User login
The Final Rule for 2022: What’s New and How Changes in the Medicare Physician Fee Schedule and Quality Payment Program Affect Dermatologists
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
Practice Points
- The Centers for Medicare & Medicaid Services (CMS) 2022 final rule contains multiple updates affecting the practice of dermatology.
- Adjustments to the conversion factor and legislative-level actions have led to changes in reimbursement for many procedures within dermatology and beyond.
- Other notable updates include refining the definition of split evaluation and management visits, clinical labor pricing, and billing for physician assistant services.
- Changes in the Merit-Based Incentive Payment System (MIPS), cost measures, and MIPS value pathways also will impact many dermatology practices.
Oncologists in malpractice suits: Less than other specialties
, notes the latest Medscape Malpractice Report.
Over a third (39%) of oncologists who were surveyed said that they had been named in at least one malpractice suit, according to the Medscape Oncologist Malpractice Report 2021.
This number is considerably lower than that reported by physicians across all specialties (51%), and it is also much lower than that reported by oncologists in past years. In the 2019 report, 54% of oncologists surveyed said they had been named in a malpractice suit, while in the 2017 report, the figure was 53%.
The dramatic decline in malpractice suits may have much to do with the COVID-19 pandemic, when oncology care was in a state of flux.
“Fewer people were seeking cancer care during the COVID pandemic, which might have impacted the number of lawsuits brought against oncologists,” says Paul Walker, a New York–based malpractice attorney at Walker Medical Law, who represents physicians and other healthcare professionals.
“Additionally, a fair number of people who died of COVID were also older,” he pointed out, and it is often older people who get cancer, so there were fewer older people who consulted an oncologist or were treated by one, he added.
However, the pandemic may be storing up trouble for future years. “Patient fears of contracting COVID-19 have led many to avoid seeking or resuming care, so delays in diagnosing new cancer cases could mean that more patients are diagnosed at a later stage of their disease, leading to potential adverse events and malpractice claims,” commented David L. Feldman, MD, MBA, chief medical officer of The Doctors Company Group.
This latest 2021 Medscape Malpractice Report was compiled from an online survey that included more than 4,300 physicians from 29 specialties. It included 106 oncologists. More than half of respondents (56%) had been in practice for more than 25 years, and 54% were aged 60 years or older. The survey was available from May 21 to August 28, 2021.
Similar to findings in previous years, complications from treatment/surgery were the most common reason for the lawsuits (31%). Failure to make a correct diagnosis or a delay in diagnosis was the second most common reason (23%), while 20% of patients sued because of a poor outcome or disease progression.
Surprise at being sued
Among the oncologists who reported involvement in a lawsuit in 2021, the majority (86%) said they were “very surprised” or “somewhat surprised” by the malpractice suit, which is similar to that of other physicians surveyed. However, fewer were surprised this year as compared to 2019 and 2017 (90% and 94%).
One reason for the surprise over the litigation was that it concerned a patient who had been treated a long time ago. One oncologist wrote that “the patient had not seen me for over 7 years and during that time, he did not call me with his new symptomatology. I was only named in the suit because I had previously been involved.”
Another common scenario reported by oncologists was being named in a lawsuit which was brought by another clinician’s patient. “I was the chairperson of the department, and one of the doctors in the practice was involved in the suit,” wrote one respondent. “I was named as an accomplice.”
More than half of surveyed oncologists said that they were able to identify the patient who bought the suit, and these figures are again comparable to those of other physicians. One oncologist commented that in the case he was involved with, the family did not understand or accept the nature of cancer and the different ways that a patient could die of complications. This patient had died of sepsis and pneumonia related to decubitus ulcers that were completely unrelated to her radiation therapy.
As in the case above, sometimes it is the family who filed the lawsuit, not the patient.
“The patient may even recognize that you did your best and be grateful for your skill and efforts, but the family can’t accept that grandma died of cancer and brings a lawsuit,” said Dennis Hursh, an attorney with Physicians Agreement Health Law in Pennsylvania.
When looking at outcomes of the lawsuit, 40% of oncologists were dismissed from the suit within the first few months, or the case was settled before going to trial. This trend is also consistent with the results from the 2019 and 2017 surveys. When the case did go to trial, 10% received a favorable verdict, which was the same in 2019.
“It seems that most of my clients end up being released from lawsuits, and many lawsuits are dismissed prior to proceeding to trial,” Mr. Hursh commented.
Murdering psychopath
Some oncologists weighed in on what they felt was the worst experience of being sued.
“Mental anguish, knowing that I did nothing wrong,” said one physician. Another reported that it was a feeling of being “inadequate and totally alone.”
Another oncologist commented that the “depositions from lawyers implied that I was worse than a murdering psychopath. My reputation was permanently damaged.”
However, the vast majority of oncologists (88%) did not believe that the lawsuit negatively affected their career, which was similar to physicians in general. That said, many did complain about the ongoing requirement to report the lawsuit to the credentialing committee, even if it was dismissed, and then having to pay increased malpractice premiums. “I still need to document this episode every single time I apply for any medical position, even more than 29 years after I was dismissed from the case,” said one respondent.
When asked if they would do anything differently, many oncologists (42%) said no, they would not have done anything differently. This is similar to the responses from physicians in general and with 2019 responses from oncologists. However, 15% of the respondents said that in retrospect, they would not have taken on that patient to begin with.
Some oncologists noted that they would have been more conscientious in relaying the information to the referring physician. Evan Lyman, an associate attorney at Voute, Lohrfink, McAndrew, Meisner & Roberts, LLP, in White Plains, N.Y., pointed out that a common reason for lawsuits is a slip-up of communication between the specialist and the referring physician.
Oncologists who had been sued have some insights to offer to colleagues, should they find themselves in a similar situation.
“Only answer with short and precise statements,” wrote one oncologist. “Attend all the depositions as much as you can; they are more likely to fabricate or exaggerate if you are not sitting in the room.”
Another physician said to base “everything on the medical record and do not answer hypothetical questions.”
“Document all interactions with patients as if a jury will be reading them, word by word,” said one respondent.
As for the public or patients, oncologists had this message: “malpractice suits should be rarely launched and only when gross errors can be absolutely proven.”
Another oncologist pointed out that communication is key. “Speak to the physicians against whom you have distrust. Lots of things could be cleared by good communication.”
A version of this article first appeared on Medscape.com.
, notes the latest Medscape Malpractice Report.
Over a third (39%) of oncologists who were surveyed said that they had been named in at least one malpractice suit, according to the Medscape Oncologist Malpractice Report 2021.
This number is considerably lower than that reported by physicians across all specialties (51%), and it is also much lower than that reported by oncologists in past years. In the 2019 report, 54% of oncologists surveyed said they had been named in a malpractice suit, while in the 2017 report, the figure was 53%.
The dramatic decline in malpractice suits may have much to do with the COVID-19 pandemic, when oncology care was in a state of flux.
“Fewer people were seeking cancer care during the COVID pandemic, which might have impacted the number of lawsuits brought against oncologists,” says Paul Walker, a New York–based malpractice attorney at Walker Medical Law, who represents physicians and other healthcare professionals.
“Additionally, a fair number of people who died of COVID were also older,” he pointed out, and it is often older people who get cancer, so there were fewer older people who consulted an oncologist or were treated by one, he added.
However, the pandemic may be storing up trouble for future years. “Patient fears of contracting COVID-19 have led many to avoid seeking or resuming care, so delays in diagnosing new cancer cases could mean that more patients are diagnosed at a later stage of their disease, leading to potential adverse events and malpractice claims,” commented David L. Feldman, MD, MBA, chief medical officer of The Doctors Company Group.
This latest 2021 Medscape Malpractice Report was compiled from an online survey that included more than 4,300 physicians from 29 specialties. It included 106 oncologists. More than half of respondents (56%) had been in practice for more than 25 years, and 54% were aged 60 years or older. The survey was available from May 21 to August 28, 2021.
Similar to findings in previous years, complications from treatment/surgery were the most common reason for the lawsuits (31%). Failure to make a correct diagnosis or a delay in diagnosis was the second most common reason (23%), while 20% of patients sued because of a poor outcome or disease progression.
Surprise at being sued
Among the oncologists who reported involvement in a lawsuit in 2021, the majority (86%) said they were “very surprised” or “somewhat surprised” by the malpractice suit, which is similar to that of other physicians surveyed. However, fewer were surprised this year as compared to 2019 and 2017 (90% and 94%).
One reason for the surprise over the litigation was that it concerned a patient who had been treated a long time ago. One oncologist wrote that “the patient had not seen me for over 7 years and during that time, he did not call me with his new symptomatology. I was only named in the suit because I had previously been involved.”
Another common scenario reported by oncologists was being named in a lawsuit which was brought by another clinician’s patient. “I was the chairperson of the department, and one of the doctors in the practice was involved in the suit,” wrote one respondent. “I was named as an accomplice.”
More than half of surveyed oncologists said that they were able to identify the patient who bought the suit, and these figures are again comparable to those of other physicians. One oncologist commented that in the case he was involved with, the family did not understand or accept the nature of cancer and the different ways that a patient could die of complications. This patient had died of sepsis and pneumonia related to decubitus ulcers that were completely unrelated to her radiation therapy.
As in the case above, sometimes it is the family who filed the lawsuit, not the patient.
“The patient may even recognize that you did your best and be grateful for your skill and efforts, but the family can’t accept that grandma died of cancer and brings a lawsuit,” said Dennis Hursh, an attorney with Physicians Agreement Health Law in Pennsylvania.
When looking at outcomes of the lawsuit, 40% of oncologists were dismissed from the suit within the first few months, or the case was settled before going to trial. This trend is also consistent with the results from the 2019 and 2017 surveys. When the case did go to trial, 10% received a favorable verdict, which was the same in 2019.
“It seems that most of my clients end up being released from lawsuits, and many lawsuits are dismissed prior to proceeding to trial,” Mr. Hursh commented.
Murdering psychopath
Some oncologists weighed in on what they felt was the worst experience of being sued.
“Mental anguish, knowing that I did nothing wrong,” said one physician. Another reported that it was a feeling of being “inadequate and totally alone.”
Another oncologist commented that the “depositions from lawyers implied that I was worse than a murdering psychopath. My reputation was permanently damaged.”
However, the vast majority of oncologists (88%) did not believe that the lawsuit negatively affected their career, which was similar to physicians in general. That said, many did complain about the ongoing requirement to report the lawsuit to the credentialing committee, even if it was dismissed, and then having to pay increased malpractice premiums. “I still need to document this episode every single time I apply for any medical position, even more than 29 years after I was dismissed from the case,” said one respondent.
When asked if they would do anything differently, many oncologists (42%) said no, they would not have done anything differently. This is similar to the responses from physicians in general and with 2019 responses from oncologists. However, 15% of the respondents said that in retrospect, they would not have taken on that patient to begin with.
Some oncologists noted that they would have been more conscientious in relaying the information to the referring physician. Evan Lyman, an associate attorney at Voute, Lohrfink, McAndrew, Meisner & Roberts, LLP, in White Plains, N.Y., pointed out that a common reason for lawsuits is a slip-up of communication between the specialist and the referring physician.
Oncologists who had been sued have some insights to offer to colleagues, should they find themselves in a similar situation.
“Only answer with short and precise statements,” wrote one oncologist. “Attend all the depositions as much as you can; they are more likely to fabricate or exaggerate if you are not sitting in the room.”
Another physician said to base “everything on the medical record and do not answer hypothetical questions.”
“Document all interactions with patients as if a jury will be reading them, word by word,” said one respondent.
As for the public or patients, oncologists had this message: “malpractice suits should be rarely launched and only when gross errors can be absolutely proven.”
Another oncologist pointed out that communication is key. “Speak to the physicians against whom you have distrust. Lots of things could be cleared by good communication.”
A version of this article first appeared on Medscape.com.
, notes the latest Medscape Malpractice Report.
Over a third (39%) of oncologists who were surveyed said that they had been named in at least one malpractice suit, according to the Medscape Oncologist Malpractice Report 2021.
This number is considerably lower than that reported by physicians across all specialties (51%), and it is also much lower than that reported by oncologists in past years. In the 2019 report, 54% of oncologists surveyed said they had been named in a malpractice suit, while in the 2017 report, the figure was 53%.
The dramatic decline in malpractice suits may have much to do with the COVID-19 pandemic, when oncology care was in a state of flux.
“Fewer people were seeking cancer care during the COVID pandemic, which might have impacted the number of lawsuits brought against oncologists,” says Paul Walker, a New York–based malpractice attorney at Walker Medical Law, who represents physicians and other healthcare professionals.
“Additionally, a fair number of people who died of COVID were also older,” he pointed out, and it is often older people who get cancer, so there were fewer older people who consulted an oncologist or were treated by one, he added.
However, the pandemic may be storing up trouble for future years. “Patient fears of contracting COVID-19 have led many to avoid seeking or resuming care, so delays in diagnosing new cancer cases could mean that more patients are diagnosed at a later stage of their disease, leading to potential adverse events and malpractice claims,” commented David L. Feldman, MD, MBA, chief medical officer of The Doctors Company Group.
This latest 2021 Medscape Malpractice Report was compiled from an online survey that included more than 4,300 physicians from 29 specialties. It included 106 oncologists. More than half of respondents (56%) had been in practice for more than 25 years, and 54% were aged 60 years or older. The survey was available from May 21 to August 28, 2021.
Similar to findings in previous years, complications from treatment/surgery were the most common reason for the lawsuits (31%). Failure to make a correct diagnosis or a delay in diagnosis was the second most common reason (23%), while 20% of patients sued because of a poor outcome or disease progression.
Surprise at being sued
Among the oncologists who reported involvement in a lawsuit in 2021, the majority (86%) said they were “very surprised” or “somewhat surprised” by the malpractice suit, which is similar to that of other physicians surveyed. However, fewer were surprised this year as compared to 2019 and 2017 (90% and 94%).
One reason for the surprise over the litigation was that it concerned a patient who had been treated a long time ago. One oncologist wrote that “the patient had not seen me for over 7 years and during that time, he did not call me with his new symptomatology. I was only named in the suit because I had previously been involved.”
Another common scenario reported by oncologists was being named in a lawsuit which was brought by another clinician’s patient. “I was the chairperson of the department, and one of the doctors in the practice was involved in the suit,” wrote one respondent. “I was named as an accomplice.”
More than half of surveyed oncologists said that they were able to identify the patient who bought the suit, and these figures are again comparable to those of other physicians. One oncologist commented that in the case he was involved with, the family did not understand or accept the nature of cancer and the different ways that a patient could die of complications. This patient had died of sepsis and pneumonia related to decubitus ulcers that were completely unrelated to her radiation therapy.
As in the case above, sometimes it is the family who filed the lawsuit, not the patient.
“The patient may even recognize that you did your best and be grateful for your skill and efforts, but the family can’t accept that grandma died of cancer and brings a lawsuit,” said Dennis Hursh, an attorney with Physicians Agreement Health Law in Pennsylvania.
When looking at outcomes of the lawsuit, 40% of oncologists were dismissed from the suit within the first few months, or the case was settled before going to trial. This trend is also consistent with the results from the 2019 and 2017 surveys. When the case did go to trial, 10% received a favorable verdict, which was the same in 2019.
“It seems that most of my clients end up being released from lawsuits, and many lawsuits are dismissed prior to proceeding to trial,” Mr. Hursh commented.
Murdering psychopath
Some oncologists weighed in on what they felt was the worst experience of being sued.
“Mental anguish, knowing that I did nothing wrong,” said one physician. Another reported that it was a feeling of being “inadequate and totally alone.”
Another oncologist commented that the “depositions from lawyers implied that I was worse than a murdering psychopath. My reputation was permanently damaged.”
However, the vast majority of oncologists (88%) did not believe that the lawsuit negatively affected their career, which was similar to physicians in general. That said, many did complain about the ongoing requirement to report the lawsuit to the credentialing committee, even if it was dismissed, and then having to pay increased malpractice premiums. “I still need to document this episode every single time I apply for any medical position, even more than 29 years after I was dismissed from the case,” said one respondent.
When asked if they would do anything differently, many oncologists (42%) said no, they would not have done anything differently. This is similar to the responses from physicians in general and with 2019 responses from oncologists. However, 15% of the respondents said that in retrospect, they would not have taken on that patient to begin with.
Some oncologists noted that they would have been more conscientious in relaying the information to the referring physician. Evan Lyman, an associate attorney at Voute, Lohrfink, McAndrew, Meisner & Roberts, LLP, in White Plains, N.Y., pointed out that a common reason for lawsuits is a slip-up of communication between the specialist and the referring physician.
Oncologists who had been sued have some insights to offer to colleagues, should they find themselves in a similar situation.
“Only answer with short and precise statements,” wrote one oncologist. “Attend all the depositions as much as you can; they are more likely to fabricate or exaggerate if you are not sitting in the room.”
Another physician said to base “everything on the medical record and do not answer hypothetical questions.”
“Document all interactions with patients as if a jury will be reading them, word by word,” said one respondent.
As for the public or patients, oncologists had this message: “malpractice suits should be rarely launched and only when gross errors can be absolutely proven.”
Another oncologist pointed out that communication is key. “Speak to the physicians against whom you have distrust. Lots of things could be cleared by good communication.”
A version of this article first appeared on Medscape.com.
10 things not to do in a medical board hearing
A Florida doctor told his patient her test result would be available in 3-4 days. When the patient didn’t hear back, she called the practice several times, but she didn’t receive a return call. So she filed a complaint against the doctor with the medical board.
When the board investigator interviewed the doctor, the physician said he wasn’t aware the patient had called. But his staff said otherwise. Because the doctor had not been truthful, the board sent him a letter of guidance and required him to attend a training program in ethics.
Miami attorney William J. Spratt Jr., who supplied this anecdote about a former client, said that
The following are some common mistakes that physicians make when dealing with a board complaint.
1. Not responding to the complaint
The complaint you get from the board – which often comes with a subpoena and a response deadline – usually asks for medical records pertinent to the case.
You can’t disregard the board’s letter, said Doug Brocker, an attorney handling board actions in Raleigh, N.C. “It’s amazing to me that some people just ignore a board complaint. Sometimes it’s because the doctor is just burnt out, which may have gotten the doctor into trouble in the first place.”
If you do not respond to a subpoena, “the board can file a court order holding you in contempt and start taking action on your license,” said Jeff Segal, MD, a neurosurgeon and attorney in Greensboro, N.C. Dr. Segal is CEO of Medical Justice Services, which protects physicians’ reputations associated with malpractice suits and board actions. “Not responding is not much different from agreeing to all of the charges.”
2. Not recognizing the seriousness of the complaint
“The biggest mistake is not taking a complaint seriously,” said Linda Stimmel, an attorney at Wilson Elser in Dallas. “Physicians who get a complaint often fire off a brief response stating that the complaint has no merit, without offering any evidence.”
According to Ms. Stimmel, “it’s really important to back up your assertions, such as using excerpts from the medical record, citations of peer-reviewed articles, or a letter of support from a colleague.”
“Weigh your answers carefully, because lack of accuracy will complicate your case,” Mr. Brocker said. “Consult the medical record rather than rely on your memory.”
“Present your version of events, in your own words, because that’s almost always better than the board’s version,” said Dr. Segal.
Even if there was a bad clinical outcome, Dr. Segal said you might point out that the patient was at high risk, or you could show that your clinical outcomes are better than the national average.
3. Thinking the board is on your side
You may be lulled into a false sense of security because the physicians on the medical board are your peers, but they can be as tough as any medical malpractice judge, said William P. Sullivan, DO, an emergency physician and attorney in Frankfort, Ill.
As per the National Practitioner Data Bank, physicians are three to four times more likely to incur an adverse board action than make a malpractice payout, Dr. Sullivan said.
Also, although a malpractice lawsuit rarely involves more than a monetary payment, a board action, like a monitoring plan, can restrict your ability to practice medicine. In fact, any kind of board action against you can make it harder to find employment.
4. Not being honest or forthcoming
“Lying to the board is the fastest way to turn what would have been a minor infraction into putting your license at risk,” Mr. Brocker said. This can happen when doctors update a medical record to support their version of events.
As per Dr. Sullivan, another way to put your license at risk is to withhold adverse information, which the board can detect by obtaining your application for hospital privileges or for licensure to another state, in which you revealed the adverse information.
