User login
For MD-IQ use only
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
Multiple Firm Papules on the Wrists and Forearms
Multiple Firm Papules on the Wrists and Forearms
THE DIAGNOSIS: Acral Persistent Papular Mucinosis
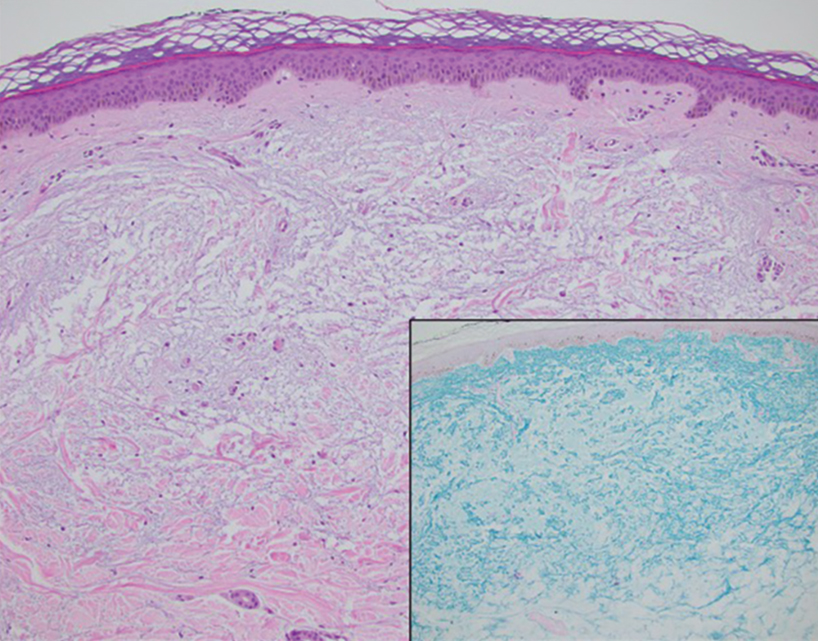
Histopathologic analysis revealed conspicuous interstitial mucin deposition throughout the upper to mid reticular dermis in the absence of a cellular infiltrate or fibroplasia. Colloidal iron staining confirmed the presence of mucin. In correlation with the clinical presentation, a diagnosis of acral persistent papular mucinosis (APPM) was made. The patient was counseled on the benign disease course and lack of associated comorbidities, and additional treatment was not pursued.
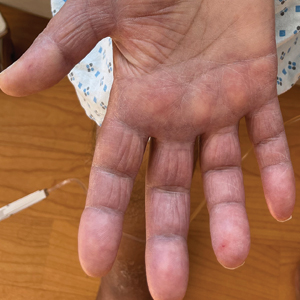
Acral persistent papular mucinosis is a rare distinct subtype of cutaneous mucinosis that initially was described by Rongioletti et al1 in 1986. As a localized form of lichen myxedematosus, APPM is characterized by mucin deposition in the dermis with no systemic involvement. The precise pathogenesis remains unclear, although some investigators have suggested that cytokine-mediated stimulation of glycosaminoglycan production may contribute to increased mucin accumulation in the dermis.2 Acral persistent papular mucinosis predominantly affects middle-aged women with a 5:1 female-to-male predominance.3 Clinically, patients present with discrete, nonfollicular, waxy papules that typically measure 2 to 5 mm and are distributed symmetrically on the extensor surfaces of the wrists and forearms. While the lesions generally are asymptomatic, some patients may report mild pruritus. The condition is chronic, with lesions seldom resolving and often increasing in number over time.3
Histologically, APPM is characterized by focal deposits of mucin in the upper reticular dermis with no evidence of increased fibroblast proliferation or fibrosis.4 This feature is pivotal in differentiating APPM from other subtypes of localized lichen myxedematosus and similar dermatoses. Diagnosis of APPM requires exclusion of systemic involvement, including thyroid abnormalities and monoclonal gammopathy, aligning with its classification as a purely cutaneous condition.5 Management of APPM is unclear due to its rarity. Reassurance for patients of its benign nature as well as clinical observation are recommended, though some reports cite benefits of treatment with topical corticosteroids or calcineurin inhibitors.6,7 The long-term prognosis for patients with APPM is favorable, although the persistence of and potential increase in lesions over time can be a cosmetic concern.
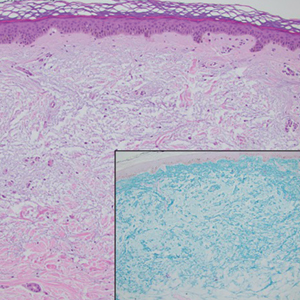
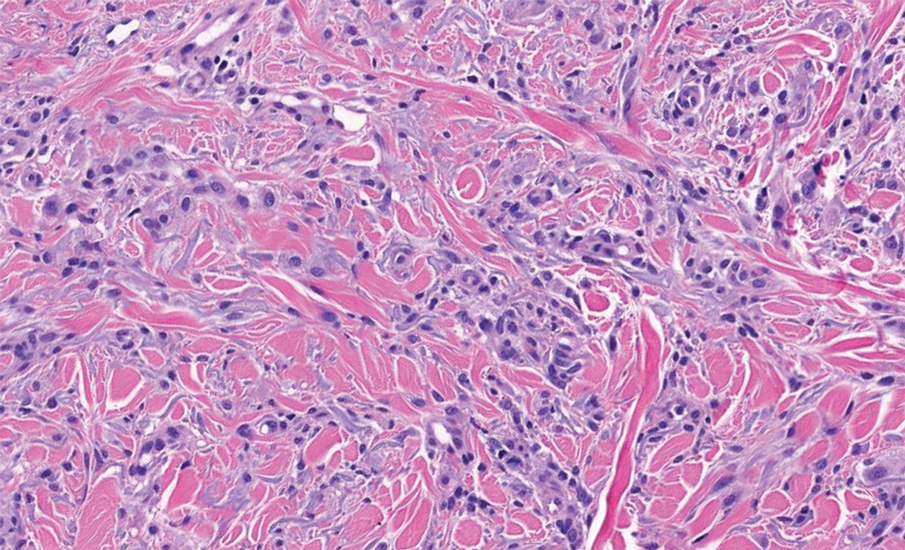
The differential diagnoses for APPM include scleromyxedema, scleredema, and other cutaneous eruptions that manifest as smooth flesh-colored papules, such as granuloma annulare and lichen nitidus.3 Scleromyxedema is a systemic cutaneous mucinosis that is part of the same disease spectrum as lichen myxedematosus. The papular eruption of scleromyxedema is much more widespread, and coalescing of the lesions may lead to characteristic skin thickening, creating leonine facies and deep furrowing over the trunk.8 Extracutaneous manifestations are frequent in scleromyxedema, and up to 90% of patients exhibit evidence of an underlying plasma cell dyscrasia.2 Histopathologically, scleromyxedema shows extensive fibroblast proliferation and fibrosis, in contrast to the findings of APPM (Figure 1).
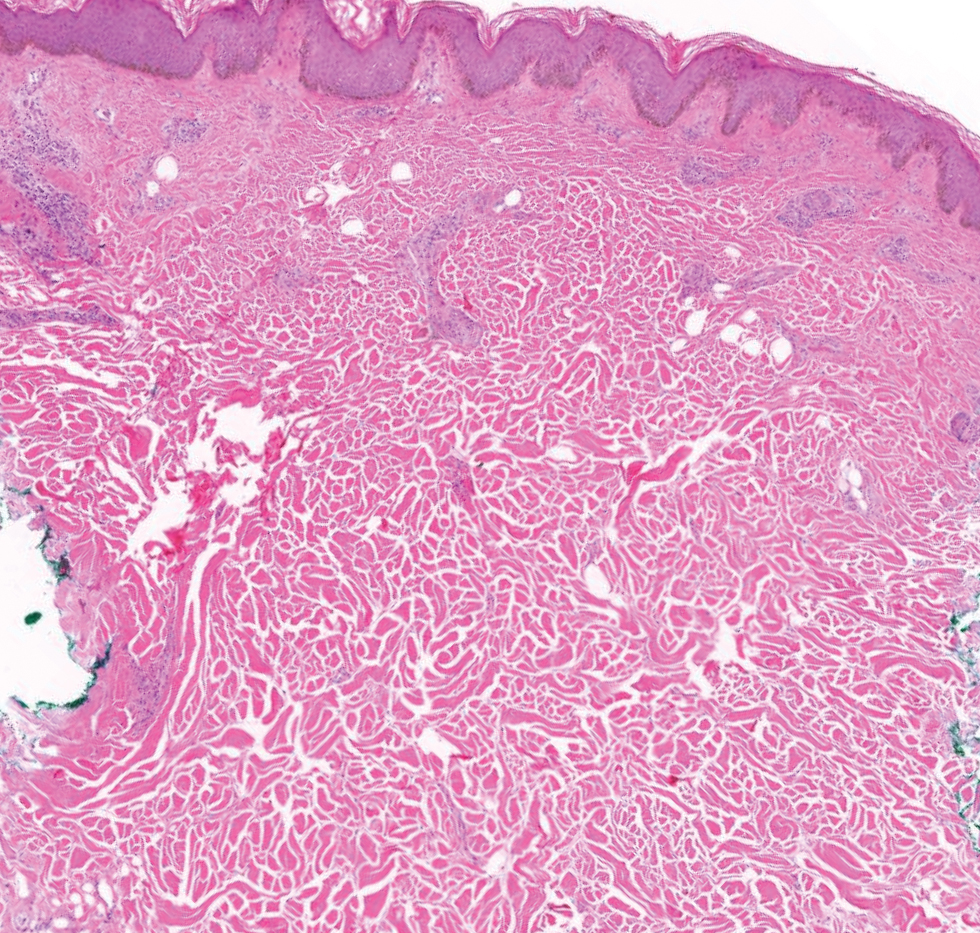
The histopathology of APPM is most similar to scleredema, a rare fibromucinous disorder of the skin associated with diabetes, infection (especially poststreptococcal), or monoclonal gammopathy.9 Biopsy evaluation of scleredema reveals a normal epidermis with mucin deposition between collagen bundles predominantly in the deep reticular dermis as well as absent fibroblast proliferation (Figure 2). Unlike APPM, scleredema manifests with diffuse woody induration with erythema and hyperpigmentation on the posterior neck and upper back.9 On physical examination, the distinct clinical features of scleredema distinguish this condition from APPM and scleromyxedema.
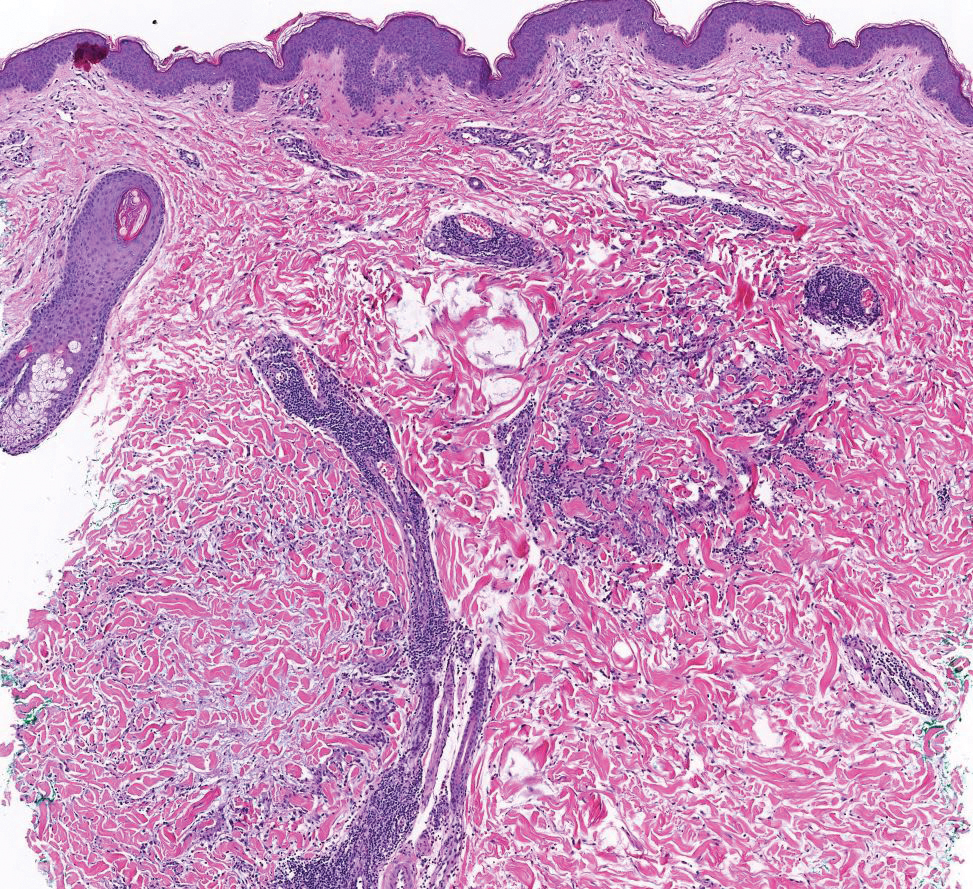
Papular granuloma annulare also was considered in our patient due to the presence of small flesh-colored papules. Histologically, granuloma annulare is characterized by palisading granulomas and mucin deposition in the dermis.10 However, the pattern of mucin deposition differs from that seen in APPM. In granuloma annulare, mucin is observed around foci of degenerated collagen (Figure 3), which was not observed in our patient.10 Additionally, the absence of an inflammatory infiltrate in our patient further ruled out this diagnosis.
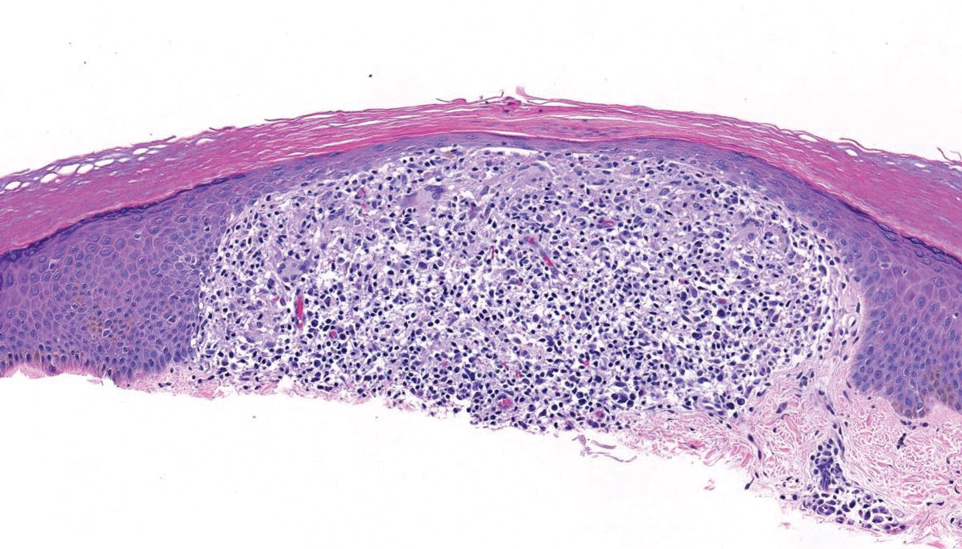
Lichen nitidus also could be considered in the differential diagnosis for ACCM. It typically manifests with minute, clustered, monomorphous papules with a predilection for the chest, abdomen, flexural forearms, and genitalia. The histology of lichen nitidus is distinct, showing a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis bordered by epidermal ridges, resembling a ball and clutch appearance (Figure 4).11
Although the clinical differential diagnosis in our patient was broad, histopathologic evaluation played a crucial role in confirming the diagnosis of APPM. This benign condition could be overlooked by patients and physicians; thorough clinical evaluation is necessary to rule out systemic mucinoses, which are associated with higher risks of morbidity and mortality.
- Rongioletti F, Rebora A. Acral persistent papular mucinosis: a new entity. Arch Dermatol. 1986;122:1237-1239. doi:10.1001 /archderm.1986.01660230027002
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:13030/qt3xp109qd.
- Rongioletti F, Ferreli C, Atzori L. Acral persistent papular mucinosis. Clin Dermatol. 2021;39:211-214. doi:10.1016/j.clindermatol.2020.10.001
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267. doi:10.1097/00000372- 200106000-00022
- Rongioletti F. Lichen myxedematosus (papular mucinosis): new concepts and perspectives for an old disease. Semin Cutan Med Surg. 2006;25:100-104. doi:10.1016/j.sder.2006.04.001
- Jun JY, Oh SH, Shim JH, et al. Acral persistent papular mucinosis with partial response to tacrolimus ointment. Ann Dermatol. 2016;28:517-519. doi:10.5021/ad.2016.28.4.517
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;58:530-532. doi:10.1016/j.jaad.2006.10.021
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72. doi:10.1016 /j.jaad.2013.01.007
- Rongioletti F, Kaiser F, Cinotti E, et al. Scleredema. a multicentre study of characteristics, comorbidities, course and therapy in 44 patients. J Eur Acad Dermatol Venereol. 2015;29:2399-2404. doi:10.1111/jdv.13272
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465. doi:10.1016/j.jaad.2015.03.054
- Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160. doi:10.1111 /j.1525-1470.2005.22215.x
THE DIAGNOSIS: Acral Persistent Papular Mucinosis
Histopathologic analysis revealed conspicuous interstitial mucin deposition throughout the upper to mid reticular dermis in the absence of a cellular infiltrate or fibroplasia. Colloidal iron staining confirmed the presence of mucin. In correlation with the clinical presentation, a diagnosis of acral persistent papular mucinosis (APPM) was made. The patient was counseled on the benign disease course and lack of associated comorbidities, and additional treatment was not pursued.
Acral persistent papular mucinosis is a rare distinct subtype of cutaneous mucinosis that initially was described by Rongioletti et al1 in 1986. As a localized form of lichen myxedematosus, APPM is characterized by mucin deposition in the dermis with no systemic involvement. The precise pathogenesis remains unclear, although some investigators have suggested that cytokine-mediated stimulation of glycosaminoglycan production may contribute to increased mucin accumulation in the dermis.2 Acral persistent papular mucinosis predominantly affects middle-aged women with a 5:1 female-to-male predominance.3 Clinically, patients present with discrete, nonfollicular, waxy papules that typically measure 2 to 5 mm and are distributed symmetrically on the extensor surfaces of the wrists and forearms. While the lesions generally are asymptomatic, some patients may report mild pruritus. The condition is chronic, with lesions seldom resolving and often increasing in number over time.3
Histologically, APPM is characterized by focal deposits of mucin in the upper reticular dermis with no evidence of increased fibroblast proliferation or fibrosis.4 This feature is pivotal in differentiating APPM from other subtypes of localized lichen myxedematosus and similar dermatoses. Diagnosis of APPM requires exclusion of systemic involvement, including thyroid abnormalities and monoclonal gammopathy, aligning with its classification as a purely cutaneous condition.5 Management of APPM is unclear due to its rarity. Reassurance for patients of its benign nature as well as clinical observation are recommended, though some reports cite benefits of treatment with topical corticosteroids or calcineurin inhibitors.6,7 The long-term prognosis for patients with APPM is favorable, although the persistence of and potential increase in lesions over time can be a cosmetic concern.
The differential diagnoses for APPM include scleromyxedema, scleredema, and other cutaneous eruptions that manifest as smooth flesh-colored papules, such as granuloma annulare and lichen nitidus.3 Scleromyxedema is a systemic cutaneous mucinosis that is part of the same disease spectrum as lichen myxedematosus. The papular eruption of scleromyxedema is much more widespread, and coalescing of the lesions may lead to characteristic skin thickening, creating leonine facies and deep furrowing over the trunk.8 Extracutaneous manifestations are frequent in scleromyxedema, and up to 90% of patients exhibit evidence of an underlying plasma cell dyscrasia.2 Histopathologically, scleromyxedema shows extensive fibroblast proliferation and fibrosis, in contrast to the findings of APPM (Figure 1).

The histopathology of APPM is most similar to scleredema, a rare fibromucinous disorder of the skin associated with diabetes, infection (especially poststreptococcal), or monoclonal gammopathy.9 Biopsy evaluation of scleredema reveals a normal epidermis with mucin deposition between collagen bundles predominantly in the deep reticular dermis as well as absent fibroblast proliferation (Figure 2). Unlike APPM, scleredema manifests with diffuse woody induration with erythema and hyperpigmentation on the posterior neck and upper back.9 On physical examination, the distinct clinical features of scleredema distinguish this condition from APPM and scleromyxedema.

Papular granuloma annulare also was considered in our patient due to the presence of small flesh-colored papules. Histologically, granuloma annulare is characterized by palisading granulomas and mucin deposition in the dermis.10 However, the pattern of mucin deposition differs from that seen in APPM. In granuloma annulare, mucin is observed around foci of degenerated collagen (Figure 3), which was not observed in our patient.10 Additionally, the absence of an inflammatory infiltrate in our patient further ruled out this diagnosis.

Lichen nitidus also could be considered in the differential diagnosis for ACCM. It typically manifests with minute, clustered, monomorphous papules with a predilection for the chest, abdomen, flexural forearms, and genitalia. The histology of lichen nitidus is distinct, showing a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis bordered by epidermal ridges, resembling a ball and clutch appearance (Figure 4).11

Although the clinical differential diagnosis in our patient was broad, histopathologic evaluation played a crucial role in confirming the diagnosis of APPM. This benign condition could be overlooked by patients and physicians; thorough clinical evaluation is necessary to rule out systemic mucinoses, which are associated with higher risks of morbidity and mortality.
THE DIAGNOSIS: Acral Persistent Papular Mucinosis
Histopathologic analysis revealed conspicuous interstitial mucin deposition throughout the upper to mid reticular dermis in the absence of a cellular infiltrate or fibroplasia. Colloidal iron staining confirmed the presence of mucin. In correlation with the clinical presentation, a diagnosis of acral persistent papular mucinosis (APPM) was made. The patient was counseled on the benign disease course and lack of associated comorbidities, and additional treatment was not pursued.
Acral persistent papular mucinosis is a rare distinct subtype of cutaneous mucinosis that initially was described by Rongioletti et al1 in 1986. As a localized form of lichen myxedematosus, APPM is characterized by mucin deposition in the dermis with no systemic involvement. The precise pathogenesis remains unclear, although some investigators have suggested that cytokine-mediated stimulation of glycosaminoglycan production may contribute to increased mucin accumulation in the dermis.2 Acral persistent papular mucinosis predominantly affects middle-aged women with a 5:1 female-to-male predominance.3 Clinically, patients present with discrete, nonfollicular, waxy papules that typically measure 2 to 5 mm and are distributed symmetrically on the extensor surfaces of the wrists and forearms. While the lesions generally are asymptomatic, some patients may report mild pruritus. The condition is chronic, with lesions seldom resolving and often increasing in number over time.3
Histologically, APPM is characterized by focal deposits of mucin in the upper reticular dermis with no evidence of increased fibroblast proliferation or fibrosis.4 This feature is pivotal in differentiating APPM from other subtypes of localized lichen myxedematosus and similar dermatoses. Diagnosis of APPM requires exclusion of systemic involvement, including thyroid abnormalities and monoclonal gammopathy, aligning with its classification as a purely cutaneous condition.5 Management of APPM is unclear due to its rarity. Reassurance for patients of its benign nature as well as clinical observation are recommended, though some reports cite benefits of treatment with topical corticosteroids or calcineurin inhibitors.6,7 The long-term prognosis for patients with APPM is favorable, although the persistence of and potential increase in lesions over time can be a cosmetic concern.
The differential diagnoses for APPM include scleromyxedema, scleredema, and other cutaneous eruptions that manifest as smooth flesh-colored papules, such as granuloma annulare and lichen nitidus.3 Scleromyxedema is a systemic cutaneous mucinosis that is part of the same disease spectrum as lichen myxedematosus. The papular eruption of scleromyxedema is much more widespread, and coalescing of the lesions may lead to characteristic skin thickening, creating leonine facies and deep furrowing over the trunk.8 Extracutaneous manifestations are frequent in scleromyxedema, and up to 90% of patients exhibit evidence of an underlying plasma cell dyscrasia.2 Histopathologically, scleromyxedema shows extensive fibroblast proliferation and fibrosis, in contrast to the findings of APPM (Figure 1).

The histopathology of APPM is most similar to scleredema, a rare fibromucinous disorder of the skin associated with diabetes, infection (especially poststreptococcal), or monoclonal gammopathy.9 Biopsy evaluation of scleredema reveals a normal epidermis with mucin deposition between collagen bundles predominantly in the deep reticular dermis as well as absent fibroblast proliferation (Figure 2). Unlike APPM, scleredema manifests with diffuse woody induration with erythema and hyperpigmentation on the posterior neck and upper back.9 On physical examination, the distinct clinical features of scleredema distinguish this condition from APPM and scleromyxedema.

Papular granuloma annulare also was considered in our patient due to the presence of small flesh-colored papules. Histologically, granuloma annulare is characterized by palisading granulomas and mucin deposition in the dermis.10 However, the pattern of mucin deposition differs from that seen in APPM. In granuloma annulare, mucin is observed around foci of degenerated collagen (Figure 3), which was not observed in our patient.10 Additionally, the absence of an inflammatory infiltrate in our patient further ruled out this diagnosis.

