User login
Frailty may affect the expression of dementia
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
FROM LANCET NEUROLOGY
Key clinical point: Frailty modifies the association between Alzheimer’s disease pathology and Alzheimer dementia.
Major finding: Frailty index score (odds ratio, 1.76) is independently associated with dementia status.
Study details: A cross-sectional analysis of 456 deceased participants in the Rush Memory and Aging Project.
Disclosures: The study had no outside funding.
Source: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
Obesity paradox applies to post-stroke mortality
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.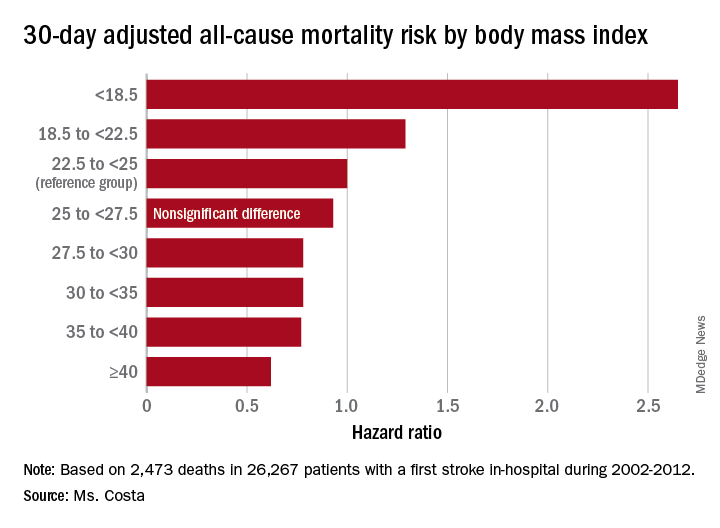
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Heavier stroke patients have lower 30-day and 1-year all-cause mortality.
Major finding: The 30-day stroke-related mortality rate after in-hospital stroke was reduced by 45% in VA patients with Class III obesity.
Study details: This retrospective study looked at the relationship between body mass index and post-stroke mortality in more than 26,000 veterans who had an inpatient stroke, with extensive adjustments made for potential confounders.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was sponsored by the Department of Veterans Affairs.
Source: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
FDA approves generic version of vigabatrin
The drug is approved for the adjunctive treatment of focal seizures in patients aged 10 years and older who have not had an adequate response to other therapies.
The approval was granted to Teva Pharmaceuticals.
An FDA announcement noted that the agency has prioritized the approval of generic versions of drugs to improve access to treatments and to lower drug costs. Vigabatrin had been included on an FDA list of off-patent, off-exclusivity branded drugs without approved generics. The approval of generic vigabatrin “demonstrates that there is an open pathway to approving products like this one,” said FDA Commissioner Scott Gottlieb, MD.
The label for vigabatrin tablets includes a boxed warning for permanent vision loss. The generic vigabatrin tablets are part of a single shared-system Risk Evaluation and Mitigation Strategy (REMS) program with other drug products containing vigabatrin.
The most common side effects associated with vigabatrin tablets include dizziness, fatigue, sleepiness, involuntary eye movement, tremor, blurred vision, memory impairment, weight gain, joint pain, upper respiratory tract infection, aggression, double vision, abnormal coordination, and a confused state. Serious side effects associated with vigabatrin tablets include permanent vision loss and risk of suicidal thoughts or actions.
The drug is approved for the adjunctive treatment of focal seizures in patients aged 10 years and older who have not had an adequate response to other therapies.
The approval was granted to Teva Pharmaceuticals.
An FDA announcement noted that the agency has prioritized the approval of generic versions of drugs to improve access to treatments and to lower drug costs. Vigabatrin had been included on an FDA list of off-patent, off-exclusivity branded drugs without approved generics. The approval of generic vigabatrin “demonstrates that there is an open pathway to approving products like this one,” said FDA Commissioner Scott Gottlieb, MD.
The label for vigabatrin tablets includes a boxed warning for permanent vision loss. The generic vigabatrin tablets are part of a single shared-system Risk Evaluation and Mitigation Strategy (REMS) program with other drug products containing vigabatrin.
The most common side effects associated with vigabatrin tablets include dizziness, fatigue, sleepiness, involuntary eye movement, tremor, blurred vision, memory impairment, weight gain, joint pain, upper respiratory tract infection, aggression, double vision, abnormal coordination, and a confused state. Serious side effects associated with vigabatrin tablets include permanent vision loss and risk of suicidal thoughts or actions.
The drug is approved for the adjunctive treatment of focal seizures in patients aged 10 years and older who have not had an adequate response to other therapies.
The approval was granted to Teva Pharmaceuticals.
