User login
Hair Repigmentation as a Melanoma Warning Sign
To the Editor:
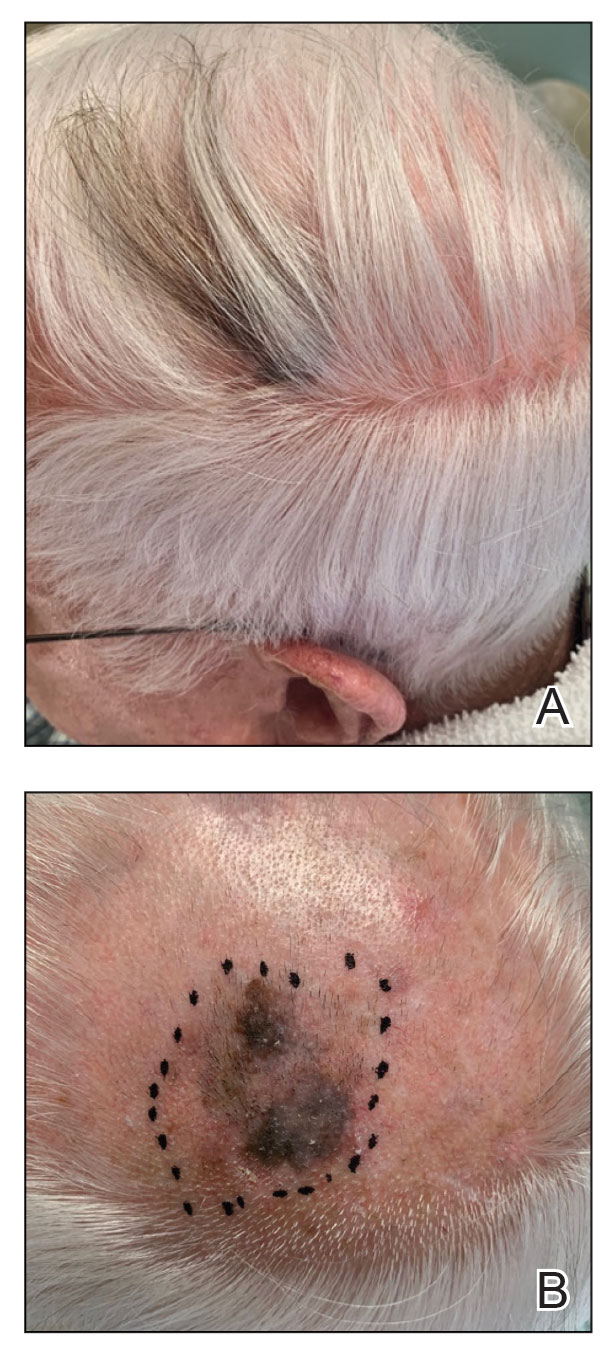
An 85-year-old man with a history of hypertension and chronic kidney disease presented with a localized darkening patch of hair on the left parietal scalp that had progressed over the last 7 years (Figure 1A). He had no prior history of skin cancer. Physical examination revealed the remainder of the hair was gray. There was an irregularly pigmented plaque on the skin underlying the darkened hair measuring 5.0 cm in diameter that was confirmed to be melanoma (Figure 1B). He underwent a staged excision to remove the lesion. The surgical defect was closed via a 5.0×6.0-cm full-thickness skin graft.
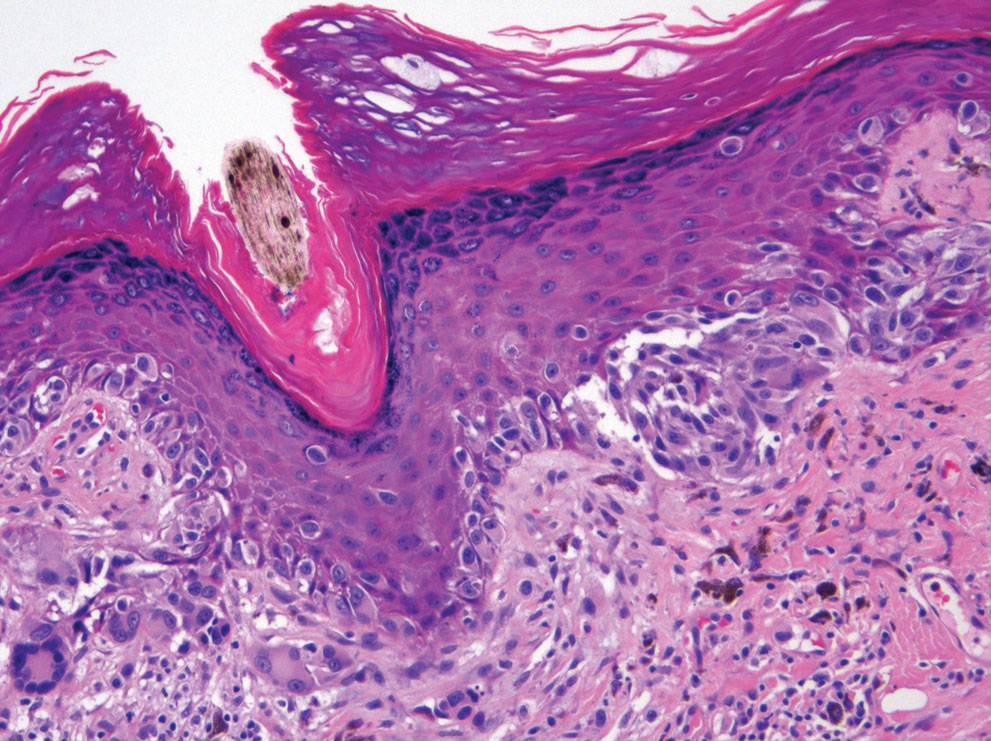
The initial biopsy showed melanoma in situ. However, the final pathology report following the excision revealed an invasive melanoma with a Breslow depth of 1.0 mm (Clark level IV; American Joint Committee on Cancer T1b).1 Histopathology showed pigment deposition with surrounding deep follicular extension of melanoma (Figure 2).
The patient declined a sentinel lymph node biopsy and agreed to a genetic profile assessment.2 The results of the test identified the patient had a low probability of a positive sentinel lymph node and the lowest risk of melanoma recurrence within 5 years. The patient was clear of disease at 12-month follow-up.
Based on a PubMed search of articles indexed for MEDLINE using the terms hair repigmentation and melanoma, there have been 11 other reported cases of hair repigmentation associated with melanoma (Table).3-13 It initially was suspected that this rare phenomenon primarily existed in the female population, as the first 5 cases were reported solely in females,3-7 possibly due to the prevalence of androgenetic alopecia in males.11 However, 6 cases of repigmentation associated with melanoma were later reported in males8-13; our patient represents an additional reported case in a male. It is unknown if there is a higher prevalence of this phenomenon among males or females.

Most previously reported cases of repigmentation were associated with melanoma in situ, lentigo maligna type. Repigmentation also has been reported in malignant melanoma, as documented in our patient, as well as desmoplastic and amelanotic melanoma.5,6 In every case, the color of the repigmentation was darker than the rest of the patient’s hair; however, the repigmentation color can be different from the patient’s original hair color from their youth.4,5,11
The exact mechanism responsible for hair repigmentation in the setting of melanoma is unclear. It has been speculated from prior cases that repigmentation may be caused by paracrine stimulation from melanoma cells activating adjacent benign hair follicle melanocytes to produce melanin.7,14,15 This process likely is due to cytokines or growth factors, such as c-kit ligand.14,15 Several neural and immune networks and mediators activate the receptor tyrosine kinase KIT, which is thought to play a role in activating melanogenesis within the hair bulb.14 These signals also could originate from changes in the microenvironment instead of the melanoma cells themselves.6 Another possible mechanism is that repigmentation was caused by melanin-producing malignant melanocytes.4
Because this phenomenon typically occurs in older patients, the cause of repigmentation also could be related to chronic sun damage, which may result in upregulation of stem cell factor and α-melanocyte–stimulating hormone, as well as other molecules associated with melanogenesis, such as c-KIT receptor and tyrosinase.15,16 Upregulation of these molecules can lead to an increased number of melanocytes within the hair bulb. In addition, UVA and narrowband UVB have been recognized as major players in melanocyte stimulation. Phototherapy with UVA or narrowband UVB has been used for repigmentation in vitiligo patients.17
In cases without invasion of hair follicles by malignant cells, repigmentation more likely results from external signals stimulating benign bulbar melanocytes to produce melanin rather than melanoma cell growth extending into the hair bulb.6 In these cases, there is an increase in the number of hair bulbar melanocytes with a lack of malignant morphology in the hair bulb.8 If the signals are directly from melanoma cells in the hair bulb, it is unknown how the malignant cells upregulated melanogenesis in adjacent benign melanocytes or which specific signals required for normal pigmentation were involved in these repigmentation cases.6
Use of medications was ruled out as an underlying cause of the repigmentation in our patient. Drug-related repigmentation of the hair typically is observed in a diffuse generalized pattern. In our case, the repigmentation was localized to the area of the underlying dark patch, and the patient was not on any medications that could cause hair hyperpigmentation. Hyperpigmentation has been associated with acitretin, lenalidomide, corticosteroids, erlotinib, latanoprost, verapamil, tamoxifen, levodopa, thalidomide, PD-1 inhibitors, and tumor necrosis α inhibitors.18-30 Repigmentation also has been reported after local radiotherapy and herpes zoster infection.31,32
The underlying melanoma in our patient was removed by staged square excision. Excision was the treatment of choice for most similar reported cases. Radiotherapy was utilized in two different cases.3,4 In one case, radiotherapy was successfully used to treat melanoma in situ, lentigo maligna type; the patient’s hair grew back to its original color, which suggests that normal hair physiology was restored once melanoma cells were eliminated.3 One reported case demonstrated successful treatment of lentigo maligna type–melanoma with imiquimod cream 5% applied 6 times weekly for 9 months with a positive cosmetic result.9 The exact mechanism of imiquimod is not fully understood. Imiquimod induces cytokines to stimulate the production of IFN-α via activation of toll-like receptor 7.33 There was complete clearing of the lesion as well as the hair pigmentation,9 which suggests that the treatment also eliminated deeper cells influencing pigmentation. A case of malignant amelanotic melanoma was successfully treated with anti–PD-1 antibody pembrolizumab (2 mg/kg every 3 weeks), with no recurrence at 12 months. Pembrolizumab acts as an immune checkpoint inhibitor by binding to the PD-1 receptor and allowing the immune system to recognize and attack melanoma cells. After 5 doses of pembrolizumab, the patient was clear of disease and his hair color returned to gray.5
In 2022, melanoma was estimated to be the fifth most commonly diagnosed cancer among men and women in the United States.34 Early melanoma detection is a critical factor in achieving positive patient outcomes. Hair repigmentation is a potentially serious phenomenon that warrants a physician visit. Melanoma lesions under the hair may be overlooked because of limited visibility. Physicians must inspect spontaneous hair repigmentation with high suspicion and interpret the change as a possible indirect result of melanoma. Overall, it is important to increase public awareness of regular skin checks and melanoma warning signs.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1–T2 melanoma using gene expression profiling. Futur Oncol. 2019;15:1207-1217.
- Dummer R. Hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
- Inzinger M, Massone C, Arzberger E, et al. Hair repigmentation in melanoma. Lancet. 2013;382:1224.
- Rahim RR, Husain A, Tobin DJ, et al. Desmoplastic melanoma presenting with localized hair repigmentation. Br J Dermatol. 2013;169:1371-1373.
- Tiger JB, Habeshian KA, Barton DT, et al. Repigmentation of hair associated with melanoma in situ of scalp. J Am Acad Dermatol. 2014;71:E144-E145.
- Amann VC, Dummer R. Localized hair repigmentation in a 91-year-old woman. JAMA Dermatol. 2016;152:81-82.
- Chan C, Magro CM, Pham AK, et al. Spontaneous hair repigmentation in an 80-year-old man: a case of melanoma-associated hair repigmentation and review of the literature. Am J Dermatopathol. 2019;41:671-674.
- Lackey AE, Glassman G, Grichnik J, et al. Repigmentation of gray hairs with lentigo maligna and response to topical imiquimod. JAAD Case Rep. 2019;5:1015-1017.
- Chew T, Pannell M, Jeeves A. Focal hair re-pigmentation associated with melanoma of the scalp. ANZ J Surg. 2019;90:1175-1176.
- López-Sánchez C, Collgros H. Hair repigmentation as a clue for scalp melanoma. Australas J Dermatol. 2019;61:179-180.
- Gessler J, Tejasvi T, Bresler SC. Repigmentation of scalp hair: a feature of early melanoma. Am J Med. 2023;136:E7-E8.
- Hasegawa T, Iino S, Kitakaze K, et al. Repigmentation of aging gray hair associated with unrecognized development and progression of amelanotic melanoma of the scalp: a physiological alert underlying hair rejuvenation. J Dermatol. 2021;48:E281-E283. doi:10.1111/1346-8138.15881
- D’Mello SAN, Finlay GJ, Baguley BC, et al. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:1144.
- Hachiya A, Kobayashi A, Ohuchi A, et al. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001;116:578-586.
- Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
- Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313.
- Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol. 2009;50:214-216.
- Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process! J Oncol Pharm Pract. 2013;19:165-169.
- Sebaratnam DF, Rodríguez Bandera AI, Lowe PM. Hair repigmentation with anti–PD-1 and anti–PD-L1 immunotherapy: a novel hypothesis. JAMA Dermatol. 2018;154:112-113. doi:10.1001/jamadermatol.2017.4420
- Tintle SJ, Dabade TS, Kalish RA, et al. Repigmentation of hair following adalimumab therapy. Dermatol Online J. 2015;21:13030/qt6fn0t1xz.
