User login
How long is it safe to delay gynecologic cancer surgery?
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
Preventable diseases could gain a foothold because of COVID-19
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
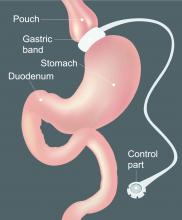
The hospitalized postbariatric surgery patient
What every hospitalist should know
With the prevalence of obesity worldwide topping 650 million people1 and nearly 40% of U.S. adults having obesity,2 bariatric surgery is increasingly used to treat this disease and its associated comorbidities.
The American Society for Metabolic & Bariatric Surgery estimates that 228,000 bariatric procedures were performed on Americans in 2017, up from 158,000 in 2011.3 Despite lowering the risks of diabetes, stroke, myocardial infarction, cancer, and all-cause mortality,4 bariatric surgery is associated with increased health care use. Neovius et al. found that people who underwent bariatric surgery used 54 mean cumulative hospital days in the 20 years following their procedures, compared with just 40 inpatient days used by controls.5
Although hospitalists are caring for increasing numbers of patients who have undergone bariatric surgery, many of us may not be aware of some of the things that can lead to hospitalization or otherwise affect inpatient medical care. Here are a few points to keep in mind the next time you care for an inpatient with prior bariatric surgery.
Pharmacokinetics change after surgery
Gastrointestinal anatomy necessarily changes after bariatric surgery and can affect the oral absorption of drugs. Because gastric motility may be impaired and the pH in the stomach is increased after bariatric surgery, the disintegration and dissolution of immediate-release solid pills or caps may be compromised.
It is therefore prudent to crush solid forms or switch to liquid or chewable formulations of immediate-release drugs for the first few weeks to months after surgery. Enteric-coated or long-acting drug formulations should not be crushed and should generally be avoided in patients who have undergone bypass procedures such as Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion with duodenal switch (BPD/DS), as they can demonstrate either enhanced or diminished absorption (depending on the drug).
Reduced intestinal transit times and changes in intestinal pH can alter the absorption of certain drugs as well, and the expression of some drug transporter proteins and enzymes such as the CYP3A4 variant of cytochrome P450 – which is estimated to metabolize up to half of currently available drugs – varies between the upper and the lower small intestine, potentially leading to increased bioavailability of medications metabolized by this enzyme in patients who have undergone bypass surgeries.
Interestingly, longer-term studies have reexamined drug absorption in patients 2-4 years after RYGB and found that initially-increased drug plasma levels often return to preoperative levels or even lower over time,6 likely because of adaptive changes in the GI tract. Because research on the pharmacokinetics of individual drugs after bariatric surgery is lacking, the hospitalist should be aware that the bioavailability of oral drugs is often altered and should monitor patients for the desired therapeutic effect as well as potential toxicities for any drug administered to postbariatric surgery patients.
Finally, note that nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and corticosteroids should be avoided after bariatric surgery unless the benefit clearly outweighs the risk, as they increase the risk of ulcers even in patients without underlying surgical disruptions to the gastric mucosa.
Micronutrient deficiencies are common and can occur at any time
While many clinicians recognize that vitamin deficiencies can occur after weight loss surgeries which bypass the duodenum, such as the RYGB or the BPD/DS, it is important to note that vitamin and mineral deficiencies occur commonly even in patients with intact intestinal absorption such as those who underwent sleeve gastrectomy (SG) and even despite regained weight due to greater volumes of food (and micronutrient) intake over time.
The most common vitamin deficiencies include iron, vitamin B12, thiamine (vitamin B1), and vitamin D, but deficiencies in other vitamins and minerals may found as well. Anemia, bone fractures, heart failure, and encephalopathy can all be related to postoperative vitamin deficiencies. Most bariatric surgery patients should have micronutrient levels monitored on a yearly basis and should be taking at least a multivitamin with minerals (including zinc, copper, selenium and iron), a form of vitamin B12, and vitamin D with calcium supplementation. Additional supplements may be appropriate depending on the type of surgery the patient had or whether a deficiency is found.