Lichen nitidus also could be considered in the differential diagnosis for ACCM. It typically manifests with minute, clustered, monomorphous papules with a predilection for the chest, abdomen, flexural forearms, and genitalia. The histology of lichen nitidus is distinct, showing a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis bordered by epidermal ridges, resembling a ball and clutch appearance (Figure 4).11

Although the clinical differential diagnosis in our patient was broad, histopathologic evaluation played a crucial role in confirming the diagnosis of APPM. This benign condition could be overlooked by patients and physicians; thorough clinical evaluation is necessary to rule out systemic mucinoses, which are associated with higher risks of morbidity and mortality.
- Rongioletti F, Rebora A. Acral persistent papular mucinosis: a new entity. Arch Dermatol. 1986;122:1237-1239. doi:10.1001 /archderm.1986.01660230027002
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:13030/qt3xp109qd.
- Rongioletti F, Ferreli C, Atzori L. Acral persistent papular mucinosis. Clin Dermatol. 2021;39:211-214. doi:10.1016/j.clindermatol.2020.10.001
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267. doi:10.1097/00000372- 200106000-00022
- Rongioletti F. Lichen myxedematosus (papular mucinosis): new concepts and perspectives for an old disease. Semin Cutan Med Surg. 2006;25:100-104. doi:10.1016/j.sder.2006.04.001
- Jun JY, Oh SH, Shim JH, et al. Acral persistent papular mucinosis with partial response to tacrolimus ointment. Ann Dermatol. 2016;28:517-519. doi:10.5021/ad.2016.28.4.517
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;58:530-532. doi:10.1016/j.jaad.2006.10.021
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72. doi:10.1016 /j.jaad.2013.01.007
- Rongioletti F, Kaiser F, Cinotti E, et al. Scleredema. a multicentre study of characteristics, comorbidities, course and therapy in 44 patients. J Eur Acad Dermatol Venereol. 2015;29:2399-2404. doi:10.1111/jdv.13272
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465. doi:10.1016/j.jaad.2015.03.054
- Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160. doi:10.1111 /j.1525-1470.2005.22215.x
- Rongioletti F, Rebora A. Acral persistent papular mucinosis: a new entity. Arch Dermatol. 1986;122:1237-1239. doi:10.1001 /archderm.1986.01660230027002
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:13030/qt3xp109qd.
- Rongioletti F, Ferreli C, Atzori L. Acral persistent papular mucinosis. Clin Dermatol. 2021;39:211-214. doi:10.1016/j.clindermatol.2020.10.001
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267. doi:10.1097/00000372- 200106000-00022
- Rongioletti F. Lichen myxedematosus (papular mucinosis): new concepts and perspectives for an old disease. Semin Cutan Med Surg. 2006;25:100-104. doi:10.1016/j.sder.2006.04.001
- Jun JY, Oh SH, Shim JH, et al. Acral persistent papular mucinosis with partial response to tacrolimus ointment. Ann Dermatol. 2016;28:517-519. doi:10.5021/ad.2016.28.4.517
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;58:530-532. doi:10.1016/j.jaad.2006.10.021
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72. doi:10.1016 /j.jaad.2013.01.007
- Rongioletti F, Kaiser F, Cinotti E, et al. Scleredema. a multicentre study of characteristics, comorbidities, course and therapy in 44 patients. J Eur Acad Dermatol Venereol. 2015;29:2399-2404. doi:10.1111/jdv.13272
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465. doi:10.1016/j.jaad.2015.03.054
- Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160. doi:10.1111 /j.1525-1470.2005.22215.x
Multiple Firm Papules on the Wrists and Forearms
Multiple Firm Papules on the Wrists and Forearms
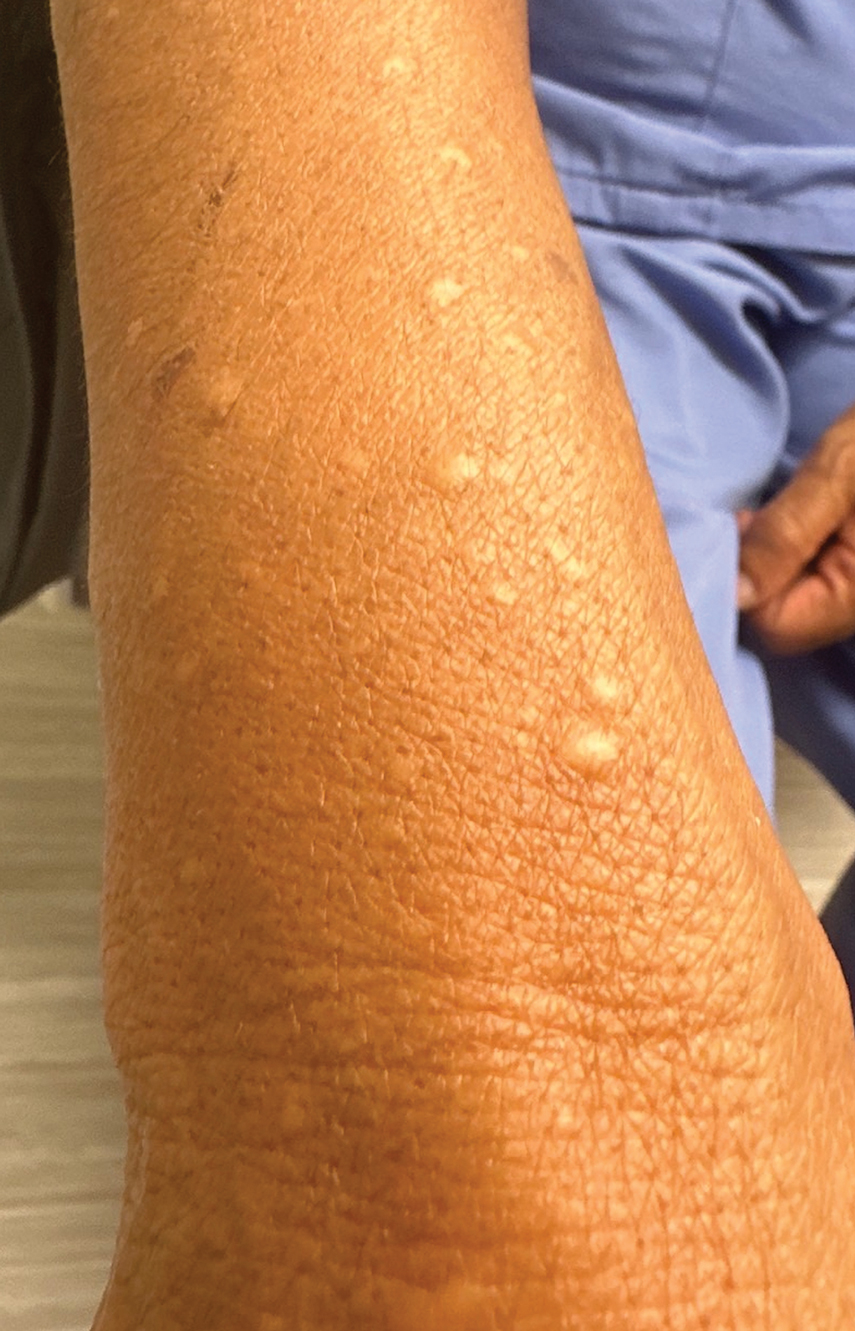
A 69-year-old woman presented to the dermatology department with persistent asymptomatic skin lesions on the wrists and forearms of several months’ duration. The lesions had slowly grown in number over the past few months with no identifiable triggers. The patient reported no known history of injury or trauma to the affected sites and was not taking any prescription medications other than daily vitamins. She denied any family history of similar lesions and was otherwise healthy. Physical examination revealed multiple waxy, firm, hypopigmented, 3- to 5-mm papules located exclusively on the dorsal wrists and forearms. No extracutaneous involvement was observed. A 4-mm punch biopsy from the forearm was obtained.
Bridging the Knowledge-Action Gap in Skin Cancer Prevention Among US Military Personnel
Bridging the Knowledge-Action Gap in Skin Cancer Prevention Among US Military Personnel
Skin cancer is a major health concern for military service members, who experience notably higher incidence rates than the general population.1 Active-duty military personnel are particularly vulnerable to prolonged sun exposure due to deployments, specialized training, and everyday outdoor duties.1 Despite skin cancer being the most commonly diagnosed malignancy in active-duty service members,2 tracking and documenting the quantity and diversity of these risk factors remain limited. This knowledge gap comes at high cost, simultaneously impairing military medicine preventive measures while burdening the military health care system with substantial expenditures.3 These findings underscore the critical need for targeted surveillance, early-detection programs, and policy-driven interventions to mitigate these medical and economic concerns.
Skin cancer has been recognized as a major health risk to the military population for decades, yet incidence and prevalence remain high. This phenomenon is closely linked to the inherent responsibilities and expectations of active-duty military members, including outdoor physical training, field exercises, standing in formation, and outdoor working environments—all of which can occur during peak sunlight hours. These risks are further elevated at duty stations in geographic regions with high levels of UV exposure, such as those in tropical and arid regions of the world. Certain military occupational specialties and missions may further introduce unique risk factors; for instance, pilots with frequent high-altitude missions experience heightened UV exposure and melanoma risk.4 Secondary to compounding determinants, the aviation, diving, and nuclear subgroups of the military community are particularly vulnerable to skin cancer.5
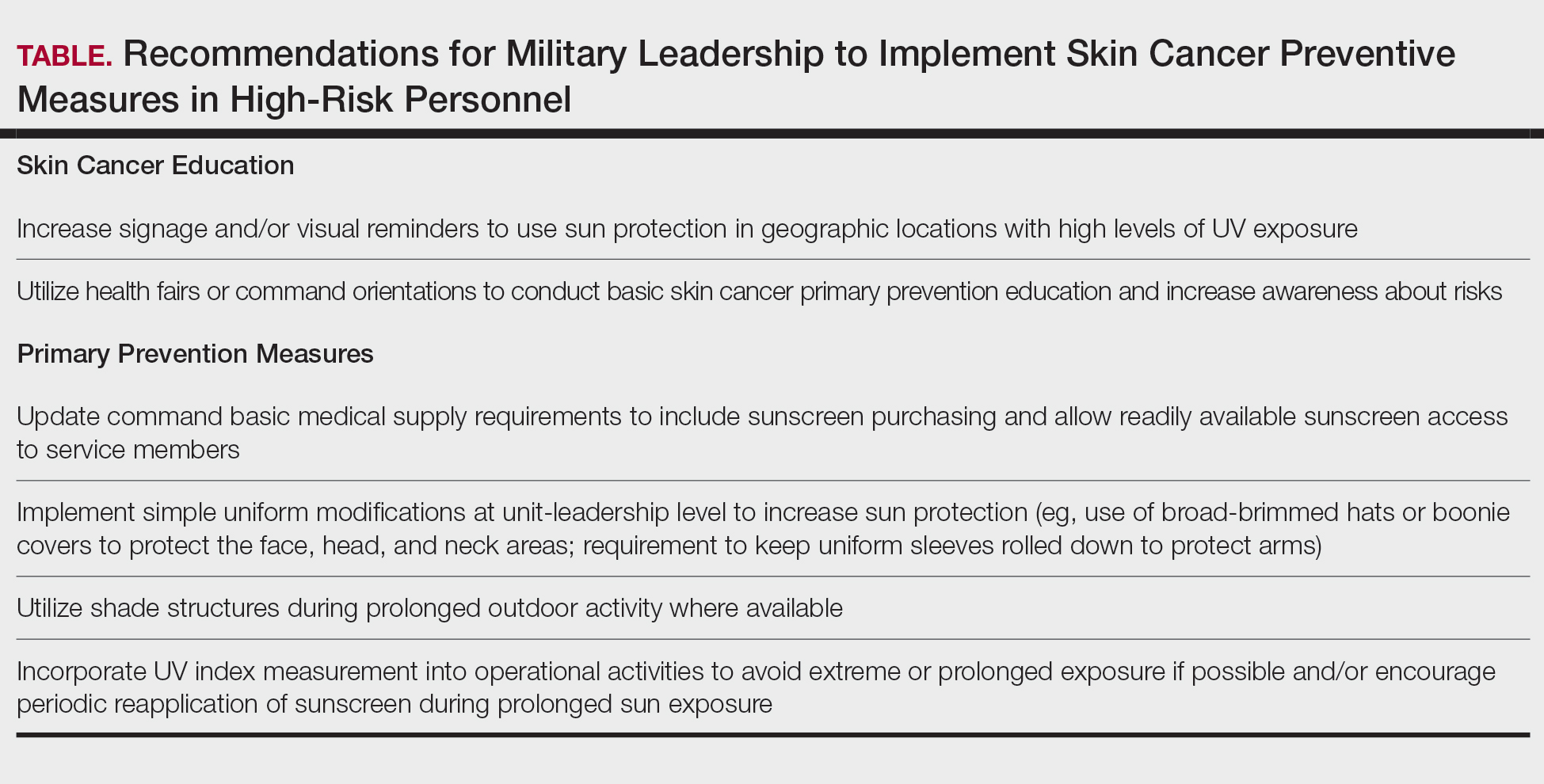
Despite well-documented risks, considerable gaps remain in quantifying and analyzing variations in UV exposure across military occupations, duty locations, and operational roles. Factors such as the existence of over 150 distinct military occupational specialties, frequent geographic relocations, and routine work in austere environments contribute to a wide range of UV exposure profiles that remain insufficiently characterized. This lack of comprehensive exposure data hinders the development of large-scale, targeted skin cancer prevention strategies. Initial approaches to addressing these challenges include enhanced surveillance, education, and policy initiatives. The Table presents practical recommendations for military leadership to consider in implementing preventive measures for skin cancer. Herein, we outline broader systemic strategies to bridge knowledge gaps and address underrecognized occupational risk factors for skin cancer in military service members; these elements include proposed modifications to the electronic Periodic Health Assessment (ePHA) and the development of standardized, military-specific screening and prevention guidelines to support early detection and resource optimization.
Skin Cancer Education for Service Members
Sunscreen and Signage—Diligent primary prevention offers a promising avenue for mitigating skin cancer incidence in military service members. Basic education and precautionary messaging on photoprotection can be widely implemented to simultaneously educate service members on the dangers of sun exposure while reinforcing healthy behaviors in real time. Simple low-cost initiatives such as strategically placed visual signage reminding service members to apply sunscreen in high UV environments can support consistent sun-safe practices. Educational efforts also should emphasize proper sunscreen use, including application on high-risk anatomic sites (eg, the face, neck, scalp, dorsal hands, and ears) and the essentiality of using sufficient quantities of broad-spectrum sunscreen for effective protection. Incorporating this guidance into training materials, briefings, and visual reminders allow seamless integration of photoprotection into service members’ daily routines without compromising operational efficiency.6 Younger service members, who may be less likely to prioritize preventive behaviors, may be particularly responsive to sun safety reminders in training areas, bases, and deployment zones.7 Health fairs and orientation briefs in high-UV regions also offer potential opportunities for targeted education.
Resources for Sun Protection in the Military
Sunscreen—Although sunscreen is critical in minimizing the risk for UV-induced skin cancer, its widespread use in the military is hindered by practical challenges related to accessibility and the need for consistent reapplication; for instance, providing free sunscreen dispensers at institutions for staff working under intense or prolonged UV exposure may improve sunscreen accessibility and use.8 Including sunscreen in standard-issue gear offers another logical way to embed its use into operational readiness as part of the routine protective measures.
Uniform Modifications—Adapting military uniforms and practices to improve sun protection plays a critical role in reducing skin cancer risk. Targeted protective gear for commonly sun-exposed areas can help mitigate UV exposure. One practical option is the use of wide-brimmed headgear (eg, boonie hats), which provide more face and neck coverage than standard-issue military caps, or covers. The wide-brimmed headgear currently is only selectively authorized during specific scenarios, such as field operations and training exercises, or at the discretion of unit-level leadership. Wide-brimmed headgear, already used by many service members, has been associated with up to a 17% reduction in UV exposure to inadequately protected areas, potentially lowering skin cancer risk.9,10 Similarly, a “sleeves-down” policy—requiring sleeves to remain unrolled and covering the forearms during outdoor activities—offers a simple way to minimize sun exposure without necessitating additional gear. Other specialized clothing items, including UV-blocking neck gaiters, photoprotective clothing, and lightweight gloves, also may be appropriate for high-risk groups and can be implemented in a relatively straightforward manner.
Shade Structures and UV Index Monitoring—Aside from uniform adaptation, physical barrier intervention can further complement skin cancer prevention efforts in the military. Shade structures offer a straightforward way to reduce UV exposure during prolonged outdoor activities. Incorporating daily UV index monitoring into operational guidance can help inform adjustments to training schedules and guide the implementation of additional sun protection measures, such as mandatory sunscreen application, use of wide-brimmed hats, or increased access to shaded rest areas during heavy sunlight hours. Currently, outdoor physical training is restricted during periods of high heat index, measured via Wet Bulb Globe Temperature, to reduce heat-related injuries. We argue that avoidance of nonoperational outdoor activity during peak UV index hours also should be incorporated into standardized policies. This intervention is of particular benefit to service members stationed in regions with a high UV index year-round, such as those stationed in the Middle East, Guam, Okinawa, and southern coastal United States bases.
Policy Changes to Support Photoprotective Measures
Annual Risk Factor Screening‐Screening—Effective secondary prevention efforts by military dermatologists remain an important measure in reducing the burden of skin cancer among military personnel; however, these efforts have become increasingly challenging due to 2 main factors—the diversity of military occupational specialties and their associated unique occupational risks as well as the limited availability of military dermatologists across all branches (approximately 100 active-duty dermatologists for nearly 3 million service members).11 Therefore, targeted interventions that enhance risk assessment, refined screening protocols, and leveraging of existing military health networks can improve early skin cancer detection while optimizing resource allocation.
The ePHA is an online screening tool used annually by all service members to evaluate their overall health. Presently, the ePHA lacks specific questions to assess sun exposure and skin cancer risks. Integrating annual skin cancer risk factor assessments into the ePHA would offer a practical and straightforward approach to identifying at-risk individuals, as suggested by Newnam et al12 in 2022. Skin cancer risk factor assessments allow for targeted data collection related to sun exposure history, family history, and personal risk factors, which can be used to determine individualized risk stratification to assess the need for early secondary prevention measures and specialist referral. These ePHA data can also support population-based analyses to inform preventive strategies and address knowledge gaps related to high-risk exposures, such as extended field exercises or assignments in high-UV regions, that may impede effective skin cancer prevention.
Development of Military-Specific Screening Guidelines—Given the limited number of military dermatologists, a standardized risk-assessment tool could enhance early detection of skin cancer and streamline the referral process. We propose a military-specific skin cancer screening algorithm or risk nomogram that could help to consolidate risk factors into a clear and actionable framework for more efficient triage and appropriate allocation of dermatologic resources and manpower. This nomogram could be developed by military dermatologists and then implemented on a command level, affording primary care providers a useful tool to expedite evaluation of individuals at higher risk for skin cancer while simultaneously promoting judicious use of limited dermatology resources.
Although the United States Preventive Services Task Force does not universally recommend routine skin cancer screenings for asymptomatic adults, military service members are exposed to higher occupational risks than the general population, as previously mentioned. Currently, there is no standardized screening guideline across all military services due to the unique nature and exposure risks for each branch of service and their varied occupations; however, we propose the development of basic standardized screening guidelines by adapting the framework of the United States Preventive Services Task Force and adjusting for military-specific UV exposure and occupational risks to improve early detection of skin cancer. These guidelines could be updated and tailored appropriately when additional population-based data are collected and analyzed through ePHA.
Critiques and Limitations of Implementation
Several challenges and limitations must be considered when attempting to integrate large-scale preventive measures for skin cancer within the US military. A primary concern is the extent to which military resources should be allocated to prevention when off-duty sun exposure remains largely beyond institutional control. Although military health initiatives can address workplace risk through education and policy, individual decisions during both work and leisure time remain a major variable that cannot be feasibly controlled. Cultural and operational barriers also pose challenges; for instance, the US Marine Corps maintains a strong cultural identity tied to uniform appearance, making it difficult to implement widespread changes to clothing-based sun-protection measures. Institutional changes, particularly those involving uniforms, likely will face substantial administrative resistance and potential operational limitations. When broad uniform modifications are unattainable, a more feasible approach may be to encourage unit-level leadership to authorize and promote the frequent use of nonuniform protective measures.
Furthermore, integrating additional skin cancer risk questions into the already extensive ePHA means extra time required to complete the assessment; this adds to service members’ administrative burden, potentially leading to reduced timely compliance, rushed responses, and survey fatigue, which threaten data quality. If new items are to be included, they should be carefully selected for efficiency and clinical relevance. Existing validated questionnaires such as those from the study by Lyford et al7 published in 2021 can serve as a foundation.
Another critical limitation is access to dermatologic care for active-duty service members. Raising awareness of skin cancer risk without ensuring adequate resources may create ethical concerns, particularly in high-risk environments such as the Middle East and Indo-Pacific. Additionally, because skin cancer often develops years or decades after exposure, securing early buy-in from service members and their leaders can be challenging. These concerns make it clear that, while skin cancer prevention is important, implementing widespread measures is not straightforward and requires a practical and balanced approach.
Final Thoughts
Implementing prevention strategies for skin cancer in the military requires balancing evidence-based recommendations with the practical realities of military culture, resource limitations, and operational demands. Challenges remain for dermatologists in providing targeted recommendations due to the multifaceted nature of military roles, including over 150 Navy Military Occupational Specialties, limited familiarity with the unique UV exposure risks associated with each occupation, and variability in local and regional policies on uniform wear, physical training requirements, and other operational practices. Although targeted prevention measures are difficult to establish in the setting of these knowledge gaps, leveraging unit-level leadership to align with existing screening guidelines and optimizing primary prevention measures can be meaningful steps toward reducing skin cancer risk for military service members while maintaining mission readiness.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancerincidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Krivda KR, Watson NL, Lyford WH, et al. The burden of skin cancer in the military health system, 2017-2022. Cutis. 2024;113:200-215. doi:10.12788/cutis.1015
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58. doi:10.1001/jamadermatol.2014.1077
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MMSR. 2017;24:8-14.
- Subramaniam P, Olsen CM, Thompson BS, et al, for the QSkin Sun and Health Study Investigators. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182. doi:10.1001/jamadermatol.2016.4070
- Lyford WH, Crotty A, Logemann NF. Sun exposure prevention practices within U.S. naval aviation. Mil Med. 2021;186:1169-1175. doi:10.1093/milmed/usab099
- Wood M, Raisanen T, Polcari I. Observational study of free public sunscreen dispenser use at a major US outdoor event. J Am Acad Dermatol. 2017;77:164-166.
- Schissel D. Operation shadow warrior: a quantitative analysis of the ultraviolet radiation protection demonstrated by various headgear. Mil Med. 2001;166:783-785.
- Milch JM, Logemann NF. Photoprotection prevents skin cancer: let’s make it fashionable to wear sun-protective clothing. Cutis. 2017;99:89-92.
- Association of Military Dermatologists. (n.d.). Military dermatology. https://militaryderm.org/military-dermatology/
- Newnam R, Le-Jenkins U, Rutledge C, et al. The association of skin cancer prevention knowledge, sun-protective attitudes, and sunprotective behaviors in a Navy population. Mil Med. 2024;189:1-7. doi:10.1093/milmed/usac285
Skin cancer is a major health concern for military service members, who experience notably higher incidence rates than the general population.1 Active-duty military personnel are particularly vulnerable to prolonged sun exposure due to deployments, specialized training, and everyday outdoor duties.1 Despite skin cancer being the most commonly diagnosed malignancy in active-duty service members,2 tracking and documenting the quantity and diversity of these risk factors remain limited. This knowledge gap comes at high cost, simultaneously impairing military medicine preventive measures while burdening the military health care system with substantial expenditures.3 These findings underscore the critical need for targeted surveillance, early-detection programs, and policy-driven interventions to mitigate these medical and economic concerns.
Skin cancer has been recognized as a major health risk to the military population for decades, yet incidence and prevalence remain high. This phenomenon is closely linked to the inherent responsibilities and expectations of active-duty military members, including outdoor physical training, field exercises, standing in formation, and outdoor working environments—all of which can occur during peak sunlight hours. These risks are further elevated at duty stations in geographic regions with high levels of UV exposure, such as those in tropical and arid regions of the world. Certain military occupational specialties and missions may further introduce unique risk factors; for instance, pilots with frequent high-altitude missions experience heightened UV exposure and melanoma risk.4 Secondary to compounding determinants, the aviation, diving, and nuclear subgroups of the military community are particularly vulnerable to skin cancer.5
Despite well-documented risks, considerable gaps remain in quantifying and analyzing variations in UV exposure across military occupations, duty locations, and operational roles. Factors such as the existence of over 150 distinct military occupational specialties, frequent geographic relocations, and routine work in austere environments contribute to a wide range of UV exposure profiles that remain insufficiently characterized. This lack of comprehensive exposure data hinders the development of large-scale, targeted skin cancer prevention strategies. Initial approaches to addressing these challenges include enhanced surveillance, education, and policy initiatives. The Table presents practical recommendations for military leadership to consider in implementing preventive measures for skin cancer. Herein, we outline broader systemic strategies to bridge knowledge gaps and address underrecognized occupational risk factors for skin cancer in military service members; these elements include proposed modifications to the electronic Periodic Health Assessment (ePHA) and the development of standardized, military-specific screening and prevention guidelines to support early detection and resource optimization.