- Penzi LR, Manatis-Lornell A, Saavedra A, et al. Hair repigmentation associated with the use of brentuximab. JAAD Case Rep. 2017;3:563-565.
- Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatology Venereol. 2008;22:1018-1020.
- Cheng YP, Chen HJ, Chiu HC. Erlotinib-induced hair repigmentation. Int J Dermatol. 2014;53:E55-E57.
- Bellandi S, Amato L, Cipollini EM, et al. Repigmentation of hair after latanoprost therapy. J Eur Acad Dermatology Venereol. 2011;25:1485-1487.
- Read GM. Verapamil and hair colour change. Lancet. 1991;338:1520.
- Hampson JP, Donnelly A, Lewis‐Jones MS, et al. Tamoxifen‐induced hair colour change. Br J Dermatol. 1995;132:483-484.
- Reynolds NJ, Crossley J, Ferguson I, et al. Darkening of white hair in Parkinson’s disease. Clin Exp Dermatol. 1989;14:317-318.
- Lovering S, Miao W, Bailie T, et al. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016;2016:bcr2016215521.
- Rivera N, Boada A, Bielsa MI, et al. Hair repigmentation during immunotherapy treatment with an anti–programmed cell death 1 and anti–programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol. 2017;153:1162-1165.
- Prasad S, Dougheney N, Hong A. Scalp hair repigmentation in the penumbral region of radiotherapy–a case series. Int J Radiol Radiat Ther. 2020;7:151-157.
- Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
- Hanna E, Abadi R, Abbas O. Imiquimod in dermatology: an overview. Int J Dermatol. 2016;55:831-844.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
To the Editor:
An 85-year-old man with a history of hypertension and chronic kidney disease presented with a localized darkening patch of hair on the left parietal scalp that had progressed over the last 7 years (Figure 1A). He had no prior history of skin cancer. Physical examination revealed the remainder of the hair was gray. There was an irregularly pigmented plaque on the skin underlying the darkened hair measuring 5.0 cm in diameter that was confirmed to be melanoma (Figure 1B). He underwent a staged excision to remove the lesion. The surgical defect was closed via a 5.0×6.0-cm full-thickness skin graft.

The initial biopsy showed melanoma in situ. However, the final pathology report following the excision revealed an invasive melanoma with a Breslow depth of 1.0 mm (Clark level IV; American Joint Committee on Cancer T1b).1 Histopathology showed pigment deposition with surrounding deep follicular extension of melanoma (Figure 2).

The patient declined a sentinel lymph node biopsy and agreed to a genetic profile assessment.2 The results of the test identified the patient had a low probability of a positive sentinel lymph node and the lowest risk of melanoma recurrence within 5 years. The patient was clear of disease at 12-month follow-up.
Based on a PubMed search of articles indexed for MEDLINE using the terms hair repigmentation and melanoma, there have been 11 other reported cases of hair repigmentation associated with melanoma (Table).3-13 It initially was suspected that this rare phenomenon primarily existed in the female population, as the first 5 cases were reported solely in females,3-7 possibly due to the prevalence of androgenetic alopecia in males.11 However, 6 cases of repigmentation associated with melanoma were later reported in males8-13; our patient represents an additional reported case in a male. It is unknown if there is a higher prevalence of this phenomenon among males or females.

Most previously reported cases of repigmentation were associated with melanoma in situ, lentigo maligna type. Repigmentation also has been reported in malignant melanoma, as documented in our patient, as well as desmoplastic and amelanotic melanoma.5,6 In every case, the color of the repigmentation was darker than the rest of the patient’s hair; however, the repigmentation color can be different from the patient’s original hair color from their youth.4,5,11
The exact mechanism responsible for hair repigmentation in the setting of melanoma is unclear. It has been speculated from prior cases that repigmentation may be caused by paracrine stimulation from melanoma cells activating adjacent benign hair follicle melanocytes to produce melanin.7,14,15 This process likely is due to cytokines or growth factors, such as c-kit ligand.14,15 Several neural and immune networks and mediators activate the receptor tyrosine kinase KIT, which is thought to play a role in activating melanogenesis within the hair bulb.14 These signals also could originate from changes in the microenvironment instead of the melanoma cells themselves.6 Another possible mechanism is that repigmentation was caused by melanin-producing malignant melanocytes.4
Because this phenomenon typically occurs in older patients, the cause of repigmentation also could be related to chronic sun damage, which may result in upregulation of stem cell factor and α-melanocyte–stimulating hormone, as well as other molecules associated with melanogenesis, such as c-KIT receptor and tyrosinase.15,16 Upregulation of these molecules can lead to an increased number of melanocytes within the hair bulb. In addition, UVA and narrowband UVB have been recognized as major players in melanocyte stimulation. Phototherapy with UVA or narrowband UVB has been used for repigmentation in vitiligo patients.17
In cases without invasion of hair follicles by malignant cells, repigmentation more likely results from external signals stimulating benign bulbar melanocytes to produce melanin rather than melanoma cell growth extending into the hair bulb.6 In these cases, there is an increase in the number of hair bulbar melanocytes with a lack of malignant morphology in the hair bulb.8 If the signals are directly from melanoma cells in the hair bulb, it is unknown how the malignant cells upregulated melanogenesis in adjacent benign melanocytes or which specific signals required for normal pigmentation were involved in these repigmentation cases.6
Use of medications was ruled out as an underlying cause of the repigmentation in our patient. Drug-related repigmentation of the hair typically is observed in a diffuse generalized pattern. In our case, the repigmentation was localized to the area of the underlying dark patch, and the patient was not on any medications that could cause hair hyperpigmentation. Hyperpigmentation has been associated with acitretin, lenalidomide, corticosteroids, erlotinib, latanoprost, verapamil, tamoxifen, levodopa, thalidomide, PD-1 inhibitors, and tumor necrosis α inhibitors.18-30 Repigmentation also has been reported after local radiotherapy and herpes zoster infection.31,32
The underlying melanoma in our patient was removed by staged square excision. Excision was the treatment of choice for most similar reported cases. Radiotherapy was utilized in two different cases.3,4 In one case, radiotherapy was successfully used to treat melanoma in situ, lentigo maligna type; the patient’s hair grew back to its original color, which suggests that normal hair physiology was restored once melanoma cells were eliminated.3 One reported case demonstrated successful treatment of lentigo maligna type–melanoma with imiquimod cream 5% applied 6 times weekly for 9 months with a positive cosmetic result.9 The exact mechanism of imiquimod is not fully understood. Imiquimod induces cytokines to stimulate the production of IFN-α via activation of toll-like receptor 7.33 There was complete clearing of the lesion as well as the hair pigmentation,9 which suggests that the treatment also eliminated deeper cells influencing pigmentation. A case of malignant amelanotic melanoma was successfully treated with anti–PD-1 antibody pembrolizumab (2 mg/kg every 3 weeks), with no recurrence at 12 months. Pembrolizumab acts as an immune checkpoint inhibitor by binding to the PD-1 receptor and allowing the immune system to recognize and attack melanoma cells. After 5 doses of pembrolizumab, the patient was clear of disease and his hair color returned to gray.5
In 2022, melanoma was estimated to be the fifth most commonly diagnosed cancer among men and women in the United States.34 Early melanoma detection is a critical factor in achieving positive patient outcomes. Hair repigmentation is a potentially serious phenomenon that warrants a physician visit. Melanoma lesions under the hair may be overlooked because of limited visibility. Physicians must inspect spontaneous hair repigmentation with high suspicion and interpret the change as a possible indirect result of melanoma. Overall, it is important to increase public awareness of regular skin checks and melanoma warning signs.
To the Editor:
An 85-year-old man with a history of hypertension and chronic kidney disease presented with a localized darkening patch of hair on the left parietal scalp that had progressed over the last 7 years (Figure 1A). He had no prior history of skin cancer. Physical examination revealed the remainder of the hair was gray. There was an irregularly pigmented plaque on the skin underlying the darkened hair measuring 5.0 cm in diameter that was confirmed to be melanoma (Figure 1B). He underwent a staged excision to remove the lesion. The surgical defect was closed via a 5.0×6.0-cm full-thickness skin graft.

The initial biopsy showed melanoma in situ. However, the final pathology report following the excision revealed an invasive melanoma with a Breslow depth of 1.0 mm (Clark level IV; American Joint Committee on Cancer T1b).1 Histopathology showed pigment deposition with surrounding deep follicular extension of melanoma (Figure 2).

The patient declined a sentinel lymph node biopsy and agreed to a genetic profile assessment.2 The results of the test identified the patient had a low probability of a positive sentinel lymph node and the lowest risk of melanoma recurrence within 5 years. The patient was clear of disease at 12-month follow-up.
Based on a PubMed search of articles indexed for MEDLINE using the terms hair repigmentation and melanoma, there have been 11 other reported cases of hair repigmentation associated with melanoma (Table).3-13 It initially was suspected that this rare phenomenon primarily existed in the female population, as the first 5 cases were reported solely in females,3-7 possibly due to the prevalence of androgenetic alopecia in males.11 However, 6 cases of repigmentation associated with melanoma were later reported in males8-13; our patient represents an additional reported case in a male. It is unknown if there is a higher prevalence of this phenomenon among males or females.

Most previously reported cases of repigmentation were associated with melanoma in situ, lentigo maligna type. Repigmentation also has been reported in malignant melanoma, as documented in our patient, as well as desmoplastic and amelanotic melanoma.5,6 In every case, the color of the repigmentation was darker than the rest of the patient’s hair; however, the repigmentation color can be different from the patient’s original hair color from their youth.4,5,11
The exact mechanism responsible for hair repigmentation in the setting of melanoma is unclear. It has been speculated from prior cases that repigmentation may be caused by paracrine stimulation from melanoma cells activating adjacent benign hair follicle melanocytes to produce melanin.7,14,15 This process likely is due to cytokines or growth factors, such as c-kit ligand.14,15 Several neural and immune networks and mediators activate the receptor tyrosine kinase KIT, which is thought to play a role in activating melanogenesis within the hair bulb.14 These signals also could originate from changes in the microenvironment instead of the melanoma cells themselves.6 Another possible mechanism is that repigmentation was caused by melanin-producing malignant melanocytes.4
Because this phenomenon typically occurs in older patients, the cause of repigmentation also could be related to chronic sun damage, which may result in upregulation of stem cell factor and α-melanocyte–stimulating hormone, as well as other molecules associated with melanogenesis, such as c-KIT receptor and tyrosinase.15,16 Upregulation of these molecules can lead to an increased number of melanocytes within the hair bulb. In addition, UVA and narrowband UVB have been recognized as major players in melanocyte stimulation. Phototherapy with UVA or narrowband UVB has been used for repigmentation in vitiligo patients.17
In cases without invasion of hair follicles by malignant cells, repigmentation more likely results from external signals stimulating benign bulbar melanocytes to produce melanin rather than melanoma cell growth extending into the hair bulb.6 In these cases, there is an increase in the number of hair bulbar melanocytes with a lack of malignant morphology in the hair bulb.8 If the signals are directly from melanoma cells in the hair bulb, it is unknown how the malignant cells upregulated melanogenesis in adjacent benign melanocytes or which specific signals required for normal pigmentation were involved in these repigmentation cases.6
Use of medications was ruled out as an underlying cause of the repigmentation in our patient. Drug-related repigmentation of the hair typically is observed in a diffuse generalized pattern. In our case, the repigmentation was localized to the area of the underlying dark patch, and the patient was not on any medications that could cause hair hyperpigmentation. Hyperpigmentation has been associated with acitretin, lenalidomide, corticosteroids, erlotinib, latanoprost, verapamil, tamoxifen, levodopa, thalidomide, PD-1 inhibitors, and tumor necrosis α inhibitors.18-30 Repigmentation also has been reported after local radiotherapy and herpes zoster infection.31,32
The underlying melanoma in our patient was removed by staged square excision. Excision was the treatment of choice for most similar reported cases. Radiotherapy was utilized in two different cases.3,4 In one case, radiotherapy was successfully used to treat melanoma in situ, lentigo maligna type; the patient’s hair grew back to its original color, which suggests that normal hair physiology was restored once melanoma cells were eliminated.3 One reported case demonstrated successful treatment of lentigo maligna type–melanoma with imiquimod cream 5% applied 6 times weekly for 9 months with a positive cosmetic result.9 The exact mechanism of imiquimod is not fully understood. Imiquimod induces cytokines to stimulate the production of IFN-α via activation of toll-like receptor 7.33 There was complete clearing of the lesion as well as the hair pigmentation,9 which suggests that the treatment also eliminated deeper cells influencing pigmentation. A case of malignant amelanotic melanoma was successfully treated with anti–PD-1 antibody pembrolizumab (2 mg/kg every 3 weeks), with no recurrence at 12 months. Pembrolizumab acts as an immune checkpoint inhibitor by binding to the PD-1 receptor and allowing the immune system to recognize and attack melanoma cells. After 5 doses of pembrolizumab, the patient was clear of disease and his hair color returned to gray.5
In 2022, melanoma was estimated to be the fifth most commonly diagnosed cancer among men and women in the United States.34 Early melanoma detection is a critical factor in achieving positive patient outcomes. Hair repigmentation is a potentially serious phenomenon that warrants a physician visit. Melanoma lesions under the hair may be overlooked because of limited visibility. Physicians must inspect spontaneous hair repigmentation with high suspicion and interpret the change as a possible indirect result of melanoma. Overall, it is important to increase public awareness of regular skin checks and melanoma warning signs.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1–T2 melanoma using gene expression profiling. Futur Oncol. 2019;15:1207-1217.
