User login
For MD-IQ use only
Is AI a Cure for Clinician Burnout?
The practice of medicine is evolving rapidly, with clinicians facing enhanced pressure to maximize productivity while managing increasingly complex patients and related clinical documentation. Indeed, clinicians are spending less time seeing patients, and more time in front of a computer screen.
Despite the many rewards of clinical medicine, rates of clinical practice attrition have increased among physicians in all specialties since 2013 with enhanced administrative burdens identified as a prominent driver. Among its many applications, artificial intelligence (AI) has immense potential to reduce the administrative and cognitive burdens that contribute to clinician burnout and attrition through tools such as AI scribes – these technologies have been rapidly adopted across healthcare systems and are already in use by ~30% of physician practices. The hope is that AI scribes will significantly reduce documentation time, leading to improvements in clinician wellbeing and expanding capacity for patient care. Indeed, some studies have shown up to a 20-30% improvement in documentation efficiency.
So, is AI a cure for physician burnout? The answer depends on what is done with these efficiency gains. If healthcare organizations respond to this enhanced efficiency by increasing patient volume expectations rather than allowing clinicians to recapture some of this time for meaningful work and professional wellbeing, it could create a so-called “workload paradox” where modest time savings are offset by greater productivity demands and the cognitive burden of reviewing AI-generated errors. that prioritizes clinician well-being and patient safety in addition to productivity.
In our final issue of 2025, we highlight a recent RCT from Annals of Internal Medicine finding that fecal microbiota transplantation is at least as effective as vancomycin in treating primary C. difficile infection. In this month’s Member Spotlight, we feature Andrew Ofosu, MD, MPH (University of Cincinnati Health), who stresses the importance of transparency and compassion in communicating effectively with patients, particularly around complex diagnoses. We hope you enjoy this and all the exciting content in our December issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
The practice of medicine is evolving rapidly, with clinicians facing enhanced pressure to maximize productivity while managing increasingly complex patients and related clinical documentation. Indeed, clinicians are spending less time seeing patients, and more time in front of a computer screen.
Despite the many rewards of clinical medicine, rates of clinical practice attrition have increased among physicians in all specialties since 2013 with enhanced administrative burdens identified as a prominent driver. Among its many applications, artificial intelligence (AI) has immense potential to reduce the administrative and cognitive burdens that contribute to clinician burnout and attrition through tools such as AI scribes – these technologies have been rapidly adopted across healthcare systems and are already in use by ~30% of physician practices. The hope is that AI scribes will significantly reduce documentation time, leading to improvements in clinician wellbeing and expanding capacity for patient care. Indeed, some studies have shown up to a 20-30% improvement in documentation efficiency.
So, is AI a cure for physician burnout? The answer depends on what is done with these efficiency gains. If healthcare organizations respond to this enhanced efficiency by increasing patient volume expectations rather than allowing clinicians to recapture some of this time for meaningful work and professional wellbeing, it could create a so-called “workload paradox” where modest time savings are offset by greater productivity demands and the cognitive burden of reviewing AI-generated errors. that prioritizes clinician well-being and patient safety in addition to productivity.
In our final issue of 2025, we highlight a recent RCT from Annals of Internal Medicine finding that fecal microbiota transplantation is at least as effective as vancomycin in treating primary C. difficile infection. In this month’s Member Spotlight, we feature Andrew Ofosu, MD, MPH (University of Cincinnati Health), who stresses the importance of transparency and compassion in communicating effectively with patients, particularly around complex diagnoses. We hope you enjoy this and all the exciting content in our December issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
The practice of medicine is evolving rapidly, with clinicians facing enhanced pressure to maximize productivity while managing increasingly complex patients and related clinical documentation. Indeed, clinicians are spending less time seeing patients, and more time in front of a computer screen.
Despite the many rewards of clinical medicine, rates of clinical practice attrition have increased among physicians in all specialties since 2013 with enhanced administrative burdens identified as a prominent driver. Among its many applications, artificial intelligence (AI) has immense potential to reduce the administrative and cognitive burdens that contribute to clinician burnout and attrition through tools such as AI scribes – these technologies have been rapidly adopted across healthcare systems and are already in use by ~30% of physician practices. The hope is that AI scribes will significantly reduce documentation time, leading to improvements in clinician wellbeing and expanding capacity for patient care. Indeed, some studies have shown up to a 20-30% improvement in documentation efficiency.
So, is AI a cure for physician burnout? The answer depends on what is done with these efficiency gains. If healthcare organizations respond to this enhanced efficiency by increasing patient volume expectations rather than allowing clinicians to recapture some of this time for meaningful work and professional wellbeing, it could create a so-called “workload paradox” where modest time savings are offset by greater productivity demands and the cognitive burden of reviewing AI-generated errors. that prioritizes clinician well-being and patient safety in addition to productivity.
In our final issue of 2025, we highlight a recent RCT from Annals of Internal Medicine finding that fecal microbiota transplantation is at least as effective as vancomycin in treating primary C. difficile infection. In this month’s Member Spotlight, we feature Andrew Ofosu, MD, MPH (University of Cincinnati Health), who stresses the importance of transparency and compassion in communicating effectively with patients, particularly around complex diagnoses. We hope you enjoy this and all the exciting content in our December issue.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Managing Adverse Effects of GLP-1 Agonists: Practical Insights From Dr. Bridget E. Shields
Managing Adverse Effects of GLP-1 Agonists: Practical Insights From Dr. Bridget E. Shields
Are you seeing any increase or trends in cutaneous adverse effects related to the use of GLP-1 agonists in your practice?
DR. SHIELDS: The use of GLP-1 agonists is increasing substantially across numerous populations. Patients are using these medications not only for weight management and diabetes control but also for blood pressure modulation and cardiovascular risk reduction. The market size is expected to grow at a rate of about 6% until 2027. While severe cutaneous adverse effects still are considered relatively rare with GLP-1 agonist use, mild adverse effects are quite common. Dermatologists should be familiar with these effects and how to manage them. Rare but serious cutaneous reactions include morbilliform drug eruptions, dermal hypersensitivity reactions, panniculitis, and bullous pemphigoid. It is thought that some GLP-1 agonists may cause more skin reactions than others; for example, exenatide extended-release has been associated with cutaneous adverse events more frequently than other GLP-1 agonists in a recent comprehensive literature review.
Do you see a role for dermatologists in monitoring or managing the downstream dermatologic effects of GLP-1 agonists over the next few years?
DR. SHIELDS: Absolutely. When patients develop a drug eruption, bullous pemphigoid, or eosinophilic panniculitis, dermatologists are going to be the ones to diagnose and manage therapy. Awareness of these adverse effects is crucial to timely and thoughtful discussions surrounding medication discontinuation vs a “treat through” approach.
Do you recommend coordinating with endocrinologists or obesity medicine specialists when managing shared patients on GLP-1s (particularly if skin concerns arise)?
DR. SHIELDS: Yes. This is crucial to patient success. Co-management can provide clarity around the indication for therapy and allow for a thoughtful risk-benefit discussion with the patient, primary care physician, endocrinologist, cardiologist, etc. In my practice, I have found that many patients do not want to stop therapy even when they develop cutaneous adverse effects. There are options to transition therapy or treat through in some cases, but having a comprehensive monitoring and therapy plan is critical.
Have you encountered cases in which rapid weight loss from GLP-1s worsened conditions such as loose skin, cellulite, or facial lipoatrophy, leading to new aesthetic concerns? How would you recommend counseling and/or treating affected patients?
DR. SHIELDS: Accelerated facial aging is a noticeable adverse effect in patients who undergo treatment with GLP-1 agonists, especially when used off-label for weight loss. Localized loss of facial fat can result in altered facial proportions and excess skin. There are multiple additional mechanisms that may underlie accelerated facial aging in patients on GLP-1s, and really we are just beginning to scratch the surface of why and how this happens. Understanding these mechanisms will open the door to downstream preventive and therapeutic options. If patients experience new aesthetic concerns, I currently work with them to adjust their medication to slow weight loss, recommend improved nutrition and hydration, encourage exercise and weight training to maintain muscle mass, and engage my cosmetic dermatology colleagues to discuss procedures such as dermal fillers.
All patients starting GLP-1 agonists should be thoroughly counseled on risks and adverse effects of their medication. These are well reported and should be considered carefully. Starting with lower medication dosing in conjunction with slow escalation and careful monitoring can be helpful in combatting these adverse effects.
Are you seeing any increase or trends in cutaneous adverse effects related to the use of GLP-1 agonists in your practice?
DR. SHIELDS: The use of GLP-1 agonists is increasing substantially across numerous populations. Patients are using these medications not only for weight management and diabetes control but also for blood pressure modulation and cardiovascular risk reduction. The market size is expected to grow at a rate of about 6% until 2027. While severe cutaneous adverse effects still are considered relatively rare with GLP-1 agonist use, mild adverse effects are quite common. Dermatologists should be familiar with these effects and how to manage them. Rare but serious cutaneous reactions include morbilliform drug eruptions, dermal hypersensitivity reactions, panniculitis, and bullous pemphigoid. It is thought that some GLP-1 agonists may cause more skin reactions than others; for example, exenatide extended-release has been associated with cutaneous adverse events more frequently than other GLP-1 agonists in a recent comprehensive literature review.
Do you see a role for dermatologists in monitoring or managing the downstream dermatologic effects of GLP-1 agonists over the next few years?
DR. SHIELDS: Absolutely. When patients develop a drug eruption, bullous pemphigoid, or eosinophilic panniculitis, dermatologists are going to be the ones to diagnose and manage therapy. Awareness of these adverse effects is crucial to timely and thoughtful discussions surrounding medication discontinuation vs a “treat through” approach.
Do you recommend coordinating with endocrinologists or obesity medicine specialists when managing shared patients on GLP-1s (particularly if skin concerns arise)?
DR. SHIELDS: Yes. This is crucial to patient success. Co-management can provide clarity around the indication for therapy and allow for a thoughtful risk-benefit discussion with the patient, primary care physician, endocrinologist, cardiologist, etc. In my practice, I have found that many patients do not want to stop therapy even when they develop cutaneous adverse effects. There are options to transition therapy or treat through in some cases, but having a comprehensive monitoring and therapy plan is critical.
Have you encountered cases in which rapid weight loss from GLP-1s worsened conditions such as loose skin, cellulite, or facial lipoatrophy, leading to new aesthetic concerns? How would you recommend counseling and/or treating affected patients?
DR. SHIELDS: Accelerated facial aging is a noticeable adverse effect in patients who undergo treatment with GLP-1 agonists, especially when used off-label for weight loss. Localized loss of facial fat can result in altered facial proportions and excess skin. There are multiple additional mechanisms that may underlie accelerated facial aging in patients on GLP-1s, and really we are just beginning to scratch the surface of why and how this happens. Understanding these mechanisms will open the door to downstream preventive and therapeutic options. If patients experience new aesthetic concerns, I currently work with them to adjust their medication to slow weight loss, recommend improved nutrition and hydration, encourage exercise and weight training to maintain muscle mass, and engage my cosmetic dermatology colleagues to discuss procedures such as dermal fillers.
All patients starting GLP-1 agonists should be thoroughly counseled on risks and adverse effects of their medication. These are well reported and should be considered carefully. Starting with lower medication dosing in conjunction with slow escalation and careful monitoring can be helpful in combatting these adverse effects.
Are you seeing any increase or trends in cutaneous adverse effects related to the use of GLP-1 agonists in your practice?
DR. SHIELDS: The use of GLP-1 agonists is increasing substantially across numerous populations. Patients are using these medications not only for weight management and diabetes control but also for blood pressure modulation and cardiovascular risk reduction. The market size is expected to grow at a rate of about 6% until 2027. While severe cutaneous adverse effects still are considered relatively rare with GLP-1 agonist use, mild adverse effects are quite common. Dermatologists should be familiar with these effects and how to manage them. Rare but serious cutaneous reactions include morbilliform drug eruptions, dermal hypersensitivity reactions, panniculitis, and bullous pemphigoid. It is thought that some GLP-1 agonists may cause more skin reactions than others; for example, exenatide extended-release has been associated with cutaneous adverse events more frequently than other GLP-1 agonists in a recent comprehensive literature review.
Do you see a role for dermatologists in monitoring or managing the downstream dermatologic effects of GLP-1 agonists over the next few years?
DR. SHIELDS: Absolutely. When patients develop a drug eruption, bullous pemphigoid, or eosinophilic panniculitis, dermatologists are going to be the ones to diagnose and manage therapy. Awareness of these adverse effects is crucial to timely and thoughtful discussions surrounding medication discontinuation vs a “treat through” approach.
Do you recommend coordinating with endocrinologists or obesity medicine specialists when managing shared patients on GLP-1s (particularly if skin concerns arise)?
DR. SHIELDS: Yes. This is crucial to patient success. Co-management can provide clarity around the indication for therapy and allow for a thoughtful risk-benefit discussion with the patient, primary care physician, endocrinologist, cardiologist, etc. In my practice, I have found that many patients do not want to stop therapy even when they develop cutaneous adverse effects. There are options to transition therapy or treat through in some cases, but having a comprehensive monitoring and therapy plan is critical.
Have you encountered cases in which rapid weight loss from GLP-1s worsened conditions such as loose skin, cellulite, or facial lipoatrophy, leading to new aesthetic concerns? How would you recommend counseling and/or treating affected patients?
DR. SHIELDS: Accelerated facial aging is a noticeable adverse effect in patients who undergo treatment with GLP-1 agonists, especially when used off-label for weight loss. Localized loss of facial fat can result in altered facial proportions and excess skin. There are multiple additional mechanisms that may underlie accelerated facial aging in patients on GLP-1s, and really we are just beginning to scratch the surface of why and how this happens. Understanding these mechanisms will open the door to downstream preventive and therapeutic options. If patients experience new aesthetic concerns, I currently work with them to adjust their medication to slow weight loss, recommend improved nutrition and hydration, encourage exercise and weight training to maintain muscle mass, and engage my cosmetic dermatology colleagues to discuss procedures such as dermal fillers.
All patients starting GLP-1 agonists should be thoroughly counseled on risks and adverse effects of their medication. These are well reported and should be considered carefully. Starting with lower medication dosing in conjunction with slow escalation and careful monitoring can be helpful in combatting these adverse effects.
Managing Adverse Effects of GLP-1 Agonists: Practical Insights From Dr. Bridget E. Shields
Managing Adverse Effects of GLP-1 Agonists: Practical Insights From Dr. Bridget E. Shields
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
Use of artificial intelligence (AI) in dermatology has increased over the past decade, likely driven by advances in deep learning algorithms, computing hardware, and machine learning.1 Studies comparing the performance of AI algorithms to dermatologists in classifying skin disorders have shown conflicting results.2,3 In this study, we aimed to analyze AI tools used for diagnosing and classifying skin disease and evaluate the role of dermatologists in the creation of AI technology. We also investigated the number of clinical images used in datasets to train AI programs and compared tools that were created with dermatologist input to those created without dermatologist/clinician involvement.
Methods
A search of PubMed articles indexed for MEDLINE using the terms machine learning, artificial intelligence, and dermatology was conducted on September 18, 2022. Articles were included if they described full-length trials; used machine learning for diagnosis of or screening for dermatologic conditions; and used dermoscopic or gross image datasets of the skin, hair, or nails. Articles were categorized into 4 groups based on the conditions covered: chronic wounds, inflammatory skin diseases, mixed conditions, and pigmented skin lesions. Algorithms were sorted into 4 categories: convolutional/convoluted neural network, deep learning model/deep neural network, AI/artificial neural network, and other. Details regarding Fitzpatrick skin type and skin of color (SoC) inclusion in the articles or AI algorithm datasets were recorded. Univariate and multivariate analyses were performed using Microsoft Excel and SAS Studio 3.8. Sensitivity and specificity were calculated for all included AI technology. Sensitivity, specificity, and the number of clinical images were compared among the included articles using analysis of variance and t tests (α=0.05; P<.05 indicated statistical significance).
Results
Our search yielded 1016 articles, 58 of which met the inclusion criteria. Overall, 25.9% (15/58) of the articles utilized AI to diagnose or classify mixed skin diseases; 22.4% (13/58) for pigmented skin lesions; 19.0% (11/58) for wounds; 17.2% (10/58) for inflammatory skin diseases; and 5.2% (3/58) each for acne, psoriasis, and onychomycosis. Overall, 24.0% (14/58) of articles provided information about Fitzpatrick skin type, and 58.7% (34/58) included clinical images depicting SoC. Furthermore, we found that only 20.7% (12/58) of articles on deep learning models included descriptions of patient ethnicity or race in at least 1 dataset, and only 10.3% (6/58) of studies included any information about skin tone in the dataset. Studies with a dermatologist as the last author (most likely to be supervising the project) were more likely to include clinical images depicting SoC than those without (82.6% [19/23] and 16.7% [3/18], respectively [P=.0411]).
The mean (SD) number of clinical images in the study articles was 28,422 (84,050). Thirty-seven (63.8%) of the study articles included gross images, 17 (29.3%) used dermoscopic images, and 4 (6.9%) used both. Twenty-seven (46.6%) articles used convolutional/convoluted neural networks, 15 (25.9%) used deep learning model/deep neural networks, 8 (13.8%) used other algorithms, 6 (10.3%) used AI/artificial neural network, and 2 (3.4%) used fuzzy algorithms. Most studies were conducted in China (29.3% [17/58]), Germany (12.1% [7/58]), India (10.3% [6/58]), multiple nations (10.3% [6/58]), and the United States (10.3% [6/58]). Overall, 82.8% (48/58) of articles included at least 1 dermatologist coauthor. Sensitivity of the AI models was 0.85, and specificity was 0.85. The average percentage of images in the dataset correctly identified by a physician was 76.87% vs 81.62% of images correctly identified by AI. Average agreement between AI and physician assessment was 77.98%, defined as AI and physician both having the same diagnosis.
Articles authored by dermatologists contained more clinical images than those without dermatologists in key authorship roles (P<.0001)(eTable). Psoriasis-related algorithms had the fewest (mean [SD]: 3173 [4203]), and pigmented skin lesions had the most clinical images (mean [SD]: 53,19l [155,579]).

Comment
Our results indicated that AI studies with dermatologist authors had significantly more images in their datasets (ie, the set of clinical images of skin lesions used to train AI algorithms in diagnosing or classifying lesions) than those with nondermatologist authors (P<.0001)(eTable). Similarly, in a study of AI technology for skin cancer diagnosis, AI studies with dermatologist authors (ie, included in the development of the AI algorithm) had more images than studies without dermatologist authors.1 Deep learning textbooks have suggested that 5000 clinical images or training input per output category are needed to produce acceptable algorithm performance, and more than 10 million are needed to produce results superior to human performance.4-10 Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists1; therefore, dermatologist involvement in AI development is necessary to facilitate collection of larger image datasets and optimal performance for image diagnosis/classification tasks.
We found that 20.7% of articles on deep learning models included descriptions of patient ethnicity or race, and only 10.3% of studies included any information about skin tone in the dataset. Furthermore, American investigators primarily trained models using clinical images of patients with lighter skin tones, whereas Chinese investigators exclusively included images depicting darker skin tones. Similarly, in a study of 52 cutaneous imaging deep learning articles, only 17.3% (9/52) reported race and/or Fitzpatrick skin type, and only 7.7% (4/52) of articles included both.2,6,8 Therefore, dermatologists are needed to contribute images representing diverse populations and collaborate in AI research studies, as their involvement is necessary to ensure the accuracy of AI models in classifying lesions or diagnosing skin lesions across all skin types.
Our search was limited to PubMed, and real-world applications could not be evaluated.
Conclusion
In summary, we found that AI studies with dermatologist authors used larger numbers of clinical images in their datasets and more images representing diverse skin types than studies without. Therefore, we advocate for greater involvement of dermatologists in AI research, which might result in better patient outcomes by improving diagnostic accuracy.
- Zakhem GA, Fakhoury JW, Motosko CC, et al. Characterizing the role of dermatologists in developing artificial intelligence for assessment of skin cancer. J Am Acad Dermatol. 2021;85:1544-1556.
- Daneshjou R, Vodrahalli K, Novoa RA, et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv. 2022;8:eabq6147.
- Wu E, Wu K, Daneshjou R, et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat Med. 2021;27:582-584.