Skin Cancer Education for Service Members
Sunscreen and Signage—Diligent primary prevention offers a promising avenue for mitigating skin cancer incidence in military service members. Basic education and precautionary messaging on photoprotection can be widely implemented to simultaneously educate service members on the dangers of sun exposure while reinforcing healthy behaviors in real time. Simple low-cost initiatives such as strategically placed visual signage reminding service members to apply sunscreen in high UV environments can support consistent sun-safe practices. Educational efforts also should emphasize proper sunscreen use, including application on high-risk anatomic sites (eg, the face, neck, scalp, dorsal hands, and ears) and the essentiality of using sufficient quantities of broad-spectrum sunscreen for effective protection. Incorporating this guidance into training materials, briefings, and visual reminders allow seamless integration of photoprotection into service members’ daily routines without compromising operational efficiency.6 Younger service members, who may be less likely to prioritize preventive behaviors, may be particularly responsive to sun safety reminders in training areas, bases, and deployment zones.7 Health fairs and orientation briefs in high-UV regions also offer potential opportunities for targeted education.
Resources for Sun Protection in the Military
Sunscreen—Although sunscreen is critical in minimizing the risk for UV-induced skin cancer, its widespread use in the military is hindered by practical challenges related to accessibility and the need for consistent reapplication; for instance, providing free sunscreen dispensers at institutions for staff working under intense or prolonged UV exposure may improve sunscreen accessibility and use.8 Including sunscreen in standard-issue gear offers another logical way to embed its use into operational readiness as part of the routine protective measures.
Uniform Modifications—Adapting military uniforms and practices to improve sun protection plays a critical role in reducing skin cancer risk. Targeted protective gear for commonly sun-exposed areas can help mitigate UV exposure. One practical option is the use of wide-brimmed headgear (eg, boonie hats), which provide more face and neck coverage than standard-issue military caps, or covers. The wide-brimmed headgear currently is only selectively authorized during specific scenarios, such as field operations and training exercises, or at the discretion of unit-level leadership. Wide-brimmed headgear, already used by many service members, has been associated with up to a 17% reduction in UV exposure to inadequately protected areas, potentially lowering skin cancer risk.9,10 Similarly, a “sleeves-down” policy—requiring sleeves to remain unrolled and covering the forearms during outdoor activities—offers a simple way to minimize sun exposure without necessitating additional gear. Other specialized clothing items, including UV-blocking neck gaiters, photoprotective clothing, and lightweight gloves, also may be appropriate for high-risk groups and can be implemented in a relatively straightforward manner.
Shade Structures and UV Index Monitoring—Aside from uniform adaptation, physical barrier intervention can further complement skin cancer prevention efforts in the military. Shade structures offer a straightforward way to reduce UV exposure during prolonged outdoor activities. Incorporating daily UV index monitoring into operational guidance can help inform adjustments to training schedules and guide the implementation of additional sun protection measures, such as mandatory sunscreen application, use of wide-brimmed hats, or increased access to shaded rest areas during heavy sunlight hours. Currently, outdoor physical training is restricted during periods of high heat index, measured via Wet Bulb Globe Temperature, to reduce heat-related injuries. We argue that avoidance of nonoperational outdoor activity during peak UV index hours also should be incorporated into standardized policies. This intervention is of particular benefit to service members stationed in regions with a high UV index year-round, such as those stationed in the Middle East, Guam, Okinawa, and southern coastal United States bases.
Policy Changes to Support Photoprotective Measures
Annual Risk Factor Screening‐Screening—Effective secondary prevention efforts by military dermatologists remain an important measure in reducing the burden of skin cancer among military personnel; however, these efforts have become increasingly challenging due to 2 main factors—the diversity of military occupational specialties and their associated unique occupational risks as well as the limited availability of military dermatologists across all branches (approximately 100 active-duty dermatologists for nearly 3 million service members).11 Therefore, targeted interventions that enhance risk assessment, refined screening protocols, and leveraging of existing military health networks can improve early skin cancer detection while optimizing resource allocation.
The ePHA is an online screening tool used annually by all service members to evaluate their overall health. Presently, the ePHA lacks specific questions to assess sun exposure and skin cancer risks. Integrating annual skin cancer risk factor assessments into the ePHA would offer a practical and straightforward approach to identifying at-risk individuals, as suggested by Newnam et al12 in 2022. Skin cancer risk factor assessments allow for targeted data collection related to sun exposure history, family history, and personal risk factors, which can be used to determine individualized risk stratification to assess the need for early secondary prevention measures and specialist referral. These ePHA data can also support population-based analyses to inform preventive strategies and address knowledge gaps related to high-risk exposures, such as extended field exercises or assignments in high-UV regions, that may impede effective skin cancer prevention.
Development of Military-Specific Screening Guidelines—Given the limited number of military dermatologists, a standardized risk-assessment tool could enhance early detection of skin cancer and streamline the referral process. We propose a military-specific skin cancer screening algorithm or risk nomogram that could help to consolidate risk factors into a clear and actionable framework for more efficient triage and appropriate allocation of dermatologic resources and manpower. This nomogram could be developed by military dermatologists and then implemented on a command level, affording primary care providers a useful tool to expedite evaluation of individuals at higher risk for skin cancer while simultaneously promoting judicious use of limited dermatology resources.
Although the United States Preventive Services Task Force does not universally recommend routine skin cancer screenings for asymptomatic adults, military service members are exposed to higher occupational risks than the general population, as previously mentioned. Currently, there is no standardized screening guideline across all military services due to the unique nature and exposure risks for each branch of service and their varied occupations; however, we propose the development of basic standardized screening guidelines by adapting the framework of the United States Preventive Services Task Force and adjusting for military-specific UV exposure and occupational risks to improve early detection of skin cancer. These guidelines could be updated and tailored appropriately when additional population-based data are collected and analyzed through ePHA.
Critiques and Limitations of Implementation
Several challenges and limitations must be considered when attempting to integrate large-scale preventive measures for skin cancer within the US military. A primary concern is the extent to which military resources should be allocated to prevention when off-duty sun exposure remains largely beyond institutional control. Although military health initiatives can address workplace risk through education and policy, individual decisions during both work and leisure time remain a major variable that cannot be feasibly controlled. Cultural and operational barriers also pose challenges; for instance, the US Marine Corps maintains a strong cultural identity tied to uniform appearance, making it difficult to implement widespread changes to clothing-based sun-protection measures. Institutional changes, particularly those involving uniforms, likely will face substantial administrative resistance and potential operational limitations. When broad uniform modifications are unattainable, a more feasible approach may be to encourage unit-level leadership to authorize and promote the frequent use of nonuniform protective measures.
Furthermore, integrating additional skin cancer risk questions into the already extensive ePHA means extra time required to complete the assessment; this adds to service members’ administrative burden, potentially leading to reduced timely compliance, rushed responses, and survey fatigue, which threaten data quality. If new items are to be included, they should be carefully selected for efficiency and clinical relevance. Existing validated questionnaires such as those from the study by Lyford et al7 published in 2021 can serve as a foundation.
Another critical limitation is access to dermatologic care for active-duty service members. Raising awareness of skin cancer risk without ensuring adequate resources may create ethical concerns, particularly in high-risk environments such as the Middle East and Indo-Pacific. Additionally, because skin cancer often develops years or decades after exposure, securing early buy-in from service members and their leaders can be challenging. These concerns make it clear that, while skin cancer prevention is important, implementing widespread measures is not straightforward and requires a practical and balanced approach.
Final Thoughts
Implementing prevention strategies for skin cancer in the military requires balancing evidence-based recommendations with the practical realities of military culture, resource limitations, and operational demands. Challenges remain for dermatologists in providing targeted recommendations due to the multifaceted nature of military roles, including over 150 Navy Military Occupational Specialties, limited familiarity with the unique UV exposure risks associated with each occupation, and variability in local and regional policies on uniform wear, physical training requirements, and other operational practices. Although targeted prevention measures are difficult to establish in the setting of these knowledge gaps, leveraging unit-level leadership to align with existing screening guidelines and optimizing primary prevention measures can be meaningful steps toward reducing skin cancer risk for military service members while maintaining mission readiness.
Skin cancer is a major health concern for military service members, who experience notably higher incidence rates than the general population.1 Active-duty military personnel are particularly vulnerable to prolonged sun exposure due to deployments, specialized training, and everyday outdoor duties.1 Despite skin cancer being the most commonly diagnosed malignancy in active-duty service members,2 tracking and documenting the quantity and diversity of these risk factors remain limited. This knowledge gap comes at high cost, simultaneously impairing military medicine preventive measures while burdening the military health care system with substantial expenditures.3 These findings underscore the critical need for targeted surveillance, early-detection programs, and policy-driven interventions to mitigate these medical and economic concerns.
Skin cancer has been recognized as a major health risk to the military population for decades, yet incidence and prevalence remain high. This phenomenon is closely linked to the inherent responsibilities and expectations of active-duty military members, including outdoor physical training, field exercises, standing in formation, and outdoor working environments—all of which can occur during peak sunlight hours. These risks are further elevated at duty stations in geographic regions with high levels of UV exposure, such as those in tropical and arid regions of the world. Certain military occupational specialties and missions may further introduce unique risk factors; for instance, pilots with frequent high-altitude missions experience heightened UV exposure and melanoma risk.4 Secondary to compounding determinants, the aviation, diving, and nuclear subgroups of the military community are particularly vulnerable to skin cancer.5
Despite well-documented risks, considerable gaps remain in quantifying and analyzing variations in UV exposure across military occupations, duty locations, and operational roles. Factors such as the existence of over 150 distinct military occupational specialties, frequent geographic relocations, and routine work in austere environments contribute to a wide range of UV exposure profiles that remain insufficiently characterized. This lack of comprehensive exposure data hinders the development of large-scale, targeted skin cancer prevention strategies. Initial approaches to addressing these challenges include enhanced surveillance, education, and policy initiatives. The Table presents practical recommendations for military leadership to consider in implementing preventive measures for skin cancer. Herein, we outline broader systemic strategies to bridge knowledge gaps and address underrecognized occupational risk factors for skin cancer in military service members; these elements include proposed modifications to the electronic Periodic Health Assessment (ePHA) and the development of standardized, military-specific screening and prevention guidelines to support early detection and resource optimization.

Skin Cancer Education for Service Members
Sunscreen and Signage—Diligent primary prevention offers a promising avenue for mitigating skin cancer incidence in military service members. Basic education and precautionary messaging on photoprotection can be widely implemented to simultaneously educate service members on the dangers of sun exposure while reinforcing healthy behaviors in real time. Simple low-cost initiatives such as strategically placed visual signage reminding service members to apply sunscreen in high UV environments can support consistent sun-safe practices. Educational efforts also should emphasize proper sunscreen use, including application on high-risk anatomic sites (eg, the face, neck, scalp, dorsal hands, and ears) and the essentiality of using sufficient quantities of broad-spectrum sunscreen for effective protection. Incorporating this guidance into training materials, briefings, and visual reminders allow seamless integration of photoprotection into service members’ daily routines without compromising operational efficiency.6 Younger service members, who may be less likely to prioritize preventive behaviors, may be particularly responsive to sun safety reminders in training areas, bases, and deployment zones.7 Health fairs and orientation briefs in high-UV regions also offer potential opportunities for targeted education.
Resources for Sun Protection in the Military
Sunscreen—Although sunscreen is critical in minimizing the risk for UV-induced skin cancer, its widespread use in the military is hindered by practical challenges related to accessibility and the need for consistent reapplication; for instance, providing free sunscreen dispensers at institutions for staff working under intense or prolonged UV exposure may improve sunscreen accessibility and use.8 Including sunscreen in standard-issue gear offers another logical way to embed its use into operational readiness as part of the routine protective measures.
Uniform Modifications—Adapting military uniforms and practices to improve sun protection plays a critical role in reducing skin cancer risk. Targeted protective gear for commonly sun-exposed areas can help mitigate UV exposure. One practical option is the use of wide-brimmed headgear (eg, boonie hats), which provide more face and neck coverage than standard-issue military caps, or covers. The wide-brimmed headgear currently is only selectively authorized during specific scenarios, such as field operations and training exercises, or at the discretion of unit-level leadership. Wide-brimmed headgear, already used by many service members, has been associated with up to a 17% reduction in UV exposure to inadequately protected areas, potentially lowering skin cancer risk.9,10 Similarly, a “sleeves-down” policy—requiring sleeves to remain unrolled and covering the forearms during outdoor activities—offers a simple way to minimize sun exposure without necessitating additional gear. Other specialized clothing items, including UV-blocking neck gaiters, photoprotective clothing, and lightweight gloves, also may be appropriate for high-risk groups and can be implemented in a relatively straightforward manner.
Shade Structures and UV Index Monitoring—Aside from uniform adaptation, physical barrier intervention can further complement skin cancer prevention efforts in the military. Shade structures offer a straightforward way to reduce UV exposure during prolonged outdoor activities. Incorporating daily UV index monitoring into operational guidance can help inform adjustments to training schedules and guide the implementation of additional sun protection measures, such as mandatory sunscreen application, use of wide-brimmed hats, or increased access to shaded rest areas during heavy sunlight hours. Currently, outdoor physical training is restricted during periods of high heat index, measured via Wet Bulb Globe Temperature, to reduce heat-related injuries. We argue that avoidance of nonoperational outdoor activity during peak UV index hours also should be incorporated into standardized policies. This intervention is of particular benefit to service members stationed in regions with a high UV index year-round, such as those stationed in the Middle East, Guam, Okinawa, and southern coastal United States bases.
Policy Changes to Support Photoprotective Measures
Annual Risk Factor Screening‐Screening—Effective secondary prevention efforts by military dermatologists remain an important measure in reducing the burden of skin cancer among military personnel; however, these efforts have become increasingly challenging due to 2 main factors—the diversity of military occupational specialties and their associated unique occupational risks as well as the limited availability of military dermatologists across all branches (approximately 100 active-duty dermatologists for nearly 3 million service members).11 Therefore, targeted interventions that enhance risk assessment, refined screening protocols, and leveraging of existing military health networks can improve early skin cancer detection while optimizing resource allocation.
The ePHA is an online screening tool used annually by all service members to evaluate their overall health. Presently, the ePHA lacks specific questions to assess sun exposure and skin cancer risks. Integrating annual skin cancer risk factor assessments into the ePHA would offer a practical and straightforward approach to identifying at-risk individuals, as suggested by Newnam et al12 in 2022. Skin cancer risk factor assessments allow for targeted data collection related to sun exposure history, family history, and personal risk factors, which can be used to determine individualized risk stratification to assess the need for early secondary prevention measures and specialist referral. These ePHA data can also support population-based analyses to inform preventive strategies and address knowledge gaps related to high-risk exposures, such as extended field exercises or assignments in high-UV regions, that may impede effective skin cancer prevention.
Development of Military-Specific Screening Guidelines—Given the limited number of military dermatologists, a standardized risk-assessment tool could enhance early detection of skin cancer and streamline the referral process. We propose a military-specific skin cancer screening algorithm or risk nomogram that could help to consolidate risk factors into a clear and actionable framework for more efficient triage and appropriate allocation of dermatologic resources and manpower. This nomogram could be developed by military dermatologists and then implemented on a command level, affording primary care providers a useful tool to expedite evaluation of individuals at higher risk for skin cancer while simultaneously promoting judicious use of limited dermatology resources.
Although the United States Preventive Services Task Force does not universally recommend routine skin cancer screenings for asymptomatic adults, military service members are exposed to higher occupational risks than the general population, as previously mentioned. Currently, there is no standardized screening guideline across all military services due to the unique nature and exposure risks for each branch of service and their varied occupations; however, we propose the development of basic standardized screening guidelines by adapting the framework of the United States Preventive Services Task Force and adjusting for military-specific UV exposure and occupational risks to improve early detection of skin cancer. These guidelines could be updated and tailored appropriately when additional population-based data are collected and analyzed through ePHA.
Critiques and Limitations of Implementation
Several challenges and limitations must be considered when attempting to integrate large-scale preventive measures for skin cancer within the US military. A primary concern is the extent to which military resources should be allocated to prevention when off-duty sun exposure remains largely beyond institutional control. Although military health initiatives can address workplace risk through education and policy, individual decisions during both work and leisure time remain a major variable that cannot be feasibly controlled. Cultural and operational barriers also pose challenges; for instance, the US Marine Corps maintains a strong cultural identity tied to uniform appearance, making it difficult to implement widespread changes to clothing-based sun-protection measures. Institutional changes, particularly those involving uniforms, likely will face substantial administrative resistance and potential operational limitations. When broad uniform modifications are unattainable, a more feasible approach may be to encourage unit-level leadership to authorize and promote the frequent use of nonuniform protective measures.
Furthermore, integrating additional skin cancer risk questions into the already extensive ePHA means extra time required to complete the assessment; this adds to service members’ administrative burden, potentially leading to reduced timely compliance, rushed responses, and survey fatigue, which threaten data quality. If new items are to be included, they should be carefully selected for efficiency and clinical relevance. Existing validated questionnaires such as those from the study by Lyford et al7 published in 2021 can serve as a foundation.
Another critical limitation is access to dermatologic care for active-duty service members. Raising awareness of skin cancer risk without ensuring adequate resources may create ethical concerns, particularly in high-risk environments such as the Middle East and Indo-Pacific. Additionally, because skin cancer often develops years or decades after exposure, securing early buy-in from service members and their leaders can be challenging. These concerns make it clear that, while skin cancer prevention is important, implementing widespread measures is not straightforward and requires a practical and balanced approach.
Final Thoughts
Implementing prevention strategies for skin cancer in the military requires balancing evidence-based recommendations with the practical realities of military culture, resource limitations, and operational demands. Challenges remain for dermatologists in providing targeted recommendations due to the multifaceted nature of military roles, including over 150 Navy Military Occupational Specialties, limited familiarity with the unique UV exposure risks associated with each occupation, and variability in local and regional policies on uniform wear, physical training requirements, and other operational practices. Although targeted prevention measures are difficult to establish in the setting of these knowledge gaps, leveraging unit-level leadership to align with existing screening guidelines and optimizing primary prevention measures can be meaningful steps toward reducing skin cancer risk for military service members while maintaining mission readiness.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancerincidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Krivda KR, Watson NL, Lyford WH, et al. The burden of skin cancer in the military health system, 2017-2022. Cutis. 2024;113:200-215. doi:10.12788/cutis.1015
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58. doi:10.1001/jamadermatol.2014.1077
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MMSR. 2017;24:8-14.
- Subramaniam P, Olsen CM, Thompson BS, et al, for the QSkin Sun and Health Study Investigators. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182. doi:10.1001/jamadermatol.2016.4070
- Lyford WH, Crotty A, Logemann NF. Sun exposure prevention practices within U.S. naval aviation. Mil Med. 2021;186:1169-1175. doi:10.1093/milmed/usab099
- Wood M, Raisanen T, Polcari I. Observational study of free public sunscreen dispenser use at a major US outdoor event. J Am Acad Dermatol. 2017;77:164-166.
- Schissel D. Operation shadow warrior: a quantitative analysis of the ultraviolet radiation protection demonstrated by various headgear. Mil Med. 2001;166:783-785.
- Milch JM, Logemann NF. Photoprotection prevents skin cancer: let’s make it fashionable to wear sun-protective clothing. Cutis. 2017;99:89-92.
- Association of Military Dermatologists. (n.d.). Military dermatology. https://militaryderm.org/military-dermatology/
- Newnam R, Le-Jenkins U, Rutledge C, et al. The association of skin cancer prevention knowledge, sun-protective attitudes, and sunprotective behaviors in a Navy population. Mil Med. 2024;189:1-7. doi:10.1093/milmed/usac285
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancerincidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Krivda KR, Watson NL, Lyford WH, et al. The burden of skin cancer in the military health system, 2017-2022. Cutis. 2024;113:200-215. doi:10.12788/cutis.1015
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58. doi:10.1001/jamadermatol.2014.1077
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MMSR. 2017;24:8-14.
- Subramaniam P, Olsen CM, Thompson BS, et al, for the QSkin Sun and Health Study Investigators. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182. doi:10.1001/jamadermatol.2016.4070
- Lyford WH, Crotty A, Logemann NF. Sun exposure prevention practices within U.S. naval aviation. Mil Med. 2021;186:1169-1175. doi:10.1093/milmed/usab099
- Wood M, Raisanen T, Polcari I. Observational study of free public sunscreen dispenser use at a major US outdoor event. J Am Acad Dermatol. 2017;77:164-166.
- Schissel D. Operation shadow warrior: a quantitative analysis of the ultraviolet radiation protection demonstrated by various headgear. Mil Med. 2001;166:783-785.
- Milch JM, Logemann NF. Photoprotection prevents skin cancer: let’s make it fashionable to wear sun-protective clothing. Cutis. 2017;99:89-92.
- Association of Military Dermatologists. (n.d.). Military dermatology. https://militaryderm.org/military-dermatology/
- Newnam R, Le-Jenkins U, Rutledge C, et al. The association of skin cancer prevention knowledge, sun-protective attitudes, and sunprotective behaviors in a Navy population. Mil Med. 2024;189:1-7. doi:10.1093/milmed/usac285
Bridging the Knowledge-Action Gap in Skin Cancer Prevention Among US Military Personnel
Bridging the Knowledge-Action Gap in Skin Cancer Prevention Among US Military Personnel
PRACTICE POINTS
- Military personnel face elevated skin cancer risks due to prolonged occupational UV exposure.
- Medical providers can partner with unit-level leadership to implement low-cost interventions such as shade structures and uniform modifications.
- Annual sun exposure risk assessments should be integrated into the military Electronic Periodic Health Assessment for targeted screening and early intervention of risk factors.
- Photoprotective gear and signage in high—UV index areas can improve service member awareness and adherence to preventive measures.
Gastroenterology Data Trends 2025
Gastroenterology Data Trends 2025
GI & Hepatology News and the American Gastroenterological Association (AGA) present Gastroenterology Data Trends 2025, a special report on hot topics in GI told through original infographics and visual storytelling.
In this issue:
The Role of Bedside Intestinal Ultrasound in IBD Management
Bincy Abraham, MD, MS
Obesity Management in the Era of GLP-1: The Role of GLP-1 RAs
Michael Camilleri, MD, MPhil, DSc
Ergonomics in Endoscopy
Amandeep K. Shergill, MD, MS
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Kira Newman, MD, PhD
New Therapeutic Frontiers in the Treatment of Eosinophilic Esophagitis
Evan S. Dellon, MD, MPH
New and Emerging Treatments for MASLD/MASH
Naim Alkhouri, MD
Advances in Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma
Joel Rubenstein, MD, MS
Alagille Syndrome: Epidemiology and Management of a Rare Genetic Disease
Alisha Mavis, MD
IBS: Mental Health Factors and Comorbidities
Lin Chang, MD, and Laurie A. Keefer, PhD
GI & Hepatology News and the American Gastroenterological Association (AGA) present Gastroenterology Data Trends 2025, a special report on hot topics in GI told through original infographics and visual storytelling.
In this issue:
The Role of Bedside Intestinal Ultrasound in IBD Management
Bincy Abraham, MD, MS
Obesity Management in the Era of GLP-1: The Role of GLP-1 RAs
Michael Camilleri, MD, MPhil, DSc
Ergonomics in Endoscopy
Amandeep K. Shergill, MD, MS
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Kira Newman, MD, PhD
New Therapeutic Frontiers in the Treatment of Eosinophilic Esophagitis
Evan S. Dellon, MD, MPH
New and Emerging Treatments for MASLD/MASH
Naim Alkhouri, MD
Advances in Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma
Joel Rubenstein, MD, MS
Alagille Syndrome: Epidemiology and Management of a Rare Genetic Disease
Alisha Mavis, MD
IBS: Mental Health Factors and Comorbidities
Lin Chang, MD, and Laurie A. Keefer, PhD
GI & Hepatology News and the American Gastroenterological Association (AGA) present Gastroenterology Data Trends 2025, a special report on hot topics in GI told through original infographics and visual storytelling.
In this issue:
The Role of Bedside Intestinal Ultrasound in IBD Management
Bincy Abraham, MD, MS
Obesity Management in the Era of GLP-1: The Role of GLP-1 RAs
Michael Camilleri, MD, MPhil, DSc
Ergonomics in Endoscopy
Amandeep K. Shergill, MD, MS
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Kira Newman, MD, PhD
New Therapeutic Frontiers in the Treatment of Eosinophilic Esophagitis
Evan S. Dellon, MD, MPH
New and Emerging Treatments for MASLD/MASH
Naim Alkhouri, MD
Advances in Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma
Joel Rubenstein, MD, MS
Alagille Syndrome: Epidemiology and Management of a Rare Genetic Disease
Alisha Mavis, MD
IBS: Mental Health Factors and Comorbidities
Lin Chang, MD, and Laurie A. Keefer, PhD
Gastroenterology Data Trends 2025
Gastroenterology Data Trends 2025
Repair of a Large Full-Thickness Conchal Bowl Defect
Repair of a Large Full-Thickness Conchal Bowl Defect
Practice Gap
Large full-thickness conchal bowl defects often pose a reconstructive challenge. Maintaining the shape and structural integrity of the concha is fundamental for optimal cosmetic and functional outcomes. Prior reports have suggested wedge excisions, composite grafts, interpolation flaps with or without cartilage struts, and hinge flaps as possible options for reconstruction.1-3 However, patients with large defects who prefer single-stage reconstruction procedures present a unique challenge. Herein, we describe a single-stage full-thickness hinge flap technique for a large conchal bowl defect.
The Technique
A 77-year-old man was referred to our dermatology clinic by an outside dermatologist for Mohs micrographic surgery of a biopsy-proven cutaneous squamous cell carcinoma on the right conchal bowl measuring 1.1×2.1 cm and extending to the edge of the external auditory canal (EAC). The excision was performed that same day and was completed in 2 stages, achieving negative margins and resulting in a full-thickness defect measuring 2.0×3.6 cm that included the posterior auricular sulcus, cavum, antitragus, and proximal EAC (Figure 1). The patient requested a single-stage procedure but emphasized that his main priority was an optimal cosmetic outcome.
To repair this large defect, a full-thickness hinge flap with Burow graft was performed. The hinge-type flap was designed in a triangular fashion emanating at the posterior auricular sulcus adjacent to the posterior aspect of the defect and extending down the lateral neck (Figure 2). The flap was incised and the surrounding tissue was undermined, maintaining a robust pedicle in the center of its body on the superolateral neck. The flap was passed through the posterior aspect of the full-thickness defect and was secured in place with 4-0 polyglactin sutures in a buried interrupted fashion, thereby recreating the anterior portion of the defect. The superficial skin edges were reapproximated using 4-0 and 5-0 polypropylene sutures in a running interrupted fashion. The distal Burow triangle created from closure of the flap’s secondary defect was aggressively thinned and was utilized as a full-thickness graft for the residual postauricular groove defect (Figure 3). At 2 weeks’ follow-up, the patient was healing well with no postoperative issues and the sutures were removed (Figure 4).
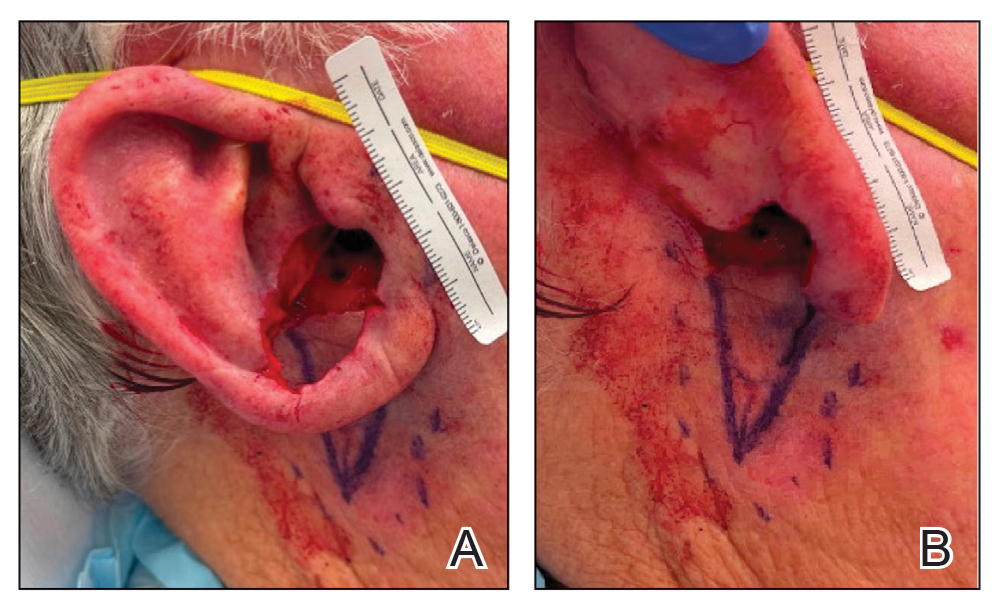
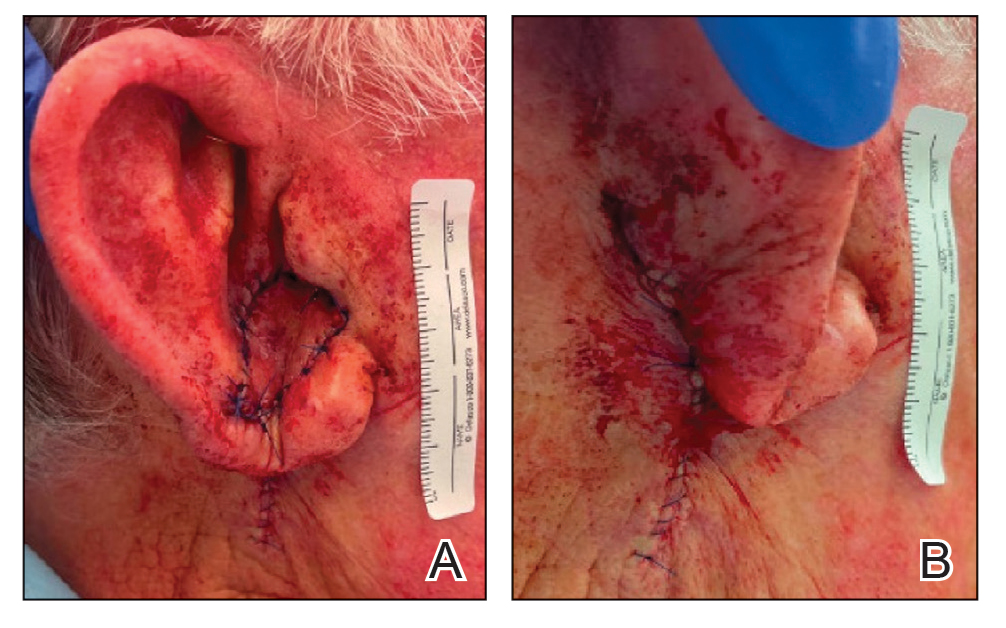
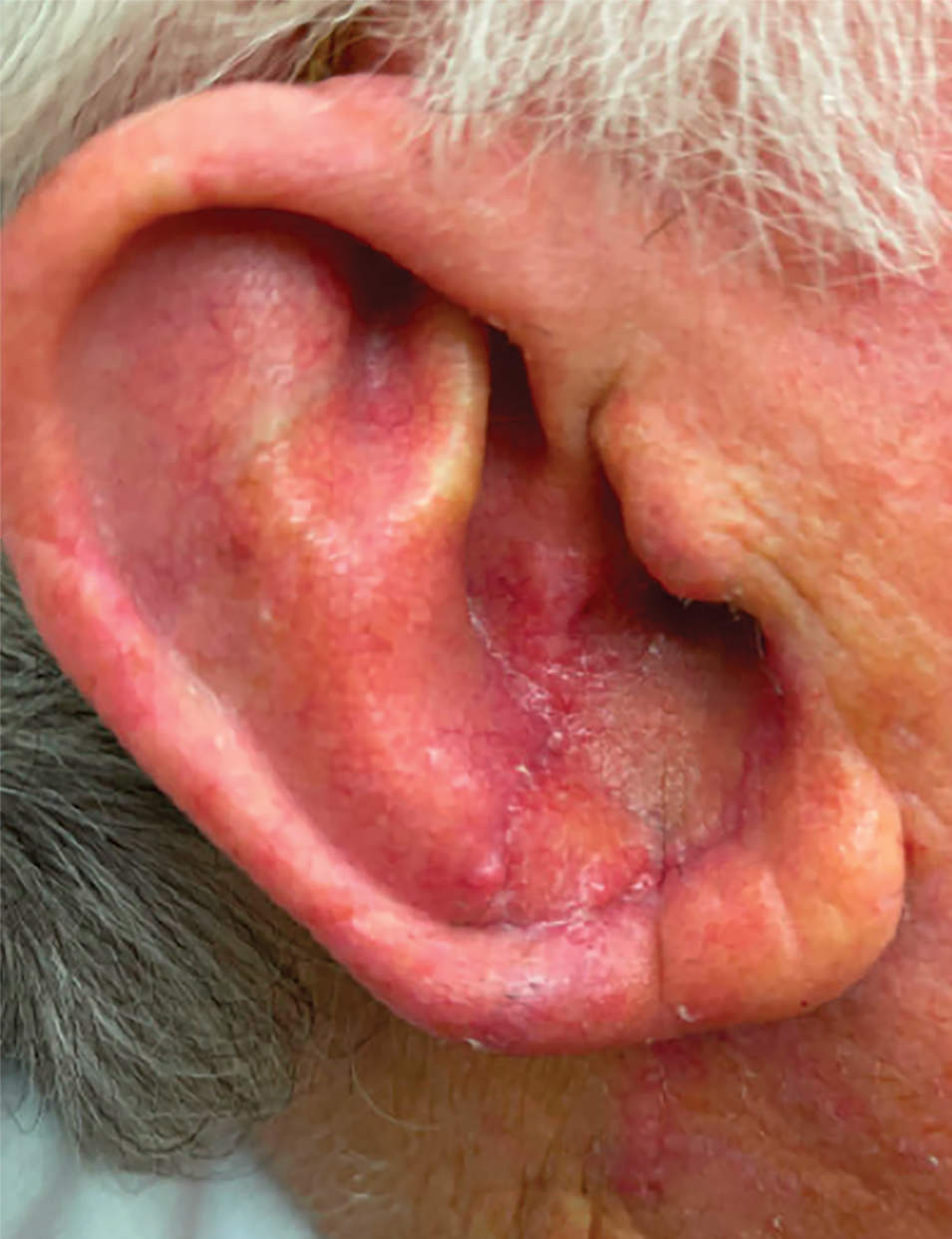
Practice Implications
There are many different reconstructive options for conchal bowl defects, including primary repair, wedge excision, composite graft and interpolation flaps with or without cartilage struts, and hinge flaps. Structural support, EAC patency, auricle symmetry, overall auricle size, and re-creation of natural contours were considered when designing the reconstruction of the defect in our patient; however, his main priority was achieving the greatest cosmetic outcome in a single-stage procedure, therefore limiting our reconstruction options.
Wedge excision, in which the residual lobule and inferior helical rim are removed, could have been considered in our patient but would have drastically altered the symmetry of the size of the ears. A folded postauricular flap, as described in the otolaryngology literature, is an interpolation flap based on the posterior auricular artery that was designed for full-thickness defects of the auricle to prevent any posterior pinning.1 This technique may have worked well in our case, but the patient preferred to avoid a multistage procedure. Additionally, the positional symmetry of the ears was maintained despite utilizing a hinge flap, which does not involve takedown of the pedicle. A composite graft from the contralateral ear could be considered for smaller conchal bowl defects but likely would have resulted in graft failure in our patient’s large defect due to its need for rich blood supply to heal and dependence on lateral wound edges. Cartilage struts in conjunction with a flap could have been considered in this scenario for greater structural support, but in our patient’s case, by maintaining the robust pedicle of our flap and having residual superior cartilage, further structural support was not necessary.
A prior case report described a partial and full-thickness defect in a similar location that was repaired with a retroauricular hinge flap, in which a portion of the flap was extensively de-epithelialized to address the varied thicknesses of the surgical defect.2 In our patient, the defect abutted the skin reservoir on the superolateral neck, and therefore no de-epithelialization was required as the entire epithelialized portion was utilized to recreate the anterior aspect of the defect. Postauricular hinge-type flaps are a reliable, single-stage surgical alternative to the 2-stage folded postauricular interpolation flap when reconstructing large conchal bowl defects. For small full-thickness defects of the ear, a composite graft may be considered; however, blood supply and other nutritional requirements limit this option for large full-thickness defects.
- Roche AM, Griffin M, Shelton R, et al. The folded postauricular flap: a novel approach to reconstruction of large full thickness defects of the conchal bowl. Am J Otolaryngol. 2017;38:706-709. doi:10.1016 /j.amjoto.2017.09.006
- Klein JC, Nijhawan RI. Retroauricular hinge flaps for full-thickness conchal bowl defects. J Am Acad Dermatol. 2024;90:E71-E72. doi:10.1016/j.jaad.2022.10.056
- Pickrell BB, Hughes CD, Maricevich RS. Partial ear defects. Semin Plast Surg. 2017 Aug;31:134-140. doi:10.1055/s-0037-1603968.
Practice Gap
Large full-thickness conchal bowl defects often pose a reconstructive challenge. Maintaining the shape and structural integrity of the concha is fundamental for optimal cosmetic and functional outcomes. Prior reports have suggested wedge excisions, composite grafts, interpolation flaps with or without cartilage struts, and hinge flaps as possible options for reconstruction.1-3 However, patients with large defects who prefer single-stage reconstruction procedures present a unique challenge. Herein, we describe a single-stage full-thickness hinge flap technique for a large conchal bowl defect.
The Technique
A 77-year-old man was referred to our dermatology clinic by an outside dermatologist for Mohs micrographic surgery of a biopsy-proven cutaneous squamous cell carcinoma on the right conchal bowl measuring 1.1×2.1 cm and extending to the edge of the external auditory canal (EAC). The excision was performed that same day and was completed in 2 stages, achieving negative margins and resulting in a full-thickness defect measuring 2.0×3.6 cm that included the posterior auricular sulcus, cavum, antitragus, and proximal EAC (Figure 1). The patient requested a single-stage procedure but emphasized that his main priority was an optimal cosmetic outcome.

