr. Van Tine has financial relationships with a wide range of pharmaceutical companies.
FROM AACR 2023
Study suggests narrow excision margins safe in early melanoma resection
Current U.S., European, and Australian or melanoma-specific mortality (MSM), results of a retrospective study suggest.
Among 1,179 patients with stage T1a melanomas near the face, scalp, external genitalia, or other critical areas, the weighted 10-year local recurrence rate for patients who underwent resection with 10-mm margins was 5.7%, compared with 6.7% for those who had resections with 5-mm margins, a nonsignificant difference.
Weighted 10-year melanoma-specific mortality was 1.8% for patients treated with wide margins, vs. 4.2% for those treated with narrow margins, also a nonsignificant difference. Patients treated with narrow margins did have significantly fewer reconstructive surgeries than patients treated with wide margins, reported Andrea Maurichi, MD, and colleagues at the National Cancer Institute of Italy in Milan.
“Because this association was found in melanomas of the head and neck, acral, and genital sites, there is no plausible reason why it could not be extrapolated to other locations. The findings also support the need for prospective randomized clinical trials to definitively answer the important question about appropriate excision margins for T1a melanoma,” they wrote in the study, published online in JAMA Dermatology.
The authors also found, however, that Breslow thickness greater than 0.4 mm and mitotic rate greater than 1/mm2 were associated with worse MSM, and that acral lentiginous melanoma, lentigo maligna melanoma, and increasing Breslow thickness were associated with a higher incidence of local recurrence.
A melanoma expert who was not involved in the study said that despite these findings, wider margins are always preferable.
“There is always a conversation around these general [critical] areas, but as a rule we try to get larger margins,” said Ryan J. Sullivan, MD, of Mass General Cancer Center in Boston.
In an interview, Dr. Sullivan said that the finding about lower frequency of reconstructive procedures in the narrow margins groups may be more of a concern for younger patients than for the elderly.
Study design
The investigators conducted a retrospective cohort study of consecutive patients aged 18 or older at the National Cancer Institute of Milan who were diagnosed with T1a cutaneous melanoma close to critical areas from 2001 through 2020.
Patients with primary cutaneous melanoma of the head and face areas with functional or cosmetic considerations, acral areas (plantar, palmar, digital and interdigital areas), external genitalia, or periumbilical and perineal areas were eligible for inclusion.
The cohort comprised 1,179 patients with a median age of 50 and equal sex distribution. Of these patients, 626 (53%) had a wide excision, of whom 434 had a linear repair, and 192 had a flap of graft reconstruction. The remaining 553 patients had narrow excisions, 491 with linear repair, and 62 with flap or graft reconstruction.
Analyses were adjusted to account for imbalances between the surgical groups.
The study was supported by the nonprofit foundation Emme Rouge. The authors and Dr. Sullivan reported having no relevant conflicts of interest to disclose.
Current U.S., European, and Australian or melanoma-specific mortality (MSM), results of a retrospective study suggest.
Among 1,179 patients with stage T1a melanomas near the face, scalp, external genitalia, or other critical areas, the weighted 10-year local recurrence rate for patients who underwent resection with 10-mm margins was 5.7%, compared with 6.7% for those who had resections with 5-mm margins, a nonsignificant difference.
Weighted 10-year melanoma-specific mortality was 1.8% for patients treated with wide margins, vs. 4.2% for those treated with narrow margins, also a nonsignificant difference. Patients treated with narrow margins did have significantly fewer reconstructive surgeries than patients treated with wide margins, reported Andrea Maurichi, MD, and colleagues at the National Cancer Institute of Italy in Milan.
“Because this association was found in melanomas of the head and neck, acral, and genital sites, there is no plausible reason why it could not be extrapolated to other locations. The findings also support the need for prospective randomized clinical trials to definitively answer the important question about appropriate excision margins for T1a melanoma,” they wrote in the study, published online in JAMA Dermatology.
The authors also found, however, that Breslow thickness greater than 0.4 mm and mitotic rate greater than 1/mm2 were associated with worse MSM, and that acral lentiginous melanoma, lentigo maligna melanoma, and increasing Breslow thickness were associated with a higher incidence of local recurrence.
A melanoma expert who was not involved in the study said that despite these findings, wider margins are always preferable.
“There is always a conversation around these general [critical] areas, but as a rule we try to get larger margins,” said Ryan J. Sullivan, MD, of Mass General Cancer Center in Boston.
In an interview, Dr. Sullivan said that the finding about lower frequency of reconstructive procedures in the narrow margins groups may be more of a concern for younger patients than for the elderly.
Study design
The investigators conducted a retrospective cohort study of consecutive patients aged 18 or older at the National Cancer Institute of Milan who were diagnosed with T1a cutaneous melanoma close to critical areas from 2001 through 2020.
Patients with primary cutaneous melanoma of the head and face areas with functional or cosmetic considerations, acral areas (plantar, palmar, digital and interdigital areas), external genitalia, or periumbilical and perineal areas were eligible for inclusion.
The cohort comprised 1,179 patients with a median age of 50 and equal sex distribution. Of these patients, 626 (53%) had a wide excision, of whom 434 had a linear repair, and 192 had a flap of graft reconstruction. The remaining 553 patients had narrow excisions, 491 with linear repair, and 62 with flap or graft reconstruction.
Analyses were adjusted to account for imbalances between the surgical groups.
The study was supported by the nonprofit foundation Emme Rouge. The authors and Dr. Sullivan reported having no relevant conflicts of interest to disclose.
Current U.S., European, and Australian or melanoma-specific mortality (MSM), results of a retrospective study suggest.
Among 1,179 patients with stage T1a melanomas near the face, scalp, external genitalia, or other critical areas, the weighted 10-year local recurrence rate for patients who underwent resection with 10-mm margins was 5.7%, compared with 6.7% for those who had resections with 5-mm margins, a nonsignificant difference.
Weighted 10-year melanoma-specific mortality was 1.8% for patients treated with wide margins, vs. 4.2% for those treated with narrow margins, also a nonsignificant difference. Patients treated with narrow margins did have significantly fewer reconstructive surgeries than patients treated with wide margins, reported Andrea Maurichi, MD, and colleagues at the National Cancer Institute of Italy in Milan.
