User login
COVID-19 experiences from the ob.gyn. front line
As the COVID-19 pandemic continues to spread across the United States, several members of the Ob.Gyn. News Editorial Advisory Board shared their experiences.
Catherine Cansino, MD, MPH, who is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, discussed the changes COVID-19 has had on local and regional practice in Sacramento and northern California.
There has been a dramatic increase in telehealth, using video, phone, and apps such as Zoom. Although ob.gyns. at the university are limiting outpatient appointments to essential visits only, we are continuing to offer telehealth to a few nonessential visits. This will be readdressed when the COVID-19 cases peak, Dr. Cansino said.
All patients admitted to labor & delivery undergo COVID-19 testing regardless of symptoms. For patients in the clinic who are expected to be induced or scheduled for cesarean delivery, we are screening them within 72 hours before admission.
In gynecology, only essential or urgent surgeries at UC Davis are being performed and include indications such as cancer, serious benign conditions unresponsive to conservative treatment (e.g., tubo-ovarian abscess, large symptomatic adnexal mass), and pregnancy termination. We are preserving access to abortion and reproductive health services since these are essential services.
We limit the number of providers involved in direct contact with inpatients to one or two, including a physician, nurse, and/or resident, Dr. Cansino said in an interview. Based on recent Liaison Committee on Medical Education policies related to concerns about educational experience during the pandemic, no medical students are allowed at the hospital at present. We also severely restrict the number of visitors in the inpatient and outpatient settings, including only two attendants (partner, doula, and such) during labor and delivery, and consider the impact on patients’ well-being when we restrict their visitors.
We are following University of California guidelines regarding face mask use, which have been in evolution over the last month. Face masks are used for patients and the health care providers primarily when patients either have known COVID-19 infection or are considered as patients under investigation or if the employee had a high-risk exposure. The use of face masks is becoming more permissive, rather than mandatory, to conserve personal protective equipment (PPE) for when the surge arrives.
Education is ongoing about caring for our families and ourselves if we get infected and need to isolate within our own homes. The department and health system is trying to balance the challenges of urgent patient care needs against the wellness concerns for the faculty, staff, and residents. Many physicians are also struggling with childcare problems, which add to our personal stress. There is anxiety among many physicians about exposure to asymptomatic carriers, including themselves, patients, and their families, Dr. Cansino said.
David Forstein, DO, dean and professor of obstetrics and gynecology at Touro College of Osteopathic Medicine, New York, said in an interview that the COVID-19 pandemic has “totally disrupted medical education. At almost all medical schools, didactics have moved completely online – ZOOM sessions abound, but labs become demonstrations, if at all, during the preclinical years. The clinical years have been put on hold, as well as student rotations suspended, out of caution for the students because hospitals needed to conserve PPE for the essential personnel and because administrators knew there would be less time for teaching. After initially requesting a pause, many hospitals now are asking students to come back because so many physicians, nurses, and residents have become ill with COVID-19 and either are quarantined or are patients in the hospital themselves.
“There has been a state-by-state call to consider graduating health professions students early, and press them into service, before their residencies actually begin. Some locations are looking for these new graduates to volunteer; some are willing to pay them a resident’s salary level. Medical schools are auditing their student records now to see which students would qualify to graduate early,” Dr. Forstein noted.
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology at the Einstein Health Care Network in Philadelphia, described in an email interview how COVID-19 has changed practice.
To minimize the number of providers on the front line, we have developed a Monday to Friday rotating schedule of three teams of five members, he explained. There will be a hospital-based team, an office-based team, and a telehealth-based team who will provide their services from home. On-call responsibilities remain the same.
The hospital team, working 7 a.m. to 5 p.m., will rotate through assignments each day:
- One person will cover labor and delivery.
- One person will cover triage and help on labor and delivery.
- One person will be assigned to the resident office.
- One person will be assigned to cover the team of the post call attending (Sunday through Thursday call).
- One person will be assigned to gynecology coverage, consults, and postpartum rounds.
To further minimize the patient interactions, when possible, each patient should be seen by the attending physician with the resident. This is a change from usual practice, where the patient is first seen by the resident, who reports back to the attending, and then both physicians see the patient together.
The network’s offices now open from 9 a.m. (many offices had been offering early-morning hours starting at 7 a.m.), and the physicians and advanced practice providers will work through the last scheduled patient appointment, Dr. Jaspan explained. “The office-based team will preferentially see in-person visits.”
Several offices have been closed so that ob.gyns. and staff can be reassigned to telehealth. The remaining five offices generally have one attending physician and one advanced practice provider.
The remaining team of ob.gyns. provides telehealth with the help of staff members. This involves an initial call to the patient by staff letting them know the doctor will be calling, checking them in, verifying insurance, and collecting payment, followed by the actual telehealth visit. If follow-up is needed, the staff member schedules the follow-up.
Dr. Jaspan called the new approach to prenatal care because of COVID-19 a “cataclysmic change in how we care for our patients. We have decided to further limit our obstetrical in-person visits. It is our feeling that these changes will enable patients to remain outside of the office and in the safety of their homes, provide appropriate social distancing, and diminish potential exposures to the office staff providers and patients.”
In-person visits will occur at: the initial visit, between 24 and 28 weeks, at 32 weeks, and at 36 or 37 weeks; if the patient at 36/37 has a blood pressure cuff, they will not have additional scheduled in-patient visits. We have partnered with the insurance companies to provide more than 88% of obstetrical patients with home blood pressure cuffs.
Obstetrical visits via telehealth will continue at our standard intervals: monthly until 26 weeks; twice monthly during 26-36 weeks; and weekly from 37 weeks to delivery. These visits should use a video component such as Zoom, Doxy.me, or FaceTime.
“If the patient has concerns or problems, we will see them at any time. However, the new standard will be telehealth visits and the exception will be the in-person visit,” Dr. Jaspan said.
In addition, we have worked our division of maternal-fetal medicine to adjust the antenatal testing schedules, and we have curtailed the frequency of ultrasound, he noted.
He emphasized the importance of documenting telehealth interactions with obstetrical patients, in addition to “providing adequate teaching and education for patients regarding kick counts to ensure fetal well-being.” It also is key to “properly document conversations with patients regarding bleeding, rupture of membranes, fetal movement, headache, visual changes, fevers, cough, nausea and vomiting, diarrhea, fatigue, muscle aches, etc.”
