User login
Practical Application of Self-Determination Theory to Achieve a Reduction in Postoperative Hypothermia Rate: A Quality Improvement Project
From Children’s Health System of Texas, Division of Pediatric Anesthesiology, Dallas, TX (Drs. Sakhai, Bocanegra, Chandran, Kimatian, and Kiss), UT Southwestern Medical Center, Department of Anesthesiology and Pain Management, Dallas, TX (Drs. Bocanegra, Chandran, Kimatian, and Kiss), and UT Southwestern Medical Center, Department of Population and Data Sciences, Dallas, TX (Dr. Reisch).
Objective: Policy-driven changes in medical practice have long been the norm. Seldom are changes in clinical practice sought to be brought about by a person’s tendency toward growth or self‐actualization. Many hospitals have instituted hypothermia bundles to help reduce the incidence of unanticipated postoperative hypothermia. Although successful in the short-term, sustained changes are difficult to maintain. We implemented a quality-improvement project focused on addressing the affective components of self-determination theory (SDT) to create sustainable behavioral change while satisfying providers’ basic psychological needs for autonomy, competence, and relatedness.
Methods: A total of 3 Plan-Do-Study-Act (PDSA) cycles were enacted over the span of 14 months at a major tertiary care pediatric hospital to recruit and motivate anesthesia providers and perioperative team members to reduce the percentage of hypothermic postsurgical patients by 50%. As an optional initial incentive for participation, anesthesiologists would qualify for American Board of Anesthesiology Maintenance of Certification in Anesthesiology (MOCA) Part 4 Quality Improvement credits for monitoring their own temperature data and participating in project-related meetings. Providers were given autonomy to develop a personal plan for achieving the desired goals.
Results: The median rate of hypothermia was reduced from 6.9% to 1.6% in July 2019 and was reduced again in July 2020 to 1.3%, an 81% reduction overall. A low hypothermia rate was successfully maintained for at least 21 subsequent months after participants received their MOCA credits in July 2019.
Conclusions: Using an approach that focused on the elements of competency, autonomy, and relatedness central to the principles of SDT, we observed the development of a new culture of vigilance for prevention of hypothermia that successfully endured beyond the project end date.
Keywords: postoperative hypothermia; self-determination theory; motivation; quality improvement.
Perioperative hypothermia, generally accepted as a core temperature less than 36 °C in clinical practice, is a common complication in the pediatric surgical population and is associated with poor postoperative outcomes.1 Hypothermic patients may develop respiratory depression, hypoglycemia, and metabolic acidosis that may lead to decreased oxygen delivery and end organ tissue hypoxia.2-4 Other potential detrimental effects of failing to maintain normal body temperature are impaired clotting factor enzyme function and platelet dysfunction, increasing the risk for postoperative bleeding.5,6 In addition, there are financial implications when hypothermic patients require care and resources postoperatively because of delayed emergence or shivering.7
The American Society of Anesthesiologists recommends intraoperative temperature monitoring for procedures when clinically significant changes in body temperature are anticipated.8 Maintenance of normothermia in the pediatric population is especially challenging owing to a larger skin-surface area compared with body mass ratio and less subcutaneous fat content than in adults. Preventing postoperative hypothermia starts preoperatively with parental education and can be as simple as covering the child with a blanket and setting the preoperative room to an acceptably warm temperature.9,10 Intraoperatively, maintaining operating room (OR) temperatures at or above 21.1 °C and using active warming devices and radiant warmers when appropriate are important techniques to preserve the child’s body temperature.11,12
Despite the knowledge of these risks and vigilant avoidance of hypothermia, unplanned perioperative hypothermia can occur in up to 70% of surgical patients.1 Beyond the clinical benefits, as health care marches toward a value-based payment methodology, quality indicators such as avoiding hypothermia may be linked directly to payment.
Self-determination theory (SDT) was first developed in 1980 by Deci and Ryan.13 The central premise of the theory states that people develop their full potential if circumstances allow them to satisfy their basic psychological needs: autonomy, competence, and relatedness. Under these conditions, people’s natural inclination toward growth can be realized, and they are more likely to internalize external goals. Under an extrinsic reward system, motivation can waver, as people may perceive rewards as controlling.
Many institutions have implemented hypothermia bundles to help decrease the rate of hypothermic patients, but while initially successful, the effectiveness of these interventions tends to fade over time as participants settle into old, comfortable routines.14 With SDT in mind, we designed our quality-improvement (QI) project with interventions to allow clinicians autonomy without instituting rigid guidelines or punitive actions. We aimed to directly address the affective components central to motivation and engagement so that we could bring about long-term meaningful changes in our practice.
Methods
Setting
The hypothermia QI intervention was instituted at a major tertiary care children’s hospital that performs more than 40 000 pediatric general anesthetics annually. Our division of pediatric anesthesiology consists of 66 fellowship-trained pediatric anesthesiologists, 15 or more rotating trainees per month, 13 anesthesiology assistants, 15 anesthesia technicians, and more than 50 perioperative nurses.
The most frequent pediatric surgeries include, but are not limited to, general surgery, otolaryngology, urology, gastroenterology, plastic surgery, neurosurgery, and dentistry. The surgeries are conducted in the hospital’s main operative floor, which consists of 15 ORs and 2 gastroenterology procedure rooms. Although the implementation of the QI project included several operating sites, we focused on collecting temperature data from surgical patients at our main campus recovery unit. We obtained the patients’ initial temperatures upon arrival to the recovery unit from a retrospective electronic health record review of all patients who underwent anesthesia from January 2016 through April 2021.
Postoperative hypothermia was identified as an area of potential improvement after several patients were reported to be hypothermic upon arrival to the recovery unit in the later part of 2018. Further review revealed significant heterogeneity of practices and lack of standardization of patient-warming methods. By comparing the temperatures pre- and postintervention, we could measure the effectiveness of the QI initiative. Prior to the start of our project, the hypothermia rate in our patient population was not actively tracked, and the effectiveness of our variable practice was not measured.
The cutoff for hypothermia for our QI project was defined as body temperature below 36 °C, since this value has been previously used in the literature and is commonly accepted in anesthesia practice as the delineation for hypothermia in patients undergoing general anesthesia.1
Interventions
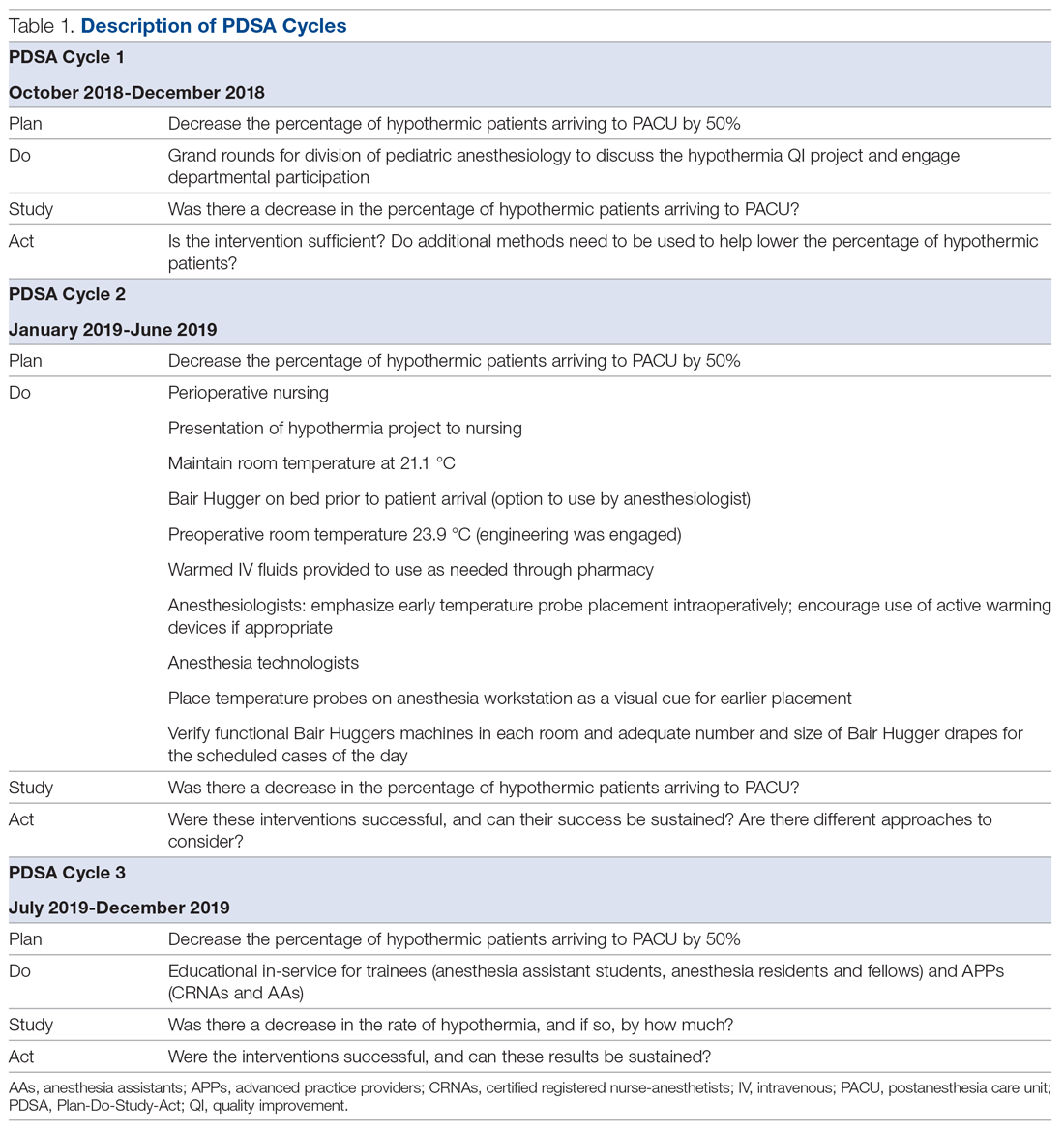
This QI project was designed and modeled after the Institute for Healthcare Improvement Model for Improvement.15 Three cycles of Plan-Do-Study-Act (PDSA) were developed and instituted over a 14-month period until December 2019 (Table 1).
A retrospective review was conducted to determine the percentage of surgical patients arriving to our recovery units with an initial temperature reading of less than 36 °C. A project key driver diagram and smart aim were created and approved by the hospital’s continuing medical education (CME) committee for credit via the American Board of Medical Specialties (ABMS) Multi-Specialty Portfolio Program, Maintenance of Certification in Anesthesiology (MOCA) Part 4.
The first PDSA cycle involved introducing the QI project and sharing the aims of the project at a department grand rounds in the latter part of October 2018. Enrollment to participate in the project was open to all anesthesiologists in the division, and participants could earn up to 20 hours of MOCA Part 4 credits. A spreadsheet was developed and maintained to track each anesthesiologist’s monthly percentage of hypothermic patients. The de-identified patient data were shared with the division via monthly emails. In addition, individual providers with a hypothermic patient in the recovery room received a notification email.
The anesthesiologists participated in the QI project by reviewing their personal percentage of hypothermic patients on an ongoing basis to earn the credit. There was no explicit requirement to decrease their own rate of patients with body temperature less than 36 °C or expectation to achieve a predetermined goal, so the participants could not “fail.”
Because of the large interest in this project, a hypothermia committee was formed that consisted of 36 anesthesiologists. This group reviewed the data and exchanged ideas for improvement in November 2018 as part of the first PDSA cycle. The committee met monthly and was responsible for actively engaging other members of the department and perioperative staff to help in this multidisciplinary effort of combating hypothermia in our surgical pediatric population.
PDSA cycle 2 involved several major initiatives, including direct incorporation of the rest of the perioperative team. The perioperative nursing team was educated on the risks of hypothermia and engaged to take an active role by maintaining the operating suite temperature at 21.1 °C and turning on the Bair Hugger (3M) blanket to 43 °C on the OR bed prior to patient arrival to the OR. Additionally, anesthesia technicians (ATs) were tasked with ensuring an adequate supply of Bair Hugger drapes for all cases of the day. The facility’s engineering team was engaged to move the preoperative room temperature controls away from families (who frequently made the rooms cold) and instead set it at a consistent temperature of 23.9 °C. ATs were also asked to place axillary and nasal temperature probes on the anesthesia workstations as a visual reminder to facilitate temperature monitoring closer to the start of anesthesia (instead of the anesthesia provider having to remember to retrieve a temperature probe out of a drawer and place it on the patient). Furthermore, anesthesiologists were instructed via the aforementioned monthly emails and at monthly department meetings to place the temperature probes as early as possible in order to recognize and respond to intraoperative hypothermia in a timelier manner. Finally, supply chain leaders were informed of our expected increase in the use of the blankets and probes and proportionally increased ordering of these supplies to make sure availability would not present an obstacle.
In PDSA cycle 3, trainees (anesthesia assistant students, anesthesia residents and fellows) and advanced practice providers (APPs) (certified registered nurse-anesthetists [CRNAs] and certified anesthesia assistants [C-AAs]) were informed of the QI project. This initiative was guided toward improving vigilance for hypothermia in the rest of the anesthesia team members. The trainees and APPs usually set up the anesthesia area prior to patient arrival, so their recruitment in support of this effort would ensure appropriate OR temperature, active warming device deployment, and the availability and early placement of the correct temperature probe for the case. To facilitate personal accountability, the trainees and APPs were also emailed their own patients’ rate of hypothermia.
Along the course of the project, quarterly committee meetings and departmental monthly meetings served as venues to express concerns and look for areas of improvement, such as specific patterns or trends leading to hypothermic patients. One specific example was the identification of the gastrointestinal endoscopic patients having a rate of hypothermia that was 2% higher than average. Directed education on the importance of Bair Hugger blankets and using warm intravenous fluids worked well to decrease the rate of hypothermia in these patients. This collection of data was shared at regular intervals during monthly department meetings as well and more frequently using departmental emails. The hospital’s secure intranet SharePoint (Microsoft) site was used to share the data among providers.
Study of the interventions and measures
To study the effectiveness and impact of the project to motivate our anesthesiologists and other team members, we compared the first temperatures obtained in the recovery unit prior to the start of the intervention with those collected after the start of the QI project in November 2018. Because of the variability of temperature monitoring intraoperatively (nasal, axillary, rectal), we decided to use the temperature obtained by the nurse in the recovery room upon the patient’s arrival. Over the years analyzed, the nurse’s technique of measuring the temperature remained consistent. All patient temperature measurements were performed using the TAT-5000 (Exergen Corporation). This temporal artery thermometer has been previously shown to correlate well with bladder temperatures (70% of measurements differ by no more than 0.5 °C, as reported by Langham et al16).
Admittedly, we could not measure the degree of motivation or internalization of the project goals by our cohort, but we could measure the reduction in the rate of hypothermia and subjectively gauge engagement in the project by the various groups of participants and the sustainability of the results. In addition, all participating anesthesiologists received MOCA Part 4 credits in July 2019. We continued our data collection until April 2021 to determine if our project had brought about sustainable changes in practice that would continue past the initial motivator of obtaining CME credit.
Analysis
Data analysis was performed using Excel (Microsoft) and SAS, version 9.4 (SAS Institute).
The median of the monthly percentage of patients with a temperature of less than 36.0 °C was also determined for the preintervention time frame. This served as our baseline hypothermia rate, and we aimed to lower it by 50%. Run charts, a well-described methodology to gauge the effectiveness of the QI project, were constructed with the collected data.17
We performed additional analysis to adjust for different time periods throughout the year. The time period between January 2016 and October 2018 was considered preintervention. We considered November 2018 the start of our intervention, or more specifically, the start of our PDSA cycles. October 2018 was analyzed as part of the preintervention data. To account for seasonal temperature variations, the statistical analysis focused on the comparisons of the same calendar quarters for before and after starting intervention using Wilcoxon Mann-Whitney U tests. To reach an overall conclusion, the probabilities for the 4 quarters were combined for each criterion separately utilizing the Fisher χ2 combined probability method.
The hypothermia QI project was reviewed by the institutional review board and determined to be exempt.
Results
The temperatures of 40 875 patients were available for analysis for the preintervention period between January 2016 and October 2018. The median percentage of patients with temperatures less than 36.0 °C was 6.9% (interquartile range [IQR], 5.8%-8.4%). The highest percentage was in February 2016 (9.9%), and the lowest was in March 2018 (3.4%). Following the start of the first PDSA cycle, the next 6 consecutive rates of hypothermia were below the median preintervention value, and a new median for these percentages was calculated at 3.4% (IQR, 2.6%-4.3%). In July 2019, the proportion of hypothermic patients decreased once more for 6 consecutive months, yielding a new median of 1.6% (IQR, 1.2%-1.8%) and again in July 2020, to yield a median of 1.3% (IQR, 1.2%-1.5%) (Figure). In all, 33 799 patients were analyzed after the start of the project from November 2018 to the end of the data collection period through April 2021.