- Murphree DH, Puri P, Shamim H, et al. Deep learning for dermatologists: part I. Fundamental concepts. J Am Acad Dermatol. 2022;87:1343-1351.
- Goodfellow I, Bengio Y, Courville A. Deep Learning. The MIT Press; 2016.
- Kim YH, Kobic A, Vidal NY. Distribution of race and Fitzpatrick skin types in data sets for deep learning in dermatology: a systematic review. J Am Acad Dermatol. 2022;87:460-461.
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908.
- Zhu CY, Wang YK, Chen HP, et al. A deep learning based framework for diagnosing multiple skin diseases in a clinical environment. Front Med (Lausanne). 2021;8:626369.
- Capurro N, Pastore VP, Touijer L, et al. A deep learning approach to direct immunofluorescence pattern recognition in autoimmune bullous diseases. Br J Dermatol. 2024;191:261-266.
- Han SS, Park I, Eun Chang S, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140:1753-1761.
Use of artificial intelligence (AI) in dermatology has increased over the past decade, likely driven by advances in deep learning algorithms, computing hardware, and machine learning.1 Studies comparing the performance of AI algorithms to dermatologists in classifying skin disorders have shown conflicting results.2,3 In this study, we aimed to analyze AI tools used for diagnosing and classifying skin disease and evaluate the role of dermatologists in the creation of AI technology. We also investigated the number of clinical images used in datasets to train AI programs and compared tools that were created with dermatologist input to those created without dermatologist/clinician involvement.
Methods
A search of PubMed articles indexed for MEDLINE using the terms machine learning, artificial intelligence, and dermatology was conducted on September 18, 2022. Articles were included if they described full-length trials; used machine learning for diagnosis of or screening for dermatologic conditions; and used dermoscopic or gross image datasets of the skin, hair, or nails. Articles were categorized into 4 groups based on the conditions covered: chronic wounds, inflammatory skin diseases, mixed conditions, and pigmented skin lesions. Algorithms were sorted into 4 categories: convolutional/convoluted neural network, deep learning model/deep neural network, AI/artificial neural network, and other. Details regarding Fitzpatrick skin type and skin of color (SoC) inclusion in the articles or AI algorithm datasets were recorded. Univariate and multivariate analyses were performed using Microsoft Excel and SAS Studio 3.8. Sensitivity and specificity were calculated for all included AI technology. Sensitivity, specificity, and the number of clinical images were compared among the included articles using analysis of variance and t tests (α=0.05; P<.05 indicated statistical significance).
Results
Our search yielded 1016 articles, 58 of which met the inclusion criteria. Overall, 25.9% (15/58) of the articles utilized AI to diagnose or classify mixed skin diseases; 22.4% (13/58) for pigmented skin lesions; 19.0% (11/58) for wounds; 17.2% (10/58) for inflammatory skin diseases; and 5.2% (3/58) each for acne, psoriasis, and onychomycosis. Overall, 24.0% (14/58) of articles provided information about Fitzpatrick skin type, and 58.7% (34/58) included clinical images depicting SoC. Furthermore, we found that only 20.7% (12/58) of articles on deep learning models included descriptions of patient ethnicity or race in at least 1 dataset, and only 10.3% (6/58) of studies included any information about skin tone in the dataset. Studies with a dermatologist as the last author (most likely to be supervising the project) were more likely to include clinical images depicting SoC than those without (82.6% [19/23] and 16.7% [3/18], respectively [P=.0411]).
The mean (SD) number of clinical images in the study articles was 28,422 (84,050). Thirty-seven (63.8%) of the study articles included gross images, 17 (29.3%) used dermoscopic images, and 4 (6.9%) used both. Twenty-seven (46.6%) articles used convolutional/convoluted neural networks, 15 (25.9%) used deep learning model/deep neural networks, 8 (13.8%) used other algorithms, 6 (10.3%) used AI/artificial neural network, and 2 (3.4%) used fuzzy algorithms. Most studies were conducted in China (29.3% [17/58]), Germany (12.1% [7/58]), India (10.3% [6/58]), multiple nations (10.3% [6/58]), and the United States (10.3% [6/58]). Overall, 82.8% (48/58) of articles included at least 1 dermatologist coauthor. Sensitivity of the AI models was 0.85, and specificity was 0.85. The average percentage of images in the dataset correctly identified by a physician was 76.87% vs 81.62% of images correctly identified by AI. Average agreement between AI and physician assessment was 77.98%, defined as AI and physician both having the same diagnosis.
Articles authored by dermatologists contained more clinical images than those without dermatologists in key authorship roles (P<.0001)(eTable). Psoriasis-related algorithms had the fewest (mean [SD]: 3173 [4203]), and pigmented skin lesions had the most clinical images (mean [SD]: 53,19l [155,579]).

Comment
Our results indicated that AI studies with dermatologist authors had significantly more images in their datasets (ie, the set of clinical images of skin lesions used to train AI algorithms in diagnosing or classifying lesions) than those with nondermatologist authors (P<.0001)(eTable). Similarly, in a study of AI technology for skin cancer diagnosis, AI studies with dermatologist authors (ie, included in the development of the AI algorithm) had more images than studies without dermatologist authors.1 Deep learning textbooks have suggested that 5000 clinical images or training input per output category are needed to produce acceptable algorithm performance, and more than 10 million are needed to produce results superior to human performance.4-10 Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists1; therefore, dermatologist involvement in AI development is necessary to facilitate collection of larger image datasets and optimal performance for image diagnosis/classification tasks.
We found that 20.7% of articles on deep learning models included descriptions of patient ethnicity or race, and only 10.3% of studies included any information about skin tone in the dataset. Furthermore, American investigators primarily trained models using clinical images of patients with lighter skin tones, whereas Chinese investigators exclusively included images depicting darker skin tones. Similarly, in a study of 52 cutaneous imaging deep learning articles, only 17.3% (9/52) reported race and/or Fitzpatrick skin type, and only 7.7% (4/52) of articles included both.2,6,8 Therefore, dermatologists are needed to contribute images representing diverse populations and collaborate in AI research studies, as their involvement is necessary to ensure the accuracy of AI models in classifying lesions or diagnosing skin lesions across all skin types.
Our search was limited to PubMed, and real-world applications could not be evaluated.
Conclusion
In summary, we found that AI studies with dermatologist authors used larger numbers of clinical images in their datasets and more images representing diverse skin types than studies without. Therefore, we advocate for greater involvement of dermatologists in AI research, which might result in better patient outcomes by improving diagnostic accuracy.
Use of artificial intelligence (AI) in dermatology has increased over the past decade, likely driven by advances in deep learning algorithms, computing hardware, and machine learning.1 Studies comparing the performance of AI algorithms to dermatologists in classifying skin disorders have shown conflicting results.2,3 In this study, we aimed to analyze AI tools used for diagnosing and classifying skin disease and evaluate the role of dermatologists in the creation of AI technology. We also investigated the number of clinical images used in datasets to train AI programs and compared tools that were created with dermatologist input to those created without dermatologist/clinician involvement.
Methods
A search of PubMed articles indexed for MEDLINE using the terms machine learning, artificial intelligence, and dermatology was conducted on September 18, 2022. Articles were included if they described full-length trials; used machine learning for diagnosis of or screening for dermatologic conditions; and used dermoscopic or gross image datasets of the skin, hair, or nails. Articles were categorized into 4 groups based on the conditions covered: chronic wounds, inflammatory skin diseases, mixed conditions, and pigmented skin lesions. Algorithms were sorted into 4 categories: convolutional/convoluted neural network, deep learning model/deep neural network, AI/artificial neural network, and other. Details regarding Fitzpatrick skin type and skin of color (SoC) inclusion in the articles or AI algorithm datasets were recorded. Univariate and multivariate analyses were performed using Microsoft Excel and SAS Studio 3.8. Sensitivity and specificity were calculated for all included AI technology. Sensitivity, specificity, and the number of clinical images were compared among the included articles using analysis of variance and t tests (α=0.05; P<.05 indicated statistical significance).
Results
Our search yielded 1016 articles, 58 of which met the inclusion criteria. Overall, 25.9% (15/58) of the articles utilized AI to diagnose or classify mixed skin diseases; 22.4% (13/58) for pigmented skin lesions; 19.0% (11/58) for wounds; 17.2% (10/58) for inflammatory skin diseases; and 5.2% (3/58) each for acne, psoriasis, and onychomycosis. Overall, 24.0% (14/58) of articles provided information about Fitzpatrick skin type, and 58.7% (34/58) included clinical images depicting SoC. Furthermore, we found that only 20.7% (12/58) of articles on deep learning models included descriptions of patient ethnicity or race in at least 1 dataset, and only 10.3% (6/58) of studies included any information about skin tone in the dataset. Studies with a dermatologist as the last author (most likely to be supervising the project) were more likely to include clinical images depicting SoC than those without (82.6% [19/23] and 16.7% [3/18], respectively [P=.0411]).
The mean (SD) number of clinical images in the study articles was 28,422 (84,050). Thirty-seven (63.8%) of the study articles included gross images, 17 (29.3%) used dermoscopic images, and 4 (6.9%) used both. Twenty-seven (46.6%) articles used convolutional/convoluted neural networks, 15 (25.9%) used deep learning model/deep neural networks, 8 (13.8%) used other algorithms, 6 (10.3%) used AI/artificial neural network, and 2 (3.4%) used fuzzy algorithms. Most studies were conducted in China (29.3% [17/58]), Germany (12.1% [7/58]), India (10.3% [6/58]), multiple nations (10.3% [6/58]), and the United States (10.3% [6/58]). Overall, 82.8% (48/58) of articles included at least 1 dermatologist coauthor. Sensitivity of the AI models was 0.85, and specificity was 0.85. The average percentage of images in the dataset correctly identified by a physician was 76.87% vs 81.62% of images correctly identified by AI. Average agreement between AI and physician assessment was 77.98%, defined as AI and physician both having the same diagnosis.
Articles authored by dermatologists contained more clinical images than those without dermatologists in key authorship roles (P<.0001)(eTable). Psoriasis-related algorithms had the fewest (mean [SD]: 3173 [4203]), and pigmented skin lesions had the most clinical images (mean [SD]: 53,19l [155,579]).

Comment
Our results indicated that AI studies with dermatologist authors had significantly more images in their datasets (ie, the set of clinical images of skin lesions used to train AI algorithms in diagnosing or classifying lesions) than those with nondermatologist authors (P<.0001)(eTable). Similarly, in a study of AI technology for skin cancer diagnosis, AI studies with dermatologist authors (ie, included in the development of the AI algorithm) had more images than studies without dermatologist authors.1 Deep learning textbooks have suggested that 5000 clinical images or training input per output category are needed to produce acceptable algorithm performance, and more than 10 million are needed to produce results superior to human performance.4-10 Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists1; therefore, dermatologist involvement in AI development is necessary to facilitate collection of larger image datasets and optimal performance for image diagnosis/classification tasks.
We found that 20.7% of articles on deep learning models included descriptions of patient ethnicity or race, and only 10.3% of studies included any information about skin tone in the dataset. Furthermore, American investigators primarily trained models using clinical images of patients with lighter skin tones, whereas Chinese investigators exclusively included images depicting darker skin tones. Similarly, in a study of 52 cutaneous imaging deep learning articles, only 17.3% (9/52) reported race and/or Fitzpatrick skin type, and only 7.7% (4/52) of articles included both.2,6,8 Therefore, dermatologists are needed to contribute images representing diverse populations and collaborate in AI research studies, as their involvement is necessary to ensure the accuracy of AI models in classifying lesions or diagnosing skin lesions across all skin types.
Our search was limited to PubMed, and real-world applications could not be evaluated.
Conclusion
In summary, we found that AI studies with dermatologist authors used larger numbers of clinical images in their datasets and more images representing diverse skin types than studies without. Therefore, we advocate for greater involvement of dermatologists in AI research, which might result in better patient outcomes by improving diagnostic accuracy.
- Zakhem GA, Fakhoury JW, Motosko CC, et al. Characterizing the role of dermatologists in developing artificial intelligence for assessment of skin cancer. J Am Acad Dermatol. 2021;85:1544-1556.
- Daneshjou R, Vodrahalli K, Novoa RA, et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv. 2022;8:eabq6147.
- Wu E, Wu K, Daneshjou R, et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat Med. 2021;27:582-584.
- Murphree DH, Puri P, Shamim H, et al. Deep learning for dermatologists: part I. Fundamental concepts. J Am Acad Dermatol. 2022;87:1343-1351.
- Goodfellow I, Bengio Y, Courville A. Deep Learning. The MIT Press; 2016.
- Kim YH, Kobic A, Vidal NY. Distribution of race and Fitzpatrick skin types in data sets for deep learning in dermatology: a systematic review. J Am Acad Dermatol. 2022;87:460-461.
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908.
- Zhu CY, Wang YK, Chen HP, et al. A deep learning based framework for diagnosing multiple skin diseases in a clinical environment. Front Med (Lausanne). 2021;8:626369.
- Capurro N, Pastore VP, Touijer L, et al. A deep learning approach to direct immunofluorescence pattern recognition in autoimmune bullous diseases. Br J Dermatol. 2024;191:261-266.
- Han SS, Park I, Eun Chang S, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140:1753-1761.
- Zakhem GA, Fakhoury JW, Motosko CC, et al. Characterizing the role of dermatologists in developing artificial intelligence for assessment of skin cancer. J Am Acad Dermatol. 2021;85:1544-1556.
- Daneshjou R, Vodrahalli K, Novoa RA, et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv. 2022;8:eabq6147.
- Wu E, Wu K, Daneshjou R, et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat Med. 2021;27:582-584.
- Murphree DH, Puri P, Shamim H, et al. Deep learning for dermatologists: part I. Fundamental concepts. J Am Acad Dermatol. 2022;87:1343-1351.
- Goodfellow I, Bengio Y, Courville A. Deep Learning. The MIT Press; 2016.
- Kim YH, Kobic A, Vidal NY. Distribution of race and Fitzpatrick skin types in data sets for deep learning in dermatology: a systematic review. J Am Acad Dermatol. 2022;87:460-461.
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908.
- Zhu CY, Wang YK, Chen HP, et al. A deep learning based framework for diagnosing multiple skin diseases in a clinical environment. Front Med (Lausanne). 2021;8:626369.
- Capurro N, Pastore VP, Touijer L, et al. A deep learning approach to direct immunofluorescence pattern recognition in autoimmune bullous diseases. Br J Dermatol. 2024;191:261-266.
- Han SS, Park I, Eun Chang S, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140:1753-1761.
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
Practice Points
- Artificial intelligence (AI) technology is emerging as a valuable tool in diagnosing and classifying dermatologic conditions.
- Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists.
The Current State of Postgraduate Dermatology Training Programs for Advanced Practice Providers
The Current State of Postgraduate Dermatology Training Programs for Advanced Practice Providers
Nurse practitioners (NPs) and physician assistants (PAs) often help provide dermatologic care but lack the same mandatory specialized postgraduate training required of board-certified dermatologists (BCDs), which includes at least 3 years of dermatology-focused education in an accredited residency program in addition to an intern year of general medicine, pediatrics, or surgery. Dermatology residency is followed by a certification examination administered by the American Board of Dermatology (ABD) or the American Osteopathic Board of Dermatology, leading to board certification. Some physicians choose to do a fellowship, which typically involves an additional 1 to 2 years of postresidency subspeciality training.
Optional postgraduate dermatology training programs for advanced practice providers (APPs) have been offered by some academic institutions and private practice groups since at least 2003, including Lahey Hospital and Medical Center (Burlington, Massachusetts) as well as the University of Rochester Medical Center (Rochester, New York). Despite a lack of accreditation or standardization, the programs can be beneficial for NPs and PAs to expand their dermatologic knowledge and skills and help bridge the care gap within the specialty. Didactics often are conducted in parallel with the educational activities of the parent institution’s traditional dermatology residency program (eg, lectures, grand rounds). While these programs often are managed by practicing dermatology NPs and PAs, dermatologists also may be involved in their education with didactic instruction, curriculum development, and clinical preceptorship.
In this cross-sectional study, we identified and evaluated 10 postgraduate dermatology training programs for APPs across the United States. With the growing number of NPs and PAs in the dermatology workforce—both in academic and private practice—it is important for BCDs to be aware of the differences in the dermatology training received in order to ensure safe and effective care is provided through supervisory or collaborative roles (depending on state independent practice laws for APPs and to be aware of the implications these programs may have on the field of dermatology.
Methods
To identify postgraduate dermatology training programs for APPs in the United States, we conducted a cross-sectional study using data obtained via a Google search of various combinations of the following terms: nurse practitioner, NP, physician assistant, PA, advance practice provider, APP, dermatology, postgraduate training, residency, and fellowship. We excluded postgraduate dermatology training programs for APPs that required tuition and did not provide a stipend, as well as programs that lacked the formal structure and credibility needed to qualify as legitimate postgraduate training. Many of the excluded programs operate in a manner that raises ethical concerns, offering pay-to-play opportunities under the guise of education. Information collected on each program included the program name, location, parent institution, program length, class size, curriculum, and any associated salary and benefits.
Results
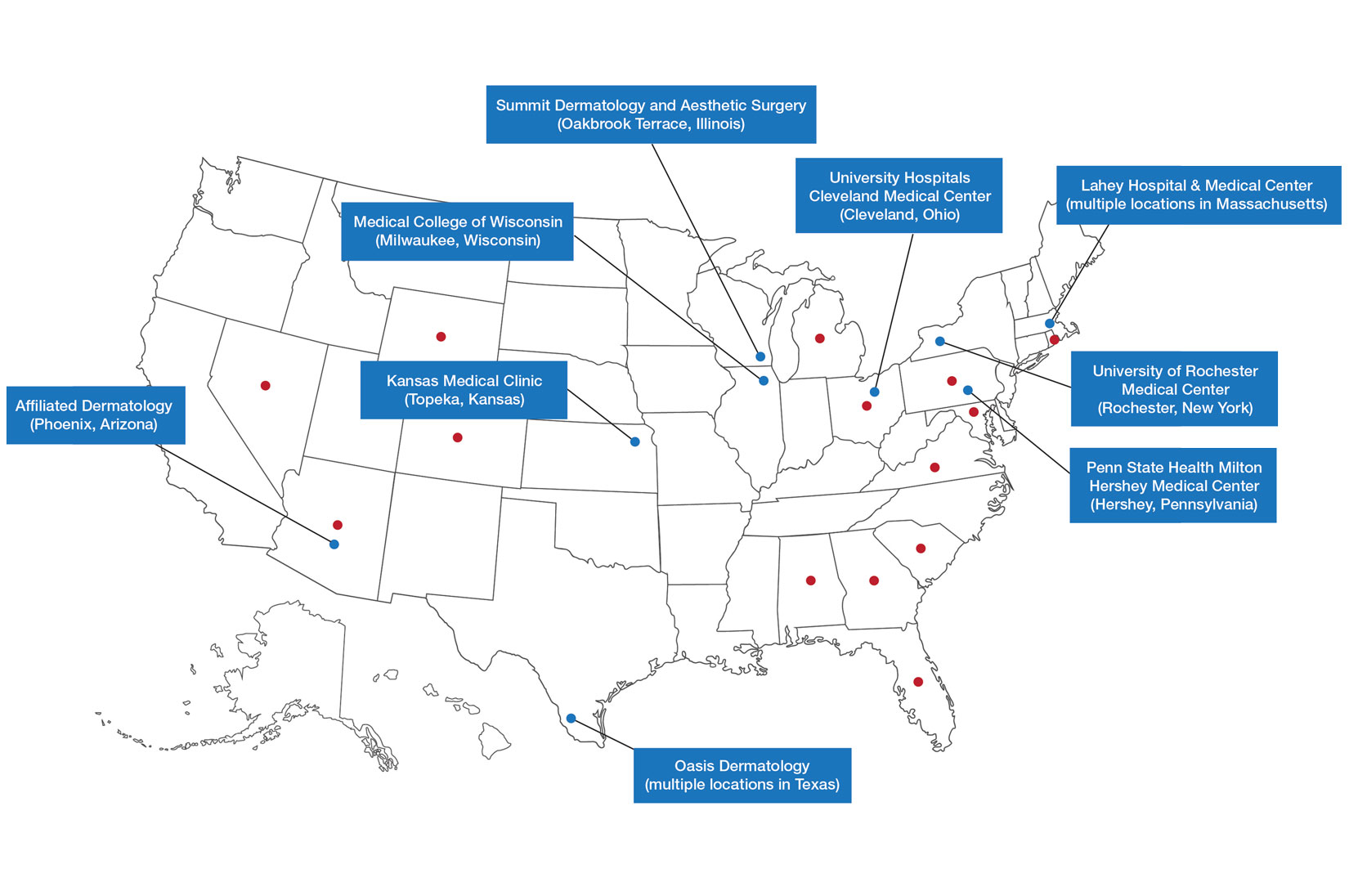
Ten academic and private practice organizations across the United States that offer postgraduate dermatologic training programs for APPs were identified (eTable). Four (40%) programs were advertised as fellowships. Six (60%) of the programs were offered at academic medical centers, and 4 (40%) were offered by private practices. Most programs were located east of the Mississippi River, and many institutions offered instruction at 1 or more locations within the same state (eFigure). The Advanced Dermatology and Cosmetic Surgery private practice group offered training opportunities in multiple states.