The residents’ schedule also has been modified to diminish their exposure. Within our new paradigm, we have scheduled video conferences to enable our program to maintain our commitment to academics.
It is imperative that we keep our patients safe, and it is critical to protect our staff members. Those who provide women’s health cannot be replaced by other nurses or physicians.
Mark P. Trolice, MD, is director of Fertility CARE: the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He related in an email interview that, on March 17, 2020, the American Society for Reproductive Medicine (ASRM) released “Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic.” This document serves as guidance on fertility care during the current crisis. Specifically, the recommendations include the following:
- Suspend initiation of new treatment cycles, including ovulation induction, intrauterine inseminations, in vitro fertilization including retrievals and frozen embryo transfers, and nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
- Minimize in-person interactions and increase utilization of telehealth.
As a member of ASRM for more than 2 decades and a participant of several of their committees, my practice immediately ceased treatment cycles to comply with this guidance.
Then on March 20, 2020, the Florida governor’s executive order 20-72 was released, stating, “All hospitals, ambulatory surgical centers, office surgery centers, dental, orthodontic and endodontic offices, and other health care practitioners’ offices in the State of Florida are prohibited from providing any medically unnecessary, nonurgent or nonemergency procedure or surgery which, if delayed, does not place a patient’s immediate health, safety, or well-being at risk, or will, if delayed, not contribute to the worsening of a serious or life-threatening medical condition.”
As a result, my practice has been limited to telemedicine consultations. While the ASRM guidance and the gubernatorial executive order pose a significant financial hardship on my center and all applicable medical clinics in my state, resulting in expected layoffs, salary reductions, and requests for government stimulus loans, the greater good takes priority and we pray for all the victims of this devastating pandemic.
The governor’s current executive order is set to expire on May 9, 2020, unless it is extended.
ASRM released an update of their guidance on March 30, 2020, offering no change from their prior recommendations. The organization plans to reevaluate the guidance at 2-week intervals.
Sangeeta Sinha, MD, an ob.gyn. in private practice at Stone Springs Hospital Center, Dulles, Va. said in an interview, “COVID 19 has put fear in all aspects of our daily activities which we are attempting to cope with.”
She related several changes made to her office and hospital environments. “In our office, we are now wearing a mask at all times, gloves to examine every patient. We have staggered physicians in the office to take televisits and in-office patients. We are screening all new patients on the phone to determine if they are sick, have traveled to high-risk, hot spot areas of the country, or have had contact with someone who tested positive for COVID-19. We are only seeing our pregnant women and have also pushed out their return appointments to 4 weeks if possible. There are several staff who are not working due to fear or are in self quarantine so we have shortage of staff in the office. At the hospital as well we are wearing a mask at all times, using personal protective equipment for deliveries and C-sections.
“We have had several scares, including a new transfer of an 18-year-old pregnant patient at 30 weeks with cough and sore throat, who later reported that her roommate is very sick and he works with someone who has tested positive for COVID-19. Thankfully she is healthy and well. We learned several lessons from this one.”
As the COVID-19 pandemic continues to spread across the United States, several members of the Ob.Gyn. News Editorial Advisory Board shared their experiences.
Catherine Cansino, MD, MPH, who is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, discussed the changes COVID-19 has had on local and regional practice in Sacramento and northern California.
There has been a dramatic increase in telehealth, using video, phone, and apps such as Zoom. Although ob.gyns. at the university are limiting outpatient appointments to essential visits only, we are continuing to offer telehealth to a few nonessential visits. This will be readdressed when the COVID-19 cases peak, Dr. Cansino said.
All patients admitted to labor & delivery undergo COVID-19 testing regardless of symptoms. For patients in the clinic who are expected to be induced or scheduled for cesarean delivery, we are screening them within 72 hours before admission.
In gynecology, only essential or urgent surgeries at UC Davis are being performed and include indications such as cancer, serious benign conditions unresponsive to conservative treatment (e.g., tubo-ovarian abscess, large symptomatic adnexal mass), and pregnancy termination. We are preserving access to abortion and reproductive health services since these are essential services.
We limit the number of providers involved in direct contact with inpatients to one or two, including a physician, nurse, and/or resident, Dr. Cansino said in an interview. Based on recent Liaison Committee on Medical Education policies related to concerns about educational experience during the pandemic, no medical students are allowed at the hospital at present. We also severely restrict the number of visitors in the inpatient and outpatient settings, including only two attendants (partner, doula, and such) during labor and delivery, and consider the impact on patients’ well-being when we restrict their visitors.
We are following University of California guidelines regarding face mask use, which have been in evolution over the last month. Face masks are used for patients and the health care providers primarily when patients either have known COVID-19 infection or are considered as patients under investigation or if the employee had a high-risk exposure. The use of face masks is becoming more permissive, rather than mandatory, to conserve personal protective equipment (PPE) for when the surge arrives.
Education is ongoing about caring for our families and ourselves if we get infected and need to isolate within our own homes. The department and health system is trying to balance the challenges of urgent patient care needs against the wellness concerns for the faculty, staff, and residents. Many physicians are also struggling with childcare problems, which add to our personal stress. There is anxiety among many physicians about exposure to asymptomatic carriers, including themselves, patients, and their families, Dr. Cansino said.
David Forstein, DO, dean and professor of obstetrics and gynecology at Touro College of Osteopathic Medicine, New York, said in an interview that the COVID-19 pandemic has “totally disrupted medical education. At almost all medical schools, didactics have moved completely online – ZOOM sessions abound, but labs become demonstrations, if at all, during the preclinical years. The clinical years have been put on hold, as well as student rotations suspended, out of caution for the students because hospitals needed to conserve PPE for the essential personnel and because administrators knew there would be less time for teaching. After initially requesting a pause, many hospitals now are asking students to come back because so many physicians, nurses, and residents have become ill with COVID-19 and either are quarantined or are patients in the hospital themselves.
“There has been a state-by-state call to consider graduating health professions students early, and press them into service, before their residencies actually begin. Some locations are looking for these new graduates to volunteer; some are willing to pay them a resident’s salary level. Medical schools are auditing their student records now to see which students would qualify to graduate early,” Dr. Forstein noted.
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology at the Einstein Health Care Network in Philadelphia, described in an email interview how COVID-19 has changed practice.
