User login
Vaccinium myrtillus (bilberry seed oil) extract
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
Combination of energy-based treatments found to improve Becker’s nevi
Denver – out to 40 weeks, results of a small retrospective case series demonstrated.
During an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery, presenting author Shelby L. Kubicki, MD, said that NAFR and LHR target the clinically bothersome Becker’s nevi features of hyperpigmentation and hypertrichosis via different mechanisms. “NAFR creates microcolumns of thermal injury in the skin, which improves hyperpigmentation,” explained Dr. Kubicki, a 3rd-year dermatology resident at University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston.
“LHR targets follicular melanocytes, which are located more deeply in the dermis,” she said. “This improves hypertrichosis and likely prevents recurrence of hyperpigmentation by targeting these melanocytes that are not reached by NAFR.”
Dr. Kubicki and her colleagues retrospectively reviewed 12 patients with Becker’s nevus who underwent a mean of 5.3 NAFR treatments at a single dermatology practice at intervals that ranged between 1 and 4 months. The long-pulsed 755-nm alexandrite laser was used for study participants with skin types I-III, while the long-pulsed 1,064-nm Nd: YAG laser was used for those with skin types IV-VI. Ten of the 12 patients underwent concomitant LHR with one of the two devices and three independent physicians used a 5-point visual analog scale (VAS) to rate clinical photographs. All patients completed a strict pre- and postoperative regimen with either 4% hydroquinone or topical 3% tranexamic acid and broad-spectrum sunscreen and postoperative treatment with a midpotency topical corticosteroid for 3 days.
The study is the largest known case series of therapy combining 1,550-nm NAFR and LHR for Becker’s nevus patients with skin types III-VI.
After comparing VAS scores at baseline and follow-up, physicians rated the cosmetic appearance of Becker’s nevus as improving by a range of 51%-75%. Two patients did not undergo LHR: one male patient with Becker’s nevus in his beard region, for whom LHR was undesirable, and a second patient with atrichotic Becker’s nevus. These two patients demonstrated improvements in VAS scores of 26%-50% and 76%-99%, respectively.
No long-term adverse events were observed during follow-up, which ranged from 6 to 40 weeks. “We do want more long-term follow-up,” Dr. Kubicki said, noting that there are more data on some patients to extend the follow-up.
She and her coinvestigators concluded that the results show that treatment with a combination of NAFR and LHR safely addresses both hyperpigmentation and hypertrichosis in Becker’s nevi. “In addition, LHR likely prevents recurrence of hyperpigmentation by targeting follicular melanocytes,” she said. “In our study, we did have one patient experience recurrence of a Becker’s nevus during follow-up, but [the rest] did not, which we considered a success.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on the study, characterized Becker’s nevus as a difficult-to-treat condition that is made even more difficult to treat in skin types III-VI.
“Combining laser hair removal using appropriate wavelengths with 1,550-nm nonablative fractional resurfacing yielded good clinical results with few recurrences,” he said in an interview with this news organization. “Though it was a small series, it definitely is an interesting option for practicing dermatologists who encounter patients interested in improving the appearance of a Becker’s nevus.”
The researchers reported having no relevant disclosures.
Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory boards for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
Denver – out to 40 weeks, results of a small retrospective case series demonstrated.
During an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery, presenting author Shelby L. Kubicki, MD, said that NAFR and LHR target the clinically bothersome Becker’s nevi features of hyperpigmentation and hypertrichosis via different mechanisms. “NAFR creates microcolumns of thermal injury in the skin, which improves hyperpigmentation,” explained Dr. Kubicki, a 3rd-year dermatology resident at University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston.
“LHR targets follicular melanocytes, which are located more deeply in the dermis,” she said. “This improves hypertrichosis and likely prevents recurrence of hyperpigmentation by targeting these melanocytes that are not reached by NAFR.”
Dr. Kubicki and her colleagues retrospectively reviewed 12 patients with Becker’s nevus who underwent a mean of 5.3 NAFR treatments at a single dermatology practice at intervals that ranged between 1 and 4 months. The long-pulsed 755-nm alexandrite laser was used for study participants with skin types I-III, while the long-pulsed 1,064-nm Nd: YAG laser was used for those with skin types IV-VI. Ten of the 12 patients underwent concomitant LHR with one of the two devices and three independent physicians used a 5-point visual analog scale (VAS) to rate clinical photographs. All patients completed a strict pre- and postoperative regimen with either 4% hydroquinone or topical 3% tranexamic acid and broad-spectrum sunscreen and postoperative treatment with a midpotency topical corticosteroid for 3 days.
The study is the largest known case series of therapy combining 1,550-nm NAFR and LHR for Becker’s nevus patients with skin types III-VI.
After comparing VAS scores at baseline and follow-up, physicians rated the cosmetic appearance of Becker’s nevus as improving by a range of 51%-75%. Two patients did not undergo LHR: one male patient with Becker’s nevus in his beard region, for whom LHR was undesirable, and a second patient with atrichotic Becker’s nevus. These two patients demonstrated improvements in VAS scores of 26%-50% and 76%-99%, respectively.
No long-term adverse events were observed during follow-up, which ranged from 6 to 40 weeks. “We do want more long-term follow-up,” Dr. Kubicki said, noting that there are more data on some patients to extend the follow-up.
She and her coinvestigators concluded that the results show that treatment with a combination of NAFR and LHR safely addresses both hyperpigmentation and hypertrichosis in Becker’s nevi. “In addition, LHR likely prevents recurrence of hyperpigmentation by targeting follicular melanocytes,” she said. “In our study, we did have one patient experience recurrence of a Becker’s nevus during follow-up, but [the rest] did not, which we considered a success.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on the study, characterized Becker’s nevus as a difficult-to-treat condition that is made even more difficult to treat in skin types III-VI.
“Combining laser hair removal using appropriate wavelengths with 1,550-nm nonablative fractional resurfacing yielded good clinical results with few recurrences,” he said in an interview with this news organization. “Though it was a small series, it definitely is an interesting option for practicing dermatologists who encounter patients interested in improving the appearance of a Becker’s nevus.”
The researchers reported having no relevant disclosures.
Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory boards for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
Denver – out to 40 weeks, results of a small retrospective case series demonstrated.
During an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery, presenting author Shelby L. Kubicki, MD, said that NAFR and LHR target the clinically bothersome Becker’s nevi features of hyperpigmentation and hypertrichosis via different mechanisms. “NAFR creates microcolumns of thermal injury in the skin, which improves hyperpigmentation,” explained Dr. Kubicki, a 3rd-year dermatology resident at University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston.
“LHR targets follicular melanocytes, which are located more deeply in the dermis,” she said. “This improves hypertrichosis and likely prevents recurrence of hyperpigmentation by targeting these melanocytes that are not reached by NAFR.”
Dr. Kubicki and her colleagues retrospectively reviewed 12 patients with Becker’s nevus who underwent a mean of 5.3 NAFR treatments at a single dermatology practice at intervals that ranged between 1 and 4 months. The long-pulsed 755-nm alexandrite laser was used for study participants with skin types I-III, while the long-pulsed 1,064-nm Nd: YAG laser was used for those with skin types IV-VI. Ten of the 12 patients underwent concomitant LHR with one of the two devices and three independent physicians used a 5-point visual analog scale (VAS) to rate clinical photographs. All patients completed a strict pre- and postoperative regimen with either 4% hydroquinone or topical 3% tranexamic acid and broad-spectrum sunscreen and postoperative treatment with a midpotency topical corticosteroid for 3 days.
The study is the largest known case series of therapy combining 1,550-nm NAFR and LHR for Becker’s nevus patients with skin types III-VI.
After comparing VAS scores at baseline and follow-up, physicians rated the cosmetic appearance of Becker’s nevus as improving by a range of 51%-75%. Two patients did not undergo LHR: one male patient with Becker’s nevus in his beard region, for whom LHR was undesirable, and a second patient with atrichotic Becker’s nevus. These two patients demonstrated improvements in VAS scores of 26%-50% and 76%-99%, respectively.
No long-term adverse events were observed during follow-up, which ranged from 6 to 40 weeks. “We do want more long-term follow-up,” Dr. Kubicki said, noting that there are more data on some patients to extend the follow-up.
She and her coinvestigators concluded that the results show that treatment with a combination of NAFR and LHR safely addresses both hyperpigmentation and hypertrichosis in Becker’s nevi. “In addition, LHR likely prevents recurrence of hyperpigmentation by targeting follicular melanocytes,” she said. “In our study, we did have one patient experience recurrence of a Becker’s nevus during follow-up, but [the rest] did not, which we considered a success.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on the study, characterized Becker’s nevus as a difficult-to-treat condition that is made even more difficult to treat in skin types III-VI.
“Combining laser hair removal using appropriate wavelengths with 1,550-nm nonablative fractional resurfacing yielded good clinical results with few recurrences,” he said in an interview with this news organization. “Though it was a small series, it definitely is an interesting option for practicing dermatologists who encounter patients interested in improving the appearance of a Becker’s nevus.”
The researchers reported having no relevant disclosures.
Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory boards for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
AT ASDS 2022
Unusual Bilateral Distribution of Neurofibromatosis Type 5 on the Distal Upper Extremities
To the Editor:
Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosis type 1 (NF1)(also known as von Recklinghausen disease). Phenotypic manifestations of NF5 include café-au-lait macules, neurofibromas, or both in 1 or more adjacent dermatomes. In contrast to the systemic features of NF1, the dermatomal distribution of NF5 demonstrates mosaicism due to a spontaneous postzygotic mutation in the neurofibromin 1 gene, NF1. We describe an atypical presentation of NF5 with bilateral features on the upper extremities.