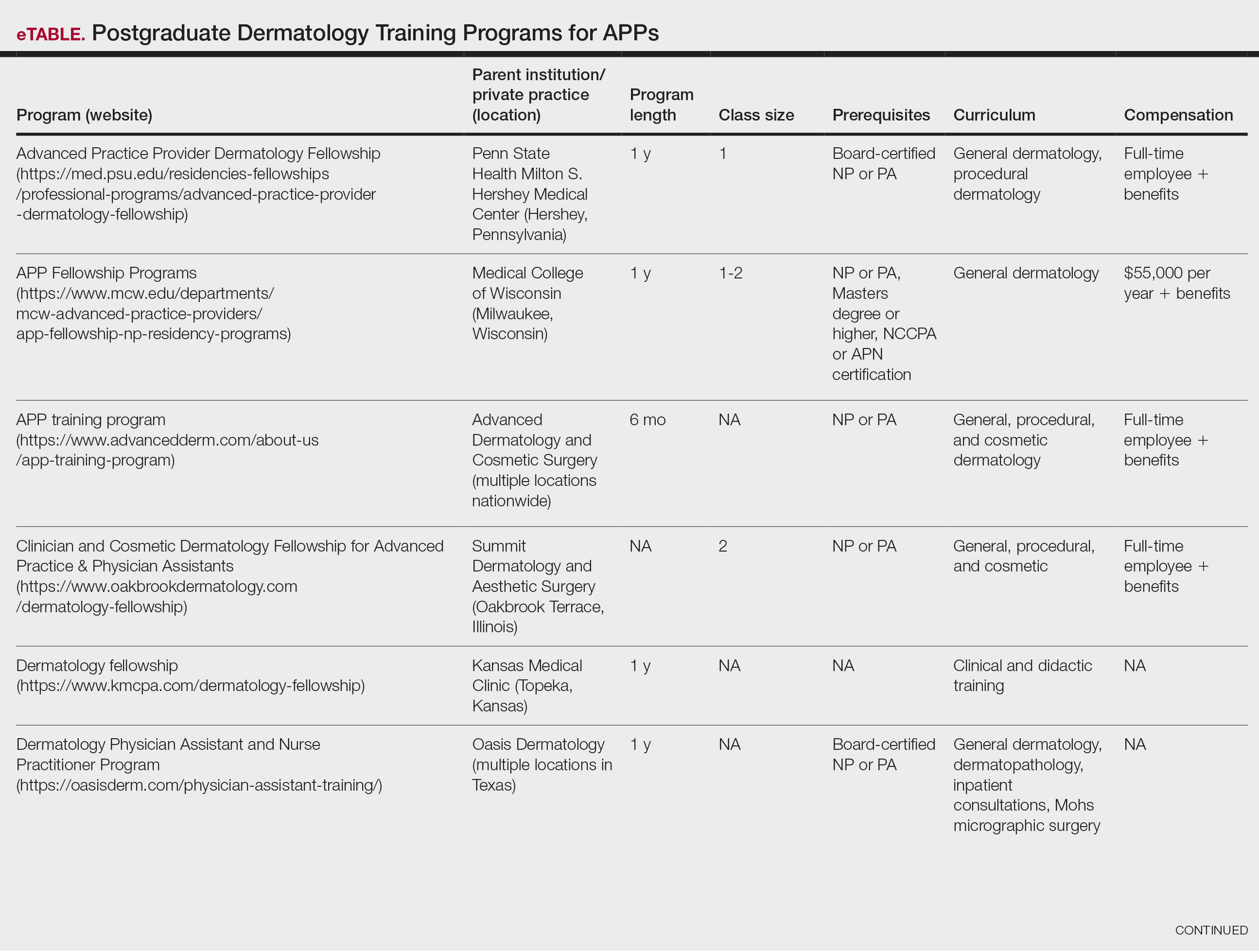
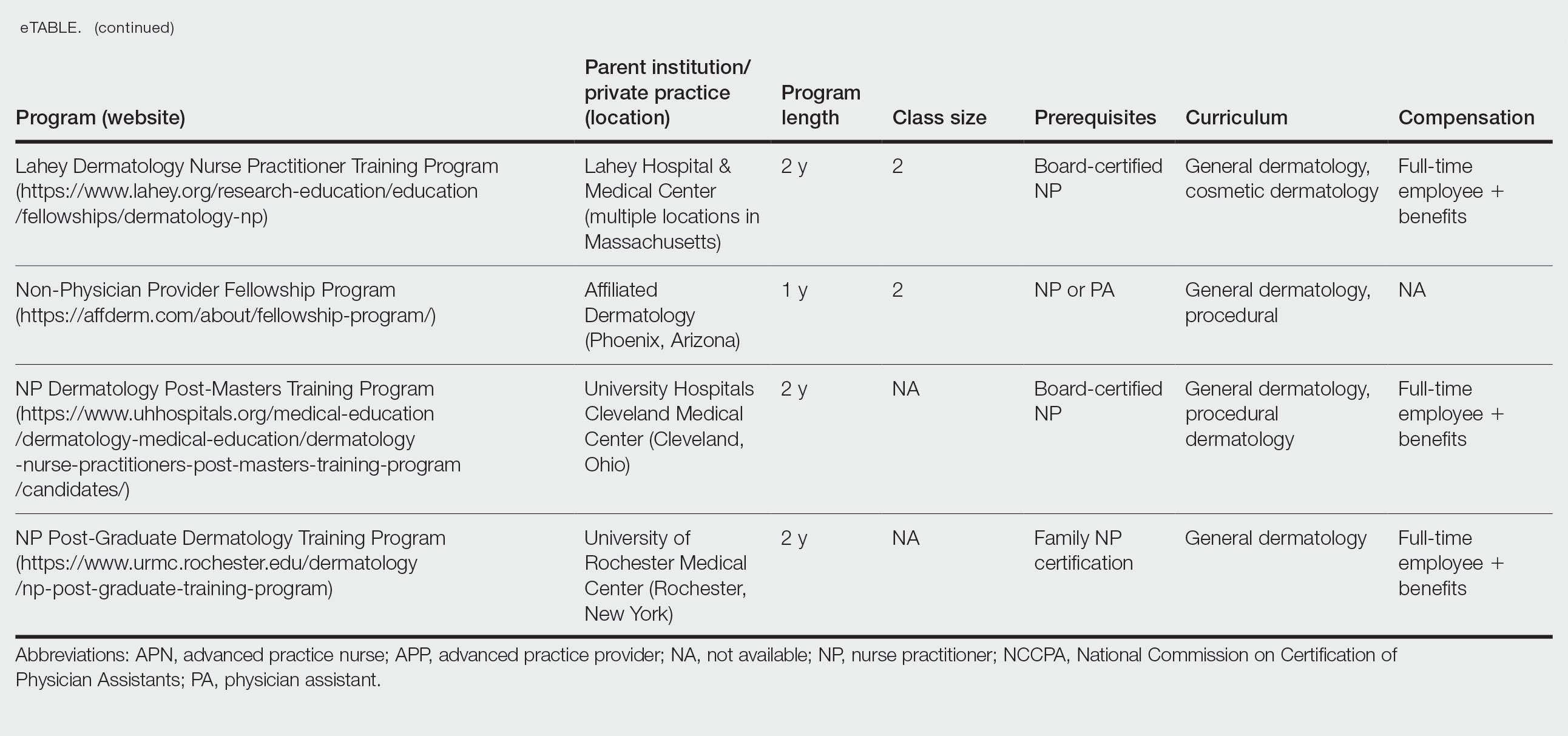
Six programs required APPs to become board-certified NPs or PAs prior to enrolling. Most programs enrolled both NPs and PAs, while some only enrolled NPs (eTable). Only 1 (10%) program required NPs to be board certified as a family NP, while another (10%) recommended that applicants have experience in urgent care, emergency medicine, or trauma medicine. Lahey Hospital & Medical Center required experience as an NP in a general setting for 1 to 2 years prior to applying. No program required prior experience in the field of dermatology.
Program length varied from 6 to 24 months, and cohort size typically was limited to 1 to 2 providers (eTable). Although the exact numbers could not be ascertained, most curricula focused on medical dermatology, including clinical and didactic components, but many offered electives such as cosmetic and procedural dermatology. Two institutions (20%) required independent research. Work typically was limited to 40 hours per week, and most paid a full-time employee salary and provided benefits such as health insurance, retirement, and paid leave (eTable). Kansas Medical Clinic (Topeka, Kansas) required at least 3 years of employment in an underserved community following program completion. The Oasis Dermatology private practice group in Texas required a 1-year teaching commitment after program completion. The Advanced Dermatology and Cosmetic Surgery group offered a full-time position upon program completion.
Comment
There is a large difference in the total number of training and credentialing hours when comparing graduate school training and postgraduate credentialing of medical and osteopathic physicians compared with APPs. A new graduate physician has at least twice as many clinical hours as a PA and 10 times as many clinical hours as an NP prior to starting residency. Physicians also typically complete at least 6 times the number of hours of certification examinations compared to NPs and PAs.1
Nurse practitioner students typically complete the 500 hours of prelicensure clinical training required for NP school in 2 to 4 years.2,3 The amount of time required for completion is dependent on the degree and experience of the student upon program entry (eg, bachelor of science in nursing vs master of science in nursing as a terminal degree). Physician assistant students are required to complete 2000 prelicensure clinical hours, and most PA programs are 3 years in duration.4 Many NP and PA programs require some degree of clinical experience prior to beginning graduate education.5
When comparing prelicensure examinations, questions assessing dermatologic knowledge comprise approximately 6% to 10% of the total questions on the United States Medical Licensing Examination Steps 1 and 2.6 The Comprehensive Osteopathic Medical Licensing Examination of the United States Level 1 and Level 2-Cognitive Evaluation both have at least 5% of questions dedicated to dermatology.7 Approximately 5% of the questions on the Physician Assistant National Certifying Examination are dedicated to dermatology.8 The dermatology content on either of the NP certification examinations is unclear.2,3 In the states of California, Indiana, and New York, national certification through the American Association of Nurse Practitioners or American Nurses Credentialing Center is not required for NPs to practice in their respective states.9
Regarding dermatologic board certification, a new graduate NP may obtain certification from the
Many of the programs we evaluated integrate APP trainees into resident education, allowing participation in equivalent didactic curricula, clinical rotations, and departmental academic activities. The salary and benefits associated with these programs are somewhat like those of resident physicians.15,16 While most tuition-based programs were excluded from our study due to their lack of credibility and alignment with our study criteria, we identified 2 specific programs that stood out as credible despite requiring students to pay tuition. These programs demonstrated a structured and rigorous curriculum with a clear focus on comprehensive dermatologic training, meeting our standards for inclusion. These programs offer dermatologic training for graduates of NP and PA programs at a cost to the student.15,16 The program at the Florida Atlantic University, Boca Raton, is largely online,15 and the program at the University of Miami, Florida, offers no direct clinical contact.16 These programs illustrate the variety of postgraduate dermatology curricula available nationally in comparison to resident salaries; however, they were not included in our formal analysis because they do not provide structured, in-person clinical training consistent with our inclusion criteria. Neither of these programs would enable participants to qualify for credentialing with the Dermatology Nurse Practitioner Certification Board after completion. While this study identified postgraduate training programs for APPs in dermatology advertised online, it is possible some were omitted or not advertised online.
While many of the postgraduate programs we evaluated provide unique educational opportunities for APPs, it is unknown if graduating providers are equipped to handle the care of patients with complex dermatologic needs. Regardless, the increased utilization of APPs by BCDs has been well documented over the past 2 decades.17-20 It has been suggested that a higher ratio of APPs to dermatologists can decrease the time it takes for a patient to be seen in a clinic.21-23 However, investigators have expressed concerns that APPs lack standardized surgical training and clinical hour requirements in the field of dermatology.24 Despite these concerns, Medicare claims data show that APPs are performing advanced surgical and cosmetic procedures at increasing rates.17,18 Other authors have questioned the cost-effectiveness of APPs, as multiple studies have shown that the number of biopsies needed to diagnose 1 case of skin cancer is higher for midlevel providers than for dermatologists.25-27
Conclusion
With the anticipated expansion of private equity in dermatology and the growth of our Medicare-eligible population, we are likely to see increased utilization of APPs to address the shortage of BCDs.28,29 Understanding the prelicensure and postlicensure clinical training requirements, examination hours, and extent of dermatology-focused education among APPs and BCDs can help dermatologists collaborate more effectively and ensure safe, high-quality patient care. Standardizing, improving, and providing high-quality education and promoting lifelong learning in the field of dermatology should be celebrated, and dermatologists are the skin experts best equipped to lead dermatologic education forward.
- Robeznieks A. Training gaps between physicians, nonphysicians are significant. American Medical Association. February 17, 2025. Accessed October 23, 2025. https://www.ama-assn.org/practice-management/scope-practice/training-gaps-between-physicians-nonphysicians-are-significant
- American Nurses Credentialing Center. Test content outline. Accessed October 6, 2025. https://www.nursingworld.org/globalassets/08282024-exam-24-npd-tco-website.pdf
- American Academy of Nurse Practitioners National Certification Board. AANPCB Family Nurse Practitioner Adult-Gerontology Primary Care Nurse Practitioner Psychiatric Mental Health Pratitioner: FNP, AGNP & PMHNP Certification Certification Handbook. American Academy of Nurse Practitioners Certification Board; 2023. Accessed October 6, 2025. https://www.aanpcert.org/resource/documents/AGNP%20FNP%20Candidate%20Handbook.pdf
- Society of Dermatology Physician Associates. SDPA Diplomate Fellowship. Accessed October 6, 2025. https://learning.dermpa.orgdiplomate-fellowship
- American Academy of Physician Associates. Become a PA. Accessed October 6, 2025. https://www.aapa.org/career-central/become-a-pa/
- United States Medical Licensing Examination. Prepare for your exam. Accessed October 6, 2025. https://www.usmle.org/prepare-your-exam
- National Board of Osteopathic Medical Examiners. Patient presentations related to the integumentary system. Accessed October 6, 2025. https://www.nbome.org/assessments/comlex-usa/comlex-usa-blueprint/d2-clinical-presentations/integumentary-system
- National Commission on Certification of Physician Assistants. PANCE content blueprint. Accessed October 6, 2025. https://prodcmsstoragesa.blob.core.windows.net/uploads/files/PANCEBlueprint.pdf
- American Association of Nurse Practitioners. Practice information by state. Accessed October 6, 2025. https://www.aanp.org/practice/practice-information-by-state
- Dermatology Nurse Practitioner Certification Board. Eligibility. Accessed October 6, 2025. https://www.dnpcb.org/eligibility.php
- National Board of Dermatology Physician Assistants. Certification. Accessed September 3, 2022.
- Society of Dermatology Physician Associates. SDPA statement regarding the ABDPA Board Certification Exam for derm PAs. October 8, 2019. Accessed October 6, 2025. https://www.dermpa.org/news/articles/2019-10/sdpa-statement-regarding-abdpa-board-certification-exam-derm-pas
- American Board of Dermatology. Residents and fellows. Accessed October 6, 2025. https://www.abderm.org/residents-and-fellows
- American Osteopathic Board of Dermatology. Primary certificaiton exam. Accessed October 6, 2025. https://certification.osteopathic.org/dermatology/certification-process/dermatology/written-exams/
- Florida Atlantic University. Christine E. Lynn College of Nursing. Dermatology nurse practitioner certificate program. Accessed October 6, 2025. https://www.fau.edu/nursing/academics/certificates/dermatology-program/
- Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery. Advanced Practitioner Program.
- Coldiron B, Ratnarathorn M. Scope of physician procedures independently billed by mid-level providers in the office setting. JAMA Dermatol. 2014;150:1153-1159.
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Resneck J Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Braun RT, Bond AM, Qian Y, et al. Private equity in dermatology: effect on price, utilization, and spending. Health Aff (Millwood). 2021;40:727-735.
- Skaljic M, Lipoff JB. Association of private equity ownership with increased employment of advanced practice professionals in outpatient dermatology offices. J Am Acad Dermatol. 2021;84:1178-1180.
- Jalian HR, Avram MM. Mid-level practitioners in dermatology: a need for further study and oversight. JAMA Dermatol. 2014;150:1149-1151.
- Sarzynski E, Barry H. Current evidence and controversies: advanced practice providers in healthcare. Am J Manag Care. 2019;25:366-368.
- Nault A, Zhang C, Kim K, et al. Biopsy use in skin cancer diagnosis: comparing dermatology physicians and advanced practice professionals. JAMA Dermatol. 2015;151:899-902.
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis by physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573.
- Sung C, Salem S, Oulee A, et al. A systematic review: landscape of private equity in dermatology from past to present. J Drugs Dermatol. 2023 Apr 1;22:404-409. doi: 10.36849/JDD.6892.
- CMS releases National Healthcare Expenditure and enrollment projections through 2031. Health Management Associates. July 13, 2023. Accessed October 23, 2025. https://www.healthmanagement.com/blog/cms-releases-national-healthcare-expenditure-and-enrollment-projections-through-2031/
Nurse practitioners (NPs) and physician assistants (PAs) often help provide dermatologic care but lack the same mandatory specialized postgraduate training required of board-certified dermatologists (BCDs), which includes at least 3 years of dermatology-focused education in an accredited residency program in addition to an intern year of general medicine, pediatrics, or surgery. Dermatology residency is followed by a certification examination administered by the American Board of Dermatology (ABD) or the American Osteopathic Board of Dermatology, leading to board certification. Some physicians choose to do a fellowship, which typically involves an additional 1 to 2 years of postresidency subspeciality training.
Optional postgraduate dermatology training programs for advanced practice providers (APPs) have been offered by some academic institutions and private practice groups since at least 2003, including Lahey Hospital and Medical Center (Burlington, Massachusetts) as well as the University of Rochester Medical Center (Rochester, New York). Despite a lack of accreditation or standardization, the programs can be beneficial for NPs and PAs to expand their dermatologic knowledge and skills and help bridge the care gap within the specialty. Didactics often are conducted in parallel with the educational activities of the parent institution’s traditional dermatology residency program (eg, lectures, grand rounds). While these programs often are managed by practicing dermatology NPs and PAs, dermatologists also may be involved in their education with didactic instruction, curriculum development, and clinical preceptorship.
In this cross-sectional study, we identified and evaluated 10 postgraduate dermatology training programs for APPs across the United States. With the growing number of NPs and PAs in the dermatology workforce—both in academic and private practice—it is important for BCDs to be aware of the differences in the dermatology training received in order to ensure safe and effective care is provided through supervisory or collaborative roles (depending on state independent practice laws for APPs and to be aware of the implications these programs may have on the field of dermatology.
Methods
To identify postgraduate dermatology training programs for APPs in the United States, we conducted a cross-sectional study using data obtained via a Google search of various combinations of the following terms: nurse practitioner, NP, physician assistant, PA, advance practice provider, APP, dermatology, postgraduate training, residency, and fellowship. We excluded postgraduate dermatology training programs for APPs that required tuition and did not provide a stipend, as well as programs that lacked the formal structure and credibility needed to qualify as legitimate postgraduate training. Many of the excluded programs operate in a manner that raises ethical concerns, offering pay-to-play opportunities under the guise of education. Information collected on each program included the program name, location, parent institution, program length, class size, curriculum, and any associated salary and benefits.
