User login
Acne stigma persists across social and professional settings
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
FROM JAMA DERMATOLOGY
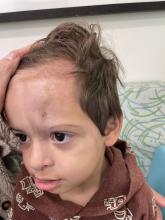
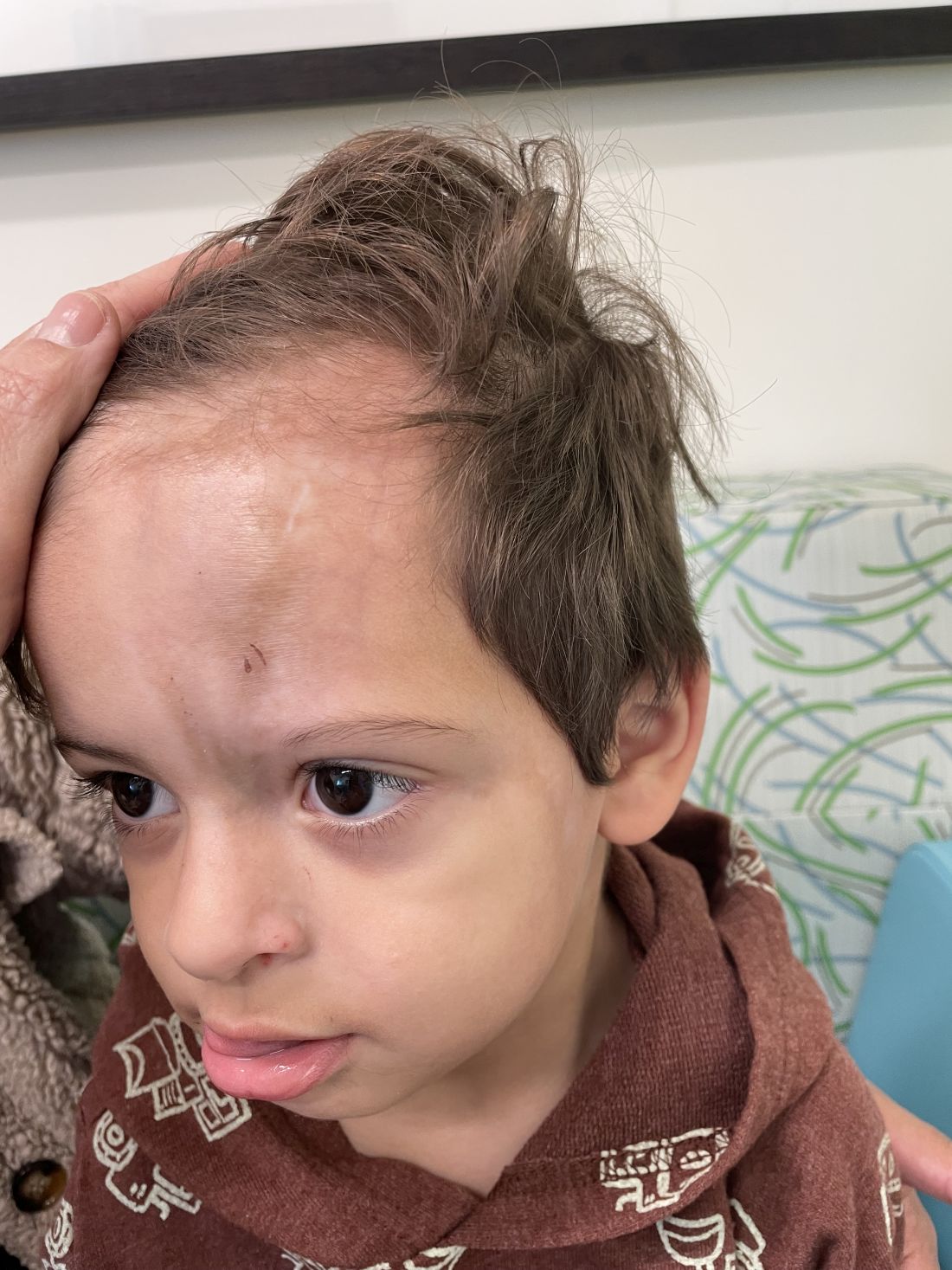
An 18-month-old male presents with a red mark on the forehead and nose
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
On examination, a faint pink patch was observed on the right forehead, frontal scalp, and nose. The lesion paled under pressure, with small areas of hair loss on the scalp. No atrophy was noted.
Just gas? New study on colic suggests some longer-term implications
Pediatricians commonly are asked to see infants presenting with symptoms of colic. The frequent and intense crying associated with colic is understandably quite distressing to parents, who often worry about a serious underlying medical cause. There also is the stress of trying to soothe an irritable infant who often does not seem to respond to the typical interventions.
Conventional wisdom about colic has been that the behaviors are the result of some gastrointestinal problem that, while not perfectly understood, tends to be mercifully self-limited and not predictive of future medical or mental health problems. This perspective then leads to pediatricians typically offering mainly sympathy and reassurance at these visits.
A new study,1 however, challenges some of this traditional thinking. The data come from a remarkable longitudinal study called the Generation R Study (R being Rotterdam in the Netherlands) that has prospectively studied a group of nearly 5,000 children from before birth into adolescence. Colic symptoms were briefly assessed when infants were about 3 months old and emotional-behavioral problems have been prospectively measured at multiple time points subsequently using well-validated rating scales.
The main finding of the study was and, to a lesser extent, in adolescence. This held for both internalizing problems (like anxiety and depressive symptoms) and externalizing problems (like defiance and aggressive behavior). At age 10, participants also underwent an MRI scan and those who were excessive criers as infants were found to have a smaller amygdala, a region known for being important in regulating emotions.
The authors concluded that colicky behavior in infancy may reflect some underlying temperamental vulnerabilities and may have more predictive value than previously thought. The connection between excessive crying and a measurable brain region difference later in life is also interesting, although these kinds of brain imaging findings have been notoriously difficult to interpret clinically.
Overall, this is a solid study that deserves to be considered. Colic may reflect a bit more than most of us have been taught and shouldn’t necessarily be “shrugged off,” as the authors state in their discussion.
At the same time, however, it is important not to overinterpret the findings. The magnitude of the effects were on the small side (about 0.2 of a standard deviation) and most children with excessive crying in early infancy did not manifest high levels of mental health problems later in life. The mothers of high crying infants also had slightly higher levels of mental health problems themselves so there could be other mechanisms at work here, such as genetic differences between the two groups.
So how could a pediatrician best use this new information without taking things too far? Regardless of the question of whether the excessive crying infancy is a true risk factor for later behavior problems (in the causal sense) or whether it represents more of a marker for something else, its presence so early in life offers an opportunity. Primary care clinicians would still likely want to provide the reassurance that has typically been given in these visits but perhaps with the caveat that some of these kids go on to struggle a bit more with mental health and that they might benefit from some additional support. We are not talking about prophylactic medications here, but something like additional parenting skills. Especially if you, as the pediatrician, suspect that the parents might benefit from expanding their parenting toolkit already, here is a nice opportunity to invite them to learn some new approaches and skills — framed in a way that focuses on the temperament of the child rather than any “deficits” you perceive in the parents. Some parents may be more receptive and less defensive to the idea of participating in parent training under the framework that they are doing this because they have a temperamentally more challenging child (rather than feeling that they are deficient in basic parenting skills).
It’s always a good idea to know about what resources are available in the community when it comes to teaching parenting skills. In addition to scientifically supported books and podcasts, there has been a steady increase in reliable websites, apps, and other digital platforms related to parenting, as well as standard in-person groups and classes. This could also be a great use of an integrated behavioral health professional for practices fortunate enough to have one.
In summary, there is some new evidence that colic can represent a little more than “just gas,” and while we shouldn’t take this one study to the extreme, there may be some good opportunities here to discuss and support good parenting practices in general.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. His latest book is “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.” You can follow him on Twitter and Facebook @PediPsych.
Reference
1. Sammallahti S et al. Excessive crying, behavior problems, and amygdala volume: A study from infancy to adolescence. J Am Acad Child Adolesc Psychiatry. 2023 Jun;62(6):675-83. doi: 10.1016/j.jaac.2023.01.014.
Pediatricians commonly are asked to see infants presenting with symptoms of colic. The frequent and intense crying associated with colic is understandably quite distressing to parents, who often worry about a serious underlying medical cause. There also is the stress of trying to soothe an irritable infant who often does not seem to respond to the typical interventions.
Conventional wisdom about colic has been that the behaviors are the result of some gastrointestinal problem that, while not perfectly understood, tends to be mercifully self-limited and not predictive of future medical or mental health problems. This perspective then leads to pediatricians typically offering mainly sympathy and reassurance at these visits.
A new study,1 however, challenges some of this traditional thinking. The data come from a remarkable longitudinal study called the Generation R Study (R being Rotterdam in the Netherlands) that has prospectively studied a group of nearly 5,000 children from before birth into adolescence. Colic symptoms were briefly assessed when infants were about 3 months old and emotional-behavioral problems have been prospectively measured at multiple time points subsequently using well-validated rating scales.
The main finding of the study was and, to a lesser extent, in adolescence. This held for both internalizing problems (like anxiety and depressive symptoms) and externalizing problems (like defiance and aggressive behavior). At age 10, participants also underwent an MRI scan and those who were excessive criers as infants were found to have a smaller amygdala, a region known for being important in regulating emotions.
The authors concluded that colicky behavior in infancy may reflect some underlying temperamental vulnerabilities and may have more predictive value than previously thought. The connection between excessive crying and a measurable brain region difference later in life is also interesting, although these kinds of brain imaging findings have been notoriously difficult to interpret clinically.
Overall, this is a solid study that deserves to be considered. Colic may reflect a bit more than most of us have been taught and shouldn’t necessarily be “shrugged off,” as the authors state in their discussion.
At the same time, however, it is important not to overinterpret the findings. The magnitude of the effects were on the small side (about 0.2 of a standard deviation) and most children with excessive crying in early infancy did not manifest high levels of mental health problems later in life. The mothers of high crying infants also had slightly higher levels of mental health problems themselves so there could be other mechanisms at work here, such as genetic differences between the two groups.
