User login
Discontinuing immunotherapy: Is the infusion bag half empty or half full?
It’s a “champagne problem” many of us have encountered over the past few years in the clinic.
A patient with advanced non–small cell lung cancer (NSCLC) is fortunate enough to continue to do well for 2 years on ongoing pembrolizumab or perhaps pemetrexed and pembrolizumab as maintenance therapy. The latest CT shows a residual but far smaller primary tumor than what she started with.
In this instance, you may be considering stopping treatment but are concerned about doing so with evidence of disease still present.
Clinical trials of immunotherapy or chemoimmunotherapy have generally terminated treatment in nonprogressing patients after 2 years. We also know that some patients in early trials of immunotherapy stopped treatment after a fixed period of 1 or 2 years and continued to show no evidence of progression many years later.
The reason some patients experience this kind of success: Unlike the mechanism of action of conventional chemotherapy or targeted therapies, where ongoing treatment would be important to continue to exert an inhibitory effect, the active substrate of immunotherapy is the patient’s immune system, which can potentially have a self-sustaining efficacy beyond the stimulatory effect of the checkpoint inhibitor.
One trial directly addressed this question of stopping vs. continuing treatment in patients on immunotherapy. The CheckMate 153 trial, published in 2020, randomly assigned 252 previously treated patients who hadn’t demonstrated progression after 1 year on nivolumab to either discontinue nivolumab or continue nivolumab on an ongoing basis. The results were strongly in favor of ongoing therapy. Both progression-free survival (PFS) and overall survival (OS) were significantly longer in patients who continued therapy: PFS of 24.7 months vs. 9.4 months and OS not reached vs. 32.5 months.
This finding is important, but there’s an important caveat. The study population included many heavily pretreated patients, but, in practice, immunotherapy has generally moved into the first-line setting, where we see dramatic responses in a significant subset of patients.
Even more recent data are emerging that may help us evaluate who will do well off therapy and who should continue treatment.
We now have a growing collection of long-term data on patients who are more likely to have good outcomes with immunotherapy, specifically those with high tumor programmed death-ligand 1 (PD-L1) expression (≥ 50%), from the KEYNOTE-024 trial. In this study, 39 of 151 (25.8%) patients assigned to pembrolizumab completed the planned maximum of 2 years of treatment, among whom 82.1% achieved an objective response; but, only 10% (4 patients) achieved a complete response. The proportion of patients without progression and remaining off therapy wasn’t reported, but the OS rate 3 years after completing treatment was 81.4%.
In addition, restarting immunotherapy after discontinuing appears to be a moderately effective strategy. In the KEYNOTE-024 trial, 12 patients received a second course of pembrolizumab because of disease progression a median of 15.2 months after discontinuing pembrolizumab. In this small cohort, eight of these patients (66.7%) were alive at the data cutoff, and six (50%) achieved stable disease.
Recently, we received additional insight in the follow-up from two chemoimmunotherapy trials that have most shaped my practice for patients with advanced NSCLC and any level of PD-L1 expression. These are the KEYNOTE-189 trial of platinum-pemetrexed with pembrolizumab vs. placebo in those with nonsquamous NSCLC, and the KEYNOTE-407 trial of carboplatin-taxane with pembrolizumab vs. placebo in patients with advanced squamous NSCLC. The National Comprehensive Cancer Network has designated each as a “preferred regimen” for patients with advanced NSCLC.
Both regimens have demonstrated sustained efficacy benefits with prolonged follow-up, including significantly superior objective response rate, PFS, and OS with the addition of pembrolizumab. These findings merely cemented the role of these regimens in our practice, but the trials also reported on the cohort of patients who completed 35 cycles of treatment over 2 years then discontinued therapy. In both, the majority of patients showed an objective response (86% in KEYNOTE-189 and 90% in KEYNOTE-407), with most patients alive at 3 years after 2 years of treatment (71.9% in KEYNOTE-189 and 69.5% in KEYNOTE-407). In addition, the proportion of patients alive without disease progression or subsequent therapy was notable – 40.4% in KEYNOTE-189 and 43.6% KEYNOTE-407.
How should we interpret these data for the patient who is in the exam room with us?
The short answer is that we don’t know. I see this as a half-empty, half-full conundrum.
I’m disappointed that more patients who responded for 2 years will experience disease progression in the 1-3 years that follow. This signals that their immune systems have not perpetuated their initial response over the long-term. But these patients may have demonstrated disease progression even if they had continued therapy.
We also know that some patients can be rechallenged and will respond again. Some of these patients will show stable disease, whereas others will progress with repeat treatment. I would love to be able to better predict which patients are destined to do well without treatment vs. those who benefit from treatment beyond 2 years.
Might the level of PD-L1 expression tell us? Can PET imaging discriminate those with residual hypermetabolism who may need continued treatment from those with no residual uptake who could be spared it? Would serial measurement of circulating tumor DNA (ctDNA) in responding patients identify when they have achieved a point of diminishing returns, potentially indicating that some can safely discontinue treatment after 2 years, whereas others need to continue to suppress on prolonged maintenance therapy?
These questions have yet to be studied systematically. In the meantime, I take an individualized approach with my patients facing this decision. Some have experienced escalating arthralgias and myalgias, cost concerns, or other issues related to immunotherapy that may dissuade us from continuing treatment. But several others have been grateful to continue with their treatment, hesitant to do anything that could change the path of their disease.
In my patients who tolerate therapy well, I’m more worried about potential undertreatment than overtreatment. I tend to favor having my patients continue therapy in the absence of problematic toxicity or practical challenges. There is certainly room for debate here while we await data to better guide these decisions. How do you approach these patients?
Dr. West is Clinical Associate Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Care, Duarte, Calif. He reported conflicts of interest with Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, Merck, and Eli Lilly.
A version of this article first appeared on Medscape.com.
It’s a “champagne problem” many of us have encountered over the past few years in the clinic.
A patient with advanced non–small cell lung cancer (NSCLC) is fortunate enough to continue to do well for 2 years on ongoing pembrolizumab or perhaps pemetrexed and pembrolizumab as maintenance therapy. The latest CT shows a residual but far smaller primary tumor than what she started with.
In this instance, you may be considering stopping treatment but are concerned about doing so with evidence of disease still present.
Clinical trials of immunotherapy or chemoimmunotherapy have generally terminated treatment in nonprogressing patients after 2 years. We also know that some patients in early trials of immunotherapy stopped treatment after a fixed period of 1 or 2 years and continued to show no evidence of progression many years later.
The reason some patients experience this kind of success: Unlike the mechanism of action of conventional chemotherapy or targeted therapies, where ongoing treatment would be important to continue to exert an inhibitory effect, the active substrate of immunotherapy is the patient’s immune system, which can potentially have a self-sustaining efficacy beyond the stimulatory effect of the checkpoint inhibitor.
One trial directly addressed this question of stopping vs. continuing treatment in patients on immunotherapy. The CheckMate 153 trial, published in 2020, randomly assigned 252 previously treated patients who hadn’t demonstrated progression after 1 year on nivolumab to either discontinue nivolumab or continue nivolumab on an ongoing basis. The results were strongly in favor of ongoing therapy. Both progression-free survival (PFS) and overall survival (OS) were significantly longer in patients who continued therapy: PFS of 24.7 months vs. 9.4 months and OS not reached vs. 32.5 months.
This finding is important, but there’s an important caveat. The study population included many heavily pretreated patients, but, in practice, immunotherapy has generally moved into the first-line setting, where we see dramatic responses in a significant subset of patients.
Even more recent data are emerging that may help us evaluate who will do well off therapy and who should continue treatment.
We now have a growing collection of long-term data on patients who are more likely to have good outcomes with immunotherapy, specifically those with high tumor programmed death-ligand 1 (PD-L1) expression (≥ 50%), from the KEYNOTE-024 trial. In this study, 39 of 151 (25.8%) patients assigned to pembrolizumab completed the planned maximum of 2 years of treatment, among whom 82.1% achieved an objective response; but, only 10% (4 patients) achieved a complete response. The proportion of patients without progression and remaining off therapy wasn’t reported, but the OS rate 3 years after completing treatment was 81.4%.
In addition, restarting immunotherapy after discontinuing appears to be a moderately effective strategy. In the KEYNOTE-024 trial, 12 patients received a second course of pembrolizumab because of disease progression a median of 15.2 months after discontinuing pembrolizumab. In this small cohort, eight of these patients (66.7%) were alive at the data cutoff, and six (50%) achieved stable disease.
Recently, we received additional insight in the follow-up from two chemoimmunotherapy trials that have most shaped my practice for patients with advanced NSCLC and any level of PD-L1 expression. These are the KEYNOTE-189 trial of platinum-pemetrexed with pembrolizumab vs. placebo in those with nonsquamous NSCLC, and the KEYNOTE-407 trial of carboplatin-taxane with pembrolizumab vs. placebo in patients with advanced squamous NSCLC. The National Comprehensive Cancer Network has designated each as a “preferred regimen” for patients with advanced NSCLC.
Both regimens have demonstrated sustained efficacy benefits with prolonged follow-up, including significantly superior objective response rate, PFS, and OS with the addition of pembrolizumab. These findings merely cemented the role of these regimens in our practice, but the trials also reported on the cohort of patients who completed 35 cycles of treatment over 2 years then discontinued therapy. In both, the majority of patients showed an objective response (86% in KEYNOTE-189 and 90% in KEYNOTE-407), with most patients alive at 3 years after 2 years of treatment (71.9% in KEYNOTE-189 and 69.5% in KEYNOTE-407). In addition, the proportion of patients alive without disease progression or subsequent therapy was notable – 40.4% in KEYNOTE-189 and 43.6% KEYNOTE-407.
How should we interpret these data for the patient who is in the exam room with us?
The short answer is that we don’t know. I see this as a half-empty, half-full conundrum.
I’m disappointed that more patients who responded for 2 years will experience disease progression in the 1-3 years that follow. This signals that their immune systems have not perpetuated their initial response over the long-term. But these patients may have demonstrated disease progression even if they had continued therapy.
We also know that some patients can be rechallenged and will respond again. Some of these patients will show stable disease, whereas others will progress with repeat treatment. I would love to be able to better predict which patients are destined to do well without treatment vs. those who benefit from treatment beyond 2 years.
Might the level of PD-L1 expression tell us? Can PET imaging discriminate those with residual hypermetabolism who may need continued treatment from those with no residual uptake who could be spared it? Would serial measurement of circulating tumor DNA (ctDNA) in responding patients identify when they have achieved a point of diminishing returns, potentially indicating that some can safely discontinue treatment after 2 years, whereas others need to continue to suppress on prolonged maintenance therapy?
These questions have yet to be studied systematically. In the meantime, I take an individualized approach with my patients facing this decision. Some have experienced escalating arthralgias and myalgias, cost concerns, or other issues related to immunotherapy that may dissuade us from continuing treatment. But several others have been grateful to continue with their treatment, hesitant to do anything that could change the path of their disease.
In my patients who tolerate therapy well, I’m more worried about potential undertreatment than overtreatment. I tend to favor having my patients continue therapy in the absence of problematic toxicity or practical challenges. There is certainly room for debate here while we await data to better guide these decisions. How do you approach these patients?
Dr. West is Clinical Associate Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Care, Duarte, Calif. He reported conflicts of interest with Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, Merck, and Eli Lilly.
A version of this article first appeared on Medscape.com.
It’s a “champagne problem” many of us have encountered over the past few years in the clinic.
A patient with advanced non–small cell lung cancer (NSCLC) is fortunate enough to continue to do well for 2 years on ongoing pembrolizumab or perhaps pemetrexed and pembrolizumab as maintenance therapy. The latest CT shows a residual but far smaller primary tumor than what she started with.
In this instance, you may be considering stopping treatment but are concerned about doing so with evidence of disease still present.
Clinical trials of immunotherapy or chemoimmunotherapy have generally terminated treatment in nonprogressing patients after 2 years. We also know that some patients in early trials of immunotherapy stopped treatment after a fixed period of 1 or 2 years and continued to show no evidence of progression many years later.
The reason some patients experience this kind of success: Unlike the mechanism of action of conventional chemotherapy or targeted therapies, where ongoing treatment would be important to continue to exert an inhibitory effect, the active substrate of immunotherapy is the patient’s immune system, which can potentially have a self-sustaining efficacy beyond the stimulatory effect of the checkpoint inhibitor.
One trial directly addressed this question of stopping vs. continuing treatment in patients on immunotherapy. The CheckMate 153 trial, published in 2020, randomly assigned 252 previously treated patients who hadn’t demonstrated progression after 1 year on nivolumab to either discontinue nivolumab or continue nivolumab on an ongoing basis. The results were strongly in favor of ongoing therapy. Both progression-free survival (PFS) and overall survival (OS) were significantly longer in patients who continued therapy: PFS of 24.7 months vs. 9.4 months and OS not reached vs. 32.5 months.
This finding is important, but there’s an important caveat. The study population included many heavily pretreated patients, but, in practice, immunotherapy has generally moved into the first-line setting, where we see dramatic responses in a significant subset of patients.
Even more recent data are emerging that may help us evaluate who will do well off therapy and who should continue treatment.
We now have a growing collection of long-term data on patients who are more likely to have good outcomes with immunotherapy, specifically those with high tumor programmed death-ligand 1 (PD-L1) expression (≥ 50%), from the KEYNOTE-024 trial. In this study, 39 of 151 (25.8%) patients assigned to pembrolizumab completed the planned maximum of 2 years of treatment, among whom 82.1% achieved an objective response; but, only 10% (4 patients) achieved a complete response. The proportion of patients without progression and remaining off therapy wasn’t reported, but the OS rate 3 years after completing treatment was 81.4%.
In addition, restarting immunotherapy after discontinuing appears to be a moderately effective strategy. In the KEYNOTE-024 trial, 12 patients received a second course of pembrolizumab because of disease progression a median of 15.2 months after discontinuing pembrolizumab. In this small cohort, eight of these patients (66.7%) were alive at the data cutoff, and six (50%) achieved stable disease.
Recently, we received additional insight in the follow-up from two chemoimmunotherapy trials that have most shaped my practice for patients with advanced NSCLC and any level of PD-L1 expression. These are the KEYNOTE-189 trial of platinum-pemetrexed with pembrolizumab vs. placebo in those with nonsquamous NSCLC, and the KEYNOTE-407 trial of carboplatin-taxane with pembrolizumab vs. placebo in patients with advanced squamous NSCLC. The National Comprehensive Cancer Network has designated each as a “preferred regimen” for patients with advanced NSCLC.
