User login
NY radiation oncologist loses license, poses ‘potential danger’
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
When physicians are the plaintiffs
Have you experienced malpractice?
No, I’m not asking whether you have experienced litigation. I’m asking whether you, as a physician, have actually experienced substandard care from a colleague. I have heard many such experiences over the years, and mistreatment doesn’t seem to be getting any less frequent.
The first is that, unlike the Pope, who has a dedicated confessor trained to minister to his spiritual needs, no one formally trains physicians to treat physicians. As a result, most of us feel slightly uneasy at treating other physicians. We naturally wish to keep our colleagues well, but at the same time realize that our clinical skills are being very closely scrutinized. What if they are found to be wanting? This discomfiture can make a physician treating a physician overly compulsive, or worse, overtly dismissive.
Second, we physicians are famously poor patients. We pretend we don’t need the advice we give others, to monitor our health and promptly seek care when something feels amiss. And, for the period during which we delay a medical encounter, we often attempt to diagnose and treat ourselves.
Sometimes we are successful, which reinforces this approach. Other times, we fail at being our own caregiver and present to someone else either too late, or with avoidable complications. In the former instance, we congratulate ourselves and learn nothing from the experience. In the latter, we may heap shame upon ourselves for our folly, and we may learn; but it could be a lethal lesson. In the worst scenario, our colleague gives in to frustration (or angst), and heaps even more shame onto their late-presenting physician patient.
Third, when we do submit to being a patient, we often demand VIP treatment. This is probably in response to our anxiety that some of the worst things we have seen happen to patients might happen to us if we are not vigilant to ensure we receive a higher level of care. But of course, such hypervigilance can lead to excessive care and testing, with all the attendant hazards, or alternatively to dilution of care if our caregivers decide we are just too much trouble.
Fourth, as a fifth-generation physician myself, I am convinced that physicians and physician family members are either prone to unusual manifestations of common diseases or unusual diseases, or that rare disease entities and complications are actually more common than literature suggests, and they simply aren’t pursued or diagnosed in nonphysician families.
No matter how we may have arrived in a position to need medical care, how often is such care substandard? And how do we respond when we suspect, or know, this to be the case? Are physicians more, or less, likely to take legal action in the face of it?
I certainly don’t know any statistics. Physicians are in an excellent position to take such action, because judges and juries will likely believe that a doctor can recognize negligence when we fall victim to it. But we may also be reluctant to publicly admit the way (or ways) in which we may have contributed to substandard care or outcome.
Based on decades of working with physician clients who have been sued, and having been sued myself (thus witnessing and also experiencing the effects of litigation), I am probably more reluctant than normal patients or physicians to consider taking legal action. This, despite the fact that I am also a lawyer and (through organized medicine) know many colleagues in all specialties who might serve as expert witnesses.
I have experienced serial substandard care, which has left me highly conflicted about the efficacy of my chosen profession. As a resident, I had my first odd pain condition and consulted an “elder statesperson” from my institution, whom I assumed to be a “doctor’s doctor” because he was a superb teacher (wrong!)
He completely missed the diagnosis and further belittled (indeed, libeled) me in the medical record. (Some years later, I learned that, during that period, he was increasingly demented and tended to view all female patients as having “wandering uterus” equivalents.) Fortunately, I found a better diagnostician, or at least one more willing to lend credence to my complaints, who successfully removed the first of several “zebra” lesions I have experienced.
As a young faculty member, I had an odd presentation of a recurring gynecologic condition, which was treated surgically, successfully, except that my fertility was cut in half – a possibility about which I had not been informed when giving operative consent. Would I have sued this fellow faculty member for that? Never, because she invariably treated me with respect as a colleague.
Later in my career after leaving academia, the same condition recurred in a new location. My old-school gynecologist desired to do an extensive procedure, to which I demurred unless specific pathology was found intraoperatively. Affronted, he subjected me to laparoscopy, did nothing but look, and then left the hospital leaving me and the PACU nurse to try to decipher his instructions (which said, basically, “I didn’t find anything; don’t bother me again.”). Several years of pain later, a younger gynecologist performed the correct procedure to address my problem, which has never recurred. Would I have sued him? No, because I believe he had a disability.
At age 59, I developed a new mole. My beloved general practitioner, in the waning years of his practice, forgot to consult a colleague to remove it for several months. When I forced the issue, the mole was removed and turned out to be a rare pediatric condition considered a precursor to melanoma. The same general practitioner had told me I needn’t worry about my “mild hypercalcemia.”
Ten years later I diagnosed my own parathyroid adenoma, in the interim losing 10% of my bone density. Would I have sued him? No, for he always showed he cared. (Though maybe, if I had fractured my spine or hip.)
If you have been the victim of physician malpractice, how did you respond?
Do we serve our profession well by how we handle substandard care – upon ourselves (or our loved ones)?
Dr. Andrew is a former assistant professor in the department of emergency medicine, Johns Hopkins University, Baltimore, and founder and principal of MDMentor, Victoria, B.C.
A version of this article first appeared on Medscape.com.
Have you experienced malpractice?
No, I’m not asking whether you have experienced litigation. I’m asking whether you, as a physician, have actually experienced substandard care from a colleague. I have heard many such experiences over the years, and mistreatment doesn’t seem to be getting any less frequent.
The first is that, unlike the Pope, who has a dedicated confessor trained to minister to his spiritual needs, no one formally trains physicians to treat physicians. As a result, most of us feel slightly uneasy at treating other physicians. We naturally wish to keep our colleagues well, but at the same time realize that our clinical skills are being very closely scrutinized. What if they are found to be wanting? This discomfiture can make a physician treating a physician overly compulsive, or worse, overtly dismissive.
Second, we physicians are famously poor patients. We pretend we don’t need the advice we give others, to monitor our health and promptly seek care when something feels amiss. And, for the period during which we delay a medical encounter, we often attempt to diagnose and treat ourselves.
Sometimes we are successful, which reinforces this approach. Other times, we fail at being our own caregiver and present to someone else either too late, or with avoidable complications. In the former instance, we congratulate ourselves and learn nothing from the experience. In the latter, we may heap shame upon ourselves for our folly, and we may learn; but it could be a lethal lesson. In the worst scenario, our colleague gives in to frustration (or angst), and heaps even more shame onto their late-presenting physician patient.
Third, when we do submit to being a patient, we often demand VIP treatment. This is probably in response to our anxiety that some of the worst things we have seen happen to patients might happen to us if we are not vigilant to ensure we receive a higher level of care. But of course, such hypervigilance can lead to excessive care and testing, with all the attendant hazards, or alternatively to dilution of care if our caregivers decide we are just too much trouble.
Fourth, as a fifth-generation physician myself, I am convinced that physicians and physician family members are either prone to unusual manifestations of common diseases or unusual diseases, or that rare disease entities and complications are actually more common than literature suggests, and they simply aren’t pursued or diagnosed in nonphysician families.
No matter how we may have arrived in a position to need medical care, how often is such care substandard? And how do we respond when we suspect, or know, this to be the case? Are physicians more, or less, likely to take legal action in the face of it?
I certainly don’t know any statistics. Physicians are in an excellent position to take such action, because judges and juries will likely believe that a doctor can recognize negligence when we fall victim to it. But we may also be reluctant to publicly admit the way (or ways) in which we may have contributed to substandard care or outcome.
Based on decades of working with physician clients who have been sued, and having been sued myself (thus witnessing and also experiencing the effects of litigation), I am probably more reluctant than normal patients or physicians to consider taking legal action. This, despite the fact that I am also a lawyer and (through organized medicine) know many colleagues in all specialties who might serve as expert witnesses.
I have experienced serial substandard care, which has left me highly conflicted about the efficacy of my chosen profession. As a resident, I had my first odd pain condition and consulted an “elder statesperson” from my institution, whom I assumed to be a “doctor’s doctor” because he was a superb teacher (wrong!)
He completely missed the diagnosis and further belittled (indeed, libeled) me in the medical record. (Some years later, I learned that, during that period, he was increasingly demented and tended to view all female patients as having “wandering uterus” equivalents.) Fortunately, I found a better diagnostician, or at least one more willing to lend credence to my complaints, who successfully removed the first of several “zebra” lesions I have experienced.
As a young faculty member, I had an odd presentation of a recurring gynecologic condition, which was treated surgically, successfully, except that my fertility was cut in half – a possibility about which I had not been informed when giving operative consent. Would I have sued this fellow faculty member for that? Never, because she invariably treated me with respect as a colleague.
Later in my career after leaving academia, the same condition recurred in a new location. My old-school gynecologist desired to do an extensive procedure, to which I demurred unless specific pathology was found intraoperatively. Affronted, he subjected me to laparoscopy, did nothing but look, and then left the hospital leaving me and the PACU nurse to try to decipher his instructions (which said, basically, “I didn’t find anything; don’t bother me again.”). Several years of pain later, a younger gynecologist performed the correct procedure to address my problem, which has never recurred. Would I have sued him? No, because I believe he had a disability.
At age 59, I developed a new mole. My beloved general practitioner, in the waning years of his practice, forgot to consult a colleague to remove it for several months. When I forced the issue, the mole was removed and turned out to be a rare pediatric condition considered a precursor to melanoma. The same general practitioner had told me I needn’t worry about my “mild hypercalcemia.”
Ten years later I diagnosed my own parathyroid adenoma, in the interim losing 10% of my bone density. Would I have sued him? No, for he always showed he cared. (Though maybe, if I had fractured my spine or hip.)
If you have been the victim of physician malpractice, how did you respond?
Do we serve our profession well by how we handle substandard care – upon ourselves (or our loved ones)?
Dr. Andrew is a former assistant professor in the department of emergency medicine, Johns Hopkins University, Baltimore, and founder and principal of MDMentor, Victoria, B.C.
A version of this article first appeared on Medscape.com.
Have you experienced malpractice?
No, I’m not asking whether you have experienced litigation. I’m asking whether you, as a physician, have actually experienced substandard care from a colleague. I have heard many such experiences over the years, and mistreatment doesn’t seem to be getting any less frequent.
The first is that, unlike the Pope, who has a dedicated confessor trained to minister to his spiritual needs, no one formally trains physicians to treat physicians. As a result, most of us feel slightly uneasy at treating other physicians. We naturally wish to keep our colleagues well, but at the same time realize that our clinical skills are being very closely scrutinized. What if they are found to be wanting? This discomfiture can make a physician treating a physician overly compulsive, or worse, overtly dismissive.
Second, we physicians are famously poor patients. We pretend we don’t need the advice we give others, to monitor our health and promptly seek care when something feels amiss. And, for the period during which we delay a medical encounter, we often attempt to diagnose and treat ourselves.