To repair this large defect, a full-thickness hinge flap with Burow graft was performed. The hinge-type flap was designed in a triangular fashion emanating at the posterior auricular sulcus adjacent to the posterior aspect of the defect and extending down the lateral neck (Figure 2). The flap was incised and the surrounding tissue was undermined, maintaining a robust pedicle in the center of its body on the superolateral neck. The flap was passed through the posterior aspect of the full-thickness defect and was secured in place with 4-0 polyglactin sutures in a buried interrupted fashion, thereby recreating the anterior portion of the defect. The superficial skin edges were reapproximated using 4-0 and 5-0 polypropylene sutures in a running interrupted fashion. The distal Burow triangle created from closure of the flap’s secondary defect was aggressively thinned and was utilized as a full-thickness graft for the residual postauricular groove defect (Figure 3). At 2 weeks’ follow-up, the patient was healing well with no postoperative issues and the sutures were removed (Figure 4).



Practice Implications
There are many different reconstructive options for conchal bowl defects, including primary repair, wedge excision, composite graft and interpolation flaps with or without cartilage struts, and hinge flaps. Structural support, EAC patency, auricle symmetry, overall auricle size, and re-creation of natural contours were considered when designing the reconstruction of the defect in our patient; however, his main priority was achieving the greatest cosmetic outcome in a single-stage procedure, therefore limiting our reconstruction options.
Wedge excision, in which the residual lobule and inferior helical rim are removed, could have been considered in our patient but would have drastically altered the symmetry of the size of the ears. A folded postauricular flap, as described in the otolaryngology literature, is an interpolation flap based on the posterior auricular artery that was designed for full-thickness defects of the auricle to prevent any posterior pinning.1 This technique may have worked well in our case, but the patient preferred to avoid a multistage procedure. Additionally, the positional symmetry of the ears was maintained despite utilizing a hinge flap, which does not involve takedown of the pedicle. A composite graft from the contralateral ear could be considered for smaller conchal bowl defects but likely would have resulted in graft failure in our patient’s large defect due to its need for rich blood supply to heal and dependence on lateral wound edges. Cartilage struts in conjunction with a flap could have been considered in this scenario for greater structural support, but in our patient’s case, by maintaining the robust pedicle of our flap and having residual superior cartilage, further structural support was not necessary.
A prior case report described a partial and full-thickness defect in a similar location that was repaired with a retroauricular hinge flap, in which a portion of the flap was extensively de-epithelialized to address the varied thicknesses of the surgical defect.2 In our patient, the defect abutted the skin reservoir on the superolateral neck, and therefore no de-epithelialization was required as the entire epithelialized portion was utilized to recreate the anterior aspect of the defect. Postauricular hinge-type flaps are a reliable, single-stage surgical alternative to the 2-stage folded postauricular interpolation flap when reconstructing large conchal bowl defects. For small full-thickness defects of the ear, a composite graft may be considered; however, blood supply and other nutritional requirements limit this option for large full-thickness defects.
Practice Gap
Large full-thickness conchal bowl defects often pose a reconstructive challenge. Maintaining the shape and structural integrity of the concha is fundamental for optimal cosmetic and functional outcomes. Prior reports have suggested wedge excisions, composite grafts, interpolation flaps with or without cartilage struts, and hinge flaps as possible options for reconstruction.1-3 However, patients with large defects who prefer single-stage reconstruction procedures present a unique challenge. Herein, we describe a single-stage full-thickness hinge flap technique for a large conchal bowl defect.
The Technique
A 77-year-old man was referred to our dermatology clinic by an outside dermatologist for Mohs micrographic surgery of a biopsy-proven cutaneous squamous cell carcinoma on the right conchal bowl measuring 1.1×2.1 cm and extending to the edge of the external auditory canal (EAC). The excision was performed that same day and was completed in 2 stages, achieving negative margins and resulting in a full-thickness defect measuring 2.0×3.6 cm that included the posterior auricular sulcus, cavum, antitragus, and proximal EAC (Figure 1). The patient requested a single-stage procedure but emphasized that his main priority was an optimal cosmetic outcome.

To repair this large defect, a full-thickness hinge flap with Burow graft was performed. The hinge-type flap was designed in a triangular fashion emanating at the posterior auricular sulcus adjacent to the posterior aspect of the defect and extending down the lateral neck (Figure 2). The flap was incised and the surrounding tissue was undermined, maintaining a robust pedicle in the center of its body on the superolateral neck. The flap was passed through the posterior aspect of the full-thickness defect and was secured in place with 4-0 polyglactin sutures in a buried interrupted fashion, thereby recreating the anterior portion of the defect. The superficial skin edges were reapproximated using 4-0 and 5-0 polypropylene sutures in a running interrupted fashion. The distal Burow triangle created from closure of the flap’s secondary defect was aggressively thinned and was utilized as a full-thickness graft for the residual postauricular groove defect (Figure 3). At 2 weeks’ follow-up, the patient was healing well with no postoperative issues and the sutures were removed (Figure 4).



Practice Implications
There are many different reconstructive options for conchal bowl defects, including primary repair, wedge excision, composite graft and interpolation flaps with or without cartilage struts, and hinge flaps. Structural support, EAC patency, auricle symmetry, overall auricle size, and re-creation of natural contours were considered when designing the reconstruction of the defect in our patient; however, his main priority was achieving the greatest cosmetic outcome in a single-stage procedure, therefore limiting our reconstruction options.
Wedge excision, in which the residual lobule and inferior helical rim are removed, could have been considered in our patient but would have drastically altered the symmetry of the size of the ears. A folded postauricular flap, as described in the otolaryngology literature, is an interpolation flap based on the posterior auricular artery that was designed for full-thickness defects of the auricle to prevent any posterior pinning.1 This technique may have worked well in our case, but the patient preferred to avoid a multistage procedure. Additionally, the positional symmetry of the ears was maintained despite utilizing a hinge flap, which does not involve takedown of the pedicle. A composite graft from the contralateral ear could be considered for smaller conchal bowl defects but likely would have resulted in graft failure in our patient’s large defect due to its need for rich blood supply to heal and dependence on lateral wound edges. Cartilage struts in conjunction with a flap could have been considered in this scenario for greater structural support, but in our patient’s case, by maintaining the robust pedicle of our flap and having residual superior cartilage, further structural support was not necessary.
A prior case report described a partial and full-thickness defect in a similar location that was repaired with a retroauricular hinge flap, in which a portion of the flap was extensively de-epithelialized to address the varied thicknesses of the surgical defect.2 In our patient, the defect abutted the skin reservoir on the superolateral neck, and therefore no de-epithelialization was required as the entire epithelialized portion was utilized to recreate the anterior aspect of the defect. Postauricular hinge-type flaps are a reliable, single-stage surgical alternative to the 2-stage folded postauricular interpolation flap when reconstructing large conchal bowl defects. For small full-thickness defects of the ear, a composite graft may be considered; however, blood supply and other nutritional requirements limit this option for large full-thickness defects.
- Roche AM, Griffin M, Shelton R, et al. The folded postauricular flap: a novel approach to reconstruction of large full thickness defects of the conchal bowl. Am J Otolaryngol. 2017;38:706-709. doi:10.1016 /j.amjoto.2017.09.006
- Klein JC, Nijhawan RI. Retroauricular hinge flaps for full-thickness conchal bowl defects. J Am Acad Dermatol. 2024;90:E71-E72. doi:10.1016/j.jaad.2022.10.056
- Pickrell BB, Hughes CD, Maricevich RS. Partial ear defects. Semin Plast Surg. 2017 Aug;31:134-140. doi:10.1055/s-0037-1603968.
- Roche AM, Griffin M, Shelton R, et al. The folded postauricular flap: a novel approach to reconstruction of large full thickness defects of the conchal bowl. Am J Otolaryngol. 2017;38:706-709. doi:10.1016 /j.amjoto.2017.09.006
- Klein JC, Nijhawan RI. Retroauricular hinge flaps for full-thickness conchal bowl defects. J Am Acad Dermatol. 2024;90:E71-E72. doi:10.1016/j.jaad.2022.10.056
- Pickrell BB, Hughes CD, Maricevich RS. Partial ear defects. Semin Plast Surg. 2017 Aug;31:134-140. doi:10.1055/s-0037-1603968.
Repair of a Large Full-Thickness Conchal Bowl Defect
Repair of a Large Full-Thickness Conchal Bowl Defect
Papulonodules on the Ankle in a Patient with Lung Cancer
Papulonodules on the Ankle in a Patient with Lung Cancer
THE DIAGNOSIS: Pembrolizumab-Induced Eruptive Squamous Proliferation
Histopathology showed a broad squamous proliferation with acanthosis of the epidermis. Large glassy keratinocytes were seen with scattered necrotic keratinocytes (Figure), and a dense lichenoid band of inflammation was present subjacent to the proliferation. Notably, no hypergranulosis, remarkable keratinocyte atypia, or increased mitotic figures were seen. Based on the patient’s medical history and biopsy results, a diagnosis of pembrolizumab-induced eruptive squamous proliferation was made. The diagnosis was supported by a growing body of evidence of this type of reaction in patients taking programmed death 1 (PD-1) inhibitors.1,2 Conservative treatment with clobetasol ointment 0.05% was initiated with complete resolution of the lesions at the 2-month follow-up appointment. Other common treatments include topical steroids, injected corticosteroids, or cryosurgery to locally control the inflammation and atypical proliferation of cells.3
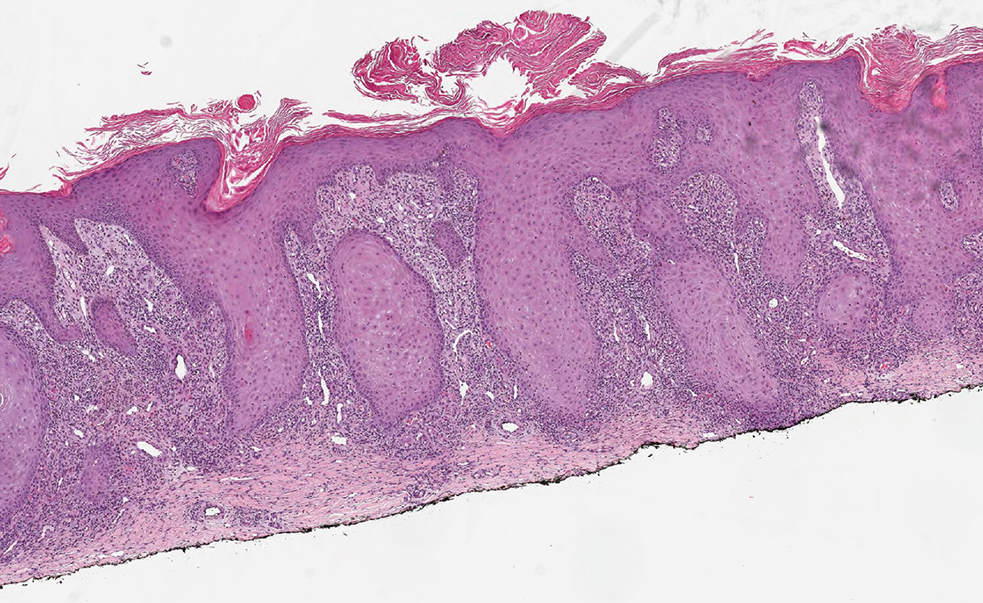
Pembrolizumab is a humanized IgG4 monoclonal antibody targeting the PD-1 receptor that has been utilized for its antitumor activity against various cancers, including unresectable and metastatic melanoma, head and neck cancers, and non–small cell lung cancer (NSCLC).1,4,5 While this drug has extended the lives of many patients with cancer, there are adverse reactions associated with PD-1 inhibitors (eg, pembrolizumab, nivolumab). Skin toxicity to PD-1 inhibitors is the one of most common immune-mediated reactions worldwide, occurring in approximately 30% of patients.6,7 Reactions can occur while a patient is taking the inciting drug and can continue up to 2 months after treatment discontinuation.8 Skin reactions associated with PD-1 inhibitors vary from lichenoid reactions and vitiligolike patches to psoriasis or eczema flares and are organized into 4 categories: inflammatory, immunobullous, alteration of keratinocytes, and alteration of melanocytes.9 Our patient demonstrated alteration of keratinocytes, which is characterized by overlapping features of hypertrophic lichen planus and early keratoacanthoma.
The differential diagnoses for pembrolizumab-induced eruptive squamous proliferation include squamous cell carcinoma (SCC), psoriasis, hypertrophic lichen planus, and cutaneous metastasis of NSCLC. Hypertrophic lupus erythematosus also is a well-documented reaction to use of immune-checkpoint inhibitors.10 Direct immunofluorescence could have helped differentiate hypertrophic lupus erythematosus from an eruptive squamous proliferation in our patient; however, due to her response to treatment, no additional workup was done.
Squamous cell carcinoma, which is the most common type of skin cancer in Black patients in the United States,11 has been shown to manifest after a PD-1 inhibitor is taken.12 Although it typically has a more chronic persistent course, the clinical appearance of SCC can be similar to the findings seen in our patient. Histologically, SCC may demonstrate necrosis, but the atypical proliferations will invade the dermis—a feature not seen in our patient’s histopathology.13
Lichen planus (LP) is an eruptive immune reaction of violaceous polygonal papules and plaques commonly seen on the ankles14 that has been shown to be an adverse effect of pembrolizumab.15 There are several subtypes of LP including hypertrophic versions, which can appear clinically similar to the findings seen in our patient. On dermoscopy, the classic finding of white lines, known as Wickham striae, is seen in all subtypes and can help diagnose this pathologic process. Under the microscope, LP can manifest with hyperkeratosis without parakeratosis, irregular thickening of the stratum granulosum, sawtooth rete ridges, and destruction of the basal layer.14
Psoriasis also has been shown to be exacerbated by anti–PD-1 therapy, although the majority of patients diagnosed with PD-1–induced psoriasis have a personal or family history of the disease.6 Clinically, psoriasis can have a hyperpigmented or violaceous appearance in patients with skin of color.16 The histopathology of psoriasis typically reveals confluent parakeratosis, neutrophils in the stratum corneum, regular acanthosis, thinning of the suprapapillary plates, and vessels in the dermal papillae.17
Although cutaneous metastasis of NSCLC may appear clinically similar to the current case, it is one of the rarer organ sites of metastasis for lung cancer.18 In our patient, biopsy quickly ruled out this diagnosis. If it had been a site of metastasis, histopathology would have shown a dermal-based proliferation of dysplastic cells without epidermal connection.19
It is important for dermatologists to recognize eruptive squamous proliferations associated with pembrolizumab, as they often respond to conservative treatment and typically do not require dose reduction or discontinuation of the inciting drug.
- Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43. doi:10.1186/s40425-017-0242-5
- Preti BTB, Pencz A, Cowger JJM, et al. Skin deep: a fascinating case report of immunotherapy-triggered, treatment-refractory autoimmune lichen planus and keratoacanthoma. Case Rep Oncol. 2021;14: 1189-1193. doi:10.1159/000518313
- Fradet M, Sibaud V, Tournier E, et al. Multiple keratoacanthoma-like lesions in a patient treated with pembrolizumab. Acta Derm Venereol. 2019;99:1301-1302. doi:10.2340/00015555-3301
- Flynn JP, Gerriets V. Pembrolizumab. StatPearls [Internet]. StatPearls Publishing; 2023. Updated June 26, 2023. Accessed April 2, 2025.
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with Nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Voudouri D, Nikolaou V, Laschos K, et al. Anti-Pd1/Pdl1 induced psoriasis. Curr Probl Cancer. 2017;41:407-412. doi:10.1016 /j.currproblcancer.2017.10.003
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Coscarart A, Martel J, Lee MP, et al. Pembrolizumab-induced pseudoepitheliomatous eruption consistent with hypertrophic lichen planus. J Cutan Pathol. 2020;47:275-279. doi:10.1111/cup.13587
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176. doi:10.1111/cup.12858
- Vitzthum von Eckstaedt H, Singh A, Reid P, et al. Immune checkpoint inhibitors and lupus erythematosus. Pharmaceuticals (Basel). 2024;2:15;17. doi:10.3390/ph17020252
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Vu M, Chapman S, Lenz B, et al. Squamous cell carcinoma or squamous proliferation associated with nivolumab treatment for metastatic melanoma. Dermatol Online J. 2022;6:28. doi:10.5070/d328357786
- Howell JY, Ramsey ML. Squamous cell skin cancer. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 2, 2024. Accessed April 2, 2025.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. StatPearls Publishing; 2024. Updated October 29, 2024. Accessed April 2, 2025.
- Yamashita A, Akasaka E, Nakano H, et al. Pembrolizumab-induced lichen planus on the scalp of a patient with non-small-cell lung carcinoma. Case Rep Dermatol. 2021;13:487-491. doi:10.1159/000519486
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Murphy M, Kerr P, Grant-Kels JM. The histopathologic spectrum of psoriasis. Clin Dermatol. 2007;25:524-528. doi:10.1016 /j.clindermatol.2007.08.005.
- Hidaka T, Ishii Y, Kitamura S. Clinical features of skin metastasis from lung cancer. Intern Med. 1996;35:459-462. doi:10.2169 /internalmedicine.35.459.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613–620. doi:10.1001/archderm.143.5.613
THE DIAGNOSIS: Pembrolizumab-Induced Eruptive Squamous Proliferation
Histopathology showed a broad squamous proliferation with acanthosis of the epidermis. Large glassy keratinocytes were seen with scattered necrotic keratinocytes (Figure), and a dense lichenoid band of inflammation was present subjacent to the proliferation. Notably, no hypergranulosis, remarkable keratinocyte atypia, or increased mitotic figures were seen. Based on the patient’s medical history and biopsy results, a diagnosis of pembrolizumab-induced eruptive squamous proliferation was made. The diagnosis was supported by a growing body of evidence of this type of reaction in patients taking programmed death 1 (PD-1) inhibitors.1,2 Conservative treatment with clobetasol ointment 0.05% was initiated with complete resolution of the lesions at the 2-month follow-up appointment. Other common treatments include topical steroids, injected corticosteroids, or cryosurgery to locally control the inflammation and atypical proliferation of cells.3