Both regimens have demonstrated sustained efficacy benefits with prolonged follow-up, including significantly superior objective response rate, PFS, and OS with the addition of pembrolizumab. These findings merely cemented the role of these regimens in our practice, but the trials also reported on the cohort of patients who completed 35 cycles of treatment over 2 years then discontinued therapy. In both, the majority of patients showed an objective response (86% in KEYNOTE-189 and 90% in KEYNOTE-407), with most patients alive at 3 years after 2 years of treatment (71.9% in KEYNOTE-189 and 69.5% in KEYNOTE-407). In addition, the proportion of patients alive without disease progression or subsequent therapy was notable – 40.4% in KEYNOTE-189 and 43.6% KEYNOTE-407.
How should we interpret these data for the patient who is in the exam room with us?
The short answer is that we don’t know. I see this as a half-empty, half-full conundrum.
I’m disappointed that more patients who responded for 2 years will experience disease progression in the 1-3 years that follow. This signals that their immune systems have not perpetuated their initial response over the long-term. But these patients may have demonstrated disease progression even if they had continued therapy.
We also know that some patients can be rechallenged and will respond again. Some of these patients will show stable disease, whereas others will progress with repeat treatment. I would love to be able to better predict which patients are destined to do well without treatment vs. those who benefit from treatment beyond 2 years.
Might the level of PD-L1 expression tell us? Can PET imaging discriminate those with residual hypermetabolism who may need continued treatment from those with no residual uptake who could be spared it? Would serial measurement of circulating tumor DNA (ctDNA) in responding patients identify when they have achieved a point of diminishing returns, potentially indicating that some can safely discontinue treatment after 2 years, whereas others need to continue to suppress on prolonged maintenance therapy?
These questions have yet to be studied systematically. In the meantime, I take an individualized approach with my patients facing this decision. Some have experienced escalating arthralgias and myalgias, cost concerns, or other issues related to immunotherapy that may dissuade us from continuing treatment. But several others have been grateful to continue with their treatment, hesitant to do anything that could change the path of their disease.
In my patients who tolerate therapy well, I’m more worried about potential undertreatment than overtreatment. I tend to favor having my patients continue therapy in the absence of problematic toxicity or practical challenges. There is certainly room for debate here while we await data to better guide these decisions. How do you approach these patients?
Dr. West is Clinical Associate Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Care, Duarte, Calif. He reported conflicts of interest with Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, Merck, and Eli Lilly.
A version of this article first appeared on Medscape.com.
Primary Malignant Melanoma of the Middle Ear
To the Editor:
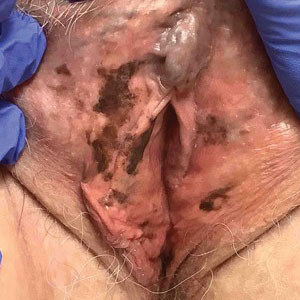
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
PRACTICE POINTS
- Primary malignant melanoma of the middle ear is rare and has poor prognosis.
- Distant metastasis, including cutaneous metastasis, results from primary middle ear melanoma.
How AI is, or will soon be, relevant in radiation oncology
Artificial intelligence (AI) is impacting many aspects of health care, and radiation oncology is no exception. It has the potential to cut costs and streamline work flows ranging from image analysis to treatment plan formulation, but its specific place in clinical practice is still being debated.
In a session at the annual meeting of the American Society for Radiation Oncology, researchers discussed some of the ways that AI is or will soon be relevant to the clinic. The general consensus was that
In his talk, Sanjay Aneja, MD focused on practical applications of AI that are in the clinic or close to being ready. One example is image classification. “There has been recent evidence that suggests in a variety of different kind of scenarios, deep-learning models can be very good at image classification in automated ways,” said Dr. Aneja, who is a professor of radiology at Yale University, New Haven, Conn. He described one study that used AI to classify 14 different pathologies on chest x-ray images.
Dr. Aneja described the open-source nnU-net tool, which automatically configures itself and segments biomedical images for research or clinical purposes, including therapy planning support, intraoperative support, and tumor growth monitoring. The researchers who developed it also created a “recipe” to systematize configuration of nnU-net, making it useful as an out-of-the-box tool for image segmentation.
He predicted that AI will improve radiology oncology by assisting in the determination of disease extent, including microscopic areas of disease. It could also help plan treatment volume and monitor treatment response. “I think that these are the types of things that will be moving toward the clinic in the future; very specific applications and models trained on very specific scenarios that will help us answer a very important clinical question,” Dr. Aneja said.
He expects AI to contribute to auto-segmenting and clinical contouring, “but I will caution everyone that these algorithms have not been proven to be better than physician contours. They very frequently fail in the specific use cases when anatomy is distorted by, I don’t know, say a tumor. And so a lot of times, we don’t actually have the ability to just make it an automated process. I think it’ll be something that physicians will use to help them but not necessarily replace their contouring ability,” Dr. Aneja said.
Another, potentially more useful application, is in adaptive radiation planning. “I think that AI auto-contouring will be very helpful in establishing contours in a situation in which a physician doing them would not be feasible. We need to have nimble and computationally efficient auto segmentation algorithms that will be able to be easily deployed at the linear accelerator,” he said.
AI in pathology and treatment selection
In another talk, Osama Mohamad, MD talked about AI in pathology, and specifically treatment selection. He described research from his group that digitized pathology data from 5,500 patients drawn from five randomized, clinical trials. They used AI on data from four of the clinical trials to identify a prognostic biomarker for distant metastasis, then validated it on data from the remaining clinical trial, which compared radiation versus radiation plus short-term hormone therapy in prostate cancer.
The results suggested that most patients should receive hormone therapy, but the AI suggested a more nuanced answer. “Patients who had AI biomarker negative do not see any benefit from adding 4 months of hormone therapy ... whereas patients who have biomarker positive have significant difference and improvement in distant metastasis at 10 years and 15 years. This means that we can save a significant proportion of patients from getting [androgen deprivation therapy], which is hormonal therapy and has very well-known side effects, because they simply they will not benefit,” said Dr. Mohamad, who is an assistant professor of radiation oncology at University of California, San Francisco.
That study relied on the ArteraAI prostate cancer test, which is available through a Clinical Laboratory Improvement Amendment–certified laboratory in Florida.
Another example of AI used to plan treatment is On-line Real-time Benchmarking Informatics Technology for Radiotherapy (ORBIT-RT), developed at the University of California, San Diego. It focuses on radiotherapy treatment plan quality control, and has two main components: creating clinically validated plan routines and a free radiotherapy plan quality control system.
No matter how impressive the technical advances may be, AI contributions won’t impact clinical practice if radiation oncologists, physicians, and patients don’t accept AI. Dr. Aneja’s group surveyed patients about which health field they would feel more comfortable with AI having an important role. Most said they were extremely uncomfortable when it came to cancer. “Now, does that mean that we can’t use AI in oncology? No, I think it just means that we have to be a little bit more nuanced in our approach and how we develop AI solutions for cancer patients,” Dr. Aneja said.
Physicians also show reluctance, according to Alejandro Berlin, MD, who is an affiliate scientist at Princess Margaret Cancer Centre in Toronto. He discussed some research looking at physician acceptance of machine learning. His group looked at physician acceptance of treatment plans for prostate cancer that were generated by physicians and in parallel by machine learning. In a theoretical phase, physicians generally agreed that the machine learning plans were better, but when it came to a phase of the study in which physicians chose which plan to implement in a real patient, the acceptance of machine learning-generated plans dropped by 20%.
This tendency to trust humans over machines is what Dr. Berlin called “automation bias,” and he called for a more collaborative approach to implement AI. “In some cases, [machine learning] is going to be good and sufficient. And in some cases, you will need the expertise of a human.”
Dr. Aneja, who also moderated the session, expressed a similar sentiment when summing up the day’s talks: “I do feel like it’s a disruptive technology ... but I think there will still be a need for us to have people who are trained in order to evaluate and make sure that these algorithms are working correctly and efficiently.”
Dr. Aneja, Dr. Mohamad, and Dr. Berlin have no relevant financial disclosures.
* This article was updated on Nov. 15, 2022.
Artificial intelligence (AI) is impacting many aspects of health care, and radiation oncology is no exception. It has the potential to cut costs and streamline work flows ranging from image analysis to treatment plan formulation, but its specific place in clinical practice is still being debated.
In a session at the annual meeting of the American Society for Radiation Oncology, researchers discussed some of the ways that AI is or will soon be relevant to the clinic. The general consensus was that
In his talk, Sanjay Aneja, MD focused on practical applications of AI that are in the clinic or close to being ready. One example is image classification. “There has been recent evidence that suggests in a variety of different kind of scenarios, deep-learning models can be very good at image classification in automated ways,” said Dr. Aneja, who is a professor of radiology at Yale University, New Haven, Conn. He described one study that used AI to classify 14 different pathologies on chest x-ray images.
Dr. Aneja described the open-source nnU-net tool, which automatically configures itself and segments biomedical images for research or clinical purposes, including therapy planning support, intraoperative support, and tumor growth monitoring. The researchers who developed it also created a “recipe” to systematize configuration of nnU-net, making it useful as an out-of-the-box tool for image segmentation.
He predicted that AI will improve radiology oncology by assisting in the determination of disease extent, including microscopic areas of disease. It could also help plan treatment volume and monitor treatment response. “I think that these are the types of things that will be moving toward the clinic in the future; very specific applications and models trained on very specific scenarios that will help us answer a very important clinical question,” Dr. Aneja said.
He expects AI to contribute to auto-segmenting and clinical contouring, “but I will caution everyone that these algorithms have not been proven to be better than physician contours. They very frequently fail in the specific use cases when anatomy is distorted by, I don’t know, say a tumor. And so a lot of times, we don’t actually have the ability to just make it an automated process. I think it’ll be something that physicians will use to help them but not necessarily replace their contouring ability,” Dr. Aneja said.
Another, potentially more useful application, is in adaptive radiation planning. “I think that AI auto-contouring will be very helpful in establishing contours in a situation in which a physician doing them would not be feasible. We need to have nimble and computationally efficient auto segmentation algorithms that will be able to be easily deployed at the linear accelerator,” he said.
AI in pathology and treatment selection
In another talk, Osama Mohamad, MD talked about AI in pathology, and specifically treatment selection. He described research from his group that digitized pathology data from 5,500 patients drawn from five randomized, clinical trials. They used AI on data from four of the clinical trials to identify a prognostic biomarker for distant metastasis, then validated it on data from the remaining clinical trial, which compared radiation versus radiation plus short-term hormone therapy in prostate cancer.
The results suggested that most patients should receive hormone therapy, but the AI suggested a more nuanced answer. “Patients who had AI biomarker negative do not see any benefit from adding 4 months of hormone therapy ... whereas patients who have biomarker positive have significant difference and improvement in distant metastasis at 10 years and 15 years. This means that we can save a significant proportion of patients from getting [androgen deprivation therapy], which is hormonal therapy and has very well-known side effects, because they simply they will not benefit,” said Dr. Mohamad, who is an assistant professor of radiation oncology at University of California, San Francisco.
That study relied on the ArteraAI prostate cancer test, which is available through a Clinical Laboratory Improvement Amendment–certified laboratory in Florida.
Another example of AI used to plan treatment is On-line Real-time Benchmarking Informatics Technology for Radiotherapy (ORBIT-RT), developed at the University of California, San Diego. It focuses on radiotherapy treatment plan quality control, and has two main components: creating clinically validated plan routines and a free radiotherapy plan quality control system.
No matter how impressive the technical advances may be, AI contributions won’t impact clinical practice if radiation oncologists, physicians, and patients don’t accept AI. Dr. Aneja’s group surveyed patients about which health field they would feel more comfortable with AI having an important role. Most said they were extremely uncomfortable when it came to cancer. “Now, does that mean that we can’t use AI in oncology? No, I think it just means that we have to be a little bit more nuanced in our approach and how we develop AI solutions for cancer patients,” Dr. Aneja said.
Physicians also show reluctance, according to Alejandro Berlin, MD, who is an affiliate scientist at Princess Margaret Cancer Centre in Toronto. He discussed some research looking at physician acceptance of machine learning. His group looked at physician acceptance of treatment plans for prostate cancer that were generated by physicians and in parallel by machine learning. In a theoretical phase, physicians generally agreed that the machine learning plans were better, but when it came to a phase of the study in which physicians chose which plan to implement in a real patient, the acceptance of machine learning-generated plans dropped by 20%.
This tendency to trust humans over machines is what Dr. Berlin called “automation bias,” and he called for a more collaborative approach to implement AI. “In some cases, [machine learning] is going to be good and sufficient. And in some cases, you will need the expertise of a human.”
Dr. Aneja, who also moderated the session, expressed a similar sentiment when summing up the day’s talks: “I do feel like it’s a disruptive technology ... but I think there will still be a need for us to have people who are trained in order to evaluate and make sure that these algorithms are working correctly and efficiently.”
Dr. Aneja, Dr. Mohamad, and Dr. Berlin have no relevant financial disclosures.
* This article was updated on Nov. 15, 2022.
Artificial intelligence (AI) is impacting many aspects of health care, and radiation oncology is no exception. It has the potential to cut costs and streamline work flows ranging from image analysis to treatment plan formulation, but its specific place in clinical practice is still being debated.
In a session at the annual meeting of the American Society for Radiation Oncology, researchers discussed some of the ways that AI is or will soon be relevant to the clinic. The general consensus was that
In his talk, Sanjay Aneja, MD focused on practical applications of AI that are in the clinic or close to being ready. One example is image classification. “There has been recent evidence that suggests in a variety of different kind of scenarios, deep-learning models can be very good at image classification in automated ways,” said Dr. Aneja, who is a professor of radiology at Yale University, New Haven, Conn. He described one study that used AI to classify 14 different pathologies on chest x-ray images.
Dr. Aneja described the open-source nnU-net tool, which automatically configures itself and segments biomedical images for research or clinical purposes, including therapy planning support, intraoperative support, and tumor growth monitoring. The researchers who developed it also created a “recipe” to systematize configuration of nnU-net, making it useful as an out-of-the-box tool for image segmentation.