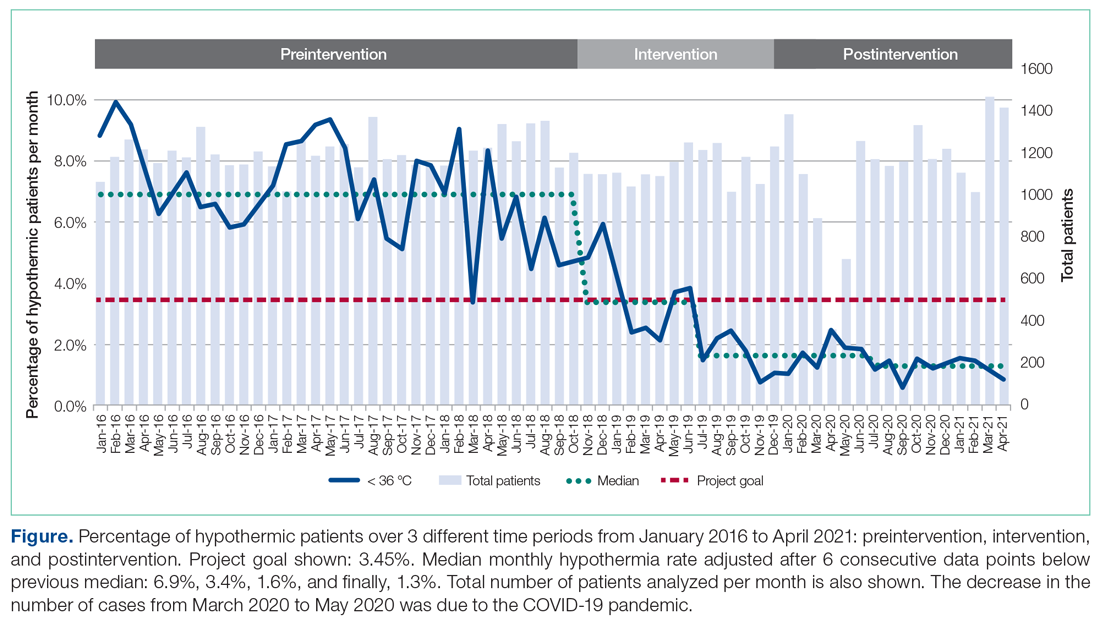
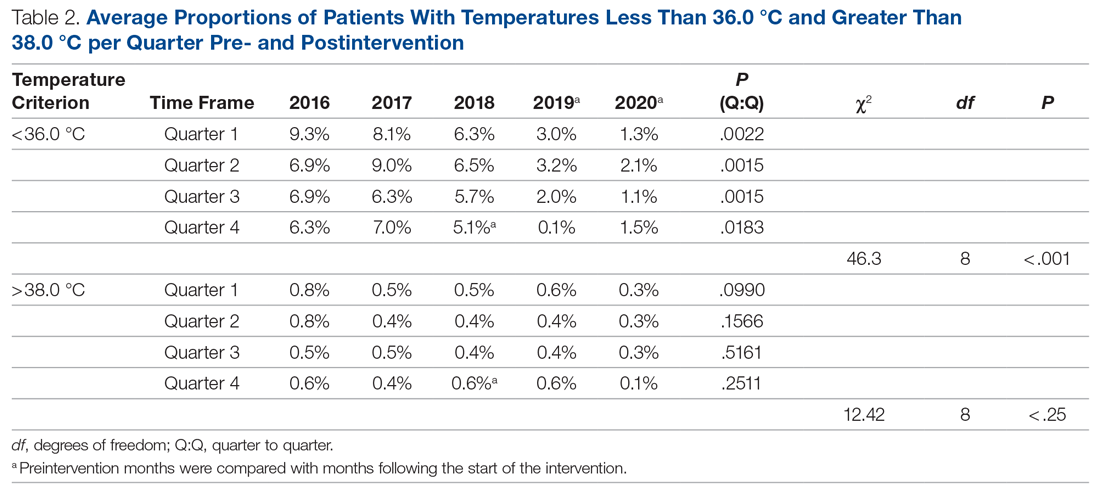
The preintervention monthly rates of hypothermia were compared, quarter to quarter, with those starting in November 2018 using the Wilcoxon Mann-Whitney U test. The decrease in proportion of hypothermic patients after the start of the intervention was statistically significant (P < .001). In addition, the percentage of patients with temperatures greater than 38 °C was not significantly different between the pre- and postintervention time periods (P < .25) (Table 2). The decrease in the number of patients available for analysis from March 2020 to May 2020 was due to the COVID-19 pandemic.
Subjectively, we did not experience any notable resistance to our efforts, and the experience was largely positive for everyone involved. Clinicians identified as having high monthly rates of hypothermia (5% or higher) corrected their numbers the following month after being notified via email or in person.
Discussion
To achieve changes in practice, the health care industry has relied on instituting guidelines, regulations, and policies, often with punitive consequences. We call into question this long-standing framework and propose a novel approach to help evolve the field of QI. Studies in human psychology have long demonstrated the demotivation power of a reward system and the negative response to attempts by authority to use incentives to control or coerce. In our QI project, we instituted 3 PDSA cycles and applied elements from SDT to motivate people’s behaviors. We demonstrate how a new culture focused on maintaining intraoperative normothermia was developed and brought about a measurable and significant decrease in the rate of hypothermia. The relevance of SDT, a widely accepted unifying theory that bridges and links social and personality psychology, should not be understated in health care. Authorities wishing to have long-standing influence should consider a person’s right to make their own decisions and, if possible, a unique way of doing things.
Positively reinforcing behavior has been shown to have a paradoxical effect by dampening an individual’s intrinsic motivation or desire to perform certain tasks.18 Deadlines, surveillance, and authoritative commands are also deterrents.19,20 We focused on providing the tools and information to the clinicians and relied on their innate need for autonomy, growth, and self-actualization to bring about change in clinical practice.21 Group meetings served as a construct for exchanging ideas and to encourage participation, but without the implementation of rigid guidelines or policies. Intraoperative active warming devices and temperature probes were made available, but their use was not mandated. The use of these devices was intentionally not audited to avoid any overbearing control. Providers were, however, given monthly temperature data to help individually assess the effectiveness of their interventions. We did not impose any negative or punitive actions for those clinicians who had high rates of hypothermic patients, and we did not reward those who had low rates of hypothermia. We wanted the participants to feel that the inner self was the source of their behavior, and this was in parallel with their own interests and values. If providers could feel their need for competency could be realized, we hoped they would continue to adhere to the measures we provided to maintain a low rate of hypothermia.
The effectiveness of our efforts was demonstrated by a decrease in the prevalence of postoperative hypothermia in our surgical patients. The initial decrease of the median rate of hypothermia from 6.9% to 3.4% occurred shortly into the start of the first PDSA cycle. The second PDSA cycle started in January 2019 with a multimodal approach and included almost all parties involved in the perioperative care of our surgical patients. Not only was this intervention responsible for a continued downward trend in the percentage of hypothermic patients, but it set the stage for the third and final PDSA cycle, which started in July 2019. The architecture was in place to integrate trainees and APPs to reinforce our initiative. Subsequently, the new median percentage of hypothermic patients was further decreased to an all-time low of 1.6% per month, satisfying and surpassing the goal of the QI project of decreasing the rate of hypothermia by only 50%. Our organization thereafter maintained a monthly hypothermia rate below 2%, except for April 2020, when it reached 2.5%. Our lowest median percentage was obtained after July 2020, reaching 1.3%.
To account for seasonal variations in temperatures and types of surgeries performed, we compared the percentage of hypothermic patients before and after the start of intervention, quarter by quarter. The decrease in the proportion of hypothermic patients after the start of intervention was statistically significant (P < .001). In addition, the data failed to prove any statistical difference for temperatures above 38 °C between the 2 periods, indicating that our interventions did not result in significant overwarming of patients. The clinical implications of decreasing the percentage of hypothermic patients from 6.9% to 1.3% is likely clinically important when considering the large number of patients who undergo surgery at large tertiary care pediatric centers. Even if simple interventions reduce hypothermia in only a handful of patients, routine applications of simple measures to keep patients normothermic is likely best clinical practice.
Anesthesiologists who participated in the hypothermia QI project by tracking the incidence of hypothermia in their patients were able to collect MOCA Part 4 credits in July 2019. There was no requirement for the individual anesthesiologist to reduce the rate of hypothermia or apply any of the encouraged strategies to obtain credit. As previously stated, there were also no rewards for obtaining low hypothermia rates for the providers. The temperature data continued to be collected through April 2021, 21 months after the credits were distributed, to demonstrate a continued, meaningful change, at least in the short-term. While the MOCA Part 4 credits likely served as an initial motivating factor to encourage participation in the QI project, they certainly were not responsible for the sustained low hypothermia rate after July 2019. We showed that the low rate of hypothermia was successfully maintained, indicating that the change in providers’ behavior was independent of the external motivator of obtaining the credit hours. Mere participation in the project by reviewing one’s temperature data was all that was required to obtain the credit. The Organismic Integration Theory, a mini-theory within SDT, best explains this phenomenon by describing any motivated behavior on a continuum ranging from controlled to autonomous.22 Do people perform the task resentfully, on their own volition because they believe it is the correct action, or somewhere in between? We explain the sustained low rates of hypothermia after the MOCA credits were distributed due to a shift to the autonomous end of the continuum with the clinician’s active willingness to meet the challenges and apply intrinsically motivated behaviors to lower the rate of hypothermia. The internalization of external motivators is difficult to prove, but the evidence supports that the methods we used to motivate individuals were effective and have resulted in a significant downward trend in our hypothermia rate.
There are several limitations to our QI project. The first involves the measuring of postoperative temperature in the recovery units. The temperatures were obtained using the same medical-grade infrared thermometer for all the patients, but other variables, such as timing and techniques, were not standardized. Secondly, overall surgical outcomes related to hypothermia were not tracked because we were unable to control for other confounding variables in our large cohort of patients, so we cannot say if the drop in the hypothermia rate had a clinically significant outcome. Thirdly, we propose that SDT offers a compellingly fitting explanation of the psychology of motivation in our efforts, but it may be possible that other theories may offer equally fitting explanations. The ability to measure the degree of motivation is lacking, and we did not explicitly ask participants what their specific source of motivation was. Aside from SDT, the reduction in hypothermia rate could also be attributed to the ease and availability of warming equipment that was made in each OR. This QI project was successfully applied to only 1 institution, so its ability to be widely applicable remains uncertain. In addition, data collection continued during the COVID-19 pandemic when case volumes decreased. However, by June 2020, the number of surgical cases at our institution had largely returned to prepandemic levels. Additional data collection beyond April 2021 would be helpful to determine if the reduction in hypothermia rates is truly sustained.
Conclusion
Overall, the importance of maintaining perioperative normothermia was well disseminated and agreed upon by all departments involved. Despite the limitations of the project, there was a significant reduction in rates of hypothermia, and sustainability of outcomes was consistently demonstrated in the poststudy period.
Using 3 cycles of the PDSA method, we successfully decreased the median rate of postoperative hypothermia in our pediatric surgical population from a preintervention value of 6.9% to 1.3%—a reduction of more than 81.2%. We provided motivation for members of our anesthesiology staff to participate by offering MOCA 2.0 Part 4 credits, but the lower rate of hypothermic patients was maintained for 15 months after the credits were distributed. Over the course of the project, there was a shift in culture, and extra vigilance was given to temperature monitoring and assessment. We attribute this sustained cultural change to the deliberate incorporation of the principles of competency, autonomy, and relatedness central to SDT to the structure of the interventions, avoiding rigid guidelines and pathways in favor of affective engagement to establish intrinsic motivation.
Acknowledgements: The authors thank Joan Reisch, PhD, for her assistance with the statistical analysis.
Corresponding author: Edgar Erold Kiss, MD, 1935 Medical District Dr, Dallas, TX 75235; [email protected].
Financial disclosures: None.
1. Leslie K, Sessler DI. Perioperative hypothermia in the high-risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17(4):485-498.
2. Sessler DI. Forced-air warming in infants and children. Paediatr Anaesth. 2013;23(6):467-468.
3. Wetzel RC. Evaluation of children. In: Longnecker DE, Tinker JH, Morgan Jr GE, eds. Principles and Practice of Anesthesiology. 2nd ed. Mosby Publishers; 1999:445-447.
4. Witt L, Dennhardt N, Eich C, et al. Prevention of intraoperative hypothermia in neonates and infants: results of a prospective multicenter observational study with a new forced-air warming system with increased warm air flow. Paediatr Anaesth. 2013;23(6):469-474.
5. Blum R, Cote C. Pediatric equipment. In: Blum R, Cote C, eds. A Practice of Anaesthesia for Infants and Children. Saunders Elsevier; 2009:1099-1101.
6. Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2003;17(4):535-549.
7. Mahoney CB, Odom J. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA J. 1999;67(2):155-163.
8. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring. Approved by the ASA House of Delegates October 21, 1986; last amended October 20, 2010; last affirmed October 28, 2015.
9. Horn E-P, Bein B, Böhm R, et al. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia. 2012;67(6):612-617.
10. Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth. 2008;101(5):627-631.
11. Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95(2):531-543.
12. Bräuer A, English MJM, Steinmetz N, et al. Efficacy of forced-air warming systems with full body blankets. Can J Anaesth. 2007;54(1):34-41.
13. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self‐determination of behavior. Psychol Inquiry. 2000;11(4):227-268.
14. Al-Shamari M, Puttha R, Yuen S, et al. G9 Can introduction of a hypothermia bundle reduce hypothermia in the newborns? Arch Dis Childhood. 2019;104(suppl 2):A4.1-A4.
15. Institute for Healthcare Improvement. How to improve. Accessed May 12, 2021. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
16. Langham GE, Meheshwari A, You J, et al. Noninvasive temperature monitoring in postanesthesia care units. Anesthesiology. 2009;111(1):90-96.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46-51.
18. Deci EL. Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol. 1971;18(1):105-115.
19. Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627-668.
20. Deci EL, Koestner R, Ryan RM. The undermining effect is a reality after all—extrinsic rewards, task interest, and self-determination: Reply to Eisenberger, Pierce, and Cameron (1999) and Lepper, Henderlong, and Gingras (1999). Psychol Bull. 1999;125(6):692-700.
21. Maslow A. The Farther Reaches of Human Nature. Viking Press; 1971.
22. Sheldon KM, Prentice M. Self-determination theory as a foundation for personality researchers. J Pers. 2019;87(1):5-14.
From Children’s Health System of Texas, Division of Pediatric Anesthesiology, Dallas, TX (Drs. Sakhai, Bocanegra, Chandran, Kimatian, and Kiss), UT Southwestern Medical Center, Department of Anesthesiology and Pain Management, Dallas, TX (Drs. Bocanegra, Chandran, Kimatian, and Kiss), and UT Southwestern Medical Center, Department of Population and Data Sciences, Dallas, TX (Dr. Reisch).
Objective: Policy-driven changes in medical practice have long been the norm. Seldom are changes in clinical practice sought to be brought about by a person’s tendency toward growth or self‐actualization. Many hospitals have instituted hypothermia bundles to help reduce the incidence of unanticipated postoperative hypothermia. Although successful in the short-term, sustained changes are difficult to maintain. We implemented a quality-improvement project focused on addressing the affective components of self-determination theory (SDT) to create sustainable behavioral change while satisfying providers’ basic psychological needs for autonomy, competence, and relatedness.
Methods: A total of 3 Plan-Do-Study-Act (PDSA) cycles were enacted over the span of 14 months at a major tertiary care pediatric hospital to recruit and motivate anesthesia providers and perioperative team members to reduce the percentage of hypothermic postsurgical patients by 50%. As an optional initial incentive for participation, anesthesiologists would qualify for American Board of Anesthesiology Maintenance of Certification in Anesthesiology (MOCA) Part 4 Quality Improvement credits for monitoring their own temperature data and participating in project-related meetings. Providers were given autonomy to develop a personal plan for achieving the desired goals.
Results: The median rate of hypothermia was reduced from 6.9% to 1.6% in July 2019 and was reduced again in July 2020 to 1.3%, an 81% reduction overall. A low hypothermia rate was successfully maintained for at least 21 subsequent months after participants received their MOCA credits in July 2019.
Conclusions: Using an approach that focused on the elements of competency, autonomy, and relatedness central to the principles of SDT, we observed the development of a new culture of vigilance for prevention of hypothermia that successfully endured beyond the project end date.
Keywords: postoperative hypothermia; self-determination theory; motivation; quality improvement.
Perioperative hypothermia, generally accepted as a core temperature less than 36 °C in clinical practice, is a common complication in the pediatric surgical population and is associated with poor postoperative outcomes.1 Hypothermic patients may develop respiratory depression, hypoglycemia, and metabolic acidosis that may lead to decreased oxygen delivery and end organ tissue hypoxia.2-4 Other potential detrimental effects of failing to maintain normal body temperature are impaired clotting factor enzyme function and platelet dysfunction, increasing the risk for postoperative bleeding.5,6 In addition, there are financial implications when hypothermic patients require care and resources postoperatively because of delayed emergence or shivering.7
The American Society of Anesthesiologists recommends intraoperative temperature monitoring for procedures when clinically significant changes in body temperature are anticipated.8 Maintenance of normothermia in the pediatric population is especially challenging owing to a larger skin-surface area compared with body mass ratio and less subcutaneous fat content than in adults. Preventing postoperative hypothermia starts preoperatively with parental education and can be as simple as covering the child with a blanket and setting the preoperative room to an acceptably warm temperature.9,10 Intraoperatively, maintaining operating room (OR) temperatures at or above 21.1 °C and using active warming devices and radiant warmers when appropriate are important techniques to preserve the child’s body temperature.11,12
Despite the knowledge of these risks and vigilant avoidance of hypothermia, unplanned perioperative hypothermia can occur in up to 70% of surgical patients.1 Beyond the clinical benefits, as health care marches toward a value-based payment methodology, quality indicators such as avoiding hypothermia may be linked directly to payment.