An FDA announcement noted that the agency has prioritized the approval of generic versions of drugs to improve access to treatments and to lower drug costs. Vigabatrin had been included on an FDA list of off-patent, off-exclusivity branded drugs without approved generics. The approval of generic vigabatrin “demonstrates that there is an open pathway to approving products like this one,” said FDA Commissioner Scott Gottlieb, MD.
The label for vigabatrin tablets includes a boxed warning for permanent vision loss. The generic vigabatrin tablets are part of a single shared-system Risk Evaluation and Mitigation Strategy (REMS) program with other drug products containing vigabatrin.
The most common side effects associated with vigabatrin tablets include dizziness, fatigue, sleepiness, involuntary eye movement, tremor, blurred vision, memory impairment, weight gain, joint pain, upper respiratory tract infection, aggression, double vision, abnormal coordination, and a confused state. Serious side effects associated with vigabatrin tablets include permanent vision loss and risk of suicidal thoughts or actions.
Prioritize oral route for inpatient opioids with subcutaneous route as alternative
Clinical question: Can adoption of a local opioid standard of practice for hospitalized patients reduce intravenous and overall opioid exposure while providing effective pain control?
Background: Inpatient use of intravenous opioids may be excessive, considering that oral opioids may provide more consistent pain control with less risk of adverse effects. If oral treatment is not possible, subcutaneous administration of opioids is an effective and possibly less addictive alternative to the intravenous route.
Study design: Historical control pilot study.
Setting: Single adult general medicine unit in an urban academic medical center.
Synopsis: A 6-month historical period with 287 patients was compared with a 3-month intervention period with 127 patients. The intervention consisted of a clinical practice standard that was presented to medical and nursing staff via didactic sessions and email. The standard recommended the oral route for opioids in patients tolerating oral intake and endorsed subcutaneous over intravenous administration.
Intravenous doses decreased by 84% (0.06 vs. 0.39 doses/patient-day; P less than .001), the daily rate of patients receiving any parenteral opioid decreased by 57% (6% vs. 14%; P less than .001), and the mean daily overall morphine-milligram equivalents decreased by 31% (6.30 vs. 9.11). Pain scores were unchanged for hospital days 1 through 3 but were significantly improved on day 4 (P = .004) and day 5 (P = .009).
Limitations of this study include the small number of patients on one unit, in one institution, with one clinician group. Attractive features of the intervention include its scalability and potential for augmentation via additional processes such as EHR changes, prescribing restrictions, and pharmacy monitoring.
Bottom line: A standard of practice intervention with peer-to-peer education was associated with decreased intravenous opioid exposure, decreased total opioid exposure, and effective pain control.
Citation: Ackerman AL et al. Association of an opioid standard of practice intervention with intravenous opioid exposure in hospitalized patients. JAMA Int Med. 2018;178(6):759-63.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Can adoption of a local opioid standard of practice for hospitalized patients reduce intravenous and overall opioid exposure while providing effective pain control?
Background: Inpatient use of intravenous opioids may be excessive, considering that oral opioids may provide more consistent pain control with less risk of adverse effects. If oral treatment is not possible, subcutaneous administration of opioids is an effective and possibly less addictive alternative to the intravenous route.
Study design: Historical control pilot study.
Setting: Single adult general medicine unit in an urban academic medical center.
Synopsis: A 6-month historical period with 287 patients was compared with a 3-month intervention period with 127 patients. The intervention consisted of a clinical practice standard that was presented to medical and nursing staff via didactic sessions and email. The standard recommended the oral route for opioids in patients tolerating oral intake and endorsed subcutaneous over intravenous administration.
Intravenous doses decreased by 84% (0.06 vs. 0.39 doses/patient-day; P less than .001), the daily rate of patients receiving any parenteral opioid decreased by 57% (6% vs. 14%; P less than .001), and the mean daily overall morphine-milligram equivalents decreased by 31% (6.30 vs. 9.11). Pain scores were unchanged for hospital days 1 through 3 but were significantly improved on day 4 (P = .004) and day 5 (P = .009).
Limitations of this study include the small number of patients on one unit, in one institution, with one clinician group. Attractive features of the intervention include its scalability and potential for augmentation via additional processes such as EHR changes, prescribing restrictions, and pharmacy monitoring.
Bottom line: A standard of practice intervention with peer-to-peer education was associated with decreased intravenous opioid exposure, decreased total opioid exposure, and effective pain control.
Citation: Ackerman AL et al. Association of an opioid standard of practice intervention with intravenous opioid exposure in hospitalized patients. JAMA Int Med. 2018;178(6):759-63.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Can adoption of a local opioid standard of practice for hospitalized patients reduce intravenous and overall opioid exposure while providing effective pain control?