A 74-year-old woman presented with soft pink- to flesh-colored growths on the left dorsal forearm and hand that were observed incidentally during a Mohs procedure for treatment of a basal cell carcinoma on the upper cutaneous lip. The patient reported that the lesions initially appeared on the left dorsal hand at approximately 16 years of age and had since spread proximally up to the mid dorsal forearm over the course of her lifetime. She denied any pain but claimed the affected area could be itchy. The lesions did not interfere with her daily activities, but they negatively impacted her social life due to their cosmetic appearance as well as her fear that they could be contagious. She denied any family history of NF1.
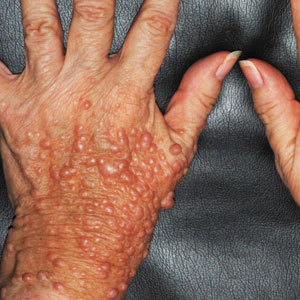
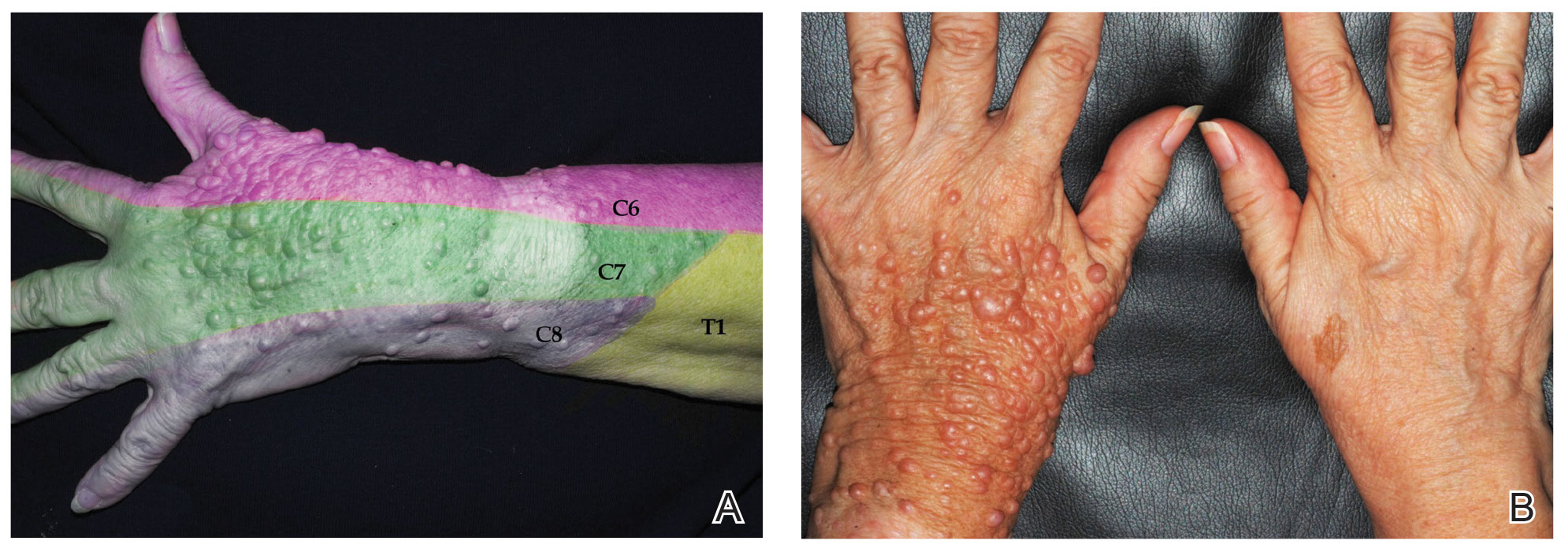
Physical examination revealed innumerable soft, pink- to flesh-colored cutaneous nodules ranging from 3 to 9 mm in diameter clustered uniformly on the left dorsal hand and lower forearm within the C6, C7, and C8 dermatomal regions (Figure, A). A singular brown patch measuring 20 mm in diameter also was observed on the right dorsal hand within the C6 dermatome, which the patient reported had been present since birth (Figure, B). The nodules and pigmented patch were clinically diagnosed as cutaneous neurofibromas on the left arm and a café-au-lait macule on the right arm, each manifesting within the C6 dermatome on separate upper extremities. Lisch nodules, axillary freckling, and acoustic schwannomas were not observed. Because of the dermatomal distribution of the lesions and lack of family history of NF1, a diagnosis of bilateral NF5 was made. The patient stated she had declined treatment of the neurofibromas from her referring general dermatologist due to possible risk for recurrence.
Segmental neurofibromatosis was first described in 1931 by Gammel,1 and in 1982, segmental neurofibromatosis was classified as NF5 by Riccardi.2 After Tinschert et al3 later demonstrated NF5 to be a somatic mutation of NF1,3 Ruggieri and Huson4 proposed the term mosaic neurofibromatosis 1 in 2001.
While the prevalence of NF1 is 1 in 3000 individuals,5 NF5 is rare with an occurrence of 1 in 40,000.6 In NF5, a spontaneous NF1 gene mutation occurs on chromosome 17 in a dividing cell after conception.7 Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the NF1 gene.8 This contrasts with the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells. Patients with NF5 generally are not expected to have affected offspring because the spontaneous mutation usually arises in somatic cells; however, a postzygotic mutation in the gonadal region could potentially affect germline cells, resulting in vertical transmission, with documented cases of offspring with systemic NF1.4 Because of the risk for malignancy with systemic neurofibromatosis, early diagnosis with genetic counseling is imperative in patients with both NF1 and NF5.
Neurofibromatosis type 5 is a clinical diagnosis based on the presence of neurofibromas and/or café-au-lait macules in a dermatomal distribution. The clinical presentation depends on when and where the NF1 gene mutation occurs in utero as cells multiply, differentiate, and migrate.8 Earlier mutations result in a broader manifestation of NF5 in comparison to late mutations, which have more localized features. An NF1 gene mutation causes a loss of function of neurofibromin, a tumor suppressor protein, in Schwann cells and fibroblasts.8 This produces neurofibromas and café-au-lait macules, respectively.8
A large literature review on segmental neurofibromatosis by Garcia-Romero et al6 identified 320 individuals who did not meet full inclusion criteria for NF1 between 1977 and 2012. Overall, 76% of cases were unilaterally distributed. The investigators identified 157 individual case reports in which the most to least common presentation was pigmentary changes only, neurofibromas only, mixed pigmentary changes with neurofibromas, and plexiform neurofibromas only; however, many of these cases were children who may have later developed both neurofibromas and pigmentary changes during puberty.6 Additional features of NF5 may include freckling, Lisch nodules, optic gliomas, malignant peripheral nerve sheath tumors, skeletal abnormalities, precocious puberty, vascular malformations, hypertension, seizures, and/or learning difficulties based on the affected anatomy.
Segmental neurofibromatosis, or NF5, is a rare subtype of NF1. Our case demonstrates an unusual bilateral distribution of NF5 with cutaneous neurofibromas and a café-au-lait macule on the upper extremities. Awareness of variations of neurofibromatosis and their genetic implications is essential in establishing earlier clinical diagnoses in cases with subtle manifestations.
- Gammel JA. Localized neurofibromatosis. Arch Dermatol. 1931;24:712-713.
- Riccardi VM. Neurofibromatosis: clinical heterogeneity. Curr Probl Cancer. 1982;7:1-34.
- Tinschert S, Naumann I, Stegmann E, et al. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur J Hum Genet. 2000;8:455-459.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Crowe FW, Schull WJ, Neel JV. A Clinical, Pathological and Genetic Study of Multiple Neurofibromatosis. Charles C Thomas; 1956.
- García-Romero MT, Parkin P, Lara-Corrales I. Mosaic neurofibromatosis type 1: a systematic review. Pediatr Dermatol. 2016;33:9-17.
- Ledbetter DH, Rich DC, O’Connell P, et al. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989;44:20-24.
- Redlick FP, Shaw JC. Segmental neurofibromatosis follows Blaschko’s lines or dermatomes depending on the cell line affected: case report and literature review. J Cutan Med Surg. 2004;8:353-356.
To the Editor:
Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosis type 1 (NF1)(also known as von Recklinghausen disease). Phenotypic manifestations of NF5 include café-au-lait macules, neurofibromas, or both in 1 or more adjacent dermatomes. In contrast to the systemic features of NF1, the dermatomal distribution of NF5 demonstrates mosaicism due to a spontaneous postzygotic mutation in the neurofibromin 1 gene, NF1. We describe an atypical presentation of NF5 with bilateral features on the upper extremities.
A 74-year-old woman presented with soft pink- to flesh-colored growths on the left dorsal forearm and hand that were observed incidentally during a Mohs procedure for treatment of a basal cell carcinoma on the upper cutaneous lip. The patient reported that the lesions initially appeared on the left dorsal hand at approximately 16 years of age and had since spread proximally up to the mid dorsal forearm over the course of her lifetime. She denied any pain but claimed the affected area could be itchy. The lesions did not interfere with her daily activities, but they negatively impacted her social life due to their cosmetic appearance as well as her fear that they could be contagious. She denied any family history of NF1.