He predicted that AI will improve radiology oncology by assisting in the determination of disease extent, including microscopic areas of disease. It could also help plan treatment volume and monitor treatment response. “I think that these are the types of things that will be moving toward the clinic in the future; very specific applications and models trained on very specific scenarios that will help us answer a very important clinical question,” Dr. Aneja said.
He expects AI to contribute to auto-segmenting and clinical contouring, “but I will caution everyone that these algorithms have not been proven to be better than physician contours. They very frequently fail in the specific use cases when anatomy is distorted by, I don’t know, say a tumor. And so a lot of times, we don’t actually have the ability to just make it an automated process. I think it’ll be something that physicians will use to help them but not necessarily replace their contouring ability,” Dr. Aneja said.
Another, potentially more useful application, is in adaptive radiation planning. “I think that AI auto-contouring will be very helpful in establishing contours in a situation in which a physician doing them would not be feasible. We need to have nimble and computationally efficient auto segmentation algorithms that will be able to be easily deployed at the linear accelerator,” he said.
AI in pathology and treatment selection
In another talk, Osama Mohamad, MD talked about AI in pathology, and specifically treatment selection. He described research from his group that digitized pathology data from 5,500 patients drawn from five randomized, clinical trials. They used AI on data from four of the clinical trials to identify a prognostic biomarker for distant metastasis, then validated it on data from the remaining clinical trial, which compared radiation versus radiation plus short-term hormone therapy in prostate cancer.
The results suggested that most patients should receive hormone therapy, but the AI suggested a more nuanced answer. “Patients who had AI biomarker negative do not see any benefit from adding 4 months of hormone therapy ... whereas patients who have biomarker positive have significant difference and improvement in distant metastasis at 10 years and 15 years. This means that we can save a significant proportion of patients from getting [androgen deprivation therapy], which is hormonal therapy and has very well-known side effects, because they simply they will not benefit,” said Dr. Mohamad, who is an assistant professor of radiation oncology at University of California, San Francisco.
That study relied on the ArteraAI prostate cancer test, which is available through a Clinical Laboratory Improvement Amendment–certified laboratory in Florida.
Another example of AI used to plan treatment is On-line Real-time Benchmarking Informatics Technology for Radiotherapy (ORBIT-RT), developed at the University of California, San Diego. It focuses on radiotherapy treatment plan quality control, and has two main components: creating clinically validated plan routines and a free radiotherapy plan quality control system.
No matter how impressive the technical advances may be, AI contributions won’t impact clinical practice if radiation oncologists, physicians, and patients don’t accept AI. Dr. Aneja’s group surveyed patients about which health field they would feel more comfortable with AI having an important role. Most said they were extremely uncomfortable when it came to cancer. “Now, does that mean that we can’t use AI in oncology? No, I think it just means that we have to be a little bit more nuanced in our approach and how we develop AI solutions for cancer patients,” Dr. Aneja said.
Physicians also show reluctance, according to Alejandro Berlin, MD, who is an affiliate scientist at Princess Margaret Cancer Centre in Toronto. He discussed some research looking at physician acceptance of machine learning. His group looked at physician acceptance of treatment plans for prostate cancer that were generated by physicians and in parallel by machine learning. In a theoretical phase, physicians generally agreed that the machine learning plans were better, but when it came to a phase of the study in which physicians chose which plan to implement in a real patient, the acceptance of machine learning-generated plans dropped by 20%.
This tendency to trust humans over machines is what Dr. Berlin called “automation bias,” and he called for a more collaborative approach to implement AI. “In some cases, [machine learning] is going to be good and sufficient. And in some cases, you will need the expertise of a human.”
Dr. Aneja, who also moderated the session, expressed a similar sentiment when summing up the day’s talks: “I do feel like it’s a disruptive technology ... but I think there will still be a need for us to have people who are trained in order to evaluate and make sure that these algorithms are working correctly and efficiently.”
Dr. Aneja, Dr. Mohamad, and Dr. Berlin have no relevant financial disclosures.
* This article was updated on Nov. 15, 2022.
FROM ASTRO 2022
Chronic stress, especially race related, may hasten cancer death
The American folk hero John Henry pitted his hammer against a mechanical steam drill, only to die of exhaustion after winning the battle. In the legend, John Henry was African American, and it’s a fitting metaphor, according to Justin Xavier Moore, PhD.
It’s a metaphor for accumulated stress over a lifetime, also known as allostatic load. Though it affects everyone, Black, Indigenous, and people of color experience it in excess. “It serves as a symbolism for the plight of African Americans within the United States, that regardless of all the triumph and trying to overcompensate and work just as hard as your counterpart, it oftentimes leads to this overtaxing or exhaustion because your competitor has an unfair advantage. You have Jim Crow laws in the South. We have the history of slavery. We have individuals of racial subgroups that are exposed daily to microaggressions, racial discrimination, stereotypes, redlining, all of these different issues that basically reduce to systemic racism,” said Dr. Moore, who is an assistant professor of medicine at the Medical College of Georgia, Augusta.
Dr. Moore is also a coauthor of a new study published online in SSM–Population Health, which examined the association between increased allostatic load and cancer outcomes among participants in the National Health and Nutrition Examination Survey (NHANES) and the National Death Index. They found that both non-Hispanic Black and non-Hispanic White adults with high allostatic load had about a doubled risk of cancer death.
To determine allostatic load, the researchers looked at nine factors collected in NHANES: abnormal values of BMI, diastolic blood pressure, glycohemoglobin, systolic blood pressure, total cholesterol, serum triglycerides, serum albumin, serum creatinine, and C-reactive protein. “The fact that we’re looking at cardiovascular, metabolic and immune function, all in one gives us a better risk assessment for morbidity and mortality. Allostatic load has actually been associated with cardiovascular disease. I think we are one of the first studies to actually look at whether allostatic load is associated with cancer mortality,” said Dr. Moore.
Previous research coauthored by Dr. Moore showed 20-year old African Americans have an allostatic load comparable with that seen in 30-year-old non-Hispanic Whites. That can lead to a proinflammatory state that might be causing increased cancer risk. But stress isn’t a simple concept to pin down, Dr. Moore said. “One of the founding fathers of public health research and epidemiology, Paracelsus, [said] ‘the dose makes the poison.’ ”
In this case, it means that not all stress is bad. Exercise is good stress. “Your heart rate goes up, you compete, and then it comes back down. That’s healthy. But then there’s those stressful situations like dealing with a horrible job, and a boss that may just be overdemanding. Deadlines, and not having a work-life balance. Too much stress, in this case, can cause cancer death,” Dr. Moore said.
In the study, both non-Hispanic Black adults and non-Hispanic White adults heightened risk of cancer death when dealing with high allostatic load, even though the cause of stress may be different. “It’s almost like the cause of the stress does not matter as much. There are millions of Americans that live in environments that are not conducive to their health. The fact of the matter is that because of racial discrimination, because all these different biases, African Americans may have higher allostatic load, which they did on an average, but high allostatic load for even White people is associated with dying from cancer,” Dr. Moore said.
After adjustment, the (adjusted subdistributed hazard ratio, 1.14; 95% CI, 1.04-1.26). After stratification by age, high allostatic load was associated with an 80% increased risk of cancer death among adults (SHR, 1.80; 95% CI, 1.35-2.41). Non-Hispanic White adults had a 95% increased risk (SHR, 1.95; 95% CI, 1.22-3.12), non-Hispanic Black adults had a twofold increased risk (SHR, 1.06; 95% CI, 1.27-3.34), and Hispanic adults had a 36% increased risk.
Dr. Moore has no relevant financial disclosures.
The American folk hero John Henry pitted his hammer against a mechanical steam drill, only to die of exhaustion after winning the battle. In the legend, John Henry was African American, and it’s a fitting metaphor, according to Justin Xavier Moore, PhD.
It’s a metaphor for accumulated stress over a lifetime, also known as allostatic load. Though it affects everyone, Black, Indigenous, and people of color experience it in excess. “It serves as a symbolism for the plight of African Americans within the United States, that regardless of all the triumph and trying to overcompensate and work just as hard as your counterpart, it oftentimes leads to this overtaxing or exhaustion because your competitor has an unfair advantage. You have Jim Crow laws in the South. We have the history of slavery. We have individuals of racial subgroups that are exposed daily to microaggressions, racial discrimination, stereotypes, redlining, all of these different issues that basically reduce to systemic racism,” said Dr. Moore, who is an assistant professor of medicine at the Medical College of Georgia, Augusta.
Dr. Moore is also a coauthor of a new study published online in SSM–Population Health, which examined the association between increased allostatic load and cancer outcomes among participants in the National Health and Nutrition Examination Survey (NHANES) and the National Death Index. They found that both non-Hispanic Black and non-Hispanic White adults with high allostatic load had about a doubled risk of cancer death.
To determine allostatic load, the researchers looked at nine factors collected in NHANES: abnormal values of BMI, diastolic blood pressure, glycohemoglobin, systolic blood pressure, total cholesterol, serum triglycerides, serum albumin, serum creatinine, and C-reactive protein. “The fact that we’re looking at cardiovascular, metabolic and immune function, all in one gives us a better risk assessment for morbidity and mortality. Allostatic load has actually been associated with cardiovascular disease. I think we are one of the first studies to actually look at whether allostatic load is associated with cancer mortality,” said Dr. Moore.
Previous research coauthored by Dr. Moore showed 20-year old African Americans have an allostatic load comparable with that seen in 30-year-old non-Hispanic Whites. That can lead to a proinflammatory state that might be causing increased cancer risk. But stress isn’t a simple concept to pin down, Dr. Moore said. “One of the founding fathers of public health research and epidemiology, Paracelsus, [said] ‘the dose makes the poison.’ ”
In this case, it means that not all stress is bad. Exercise is good stress. “Your heart rate goes up, you compete, and then it comes back down. That’s healthy. But then there’s those stressful situations like dealing with a horrible job, and a boss that may just be overdemanding. Deadlines, and not having a work-life balance. Too much stress, in this case, can cause cancer death,” Dr. Moore said.
In the study, both non-Hispanic Black adults and non-Hispanic White adults heightened risk of cancer death when dealing with high allostatic load, even though the cause of stress may be different. “It’s almost like the cause of the stress does not matter as much. There are millions of Americans that live in environments that are not conducive to their health. The fact of the matter is that because of racial discrimination, because all these different biases, African Americans may have higher allostatic load, which they did on an average, but high allostatic load for even White people is associated with dying from cancer,” Dr. Moore said.
After adjustment, the (adjusted subdistributed hazard ratio, 1.14; 95% CI, 1.04-1.26). After stratification by age, high allostatic load was associated with an 80% increased risk of cancer death among adults (SHR, 1.80; 95% CI, 1.35-2.41). Non-Hispanic White adults had a 95% increased risk (SHR, 1.95; 95% CI, 1.22-3.12), non-Hispanic Black adults had a twofold increased risk (SHR, 1.06; 95% CI, 1.27-3.34), and Hispanic adults had a 36% increased risk.
Dr. Moore has no relevant financial disclosures.
The American folk hero John Henry pitted his hammer against a mechanical steam drill, only to die of exhaustion after winning the battle. In the legend, John Henry was African American, and it’s a fitting metaphor, according to Justin Xavier Moore, PhD.
It’s a metaphor for accumulated stress over a lifetime, also known as allostatic load. Though it affects everyone, Black, Indigenous, and people of color experience it in excess. “It serves as a symbolism for the plight of African Americans within the United States, that regardless of all the triumph and trying to overcompensate and work just as hard as your counterpart, it oftentimes leads to this overtaxing or exhaustion because your competitor has an unfair advantage. You have Jim Crow laws in the South. We have the history of slavery. We have individuals of racial subgroups that are exposed daily to microaggressions, racial discrimination, stereotypes, redlining, all of these different issues that basically reduce to systemic racism,” said Dr. Moore, who is an assistant professor of medicine at the Medical College of Georgia, Augusta.
Dr. Moore is also a coauthor of a new study published online in SSM–Population Health, which examined the association between increased allostatic load and cancer outcomes among participants in the National Health and Nutrition Examination Survey (NHANES) and the National Death Index. They found that both non-Hispanic Black and non-Hispanic White adults with high allostatic load had about a doubled risk of cancer death.
To determine allostatic load, the researchers looked at nine factors collected in NHANES: abnormal values of BMI, diastolic blood pressure, glycohemoglobin, systolic blood pressure, total cholesterol, serum triglycerides, serum albumin, serum creatinine, and C-reactive protein. “The fact that we’re looking at cardiovascular, metabolic and immune function, all in one gives us a better risk assessment for morbidity and mortality. Allostatic load has actually been associated with cardiovascular disease. I think we are one of the first studies to actually look at whether allostatic load is associated with cancer mortality,” said Dr. Moore.
Previous research coauthored by Dr. Moore showed 20-year old African Americans have an allostatic load comparable with that seen in 30-year-old non-Hispanic Whites. That can lead to a proinflammatory state that might be causing increased cancer risk. But stress isn’t a simple concept to pin down, Dr. Moore said. “One of the founding fathers of public health research and epidemiology, Paracelsus, [said] ‘the dose makes the poison.’ ”
In this case, it means that not all stress is bad. Exercise is good stress. “Your heart rate goes up, you compete, and then it comes back down. That’s healthy. But then there’s those stressful situations like dealing with a horrible job, and a boss that may just be overdemanding. Deadlines, and not having a work-life balance. Too much stress, in this case, can cause cancer death,” Dr. Moore said.
In the study, both non-Hispanic Black adults and non-Hispanic White adults heightened risk of cancer death when dealing with high allostatic load, even though the cause of stress may be different. “It’s almost like the cause of the stress does not matter as much. There are millions of Americans that live in environments that are not conducive to their health. The fact of the matter is that because of racial discrimination, because all these different biases, African Americans may have higher allostatic load, which they did on an average, but high allostatic load for even White people is associated with dying from cancer,” Dr. Moore said.
After adjustment, the (adjusted subdistributed hazard ratio, 1.14; 95% CI, 1.04-1.26). After stratification by age, high allostatic load was associated with an 80% increased risk of cancer death among adults (SHR, 1.80; 95% CI, 1.35-2.41). Non-Hispanic White adults had a 95% increased risk (SHR, 1.95; 95% CI, 1.22-3.12), non-Hispanic Black adults had a twofold increased risk (SHR, 1.06; 95% CI, 1.27-3.34), and Hispanic adults had a 36% increased risk.
Dr. Moore has no relevant financial disclosures.
