User login
How we treat acute pain could be wrong
In a surprising discovery that flies in the face of conventional medicine,
The paper, published in Science Translational Medicine, suggests that inflammation, a normal part of injury recovery, helps resolve acute pain and prevents it from becoming chronic. Blocking that inflammation may interfere with this process, leading to harder-to-treat pain.
“What we’ve been doing for decades not only appears to be wrong, but appears to be 180 degrees wrong,” says senior study author Jeffrey Mogil, PhD, a professor in the department of psychology at McGill University in Montreal. “You should not be blocking inflammation. You should be letting inflammation happen. That’s what stops chronic pain.”
Inflammation: Nature’s pain reliever
Wanting to know why pain goes away for some but drags on (and on) for others, the researchers looked at pain mechanisms in both humans and mice. They found that a type of white blood cell known as a neutrophil seems to play a key role.
“In analyzing the genes of people suffering from lower back pain, we observed active changes in genes over time in people whose pain went away,” says Luda Diatchenko, PhD, a professor in the faculty of medicine and Canada excellence research chair in human pain genetics at McGill. “Changes in the blood cells and their activity seemed to be the most important factor, especially in cells called neutrophils.”
To test this link, the researchers blocked neutrophils in mice and found the pain lasted 2-10 times longer than normal. Anti-inflammatory drugs, despite providing short-term relief, had the same pain-prolonging effect – though injecting neutrophils into the mice seemed to keep that from happening.
The findings are supported by a separate analysis of 500,000 people in the United Kingdom that showed those taking anti-inflammatory drugs to treat their pain were more likely to have pain 2-10 years later.
“Inflammation occurs for a reason,” says Dr. Mogil, “and it looks like it’s dangerous to interfere with it.”
Rethinking how we treat pain
Neutrophils arrive early during inflammation, at the onset of injury – just when many of us reach for pain medication. This research suggests it might be better not to block inflammation, instead letting the neutrophils “do their thing.” Taking an analgesic that alleviates pain without blocking neutrophils, like acetaminophen, may be better than taking an anti-inflammatory drug or steroid, says Dr. Mogil.
Still, while the findings are compelling, clinical trials are needed to directly compare anti-inflammatory drugs to other painkillers, the researchers said. This research may also lay the groundwork for new drug development for chronic pain patients, Dr. Mogil says.
“Our data strongly suggests that neutrophils act like analgesics themselves, which is potentially useful in terms of analgesic development,” Dr. Mogil says. “And of course, we need new analgesics.”
A version of this article first appeared on WebMD.com.
In a surprising discovery that flies in the face of conventional medicine,
The paper, published in Science Translational Medicine, suggests that inflammation, a normal part of injury recovery, helps resolve acute pain and prevents it from becoming chronic. Blocking that inflammation may interfere with this process, leading to harder-to-treat pain.
“What we’ve been doing for decades not only appears to be wrong, but appears to be 180 degrees wrong,” says senior study author Jeffrey Mogil, PhD, a professor in the department of psychology at McGill University in Montreal. “You should not be blocking inflammation. You should be letting inflammation happen. That’s what stops chronic pain.”
Inflammation: Nature’s pain reliever
Wanting to know why pain goes away for some but drags on (and on) for others, the researchers looked at pain mechanisms in both humans and mice. They found that a type of white blood cell known as a neutrophil seems to play a key role.
“In analyzing the genes of people suffering from lower back pain, we observed active changes in genes over time in people whose pain went away,” says Luda Diatchenko, PhD, a professor in the faculty of medicine and Canada excellence research chair in human pain genetics at McGill. “Changes in the blood cells and their activity seemed to be the most important factor, especially in cells called neutrophils.”
To test this link, the researchers blocked neutrophils in mice and found the pain lasted 2-10 times longer than normal. Anti-inflammatory drugs, despite providing short-term relief, had the same pain-prolonging effect – though injecting neutrophils into the mice seemed to keep that from happening.
The findings are supported by a separate analysis of 500,000 people in the United Kingdom that showed those taking anti-inflammatory drugs to treat their pain were more likely to have pain 2-10 years later.
“Inflammation occurs for a reason,” says Dr. Mogil, “and it looks like it’s dangerous to interfere with it.”
Rethinking how we treat pain
Neutrophils arrive early during inflammation, at the onset of injury – just when many of us reach for pain medication. This research suggests it might be better not to block inflammation, instead letting the neutrophils “do their thing.” Taking an analgesic that alleviates pain without blocking neutrophils, like acetaminophen, may be better than taking an anti-inflammatory drug or steroid, says Dr. Mogil.
Still, while the findings are compelling, clinical trials are needed to directly compare anti-inflammatory drugs to other painkillers, the researchers said. This research may also lay the groundwork for new drug development for chronic pain patients, Dr. Mogil says.
“Our data strongly suggests that neutrophils act like analgesics themselves, which is potentially useful in terms of analgesic development,” Dr. Mogil says. “And of course, we need new analgesics.”
A version of this article first appeared on WebMD.com.
In a surprising discovery that flies in the face of conventional medicine,
The paper, published in Science Translational Medicine, suggests that inflammation, a normal part of injury recovery, helps resolve acute pain and prevents it from becoming chronic. Blocking that inflammation may interfere with this process, leading to harder-to-treat pain.
“What we’ve been doing for decades not only appears to be wrong, but appears to be 180 degrees wrong,” says senior study author Jeffrey Mogil, PhD, a professor in the department of psychology at McGill University in Montreal. “You should not be blocking inflammation. You should be letting inflammation happen. That’s what stops chronic pain.”
Inflammation: Nature’s pain reliever
Wanting to know why pain goes away for some but drags on (and on) for others, the researchers looked at pain mechanisms in both humans and mice. They found that a type of white blood cell known as a neutrophil seems to play a key role.
“In analyzing the genes of people suffering from lower back pain, we observed active changes in genes over time in people whose pain went away,” says Luda Diatchenko, PhD, a professor in the faculty of medicine and Canada excellence research chair in human pain genetics at McGill. “Changes in the blood cells and their activity seemed to be the most important factor, especially in cells called neutrophils.”
To test this link, the researchers blocked neutrophils in mice and found the pain lasted 2-10 times longer than normal. Anti-inflammatory drugs, despite providing short-term relief, had the same pain-prolonging effect – though injecting neutrophils into the mice seemed to keep that from happening.
The findings are supported by a separate analysis of 500,000 people in the United Kingdom that showed those taking anti-inflammatory drugs to treat their pain were more likely to have pain 2-10 years later.
“Inflammation occurs for a reason,” says Dr. Mogil, “and it looks like it’s dangerous to interfere with it.”
Rethinking how we treat pain
Neutrophils arrive early during inflammation, at the onset of injury – just when many of us reach for pain medication. This research suggests it might be better not to block inflammation, instead letting the neutrophils “do their thing.” Taking an analgesic that alleviates pain without blocking neutrophils, like acetaminophen, may be better than taking an anti-inflammatory drug or steroid, says Dr. Mogil.
Still, while the findings are compelling, clinical trials are needed to directly compare anti-inflammatory drugs to other painkillers, the researchers said. This research may also lay the groundwork for new drug development for chronic pain patients, Dr. Mogil says.
“Our data strongly suggests that neutrophils act like analgesics themselves, which is potentially useful in terms of analgesic development,” Dr. Mogil says. “And of course, we need new analgesics.”
A version of this article first appeared on WebMD.com.
FROM SCIENCE TRANSLATIONAL MEDICINE
Migraine relief in 20 minutes using eyedrops?
ILLUSTRATIVE CASE
A 35-year-old woman with no significant past medical history presents for follow-up of migraine. At the previous visit, she was prescribed sumatriptan for abortive therapy. However, she has been having significant adverse effect intolerance from the oral formulation, and the nasal formulation is cost prohibitive. What can you recommend as an alternative abortive therapy for this patient’s migraine?
Migraine is among the most common causes of disability worldwide, affecting more than 10% of the global population.2 The prevalence of migraine is between 2.6% and 21.7% across multiple countries.3 On a scale of 0% to 100%, disability caused by migraine is 43.3%, comparable to the first 2 days after an acute myocardial infarction (42.2%) and severe dementia (43.8%).4
Abortive therapy for acute migraine includes nonsteroidal anti-inflammatory drugs (NSAIDs),
Nausea and vomiting, common components of migraine (that are included in International Classification of Headache Disorders, 3rd edition [ICHD-3] criteria for migraine5) present obstacles to effective oral administration if experienced by the patient. In addition, for migraine refractory to first-line treatments, abortive options—including the recently approved
Two oral beta-blockers, propranolol and timolol, are approved by the US Food and Drug Administration for migraine prophylaxis. Unfortunately, oral beta-blockers are ineffective for abortive treatment.7 Ophthalmic timolol is typically used in the treatment of glaucoma, but there have been case reports describing its benefits in acute migraine treatment.8,9 In addition, ophthalmic timolol is far cheaper than medications such as ubrogepant.10 A 2014 case series of 7 patients discussed ophthalmic beta-blockers as an effective and possibly cheaper option for acute migraine treatment.8 A randomized, crossover, placebo-controlled pilot study of 198 migraine attacks in 10 participants using timolol eyedrops for abortive therapy found timolol was not significantly more effective than placebo.9 However, it was an underpowered pilot study, with a lack of masking and an imperfect placebo. The trial discussed here was a controlled, prospective study investigating topical beta-blockers for acute migraine treatment.
STUDY SUMMARY
Crossover study achieved primary endpoint in pain reduction
This randomized, single-center, double-masked, crossover trial compared timolol maleate ophthalmic solution 0.5% with placebo among 43 patients ages 12 or older presenting with a diagnosis of migraine based on ICHD-3 (beta) criteria. Patients were eligible if they had not taken any antimigraine medications for at least 1 month prior to the study and were excluded if they had taken systemic beta-blockers at baseline, or had asthma, bradyarrhythmias, or cardiac dysfunction.
Patients were randomized 1:1 to treatment with timolol maleate 0.5% eyedrops or placebo. At the earliest onset of migraine, patients used 1 drop of timolol maleate 0.5% or placebo in each eye; if they experienced no relief after 10 minutes, they used a second drop or matching placebo. Patients were instructed to score their headache pain on a 10-point scale prior to using the eyedrops and then again 20 minutes after treatment. If a patient had migraine with aura, they were asked to use the eyedrops at the onset of the aura but measure their score at headache onset. If no headaches developed within 20 minutes of the aura, the episode was not included for analysis. All patients were permitted to use their standard oral rescue medication if no relief occurred after 20 minutes of pain onset.
Continue to: The groups were observed...
The groups were observed for 3 months and then followed for a 1-month washout period, during which they received no study medications. The groups were then crossed over to the other treatment and were observed for another 3 months. The primary outcome was a reduction in pain score by 4 or more points, or to 0 on a 10-point pain scale, 20 minutes after treatment. The secondary outcome was nonuse of oral rescue medication.
Forty-three patients were included in a modified intention-to-treat analysis. The primary outcome was achieved in 233 of 284 (82%) timolol-treated migraines, compared to 38 of 271 (14%) placebo-treated migraines (percentage difference = 68 percentage points; 95% CI, 62-74 percentage points; P < .001). The mean pain score at the onset of migraine attacks was 6.01 for those treated with timolol and 5.93 for those treated with placebo. Patients treated with timolol had a reduction in pain of 5.98 points, compared with 0.93 points after using placebo (difference = 5.05; 95% CI, 4.19-5.91). No attacks included in the data required oral rescue medications, and there were no systemic adverse effects from the timolol eyedrops.
WHAT’S NEW
Evidence of benefit as abortive therapy for acute migraine
This randomized controlled trial (RCT) showed evidence to support timolol maleate ophthalmic solution 0.5% vs placebo for treatment of acute migraine by significantly reducing pain when taken at the onset of an acute migraine attack.
CAVEATS
Single-center trial, measuring limited response time
The generalizability of this RCT is limited because it was a single-center trial with a study population from a single region in India. It is unknown whether pain relief, adverse effects, or adherence would differ for the global population. Additionally, only migraines with headache were included in the analysis, limiting non-headache migraine subgroup-directed treatment. Also, this trial evaluated only the response to treatment at 20 minutes, and it is unknown if pain response continued for several hours. Headaches that began more than 20 minutes after the onset of aura were not evaluated.
CHALLENGES TO IMPLEMENTATION
Timolol’s systemic adverse effects require caution
Systemic beta-blocker effects (eg, bradycardia, hypotension, drowsiness, and bronchospasm) from topical timolol have been reported. Caution should be used when prescribing timolol for patients with current cardiovascular and pulmonary conditions.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
- Kurian A, Reghunadhan I, Thilak P, et al. Short-term efficacy and safety of topical β-blockers (timolol maleate ophthalmic solution, 0.5%) in acute migraine: a randomized crossover trial. JAMA Ophthalmol. 2020;138:1160-1166. doi: 10.1001/jamaophthalmol.2020.3676
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743-800. doi: 10.1016/S0140-6736(15)60692-4
- Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav. 2018;8:e00950. doi: 10.1002/brb3.950
- Leonardi M, Raggi A. Burden of migraine: international perspectives. Neurol Sci. 2013;34(suppl 1):S117-S118. doi: 10.1007/s10072-013-1387-8
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629-808. doi: 10.1177/0333102413485658
- Ubrogepant. GoodRx. Accessed May 23, 2022. www.goodrx.com/ubrogepant
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache. 2016;56:911-940. doi: 10.1111/head.12835
- 8. Migliazzo CV, Hagan JC III. Beta blocker eye drops for treatment of acute migraine. Mo Med. 2014;111:283-288.
- 9. Cossack M, Nabrinsky E, Turner H, et al. Timolol eyedrops in the treatment of acute migraine attacks: a randomized crossover study. JAMA Neurol. 2018;75:1024-1025. doi: 10.1001/jamaneurol.2018.0970
- 10. Timolol. GoodRx. Accessed May 23, 2022. www.goodrx.com/timolol
ILLUSTRATIVE CASE
A 35-year-old woman with no significant past medical history presents for follow-up of migraine. At the previous visit, she was prescribed sumatriptan for abortive therapy. However, she has been having significant adverse effect intolerance from the oral formulation, and the nasal formulation is cost prohibitive. What can you recommend as an alternative abortive therapy for this patient’s migraine?
Migraine is among the most common causes of disability worldwide, affecting more than 10% of the global population.2 The prevalence of migraine is between 2.6% and 21.7% across multiple countries.3 On a scale of 0% to 100%, disability caused by migraine is 43.3%, comparable to the first 2 days after an acute myocardial infarction (42.2%) and severe dementia (43.8%).4
Abortive therapy for acute migraine includes nonsteroidal anti-inflammatory drugs (NSAIDs),
Nausea and vomiting, common components of migraine (that are included in International Classification of Headache Disorders, 3rd edition [ICHD-3] criteria for migraine5) present obstacles to effective oral administration if experienced by the patient. In addition, for migraine refractory to first-line treatments, abortive options—including the recently approved
Two oral beta-blockers, propranolol and timolol, are approved by the US Food and Drug Administration for migraine prophylaxis. Unfortunately, oral beta-blockers are ineffective for abortive treatment.7 Ophthalmic timolol is typically used in the treatment of glaucoma, but there have been case reports describing its benefits in acute migraine treatment.8,9 In addition, ophthalmic timolol is far cheaper than medications such as ubrogepant.10 A 2014 case series of 7 patients discussed ophthalmic beta-blockers as an effective and possibly cheaper option for acute migraine treatment.8 A randomized, crossover, placebo-controlled pilot study of 198 migraine attacks in 10 participants using timolol eyedrops for abortive therapy found timolol was not significantly more effective than placebo.9 However, it was an underpowered pilot study, with a lack of masking and an imperfect placebo. The trial discussed here was a controlled, prospective study investigating topical beta-blockers for acute migraine treatment.
STUDY SUMMARY
Crossover study achieved primary endpoint in pain reduction
This randomized, single-center, double-masked, crossover trial compared timolol maleate ophthalmic solution 0.5% with placebo among 43 patients ages 12 or older presenting with a diagnosis of migraine based on ICHD-3 (beta) criteria. Patients were eligible if they had not taken any antimigraine medications for at least 1 month prior to the study and were excluded if they had taken systemic beta-blockers at baseline, or had asthma, bradyarrhythmias, or cardiac dysfunction.
Patients were randomized 1:1 to treatment with timolol maleate 0.5% eyedrops or placebo. At the earliest onset of migraine, patients used 1 drop of timolol maleate 0.5% or placebo in each eye; if they experienced no relief after 10 minutes, they used a second drop or matching placebo. Patients were instructed to score their headache pain on a 10-point scale prior to using the eyedrops and then again 20 minutes after treatment. If a patient had migraine with aura, they were asked to use the eyedrops at the onset of the aura but measure their score at headache onset. If no headaches developed within 20 minutes of the aura, the episode was not included for analysis. All patients were permitted to use their standard oral rescue medication if no relief occurred after 20 minutes of pain onset.
Continue to: The groups were observed...
The groups were observed for 3 months and then followed for a 1-month washout period, during which they received no study medications. The groups were then crossed over to the other treatment and were observed for another 3 months. The primary outcome was a reduction in pain score by 4 or more points, or to 0 on a 10-point pain scale, 20 minutes after treatment. The secondary outcome was nonuse of oral rescue medication.
Forty-three patients were included in a modified intention-to-treat analysis. The primary outcome was achieved in 233 of 284 (82%) timolol-treated migraines, compared to 38 of 271 (14%) placebo-treated migraines (percentage difference = 68 percentage points; 95% CI, 62-74 percentage points; P < .001). The mean pain score at the onset of migraine attacks was 6.01 for those treated with timolol and 5.93 for those treated with placebo. Patients treated with timolol had a reduction in pain of 5.98 points, compared with 0.93 points after using placebo (difference = 5.05; 95% CI, 4.19-5.91). No attacks included in the data required oral rescue medications, and there were no systemic adverse effects from the timolol eyedrops.
WHAT’S NEW
Evidence of benefit as abortive therapy for acute migraine
This randomized controlled trial (RCT) showed evidence to support timolol maleate ophthalmic solution 0.5% vs placebo for treatment of acute migraine by significantly reducing pain when taken at the onset of an acute migraine attack.
CAVEATS
Single-center trial, measuring limited response time
The generalizability of this RCT is limited because it was a single-center trial with a study population from a single region in India. It is unknown whether pain relief, adverse effects, or adherence would differ for the global population. Additionally, only migraines with headache were included in the analysis, limiting non-headache migraine subgroup-directed treatment. Also, this trial evaluated only the response to treatment at 20 minutes, and it is unknown if pain response continued for several hours. Headaches that began more than 20 minutes after the onset of aura were not evaluated.
CHALLENGES TO IMPLEMENTATION
Timolol’s systemic adverse effects require caution
Systemic beta-blocker effects (eg, bradycardia, hypotension, drowsiness, and bronchospasm) from topical timolol have been reported. Caution should be used when prescribing timolol for patients with current cardiovascular and pulmonary conditions.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 35-year-old woman with no significant past medical history presents for follow-up of migraine. At the previous visit, she was prescribed sumatriptan for abortive therapy. However, she has been having significant adverse effect intolerance from the oral formulation, and the nasal formulation is cost prohibitive. What can you recommend as an alternative abortive therapy for this patient’s migraine?