The differential diagnosis for abdominal pain after bariatric surgery is unique
While the usual suspects such as diverticulitis or gastritis should be considered in postbariatric surgery patients just as in others, a few specific complications can arise after weight loss surgery.
Marginal ulcerations (ulcers at the surgical anastomotic sites) have been reported in up to a third of patients complaining of abdominal pain or dysphagia after RYGB, with tobacco, alcohol, or NSAID use conferring even greater risk.7 Early upper endoscopy may be warranted in symptomatic patients.
Small bowel obstruction (SBO) may occur due to surgical adhesions as in other patients, but catastrophic internal hernias with associated volvulus can occur due to specific anatomical defects that are created by the RYGB and BPD/DS procedures. CT imaging is insensitive and can miss up to 30% of these cases,8 and nasogastric tubes placed blindly for decompression of an SBO can lead to perforation of the end of the alimentary limb at the gastric pouch outlet, so post-RYGB or BPD/DS patients presenting with signs of small bowel obstruction should have an early surgical consult for expeditious surgical management rather than a trial of conservative medical management.9
Cholelithiasis is a very common postoperative complication, occurring in about 25% of SG patients and 32% of RYGB patients in the first year following surgery. The risk of gallstone formation can be significantly reduced with the postoperative use of ursodeoxycholic acid.10
Onset of abdominal cramping, nausea and diarrhea (sometimes accompanied by vasomotor symptoms) within 15-60 minutes of eating may be due to early dumping syndrome. Rapid delivery of food from the gastric pouch into the small intestine causes the release of gut peptides and an osmotic fluid shift into the intestinal lumen that can trigger these symptoms even in patients with a preserved pyloric sphincter, such as those who underwent SG. Simply eliminating sugars and simple carbohydrates from the diet usually resolves the problem, and eliminating lactose can often be helpful as well.
Postprandial hyperinsulinemic hypoglycemia (“late dumping syndrome”) can develop years after surgery
Vasomotor symptoms such as flushing/sweating, shaking, tachycardia/palpitations, lightheadedness, or difficulty concentrating occurring 1-3 hours after a meal should prompt blood glucose testing, as delayed hypoglycemia can occur after a large insulin surge.
Most commonly seen after RYGB, late dumping syndrome, like early dumping syndrome, can often be managed by eliminating sugars and simple carbohydrates from the diet. The onset of late dumping syndrome has been reported as late as 8 years after surgery,11 so the etiology of symptoms can be elusive. If the diagnosis is unclear, an oral glucose tolerance test may be helpful.
Alcohol use disorder is more prevalent after weight loss surgery
Changes to the gastrointestinal anatomy allow for more rapid absorption of ethanol into the bloodstream, making the drug more potent in postop patients. Simultaneously, many patients who undergo bariatric surgery have a history of using food to buffer negative emotions. Abruptly depriving them of that comfort in the context of the increased potency of alcohol could potentially leave bariatric surgery patients vulnerable to the development of alcohol use disorder, even when they did not misuse alcohol preoperatively.
Of note, alcohol misuse becomes more prevalent after the first postoperative year.12 Screening for alcohol misuse on admission to the hospital is wise in all cases, but perhaps even more so in the postbariatric surgery patient. If a patient does report excessive alcohol use, keep possible thiamine deficiency in mind.
The risk of suicide and self-harm increases after bariatric surgery
While all-cause mortality rates decrease after bariatric surgery compared with matched controls, the risk of suicide and nonfatal self-harm increases.
About half of bariatric surgery patients with nonfatal events have substance misuse.13 Notably, several studies have found reduced plasma levels of SSRIs in patients after RYGB,6 so pharmacotherapy for mood disorders could be less effective after bariatric surgery as well. The hospitalist could positively impact patients by screening for both substance misuse and depression and by having a low threshold for referral to a mental health professional.