Sometimes we are successful, which reinforces this approach. Other times, we fail at being our own caregiver and present to someone else either too late, or with avoidable complications. In the former instance, we congratulate ourselves and learn nothing from the experience. In the latter, we may heap shame upon ourselves for our folly, and we may learn; but it could be a lethal lesson. In the worst scenario, our colleague gives in to frustration (or angst), and heaps even more shame onto their late-presenting physician patient.
Third, when we do submit to being a patient, we often demand VIP treatment. This is probably in response to our anxiety that some of the worst things we have seen happen to patients might happen to us if we are not vigilant to ensure we receive a higher level of care. But of course, such hypervigilance can lead to excessive care and testing, with all the attendant hazards, or alternatively to dilution of care if our caregivers decide we are just too much trouble.
Fourth, as a fifth-generation physician myself, I am convinced that physicians and physician family members are either prone to unusual manifestations of common diseases or unusual diseases, or that rare disease entities and complications are actually more common than literature suggests, and they simply aren’t pursued or diagnosed in nonphysician families.
No matter how we may have arrived in a position to need medical care, how often is such care substandard? And how do we respond when we suspect, or know, this to be the case? Are physicians more, or less, likely to take legal action in the face of it?
I certainly don’t know any statistics. Physicians are in an excellent position to take such action, because judges and juries will likely believe that a doctor can recognize negligence when we fall victim to it. But we may also be reluctant to publicly admit the way (or ways) in which we may have contributed to substandard care or outcome.
Based on decades of working with physician clients who have been sued, and having been sued myself (thus witnessing and also experiencing the effects of litigation), I am probably more reluctant than normal patients or physicians to consider taking legal action. This, despite the fact that I am also a lawyer and (through organized medicine) know many colleagues in all specialties who might serve as expert witnesses.
I have experienced serial substandard care, which has left me highly conflicted about the efficacy of my chosen profession. As a resident, I had my first odd pain condition and consulted an “elder statesperson” from my institution, whom I assumed to be a “doctor’s doctor” because he was a superb teacher (wrong!)
He completely missed the diagnosis and further belittled (indeed, libeled) me in the medical record. (Some years later, I learned that, during that period, he was increasingly demented and tended to view all female patients as having “wandering uterus” equivalents.) Fortunately, I found a better diagnostician, or at least one more willing to lend credence to my complaints, who successfully removed the first of several “zebra” lesions I have experienced.
As a young faculty member, I had an odd presentation of a recurring gynecologic condition, which was treated surgically, successfully, except that my fertility was cut in half – a possibility about which I had not been informed when giving operative consent. Would I have sued this fellow faculty member for that? Never, because she invariably treated me with respect as a colleague.
Later in my career after leaving academia, the same condition recurred in a new location. My old-school gynecologist desired to do an extensive procedure, to which I demurred unless specific pathology was found intraoperatively. Affronted, he subjected me to laparoscopy, did nothing but look, and then left the hospital leaving me and the PACU nurse to try to decipher his instructions (which said, basically, “I didn’t find anything; don’t bother me again.”). Several years of pain later, a younger gynecologist performed the correct procedure to address my problem, which has never recurred. Would I have sued him? No, because I believe he had a disability.
At age 59, I developed a new mole. My beloved general practitioner, in the waning years of his practice, forgot to consult a colleague to remove it for several months. When I forced the issue, the mole was removed and turned out to be a rare pediatric condition considered a precursor to melanoma. The same general practitioner had told me I needn’t worry about my “mild hypercalcemia.”
Ten years later I diagnosed my own parathyroid adenoma, in the interim losing 10% of my bone density. Would I have sued him? No, for he always showed he cared. (Though maybe, if I had fractured my spine or hip.)
If you have been the victim of physician malpractice, how did you respond?
Do we serve our profession well by how we handle substandard care – upon ourselves (or our loved ones)?
Dr. Andrew is a former assistant professor in the department of emergency medicine, Johns Hopkins University, Baltimore, and founder and principal of MDMentor, Victoria, B.C.
A version of this article first appeared on Medscape.com.
Twenty-three percent of health care workers likely to leave industry soon: Poll
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.
CMS updates lung screening criteria, more aligned with USPSTF
for Medicare recipients.
According to the final decision, announced February 10, CMS will lower the age for screening from 55 to 50 years up to 77 years and reduce criteria for tobacco smoking history from at least 30 pack-years to 20 pack-years. The expanded Medicare recommendation will address racial disparities associated with lung cancer, given evidence that one third of Black patients are diagnosed with lung cancer before age 55.
The updated CMS guidelines align closely with recommendations made by the U.S. Preventive Services Task Force (USPSTF) in March 2021. The USPSTF expanded its guidelines for screening to include individuals ages 50 to 80 years, as well as those who have a 20–pack-year smoking history and who currently smoke or have quit within the past 15 years.
Overall, the expanded guidelines will nearly double the number of individuals who are eligible for screening and have the potential to save significantly more lives by identifying cancers at an earlier, more treatable stage.
“Expanding coverage broadens access for lung cancer screening to at-risk populations,” said Lee Felisher, MD, CMS chief medical officer and director of the Center for Clinical Standards and Quality, in a statement. “Today’s decision not only expands access to quality care but is also critical to improving health outcomes for people by helping to detect lung cancer earlier.”
CMS’s decision also simplifies requirements for counseling and shared decision-making visits and removes an initial requirement for the reading radiologist to document participation in continuing medical education, which will reduce administrative burden. CMS also added a requirement back to the National Coverage Determination criteria that requires radiology imaging facilities to use a standardized lung nodule identification, classification, and reporting system.
The American Lung Association applauds the decision to update eligibility.
“[The] announcement from CMS will give more people enrolled in Medicare access to lifesaving lung cancer screening. Screening for individuals at high risk is the only tool to catch this disease early when it is more curable,” Harold Wimmer, president and CEO of the American Lung Association, said in a statement. “Unfortunately, only 5.7% of people who are eligible have been screened, so it’s important that we talk with our friends and family who are at high risk about getting screened.”
While access to screening will significantly increase, the American Lung Association recommends CMS go a step further and expand eligibility to individuals up to 80 years of age, as the USPSTF recommendations do, as well as remove the recommendation that individuals cease screening once they have stopped smoking for 15 years.
Given the new guidelines, most private insurance plans will need to update screening coverage policies to reflect the updated guidelines for plan years beginning after March 31.
To read the final decision, visit the CMS website.
A version of this article first appeared on Medscape.com.
for Medicare recipients.
According to the final decision, announced February 10, CMS will lower the age for screening from 55 to 50 years up to 77 years and reduce criteria for tobacco smoking history from at least 30 pack-years to 20 pack-years. The expanded Medicare recommendation will address racial disparities associated with lung cancer, given evidence that one third of Black patients are diagnosed with lung cancer before age 55.
The updated CMS guidelines align closely with recommendations made by the U.S. Preventive Services Task Force (USPSTF) in March 2021. The USPSTF expanded its guidelines for screening to include individuals ages 50 to 80 years, as well as those who have a 20–pack-year smoking history and who currently smoke or have quit within the past 15 years.
Overall, the expanded guidelines will nearly double the number of individuals who are eligible for screening and have the potential to save significantly more lives by identifying cancers at an earlier, more treatable stage.
“Expanding coverage broadens access for lung cancer screening to at-risk populations,” said Lee Felisher, MD, CMS chief medical officer and director of the Center for Clinical Standards and Quality, in a statement. “Today’s decision not only expands access to quality care but is also critical to improving health outcomes for people by helping to detect lung cancer earlier.”
CMS’s decision also simplifies requirements for counseling and shared decision-making visits and removes an initial requirement for the reading radiologist to document participation in continuing medical education, which will reduce administrative burden. CMS also added a requirement back to the National Coverage Determination criteria that requires radiology imaging facilities to use a standardized lung nodule identification, classification, and reporting system.
The American Lung Association applauds the decision to update eligibility.
“[The] announcement from CMS will give more people enrolled in Medicare access to lifesaving lung cancer screening. Screening for individuals at high risk is the only tool to catch this disease early when it is more curable,” Harold Wimmer, president and CEO of the American Lung Association, said in a statement. “Unfortunately, only 5.7% of people who are eligible have been screened, so it’s important that we talk with our friends and family who are at high risk about getting screened.”
While access to screening will significantly increase, the American Lung Association recommends CMS go a step further and expand eligibility to individuals up to 80 years of age, as the USPSTF recommendations do, as well as remove the recommendation that individuals cease screening once they have stopped smoking for 15 years.
Given the new guidelines, most private insurance plans will need to update screening coverage policies to reflect the updated guidelines for plan years beginning after March 31.
To read the final decision, visit the CMS website.
A version of this article first appeared on Medscape.com.
for Medicare recipients.
According to the final decision, announced February 10, CMS will lower the age for screening from 55 to 50 years up to 77 years and reduce criteria for tobacco smoking history from at least 30 pack-years to 20 pack-years. The expanded Medicare recommendation will address racial disparities associated with lung cancer, given evidence that one third of Black patients are diagnosed with lung cancer before age 55.
The updated CMS guidelines align closely with recommendations made by the U.S. Preventive Services Task Force (USPSTF) in March 2021. The USPSTF expanded its guidelines for screening to include individuals ages 50 to 80 years, as well as those who have a 20–pack-year smoking history and who currently smoke or have quit within the past 15 years.
Overall, the expanded guidelines will nearly double the number of individuals who are eligible for screening and have the potential to save significantly more lives by identifying cancers at an earlier, more treatable stage.
“Expanding coverage broadens access for lung cancer screening to at-risk populations,” said Lee Felisher, MD, CMS chief medical officer and director of the Center for Clinical Standards and Quality, in a statement. “Today’s decision not only expands access to quality care but is also critical to improving health outcomes for people by helping to detect lung cancer earlier.”
CMS’s decision also simplifies requirements for counseling and shared decision-making visits and removes an initial requirement for the reading radiologist to document participation in continuing medical education, which will reduce administrative burden. CMS also added a requirement back to the National Coverage Determination criteria that requires radiology imaging facilities to use a standardized lung nodule identification, classification, and reporting system.
The American Lung Association applauds the decision to update eligibility.
“[The] announcement from CMS will give more people enrolled in Medicare access to lifesaving lung cancer screening. Screening for individuals at high risk is the only tool to catch this disease early when it is more curable,” Harold Wimmer, president and CEO of the American Lung Association, said in a statement. “Unfortunately, only 5.7% of people who are eligible have been screened, so it’s important that we talk with our friends and family who are at high risk about getting screened.”
While access to screening will significantly increase, the American Lung Association recommends CMS go a step further and expand eligibility to individuals up to 80 years of age, as the USPSTF recommendations do, as well as remove the recommendation that individuals cease screening once they have stopped smoking for 15 years.
Given the new guidelines, most private insurance plans will need to update screening coverage policies to reflect the updated guidelines for plan years beginning after March 31.
To read the final decision, visit the CMS website.