Pembrolizumab is a humanized IgG4 monoclonal antibody targeting the PD-1 receptor that has been utilized for its antitumor activity against various cancers, including unresectable and metastatic melanoma, head and neck cancers, and non–small cell lung cancer (NSCLC).1,4,5 While this drug has extended the lives of many patients with cancer, there are adverse reactions associated with PD-1 inhibitors (eg, pembrolizumab, nivolumab). Skin toxicity to PD-1 inhibitors is the one of most common immune-mediated reactions worldwide, occurring in approximately 30% of patients.6,7 Reactions can occur while a patient is taking the inciting drug and can continue up to 2 months after treatment discontinuation.8 Skin reactions associated with PD-1 inhibitors vary from lichenoid reactions and vitiligolike patches to psoriasis or eczema flares and are organized into 4 categories: inflammatory, immunobullous, alteration of keratinocytes, and alteration of melanocytes.9 Our patient demonstrated alteration of keratinocytes, which is characterized by overlapping features of hypertrophic lichen planus and early keratoacanthoma.
The differential diagnoses for pembrolizumab-induced eruptive squamous proliferation include squamous cell carcinoma (SCC), psoriasis, hypertrophic lichen planus, and cutaneous metastasis of NSCLC. Hypertrophic lupus erythematosus also is a well-documented reaction to use of immune-checkpoint inhibitors.10 Direct immunofluorescence could have helped differentiate hypertrophic lupus erythematosus from an eruptive squamous proliferation in our patient; however, due to her response to treatment, no additional workup was done.
Squamous cell carcinoma, which is the most common type of skin cancer in Black patients in the United States,11 has been shown to manifest after a PD-1 inhibitor is taken.12 Although it typically has a more chronic persistent course, the clinical appearance of SCC can be similar to the findings seen in our patient. Histologically, SCC may demonstrate necrosis, but the atypical proliferations will invade the dermis—a feature not seen in our patient’s histopathology.13
Lichen planus (LP) is an eruptive immune reaction of violaceous polygonal papules and plaques commonly seen on the ankles14 that has been shown to be an adverse effect of pembrolizumab.15 There are several subtypes of LP including hypertrophic versions, which can appear clinically similar to the findings seen in our patient. On dermoscopy, the classic finding of white lines, known as Wickham striae, is seen in all subtypes and can help diagnose this pathologic process. Under the microscope, LP can manifest with hyperkeratosis without parakeratosis, irregular thickening of the stratum granulosum, sawtooth rete ridges, and destruction of the basal layer.14
Psoriasis also has been shown to be exacerbated by anti–PD-1 therapy, although the majority of patients diagnosed with PD-1–induced psoriasis have a personal or family history of the disease.6 Clinically, psoriasis can have a hyperpigmented or violaceous appearance in patients with skin of color.16 The histopathology of psoriasis typically reveals confluent parakeratosis, neutrophils in the stratum corneum, regular acanthosis, thinning of the suprapapillary plates, and vessels in the dermal papillae.17
Although cutaneous metastasis of NSCLC may appear clinically similar to the current case, it is one of the rarer organ sites of metastasis for lung cancer.18 In our patient, biopsy quickly ruled out this diagnosis. If it had been a site of metastasis, histopathology would have shown a dermal-based proliferation of dysplastic cells without epidermal connection.19
It is important for dermatologists to recognize eruptive squamous proliferations associated with pembrolizumab, as they often respond to conservative treatment and typically do not require dose reduction or discontinuation of the inciting drug.
THE DIAGNOSIS: Pembrolizumab-Induced Eruptive Squamous Proliferation
Histopathology showed a broad squamous proliferation with acanthosis of the epidermis. Large glassy keratinocytes were seen with scattered necrotic keratinocytes (Figure), and a dense lichenoid band of inflammation was present subjacent to the proliferation. Notably, no hypergranulosis, remarkable keratinocyte atypia, or increased mitotic figures were seen. Based on the patient’s medical history and biopsy results, a diagnosis of pembrolizumab-induced eruptive squamous proliferation was made. The diagnosis was supported by a growing body of evidence of this type of reaction in patients taking programmed death 1 (PD-1) inhibitors.1,2 Conservative treatment with clobetasol ointment 0.05% was initiated with complete resolution of the lesions at the 2-month follow-up appointment. Other common treatments include topical steroids, injected corticosteroids, or cryosurgery to locally control the inflammation and atypical proliferation of cells.3