FROM SSM–POPULATION HEALTH
Third COVID booster benefits cancer patients
though this population still suffers higher risks than those of the general population, according to a new large-scale observational study out of the United Kingdom.
People living with lymphoma and those who underwent recent systemic anti-cancer treatment or radiotherapy are at the highest risk, according to study author Lennard Y.W. Lee, PhD. “Our study is the largest evaluation of a coronavirus third dose vaccine booster effectiveness in people living with cancer in the world. For the first time we have quantified the benefits of boosters for COVID-19 in cancer patients,” said Dr. Lee, UK COVID Cancer program lead and a medical oncologist at the University of Oxford, England.
The research was published in the November issue of the European Journal of Cancer.
Despite the encouraging numbers, those with cancer continue to have a more than threefold increased risk of both hospitalization and death from coronavirus compared to the general population. “More needs to be done to reduce this excess risk, like prophylactic antibody therapies,” Dr. Lee said.
Third dose efficacy was lower among cancer patients who had been diagnosed within the past 12 months, as well as those with lymphoma, and those who had undergone systemic anti-cancer therapy or radiotherapy within the past 12 months.
The increased vulnerability among individuals with cancer is likely due to compromised immune systems. “Patients with cancer often have impaired B and T cell function and this study provides the largest global clinical study showing the definitive meaningful clinical impact of this,” Dr. Lee said. The greater risk among those with lymphoma likely traces to aberrant white cells or immunosuppressant regimens, he said.
“Vaccination probably should be used in combination with new forms of prevention and in Europe the strategy of using prophylactic antibodies is going to provide additional levels of protection,” Dr. Lee said.
Overall, the study reveals the challenges that cancer patients face in a pandemic that remains a critical health concern, one that can seriously affect quality of life. “Many are still shielding, unable to see family or hug loved ones. Furthermore, looking beyond the direct health risks, there is also the mental health impact. Shielding for nearly 3 years is very difficult. It is important to realize that behind this large-scale study, which is the biggest in the world, there are real people. The pandemic still goes on for them as they remain at higher risk from COVID-19 and we must be aware of the impact on them,” Dr. Lee said.
The study included data from the United Kingdom’s third dose booster vaccine program, representing 361,098 individuals who participated from December 2020 through December 2021. It also include results from all coronavirus tests conducted in the United Kingdom during that period. Among the participants, 97.8% got the Pfizer-BioNTech vaccine as a booster, while 1.5% received the Moderna vaccine. Overall, 8,371,139 individuals received a third dose booster, including 230,666 living with cancer. The researchers used a test-negative case-controlled analysis to estimate vaccine efficacy.
The booster shot had a 59.1% efficacy against breakthrough infections, 62.8% efficacy against symptomatic infections, 80.5% efficacy versus coronavirus hospitalization, and 94.5% efficacy against coronavirus death. Patients with solid tumors benefited from higher efficacy versus breakthrough infections 66.0% versus 53.2%) and symptomatic infections (69.6% versus 56.0%).
Patients with lymphoma experienced just a 10.5% efficacy of the primary dose vaccine versus breakthrough infections and 13.6% versus symptomatic infections, and this did not improve with a third dose. The benefit was greater for hospitalization (23.2%) and death (80.1%).
Despite the additional protection of a third dose, patients with cancer had a higher risk than the population control for coronavirus hospitalization (odds ratio, 3.38; P < .000001) and death (odds ratio, 3.01; P < .000001).
Dr. Lee has no relevant financial disclosures.
though this population still suffers higher risks than those of the general population, according to a new large-scale observational study out of the United Kingdom.
People living with lymphoma and those who underwent recent systemic anti-cancer treatment or radiotherapy are at the highest risk, according to study author Lennard Y.W. Lee, PhD. “Our study is the largest evaluation of a coronavirus third dose vaccine booster effectiveness in people living with cancer in the world. For the first time we have quantified the benefits of boosters for COVID-19 in cancer patients,” said Dr. Lee, UK COVID Cancer program lead and a medical oncologist at the University of Oxford, England.
The research was published in the November issue of the European Journal of Cancer.
Despite the encouraging numbers, those with cancer continue to have a more than threefold increased risk of both hospitalization and death from coronavirus compared to the general population. “More needs to be done to reduce this excess risk, like prophylactic antibody therapies,” Dr. Lee said.
Third dose efficacy was lower among cancer patients who had been diagnosed within the past 12 months, as well as those with lymphoma, and those who had undergone systemic anti-cancer therapy or radiotherapy within the past 12 months.
The increased vulnerability among individuals with cancer is likely due to compromised immune systems. “Patients with cancer often have impaired B and T cell function and this study provides the largest global clinical study showing the definitive meaningful clinical impact of this,” Dr. Lee said. The greater risk among those with lymphoma likely traces to aberrant white cells or immunosuppressant regimens, he said.
“Vaccination probably should be used in combination with new forms of prevention and in Europe the strategy of using prophylactic antibodies is going to provide additional levels of protection,” Dr. Lee said.
Overall, the study reveals the challenges that cancer patients face in a pandemic that remains a critical health concern, one that can seriously affect quality of life. “Many are still shielding, unable to see family or hug loved ones. Furthermore, looking beyond the direct health risks, there is also the mental health impact. Shielding for nearly 3 years is very difficult. It is important to realize that behind this large-scale study, which is the biggest in the world, there are real people. The pandemic still goes on for them as they remain at higher risk from COVID-19 and we must be aware of the impact on them,” Dr. Lee said.
The study included data from the United Kingdom’s third dose booster vaccine program, representing 361,098 individuals who participated from December 2020 through December 2021. It also include results from all coronavirus tests conducted in the United Kingdom during that period. Among the participants, 97.8% got the Pfizer-BioNTech vaccine as a booster, while 1.5% received the Moderna vaccine. Overall, 8,371,139 individuals received a third dose booster, including 230,666 living with cancer. The researchers used a test-negative case-controlled analysis to estimate vaccine efficacy.
The booster shot had a 59.1% efficacy against breakthrough infections, 62.8% efficacy against symptomatic infections, 80.5% efficacy versus coronavirus hospitalization, and 94.5% efficacy against coronavirus death. Patients with solid tumors benefited from higher efficacy versus breakthrough infections 66.0% versus 53.2%) and symptomatic infections (69.6% versus 56.0%).
Patients with lymphoma experienced just a 10.5% efficacy of the primary dose vaccine versus breakthrough infections and 13.6% versus symptomatic infections, and this did not improve with a third dose. The benefit was greater for hospitalization (23.2%) and death (80.1%).
Despite the additional protection of a third dose, patients with cancer had a higher risk than the population control for coronavirus hospitalization (odds ratio, 3.38; P < .000001) and death (odds ratio, 3.01; P < .000001).
Dr. Lee has no relevant financial disclosures.
though this population still suffers higher risks than those of the general population, according to a new large-scale observational study out of the United Kingdom.
People living with lymphoma and those who underwent recent systemic anti-cancer treatment or radiotherapy are at the highest risk, according to study author Lennard Y.W. Lee, PhD. “Our study is the largest evaluation of a coronavirus third dose vaccine booster effectiveness in people living with cancer in the world. For the first time we have quantified the benefits of boosters for COVID-19 in cancer patients,” said Dr. Lee, UK COVID Cancer program lead and a medical oncologist at the University of Oxford, England.
The research was published in the November issue of the European Journal of Cancer.
Despite the encouraging numbers, those with cancer continue to have a more than threefold increased risk of both hospitalization and death from coronavirus compared to the general population. “More needs to be done to reduce this excess risk, like prophylactic antibody therapies,” Dr. Lee said.
Third dose efficacy was lower among cancer patients who had been diagnosed within the past 12 months, as well as those with lymphoma, and those who had undergone systemic anti-cancer therapy or radiotherapy within the past 12 months.
The increased vulnerability among individuals with cancer is likely due to compromised immune systems. “Patients with cancer often have impaired B and T cell function and this study provides the largest global clinical study showing the definitive meaningful clinical impact of this,” Dr. Lee said. The greater risk among those with lymphoma likely traces to aberrant white cells or immunosuppressant regimens, he said.
“Vaccination probably should be used in combination with new forms of prevention and in Europe the strategy of using prophylactic antibodies is going to provide additional levels of protection,” Dr. Lee said.
Overall, the study reveals the challenges that cancer patients face in a pandemic that remains a critical health concern, one that can seriously affect quality of life. “Many are still shielding, unable to see family or hug loved ones. Furthermore, looking beyond the direct health risks, there is also the mental health impact. Shielding for nearly 3 years is very difficult. It is important to realize that behind this large-scale study, which is the biggest in the world, there are real people. The pandemic still goes on for them as they remain at higher risk from COVID-19 and we must be aware of the impact on them,” Dr. Lee said.
The study included data from the United Kingdom’s third dose booster vaccine program, representing 361,098 individuals who participated from December 2020 through December 2021. It also include results from all coronavirus tests conducted in the United Kingdom during that period. Among the participants, 97.8% got the Pfizer-BioNTech vaccine as a booster, while 1.5% received the Moderna vaccine. Overall, 8,371,139 individuals received a third dose booster, including 230,666 living with cancer. The researchers used a test-negative case-controlled analysis to estimate vaccine efficacy.
The booster shot had a 59.1% efficacy against breakthrough infections, 62.8% efficacy against symptomatic infections, 80.5% efficacy versus coronavirus hospitalization, and 94.5% efficacy against coronavirus death. Patients with solid tumors benefited from higher efficacy versus breakthrough infections 66.0% versus 53.2%) and symptomatic infections (69.6% versus 56.0%).
Patients with lymphoma experienced just a 10.5% efficacy of the primary dose vaccine versus breakthrough infections and 13.6% versus symptomatic infections, and this did not improve with a third dose. The benefit was greater for hospitalization (23.2%) and death (80.1%).
Despite the additional protection of a third dose, patients with cancer had a higher risk than the population control for coronavirus hospitalization (odds ratio, 3.38; P < .000001) and death (odds ratio, 3.01; P < .000001).
Dr. Lee has no relevant financial disclosures.
FROM THE EUROPEAN JOURNAL OF CANCER
A cost-effective de-escalation strategy in advanced melanoma
Response-adapted , an economic analysis found.
“The rising costs of cancer therapies are becoming untenable for both patients and payers, and there is both clinical and economic benefit to finding less expensive treatment alternatives,” Wolfgang Kunz, MD, University Hospital, Ludwig Maximilian University of Munich, told this news organization.
This economic analysis “highlights that leveraging modern diagnostic capabilities can do just that: Pairing drug regimens with CT-image analysis to optimize dosages can reduce health care costs and improve clinical outcomes,” Dr. Kunz said.
The study was published online in JAMA Dermatology.
While the use of immunotherapies over the past decade has improved the prognosis for patients with advanced melanoma, these drugs come with a hefty price tag.
One potential way to help reduce costs: de-escalate therapy. The ADAPT-IT trial demonstrated similar progression-free and overall survival among patients who received response-adapted ipilimumab discontinuation and those who received standard of care.
In the current analysis, Dr. Kunz and colleagues wanted to understand whether this response-adapted approach was also cost effective.
The team applied economic modeling to data from the ADAPT-IT trial as well as CheckMate 067, in which patients received standard of care four doses of combination ipilimumab-nivolumab followed by nivolumab monotherapy. In the ADAPT-IT trial, patients also initially received the immunotherapy combination but had CT scans to determine their response after two doses; if they responded, patients discontinued ipilimumab and continued with nivolumab monotherapy.
Overall, ADAPT-IT showed that responders could forgo the additional two doses of ipilimumab plus nivolumab while maintaining similar progression-free survival and overall survival seen at 18 months in the CheckMate 067 trial.
The current economic analysis, based on 41 patients from ADAPT-IT and 314 from CheckMate 067, showed a potential reduction in health care costs of $19,891 per patient with the response-adapted approach.
Response-adapted treatment was the cost-effective option in 94% of simulated scenarios.
When extrapolated to 2019 incidence rates of distant melanoma cases, yearly national savings could reach about $58 million.
“In the relatively small space of immunotherapies in advanced melanoma, we hope this analysis motivates clinicians to consider response-adapted treatment,” Dr. Kunz told this news organization.
“On the larger scale, this analysis serves as a stepping stone to more response-guided treatment protocols,” Dr. Kunz added. “With drug costs rising and imaging capabilities growing, more frequent image-guided adjustments are a perfect fit into the personalized care model.”
When applying the cost savings noted in this analysis across all treated patients, “the economic impact may be profound,” said Joseph Skitzki, MD, surgical oncologist, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., who wasn’t involved in the study. The “financial toxicity of cancer care is increasingly recognized as a potential barrier to optimal outcomes and any measures to mitigate cost may be impactful.”
However, Dr. Skitzki said several caveats need to be considered.
One is that the data included from ADAPT-IT only included 41 patients, compared with 314 patients from CheckMate 067.
“It is possible that a larger real-world study utilizing the ADAPT-IT protocol may not be as favorable in terms of outcomes and could lessen the economic impact of de-escalation, although any form of de-escalation is likely to have a cost benefit,” Dr. Skitzki said in an interview.
A real-world response–adapted de-escalation clinical trial, with an emphasis on costs and a benchmark of similar progression-free and overall survival, should be conducted before the de-escalated option becomes “practice changing,” Dr. Skitzki said.
Jeffrey Weber, MD, PhD, deputy director, Perlmutter Cancer Center, NYU Langone Health, New York, also urged caution in interpreting the results.
“I would not base treatment decisions on a small sampling of 41 patients in the absence of a randomized comparison,” Dr. Weber told this news organization. “Without a proper comparison, I would not advocate using only two doses of ipilimumab-nivolumab to make decisions on treatment.”
Dr. Skitzki added that, while “studies like this one are desperately needed to lessen the economic impact of new and emerging combination immunotherapies,” there is likely also a “disincentive for pharmaceutical companies to conduct this type of research.”
This research had no specific funding. Dr. Kunz and Dr. Skitzki reported no relevant conflicts of interest. Dr. Weber disclosed relationships with Merck, Genentech, AstraZeneca, Pfizer, Regeneron, and GSK, among others, and holds equity in Cytomx, Biond, NexImmune, and Immunomax.
A version of this article first appeared on Medscape.com.
Response-adapted , an economic analysis found.
