User login
For MD-IQ use only
Treatment Options in MCL: What Are the Best Practices?
In the frontline setting, findings suggest that regimens should differ significantly on the basis of whether patients are older or younger, whereas more data are needed to understand whether treatment can overcome poor prognoses in patients with TP53 mutations, lymphoma specialist Nina Wagner-Johnston, MD, of Johns Hopkins University School of Medicine, Baltimore, said in a presentation at the annual meeting of the Society of Hematologic Oncology (SOHO) 2024 in Houston, Texas.
On the relapsed/refractory front, patients need better options after treatment with Bruton tyrosine kinase inhibitors or chimeric antigen receptor (CAR) T-cell therapy, Krish Patel, MD, a lymphoma specialist with Swedish Cancer Institute in Seattle, said in an adjoining presentation. Fortunately, he said, some treatments are showing early promise.
Here’s a closer look at the presentations by Dr. Wagner-Johnston and Dr. Patel.
Frontline MCL: Age Helps Determine Best Approach
“For older and less fit patients, the standard approach has typically been bendamustine (Bendeka, Treanda) and rituximab (Rituxan), and the median progression-free survival is about 4 years, with overall survival not reached at a median 5 years of follow-up,” Dr. Wagner-Johnston said.
Low doses of the chemotherapy drug cytarabine have been added to the bendamustine-rituximab regimen, with encouraging results, she said. “Certainly there’s more toxicity associated even with lower doses, but those data look fairly promising.”
For younger and fit patients, “the standard of care approach has been to administer intensive chemoimmunotherapy that contains high-dose cytarabine, and then that’s typically followed with an autologous stem cell transplant,” she said. A 2016 study reported median progression-free survival of 8.5 years and median overall survival of 12.7 years.
Now, second-generation Bruton tyrosine kinase inhibitors “look very promising” in the frontline setting, Dr. Wagner-Johnston said.
The road has been rocky, however. The SHINE trial of more than 500 patients aged over 65 found that adding ibrutinib to bendamustine-rituximab improved progression-free survival. “However, progression-free survival did not [connect] to an overall survival benefit, and that’s likely due to the toxicity seen with ibrutinib,” she said.
“It’s not surprising to many of you that ibrutinib has been removed from the FDA label for mantle cell lymphoma,” she said. However, “second-generation [Bruton tyrosine kinase inhibitors] are known to be associated with less toxicity and potentially increased potency.”
What about Bruton tyrosine kinase inhibitors in younger and fitter patients? The TRIANGLE trial demonstrated their benefit, Dr. Wagner-Johnston said, linking ibrutinib to improvement in progression-free survival.
However, “it’s really too early to evaluate the statistical significance for overall survival.” And while the study looks at therapy without stem cell transplant, she believes it’s too early to know whether that’s a good option.
Dr. Wagner-Johnston tackled another topic: Can Bruton tyrosine kinase inhibitors overcome the poor prognosis seen with MCL with TP53 mutation? For now, the limitations of research makes it “hard to know,” she said, although early results of the BOVen trial are promising.
Relapsed/Refractory MCL: Better Options Are Still Needed
In his presentation, Dr. Patel spoke about therapy in patients with MCL and relapsed/refractory disease. “We know that outcomes for patients who progress on covalent [Bruton tyrosine kinase inhibitors] is really dismal,” he said. “This has been shown by multiple groups now across the globe.”
Noncovalent Bruton tyrosine kinase inhibitors are now an option, he noted. “We do understand that they work for some patients, and it can be quite useful, but even noncovalent [Bruton tyrosine kinase inhibitors] themselves are susceptible to resistance mutations. We’ve seen that in the [chronic lymphocytic leukemia] world.”
Dr. Patel asked the audience, “Why not just give everybody CAR T-cells, post-[Bruton tyrosine kinase inhibitors]? You get a CAR T-cell! You get a CAR T-cell! Everybody gets one.”
However, he noted, “Unfortunately, mantle cell lymphoma patients experience the worst high-grade toxicity when receiving CD19[-targeted] CAR T-cells.”
Are there better options? At the moment, “really, really early data” suggest benefits from molecular glues and degraders, novel inhibitors, antibody-drug conjugates, novel CAR T-cells, and bispecific antibodies, Dr. Patel said.
“All of these tools are in clinical trials, and hopefully some of them will help,” he said.
Disclosures were not provided. Dr. Wagner-Johnston recently disclosed advisory committee/board of directors’ relationships with ADC Therapeutics, Regeneron, Calibr, and Verastem. Dr. Patel recently disclosed ties with a long list of pharmaceutical companies, including AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Genentech, Janssen, Merck, and others.
A version of this article first appeared on Medscape.com.
In the frontline setting, findings suggest that regimens should differ significantly on the basis of whether patients are older or younger, whereas more data are needed to understand whether treatment can overcome poor prognoses in patients with TP53 mutations, lymphoma specialist Nina Wagner-Johnston, MD, of Johns Hopkins University School of Medicine, Baltimore, said in a presentation at the annual meeting of the Society of Hematologic Oncology (SOHO) 2024 in Houston, Texas.
On the relapsed/refractory front, patients need better options after treatment with Bruton tyrosine kinase inhibitors or chimeric antigen receptor (CAR) T-cell therapy, Krish Patel, MD, a lymphoma specialist with Swedish Cancer Institute in Seattle, said in an adjoining presentation. Fortunately, he said, some treatments are showing early promise.
Here’s a closer look at the presentations by Dr. Wagner-Johnston and Dr. Patel.
Frontline MCL: Age Helps Determine Best Approach
“For older and less fit patients, the standard approach has typically been bendamustine (Bendeka, Treanda) and rituximab (Rituxan), and the median progression-free survival is about 4 years, with overall survival not reached at a median 5 years of follow-up,” Dr. Wagner-Johnston said.
Low doses of the chemotherapy drug cytarabine have been added to the bendamustine-rituximab regimen, with encouraging results, she said. “Certainly there’s more toxicity associated even with lower doses, but those data look fairly promising.”
For younger and fit patients, “the standard of care approach has been to administer intensive chemoimmunotherapy that contains high-dose cytarabine, and then that’s typically followed with an autologous stem cell transplant,” she said. A 2016 study reported median progression-free survival of 8.5 years and median overall survival of 12.7 years.
Now, second-generation Bruton tyrosine kinase inhibitors “look very promising” in the frontline setting, Dr. Wagner-Johnston said.
The road has been rocky, however. The SHINE trial of more than 500 patients aged over 65 found that adding ibrutinib to bendamustine-rituximab improved progression-free survival. “However, progression-free survival did not [connect] to an overall survival benefit, and that’s likely due to the toxicity seen with ibrutinib,” she said.
“It’s not surprising to many of you that ibrutinib has been removed from the FDA label for mantle cell lymphoma,” she said. However, “second-generation [Bruton tyrosine kinase inhibitors] are known to be associated with less toxicity and potentially increased potency.”
What about Bruton tyrosine kinase inhibitors in younger and fitter patients? The TRIANGLE trial demonstrated their benefit, Dr. Wagner-Johnston said, linking ibrutinib to improvement in progression-free survival.
However, “it’s really too early to evaluate the statistical significance for overall survival.” And while the study looks at therapy without stem cell transplant, she believes it’s too early to know whether that’s a good option.
Dr. Wagner-Johnston tackled another topic: Can Bruton tyrosine kinase inhibitors overcome the poor prognosis seen with MCL with TP53 mutation? For now, the limitations of research makes it “hard to know,” she said, although early results of the BOVen trial are promising.
Relapsed/Refractory MCL: Better Options Are Still Needed
In his presentation, Dr. Patel spoke about therapy in patients with MCL and relapsed/refractory disease. “We know that outcomes for patients who progress on covalent [Bruton tyrosine kinase inhibitors] is really dismal,” he said. “This has been shown by multiple groups now across the globe.”
Noncovalent Bruton tyrosine kinase inhibitors are now an option, he noted. “We do understand that they work for some patients, and it can be quite useful, but even noncovalent [Bruton tyrosine kinase inhibitors] themselves are susceptible to resistance mutations. We’ve seen that in the [chronic lymphocytic leukemia] world.”
Dr. Patel asked the audience, “Why not just give everybody CAR T-cells, post-[Bruton tyrosine kinase inhibitors]? You get a CAR T-cell! You get a CAR T-cell! Everybody gets one.”
However, he noted, “Unfortunately, mantle cell lymphoma patients experience the worst high-grade toxicity when receiving CD19[-targeted] CAR T-cells.”
Are there better options? At the moment, “really, really early data” suggest benefits from molecular glues and degraders, novel inhibitors, antibody-drug conjugates, novel CAR T-cells, and bispecific antibodies, Dr. Patel said.
“All of these tools are in clinical trials, and hopefully some of them will help,” he said.
Disclosures were not provided. Dr. Wagner-Johnston recently disclosed advisory committee/board of directors’ relationships with ADC Therapeutics, Regeneron, Calibr, and Verastem. Dr. Patel recently disclosed ties with a long list of pharmaceutical companies, including AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Genentech, Janssen, Merck, and others.
A version of this article first appeared on Medscape.com.
In the frontline setting, findings suggest that regimens should differ significantly on the basis of whether patients are older or younger, whereas more data are needed to understand whether treatment can overcome poor prognoses in patients with TP53 mutations, lymphoma specialist Nina Wagner-Johnston, MD, of Johns Hopkins University School of Medicine, Baltimore, said in a presentation at the annual meeting of the Society of Hematologic Oncology (SOHO) 2024 in Houston, Texas.
On the relapsed/refractory front, patients need better options after treatment with Bruton tyrosine kinase inhibitors or chimeric antigen receptor (CAR) T-cell therapy, Krish Patel, MD, a lymphoma specialist with Swedish Cancer Institute in Seattle, said in an adjoining presentation. Fortunately, he said, some treatments are showing early promise.
Here’s a closer look at the presentations by Dr. Wagner-Johnston and Dr. Patel.
Frontline MCL: Age Helps Determine Best Approach
“For older and less fit patients, the standard approach has typically been bendamustine (Bendeka, Treanda) and rituximab (Rituxan), and the median progression-free survival is about 4 years, with overall survival not reached at a median 5 years of follow-up,” Dr. Wagner-Johnston said.
Low doses of the chemotherapy drug cytarabine have been added to the bendamustine-rituximab regimen, with encouraging results, she said. “Certainly there’s more toxicity associated even with lower doses, but those data look fairly promising.”
For younger and fit patients, “the standard of care approach has been to administer intensive chemoimmunotherapy that contains high-dose cytarabine, and then that’s typically followed with an autologous stem cell transplant,” she said. A 2016 study reported median progression-free survival of 8.5 years and median overall survival of 12.7 years.
Now, second-generation Bruton tyrosine kinase inhibitors “look very promising” in the frontline setting, Dr. Wagner-Johnston said.
The road has been rocky, however. The SHINE trial of more than 500 patients aged over 65 found that adding ibrutinib to bendamustine-rituximab improved progression-free survival. “However, progression-free survival did not [connect] to an overall survival benefit, and that’s likely due to the toxicity seen with ibrutinib,” she said.
“It’s not surprising to many of you that ibrutinib has been removed from the FDA label for mantle cell lymphoma,” she said. However, “second-generation [Bruton tyrosine kinase inhibitors] are known to be associated with less toxicity and potentially increased potency.”
What about Bruton tyrosine kinase inhibitors in younger and fitter patients? The TRIANGLE trial demonstrated their benefit, Dr. Wagner-Johnston said, linking ibrutinib to improvement in progression-free survival.
However, “it’s really too early to evaluate the statistical significance for overall survival.” And while the study looks at therapy without stem cell transplant, she believes it’s too early to know whether that’s a good option.
Dr. Wagner-Johnston tackled another topic: Can Bruton tyrosine kinase inhibitors overcome the poor prognosis seen with MCL with TP53 mutation? For now, the limitations of research makes it “hard to know,” she said, although early results of the BOVen trial are promising.
Relapsed/Refractory MCL: Better Options Are Still Needed
In his presentation, Dr. Patel spoke about therapy in patients with MCL and relapsed/refractory disease. “We know that outcomes for patients who progress on covalent [Bruton tyrosine kinase inhibitors] is really dismal,” he said. “This has been shown by multiple groups now across the globe.”
Noncovalent Bruton tyrosine kinase inhibitors are now an option, he noted. “We do understand that they work for some patients, and it can be quite useful, but even noncovalent [Bruton tyrosine kinase inhibitors] themselves are susceptible to resistance mutations. We’ve seen that in the [chronic lymphocytic leukemia] world.”
Dr. Patel asked the audience, “Why not just give everybody CAR T-cells, post-[Bruton tyrosine kinase inhibitors]? You get a CAR T-cell! You get a CAR T-cell! Everybody gets one.”
However, he noted, “Unfortunately, mantle cell lymphoma patients experience the worst high-grade toxicity when receiving CD19[-targeted] CAR T-cells.”
Are there better options? At the moment, “really, really early data” suggest benefits from molecular glues and degraders, novel inhibitors, antibody-drug conjugates, novel CAR T-cells, and bispecific antibodies, Dr. Patel said.
“All of these tools are in clinical trials, and hopefully some of them will help,” he said.
Disclosures were not provided. Dr. Wagner-Johnston recently disclosed advisory committee/board of directors’ relationships with ADC Therapeutics, Regeneron, Calibr, and Verastem. Dr. Patel recently disclosed ties with a long list of pharmaceutical companies, including AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Genentech, Janssen, Merck, and others.
A version of this article first appeared on Medscape.com.
FROM SOHO 2024
Navigating Ethical and Clinical Considerations Relating to Percutaneous Gastrostomy (PEG) Tubes
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
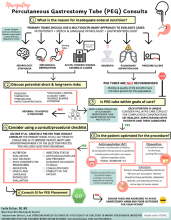
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
Benefits of High-Dose Vitamin D in Managing Cutaneous Adverse Events Induced by Chemotherapy and Radiation Therapy
Vitamin D (VD) regulates keratinocyte proliferation and differentiation, modulates inflammatory pathways, and protects against cellular damage in the skin. 1 In the setting of tissue injury and acute skin inflammation, active vitamin D—1,25(OH) 2 D—suppresses signaling from pro-inflammatory chemokines and cytokines such as IFN- γ and IL-17. 2,3 This suppression reduces proliferation of helper T cells (T H 1, T H 17) and B cells, decreasing tissue damage from reactive oxygen species release while enhancing secretion of the anti-inflammatory cytokine IL-10 by antigen-presenting cells. 2-4
Suboptimal VD levels have been associated with numerous health consequences including malignancy, prompting interest in VD supplementation for improving cancer-related outcomes.5 Beyond disease prognosis, high-dose VD supplementation has been suggested as a potential therapy for adverse events (AEs) related to cancer treatments. In one study, mice that received oral vitamin D3 supplementation of 11,500 IU/kg daily had fewer doxorubicin-induced cardiotoxic effects on ejection fraction (P<.0001) and stroke volume (P<.01) than mice that received VD supplementation of 1500 IU/kg daily.6
In this review, we examine the impact of chemoradiation on 25(OH)D levels—which more accurately reflects VD stores than 1,25(OH)2D levels—and the impact of suboptimal VD on cutaneous toxicities related to chemoradiation. To define the suboptimal VD threshold, we used the Endocrine Society’s clinical practice guidelines, which characterize suboptimal 25(OH)D levels as insufficiency (21–29 ng/mL [52.5–72.5 nmol/L]) or deficiency (<20 ng/mL [50 nmol/L])7; deficiency can be further categorized as severe deficiency (<12 ng/mL [30 nmol/L]).8 This review also evaluates the evidence for vitamin D3 supplementation to alleviate the cutaneous AEs of chemotherapy and radiation treatments.
Effects of Chemotherapy on Vitamin D Levels
A high prevalence of VD deficiency is seen in various cancers. In a retrospective review of 25(OH)D levels in 2098 adults with solid tumors of any stage (6% had metastatic disease [n=124]), suboptimal levels were found in 69% of patients with breast cancer (n=617), 75% with colorectal cancer (n=84), 72% with gynecologic cancer (n=65), 79% with kidney and bladder cancer (n=145), 83% with pancreatic and upper gastrointestinal tract cancer (n=178), 73% with lung cancer (n=73), 69% with prostate cancer (n=225), 61% with skin cancer (n=399), and 63% with thyroid cancer (n=172).5 Suboptimal VD also has been found in hematologic malignancies. In a prospective cohort study, mean serum 25(OH)D levels in 23 patients with recently diagnosed acute myeloid leukemia demonstrated VD deficiency (mean [SD], 18.6 [6.6] nmol/L).9 Given that many patients already exhibit a baseline VD deficiency at cancer diagnosis, it is important to understand the relationship between VD and cancer treatment modalities.5
In the United States, breast and colorectal cancers were estimated to be the first and fourth most common cancers, with 313,510 and 152,810 predicted new cases in 2024, respectively.10 This review will focus on breast and colorectal cancer when describing VD variation associated with chemotherapy exposure due to their high prevalence.
Effects of Chemotherapy on Vitamin D Levels in Breast Cancer—Breast cancer studies have shown suboptimal VD levels in 76% of females 75 years or younger with any T1, T2, or T3; N0 or N1; and M0 breast cancer, in which 38.5% (n=197) had insufficient and 37.5% (n=192) had deficient 25(OH)D levels.11 In a study of female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), VD deficiency was seen in 60% of patients not receiving VD supplementation.12,13 A systematic review that included 7 studies of different types of breast cancer suggested that circulating 25(OH)D may be associated with improved prognosis.14 Thus, studies have investigated risk factors associated with poor or worsening VD status in individuals with breast cancer, including exposure to chemotherapy and/or radiation treatment.12,15-18
A prospective cohort study assessed 25(OH)D levels in 95 patients with any breast cancer (stages I, II, IIIA, IIIB) before and after initiating chemotherapy with docetaxel, doxorubicin, epirubicin, 5-fluorouracil, or cyclophosphamide, compared with a group of 52 females without cancer.17 In the breast cancer group, approximately 80% (76/95) had suboptimal and 50% (47/95) had deficient VD levels before chemotherapy initiation (mean [SD], 54.1 [22.8] nmol/L). In the comparison group, 60% (31/52) had suboptimal and 30% (15/52) had deficient VD at baseline (mean [SD], 66.1 [23.5] nmol/L), which was higher than the breast cancer group (P=.03). A subgroup analysis excluded participants who started, stopped, or lacked data on dietary supplements containing VD (n=39); in the remaining 56 participants, a significant decrease in 25(OH)D levels was observed shortly after finishing chemotherapy compared with the prechemotherapy baseline value (mean, −7.9 nmol/L; P=.004). Notably, 6 months after chemotherapy completion, 25(OH)D levels increased (mean, +12.8 nmol/L; P<.001). Vitamin D levels remained stable in the comparison group (P=.987).17
Consistent with these findings, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of chemotherapy was associated with increased odds of 25(OH)D levels less than 20 ng/mL compared with breast cancer patients with no prior chemotherapy (odds ratio, 1.86; 95% CI, 1.03-3.38).12 Although the study data included chemotherapy history, no information was provided on specific chemotherapy agents or regimens used in this cohort, limiting the ability to detect the drugs most often implicated.
Both studies indicated a complex interplay between chemotherapy and VD levels in breast cancer patients. Although Kok et al17 suggested a transient decrease in VD levels during chemotherapy with a subsequent recovery after cessation, Fassio et al12 highlighted the increased odds of VD deficiency associated with chemotherapy. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status in breast cancer patients.
Effects of Chemotherapy on Vitamin D Levels in Colorectal Cancer—Similar to patterns seen in breast cancer, a systematic review with 6 studies of different types of colorectal cancer suggested that circulating 25(OH)D levels may be associated with prognosis.14 Studies also have investigated the relationship between colorectal chemotherapy regimens and VD status.15,16,18,19
A retrospective study assessed 25(OH)D levels in 315 patients with any colorectal cancer (stage I–IV).15 Patients were included in the analysis if they received less than 400 IU daily of VD supplementation at baseline. For the whole study sample, the mean (SD) VD level was 23.7 (13.71) ng/mL. Patients who had not received chemotherapy within 3 months of the VD level assessment were categorized as the no chemotherapy group, and the others were designated as the chemotherapy group; the latter group was exposed to various chemotherapy regimens, including combinations of irinotecan, oxaliplatin, 5-fluorouracil, leucovorin, bevacizumab, or cetuximab. Multivariate analysis showed that the chemotherapy group was 3.7 times more likely to have very low VD levels (≤15 ng/mL) compared with those in the no chemotherapy group (P<.0001).15
A separate cross-sectional study examined serum 25(OH)D concentrations in 1201 patients with any newly diagnosed colorectal carcinoma (stage I–III); 91% of cases were adenocarcinoma.18 In a multivariate analysis, chemotherapy plus surgery was associated with lower VD levels than surgery alone 6 months after diagnosis (mean, −8.74 nmol/L; 95% CI, −11.30 to −6.18 nmol/L), specifically decreasing by a mean of 6.7 nmol/L (95% CI, −9.8 to −3.8 nmol/L) after adjusting for demographic and lifestyle factors.18 However, a prospective cohort study demonstrated different findings.19 Comparing 58 patients with newly diagnosed colorectal adenocarcinoma (stages I–IV) who underwent chemotherapy and 36 patients who did not receive chemotherapy, there was no significant change in 25(OH)D levels from the time of diagnosis to 6 months later. Median VD levels decreased by 0.7 ng/mL in those who received chemotherapy, while a minimal (and not significant) increase of 1.6 ng/mL was observed in those without chemotherapy intervention (P=.26). Notably, supplementation was not restricted in this cohort, which may have resulted in higher VD levels in those taking supplements.19
Since time of year and geographic location can influence VD levels, one prospective cohort study controlled for differential sun exposure due to these factors in their analysis.16 Assessment of 25(OH)D levels was completed in 81 chemotherapy-naïve cancer patients immediately before beginning chemotherapy as well as 6 and 12 weeks into treatment. More than 8 primary cancer types were represented in this study, with breast (34% [29/81]) and colorectal (14% [12/81]) cancer being the most common, but the cancer stages of the participants were not detailed. Vitamin D levels decreased after commencing chemotherapy, with the largest drop occurring 6 weeks into treatment. From the 6- to 12-week end points, VD increased but remained below the original baseline level (baseline: mean [SD], 49.2 [22.3] nmol/L; 6 weeks: mean [SD], 40.9 [19.0] nmol/L; 12 weeks: mean [SD], 45.9 [19.7] nmol/L; P=.05).16
Although focused on breast and colorectal cancers, these studies suggest that various chemotherapy regimens may confer a higher risk for VD deficiency compared with VD status at diagnosis and/or prior to chemotherapy treatment. However, most of these studies only discussed stage-based differences, excluding analysis of the variety of cancer subtypes that comprise breast and colorectal malignancies, which may limit our ability to extrapolate from these data. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status across various primary cancer types.