Migraine is among the most common causes of disability worldwide, affecting more than 10% of the global population.2 The prevalence of migraine is between 2.6% and 21.7% across multiple countries.3 On a scale of 0% to 100%, disability caused by migraine is 43.3%, comparable to the first 2 days after an acute myocardial infarction (42.2%) and severe dementia (43.8%).4
Abortive therapy for acute migraine includes nonsteroidal anti-inflammatory drugs (NSAIDs),
Nausea and vomiting, common components of migraine (that are included in International Classification of Headache Disorders, 3rd edition [ICHD-3] criteria for migraine5) present obstacles to effective oral administration if experienced by the patient. In addition, for migraine refractory to first-line treatments, abortive options—including the recently approved
Two oral beta-blockers, propranolol and timolol, are approved by the US Food and Drug Administration for migraine prophylaxis. Unfortunately, oral beta-blockers are ineffective for abortive treatment.7 Ophthalmic timolol is typically used in the treatment of glaucoma, but there have been case reports describing its benefits in acute migraine treatment.8,9 In addition, ophthalmic timolol is far cheaper than medications such as ubrogepant.10 A 2014 case series of 7 patients discussed ophthalmic beta-blockers as an effective and possibly cheaper option for acute migraine treatment.8 A randomized, crossover, placebo-controlled pilot study of 198 migraine attacks in 10 participants using timolol eyedrops for abortive therapy found timolol was not significantly more effective than placebo.9 However, it was an underpowered pilot study, with a lack of masking and an imperfect placebo. The trial discussed here was a controlled, prospective study investigating topical beta-blockers for acute migraine treatment.
STUDY SUMMARY
Crossover study achieved primary endpoint in pain reduction
This randomized, single-center, double-masked, crossover trial compared timolol maleate ophthalmic solution 0.5% with placebo among 43 patients ages 12 or older presenting with a diagnosis of migraine based on ICHD-3 (beta) criteria. Patients were eligible if they had not taken any antimigraine medications for at least 1 month prior to the study and were excluded if they had taken systemic beta-blockers at baseline, or had asthma, bradyarrhythmias, or cardiac dysfunction.
Patients were randomized 1:1 to treatment with timolol maleate 0.5% eyedrops or placebo. At the earliest onset of migraine, patients used 1 drop of timolol maleate 0.5% or placebo in each eye; if they experienced no relief after 10 minutes, they used a second drop or matching placebo. Patients were instructed to score their headache pain on a 10-point scale prior to using the eyedrops and then again 20 minutes after treatment. If a patient had migraine with aura, they were asked to use the eyedrops at the onset of the aura but measure their score at headache onset. If no headaches developed within 20 minutes of the aura, the episode was not included for analysis. All patients were permitted to use their standard oral rescue medication if no relief occurred after 20 minutes of pain onset.
Continue to: The groups were observed...
The groups were observed for 3 months and then followed for a 1-month washout period, during which they received no study medications. The groups were then crossed over to the other treatment and were observed for another 3 months. The primary outcome was a reduction in pain score by 4 or more points, or to 0 on a 10-point pain scale, 20 minutes after treatment. The secondary outcome was nonuse of oral rescue medication.
Forty-three patients were included in a modified intention-to-treat analysis. The primary outcome was achieved in 233 of 284 (82%) timolol-treated migraines, compared to 38 of 271 (14%) placebo-treated migraines (percentage difference = 68 percentage points; 95% CI, 62-74 percentage points; P < .001). The mean pain score at the onset of migraine attacks was 6.01 for those treated with timolol and 5.93 for those treated with placebo. Patients treated with timolol had a reduction in pain of 5.98 points, compared with 0.93 points after using placebo (difference = 5.05; 95% CI, 4.19-5.91). No attacks included in the data required oral rescue medications, and there were no systemic adverse effects from the timolol eyedrops.
WHAT’S NEW
Evidence of benefit as abortive therapy for acute migraine
This randomized controlled trial (RCT) showed evidence to support timolol maleate ophthalmic solution 0.5% vs placebo for treatment of acute migraine by significantly reducing pain when taken at the onset of an acute migraine attack.
CAVEATS
Single-center trial, measuring limited response time
The generalizability of this RCT is limited because it was a single-center trial with a study population from a single region in India. It is unknown whether pain relief, adverse effects, or adherence would differ for the global population. Additionally, only migraines with headache were included in the analysis, limiting non-headache migraine subgroup-directed treatment. Also, this trial evaluated only the response to treatment at 20 minutes, and it is unknown if pain response continued for several hours. Headaches that began more than 20 minutes after the onset of aura were not evaluated.
CHALLENGES TO IMPLEMENTATION
Timolol’s systemic adverse effects require caution
Systemic beta-blocker effects (eg, bradycardia, hypotension, drowsiness, and bronchospasm) from topical timolol have been reported. Caution should be used when prescribing timolol for patients with current cardiovascular and pulmonary conditions.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
- Kurian A, Reghunadhan I, Thilak P, et al. Short-term efficacy and safety of topical β-blockers (timolol maleate ophthalmic solution, 0.5%) in acute migraine: a randomized crossover trial. JAMA Ophthalmol. 2020;138:1160-1166. doi: 10.1001/jamaophthalmol.2020.3676
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743-800. doi: 10.1016/S0140-6736(15)60692-4
- Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav. 2018;8:e00950. doi: 10.1002/brb3.950
- Leonardi M, Raggi A. Burden of migraine: international perspectives. Neurol Sci. 2013;34(suppl 1):S117-S118. doi: 10.1007/s10072-013-1387-8
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629-808. doi: 10.1177/0333102413485658
- Ubrogepant. GoodRx. Accessed May 23, 2022. www.goodrx.com/ubrogepant
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache. 2016;56:911-940. doi: 10.1111/head.12835
- 8. Migliazzo CV, Hagan JC III. Beta blocker eye drops for treatment of acute migraine. Mo Med. 2014;111:283-288.
- 9. Cossack M, Nabrinsky E, Turner H, et al. Timolol eyedrops in the treatment of acute migraine attacks: a randomized crossover study. JAMA Neurol. 2018;75:1024-1025. doi: 10.1001/jamaneurol.2018.0970
- 10. Timolol. GoodRx. Accessed May 23, 2022. www.goodrx.com/timolol
- Kurian A, Reghunadhan I, Thilak P, et al. Short-term efficacy and safety of topical β-blockers (timolol maleate ophthalmic solution, 0.5%) in acute migraine: a randomized crossover trial. JAMA Ophthalmol. 2020;138:1160-1166. doi: 10.1001/jamaophthalmol.2020.3676
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743-800. doi: 10.1016/S0140-6736(15)60692-4
- Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav. 2018;8:e00950. doi: 10.1002/brb3.950
- Leonardi M, Raggi A. Burden of migraine: international perspectives. Neurol Sci. 2013;34(suppl 1):S117-S118. doi: 10.1007/s10072-013-1387-8
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629-808. doi: 10.1177/0333102413485658
- Ubrogepant. GoodRx. Accessed May 23, 2022. www.goodrx.com/ubrogepant
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache. 2016;56:911-940. doi: 10.1111/head.12835
- 8. Migliazzo CV, Hagan JC III. Beta blocker eye drops for treatment of acute migraine. Mo Med. 2014;111:283-288.
- 9. Cossack M, Nabrinsky E, Turner H, et al. Timolol eyedrops in the treatment of acute migraine attacks: a randomized crossover study. JAMA Neurol. 2018;75:1024-1025. doi: 10.1001/jamaneurol.2018.0970
- 10. Timolol. GoodRx. Accessed May 23, 2022. www.goodrx.com/timolol
PRACTICE CHANGER
Consider timolol maleate 0.5% eyedrops as a quick and effective abortive therapy for migraine.1
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial.1
Kurian A, Reghunadhan I, Thilak P, et al. Short-term efficacy and safety of topical β-blockers (timolol maleate ophthalmic solution, 0.5%) in acute migraine: a randomized crossover trial. JAMA Ophthalmol. 2020;138:1160-1166.
New—and surprising—ways to approach migraine pain
Migraine headaches pose a challenge for many patients and their physicians, so new, effective approaches are always welcome. Sometimes new treatments come as total surprises. For example, who would have guessed that timolol eyedrops could be effective for acute migraine?1 Granted, the results (discussed in this issue's PURLs) are from a single randomized trial, but they look very promising.
This is not the only new and innovative treatment for migraine. Everyone knows about the heavily marketed calcium gene-related peptide antagonists, which include monoclonal antibodies and the so-called “gepants.” The monoclonal antibodies and atogepant are approved for migraine prevention, and they do a decent job (although at a high price). In randomized trials, these agents reduced migraine days per month by an average of about 1.5 to 2.5 days compared to placebo.2-5
Ubrogepant and rimegepant are approved for acute migraine treatment. In clinical trials, about 20% of patients taking ubrogepant or rimegepant were pain free at 2 hours post dose, compared to 12% to 14% taking placebo.6,7 Unfortunately, that means 80% of patients still have some pain at 2 hours. By comparison, zolmitriptan performs a bit better, with 34% of patients pain free at 2 hours.8 However, for those who can’t tolerate zolmitriptan, these newer options provide an alternative.
We also now have nonpharmacologic options. The caloric vestibular stimulation device is essentially a headset with ear probes that change temperature, alternating warm and cold. In a randomized controlled trial, it reduced monthly migraine days by 1.1 compared to placebo, from a baseline of 7.7 to 3.9 days.9 It can also be used to treat acute migraine. There is also a vagus nerve–stimulating device that reduced migraine headache severity by 20% on average in 32.2% of patients in 30 minutes. Sham treatment was as effective for 18.5% of patients, giving a number needed to treat of 6 compared to sham.10
And finally, there are complementary and alternative medicine options. Two recent randomized trials demonstrated that ≥ 2000 IU/d of vitamin D reduced monthly migraine days an average of 2 days, which is comparable to the effectiveness of the calcium gene-related peptide antagonists at a fraction of the cost.11,12 In another randomized trial, intranasal 1.5% peppermint oil was as effective as topical 4% lidocaine in providing substantial pain relief for acute migraine; about 42% of patients achieved significant relief with either treatment.13
While we may not have a perfect treatment for our patients with migraine headache, we certainly have many options to choose from.
1. Ge Y, Castelli G. Migraine relief in 20 minutes using eyedrops? J Fam Pract. 2022;71:222-223, 226.
2. Loder E, Renthal W. Calcitonin gene-related peptide monoclonal antibody treatments for migraine. JAMA Intern Med. 2019;179:421-422. doi: 10.1001/jamainternmed.2018.7536
3. Silberstein S, Diamond M, Hindiyeh NA, et al. Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (Prevention of migraine via intravenous ALD403 safety and efficacy-2) study. J Headache Pain. 2020;21:120. doi: 10.1186/s10194-020-01186-3
4. Ament M, Day K, Stauffer VL, et al. Effect of galcanezumab on severity and symptoms of migraine in phase 3 trials in patients with episodic or chronic migraine. J Headache Pain. 2021;22:6. doi: 10.1186/s10194-021-01215-9
5. Goadsby PJ, Dodick DW, Ailani J, et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol. 2020;19:727-737. doi: 10.1016/S1474-4422(20)30234-9
6. Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381:142-149. doi: 10.1056/NEJMoa1811090
7. Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887-1898. doi: 10.1001/jama.2019.16711
8. Bird S, Derry S, Moore R. Zolmitriptan for acute migraine attacks in adults. Cochrane Database Syst Rev. 2014;2014:CD008616. doi: 10.1002/14651858.CD008616.pub2
9. Wilkinson D, Ade KK, Rogers LL, et al. Preventing episodic migraine with caloric vestibular stimulation: a randomized controlled trial. Headache. 2017;57:1065-1087. doi: 10.1111/head.13120
10. Grazzi L, Tassorelli C, de Tommaso M, et al; PRESTO Study Group. Practical and clinical utility of non-invasive vagus nerve stimulation (nVNS) for the acute treatment of migraine: a post hoc analysis of the randomized, sham-controlled, double-blind PRESTO trial. J Headache Pain. 2018;19:98. doi: 10.1186/s10194-018-0928-1
11. Gazerani P, Fuglsang R, Pedersen JG, et al. A randomized, double-blinded, placebo-controlled, parallel trial of vitamin D3 supplementation in adult patients with migraine. Curr Med Res Opin. 2019;35:715-723. doi: 10.1080/03007995.2018.1519503
12. Ghorbani Z, Togha M, Rafiee P, et al. Vitamin D3 might improve headache characteristics and protect against inflammation in migraine: a randomized clinical trial. Neurol Sci. 2020;41:1183-1192. doi: 10.1007/s10072-019-04220-8
13. Rafieian-Kopaei M, Hasanpour-Dehkordi A, Lorigooini Z, et al. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: a double-blind clinical trial. Int J Prev Med. 2019;10:121. doi: 10.4103/ijpvm.IJPVM_530_17
Migraine headaches pose a challenge for many patients and their physicians, so new, effective approaches are always welcome. Sometimes new treatments come as total surprises. For example, who would have guessed that timolol eyedrops could be effective for acute migraine?1 Granted, the results (discussed in this issue's PURLs) are from a single randomized trial, but they look very promising.
This is not the only new and innovative treatment for migraine. Everyone knows about the heavily marketed calcium gene-related peptide antagonists, which include monoclonal antibodies and the so-called “gepants.” The monoclonal antibodies and atogepant are approved for migraine prevention, and they do a decent job (although at a high price). In randomized trials, these agents reduced migraine days per month by an average of about 1.5 to 2.5 days compared to placebo.2-5
Ubrogepant and rimegepant are approved for acute migraine treatment. In clinical trials, about 20% of patients taking ubrogepant or rimegepant were pain free at 2 hours post dose, compared to 12% to 14% taking placebo.6,7 Unfortunately, that means 80% of patients still have some pain at 2 hours. By comparison, zolmitriptan performs a bit better, with 34% of patients pain free at 2 hours.8 However, for those who can’t tolerate zolmitriptan, these newer options provide an alternative.
We also now have nonpharmacologic options. The caloric vestibular stimulation device is essentially a headset with ear probes that change temperature, alternating warm and cold. In a randomized controlled trial, it reduced monthly migraine days by 1.1 compared to placebo, from a baseline of 7.7 to 3.9 days.9 It can also be used to treat acute migraine. There is also a vagus nerve–stimulating device that reduced migraine headache severity by 20% on average in 32.2% of patients in 30 minutes. Sham treatment was as effective for 18.5% of patients, giving a number needed to treat of 6 compared to sham.10
And finally, there are complementary and alternative medicine options. Two recent randomized trials demonstrated that ≥ 2000 IU/d of vitamin D reduced monthly migraine days an average of 2 days, which is comparable to the effectiveness of the calcium gene-related peptide antagonists at a fraction of the cost.11,12 In another randomized trial, intranasal 1.5% peppermint oil was as effective as topical 4% lidocaine in providing substantial pain relief for acute migraine; about 42% of patients achieved significant relief with either treatment.13
While we may not have a perfect treatment for our patients with migraine headache, we certainly have many options to choose from.
Migraine headaches pose a challenge for many patients and their physicians, so new, effective approaches are always welcome. Sometimes new treatments come as total surprises. For example, who would have guessed that timolol eyedrops could be effective for acute migraine?1 Granted, the results (discussed in this issue's PURLs) are from a single randomized trial, but they look very promising.
This is not the only new and innovative treatment for migraine. Everyone knows about the heavily marketed calcium gene-related peptide antagonists, which include monoclonal antibodies and the so-called “gepants.” The monoclonal antibodies and atogepant are approved for migraine prevention, and they do a decent job (although at a high price). In randomized trials, these agents reduced migraine days per month by an average of about 1.5 to 2.5 days compared to placebo.2-5
Ubrogepant and rimegepant are approved for acute migraine treatment. In clinical trials, about 20% of patients taking ubrogepant or rimegepant were pain free at 2 hours post dose, compared to 12% to 14% taking placebo.6,7 Unfortunately, that means 80% of patients still have some pain at 2 hours. By comparison, zolmitriptan performs a bit better, with 34% of patients pain free at 2 hours.8 However, for those who can’t tolerate zolmitriptan, these newer options provide an alternative.
We also now have nonpharmacologic options. The caloric vestibular stimulation device is essentially a headset with ear probes that change temperature, alternating warm and cold. In a randomized controlled trial, it reduced monthly migraine days by 1.1 compared to placebo, from a baseline of 7.7 to 3.9 days.9 It can also be used to treat acute migraine. There is also a vagus nerve–stimulating device that reduced migraine headache severity by 20% on average in 32.2% of patients in 30 minutes. Sham treatment was as effective for 18.5% of patients, giving a number needed to treat of 6 compared to sham.10
And finally, there are complementary and alternative medicine options. Two recent randomized trials demonstrated that ≥ 2000 IU/d of vitamin D reduced monthly migraine days an average of 2 days, which is comparable to the effectiveness of the calcium gene-related peptide antagonists at a fraction of the cost.11,12 In another randomized trial, intranasal 1.5% peppermint oil was as effective as topical 4% lidocaine in providing substantial pain relief for acute migraine; about 42% of patients achieved significant relief with either treatment.13
While we may not have a perfect treatment for our patients with migraine headache, we certainly have many options to choose from.