Background: Inpatient use of intravenous opioids may be excessive, considering that oral opioids may provide more consistent pain control with less risk of adverse effects. If oral treatment is not possible, subcutaneous administration of opioids is an effective and possibly less addictive alternative to the intravenous route.
Study design: Historical control pilot study.
Setting: Single adult general medicine unit in an urban academic medical center.
Synopsis: A 6-month historical period with 287 patients was compared with a 3-month intervention period with 127 patients. The intervention consisted of a clinical practice standard that was presented to medical and nursing staff via didactic sessions and email. The standard recommended the oral route for opioids in patients tolerating oral intake and endorsed subcutaneous over intravenous administration.
Intravenous doses decreased by 84% (0.06 vs. 0.39 doses/patient-day; P less than .001), the daily rate of patients receiving any parenteral opioid decreased by 57% (6% vs. 14%; P less than .001), and the mean daily overall morphine-milligram equivalents decreased by 31% (6.30 vs. 9.11). Pain scores were unchanged for hospital days 1 through 3 but were significantly improved on day 4 (P = .004) and day 5 (P = .009).
Limitations of this study include the small number of patients on one unit, in one institution, with one clinician group. Attractive features of the intervention include its scalability and potential for augmentation via additional processes such as EHR changes, prescribing restrictions, and pharmacy monitoring.
Bottom line: A standard of practice intervention with peer-to-peer education was associated with decreased intravenous opioid exposure, decreased total opioid exposure, and effective pain control.
Citation: Ackerman AL et al. Association of an opioid standard of practice intervention with intravenous opioid exposure in hospitalized patients. JAMA Int Med. 2018;178(6):759-63.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
DMTs, stem cell transplants both reduce disease progression in MS
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
FROM JAMA
Know the red flags for synaptic autoimmune psychosis
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 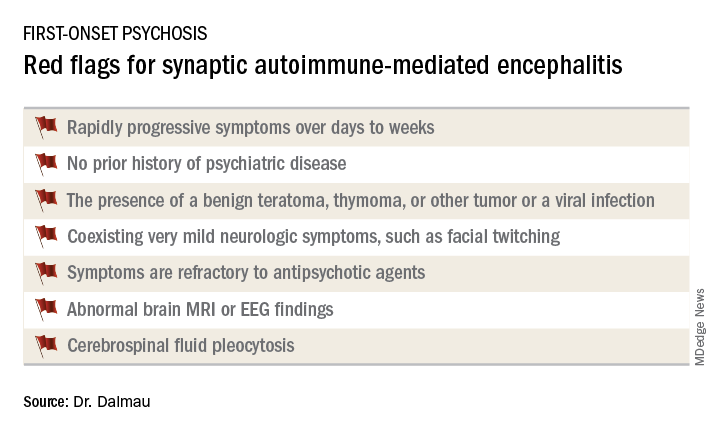
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
REPORTING FROM THE ECNP CONGRESS
Tic disorders are associated with obesity and diabetes
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
FROM JAMA NEUROLOGY
Key clinical point: Monitor cardiometabolic health in patients with Tourette syndrome or chronic tic disorder.
Major finding: Patients with Tourette syndrome or chronic tic disorder have a higher risk of metabolic or cardiovascular disorders, compared with the general population (adjusted hazard ratio, 1.99) and sibling controls (adjusted hazard ratio, 1.37).
Study details: A Swedish longitudinal, population-based cohort study of 7,804 individuals with Tourette syndrome or chronic tic disorder.
Disclosures: The study was supported by a research grant from Tourettes Action. Authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
Source: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
Population-level rate of SUDEP may have decreased
NEW ORLEANS – according to data described at the annual meeting of the American Epilepsy Society. Whether this decrease resulted from an improved understanding of SUDEP risk or a focus on risk-reduction strategies is unknown, said Daniel Friedman, MD, associate professor of neurology at the New York University Langone Health.
In addition, the rates of SUDEP in various populations differ according to their socioeconomic status. Differences in access to care are a potential, but unconfirmed, explanation for this association, said Dr. Friedman. Another possible explanation is that confounders such as mental health disorders, substance abuse, and insufficient social support affect individuals’ ability to manage their disorder.
Dr. Friedman and colleagues initially examined SUDEP rates over time in a cohort of patients who received vagus nerve stimulator (VNS) implantation for drug-resistant epilepsy. They analyzed data for 40,443 patients who underwent surgery during 1988-2012. The age-adjusted SUDEP rate per 1,000 person-years of follow-up decreased significantly from 2.47 in years 1-2 to 1.68 in years 3-10. “There was no control group, so we couldn’t necessarily attribute the SUDEP rate reduction to the intervention,” said Dr. Friedman. A study by Tomson et al of patients with epilepsy who received VNS implantation had similar findings.