“Because this association was found in melanomas of the head and neck, acral, and genital sites, there is no plausible reason why it could not be extrapolated to other locations. The findings also support the need for prospective randomized clinical trials to definitively answer the important question about appropriate excision margins for T1a melanoma,” they wrote in the study, published online in JAMA Dermatology.
The authors also found, however, that Breslow thickness greater than 0.4 mm and mitotic rate greater than 1/mm2 were associated with worse MSM, and that acral lentiginous melanoma, lentigo maligna melanoma, and increasing Breslow thickness were associated with a higher incidence of local recurrence.
A melanoma expert who was not involved in the study said that despite these findings, wider margins are always preferable.
“There is always a conversation around these general [critical] areas, but as a rule we try to get larger margins,” said Ryan J. Sullivan, MD, of Mass General Cancer Center in Boston.
In an interview, Dr. Sullivan said that the finding about lower frequency of reconstructive procedures in the narrow margins groups may be more of a concern for younger patients than for the elderly.
Study design
The investigators conducted a retrospective cohort study of consecutive patients aged 18 or older at the National Cancer Institute of Milan who were diagnosed with T1a cutaneous melanoma close to critical areas from 2001 through 2020.
Patients with primary cutaneous melanoma of the head and face areas with functional or cosmetic considerations, acral areas (plantar, palmar, digital and interdigital areas), external genitalia, or periumbilical and perineal areas were eligible for inclusion.
The cohort comprised 1,179 patients with a median age of 50 and equal sex distribution. Of these patients, 626 (53%) had a wide excision, of whom 434 had a linear repair, and 192 had a flap of graft reconstruction. The remaining 553 patients had narrow excisions, 491 with linear repair, and 62 with flap or graft reconstruction.
Analyses were adjusted to account for imbalances between the surgical groups.
The study was supported by the nonprofit foundation Emme Rouge. The authors and Dr. Sullivan reported having no relevant conflicts of interest to disclose.
FROM JAMA DERMATOLOGY
What happens to melanocytic nevi during laser hair removal?
PHOENIX – , while common histologic changes include mild atypia and thermal damage, according to results from a systematic review of literature on the topic. To date, no severe cases of severe dysplasia or melanoma have been reported.
“That’s reassuring,” study author Ahuva Cices, MD, said in an interview at the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. “But, with that in mind, we want to avoid treating nevi with laser hair removal to avoid changes that could be concerning. We also recommend baseline skin exams so we know what we’re looking at before we start treating with lasers, and any changes can be recognized from that baseline status. It’s important to keep an eye out for changes and always be evaluating.”
In December of 2022, Dr. Cices, chief dermatology resident at Mount Sinai Health System, New York, searched PubMed for articles that evaluated changes in melanocytic nevi after laser hair removal procedures. She used the search terms “nevi laser hair removal,” “nevi diode,” “nevi long pulse alexandrite,” “nevi long pulse neodymium doped yttrium aluminum garnet,” and “melanoma laser hair removal,” and limited the analysis to English language patient-based reports that discussed incidental treatment of melanocytic nevi while undergoing hair removal with a laser.
Reports excluded from the analysis were those that focused on changes following hair removal with nonlaser devices such as intense pulsed light (IPL), those evaluating nonmelanocytic nevi such as Becker’s nevus or nevus of Ota, and those evaluating the intentional ablation or removal of melanocytic lesions.
The search yielded 10 relevant studies for systematic review: seven case reports or series and three observational trials, two of which were prospective and one retrospective.
The results of the review, according to Dr. Cices, revealed that clinical and dermoscopic changes were noted to present as early as 15 days after treatment and persist to the maximum follow up time, at 3 years. Commonly reported changes included regression, decreased size, laser-induced asymmetry, bleaching, darkening, and altered pattern on dermoscopy. Histologic changes included mild atypia, thermal damage, scar formation, and regression.
“Although some of the clinical and dermoscopic alterations may be concerning for malignancy, to our knowledge, there are no documented cases of malignant transformation of nevi following treatment with laser hair removal,” she wrote in the abstract.
Dr. Cices acknowledged certain limitations of the systematic review, including the low number of relevant reports and their generally small sample size, many of which were limited to single cases.
Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was asked to comment on the review, characterized the findings as important because laser hair removal is such a commonly performed procedure.
While the study is limited by the small number of studies on the subject matter, “it brings up an important discussion,” Dr. Ibrahimi said in an interview. “Generally speaking, we know that most hair removal lasers do indeed target melanin pigment and can be absorbed by melanocytes. While the wavelengths used for LHR [laser hair removal] will not result in DNA damage or cause mutations that can lead to melanoma, they can sometimes alter the appearance of pigmented lesions and that may change the dermatologist’s ability to monitor them for atypia,” he noted.
“For that reason, I would recommend all patients see a dermatologist for evaluation of their nevi prior to any treatments and they consider very carefully where they get their laser treatments. If they have any atypical pigmented lesions, then that information should be disclosed with the person performing the laser hair removal procedure particularly if there are lesions that are being specifically monitored.”
Dr. Cices reported having no disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
PHOENIX – , while common histologic changes include mild atypia and thermal damage, according to results from a systematic review of literature on the topic. To date, no severe cases of severe dysplasia or melanoma have been reported.
“That’s reassuring,” study author Ahuva Cices, MD, said in an interview at the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. “But, with that in mind, we want to avoid treating nevi with laser hair removal to avoid changes that could be concerning. We also recommend baseline skin exams so we know what we’re looking at before we start treating with lasers, and any changes can be recognized from that baseline status. It’s important to keep an eye out for changes and always be evaluating.”
In December of 2022, Dr. Cices, chief dermatology resident at Mount Sinai Health System, New York, searched PubMed for articles that evaluated changes in melanocytic nevi after laser hair removal procedures. She used the search terms “nevi laser hair removal,” “nevi diode,” “nevi long pulse alexandrite,” “nevi long pulse neodymium doped yttrium aluminum garnet,” and “melanoma laser hair removal,” and limited the analysis to English language patient-based reports that discussed incidental treatment of melanocytic nevi while undergoing hair removal with a laser.