“The rising costs of cancer therapies are becoming untenable for both patients and payers, and there is both clinical and economic benefit to finding less expensive treatment alternatives,” Wolfgang Kunz, MD, University Hospital, Ludwig Maximilian University of Munich, told this news organization.
This economic analysis “highlights that leveraging modern diagnostic capabilities can do just that: Pairing drug regimens with CT-image analysis to optimize dosages can reduce health care costs and improve clinical outcomes,” Dr. Kunz said.
The study was published online in JAMA Dermatology.
While the use of immunotherapies over the past decade has improved the prognosis for patients with advanced melanoma, these drugs come with a hefty price tag.
One potential way to help reduce costs: de-escalate therapy. The ADAPT-IT trial demonstrated similar progression-free and overall survival among patients who received response-adapted ipilimumab discontinuation and those who received standard of care.
In the current analysis, Dr. Kunz and colleagues wanted to understand whether this response-adapted approach was also cost effective.
The team applied economic modeling to data from the ADAPT-IT trial as well as CheckMate 067, in which patients received standard of care four doses of combination ipilimumab-nivolumab followed by nivolumab monotherapy. In the ADAPT-IT trial, patients also initially received the immunotherapy combination but had CT scans to determine their response after two doses; if they responded, patients discontinued ipilimumab and continued with nivolumab monotherapy.
Overall, ADAPT-IT showed that responders could forgo the additional two doses of ipilimumab plus nivolumab while maintaining similar progression-free survival and overall survival seen at 18 months in the CheckMate 067 trial.
The current economic analysis, based on 41 patients from ADAPT-IT and 314 from CheckMate 067, showed a potential reduction in health care costs of $19,891 per patient with the response-adapted approach.
Response-adapted treatment was the cost-effective option in 94% of simulated scenarios.
When extrapolated to 2019 incidence rates of distant melanoma cases, yearly national savings could reach about $58 million.
“In the relatively small space of immunotherapies in advanced melanoma, we hope this analysis motivates clinicians to consider response-adapted treatment,” Dr. Kunz told this news organization.
“On the larger scale, this analysis serves as a stepping stone to more response-guided treatment protocols,” Dr. Kunz added. “With drug costs rising and imaging capabilities growing, more frequent image-guided adjustments are a perfect fit into the personalized care model.”
When applying the cost savings noted in this analysis across all treated patients, “the economic impact may be profound,” said Joseph Skitzki, MD, surgical oncologist, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., who wasn’t involved in the study. The “financial toxicity of cancer care is increasingly recognized as a potential barrier to optimal outcomes and any measures to mitigate cost may be impactful.”
However, Dr. Skitzki said several caveats need to be considered.
One is that the data included from ADAPT-IT only included 41 patients, compared with 314 patients from CheckMate 067.
“It is possible that a larger real-world study utilizing the ADAPT-IT protocol may not be as favorable in terms of outcomes and could lessen the economic impact of de-escalation, although any form of de-escalation is likely to have a cost benefit,” Dr. Skitzki said in an interview.
A real-world response–adapted de-escalation clinical trial, with an emphasis on costs and a benchmark of similar progression-free and overall survival, should be conducted before the de-escalated option becomes “practice changing,” Dr. Skitzki said.
Jeffrey Weber, MD, PhD, deputy director, Perlmutter Cancer Center, NYU Langone Health, New York, also urged caution in interpreting the results.
“I would not base treatment decisions on a small sampling of 41 patients in the absence of a randomized comparison,” Dr. Weber told this news organization. “Without a proper comparison, I would not advocate using only two doses of ipilimumab-nivolumab to make decisions on treatment.”
Dr. Skitzki added that, while “studies like this one are desperately needed to lessen the economic impact of new and emerging combination immunotherapies,” there is likely also a “disincentive for pharmaceutical companies to conduct this type of research.”
This research had no specific funding. Dr. Kunz and Dr. Skitzki reported no relevant conflicts of interest. Dr. Weber disclosed relationships with Merck, Genentech, AstraZeneca, Pfizer, Regeneron, and GSK, among others, and holds equity in Cytomx, Biond, NexImmune, and Immunomax.
A version of this article first appeared on Medscape.com.
Response-adapted , an economic analysis found.
“The rising costs of cancer therapies are becoming untenable for both patients and payers, and there is both clinical and economic benefit to finding less expensive treatment alternatives,” Wolfgang Kunz, MD, University Hospital, Ludwig Maximilian University of Munich, told this news organization.
This economic analysis “highlights that leveraging modern diagnostic capabilities can do just that: Pairing drug regimens with CT-image analysis to optimize dosages can reduce health care costs and improve clinical outcomes,” Dr. Kunz said.
The study was published online in JAMA Dermatology.
While the use of immunotherapies over the past decade has improved the prognosis for patients with advanced melanoma, these drugs come with a hefty price tag.
One potential way to help reduce costs: de-escalate therapy. The ADAPT-IT trial demonstrated similar progression-free and overall survival among patients who received response-adapted ipilimumab discontinuation and those who received standard of care.
In the current analysis, Dr. Kunz and colleagues wanted to understand whether this response-adapted approach was also cost effective.
The team applied economic modeling to data from the ADAPT-IT trial as well as CheckMate 067, in which patients received standard of care four doses of combination ipilimumab-nivolumab followed by nivolumab monotherapy. In the ADAPT-IT trial, patients also initially received the immunotherapy combination but had CT scans to determine their response after two doses; if they responded, patients discontinued ipilimumab and continued with nivolumab monotherapy.
Overall, ADAPT-IT showed that responders could forgo the additional two doses of ipilimumab plus nivolumab while maintaining similar progression-free survival and overall survival seen at 18 months in the CheckMate 067 trial.
The current economic analysis, based on 41 patients from ADAPT-IT and 314 from CheckMate 067, showed a potential reduction in health care costs of $19,891 per patient with the response-adapted approach.
Response-adapted treatment was the cost-effective option in 94% of simulated scenarios.
When extrapolated to 2019 incidence rates of distant melanoma cases, yearly national savings could reach about $58 million.
“In the relatively small space of immunotherapies in advanced melanoma, we hope this analysis motivates clinicians to consider response-adapted treatment,” Dr. Kunz told this news organization.
“On the larger scale, this analysis serves as a stepping stone to more response-guided treatment protocols,” Dr. Kunz added. “With drug costs rising and imaging capabilities growing, more frequent image-guided adjustments are a perfect fit into the personalized care model.”
When applying the cost savings noted in this analysis across all treated patients, “the economic impact may be profound,” said Joseph Skitzki, MD, surgical oncologist, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., who wasn’t involved in the study. The “financial toxicity of cancer care is increasingly recognized as a potential barrier to optimal outcomes and any measures to mitigate cost may be impactful.”
However, Dr. Skitzki said several caveats need to be considered.
One is that the data included from ADAPT-IT only included 41 patients, compared with 314 patients from CheckMate 067.
“It is possible that a larger real-world study utilizing the ADAPT-IT protocol may not be as favorable in terms of outcomes and could lessen the economic impact of de-escalation, although any form of de-escalation is likely to have a cost benefit,” Dr. Skitzki said in an interview.
A real-world response–adapted de-escalation clinical trial, with an emphasis on costs and a benchmark of similar progression-free and overall survival, should be conducted before the de-escalated option becomes “practice changing,” Dr. Skitzki said.
Jeffrey Weber, MD, PhD, deputy director, Perlmutter Cancer Center, NYU Langone Health, New York, also urged caution in interpreting the results.
“I would not base treatment decisions on a small sampling of 41 patients in the absence of a randomized comparison,” Dr. Weber told this news organization. “Without a proper comparison, I would not advocate using only two doses of ipilimumab-nivolumab to make decisions on treatment.”
Dr. Skitzki added that, while “studies like this one are desperately needed to lessen the economic impact of new and emerging combination immunotherapies,” there is likely also a “disincentive for pharmaceutical companies to conduct this type of research.”
This research had no specific funding. Dr. Kunz and Dr. Skitzki reported no relevant conflicts of interest. Dr. Weber disclosed relationships with Merck, Genentech, AstraZeneca, Pfizer, Regeneron, and GSK, among others, and holds equity in Cytomx, Biond, NexImmune, and Immunomax.
A version of this article first appeared on Medscape.com.
Genital Lentiginosis: A Benign Pigmentary Abnormality Often Raising Concern for Melanoma
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.
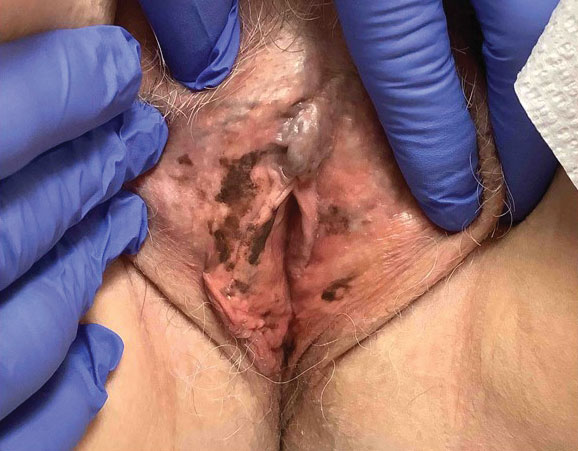
Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.
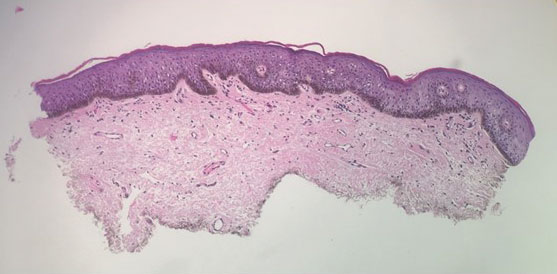
A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.
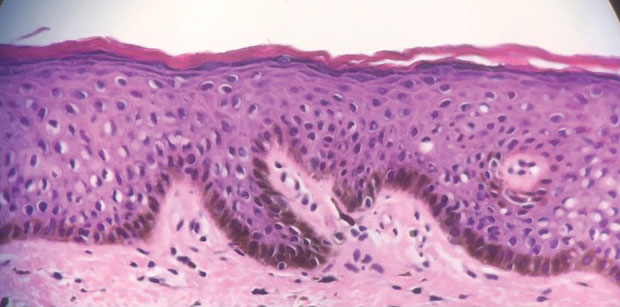
Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.

Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.

A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.

Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.

Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.

A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.

Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
Practice Points
- The irregular appearance of genital lentiginosis—multifocal, asymmetric, irregular, and darkly pigmented patches—often raises concern for melanoma but is benign.
- Certain genetic conditions can present with genital lentiginosis.
- Dermoscopic assessment of the lesion color is highly helpful in narrowing the differential diagnosis; seeing no gray, red, blue, or white makes melanoma less likely.
- Be aware of genital lentigines and their characteristic presentation in adulthood to avoid unwarranted concern and unneeded surgery.
Reminder that COVID-19 and cancer can be a deadly combo
A new study underscores the importance of COVID-19 and regular COVID-19 testing among adults with a recent cancer diagnosis.
The Indiana statewide study, conducted at the beginning of the pandemic, found that
“This analysis provides additional empirical evidence on the magnitude of risk to patients with cancer whose immune systems are often weakened either by the disease or treatment,” the study team wrote.
The study was published online in JMIR Cancer.
Although evidence has consistently revealed similar findings, the risk of death among unvaccinated people with cancer and COVID-19 has not been nearly as high in previous studies, lead author Brian E. Dixon, PhD, MBA, with Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, said in a statement. Previous studies from China, for instance, reported a two- to threefold greater risk of all-cause mortality among unvaccinated adults with cancer and COVID-19.
A potential reason for this discrepancy, Dr. Dixon noted, is that earlier studies were “generally smaller and made calculations based on data from a single cancer center or health system.”
Another reason is testing for COVID-19 early in the pandemic was limited to symptomatic individuals who may have had more severe infections, possibly leading to an overestimate of the association between SARS-CoV-2 infection, cancer, and all-cause mortality.
In the current analysis, researchers used electronic health records linked to Indiana’s statewide SARS-CoV-2 testing database and state vital records to evaluate the association between SARS-CoV-2 infection and all-cause mortality among 41,924 adults newly diagnosed with cancer between Jan. 1, 2019, and Dec. 31, 2020.
Most people with cancer were White (78.4%) and about half were male. At the time of diagnosis, 17% had one comorbid condition and about 10% had two or more. Most patients had breast cancer (14%), prostate cancer (13%), or melanoma (13%).
During the study period, 2,894 patients (7%) tested positive for SARS-CoV-2.
In multivariate adjusted analysis, the risk of death among those newly diagnosed with cancer increased by 91% (adjusted hazard ratio, 1.91) during the first year of the pandemic before vaccines were available, compared with the year before (January 2019 to Jan. 14, 2020).
During the pandemic period, the risk of death was roughly threefold higher among adults 65 years old and older, compared with adults 18-44 years old (aHR, 3.35).
When looking at the time from a cancer diagnosis to SARS-CoV-2 infection, infection was associated with an almost sevenfold increase in all-cause mortality (aHR, 6.91). Adults 65 years old and older had an almost threefold increased risk of dying, compared with their younger peers (aHR, 2.74).
Dr. Dixon and colleagues also observed an increased risk of death in men with cancer and COVID, compared with women (aHR, 1.23) and those with at least two comorbid conditions versus none (aHR, 2.12). In addition, the risk of dying was 9% higher among Indiana’s rural population than urban dwellers.
Compared with other cancer types, individuals with lung cancer and other digestive cancers had the highest risk of death after SARS-CoV-2 infection (aHR, 1.45 and 1.80, respectively).
“Our findings highlight the increased risk of death for adult cancer patients who test positive for COVID and underscore the importance to cancer patients – including those in remission – of vaccinations, boosters, and regular COVID testing,” Dr. Dixon commented.
“Our results should encourage individuals diagnosed with cancer not only to take preventive action, but also to expeditiously seek out treatments available in the marketplace should they test positive for COVID,” he added.
Support for the study was provided by Indiana University Simon Cancer Center and the Centers for Disease Control and Prevention. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study underscores the importance of COVID-19 and regular COVID-19 testing among adults with a recent cancer diagnosis.
The Indiana statewide study, conducted at the beginning of the pandemic, found that
“This analysis provides additional empirical evidence on the magnitude of risk to patients with cancer whose immune systems are often weakened either by the disease or treatment,” the study team wrote.
The study was published online in JMIR Cancer.