To minimize the number of providers on the front line, we have developed a Monday to Friday rotating schedule of three teams of five members, he explained. There will be a hospital-based team, an office-based team, and a telehealth-based team who will provide their services from home. On-call responsibilities remain the same.
The hospital team, working 7 a.m. to 5 p.m., will rotate through assignments each day:
- One person will cover labor and delivery.
- One person will cover triage and help on labor and delivery.
- One person will be assigned to the resident office.
- One person will be assigned to cover the team of the post call attending (Sunday through Thursday call).
- One person will be assigned to gynecology coverage, consults, and postpartum rounds.
To further minimize the patient interactions, when possible, each patient should be seen by the attending physician with the resident. This is a change from usual practice, where the patient is first seen by the resident, who reports back to the attending, and then both physicians see the patient together.
The network’s offices now open from 9 a.m. (many offices had been offering early-morning hours starting at 7 a.m.), and the physicians and advanced practice providers will work through the last scheduled patient appointment, Dr. Jaspan explained. “The office-based team will preferentially see in-person visits.”
Several offices have been closed so that ob.gyns. and staff can be reassigned to telehealth. The remaining five offices generally have one attending physician and one advanced practice provider.
The remaining team of ob.gyns. provides telehealth with the help of staff members. This involves an initial call to the patient by staff letting them know the doctor will be calling, checking them in, verifying insurance, and collecting payment, followed by the actual telehealth visit. If follow-up is needed, the staff member schedules the follow-up.
Dr. Jaspan called the new approach to prenatal care because of COVID-19 a “cataclysmic change in how we care for our patients. We have decided to further limit our obstetrical in-person visits. It is our feeling that these changes will enable patients to remain outside of the office and in the safety of their homes, provide appropriate social distancing, and diminish potential exposures to the office staff providers and patients.”
In-person visits will occur at: the initial visit, between 24 and 28 weeks, at 32 weeks, and at 36 or 37 weeks; if the patient at 36/37 has a blood pressure cuff, they will not have additional scheduled in-patient visits. We have partnered with the insurance companies to provide more than 88% of obstetrical patients with home blood pressure cuffs.
Obstetrical visits via telehealth will continue at our standard intervals: monthly until 26 weeks; twice monthly during 26-36 weeks; and weekly from 37 weeks to delivery. These visits should use a video component such as Zoom, Doxy.me, or FaceTime.
“If the patient has concerns or problems, we will see them at any time. However, the new standard will be telehealth visits and the exception will be the in-person visit,” Dr. Jaspan said.
In addition, we have worked our division of maternal-fetal medicine to adjust the antenatal testing schedules, and we have curtailed the frequency of ultrasound, he noted.
He emphasized the importance of documenting telehealth interactions with obstetrical patients, in addition to “providing adequate teaching and education for patients regarding kick counts to ensure fetal well-being.” It also is key to “properly document conversations with patients regarding bleeding, rupture of membranes, fetal movement, headache, visual changes, fevers, cough, nausea and vomiting, diarrhea, fatigue, muscle aches, etc.”
The residents’ schedule also has been modified to diminish their exposure. Within our new paradigm, we have scheduled video conferences to enable our program to maintain our commitment to academics.
It is imperative that we keep our patients safe, and it is critical to protect our staff members. Those who provide women’s health cannot be replaced by other nurses or physicians.
Mark P. Trolice, MD, is director of Fertility CARE: the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He related in an email interview that, on March 17, 2020, the American Society for Reproductive Medicine (ASRM) released “Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic.” This document serves as guidance on fertility care during the current crisis. Specifically, the recommendations include the following:
- Suspend initiation of new treatment cycles, including ovulation induction, intrauterine inseminations, in vitro fertilization including retrievals and frozen embryo transfers, and nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
- Minimize in-person interactions and increase utilization of telehealth.
As a member of ASRM for more than 2 decades and a participant of several of their committees, my practice immediately ceased treatment cycles to comply with this guidance.
Then on March 20, 2020, the Florida governor’s executive order 20-72 was released, stating, “All hospitals, ambulatory surgical centers, office surgery centers, dental, orthodontic and endodontic offices, and other health care practitioners’ offices in the State of Florida are prohibited from providing any medically unnecessary, nonurgent or nonemergency procedure or surgery which, if delayed, does not place a patient’s immediate health, safety, or well-being at risk, or will, if delayed, not contribute to the worsening of a serious or life-threatening medical condition.”
As a result, my practice has been limited to telemedicine consultations. While the ASRM guidance and the gubernatorial executive order pose a significant financial hardship on my center and all applicable medical clinics in my state, resulting in expected layoffs, salary reductions, and requests for government stimulus loans, the greater good takes priority and we pray for all the victims of this devastating pandemic.
The governor’s current executive order is set to expire on May 9, 2020, unless it is extended.
ASRM released an update of their guidance on March 30, 2020, offering no change from their prior recommendations. The organization plans to reevaluate the guidance at 2-week intervals.
Sangeeta Sinha, MD, an ob.gyn. in private practice at Stone Springs Hospital Center, Dulles, Va. said in an interview, “COVID 19 has put fear in all aspects of our daily activities which we are attempting to cope with.”
She related several changes made to her office and hospital environments. “In our office, we are now wearing a mask at all times, gloves to examine every patient. We have staggered physicians in the office to take televisits and in-office patients. We are screening all new patients on the phone to determine if they are sick, have traveled to high-risk, hot spot areas of the country, or have had contact with someone who tested positive for COVID-19. We are only seeing our pregnant women and have also pushed out their return appointments to 4 weeks if possible. There are several staff who are not working due to fear or are in self quarantine so we have shortage of staff in the office. At the hospital as well we are wearing a mask at all times, using personal protective equipment for deliveries and C-sections.
“We have had several scares, including a new transfer of an 18-year-old pregnant patient at 30 weeks with cough and sore throat, who later reported that her roommate is very sick and he works with someone who has tested positive for COVID-19. Thankfully she is healthy and well. We learned several lessons from this one.”
As the COVID-19 pandemic continues to spread across the United States, several members of the Ob.Gyn. News Editorial Advisory Board shared their experiences.
Catherine Cansino, MD, MPH, who is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, discussed the changes COVID-19 has had on local and regional practice in Sacramento and northern California.
There has been a dramatic increase in telehealth, using video, phone, and apps such as Zoom. Although ob.gyns. at the university are limiting outpatient appointments to essential visits only, we are continuing to offer telehealth to a few nonessential visits. This will be readdressed when the COVID-19 cases peak, Dr. Cansino said.