Reports excluded from the analysis were those that focused on changes following hair removal with nonlaser devices such as intense pulsed light (IPL), those evaluating nonmelanocytic nevi such as Becker’s nevus or nevus of Ota, and those evaluating the intentional ablation or removal of melanocytic lesions.
The search yielded 10 relevant studies for systematic review: seven case reports or series and three observational trials, two of which were prospective and one retrospective.
The results of the review, according to Dr. Cices, revealed that clinical and dermoscopic changes were noted to present as early as 15 days after treatment and persist to the maximum follow up time, at 3 years. Commonly reported changes included regression, decreased size, laser-induced asymmetry, bleaching, darkening, and altered pattern on dermoscopy. Histologic changes included mild atypia, thermal damage, scar formation, and regression.
“Although some of the clinical and dermoscopic alterations may be concerning for malignancy, to our knowledge, there are no documented cases of malignant transformation of nevi following treatment with laser hair removal,” she wrote in the abstract.
Dr. Cices acknowledged certain limitations of the systematic review, including the low number of relevant reports and their generally small sample size, many of which were limited to single cases.
Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was asked to comment on the review, characterized the findings as important because laser hair removal is such a commonly performed procedure.
While the study is limited by the small number of studies on the subject matter, “it brings up an important discussion,” Dr. Ibrahimi said in an interview. “Generally speaking, we know that most hair removal lasers do indeed target melanin pigment and can be absorbed by melanocytes. While the wavelengths used for LHR [laser hair removal] will not result in DNA damage or cause mutations that can lead to melanoma, they can sometimes alter the appearance of pigmented lesions and that may change the dermatologist’s ability to monitor them for atypia,” he noted.
“For that reason, I would recommend all patients see a dermatologist for evaluation of their nevi prior to any treatments and they consider very carefully where they get their laser treatments. If they have any atypical pigmented lesions, then that information should be disclosed with the person performing the laser hair removal procedure particularly if there are lesions that are being specifically monitored.”
Dr. Cices reported having no disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
PHOENIX – , while common histologic changes include mild atypia and thermal damage, according to results from a systematic review of literature on the topic. To date, no severe cases of severe dysplasia or melanoma have been reported.
“That’s reassuring,” study author Ahuva Cices, MD, said in an interview at the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. “But, with that in mind, we want to avoid treating nevi with laser hair removal to avoid changes that could be concerning. We also recommend baseline skin exams so we know what we’re looking at before we start treating with lasers, and any changes can be recognized from that baseline status. It’s important to keep an eye out for changes and always be evaluating.”
In December of 2022, Dr. Cices, chief dermatology resident at Mount Sinai Health System, New York, searched PubMed for articles that evaluated changes in melanocytic nevi after laser hair removal procedures. She used the search terms “nevi laser hair removal,” “nevi diode,” “nevi long pulse alexandrite,” “nevi long pulse neodymium doped yttrium aluminum garnet,” and “melanoma laser hair removal,” and limited the analysis to English language patient-based reports that discussed incidental treatment of melanocytic nevi while undergoing hair removal with a laser.
Reports excluded from the analysis were those that focused on changes following hair removal with nonlaser devices such as intense pulsed light (IPL), those evaluating nonmelanocytic nevi such as Becker’s nevus or nevus of Ota, and those evaluating the intentional ablation or removal of melanocytic lesions.
The search yielded 10 relevant studies for systematic review: seven case reports or series and three observational trials, two of which were prospective and one retrospective.
The results of the review, according to Dr. Cices, revealed that clinical and dermoscopic changes were noted to present as early as 15 days after treatment and persist to the maximum follow up time, at 3 years. Commonly reported changes included regression, decreased size, laser-induced asymmetry, bleaching, darkening, and altered pattern on dermoscopy. Histologic changes included mild atypia, thermal damage, scar formation, and regression.
“Although some of the clinical and dermoscopic alterations may be concerning for malignancy, to our knowledge, there are no documented cases of malignant transformation of nevi following treatment with laser hair removal,” she wrote in the abstract.
Dr. Cices acknowledged certain limitations of the systematic review, including the low number of relevant reports and their generally small sample size, many of which were limited to single cases.
Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was asked to comment on the review, characterized the findings as important because laser hair removal is such a commonly performed procedure.
While the study is limited by the small number of studies on the subject matter, “it brings up an important discussion,” Dr. Ibrahimi said in an interview. “Generally speaking, we know that most hair removal lasers do indeed target melanin pigment and can be absorbed by melanocytes. While the wavelengths used for LHR [laser hair removal] will not result in DNA damage or cause mutations that can lead to melanoma, they can sometimes alter the appearance of pigmented lesions and that may change the dermatologist’s ability to monitor them for atypia,” he noted.
“For that reason, I would recommend all patients see a dermatologist for evaluation of their nevi prior to any treatments and they consider very carefully where they get their laser treatments. If they have any atypical pigmented lesions, then that information should be disclosed with the person performing the laser hair removal procedure particularly if there are lesions that are being specifically monitored.”
Dr. Cices reported having no disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
AT ASLMS 2023
Study highlights potential skin cancer risk of UV nail polish dryers
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Survival improved for some patients with metastatic cancers
Over the past 30 years, more than 80 new systemic therapies for cancer have been approved, and many patients diagnosed with localized disease have benefited with improved progression-free and overall survival. The same can be said for some – but by no means all – patients with metastatic disease at diagnosis, a new study indicates.
comment the authors, led by Marianne Luyendijk, MSc, from the Netherlands Comprehensive Cancer Organization, Utrecht.
The study was published online in the Journal of the National Cancer Institute.
The retrospective study compared survival data of patients with de novo metastatic disease diagnosed from 1989 through 1993 with those of patients diagnosed from 2014 to 2018.
The results show that 5-year survival increased by 15% or more among patients with metastatic gastrointestinal stromal tumors; neuroendocrine tumors; melanoma; and cancers of the prostate, breast, thyroid, and testes.
For patients with other cancers, however, the gains in survival were more modest. For example, over the study period, 5-year survival of patients with metastatic non–small cell lung cancer increased by only 6%, a disappointing finding, given the advent of targeted therapies and immunotherapy during the most recent period, the authors note.