Physical examination revealed innumerable soft, pink- to flesh-colored cutaneous nodules ranging from 3 to 9 mm in diameter clustered uniformly on the left dorsal hand and lower forearm within the C6, C7, and C8 dermatomal regions (Figure, A). A singular brown patch measuring 20 mm in diameter also was observed on the right dorsal hand within the C6 dermatome, which the patient reported had been present since birth (Figure, B). The nodules and pigmented patch were clinically diagnosed as cutaneous neurofibromas on the left arm and a café-au-lait macule on the right arm, each manifesting within the C6 dermatome on separate upper extremities. Lisch nodules, axillary freckling, and acoustic schwannomas were not observed. Because of the dermatomal distribution of the lesions and lack of family history of NF1, a diagnosis of bilateral NF5 was made. The patient stated she had declined treatment of the neurofibromas from her referring general dermatologist due to possible risk for recurrence.

Segmental neurofibromatosis was first described in 1931 by Gammel,1 and in 1982, segmental neurofibromatosis was classified as NF5 by Riccardi.2 After Tinschert et al3 later demonstrated NF5 to be a somatic mutation of NF1,3 Ruggieri and Huson4 proposed the term mosaic neurofibromatosis 1 in 2001.
While the prevalence of NF1 is 1 in 3000 individuals,5 NF5 is rare with an occurrence of 1 in 40,000.6 In NF5, a spontaneous NF1 gene mutation occurs on chromosome 17 in a dividing cell after conception.7 Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the NF1 gene.8 This contrasts with the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells. Patients with NF5 generally are not expected to have affected offspring because the spontaneous mutation usually arises in somatic cells; however, a postzygotic mutation in the gonadal region could potentially affect germline cells, resulting in vertical transmission, with documented cases of offspring with systemic NF1.4 Because of the risk for malignancy with systemic neurofibromatosis, early diagnosis with genetic counseling is imperative in patients with both NF1 and NF5.
Neurofibromatosis type 5 is a clinical diagnosis based on the presence of neurofibromas and/or café-au-lait macules in a dermatomal distribution. The clinical presentation depends on when and where the NF1 gene mutation occurs in utero as cells multiply, differentiate, and migrate.8 Earlier mutations result in a broader manifestation of NF5 in comparison to late mutations, which have more localized features. An NF1 gene mutation causes a loss of function of neurofibromin, a tumor suppressor protein, in Schwann cells and fibroblasts.8 This produces neurofibromas and café-au-lait macules, respectively.8
A large literature review on segmental neurofibromatosis by Garcia-Romero et al6 identified 320 individuals who did not meet full inclusion criteria for NF1 between 1977 and 2012. Overall, 76% of cases were unilaterally distributed. The investigators identified 157 individual case reports in which the most to least common presentation was pigmentary changes only, neurofibromas only, mixed pigmentary changes with neurofibromas, and plexiform neurofibromas only; however, many of these cases were children who may have later developed both neurofibromas and pigmentary changes during puberty.6 Additional features of NF5 may include freckling, Lisch nodules, optic gliomas, malignant peripheral nerve sheath tumors, skeletal abnormalities, precocious puberty, vascular malformations, hypertension, seizures, and/or learning difficulties based on the affected anatomy.
Segmental neurofibromatosis, or NF5, is a rare subtype of NF1. Our case demonstrates an unusual bilateral distribution of NF5 with cutaneous neurofibromas and a café-au-lait macule on the upper extremities. Awareness of variations of neurofibromatosis and their genetic implications is essential in establishing earlier clinical diagnoses in cases with subtle manifestations.
To the Editor:
Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosis type 1 (NF1)(also known as von Recklinghausen disease). Phenotypic manifestations of NF5 include café-au-lait macules, neurofibromas, or both in 1 or more adjacent dermatomes. In contrast to the systemic features of NF1, the dermatomal distribution of NF5 demonstrates mosaicism due to a spontaneous postzygotic mutation in the neurofibromin 1 gene, NF1. We describe an atypical presentation of NF5 with bilateral features on the upper extremities.
A 74-year-old woman presented with soft pink- to flesh-colored growths on the left dorsal forearm and hand that were observed incidentally during a Mohs procedure for treatment of a basal cell carcinoma on the upper cutaneous lip. The patient reported that the lesions initially appeared on the left dorsal hand at approximately 16 years of age and had since spread proximally up to the mid dorsal forearm over the course of her lifetime. She denied any pain but claimed the affected area could be itchy. The lesions did not interfere with her daily activities, but they negatively impacted her social life due to their cosmetic appearance as well as her fear that they could be contagious. She denied any family history of NF1.
Physical examination revealed innumerable soft, pink- to flesh-colored cutaneous nodules ranging from 3 to 9 mm in diameter clustered uniformly on the left dorsal hand and lower forearm within the C6, C7, and C8 dermatomal regions (Figure, A). A singular brown patch measuring 20 mm in diameter also was observed on the right dorsal hand within the C6 dermatome, which the patient reported had been present since birth (Figure, B). The nodules and pigmented patch were clinically diagnosed as cutaneous neurofibromas on the left arm and a café-au-lait macule on the right arm, each manifesting within the C6 dermatome on separate upper extremities. Lisch nodules, axillary freckling, and acoustic schwannomas were not observed. Because of the dermatomal distribution of the lesions and lack of family history of NF1, a diagnosis of bilateral NF5 was made. The patient stated she had declined treatment of the neurofibromas from her referring general dermatologist due to possible risk for recurrence.

Segmental neurofibromatosis was first described in 1931 by Gammel,1 and in 1982, segmental neurofibromatosis was classified as NF5 by Riccardi.2 After Tinschert et al3 later demonstrated NF5 to be a somatic mutation of NF1,3 Ruggieri and Huson4 proposed the term mosaic neurofibromatosis 1 in 2001.
While the prevalence of NF1 is 1 in 3000 individuals,5 NF5 is rare with an occurrence of 1 in 40,000.6 In NF5, a spontaneous NF1 gene mutation occurs on chromosome 17 in a dividing cell after conception.7 Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the NF1 gene.8 This contrasts with the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells. Patients with NF5 generally are not expected to have affected offspring because the spontaneous mutation usually arises in somatic cells; however, a postzygotic mutation in the gonadal region could potentially affect germline cells, resulting in vertical transmission, with documented cases of offspring with systemic NF1.4 Because of the risk for malignancy with systemic neurofibromatosis, early diagnosis with genetic counseling is imperative in patients with both NF1 and NF5.
Neurofibromatosis type 5 is a clinical diagnosis based on the presence of neurofibromas and/or café-au-lait macules in a dermatomal distribution. The clinical presentation depends on when and where the NF1 gene mutation occurs in utero as cells multiply, differentiate, and migrate.8 Earlier mutations result in a broader manifestation of NF5 in comparison to late mutations, which have more localized features. An NF1 gene mutation causes a loss of function of neurofibromin, a tumor suppressor protein, in Schwann cells and fibroblasts.8 This produces neurofibromas and café-au-lait macules, respectively.8
A large literature review on segmental neurofibromatosis by Garcia-Romero et al6 identified 320 individuals who did not meet full inclusion criteria for NF1 between 1977 and 2012. Overall, 76% of cases were unilaterally distributed. The investigators identified 157 individual case reports in which the most to least common presentation was pigmentary changes only, neurofibromas only, mixed pigmentary changes with neurofibromas, and plexiform neurofibromas only; however, many of these cases were children who may have later developed both neurofibromas and pigmentary changes during puberty.6 Additional features of NF5 may include freckling, Lisch nodules, optic gliomas, malignant peripheral nerve sheath tumors, skeletal abnormalities, precocious puberty, vascular malformations, hypertension, seizures, and/or learning difficulties based on the affected anatomy.
Segmental neurofibromatosis, or NF5, is a rare subtype of NF1. Our case demonstrates an unusual bilateral distribution of NF5 with cutaneous neurofibromas and a café-au-lait macule on the upper extremities. Awareness of variations of neurofibromatosis and their genetic implications is essential in establishing earlier clinical diagnoses in cases with subtle manifestations.
- Gammel JA. Localized neurofibromatosis. Arch Dermatol. 1931;24:712-713.
- Riccardi VM. Neurofibromatosis: clinical heterogeneity. Curr Probl Cancer. 1982;7:1-34.
- Tinschert S, Naumann I, Stegmann E, et al. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur J Hum Genet. 2000;8:455-459.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Crowe FW, Schull WJ, Neel JV. A Clinical, Pathological and Genetic Study of Multiple Neurofibromatosis. Charles C Thomas; 1956.
- García-Romero MT, Parkin P, Lara-Corrales I. Mosaic neurofibromatosis type 1: a systematic review. Pediatr Dermatol. 2016;33:9-17.
- Ledbetter DH, Rich DC, O’Connell P, et al. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989;44:20-24.
- Redlick FP, Shaw JC. Segmental neurofibromatosis follows Blaschko’s lines or dermatomes depending on the cell line affected: case report and literature review. J Cutan Med Surg. 2004;8:353-356.
- Gammel JA. Localized neurofibromatosis. Arch Dermatol. 1931;24:712-713.
- Riccardi VM. Neurofibromatosis: clinical heterogeneity. Curr Probl Cancer. 1982;7:1-34.
- Tinschert S, Naumann I, Stegmann E, et al. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur J Hum Genet. 2000;8:455-459.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Crowe FW, Schull WJ, Neel JV. A Clinical, Pathological and Genetic Study of Multiple Neurofibromatosis. Charles C Thomas; 1956.
- García-Romero MT, Parkin P, Lara-Corrales I. Mosaic neurofibromatosis type 1: a systematic review. Pediatr Dermatol. 2016;33:9-17.