Effects of Radiation Therapy on Vitamin D Levels
Unlike chemotherapy, studies on the association between radiation therapy and VD levels are minimal, with most reports in the literature discussing the use of VD to potentiate the effects of radiation therapy. In one cross-sectional analysis of 1201 patients with newly diagnosed stage I, II, or III colorectal cancer of any type (94% were adenocarcinoma), radiation plus surgery was associated with slightly lower 25(OH)D levels than surgery alone for tumor treatment 6 months after diagnosis (mean, −3.17; 95% CI, −6.07 to −0.28 nmol/L). However, after adjustment for demographic and lifestyle factors, this decrease in VD levels attributable to radiotherapy was not statistically significant compared with the surgery-only cohort (mean, −1.78; 95% CI, −5.07 to 1.52 nmol/L).18
Similarly, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of radiotherapy was not associated with a difference in serum 25(OH)D levels compared with those with breast cancer without prior radiotherapy (odds ratio, 0.90; 95% CI, 0.52-1.54).12 From the limited existing literature specifically addressing variations of VD levels with radiation, radiation therapy does not appear to significantly impact VD levels.
Vitamin D Levels and the Severity of Chemotherapy- or Radiation Therapy–Induced AEs
A prospective cohort of 241 patients did not find an increase in the incidence or severity of chemotherapy-induced cutaneous toxicities in those with suboptimal 1,25(OH)2D3 levels (≤75 nmol/L).20 Eight different primary cancer types were represented, including breast and colorectal cancer; the tumor stages of the participants were not detailed. Forty-one patients had normal 1,25(OH)2D3 levels, while the remaining 200 had suboptimal levels. There was no significant association between serum VD levels and the following dermatologic toxicities: desquamation (P=.26), xerosis (P=.15), mucositis (P=.30), or painful rash (P=.87). Surprisingly, nail changes and hand-foot reactions occurred with greater frequency in patients with normal VD levels (P=.01 and P=.03, respectively).20 Hand-foot reaction is part of the toxic erythema of chemotherapy (TEC) spectrum, which is comprised of a range of cytotoxic skin injuries that typically manifest within 2 to 3 weeks of exposure to the offending chemotherapeutic agents, often characterized by erythema, pain, swelling, and blistering, particularly in intertriginous and acral areas.21-23 Recovery from TEC generally takes at least 2 to 4 weeks and may necessitate cessation of the offending chemotherapeutic agent.21,24 Notably, this study measured 1,25(OH)2D3 levels instead of 25(OH)D levels, which may not reliably indicate body stores of VD.7,20 These results underscore the complex nature between chemotherapy and VD; however, VD levels alone do not appear to be a sufficient biomarker for predicting chemotherapy-associated cutaneous AEs.
Interestingly, radiation therapy–induced AEs may be associated with VD levels. A prospective cohort study of 98 patients with prostate, bladder, or gynecologic cancers (tumor stages were not detailed) undergoing pelvic radiotherapy found that females and males with 25(OH)D levels below a threshold of 35 and 40 nmol/L, respectively, were more likely to experience higher Radiation Therapy Oncology Group (RTOG) grade acute proctitis compared with those with VD above these thresholds.25 Specifically, VD below these thresholds was associated with increased odds of RTOG grade 2 or higher radiation-induced proctitis (OR, 3.07; 95% CI, 1.27-7.50 [P=.013]). Additionally, a weak correlation was noted between VD below these thresholds and the RTOG grade, with a Spearman correlation value of −0.189 (P=.031).25
One prospective cohort study included 28 patients with any cancer of the oral cavity, oropharynx, hypopharynx, or larynx stages II, III, or IVA; 93% (26/28) were stage III or IVA.26 The 20 (71%) patients with suboptimal 25(OH)D levels (≤75 nmol/L) experienced a higher prevalence of grade II radiation dermatitis compared with the 8 (29%) patients with optimal VD levels (χ22 =5.973; P=.0505). This pattern persisted with the severity of mucositis; patients from the suboptimal VD group presented with higher rates of grades II and III mucositis compared with the VD optimal group (χ22 =13.627; P=.0011).26 Recognizing the small cohort evaluated in the study, we highlight the importance of further studies to clarify these associations.
Chemotherapy-Induced Cutaneous Events Treated with High-Dose Vitamin D
Chemotherapeutic agents are known to induce cellular damage, resulting in a range of cutaneous AEs that can invoke discontinuation of otherwise effective chemotherapeutic interventions.27,28 Recent research has explored the potential of high-dose vitamin D3 as a therapeutic agent to mitigate cutaneous reactions.29,30
A randomized, double-blind, placebo-controlled trial investigated the use of a single high dose of oral 25(OH)D to treat topical nitrogen mustard (NM)–induced rash.29 To characterize baseline inflammatory responses from NM injury without intervention, clinical measures, serum studies, and tissue analyses from skin biopsies were performed on 28 healthy adults after exposure to topical NM—a chemotherapeutic agent classified as a DNA alkylator. Two weeks later, participants were exposed to topical NM a second time and were split into 2 groups: 14 patients received a single 200,000-IU dose of oral 25(OH)D while the other 14 participants were given a placebo. Using the inflammatory markers induced from baseline exposure to NM alone, posttreatment analysis revealed that the punch biopsies from
Although Ernst et al29 did not observe any clinically significant improvements with VD treatment, a case series of 6 patients with either glioblastoma multiforme, acute myeloid leukemia, or aplastic anemia did demonstrate clinical improvement of TEC after receiving high-dose vitamin D3.30 The mean time to onset of TEC was noted at 8.5 days following administration of the inciting chemotherapeutic agent, which included combinations of anthracycline, antimetabolite, kinase inhibitor, B-cell lymphoma 2 inhibitor, purine analogue, and alkylating agents. A combination of clinical and histologic findings was used to diagnose TEC. Baseline 25(OH)D levels were not established prior to treatment. The treatment regimen for 1 patient included 2 doses of 50,000 IU of VD spaced 1 week apart, totaling 100,000 IU, while the remaining 5 patients received a total of 200,000 IU, also split into 2 doses given 1 week apart. All patients received their first dose of VD within a week of the cutaneous eruption. Following the initial VD dose, there was a notable improvement in pain, pruritus, or swelling by the next day. Reduction in erythema also was observed within 1 to 4 days.30
No AEs associated with VD supplementation were reported, suggesting a potential beneficial role of high-dose VD in accelerating recovery from chemotherapy-induced rashes without evident safety concerns.
Radiation Therapy–Induced Cutaneous Events Treated with High-Dose Vitamin D
Radiation dermatitis is a common and often severe complication of radiation therapy that affects more than 90% of patients undergoing treatment, with half of these individuals experiencing grade 2 toxicity, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events.31,32 Radiation damage to basal keratinocytes and hair follicle stem cells disrupts the renewal of the skin’s outer layer, while a surge of free radicals causes irreversible DNA damage.33 Symptoms of radiation dermatitis can vary from mild pink erythema to tissue ulceration and necrosis, typically within 1 to 4 weeks of radiation exposure.34 The resulting dermatitis can take 2 to 4 weeks to heal, notably impacting patient quality of life and often necessitating modifications or interruptions in cancer therapy.33
Prior studies have demonstrated the use of high-dose VD to improve the healing of UV-irradiated skin. A randomized controlled trial investigated high-dose vitamin D3 to treat experimentally induced sunburn in 20 healthy adults. Compared with those who received a placebo, participants receiving the oral dose of 200,000 IU of vitamin D3 demonstrated suppression of the pro-inflammatory mediators tumor necrosis factor α (P=.04) and inducible nitric oxide synthase (P=.02), while expression of tissue repair enhancer arginase 1 was increased (P<.005).35 The mechanism of this enhanced tissue repair was investigated using a mouse model, in which intraperitoneal 25(OH)D was administered following severe UV-induced skin injury. On immunofluorescence microscopy, mice treated with VD showed enhanced autophagy within the macrophages infiltrating UV-irradiated skin.36 The use of high-dose VD to treat UV-irradiated skin in these studies established a precedent for using VD to heal cutaneous injury caused by ionizing radiation therapy.
Some studies have focused on the role of VD for treating acute radiation dermatitis. A study of 23 patients with ductal carcinoma in situ or localized invasive ductal carcinoma breast cancer compared the effectiveness of topical calcipotriol to that of a standard hydrating ointment.37 Participants were randomized to 1 of 2 treatments before starting adjuvant radiotherapy to evaluate their potential in preventing radiation dermatitis. In 87% (20/23) of these patients, no difference in skin reaction was observed between the 2 treatments, suggesting that topical VD application may not offer any advantage over the standard hydrating ointment for the prevention of radiation dermatitis.37
Benefits of high-dose oral VD for treating radiation dermatitis also have been reported. Nguyen et al38 documented 3 cases in which patients with neuroendocrine carcinoma of the pancreas, tonsillar carcinoma, and breast cancer received 200,000 IU of oral ergocalciferol distributed over 2 doses given 7 days apart for radiation dermatitis. These patients experienced substantial improvements in pain, swelling, and redness within a week of the initial dose. Additionally, a case of radiation recall dermatitis, which occurred a week after vinorelbine chemotherapy, was treated with 2 doses totaling 100,000 IU of oral ergocalciferol. This patient also had improvement in pain and swelling but continued to have tumor-related induration and ulceration.39
Although topical VD did not show significant benefits over standard treatments for radiation dermatitis, high-dose oral VD appears promising in improving patient outcomes of pain and swelling more rapidly than current practices. Further research is needed to confirm these findings and establish standardized treatment protocols.
Final Thoughts
Suboptimal VD levels are prevalent in numerous cancer types. Chemotherapy often is associated with acute, potentially transient worsening of VD status in patients with breast and colorectal cancer. Although 25(OH)D levels have not corresponded with increased frequency of chemotherapy-related dermatologic AEs, suboptimal 25(OH)D levels appear to be associated with increased severity of radiation-induced mucositis and dermatitis.20,25,26 The use of high-dose VD as a therapeutic agent shows promise in mitigating chemotherapy-induced and radiation therapy–induced rashes in multiple cancer types with reduction of inflammatory markers and a durable anti-inflammatory impact. Although the mechanisms of cellular injury vary among chemotherapeutic agents, the anti-inflammatory and tissue repair properties of VD may make it an effective treatment for chemotherapy-induced cutaneous damage regardless of injury mechanism.2-4,35 However, reports of clinical improvement vary, and further objective studies to classify optimal dosing, administration, and outcome measures are needed. The absence of reported AEs associated with high-dose VD supplementation is encouraging, but selection of a safe and optimal dosing regimen can only occur with dedicated clinical trials.
- Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3-19. doi:10.1007/s11154-011-9194-0
- Penna G, Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405-2411. doi:10.4049/jimmunol.164.5.2405
- Penna G, Amuchastegui S, Cossetti C, et al. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177:8504-8511. doi:10.4049/jimmunol.177.12.8504
- Heine G, Niesner U, Chang HD, et al. 1,25-dihydroxyvitamin D3 promotes IL-10 production in human B cells. Eur J Immunol. 2008;38:2210-2218. doi:10.1002/eji.200838216
- Hauser K, Walsh D, Shrotriya S, et al. Low 25-hydroxyvitamin D levels in people with a solid tumor cancer diagnosis: the tip of the iceberg? Support Care Cancer. 2014;22:1931-1939. doi:10.1007/s00520-014-2154-y
- Lee KJ, Wright G, Bryant H, et al. Cytoprotective effect of vitamin D on doxorubicin-induced cardiac toxicity in triple negative breast cancer. Int J Mol Sci. 2021;22:7439. doi:10.3390/ijms22147439
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. doi:10.1210/jc.2011-0385
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Thomas X, Chelghoum Y, Fanari N, et al. Serum 25-hydroxyvitamin D levels are associated with prognosis in hematological malignancies. Hematology. 2011;16:278-283. doi:10.1179/102453311X13085644679908
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. doi:10.3322/caac.21820
- Goodwin PJ, Ennis M, Pritchard KI, et al. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757-3763. doi:10.1200/JCO.2008.20.0725
- Fassio A, Porciello G, Carioli G, et al. Post-diagnosis serum 25-hydroxyvitamin D concentrations in women treated for breast cancer participating in a lifestyle trial in Italy. Reumatismo. 2024;76:21-34.
- Augustin LSA, Libra M, Crispo A, et al. Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence (DEDiCa): design of a clinical trial. BMC Cancer. 2017;17:69. doi:10.1186/s12885-017-3064-4
- Toriola AT, Nguyen N, Scheitler-Ring K, et al. Circulating 25-hydroxyvitamin D levels and prognosis among cancer patients: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23:917-933. doi:10.1158/1055-9965.EPI-14-0053
- Fakih MG, Trump DL, Johnson CS, et al. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis. 2009;24:219-224. doi:10.1007/s00384-008-0593-y
- Isenring EA, Teleni L, Woodman RJ, et al. Serum vitamin D decreases during chemotherapy: an Australian prospective cohort study. Asia Pac J Clin Nutr. 2018;27:962-967. doi:10.6133/apjcn.042018.01
- Kok DE, van den Berg MMGA, Posthuma L, et al. Changes in circulating levels of 25-hydroxyvitamin D3 in breast cancer patients receiving chemotherapy. Nutr Cancer. 2019;71:756-766. doi:10.1080/01635581.2018.1559938
- Wesselink E, Bours MJL, de Wilt JHW, et al. Chemotherapy and vitamin D supplement use are determinants of serum 25-hydroxyvitamin D levels during the first six months after colorectal cancer diagnosis. J Steroid Biochem Mol Biol. 2020;199:105577. doi:10.1016/j.jsbmb.2020.105577
- Savoie MB, Paciorek A, Zhang L, et al. Vitamin D levels in patients with colorectal cancer before and after treatment initiation. J Gastrointest Cancer. 2019;50:769-779. doi:10.1007/s12029-018-0147-7
- Kitchen D, Hughes B, Gill I, et al. The relationship between vitamin D and chemotherapy-induced toxicity—a pilot study. Br J Cancer. 2012;107:158-160. doi:10.1038/bjc.2012.194
- Demircay Z, Gürbüz O, Alpdogan TB, et al. Chemotherapy-induced acral erythema in leukemic patients: a report of 15 cases. Int J Dermatol. 1997;36:593-598. doi:10.1046/j.1365-4362.1997.00040.x
- Valks R, Fraga J, Porras-Luque J, et al. Chemotherapy-induced eccrine squamous syringometaplasia. a distinctive eruption in patients receiving hematopoietic progenitor cells. Arch Dermatol. 1997;133;873-878. doi:10.1001/archderm.133.7.873
- Webber KA, Kos L, Holland KE, et al. Intertriginous eruption associated with chemotherapy in pediatric patients. Arch Dermatol. 2007;143:67-71. doi:10.1001/archderm.143.1.67
- Hunjan MK, Nowsheen S, Ramos-Rodriguez AJ, et al. Clinical and histopathological spectrum of toxic erythema of chemotherapy in patients who have undergone allogeneic hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2019;12:19-25. doi:10.1016/j.hemonc.2018.09.001
- Ghorbanzadeh-Moghaddam A, Gholamrezaei A, Hemati S. Vitamin D deficiency is associated with the severity of radiation-induced proctitis in cancer patients. Int J Radiat Oncol Biol Phys. 2015;92:613-618. doi:10.1016/j.ijrobp.2015.02.011
- Bhanu A, Waghmare CM, Jain VS, et al. Serum 25-hydroxy vitamin-D levels in head and neck cancer chemoradiation therapy: potential in cancer therapeutics. Indian J Cancer. Published online February 27, 2003. doi:10.4103/ijc.ijc_358_20
- Yang B, Xie X, Wu Z, et al. DNA damage-mediated cellular senescence promotes hand-foot syndrome that can be relieved by thymidine prodrug. Genes Dis. 2022;10:2557-2571. doi:10.1016/j.gendis.2022.10.004
- Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda®). Eur J Oncol Nurs. 2004;8(suppl 1):S31-S40. doi:10.1016/j.ejon.2004.06.007
- Ernst MK, Evans ST, Techner JM, et al. Vitamin D3 and deconvoluting a rash. JCI Insight. 2023;8:E163789.
- Nguyen CV, Zheng L, Zhou XA, et al. High-dose vitamin d for the management of toxic erythema of chemotherapy in hospitalized patients. JAMA Dermatol. 2023;159:219-221. doi:10.1001/jamadermatol.2022.5397
- Fisher J, Scott C, Stevens R, et al. Randomized phase III study comparing best supportive care to biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48:1307-1310. doi:10.1016/s0360-3016(00)00782-3
- Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085-2092. doi:10.1200/JCO.2007.15.2488
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3 pt 2):985-993. doi:10.1038/jid.2011.411
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086. doi:10.1016/j.jid.2017.04.040
- Das LM, Binko AM, Traylor ZP, et al. Vitamin D improves sunburns by increasing autophagy in M2 macrophages. Autophagy. 2019;15:813-826. doi:10.1080/15548627.2019.1569298
- Nasser NJ, Fenig S, Ravid A, et al. Vitamin D ointment for prevention of radiation dermatitis in breast cancer patients. NPJ Breast Cancer. 2017;3:10. doi:10.1038/s41523-017-0006-x
- Nguyen CV, Zheng L, Lu KQ. High-dose vitamin D for the management acute radiation dermatitis. JAAD Case Rep. 2023;39:47-50. doi:10.1016/j.jdcr.2023.07.001
- Nguyen CV, Lu KQ. Vitamin D3 and its potential to ameliorate chemical and radiation-induced skin injury during cancer therapy. Disaster Med Public Health Prep. 2024;18:E4. doi:10.1017/dmp.2023.211
Vitamin D (VD) regulates keratinocyte proliferation and differentiation, modulates inflammatory pathways, and protects against cellular damage in the skin. 1 In the setting of tissue injury and acute skin inflammation, active vitamin D—1,25(OH) 2 D—suppresses signaling from pro-inflammatory chemokines and cytokines such as IFN- γ and IL-17. 2,3 This suppression reduces proliferation of helper T cells (T H 1, T H 17) and B cells, decreasing tissue damage from reactive oxygen species release while enhancing secretion of the anti-inflammatory cytokine IL-10 by antigen-presenting cells. 2-4
Suboptimal VD levels have been associated with numerous health consequences including malignancy, prompting interest in VD supplementation for improving cancer-related outcomes.5 Beyond disease prognosis, high-dose VD supplementation has been suggested as a potential therapy for adverse events (AEs) related to cancer treatments. In one study, mice that received oral vitamin D3 supplementation of 11,500 IU/kg daily had fewer doxorubicin-induced cardiotoxic effects on ejection fraction (P<.0001) and stroke volume (P<.01) than mice that received VD supplementation of 1500 IU/kg daily.6
In this review, we examine the impact of chemoradiation on 25(OH)D levels—which more accurately reflects VD stores than 1,25(OH)2D levels—and the impact of suboptimal VD on cutaneous toxicities related to chemoradiation. To define the suboptimal VD threshold, we used the Endocrine Society’s clinical practice guidelines, which characterize suboptimal 25(OH)D levels as insufficiency (21–29 ng/mL [52.5–72.5 nmol/L]) or deficiency (<20 ng/mL [50 nmol/L])7; deficiency can be further categorized as severe deficiency (<12 ng/mL [30 nmol/L]).8 This review also evaluates the evidence for vitamin D3 supplementation to alleviate the cutaneous AEs of chemotherapy and radiation treatments.
Effects of Chemotherapy on Vitamin D Levels
A high prevalence of VD deficiency is seen in various cancers. In a retrospective review of 25(OH)D levels in 2098 adults with solid tumors of any stage (6% had metastatic disease [n=124]), suboptimal levels were found in 69% of patients with breast cancer (n=617), 75% with colorectal cancer (n=84), 72% with gynecologic cancer (n=65), 79% with kidney and bladder cancer (n=145), 83% with pancreatic and upper gastrointestinal tract cancer (n=178), 73% with lung cancer (n=73), 69% with prostate cancer (n=225), 61% with skin cancer (n=399), and 63% with thyroid cancer (n=172).5 Suboptimal VD also has been found in hematologic malignancies. In a prospective cohort study, mean serum 25(OH)D levels in 23 patients with recently diagnosed acute myeloid leukemia demonstrated VD deficiency (mean [SD], 18.6 [6.6] nmol/L).9 Given that many patients already exhibit a baseline VD deficiency at cancer diagnosis, it is important to understand the relationship between VD and cancer treatment modalities.5
In the United States, breast and colorectal cancers were estimated to be the first and fourth most common cancers, with 313,510 and 152,810 predicted new cases in 2024, respectively.10 This review will focus on breast and colorectal cancer when describing VD variation associated with chemotherapy exposure due to their high prevalence.
Effects of Chemotherapy on Vitamin D Levels in Breast Cancer—Breast cancer studies have shown suboptimal VD levels in 76% of females 75 years or younger with any T1, T2, or T3; N0 or N1; and M0 breast cancer, in which 38.5% (n=197) had insufficient and 37.5% (n=192) had deficient 25(OH)D levels.11 In a study of female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), VD deficiency was seen in 60% of patients not receiving VD supplementation.12,13 A systematic review that included 7 studies of different types of breast cancer suggested that circulating 25(OH)D may be associated with improved prognosis.14 Thus, studies have investigated risk factors associated with poor or worsening VD status in individuals with breast cancer, including exposure to chemotherapy and/or radiation treatment.12,15-18
A prospective cohort study assessed 25(OH)D levels in 95 patients with any breast cancer (stages I, II, IIIA, IIIB) before and after initiating chemotherapy with docetaxel, doxorubicin, epirubicin, 5-fluorouracil, or cyclophosphamide, compared with a group of 52 females without cancer.17 In the breast cancer group, approximately 80% (76/95) had suboptimal and 50% (47/95) had deficient VD levels before chemotherapy initiation (mean [SD], 54.1 [22.8] nmol/L). In the comparison group, 60% (31/52) had suboptimal and 30% (15/52) had deficient VD at baseline (mean [SD], 66.1 [23.5] nmol/L), which was higher than the breast cancer group (P=.03). A subgroup analysis excluded participants who started, stopped, or lacked data on dietary supplements containing VD (n=39); in the remaining 56 participants, a significant decrease in 25(OH)D levels was observed shortly after finishing chemotherapy compared with the prechemotherapy baseline value (mean, −7.9 nmol/L; P=.004). Notably, 6 months after chemotherapy completion, 25(OH)D levels increased (mean, +12.8 nmol/L; P<.001). Vitamin D levels remained stable in the comparison group (P=.987).17
Consistent with these findings, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of chemotherapy was associated with increased odds of 25(OH)D levels less than 20 ng/mL compared with breast cancer patients with no prior chemotherapy (odds ratio, 1.86; 95% CI, 1.03-3.38).12 Although the study data included chemotherapy history, no information was provided on specific chemotherapy agents or regimens used in this cohort, limiting the ability to detect the drugs most often implicated.