All patients admitted to labor & delivery undergo COVID-19 testing regardless of symptoms. For patients in the clinic who are expected to be induced or scheduled for cesarean delivery, we are screening them within 72 hours before admission.
In gynecology, only essential or urgent surgeries at UC Davis are being performed and include indications such as cancer, serious benign conditions unresponsive to conservative treatment (e.g., tubo-ovarian abscess, large symptomatic adnexal mass), and pregnancy termination. We are preserving access to abortion and reproductive health services since these are essential services.
We limit the number of providers involved in direct contact with inpatients to one or two, including a physician, nurse, and/or resident, Dr. Cansino said in an interview. Based on recent Liaison Committee on Medical Education policies related to concerns about educational experience during the pandemic, no medical students are allowed at the hospital at present. We also severely restrict the number of visitors in the inpatient and outpatient settings, including only two attendants (partner, doula, and such) during labor and delivery, and consider the impact on patients’ well-being when we restrict their visitors.
We are following University of California guidelines regarding face mask use, which have been in evolution over the last month. Face masks are used for patients and the health care providers primarily when patients either have known COVID-19 infection or are considered as patients under investigation or if the employee had a high-risk exposure. The use of face masks is becoming more permissive, rather than mandatory, to conserve personal protective equipment (PPE) for when the surge arrives.
Education is ongoing about caring for our families and ourselves if we get infected and need to isolate within our own homes. The department and health system is trying to balance the challenges of urgent patient care needs against the wellness concerns for the faculty, staff, and residents. Many physicians are also struggling with childcare problems, which add to our personal stress. There is anxiety among many physicians about exposure to asymptomatic carriers, including themselves, patients, and their families, Dr. Cansino said.
David Forstein, DO, dean and professor of obstetrics and gynecology at Touro College of Osteopathic Medicine, New York, said in an interview that the COVID-19 pandemic has “totally disrupted medical education. At almost all medical schools, didactics have moved completely online – ZOOM sessions abound, but labs become demonstrations, if at all, during the preclinical years. The clinical years have been put on hold, as well as student rotations suspended, out of caution for the students because hospitals needed to conserve PPE for the essential personnel and because administrators knew there would be less time for teaching. After initially requesting a pause, many hospitals now are asking students to come back because so many physicians, nurses, and residents have become ill with COVID-19 and either are quarantined or are patients in the hospital themselves.
“There has been a state-by-state call to consider graduating health professions students early, and press them into service, before their residencies actually begin. Some locations are looking for these new graduates to volunteer; some are willing to pay them a resident’s salary level. Medical schools are auditing their student records now to see which students would qualify to graduate early,” Dr. Forstein noted.
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology at the Einstein Health Care Network in Philadelphia, described in an email interview how COVID-19 has changed practice.
To minimize the number of providers on the front line, we have developed a Monday to Friday rotating schedule of three teams of five members, he explained. There will be a hospital-based team, an office-based team, and a telehealth-based team who will provide their services from home. On-call responsibilities remain the same.
The hospital team, working 7 a.m. to 5 p.m., will rotate through assignments each day:
- One person will cover labor and delivery.
- One person will cover triage and help on labor and delivery.
- One person will be assigned to the resident office.
- One person will be assigned to cover the team of the post call attending (Sunday through Thursday call).
- One person will be assigned to gynecology coverage, consults, and postpartum rounds.
To further minimize the patient interactions, when possible, each patient should be seen by the attending physician with the resident. This is a change from usual practice, where the patient is first seen by the resident, who reports back to the attending, and then both physicians see the patient together.
The network’s offices now open from 9 a.m. (many offices had been offering early-morning hours starting at 7 a.m.), and the physicians and advanced practice providers will work through the last scheduled patient appointment, Dr. Jaspan explained. “The office-based team will preferentially see in-person visits.”
Several offices have been closed so that ob.gyns. and staff can be reassigned to telehealth. The remaining five offices generally have one attending physician and one advanced practice provider.
The remaining team of ob.gyns. provides telehealth with the help of staff members. This involves an initial call to the patient by staff letting them know the doctor will be calling, checking them in, verifying insurance, and collecting payment, followed by the actual telehealth visit. If follow-up is needed, the staff member schedules the follow-up.
Dr. Jaspan called the new approach to prenatal care because of COVID-19 a “cataclysmic change in how we care for our patients. We have decided to further limit our obstetrical in-person visits. It is our feeling that these changes will enable patients to remain outside of the office and in the safety of their homes, provide appropriate social distancing, and diminish potential exposures to the office staff providers and patients.”
In-person visits will occur at: the initial visit, between 24 and 28 weeks, at 32 weeks, and at 36 or 37 weeks; if the patient at 36/37 has a blood pressure cuff, they will not have additional scheduled in-patient visits. We have partnered with the insurance companies to provide more than 88% of obstetrical patients with home blood pressure cuffs.
Obstetrical visits via telehealth will continue at our standard intervals: monthly until 26 weeks; twice monthly during 26-36 weeks; and weekly from 37 weeks to delivery. These visits should use a video component such as Zoom, Doxy.me, or FaceTime.
“If the patient has concerns or problems, we will see them at any time. However, the new standard will be telehealth visits and the exception will be the in-person visit,” Dr. Jaspan said.
In addition, we have worked our division of maternal-fetal medicine to adjust the antenatal testing schedules, and we have curtailed the frequency of ultrasound, he noted.
He emphasized the importance of documenting telehealth interactions with obstetrical patients, in addition to “providing adequate teaching and education for patients regarding kick counts to ensure fetal well-being.” It also is key to “properly document conversations with patients regarding bleeding, rupture of membranes, fetal movement, headache, visual changes, fevers, cough, nausea and vomiting, diarrhea, fatigue, muscle aches, etc.”
The residents’ schedule also has been modified to diminish their exposure. Within our new paradigm, we have scheduled video conferences to enable our program to maintain our commitment to academics.
It is imperative that we keep our patients safe, and it is critical to protect our staff members. Those who provide women’s health cannot be replaced by other nurses or physicians.
Mark P. Trolice, MD, is director of Fertility CARE: the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He related in an email interview that, on March 17, 2020, the American Society for Reproductive Medicine (ASRM) released “Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic.” This document serves as guidance on fertility care during the current crisis. Specifically, the recommendations include the following:
- Suspend initiation of new treatment cycles, including ovulation induction, intrauterine inseminations, in vitro fertilization including retrievals and frozen embryo transfers, and nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
- Minimize in-person interactions and increase utilization of telehealth.