Although evidence has consistently revealed similar findings, the risk of death among unvaccinated people with cancer and COVID-19 has not been nearly as high in previous studies, lead author Brian E. Dixon, PhD, MBA, with Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, said in a statement. Previous studies from China, for instance, reported a two- to threefold greater risk of all-cause mortality among unvaccinated adults with cancer and COVID-19.
A potential reason for this discrepancy, Dr. Dixon noted, is that earlier studies were “generally smaller and made calculations based on data from a single cancer center or health system.”
Another reason is testing for COVID-19 early in the pandemic was limited to symptomatic individuals who may have had more severe infections, possibly leading to an overestimate of the association between SARS-CoV-2 infection, cancer, and all-cause mortality.
In the current analysis, researchers used electronic health records linked to Indiana’s statewide SARS-CoV-2 testing database and state vital records to evaluate the association between SARS-CoV-2 infection and all-cause mortality among 41,924 adults newly diagnosed with cancer between Jan. 1, 2019, and Dec. 31, 2020.
Most people with cancer were White (78.4%) and about half were male. At the time of diagnosis, 17% had one comorbid condition and about 10% had two or more. Most patients had breast cancer (14%), prostate cancer (13%), or melanoma (13%).
During the study period, 2,894 patients (7%) tested positive for SARS-CoV-2.
In multivariate adjusted analysis, the risk of death among those newly diagnosed with cancer increased by 91% (adjusted hazard ratio, 1.91) during the first year of the pandemic before vaccines were available, compared with the year before (January 2019 to Jan. 14, 2020).
During the pandemic period, the risk of death was roughly threefold higher among adults 65 years old and older, compared with adults 18-44 years old (aHR, 3.35).
When looking at the time from a cancer diagnosis to SARS-CoV-2 infection, infection was associated with an almost sevenfold increase in all-cause mortality (aHR, 6.91). Adults 65 years old and older had an almost threefold increased risk of dying, compared with their younger peers (aHR, 2.74).
Dr. Dixon and colleagues also observed an increased risk of death in men with cancer and COVID, compared with women (aHR, 1.23) and those with at least two comorbid conditions versus none (aHR, 2.12). In addition, the risk of dying was 9% higher among Indiana’s rural population than urban dwellers.
Compared with other cancer types, individuals with lung cancer and other digestive cancers had the highest risk of death after SARS-CoV-2 infection (aHR, 1.45 and 1.80, respectively).
“Our findings highlight the increased risk of death for adult cancer patients who test positive for COVID and underscore the importance to cancer patients – including those in remission – of vaccinations, boosters, and regular COVID testing,” Dr. Dixon commented.
“Our results should encourage individuals diagnosed with cancer not only to take preventive action, but also to expeditiously seek out treatments available in the marketplace should they test positive for COVID,” he added.
Support for the study was provided by Indiana University Simon Cancer Center and the Centers for Disease Control and Prevention. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study underscores the importance of COVID-19 and regular COVID-19 testing among adults with a recent cancer diagnosis.
The Indiana statewide study, conducted at the beginning of the pandemic, found that
“This analysis provides additional empirical evidence on the magnitude of risk to patients with cancer whose immune systems are often weakened either by the disease or treatment,” the study team wrote.
The study was published online in JMIR Cancer.
Although evidence has consistently revealed similar findings, the risk of death among unvaccinated people with cancer and COVID-19 has not been nearly as high in previous studies, lead author Brian E. Dixon, PhD, MBA, with Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, said in a statement. Previous studies from China, for instance, reported a two- to threefold greater risk of all-cause mortality among unvaccinated adults with cancer and COVID-19.
A potential reason for this discrepancy, Dr. Dixon noted, is that earlier studies were “generally smaller and made calculations based on data from a single cancer center or health system.”
Another reason is testing for COVID-19 early in the pandemic was limited to symptomatic individuals who may have had more severe infections, possibly leading to an overestimate of the association between SARS-CoV-2 infection, cancer, and all-cause mortality.
In the current analysis, researchers used electronic health records linked to Indiana’s statewide SARS-CoV-2 testing database and state vital records to evaluate the association between SARS-CoV-2 infection and all-cause mortality among 41,924 adults newly diagnosed with cancer between Jan. 1, 2019, and Dec. 31, 2020.
Most people with cancer were White (78.4%) and about half were male. At the time of diagnosis, 17% had one comorbid condition and about 10% had two or more. Most patients had breast cancer (14%), prostate cancer (13%), or melanoma (13%).
During the study period, 2,894 patients (7%) tested positive for SARS-CoV-2.
In multivariate adjusted analysis, the risk of death among those newly diagnosed with cancer increased by 91% (adjusted hazard ratio, 1.91) during the first year of the pandemic before vaccines were available, compared with the year before (January 2019 to Jan. 14, 2020).
During the pandemic period, the risk of death was roughly threefold higher among adults 65 years old and older, compared with adults 18-44 years old (aHR, 3.35).
When looking at the time from a cancer diagnosis to SARS-CoV-2 infection, infection was associated with an almost sevenfold increase in all-cause mortality (aHR, 6.91). Adults 65 years old and older had an almost threefold increased risk of dying, compared with their younger peers (aHR, 2.74).
Dr. Dixon and colleagues also observed an increased risk of death in men with cancer and COVID, compared with women (aHR, 1.23) and those with at least two comorbid conditions versus none (aHR, 2.12). In addition, the risk of dying was 9% higher among Indiana’s rural population than urban dwellers.
Compared with other cancer types, individuals with lung cancer and other digestive cancers had the highest risk of death after SARS-CoV-2 infection (aHR, 1.45 and 1.80, respectively).
“Our findings highlight the increased risk of death for adult cancer patients who test positive for COVID and underscore the importance to cancer patients – including those in remission – of vaccinations, boosters, and regular COVID testing,” Dr. Dixon commented.
“Our results should encourage individuals diagnosed with cancer not only to take preventive action, but also to expeditiously seek out treatments available in the marketplace should they test positive for COVID,” he added.
Support for the study was provided by Indiana University Simon Cancer Center and the Centers for Disease Control and Prevention. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JMIR CANCER
Margin Size for Unique Skin Tumors Treated With Mohs Micrographic Surgery: A Survey of Practice Patterns
Mohs micrographic surgery (MMS) is most commonly used for the surgical management of squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) in high-risk locations. The ability for 100% margin evaluation with MMS also has shown lower recurrence rates compared with wide local excision for less common and/or more aggressive tumors. However, there is a lack of standardization on initial and subsequent margin size when treating these less common skin tumors, such as dermatofibrosarcoma protuberans (DFSP), atypical fibroxanthoma (AFX), and sebaceous carcinoma.
Because Mohs surgeons must balance normal tissue preservation with the importance of tumor clearance in the context of comprehensive margin control, we aimed to assess the practice patterns of Mohs surgeons regarding margin size for these unique tumors. The average margin size for each Mohs layer has been reported to be 1 to 3 mm for BCC compared with 3 to 6 mm or larger for other skin cancers, such as melanoma in situ (MIS).1-3 We hypothesized that the initial margin size would vary among surgeons and likely be greater for more aggressive and rarer malignancies as well as for lesions on the trunk and extremities.
Methods
A descriptive survey was created using SurveyMonkey and distributed to members of the American College of Mohs Surgery (ACMS). Survey participants and their responses were anonymous. Demographic information on survey participants was collected in addition to initial and subsequent MMS margin size for DFSP, AFX, MIS, invasive melanoma, sebaceous carcinoma, microcystic adnexal carcinoma (MAC), poorly differentiated SCC, Merkel cell carcinoma, extramammary Paget disease, leiomyosarcoma, and endocrine mucin-producing sweat gland carcinoma. Survey participants were asked to choose from a range of margin sizes: 1 to 3 mm, 4 to 6 mm, 7 to 9 mm, and greater than 9 mm. This study was approved by the University of Texas Southwest Medical Center (Dallas, Texas) institutional review board.
Results
Eighty-seven respondents from the ACMS listserve completed the survey (response rate <10%). Of these, 58 respondents (66.7%) reported practicing for more than 5 years, and 58 (66.7%) were male. Practice setting was primarily private/community (71.3% [62/87]), and survey respondents were located across the United States. More than 50% of survey respondents treated the following tumors on the head and neck in their respective practices: DFSP (80.9% [55/68]), AFX (95.6% [65/68]), MIS (67.7% [46/68]), sebaceous carcinoma (92.7% [63/68]), MAC (83.8% [57/68]), poorly differentiated SCC (97.1% [66/68]), and endocrine mucin-producing sweat gland carcinoma (51.5% [35/68]). More than 50% of survey respondents treated the following tumors on the trunk and extremities: DFSP (90.3% [47/52]), AFX (86.4% [45/52]), MIS (55.8% [29/52]), sebaceous carcinoma (80.8% [42/52]), MAC (73.1% [38/52]), poorly differentiated SCC (94.2% [49/52]), and extramammary Paget disease (53.9% [28/52]). Invasive melanoma, Merkel cell carcinoma, and leiomyosarcoma were overall less commonly treated.
In general, respondent Mohs surgeons were more likely to take larger initial and subsequent margins for tumors treated on the trunk and extremities compared with the head and neck (Table). In addition, initial margin size often was larger than the 1- to 3-mm margin commonly used in Mohs surgery for BCCs and less aggressive SCCs (Table). A larger initial margin size (>9 mm) and subsequent margin size (4–6 mm) was more commonly reported for certain tumors known to be more aggressive and/or have extensive subclinical extension, such as DFSP and invasive melanoma. Of note, most respondents performed 4- to 6-mm margins (37/67 [55.2%]) for poorly differentiated SCC. Overall, there was a high range of margin size variability among Mohs surgeons for these unique and/or more aggressive skin tumors.
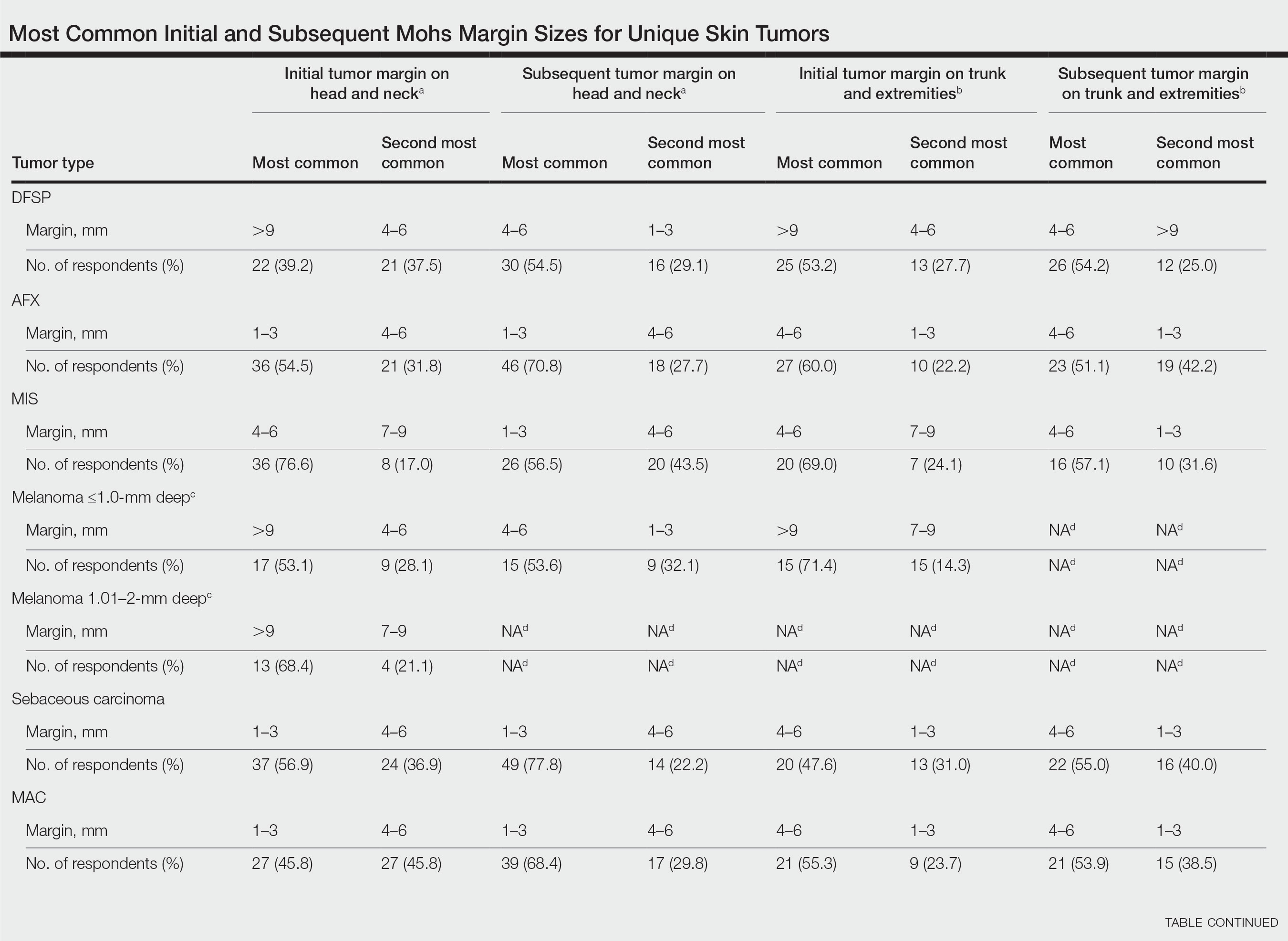
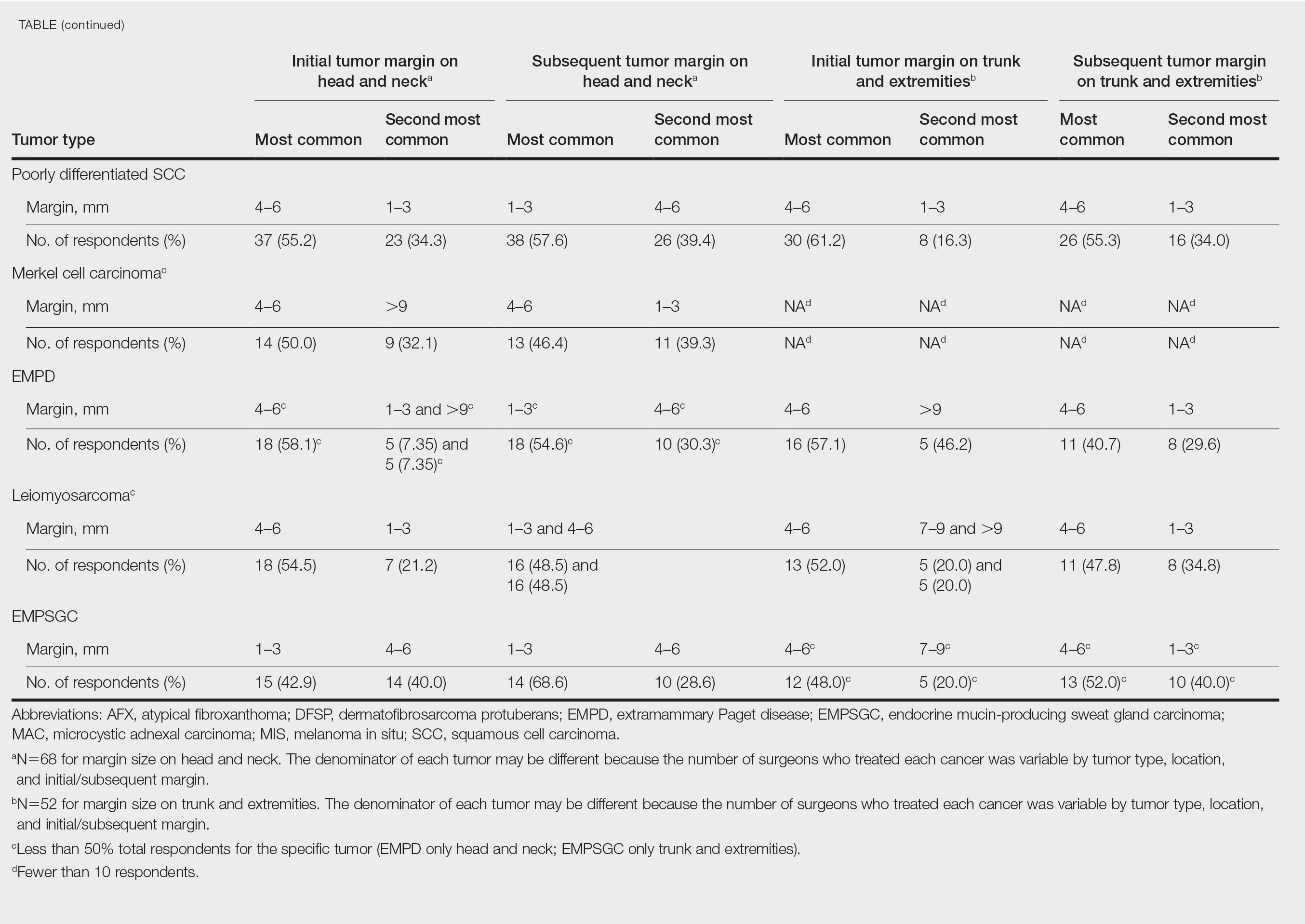
Comment
Given that no guidelines exist on margins with MMS for less commonly treated skin tumors, this study helps give Mohs surgeons perspective on current practice patterns for both initial and subsequent Mohs margin sizes. High margin-size variability among Mohs surgeons is expected, as surgeons also need to account for high-risk features of the tumor or specific locations where tissue sparing is critical. Overall, Mohs surgeons are more likely to take larger initial margins for these less common skin tumors compared with BCCs or SCCs. Initial margin size was consistently larger on the trunk and extremities where tissue sparing often is less critical.