Both studies indicated a complex interplay between chemotherapy and VD levels in breast cancer patients. Although Kok et al17 suggested a transient decrease in VD levels during chemotherapy with a subsequent recovery after cessation, Fassio et al12 highlighted the increased odds of VD deficiency associated with chemotherapy. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status in breast cancer patients.
Effects of Chemotherapy on Vitamin D Levels in Colorectal Cancer—Similar to patterns seen in breast cancer, a systematic review with 6 studies of different types of colorectal cancer suggested that circulating 25(OH)D levels may be associated with prognosis.14 Studies also have investigated the relationship between colorectal chemotherapy regimens and VD status.15,16,18,19
A retrospective study assessed 25(OH)D levels in 315 patients with any colorectal cancer (stage I–IV).15 Patients were included in the analysis if they received less than 400 IU daily of VD supplementation at baseline. For the whole study sample, the mean (SD) VD level was 23.7 (13.71) ng/mL. Patients who had not received chemotherapy within 3 months of the VD level assessment were categorized as the no chemotherapy group, and the others were designated as the chemotherapy group; the latter group was exposed to various chemotherapy regimens, including combinations of irinotecan, oxaliplatin, 5-fluorouracil, leucovorin, bevacizumab, or cetuximab. Multivariate analysis showed that the chemotherapy group was 3.7 times more likely to have very low VD levels (≤15 ng/mL) compared with those in the no chemotherapy group (P<.0001).15
A separate cross-sectional study examined serum 25(OH)D concentrations in 1201 patients with any newly diagnosed colorectal carcinoma (stage I–III); 91% of cases were adenocarcinoma.18 In a multivariate analysis, chemotherapy plus surgery was associated with lower VD levels than surgery alone 6 months after diagnosis (mean, −8.74 nmol/L; 95% CI, −11.30 to −6.18 nmol/L), specifically decreasing by a mean of 6.7 nmol/L (95% CI, −9.8 to −3.8 nmol/L) after adjusting for demographic and lifestyle factors.18 However, a prospective cohort study demonstrated different findings.19 Comparing 58 patients with newly diagnosed colorectal adenocarcinoma (stages I–IV) who underwent chemotherapy and 36 patients who did not receive chemotherapy, there was no significant change in 25(OH)D levels from the time of diagnosis to 6 months later. Median VD levels decreased by 0.7 ng/mL in those who received chemotherapy, while a minimal (and not significant) increase of 1.6 ng/mL was observed in those without chemotherapy intervention (P=.26). Notably, supplementation was not restricted in this cohort, which may have resulted in higher VD levels in those taking supplements.19
Since time of year and geographic location can influence VD levels, one prospective cohort study controlled for differential sun exposure due to these factors in their analysis.16 Assessment of 25(OH)D levels was completed in 81 chemotherapy-naïve cancer patients immediately before beginning chemotherapy as well as 6 and 12 weeks into treatment. More than 8 primary cancer types were represented in this study, with breast (34% [29/81]) and colorectal (14% [12/81]) cancer being the most common, but the cancer stages of the participants were not detailed. Vitamin D levels decreased after commencing chemotherapy, with the largest drop occurring 6 weeks into treatment. From the 6- to 12-week end points, VD increased but remained below the original baseline level (baseline: mean [SD], 49.2 [22.3] nmol/L; 6 weeks: mean [SD], 40.9 [19.0] nmol/L; 12 weeks: mean [SD], 45.9 [19.7] nmol/L; P=.05).16
Although focused on breast and colorectal cancers, these studies suggest that various chemotherapy regimens may confer a higher risk for VD deficiency compared with VD status at diagnosis and/or prior to chemotherapy treatment. However, most of these studies only discussed stage-based differences, excluding analysis of the variety of cancer subtypes that comprise breast and colorectal malignancies, which may limit our ability to extrapolate from these data. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status across various primary cancer types.
Effects of Radiation Therapy on Vitamin D Levels
Unlike chemotherapy, studies on the association between radiation therapy and VD levels are minimal, with most reports in the literature discussing the use of VD to potentiate the effects of radiation therapy. In one cross-sectional analysis of 1201 patients with newly diagnosed stage I, II, or III colorectal cancer of any type (94% were adenocarcinoma), radiation plus surgery was associated with slightly lower 25(OH)D levels than surgery alone for tumor treatment 6 months after diagnosis (mean, −3.17; 95% CI, −6.07 to −0.28 nmol/L). However, after adjustment for demographic and lifestyle factors, this decrease in VD levels attributable to radiotherapy was not statistically significant compared with the surgery-only cohort (mean, −1.78; 95% CI, −5.07 to 1.52 nmol/L).18
Similarly, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of radiotherapy was not associated with a difference in serum 25(OH)D levels compared with those with breast cancer without prior radiotherapy (odds ratio, 0.90; 95% CI, 0.52-1.54).12 From the limited existing literature specifically addressing variations of VD levels with radiation, radiation therapy does not appear to significantly impact VD levels.
Vitamin D Levels and the Severity of Chemotherapy- or Radiation Therapy–Induced AEs
A prospective cohort of 241 patients did not find an increase in the incidence or severity of chemotherapy-induced cutaneous toxicities in those with suboptimal 1,25(OH)2D3 levels (≤75 nmol/L).20 Eight different primary cancer types were represented, including breast and colorectal cancer; the tumor stages of the participants were not detailed. Forty-one patients had normal 1,25(OH)2D3 levels, while the remaining 200 had suboptimal levels. There was no significant association between serum VD levels and the following dermatologic toxicities: desquamation (P=.26), xerosis (P=.15), mucositis (P=.30), or painful rash (P=.87). Surprisingly, nail changes and hand-foot reactions occurred with greater frequency in patients with normal VD levels (P=.01 and P=.03, respectively).20 Hand-foot reaction is part of the toxic erythema of chemotherapy (TEC) spectrum, which is comprised of a range of cytotoxic skin injuries that typically manifest within 2 to 3 weeks of exposure to the offending chemotherapeutic agents, often characterized by erythema, pain, swelling, and blistering, particularly in intertriginous and acral areas.21-23 Recovery from TEC generally takes at least 2 to 4 weeks and may necessitate cessation of the offending chemotherapeutic agent.21,24 Notably, this study measured 1,25(OH)2D3 levels instead of 25(OH)D levels, which may not reliably indicate body stores of VD.7,20 These results underscore the complex nature between chemotherapy and VD; however, VD levels alone do not appear to be a sufficient biomarker for predicting chemotherapy-associated cutaneous AEs.
Interestingly, radiation therapy–induced AEs may be associated with VD levels. A prospective cohort study of 98 patients with prostate, bladder, or gynecologic cancers (tumor stages were not detailed) undergoing pelvic radiotherapy found that females and males with 25(OH)D levels below a threshold of 35 and 40 nmol/L, respectively, were more likely to experience higher Radiation Therapy Oncology Group (RTOG) grade acute proctitis compared with those with VD above these thresholds.25 Specifically, VD below these thresholds was associated with increased odds of RTOG grade 2 or higher radiation-induced proctitis (OR, 3.07; 95% CI, 1.27-7.50 [P=.013]). Additionally, a weak correlation was noted between VD below these thresholds and the RTOG grade, with a Spearman correlation value of −0.189 (P=.031).25
One prospective cohort study included 28 patients with any cancer of the oral cavity, oropharynx, hypopharynx, or larynx stages II, III, or IVA; 93% (26/28) were stage III or IVA.26 The 20 (71%) patients with suboptimal 25(OH)D levels (≤75 nmol/L) experienced a higher prevalence of grade II radiation dermatitis compared with the 8 (29%) patients with optimal VD levels (χ22 =5.973; P=.0505). This pattern persisted with the severity of mucositis; patients from the suboptimal VD group presented with higher rates of grades II and III mucositis compared with the VD optimal group (χ22 =13.627; P=.0011).26 Recognizing the small cohort evaluated in the study, we highlight the importance of further studies to clarify these associations.
Chemotherapy-Induced Cutaneous Events Treated with High-Dose Vitamin D
Chemotherapeutic agents are known to induce cellular damage, resulting in a range of cutaneous AEs that can invoke discontinuation of otherwise effective chemotherapeutic interventions.27,28 Recent research has explored the potential of high-dose vitamin D3 as a therapeutic agent to mitigate cutaneous reactions.29,30
A randomized, double-blind, placebo-controlled trial investigated the use of a single high dose of oral 25(OH)D to treat topical nitrogen mustard (NM)–induced rash.29 To characterize baseline inflammatory responses from NM injury without intervention, clinical measures, serum studies, and tissue analyses from skin biopsies were performed on 28 healthy adults after exposure to topical NM—a chemotherapeutic agent classified as a DNA alkylator. Two weeks later, participants were exposed to topical NM a second time and were split into 2 groups: 14 patients received a single 200,000-IU dose of oral 25(OH)D while the other 14 participants were given a placebo. Using the inflammatory markers induced from baseline exposure to NM alone, posttreatment analysis revealed that the punch biopsies from
Although Ernst et al29 did not observe any clinically significant improvements with VD treatment, a case series of 6 patients with either glioblastoma multiforme, acute myeloid leukemia, or aplastic anemia did demonstrate clinical improvement of TEC after receiving high-dose vitamin D3.30 The mean time to onset of TEC was noted at 8.5 days following administration of the inciting chemotherapeutic agent, which included combinations of anthracycline, antimetabolite, kinase inhibitor, B-cell lymphoma 2 inhibitor, purine analogue, and alkylating agents. A combination of clinical and histologic findings was used to diagnose TEC. Baseline 25(OH)D levels were not established prior to treatment. The treatment regimen for 1 patient included 2 doses of 50,000 IU of VD spaced 1 week apart, totaling 100,000 IU, while the remaining 5 patients received a total of 200,000 IU, also split into 2 doses given 1 week apart. All patients received their first dose of VD within a week of the cutaneous eruption. Following the initial VD dose, there was a notable improvement in pain, pruritus, or swelling by the next day. Reduction in erythema also was observed within 1 to 4 days.30
No AEs associated with VD supplementation were reported, suggesting a potential beneficial role of high-dose VD in accelerating recovery from chemotherapy-induced rashes without evident safety concerns.
Radiation Therapy–Induced Cutaneous Events Treated with High-Dose Vitamin D
Radiation dermatitis is a common and often severe complication of radiation therapy that affects more than 90% of patients undergoing treatment, with half of these individuals experiencing grade 2 toxicity, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events.31,32 Radiation damage to basal keratinocytes and hair follicle stem cells disrupts the renewal of the skin’s outer layer, while a surge of free radicals causes irreversible DNA damage.33 Symptoms of radiation dermatitis can vary from mild pink erythema to tissue ulceration and necrosis, typically within 1 to 4 weeks of radiation exposure.34 The resulting dermatitis can take 2 to 4 weeks to heal, notably impacting patient quality of life and often necessitating modifications or interruptions in cancer therapy.33
Prior studies have demonstrated the use of high-dose VD to improve the healing of UV-irradiated skin. A randomized controlled trial investigated high-dose vitamin D3 to treat experimentally induced sunburn in 20 healthy adults. Compared with those who received a placebo, participants receiving the oral dose of 200,000 IU of vitamin D3 demonstrated suppression of the pro-inflammatory mediators tumor necrosis factor α (P=.04) and inducible nitric oxide synthase (P=.02), while expression of tissue repair enhancer arginase 1 was increased (P<.005).35 The mechanism of this enhanced tissue repair was investigated using a mouse model, in which intraperitoneal 25(OH)D was administered following severe UV-induced skin injury. On immunofluorescence microscopy, mice treated with VD showed enhanced autophagy within the macrophages infiltrating UV-irradiated skin.36 The use of high-dose VD to treat UV-irradiated skin in these studies established a precedent for using VD to heal cutaneous injury caused by ionizing radiation therapy.
Some studies have focused on the role of VD for treating acute radiation dermatitis. A study of 23 patients with ductal carcinoma in situ or localized invasive ductal carcinoma breast cancer compared the effectiveness of topical calcipotriol to that of a standard hydrating ointment.37 Participants were randomized to 1 of 2 treatments before starting adjuvant radiotherapy to evaluate their potential in preventing radiation dermatitis. In 87% (20/23) of these patients, no difference in skin reaction was observed between the 2 treatments, suggesting that topical VD application may not offer any advantage over the standard hydrating ointment for the prevention of radiation dermatitis.37
Benefits of high-dose oral VD for treating radiation dermatitis also have been reported. Nguyen et al38 documented 3 cases in which patients with neuroendocrine carcinoma of the pancreas, tonsillar carcinoma, and breast cancer received 200,000 IU of oral ergocalciferol distributed over 2 doses given 7 days apart for radiation dermatitis. These patients experienced substantial improvements in pain, swelling, and redness within a week of the initial dose. Additionally, a case of radiation recall dermatitis, which occurred a week after vinorelbine chemotherapy, was treated with 2 doses totaling 100,000 IU of oral ergocalciferol. This patient also had improvement in pain and swelling but continued to have tumor-related induration and ulceration.39
Although topical VD did not show significant benefits over standard treatments for radiation dermatitis, high-dose oral VD appears promising in improving patient outcomes of pain and swelling more rapidly than current practices. Further research is needed to confirm these findings and establish standardized treatment protocols.
Final Thoughts
Suboptimal VD levels are prevalent in numerous cancer types. Chemotherapy often is associated with acute, potentially transient worsening of VD status in patients with breast and colorectal cancer. Although 25(OH)D levels have not corresponded with increased frequency of chemotherapy-related dermatologic AEs, suboptimal 25(OH)D levels appear to be associated with increased severity of radiation-induced mucositis and dermatitis.20,25,26 The use of high-dose VD as a therapeutic agent shows promise in mitigating chemotherapy-induced and radiation therapy–induced rashes in multiple cancer types with reduction of inflammatory markers and a durable anti-inflammatory impact. Although the mechanisms of cellular injury vary among chemotherapeutic agents, the anti-inflammatory and tissue repair properties of VD may make it an effective treatment for chemotherapy-induced cutaneous damage regardless of injury mechanism.2-4,35 However, reports of clinical improvement vary, and further objective studies to classify optimal dosing, administration, and outcome measures are needed. The absence of reported AEs associated with high-dose VD supplementation is encouraging, but selection of a safe and optimal dosing regimen can only occur with dedicated clinical trials.
Vitamin D (VD) regulates keratinocyte proliferation and differentiation, modulates inflammatory pathways, and protects against cellular damage in the skin. 1 In the setting of tissue injury and acute skin inflammation, active vitamin D—1,25(OH) 2 D—suppresses signaling from pro-inflammatory chemokines and cytokines such as IFN- γ and IL-17. 2,3 This suppression reduces proliferation of helper T cells (T H 1, T H 17) and B cells, decreasing tissue damage from reactive oxygen species release while enhancing secretion of the anti-inflammatory cytokine IL-10 by antigen-presenting cells. 2-4
Suboptimal VD levels have been associated with numerous health consequences including malignancy, prompting interest in VD supplementation for improving cancer-related outcomes.5 Beyond disease prognosis, high-dose VD supplementation has been suggested as a potential therapy for adverse events (AEs) related to cancer treatments. In one study, mice that received oral vitamin D3 supplementation of 11,500 IU/kg daily had fewer doxorubicin-induced cardiotoxic effects on ejection fraction (P<.0001) and stroke volume (P<.01) than mice that received VD supplementation of 1500 IU/kg daily.6
In this review, we examine the impact of chemoradiation on 25(OH)D levels—which more accurately reflects VD stores than 1,25(OH)2D levels—and the impact of suboptimal VD on cutaneous toxicities related to chemoradiation. To define the suboptimal VD threshold, we used the Endocrine Society’s clinical practice guidelines, which characterize suboptimal 25(OH)D levels as insufficiency (21–29 ng/mL [52.5–72.5 nmol/L]) or deficiency (<20 ng/mL [50 nmol/L])7; deficiency can be further categorized as severe deficiency (<12 ng/mL [30 nmol/L]).8 This review also evaluates the evidence for vitamin D3 supplementation to alleviate the cutaneous AEs of chemotherapy and radiation treatments.
Effects of Chemotherapy on Vitamin D Levels
A high prevalence of VD deficiency is seen in various cancers. In a retrospective review of 25(OH)D levels in 2098 adults with solid tumors of any stage (6% had metastatic disease [n=124]), suboptimal levels were found in 69% of patients with breast cancer (n=617), 75% with colorectal cancer (n=84), 72% with gynecologic cancer (n=65), 79% with kidney and bladder cancer (n=145), 83% with pancreatic and upper gastrointestinal tract cancer (n=178), 73% with lung cancer (n=73), 69% with prostate cancer (n=225), 61% with skin cancer (n=399), and 63% with thyroid cancer (n=172).5 Suboptimal VD also has been found in hematologic malignancies. In a prospective cohort study, mean serum 25(OH)D levels in 23 patients with recently diagnosed acute myeloid leukemia demonstrated VD deficiency (mean [SD], 18.6 [6.6] nmol/L).9 Given that many patients already exhibit a baseline VD deficiency at cancer diagnosis, it is important to understand the relationship between VD and cancer treatment modalities.5
In the United States, breast and colorectal cancers were estimated to be the first and fourth most common cancers, with 313,510 and 152,810 predicted new cases in 2024, respectively.10 This review will focus on breast and colorectal cancer when describing VD variation associated with chemotherapy exposure due to their high prevalence.
Effects of Chemotherapy on Vitamin D Levels in Breast Cancer—Breast cancer studies have shown suboptimal VD levels in 76% of females 75 years or younger with any T1, T2, or T3; N0 or N1; and M0 breast cancer, in which 38.5% (n=197) had insufficient and 37.5% (n=192) had deficient 25(OH)D levels.11 In a study of female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), VD deficiency was seen in 60% of patients not receiving VD supplementation.12,13 A systematic review that included 7 studies of different types of breast cancer suggested that circulating 25(OH)D may be associated with improved prognosis.14 Thus, studies have investigated risk factors associated with poor or worsening VD status in individuals with breast cancer, including exposure to chemotherapy and/or radiation treatment.12,15-18
A prospective cohort study assessed 25(OH)D levels in 95 patients with any breast cancer (stages I, II, IIIA, IIIB) before and after initiating chemotherapy with docetaxel, doxorubicin, epirubicin, 5-fluorouracil, or cyclophosphamide, compared with a group of 52 females without cancer.17 In the breast cancer group, approximately 80% (76/95) had suboptimal and 50% (47/95) had deficient VD levels before chemotherapy initiation (mean [SD], 54.1 [22.8] nmol/L). In the comparison group, 60% (31/52) had suboptimal and 30% (15/52) had deficient VD at baseline (mean [SD], 66.1 [23.5] nmol/L), which was higher than the breast cancer group (P=.03). A subgroup analysis excluded participants who started, stopped, or lacked data on dietary supplements containing VD (n=39); in the remaining 56 participants, a significant decrease in 25(OH)D levels was observed shortly after finishing chemotherapy compared with the prechemotherapy baseline value (mean, −7.9 nmol/L; P=.004). Notably, 6 months after chemotherapy completion, 25(OH)D levels increased (mean, +12.8 nmol/L; P<.001). Vitamin D levels remained stable in the comparison group (P=.987).17
Consistent with these findings, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of chemotherapy was associated with increased odds of 25(OH)D levels less than 20 ng/mL compared with breast cancer patients with no prior chemotherapy (odds ratio, 1.86; 95% CI, 1.03-3.38).12 Although the study data included chemotherapy history, no information was provided on specific chemotherapy agents or regimens used in this cohort, limiting the ability to detect the drugs most often implicated.
Both studies indicated a complex interplay between chemotherapy and VD levels in breast cancer patients. Although Kok et al17 suggested a transient decrease in VD levels during chemotherapy with a subsequent recovery after cessation, Fassio et al12 highlighted the increased odds of VD deficiency associated with chemotherapy. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status in breast cancer patients.
Effects of Chemotherapy on Vitamin D Levels in Colorectal Cancer—Similar to patterns seen in breast cancer, a systematic review with 6 studies of different types of colorectal cancer suggested that circulating 25(OH)D levels may be associated with prognosis.14 Studies also have investigated the relationship between colorectal chemotherapy regimens and VD status.15,16,18,19
A retrospective study assessed 25(OH)D levels in 315 patients with any colorectal cancer (stage I–IV).15 Patients were included in the analysis if they received less than 400 IU daily of VD supplementation at baseline. For the whole study sample, the mean (SD) VD level was 23.7 (13.71) ng/mL. Patients who had not received chemotherapy within 3 months of the VD level assessment were categorized as the no chemotherapy group, and the others were designated as the chemotherapy group; the latter group was exposed to various chemotherapy regimens, including combinations of irinotecan, oxaliplatin, 5-fluorouracil, leucovorin, bevacizumab, or cetuximab. Multivariate analysis showed that the chemotherapy group was 3.7 times more likely to have very low VD levels (≤15 ng/mL) compared with those in the no chemotherapy group (P<.0001).15
A separate cross-sectional study examined serum 25(OH)D concentrations in 1201 patients with any newly diagnosed colorectal carcinoma (stage I–III); 91% of cases were adenocarcinoma.18 In a multivariate analysis, chemotherapy plus surgery was associated with lower VD levels than surgery alone 6 months after diagnosis (mean, −8.74 nmol/L; 95% CI, −11.30 to −6.18 nmol/L), specifically decreasing by a mean of 6.7 nmol/L (95% CI, −9.8 to −3.8 nmol/L) after adjusting for demographic and lifestyle factors.18 However, a prospective cohort study demonstrated different findings.19 Comparing 58 patients with newly diagnosed colorectal adenocarcinoma (stages I–IV) who underwent chemotherapy and 36 patients who did not receive chemotherapy, there was no significant change in 25(OH)D levels from the time of diagnosis to 6 months later. Median VD levels decreased by 0.7 ng/mL in those who received chemotherapy, while a minimal (and not significant) increase of 1.6 ng/mL was observed in those without chemotherapy intervention (P=.26). Notably, supplementation was not restricted in this cohort, which may have resulted in higher VD levels in those taking supplements.19
Since time of year and geographic location can influence VD levels, one prospective cohort study controlled for differential sun exposure due to these factors in their analysis.16 Assessment of 25(OH)D levels was completed in 81 chemotherapy-naïve cancer patients immediately before beginning chemotherapy as well as 6 and 12 weeks into treatment. More than 8 primary cancer types were represented in this study, with breast (34% [29/81]) and colorectal (14% [12/81]) cancer being the most common, but the cancer stages of the participants were not detailed. Vitamin D levels decreased after commencing chemotherapy, with the largest drop occurring 6 weeks into treatment. From the 6- to 12-week end points, VD increased but remained below the original baseline level (baseline: mean [SD], 49.2 [22.3] nmol/L; 6 weeks: mean [SD], 40.9 [19.0] nmol/L; 12 weeks: mean [SD], 45.9 [19.7] nmol/L; P=.05).16
Although focused on breast and colorectal cancers, these studies suggest that various chemotherapy regimens may confer a higher risk for VD deficiency compared with VD status at diagnosis and/or prior to chemotherapy treatment. However, most of these studies only discussed stage-based differences, excluding analysis of the variety of cancer subtypes that comprise breast and colorectal malignancies, which may limit our ability to extrapolate from these data. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status across various primary cancer types.