- Dummer R. Hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
- Inzinger M, Massone C, Arzberger E, et al. Hair repigmentation in melanoma. Lancet. 2013;382:1224.
- Rahim RR, Husain A, Tobin DJ, et al. Desmoplastic melanoma presenting with localized hair repigmentation. Br J Dermatol. 2013;169:1371-1373.
- Tiger JB, Habeshian KA, Barton DT, et al. Repigmentation of hair associated with melanoma in situ of scalp. J Am Acad Dermatol. 2014;71:E144-E145.
- Amann VC, Dummer R. Localized hair repigmentation in a 91-year-old woman. JAMA Dermatol. 2016;152:81-82.
- Chan C, Magro CM, Pham AK, et al. Spontaneous hair repigmentation in an 80-year-old man: a case of melanoma-associated hair repigmentation and review of the literature. Am J Dermatopathol. 2019;41:671-674.
- Lackey AE, Glassman G, Grichnik J, et al. Repigmentation of gray hairs with lentigo maligna and response to topical imiquimod. JAAD Case Rep. 2019;5:1015-1017.
- Chew T, Pannell M, Jeeves A. Focal hair re-pigmentation associated with melanoma of the scalp. ANZ J Surg. 2019;90:1175-1176.
- López-Sánchez C, Collgros H. Hair repigmentation as a clue for scalp melanoma. Australas J Dermatol. 2019;61:179-180.
- Gessler J, Tejasvi T, Bresler SC. Repigmentation of scalp hair: a feature of early melanoma. Am J Med. 2023;136:E7-E8.
- Hasegawa T, Iino S, Kitakaze K, et al. Repigmentation of aging gray hair associated with unrecognized development and progression of amelanotic melanoma of the scalp: a physiological alert underlying hair rejuvenation. J Dermatol. 2021;48:E281-E283. doi:10.1111/1346-8138.15881
- D’Mello SAN, Finlay GJ, Baguley BC, et al. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:1144.
- Hachiya A, Kobayashi A, Ohuchi A, et al. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001;116:578-586.
- Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
- Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313.
- Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol. 2009;50:214-216.
- Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process! J Oncol Pharm Pract. 2013;19:165-169.
- Sebaratnam DF, Rodríguez Bandera AI, Lowe PM. Hair repigmentation with anti–PD-1 and anti–PD-L1 immunotherapy: a novel hypothesis. JAMA Dermatol. 2018;154:112-113. doi:10.1001/jamadermatol.2017.4420
- Tintle SJ, Dabade TS, Kalish RA, et al. Repigmentation of hair following adalimumab therapy. Dermatol Online J. 2015;21:13030/qt6fn0t1xz.
- Penzi LR, Manatis-Lornell A, Saavedra A, et al. Hair repigmentation associated with the use of brentuximab. JAAD Case Rep. 2017;3:563-565.
- Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatology Venereol. 2008;22:1018-1020.
- Cheng YP, Chen HJ, Chiu HC. Erlotinib-induced hair repigmentation. Int J Dermatol. 2014;53:E55-E57.
- Bellandi S, Amato L, Cipollini EM, et al. Repigmentation of hair after latanoprost therapy. J Eur Acad Dermatology Venereol. 2011;25:1485-1487.
- Read GM. Verapamil and hair colour change. Lancet. 1991;338:1520.
- Hampson JP, Donnelly A, Lewis‐Jones MS, et al. Tamoxifen‐induced hair colour change. Br J Dermatol. 1995;132:483-484.
- Reynolds NJ, Crossley J, Ferguson I, et al. Darkening of white hair in Parkinson’s disease. Clin Exp Dermatol. 1989;14:317-318.
- Lovering S, Miao W, Bailie T, et al. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016;2016:bcr2016215521.
- Rivera N, Boada A, Bielsa MI, et al. Hair repigmentation during immunotherapy treatment with an anti–programmed cell death 1 and anti–programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol. 2017;153:1162-1165.
- Prasad S, Dougheney N, Hong A. Scalp hair repigmentation in the penumbral region of radiotherapy–a case series. Int J Radiol Radiat Ther. 2020;7:151-157.
- Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
- Hanna E, Abadi R, Abbas O. Imiquimod in dermatology: an overview. Int J Dermatol. 2016;55:831-844.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1–T2 melanoma using gene expression profiling. Futur Oncol. 2019;15:1207-1217.
- Dummer R. Hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
- Inzinger M, Massone C, Arzberger E, et al. Hair repigmentation in melanoma. Lancet. 2013;382:1224.
- Rahim RR, Husain A, Tobin DJ, et al. Desmoplastic melanoma presenting with localized hair repigmentation. Br J Dermatol. 2013;169:1371-1373.
- Tiger JB, Habeshian KA, Barton DT, et al. Repigmentation of hair associated with melanoma in situ of scalp. J Am Acad Dermatol. 2014;71:E144-E145.
- Amann VC, Dummer R. Localized hair repigmentation in a 91-year-old woman. JAMA Dermatol. 2016;152:81-82.
- Chan C, Magro CM, Pham AK, et al. Spontaneous hair repigmentation in an 80-year-old man: a case of melanoma-associated hair repigmentation and review of the literature. Am J Dermatopathol. 2019;41:671-674.
- Lackey AE, Glassman G, Grichnik J, et al. Repigmentation of gray hairs with lentigo maligna and response to topical imiquimod. JAAD Case Rep. 2019;5:1015-1017.
- Chew T, Pannell M, Jeeves A. Focal hair re-pigmentation associated with melanoma of the scalp. ANZ J Surg. 2019;90:1175-1176.
- López-Sánchez C, Collgros H. Hair repigmentation as a clue for scalp melanoma. Australas J Dermatol. 2019;61:179-180.
- Gessler J, Tejasvi T, Bresler SC. Repigmentation of scalp hair: a feature of early melanoma. Am J Med. 2023;136:E7-E8.
- Hasegawa T, Iino S, Kitakaze K, et al. Repigmentation of aging gray hair associated with unrecognized development and progression of amelanotic melanoma of the scalp: a physiological alert underlying hair rejuvenation. J Dermatol. 2021;48:E281-E283. doi:10.1111/1346-8138.15881
- D’Mello SAN, Finlay GJ, Baguley BC, et al. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:1144.
- Hachiya A, Kobayashi A, Ohuchi A, et al. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001;116:578-586.
- Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
- Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313.
- Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol. 2009;50:214-216.
- Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process! J Oncol Pharm Pract. 2013;19:165-169.
- Sebaratnam DF, Rodríguez Bandera AI, Lowe PM. Hair repigmentation with anti–PD-1 and anti–PD-L1 immunotherapy: a novel hypothesis. JAMA Dermatol. 2018;154:112-113. doi:10.1001/jamadermatol.2017.4420
- Tintle SJ, Dabade TS, Kalish RA, et al. Repigmentation of hair following adalimumab therapy. Dermatol Online J. 2015;21:13030/qt6fn0t1xz.
- Penzi LR, Manatis-Lornell A, Saavedra A, et al. Hair repigmentation associated with the use of brentuximab. JAAD Case Rep. 2017;3:563-565.
- Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatology Venereol. 2008;22:1018-1020.
- Cheng YP, Chen HJ, Chiu HC. Erlotinib-induced hair repigmentation. Int J Dermatol. 2014;53:E55-E57.
- Bellandi S, Amato L, Cipollini EM, et al. Repigmentation of hair after latanoprost therapy. J Eur Acad Dermatology Venereol. 2011;25:1485-1487.
- Read GM. Verapamil and hair colour change. Lancet. 1991;338:1520.
- Hampson JP, Donnelly A, Lewis‐Jones MS, et al. Tamoxifen‐induced hair colour change. Br J Dermatol. 1995;132:483-484.
- Reynolds NJ, Crossley J, Ferguson I, et al. Darkening of white hair in Parkinson’s disease. Clin Exp Dermatol. 1989;14:317-318.
- Lovering S, Miao W, Bailie T, et al. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016;2016:bcr2016215521.
- Rivera N, Boada A, Bielsa MI, et al. Hair repigmentation during immunotherapy treatment with an anti–programmed cell death 1 and anti–programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol. 2017;153:1162-1165.
- Prasad S, Dougheney N, Hong A. Scalp hair repigmentation in the penumbral region of radiotherapy–a case series. Int J Radiol Radiat Ther. 2020;7:151-157.
- Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
- Hanna E, Abadi R, Abbas O. Imiquimod in dermatology: an overview. Int J Dermatol. 2016;55:831-844.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
Practice Points
- Localized repigmentation of the hair is a rare phenomenon that may indicate underlying melanoma.
- Careful clinicopathologic correlation is necessary to appropriately diagnose and manage this unusual presentation of melanoma.
Increased cancer in military pilots and ground crew: Pentagon
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.
Melanoma screening: Consensus statement offers greater clarity
That is why a group of expert panelists evaluated the existing evidence and a range of clinical scenarios to help clarify the optimal strategies for early detection and assessment of cutaneous melanoma.
Overall, the panelists agreed that a risk-stratified approach is likely the most appropriate strategy for melanoma screening and follow-up and supported the use of visual and dermoscopic examination. However, the panelists did not reach consensus on the role for gene expression profile (GEP) testing in clinical decision-making, citing the need for these assays to be validated in large randomized clinical trials.
In an accompanying editorial, two experts highlighted the importance of carefully evaluating the role of diagnostic tests.
“Diagnostic tests such as GEP must face critical scrutiny; if not, there are immediate concerns for patient care, such as the patient being erroneously informed that they do not have cancer or told that they do have cancer when they do not,” write Alan C. Geller, MPH, RN, from the Harvard T.H. Chan School of Public Health, Boston, and Marvin A. Weinstock, MD, PhD, from Brown University, Providence, R.I.
The consensus statement was published online in JAMA Dermatology.
The need for guidance
Although focusing melanoma screening on higher-risk populations may be cost effective, compared with population-based screening, the major guidelines lack consistent guidance to support a risk-stratified approach to skin cancer screening and best practices on diagnosing cutaneous melanoma.
In the prebiopsy setting, the appropriate use of diagnostic tools for evaluating the need for biopsy remain poorly defined, and, in the post-biopsy setting, questions remain concerning the diagnostic accuracy of molecular techniques, diagnostic GEP testing, next-generation sequencing, and immunohistochemical assessment for various markers of melanoma.
To provide consensus recommendations on optimal screening practices, prebiopsy and postbiopsy diagnostics, and prognostic assessment of cutaneous melanoma, a group of 42 panelists voted on hypothetical scenarios via an emailed survey. The panel then came together for a consensus conference, which included 51 experts who discussed their approach to the various clinical case scenarios. Most attendees (45 of the 51) answered a follow-up survey for their final recommendations.
The panelists reached a consensus, with 70% agreement, to support a risk-stratified approach to melanoma screening in clinical settings and public screening events. The experts agreed that higher-risk individuals (those with a relative risk of 5 or greater) could be appropriately screened by a general dermatologist or pigmented lesion evaluation. Higher-risk individuals included those with severe skin damage from the sun, systemic immunosuppression, or a personal history of nonmelanoma or melanoma skin cancer.
Panelists agreed that those at general or lower risk (RR < 2) could be screened by a primary care provider or through regular self- or partner examinations, whereas those at moderate risk could be screened by their primary care clinician or general dermatologist. The experts observed “a shift in acceptance” of primary care physicians screening the general population, and an acknowledgement of the importance of self- and partner examinations as screening adjuncts for all populations.
In the prebiopsy setting, panelists reached consensus that visual and dermoscopic examination was appropriate for evaluating patients with “no new, changing, or unusual skin lesions or with a new lesion that is not visually concerning.”
The panelists also reached consensus that lesions deemed clinically suspicious for cancer or showing features of cancer on reflectance confocal microscopy should be biopsied. Although most respondents (86%) did not currently use epidermal tape stripping routinely, they agreed that, in a hypothetical situation where epidermal tape stripping was used, that lesions positive for PRAME or LINC should be biopsied.
In the postbiopsy setting, views on the use of GEP scores varied. Although panelists agreed that a low-risk prognostic GEP score should not outweigh concerning histologic features when patients are selected to undergo sentinel lymph node biopsy (SLNB), they did not reach consensus for imaging recommendations in the setting of a high-risk prognostic GEP score and low-risk histology and/or negative nodal status.
“The panelists await future, well-designed prospective studies to determine if use of these and newer technologies improves the care of patients with melanoma,” the panelists write.
In the editorial, Mr. Geller and Dr. Weinstock highlighted concerns about the cost and potential access issues associated with these newer technologies, given that the current cost of GEP testing exceeds $7,000.
The editorialists also emphasize that “going forward, the field should be advanced by tackling one of the more pressing, common, potentially morbid, and costly procedures – the prognostic use of sentinel lymph node biopsy.”
Of critical importance is “whether GEP can reduce morbidity and cost by safely reducing the number of SLNBs performed,” Mr. Geller and Dr. Weinstock write.
The funding for the administration and facilitation of the consensus development conference and the development of the manuscript was provided by Dermtech, in an unrestricted award overseen by the Melanoma Research Foundation and managed and executed at UPMC by the principal investigator. Several of the coauthors disclosed relationships with industry. Mr. Geller is a contributor to UptoDate for which he receives royalties. Dr. Weinstock receives consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
That is why a group of expert panelists evaluated the existing evidence and a range of clinical scenarios to help clarify the optimal strategies for early detection and assessment of cutaneous melanoma.