A version of this article first appeared on Medscape.com.
FDA hints at deadlines to meet accelerated approval requirements
The FDA launched its accelerated approval program in 1992 in response to the AIDS crisis, but the bulk of approvals since then have been for cancer drugs, wrote the authors who included Gautam U. Mehta, MD, a clinical reviewer with the FDA’s Center for Drug Evaluation and Research; R. Angelo de Claro, MD, associate director of the FDA’s Global Clinical Sciences division within the Oncology Center of Excellence; and Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
Accelerated approvals are typically granted in oncology based on overall response rate with a requirement that companies confirm that there’s actually a clinical benefit in postmarketing studies.
The system has inspired European nations and Australia to launch their own programs, but with a key difference: Conditional approvals expire within 1 year or 2.
To be reinstated and remain on the market, companies have to submit a timeline for when they’ll meet their outstanding obligations and demonstrate that the benefit of leaving their product on the market outweighs the risk.
The approach puts “the onus of timely completion of confirmatory trials and verification of benefit” on the drug maker. In the meantime, the system limits “public exposure to stale claims of effectiveness that cannot be expeditiously substantiated,” Dr. Mehta and colleagues wrote.
There aren’t any deadlines in the United States, however, so the FDA has “to initiate a resource-intensive withdrawal process” when proof of clinical benefit is not forthcoming, they said.
In the United States, only 14 of 167 oncology indications granted accelerated approval since 1992 were withdrawn voluntarily and one was withdrawn by FDA request, and one was forced by the agency. The median time from accelerated approval to withdrawal was 8.8 years.
The actual withdrawal process itself took 11 months when bevacizumab’s breast cancer indication was canceled in 2011.
The authors didn’t call for change outright, but they did say that “future discussions of the accelerated approval program in the U.S.” will seek “to coordinate regulatory processes” with other countries, with an eye towards building “harmony between” agencies.
Among other targets for possible harmonization, they noted that only new molecular entities are eligible for accelerated approval in Europe, whereas supplemental indications are also eligible in the United States
Europe also requires a risk-benefit assessment for conditional approvals, which “has led to relatively few approvals based on single-arm clinical trial data,” the authors said.
Dr. Mehta, Dr. de Claro, and Dr. Pazdur had no conflicts of interest.
The FDA launched its accelerated approval program in 1992 in response to the AIDS crisis, but the bulk of approvals since then have been for cancer drugs, wrote the authors who included Gautam U. Mehta, MD, a clinical reviewer with the FDA’s Center for Drug Evaluation and Research; R. Angelo de Claro, MD, associate director of the FDA’s Global Clinical Sciences division within the Oncology Center of Excellence; and Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
Accelerated approvals are typically granted in oncology based on overall response rate with a requirement that companies confirm that there’s actually a clinical benefit in postmarketing studies.
The system has inspired European nations and Australia to launch their own programs, but with a key difference: Conditional approvals expire within 1 year or 2.
To be reinstated and remain on the market, companies have to submit a timeline for when they’ll meet their outstanding obligations and demonstrate that the benefit of leaving their product on the market outweighs the risk.
The approach puts “the onus of timely completion of confirmatory trials and verification of benefit” on the drug maker. In the meantime, the system limits “public exposure to stale claims of effectiveness that cannot be expeditiously substantiated,” Dr. Mehta and colleagues wrote.
There aren’t any deadlines in the United States, however, so the FDA has “to initiate a resource-intensive withdrawal process” when proof of clinical benefit is not forthcoming, they said.
In the United States, only 14 of 167 oncology indications granted accelerated approval since 1992 were withdrawn voluntarily and one was withdrawn by FDA request, and one was forced by the agency. The median time from accelerated approval to withdrawal was 8.8 years.
The actual withdrawal process itself took 11 months when bevacizumab’s breast cancer indication was canceled in 2011.
The authors didn’t call for change outright, but they did say that “future discussions of the accelerated approval program in the U.S.” will seek “to coordinate regulatory processes” with other countries, with an eye towards building “harmony between” agencies.
Among other targets for possible harmonization, they noted that only new molecular entities are eligible for accelerated approval in Europe, whereas supplemental indications are also eligible in the United States
Europe also requires a risk-benefit assessment for conditional approvals, which “has led to relatively few approvals based on single-arm clinical trial data,” the authors said.
Dr. Mehta, Dr. de Claro, and Dr. Pazdur had no conflicts of interest.
The FDA launched its accelerated approval program in 1992 in response to the AIDS crisis, but the bulk of approvals since then have been for cancer drugs, wrote the authors who included Gautam U. Mehta, MD, a clinical reviewer with the FDA’s Center for Drug Evaluation and Research; R. Angelo de Claro, MD, associate director of the FDA’s Global Clinical Sciences division within the Oncology Center of Excellence; and Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
Accelerated approvals are typically granted in oncology based on overall response rate with a requirement that companies confirm that there’s actually a clinical benefit in postmarketing studies.
The system has inspired European nations and Australia to launch their own programs, but with a key difference: Conditional approvals expire within 1 year or 2.
To be reinstated and remain on the market, companies have to submit a timeline for when they’ll meet their outstanding obligations and demonstrate that the benefit of leaving their product on the market outweighs the risk.
The approach puts “the onus of timely completion of confirmatory trials and verification of benefit” on the drug maker. In the meantime, the system limits “public exposure to stale claims of effectiveness that cannot be expeditiously substantiated,” Dr. Mehta and colleagues wrote.
There aren’t any deadlines in the United States, however, so the FDA has “to initiate a resource-intensive withdrawal process” when proof of clinical benefit is not forthcoming, they said.
In the United States, only 14 of 167 oncology indications granted accelerated approval since 1992 were withdrawn voluntarily and one was withdrawn by FDA request, and one was forced by the agency. The median time from accelerated approval to withdrawal was 8.8 years.
The actual withdrawal process itself took 11 months when bevacizumab’s breast cancer indication was canceled in 2011.
The authors didn’t call for change outright, but they did say that “future discussions of the accelerated approval program in the U.S.” will seek “to coordinate regulatory processes” with other countries, with an eye towards building “harmony between” agencies.
Among other targets for possible harmonization, they noted that only new molecular entities are eligible for accelerated approval in Europe, whereas supplemental indications are also eligible in the United States
Europe also requires a risk-benefit assessment for conditional approvals, which “has led to relatively few approvals based on single-arm clinical trial data,” the authors said.
Dr. Mehta, Dr. de Claro, and Dr. Pazdur had no conflicts of interest.
FROM JAMA ONCOLOGY
Tips for connecting with your patients
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
City of Hope completes acquisition of CTCA
The combined group now has 575 physicians and more than 11,000 employees and is expected to care for approximately 115,000 patients each year.
City of Hope, a National Cancer Institute–designated comprehensive cancer center, is located near Los Angeles. It currently comprises its main campus and a network of clinical care locations across Southern California. A new campus is scheduled to open this year in Irvine, California, about 50 miles south of the main center.
The acquisition of CTCA expands its reach into three new states – Arizona, Illinois, and Georgia – with an additional 41 clinical network locations.
Commenting on the completion of the deal, Robert Stone, president and CEO of City of Hope, said in a statement: “With the completion of this acquisition, City of Hope and Cancer Treatment Centers of America are combining complementary strengths. ... Together, we are creating a new model for how cancer care is delivered, leveraging real-world cancer care experience to inform scientific innovation and making tomorrow’s new discoveries available to the people who need them today.”
City of Hope announced in December 2020 that it would acquire CTCA for $390 million, as previously reported by this news organization.
At the time, Pat Basu MD, MBA, president and CEO of CTCA, said that they were excited about the deal. “Through the shared, patient-centric values of both organizations and expanded access as a result of the collaboration, cancer patients across the nation will be the ultimate beneficiaries of this relationship,” he said. Dr. Basu will remain CEO of CTCA and report to Robert Stone.
Controversies and closures
CTCA is a national oncology network of hospitals and outpatient care centers that offers an integrated approach to care, including surgery, radiotherapy, chemotherapy, immunotherapy, and advancements in precision medicine with supportive therapies to manage side effects and enhance quality of life during treatment and into survivorship.
However, it appears to have run into financial problems. During 2021, CTCA closed a center in Tulsa, Oklahoma, and sold off assets from a Philadelphia-based hospital.
In addition, for the past 10 years, CTCA had been involved in a series of controversies. These include a 2013 investigation into alleged questionable practices designed to boost its mortality statistics, as well as an analysis of cancer center advertising practices that showed that CTCA spent $101.7 million on advertising in 2014. More recently, a 2019 report showed that CTCA’s high advertising expenditures did not correlate with better patient outcomes in comparison with other centers.
Now that it has been acquired, CTCA will transition from a private for-profit company to a nonprofit organization, according to City of Hope.
A version of this article first appeared on Medscape.com.
The combined group now has 575 physicians and more than 11,000 employees and is expected to care for approximately 115,000 patients each year.
City of Hope, a National Cancer Institute–designated comprehensive cancer center, is located near Los Angeles. It currently comprises its main campus and a network of clinical care locations across Southern California. A new campus is scheduled to open this year in Irvine, California, about 50 miles south of the main center.
The acquisition of CTCA expands its reach into three new states – Arizona, Illinois, and Georgia – with an additional 41 clinical network locations.
Commenting on the completion of the deal, Robert Stone, president and CEO of City of Hope, said in a statement: “With the completion of this acquisition, City of Hope and Cancer Treatment Centers of America are combining complementary strengths. ... Together, we are creating a new model for how cancer care is delivered, leveraging real-world cancer care experience to inform scientific innovation and making tomorrow’s new discoveries available to the people who need them today.”
City of Hope announced in December 2020 that it would acquire CTCA for $390 million, as previously reported by this news organization.
At the time, Pat Basu MD, MBA, president and CEO of CTCA, said that they were excited about the deal. “Through the shared, patient-centric values of both organizations and expanded access as a result of the collaboration, cancer patients across the nation will be the ultimate beneficiaries of this relationship,” he said. Dr. Basu will remain CEO of CTCA and report to Robert Stone.
Controversies and closures
CTCA is a national oncology network of hospitals and outpatient care centers that offers an integrated approach to care, including surgery, radiotherapy, chemotherapy, immunotherapy, and advancements in precision medicine with supportive therapies to manage side effects and enhance quality of life during treatment and into survivorship.
However, it appears to have run into financial problems. During 2021, CTCA closed a center in Tulsa, Oklahoma, and sold off assets from a Philadelphia-based hospital.
In addition, for the past 10 years, CTCA had been involved in a series of controversies. These include a 2013 investigation into alleged questionable practices designed to boost its mortality statistics, as well as an analysis of cancer center advertising practices that showed that CTCA spent $101.7 million on advertising in 2014. More recently, a 2019 report showed that CTCA’s high advertising expenditures did not correlate with better patient outcomes in comparison with other centers.