In contrast, there was a 16% improvement in long-term survival of patients with metastatic melanoma, likely owing to the introduction of immune checkpoint inhibitors and targeted therapies, such as tyrosine kinase inhibitors.
The data also showed differences over time in the proportion of patients diagnosed with de novo metastatic disease; some cancers, such as NSCLC and small cell lung cancer, were more frequently diagnosed at late stages in the more recent era, possibly owing to increased screening and the use of technology such as FDG-PET imaging.
On the other end of the spectrum, cancers of the prostate, rectum, uterine cervix, breast, gallbladder, and bile ducts were more likely to be caught at an earlier stage during later years of the study period.
The authors say that among the possible explanations for a less than robust reduction over time in metastatic disease is that new drugs do not always translate into improved survival. They cite a 2017 study showing that among 53 new cancer drugs approved by U.S., European, or Australian drug regulators, fewer than half improved overall survival by at least 3 months, and an additional 26% offered survival advantages that were either shorter than 3 months or of unknown benefit.
“This may also explain why the 1- and 5-year survival rates of some cancers have changed little in the last 30 years,” they write. “Nevertheless, even minor benefits in survival or other outcomes (for example, quality of life) may represent progress in treating patients with metastatic cancer.”
The investigators recommend that to improve understanding of the effect of new therapies on survival of metastatic disease, cancer registries include data on therapies used beyond the first line, as well as comorbidities and quality-of-life measures.
The authors did not report a study funding source. Ms. Luyendijk has disclosed no relevant financial relationships. Several co-authors reported financial relationships with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Over the past 30 years, more than 80 new systemic therapies for cancer have been approved, and many patients diagnosed with localized disease have benefited with improved progression-free and overall survival. The same can be said for some – but by no means all – patients with metastatic disease at diagnosis, a new study indicates.
comment the authors, led by Marianne Luyendijk, MSc, from the Netherlands Comprehensive Cancer Organization, Utrecht.
The study was published online in the Journal of the National Cancer Institute.
The retrospective study compared survival data of patients with de novo metastatic disease diagnosed from 1989 through 1993 with those of patients diagnosed from 2014 to 2018.
The results show that 5-year survival increased by 15% or more among patients with metastatic gastrointestinal stromal tumors; neuroendocrine tumors; melanoma; and cancers of the prostate, breast, thyroid, and testes.
For patients with other cancers, however, the gains in survival were more modest. For example, over the study period, 5-year survival of patients with metastatic non–small cell lung cancer increased by only 6%, a disappointing finding, given the advent of targeted therapies and immunotherapy during the most recent period, the authors note.
In contrast, there was a 16% improvement in long-term survival of patients with metastatic melanoma, likely owing to the introduction of immune checkpoint inhibitors and targeted therapies, such as tyrosine kinase inhibitors.
The data also showed differences over time in the proportion of patients diagnosed with de novo metastatic disease; some cancers, such as NSCLC and small cell lung cancer, were more frequently diagnosed at late stages in the more recent era, possibly owing to increased screening and the use of technology such as FDG-PET imaging.
On the other end of the spectrum, cancers of the prostate, rectum, uterine cervix, breast, gallbladder, and bile ducts were more likely to be caught at an earlier stage during later years of the study period.
The authors say that among the possible explanations for a less than robust reduction over time in metastatic disease is that new drugs do not always translate into improved survival. They cite a 2017 study showing that among 53 new cancer drugs approved by U.S., European, or Australian drug regulators, fewer than half improved overall survival by at least 3 months, and an additional 26% offered survival advantages that were either shorter than 3 months or of unknown benefit.
“This may also explain why the 1- and 5-year survival rates of some cancers have changed little in the last 30 years,” they write. “Nevertheless, even minor benefits in survival or other outcomes (for example, quality of life) may represent progress in treating patients with metastatic cancer.”
The investigators recommend that to improve understanding of the effect of new therapies on survival of metastatic disease, cancer registries include data on therapies used beyond the first line, as well as comorbidities and quality-of-life measures.
The authors did not report a study funding source. Ms. Luyendijk has disclosed no relevant financial relationships. Several co-authors reported financial relationships with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Over the past 30 years, more than 80 new systemic therapies for cancer have been approved, and many patients diagnosed with localized disease have benefited with improved progression-free and overall survival. The same can be said for some – but by no means all – patients with metastatic disease at diagnosis, a new study indicates.
comment the authors, led by Marianne Luyendijk, MSc, from the Netherlands Comprehensive Cancer Organization, Utrecht.
The study was published online in the Journal of the National Cancer Institute.
The retrospective study compared survival data of patients with de novo metastatic disease diagnosed from 1989 through 1993 with those of patients diagnosed from 2014 to 2018.
The results show that 5-year survival increased by 15% or more among patients with metastatic gastrointestinal stromal tumors; neuroendocrine tumors; melanoma; and cancers of the prostate, breast, thyroid, and testes.
For patients with other cancers, however, the gains in survival were more modest. For example, over the study period, 5-year survival of patients with metastatic non–small cell lung cancer increased by only 6%, a disappointing finding, given the advent of targeted therapies and immunotherapy during the most recent period, the authors note.
In contrast, there was a 16% improvement in long-term survival of patients with metastatic melanoma, likely owing to the introduction of immune checkpoint inhibitors and targeted therapies, such as tyrosine kinase inhibitors.
The data also showed differences over time in the proportion of patients diagnosed with de novo metastatic disease; some cancers, such as NSCLC and small cell lung cancer, were more frequently diagnosed at late stages in the more recent era, possibly owing to increased screening and the use of technology such as FDG-PET imaging.
On the other end of the spectrum, cancers of the prostate, rectum, uterine cervix, breast, gallbladder, and bile ducts were more likely to be caught at an earlier stage during later years of the study period.
The authors say that among the possible explanations for a less than robust reduction over time in metastatic disease is that new drugs do not always translate into improved survival. They cite a 2017 study showing that among 53 new cancer drugs approved by U.S., European, or Australian drug regulators, fewer than half improved overall survival by at least 3 months, and an additional 26% offered survival advantages that were either shorter than 3 months or of unknown benefit.