Pembrolizumab is a humanized IgG4 monoclonal antibody targeting the PD-1 receptor that has been utilized for its antitumor activity against various cancers, including unresectable and metastatic melanoma, head and neck cancers, and non–small cell lung cancer (NSCLC).1,4,5 While this drug has extended the lives of many patients with cancer, there are adverse reactions associated with PD-1 inhibitors (eg, pembrolizumab, nivolumab). Skin toxicity to PD-1 inhibitors is the one of most common immune-mediated reactions worldwide, occurring in approximately 30% of patients.6,7 Reactions can occur while a patient is taking the inciting drug and can continue up to 2 months after treatment discontinuation.8 Skin reactions associated with PD-1 inhibitors vary from lichenoid reactions and vitiligolike patches to psoriasis or eczema flares and are organized into 4 categories: inflammatory, immunobullous, alteration of keratinocytes, and alteration of melanocytes.9 Our patient demonstrated alteration of keratinocytes, which is characterized by overlapping features of hypertrophic lichen planus and early keratoacanthoma.
The differential diagnoses for pembrolizumab-induced eruptive squamous proliferation include squamous cell carcinoma (SCC), psoriasis, hypertrophic lichen planus, and cutaneous metastasis of NSCLC. Hypertrophic lupus erythematosus also is a well-documented reaction to use of immune-checkpoint inhibitors.10 Direct immunofluorescence could have helped differentiate hypertrophic lupus erythematosus from an eruptive squamous proliferation in our patient; however, due to her response to treatment, no additional workup was done.
Squamous cell carcinoma, which is the most common type of skin cancer in Black patients in the United States,11 has been shown to manifest after a PD-1 inhibitor is taken.12 Although it typically has a more chronic persistent course, the clinical appearance of SCC can be similar to the findings seen in our patient. Histologically, SCC may demonstrate necrosis, but the atypical proliferations will invade the dermis—a feature not seen in our patient’s histopathology.13
Lichen planus (LP) is an eruptive immune reaction of violaceous polygonal papules and plaques commonly seen on the ankles14 that has been shown to be an adverse effect of pembrolizumab.15 There are several subtypes of LP including hypertrophic versions, which can appear clinically similar to the findings seen in our patient. On dermoscopy, the classic finding of white lines, known as Wickham striae, is seen in all subtypes and can help diagnose this pathologic process. Under the microscope, LP can manifest with hyperkeratosis without parakeratosis, irregular thickening of the stratum granulosum, sawtooth rete ridges, and destruction of the basal layer.14
Psoriasis also has been shown to be exacerbated by anti–PD-1 therapy, although the majority of patients diagnosed with PD-1–induced psoriasis have a personal or family history of the disease.6 Clinically, psoriasis can have a hyperpigmented or violaceous appearance in patients with skin of color.16 The histopathology of psoriasis typically reveals confluent parakeratosis, neutrophils in the stratum corneum, regular acanthosis, thinning of the suprapapillary plates, and vessels in the dermal papillae.17
Although cutaneous metastasis of NSCLC may appear clinically similar to the current case, it is one of the rarer organ sites of metastasis for lung cancer.18 In our patient, biopsy quickly ruled out this diagnosis. If it had been a site of metastasis, histopathology would have shown a dermal-based proliferation of dysplastic cells without epidermal connection.19
It is important for dermatologists to recognize eruptive squamous proliferations associated with pembrolizumab, as they often respond to conservative treatment and typically do not require dose reduction or discontinuation of the inciting drug.
- Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43. doi:10.1186/s40425-017-0242-5
- Preti BTB, Pencz A, Cowger JJM, et al. Skin deep: a fascinating case report of immunotherapy-triggered, treatment-refractory autoimmune lichen planus and keratoacanthoma. Case Rep Oncol. 2021;14: 1189-1193. doi:10.1159/000518313
- Fradet M, Sibaud V, Tournier E, et al. Multiple keratoacanthoma-like lesions in a patient treated with pembrolizumab. Acta Derm Venereol. 2019;99:1301-1302. doi:10.2340/00015555-3301
- Flynn JP, Gerriets V. Pembrolizumab. StatPearls [Internet]. StatPearls Publishing; 2023. Updated June 26, 2023. Accessed April 2, 2025.
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with Nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Voudouri D, Nikolaou V, Laschos K, et al. Anti-Pd1/Pdl1 induced psoriasis. Curr Probl Cancer. 2017;41:407-412. doi:10.1016 /j.currproblcancer.2017.10.003
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Coscarart A, Martel J, Lee MP, et al. Pembrolizumab-induced pseudoepitheliomatous eruption consistent with hypertrophic lichen planus. J Cutan Pathol. 2020;47:275-279. doi:10.1111/cup.13587
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176. doi:10.1111/cup.12858
- Vitzthum von Eckstaedt H, Singh A, Reid P, et al. Immune checkpoint inhibitors and lupus erythematosus. Pharmaceuticals (Basel). 2024;2:15;17. doi:10.3390/ph17020252
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Vu M, Chapman S, Lenz B, et al. Squamous cell carcinoma or squamous proliferation associated with nivolumab treatment for metastatic melanoma. Dermatol Online J. 2022;6:28. doi:10.5070/d328357786
- Howell JY, Ramsey ML. Squamous cell skin cancer. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 2, 2024. Accessed April 2, 2025.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. StatPearls Publishing; 2024. Updated October 29, 2024. Accessed April 2, 2025.
- Yamashita A, Akasaka E, Nakano H, et al. Pembrolizumab-induced lichen planus on the scalp of a patient with non-small-cell lung carcinoma. Case Rep Dermatol. 2021;13:487-491. doi:10.1159/000519486
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Murphy M, Kerr P, Grant-Kels JM. The histopathologic spectrum of psoriasis. Clin Dermatol. 2007;25:524-528. doi:10.1016 /j.clindermatol.2007.08.005.
- Hidaka T, Ishii Y, Kitamura S. Clinical features of skin metastasis from lung cancer. Intern Med. 1996;35:459-462. doi:10.2169 /internalmedicine.35.459.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613–620. doi:10.1001/archderm.143.5.613
- Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43. doi:10.1186/s40425-017-0242-5
- Preti BTB, Pencz A, Cowger JJM, et al. Skin deep: a fascinating case report of immunotherapy-triggered, treatment-refractory autoimmune lichen planus and keratoacanthoma. Case Rep Oncol. 2021;14: 1189-1193. doi:10.1159/000518313
- Fradet M, Sibaud V, Tournier E, et al. Multiple keratoacanthoma-like lesions in a patient treated with pembrolizumab. Acta Derm Venereol. 2019;99:1301-1302. doi:10.2340/00015555-3301
- Flynn JP, Gerriets V. Pembrolizumab. StatPearls [Internet]. StatPearls Publishing; 2023. Updated June 26, 2023. Accessed April 2, 2025.
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with Nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Voudouri D, Nikolaou V, Laschos K, et al. Anti-Pd1/Pdl1 induced psoriasis. Curr Probl Cancer. 2017;41:407-412. doi:10.1016 /j.currproblcancer.2017.10.003
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Coscarart A, Martel J, Lee MP, et al. Pembrolizumab-induced pseudoepitheliomatous eruption consistent with hypertrophic lichen planus. J Cutan Pathol. 2020;47:275-279. doi:10.1111/cup.13587
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176. doi:10.1111/cup.12858
- Vitzthum von Eckstaedt H, Singh A, Reid P, et al. Immune checkpoint inhibitors and lupus erythematosus. Pharmaceuticals (Basel). 2024;2:15;17. doi:10.3390/ph17020252
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Vu M, Chapman S, Lenz B, et al. Squamous cell carcinoma or squamous proliferation associated with nivolumab treatment for metastatic melanoma. Dermatol Online J. 2022;6:28. doi:10.5070/d328357786
- Howell JY, Ramsey ML. Squamous cell skin cancer. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 2, 2024. Accessed April 2, 2025.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. StatPearls Publishing; 2024. Updated October 29, 2024. Accessed April 2, 2025.
- Yamashita A, Akasaka E, Nakano H, et al. Pembrolizumab-induced lichen planus on the scalp of a patient with non-small-cell lung carcinoma. Case Rep Dermatol. 2021;13:487-491. doi:10.1159/000519486
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Murphy M, Kerr P, Grant-Kels JM. The histopathologic spectrum of psoriasis. Clin Dermatol. 2007;25:524-528. doi:10.1016 /j.clindermatol.2007.08.005.
- Hidaka T, Ishii Y, Kitamura S. Clinical features of skin metastasis from lung cancer. Intern Med. 1996;35:459-462. doi:10.2169 /internalmedicine.35.459.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613–620. doi:10.1001/archderm.143.5.613
Papulonodules on the Ankle in a Patient with Lung Cancer
Papulonodules on the Ankle in a Patient with Lung Cancer
A 75-year-old woman presented to the dermatology department with well-circumscribed, round, hyperkeratotic papulonodules on the ankle of 3 months’ duration (top). The papulonodules also were evaluated by dermoscopy, which highlighted in greater detail the hyperkeratosis seen grossly (bottom). The patient had a history of chronic obstructive pulmonary disease and metastatic lung cancer and had been taking pembrolizumab for the past 2 years. The lesions initially appeared on the medial right foot and slowly spread proximally. Most of the lesions resolved spontaneously except for 2 on the right ankle. At the current presentation, one lesion was slightly tender to palpation, but both were otherwise asymptomatic. A lesion was biopsied and sent for dermatopathologic evaluation.
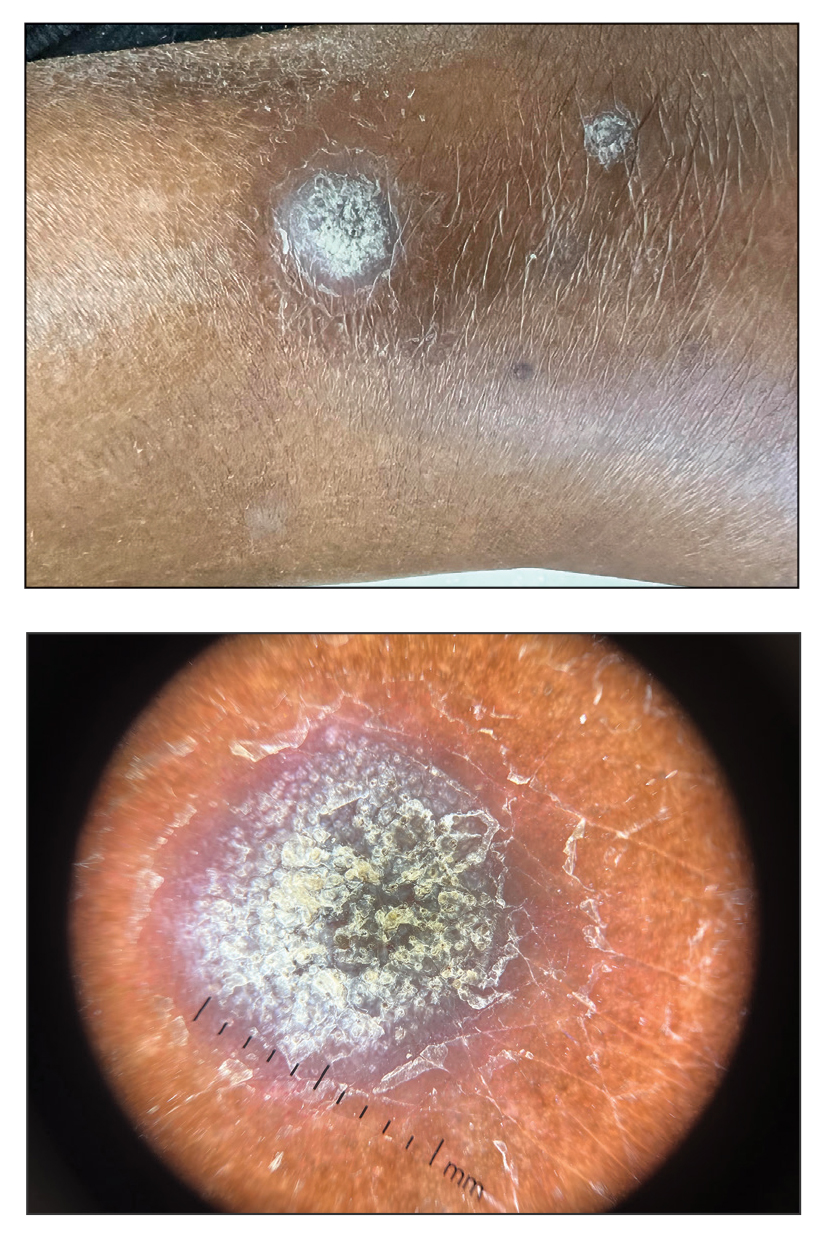
UK Funds AI Blood Test for Early Cancer Detection
A clinical trial of a promising blood test that could offer faster and more accurate diagnoses for common cancers has received funding from the Department of Health and Social Care (DHSC).
The miONCO-Dx test detects cancer at an early stage by analysing microRNA expression in blood.
It uses artificial intelligence to identify the presence and origin of the disease.
The test was developed by Xgenera, a University of Southampton spinout, in collaboration with the National Institute for Health and Care Research.
Initial analysis of data from more than 20,000 patients showed that the test detected 12 of the most common and lethal cancers at an early stage and with over 99% accuracy.
Bowel Cancer Among Key Targets
Bowel cancer, the fourth most common cancer in the United Kingdom, is a principal target for the test.
Around 44,000 people in the UK are diagnosed with bowel cancer each year. At stage 1, approximately 90% of people survive for 5 years or more, but this drops to around 10% at stage 4.
Wes Streeting, Secretary of State for Health and Social Care, said in a press release, “The key to surviving cancer is catching it as early as possible, so this government is taking the urgent action needed to make sure that happens.”
£2.4 Million Awarded for Clinical Trial
The DHSC has awarded Xgenera £2.4 million to advance development of the test, which has now been refined into a cheaper, faster, and more scalable version.
The funding will support a clinical trial involving 8000 patients. The DHSC described this as “a formal and significant step towards bringing the test closer to patients by ensuring it is fit for purpose in the NHS.”
The trial will be run by Cancer Research UK Southampton Clinical Trials Unit.
Potential for NHS Use
Dr Victoria Goss, head of early diagnosis and translational research at the trials unit, said in a press release, “A reliable test such as this could have the potential to see a major shift in cancer screening, making it easier and cheaper to provide on the NHS, cutting health inequalities, and ultimately reducing the number of people who die from the disease.”
Xgenera co-founder Dr Andy Shapanis, a research fellow at the University of Southampton, said that the new study would evaluate the useability, accuracy, and cost-effectiveness of the test for use within the NHS in future.
“The hope is that if the test is shown to be successful in the early diagnosis of the 12 cancers we have currently identified biomarkers for, then it could be expanded to look at over 50 other cancers in the future,” he said.
Comparison With Other Tests
The miONCO-Dx test follows other attempts at multicancer early detection, such as the Galleri test from Grail, which is already being trialled in the NHS.
Galleri screens for altered DNA methylation patterns in blood and claims to detect more than 50 types of cancer. It raised hopes for earlier diagnosis, less invasive treatment, and potential cost savings.
However, critics have raised concerns about low detection rates in early-stage cancers, high false-positive rates, imprecise cancer origin analysis, cost, and unproven mortality gains. Questions have also been expressed about possible political influence in its selection for NHS trials.
A Broader Screening Platform
Xgenera co-founder Professor Paul Skipp, director of the Centre for Proteomic Research at the University of Southampton, said earlier this year that the miONCO-Dx test was “a real game-changer.”
The test can detect lung, breast, prostate, pancreatic, colorectal, ovarian, liver, brain, oesophageal, bladder, and gastric cancer and bone and soft-tissue sarcoma. It works by identifying imbalances in microRNAs, a class of small noncoding RNAs with functions in posttranscriptional regulation of gene expression, influencing cellular activities including cell growth, differentiation, development, and apoptosis.
The presence of microRNA imbalances can be identified from just 10-15 drops of blood, across all stages of tumour growth.
In comparison, according to Skipp, screening is only available currently for three types of cancer in the UK, and each test targets a single type.
Xgenera has also received external investment from the innovation investment companies Qantx, Empirical Ventures, and Ascension Ventures to further develop the test.
Dr Sheena Meredith is an established medical writer, editor, and consultant in healthcare communications, with extensive experience writing for medical professionals and the general public. She is qualified in medicine and in law and medical ethics.
A version of this article first appeared on Medscape.com.
A clinical trial of a promising blood test that could offer faster and more accurate diagnoses for common cancers has received funding from the Department of Health and Social Care (DHSC).
The miONCO-Dx test detects cancer at an early stage by analysing microRNA expression in blood.
It uses artificial intelligence to identify the presence and origin of the disease.
The test was developed by Xgenera, a University of Southampton spinout, in collaboration with the National Institute for Health and Care Research.
Initial analysis of data from more than 20,000 patients showed that the test detected 12 of the most common and lethal cancers at an early stage and with over 99% accuracy.
Bowel Cancer Among Key Targets
Bowel cancer, the fourth most common cancer in the United Kingdom, is a principal target for the test.
Around 44,000 people in the UK are diagnosed with bowel cancer each year. At stage 1, approximately 90% of people survive for 5 years or more, but this drops to around 10% at stage 4.
Wes Streeting, Secretary of State for Health and Social Care, said in a press release, “The key to surviving cancer is catching it as early as possible, so this government is taking the urgent action needed to make sure that happens.”
£2.4 Million Awarded for Clinical Trial
The DHSC has awarded Xgenera £2.4 million to advance development of the test, which has now been refined into a cheaper, faster, and more scalable version.
The funding will support a clinical trial involving 8000 patients. The DHSC described this as “a formal and significant step towards bringing the test closer to patients by ensuring it is fit for purpose in the NHS.”
The trial will be run by Cancer Research UK Southampton Clinical Trials Unit.
Potential for NHS Use
Dr Victoria Goss, head of early diagnosis and translational research at the trials unit, said in a press release, “A reliable test such as this could have the potential to see a major shift in cancer screening, making it easier and cheaper to provide on the NHS, cutting health inequalities, and ultimately reducing the number of people who die from the disease.”
Xgenera co-founder Dr Andy Shapanis, a research fellow at the University of Southampton, said that the new study would evaluate the useability, accuracy, and cost-effectiveness of the test for use within the NHS in future.
“The hope is that if the test is shown to be successful in the early diagnosis of the 12 cancers we have currently identified biomarkers for, then it could be expanded to look at over 50 other cancers in the future,” he said.
Comparison With Other Tests
The miONCO-Dx test follows other attempts at multicancer early detection, such as the Galleri test from Grail, which is already being trialled in the NHS.
Galleri screens for altered DNA methylation patterns in blood and claims to detect more than 50 types of cancer. It raised hopes for earlier diagnosis, less invasive treatment, and potential cost savings.
However, critics have raised concerns about low detection rates in early-stage cancers, high false-positive rates, imprecise cancer origin analysis, cost, and unproven mortality gains. Questions have also been expressed about possible political influence in its selection for NHS trials.
A Broader Screening Platform
Xgenera co-founder Professor Paul Skipp, director of the Centre for Proteomic Research at the University of Southampton, said earlier this year that the miONCO-Dx test was “a real game-changer.”
The test can detect lung, breast, prostate, pancreatic, colorectal, ovarian, liver, brain, oesophageal, bladder, and gastric cancer and bone and soft-tissue sarcoma. It works by identifying imbalances in microRNAs, a class of small noncoding RNAs with functions in posttranscriptional regulation of gene expression, influencing cellular activities including cell growth, differentiation, development, and apoptosis.
The presence of microRNA imbalances can be identified from just 10-15 drops of blood, across all stages of tumour growth.
In comparison, according to Skipp, screening is only available currently for three types of cancer in the UK, and each test targets a single type.
Xgenera has also received external investment from the innovation investment companies Qantx, Empirical Ventures, and Ascension Ventures to further develop the test.
Dr Sheena Meredith is an established medical writer, editor, and consultant in healthcare communications, with extensive experience writing for medical professionals and the general public. She is qualified in medicine and in law and medical ethics.
A version of this article first appeared on Medscape.com.
A clinical trial of a promising blood test that could offer faster and more accurate diagnoses for common cancers has received funding from the Department of Health and Social Care (DHSC).
The miONCO-Dx test detects cancer at an early stage by analysing microRNA expression in blood.
It uses artificial intelligence to identify the presence and origin of the disease.
The test was developed by Xgenera, a University of Southampton spinout, in collaboration with the National Institute for Health and Care Research.
Initial analysis of data from more than 20,000 patients showed that the test detected 12 of the most common and lethal cancers at an early stage and with over 99% accuracy.
Bowel Cancer Among Key Targets
Bowel cancer, the fourth most common cancer in the United Kingdom, is a principal target for the test.
Around 44,000 people in the UK are diagnosed with bowel cancer each year. At stage 1, approximately 90% of people survive for 5 years or more, but this drops to around 10% at stage 4.
Wes Streeting, Secretary of State for Health and Social Care, said in a press release, “The key to surviving cancer is catching it as early as possible, so this government is taking the urgent action needed to make sure that happens.”
£2.4 Million Awarded for Clinical Trial
The DHSC has awarded Xgenera £2.4 million to advance development of the test, which has now been refined into a cheaper, faster, and more scalable version.
The funding will support a clinical trial involving 8000 patients. The DHSC described this as “a formal and significant step towards bringing the test closer to patients by ensuring it is fit for purpose in the NHS.”
The trial will be run by Cancer Research UK Southampton Clinical Trials Unit.
Potential for NHS Use
Dr Victoria Goss, head of early diagnosis and translational research at the trials unit, said in a press release, “A reliable test such as this could have the potential to see a major shift in cancer screening, making it easier and cheaper to provide on the NHS, cutting health inequalities, and ultimately reducing the number of people who die from the disease.”
Xgenera co-founder Dr Andy Shapanis, a research fellow at the University of Southampton, said that the new study would evaluate the useability, accuracy, and cost-effectiveness of the test for use within the NHS in future.
“The hope is that if the test is shown to be successful in the early diagnosis of the 12 cancers we have currently identified biomarkers for, then it could be expanded to look at over 50 other cancers in the future,” he said.
Comparison With Other Tests
The miONCO-Dx test follows other attempts at multicancer early detection, such as the Galleri test from Grail, which is already being trialled in the NHS.
Galleri screens for altered DNA methylation patterns in blood and claims to detect more than 50 types of cancer. It raised hopes for earlier diagnosis, less invasive treatment, and potential cost savings.
However, critics have raised concerns about low detection rates in early-stage cancers, high false-positive rates, imprecise cancer origin analysis, cost, and unproven mortality gains. Questions have also been expressed about possible political influence in its selection for NHS trials.
A Broader Screening Platform
Xgenera co-founder Professor Paul Skipp, director of the Centre for Proteomic Research at the University of Southampton, said earlier this year that the miONCO-Dx test was “a real game-changer.”
The test can detect lung, breast, prostate, pancreatic, colorectal, ovarian, liver, brain, oesophageal, bladder, and gastric cancer and bone and soft-tissue sarcoma. It works by identifying imbalances in microRNAs, a class of small noncoding RNAs with functions in posttranscriptional regulation of gene expression, influencing cellular activities including cell growth, differentiation, development, and apoptosis.
The presence of microRNA imbalances can be identified from just 10-15 drops of blood, across all stages of tumour growth.
In comparison, according to Skipp, screening is only available currently for three types of cancer in the UK, and each test targets a single type.
Xgenera has also received external investment from the innovation investment companies Qantx, Empirical Ventures, and Ascension Ventures to further develop the test.
Dr Sheena Meredith is an established medical writer, editor, and consultant in healthcare communications, with extensive experience writing for medical professionals and the general public. She is qualified in medicine and in law and medical ethics.
A version of this article first appeared on Medscape.com.
Can a Polygenic Risk Score Turn the Tide on Prostate Cancer Screening?
Incorporating a polygenic risk score into prostate cancer screening could enhance the detection of clinically significant prostate cancer that conventional screening may miss, according to results of the BARCODE 1 clinical trial conducted in the United Kingdom.
The study found that about 72% of participants with high polygenic risk scores were diagnosed with clinically significant prostate cancers, which would not have been detected with prostate-specific antigen (PSA) testing or MRI.
“With this test, it could be possible to turn the tide on prostate cancer,” study author Ros Eeles, PhD, professor of oncogenetics at The Institute of Cancer Research, London, England, said in a statement following the publication of the analysis in The New England Journal of Medicine.
Prostate cancer remains the second most commonly diagnosed cancer among men. As a screening tool, PSA testing has been criticized for leading to a high rate of false positive results and overdiagnosis — defined as a screen-detected cancer that would take longer to progress to clinical cancer than the patient’s lifetime. Both issues can result in overtreatment.
Given prostate cancer’s high heritability and the proliferation of genome-wide association studies identifying common genetic variants, there has been growing interest in using polygenic risk scores to improve risk stratification and guide screening.
“Building on decades of research into the genetic markers of prostate cancer, our study shows that the theory does work in practice — we can identify men at risk of aggressive cancers who need further tests and spare the men who are at lower risk from unnecessary treatments,” said Eeles.
An Adjunct to Screening?
The BARCODE 1 study, conducted in the United Kingdom, tested the clinical utility of a polygenic risk score as an adjunct to screening.
The researchers recruited men aged 55-69 years from primary care centers in the United Kingdom. Using germline DNA extracted from saliva, they derived polygenic risk scores from 130 genetic variants known to be associated with an increased risk for prostate cancer.
Among a total of 6393 men who had their scores calculated, 745 (12%) had a score in the top 10% of genetic risk (≥ 90th percentile) and were invited to undergo further screening.
Of these, 468 (63%) accepted the invite and underwent multiparametric MRI and transperineal prostate biopsy, irrespective of the PSA level. Overall, 187 (40%) were diagnosed with prostate cancer following biopsy. Of the 187 men with prostate cancer, 55% (n = 103) had disease classified as intermediate or high risk (Gleason score ≥ 7) per National Comprehensive Cancer Network criteria and therefore warranted further treatment.
Researchers then compared screening that incorporated polygenic risk scores with standard screening with PSA levels and MRI.
When participants’ risk was stratified by their polygenic risk score, 103 patients (55%) with prostate cancer could be classified as intermediate or higher risk, thus warranting treatment. Overall, 74 (71.8%) of those cancers would have been missed using the standard diagnostic pathway in the United Kingdom, which requires patients to have a high PSA level (> 3.0 μg/L) as well as a positive MRI result. These 74 patients either had PSA levels ≤ 3.0 μg/L or negative MRIs, which would mean these patients would typically fall below the action threshold for further testing.
Of the 103 participants warranting treatment, 40 of these men would have been classified as unfavorable intermediate, high, or very high risk, which would require radical treatment. Among this group, roughly 43% would have been missed using the UK diagnostic pathway.
However, the investigators estimated a rate of overdiagnosis with the use of polygenic risk scores of 16%-21%, similar to the overdiagnosis estimates in two prior PSA-based screening studies, signaling that the addition of polygenic risk scores does not necessarily reduce the risk for overdiagnosis.
Overall, “this study is the strongest evidence to date on the clinical utility of a polygenic score for prostate cancer screening,” commented Michael Inouye, professor of systems genomics & population health, University of Cambridge, Cambridge, England, in a statement from the UK nonprofit Science Media Centre (SMC).
“I suspect we will look back on this as a landmark study that really made the clinical case for polygenic scores as a new tool that moved health systems from disease management to early detection and prevention,” said Inouye, who was not involved in the study.
However, other experts were more cautious about the findings.
Dusko Ilic, MD, professor of stem cell sciences, King’s College London, London, England, said the results are “promising, especially in identifying significant cancers that would otherwise be missed,” but cautioned that “there is no direct evidence yet that using [polygenic risk scores] improves long-term outcomes such as mortality or quality-adjusted life years.”
“Modeling suggests benefit, but empirical confirmation is needed,” Ilic said in the SMC statement.
The hope is that the recently launched TRANSFORM trial will help answer some of these outstanding questions.
The current study suggests that polygenic risk scores for prostate cancer “would be a useful component of a multimodality screening program that assesses age, family history of prostate cancer, PSA, and MRI results as triage tools before biopsy is recommended,” David Hunter, MPH, ScD, with Harvard T. H. Chan School of Public Health, Boston, and University of Oxford, Oxford, England, wrote in an editorial accompanying the study.
“To make this integrated program a reality, however, changes to infrastructure would be needed to make running and analyzing a regulated genome array as easy as requesting a PSA level or ordering an MRI. Clearly, we are far from that future,” Hunter cautioned.
“A possible first step that would require less infrastructure could be to order a polygenic risk score only for men with a positive PSA result, then use the polygenic risk score to determine who should undergo an MRI, and then use all the information to determine whether biopsy is recommended,” Hunter said.
In his view, the current study is a “first step on a long road to evaluating new components of any disease screening pathway.”
The research received funding from the European Research Council, the Bob Willis Fund, Cancer Research UK, the Peacock Trust, and the National Institute for Health and Care Research Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research. Disclosures for authors and editorialists are available with the original article. Inouye and Ilic reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Incorporating a polygenic risk score into prostate cancer screening could enhance the detection of clinically significant prostate cancer that conventional screening may miss, according to results of the BARCODE 1 clinical trial conducted in the United Kingdom.
The study found that about 72% of participants with high polygenic risk scores were diagnosed with clinically significant prostate cancers, which would not have been detected with prostate-specific antigen (PSA) testing or MRI.
“With this test, it could be possible to turn the tide on prostate cancer,” study author Ros Eeles, PhD, professor of oncogenetics at The Institute of Cancer Research, London, England, said in a statement following the publication of the analysis in The New England Journal of Medicine.
Prostate cancer remains the second most commonly diagnosed cancer among men. As a screening tool, PSA testing has been criticized for leading to a high rate of false positive results and overdiagnosis — defined as a screen-detected cancer that would take longer to progress to clinical cancer than the patient’s lifetime. Both issues can result in overtreatment.
Given prostate cancer’s high heritability and the proliferation of genome-wide association studies identifying common genetic variants, there has been growing interest in using polygenic risk scores to improve risk stratification and guide screening.
“Building on decades of research into the genetic markers of prostate cancer, our study shows that the theory does work in practice — we can identify men at risk of aggressive cancers who need further tests and spare the men who are at lower risk from unnecessary treatments,” said Eeles.
An Adjunct to Screening?
The BARCODE 1 study, conducted in the United Kingdom, tested the clinical utility of a polygenic risk score as an adjunct to screening.
The researchers recruited men aged 55-69 years from primary care centers in the United Kingdom. Using germline DNA extracted from saliva, they derived polygenic risk scores from 130 genetic variants known to be associated with an increased risk for prostate cancer.
Among a total of 6393 men who had their scores calculated, 745 (12%) had a score in the top 10% of genetic risk (≥ 90th percentile) and were invited to undergo further screening.
Of these, 468 (63%) accepted the invite and underwent multiparametric MRI and transperineal prostate biopsy, irrespective of the PSA level. Overall, 187 (40%) were diagnosed with prostate cancer following biopsy. Of the 187 men with prostate cancer, 55% (n = 103) had disease classified as intermediate or high risk (Gleason score ≥ 7) per National Comprehensive Cancer Network criteria and therefore warranted further treatment.
Researchers then compared screening that incorporated polygenic risk scores with standard screening with PSA levels and MRI.
When participants’ risk was stratified by their polygenic risk score, 103 patients (55%) with prostate cancer could be classified as intermediate or higher risk, thus warranting treatment. Overall, 74 (71.8%) of those cancers would have been missed using the standard diagnostic pathway in the United Kingdom, which requires patients to have a high PSA level (> 3.0 μg/L) as well as a positive MRI result. These 74 patients either had PSA levels ≤ 3.0 μg/L or negative MRIs, which would mean these patients would typically fall below the action threshold for further testing.
Of the 103 participants warranting treatment, 40 of these men would have been classified as unfavorable intermediate, high, or very high risk, which would require radical treatment. Among this group, roughly 43% would have been missed using the UK diagnostic pathway.
However, the investigators estimated a rate of overdiagnosis with the use of polygenic risk scores of 16%-21%, similar to the overdiagnosis estimates in two prior PSA-based screening studies, signaling that the addition of polygenic risk scores does not necessarily reduce the risk for overdiagnosis.
Overall, “this study is the strongest evidence to date on the clinical utility of a polygenic score for prostate cancer screening,” commented Michael Inouye, professor of systems genomics & population health, University of Cambridge, Cambridge, England, in a statement from the UK nonprofit Science Media Centre (SMC).
“I suspect we will look back on this as a landmark study that really made the clinical case for polygenic scores as a new tool that moved health systems from disease management to early detection and prevention,” said Inouye, who was not involved in the study.
However, other experts were more cautious about the findings.
Dusko Ilic, MD, professor of stem cell sciences, King’s College London, London, England, said the results are “promising, especially in identifying significant cancers that would otherwise be missed,” but cautioned that “there is no direct evidence yet that using [polygenic risk scores] improves long-term outcomes such as mortality or quality-adjusted life years.”
“Modeling suggests benefit, but empirical confirmation is needed,” Ilic said in the SMC statement.
The hope is that the recently launched TRANSFORM trial will help answer some of these outstanding questions.
The current study suggests that polygenic risk scores for prostate cancer “would be a useful component of a multimodality screening program that assesses age, family history of prostate cancer, PSA, and MRI results as triage tools before biopsy is recommended,” David Hunter, MPH, ScD, with Harvard T. H. Chan School of Public Health, Boston, and University of Oxford, Oxford, England, wrote in an editorial accompanying the study.
“To make this integrated program a reality, however, changes to infrastructure would be needed to make running and analyzing a regulated genome array as easy as requesting a PSA level or ordering an MRI. Clearly, we are far from that future,” Hunter cautioned.
“A possible first step that would require less infrastructure could be to order a polygenic risk score only for men with a positive PSA result, then use the polygenic risk score to determine who should undergo an MRI, and then use all the information to determine whether biopsy is recommended,” Hunter said.
In his view, the current study is a “first step on a long road to evaluating new components of any disease screening pathway.”
The research received funding from the European Research Council, the Bob Willis Fund, Cancer Research UK, the Peacock Trust, and the National Institute for Health and Care Research Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research. Disclosures for authors and editorialists are available with the original article. Inouye and Ilic reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Incorporating a polygenic risk score into prostate cancer screening could enhance the detection of clinically significant prostate cancer that conventional screening may miss, according to results of the BARCODE 1 clinical trial conducted in the United Kingdom.
The study found that about 72% of participants with high polygenic risk scores were diagnosed with clinically significant prostate cancers, which would not have been detected with prostate-specific antigen (PSA) testing or MRI.
“With this test, it could be possible to turn the tide on prostate cancer,” study author Ros Eeles, PhD, professor of oncogenetics at The Institute of Cancer Research, London, England, said in a statement following the publication of the analysis in The New England Journal of Medicine.
Prostate cancer remains the second most commonly diagnosed cancer among men. As a screening tool, PSA testing has been criticized for leading to a high rate of false positive results and overdiagnosis — defined as a screen-detected cancer that would take longer to progress to clinical cancer than the patient’s lifetime. Both issues can result in overtreatment.
Given prostate cancer’s high heritability and the proliferation of genome-wide association studies identifying common genetic variants, there has been growing interest in using polygenic risk scores to improve risk stratification and guide screening.
“Building on decades of research into the genetic markers of prostate cancer, our study shows that the theory does work in practice — we can identify men at risk of aggressive cancers who need further tests and spare the men who are at lower risk from unnecessary treatments,” said Eeles.
An Adjunct to Screening?
The BARCODE 1 study, conducted in the United Kingdom, tested the clinical utility of a polygenic risk score as an adjunct to screening.
The researchers recruited men aged 55-69 years from primary care centers in the United Kingdom. Using germline DNA extracted from saliva, they derived polygenic risk scores from 130 genetic variants known to be associated with an increased risk for prostate cancer.
Among a total of 6393 men who had their scores calculated, 745 (12%) had a score in the top 10% of genetic risk (≥ 90th percentile) and were invited to undergo further screening.
Of these, 468 (63%) accepted the invite and underwent multiparametric MRI and transperineal prostate biopsy, irrespective of the PSA level. Overall, 187 (40%) were diagnosed with prostate cancer following biopsy. Of the 187 men with prostate cancer, 55% (n = 103) had disease classified as intermediate or high risk (Gleason score ≥ 7) per National Comprehensive Cancer Network criteria and therefore warranted further treatment.
Researchers then compared screening that incorporated polygenic risk scores with standard screening with PSA levels and MRI.
When participants’ risk was stratified by their polygenic risk score, 103 patients (55%) with prostate cancer could be classified as intermediate or higher risk, thus warranting treatment. Overall, 74 (71.8%) of those cancers would have been missed using the standard diagnostic pathway in the United Kingdom, which requires patients to have a high PSA level (> 3.0 μg/L) as well as a positive MRI result. These 74 patients either had PSA levels ≤ 3.0 μg/L or negative MRIs, which would mean these patients would typically fall below the action threshold for further testing.
Of the 103 participants warranting treatment, 40 of these men would have been classified as unfavorable intermediate, high, or very high risk, which would require radical treatment. Among this group, roughly 43% would have been missed using the UK diagnostic pathway.
However, the investigators estimated a rate of overdiagnosis with the use of polygenic risk scores of 16%-21%, similar to the overdiagnosis estimates in two prior PSA-based screening studies, signaling that the addition of polygenic risk scores does not necessarily reduce the risk for overdiagnosis.
Overall, “this study is the strongest evidence to date on the clinical utility of a polygenic score for prostate cancer screening,” commented Michael Inouye, professor of systems genomics & population health, University of Cambridge, Cambridge, England, in a statement from the UK nonprofit Science Media Centre (SMC).
“I suspect we will look back on this as a landmark study that really made the clinical case for polygenic scores as a new tool that moved health systems from disease management to early detection and prevention,” said Inouye, who was not involved in the study.
However, other experts were more cautious about the findings.
Dusko Ilic, MD, professor of stem cell sciences, King’s College London, London, England, said the results are “promising, especially in identifying significant cancers that would otherwise be missed,” but cautioned that “there is no direct evidence yet that using [polygenic risk scores] improves long-term outcomes such as mortality or quality-adjusted life years.”
“Modeling suggests benefit, but empirical confirmation is needed,” Ilic said in the SMC statement.
The hope is that the recently launched TRANSFORM trial will help answer some of these outstanding questions.
The current study suggests that polygenic risk scores for prostate cancer “would be a useful component of a multimodality screening program that assesses age, family history of prostate cancer, PSA, and MRI results as triage tools before biopsy is recommended,” David Hunter, MPH, ScD, with Harvard T. H. Chan School of Public Health, Boston, and University of Oxford, Oxford, England, wrote in an editorial accompanying the study.
“To make this integrated program a reality, however, changes to infrastructure would be needed to make running and analyzing a regulated genome array as easy as requesting a PSA level or ordering an MRI. Clearly, we are far from that future,” Hunter cautioned.
“A possible first step that would require less infrastructure could be to order a polygenic risk score only for men with a positive PSA result, then use the polygenic risk score to determine who should undergo an MRI, and then use all the information to determine whether biopsy is recommended,” Hunter said.
In his view, the current study is a “first step on a long road to evaluating new components of any disease screening pathway.”
The research received funding from the European Research Council, the Bob Willis Fund, Cancer Research UK, the Peacock Trust, and the National Institute for Health and Care Research Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research. Disclosures for authors and editorialists are available with the original article. Inouye and Ilic reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Acral Erythema, Edema, and Scaly Plaques in a Patient With Polyneuropathy
Acral Erythema, Edema, and Scaly Plaques in a Patient With Polyneuropathy
THE DIAGNOSIS: Borderline-Borderline Leprosy With Type 1 Lepra Reaction
Punch biopsies from plaques on the right elbow and right shin revealed diffuse granulomatous dermatitis (Figure 1) with a narrow Grenz zone in the superficial dermis. The upper dermis contained a dense bandlike infiltrate of histiocytes with abundant foamy-gray cytoplasm and a moderate admixture of lymphocytes. The mid and deep dermis contained a nodular, perivascular, periadnexal, and perineural infiltrate of histiocytes and a dense admixture of lymphocytes. Periodic acid-Schiff and Gram stains were negative for microorganisms. Fite stain was positive for numerous organisms in histiocytes and small dermal nerves (Figure 2). These findings and the clinical examination confirmed a diagnosis of borderline-borderline leprosy with type 1 lepra reaction. The patient was started on dapsone 100 mg, rifampin 600 mg, and clofazimine 100 mg once daily and experienced clinical improvement within 6 months.
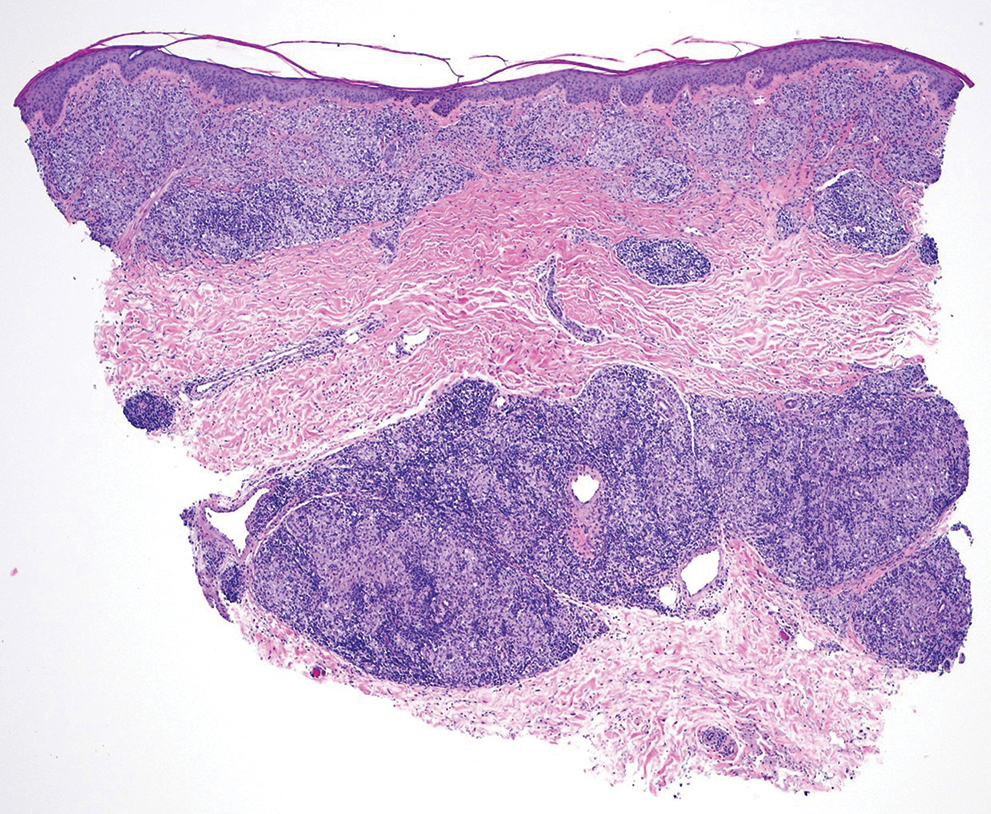
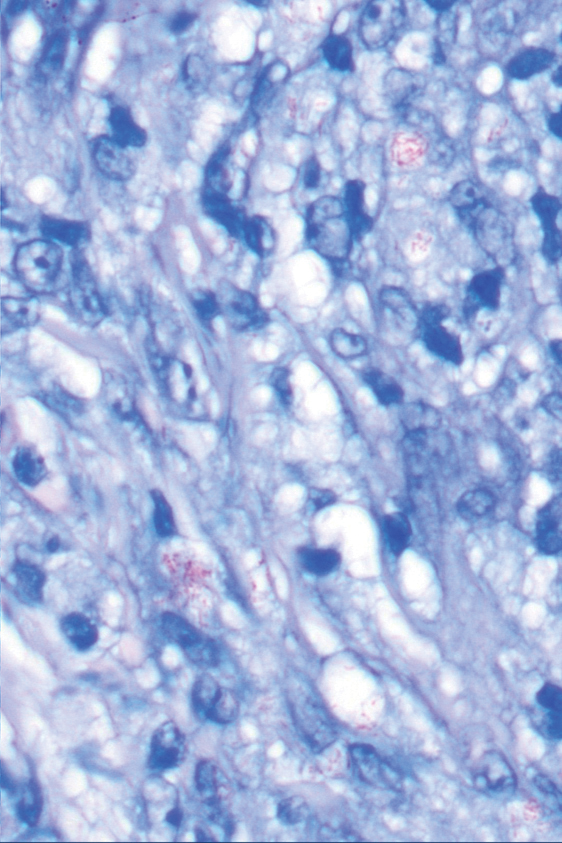
The World Health Organization reported more than 200,000 new leprosy cases globally in 2019, with most occurring in India, Brazil, and Indonesia.1 About 150 to 250 new cases are detected in the United States annually.1 The Ridley-Jopling classification of leprosy divides the condition into 5 categories: tuberculoid, borderline tuberculoid, borderline-borderline (BB), borderline lepromatous, and lepromatous. At one end of the spectrum, tuberculoid leprosy—a predominant Th1 immune response mediated by CD4 lymphocytes, interleukin (IL) 2, and interferon gamma2—is characterized by sharply demarcated erythematous and hypopigmented plaques with raised borders and an annular appearance.2,3 Lesions typically have atrophic and hypopigmented centers that often appear in an asymmetric distribution on the arms and legs.2,3 Histologic features include dermal tuberculoid granulomas with epithelioid cells—some located directly beneath the epidermis and others around deep vessels and nerves3—multinucleated Langerhans giant cells, thickened peripheral nerves with intraneural lymphocytic infiltrates, and granulomas with central necrosis. Fite-Faraco staining exhibits few bacteria.2
Lepromatous leprosy occurs in individuals with impaired T-cell immunity, leading to multiple red-brown nodular infiltrates in the skin and mucous membranes.2,3 Lesions typically are symmetric and favor the face and auricle of the ear.2,3 Histologically, there are bluish-gray foamy macrophages that form diffuse or nodular infiltrates with few lymphocytes,2 with a Grenz zone between the epidermis and dermis. Nerves may show lamination of the perineurium resembling an onion skin.2,3 Immunohistochemistry shows predominant CD8-positive infiltrates with a Th2 response and positive IL-4 and IL-10. Fite-Faraco stain shows numerous mycobacteria arranged in clusters and in histiocytes.2
Tuberculoid leprosy is treated with dapsone 100 mg and rifampin 600 mg once daily for 6 months,4 and lepromatous leprosy is treated with dapsone 100 mg, rifampin 600 mg, and clofazimine 50 mg once daily for 12 months.4 The prognosis for both is good with treatment; erythema and induration of skin lesions may improve within a few months, but residual nerve damage is common, especially in those with advanced disease prior to treatment.2 For direct contacts, a single dose of rifampin may be given.4
Borderline-borderline leprosy manifests with numerous asymmetric annular plaques, as seen in our patient (Figure 3). Histology findings can be variable and often overlap with other forms of leprosy. There can be epithelioid granulomas and only a few acid-fast bacilli (AFB) or diffuse histiocytic aggregates with foamy histiocytes containing large numbers of AFB.3 Nerve involvement is variable but can be severe in the setting of type 1 lepra reaction, which was present in our patient. Type 1 lepra reaction—a type IV cell-mediated allergic hypersensitivity reaction to Mycobacterium leprae antigens—manifests clinically with hyperesthesia, erythema, edema, and subsequent scaling.2 It occurs in up to 30% of patients with borderline leprosy, usually within 12 months of treatment initiation.2 Our patient had considerable edema and erythema of the hands and feet (Figure 4) along with extensive polyneuropathy prior to starting therapy.
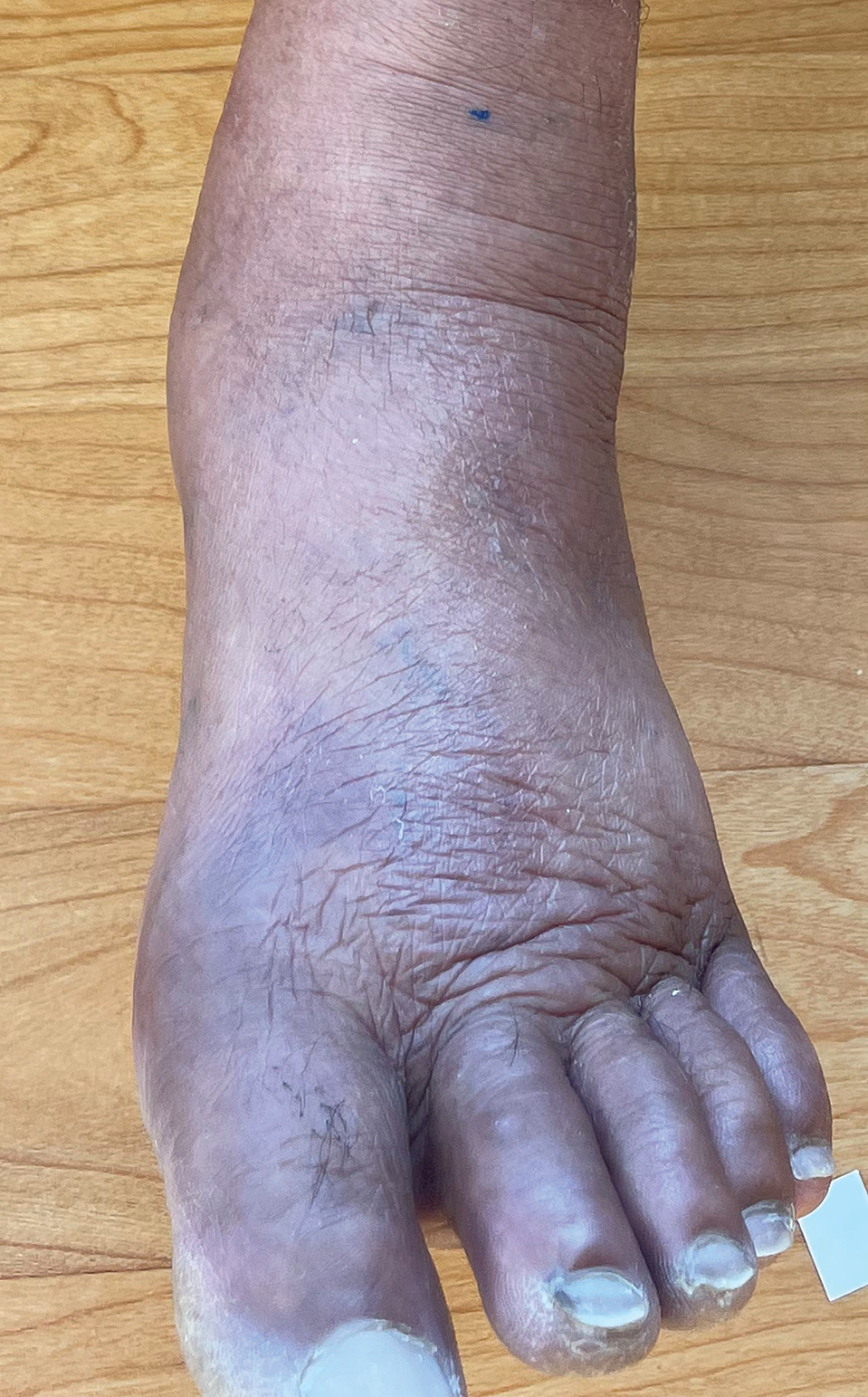
Lucio phenomenon is a rare leprosy reaction found in patients with untreated lepromatous leprosy characterized by erythematous to violaceous macules that lead to ulceronecrotic lesions.5 Histologically, there are many AFB in the vascular endothelium, leukocytoclastic vasculitis, and ischemic epidermal necrosis.5 Our patient did not have ulcerative or necrotic lesions.
The classic skin lesions of psoriasis vulgaris can be described as well-demarcated pink plaques with white or silvery scales that usually are distributed symmetrically and often are found on extensor surfaces.6 Rapidly progressive lesions can be annular with normal skin in the center, mimicking the lesions seen in tuberculoid leprosy. Clinically, both psoriasis and tuberculoid forms of leprosy are sharply demarcated; however, psoriatic lesions often have micaceous overlying scale that is not present in leprosy. Characteristic histologic findings of psoriasis are hyperkeratosis, parakeratosis, and acanthosis of the epidermis with dilated blood vessels and a lymphocytic infiltrate, predominantly into the dermis.7 Psoriatic arthritis has a variable clinical course but tends to emerge 5 to 12 years after initial skin manifestation.8 Classic clinical symptoms include swelling, tenderness, stiffness, and pain in joints and surrounding tissues.8 Other than edema, our patient did not exhibit signs of psoriatic arthritis.
Sarcoidosis is a systemic autoimmune disease characterized by noncaseating epithelioid granulomas affecting various organs, with cutaneous manifestations present in approximately 30% of all cases. Cutaneous manifestations can be variable, including maculopapular lesions, plaques, and nodules.9 Differentiating between cutaneous sarcoidosis and tuberculoid leprosy can be challenging, as both are granulomatous processes; however, histology of sarcoidosis demonstrates noncaseating granulomas in the dermis and/or subcutaneous tissues without AFB9 compared to granulomas with necrotic centers in tuberculoid leprosy.
Cutaneous tuberculosis has variable morphologies. One subtype, lupus vulgaris, can manifest with violaceous, scaly, eroded plaques that could be confused for leprosy. Lupus vulgaris usually results from hematogenous or lymphatic seeding in individuals with high or moderate immunity to M tuberculosis.10
Histologically, the dermis has tuberculoid granulomas containing multinucleated giant cells,10 which can mimic those seen in BB leprosy. Tuberculin skin test results often are positive10; while this test was not performed in our patient, chest radiography was unremarkable, making this diagnosis less likely.
Mycobacterium leprae infections should be considered in a patient with a worsening rash and progressive polyneuropathy. Clinical diagnosis can be challenging due to similarities with other diseases; however, histopathologic findings can help differentiate M leprae from other conditions. This infection is treatable, and early detection can minimize long-term patient morbidity.
- CDC. Hansen’s disease (leprosy). Accessed April 23, 2025. https://www.cdc.gov/leprosy/about/index.html
- Fischer M. Leprosy—an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827.
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020; 83:1-14.
- World Health Organization. Guidelines for the diagnosis, treatment and prevention of leprosy. October 6, 2018. Accessed April 2, 2025. https://www.who.int/publications/i/item/9789290226383
- Frade MAC, Coltro PS, Filho FB, et al. Lucio’s phenomenon: a systematic literature review of definition, clinical features, histopathogenesis and management. Indian J Dermatol Venereol Leprol. 2022;88:464-477.
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Evidence-Based Psoriasis. Springer International Publishing; 2018:1-16.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271.
- Menter A. Psoriasis and psoriatic arthritis overview. Am J Manag Care. 2016;22(8 suppl):S216-S224.
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514.
- Hill MK, Sanders CV. Cutaneous tuberculosis. Microbiol Spectr. 2017;5:1-6.
THE DIAGNOSIS: Borderline-Borderline Leprosy With Type 1 Lepra Reaction
Punch biopsies from plaques on the right elbow and right shin revealed diffuse granulomatous dermatitis (Figure 1) with a narrow Grenz zone in the superficial dermis. The upper dermis contained a dense bandlike infiltrate of histiocytes with abundant foamy-gray cytoplasm and a moderate admixture of lymphocytes. The mid and deep dermis contained a nodular, perivascular, periadnexal, and perineural infiltrate of histiocytes and a dense admixture of lymphocytes. Periodic acid-Schiff and Gram stains were negative for microorganisms. Fite stain was positive for numerous organisms in histiocytes and small dermal nerves (Figure 2). These findings and the clinical examination confirmed a diagnosis of borderline-borderline leprosy with type 1 lepra reaction. The patient was started on dapsone 100 mg, rifampin 600 mg, and clofazimine 100 mg once daily and experienced clinical improvement within 6 months.