Overall, the panelists agreed that a risk-stratified approach is likely the most appropriate strategy for melanoma screening and follow-up and supported the use of visual and dermoscopic examination. However, the panelists did not reach consensus on the role for gene expression profile (GEP) testing in clinical decision-making, citing the need for these assays to be validated in large randomized clinical trials.
In an accompanying editorial, two experts highlighted the importance of carefully evaluating the role of diagnostic tests.
“Diagnostic tests such as GEP must face critical scrutiny; if not, there are immediate concerns for patient care, such as the patient being erroneously informed that they do not have cancer or told that they do have cancer when they do not,” write Alan C. Geller, MPH, RN, from the Harvard T.H. Chan School of Public Health, Boston, and Marvin A. Weinstock, MD, PhD, from Brown University, Providence, R.I.
The consensus statement was published online in JAMA Dermatology.
The need for guidance
Although focusing melanoma screening on higher-risk populations may be cost effective, compared with population-based screening, the major guidelines lack consistent guidance to support a risk-stratified approach to skin cancer screening and best practices on diagnosing cutaneous melanoma.
In the prebiopsy setting, the appropriate use of diagnostic tools for evaluating the need for biopsy remain poorly defined, and, in the post-biopsy setting, questions remain concerning the diagnostic accuracy of molecular techniques, diagnostic GEP testing, next-generation sequencing, and immunohistochemical assessment for various markers of melanoma.
To provide consensus recommendations on optimal screening practices, prebiopsy and postbiopsy diagnostics, and prognostic assessment of cutaneous melanoma, a group of 42 panelists voted on hypothetical scenarios via an emailed survey. The panel then came together for a consensus conference, which included 51 experts who discussed their approach to the various clinical case scenarios. Most attendees (45 of the 51) answered a follow-up survey for their final recommendations.
The panelists reached a consensus, with 70% agreement, to support a risk-stratified approach to melanoma screening in clinical settings and public screening events. The experts agreed that higher-risk individuals (those with a relative risk of 5 or greater) could be appropriately screened by a general dermatologist or pigmented lesion evaluation. Higher-risk individuals included those with severe skin damage from the sun, systemic immunosuppression, or a personal history of nonmelanoma or melanoma skin cancer.
Panelists agreed that those at general or lower risk (RR < 2) could be screened by a primary care provider or through regular self- or partner examinations, whereas those at moderate risk could be screened by their primary care clinician or general dermatologist. The experts observed “a shift in acceptance” of primary care physicians screening the general population, and an acknowledgement of the importance of self- and partner examinations as screening adjuncts for all populations.
In the prebiopsy setting, panelists reached consensus that visual and dermoscopic examination was appropriate for evaluating patients with “no new, changing, or unusual skin lesions or with a new lesion that is not visually concerning.”
The panelists also reached consensus that lesions deemed clinically suspicious for cancer or showing features of cancer on reflectance confocal microscopy should be biopsied. Although most respondents (86%) did not currently use epidermal tape stripping routinely, they agreed that, in a hypothetical situation where epidermal tape stripping was used, that lesions positive for PRAME or LINC should be biopsied.
In the postbiopsy setting, views on the use of GEP scores varied. Although panelists agreed that a low-risk prognostic GEP score should not outweigh concerning histologic features when patients are selected to undergo sentinel lymph node biopsy (SLNB), they did not reach consensus for imaging recommendations in the setting of a high-risk prognostic GEP score and low-risk histology and/or negative nodal status.
“The panelists await future, well-designed prospective studies to determine if use of these and newer technologies improves the care of patients with melanoma,” the panelists write.
In the editorial, Mr. Geller and Dr. Weinstock highlighted concerns about the cost and potential access issues associated with these newer technologies, given that the current cost of GEP testing exceeds $7,000.
The editorialists also emphasize that “going forward, the field should be advanced by tackling one of the more pressing, common, potentially morbid, and costly procedures – the prognostic use of sentinel lymph node biopsy.”
Of critical importance is “whether GEP can reduce morbidity and cost by safely reducing the number of SLNBs performed,” Mr. Geller and Dr. Weinstock write.
The funding for the administration and facilitation of the consensus development conference and the development of the manuscript was provided by Dermtech, in an unrestricted award overseen by the Melanoma Research Foundation and managed and executed at UPMC by the principal investigator. Several of the coauthors disclosed relationships with industry. Mr. Geller is a contributor to UptoDate for which he receives royalties. Dr. Weinstock receives consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
That is why a group of expert panelists evaluated the existing evidence and a range of clinical scenarios to help clarify the optimal strategies for early detection and assessment of cutaneous melanoma.
Overall, the panelists agreed that a risk-stratified approach is likely the most appropriate strategy for melanoma screening and follow-up and supported the use of visual and dermoscopic examination. However, the panelists did not reach consensus on the role for gene expression profile (GEP) testing in clinical decision-making, citing the need for these assays to be validated in large randomized clinical trials.
In an accompanying editorial, two experts highlighted the importance of carefully evaluating the role of diagnostic tests.
“Diagnostic tests such as GEP must face critical scrutiny; if not, there are immediate concerns for patient care, such as the patient being erroneously informed that they do not have cancer or told that they do have cancer when they do not,” write Alan C. Geller, MPH, RN, from the Harvard T.H. Chan School of Public Health, Boston, and Marvin A. Weinstock, MD, PhD, from Brown University, Providence, R.I.
The consensus statement was published online in JAMA Dermatology.
The need for guidance
Although focusing melanoma screening on higher-risk populations may be cost effective, compared with population-based screening, the major guidelines lack consistent guidance to support a risk-stratified approach to skin cancer screening and best practices on diagnosing cutaneous melanoma.
In the prebiopsy setting, the appropriate use of diagnostic tools for evaluating the need for biopsy remain poorly defined, and, in the post-biopsy setting, questions remain concerning the diagnostic accuracy of molecular techniques, diagnostic GEP testing, next-generation sequencing, and immunohistochemical assessment for various markers of melanoma.
To provide consensus recommendations on optimal screening practices, prebiopsy and postbiopsy diagnostics, and prognostic assessment of cutaneous melanoma, a group of 42 panelists voted on hypothetical scenarios via an emailed survey. The panel then came together for a consensus conference, which included 51 experts who discussed their approach to the various clinical case scenarios. Most attendees (45 of the 51) answered a follow-up survey for their final recommendations.
The panelists reached a consensus, with 70% agreement, to support a risk-stratified approach to melanoma screening in clinical settings and public screening events. The experts agreed that higher-risk individuals (those with a relative risk of 5 or greater) could be appropriately screened by a general dermatologist or pigmented lesion evaluation. Higher-risk individuals included those with severe skin damage from the sun, systemic immunosuppression, or a personal history of nonmelanoma or melanoma skin cancer.
Panelists agreed that those at general or lower risk (RR < 2) could be screened by a primary care provider or through regular self- or partner examinations, whereas those at moderate risk could be screened by their primary care clinician or general dermatologist. The experts observed “a shift in acceptance” of primary care physicians screening the general population, and an acknowledgement of the importance of self- and partner examinations as screening adjuncts for all populations.
In the prebiopsy setting, panelists reached consensus that visual and dermoscopic examination was appropriate for evaluating patients with “no new, changing, or unusual skin lesions or with a new lesion that is not visually concerning.”
The panelists also reached consensus that lesions deemed clinically suspicious for cancer or showing features of cancer on reflectance confocal microscopy should be biopsied. Although most respondents (86%) did not currently use epidermal tape stripping routinely, they agreed that, in a hypothetical situation where epidermal tape stripping was used, that lesions positive for PRAME or LINC should be biopsied.
In the postbiopsy setting, views on the use of GEP scores varied. Although panelists agreed that a low-risk prognostic GEP score should not outweigh concerning histologic features when patients are selected to undergo sentinel lymph node biopsy (SLNB), they did not reach consensus for imaging recommendations in the setting of a high-risk prognostic GEP score and low-risk histology and/or negative nodal status.
“The panelists await future, well-designed prospective studies to determine if use of these and newer technologies improves the care of patients with melanoma,” the panelists write.
In the editorial, Mr. Geller and Dr. Weinstock highlighted concerns about the cost and potential access issues associated with these newer technologies, given that the current cost of GEP testing exceeds $7,000.
The editorialists also emphasize that “going forward, the field should be advanced by tackling one of the more pressing, common, potentially morbid, and costly procedures – the prognostic use of sentinel lymph node biopsy.”
Of critical importance is “whether GEP can reduce morbidity and cost by safely reducing the number of SLNBs performed,” Mr. Geller and Dr. Weinstock write.
The funding for the administration and facilitation of the consensus development conference and the development of the manuscript was provided by Dermtech, in an unrestricted award overseen by the Melanoma Research Foundation and managed and executed at UPMC by the principal investigator. Several of the coauthors disclosed relationships with industry. Mr. Geller is a contributor to UptoDate for which he receives royalties. Dr. Weinstock receives consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Dermatologic Implications of Sleep Deprivation in the US Military
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Practice Points
- Sleep deprivation may have negative effects on skin function and worsen dermatologic conditions.
- Proposed mechanisms of action for these negative effects include dysregulation of the hypothalamic-pituitary-adrenal axis, impairment of cutaneous barrier function, and alteration of cutaneous immune function.
- Members of the US Military are at an increased risk for sleep deprivation, especially during training and overseas deployments.
Pembrolizumab before and after melanoma surgery boosts outcomes
, show results from the phase 2 SWOG S1801 trial.
The trial involved 319 patients with operable stage IIIB to stage IV melanoma. The investigators found that patients who received pembrolizumab both before and after surgery (i.e., neoadjuvant and adjuvant therapy) fared better than those who received the drug only after surgery: The 2-year event-free survival rates were 72% vs. 49%, respectively.
The research was published in the New England Journal of Medicine, but similar results had already been presented at the European Society for Medical Oncology 2022 annual Meeting.
“It’s not just what you give; it’s when you give it,” said lead author Sapna Patel, MD, in a press release issued by the University of Texas MD Anderson Cancer Center, echoing comments she gave at ESMO 2022.
The study, she continued, “demonstrates the same treatment for resectable melanoma given before surgery can generate better outcomes.”
On the basis of their findings, Dr. Patel, who is associate professor of melanoma medical oncology at the University of Texas MD Anderson Cancer Center, Houston, said that patients with high-risk melanoma “should start immunotherapy prior to surgery to generate an immune response while the bulk of the melanoma and the anti-tumor T cells are intact.”
The mechanism of action of PD-1 blockade “relies on the presence of preexisting anti-tumor T cells attempting to attack cancer cells,” with the immunotherapy allowing the anti-tumor cells to proliferate and mediate clinical responses.
Resection of the bulk of the tumor is therefore “likely to take away some or even most of the potential anti-tumor T cells that would proliferate after PD-1 blockade,” they write.
Likely to apply also to nivolumab
Approached for comment, Jeffrey S. Weber, MD, PhD, professor of medicine, NYU Langone Medical Center, New York, said that outside of trials, both pembrolizumab and ipilimumab (Yervoy)/nivolumab (Opdivo) are already being used neoadjuvantly.
He thinks that the findings for neoadjuvant and adjuvant pembrolizumab could also apply to nivolumab because “the drugs are quite similar in efficacy.”
Dr. Weber told this news organization that, “even though the S1801 trial was not accepted as a registration trial by the FDA, I think that its results could very well change practice and confirm it for others who already use neoadjuvant therapy for palpable stage III melanoma.”
One question that is being addressed to an extent in the NADINA trial is whether adjuvant immunotherapy can be avoided all together and patients receive only neoadjuvant therapy, although Dr. Weber said, “I doubt that will be the case.”
Study details
In this study, patients were randomly assigned to either surgery followed by 18 doses of adjuvant pembrolizumab, or to receive 3 doses of neoadjuvant pembrolizumab followed by surgery and then 15 additional doses of adjuvant pembrolizumab.
After a median duration of follow-up of 14.7 months, there were 38 events in the neoadjuvant-adjuvant group and 67 in the adjuvant-only group.
“Events” were defined as disease progression, toxic effects, or complications that precluded surgery or the initiation of adjuvant therapy within 84 days of surgery, as well as the inability to fully resect the gross disease, melanoma recurrence, and death.
The team calculated that event-free survival was significantly longer in the neoadjuvant-adjuvant group (P = .004), with 2-year event-free survival at 72% vs. 49% in the adjuvant-only group.
“The benefit of neoadjuvant pembrolizumab was seen across all subgroups of patients,” the investigators note.
At the data cut-off, there were 14 deaths in the neoadjuvant-adjuvant group vs. 22 in the adjuvant-only group, which the researchers say is too few to allow “definitive comparison” in terms of overall survival.
Definitive surgery had been performed in 88% of neoadjuvant-adjuvant patients and in 95% of those assigned to adjuvant-only pembrolizumab. The most common reason for not undergoing surgery was disease progression.
Among the patients for whom safety data were available, 7% in the neoadjuvant-adjuvant group had at least one grade 3 or 4 adverse event related to pembrolizumab, whereas 7% had at least one grade 3 or 4 adverse event related to surgery.
In the adjuvant-only arm, 4% of patients had at least one grade 3 adverse event related to surgery, with no grade 4 adverse events reported.
The rates of grade 3 or 4 adverse events during adjuvant therapy were similar in the two groups, at 12% in patients assigned to neoadjuvant-adjuvant therapy and 14% in those given adjuvant-only pembrolizumab.