Dr. Sullivan also advised against claiming you “always” take a certain precautionary measure. “In reality, we doctors don’t always do what we would like to have done. By saying you always do it when you didn’t, you appear less than truthful to the board, and boards have a hard time with that.”
Similarly, “when doctors don’t want to recognize that they could have handled things better, they tend to dance around the issue,” Mr. Brocker said. “This does not sit well with the board.” Insisting that you did everything right when it’s obvious that you didn’t can lead to harsher sanctions. “The board wants to make sure doctors recognize their mistakes and are willing to learn from them.”
5. Providing too much information
You may think that providing a great deal of information strengthens your case, but it can actually weaken it, Mr. Brocker said. Irrelevant information makes your response hard to follow, and it may contain evidence that could prompt another line of inquiry.
“Less is more,” Dr. Segal advised. “Present a coherent argument and keep to the most salient points.” Being concise is also good advice if your complaint proceeds to the board and you have to present your case.
Dr. Segal said the board will stop paying attention to long-winded presentations. He tells his clients to imagine the board is watching a movie. “If your presentation is tedious or hard to follow, you will lose them.”
6. Trying to contact the complainant
Complaints are kept anonymous, but in many cases, the doctor has an idea who the complainant was and may try to contact that person. “It’s natural to wonder why a patient would file a complaint against you,” Mr. Brocker said, but if you reach out to the patient to ask why, “it could look like you’re trying to persuade the patient to drop the complaint.”
Doctors who are involved in a practice breakup or a divorce can be victims of false and malicious complaints, but Beth Y. Collis, a partner at the law firm of Dinsmore & Shohl in Columbus, said boards are onto this tactic and usually reject these complaints.
The doctor may be tempted to sue the complainant, but Mr. Brocker said this won’t stop the complaint and could strengthen it. “Most statements to the medical board are protected from defamation lawsuits, and any lawsuit could appear to be intimidation.”
7. Simply signing a consent agreement
A small minority of complaints may result in the board taking action against the doctor. Typically, this involves getting the doctor to sign a consent agreement stating that he or she agrees with the board’s decision and its remedy, such as continuing education, a fine, or being placed under another doctor’s supervision.
“When the board sends you a consent agreement, it’s usually about something fairly minor,” Ms. Collis said. “You can make a counteroffer and see if they accept that. But once you enter into the agreement, you waive any right to appeal the board’s decision.”
8. Not hiring an attorney
Although some doctors manage to deal with a board complaint on their own, many will need to get an attorney, Mr. Brocker said. “An experienced attorney can help you navigate the board’s process.”
Clients often look for attorneys at the end of the process, when formal charges have already been filed, Mr. Brocker said. At that point, “it’s harder to get things moving in the right direction. You can’t unring the bell.”
Even if you don’t think you need an attorney throughout the case, “it helps to get advice from an attorney at the beginning,” Dr. Segal said. Doctors may think they can’t afford an attorney, but many malpractice carriers pay attorneys’ fees in medical board investigations.
Mr. Brocker advised finding an attorney who is familiar with licensing boards. “Malpractice attorneys may think they can deal with medical boards, but boards are quite different.” For example, “malpractice cases involve an adversarial approach, but licensing boards normally require working collaboratively.”
9. Not requesting a hearing
When the board takes action against you, it can be tempting to just accept the allegations and move on with your life, but it may be possible to undo the action, Dr. Sullivan said. “The board still has to prove its allegations, and it may not have a strong case against you.”
In some states, the medical board has to meet a very high standard of proof, Dr. Sullivan said. In Illinois, for example, the board must show “clear and convincing evidence,” while a malpractice plaintiff must only prove that it’s “more likely than not” that a physician violated the standard of care.
A hearing can especially help doctors facing harsh sanctions for minor offenses. For example, in a case handled by the law firm of Ray & Bishop in Newport Beach, Calif., a doctor who was stopped by police while driving home after having wine at a family gathering was found to have a blood alcohol level of 0.11%. Noting that the physician was on call at the time, the Medical Board of California decided to give him 5 years of probation.
Ray & Bishop asked for a judicial hearing to contest the decision. At the hearing, the physician noted that other physicians were also available to take call that night, and an expert stated that the doctor was not an alcohol abuser. The judge ruled that the board’s action was unduly harsh, and the physician received a public reprimand with no further penalties.
10. Getting upset with board officials
A board investigator may show up at your office uninvited and ask you to answer some questions, but you aren’t required to answer then and there, said Ms. Collis.
In fact, she noted, it’s never a good idea to let investigators into your office. “They can walk around, look through your records, and find more things to investigate.” For this reason, Ms. Collis makes it a point to schedule meetings with investigators at her office.
When you have to interact with board officials, such as during hearings, expressing anger is a mistake. “Some board members may raise their voices and make untrue assertions about your medical care,” Dr. Sullivan said. “You may wish you could respond in kind, but that will not help you.” Instead, calmly provide studies or guidelines supporting the care you provided.
Taking board investigators to task is also a mistake, Mr. Brocker pointed out. In his words, “investigators have to follow the rules. Getting mad at them will only make your case more difficult. Even if you believe the complaint against you is totally without merit, the process needs to run its course.”
A version of this article first appeared on Medscape.com.
A Florida doctor told his patient her test result would be available in 3-4 days. When the patient didn’t hear back, she called the practice several times, but she didn’t receive a return call. So she filed a complaint against the doctor with the medical board.
When the board investigator interviewed the doctor, the physician said he wasn’t aware the patient had called. But his staff said otherwise. Because the doctor had not been truthful, the board sent him a letter of guidance and required him to attend a training program in ethics.
Miami attorney William J. Spratt Jr., who supplied this anecdote about a former client, said that
The following are some common mistakes that physicians make when dealing with a board complaint.
1. Not responding to the complaint
The complaint you get from the board – which often comes with a subpoena and a response deadline – usually asks for medical records pertinent to the case.
You can’t disregard the board’s letter, said Doug Brocker, an attorney handling board actions in Raleigh, N.C. “It’s amazing to me that some people just ignore a board complaint. Sometimes it’s because the doctor is just burnt out, which may have gotten the doctor into trouble in the first place.”
If you do not respond to a subpoena, “the board can file a court order holding you in contempt and start taking action on your license,” said Jeff Segal, MD, a neurosurgeon and attorney in Greensboro, N.C. Dr. Segal is CEO of Medical Justice Services, which protects physicians’ reputations associated with malpractice suits and board actions. “Not responding is not much different from agreeing to all of the charges.”
2. Not recognizing the seriousness of the complaint
“The biggest mistake is not taking a complaint seriously,” said Linda Stimmel, an attorney at Wilson Elser in Dallas. “Physicians who get a complaint often fire off a brief response stating that the complaint has no merit, without offering any evidence.”
According to Ms. Stimmel, “it’s really important to back up your assertions, such as using excerpts from the medical record, citations of peer-reviewed articles, or a letter of support from a colleague.”
“Weigh your answers carefully, because lack of accuracy will complicate your case,” Mr. Brocker said. “Consult the medical record rather than rely on your memory.”
“Present your version of events, in your own words, because that’s almost always better than the board’s version,” said Dr. Segal.
Even if there was a bad clinical outcome, Dr. Segal said you might point out that the patient was at high risk, or you could show that your clinical outcomes are better than the national average.
3. Thinking the board is on your side
You may be lulled into a false sense of security because the physicians on the medical board are your peers, but they can be as tough as any medical malpractice judge, said William P. Sullivan, DO, an emergency physician and attorney in Frankfort, Ill.
As per the National Practitioner Data Bank, physicians are three to four times more likely to incur an adverse board action than make a malpractice payout, Dr. Sullivan said.
Also, although a malpractice lawsuit rarely involves more than a monetary payment, a board action, like a monitoring plan, can restrict your ability to practice medicine. In fact, any kind of board action against you can make it harder to find employment.
4. Not being honest or forthcoming
“Lying to the board is the fastest way to turn what would have been a minor infraction into putting your license at risk,” Mr. Brocker said. This can happen when doctors update a medical record to support their version of events.
As per Dr. Sullivan, another way to put your license at risk is to withhold adverse information, which the board can detect by obtaining your application for hospital privileges or for licensure to another state, in which you revealed the adverse information.
Dr. Sullivan also advised against claiming you “always” take a certain precautionary measure. “In reality, we doctors don’t always do what we would like to have done. By saying you always do it when you didn’t, you appear less than truthful to the board, and boards have a hard time with that.”
Similarly, “when doctors don’t want to recognize that they could have handled things better, they tend to dance around the issue,” Mr. Brocker said. “This does not sit well with the board.” Insisting that you did everything right when it’s obvious that you didn’t can lead to harsher sanctions. “The board wants to make sure doctors recognize their mistakes and are willing to learn from them.”
5. Providing too much information
You may think that providing a great deal of information strengthens your case, but it can actually weaken it, Mr. Brocker said. Irrelevant information makes your response hard to follow, and it may contain evidence that could prompt another line of inquiry.
“Less is more,” Dr. Segal advised. “Present a coherent argument and keep to the most salient points.” Being concise is also good advice if your complaint proceeds to the board and you have to present your case.
Dr. Segal said the board will stop paying attention to long-winded presentations. He tells his clients to imagine the board is watching a movie. “If your presentation is tedious or hard to follow, you will lose them.”
6. Trying to contact the complainant
Complaints are kept anonymous, but in many cases, the doctor has an idea who the complainant was and may try to contact that person. “It’s natural to wonder why a patient would file a complaint against you,” Mr. Brocker said, but if you reach out to the patient to ask why, “it could look like you’re trying to persuade the patient to drop the complaint.”
Doctors who are involved in a practice breakup or a divorce can be victims of false and malicious complaints, but Beth Y. Collis, a partner at the law firm of Dinsmore & Shohl in Columbus, said boards are onto this tactic and usually reject these complaints.
The doctor may be tempted to sue the complainant, but Mr. Brocker said this won’t stop the complaint and could strengthen it. “Most statements to the medical board are protected from defamation lawsuits, and any lawsuit could appear to be intimidation.”
7. Simply signing a consent agreement
A small minority of complaints may result in the board taking action against the doctor. Typically, this involves getting the doctor to sign a consent agreement stating that he or she agrees with the board’s decision and its remedy, such as continuing education, a fine, or being placed under another doctor’s supervision.
“When the board sends you a consent agreement, it’s usually about something fairly minor,” Ms. Collis said. “You can make a counteroffer and see if they accept that. But once you enter into the agreement, you waive any right to appeal the board’s decision.”
8. Not hiring an attorney
Although some doctors manage to deal with a board complaint on their own, many will need to get an attorney, Mr. Brocker said. “An experienced attorney can help you navigate the board’s process.”
Clients often look for attorneys at the end of the process, when formal charges have already been filed, Mr. Brocker said. At that point, “it’s harder to get things moving in the right direction. You can’t unring the bell.”
Even if you don’t think you need an attorney throughout the case, “it helps to get advice from an attorney at the beginning,” Dr. Segal said. Doctors may think they can’t afford an attorney, but many malpractice carriers pay attorneys’ fees in medical board investigations.
Mr. Brocker advised finding an attorney who is familiar with licensing boards. “Malpractice attorneys may think they can deal with medical boards, but boards are quite different.” For example, “malpractice cases involve an adversarial approach, but licensing boards normally require working collaboratively.”
9. Not requesting a hearing
When the board takes action against you, it can be tempting to just accept the allegations and move on with your life, but it may be possible to undo the action, Dr. Sullivan said. “The board still has to prove its allegations, and it may not have a strong case against you.”
In some states, the medical board has to meet a very high standard of proof, Dr. Sullivan said. In Illinois, for example, the board must show “clear and convincing evidence,” while a malpractice plaintiff must only prove that it’s “more likely than not” that a physician violated the standard of care.
A hearing can especially help doctors facing harsh sanctions for minor offenses. For example, in a case handled by the law firm of Ray & Bishop in Newport Beach, Calif., a doctor who was stopped by police while driving home after having wine at a family gathering was found to have a blood alcohol level of 0.11%. Noting that the physician was on call at the time, the Medical Board of California decided to give him 5 years of probation.
Ray & Bishop asked for a judicial hearing to contest the decision. At the hearing, the physician noted that other physicians were also available to take call that night, and an expert stated that the doctor was not an alcohol abuser. The judge ruled that the board’s action was unduly harsh, and the physician received a public reprimand with no further penalties.
10. Getting upset with board officials
A board investigator may show up at your office uninvited and ask you to answer some questions, but you aren’t required to answer then and there, said Ms. Collis.
In fact, she noted, it’s never a good idea to let investigators into your office. “They can walk around, look through your records, and find more things to investigate.” For this reason, Ms. Collis makes it a point to schedule meetings with investigators at her office.
When you have to interact with board officials, such as during hearings, expressing anger is a mistake. “Some board members may raise their voices and make untrue assertions about your medical care,” Dr. Sullivan said. “You may wish you could respond in kind, but that will not help you.” Instead, calmly provide studies or guidelines supporting the care you provided.
Taking board investigators to task is also a mistake, Mr. Brocker pointed out. In his words, “investigators have to follow the rules. Getting mad at them will only make your case more difficult. Even if you believe the complaint against you is totally without merit, the process needs to run its course.”
A version of this article first appeared on Medscape.com.
A Florida doctor told his patient her test result would be available in 3-4 days. When the patient didn’t hear back, she called the practice several times, but she didn’t receive a return call. So she filed a complaint against the doctor with the medical board.
When the board investigator interviewed the doctor, the physician said he wasn’t aware the patient had called. But his staff said otherwise. Because the doctor had not been truthful, the board sent him a letter of guidance and required him to attend a training program in ethics.
Miami attorney William J. Spratt Jr., who supplied this anecdote about a former client, said that
The following are some common mistakes that physicians make when dealing with a board complaint.
1. Not responding to the complaint
The complaint you get from the board – which often comes with a subpoena and a response deadline – usually asks for medical records pertinent to the case.
You can’t disregard the board’s letter, said Doug Brocker, an attorney handling board actions in Raleigh, N.C. “It’s amazing to me that some people just ignore a board complaint. Sometimes it’s because the doctor is just burnt out, which may have gotten the doctor into trouble in the first place.”
If you do not respond to a subpoena, “the board can file a court order holding you in contempt and start taking action on your license,” said Jeff Segal, MD, a neurosurgeon and attorney in Greensboro, N.C. Dr. Segal is CEO of Medical Justice Services, which protects physicians’ reputations associated with malpractice suits and board actions. “Not responding is not much different from agreeing to all of the charges.”
2. Not recognizing the seriousness of the complaint
“The biggest mistake is not taking a complaint seriously,” said Linda Stimmel, an attorney at Wilson Elser in Dallas. “Physicians who get a complaint often fire off a brief response stating that the complaint has no merit, without offering any evidence.”
According to Ms. Stimmel, “it’s really important to back up your assertions, such as using excerpts from the medical record, citations of peer-reviewed articles, or a letter of support from a colleague.”
“Weigh your answers carefully, because lack of accuracy will complicate your case,” Mr. Brocker said. “Consult the medical record rather than rely on your memory.”
“Present your version of events, in your own words, because that’s almost always better than the board’s version,” said Dr. Segal.
Even if there was a bad clinical outcome, Dr. Segal said you might point out that the patient was at high risk, or you could show that your clinical outcomes are better than the national average.
3. Thinking the board is on your side
You may be lulled into a false sense of security because the physicians on the medical board are your peers, but they can be as tough as any medical malpractice judge, said William P. Sullivan, DO, an emergency physician and attorney in Frankfort, Ill.
As per the National Practitioner Data Bank, physicians are three to four times more likely to incur an adverse board action than make a malpractice payout, Dr. Sullivan said.
Also, although a malpractice lawsuit rarely involves more than a monetary payment, a board action, like a monitoring plan, can restrict your ability to practice medicine. In fact, any kind of board action against you can make it harder to find employment.
4. Not being honest or forthcoming
“Lying to the board is the fastest way to turn what would have been a minor infraction into putting your license at risk,” Mr. Brocker said. This can happen when doctors update a medical record to support their version of events.
As per Dr. Sullivan, another way to put your license at risk is to withhold adverse information, which the board can detect by obtaining your application for hospital privileges or for licensure to another state, in which you revealed the adverse information.
Dr. Sullivan also advised against claiming you “always” take a certain precautionary measure. “In reality, we doctors don’t always do what we would like to have done. By saying you always do it when you didn’t, you appear less than truthful to the board, and boards have a hard time with that.”
Similarly, “when doctors don’t want to recognize that they could have handled things better, they tend to dance around the issue,” Mr. Brocker said. “This does not sit well with the board.” Insisting that you did everything right when it’s obvious that you didn’t can lead to harsher sanctions. “The board wants to make sure doctors recognize their mistakes and are willing to learn from them.”
5. Providing too much information
You may think that providing a great deal of information strengthens your case, but it can actually weaken it, Mr. Brocker said. Irrelevant information makes your response hard to follow, and it may contain evidence that could prompt another line of inquiry.
“Less is more,” Dr. Segal advised. “Present a coherent argument and keep to the most salient points.” Being concise is also good advice if your complaint proceeds to the board and you have to present your case.
Dr. Segal said the board will stop paying attention to long-winded presentations. He tells his clients to imagine the board is watching a movie. “If your presentation is tedious or hard to follow, you will lose them.”
6. Trying to contact the complainant
Complaints are kept anonymous, but in many cases, the doctor has an idea who the complainant was and may try to contact that person. “It’s natural to wonder why a patient would file a complaint against you,” Mr. Brocker said, but if you reach out to the patient to ask why, “it could look like you’re trying to persuade the patient to drop the complaint.”
Doctors who are involved in a practice breakup or a divorce can be victims of false and malicious complaints, but Beth Y. Collis, a partner at the law firm of Dinsmore & Shohl in Columbus, said boards are onto this tactic and usually reject these complaints.
The doctor may be tempted to sue the complainant, but Mr. Brocker said this won’t stop the complaint and could strengthen it. “Most statements to the medical board are protected from defamation lawsuits, and any lawsuit could appear to be intimidation.”
7. Simply signing a consent agreement
A small minority of complaints may result in the board taking action against the doctor. Typically, this involves getting the doctor to sign a consent agreement stating that he or she agrees with the board’s decision and its remedy, such as continuing education, a fine, or being placed under another doctor’s supervision.
“When the board sends you a consent agreement, it’s usually about something fairly minor,” Ms. Collis said. “You can make a counteroffer and see if they accept that. But once you enter into the agreement, you waive any right to appeal the board’s decision.”
8. Not hiring an attorney
Although some doctors manage to deal with a board complaint on their own, many will need to get an attorney, Mr. Brocker said. “An experienced attorney can help you navigate the board’s process.”
Clients often look for attorneys at the end of the process, when formal charges have already been filed, Mr. Brocker said. At that point, “it’s harder to get things moving in the right direction. You can’t unring the bell.”
Even if you don’t think you need an attorney throughout the case, “it helps to get advice from an attorney at the beginning,” Dr. Segal said. Doctors may think they can’t afford an attorney, but many malpractice carriers pay attorneys’ fees in medical board investigations.
Mr. Brocker advised finding an attorney who is familiar with licensing boards. “Malpractice attorneys may think they can deal with medical boards, but boards are quite different.” For example, “malpractice cases involve an adversarial approach, but licensing boards normally require working collaboratively.”
9. Not requesting a hearing
When the board takes action against you, it can be tempting to just accept the allegations and move on with your life, but it may be possible to undo the action, Dr. Sullivan said. “The board still has to prove its allegations, and it may not have a strong case against you.”
In some states, the medical board has to meet a very high standard of proof, Dr. Sullivan said. In Illinois, for example, the board must show “clear and convincing evidence,” while a malpractice plaintiff must only prove that it’s “more likely than not” that a physician violated the standard of care.
A hearing can especially help doctors facing harsh sanctions for minor offenses. For example, in a case handled by the law firm of Ray & Bishop in Newport Beach, Calif., a doctor who was stopped by police while driving home after having wine at a family gathering was found to have a blood alcohol level of 0.11%. Noting that the physician was on call at the time, the Medical Board of California decided to give him 5 years of probation.
Ray & Bishop asked for a judicial hearing to contest the decision. At the hearing, the physician noted that other physicians were also available to take call that night, and an expert stated that the doctor was not an alcohol abuser. The judge ruled that the board’s action was unduly harsh, and the physician received a public reprimand with no further penalties.