- Ledbetter DH, Rich DC, O’Connell P, et al. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989;44:20-24.
- Redlick FP, Shaw JC. Segmental neurofibromatosis follows Blaschko’s lines or dermatomes depending on the cell line affected: case report and literature review. J Cutan Med Surg. 2004;8:353-356.
Practice Points
- Segmental neurofibromatosis, or neurofibromatosis type 5 (NF5), is a rare subtype of neurofibromatosistype 1 (NF1)(also known as von Recklinghausen disease).
- Individuals with NF5 are born mosaic with 2 genotypes—one normal and one abnormal—for the neurofibromin 1 gene, NF1. This is in contrast to the autosomal-dominant and systemic characteristics of NF1, which has the NF1 gene mutation in all cells.
Ruxolitinib repigments many vitiligo-affected body areas
.
Those difficult areas include the hands and feet, said Thierry Passeron, MD, PhD, of Université Côte d’Azur and Centre Hospitalier Universitaire de Nice (France).
Indeed, a 50% or greater improvement in the Vitiligo Area Scoring Index (VASI-50) of the hands and feet was achieved with ruxolitinib cream (Opzelura) in around one-third of patients after 52 weeks’ treatment, and more than half of patients showed improvement in the upper and lower extremities.
During one of the late-breaking news sessions, Dr. Passeron presented a pooled analysis of the Topical Ruxolitinib Evaluation in Vitiligo Study 1 (TRuE-V1) and Study 2 (TruE-V2), which assessed VASI-50 data by body regions.
Similarly positive results were seen on the head and neck and the trunk, with VASI-50 being reached in a respective 68% and 48% of patients after a full year of treatment.
“VASI-50 response rates rose steadily through 52 weeks for both the head and trunk,” said Dr. Passeron. He noted that the trials were initially double-blinded for 24 weeks and that there was a further open-label extension phase through week 52.
In the latter phase, all patients were treated with ruxolitinib; those who originally received a vehicle agent as placebo crossed over to the active treatment.
First FDA-approved treatment for adults and adolescents with vitiligo
Ruxolitinib is a Janus kinase 1/2 inhibitor that has been available for the treatment for atopic dermatitis for more than a year. It was recently approved by the U.S. Food and Drug Administration for the treatment of vitiligo in adults and pediatric patients aged 12 years and older.
This approval was based on the positive findings of the TRuE-V1 and TRuE-V2 studies, which showed that after 24 weeks, 30% of patients treated with ruxolitinib had at least 75% improvement in the facial VASI, compared with 10% of placebo-treated patients.
“These studies demonstrated very nice results, especially on the face, which is the easiest part to repigment in vitiligo,” Dr. Passeron said.
“We know that the location is very important when it comes to repigmentation of vitiligo,” he added. He noted that other body areas, “the extremities, for example, are much more difficult.”
The analysis he presented specifically assessed the effect of ruxolitinib cream on repigmentation in other areas.
Pooled analysis performed
Data from the two TRuE-V trials were pooled. The new analysis included a total of 661 individuals; of those patients, 443 had been treated with topical ruxolitinib, and 218 had received a vehicle cream as a placebo.
For the first 24 weeks, patients received twice-daily 1.5% ruxolitinib cream or vehicle cream. This was followed by a 28-week extension phase in which everyone was treated with ruxolitinib cream, after which there was a 30-day final follow-up period.
Dr. Passeron reported data by body region for weeks 12, 24, and 52, which showed an increasing percentage of patients with VASI-50.
“We didn’t look at the face; that we know well, that is a very good result,” he said.
The best results were seen for the head and neck. VASI-50 was reached by 28.3%, 45.3%, and 68.1% of patients treated with ruxolitinib cream at weeks 12, 24, and 52, respectively. Corresponding rates for the placebo-crossover group were 19.8%, 23.8%, and 51%.
Repigmentation rates of the hand, upper extremities, trunk, lower extremities, and feet were about 9%-15% for both ruxolitinib and placebo at 12 weeks, but by 24 weeks, there was a clear increase in repigmentation rates in the ruxolitinib group for all body areas.
The 24-week VASI-50 rates for hand repigmentation were 24.9% for ruxolitinib cream and 14.4% for placebo. Corresponding rates for upper extremity repigmentation were 33.2% and 8.2%; for the trunk, 26.4% and 12.2%; for the lower extremities, 29.5% and 12.2%; and for the feet, 18.5% and 12.5%.
“The results are quite poor at 12 weeks,” Dr. Passeron said. “It’s very important to keep this in mind; it takes time to repigment vitiligo, it takes to 6-24 months. We have to explain to our patients that they will have to wait to see the results.”
Steady improvements, no new safety concerns
Regarding VASI-50 over time, there was a steady increase in total body scores; 47.7% of patients who received ruxolitinib and 23.3% of placebo-treated patients hit this target at 52 weeks.
“And what is also very important to see is that we didn’t reach the plateau,” Dr. Passeron reported.
Similar patterns were seen for all the other body areas. Again there was a suggestion that rates may continue to rise with continued long-term treatment.
“About one-third of the patients reached at least 50% repigmentation after 1 year of treatment in the hands and feet,” Dr. Passeron said. He noted that certain areas, such as the back of the hand or tips of the fingers, may be unresponsive.
“So, we have to also to warn the patient that probably on these areas we have to combine it with other treatment because it remains very, very difficult to treat.”
There were no new safety concerns regarding treatment-emergent adverse events, which were reported in 52% of patients who received ruxolitinib and in 36% of placebo-treated patients.
The most common adverse reactions included COVID-19 (6.1% vs. 3.1%), acne at the application site (5.3% vs. 1.3%), and pruritus at the application site (3.9% vs. 2.7%), although cases were “mild or moderate,” said Dr. Passeron.
An expert’s take-home
“The results of TRuE-V phase 3 studies are encouraging and exciting,” Viktoria Eleftheriadou, MD, MRCP(UK), SCE(Derm), PhD, said in providing an independent comment for this news organization.
“Although ruxolitinib cream is applied on the skin, this novel treatment for vitiligo is not without risks; therefore, careful monitoring of patients who are started on this topical treatment would be prudent,” said Dr. Eleftheriadou, who is a consultant dermatologist for Walsall Healthcare NHS Trust and the Royal Wolverhampton NHS Trust, Birmingham, United Kingdom.
“I would like to see how many patients achieved VASI-75 or VASI-80 score, which from patients’ perspectives is a more meaningful outcome, as well as how long these results will last for,” she added.
The study was funded by Incyte Corporation. Dr. Passeron has received grants, honoraria, or both from AbbVie, ACM Pharma, Almirall, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte Corporation, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceuticals, and UCB. Dr. Passeron is the cofounder of YUKIN Therapeutics and has patents on WNT agonists or GSK2b antagonist for repigmentation of vitiligo and on the use of CXCR3B blockers in vitiligo. Dr. Eleftheriadou is an investigator and trial development group member on the HI-Light Vitiligo Trial (specific), a lead investigator on the pilot HI-Light Vitiligo Trial, and a medical advisory panel member of the Vitiligo Society UK. Dr. Eleftheriadou also provides consultancy services to Incyte and Pfizer.
A version of this article first appeared on Medscape.com.
.
Those difficult areas include the hands and feet, said Thierry Passeron, MD, PhD, of Université Côte d’Azur and Centre Hospitalier Universitaire de Nice (France).
Indeed, a 50% or greater improvement in the Vitiligo Area Scoring Index (VASI-50) of the hands and feet was achieved with ruxolitinib cream (Opzelura) in around one-third of patients after 52 weeks’ treatment, and more than half of patients showed improvement in the upper and lower extremities.
During one of the late-breaking news sessions, Dr. Passeron presented a pooled analysis of the Topical Ruxolitinib Evaluation in Vitiligo Study 1 (TRuE-V1) and Study 2 (TruE-V2), which assessed VASI-50 data by body regions.
Similarly positive results were seen on the head and neck and the trunk, with VASI-50 being reached in a respective 68% and 48% of patients after a full year of treatment.
“VASI-50 response rates rose steadily through 52 weeks for both the head and trunk,” said Dr. Passeron. He noted that the trials were initially double-blinded for 24 weeks and that there was a further open-label extension phase through week 52.
In the latter phase, all patients were treated with ruxolitinib; those who originally received a vehicle agent as placebo crossed over to the active treatment.
First FDA-approved treatment for adults and adolescents with vitiligo
Ruxolitinib is a Janus kinase 1/2 inhibitor that has been available for the treatment for atopic dermatitis for more than a year. It was recently approved by the U.S. Food and Drug Administration for the treatment of vitiligo in adults and pediatric patients aged 12 years and older.
This approval was based on the positive findings of the TRuE-V1 and TRuE-V2 studies, which showed that after 24 weeks, 30% of patients treated with ruxolitinib had at least 75% improvement in the facial VASI, compared with 10% of placebo-treated patients.
“These studies demonstrated very nice results, especially on the face, which is the easiest part to repigment in vitiligo,” Dr. Passeron said.
“We know that the location is very important when it comes to repigmentation of vitiligo,” he added. He noted that other body areas, “the extremities, for example, are much more difficult.”
The analysis he presented specifically assessed the effect of ruxolitinib cream on repigmentation in other areas.
Pooled analysis performed
Data from the two TRuE-V trials were pooled. The new analysis included a total of 661 individuals; of those patients, 443 had been treated with topical ruxolitinib, and 218 had received a vehicle cream as a placebo.