Self-determination theory (SDT) was first developed in 1980 by Deci and Ryan.13 The central premise of the theory states that people develop their full potential if circumstances allow them to satisfy their basic psychological needs: autonomy, competence, and relatedness. Under these conditions, people’s natural inclination toward growth can be realized, and they are more likely to internalize external goals. Under an extrinsic reward system, motivation can waver, as people may perceive rewards as controlling.
Many institutions have implemented hypothermia bundles to help decrease the rate of hypothermic patients, but while initially successful, the effectiveness of these interventions tends to fade over time as participants settle into old, comfortable routines.14 With SDT in mind, we designed our quality-improvement (QI) project with interventions to allow clinicians autonomy without instituting rigid guidelines or punitive actions. We aimed to directly address the affective components central to motivation and engagement so that we could bring about long-term meaningful changes in our practice.
Methods
Setting
The hypothermia QI intervention was instituted at a major tertiary care children’s hospital that performs more than 40 000 pediatric general anesthetics annually. Our division of pediatric anesthesiology consists of 66 fellowship-trained pediatric anesthesiologists, 15 or more rotating trainees per month, 13 anesthesiology assistants, 15 anesthesia technicians, and more than 50 perioperative nurses.
The most frequent pediatric surgeries include, but are not limited to, general surgery, otolaryngology, urology, gastroenterology, plastic surgery, neurosurgery, and dentistry. The surgeries are conducted in the hospital’s main operative floor, which consists of 15 ORs and 2 gastroenterology procedure rooms. Although the implementation of the QI project included several operating sites, we focused on collecting temperature data from surgical patients at our main campus recovery unit. We obtained the patients’ initial temperatures upon arrival to the recovery unit from a retrospective electronic health record review of all patients who underwent anesthesia from January 2016 through April 2021.
Postoperative hypothermia was identified as an area of potential improvement after several patients were reported to be hypothermic upon arrival to the recovery unit in the later part of 2018. Further review revealed significant heterogeneity of practices and lack of standardization of patient-warming methods. By comparing the temperatures pre- and postintervention, we could measure the effectiveness of the QI initiative. Prior to the start of our project, the hypothermia rate in our patient population was not actively tracked, and the effectiveness of our variable practice was not measured.
The cutoff for hypothermia for our QI project was defined as body temperature below 36 °C, since this value has been previously used in the literature and is commonly accepted in anesthesia practice as the delineation for hypothermia in patients undergoing general anesthesia.1
Interventions
This QI project was designed and modeled after the Institute for Healthcare Improvement Model for Improvement.15 Three cycles of Plan-Do-Study-Act (PDSA) were developed and instituted over a 14-month period until December 2019 (Table 1).

A retrospective review was conducted to determine the percentage of surgical patients arriving to our recovery units with an initial temperature reading of less than 36 °C. A project key driver diagram and smart aim were created and approved by the hospital’s continuing medical education (CME) committee for credit via the American Board of Medical Specialties (ABMS) Multi-Specialty Portfolio Program, Maintenance of Certification in Anesthesiology (MOCA) Part 4.
The first PDSA cycle involved introducing the QI project and sharing the aims of the project at a department grand rounds in the latter part of October 2018. Enrollment to participate in the project was open to all anesthesiologists in the division, and participants could earn up to 20 hours of MOCA Part 4 credits. A spreadsheet was developed and maintained to track each anesthesiologist’s monthly percentage of hypothermic patients. The de-identified patient data were shared with the division via monthly emails. In addition, individual providers with a hypothermic patient in the recovery room received a notification email.
The anesthesiologists participated in the QI project by reviewing their personal percentage of hypothermic patients on an ongoing basis to earn the credit. There was no explicit requirement to decrease their own rate of patients with body temperature less than 36 °C or expectation to achieve a predetermined goal, so the participants could not “fail.”
Because of the large interest in this project, a hypothermia committee was formed that consisted of 36 anesthesiologists. This group reviewed the data and exchanged ideas for improvement in November 2018 as part of the first PDSA cycle. The committee met monthly and was responsible for actively engaging other members of the department and perioperative staff to help in this multidisciplinary effort of combating hypothermia in our surgical pediatric population.
PDSA cycle 2 involved several major initiatives, including direct incorporation of the rest of the perioperative team. The perioperative nursing team was educated on the risks of hypothermia and engaged to take an active role by maintaining the operating suite temperature at 21.1 °C and turning on the Bair Hugger (3M) blanket to 43 °C on the OR bed prior to patient arrival to the OR. Additionally, anesthesia technicians (ATs) were tasked with ensuring an adequate supply of Bair Hugger drapes for all cases of the day. The facility’s engineering team was engaged to move the preoperative room temperature controls away from families (who frequently made the rooms cold) and instead set it at a consistent temperature of 23.9 °C. ATs were also asked to place axillary and nasal temperature probes on the anesthesia workstations as a visual reminder to facilitate temperature monitoring closer to the start of anesthesia (instead of the anesthesia provider having to remember to retrieve a temperature probe out of a drawer and place it on the patient). Furthermore, anesthesiologists were instructed via the aforementioned monthly emails and at monthly department meetings to place the temperature probes as early as possible in order to recognize and respond to intraoperative hypothermia in a timelier manner. Finally, supply chain leaders were informed of our expected increase in the use of the blankets and probes and proportionally increased ordering of these supplies to make sure availability would not present an obstacle.
In PDSA cycle 3, trainees (anesthesia assistant students, anesthesia residents and fellows) and advanced practice providers (APPs) (certified registered nurse-anesthetists [CRNAs] and certified anesthesia assistants [C-AAs]) were informed of the QI project. This initiative was guided toward improving vigilance for hypothermia in the rest of the anesthesia team members. The trainees and APPs usually set up the anesthesia area prior to patient arrival, so their recruitment in support of this effort would ensure appropriate OR temperature, active warming device deployment, and the availability and early placement of the correct temperature probe for the case. To facilitate personal accountability, the trainees and APPs were also emailed their own patients’ rate of hypothermia.
Along the course of the project, quarterly committee meetings and departmental monthly meetings served as venues to express concerns and look for areas of improvement, such as specific patterns or trends leading to hypothermic patients. One specific example was the identification of the gastrointestinal endoscopic patients having a rate of hypothermia that was 2% higher than average. Directed education on the importance of Bair Hugger blankets and using warm intravenous fluids worked well to decrease the rate of hypothermia in these patients. This collection of data was shared at regular intervals during monthly department meetings as well and more frequently using departmental emails. The hospital’s secure intranet SharePoint (Microsoft) site was used to share the data among providers.
Study of the interventions and measures
To study the effectiveness and impact of the project to motivate our anesthesiologists and other team members, we compared the first temperatures obtained in the recovery unit prior to the start of the intervention with those collected after the start of the QI project in November 2018. Because of the variability of temperature monitoring intraoperatively (nasal, axillary, rectal), we decided to use the temperature obtained by the nurse in the recovery room upon the patient’s arrival. Over the years analyzed, the nurse’s technique of measuring the temperature remained consistent. All patient temperature measurements were performed using the TAT-5000 (Exergen Corporation). This temporal artery thermometer has been previously shown to correlate well with bladder temperatures (70% of measurements differ by no more than 0.5 °C, as reported by Langham et al16).
Admittedly, we could not measure the degree of motivation or internalization of the project goals by our cohort, but we could measure the reduction in the rate of hypothermia and subjectively gauge engagement in the project by the various groups of participants and the sustainability of the results. In addition, all participating anesthesiologists received MOCA Part 4 credits in July 2019. We continued our data collection until April 2021 to determine if our project had brought about sustainable changes in practice that would continue past the initial motivator of obtaining CME credit.
Analysis
Data analysis was performed using Excel (Microsoft) and SAS, version 9.4 (SAS Institute).
The median of the monthly percentage of patients with a temperature of less than 36.0 °C was also determined for the preintervention time frame. This served as our baseline hypothermia rate, and we aimed to lower it by 50%. Run charts, a well-described methodology to gauge the effectiveness of the QI project, were constructed with the collected data.17
We performed additional analysis to adjust for different time periods throughout the year. The time period between January 2016 and October 2018 was considered preintervention. We considered November 2018 the start of our intervention, or more specifically, the start of our PDSA cycles. October 2018 was analyzed as part of the preintervention data. To account for seasonal temperature variations, the statistical analysis focused on the comparisons of the same calendar quarters for before and after starting intervention using Wilcoxon Mann-Whitney U tests. To reach an overall conclusion, the probabilities for the 4 quarters were combined for each criterion separately utilizing the Fisher χ2 combined probability method.
The hypothermia QI project was reviewed by the institutional review board and determined to be exempt.
Results
The temperatures of 40 875 patients were available for analysis for the preintervention period between January 2016 and October 2018. The median percentage of patients with temperatures less than 36.0 °C was 6.9% (interquartile range [IQR], 5.8%-8.4%). The highest percentage was in February 2016 (9.9%), and the lowest was in March 2018 (3.4%). Following the start of the first PDSA cycle, the next 6 consecutive rates of hypothermia were below the median preintervention value, and a new median for these percentages was calculated at 3.4% (IQR, 2.6%-4.3%). In July 2019, the proportion of hypothermic patients decreased once more for 6 consecutive months, yielding a new median of 1.6% (IQR, 1.2%-1.8%) and again in July 2020, to yield a median of 1.3% (IQR, 1.2%-1.5%) (Figure). In all, 33 799 patients were analyzed after the start of the project from November 2018 to the end of the data collection period through April 2021.

The preintervention monthly rates of hypothermia were compared, quarter to quarter, with those starting in November 2018 using the Wilcoxon Mann-Whitney U test. The decrease in proportion of hypothermic patients after the start of the intervention was statistically significant (P < .001). In addition, the percentage of patients with temperatures greater than 38 °C was not significantly different between the pre- and postintervention time periods (P < .25) (Table 2). The decrease in the number of patients available for analysis from March 2020 to May 2020 was due to the COVID-19 pandemic.

Subjectively, we did not experience any notable resistance to our efforts, and the experience was largely positive for everyone involved. Clinicians identified as having high monthly rates of hypothermia (5% or higher) corrected their numbers the following month after being notified via email or in person.
Discussion
To achieve changes in practice, the health care industry has relied on instituting guidelines, regulations, and policies, often with punitive consequences. We call into question this long-standing framework and propose a novel approach to help evolve the field of QI. Studies in human psychology have long demonstrated the demotivation power of a reward system and the negative response to attempts by authority to use incentives to control or coerce. In our QI project, we instituted 3 PDSA cycles and applied elements from SDT to motivate people’s behaviors. We demonstrate how a new culture focused on maintaining intraoperative normothermia was developed and brought about a measurable and significant decrease in the rate of hypothermia. The relevance of SDT, a widely accepted unifying theory that bridges and links social and personality psychology, should not be understated in health care. Authorities wishing to have long-standing influence should consider a person’s right to make their own decisions and, if possible, a unique way of doing things.
Positively reinforcing behavior has been shown to have a paradoxical effect by dampening an individual’s intrinsic motivation or desire to perform certain tasks.18 Deadlines, surveillance, and authoritative commands are also deterrents.19,20 We focused on providing the tools and information to the clinicians and relied on their innate need for autonomy, growth, and self-actualization to bring about change in clinical practice.21 Group meetings served as a construct for exchanging ideas and to encourage participation, but without the implementation of rigid guidelines or policies. Intraoperative active warming devices and temperature probes were made available, but their use was not mandated. The use of these devices was intentionally not audited to avoid any overbearing control. Providers were, however, given monthly temperature data to help individually assess the effectiveness of their interventions. We did not impose any negative or punitive actions for those clinicians who had high rates of hypothermic patients, and we did not reward those who had low rates of hypothermia. We wanted the participants to feel that the inner self was the source of their behavior, and this was in parallel with their own interests and values. If providers could feel their need for competency could be realized, we hoped they would continue to adhere to the measures we provided to maintain a low rate of hypothermia.
The effectiveness of our efforts was demonstrated by a decrease in the prevalence of postoperative hypothermia in our surgical patients. The initial decrease of the median rate of hypothermia from 6.9% to 3.4% occurred shortly into the start of the first PDSA cycle. The second PDSA cycle started in January 2019 with a multimodal approach and included almost all parties involved in the perioperative care of our surgical patients. Not only was this intervention responsible for a continued downward trend in the percentage of hypothermic patients, but it set the stage for the third and final PDSA cycle, which started in July 2019. The architecture was in place to integrate trainees and APPs to reinforce our initiative. Subsequently, the new median percentage of hypothermic patients was further decreased to an all-time low of 1.6% per month, satisfying and surpassing the goal of the QI project of decreasing the rate of hypothermia by only 50%. Our organization thereafter maintained a monthly hypothermia rate below 2%, except for April 2020, when it reached 2.5%. Our lowest median percentage was obtained after July 2020, reaching 1.3%.
To account for seasonal variations in temperatures and types of surgeries performed, we compared the percentage of hypothermic patients before and after the start of intervention, quarter by quarter. The decrease in the proportion of hypothermic patients after the start of intervention was statistically significant (P < .001). In addition, the data failed to prove any statistical difference for temperatures above 38 °C between the 2 periods, indicating that our interventions did not result in significant overwarming of patients. The clinical implications of decreasing the percentage of hypothermic patients from 6.9% to 1.3% is likely clinically important when considering the large number of patients who undergo surgery at large tertiary care pediatric centers. Even if simple interventions reduce hypothermia in only a handful of patients, routine applications of simple measures to keep patients normothermic is likely best clinical practice.
Anesthesiologists who participated in the hypothermia QI project by tracking the incidence of hypothermia in their patients were able to collect MOCA Part 4 credits in July 2019. There was no requirement for the individual anesthesiologist to reduce the rate of hypothermia or apply any of the encouraged strategies to obtain credit. As previously stated, there were also no rewards for obtaining low hypothermia rates for the providers. The temperature data continued to be collected through April 2021, 21 months after the credits were distributed, to demonstrate a continued, meaningful change, at least in the short-term. While the MOCA Part 4 credits likely served as an initial motivating factor to encourage participation in the QI project, they certainly were not responsible for the sustained low hypothermia rate after July 2019. We showed that the low rate of hypothermia was successfully maintained, indicating that the change in providers’ behavior was independent of the external motivator of obtaining the credit hours. Mere participation in the project by reviewing one’s temperature data was all that was required to obtain the credit. The Organismic Integration Theory, a mini-theory within SDT, best explains this phenomenon by describing any motivated behavior on a continuum ranging from controlled to autonomous.22 Do people perform the task resentfully, on their own volition because they believe it is the correct action, or somewhere in between? We explain the sustained low rates of hypothermia after the MOCA credits were distributed due to a shift to the autonomous end of the continuum with the clinician’s active willingness to meet the challenges and apply intrinsically motivated behaviors to lower the rate of hypothermia. The internalization of external motivators is difficult to prove, but the evidence supports that the methods we used to motivate individuals were effective and have resulted in a significant downward trend in our hypothermia rate.
There are several limitations to our QI project. The first involves the measuring of postoperative temperature in the recovery units. The temperatures were obtained using the same medical-grade infrared thermometer for all the patients, but other variables, such as timing and techniques, were not standardized. Secondly, overall surgical outcomes related to hypothermia were not tracked because we were unable to control for other confounding variables in our large cohort of patients, so we cannot say if the drop in the hypothermia rate had a clinically significant outcome. Thirdly, we propose that SDT offers a compellingly fitting explanation of the psychology of motivation in our efforts, but it may be possible that other theories may offer equally fitting explanations. The ability to measure the degree of motivation is lacking, and we did not explicitly ask participants what their specific source of motivation was. Aside from SDT, the reduction in hypothermia rate could also be attributed to the ease and availability of warming equipment that was made in each OR. This QI project was successfully applied to only 1 institution, so its ability to be widely applicable remains uncertain. In addition, data collection continued during the COVID-19 pandemic when case volumes decreased. However, by June 2020, the number of surgical cases at our institution had largely returned to prepandemic levels. Additional data collection beyond April 2021 would be helpful to determine if the reduction in hypothermia rates is truly sustained.
Conclusion
Overall, the importance of maintaining perioperative normothermia was well disseminated and agreed upon by all departments involved. Despite the limitations of the project, there was a significant reduction in rates of hypothermia, and sustainability of outcomes was consistently demonstrated in the poststudy period.