Now that it has been acquired, CTCA will transition from a private for-profit company to a nonprofit organization, according to City of Hope.
A version of this article first appeared on Medscape.com.
The combined group now has 575 physicians and more than 11,000 employees and is expected to care for approximately 115,000 patients each year.
City of Hope, a National Cancer Institute–designated comprehensive cancer center, is located near Los Angeles. It currently comprises its main campus and a network of clinical care locations across Southern California. A new campus is scheduled to open this year in Irvine, California, about 50 miles south of the main center.
The acquisition of CTCA expands its reach into three new states – Arizona, Illinois, and Georgia – with an additional 41 clinical network locations.
Commenting on the completion of the deal, Robert Stone, president and CEO of City of Hope, said in a statement: “With the completion of this acquisition, City of Hope and Cancer Treatment Centers of America are combining complementary strengths. ... Together, we are creating a new model for how cancer care is delivered, leveraging real-world cancer care experience to inform scientific innovation and making tomorrow’s new discoveries available to the people who need them today.”
City of Hope announced in December 2020 that it would acquire CTCA for $390 million, as previously reported by this news organization.
At the time, Pat Basu MD, MBA, president and CEO of CTCA, said that they were excited about the deal. “Through the shared, patient-centric values of both organizations and expanded access as a result of the collaboration, cancer patients across the nation will be the ultimate beneficiaries of this relationship,” he said. Dr. Basu will remain CEO of CTCA and report to Robert Stone.
Controversies and closures
CTCA is a national oncology network of hospitals and outpatient care centers that offers an integrated approach to care, including surgery, radiotherapy, chemotherapy, immunotherapy, and advancements in precision medicine with supportive therapies to manage side effects and enhance quality of life during treatment and into survivorship.
However, it appears to have run into financial problems. During 2021, CTCA closed a center in Tulsa, Oklahoma, and sold off assets from a Philadelphia-based hospital.
In addition, for the past 10 years, CTCA had been involved in a series of controversies. These include a 2013 investigation into alleged questionable practices designed to boost its mortality statistics, as well as an analysis of cancer center advertising practices that showed that CTCA spent $101.7 million on advertising in 2014. More recently, a 2019 report showed that CTCA’s high advertising expenditures did not correlate with better patient outcomes in comparison with other centers.
Now that it has been acquired, CTCA will transition from a private for-profit company to a nonprofit organization, according to City of Hope.
A version of this article first appeared on Medscape.com.
President Biden’s ‘Cancer Moonshot’ to be relaunched
The “Cancer Moonshot” is about to be relaunched.
In a White House briefing, President Joe Biden announced that he is “reigniting” the initiative he spearheaded when he was vice president during the Obama administration.
During the livestreamed event, the president discussed his plans to bring a “fierce sense of urgency” to the fight against cancer and better support patients with cancer and their families.
He emphasized that cancer is one of the truly bipartisan issues. There is strong support from both “sides of the aisle,” he said, and he sees it as an issue that can bring the country together.
“We can do this. I promise you, we can do this. For all those we lost, for all those we miss. We can end cancer as we know it,” he said. “This is a presidential White House priority.”
The aim is to reduce the death rate from cancer by at least 50% over the next 25 years.
There is also a proposal to create the Advanced Research Projects Agency for Health, which would focus on driving cutting-edge innovation in health research.
Part of the plan is to assemble a “cancer cabinet” that includes 18 federal departments, agencies, and offices, including leaders from the departments of Health & Human Services, Veterans Affairs, Defense, Energy, and Agriculture.
At present, there are few details about the new program or how it will be funded.
Presumably more will be revealed at the Cancer Moonshot Summit being planned, as well as on a planned new website where people can track its progress.
President priority
Cancer Moonshot began back in 2016, when during his last State of the Union Address, former President Barack Obama announced the ambitious initiative. A few days later, Obama asked Congress for $1 billion to send cancer to the moon, and he put Biden, then vice president, in charge of “mission control” in the remaining months of the administration.
The new initiative will be headed by Danielle Carnival, PhD, who serves in the White House Office of Science and Technology Policy and has been appointed as White House Cancer Moonshot coordinator.
At the briefing, Mr. Biden and Vice President Kamala Harris spoke about losing family members to cancer. The president spoke about his eldest son, Beau, who died from brain cancer when he was 46 years old, while Ms. Harris spoke about her mother, Shyamala Gopalan, a breast cancer researcher who died of colon cancer in 2009.
Accolades but a bit of caution
The president’s speech was applauded by many cancer groups, both professional organizations and patient advocacy groups.
Karen E. Knudsen, PhD, chief executive officer of the American Cancer Society and its advocacy affiliate, the American Cancer Society Cancer Action Network, commended Mr. Biden for reigniting Cancer Moonshot.
“In 2022 alone, there will be an estimated 1.9 million people diagnosed with cancer and more than 600,000 people in the U.S. will die. Marshaling the resources of the federal government will be critical in our ability to reduce death and suffering from this disease,” she said.
The American Society for Radiation Oncology issued a press release, saying: “On behalf of radiation oncologists who treat people with cancer every day, we support the Biden-Harris administration’s move to drastically reduce the number of cancer deaths in the United States and improve the lives of people diagnosed with this disease.
“We believe the administration’s commitment to expand cancer prevention efforts and to increase equitable access to screenings and treatments will help mitigate some of the negative impact of the COVID-19 pandemic,” the society added.
At the American Association for Cancer Research, Chief Executive Officer Margaret Foti, MD, PhD, said she was thrilled to hear the announcement after the devastating interruptions in cancer research and patient care over the past 2 years.
“The reignited Cancer Moonshot will provide an important framework to help improve cancer prevention strategies, increase cancer screenings and early detection, reduce cancer disparities, and propel new lifesaving cures for patients with cancer,” she said.
However, increased funding from Congress will be needed for these goals to be achieved, she emphasized.
A version of this article first appeared on Medscape.com.
The “Cancer Moonshot” is about to be relaunched.
In a White House briefing, President Joe Biden announced that he is “reigniting” the initiative he spearheaded when he was vice president during the Obama administration.
During the livestreamed event, the president discussed his plans to bring a “fierce sense of urgency” to the fight against cancer and better support patients with cancer and their families.
He emphasized that cancer is one of the truly bipartisan issues. There is strong support from both “sides of the aisle,” he said, and he sees it as an issue that can bring the country together.
“We can do this. I promise you, we can do this. For all those we lost, for all those we miss. We can end cancer as we know it,” he said. “This is a presidential White House priority.”
The aim is to reduce the death rate from cancer by at least 50% over the next 25 years.
There is also a proposal to create the Advanced Research Projects Agency for Health, which would focus on driving cutting-edge innovation in health research.
Part of the plan is to assemble a “cancer cabinet” that includes 18 federal departments, agencies, and offices, including leaders from the departments of Health & Human Services, Veterans Affairs, Defense, Energy, and Agriculture.
At present, there are few details about the new program or how it will be funded.
Presumably more will be revealed at the Cancer Moonshot Summit being planned, as well as on a planned new website where people can track its progress.
President priority
Cancer Moonshot began back in 2016, when during his last State of the Union Address, former President Barack Obama announced the ambitious initiative. A few days later, Obama asked Congress for $1 billion to send cancer to the moon, and he put Biden, then vice president, in charge of “mission control” in the remaining months of the administration.
The new initiative will be headed by Danielle Carnival, PhD, who serves in the White House Office of Science and Technology Policy and has been appointed as White House Cancer Moonshot coordinator.
At the briefing, Mr. Biden and Vice President Kamala Harris spoke about losing family members to cancer. The president spoke about his eldest son, Beau, who died from brain cancer when he was 46 years old, while Ms. Harris spoke about her mother, Shyamala Gopalan, a breast cancer researcher who died of colon cancer in 2009.
Accolades but a bit of caution
The president’s speech was applauded by many cancer groups, both professional organizations and patient advocacy groups.
Karen E. Knudsen, PhD, chief executive officer of the American Cancer Society and its advocacy affiliate, the American Cancer Society Cancer Action Network, commended Mr. Biden for reigniting Cancer Moonshot.
“In 2022 alone, there will be an estimated 1.9 million people diagnosed with cancer and more than 600,000 people in the U.S. will die. Marshaling the resources of the federal government will be critical in our ability to reduce death and suffering from this disease,” she said.
The American Society for Radiation Oncology issued a press release, saying: “On behalf of radiation oncologists who treat people with cancer every day, we support the Biden-Harris administration’s move to drastically reduce the number of cancer deaths in the United States and improve the lives of people diagnosed with this disease.
“We believe the administration’s commitment to expand cancer prevention efforts and to increase equitable access to screenings and treatments will help mitigate some of the negative impact of the COVID-19 pandemic,” the society added.
At the American Association for Cancer Research, Chief Executive Officer Margaret Foti, MD, PhD, said she was thrilled to hear the announcement after the devastating interruptions in cancer research and patient care over the past 2 years.
“The reignited Cancer Moonshot will provide an important framework to help improve cancer prevention strategies, increase cancer screenings and early detection, reduce cancer disparities, and propel new lifesaving cures for patients with cancer,” she said.
However, increased funding from Congress will be needed for these goals to be achieved, she emphasized.
A version of this article first appeared on Medscape.com.
The “Cancer Moonshot” is about to be relaunched.
In a White House briefing, President Joe Biden announced that he is “reigniting” the initiative he spearheaded when he was vice president during the Obama administration.
During the livestreamed event, the president discussed his plans to bring a “fierce sense of urgency” to the fight against cancer and better support patients with cancer and their families.
He emphasized that cancer is one of the truly bipartisan issues. There is strong support from both “sides of the aisle,” he said, and he sees it as an issue that can bring the country together.
“We can do this. I promise you, we can do this. For all those we lost, for all those we miss. We can end cancer as we know it,” he said. “This is a presidential White House priority.”
The aim is to reduce the death rate from cancer by at least 50% over the next 25 years.
There is also a proposal to create the Advanced Research Projects Agency for Health, which would focus on driving cutting-edge innovation in health research.
Part of the plan is to assemble a “cancer cabinet” that includes 18 federal departments, agencies, and offices, including leaders from the departments of Health & Human Services, Veterans Affairs, Defense, Energy, and Agriculture.
At present, there are few details about the new program or how it will be funded.
Presumably more will be revealed at the Cancer Moonshot Summit being planned, as well as on a planned new website where people can track its progress.
President priority
Cancer Moonshot began back in 2016, when during his last State of the Union Address, former President Barack Obama announced the ambitious initiative. A few days later, Obama asked Congress for $1 billion to send cancer to the moon, and he put Biden, then vice president, in charge of “mission control” in the remaining months of the administration.
The new initiative will be headed by Danielle Carnival, PhD, who serves in the White House Office of Science and Technology Policy and has been appointed as White House Cancer Moonshot coordinator.