Our survey was limited by a small sample size and incomplete response of the ACMS membership. In addition, most respondents practiced in a private/community setting, which may have led to bias, as academic centers may manage rare malignancies more commonly and/or have increased access to immunostains and multispecialty care. Future registries for rare skin malignancies will hopefully be developed that will allow for further consensus on standardized margins. Additional studies on the average number of stages required to clear these less common tumors also are warranted.
- Muller FM, Dawe RS, Moseley H, et al. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma: tissue‐sparing outcome. Dermatol Surg. 2009;35:1349-1354.
- van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
- Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: a prospective multicenter study. J Am Acad Dermatol. 2019;81:767-774.
Mohs micrographic surgery (MMS) is most commonly used for the surgical management of squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) in high-risk locations. The ability for 100% margin evaluation with MMS also has shown lower recurrence rates compared with wide local excision for less common and/or more aggressive tumors. However, there is a lack of standardization on initial and subsequent margin size when treating these less common skin tumors, such as dermatofibrosarcoma protuberans (DFSP), atypical fibroxanthoma (AFX), and sebaceous carcinoma.
Because Mohs surgeons must balance normal tissue preservation with the importance of tumor clearance in the context of comprehensive margin control, we aimed to assess the practice patterns of Mohs surgeons regarding margin size for these unique tumors. The average margin size for each Mohs layer has been reported to be 1 to 3 mm for BCC compared with 3 to 6 mm or larger for other skin cancers, such as melanoma in situ (MIS).1-3 We hypothesized that the initial margin size would vary among surgeons and likely be greater for more aggressive and rarer malignancies as well as for lesions on the trunk and extremities.
Methods
A descriptive survey was created using SurveyMonkey and distributed to members of the American College of Mohs Surgery (ACMS). Survey participants and their responses were anonymous. Demographic information on survey participants was collected in addition to initial and subsequent MMS margin size for DFSP, AFX, MIS, invasive melanoma, sebaceous carcinoma, microcystic adnexal carcinoma (MAC), poorly differentiated SCC, Merkel cell carcinoma, extramammary Paget disease, leiomyosarcoma, and endocrine mucin-producing sweat gland carcinoma. Survey participants were asked to choose from a range of margin sizes: 1 to 3 mm, 4 to 6 mm, 7 to 9 mm, and greater than 9 mm. This study was approved by the University of Texas Southwest Medical Center (Dallas, Texas) institutional review board.
Results
Eighty-seven respondents from the ACMS listserve completed the survey (response rate <10%). Of these, 58 respondents (66.7%) reported practicing for more than 5 years, and 58 (66.7%) were male. Practice setting was primarily private/community (71.3% [62/87]), and survey respondents were located across the United States. More than 50% of survey respondents treated the following tumors on the head and neck in their respective practices: DFSP (80.9% [55/68]), AFX (95.6% [65/68]), MIS (67.7% [46/68]), sebaceous carcinoma (92.7% [63/68]), MAC (83.8% [57/68]), poorly differentiated SCC (97.1% [66/68]), and endocrine mucin-producing sweat gland carcinoma (51.5% [35/68]). More than 50% of survey respondents treated the following tumors on the trunk and extremities: DFSP (90.3% [47/52]), AFX (86.4% [45/52]), MIS (55.8% [29/52]), sebaceous carcinoma (80.8% [42/52]), MAC (73.1% [38/52]), poorly differentiated SCC (94.2% [49/52]), and extramammary Paget disease (53.9% [28/52]). Invasive melanoma, Merkel cell carcinoma, and leiomyosarcoma were overall less commonly treated.
In general, respondent Mohs surgeons were more likely to take larger initial and subsequent margins for tumors treated on the trunk and extremities compared with the head and neck (Table). In addition, initial margin size often was larger than the 1- to 3-mm margin commonly used in Mohs surgery for BCCs and less aggressive SCCs (Table). A larger initial margin size (>9 mm) and subsequent margin size (4–6 mm) was more commonly reported for certain tumors known to be more aggressive and/or have extensive subclinical extension, such as DFSP and invasive melanoma. Of note, most respondents performed 4- to 6-mm margins (37/67 [55.2%]) for poorly differentiated SCC. Overall, there was a high range of margin size variability among Mohs surgeons for these unique and/or more aggressive skin tumors.


Comment
Given that no guidelines exist on margins with MMS for less commonly treated skin tumors, this study helps give Mohs surgeons perspective on current practice patterns for both initial and subsequent Mohs margin sizes. High margin-size variability among Mohs surgeons is expected, as surgeons also need to account for high-risk features of the tumor or specific locations where tissue sparing is critical. Overall, Mohs surgeons are more likely to take larger initial margins for these less common skin tumors compared with BCCs or SCCs. Initial margin size was consistently larger on the trunk and extremities where tissue sparing often is less critical.
Our survey was limited by a small sample size and incomplete response of the ACMS membership. In addition, most respondents practiced in a private/community setting, which may have led to bias, as academic centers may manage rare malignancies more commonly and/or have increased access to immunostains and multispecialty care. Future registries for rare skin malignancies will hopefully be developed that will allow for further consensus on standardized margins. Additional studies on the average number of stages required to clear these less common tumors also are warranted.
Mohs micrographic surgery (MMS) is most commonly used for the surgical management of squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) in high-risk locations. The ability for 100% margin evaluation with MMS also has shown lower recurrence rates compared with wide local excision for less common and/or more aggressive tumors. However, there is a lack of standardization on initial and subsequent margin size when treating these less common skin tumors, such as dermatofibrosarcoma protuberans (DFSP), atypical fibroxanthoma (AFX), and sebaceous carcinoma.
Because Mohs surgeons must balance normal tissue preservation with the importance of tumor clearance in the context of comprehensive margin control, we aimed to assess the practice patterns of Mohs surgeons regarding margin size for these unique tumors. The average margin size for each Mohs layer has been reported to be 1 to 3 mm for BCC compared with 3 to 6 mm or larger for other skin cancers, such as melanoma in situ (MIS).1-3 We hypothesized that the initial margin size would vary among surgeons and likely be greater for more aggressive and rarer malignancies as well as for lesions on the trunk and extremities.
Methods
A descriptive survey was created using SurveyMonkey and distributed to members of the American College of Mohs Surgery (ACMS). Survey participants and their responses were anonymous. Demographic information on survey participants was collected in addition to initial and subsequent MMS margin size for DFSP, AFX, MIS, invasive melanoma, sebaceous carcinoma, microcystic adnexal carcinoma (MAC), poorly differentiated SCC, Merkel cell carcinoma, extramammary Paget disease, leiomyosarcoma, and endocrine mucin-producing sweat gland carcinoma. Survey participants were asked to choose from a range of margin sizes: 1 to 3 mm, 4 to 6 mm, 7 to 9 mm, and greater than 9 mm. This study was approved by the University of Texas Southwest Medical Center (Dallas, Texas) institutional review board.
Results
Eighty-seven respondents from the ACMS listserve completed the survey (response rate <10%). Of these, 58 respondents (66.7%) reported practicing for more than 5 years, and 58 (66.7%) were male. Practice setting was primarily private/community (71.3% [62/87]), and survey respondents were located across the United States. More than 50% of survey respondents treated the following tumors on the head and neck in their respective practices: DFSP (80.9% [55/68]), AFX (95.6% [65/68]), MIS (67.7% [46/68]), sebaceous carcinoma (92.7% [63/68]), MAC (83.8% [57/68]), poorly differentiated SCC (97.1% [66/68]), and endocrine mucin-producing sweat gland carcinoma (51.5% [35/68]). More than 50% of survey respondents treated the following tumors on the trunk and extremities: DFSP (90.3% [47/52]), AFX (86.4% [45/52]), MIS (55.8% [29/52]), sebaceous carcinoma (80.8% [42/52]), MAC (73.1% [38/52]), poorly differentiated SCC (94.2% [49/52]), and extramammary Paget disease (53.9% [28/52]). Invasive melanoma, Merkel cell carcinoma, and leiomyosarcoma were overall less commonly treated.
In general, respondent Mohs surgeons were more likely to take larger initial and subsequent margins for tumors treated on the trunk and extremities compared with the head and neck (Table). In addition, initial margin size often was larger than the 1- to 3-mm margin commonly used in Mohs surgery for BCCs and less aggressive SCCs (Table). A larger initial margin size (>9 mm) and subsequent margin size (4–6 mm) was more commonly reported for certain tumors known to be more aggressive and/or have extensive subclinical extension, such as DFSP and invasive melanoma. Of note, most respondents performed 4- to 6-mm margins (37/67 [55.2%]) for poorly differentiated SCC. Overall, there was a high range of margin size variability among Mohs surgeons for these unique and/or more aggressive skin tumors.


Comment
Given that no guidelines exist on margins with MMS for less commonly treated skin tumors, this study helps give Mohs surgeons perspective on current practice patterns for both initial and subsequent Mohs margin sizes. High margin-size variability among Mohs surgeons is expected, as surgeons also need to account for high-risk features of the tumor or specific locations where tissue sparing is critical. Overall, Mohs surgeons are more likely to take larger initial margins for these less common skin tumors compared with BCCs or SCCs. Initial margin size was consistently larger on the trunk and extremities where tissue sparing often is less critical.
Our survey was limited by a small sample size and incomplete response of the ACMS membership. In addition, most respondents practiced in a private/community setting, which may have led to bias, as academic centers may manage rare malignancies more commonly and/or have increased access to immunostains and multispecialty care. Future registries for rare skin malignancies will hopefully be developed that will allow for further consensus on standardized margins. Additional studies on the average number of stages required to clear these less common tumors also are warranted.
- Muller FM, Dawe RS, Moseley H, et al. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma: tissue‐sparing outcome. Dermatol Surg. 2009;35:1349-1354.
- van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
- Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: a prospective multicenter study. J Am Acad Dermatol. 2019;81:767-774.
- Muller FM, Dawe RS, Moseley H, et al. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma: tissue‐sparing outcome. Dermatol Surg. 2009;35:1349-1354.
- van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
- Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: a prospective multicenter study. J Am Acad Dermatol. 2019;81:767-774.
Practice Points
- It is common for initial margin size for uncommon skin tumors to be larger than the 1 to 3 mm commonly used in Mohs surgery for basal cell carcinomas and less aggressive squamous cell carcinomas.
- Mohs surgeons commonly take larger starting and subsequent margins for uncommon skin tumors treated on the trunk and extremities compared with the head and neck.
Cancer as a full contact sport
John worked as a handyman and lived on a small sailboat in a marina. When he was diagnosed with metastatic kidney cancer at age 48, he quickly fell through the cracks. He failed to show to appointments and took oral anticancer treatments, but just sporadically. He had Medicaid, so insurance wasn’t the issue. It was everything else.