Using 3 cycles of the PDSA method, we successfully decreased the median rate of postoperative hypothermia in our pediatric surgical population from a preintervention value of 6.9% to 1.3%—a reduction of more than 81.2%. We provided motivation for members of our anesthesiology staff to participate by offering MOCA 2.0 Part 4 credits, but the lower rate of hypothermic patients was maintained for 15 months after the credits were distributed. Over the course of the project, there was a shift in culture, and extra vigilance was given to temperature monitoring and assessment. We attribute this sustained cultural change to the deliberate incorporation of the principles of competency, autonomy, and relatedness central to SDT to the structure of the interventions, avoiding rigid guidelines and pathways in favor of affective engagement to establish intrinsic motivation.
Acknowledgements: The authors thank Joan Reisch, PhD, for her assistance with the statistical analysis.
Corresponding author: Edgar Erold Kiss, MD, 1935 Medical District Dr, Dallas, TX 75235; [email protected].
Financial disclosures: None.
From Children’s Health System of Texas, Division of Pediatric Anesthesiology, Dallas, TX (Drs. Sakhai, Bocanegra, Chandran, Kimatian, and Kiss), UT Southwestern Medical Center, Department of Anesthesiology and Pain Management, Dallas, TX (Drs. Bocanegra, Chandran, Kimatian, and Kiss), and UT Southwestern Medical Center, Department of Population and Data Sciences, Dallas, TX (Dr. Reisch).
Objective: Policy-driven changes in medical practice have long been the norm. Seldom are changes in clinical practice sought to be brought about by a person’s tendency toward growth or self‐actualization. Many hospitals have instituted hypothermia bundles to help reduce the incidence of unanticipated postoperative hypothermia. Although successful in the short-term, sustained changes are difficult to maintain. We implemented a quality-improvement project focused on addressing the affective components of self-determination theory (SDT) to create sustainable behavioral change while satisfying providers’ basic psychological needs for autonomy, competence, and relatedness.
Methods: A total of 3 Plan-Do-Study-Act (PDSA) cycles were enacted over the span of 14 months at a major tertiary care pediatric hospital to recruit and motivate anesthesia providers and perioperative team members to reduce the percentage of hypothermic postsurgical patients by 50%. As an optional initial incentive for participation, anesthesiologists would qualify for American Board of Anesthesiology Maintenance of Certification in Anesthesiology (MOCA) Part 4 Quality Improvement credits for monitoring their own temperature data and participating in project-related meetings. Providers were given autonomy to develop a personal plan for achieving the desired goals.
Results: The median rate of hypothermia was reduced from 6.9% to 1.6% in July 2019 and was reduced again in July 2020 to 1.3%, an 81% reduction overall. A low hypothermia rate was successfully maintained for at least 21 subsequent months after participants received their MOCA credits in July 2019.
Conclusions: Using an approach that focused on the elements of competency, autonomy, and relatedness central to the principles of SDT, we observed the development of a new culture of vigilance for prevention of hypothermia that successfully endured beyond the project end date.
Keywords: postoperative hypothermia; self-determination theory; motivation; quality improvement.
Perioperative hypothermia, generally accepted as a core temperature less than 36 °C in clinical practice, is a common complication in the pediatric surgical population and is associated with poor postoperative outcomes.1 Hypothermic patients may develop respiratory depression, hypoglycemia, and metabolic acidosis that may lead to decreased oxygen delivery and end organ tissue hypoxia.2-4 Other potential detrimental effects of failing to maintain normal body temperature are impaired clotting factor enzyme function and platelet dysfunction, increasing the risk for postoperative bleeding.5,6 In addition, there are financial implications when hypothermic patients require care and resources postoperatively because of delayed emergence or shivering.7
The American Society of Anesthesiologists recommends intraoperative temperature monitoring for procedures when clinically significant changes in body temperature are anticipated.8 Maintenance of normothermia in the pediatric population is especially challenging owing to a larger skin-surface area compared with body mass ratio and less subcutaneous fat content than in adults. Preventing postoperative hypothermia starts preoperatively with parental education and can be as simple as covering the child with a blanket and setting the preoperative room to an acceptably warm temperature.9,10 Intraoperatively, maintaining operating room (OR) temperatures at or above 21.1 °C and using active warming devices and radiant warmers when appropriate are important techniques to preserve the child’s body temperature.11,12
Despite the knowledge of these risks and vigilant avoidance of hypothermia, unplanned perioperative hypothermia can occur in up to 70% of surgical patients.1 Beyond the clinical benefits, as health care marches toward a value-based payment methodology, quality indicators such as avoiding hypothermia may be linked directly to payment.
Self-determination theory (SDT) was first developed in 1980 by Deci and Ryan.13 The central premise of the theory states that people develop their full potential if circumstances allow them to satisfy their basic psychological needs: autonomy, competence, and relatedness. Under these conditions, people’s natural inclination toward growth can be realized, and they are more likely to internalize external goals. Under an extrinsic reward system, motivation can waver, as people may perceive rewards as controlling.
Many institutions have implemented hypothermia bundles to help decrease the rate of hypothermic patients, but while initially successful, the effectiveness of these interventions tends to fade over time as participants settle into old, comfortable routines.14 With SDT in mind, we designed our quality-improvement (QI) project with interventions to allow clinicians autonomy without instituting rigid guidelines or punitive actions. We aimed to directly address the affective components central to motivation and engagement so that we could bring about long-term meaningful changes in our practice.
Methods
Setting
The hypothermia QI intervention was instituted at a major tertiary care children’s hospital that performs more than 40 000 pediatric general anesthetics annually. Our division of pediatric anesthesiology consists of 66 fellowship-trained pediatric anesthesiologists, 15 or more rotating trainees per month, 13 anesthesiology assistants, 15 anesthesia technicians, and more than 50 perioperative nurses.
The most frequent pediatric surgeries include, but are not limited to, general surgery, otolaryngology, urology, gastroenterology, plastic surgery, neurosurgery, and dentistry. The surgeries are conducted in the hospital’s main operative floor, which consists of 15 ORs and 2 gastroenterology procedure rooms. Although the implementation of the QI project included several operating sites, we focused on collecting temperature data from surgical patients at our main campus recovery unit. We obtained the patients’ initial temperatures upon arrival to the recovery unit from a retrospective electronic health record review of all patients who underwent anesthesia from January 2016 through April 2021.
Postoperative hypothermia was identified as an area of potential improvement after several patients were reported to be hypothermic upon arrival to the recovery unit in the later part of 2018. Further review revealed significant heterogeneity of practices and lack of standardization of patient-warming methods. By comparing the temperatures pre- and postintervention, we could measure the effectiveness of the QI initiative. Prior to the start of our project, the hypothermia rate in our patient population was not actively tracked, and the effectiveness of our variable practice was not measured.
The cutoff for hypothermia for our QI project was defined as body temperature below 36 °C, since this value has been previously used in the literature and is commonly accepted in anesthesia practice as the delineation for hypothermia in patients undergoing general anesthesia.1
Interventions
This QI project was designed and modeled after the Institute for Healthcare Improvement Model for Improvement.15 Three cycles of Plan-Do-Study-Act (PDSA) were developed and instituted over a 14-month period until December 2019 (Table 1).

A retrospective review was conducted to determine the percentage of surgical patients arriving to our recovery units with an initial temperature reading of less than 36 °C. A project key driver diagram and smart aim were created and approved by the hospital’s continuing medical education (CME) committee for credit via the American Board of Medical Specialties (ABMS) Multi-Specialty Portfolio Program, Maintenance of Certification in Anesthesiology (MOCA) Part 4.
The first PDSA cycle involved introducing the QI project and sharing the aims of the project at a department grand rounds in the latter part of October 2018. Enrollment to participate in the project was open to all anesthesiologists in the division, and participants could earn up to 20 hours of MOCA Part 4 credits. A spreadsheet was developed and maintained to track each anesthesiologist’s monthly percentage of hypothermic patients. The de-identified patient data were shared with the division via monthly emails. In addition, individual providers with a hypothermic patient in the recovery room received a notification email.
The anesthesiologists participated in the QI project by reviewing their personal percentage of hypothermic patients on an ongoing basis to earn the credit. There was no explicit requirement to decrease their own rate of patients with body temperature less than 36 °C or expectation to achieve a predetermined goal, so the participants could not “fail.”
Because of the large interest in this project, a hypothermia committee was formed that consisted of 36 anesthesiologists. This group reviewed the data and exchanged ideas for improvement in November 2018 as part of the first PDSA cycle. The committee met monthly and was responsible for actively engaging other members of the department and perioperative staff to help in this multidisciplinary effort of combating hypothermia in our surgical pediatric population.
PDSA cycle 2 involved several major initiatives, including direct incorporation of the rest of the perioperative team. The perioperative nursing team was educated on the risks of hypothermia and engaged to take an active role by maintaining the operating suite temperature at 21.1 °C and turning on the Bair Hugger (3M) blanket to 43 °C on the OR bed prior to patient arrival to the OR. Additionally, anesthesia technicians (ATs) were tasked with ensuring an adequate supply of Bair Hugger drapes for all cases of the day. The facility’s engineering team was engaged to move the preoperative room temperature controls away from families (who frequently made the rooms cold) and instead set it at a consistent temperature of 23.9 °C. ATs were also asked to place axillary and nasal temperature probes on the anesthesia workstations as a visual reminder to facilitate temperature monitoring closer to the start of anesthesia (instead of the anesthesia provider having to remember to retrieve a temperature probe out of a drawer and place it on the patient). Furthermore, anesthesiologists were instructed via the aforementioned monthly emails and at monthly department meetings to place the temperature probes as early as possible in order to recognize and respond to intraoperative hypothermia in a timelier manner. Finally, supply chain leaders were informed of our expected increase in the use of the blankets and probes and proportionally increased ordering of these supplies to make sure availability would not present an obstacle.
In PDSA cycle 3, trainees (anesthesia assistant students, anesthesia residents and fellows) and advanced practice providers (APPs) (certified registered nurse-anesthetists [CRNAs] and certified anesthesia assistants [C-AAs]) were informed of the QI project. This initiative was guided toward improving vigilance for hypothermia in the rest of the anesthesia team members. The trainees and APPs usually set up the anesthesia area prior to patient arrival, so their recruitment in support of this effort would ensure appropriate OR temperature, active warming device deployment, and the availability and early placement of the correct temperature probe for the case. To facilitate personal accountability, the trainees and APPs were also emailed their own patients’ rate of hypothermia.
Along the course of the project, quarterly committee meetings and departmental monthly meetings served as venues to express concerns and look for areas of improvement, such as specific patterns or trends leading to hypothermic patients. One specific example was the identification of the gastrointestinal endoscopic patients having a rate of hypothermia that was 2% higher than average. Directed education on the importance of Bair Hugger blankets and using warm intravenous fluids worked well to decrease the rate of hypothermia in these patients. This collection of data was shared at regular intervals during monthly department meetings as well and more frequently using departmental emails. The hospital’s secure intranet SharePoint (Microsoft) site was used to share the data among providers.
Study of the interventions and measures
To study the effectiveness and impact of the project to motivate our anesthesiologists and other team members, we compared the first temperatures obtained in the recovery unit prior to the start of the intervention with those collected after the start of the QI project in November 2018. Because of the variability of temperature monitoring intraoperatively (nasal, axillary, rectal), we decided to use the temperature obtained by the nurse in the recovery room upon the patient’s arrival. Over the years analyzed, the nurse’s technique of measuring the temperature remained consistent. All patient temperature measurements were performed using the TAT-5000 (Exergen Corporation). This temporal artery thermometer has been previously shown to correlate well with bladder temperatures (70% of measurements differ by no more than 0.5 °C, as reported by Langham et al16).
Admittedly, we could not measure the degree of motivation or internalization of the project goals by our cohort, but we could measure the reduction in the rate of hypothermia and subjectively gauge engagement in the project by the various groups of participants and the sustainability of the results. In addition, all participating anesthesiologists received MOCA Part 4 credits in July 2019. We continued our data collection until April 2021 to determine if our project had brought about sustainable changes in practice that would continue past the initial motivator of obtaining CME credit.
Analysis
Data analysis was performed using Excel (Microsoft) and SAS, version 9.4 (SAS Institute).
The median of the monthly percentage of patients with a temperature of less than 36.0 °C was also determined for the preintervention time frame. This served as our baseline hypothermia rate, and we aimed to lower it by 50%. Run charts, a well-described methodology to gauge the effectiveness of the QI project, were constructed with the collected data.17
We performed additional analysis to adjust for different time periods throughout the year. The time period between January 2016 and October 2018 was considered preintervention. We considered November 2018 the start of our intervention, or more specifically, the start of our PDSA cycles. October 2018 was analyzed as part of the preintervention data. To account for seasonal temperature variations, the statistical analysis focused on the comparisons of the same calendar quarters for before and after starting intervention using Wilcoxon Mann-Whitney U tests. To reach an overall conclusion, the probabilities for the 4 quarters were combined for each criterion separately utilizing the Fisher χ2 combined probability method.
The hypothermia QI project was reviewed by the institutional review board and determined to be exempt.
Results
The temperatures of 40 875 patients were available for analysis for the preintervention period between January 2016 and October 2018. The median percentage of patients with temperatures less than 36.0 °C was 6.9% (interquartile range [IQR], 5.8%-8.4%). The highest percentage was in February 2016 (9.9%), and the lowest was in March 2018 (3.4%). Following the start of the first PDSA cycle, the next 6 consecutive rates of hypothermia were below the median preintervention value, and a new median for these percentages was calculated at 3.4% (IQR, 2.6%-4.3%). In July 2019, the proportion of hypothermic patients decreased once more for 6 consecutive months, yielding a new median of 1.6% (IQR, 1.2%-1.8%) and again in July 2020, to yield a median of 1.3% (IQR, 1.2%-1.5%) (Figure). In all, 33 799 patients were analyzed after the start of the project from November 2018 to the end of the data collection period through April 2021.

The preintervention monthly rates of hypothermia were compared, quarter to quarter, with those starting in November 2018 using the Wilcoxon Mann-Whitney U test. The decrease in proportion of hypothermic patients after the start of the intervention was statistically significant (P < .001). In addition, the percentage of patients with temperatures greater than 38 °C was not significantly different between the pre- and postintervention time periods (P < .25) (Table 2). The decrease in the number of patients available for analysis from March 2020 to May 2020 was due to the COVID-19 pandemic.

Subjectively, we did not experience any notable resistance to our efforts, and the experience was largely positive for everyone involved. Clinicians identified as having high monthly rates of hypothermia (5% or higher) corrected their numbers the following month after being notified via email or in person.
Discussion
To achieve changes in practice, the health care industry has relied on instituting guidelines, regulations, and policies, often with punitive consequences. We call into question this long-standing framework and propose a novel approach to help evolve the field of QI. Studies in human psychology have long demonstrated the demotivation power of a reward system and the negative response to attempts by authority to use incentives to control or coerce. In our QI project, we instituted 3 PDSA cycles and applied elements from SDT to motivate people’s behaviors. We demonstrate how a new culture focused on maintaining intraoperative normothermia was developed and brought about a measurable and significant decrease in the rate of hypothermia. The relevance of SDT, a widely accepted unifying theory that bridges and links social and personality psychology, should not be understated in health care. Authorities wishing to have long-standing influence should consider a person’s right to make their own decisions and, if possible, a unique way of doing things.
Positively reinforcing behavior has been shown to have a paradoxical effect by dampening an individual’s intrinsic motivation or desire to perform certain tasks.18 Deadlines, surveillance, and authoritative commands are also deterrents.19,20 We focused on providing the tools and information to the clinicians and relied on their innate need for autonomy, growth, and self-actualization to bring about change in clinical practice.21 Group meetings served as a construct for exchanging ideas and to encourage participation, but without the implementation of rigid guidelines or policies. Intraoperative active warming devices and temperature probes were made available, but their use was not mandated. The use of these devices was intentionally not audited to avoid any overbearing control. Providers were, however, given monthly temperature data to help individually assess the effectiveness of their interventions. We did not impose any negative or punitive actions for those clinicians who had high rates of hypothermic patients, and we did not reward those who had low rates of hypothermia. We wanted the participants to feel that the inner self was the source of their behavior, and this was in parallel with their own interests and values. If providers could feel their need for competency could be realized, we hoped they would continue to adhere to the measures we provided to maintain a low rate of hypothermia.
The effectiveness of our efforts was demonstrated by a decrease in the prevalence of postoperative hypothermia in our surgical patients. The initial decrease of the median rate of hypothermia from 6.9% to 3.4% occurred shortly into the start of the first PDSA cycle. The second PDSA cycle started in January 2019 with a multimodal approach and included almost all parties involved in the perioperative care of our surgical patients. Not only was this intervention responsible for a continued downward trend in the percentage of hypothermic patients, but it set the stage for the third and final PDSA cycle, which started in July 2019. The architecture was in place to integrate trainees and APPs to reinforce our initiative. Subsequently, the new median percentage of hypothermic patients was further decreased to an all-time low of 1.6% per month, satisfying and surpassing the goal of the QI project of decreasing the rate of hypothermia by only 50%. Our organization thereafter maintained a monthly hypothermia rate below 2%, except for April 2020, when it reached 2.5%. Our lowest median percentage was obtained after July 2020, reaching 1.3%.