Effects of Radiation Therapy on Vitamin D Levels
Unlike chemotherapy, studies on the association between radiation therapy and VD levels are minimal, with most reports in the literature discussing the use of VD to potentiate the effects of radiation therapy. In one cross-sectional analysis of 1201 patients with newly diagnosed stage I, II, or III colorectal cancer of any type (94% were adenocarcinoma), radiation plus surgery was associated with slightly lower 25(OH)D levels than surgery alone for tumor treatment 6 months after diagnosis (mean, −3.17; 95% CI, −6.07 to −0.28 nmol/L). However, after adjustment for demographic and lifestyle factors, this decrease in VD levels attributable to radiotherapy was not statistically significant compared with the surgery-only cohort (mean, −1.78; 95% CI, −5.07 to 1.52 nmol/L).18
Similarly, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of radiotherapy was not associated with a difference in serum 25(OH)D levels compared with those with breast cancer without prior radiotherapy (odds ratio, 0.90; 95% CI, 0.52-1.54).12 From the limited existing literature specifically addressing variations of VD levels with radiation, radiation therapy does not appear to significantly impact VD levels.
Vitamin D Levels and the Severity of Chemotherapy- or Radiation Therapy–Induced AEs
A prospective cohort of 241 patients did not find an increase in the incidence or severity of chemotherapy-induced cutaneous toxicities in those with suboptimal 1,25(OH)2D3 levels (≤75 nmol/L).20 Eight different primary cancer types were represented, including breast and colorectal cancer; the tumor stages of the participants were not detailed. Forty-one patients had normal 1,25(OH)2D3 levels, while the remaining 200 had suboptimal levels. There was no significant association between serum VD levels and the following dermatologic toxicities: desquamation (P=.26), xerosis (P=.15), mucositis (P=.30), or painful rash (P=.87). Surprisingly, nail changes and hand-foot reactions occurred with greater frequency in patients with normal VD levels (P=.01 and P=.03, respectively).20 Hand-foot reaction is part of the toxic erythema of chemotherapy (TEC) spectrum, which is comprised of a range of cytotoxic skin injuries that typically manifest within 2 to 3 weeks of exposure to the offending chemotherapeutic agents, often characterized by erythema, pain, swelling, and blistering, particularly in intertriginous and acral areas.21-23 Recovery from TEC generally takes at least 2 to 4 weeks and may necessitate cessation of the offending chemotherapeutic agent.21,24 Notably, this study measured 1,25(OH)2D3 levels instead of 25(OH)D levels, which may not reliably indicate body stores of VD.7,20 These results underscore the complex nature between chemotherapy and VD; however, VD levels alone do not appear to be a sufficient biomarker for predicting chemotherapy-associated cutaneous AEs.
Interestingly, radiation therapy–induced AEs may be associated with VD levels. A prospective cohort study of 98 patients with prostate, bladder, or gynecologic cancers (tumor stages were not detailed) undergoing pelvic radiotherapy found that females and males with 25(OH)D levels below a threshold of 35 and 40 nmol/L, respectively, were more likely to experience higher Radiation Therapy Oncology Group (RTOG) grade acute proctitis compared with those with VD above these thresholds.25 Specifically, VD below these thresholds was associated with increased odds of RTOG grade 2 or higher radiation-induced proctitis (OR, 3.07; 95% CI, 1.27-7.50 [P=.013]). Additionally, a weak correlation was noted between VD below these thresholds and the RTOG grade, with a Spearman correlation value of −0.189 (P=.031).25
One prospective cohort study included 28 patients with any cancer of the oral cavity, oropharynx, hypopharynx, or larynx stages II, III, or IVA; 93% (26/28) were stage III or IVA.26 The 20 (71%) patients with suboptimal 25(OH)D levels (≤75 nmol/L) experienced a higher prevalence of grade II radiation dermatitis compared with the 8 (29%) patients with optimal VD levels (χ22 =5.973; P=.0505). This pattern persisted with the severity of mucositis; patients from the suboptimal VD group presented with higher rates of grades II and III mucositis compared with the VD optimal group (χ22 =13.627; P=.0011).26 Recognizing the small cohort evaluated in the study, we highlight the importance of further studies to clarify these associations.
Chemotherapy-Induced Cutaneous Events Treated with High-Dose Vitamin D
Chemotherapeutic agents are known to induce cellular damage, resulting in a range of cutaneous AEs that can invoke discontinuation of otherwise effective chemotherapeutic interventions.27,28 Recent research has explored the potential of high-dose vitamin D3 as a therapeutic agent to mitigate cutaneous reactions.29,30
A randomized, double-blind, placebo-controlled trial investigated the use of a single high dose of oral 25(OH)D to treat topical nitrogen mustard (NM)–induced rash.29 To characterize baseline inflammatory responses from NM injury without intervention, clinical measures, serum studies, and tissue analyses from skin biopsies were performed on 28 healthy adults after exposure to topical NM—a chemotherapeutic agent classified as a DNA alkylator. Two weeks later, participants were exposed to topical NM a second time and were split into 2 groups: 14 patients received a single 200,000-IU dose of oral 25(OH)D while the other 14 participants were given a placebo. Using the inflammatory markers induced from baseline exposure to NM alone, posttreatment analysis revealed that the punch biopsies from
Although Ernst et al29 did not observe any clinically significant improvements with VD treatment, a case series of 6 patients with either glioblastoma multiforme, acute myeloid leukemia, or aplastic anemia did demonstrate clinical improvement of TEC after receiving high-dose vitamin D3.30 The mean time to onset of TEC was noted at 8.5 days following administration of the inciting chemotherapeutic agent, which included combinations of anthracycline, antimetabolite, kinase inhibitor, B-cell lymphoma 2 inhibitor, purine analogue, and alkylating agents. A combination of clinical and histologic findings was used to diagnose TEC. Baseline 25(OH)D levels were not established prior to treatment. The treatment regimen for 1 patient included 2 doses of 50,000 IU of VD spaced 1 week apart, totaling 100,000 IU, while the remaining 5 patients received a total of 200,000 IU, also split into 2 doses given 1 week apart. All patients received their first dose of VD within a week of the cutaneous eruption. Following the initial VD dose, there was a notable improvement in pain, pruritus, or swelling by the next day. Reduction in erythema also was observed within 1 to 4 days.30
No AEs associated with VD supplementation were reported, suggesting a potential beneficial role of high-dose VD in accelerating recovery from chemotherapy-induced rashes without evident safety concerns.
Radiation Therapy–Induced Cutaneous Events Treated with High-Dose Vitamin D
Radiation dermatitis is a common and often severe complication of radiation therapy that affects more than 90% of patients undergoing treatment, with half of these individuals experiencing grade 2 toxicity, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events.31,32 Radiation damage to basal keratinocytes and hair follicle stem cells disrupts the renewal of the skin’s outer layer, while a surge of free radicals causes irreversible DNA damage.33 Symptoms of radiation dermatitis can vary from mild pink erythema to tissue ulceration and necrosis, typically within 1 to 4 weeks of radiation exposure.34 The resulting dermatitis can take 2 to 4 weeks to heal, notably impacting patient quality of life and often necessitating modifications or interruptions in cancer therapy.33
Prior studies have demonstrated the use of high-dose VD to improve the healing of UV-irradiated skin. A randomized controlled trial investigated high-dose vitamin D3 to treat experimentally induced sunburn in 20 healthy adults. Compared with those who received a placebo, participants receiving the oral dose of 200,000 IU of vitamin D3 demonstrated suppression of the pro-inflammatory mediators tumor necrosis factor α (P=.04) and inducible nitric oxide synthase (P=.02), while expression of tissue repair enhancer arginase 1 was increased (P<.005).35 The mechanism of this enhanced tissue repair was investigated using a mouse model, in which intraperitoneal 25(OH)D was administered following severe UV-induced skin injury. On immunofluorescence microscopy, mice treated with VD showed enhanced autophagy within the macrophages infiltrating UV-irradiated skin.36 The use of high-dose VD to treat UV-irradiated skin in these studies established a precedent for using VD to heal cutaneous injury caused by ionizing radiation therapy.
Some studies have focused on the role of VD for treating acute radiation dermatitis. A study of 23 patients with ductal carcinoma in situ or localized invasive ductal carcinoma breast cancer compared the effectiveness of topical calcipotriol to that of a standard hydrating ointment.37 Participants were randomized to 1 of 2 treatments before starting adjuvant radiotherapy to evaluate their potential in preventing radiation dermatitis. In 87% (20/23) of these patients, no difference in skin reaction was observed between the 2 treatments, suggesting that topical VD application may not offer any advantage over the standard hydrating ointment for the prevention of radiation dermatitis.37
Benefits of high-dose oral VD for treating radiation dermatitis also have been reported. Nguyen et al38 documented 3 cases in which patients with neuroendocrine carcinoma of the pancreas, tonsillar carcinoma, and breast cancer received 200,000 IU of oral ergocalciferol distributed over 2 doses given 7 days apart for radiation dermatitis. These patients experienced substantial improvements in pain, swelling, and redness within a week of the initial dose. Additionally, a case of radiation recall dermatitis, which occurred a week after vinorelbine chemotherapy, was treated with 2 doses totaling 100,000 IU of oral ergocalciferol. This patient also had improvement in pain and swelling but continued to have tumor-related induration and ulceration.39
Although topical VD did not show significant benefits over standard treatments for radiation dermatitis, high-dose oral VD appears promising in improving patient outcomes of pain and swelling more rapidly than current practices. Further research is needed to confirm these findings and establish standardized treatment protocols.
Final Thoughts
Suboptimal VD levels are prevalent in numerous cancer types. Chemotherapy often is associated with acute, potentially transient worsening of VD status in patients with breast and colorectal cancer. Although 25(OH)D levels have not corresponded with increased frequency of chemotherapy-related dermatologic AEs, suboptimal 25(OH)D levels appear to be associated with increased severity of radiation-induced mucositis and dermatitis.20,25,26 The use of high-dose VD as a therapeutic agent shows promise in mitigating chemotherapy-induced and radiation therapy–induced rashes in multiple cancer types with reduction of inflammatory markers and a durable anti-inflammatory impact. Although the mechanisms of cellular injury vary among chemotherapeutic agents, the anti-inflammatory and tissue repair properties of VD may make it an effective treatment for chemotherapy-induced cutaneous damage regardless of injury mechanism.2-4,35 However, reports of clinical improvement vary, and further objective studies to classify optimal dosing, administration, and outcome measures are needed. The absence of reported AEs associated with high-dose VD supplementation is encouraging, but selection of a safe and optimal dosing regimen can only occur with dedicated clinical trials.
- Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3-19. doi:10.1007/s11154-011-9194-0
- Penna G, Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405-2411. doi:10.4049/jimmunol.164.5.2405
- Penna G, Amuchastegui S, Cossetti C, et al. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177:8504-8511. doi:10.4049/jimmunol.177.12.8504
- Heine G, Niesner U, Chang HD, et al. 1,25-dihydroxyvitamin D3 promotes IL-10 production in human B cells. Eur J Immunol. 2008;38:2210-2218. doi:10.1002/eji.200838216
- Hauser K, Walsh D, Shrotriya S, et al. Low 25-hydroxyvitamin D levels in people with a solid tumor cancer diagnosis: the tip of the iceberg? Support Care Cancer. 2014;22:1931-1939. doi:10.1007/s00520-014-2154-y
- Lee KJ, Wright G, Bryant H, et al. Cytoprotective effect of vitamin D on doxorubicin-induced cardiac toxicity in triple negative breast cancer. Int J Mol Sci. 2021;22:7439. doi:10.3390/ijms22147439
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. doi:10.1210/jc.2011-0385
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Thomas X, Chelghoum Y, Fanari N, et al. Serum 25-hydroxyvitamin D levels are associated with prognosis in hematological malignancies. Hematology. 2011;16:278-283. doi:10.1179/102453311X13085644679908
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. doi:10.3322/caac.21820
- Goodwin PJ, Ennis M, Pritchard KI, et al. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757-3763. doi:10.1200/JCO.2008.20.0725
- Fassio A, Porciello G, Carioli G, et al. Post-diagnosis serum 25-hydroxyvitamin D concentrations in women treated for breast cancer participating in a lifestyle trial in Italy. Reumatismo. 2024;76:21-34.
- Augustin LSA, Libra M, Crispo A, et al. Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence (DEDiCa): design of a clinical trial. BMC Cancer. 2017;17:69. doi:10.1186/s12885-017-3064-4
- Toriola AT, Nguyen N, Scheitler-Ring K, et al. Circulating 25-hydroxyvitamin D levels and prognosis among cancer patients: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23:917-933. doi:10.1158/1055-9965.EPI-14-0053
- Fakih MG, Trump DL, Johnson CS, et al. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis. 2009;24:219-224. doi:10.1007/s00384-008-0593-y
- Isenring EA, Teleni L, Woodman RJ, et al. Serum vitamin D decreases during chemotherapy: an Australian prospective cohort study. Asia Pac J Clin Nutr. 2018;27:962-967. doi:10.6133/apjcn.042018.01
- Kok DE, van den Berg MMGA, Posthuma L, et al. Changes in circulating levels of 25-hydroxyvitamin D3 in breast cancer patients receiving chemotherapy. Nutr Cancer. 2019;71:756-766. doi:10.1080/01635581.2018.1559938
- Wesselink E, Bours MJL, de Wilt JHW, et al. Chemotherapy and vitamin D supplement use are determinants of serum 25-hydroxyvitamin D levels during the first six months after colorectal cancer diagnosis. J Steroid Biochem Mol Biol. 2020;199:105577. doi:10.1016/j.jsbmb.2020.105577
- Savoie MB, Paciorek A, Zhang L, et al. Vitamin D levels in patients with colorectal cancer before and after treatment initiation. J Gastrointest Cancer. 2019;50:769-779. doi:10.1007/s12029-018-0147-7
- Kitchen D, Hughes B, Gill I, et al. The relationship between vitamin D and chemotherapy-induced toxicity—a pilot study. Br J Cancer. 2012;107:158-160. doi:10.1038/bjc.2012.194
- Demircay Z, Gürbüz O, Alpdogan TB, et al. Chemotherapy-induced acral erythema in leukemic patients: a report of 15 cases. Int J Dermatol. 1997;36:593-598. doi:10.1046/j.1365-4362.1997.00040.x
- Valks R, Fraga J, Porras-Luque J, et al. Chemotherapy-induced eccrine squamous syringometaplasia. a distinctive eruption in patients receiving hematopoietic progenitor cells. Arch Dermatol. 1997;133;873-878. doi:10.1001/archderm.133.7.873
- Webber KA, Kos L, Holland KE, et al. Intertriginous eruption associated with chemotherapy in pediatric patients. Arch Dermatol. 2007;143:67-71. doi:10.1001/archderm.143.1.67
- Hunjan MK, Nowsheen S, Ramos-Rodriguez AJ, et al. Clinical and histopathological spectrum of toxic erythema of chemotherapy in patients who have undergone allogeneic hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2019;12:19-25. doi:10.1016/j.hemonc.2018.09.001
- Ghorbanzadeh-Moghaddam A, Gholamrezaei A, Hemati S. Vitamin D deficiency is associated with the severity of radiation-induced proctitis in cancer patients. Int J Radiat Oncol Biol Phys. 2015;92:613-618. doi:10.1016/j.ijrobp.2015.02.011
- Bhanu A, Waghmare CM, Jain VS, et al. Serum 25-hydroxy vitamin-D levels in head and neck cancer chemoradiation therapy: potential in cancer therapeutics. Indian J Cancer. Published online February 27, 2003. doi:10.4103/ijc.ijc_358_20
- Yang B, Xie X, Wu Z, et al. DNA damage-mediated cellular senescence promotes hand-foot syndrome that can be relieved by thymidine prodrug. Genes Dis. 2022;10:2557-2571. doi:10.1016/j.gendis.2022.10.004
- Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda®). Eur J Oncol Nurs. 2004;8(suppl 1):S31-S40. doi:10.1016/j.ejon.2004.06.007
- Ernst MK, Evans ST, Techner JM, et al. Vitamin D3 and deconvoluting a rash. JCI Insight. 2023;8:E163789.
- Nguyen CV, Zheng L, Zhou XA, et al. High-dose vitamin d for the management of toxic erythema of chemotherapy in hospitalized patients. JAMA Dermatol. 2023;159:219-221. doi:10.1001/jamadermatol.2022.5397
- Fisher J, Scott C, Stevens R, et al. Randomized phase III study comparing best supportive care to biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48:1307-1310. doi:10.1016/s0360-3016(00)00782-3
- Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085-2092. doi:10.1200/JCO.2007.15.2488
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3 pt 2):985-993. doi:10.1038/jid.2011.411
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086. doi:10.1016/j.jid.2017.04.040
- Das LM, Binko AM, Traylor ZP, et al. Vitamin D improves sunburns by increasing autophagy in M2 macrophages. Autophagy. 2019;15:813-826. doi:10.1080/15548627.2019.1569298
- Nasser NJ, Fenig S, Ravid A, et al. Vitamin D ointment for prevention of radiation dermatitis in breast cancer patients. NPJ Breast Cancer. 2017;3:10. doi:10.1038/s41523-017-0006-x
- Nguyen CV, Zheng L, Lu KQ. High-dose vitamin D for the management acute radiation dermatitis. JAAD Case Rep. 2023;39:47-50. doi:10.1016/j.jdcr.2023.07.001
- Nguyen CV, Lu KQ. Vitamin D3 and its potential to ameliorate chemical and radiation-induced skin injury during cancer therapy. Disaster Med Public Health Prep. 2024;18:E4. doi:10.1017/dmp.2023.211
- Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3-19. doi:10.1007/s11154-011-9194-0
- Penna G, Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405-2411. doi:10.4049/jimmunol.164.5.2405
- Penna G, Amuchastegui S, Cossetti C, et al. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177:8504-8511. doi:10.4049/jimmunol.177.12.8504
- Heine G, Niesner U, Chang HD, et al. 1,25-dihydroxyvitamin D3 promotes IL-10 production in human B cells. Eur J Immunol. 2008;38:2210-2218. doi:10.1002/eji.200838216
- Hauser K, Walsh D, Shrotriya S, et al. Low 25-hydroxyvitamin D levels in people with a solid tumor cancer diagnosis: the tip of the iceberg? Support Care Cancer. 2014;22:1931-1939. doi:10.1007/s00520-014-2154-y
- Lee KJ, Wright G, Bryant H, et al. Cytoprotective effect of vitamin D on doxorubicin-induced cardiac toxicity in triple negative breast cancer. Int J Mol Sci. 2021;22:7439. doi:10.3390/ijms22147439
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. doi:10.1210/jc.2011-0385
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Thomas X, Chelghoum Y, Fanari N, et al. Serum 25-hydroxyvitamin D levels are associated with prognosis in hematological malignancies. Hematology. 2011;16:278-283. doi:10.1179/102453311X13085644679908
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. doi:10.3322/caac.21820
- Goodwin PJ, Ennis M, Pritchard KI, et al. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757-3763. doi:10.1200/JCO.2008.20.0725
- Fassio A, Porciello G, Carioli G, et al. Post-diagnosis serum 25-hydroxyvitamin D concentrations in women treated for breast cancer participating in a lifestyle trial in Italy. Reumatismo. 2024;76:21-34.
- Augustin LSA, Libra M, Crispo A, et al. Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence (DEDiCa): design of a clinical trial. BMC Cancer. 2017;17:69. doi:10.1186/s12885-017-3064-4
- Toriola AT, Nguyen N, Scheitler-Ring K, et al. Circulating 25-hydroxyvitamin D levels and prognosis among cancer patients: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23:917-933. doi:10.1158/1055-9965.EPI-14-0053
- Fakih MG, Trump DL, Johnson CS, et al. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis. 2009;24:219-224. doi:10.1007/s00384-008-0593-y
- Isenring EA, Teleni L, Woodman RJ, et al. Serum vitamin D decreases during chemotherapy: an Australian prospective cohort study. Asia Pac J Clin Nutr. 2018;27:962-967. doi:10.6133/apjcn.042018.01
- Kok DE, van den Berg MMGA, Posthuma L, et al. Changes in circulating levels of 25-hydroxyvitamin D3 in breast cancer patients receiving chemotherapy. Nutr Cancer. 2019;71:756-766. doi:10.1080/01635581.2018.1559938
- Wesselink E, Bours MJL, de Wilt JHW, et al. Chemotherapy and vitamin D supplement use are determinants of serum 25-hydroxyvitamin D levels during the first six months after colorectal cancer diagnosis. J Steroid Biochem Mol Biol. 2020;199:105577. doi:10.1016/j.jsbmb.2020.105577
- Savoie MB, Paciorek A, Zhang L, et al. Vitamin D levels in patients with colorectal cancer before and after treatment initiation. J Gastrointest Cancer. 2019;50:769-779. doi:10.1007/s12029-018-0147-7
- Kitchen D, Hughes B, Gill I, et al. The relationship between vitamin D and chemotherapy-induced toxicity—a pilot study. Br J Cancer. 2012;107:158-160. doi:10.1038/bjc.2012.194
- Demircay Z, Gürbüz O, Alpdogan TB, et al. Chemotherapy-induced acral erythema in leukemic patients: a report of 15 cases. Int J Dermatol. 1997;36:593-598. doi:10.1046/j.1365-4362.1997.00040.x
- Valks R, Fraga J, Porras-Luque J, et al. Chemotherapy-induced eccrine squamous syringometaplasia. a distinctive eruption in patients receiving hematopoietic progenitor cells. Arch Dermatol. 1997;133;873-878. doi:10.1001/archderm.133.7.873
- Webber KA, Kos L, Holland KE, et al. Intertriginous eruption associated with chemotherapy in pediatric patients. Arch Dermatol. 2007;143:67-71. doi:10.1001/archderm.143.1.67
- Hunjan MK, Nowsheen S, Ramos-Rodriguez AJ, et al. Clinical and histopathological spectrum of toxic erythema of chemotherapy in patients who have undergone allogeneic hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2019;12:19-25. doi:10.1016/j.hemonc.2018.09.001
- Ghorbanzadeh-Moghaddam A, Gholamrezaei A, Hemati S. Vitamin D deficiency is associated with the severity of radiation-induced proctitis in cancer patients. Int J Radiat Oncol Biol Phys. 2015;92:613-618. doi:10.1016/j.ijrobp.2015.02.011
- Bhanu A, Waghmare CM, Jain VS, et al. Serum 25-hydroxy vitamin-D levels in head and neck cancer chemoradiation therapy: potential in cancer therapeutics. Indian J Cancer. Published online February 27, 2003. doi:10.4103/ijc.ijc_358_20
- Yang B, Xie X, Wu Z, et al. DNA damage-mediated cellular senescence promotes hand-foot syndrome that can be relieved by thymidine prodrug. Genes Dis. 2022;10:2557-2571. doi:10.1016/j.gendis.2022.10.004
- Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda®). Eur J Oncol Nurs. 2004;8(suppl 1):S31-S40. doi:10.1016/j.ejon.2004.06.007
- Ernst MK, Evans ST, Techner JM, et al. Vitamin D3 and deconvoluting a rash. JCI Insight. 2023;8:E163789.