At the briefing, Mr. Biden and Vice President Kamala Harris spoke about losing family members to cancer. The president spoke about his eldest son, Beau, who died from brain cancer when he was 46 years old, while Ms. Harris spoke about her mother, Shyamala Gopalan, a breast cancer researcher who died of colon cancer in 2009.
Accolades but a bit of caution
The president’s speech was applauded by many cancer groups, both professional organizations and patient advocacy groups.
Karen E. Knudsen, PhD, chief executive officer of the American Cancer Society and its advocacy affiliate, the American Cancer Society Cancer Action Network, commended Mr. Biden for reigniting Cancer Moonshot.
“In 2022 alone, there will be an estimated 1.9 million people diagnosed with cancer and more than 600,000 people in the U.S. will die. Marshaling the resources of the federal government will be critical in our ability to reduce death and suffering from this disease,” she said.
The American Society for Radiation Oncology issued a press release, saying: “On behalf of radiation oncologists who treat people with cancer every day, we support the Biden-Harris administration’s move to drastically reduce the number of cancer deaths in the United States and improve the lives of people diagnosed with this disease.
“We believe the administration’s commitment to expand cancer prevention efforts and to increase equitable access to screenings and treatments will help mitigate some of the negative impact of the COVID-19 pandemic,” the society added.
At the American Association for Cancer Research, Chief Executive Officer Margaret Foti, MD, PhD, said she was thrilled to hear the announcement after the devastating interruptions in cancer research and patient care over the past 2 years.
“The reignited Cancer Moonshot will provide an important framework to help improve cancer prevention strategies, increase cancer screenings and early detection, reduce cancer disparities, and propel new lifesaving cures for patients with cancer,” she said.
However, increased funding from Congress will be needed for these goals to be achieved, she emphasized.
A version of this article first appeared on Medscape.com.
Bullous Dermatoses and Quality of Life: A Summary of Tools to Assess Psychosocial Health
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.
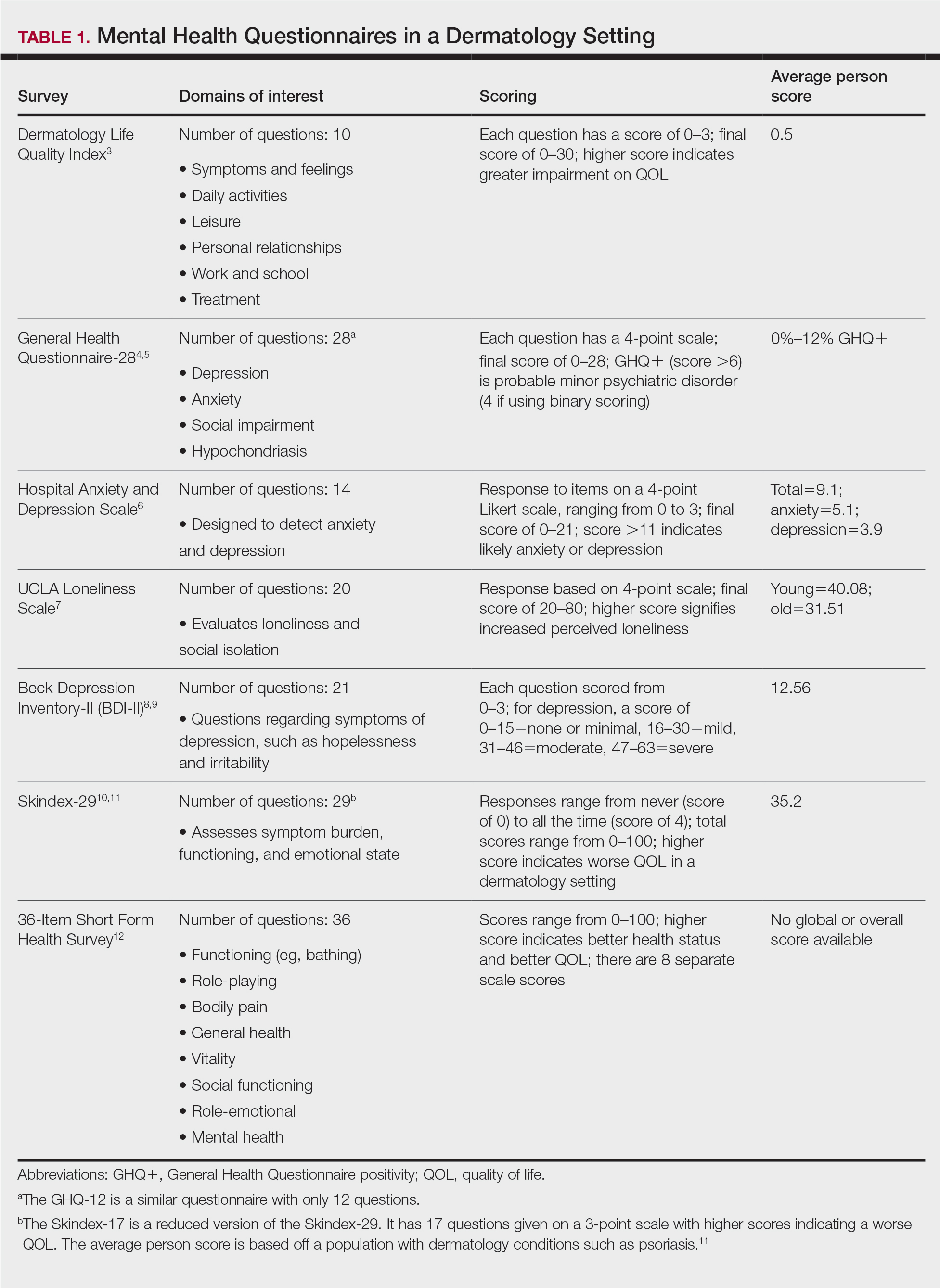
Results
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.
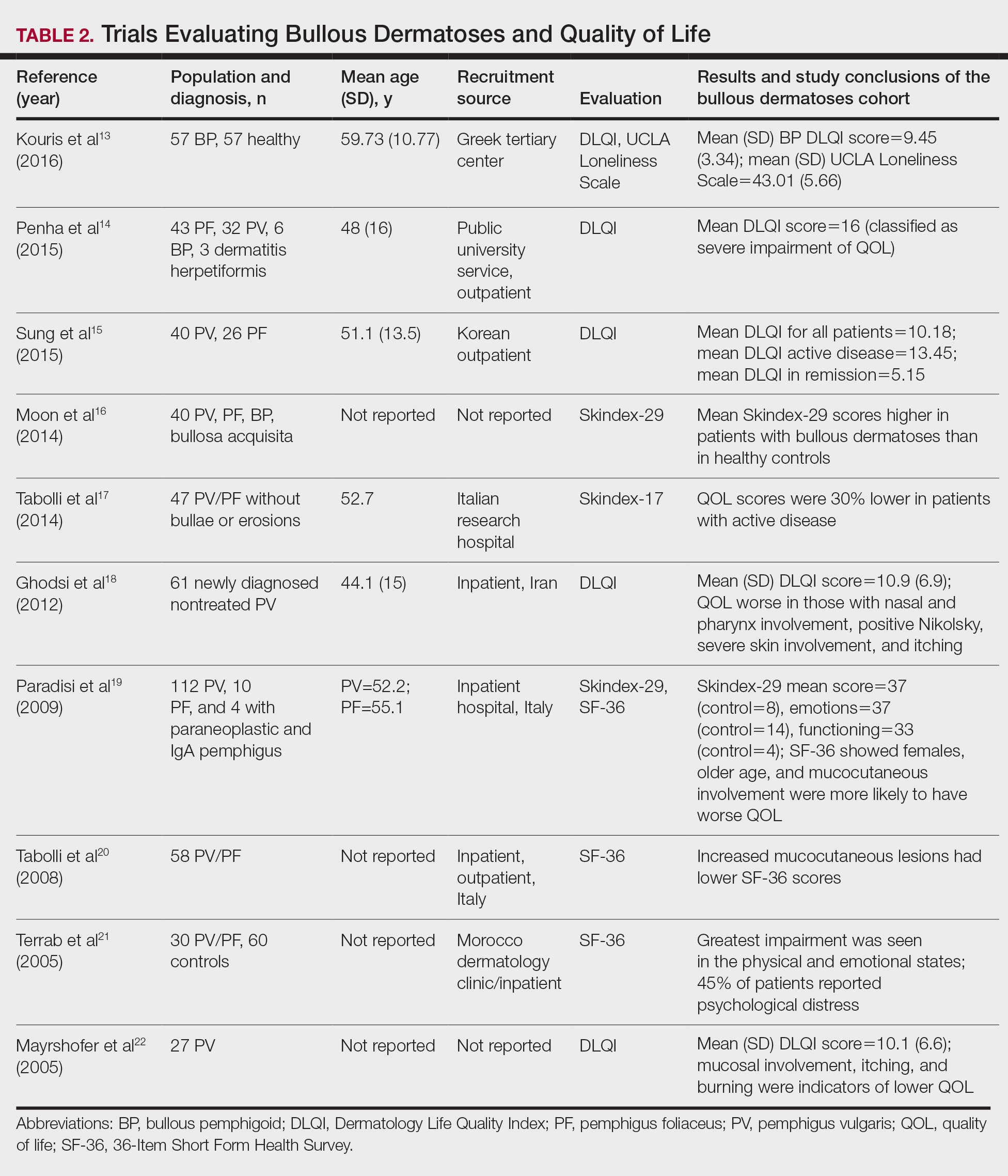
The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.