Results
Ten academic and private practice organizations across the United States that offer postgraduate dermatologic training programs for APPs were identified (eTable). Four (40%) programs were advertised as fellowships. Six (60%) of the programs were offered at academic medical centers, and 4 (40%) were offered by private practices. Most programs were located east of the Mississippi River, and many institutions offered instruction at 1 or more locations within the same state (eFigure). The Advanced Dermatology and Cosmetic Surgery private practice group offered training opportunities in multiple states.



Six programs required APPs to become board-certified NPs or PAs prior to enrolling. Most programs enrolled both NPs and PAs, while some only enrolled NPs (eTable). Only 1 (10%) program required NPs to be board certified as a family NP, while another (10%) recommended that applicants have experience in urgent care, emergency medicine, or trauma medicine. Lahey Hospital & Medical Center required experience as an NP in a general setting for 1 to 2 years prior to applying. No program required prior experience in the field of dermatology.
Program length varied from 6 to 24 months, and cohort size typically was limited to 1 to 2 providers (eTable). Although the exact numbers could not be ascertained, most curricula focused on medical dermatology, including clinical and didactic components, but many offered electives such as cosmetic and procedural dermatology. Two institutions (20%) required independent research. Work typically was limited to 40 hours per week, and most paid a full-time employee salary and provided benefits such as health insurance, retirement, and paid leave (eTable). Kansas Medical Clinic (Topeka, Kansas) required at least 3 years of employment in an underserved community following program completion. The Oasis Dermatology private practice group in Texas required a 1-year teaching commitment after program completion. The Advanced Dermatology and Cosmetic Surgery group offered a full-time position upon program completion.
Comment
There is a large difference in the total number of training and credentialing hours when comparing graduate school training and postgraduate credentialing of medical and osteopathic physicians compared with APPs. A new graduate physician has at least twice as many clinical hours as a PA and 10 times as many clinical hours as an NP prior to starting residency. Physicians also typically complete at least 6 times the number of hours of certification examinations compared to NPs and PAs.1
Nurse practitioner students typically complete the 500 hours of prelicensure clinical training required for NP school in 2 to 4 years.2,3 The amount of time required for completion is dependent on the degree and experience of the student upon program entry (eg, bachelor of science in nursing vs master of science in nursing as a terminal degree). Physician assistant students are required to complete 2000 prelicensure clinical hours, and most PA programs are 3 years in duration.4 Many NP and PA programs require some degree of clinical experience prior to beginning graduate education.5
When comparing prelicensure examinations, questions assessing dermatologic knowledge comprise approximately 6% to 10% of the total questions on the United States Medical Licensing Examination Steps 1 and 2.6 The Comprehensive Osteopathic Medical Licensing Examination of the United States Level 1 and Level 2-Cognitive Evaluation both have at least 5% of questions dedicated to dermatology.7 Approximately 5% of the questions on the Physician Assistant National Certifying Examination are dedicated to dermatology.8 The dermatology content on either of the NP certification examinations is unclear.2,3 In the states of California, Indiana, and New York, national certification through the American Association of Nurse Practitioners or American Nurses Credentialing Center is not required for NPs to practice in their respective states.9
Regarding dermatologic board certification, a new graduate NP may obtain certification from the
Many of the programs we evaluated integrate APP trainees into resident education, allowing participation in equivalent didactic curricula, clinical rotations, and departmental academic activities. The salary and benefits associated with these programs are somewhat like those of resident physicians.15,16 While most tuition-based programs were excluded from our study due to their lack of credibility and alignment with our study criteria, we identified 2 specific programs that stood out as credible despite requiring students to pay tuition. These programs demonstrated a structured and rigorous curriculum with a clear focus on comprehensive dermatologic training, meeting our standards for inclusion. These programs offer dermatologic training for graduates of NP and PA programs at a cost to the student.15,16 The program at the Florida Atlantic University, Boca Raton, is largely online,15 and the program at the University of Miami, Florida, offers no direct clinical contact.16 These programs illustrate the variety of postgraduate dermatology curricula available nationally in comparison to resident salaries; however, they were not included in our formal analysis because they do not provide structured, in-person clinical training consistent with our inclusion criteria. Neither of these programs would enable participants to qualify for credentialing with the Dermatology Nurse Practitioner Certification Board after completion. While this study identified postgraduate training programs for APPs in dermatology advertised online, it is possible some were omitted or not advertised online.
While many of the postgraduate programs we evaluated provide unique educational opportunities for APPs, it is unknown if graduating providers are equipped to handle the care of patients with complex dermatologic needs. Regardless, the increased utilization of APPs by BCDs has been well documented over the past 2 decades.17-20 It has been suggested that a higher ratio of APPs to dermatologists can decrease the time it takes for a patient to be seen in a clinic.21-23 However, investigators have expressed concerns that APPs lack standardized surgical training and clinical hour requirements in the field of dermatology.24 Despite these concerns, Medicare claims data show that APPs are performing advanced surgical and cosmetic procedures at increasing rates.17,18 Other authors have questioned the cost-effectiveness of APPs, as multiple studies have shown that the number of biopsies needed to diagnose 1 case of skin cancer is higher for midlevel providers than for dermatologists.25-27
Conclusion
With the anticipated expansion of private equity in dermatology and the growth of our Medicare-eligible population, we are likely to see increased utilization of APPs to address the shortage of BCDs.28,29 Understanding the prelicensure and postlicensure clinical training requirements, examination hours, and extent of dermatology-focused education among APPs and BCDs can help dermatologists collaborate more effectively and ensure safe, high-quality patient care. Standardizing, improving, and providing high-quality education and promoting lifelong learning in the field of dermatology should be celebrated, and dermatologists are the skin experts best equipped to lead dermatologic education forward.
Nurse practitioners (NPs) and physician assistants (PAs) often help provide dermatologic care but lack the same mandatory specialized postgraduate training required of board-certified dermatologists (BCDs), which includes at least 3 years of dermatology-focused education in an accredited residency program in addition to an intern year of general medicine, pediatrics, or surgery. Dermatology residency is followed by a certification examination administered by the American Board of Dermatology (ABD) or the American Osteopathic Board of Dermatology, leading to board certification. Some physicians choose to do a fellowship, which typically involves an additional 1 to 2 years of postresidency subspeciality training.
Optional postgraduate dermatology training programs for advanced practice providers (APPs) have been offered by some academic institutions and private practice groups since at least 2003, including Lahey Hospital and Medical Center (Burlington, Massachusetts) as well as the University of Rochester Medical Center (Rochester, New York). Despite a lack of accreditation or standardization, the programs can be beneficial for NPs and PAs to expand their dermatologic knowledge and skills and help bridge the care gap within the specialty. Didactics often are conducted in parallel with the educational activities of the parent institution’s traditional dermatology residency program (eg, lectures, grand rounds). While these programs often are managed by practicing dermatology NPs and PAs, dermatologists also may be involved in their education with didactic instruction, curriculum development, and clinical preceptorship.
In this cross-sectional study, we identified and evaluated 10 postgraduate dermatology training programs for APPs across the United States. With the growing number of NPs and PAs in the dermatology workforce—both in academic and private practice—it is important for BCDs to be aware of the differences in the dermatology training received in order to ensure safe and effective care is provided through supervisory or collaborative roles (depending on state independent practice laws for APPs and to be aware of the implications these programs may have on the field of dermatology.
Methods
To identify postgraduate dermatology training programs for APPs in the United States, we conducted a cross-sectional study using data obtained via a Google search of various combinations of the following terms: nurse practitioner, NP, physician assistant, PA, advance practice provider, APP, dermatology, postgraduate training, residency, and fellowship. We excluded postgraduate dermatology training programs for APPs that required tuition and did not provide a stipend, as well as programs that lacked the formal structure and credibility needed to qualify as legitimate postgraduate training. Many of the excluded programs operate in a manner that raises ethical concerns, offering pay-to-play opportunities under the guise of education. Information collected on each program included the program name, location, parent institution, program length, class size, curriculum, and any associated salary and benefits.
Results
Ten academic and private practice organizations across the United States that offer postgraduate dermatologic training programs for APPs were identified (eTable). Four (40%) programs were advertised as fellowships. Six (60%) of the programs were offered at academic medical centers, and 4 (40%) were offered by private practices. Most programs were located east of the Mississippi River, and many institutions offered instruction at 1 or more locations within the same state (eFigure). The Advanced Dermatology and Cosmetic Surgery private practice group offered training opportunities in multiple states.



Six programs required APPs to become board-certified NPs or PAs prior to enrolling. Most programs enrolled both NPs and PAs, while some only enrolled NPs (eTable). Only 1 (10%) program required NPs to be board certified as a family NP, while another (10%) recommended that applicants have experience in urgent care, emergency medicine, or trauma medicine. Lahey Hospital & Medical Center required experience as an NP in a general setting for 1 to 2 years prior to applying. No program required prior experience in the field of dermatology.
Program length varied from 6 to 24 months, and cohort size typically was limited to 1 to 2 providers (eTable). Although the exact numbers could not be ascertained, most curricula focused on medical dermatology, including clinical and didactic components, but many offered electives such as cosmetic and procedural dermatology. Two institutions (20%) required independent research. Work typically was limited to 40 hours per week, and most paid a full-time employee salary and provided benefits such as health insurance, retirement, and paid leave (eTable). Kansas Medical Clinic (Topeka, Kansas) required at least 3 years of employment in an underserved community following program completion. The Oasis Dermatology private practice group in Texas required a 1-year teaching commitment after program completion. The Advanced Dermatology and Cosmetic Surgery group offered a full-time position upon program completion.
Comment
There is a large difference in the total number of training and credentialing hours when comparing graduate school training and postgraduate credentialing of medical and osteopathic physicians compared with APPs. A new graduate physician has at least twice as many clinical hours as a PA and 10 times as many clinical hours as an NP prior to starting residency. Physicians also typically complete at least 6 times the number of hours of certification examinations compared to NPs and PAs.1
Nurse practitioner students typically complete the 500 hours of prelicensure clinical training required for NP school in 2 to 4 years.2,3 The amount of time required for completion is dependent on the degree and experience of the student upon program entry (eg, bachelor of science in nursing vs master of science in nursing as a terminal degree). Physician assistant students are required to complete 2000 prelicensure clinical hours, and most PA programs are 3 years in duration.4 Many NP and PA programs require some degree of clinical experience prior to beginning graduate education.5
When comparing prelicensure examinations, questions assessing dermatologic knowledge comprise approximately 6% to 10% of the total questions on the United States Medical Licensing Examination Steps 1 and 2.6 The Comprehensive Osteopathic Medical Licensing Examination of the United States Level 1 and Level 2-Cognitive Evaluation both have at least 5% of questions dedicated to dermatology.7 Approximately 5% of the questions on the Physician Assistant National Certifying Examination are dedicated to dermatology.8 The dermatology content on either of the NP certification examinations is unclear.2,3 In the states of California, Indiana, and New York, national certification through the American Association of Nurse Practitioners or American Nurses Credentialing Center is not required for NPs to practice in their respective states.9
Regarding dermatologic board certification, a new graduate NP may obtain certification from the
Many of the programs we evaluated integrate APP trainees into resident education, allowing participation in equivalent didactic curricula, clinical rotations, and departmental academic activities. The salary and benefits associated with these programs are somewhat like those of resident physicians.15,16 While most tuition-based programs were excluded from our study due to their lack of credibility and alignment with our study criteria, we identified 2 specific programs that stood out as credible despite requiring students to pay tuition. These programs demonstrated a structured and rigorous curriculum with a clear focus on comprehensive dermatologic training, meeting our standards for inclusion. These programs offer dermatologic training for graduates of NP and PA programs at a cost to the student.15,16 The program at the Florida Atlantic University, Boca Raton, is largely online,15 and the program at the University of Miami, Florida, offers no direct clinical contact.16 These programs illustrate the variety of postgraduate dermatology curricula available nationally in comparison to resident salaries; however, they were not included in our formal analysis because they do not provide structured, in-person clinical training consistent with our inclusion criteria. Neither of these programs would enable participants to qualify for credentialing with the Dermatology Nurse Practitioner Certification Board after completion. While this study identified postgraduate training programs for APPs in dermatology advertised online, it is possible some were omitted or not advertised online.
While many of the postgraduate programs we evaluated provide unique educational opportunities for APPs, it is unknown if graduating providers are equipped to handle the care of patients with complex dermatologic needs. Regardless, the increased utilization of APPs by BCDs has been well documented over the past 2 decades.17-20 It has been suggested that a higher ratio of APPs to dermatologists can decrease the time it takes for a patient to be seen in a clinic.21-23 However, investigators have expressed concerns that APPs lack standardized surgical training and clinical hour requirements in the field of dermatology.24 Despite these concerns, Medicare claims data show that APPs are performing advanced surgical and cosmetic procedures at increasing rates.17,18 Other authors have questioned the cost-effectiveness of APPs, as multiple studies have shown that the number of biopsies needed to diagnose 1 case of skin cancer is higher for midlevel providers than for dermatologists.25-27
Conclusion
With the anticipated expansion of private equity in dermatology and the growth of our Medicare-eligible population, we are likely to see increased utilization of APPs to address the shortage of BCDs.28,29 Understanding the prelicensure and postlicensure clinical training requirements, examination hours, and extent of dermatology-focused education among APPs and BCDs can help dermatologists collaborate more effectively and ensure safe, high-quality patient care. Standardizing, improving, and providing high-quality education and promoting lifelong learning in the field of dermatology should be celebrated, and dermatologists are the skin experts best equipped to lead dermatologic education forward.
- Robeznieks A. Training gaps between physicians, nonphysicians are significant. American Medical Association. February 17, 2025. Accessed October 23, 2025. https://www.ama-assn.org/practice-management/scope-practice/training-gaps-between-physicians-nonphysicians-are-significant
- American Nurses Credentialing Center. Test content outline. Accessed October 6, 2025. https://www.nursingworld.org/globalassets/08282024-exam-24-npd-tco-website.pdf
- American Academy of Nurse Practitioners National Certification Board. AANPCB Family Nurse Practitioner Adult-Gerontology Primary Care Nurse Practitioner Psychiatric Mental Health Pratitioner: FNP, AGNP & PMHNP Certification Certification Handbook. American Academy of Nurse Practitioners Certification Board; 2023. Accessed October 6, 2025. https://www.aanpcert.org/resource/documents/AGNP%20FNP%20Candidate%20Handbook.pdf
- Society of Dermatology Physician Associates. SDPA Diplomate Fellowship. Accessed October 6, 2025. https://learning.dermpa.orgdiplomate-fellowship
- American Academy of Physician Associates. Become a PA. Accessed October 6, 2025. https://www.aapa.org/career-central/become-a-pa/
- United States Medical Licensing Examination. Prepare for your exam. Accessed October 6, 2025. https://www.usmle.org/prepare-your-exam
- National Board of Osteopathic Medical Examiners. Patient presentations related to the integumentary system. Accessed October 6, 2025. https://www.nbome.org/assessments/comlex-usa/comlex-usa-blueprint/d2-clinical-presentations/integumentary-system
- National Commission on Certification of Physician Assistants. PANCE content blueprint. Accessed October 6, 2025. https://prodcmsstoragesa.blob.core.windows.net/uploads/files/PANCEBlueprint.pdf
- American Association of Nurse Practitioners. Practice information by state. Accessed October 6, 2025. https://www.aanp.org/practice/practice-information-by-state
- Dermatology Nurse Practitioner Certification Board. Eligibility. Accessed October 6, 2025. https://www.dnpcb.org/eligibility.php
- National Board of Dermatology Physician Assistants. Certification. Accessed September 3, 2022.
- Society of Dermatology Physician Associates. SDPA statement regarding the ABDPA Board Certification Exam for derm PAs. October 8, 2019. Accessed October 6, 2025. https://www.dermpa.org/news/articles/2019-10/sdpa-statement-regarding-abdpa-board-certification-exam-derm-pas
- American Board of Dermatology. Residents and fellows. Accessed October 6, 2025. https://www.abderm.org/residents-and-fellows
- American Osteopathic Board of Dermatology. Primary certificaiton exam. Accessed October 6, 2025. https://certification.osteopathic.org/dermatology/certification-process/dermatology/written-exams/
- Florida Atlantic University. Christine E. Lynn College of Nursing. Dermatology nurse practitioner certificate program. Accessed October 6, 2025. https://www.fau.edu/nursing/academics/certificates/dermatology-program/
- Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery. Advanced Practitioner Program.
- Coldiron B, Ratnarathorn M. Scope of physician procedures independently billed by mid-level providers in the office setting. JAMA Dermatol. 2014;150:1153-1159.
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Resneck J Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Braun RT, Bond AM, Qian Y, et al. Private equity in dermatology: effect on price, utilization, and spending. Health Aff (Millwood). 2021;40:727-735.
- Skaljic M, Lipoff JB. Association of private equity ownership with increased employment of advanced practice professionals in outpatient dermatology offices. J Am Acad Dermatol. 2021;84:1178-1180.
- Jalian HR, Avram MM. Mid-level practitioners in dermatology: a need for further study and oversight. JAMA Dermatol. 2014;150:1149-1151.
- Sarzynski E, Barry H. Current evidence and controversies: advanced practice providers in healthcare. Am J Manag Care. 2019;25:366-368.
- Nault A, Zhang C, Kim K, et al. Biopsy use in skin cancer diagnosis: comparing dermatology physicians and advanced practice professionals. JAMA Dermatol. 2015;151:899-902.
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis by physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573.
- Sung C, Salem S, Oulee A, et al. A systematic review: landscape of private equity in dermatology from past to present. J Drugs Dermatol. 2023 Apr 1;22:404-409. doi: 10.36849/JDD.6892.
- CMS releases National Healthcare Expenditure and enrollment projections through 2031. Health Management Associates. July 13, 2023. Accessed October 23, 2025. https://www.healthmanagement.com/blog/cms-releases-national-healthcare-expenditure-and-enrollment-projections-through-2031/
- Robeznieks A. Training gaps between physicians, nonphysicians are significant. American Medical Association. February 17, 2025. Accessed October 23, 2025. https://www.ama-assn.org/practice-management/scope-practice/training-gaps-between-physicians-nonphysicians-are-significant
- American Nurses Credentialing Center. Test content outline. Accessed October 6, 2025. https://www.nursingworld.org/globalassets/08282024-exam-24-npd-tco-website.pdf
- American Academy of Nurse Practitioners National Certification Board. AANPCB Family Nurse Practitioner Adult-Gerontology Primary Care Nurse Practitioner Psychiatric Mental Health Pratitioner: FNP, AGNP & PMHNP Certification Certification Handbook. American Academy of Nurse Practitioners Certification Board; 2023. Accessed October 6, 2025. https://www.aanpcert.org/resource/documents/AGNP%20FNP%20Candidate%20Handbook.pdf
- Society of Dermatology Physician Associates. SDPA Diplomate Fellowship. Accessed October 6, 2025. https://learning.dermpa.orgdiplomate-fellowship
- American Academy of Physician Associates. Become a PA. Accessed October 6, 2025. https://www.aapa.org/career-central/become-a-pa/
- United States Medical Licensing Examination. Prepare for your exam. Accessed October 6, 2025. https://www.usmle.org/prepare-your-exam
- National Board of Osteopathic Medical Examiners. Patient presentations related to the integumentary system. Accessed October 6, 2025. https://www.nbome.org/assessments/comlex-usa/comlex-usa-blueprint/d2-clinical-presentations/integumentary-system
- National Commission on Certification of Physician Assistants. PANCE content blueprint. Accessed October 6, 2025. https://prodcmsstoragesa.blob.core.windows.net/uploads/files/PANCEBlueprint.pdf
- American Association of Nurse Practitioners. Practice information by state. Accessed October 6, 2025. https://www.aanp.org/practice/practice-information-by-state
- Dermatology Nurse Practitioner Certification Board. Eligibility. Accessed October 6, 2025. https://www.dnpcb.org/eligibility.php
- National Board of Dermatology Physician Assistants. Certification. Accessed September 3, 2022.