- Nguyen CV, Zheng L, Zhou XA, et al. High-dose vitamin d for the management of toxic erythema of chemotherapy in hospitalized patients. JAMA Dermatol. 2023;159:219-221. doi:10.1001/jamadermatol.2022.5397
- Fisher J, Scott C, Stevens R, et al. Randomized phase III study comparing best supportive care to biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48:1307-1310. doi:10.1016/s0360-3016(00)00782-3
- Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085-2092. doi:10.1200/JCO.2007.15.2488
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3 pt 2):985-993. doi:10.1038/jid.2011.411
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086. doi:10.1016/j.jid.2017.04.040
- Das LM, Binko AM, Traylor ZP, et al. Vitamin D improves sunburns by increasing autophagy in M2 macrophages. Autophagy. 2019;15:813-826. doi:10.1080/15548627.2019.1569298
- Nasser NJ, Fenig S, Ravid A, et al. Vitamin D ointment for prevention of radiation dermatitis in breast cancer patients. NPJ Breast Cancer. 2017;3:10. doi:10.1038/s41523-017-0006-x
- Nguyen CV, Zheng L, Lu KQ. High-dose vitamin D for the management acute radiation dermatitis. JAAD Case Rep. 2023;39:47-50. doi:10.1016/j.jdcr.2023.07.001
- Nguyen CV, Lu KQ. Vitamin D3 and its potential to ameliorate chemical and radiation-induced skin injury during cancer therapy. Disaster Med Public Health Prep. 2024;18:E4. doi:10.1017/dmp.2023.211
Practice Points
- High-dose vitamin D supplementation may be considered in the management of cutaneous injury from chemotherapy or ionizing radiation.
- Optimal dosing has not been established, so patients given high-dose vitamin D supplementation should have close clinical follow-up; however, adverse events from high-dose vitamin D supplementation have not been reported.
Metformin Led to Improvements in Women with Central Centrifugal Cicatricial Alopecia
TOPLINE:
, in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 upregulated genes, which included upregulated of 23 hair keratin-associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were downregulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. In addition, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 upregulated genes, which included upregulated of 23 hair keratin-associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were downregulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. In addition, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 upregulated genes, which included upregulated of 23 hair keratin-associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were downregulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. In addition, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
High Breast Cancer Risk With Menopausal Hormone Therapy & Strong Family History
TOPLINE:
These women have a striking cumulative risk of developing breast cancer (age, 50-80 years) of 22.4%, according to a new modelling study of UK women.
METHODOLOGY:
This was a modeling study integrating two data-sets of UK women: the BOADICEA dataset of age-specific breast cancer risk with family history and the Collaborative Group on Hormonal Factors in Breast Cancer, which covers relative risk for breast cancer with different types and durations of MHT.
Four different breast cancer family history profiles were:
- “Average” family history of breast cancer has unknown affected family members;
- “Modest” family history comprises a single first-degree relative with breast cancer at the age of 60 years.
- “Intermediate” family history comprises a single first-degree relative who developed breast cancer at the age of 40 years.
- “Strong” family history comprises two first-degree relatives who developed breast cancer at the age of 50 years.
TAKEAWAY:
- The lowest risk category: “Average” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 9.8% and a risk of dying from breast cancer of 1.7%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 11.0% and 1.8%, respectively.
- The highest risk category: “Strong” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 19.6% and a risk of dying from breast cancer of 3.2%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 22.4% and 3.5%, respectively.
IN PRACTICE:
The authors concluded that, “These integrated data will enable more accurate estimates of absolute and attributable risk associated with MHT exposure for women with a family history of breast cancer, informing shared decision-making.”
SOURCE:
The lead author is Catherine Huntley of the Institute of Cancer Research, London, England. The study appeared in the British Journal of General Practice.
LIMITATIONS:
Limitations included modeling study that did not directly measure individuals with combined risks.
DISCLOSURES:
The study was funded by several sources including Cancer Research UK. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
These women have a striking cumulative risk of developing breast cancer (age, 50-80 years) of 22.4%, according to a new modelling study of UK women.
METHODOLOGY:
This was a modeling study integrating two data-sets of UK women: the BOADICEA dataset of age-specific breast cancer risk with family history and the Collaborative Group on Hormonal Factors in Breast Cancer, which covers relative risk for breast cancer with different types and durations of MHT.
Four different breast cancer family history profiles were:
- “Average” family history of breast cancer has unknown affected family members;
- “Modest” family history comprises a single first-degree relative with breast cancer at the age of 60 years.
- “Intermediate” family history comprises a single first-degree relative who developed breast cancer at the age of 40 years.
- “Strong” family history comprises two first-degree relatives who developed breast cancer at the age of 50 years.
TAKEAWAY:
- The lowest risk category: “Average” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 9.8% and a risk of dying from breast cancer of 1.7%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 11.0% and 1.8%, respectively.
- The highest risk category: “Strong” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 19.6% and a risk of dying from breast cancer of 3.2%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 22.4% and 3.5%, respectively.
IN PRACTICE:
The authors concluded that, “These integrated data will enable more accurate estimates of absolute and attributable risk associated with MHT exposure for women with a family history of breast cancer, informing shared decision-making.”
SOURCE:
The lead author is Catherine Huntley of the Institute of Cancer Research, London, England. The study appeared in the British Journal of General Practice.
LIMITATIONS:
Limitations included modeling study that did not directly measure individuals with combined risks.
DISCLOSURES:
The study was funded by several sources including Cancer Research UK. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
These women have a striking cumulative risk of developing breast cancer (age, 50-80 years) of 22.4%, according to a new modelling study of UK women.
METHODOLOGY:
This was a modeling study integrating two data-sets of UK women: the BOADICEA dataset of age-specific breast cancer risk with family history and the Collaborative Group on Hormonal Factors in Breast Cancer, which covers relative risk for breast cancer with different types and durations of MHT.
Four different breast cancer family history profiles were:
- “Average” family history of breast cancer has unknown affected family members;
- “Modest” family history comprises a single first-degree relative with breast cancer at the age of 60 years.
- “Intermediate” family history comprises a single first-degree relative who developed breast cancer at the age of 40 years.
- “Strong” family history comprises two first-degree relatives who developed breast cancer at the age of 50 years.
TAKEAWAY:
- The lowest risk category: “Average” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 9.8% and a risk of dying from breast cancer of 1.7%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 11.0% and 1.8%, respectively.
- The highest risk category: “Strong” family history with no MHT use has a cumulative breast cancer risk (age, 50-80 years) of 19.6% and a risk of dying from breast cancer of 3.2%. These risks rise with 5 years’ exposure to MHT (age, 50-55 years) to 22.4% and 3.5%, respectively.
IN PRACTICE:
The authors concluded that, “These integrated data will enable more accurate estimates of absolute and attributable risk associated with MHT exposure for women with a family history of breast cancer, informing shared decision-making.”
SOURCE:
The lead author is Catherine Huntley of the Institute of Cancer Research, London, England. The study appeared in the British Journal of General Practice.
LIMITATIONS:
Limitations included modeling study that did not directly measure individuals with combined risks.
DISCLOSURES:
The study was funded by several sources including Cancer Research UK. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Do Clonal Hematopoiesis and Mosaic Chromosomal Alterations Increase Solid Tumor Risk?
Clonal hematopoiesis of indeterminate potential (CHIP) and mosaic chromosomal alterations (mCAs) are associated with an increased risk for breast cancer, and CHIP is associated with increased mortality in patients with colon cancer, according to the authors of new research.
These findings, drawn from almost 11,000 patients in the Women’s Health Initiative (WHI) study, add further evidence that CHIP and mCA drive solid tumor risk, alongside known associations with hematologic malignancies, reported lead author Pinkal Desai, MD, associate professor of medicine and clinical director of molecular aging at Englander Institute for Precision Medicine, Weill Cornell Medical College, New York City, and colleagues.
How This Study Differs From Others of Breast Cancer Risk Factors
“The independent effect of CHIP and mCA on risk and mortality from solid tumors has not been elucidated due to lack of detailed data on mortality outcomes and risk factors,” the investigators wrote in Cancer, although some previous studies have suggested a link.
In particular, the investigators highlighted a 2022 UK Biobank study, which reported an association between CHIP and lung cancer and a borderline association with breast cancer that did not quite reach statistical significance.
But the UK Biobank study was confined to a UK population, Dr. Desai noted in an interview, and the data were less detailed than those in the present investigation.
“In terms of risk, the part that was lacking in previous studies was a comprehensive assessment of risk factors that increase risk for all these cancers,” Dr. Desai said. “For example, for breast cancer, we had very detailed data on [participants’] Gail risk score, which is known to impact breast cancer risk. We also had mammogram data and colonoscopy data.”
In an accompanying editorial, Koichi Takahashi, MD, PhD , and Nehali Shah, BS, of The University of Texas MD Anderson Cancer Center, Houston, Texas, pointed out the same UK Biobank findings, then noted that CHIP has also been linked with worse overall survival in unselected cancer patients. Still, they wrote, “the impact of CH on cancer risk and mortality remains controversial due to conflicting data and context‐dependent effects,” necessitating studies like this one by Dr. Desai and colleagues.
How Was the Relationship Between CHIP, MCA, and Solid Tumor Risk Assessed?
To explore possible associations between CHIP, mCA, and solid tumors, the investigators analyzed whole genome sequencing data from 10,866 women in the WHI, a multi-study program that began in 1992 and involved 161,808 women in both observational and clinical trial cohorts.
In 2002, the first big data release from the WHI suggested that hormone replacement therapy (HRT) increased breast cancer risk, leading to widespread reduction in HRT use.
More recent reports continue to shape our understanding of these risks, suggesting differences across cancer types. For breast cancer, the WHI data suggested that HRT-associated risk was largely driven by formulations involving progesterone and estrogen, whereas estrogen-only formulations, now more common, are generally considered to present an acceptable risk profile for suitable patients.
The new study accounted for this potential HRT-associated risk, including by adjusting for patients who received HRT, type of HRT received, and duration of HRT received. According to Desai, this approach is commonly used when analyzing data from the WHI, nullifying concerns about the potentially deleterious effects of the hormones used in the study.
“Our question was not ‘does HRT cause cancer?’ ” Dr. Desai said in an interview. “But HRT can be linked to breast cancer risk and has a potential to be a confounder, and hence the above methodology.
“So I can say that the confounding/effect modification that HRT would have contributed to in the relationship between exposure (CH and mCA) and outcome (cancer) is well adjusted for as described above. This is standard in WHI analyses,” she continued.
“Every Women’s Health Initiative analysis that comes out — not just for our study — uses a standard method ... where you account for hormonal therapy,” Dr. Desai added, again noting that many other potential risk factors were considered, enabling a “detailed, robust” analysis.
Dr. Takahashi and Ms. Shah agreed. “A notable strength of this study is its adjustment for many confounding factors,” they wrote. “The cohort’s well‐annotated data on other known cancer risk factors allowed for a robust assessment of CH’s independent risk.”
How Do Findings Compare With Those of the UK Biobank Study?
CHIP was associated with a 30% increased risk for breast cancer (hazard ratio [HR], 1.30; 95% CI, 1.03-1.64; P = .02), strengthening the borderline association reported by the UK Biobank study.
In contrast with the UK Biobank study, CHIP was not associated with lung cancer risk, although this may have been caused by fewer cases of lung cancer and a lack of male patients, Dr. Desai suggested.
“The discrepancy between the studies lies in the risk of lung cancer, although the point estimate in the current study suggested a positive association,” wrote Dr. Takahashi and Ms. Shah.
As in the UK Biobank study, CHIP was not associated with increased risk of developing colorectal cancer.
Mortality analysis, however, which was not conducted in the UK Biobank study, offered a new insight: Patients with existing colorectal cancer and CHIP had a significantly higher mortality risk than those without CHIP. Before stage adjustment, risk for mortality among those with colorectal cancer and CHIP was fourfold higher than those without CHIP (HR, 3.99; 95% CI, 2.41-6.62; P < .001). After stage adjustment, CHIP was still associated with a twofold higher mortality risk (HR, 2.50; 95% CI, 1.32-4.72; P = .004).
The investigators’ first mCA analyses, which employed a cell fraction cutoff greater than 3%, were unfruitful. But raising the cell fraction threshold to 5% in an exploratory analysis showed that autosomal mCA was associated with a 39% increased risk for breast cancer (HR, 1.39; 95% CI, 1.06-1.83; P = .01). No such associations were found between mCA and colorectal or lung cancer, regardless of cell fraction threshold.
The original 3% cell fraction threshold was selected on the basis of previous studies reporting a link between mCA and hematologic malignancies at this cutoff, Dr. Desai said.
She and her colleagues said a higher 5% cutoff might be needed, as they suspected that the link between mCA and solid tumors may not be causal, requiring a higher mutation rate.
Why Do Results Differ Between These Types of Studies?
Dr. Takahashi and Ms. Shah suggested that one possible limitation of the new study, and an obstacle to comparing results with the UK Biobank study and others like it, goes beyond population heterogeneity; incongruent findings could also be explained by differences in whole genome sequencing (WGS) technique.
“Although WGS allows sensitive detection of mCA through broad genomic coverage, it is less effective at detecting CHIP with low variant allele frequency (VAF) due to its relatively shallow depth (30x),” they wrote. “Consequently, the prevalence of mCA (18.8%) was much higher than that of CHIP (8.3%) in this cohort, contrasting with other studies using deeper sequencing.” As a result, the present study may have underestimated CHIP prevalence because of shallow sequencing depth.
“This inconsistency is a common challenge in CH population studies due to the lack of standardized methodologies and the frequent reliance on preexisting data not originally intended for CH detection,” Dr. Takahashi and Ms. Shah said.
Even so, despite the “heavily context-dependent” nature of these reported risks, the body of evidence to date now offers a convincing biological rationale linking CH with cancer development and outcomes, they added.
How Do the CHIP- and mCA-associated Risks Differ Between Solid Tumors and Blood Cancers?
“[These solid tumor risks are] not causal in the way CHIP mutations are causal for blood cancers,” Dr. Desai said. “Here we are talking about solid tumor risk, and it’s kind of scattered. It’s not just breast cancer ... there’s also increased colon cancer mortality. So I feel these mutations are doing something different ... they are sort of an added factor.”
Specific mechanisms remain unclear, Dr. Desai said, although she speculated about possible impacts on the inflammatory state or alterations to the tumor microenvironment.
“These are blood cells, right?” Dr. Desai asked. “They’re everywhere, and they’re changing something inherently in these tumors.”
Future research and therapeutic development
Siddhartha Jaiswal, MD, PhD, assistant professor in the Department of Pathology at Stanford University in California, whose lab focuses on clonal hematopoiesis, said the causality question is central to future research.
“The key question is, are these mutations acting because they alter the function of blood cells in some way to promote cancer risk, or is it reflective of some sort of shared etiology that’s not causal?” Dr. Jaiswal said in an interview.
Available data support both possibilities.
On one side, “reasonable evidence” supports the noncausal view, Dr. Jaiswal noted, because telomere length is one of the most common genetic risk factors for clonal hematopoiesis and also for solid tumors, suggesting a shared genetic factor. On the other hand, CHIP and mCA could be directly protumorigenic via conferred disturbances of immune cell function.
When asked if both causal and noncausal factors could be at play, Dr. Jaiswal said, “yeah, absolutely.”
The presence of a causal association could be promising from a therapeutic standpoint.
“If it turns out that this association is driven by a direct causal effect of the mutations, perhaps related to immune cell function or dysfunction, then targeting that dysfunction could be a therapeutic path to improve outcomes in people, and there’s a lot of interest in this,” Dr. Jaiswal said. He went on to explain how a trial exploring this approach via interleukin-8 inhibition in lung cancer fell short.
Yet earlier intervention may still hold promise, according to experts.
“[This study] provokes the hypothesis that CH‐targeted interventions could potentially reduce cancer risk in the future,” Dr. Takahashi and Ms. Shah said in their editorial.
The WHI program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The investigators disclosed relationships with Eli Lilly, AbbVie, Celgene, and others. Dr. Jaiswal reported stock equity in a company that has an interest in clonal hematopoiesis.
A version of this article first appeared on Medscape.com.
Clonal hematopoiesis of indeterminate potential (CHIP) and mosaic chromosomal alterations (mCAs) are associated with an increased risk for breast cancer, and CHIP is associated with increased mortality in patients with colon cancer, according to the authors of new research.
These findings, drawn from almost 11,000 patients in the Women’s Health Initiative (WHI) study, add further evidence that CHIP and mCA drive solid tumor risk, alongside known associations with hematologic malignancies, reported lead author Pinkal Desai, MD, associate professor of medicine and clinical director of molecular aging at Englander Institute for Precision Medicine, Weill Cornell Medical College, New York City, and colleagues.
How This Study Differs From Others of Breast Cancer Risk Factors
“The independent effect of CHIP and mCA on risk and mortality from solid tumors has not been elucidated due to lack of detailed data on mortality outcomes and risk factors,” the investigators wrote in Cancer, although some previous studies have suggested a link.
In particular, the investigators highlighted a 2022 UK Biobank study, which reported an association between CHIP and lung cancer and a borderline association with breast cancer that did not quite reach statistical significance.
But the UK Biobank study was confined to a UK population, Dr. Desai noted in an interview, and the data were less detailed than those in the present investigation.
“In terms of risk, the part that was lacking in previous studies was a comprehensive assessment of risk factors that increase risk for all these cancers,” Dr. Desai said. “For example, for breast cancer, we had very detailed data on [participants’] Gail risk score, which is known to impact breast cancer risk. We also had mammogram data and colonoscopy data.”
In an accompanying editorial, Koichi Takahashi, MD, PhD , and Nehali Shah, BS, of The University of Texas MD Anderson Cancer Center, Houston, Texas, pointed out the same UK Biobank findings, then noted that CHIP has also been linked with worse overall survival in unselected cancer patients. Still, they wrote, “the impact of CH on cancer risk and mortality remains controversial due to conflicting data and context‐dependent effects,” necessitating studies like this one by Dr. Desai and colleagues.
How Was the Relationship Between CHIP, MCA, and Solid Tumor Risk Assessed?
To explore possible associations between CHIP, mCA, and solid tumors, the investigators analyzed whole genome sequencing data from 10,866 women in the WHI, a multi-study program that began in 1992 and involved 161,808 women in both observational and clinical trial cohorts.
In 2002, the first big data release from the WHI suggested that hormone replacement therapy (HRT) increased breast cancer risk, leading to widespread reduction in HRT use.
More recent reports continue to shape our understanding of these risks, suggesting differences across cancer types. For breast cancer, the WHI data suggested that HRT-associated risk was largely driven by formulations involving progesterone and estrogen, whereas estrogen-only formulations, now more common, are generally considered to present an acceptable risk profile for suitable patients.
The new study accounted for this potential HRT-associated risk, including by adjusting for patients who received HRT, type of HRT received, and duration of HRT received. According to Desai, this approach is commonly used when analyzing data from the WHI, nullifying concerns about the potentially deleterious effects of the hormones used in the study.
“Our question was not ‘does HRT cause cancer?’ ” Dr. Desai said in an interview. “But HRT can be linked to breast cancer risk and has a potential to be a confounder, and hence the above methodology.
“So I can say that the confounding/effect modification that HRT would have contributed to in the relationship between exposure (CH and mCA) and outcome (cancer) is well adjusted for as described above. This is standard in WHI analyses,” she continued.
“Every Women’s Health Initiative analysis that comes out — not just for our study — uses a standard method ... where you account for hormonal therapy,” Dr. Desai added, again noting that many other potential risk factors were considered, enabling a “detailed, robust” analysis.
Dr. Takahashi and Ms. Shah agreed. “A notable strength of this study is its adjustment for many confounding factors,” they wrote. “The cohort’s well‐annotated data on other known cancer risk factors allowed for a robust assessment of CH’s independent risk.”
How Do Findings Compare With Those of the UK Biobank Study?
CHIP was associated with a 30% increased risk for breast cancer (hazard ratio [HR], 1.30; 95% CI, 1.03-1.64; P = .02), strengthening the borderline association reported by the UK Biobank study.
In contrast with the UK Biobank study, CHIP was not associated with lung cancer risk, although this may have been caused by fewer cases of lung cancer and a lack of male patients, Dr. Desai suggested.
“The discrepancy between the studies lies in the risk of lung cancer, although the point estimate in the current study suggested a positive association,” wrote Dr. Takahashi and Ms. Shah.
As in the UK Biobank study, CHIP was not associated with increased risk of developing colorectal cancer.
Mortality analysis, however, which was not conducted in the UK Biobank study, offered a new insight: Patients with existing colorectal cancer and CHIP had a significantly higher mortality risk than those without CHIP. Before stage adjustment, risk for mortality among those with colorectal cancer and CHIP was fourfold higher than those without CHIP (HR, 3.99; 95% CI, 2.41-6.62; P < .001). After stage adjustment, CHIP was still associated with a twofold higher mortality risk (HR, 2.50; 95% CI, 1.32-4.72; P = .004).
The investigators’ first mCA analyses, which employed a cell fraction cutoff greater than 3%, were unfruitful. But raising the cell fraction threshold to 5% in an exploratory analysis showed that autosomal mCA was associated with a 39% increased risk for breast cancer (HR, 1.39; 95% CI, 1.06-1.83; P = .01). No such associations were found between mCA and colorectal or lung cancer, regardless of cell fraction threshold.
The original 3% cell fraction threshold was selected on the basis of previous studies reporting a link between mCA and hematologic malignancies at this cutoff, Dr. Desai said.