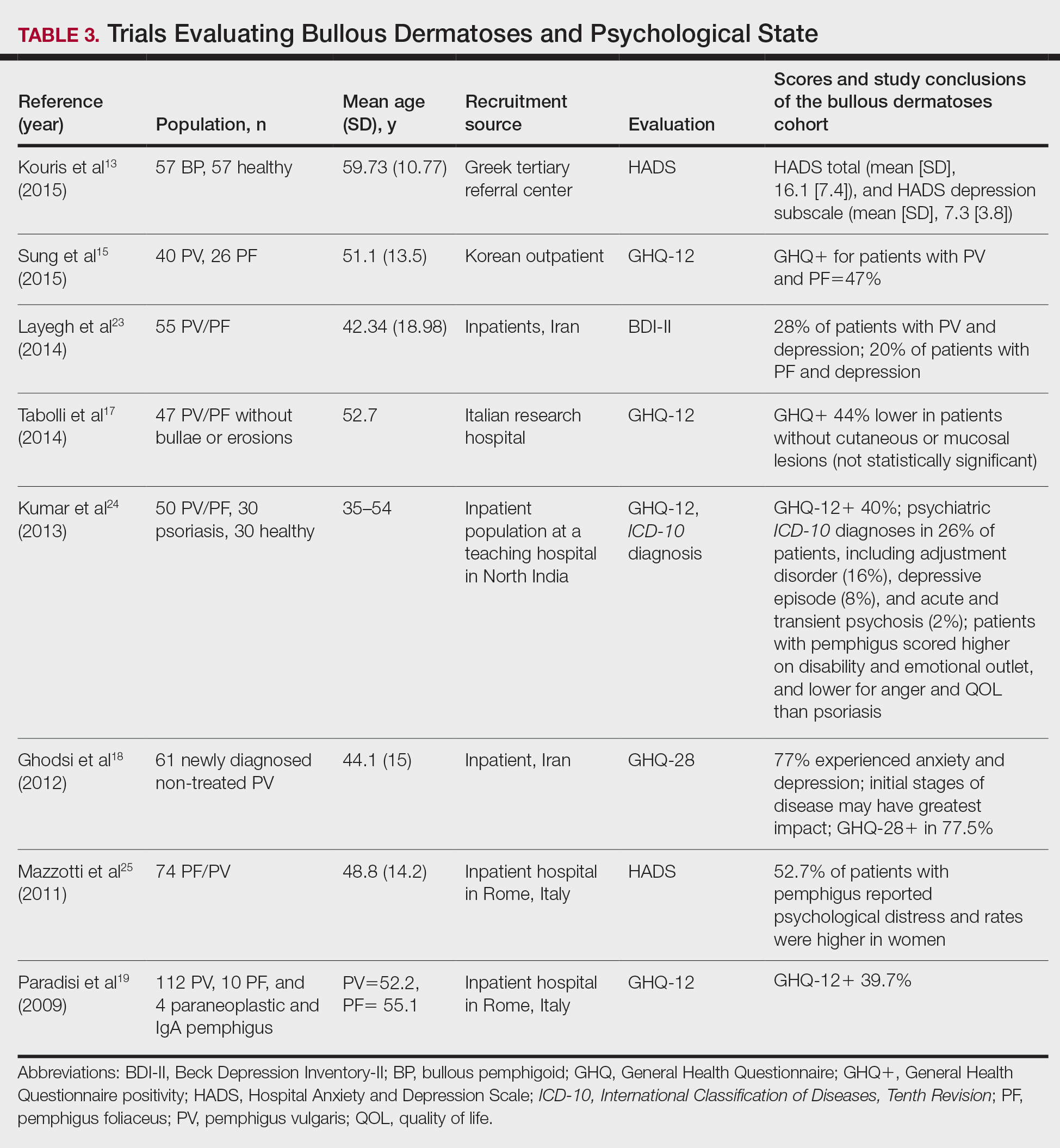
To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.

Results
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.

The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.

To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.

Results
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.

The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.

To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
Practice Points
- Autoimmune bullous dermatoses cause cutaneous lesions that are painful and disfiguring. These conditions affect a patient’s ability to perform everyday tasks, and individual lesions can take years to heal.
- Providers should take necessary steps to address patient well-being, especially at disease onset in patients with bullous dermatoses.
Nuances in Training During the Age of Teledermatology
The COVID-19 pandemic largely altered the practice of medicine, including a rapid expansion of telemedicine following the March 2020 World Health Organization guidelines for social distancing, which recommended suspension of all nonurgent in-person visits.1 Expectedly, COVID-related urgent care visits initially comprised the bulk of the new telemedicine wave: NYU Langone Health (New York, New York), for example, saw a 683% increase in virtual visits between March and April 2020, most (55.3%) of which were for respiratory concerns. In-person visits, on the other hand, concurrently fell by more than 80%. Interestingly, nonurgent ambulatory care specialties also saw a considerable uptick in virtual encounters, from less than 50 visits in a typical day to an average of 7000 in a 10-day stretch.2
As a largely ambulatory specialty that relies on visual examination, dermatology was no exception to the swing toward telemedicine, or teledermatology (TD). Before the COVID-19 pandemic, 14.1% (82 of 582 respondents) of practicing US dermatologists reported having used teledermatology, compared to 96.9% (572/591) during the pandemic.3 Even at my home institution (Massachusetts General Hospital [Boston, Massachusetts] and its 12 affiliated dermatology clinics), the number of in-person visits in April 2020 (n=67) was less than 1% of that in April 2019 (n=7919), whereas there was a total of 1564 virtual visits in April 2020 compared to zero the year prior. Virtual provider-to-provider consults (e-consultations) also saw an increase of more than 20%, suggesting that dermatology’s avid adoption of TD also had improved the perceived accessibility of our specialty.4
The adoption and adaptation of TD are projected to continue to grow rapidly across the globe, as digitalization has enhanced access without increasing costs, shortened wait times, and even created opportunities for primary care providers based in rural or overseas locations to learn the diagnosis and treatment of skin disease.5 Residents and fellows should be privy to the nuances of training and practicing in this digital era, as our careers inevitably will involve some facet of TD.
The Art of Medicine
Touch, a sense that perhaps ranks second to sight in dermatology, is absent in TD. In either synchronous (live-interactive, face video visits) or asynchronous (store-and-forward, where digital photographs and clinical information sent by patients or referring physicians are assessed at a later time) TD, the skin cannot be rubbed for texture, pinched for thickness, or pushed for blanching. Instead, all we have is vision. Irwin Braverman, MD, Professor Emeritus of Dermatology at Yale University (New Haven, Connecticut), alongside Jacqueline Dolev, MD, dermatologist and Yale graduate, and Linda Friedlaender, curator at the Yale Center for British Art, founded an observational skills workshop in which trainees learn to observe and describe the paintings housed in the museum, noting all memorable details: the color of the sky, the actions of the animals, and the facial expressions of the people. A study of 90 participants over a 2-year period found that following the workshop, the ability to identify key diagnostic details from clinical photography improved by more than 10%.6 Other studies also utilizing fine art as a medical training tool to improve “visual literacy” saw similarly increased sophistication in the description of clinical imagery, which translated to better diagnostic acumen.7 Confined to video and photographs, TD necessitates trainees and practicing dermatologists to be excellent visual diagnosticians. Although surveyed dermatologists believe TD is presently appropriate for acne, benign lesions, or follow-up appointments,3 conditions for which patients have been examined via TD have included drug eruptions, premalignant or malignant neoplasms, infections, and papulosquamous or inflammatory dermatoses.8 At the very least, clinicians should be versed in identifying those conditions that require in-person evaluation, as patients cannot be held responsible to distinguish which situations can and cannot be addressed virtually.
Issues of Patient-Physician Confidentiality
Teledermatology is not without its shortcomings; critics have noted diagnostic challenges with poor quality photographs or videos, inability to perform total-body skin examinations, and socioeconomic limitations due to broadband availability and speed.5,9 Although most of these shortcomings are outside of our control, a key challenge within the purview of the provider is the protection of patient privacy.
Much of the salient concerns regarding patient-physician confidentiality involve asynchronous TD, where store-and-forward data sharing allows physicians to download patient photographs or information onto their personal email or smartphones.10 Although some hospital systems provide encryption software or hospital-sponsored devices to ensure security, physicians may opt to use their personal phones or laptops out of convenience or to save time.10,11 One study found that less than 30% of smartphone users choose to activate user authentication on their devices, even ones as simple as a passphrase.11 The digital exchange of information thus poses an immense risk for compromising protected health information (PHI), as personal devices can be easily lost, stolen, or hacked. Indeed, in 2015, more than 113 million individuals were affected by a breach of PHI, the majority over hacked network servers.12 With the growing diversity of mediums through which PHI is exchanged, such as videoconferencing and instant messaging, the potential medicolegal risks of information breach continue to climb. The US Department of Health & Human Services urges health care providers to uphold best practices for security, including encrypting data, updating all software including antivirus software, using multifactor authentication, and following local cybersecurity regulations or recommendations.13 For synchronous TD, suggested best practices include utilizing headphones during live appointments, avoiding public wireless networks, and ensuring the provider and patient both scan the room with their device’s camera before the start of the visit.14
On the Horizon of Teledermatology
What can we expect in the coming years? Increased utilization of telemedicine will translate into data that will help address questions surrounding safety, diagnostic accuracy, privacy, and accessibility. One aspect of TD in need of clarity is a guideline on payment and reimbursement, and whether TD can continue to be financially attractive to providers. Starting in 2020, the Centers for Medicare & Medicaid Services removed geographic restrictions for reimbursement of telemedicine visits, enabling even urban-residing patients to enjoy the convenience of TD. This followed a prior relaxation of restrictions, where even prerecorded patient information became eligible for Medicare reimbursement.9 However, as virtual visits tend to be shorter with fewer diagnostic services compared to in-person visits, the reimbursement structure of TD must be nuanced, which is the subject of ongoing study and modification in the wake of the COVID-19 pandemic.15
Another point to consider is the explosion of direct-to-consumer TD, which allows patients to receive virtual dermatologic care or prescription medication without a pre-established relationship with any physician. In 2017, there were 22 direct-to-consumer TD services available to US patients in 45 states, 16 (73%) of which provided dermatologic care for any concern while 6 (27%) were limited to acne or antiaging and were largely prescription oriented. Orchestrated mostly by the for-profit private sector, direct-to-consumer companies are poorly regulated and have raised concerns over questionable practices, such as the use of non–US board-certified physicians, exorbitant fees, and failure to disclose medication side effects.16 A study of 16 direct-to-consumer telemedicine sites found substantial discordance in the suggested management of the same patient, and many of the services relied heavily on patient-provided self-diagnoses, such as a case where psoriasis medication was dispensed for a psoriasis patient who submitted a photograph of his syphilitic rash.17 Despite these problems, consumers show a willingness to pay out of pocket to access these services for their shorter waiting times and convenience.18 Hence, we must learn to ask about direct-to-consumer service use when obtaining a thorough history and be open to counseling our patients on the proper use and potential risks of direct-to-consumer TD.
Final Thoughts
The telemedicine industry is expected to reach more than $130 billion by 2025, with more than 90% of surveyed health care executives planning for the adoption and incorporation of telemedicine into their business models.19 The COVID-19 pandemic was an impetus for an exponential adoption of TD, and it would behoove current residents to realize that the practice of dermatology will continue to be increasingly digitalized within the coming years. Whether through formal training or self-assessment, we must strive to grow as proficient virtual dermatologists while upholding professionalism, patient safety, and health information privacy.
- Yeboah CB, Harvey N, Krishnan R, et al. The impact of COVID-19 on teledermatology: a review. Dermatol Clin. 2021;39:599-608.
- Mann DM, Chen J, Chunara R, et al. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132-1135.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Su MY, Das S. Expansion of asynchronous teledermatology during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E471-E472.