So how could a pediatrician best use this new information without taking things too far? Regardless of the question of whether the excessive crying infancy is a true risk factor for later behavior problems (in the causal sense) or whether it represents more of a marker for something else, its presence so early in life offers an opportunity. Primary care clinicians would still likely want to provide the reassurance that has typically been given in these visits but perhaps with the caveat that some of these kids go on to struggle a bit more with mental health and that they might benefit from some additional support. We are not talking about prophylactic medications here, but something like additional parenting skills. Especially if you, as the pediatrician, suspect that the parents might benefit from expanding their parenting toolkit already, here is a nice opportunity to invite them to learn some new approaches and skills — framed in a way that focuses on the temperament of the child rather than any “deficits” you perceive in the parents. Some parents may be more receptive and less defensive to the idea of participating in parent training under the framework that they are doing this because they have a temperamentally more challenging child (rather than feeling that they are deficient in basic parenting skills).
It’s always a good idea to know about what resources are available in the community when it comes to teaching parenting skills. In addition to scientifically supported books and podcasts, there has been a steady increase in reliable websites, apps, and other digital platforms related to parenting, as well as standard in-person groups and classes. This could also be a great use of an integrated behavioral health professional for practices fortunate enough to have one.
In summary, there is some new evidence that colic can represent a little more than “just gas,” and while we shouldn’t take this one study to the extreme, there may be some good opportunities here to discuss and support good parenting practices in general.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. His latest book is “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.” You can follow him on Twitter and Facebook @PediPsych.
Reference
1. Sammallahti S et al. Excessive crying, behavior problems, and amygdala volume: A study from infancy to adolescence. J Am Acad Child Adolesc Psychiatry. 2023 Jun;62(6):675-83. doi: 10.1016/j.jaac.2023.01.014.
Pediatricians commonly are asked to see infants presenting with symptoms of colic. The frequent and intense crying associated with colic is understandably quite distressing to parents, who often worry about a serious underlying medical cause. There also is the stress of trying to soothe an irritable infant who often does not seem to respond to the typical interventions.
Conventional wisdom about colic has been that the behaviors are the result of some gastrointestinal problem that, while not perfectly understood, tends to be mercifully self-limited and not predictive of future medical or mental health problems. This perspective then leads to pediatricians typically offering mainly sympathy and reassurance at these visits.
A new study,1 however, challenges some of this traditional thinking. The data come from a remarkable longitudinal study called the Generation R Study (R being Rotterdam in the Netherlands) that has prospectively studied a group of nearly 5,000 children from before birth into adolescence. Colic symptoms were briefly assessed when infants were about 3 months old and emotional-behavioral problems have been prospectively measured at multiple time points subsequently using well-validated rating scales.
The main finding of the study was and, to a lesser extent, in adolescence. This held for both internalizing problems (like anxiety and depressive symptoms) and externalizing problems (like defiance and aggressive behavior). At age 10, participants also underwent an MRI scan and those who were excessive criers as infants were found to have a smaller amygdala, a region known for being important in regulating emotions.
The authors concluded that colicky behavior in infancy may reflect some underlying temperamental vulnerabilities and may have more predictive value than previously thought. The connection between excessive crying and a measurable brain region difference later in life is also interesting, although these kinds of brain imaging findings have been notoriously difficult to interpret clinically.
Overall, this is a solid study that deserves to be considered. Colic may reflect a bit more than most of us have been taught and shouldn’t necessarily be “shrugged off,” as the authors state in their discussion.
At the same time, however, it is important not to overinterpret the findings. The magnitude of the effects were on the small side (about 0.2 of a standard deviation) and most children with excessive crying in early infancy did not manifest high levels of mental health problems later in life. The mothers of high crying infants also had slightly higher levels of mental health problems themselves so there could be other mechanisms at work here, such as genetic differences between the two groups.
So how could a pediatrician best use this new information without taking things too far? Regardless of the question of whether the excessive crying infancy is a true risk factor for later behavior problems (in the causal sense) or whether it represents more of a marker for something else, its presence so early in life offers an opportunity. Primary care clinicians would still likely want to provide the reassurance that has typically been given in these visits but perhaps with the caveat that some of these kids go on to struggle a bit more with mental health and that they might benefit from some additional support. We are not talking about prophylactic medications here, but something like additional parenting skills. Especially if you, as the pediatrician, suspect that the parents might benefit from expanding their parenting toolkit already, here is a nice opportunity to invite them to learn some new approaches and skills — framed in a way that focuses on the temperament of the child rather than any “deficits” you perceive in the parents. Some parents may be more receptive and less defensive to the idea of participating in parent training under the framework that they are doing this because they have a temperamentally more challenging child (rather than feeling that they are deficient in basic parenting skills).
It’s always a good idea to know about what resources are available in the community when it comes to teaching parenting skills. In addition to scientifically supported books and podcasts, there has been a steady increase in reliable websites, apps, and other digital platforms related to parenting, as well as standard in-person groups and classes. This could also be a great use of an integrated behavioral health professional for practices fortunate enough to have one.
In summary, there is some new evidence that colic can represent a little more than “just gas,” and while we shouldn’t take this one study to the extreme, there may be some good opportunities here to discuss and support good parenting practices in general.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. His latest book is “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.” You can follow him on Twitter and Facebook @PediPsych.
Reference
1. Sammallahti S et al. Excessive crying, behavior problems, and amygdala volume: A study from infancy to adolescence. J Am Acad Child Adolesc Psychiatry. 2023 Jun;62(6):675-83. doi: 10.1016/j.jaac.2023.01.014.
Adverse events in childhood alter brain function
In a meta-analysis of 83 functional magnetic resonance imaging (fMRI) studies that included more than 5000 patients, exposure to adversity was associated with higher amygdala reactivity and lower prefrontal cortical reactivity across a range of task domains.
The altered responses were only observed in studies including adult participants and were clearest in participants who had been exposed to severe threat and trauma. Children and adolescents did not show significant adversity-related differences in brain function.
“By integrating the results from 83 previous brain imaging studies, we were able to provide what is arguably the clearest evidence to date that adults who have been exposed to early life trauma have different brain responses to psychological challenges,” senior author Marco Leyton, PhD, professor of psychiatry and director of the Temperament Adversity Biology Lab at McGill University in Montreal, Quebec, Canada, said in a press release. “This includes exaggerated responses in a region that processes emotionally intense information (the amygdala) and reduced responses in a region that helps people regulate emotions and associated behaviors (the frontal cortex).”
The findings were published in JAMA Network Open.
Changes in Reactivity
“One big issue we have in psychology, and especially in neuroscience, is that single-study results are often not reproducible,” lead author Niki Hosseini-Kamkar, PhD, neuroimaging research associate at Atlas Institute for Veterans and Families at Royal Ottawa Hospital, said in an interview.
“It was very important to me to use a meta-analysis to get an overall picture of what brain regions are consistently reported across all these different studies. That is what we did here,” she added. Dr. Hosseini-Kamkar conducted this analysis while she was a postdoctoral research fellow at McGill University in Montreal.
She and her group examined adversity exposure and brain function in the following four domains of task-based fMRI: emotion processing, memory processing, inhibitory control, and reward processing. Their study included 5242 participants. The researchers used multilevel kernel density analyses (MKDA) to analyze the data more accurately.
Adversity exposure was associated with higher amygdala reactivity (P < .001) and lower prefrontal cortical reactivity (P < .001), compared with controls with no adversity exposure.
Threat types of adversity were associated with greater blood-oxygen-level-dependent (BOLD) responses in the superior temporal gyrus and lower prefrontal cortex activity in participants exposed to threat, compared with controls.
Analysis of studies of inhibitory control tasks found greater activity in the claustrum, anterior cingulate cortex, and insula in the adversity-exposed participants, compared with controls.
In addition, studies that administered emotion processing tasks showed greater amygdala reactivity and lower prefrontal cortex (superior frontal gyrus) reactivity in the adversity exposure group, compared with controls.
“The main takeaway is that there’s an exaggerated activity in the amygdala, and diminished prefrontal cortex activity, and together, this might point to a mechanism for how a history of adversity diminishes the ability to cope with later stressors and can therefore heighten susceptibility to mental illness,” said Dr. Hosseini-Kamkar.
‘Important Next Step’
“Overall, the meta-analysis by Dr. Hosseini-Kamkar and colleagues represents an important next step in understanding associations of adversity exposure with brain function while highlighting the importance of considering the role of development,” wrote Dylan G. Gee, PhD, associate professor of psychology at Yale University in New Haven, Connecticut, and Alexis Brieant, PhD, assistant professor of research or creative works at the University of Vermont in Burlington, in an accompanying commentary.
They also applauded the authors for their use of MKDA. They noted that the technique “allows inferences about the consistency and specificity of brain activation across studies and is thought to be more robust to small sample sizes than activation likelihood estimation (ALE) meta-analysis.”
Dr. Gee and Dr. Brieant also observed that a recent ALE meta-analysis failed to find a link between adversity and brain function. “Although it is important to note that the file drawer problem — by which researchers are less likely to publish null results — presents challenges to the inferences that can be drawn in the current work, the current study may provide complementary information to prior ALE meta-analyses.”
Epigenetic Changes?
Commenting on the findings for this article, Victor Fornari, MD, director of child and adolescent psychiatry at Northwell Health in Glen Oaks, New York, said, “Historically, when someone went through a traumatic event, they were told to just get over it, because somehow trauma doesn’t have a lasting impact on the brain.” Dr. Fornari was not involved in the research.
“We have certainly learned so much more over the past decade about early adversity and that it does have a profound impact on the brain and probably even epigenetic changes in our genes,” Dr. Fornari said.
“This is a very important avenue of investigation. People are really trying to understand if there are biological markers that we can actually measure in the brain that will offer us a window to better understand the consequence of adversity, as well as possible avenues of treatment.”
No funding source for this study was reported. Dr. Leyton, Dr. Hosseini-Kamkar, and Dr. Fornari report no relevant financial relationships. Gee reports receiving grants from the National Science Foundation and National Institutes of Health outside the submitted work. Dr. Brieant reports receiving grants from the National Institute of Mental Health outside the submitted work.
A version of this article appeared on Medscape.com.
In a meta-analysis of 83 functional magnetic resonance imaging (fMRI) studies that included more than 5000 patients, exposure to adversity was associated with higher amygdala reactivity and lower prefrontal cortical reactivity across a range of task domains.
The altered responses were only observed in studies including adult participants and were clearest in participants who had been exposed to severe threat and trauma. Children and adolescents did not show significant adversity-related differences in brain function.