As we see ever-increasing numbers of inpatients who have a history of bariatric surgery, being aware of these common and important complications can help today’s hospitalist provide the best care possible.
Dr. Kerns is a hospitalist and codirector of bariatric surgery at the Washington DC VA Medical Center.
References
1. Obesity and overweight. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published Feb 16, 2018.
2. Hales CM et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS data brief, no 288. Hyattsville, MD: National Center for Health Statistics. 2017.
3. Estimate of Bariatric Surgery Numbers, 2011-2018. ASMBS.org. Published June 2018.
4. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013 Mar;273(3):219-34. doi: 10.1111/joim.12012.
5. Neovius M et al. Health care use during 20 years following bariatric surgery. JAMA. 2012 Sep 19; 308(11):1132-41. doi: 10.1001/2012.jama.11792.
6. Azran C. et al. Oral drug therapy following bariatric surgery: An overview of fundamentals, literature and clinical recommendations. Obes Rev. 2016 Nov;17(11):1050-66. doi: 10.1111/obr.12434.
7. El-hayek KM et al. Marginal ulcer after Roux-en-Y gastric bypass: What have we really learned? Surg Endosc. 2012 Oct;26(10):2789-96. Epub 2012 Apr 28. (Abstract presented at Society of American Gastrointestinal and Endoscopic Surgeons 2012 annual meeting, San Diego.) 8. Iannelli A et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2006;16:1265-71. doi: 10.1381/096089206778663689.
9. Lim R et al. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018 Oct 9;3(1): e000219. doi: 10.1136/tsaco-2018-000219.
10. Coupaye M et al. Evaluation of incidence of cholelithiasis after bariatric surgery in subjects treated or not treated with ursodeoxycholic acid. Surg Obes Relat Dis. 2017;13(4):681-5. doi: 10.1016/j.soard.2016.11.022.
11. Eisenberg D et al. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2017 Mar;13(3):371-8. doi: 10.1016/j.soard.2016.12.005.
12. King WC et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012 Jun 20;307(23):2516-25. doi: 10.1001/jama.2012.6147.
13. Neovius M et al. Risk of suicide and non-fatal self-harm after bariatric surgery: Results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018 Mar;6(3):197-207. doi: 10.1016/S2213-8587(17)30437-0.
What every hospitalist should know
What every hospitalist should know
With the prevalence of obesity worldwide topping 650 million people1 and nearly 40% of U.S. adults having obesity,2 bariatric surgery is increasingly used to treat this disease and its associated comorbidities.
The American Society for Metabolic & Bariatric Surgery estimates that 228,000 bariatric procedures were performed on Americans in 2017, up from 158,000 in 2011.3 Despite lowering the risks of diabetes, stroke, myocardial infarction, cancer, and all-cause mortality,4 bariatric surgery is associated with increased health care use. Neovius et al. found that people who underwent bariatric surgery used 54 mean cumulative hospital days in the 20 years following their procedures, compared with just 40 inpatient days used by controls.5
Although hospitalists are caring for increasing numbers of patients who have undergone bariatric surgery, many of us may not be aware of some of the things that can lead to hospitalization or otherwise affect inpatient medical care. Here are a few points to keep in mind the next time you care for an inpatient with prior bariatric surgery.
Pharmacokinetics change after surgery
Gastrointestinal anatomy necessarily changes after bariatric surgery and can affect the oral absorption of drugs. Because gastric motility may be impaired and the pH in the stomach is increased after bariatric surgery, the disintegration and dissolution of immediate-release solid pills or caps may be compromised.
It is therefore prudent to crush solid forms or switch to liquid or chewable formulations of immediate-release drugs for the first few weeks to months after surgery. Enteric-coated or long-acting drug formulations should not be crushed and should generally be avoided in patients who have undergone bypass procedures such as Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion with duodenal switch (BPD/DS), as they can demonstrate either enhanced or diminished absorption (depending on the drug).