For the first 24 weeks, patients received twice-daily 1.5% ruxolitinib cream or vehicle cream. This was followed by a 28-week extension phase in which everyone was treated with ruxolitinib cream, after which there was a 30-day final follow-up period.
Dr. Passeron reported data by body region for weeks 12, 24, and 52, which showed an increasing percentage of patients with VASI-50.
“We didn’t look at the face; that we know well, that is a very good result,” he said.
The best results were seen for the head and neck. VASI-50 was reached by 28.3%, 45.3%, and 68.1% of patients treated with ruxolitinib cream at weeks 12, 24, and 52, respectively. Corresponding rates for the placebo-crossover group were 19.8%, 23.8%, and 51%.
Repigmentation rates of the hand, upper extremities, trunk, lower extremities, and feet were about 9%-15% for both ruxolitinib and placebo at 12 weeks, but by 24 weeks, there was a clear increase in repigmentation rates in the ruxolitinib group for all body areas.
The 24-week VASI-50 rates for hand repigmentation were 24.9% for ruxolitinib cream and 14.4% for placebo. Corresponding rates for upper extremity repigmentation were 33.2% and 8.2%; for the trunk, 26.4% and 12.2%; for the lower extremities, 29.5% and 12.2%; and for the feet, 18.5% and 12.5%.
“The results are quite poor at 12 weeks,” Dr. Passeron said. “It’s very important to keep this in mind; it takes time to repigment vitiligo, it takes to 6-24 months. We have to explain to our patients that they will have to wait to see the results.”
Steady improvements, no new safety concerns
Regarding VASI-50 over time, there was a steady increase in total body scores; 47.7% of patients who received ruxolitinib and 23.3% of placebo-treated patients hit this target at 52 weeks.
“And what is also very important to see is that we didn’t reach the plateau,” Dr. Passeron reported.
Similar patterns were seen for all the other body areas. Again there was a suggestion that rates may continue to rise with continued long-term treatment.
“About one-third of the patients reached at least 50% repigmentation after 1 year of treatment in the hands and feet,” Dr. Passeron said. He noted that certain areas, such as the back of the hand or tips of the fingers, may be unresponsive.
“So, we have to also to warn the patient that probably on these areas we have to combine it with other treatment because it remains very, very difficult to treat.”
There were no new safety concerns regarding treatment-emergent adverse events, which were reported in 52% of patients who received ruxolitinib and in 36% of placebo-treated patients.
The most common adverse reactions included COVID-19 (6.1% vs. 3.1%), acne at the application site (5.3% vs. 1.3%), and pruritus at the application site (3.9% vs. 2.7%), although cases were “mild or moderate,” said Dr. Passeron.
An expert’s take-home
“The results of TRuE-V phase 3 studies are encouraging and exciting,” Viktoria Eleftheriadou, MD, MRCP(UK), SCE(Derm), PhD, said in providing an independent comment for this news organization.
“Although ruxolitinib cream is applied on the skin, this novel treatment for vitiligo is not without risks; therefore, careful monitoring of patients who are started on this topical treatment would be prudent,” said Dr. Eleftheriadou, who is a consultant dermatologist for Walsall Healthcare NHS Trust and the Royal Wolverhampton NHS Trust, Birmingham, United Kingdom.
“I would like to see how many patients achieved VASI-75 or VASI-80 score, which from patients’ perspectives is a more meaningful outcome, as well as how long these results will last for,” she added.
The study was funded by Incyte Corporation. Dr. Passeron has received grants, honoraria, or both from AbbVie, ACM Pharma, Almirall, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte Corporation, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceuticals, and UCB. Dr. Passeron is the cofounder of YUKIN Therapeutics and has patents on WNT agonists or GSK2b antagonist for repigmentation of vitiligo and on the use of CXCR3B blockers in vitiligo. Dr. Eleftheriadou is an investigator and trial development group member on the HI-Light Vitiligo Trial (specific), a lead investigator on the pilot HI-Light Vitiligo Trial, and a medical advisory panel member of the Vitiligo Society UK. Dr. Eleftheriadou also provides consultancy services to Incyte and Pfizer.
A version of this article first appeared on Medscape.com.
.
Those difficult areas include the hands and feet, said Thierry Passeron, MD, PhD, of Université Côte d’Azur and Centre Hospitalier Universitaire de Nice (France).
Indeed, a 50% or greater improvement in the Vitiligo Area Scoring Index (VASI-50) of the hands and feet was achieved with ruxolitinib cream (Opzelura) in around one-third of patients after 52 weeks’ treatment, and more than half of patients showed improvement in the upper and lower extremities.
During one of the late-breaking news sessions, Dr. Passeron presented a pooled analysis of the Topical Ruxolitinib Evaluation in Vitiligo Study 1 (TRuE-V1) and Study 2 (TruE-V2), which assessed VASI-50 data by body regions.
Similarly positive results were seen on the head and neck and the trunk, with VASI-50 being reached in a respective 68% and 48% of patients after a full year of treatment.
“VASI-50 response rates rose steadily through 52 weeks for both the head and trunk,” said Dr. Passeron. He noted that the trials were initially double-blinded for 24 weeks and that there was a further open-label extension phase through week 52.
In the latter phase, all patients were treated with ruxolitinib; those who originally received a vehicle agent as placebo crossed over to the active treatment.
First FDA-approved treatment for adults and adolescents with vitiligo
Ruxolitinib is a Janus kinase 1/2 inhibitor that has been available for the treatment for atopic dermatitis for more than a year. It was recently approved by the U.S. Food and Drug Administration for the treatment of vitiligo in adults and pediatric patients aged 12 years and older.
This approval was based on the positive findings of the TRuE-V1 and TRuE-V2 studies, which showed that after 24 weeks, 30% of patients treated with ruxolitinib had at least 75% improvement in the facial VASI, compared with 10% of placebo-treated patients.
“These studies demonstrated very nice results, especially on the face, which is the easiest part to repigment in vitiligo,” Dr. Passeron said.
“We know that the location is very important when it comes to repigmentation of vitiligo,” he added. He noted that other body areas, “the extremities, for example, are much more difficult.”
The analysis he presented specifically assessed the effect of ruxolitinib cream on repigmentation in other areas.
Pooled analysis performed
Data from the two TRuE-V trials were pooled. The new analysis included a total of 661 individuals; of those patients, 443 had been treated with topical ruxolitinib, and 218 had received a vehicle cream as a placebo.
For the first 24 weeks, patients received twice-daily 1.5% ruxolitinib cream or vehicle cream. This was followed by a 28-week extension phase in which everyone was treated with ruxolitinib cream, after which there was a 30-day final follow-up period.
Dr. Passeron reported data by body region for weeks 12, 24, and 52, which showed an increasing percentage of patients with VASI-50.
“We didn’t look at the face; that we know well, that is a very good result,” he said.
The best results were seen for the head and neck. VASI-50 was reached by 28.3%, 45.3%, and 68.1% of patients treated with ruxolitinib cream at weeks 12, 24, and 52, respectively. Corresponding rates for the placebo-crossover group were 19.8%, 23.8%, and 51%.
Repigmentation rates of the hand, upper extremities, trunk, lower extremities, and feet were about 9%-15% for both ruxolitinib and placebo at 12 weeks, but by 24 weeks, there was a clear increase in repigmentation rates in the ruxolitinib group for all body areas.
The 24-week VASI-50 rates for hand repigmentation were 24.9% for ruxolitinib cream and 14.4% for placebo. Corresponding rates for upper extremity repigmentation were 33.2% and 8.2%; for the trunk, 26.4% and 12.2%; for the lower extremities, 29.5% and 12.2%; and for the feet, 18.5% and 12.5%.
“The results are quite poor at 12 weeks,” Dr. Passeron said. “It’s very important to keep this in mind; it takes time to repigment vitiligo, it takes to 6-24 months. We have to explain to our patients that they will have to wait to see the results.”
Steady improvements, no new safety concerns
Regarding VASI-50 over time, there was a steady increase in total body scores; 47.7% of patients who received ruxolitinib and 23.3% of placebo-treated patients hit this target at 52 weeks.
“And what is also very important to see is that we didn’t reach the plateau,” Dr. Passeron reported.
Similar patterns were seen for all the other body areas. Again there was a suggestion that rates may continue to rise with continued long-term treatment.
“About one-third of the patients reached at least 50% repigmentation after 1 year of treatment in the hands and feet,” Dr. Passeron said. He noted that certain areas, such as the back of the hand or tips of the fingers, may be unresponsive.
“So, we have to also to warn the patient that probably on these areas we have to combine it with other treatment because it remains very, very difficult to treat.”
There were no new safety concerns regarding treatment-emergent adverse events, which were reported in 52% of patients who received ruxolitinib and in 36% of placebo-treated patients.
The most common adverse reactions included COVID-19 (6.1% vs. 3.1%), acne at the application site (5.3% vs. 1.3%), and pruritus at the application site (3.9% vs. 2.7%), although cases were “mild or moderate,” said Dr. Passeron.
An expert’s take-home
“The results of TRuE-V phase 3 studies are encouraging and exciting,” Viktoria Eleftheriadou, MD, MRCP(UK), SCE(Derm), PhD, said in providing an independent comment for this news organization.
“Although ruxolitinib cream is applied on the skin, this novel treatment for vitiligo is not without risks; therefore, careful monitoring of patients who are started on this topical treatment would be prudent,” said Dr. Eleftheriadou, who is a consultant dermatologist for Walsall Healthcare NHS Trust and the Royal Wolverhampton NHS Trust, Birmingham, United Kingdom.