“This may also explain why the 1- and 5-year survival rates of some cancers have changed little in the last 30 years,” they write. “Nevertheless, even minor benefits in survival or other outcomes (for example, quality of life) may represent progress in treating patients with metastatic cancer.”
The investigators recommend that to improve understanding of the effect of new therapies on survival of metastatic disease, cancer registries include data on therapies used beyond the first line, as well as comorbidities and quality-of-life measures.
The authors did not report a study funding source. Ms. Luyendijk has disclosed no relevant financial relationships. Several co-authors reported financial relationships with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
Symmetric Palmoplantar Papules With a Keratotic Border
The Diagnosis: Porokeratosis Plantaris Palmaris et Disseminata
A 3-mm punch biopsy of the right upper arm showed incipient cornoid lamellae formation, pigment incontinence, and sparse dermal lymphocytic inflammation (Figure), suggestive of porokeratosis plantaris palmaris et disseminata (PPPD). The dermatopathologist recommended a second biopsy to confirm the diagnosis and to confirm that the lesions on the palms and soles also were suggestive of porokeratosis. A second 4-mm punch biopsy of the left palm was consistent with PPPD.
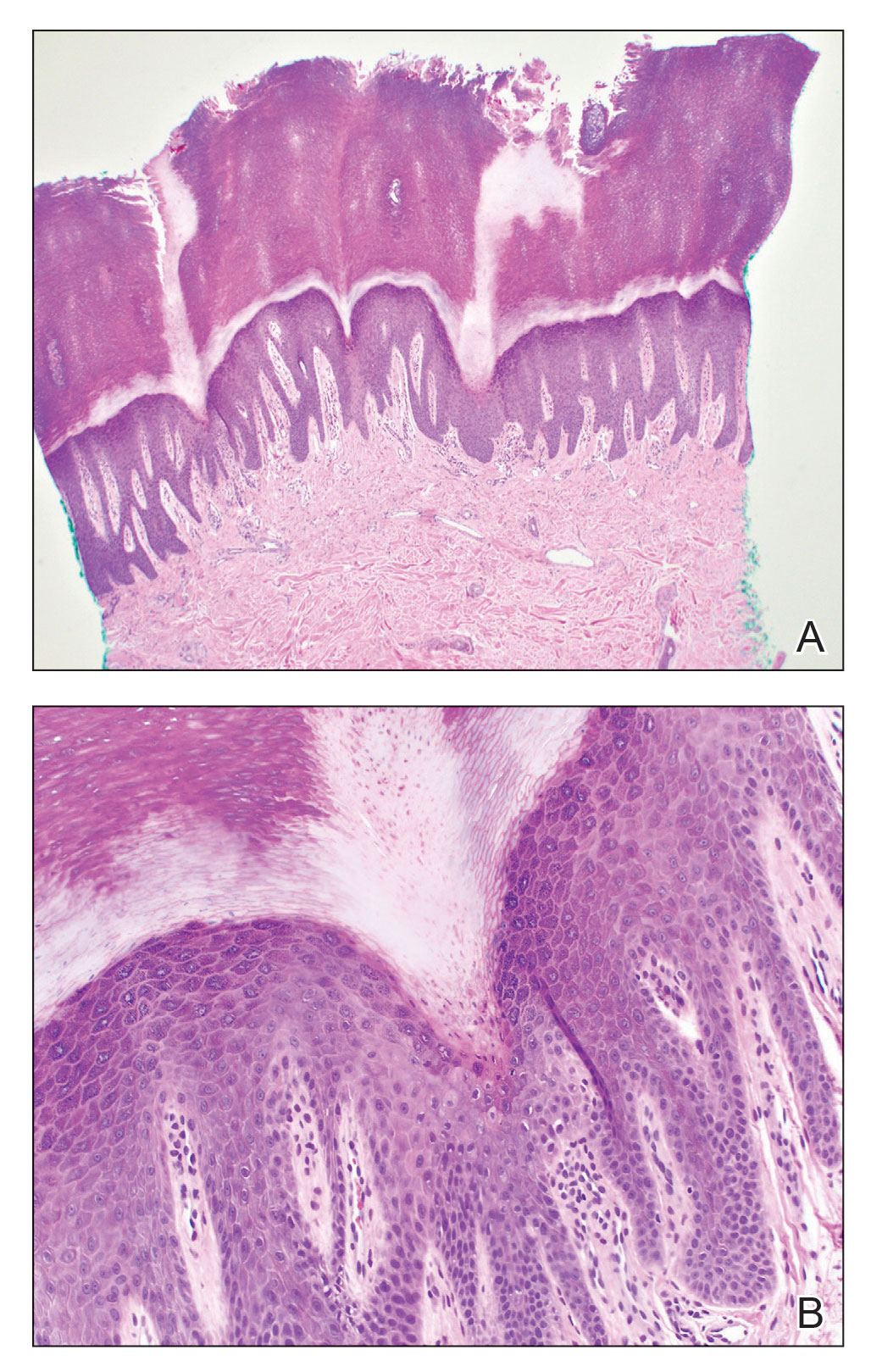
The risks of PPPD as a precancerous entity along with the benefits and side effects of the various management options were discussed with our patient. We recommended that he start low-dose isotretinoin (20 mg/d) due to the large body surface area affected, making focal and field treatments likely insufficient. However, our patient opted not to treat and did not return for follow-up.