“By integrating the results from 83 previous brain imaging studies, we were able to provide what is arguably the clearest evidence to date that adults who have been exposed to early life trauma have different brain responses to psychological challenges,” senior author Marco Leyton, PhD, professor of psychiatry and director of the Temperament Adversity Biology Lab at McGill University in Montreal, Quebec, Canada, said in a press release. “This includes exaggerated responses in a region that processes emotionally intense information (the amygdala) and reduced responses in a region that helps people regulate emotions and associated behaviors (the frontal cortex).”
The findings were published in JAMA Network Open.
Changes in Reactivity
“One big issue we have in psychology, and especially in neuroscience, is that single-study results are often not reproducible,” lead author Niki Hosseini-Kamkar, PhD, neuroimaging research associate at Atlas Institute for Veterans and Families at Royal Ottawa Hospital, said in an interview.
“It was very important to me to use a meta-analysis to get an overall picture of what brain regions are consistently reported across all these different studies. That is what we did here,” she added. Dr. Hosseini-Kamkar conducted this analysis while she was a postdoctoral research fellow at McGill University in Montreal.
She and her group examined adversity exposure and brain function in the following four domains of task-based fMRI: emotion processing, memory processing, inhibitory control, and reward processing. Their study included 5242 participants. The researchers used multilevel kernel density analyses (MKDA) to analyze the data more accurately.
Adversity exposure was associated with higher amygdala reactivity (P < .001) and lower prefrontal cortical reactivity (P < .001), compared with controls with no adversity exposure.
Threat types of adversity were associated with greater blood-oxygen-level-dependent (BOLD) responses in the superior temporal gyrus and lower prefrontal cortex activity in participants exposed to threat, compared with controls.
Analysis of studies of inhibitory control tasks found greater activity in the claustrum, anterior cingulate cortex, and insula in the adversity-exposed participants, compared with controls.
In addition, studies that administered emotion processing tasks showed greater amygdala reactivity and lower prefrontal cortex (superior frontal gyrus) reactivity in the adversity exposure group, compared with controls.
“The main takeaway is that there’s an exaggerated activity in the amygdala, and diminished prefrontal cortex activity, and together, this might point to a mechanism for how a history of adversity diminishes the ability to cope with later stressors and can therefore heighten susceptibility to mental illness,” said Dr. Hosseini-Kamkar.
‘Important Next Step’
“Overall, the meta-analysis by Dr. Hosseini-Kamkar and colleagues represents an important next step in understanding associations of adversity exposure with brain function while highlighting the importance of considering the role of development,” wrote Dylan G. Gee, PhD, associate professor of psychology at Yale University in New Haven, Connecticut, and Alexis Brieant, PhD, assistant professor of research or creative works at the University of Vermont in Burlington, in an accompanying commentary.
They also applauded the authors for their use of MKDA. They noted that the technique “allows inferences about the consistency and specificity of brain activation across studies and is thought to be more robust to small sample sizes than activation likelihood estimation (ALE) meta-analysis.”
Dr. Gee and Dr. Brieant also observed that a recent ALE meta-analysis failed to find a link between adversity and brain function. “Although it is important to note that the file drawer problem — by which researchers are less likely to publish null results — presents challenges to the inferences that can be drawn in the current work, the current study may provide complementary information to prior ALE meta-analyses.”
Epigenetic Changes?
Commenting on the findings for this article, Victor Fornari, MD, director of child and adolescent psychiatry at Northwell Health in Glen Oaks, New York, said, “Historically, when someone went through a traumatic event, they were told to just get over it, because somehow trauma doesn’t have a lasting impact on the brain.” Dr. Fornari was not involved in the research.
“We have certainly learned so much more over the past decade about early adversity and that it does have a profound impact on the brain and probably even epigenetic changes in our genes,” Dr. Fornari said.
“This is a very important avenue of investigation. People are really trying to understand if there are biological markers that we can actually measure in the brain that will offer us a window to better understand the consequence of adversity, as well as possible avenues of treatment.”
No funding source for this study was reported. Dr. Leyton, Dr. Hosseini-Kamkar, and Dr. Fornari report no relevant financial relationships. Gee reports receiving grants from the National Science Foundation and National Institutes of Health outside the submitted work. Dr. Brieant reports receiving grants from the National Institute of Mental Health outside the submitted work.
A version of this article appeared on Medscape.com.
In a meta-analysis of 83 functional magnetic resonance imaging (fMRI) studies that included more than 5000 patients, exposure to adversity was associated with higher amygdala reactivity and lower prefrontal cortical reactivity across a range of task domains.
The altered responses were only observed in studies including adult participants and were clearest in participants who had been exposed to severe threat and trauma. Children and adolescents did not show significant adversity-related differences in brain function.
“By integrating the results from 83 previous brain imaging studies, we were able to provide what is arguably the clearest evidence to date that adults who have been exposed to early life trauma have different brain responses to psychological challenges,” senior author Marco Leyton, PhD, professor of psychiatry and director of the Temperament Adversity Biology Lab at McGill University in Montreal, Quebec, Canada, said in a press release. “This includes exaggerated responses in a region that processes emotionally intense information (the amygdala) and reduced responses in a region that helps people regulate emotions and associated behaviors (the frontal cortex).”
The findings were published in JAMA Network Open.
Changes in Reactivity
“One big issue we have in psychology, and especially in neuroscience, is that single-study results are often not reproducible,” lead author Niki Hosseini-Kamkar, PhD, neuroimaging research associate at Atlas Institute for Veterans and Families at Royal Ottawa Hospital, said in an interview.
“It was very important to me to use a meta-analysis to get an overall picture of what brain regions are consistently reported across all these different studies. That is what we did here,” she added. Dr. Hosseini-Kamkar conducted this analysis while she was a postdoctoral research fellow at McGill University in Montreal.
She and her group examined adversity exposure and brain function in the following four domains of task-based fMRI: emotion processing, memory processing, inhibitory control, and reward processing. Their study included 5242 participants. The researchers used multilevel kernel density analyses (MKDA) to analyze the data more accurately.
Adversity exposure was associated with higher amygdala reactivity (P < .001) and lower prefrontal cortical reactivity (P < .001), compared with controls with no adversity exposure.
Threat types of adversity were associated with greater blood-oxygen-level-dependent (BOLD) responses in the superior temporal gyrus and lower prefrontal cortex activity in participants exposed to threat, compared with controls.
Analysis of studies of inhibitory control tasks found greater activity in the claustrum, anterior cingulate cortex, and insula in the adversity-exposed participants, compared with controls.
In addition, studies that administered emotion processing tasks showed greater amygdala reactivity and lower prefrontal cortex (superior frontal gyrus) reactivity in the adversity exposure group, compared with controls.
“The main takeaway is that there’s an exaggerated activity in the amygdala, and diminished prefrontal cortex activity, and together, this might point to a mechanism for how a history of adversity diminishes the ability to cope with later stressors and can therefore heighten susceptibility to mental illness,” said Dr. Hosseini-Kamkar.
‘Important Next Step’
“Overall, the meta-analysis by Dr. Hosseini-Kamkar and colleagues represents an important next step in understanding associations of adversity exposure with brain function while highlighting the importance of considering the role of development,” wrote Dylan G. Gee, PhD, associate professor of psychology at Yale University in New Haven, Connecticut, and Alexis Brieant, PhD, assistant professor of research or creative works at the University of Vermont in Burlington, in an accompanying commentary.
They also applauded the authors for their use of MKDA. They noted that the technique “allows inferences about the consistency and specificity of brain activation across studies and is thought to be more robust to small sample sizes than activation likelihood estimation (ALE) meta-analysis.”
Dr. Gee and Dr. Brieant also observed that a recent ALE meta-analysis failed to find a link between adversity and brain function. “Although it is important to note that the file drawer problem — by which researchers are less likely to publish null results — presents challenges to the inferences that can be drawn in the current work, the current study may provide complementary information to prior ALE meta-analyses.”
Epigenetic Changes?
Commenting on the findings for this article, Victor Fornari, MD, director of child and adolescent psychiatry at Northwell Health in Glen Oaks, New York, said, “Historically, when someone went through a traumatic event, they were told to just get over it, because somehow trauma doesn’t have a lasting impact on the brain.” Dr. Fornari was not involved in the research.
“We have certainly learned so much more over the past decade about early adversity and that it does have a profound impact on the brain and probably even epigenetic changes in our genes,” Dr. Fornari said.
“This is a very important avenue of investigation. People are really trying to understand if there are biological markers that we can actually measure in the brain that will offer us a window to better understand the consequence of adversity, as well as possible avenues of treatment.”
No funding source for this study was reported. Dr. Leyton, Dr. Hosseini-Kamkar, and Dr. Fornari report no relevant financial relationships. Gee reports receiving grants from the National Science Foundation and National Institutes of Health outside the submitted work. Dr. Brieant reports receiving grants from the National Institute of Mental Health outside the submitted work.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Patients with hypermobile Ehlers-Danlos syndrome report skin laxity, scarring
.
The genetic cause of hEDS, a common inherited connective tissue disorder, remains unknown, wrote Alan Snyder, MD, of the department of dermatology and dermatologic surgery at Medical University of South Carolina, Charleston, and colleagues.
Previous research suggests that changes in dermal mechanics predispose these patients to a range of skin conditions including mast cell activation disorder (MCAD) spectrum and chronic spontaneous urticaria, abnormal scars or wound healing, piezogenic papules, dyshidrosis, skin laxity or softness, easy bruising, local anesthesia resistance, keratosis pilaris, striae, and hidradenitis suppurativa, the researchers wrote.
However, data on these and other dermatologic manifestations of hEDS are limited, they said.
The diagnosis of hEDS will continue to be made more frequently and carefully, as the condition becomes more recognized and understood in the medical community, especially with anticipated capabilities of genetic testing, Dr. Snyder said in an interview.
“Being able to be aware of disease-specific comorbidities, such as those discovered in this study, allows providers to better stratify phenotypes and improve patient disease co-management,” he said.
In the study, published in the Journal of the American Academy of Dermatology, the researchers reviewed data on 1,364 patients with ICD-10 or ICD-9 codes for hEDS or EDS unspecified who were seen at a single institution between June 2005 and May 2022. Most of the patients were White (95.4%) and female (86.7%); the average age was 29.2 years.
Of the 1,364 patients included in the chart review, 497 (36.4%) had documented skin manifestations. Of these, 118 (24.2%) had disorders of follicular occlusion (12 had hidradenitis suppurativa, 32 had folliculitis, and 74 had acne); 112 (23%) had eczema or atopic dermatitis, 98 (19.7%) had mast cell disorder, 32 (6.4%) had psoriasis, and 32 (6.4%) had wound healing issues (16 had hypertrophic keloids/scarring, 5 had abscesses, 3 had abnormal bruising, and 8 had other would healing issues).