Reduced intestinal transit times and changes in intestinal pH can alter the absorption of certain drugs as well, and the expression of some drug transporter proteins and enzymes such as the CYP3A4 variant of cytochrome P450 – which is estimated to metabolize up to half of currently available drugs – varies between the upper and the lower small intestine, potentially leading to increased bioavailability of medications metabolized by this enzyme in patients who have undergone bypass surgeries.
Interestingly, longer-term studies have reexamined drug absorption in patients 2-4 years after RYGB and found that initially-increased drug plasma levels often return to preoperative levels or even lower over time,6 likely because of adaptive changes in the GI tract. Because research on the pharmacokinetics of individual drugs after bariatric surgery is lacking, the hospitalist should be aware that the bioavailability of oral drugs is often altered and should monitor patients for the desired therapeutic effect as well as potential toxicities for any drug administered to postbariatric surgery patients.
Finally, note that nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and corticosteroids should be avoided after bariatric surgery unless the benefit clearly outweighs the risk, as they increase the risk of ulcers even in patients without underlying surgical disruptions to the gastric mucosa.
Micronutrient deficiencies are common and can occur at any time
While many clinicians recognize that vitamin deficiencies can occur after weight loss surgeries which bypass the duodenum, such as the RYGB or the BPD/DS, it is important to note that vitamin and mineral deficiencies occur commonly even in patients with intact intestinal absorption such as those who underwent sleeve gastrectomy (SG) and even despite regained weight due to greater volumes of food (and micronutrient) intake over time.
The most common vitamin deficiencies include iron, vitamin B12, thiamine (vitamin B1), and vitamin D, but deficiencies in other vitamins and minerals may found as well. Anemia, bone fractures, heart failure, and encephalopathy can all be related to postoperative vitamin deficiencies. Most bariatric surgery patients should have micronutrient levels monitored on a yearly basis and should be taking at least a multivitamin with minerals (including zinc, copper, selenium and iron), a form of vitamin B12, and vitamin D with calcium supplementation. Additional supplements may be appropriate depending on the type of surgery the patient had or whether a deficiency is found.
The differential diagnosis for abdominal pain after bariatric surgery is unique
While the usual suspects such as diverticulitis or gastritis should be considered in postbariatric surgery patients just as in others, a few specific complications can arise after weight loss surgery.
Marginal ulcerations (ulcers at the surgical anastomotic sites) have been reported in up to a third of patients complaining of abdominal pain or dysphagia after RYGB, with tobacco, alcohol, or NSAID use conferring even greater risk.7 Early upper endoscopy may be warranted in symptomatic patients.
Small bowel obstruction (SBO) may occur due to surgical adhesions as in other patients, but catastrophic internal hernias with associated volvulus can occur due to specific anatomical defects that are created by the RYGB and BPD/DS procedures. CT imaging is insensitive and can miss up to 30% of these cases,8 and nasogastric tubes placed blindly for decompression of an SBO can lead to perforation of the end of the alimentary limb at the gastric pouch outlet, so post-RYGB or BPD/DS patients presenting with signs of small bowel obstruction should have an early surgical consult for expeditious surgical management rather than a trial of conservative medical management.9
Cholelithiasis is a very common postoperative complication, occurring in about 25% of SG patients and 32% of RYGB patients in the first year following surgery. The risk of gallstone formation can be significantly reduced with the postoperative use of ursodeoxycholic acid.10
Onset of abdominal cramping, nausea and diarrhea (sometimes accompanied by vasomotor symptoms) within 15-60 minutes of eating may be due to early dumping syndrome. Rapid delivery of food from the gastric pouch into the small intestine causes the release of gut peptides and an osmotic fluid shift into the intestinal lumen that can trigger these symptoms even in patients with a preserved pyloric sphincter, such as those who underwent SG. Simply eliminating sugars and simple carbohydrates from the diet usually resolves the problem, and eliminating lactose can often be helpful as well.