Subtypes of porokeratosis, including disseminated superficial actinic porokeratosis (DSAP) and PPPD, are conditions that disrupt the normal maturation of keratin and present clinically with symmetric, crusted, annular papules.1 The signature but nonspecific histopathologic feature shared among the subtypes is the presence of a cornoid lamellae.2 Several triggers of porokeratosis have been proposed, including trauma and exposure to UV and ionizing radiation.2,3 The clinical variants of porokeratosis are important conditions to diagnose correctly because they portend a risk for Bowen disease and invasive squamous cell carcinoma and may indicate the presence of an underlying hematologic and/or solid organ malignancy.4 Management of porokeratosis is difficult, as treatments have shown limited efficacy and variable recurrence rates. Treatment options include focal, field, and systemic options, such as 5-fluorouracil, topical compound of cholesterol and lovastatin, isotretinoin, and acitretin.1,2
Porokeratoses may arise from gene mutations in the mevalonate pathway,5 which is essential for the production of cholesterol.6 Topical cholesterol alone has not been shown to improve porokeratosis, but the combination topical therapy of cholesterol and lovastatin is promising. It is theorized to deliver benefit by both providing the essential end product of the pathway and simultaneously reducing the number of potentially toxic intermediates.6
Porokeratosis plantaris palmaris et disseminata (also known as porokeratosis plantaris) is unique among the subtypes of porokeratosis in that its annular, red-pink, papular rash with scaling and a keratotic border tends to start distally, involving the palms and soles, and progresses proximally to the trunk with smaller lesions.1,7 This centripetal progression can take years, as was seen in our patient.1 The disease is uncommon, with a dearth of published reports on PPPD.2 However, case reports have shown that PPPD is strongly linked to family history and may have an autosomal-dominant inheritance pattern. Penetrance is greater in men than in women, as PPPD is twice as common in men.8 Most cases of PPPD have been diagnosed in patients in their 20s and 30s, but Hartman et al9 reported a case wherein a patient was diagnosed with PPPD after 65 years of age, similar to our patient.
Although the lesions in DSAP can appear similar to those in PPPD, DSAP is more common among the family of porokeratotic conditions, affecting women twice as often as men, with a sporadic pattern of inheritance.2 These same features are present in some other types of porokeratosis but not PPPD. Furthermore, DSAP progresses proximally to distally but often with truncal sparing.2
Akin to PPPD, pityriasis rubra pilaris (PRP) often presents with palmoplantar keratoderma.10 There are at least 6 types of PRP with varying degrees of similarity to PPPD. However, in many cases PRP is associated with a background of diffuse erythema on the body with islands of spared skin. In addition, cases of PRP have been linked to extracutaneous findings such as ectropion and joint pain.11
Darier disease, especially the acrokeratosis verruciformis of Hopf variant, is more common in men and involves younger populations, as in PPPD.11 However, the crusted lesions seen in Darier disease frequently involve the skin folds. These intertriginous lesions may coalesce, mimicking warts in appearance, and are at risk for secondary infection. Nail findings in Darier disease also are distinct and include longitudinal white or red stripes running along the nail bed, in addition to V-shaped nicks at the nail tips.
Psoriasis can occur anywhere on the body and is associated with silver scaling atop a salmon-colored dermatitis.12 It results from aberrant proliferation of keratinocytes. Some distinguishing features of psoriasis include a disease course that waxes and wanes as well as pitting of the nails.
Although PPPD typically affects young adults, we presented a case of PPPD in an older man. Porokeratosis plantaris palmaris et disseminata in older adults may represent a delayed diagnosis, imply a broader range for the age of onset, or suggest its manifestation secondary to radiation treatment or another phenomenon. For example, our patient received 35 radiotherapy cycles for tongue cancer more than 5 years prior to the onset of PPPD.
- Irisawa R, Yamazaki M, Yamamoto T, et al. A case of porokeratosis plantaris palmaris et disseminata and literature review. Dermatol Online J. 2012;18:5.
- Vargas-Mora P, Morgado-Carrasco D, Fusta-Novell X. Porokeratosis: a review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr. 2020;111:545-560.
- James AJ, Clarke LE, Elenitsas R, et al. Segmental porokeratosis after radiation therapy for follicular lymphoma. J Am Acad Dermatol. 2008;58(2 suppl):S49-S50.
- Schena D, Papagrigoraki A, Frigo A, et al. Eruptive disseminated porokeratosis associated with internal malignancies: a case report. Cutis. 2010;85:156-159.
- Zhang Z, Li C, Wu F, et al. Genomic variations of the mevalonate pathway in porokeratosis. Elife. 2015;4:E06322. doi:10.7554/eLife.06322
- Atzmony L, Lim YH, Hamilton C, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82:123-131. doi:10.1016/j.jaad.2019.08.043
- Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris, et disseminata. a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
- Kanitakis J. Porokeratoses: an update of clinical, aetiopathogenic and therapeutic features. Eur J Dermatol. 2014;24:533-544.
- Hartman R, Mandal R, Sanchez M, et al. Porokeratosis plantaris, palmaris, et disseminata. Dermatol Online J. 2010;16:22.
- Suryawanshi H, Dhobley A, Sharma A, et al. Darier disease: a rare genodermatosis. J Oral Maxillofac Pathol. 2017;21:321. doi:10.4103/jomfp.JOMFP_170_16
- Eastham AB. Pityriasis rubra pilaris. JAMA Dermatol. 2019;155:404. doi:10.1001/jamadermatol.2018.5030
- Nair PA, Badri T. Psoriasis. StatPearls Publishing; 2022. Updated April 6, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK448194/
The Diagnosis: Porokeratosis Plantaris Palmaris et Disseminata
A 3-mm punch biopsy of the right upper arm showed incipient cornoid lamellae formation, pigment incontinence, and sparse dermal lymphocytic inflammation (Figure), suggestive of porokeratosis plantaris palmaris et disseminata (PPPD). The dermatopathologist recommended a second biopsy to confirm the diagnosis and to confirm that the lesions on the palms and soles also were suggestive of porokeratosis. A second 4-mm punch biopsy of the left palm was consistent with PPPD.

The risks of PPPD as a precancerous entity along with the benefits and side effects of the various management options were discussed with our patient. We recommended that he start low-dose isotretinoin (20 mg/d) due to the large body surface area affected, making focal and field treatments likely insufficient. However, our patient opted not to treat and did not return for follow-up.