“I would like to see how many patients achieved VASI-75 or VASI-80 score, which from patients’ perspectives is a more meaningful outcome, as well as how long these results will last for,” she added.
The study was funded by Incyte Corporation. Dr. Passeron has received grants, honoraria, or both from AbbVie, ACM Pharma, Almirall, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte Corporation, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceuticals, and UCB. Dr. Passeron is the cofounder of YUKIN Therapeutics and has patents on WNT agonists or GSK2b antagonist for repigmentation of vitiligo and on the use of CXCR3B blockers in vitiligo. Dr. Eleftheriadou is an investigator and trial development group member on the HI-Light Vitiligo Trial (specific), a lead investigator on the pilot HI-Light Vitiligo Trial, and a medical advisory panel member of the Vitiligo Society UK. Dr. Eleftheriadou also provides consultancy services to Incyte and Pfizer.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Artemisia capillaris extract
Melasma is a difficult disorder to treat. With the removal of hydroquinone from the cosmetic market and the prevalence of dyschromia, new skin lightening ingredients are being sought and many new discoveries are coming from Asia.
There are more than 500 species of the genus Artemisia (of the Astraceae or Compositae family) dispersed throughout the temperate areas of Asia, Europe, and North America.1 Various parts of the shrub Artemisia capillaris, found abundantly in China, Japan, and Korea, have been used in traditional medicine in Asia for hundreds of years. A. capillaris (Yin-Chen in Chinese) has been deployed in traditional Chinese medicine as a diuretic, to protect the liver, and to treat skin inflammation.2,3 Antioxidant, anti-inflammatory, antisteatotic, antitumor, and antiviral properties have been associated with this plant,3 and hydrating effects have been recently attributed to it. In Korean medicine, A. capillaris (InJin in Korean) has been used for its hepatoprotective, analgesic, and antipyretic activities.4,5 In this column, the focus will be on recent evidence that suggests possible applications in skin care.
Chemical constituents
In 2008, Kim et al. studied the anticarcinogenic activity of A. capillaris, among other medicinal herbs, using the 7,12-dimethylbenz[a]anthracene (DMBA)-induced mouse skin carcinogenesis model. The researchers found that A. capillaris exhibited the most effective anticarcinogenic activity compared to the other herbs tested, with such properties ascribed to its constituent camphor, 1-borneol, coumarin, and achillin. Notably, the chloroform fraction of A. capillaris significantly lowered the number of tumors/mouse and tumor incidence compared with the other tested herbs.6
The wide range of biological functions associated with A. capillaris, including anti-inflammatory, antioxidant, antidiabetic, antisteatotic, and antitumor activities have, in various studies, been attributed to the bioactive constituents scoparone, scopoletin, capillarisin, capillin, and chlorogenic acids.3
Tyrosinase-related protein 1 (TYRP-1) and its role in skin pigmentation
Tyrosinase related protein 1 (TYRP-1) is structurally similar to tyrosinase, but its role is still being elucidated. Mutations in TYR-1 results in oculocutaneous albinism. TYRP-1 is involved in eumelanin synthesis, but not in pheomelanin synthesis. Mutations in TYRP-1 affect the quality of melanin synthesized rather than the quantity.4 TYRP-1 is being looked at as a target for treatment of hyperpigmentation disorders such as melasma.
Effects on melanin synthesis
A. capillaris reduces the expression of TYRP-1, making it attractive for use in skin lightening products. Although there are not a lot of data, this is a developing area of interest and the following will discuss what is known so far.
Kim et al. investigated the antimelanogenic activity of 10 essential oils, including A. capillaris, utilizing the B16F10 cell line model. A. capillaris was among four extracts found to hinder melanogenesis, and the only one that improved cell proliferation, displayed anti-H2O2 activity, and reduced tyrosinase-related protein (TRP)-1 expression. The researchers determined that A. capillaris extract suppressed melanin production through the downregulation of the TRP 1 translational level. They concluded that while investigations using in vivo models are necessary to buttress and validate these results, A. capillaris extract appears to be suitable as a natural therapeutic antimelanogenic agent as well as a skin-whitening ingredient in cosmeceutical products.7
Tabassum et al. screened A. capillaris for antipigmentary functions using murine cultured cells (B16-F10 malignant melanocytes). They found that the A. capillaris constituent 4,5-O-dicaffeoylquinic acid significantly and dose-dependently diminished melanin production and tyrosinase activity in the melanocytes. The expression of tyrosinase-related protein-1 was also decreased. Further, the researchers observed antipigmentary activity in a zebrafish model, with no toxicity demonstrated by either A. capillaris or its component 4,5-O-dicaffeoylquinic acid. They concluded that this compound could be included as an active ingredient in products intended to address pigmentation disorders.8
Anti-inflammatory activity
Inflammation is well known to trigger the production of melanin. This is why anti-inflammatory ingredients are often included in skin lighting products. A. capillaris displays anti-inflammatory activity and has shown some antioxidant activity.
In 2018, Lee et al. confirmed the therapeutic potential of A. capillaris extract to treat psoriasis in HaCaT cells and imiquimod-induced psoriasis-like mouse models. In the murine models, those treated with the ethanol extract of A. capillaris had a significantly lower Psoriasis Area and Severity Index score than that of the mice not given the topical application of the botanical. Epidermal thickness was noted to be significantly lower compared with the mice not treated with A. capillaris.9 Further studies in mice by the same team later that year supported the use of a cream formulation containing A. capillaris that they developed to treat psoriasis, warranting new investigations in human skin.10
Yeo et al. reported, earlier in 2018, on other anti-inflammatory activity of the herb, finding that the aqueous extract from A. capillaris blocked acute gastric mucosal injury by hindering reactive oxygen species and nuclear factor kappa B. They added that A. capillaris maintains oxidant/antioxidant homeostasis and displays potential as a nutraceutical agent for treating gastric ulcers and gastritis.5
In 2011, Kwon et al. studied the 5-lipoxygenase inhibitory action of a 70% ethanol extract of aerial parts of A. capillaris. They identified esculetin and quercetin as strong inhibitors of 5-lipoxygenase. The botanical agent, and esculetin in particular, robustly suppressed arachidonic acid-induced ear edema in mice as well as delayed-type hypersensitivity reactions. Further, A. capillaris potently blocked 5-lipoxygenase-catalyzed leukotriene synthesis by ionophore-induced rat basophilic leukemia-1 cells. The researchers concluded that their findings may partially account for the use of A. capillaris as a traditional medical treatment for cutaneous inflammatory conditions.2
Atopic dermatitis and A. capillaris
In 2014, Ha et al. used in vitro and in vivo systems to assess the anti-inflammatory effects of A. capillaris as well as its activity against atopic dermatitis. The in vitro studies revealed that A. capillaris hampered NO and cellular histamine synthesis. In Nc/Nga mice sensitized by Dermatophagoides farinae, dermatitis scores as well as hemorrhage, hypertrophy, and hyperkeratosis of the epidermis in the dorsal skin and ear all declined after the topical application of A. capillaris. Plasma levels of histamine and IgE also significantly decreased after treatment with A. capillaris. The investigators concluded that further study of A. capillaris is warranted as a potential therapeutic option for atopic dermatitis.11
Summary
Many botanical ingredients from Asia are making their way into skin care products in the USA. A. capillaris extract is an example and may have utility in treating hyperpigmentation-associated skin issues such as melasma. Its inhibitory effects on both inflammation and melanin production in addition to possible antioxidant activity make it an interesting compound worthy of more scrutiny.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Bora KS and Sharma A. Pharm Biol. 2011 Jan;49(1):101-9.
2. Kwon OS et al. Arch Pharm Res. 2011 Sep;34(9):1561-9.
3. Hsueh TP et al. Biomedicines. 2021 Oct 8;9(10):1412.
4. Dolinska MB et al. Int J Mol Sci. 2020 Jan 3;21(1):331.
5. Yeo D et al. Biomed Pharmacother. 2018 Mar;99:681-7.
6. Kim YS et al. J Food Sci. 2008 Jan;73(1):T16-20.
7. Kim MJ et al. Mol Med Rep. 2022 Apr;25(4):113.
8. Tabassum N et al. Evid Based Complement Alternat Med. 2016;2016:7823541.
9. Lee SY et al. Phytother Res. 2018 May;32(5):923-2.
10. Lee SY et al. Evid Based Complement Alternat Med. 2018 Aug 19;2018:3610494.
11. Ha H et al. BMC Complement Altern Med. 2014 Mar 14;14:100.
Melasma is a difficult disorder to treat. With the removal of hydroquinone from the cosmetic market and the prevalence of dyschromia, new skin lightening ingredients are being sought and many new discoveries are coming from Asia.
There are more than 500 species of the genus Artemisia (of the Astraceae or Compositae family) dispersed throughout the temperate areas of Asia, Europe, and North America.1 Various parts of the shrub Artemisia capillaris, found abundantly in China, Japan, and Korea, have been used in traditional medicine in Asia for hundreds of years. A. capillaris (Yin-Chen in Chinese) has been deployed in traditional Chinese medicine as a diuretic, to protect the liver, and to treat skin inflammation.2,3 Antioxidant, anti-inflammatory, antisteatotic, antitumor, and antiviral properties have been associated with this plant,3 and hydrating effects have been recently attributed to it. In Korean medicine, A. capillaris (InJin in Korean) has been used for its hepatoprotective, analgesic, and antipyretic activities.4,5 In this column, the focus will be on recent evidence that suggests possible applications in skin care.