- Society of Dermatology Physician Associates. SDPA statement regarding the ABDPA Board Certification Exam for derm PAs. October 8, 2019. Accessed October 6, 2025. https://www.dermpa.org/news/articles/2019-10/sdpa-statement-regarding-abdpa-board-certification-exam-derm-pas
- American Board of Dermatology. Residents and fellows. Accessed October 6, 2025. https://www.abderm.org/residents-and-fellows
- American Osteopathic Board of Dermatology. Primary certificaiton exam. Accessed October 6, 2025. https://certification.osteopathic.org/dermatology/certification-process/dermatology/written-exams/
- Florida Atlantic University. Christine E. Lynn College of Nursing. Dermatology nurse practitioner certificate program. Accessed October 6, 2025. https://www.fau.edu/nursing/academics/certificates/dermatology-program/
- Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery. Advanced Practitioner Program.
- Coldiron B, Ratnarathorn M. Scope of physician procedures independently billed by mid-level providers in the office setting. JAMA Dermatol. 2014;150:1153-1159.
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Resneck J Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Braun RT, Bond AM, Qian Y, et al. Private equity in dermatology: effect on price, utilization, and spending. Health Aff (Millwood). 2021;40:727-735.
- Skaljic M, Lipoff JB. Association of private equity ownership with increased employment of advanced practice professionals in outpatient dermatology offices. J Am Acad Dermatol. 2021;84:1178-1180.
- Jalian HR, Avram MM. Mid-level practitioners in dermatology: a need for further study and oversight. JAMA Dermatol. 2014;150:1149-1151.
- Sarzynski E, Barry H. Current evidence and controversies: advanced practice providers in healthcare. Am J Manag Care. 2019;25:366-368.
- Nault A, Zhang C, Kim K, et al. Biopsy use in skin cancer diagnosis: comparing dermatology physicians and advanced practice professionals. JAMA Dermatol. 2015;151:899-902.
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis by physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573.
- Sung C, Salem S, Oulee A, et al. A systematic review: landscape of private equity in dermatology from past to present. J Drugs Dermatol. 2023 Apr 1;22:404-409. doi: 10.36849/JDD.6892.
- CMS releases National Healthcare Expenditure and enrollment projections through 2031. Health Management Associates. July 13, 2023. Accessed October 23, 2025. https://www.healthmanagement.com/blog/cms-releases-national-healthcare-expenditure-and-enrollment-projections-through-2031/
The Current State of Postgraduate Dermatology Training Programs for Advanced Practice Providers
The Current State of Postgraduate Dermatology Training Programs for Advanced Practice Providers
Practice Points
- Postgraduate dermatology training programs are available for advanced practice providers (APPs), but they are optional and lack a formal accreditation process.
- Awareness of these programs and the differences between APPs and physician training may help dermatologists provide safe and effective care in collaborative or supervisory roles.
Colon Cleanses: How to Discourage Patients
Social media is rife with content promoting colon cleansing as a way to shed toxins and fix everything from chronic fatigue and overweight to weak immunity and skin problems.
Even doctors who aren’t hip to the latest TikTok trends may not be able to avoid the hype. That’s because patients are bringing up colon cleansing during their office visit.
“Patients often raise questions about colonics or detox teas, especially when these gain traction on social media platforms like TikTok,” said Tauseef Ali, MD, AGAF, medical executive director of SSM Health Digestive Care at St. Anthony Hospital in Oklahoma City. “Interest typically comes in waves, closely tied to the latest online trends.”
That means . And it’s not just patients who are asking.
“Sometimes we’ll get a message from primary care,” Mohammad Bilal, MD, associate professor of medicine and director of Bariatric and Third Space Endoscopy at the University of Colorado Anschutz Medical Campus in Aurora, Colorado, told GI & Hepatology News. They’re getting the same questions from patients, and they want to know if colon cleansing that’s not connected with a colonoscopy exam has any benefits for overall health or specific health conditions.
The answer is no, and patients are more likely to believe that when physicians explain it using good information. Here is how Ali, Bilal, and professional organizations advise doctors to approach the issue.
What Exactly Is a Colon Cleanse?
Colon cleanses come in a variety of forms. Colonic irrigation, also called colon hydrotherapy, involves inserting a tube into the rectum and flushing out the colon with a large amount of fluid. Enemas do the same but use a small amount of liquid, and some product instructions tell the user to “hold it” for a designated amount of time before expelling colon contents.
Other cleanses, often called detoxing cleanses, are laxatives or herbal teas that users drink — and then stay close to the bathroom. Detox regimens and diets also are mentioned as a way to remove toxins from the body, improve health, and promote well-being.
Why Do Patients Use Them?
“Many patients describe a desire for ‘cleanliness,’ ‘detoxification,’ or to ‘feel lighter,’” Ali told GI & Hepatology News.
The claims on social media promote all of this and more — and well-known influencers make it all sound even more attractive.
“These motivations are often rooted in the cultural belief that the colon accumulates harmful toxins that must be flushed out,” Ali said. “This idea is not supported by scientific evidence. The body’s natural detox systems, primarily the liver and kidneys, already perform this function effectively.”
Bilal said that in recent years, he has noticed more awareness in general about the importance of gut health. “When there’s awareness, people often go to the other extreme,” he said.
Where Is the Evidence?
The National Center for Complementary and Integrative Health (NCCIH), part of the National Institutes of Health, warns on an information page that both cleansing and detox programs can be unsafe and falsely advertised.
While searches of medical literature turn up few studies, the NCCIH information points to a 2014 review that concluded that there is no compelling research to support the use of detox diets for managing weight or eliminating toxins. A 2017 review found juicing and detox regimens can cause weight loss initially but then lead to weight gain once a normal diet is resumed.
A systematic review of research on the safety and effectiveness of self-administered coffee enemas found nine case reports describing adverse events: seven reported colitis after the enema, and two reported more critical adverse events. All nine reports warned against the procedure. The researchers found no study reporting the effectiveness of coffee enemas.
The NCCIH information also notes that there is “limited clinical evidence validating colonic irrigation and insufficient evidence for its prescribed uses.”
Are Cleanses Regulated?
Some over-the-counter colon cleansing products are viewed as dietary supplements, giving the FDA authority to regulate them and take action under the Dietary Supplement Health and Education Act of 1994.
Certain products promoted as colon cleanses, such as laxatives, are regulated by the FDA as over-the-counter drugs and must meet safety and other requirements.
Colonic irrigation systems meant for cleansing before radiologic or endoscopic exams are class II devices — subject to 510(k) premarket notification requirements before marketing — whereas systems intended for other uses, such as routine colon cleansing for general well-being, are regarded as class III devices — subject to premarket approval requirements — according to an FDA spokesperson. To date, the FDA has not approved any colonic irrigation devices for the latter use, the spokesperson said.
For instance, the FDA warned consumers not to use a product promoted for colon cleansing after finding it contained tadalafil, the active ingredient in an FDA-approved drug for erectile dysfunction. The FDA has also issued numerous warning letters to the makers of colon cleansing devices, as they are not approved for this purpose.
The Federal Trade Commission can also take action specifically if the claims about the benefits and safety of products — including supplements, foods, over-the-counter drugs, or health equipment — are false, misleading, or not supported by science.
What Are the Dangers?
Cleanse and detox products come with many risks, including electrolyte imbalances, dehydration, and infections, Ali said. With colonic irrigation, there is a risk for rectal perforation. Products also may disrupt the gut microbiome, and some can interact with medications or worsen underlying health conditions, he added.
“It’s important for patients to be aware of these risks before considering nonmedical ‘cleaning’ methods,” he said.
At worst, patients risk fatality, Ali noted. He recalled a young patient who began using a vegetable enema as a detox. As it was being administered, the colon ruptured. The patient was admitted as a medical emergency and required surgery. Fortunately, the patient survived, but the incident could have proven fatal, Ali said.
Educating Patients
Because patients often don’t think of herbal cleanses, detox teas, and over-the-counter powders as supplements, Ali said it’s important to ask them about everything they take.
One way to frame this question is to ask if they are consuming any over-the-counter supplements or any other remedies, he said, and perhaps ask directly about any cleanses they are doing.
When patients ask him about colon cleanses, Ali explains the difference between evidence-based colonoscopy preparation and unregulated “cleanses.” Most patients respond to that approach, he said. Indeed, AGA and other GI societies updated their recommendations on optimizing bowel preparation quality for colonoscopy.
“Still, the appeal of quick fixes of social media trends can sometimes outweigh medical advice,” Ali said. He depends on building trusted relationships and reinforcing the message over time and finds that helps patients make informed and healthier choices.
Bilal, too, explains to patients that cleanses are unnecessary and educates them about what to do instead:
- Eat a containing the recommended amount of (22-34 g, depending on age and gender).
- For , follow a bowel regimen advised by your doctor.
- If gastrointestinal issues persist, get a medical checkup.
- Get any unexplained constipation or checked out by a doctor.
Taking a careful history can pay off, Ali has found. He questioned a patient complaining of abdominal discomfort whose testing found unexpectedly elevated liver enzymes and found she had been using an herbal “cleanse tea” found online. Within 4 weeks of stopping it, her liver enzymes normalized. “Thankfully, she made a full recovery — and she never touched those remedies again,” he said.
Ali had no relevant disclosures. Bilal reported consulting for Boston Scientific, Cook Medical, and Steris.
A version of this article appeared on Medscape.com.
Social media is rife with content promoting colon cleansing as a way to shed toxins and fix everything from chronic fatigue and overweight to weak immunity and skin problems.
Even doctors who aren’t hip to the latest TikTok trends may not be able to avoid the hype. That’s because patients are bringing up colon cleansing during their office visit.
“Patients often raise questions about colonics or detox teas, especially when these gain traction on social media platforms like TikTok,” said Tauseef Ali, MD, AGAF, medical executive director of SSM Health Digestive Care at St. Anthony Hospital in Oklahoma City. “Interest typically comes in waves, closely tied to the latest online trends.”
That means . And it’s not just patients who are asking.
“Sometimes we’ll get a message from primary care,” Mohammad Bilal, MD, associate professor of medicine and director of Bariatric and Third Space Endoscopy at the University of Colorado Anschutz Medical Campus in Aurora, Colorado, told GI & Hepatology News. They’re getting the same questions from patients, and they want to know if colon cleansing that’s not connected with a colonoscopy exam has any benefits for overall health or specific health conditions.
The answer is no, and patients are more likely to believe that when physicians explain it using good information. Here is how Ali, Bilal, and professional organizations advise doctors to approach the issue.
What Exactly Is a Colon Cleanse?
Colon cleanses come in a variety of forms. Colonic irrigation, also called colon hydrotherapy, involves inserting a tube into the rectum and flushing out the colon with a large amount of fluid. Enemas do the same but use a small amount of liquid, and some product instructions tell the user to “hold it” for a designated amount of time before expelling colon contents.
Other cleanses, often called detoxing cleanses, are laxatives or herbal teas that users drink — and then stay close to the bathroom. Detox regimens and diets also are mentioned as a way to remove toxins from the body, improve health, and promote well-being.
Why Do Patients Use Them?
“Many patients describe a desire for ‘cleanliness,’ ‘detoxification,’ or to ‘feel lighter,’” Ali told GI & Hepatology News.
The claims on social media promote all of this and more — and well-known influencers make it all sound even more attractive.
“These motivations are often rooted in the cultural belief that the colon accumulates harmful toxins that must be flushed out,” Ali said. “This idea is not supported by scientific evidence. The body’s natural detox systems, primarily the liver and kidneys, already perform this function effectively.”
Bilal said that in recent years, he has noticed more awareness in general about the importance of gut health. “When there’s awareness, people often go to the other extreme,” he said.
Where Is the Evidence?
The National Center for Complementary and Integrative Health (NCCIH), part of the National Institutes of Health, warns on an information page that both cleansing and detox programs can be unsafe and falsely advertised.
While searches of medical literature turn up few studies, the NCCIH information points to a 2014 review that concluded that there is no compelling research to support the use of detox diets for managing weight or eliminating toxins. A 2017 review found juicing and detox regimens can cause weight loss initially but then lead to weight gain once a normal diet is resumed.
A systematic review of research on the safety and effectiveness of self-administered coffee enemas found nine case reports describing adverse events: seven reported colitis after the enema, and two reported more critical adverse events. All nine reports warned against the procedure. The researchers found no study reporting the effectiveness of coffee enemas.
The NCCIH information also notes that there is “limited clinical evidence validating colonic irrigation and insufficient evidence for its prescribed uses.”
Are Cleanses Regulated?
Some over-the-counter colon cleansing products are viewed as dietary supplements, giving the FDA authority to regulate them and take action under the Dietary Supplement Health and Education Act of 1994.
Certain products promoted as colon cleanses, such as laxatives, are regulated by the FDA as over-the-counter drugs and must meet safety and other requirements.
Colonic irrigation systems meant for cleansing before radiologic or endoscopic exams are class II devices — subject to 510(k) premarket notification requirements before marketing — whereas systems intended for other uses, such as routine colon cleansing for general well-being, are regarded as class III devices — subject to premarket approval requirements — according to an FDA spokesperson. To date, the FDA has not approved any colonic irrigation devices for the latter use, the spokesperson said.
For instance, the FDA warned consumers not to use a product promoted for colon cleansing after finding it contained tadalafil, the active ingredient in an FDA-approved drug for erectile dysfunction. The FDA has also issued numerous warning letters to the makers of colon cleansing devices, as they are not approved for this purpose.
The Federal Trade Commission can also take action specifically if the claims about the benefits and safety of products — including supplements, foods, over-the-counter drugs, or health equipment — are false, misleading, or not supported by science.
What Are the Dangers?
Cleanse and detox products come with many risks, including electrolyte imbalances, dehydration, and infections, Ali said. With colonic irrigation, there is a risk for rectal perforation. Products also may disrupt the gut microbiome, and some can interact with medications or worsen underlying health conditions, he added.
“It’s important for patients to be aware of these risks before considering nonmedical ‘cleaning’ methods,” he said.
At worst, patients risk fatality, Ali noted. He recalled a young patient who began using a vegetable enema as a detox. As it was being administered, the colon ruptured. The patient was admitted as a medical emergency and required surgery. Fortunately, the patient survived, but the incident could have proven fatal, Ali said.
Educating Patients
Because patients often don’t think of herbal cleanses, detox teas, and over-the-counter powders as supplements, Ali said it’s important to ask them about everything they take.
One way to frame this question is to ask if they are consuming any over-the-counter supplements or any other remedies, he said, and perhaps ask directly about any cleanses they are doing.
When patients ask him about colon cleanses, Ali explains the difference between evidence-based colonoscopy preparation and unregulated “cleanses.” Most patients respond to that approach, he said. Indeed, AGA and other GI societies updated their recommendations on optimizing bowel preparation quality for colonoscopy.
“Still, the appeal of quick fixes of social media trends can sometimes outweigh medical advice,” Ali said. He depends on building trusted relationships and reinforcing the message over time and finds that helps patients make informed and healthier choices.
Bilal, too, explains to patients that cleanses are unnecessary and educates them about what to do instead:
- Eat a containing the recommended amount of (22-34 g, depending on age and gender).
- For , follow a bowel regimen advised by your doctor.
- If gastrointestinal issues persist, get a medical checkup.
- Get any unexplained constipation or checked out by a doctor.
Taking a careful history can pay off, Ali has found. He questioned a patient complaining of abdominal discomfort whose testing found unexpectedly elevated liver enzymes and found she had been using an herbal “cleanse tea” found online. Within 4 weeks of stopping it, her liver enzymes normalized. “Thankfully, she made a full recovery — and she never touched those remedies again,” he said.
Ali had no relevant disclosures. Bilal reported consulting for Boston Scientific, Cook Medical, and Steris.
A version of this article appeared on Medscape.com.
Social media is rife with content promoting colon cleansing as a way to shed toxins and fix everything from chronic fatigue and overweight to weak immunity and skin problems.
Even doctors who aren’t hip to the latest TikTok trends may not be able to avoid the hype. That’s because patients are bringing up colon cleansing during their office visit.
“Patients often raise questions about colonics or detox teas, especially when these gain traction on social media platforms like TikTok,” said Tauseef Ali, MD, AGAF, medical executive director of SSM Health Digestive Care at St. Anthony Hospital in Oklahoma City. “Interest typically comes in waves, closely tied to the latest online trends.”
That means . And it’s not just patients who are asking.
“Sometimes we’ll get a message from primary care,” Mohammad Bilal, MD, associate professor of medicine and director of Bariatric and Third Space Endoscopy at the University of Colorado Anschutz Medical Campus in Aurora, Colorado, told GI & Hepatology News. They’re getting the same questions from patients, and they want to know if colon cleansing that’s not connected with a colonoscopy exam has any benefits for overall health or specific health conditions.
The answer is no, and patients are more likely to believe that when physicians explain it using good information. Here is how Ali, Bilal, and professional organizations advise doctors to approach the issue.
What Exactly Is a Colon Cleanse?
Colon cleanses come in a variety of forms. Colonic irrigation, also called colon hydrotherapy, involves inserting a tube into the rectum and flushing out the colon with a large amount of fluid. Enemas do the same but use a small amount of liquid, and some product instructions tell the user to “hold it” for a designated amount of time before expelling colon contents.
Other cleanses, often called detoxing cleanses, are laxatives or herbal teas that users drink — and then stay close to the bathroom. Detox regimens and diets also are mentioned as a way to remove toxins from the body, improve health, and promote well-being.
Why Do Patients Use Them?
“Many patients describe a desire for ‘cleanliness,’ ‘detoxification,’ or to ‘feel lighter,’” Ali told GI & Hepatology News.
The claims on social media promote all of this and more — and well-known influencers make it all sound even more attractive.
“These motivations are often rooted in the cultural belief that the colon accumulates harmful toxins that must be flushed out,” Ali said. “This idea is not supported by scientific evidence. The body’s natural detox systems, primarily the liver and kidneys, already perform this function effectively.”
Bilal said that in recent years, he has noticed more awareness in general about the importance of gut health. “When there’s awareness, people often go to the other extreme,” he said.
Where Is the Evidence?
The National Center for Complementary and Integrative Health (NCCIH), part of the National Institutes of Health, warns on an information page that both cleansing and detox programs can be unsafe and falsely advertised.
While searches of medical literature turn up few studies, the NCCIH information points to a 2014 review that concluded that there is no compelling research to support the use of detox diets for managing weight or eliminating toxins. A 2017 review found juicing and detox regimens can cause weight loss initially but then lead to weight gain once a normal diet is resumed.
A systematic review of research on the safety and effectiveness of self-administered coffee enemas found nine case reports describing adverse events: seven reported colitis after the enema, and two reported more critical adverse events. All nine reports warned against the procedure. The researchers found no study reporting the effectiveness of coffee enemas.
The NCCIH information also notes that there is “limited clinical evidence validating colonic irrigation and insufficient evidence for its prescribed uses.”
Are Cleanses Regulated?
Some over-the-counter colon cleansing products are viewed as dietary supplements, giving the FDA authority to regulate them and take action under the Dietary Supplement Health and Education Act of 1994.
Certain products promoted as colon cleanses, such as laxatives, are regulated by the FDA as over-the-counter drugs and must meet safety and other requirements.
Colonic irrigation systems meant for cleansing before radiologic or endoscopic exams are class II devices — subject to 510(k) premarket notification requirements before marketing — whereas systems intended for other uses, such as routine colon cleansing for general well-being, are regarded as class III devices — subject to premarket approval requirements — according to an FDA spokesperson. To date, the FDA has not approved any colonic irrigation devices for the latter use, the spokesperson said.
For instance, the FDA warned consumers not to use a product promoted for colon cleansing after finding it contained tadalafil, the active ingredient in an FDA-approved drug for erectile dysfunction. The FDA has also issued numerous warning letters to the makers of colon cleansing devices, as they are not approved for this purpose.
The Federal Trade Commission can also take action specifically if the claims about the benefits and safety of products — including supplements, foods, over-the-counter drugs, or health equipment — are false, misleading, or not supported by science.
What Are the Dangers?
Cleanse and detox products come with many risks, including electrolyte imbalances, dehydration, and infections, Ali said. With colonic irrigation, there is a risk for rectal perforation. Products also may disrupt the gut microbiome, and some can interact with medications or worsen underlying health conditions, he added.
“It’s important for patients to be aware of these risks before considering nonmedical ‘cleaning’ methods,” he said.
At worst, patients risk fatality, Ali noted. He recalled a young patient who began using a vegetable enema as a detox. As it was being administered, the colon ruptured. The patient was admitted as a medical emergency and required surgery. Fortunately, the patient survived, but the incident could have proven fatal, Ali said.