As a member of ASRM for more than 2 decades and a participant of several of their committees, my practice immediately ceased treatment cycles to comply with this guidance.
Then on March 20, 2020, the Florida governor’s executive order 20-72 was released, stating, “All hospitals, ambulatory surgical centers, office surgery centers, dental, orthodontic and endodontic offices, and other health care practitioners’ offices in the State of Florida are prohibited from providing any medically unnecessary, nonurgent or nonemergency procedure or surgery which, if delayed, does not place a patient’s immediate health, safety, or well-being at risk, or will, if delayed, not contribute to the worsening of a serious or life-threatening medical condition.”
As a result, my practice has been limited to telemedicine consultations. While the ASRM guidance and the gubernatorial executive order pose a significant financial hardship on my center and all applicable medical clinics in my state, resulting in expected layoffs, salary reductions, and requests for government stimulus loans, the greater good takes priority and we pray for all the victims of this devastating pandemic.
The governor’s current executive order is set to expire on May 9, 2020, unless it is extended.
ASRM released an update of their guidance on March 30, 2020, offering no change from their prior recommendations. The organization plans to reevaluate the guidance at 2-week intervals.
Sangeeta Sinha, MD, an ob.gyn. in private practice at Stone Springs Hospital Center, Dulles, Va. said in an interview, “COVID 19 has put fear in all aspects of our daily activities which we are attempting to cope with.”
She related several changes made to her office and hospital environments. “In our office, we are now wearing a mask at all times, gloves to examine every patient. We have staggered physicians in the office to take televisits and in-office patients. We are screening all new patients on the phone to determine if they are sick, have traveled to high-risk, hot spot areas of the country, or have had contact with someone who tested positive for COVID-19. We are only seeing our pregnant women and have also pushed out their return appointments to 4 weeks if possible. There are several staff who are not working due to fear or are in self quarantine so we have shortage of staff in the office. At the hospital as well we are wearing a mask at all times, using personal protective equipment for deliveries and C-sections.
“We have had several scares, including a new transfer of an 18-year-old pregnant patient at 30 weeks with cough and sore throat, who later reported that her roommate is very sick and he works with someone who has tested positive for COVID-19. Thankfully she is healthy and well. We learned several lessons from this one.”
Patients with preexisting diabetes benefit less from bariatric surgery
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
FROM ENDO 2020
Maintaining cancer care in the face of COVID-19
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Flu activity measures continue COVID-19–related divergence
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
Use of an Electronic Alert Tool to Prevent Readmissions Following Coronary Artery Bypass Graft Surgery
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
Factors Associated With Lower-Extremity Amputation in Patients With Diabetic Foot Ulcers
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1). 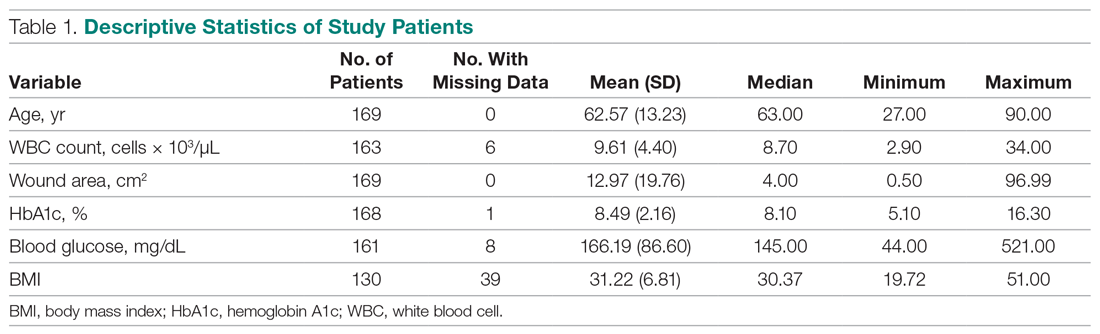
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.
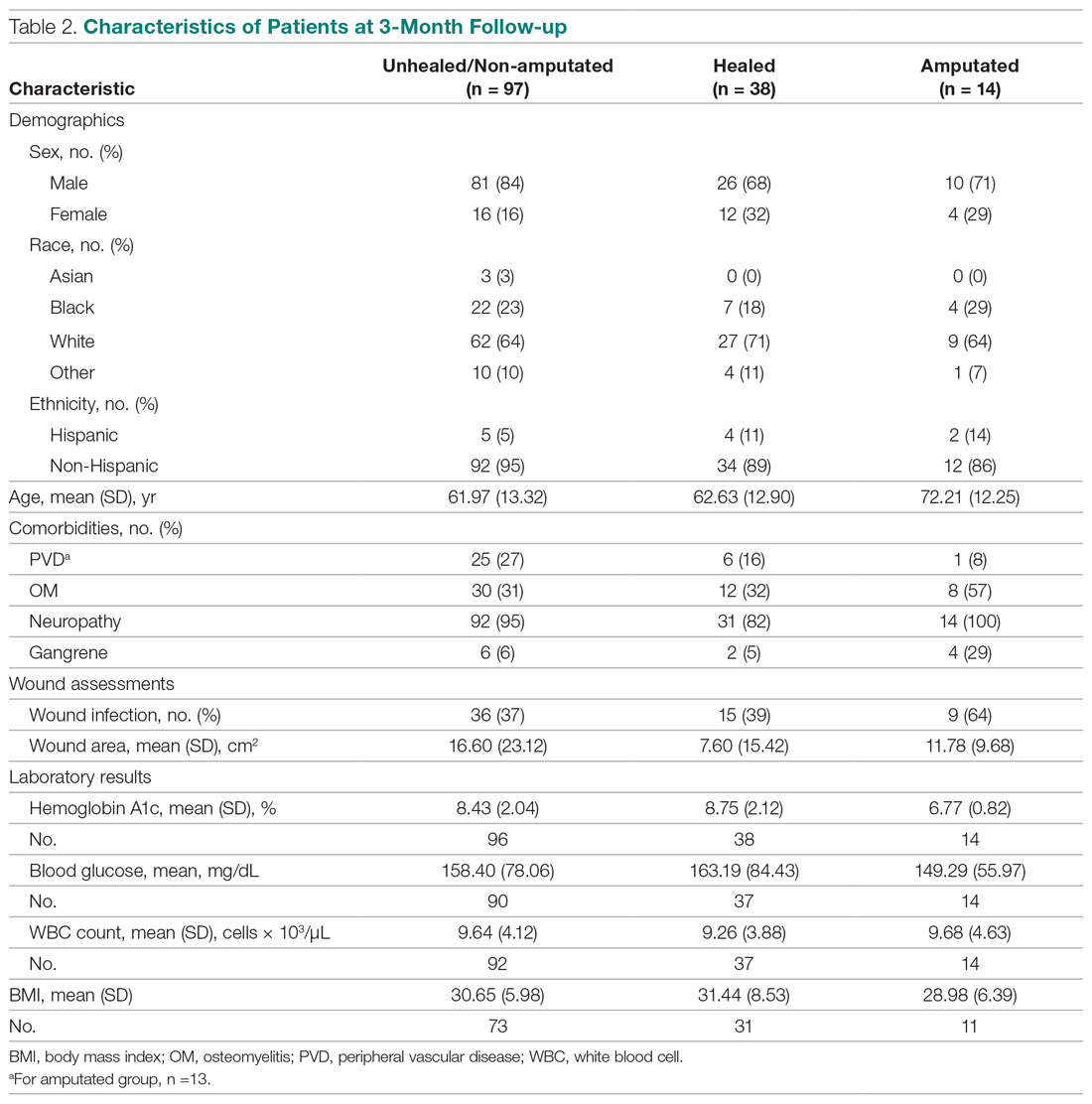
The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).