The study also included results of a multiple-choice patient survey from 1,354 individuals. In the survey, approximately two-thirds of patients reported abnormal scarring, abnormal wound healing, and cutaneous laxity (61.7%, 69.0%, and 71.0%, respectively).
The findings were limited by several factors including the retrospective study design, lack of testing to confirm hEDS diagnosis, and the potential interdisciplinary selection bias for diagnoses, the authors noted.
However, the results support previous studies showing increased rates of occlusive conditions in hEDS and higher rates of acne, folliculitis, and psoriasis, and highlight the need for clinician education to manage patients and promote better outcomes, the researchers concluded.
Data Enhance Clinical Awareness
“Given the increasingly understood relation between TH2-directed and mast-cell mediated diseases and hEDS, it was not necessarily a surprise to find the increased prevalence of atopy and mast cell disease, but rather an interesting confirmation, within the limitations that exist with retrospective chart review,” Dr. Snyder told this news organization. “While it may make some intuitive sense that certain cohorts with higher risk of HS may have a higher risk of acne, this had not been reported in the literature to date,” he noted. “Given the high levels of patient reported issues with scarring and wound healing, I was surprised that so few analogous diagnoses were physician-reported in the medical records.”
In clinical practice, “health care professionals and patients need to be aware hEDS is associated with high rates of eczematous, mast-cell mediated and follicular occlusive cutaneous disorders,” Dr. Snyder said in an interview. “There seems to be a discrepancy between patients and physician awareness of scarring or wound healing issues in this patient population,” he added.
Looking ahead, “we need to better research and characterize the various hEDS phenotypes to understand who is at highest risk for various TH2-mediated or follicular occlusive disorders,” said Dr. Snyder. “Moreover, a greater understanding is needed of the wound healing inadequacies that predispose these patients to poor outcomes during dermatologic surgery,” he said.
The study was supported by the Ehlers-Danlos Society and the Milton and Tamar Maltz Family Foundation. The researchers had no financial conflicts to disclose.
.
The genetic cause of hEDS, a common inherited connective tissue disorder, remains unknown, wrote Alan Snyder, MD, of the department of dermatology and dermatologic surgery at Medical University of South Carolina, Charleston, and colleagues.
Previous research suggests that changes in dermal mechanics predispose these patients to a range of skin conditions including mast cell activation disorder (MCAD) spectrum and chronic spontaneous urticaria, abnormal scars or wound healing, piezogenic papules, dyshidrosis, skin laxity or softness, easy bruising, local anesthesia resistance, keratosis pilaris, striae, and hidradenitis suppurativa, the researchers wrote.
However, data on these and other dermatologic manifestations of hEDS are limited, they said.
The diagnosis of hEDS will continue to be made more frequently and carefully, as the condition becomes more recognized and understood in the medical community, especially with anticipated capabilities of genetic testing, Dr. Snyder said in an interview.
“Being able to be aware of disease-specific comorbidities, such as those discovered in this study, allows providers to better stratify phenotypes and improve patient disease co-management,” he said.
In the study, published in the Journal of the American Academy of Dermatology, the researchers reviewed data on 1,364 patients with ICD-10 or ICD-9 codes for hEDS or EDS unspecified who were seen at a single institution between June 2005 and May 2022. Most of the patients were White (95.4%) and female (86.7%); the average age was 29.2 years.
Of the 1,364 patients included in the chart review, 497 (36.4%) had documented skin manifestations. Of these, 118 (24.2%) had disorders of follicular occlusion (12 had hidradenitis suppurativa, 32 had folliculitis, and 74 had acne); 112 (23%) had eczema or atopic dermatitis, 98 (19.7%) had mast cell disorder, 32 (6.4%) had psoriasis, and 32 (6.4%) had wound healing issues (16 had hypertrophic keloids/scarring, 5 had abscesses, 3 had abnormal bruising, and 8 had other would healing issues).
The study also included results of a multiple-choice patient survey from 1,354 individuals. In the survey, approximately two-thirds of patients reported abnormal scarring, abnormal wound healing, and cutaneous laxity (61.7%, 69.0%, and 71.0%, respectively).
The findings were limited by several factors including the retrospective study design, lack of testing to confirm hEDS diagnosis, and the potential interdisciplinary selection bias for diagnoses, the authors noted.
However, the results support previous studies showing increased rates of occlusive conditions in hEDS and higher rates of acne, folliculitis, and psoriasis, and highlight the need for clinician education to manage patients and promote better outcomes, the researchers concluded.
Data Enhance Clinical Awareness
“Given the increasingly understood relation between TH2-directed and mast-cell mediated diseases and hEDS, it was not necessarily a surprise to find the increased prevalence of atopy and mast cell disease, but rather an interesting confirmation, within the limitations that exist with retrospective chart review,” Dr. Snyder told this news organization. “While it may make some intuitive sense that certain cohorts with higher risk of HS may have a higher risk of acne, this had not been reported in the literature to date,” he noted. “Given the high levels of patient reported issues with scarring and wound healing, I was surprised that so few analogous diagnoses were physician-reported in the medical records.”
In clinical practice, “health care professionals and patients need to be aware hEDS is associated with high rates of eczematous, mast-cell mediated and follicular occlusive cutaneous disorders,” Dr. Snyder said in an interview. “There seems to be a discrepancy between patients and physician awareness of scarring or wound healing issues in this patient population,” he added.
Looking ahead, “we need to better research and characterize the various hEDS phenotypes to understand who is at highest risk for various TH2-mediated or follicular occlusive disorders,” said Dr. Snyder. “Moreover, a greater understanding is needed of the wound healing inadequacies that predispose these patients to poor outcomes during dermatologic surgery,” he said.
The study was supported by the Ehlers-Danlos Society and the Milton and Tamar Maltz Family Foundation. The researchers had no financial conflicts to disclose.
.
The genetic cause of hEDS, a common inherited connective tissue disorder, remains unknown, wrote Alan Snyder, MD, of the department of dermatology and dermatologic surgery at Medical University of South Carolina, Charleston, and colleagues.
Previous research suggests that changes in dermal mechanics predispose these patients to a range of skin conditions including mast cell activation disorder (MCAD) spectrum and chronic spontaneous urticaria, abnormal scars or wound healing, piezogenic papules, dyshidrosis, skin laxity or softness, easy bruising, local anesthesia resistance, keratosis pilaris, striae, and hidradenitis suppurativa, the researchers wrote.
However, data on these and other dermatologic manifestations of hEDS are limited, they said.
The diagnosis of hEDS will continue to be made more frequently and carefully, as the condition becomes more recognized and understood in the medical community, especially with anticipated capabilities of genetic testing, Dr. Snyder said in an interview.
“Being able to be aware of disease-specific comorbidities, such as those discovered in this study, allows providers to better stratify phenotypes and improve patient disease co-management,” he said.
In the study, published in the Journal of the American Academy of Dermatology, the researchers reviewed data on 1,364 patients with ICD-10 or ICD-9 codes for hEDS or EDS unspecified who were seen at a single institution between June 2005 and May 2022. Most of the patients were White (95.4%) and female (86.7%); the average age was 29.2 years.
Of the 1,364 patients included in the chart review, 497 (36.4%) had documented skin manifestations. Of these, 118 (24.2%) had disorders of follicular occlusion (12 had hidradenitis suppurativa, 32 had folliculitis, and 74 had acne); 112 (23%) had eczema or atopic dermatitis, 98 (19.7%) had mast cell disorder, 32 (6.4%) had psoriasis, and 32 (6.4%) had wound healing issues (16 had hypertrophic keloids/scarring, 5 had abscesses, 3 had abnormal bruising, and 8 had other would healing issues).
The study also included results of a multiple-choice patient survey from 1,354 individuals. In the survey, approximately two-thirds of patients reported abnormal scarring, abnormal wound healing, and cutaneous laxity (61.7%, 69.0%, and 71.0%, respectively).
The findings were limited by several factors including the retrospective study design, lack of testing to confirm hEDS diagnosis, and the potential interdisciplinary selection bias for diagnoses, the authors noted.
However, the results support previous studies showing increased rates of occlusive conditions in hEDS and higher rates of acne, folliculitis, and psoriasis, and highlight the need for clinician education to manage patients and promote better outcomes, the researchers concluded.
Data Enhance Clinical Awareness
“Given the increasingly understood relation between TH2-directed and mast-cell mediated diseases and hEDS, it was not necessarily a surprise to find the increased prevalence of atopy and mast cell disease, but rather an interesting confirmation, within the limitations that exist with retrospective chart review,” Dr. Snyder told this news organization. “While it may make some intuitive sense that certain cohorts with higher risk of HS may have a higher risk of acne, this had not been reported in the literature to date,” he noted. “Given the high levels of patient reported issues with scarring and wound healing, I was surprised that so few analogous diagnoses were physician-reported in the medical records.”
In clinical practice, “health care professionals and patients need to be aware hEDS is associated with high rates of eczematous, mast-cell mediated and follicular occlusive cutaneous disorders,” Dr. Snyder said in an interview. “There seems to be a discrepancy between patients and physician awareness of scarring or wound healing issues in this patient population,” he added.
Looking ahead, “we need to better research and characterize the various hEDS phenotypes to understand who is at highest risk for various TH2-mediated or follicular occlusive disorders,” said Dr. Snyder. “Moreover, a greater understanding is needed of the wound healing inadequacies that predispose these patients to poor outcomes during dermatologic surgery,” he said.
The study was supported by the Ehlers-Danlos Society and the Milton and Tamar Maltz Family Foundation. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
FDA mandates five changes to iPLEDGE program for isotretinoin
In a letter dated Nov. 30, 2023, the .
The development follows a March 2023 joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee about iPLEDGE REMS requirements, which included feedback from patients and dermatologists and recommendations for changes to the REMS program, aimed at minimizing the burden of the program on patients, pharmacies, and prescribers while continuing to maintain safe use of the highly teratogenic drug for patients.
The five changes include the following:
- Remove the requirement that pregnancy tests must be performed in a specially certified (i.e., Clinical Laboratory Improvement Amendments [CLIA]) laboratory. In the opinion of John S. Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, this change “may make it easier to perform pregnancy tests in a clinic setting without needing to send the patient to a separate lab,” he said in an interview.