She and her colleagues said a higher 5% cutoff might be needed, as they suspected that the link between mCA and solid tumors may not be causal, requiring a higher mutation rate.
Why Do Results Differ Between These Types of Studies?
Dr. Takahashi and Ms. Shah suggested that one possible limitation of the new study, and an obstacle to comparing results with the UK Biobank study and others like it, goes beyond population heterogeneity; incongruent findings could also be explained by differences in whole genome sequencing (WGS) technique.
“Although WGS allows sensitive detection of mCA through broad genomic coverage, it is less effective at detecting CHIP with low variant allele frequency (VAF) due to its relatively shallow depth (30x),” they wrote. “Consequently, the prevalence of mCA (18.8%) was much higher than that of CHIP (8.3%) in this cohort, contrasting with other studies using deeper sequencing.” As a result, the present study may have underestimated CHIP prevalence because of shallow sequencing depth.
“This inconsistency is a common challenge in CH population studies due to the lack of standardized methodologies and the frequent reliance on preexisting data not originally intended for CH detection,” Dr. Takahashi and Ms. Shah said.
Even so, despite the “heavily context-dependent” nature of these reported risks, the body of evidence to date now offers a convincing biological rationale linking CH with cancer development and outcomes, they added.
How Do the CHIP- and mCA-associated Risks Differ Between Solid Tumors and Blood Cancers?
“[These solid tumor risks are] not causal in the way CHIP mutations are causal for blood cancers,” Dr. Desai said. “Here we are talking about solid tumor risk, and it’s kind of scattered. It’s not just breast cancer ... there’s also increased colon cancer mortality. So I feel these mutations are doing something different ... they are sort of an added factor.”
Specific mechanisms remain unclear, Dr. Desai said, although she speculated about possible impacts on the inflammatory state or alterations to the tumor microenvironment.
“These are blood cells, right?” Dr. Desai asked. “They’re everywhere, and they’re changing something inherently in these tumors.”
Future research and therapeutic development
Siddhartha Jaiswal, MD, PhD, assistant professor in the Department of Pathology at Stanford University in California, whose lab focuses on clonal hematopoiesis, said the causality question is central to future research.
“The key question is, are these mutations acting because they alter the function of blood cells in some way to promote cancer risk, or is it reflective of some sort of shared etiology that’s not causal?” Dr. Jaiswal said in an interview.
Available data support both possibilities.
On one side, “reasonable evidence” supports the noncausal view, Dr. Jaiswal noted, because telomere length is one of the most common genetic risk factors for clonal hematopoiesis and also for solid tumors, suggesting a shared genetic factor. On the other hand, CHIP and mCA could be directly protumorigenic via conferred disturbances of immune cell function.
When asked if both causal and noncausal factors could be at play, Dr. Jaiswal said, “yeah, absolutely.”
The presence of a causal association could be promising from a therapeutic standpoint.
“If it turns out that this association is driven by a direct causal effect of the mutations, perhaps related to immune cell function or dysfunction, then targeting that dysfunction could be a therapeutic path to improve outcomes in people, and there’s a lot of interest in this,” Dr. Jaiswal said. He went on to explain how a trial exploring this approach via interleukin-8 inhibition in lung cancer fell short.
Yet earlier intervention may still hold promise, according to experts.
“[This study] provokes the hypothesis that CH‐targeted interventions could potentially reduce cancer risk in the future,” Dr. Takahashi and Ms. Shah said in their editorial.
The WHI program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The investigators disclosed relationships with Eli Lilly, AbbVie, Celgene, and others. Dr. Jaiswal reported stock equity in a company that has an interest in clonal hematopoiesis.
A version of this article first appeared on Medscape.com.
Clonal hematopoiesis of indeterminate potential (CHIP) and mosaic chromosomal alterations (mCAs) are associated with an increased risk for breast cancer, and CHIP is associated with increased mortality in patients with colon cancer, according to the authors of new research.
These findings, drawn from almost 11,000 patients in the Women’s Health Initiative (WHI) study, add further evidence that CHIP and mCA drive solid tumor risk, alongside known associations with hematologic malignancies, reported lead author Pinkal Desai, MD, associate professor of medicine and clinical director of molecular aging at Englander Institute for Precision Medicine, Weill Cornell Medical College, New York City, and colleagues.
How This Study Differs From Others of Breast Cancer Risk Factors
“The independent effect of CHIP and mCA on risk and mortality from solid tumors has not been elucidated due to lack of detailed data on mortality outcomes and risk factors,” the investigators wrote in Cancer, although some previous studies have suggested a link.
In particular, the investigators highlighted a 2022 UK Biobank study, which reported an association between CHIP and lung cancer and a borderline association with breast cancer that did not quite reach statistical significance.
But the UK Biobank study was confined to a UK population, Dr. Desai noted in an interview, and the data were less detailed than those in the present investigation.
“In terms of risk, the part that was lacking in previous studies was a comprehensive assessment of risk factors that increase risk for all these cancers,” Dr. Desai said. “For example, for breast cancer, we had very detailed data on [participants’] Gail risk score, which is known to impact breast cancer risk. We also had mammogram data and colonoscopy data.”
In an accompanying editorial, Koichi Takahashi, MD, PhD , and Nehali Shah, BS, of The University of Texas MD Anderson Cancer Center, Houston, Texas, pointed out the same UK Biobank findings, then noted that CHIP has also been linked with worse overall survival in unselected cancer patients. Still, they wrote, “the impact of CH on cancer risk and mortality remains controversial due to conflicting data and context‐dependent effects,” necessitating studies like this one by Dr. Desai and colleagues.
How Was the Relationship Between CHIP, MCA, and Solid Tumor Risk Assessed?
To explore possible associations between CHIP, mCA, and solid tumors, the investigators analyzed whole genome sequencing data from 10,866 women in the WHI, a multi-study program that began in 1992 and involved 161,808 women in both observational and clinical trial cohorts.
In 2002, the first big data release from the WHI suggested that hormone replacement therapy (HRT) increased breast cancer risk, leading to widespread reduction in HRT use.
More recent reports continue to shape our understanding of these risks, suggesting differences across cancer types. For breast cancer, the WHI data suggested that HRT-associated risk was largely driven by formulations involving progesterone and estrogen, whereas estrogen-only formulations, now more common, are generally considered to present an acceptable risk profile for suitable patients.
The new study accounted for this potential HRT-associated risk, including by adjusting for patients who received HRT, type of HRT received, and duration of HRT received. According to Desai, this approach is commonly used when analyzing data from the WHI, nullifying concerns about the potentially deleterious effects of the hormones used in the study.
“Our question was not ‘does HRT cause cancer?’ ” Dr. Desai said in an interview. “But HRT can be linked to breast cancer risk and has a potential to be a confounder, and hence the above methodology.
“So I can say that the confounding/effect modification that HRT would have contributed to in the relationship between exposure (CH and mCA) and outcome (cancer) is well adjusted for as described above. This is standard in WHI analyses,” she continued.
“Every Women’s Health Initiative analysis that comes out — not just for our study — uses a standard method ... where you account for hormonal therapy,” Dr. Desai added, again noting that many other potential risk factors were considered, enabling a “detailed, robust” analysis.
Dr. Takahashi and Ms. Shah agreed. “A notable strength of this study is its adjustment for many confounding factors,” they wrote. “The cohort’s well‐annotated data on other known cancer risk factors allowed for a robust assessment of CH’s independent risk.”
How Do Findings Compare With Those of the UK Biobank Study?
CHIP was associated with a 30% increased risk for breast cancer (hazard ratio [HR], 1.30; 95% CI, 1.03-1.64; P = .02), strengthening the borderline association reported by the UK Biobank study.
In contrast with the UK Biobank study, CHIP was not associated with lung cancer risk, although this may have been caused by fewer cases of lung cancer and a lack of male patients, Dr. Desai suggested.
“The discrepancy between the studies lies in the risk of lung cancer, although the point estimate in the current study suggested a positive association,” wrote Dr. Takahashi and Ms. Shah.
As in the UK Biobank study, CHIP was not associated with increased risk of developing colorectal cancer.
Mortality analysis, however, which was not conducted in the UK Biobank study, offered a new insight: Patients with existing colorectal cancer and CHIP had a significantly higher mortality risk than those without CHIP. Before stage adjustment, risk for mortality among those with colorectal cancer and CHIP was fourfold higher than those without CHIP (HR, 3.99; 95% CI, 2.41-6.62; P < .001). After stage adjustment, CHIP was still associated with a twofold higher mortality risk (HR, 2.50; 95% CI, 1.32-4.72; P = .004).
The investigators’ first mCA analyses, which employed a cell fraction cutoff greater than 3%, were unfruitful. But raising the cell fraction threshold to 5% in an exploratory analysis showed that autosomal mCA was associated with a 39% increased risk for breast cancer (HR, 1.39; 95% CI, 1.06-1.83; P = .01). No such associations were found between mCA and colorectal or lung cancer, regardless of cell fraction threshold.
The original 3% cell fraction threshold was selected on the basis of previous studies reporting a link between mCA and hematologic malignancies at this cutoff, Dr. Desai said.
She and her colleagues said a higher 5% cutoff might be needed, as they suspected that the link between mCA and solid tumors may not be causal, requiring a higher mutation rate.
Why Do Results Differ Between These Types of Studies?
Dr. Takahashi and Ms. Shah suggested that one possible limitation of the new study, and an obstacle to comparing results with the UK Biobank study and others like it, goes beyond population heterogeneity; incongruent findings could also be explained by differences in whole genome sequencing (WGS) technique.
“Although WGS allows sensitive detection of mCA through broad genomic coverage, it is less effective at detecting CHIP with low variant allele frequency (VAF) due to its relatively shallow depth (30x),” they wrote. “Consequently, the prevalence of mCA (18.8%) was much higher than that of CHIP (8.3%) in this cohort, contrasting with other studies using deeper sequencing.” As a result, the present study may have underestimated CHIP prevalence because of shallow sequencing depth.
“This inconsistency is a common challenge in CH population studies due to the lack of standardized methodologies and the frequent reliance on preexisting data not originally intended for CH detection,” Dr. Takahashi and Ms. Shah said.
Even so, despite the “heavily context-dependent” nature of these reported risks, the body of evidence to date now offers a convincing biological rationale linking CH with cancer development and outcomes, they added.
How Do the CHIP- and mCA-associated Risks Differ Between Solid Tumors and Blood Cancers?
“[These solid tumor risks are] not causal in the way CHIP mutations are causal for blood cancers,” Dr. Desai said. “Here we are talking about solid tumor risk, and it’s kind of scattered. It’s not just breast cancer ... there’s also increased colon cancer mortality. So I feel these mutations are doing something different ... they are sort of an added factor.”
Specific mechanisms remain unclear, Dr. Desai said, although she speculated about possible impacts on the inflammatory state or alterations to the tumor microenvironment.
“These are blood cells, right?” Dr. Desai asked. “They’re everywhere, and they’re changing something inherently in these tumors.”
Future research and therapeutic development
Siddhartha Jaiswal, MD, PhD, assistant professor in the Department of Pathology at Stanford University in California, whose lab focuses on clonal hematopoiesis, said the causality question is central to future research.
“The key question is, are these mutations acting because they alter the function of blood cells in some way to promote cancer risk, or is it reflective of some sort of shared etiology that’s not causal?” Dr. Jaiswal said in an interview.
Available data support both possibilities.
On one side, “reasonable evidence” supports the noncausal view, Dr. Jaiswal noted, because telomere length is one of the most common genetic risk factors for clonal hematopoiesis and also for solid tumors, suggesting a shared genetic factor. On the other hand, CHIP and mCA could be directly protumorigenic via conferred disturbances of immune cell function.
When asked if both causal and noncausal factors could be at play, Dr. Jaiswal said, “yeah, absolutely.”
The presence of a causal association could be promising from a therapeutic standpoint.
“If it turns out that this association is driven by a direct causal effect of the mutations, perhaps related to immune cell function or dysfunction, then targeting that dysfunction could be a therapeutic path to improve outcomes in people, and there’s a lot of interest in this,” Dr. Jaiswal said. He went on to explain how a trial exploring this approach via interleukin-8 inhibition in lung cancer fell short.
Yet earlier intervention may still hold promise, according to experts.
“[This study] provokes the hypothesis that CH‐targeted interventions could potentially reduce cancer risk in the future,” Dr. Takahashi and Ms. Shah said in their editorial.
The WHI program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The investigators disclosed relationships with Eli Lilly, AbbVie, Celgene, and others. Dr. Jaiswal reported stock equity in a company that has an interest in clonal hematopoiesis.
A version of this article first appeared on Medscape.com.
FROM CANCER
Pulmonology Data Trends 2024
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Noninvasive Ventilation in Neuromuscular Disease
- Gong Y, Sankari A. Noninvasive ventilation. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024.Updated December 11, 2022. Accessed June 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK578188/
- Khan A, Frazer-Green L, Amin R, et al. Respiratory management of patients with neuromuscular weakness: an American College of Chest Physicians clinical practice guideline and expert panel report. Chest. 2023;164(2):394-413. doi:10.1016/j.chest.2023.03.011
- Taran S, McCredie VA, Goligher EC. Noninvasive and invasive mechanical ventilation for neurologic disorders. Handb Clin Neurol. 2022;189:361-386. doi:10.1016/B978-0-323-91532-8.00015-X
- Rao F, Garuti G, Vitacca M, et al; for the UILDM Respiratory Group. Management of respiratory complications and rehabilitation in individuals with muscular dystrophies: 1st Consensus Conference report from UILDM - Italian Muscular Dystrophy Association (Milan, January 25-26, 2019). Acta Myol. 2021;40(1):8-42. doi:10.36185/2532-1900-045
- Respiratory assist devices. Centers for Medicare & Medicaid Services. Revised January 1, 2024. Accessed June 19, 2024. https://www.cms.gov/ medicare-coverage-database/view/lcd.aspx?lcdid=33800
- What you need to know about the Philips PAP device recalls. American College of Chest Physicians. February 1, 2024. Accessed June 19, 2024. https://www.chestnet.org/Newsroom/CHEST-News/2021/07/What-YouNeed-to-Know-About-the-Philips-PAP-Device-Recall
- Orr JE, Chen K, Vaida F, et al. Effectiveness of long-term noninvasive ventilation measured by remote monitoring in neuromuscular disease. ERJ Open Res. 2023;9(5):00163-2023. doi:10.1183/23120541.00163-2023
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504. doi:10.5664/jcsm.6506
- Phillips Respironics. Trilogy Evo Clinical Manual. 2019
- ResMed. Astral Series Clinical Guide. 2018
- Breas. Vivo 45 LS User Manual. 2023
- Lowenstein Medical. Luisa Life Support Ventilation.
- Ventec Life Systems. VOCSN Clinical and Technical Manual. 2019
- Gong Y, Sankari A. Noninvasive ventilation. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024.Updated December 11, 2022. Accessed June 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK578188/
- Khan A, Frazer-Green L, Amin R, et al. Respiratory management of patients with neuromuscular weakness: an American College of Chest Physicians clinical practice guideline and expert panel report. Chest. 2023;164(2):394-413. doi:10.1016/j.chest.2023.03.011
- Taran S, McCredie VA, Goligher EC. Noninvasive and invasive mechanical ventilation for neurologic disorders. Handb Clin Neurol. 2022;189:361-386. doi:10.1016/B978-0-323-91532-8.00015-X
- Rao F, Garuti G, Vitacca M, et al; for the UILDM Respiratory Group. Management of respiratory complications and rehabilitation in individuals with muscular dystrophies: 1st Consensus Conference report from UILDM - Italian Muscular Dystrophy Association (Milan, January 25-26, 2019). Acta Myol. 2021;40(1):8-42. doi:10.36185/2532-1900-045
- Respiratory assist devices. Centers for Medicare & Medicaid Services. Revised January 1, 2024. Accessed June 19, 2024. https://www.cms.gov/ medicare-coverage-database/view/lcd.aspx?lcdid=33800
- What you need to know about the Philips PAP device recalls. American College of Chest Physicians. February 1, 2024. Accessed June 19, 2024. https://www.chestnet.org/Newsroom/CHEST-News/2021/07/What-YouNeed-to-Know-About-the-Philips-PAP-Device-Recall
- Orr JE, Chen K, Vaida F, et al. Effectiveness of long-term noninvasive ventilation measured by remote monitoring in neuromuscular disease. ERJ Open Res. 2023;9(5):00163-2023. doi:10.1183/23120541.00163-2023
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504. doi:10.5664/jcsm.6506
- Phillips Respironics. Trilogy Evo Clinical Manual. 2019
- ResMed. Astral Series Clinical Guide. 2018
- Breas. Vivo 45 LS User Manual. 2023
- Lowenstein Medical. Luisa Life Support Ventilation.
- Ventec Life Systems. VOCSN Clinical and Technical Manual. 2019
- Gong Y, Sankari A. Noninvasive ventilation. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024.Updated December 11, 2022. Accessed June 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK578188/
- Khan A, Frazer-Green L, Amin R, et al. Respiratory management of patients with neuromuscular weakness: an American College of Chest Physicians clinical practice guideline and expert panel report. Chest. 2023;164(2):394-413. doi:10.1016/j.chest.2023.03.011
- Taran S, McCredie VA, Goligher EC. Noninvasive and invasive mechanical ventilation for neurologic disorders. Handb Clin Neurol. 2022;189:361-386. doi:10.1016/B978-0-323-91532-8.00015-X
- Rao F, Garuti G, Vitacca M, et al; for the UILDM Respiratory Group. Management of respiratory complications and rehabilitation in individuals with muscular dystrophies: 1st Consensus Conference report from UILDM - Italian Muscular Dystrophy Association (Milan, January 25-26, 2019). Acta Myol. 2021;40(1):8-42. doi:10.36185/2532-1900-045
- Respiratory assist devices. Centers for Medicare & Medicaid Services. Revised January 1, 2024. Accessed June 19, 2024. https://www.cms.gov/ medicare-coverage-database/view/lcd.aspx?lcdid=33800
- What you need to know about the Philips PAP device recalls. American College of Chest Physicians. February 1, 2024. Accessed June 19, 2024. https://www.chestnet.org/Newsroom/CHEST-News/2021/07/What-YouNeed-to-Know-About-the-Philips-PAP-Device-Recall
- Orr JE, Chen K, Vaida F, et al. Effectiveness of long-term noninvasive ventilation measured by remote monitoring in neuromuscular disease. ERJ Open Res. 2023;9(5):00163-2023. doi:10.1183/23120541.00163-2023
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504. doi:10.5664/jcsm.6506
- Phillips Respironics. Trilogy Evo Clinical Manual. 2019
- ResMed. Astral Series Clinical Guide. 2018
- Breas. Vivo 45 LS User Manual. 2023
- Lowenstein Medical. Luisa Life Support Ventilation.
- Ventec Life Systems. VOCSN Clinical and Technical Manual. 2019
In Colorectal Cancer, Donating Half a Liver Could Save Lives
This transcript has been edited for clarity.
Benjamin L. Schlechter, MD: Dr. Dib is the director of the Hepatobiliary Surgery and Living Donor Program at Beth Israel Deaconess Medical Center here in Boston, and a Harvard Medical School faculty member.
He was previously at the Pontificia Universidad Católica de Chile, a leading international institution investigating the role of liver transplant in colorectal cancer, among other diseases. Dr. Dib, before we move to our discussion, I’d like to hear a bit about your pathway to becoming a transplant surgeon. How did you end up working on colorectal cancer and liver transplants in this field?
Martin J. Dib, MD: Thank you so much, Dr. Schlechter. I am originally from Chile. I had an opportunity to come to Beth Israel Deaconess Medical Center after medical school and I did liver regeneration research at the transplant center. After that, I was lucky enough to match as a general surgery resident at Beth Israel Deaconess.
This is my alma mater and I was able to graduate as a surgeon here. You and I had some paths together. After graduating from Harvard as a surgeon, I was trained in liver transplant, abdominal transplant, surgical oncology, and hepatobiliary surgery at the University of Toronto.
I have been developing this passion for being able to transplant cancer patients and use organ transplant techniques to be able to do complex resections for cancer.
Dr. Schlechter: Let’s talk about the topic for today, which is liver transplant and colorectal cancer. I’ll be honest — this is not a very familiar topic for a lot of oncologists. There are a lot of details that I think are new to us as oncologists. We need to expand this conversation to get access to patients for this.
First and foremost, can you talk about some of the parameters for a resectable liver metastasis vs unresectable disease that would be an indication for a liver transplant?
Dr. Dib: I think this is a very interesting topic because liver transplantation for cancer is not new. Liver transplantation started in the 1960s when people started doing liver transplants for advanced liver tumors. The problem is that they were selecting patients who had very advanced — and poor tumor biology — tumors. The outcomes were not good.
It was only in 1996 when the Milan criteria started. Mazzaferro and colleagues, using strict patient selection, were able to do liver transplant for selected hepatocellular carcinoma patients. Having those excellent outcomes in selecting patients opened the field for what we now call transplant oncology, which is using selection criteria and using other methods to be able to select which patients will do well after transplantation, even with immunosuppression.
Liver transplantation for colorectal metastasis was used at the very beginning of the era of liver transplantation, but with very poor outcomes. It was abandoned because of the outcomes. It is exciting to see that after 20 years of not doing it, there was a group in Norway that started again. They are doing liver transplants for colorectal metastases (mets), but with very selected patients.
In Norway, they had a very unusual setting where they had more liver donors than patients on the list waiting for liver transplant. So they can’t share these livers and we’re all jealous, right? Every single country in the West struggles because we don’t have enough livers for the rest of the list. And they had a lot of livers to be able to transplant people.
They decided to transplant some selected patients with colorectal mets that were unresectable. And the surprise was that they found that they were able to get a 60% survival at 5 years. And so that was new. After that, in Norway, they started showing this data to other centers in the world. It wasn’t until this year that we could see not only the long-term data and long-term outcomes of using liver transplantation for unresectable colorectal mets, but also we’re now having data from a prospective clinical trial from France.
It was three countries in the prospective clinical trial: France, Belgium, and Italy. We now see that we have a little stronger data to support the use of liver transplants for unresectable colorectal mets.
Dr. Schlechter: That’s the TRANSMET study you’re referencing that was presented at ASCO in the late-breaking abstract session in 2024, and then more recently in The Lancet’s eClinicalMedicine. Both of those papers were led by René Adam. That was a cool presentation to sit through. I was in the room, and I was taking a ton of notes and there was a lot of info that came out of that.
First of all, it showed that patients who had received chemotherapy and were responding could then go on to liver transplant in that population. Impressively, 81% of the patients who were randomized to transplant received it. Frankly, that’s a big number, especially compared with the West, as you said, and in particular the US and here in New England where livers are a very precious commodity.