1. Ge Y, Castelli G. Migraine relief in 20 minutes using eyedrops? J Fam Pract. 2022;71:222-223, 226.
2. Loder E, Renthal W. Calcitonin gene-related peptide monoclonal antibody treatments for migraine. JAMA Intern Med. 2019;179:421-422. doi: 10.1001/jamainternmed.2018.7536
3. Silberstein S, Diamond M, Hindiyeh NA, et al. Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (Prevention of migraine via intravenous ALD403 safety and efficacy-2) study. J Headache Pain. 2020;21:120. doi: 10.1186/s10194-020-01186-3
4. Ament M, Day K, Stauffer VL, et al. Effect of galcanezumab on severity and symptoms of migraine in phase 3 trials in patients with episodic or chronic migraine. J Headache Pain. 2021;22:6. doi: 10.1186/s10194-021-01215-9
5. Goadsby PJ, Dodick DW, Ailani J, et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol. 2020;19:727-737. doi: 10.1016/S1474-4422(20)30234-9
6. Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381:142-149. doi: 10.1056/NEJMoa1811090
7. Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887-1898. doi: 10.1001/jama.2019.16711
8. Bird S, Derry S, Moore R. Zolmitriptan for acute migraine attacks in adults. Cochrane Database Syst Rev. 2014;2014:CD008616. doi: 10.1002/14651858.CD008616.pub2
9. Wilkinson D, Ade KK, Rogers LL, et al. Preventing episodic migraine with caloric vestibular stimulation: a randomized controlled trial. Headache. 2017;57:1065-1087. doi: 10.1111/head.13120
10. Grazzi L, Tassorelli C, de Tommaso M, et al; PRESTO Study Group. Practical and clinical utility of non-invasive vagus nerve stimulation (nVNS) for the acute treatment of migraine: a post hoc analysis of the randomized, sham-controlled, double-blind PRESTO trial. J Headache Pain. 2018;19:98. doi: 10.1186/s10194-018-0928-1
11. Gazerani P, Fuglsang R, Pedersen JG, et al. A randomized, double-blinded, placebo-controlled, parallel trial of vitamin D3 supplementation in adult patients with migraine. Curr Med Res Opin. 2019;35:715-723. doi: 10.1080/03007995.2018.1519503
12. Ghorbani Z, Togha M, Rafiee P, et al. Vitamin D3 might improve headache characteristics and protect against inflammation in migraine: a randomized clinical trial. Neurol Sci. 2020;41:1183-1192. doi: 10.1007/s10072-019-04220-8
13. Rafieian-Kopaei M, Hasanpour-Dehkordi A, Lorigooini Z, et al. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: a double-blind clinical trial. Int J Prev Med. 2019;10:121. doi: 10.4103/ijpvm.IJPVM_530_17
1. Ge Y, Castelli G. Migraine relief in 20 minutes using eyedrops? J Fam Pract. 2022;71:222-223, 226.
2. Loder E, Renthal W. Calcitonin gene-related peptide monoclonal antibody treatments for migraine. JAMA Intern Med. 2019;179:421-422. doi: 10.1001/jamainternmed.2018.7536
3. Silberstein S, Diamond M, Hindiyeh NA, et al. Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (Prevention of migraine via intravenous ALD403 safety and efficacy-2) study. J Headache Pain. 2020;21:120. doi: 10.1186/s10194-020-01186-3
4. Ament M, Day K, Stauffer VL, et al. Effect of galcanezumab on severity and symptoms of migraine in phase 3 trials in patients with episodic or chronic migraine. J Headache Pain. 2021;22:6. doi: 10.1186/s10194-021-01215-9
5. Goadsby PJ, Dodick DW, Ailani J, et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol. 2020;19:727-737. doi: 10.1016/S1474-4422(20)30234-9
6. Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381:142-149. doi: 10.1056/NEJMoa1811090
7. Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887-1898. doi: 10.1001/jama.2019.16711
8. Bird S, Derry S, Moore R. Zolmitriptan for acute migraine attacks in adults. Cochrane Database Syst Rev. 2014;2014:CD008616. doi: 10.1002/14651858.CD008616.pub2
9. Wilkinson D, Ade KK, Rogers LL, et al. Preventing episodic migraine with caloric vestibular stimulation: a randomized controlled trial. Headache. 2017;57:1065-1087. doi: 10.1111/head.13120
10. Grazzi L, Tassorelli C, de Tommaso M, et al; PRESTO Study Group. Practical and clinical utility of non-invasive vagus nerve stimulation (nVNS) for the acute treatment of migraine: a post hoc analysis of the randomized, sham-controlled, double-blind PRESTO trial. J Headache Pain. 2018;19:98. doi: 10.1186/s10194-018-0928-1
11. Gazerani P, Fuglsang R, Pedersen JG, et al. A randomized, double-blinded, placebo-controlled, parallel trial of vitamin D3 supplementation in adult patients with migraine. Curr Med Res Opin. 2019;35:715-723. doi: 10.1080/03007995.2018.1519503
12. Ghorbani Z, Togha M, Rafiee P, et al. Vitamin D3 might improve headache characteristics and protect against inflammation in migraine: a randomized clinical trial. Neurol Sci. 2020;41:1183-1192. doi: 10.1007/s10072-019-04220-8
13. Rafieian-Kopaei M, Hasanpour-Dehkordi A, Lorigooini Z, et al. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: a double-blind clinical trial. Int J Prev Med. 2019;10:121. doi: 10.4103/ijpvm.IJPVM_530_17
Tips for managing 4 common soft-tissue finger and thumb injuries
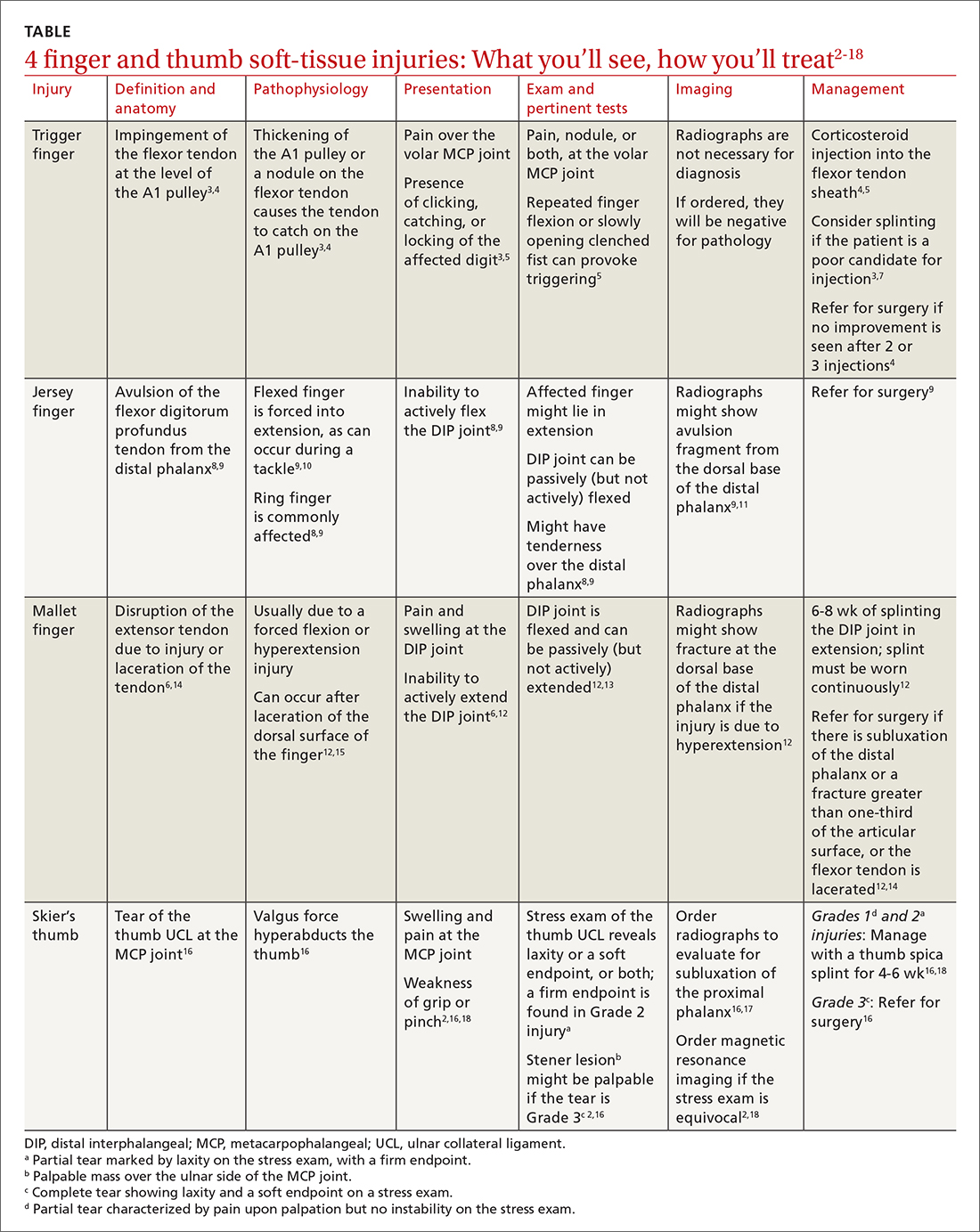
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).
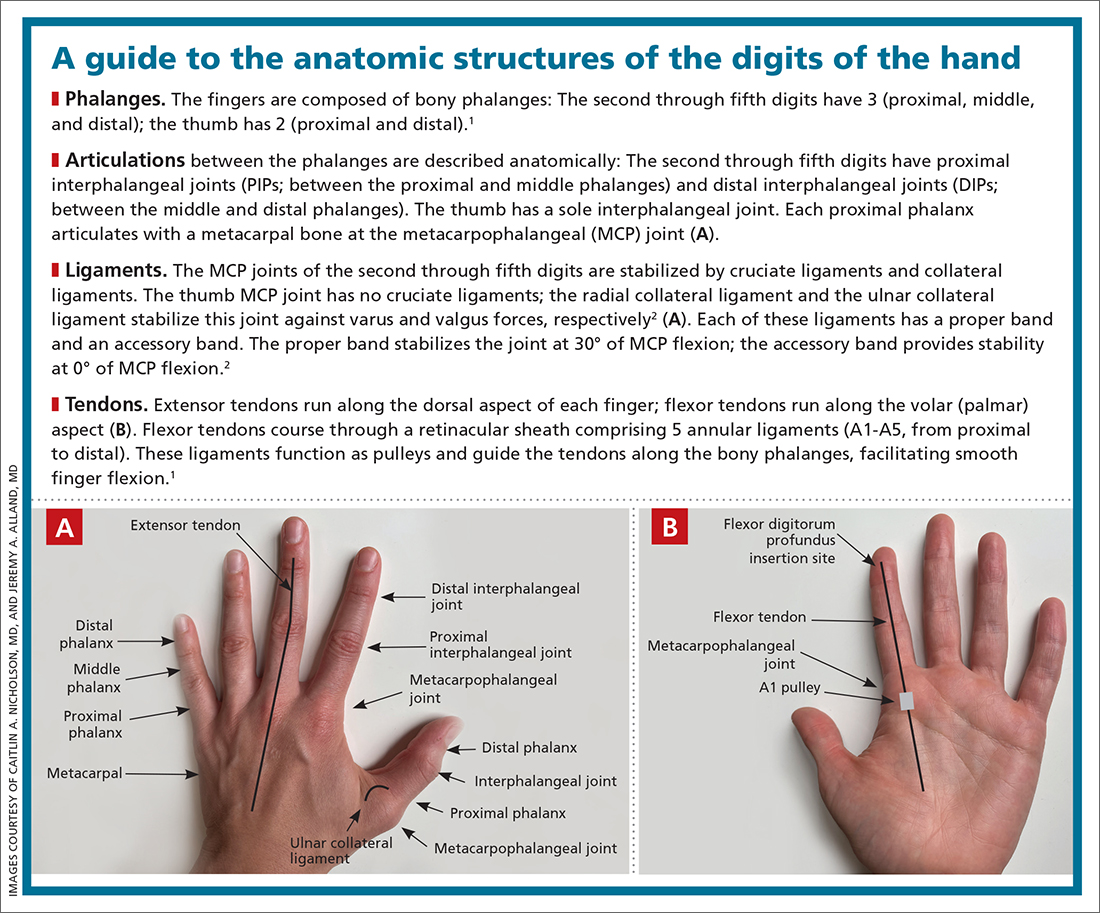
Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6
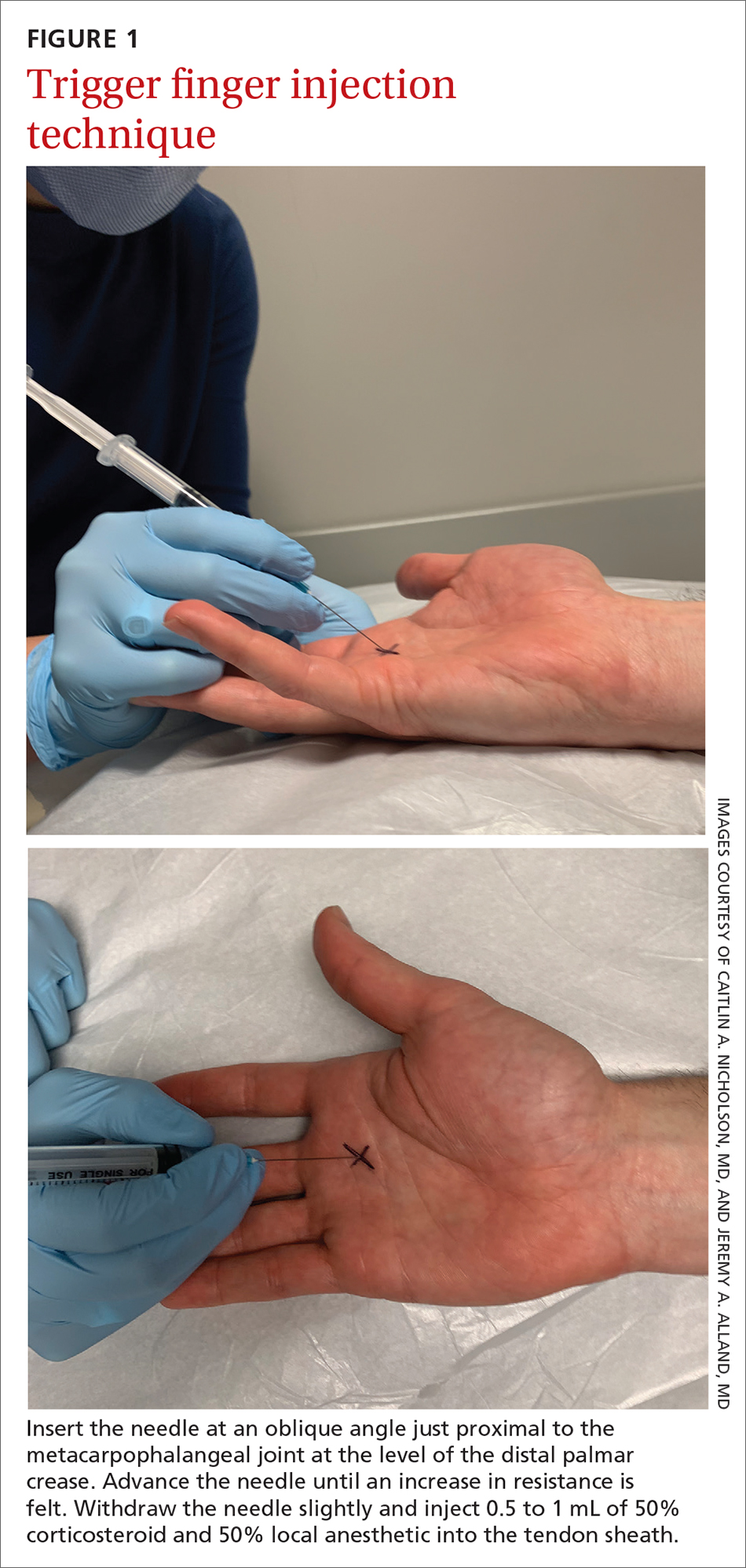
The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
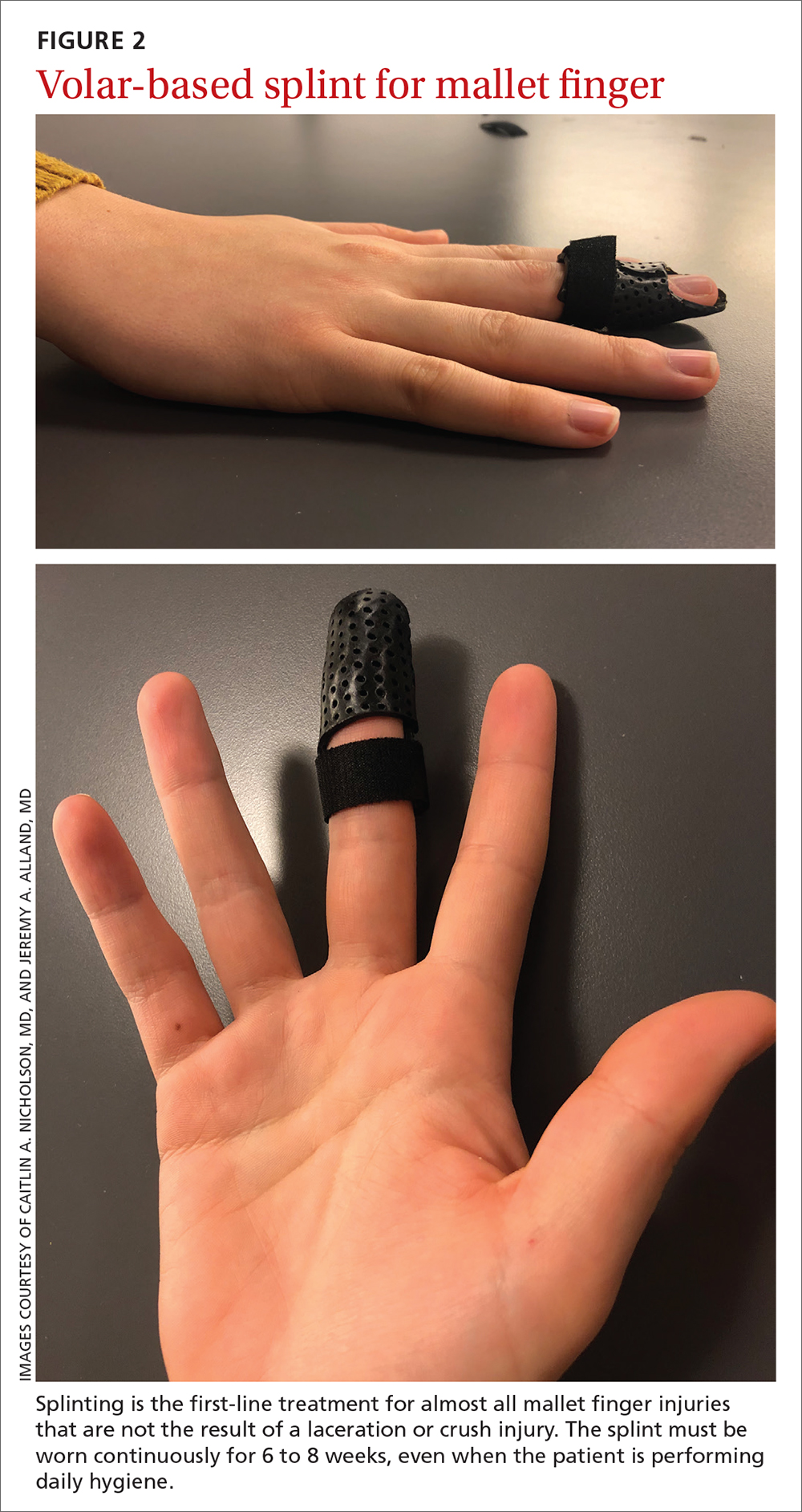
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23
Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
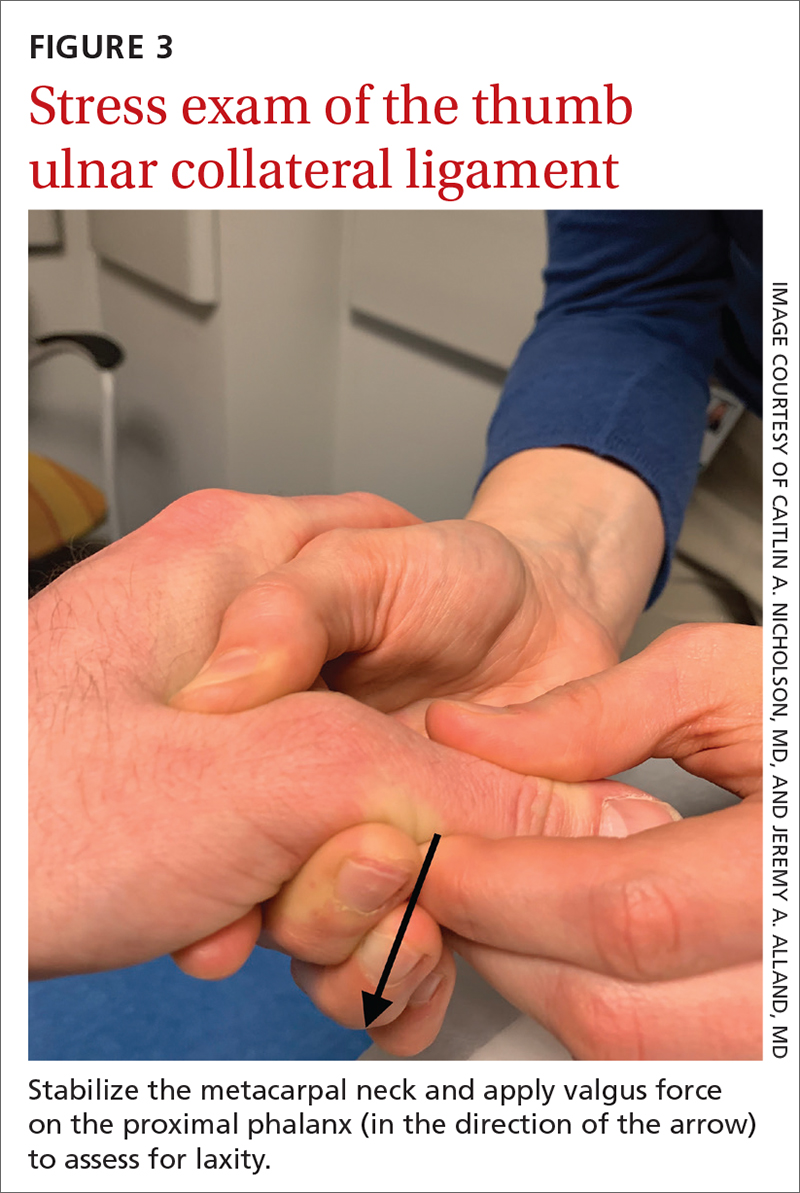
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17
Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).


Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6

The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23

Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17

Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).


Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6

The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23

Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17

Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
PRACTICE RECOMMENDATIONS
› Treat trigger finger with a corticosteroid injection into the flexor tendon sheath. A
› Refer a case of jersey finger to a hand surgeon within 1 week after injury for flexor tendon repair. C
› Treat mallet finger with strict distal interphalangeal joint immobilization for 6 to 8 weeks. A
› Treat Grades 1 and 2 skier’s thumb with immobilization in a thumb spica splint or a cast for 4 to 6 weeks. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
‘Genetic’ height linked to peripheral neuropathy and certain skin and bone infections
, according to a study published in PLOS Genetics.
Prior studies have investigated height as a risk factor for chronic diseases, such as a higher risk for atrial fibrillation and a reduced risk of cardiovascular disease. It’s been consistently difficult, however, to eliminate the confounding influences of diet, socioeconomics, lifestyle behaviors, and other environmental factors that may interfere with a person’s reaching their expected height based on their genes.
This study, however, was able to better parse those differences by using Mendelian randomization within the comprehensive clinical and genetic dataset of a national health care system biobank. Mendelian randomization uses “genetic instruments for exposures of interest under the assumption that genotype is less susceptible to confounding than measured exposures,” the authors explained. The findings confirmed previously suspected associations between height and a range of cardiovascular and metabolic conditions as well as revealing new associations with several other conditions.
Prior associations confirmed, new associations uncovered
The results confirmed that being tall is linked to a higher risk of atrial fibrillation and varicose veins, and a lower risk of coronary heart disease, high blood pressure, and high cholesterol. The study also uncovered new associations between greater height and a higher risk of peripheral neuropathy, which is caused by damage to nerves on the extremities, as well as skin and bone infections, such as leg and foot ulcers.
The meta-analysis “identified five additional traits associated with genetically-predicted height,” wrote Sridharan Raghavan, MD, assistant professor of medicine at the University of Colorado Anschutz Medical Campus, and colleagues. “Two were genitourinary conditions – erectile dysfunction and urinary retention – that can be associated with neuropathy, and a third was a phecode for nonspecific skin disorders that may be related to skin infections – consistent with the race/ethnicity stratified results.”
Removing potential confounders
F. Perry Wilson, MD, associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study, said the findings were not particularly surprising overall, but it’s striking that the researchers had ”such a large cohort with such detailed electronic health records allowing for the comparison of genetic height with a variety of clinical outcomes.” He also noted the study’s strength in using Mendelian randomization so that the exposure is the predicted genetic height instead of a person’s measured height.
“This is key, since lots of things affect actual height – nutrition is an important one that could certainly be linked to disease as well,” Dr. Wilson said. ”By using genetic height, the authors remove these potential confounders. Since genetic height is “assigned” at birth (or conception), there is little opportunity for confounding. Of course, it is possible that some of the gene variants used to predict genetic height actually do something else, such as make you seek out less nutritious meals, but by and large this is how these types of studies need to be done.”
Height may impact over 100 clinical traits
The study relied on data from the U.S. Veteran Affairs Million Veteran Program with 222,300 non-Hispanic White and 58,151 non-Hispanic Black participants. The researchers first estimated the likelihood of participants’ genetic height based on 3,290 genetic variants determined to affect genetic height in a recent European-ancestry genome-wide meta-analysis. Then they compared these estimates with participants’ actual height in the VA medical record, adjusting for age, sex, and other genetic characteristics.
In doing so, the researchers found 345 clinical traits that were associated with the actual measured height in White participants plus another 17 clinical trials linked to actual measured height in Black participants. An overall 127 of these clinical traits were significantly associated with White participants’ genetically predicted height, and two of them were significantly associated with Black participants’ genetically predicted height.
In analyzing all these data together, the researchers were largely able to separate out those associations between genetically predicted height and certain health conditions from those associations between health conditions and a person’s actual measured height. They also determined that including body mass index as a covariate had little impact on the results. The researchers conducted the appropriate statistical correction to ensure the use of so many variables did not result in spurious statistical significance in some associations.
“Using genetic methods applied to the VA Million Veteran Program, we found evidence that adult height may impact over 100 clinical traits, including several conditions associated with poor outcomes and quality of life – peripheral neuropathy, lower extremity ulcers, and chronic venous insufficiency. We conclude that height may be an unrecognized nonmodifiable risk factor for several common conditions in adults.”
Height linked with health conditions
Genetically predicted height predicted a reduced risk of hyperlipidemia and hypertension independent of coronary heart disease, the analysis revealed. Genetically predicted height was also linked to an approximately 51% increased risk of atrial fibrillation in participants without coronary heart disease but, paradoxically, only a 39% increased risk in those with coronary heart disease, despite coronary heart disease being a risk factor for atrial fibrillation. Genetically predicted height was also associated with a greater risk of varicose veins in the legs and deep vein thrombosis.
Another novel association uncovered by the analysis was between women’s genetically predicted height and both asthma and nonspecific peripheral nerve disorders. “Whether these associations reflect differences by sex in disease pathophysiology related to height may warrant exploration in a sample with better balance between men and women,” the authors wrote. “In sum, our results suggest that an individual’s height may warrant consideration as a nonmodifiable predictor for several common conditions, particularly those affecting peripheral/distal extremities that are most physically impacted by tall stature.”
A substantial limitation of the study was its homogeneity of participants, who were 92% male with an average height of 176 cm and an average BMI of 30.1. The Black participants tended to be younger, with an average age of 58 compared with 64 years in the White participants, but the groups were otherwise similar in height and weight.* The database included data from Hispanic participants, but the researchers excluded these data because of the small sample size.
The smaller dataset for Black participants was a limitation as well as the fact that the genome-wide association study the researchers relied on came from a European population, which may not be as accurate in people with other ancestry, Dr. Wilson said. The bigger limitation, however, is what the findings’ clinical relevance is.
What does it all mean?
“Genetic height is in your genes – there is nothing to be done about it – so it is more of academic interest than clinical interest,” Dr. Wilson said. It’s not even clear whether incorporating a person’s height – actual or genetically predicted, if it could be easily determined for each person – into risk calculators. ”To know whether it would be beneficial to use height (or genetic height) as a risk factor, you’d need to examine each condition of interest, adjusting for all known risk factors, to see if height improved the prediction,” Dr. Wilson said. “I suspect for most conditions, the well-known risk factors would swamp height. For example, high genetic height might truly increase risk for neuropathy. But diabetes might increase the risk so much more that height is not particularly relevant.”
On the other hand, the fact that height in general has any potential influence at all on disease risk may inspire physicians to consider other risk factors in especially tall individuals.
”Physicians may find it interesting that we have some confirmation that height does increase the risk of certain conditions,” Dr. Wilson said. “While this is unlikely to dramatically change practice, they may be a bit more diligent in looking for other relevant risk factors for the diseases found in this study in their very tall patients.”
The research was funded by the U.S. Department of Veteran Affairs, the Boettcher Foundation’s Webb-Waring Biomedical Research Program, the National Institutes of Health, and a Linda Pechenik Montague Investigator award. One study coauthor is a full-time employee of Novartis Institutes of Biomedical Research. The other authors and Dr. Wilson had no disclosures.
*Correction, 6/29/22: An earlier version of this article misstated the average age of Black participants.
, according to a study published in PLOS Genetics.
Prior studies have investigated height as a risk factor for chronic diseases, such as a higher risk for atrial fibrillation and a reduced risk of cardiovascular disease. It’s been consistently difficult, however, to eliminate the confounding influences of diet, socioeconomics, lifestyle behaviors, and other environmental factors that may interfere with a person’s reaching their expected height based on their genes.
This study, however, was able to better parse those differences by using Mendelian randomization within the comprehensive clinical and genetic dataset of a national health care system biobank. Mendelian randomization uses “genetic instruments for exposures of interest under the assumption that genotype is less susceptible to confounding than measured exposures,” the authors explained. The findings confirmed previously suspected associations between height and a range of cardiovascular and metabolic conditions as well as revealing new associations with several other conditions.
Prior associations confirmed, new associations uncovered
The results confirmed that being tall is linked to a higher risk of atrial fibrillation and varicose veins, and a lower risk of coronary heart disease, high blood pressure, and high cholesterol. The study also uncovered new associations between greater height and a higher risk of peripheral neuropathy, which is caused by damage to nerves on the extremities, as well as skin and bone infections, such as leg and foot ulcers.
The meta-analysis “identified five additional traits associated with genetically-predicted height,” wrote Sridharan Raghavan, MD, assistant professor of medicine at the University of Colorado Anschutz Medical Campus, and colleagues. “Two were genitourinary conditions – erectile dysfunction and urinary retention – that can be associated with neuropathy, and a third was a phecode for nonspecific skin disorders that may be related to skin infections – consistent with the race/ethnicity stratified results.”
Removing potential confounders
F. Perry Wilson, MD, associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study, said the findings were not particularly surprising overall, but it’s striking that the researchers had ”such a large cohort with such detailed electronic health records allowing for the comparison of genetic height with a variety of clinical outcomes.” He also noted the study’s strength in using Mendelian randomization so that the exposure is the predicted genetic height instead of a person’s measured height.
“This is key, since lots of things affect actual height – nutrition is an important one that could certainly be linked to disease as well,” Dr. Wilson said. ”By using genetic height, the authors remove these potential confounders. Since genetic height is “assigned” at birth (or conception), there is little opportunity for confounding. Of course, it is possible that some of the gene variants used to predict genetic height actually do something else, such as make you seek out less nutritious meals, but by and large this is how these types of studies need to be done.”
Height may impact over 100 clinical traits
The study relied on data from the U.S. Veteran Affairs Million Veteran Program with 222,300 non-Hispanic White and 58,151 non-Hispanic Black participants. The researchers first estimated the likelihood of participants’ genetic height based on 3,290 genetic variants determined to affect genetic height in a recent European-ancestry genome-wide meta-analysis. Then they compared these estimates with participants’ actual height in the VA medical record, adjusting for age, sex, and other genetic characteristics.
In doing so, the researchers found 345 clinical traits that were associated with the actual measured height in White participants plus another 17 clinical trials linked to actual measured height in Black participants. An overall 127 of these clinical traits were significantly associated with White participants’ genetically predicted height, and two of them were significantly associated with Black participants’ genetically predicted height.
In analyzing all these data together, the researchers were largely able to separate out those associations between genetically predicted height and certain health conditions from those associations between health conditions and a person’s actual measured height. They also determined that including body mass index as a covariate had little impact on the results. The researchers conducted the appropriate statistical correction to ensure the use of so many variables did not result in spurious statistical significance in some associations.
“Using genetic methods applied to the VA Million Veteran Program, we found evidence that adult height may impact over 100 clinical traits, including several conditions associated with poor outcomes and quality of life – peripheral neuropathy, lower extremity ulcers, and chronic venous insufficiency. We conclude that height may be an unrecognized nonmodifiable risk factor for several common conditions in adults.”
Height linked with health conditions
Genetically predicted height predicted a reduced risk of hyperlipidemia and hypertension independent of coronary heart disease, the analysis revealed. Genetically predicted height was also linked to an approximately 51% increased risk of atrial fibrillation in participants without coronary heart disease but, paradoxically, only a 39% increased risk in those with coronary heart disease, despite coronary heart disease being a risk factor for atrial fibrillation. Genetically predicted height was also associated with a greater risk of varicose veins in the legs and deep vein thrombosis.
Another novel association uncovered by the analysis was between women’s genetically predicted height and both asthma and nonspecific peripheral nerve disorders. “Whether these associations reflect differences by sex in disease pathophysiology related to height may warrant exploration in a sample with better balance between men and women,” the authors wrote. “In sum, our results suggest that an individual’s height may warrant consideration as a nonmodifiable predictor for several common conditions, particularly those affecting peripheral/distal extremities that are most physically impacted by tall stature.”
A substantial limitation of the study was its homogeneity of participants, who were 92% male with an average height of 176 cm and an average BMI of 30.1. The Black participants tended to be younger, with an average age of 58 compared with 64 years in the White participants, but the groups were otherwise similar in height and weight.* The database included data from Hispanic participants, but the researchers excluded these data because of the small sample size.
The smaller dataset for Black participants was a limitation as well as the fact that the genome-wide association study the researchers relied on came from a European population, which may not be as accurate in people with other ancestry, Dr. Wilson said. The bigger limitation, however, is what the findings’ clinical relevance is.
What does it all mean?
“Genetic height is in your genes – there is nothing to be done about it – so it is more of academic interest than clinical interest,” Dr. Wilson said. It’s not even clear whether incorporating a person’s height – actual or genetically predicted, if it could be easily determined for each person – into risk calculators. ”To know whether it would be beneficial to use height (or genetic height) as a risk factor, you’d need to examine each condition of interest, adjusting for all known risk factors, to see if height improved the prediction,” Dr. Wilson said. “I suspect for most conditions, the well-known risk factors would swamp height. For example, high genetic height might truly increase risk for neuropathy. But diabetes might increase the risk so much more that height is not particularly relevant.”
On the other hand, the fact that height in general has any potential influence at all on disease risk may inspire physicians to consider other risk factors in especially tall individuals.
”Physicians may find it interesting that we have some confirmation that height does increase the risk of certain conditions,” Dr. Wilson said. “While this is unlikely to dramatically change practice, they may be a bit more diligent in looking for other relevant risk factors for the diseases found in this study in their very tall patients.”
The research was funded by the U.S. Department of Veteran Affairs, the Boettcher Foundation’s Webb-Waring Biomedical Research Program, the National Institutes of Health, and a Linda Pechenik Montague Investigator award. One study coauthor is a full-time employee of Novartis Institutes of Biomedical Research. The other authors and Dr. Wilson had no disclosures.
*Correction, 6/29/22: An earlier version of this article misstated the average age of Black participants.
, according to a study published in PLOS Genetics.
Prior studies have investigated height as a risk factor for chronic diseases, such as a higher risk for atrial fibrillation and a reduced risk of cardiovascular disease. It’s been consistently difficult, however, to eliminate the confounding influences of diet, socioeconomics, lifestyle behaviors, and other environmental factors that may interfere with a person’s reaching their expected height based on their genes.
This study, however, was able to better parse those differences by using Mendelian randomization within the comprehensive clinical and genetic dataset of a national health care system biobank. Mendelian randomization uses “genetic instruments for exposures of interest under the assumption that genotype is less susceptible to confounding than measured exposures,” the authors explained. The findings confirmed previously suspected associations between height and a range of cardiovascular and metabolic conditions as well as revealing new associations with several other conditions.
Prior associations confirmed, new associations uncovered
The results confirmed that being tall is linked to a higher risk of atrial fibrillation and varicose veins, and a lower risk of coronary heart disease, high blood pressure, and high cholesterol. The study also uncovered new associations between greater height and a higher risk of peripheral neuropathy, which is caused by damage to nerves on the extremities, as well as skin and bone infections, such as leg and foot ulcers.
The meta-analysis “identified five additional traits associated with genetically-predicted height,” wrote Sridharan Raghavan, MD, assistant professor of medicine at the University of Colorado Anschutz Medical Campus, and colleagues. “Two were genitourinary conditions – erectile dysfunction and urinary retention – that can be associated with neuropathy, and a third was a phecode for nonspecific skin disorders that may be related to skin infections – consistent with the race/ethnicity stratified results.”
Removing potential confounders
F. Perry Wilson, MD, associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study, said the findings were not particularly surprising overall, but it’s striking that the researchers had ”such a large cohort with such detailed electronic health records allowing for the comparison of genetic height with a variety of clinical outcomes.” He also noted the study’s strength in using Mendelian randomization so that the exposure is the predicted genetic height instead of a person’s measured height.
“This is key, since lots of things affect actual height – nutrition is an important one that could certainly be linked to disease as well,” Dr. Wilson said. ”By using genetic height, the authors remove these potential confounders. Since genetic height is “assigned” at birth (or conception), there is little opportunity for confounding. Of course, it is possible that some of the gene variants used to predict genetic height actually do something else, such as make you seek out less nutritious meals, but by and large this is how these types of studies need to be done.”
Height may impact over 100 clinical traits
The study relied on data from the U.S. Veteran Affairs Million Veteran Program with 222,300 non-Hispanic White and 58,151 non-Hispanic Black participants. The researchers first estimated the likelihood of participants’ genetic height based on 3,290 genetic variants determined to affect genetic height in a recent European-ancestry genome-wide meta-analysis. Then they compared these estimates with participants’ actual height in the VA medical record, adjusting for age, sex, and other genetic characteristics.
In doing so, the researchers found 345 clinical traits that were associated with the actual measured height in White participants plus another 17 clinical trials linked to actual measured height in Black participants. An overall 127 of these clinical traits were significantly associated with White participants’ genetically predicted height, and two of them were significantly associated with Black participants’ genetically predicted height.
In analyzing all these data together, the researchers were largely able to separate out those associations between genetically predicted height and certain health conditions from those associations between health conditions and a person’s actual measured height. They also determined that including body mass index as a covariate had little impact on the results. The researchers conducted the appropriate statistical correction to ensure the use of so many variables did not result in spurious statistical significance in some associations.
“Using genetic methods applied to the VA Million Veteran Program, we found evidence that adult height may impact over 100 clinical traits, including several conditions associated with poor outcomes and quality of life – peripheral neuropathy, lower extremity ulcers, and chronic venous insufficiency. We conclude that height may be an unrecognized nonmodifiable risk factor for several common conditions in adults.”
Height linked with health conditions
Genetically predicted height predicted a reduced risk of hyperlipidemia and hypertension independent of coronary heart disease, the analysis revealed. Genetically predicted height was also linked to an approximately 51% increased risk of atrial fibrillation in participants without coronary heart disease but, paradoxically, only a 39% increased risk in those with coronary heart disease, despite coronary heart disease being a risk factor for atrial fibrillation. Genetically predicted height was also associated with a greater risk of varicose veins in the legs and deep vein thrombosis.
Another novel association uncovered by the analysis was between women’s genetically predicted height and both asthma and nonspecific peripheral nerve disorders. “Whether these associations reflect differences by sex in disease pathophysiology related to height may warrant exploration in a sample with better balance between men and women,” the authors wrote. “In sum, our results suggest that an individual’s height may warrant consideration as a nonmodifiable predictor for several common conditions, particularly those affecting peripheral/distal extremities that are most physically impacted by tall stature.”
A substantial limitation of the study was its homogeneity of participants, who were 92% male with an average height of 176 cm and an average BMI of 30.1. The Black participants tended to be younger, with an average age of 58 compared with 64 years in the White participants, but the groups were otherwise similar in height and weight.* The database included data from Hispanic participants, but the researchers excluded these data because of the small sample size.
The smaller dataset for Black participants was a limitation as well as the fact that the genome-wide association study the researchers relied on came from a European population, which may not be as accurate in people with other ancestry, Dr. Wilson said. The bigger limitation, however, is what the findings’ clinical relevance is.