The literature about the mechanisms of SUDEP and reduction of SUDEP risk has increased in recent years. Neurologists have advocated for greater disclosure to patients of SUDEP risk, as well as better risk counseling. Dr. Friedman and his colleagues decided to investigate whether these factors have affected the risk of SUDEP during the past decade.
They retrospectively examined data for people whose deaths had been investigated at medical examiner’s offices in New York City, San Diego County, and Maryland. They focused on decedents for whom epilepsy or seizure was listed as a cause or contributor to death or as a comorbid condition on the death certificate. They reviewed all available reports, including investigator notes, autopsy reports, and medical records. Next, Dr. Friedman and his colleagues calculated the annual SUDEP rate as a proportion of the general population, estimated using annual Census and American Community Survey data. They used the Mann-Kendall test to analyze the trends in SUDEP rate during 2009-2015.
Of 1,466 deaths in people with epilepsy during this period, 1,124 were classified as definite SUDEP, probable SUDEP, or near SUDEP. Approximately 63% of SUDEP cases were male, and 45% were African-American. The mean age at death was 38 years.
Dr. Friedman’s group found a significant decrease in the overall incidence of SUDEP in the total population during 2009-2015. When they examined the three regions separately, they found decreases in SUDEP incidence in New York City and Maryland, but not in San Diego County. They found no difference in SUDEP rates by season or by day of the week.
In a subsequent analysis, Dr. Friedman and his colleagues adjudicated all deaths related to seizure and epilepsy in the three regions during 2009-2010 and 2014-2015 and identified all cases of definite and probable SUDEP. The estimated rate of SUDEP decreased by about 36% from the first period to the second period. SUDEP rates as a proportion of the total population in those regions also declined.
The investigators also examined differences in estimated SUDEP rates in the United States according to median household income. In New York, the zip codes with the highest SUDEP rates tended to have the lowest median household incomes. The zip codes in the lowest quartile of family household income had a SUDEP rate more than twice as high as that in the zip codes in the highest income quartile. This association held true for the period from 2009-2010 and for 2014-2015.
Dr. Friedman and colleagues received funding from Finding a Cure for Epilepsy and Seizures, which is affiliated with the NYU Comprehensive Epilepsy Center and NYU Langone Health.
SOURCE: Cihan E et al. AES 2018, Abstract 2.419.
NEW ORLEANS – according to data described at the annual meeting of the American Epilepsy Society. Whether this decrease resulted from an improved understanding of SUDEP risk or a focus on risk-reduction strategies is unknown, said Daniel Friedman, MD, associate professor of neurology at the New York University Langone Health.
In addition, the rates of SUDEP in various populations differ according to their socioeconomic status. Differences in access to care are a potential, but unconfirmed, explanation for this association, said Dr. Friedman. Another possible explanation is that confounders such as mental health disorders, substance abuse, and insufficient social support affect individuals’ ability to manage their disorder.
Dr. Friedman and colleagues initially examined SUDEP rates over time in a cohort of patients who received vagus nerve stimulator (VNS) implantation for drug-resistant epilepsy. They analyzed data for 40,443 patients who underwent surgery during 1988-2012. The age-adjusted SUDEP rate per 1,000 person-years of follow-up decreased significantly from 2.47 in years 1-2 to 1.68 in years 3-10. “There was no control group, so we couldn’t necessarily attribute the SUDEP rate reduction to the intervention,” said Dr. Friedman. A study by Tomson et al of patients with epilepsy who received VNS implantation had similar findings.
The literature about the mechanisms of SUDEP and reduction of SUDEP risk has increased in recent years. Neurologists have advocated for greater disclosure to patients of SUDEP risk, as well as better risk counseling. Dr. Friedman and his colleagues decided to investigate whether these factors have affected the risk of SUDEP during the past decade.
They retrospectively examined data for people whose deaths had been investigated at medical examiner’s offices in New York City, San Diego County, and Maryland. They focused on decedents for whom epilepsy or seizure was listed as a cause or contributor to death or as a comorbid condition on the death certificate. They reviewed all available reports, including investigator notes, autopsy reports, and medical records. Next, Dr. Friedman and his colleagues calculated the annual SUDEP rate as a proportion of the general population, estimated using annual Census and American Community Survey data. They used the Mann-Kendall test to analyze the trends in SUDEP rate during 2009-2015.
Of 1,466 deaths in people with epilepsy during this period, 1,124 were classified as definite SUDEP, probable SUDEP, or near SUDEP. Approximately 63% of SUDEP cases were male, and 45% were African-American. The mean age at death was 38 years.
Dr. Friedman’s group found a significant decrease in the overall incidence of SUDEP in the total population during 2009-2015. When they examined the three regions separately, they found decreases in SUDEP incidence in New York City and Maryland, but not in San Diego County. They found no difference in SUDEP rates by season or by day of the week.