And even accounting for that, if you look at the intention-to-treat analysis, the 5-year overall survival in that population was 57% compared with 13% with chemotherapy. And that feels like a real number for chemotherapy. If you look at the per-protocol analysis, frankly, the numbers are higher.
It’s always a challenging assessment. What was also interesting to me was the pattern of recurrence, which in general was that recurrences were extrahepatic. So not only were patients rendered disease-free, but in general, the liver remained disease-free and only 3% of patients had liver-only recurrence and 11% had widespread metastatic disease.
The biggest group was lung metastases, at about 40%. Ultimately, they reported a progression-free survival of 17. 4 months for transplant compared with 6. 4 months with chemotherapy. On every parameter, it looks like liver transplant wins for these people. Help me out. Who are these people? How do we find these people?
What are the inclusions and exclusions for this population?
Dr. Dib: I think that’s very important. This is not a therapy that will be for every patient. These are selected patients who have liver-only unresectable colorectal mets. These are patients that don’t have any extrahepatic disease and that either the primary has been taken out already or that they have the primary present, but the plan is to take the primary and then do a liver transplantation after 3 months, hopefully after 6 months, of removing the primary.
These are patients who meet all the criteria that we have seen in terms of the best outcomes — patients that have Oslo scores of less than three. The Oslo trial, which included the SECA (Secondary Cancer)-I and SECA-II trials, basically showed that patients with a maximal tumor diameter of less than 5.5 with a pretransplant CEA (carcinoembryonic antigen) of less than 80 that do not have progression on chemotherapy, among other variables, do better. But the concept is that this is a therapy that will apply only to selected patients. That way we can continue to have adequate overall survival post-transplant that would be comparable to other diseases that we do liver transplants for.
Dr. Schlechter: Were there other biomarkers, any mutations that were included or excluded?
Dr. Dib: Yes. If you look at SECA-I, SECA-II trial outcomes, and also TRANSMET, they all say patients with BRAF mutations shouldn’t be transplanted. There are other parameters, including, for example, the site of the primary tumor. Patients with a left-sided colon primary tumor do much better than patients who have a right-sided primary tumor.
That’s not a complete contraindication, but if you look at the most updated inclusion criteria of programs, like the ones that the one that we have here at Beth Israel Deaconess and many others, the inclusion criteria protocols include patients who have only hepatic disease.
So, if there are no extrahepatic mets, the resection of the primary has been done or will be done after a multidisciplinary discussion. We want to make sure they have the absence of BRAF mutation, and that they don’t have disease progression while on chemotherapy. So hopefully we have data from enough months to be able to make sure that there’s no intrahepatic or extrahepatic progression while on chemotherapy.
And that’s including CEA and also looking at the imaging.
Dr. Schlechter: When you’re seeing a patient, how much chemo do you think they should have? What’s a good run chemotherapy-wise for these patients? Let’s say, before I refer a patient to you, how much chemo should they have? And then what should I do? Do I get a PET scan? Do I get MRI? What’s the right scanning I should do to prove there’s no extrahepatic disease before sending a patient in for consideration?
Dr. Dib: First, we need to confirm unresectability. Referring patients early is always a good measure to make sure that we’re all in agreement that it’s an unresectable patient. Having a PET scan from the very beginning is helpful because it shows the disease before doing chemotherapy.
In terms of the lines of chemotherapy, ideally in the TRANSMET trial, for example, the idea was to show tumor control for at least 3 months, with less than three lines of chemotherapy. Some patients will do that with FOLFIRI. It depends on the case.
I think some of those evaluations will need a multidisciplinary discussion. In our case, we are connected to the Norway team. We frequently talk with the Oslo team and an international community of transplant centers to get opinions on particular cases.
But I think referring patients early is a good measure. If we don’t think that they qualify, we will let the team know. We’re strictly looking at patients who have unresectable liver mets that don’t have extrahepatic disease. The idea is to do a primary tumor resection, and then get to transplantation, hopefully after 6 months. In some cases that have some concerns in terms of tumor biology, we may even extend the time from diagnosis to transplant to over 1.5 years.
Dr. Schlechter: Excellent. And what’s the experience like for these patients? In training as a resident many years ago, I saw patients with cirrhosis who went on to have a liver transplant, and that was sort of trading one disease for another. What is the posttransplant, or the remission, experience of a liver transplant for colorectal cancer like for the patient?
Dr. Dib: That’s a very important point. I think that transplantation has gotten better and better, as has chemotherapy systemic therapy. The liver transplantation experience from 20 years ago has improved dramatically. I think the quality of life of liver transplant patients after transplantation has increased quite a bit.
At Beth Israel Deaconess, we have a liver transplant program that is doing over a 100 livers a year. And when you have a high-volume center, usually the experience gets better. The time in the hospital post-transplant decreases.
In general, when we’re doing liver transplants, patients are getting extubated in the OR 30% of the time. The vast majority of patients are going home within 1 or 2 weeks. They need to have immunosuppression for the rest of their lives. We have a very good program of transplant coordinators that will help the family and the patient to live with immunosuppression and live with a transplanted organ.
But I would say that we have many, many patients, especially these patients who are not patients with cirrhosis. Their health is not as deteriorated as patients who have low MELD (model for end-stage liver disease) scores. They don’t have liver disease. They have cancer. So usually patients like that, many of them can go back to work and live a quality of life that is fairly reasonable.
Dr. Schlechter: That’s good to hear. When we hear statements like liver transplant for colon cancer, a lot of us have this picture of a much sicker population, but it’s interesting and true that the colorectal cancer population as a candidate for liver transplant is a much healthier population than the population with cirrhosis.
Let’s talk about organs and donors. Largely in the TRANSMET study, for example, that was cadaveric donors. Those were not living donors and you’ve done a lot of work on living donors. If the answer in the United States, because of limited access to organs, is going to be living donors, who are those donors?
What is that like? How do you identify them?
Dr. Dib: There’s a lot of advantages to using living donors for these patients. In any type of patient that needs a liver transplant, cadaveric donors or deceased donors is the same concept. There are two types of deceased donors: the brain-dead donors and donors after cardiac death. Those are hard to come by.
We still have 15%-20% mortality on the waiting list in the United States. We’re already still struggling to get enough donors to transplant the patients that are on the list. Now, if you add a new indication, which is unresectable colorectal mets, we need to make sure that the outcomes are equivalent to the patients who are going to be transplanted for other reasons.
Right now, for example, the 5-year overall survival of a patient with cirrhosis, or a patient with hepatocellular carcinoma, is over 80% 5-year survival. In the SECA trials and TRANSMET trial, if we do a good selection, I think we can get to 70% 5-year survival. But until we have more data, I think it’s a cautious measure to, as a field, try to use living donors and not compete with the rest of the list of patients who are already dying on the list for liver transplants.
Once we get more data, it’s going to be something that, in the transplant community, we may be able to use deceased donors. Especially deceased donors with maybe extended criteria that are not going to be used for other patients. We can do living-unrelated or living-related donations. Family members or also friends or neighbors or part of the community, even altruistic donors, can donate to a potential recipient. And that enables us to not only time the transplant in an adequate manner, because we’re able to transplant the patient early, but also time it so we can give the number of chemotherapy cycles that we want to give.
That’s a huge advantage. You don’t compete for a liver with the cadaveric waiting list of patients that are waiting for other reasons, and you can select the tumor biology very well because you know exactly when the surgery is going to be. For instance, we can say, okay, this patient has KRAS mutation, left-sided colon cancer, and has been having good tumor biology with no progression. We will wait 6 months from the primary tumor to the transplant, which is going to be 1 year from diagnosis to transplant. And we can see during that time whether they continue to have good tumor biology.
But if you have a deceased donor liver transplant, sometimes you can’t time that well and schedule it. It becomes a bit more tricky in terms of patient selection and making sure that we do this for the people who are going to benefit.
Dr. Schlechter: And how does donor matching work? Is it HLA (human leukocyte antigen) matched or ABO-matched? Who can donate when you say a living-related? For example, when we think about bone marrow transplantation, which we’re all familiar with in the oncology population, it’s an incredibly complex match process. Is this the same challenge?
Dr. Dib: No, it’s a little bit simpler. Living donors for liver transplants need to be between the ages of 18 and 60. They need to be relatively healthy, relatively fit, with a BMI hopefully less than 30, definitely less than 35. The compatibility is ABO compatibility. So, if they’re ABO-compatible, relatively young, relatively healthy, they would be a potential donor and we will go ahead and do a CT scan.
If the CT scan shows that they have a good, adequate anatomy, more than 90% of those will be good donors. I would say that out of 100 people who want to be donors, 25 of them will be adequate. One out of four people who want to save their family member and want to have this operation are able to donate half of their liver to their family member or loved one.
Dr. Schlechter: Excellent. And it’s helpful to know that the matching process is simpler. During his discussion, René Adam unequivocally stated that liver transplants are a new standard of care for colorectal cancer. And I guess my question is, do you agree with this statement? How do we balance the demand for living donors and the demand for deceased donors? Especially in a time of increasing fatty liver disease and obesity, other indications for liver transplant, causes of cirrhosis, and also in an era of young-onset colorectal cancer. Patients are younger. Is this a new standard of care? Do you agree with that statement?
Dr. Dib: I do agree with that statement. I think it’s important to understand that not all patients with colorectal mets are the same. Of the number of patients in the United States who have colorectal cancer, let’s say 50% of them will have liver metastasis. Only 15%-20% of them will have liver-only metastasis.
This is only for patients who have liver-only metastasis without extrahepatic disease. And only maybe 15%-20% of them will meet all the criteria to be able to undergo liver transplantation. I think it’s for a very selective subset of patients who have very good tumor biology, generally young patients who don’t have any other alternative to having even a complex liver resection and are not able to get R0 resection. That is when we could think about doing liver transplantation.
It’s one more of the skills that we can have. It doesn’t mean that it will be the only skill, or the best skill, for all of the patients.
Dr. Schlechter: When a patient volunteers to be a living donor for a loved one or a family member, and they go through all the screening and they’re found to be a candidate, what is the surgical experience for that patient?
How long are they in the hospital? What sort of operation is that?
Dr. Dib: Living donors are very special patients. These are patients who do not need an operation. And the only reason they’re doing this is to save the life of their loved one. Donor safety is our priority number one, two, three, and four. The donor operation needs to be perfect.
And so we take good care of, first of all, selecting the living donors, making sure that they’re young and they don’t have any big contraindications. We also ensure that they are well informed of the process. The living donor surgery that we’re now doing is laparoscopic and minimally invasive. Here at Beth Israel Deaconess, we have done it laparoscopically with very good results.
I think that experience before and after the surgery gets so much better because of the better recovery. They’re able to go home, in general, within 4 or 5 days, and they get on with their normal life within 6-8 weeks. I think it’s important for them to know all the processes and the actual risks and benefits for the recipient.
Among those risks, I think it’s important for them to understand that this is a complex operation. Even if we do it laparoscopically or robotically, so that the scar is less, inside we’re still taking out half of the liver. That is a surgery that needs to be undertaken very meticulously, with a focus on minimizing any bleeding.
It’s a surgery that takes a long time. It takes about 6 hours. We do our best to try to minimize any risks.
Dr. Schlechter: Excellent. Thanks for that. Today we had Dr. Martin Dib joining us to discuss liver transplant for metastatic colorectal cancer. We discussed the various important criteria. We discussed that early referral to multidisciplinary centers that manage these is important to help get patients set up.
We discussed the fact that there are certain inclusion and exclusion criteria to consider. Obviously, unresectable disease is a critical determination that should be made by a liver surgeon. The absence of extrahepatic disease is important in staging with PET or other imaging. We discussed certain other biological exclusions.
There’s a relative contraindication of right-sided vs left-sided cancers, but right-sided cancers can be transplanted. We discussed that an elevated CEA greater than 80 is a contraindication, as are mutations in BRAF. We reviewed data from both the TRANSMET trial recently published in The Lancet and presented at ASCO in 2024, as well as the older Oslo criteria and Oslo trials and SECA trials.
And finally, we heard that donors can now come as living donors, a laparoscopic robotic surgery with a better safety profile, and greater access to organs that are ABO matched and not HLA matched because of the nature of the biology. Thank you again for joining us.
Benjamin L. Schlechter, MD, is senior physician, Gastrointestinal Cancer Center, Dana-Farber Cancer Institute, Boston, Massachusetts. He has disclosed no relevant financial relationships. Martin J. Dib, MD, is member of the faculty, Department of Surgery, Harvard Medical School; director of Hepatobiliary Surgery, Division of Transplantation, Beth Israel Deaconess Medical Center, Boston. He has disclosed no relevant financial relationships.
A version of this transcript appeared on Medscape.com.
This transcript has been edited for clarity.
Benjamin L. Schlechter, MD: Dr. Dib is the director of the Hepatobiliary Surgery and Living Donor Program at Beth Israel Deaconess Medical Center here in Boston, and a Harvard Medical School faculty member.
He was previously at the Pontificia Universidad Católica de Chile, a leading international institution investigating the role of liver transplant in colorectal cancer, among other diseases. Dr. Dib, before we move to our discussion, I’d like to hear a bit about your pathway to becoming a transplant surgeon. How did you end up working on colorectal cancer and liver transplants in this field?
Martin J. Dib, MD: Thank you so much, Dr. Schlechter. I am originally from Chile. I had an opportunity to come to Beth Israel Deaconess Medical Center after medical school and I did liver regeneration research at the transplant center. After that, I was lucky enough to match as a general surgery resident at Beth Israel Deaconess.
This is my alma mater and I was able to graduate as a surgeon here. You and I had some paths together. After graduating from Harvard as a surgeon, I was trained in liver transplant, abdominal transplant, surgical oncology, and hepatobiliary surgery at the University of Toronto.
I have been developing this passion for being able to transplant cancer patients and use organ transplant techniques to be able to do complex resections for cancer.
Dr. Schlechter: Let’s talk about the topic for today, which is liver transplant and colorectal cancer. I’ll be honest — this is not a very familiar topic for a lot of oncologists. There are a lot of details that I think are new to us as oncologists. We need to expand this conversation to get access to patients for this.
First and foremost, can you talk about some of the parameters for a resectable liver metastasis vs unresectable disease that would be an indication for a liver transplant?
Dr. Dib: I think this is a very interesting topic because liver transplantation for cancer is not new. Liver transplantation started in the 1960s when people started doing liver transplants for advanced liver tumors. The problem is that they were selecting patients who had very advanced — and poor tumor biology — tumors. The outcomes were not good.
It was only in 1996 when the Milan criteria started. Mazzaferro and colleagues, using strict patient selection, were able to do liver transplant for selected hepatocellular carcinoma patients. Having those excellent outcomes in selecting patients opened the field for what we now call transplant oncology, which is using selection criteria and using other methods to be able to select which patients will do well after transplantation, even with immunosuppression.
Liver transplantation for colorectal metastasis was used at the very beginning of the era of liver transplantation, but with very poor outcomes. It was abandoned because of the outcomes. It is exciting to see that after 20 years of not doing it, there was a group in Norway that started again. They are doing liver transplants for colorectal metastases (mets), but with very selected patients.
In Norway, they had a very unusual setting where they had more liver donors than patients on the list waiting for liver transplant. So they can’t share these livers and we’re all jealous, right? Every single country in the West struggles because we don’t have enough livers for the rest of the list. And they had a lot of livers to be able to transplant people.
They decided to transplant some selected patients with colorectal mets that were unresectable. And the surprise was that they found that they were able to get a 60% survival at 5 years. And so that was new. After that, in Norway, they started showing this data to other centers in the world. It wasn’t until this year that we could see not only the long-term data and long-term outcomes of using liver transplantation for unresectable colorectal mets, but also we’re now having data from a prospective clinical trial from France.
It was three countries in the prospective clinical trial: France, Belgium, and Italy. We now see that we have a little stronger data to support the use of liver transplants for unresectable colorectal mets.
Dr. Schlechter: That’s the TRANSMET study you’re referencing that was presented at ASCO in the late-breaking abstract session in 2024, and then more recently in The Lancet’s eClinicalMedicine. Both of those papers were led by René Adam. That was a cool presentation to sit through. I was in the room, and I was taking a ton of notes and there was a lot of info that came out of that.
First of all, it showed that patients who had received chemotherapy and were responding could then go on to liver transplant in that population. Impressively, 81% of the patients who were randomized to transplant received it. Frankly, that’s a big number, especially compared with the West, as you said, and in particular the US and here in New England where livers are a very precious commodity.
And even accounting for that, if you look at the intention-to-treat analysis, the 5-year overall survival in that population was 57% compared with 13% with chemotherapy. And that feels like a real number for chemotherapy. If you look at the per-protocol analysis, frankly, the numbers are higher.
It’s always a challenging assessment. What was also interesting to me was the pattern of recurrence, which in general was that recurrences were extrahepatic. So not only were patients rendered disease-free, but in general, the liver remained disease-free and only 3% of patients had liver-only recurrence and 11% had widespread metastatic disease.
The biggest group was lung metastases, at about 40%. Ultimately, they reported a progression-free survival of 17. 4 months for transplant compared with 6. 4 months with chemotherapy. On every parameter, it looks like liver transplant wins for these people. Help me out. Who are these people? How do we find these people?
What are the inclusions and exclusions for this population?
Dr. Dib: I think that’s very important. This is not a therapy that will be for every patient. These are selected patients who have liver-only unresectable colorectal mets. These are patients that don’t have any extrahepatic disease and that either the primary has been taken out already or that they have the primary present, but the plan is to take the primary and then do a liver transplantation after 3 months, hopefully after 6 months, of removing the primary.
These are patients who meet all the criteria that we have seen in terms of the best outcomes — patients that have Oslo scores of less than three. The Oslo trial, which included the SECA (Secondary Cancer)-I and SECA-II trials, basically showed that patients with a maximal tumor diameter of less than 5.5 with a pretransplant CEA (carcinoembryonic antigen) of less than 80 that do not have progression on chemotherapy, among other variables, do better. But the concept is that this is a therapy that will apply only to selected patients. That way we can continue to have adequate overall survival post-transplant that would be comparable to other diseases that we do liver transplants for.
Dr. Schlechter: Were there other biomarkers, any mutations that were included or excluded?
Dr. Dib: Yes. If you look at SECA-I, SECA-II trial outcomes, and also TRANSMET, they all say patients with BRAF mutations shouldn’t be transplanted. There are other parameters, including, for example, the site of the primary tumor. Patients with a left-sided colon primary tumor do much better than patients who have a right-sided primary tumor.
That’s not a complete contraindication, but if you look at the most updated inclusion criteria of programs, like the ones that the one that we have here at Beth Israel Deaconess and many others, the inclusion criteria protocols include patients who have only hepatic disease.
So, if there are no extrahepatic mets, the resection of the primary has been done or will be done after a multidisciplinary discussion. We want to make sure they have the absence of BRAF mutation, and that they don’t have disease progression while on chemotherapy. So hopefully we have data from enough months to be able to make sure that there’s no intrahepatic or extrahepatic progression while on chemotherapy.
And that’s including CEA and also looking at the imaging.
Dr. Schlechter: When you’re seeing a patient, how much chemo do you think they should have? What’s a good run chemotherapy-wise for these patients? Let’s say, before I refer a patient to you, how much chemo should they have? And then what should I do? Do I get a PET scan? Do I get MRI? What’s the right scanning I should do to prove there’s no extrahepatic disease before sending a patient in for consideration?
Dr. Dib: First, we need to confirm unresectability. Referring patients early is always a good measure to make sure that we’re all in agreement that it’s an unresectable patient. Having a PET scan from the very beginning is helpful because it shows the disease before doing chemotherapy.
In terms of the lines of chemotherapy, ideally in the TRANSMET trial, for example, the idea was to show tumor control for at least 3 months, with less than three lines of chemotherapy. Some patients will do that with FOLFIRI. It depends on the case.
I think some of those evaluations will need a multidisciplinary discussion. In our case, we are connected to the Norway team. We frequently talk with the Oslo team and an international community of transplant centers to get opinions on particular cases.
But I think referring patients early is a good measure. If we don’t think that they qualify, we will let the team know. We’re strictly looking at patients who have unresectable liver mets that don’t have extrahepatic disease. The idea is to do a primary tumor resection, and then get to transplantation, hopefully after 6 months. In some cases that have some concerns in terms of tumor biology, we may even extend the time from diagnosis to transplant to over 1.5 years.
Dr. Schlechter: Excellent. And what’s the experience like for these patients? In training as a resident many years ago, I saw patients with cirrhosis who went on to have a liver transplant, and that was sort of trading one disease for another. What is the posttransplant, or the remission, experience of a liver transplant for colorectal cancer like for the patient?
Dr. Dib: That’s a very important point. I think that transplantation has gotten better and better, as has chemotherapy systemic therapy. The liver transplantation experience from 20 years ago has improved dramatically. I think the quality of life of liver transplant patients after transplantation has increased quite a bit.
At Beth Israel Deaconess, we have a liver transplant program that is doing over a 100 livers a year. And when you have a high-volume center, usually the experience gets better. The time in the hospital post-transplant decreases.
In general, when we’re doing liver transplants, patients are getting extubated in the OR 30% of the time. The vast majority of patients are going home within 1 or 2 weeks. They need to have immunosuppression for the rest of their lives. We have a very good program of transplant coordinators that will help the family and the patient to live with immunosuppression and live with a transplanted organ.
But I would say that we have many, many patients, especially these patients who are not patients with cirrhosis. Their health is not as deteriorated as patients who have low MELD (model for end-stage liver disease) scores. They don’t have liver disease. They have cancer. So usually patients like that, many of them can go back to work and live a quality of life that is fairly reasonable.
Dr. Schlechter: That’s good to hear. When we hear statements like liver transplant for colon cancer, a lot of us have this picture of a much sicker population, but it’s interesting and true that the colorectal cancer population as a candidate for liver transplant is a much healthier population than the population with cirrhosis.
Let’s talk about organs and donors. Largely in the TRANSMET study, for example, that was cadaveric donors. Those were not living donors and you’ve done a lot of work on living donors. If the answer in the United States, because of limited access to organs, is going to be living donors, who are those donors?
What is that like? How do you identify them?
Dr. Dib: There’s a lot of advantages to using living donors for these patients. In any type of patient that needs a liver transplant, cadaveric donors or deceased donors is the same concept. There are two types of deceased donors: the brain-dead donors and donors after cardiac death. Those are hard to come by.
We still have 15%-20% mortality on the waiting list in the United States. We’re already still struggling to get enough donors to transplant the patients that are on the list. Now, if you add a new indication, which is unresectable colorectal mets, we need to make sure that the outcomes are equivalent to the patients who are going to be transplanted for other reasons.