To account for seasonal variations in temperatures and types of surgeries performed, we compared the percentage of hypothermic patients before and after the start of intervention, quarter by quarter. The decrease in the proportion of hypothermic patients after the start of intervention was statistically significant (P < .001). In addition, the data failed to prove any statistical difference for temperatures above 38 °C between the 2 periods, indicating that our interventions did not result in significant overwarming of patients. The clinical implications of decreasing the percentage of hypothermic patients from 6.9% to 1.3% is likely clinically important when considering the large number of patients who undergo surgery at large tertiary care pediatric centers. Even if simple interventions reduce hypothermia in only a handful of patients, routine applications of simple measures to keep patients normothermic is likely best clinical practice.
Anesthesiologists who participated in the hypothermia QI project by tracking the incidence of hypothermia in their patients were able to collect MOCA Part 4 credits in July 2019. There was no requirement for the individual anesthesiologist to reduce the rate of hypothermia or apply any of the encouraged strategies to obtain credit. As previously stated, there were also no rewards for obtaining low hypothermia rates for the providers. The temperature data continued to be collected through April 2021, 21 months after the credits were distributed, to demonstrate a continued, meaningful change, at least in the short-term. While the MOCA Part 4 credits likely served as an initial motivating factor to encourage participation in the QI project, they certainly were not responsible for the sustained low hypothermia rate after July 2019. We showed that the low rate of hypothermia was successfully maintained, indicating that the change in providers’ behavior was independent of the external motivator of obtaining the credit hours. Mere participation in the project by reviewing one’s temperature data was all that was required to obtain the credit. The Organismic Integration Theory, a mini-theory within SDT, best explains this phenomenon by describing any motivated behavior on a continuum ranging from controlled to autonomous.22 Do people perform the task resentfully, on their own volition because they believe it is the correct action, or somewhere in between? We explain the sustained low rates of hypothermia after the MOCA credits were distributed due to a shift to the autonomous end of the continuum with the clinician’s active willingness to meet the challenges and apply intrinsically motivated behaviors to lower the rate of hypothermia. The internalization of external motivators is difficult to prove, but the evidence supports that the methods we used to motivate individuals were effective and have resulted in a significant downward trend in our hypothermia rate.
There are several limitations to our QI project. The first involves the measuring of postoperative temperature in the recovery units. The temperatures were obtained using the same medical-grade infrared thermometer for all the patients, but other variables, such as timing and techniques, were not standardized. Secondly, overall surgical outcomes related to hypothermia were not tracked because we were unable to control for other confounding variables in our large cohort of patients, so we cannot say if the drop in the hypothermia rate had a clinically significant outcome. Thirdly, we propose that SDT offers a compellingly fitting explanation of the psychology of motivation in our efforts, but it may be possible that other theories may offer equally fitting explanations. The ability to measure the degree of motivation is lacking, and we did not explicitly ask participants what their specific source of motivation was. Aside from SDT, the reduction in hypothermia rate could also be attributed to the ease and availability of warming equipment that was made in each OR. This QI project was successfully applied to only 1 institution, so its ability to be widely applicable remains uncertain. In addition, data collection continued during the COVID-19 pandemic when case volumes decreased. However, by June 2020, the number of surgical cases at our institution had largely returned to prepandemic levels. Additional data collection beyond April 2021 would be helpful to determine if the reduction in hypothermia rates is truly sustained.
Conclusion
Overall, the importance of maintaining perioperative normothermia was well disseminated and agreed upon by all departments involved. Despite the limitations of the project, there was a significant reduction in rates of hypothermia, and sustainability of outcomes was consistently demonstrated in the poststudy period.
Using 3 cycles of the PDSA method, we successfully decreased the median rate of postoperative hypothermia in our pediatric surgical population from a preintervention value of 6.9% to 1.3%—a reduction of more than 81.2%. We provided motivation for members of our anesthesiology staff to participate by offering MOCA 2.0 Part 4 credits, but the lower rate of hypothermic patients was maintained for 15 months after the credits were distributed. Over the course of the project, there was a shift in culture, and extra vigilance was given to temperature monitoring and assessment. We attribute this sustained cultural change to the deliberate incorporation of the principles of competency, autonomy, and relatedness central to SDT to the structure of the interventions, avoiding rigid guidelines and pathways in favor of affective engagement to establish intrinsic motivation.
Acknowledgements: The authors thank Joan Reisch, PhD, for her assistance with the statistical analysis.
Corresponding author: Edgar Erold Kiss, MD, 1935 Medical District Dr, Dallas, TX 75235; [email protected].
Financial disclosures: None.
1. Leslie K, Sessler DI. Perioperative hypothermia in the high-risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17(4):485-498.
2. Sessler DI. Forced-air warming in infants and children. Paediatr Anaesth. 2013;23(6):467-468.
3. Wetzel RC. Evaluation of children. In: Longnecker DE, Tinker JH, Morgan Jr GE, eds. Principles and Practice of Anesthesiology. 2nd ed. Mosby Publishers; 1999:445-447.
4. Witt L, Dennhardt N, Eich C, et al. Prevention of intraoperative hypothermia in neonates and infants: results of a prospective multicenter observational study with a new forced-air warming system with increased warm air flow. Paediatr Anaesth. 2013;23(6):469-474.
5. Blum R, Cote C. Pediatric equipment. In: Blum R, Cote C, eds. A Practice of Anaesthesia for Infants and Children. Saunders Elsevier; 2009:1099-1101.
6. Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2003;17(4):535-549.
7. Mahoney CB, Odom J. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA J. 1999;67(2):155-163.
8. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring. Approved by the ASA House of Delegates October 21, 1986; last amended October 20, 2010; last affirmed October 28, 2015.
9. Horn E-P, Bein B, Böhm R, et al. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia. 2012;67(6):612-617.
10. Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth. 2008;101(5):627-631.
11. Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95(2):531-543.
12. Bräuer A, English MJM, Steinmetz N, et al. Efficacy of forced-air warming systems with full body blankets. Can J Anaesth. 2007;54(1):34-41.
13. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self‐determination of behavior. Psychol Inquiry. 2000;11(4):227-268.
14. Al-Shamari M, Puttha R, Yuen S, et al. G9 Can introduction of a hypothermia bundle reduce hypothermia in the newborns? Arch Dis Childhood. 2019;104(suppl 2):A4.1-A4.
15. Institute for Healthcare Improvement. How to improve. Accessed May 12, 2021. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
16. Langham GE, Meheshwari A, You J, et al. Noninvasive temperature monitoring in postanesthesia care units. Anesthesiology. 2009;111(1):90-96.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46-51.
18. Deci EL. Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol. 1971;18(1):105-115.
19. Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627-668.
20. Deci EL, Koestner R, Ryan RM. The undermining effect is a reality after all—extrinsic rewards, task interest, and self-determination: Reply to Eisenberger, Pierce, and Cameron (1999) and Lepper, Henderlong, and Gingras (1999). Psychol Bull. 1999;125(6):692-700.
21. Maslow A. The Farther Reaches of Human Nature. Viking Press; 1971.
22. Sheldon KM, Prentice M. Self-determination theory as a foundation for personality researchers. J Pers. 2019;87(1):5-14.
1. Leslie K, Sessler DI. Perioperative hypothermia in the high-risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17(4):485-498.
2. Sessler DI. Forced-air warming in infants and children. Paediatr Anaesth. 2013;23(6):467-468.
3. Wetzel RC. Evaluation of children. In: Longnecker DE, Tinker JH, Morgan Jr GE, eds. Principles and Practice of Anesthesiology. 2nd ed. Mosby Publishers; 1999:445-447.
4. Witt L, Dennhardt N, Eich C, et al. Prevention of intraoperative hypothermia in neonates and infants: results of a prospective multicenter observational study with a new forced-air warming system with increased warm air flow. Paediatr Anaesth. 2013;23(6):469-474.
5. Blum R, Cote C. Pediatric equipment. In: Blum R, Cote C, eds. A Practice of Anaesthesia for Infants and Children. Saunders Elsevier; 2009:1099-1101.
6. Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2003;17(4):535-549.
7. Mahoney CB, Odom J. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA J. 1999;67(2):155-163.
8. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring. Approved by the ASA House of Delegates October 21, 1986; last amended October 20, 2010; last affirmed October 28, 2015.
9. Horn E-P, Bein B, Böhm R, et al. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia. 2012;67(6):612-617.
10. Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth. 2008;101(5):627-631.
11. Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95(2):531-543.
12. Bräuer A, English MJM, Steinmetz N, et al. Efficacy of forced-air warming systems with full body blankets. Can J Anaesth. 2007;54(1):34-41.
13. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self‐determination of behavior. Psychol Inquiry. 2000;11(4):227-268.
14. Al-Shamari M, Puttha R, Yuen S, et al. G9 Can introduction of a hypothermia bundle reduce hypothermia in the newborns? Arch Dis Childhood. 2019;104(suppl 2):A4.1-A4.
15. Institute for Healthcare Improvement. How to improve. Accessed May 12, 2021. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
16. Langham GE, Meheshwari A, You J, et al. Noninvasive temperature monitoring in postanesthesia care units. Anesthesiology. 2009;111(1):90-96.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46-51.
18. Deci EL. Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol. 1971;18(1):105-115.
19. Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627-668.
20. Deci EL, Koestner R, Ryan RM. The undermining effect is a reality after all—extrinsic rewards, task interest, and self-determination: Reply to Eisenberger, Pierce, and Cameron (1999) and Lepper, Henderlong, and Gingras (1999). Psychol Bull. 1999;125(6):692-700.
21. Maslow A. The Farther Reaches of Human Nature. Viking Press; 1971.
22. Sheldon KM, Prentice M. Self-determination theory as a foundation for personality researchers. J Pers. 2019;87(1):5-14.
As common respiratory viruses resurface, children are at serious risk
Younger children may be vulnerable to the reemergence of common respiratory viruses such as influenza and respiratory syncytial virus (RSV) as COVID-19 restrictions wane, experts say. The impact could be detrimental.
The COVID-19 pandemic and the implementation of preventative measures such as social distancing, travel restrictions, mask use, and shelter in place, reduced the transmission of respiratory viruses, according to the Centers for Disease Control and Prevention. However, because older infants and toddlers have not been exposed to these bugs during the pandemic, they are vulnerable to suffering severe viral infections.
“[We’ve] been in the honeymoon for 18 months,” said Christopher J. Harrison, MD, professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. “We are going to be coming out of the honeymoon and the children who didn’t get sick are going to start packing 2 years’ worth of infections into the next 9 months so there’s going to be twice as many as would be normal.”
The CDC issued a health advisory in June for parts of the southern United States, such as Texas, the Carolinas, and Oklahoma, encouraging broader testing for RSV – a virus that usually causes mild, cold-like symptoms and is the most common cause of bronchiolitis and pneumonia in children – among those who test negative for COVID-19. Virtually all children get an RSV infection by the time they are 2 years old, according to the CDC.
In previous years, RSV usually spread during the fall and spring seasons and usually peaked late December to mid-February. However, there’s been an offseason spike in the common illness this year, with nearly 2,000 confirmed cases each week of July.
Richard J. Webby, PhD, of the infectious diseases department at St. Jude Children’s Research Hospital, Memphis, Tenn., said that although RSV transmits more easily during the winter, the virus is able to thrive during this summer because many children have limited immunity and are more vulnerable to catching the virus than before. Population immunity normally limits a virus to circulating under its most favorable conditions, which is usually the winter. However, because there are a few more “susceptible hosts,” it gives the virus the ability to spread during a time when it typically wouldn’t be able to.
“Now we have a wider range of susceptible kids because they haven’t had that exposure over the past 18 months,” said Dr. Webby, who is on the World Health Organization’s Influenza Vaccine Composition Advisory Team. “It gives the virus more chances to transmit during conditions that are less favorable.”
Dr. Harrison said that, if children continue to take preventative measures such as wearing masks and sanitizing, they can delay catching the RSV – which can be severe in infants and young children – until they’re older and symptoms won’t be as severe.
“The swelling that these viruses cause in the trachea and the bronchial tubes is much bigger in proportion to the overall size of the tubes, so it takes less swelling to clog up the trachea or bronchial tube for the 9-month-old than it does of a 9-year-old,” Dr. Harrison said. “So if a 9-year-old was to get RSV, they’re not going to have nearly the same amount symptoms as the 9-month-old.
Dr. Harrison said delaying RSV in children was never an option before because it’s a virus that’s almost impossible to avoid.
“Hopefully, the mask means that if you get exposed, instead of getting a million virus particles from your classmate or your playmate, you may only get a couple thousand,” Dr. Harrison explained. “And maybe that’s enough that you can fight it off or it may be small enough that you get a mild infection instead of a severe infection.”
A summer surge of RSV has also occurred in Australia. A study published in Clinical Infectious Diseases found that Western Australia saw a 98% reduction in RSV cases. This suggests that COVID-19 restrictions also delayed the RSV season.
Dr. Webby said the lax in penetrative measures against COVID-19 may also affect this upcoming flu season. Usually, around 10%-30% of the population gets infected with the flu each year, but that hasn’t happened the past couple of seasons, he said.
“There might be slightly less overall immunity to these viruses,” Dr. Webby said. “When these viruses do come back, there’s a little bit more room for them to take off.”
Although a severe influenza season rebound this winter is a possibility, Australia continues to experience a historically low flu season. Dr. Harrison, who said the northern hemisphere looks at what’s happening in Australia and the rest of the “southern half of the world because their influenza season is during our summer,” hopes this is an indication that the northern hemisphere will also experience a mild season.
However, there’s no indication of how this upcoming flu season will hit the United States and there isn’t any guidance on what could happen because these historically low levels of respiratory viruses have never happened before, Dr. Webby explained.
He said that, if COVID-19’s delta variant continues to circulate during the fall and winter seasons, it will keep other viruses at low levels. This is because there is rarely a peak of activity of different viruses at the same time.
“When you get infected with the virus, your body’s immune response has this nonspecific reaction that protects you from anything else for a short period of time,” Dr. Webby explained. “When you get a lot of one virus circulating, it’s really hard for these other viruses to get into that population and sort of set off an epidemic of their own.”
To prepare for an unsure influenza season, Dr. Harrison suggests making the influenza vaccine available in August as opposed to October.
Dr. Harrison and Dr. Webby reported no conflicts of interest.
Younger children may be vulnerable to the reemergence of common respiratory viruses such as influenza and respiratory syncytial virus (RSV) as COVID-19 restrictions wane, experts say. The impact could be detrimental.
The COVID-19 pandemic and the implementation of preventative measures such as social distancing, travel restrictions, mask use, and shelter in place, reduced the transmission of respiratory viruses, according to the Centers for Disease Control and Prevention. However, because older infants and toddlers have not been exposed to these bugs during the pandemic, they are vulnerable to suffering severe viral infections.
“[We’ve] been in the honeymoon for 18 months,” said Christopher J. Harrison, MD, professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. “We are going to be coming out of the honeymoon and the children who didn’t get sick are going to start packing 2 years’ worth of infections into the next 9 months so there’s going to be twice as many as would be normal.”
The CDC issued a health advisory in June for parts of the southern United States, such as Texas, the Carolinas, and Oklahoma, encouraging broader testing for RSV – a virus that usually causes mild, cold-like symptoms and is the most common cause of bronchiolitis and pneumonia in children – among those who test negative for COVID-19. Virtually all children get an RSV infection by the time they are 2 years old, according to the CDC.
In previous years, RSV usually spread during the fall and spring seasons and usually peaked late December to mid-February. However, there’s been an offseason spike in the common illness this year, with nearly 2,000 confirmed cases each week of July.
Richard J. Webby, PhD, of the infectious diseases department at St. Jude Children’s Research Hospital, Memphis, Tenn., said that although RSV transmits more easily during the winter, the virus is able to thrive during this summer because many children have limited immunity and are more vulnerable to catching the virus than before. Population immunity normally limits a virus to circulating under its most favorable conditions, which is usually the winter. However, because there are a few more “susceptible hosts,” it gives the virus the ability to spread during a time when it typically wouldn’t be able to.
“Now we have a wider range of susceptible kids because they haven’t had that exposure over the past 18 months,” said Dr. Webby, who is on the World Health Organization’s Influenza Vaccine Composition Advisory Team. “It gives the virus more chances to transmit during conditions that are less favorable.”
Dr. Harrison said that, if children continue to take preventative measures such as wearing masks and sanitizing, they can delay catching the RSV – which can be severe in infants and young children – until they’re older and symptoms won’t be as severe.
“The swelling that these viruses cause in the trachea and the bronchial tubes is much bigger in proportion to the overall size of the tubes, so it takes less swelling to clog up the trachea or bronchial tube for the 9-month-old than it does of a 9-year-old,” Dr. Harrison said. “So if a 9-year-old was to get RSV, they’re not going to have nearly the same amount symptoms as the 9-month-old.
Dr. Harrison said delaying RSV in children was never an option before because it’s a virus that’s almost impossible to avoid.
“Hopefully, the mask means that if you get exposed, instead of getting a million virus particles from your classmate or your playmate, you may only get a couple thousand,” Dr. Harrison explained. “And maybe that’s enough that you can fight it off or it may be small enough that you get a mild infection instead of a severe infection.”