The World Health Organization reported more than 200,000 new leprosy cases globally in 2019, with most occurring in India, Brazil, and Indonesia.1 About 150 to 250 new cases are detected in the United States annually.1 The Ridley-Jopling classification of leprosy divides the condition into 5 categories: tuberculoid, borderline tuberculoid, borderline-borderline (BB), borderline lepromatous, and lepromatous. At one end of the spectrum, tuberculoid leprosy—a predominant Th1 immune response mediated by CD4 lymphocytes, interleukin (IL) 2, and interferon gamma2—is characterized by sharply demarcated erythematous and hypopigmented plaques with raised borders and an annular appearance.2,3 Lesions typically have atrophic and hypopigmented centers that often appear in an asymmetric distribution on the arms and legs.2,3 Histologic features include dermal tuberculoid granulomas with epithelioid cells—some located directly beneath the epidermis and others around deep vessels and nerves3—multinucleated Langerhans giant cells, thickened peripheral nerves with intraneural lymphocytic infiltrates, and granulomas with central necrosis. Fite-Faraco staining exhibits few bacteria.2
Lepromatous leprosy occurs in individuals with impaired T-cell immunity, leading to multiple red-brown nodular infiltrates in the skin and mucous membranes.2,3 Lesions typically are symmetric and favor the face and auricle of the ear.2,3 Histologically, there are bluish-gray foamy macrophages that form diffuse or nodular infiltrates with few lymphocytes,2 with a Grenz zone between the epidermis and dermis. Nerves may show lamination of the perineurium resembling an onion skin.2,3 Immunohistochemistry shows predominant CD8-positive infiltrates with a Th2 response and positive IL-4 and IL-10. Fite-Faraco stain shows numerous mycobacteria arranged in clusters and in histiocytes.2
Tuberculoid leprosy is treated with dapsone 100 mg and rifampin 600 mg once daily for 6 months,4 and lepromatous leprosy is treated with dapsone 100 mg, rifampin 600 mg, and clofazimine 50 mg once daily for 12 months.4 The prognosis for both is good with treatment; erythema and induration of skin lesions may improve within a few months, but residual nerve damage is common, especially in those with advanced disease prior to treatment.2 For direct contacts, a single dose of rifampin may be given.4
Borderline-borderline leprosy manifests with numerous asymmetric annular plaques, as seen in our patient (Figure 3). Histology findings can be variable and often overlap with other forms of leprosy. There can be epithelioid granulomas and only a few acid-fast bacilli (AFB) or diffuse histiocytic aggregates with foamy histiocytes containing large numbers of AFB.3 Nerve involvement is variable but can be severe in the setting of type 1 lepra reaction, which was present in our patient. Type 1 lepra reaction—a type IV cell-mediated allergic hypersensitivity reaction to Mycobacterium leprae antigens—manifests clinically with hyperesthesia, erythema, edema, and subsequent scaling.2 It occurs in up to 30% of patients with borderline leprosy, usually within 12 months of treatment initiation.2 Our patient had considerable edema and erythema of the hands and feet (Figure 4) along with extensive polyneuropathy prior to starting therapy.


Lucio phenomenon is a rare leprosy reaction found in patients with untreated lepromatous leprosy characterized by erythematous to violaceous macules that lead to ulceronecrotic lesions.5 Histologically, there are many AFB in the vascular endothelium, leukocytoclastic vasculitis, and ischemic epidermal necrosis.5 Our patient did not have ulcerative or necrotic lesions.
The classic skin lesions of psoriasis vulgaris can be described as well-demarcated pink plaques with white or silvery scales that usually are distributed symmetrically and often are found on extensor surfaces.6 Rapidly progressive lesions can be annular with normal skin in the center, mimicking the lesions seen in tuberculoid leprosy. Clinically, both psoriasis and tuberculoid forms of leprosy are sharply demarcated; however, psoriatic lesions often have micaceous overlying scale that is not present in leprosy. Characteristic histologic findings of psoriasis are hyperkeratosis, parakeratosis, and acanthosis of the epidermis with dilated blood vessels and a lymphocytic infiltrate, predominantly into the dermis.7 Psoriatic arthritis has a variable clinical course but tends to emerge 5 to 12 years after initial skin manifestation.8 Classic clinical symptoms include swelling, tenderness, stiffness, and pain in joints and surrounding tissues.8 Other than edema, our patient did not exhibit signs of psoriatic arthritis.
Sarcoidosis is a systemic autoimmune disease characterized by noncaseating epithelioid granulomas affecting various organs, with cutaneous manifestations present in approximately 30% of all cases. Cutaneous manifestations can be variable, including maculopapular lesions, plaques, and nodules.9 Differentiating between cutaneous sarcoidosis and tuberculoid leprosy can be challenging, as both are granulomatous processes; however, histology of sarcoidosis demonstrates noncaseating granulomas in the dermis and/or subcutaneous tissues without AFB9 compared to granulomas with necrotic centers in tuberculoid leprosy.
Cutaneous tuberculosis has variable morphologies. One subtype, lupus vulgaris, can manifest with violaceous, scaly, eroded plaques that could be confused for leprosy. Lupus vulgaris usually results from hematogenous or lymphatic seeding in individuals with high or moderate immunity to M tuberculosis.10
Histologically, the dermis has tuberculoid granulomas containing multinucleated giant cells,10 which can mimic those seen in BB leprosy. Tuberculin skin test results often are positive10; while this test was not performed in our patient, chest radiography was unremarkable, making this diagnosis less likely.
Mycobacterium leprae infections should be considered in a patient with a worsening rash and progressive polyneuropathy. Clinical diagnosis can be challenging due to similarities with other diseases; however, histopathologic findings can help differentiate M leprae from other conditions. This infection is treatable, and early detection can minimize long-term patient morbidity.
THE DIAGNOSIS: Borderline-Borderline Leprosy With Type 1 Lepra Reaction
Punch biopsies from plaques on the right elbow and right shin revealed diffuse granulomatous dermatitis (Figure 1) with a narrow Grenz zone in the superficial dermis. The upper dermis contained a dense bandlike infiltrate of histiocytes with abundant foamy-gray cytoplasm and a moderate admixture of lymphocytes. The mid and deep dermis contained a nodular, perivascular, periadnexal, and perineural infiltrate of histiocytes and a dense admixture of lymphocytes. Periodic acid-Schiff and Gram stains were negative for microorganisms. Fite stain was positive for numerous organisms in histiocytes and small dermal nerves (Figure 2). These findings and the clinical examination confirmed a diagnosis of borderline-borderline leprosy with type 1 lepra reaction. The patient was started on dapsone 100 mg, rifampin 600 mg, and clofazimine 100 mg once daily and experienced clinical improvement within 6 months.


The World Health Organization reported more than 200,000 new leprosy cases globally in 2019, with most occurring in India, Brazil, and Indonesia.1 About 150 to 250 new cases are detected in the United States annually.1 The Ridley-Jopling classification of leprosy divides the condition into 5 categories: tuberculoid, borderline tuberculoid, borderline-borderline (BB), borderline lepromatous, and lepromatous. At one end of the spectrum, tuberculoid leprosy—a predominant Th1 immune response mediated by CD4 lymphocytes, interleukin (IL) 2, and interferon gamma2—is characterized by sharply demarcated erythematous and hypopigmented plaques with raised borders and an annular appearance.2,3 Lesions typically have atrophic and hypopigmented centers that often appear in an asymmetric distribution on the arms and legs.2,3 Histologic features include dermal tuberculoid granulomas with epithelioid cells—some located directly beneath the epidermis and others around deep vessels and nerves3—multinucleated Langerhans giant cells, thickened peripheral nerves with intraneural lymphocytic infiltrates, and granulomas with central necrosis. Fite-Faraco staining exhibits few bacteria.2
Lepromatous leprosy occurs in individuals with impaired T-cell immunity, leading to multiple red-brown nodular infiltrates in the skin and mucous membranes.2,3 Lesions typically are symmetric and favor the face and auricle of the ear.2,3 Histologically, there are bluish-gray foamy macrophages that form diffuse or nodular infiltrates with few lymphocytes,2 with a Grenz zone between the epidermis and dermis. Nerves may show lamination of the perineurium resembling an onion skin.2,3 Immunohistochemistry shows predominant CD8-positive infiltrates with a Th2 response and positive IL-4 and IL-10. Fite-Faraco stain shows numerous mycobacteria arranged in clusters and in histiocytes.2
Tuberculoid leprosy is treated with dapsone 100 mg and rifampin 600 mg once daily for 6 months,4 and lepromatous leprosy is treated with dapsone 100 mg, rifampin 600 mg, and clofazimine 50 mg once daily for 12 months.4 The prognosis for both is good with treatment; erythema and induration of skin lesions may improve within a few months, but residual nerve damage is common, especially in those with advanced disease prior to treatment.2 For direct contacts, a single dose of rifampin may be given.4
Borderline-borderline leprosy manifests with numerous asymmetric annular plaques, as seen in our patient (Figure 3). Histology findings can be variable and often overlap with other forms of leprosy. There can be epithelioid granulomas and only a few acid-fast bacilli (AFB) or diffuse histiocytic aggregates with foamy histiocytes containing large numbers of AFB.3 Nerve involvement is variable but can be severe in the setting of type 1 lepra reaction, which was present in our patient. Type 1 lepra reaction—a type IV cell-mediated allergic hypersensitivity reaction to Mycobacterium leprae antigens—manifests clinically with hyperesthesia, erythema, edema, and subsequent scaling.2 It occurs in up to 30% of patients with borderline leprosy, usually within 12 months of treatment initiation.2 Our patient had considerable edema and erythema of the hands and feet (Figure 4) along with extensive polyneuropathy prior to starting therapy.


Lucio phenomenon is a rare leprosy reaction found in patients with untreated lepromatous leprosy characterized by erythematous to violaceous macules that lead to ulceronecrotic lesions.5 Histologically, there are many AFB in the vascular endothelium, leukocytoclastic vasculitis, and ischemic epidermal necrosis.5 Our patient did not have ulcerative or necrotic lesions.
The classic skin lesions of psoriasis vulgaris can be described as well-demarcated pink plaques with white or silvery scales that usually are distributed symmetrically and often are found on extensor surfaces.6 Rapidly progressive lesions can be annular with normal skin in the center, mimicking the lesions seen in tuberculoid leprosy. Clinically, both psoriasis and tuberculoid forms of leprosy are sharply demarcated; however, psoriatic lesions often have micaceous overlying scale that is not present in leprosy. Characteristic histologic findings of psoriasis are hyperkeratosis, parakeratosis, and acanthosis of the epidermis with dilated blood vessels and a lymphocytic infiltrate, predominantly into the dermis.7 Psoriatic arthritis has a variable clinical course but tends to emerge 5 to 12 years after initial skin manifestation.8 Classic clinical symptoms include swelling, tenderness, stiffness, and pain in joints and surrounding tissues.8 Other than edema, our patient did not exhibit signs of psoriatic arthritis.
Sarcoidosis is a systemic autoimmune disease characterized by noncaseating epithelioid granulomas affecting various organs, with cutaneous manifestations present in approximately 30% of all cases. Cutaneous manifestations can be variable, including maculopapular lesions, plaques, and nodules.9 Differentiating between cutaneous sarcoidosis and tuberculoid leprosy can be challenging, as both are granulomatous processes; however, histology of sarcoidosis demonstrates noncaseating granulomas in the dermis and/or subcutaneous tissues without AFB9 compared to granulomas with necrotic centers in tuberculoid leprosy.
Cutaneous tuberculosis has variable morphologies. One subtype, lupus vulgaris, can manifest with violaceous, scaly, eroded plaques that could be confused for leprosy. Lupus vulgaris usually results from hematogenous or lymphatic seeding in individuals with high or moderate immunity to M tuberculosis.10
Histologically, the dermis has tuberculoid granulomas containing multinucleated giant cells,10 which can mimic those seen in BB leprosy. Tuberculin skin test results often are positive10; while this test was not performed in our patient, chest radiography was unremarkable, making this diagnosis less likely.
Mycobacterium leprae infections should be considered in a patient with a worsening rash and progressive polyneuropathy. Clinical diagnosis can be challenging due to similarities with other diseases; however, histopathologic findings can help differentiate M leprae from other conditions. This infection is treatable, and early detection can minimize long-term patient morbidity.
- CDC. Hansen’s disease (leprosy). Accessed April 23, 2025. https://www.cdc.gov/leprosy/about/index.html
- Fischer M. Leprosy—an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827.
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020; 83:1-14.
- World Health Organization. Guidelines for the diagnosis, treatment and prevention of leprosy. October 6, 2018. Accessed April 2, 2025. https://www.who.int/publications/i/item/9789290226383
- Frade MAC, Coltro PS, Filho FB, et al. Lucio’s phenomenon: a systematic literature review of definition, clinical features, histopathogenesis and management. Indian J Dermatol Venereol Leprol. 2022;88:464-477.
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Evidence-Based Psoriasis. Springer International Publishing; 2018:1-16.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271.
- Menter A. Psoriasis and psoriatic arthritis overview. Am J Manag Care. 2016;22(8 suppl):S216-S224.
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514.
- Hill MK, Sanders CV. Cutaneous tuberculosis. Microbiol Spectr. 2017;5:1-6.
- CDC. Hansen’s disease (leprosy). Accessed April 23, 2025. https://www.cdc.gov/leprosy/about/index.html
- Fischer M. Leprosy—an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827.
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020; 83:1-14.
- World Health Organization. Guidelines for the diagnosis, treatment and prevention of leprosy. October 6, 2018. Accessed April 2, 2025. https://www.who.int/publications/i/item/9789290226383
- Frade MAC, Coltro PS, Filho FB, et al. Lucio’s phenomenon: a systematic literature review of definition, clinical features, histopathogenesis and management. Indian J Dermatol Venereol Leprol. 2022;88:464-477.
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Evidence-Based Psoriasis. Springer International Publishing; 2018:1-16.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271.
- Menter A. Psoriasis and psoriatic arthritis overview. Am J Manag Care. 2016;22(8 suppl):S216-S224.
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514.
- Hill MK, Sanders CV. Cutaneous tuberculosis. Microbiol Spectr. 2017;5:1-6.
Acral Erythema, Edema, and Scaly Plaques in a Patient With Polyneuropathy
Acral Erythema, Edema, and Scaly Plaques in a Patient With Polyneuropathy
A 67-year-old man presented to his primary care physician with scaly plaques on the extensor surfaces along with distal neuropathy that had been slowly worsening over the past 6 months. The patient was prescribed triamcinolone cream 0.1% twice daily for presumed atopic dermatitis. Three months later, his symptoms rapidly worsened and he developed edema of the hands and feet. He was seen by neurology, and electromyography revealed severe distal sensorimotor neuropathy, prompting hospital admission for further evaluation of a potential rapidly progressive autoimmune disease. Laboratory workup and imaging were ordered, and the patient began an intravenous course of methylprednisolone. Minimal improvement in his symptoms was noted after 1 day, at which time dermatology was consulted.
Physical examination by dermatology revealed well-defined plaques with annular scale on extensor surfaces of the arms and legs, and edema on the hands and feet as well as distal sensorimotor neuropathy. The patient reported associated unspecified weight loss but denied any chest pain, shortness of breath, fevers, chills, cough, night sweats, exposure to chemicals, or recent travel. He reported that he had immigrated from India 37 years prior; his last visit to India was 6 years ago. He currently was taking famotidine for gastrointestinal reflux disease and losartan for hypertension. There was no personal or family history of autoimmune diseases. A complete workup for hematologic, thyroid, liver, and renal function was unremarkable. Initial autoimmune workup was negative for antinuclear antibodies and rheumatoid factor. Serum protein electrophoresis was normal. Results of testing for HIV, hepatitis B, and hepatitis C were negative. Chest radiography was unremarkable. Erythrocyte sedimentation rate and C-reactive protein level were elevated.
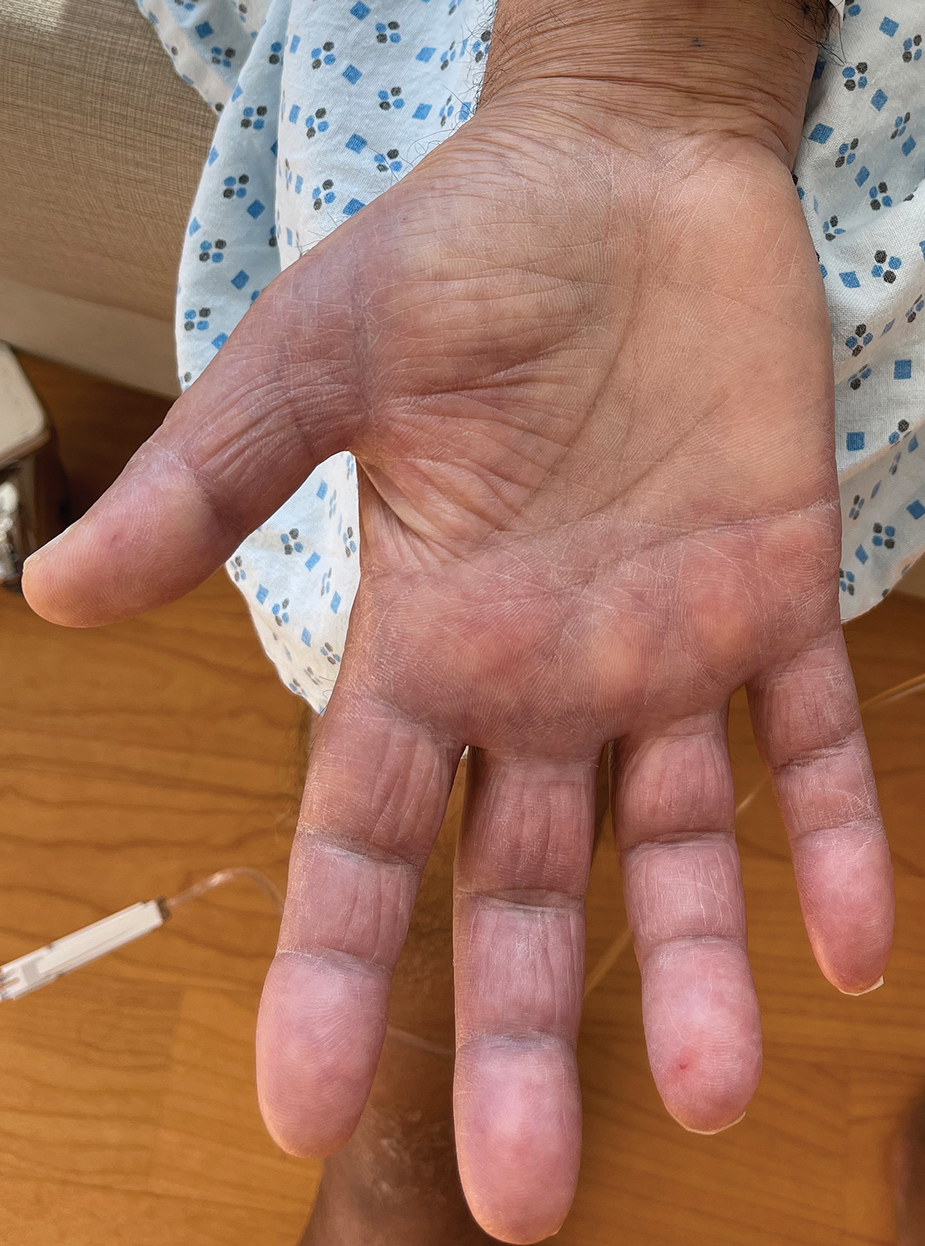
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
To the Editor:
Cutaneous medical conditions can have a substantial impact on patients’ functioning and quality of life. Many patients with severe skin disease are eligible to receive disability assistance that can provide them with essential income and health care. Previous research has highlighted disability assessment as one of the most important ways physicians can help mitigate the health consequences of poverty.1 Dermatologists can play an important role in the disability assessment process by documenting the facts associated with patients’ skin conditions.
Although skin conditions have a relatively high prevalence, they remain underrepresented in disability claims. Between 1997 and 2004, occupational skin diseases accounted for 12% to 17% of nonfatal work-related illnesses; however, during that same period, skin conditions comprised only 0.21% of disability claims in the United States.2,3 Historically, there has been hesitancy among dermatologists to complete disability paperwork; a 1976 survey of dermatologists cited extensive paperwork, “troublesome patients,” and fee schedule issues as reasons.4 The lack of training regarding disability assessment in medical school and residency also has been noted.5
To characterize modern attitudes toward disability assessments, we conducted a survey of dermatologists across the United States. Our study was reviewed and declared exempt by the institutional review board of the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (Torrance, California)(approval #18CR-32242-01). Using convenience sampling, we emailed dermatologists from the Association of Professors of Dermatology and dermatology state societies in all 50 states inviting them to participate in our voluntary and anonymous survey, which was administered using SurveyMonkey. The use of all society mailing lists was approved by the respective owners. The 15-question survey included multiple choice, Likert scale, and free response sections. Summary and descriptive statistics were used to describe respondent demographics and identify any patterns in responses.
For each Likert-based question, participants ranked their degree of agreement with a statement as: 1=strongly disagree, 2=somewhat disagree, 3=neither agree nor disagree/neutral, 4=somewhat agree, and 5=strongly agree. The mean response and standard deviation were reported for each Likert scale prompt. Preplanned 1-sample t testing was used to analyze Likert scale data, in which the mean response for each prompt was compared to a baseline response of 3 (neutral). A P value <.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics for MacOS, version 27 (IBM).
Seventy-eight dermatologists agreed to participate, and 70 completed the survey, for a response rate of 89.7% (Table 1). The dermatologists we surveyed practiced in a variety of clinical settings, including academic public hospitals (46.2% [36/78]), academic private hospitals (33.3% [26/78]), and private practices (32.1% [25/78]), and 60.3% (47/78) reported providing disability documentation at some point. Most of the respondents (64.3% [45/70]) did not perform assessments in an average month (Table 2). Medical assessment documentation was provided most frequently for workers’ compensation (50.0% [35/70]), private insurance (27.1% [19/70]), and Social Security Disability Insurance (25.7% [18/70]). Dermatologists overwhelmingly reported no formal training for disability assessment in medical school (94.3% [66/70]), residency (97.1% [68/70]), or clinical practice (81.4% [57/70]).