- Allow prescribers the option of using home pregnancy testing for their patients during and after isotretinoin treatment. Prescribers who rely on the patient to perform a home pregnancy test need to take steps to minimize patients falsifying the results of these tests. According to Dr. Barbieri, this means that two pregnancy tests prior to starting isotretinoin must be done in a lab or office setting. “However, all the pregnancy tests on therapy can be either in a medical setting or using a home pregnancy test,” he told this news organization. “This option facilitates the use of telemedicine so that patients would not need to come in; they can just share a pregnancy test with their name and date with their dermatologist.”
- Remove the waiting period requirement — also known as the “19-day lockout” — for patients if they do not obtain isotretinoin within the first 7-day prescription window. According to Dr. Barbieri, this change helps to ensure that patients can begin isotretinoin in a timely manner. “Insurance and pharmacy delays that are no fault of the patient can commonly cause missed initial window periods,” he said. “Allowing for immediate repeat of a pregnancy test to start a new window period, rather than requiring the patient to wait 19 more days, can ensure patient safety and pregnancy prevention without negatively impacting access.”
- Revise the pregnancy registry requirement to remove the objective to document the pregnancy and fetal outcomes for each pregnancy.
- Revise the requirement for prescribers to document patient counseling in patients who cannot become pregnant from monthly to only at enrollment. Dr. Barbieri characterized this change as “major” and said that it could eliminate the need for monthly visits for persons of non–childbearing potential. “This could substantially reduce logistical burdens for patients and reduce wait times to see a dermatologist,” he said.
Future changes to iPLEDGE that Dr. Barbieri would like to see include allowing for home pregnancy tests prior to starting therapy — particularly the test after the 30-day window period. “In addition, it would be good to be able to reduce the 30-day waiting period prior to therapy to something shorter,” such as 14 days, which would still “reliably exclude pregnancy, particularly for those on stable long-acting reversible contraception,” he said. There are also opportunities to improve the iPLEDGE website functionality and to ensure that the website is accessible to patients with limited English proficiency, he added.
He also recommended greater transparency by the Isotretinoin Products Manufacturers Group and inclusion of input from diverse stakeholders such as dermatologists, patients, and pharmacists.
Dr. Barbieri reported personal fees from Dexcel Pharma.
In a letter dated Nov. 30, 2023, the .
The development follows a March 2023 joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee about iPLEDGE REMS requirements, which included feedback from patients and dermatologists and recommendations for changes to the REMS program, aimed at minimizing the burden of the program on patients, pharmacies, and prescribers while continuing to maintain safe use of the highly teratogenic drug for patients.
The five changes include the following:
- Remove the requirement that pregnancy tests must be performed in a specially certified (i.e., Clinical Laboratory Improvement Amendments [CLIA]) laboratory. In the opinion of John S. Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, this change “may make it easier to perform pregnancy tests in a clinic setting without needing to send the patient to a separate lab,” he said in an interview.
- Allow prescribers the option of using home pregnancy testing for their patients during and after isotretinoin treatment. Prescribers who rely on the patient to perform a home pregnancy test need to take steps to minimize patients falsifying the results of these tests. According to Dr. Barbieri, this means that two pregnancy tests prior to starting isotretinoin must be done in a lab or office setting. “However, all the pregnancy tests on therapy can be either in a medical setting or using a home pregnancy test,” he told this news organization. “This option facilitates the use of telemedicine so that patients would not need to come in; they can just share a pregnancy test with their name and date with their dermatologist.”
- Remove the waiting period requirement — also known as the “19-day lockout” — for patients if they do not obtain isotretinoin within the first 7-day prescription window. According to Dr. Barbieri, this change helps to ensure that patients can begin isotretinoin in a timely manner. “Insurance and pharmacy delays that are no fault of the patient can commonly cause missed initial window periods,” he said. “Allowing for immediate repeat of a pregnancy test to start a new window period, rather than requiring the patient to wait 19 more days, can ensure patient safety and pregnancy prevention without negatively impacting access.”
- Revise the pregnancy registry requirement to remove the objective to document the pregnancy and fetal outcomes for each pregnancy.
- Revise the requirement for prescribers to document patient counseling in patients who cannot become pregnant from monthly to only at enrollment. Dr. Barbieri characterized this change as “major” and said that it could eliminate the need for monthly visits for persons of non–childbearing potential. “This could substantially reduce logistical burdens for patients and reduce wait times to see a dermatologist,” he said.
Future changes to iPLEDGE that Dr. Barbieri would like to see include allowing for home pregnancy tests prior to starting therapy — particularly the test after the 30-day window period. “In addition, it would be good to be able to reduce the 30-day waiting period prior to therapy to something shorter,” such as 14 days, which would still “reliably exclude pregnancy, particularly for those on stable long-acting reversible contraception,” he said. There are also opportunities to improve the iPLEDGE website functionality and to ensure that the website is accessible to patients with limited English proficiency, he added.
He also recommended greater transparency by the Isotretinoin Products Manufacturers Group and inclusion of input from diverse stakeholders such as dermatologists, patients, and pharmacists.
Dr. Barbieri reported personal fees from Dexcel Pharma.
In a letter dated Nov. 30, 2023, the .
The development follows a March 2023 joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee about iPLEDGE REMS requirements, which included feedback from patients and dermatologists and recommendations for changes to the REMS program, aimed at minimizing the burden of the program on patients, pharmacies, and prescribers while continuing to maintain safe use of the highly teratogenic drug for patients.
The five changes include the following:
- Remove the requirement that pregnancy tests must be performed in a specially certified (i.e., Clinical Laboratory Improvement Amendments [CLIA]) laboratory. In the opinion of John S. Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, this change “may make it easier to perform pregnancy tests in a clinic setting without needing to send the patient to a separate lab,” he said in an interview.
- Allow prescribers the option of using home pregnancy testing for their patients during and after isotretinoin treatment. Prescribers who rely on the patient to perform a home pregnancy test need to take steps to minimize patients falsifying the results of these tests. According to Dr. Barbieri, this means that two pregnancy tests prior to starting isotretinoin must be done in a lab or office setting. “However, all the pregnancy tests on therapy can be either in a medical setting or using a home pregnancy test,” he told this news organization. “This option facilitates the use of telemedicine so that patients would not need to come in; they can just share a pregnancy test with their name and date with their dermatologist.”
- Remove the waiting period requirement — also known as the “19-day lockout” — for patients if they do not obtain isotretinoin within the first 7-day prescription window. According to Dr. Barbieri, this change helps to ensure that patients can begin isotretinoin in a timely manner. “Insurance and pharmacy delays that are no fault of the patient can commonly cause missed initial window periods,” he said. “Allowing for immediate repeat of a pregnancy test to start a new window period, rather than requiring the patient to wait 19 more days, can ensure patient safety and pregnancy prevention without negatively impacting access.”
- Revise the pregnancy registry requirement to remove the objective to document the pregnancy and fetal outcomes for each pregnancy.
- Revise the requirement for prescribers to document patient counseling in patients who cannot become pregnant from monthly to only at enrollment. Dr. Barbieri characterized this change as “major” and said that it could eliminate the need for monthly visits for persons of non–childbearing potential. “This could substantially reduce logistical burdens for patients and reduce wait times to see a dermatologist,” he said.
Future changes to iPLEDGE that Dr. Barbieri would like to see include allowing for home pregnancy tests prior to starting therapy — particularly the test after the 30-day window period. “In addition, it would be good to be able to reduce the 30-day waiting period prior to therapy to something shorter,” such as 14 days, which would still “reliably exclude pregnancy, particularly for those on stable long-acting reversible contraception,” he said. There are also opportunities to improve the iPLEDGE website functionality and to ensure that the website is accessible to patients with limited English proficiency, he added.
He also recommended greater transparency by the Isotretinoin Products Manufacturers Group and inclusion of input from diverse stakeholders such as dermatologists, patients, and pharmacists.
Dr. Barbieri reported personal fees from Dexcel Pharma.
ASH 2023: Equity, Sickle Cell, and Real-Life Outcomes
Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute and secretary of ASH, added that insight into actual patient experiences also will be a major theme at ASH 2023.
“There is a huge growth in research on outcomes and focusing on using real-world data and how important that is,” Dr. Dunbar said. “Academic research and hematology is really focusing on patient-reported outcomes and how care is delivered in a real-world setting – actually looking at what matters to patients. Are they alive in a certain number of years? And how are they feeling?”
As an example, Dr. Dunbar pointed to an abstract that examined clinical databases in Canada and found that real-world outcomes in multiple myeloma treatments were much worse than those in the original clinical trials for the therapies. Patients reached relapse 44% faster and their overall survival was 75% worse.
In the media briefing, ASH chair of communications Mikkael A. Sekeres, MD, MS, of the Sylvester Comprehensive Cancer Center at the University of Miami, noted that patients in these types of clinical trials “are just these pristine specimens of human beings except for the cancer that’s being treated.”
Dr. Dunbar agreed, noting that “patients who are able to enroll in clinical trials are more likely to be able to show up at the treatment center at the right time and for every dose, have transportation, and afford drugs to prevent side effects. They might stay on the drug for longer, or they have nurses who are always encouraging them of how to make it through a toxicity.”
Hematologists and patients should consider randomized controlled trials to be “the best possible outcome, and perhaps adjust their thinking if an individual patient is older, sicker, or less able to follow a regimen exactly,” she said.
Another highlighted study linked worse outcomes in African-Americans with pediatric acute myeloid leukemia to genetic traits that are more common in that population. The traits “likely explain at least in part the worst outcomes in Black patients in prior studies and on some regimens,” Dr. Dunbar said.
She added that the findings emphasize how testing for genetic variants and biomarkers that impact outcomes should be performed “instead of assuming that a certain dose should be given simply based on perceived or reported race or ethnicity.”
ASH President Robert A. Brodsky, MD, of Johns Hopkins University School of Medicine, Baltimore, highlighted an abstract that reported on the use of AI as a clinical decision support tool to differentiate two easily confused conditions — prefibrotic primary myelofibrosis and essential thrombocythemia.
AI “is a tool that’s going to help pathologists make more accurate and faster diagnoses,” he said. He also spotlighted an abstract about the use of “social media listening” to understand the experiences of patients with SCD and their caregivers. “There can be a lot of misuse and waste of time with social media, but they used this in a way to try and gain insight as to what’s really important to the patients and the caregiver.”