10. Getting upset with board officials
A board investigator may show up at your office uninvited and ask you to answer some questions, but you aren’t required to answer then and there, said Ms. Collis.
In fact, she noted, it’s never a good idea to let investigators into your office. “They can walk around, look through your records, and find more things to investigate.” For this reason, Ms. Collis makes it a point to schedule meetings with investigators at her office.
When you have to interact with board officials, such as during hearings, expressing anger is a mistake. “Some board members may raise their voices and make untrue assertions about your medical care,” Dr. Sullivan said. “You may wish you could respond in kind, but that will not help you.” Instead, calmly provide studies or guidelines supporting the care you provided.
Taking board investigators to task is also a mistake, Mr. Brocker pointed out. In his words, “investigators have to follow the rules. Getting mad at them will only make your case more difficult. Even if you believe the complaint against you is totally without merit, the process needs to run its course.”
A version of this article first appeared on Medscape.com.
Differences in COVID-19 Outcomes Among Patients With Type 1 Diabetes: First vs Later Surges
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.
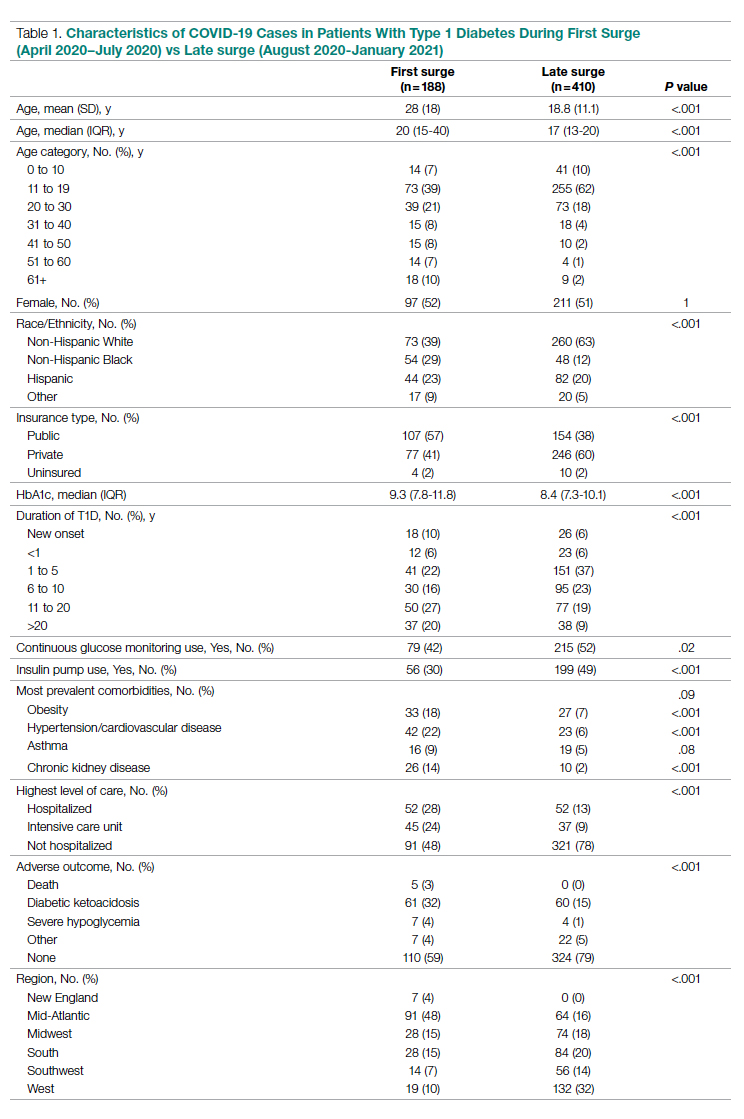
Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.
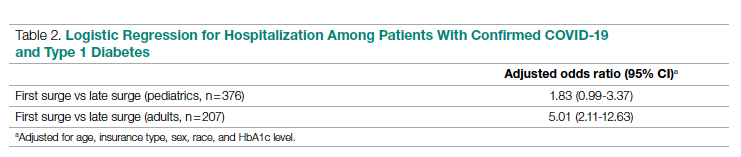
Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.

Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.

Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.

Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.

Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
Role and Experience of a Subintensive Care Unit in Caring for Patients With COVID-19 in Italy: The CO-RESP Study
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.
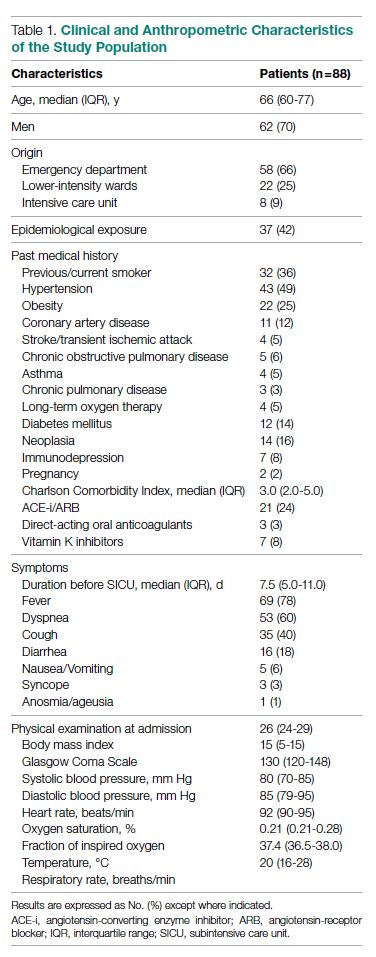
Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).
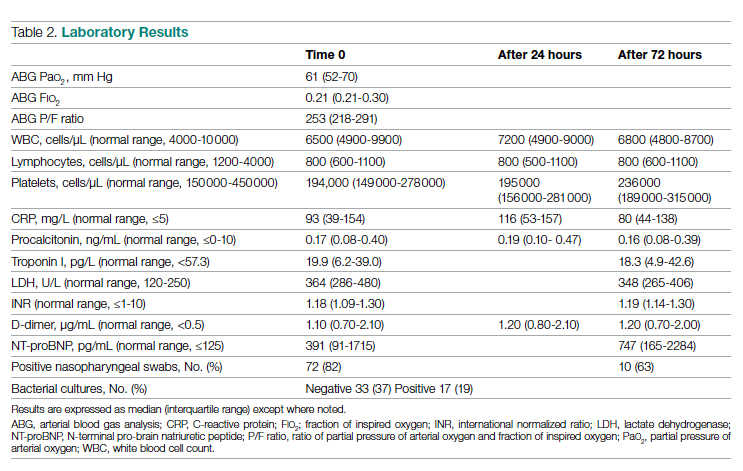
Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.
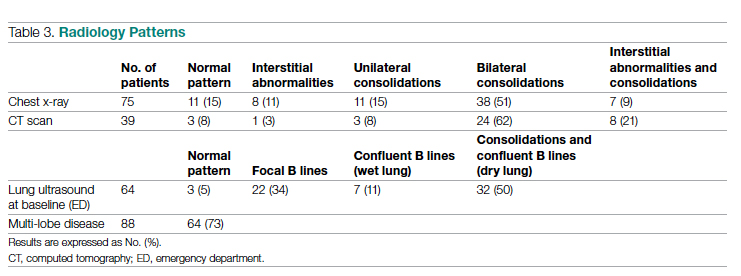
Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.
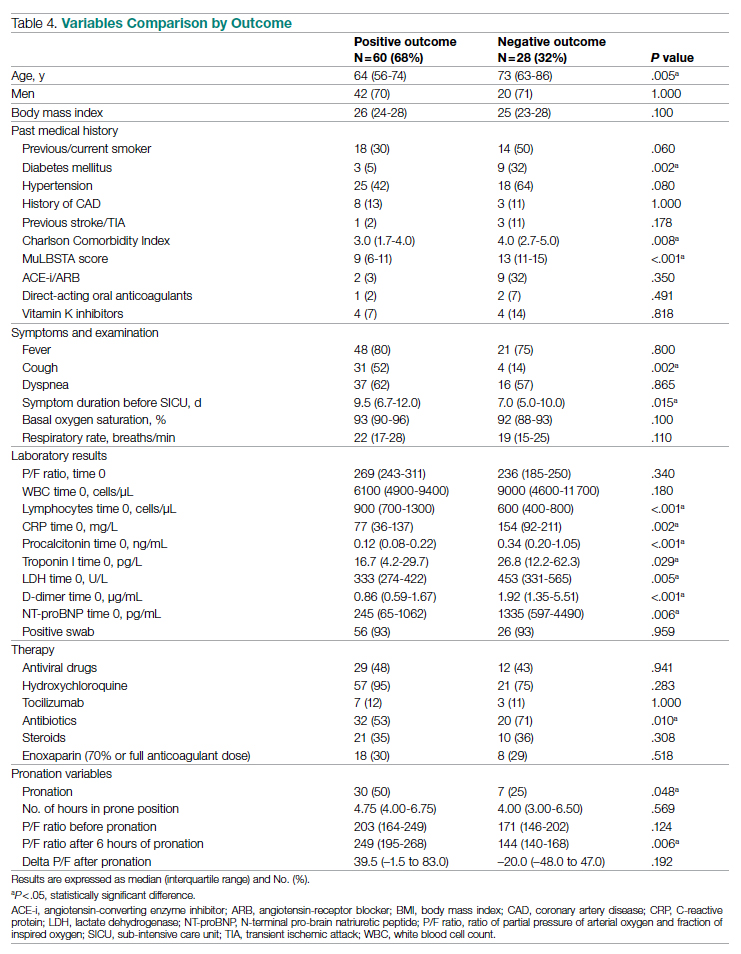
Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.

Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).

Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.

Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.

Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.

Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).

Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.

Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.

Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
Structural Ableism: Defining Standards of Care Amid Crisis and Inequity
Equitable Standards for All Patients in a Crisis
Health care delivered during a pandemic instantiates medicine’s perspectives on the value of human life in clinical scenarios where resource allocation is limited. The COVID-19 pandemic has fostered dialogue and debate around the ethical principles that underly such resource allocation, which generally balance (1) utilitarian optimization of resources, (2) equality or equity in health access, (3) the instrumental value of individuals as agents in society, and (4) prioritizing the “worst off” in their natural history of disease.1,2 State legislatures and health systems have responded to the challeges posed by COVID-19 by considering both the scarcity of intensive care resources, such as mechanical ventilation and hemodialysis, and the clinical criteria to be used for determining which patients should receive said resources. These crisis guidelines have yielded several concerning themes vis-à-vis equitable distribution of health care resources, particularly when the disability status of patients is considered alongside life-expectancy or quality of life.3
Crisis standards of care (CSC) prioritize population-level health under a utilitarian paradigm, explicitly maximizing “life-years” within a population of patients rather than the life of any individual patient.4 Debated during initial COVID surges, these CSC guidelines have recently been enacted at the state level in several settings, including Alaska and Idaho.5 In a setting with scarce intensive care resources, balancing health equity in access to these resources against population-based survival metrics has been a challenge for commissions considering CSC.6,7 This need for balance has further promoted systemic views of “disability,” raising concern for structural “ableism” and highlighting the need for greater “ability awareness” in clinicians’ continued professional learning.
Structural Ableism: Defining Perspectives to Address Health Equity
Ableism has been defined as “a system that places value on people’s bodies and minds, based on societally constructed ideas of normalcy, intelligence, excellence, and productivity…[and] leads to people and society determining who is valuable and worthy based on their appearance and/or their ability to satisfactorily [re]produce, excel, and ‘behave.’”8 Regarding CSC, concerns about systemic bias in guideline design were raised early by disability advocacy groups during comment periods.9,10 More broadly, concerns about ableism sit alongside many deeply rooted societal perspectives of disabled individuals as pitiable or, conversely, heroic for having “overcome” their disability in some way. As a physician who sits in a manual wheelchair with paraplegia and mobility impairment, I have equally been subject to inappropriate bias and inappropriate praise for living in a wheelchair. I have also wondered, alongside my patients living with different levels of mobility or ability, why others often view us as “worse off.” Addressing directly whether disabled individuals are “worse off,” disability rights attorney and advocate Harriet McBryde Johnson has articulated a predominant sentiment among persons living with unique or different abilities:
Are we “worse off”? I don’t think so. Not in any meaningful way. There are too many variables. For those of us with congenital conditions, disability shapes all we are. Those disabled later in life adapt. We take constraints that no one would choose and build rich and satisfying lives within them. We enjoy pleasures other people enjoy and pleasures peculiarly our own. We have something the world needs.11
Many physician colleagues have common, invisible diseases such as diabetes and heart disease; fewer colleagues share conditions that are as visible as my spinal cord injury, as readily apparent to patients upon my entry to their hospital rooms. This simultaneous and inescapable identity as both patient and provider has afforded me wonderful doctor-patient interactions, particularly with those patients who appreciate how my patient experience impacts my ability to partially understand theirs. However, this simultaneous identity as doctor and patient also informed my personal and professional concerns regarding structural ableism as I considered scoring my own acutely ill hospital medicine patients with CSC triage scores in April 2020.
As a practicing hospital medicine physician, I have been emboldened by the efforts of my fellow clinicians amid COVID-19; their efforts have reaffirmed all the reasons I pursued a career in medicine. However, when I heard my clinical colleagues’ first explanation of the Massachusetts CSC guidelines in April 2020, I raised my hand to ask whether the “life-years” to which the guidelines referred were quality-adjusted. My concern regarding the implicit use of quality-adjusted life years (QALY) or disability-adjusted life years in clinical decision-making and implementation of these guidelines was validated when no clinical leaders could address this question directly. Sitting on the CSC committee for my hospital during this time was an honor. However, it was disconcerting to hear many clinicians’ unease when estimating mean survival for common chronic diseases, ranging from end-stage renal disease to advanced heart failure. If my expert colleagues, clinical specialists in kidney and heart disease, could not confidently apply mean survival estimates to multimorbid hospital patients, then idiosyncratic clinical judgment was sure to have a heavy hand in any calculation of “life-years.” Thus, my primary concern was that clinicians using triage heuristics would be subject to bias, regardless of their intention, and negatively adjust for the quality of a disabled life in their CSC triage scoring. My secondary concern was that the CSC guidelines themselves included systemic bias against disabled individuals.
According to CSC schema, triage scores index heavily on Sequential Organ Failure Assessment (SOFA) scores to define short-term survival; SOFA scores are partially driven by the Glasgow Coma Scale (GCS). Following professional and public comment periods, CSC guidelines in Massachusetts were revised to, among other critical points of revision, change prognostic estimation via “life years” in favor of generic estimation of short-term survival (Table). I wondered, if I presented to an emergency department with severe COVID-19 and was scored with the GCS for the purpose of making a CSC ventilator triage decision, how would my complete paraplegia and lower-extremity motor impairment be accounted for by a clinician assessing “best motor response” in the GCS? The purpose of these scores is to act algorithmically, to guide clinicians whose cognitive load and time limitations may not allow for adjustment of these algorithms based on the individual patient in front of them. Individualization of clinical decisions is part of medicine’s art, but is difficult in the best of times and no easier during a crisis in care delivery. As CSC triage scores were amended and addended throughout 2020, I returned to the COVID wards, time and again wondering, “What have we learned about systemic bias and health inequity in the CSC process and the pandemic broadly, with specific regard to disability?”
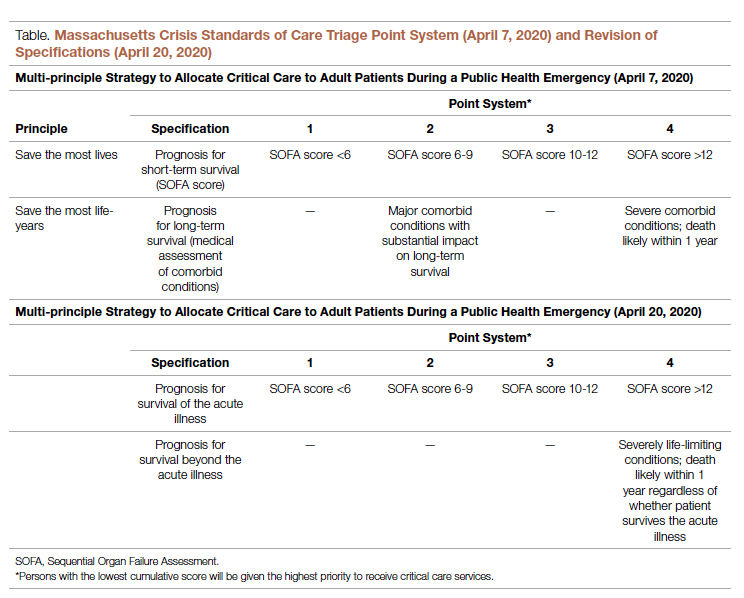
Ability Awareness: Room for Our Improvement
Unfortunately, there is reason to believe that clinical judgment is impaired by structural ableism. In seminal work on this topic, Gerhart et al12 demonstrated that clinicians considered spinal cord injury (SCI) survivors to have low self-perceptions of worthiness, overall negative attitudes, and low self-esteem as compared to able-bodied individuals. However, surveyed SCI survivors generally had similar self-perceptions of worth and positivity as compared to ”able-bodied” clinicians.12 For providers who care for persons with disabilities, the majority (82.4%) have rated their disabled patients’ quality of life as worse.13 It is no wonder that patients with disabilities are more likely to feel that their doctor-patient relationship is impacted by lack of understanding, negative sentiment, or simple lack of listening.14 Generally, this poor doctor-patient relationship with disabled patients is exacerbated by poor exposure of medical trainees to disability education; only 34.2% of internal medicine residents recall any form of disability education in medical school, while only 52% of medical school deans report having disability educational content in their curricula.15,16 There is a similar lack of disability representation in the population of medical trainees themselves. While approximately 20% of the American population lives with a disability, less than 2% of American medical students have a disability.17-19
While representation of disabled populations in medical practice remains poor, disabled patients are generally less likely to receive age-appropriate prevention, appropriate access to care, and equal access to treatment.20-22 “Diagnostic overshadowing” refers to clinicians’ attribution of nonspecific signs or symptoms to a patient’s chronic disability as opposed to acute illness.23 This phenomenon has led to higher rates of preventable malignancy in disabled patients and misattribution of common somatic symptoms to intellectual disability.24,25 With this disparity in place as status quo for health care delivery to disabled populations, it is no surprise that certain portions of the disabled population have accounted for disproportionate mortality due to COVID-19.26,27Disability advocates have called for “nothing about us without us,” a phrase associated with the United Nations Convention on the Rights of Persons with Disabilities. Understanding the profound neurodiversity among several forms of sensory and cognitive disabilities, as well as the functional difference between cognitive disabilities, mobility impairment, and inability to meet one’s instrumental activities of daily living independently, others have proposed a unique approach to certain disabled populations in COVID care.28 My own perspective is that definite progress may require a more general understanding of the prevalence of disability by clinicians, both via medical training and by directly addressing health equity for disabled populations in such calculations as the CSC. Systemic ableism is apparent in our most common clinical scoring systems, ranging from the GCS and Functional Assessment Staging Table to the Eastern Cooperative Oncology Group and Karnofsky Performance Status scales. I have reexamined these scoring systems in my own understanding given their general equation of ambulation with ability or normalcy. As a doctor in a manual wheelchair who values greatly my personal quality of life and professional contribution to patient care, I worry that these scoring systems inherently discount my own equitable access to care. Individualization of patients’ particular abilities in the context of these scales must occur alongside evidence-based, guideline-directed management via these scoring systems.
Conclusion: Future Orientation
Updated CSC guidelines have accounted for the unique considerations of disabled patients by effectively caveating their scoring algorithms, directing clinicians via disclaimers to uniquely consider their disabled patients in clinical judgement. This is a first step, but it is also one that erodes the value of algorithms, which generally obviate more deliberative thinking and individualization. For our patients who lack certain abilities, as CSC continue to be activated in several states, we have an opportunity to pursue more inherently equitable solutions before further suffering accrues.29 By way of example, adaptations to scoring systems that leverage QALYs for value-based drug pricing indices have been proposed by organizations like the Institute for Clinical and Economic Review, which proposed the Equal-Value-of Life-Years-Gained framework to inform QALY-based arbitration of drug pricing.30 This is not a perfect rubric but instead represents an attempt to balance consideration of drugs, as has been done with ventilators during the pandemic, as a scare and expensive resource while addressing the just concerns of advocacy groups in structural ableism.