In the Likert scale prompts, respondents agreed that they were uncertain of their role in disability assessment (mean response, 3.6; P<.001). Moreover, they were uncomfortable providing assessments (mean response, 3.5; P<.001) and felt that they did not have sufficient time to perform them (mean response, 3.6; P<.001). Dermatologists disagreed that they received adequate compensation for performing assessments (mean response, 2.2; P<.001) and felt that they did not have enough time to participate in assessments (mean response, 3.6; P<.001). Respondents generally did not feel distrustful of patients seeking disability assessment (mean response, 2.8; P=.043). Dermatologists neither agreed nor disagreed when asked if they thought that physicians can determine disability status (mean response, 3.2; P=.118). The details of the Likert scale responses are described in Table 3. Respondents also were uncertain as to which dermatologic conditions were eligible for disability. When asked to select which conditions from a list of 10 were eligible per the Social Security Administration listing of disability impairments, only 15.4% (12/70) of respondents correctly identified that all the conditions qualified; these included ichthyosis, pemphigus vulgaris, allergic contact dermatitis, hidradenitis suppurativa, systemic lupus erythematosus, chromoblastomycosis, xeroderma pigmentosum, burns, malignant melanoma, and scleroderma.6

In the free-response prompts, respondents frequently described extensive paperwork, inadequate time, and lack of reimbursement as barriers to providing documentation. Often, dermatologists found that the forms were not well matched to the skin conditions they were evaluating and rather had a musculoskeletal focus. Multiple individuals commented on the challenge in assessing the percentage of disability and functional/psychosocial impairment in skin conditions. One respondent noted that workers’ compensation forms ask if the patient is “…permanent and stationary…for most conditions this has no meaning in dermatology.” Some felt hesitant to provide documentation because they had insufficient patient history, especially regarding employment, and opted to defer to primary care providers who might be more familiar with the full patient history.
A dermatologist described their perspective as follows:
“…As a specialist I feel that I don’t have a complete look into all the factors that could contribute to a patient[’]s need to go on disability, and I don’t have experience with filling out disability requests. That being said, if a patient[’]s request for disability was due to a skin disease that I know way more about than [a] primary care [physician] would, I would do the disability assessment.”
Another respondent noted the complexity in “establishing causality” for workers’ compensation. Another dermatologist reported,
“The most frequent challenging situation I encounter is being asked to evaluate for maximum medical improvement after patch testing. If the patient is not fully avoiding contact allergens either at home or at work, then I typically document that they are not at [maximum medical improvement]. The reality is that most frequently it is due to exposure to allergens at home so the line between what is a legitimate worker’s comp[ensation] issue and what is a home life choice is blurry.”
Nevertheless, respondents expressed interest in learning more about disability assessment procedures. Summary guides, lectures, and prefilled paperwork were the most popular initiatives that respondents agreed would be beneficial toward becoming educated regarding disability assessment (78.6%, 58.6%, and 58.6%, respectively)(Table 2). One respondent noted that “previous [internal medicine] history help[ed]” them in performing cutaneous disability assessments.
As with any survey, our study did have some inherent limitations. Only a relatively small sample size was willing to complete the survey. There was a predominance of respondents from California (34.6% [27/78]), as well as those practicing for less than 15 years (58.9% [46/78])(Figure). This could limit generalizability to the national population of dermatologists. In addition, there was potential for recall bias and errors in responding given the self-reported nature of the study. Different individuals may interpret the Likert scale options in various ways, which could skew results unintentionally. However, the survey was largely qualitative in nature, making it a legitimate tool for answering our research questions. Moreover, we were able to hear the perspectives of dermatologists across diverse practice settings, with free response prompts to increase the depth of the survey.
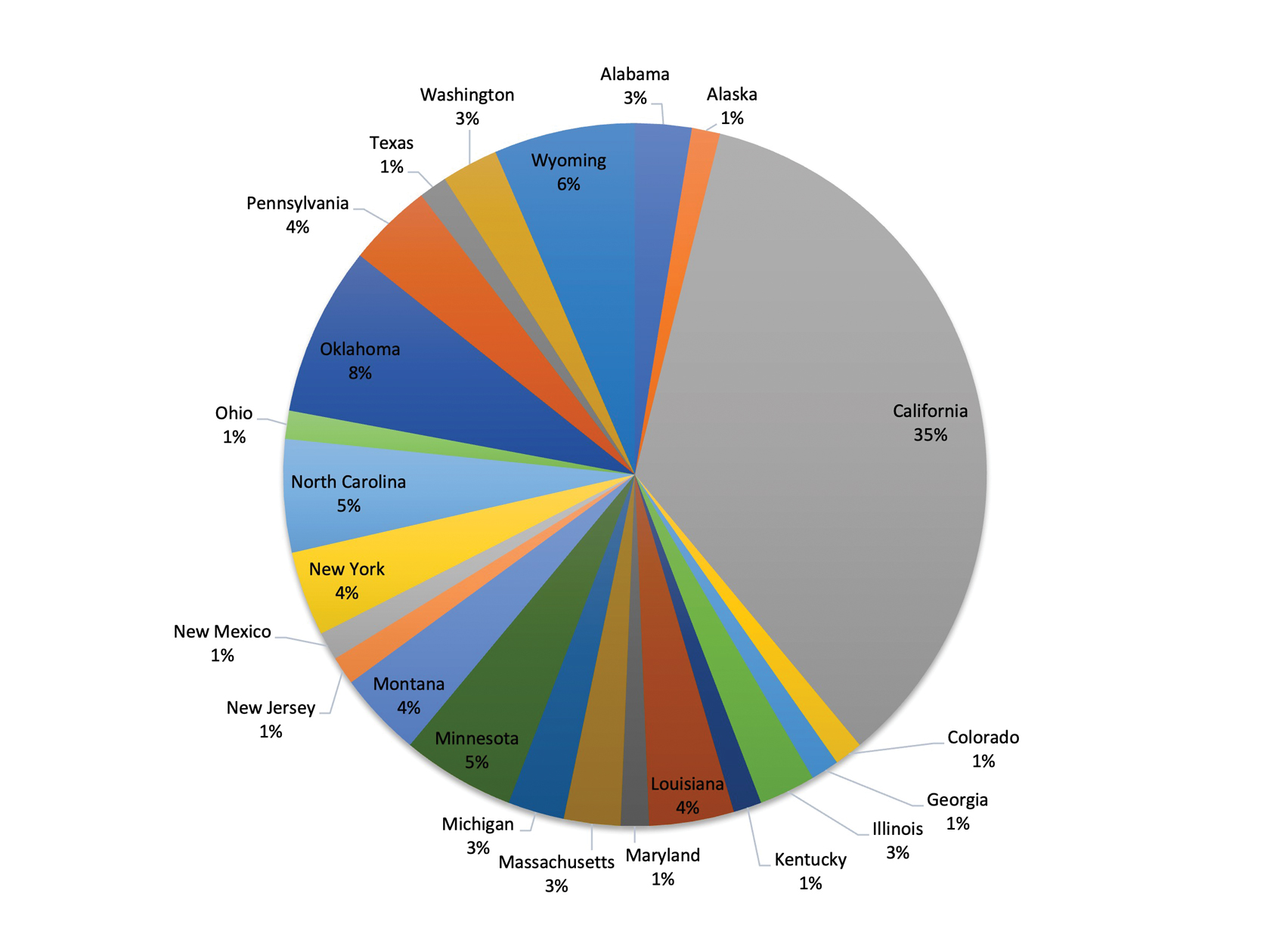
Almost 50 years later, our survey echoes common themes from Adams’ 1976 survey.4 Inadequate compensation, limited time, and burdensome paperwork all continue to hinder dermatologists’ ability to perform disability assessments. Our participants frequently commented that the current disability forms are not congruent with the nature of skin conditions, making it challenging to accurately document the facts.
Moreover, respondents felt uncertain in their role in disability assessment and occasionally noted distrust of patients or insufficient patient history as barriers to completing assessments. They also were unsure if physicians can grant disability status. This is a common misconception among physicians that leads to discomfort in helping with disability assessment.7 The role of physicians in disability assessment is to document the facts of a patient’s illness, not to determine whether they are eligible for benefits. We discovered uncertainty in our respondents’ ability to identify conditions eligible for disability, highlighting an area in need of greater education for physicians.
Despite these obstacles, respondents were interested in learning more about disability assessment and highlighted several practical approaches that could help them better perform this task. As skin specialists, dermatologists are the best-equipped physicians to assess cutaneous conditions and should play a greater role in performing disability assessments, which could be achieved through increased educational initiatives and individual physician motivation.7 We call for greater collaboration and reflection on the importance of disability assistance among dermatologists to increase participation in the disability-assessment process.
- O’Connell JJ, Zevin BD, Quick PD, et al. Documenting disability: simple strategies for medical providers. Health Care for the Homeless Clinicians’ Network. September 2007. Accessed March 31, 2025. https://nhchc.org/wp-content/uploads/2019/08/DocumentingDisability2007.pdf
- US Bureau of Labor Statistics. Injuries, illnesses, and fatalities. Accessed March 31, 2025. https://www.bls.gov/iif/
- Meseguer J. Outcome variation in the Social Security Disability Insurance Program: the role of primary diagnoses. Soc Secur Bull. 2013;73:39-75.
- Adams RM. Attitudes of California dermatologists toward Worker’s Compensation: results of a survey. West J Med. 1976;125:169-175.
- Talmage J, Melhorn J, Hyman M. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
- Social Security Administration. Disability evaluation under Social Security. 8.00 skin disorders - adult. March 31, 2025. https://www.ssa.gov/disability/professionals/bluebook/8.00-Skin-Adult.htm
- Dawson J, Smogorzewski J. Demystifying disability assessments for dermatologists—a call to action. JAMA Dermatol. 2021;157:903-904. doi:10.1001/jamadermatol.2021.1767
To the Editor:
Cutaneous medical conditions can have a substantial impact on patients’ functioning and quality of life. Many patients with severe skin disease are eligible to receive disability assistance that can provide them with essential income and health care. Previous research has highlighted disability assessment as one of the most important ways physicians can help mitigate the health consequences of poverty.1 Dermatologists can play an important role in the disability assessment process by documenting the facts associated with patients’ skin conditions.
Although skin conditions have a relatively high prevalence, they remain underrepresented in disability claims. Between 1997 and 2004, occupational skin diseases accounted for 12% to 17% of nonfatal work-related illnesses; however, during that same period, skin conditions comprised only 0.21% of disability claims in the United States.2,3 Historically, there has been hesitancy among dermatologists to complete disability paperwork; a 1976 survey of dermatologists cited extensive paperwork, “troublesome patients,” and fee schedule issues as reasons.4 The lack of training regarding disability assessment in medical school and residency also has been noted.5
To characterize modern attitudes toward disability assessments, we conducted a survey of dermatologists across the United States. Our study was reviewed and declared exempt by the institutional review board of the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (Torrance, California)(approval #18CR-32242-01). Using convenience sampling, we emailed dermatologists from the Association of Professors of Dermatology and dermatology state societies in all 50 states inviting them to participate in our voluntary and anonymous survey, which was administered using SurveyMonkey. The use of all society mailing lists was approved by the respective owners. The 15-question survey included multiple choice, Likert scale, and free response sections. Summary and descriptive statistics were used to describe respondent demographics and identify any patterns in responses.
For each Likert-based question, participants ranked their degree of agreement with a statement as: 1=strongly disagree, 2=somewhat disagree, 3=neither agree nor disagree/neutral, 4=somewhat agree, and 5=strongly agree. The mean response and standard deviation were reported for each Likert scale prompt. Preplanned 1-sample t testing was used to analyze Likert scale data, in which the mean response for each prompt was compared to a baseline response of 3 (neutral). A P value <.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics for MacOS, version 27 (IBM).
Seventy-eight dermatologists agreed to participate, and 70 completed the survey, for a response rate of 89.7% (Table 1). The dermatologists we surveyed practiced in a variety of clinical settings, including academic public hospitals (46.2% [36/78]), academic private hospitals (33.3% [26/78]), and private practices (32.1% [25/78]), and 60.3% (47/78) reported providing disability documentation at some point. Most of the respondents (64.3% [45/70]) did not perform assessments in an average month (Table 2). Medical assessment documentation was provided most frequently for workers’ compensation (50.0% [35/70]), private insurance (27.1% [19/70]), and Social Security Disability Insurance (25.7% [18/70]). Dermatologists overwhelmingly reported no formal training for disability assessment in medical school (94.3% [66/70]), residency (97.1% [68/70]), or clinical practice (81.4% [57/70]).


In the Likert scale prompts, respondents agreed that they were uncertain of their role in disability assessment (mean response, 3.6; P<.001). Moreover, they were uncomfortable providing assessments (mean response, 3.5; P<.001) and felt that they did not have sufficient time to perform them (mean response, 3.6; P<.001). Dermatologists disagreed that they received adequate compensation for performing assessments (mean response, 2.2; P<.001) and felt that they did not have enough time to participate in assessments (mean response, 3.6; P<.001). Respondents generally did not feel distrustful of patients seeking disability assessment (mean response, 2.8; P=.043). Dermatologists neither agreed nor disagreed when asked if they thought that physicians can determine disability status (mean response, 3.2; P=.118). The details of the Likert scale responses are described in Table 3. Respondents also were uncertain as to which dermatologic conditions were eligible for disability. When asked to select which conditions from a list of 10 were eligible per the Social Security Administration listing of disability impairments, only 15.4% (12/70) of respondents correctly identified that all the conditions qualified; these included ichthyosis, pemphigus vulgaris, allergic contact dermatitis, hidradenitis suppurativa, systemic lupus erythematosus, chromoblastomycosis, xeroderma pigmentosum, burns, malignant melanoma, and scleroderma.6

In the free-response prompts, respondents frequently described extensive paperwork, inadequate time, and lack of reimbursement as barriers to providing documentation. Often, dermatologists found that the forms were not well matched to the skin conditions they were evaluating and rather had a musculoskeletal focus. Multiple individuals commented on the challenge in assessing the percentage of disability and functional/psychosocial impairment in skin conditions. One respondent noted that workers’ compensation forms ask if the patient is “…permanent and stationary…for most conditions this has no meaning in dermatology.” Some felt hesitant to provide documentation because they had insufficient patient history, especially regarding employment, and opted to defer to primary care providers who might be more familiar with the full patient history.
A dermatologist described their perspective as follows:
“…As a specialist I feel that I don’t have a complete look into all the factors that could contribute to a patient[’]s need to go on disability, and I don’t have experience with filling out disability requests. That being said, if a patient[’]s request for disability was due to a skin disease that I know way more about than [a] primary care [physician] would, I would do the disability assessment.”
Another respondent noted the complexity in “establishing causality” for workers’ compensation. Another dermatologist reported,
“The most frequent challenging situation I encounter is being asked to evaluate for maximum medical improvement after patch testing. If the patient is not fully avoiding contact allergens either at home or at work, then I typically document that they are not at [maximum medical improvement]. The reality is that most frequently it is due to exposure to allergens at home so the line between what is a legitimate worker’s comp[ensation] issue and what is a home life choice is blurry.”
Nevertheless, respondents expressed interest in learning more about disability assessment procedures. Summary guides, lectures, and prefilled paperwork were the most popular initiatives that respondents agreed would be beneficial toward becoming educated regarding disability assessment (78.6%, 58.6%, and 58.6%, respectively)(Table 2). One respondent noted that “previous [internal medicine] history help[ed]” them in performing cutaneous disability assessments.
As with any survey, our study did have some inherent limitations. Only a relatively small sample size was willing to complete the survey. There was a predominance of respondents from California (34.6% [27/78]), as well as those practicing for less than 15 years (58.9% [46/78])(Figure). This could limit generalizability to the national population of dermatologists. In addition, there was potential for recall bias and errors in responding given the self-reported nature of the study. Different individuals may interpret the Likert scale options in various ways, which could skew results unintentionally. However, the survey was largely qualitative in nature, making it a legitimate tool for answering our research questions. Moreover, we were able to hear the perspectives of dermatologists across diverse practice settings, with free response prompts to increase the depth of the survey.

Almost 50 years later, our survey echoes common themes from Adams’ 1976 survey.4 Inadequate compensation, limited time, and burdensome paperwork all continue to hinder dermatologists’ ability to perform disability assessments. Our participants frequently commented that the current disability forms are not congruent with the nature of skin conditions, making it challenging to accurately document the facts.
Moreover, respondents felt uncertain in their role in disability assessment and occasionally noted distrust of patients or insufficient patient history as barriers to completing assessments. They also were unsure if physicians can grant disability status. This is a common misconception among physicians that leads to discomfort in helping with disability assessment.7 The role of physicians in disability assessment is to document the facts of a patient’s illness, not to determine whether they are eligible for benefits. We discovered uncertainty in our respondents’ ability to identify conditions eligible for disability, highlighting an area in need of greater education for physicians.
Despite these obstacles, respondents were interested in learning more about disability assessment and highlighted several practical approaches that could help them better perform this task. As skin specialists, dermatologists are the best-equipped physicians to assess cutaneous conditions and should play a greater role in performing disability assessments, which could be achieved through increased educational initiatives and individual physician motivation.7 We call for greater collaboration and reflection on the importance of disability assistance among dermatologists to increase participation in the disability-assessment process.
To the Editor:
Cutaneous medical conditions can have a substantial impact on patients’ functioning and quality of life. Many patients with severe skin disease are eligible to receive disability assistance that can provide them with essential income and health care. Previous research has highlighted disability assessment as one of the most important ways physicians can help mitigate the health consequences of poverty.1 Dermatologists can play an important role in the disability assessment process by documenting the facts associated with patients’ skin conditions.
Although skin conditions have a relatively high prevalence, they remain underrepresented in disability claims. Between 1997 and 2004, occupational skin diseases accounted for 12% to 17% of nonfatal work-related illnesses; however, during that same period, skin conditions comprised only 0.21% of disability claims in the United States.2,3 Historically, there has been hesitancy among dermatologists to complete disability paperwork; a 1976 survey of dermatologists cited extensive paperwork, “troublesome patients,” and fee schedule issues as reasons.4 The lack of training regarding disability assessment in medical school and residency also has been noted.5
To characterize modern attitudes toward disability assessments, we conducted a survey of dermatologists across the United States. Our study was reviewed and declared exempt by the institutional review board of the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (Torrance, California)(approval #18CR-32242-01). Using convenience sampling, we emailed dermatologists from the Association of Professors of Dermatology and dermatology state societies in all 50 states inviting them to participate in our voluntary and anonymous survey, which was administered using SurveyMonkey. The use of all society mailing lists was approved by the respective owners. The 15-question survey included multiple choice, Likert scale, and free response sections. Summary and descriptive statistics were used to describe respondent demographics and identify any patterns in responses.
For each Likert-based question, participants ranked their degree of agreement with a statement as: 1=strongly disagree, 2=somewhat disagree, 3=neither agree nor disagree/neutral, 4=somewhat agree, and 5=strongly agree. The mean response and standard deviation were reported for each Likert scale prompt. Preplanned 1-sample t testing was used to analyze Likert scale data, in which the mean response for each prompt was compared to a baseline response of 3 (neutral). A P value <.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics for MacOS, version 27 (IBM).
Seventy-eight dermatologists agreed to participate, and 70 completed the survey, for a response rate of 89.7% (Table 1). The dermatologists we surveyed practiced in a variety of clinical settings, including academic public hospitals (46.2% [36/78]), academic private hospitals (33.3% [26/78]), and private practices (32.1% [25/78]), and 60.3% (47/78) reported providing disability documentation at some point. Most of the respondents (64.3% [45/70]) did not perform assessments in an average month (Table 2). Medical assessment documentation was provided most frequently for workers’ compensation (50.0% [35/70]), private insurance (27.1% [19/70]), and Social Security Disability Insurance (25.7% [18/70]). Dermatologists overwhelmingly reported no formal training for disability assessment in medical school (94.3% [66/70]), residency (97.1% [68/70]), or clinical practice (81.4% [57/70]).


In the Likert scale prompts, respondents agreed that they were uncertain of their role in disability assessment (mean response, 3.6; P<.001). Moreover, they were uncomfortable providing assessments (mean response, 3.5; P<.001) and felt that they did not have sufficient time to perform them (mean response, 3.6; P<.001). Dermatologists disagreed that they received adequate compensation for performing assessments (mean response, 2.2; P<.001) and felt that they did not have enough time to participate in assessments (mean response, 3.6; P<.001). Respondents generally did not feel distrustful of patients seeking disability assessment (mean response, 2.8; P=.043). Dermatologists neither agreed nor disagreed when asked if they thought that physicians can determine disability status (mean response, 3.2; P=.118). The details of the Likert scale responses are described in Table 3. Respondents also were uncertain as to which dermatologic conditions were eligible for disability. When asked to select which conditions from a list of 10 were eligible per the Social Security Administration listing of disability impairments, only 15.4% (12/70) of respondents correctly identified that all the conditions qualified; these included ichthyosis, pemphigus vulgaris, allergic contact dermatitis, hidradenitis suppurativa, systemic lupus erythematosus, chromoblastomycosis, xeroderma pigmentosum, burns, malignant melanoma, and scleroderma.6

In the free-response prompts, respondents frequently described extensive paperwork, inadequate time, and lack of reimbursement as barriers to providing documentation. Often, dermatologists found that the forms were not well matched to the skin conditions they were evaluating and rather had a musculoskeletal focus. Multiple individuals commented on the challenge in assessing the percentage of disability and functional/psychosocial impairment in skin conditions. One respondent noted that workers’ compensation forms ask if the patient is “…permanent and stationary…for most conditions this has no meaning in dermatology.” Some felt hesitant to provide documentation because they had insufficient patient history, especially regarding employment, and opted to defer to primary care providers who might be more familiar with the full patient history.
A dermatologist described their perspective as follows:
“…As a specialist I feel that I don’t have a complete look into all the factors that could contribute to a patient[’]s need to go on disability, and I don’t have experience with filling out disability requests. That being said, if a patient[’]s request for disability was due to a skin disease that I know way more about than [a] primary care [physician] would, I would do the disability assessment.”
Another respondent noted the complexity in “establishing causality” for workers’ compensation. Another dermatologist reported,
“The most frequent challenging situation I encounter is being asked to evaluate for maximum medical improvement after patch testing. If the patient is not fully avoiding contact allergens either at home or at work, then I typically document that they are not at [maximum medical improvement]. The reality is that most frequently it is due to exposure to allergens at home so the line between what is a legitimate worker’s comp[ensation] issue and what is a home life choice is blurry.”
Nevertheless, respondents expressed interest in learning more about disability assessment procedures. Summary guides, lectures, and prefilled paperwork were the most popular initiatives that respondents agreed would be beneficial toward becoming educated regarding disability assessment (78.6%, 58.6%, and 58.6%, respectively)(Table 2). One respondent noted that “previous [internal medicine] history help[ed]” them in performing cutaneous disability assessments.
As with any survey, our study did have some inherent limitations. Only a relatively small sample size was willing to complete the survey. There was a predominance of respondents from California (34.6% [27/78]), as well as those practicing for less than 15 years (58.9% [46/78])(Figure). This could limit generalizability to the national population of dermatologists. In addition, there was potential for recall bias and errors in responding given the self-reported nature of the study. Different individuals may interpret the Likert scale options in various ways, which could skew results unintentionally. However, the survey was largely qualitative in nature, making it a legitimate tool for answering our research questions. Moreover, we were able to hear the perspectives of dermatologists across diverse practice settings, with free response prompts to increase the depth of the survey.

Almost 50 years later, our survey echoes common themes from Adams’ 1976 survey.4 Inadequate compensation, limited time, and burdensome paperwork all continue to hinder dermatologists’ ability to perform disability assessments. Our participants frequently commented that the current disability forms are not congruent with the nature of skin conditions, making it challenging to accurately document the facts.
Moreover, respondents felt uncertain in their role in disability assessment and occasionally noted distrust of patients or insufficient patient history as barriers to completing assessments. They also were unsure if physicians can grant disability status. This is a common misconception among physicians that leads to discomfort in helping with disability assessment.7 The role of physicians in disability assessment is to document the facts of a patient’s illness, not to determine whether they are eligible for benefits. We discovered uncertainty in our respondents’ ability to identify conditions eligible for disability, highlighting an area in need of greater education for physicians.
Despite these obstacles, respondents were interested in learning more about disability assessment and highlighted several practical approaches that could help them better perform this task. As skin specialists, dermatologists are the best-equipped physicians to assess cutaneous conditions and should play a greater role in performing disability assessments, which could be achieved through increased educational initiatives and individual physician motivation.7 We call for greater collaboration and reflection on the importance of disability assistance among dermatologists to increase participation in the disability-assessment process.
- O’Connell JJ, Zevin BD, Quick PD, et al. Documenting disability: simple strategies for medical providers. Health Care for the Homeless Clinicians’ Network. September 2007. Accessed March 31, 2025. https://nhchc.org/wp-content/uploads/2019/08/DocumentingDisability2007.pdf
- US Bureau of Labor Statistics. Injuries, illnesses, and fatalities. Accessed March 31, 2025. https://www.bls.gov/iif/
- Meseguer J. Outcome variation in the Social Security Disability Insurance Program: the role of primary diagnoses. Soc Secur Bull. 2013;73:39-75.
- Adams RM. Attitudes of California dermatologists toward Worker’s Compensation: results of a survey. West J Med. 1976;125:169-175.
- Talmage J, Melhorn J, Hyman M. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
- Social Security Administration. Disability evaluation under Social Security. 8.00 skin disorders - adult. March 31, 2025. https://www.ssa.gov/disability/professionals/bluebook/8.00-Skin-Adult.htm
- Dawson J, Smogorzewski J. Demystifying disability assessments for dermatologists—a call to action. JAMA Dermatol. 2021;157:903-904. doi:10.1001/jamadermatol.2021.1767
- O’Connell JJ, Zevin BD, Quick PD, et al. Documenting disability: simple strategies for medical providers. Health Care for the Homeless Clinicians’ Network. September 2007. Accessed March 31, 2025. https://nhchc.org/wp-content/uploads/2019/08/DocumentingDisability2007.pdf
- US Bureau of Labor Statistics. Injuries, illnesses, and fatalities. Accessed March 31, 2025. https://www.bls.gov/iif/
- Meseguer J. Outcome variation in the Social Security Disability Insurance Program: the role of primary diagnoses. Soc Secur Bull. 2013;73:39-75.
- Adams RM. Attitudes of California dermatologists toward Worker’s Compensation: results of a survey. West J Med. 1976;125:169-175.
- Talmage J, Melhorn J, Hyman M. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
- Social Security Administration. Disability evaluation under Social Security. 8.00 skin disorders - adult. March 31, 2025. https://www.ssa.gov/disability/professionals/bluebook/8.00-Skin-Adult.htm
- Dawson J, Smogorzewski J. Demystifying disability assessments for dermatologists—a call to action. JAMA Dermatol. 2021;157:903-904. doi:10.1001/jamadermatol.2021.1767
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
PRACTICE POINTS
- As experts in skin conditions, dermatologists are most qualified to assist with disability assessment for dermatologic concerns.
- There are several barriers to dermatologists participating in the disability assessment process, including lack of time, compensation, and education on the subject.
- Many dermatologists may be interested in learning more about disability assessment, and education could be provided in the form of summary guides, lectures, and prefilled paperwork.