A summer surge of RSV has also occurred in Australia. A study published in Clinical Infectious Diseases found that Western Australia saw a 98% reduction in RSV cases. This suggests that COVID-19 restrictions also delayed the RSV season.
Dr. Webby said the lax in penetrative measures against COVID-19 may also affect this upcoming flu season. Usually, around 10%-30% of the population gets infected with the flu each year, but that hasn’t happened the past couple of seasons, he said.
“There might be slightly less overall immunity to these viruses,” Dr. Webby said. “When these viruses do come back, there’s a little bit more room for them to take off.”
Although a severe influenza season rebound this winter is a possibility, Australia continues to experience a historically low flu season. Dr. Harrison, who said the northern hemisphere looks at what’s happening in Australia and the rest of the “southern half of the world because their influenza season is during our summer,” hopes this is an indication that the northern hemisphere will also experience a mild season.
However, there’s no indication of how this upcoming flu season will hit the United States and there isn’t any guidance on what could happen because these historically low levels of respiratory viruses have never happened before, Dr. Webby explained.
He said that, if COVID-19’s delta variant continues to circulate during the fall and winter seasons, it will keep other viruses at low levels. This is because there is rarely a peak of activity of different viruses at the same time.
“When you get infected with the virus, your body’s immune response has this nonspecific reaction that protects you from anything else for a short period of time,” Dr. Webby explained. “When you get a lot of one virus circulating, it’s really hard for these other viruses to get into that population and sort of set off an epidemic of their own.”
To prepare for an unsure influenza season, Dr. Harrison suggests making the influenza vaccine available in August as opposed to October.
Dr. Harrison and Dr. Webby reported no conflicts of interest.
Younger children may be vulnerable to the reemergence of common respiratory viruses such as influenza and respiratory syncytial virus (RSV) as COVID-19 restrictions wane, experts say. The impact could be detrimental.
The COVID-19 pandemic and the implementation of preventative measures such as social distancing, travel restrictions, mask use, and shelter in place, reduced the transmission of respiratory viruses, according to the Centers for Disease Control and Prevention. However, because older infants and toddlers have not been exposed to these bugs during the pandemic, they are vulnerable to suffering severe viral infections.
“[We’ve] been in the honeymoon for 18 months,” said Christopher J. Harrison, MD, professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. “We are going to be coming out of the honeymoon and the children who didn’t get sick are going to start packing 2 years’ worth of infections into the next 9 months so there’s going to be twice as many as would be normal.”
The CDC issued a health advisory in June for parts of the southern United States, such as Texas, the Carolinas, and Oklahoma, encouraging broader testing for RSV – a virus that usually causes mild, cold-like symptoms and is the most common cause of bronchiolitis and pneumonia in children – among those who test negative for COVID-19. Virtually all children get an RSV infection by the time they are 2 years old, according to the CDC.
In previous years, RSV usually spread during the fall and spring seasons and usually peaked late December to mid-February. However, there’s been an offseason spike in the common illness this year, with nearly 2,000 confirmed cases each week of July.
Richard J. Webby, PhD, of the infectious diseases department at St. Jude Children’s Research Hospital, Memphis, Tenn., said that although RSV transmits more easily during the winter, the virus is able to thrive during this summer because many children have limited immunity and are more vulnerable to catching the virus than before. Population immunity normally limits a virus to circulating under its most favorable conditions, which is usually the winter. However, because there are a few more “susceptible hosts,” it gives the virus the ability to spread during a time when it typically wouldn’t be able to.
“Now we have a wider range of susceptible kids because they haven’t had that exposure over the past 18 months,” said Dr. Webby, who is on the World Health Organization’s Influenza Vaccine Composition Advisory Team. “It gives the virus more chances to transmit during conditions that are less favorable.”
Dr. Harrison said that, if children continue to take preventative measures such as wearing masks and sanitizing, they can delay catching the RSV – which can be severe in infants and young children – until they’re older and symptoms won’t be as severe.
“The swelling that these viruses cause in the trachea and the bronchial tubes is much bigger in proportion to the overall size of the tubes, so it takes less swelling to clog up the trachea or bronchial tube for the 9-month-old than it does of a 9-year-old,” Dr. Harrison said. “So if a 9-year-old was to get RSV, they’re not going to have nearly the same amount symptoms as the 9-month-old.
Dr. Harrison said delaying RSV in children was never an option before because it’s a virus that’s almost impossible to avoid.
“Hopefully, the mask means that if you get exposed, instead of getting a million virus particles from your classmate or your playmate, you may only get a couple thousand,” Dr. Harrison explained. “And maybe that’s enough that you can fight it off or it may be small enough that you get a mild infection instead of a severe infection.”
A summer surge of RSV has also occurred in Australia. A study published in Clinical Infectious Diseases found that Western Australia saw a 98% reduction in RSV cases. This suggests that COVID-19 restrictions also delayed the RSV season.
Dr. Webby said the lax in penetrative measures against COVID-19 may also affect this upcoming flu season. Usually, around 10%-30% of the population gets infected with the flu each year, but that hasn’t happened the past couple of seasons, he said.
“There might be slightly less overall immunity to these viruses,” Dr. Webby said. “When these viruses do come back, there’s a little bit more room for them to take off.”
Although a severe influenza season rebound this winter is a possibility, Australia continues to experience a historically low flu season. Dr. Harrison, who said the northern hemisphere looks at what’s happening in Australia and the rest of the “southern half of the world because their influenza season is during our summer,” hopes this is an indication that the northern hemisphere will also experience a mild season.
However, there’s no indication of how this upcoming flu season will hit the United States and there isn’t any guidance on what could happen because these historically low levels of respiratory viruses have never happened before, Dr. Webby explained.
He said that, if COVID-19’s delta variant continues to circulate during the fall and winter seasons, it will keep other viruses at low levels. This is because there is rarely a peak of activity of different viruses at the same time.
“When you get infected with the virus, your body’s immune response has this nonspecific reaction that protects you from anything else for a short period of time,” Dr. Webby explained. “When you get a lot of one virus circulating, it’s really hard for these other viruses to get into that population and sort of set off an epidemic of their own.”
To prepare for an unsure influenza season, Dr. Harrison suggests making the influenza vaccine available in August as opposed to October.
Dr. Harrison and Dr. Webby reported no conflicts of interest.
When is MRI useful in the management of congenital melanocytic nevi?
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
FROM SPD 2021
More children with high-risk brain cancer now surviving
A practice-changing study that used molecular testing to distinguish between subtypes of medulloblastoma has shown a significant improvement in survival for children with high-risk disease who underwent treatment intensification with carboplatin.
“Each of the four subgroups of medulloblastoma has a different prognosis, but for this particular subgroup, 20 fewer children out of every 100 would have survived prior to this study,” James Olson, MD, professor of medicine, French Hutchinson Cancer Research Center, University of Washington, Seattle, said in an interview.
“This is the reason for celebration – for now and forevermore, we can expect 20 more children with high-risk, group 3 medulloblastoma to survive,” he said.
“We recommend that all children with high-risk, group 3 medulloblastoma receive carboplatin and all children in the other subgroups do not, because we don’t want them to experience the toxicity without benefit,” Dr. Olson said.
The study was published online July 22, 2021, in JAMA Oncology.
Hematologic toxicity was more pronounced in the carboplatin arm in the induction phase of the protocol, and toxicity persisted into the first cycles of maintenance therapy. On the other hand, “there weren’t enough additional side effects to recommend children not get carboplatin if they would benefit from it,” Dr. Olson noted.
At least 75% of children with newly diagnosed medulloblastoma survive, although those with high-risk, group 3 disease have a substantially poorer prognosis than those with other molecular subtypes.
However, if a child with medulloblastoma experiences relapse, “the likelihood of survival is near zero, so it’s important to get it right the first time,” Dr. Olson said.
One of the patients who took part in this trial, Sammy Loch of Seattle, is now 27 years old and has been cancer free for 11 years.
She was diagnosed with medulloblastoma when in high school. At the time of her diagnosis, she was asked by her pediatric oncologist at Seattle Children’s Hospital about taking part in the study. After careful consideration, she agreed.
“Participating in research was my way to give back and pay it forward,” Ms. Loch said in a statement. “It’s really exciting to know more people will survive because of the research I was involved in,” she added. She continues to pay her debt forward, serving as a therapist for people with chronic health conditions and raising funds for pediatric cancer research.
Patients had high-risk features
The study involved 261 evaluable patients (median age, 8.6 years). All patients had high-risk features, including metastatic disease (72.4% of the group), diffuse anaplastic histologic characteristics (22.2%), and incomplete surgical resection (5.4%), defined as residual tumor greater than 1.5 cm2.
“All patients received 36 Gy craniospinal radiotherapy with boost to the posterior fossa of 55.8 Gy cumulative dose with conventional fractionation of 1.8 Gy/d,” Dr. Olson and colleagues explain. Patients also received six doses of vincristine 1.5 mg/m2 weekly during radiotherapy and were randomly assigned to receive carboplatin 35 mg/m2 for a total of 30 doses given daily prior to radiotherapy or placebo.
This regimen was followed by maintenance therapy, which consisted of six 28-day cycles of the combination of cisplatin 75 mg/m2 on day 1; cyclophosphamide 1,000 mg/m2 on days 2 and 3; and vincristine 1.5 mg/m2 on days 1 and 8.
Patients were originally assigned to receive an additional 12 cycles of isotretinoin or placebo, to be given during and after maintenance therapy. However, randomization to isotretinoin was discontinued early because of futility.
The study was initially powered to evaluate medulloblastoma as a single disease. However, as a result of biologic insights gained after the study was conceived, it was amended to include a molecular subgroup analysis to better distinguish patients who might truly benefit from intensified therapy, the authors explained.
Study results
The World Health Organization categorizes tumors of the central nervous system into four groups. The authors followed this system of categorization for their patients with medulloblastoma. The four groups are WNT, in which WNT signaling pathway is activated; SHH, in which the SHH signaling pathway is activated; with or without TP53 mutation (provisionally designated group 3); and non-WNT/non-SHH (provisionally designed group 4)
The primary endpoint of the trial was event-free survival (EFS). In the patient population overall, there was no significant difference regarding this endpoint among those who received carboplatin and those who did not (EFS at 5 years, 66.4% vs. 59.2%).
However, there was a significant improvement among the patients in subgroup 3. Among those patients, EFS at 5 years was 73.2% with carboplatin versus 53.7% without (P = .047).
Similarly, in the overall group, there was no significant improvement in overall survival (OS) at 5 years from the addition of carboplatin (77.6% vs. 68.8% without carboplatin). However, the OS at 5 years varied widely between the different subtypes. There was again a significant improvement in OS at 5 years among the patients in subgroup 3 (82% with carboplation vs 63.7% without).
The beneficial effects from the addition of carboplatin on both endpoints were seen exclusively in patients in group 3, the authors emphasized.
“The WNT group does really well with less therapy, so if we treated all children the same, we would likely be overtreating WNT children and undertreating group 3 children,” Dr. Olson observed. “Genetic analysis is essential.”
In an earlier study, Dr. Olson and colleagues found that 70% of children with primitive neuroectodermal tumor of the CNS and pineoblastoma had been misdiagnosed even by outstanding children’s oncology centers because clinicians were relying on microscopic diagnosis.
“With molecular diagnosis, we were able to learn that many of these children had completely different diseases that require complexly different treatments, so doing diagnosis by molecular classification is absolutely essential,” he reemphasized.
“Glimmers of hope”
This study provides clinicians with “glimmers of hope” that children with high-risk, group 3 medulloblastoma will experience improvements in survival, wrote Allison Martin, MD, Albert Einstein College of Medicine, New York, and Sadhana Jackson, MD, National Institutes of Health, Bethesda, M.d., in an accompanying editorial.
The editorialists hope that “the treatment paradigm for all patients with high-risk disease can be improved through incorporation of detailed molecular analyses.”
However, they pointed out that DNA methylation and other advanced testing methods used to distinguish subgroups 3 and 4 in this study are not widely available, even at most Children’s Oncology Group member institutions. (Dr. Olson countered that, even if these sophisticated tests are not available at all pediatric oncology centers, tests will be performed if clinicians send tissue to the few sites that are equipped to conduct them.)
The editorialists also noted that therapy intensification with carboplatin is associated with an increased risk for adverse effects – “underscoring the importance of correctly identifying patients who could benefit from this intervention and avoid unnecessary toxic effects.”
The study was funded by the National Cancer Institute. Dr. Olson has disclosed no relevant financial relationships. Dr. Martin reported that she previously owed shares in Celgene, which she has subsequently sold.
A version of this article first appeared on Medscape.com.
A practice-changing study that used molecular testing to distinguish between subtypes of medulloblastoma has shown a significant improvement in survival for children with high-risk disease who underwent treatment intensification with carboplatin.
“Each of the four subgroups of medulloblastoma has a different prognosis, but for this particular subgroup, 20 fewer children out of every 100 would have survived prior to this study,” James Olson, MD, professor of medicine, French Hutchinson Cancer Research Center, University of Washington, Seattle, said in an interview.
“This is the reason for celebration – for now and forevermore, we can expect 20 more children with high-risk, group 3 medulloblastoma to survive,” he said.
“We recommend that all children with high-risk, group 3 medulloblastoma receive carboplatin and all children in the other subgroups do not, because we don’t want them to experience the toxicity without benefit,” Dr. Olson said.
The study was published online July 22, 2021, in JAMA Oncology.
Hematologic toxicity was more pronounced in the carboplatin arm in the induction phase of the protocol, and toxicity persisted into the first cycles of maintenance therapy. On the other hand, “there weren’t enough additional side effects to recommend children not get carboplatin if they would benefit from it,” Dr. Olson noted.
At least 75% of children with newly diagnosed medulloblastoma survive, although those with high-risk, group 3 disease have a substantially poorer prognosis than those with other molecular subtypes.
However, if a child with medulloblastoma experiences relapse, “the likelihood of survival is near zero, so it’s important to get it right the first time,” Dr. Olson said.
One of the patients who took part in this trial, Sammy Loch of Seattle, is now 27 years old and has been cancer free for 11 years.
She was diagnosed with medulloblastoma when in high school. At the time of her diagnosis, she was asked by her pediatric oncologist at Seattle Children’s Hospital about taking part in the study. After careful consideration, she agreed.
“Participating in research was my way to give back and pay it forward,” Ms. Loch said in a statement. “It’s really exciting to know more people will survive because of the research I was involved in,” she added. She continues to pay her debt forward, serving as a therapist for people with chronic health conditions and raising funds for pediatric cancer research.
Patients had high-risk features
The study involved 261 evaluable patients (median age, 8.6 years). All patients had high-risk features, including metastatic disease (72.4% of the group), diffuse anaplastic histologic characteristics (22.2%), and incomplete surgical resection (5.4%), defined as residual tumor greater than 1.5 cm2.
“All patients received 36 Gy craniospinal radiotherapy with boost to the posterior fossa of 55.8 Gy cumulative dose with conventional fractionation of 1.8 Gy/d,” Dr. Olson and colleagues explain. Patients also received six doses of vincristine 1.5 mg/m2 weekly during radiotherapy and were randomly assigned to receive carboplatin 35 mg/m2 for a total of 30 doses given daily prior to radiotherapy or placebo.
This regimen was followed by maintenance therapy, which consisted of six 28-day cycles of the combination of cisplatin 75 mg/m2 on day 1; cyclophosphamide 1,000 mg/m2 on days 2 and 3; and vincristine 1.5 mg/m2 on days 1 and 8.
Patients were originally assigned to receive an additional 12 cycles of isotretinoin or placebo, to be given during and after maintenance therapy. However, randomization to isotretinoin was discontinued early because of futility.
The study was initially powered to evaluate medulloblastoma as a single disease. However, as a result of biologic insights gained after the study was conceived, it was amended to include a molecular subgroup analysis to better distinguish patients who might truly benefit from intensified therapy, the authors explained.
Study results
The World Health Organization categorizes tumors of the central nervous system into four groups. The authors followed this system of categorization for their patients with medulloblastoma. The four groups are WNT, in which WNT signaling pathway is activated; SHH, in which the SHH signaling pathway is activated; with or without TP53 mutation (provisionally designated group 3); and non-WNT/non-SHH (provisionally designed group 4)
The primary endpoint of the trial was event-free survival (EFS). In the patient population overall, there was no significant difference regarding this endpoint among those who received carboplatin and those who did not (EFS at 5 years, 66.4% vs. 59.2%).
However, there was a significant improvement among the patients in subgroup 3. Among those patients, EFS at 5 years was 73.2% with carboplatin versus 53.7% without (P = .047).
Similarly, in the overall group, there was no significant improvement in overall survival (OS) at 5 years from the addition of carboplatin (77.6% vs. 68.8% without carboplatin). However, the OS at 5 years varied widely between the different subtypes. There was again a significant improvement in OS at 5 years among the patients in subgroup 3 (82% with carboplation vs 63.7% without).
The beneficial effects from the addition of carboplatin on both endpoints were seen exclusively in patients in group 3, the authors emphasized.
“The WNT group does really well with less therapy, so if we treated all children the same, we would likely be overtreating WNT children and undertreating group 3 children,” Dr. Olson observed. “Genetic analysis is essential.”