Chemical constituents
In 2008, Kim et al. studied the anticarcinogenic activity of A. capillaris, among other medicinal herbs, using the 7,12-dimethylbenz[a]anthracene (DMBA)-induced mouse skin carcinogenesis model. The researchers found that A. capillaris exhibited the most effective anticarcinogenic activity compared to the other herbs tested, with such properties ascribed to its constituent camphor, 1-borneol, coumarin, and achillin. Notably, the chloroform fraction of A. capillaris significantly lowered the number of tumors/mouse and tumor incidence compared with the other tested herbs.6
The wide range of biological functions associated with A. capillaris, including anti-inflammatory, antioxidant, antidiabetic, antisteatotic, and antitumor activities have, in various studies, been attributed to the bioactive constituents scoparone, scopoletin, capillarisin, capillin, and chlorogenic acids.3
Tyrosinase-related protein 1 (TYRP-1) and its role in skin pigmentation
Tyrosinase related protein 1 (TYRP-1) is structurally similar to tyrosinase, but its role is still being elucidated. Mutations in TYR-1 results in oculocutaneous albinism. TYRP-1 is involved in eumelanin synthesis, but not in pheomelanin synthesis. Mutations in TYRP-1 affect the quality of melanin synthesized rather than the quantity.4 TYRP-1 is being looked at as a target for treatment of hyperpigmentation disorders such as melasma.
Effects on melanin synthesis
A. capillaris reduces the expression of TYRP-1, making it attractive for use in skin lightening products. Although there are not a lot of data, this is a developing area of interest and the following will discuss what is known so far.
Kim et al. investigated the antimelanogenic activity of 10 essential oils, including A. capillaris, utilizing the B16F10 cell line model. A. capillaris was among four extracts found to hinder melanogenesis, and the only one that improved cell proliferation, displayed anti-H2O2 activity, and reduced tyrosinase-related protein (TRP)-1 expression. The researchers determined that A. capillaris extract suppressed melanin production through the downregulation of the TRP 1 translational level. They concluded that while investigations using in vivo models are necessary to buttress and validate these results, A. capillaris extract appears to be suitable as a natural therapeutic antimelanogenic agent as well as a skin-whitening ingredient in cosmeceutical products.7
Tabassum et al. screened A. capillaris for antipigmentary functions using murine cultured cells (B16-F10 malignant melanocytes). They found that the A. capillaris constituent 4,5-O-dicaffeoylquinic acid significantly and dose-dependently diminished melanin production and tyrosinase activity in the melanocytes. The expression of tyrosinase-related protein-1 was also decreased. Further, the researchers observed antipigmentary activity in a zebrafish model, with no toxicity demonstrated by either A. capillaris or its component 4,5-O-dicaffeoylquinic acid. They concluded that this compound could be included as an active ingredient in products intended to address pigmentation disorders.8
Anti-inflammatory activity
Inflammation is well known to trigger the production of melanin. This is why anti-inflammatory ingredients are often included in skin lighting products. A. capillaris displays anti-inflammatory activity and has shown some antioxidant activity.
In 2018, Lee et al. confirmed the therapeutic potential of A. capillaris extract to treat psoriasis in HaCaT cells and imiquimod-induced psoriasis-like mouse models. In the murine models, those treated with the ethanol extract of A. capillaris had a significantly lower Psoriasis Area and Severity Index score than that of the mice not given the topical application of the botanical. Epidermal thickness was noted to be significantly lower compared with the mice not treated with A. capillaris.9 Further studies in mice by the same team later that year supported the use of a cream formulation containing A. capillaris that they developed to treat psoriasis, warranting new investigations in human skin.10
Yeo et al. reported, earlier in 2018, on other anti-inflammatory activity of the herb, finding that the aqueous extract from A. capillaris blocked acute gastric mucosal injury by hindering reactive oxygen species and nuclear factor kappa B. They added that A. capillaris maintains oxidant/antioxidant homeostasis and displays potential as a nutraceutical agent for treating gastric ulcers and gastritis.5
In 2011, Kwon et al. studied the 5-lipoxygenase inhibitory action of a 70% ethanol extract of aerial parts of A. capillaris. They identified esculetin and quercetin as strong inhibitors of 5-lipoxygenase. The botanical agent, and esculetin in particular, robustly suppressed arachidonic acid-induced ear edema in mice as well as delayed-type hypersensitivity reactions. Further, A. capillaris potently blocked 5-lipoxygenase-catalyzed leukotriene synthesis by ionophore-induced rat basophilic leukemia-1 cells. The researchers concluded that their findings may partially account for the use of A. capillaris as a traditional medical treatment for cutaneous inflammatory conditions.2
Atopic dermatitis and A. capillaris
In 2014, Ha et al. used in vitro and in vivo systems to assess the anti-inflammatory effects of A. capillaris as well as its activity against atopic dermatitis. The in vitro studies revealed that A. capillaris hampered NO and cellular histamine synthesis. In Nc/Nga mice sensitized by Dermatophagoides farinae, dermatitis scores as well as hemorrhage, hypertrophy, and hyperkeratosis of the epidermis in the dorsal skin and ear all declined after the topical application of A. capillaris. Plasma levels of histamine and IgE also significantly decreased after treatment with A. capillaris. The investigators concluded that further study of A. capillaris is warranted as a potential therapeutic option for atopic dermatitis.11
Summary
Many botanical ingredients from Asia are making their way into skin care products in the USA. A. capillaris extract is an example and may have utility in treating hyperpigmentation-associated skin issues such as melasma. Its inhibitory effects on both inflammation and melanin production in addition to possible antioxidant activity make it an interesting compound worthy of more scrutiny.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Bora KS and Sharma A. Pharm Biol. 2011 Jan;49(1):101-9.
2. Kwon OS et al. Arch Pharm Res. 2011 Sep;34(9):1561-9.
3. Hsueh TP et al. Biomedicines. 2021 Oct 8;9(10):1412.
4. Dolinska MB et al. Int J Mol Sci. 2020 Jan 3;21(1):331.
5. Yeo D et al. Biomed Pharmacother. 2018 Mar;99:681-7.
6. Kim YS et al. J Food Sci. 2008 Jan;73(1):T16-20.
7. Kim MJ et al. Mol Med Rep. 2022 Apr;25(4):113.
8. Tabassum N et al. Evid Based Complement Alternat Med. 2016;2016:7823541.
9. Lee SY et al. Phytother Res. 2018 May;32(5):923-2.
10. Lee SY et al. Evid Based Complement Alternat Med. 2018 Aug 19;2018:3610494.
11. Ha H et al. BMC Complement Altern Med. 2014 Mar 14;14:100.
Melasma is a difficult disorder to treat. With the removal of hydroquinone from the cosmetic market and the prevalence of dyschromia, new skin lightening ingredients are being sought and many new discoveries are coming from Asia.
There are more than 500 species of the genus Artemisia (of the Astraceae or Compositae family) dispersed throughout the temperate areas of Asia, Europe, and North America.1 Various parts of the shrub Artemisia capillaris, found abundantly in China, Japan, and Korea, have been used in traditional medicine in Asia for hundreds of years. A. capillaris (Yin-Chen in Chinese) has been deployed in traditional Chinese medicine as a diuretic, to protect the liver, and to treat skin inflammation.2,3 Antioxidant, anti-inflammatory, antisteatotic, antitumor, and antiviral properties have been associated with this plant,3 and hydrating effects have been recently attributed to it. In Korean medicine, A. capillaris (InJin in Korean) has been used for its hepatoprotective, analgesic, and antipyretic activities.4,5 In this column, the focus will be on recent evidence that suggests possible applications in skin care.
Chemical constituents
In 2008, Kim et al. studied the anticarcinogenic activity of A. capillaris, among other medicinal herbs, using the 7,12-dimethylbenz[a]anthracene (DMBA)-induced mouse skin carcinogenesis model. The researchers found that A. capillaris exhibited the most effective anticarcinogenic activity compared to the other herbs tested, with such properties ascribed to its constituent camphor, 1-borneol, coumarin, and achillin. Notably, the chloroform fraction of A. capillaris significantly lowered the number of tumors/mouse and tumor incidence compared with the other tested herbs.6
The wide range of biological functions associated with A. capillaris, including anti-inflammatory, antioxidant, antidiabetic, antisteatotic, and antitumor activities have, in various studies, been attributed to the bioactive constituents scoparone, scopoletin, capillarisin, capillin, and chlorogenic acids.3
Tyrosinase-related protein 1 (TYRP-1) and its role in skin pigmentation
Tyrosinase related protein 1 (TYRP-1) is structurally similar to tyrosinase, but its role is still being elucidated. Mutations in TYR-1 results in oculocutaneous albinism. TYRP-1 is involved in eumelanin synthesis, but not in pheomelanin synthesis. Mutations in TYRP-1 affect the quality of melanin synthesized rather than the quantity.4 TYRP-1 is being looked at as a target for treatment of hyperpigmentation disorders such as melasma.
Effects on melanin synthesis
A. capillaris reduces the expression of TYRP-1, making it attractive for use in skin lightening products. Although there are not a lot of data, this is a developing area of interest and the following will discuss what is known so far.