Resource stewardship during a crisis should not discount those states of human life that are perceived to be less desirable, particularly if they are not experienced as less desirable but are experienced uniquely. Instead, we should consider equitably measuring our intervention to match a patient’s needs, as we would dose-adjust a medication for renal function or consider minimally invasive procedures for multimorbid patients. COVID-19 has reflected our profession’s ethical adaptation during crisis as resources have become scarce; there is no better time to define solutions for health equity. We should now be concerned equally by the influence our personal biases have on our clinical practice and by the way in which these crisis standards will influence patients’ perception of and trust in their care providers during periods of perceived plentiful resources in the future. Health care resources are always limited, allocated according to societal values; if we value health equity for people of all abilities, then we will consider these abilities equitably as we pursue new standards for health care delivery.
Corresponding author: Gregory D. Snyder, MD, MBA, 2014 Washington Street, Newton, MA 02462; [email protected].
Disclosures: None.
1. Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. doi:10.1056/NEJMsb2005114
2. Savulescu J, Persson I, Wilkinson D. Utilitarianism and the pandemic. Bioethics. 2020;34(6):620-632. doi:10.1111/bioe.12771
3. Mello MM, Persad G, White DB. Respecting disability rights - toward improved crisis standards of care. N Engl J Med. 2020;383(5):e26. doi: 10.1056/NEJMp2011997
4. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care Planning Guidance for the COVID-19 Pandemic. April 7, 2020. https://d279m997dpfwgl.cloudfront.net/wp/2020/04/CSC_April-7_2020.pdf
5. Knowles H. Hospitals overwhelmed by covid are turning to ‘crisis standards of care.’ What does that mean? The Washington Post. September 21, 2021. Accessed January 24, 2022. https://www.washingtonpost.com/health/2021/09/22/crisis-standards-of-care/
6. Hick JL, Hanfling D, Wynia MK, Toner E. Crisis standards of care and COVID-19: What did we learn? How do we ensure equity? What should we do? NAM Perspect. 2021;2021:10.31478/202108e. doi:10.31478/202108e
7. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
8. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
9. Kukla E. My life is more ‘disposable’ during this pandemic. The New York Times. March 19, 2020. Accessed January 24, 2022. https://www.nytimes.com/2020/03/19/opinion/coronavirus-disabled-health-care.html
10. CPR and Coalition Partners Secure Important Changes in Massachusetts’ Crisis Standards of Care. Center for Public Representation. December 1, 2020. Accessed January 24, 2022. https://www.centerforpublicrep.org/news/cpr-and-coalition-partners-secure-important-changes-in-massachusetts-crisis-standards-of-care/
11. Johnson HM. Unspeakable conversations. The New York Times. February 16, 2003. Accessed January 24, 2022. https://www.nytimes.com/2003/02/16/magazine/unspeakable-conversations.html
12. Gerhart KA, Koziol-McLain J, Lowenstein SR, Whiteneck GG. Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann Emerg Med. 1994;23(4):807-812. doi:10.1016/s0196-0644(94)70318-3
13. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. doi:10.1377/hlthaff.2020.01452
14. Smith DL. Disparities in patient-physician communication for persons with a disability from the 2006 Medical Expenditure Panel Survey (MEPS). Disabil Health J. 2009;2(4):206-215. doi:10.1016/j.dhjo.2009.06.002
15. Stillman MD, Ankam N, Mallow M, Capron M, Williams S. A survey of internal and family medicine residents: Assessment of disability-specific education and knowledge. Disabil Health J. 2021;14(2):101011. doi:10.1016/j.dhjo.2020.101011
16. Seidel E, Crowe S. The state of disability awareness in American medical schools. Am J Phys Med Rehabil. 2017;96(9):673-676. doi:10.1097/PHM.0000000000000719
17. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. doi:10.15585/mmwr.mm6732a3
18. Peacock G, Iezzoni LI, Harkin TR. Health care for Americans with disabilities--25 years after the ADA. N Engl J Med. 2015;373(10):892-893. doi:10.1056/NEJMp1508854
19. DeLisa JA, Thomas P. Physicians with disabilities and the physician workforce: a need to reassess our policies. Am J Phys Med Rehabil. 2005;84(1):5-11. doi:10.1097/01.phm.0000153323.28396.de
20. Disability and Health. Healthy People 2020. Accessed January 24, 2022. https://www.healthypeople.gov/2020/topics-objectives/topic/disability-and-health
21. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: a survey. Ann Intern Med. 2013;158(6):441-446. doi: 10.7326/0003-4819-158-6-201303190-00003
22. McCarthy EP, Ngo LH, Roetzheim RG, et al. Disparities in breast cancer treatment and survival for women with disabilities. Ann Intern Med. 2006;145(9):637-645. doi: 10.7326/0003-4819-145-9-200611070-00005
23. Javaid A, Nakata V, Michael D. Diagnostic overshadowing in learning disability: think beyond the disability. Prog Neurol Psychiatry. 2019;23:8-10.
24. Iezzoni LI, Rao SR, Agaronnik ND, El-Jawahri A. Cross-sectional analysis of the associations between four common cancers and disability. J Natl Compr Canc Netw. 2020;18(8):1031-1044. doi:10.6004/jnccn.2020.7551
25. Sanders JS, Keller S, Aravamuthan BR. Caring for individuals with intellectual and developmental disabilities in the COVID-19 crisis. Neurol Clin Pract. 2021;11(2):e174-e178. doi:10.1212/CPJ.0000000000000886
26. Landes SD, Turk MA, Formica MK, McDonald KE, Stevens JD. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York State. Disabil Health J. 2020;13(4):100969. doi:10.1016/j.dhjo.2020.100969
27. Gleason J, Ross W, Fossi A, Blonksy H, Tobias J, Stephens M. The devastating impact of Covid-19 on individuals with intellectual disabilities in the United States. NEJM Catalyst. 2021.doi.org/10.1056/CAT.21.0051
28. Nankervis K, Chan J. Applying the CRPD to people with intellectual and developmental disability with behaviors of concern during COVID-19. J Policy Pract Intellect Disabil. 2021:10.1111/jppi.12374. doi:10.1111/jppi.12374
29. Alaska Department of Health and Social Services, Division of Public Health, Rural and Community Health Systems. Patient care strategies for scarce resource situations. Version 1. August 2021. Accessed November 11, 2021, https://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/HumanCoV/SOA_DHSS_CrisisStandardsOfCare.pdf
30. Cost-effectiveness, the QALY, and the evlyg. ICER. May 21, 2021. Accessed January 24, 2022. https://icer.org/our-approach/methods-process/cost-effectiveness-the-qaly-and-the-evlyg/
Equitable Standards for All Patients in a Crisis
Health care delivered during a pandemic instantiates medicine’s perspectives on the value of human life in clinical scenarios where resource allocation is limited. The COVID-19 pandemic has fostered dialogue and debate around the ethical principles that underly such resource allocation, which generally balance (1) utilitarian optimization of resources, (2) equality or equity in health access, (3) the instrumental value of individuals as agents in society, and (4) prioritizing the “worst off” in their natural history of disease.1,2 State legislatures and health systems have responded to the challeges posed by COVID-19 by considering both the scarcity of intensive care resources, such as mechanical ventilation and hemodialysis, and the clinical criteria to be used for determining which patients should receive said resources. These crisis guidelines have yielded several concerning themes vis-à-vis equitable distribution of health care resources, particularly when the disability status of patients is considered alongside life-expectancy or quality of life.3
Crisis standards of care (CSC) prioritize population-level health under a utilitarian paradigm, explicitly maximizing “life-years” within a population of patients rather than the life of any individual patient.4 Debated during initial COVID surges, these CSC guidelines have recently been enacted at the state level in several settings, including Alaska and Idaho.5 In a setting with scarce intensive care resources, balancing health equity in access to these resources against population-based survival metrics has been a challenge for commissions considering CSC.6,7 This need for balance has further promoted systemic views of “disability,” raising concern for structural “ableism” and highlighting the need for greater “ability awareness” in clinicians’ continued professional learning.
Structural Ableism: Defining Perspectives to Address Health Equity
Ableism has been defined as “a system that places value on people’s bodies and minds, based on societally constructed ideas of normalcy, intelligence, excellence, and productivity…[and] leads to people and society determining who is valuable and worthy based on their appearance and/or their ability to satisfactorily [re]produce, excel, and ‘behave.’”8 Regarding CSC, concerns about systemic bias in guideline design were raised early by disability advocacy groups during comment periods.9,10 More broadly, concerns about ableism sit alongside many deeply rooted societal perspectives of disabled individuals as pitiable or, conversely, heroic for having “overcome” their disability in some way. As a physician who sits in a manual wheelchair with paraplegia and mobility impairment, I have equally been subject to inappropriate bias and inappropriate praise for living in a wheelchair. I have also wondered, alongside my patients living with different levels of mobility or ability, why others often view us as “worse off.” Addressing directly whether disabled individuals are “worse off,” disability rights attorney and advocate Harriet McBryde Johnson has articulated a predominant sentiment among persons living with unique or different abilities:
Are we “worse off”? I don’t think so. Not in any meaningful way. There are too many variables. For those of us with congenital conditions, disability shapes all we are. Those disabled later in life adapt. We take constraints that no one would choose and build rich and satisfying lives within them. We enjoy pleasures other people enjoy and pleasures peculiarly our own. We have something the world needs.11
Many physician colleagues have common, invisible diseases such as diabetes and heart disease; fewer colleagues share conditions that are as visible as my spinal cord injury, as readily apparent to patients upon my entry to their hospital rooms. This simultaneous and inescapable identity as both patient and provider has afforded me wonderful doctor-patient interactions, particularly with those patients who appreciate how my patient experience impacts my ability to partially understand theirs. However, this simultaneous identity as doctor and patient also informed my personal and professional concerns regarding structural ableism as I considered scoring my own acutely ill hospital medicine patients with CSC triage scores in April 2020.
As a practicing hospital medicine physician, I have been emboldened by the efforts of my fellow clinicians amid COVID-19; their efforts have reaffirmed all the reasons I pursued a career in medicine. However, when I heard my clinical colleagues’ first explanation of the Massachusetts CSC guidelines in April 2020, I raised my hand to ask whether the “life-years” to which the guidelines referred were quality-adjusted. My concern regarding the implicit use of quality-adjusted life years (QALY) or disability-adjusted life years in clinical decision-making and implementation of these guidelines was validated when no clinical leaders could address this question directly. Sitting on the CSC committee for my hospital during this time was an honor. However, it was disconcerting to hear many clinicians’ unease when estimating mean survival for common chronic diseases, ranging from end-stage renal disease to advanced heart failure. If my expert colleagues, clinical specialists in kidney and heart disease, could not confidently apply mean survival estimates to multimorbid hospital patients, then idiosyncratic clinical judgment was sure to have a heavy hand in any calculation of “life-years.” Thus, my primary concern was that clinicians using triage heuristics would be subject to bias, regardless of their intention, and negatively adjust for the quality of a disabled life in their CSC triage scoring. My secondary concern was that the CSC guidelines themselves included systemic bias against disabled individuals.
According to CSC schema, triage scores index heavily on Sequential Organ Failure Assessment (SOFA) scores to define short-term survival; SOFA scores are partially driven by the Glasgow Coma Scale (GCS). Following professional and public comment periods, CSC guidelines in Massachusetts were revised to, among other critical points of revision, change prognostic estimation via “life years” in favor of generic estimation of short-term survival (Table). I wondered, if I presented to an emergency department with severe COVID-19 and was scored with the GCS for the purpose of making a CSC ventilator triage decision, how would my complete paraplegia and lower-extremity motor impairment be accounted for by a clinician assessing “best motor response” in the GCS? The purpose of these scores is to act algorithmically, to guide clinicians whose cognitive load and time limitations may not allow for adjustment of these algorithms based on the individual patient in front of them. Individualization of clinical decisions is part of medicine’s art, but is difficult in the best of times and no easier during a crisis in care delivery. As CSC triage scores were amended and addended throughout 2020, I returned to the COVID wards, time and again wondering, “What have we learned about systemic bias and health inequity in the CSC process and the pandemic broadly, with specific regard to disability?”

Ability Awareness: Room for Our Improvement
Unfortunately, there is reason to believe that clinical judgment is impaired by structural ableism. In seminal work on this topic, Gerhart et al12 demonstrated that clinicians considered spinal cord injury (SCI) survivors to have low self-perceptions of worthiness, overall negative attitudes, and low self-esteem as compared to able-bodied individuals. However, surveyed SCI survivors generally had similar self-perceptions of worth and positivity as compared to ”able-bodied” clinicians.12 For providers who care for persons with disabilities, the majority (82.4%) have rated their disabled patients’ quality of life as worse.13 It is no wonder that patients with disabilities are more likely to feel that their doctor-patient relationship is impacted by lack of understanding, negative sentiment, or simple lack of listening.14 Generally, this poor doctor-patient relationship with disabled patients is exacerbated by poor exposure of medical trainees to disability education; only 34.2% of internal medicine residents recall any form of disability education in medical school, while only 52% of medical school deans report having disability educational content in their curricula.15,16 There is a similar lack of disability representation in the population of medical trainees themselves. While approximately 20% of the American population lives with a disability, less than 2% of American medical students have a disability.17-19
While representation of disabled populations in medical practice remains poor, disabled patients are generally less likely to receive age-appropriate prevention, appropriate access to care, and equal access to treatment.20-22 “Diagnostic overshadowing” refers to clinicians’ attribution of nonspecific signs or symptoms to a patient’s chronic disability as opposed to acute illness.23 This phenomenon has led to higher rates of preventable malignancy in disabled patients and misattribution of common somatic symptoms to intellectual disability.24,25 With this disparity in place as status quo for health care delivery to disabled populations, it is no surprise that certain portions of the disabled population have accounted for disproportionate mortality due to COVID-19.26,27Disability advocates have called for “nothing about us without us,” a phrase associated with the United Nations Convention on the Rights of Persons with Disabilities. Understanding the profound neurodiversity among several forms of sensory and cognitive disabilities, as well as the functional difference between cognitive disabilities, mobility impairment, and inability to meet one’s instrumental activities of daily living independently, others have proposed a unique approach to certain disabled populations in COVID care.28 My own perspective is that definite progress may require a more general understanding of the prevalence of disability by clinicians, both via medical training and by directly addressing health equity for disabled populations in such calculations as the CSC. Systemic ableism is apparent in our most common clinical scoring systems, ranging from the GCS and Functional Assessment Staging Table to the Eastern Cooperative Oncology Group and Karnofsky Performance Status scales. I have reexamined these scoring systems in my own understanding given their general equation of ambulation with ability or normalcy. As a doctor in a manual wheelchair who values greatly my personal quality of life and professional contribution to patient care, I worry that these scoring systems inherently discount my own equitable access to care. Individualization of patients’ particular abilities in the context of these scales must occur alongside evidence-based, guideline-directed management via these scoring systems.
Conclusion: Future Orientation
Updated CSC guidelines have accounted for the unique considerations of disabled patients by effectively caveating their scoring algorithms, directing clinicians via disclaimers to uniquely consider their disabled patients in clinical judgement. This is a first step, but it is also one that erodes the value of algorithms, which generally obviate more deliberative thinking and individualization. For our patients who lack certain abilities, as CSC continue to be activated in several states, we have an opportunity to pursue more inherently equitable solutions before further suffering accrues.29 By way of example, adaptations to scoring systems that leverage QALYs for value-based drug pricing indices have been proposed by organizations like the Institute for Clinical and Economic Review, which proposed the Equal-Value-of Life-Years-Gained framework to inform QALY-based arbitration of drug pricing.30 This is not a perfect rubric but instead represents an attempt to balance consideration of drugs, as has been done with ventilators during the pandemic, as a scare and expensive resource while addressing the just concerns of advocacy groups in structural ableism.
Resource stewardship during a crisis should not discount those states of human life that are perceived to be less desirable, particularly if they are not experienced as less desirable but are experienced uniquely. Instead, we should consider equitably measuring our intervention to match a patient’s needs, as we would dose-adjust a medication for renal function or consider minimally invasive procedures for multimorbid patients. COVID-19 has reflected our profession’s ethical adaptation during crisis as resources have become scarce; there is no better time to define solutions for health equity. We should now be concerned equally by the influence our personal biases have on our clinical practice and by the way in which these crisis standards will influence patients’ perception of and trust in their care providers during periods of perceived plentiful resources in the future. Health care resources are always limited, allocated according to societal values; if we value health equity for people of all abilities, then we will consider these abilities equitably as we pursue new standards for health care delivery.
Corresponding author: Gregory D. Snyder, MD, MBA, 2014 Washington Street, Newton, MA 02462; [email protected].
Disclosures: None.
Equitable Standards for All Patients in a Crisis
Health care delivered during a pandemic instantiates medicine’s perspectives on the value of human life in clinical scenarios where resource allocation is limited. The COVID-19 pandemic has fostered dialogue and debate around the ethical principles that underly such resource allocation, which generally balance (1) utilitarian optimization of resources, (2) equality or equity in health access, (3) the instrumental value of individuals as agents in society, and (4) prioritizing the “worst off” in their natural history of disease.1,2 State legislatures and health systems have responded to the challeges posed by COVID-19 by considering both the scarcity of intensive care resources, such as mechanical ventilation and hemodialysis, and the clinical criteria to be used for determining which patients should receive said resources. These crisis guidelines have yielded several concerning themes vis-à-vis equitable distribution of health care resources, particularly when the disability status of patients is considered alongside life-expectancy or quality of life.3
Crisis standards of care (CSC) prioritize population-level health under a utilitarian paradigm, explicitly maximizing “life-years” within a population of patients rather than the life of any individual patient.4 Debated during initial COVID surges, these CSC guidelines have recently been enacted at the state level in several settings, including Alaska and Idaho.5 In a setting with scarce intensive care resources, balancing health equity in access to these resources against population-based survival metrics has been a challenge for commissions considering CSC.6,7 This need for balance has further promoted systemic views of “disability,” raising concern for structural “ableism” and highlighting the need for greater “ability awareness” in clinicians’ continued professional learning.
Structural Ableism: Defining Perspectives to Address Health Equity
Ableism has been defined as “a system that places value on people’s bodies and minds, based on societally constructed ideas of normalcy, intelligence, excellence, and productivity…[and] leads to people and society determining who is valuable and worthy based on their appearance and/or their ability to satisfactorily [re]produce, excel, and ‘behave.’”8 Regarding CSC, concerns about systemic bias in guideline design were raised early by disability advocacy groups during comment periods.9,10 More broadly, concerns about ableism sit alongside many deeply rooted societal perspectives of disabled individuals as pitiable or, conversely, heroic for having “overcome” their disability in some way. As a physician who sits in a manual wheelchair with paraplegia and mobility impairment, I have equally been subject to inappropriate bias and inappropriate praise for living in a wheelchair. I have also wondered, alongside my patients living with different levels of mobility or ability, why others often view us as “worse off.” Addressing directly whether disabled individuals are “worse off,” disability rights attorney and advocate Harriet McBryde Johnson has articulated a predominant sentiment among persons living with unique or different abilities:
Are we “worse off”? I don’t think so. Not in any meaningful way. There are too many variables. For those of us with congenital conditions, disability shapes all we are. Those disabled later in life adapt. We take constraints that no one would choose and build rich and satisfying lives within them. We enjoy pleasures other people enjoy and pleasures peculiarly our own. We have something the world needs.11
Many physician colleagues have common, invisible diseases such as diabetes and heart disease; fewer colleagues share conditions that are as visible as my spinal cord injury, as readily apparent to patients upon my entry to their hospital rooms. This simultaneous and inescapable identity as both patient and provider has afforded me wonderful doctor-patient interactions, particularly with those patients who appreciate how my patient experience impacts my ability to partially understand theirs. However, this simultaneous identity as doctor and patient also informed my personal and professional concerns regarding structural ableism as I considered scoring my own acutely ill hospital medicine patients with CSC triage scores in April 2020.