“Future studies can explore deescalation strategies for both surgery and adjuvant therapy, as well as approaches for patients whose melanoma does not respond to neoadjuvant therapy,” the researchers commented.
The study was funded by the National Cancer Institute and Merck Sharp and Dohme.
Dr. Patel reports numerous relationships with industry, including with Merck, manufacturer of pembrolizumab; other coauthors also have numerous relationships with industry. Dr. Weber is a regular columnist for this news organization and lists his disclosures in his Weber on Oncology column.
A version of this article first appeared on Medscape.com.
, show results from the phase 2 SWOG S1801 trial.
The trial involved 319 patients with operable stage IIIB to stage IV melanoma. The investigators found that patients who received pembrolizumab both before and after surgery (i.e., neoadjuvant and adjuvant therapy) fared better than those who received the drug only after surgery: The 2-year event-free survival rates were 72% vs. 49%, respectively.
The research was published in the New England Journal of Medicine, but similar results had already been presented at the European Society for Medical Oncology 2022 annual Meeting.
“It’s not just what you give; it’s when you give it,” said lead author Sapna Patel, MD, in a press release issued by the University of Texas MD Anderson Cancer Center, echoing comments she gave at ESMO 2022.
The study, she continued, “demonstrates the same treatment for resectable melanoma given before surgery can generate better outcomes.”
On the basis of their findings, Dr. Patel, who is associate professor of melanoma medical oncology at the University of Texas MD Anderson Cancer Center, Houston, said that patients with high-risk melanoma “should start immunotherapy prior to surgery to generate an immune response while the bulk of the melanoma and the anti-tumor T cells are intact.”
The mechanism of action of PD-1 blockade “relies on the presence of preexisting anti-tumor T cells attempting to attack cancer cells,” with the immunotherapy allowing the anti-tumor cells to proliferate and mediate clinical responses.
Resection of the bulk of the tumor is therefore “likely to take away some or even most of the potential anti-tumor T cells that would proliferate after PD-1 blockade,” they write.
Likely to apply also to nivolumab
Approached for comment, Jeffrey S. Weber, MD, PhD, professor of medicine, NYU Langone Medical Center, New York, said that outside of trials, both pembrolizumab and ipilimumab (Yervoy)/nivolumab (Opdivo) are already being used neoadjuvantly.
He thinks that the findings for neoadjuvant and adjuvant pembrolizumab could also apply to nivolumab because “the drugs are quite similar in efficacy.”
Dr. Weber told this news organization that, “even though the S1801 trial was not accepted as a registration trial by the FDA, I think that its results could very well change practice and confirm it for others who already use neoadjuvant therapy for palpable stage III melanoma.”
One question that is being addressed to an extent in the NADINA trial is whether adjuvant immunotherapy can be avoided all together and patients receive only neoadjuvant therapy, although Dr. Weber said, “I doubt that will be the case.”
Study details
In this study, patients were randomly assigned to either surgery followed by 18 doses of adjuvant pembrolizumab, or to receive 3 doses of neoadjuvant pembrolizumab followed by surgery and then 15 additional doses of adjuvant pembrolizumab.
After a median duration of follow-up of 14.7 months, there were 38 events in the neoadjuvant-adjuvant group and 67 in the adjuvant-only group.
“Events” were defined as disease progression, toxic effects, or complications that precluded surgery or the initiation of adjuvant therapy within 84 days of surgery, as well as the inability to fully resect the gross disease, melanoma recurrence, and death.
The team calculated that event-free survival was significantly longer in the neoadjuvant-adjuvant group (P = .004), with 2-year event-free survival at 72% vs. 49% in the adjuvant-only group.
“The benefit of neoadjuvant pembrolizumab was seen across all subgroups of patients,” the investigators note.
At the data cut-off, there were 14 deaths in the neoadjuvant-adjuvant group vs. 22 in the adjuvant-only group, which the researchers say is too few to allow “definitive comparison” in terms of overall survival.
Definitive surgery had been performed in 88% of neoadjuvant-adjuvant patients and in 95% of those assigned to adjuvant-only pembrolizumab. The most common reason for not undergoing surgery was disease progression.
Among the patients for whom safety data were available, 7% in the neoadjuvant-adjuvant group had at least one grade 3 or 4 adverse event related to pembrolizumab, whereas 7% had at least one grade 3 or 4 adverse event related to surgery.
In the adjuvant-only arm, 4% of patients had at least one grade 3 adverse event related to surgery, with no grade 4 adverse events reported.
The rates of grade 3 or 4 adverse events during adjuvant therapy were similar in the two groups, at 12% in patients assigned to neoadjuvant-adjuvant therapy and 14% in those given adjuvant-only pembrolizumab.
“Future studies can explore deescalation strategies for both surgery and adjuvant therapy, as well as approaches for patients whose melanoma does not respond to neoadjuvant therapy,” the researchers commented.
The study was funded by the National Cancer Institute and Merck Sharp and Dohme.
Dr. Patel reports numerous relationships with industry, including with Merck, manufacturer of pembrolizumab; other coauthors also have numerous relationships with industry. Dr. Weber is a regular columnist for this news organization and lists his disclosures in his Weber on Oncology column.
A version of this article first appeared on Medscape.com.
, show results from the phase 2 SWOG S1801 trial.
The trial involved 319 patients with operable stage IIIB to stage IV melanoma. The investigators found that patients who received pembrolizumab both before and after surgery (i.e., neoadjuvant and adjuvant therapy) fared better than those who received the drug only after surgery: The 2-year event-free survival rates were 72% vs. 49%, respectively.
The research was published in the New England Journal of Medicine, but similar results had already been presented at the European Society for Medical Oncology 2022 annual Meeting.
“It’s not just what you give; it’s when you give it,” said lead author Sapna Patel, MD, in a press release issued by the University of Texas MD Anderson Cancer Center, echoing comments she gave at ESMO 2022.
The study, she continued, “demonstrates the same treatment for resectable melanoma given before surgery can generate better outcomes.”
On the basis of their findings, Dr. Patel, who is associate professor of melanoma medical oncology at the University of Texas MD Anderson Cancer Center, Houston, said that patients with high-risk melanoma “should start immunotherapy prior to surgery to generate an immune response while the bulk of the melanoma and the anti-tumor T cells are intact.”
The mechanism of action of PD-1 blockade “relies on the presence of preexisting anti-tumor T cells attempting to attack cancer cells,” with the immunotherapy allowing the anti-tumor cells to proliferate and mediate clinical responses.
Resection of the bulk of the tumor is therefore “likely to take away some or even most of the potential anti-tumor T cells that would proliferate after PD-1 blockade,” they write.
Likely to apply also to nivolumab
Approached for comment, Jeffrey S. Weber, MD, PhD, professor of medicine, NYU Langone Medical Center, New York, said that outside of trials, both pembrolizumab and ipilimumab (Yervoy)/nivolumab (Opdivo) are already being used neoadjuvantly.
He thinks that the findings for neoadjuvant and adjuvant pembrolizumab could also apply to nivolumab because “the drugs are quite similar in efficacy.”
Dr. Weber told this news organization that, “even though the S1801 trial was not accepted as a registration trial by the FDA, I think that its results could very well change practice and confirm it for others who already use neoadjuvant therapy for palpable stage III melanoma.”
One question that is being addressed to an extent in the NADINA trial is whether adjuvant immunotherapy can be avoided all together and patients receive only neoadjuvant therapy, although Dr. Weber said, “I doubt that will be the case.”
Study details
In this study, patients were randomly assigned to either surgery followed by 18 doses of adjuvant pembrolizumab, or to receive 3 doses of neoadjuvant pembrolizumab followed by surgery and then 15 additional doses of adjuvant pembrolizumab.
After a median duration of follow-up of 14.7 months, there were 38 events in the neoadjuvant-adjuvant group and 67 in the adjuvant-only group.
“Events” were defined as disease progression, toxic effects, or complications that precluded surgery or the initiation of adjuvant therapy within 84 days of surgery, as well as the inability to fully resect the gross disease, melanoma recurrence, and death.
The team calculated that event-free survival was significantly longer in the neoadjuvant-adjuvant group (P = .004), with 2-year event-free survival at 72% vs. 49% in the adjuvant-only group.
“The benefit of neoadjuvant pembrolizumab was seen across all subgroups of patients,” the investigators note.
At the data cut-off, there were 14 deaths in the neoadjuvant-adjuvant group vs. 22 in the adjuvant-only group, which the researchers say is too few to allow “definitive comparison” in terms of overall survival.
Definitive surgery had been performed in 88% of neoadjuvant-adjuvant patients and in 95% of those assigned to adjuvant-only pembrolizumab. The most common reason for not undergoing surgery was disease progression.
Among the patients for whom safety data were available, 7% in the neoadjuvant-adjuvant group had at least one grade 3 or 4 adverse event related to pembrolizumab, whereas 7% had at least one grade 3 or 4 adverse event related to surgery.
In the adjuvant-only arm, 4% of patients had at least one grade 3 adverse event related to surgery, with no grade 4 adverse events reported.
The rates of grade 3 or 4 adverse events during adjuvant therapy were similar in the two groups, at 12% in patients assigned to neoadjuvant-adjuvant therapy and 14% in those given adjuvant-only pembrolizumab.
“Future studies can explore deescalation strategies for both surgery and adjuvant therapy, as well as approaches for patients whose melanoma does not respond to neoadjuvant therapy,” the researchers commented.
The study was funded by the National Cancer Institute and Merck Sharp and Dohme.
Dr. Patel reports numerous relationships with industry, including with Merck, manufacturer of pembrolizumab; other coauthors also have numerous relationships with industry. Dr. Weber is a regular columnist for this news organization and lists his disclosures in his Weber on Oncology column.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Skin reactions from melanoma targeted and immune therapies range from pruritus to SJS
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
AT MELANOMA 2023
Expert discusses pros, cons of molecular tests for melanoma
SAN DIEGO – , according to Gregory A. Hosler, MD, PhD.
At the annual Cutaneous Malignancy Update, Dr. Hosler, director of dermatopathology for ProPath, highlighted the following molecular tests currently used for the diagnosis of challenging melanocytic lesions:
Comparative genomic hybridization (CGH). This technique allows for the detection of chromosomal copy number changes throughout the tumor genome. “With CGH, test (tumor) DNA and normal DNA are differentially labeled and compared to a reference library. Gains and losses of portions of the tumor genome are determined by comparing the relative signals from these two groups,” said Dr. Hosler, clinical professor of pathology and dermatology at the University of Texas Southwestern Medical Center, Dallas.
“In the past, your library was a metaphase of spread of chromosomes, which introduced technical challenges and made performance of the assay labor intensive. Because of this, CGH is not routinely performed by clinical laboratories and is used more as an exploratory/research technique.”
Array CGH (also known as SNP array). Newer versions of CGH use short DNA sequences that are tiled onto a chip. “The interesting thing about these chips is that you can purchase them or design them on your own,” Dr. Hosler said. “The chips may cover the entire genome or cover specific areas of the genome at higher resolution.” One upside of array CGH, he continued, is that it allows one to detect essentially all gains or losses of chromosomal material in a single reaction. “It is not subject to the artifacts associated with cutting thin sections like with fluorescence in situ hybridization (FISH); it can detect copy number neutral loss of heterozygosity, and it is more scalable,” Dr. Hosler said at the meeting, which was hosted by Scripps MD Anderson Cancer Center.
One downside of array CGH is that does not allow one to analyze specific cells, “so if you have a tumor that’s heterogeneous, the assay is agnostic to this and spits out a result based on all the material provided,” he said. “You can’t parse out different areas of the lesion. It also does not track balanced translocations.” In addition, he said, “there are also questions about reimbursement and these are lab-developed tests, so each lab’s assay is different. Finally, it requires specialized equipment and expertise for interpretation.”
FISH. First-generation melanoma FISH assays, which became available in 2009, used six probes and four colors and had a sensitivity of about 87% and specificity of about 95%, Dr. Hosler said, but there were problems with those assays, particularly related to Spitz nevi. Spitz nevi often duplicate their chromosomes, “so instead of being diploid they’re tetraploid,” he said.
“The second-generation melanoma FISH assays addressed this by adding centromeres to the assay, and targeted probes could be compared to the centromeres on the same chromosome to determine if these were true copy number gains, due to genetic instability, or gains or losses of entire arms or whole chromosomes. This modification and the addition of new targets really improved upon the sensitivity and specificity (94% and 98%, respectively),” he said, noting that this assay is widely used.
Upsides of melanoma FISH assays are that they are a “fairly routine methodology” in large clinical laboratories, he said, and that many labs are familiar with interpretation. “I would say the biggest advantage to FISH is its ability to analyze specific cells, which is useful with small or heterogeneous tumors,” Dr. Hosler said. “Also, there is a genetic reimbursement code for it, and it yields diagnostic and potentially prognostic information.” For example, certain copy number changes have shown to portend a worse prognosis if they’re present in a melanocytic tumor, including alterations in CDKN2A, CCND1, MYC, topoisomerase, and BAP1.
Downsides of melanoma FISH assays are that they are expensive, labor-intensive, and require experts to interpret the results. “The stacking and truncation of cell nuclei innate to paraffin-embedded FISH make interpretation difficult,” he said. “Also, all colors cannot be viewed simultaneously, and each lab’s assay potentially is different, requiring validation. These are not [Food and Drug Administration]-approved tests.”