In a subsequent analysis, Dr. Friedman and his colleagues adjudicated all deaths related to seizure and epilepsy in the three regions during 2009-2010 and 2014-2015 and identified all cases of definite and probable SUDEP. The estimated rate of SUDEP decreased by about 36% from the first period to the second period. SUDEP rates as a proportion of the total population in those regions also declined.
The investigators also examined differences in estimated SUDEP rates in the United States according to median household income. In New York, the zip codes with the highest SUDEP rates tended to have the lowest median household incomes. The zip codes in the lowest quartile of family household income had a SUDEP rate more than twice as high as that in the zip codes in the highest income quartile. This association held true for the period from 2009-2010 and for 2014-2015.
Dr. Friedman and colleagues received funding from Finding a Cure for Epilepsy and Seizures, which is affiliated with the NYU Comprehensive Epilepsy Center and NYU Langone Health.
SOURCE: Cihan E et al. AES 2018, Abstract 2.419.
NEW ORLEANS – according to data described at the annual meeting of the American Epilepsy Society. Whether this decrease resulted from an improved understanding of SUDEP risk or a focus on risk-reduction strategies is unknown, said Daniel Friedman, MD, associate professor of neurology at the New York University Langone Health.
In addition, the rates of SUDEP in various populations differ according to their socioeconomic status. Differences in access to care are a potential, but unconfirmed, explanation for this association, said Dr. Friedman. Another possible explanation is that confounders such as mental health disorders, substance abuse, and insufficient social support affect individuals’ ability to manage their disorder.
Dr. Friedman and colleagues initially examined SUDEP rates over time in a cohort of patients who received vagus nerve stimulator (VNS) implantation for drug-resistant epilepsy. They analyzed data for 40,443 patients who underwent surgery during 1988-2012. The age-adjusted SUDEP rate per 1,000 person-years of follow-up decreased significantly from 2.47 in years 1-2 to 1.68 in years 3-10. “There was no control group, so we couldn’t necessarily attribute the SUDEP rate reduction to the intervention,” said Dr. Friedman. A study by Tomson et al of patients with epilepsy who received VNS implantation had similar findings.
The literature about the mechanisms of SUDEP and reduction of SUDEP risk has increased in recent years. Neurologists have advocated for greater disclosure to patients of SUDEP risk, as well as better risk counseling. Dr. Friedman and his colleagues decided to investigate whether these factors have affected the risk of SUDEP during the past decade.
They retrospectively examined data for people whose deaths had been investigated at medical examiner’s offices in New York City, San Diego County, and Maryland. They focused on decedents for whom epilepsy or seizure was listed as a cause or contributor to death or as a comorbid condition on the death certificate. They reviewed all available reports, including investigator notes, autopsy reports, and medical records. Next, Dr. Friedman and his colleagues calculated the annual SUDEP rate as a proportion of the general population, estimated using annual Census and American Community Survey data. They used the Mann-Kendall test to analyze the trends in SUDEP rate during 2009-2015.
Of 1,466 deaths in people with epilepsy during this period, 1,124 were classified as definite SUDEP, probable SUDEP, or near SUDEP. Approximately 63% of SUDEP cases were male, and 45% were African-American. The mean age at death was 38 years.
Dr. Friedman’s group found a significant decrease in the overall incidence of SUDEP in the total population during 2009-2015. When they examined the three regions separately, they found decreases in SUDEP incidence in New York City and Maryland, but not in San Diego County. They found no difference in SUDEP rates by season or by day of the week.
In a subsequent analysis, Dr. Friedman and his colleagues adjudicated all deaths related to seizure and epilepsy in the three regions during 2009-2010 and 2014-2015 and identified all cases of definite and probable SUDEP. The estimated rate of SUDEP decreased by about 36% from the first period to the second period. SUDEP rates as a proportion of the total population in those regions also declined.
The investigators also examined differences in estimated SUDEP rates in the United States according to median household income. In New York, the zip codes with the highest SUDEP rates tended to have the lowest median household incomes. The zip codes in the lowest quartile of family household income had a SUDEP rate more than twice as high as that in the zip codes in the highest income quartile. This association held true for the period from 2009-2010 and for 2014-2015.
Dr. Friedman and colleagues received funding from Finding a Cure for Epilepsy and Seizures, which is affiliated with the NYU Comprehensive Epilepsy Center and NYU Langone Health.
SOURCE: Cihan E et al. AES 2018, Abstract 2.419.
REPORTING FROM AES 2018
Key clinical point: Data indicate a decline over time in the incidence of SUDEP.
Major finding: The incidence of SUDEP declined by 36% from 2009-2010 to 2014-2015.