As a practicing hospital medicine physician, I have been emboldened by the efforts of my fellow clinicians amid COVID-19; their efforts have reaffirmed all the reasons I pursued a career in medicine. However, when I heard my clinical colleagues’ first explanation of the Massachusetts CSC guidelines in April 2020, I raised my hand to ask whether the “life-years” to which the guidelines referred were quality-adjusted. My concern regarding the implicit use of quality-adjusted life years (QALY) or disability-adjusted life years in clinical decision-making and implementation of these guidelines was validated when no clinical leaders could address this question directly. Sitting on the CSC committee for my hospital during this time was an honor. However, it was disconcerting to hear many clinicians’ unease when estimating mean survival for common chronic diseases, ranging from end-stage renal disease to advanced heart failure. If my expert colleagues, clinical specialists in kidney and heart disease, could not confidently apply mean survival estimates to multimorbid hospital patients, then idiosyncratic clinical judgment was sure to have a heavy hand in any calculation of “life-years.” Thus, my primary concern was that clinicians using triage heuristics would be subject to bias, regardless of their intention, and negatively adjust for the quality of a disabled life in their CSC triage scoring. My secondary concern was that the CSC guidelines themselves included systemic bias against disabled individuals.
According to CSC schema, triage scores index heavily on Sequential Organ Failure Assessment (SOFA) scores to define short-term survival; SOFA scores are partially driven by the Glasgow Coma Scale (GCS). Following professional and public comment periods, CSC guidelines in Massachusetts were revised to, among other critical points of revision, change prognostic estimation via “life years” in favor of generic estimation of short-term survival (Table). I wondered, if I presented to an emergency department with severe COVID-19 and was scored with the GCS for the purpose of making a CSC ventilator triage decision, how would my complete paraplegia and lower-extremity motor impairment be accounted for by a clinician assessing “best motor response” in the GCS? The purpose of these scores is to act algorithmically, to guide clinicians whose cognitive load and time limitations may not allow for adjustment of these algorithms based on the individual patient in front of them. Individualization of clinical decisions is part of medicine’s art, but is difficult in the best of times and no easier during a crisis in care delivery. As CSC triage scores were amended and addended throughout 2020, I returned to the COVID wards, time and again wondering, “What have we learned about systemic bias and health inequity in the CSC process and the pandemic broadly, with specific regard to disability?”

Ability Awareness: Room for Our Improvement
Unfortunately, there is reason to believe that clinical judgment is impaired by structural ableism. In seminal work on this topic, Gerhart et al12 demonstrated that clinicians considered spinal cord injury (SCI) survivors to have low self-perceptions of worthiness, overall negative attitudes, and low self-esteem as compared to able-bodied individuals. However, surveyed SCI survivors generally had similar self-perceptions of worth and positivity as compared to ”able-bodied” clinicians.12 For providers who care for persons with disabilities, the majority (82.4%) have rated their disabled patients’ quality of life as worse.13 It is no wonder that patients with disabilities are more likely to feel that their doctor-patient relationship is impacted by lack of understanding, negative sentiment, or simple lack of listening.14 Generally, this poor doctor-patient relationship with disabled patients is exacerbated by poor exposure of medical trainees to disability education; only 34.2% of internal medicine residents recall any form of disability education in medical school, while only 52% of medical school deans report having disability educational content in their curricula.15,16 There is a similar lack of disability representation in the population of medical trainees themselves. While approximately 20% of the American population lives with a disability, less than 2% of American medical students have a disability.17-19
While representation of disabled populations in medical practice remains poor, disabled patients are generally less likely to receive age-appropriate prevention, appropriate access to care, and equal access to treatment.20-22 “Diagnostic overshadowing” refers to clinicians’ attribution of nonspecific signs or symptoms to a patient’s chronic disability as opposed to acute illness.23 This phenomenon has led to higher rates of preventable malignancy in disabled patients and misattribution of common somatic symptoms to intellectual disability.24,25 With this disparity in place as status quo for health care delivery to disabled populations, it is no surprise that certain portions of the disabled population have accounted for disproportionate mortality due to COVID-19.26,27Disability advocates have called for “nothing about us without us,” a phrase associated with the United Nations Convention on the Rights of Persons with Disabilities. Understanding the profound neurodiversity among several forms of sensory and cognitive disabilities, as well as the functional difference between cognitive disabilities, mobility impairment, and inability to meet one’s instrumental activities of daily living independently, others have proposed a unique approach to certain disabled populations in COVID care.28 My own perspective is that definite progress may require a more general understanding of the prevalence of disability by clinicians, both via medical training and by directly addressing health equity for disabled populations in such calculations as the CSC. Systemic ableism is apparent in our most common clinical scoring systems, ranging from the GCS and Functional Assessment Staging Table to the Eastern Cooperative Oncology Group and Karnofsky Performance Status scales. I have reexamined these scoring systems in my own understanding given their general equation of ambulation with ability or normalcy. As a doctor in a manual wheelchair who values greatly my personal quality of life and professional contribution to patient care, I worry that these scoring systems inherently discount my own equitable access to care. Individualization of patients’ particular abilities in the context of these scales must occur alongside evidence-based, guideline-directed management via these scoring systems.
Conclusion: Future Orientation
Updated CSC guidelines have accounted for the unique considerations of disabled patients by effectively caveating their scoring algorithms, directing clinicians via disclaimers to uniquely consider their disabled patients in clinical judgement. This is a first step, but it is also one that erodes the value of algorithms, which generally obviate more deliberative thinking and individualization. For our patients who lack certain abilities, as CSC continue to be activated in several states, we have an opportunity to pursue more inherently equitable solutions before further suffering accrues.29 By way of example, adaptations to scoring systems that leverage QALYs for value-based drug pricing indices have been proposed by organizations like the Institute for Clinical and Economic Review, which proposed the Equal-Value-of Life-Years-Gained framework to inform QALY-based arbitration of drug pricing.30 This is not a perfect rubric but instead represents an attempt to balance consideration of drugs, as has been done with ventilators during the pandemic, as a scare and expensive resource while addressing the just concerns of advocacy groups in structural ableism.
Resource stewardship during a crisis should not discount those states of human life that are perceived to be less desirable, particularly if they are not experienced as less desirable but are experienced uniquely. Instead, we should consider equitably measuring our intervention to match a patient’s needs, as we would dose-adjust a medication for renal function or consider minimally invasive procedures for multimorbid patients. COVID-19 has reflected our profession’s ethical adaptation during crisis as resources have become scarce; there is no better time to define solutions for health equity. We should now be concerned equally by the influence our personal biases have on our clinical practice and by the way in which these crisis standards will influence patients’ perception of and trust in their care providers during periods of perceived plentiful resources in the future. Health care resources are always limited, allocated according to societal values; if we value health equity for people of all abilities, then we will consider these abilities equitably as we pursue new standards for health care delivery.
Corresponding author: Gregory D. Snyder, MD, MBA, 2014 Washington Street, Newton, MA 02462; [email protected].
Disclosures: None.
1. Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. doi:10.1056/NEJMsb2005114
2. Savulescu J, Persson I, Wilkinson D. Utilitarianism and the pandemic. Bioethics. 2020;34(6):620-632. doi:10.1111/bioe.12771
3. Mello MM, Persad G, White DB. Respecting disability rights - toward improved crisis standards of care. N Engl J Med. 2020;383(5):e26. doi: 10.1056/NEJMp2011997
4. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care Planning Guidance for the COVID-19 Pandemic. April 7, 2020. https://d279m997dpfwgl.cloudfront.net/wp/2020/04/CSC_April-7_2020.pdf
5. Knowles H. Hospitals overwhelmed by covid are turning to ‘crisis standards of care.’ What does that mean? The Washington Post. September 21, 2021. Accessed January 24, 2022. https://www.washingtonpost.com/health/2021/09/22/crisis-standards-of-care/
6. Hick JL, Hanfling D, Wynia MK, Toner E. Crisis standards of care and COVID-19: What did we learn? How do we ensure equity? What should we do? NAM Perspect. 2021;2021:10.31478/202108e. doi:10.31478/202108e
7. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
8. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
9. Kukla E. My life is more ‘disposable’ during this pandemic. The New York Times. March 19, 2020. Accessed January 24, 2022. https://www.nytimes.com/2020/03/19/opinion/coronavirus-disabled-health-care.html
10. CPR and Coalition Partners Secure Important Changes in Massachusetts’ Crisis Standards of Care. Center for Public Representation. December 1, 2020. Accessed January 24, 2022. https://www.centerforpublicrep.org/news/cpr-and-coalition-partners-secure-important-changes-in-massachusetts-crisis-standards-of-care/
11. Johnson HM. Unspeakable conversations. The New York Times. February 16, 2003. Accessed January 24, 2022. https://www.nytimes.com/2003/02/16/magazine/unspeakable-conversations.html
12. Gerhart KA, Koziol-McLain J, Lowenstein SR, Whiteneck GG. Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann Emerg Med. 1994;23(4):807-812. doi:10.1016/s0196-0644(94)70318-3
13. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. doi:10.1377/hlthaff.2020.01452
14. Smith DL. Disparities in patient-physician communication for persons with a disability from the 2006 Medical Expenditure Panel Survey (MEPS). Disabil Health J. 2009;2(4):206-215. doi:10.1016/j.dhjo.2009.06.002
15. Stillman MD, Ankam N, Mallow M, Capron M, Williams S. A survey of internal and family medicine residents: Assessment of disability-specific education and knowledge. Disabil Health J. 2021;14(2):101011. doi:10.1016/j.dhjo.2020.101011
16. Seidel E, Crowe S. The state of disability awareness in American medical schools. Am J Phys Med Rehabil. 2017;96(9):673-676. doi:10.1097/PHM.0000000000000719
17. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. doi:10.15585/mmwr.mm6732a3
18. Peacock G, Iezzoni LI, Harkin TR. Health care for Americans with disabilities--25 years after the ADA. N Engl J Med. 2015;373(10):892-893. doi:10.1056/NEJMp1508854
19. DeLisa JA, Thomas P. Physicians with disabilities and the physician workforce: a need to reassess our policies. Am J Phys Med Rehabil. 2005;84(1):5-11. doi:10.1097/01.phm.0000153323.28396.de
20. Disability and Health. Healthy People 2020. Accessed January 24, 2022. https://www.healthypeople.gov/2020/topics-objectives/topic/disability-and-health
21. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: a survey. Ann Intern Med. 2013;158(6):441-446. doi: 10.7326/0003-4819-158-6-201303190-00003
22. McCarthy EP, Ngo LH, Roetzheim RG, et al. Disparities in breast cancer treatment and survival for women with disabilities. Ann Intern Med. 2006;145(9):637-645. doi: 10.7326/0003-4819-145-9-200611070-00005
23. Javaid A, Nakata V, Michael D. Diagnostic overshadowing in learning disability: think beyond the disability. Prog Neurol Psychiatry. 2019;23:8-10.
24. Iezzoni LI, Rao SR, Agaronnik ND, El-Jawahri A. Cross-sectional analysis of the associations between four common cancers and disability. J Natl Compr Canc Netw. 2020;18(8):1031-1044. doi:10.6004/jnccn.2020.7551
25. Sanders JS, Keller S, Aravamuthan BR. Caring for individuals with intellectual and developmental disabilities in the COVID-19 crisis. Neurol Clin Pract. 2021;11(2):e174-e178. doi:10.1212/CPJ.0000000000000886
26. Landes SD, Turk MA, Formica MK, McDonald KE, Stevens JD. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York State. Disabil Health J. 2020;13(4):100969. doi:10.1016/j.dhjo.2020.100969
27. Gleason J, Ross W, Fossi A, Blonksy H, Tobias J, Stephens M. The devastating impact of Covid-19 on individuals with intellectual disabilities in the United States. NEJM Catalyst. 2021.doi.org/10.1056/CAT.21.0051
28. Nankervis K, Chan J. Applying the CRPD to people with intellectual and developmental disability with behaviors of concern during COVID-19. J Policy Pract Intellect Disabil. 2021:10.1111/jppi.12374. doi:10.1111/jppi.12374
29. Alaska Department of Health and Social Services, Division of Public Health, Rural and Community Health Systems. Patient care strategies for scarce resource situations. Version 1. August 2021. Accessed November 11, 2021, https://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/HumanCoV/SOA_DHSS_CrisisStandardsOfCare.pdf
30. Cost-effectiveness, the QALY, and the evlyg. ICER. May 21, 2021. Accessed January 24, 2022. https://icer.org/our-approach/methods-process/cost-effectiveness-the-qaly-and-the-evlyg/
1. Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. doi:10.1056/NEJMsb2005114
2. Savulescu J, Persson I, Wilkinson D. Utilitarianism and the pandemic. Bioethics. 2020;34(6):620-632. doi:10.1111/bioe.12771
3. Mello MM, Persad G, White DB. Respecting disability rights - toward improved crisis standards of care. N Engl J Med. 2020;383(5):e26. doi: 10.1056/NEJMp2011997
4. The Commonwealth of Massachusetts Executive Office of Health and Human Services Department of Public Health. Crisis Standards of Care Planning Guidance for the COVID-19 Pandemic. April 7, 2020. https://d279m997dpfwgl.cloudfront.net/wp/2020/04/CSC_April-7_2020.pdf
5. Knowles H. Hospitals overwhelmed by covid are turning to ‘crisis standards of care.’ What does that mean? The Washington Post. September 21, 2021. Accessed January 24, 2022. https://www.washingtonpost.com/health/2021/09/22/crisis-standards-of-care/
6. Hick JL, Hanfling D, Wynia MK, Toner E. Crisis standards of care and COVID-19: What did we learn? How do we ensure equity? What should we do? NAM Perspect. 2021;2021:10.31478/202108e. doi:10.31478/202108e
7. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
8. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2021;8(4):824-836. doi:10.1007/s40615-020-00840-5
9. Kukla E. My life is more ‘disposable’ during this pandemic. The New York Times. March 19, 2020. Accessed January 24, 2022. https://www.nytimes.com/2020/03/19/opinion/coronavirus-disabled-health-care.html
10. CPR and Coalition Partners Secure Important Changes in Massachusetts’ Crisis Standards of Care. Center for Public Representation. December 1, 2020. Accessed January 24, 2022. https://www.centerforpublicrep.org/news/cpr-and-coalition-partners-secure-important-changes-in-massachusetts-crisis-standards-of-care/
11. Johnson HM. Unspeakable conversations. The New York Times. February 16, 2003. Accessed January 24, 2022. https://www.nytimes.com/2003/02/16/magazine/unspeakable-conversations.html
12. Gerhart KA, Koziol-McLain J, Lowenstein SR, Whiteneck GG. Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann Emerg Med. 1994;23(4):807-812. doi:10.1016/s0196-0644(94)70318-3
13. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. doi:10.1377/hlthaff.2020.01452
14. Smith DL. Disparities in patient-physician communication for persons with a disability from the 2006 Medical Expenditure Panel Survey (MEPS). Disabil Health J. 2009;2(4):206-215. doi:10.1016/j.dhjo.2009.06.002
15. Stillman MD, Ankam N, Mallow M, Capron M, Williams S. A survey of internal and family medicine residents: Assessment of disability-specific education and knowledge. Disabil Health J. 2021;14(2):101011. doi:10.1016/j.dhjo.2020.101011
16. Seidel E, Crowe S. The state of disability awareness in American medical schools. Am J Phys Med Rehabil. 2017;96(9):673-676. doi:10.1097/PHM.0000000000000719
17. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. doi:10.15585/mmwr.mm6732a3
18. Peacock G, Iezzoni LI, Harkin TR. Health care for Americans with disabilities--25 years after the ADA. N Engl J Med. 2015;373(10):892-893. doi:10.1056/NEJMp1508854
19. DeLisa JA, Thomas P. Physicians with disabilities and the physician workforce: a need to reassess our policies. Am J Phys Med Rehabil. 2005;84(1):5-11. doi:10.1097/01.phm.0000153323.28396.de
20. Disability and Health. Healthy People 2020. Accessed January 24, 2022. https://www.healthypeople.gov/2020/topics-objectives/topic/disability-and-health
21. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: a survey. Ann Intern Med. 2013;158(6):441-446. doi: 10.7326/0003-4819-158-6-201303190-00003
22. McCarthy EP, Ngo LH, Roetzheim RG, et al. Disparities in breast cancer treatment and survival for women with disabilities. Ann Intern Med. 2006;145(9):637-645. doi: 10.7326/0003-4819-145-9-200611070-00005
23. Javaid A, Nakata V, Michael D. Diagnostic overshadowing in learning disability: think beyond the disability. Prog Neurol Psychiatry. 2019;23:8-10.
24. Iezzoni LI, Rao SR, Agaronnik ND, El-Jawahri A. Cross-sectional analysis of the associations between four common cancers and disability. J Natl Compr Canc Netw. 2020;18(8):1031-1044. doi:10.6004/jnccn.2020.7551
25. Sanders JS, Keller S, Aravamuthan BR. Caring for individuals with intellectual and developmental disabilities in the COVID-19 crisis. Neurol Clin Pract. 2021;11(2):e174-e178. doi:10.1212/CPJ.0000000000000886
26. Landes SD, Turk MA, Formica MK, McDonald KE, Stevens JD. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York State. Disabil Health J. 2020;13(4):100969. doi:10.1016/j.dhjo.2020.100969
27. Gleason J, Ross W, Fossi A, Blonksy H, Tobias J, Stephens M. The devastating impact of Covid-19 on individuals with intellectual disabilities in the United States. NEJM Catalyst. 2021.doi.org/10.1056/CAT.21.0051
28. Nankervis K, Chan J. Applying the CRPD to people with intellectual and developmental disability with behaviors of concern during COVID-19. J Policy Pract Intellect Disabil. 2021:10.1111/jppi.12374. doi:10.1111/jppi.12374
29. Alaska Department of Health and Social Services, Division of Public Health, Rural and Community Health Systems. Patient care strategies for scarce resource situations. Version 1. August 2021. Accessed November 11, 2021, https://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/HumanCoV/SOA_DHSS_CrisisStandardsOfCare.pdf
30. Cost-effectiveness, the QALY, and the evlyg. ICER. May 21, 2021. Accessed January 24, 2022. https://icer.org/our-approach/methods-process/cost-effectiveness-the-qaly-and-the-evlyg/
Intervention in Acute Hospital Unit Reduces Delirium Incidence for Older Adults, Has No Effect on Length of Stay, Other Complications
Study Overview
Objective: To examine the effect of the intervention “Eat Walk Engage,” a program that is designed to more consistently deliver age-friendly principles of care to older individuals in acute medical and surgical wards.
Design: This cluster randomized trial to examine the effect of an intervention in acute medical and surgical wards on older adults was conducted in 8 acute medical and surgical wards in 4 public hospitals in Australia from 2016 to 2017. To be eligible to participate in this trial, wards had to have the following: a patient population with 50% of patients aged 65 years and older; perceived alignment with hospital priorities; and nurse manager agreement to participation. Randomization was stratified by hospital, resulting in 4 wards with the intervention (a general medicine ward, an orthopedic ward, a general surgery ward, and a respiratory medicine ward) and 4 control wards (2 general medicine wards, a respiratory medicine ward, and a general surgery ward). Participants were consecutive inpatients aged 65 years or older who were admitted to the ward for at least 3 consecutive days during the study time period. Exclusion criteria included terminal or critical illness, severe cognitive impairment without a surrogate decision-maker, non-English speaking, or previously enrolled in the trial. Of a total of 453 patients who were eligible from the intervention wards, 188 were excluded and 6 died, yielding 259 participants in the intervention group. There were 413 patients eligible from the control wards, with 139 excluded and 3 deaths, yielding 271 participants in the control group.
Intervention: The intervention, called “Eat Walk Engage,” was developed to target older adults at risk for hospital-associated complications of delirium, functional decline, pressure injuries, falls, and incontinence, and aimed to improve care practices, environment, and culture to support age-friendly principles. This ward-based program delivered a structured improvement intervention through a site facilitator who is a nurse or allied health professional. The site facilitator identified opportunities for improvement using structured assessments of context, patient-experience interviews, and audits of care processes, and engaged an interdisciplinary working group from the intervention wards to participate in an hour-per-month meeting to develop plans for iterative improvements. Each site developed their own intervention plan; examples of interventions include shifting priorities to enable staff to increase the proportion of patients sitting in a chair for meals; designating the patient lounge as a walking destination to increase the proportion of time patients spend mobile; and using orientation boards and small groups to engage older patients in meaningful activities.