Next generation sequencing (NGS). Also known as high-throughput sequencing, this technique allows for the generation of millions of sequencing reads that are aligned to a standard human genome, and likely represents the wave of the future. “With NGS you can increase breadth, so you can sequence the entire genome if you want, but you can also increase depth, meaning increasing the number of reads over a single target of the genome,” Dr. Hosler said. “That’s useful if you’re looking for a low frequency mutation.”
For example, NGS allows one to detect alterations of BRAF and KIT and other potentially actionable alterations. It can also be used to detect mutations in benign and malignant melanocytic lesions, including historically diagnostically challenging Spitz and desmoplastic subgroups. Several different NGS technologies exist, and there are different strategies behind each assay, including whole genome sequencing, whole exome sequencing, transcriptome sequencing, and targeted panels. “I’ve seen panels of 10 and I’ve seen panels of 1,500; there’s a wide range,” Dr. Hosler said. “The biggest challenge with NGS, currently, is that it’s difficult to interpret. Trying to figure out what’s important and what’s not important can be challenging. Often you need a team of people who are experts in bioinformatics to interpret these results.”
Slow turnaround time is another downside. “It can take a month to get results, and sometimes clinicians don’t want to wait that long, especially if they think a lesion is melanoma, so that’s an area of focus for NGS laboratories,” he said. “And there are questions on reimbursement. If you run NGS on every unusual melanocytic lesion, that’s not a good use of health care dollars. Who’s paying for it? I don’t have an answer for you. It’s all over the map right now. Each lab’s test and billing practice is different.”
Dr. Hosler reported having no relevant financial disclosures. ProPath is a nationwide pathology practice.
SAN DIEGO – , according to Gregory A. Hosler, MD, PhD.
At the annual Cutaneous Malignancy Update, Dr. Hosler, director of dermatopathology for ProPath, highlighted the following molecular tests currently used for the diagnosis of challenging melanocytic lesions:
Comparative genomic hybridization (CGH). This technique allows for the detection of chromosomal copy number changes throughout the tumor genome. “With CGH, test (tumor) DNA and normal DNA are differentially labeled and compared to a reference library. Gains and losses of portions of the tumor genome are determined by comparing the relative signals from these two groups,” said Dr. Hosler, clinical professor of pathology and dermatology at the University of Texas Southwestern Medical Center, Dallas.
“In the past, your library was a metaphase of spread of chromosomes, which introduced technical challenges and made performance of the assay labor intensive. Because of this, CGH is not routinely performed by clinical laboratories and is used more as an exploratory/research technique.”
Array CGH (also known as SNP array). Newer versions of CGH use short DNA sequences that are tiled onto a chip. “The interesting thing about these chips is that you can purchase them or design them on your own,” Dr. Hosler said. “The chips may cover the entire genome or cover specific areas of the genome at higher resolution.” One upside of array CGH, he continued, is that it allows one to detect essentially all gains or losses of chromosomal material in a single reaction. “It is not subject to the artifacts associated with cutting thin sections like with fluorescence in situ hybridization (FISH); it can detect copy number neutral loss of heterozygosity, and it is more scalable,” Dr. Hosler said at the meeting, which was hosted by Scripps MD Anderson Cancer Center.
One downside of array CGH is that does not allow one to analyze specific cells, “so if you have a tumor that’s heterogeneous, the assay is agnostic to this and spits out a result based on all the material provided,” he said. “You can’t parse out different areas of the lesion. It also does not track balanced translocations.” In addition, he said, “there are also questions about reimbursement and these are lab-developed tests, so each lab’s assay is different. Finally, it requires specialized equipment and expertise for interpretation.”
FISH. First-generation melanoma FISH assays, which became available in 2009, used six probes and four colors and had a sensitivity of about 87% and specificity of about 95%, Dr. Hosler said, but there were problems with those assays, particularly related to Spitz nevi. Spitz nevi often duplicate their chromosomes, “so instead of being diploid they’re tetraploid,” he said.
“The second-generation melanoma FISH assays addressed this by adding centromeres to the assay, and targeted probes could be compared to the centromeres on the same chromosome to determine if these were true copy number gains, due to genetic instability, or gains or losses of entire arms or whole chromosomes. This modification and the addition of new targets really improved upon the sensitivity and specificity (94% and 98%, respectively),” he said, noting that this assay is widely used.
Upsides of melanoma FISH assays are that they are a “fairly routine methodology” in large clinical laboratories, he said, and that many labs are familiar with interpretation. “I would say the biggest advantage to FISH is its ability to analyze specific cells, which is useful with small or heterogeneous tumors,” Dr. Hosler said. “Also, there is a genetic reimbursement code for it, and it yields diagnostic and potentially prognostic information.” For example, certain copy number changes have shown to portend a worse prognosis if they’re present in a melanocytic tumor, including alterations in CDKN2A, CCND1, MYC, topoisomerase, and BAP1.
Downsides of melanoma FISH assays are that they are expensive, labor-intensive, and require experts to interpret the results. “The stacking and truncation of cell nuclei innate to paraffin-embedded FISH make interpretation difficult,” he said. “Also, all colors cannot be viewed simultaneously, and each lab’s assay potentially is different, requiring validation. These are not [Food and Drug Administration]-approved tests.”
Next generation sequencing (NGS). Also known as high-throughput sequencing, this technique allows for the generation of millions of sequencing reads that are aligned to a standard human genome, and likely represents the wave of the future. “With NGS you can increase breadth, so you can sequence the entire genome if you want, but you can also increase depth, meaning increasing the number of reads over a single target of the genome,” Dr. Hosler said. “That’s useful if you’re looking for a low frequency mutation.”
For example, NGS allows one to detect alterations of BRAF and KIT and other potentially actionable alterations. It can also be used to detect mutations in benign and malignant melanocytic lesions, including historically diagnostically challenging Spitz and desmoplastic subgroups. Several different NGS technologies exist, and there are different strategies behind each assay, including whole genome sequencing, whole exome sequencing, transcriptome sequencing, and targeted panels. “I’ve seen panels of 10 and I’ve seen panels of 1,500; there’s a wide range,” Dr. Hosler said. “The biggest challenge with NGS, currently, is that it’s difficult to interpret. Trying to figure out what’s important and what’s not important can be challenging. Often you need a team of people who are experts in bioinformatics to interpret these results.”
Slow turnaround time is another downside. “It can take a month to get results, and sometimes clinicians don’t want to wait that long, especially if they think a lesion is melanoma, so that’s an area of focus for NGS laboratories,” he said. “And there are questions on reimbursement. If you run NGS on every unusual melanocytic lesion, that’s not a good use of health care dollars. Who’s paying for it? I don’t have an answer for you. It’s all over the map right now. Each lab’s test and billing practice is different.”
Dr. Hosler reported having no relevant financial disclosures. ProPath is a nationwide pathology practice.
SAN DIEGO – , according to Gregory A. Hosler, MD, PhD.
At the annual Cutaneous Malignancy Update, Dr. Hosler, director of dermatopathology for ProPath, highlighted the following molecular tests currently used for the diagnosis of challenging melanocytic lesions:
Comparative genomic hybridization (CGH). This technique allows for the detection of chromosomal copy number changes throughout the tumor genome. “With CGH, test (tumor) DNA and normal DNA are differentially labeled and compared to a reference library. Gains and losses of portions of the tumor genome are determined by comparing the relative signals from these two groups,” said Dr. Hosler, clinical professor of pathology and dermatology at the University of Texas Southwestern Medical Center, Dallas.
“In the past, your library was a metaphase of spread of chromosomes, which introduced technical challenges and made performance of the assay labor intensive. Because of this, CGH is not routinely performed by clinical laboratories and is used more as an exploratory/research technique.”
Array CGH (also known as SNP array). Newer versions of CGH use short DNA sequences that are tiled onto a chip. “The interesting thing about these chips is that you can purchase them or design them on your own,” Dr. Hosler said. “The chips may cover the entire genome or cover specific areas of the genome at higher resolution.” One upside of array CGH, he continued, is that it allows one to detect essentially all gains or losses of chromosomal material in a single reaction. “It is not subject to the artifacts associated with cutting thin sections like with fluorescence in situ hybridization (FISH); it can detect copy number neutral loss of heterozygosity, and it is more scalable,” Dr. Hosler said at the meeting, which was hosted by Scripps MD Anderson Cancer Center.
One downside of array CGH is that does not allow one to analyze specific cells, “so if you have a tumor that’s heterogeneous, the assay is agnostic to this and spits out a result based on all the material provided,” he said. “You can’t parse out different areas of the lesion. It also does not track balanced translocations.” In addition, he said, “there are also questions about reimbursement and these are lab-developed tests, so each lab’s assay is different. Finally, it requires specialized equipment and expertise for interpretation.”
FISH. First-generation melanoma FISH assays, which became available in 2009, used six probes and four colors and had a sensitivity of about 87% and specificity of about 95%, Dr. Hosler said, but there were problems with those assays, particularly related to Spitz nevi. Spitz nevi often duplicate their chromosomes, “so instead of being diploid they’re tetraploid,” he said.
“The second-generation melanoma FISH assays addressed this by adding centromeres to the assay, and targeted probes could be compared to the centromeres on the same chromosome to determine if these were true copy number gains, due to genetic instability, or gains or losses of entire arms or whole chromosomes. This modification and the addition of new targets really improved upon the sensitivity and specificity (94% and 98%, respectively),” he said, noting that this assay is widely used.
Upsides of melanoma FISH assays are that they are a “fairly routine methodology” in large clinical laboratories, he said, and that many labs are familiar with interpretation. “I would say the biggest advantage to FISH is its ability to analyze specific cells, which is useful with small or heterogeneous tumors,” Dr. Hosler said. “Also, there is a genetic reimbursement code for it, and it yields diagnostic and potentially prognostic information.” For example, certain copy number changes have shown to portend a worse prognosis if they’re present in a melanocytic tumor, including alterations in CDKN2A, CCND1, MYC, topoisomerase, and BAP1.
Downsides of melanoma FISH assays are that they are expensive, labor-intensive, and require experts to interpret the results. “The stacking and truncation of cell nuclei innate to paraffin-embedded FISH make interpretation difficult,” he said. “Also, all colors cannot be viewed simultaneously, and each lab’s assay potentially is different, requiring validation. These are not [Food and Drug Administration]-approved tests.”
Next generation sequencing (NGS). Also known as high-throughput sequencing, this technique allows for the generation of millions of sequencing reads that are aligned to a standard human genome, and likely represents the wave of the future. “With NGS you can increase breadth, so you can sequence the entire genome if you want, but you can also increase depth, meaning increasing the number of reads over a single target of the genome,” Dr. Hosler said. “That’s useful if you’re looking for a low frequency mutation.”
For example, NGS allows one to detect alterations of BRAF and KIT and other potentially actionable alterations. It can also be used to detect mutations in benign and malignant melanocytic lesions, including historically diagnostically challenging Spitz and desmoplastic subgroups. Several different NGS technologies exist, and there are different strategies behind each assay, including whole genome sequencing, whole exome sequencing, transcriptome sequencing, and targeted panels. “I’ve seen panels of 10 and I’ve seen panels of 1,500; there’s a wide range,” Dr. Hosler said. “The biggest challenge with NGS, currently, is that it’s difficult to interpret. Trying to figure out what’s important and what’s not important can be challenging. Often you need a team of people who are experts in bioinformatics to interpret these results.”
Slow turnaround time is another downside. “It can take a month to get results, and sometimes clinicians don’t want to wait that long, especially if they think a lesion is melanoma, so that’s an area of focus for NGS laboratories,” he said. “And there are questions on reimbursement. If you run NGS on every unusual melanocytic lesion, that’s not a good use of health care dollars. Who’s paying for it? I don’t have an answer for you. It’s all over the map right now. Each lab’s test and billing practice is different.”
Dr. Hosler reported having no relevant financial disclosures. ProPath is a nationwide pathology practice.
AT MELANOMA 2023
How prevalent is pediatric melanoma?
SAN DIEGO – When parents bring their children to Caroline Piggott, MD, to evaluate a suspicious mole on the scalp or other body location, the vast majority turn out to be benign, because the incidence of melanoma is rare, especially before puberty.
“Only 1%-2% of all melanomas in the world are in children, so most of my job is to provide reassurance,” Dr. Piggott, a pediatric dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “
To help parents identify melanoma, clinicians typically recommend the “ABCDE” rule, for Asymmetry, Border irregularity, Color variation (especially dark or multiple colors), Diameter greater than 6 mm, and Evolving (is it changing, bleeding or painful?).
While Dr. Piggott considers the standard ABCDE rules as important – especially in older children and teenagers – researchers led by Kelly M. Cordoro, MD, professor of dermatology at the University of California, San Francisco, proposed a modified ABCD criteria based on evaluating a cohort of 60 children who were diagnosed with melanoma and 10 who were diagnosed with ambiguous melanocytic tumors treated as melanoma before age 20 years at UCSF from 1984 to 2009.
The researchers divided patients into two groups: those aged 0-10 years (19; group A) and those aged 11-19 years (51; group B), and found that 60% of children in group A and 40% of those in group B did not present with conventional ABCDE criteria for children. Of the 60 melanoma patients, 10 died. Of these, 9 were older than age 10, and 70% had amelanotic lesions. Based on their analysis of clinical, histopathologic, and outcomes data, Dr. Cordoro and colleagues proposed additional ABCD criteria in which A stands for stands Amelanotic; B for Bleeding or Bump; C for Color uniformity, and D for De novo or any Diameter.