Educating Patients
Because patients often don’t think of herbal cleanses, detox teas, and over-the-counter powders as supplements, Ali said it’s important to ask them about everything they take.
One way to frame this question is to ask if they are consuming any over-the-counter supplements or any other remedies, he said, and perhaps ask directly about any cleanses they are doing.
When patients ask him about colon cleanses, Ali explains the difference between evidence-based colonoscopy preparation and unregulated “cleanses.” Most patients respond to that approach, he said. Indeed, AGA and other GI societies updated their recommendations on optimizing bowel preparation quality for colonoscopy.
“Still, the appeal of quick fixes of social media trends can sometimes outweigh medical advice,” Ali said. He depends on building trusted relationships and reinforcing the message over time and finds that helps patients make informed and healthier choices.
Bilal, too, explains to patients that cleanses are unnecessary and educates them about what to do instead:
- Eat a containing the recommended amount of (22-34 g, depending on age and gender).
- For , follow a bowel regimen advised by your doctor.
- If gastrointestinal issues persist, get a medical checkup.
- Get any unexplained constipation or checked out by a doctor.
Taking a careful history can pay off, Ali has found. He questioned a patient complaining of abdominal discomfort whose testing found unexpectedly elevated liver enzymes and found she had been using an herbal “cleanse tea” found online. Within 4 weeks of stopping it, her liver enzymes normalized. “Thankfully, she made a full recovery — and she never touched those remedies again,” he said.
Ali had no relevant disclosures. Bilal reported consulting for Boston Scientific, Cook Medical, and Steris.
A version of this article appeared on Medscape.com.
Flesh-Colored Lesion on the Ear
Flesh-Colored Lesion on the Ear
THE DIAGNOSIS: Gouty Tophus
The lesion was excised and sent for histopathologic examination (eFigures 1 and 2), revealing aggregates of feathery, amorphous, pale-pink material, which confirmed the diagnosis of gouty tophus. The surgical site was left to heal by secondary intention. Upon further evaluation, the patient reported recurrent monoarticular joint pain in the ankles and feet, and laboratory workup revealed elevated serum uric acid. He was advised to follow up with his primary care physician to discuss systemic treatment options for gout.
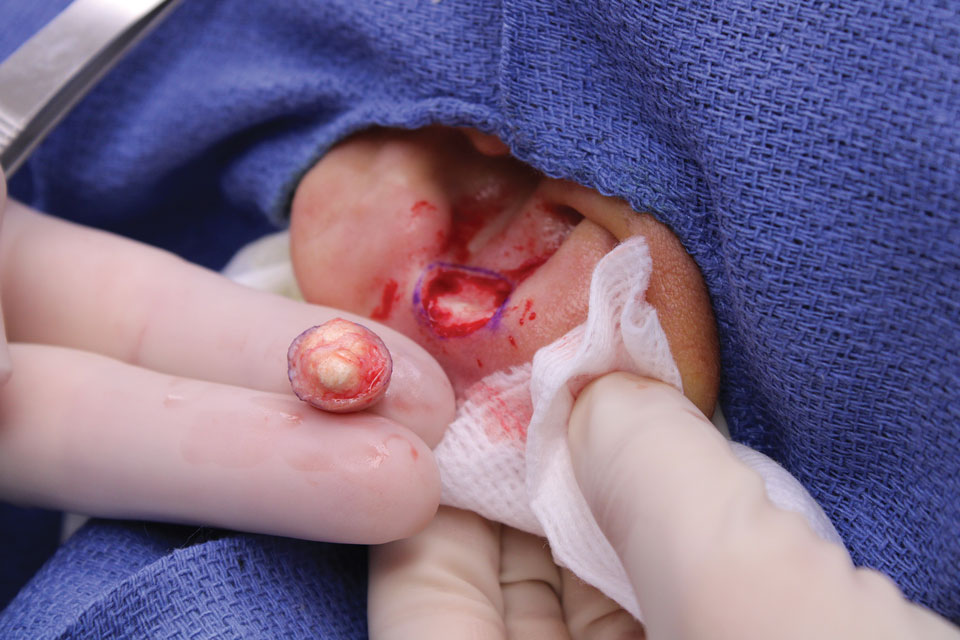
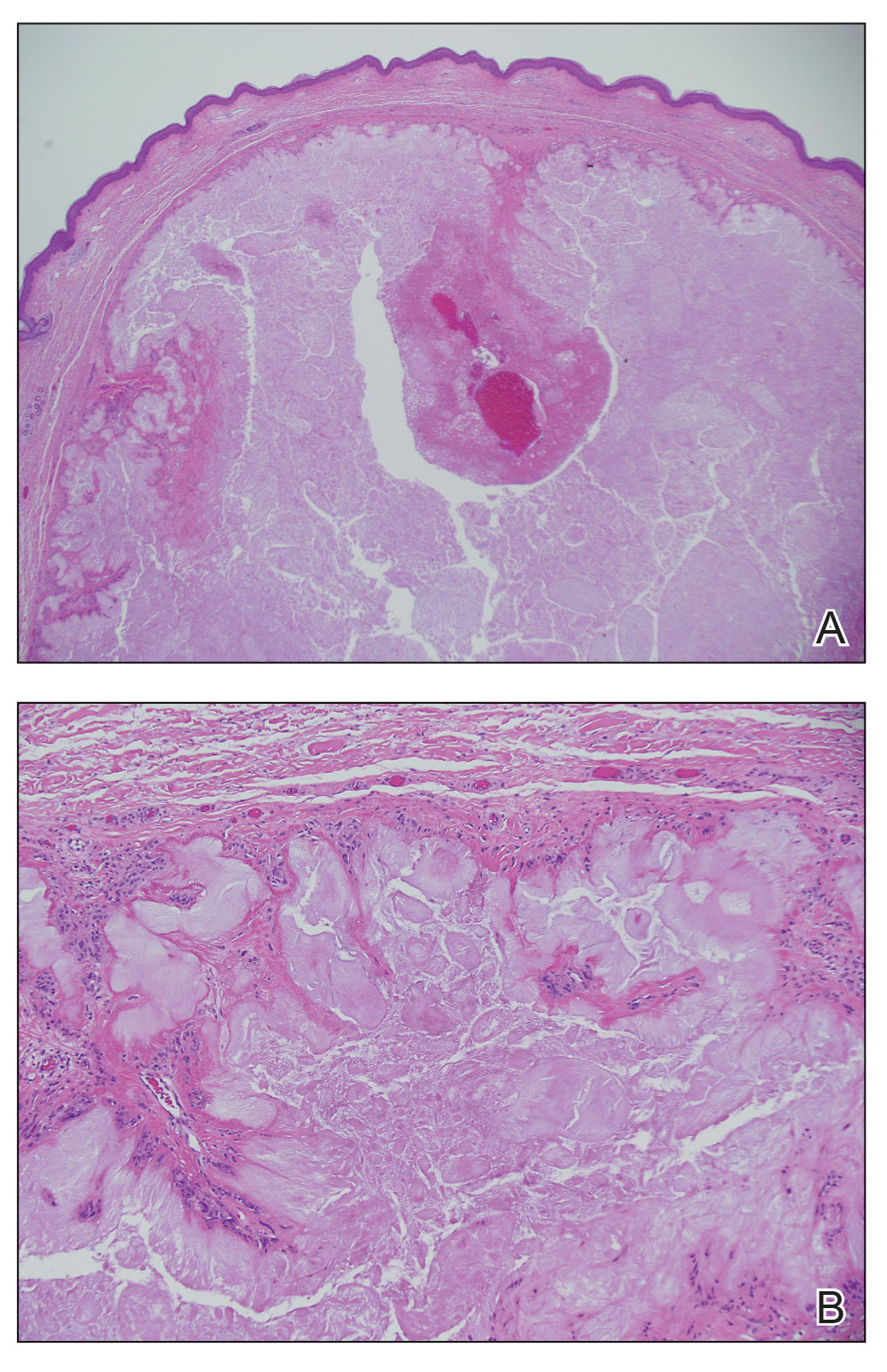
Gout is an inflammatory arthritis characterized by the deposition of monosodium urate monohydrate crystals in the joints, soft tissue, and bone due to elevated serum uric acid. Uric acid is the final product of purine metabolism, and serum levels may be elevated due to excess production or underexcretion. Multiple genetic, environmental, and metabolic factors influence these processes.1 Collections of monosodium urate crystals may develop intra- or extra-articularly, the latter of which are known as gouty tophi. These nodules have a classic chalklike consistency and typically are seen in patients with untreated gout starting approximately 10 years after the first flare. The most common locations for subcutaneous gouty tophi are acral sites (eg, fingertips, ears) as well as the wrists, knees, and elbows (olecranon bursae). Rarely, gouty panniculitis also may develop.2
Histopathology of gouty tophi reveals nodular aggregates of acellular, amorphous, pale-pink material surrounded by palisading histiocytes and multinucleated giant cells. The presence of needlelike monosodium urate crystals, which display negative birefringence, is diagnostic. Unfortunately, these crystals are destroyed in routine formalin processing.3
There are limited data regarding treatment of gouty tophi. Urate-lowering systemic medications such as pegloticase may be beneficial, but more data are needed.4 We pursued surgical excision in our case for definitive diagnosis; however, it is not a common treatment for gouty tophi. Typically, urate-lowering therapy is utilized to resolve or shrink lesions over time.5
The differential diagnosis for gouty tophi includes epidermal inclusion cyst (EIC), the most common type of cutaneous cyst. Though EICs can manifest anywhere on the body, they are not as common on the ears as gouty tophi. Epidermal inclusion cysts clinically manifest as soft subcutaneous nodules, and a central punctum often is noted. These lesions are derived from the follicular infundibulum and histologically are characterized by a cystic cavity lined by a stratified squamous epithelium with a granular layer. The cavity contains loose laminated keratin material.6
Pseudocyst of the auricle is a benign cystic swelling of the pinna that can develop spontaneously but most often manifests following trauma to the area, which is believed to separate the tissue planes in the cartilage, allowing fluid to accumulate. This lesion typically is asymptomatic, though some patients report mild tenderness.7 Histology shows a cystic structure within the cartilage without an epithelial lining, and a perivascular inflammatory response often is observed.8
Pilomatricoma, also known as pilomatrixoma, is a benign tumor derived from the hair follicle matrix that manifests as a firm, slow-growing, painless subcutaneous nodule. It most often is found on the head and neck, commonly in the periauricular area.9 Though rare, it has been found on the auricle and external auditory canal.10 Histologically, pilomatricomas are well-defined tumors containing internal trabeculae. They contain populations of basaloid and ghost cells and often calcify, sometimes with resultant bone formation.9
Dermoid cysts are benign tumors that develop along lines of embryonic closure and often are diagnosed at birth or in early childhood. They most commonly manifest on the head and neck, typically in the supraorbital area. Rarely, they have been reported on the ear.6 Dermoid cysts may resemble EICs clinically and histopathologically, except that the cyst wall contains mature adnexal structures such as hair follicles and sebaceous glands.
- Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039-2052. doi:10.1016/S0140-6736(16)00346-9
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445. doi:10.1097/GOX.0000000000000420
- Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20:140. doi:10.1186/s12891-019-2519-y
- Sriranganathan MK, Vinik O, Pardo Pardo J, et al. Interventions for tophi in gout. Cochrane Database Syst Rev. 2021;8:CD010069. doi:10.1002/14651858.CD010069.pub3
- Evidence review for surgical excision of tophi. Gout: diagnosis and management. National Institute for Health and Care Excellence (NICE). June 2022. Accessed October 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK583526/
- Cho Y, Lee DH. Clinical characteristics of idiopathic epidermoid and dermoid cysts of the ear. J Audiol Otol. 2017;21:77-80. doi:10.7874 /jao.2017.21.2.77
- Ballan A, Zogheib S, Hanna C, et al. Auricular pseudocysts: a systematic review of the literature. Int J Dermatol. 2022;61:109-117. doi:10.1111/ijd.15816
- Lim CM, Goh YH, Chao SS, et al. Pseudocyst of the auricle: a histologic perspective. Laryngoscope. 2004;114:1281-1284. doi:10.1097/00005537-200407000-00026
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018; 40:631-641. doi:10.1097/DAD.0000000000001118
- McInerney NJ, Nae A, Brennan S, et al. Pilomatricoma of the external auditory canal. Royal College of Surgeons in Ireland. 2023. doi:10.1016/j.xocr.2023.10053
THE DIAGNOSIS: Gouty Tophus
The lesion was excised and sent for histopathologic examination (eFigures 1 and 2), revealing aggregates of feathery, amorphous, pale-pink material, which confirmed the diagnosis of gouty tophus. The surgical site was left to heal by secondary intention. Upon further evaluation, the patient reported recurrent monoarticular joint pain in the ankles and feet, and laboratory workup revealed elevated serum uric acid. He was advised to follow up with his primary care physician to discuss systemic treatment options for gout.


Gout is an inflammatory arthritis characterized by the deposition of monosodium urate monohydrate crystals in the joints, soft tissue, and bone due to elevated serum uric acid. Uric acid is the final product of purine metabolism, and serum levels may be elevated due to excess production or underexcretion. Multiple genetic, environmental, and metabolic factors influence these processes.1 Collections of monosodium urate crystals may develop intra- or extra-articularly, the latter of which are known as gouty tophi. These nodules have a classic chalklike consistency and typically are seen in patients with untreated gout starting approximately 10 years after the first flare. The most common locations for subcutaneous gouty tophi are acral sites (eg, fingertips, ears) as well as the wrists, knees, and elbows (olecranon bursae). Rarely, gouty panniculitis also may develop.2
Histopathology of gouty tophi reveals nodular aggregates of acellular, amorphous, pale-pink material surrounded by palisading histiocytes and multinucleated giant cells. The presence of needlelike monosodium urate crystals, which display negative birefringence, is diagnostic. Unfortunately, these crystals are destroyed in routine formalin processing.3
There are limited data regarding treatment of gouty tophi. Urate-lowering systemic medications such as pegloticase may be beneficial, but more data are needed.4 We pursued surgical excision in our case for definitive diagnosis; however, it is not a common treatment for gouty tophi. Typically, urate-lowering therapy is utilized to resolve or shrink lesions over time.5
The differential diagnosis for gouty tophi includes epidermal inclusion cyst (EIC), the most common type of cutaneous cyst. Though EICs can manifest anywhere on the body, they are not as common on the ears as gouty tophi. Epidermal inclusion cysts clinically manifest as soft subcutaneous nodules, and a central punctum often is noted. These lesions are derived from the follicular infundibulum and histologically are characterized by a cystic cavity lined by a stratified squamous epithelium with a granular layer. The cavity contains loose laminated keratin material.6
Pseudocyst of the auricle is a benign cystic swelling of the pinna that can develop spontaneously but most often manifests following trauma to the area, which is believed to separate the tissue planes in the cartilage, allowing fluid to accumulate. This lesion typically is asymptomatic, though some patients report mild tenderness.7 Histology shows a cystic structure within the cartilage without an epithelial lining, and a perivascular inflammatory response often is observed.8
Pilomatricoma, also known as pilomatrixoma, is a benign tumor derived from the hair follicle matrix that manifests as a firm, slow-growing, painless subcutaneous nodule. It most often is found on the head and neck, commonly in the periauricular area.9 Though rare, it has been found on the auricle and external auditory canal.10 Histologically, pilomatricomas are well-defined tumors containing internal trabeculae. They contain populations of basaloid and ghost cells and often calcify, sometimes with resultant bone formation.9
Dermoid cysts are benign tumors that develop along lines of embryonic closure and often are diagnosed at birth or in early childhood. They most commonly manifest on the head and neck, typically in the supraorbital area. Rarely, they have been reported on the ear.6 Dermoid cysts may resemble EICs clinically and histopathologically, except that the cyst wall contains mature adnexal structures such as hair follicles and sebaceous glands.
THE DIAGNOSIS: Gouty Tophus
The lesion was excised and sent for histopathologic examination (eFigures 1 and 2), revealing aggregates of feathery, amorphous, pale-pink material, which confirmed the diagnosis of gouty tophus. The surgical site was left to heal by secondary intention. Upon further evaluation, the patient reported recurrent monoarticular joint pain in the ankles and feet, and laboratory workup revealed elevated serum uric acid. He was advised to follow up with his primary care physician to discuss systemic treatment options for gout.


Gout is an inflammatory arthritis characterized by the deposition of monosodium urate monohydrate crystals in the joints, soft tissue, and bone due to elevated serum uric acid. Uric acid is the final product of purine metabolism, and serum levels may be elevated due to excess production or underexcretion. Multiple genetic, environmental, and metabolic factors influence these processes.1 Collections of monosodium urate crystals may develop intra- or extra-articularly, the latter of which are known as gouty tophi. These nodules have a classic chalklike consistency and typically are seen in patients with untreated gout starting approximately 10 years after the first flare. The most common locations for subcutaneous gouty tophi are acral sites (eg, fingertips, ears) as well as the wrists, knees, and elbows (olecranon bursae). Rarely, gouty panniculitis also may develop.2
Histopathology of gouty tophi reveals nodular aggregates of acellular, amorphous, pale-pink material surrounded by palisading histiocytes and multinucleated giant cells. The presence of needlelike monosodium urate crystals, which display negative birefringence, is diagnostic. Unfortunately, these crystals are destroyed in routine formalin processing.3
There are limited data regarding treatment of gouty tophi. Urate-lowering systemic medications such as pegloticase may be beneficial, but more data are needed.4 We pursued surgical excision in our case for definitive diagnosis; however, it is not a common treatment for gouty tophi. Typically, urate-lowering therapy is utilized to resolve or shrink lesions over time.5
The differential diagnosis for gouty tophi includes epidermal inclusion cyst (EIC), the most common type of cutaneous cyst. Though EICs can manifest anywhere on the body, they are not as common on the ears as gouty tophi. Epidermal inclusion cysts clinically manifest as soft subcutaneous nodules, and a central punctum often is noted. These lesions are derived from the follicular infundibulum and histologically are characterized by a cystic cavity lined by a stratified squamous epithelium with a granular layer. The cavity contains loose laminated keratin material.6
Pseudocyst of the auricle is a benign cystic swelling of the pinna that can develop spontaneously but most often manifests following trauma to the area, which is believed to separate the tissue planes in the cartilage, allowing fluid to accumulate. This lesion typically is asymptomatic, though some patients report mild tenderness.7 Histology shows a cystic structure within the cartilage without an epithelial lining, and a perivascular inflammatory response often is observed.8
Pilomatricoma, also known as pilomatrixoma, is a benign tumor derived from the hair follicle matrix that manifests as a firm, slow-growing, painless subcutaneous nodule. It most often is found on the head and neck, commonly in the periauricular area.9 Though rare, it has been found on the auricle and external auditory canal.10 Histologically, pilomatricomas are well-defined tumors containing internal trabeculae. They contain populations of basaloid and ghost cells and often calcify, sometimes with resultant bone formation.9
Dermoid cysts are benign tumors that develop along lines of embryonic closure and often are diagnosed at birth or in early childhood. They most commonly manifest on the head and neck, typically in the supraorbital area. Rarely, they have been reported on the ear.6 Dermoid cysts may resemble EICs clinically and histopathologically, except that the cyst wall contains mature adnexal structures such as hair follicles and sebaceous glands.
- Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039-2052. doi:10.1016/S0140-6736(16)00346-9
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445. doi:10.1097/GOX.0000000000000420
- Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20:140. doi:10.1186/s12891-019-2519-y
- Sriranganathan MK, Vinik O, Pardo Pardo J, et al. Interventions for tophi in gout. Cochrane Database Syst Rev. 2021;8:CD010069. doi:10.1002/14651858.CD010069.pub3
- Evidence review for surgical excision of tophi. Gout: diagnosis and management. National Institute for Health and Care Excellence (NICE). June 2022. Accessed October 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK583526/
- Cho Y, Lee DH. Clinical characteristics of idiopathic epidermoid and dermoid cysts of the ear. J Audiol Otol. 2017;21:77-80. doi:10.7874 /jao.2017.21.2.77
- Ballan A, Zogheib S, Hanna C, et al. Auricular pseudocysts: a systematic review of the literature. Int J Dermatol. 2022;61:109-117. doi:10.1111/ijd.15816
- Lim CM, Goh YH, Chao SS, et al. Pseudocyst of the auricle: a histologic perspective. Laryngoscope. 2004;114:1281-1284. doi:10.1097/00005537-200407000-00026
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018; 40:631-641. doi:10.1097/DAD.0000000000001118
- McInerney NJ, Nae A, Brennan S, et al. Pilomatricoma of the external auditory canal. Royal College of Surgeons in Ireland. 2023. doi:10.1016/j.xocr.2023.10053
- Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039-2052. doi:10.1016/S0140-6736(16)00346-9
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445. doi:10.1097/GOX.0000000000000420
- Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20:140. doi:10.1186/s12891-019-2519-y
- Sriranganathan MK, Vinik O, Pardo Pardo J, et al. Interventions for tophi in gout. Cochrane Database Syst Rev. 2021;8:CD010069. doi:10.1002/14651858.CD010069.pub3
- Evidence review for surgical excision of tophi. Gout: diagnosis and management. National Institute for Health and Care Excellence (NICE). June 2022. Accessed October 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK583526/
- Cho Y, Lee DH. Clinical characteristics of idiopathic epidermoid and dermoid cysts of the ear. J Audiol Otol. 2017;21:77-80. doi:10.7874 /jao.2017.21.2.77
- Ballan A, Zogheib S, Hanna C, et al. Auricular pseudocysts: a systematic review of the literature. Int J Dermatol. 2022;61:109-117. doi:10.1111/ijd.15816
- Lim CM, Goh YH, Chao SS, et al. Pseudocyst of the auricle: a histologic perspective. Laryngoscope. 2004;114:1281-1284. doi:10.1097/00005537-200407000-00026
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018; 40:631-641. doi:10.1097/DAD.0000000000001118
- McInerney NJ, Nae A, Brennan S, et al. Pilomatricoma of the external auditory canal. Royal College of Surgeons in Ireland. 2023. doi:10.1016/j.xocr.2023.10053
Flesh-Colored Lesion on the Ear
Flesh-Colored Lesion on the Ear
A 46-year-old man with a history of hypertension, hyperlipidemia, and type 2 diabetes presented to the dermatology clinic with a painless nodule on the left ear of 2 years’ duration. The patient denied any bleeding, drainage, or prior trauma to the area. He noted that the lesion had grown slowly over time. Physical examination revealed a 1.5×1.5-cm, flesh-colored, subcutaneous nodule with overlying telangiectasias on the left antihelix.
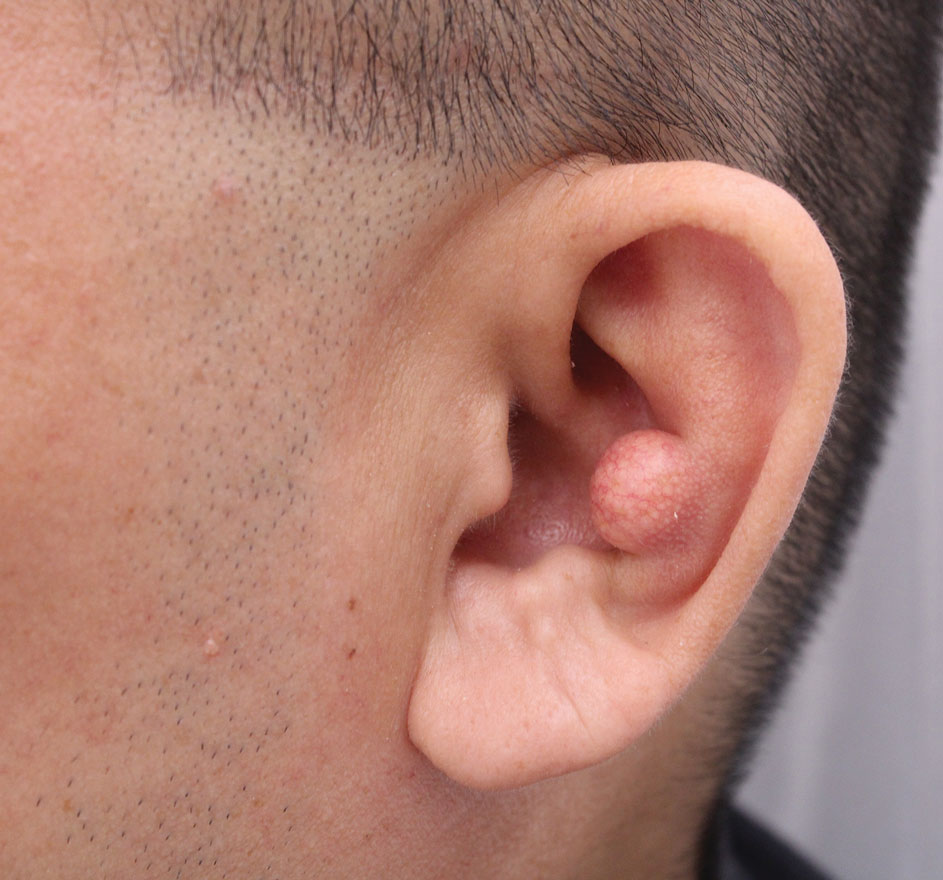
COVID-19 Vaccines: Navigating the Chaos of Conflicting Guidance
Hi, everyone. I’m Dr Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
The receding of the pandemic and the understandable desire to return to normalcy has made COVID-19 vaccines a lower priority for many of our patients. However, family physicians should keep in mind that from October 1, 2024, to September 6, 2025, COVID-19 was responsible for an estimated 3.2 to 4.6 million outpatient visits, 360,000 to 520,000 hospitalizations, and 42,000 to 60,000 deaths.
In a previous commentary, I discussed the worsening disconnect between the evidence supporting the effectiveness and safety of vaccinations and increasing reluctance of patients and parents to receive them, fueled by misinformation from federal health agencies and the packing of the Advisory Committee on Immunization Practices (ACIP) with vaccine skeptics. Since then, Secretary of Health and Human Services (HHS), Robert F. Kennedy, Jr, has fired Dr Susan Monarez, his handpicked director of the CDC. This caused three senior CDC officials to resign in protest and precipitated further turmoil at the embattled agency.
The FDA has approved 3 updated COVID-19 vaccines targeted to currently circulating strains: an mRNA vaccine from Moderna (Spikevax) for those aged 6 months or older; an mRNA vaccine from Pfizer/BioNTech (Comirnaty) for those aged ≥ 5 years; and a protein subunit vaccine from Novavax (Nuvaxovid) for those aged ≥ 12 years. However, approvals restricting the scope of these approvals to certain high-risk groups, combined with the ACIP’s recent decision to not explicitly recommend them for any group, have complicated access for many patients.
Medical groups, including the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American College of Obstetricians and Gynecologists (ACOG), have published their own recommendations (Table). Of note, in opposition to the FDA and ACIP, the AAP and AAFP strongly recommend routine vaccination for children aged 6 to 23 months because they have the highest risk for hospitalization. The AAFP and ACOG both recommend COVID-19 vaccination in pregnancy to protect the pregnant patient and provide passive antibody protection to their infants up to 6 months of age. The Vaccine Integrity Project’s review of 12 safety studies published since June 2024 found that mRNA vaccines were not associated with increases in any adverse maternal or infant outcomes and had a possible protective effect against preterm birth.
In my previous commentary, 70% of Medscape readers indicated that they would follow vaccination recommendations from AAP even if they differed from CDC guidance. Administering vaccines outside of FDA labeling indications (i.e., “off label”) typically requires a physician’s prescription, which will almost certainly reduce COVID-19 vaccine uptake in children and pregnant patients, given that most people received these shots in pharmacies during the 2024-25 season. CVS and Walgreens, the country’s two largest pharmacy chains, are requiring physician prescriptions or waiting for ACIP guidance to make the new vaccines available in many states. However, an increasing number of states have implemented executive orders or passed legislation to permit pharmacists to provide vaccines to anyone who wants them. For example, the Pennsylvania State Board of Pharmacy voted unanimously to issue guidance that would allow pharmacists to administer any vaccines recommended by AAFP, AAP, or ACOG.
Erosion of vaccine uptake could easily worsen the burden of illness for our patients and the health system. Navigating the unnecessarily complex landscape of COVID-19 vaccines will be challenging, but it remains worthwhile.
Risk group | FDA | ACIP/HHS | AAFP | AAP | ACOG |
|---|---|---|---|---|---|
Adults aged > 65 | Approved | Shared decision-making | Recommend | N/A | N/A |
6 months to 64 years with high-risk condition | Approved | Shared decision-making | Recommend | Recommend | NA |
Pregnant patients | Unclear, but pregnancy included as high-risk condition | Not approved | Recommend | NA | Recommend |
Children and adults without risk factors | Not approved | Shared decision-making | Recommend for age 6-23 months and administer to all others who desire it | Recommend for age 6-23 months and administer to all others who desire it | NA |
Kenneth W. Lin, MD, MPH, Associate Director, Department of Family Medicine, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: UpToDate; American Academy of Family Physicians; Archdiocese of Washington; Association of Prevention Teaching and Research.
A version of this article appeared on Medscape.com.
Hi, everyone. I’m Dr Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
The receding of the pandemic and the understandable desire to return to normalcy has made COVID-19 vaccines a lower priority for many of our patients. However, family physicians should keep in mind that from October 1, 2024, to September 6, 2025, COVID-19 was responsible for an estimated 3.2 to 4.6 million outpatient visits, 360,000 to 520,000 hospitalizations, and 42,000 to 60,000 deaths.
In a previous commentary, I discussed the worsening disconnect between the evidence supporting the effectiveness and safety of vaccinations and increasing reluctance of patients and parents to receive them, fueled by misinformation from federal health agencies and the packing of the Advisory Committee on Immunization Practices (ACIP) with vaccine skeptics. Since then, Secretary of Health and Human Services (HHS), Robert F. Kennedy, Jr, has fired Dr Susan Monarez, his handpicked director of the CDC. This caused three senior CDC officials to resign in protest and precipitated further turmoil at the embattled agency.
The FDA has approved 3 updated COVID-19 vaccines targeted to currently circulating strains: an mRNA vaccine from Moderna (Spikevax) for those aged 6 months or older; an mRNA vaccine from Pfizer/BioNTech (Comirnaty) for those aged ≥ 5 years; and a protein subunit vaccine from Novavax (Nuvaxovid) for those aged ≥ 12 years. However, approvals restricting the scope of these approvals to certain high-risk groups, combined with the ACIP’s recent decision to not explicitly recommend them for any group, have complicated access for many patients.
Medical groups, including the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American College of Obstetricians and Gynecologists (ACOG), have published their own recommendations (Table). Of note, in opposition to the FDA and ACIP, the AAP and AAFP strongly recommend routine vaccination for children aged 6 to 23 months because they have the highest risk for hospitalization. The AAFP and ACOG both recommend COVID-19 vaccination in pregnancy to protect the pregnant patient and provide passive antibody protection to their infants up to 6 months of age. The Vaccine Integrity Project’s review of 12 safety studies published since June 2024 found that mRNA vaccines were not associated with increases in any adverse maternal or infant outcomes and had a possible protective effect against preterm birth.
In my previous commentary, 70% of Medscape readers indicated that they would follow vaccination recommendations from AAP even if they differed from CDC guidance. Administering vaccines outside of FDA labeling indications (i.e., “off label”) typically requires a physician’s prescription, which will almost certainly reduce COVID-19 vaccine uptake in children and pregnant patients, given that most people received these shots in pharmacies during the 2024-25 season. CVS and Walgreens, the country’s two largest pharmacy chains, are requiring physician prescriptions or waiting for ACIP guidance to make the new vaccines available in many states. However, an increasing number of states have implemented executive orders or passed legislation to permit pharmacists to provide vaccines to anyone who wants them. For example, the Pennsylvania State Board of Pharmacy voted unanimously to issue guidance that would allow pharmacists to administer any vaccines recommended by AAFP, AAP, or ACOG.
Erosion of vaccine uptake could easily worsen the burden of illness for our patients and the health system. Navigating the unnecessarily complex landscape of COVID-19 vaccines will be challenging, but it remains worthwhile.
Risk group | FDA | ACIP/HHS | AAFP | AAP | ACOG |
|---|---|---|---|---|---|
Adults aged > 65 | Approved | Shared decision-making | Recommend | N/A | N/A |
6 months to 64 years with high-risk condition | Approved | Shared decision-making | Recommend | Recommend | NA |
Pregnant patients | Unclear, but pregnancy included as high-risk condition | Not approved | Recommend | NA | Recommend |
Children and adults without risk factors | Not approved | Shared decision-making | Recommend for age 6-23 months and administer to all others who desire it | Recommend for age 6-23 months and administer to all others who desire it | NA |
Kenneth W. Lin, MD, MPH, Associate Director, Department of Family Medicine, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: UpToDate; American Academy of Family Physicians; Archdiocese of Washington; Association of Prevention Teaching and Research.
A version of this article appeared on Medscape.com.
Hi, everyone. I’m Dr Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
The receding of the pandemic and the understandable desire to return to normalcy has made COVID-19 vaccines a lower priority for many of our patients. However, family physicians should keep in mind that from October 1, 2024, to September 6, 2025, COVID-19 was responsible for an estimated 3.2 to 4.6 million outpatient visits, 360,000 to 520,000 hospitalizations, and 42,000 to 60,000 deaths.
In a previous commentary, I discussed the worsening disconnect between the evidence supporting the effectiveness and safety of vaccinations and increasing reluctance of patients and parents to receive them, fueled by misinformation from federal health agencies and the packing of the Advisory Committee on Immunization Practices (ACIP) with vaccine skeptics. Since then, Secretary of Health and Human Services (HHS), Robert F. Kennedy, Jr, has fired Dr Susan Monarez, his handpicked director of the CDC. This caused three senior CDC officials to resign in protest and precipitated further turmoil at the embattled agency.
The FDA has approved 3 updated COVID-19 vaccines targeted to currently circulating strains: an mRNA vaccine from Moderna (Spikevax) for those aged 6 months or older; an mRNA vaccine from Pfizer/BioNTech (Comirnaty) for those aged ≥ 5 years; and a protein subunit vaccine from Novavax (Nuvaxovid) for those aged ≥ 12 years. However, approvals restricting the scope of these approvals to certain high-risk groups, combined with the ACIP’s recent decision to not explicitly recommend them for any group, have complicated access for many patients.
Medical groups, including the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American College of Obstetricians and Gynecologists (ACOG), have published their own recommendations (Table). Of note, in opposition to the FDA and ACIP, the AAP and AAFP strongly recommend routine vaccination for children aged 6 to 23 months because they have the highest risk for hospitalization. The AAFP and ACOG both recommend COVID-19 vaccination in pregnancy to protect the pregnant patient and provide passive antibody protection to their infants up to 6 months of age. The Vaccine Integrity Project’s review of 12 safety studies published since June 2024 found that mRNA vaccines were not associated with increases in any adverse maternal or infant outcomes and had a possible protective effect against preterm birth.
In my previous commentary, 70% of Medscape readers indicated that they would follow vaccination recommendations from AAP even if they differed from CDC guidance. Administering vaccines outside of FDA labeling indications (i.e., “off label”) typically requires a physician’s prescription, which will almost certainly reduce COVID-19 vaccine uptake in children and pregnant patients, given that most people received these shots in pharmacies during the 2024-25 season. CVS and Walgreens, the country’s two largest pharmacy chains, are requiring physician prescriptions or waiting for ACIP guidance to make the new vaccines available in many states. However, an increasing number of states have implemented executive orders or passed legislation to permit pharmacists to provide vaccines to anyone who wants them. For example, the Pennsylvania State Board of Pharmacy voted unanimously to issue guidance that would allow pharmacists to administer any vaccines recommended by AAFP, AAP, or ACOG.
Erosion of vaccine uptake could easily worsen the burden of illness for our patients and the health system. Navigating the unnecessarily complex landscape of COVID-19 vaccines will be challenging, but it remains worthwhile.
Risk group | FDA | ACIP/HHS | AAFP | AAP | ACOG |
|---|---|---|---|---|---|
Adults aged > 65 | Approved | Shared decision-making | Recommend | N/A | N/A |
6 months to 64 years with high-risk condition | Approved | Shared decision-making | Recommend | Recommend | NA |
Pregnant patients | Unclear, but pregnancy included as high-risk condition | Not approved | Recommend | NA | Recommend |
Children and adults without risk factors | Not approved | Shared decision-making | Recommend for age 6-23 months and administer to all others who desire it | Recommend for age 6-23 months and administer to all others who desire it | NA |
Kenneth W. Lin, MD, MPH, Associate Director, Department of Family Medicine, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: UpToDate; American Academy of Family Physicians; Archdiocese of Washington; Association of Prevention Teaching and Research.
A version of this article appeared on Medscape.com.
Hospitalists Must Encourage Mental Stimulation for Patients
As a hospitalist, you are in a unique position to notice changes in your hospitalized patients. This frontline perspective can be used to improve inpatient attention and care, and differs from primary care, where a clinician might only see a patient once or twice a year, and subtle, gradual changes may be missed, said George Cao, MD, MBA, a hospitalist at the University of Vermont Medical Center in Burlington and assistant professor at UVM’s Larner College of Medicine.
But in the hospital, Cao said even small shifts — like becoming less active, eating less, or changes in personality — can become much more obvious.
“As hospitalists…we see patients throughout the day, in different situations, and often end up spending more time with them over the course of a week than their primary care provider might in a year,” Cao explained. “This gives us a real advantage in picking up on subtle changes in mental awareness.”
These assessments can also be evaluated with the benefit of daily labs, frequent bedside interactions, and 24–hour observations.
With older adults, Cao said it’s important to go beyond just what’s in the chart.
“I always start by reviewing notes from the primary care provider and previous admissions, but some of the most valuable insights come from talking with family and close friends to get a true sense of the patient’s baseline — how they usually think, move, and interact,” he said.
Why to Watch for Declining Mental Awareness
Declining mental awareness in the inpatient setting is often a sign of an underlying problem — whether that’s a reversible medical condition, unrecognized dementia, or the development of delirium, Cao said.
“On the inpatient side, I pay close attention to more than just memory loss,” he said.
Changes in how patients function day–to–day, shifts in their behavior, or even something as simple as not wanting to get out of bed can be early signs of an aging mind or untreated psychiatric issues, he noted.
“Of course, we always rule out infections and medication side effects, but I also look for other reversible causes like thyroid problems, electrolyte imbalances, low oxygen, pain, urinary retention, constipation, and nutritional deficiencies,” Cao said.
Of note, delirium is the most common cause of sudden mental status changes in the hospital, and “it’s easy to miss if you’re not looking for it.”
He summarized that classic signs are an acute and fluctuating course with changes in alertness, but added there are other red flags too: disorientation, hallucinations, changes in sleep patterns, sporadic unsafe behaviors, mood swings, and changes in activity level, whether that’s agitation or just being unusually quiet.
By combining what he notices bedside and what is learned from the medical record (and from the people who know the patient best), Cao said he’s able to catch these changes early, identify the underlying cause, and work toward the best possible outcome.
“One of the main interventions is providing mental stimulation,” he said.
Why Mental Stimulation Is So Vital
Mental stimulation of the patient is critical to recovery and may prevent prolonged illness, said Meghana R. Medavaram, MD, associate director of consultation liaison and emergency psychiatry at Montefiore Medical Center’s Weiler Hospital in New York City. “Keeping a patient active both physically and mentally can help prevent deconditioning and risks of prolonged immobility,” she said.
It’s important to note that when patients are out of their familiar routines, away from their usual environment and people, and their sleep is fragmented, this can make them even more vulnerable. Keeping patients mentally stimulated during their hospital stay can help maintain their attention, orientation, and a healthy sleep-wake cycle — all things that are easily thrown off in the hospital, Cao said.
“These disruptions hit the pathways that control attention, wakefulness, and the sleep–wake cycle. That’s when you see attention drifting, orientation fading, and circadian rhythms unraveling, especially at night, which is why “sundowning” is so common, Cao said, referring to the syndrome where older adults or people with dementia experience behavioral changes in late afternoon or evening. “Mental stimulation is critical in the hospital because when the brain isn’t active and gets disoriented, it becomes an easy target for delirium.”
He said delirium often develops in older adults when acute stressors like inflammation, low oxygen, metabolic imbalances, or sedating medications disrupt the brain’s arousal systems and networks, especially in older adults.