Main outcome measures: Study outcome measures included hospital-associated complications for older people, which is a composite of hospital-associated delirium, hospital-associated disability, hospital-associated incontinence, and fall or pressure injury during hospitalization. Delirium was assessed using the 3-minute diagnostic interview for Confusion Assessment Method (3D-CAM); hospital-associated disability was defined as new disability at discharge compared to 2 weeks prior to hospitalization. The primary outcome was defined as incidence of any complications and hospital length of stay. Secondary outcomes included incidence of individual complications, hospital discharge to facility, mortality at 6 months, and readmission for any cause at 6 months.
Main results: Patient characteristics for the intervention and control groups, respectively, were: 47% women with a mean age of 75.9 years (SD, 7.3), and 53% women with a mean age of 78.0 years (SD, 8.2). For the primary outcome, 46.4% of participants in the intervention group experienced any hospital complications compared with 51.8% in the control group (odds ratio [OR], 1.07; 95% CI, 0.71-1.61). The incidence of delirium was lower in the intervention group as compared with the control group (15.9% vs 31.4%; OR, 0.53; 95% CI, 0.31-0.90), while there were no other differences in the incidence rates of other complications. There was also no difference in hospital length of stay; median length of stay in the intervention group was 6 days (interquartile range [IQR], 4-9 days) compared with 7 days in the control group (IQR, 5-10), with an estimated mean difference in length of stay of 0.16 days (95% CI, –0.43 to 0.78 days). There was also no significant difference in mortality or all-cause readmission at 6 months.
Conclusion: The intervention “Eat Walk Engage” did not reduce hospital-associated complications overall or hospital length of stay, but it did reduce the incidence of hospital-associated delirium.
Commentary
Older adults, often with reduced physiologic reserve, when admitted to the hospital with an acute illness may be vulnerable to potential hazards of hospitalization, such as complications from prolonged periods of immobility, pressure injury, and delirium.1 Models of care in the inpatient setting to reduce these hazards, including the Acute Care for the Elderly model and the Mobile Acute Care for the Elderly Team model, have been examined in clinical trials.2,3 Specifically, models of care to prevent and treat delirium have been developed and tested over the past decade.4 The effect of these models in improving function, reducing complications, and reducing delirium incidence has been well documented. The present study adds to the literature by testing a model that utilizes implementation science methods to take into account real-world settings. In contrast with prior models-of-care studies, the implementation of the intervention at each ward was not prescriptive, but rather was developed in each ward in an iterative manner with stakeholder input. The advantage of this approach is that engagement of stakeholders at each intervention ward obtains buy-in from staff, mobilizing staff in a way that a prescriptive model of care may not; this ultimately may lead to longer-lasting change. The iterative approach also allows for the intervention to be adapted to conditions and settings over time. Other studies have taken this approach of using implementation science to drive change.5 Although the intervention in the present study failed to improve the primary outcome, it did reduce the incidence of delirium, which is a significant outcome and one that may confer considerable benefits to older adults under the model’s care.
A limitation of the intervention’s nonprescriptive approach is that, because of the variation of the interventions across sites, it is difficult to discern what elements drove the clinical outcomes. In addition, it would be challenging to consider what aspects of the intervention did not work should refinement or changes be needed. How one may measure fidelity to the intervention or how well a site implements the intervention and its relationship with clinical outcomes will need to be examined further.
Application for Clinical Practice
Clinicians look to effective models of care to improve clinical outcomes for older adults in the hospital. The intervention described in this study offers a real-world approach that may need less upfront investment than other recently studied models, such as the Acute Care for the Elderly model, which requires structural and staffing enhancements. Clinicians and health system leaders may consider implementing this model to improve the care delivered to older adults in the hospital as it may help reduce the incidence of delirium among the older adults they serve.
–William W. Hung, MD, MPH
Disclosures: None.
1. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi:10.7326/0003-4819-118-3-199302010-00011
2. Fox MT, Persaud M, Maimets I, et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc. 2012;60(12):2237-2245. doi:10.1111/jgs.12028
3. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. doi:10.1001/jamainternmed.2013.478
4. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015-1033. doi:10.1016/j.jagp.2018.06.007
5. Naughton C, Cummins H, de Foubert M, et al. Implementation of the Frailty Care Bundle (FCB) to promote mobilisation, nutrition and cognitive engagement in older people in acute care settings: protocol for an implementation science study. [version 1; peer review: 1 approved]. HRB Open Res. 2022;5:3. doi:10.12688/hrbopenres.134731
Study Overview
Objective: To examine the effect of the intervention “Eat Walk Engage,” a program that is designed to more consistently deliver age-friendly principles of care to older individuals in acute medical and surgical wards.
Design: This cluster randomized trial to examine the effect of an intervention in acute medical and surgical wards on older adults was conducted in 8 acute medical and surgical wards in 4 public hospitals in Australia from 2016 to 2017. To be eligible to participate in this trial, wards had to have the following: a patient population with 50% of patients aged 65 years and older; perceived alignment with hospital priorities; and nurse manager agreement to participation. Randomization was stratified by hospital, resulting in 4 wards with the intervention (a general medicine ward, an orthopedic ward, a general surgery ward, and a respiratory medicine ward) and 4 control wards (2 general medicine wards, a respiratory medicine ward, and a general surgery ward). Participants were consecutive inpatients aged 65 years or older who were admitted to the ward for at least 3 consecutive days during the study time period. Exclusion criteria included terminal or critical illness, severe cognitive impairment without a surrogate decision-maker, non-English speaking, or previously enrolled in the trial. Of a total of 453 patients who were eligible from the intervention wards, 188 were excluded and 6 died, yielding 259 participants in the intervention group. There were 413 patients eligible from the control wards, with 139 excluded and 3 deaths, yielding 271 participants in the control group.
Intervention: The intervention, called “Eat Walk Engage,” was developed to target older adults at risk for hospital-associated complications of delirium, functional decline, pressure injuries, falls, and incontinence, and aimed to improve care practices, environment, and culture to support age-friendly principles. This ward-based program delivered a structured improvement intervention through a site facilitator who is a nurse or allied health professional. The site facilitator identified opportunities for improvement using structured assessments of context, patient-experience interviews, and audits of care processes, and engaged an interdisciplinary working group from the intervention wards to participate in an hour-per-month meeting to develop plans for iterative improvements. Each site developed their own intervention plan; examples of interventions include shifting priorities to enable staff to increase the proportion of patients sitting in a chair for meals; designating the patient lounge as a walking destination to increase the proportion of time patients spend mobile; and using orientation boards and small groups to engage older patients in meaningful activities.
Main outcome measures: Study outcome measures included hospital-associated complications for older people, which is a composite of hospital-associated delirium, hospital-associated disability, hospital-associated incontinence, and fall or pressure injury during hospitalization. Delirium was assessed using the 3-minute diagnostic interview for Confusion Assessment Method (3D-CAM); hospital-associated disability was defined as new disability at discharge compared to 2 weeks prior to hospitalization. The primary outcome was defined as incidence of any complications and hospital length of stay. Secondary outcomes included incidence of individual complications, hospital discharge to facility, mortality at 6 months, and readmission for any cause at 6 months.
Main results: Patient characteristics for the intervention and control groups, respectively, were: 47% women with a mean age of 75.9 years (SD, 7.3), and 53% women with a mean age of 78.0 years (SD, 8.2). For the primary outcome, 46.4% of participants in the intervention group experienced any hospital complications compared with 51.8% in the control group (odds ratio [OR], 1.07; 95% CI, 0.71-1.61). The incidence of delirium was lower in the intervention group as compared with the control group (15.9% vs 31.4%; OR, 0.53; 95% CI, 0.31-0.90), while there were no other differences in the incidence rates of other complications. There was also no difference in hospital length of stay; median length of stay in the intervention group was 6 days (interquartile range [IQR], 4-9 days) compared with 7 days in the control group (IQR, 5-10), with an estimated mean difference in length of stay of 0.16 days (95% CI, –0.43 to 0.78 days). There was also no significant difference in mortality or all-cause readmission at 6 months.
Conclusion: The intervention “Eat Walk Engage” did not reduce hospital-associated complications overall or hospital length of stay, but it did reduce the incidence of hospital-associated delirium.
Commentary
Older adults, often with reduced physiologic reserve, when admitted to the hospital with an acute illness may be vulnerable to potential hazards of hospitalization, such as complications from prolonged periods of immobility, pressure injury, and delirium.1 Models of care in the inpatient setting to reduce these hazards, including the Acute Care for the Elderly model and the Mobile Acute Care for the Elderly Team model, have been examined in clinical trials.2,3 Specifically, models of care to prevent and treat delirium have been developed and tested over the past decade.4 The effect of these models in improving function, reducing complications, and reducing delirium incidence has been well documented. The present study adds to the literature by testing a model that utilizes implementation science methods to take into account real-world settings. In contrast with prior models-of-care studies, the implementation of the intervention at each ward was not prescriptive, but rather was developed in each ward in an iterative manner with stakeholder input. The advantage of this approach is that engagement of stakeholders at each intervention ward obtains buy-in from staff, mobilizing staff in a way that a prescriptive model of care may not; this ultimately may lead to longer-lasting change. The iterative approach also allows for the intervention to be adapted to conditions and settings over time. Other studies have taken this approach of using implementation science to drive change.5 Although the intervention in the present study failed to improve the primary outcome, it did reduce the incidence of delirium, which is a significant outcome and one that may confer considerable benefits to older adults under the model’s care.
A limitation of the intervention’s nonprescriptive approach is that, because of the variation of the interventions across sites, it is difficult to discern what elements drove the clinical outcomes. In addition, it would be challenging to consider what aspects of the intervention did not work should refinement or changes be needed. How one may measure fidelity to the intervention or how well a site implements the intervention and its relationship with clinical outcomes will need to be examined further.
Application for Clinical Practice
Clinicians look to effective models of care to improve clinical outcomes for older adults in the hospital. The intervention described in this study offers a real-world approach that may need less upfront investment than other recently studied models, such as the Acute Care for the Elderly model, which requires structural and staffing enhancements. Clinicians and health system leaders may consider implementing this model to improve the care delivered to older adults in the hospital as it may help reduce the incidence of delirium among the older adults they serve.
–William W. Hung, MD, MPH
Disclosures: None.
Study Overview
Objective: To examine the effect of the intervention “Eat Walk Engage,” a program that is designed to more consistently deliver age-friendly principles of care to older individuals in acute medical and surgical wards.
Design: This cluster randomized trial to examine the effect of an intervention in acute medical and surgical wards on older adults was conducted in 8 acute medical and surgical wards in 4 public hospitals in Australia from 2016 to 2017. To be eligible to participate in this trial, wards had to have the following: a patient population with 50% of patients aged 65 years and older; perceived alignment with hospital priorities; and nurse manager agreement to participation. Randomization was stratified by hospital, resulting in 4 wards with the intervention (a general medicine ward, an orthopedic ward, a general surgery ward, and a respiratory medicine ward) and 4 control wards (2 general medicine wards, a respiratory medicine ward, and a general surgery ward). Participants were consecutive inpatients aged 65 years or older who were admitted to the ward for at least 3 consecutive days during the study time period. Exclusion criteria included terminal or critical illness, severe cognitive impairment without a surrogate decision-maker, non-English speaking, or previously enrolled in the trial. Of a total of 453 patients who were eligible from the intervention wards, 188 were excluded and 6 died, yielding 259 participants in the intervention group. There were 413 patients eligible from the control wards, with 139 excluded and 3 deaths, yielding 271 participants in the control group.
Intervention: The intervention, called “Eat Walk Engage,” was developed to target older adults at risk for hospital-associated complications of delirium, functional decline, pressure injuries, falls, and incontinence, and aimed to improve care practices, environment, and culture to support age-friendly principles. This ward-based program delivered a structured improvement intervention through a site facilitator who is a nurse or allied health professional. The site facilitator identified opportunities for improvement using structured assessments of context, patient-experience interviews, and audits of care processes, and engaged an interdisciplinary working group from the intervention wards to participate in an hour-per-month meeting to develop plans for iterative improvements. Each site developed their own intervention plan; examples of interventions include shifting priorities to enable staff to increase the proportion of patients sitting in a chair for meals; designating the patient lounge as a walking destination to increase the proportion of time patients spend mobile; and using orientation boards and small groups to engage older patients in meaningful activities.
Main outcome measures: Study outcome measures included hospital-associated complications for older people, which is a composite of hospital-associated delirium, hospital-associated disability, hospital-associated incontinence, and fall or pressure injury during hospitalization. Delirium was assessed using the 3-minute diagnostic interview for Confusion Assessment Method (3D-CAM); hospital-associated disability was defined as new disability at discharge compared to 2 weeks prior to hospitalization. The primary outcome was defined as incidence of any complications and hospital length of stay. Secondary outcomes included incidence of individual complications, hospital discharge to facility, mortality at 6 months, and readmission for any cause at 6 months.
Main results: Patient characteristics for the intervention and control groups, respectively, were: 47% women with a mean age of 75.9 years (SD, 7.3), and 53% women with a mean age of 78.0 years (SD, 8.2). For the primary outcome, 46.4% of participants in the intervention group experienced any hospital complications compared with 51.8% in the control group (odds ratio [OR], 1.07; 95% CI, 0.71-1.61). The incidence of delirium was lower in the intervention group as compared with the control group (15.9% vs 31.4%; OR, 0.53; 95% CI, 0.31-0.90), while there were no other differences in the incidence rates of other complications. There was also no difference in hospital length of stay; median length of stay in the intervention group was 6 days (interquartile range [IQR], 4-9 days) compared with 7 days in the control group (IQR, 5-10), with an estimated mean difference in length of stay of 0.16 days (95% CI, –0.43 to 0.78 days). There was also no significant difference in mortality or all-cause readmission at 6 months.
Conclusion: The intervention “Eat Walk Engage” did not reduce hospital-associated complications overall or hospital length of stay, but it did reduce the incidence of hospital-associated delirium.
Commentary
Older adults, often with reduced physiologic reserve, when admitted to the hospital with an acute illness may be vulnerable to potential hazards of hospitalization, such as complications from prolonged periods of immobility, pressure injury, and delirium.1 Models of care in the inpatient setting to reduce these hazards, including the Acute Care for the Elderly model and the Mobile Acute Care for the Elderly Team model, have been examined in clinical trials.2,3 Specifically, models of care to prevent and treat delirium have been developed and tested over the past decade.4 The effect of these models in improving function, reducing complications, and reducing delirium incidence has been well documented. The present study adds to the literature by testing a model that utilizes implementation science methods to take into account real-world settings. In contrast with prior models-of-care studies, the implementation of the intervention at each ward was not prescriptive, but rather was developed in each ward in an iterative manner with stakeholder input. The advantage of this approach is that engagement of stakeholders at each intervention ward obtains buy-in from staff, mobilizing staff in a way that a prescriptive model of care may not; this ultimately may lead to longer-lasting change. The iterative approach also allows for the intervention to be adapted to conditions and settings over time. Other studies have taken this approach of using implementation science to drive change.5 Although the intervention in the present study failed to improve the primary outcome, it did reduce the incidence of delirium, which is a significant outcome and one that may confer considerable benefits to older adults under the model’s care.
A limitation of the intervention’s nonprescriptive approach is that, because of the variation of the interventions across sites, it is difficult to discern what elements drove the clinical outcomes. In addition, it would be challenging to consider what aspects of the intervention did not work should refinement or changes be needed. How one may measure fidelity to the intervention or how well a site implements the intervention and its relationship with clinical outcomes will need to be examined further.
Application for Clinical Practice
Clinicians look to effective models of care to improve clinical outcomes for older adults in the hospital. The intervention described in this study offers a real-world approach that may need less upfront investment than other recently studied models, such as the Acute Care for the Elderly model, which requires structural and staffing enhancements. Clinicians and health system leaders may consider implementing this model to improve the care delivered to older adults in the hospital as it may help reduce the incidence of delirium among the older adults they serve.
–William W. Hung, MD, MPH
Disclosures: None.
1. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi:10.7326/0003-4819-118-3-199302010-00011
2. Fox MT, Persaud M, Maimets I, et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc. 2012;60(12):2237-2245. doi:10.1111/jgs.12028
3. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. doi:10.1001/jamainternmed.2013.478
4. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015-1033. doi:10.1016/j.jagp.2018.06.007
5. Naughton C, Cummins H, de Foubert M, et al. Implementation of the Frailty Care Bundle (FCB) to promote mobilisation, nutrition and cognitive engagement in older people in acute care settings: protocol for an implementation science study. [version 1; peer review: 1 approved]. HRB Open Res. 2022;5:3. doi:10.12688/hrbopenres.134731
1. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi:10.7326/0003-4819-118-3-199302010-00011
2. Fox MT, Persaud M, Maimets I, et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc. 2012;60(12):2237-2245. doi:10.1111/jgs.12028
3. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. doi:10.1001/jamainternmed.2013.478
4. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015-1033. doi:10.1016/j.jagp.2018.06.007
5. Naughton C, Cummins H, de Foubert M, et al. Implementation of the Frailty Care Bundle (FCB) to promote mobilisation, nutrition and cognitive engagement in older people in acute care settings: protocol for an implementation science study. [version 1; peer review: 1 approved]. HRB Open Res. 2022;5:3. doi:10.12688/hrbopenres.134731
Comparison of Fractional Flow Reserve–Guided PCI and Coronary Bypass Surgery in 3-Vessel Disease
Study Overview
Objective: To determine whether fractional flow reserve (FFR)–guided percutaneous coronary intervention (PCI) is noninferior to coronary-artery bypass grafting (CABG) in patients with 3-vessel coronary artery disease (CAD).
Design: Investigator-initiated, multicenter, international, randomized, controlled trial conducted at 48 sites.
Setting and participants: A total of 1500 patients with angiographically identified 3-vessel CAD not involving the left main coronary artery were randomly assigned to receive FFR-guided PCI with zotarolimus-eluting stents or CABG in a 1:1 ratio. Randomization was stratified according to trial site and diabetes status.
Main outcome measures: The primary end point was major adverse cardiac or cerebrovascular event, defined as death from any cause, myocardial infarction (MI), stroke, or repeat revascularization. The secondary end point was defined as a composite of death, MI, or stroke.
Results: At 1 year, the incidence of the composite primary end point was 10.6% for patients with FFR-guided PCI and 6.9% for patients with CABG (hazard ratio [HR], 1.5; 95% CI, 1.1-2.2; P = .35 for noninferiority), which was not consistent with noninferiority of FFR-guided PCI compared to CABG. The secondary end point occurred in 7.3% of patients in the FFR-guided PCI group compared with 5.2% in the CABG group (HR, 1.4; 95% CI, 0.9-2.1). Individual findings for the outcomes comprising the primary end point for the FFR-guided PCI group vs the CABG group were as follows: death, 1.6% vs 0.9%; MI, 5.2% vs 3.5%; stroke, 0.9% vs 1.1%; and repeat revascularization, 5.9% vs 3.9%. The CABG group had more extended hospital stays and higher incidences of major bleeding, arrhythmia, acute kidney injury, and rehospitalization within 30 days than the FFR-guided PCI group.
Conclusion: FFR-guided PCI was not found to be noninferior to CABG with respect to the incidence of a composite of death, MI, stroke, or repeat revascularization at 1 year.
Commentary
Revascularization for multivessel CAD can be performed by CABG or PCI. Previous studies have shown superior outcomes in patients with multivessel CAD who were treated with CABG compared to PCI.1-3 The Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) trial, which compared CABG to PCI in patients with multivessel disease or unprotected left main CAD, stratified the anatomic complexity based on SYNTAX score and found that patients with higher anatomic complexity with a high SYNTAX score derive larger benefit from CABG compared to PCI.4 Therefore, the current guidelines favor CABG over PCI in patients with severe 3-vessel disease, except for patients with a lower SYNTAX score (<22) without diabetes.5,6 However, except for a smaller size study,3 the previous trials that led to this recommendation used mostly first-generation drug-eluting stents and have not evaluated second-generation stents that have lower rates of in-stent restenosis and stent thrombosis. In addition, there have been significant improvements in PCI techniques since the study period, including the adoption of a radial approach and superior adjunct pharmacologic therapy. Furthermore, previous studies have not systematically investigated the use of FFR-guided PCI, which has been shown to be superior to angiography-guided PCI or medical treatment alone.7-9
In this context, Fearon and the FAME-3 trial investigators studied the use of FFR-guided PCI with second-generation zotarolimus drug-eluting stents compared to CABG in patients with 3-vessel CAD. They randomized patients with angiographically identified 3-vessel CAD in a 1:1 ratio to receive FFR-guided PCI or CABG at 48 sites internationally. Patients with left main CAD, recent ST-elevation MI, cardiogenic shock, and left-ventricular ejection fraction <30% were excluded. The study results (composite primary end point incidence of 10.6% for patients with FFR-guided PCI vs 6.9% in the CABG group [HR, 1.5; 95% CI, 1.1-2.2; P = 0.35 for noninferiority]) showed that FFR-guided PCI did not meet the noninferiority criterion.