- Maddukuri S, Patel J, Lipoff JB. Teledermatology addressing disparities in health care access: a review [published online March 12, 2021]. Curr Dermatol Rep. doi:10.1007/s13671-021-00329-2
- Dolev JC, Friedlaender LK, Braverman IM. Use of fine art to enhance visual diagnostic skills. JAMA. 2001;286:1020-1021.
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med. 2008;23:991-997.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Wang RH, Barbieri JS, Nguyen HP, et al. Clinical effectiveness and cost-effectiveness of teledermatology: where are we now, and what are the barriers to adoption? J Am Acad Dermatol. 2020;83:299-307.
- Stevenson P, Finnane AR, Soyer HP. Teledermatology and clinical photography: safeguarding patient privacy and mitigating medico-legal risk. Med J Aust. 2016;204:198-200e1.
- Smith KA, Zhou L, Watzlaf VJM. User authentication in smartphones for telehealth. Int J Telerehabil. 2017;9:3-12.
- Breaches of unsecured protected health information. Health IT website. Updated July 22, 2021. Accessed January 16, 2022. https://www.healthit.gov/data/quickstats/breaches-unsecured-protected-health-information
- Jalali MS, Landman A, Gordon WJ. Telemedicine, privacy, and information security in the age of COVID-19. J Am Med Inform Assoc. 2021;28:671-672.
- Telehealth for behavioral health care: protecting patients’ privacy. United States Department of Health and Human Services website. Updated July 2, 2021. Accessed January 16, 2022. https://telehealth.hhs.gov/providers/telehealth-for-behavioral-health/preparing-patients-for-telebehavioral-health/protecting-patients-privacy/
- Shachar C, Engel J, Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020;323:2375-2376.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Snoswell CL, Whitty JA, Caffery LJ, et al. Consumer preference and willingness to pay for direct-to-consumer mobile teledermoscopy services in Australia [published online August 13, 2021]. Dermatology. doi:10.1159/000517257
- Elliott T, Yopes MC. Direct-to-consumer telemedicine. J Allergy Clin Immunol Pract. 2019;7:2546-2552.
The COVID-19 pandemic largely altered the practice of medicine, including a rapid expansion of telemedicine following the March 2020 World Health Organization guidelines for social distancing, which recommended suspension of all nonurgent in-person visits.1 Expectedly, COVID-related urgent care visits initially comprised the bulk of the new telemedicine wave: NYU Langone Health (New York, New York), for example, saw a 683% increase in virtual visits between March and April 2020, most (55.3%) of which were for respiratory concerns. In-person visits, on the other hand, concurrently fell by more than 80%. Interestingly, nonurgent ambulatory care specialties also saw a considerable uptick in virtual encounters, from less than 50 visits in a typical day to an average of 7000 in a 10-day stretch.2
As a largely ambulatory specialty that relies on visual examination, dermatology was no exception to the swing toward telemedicine, or teledermatology (TD). Before the COVID-19 pandemic, 14.1% (82 of 582 respondents) of practicing US dermatologists reported having used teledermatology, compared to 96.9% (572/591) during the pandemic.3 Even at my home institution (Massachusetts General Hospital [Boston, Massachusetts] and its 12 affiliated dermatology clinics), the number of in-person visits in April 2020 (n=67) was less than 1% of that in April 2019 (n=7919), whereas there was a total of 1564 virtual visits in April 2020 compared to zero the year prior. Virtual provider-to-provider consults (e-consultations) also saw an increase of more than 20%, suggesting that dermatology’s avid adoption of TD also had improved the perceived accessibility of our specialty.4
The adoption and adaptation of TD are projected to continue to grow rapidly across the globe, as digitalization has enhanced access without increasing costs, shortened wait times, and even created opportunities for primary care providers based in rural or overseas locations to learn the diagnosis and treatment of skin disease.5 Residents and fellows should be privy to the nuances of training and practicing in this digital era, as our careers inevitably will involve some facet of TD.
The Art of Medicine
Touch, a sense that perhaps ranks second to sight in dermatology, is absent in TD. In either synchronous (live-interactive, face video visits) or asynchronous (store-and-forward, where digital photographs and clinical information sent by patients or referring physicians are assessed at a later time) TD, the skin cannot be rubbed for texture, pinched for thickness, or pushed for blanching. Instead, all we have is vision. Irwin Braverman, MD, Professor Emeritus of Dermatology at Yale University (New Haven, Connecticut), alongside Jacqueline Dolev, MD, dermatologist and Yale graduate, and Linda Friedlaender, curator at the Yale Center for British Art, founded an observational skills workshop in which trainees learn to observe and describe the paintings housed in the museum, noting all memorable details: the color of the sky, the actions of the animals, and the facial expressions of the people. A study of 90 participants over a 2-year period found that following the workshop, the ability to identify key diagnostic details from clinical photography improved by more than 10%.6 Other studies also utilizing fine art as a medical training tool to improve “visual literacy” saw similarly increased sophistication in the description of clinical imagery, which translated to better diagnostic acumen.7 Confined to video and photographs, TD necessitates trainees and practicing dermatologists to be excellent visual diagnosticians. Although surveyed dermatologists believe TD is presently appropriate for acne, benign lesions, or follow-up appointments,3 conditions for which patients have been examined via TD have included drug eruptions, premalignant or malignant neoplasms, infections, and papulosquamous or inflammatory dermatoses.8 At the very least, clinicians should be versed in identifying those conditions that require in-person evaluation, as patients cannot be held responsible to distinguish which situations can and cannot be addressed virtually.
Issues of Patient-Physician Confidentiality
Teledermatology is not without its shortcomings; critics have noted diagnostic challenges with poor quality photographs or videos, inability to perform total-body skin examinations, and socioeconomic limitations due to broadband availability and speed.5,9 Although most of these shortcomings are outside of our control, a key challenge within the purview of the provider is the protection of patient privacy.
Much of the salient concerns regarding patient-physician confidentiality involve asynchronous TD, where store-and-forward data sharing allows physicians to download patient photographs or information onto their personal email or smartphones.10 Although some hospital systems provide encryption software or hospital-sponsored devices to ensure security, physicians may opt to use their personal phones or laptops out of convenience or to save time.10,11 One study found that less than 30% of smartphone users choose to activate user authentication on their devices, even ones as simple as a passphrase.11 The digital exchange of information thus poses an immense risk for compromising protected health information (PHI), as personal devices can be easily lost, stolen, or hacked. Indeed, in 2015, more than 113 million individuals were affected by a breach of PHI, the majority over hacked network servers.12 With the growing diversity of mediums through which PHI is exchanged, such as videoconferencing and instant messaging, the potential medicolegal risks of information breach continue to climb. The US Department of Health & Human Services urges health care providers to uphold best practices for security, including encrypting data, updating all software including antivirus software, using multifactor authentication, and following local cybersecurity regulations or recommendations.13 For synchronous TD, suggested best practices include utilizing headphones during live appointments, avoiding public wireless networks, and ensuring the provider and patient both scan the room with their device’s camera before the start of the visit.14
On the Horizon of Teledermatology
What can we expect in the coming years? Increased utilization of telemedicine will translate into data that will help address questions surrounding safety, diagnostic accuracy, privacy, and accessibility. One aspect of TD in need of clarity is a guideline on payment and reimbursement, and whether TD can continue to be financially attractive to providers. Starting in 2020, the Centers for Medicare & Medicaid Services removed geographic restrictions for reimbursement of telemedicine visits, enabling even urban-residing patients to enjoy the convenience of TD. This followed a prior relaxation of restrictions, where even prerecorded patient information became eligible for Medicare reimbursement.9 However, as virtual visits tend to be shorter with fewer diagnostic services compared to in-person visits, the reimbursement structure of TD must be nuanced, which is the subject of ongoing study and modification in the wake of the COVID-19 pandemic.15
Another point to consider is the explosion of direct-to-consumer TD, which allows patients to receive virtual dermatologic care or prescription medication without a pre-established relationship with any physician. In 2017, there were 22 direct-to-consumer TD services available to US patients in 45 states, 16 (73%) of which provided dermatologic care for any concern while 6 (27%) were limited to acne or antiaging and were largely prescription oriented. Orchestrated mostly by the for-profit private sector, direct-to-consumer companies are poorly regulated and have raised concerns over questionable practices, such as the use of non–US board-certified physicians, exorbitant fees, and failure to disclose medication side effects.16 A study of 16 direct-to-consumer telemedicine sites found substantial discordance in the suggested management of the same patient, and many of the services relied heavily on patient-provided self-diagnoses, such as a case where psoriasis medication was dispensed for a psoriasis patient who submitted a photograph of his syphilitic rash.17 Despite these problems, consumers show a willingness to pay out of pocket to access these services for their shorter waiting times and convenience.18 Hence, we must learn to ask about direct-to-consumer service use when obtaining a thorough history and be open to counseling our patients on the proper use and potential risks of direct-to-consumer TD.
Final Thoughts
The telemedicine industry is expected to reach more than $130 billion by 2025, with more than 90% of surveyed health care executives planning for the adoption and incorporation of telemedicine into their business models.19 The COVID-19 pandemic was an impetus for an exponential adoption of TD, and it would behoove current residents to realize that the practice of dermatology will continue to be increasingly digitalized within the coming years. Whether through formal training or self-assessment, we must strive to grow as proficient virtual dermatologists while upholding professionalism, patient safety, and health information privacy.
The COVID-19 pandemic largely altered the practice of medicine, including a rapid expansion of telemedicine following the March 2020 World Health Organization guidelines for social distancing, which recommended suspension of all nonurgent in-person visits.1 Expectedly, COVID-related urgent care visits initially comprised the bulk of the new telemedicine wave: NYU Langone Health (New York, New York), for example, saw a 683% increase in virtual visits between March and April 2020, most (55.3%) of which were for respiratory concerns. In-person visits, on the other hand, concurrently fell by more than 80%. Interestingly, nonurgent ambulatory care specialties also saw a considerable uptick in virtual encounters, from less than 50 visits in a typical day to an average of 7000 in a 10-day stretch.2
As a largely ambulatory specialty that relies on visual examination, dermatology was no exception to the swing toward telemedicine, or teledermatology (TD). Before the COVID-19 pandemic, 14.1% (82 of 582 respondents) of practicing US dermatologists reported having used teledermatology, compared to 96.9% (572/591) during the pandemic.3 Even at my home institution (Massachusetts General Hospital [Boston, Massachusetts] and its 12 affiliated dermatology clinics), the number of in-person visits in April 2020 (n=67) was less than 1% of that in April 2019 (n=7919), whereas there was a total of 1564 virtual visits in April 2020 compared to zero the year prior. Virtual provider-to-provider consults (e-consultations) also saw an increase of more than 20%, suggesting that dermatology’s avid adoption of TD also had improved the perceived accessibility of our specialty.4
The adoption and adaptation of TD are projected to continue to grow rapidly across the globe, as digitalization has enhanced access without increasing costs, shortened wait times, and even created opportunities for primary care providers based in rural or overseas locations to learn the diagnosis and treatment of skin disease.5 Residents and fellows should be privy to the nuances of training and practicing in this digital era, as our careers inevitably will involve some facet of TD.