Subtypes of porokeratosis, including disseminated superficial actinic porokeratosis (DSAP) and PPPD, are conditions that disrupt the normal maturation of keratin and present clinically with symmetric, crusted, annular papules.1 The signature but nonspecific histopathologic feature shared among the subtypes is the presence of a cornoid lamellae.2 Several triggers of porokeratosis have been proposed, including trauma and exposure to UV and ionizing radiation.2,3 The clinical variants of porokeratosis are important conditions to diagnose correctly because they portend a risk for Bowen disease and invasive squamous cell carcinoma and may indicate the presence of an underlying hematologic and/or solid organ malignancy.4 Management of porokeratosis is difficult, as treatments have shown limited efficacy and variable recurrence rates. Treatment options include focal, field, and systemic options, such as 5-fluorouracil, topical compound of cholesterol and lovastatin, isotretinoin, and acitretin.1,2
Porokeratoses may arise from gene mutations in the mevalonate pathway,5 which is essential for the production of cholesterol.6 Topical cholesterol alone has not been shown to improve porokeratosis, but the combination topical therapy of cholesterol and lovastatin is promising. It is theorized to deliver benefit by both providing the essential end product of the pathway and simultaneously reducing the number of potentially toxic intermediates.6
Porokeratosis plantaris palmaris et disseminata (also known as porokeratosis plantaris) is unique among the subtypes of porokeratosis in that its annular, red-pink, papular rash with scaling and a keratotic border tends to start distally, involving the palms and soles, and progresses proximally to the trunk with smaller lesions.1,7 This centripetal progression can take years, as was seen in our patient.1 The disease is uncommon, with a dearth of published reports on PPPD.2 However, case reports have shown that PPPD is strongly linked to family history and may have an autosomal-dominant inheritance pattern. Penetrance is greater in men than in women, as PPPD is twice as common in men.8 Most cases of PPPD have been diagnosed in patients in their 20s and 30s, but Hartman et al9 reported a case wherein a patient was diagnosed with PPPD after 65 years of age, similar to our patient.
Although the lesions in DSAP can appear similar to those in PPPD, DSAP is more common among the family of porokeratotic conditions, affecting women twice as often as men, with a sporadic pattern of inheritance.2 These same features are present in some other types of porokeratosis but not PPPD. Furthermore, DSAP progresses proximally to distally but often with truncal sparing.2
Akin to PPPD, pityriasis rubra pilaris (PRP) often presents with palmoplantar keratoderma.10 There are at least 6 types of PRP with varying degrees of similarity to PPPD. However, in many cases PRP is associated with a background of diffuse erythema on the body with islands of spared skin. In addition, cases of PRP have been linked to extracutaneous findings such as ectropion and joint pain.11
Darier disease, especially the acrokeratosis verruciformis of Hopf variant, is more common in men and involves younger populations, as in PPPD.11 However, the crusted lesions seen in Darier disease frequently involve the skin folds. These intertriginous lesions may coalesce, mimicking warts in appearance, and are at risk for secondary infection. Nail findings in Darier disease also are distinct and include longitudinal white or red stripes running along the nail bed, in addition to V-shaped nicks at the nail tips.
Psoriasis can occur anywhere on the body and is associated with silver scaling atop a salmon-colored dermatitis.12 It results from aberrant proliferation of keratinocytes. Some distinguishing features of psoriasis include a disease course that waxes and wanes as well as pitting of the nails.
Although PPPD typically affects young adults, we presented a case of PPPD in an older man. Porokeratosis plantaris palmaris et disseminata in older adults may represent a delayed diagnosis, imply a broader range for the age of onset, or suggest its manifestation secondary to radiation treatment or another phenomenon. For example, our patient received 35 radiotherapy cycles for tongue cancer more than 5 years prior to the onset of PPPD.
The Diagnosis: Porokeratosis Plantaris Palmaris et Disseminata
A 3-mm punch biopsy of the right upper arm showed incipient cornoid lamellae formation, pigment incontinence, and sparse dermal lymphocytic inflammation (Figure), suggestive of porokeratosis plantaris palmaris et disseminata (PPPD). The dermatopathologist recommended a second biopsy to confirm the diagnosis and to confirm that the lesions on the palms and soles also were suggestive of porokeratosis. A second 4-mm punch biopsy of the left palm was consistent with PPPD.

The risks of PPPD as a precancerous entity along with the benefits and side effects of the various management options were discussed with our patient. We recommended that he start low-dose isotretinoin (20 mg/d) due to the large body surface area affected, making focal and field treatments likely insufficient. However, our patient opted not to treat and did not return for follow-up.
Subtypes of porokeratosis, including disseminated superficial actinic porokeratosis (DSAP) and PPPD, are conditions that disrupt the normal maturation of keratin and present clinically with symmetric, crusted, annular papules.1 The signature but nonspecific histopathologic feature shared among the subtypes is the presence of a cornoid lamellae.2 Several triggers of porokeratosis have been proposed, including trauma and exposure to UV and ionizing radiation.2,3 The clinical variants of porokeratosis are important conditions to diagnose correctly because they portend a risk for Bowen disease and invasive squamous cell carcinoma and may indicate the presence of an underlying hematologic and/or solid organ malignancy.4 Management of porokeratosis is difficult, as treatments have shown limited efficacy and variable recurrence rates. Treatment options include focal, field, and systemic options, such as 5-fluorouracil, topical compound of cholesterol and lovastatin, isotretinoin, and acitretin.1,2
Porokeratoses may arise from gene mutations in the mevalonate pathway,5 which is essential for the production of cholesterol.6 Topical cholesterol alone has not been shown to improve porokeratosis, but the combination topical therapy of cholesterol and lovastatin is promising. It is theorized to deliver benefit by both providing the essential end product of the pathway and simultaneously reducing the number of potentially toxic intermediates.6
Porokeratosis plantaris palmaris et disseminata (also known as porokeratosis plantaris) is unique among the subtypes of porokeratosis in that its annular, red-pink, papular rash with scaling and a keratotic border tends to start distally, involving the palms and soles, and progresses proximally to the trunk with smaller lesions.1,7 This centripetal progression can take years, as was seen in our patient.1 The disease is uncommon, with a dearth of published reports on PPPD.2 However, case reports have shown that PPPD is strongly linked to family history and may have an autosomal-dominant inheritance pattern. Penetrance is greater in men than in women, as PPPD is twice as common in men.8 Most cases of PPPD have been diagnosed in patients in their 20s and 30s, but Hartman et al9 reported a case wherein a patient was diagnosed with PPPD after 65 years of age, similar to our patient.