In an earlier study, Dr. Olson and colleagues found that 70% of children with primitive neuroectodermal tumor of the CNS and pineoblastoma had been misdiagnosed even by outstanding children’s oncology centers because clinicians were relying on microscopic diagnosis.
“With molecular diagnosis, we were able to learn that many of these children had completely different diseases that require complexly different treatments, so doing diagnosis by molecular classification is absolutely essential,” he reemphasized.
“Glimmers of hope”
This study provides clinicians with “glimmers of hope” that children with high-risk, group 3 medulloblastoma will experience improvements in survival, wrote Allison Martin, MD, Albert Einstein College of Medicine, New York, and Sadhana Jackson, MD, National Institutes of Health, Bethesda, M.d., in an accompanying editorial.
The editorialists hope that “the treatment paradigm for all patients with high-risk disease can be improved through incorporation of detailed molecular analyses.”
However, they pointed out that DNA methylation and other advanced testing methods used to distinguish subgroups 3 and 4 in this study are not widely available, even at most Children’s Oncology Group member institutions. (Dr. Olson countered that, even if these sophisticated tests are not available at all pediatric oncology centers, tests will be performed if clinicians send tissue to the few sites that are equipped to conduct them.)
The editorialists also noted that therapy intensification with carboplatin is associated with an increased risk for adverse effects – “underscoring the importance of correctly identifying patients who could benefit from this intervention and avoid unnecessary toxic effects.”
The study was funded by the National Cancer Institute. Dr. Olson has disclosed no relevant financial relationships. Dr. Martin reported that she previously owed shares in Celgene, which she has subsequently sold.
A version of this article first appeared on Medscape.com.
A practice-changing study that used molecular testing to distinguish between subtypes of medulloblastoma has shown a significant improvement in survival for children with high-risk disease who underwent treatment intensification with carboplatin.
“Each of the four subgroups of medulloblastoma has a different prognosis, but for this particular subgroup, 20 fewer children out of every 100 would have survived prior to this study,” James Olson, MD, professor of medicine, French Hutchinson Cancer Research Center, University of Washington, Seattle, said in an interview.
“This is the reason for celebration – for now and forevermore, we can expect 20 more children with high-risk, group 3 medulloblastoma to survive,” he said.
“We recommend that all children with high-risk, group 3 medulloblastoma receive carboplatin and all children in the other subgroups do not, because we don’t want them to experience the toxicity without benefit,” Dr. Olson said.
The study was published online July 22, 2021, in JAMA Oncology.
Hematologic toxicity was more pronounced in the carboplatin arm in the induction phase of the protocol, and toxicity persisted into the first cycles of maintenance therapy. On the other hand, “there weren’t enough additional side effects to recommend children not get carboplatin if they would benefit from it,” Dr. Olson noted.
At least 75% of children with newly diagnosed medulloblastoma survive, although those with high-risk, group 3 disease have a substantially poorer prognosis than those with other molecular subtypes.
However, if a child with medulloblastoma experiences relapse, “the likelihood of survival is near zero, so it’s important to get it right the first time,” Dr. Olson said.
One of the patients who took part in this trial, Sammy Loch of Seattle, is now 27 years old and has been cancer free for 11 years.
She was diagnosed with medulloblastoma when in high school. At the time of her diagnosis, she was asked by her pediatric oncologist at Seattle Children’s Hospital about taking part in the study. After careful consideration, she agreed.
“Participating in research was my way to give back and pay it forward,” Ms. Loch said in a statement. “It’s really exciting to know more people will survive because of the research I was involved in,” she added. She continues to pay her debt forward, serving as a therapist for people with chronic health conditions and raising funds for pediatric cancer research.
Patients had high-risk features
The study involved 261 evaluable patients (median age, 8.6 years). All patients had high-risk features, including metastatic disease (72.4% of the group), diffuse anaplastic histologic characteristics (22.2%), and incomplete surgical resection (5.4%), defined as residual tumor greater than 1.5 cm2.
“All patients received 36 Gy craniospinal radiotherapy with boost to the posterior fossa of 55.8 Gy cumulative dose with conventional fractionation of 1.8 Gy/d,” Dr. Olson and colleagues explain. Patients also received six doses of vincristine 1.5 mg/m2 weekly during radiotherapy and were randomly assigned to receive carboplatin 35 mg/m2 for a total of 30 doses given daily prior to radiotherapy or placebo.
This regimen was followed by maintenance therapy, which consisted of six 28-day cycles of the combination of cisplatin 75 mg/m2 on day 1; cyclophosphamide 1,000 mg/m2 on days 2 and 3; and vincristine 1.5 mg/m2 on days 1 and 8.
Patients were originally assigned to receive an additional 12 cycles of isotretinoin or placebo, to be given during and after maintenance therapy. However, randomization to isotretinoin was discontinued early because of futility.
The study was initially powered to evaluate medulloblastoma as a single disease. However, as a result of biologic insights gained after the study was conceived, it was amended to include a molecular subgroup analysis to better distinguish patients who might truly benefit from intensified therapy, the authors explained.
Study results
The World Health Organization categorizes tumors of the central nervous system into four groups. The authors followed this system of categorization for their patients with medulloblastoma. The four groups are WNT, in which WNT signaling pathway is activated; SHH, in which the SHH signaling pathway is activated; with or without TP53 mutation (provisionally designated group 3); and non-WNT/non-SHH (provisionally designed group 4)
The primary endpoint of the trial was event-free survival (EFS). In the patient population overall, there was no significant difference regarding this endpoint among those who received carboplatin and those who did not (EFS at 5 years, 66.4% vs. 59.2%).
However, there was a significant improvement among the patients in subgroup 3. Among those patients, EFS at 5 years was 73.2% with carboplatin versus 53.7% without (P = .047).
Similarly, in the overall group, there was no significant improvement in overall survival (OS) at 5 years from the addition of carboplatin (77.6% vs. 68.8% without carboplatin). However, the OS at 5 years varied widely between the different subtypes. There was again a significant improvement in OS at 5 years among the patients in subgroup 3 (82% with carboplation vs 63.7% without).
The beneficial effects from the addition of carboplatin on both endpoints were seen exclusively in patients in group 3, the authors emphasized.
“The WNT group does really well with less therapy, so if we treated all children the same, we would likely be overtreating WNT children and undertreating group 3 children,” Dr. Olson observed. “Genetic analysis is essential.”
In an earlier study, Dr. Olson and colleagues found that 70% of children with primitive neuroectodermal tumor of the CNS and pineoblastoma had been misdiagnosed even by outstanding children’s oncology centers because clinicians were relying on microscopic diagnosis.
“With molecular diagnosis, we were able to learn that many of these children had completely different diseases that require complexly different treatments, so doing diagnosis by molecular classification is absolutely essential,” he reemphasized.
“Glimmers of hope”
This study provides clinicians with “glimmers of hope” that children with high-risk, group 3 medulloblastoma will experience improvements in survival, wrote Allison Martin, MD, Albert Einstein College of Medicine, New York, and Sadhana Jackson, MD, National Institutes of Health, Bethesda, M.d., in an accompanying editorial.
The editorialists hope that “the treatment paradigm for all patients with high-risk disease can be improved through incorporation of detailed molecular analyses.”
However, they pointed out that DNA methylation and other advanced testing methods used to distinguish subgroups 3 and 4 in this study are not widely available, even at most Children’s Oncology Group member institutions. (Dr. Olson countered that, even if these sophisticated tests are not available at all pediatric oncology centers, tests will be performed if clinicians send tissue to the few sites that are equipped to conduct them.)
The editorialists also noted that therapy intensification with carboplatin is associated with an increased risk for adverse effects – “underscoring the importance of correctly identifying patients who could benefit from this intervention and avoid unnecessary toxic effects.”
The study was funded by the National Cancer Institute. Dr. Olson has disclosed no relevant financial relationships. Dr. Martin reported that she previously owed shares in Celgene, which she has subsequently sold.
A version of this article first appeared on Medscape.com.
Study estimates carbon footprint reduction of virtual isotretinoin visits
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
FROM PEDIATRIC DERMATOLOGY
Children and COVID: Vaccinations, new cases both rising
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.
the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.

the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.

the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
Nowhere to run and nowhere to hide
Not surprisingly, the pandemic has torn at the already fraying fabric of many families. Cooped up away from friends and the emotional relief valve of school, even children who had been relatively easy to manage in the past have posed disciplinary challenges beyond their parents’ abilities to cope.
In a recent study from the Parenting in Context Lab of the University of Michigan (“Child Discipline During the Covid-19 Pandemic,” Family Snapshots: Life During the Pandemic, American Academy of Pediatrics, June 8 2021) researchers found that one in six parents surveyed (n = 3,000 adults) admitted to spanking. Nearly half of the parents said that they had yelled at or threatened their children.
Five out of six parents reported using what the investigators described as less harsh “positive discipline measures.” Three-quarters of these parents used “explaining” as a strategy and nearly the same number used either time-outs or sent the children to their rooms.
Again, not surprisingly, parents who had experienced at least one adverse childhood experience (ACE) were more than twice as likely to spank. And parents who reported an episode of intimate partner violence (IPV) were more likely to resort to a harsh discipline strategy (yelling, threatening, or spanking).
Over my professional career I’ve spent a lot of time thinking about discipline and I have attempted to summarize my thoughts in a book titled, “How to Say No to Your Toddler” (Simon and Schuster, 2003), that has been published in four languages. Based on my observations, trying to explain to a misbehaving child the error of his ways is generally parental time not well spent. A well-structured time-out, preferably in a separate room with a door closed, is the most effective and safest discipline strategy.
However, as in all of my books, this advice on discipline was colored by the families in my practice and the audience for which I was writing, primarily middle class and upper middle class, reasonably affluent parents who buy books. These are usually folks who have homes in which children often have their own rooms, or where at least there are multiple rooms with doors – spaces to escape when tensions rise. Few of these parents have endured ACEs. Few have they experienced – nor have their children witnessed – IPV.
My advice that parents make only threats that can be safely carried, out such as time-out, and to always follow up on threats and promises, is valid regardless of a family’s socioeconomic situation. However, when it comes to choosing a consequence, my standard recommendation of a time-out can be difficult to follow for a family of six living in a three-room apartment, particularly during pandemic-dictated restrictions and lockdowns.
Of course there are alternatives to time-outs in a separate space, including an extended hug in a parental lap, but these responses require that the parents have been able to compose themselves well enough, and that they have the time. One of the important benefits of time-outs is that they can provide parents the time and space to reassess the situation and consider their role in the conflict. The bottom line is that a time-out is the safest and most effective form of discipline, but it requires space and a parent relatively unburdened of financial or emotional stress. Families without these luxuries are left with few alternatives other than physical or verbal abuse.
The AAP’s Family Snapshot concludes with the observation that “pediatricians and pediatric health care providers can continue to play an important role in supporting positive discipline strategies.” That is a difficult assignment even in prepandemic times, but for those of you working with families who lack the space and time to defuse disciplinary tensions, it is a heroic task.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Not surprisingly, the pandemic has torn at the already fraying fabric of many families. Cooped up away from friends and the emotional relief valve of school, even children who had been relatively easy to manage in the past have posed disciplinary challenges beyond their parents’ abilities to cope.
In a recent study from the Parenting in Context Lab of the University of Michigan (“Child Discipline During the Covid-19 Pandemic,” Family Snapshots: Life During the Pandemic, American Academy of Pediatrics, June 8 2021) researchers found that one in six parents surveyed (n = 3,000 adults) admitted to spanking. Nearly half of the parents said that they had yelled at or threatened their children.
Five out of six parents reported using what the investigators described as less harsh “positive discipline measures.” Three-quarters of these parents used “explaining” as a strategy and nearly the same number used either time-outs or sent the children to their rooms.
Again, not surprisingly, parents who had experienced at least one adverse childhood experience (ACE) were more than twice as likely to spank. And parents who reported an episode of intimate partner violence (IPV) were more likely to resort to a harsh discipline strategy (yelling, threatening, or spanking).
Over my professional career I’ve spent a lot of time thinking about discipline and I have attempted to summarize my thoughts in a book titled, “How to Say No to Your Toddler” (Simon and Schuster, 2003), that has been published in four languages. Based on my observations, trying to explain to a misbehaving child the error of his ways is generally parental time not well spent. A well-structured time-out, preferably in a separate room with a door closed, is the most effective and safest discipline strategy.
However, as in all of my books, this advice on discipline was colored by the families in my practice and the audience for which I was writing, primarily middle class and upper middle class, reasonably affluent parents who buy books. These are usually folks who have homes in which children often have their own rooms, or where at least there are multiple rooms with doors – spaces to escape when tensions rise. Few of these parents have endured ACEs. Few have they experienced – nor have their children witnessed – IPV.
My advice that parents make only threats that can be safely carried, out such as time-out, and to always follow up on threats and promises, is valid regardless of a family’s socioeconomic situation. However, when it comes to choosing a consequence, my standard recommendation of a time-out can be difficult to follow for a family of six living in a three-room apartment, particularly during pandemic-dictated restrictions and lockdowns.
Of course there are alternatives to time-outs in a separate space, including an extended hug in a parental lap, but these responses require that the parents have been able to compose themselves well enough, and that they have the time. One of the important benefits of time-outs is that they can provide parents the time and space to reassess the situation and consider their role in the conflict. The bottom line is that a time-out is the safest and most effective form of discipline, but it requires space and a parent relatively unburdened of financial or emotional stress. Families without these luxuries are left with few alternatives other than physical or verbal abuse.
The AAP’s Family Snapshot concludes with the observation that “pediatricians and pediatric health care providers can continue to play an important role in supporting positive discipline strategies.” That is a difficult assignment even in prepandemic times, but for those of you working with families who lack the space and time to defuse disciplinary tensions, it is a heroic task.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Not surprisingly, the pandemic has torn at the already fraying fabric of many families. Cooped up away from friends and the emotional relief valve of school, even children who had been relatively easy to manage in the past have posed disciplinary challenges beyond their parents’ abilities to cope.
In a recent study from the Parenting in Context Lab of the University of Michigan (“Child Discipline During the Covid-19 Pandemic,” Family Snapshots: Life During the Pandemic, American Academy of Pediatrics, June 8 2021) researchers found that one in six parents surveyed (n = 3,000 adults) admitted to spanking. Nearly half of the parents said that they had yelled at or threatened their children.
Five out of six parents reported using what the investigators described as less harsh “positive discipline measures.” Three-quarters of these parents used “explaining” as a strategy and nearly the same number used either time-outs or sent the children to their rooms.
Again, not surprisingly, parents who had experienced at least one adverse childhood experience (ACE) were more than twice as likely to spank. And parents who reported an episode of intimate partner violence (IPV) were more likely to resort to a harsh discipline strategy (yelling, threatening, or spanking).
Over my professional career I’ve spent a lot of time thinking about discipline and I have attempted to summarize my thoughts in a book titled, “How to Say No to Your Toddler” (Simon and Schuster, 2003), that has been published in four languages. Based on my observations, trying to explain to a misbehaving child the error of his ways is generally parental time not well spent. A well-structured time-out, preferably in a separate room with a door closed, is the most effective and safest discipline strategy.
However, as in all of my books, this advice on discipline was colored by the families in my practice and the audience for which I was writing, primarily middle class and upper middle class, reasonably affluent parents who buy books. These are usually folks who have homes in which children often have their own rooms, or where at least there are multiple rooms with doors – spaces to escape when tensions rise. Few of these parents have endured ACEs. Few have they experienced – nor have their children witnessed – IPV.
My advice that parents make only threats that can be safely carried, out such as time-out, and to always follow up on threats and promises, is valid regardless of a family’s socioeconomic situation. However, when it comes to choosing a consequence, my standard recommendation of a time-out can be difficult to follow for a family of six living in a three-room apartment, particularly during pandemic-dictated restrictions and lockdowns.
Of course there are alternatives to time-outs in a separate space, including an extended hug in a parental lap, but these responses require that the parents have been able to compose themselves well enough, and that they have the time. One of the important benefits of time-outs is that they can provide parents the time and space to reassess the situation and consider their role in the conflict. The bottom line is that a time-out is the safest and most effective form of discipline, but it requires space and a parent relatively unburdened of financial or emotional stress. Families without these luxuries are left with few alternatives other than physical or verbal abuse.
The AAP’s Family Snapshot concludes with the observation that “pediatricians and pediatric health care providers can continue to play an important role in supporting positive discipline strategies.” That is a difficult assignment even in prepandemic times, but for those of you working with families who lack the space and time to defuse disciplinary tensions, it is a heroic task.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Genetic testing for neurofibromatosis 1: An imperfect science
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
FROM SPD 2021
Childhood deprivation affects later executive function
Exposure to deprivation in early life was significantly associated with impaired executive functioning in children and adolescents, based on data from a systematic review and meta-analysis of 91 studies.
Previous research has shown connections between early-life adversity (ELA) and changes in psychological, cognitive, and neurobiological development, including increased risk of anxiety, depression, attention-deficit/hyperactivity disorder, conduct disorder, suicidality, and substance use disorder; however, research focusing on the associations between different types of ELA and specific processes is limited, wrote Dylan Johnson, MSc, of the University of Toronto and colleagues.