Postprandial hyperinsulinemic hypoglycemia (“late dumping syndrome”) can develop years after surgery
Vasomotor symptoms such as flushing/sweating, shaking, tachycardia/palpitations, lightheadedness, or difficulty concentrating occurring 1-3 hours after a meal should prompt blood glucose testing, as delayed hypoglycemia can occur after a large insulin surge.
Most commonly seen after RYGB, late dumping syndrome, like early dumping syndrome, can often be managed by eliminating sugars and simple carbohydrates from the diet. The onset of late dumping syndrome has been reported as late as 8 years after surgery,11 so the etiology of symptoms can be elusive. If the diagnosis is unclear, an oral glucose tolerance test may be helpful.
Alcohol use disorder is more prevalent after weight loss surgery
Changes to the gastrointestinal anatomy allow for more rapid absorption of ethanol into the bloodstream, making the drug more potent in postop patients. Simultaneously, many patients who undergo bariatric surgery have a history of using food to buffer negative emotions. Abruptly depriving them of that comfort in the context of the increased potency of alcohol could potentially leave bariatric surgery patients vulnerable to the development of alcohol use disorder, even when they did not misuse alcohol preoperatively.
Of note, alcohol misuse becomes more prevalent after the first postoperative year.12 Screening for alcohol misuse on admission to the hospital is wise in all cases, but perhaps even more so in the postbariatric surgery patient. If a patient does report excessive alcohol use, keep possible thiamine deficiency in mind.
The risk of suicide and self-harm increases after bariatric surgery
While all-cause mortality rates decrease after bariatric surgery compared with matched controls, the risk of suicide and nonfatal self-harm increases.
About half of bariatric surgery patients with nonfatal events have substance misuse.13 Notably, several studies have found reduced plasma levels of SSRIs in patients after RYGB,6 so pharmacotherapy for mood disorders could be less effective after bariatric surgery as well. The hospitalist could positively impact patients by screening for both substance misuse and depression and by having a low threshold for referral to a mental health professional.
As we see ever-increasing numbers of inpatients who have a history of bariatric surgery, being aware of these common and important complications can help today’s hospitalist provide the best care possible.
Dr. Kerns is a hospitalist and codirector of bariatric surgery at the Washington DC VA Medical Center.
References
1. Obesity and overweight. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published Feb 16, 2018.
2. Hales CM et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS data brief, no 288. Hyattsville, MD: National Center for Health Statistics. 2017.
3. Estimate of Bariatric Surgery Numbers, 2011-2018. ASMBS.org. Published June 2018.
4. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013 Mar;273(3):219-34. doi: 10.1111/joim.12012.
5. Neovius M et al. Health care use during 20 years following bariatric surgery. JAMA. 2012 Sep 19; 308(11):1132-41. doi: 10.1001/2012.jama.11792.
6. Azran C. et al. Oral drug therapy following bariatric surgery: An overview of fundamentals, literature and clinical recommendations. Obes Rev. 2016 Nov;17(11):1050-66. doi: 10.1111/obr.12434.
7. El-hayek KM et al. Marginal ulcer after Roux-en-Y gastric bypass: What have we really learned? Surg Endosc. 2012 Oct;26(10):2789-96. Epub 2012 Apr 28. (Abstract presented at Society of American Gastrointestinal and Endoscopic Surgeons 2012 annual meeting, San Diego.) 8. Iannelli A et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2006;16:1265-71. doi: 10.1381/096089206778663689.
9. Lim R et al. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018 Oct 9;3(1): e000219. doi: 10.1136/tsaco-2018-000219.
10. Coupaye M et al. Evaluation of incidence of cholelithiasis after bariatric surgery in subjects treated or not treated with ursodeoxycholic acid. Surg Obes Relat Dis. 2017;13(4):681-5. doi: 10.1016/j.soard.2016.11.022.