Although the lesions in DSAP can appear similar to those in PPPD, DSAP is more common among the family of porokeratotic conditions, affecting women twice as often as men, with a sporadic pattern of inheritance.2 These same features are present in some other types of porokeratosis but not PPPD. Furthermore, DSAP progresses proximally to distally but often with truncal sparing.2
Akin to PPPD, pityriasis rubra pilaris (PRP) often presents with palmoplantar keratoderma.10 There are at least 6 types of PRP with varying degrees of similarity to PPPD. However, in many cases PRP is associated with a background of diffuse erythema on the body with islands of spared skin. In addition, cases of PRP have been linked to extracutaneous findings such as ectropion and joint pain.11
Darier disease, especially the acrokeratosis verruciformis of Hopf variant, is more common in men and involves younger populations, as in PPPD.11 However, the crusted lesions seen in Darier disease frequently involve the skin folds. These intertriginous lesions may coalesce, mimicking warts in appearance, and are at risk for secondary infection. Nail findings in Darier disease also are distinct and include longitudinal white or red stripes running along the nail bed, in addition to V-shaped nicks at the nail tips.
Psoriasis can occur anywhere on the body and is associated with silver scaling atop a salmon-colored dermatitis.12 It results from aberrant proliferation of keratinocytes. Some distinguishing features of psoriasis include a disease course that waxes and wanes as well as pitting of the nails.
Although PPPD typically affects young adults, we presented a case of PPPD in an older man. Porokeratosis plantaris palmaris et disseminata in older adults may represent a delayed diagnosis, imply a broader range for the age of onset, or suggest its manifestation secondary to radiation treatment or another phenomenon. For example, our patient received 35 radiotherapy cycles for tongue cancer more than 5 years prior to the onset of PPPD.
- Irisawa R, Yamazaki M, Yamamoto T, et al. A case of porokeratosis plantaris palmaris et disseminata and literature review. Dermatol Online J. 2012;18:5.
- Vargas-Mora P, Morgado-Carrasco D, Fusta-Novell X. Porokeratosis: a review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr. 2020;111:545-560.
- James AJ, Clarke LE, Elenitsas R, et al. Segmental porokeratosis after radiation therapy for follicular lymphoma. J Am Acad Dermatol. 2008;58(2 suppl):S49-S50.
- Schena D, Papagrigoraki A, Frigo A, et al. Eruptive disseminated porokeratosis associated with internal malignancies: a case report. Cutis. 2010;85:156-159.
- Zhang Z, Li C, Wu F, et al. Genomic variations of the mevalonate pathway in porokeratosis. Elife. 2015;4:E06322. doi:10.7554/eLife.06322
- Atzmony L, Lim YH, Hamilton C, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82:123-131. doi:10.1016/j.jaad.2019.08.043
- Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris, et disseminata. a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
- Kanitakis J. Porokeratoses: an update of clinical, aetiopathogenic and therapeutic features. Eur J Dermatol. 2014;24:533-544.
- Hartman R, Mandal R, Sanchez M, et al. Porokeratosis plantaris, palmaris, et disseminata. Dermatol Online J. 2010;16:22.
- Suryawanshi H, Dhobley A, Sharma A, et al. Darier disease: a rare genodermatosis. J Oral Maxillofac Pathol. 2017;21:321. doi:10.4103/jomfp.JOMFP_170_16
- Eastham AB. Pityriasis rubra pilaris. JAMA Dermatol. 2019;155:404. doi:10.1001/jamadermatol.2018.5030
- Nair PA, Badri T. Psoriasis. StatPearls Publishing; 2022. Updated April 6, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK448194/
- Irisawa R, Yamazaki M, Yamamoto T, et al. A case of porokeratosis plantaris palmaris et disseminata and literature review. Dermatol Online J. 2012;18:5.
- Vargas-Mora P, Morgado-Carrasco D, Fusta-Novell X. Porokeratosis: a review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr. 2020;111:545-560.
- James AJ, Clarke LE, Elenitsas R, et al. Segmental porokeratosis after radiation therapy for follicular lymphoma. J Am Acad Dermatol. 2008;58(2 suppl):S49-S50.
- Schena D, Papagrigoraki A, Frigo A, et al. Eruptive disseminated porokeratosis associated with internal malignancies: a case report. Cutis. 2010;85:156-159.
- Zhang Z, Li C, Wu F, et al. Genomic variations of the mevalonate pathway in porokeratosis. Elife. 2015;4:E06322. doi:10.7554/eLife.06322
- Atzmony L, Lim YH, Hamilton C, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82:123-131. doi:10.1016/j.jaad.2019.08.043
- Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris, et disseminata. a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
- Kanitakis J. Porokeratoses: an update of clinical, aetiopathogenic and therapeutic features. Eur J Dermatol. 2014;24:533-544.
- Hartman R, Mandal R, Sanchez M, et al. Porokeratosis plantaris, palmaris, et disseminata. Dermatol Online J. 2010;16:22.
- Suryawanshi H, Dhobley A, Sharma A, et al. Darier disease: a rare genodermatosis. J Oral Maxillofac Pathol. 2017;21:321. doi:10.4103/jomfp.JOMFP_170_16
- Eastham AB. Pityriasis rubra pilaris. JAMA Dermatol. 2019;155:404. doi:10.1001/jamadermatol.2018.5030
- Nair PA, Badri T. Psoriasis. StatPearls Publishing; 2022. Updated April 6, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK448194/
A 67-year-old man presented to our office with a rash on the hands, feet, and periungual skin that began with wartlike growths many years prior and recently had started to involve the proximal arms and legs up to the thighs as well as the trunk. He had a medical history of essential hypertension and chronic obstructive pulmonary disease. He had an 18-year smoking history and had quit more than 25 years prior, with tongue cancer diagnosed more than 5 years prior that was treated with surgery, chemotherapy, and radiation. The lesions occasionally were itchy but not painful. He also reported that his nails frequently split down the middle. He denied any oral lesions and was not using any treatments for the rash. He had no history of skin cancer or other skin conditions. His family history was unclear. Physical examination revealed annular red-pink scaling with a keratotic border on the soles of the feet, palms, and periungual skin. There also were small hyperpigmented papules on the arms, legs, thighs, and trunk over a background of dry and discolored skin, as well as dystrophy of all nails.