Although the FAME-3 study is an important study, there are a few points to consider. First, 24% of the lesions had a FFR measured at >0.80. The benefit of FFR-guided PCI lies in the number of lesions that are safely deferred compared to angiography-guided PCI. The small number of deferred lesions could have limited the benefit of FFR guidance compared with angiography. Second, this study did not include all comers who had angiographic 3-vessel disease. Patients who had FFR assessment of moderate lesions at the time of diagnostic angiogram and were found to have FFR >0.80 or were deemed single- or 2-vessel disease were likely treated with PCI. Therefore, as the authors point out, the patients included in this study may have been skewed to a higher-risk population compared to previous studies.
Third, the study may not reflect contemporary interventional practice, as the use of intravascular ultrasound was very low (12%). Intravascular ultrasound–guided PCI has been associated with increased luminal gain and improved outcomes compared to angiography-guided PCI.10 Although 20% of the patients in each arm were found to have chronic total occlusions, the completeness of revascularization has not yet been reported. It is possible that the PCI arm had fewer complete revascularizations, which has been shown in previous observational studies to be associated with worse clinical outcomes.11,12
Although the current guidelines favor CABG over PCI in patients with multivessel disease, this recommendation is stratified by anatomic complexity.6 In fact, in the European guidelines, CABG and PCI are both class I recommendations for the treatment of 3-vessel disease with low SYNTAX score in patients without diabetes.5 Although the FAME-3 study failed to show noninferiority in the overall population, when stratified by the SYNTAX score, the major adverse cardiac event rate for the PCI group was numerically lower than that of the CABG group. The results from the FAME-3 study are overall in line with the previous studies and the current guidelines. Future studies are necessary to assess the outcomes of multivessel PCI compared to CABG using the most contemporary interventional practice and achieving complete revascularization in the PCI arm.
Applications for Clinical Practice
In patients with 3-vessel disease, FFR-guided PCI was not found to be noninferior to CABG; this finding is consistent with previous studies.
—Shubham Kanake, BS, Chirag Bavishi, MD, MPH, and Taishi Hirai, MD, University of Missouri, Columbia, MO
Disclosures: None.
1. Farkouh ME, Domanski M, Sleeper LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375-2384. doi:10.1056/NEJMoa1211585
2. Serruys PW, Morice MC, Kappetein AP, et al; SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961-972. doi:10.1056/NEJMoa0804626
3. Park SJ, Ahn JM, Kim YH, et al; BEST Trial Investigators. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372(13):1204-1212. doi:10.1056/NEJMoa1415447
4. Stone GW, Kappetein AP, Sabik JF, et al; EXCEL Trial Investigators. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019; 381(19):1820-1830. doi:10.1056/NEJMoa1909406
5. Neumann FJ, Sousa-Uva M, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi:10.1093/eurheartj/ehy394
6. Writing Committee Members, Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
7. Tonino PAL, De Bruyne B, Pijls NHJ, et al; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-224. doi:10.1056/NEJMoa0807611
8. De Bruyne B, Fearon WF, Pijls NHJ, et al; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208-1217. doi:10.1056/NEJMoa1408758
9. Xaplanteris P, Fournier S, Pijls NHJ, et al; FAME 2 Investigators. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379(3):250-259. doi:10.1056/NEJMoa1803538
10. Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: The ULTIMATE trial. J Am Coll Cardiol. 2018;72:3126-3137. doi:10.1016/j.jacc.2018.09.013
11. Garcia S, Sandoval Y, Roukoz H, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. 2013;62:1421-1431. doi:10.1016/j.jacc.2013.05.033
12. Farooq V, Serruys PW, Garcia-Garcia HM et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol. 2013;61:282-294. doi: 10.1016/j.jacc.2012.10.017
Study Overview
Objective: To determine whether fractional flow reserve (FFR)–guided percutaneous coronary intervention (PCI) is noninferior to coronary-artery bypass grafting (CABG) in patients with 3-vessel coronary artery disease (CAD).
Design: Investigator-initiated, multicenter, international, randomized, controlled trial conducted at 48 sites.
Setting and participants: A total of 1500 patients with angiographically identified 3-vessel CAD not involving the left main coronary artery were randomly assigned to receive FFR-guided PCI with zotarolimus-eluting stents or CABG in a 1:1 ratio. Randomization was stratified according to trial site and diabetes status.
Main outcome measures: The primary end point was major adverse cardiac or cerebrovascular event, defined as death from any cause, myocardial infarction (MI), stroke, or repeat revascularization. The secondary end point was defined as a composite of death, MI, or stroke.
Results: At 1 year, the incidence of the composite primary end point was 10.6% for patients with FFR-guided PCI and 6.9% for patients with CABG (hazard ratio [HR], 1.5; 95% CI, 1.1-2.2; P = .35 for noninferiority), which was not consistent with noninferiority of FFR-guided PCI compared to CABG. The secondary end point occurred in 7.3% of patients in the FFR-guided PCI group compared with 5.2% in the CABG group (HR, 1.4; 95% CI, 0.9-2.1). Individual findings for the outcomes comprising the primary end point for the FFR-guided PCI group vs the CABG group were as follows: death, 1.6% vs 0.9%; MI, 5.2% vs 3.5%; stroke, 0.9% vs 1.1%; and repeat revascularization, 5.9% vs 3.9%. The CABG group had more extended hospital stays and higher incidences of major bleeding, arrhythmia, acute kidney injury, and rehospitalization within 30 days than the FFR-guided PCI group.
Conclusion: FFR-guided PCI was not found to be noninferior to CABG with respect to the incidence of a composite of death, MI, stroke, or repeat revascularization at 1 year.
Commentary
Revascularization for multivessel CAD can be performed by CABG or PCI. Previous studies have shown superior outcomes in patients with multivessel CAD who were treated with CABG compared to PCI.1-3 The Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) trial, which compared CABG to PCI in patients with multivessel disease or unprotected left main CAD, stratified the anatomic complexity based on SYNTAX score and found that patients with higher anatomic complexity with a high SYNTAX score derive larger benefit from CABG compared to PCI.4 Therefore, the current guidelines favor CABG over PCI in patients with severe 3-vessel disease, except for patients with a lower SYNTAX score (<22) without diabetes.5,6 However, except for a smaller size study,3 the previous trials that led to this recommendation used mostly first-generation drug-eluting stents and have not evaluated second-generation stents that have lower rates of in-stent restenosis and stent thrombosis. In addition, there have been significant improvements in PCI techniques since the study period, including the adoption of a radial approach and superior adjunct pharmacologic therapy. Furthermore, previous studies have not systematically investigated the use of FFR-guided PCI, which has been shown to be superior to angiography-guided PCI or medical treatment alone.7-9
In this context, Fearon and the FAME-3 trial investigators studied the use of FFR-guided PCI with second-generation zotarolimus drug-eluting stents compared to CABG in patients with 3-vessel CAD. They randomized patients with angiographically identified 3-vessel CAD in a 1:1 ratio to receive FFR-guided PCI or CABG at 48 sites internationally. Patients with left main CAD, recent ST-elevation MI, cardiogenic shock, and left-ventricular ejection fraction <30% were excluded. The study results (composite primary end point incidence of 10.6% for patients with FFR-guided PCI vs 6.9% in the CABG group [HR, 1.5; 95% CI, 1.1-2.2; P = 0.35 for noninferiority]) showed that FFR-guided PCI did not meet the noninferiority criterion.
Although the FAME-3 study is an important study, there are a few points to consider. First, 24% of the lesions had a FFR measured at >0.80. The benefit of FFR-guided PCI lies in the number of lesions that are safely deferred compared to angiography-guided PCI. The small number of deferred lesions could have limited the benefit of FFR guidance compared with angiography. Second, this study did not include all comers who had angiographic 3-vessel disease. Patients who had FFR assessment of moderate lesions at the time of diagnostic angiogram and were found to have FFR >0.80 or were deemed single- or 2-vessel disease were likely treated with PCI. Therefore, as the authors point out, the patients included in this study may have been skewed to a higher-risk population compared to previous studies.
Third, the study may not reflect contemporary interventional practice, as the use of intravascular ultrasound was very low (12%). Intravascular ultrasound–guided PCI has been associated with increased luminal gain and improved outcomes compared to angiography-guided PCI.10 Although 20% of the patients in each arm were found to have chronic total occlusions, the completeness of revascularization has not yet been reported. It is possible that the PCI arm had fewer complete revascularizations, which has been shown in previous observational studies to be associated with worse clinical outcomes.11,12
Although the current guidelines favor CABG over PCI in patients with multivessel disease, this recommendation is stratified by anatomic complexity.6 In fact, in the European guidelines, CABG and PCI are both class I recommendations for the treatment of 3-vessel disease with low SYNTAX score in patients without diabetes.5 Although the FAME-3 study failed to show noninferiority in the overall population, when stratified by the SYNTAX score, the major adverse cardiac event rate for the PCI group was numerically lower than that of the CABG group. The results from the FAME-3 study are overall in line with the previous studies and the current guidelines. Future studies are necessary to assess the outcomes of multivessel PCI compared to CABG using the most contemporary interventional practice and achieving complete revascularization in the PCI arm.
Applications for Clinical Practice
In patients with 3-vessel disease, FFR-guided PCI was not found to be noninferior to CABG; this finding is consistent with previous studies.
—Shubham Kanake, BS, Chirag Bavishi, MD, MPH, and Taishi Hirai, MD, University of Missouri, Columbia, MO
Disclosures: None.
Study Overview
Objective: To determine whether fractional flow reserve (FFR)–guided percutaneous coronary intervention (PCI) is noninferior to coronary-artery bypass grafting (CABG) in patients with 3-vessel coronary artery disease (CAD).
Design: Investigator-initiated, multicenter, international, randomized, controlled trial conducted at 48 sites.
Setting and participants: A total of 1500 patients with angiographically identified 3-vessel CAD not involving the left main coronary artery were randomly assigned to receive FFR-guided PCI with zotarolimus-eluting stents or CABG in a 1:1 ratio. Randomization was stratified according to trial site and diabetes status.
Main outcome measures: The primary end point was major adverse cardiac or cerebrovascular event, defined as death from any cause, myocardial infarction (MI), stroke, or repeat revascularization. The secondary end point was defined as a composite of death, MI, or stroke.
Results: At 1 year, the incidence of the composite primary end point was 10.6% for patients with FFR-guided PCI and 6.9% for patients with CABG (hazard ratio [HR], 1.5; 95% CI, 1.1-2.2; P = .35 for noninferiority), which was not consistent with noninferiority of FFR-guided PCI compared to CABG. The secondary end point occurred in 7.3% of patients in the FFR-guided PCI group compared with 5.2% in the CABG group (HR, 1.4; 95% CI, 0.9-2.1). Individual findings for the outcomes comprising the primary end point for the FFR-guided PCI group vs the CABG group were as follows: death, 1.6% vs 0.9%; MI, 5.2% vs 3.5%; stroke, 0.9% vs 1.1%; and repeat revascularization, 5.9% vs 3.9%. The CABG group had more extended hospital stays and higher incidences of major bleeding, arrhythmia, acute kidney injury, and rehospitalization within 30 days than the FFR-guided PCI group.
Conclusion: FFR-guided PCI was not found to be noninferior to CABG with respect to the incidence of a composite of death, MI, stroke, or repeat revascularization at 1 year.
Commentary
Revascularization for multivessel CAD can be performed by CABG or PCI. Previous studies have shown superior outcomes in patients with multivessel CAD who were treated with CABG compared to PCI.1-3 The Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) trial, which compared CABG to PCI in patients with multivessel disease or unprotected left main CAD, stratified the anatomic complexity based on SYNTAX score and found that patients with higher anatomic complexity with a high SYNTAX score derive larger benefit from CABG compared to PCI.4 Therefore, the current guidelines favor CABG over PCI in patients with severe 3-vessel disease, except for patients with a lower SYNTAX score (<22) without diabetes.5,6 However, except for a smaller size study,3 the previous trials that led to this recommendation used mostly first-generation drug-eluting stents and have not evaluated second-generation stents that have lower rates of in-stent restenosis and stent thrombosis. In addition, there have been significant improvements in PCI techniques since the study period, including the adoption of a radial approach and superior adjunct pharmacologic therapy. Furthermore, previous studies have not systematically investigated the use of FFR-guided PCI, which has been shown to be superior to angiography-guided PCI or medical treatment alone.7-9
In this context, Fearon and the FAME-3 trial investigators studied the use of FFR-guided PCI with second-generation zotarolimus drug-eluting stents compared to CABG in patients with 3-vessel CAD. They randomized patients with angiographically identified 3-vessel CAD in a 1:1 ratio to receive FFR-guided PCI or CABG at 48 sites internationally. Patients with left main CAD, recent ST-elevation MI, cardiogenic shock, and left-ventricular ejection fraction <30% were excluded. The study results (composite primary end point incidence of 10.6% for patients with FFR-guided PCI vs 6.9% in the CABG group [HR, 1.5; 95% CI, 1.1-2.2; P = 0.35 for noninferiority]) showed that FFR-guided PCI did not meet the noninferiority criterion.
Although the FAME-3 study is an important study, there are a few points to consider. First, 24% of the lesions had a FFR measured at >0.80. The benefit of FFR-guided PCI lies in the number of lesions that are safely deferred compared to angiography-guided PCI. The small number of deferred lesions could have limited the benefit of FFR guidance compared with angiography. Second, this study did not include all comers who had angiographic 3-vessel disease. Patients who had FFR assessment of moderate lesions at the time of diagnostic angiogram and were found to have FFR >0.80 or were deemed single- or 2-vessel disease were likely treated with PCI. Therefore, as the authors point out, the patients included in this study may have been skewed to a higher-risk population compared to previous studies.
Third, the study may not reflect contemporary interventional practice, as the use of intravascular ultrasound was very low (12%). Intravascular ultrasound–guided PCI has been associated with increased luminal gain and improved outcomes compared to angiography-guided PCI.10 Although 20% of the patients in each arm were found to have chronic total occlusions, the completeness of revascularization has not yet been reported. It is possible that the PCI arm had fewer complete revascularizations, which has been shown in previous observational studies to be associated with worse clinical outcomes.11,12
Although the current guidelines favor CABG over PCI in patients with multivessel disease, this recommendation is stratified by anatomic complexity.6 In fact, in the European guidelines, CABG and PCI are both class I recommendations for the treatment of 3-vessel disease with low SYNTAX score in patients without diabetes.5 Although the FAME-3 study failed to show noninferiority in the overall population, when stratified by the SYNTAX score, the major adverse cardiac event rate for the PCI group was numerically lower than that of the CABG group. The results from the FAME-3 study are overall in line with the previous studies and the current guidelines. Future studies are necessary to assess the outcomes of multivessel PCI compared to CABG using the most contemporary interventional practice and achieving complete revascularization in the PCI arm.
Applications for Clinical Practice
In patients with 3-vessel disease, FFR-guided PCI was not found to be noninferior to CABG; this finding is consistent with previous studies.
—Shubham Kanake, BS, Chirag Bavishi, MD, MPH, and Taishi Hirai, MD, University of Missouri, Columbia, MO
Disclosures: None.
1. Farkouh ME, Domanski M, Sleeper LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375-2384. doi:10.1056/NEJMoa1211585
2. Serruys PW, Morice MC, Kappetein AP, et al; SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961-972. doi:10.1056/NEJMoa0804626
3. Park SJ, Ahn JM, Kim YH, et al; BEST Trial Investigators. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372(13):1204-1212. doi:10.1056/NEJMoa1415447
4. Stone GW, Kappetein AP, Sabik JF, et al; EXCEL Trial Investigators. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019; 381(19):1820-1830. doi:10.1056/NEJMoa1909406
5. Neumann FJ, Sousa-Uva M, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi:10.1093/eurheartj/ehy394
6. Writing Committee Members, Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
7. Tonino PAL, De Bruyne B, Pijls NHJ, et al; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-224. doi:10.1056/NEJMoa0807611
8. De Bruyne B, Fearon WF, Pijls NHJ, et al; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208-1217. doi:10.1056/NEJMoa1408758
9. Xaplanteris P, Fournier S, Pijls NHJ, et al; FAME 2 Investigators. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379(3):250-259. doi:10.1056/NEJMoa1803538
10. Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: The ULTIMATE trial. J Am Coll Cardiol. 2018;72:3126-3137. doi:10.1016/j.jacc.2018.09.013
11. Garcia S, Sandoval Y, Roukoz H, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. 2013;62:1421-1431. doi:10.1016/j.jacc.2013.05.033
12. Farooq V, Serruys PW, Garcia-Garcia HM et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol. 2013;61:282-294. doi: 10.1016/j.jacc.2012.10.017
1. Farkouh ME, Domanski M, Sleeper LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375-2384. doi:10.1056/NEJMoa1211585
2. Serruys PW, Morice MC, Kappetein AP, et al; SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961-972. doi:10.1056/NEJMoa0804626
3. Park SJ, Ahn JM, Kim YH, et al; BEST Trial Investigators. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372(13):1204-1212. doi:10.1056/NEJMoa1415447
4. Stone GW, Kappetein AP, Sabik JF, et al; EXCEL Trial Investigators. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019; 381(19):1820-1830. doi:10.1056/NEJMoa1909406
5. Neumann FJ, Sousa-Uva M, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi:10.1093/eurheartj/ehy394
6. Writing Committee Members, Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
7. Tonino PAL, De Bruyne B, Pijls NHJ, et al; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-224. doi:10.1056/NEJMoa0807611
8. De Bruyne B, Fearon WF, Pijls NHJ, et al; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208-1217. doi:10.1056/NEJMoa1408758
9. Xaplanteris P, Fournier S, Pijls NHJ, et al; FAME 2 Investigators. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379(3):250-259. doi:10.1056/NEJMoa1803538
10. Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: The ULTIMATE trial. J Am Coll Cardiol. 2018;72:3126-3137. doi:10.1016/j.jacc.2018.09.013
11. Garcia S, Sandoval Y, Roukoz H, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. 2013;62:1421-1431. doi:10.1016/j.jacc.2013.05.033
12. Farooq V, Serruys PW, Garcia-Garcia HM et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol. 2013;61:282-294. doi: 10.1016/j.jacc.2012.10.017
What docs don’t know about the Disabilities Act can hurt them and patients
Lisa Iezzoni, MD, a professor of medicine at Harvard Medical School and a disability researcher at Massachusetts General Hospital, both in Boston, has used a wheelchair for more than 30 years because of multiple sclerosis. When she visits her primary care doctor, she doesn’t get weighed because the scales are not wheelchair accessible.
This failure to weigh her and other patients in wheelchairs could lead to serious medical problems. Weight is used to monitor a person’s overall health and prenatal health and to determine accurate doses for medications such as some chemotherapies, said Dr. Iezzoni.
In another situation, a man who used a wheelchair said that his primary care doctor never got him out of it for a complete physical exam. The patient later developed lymphoma, which first appeared in his groin. The doctor should have accommodated his disability and used a height-adjustable exam table or a portable lift to transfer him onto the table.
When physicians don’t provide access to medical care that patients with disabilities need, they put themselves at greater risk of lawsuits, fines, and settlements.
Yet, a new study in Health Affairs suggests that a large percentage of doctors are not fully aware of what they are legally required to do.
Under federal nondiscrimination laws (Americans With Disabilities Act, American Rehabilitation Act, and ADA Amendments Act), medical practices must provide equal access to people with disabilities, accommodate their disability-related needs, and not refuse them medical services because of their disabilities, say disability experts.
Where doctors go wrong with disability laws
What doctors don’t know about providing reasonable accommodations makes them vulnerable to lawsuits, which worries more than two-thirds of the 714 outpatient doctors surveyed.
Not only are they required to provide reasonable accommodations, but they also have to pay for them, the researchers said. One-fifth of the surveyed doctors said they didn’t know that practice owners have to pay.
More than one practice has made patients pay for services needed for their disability, such as sign language interpreters – the patients later complained this violated the ADA to enforcement agencies.