Also, in regard to SCD, Dr. Dunbar pointed to a study that reports on outcomes in patients who received lovotibeglogene autotemcel (lovo-cel) gene therapy for up to 60 months. Both this treatment and a CRISPR-based therapy called exa-cel “appear to result in comparable very impressive efficacy in terms of pain crises and organ dysfunction,” she said. “The hurdle is going to be figuring out how to deliver what will be very expensive and complicated therapies — but likely curative — therapies to patients.”
Another study to be presented at ASH — coauthored by Dr. Brodsky — shows promising results from reduced-intensity haploidentical bone marrow transplantation in adults with severe SCD. Results were similar to those seen with bone marrow from matched siblings, Dr. Sekeres said.
He added that more clarity is needed about new treatment options for SCD, perhaps through a “randomized trial where patients upfront get a haploidentical bone marrow transplant or fully matched bone marrow transplant. Then other patients are randomized to some of these other, newer technology therapies, and we follow them over time. We’re looking not only for overall survival but complications of the therapy itself and how many patients relapse from the treatment.”
Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute and secretary of ASH, added that insight into actual patient experiences also will be a major theme at ASH 2023.
“There is a huge growth in research on outcomes and focusing on using real-world data and how important that is,” Dr. Dunbar said. “Academic research and hematology is really focusing on patient-reported outcomes and how care is delivered in a real-world setting – actually looking at what matters to patients. Are they alive in a certain number of years? And how are they feeling?”
As an example, Dr. Dunbar pointed to an abstract that examined clinical databases in Canada and found that real-world outcomes in multiple myeloma treatments were much worse than those in the original clinical trials for the therapies. Patients reached relapse 44% faster and their overall survival was 75% worse.
In the media briefing, ASH chair of communications Mikkael A. Sekeres, MD, MS, of the Sylvester Comprehensive Cancer Center at the University of Miami, noted that patients in these types of clinical trials “are just these pristine specimens of human beings except for the cancer that’s being treated.”
Dr. Dunbar agreed, noting that “patients who are able to enroll in clinical trials are more likely to be able to show up at the treatment center at the right time and for every dose, have transportation, and afford drugs to prevent side effects. They might stay on the drug for longer, or they have nurses who are always encouraging them of how to make it through a toxicity.”
Hematologists and patients should consider randomized controlled trials to be “the best possible outcome, and perhaps adjust their thinking if an individual patient is older, sicker, or less able to follow a regimen exactly,” she said.
Another highlighted study linked worse outcomes in African-Americans with pediatric acute myeloid leukemia to genetic traits that are more common in that population. The traits “likely explain at least in part the worst outcomes in Black patients in prior studies and on some regimens,” Dr. Dunbar said.
She added that the findings emphasize how testing for genetic variants and biomarkers that impact outcomes should be performed “instead of assuming that a certain dose should be given simply based on perceived or reported race or ethnicity.”
ASH President Robert A. Brodsky, MD, of Johns Hopkins University School of Medicine, Baltimore, highlighted an abstract that reported on the use of AI as a clinical decision support tool to differentiate two easily confused conditions — prefibrotic primary myelofibrosis and essential thrombocythemia.
AI “is a tool that’s going to help pathologists make more accurate and faster diagnoses,” he said. He also spotlighted an abstract about the use of “social media listening” to understand the experiences of patients with SCD and their caregivers. “There can be a lot of misuse and waste of time with social media, but they used this in a way to try and gain insight as to what’s really important to the patients and the caregiver.”
Also, in regard to SCD, Dr. Dunbar pointed to a study that reports on outcomes in patients who received lovotibeglogene autotemcel (lovo-cel) gene therapy for up to 60 months. Both this treatment and a CRISPR-based therapy called exa-cel “appear to result in comparable very impressive efficacy in terms of pain crises and organ dysfunction,” she said. “The hurdle is going to be figuring out how to deliver what will be very expensive and complicated therapies — but likely curative — therapies to patients.”
Another study to be presented at ASH — coauthored by Dr. Brodsky — shows promising results from reduced-intensity haploidentical bone marrow transplantation in adults with severe SCD. Results were similar to those seen with bone marrow from matched siblings, Dr. Sekeres said.
He added that more clarity is needed about new treatment options for SCD, perhaps through a “randomized trial where patients upfront get a haploidentical bone marrow transplant or fully matched bone marrow transplant. Then other patients are randomized to some of these other, newer technology therapies, and we follow them over time. We’re looking not only for overall survival but complications of the therapy itself and how many patients relapse from the treatment.”
Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute and secretary of ASH, added that insight into actual patient experiences also will be a major theme at ASH 2023.
“There is a huge growth in research on outcomes and focusing on using real-world data and how important that is,” Dr. Dunbar said. “Academic research and hematology is really focusing on patient-reported outcomes and how care is delivered in a real-world setting – actually looking at what matters to patients. Are they alive in a certain number of years? And how are they feeling?”
As an example, Dr. Dunbar pointed to an abstract that examined clinical databases in Canada and found that real-world outcomes in multiple myeloma treatments were much worse than those in the original clinical trials for the therapies. Patients reached relapse 44% faster and their overall survival was 75% worse.
In the media briefing, ASH chair of communications Mikkael A. Sekeres, MD, MS, of the Sylvester Comprehensive Cancer Center at the University of Miami, noted that patients in these types of clinical trials “are just these pristine specimens of human beings except for the cancer that’s being treated.”
Dr. Dunbar agreed, noting that “patients who are able to enroll in clinical trials are more likely to be able to show up at the treatment center at the right time and for every dose, have transportation, and afford drugs to prevent side effects. They might stay on the drug for longer, or they have nurses who are always encouraging them of how to make it through a toxicity.”
Hematologists and patients should consider randomized controlled trials to be “the best possible outcome, and perhaps adjust their thinking if an individual patient is older, sicker, or less able to follow a regimen exactly,” she said.
Another highlighted study linked worse outcomes in African-Americans with pediatric acute myeloid leukemia to genetic traits that are more common in that population. The traits “likely explain at least in part the worst outcomes in Black patients in prior studies and on some regimens,” Dr. Dunbar said.
She added that the findings emphasize how testing for genetic variants and biomarkers that impact outcomes should be performed “instead of assuming that a certain dose should be given simply based on perceived or reported race or ethnicity.”
ASH President Robert A. Brodsky, MD, of Johns Hopkins University School of Medicine, Baltimore, highlighted an abstract that reported on the use of AI as a clinical decision support tool to differentiate two easily confused conditions — prefibrotic primary myelofibrosis and essential thrombocythemia.
AI “is a tool that’s going to help pathologists make more accurate and faster diagnoses,” he said. He also spotlighted an abstract about the use of “social media listening” to understand the experiences of patients with SCD and their caregivers. “There can be a lot of misuse and waste of time with social media, but they used this in a way to try and gain insight as to what’s really important to the patients and the caregiver.”
Also, in regard to SCD, Dr. Dunbar pointed to a study that reports on outcomes in patients who received lovotibeglogene autotemcel (lovo-cel) gene therapy for up to 60 months. Both this treatment and a CRISPR-based therapy called exa-cel “appear to result in comparable very impressive efficacy in terms of pain crises and organ dysfunction,” she said. “The hurdle is going to be figuring out how to deliver what will be very expensive and complicated therapies — but likely curative — therapies to patients.”
Another study to be presented at ASH — coauthored by Dr. Brodsky — shows promising results from reduced-intensity haploidentical bone marrow transplantation in adults with severe SCD. Results were similar to those seen with bone marrow from matched siblings, Dr. Sekeres said.
He added that more clarity is needed about new treatment options for SCD, perhaps through a “randomized trial where patients upfront get a haploidentical bone marrow transplant or fully matched bone marrow transplant. Then other patients are randomized to some of these other, newer technology therapies, and we follow them over time. We’re looking not only for overall survival but complications of the therapy itself and how many patients relapse from the treatment.”
AT ASH 2023
COVID vaccines lower risk of serious illness in children
TOPLINE:
, according to a new study by the Centers for Disease Control and Prevention (CDC).
METHODOLOGY:
- SARS-CoV-2 infection can severely affect children who have certain chronic conditions.
- Researchers assessed the effectiveness of COVID-19 vaccines in preventing emergency ED visits and hospitalizations associated with the illness from July 2022 to September 2023.
- They drew data from the New Vaccine Surveillance Network, which conducts population-based, prospective surveillance for acute respiratory illness in children at seven pediatric medical centers.
- The period assessed was the first year vaccines were authorized for children aged 6 months to 4 years; during that period, several Omicron subvariants arose.
- Researchers used data from 7,434 infants and children; data included patients’ vaccine status and their test results for SARS-CoV-2.
TAKEAWAY:
- Of the 7,434 infants and children who had an acute respiratory illness and were hospitalized or visited the ED, 387 had COVID-19.
- Children who received two doses of a COVID-19 vaccine were 40% less likely to have a COVID-19-associated hospitalization or ED visit compared with unvaccinated youth.
- One dose of a COVID-19 vaccine reduced ED visits and hospitalizations by 31%.
IN PRACTICE:
“The findings in this report support the recommendation for COVID-19 vaccination for all children aged ≥6 months and highlight the importance of completion of a primary series for young children,” the researchers reported.
SOURCE:
The study was led by Heidi L. Moline, MD, of the CDC.
LIMITATIONS:
Because the number of children with antibodies and immunity against SARS-CoV-2 has grown, vaccine effectiveness rates in the study may no longer be as relevant. Children with preexisting chronic conditions may be more likely to be vaccinated and receive medical attention. The low rates of vaccination may have prevented researchers from conducting a more detailed analysis. The Pfizer-BioNTech vaccine requires three doses, whereas Moderna’s requires two doses; this may have skewed the estimated efficacy of the Pfizer-BioNTech vaccine.
DISCLOSURES:
The authors report a variety of potential conflicts of interest, which are detailed in the article.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a new study by the Centers for Disease Control and Prevention (CDC).
METHODOLOGY:
- SARS-CoV-2 infection can severely affect children who have certain chronic conditions.
- Researchers assessed the effectiveness of COVID-19 vaccines in preventing emergency ED visits and hospitalizations associated with the illness from July 2022 to September 2023.
- They drew data from the New Vaccine Surveillance Network, which conducts population-based, prospective surveillance for acute respiratory illness in children at seven pediatric medical centers.
- The period assessed was the first year vaccines were authorized for children aged 6 months to 4 years; during that period, several Omicron subvariants arose.
- Researchers used data from 7,434 infants and children; data included patients’ vaccine status and their test results for SARS-CoV-2.