Therefore, Cao said, encourage your patients to be more engaged during the day through conversation, activities, or regular reorientation. “This supports the brain networks that help prevent inattention and confusion, which are the hallmarks of delirium. Daytime stimulation also helps build up the natural drive for nighttime sleep, so patients are less likely to nap during the day and be awake and disoriented at night.”
To support this, it’s helpful to schedule medications during waking hours instead of around–the–clock dosing that interrupts sleep, and to cluster nighttime care activities to minimize disturbances, Cao explained. Ensuring patients have their glasses, hearing aids, and familiar routines, along with encouraging mobility and hydration, further protects against delirium and supports patients’ cognitive health during hospitalization. “These same principles are just as important in outpatient subacute rehab settings and at home, so it’s essential to take home these strategies after discharge,” he said.
A Family Member or Friend May Help
Hospitalists can suggest straightforward ways to encourage families and friends to keep patients engaged during a hospital stay. Visits and chats can go a long way as conversations are incredibly grounding, Cao said. Other methods could be bringing in favorite foods or snacks, a phone chat or video call, or even showing prerecorded video messages. “These can be effective. Patients respond well to seeing and hearing familiar faces and voices, even if it’s just on a screen,” Cao said.
Beyond that, he said, activities such as watching and discussing the news, reading aloud, using tablets for games, watching movies, doing crossword puzzles, knitting, reminiscing, and playing word games can also be mentally stimulating for patients.
In addition, safe exercises/activities that patients can do in bed — with advice from physical therapy and occupational therapy — are beneficial, Medavaram said. “These often include gentle range–of-motion activities,” she said.
Share Importance of Mental Stimulation With Patients and Caregivers
If a hospitalist wants to motivate patients to keep their minds active, the framing should be simple, positive, and tied directly to their goals of getting better and getting home, said Medavaram. She provided this script suggestion:
“One of the best ways to help your recovery isn’t just taking your medicine, it’s keeping your mind active. When you’re in the hospital, it’s easy to spend the day lying in bed and staring at the TV in your room, but that can make your brain slow down and even cause confusion. Simple things — like reading, talking with visitors, doing puzzles, listening to music you enjoy, or telling a nurse about your favorite memories — can keep your brain sharp. Staying mentally active helps your thinking stay clear and can even help you get home sooner. Think of it like physical therapy for your brain.”
A version of this article first appeared on Medscape.com.
As a hospitalist, you are in a unique position to notice changes in your hospitalized patients. This frontline perspective can be used to improve inpatient attention and care, and differs from primary care, where a clinician might only see a patient once or twice a year, and subtle, gradual changes may be missed, said George Cao, MD, MBA, a hospitalist at the University of Vermont Medical Center in Burlington and assistant professor at UVM’s Larner College of Medicine.
But in the hospital, Cao said even small shifts — like becoming less active, eating less, or changes in personality — can become much more obvious.
“As hospitalists…we see patients throughout the day, in different situations, and often end up spending more time with them over the course of a week than their primary care provider might in a year,” Cao explained. “This gives us a real advantage in picking up on subtle changes in mental awareness.”
These assessments can also be evaluated with the benefit of daily labs, frequent bedside interactions, and 24–hour observations.
With older adults, Cao said it’s important to go beyond just what’s in the chart.
“I always start by reviewing notes from the primary care provider and previous admissions, but some of the most valuable insights come from talking with family and close friends to get a true sense of the patient’s baseline — how they usually think, move, and interact,” he said.
Why to Watch for Declining Mental Awareness
Declining mental awareness in the inpatient setting is often a sign of an underlying problem — whether that’s a reversible medical condition, unrecognized dementia, or the development of delirium, Cao said.
“On the inpatient side, I pay close attention to more than just memory loss,” he said.
Changes in how patients function day–to–day, shifts in their behavior, or even something as simple as not wanting to get out of bed can be early signs of an aging mind or untreated psychiatric issues, he noted.
“Of course, we always rule out infections and medication side effects, but I also look for other reversible causes like thyroid problems, electrolyte imbalances, low oxygen, pain, urinary retention, constipation, and nutritional deficiencies,” Cao said.
Of note, delirium is the most common cause of sudden mental status changes in the hospital, and “it’s easy to miss if you’re not looking for it.”
He summarized that classic signs are an acute and fluctuating course with changes in alertness, but added there are other red flags too: disorientation, hallucinations, changes in sleep patterns, sporadic unsafe behaviors, mood swings, and changes in activity level, whether that’s agitation or just being unusually quiet.
By combining what he notices bedside and what is learned from the medical record (and from the people who know the patient best), Cao said he’s able to catch these changes early, identify the underlying cause, and work toward the best possible outcome.
“One of the main interventions is providing mental stimulation,” he said.
Why Mental Stimulation Is So Vital
Mental stimulation of the patient is critical to recovery and may prevent prolonged illness, said Meghana R. Medavaram, MD, associate director of consultation liaison and emergency psychiatry at Montefiore Medical Center’s Weiler Hospital in New York City. “Keeping a patient active both physically and mentally can help prevent deconditioning and risks of prolonged immobility,” she said.
It’s important to note that when patients are out of their familiar routines, away from their usual environment and people, and their sleep is fragmented, this can make them even more vulnerable. Keeping patients mentally stimulated during their hospital stay can help maintain their attention, orientation, and a healthy sleep-wake cycle — all things that are easily thrown off in the hospital, Cao said.
“These disruptions hit the pathways that control attention, wakefulness, and the sleep–wake cycle. That’s when you see attention drifting, orientation fading, and circadian rhythms unraveling, especially at night, which is why “sundowning” is so common, Cao said, referring to the syndrome where older adults or people with dementia experience behavioral changes in late afternoon or evening. “Mental stimulation is critical in the hospital because when the brain isn’t active and gets disoriented, it becomes an easy target for delirium.”
He said delirium often develops in older adults when acute stressors like inflammation, low oxygen, metabolic imbalances, or sedating medications disrupt the brain’s arousal systems and networks, especially in older adults.
Therefore, Cao said, encourage your patients to be more engaged during the day through conversation, activities, or regular reorientation. “This supports the brain networks that help prevent inattention and confusion, which are the hallmarks of delirium. Daytime stimulation also helps build up the natural drive for nighttime sleep, so patients are less likely to nap during the day and be awake and disoriented at night.”
To support this, it’s helpful to schedule medications during waking hours instead of around–the–clock dosing that interrupts sleep, and to cluster nighttime care activities to minimize disturbances, Cao explained. Ensuring patients have their glasses, hearing aids, and familiar routines, along with encouraging mobility and hydration, further protects against delirium and supports patients’ cognitive health during hospitalization. “These same principles are just as important in outpatient subacute rehab settings and at home, so it’s essential to take home these strategies after discharge,” he said.
A Family Member or Friend May Help
Hospitalists can suggest straightforward ways to encourage families and friends to keep patients engaged during a hospital stay. Visits and chats can go a long way as conversations are incredibly grounding, Cao said. Other methods could be bringing in favorite foods or snacks, a phone chat or video call, or even showing prerecorded video messages. “These can be effective. Patients respond well to seeing and hearing familiar faces and voices, even if it’s just on a screen,” Cao said.
Beyond that, he said, activities such as watching and discussing the news, reading aloud, using tablets for games, watching movies, doing crossword puzzles, knitting, reminiscing, and playing word games can also be mentally stimulating for patients.
In addition, safe exercises/activities that patients can do in bed — with advice from physical therapy and occupational therapy — are beneficial, Medavaram said. “These often include gentle range–of-motion activities,” she said.
Share Importance of Mental Stimulation With Patients and Caregivers
If a hospitalist wants to motivate patients to keep their minds active, the framing should be simple, positive, and tied directly to their goals of getting better and getting home, said Medavaram. She provided this script suggestion:
“One of the best ways to help your recovery isn’t just taking your medicine, it’s keeping your mind active. When you’re in the hospital, it’s easy to spend the day lying in bed and staring at the TV in your room, but that can make your brain slow down and even cause confusion. Simple things — like reading, talking with visitors, doing puzzles, listening to music you enjoy, or telling a nurse about your favorite memories — can keep your brain sharp. Staying mentally active helps your thinking stay clear and can even help you get home sooner. Think of it like physical therapy for your brain.”
A version of this article first appeared on Medscape.com.
As a hospitalist, you are in a unique position to notice changes in your hospitalized patients. This frontline perspective can be used to improve inpatient attention and care, and differs from primary care, where a clinician might only see a patient once or twice a year, and subtle, gradual changes may be missed, said George Cao, MD, MBA, a hospitalist at the University of Vermont Medical Center in Burlington and assistant professor at UVM’s Larner College of Medicine.
But in the hospital, Cao said even small shifts — like becoming less active, eating less, or changes in personality — can become much more obvious.
“As hospitalists…we see patients throughout the day, in different situations, and often end up spending more time with them over the course of a week than their primary care provider might in a year,” Cao explained. “This gives us a real advantage in picking up on subtle changes in mental awareness.”
These assessments can also be evaluated with the benefit of daily labs, frequent bedside interactions, and 24–hour observations.
With older adults, Cao said it’s important to go beyond just what’s in the chart.
“I always start by reviewing notes from the primary care provider and previous admissions, but some of the most valuable insights come from talking with family and close friends to get a true sense of the patient’s baseline — how they usually think, move, and interact,” he said.
Why to Watch for Declining Mental Awareness
Declining mental awareness in the inpatient setting is often a sign of an underlying problem — whether that’s a reversible medical condition, unrecognized dementia, or the development of delirium, Cao said.
“On the inpatient side, I pay close attention to more than just memory loss,” he said.
Changes in how patients function day–to–day, shifts in their behavior, or even something as simple as not wanting to get out of bed can be early signs of an aging mind or untreated psychiatric issues, he noted.
“Of course, we always rule out infections and medication side effects, but I also look for other reversible causes like thyroid problems, electrolyte imbalances, low oxygen, pain, urinary retention, constipation, and nutritional deficiencies,” Cao said.
Of note, delirium is the most common cause of sudden mental status changes in the hospital, and “it’s easy to miss if you’re not looking for it.”
He summarized that classic signs are an acute and fluctuating course with changes in alertness, but added there are other red flags too: disorientation, hallucinations, changes in sleep patterns, sporadic unsafe behaviors, mood swings, and changes in activity level, whether that’s agitation or just being unusually quiet.
By combining what he notices bedside and what is learned from the medical record (and from the people who know the patient best), Cao said he’s able to catch these changes early, identify the underlying cause, and work toward the best possible outcome.
“One of the main interventions is providing mental stimulation,” he said.
Why Mental Stimulation Is So Vital
Mental stimulation of the patient is critical to recovery and may prevent prolonged illness, said Meghana R. Medavaram, MD, associate director of consultation liaison and emergency psychiatry at Montefiore Medical Center’s Weiler Hospital in New York City. “Keeping a patient active both physically and mentally can help prevent deconditioning and risks of prolonged immobility,” she said.
It’s important to note that when patients are out of their familiar routines, away from their usual environment and people, and their sleep is fragmented, this can make them even more vulnerable. Keeping patients mentally stimulated during their hospital stay can help maintain their attention, orientation, and a healthy sleep-wake cycle — all things that are easily thrown off in the hospital, Cao said.
“These disruptions hit the pathways that control attention, wakefulness, and the sleep–wake cycle. That’s when you see attention drifting, orientation fading, and circadian rhythms unraveling, especially at night, which is why “sundowning” is so common, Cao said, referring to the syndrome where older adults or people with dementia experience behavioral changes in late afternoon or evening. “Mental stimulation is critical in the hospital because when the brain isn’t active and gets disoriented, it becomes an easy target for delirium.”
He said delirium often develops in older adults when acute stressors like inflammation, low oxygen, metabolic imbalances, or sedating medications disrupt the brain’s arousal systems and networks, especially in older adults.
Therefore, Cao said, encourage your patients to be more engaged during the day through conversation, activities, or regular reorientation. “This supports the brain networks that help prevent inattention and confusion, which are the hallmarks of delirium. Daytime stimulation also helps build up the natural drive for nighttime sleep, so patients are less likely to nap during the day and be awake and disoriented at night.”
To support this, it’s helpful to schedule medications during waking hours instead of around–the–clock dosing that interrupts sleep, and to cluster nighttime care activities to minimize disturbances, Cao explained. Ensuring patients have their glasses, hearing aids, and familiar routines, along with encouraging mobility and hydration, further protects against delirium and supports patients’ cognitive health during hospitalization. “These same principles are just as important in outpatient subacute rehab settings and at home, so it’s essential to take home these strategies after discharge,” he said.
A Family Member or Friend May Help
Hospitalists can suggest straightforward ways to encourage families and friends to keep patients engaged during a hospital stay. Visits and chats can go a long way as conversations are incredibly grounding, Cao said. Other methods could be bringing in favorite foods or snacks, a phone chat or video call, or even showing prerecorded video messages. “These can be effective. Patients respond well to seeing and hearing familiar faces and voices, even if it’s just on a screen,” Cao said.
Beyond that, he said, activities such as watching and discussing the news, reading aloud, using tablets for games, watching movies, doing crossword puzzles, knitting, reminiscing, and playing word games can also be mentally stimulating for patients.
In addition, safe exercises/activities that patients can do in bed — with advice from physical therapy and occupational therapy — are beneficial, Medavaram said. “These often include gentle range–of-motion activities,” she said.
Share Importance of Mental Stimulation With Patients and Caregivers
If a hospitalist wants to motivate patients to keep their minds active, the framing should be simple, positive, and tied directly to their goals of getting better and getting home, said Medavaram. She provided this script suggestion:
“One of the best ways to help your recovery isn’t just taking your medicine, it’s keeping your mind active. When you’re in the hospital, it’s easy to spend the day lying in bed and staring at the TV in your room, but that can make your brain slow down and even cause confusion. Simple things — like reading, talking with visitors, doing puzzles, listening to music you enjoy, or telling a nurse about your favorite memories — can keep your brain sharp. Staying mentally active helps your thinking stay clear and can even help you get home sooner. Think of it like physical therapy for your brain.”
A version of this article first appeared on Medscape.com.
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.


The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).

The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.


The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).

The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.


The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).

The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
PRACTICE POINTS
- Tapinarof cream 1% can be absorbed systemically and cause acute generalized exanthematous pustulosis (AGEP).
- Clinical configuration and histology can be useful to distinguish AGEP from mimickers.
- Topical application of drugs in general, particularly over large body surface areas, may lead to systemic drug eruptions.
Celebrating VA Physicians in Gastroenterology
Last month, I had the privilege of joining more than one hundred physician colleagues in Washington, DC, for AGA Advocacy Day. While standing amidst the majesty of the Capital, I found myself deeply appreciative for those who dedicate their time and energy to public service. Many of these dedicated federal workers choose to be in DC because of a sincere belief in their mission.
Among these mission-driven public servants are federal employees who work in the Department of Veterans Affairs (VA). As a member of this group, I come to work energized by the mission to care for those who have served in our military. In my clinical practice, I am reminded regularly of the sacrifices of veterans and their families. This month, and especially on Veterans Day, I hope we will take a moment to express gratitude to veterans for their service to our country.
Many young gastroenterologists may not know that it was the landmark VA Cooperative Study #380, led by Dr. David Lieberman (Portland VA) that helped push Medicare to cover reimbursement for screening colonoscopy. Today, one of the most important ongoing studies in our field – VA Cooperative Study #577 – continues the VA tradition of high-impact health services research. Launched in 2012, the study has enrolled 50,000 veterans to compare FIT and colonoscopy. It is led by Dr. Jason Dominitz (Seattle VA) and Dr. Doug Robertson (White River Junction VA).
Beyond research, VA gastroenterologists play a critical role in training the next generation of clinicians. Over 700 gastroenterologists count the VA as a clinical home, making it the largest GI group practice in the country. Many of us — myself included — were trained or mentored by VA physicians whose dedication to service and science has shaped our careers and the field at large.
This month’s issue of GI & Hepatology News has stories about other important contributions to our field. The stories and perspective pieces on Artificial Intelligence are particularly poignant given the announcement last month on the awarding of the Nobel Prize in economics to researchers who study “creative destruction,” the way in which one technological innovation renders others obsolete. Perhaps this award offers another reason to reemphasize and embrace the “art” of medicine.
The views expressed here are my own and do not necessarily reflect the official policy or position of the U.S. Department of Veterans Affairs or the United States Government.
Ziad Gellad, MD, MPH, AGAF
Associate Editor
Last month, I had the privilege of joining more than one hundred physician colleagues in Washington, DC, for AGA Advocacy Day. While standing amidst the majesty of the Capital, I found myself deeply appreciative for those who dedicate their time and energy to public service. Many of these dedicated federal workers choose to be in DC because of a sincere belief in their mission.
Among these mission-driven public servants are federal employees who work in the Department of Veterans Affairs (VA). As a member of this group, I come to work energized by the mission to care for those who have served in our military. In my clinical practice, I am reminded regularly of the sacrifices of veterans and their families. This month, and especially on Veterans Day, I hope we will take a moment to express gratitude to veterans for their service to our country.
Many young gastroenterologists may not know that it was the landmark VA Cooperative Study #380, led by Dr. David Lieberman (Portland VA) that helped push Medicare to cover reimbursement for screening colonoscopy. Today, one of the most important ongoing studies in our field – VA Cooperative Study #577 – continues the VA tradition of high-impact health services research. Launched in 2012, the study has enrolled 50,000 veterans to compare FIT and colonoscopy. It is led by Dr. Jason Dominitz (Seattle VA) and Dr. Doug Robertson (White River Junction VA).
Beyond research, VA gastroenterologists play a critical role in training the next generation of clinicians. Over 700 gastroenterologists count the VA as a clinical home, making it the largest GI group practice in the country. Many of us — myself included — were trained or mentored by VA physicians whose dedication to service and science has shaped our careers and the field at large.
This month’s issue of GI & Hepatology News has stories about other important contributions to our field. The stories and perspective pieces on Artificial Intelligence are particularly poignant given the announcement last month on the awarding of the Nobel Prize in economics to researchers who study “creative destruction,” the way in which one technological innovation renders others obsolete. Perhaps this award offers another reason to reemphasize and embrace the “art” of medicine.
The views expressed here are my own and do not necessarily reflect the official policy or position of the U.S. Department of Veterans Affairs or the United States Government.
Ziad Gellad, MD, MPH, AGAF
Associate Editor
Last month, I had the privilege of joining more than one hundred physician colleagues in Washington, DC, for AGA Advocacy Day. While standing amidst the majesty of the Capital, I found myself deeply appreciative for those who dedicate their time and energy to public service. Many of these dedicated federal workers choose to be in DC because of a sincere belief in their mission.
Among these mission-driven public servants are federal employees who work in the Department of Veterans Affairs (VA). As a member of this group, I come to work energized by the mission to care for those who have served in our military. In my clinical practice, I am reminded regularly of the sacrifices of veterans and their families. This month, and especially on Veterans Day, I hope we will take a moment to express gratitude to veterans for their service to our country.
Many young gastroenterologists may not know that it was the landmark VA Cooperative Study #380, led by Dr. David Lieberman (Portland VA) that helped push Medicare to cover reimbursement for screening colonoscopy. Today, one of the most important ongoing studies in our field – VA Cooperative Study #577 – continues the VA tradition of high-impact health services research. Launched in 2012, the study has enrolled 50,000 veterans to compare FIT and colonoscopy. It is led by Dr. Jason Dominitz (Seattle VA) and Dr. Doug Robertson (White River Junction VA).
Beyond research, VA gastroenterologists play a critical role in training the next generation of clinicians. Over 700 gastroenterologists count the VA as a clinical home, making it the largest GI group practice in the country. Many of us — myself included — were trained or mentored by VA physicians whose dedication to service and science has shaped our careers and the field at large.
This month’s issue of GI & Hepatology News has stories about other important contributions to our field. The stories and perspective pieces on Artificial Intelligence are particularly poignant given the announcement last month on the awarding of the Nobel Prize in economics to researchers who study “creative destruction,” the way in which one technological innovation renders others obsolete. Perhaps this award offers another reason to reemphasize and embrace the “art” of medicine.
The views expressed here are my own and do not necessarily reflect the official policy or position of the U.S. Department of Veterans Affairs or the United States Government.
Ziad Gellad, MD, MPH, AGAF
Associate Editor