What does it all mean?
“Genetic height is in your genes – there is nothing to be done about it – so it is more of academic interest than clinical interest,” Dr. Wilson said. It’s not even clear whether incorporating a person’s height – actual or genetically predicted, if it could be easily determined for each person – into risk calculators. ”To know whether it would be beneficial to use height (or genetic height) as a risk factor, you’d need to examine each condition of interest, adjusting for all known risk factors, to see if height improved the prediction,” Dr. Wilson said. “I suspect for most conditions, the well-known risk factors would swamp height. For example, high genetic height might truly increase risk for neuropathy. But diabetes might increase the risk so much more that height is not particularly relevant.”
On the other hand, the fact that height in general has any potential influence at all on disease risk may inspire physicians to consider other risk factors in especially tall individuals.
”Physicians may find it interesting that we have some confirmation that height does increase the risk of certain conditions,” Dr. Wilson said. “While this is unlikely to dramatically change practice, they may be a bit more diligent in looking for other relevant risk factors for the diseases found in this study in their very tall patients.”
The research was funded by the U.S. Department of Veteran Affairs, the Boettcher Foundation’s Webb-Waring Biomedical Research Program, the National Institutes of Health, and a Linda Pechenik Montague Investigator award. One study coauthor is a full-time employee of Novartis Institutes of Biomedical Research. The other authors and Dr. Wilson had no disclosures.
*Correction, 6/29/22: An earlier version of this article misstated the average age of Black participants.
FROM PLOS GENETICS
Hope for quicker and more accurate endometriosis diagnosis
A new imaging study hopes to make diagnosing endometriosis quicker, more accurate and reduce the need for invasive surgery.
In October 2020 the All Party Parliamentary Group on Endometriosis published a report that included within its recommendations “a commitment to drive down diagnosis times” for women with the condition. On average, it takes around 8 years for a woman to get a diagnosis of endometriosis, a figure, said the authors of the report, that had “not improved in the last decade.”
Indeed, in its report the APPG said that it was seeking a commitment from Governments in all four nations to reduce average diagnosis times with “targets of 4 years or less by 2025, and a year or less by 2030.”
Surgery often needed for endometriosis diagnosis
Endometriosis affects 1 in 10 women between puberty and menopause – 1.5 million in the United Kingdom – often results in multiple general practitioner and accident and emergency department visits, multiple scans, and often laparoscopic surgery to confirm the diagnosis, as there is currently no simple diagnostic test for the condition. One of the main reasons for the delay in diagnosis is the lack of noninvasive tests capable of detecting all endometriosis subtypes – ovarian, superficial, and deep disease.
Now, experts at the Endometriosis CaRe Centre and Nuffield Department of Women’s and Reproductive Health, University of Oxford (England), in collaboration with British life sciences company Serac Healthcare, hope to establish a faster process for diagnosing endometriosis.
Christian Becker, codirector of the Endometriosis CaRe Centre in Oxford, and a study lead, said: “There is an urgent unmet clinical need for a noninvasive marker to identify or rule out endometriosis as it is such a very common disease affecting more than 190 million women worldwide.”
In the study, researchers will investigate whether a 20-minute imaging scan can detect the most common types of endometriosis, which currently require surgery to diagnose. In turn, they hope that earlier diagnosis of the condition will allow women to seek appropriate treatment sooner. They will use an experimental imaging marker – 99mTc-maraciclatide – that binds to areas of inflammation and that can be used in endometriosis to visualize the disease on a scan. The imaging marker has already been used for detecting inflammation in conditions such as rheumatoid arthritis.
Between 2 and 7 days before planned surgery for suspected endometriosis, participants will be invited for an imaging scan, and the team will compare the suspected locations of disease detected on the scan with those seen during surgery to confirm whether this imaging test could be an effective noninvasive method of detecting all endometriosis subtypes.
Doctor visits and repeated investigations reduced
The researchers commented that the potential strengths of the scan lie in the way the imaging marker binds to areas of inflammation, which may allow doctors to distinguish between new and old lesions and detect endometriosis in areas not easily seen during surgery, such as the lung.
They added that the development of a 20-minute imaging test would reduce the need for repeated visits to doctors, for repeated investigations, and for invasive surgery to obtain a diagnosis. This would ultimately “reduce the time taken to confirm or exclude endometriosis,” they pointed out.
Following the publication of the APPG report in October 2020 the group’s then chair, the late Sir David Amess, said: “Without investment in research, a reduction in diagnosis time, and appropriate NHS pathways, those with endometriosis will continue to face huge barriers in accessing the appropriate support at the right time.”
Krina Zondervan, head of department at the Nuffield Department of Women’s and Reproductive Health, University of Oxford, and a study lead, said: “This study highlights that close collaborations between academics, clinicians and industry are important to combine and accelerate discovery and innovation in addressing high-priority areas in women’s health such as endometriosis.”
David Hail, CEO of Serac Healthcare, said: “We are excited about the potential of 99mTc-maraciclatide to diagnose endometriosis noninvasively and delighted to be working with the internationally renowned team at Oxford on this important first study.”
A version of this article first appeared on Medscape UK.
A new imaging study hopes to make diagnosing endometriosis quicker, more accurate and reduce the need for invasive surgery.
In October 2020 the All Party Parliamentary Group on Endometriosis published a report that included within its recommendations “a commitment to drive down diagnosis times” for women with the condition. On average, it takes around 8 years for a woman to get a diagnosis of endometriosis, a figure, said the authors of the report, that had “not improved in the last decade.”
Indeed, in its report the APPG said that it was seeking a commitment from Governments in all four nations to reduce average diagnosis times with “targets of 4 years or less by 2025, and a year or less by 2030.”
Surgery often needed for endometriosis diagnosis
Endometriosis affects 1 in 10 women between puberty and menopause – 1.5 million in the United Kingdom – often results in multiple general practitioner and accident and emergency department visits, multiple scans, and often laparoscopic surgery to confirm the diagnosis, as there is currently no simple diagnostic test for the condition. One of the main reasons for the delay in diagnosis is the lack of noninvasive tests capable of detecting all endometriosis subtypes – ovarian, superficial, and deep disease.
Now, experts at the Endometriosis CaRe Centre and Nuffield Department of Women’s and Reproductive Health, University of Oxford (England), in collaboration with British life sciences company Serac Healthcare, hope to establish a faster process for diagnosing endometriosis.
Christian Becker, codirector of the Endometriosis CaRe Centre in Oxford, and a study lead, said: “There is an urgent unmet clinical need for a noninvasive marker to identify or rule out endometriosis as it is such a very common disease affecting more than 190 million women worldwide.”
In the study, researchers will investigate whether a 20-minute imaging scan can detect the most common types of endometriosis, which currently require surgery to diagnose. In turn, they hope that earlier diagnosis of the condition will allow women to seek appropriate treatment sooner. They will use an experimental imaging marker – 99mTc-maraciclatide – that binds to areas of inflammation and that can be used in endometriosis to visualize the disease on a scan. The imaging marker has already been used for detecting inflammation in conditions such as rheumatoid arthritis.
Between 2 and 7 days before planned surgery for suspected endometriosis, participants will be invited for an imaging scan, and the team will compare the suspected locations of disease detected on the scan with those seen during surgery to confirm whether this imaging test could be an effective noninvasive method of detecting all endometriosis subtypes.
Doctor visits and repeated investigations reduced
The researchers commented that the potential strengths of the scan lie in the way the imaging marker binds to areas of inflammation, which may allow doctors to distinguish between new and old lesions and detect endometriosis in areas not easily seen during surgery, such as the lung.
They added that the development of a 20-minute imaging test would reduce the need for repeated visits to doctors, for repeated investigations, and for invasive surgery to obtain a diagnosis. This would ultimately “reduce the time taken to confirm or exclude endometriosis,” they pointed out.
Following the publication of the APPG report in October 2020 the group’s then chair, the late Sir David Amess, said: “Without investment in research, a reduction in diagnosis time, and appropriate NHS pathways, those with endometriosis will continue to face huge barriers in accessing the appropriate support at the right time.”
Krina Zondervan, head of department at the Nuffield Department of Women’s and Reproductive Health, University of Oxford, and a study lead, said: “This study highlights that close collaborations between academics, clinicians and industry are important to combine and accelerate discovery and innovation in addressing high-priority areas in women’s health such as endometriosis.”
David Hail, CEO of Serac Healthcare, said: “We are excited about the potential of 99mTc-maraciclatide to diagnose endometriosis noninvasively and delighted to be working with the internationally renowned team at Oxford on this important first study.”
A version of this article first appeared on Medscape UK.
A new imaging study hopes to make diagnosing endometriosis quicker, more accurate and reduce the need for invasive surgery.
In October 2020 the All Party Parliamentary Group on Endometriosis published a report that included within its recommendations “a commitment to drive down diagnosis times” for women with the condition. On average, it takes around 8 years for a woman to get a diagnosis of endometriosis, a figure, said the authors of the report, that had “not improved in the last decade.”
Indeed, in its report the APPG said that it was seeking a commitment from Governments in all four nations to reduce average diagnosis times with “targets of 4 years or less by 2025, and a year or less by 2030.”
Surgery often needed for endometriosis diagnosis
Endometriosis affects 1 in 10 women between puberty and menopause – 1.5 million in the United Kingdom – often results in multiple general practitioner and accident and emergency department visits, multiple scans, and often laparoscopic surgery to confirm the diagnosis, as there is currently no simple diagnostic test for the condition. One of the main reasons for the delay in diagnosis is the lack of noninvasive tests capable of detecting all endometriosis subtypes – ovarian, superficial, and deep disease.
Now, experts at the Endometriosis CaRe Centre and Nuffield Department of Women’s and Reproductive Health, University of Oxford (England), in collaboration with British life sciences company Serac Healthcare, hope to establish a faster process for diagnosing endometriosis.
Christian Becker, codirector of the Endometriosis CaRe Centre in Oxford, and a study lead, said: “There is an urgent unmet clinical need for a noninvasive marker to identify or rule out endometriosis as it is such a very common disease affecting more than 190 million women worldwide.”
In the study, researchers will investigate whether a 20-minute imaging scan can detect the most common types of endometriosis, which currently require surgery to diagnose. In turn, they hope that earlier diagnosis of the condition will allow women to seek appropriate treatment sooner. They will use an experimental imaging marker – 99mTc-maraciclatide – that binds to areas of inflammation and that can be used in endometriosis to visualize the disease on a scan. The imaging marker has already been used for detecting inflammation in conditions such as rheumatoid arthritis.
Between 2 and 7 days before planned surgery for suspected endometriosis, participants will be invited for an imaging scan, and the team will compare the suspected locations of disease detected on the scan with those seen during surgery to confirm whether this imaging test could be an effective noninvasive method of detecting all endometriosis subtypes.
Doctor visits and repeated investigations reduced
The researchers commented that the potential strengths of the scan lie in the way the imaging marker binds to areas of inflammation, which may allow doctors to distinguish between new and old lesions and detect endometriosis in areas not easily seen during surgery, such as the lung.
They added that the development of a 20-minute imaging test would reduce the need for repeated visits to doctors, for repeated investigations, and for invasive surgery to obtain a diagnosis. This would ultimately “reduce the time taken to confirm or exclude endometriosis,” they pointed out.
Following the publication of the APPG report in October 2020 the group’s then chair, the late Sir David Amess, said: “Without investment in research, a reduction in diagnosis time, and appropriate NHS pathways, those with endometriosis will continue to face huge barriers in accessing the appropriate support at the right time.”
Krina Zondervan, head of department at the Nuffield Department of Women’s and Reproductive Health, University of Oxford, and a study lead, said: “This study highlights that close collaborations between academics, clinicians and industry are important to combine and accelerate discovery and innovation in addressing high-priority areas in women’s health such as endometriosis.”
David Hail, CEO of Serac Healthcare, said: “We are excited about the potential of 99mTc-maraciclatide to diagnose endometriosis noninvasively and delighted to be working with the internationally renowned team at Oxford on this important first study.”
A version of this article first appeared on Medscape UK.
Cannabis may relieve pain as effectively as opioids, but more research is needed
Several other systematic reviews have recently evaluated cannabinoids for treating chronic pain, but the new study’s methodology was “distinct” in “important ways,” leading to “conclusions that differ from other reviews,” according to the authors of the paper published in the Annals of Internal Medicine.
In the new systematic review, synthetic products with high THC:CBD ratios were associated with moderate improvements in pain, whereas plant-based products with comparable THC:CBD ratios offered less relief, said study author Marian S. McDonagh, PharmD, professor of medical informatics and clinical epidemiology, and codirector of the Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues.
Specifically, the investigators stratified cannabis-based interventions according to relative content of two key cannabinoids: THC and CBD. Products were sorted into five categories: high THC:CBD ratio (at least 2:1), comparable THC:CBD ratio (less than 2:1 but more than 1:2), low THC:CBD ratio (no more than 1:2), whole-plant cannabis products, and other cannabinoids.
“In preclinical studies, THC and related compounds have demonstrated analgesic properties, although its psychoactive effects and addiction potential may limit its suitability as an analgesic,” the investigators wrote. “CBD and other cannabinoids may also have some analgesic or anti-inflammatory properties and are not believed to be psychoactive or addictive. Given the variation in analgesic effect with THC and CBD, response may differ according to the ratio of THC to CBD in products used to treat pain.”
The final analysis included 18 randomized placebo-controlled trials involving 1,740 individuals and 7 cohort studies involving 13,095 individuals. Most of the studies were short-term, lasting 1-6 months.
Pain was scored on a ten-point scale, with improvements reported as the mean difference from baseline to post treatment. A mean difference in pain score of 0.5-1.0 was considered a “small effect,” an improvement of 1-2 points was considered a “moderate effect,” and an improvement greater than 2 points was considered a “large effect.”
Cannabis-based products with relatively high THC:CBD ratios showed efficacy
Synthetic products with high THC:CBD ratios offered moderate pain relief, based on a mean difference in pain score of –1.15 (95% confidence interval, –1.99 to –0.54), whereas products with comparable THC:CBD ratios were associated with a small effect on pain, with a mean difference of –0.52 (95% CI, –0.95 to –0.19).
According to Dr. McDonagh, treatment response rates were on par with response rates for more conventional treatments, “such as opioids or specific antidepressant drugs,” but data for the cannabis-based products are weaker.
“The amount of evidence available for cannabis-related products is very limited for [response rates], and therefore less certain,” Dr. McDonagh said in an interview. “The average reduction in pain severity is also similar to some other treatments, but we do not have studies directly comparing these treatments to draw conclusions.”
Although the cannabis-based products with relatively high and comparable THC:CBD ratios showed efficacy, they were also associated with “moderate to large increased risk for dizziness, sedation, and nausea,” the investigators wrote, noting that evidence was insufficient to characterize other “key adverse event outcomes” that may occur with long-term use, such as “psychosis, cannabis use disorder, and cognitive deficits.”
For products with low THC:CBD ratios, or without reported THC:CBD ratios, data were too scarce to reach any conclusions at all about safety or efficacy, highlighting the sizable knowledge gaps that remain in the area, the authors said.
“The current evidence on cannabis-related products for chronic pain is quite limited,” Dr. McDonagh said in an interview. “Patients with chronic pain should consult with their doctor to discuss which of the many options for treating chronic pain is best for them to start with.”
Patients may face resistance when asking about cannabis
According to Kevin F. Boehnke, PhD, and Daniel J. Clauw, MD, of the anesthesiology department and Chronic Pain and Fatigue Research Center at the University of Michigan, Ann Arbor, patients with chronic pain may face resistance, or even risk of being reported, when asking about cannabis-based products.
“Some physicians cite lack of data as rationale for not engaging with patients who wish to use or currently use cannabis,” Dr. Boehnke and Dr. Clauw wrote in an accompanying editorial. “Such practices may reflect consideration of cannabis solely as a drug of misuse (even in the 37 states where medical cannabis is legal) and requirements to refer patients who disclose or test positive for cannabis use to addiction services or decline to refill opioid prescriptions.”
Instead of shutting patients out, Dr. Boehnke and Dr. Clauw suggested clinicians engage in an “open information exchange” with their patients that focuses on “pragmatism, patient experience, known cannabinoid effects, and harm reduction.” In these conversations, the editorialists recommend noting that, “as with other analgesics, some persons will benefit, and others will not.”
They also offered some practical guidance: “Clinicians could suggest using tinctures (effect onset, 15-45 minutes) for breakthrough pain and edibles or capsules (which last about 6-8 hours) for extended relief. ... The scientific literature suggests that CBD doses could start at 5-10 mg twice daily and increase to 40-50 mg daily, whereas THC doses could start at 0.5-3 mg (initially at night) and increase to 30-40 mg/day.”
David Copenhaver, MD, MPH, clinical professor and chief of the division of pain medicine at UC Davis Health, Sacramento, shared a similar clinical mindset for patients choosing between opioids and cannabis-based products, specifically, CBD.
Compared with opioids, “the side-effect profile for CBD is less and the risk of mortality is less,” Dr. Copenhaver said in an interview, pointing out that nobody, to his knowledge, has ever died from an overdose of cannabis alone, and that CBD doses up to 1,000 mg/kg have been safely tolerated in people. “You present that, and most patients will say, ‘You know, I’d like to give this a try.’”
If so, Dr. Copenhaver makes sure patients know about a nonmedical risk: “The risk to the pocketbook.” Unlike opioids, which are covered under most insurance policies, most cannabis-based therapies are self-pay.
Buyers may get what they pay for, Dr. Copenhaver said, since products vary in quality, as do the dispensaries, from “very modest,” to highly sophisticated, with some even using chromatographic datasets to support the purity of their products.
Dr. Copenhaver steers his patients toward these more sophisticated retailers. Their expertise appears to be paying off, he said, not only in relief for patients, but also in market share. “Survival of the most fit will occur in the marketplace based on the results,” he said. “Unfortunately, some of that information doesn’t get percolated out into the literature.”
For investigators to fully uncover what cannabis-based products can do for chronic pain, Dr. Copenhaver said they need to get as “granular” as the leading dispensaries, which may first require recognition of the “very expansive opportunity” that less-studied cannabinoids may provide.
The study was supported by the Agency for Healthcare Research and Quality, U.S. Department of Health & Human Services. The investigators, Dr. Boehnke, Dr. Clauw, and Dr. Copenhaver, disclosed no conflicts of interest.
Several other systematic reviews have recently evaluated cannabinoids for treating chronic pain, but the new study’s methodology was “distinct” in “important ways,” leading to “conclusions that differ from other reviews,” according to the authors of the paper published in the Annals of Internal Medicine.
In the new systematic review, synthetic products with high THC:CBD ratios were associated with moderate improvements in pain, whereas plant-based products with comparable THC:CBD ratios offered less relief, said study author Marian S. McDonagh, PharmD, professor of medical informatics and clinical epidemiology, and codirector of the Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues.
Specifically, the investigators stratified cannabis-based interventions according to relative content of two key cannabinoids: THC and CBD. Products were sorted into five categories: high THC:CBD ratio (at least 2:1), comparable THC:CBD ratio (less than 2:1 but more than 1:2), low THC:CBD ratio (no more than 1:2), whole-plant cannabis products, and other cannabinoids.
“In preclinical studies, THC and related compounds have demonstrated analgesic properties, although its psychoactive effects and addiction potential may limit its suitability as an analgesic,” the investigators wrote. “CBD and other cannabinoids may also have some analgesic or anti-inflammatory properties and are not believed to be psychoactive or addictive. Given the variation in analgesic effect with THC and CBD, response may differ according to the ratio of THC to CBD in products used to treat pain.”