Kim et al. investigated the antimelanogenic activity of 10 essential oils, including A. capillaris, utilizing the B16F10 cell line model. A. capillaris was among four extracts found to hinder melanogenesis, and the only one that improved cell proliferation, displayed anti-H2O2 activity, and reduced tyrosinase-related protein (TRP)-1 expression. The researchers determined that A. capillaris extract suppressed melanin production through the downregulation of the TRP 1 translational level. They concluded that while investigations using in vivo models are necessary to buttress and validate these results, A. capillaris extract appears to be suitable as a natural therapeutic antimelanogenic agent as well as a skin-whitening ingredient in cosmeceutical products.7
Tabassum et al. screened A. capillaris for antipigmentary functions using murine cultured cells (B16-F10 malignant melanocytes). They found that the A. capillaris constituent 4,5-O-dicaffeoylquinic acid significantly and dose-dependently diminished melanin production and tyrosinase activity in the melanocytes. The expression of tyrosinase-related protein-1 was also decreased. Further, the researchers observed antipigmentary activity in a zebrafish model, with no toxicity demonstrated by either A. capillaris or its component 4,5-O-dicaffeoylquinic acid. They concluded that this compound could be included as an active ingredient in products intended to address pigmentation disorders.8
Anti-inflammatory activity
Inflammation is well known to trigger the production of melanin. This is why anti-inflammatory ingredients are often included in skin lighting products. A. capillaris displays anti-inflammatory activity and has shown some antioxidant activity.
In 2018, Lee et al. confirmed the therapeutic potential of A. capillaris extract to treat psoriasis in HaCaT cells and imiquimod-induced psoriasis-like mouse models. In the murine models, those treated with the ethanol extract of A. capillaris had a significantly lower Psoriasis Area and Severity Index score than that of the mice not given the topical application of the botanical. Epidermal thickness was noted to be significantly lower compared with the mice not treated with A. capillaris.9 Further studies in mice by the same team later that year supported the use of a cream formulation containing A. capillaris that they developed to treat psoriasis, warranting new investigations in human skin.10
Yeo et al. reported, earlier in 2018, on other anti-inflammatory activity of the herb, finding that the aqueous extract from A. capillaris blocked acute gastric mucosal injury by hindering reactive oxygen species and nuclear factor kappa B. They added that A. capillaris maintains oxidant/antioxidant homeostasis and displays potential as a nutraceutical agent for treating gastric ulcers and gastritis.5
In 2011, Kwon et al. studied the 5-lipoxygenase inhibitory action of a 70% ethanol extract of aerial parts of A. capillaris. They identified esculetin and quercetin as strong inhibitors of 5-lipoxygenase. The botanical agent, and esculetin in particular, robustly suppressed arachidonic acid-induced ear edema in mice as well as delayed-type hypersensitivity reactions. Further, A. capillaris potently blocked 5-lipoxygenase-catalyzed leukotriene synthesis by ionophore-induced rat basophilic leukemia-1 cells. The researchers concluded that their findings may partially account for the use of A. capillaris as a traditional medical treatment for cutaneous inflammatory conditions.2
Atopic dermatitis and A. capillaris
In 2014, Ha et al. used in vitro and in vivo systems to assess the anti-inflammatory effects of A. capillaris as well as its activity against atopic dermatitis. The in vitro studies revealed that A. capillaris hampered NO and cellular histamine synthesis. In Nc/Nga mice sensitized by Dermatophagoides farinae, dermatitis scores as well as hemorrhage, hypertrophy, and hyperkeratosis of the epidermis in the dorsal skin and ear all declined after the topical application of A. capillaris. Plasma levels of histamine and IgE also significantly decreased after treatment with A. capillaris. The investigators concluded that further study of A. capillaris is warranted as a potential therapeutic option for atopic dermatitis.11
Summary
Many botanical ingredients from Asia are making their way into skin care products in the USA. A. capillaris extract is an example and may have utility in treating hyperpigmentation-associated skin issues such as melasma. Its inhibitory effects on both inflammation and melanin production in addition to possible antioxidant activity make it an interesting compound worthy of more scrutiny.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Bora KS and Sharma A. Pharm Biol. 2011 Jan;49(1):101-9.
2. Kwon OS et al. Arch Pharm Res. 2011 Sep;34(9):1561-9.
3. Hsueh TP et al. Biomedicines. 2021 Oct 8;9(10):1412.
4. Dolinska MB et al. Int J Mol Sci. 2020 Jan 3;21(1):331.
5. Yeo D et al. Biomed Pharmacother. 2018 Mar;99:681-7.
6. Kim YS et al. J Food Sci. 2008 Jan;73(1):T16-20.
7. Kim MJ et al. Mol Med Rep. 2022 Apr;25(4):113.
8. Tabassum N et al. Evid Based Complement Alternat Med. 2016;2016:7823541.
9. Lee SY et al. Phytother Res. 2018 May;32(5):923-2.
10. Lee SY et al. Evid Based Complement Alternat Med. 2018 Aug 19;2018:3610494.
11. Ha H et al. BMC Complement Altern Med. 2014 Mar 14;14:100.
Linear Hypopigmentation on the Right Arm
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
A 73-year-old woman presented to the dermatology clinic with hypopigmentation along the right arm. Her medical history was notable for prior treatment with intralesional triamcinolone injections for De Quervain tenosynovitis. Two months after receiving the steroid injections she noted progressive spreading of an asymptomatic white discoloration originating on the right wrist. Physical examination revealed a hypopigmented atrophic patch on the medial aspect of the right wrist (left) with linear hypopigmented patches extending proximally up the forearm (right).
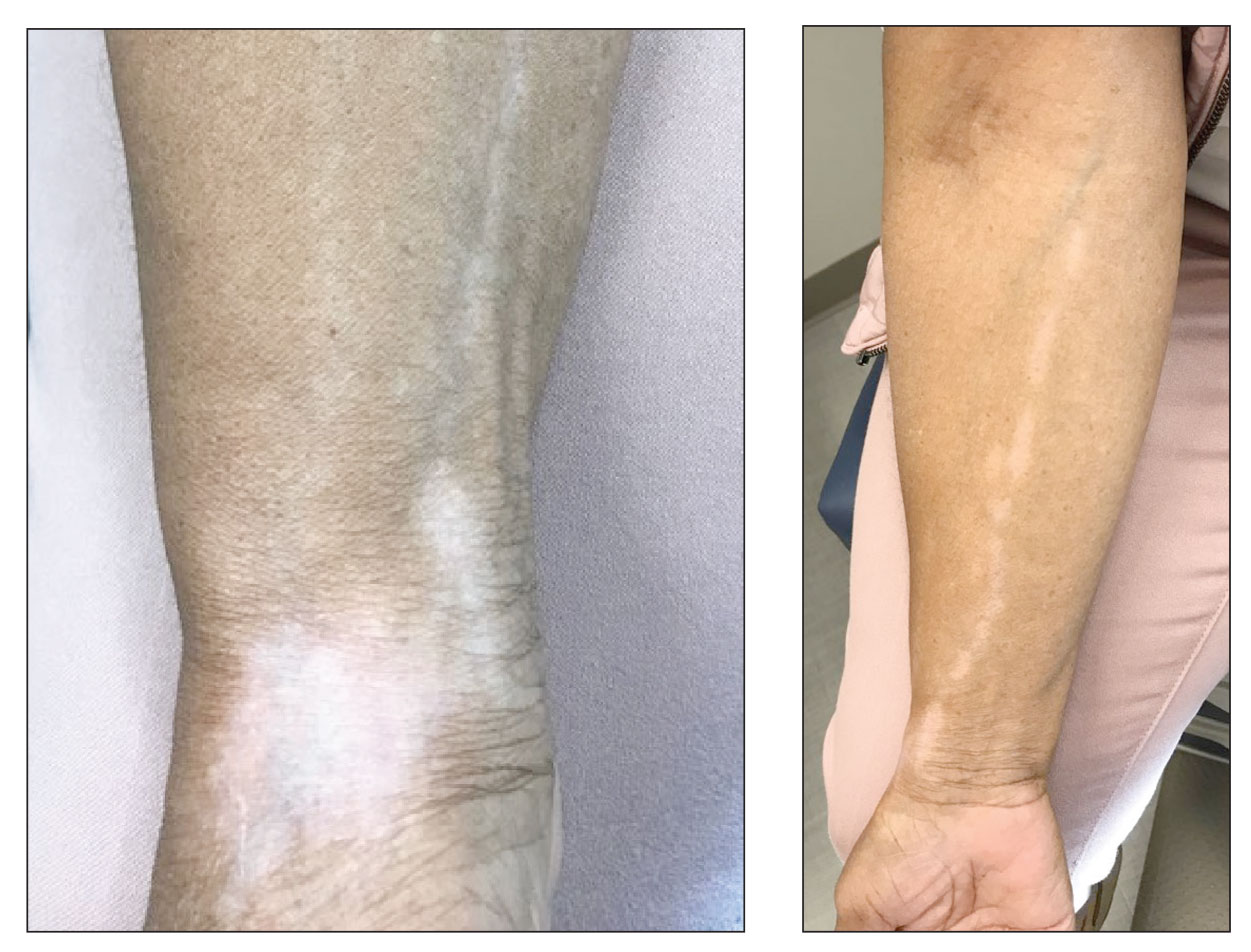
Hydroquinone, found in skin-lightening agents worldwide, linked with increased skin cancer risk
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
FROM SID 2022
VTE risk not elevated in AD patients on JAK inhibitors: Study
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
FROM JAMA DERMATOLOGY
Why it’s important for dermatologists to learn about JAK inhibitors
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
AT PDA 2022
Dermatologists share vitiligo breakthrough news with patients
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.