Right now, for example, the 5-year overall survival of a patient with cirrhosis, or a patient with hepatocellular carcinoma, is over 80% 5-year survival. In the SECA trials and TRANSMET trial, if we do a good selection, I think we can get to 70% 5-year survival. But until we have more data, I think it’s a cautious measure to, as a field, try to use living donors and not compete with the rest of the list of patients who are already dying on the list for liver transplants.
Once we get more data, it’s going to be something that, in the transplant community, we may be able to use deceased donors. Especially deceased donors with maybe extended criteria that are not going to be used for other patients. We can do living-unrelated or living-related donations. Family members or also friends or neighbors or part of the community, even altruistic donors, can donate to a potential recipient. And that enables us to not only time the transplant in an adequate manner, because we’re able to transplant the patient early, but also time it so we can give the number of chemotherapy cycles that we want to give.
That’s a huge advantage. You don’t compete for a liver with the cadaveric waiting list of patients that are waiting for other reasons, and you can select the tumor biology very well because you know exactly when the surgery is going to be. For instance, we can say, okay, this patient has KRAS mutation, left-sided colon cancer, and has been having good tumor biology with no progression. We will wait 6 months from the primary tumor to the transplant, which is going to be 1 year from diagnosis to transplant. And we can see during that time whether they continue to have good tumor biology.
But if you have a deceased donor liver transplant, sometimes you can’t time that well and schedule it. It becomes a bit more tricky in terms of patient selection and making sure that we do this for the people who are going to benefit.
Dr. Schlechter: And how does donor matching work? Is it HLA (human leukocyte antigen) matched or ABO-matched? Who can donate when you say a living-related? For example, when we think about bone marrow transplantation, which we’re all familiar with in the oncology population, it’s an incredibly complex match process. Is this the same challenge?
Dr. Dib: No, it’s a little bit simpler. Living donors for liver transplants need to be between the ages of 18 and 60. They need to be relatively healthy, relatively fit, with a BMI hopefully less than 30, definitely less than 35. The compatibility is ABO compatibility. So, if they’re ABO-compatible, relatively young, relatively healthy, they would be a potential donor and we will go ahead and do a CT scan.
If the CT scan shows that they have a good, adequate anatomy, more than 90% of those will be good donors. I would say that out of 100 people who want to be donors, 25 of them will be adequate. One out of four people who want to save their family member and want to have this operation are able to donate half of their liver to their family member or loved one.
Dr. Schlechter: Excellent. And it’s helpful to know that the matching process is simpler. During his discussion, René Adam unequivocally stated that liver transplants are a new standard of care for colorectal cancer. And I guess my question is, do you agree with this statement? How do we balance the demand for living donors and the demand for deceased donors? Especially in a time of increasing fatty liver disease and obesity, other indications for liver transplant, causes of cirrhosis, and also in an era of young-onset colorectal cancer. Patients are younger. Is this a new standard of care? Do you agree with that statement?
Dr. Dib: I do agree with that statement. I think it’s important to understand that not all patients with colorectal mets are the same. Of the number of patients in the United States who have colorectal cancer, let’s say 50% of them will have liver metastasis. Only 15%-20% of them will have liver-only metastasis.
This is only for patients who have liver-only metastasis without extrahepatic disease. And only maybe 15%-20% of them will meet all the criteria to be able to undergo liver transplantation. I think it’s for a very selective subset of patients who have very good tumor biology, generally young patients who don’t have any other alternative to having even a complex liver resection and are not able to get R0 resection. That is when we could think about doing liver transplantation.
It’s one more of the skills that we can have. It doesn’t mean that it will be the only skill, or the best skill, for all of the patients.
Dr. Schlechter: When a patient volunteers to be a living donor for a loved one or a family member, and they go through all the screening and they’re found to be a candidate, what is the surgical experience for that patient?
How long are they in the hospital? What sort of operation is that?
Dr. Dib: Living donors are very special patients. These are patients who do not need an operation. And the only reason they’re doing this is to save the life of their loved one. Donor safety is our priority number one, two, three, and four. The donor operation needs to be perfect.
And so we take good care of, first of all, selecting the living donors, making sure that they’re young and they don’t have any big contraindications. We also ensure that they are well informed of the process. The living donor surgery that we’re now doing is laparoscopic and minimally invasive. Here at Beth Israel Deaconess, we have done it laparoscopically with very good results.
I think that experience before and after the surgery gets so much better because of the better recovery. They’re able to go home, in general, within 4 or 5 days, and they get on with their normal life within 6-8 weeks. I think it’s important for them to know all the processes and the actual risks and benefits for the recipient.
Among those risks, I think it’s important for them to understand that this is a complex operation. Even if we do it laparoscopically or robotically, so that the scar is less, inside we’re still taking out half of the liver. That is a surgery that needs to be undertaken very meticulously, with a focus on minimizing any bleeding.
It’s a surgery that takes a long time. It takes about 6 hours. We do our best to try to minimize any risks.
Dr. Schlechter: Excellent. Thanks for that. Today we had Dr. Martin Dib joining us to discuss liver transplant for metastatic colorectal cancer. We discussed the various important criteria. We discussed that early referral to multidisciplinary centers that manage these is important to help get patients set up.
We discussed the fact that there are certain inclusion and exclusion criteria to consider. Obviously, unresectable disease is a critical determination that should be made by a liver surgeon. The absence of extrahepatic disease is important in staging with PET or other imaging. We discussed certain other biological exclusions.
There’s a relative contraindication of right-sided vs left-sided cancers, but right-sided cancers can be transplanted. We discussed that an elevated CEA greater than 80 is a contraindication, as are mutations in BRAF. We reviewed data from both the TRANSMET trial recently published in The Lancet and presented at ASCO in 2024, as well as the older Oslo criteria and Oslo trials and SECA trials.
And finally, we heard that donors can now come as living donors, a laparoscopic robotic surgery with a better safety profile, and greater access to organs that are ABO matched and not HLA matched because of the nature of the biology. Thank you again for joining us.
Benjamin L. Schlechter, MD, is senior physician, Gastrointestinal Cancer Center, Dana-Farber Cancer Institute, Boston, Massachusetts. He has disclosed no relevant financial relationships. Martin J. Dib, MD, is member of the faculty, Department of Surgery, Harvard Medical School; director of Hepatobiliary Surgery, Division of Transplantation, Beth Israel Deaconess Medical Center, Boston. He has disclosed no relevant financial relationships.
A version of this transcript appeared on Medscape.com.
This transcript has been edited for clarity.
Benjamin L. Schlechter, MD: Dr. Dib is the director of the Hepatobiliary Surgery and Living Donor Program at Beth Israel Deaconess Medical Center here in Boston, and a Harvard Medical School faculty member.
He was previously at the Pontificia Universidad Católica de Chile, a leading international institution investigating the role of liver transplant in colorectal cancer, among other diseases. Dr. Dib, before we move to our discussion, I’d like to hear a bit about your pathway to becoming a transplant surgeon. How did you end up working on colorectal cancer and liver transplants in this field?
Martin J. Dib, MD: Thank you so much, Dr. Schlechter. I am originally from Chile. I had an opportunity to come to Beth Israel Deaconess Medical Center after medical school and I did liver regeneration research at the transplant center. After that, I was lucky enough to match as a general surgery resident at Beth Israel Deaconess.
This is my alma mater and I was able to graduate as a surgeon here. You and I had some paths together. After graduating from Harvard as a surgeon, I was trained in liver transplant, abdominal transplant, surgical oncology, and hepatobiliary surgery at the University of Toronto.
I have been developing this passion for being able to transplant cancer patients and use organ transplant techniques to be able to do complex resections for cancer.
Dr. Schlechter: Let’s talk about the topic for today, which is liver transplant and colorectal cancer. I’ll be honest — this is not a very familiar topic for a lot of oncologists. There are a lot of details that I think are new to us as oncologists. We need to expand this conversation to get access to patients for this.
First and foremost, can you talk about some of the parameters for a resectable liver metastasis vs unresectable disease that would be an indication for a liver transplant?
Dr. Dib: I think this is a very interesting topic because liver transplantation for cancer is not new. Liver transplantation started in the 1960s when people started doing liver transplants for advanced liver tumors. The problem is that they were selecting patients who had very advanced — and poor tumor biology — tumors. The outcomes were not good.
It was only in 1996 when the Milan criteria started. Mazzaferro and colleagues, using strict patient selection, were able to do liver transplant for selected hepatocellular carcinoma patients. Having those excellent outcomes in selecting patients opened the field for what we now call transplant oncology, which is using selection criteria and using other methods to be able to select which patients will do well after transplantation, even with immunosuppression.
Liver transplantation for colorectal metastasis was used at the very beginning of the era of liver transplantation, but with very poor outcomes. It was abandoned because of the outcomes. It is exciting to see that after 20 years of not doing it, there was a group in Norway that started again. They are doing liver transplants for colorectal metastases (mets), but with very selected patients.
In Norway, they had a very unusual setting where they had more liver donors than patients on the list waiting for liver transplant. So they can’t share these livers and we’re all jealous, right? Every single country in the West struggles because we don’t have enough livers for the rest of the list. And they had a lot of livers to be able to transplant people.
They decided to transplant some selected patients with colorectal mets that were unresectable. And the surprise was that they found that they were able to get a 60% survival at 5 years. And so that was new. After that, in Norway, they started showing this data to other centers in the world. It wasn’t until this year that we could see not only the long-term data and long-term outcomes of using liver transplantation for unresectable colorectal mets, but also we’re now having data from a prospective clinical trial from France.
It was three countries in the prospective clinical trial: France, Belgium, and Italy. We now see that we have a little stronger data to support the use of liver transplants for unresectable colorectal mets.
Dr. Schlechter: That’s the TRANSMET study you’re referencing that was presented at ASCO in the late-breaking abstract session in 2024, and then more recently in The Lancet’s eClinicalMedicine. Both of those papers were led by René Adam. That was a cool presentation to sit through. I was in the room, and I was taking a ton of notes and there was a lot of info that came out of that.
First of all, it showed that patients who had received chemotherapy and were responding could then go on to liver transplant in that population. Impressively, 81% of the patients who were randomized to transplant received it. Frankly, that’s a big number, especially compared with the West, as you said, and in particular the US and here in New England where livers are a very precious commodity.
And even accounting for that, if you look at the intention-to-treat analysis, the 5-year overall survival in that population was 57% compared with 13% with chemotherapy. And that feels like a real number for chemotherapy. If you look at the per-protocol analysis, frankly, the numbers are higher.
It’s always a challenging assessment. What was also interesting to me was the pattern of recurrence, which in general was that recurrences were extrahepatic. So not only were patients rendered disease-free, but in general, the liver remained disease-free and only 3% of patients had liver-only recurrence and 11% had widespread metastatic disease.
The biggest group was lung metastases, at about 40%. Ultimately, they reported a progression-free survival of 17. 4 months for transplant compared with 6. 4 months with chemotherapy. On every parameter, it looks like liver transplant wins for these people. Help me out. Who are these people? How do we find these people?
What are the inclusions and exclusions for this population?
Dr. Dib: I think that’s very important. This is not a therapy that will be for every patient. These are selected patients who have liver-only unresectable colorectal mets. These are patients that don’t have any extrahepatic disease and that either the primary has been taken out already or that they have the primary present, but the plan is to take the primary and then do a liver transplantation after 3 months, hopefully after 6 months, of removing the primary.
These are patients who meet all the criteria that we have seen in terms of the best outcomes — patients that have Oslo scores of less than three. The Oslo trial, which included the SECA (Secondary Cancer)-I and SECA-II trials, basically showed that patients with a maximal tumor diameter of less than 5.5 with a pretransplant CEA (carcinoembryonic antigen) of less than 80 that do not have progression on chemotherapy, among other variables, do better. But the concept is that this is a therapy that will apply only to selected patients. That way we can continue to have adequate overall survival post-transplant that would be comparable to other diseases that we do liver transplants for.
Dr. Schlechter: Were there other biomarkers, any mutations that were included or excluded?
Dr. Dib: Yes. If you look at SECA-I, SECA-II trial outcomes, and also TRANSMET, they all say patients with BRAF mutations shouldn’t be transplanted. There are other parameters, including, for example, the site of the primary tumor. Patients with a left-sided colon primary tumor do much better than patients who have a right-sided primary tumor.
That’s not a complete contraindication, but if you look at the most updated inclusion criteria of programs, like the ones that the one that we have here at Beth Israel Deaconess and many others, the inclusion criteria protocols include patients who have only hepatic disease.
So, if there are no extrahepatic mets, the resection of the primary has been done or will be done after a multidisciplinary discussion. We want to make sure they have the absence of BRAF mutation, and that they don’t have disease progression while on chemotherapy. So hopefully we have data from enough months to be able to make sure that there’s no intrahepatic or extrahepatic progression while on chemotherapy.
And that’s including CEA and also looking at the imaging.
Dr. Schlechter: When you’re seeing a patient, how much chemo do you think they should have? What’s a good run chemotherapy-wise for these patients? Let’s say, before I refer a patient to you, how much chemo should they have? And then what should I do? Do I get a PET scan? Do I get MRI? What’s the right scanning I should do to prove there’s no extrahepatic disease before sending a patient in for consideration?
Dr. Dib: First, we need to confirm unresectability. Referring patients early is always a good measure to make sure that we’re all in agreement that it’s an unresectable patient. Having a PET scan from the very beginning is helpful because it shows the disease before doing chemotherapy.
In terms of the lines of chemotherapy, ideally in the TRANSMET trial, for example, the idea was to show tumor control for at least 3 months, with less than three lines of chemotherapy. Some patients will do that with FOLFIRI. It depends on the case.
I think some of those evaluations will need a multidisciplinary discussion. In our case, we are connected to the Norway team. We frequently talk with the Oslo team and an international community of transplant centers to get opinions on particular cases.
But I think referring patients early is a good measure. If we don’t think that they qualify, we will let the team know. We’re strictly looking at patients who have unresectable liver mets that don’t have extrahepatic disease. The idea is to do a primary tumor resection, and then get to transplantation, hopefully after 6 months. In some cases that have some concerns in terms of tumor biology, we may even extend the time from diagnosis to transplant to over 1.5 years.
Dr. Schlechter: Excellent. And what’s the experience like for these patients? In training as a resident many years ago, I saw patients with cirrhosis who went on to have a liver transplant, and that was sort of trading one disease for another. What is the posttransplant, or the remission, experience of a liver transplant for colorectal cancer like for the patient?
Dr. Dib: That’s a very important point. I think that transplantation has gotten better and better, as has chemotherapy systemic therapy. The liver transplantation experience from 20 years ago has improved dramatically. I think the quality of life of liver transplant patients after transplantation has increased quite a bit.
At Beth Israel Deaconess, we have a liver transplant program that is doing over a 100 livers a year. And when you have a high-volume center, usually the experience gets better. The time in the hospital post-transplant decreases.
In general, when we’re doing liver transplants, patients are getting extubated in the OR 30% of the time. The vast majority of patients are going home within 1 or 2 weeks. They need to have immunosuppression for the rest of their lives. We have a very good program of transplant coordinators that will help the family and the patient to live with immunosuppression and live with a transplanted organ.
But I would say that we have many, many patients, especially these patients who are not patients with cirrhosis. Their health is not as deteriorated as patients who have low MELD (model for end-stage liver disease) scores. They don’t have liver disease. They have cancer. So usually patients like that, many of them can go back to work and live a quality of life that is fairly reasonable.
Dr. Schlechter: That’s good to hear. When we hear statements like liver transplant for colon cancer, a lot of us have this picture of a much sicker population, but it’s interesting and true that the colorectal cancer population as a candidate for liver transplant is a much healthier population than the population with cirrhosis.
Let’s talk about organs and donors. Largely in the TRANSMET study, for example, that was cadaveric donors. Those were not living donors and you’ve done a lot of work on living donors. If the answer in the United States, because of limited access to organs, is going to be living donors, who are those donors?
What is that like? How do you identify them?
Dr. Dib: There’s a lot of advantages to using living donors for these patients. In any type of patient that needs a liver transplant, cadaveric donors or deceased donors is the same concept. There are two types of deceased donors: the brain-dead donors and donors after cardiac death. Those are hard to come by.
We still have 15%-20% mortality on the waiting list in the United States. We’re already still struggling to get enough donors to transplant the patients that are on the list. Now, if you add a new indication, which is unresectable colorectal mets, we need to make sure that the outcomes are equivalent to the patients who are going to be transplanted for other reasons.
Right now, for example, the 5-year overall survival of a patient with cirrhosis, or a patient with hepatocellular carcinoma, is over 80% 5-year survival. In the SECA trials and TRANSMET trial, if we do a good selection, I think we can get to 70% 5-year survival. But until we have more data, I think it’s a cautious measure to, as a field, try to use living donors and not compete with the rest of the list of patients who are already dying on the list for liver transplants.
Once we get more data, it’s going to be something that, in the transplant community, we may be able to use deceased donors. Especially deceased donors with maybe extended criteria that are not going to be used for other patients. We can do living-unrelated or living-related donations. Family members or also friends or neighbors or part of the community, even altruistic donors, can donate to a potential recipient. And that enables us to not only time the transplant in an adequate manner, because we’re able to transplant the patient early, but also time it so we can give the number of chemotherapy cycles that we want to give.
That’s a huge advantage. You don’t compete for a liver with the cadaveric waiting list of patients that are waiting for other reasons, and you can select the tumor biology very well because you know exactly when the surgery is going to be. For instance, we can say, okay, this patient has KRAS mutation, left-sided colon cancer, and has been having good tumor biology with no progression. We will wait 6 months from the primary tumor to the transplant, which is going to be 1 year from diagnosis to transplant. And we can see during that time whether they continue to have good tumor biology.
But if you have a deceased donor liver transplant, sometimes you can’t time that well and schedule it. It becomes a bit more tricky in terms of patient selection and making sure that we do this for the people who are going to benefit.
Dr. Schlechter: And how does donor matching work? Is it HLA (human leukocyte antigen) matched or ABO-matched? Who can donate when you say a living-related? For example, when we think about bone marrow transplantation, which we’re all familiar with in the oncology population, it’s an incredibly complex match process. Is this the same challenge?
Dr. Dib: No, it’s a little bit simpler. Living donors for liver transplants need to be between the ages of 18 and 60. They need to be relatively healthy, relatively fit, with a BMI hopefully less than 30, definitely less than 35. The compatibility is ABO compatibility. So, if they’re ABO-compatible, relatively young, relatively healthy, they would be a potential donor and we will go ahead and do a CT scan.
If the CT scan shows that they have a good, adequate anatomy, more than 90% of those will be good donors. I would say that out of 100 people who want to be donors, 25 of them will be adequate. One out of four people who want to save their family member and want to have this operation are able to donate half of their liver to their family member or loved one.
Dr. Schlechter: Excellent. And it’s helpful to know that the matching process is simpler. During his discussion, René Adam unequivocally stated that liver transplants are a new standard of care for colorectal cancer. And I guess my question is, do you agree with this statement? How do we balance the demand for living donors and the demand for deceased donors? Especially in a time of increasing fatty liver disease and obesity, other indications for liver transplant, causes of cirrhosis, and also in an era of young-onset colorectal cancer. Patients are younger. Is this a new standard of care? Do you agree with that statement?
Dr. Dib: I do agree with that statement. I think it’s important to understand that not all patients with colorectal mets are the same. Of the number of patients in the United States who have colorectal cancer, let’s say 50% of them will have liver metastasis. Only 15%-20% of them will have liver-only metastasis.
This is only for patients who have liver-only metastasis without extrahepatic disease. And only maybe 15%-20% of them will meet all the criteria to be able to undergo liver transplantation. I think it’s for a very selective subset of patients who have very good tumor biology, generally young patients who don’t have any other alternative to having even a complex liver resection and are not able to get R0 resection. That is when we could think about doing liver transplantation.
It’s one more of the skills that we can have. It doesn’t mean that it will be the only skill, or the best skill, for all of the patients.
Dr. Schlechter: When a patient volunteers to be a living donor for a loved one or a family member, and they go through all the screening and they’re found to be a candidate, what is the surgical experience for that patient?
How long are they in the hospital? What sort of operation is that?
Dr. Dib: Living donors are very special patients. These are patients who do not need an operation. And the only reason they’re doing this is to save the life of their loved one. Donor safety is our priority number one, two, three, and four. The donor operation needs to be perfect.
And so we take good care of, first of all, selecting the living donors, making sure that they’re young and they don’t have any big contraindications. We also ensure that they are well informed of the process. The living donor surgery that we’re now doing is laparoscopic and minimally invasive. Here at Beth Israel Deaconess, we have done it laparoscopically with very good results.
I think that experience before and after the surgery gets so much better because of the better recovery. They’re able to go home, in general, within 4 or 5 days, and they get on with their normal life within 6-8 weeks. I think it’s important for them to know all the processes and the actual risks and benefits for the recipient.
Among those risks, I think it’s important for them to understand that this is a complex operation. Even if we do it laparoscopically or robotically, so that the scar is less, inside we’re still taking out half of the liver. That is a surgery that needs to be undertaken very meticulously, with a focus on minimizing any bleeding.
It’s a surgery that takes a long time. It takes about 6 hours. We do our best to try to minimize any risks.
Dr. Schlechter: Excellent. Thanks for that. Today we had Dr. Martin Dib joining us to discuss liver transplant for metastatic colorectal cancer. We discussed the various important criteria. We discussed that early referral to multidisciplinary centers that manage these is important to help get patients set up.
We discussed the fact that there are certain inclusion and exclusion criteria to consider. Obviously, unresectable disease is a critical determination that should be made by a liver surgeon. The absence of extrahepatic disease is important in staging with PET or other imaging. We discussed certain other biological exclusions.
There’s a relative contraindication of right-sided vs left-sided cancers, but right-sided cancers can be transplanted. We discussed that an elevated CEA greater than 80 is a contraindication, as are mutations in BRAF. We reviewed data from both the TRANSMET trial recently published in The Lancet and presented at ASCO in 2024, as well as the older Oslo criteria and Oslo trials and SECA trials.
And finally, we heard that donors can now come as living donors, a laparoscopic robotic surgery with a better safety profile, and greater access to organs that are ABO matched and not HLA matched because of the nature of the biology. Thank you again for joining us.
Benjamin L. Schlechter, MD, is senior physician, Gastrointestinal Cancer Center, Dana-Farber Cancer Institute, Boston, Massachusetts. He has disclosed no relevant financial relationships. Martin J. Dib, MD, is member of the faculty, Department of Surgery, Harvard Medical School; director of Hepatobiliary Surgery, Division of Transplantation, Beth Israel Deaconess Medical Center, Boston. He has disclosed no relevant financial relationships.
A version of this transcript appeared on Medscape.com.
Black Children With Vitiligo at Increased Risk for Psychiatric Disorders: Study
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.