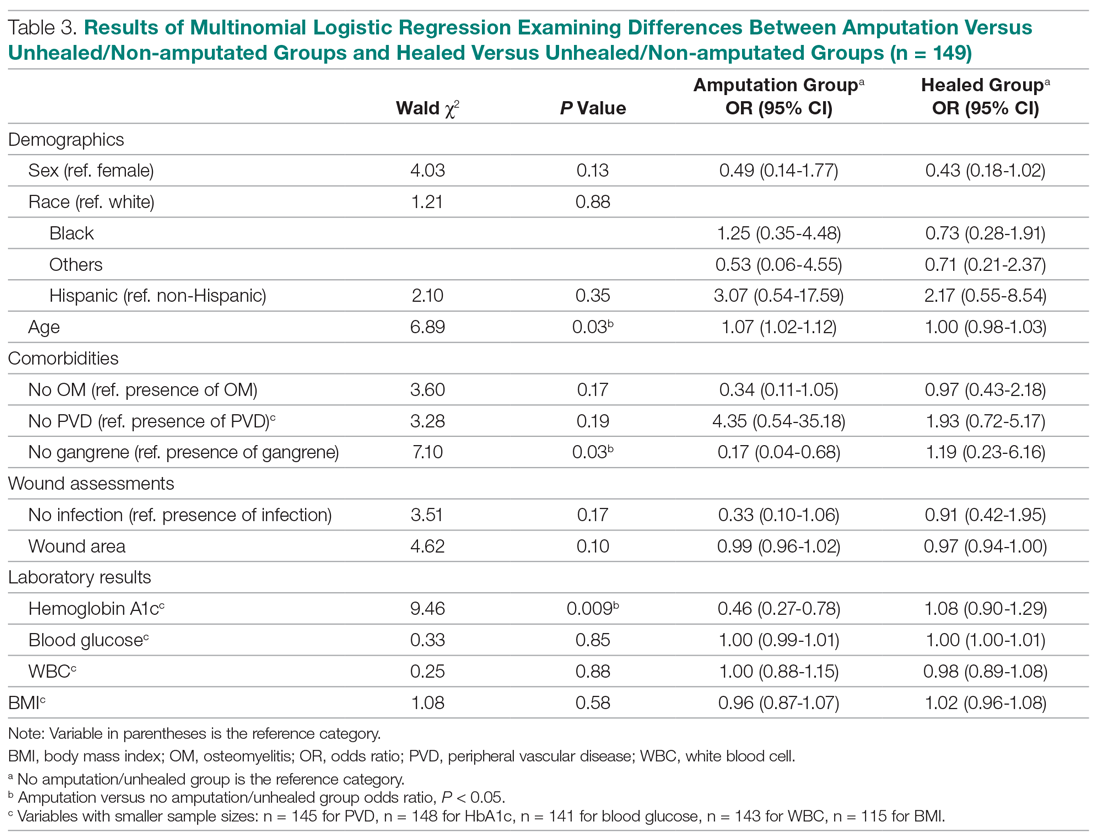
The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; [email protected].
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1). 
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.

The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).

The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; [email protected].
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1). 
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.

The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).

The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; [email protected].
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
Outcomes-based measurement of TAVR program quality goes live
The long-sought goal of measuring the quality of U.S. transcatheter aortic valve replacement (TAVR) programs by patient outcomes rather than by the surrogate measure of case volume is about to be realized.
Starting more or less immediately, the U.S. national register for all TAVR cases that’s mandated by Food and Drug Administration labeling of these devices and run through a collaboration of the American College of Cardiology and the Society of Thoracic Surgeons will start applying a newly developed and validated five-item metric for measuring 30-day patient outcomes and designed to gauge the quality of TAVR programs.
At first, the only recipients of the data will be the programs themselves, but starting in about a year, by sometime in 2021, the STS/ACC TVT (transcatheter valve therapy) Registry will start to make its star-based rating of TAVR programs available to the public, Nimesh D. Desai, MD, said on March 29 at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. These societies already make star-based ratings of U.S. programs available to the public for several other types of cardiac interventions, including coronary artery bypass surgery and MI management.
The new, composite metric based on 30-day outcome data “is appropriate for high-stakes applications such as public reporting,” said Dr. Desai, a thoracic surgeon and director of thoracic aortic surgery research at the University of Pennsylvania in Philadelphia. “We’re confident this model can be used for public reporting. It’s undergone extensive testing of its validity.” The steering committee of the STS/ACC TVT Registry commissioned development of the metric, and it’s now “considered approved,” and ready for use, he explained.