Study details: A retrospective analysis of medical examiner data on 1,466 deaths in people with epilepsy.
Disclosures: Finding a Cure for Epilepsy and Seizures provided funding for the study.
Source: Cihan E et al. AES 2018, Abstract 2.419.
Deep sleep decreases, Alzheimer’s increases
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Sleep disorders in children with ADHD treated with off-label medications
Sleep problems in children diagnosed with attention-deficit/hyperactivity disorder are treated with a variety of medications, many off label for sleep and unstudied for safety and effectiveness in children, a study of Medicaid prescriptions has found.
“Sleep disorders coexist with attention-deficit/hyperactivity disorder (ADHD) for many children and are associated with neuropsychiatric, physiologic, and medication-related outcomes,” wrote Tracy Klein, PhD, of Washington State University, Vancouver, and her colleagues. The report is in the Journal of Pediatric Health Care. These patients can have sleep disordered breathing and behavioral issues occurring around bedtime. Known adverse effects of the stimulant and nonstimulant medications used to treat ADHD can include sleep disturbance, delayed circadian rhythm, insomnia, and somnolence. Yet, research on both sleep problems in children with ADHD and prescribing patterns is scanty, according to the investigators.
Dr. Klein and her colleagues conducted a study aimed at identifying the off-label medications being prescribed to potentiate sleep in children with ADHD, and the characteristics of the children and their prescribers. They used 5 years of pharmacy claims for children in Oregon insured through Medicaid and had a provider diagnosis of ADHD during Jan. 1, 2012, to Dec. 31, 2016. The children were aged 3-18 years and the prescriptions measured were the number of 30-day prescriptions. Prescribers were identified by national provider identifier taxonomies (nurse, physician, other prescriber), and classified as either generalist or specialist. The medications were classified as controlled or uncontrolled as determined by Title 21 of the U.S. Controlled Substances Act.
The data yielded 14,567 prescriptions for 2,518 children for a 30-day supply of medication known to potentiate sleep but off-label for children. Children aged 3-11 years comprised about 38% of these patients. Some children were prescribed more than one of these medications. Medications specifically on label for sleep but not indicated for children were not included. Those medications indicated for comorbid conditions and those indicated for ADHD that specifically cause somnolence were excluded.
The uncontrolled medications prescribed in this sample were amitriptyline, doxepin, hydroxyzine, low-dose quetiapine, and trazodone. The controlled medications identified were clonazepam and lorazepam, and a few prescriptions for phenobarbital.
Most of the prescriptions (63.8%) went to older children aged 12-18 years and most prescriptions (66.3%) went to males. The most commonly prescribed noncontrolled medication was trazodone (5,190 prescriptions), followed by hydroxyzine (2,539), and quetiapine (2,402). The most frequently prescribed controlled medication was clonazepam (2,145), followed by lorazepam (534).
Specialist prescribers wrote most of the prescriptions for this patient group, but no differences were found in prescribing patterns between specialists and generalists.
Dr. Klein and her colleagues noted that 871 unique children were prescribed 5,190 30-day−supply prescriptions for trazodone, including 23 children under age 5. Trazodone is a serotonin modulator indicated for the treatment of major depressive disorder, but has not been studied for safety and efficacy in children and has no Food and Drug Administration indication for children. “Hydroxyzine, quetiapine, and amitriptyline also were prescribed for a large number of children, including some for children as young as 3 years, despite lack of approval for use to induce to sleep and increased potential for significant adverse reactions in children,” they wrote.
Dr. Klein suggested that prescribers receive pressure from families to “do something” for their children, who may be disruptive day and night. “Prescribers may be unaware that trazodone, which is commonly used in practice, has never been approved for treatment of insomnia in children or adults. Insurance may not adequately fund other options, such as extensive behavioral therapy,” she stated in an interview. These medications come with some risk for children, Dr. Klein noted.
especially if their reaction to it is behavioral.” There is also potential for unanticipated drug interactions between off-label medications prescribed for sleep and drugs prescribed to treat ADHD.
This study has limitations related to the absence of detailed clinical explanatory information found in claims data. Information on adherence to treatment and adverse events, for example, is not contained in claims data. The study does not address the overall rates of sleep disorders in children with ADHD nor the percentage of children with ADHD who are prescribed any medication to potentiate sleep but looks at which off-label drugs are being prescribed, to which children, and by whom.
“Most medications prescribed in this study, used to induce sleep or treat insomnia, have not been studied for safety and efficacy in children, and their use should not be extrapolated from adult studies,” the researchers concluded.
They reported having no disclosures.
SOURCE: Klein T et al. J Pediatr Health Care. 2018 Jan 8. doi: 10.1016/j.pedhc.2018.10.002.