“We directly addressed this gap in the literature by examining the association between the type of ELA and executive functioning in children and youth,” they said.
In a study published in JAMA Pediatrics, the researchers identified 91 articles including 82 unique cohorts and 31,188 unique individuals aged 1-18 years.
The articles were selected from Embase, ERIC, MEDLINE, and PsycInfo databases and published up to Dec. 31, 2020. The primary outcomes were measures of the three domains of executive functioning: cognitive flexibility, inhibitory control, and working memory. To correct for small sample sizes in some studies, the researchers standardized their measures of association into Hedges g effect sizes.
Overall, the pooled estimates of the association of any childhood adversity with the three domains of executive functioning showed significant heterogeneity, with Hedges g effects of –0.49 for cognitive flexibility, –0.39 for inhibitory control, and –0.47 for working memory.
The researchers also examined a subsample of ELA–executive functioning associations in categories of early-life exposure to threat, compared with early-life deprivation, including 56 of the original 91 articles. In this analysis, significantly lower inhibitory control was associated with deprivation compared to threat (Hedges g –0.43 vs. –0.27). Similarly, significantly lower working memory was associated with deprivation, compared with threat (Hedges g –0.54 vs. Hedges g –0.28). For both inhibitory control and working memory, the association of adversity was not moderated by the age or sex of the study participants, study design, outcome quality, or selection quality, the researchers noted.
No significant difference in affect of exposure threat vs. deprivation was noted for the association with cognitive flexibility. The reason for this discrepancy remains unclear, the researchers said. “Some evidence suggests that individuals who grow up in unpredictable environments may have reduced inhibitory control but enhanced cognitive flexibility,” they noted.
However, the overall results suggest that exposure to deprivation may be associated with neurodevelopmental changes that support the development of executive functioning, they said.
The study findings were limited by several factors, including the substantial heterogeneity in the pooled estimates and the need to consider variation in study design, the researchers noted. In addition, the cross-sectional design of many studies prevented conclusions about causality between ELA and executive functioning, they said.
“Future research should explore the differences between threat and deprivation when emotionally salient executive functioning measures are used,” the researchers emphasized. “Threat experiences are often associated with alterations in emotional processing, and different findings may be observed when investigating emotionally salient executive functioning outcomes,” they concluded.
Prevention and intervention plans needed
“Although numerous studies have examined associations between ELA and executive functioning, the associations of threat and deprivation with specific executive functioning domains (e.g., cognitive flexibility, inhibitory control, and working memory) have not been explored comprehensively,” wrote Beth S. Slomine, PhD, and Nikeea Copeland-Linder, PhD, of the Kennedy Krieger Institute, Johns Hopkins University School, Baltimore, in an accompanying editorial.
The study is “critical and timely” because of the impact of the COVID-19 pandemic on children’s exposure to deprivation, the authors said. “Many children have experienced the death of family members or friends, food and housing insecurity owing to the economic recession, school closures, loss of critical support services, and increased isolation because of social distancing measures,” and these effects are even greater for children already living in poverty and those with developmental disabilities, they noted.
More resources are needed to develop and implement ELA prevention policies, as well as early intervention plans, the editorialists said.
“Early intervention programs have a great potential to reduce the risk of ELA and promote executive functioning development,” they said. “These programs, such as family support and preschool services, are viable solutions for children and their families,” they added. Although the pandemic prevented the use of many support services for children at risk, the adoption of telehealth technology means that “it is now more feasible for cognitive rehabilitation experts to implement the telehealth technology to train parents and school staff on how to assist with the delivery of interventions in real-world settings and how to promote executive functioning in daily life,” they noted.
Overall, the study findings highlight the urgency of identifying ELA and implementing strategies to reduce and prevent ELA, and to provide early intervention to mitigate the impact of ELA on executive function in children, the editorialists emphasized.
Data bring understanding, but barriers remain
“At this point, there are data demonstrating the significant impact that adverse childhood experiences have on health outcomes – from worsened mental health to an increased risk for cancer and diabetes,” said Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, in an interview.
“Physicians – myself included – tend to lump all these experiences together when thinking about future health outcomes,” Dr. Curran said. “However, there are evolving data that neurocognitive outcomes may be different based on the type of early-life adversity experienced. This meta-analysis examines the risk of different neurocognitive impact of threat versus deprivation types of adversity, which is important to pediatricians because it helps us to better understand the risks that our patients may experience,” she explained.
“The results of this meta-analysis were especially intriguing because I hadn’t previously considered the impact that different types of adversity had on neurocognitive development,” said Dr. Curran. “This study caused me to think about these experiences differently, and as I reflect on the patients I have cared for over the years, I can see the difference in their outcomes,” she said.
Many barriers persist in addressing the effects of early-life deprivation on executive function, Dr. Curran said.
“First are barriers around identification of these children and adolescents, who may not have regular contact with the medical system. Additionally, it’s important to provide resources for parents and caregivers – this includes creating a strong support network and providing education about the impact of these experiences,” she noted. “There are also barriers to identifying and connecting with what resources will help children at risk of poor neurodevelopmental outcomes,” she added.
“Now that we know that children who have experienced early-life deprivation are at increased risk of worsened neurodevelopmental outcomes, it will be important to understand what interventions can help improve their outcomes,” Dr. Curran said.
The study was supported by a Connaught New Researcher Award from the University of Toronto. The researchers had no financial conflicts to disclose.
Dr. Slomine disclosed book royalties from Cambridge University Press unrelated to this study. Dr. Curran had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Exposure to deprivation in early life was significantly associated with impaired executive functioning in children and adolescents, based on data from a systematic review and meta-analysis of 91 studies.
Previous research has shown connections between early-life adversity (ELA) and changes in psychological, cognitive, and neurobiological development, including increased risk of anxiety, depression, attention-deficit/hyperactivity disorder, conduct disorder, suicidality, and substance use disorder; however, research focusing on the associations between different types of ELA and specific processes is limited, wrote Dylan Johnson, MSc, of the University of Toronto and colleagues.
“We directly addressed this gap in the literature by examining the association between the type of ELA and executive functioning in children and youth,” they said.
In a study published in JAMA Pediatrics, the researchers identified 91 articles including 82 unique cohorts and 31,188 unique individuals aged 1-18 years.
The articles were selected from Embase, ERIC, MEDLINE, and PsycInfo databases and published up to Dec. 31, 2020. The primary outcomes were measures of the three domains of executive functioning: cognitive flexibility, inhibitory control, and working memory. To correct for small sample sizes in some studies, the researchers standardized their measures of association into Hedges g effect sizes.
Overall, the pooled estimates of the association of any childhood adversity with the three domains of executive functioning showed significant heterogeneity, with Hedges g effects of –0.49 for cognitive flexibility, –0.39 for inhibitory control, and –0.47 for working memory.
The researchers also examined a subsample of ELA–executive functioning associations in categories of early-life exposure to threat, compared with early-life deprivation, including 56 of the original 91 articles. In this analysis, significantly lower inhibitory control was associated with deprivation compared to threat (Hedges g –0.43 vs. –0.27). Similarly, significantly lower working memory was associated with deprivation, compared with threat (Hedges g –0.54 vs. Hedges g –0.28). For both inhibitory control and working memory, the association of adversity was not moderated by the age or sex of the study participants, study design, outcome quality, or selection quality, the researchers noted.
No significant difference in affect of exposure threat vs. deprivation was noted for the association with cognitive flexibility. The reason for this discrepancy remains unclear, the researchers said. “Some evidence suggests that individuals who grow up in unpredictable environments may have reduced inhibitory control but enhanced cognitive flexibility,” they noted.
However, the overall results suggest that exposure to deprivation may be associated with neurodevelopmental changes that support the development of executive functioning, they said.
The study findings were limited by several factors, including the substantial heterogeneity in the pooled estimates and the need to consider variation in study design, the researchers noted. In addition, the cross-sectional design of many studies prevented conclusions about causality between ELA and executive functioning, they said.
“Future research should explore the differences between threat and deprivation when emotionally salient executive functioning measures are used,” the researchers emphasized. “Threat experiences are often associated with alterations in emotional processing, and different findings may be observed when investigating emotionally salient executive functioning outcomes,” they concluded.
Prevention and intervention plans needed
“Although numerous studies have examined associations between ELA and executive functioning, the associations of threat and deprivation with specific executive functioning domains (e.g., cognitive flexibility, inhibitory control, and working memory) have not been explored comprehensively,” wrote Beth S. Slomine, PhD, and Nikeea Copeland-Linder, PhD, of the Kennedy Krieger Institute, Johns Hopkins University School, Baltimore, in an accompanying editorial.
The study is “critical and timely” because of the impact of the COVID-19 pandemic on children’s exposure to deprivation, the authors said. “Many children have experienced the death of family members or friends, food and housing insecurity owing to the economic recession, school closures, loss of critical support services, and increased isolation because of social distancing measures,” and these effects are even greater for children already living in poverty and those with developmental disabilities, they noted.
More resources are needed to develop and implement ELA prevention policies, as well as early intervention plans, the editorialists said.
“Early intervention programs have a great potential to reduce the risk of ELA and promote executive functioning development,” they said. “These programs, such as family support and preschool services, are viable solutions for children and their families,” they added. Although the pandemic prevented the use of many support services for children at risk, the adoption of telehealth technology means that “it is now more feasible for cognitive rehabilitation experts to implement the telehealth technology to train parents and school staff on how to assist with the delivery of interventions in real-world settings and how to promote executive functioning in daily life,” they noted.
Overall, the study findings highlight the urgency of identifying ELA and implementing strategies to reduce and prevent ELA, and to provide early intervention to mitigate the impact of ELA on executive function in children, the editorialists emphasized.
Data bring understanding, but barriers remain
“At this point, there are data demonstrating the significant impact that adverse childhood experiences have on health outcomes – from worsened mental health to an increased risk for cancer and diabetes,” said Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, in an interview.
“Physicians – myself included – tend to lump all these experiences together when thinking about future health outcomes,” Dr. Curran said. “However, there are evolving data that neurocognitive outcomes may be different based on the type of early-life adversity experienced. This meta-analysis examines the risk of different neurocognitive impact of threat versus deprivation types of adversity, which is important to pediatricians because it helps us to better understand the risks that our patients may experience,” she explained.
“The results of this meta-analysis were especially intriguing because I hadn’t previously considered the impact that different types of adversity had on neurocognitive development,” said Dr. Curran. “This study caused me to think about these experiences differently, and as I reflect on the patients I have cared for over the years, I can see the difference in their outcomes,” she said.
Many barriers persist in addressing the effects of early-life deprivation on executive function, Dr. Curran said.
“First are barriers around identification of these children and adolescents, who may not have regular contact with the medical system. Additionally, it’s important to provide resources for parents and caregivers – this includes creating a strong support network and providing education about the impact of these experiences,” she noted. “There are also barriers to identifying and connecting with what resources will help children at risk of poor neurodevelopmental outcomes,” she added.
“Now that we know that children who have experienced early-life deprivation are at increased risk of worsened neurodevelopmental outcomes, it will be important to understand what interventions can help improve their outcomes,” Dr. Curran said.
The study was supported by a Connaught New Researcher Award from the University of Toronto. The researchers had no financial conflicts to disclose.
Dr. Slomine disclosed book royalties from Cambridge University Press unrelated to this study. Dr. Curran had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Exposure to deprivation in early life was significantly associated with impaired executive functioning in children and adolescents, based on data from a systematic review and meta-analysis of 91 studies.
Previous research has shown connections between early-life adversity (ELA) and changes in psychological, cognitive, and neurobiological development, including increased risk of anxiety, depression, attention-deficit/hyperactivity disorder, conduct disorder, suicidality, and substance use disorder; however, research focusing on the associations between different types of ELA and specific processes is limited, wrote Dylan Johnson, MSc, of the University of Toronto and colleagues.
“We directly addressed this gap in the literature by examining the association between the type of ELA and executive functioning in children and youth,” they said.
In a study published in JAMA Pediatrics, the researchers identified 91 articles including 82 unique cohorts and 31,188 unique individuals aged 1-18 years.
The articles were selected from Embase, ERIC, MEDLINE, and PsycInfo databases and published up to Dec. 31, 2020. The primary outcomes were measures of the three domains of executive functioning: cognitive flexibility, inhibitory control, and working memory. To correct for small sample sizes in some studies, the researchers standardized their measures of association into Hedges g effect sizes.
Overall, the pooled estimates of the association of any childhood adversity with the three domains of executive functioning showed significant heterogeneity, with Hedges g effects of –0.49 for cognitive flexibility, –0.39 for inhibitory control, and –0.47 for working memory.
The researchers also examined a subsample of ELA–executive functioning associations in categories of early-life exposure to threat, compared with early-life deprivation, including 56 of the original 91 articles. In this analysis, significantly lower inhibitory control was associated with deprivation compared to threat (Hedges g –0.43 vs. –0.27). Similarly, significantly lower working memory was associated with deprivation, compared with threat (Hedges g –0.54 vs. Hedges g –0.28). For both inhibitory control and working memory, the association of adversity was not moderated by the age or sex of the study participants, study design, outcome quality, or selection quality, the researchers noted.
No significant difference in affect of exposure threat vs. deprivation was noted for the association with cognitive flexibility. The reason for this discrepancy remains unclear, the researchers said. “Some evidence suggests that individuals who grow up in unpredictable environments may have reduced inhibitory control but enhanced cognitive flexibility,” they noted.
However, the overall results suggest that exposure to deprivation may be associated with neurodevelopmental changes that support the development of executive functioning, they said.
The study findings were limited by several factors, including the substantial heterogeneity in the pooled estimates and the need to consider variation in study design, the researchers noted. In addition, the cross-sectional design of many studies prevented conclusions about causality between ELA and executive functioning, they said.
“Future research should explore the differences between threat and deprivation when emotionally salient executive functioning measures are used,” the researchers emphasized. “Threat experiences are often associated with alterations in emotional processing, and different findings may be observed when investigating emotionally salient executive functioning outcomes,” they concluded.
Prevention and intervention plans needed
“Although numerous studies have examined associations between ELA and executive functioning, the associations of threat and deprivation with specific executive functioning domains (e.g., cognitive flexibility, inhibitory control, and working memory) have not been explored comprehensively,” wrote Beth S. Slomine, PhD, and Nikeea Copeland-Linder, PhD, of the Kennedy Krieger Institute, Johns Hopkins University School, Baltimore, in an accompanying editorial.
The study is “critical and timely” because of the impact of the COVID-19 pandemic on children’s exposure to deprivation, the authors said. “Many children have experienced the death of family members or friends, food and housing insecurity owing to the economic recession, school closures, loss of critical support services, and increased isolation because of social distancing measures,” and these effects are even greater for children already living in poverty and those with developmental disabilities, they noted.
More resources are needed to develop and implement ELA prevention policies, as well as early intervention plans, the editorialists said.
“Early intervention programs have a great potential to reduce the risk of ELA and promote executive functioning development,” they said. “These programs, such as family support and preschool services, are viable solutions for children and their families,” they added. Although the pandemic prevented the use of many support services for children at risk, the adoption of telehealth technology means that “it is now more feasible for cognitive rehabilitation experts to implement the telehealth technology to train parents and school staff on how to assist with the delivery of interventions in real-world settings and how to promote executive functioning in daily life,” they noted.
Overall, the study findings highlight the urgency of identifying ELA and implementing strategies to reduce and prevent ELA, and to provide early intervention to mitigate the impact of ELA on executive function in children, the editorialists emphasized.
Data bring understanding, but barriers remain
“At this point, there are data demonstrating the significant impact that adverse childhood experiences have on health outcomes – from worsened mental health to an increased risk for cancer and diabetes,” said Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, in an interview.
“Physicians – myself included – tend to lump all these experiences together when thinking about future health outcomes,” Dr. Curran said. “However, there are evolving data that neurocognitive outcomes may be different based on the type of early-life adversity experienced. This meta-analysis examines the risk of different neurocognitive impact of threat versus deprivation types of adversity, which is important to pediatricians because it helps us to better understand the risks that our patients may experience,” she explained.
“The results of this meta-analysis were especially intriguing because I hadn’t previously considered the impact that different types of adversity had on neurocognitive development,” said Dr. Curran. “This study caused me to think about these experiences differently, and as I reflect on the patients I have cared for over the years, I can see the difference in their outcomes,” she said.
Many barriers persist in addressing the effects of early-life deprivation on executive function, Dr. Curran said.
“First are barriers around identification of these children and adolescents, who may not have regular contact with the medical system. Additionally, it’s important to provide resources for parents and caregivers – this includes creating a strong support network and providing education about the impact of these experiences,” she noted. “There are also barriers to identifying and connecting with what resources will help children at risk of poor neurodevelopmental outcomes,” she added.
“Now that we know that children who have experienced early-life deprivation are at increased risk of worsened neurodevelopmental outcomes, it will be important to understand what interventions can help improve their outcomes,” Dr. Curran said.
The study was supported by a Connaught New Researcher Award from the University of Toronto. The researchers had no financial conflicts to disclose.
Dr. Slomine disclosed book royalties from Cambridge University Press unrelated to this study. Dr. Curran had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
FROM JAMA PEDIATRICS
No link between childhood vaccinations and allergies or asthma
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.