To create the new metric, Dr. Desai and his associates used data from 52,561 patients who underwent transfemoral TAVR during 2015-2017 at any of 301 U.S. sites. These data came from a total pool of more than 114,000 patients at 556 sites, but data from many sites weren’t usable because they were not adequately complete. The researchers then identified the top four measures taken during the 30 days following intervention (hospitalization included) that best correlated with 1-year survival and patients’ quality-of-life scores on the Kansas City Cardiomyopathy Questionnaire: stroke; major, life-threatening, or disabling bleed; acute kidney injury (stage III); and moderate or severe paravalvular leak. These outcomes “matter most to patients,” Dr. Desai said.
They used these four outcomes plus 30-day mortality to calculate the programs’ ratings. Among the 52,561 patients, 14% had at least one of these adverse outcomes. The researchers then used a logistic regression model that adjusted for 46 measured variables to calculate how each program performed relative to the average performance of all the programs. Programs with outcomes that fell within the 95% confidence intervals of average performance were rated as performing as expected; those outside rated as performing either better or worse than expected. The results showed 34 centers (11%) had worse than expected outcomes and 25 (8%) had better than expected outcomes, Dr. Desai said.
“This is a major step forward in measuring TAVR quality,” commented Michael Mack, MD, a cardiac surgeon with Baylor Scott & White Health in Dallas who has been very active in studying TAVR. “Until now, we used volume as a surrogate for quality, but the precision was not great. This is an extremely welcome metric.” The next step is to eventually use 1-year follow-up data instead of 30-day outcomes, he added.
“With the rapid expansion of TAVR over the past 6-8 years, we’re now at the point to start to do this. It’s an ethical obligation This will be one of the most high-fidelity, valid models for public reporting” of clinical outcomes,” said Joseph Cleveland, MD, a professor of surgery at the University of Colorado at Denver in Aurora. “It’s reassuring that about 90% of the program performed as expected or better than expected,” he added.
“Transparency and outcomes should drive how TAVR is delivered,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medicine Heart Institute in Phoenix who estimated that he performs about 150 TAVR procedures annually. “This is a step forward. I’ve been waiting for this for a long time. Until now, volume has been used as a surrogate outcome, but we know it’s not accurate. I’m confident that this model is a good starting point.” But Dr. Pershad also had concern that this new approach “can lend itself to some degree of gaming,” like a bleeding event getting classified as minor when it was really major, or outlier patients getting dropped from reports.
The temptation to cut corners may be higher for TAVR than it’s been for the cardiac-disease metrics that already get publicly reported, like bypass surgery and myocardial infarction management, because of TAVR’s higher cost and higher profile, Dr. Pershad said. Existing measures “have not been as linked to financial disincentive as TAVR might be” because TAVR reimbursements can run as high as $50,000 per case. “The stakes with TAVR are higher,” he said.
Ultimately, the reliable examination of TAVR outcomes that this new metric allows may lead to a shake-up of TAVR programs, Dr. Pershad predicted. “This is clearly a step toward closing down some programs that [consistently] underperform.”
The STS/ACC TVT Registry receives no commercial funding. Dr. Desai has been a consultant to, speaker on behalf of, and received research funding from Gore, and he has also spoken on behalf of Cook, Medtronic, and Terumo Aortic. Dr. Cleveland, Dr. Mack, and Dr. Pershad had no disclosures.
The long-sought goal of measuring the quality of U.S. transcatheter aortic valve replacement (TAVR) programs by patient outcomes rather than by the surrogate measure of case volume is about to be realized.
Starting more or less immediately, the U.S. national register for all TAVR cases that’s mandated by Food and Drug Administration labeling of these devices and run through a collaboration of the American College of Cardiology and the Society of Thoracic Surgeons will start applying a newly developed and validated five-item metric for measuring 30-day patient outcomes and designed to gauge the quality of TAVR programs.
At first, the only recipients of the data will be the programs themselves, but starting in about a year, by sometime in 2021, the STS/ACC TVT (transcatheter valve therapy) Registry will start to make its star-based rating of TAVR programs available to the public, Nimesh D. Desai, MD, said on March 29 at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. These societies already make star-based ratings of U.S. programs available to the public for several other types of cardiac interventions, including coronary artery bypass surgery and MI management.
The new, composite metric based on 30-day outcome data “is appropriate for high-stakes applications such as public reporting,” said Dr. Desai, a thoracic surgeon and director of thoracic aortic surgery research at the University of Pennsylvania in Philadelphia. “We’re confident this model can be used for public reporting. It’s undergone extensive testing of its validity.” The steering committee of the STS/ACC TVT Registry commissioned development of the metric, and it’s now “considered approved,” and ready for use, he explained.
To create the new metric, Dr. Desai and his associates used data from 52,561 patients who underwent transfemoral TAVR during 2015-2017 at any of 301 U.S. sites. These data came from a total pool of more than 114,000 patients at 556 sites, but data from many sites weren’t usable because they were not adequately complete. The researchers then identified the top four measures taken during the 30 days following intervention (hospitalization included) that best correlated with 1-year survival and patients’ quality-of-life scores on the Kansas City Cardiomyopathy Questionnaire: stroke; major, life-threatening, or disabling bleed; acute kidney injury (stage III); and moderate or severe paravalvular leak. These outcomes “matter most to patients,” Dr. Desai said.
They used these four outcomes plus 30-day mortality to calculate the programs’ ratings. Among the 52,561 patients, 14% had at least one of these adverse outcomes. The researchers then used a logistic regression model that adjusted for 46 measured variables to calculate how each program performed relative to the average performance of all the programs. Programs with outcomes that fell within the 95% confidence intervals of average performance were rated as performing as expected; those outside rated as performing either better or worse than expected. The results showed 34 centers (11%) had worse than expected outcomes and 25 (8%) had better than expected outcomes, Dr. Desai said.
“This is a major step forward in measuring TAVR quality,” commented Michael Mack, MD, a cardiac surgeon with Baylor Scott & White Health in Dallas who has been very active in studying TAVR. “Until now, we used volume as a surrogate for quality, but the precision was not great. This is an extremely welcome metric.” The next step is to eventually use 1-year follow-up data instead of 30-day outcomes, he added.
“With the rapid expansion of TAVR over the past 6-8 years, we’re now at the point to start to do this. It’s an ethical obligation This will be one of the most high-fidelity, valid models for public reporting” of clinical outcomes,” said Joseph Cleveland, MD, a professor of surgery at the University of Colorado at Denver in Aurora. “It’s reassuring that about 90% of the program performed as expected or better than expected,” he added.