The final analysis included 18 randomized placebo-controlled trials involving 1,740 individuals and 7 cohort studies involving 13,095 individuals. Most of the studies were short-term, lasting 1-6 months.
Pain was scored on a ten-point scale, with improvements reported as the mean difference from baseline to post treatment. A mean difference in pain score of 0.5-1.0 was considered a “small effect,” an improvement of 1-2 points was considered a “moderate effect,” and an improvement greater than 2 points was considered a “large effect.”
Cannabis-based products with relatively high THC:CBD ratios showed efficacy
Synthetic products with high THC:CBD ratios offered moderate pain relief, based on a mean difference in pain score of –1.15 (95% confidence interval, –1.99 to –0.54), whereas products with comparable THC:CBD ratios were associated with a small effect on pain, with a mean difference of –0.52 (95% CI, –0.95 to –0.19).
According to Dr. McDonagh, treatment response rates were on par with response rates for more conventional treatments, “such as opioids or specific antidepressant drugs,” but data for the cannabis-based products are weaker.
“The amount of evidence available for cannabis-related products is very limited for [response rates], and therefore less certain,” Dr. McDonagh said in an interview. “The average reduction in pain severity is also similar to some other treatments, but we do not have studies directly comparing these treatments to draw conclusions.”
Although the cannabis-based products with relatively high and comparable THC:CBD ratios showed efficacy, they were also associated with “moderate to large increased risk for dizziness, sedation, and nausea,” the investigators wrote, noting that evidence was insufficient to characterize other “key adverse event outcomes” that may occur with long-term use, such as “psychosis, cannabis use disorder, and cognitive deficits.”
For products with low THC:CBD ratios, or without reported THC:CBD ratios, data were too scarce to reach any conclusions at all about safety or efficacy, highlighting the sizable knowledge gaps that remain in the area, the authors said.
“The current evidence on cannabis-related products for chronic pain is quite limited,” Dr. McDonagh said in an interview. “Patients with chronic pain should consult with their doctor to discuss which of the many options for treating chronic pain is best for them to start with.”
Patients may face resistance when asking about cannabis
According to Kevin F. Boehnke, PhD, and Daniel J. Clauw, MD, of the anesthesiology department and Chronic Pain and Fatigue Research Center at the University of Michigan, Ann Arbor, patients with chronic pain may face resistance, or even risk of being reported, when asking about cannabis-based products.
“Some physicians cite lack of data as rationale for not engaging with patients who wish to use or currently use cannabis,” Dr. Boehnke and Dr. Clauw wrote in an accompanying editorial. “Such practices may reflect consideration of cannabis solely as a drug of misuse (even in the 37 states where medical cannabis is legal) and requirements to refer patients who disclose or test positive for cannabis use to addiction services or decline to refill opioid prescriptions.”
Instead of shutting patients out, Dr. Boehnke and Dr. Clauw suggested clinicians engage in an “open information exchange” with their patients that focuses on “pragmatism, patient experience, known cannabinoid effects, and harm reduction.” In these conversations, the editorialists recommend noting that, “as with other analgesics, some persons will benefit, and others will not.”
They also offered some practical guidance: “Clinicians could suggest using tinctures (effect onset, 15-45 minutes) for breakthrough pain and edibles or capsules (which last about 6-8 hours) for extended relief. ... The scientific literature suggests that CBD doses could start at 5-10 mg twice daily and increase to 40-50 mg daily, whereas THC doses could start at 0.5-3 mg (initially at night) and increase to 30-40 mg/day.”
David Copenhaver, MD, MPH, clinical professor and chief of the division of pain medicine at UC Davis Health, Sacramento, shared a similar clinical mindset for patients choosing between opioids and cannabis-based products, specifically, CBD.
Compared with opioids, “the side-effect profile for CBD is less and the risk of mortality is less,” Dr. Copenhaver said in an interview, pointing out that nobody, to his knowledge, has ever died from an overdose of cannabis alone, and that CBD doses up to 1,000 mg/kg have been safely tolerated in people. “You present that, and most patients will say, ‘You know, I’d like to give this a try.’”
If so, Dr. Copenhaver makes sure patients know about a nonmedical risk: “The risk to the pocketbook.” Unlike opioids, which are covered under most insurance policies, most cannabis-based therapies are self-pay.
Buyers may get what they pay for, Dr. Copenhaver said, since products vary in quality, as do the dispensaries, from “very modest,” to highly sophisticated, with some even using chromatographic datasets to support the purity of their products.
Dr. Copenhaver steers his patients toward these more sophisticated retailers. Their expertise appears to be paying off, he said, not only in relief for patients, but also in market share. “Survival of the most fit will occur in the marketplace based on the results,” he said. “Unfortunately, some of that information doesn’t get percolated out into the literature.”
For investigators to fully uncover what cannabis-based products can do for chronic pain, Dr. Copenhaver said they need to get as “granular” as the leading dispensaries, which may first require recognition of the “very expansive opportunity” that less-studied cannabinoids may provide.
The study was supported by the Agency for Healthcare Research and Quality, U.S. Department of Health & Human Services. The investigators, Dr. Boehnke, Dr. Clauw, and Dr. Copenhaver, disclosed no conflicts of interest.
Several other systematic reviews have recently evaluated cannabinoids for treating chronic pain, but the new study’s methodology was “distinct” in “important ways,” leading to “conclusions that differ from other reviews,” according to the authors of the paper published in the Annals of Internal Medicine.
In the new systematic review, synthetic products with high THC:CBD ratios were associated with moderate improvements in pain, whereas plant-based products with comparable THC:CBD ratios offered less relief, said study author Marian S. McDonagh, PharmD, professor of medical informatics and clinical epidemiology, and codirector of the Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues.
Specifically, the investigators stratified cannabis-based interventions according to relative content of two key cannabinoids: THC and CBD. Products were sorted into five categories: high THC:CBD ratio (at least 2:1), comparable THC:CBD ratio (less than 2:1 but more than 1:2), low THC:CBD ratio (no more than 1:2), whole-plant cannabis products, and other cannabinoids.
“In preclinical studies, THC and related compounds have demonstrated analgesic properties, although its psychoactive effects and addiction potential may limit its suitability as an analgesic,” the investigators wrote. “CBD and other cannabinoids may also have some analgesic or anti-inflammatory properties and are not believed to be psychoactive or addictive. Given the variation in analgesic effect with THC and CBD, response may differ according to the ratio of THC to CBD in products used to treat pain.”
The final analysis included 18 randomized placebo-controlled trials involving 1,740 individuals and 7 cohort studies involving 13,095 individuals. Most of the studies were short-term, lasting 1-6 months.
Pain was scored on a ten-point scale, with improvements reported as the mean difference from baseline to post treatment. A mean difference in pain score of 0.5-1.0 was considered a “small effect,” an improvement of 1-2 points was considered a “moderate effect,” and an improvement greater than 2 points was considered a “large effect.”
Cannabis-based products with relatively high THC:CBD ratios showed efficacy
Synthetic products with high THC:CBD ratios offered moderate pain relief, based on a mean difference in pain score of –1.15 (95% confidence interval, –1.99 to –0.54), whereas products with comparable THC:CBD ratios were associated with a small effect on pain, with a mean difference of –0.52 (95% CI, –0.95 to –0.19).
According to Dr. McDonagh, treatment response rates were on par with response rates for more conventional treatments, “such as opioids or specific antidepressant drugs,” but data for the cannabis-based products are weaker.
“The amount of evidence available for cannabis-related products is very limited for [response rates], and therefore less certain,” Dr. McDonagh said in an interview. “The average reduction in pain severity is also similar to some other treatments, but we do not have studies directly comparing these treatments to draw conclusions.”
Although the cannabis-based products with relatively high and comparable THC:CBD ratios showed efficacy, they were also associated with “moderate to large increased risk for dizziness, sedation, and nausea,” the investigators wrote, noting that evidence was insufficient to characterize other “key adverse event outcomes” that may occur with long-term use, such as “psychosis, cannabis use disorder, and cognitive deficits.”
For products with low THC:CBD ratios, or without reported THC:CBD ratios, data were too scarce to reach any conclusions at all about safety or efficacy, highlighting the sizable knowledge gaps that remain in the area, the authors said.
“The current evidence on cannabis-related products for chronic pain is quite limited,” Dr. McDonagh said in an interview. “Patients with chronic pain should consult with their doctor to discuss which of the many options for treating chronic pain is best for them to start with.”
Patients may face resistance when asking about cannabis
According to Kevin F. Boehnke, PhD, and Daniel J. Clauw, MD, of the anesthesiology department and Chronic Pain and Fatigue Research Center at the University of Michigan, Ann Arbor, patients with chronic pain may face resistance, or even risk of being reported, when asking about cannabis-based products.
“Some physicians cite lack of data as rationale for not engaging with patients who wish to use or currently use cannabis,” Dr. Boehnke and Dr. Clauw wrote in an accompanying editorial. “Such practices may reflect consideration of cannabis solely as a drug of misuse (even in the 37 states where medical cannabis is legal) and requirements to refer patients who disclose or test positive for cannabis use to addiction services or decline to refill opioid prescriptions.”
Instead of shutting patients out, Dr. Boehnke and Dr. Clauw suggested clinicians engage in an “open information exchange” with their patients that focuses on “pragmatism, patient experience, known cannabinoid effects, and harm reduction.” In these conversations, the editorialists recommend noting that, “as with other analgesics, some persons will benefit, and others will not.”
They also offered some practical guidance: “Clinicians could suggest using tinctures (effect onset, 15-45 minutes) for breakthrough pain and edibles or capsules (which last about 6-8 hours) for extended relief. ... The scientific literature suggests that CBD doses could start at 5-10 mg twice daily and increase to 40-50 mg daily, whereas THC doses could start at 0.5-3 mg (initially at night) and increase to 30-40 mg/day.”
David Copenhaver, MD, MPH, clinical professor and chief of the division of pain medicine at UC Davis Health, Sacramento, shared a similar clinical mindset for patients choosing between opioids and cannabis-based products, specifically, CBD.
Compared with opioids, “the side-effect profile for CBD is less and the risk of mortality is less,” Dr. Copenhaver said in an interview, pointing out that nobody, to his knowledge, has ever died from an overdose of cannabis alone, and that CBD doses up to 1,000 mg/kg have been safely tolerated in people. “You present that, and most patients will say, ‘You know, I’d like to give this a try.’”
If so, Dr. Copenhaver makes sure patients know about a nonmedical risk: “The risk to the pocketbook.” Unlike opioids, which are covered under most insurance policies, most cannabis-based therapies are self-pay.
Buyers may get what they pay for, Dr. Copenhaver said, since products vary in quality, as do the dispensaries, from “very modest,” to highly sophisticated, with some even using chromatographic datasets to support the purity of their products.
Dr. Copenhaver steers his patients toward these more sophisticated retailers. Their expertise appears to be paying off, he said, not only in relief for patients, but also in market share. “Survival of the most fit will occur in the marketplace based on the results,” he said. “Unfortunately, some of that information doesn’t get percolated out into the literature.”
For investigators to fully uncover what cannabis-based products can do for chronic pain, Dr. Copenhaver said they need to get as “granular” as the leading dispensaries, which may first require recognition of the “very expansive opportunity” that less-studied cannabinoids may provide.
The study was supported by the Agency for Healthcare Research and Quality, U.S. Department of Health & Human Services. The investigators, Dr. Boehnke, Dr. Clauw, and Dr. Copenhaver, disclosed no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Can lasers be used to measure nerve sensitivity in the skin?
SAN DIEGO – In a 2006 report of complications from laser dermatologic surgery, one of the authors, Dieter Manstein, MD, PhD, who had subjected his forearm to treatment with a fractional laser skin resurfacing prototype device, was included as 1 of the 19 featured cases.
Dr. Manstein, of the Cutaneous Biology Research Center in the department of dermatology at Massachusetts General Hospital, Boston, was exposed to three test spots in the evaluation of the effects of different microscopic thermal zone densities for the prototype device, emitting at 1,450 nm and an energy per MTZ of 3 mJ.
Two years later, hypopigmentation persisted at the test site treated with the highest MTZ density, while two other sites treated with the lower MTZ densities did not show any dyspigmentation. But he noticed something else during the experiment: He felt minimal to no pain as each test site was being treated.
“It took 7 minutes without any cooling or anesthesia,” Dr. Manstein recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “It was not completely painless, but each time the laser was applied, sometimes I felt a little prick, sometimes I felt nothing.” Essentially, he added, “we created cell injury with a focused laser beam without anesthesia,” but this could also indicate that if skin is treated with a fractional laser very slowly, anesthesia is not needed. “Current devices are meant to treat very quickly, but if we [treat] slowly, maybe you could remove lesions painlessly without anesthesia.”
The observation from that experiment also led Dr. Manstein and colleagues to wonder: Could a focused laser beam pattern be used to assess cutaneous innervation? If so, they postulated, perhaps it could be used to not only assess nerve sensitivity of candidates for dermatologic surgery, but as a tool to help diagnose small fiber neuropathies such as diabetic neuropathy, and neuropathies in patients with HIV and sarcoidosis.
The current gold standard for making these diagnoses involves a skin biopsy, immunohistochemical analysis, and nerve fiber quantification, which is not widely available. It also requires strict histologic processing and nerve counting rules. Confocal microscopy of nerve fibers in the cornea is another approach, but is very difficult to perform, “so it would be nice if there was a simple way” to determine nerve fiber density in the skin using a focused laser beam, Dr. Manstein said.
With help from Payal Patel, MD, a dermatology research fellow at MGH, records each subject’s perception of a stimulus, and maps the areas of stimulus response. Current diameters being studied range from 0.076-1.15 mm and depths less than 0.71 mm. “We can focus the laser beam, preset the beam diameter, and very slowly, in a controlled manner, make a rectangular pattern, and after each time, inquire if the subject felt the pulse or not,” Dr. Manstein explained.
“This laser could become a new method for diagnosing nerve fiber neuropathies. If this works well, I think we can miniaturize the device,” he added.
Dr. Manstein disclosed that he is a consultant for Blossom Innovations, R2 Dermatology, and AVAVA. He is also a member of the advisory board for Blossom Innovations.
SAN DIEGO – In a 2006 report of complications from laser dermatologic surgery, one of the authors, Dieter Manstein, MD, PhD, who had subjected his forearm to treatment with a fractional laser skin resurfacing prototype device, was included as 1 of the 19 featured cases.
Dr. Manstein, of the Cutaneous Biology Research Center in the department of dermatology at Massachusetts General Hospital, Boston, was exposed to three test spots in the evaluation of the effects of different microscopic thermal zone densities for the prototype device, emitting at 1,450 nm and an energy per MTZ of 3 mJ.
Two years later, hypopigmentation persisted at the test site treated with the highest MTZ density, while two other sites treated with the lower MTZ densities did not show any dyspigmentation. But he noticed something else during the experiment: He felt minimal to no pain as each test site was being treated.
“It took 7 minutes without any cooling or anesthesia,” Dr. Manstein recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “It was not completely painless, but each time the laser was applied, sometimes I felt a little prick, sometimes I felt nothing.” Essentially, he added, “we created cell injury with a focused laser beam without anesthesia,” but this could also indicate that if skin is treated with a fractional laser very slowly, anesthesia is not needed. “Current devices are meant to treat very quickly, but if we [treat] slowly, maybe you could remove lesions painlessly without anesthesia.”
The observation from that experiment also led Dr. Manstein and colleagues to wonder: Could a focused laser beam pattern be used to assess cutaneous innervation? If so, they postulated, perhaps it could be used to not only assess nerve sensitivity of candidates for dermatologic surgery, but as a tool to help diagnose small fiber neuropathies such as diabetic neuropathy, and neuropathies in patients with HIV and sarcoidosis.
The current gold standard for making these diagnoses involves a skin biopsy, immunohistochemical analysis, and nerve fiber quantification, which is not widely available. It also requires strict histologic processing and nerve counting rules. Confocal microscopy of nerve fibers in the cornea is another approach, but is very difficult to perform, “so it would be nice if there was a simple way” to determine nerve fiber density in the skin using a focused laser beam, Dr. Manstein said.
With help from Payal Patel, MD, a dermatology research fellow at MGH, records each subject’s perception of a stimulus, and maps the areas of stimulus response. Current diameters being studied range from 0.076-1.15 mm and depths less than 0.71 mm. “We can focus the laser beam, preset the beam diameter, and very slowly, in a controlled manner, make a rectangular pattern, and after each time, inquire if the subject felt the pulse or not,” Dr. Manstein explained.
“This laser could become a new method for diagnosing nerve fiber neuropathies. If this works well, I think we can miniaturize the device,” he added.
Dr. Manstein disclosed that he is a consultant for Blossom Innovations, R2 Dermatology, and AVAVA. He is also a member of the advisory board for Blossom Innovations.
SAN DIEGO – In a 2006 report of complications from laser dermatologic surgery, one of the authors, Dieter Manstein, MD, PhD, who had subjected his forearm to treatment with a fractional laser skin resurfacing prototype device, was included as 1 of the 19 featured cases.
Dr. Manstein, of the Cutaneous Biology Research Center in the department of dermatology at Massachusetts General Hospital, Boston, was exposed to three test spots in the evaluation of the effects of different microscopic thermal zone densities for the prototype device, emitting at 1,450 nm and an energy per MTZ of 3 mJ.
Two years later, hypopigmentation persisted at the test site treated with the highest MTZ density, while two other sites treated with the lower MTZ densities did not show any dyspigmentation. But he noticed something else during the experiment: He felt minimal to no pain as each test site was being treated.
“It took 7 minutes without any cooling or anesthesia,” Dr. Manstein recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “It was not completely painless, but each time the laser was applied, sometimes I felt a little prick, sometimes I felt nothing.” Essentially, he added, “we created cell injury with a focused laser beam without anesthesia,” but this could also indicate that if skin is treated with a fractional laser very slowly, anesthesia is not needed. “Current devices are meant to treat very quickly, but if we [treat] slowly, maybe you could remove lesions painlessly without anesthesia.”
The observation from that experiment also led Dr. Manstein and colleagues to wonder: Could a focused laser beam pattern be used to assess cutaneous innervation? If so, they postulated, perhaps it could be used to not only assess nerve sensitivity of candidates for dermatologic surgery, but as a tool to help diagnose small fiber neuropathies such as diabetic neuropathy, and neuropathies in patients with HIV and sarcoidosis.
The current gold standard for making these diagnoses involves a skin biopsy, immunohistochemical analysis, and nerve fiber quantification, which is not widely available. It also requires strict histologic processing and nerve counting rules. Confocal microscopy of nerve fibers in the cornea is another approach, but is very difficult to perform, “so it would be nice if there was a simple way” to determine nerve fiber density in the skin using a focused laser beam, Dr. Manstein said.
With help from Payal Patel, MD, a dermatology research fellow at MGH, records each subject’s perception of a stimulus, and maps the areas of stimulus response. Current diameters being studied range from 0.076-1.15 mm and depths less than 0.71 mm. “We can focus the laser beam, preset the beam diameter, and very slowly, in a controlled manner, make a rectangular pattern, and after each time, inquire if the subject felt the pulse or not,” Dr. Manstein explained.
“This laser could become a new method for diagnosing nerve fiber neuropathies. If this works well, I think we can miniaturize the device,” he added.
Dr. Manstein disclosed that he is a consultant for Blossom Innovations, R2 Dermatology, and AVAVA. He is also a member of the advisory board for Blossom Innovations.
AT ASLMS 2022
Refugees have a high burden of chronic pain associated with mental illness
The study covered in this summary was published in researchsquare.com and has not yet been peer reviewed.