TAKEAWAY:
- Of the 7,434 infants and children who had an acute respiratory illness and were hospitalized or visited the ED, 387 had COVID-19.
- Children who received two doses of a COVID-19 vaccine were 40% less likely to have a COVID-19-associated hospitalization or ED visit compared with unvaccinated youth.
- One dose of a COVID-19 vaccine reduced ED visits and hospitalizations by 31%.
IN PRACTICE:
“The findings in this report support the recommendation for COVID-19 vaccination for all children aged ≥6 months and highlight the importance of completion of a primary series for young children,” the researchers reported.
SOURCE:
The study was led by Heidi L. Moline, MD, of the CDC.
LIMITATIONS:
Because the number of children with antibodies and immunity against SARS-CoV-2 has grown, vaccine effectiveness rates in the study may no longer be as relevant. Children with preexisting chronic conditions may be more likely to be vaccinated and receive medical attention. The low rates of vaccination may have prevented researchers from conducting a more detailed analysis. The Pfizer-BioNTech vaccine requires three doses, whereas Moderna’s requires two doses; this may have skewed the estimated efficacy of the Pfizer-BioNTech vaccine.
DISCLOSURES:
The authors report a variety of potential conflicts of interest, which are detailed in the article.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a new study by the Centers for Disease Control and Prevention (CDC).
METHODOLOGY:
- SARS-CoV-2 infection can severely affect children who have certain chronic conditions.
- Researchers assessed the effectiveness of COVID-19 vaccines in preventing emergency ED visits and hospitalizations associated with the illness from July 2022 to September 2023.
- They drew data from the New Vaccine Surveillance Network, which conducts population-based, prospective surveillance for acute respiratory illness in children at seven pediatric medical centers.
- The period assessed was the first year vaccines were authorized for children aged 6 months to 4 years; during that period, several Omicron subvariants arose.
- Researchers used data from 7,434 infants and children; data included patients’ vaccine status and their test results for SARS-CoV-2.
TAKEAWAY:
- Of the 7,434 infants and children who had an acute respiratory illness and were hospitalized or visited the ED, 387 had COVID-19.
- Children who received two doses of a COVID-19 vaccine were 40% less likely to have a COVID-19-associated hospitalization or ED visit compared with unvaccinated youth.
- One dose of a COVID-19 vaccine reduced ED visits and hospitalizations by 31%.
IN PRACTICE:
“The findings in this report support the recommendation for COVID-19 vaccination for all children aged ≥6 months and highlight the importance of completion of a primary series for young children,” the researchers reported.
SOURCE:
The study was led by Heidi L. Moline, MD, of the CDC.
LIMITATIONS:
Because the number of children with antibodies and immunity against SARS-CoV-2 has grown, vaccine effectiveness rates in the study may no longer be as relevant. Children with preexisting chronic conditions may be more likely to be vaccinated and receive medical attention. The low rates of vaccination may have prevented researchers from conducting a more detailed analysis. The Pfizer-BioNTech vaccine requires three doses, whereas Moderna’s requires two doses; this may have skewed the estimated efficacy of the Pfizer-BioNTech vaccine.
DISCLOSURES:
The authors report a variety of potential conflicts of interest, which are detailed in the article.
A version of this article appeared on Medscape.com.
AHA, AAP update neonatal resuscitation guidelines
The 2023 focused update was prompted by four systematic literature reviews by the International Liaison Committee on Resuscitation (ILCOR) Neonatal Life Support Task Force.
“Evidence evaluations by the ILCOR play a large role in the group’s process and timing of updates,” Henry Lee, MD, co-chair of the writing group, said in an interview.
He noted that updated recommendations do not change prior recommendations from the 2020 guidelines.
“However, they provide additional details to consider in neonatal resuscitation that could lead to changes in some practice in various settings,” said Dr. Lee, medical director of the University of California San Diego neonatal intensive care unit.
The focused update was simultaneously published online November 16 in Circulation and in Pediatrics.
Dr. Lee noted that effective positive-pressure ventilation (PPV) is the priority in newborn infants who need support after birth.
And while the 2020 update provided some details on devices to be used for PPV, the 2023 focused update gives guidance on use of T-piece resuscitators for providing PPV, which may be particularly helpful for preterm infants, and the use of supraglottic airways as a primary interface to deliver PPV, he explained.
Specifically, the updated guidelines state that use of a T-piece resuscitator to deliver PPV is preferred to the use of a self-inflating bag.
Because both T-piece resuscitators and flow-inflating bags require a compressed gas source to function, a self-inflating bag should be available as a backup in the event of compressed gas failure when using either of these devices.
Use of a supraglottic airway may be considered as the primary interface to administer PPV instead of a face mask for newborn infants delivered at 34 0/7 weeks’ gestation or later.
Continued Emphasis on Delayed Cord Clamping
The updated guidelines “continue to emphasize delayed cord clamping for both term and preterm newborn infants when clinically possible. There is also a new recommendation for nonvigorous infants born 35-42 weeks’ gestational age to consider umbilical cord milking,” Dr. Lee said in an interview.
Specifically, the guidelines state:
- For term and late preterm newborn infants ≥34 weeks’ gestation, and preterm newborn infants <34 weeks’ gestation, who do not require resuscitation, delayed cord clamping (≥30 seconds) can be beneficial compared with early cord clamping (<30 seconds).
- For term and late preterm newborn infants ≥34 weeks’ gestation who do not require resuscitation, intact cord milking is not known to be beneficial compared with delayed cord clamping (≥30 seconds).
- For preterm newborn infants between 28- and 34-weeks’ gestation who do not require resuscitation and in whom delayed cord clamping cannot be performed, intact cord milking may be reasonable.
- For preterm newborn infants <28 weeks’ gestation, intact cord milking is not recommended.
- For nonvigorous term and late preterm infants (35-42 weeks’ gestation), intact cord milking may be reasonable compared with early cord clamping (<30 seconds).
The guidelines also highlight the following knowledge gaps that require further research:
- Optimal management of the umbilical cord in term, late preterm, and preterm infants who require resuscitation at delivery
- Longer-term outcome data, such as anemia during infancy and neurodevelopmental outcomes, for all umbilical cord management strategies
- Cost-effectiveness of a T-piece resuscitator compared with a self-inflating bag
- The effect of a self-inflating bag with a positive end-expiratory pressure valve on outcomes in preterm newborn infants
- Comparison of either a T-piece resuscitator or a self-inflating bag with a flow-inflating bag for administering PPV
- Comparison of clinical outcomes by gestational age for any PPV device
- Comparison of supraglottic airway devices and face masks as the primary interface for PPV in high-resourced settings
- The amount and type of training required for successful supraglottic airway insertion and the potential for skill decay
- The utility of supraglottic airway devices for suctioning secretions from the airway
- The efficacy of a supraglottic airway during advanced neonatal resuscitation requiring chest compressions or the delivery of intratracheal medications
This research had no commercial funding. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
The 2023 focused update was prompted by four systematic literature reviews by the International Liaison Committee on Resuscitation (ILCOR) Neonatal Life Support Task Force.
“Evidence evaluations by the ILCOR play a large role in the group’s process and timing of updates,” Henry Lee, MD, co-chair of the writing group, said in an interview.
He noted that updated recommendations do not change prior recommendations from the 2020 guidelines.
“However, they provide additional details to consider in neonatal resuscitation that could lead to changes in some practice in various settings,” said Dr. Lee, medical director of the University of California San Diego neonatal intensive care unit.
The focused update was simultaneously published online November 16 in Circulation and in Pediatrics.
Dr. Lee noted that effective positive-pressure ventilation (PPV) is the priority in newborn infants who need support after birth.
And while the 2020 update provided some details on devices to be used for PPV, the 2023 focused update gives guidance on use of T-piece resuscitators for providing PPV, which may be particularly helpful for preterm infants, and the use of supraglottic airways as a primary interface to deliver PPV, he explained.
Specifically, the updated guidelines state that use of a T-piece resuscitator to deliver PPV is preferred to the use of a self-inflating bag.
Because both T-piece resuscitators and flow-inflating bags require a compressed gas source to function, a self-inflating bag should be available as a backup in the event of compressed gas failure when using either of these devices.
Use of a supraglottic airway may be considered as the primary interface to administer PPV instead of a face mask for newborn infants delivered at 34 0/7 weeks’ gestation or later.
Continued Emphasis on Delayed Cord Clamping
The updated guidelines “continue to emphasize delayed cord clamping for both term and preterm newborn infants when clinically possible. There is also a new recommendation for nonvigorous infants born 35-42 weeks’ gestational age to consider umbilical cord milking,” Dr. Lee said in an interview.
Specifically, the guidelines state:
- For term and late preterm newborn infants ≥34 weeks’ gestation, and preterm newborn infants <34 weeks’ gestation, who do not require resuscitation, delayed cord clamping (≥30 seconds) can be beneficial compared with early cord clamping (<30 seconds).
- For term and late preterm newborn infants ≥34 weeks’ gestation who do not require resuscitation, intact cord milking is not known to be beneficial compared with delayed cord clamping (≥30 seconds).
- For preterm newborn infants between 28- and 34-weeks’ gestation who do not require resuscitation and in whom delayed cord clamping cannot be performed, intact cord milking may be reasonable.
- For preterm newborn infants <28 weeks’ gestation, intact cord milking is not recommended.
- For nonvigorous term and late preterm infants (35-42 weeks’ gestation), intact cord milking may be reasonable compared with early cord clamping (<30 seconds).
The guidelines also highlight the following knowledge gaps that require further research:
- Optimal management of the umbilical cord in term, late preterm, and preterm infants who require resuscitation at delivery
- Longer-term outcome data, such as anemia during infancy and neurodevelopmental outcomes, for all umbilical cord management strategies
- Cost-effectiveness of a T-piece resuscitator compared with a self-inflating bag
- The effect of a self-inflating bag with a positive end-expiratory pressure valve on outcomes in preterm newborn infants
- Comparison of either a T-piece resuscitator or a self-inflating bag with a flow-inflating bag for administering PPV
- Comparison of clinical outcomes by gestational age for any PPV device
- Comparison of supraglottic airway devices and face masks as the primary interface for PPV in high-resourced settings
- The amount and type of training required for successful supraglottic airway insertion and the potential for skill decay
- The utility of supraglottic airway devices for suctioning secretions from the airway
- The efficacy of a supraglottic airway during advanced neonatal resuscitation requiring chest compressions or the delivery of intratracheal medications
This research had no commercial funding. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
The 2023 focused update was prompted by four systematic literature reviews by the International Liaison Committee on Resuscitation (ILCOR) Neonatal Life Support Task Force.