The Art of Medicine
Touch, a sense that perhaps ranks second to sight in dermatology, is absent in TD. In either synchronous (live-interactive, face video visits) or asynchronous (store-and-forward, where digital photographs and clinical information sent by patients or referring physicians are assessed at a later time) TD, the skin cannot be rubbed for texture, pinched for thickness, or pushed for blanching. Instead, all we have is vision. Irwin Braverman, MD, Professor Emeritus of Dermatology at Yale University (New Haven, Connecticut), alongside Jacqueline Dolev, MD, dermatologist and Yale graduate, and Linda Friedlaender, curator at the Yale Center for British Art, founded an observational skills workshop in which trainees learn to observe and describe the paintings housed in the museum, noting all memorable details: the color of the sky, the actions of the animals, and the facial expressions of the people. A study of 90 participants over a 2-year period found that following the workshop, the ability to identify key diagnostic details from clinical photography improved by more than 10%.6 Other studies also utilizing fine art as a medical training tool to improve “visual literacy” saw similarly increased sophistication in the description of clinical imagery, which translated to better diagnostic acumen.7 Confined to video and photographs, TD necessitates trainees and practicing dermatologists to be excellent visual diagnosticians. Although surveyed dermatologists believe TD is presently appropriate for acne, benign lesions, or follow-up appointments,3 conditions for which patients have been examined via TD have included drug eruptions, premalignant or malignant neoplasms, infections, and papulosquamous or inflammatory dermatoses.8 At the very least, clinicians should be versed in identifying those conditions that require in-person evaluation, as patients cannot be held responsible to distinguish which situations can and cannot be addressed virtually.
Issues of Patient-Physician Confidentiality
Teledermatology is not without its shortcomings; critics have noted diagnostic challenges with poor quality photographs or videos, inability to perform total-body skin examinations, and socioeconomic limitations due to broadband availability and speed.5,9 Although most of these shortcomings are outside of our control, a key challenge within the purview of the provider is the protection of patient privacy.
Much of the salient concerns regarding patient-physician confidentiality involve asynchronous TD, where store-and-forward data sharing allows physicians to download patient photographs or information onto their personal email or smartphones.10 Although some hospital systems provide encryption software or hospital-sponsored devices to ensure security, physicians may opt to use their personal phones or laptops out of convenience or to save time.10,11 One study found that less than 30% of smartphone users choose to activate user authentication on their devices, even ones as simple as a passphrase.11 The digital exchange of information thus poses an immense risk for compromising protected health information (PHI), as personal devices can be easily lost, stolen, or hacked. Indeed, in 2015, more than 113 million individuals were affected by a breach of PHI, the majority over hacked network servers.12 With the growing diversity of mediums through which PHI is exchanged, such as videoconferencing and instant messaging, the potential medicolegal risks of information breach continue to climb. The US Department of Health & Human Services urges health care providers to uphold best practices for security, including encrypting data, updating all software including antivirus software, using multifactor authentication, and following local cybersecurity regulations or recommendations.13 For synchronous TD, suggested best practices include utilizing headphones during live appointments, avoiding public wireless networks, and ensuring the provider and patient both scan the room with their device’s camera before the start of the visit.14
On the Horizon of Teledermatology
What can we expect in the coming years? Increased utilization of telemedicine will translate into data that will help address questions surrounding safety, diagnostic accuracy, privacy, and accessibility. One aspect of TD in need of clarity is a guideline on payment and reimbursement, and whether TD can continue to be financially attractive to providers. Starting in 2020, the Centers for Medicare & Medicaid Services removed geographic restrictions for reimbursement of telemedicine visits, enabling even urban-residing patients to enjoy the convenience of TD. This followed a prior relaxation of restrictions, where even prerecorded patient information became eligible for Medicare reimbursement.9 However, as virtual visits tend to be shorter with fewer diagnostic services compared to in-person visits, the reimbursement structure of TD must be nuanced, which is the subject of ongoing study and modification in the wake of the COVID-19 pandemic.15
Another point to consider is the explosion of direct-to-consumer TD, which allows patients to receive virtual dermatologic care or prescription medication without a pre-established relationship with any physician. In 2017, there were 22 direct-to-consumer TD services available to US patients in 45 states, 16 (73%) of which provided dermatologic care for any concern while 6 (27%) were limited to acne or antiaging and were largely prescription oriented. Orchestrated mostly by the for-profit private sector, direct-to-consumer companies are poorly regulated and have raised concerns over questionable practices, such as the use of non–US board-certified physicians, exorbitant fees, and failure to disclose medication side effects.16 A study of 16 direct-to-consumer telemedicine sites found substantial discordance in the suggested management of the same patient, and many of the services relied heavily on patient-provided self-diagnoses, such as a case where psoriasis medication was dispensed for a psoriasis patient who submitted a photograph of his syphilitic rash.17 Despite these problems, consumers show a willingness to pay out of pocket to access these services for their shorter waiting times and convenience.18 Hence, we must learn to ask about direct-to-consumer service use when obtaining a thorough history and be open to counseling our patients on the proper use and potential risks of direct-to-consumer TD.
Final Thoughts
The telemedicine industry is expected to reach more than $130 billion by 2025, with more than 90% of surveyed health care executives planning for the adoption and incorporation of telemedicine into their business models.19 The COVID-19 pandemic was an impetus for an exponential adoption of TD, and it would behoove current residents to realize that the practice of dermatology will continue to be increasingly digitalized within the coming years. Whether through formal training or self-assessment, we must strive to grow as proficient virtual dermatologists while upholding professionalism, patient safety, and health information privacy.
- Yeboah CB, Harvey N, Krishnan R, et al. The impact of COVID-19 on teledermatology: a review. Dermatol Clin. 2021;39:599-608.
- Mann DM, Chen J, Chunara R, et al. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132-1135.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Su MY, Das S. Expansion of asynchronous teledermatology during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E471-E472.
- Maddukuri S, Patel J, Lipoff JB. Teledermatology addressing disparities in health care access: a review [published online March 12, 2021]. Curr Dermatol Rep. doi:10.1007/s13671-021-00329-2
- Dolev JC, Friedlaender LK, Braverman IM. Use of fine art to enhance visual diagnostic skills. JAMA. 2001;286:1020-1021.
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med. 2008;23:991-997.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Wang RH, Barbieri JS, Nguyen HP, et al. Clinical effectiveness and cost-effectiveness of teledermatology: where are we now, and what are the barriers to adoption? J Am Acad Dermatol. 2020;83:299-307.
- Stevenson P, Finnane AR, Soyer HP. Teledermatology and clinical photography: safeguarding patient privacy and mitigating medico-legal risk. Med J Aust. 2016;204:198-200e1.
- Smith KA, Zhou L, Watzlaf VJM. User authentication in smartphones for telehealth. Int J Telerehabil. 2017;9:3-12.
- Breaches of unsecured protected health information. Health IT website. Updated July 22, 2021. Accessed January 16, 2022. https://www.healthit.gov/data/quickstats/breaches-unsecured-protected-health-information
- Jalali MS, Landman A, Gordon WJ. Telemedicine, privacy, and information security in the age of COVID-19. J Am Med Inform Assoc. 2021;28:671-672.
- Telehealth for behavioral health care: protecting patients’ privacy. United States Department of Health and Human Services website. Updated July 2, 2021. Accessed January 16, 2022. https://telehealth.hhs.gov/providers/telehealth-for-behavioral-health/preparing-patients-for-telebehavioral-health/protecting-patients-privacy/
- Shachar C, Engel J, Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020;323:2375-2376.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Snoswell CL, Whitty JA, Caffery LJ, et al. Consumer preference and willingness to pay for direct-to-consumer mobile teledermoscopy services in Australia [published online August 13, 2021]. Dermatology. doi:10.1159/000517257
- Elliott T, Yopes MC. Direct-to-consumer telemedicine. J Allergy Clin Immunol Pract. 2019;7:2546-2552.
- Yeboah CB, Harvey N, Krishnan R, et al. The impact of COVID-19 on teledermatology: a review. Dermatol Clin. 2021;39:599-608.
- Mann DM, Chen J, Chunara R, et al. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132-1135.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Su MY, Das S. Expansion of asynchronous teledermatology during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E471-E472.
- Maddukuri S, Patel J, Lipoff JB. Teledermatology addressing disparities in health care access: a review [published online March 12, 2021]. Curr Dermatol Rep. doi:10.1007/s13671-021-00329-2
- Dolev JC, Friedlaender LK, Braverman IM. Use of fine art to enhance visual diagnostic skills. JAMA. 2001;286:1020-1021.
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med. 2008;23:991-997.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Wang RH, Barbieri JS, Nguyen HP, et al. Clinical effectiveness and cost-effectiveness of teledermatology: where are we now, and what are the barriers to adoption? J Am Acad Dermatol. 2020;83:299-307.
- Stevenson P, Finnane AR, Soyer HP. Teledermatology and clinical photography: safeguarding patient privacy and mitigating medico-legal risk. Med J Aust. 2016;204:198-200e1.
- Smith KA, Zhou L, Watzlaf VJM. User authentication in smartphones for telehealth. Int J Telerehabil. 2017;9:3-12.
- Breaches of unsecured protected health information. Health IT website. Updated July 22, 2021. Accessed January 16, 2022. https://www.healthit.gov/data/quickstats/breaches-unsecured-protected-health-information
- Jalali MS, Landman A, Gordon WJ. Telemedicine, privacy, and information security in the age of COVID-19. J Am Med Inform Assoc. 2021;28:671-672.
- Telehealth for behavioral health care: protecting patients’ privacy. United States Department of Health and Human Services website. Updated July 2, 2021. Accessed January 16, 2022. https://telehealth.hhs.gov/providers/telehealth-for-behavioral-health/preparing-patients-for-telebehavioral-health/protecting-patients-privacy/
- Shachar C, Engel J, Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020;323:2375-2376.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Snoswell CL, Whitty JA, Caffery LJ, et al. Consumer preference and willingness to pay for direct-to-consumer mobile teledermoscopy services in Australia [published online August 13, 2021]. Dermatology. doi:10.1159/000517257
- Elliott T, Yopes MC. Direct-to-consumer telemedicine. J Allergy Clin Immunol Pract. 2019;7:2546-2552.
Resident Pearl
- The COVID-19 pandemic has accelerated the adoption of teledermatology, enhancing patient access to dermatologic care while also facilitating multidisciplinary discourse and providing opportunities for education and training. However, these virtual interactions require a vigilance for patient privacy and security with an added emphasis on visual diagnostics to deliver high-quality care.