Key takeaways
- Anxiety, , and PTSD are associated with higher levels of chronic pain in the refugee population studied.
- Being a male refugee is associated more strongly with anxiety and depression leading to functional impairment than being a woman. Being a woman is associated with higher odds of chronic pain. Gender acted as an effect modifier between mental illness and functional impairment.
- Future research aimed toward harmonizing and standardizing pain measurement to measure its effect on health burden is needed. Pain should be understood under an ethnocultural construct to enhance transcultural validity.
Why this matters
- The present cross-sectional survey of adult refugees from Syria resettled in Norway is only one of a few studies investigating the burden of chronic pain and how it relates to mental ill health in a general refugee population. Elevated rates of PTSD, depression, and anxiety have been repeatedly found in refugee populations, and high levels of pain have also been documented.
- Attention to the association between chronic pain and mental health should be made by personnel working with refugees. Because of the gender-specific associations between mental illness and functional impairment, initiatives addressing mental health, chronic pain, or functional impairment in refugee populations should consider gender when tailoring their content and outreach.
Study design
- The study involved a cross-sectional, postal survey questionnaire of participants randomly drawn from full population registries in Norway. There was an initial low response. Invitations were sent out in November 2018 and did not close until September 2019. Several efforts were made to boost participation, including one postal or telephone reminder to all nonresponders.
- Participants were refugee adults from Syria aged 18 and older who arrived in Norway between 2015 and 2017. Gender was tested as an effect modifier.
- Chronic pain was measured with 10 items on the questionnaire and was defined as pain for 3 or more consecutive months in the last year. It included both musculoskeletal pain and pain in five other body regions (stomach, head, genital area, chest, other).
- Anxiety, depression, and PTSD symptoms were measured with the 25-item Hopkins Symptom Checklist, the Harvard Trauma Questionnaire, and the Refugee Trauma History Checklist.
- Questionnaires on perceived general health regarding refugee perceptions of their own health, and functional impairment affecting daily activities because of illness, disability, and mental health were adapted from the European Social Survey 2010.
Key results
- A total of 902 participants who responded to the questionnaire were included in the study from roughly 10,000 invitations, giving a participation rate of about 10%, with no differences in gender distribution.
- The overall prevalence of severe chronic pain was 43.1%, and overall perception of poor general health was 39.9%.
- There was a strong association of chronic pain with all mental illness measured, poor perceived general health, and functional impairment (P < .001). All mental health variables were associated with increased odds of chronic pain (anxiety odds ratio), 2.42; depression, OR, 2.28; PTSD, OR, 1.97; all OR fully adjusted).
- Chronic pain was associated with poor perceived general health and functional impairment with no difference across gender. Mental health showed weaker association with poor perceived general health than chronic pain.
- Syrian men with mental health had three times higher odds of functional impairment. For women, there was no evidence of association between any of the mental ill health variables and functional impairment. Being a woman was associated with chronic pain and poor perceived general health but not functional impairment.
- Being a woman was associated with 50% higher odds of chronic pain in both unadjusted and adjusted models.
Limitations
- With a 10% response rate, selection bias in this cross-sectional study may have been present.
- The cross-sectional design of the study limits causality.
- The validity of the survey is questionable because of transcultural construct regarding pain and mental illness.
- Regression models were built with data at hand. Without preregistered plans for data handling, the findings should be viewed as exploratory with a risk for false-positive findings.
Disclosures
- No external funding was received. The study was funded by the Norwegian Center for Violence and Traumatic Stress Studies.
- None of the authors disclosed relevant financial relationships.
This is a summary of a preprint research study, “Chronic pain, mental health and functional impairment in adult refugees from Syria resettled in Norway: a cross-sectional study,” written by researchers at the Norwegian Centre for Violence and Traumatic Stress Studies in Oslo, the Norwegian Institute of Public Health in Oslo, and the Weill Cornell Medicine in New York City on Research Square. This study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com. A version of this article first appeared on Medscape.com.
The study covered in this summary was published in researchsquare.com and has not yet been peer reviewed.
Key takeaways
- Anxiety, , and PTSD are associated with higher levels of chronic pain in the refugee population studied.
- Being a male refugee is associated more strongly with anxiety and depression leading to functional impairment than being a woman. Being a woman is associated with higher odds of chronic pain. Gender acted as an effect modifier between mental illness and functional impairment.
- Future research aimed toward harmonizing and standardizing pain measurement to measure its effect on health burden is needed. Pain should be understood under an ethnocultural construct to enhance transcultural validity.
Why this matters
- The present cross-sectional survey of adult refugees from Syria resettled in Norway is only one of a few studies investigating the burden of chronic pain and how it relates to mental ill health in a general refugee population. Elevated rates of PTSD, depression, and anxiety have been repeatedly found in refugee populations, and high levels of pain have also been documented.
- Attention to the association between chronic pain and mental health should be made by personnel working with refugees. Because of the gender-specific associations between mental illness and functional impairment, initiatives addressing mental health, chronic pain, or functional impairment in refugee populations should consider gender when tailoring their content and outreach.
Study design
- The study involved a cross-sectional, postal survey questionnaire of participants randomly drawn from full population registries in Norway. There was an initial low response. Invitations were sent out in November 2018 and did not close until September 2019. Several efforts were made to boost participation, including one postal or telephone reminder to all nonresponders.
- Participants were refugee adults from Syria aged 18 and older who arrived in Norway between 2015 and 2017. Gender was tested as an effect modifier.
- Chronic pain was measured with 10 items on the questionnaire and was defined as pain for 3 or more consecutive months in the last year. It included both musculoskeletal pain and pain in five other body regions (stomach, head, genital area, chest, other).
- Anxiety, depression, and PTSD symptoms were measured with the 25-item Hopkins Symptom Checklist, the Harvard Trauma Questionnaire, and the Refugee Trauma History Checklist.
- Questionnaires on perceived general health regarding refugee perceptions of their own health, and functional impairment affecting daily activities because of illness, disability, and mental health were adapted from the European Social Survey 2010.
Key results
- A total of 902 participants who responded to the questionnaire were included in the study from roughly 10,000 invitations, giving a participation rate of about 10%, with no differences in gender distribution.
- The overall prevalence of severe chronic pain was 43.1%, and overall perception of poor general health was 39.9%.
- There was a strong association of chronic pain with all mental illness measured, poor perceived general health, and functional impairment (P < .001). All mental health variables were associated with increased odds of chronic pain (anxiety odds ratio), 2.42; depression, OR, 2.28; PTSD, OR, 1.97; all OR fully adjusted).
- Chronic pain was associated with poor perceived general health and functional impairment with no difference across gender. Mental health showed weaker association with poor perceived general health than chronic pain.
- Syrian men with mental health had three times higher odds of functional impairment. For women, there was no evidence of association between any of the mental ill health variables and functional impairment. Being a woman was associated with chronic pain and poor perceived general health but not functional impairment.
- Being a woman was associated with 50% higher odds of chronic pain in both unadjusted and adjusted models.
Limitations
- With a 10% response rate, selection bias in this cross-sectional study may have been present.
- The cross-sectional design of the study limits causality.
- The validity of the survey is questionable because of transcultural construct regarding pain and mental illness.
- Regression models were built with data at hand. Without preregistered plans for data handling, the findings should be viewed as exploratory with a risk for false-positive findings.
Disclosures
- No external funding was received. The study was funded by the Norwegian Center for Violence and Traumatic Stress Studies.
- None of the authors disclosed relevant financial relationships.
This is a summary of a preprint research study, “Chronic pain, mental health and functional impairment in adult refugees from Syria resettled in Norway: a cross-sectional study,” written by researchers at the Norwegian Centre for Violence and Traumatic Stress Studies in Oslo, the Norwegian Institute of Public Health in Oslo, and the Weill Cornell Medicine in New York City on Research Square. This study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com. A version of this article first appeared on Medscape.com.
The study covered in this summary was published in researchsquare.com and has not yet been peer reviewed.
Key takeaways
- Anxiety, , and PTSD are associated with higher levels of chronic pain in the refugee population studied.
- Being a male refugee is associated more strongly with anxiety and depression leading to functional impairment than being a woman. Being a woman is associated with higher odds of chronic pain. Gender acted as an effect modifier between mental illness and functional impairment.
- Future research aimed toward harmonizing and standardizing pain measurement to measure its effect on health burden is needed. Pain should be understood under an ethnocultural construct to enhance transcultural validity.
Why this matters
- The present cross-sectional survey of adult refugees from Syria resettled in Norway is only one of a few studies investigating the burden of chronic pain and how it relates to mental ill health in a general refugee population. Elevated rates of PTSD, depression, and anxiety have been repeatedly found in refugee populations, and high levels of pain have also been documented.
- Attention to the association between chronic pain and mental health should be made by personnel working with refugees. Because of the gender-specific associations between mental illness and functional impairment, initiatives addressing mental health, chronic pain, or functional impairment in refugee populations should consider gender when tailoring their content and outreach.
Study design
- The study involved a cross-sectional, postal survey questionnaire of participants randomly drawn from full population registries in Norway. There was an initial low response. Invitations were sent out in November 2018 and did not close until September 2019. Several efforts were made to boost participation, including one postal or telephone reminder to all nonresponders.
- Participants were refugee adults from Syria aged 18 and older who arrived in Norway between 2015 and 2017. Gender was tested as an effect modifier.
- Chronic pain was measured with 10 items on the questionnaire and was defined as pain for 3 or more consecutive months in the last year. It included both musculoskeletal pain and pain in five other body regions (stomach, head, genital area, chest, other).
- Anxiety, depression, and PTSD symptoms were measured with the 25-item Hopkins Symptom Checklist, the Harvard Trauma Questionnaire, and the Refugee Trauma History Checklist.
- Questionnaires on perceived general health regarding refugee perceptions of their own health, and functional impairment affecting daily activities because of illness, disability, and mental health were adapted from the European Social Survey 2010.
Key results
- A total of 902 participants who responded to the questionnaire were included in the study from roughly 10,000 invitations, giving a participation rate of about 10%, with no differences in gender distribution.
- The overall prevalence of severe chronic pain was 43.1%, and overall perception of poor general health was 39.9%.
- There was a strong association of chronic pain with all mental illness measured, poor perceived general health, and functional impairment (P < .001). All mental health variables were associated with increased odds of chronic pain (anxiety odds ratio), 2.42; depression, OR, 2.28; PTSD, OR, 1.97; all OR fully adjusted).
- Chronic pain was associated with poor perceived general health and functional impairment with no difference across gender. Mental health showed weaker association with poor perceived general health than chronic pain.
- Syrian men with mental health had three times higher odds of functional impairment. For women, there was no evidence of association between any of the mental ill health variables and functional impairment. Being a woman was associated with chronic pain and poor perceived general health but not functional impairment.
- Being a woman was associated with 50% higher odds of chronic pain in both unadjusted and adjusted models.
Limitations
- With a 10% response rate, selection bias in this cross-sectional study may have been present.
- The cross-sectional design of the study limits causality.
- The validity of the survey is questionable because of transcultural construct regarding pain and mental illness.
- Regression models were built with data at hand. Without preregistered plans for data handling, the findings should be viewed as exploratory with a risk for false-positive findings.
Disclosures
- No external funding was received. The study was funded by the Norwegian Center for Violence and Traumatic Stress Studies.
- None of the authors disclosed relevant financial relationships.
This is a summary of a preprint research study, “Chronic pain, mental health and functional impairment in adult refugees from Syria resettled in Norway: a cross-sectional study,” written by researchers at the Norwegian Centre for Violence and Traumatic Stress Studies in Oslo, the Norwegian Institute of Public Health in Oslo, and the Weill Cornell Medicine in New York City on Research Square. This study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com. A version of this article first appeared on Medscape.com.
Coronary CT Angiography Compared to Coronary Angiography or Standard of Care in Patients With Intermediate-Risk Stable Chest Pain
Study 1 Overview (SCOT-HEART Investigators)
Objective: To assess cardiovascular mortality and nonfatal myocardial infarction at 5 years in patients with stable chest pain referred to cardiology clinic for management with either standard care plus computed tomography angiography (CTA) or standard care alone.
Design: Multicenter, randomized, open-label prospective study.
Setting and participants: A total of 4146 patients with stable chest pain were randomized to standard care or standard care plus CTA at 12 centers across Scotland and were followed for 5 years.
Main outcome measures: The primary end point was a composite of death from coronary heart disease or nonfatal myocardial infarction. Main secondary end points were nonfatal myocardial infarction, nonfatal stroke, and frequency of invasive coronary angiography (ICA) and coronary revascularization with percutaneous coronary intervention or coronary artery bypass grafting.
Main results: The primary outcome including the composite of cardiovascular death or nonfatal myocardial infarction was lower in the CTA group than in the standard-care group at 2.3% (48 of 2073 patients) vs 3.9% (81 of 2073 patients), respectively (hazard ratio, 0.59; 95% CI, 0.41-0.84; P = .004). Although there was a higher rate of ICA and coronary revascularization in the CTA group than in the standard-care group in the first few months of follow-up, the overall rates were similar at 5 years, with ICA performed in 491 patients and 502 patients in the CTA vs standard-care groups, respectively (hazard ratio, 1.00; 95% CI, 0.88-1.13). Similarly, coronary revascularization was performed in 279 patients in the CTA group and in 267 patients in the standard-care group (hazard ratio, 1.07; 95% CI, 0.91-1.27). There were, however, more preventive therapies initiated in patients in the CTA group than in the standard-care group (odds ratio, 1.40; 95% CI, 1.19-1.65).
Conclusion: In patients with stable chest pain, the use of CTA in addition to standard care resulted in a significantly lower rate of death from coronary heart disease or nonfatal myocardial infarction at 5 years; the main contributor to this outcome was a reduced nonfatal myocardial infarction rate. There was no difference in the rate of coronary angiography or coronary revascularization between the 2 groups at 5 years.
Study 2 Overview (DISCHARGE Trial Group)
Objective: To compare the effectiveness of computed tomography (CT) with ICA as a diagnostic tool in patients with stable chest pain and intermediate pretest probability of coronary artery disease (CAD).
Design: Multicenter, randomized, assessor-blinded pragmatic prospective study.
Setting and participants: A total of 3667 patients with stable chest pain and intermediate pretest probability of CAD were enrolled at 26 centers and randomized into CT or ICA groups. Only 3561 patients were included in the modified intention-to-treat analysis, with 1808 patients and 1753 patients in the CT and ICA groups, respectively.
Main outcome measures: The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke over 3.5 years. The main secondary outcomes were major procedure-related complications and patient-reported angina pectoris during the last 4 weeks of follow up.
Main results: The primary outcome occurred in 38 of 1808 patients (2.1%) in the CT group and in 52 of 1753 patients (3.0%) in the ICA group (hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). The secondary outcomes showed that major procedure-related complications occurred in 9 patients (0.5%) in the CT group and in 33 patients (1.9%) in the ICA group (hazard ratio, 0.26; 95% CI, 0.13-0.55). Rates of patient-reported angina in the final 4 weeks of follow-up were 8.8% in the CT group and 7.5% in the ICA group (odds ratio, 1.17; 95% CI, 0.92-1.48).
Conclusion: Risk of major adverse cardiovascular events from the primary outcome were similar in both the CT and ICA groups among patients with stable chest pain and intermediate pretest probability of CAD. Patients referred for CT had a lower rate of coronary angiography leading to fewer major procedure-related complications in these patients than in those referred for ICA.
Commentary
Evaluation and treatment of obstructive atherosclerosis is an important part of clinical care in patients presenting with angina symptoms.1 Thus, the initial investigation for patients with suspected obstructive CAD includes ruling out acute coronary syndrome and assessing quality of life.1 The diagnostic test should be tailored to the pretest probability for the diagnosis of obstructive CAD.2
In the United States, stress testing traditionally has been used for the initial assessment in patients with suspected CAD,3 but recently CTA has been utilized more frequently for this purpose. Compared to a stress test, which often helps identify and assess ischemia, CTA can provide anatomical assessment, with higher sensitivity to identify CAD.4 Furthermore, it can distinguish nonobstructive plaques that can be challenging to identify with stress test alone.
Whether CTA is superior to stress testing as the initial assessment for CAD has been debated. The randomized PROMISE trial compared patients with stable angina who underwent functional stress testing or CTA as an initial strategy.5 They reported a similar outcome between the 2 groups at a median follow-up of 2 years. However, in the original SCOT-HEART trial (CT coronary angiography in patients with suspected angina due to coronary heart disease), which was published in the same year as the PROMISE trial, the patients who underwent initial assessment with CTA had a numerically lower composite end point of cardiac death and myocardial infarction at a median follow-up of 1.7 years (1.3% vs 2.0%, P = .053).6
Given this result, the SCOT-HEART investigators extended the follow-up to evaluate the composite end point of death from coronary heart disease or nonfatal myocardial infarction at 5 years.7 This trial enrolled patients who were initially referred to a cardiology clinic for evaluation of chest pain, and they were randomized to standard care plus CTA or standard care alone. At a median duration of 4.8 years, the primary outcome was lower in the CTA group (2.3%, 48 patients) than in the standard-care group (3.9%, 81 patients) (hazard ratio, 0.58; 95% CI, 0.41-0.84; P = .004). Both groups had similar rates of invasive coronary angiography and had similar coronary revascularization rates.
It is hypothesized that this lower rate of nonfatal myocardial infarction in patients with CTA plus standard care is associated with a higher rate of preventive therapies initiated in patients in the CTA-plus-standard-care group compared to standard care alone. However, the difference in the standard-care group should be noted when compared to the PROMISE trial. In the PROMISE trial, the comparator group had predominantly stress imaging (either nuclear stress test or echocardiography), while in the SCOT-HEART trial, the group had predominantly stress electrocardiogram (ECG), and only 10% of the patients underwent stress imaging. It is possible the difference seen in the rate of nonfatal myocardial infarction was due to suboptimal diagnosis of CAD with stress ECG, which has lower sensitivity compared to stress imaging.
The DISCHARGE trial investigated the effectiveness of CTA vs ICA as the initial diagnostic test in the management of patients with stable chest pain and an intermediate pretest probability of obstructive CAD.8 At 3.5 years of follow-up, the primary composite of cardiovascular death, myocardial infarction, or stroke was similar in both groups (2.1% vs 3.0; hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). Importantly, as fewer patients underwent ICA, the risk of procedure-related complication was lower in the CTA group than in the ICA group. However, it is important to note that only 25% of the patients diagnosed with obstructive CAD had greater than 50% vessel stenosis, which raises the question of whether an initial invasive strategy is appropriate for this population.
The strengths of these 2 studies include the large number of patients enrolled along with adequate follow-up, 5 years in the SCOT-HEART trial and 3.5 years in the DISCHARGE trial. The 2 studies overall suggest the usefulness of CTA for assessment of CAD. However, the control groups were very different in these 2 trials. In the SCOT-HEART study, the comparator group was primarily assessed by stress ECG, while in the DISCHARGE study, the comparator group was primary assessed by ICA. In the PROMISE trial, the composite end point of death, myocardial infarction, hospitalization for unstable angina, or major procedural complication was similar when the strategy of initial CTA was compared to functional testing with imaging (exercise ECG, nuclear stress testing, or echocardiography).5 Thus, clinical assessment is still needed when clinicians are selecting the appropriate diagnostic test for patients with suspected CAD. The most recent guidelines give similar recommendations for CTA compared to stress imaging.9 Whether further improvement in CTA acquisition or the addition of CT fractional flow reserve can further improve outcomes requires additional study.