Sleep problems in children diagnosed with attention-deficit/hyperactivity disorder are treated with a variety of medications, many off label for sleep and unstudied for safety and effectiveness in children, a study of Medicaid prescriptions has found.
“Sleep disorders coexist with attention-deficit/hyperactivity disorder (ADHD) for many children and are associated with neuropsychiatric, physiologic, and medication-related outcomes,” wrote Tracy Klein, PhD, of Washington State University, Vancouver, and her colleagues. The report is in the Journal of Pediatric Health Care. These patients can have sleep disordered breathing and behavioral issues occurring around bedtime. Known adverse effects of the stimulant and nonstimulant medications used to treat ADHD can include sleep disturbance, delayed circadian rhythm, insomnia, and somnolence. Yet, research on both sleep problems in children with ADHD and prescribing patterns is scanty, according to the investigators.
Dr. Klein and her colleagues conducted a study aimed at identifying the off-label medications being prescribed to potentiate sleep in children with ADHD, and the characteristics of the children and their prescribers. They used 5 years of pharmacy claims for children in Oregon insured through Medicaid and had a provider diagnosis of ADHD during Jan. 1, 2012, to Dec. 31, 2016. The children were aged 3-18 years and the prescriptions measured were the number of 30-day prescriptions. Prescribers were identified by national provider identifier taxonomies (nurse, physician, other prescriber), and classified as either generalist or specialist. The medications were classified as controlled or uncontrolled as determined by Title 21 of the U.S. Controlled Substances Act.
The data yielded 14,567 prescriptions for 2,518 children for a 30-day supply of medication known to potentiate sleep but off-label for children. Children aged 3-11 years comprised about 38% of these patients. Some children were prescribed more than one of these medications. Medications specifically on label for sleep but not indicated for children were not included. Those medications indicated for comorbid conditions and those indicated for ADHD that specifically cause somnolence were excluded.
The uncontrolled medications prescribed in this sample were amitriptyline, doxepin, hydroxyzine, low-dose quetiapine, and trazodone. The controlled medications identified were clonazepam and lorazepam, and a few prescriptions for phenobarbital.
Most of the prescriptions (63.8%) went to older children aged 12-18 years and most prescriptions (66.3%) went to males. The most commonly prescribed noncontrolled medication was trazodone (5,190 prescriptions), followed by hydroxyzine (2,539), and quetiapine (2,402). The most frequently prescribed controlled medication was clonazepam (2,145), followed by lorazepam (534).
Specialist prescribers wrote most of the prescriptions for this patient group, but no differences were found in prescribing patterns between specialists and generalists.
Dr. Klein and her colleagues noted that 871 unique children were prescribed 5,190 30-day−supply prescriptions for trazodone, including 23 children under age 5. Trazodone is a serotonin modulator indicated for the treatment of major depressive disorder, but has not been studied for safety and efficacy in children and has no Food and Drug Administration indication for children. “Hydroxyzine, quetiapine, and amitriptyline also were prescribed for a large number of children, including some for children as young as 3 years, despite lack of approval for use to induce to sleep and increased potential for significant adverse reactions in children,” they wrote.
Dr. Klein suggested that prescribers receive pressure from families to “do something” for their children, who may be disruptive day and night. “Prescribers may be unaware that trazodone, which is commonly used in practice, has never been approved for treatment of insomnia in children or adults. Insurance may not adequately fund other options, such as extensive behavioral therapy,” she stated in an interview. These medications come with some risk for children, Dr. Klein noted.
especially if their reaction to it is behavioral.” There is also potential for unanticipated drug interactions between off-label medications prescribed for sleep and drugs prescribed to treat ADHD.
This study has limitations related to the absence of detailed clinical explanatory information found in claims data. Information on adherence to treatment and adverse events, for example, is not contained in claims data. The study does not address the overall rates of sleep disorders in children with ADHD nor the percentage of children with ADHD who are prescribed any medication to potentiate sleep but looks at which off-label drugs are being prescribed, to which children, and by whom.
“Most medications prescribed in this study, used to induce sleep or treat insomnia, have not been studied for safety and efficacy in children, and their use should not be extrapolated from adult studies,” the researchers concluded.
They reported having no disclosures.
SOURCE: Klein T et al. J Pediatr Health Care. 2018 Jan 8. doi: 10.1016/j.pedhc.2018.10.002.
Sleep problems in children diagnosed with attention-deficit/hyperactivity disorder are treated with a variety of medications, many off label for sleep and unstudied for safety and effectiveness in children, a study of Medicaid prescriptions has found.