John was behind on his slip fees; he hadn’t been able to work for some time because of his progressive weakness and pain. He was chronically in danger of getting kicked out of his makeshift home aboard the boat. He had no reliable transportation to the clinic and so he didn’t come to appointments regularly. The specialty pharmacy refused to deliver his expensive oral chemotherapy to his address at the marina. He went days without eating full meals because he was too weak to cook for himself. Plus, he was estranged from his family who were unaware of his illness. His oncologist was overwhelmed trying to take care of him. He had a reasonable chance of achieving disease control on first-line oral therapy, but his problems seemed to hinder these chances at every turn. She was distraught – what could she do?
Enter the team approach. John’s oncologist reached out to our palliative care program for help. We recognized that this was a job too big for us alone so we connected John with the Extensivist Medicine program at UCLA Health, a high-intensity primary care program led by a physician specializing in primary care for high-risk individuals. The program provides wraparound outpatient services for chronically and seriously ill patients, like John, who are at risk for falling through the cracks. John went from receiving very little support to now having an entire team of caring professionals focused on helping him achieve his best possible outcome despite the seriousness of his disease.
He now had the support of a high-functioning team with clearly defined roles. Social work connected him with housing, food, and transportation resources. A nurse called him every day to check in and make sure he was taking medications and reminded him about his upcoming appointments. Case management helped him get needed equipment, such as grab bars and a walker. As his palliative care nurse practitioner, I counseled him on understanding his prognosis and planning ahead for medical emergencies. Our psycho-oncology clinicians helped John reconcile with his family, who were more than willing to take him in once they realized how ill he was. Once these social factors were addressed, John could more easily stay current with his oral chemotherapy, giving him the best chance possible to achieve a robust treatment response that could buy him more time.
And, John did get that time – he got 6 months of improved quality of life, during which he reconnected with his family, including his children, and rebuilt these important relationships. Eventually treatment failed him. His disease, already widely metastatic, became more active and painful. He accepted hospice care at his sister’s house and we transitioned him from our team to the hospice team. He died peacefully surrounded by family.
Interprofessional teamwork is fundamental to treat ‘total pain’
None of this would have been possible without the work of high-functioning teams. It is a commonly held belief that interprofessional teamwork is fundamental to the care of patients and families living with serious illness. But why? How did this idea come about? And what evidence is there to support teamwork?
Dame Cicely Saunders, who founded the modern hospice movement in mid-20th century England, embodied the interdisciplinary team by working first as a nurse, then a social worker, and finally as a physician. She wrote about patients’ “total pain,” the crisis of physical, spiritual, social, and emotional distress that many people have at the end of life. She understood that no single health care discipline was adequate to the task of addressing each of these domains equally well. Thus, hospice became synonymous with care provided by a quartet of specialists – physicians, nurses, social workers, and chaplains. Nowadays, there are other specialists that are added to the mix – home health aides, pharmacists, physical and occupational therapists, music and pet therapists, and so on.
But in medicine, like all areas of science, convention and tradition only go so far. What evidence is there to support the work of an interdisciplinary team in managing the distress of patients and families living with advanced illnesses? It turns out that there is good evidence to support the use of high-functioning interdisciplinary teams in the care of the seriously ill. Palliative care is associated with improved patient outcomes, including improvements in symptom control, quality of life, and end of life care, when it is delivered by an interdisciplinary team rather than by a solo practitioner.
You may think that teamwork is most useful for patients like John who have seemingly intractable social barriers. But it is also true that for even patients with many more social advantages teamwork improves quality of life. I got to see this up close recently in my own life.
Teamwork improves quality of life
My father recently passed away after a 9-month battle with advanced cancer. He had every advantage possible – financial stability, high health literacy, an incredibly devoted spouse who happens to be an RN, good insurance, and access to top-notch medical care. Yet, even he benefited from a team approach. It started small, with the oncologist and oncology NP providing excellent, patient-centered care. Then it grew to include myself as the daughter/palliative care nurse practitioner who made recommendations for treating his nausea and ensured that his advance directive was completed and uploaded to his chart. When my dad needed physical therapy, the home health agency sent a wonderful physical therapist, who brought all sorts of equipment that kept him more functional than he would have been otherwise. Other family members helped out – my sisters helped connect my dad with a priest who came to the home to provide spiritual care, which was crucial to ensuring that he was at peace. And, in his final days, my dad had the hospice team to help manage his symptoms and his family members to provide hands-on care.
The complexity of cancer care has long necessitated a team approach to planning cancer treatment – known as a tumor board – with medical oncology, radiation oncology, surgery, and pathology all weighing in. It makes sense that patients and their families would also need a team of clinicians representing different specialty areas to assist with the wide array of physical, psychosocial, practical, and spiritual concerns that arise throughout the cancer disease trajectory.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
John worked as a handyman and lived on a small sailboat in a marina. When he was diagnosed with metastatic kidney cancer at age 48, he quickly fell through the cracks. He failed to show to appointments and took oral anticancer treatments, but just sporadically. He had Medicaid, so insurance wasn’t the issue. It was everything else.
John was behind on his slip fees; he hadn’t been able to work for some time because of his progressive weakness and pain. He was chronically in danger of getting kicked out of his makeshift home aboard the boat. He had no reliable transportation to the clinic and so he didn’t come to appointments regularly. The specialty pharmacy refused to deliver his expensive oral chemotherapy to his address at the marina. He went days without eating full meals because he was too weak to cook for himself. Plus, he was estranged from his family who were unaware of his illness. His oncologist was overwhelmed trying to take care of him. He had a reasonable chance of achieving disease control on first-line oral therapy, but his problems seemed to hinder these chances at every turn. She was distraught – what could she do?
Enter the team approach. John’s oncologist reached out to our palliative care program for help. We recognized that this was a job too big for us alone so we connected John with the Extensivist Medicine program at UCLA Health, a high-intensity primary care program led by a physician specializing in primary care for high-risk individuals. The program provides wraparound outpatient services for chronically and seriously ill patients, like John, who are at risk for falling through the cracks. John went from receiving very little support to now having an entire team of caring professionals focused on helping him achieve his best possible outcome despite the seriousness of his disease.
He now had the support of a high-functioning team with clearly defined roles. Social work connected him with housing, food, and transportation resources. A nurse called him every day to check in and make sure he was taking medications and reminded him about his upcoming appointments. Case management helped him get needed equipment, such as grab bars and a walker. As his palliative care nurse practitioner, I counseled him on understanding his prognosis and planning ahead for medical emergencies. Our psycho-oncology clinicians helped John reconcile with his family, who were more than willing to take him in once they realized how ill he was. Once these social factors were addressed, John could more easily stay current with his oral chemotherapy, giving him the best chance possible to achieve a robust treatment response that could buy him more time.
And, John did get that time – he got 6 months of improved quality of life, during which he reconnected with his family, including his children, and rebuilt these important relationships. Eventually treatment failed him. His disease, already widely metastatic, became more active and painful. He accepted hospice care at his sister’s house and we transitioned him from our team to the hospice team. He died peacefully surrounded by family.
Interprofessional teamwork is fundamental to treat ‘total pain’
None of this would have been possible without the work of high-functioning teams. It is a commonly held belief that interprofessional teamwork is fundamental to the care of patients and families living with serious illness. But why? How did this idea come about? And what evidence is there to support teamwork?
Dame Cicely Saunders, who founded the modern hospice movement in mid-20th century England, embodied the interdisciplinary team by working first as a nurse, then a social worker, and finally as a physician. She wrote about patients’ “total pain,” the crisis of physical, spiritual, social, and emotional distress that many people have at the end of life. She understood that no single health care discipline was adequate to the task of addressing each of these domains equally well. Thus, hospice became synonymous with care provided by a quartet of specialists – physicians, nurses, social workers, and chaplains. Nowadays, there are other specialists that are added to the mix – home health aides, pharmacists, physical and occupational therapists, music and pet therapists, and so on.
But in medicine, like all areas of science, convention and tradition only go so far. What evidence is there to support the work of an interdisciplinary team in managing the distress of patients and families living with advanced illnesses? It turns out that there is good evidence to support the use of high-functioning interdisciplinary teams in the care of the seriously ill. Palliative care is associated with improved patient outcomes, including improvements in symptom control, quality of life, and end of life care, when it is delivered by an interdisciplinary team rather than by a solo practitioner.
You may think that teamwork is most useful for patients like John who have seemingly intractable social barriers. But it is also true that for even patients with many more social advantages teamwork improves quality of life. I got to see this up close recently in my own life.
Teamwork improves quality of life
My father recently passed away after a 9-month battle with advanced cancer. He had every advantage possible – financial stability, high health literacy, an incredibly devoted spouse who happens to be an RN, good insurance, and access to top-notch medical care. Yet, even he benefited from a team approach. It started small, with the oncologist and oncology NP providing excellent, patient-centered care. Then it grew to include myself as the daughter/palliative care nurse practitioner who made recommendations for treating his nausea and ensured that his advance directive was completed and uploaded to his chart. When my dad needed physical therapy, the home health agency sent a wonderful physical therapist, who brought all sorts of equipment that kept him more functional than he would have been otherwise. Other family members helped out – my sisters helped connect my dad with a priest who came to the home to provide spiritual care, which was crucial to ensuring that he was at peace. And, in his final days, my dad had the hospice team to help manage his symptoms and his family members to provide hands-on care.
The complexity of cancer care has long necessitated a team approach to planning cancer treatment – known as a tumor board – with medical oncology, radiation oncology, surgery, and pathology all weighing in. It makes sense that patients and their families would also need a team of clinicians representing different specialty areas to assist with the wide array of physical, psychosocial, practical, and spiritual concerns that arise throughout the cancer disease trajectory.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
John worked as a handyman and lived on a small sailboat in a marina. When he was diagnosed with metastatic kidney cancer at age 48, he quickly fell through the cracks. He failed to show to appointments and took oral anticancer treatments, but just sporadically. He had Medicaid, so insurance wasn’t the issue. It was everything else.
John was behind on his slip fees; he hadn’t been able to work for some time because of his progressive weakness and pain. He was chronically in danger of getting kicked out of his makeshift home aboard the boat. He had no reliable transportation to the clinic and so he didn’t come to appointments regularly. The specialty pharmacy refused to deliver his expensive oral chemotherapy to his address at the marina. He went days without eating full meals because he was too weak to cook for himself. Plus, he was estranged from his family who were unaware of his illness. His oncologist was overwhelmed trying to take care of him. He had a reasonable chance of achieving disease control on first-line oral therapy, but his problems seemed to hinder these chances at every turn. She was distraught – what could she do?
Enter the team approach. John’s oncologist reached out to our palliative care program for help. We recognized that this was a job too big for us alone so we connected John with the Extensivist Medicine program at UCLA Health, a high-intensity primary care program led by a physician specializing in primary care for high-risk individuals. The program provides wraparound outpatient services for chronically and seriously ill patients, like John, who are at risk for falling through the cracks. John went from receiving very little support to now having an entire team of caring professionals focused on helping him achieve his best possible outcome despite the seriousness of his disease.
He now had the support of a high-functioning team with clearly defined roles. Social work connected him with housing, food, and transportation resources. A nurse called him every day to check in and make sure he was taking medications and reminded him about his upcoming appointments. Case management helped him get needed equipment, such as grab bars and a walker. As his palliative care nurse practitioner, I counseled him on understanding his prognosis and planning ahead for medical emergencies. Our psycho-oncology clinicians helped John reconcile with his family, who were more than willing to take him in once they realized how ill he was. Once these social factors were addressed, John could more easily stay current with his oral chemotherapy, giving him the best chance possible to achieve a robust treatment response that could buy him more time.
And, John did get that time – he got 6 months of improved quality of life, during which he reconnected with his family, including his children, and rebuilt these important relationships. Eventually treatment failed him. His disease, already widely metastatic, became more active and painful. He accepted hospice care at his sister’s house and we transitioned him from our team to the hospice team. He died peacefully surrounded by family.
Interprofessional teamwork is fundamental to treat ‘total pain’
None of this would have been possible without the work of high-functioning teams. It is a commonly held belief that interprofessional teamwork is fundamental to the care of patients and families living with serious illness. But why? How did this idea come about? And what evidence is there to support teamwork?
Dame Cicely Saunders, who founded the modern hospice movement in mid-20th century England, embodied the interdisciplinary team by working first as a nurse, then a social worker, and finally as a physician. She wrote about patients’ “total pain,” the crisis of physical, spiritual, social, and emotional distress that many people have at the end of life. She understood that no single health care discipline was adequate to the task of addressing each of these domains equally well. Thus, hospice became synonymous with care provided by a quartet of specialists – physicians, nurses, social workers, and chaplains. Nowadays, there are other specialists that are added to the mix – home health aides, pharmacists, physical and occupational therapists, music and pet therapists, and so on.
But in medicine, like all areas of science, convention and tradition only go so far. What evidence is there to support the work of an interdisciplinary team in managing the distress of patients and families living with advanced illnesses? It turns out that there is good evidence to support the use of high-functioning interdisciplinary teams in the care of the seriously ill. Palliative care is associated with improved patient outcomes, including improvements in symptom control, quality of life, and end of life care, when it is delivered by an interdisciplinary team rather than by a solo practitioner.
You may think that teamwork is most useful for patients like John who have seemingly intractable social barriers. But it is also true that for even patients with many more social advantages teamwork improves quality of life. I got to see this up close recently in my own life.
Teamwork improves quality of life
My father recently passed away after a 9-month battle with advanced cancer. He had every advantage possible – financial stability, high health literacy, an incredibly devoted spouse who happens to be an RN, good insurance, and access to top-notch medical care. Yet, even he benefited from a team approach. It started small, with the oncologist and oncology NP providing excellent, patient-centered care. Then it grew to include myself as the daughter/palliative care nurse practitioner who made recommendations for treating his nausea and ensured that his advance directive was completed and uploaded to his chart. When my dad needed physical therapy, the home health agency sent a wonderful physical therapist, who brought all sorts of equipment that kept him more functional than he would have been otherwise. Other family members helped out – my sisters helped connect my dad with a priest who came to the home to provide spiritual care, which was crucial to ensuring that he was at peace. And, in his final days, my dad had the hospice team to help manage his symptoms and his family members to provide hands-on care.
The complexity of cancer care has long necessitated a team approach to planning cancer treatment – known as a tumor board – with medical oncology, radiation oncology, surgery, and pathology all weighing in. It makes sense that patients and their families would also need a team of clinicians representing different specialty areas to assist with the wide array of physical, psychosocial, practical, and spiritual concerns that arise throughout the cancer disease trajectory.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.