Applications for Clinical Practice and System Implementation
In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful in diagnosis compared to stress ECG and in reducing utilization of low-yield ICA. Whether CTA is more useful compared to the other noninvasive stress imaging modalities in this population requires further study.
Practice Points
- In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful compared to stress ECG.
- Use of CTA can potentially reduce the use of low-yield coronary angiography.
–Thai Nguyen, MD, Albert Chan, MD, Taishi Hirai, MD
University of Missouri, Columbia, MO
1. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477. doi:10.1093/eurheartj/ehz425
2. Nakano S, Kohsaka S, Chikamori T et al. JCS 2022 guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J. 2022;86(5):882-915. doi:10.1253/circj.CJ-21-1041.
3. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164. doi:10.1016/j.jacc.2012.07.013
4. Arbab-Zadeh A, Di Carli MF, Cerci R, et al. Accuracy of computed tomographic angiography and single-photon emission computed tomography-acquired myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ Cardiovasc Imaging. 2015;8(10):e003533. doi:10.1161/CIRCIMAGING
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-300. doi:10.1056/NEJMoa1415516
6. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383-2391. doi:10.1016/S0140-6736(15)60291-4
7. SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924-933. doi:10.1056/NEJMoa1805971
8. DISCHARGE Trial Group, Maurovich-Horvat P, Bosserdt M, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med. 2022;386(17):1591-1602. doi:10.1056/NEJMoa2200963
9. Writing Committee Members, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
Study 1 Overview (SCOT-HEART Investigators)
Objective: To assess cardiovascular mortality and nonfatal myocardial infarction at 5 years in patients with stable chest pain referred to cardiology clinic for management with either standard care plus computed tomography angiography (CTA) or standard care alone.
Design: Multicenter, randomized, open-label prospective study.
Setting and participants: A total of 4146 patients with stable chest pain were randomized to standard care or standard care plus CTA at 12 centers across Scotland and were followed for 5 years.
Main outcome measures: The primary end point was a composite of death from coronary heart disease or nonfatal myocardial infarction. Main secondary end points were nonfatal myocardial infarction, nonfatal stroke, and frequency of invasive coronary angiography (ICA) and coronary revascularization with percutaneous coronary intervention or coronary artery bypass grafting.
Main results: The primary outcome including the composite of cardiovascular death or nonfatal myocardial infarction was lower in the CTA group than in the standard-care group at 2.3% (48 of 2073 patients) vs 3.9% (81 of 2073 patients), respectively (hazard ratio, 0.59; 95% CI, 0.41-0.84; P = .004). Although there was a higher rate of ICA and coronary revascularization in the CTA group than in the standard-care group in the first few months of follow-up, the overall rates were similar at 5 years, with ICA performed in 491 patients and 502 patients in the CTA vs standard-care groups, respectively (hazard ratio, 1.00; 95% CI, 0.88-1.13). Similarly, coronary revascularization was performed in 279 patients in the CTA group and in 267 patients in the standard-care group (hazard ratio, 1.07; 95% CI, 0.91-1.27). There were, however, more preventive therapies initiated in patients in the CTA group than in the standard-care group (odds ratio, 1.40; 95% CI, 1.19-1.65).
Conclusion: In patients with stable chest pain, the use of CTA in addition to standard care resulted in a significantly lower rate of death from coronary heart disease or nonfatal myocardial infarction at 5 years; the main contributor to this outcome was a reduced nonfatal myocardial infarction rate. There was no difference in the rate of coronary angiography or coronary revascularization between the 2 groups at 5 years.
Study 2 Overview (DISCHARGE Trial Group)
Objective: To compare the effectiveness of computed tomography (CT) with ICA as a diagnostic tool in patients with stable chest pain and intermediate pretest probability of coronary artery disease (CAD).
Design: Multicenter, randomized, assessor-blinded pragmatic prospective study.
Setting and participants: A total of 3667 patients with stable chest pain and intermediate pretest probability of CAD were enrolled at 26 centers and randomized into CT or ICA groups. Only 3561 patients were included in the modified intention-to-treat analysis, with 1808 patients and 1753 patients in the CT and ICA groups, respectively.
Main outcome measures: The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke over 3.5 years. The main secondary outcomes were major procedure-related complications and patient-reported angina pectoris during the last 4 weeks of follow up.
Main results: The primary outcome occurred in 38 of 1808 patients (2.1%) in the CT group and in 52 of 1753 patients (3.0%) in the ICA group (hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). The secondary outcomes showed that major procedure-related complications occurred in 9 patients (0.5%) in the CT group and in 33 patients (1.9%) in the ICA group (hazard ratio, 0.26; 95% CI, 0.13-0.55). Rates of patient-reported angina in the final 4 weeks of follow-up were 8.8% in the CT group and 7.5% in the ICA group (odds ratio, 1.17; 95% CI, 0.92-1.48).
Conclusion: Risk of major adverse cardiovascular events from the primary outcome were similar in both the CT and ICA groups among patients with stable chest pain and intermediate pretest probability of CAD. Patients referred for CT had a lower rate of coronary angiography leading to fewer major procedure-related complications in these patients than in those referred for ICA.
Commentary
Evaluation and treatment of obstructive atherosclerosis is an important part of clinical care in patients presenting with angina symptoms.1 Thus, the initial investigation for patients with suspected obstructive CAD includes ruling out acute coronary syndrome and assessing quality of life.1 The diagnostic test should be tailored to the pretest probability for the diagnosis of obstructive CAD.2
In the United States, stress testing traditionally has been used for the initial assessment in patients with suspected CAD,3 but recently CTA has been utilized more frequently for this purpose. Compared to a stress test, which often helps identify and assess ischemia, CTA can provide anatomical assessment, with higher sensitivity to identify CAD.4 Furthermore, it can distinguish nonobstructive plaques that can be challenging to identify with stress test alone.
Whether CTA is superior to stress testing as the initial assessment for CAD has been debated. The randomized PROMISE trial compared patients with stable angina who underwent functional stress testing or CTA as an initial strategy.5 They reported a similar outcome between the 2 groups at a median follow-up of 2 years. However, in the original SCOT-HEART trial (CT coronary angiography in patients with suspected angina due to coronary heart disease), which was published in the same year as the PROMISE trial, the patients who underwent initial assessment with CTA had a numerically lower composite end point of cardiac death and myocardial infarction at a median follow-up of 1.7 years (1.3% vs 2.0%, P = .053).6
Given this result, the SCOT-HEART investigators extended the follow-up to evaluate the composite end point of death from coronary heart disease or nonfatal myocardial infarction at 5 years.7 This trial enrolled patients who were initially referred to a cardiology clinic for evaluation of chest pain, and they were randomized to standard care plus CTA or standard care alone. At a median duration of 4.8 years, the primary outcome was lower in the CTA group (2.3%, 48 patients) than in the standard-care group (3.9%, 81 patients) (hazard ratio, 0.58; 95% CI, 0.41-0.84; P = .004). Both groups had similar rates of invasive coronary angiography and had similar coronary revascularization rates.
It is hypothesized that this lower rate of nonfatal myocardial infarction in patients with CTA plus standard care is associated with a higher rate of preventive therapies initiated in patients in the CTA-plus-standard-care group compared to standard care alone. However, the difference in the standard-care group should be noted when compared to the PROMISE trial. In the PROMISE trial, the comparator group had predominantly stress imaging (either nuclear stress test or echocardiography), while in the SCOT-HEART trial, the group had predominantly stress electrocardiogram (ECG), and only 10% of the patients underwent stress imaging. It is possible the difference seen in the rate of nonfatal myocardial infarction was due to suboptimal diagnosis of CAD with stress ECG, which has lower sensitivity compared to stress imaging.
The DISCHARGE trial investigated the effectiveness of CTA vs ICA as the initial diagnostic test in the management of patients with stable chest pain and an intermediate pretest probability of obstructive CAD.8 At 3.5 years of follow-up, the primary composite of cardiovascular death, myocardial infarction, or stroke was similar in both groups (2.1% vs 3.0; hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). Importantly, as fewer patients underwent ICA, the risk of procedure-related complication was lower in the CTA group than in the ICA group. However, it is important to note that only 25% of the patients diagnosed with obstructive CAD had greater than 50% vessel stenosis, which raises the question of whether an initial invasive strategy is appropriate for this population.
The strengths of these 2 studies include the large number of patients enrolled along with adequate follow-up, 5 years in the SCOT-HEART trial and 3.5 years in the DISCHARGE trial. The 2 studies overall suggest the usefulness of CTA for assessment of CAD. However, the control groups were very different in these 2 trials. In the SCOT-HEART study, the comparator group was primarily assessed by stress ECG, while in the DISCHARGE study, the comparator group was primary assessed by ICA. In the PROMISE trial, the composite end point of death, myocardial infarction, hospitalization for unstable angina, or major procedural complication was similar when the strategy of initial CTA was compared to functional testing with imaging (exercise ECG, nuclear stress testing, or echocardiography).5 Thus, clinical assessment is still needed when clinicians are selecting the appropriate diagnostic test for patients with suspected CAD. The most recent guidelines give similar recommendations for CTA compared to stress imaging.9 Whether further improvement in CTA acquisition or the addition of CT fractional flow reserve can further improve outcomes requires additional study.
Applications for Clinical Practice and System Implementation
In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful in diagnosis compared to stress ECG and in reducing utilization of low-yield ICA. Whether CTA is more useful compared to the other noninvasive stress imaging modalities in this population requires further study.
Practice Points
- In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful compared to stress ECG.
- Use of CTA can potentially reduce the use of low-yield coronary angiography.
–Thai Nguyen, MD, Albert Chan, MD, Taishi Hirai, MD
University of Missouri, Columbia, MO
Study 1 Overview (SCOT-HEART Investigators)
Objective: To assess cardiovascular mortality and nonfatal myocardial infarction at 5 years in patients with stable chest pain referred to cardiology clinic for management with either standard care plus computed tomography angiography (CTA) or standard care alone.
Design: Multicenter, randomized, open-label prospective study.
Setting and participants: A total of 4146 patients with stable chest pain were randomized to standard care or standard care plus CTA at 12 centers across Scotland and were followed for 5 years.
Main outcome measures: The primary end point was a composite of death from coronary heart disease or nonfatal myocardial infarction. Main secondary end points were nonfatal myocardial infarction, nonfatal stroke, and frequency of invasive coronary angiography (ICA) and coronary revascularization with percutaneous coronary intervention or coronary artery bypass grafting.
Main results: The primary outcome including the composite of cardiovascular death or nonfatal myocardial infarction was lower in the CTA group than in the standard-care group at 2.3% (48 of 2073 patients) vs 3.9% (81 of 2073 patients), respectively (hazard ratio, 0.59; 95% CI, 0.41-0.84; P = .004). Although there was a higher rate of ICA and coronary revascularization in the CTA group than in the standard-care group in the first few months of follow-up, the overall rates were similar at 5 years, with ICA performed in 491 patients and 502 patients in the CTA vs standard-care groups, respectively (hazard ratio, 1.00; 95% CI, 0.88-1.13). Similarly, coronary revascularization was performed in 279 patients in the CTA group and in 267 patients in the standard-care group (hazard ratio, 1.07; 95% CI, 0.91-1.27). There were, however, more preventive therapies initiated in patients in the CTA group than in the standard-care group (odds ratio, 1.40; 95% CI, 1.19-1.65).
Conclusion: In patients with stable chest pain, the use of CTA in addition to standard care resulted in a significantly lower rate of death from coronary heart disease or nonfatal myocardial infarction at 5 years; the main contributor to this outcome was a reduced nonfatal myocardial infarction rate. There was no difference in the rate of coronary angiography or coronary revascularization between the 2 groups at 5 years.
Study 2 Overview (DISCHARGE Trial Group)
Objective: To compare the effectiveness of computed tomography (CT) with ICA as a diagnostic tool in patients with stable chest pain and intermediate pretest probability of coronary artery disease (CAD).
Design: Multicenter, randomized, assessor-blinded pragmatic prospective study.
Setting and participants: A total of 3667 patients with stable chest pain and intermediate pretest probability of CAD were enrolled at 26 centers and randomized into CT or ICA groups. Only 3561 patients were included in the modified intention-to-treat analysis, with 1808 patients and 1753 patients in the CT and ICA groups, respectively.
Main outcome measures: The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke over 3.5 years. The main secondary outcomes were major procedure-related complications and patient-reported angina pectoris during the last 4 weeks of follow up.
Main results: The primary outcome occurred in 38 of 1808 patients (2.1%) in the CT group and in 52 of 1753 patients (3.0%) in the ICA group (hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). The secondary outcomes showed that major procedure-related complications occurred in 9 patients (0.5%) in the CT group and in 33 patients (1.9%) in the ICA group (hazard ratio, 0.26; 95% CI, 0.13-0.55). Rates of patient-reported angina in the final 4 weeks of follow-up were 8.8% in the CT group and 7.5% in the ICA group (odds ratio, 1.17; 95% CI, 0.92-1.48).
Conclusion: Risk of major adverse cardiovascular events from the primary outcome were similar in both the CT and ICA groups among patients with stable chest pain and intermediate pretest probability of CAD. Patients referred for CT had a lower rate of coronary angiography leading to fewer major procedure-related complications in these patients than in those referred for ICA.
Commentary
Evaluation and treatment of obstructive atherosclerosis is an important part of clinical care in patients presenting with angina symptoms.1 Thus, the initial investigation for patients with suspected obstructive CAD includes ruling out acute coronary syndrome and assessing quality of life.1 The diagnostic test should be tailored to the pretest probability for the diagnosis of obstructive CAD.2
In the United States, stress testing traditionally has been used for the initial assessment in patients with suspected CAD,3 but recently CTA has been utilized more frequently for this purpose. Compared to a stress test, which often helps identify and assess ischemia, CTA can provide anatomical assessment, with higher sensitivity to identify CAD.4 Furthermore, it can distinguish nonobstructive plaques that can be challenging to identify with stress test alone.
Whether CTA is superior to stress testing as the initial assessment for CAD has been debated. The randomized PROMISE trial compared patients with stable angina who underwent functional stress testing or CTA as an initial strategy.5 They reported a similar outcome between the 2 groups at a median follow-up of 2 years. However, in the original SCOT-HEART trial (CT coronary angiography in patients with suspected angina due to coronary heart disease), which was published in the same year as the PROMISE trial, the patients who underwent initial assessment with CTA had a numerically lower composite end point of cardiac death and myocardial infarction at a median follow-up of 1.7 years (1.3% vs 2.0%, P = .053).6
Given this result, the SCOT-HEART investigators extended the follow-up to evaluate the composite end point of death from coronary heart disease or nonfatal myocardial infarction at 5 years.7 This trial enrolled patients who were initially referred to a cardiology clinic for evaluation of chest pain, and they were randomized to standard care plus CTA or standard care alone. At a median duration of 4.8 years, the primary outcome was lower in the CTA group (2.3%, 48 patients) than in the standard-care group (3.9%, 81 patients) (hazard ratio, 0.58; 95% CI, 0.41-0.84; P = .004). Both groups had similar rates of invasive coronary angiography and had similar coronary revascularization rates.
It is hypothesized that this lower rate of nonfatal myocardial infarction in patients with CTA plus standard care is associated with a higher rate of preventive therapies initiated in patients in the CTA-plus-standard-care group compared to standard care alone. However, the difference in the standard-care group should be noted when compared to the PROMISE trial. In the PROMISE trial, the comparator group had predominantly stress imaging (either nuclear stress test or echocardiography), while in the SCOT-HEART trial, the group had predominantly stress electrocardiogram (ECG), and only 10% of the patients underwent stress imaging. It is possible the difference seen in the rate of nonfatal myocardial infarction was due to suboptimal diagnosis of CAD with stress ECG, which has lower sensitivity compared to stress imaging.
The DISCHARGE trial investigated the effectiveness of CTA vs ICA as the initial diagnostic test in the management of patients with stable chest pain and an intermediate pretest probability of obstructive CAD.8 At 3.5 years of follow-up, the primary composite of cardiovascular death, myocardial infarction, or stroke was similar in both groups (2.1% vs 3.0; hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). Importantly, as fewer patients underwent ICA, the risk of procedure-related complication was lower in the CTA group than in the ICA group. However, it is important to note that only 25% of the patients diagnosed with obstructive CAD had greater than 50% vessel stenosis, which raises the question of whether an initial invasive strategy is appropriate for this population.
The strengths of these 2 studies include the large number of patients enrolled along with adequate follow-up, 5 years in the SCOT-HEART trial and 3.5 years in the DISCHARGE trial. The 2 studies overall suggest the usefulness of CTA for assessment of CAD. However, the control groups were very different in these 2 trials. In the SCOT-HEART study, the comparator group was primarily assessed by stress ECG, while in the DISCHARGE study, the comparator group was primary assessed by ICA. In the PROMISE trial, the composite end point of death, myocardial infarction, hospitalization for unstable angina, or major procedural complication was similar when the strategy of initial CTA was compared to functional testing with imaging (exercise ECG, nuclear stress testing, or echocardiography).5 Thus, clinical assessment is still needed when clinicians are selecting the appropriate diagnostic test for patients with suspected CAD. The most recent guidelines give similar recommendations for CTA compared to stress imaging.9 Whether further improvement in CTA acquisition or the addition of CT fractional flow reserve can further improve outcomes requires additional study.
Applications for Clinical Practice and System Implementation
In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful in diagnosis compared to stress ECG and in reducing utilization of low-yield ICA. Whether CTA is more useful compared to the other noninvasive stress imaging modalities in this population requires further study.
Practice Points
- In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful compared to stress ECG.
- Use of CTA can potentially reduce the use of low-yield coronary angiography.
–Thai Nguyen, MD, Albert Chan, MD, Taishi Hirai, MD
University of Missouri, Columbia, MO
1. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477. doi:10.1093/eurheartj/ehz425
2. Nakano S, Kohsaka S, Chikamori T et al. JCS 2022 guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J. 2022;86(5):882-915. doi:10.1253/circj.CJ-21-1041.
3. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164. doi:10.1016/j.jacc.2012.07.013
4. Arbab-Zadeh A, Di Carli MF, Cerci R, et al. Accuracy of computed tomographic angiography and single-photon emission computed tomography-acquired myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ Cardiovasc Imaging. 2015;8(10):e003533. doi:10.1161/CIRCIMAGING
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-300. doi:10.1056/NEJMoa1415516
6. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383-2391. doi:10.1016/S0140-6736(15)60291-4
7. SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924-933. doi:10.1056/NEJMoa1805971
8. DISCHARGE Trial Group, Maurovich-Horvat P, Bosserdt M, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med. 2022;386(17):1591-1602. doi:10.1056/NEJMoa2200963
9. Writing Committee Members, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
1. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477. doi:10.1093/eurheartj/ehz425
2. Nakano S, Kohsaka S, Chikamori T et al. JCS 2022 guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J. 2022;86(5):882-915. doi:10.1253/circj.CJ-21-1041.
3. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164. doi:10.1016/j.jacc.2012.07.013
4. Arbab-Zadeh A, Di Carli MF, Cerci R, et al. Accuracy of computed tomographic angiography and single-photon emission computed tomography-acquired myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ Cardiovasc Imaging. 2015;8(10):e003533. doi:10.1161/CIRCIMAGING
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-300. doi:10.1056/NEJMoa1415516
6. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383-2391. doi:10.1016/S0140-6736(15)60291-4
7. SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924-933. doi:10.1056/NEJMoa1805971
8. DISCHARGE Trial Group, Maurovich-Horvat P, Bosserdt M, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med. 2022;386(17):1591-1602. doi:10.1056/NEJMoa2200963
9. Writing Committee Members, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006