“This doesn’t mean you throw the old ABCDE criteria out the window,” Dr. Piggott said. “It means that you use this modified criteria in conjunction with the conventional ABCDE rules.”
Risk factors for melanoma in children are like those in adults, and include a family history of melanoma, large/giant congenital nevi, the presence of many atypical appearing nevi, having Fitzpatrick skin types I or II, a history of blistering sunburns, and the presence of genetic anomalies such as xeroderma pigmentosum.
According to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program, melanoma incidence increased in all individuals in the United States aged 0-19 years from 1973 to 2009. Key risk factors included White race, female sex, and living in a SEER registry categorized as low UVB exposure. Over the study period, boys experienced increased incidence rates of melanoma on the face and trunk, while girls experienced increased incidence rates of melanoma on the lower limbs and hip.
More recently, researchers extracted data from 988,103 cases of invasive melanoma in the 2001-2015 SEER database to determine the age-specific incidence of melanoma in the United States. In 2015, 83,362 cases of invasive melanoma were reported for all ages. Of these, only 67 cases were younger than age 10, while 251 were between the ages of 10 and 19 and 1,973 were young adults between the ages of 20 and 29.
In other findings, between 2006 and 2015, the overall incidence of invasive melanoma for all ages increased from 200 million to 229 cases per million person-years. “However, there were statistically significant decreases in melanoma incidence for individuals aged 10-19 years and for those aged 10-29 years,” said Dr. Piggott, who was not involved with the study. “The hypothesis is that public health efforts encouraging against sun exposure and tanning bed use may be influencing melanoma incidence in younger populations. What is interesting, though, is that young adult women have twice the melanoma risk as young adult men.”
In a separate study, researchers prospectively followed 60 melanoma-prone families for up to 40 years to evaluate the risk of pediatric melanoma in those with and without cyclin-dependent kinase inhibitor 2A (CDKN2A) mutations. Regardless of their CDKN2A status, the percentage of pediatric melanoma cases was 6- to 28-fold higher among melanoma-prone families, compared with the general population. In addition, families who were CDKN2A positive had a significantly higher rate of pediatric melanoma cases compared with those who were CDKN2A negative (11.1% vs. 2.5%; P = .004).
As for treating pediatric melanoma, the standard of care is similar to that for adults: usually wide local surgical excision of the primary lesion, depending on depth. Clinicians typically follow adult parameters for sentinel lymph node biopsy, such as lesion depth and ulceration.
“We know that a positive sentinel node does have prognostic value, but there is great debate on whether to do a lymph node dissection if the sentinel lymph node is positive,” Dr. Piggott said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “This is determined on a case-by-case basis. We consider factors such as, are the nodes palpable? Is there evidence on ultrasound? But there are no formal guidelines.”
Limited studies of systemic therapy in children exist because this population is excluded from most melanoma clinical trials. “In the past, interferon was sometimes used,” she said. “But in recent years, as with adults, we have started to use targeted immunologic therapy. This is usually managed by a tertiary academic oncology center.”
The chance of surviving pediatric melanoma is good if caught early. As in adults, the stage correlates strongly with survival, and distant metastases carry a poor prognosis.
In 2020, researchers published a retrospective, multicenter review of 38 cases of fatal pediatric melanoma between 1994 and 2017. The analysis was limited to individuals 20 years of age and younger who were cared for at 12 academic medical centers. Of the 38 patients, 42% were male, 58% were female, and 57% were White. In addition, 19% were Hispanic, “which is a larger percentage than fatalities in adult [Hispanic] populations with melanoma,” said Dr. Piggott, who was not involved in the study.
The mean age at diagnosis was 12.7 years, the mean age at death was 15.6 , and the mean survival time after diagnosis was about 35 months. Of the 16 cases with known identifiable subtypes, 50% were nodular, 31% were superficial spreading, and 19% were spitzoid melanoma. In addition, one-quarter of melanomas arose in association with congenital melanocytic nevi.
“The good news is that there are only 38 total cases of fatal pediatric melanoma between 12 academic centers over a 23-year period,” Dr. Piggott said. “Thanks goodness the number is that low.”
Dr. Piggott reported having no relevant disclosures.
SAN DIEGO – When parents bring their children to Caroline Piggott, MD, to evaluate a suspicious mole on the scalp or other body location, the vast majority turn out to be benign, because the incidence of melanoma is rare, especially before puberty.
“Only 1%-2% of all melanomas in the world are in children, so most of my job is to provide reassurance,” Dr. Piggott, a pediatric dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “
To help parents identify melanoma, clinicians typically recommend the “ABCDE” rule, for Asymmetry, Border irregularity, Color variation (especially dark or multiple colors), Diameter greater than 6 mm, and Evolving (is it changing, bleeding or painful?).
While Dr. Piggott considers the standard ABCDE rules as important – especially in older children and teenagers – researchers led by Kelly M. Cordoro, MD, professor of dermatology at the University of California, San Francisco, proposed a modified ABCD criteria based on evaluating a cohort of 60 children who were diagnosed with melanoma and 10 who were diagnosed with ambiguous melanocytic tumors treated as melanoma before age 20 years at UCSF from 1984 to 2009.
The researchers divided patients into two groups: those aged 0-10 years (19; group A) and those aged 11-19 years (51; group B), and found that 60% of children in group A and 40% of those in group B did not present with conventional ABCDE criteria for children. Of the 60 melanoma patients, 10 died. Of these, 9 were older than age 10, and 70% had amelanotic lesions. Based on their analysis of clinical, histopathologic, and outcomes data, Dr. Cordoro and colleagues proposed additional ABCD criteria in which A stands for stands Amelanotic; B for Bleeding or Bump; C for Color uniformity, and D for De novo or any Diameter.
“This doesn’t mean you throw the old ABCDE criteria out the window,” Dr. Piggott said. “It means that you use this modified criteria in conjunction with the conventional ABCDE rules.”
Risk factors for melanoma in children are like those in adults, and include a family history of melanoma, large/giant congenital nevi, the presence of many atypical appearing nevi, having Fitzpatrick skin types I or II, a history of blistering sunburns, and the presence of genetic anomalies such as xeroderma pigmentosum.
According to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program, melanoma incidence increased in all individuals in the United States aged 0-19 years from 1973 to 2009. Key risk factors included White race, female sex, and living in a SEER registry categorized as low UVB exposure. Over the study period, boys experienced increased incidence rates of melanoma on the face and trunk, while girls experienced increased incidence rates of melanoma on the lower limbs and hip.
More recently, researchers extracted data from 988,103 cases of invasive melanoma in the 2001-2015 SEER database to determine the age-specific incidence of melanoma in the United States. In 2015, 83,362 cases of invasive melanoma were reported for all ages. Of these, only 67 cases were younger than age 10, while 251 were between the ages of 10 and 19 and 1,973 were young adults between the ages of 20 and 29.
In other findings, between 2006 and 2015, the overall incidence of invasive melanoma for all ages increased from 200 million to 229 cases per million person-years. “However, there were statistically significant decreases in melanoma incidence for individuals aged 10-19 years and for those aged 10-29 years,” said Dr. Piggott, who was not involved with the study. “The hypothesis is that public health efforts encouraging against sun exposure and tanning bed use may be influencing melanoma incidence in younger populations. What is interesting, though, is that young adult women have twice the melanoma risk as young adult men.”
In a separate study, researchers prospectively followed 60 melanoma-prone families for up to 40 years to evaluate the risk of pediatric melanoma in those with and without cyclin-dependent kinase inhibitor 2A (CDKN2A) mutations. Regardless of their CDKN2A status, the percentage of pediatric melanoma cases was 6- to 28-fold higher among melanoma-prone families, compared with the general population. In addition, families who were CDKN2A positive had a significantly higher rate of pediatric melanoma cases compared with those who were CDKN2A negative (11.1% vs. 2.5%; P = .004).
As for treating pediatric melanoma, the standard of care is similar to that for adults: usually wide local surgical excision of the primary lesion, depending on depth. Clinicians typically follow adult parameters for sentinel lymph node biopsy, such as lesion depth and ulceration.
“We know that a positive sentinel node does have prognostic value, but there is great debate on whether to do a lymph node dissection if the sentinel lymph node is positive,” Dr. Piggott said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “This is determined on a case-by-case basis. We consider factors such as, are the nodes palpable? Is there evidence on ultrasound? But there are no formal guidelines.”
Limited studies of systemic therapy in children exist because this population is excluded from most melanoma clinical trials. “In the past, interferon was sometimes used,” she said. “But in recent years, as with adults, we have started to use targeted immunologic therapy. This is usually managed by a tertiary academic oncology center.”
The chance of surviving pediatric melanoma is good if caught early. As in adults, the stage correlates strongly with survival, and distant metastases carry a poor prognosis.
In 2020, researchers published a retrospective, multicenter review of 38 cases of fatal pediatric melanoma between 1994 and 2017. The analysis was limited to individuals 20 years of age and younger who were cared for at 12 academic medical centers. Of the 38 patients, 42% were male, 58% were female, and 57% were White. In addition, 19% were Hispanic, “which is a larger percentage than fatalities in adult [Hispanic] populations with melanoma,” said Dr. Piggott, who was not involved in the study.
The mean age at diagnosis was 12.7 years, the mean age at death was 15.6 , and the mean survival time after diagnosis was about 35 months. Of the 16 cases with known identifiable subtypes, 50% were nodular, 31% were superficial spreading, and 19% were spitzoid melanoma. In addition, one-quarter of melanomas arose in association with congenital melanocytic nevi.
“The good news is that there are only 38 total cases of fatal pediatric melanoma between 12 academic centers over a 23-year period,” Dr. Piggott said. “Thanks goodness the number is that low.”
Dr. Piggott reported having no relevant disclosures.
SAN DIEGO – When parents bring their children to Caroline Piggott, MD, to evaluate a suspicious mole on the scalp or other body location, the vast majority turn out to be benign, because the incidence of melanoma is rare, especially before puberty.
“Only 1%-2% of all melanomas in the world are in children, so most of my job is to provide reassurance,” Dr. Piggott, a pediatric dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “
To help parents identify melanoma, clinicians typically recommend the “ABCDE” rule, for Asymmetry, Border irregularity, Color variation (especially dark or multiple colors), Diameter greater than 6 mm, and Evolving (is it changing, bleeding or painful?).
While Dr. Piggott considers the standard ABCDE rules as important – especially in older children and teenagers – researchers led by Kelly M. Cordoro, MD, professor of dermatology at the University of California, San Francisco, proposed a modified ABCD criteria based on evaluating a cohort of 60 children who were diagnosed with melanoma and 10 who were diagnosed with ambiguous melanocytic tumors treated as melanoma before age 20 years at UCSF from 1984 to 2009.
The researchers divided patients into two groups: those aged 0-10 years (19; group A) and those aged 11-19 years (51; group B), and found that 60% of children in group A and 40% of those in group B did not present with conventional ABCDE criteria for children. Of the 60 melanoma patients, 10 died. Of these, 9 were older than age 10, and 70% had amelanotic lesions. Based on their analysis of clinical, histopathologic, and outcomes data, Dr. Cordoro and colleagues proposed additional ABCD criteria in which A stands for stands Amelanotic; B for Bleeding or Bump; C for Color uniformity, and D for De novo or any Diameter.
“This doesn’t mean you throw the old ABCDE criteria out the window,” Dr. Piggott said. “It means that you use this modified criteria in conjunction with the conventional ABCDE rules.”
Risk factors for melanoma in children are like those in adults, and include a family history of melanoma, large/giant congenital nevi, the presence of many atypical appearing nevi, having Fitzpatrick skin types I or II, a history of blistering sunburns, and the presence of genetic anomalies such as xeroderma pigmentosum.
According to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program, melanoma incidence increased in all individuals in the United States aged 0-19 years from 1973 to 2009. Key risk factors included White race, female sex, and living in a SEER registry categorized as low UVB exposure. Over the study period, boys experienced increased incidence rates of melanoma on the face and trunk, while girls experienced increased incidence rates of melanoma on the lower limbs and hip.
More recently, researchers extracted data from 988,103 cases of invasive melanoma in the 2001-2015 SEER database to determine the age-specific incidence of melanoma in the United States. In 2015, 83,362 cases of invasive melanoma were reported for all ages. Of these, only 67 cases were younger than age 10, while 251 were between the ages of 10 and 19 and 1,973 were young adults between the ages of 20 and 29.
In other findings, between 2006 and 2015, the overall incidence of invasive melanoma for all ages increased from 200 million to 229 cases per million person-years. “However, there were statistically significant decreases in melanoma incidence for individuals aged 10-19 years and for those aged 10-29 years,” said Dr. Piggott, who was not involved with the study. “The hypothesis is that public health efforts encouraging against sun exposure and tanning bed use may be influencing melanoma incidence in younger populations. What is interesting, though, is that young adult women have twice the melanoma risk as young adult men.”
In a separate study, researchers prospectively followed 60 melanoma-prone families for up to 40 years to evaluate the risk of pediatric melanoma in those with and without cyclin-dependent kinase inhibitor 2A (CDKN2A) mutations. Regardless of their CDKN2A status, the percentage of pediatric melanoma cases was 6- to 28-fold higher among melanoma-prone families, compared with the general population. In addition, families who were CDKN2A positive had a significantly higher rate of pediatric melanoma cases compared with those who were CDKN2A negative (11.1% vs. 2.5%; P = .004).
As for treating pediatric melanoma, the standard of care is similar to that for adults: usually wide local surgical excision of the primary lesion, depending on depth. Clinicians typically follow adult parameters for sentinel lymph node biopsy, such as lesion depth and ulceration.