“Transparency and outcomes should drive how TAVR is delivered,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medicine Heart Institute in Phoenix who estimated that he performs about 150 TAVR procedures annually. “This is a step forward. I’ve been waiting for this for a long time. Until now, volume has been used as a surrogate outcome, but we know it’s not accurate. I’m confident that this model is a good starting point.” But Dr. Pershad also had concern that this new approach “can lend itself to some degree of gaming,” like a bleeding event getting classified as minor when it was really major, or outlier patients getting dropped from reports.
The temptation to cut corners may be higher for TAVR than it’s been for the cardiac-disease metrics that already get publicly reported, like bypass surgery and myocardial infarction management, because of TAVR’s higher cost and higher profile, Dr. Pershad said. Existing measures “have not been as linked to financial disincentive as TAVR might be” because TAVR reimbursements can run as high as $50,000 per case. “The stakes with TAVR are higher,” he said.
Ultimately, the reliable examination of TAVR outcomes that this new metric allows may lead to a shake-up of TAVR programs, Dr. Pershad predicted. “This is clearly a step toward closing down some programs that [consistently] underperform.”
The STS/ACC TVT Registry receives no commercial funding. Dr. Desai has been a consultant to, speaker on behalf of, and received research funding from Gore, and he has also spoken on behalf of Cook, Medtronic, and Terumo Aortic. Dr. Cleveland, Dr. Mack, and Dr. Pershad had no disclosures.
The long-sought goal of measuring the quality of U.S. transcatheter aortic valve replacement (TAVR) programs by patient outcomes rather than by the surrogate measure of case volume is about to be realized.
Starting more or less immediately, the U.S. national register for all TAVR cases that’s mandated by Food and Drug Administration labeling of these devices and run through a collaboration of the American College of Cardiology and the Society of Thoracic Surgeons will start applying a newly developed and validated five-item metric for measuring 30-day patient outcomes and designed to gauge the quality of TAVR programs.
At first, the only recipients of the data will be the programs themselves, but starting in about a year, by sometime in 2021, the STS/ACC TVT (transcatheter valve therapy) Registry will start to make its star-based rating of TAVR programs available to the public, Nimesh D. Desai, MD, said on March 29 at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. These societies already make star-based ratings of U.S. programs available to the public for several other types of cardiac interventions, including coronary artery bypass surgery and MI management.
The new, composite metric based on 30-day outcome data “is appropriate for high-stakes applications such as public reporting,” said Dr. Desai, a thoracic surgeon and director of thoracic aortic surgery research at the University of Pennsylvania in Philadelphia. “We’re confident this model can be used for public reporting. It’s undergone extensive testing of its validity.” The steering committee of the STS/ACC TVT Registry commissioned development of the metric, and it’s now “considered approved,” and ready for use, he explained.
To create the new metric, Dr. Desai and his associates used data from 52,561 patients who underwent transfemoral TAVR during 2015-2017 at any of 301 U.S. sites. These data came from a total pool of more than 114,000 patients at 556 sites, but data from many sites weren’t usable because they were not adequately complete. The researchers then identified the top four measures taken during the 30 days following intervention (hospitalization included) that best correlated with 1-year survival and patients’ quality-of-life scores on the Kansas City Cardiomyopathy Questionnaire: stroke; major, life-threatening, or disabling bleed; acute kidney injury (stage III); and moderate or severe paravalvular leak. These outcomes “matter most to patients,” Dr. Desai said.
They used these four outcomes plus 30-day mortality to calculate the programs’ ratings. Among the 52,561 patients, 14% had at least one of these adverse outcomes. The researchers then used a logistic regression model that adjusted for 46 measured variables to calculate how each program performed relative to the average performance of all the programs. Programs with outcomes that fell within the 95% confidence intervals of average performance were rated as performing as expected; those outside rated as performing either better or worse than expected. The results showed 34 centers (11%) had worse than expected outcomes and 25 (8%) had better than expected outcomes, Dr. Desai said.
“This is a major step forward in measuring TAVR quality,” commented Michael Mack, MD, a cardiac surgeon with Baylor Scott & White Health in Dallas who has been very active in studying TAVR. “Until now, we used volume as a surrogate for quality, but the precision was not great. This is an extremely welcome metric.” The next step is to eventually use 1-year follow-up data instead of 30-day outcomes, he added.
“With the rapid expansion of TAVR over the past 6-8 years, we’re now at the point to start to do this. It’s an ethical obligation This will be one of the most high-fidelity, valid models for public reporting” of clinical outcomes,” said Joseph Cleveland, MD, a professor of surgery at the University of Colorado at Denver in Aurora. “It’s reassuring that about 90% of the program performed as expected or better than expected,” he added.
“Transparency and outcomes should drive how TAVR is delivered,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medicine Heart Institute in Phoenix who estimated that he performs about 150 TAVR procedures annually. “This is a step forward. I’ve been waiting for this for a long time. Until now, volume has been used as a surrogate outcome, but we know it’s not accurate. I’m confident that this model is a good starting point.” But Dr. Pershad also had concern that this new approach “can lend itself to some degree of gaming,” like a bleeding event getting classified as minor when it was really major, or outlier patients getting dropped from reports.
The temptation to cut corners may be higher for TAVR than it’s been for the cardiac-disease metrics that already get publicly reported, like bypass surgery and myocardial infarction management, because of TAVR’s higher cost and higher profile, Dr. Pershad said. Existing measures “have not been as linked to financial disincentive as TAVR might be” because TAVR reimbursements can run as high as $50,000 per case. “The stakes with TAVR are higher,” he said.
Ultimately, the reliable examination of TAVR outcomes that this new metric allows may lead to a shake-up of TAVR programs, Dr. Pershad predicted. “This is clearly a step toward closing down some programs that [consistently] underperform.”
The STS/ACC TVT Registry receives no commercial funding. Dr. Desai has been a consultant to, speaker on behalf of, and received research funding from Gore, and he has also spoken on behalf of Cook, Medtronic, and Terumo Aortic. Dr. Cleveland, Dr. Mack, and Dr. Pershad had no disclosures.
REPORTING FROM ACC 20
Reports suggest possible in utero transmission of novel coronavirus 2019
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
FROM JAMA
Despite strict controls, some infants born to mothers with COVID-19 appear infected
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
FROM JAMA PEDIATRICS
Guidelines on delaying cancer surgery during COVID-19
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.