Doctors also don’t know that they have to collaborate with patients to determine what reasonable accommodations they need – over two-thirds of those surveyed said they didn’t know it was a joint responsibility, the study found.
When doctors fail to accommodate patients’ disability needs, they engage in discrimination and violate the ADA, says Elizabeth Pendo, JD, a coauthor of the study and the Joseph J. Simeone Professor of Law at Saint Louis University.
The Department of Justice has investigated several patient complaints of alleged disability discrimination recently and resolved the disputes with agreements and small fines in some cases. “The goal is not to get large financial settlements but to work with practices to get the correct procedures in place to be compliant,” said Ms. Pendo.
Physicians would be wise to check out whether their practices are as accessible as they think. Even if there’s a ramp to the office building, the parking lot may not have a van-accessible space or enough handicapped parking signs, or the exam room may be too narrow for a wheelchair to navigate.
These practices violated the ADA and agreed to make changes:
- Hamden, Conn., has two buildings that patients with physical disabilities couldn’t easily enter. The physician owners agreed to change the buildings’ entrances and access routes and add features to make it easier to use examination rooms and restrooms and the check-in and check-out areas.
- Seven medical offices in Riverside, Calif., failed to communicate effectively with deaf and hard-of-hearing patients. They should have had a qualified sign language interpreter, an assistive listening device, or another appropriate aid or service available to a deaf patient and her family. Instead, the office relied on a video remote interpretation system that often failed to work. The agreement requires the clinic to provide those aids and services to patients and their companions who are deaf or hard of hearing, advertise their availability, assess each patient who is deaf or hard of hearing to determine the best aids and services for their needs, and pay $5,000 in compensation to the complainant and a $1,000 civil penalty to the United States.
- Springfield, Mass., refused to provide full joint replacements to two patients being treated with buprenorphine, a medication used to treat opioid use disorder. Rather than accommodate the patients, the surgeons referred them elsewhere because they were uncomfortable with the postoperative pain management protocol for patients prescribed buprenorphine. “The Americans With Disabilities Act protects health care access for people under medical treatment for opioid use disorder,” said Acting U.S. Attorney Nathaniel R. Mendell. “Health care providers must comply with the ADA, even when doing so is inconvenient or makes them uncomfortable.” The agreement requires the practice to adopt a nondiscrimination policy, provide training on the ADA and opioid use disorder, and pay two complainants $15,000 each for pain and suffering.
The DOJ has filed civil lawsuits against medical practices when they failed to resolve the allegations. Recent cases include an ophthalmology practice with 24 facilities in Arizona that refused to help transfer patients in wheelchairs to surgery tables for eye surgery and required them to pay for transfer support services and two obstetricians-gynecologists in Bakersfield, Calif., who refused to provide routine medical care to a patient because of her HIV status.
What doctors should know
Many people tend to think of a person with a disability as being in a wheelchair. But the ADA has a very broad definition of disability, which includes any physical or mental impairment that substantially limits any major life activity, said Ms. Pendo.
“It was amended in 2008 to clarify that the definition includes people with chronic diseases such as diabetes and cancer, cognitive and neurological disorders, substance abuse disorders, vision and hearing loss, and learning and other disabilities,” she said.
That means that doctors have to accommodate many types of disabilities, which can be challenging. The ADA only specifies that fixed structures need to be accessible, such as parking lots, driveways, and buildings, said Dr. Iezzoni.
When it comes to “reasonable accommodations,” doctors should decide that on a case-by-case basis, she said.
“We can say based on our study that 71% of doctors don’t know the right way to think about the accommodations – they don’t know they need to talk to patients so they can explain to them exactly what they need to accommodate their disability,” said Dr. Iezzoni.
Doctors are also required to provide effective communication for patients with sensory or cognitive disabilities, which can depend on the severity, said Ms. Pendo. Is the person deaf or hard of hearing, blind or partially sighted – is the dementia mild or severe?
“The requirement is there, but what that looks like will vary by patient. That’s what’s challenging,” said Ms. Pendo.
Dr. Iezzoni recommends that doctor’s offices ask patients whether they need special help or individual assistance when they make appointments and enter their responses in their records. She also suggests that patients be asked at follow-up appointments whether they still need the same help or not.
“Disabilities can change over time – a person with bad arthritis may need help getting onto an exam table, but later get a knee or hip replacement that is effective and no longer need that help,” said Dr. Iezzoni.
Benefits outweigh costs
Physicians have made progress in meeting the ADA’s physical accessibility requirements, said Dr. Iezzoni. “The literature suggests that doctors have done a good job at fixing the structural barriers people with mobility issues face, such as ramps and bathrooms.”
However, there are exceptions in rural older buildings which can be harder to retrofit for wheelchair accessibility, she said. “I recall interviewing a rural doctor several years ago who said that he knew his patients well and when a patient visits with mobility problems, he goes down and carries the patient up the steps to his office. My response was that is not respectful of the patient or safe for the patient or you. That doctor has since changed the location of his practice,” said Dr. Iezzoni.
Some doctors may resist paying for accessible medical equipment because of cost, but she said the benefits are worth it. These include preventing staff injuries when they transfer patients and being used by patients with temporary disabilities and aging people with bad knees, backs, hearing and sight. In addition, businesses may be eligible for federal and state tax credits.
Dr. Iezzoni recently visited her doctor where they finally got height-adjustable exam tables. “I asked the assistant, who really likes these tables? She said it’s the elderly ladies of short stature – the table is lowered and they sit down and get on it.”
But, Dr. Iezonni’s main message to doctors is that patients with disabilities deserve equal quality of care. “Just because we have a disability doesn’t mean we should get worse care than other people. It’s a matter of professionalism that doctors should want to give the same quality care to all their patients.”
A version of this article first appeared on Medscape.com.
Lisa Iezzoni, MD, a professor of medicine at Harvard Medical School and a disability researcher at Massachusetts General Hospital, both in Boston, has used a wheelchair for more than 30 years because of multiple sclerosis. When she visits her primary care doctor, she doesn’t get weighed because the scales are not wheelchair accessible.
This failure to weigh her and other patients in wheelchairs could lead to serious medical problems. Weight is used to monitor a person’s overall health and prenatal health and to determine accurate doses for medications such as some chemotherapies, said Dr. Iezzoni.
In another situation, a man who used a wheelchair said that his primary care doctor never got him out of it for a complete physical exam. The patient later developed lymphoma, which first appeared in his groin. The doctor should have accommodated his disability and used a height-adjustable exam table or a portable lift to transfer him onto the table.
When physicians don’t provide access to medical care that patients with disabilities need, they put themselves at greater risk of lawsuits, fines, and settlements.
Yet, a new study in Health Affairs suggests that a large percentage of doctors are not fully aware of what they are legally required to do.
Under federal nondiscrimination laws (Americans With Disabilities Act, American Rehabilitation Act, and ADA Amendments Act), medical practices must provide equal access to people with disabilities, accommodate their disability-related needs, and not refuse them medical services because of their disabilities, say disability experts.
Where doctors go wrong with disability laws
What doctors don’t know about providing reasonable accommodations makes them vulnerable to lawsuits, which worries more than two-thirds of the 714 outpatient doctors surveyed.
Not only are they required to provide reasonable accommodations, but they also have to pay for them, the researchers said. One-fifth of the surveyed doctors said they didn’t know that practice owners have to pay.
More than one practice has made patients pay for services needed for their disability, such as sign language interpreters – the patients later complained this violated the ADA to enforcement agencies.
Doctors also don’t know that they have to collaborate with patients to determine what reasonable accommodations they need – over two-thirds of those surveyed said they didn’t know it was a joint responsibility, the study found.
When doctors fail to accommodate patients’ disability needs, they engage in discrimination and violate the ADA, says Elizabeth Pendo, JD, a coauthor of the study and the Joseph J. Simeone Professor of Law at Saint Louis University.
The Department of Justice has investigated several patient complaints of alleged disability discrimination recently and resolved the disputes with agreements and small fines in some cases. “The goal is not to get large financial settlements but to work with practices to get the correct procedures in place to be compliant,” said Ms. Pendo.
Physicians would be wise to check out whether their practices are as accessible as they think. Even if there’s a ramp to the office building, the parking lot may not have a van-accessible space or enough handicapped parking signs, or the exam room may be too narrow for a wheelchair to navigate.
These practices violated the ADA and agreed to make changes:
- Hamden, Conn., has two buildings that patients with physical disabilities couldn’t easily enter. The physician owners agreed to change the buildings’ entrances and access routes and add features to make it easier to use examination rooms and restrooms and the check-in and check-out areas.
- Seven medical offices in Riverside, Calif., failed to communicate effectively with deaf and hard-of-hearing patients. They should have had a qualified sign language interpreter, an assistive listening device, or another appropriate aid or service available to a deaf patient and her family. Instead, the office relied on a video remote interpretation system that often failed to work. The agreement requires the clinic to provide those aids and services to patients and their companions who are deaf or hard of hearing, advertise their availability, assess each patient who is deaf or hard of hearing to determine the best aids and services for their needs, and pay $5,000 in compensation to the complainant and a $1,000 civil penalty to the United States.
- Springfield, Mass., refused to provide full joint replacements to two patients being treated with buprenorphine, a medication used to treat opioid use disorder. Rather than accommodate the patients, the surgeons referred them elsewhere because they were uncomfortable with the postoperative pain management protocol for patients prescribed buprenorphine. “The Americans With Disabilities Act protects health care access for people under medical treatment for opioid use disorder,” said Acting U.S. Attorney Nathaniel R. Mendell. “Health care providers must comply with the ADA, even when doing so is inconvenient or makes them uncomfortable.” The agreement requires the practice to adopt a nondiscrimination policy, provide training on the ADA and opioid use disorder, and pay two complainants $15,000 each for pain and suffering.
The DOJ has filed civil lawsuits against medical practices when they failed to resolve the allegations. Recent cases include an ophthalmology practice with 24 facilities in Arizona that refused to help transfer patients in wheelchairs to surgery tables for eye surgery and required them to pay for transfer support services and two obstetricians-gynecologists in Bakersfield, Calif., who refused to provide routine medical care to a patient because of her HIV status.
What doctors should know
Many people tend to think of a person with a disability as being in a wheelchair. But the ADA has a very broad definition of disability, which includes any physical or mental impairment that substantially limits any major life activity, said Ms. Pendo.
“It was amended in 2008 to clarify that the definition includes people with chronic diseases such as diabetes and cancer, cognitive and neurological disorders, substance abuse disorders, vision and hearing loss, and learning and other disabilities,” she said.
That means that doctors have to accommodate many types of disabilities, which can be challenging. The ADA only specifies that fixed structures need to be accessible, such as parking lots, driveways, and buildings, said Dr. Iezzoni.
When it comes to “reasonable accommodations,” doctors should decide that on a case-by-case basis, she said.
“We can say based on our study that 71% of doctors don’t know the right way to think about the accommodations – they don’t know they need to talk to patients so they can explain to them exactly what they need to accommodate their disability,” said Dr. Iezzoni.
Doctors are also required to provide effective communication for patients with sensory or cognitive disabilities, which can depend on the severity, said Ms. Pendo. Is the person deaf or hard of hearing, blind or partially sighted – is the dementia mild or severe?
“The requirement is there, but what that looks like will vary by patient. That’s what’s challenging,” said Ms. Pendo.
Dr. Iezzoni recommends that doctor’s offices ask patients whether they need special help or individual assistance when they make appointments and enter their responses in their records. She also suggests that patients be asked at follow-up appointments whether they still need the same help or not.
“Disabilities can change over time – a person with bad arthritis may need help getting onto an exam table, but later get a knee or hip replacement that is effective and no longer need that help,” said Dr. Iezzoni.
Benefits outweigh costs
Physicians have made progress in meeting the ADA’s physical accessibility requirements, said Dr. Iezzoni. “The literature suggests that doctors have done a good job at fixing the structural barriers people with mobility issues face, such as ramps and bathrooms.”
However, there are exceptions in rural older buildings which can be harder to retrofit for wheelchair accessibility, she said. “I recall interviewing a rural doctor several years ago who said that he knew his patients well and when a patient visits with mobility problems, he goes down and carries the patient up the steps to his office. My response was that is not respectful of the patient or safe for the patient or you. That doctor has since changed the location of his practice,” said Dr. Iezzoni.
Some doctors may resist paying for accessible medical equipment because of cost, but she said the benefits are worth it. These include preventing staff injuries when they transfer patients and being used by patients with temporary disabilities and aging people with bad knees, backs, hearing and sight. In addition, businesses may be eligible for federal and state tax credits.
Dr. Iezzoni recently visited her doctor where they finally got height-adjustable exam tables. “I asked the assistant, who really likes these tables? She said it’s the elderly ladies of short stature – the table is lowered and they sit down and get on it.”
But, Dr. Iezonni’s main message to doctors is that patients with disabilities deserve equal quality of care. “Just because we have a disability doesn’t mean we should get worse care than other people. It’s a matter of professionalism that doctors should want to give the same quality care to all their patients.”
A version of this article first appeared on Medscape.com.
Lisa Iezzoni, MD, a professor of medicine at Harvard Medical School and a disability researcher at Massachusetts General Hospital, both in Boston, has used a wheelchair for more than 30 years because of multiple sclerosis. When she visits her primary care doctor, she doesn’t get weighed because the scales are not wheelchair accessible.
This failure to weigh her and other patients in wheelchairs could lead to serious medical problems. Weight is used to monitor a person’s overall health and prenatal health and to determine accurate doses for medications such as some chemotherapies, said Dr. Iezzoni.
In another situation, a man who used a wheelchair said that his primary care doctor never got him out of it for a complete physical exam. The patient later developed lymphoma, which first appeared in his groin. The doctor should have accommodated his disability and used a height-adjustable exam table or a portable lift to transfer him onto the table.
When physicians don’t provide access to medical care that patients with disabilities need, they put themselves at greater risk of lawsuits, fines, and settlements.
Yet, a new study in Health Affairs suggests that a large percentage of doctors are not fully aware of what they are legally required to do.
Under federal nondiscrimination laws (Americans With Disabilities Act, American Rehabilitation Act, and ADA Amendments Act), medical practices must provide equal access to people with disabilities, accommodate their disability-related needs, and not refuse them medical services because of their disabilities, say disability experts.
Where doctors go wrong with disability laws
What doctors don’t know about providing reasonable accommodations makes them vulnerable to lawsuits, which worries more than two-thirds of the 714 outpatient doctors surveyed.
Not only are they required to provide reasonable accommodations, but they also have to pay for them, the researchers said. One-fifth of the surveyed doctors said they didn’t know that practice owners have to pay.
More than one practice has made patients pay for services needed for their disability, such as sign language interpreters – the patients later complained this violated the ADA to enforcement agencies.
Doctors also don’t know that they have to collaborate with patients to determine what reasonable accommodations they need – over two-thirds of those surveyed said they didn’t know it was a joint responsibility, the study found.
When doctors fail to accommodate patients’ disability needs, they engage in discrimination and violate the ADA, says Elizabeth Pendo, JD, a coauthor of the study and the Joseph J. Simeone Professor of Law at Saint Louis University.
The Department of Justice has investigated several patient complaints of alleged disability discrimination recently and resolved the disputes with agreements and small fines in some cases. “The goal is not to get large financial settlements but to work with practices to get the correct procedures in place to be compliant,” said Ms. Pendo.
Physicians would be wise to check out whether their practices are as accessible as they think. Even if there’s a ramp to the office building, the parking lot may not have a van-accessible space or enough handicapped parking signs, or the exam room may be too narrow for a wheelchair to navigate.
These practices violated the ADA and agreed to make changes:
- Hamden, Conn., has two buildings that patients with physical disabilities couldn’t easily enter. The physician owners agreed to change the buildings’ entrances and access routes and add features to make it easier to use examination rooms and restrooms and the check-in and check-out areas.
- Seven medical offices in Riverside, Calif., failed to communicate effectively with deaf and hard-of-hearing patients. They should have had a qualified sign language interpreter, an assistive listening device, or another appropriate aid or service available to a deaf patient and her family. Instead, the office relied on a video remote interpretation system that often failed to work. The agreement requires the clinic to provide those aids and services to patients and their companions who are deaf or hard of hearing, advertise their availability, assess each patient who is deaf or hard of hearing to determine the best aids and services for their needs, and pay $5,000 in compensation to the complainant and a $1,000 civil penalty to the United States.
- Springfield, Mass., refused to provide full joint replacements to two patients being treated with buprenorphine, a medication used to treat opioid use disorder. Rather than accommodate the patients, the surgeons referred them elsewhere because they were uncomfortable with the postoperative pain management protocol for patients prescribed buprenorphine. “The Americans With Disabilities Act protects health care access for people under medical treatment for opioid use disorder,” said Acting U.S. Attorney Nathaniel R. Mendell. “Health care providers must comply with the ADA, even when doing so is inconvenient or makes them uncomfortable.” The agreement requires the practice to adopt a nondiscrimination policy, provide training on the ADA and opioid use disorder, and pay two complainants $15,000 each for pain and suffering.
The DOJ has filed civil lawsuits against medical practices when they failed to resolve the allegations. Recent cases include an ophthalmology practice with 24 facilities in Arizona that refused to help transfer patients in wheelchairs to surgery tables for eye surgery and required them to pay for transfer support services and two obstetricians-gynecologists in Bakersfield, Calif., who refused to provide routine medical care to a patient because of her HIV status.
What doctors should know
Many people tend to think of a person with a disability as being in a wheelchair. But the ADA has a very broad definition of disability, which includes any physical or mental impairment that substantially limits any major life activity, said Ms. Pendo.
“It was amended in 2008 to clarify that the definition includes people with chronic diseases such as diabetes and cancer, cognitive and neurological disorders, substance abuse disorders, vision and hearing loss, and learning and other disabilities,” she said.
That means that doctors have to accommodate many types of disabilities, which can be challenging. The ADA only specifies that fixed structures need to be accessible, such as parking lots, driveways, and buildings, said Dr. Iezzoni.
When it comes to “reasonable accommodations,” doctors should decide that on a case-by-case basis, she said.
“We can say based on our study that 71% of doctors don’t know the right way to think about the accommodations – they don’t know they need to talk to patients so they can explain to them exactly what they need to accommodate their disability,” said Dr. Iezzoni.
Doctors are also required to provide effective communication for patients with sensory or cognitive disabilities, which can depend on the severity, said Ms. Pendo. Is the person deaf or hard of hearing, blind or partially sighted – is the dementia mild or severe?
“The requirement is there, but what that looks like will vary by patient. That’s what’s challenging,” said Ms. Pendo.
Dr. Iezzoni recommends that doctor’s offices ask patients whether they need special help or individual assistance when they make appointments and enter their responses in their records. She also suggests that patients be asked at follow-up appointments whether they still need the same help or not.
“Disabilities can change over time – a person with bad arthritis may need help getting onto an exam table, but later get a knee or hip replacement that is effective and no longer need that help,” said Dr. Iezzoni.
Benefits outweigh costs
Physicians have made progress in meeting the ADA’s physical accessibility requirements, said Dr. Iezzoni. “The literature suggests that doctors have done a good job at fixing the structural barriers people with mobility issues face, such as ramps and bathrooms.”
However, there are exceptions in rural older buildings which can be harder to retrofit for wheelchair accessibility, she said. “I recall interviewing a rural doctor several years ago who said that he knew his patients well and when a patient visits with mobility problems, he goes down and carries the patient up the steps to his office. My response was that is not respectful of the patient or safe for the patient or you. That doctor has since changed the location of his practice,” said Dr. Iezzoni.
Some doctors may resist paying for accessible medical equipment because of cost, but she said the benefits are worth it. These include preventing staff injuries when they transfer patients and being used by patients with temporary disabilities and aging people with bad knees, backs, hearing and sight. In addition, businesses may be eligible for federal and state tax credits.
Dr. Iezzoni recently visited her doctor where they finally got height-adjustable exam tables. “I asked the assistant, who really likes these tables? She said it’s the elderly ladies of short stature – the table is lowered and they sit down and get on it.”
But, Dr. Iezonni’s main message to doctors is that patients with disabilities deserve equal quality of care. “Just because we have a disability doesn’t mean we should get worse care than other people. It’s a matter of professionalism that doctors should want to give the same quality care to all their patients.”
A version of this article first appeared on Medscape.com.
Ways to make sure 2022 doesn’t stink for docs
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.