“Evidence evaluations by the ILCOR play a large role in the group’s process and timing of updates,” Henry Lee, MD, co-chair of the writing group, said in an interview.
He noted that updated recommendations do not change prior recommendations from the 2020 guidelines.
“However, they provide additional details to consider in neonatal resuscitation that could lead to changes in some practice in various settings,” said Dr. Lee, medical director of the University of California San Diego neonatal intensive care unit.
The focused update was simultaneously published online November 16 in Circulation and in Pediatrics.
Dr. Lee noted that effective positive-pressure ventilation (PPV) is the priority in newborn infants who need support after birth.
And while the 2020 update provided some details on devices to be used for PPV, the 2023 focused update gives guidance on use of T-piece resuscitators for providing PPV, which may be particularly helpful for preterm infants, and the use of supraglottic airways as a primary interface to deliver PPV, he explained.
Specifically, the updated guidelines state that use of a T-piece resuscitator to deliver PPV is preferred to the use of a self-inflating bag.
Because both T-piece resuscitators and flow-inflating bags require a compressed gas source to function, a self-inflating bag should be available as a backup in the event of compressed gas failure when using either of these devices.
Use of a supraglottic airway may be considered as the primary interface to administer PPV instead of a face mask for newborn infants delivered at 34 0/7 weeks’ gestation or later.
Continued Emphasis on Delayed Cord Clamping
The updated guidelines “continue to emphasize delayed cord clamping for both term and preterm newborn infants when clinically possible. There is also a new recommendation for nonvigorous infants born 35-42 weeks’ gestational age to consider umbilical cord milking,” Dr. Lee said in an interview.
Specifically, the guidelines state:
- For term and late preterm newborn infants ≥34 weeks’ gestation, and preterm newborn infants <34 weeks’ gestation, who do not require resuscitation, delayed cord clamping (≥30 seconds) can be beneficial compared with early cord clamping (<30 seconds).
- For term and late preterm newborn infants ≥34 weeks’ gestation who do not require resuscitation, intact cord milking is not known to be beneficial compared with delayed cord clamping (≥30 seconds).
- For preterm newborn infants between 28- and 34-weeks’ gestation who do not require resuscitation and in whom delayed cord clamping cannot be performed, intact cord milking may be reasonable.
- For preterm newborn infants <28 weeks’ gestation, intact cord milking is not recommended.
- For nonvigorous term and late preterm infants (35-42 weeks’ gestation), intact cord milking may be reasonable compared with early cord clamping (<30 seconds).
The guidelines also highlight the following knowledge gaps that require further research:
- Optimal management of the umbilical cord in term, late preterm, and preterm infants who require resuscitation at delivery
- Longer-term outcome data, such as anemia during infancy and neurodevelopmental outcomes, for all umbilical cord management strategies
- Cost-effectiveness of a T-piece resuscitator compared with a self-inflating bag
- The effect of a self-inflating bag with a positive end-expiratory pressure valve on outcomes in preterm newborn infants
- Comparison of either a T-piece resuscitator or a self-inflating bag with a flow-inflating bag for administering PPV
- Comparison of clinical outcomes by gestational age for any PPV device
- Comparison of supraglottic airway devices and face masks as the primary interface for PPV in high-resourced settings
- The amount and type of training required for successful supraglottic airway insertion and the potential for skill decay
- The utility of supraglottic airway devices for suctioning secretions from the airway
- The efficacy of a supraglottic airway during advanced neonatal resuscitation requiring chest compressions or the delivery of intratracheal medications
This research had no commercial funding. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Psychosocial environmental factors may drive persistent childhood asthma
TOPLINE:
Children with asthma exposed to worsening psychosocial environmental factors during childhood were more likely to have more severe asthma symptoms than those without such exposures.
METHODOLOGY:
- The researchers reviewed data from the Longitudinal Study of Australian Children, a nationally representative cohort that also collects data on the health, psychosocial, and environmental status of parents, and used three multivariate models to assess the impact of psychosocial environmental factors on asthma symptoms at ages 1 year, 4-5 years, and 14-15 years.
- The study population included 3,917 children aged 0-15 years who were sorted into three asthma symptom trajectory groups (low/no asthma, transient high asthma, and persistent high asthma); asthma symptoms were defined as a history of chest wheezing lasting at least a week within the past 12 months.
- The researchers identified several psychosocial environmental factors as exposure variables on the basis of literature reviews; these factors were maternal depression, parents’ financial hardship, parental availability, and parental stressful life events.
TAKEAWAY:
- The mean scores of psychosocial factors for the overall study population remained stable over time, but groups of children exposed to bad trajectories of psychosocial factors were significantly more likely to have transient high and persistent high asthma symptoms.
- In the first year of life, only parents’ stressful life events were significantly associated with the persistent high asthma symptom trajectory group in an adjusted analysis.
- At age 4-5 years, maternal depression, low parental availability, and parents’ stressful life events were significantly associated with persistent high asthma; parents’ financial hardship was significantly associated with transient high asthma symptoms.
- At age 14-15 years, children exposed to “moderate and increasing” maternal depression, “moderate and declining” parents’ financial hardship, and “moderate and increasing” parents’ stressful life events were significantly associated with persistent high asthma versus no or low asthma, with relative risk ratios of 1.55, 1.40, and 1.77, respectively.
IN PRACTICE:
The study findings highlight the need for policy makers to take action to improve asthma control in children by reducing exposure to harmful psychosocial environmental factors, the researchers concluded.
SOURCE:
The lead author of the study was K.M. Shahunja, MBBS, PhD candidate at the University of Queensland, Brisbane, Australia. The study was published online in Pediatric Pulmonology.
LIMITATIONS:
The study is the first known to examine asthma symptom trajectories at different developmental stages, but participant attrition and missing values were limiting factors, as was the inability to account for all potential psychosocial environmental factors that might influence asthma symptoms in childhood.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
Children with asthma exposed to worsening psychosocial environmental factors during childhood were more likely to have more severe asthma symptoms than those without such exposures.
METHODOLOGY:
- The researchers reviewed data from the Longitudinal Study of Australian Children, a nationally representative cohort that also collects data on the health, psychosocial, and environmental status of parents, and used three multivariate models to assess the impact of psychosocial environmental factors on asthma symptoms at ages 1 year, 4-5 years, and 14-15 years.
- The study population included 3,917 children aged 0-15 years who were sorted into three asthma symptom trajectory groups (low/no asthma, transient high asthma, and persistent high asthma); asthma symptoms were defined as a history of chest wheezing lasting at least a week within the past 12 months.
- The researchers identified several psychosocial environmental factors as exposure variables on the basis of literature reviews; these factors were maternal depression, parents’ financial hardship, parental availability, and parental stressful life events.
TAKEAWAY:
- The mean scores of psychosocial factors for the overall study population remained stable over time, but groups of children exposed to bad trajectories of psychosocial factors were significantly more likely to have transient high and persistent high asthma symptoms.
- In the first year of life, only parents’ stressful life events were significantly associated with the persistent high asthma symptom trajectory group in an adjusted analysis.
- At age 4-5 years, maternal depression, low parental availability, and parents’ stressful life events were significantly associated with persistent high asthma; parents’ financial hardship was significantly associated with transient high asthma symptoms.
- At age 14-15 years, children exposed to “moderate and increasing” maternal depression, “moderate and declining” parents’ financial hardship, and “moderate and increasing” parents’ stressful life events were significantly associated with persistent high asthma versus no or low asthma, with relative risk ratios of 1.55, 1.40, and 1.77, respectively.
IN PRACTICE:
The study findings highlight the need for policy makers to take action to improve asthma control in children by reducing exposure to harmful psychosocial environmental factors, the researchers concluded.
SOURCE:
The lead author of the study was K.M. Shahunja, MBBS, PhD candidate at the University of Queensland, Brisbane, Australia. The study was published online in Pediatric Pulmonology.
LIMITATIONS:
The study is the first known to examine asthma symptom trajectories at different developmental stages, but participant attrition and missing values were limiting factors, as was the inability to account for all potential psychosocial environmental factors that might influence asthma symptoms in childhood.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
Children with asthma exposed to worsening psychosocial environmental factors during childhood were more likely to have more severe asthma symptoms than those without such exposures.
METHODOLOGY:
- The researchers reviewed data from the Longitudinal Study of Australian Children, a nationally representative cohort that also collects data on the health, psychosocial, and environmental status of parents, and used three multivariate models to assess the impact of psychosocial environmental factors on asthma symptoms at ages 1 year, 4-5 years, and 14-15 years.
- The study population included 3,917 children aged 0-15 years who were sorted into three asthma symptom trajectory groups (low/no asthma, transient high asthma, and persistent high asthma); asthma symptoms were defined as a history of chest wheezing lasting at least a week within the past 12 months.
- The researchers identified several psychosocial environmental factors as exposure variables on the basis of literature reviews; these factors were maternal depression, parents’ financial hardship, parental availability, and parental stressful life events.
TAKEAWAY:
- The mean scores of psychosocial factors for the overall study population remained stable over time, but groups of children exposed to bad trajectories of psychosocial factors were significantly more likely to have transient high and persistent high asthma symptoms.
- In the first year of life, only parents’ stressful life events were significantly associated with the persistent high asthma symptom trajectory group in an adjusted analysis.
- At age 4-5 years, maternal depression, low parental availability, and parents’ stressful life events were significantly associated with persistent high asthma; parents’ financial hardship was significantly associated with transient high asthma symptoms.
- At age 14-15 years, children exposed to “moderate and increasing” maternal depression, “moderate and declining” parents’ financial hardship, and “moderate and increasing” parents’ stressful life events were significantly associated with persistent high asthma versus no or low asthma, with relative risk ratios of 1.55, 1.40, and 1.77, respectively.
IN PRACTICE:
The study findings highlight the need for policy makers to take action to improve asthma control in children by reducing exposure to harmful psychosocial environmental factors, the researchers concluded.
SOURCE:
The lead author of the study was K.M. Shahunja, MBBS, PhD candidate at the University of Queensland, Brisbane, Australia. The study was published online in Pediatric Pulmonology.
LIMITATIONS:
The study is the first known to examine asthma symptom trajectories at different developmental stages, but participant attrition and missing values were limiting factors, as was the inability to account for all potential psychosocial environmental factors that might influence asthma symptoms in childhood.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.