“Sleep disorders coexist with attention-deficit/hyperactivity disorder (ADHD) for many children and are associated with neuropsychiatric, physiologic, and medication-related outcomes,” wrote Tracy Klein, PhD, of Washington State University, Vancouver, and her colleagues. The report is in the Journal of Pediatric Health Care. These patients can have sleep disordered breathing and behavioral issues occurring around bedtime. Known adverse effects of the stimulant and nonstimulant medications used to treat ADHD can include sleep disturbance, delayed circadian rhythm, insomnia, and somnolence. Yet, research on both sleep problems in children with ADHD and prescribing patterns is scanty, according to the investigators.
Dr. Klein and her colleagues conducted a study aimed at identifying the off-label medications being prescribed to potentiate sleep in children with ADHD, and the characteristics of the children and their prescribers. They used 5 years of pharmacy claims for children in Oregon insured through Medicaid and had a provider diagnosis of ADHD during Jan. 1, 2012, to Dec. 31, 2016. The children were aged 3-18 years and the prescriptions measured were the number of 30-day prescriptions. Prescribers were identified by national provider identifier taxonomies (nurse, physician, other prescriber), and classified as either generalist or specialist. The medications were classified as controlled or uncontrolled as determined by Title 21 of the U.S. Controlled Substances Act.
The data yielded 14,567 prescriptions for 2,518 children for a 30-day supply of medication known to potentiate sleep but off-label for children. Children aged 3-11 years comprised about 38% of these patients. Some children were prescribed more than one of these medications. Medications specifically on label for sleep but not indicated for children were not included. Those medications indicated for comorbid conditions and those indicated for ADHD that specifically cause somnolence were excluded.
The uncontrolled medications prescribed in this sample were amitriptyline, doxepin, hydroxyzine, low-dose quetiapine, and trazodone. The controlled medications identified were clonazepam and lorazepam, and a few prescriptions for phenobarbital.
Most of the prescriptions (63.8%) went to older children aged 12-18 years and most prescriptions (66.3%) went to males. The most commonly prescribed noncontrolled medication was trazodone (5,190 prescriptions), followed by hydroxyzine (2,539), and quetiapine (2,402). The most frequently prescribed controlled medication was clonazepam (2,145), followed by lorazepam (534).
Specialist prescribers wrote most of the prescriptions for this patient group, but no differences were found in prescribing patterns between specialists and generalists.
Dr. Klein and her colleagues noted that 871 unique children were prescribed 5,190 30-day−supply prescriptions for trazodone, including 23 children under age 5. Trazodone is a serotonin modulator indicated for the treatment of major depressive disorder, but has not been studied for safety and efficacy in children and has no Food and Drug Administration indication for children. “Hydroxyzine, quetiapine, and amitriptyline also were prescribed for a large number of children, including some for children as young as 3 years, despite lack of approval for use to induce to sleep and increased potential for significant adverse reactions in children,” they wrote.
Dr. Klein suggested that prescribers receive pressure from families to “do something” for their children, who may be disruptive day and night. “Prescribers may be unaware that trazodone, which is commonly used in practice, has never been approved for treatment of insomnia in children or adults. Insurance may not adequately fund other options, such as extensive behavioral therapy,” she stated in an interview. These medications come with some risk for children, Dr. Klein noted.
especially if their reaction to it is behavioral.” There is also potential for unanticipated drug interactions between off-label medications prescribed for sleep and drugs prescribed to treat ADHD.
This study has limitations related to the absence of detailed clinical explanatory information found in claims data. Information on adherence to treatment and adverse events, for example, is not contained in claims data. The study does not address the overall rates of sleep disorders in children with ADHD nor the percentage of children with ADHD who are prescribed any medication to potentiate sleep but looks at which off-label drugs are being prescribed, to which children, and by whom.
“Most medications prescribed in this study, used to induce sleep or treat insomnia, have not been studied for safety and efficacy in children, and their use should not be extrapolated from adult studies,” the researchers concluded.
They reported having no disclosures.
SOURCE: Klein T et al. J Pediatr Health Care. 2018 Jan 8. doi: 10.1016/j.pedhc.2018.10.002.
FROM THE JOURNAL OF PEDIATRIC HEALTH CARE
Key clinical point: The most commonly prescribed off-label medications prescribed to children were trazodone (5,190), hydroxyzine (2,539), quetiapine (2,402), and clonazepam (2,145).
Major finding: Most of the prescriptions (63.8%) went to older children aged 12-18 years, and most prescriptions (66.3%) went to males.
Study details: Medicaid claims data for Jan. 1, 2012, to Dec. 31, 2016, yielding 14,567 prescriptions of off-label medications for 2,518 children.
Disclosures: The investigators reported no disclosures.
Source: Klein T et al. J Pediatr Health Care. 2018 Jan 8. doi: 10.1016/j.pedhc.2018.10.002.