“We know that a positive sentinel node does have prognostic value, but there is great debate on whether to do a lymph node dissection if the sentinel lymph node is positive,” Dr. Piggott said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “This is determined on a case-by-case basis. We consider factors such as, are the nodes palpable? Is there evidence on ultrasound? But there are no formal guidelines.”
Limited studies of systemic therapy in children exist because this population is excluded from most melanoma clinical trials. “In the past, interferon was sometimes used,” she said. “But in recent years, as with adults, we have started to use targeted immunologic therapy. This is usually managed by a tertiary academic oncology center.”
The chance of surviving pediatric melanoma is good if caught early. As in adults, the stage correlates strongly with survival, and distant metastases carry a poor prognosis.
In 2020, researchers published a retrospective, multicenter review of 38 cases of fatal pediatric melanoma between 1994 and 2017. The analysis was limited to individuals 20 years of age and younger who were cared for at 12 academic medical centers. Of the 38 patients, 42% were male, 58% were female, and 57% were White. In addition, 19% were Hispanic, “which is a larger percentage than fatalities in adult [Hispanic] populations with melanoma,” said Dr. Piggott, who was not involved in the study.
The mean age at diagnosis was 12.7 years, the mean age at death was 15.6 , and the mean survival time after diagnosis was about 35 months. Of the 16 cases with known identifiable subtypes, 50% were nodular, 31% were superficial spreading, and 19% were spitzoid melanoma. In addition, one-quarter of melanomas arose in association with congenital melanocytic nevi.
“The good news is that there are only 38 total cases of fatal pediatric melanoma between 12 academic centers over a 23-year period,” Dr. Piggott said. “Thanks goodness the number is that low.”
Dr. Piggott reported having no relevant disclosures.
AT MELANOMA 2023
Optimal management of dysplastic nevi continues to evolve
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
AT MELANOMA 2023
More than 97K new cutaneous melanoma diagnoses expected in 2023
SAN DIEGO – , following cancer of the colorectal area, lung and bronchus, prostate, and breast.
“The incidence of melanoma seems to have continued to go up since the early 1990s,” David E. Kent, MD, a dermatologist who practices in Macon, Ga., said at the annual Cutaneous Malignancy Update. “The death rates have been flat and may have slightly decreased.”
In 2023, the ACS estimates that about 97,610 new melanomas will be diagnosed in the United States (58,120 men and 39,490 women), and about 7,990 people are expected to die of melanoma (5,420 men and 2,570 women). In addition, ACS data from 2017-2019 project that about 2.1% of men and women will be diagnosed with cutaneous melanoma in their lifetime. To date, more than 1.3 million people in the United States live with cutaneous melanoma, and the overall 5-year survival is 93.7%.
Epidemiologic studies show an increase in melanoma incidence, primarily among White populations. “This is believed to be due primarily to sun exposure and to changing recreational behaviors and tanning bed exposures,” said Dr. Kent, who holds a faculty position in the department of dermatology at the Medical College of Georgia, Augusta. Increased surveillance and diagnosis also play a role. In the medical literature, annual increases in melanoma incidence vary from 3% to 7% per year, “which translates into a doubling of rates every 10-20 years,” he said, noting that annual melanoma costs are approximately $3.3 billion.
While incidence rates are lower in non-White, non-Hispanic populations, poor outcomes are disproportionately higher in persons of color. Blacks present at diagnosis with more advanced stage disease and are 1.5 times more likely to die from melanoma, he said, while Hispanics are 2.4 times more likely to present with stage III disease and 3.6 times more likely to have distant metastases. Persons of color also have higher rates of mucosal, acral lentiginous, and subungual melanoma.
Known genetic risk factors for melanoma include having skin types I and II, particularly those with light hair, light eyes, and freckling, and those with a family history have a twofold increased risk. Also, up to 40% of genetic cases are from inherited mutations in CDKN2A, CDK4, BAP1, and MCR1. Other genetic-related risk factors include the number and size of nevi, having atypical nevus syndrome, DNA repair defects, large congenital nevi, and a personal history of melanoma.
The main environmental risk factor for melanoma is exposure to ultraviolet radiation. “You can break it down in terms of whether this exposure is lifetime, intermittent intense UV exposure, from the use of tanning beds, or due to sunburns during childhood,” Dr. Kent said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. Other environmental risk factors include distance from the equator, having a high socioeconomic status, being immunosuppressed, and exposure to heavy metals, insecticides, or hormones.
In a recently published study, researchers investigated the risk factors associated with first and second primary melanomas in 38,845 patients who were followed in Australia between 2011 and 2018. During a median follow-up of 7.4 years, 1,212 patients (3.1%) had a single primary melanoma diagnosis and 245 (0.6%) had a secondary primary melanoma diagnosis. The researchers found that second melanomas were more likely than were first melanomas to be in situ; for invasive tumors, second melanomas were more likely to be thin (defined as 1 mm or less) than were first melanomas.
In addition, having many self-reported moles at age 21 years was more strongly associated with second melanomas compared with first melanomas (hazard ratio [HR], 6.36 vs. 3.46, respectively; P = .01), as was having a high genetic predisposition (HR, 3.28 vs. 2.06; P = .03).
Second melanomas were also more strongly associated with a history of multiple skin cancer excisions than were first melanomas (HR, 2.63 vs. 1.86; P = .05). “Interestingly, there were no differences in UV exposure between the first primary and second primary melanoma groups,” said Dr. Kent, who was not involved with the study.
He noted that while sunscreen use protects against melanoma, a National Ambulatory Medical Care Survey (NAMCS) found that internists and pediatricians mentioned sunscreen at fewer than 0.1% of visits – even those with patients who have a diagnosis of skin disease. “Physicians need to do better,” he said. “We as dermatologists have work to do to help educate them.”
Dr. Kent reported having no relevant disclosures.
SAN DIEGO – , following cancer of the colorectal area, lung and bronchus, prostate, and breast.
“The incidence of melanoma seems to have continued to go up since the early 1990s,” David E. Kent, MD, a dermatologist who practices in Macon, Ga., said at the annual Cutaneous Malignancy Update. “The death rates have been flat and may have slightly decreased.”
In 2023, the ACS estimates that about 97,610 new melanomas will be diagnosed in the United States (58,120 men and 39,490 women), and about 7,990 people are expected to die of melanoma (5,420 men and 2,570 women). In addition, ACS data from 2017-2019 project that about 2.1% of men and women will be diagnosed with cutaneous melanoma in their lifetime. To date, more than 1.3 million people in the United States live with cutaneous melanoma, and the overall 5-year survival is 93.7%.
Epidemiologic studies show an increase in melanoma incidence, primarily among White populations. “This is believed to be due primarily to sun exposure and to changing recreational behaviors and tanning bed exposures,” said Dr. Kent, who holds a faculty position in the department of dermatology at the Medical College of Georgia, Augusta. Increased surveillance and diagnosis also play a role. In the medical literature, annual increases in melanoma incidence vary from 3% to 7% per year, “which translates into a doubling of rates every 10-20 years,” he said, noting that annual melanoma costs are approximately $3.3 billion.
While incidence rates are lower in non-White, non-Hispanic populations, poor outcomes are disproportionately higher in persons of color. Blacks present at diagnosis with more advanced stage disease and are 1.5 times more likely to die from melanoma, he said, while Hispanics are 2.4 times more likely to present with stage III disease and 3.6 times more likely to have distant metastases. Persons of color also have higher rates of mucosal, acral lentiginous, and subungual melanoma.
Known genetic risk factors for melanoma include having skin types I and II, particularly those with light hair, light eyes, and freckling, and those with a family history have a twofold increased risk. Also, up to 40% of genetic cases are from inherited mutations in CDKN2A, CDK4, BAP1, and MCR1. Other genetic-related risk factors include the number and size of nevi, having atypical nevus syndrome, DNA repair defects, large congenital nevi, and a personal history of melanoma.
The main environmental risk factor for melanoma is exposure to ultraviolet radiation. “You can break it down in terms of whether this exposure is lifetime, intermittent intense UV exposure, from the use of tanning beds, or due to sunburns during childhood,” Dr. Kent said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. Other environmental risk factors include distance from the equator, having a high socioeconomic status, being immunosuppressed, and exposure to heavy metals, insecticides, or hormones.
In a recently published study, researchers investigated the risk factors associated with first and second primary melanomas in 38,845 patients who were followed in Australia between 2011 and 2018. During a median follow-up of 7.4 years, 1,212 patients (3.1%) had a single primary melanoma diagnosis and 245 (0.6%) had a secondary primary melanoma diagnosis. The researchers found that second melanomas were more likely than were first melanomas to be in situ; for invasive tumors, second melanomas were more likely to be thin (defined as 1 mm or less) than were first melanomas.
In addition, having many self-reported moles at age 21 years was more strongly associated with second melanomas compared with first melanomas (hazard ratio [HR], 6.36 vs. 3.46, respectively; P = .01), as was having a high genetic predisposition (HR, 3.28 vs. 2.06; P = .03).
Second melanomas were also more strongly associated with a history of multiple skin cancer excisions than were first melanomas (HR, 2.63 vs. 1.86; P = .05). “Interestingly, there were no differences in UV exposure between the first primary and second primary melanoma groups,” said Dr. Kent, who was not involved with the study.
He noted that while sunscreen use protects against melanoma, a National Ambulatory Medical Care Survey (NAMCS) found that internists and pediatricians mentioned sunscreen at fewer than 0.1% of visits – even those with patients who have a diagnosis of skin disease. “Physicians need to do better,” he said. “We as dermatologists have work to do to help educate them.”
Dr. Kent reported having no relevant disclosures.
SAN DIEGO – , following cancer of the colorectal area, lung and bronchus, prostate, and breast.
“The incidence of melanoma seems to have continued to go up since the early 1990s,” David E. Kent, MD, a dermatologist who practices in Macon, Ga., said at the annual Cutaneous Malignancy Update. “The death rates have been flat and may have slightly decreased.”
In 2023, the ACS estimates that about 97,610 new melanomas will be diagnosed in the United States (58,120 men and 39,490 women), and about 7,990 people are expected to die of melanoma (5,420 men and 2,570 women). In addition, ACS data from 2017-2019 project that about 2.1% of men and women will be diagnosed with cutaneous melanoma in their lifetime. To date, more than 1.3 million people in the United States live with cutaneous melanoma, and the overall 5-year survival is 93.7%.
Epidemiologic studies show an increase in melanoma incidence, primarily among White populations. “This is believed to be due primarily to sun exposure and to changing recreational behaviors and tanning bed exposures,” said Dr. Kent, who holds a faculty position in the department of dermatology at the Medical College of Georgia, Augusta. Increased surveillance and diagnosis also play a role. In the medical literature, annual increases in melanoma incidence vary from 3% to 7% per year, “which translates into a doubling of rates every 10-20 years,” he said, noting that annual melanoma costs are approximately $3.3 billion.
While incidence rates are lower in non-White, non-Hispanic populations, poor outcomes are disproportionately higher in persons of color. Blacks present at diagnosis with more advanced stage disease and are 1.5 times more likely to die from melanoma, he said, while Hispanics are 2.4 times more likely to present with stage III disease and 3.6 times more likely to have distant metastases. Persons of color also have higher rates of mucosal, acral lentiginous, and subungual melanoma.
Known genetic risk factors for melanoma include having skin types I and II, particularly those with light hair, light eyes, and freckling, and those with a family history have a twofold increased risk. Also, up to 40% of genetic cases are from inherited mutations in CDKN2A, CDK4, BAP1, and MCR1. Other genetic-related risk factors include the number and size of nevi, having atypical nevus syndrome, DNA repair defects, large congenital nevi, and a personal history of melanoma.
The main environmental risk factor for melanoma is exposure to ultraviolet radiation. “You can break it down in terms of whether this exposure is lifetime, intermittent intense UV exposure, from the use of tanning beds, or due to sunburns during childhood,” Dr. Kent said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. Other environmental risk factors include distance from the equator, having a high socioeconomic status, being immunosuppressed, and exposure to heavy metals, insecticides, or hormones.
In a recently published study, researchers investigated the risk factors associated with first and second primary melanomas in 38,845 patients who were followed in Australia between 2011 and 2018. During a median follow-up of 7.4 years, 1,212 patients (3.1%) had a single primary melanoma diagnosis and 245 (0.6%) had a secondary primary melanoma diagnosis. The researchers found that second melanomas were more likely than were first melanomas to be in situ; for invasive tumors, second melanomas were more likely to be thin (defined as 1 mm or less) than were first melanomas.
In addition, having many self-reported moles at age 21 years was more strongly associated with second melanomas compared with first melanomas (hazard ratio [HR], 6.36 vs. 3.46, respectively; P = .01), as was having a high genetic predisposition (HR, 3.28 vs. 2.06; P = .03).
Second melanomas were also more strongly associated with a history of multiple skin cancer excisions than were first melanomas (HR, 2.63 vs. 1.86; P = .05). “Interestingly, there were no differences in UV exposure between the first primary and second primary melanoma groups,” said Dr. Kent, who was not involved with the study.
He noted that while sunscreen use protects against melanoma, a National Ambulatory Medical Care Survey (NAMCS) found that internists and pediatricians mentioned sunscreen at fewer than 0.1% of visits – even those with patients who have a diagnosis of skin disease. “Physicians need to do better,” he said. “We as dermatologists have work to do to help educate them.”
Dr. Kent reported having no relevant disclosures.
AT MELANOMA 2023