11. Eisenberg D et al. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2017 Mar;13(3):371-8. doi: 10.1016/j.soard.2016.12.005.
12. King WC et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012 Jun 20;307(23):2516-25. doi: 10.1001/jama.2012.6147.
13. Neovius M et al. Risk of suicide and non-fatal self-harm after bariatric surgery: Results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018 Mar;6(3):197-207. doi: 10.1016/S2213-8587(17)30437-0.
With the prevalence of obesity worldwide topping 650 million people1 and nearly 40% of U.S. adults having obesity,2 bariatric surgery is increasingly used to treat this disease and its associated comorbidities.
The American Society for Metabolic & Bariatric Surgery estimates that 228,000 bariatric procedures were performed on Americans in 2017, up from 158,000 in 2011.3 Despite lowering the risks of diabetes, stroke, myocardial infarction, cancer, and all-cause mortality,4 bariatric surgery is associated with increased health care use. Neovius et al. found that people who underwent bariatric surgery used 54 mean cumulative hospital days in the 20 years following their procedures, compared with just 40 inpatient days used by controls.5
Although hospitalists are caring for increasing numbers of patients who have undergone bariatric surgery, many of us may not be aware of some of the things that can lead to hospitalization or otherwise affect inpatient medical care. Here are a few points to keep in mind the next time you care for an inpatient with prior bariatric surgery.
Pharmacokinetics change after surgery
Gastrointestinal anatomy necessarily changes after bariatric surgery and can affect the oral absorption of drugs. Because gastric motility may be impaired and the pH in the stomach is increased after bariatric surgery, the disintegration and dissolution of immediate-release solid pills or caps may be compromised.
It is therefore prudent to crush solid forms or switch to liquid or chewable formulations of immediate-release drugs for the first few weeks to months after surgery. Enteric-coated or long-acting drug formulations should not be crushed and should generally be avoided in patients who have undergone bypass procedures such as Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion with duodenal switch (BPD/DS), as they can demonstrate either enhanced or diminished absorption (depending on the drug).
Reduced intestinal transit times and changes in intestinal pH can alter the absorption of certain drugs as well, and the expression of some drug transporter proteins and enzymes such as the CYP3A4 variant of cytochrome P450 – which is estimated to metabolize up to half of currently available drugs – varies between the upper and the lower small intestine, potentially leading to increased bioavailability of medications metabolized by this enzyme in patients who have undergone bypass surgeries.
Interestingly, longer-term studies have reexamined drug absorption in patients 2-4 years after RYGB and found that initially-increased drug plasma levels often return to preoperative levels or even lower over time,6 likely because of adaptive changes in the GI tract. Because research on the pharmacokinetics of individual drugs after bariatric surgery is lacking, the hospitalist should be aware that the bioavailability of oral drugs is often altered and should monitor patients for the desired therapeutic effect as well as potential toxicities for any drug administered to postbariatric surgery patients.
Finally, note that nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and corticosteroids should be avoided after bariatric surgery unless the benefit clearly outweighs the risk, as they increase the risk of ulcers even in patients without underlying surgical disruptions to the gastric mucosa.
Micronutrient deficiencies are common and can occur at any time
While many clinicians recognize that vitamin deficiencies can occur after weight loss surgeries which bypass the duodenum, such as the RYGB or the BPD/DS, it is important to note that vitamin and mineral deficiencies occur commonly even in patients with intact intestinal absorption such as those who underwent sleeve gastrectomy (SG) and even despite regained weight due to greater volumes of food (and micronutrient) intake over time.
The most common vitamin deficiencies include iron, vitamin B12, thiamine (vitamin B1), and vitamin D, but deficiencies in other vitamins and minerals may found as well. Anemia, bone fractures, heart failure, and encephalopathy can all be related to postoperative vitamin deficiencies. Most bariatric surgery patients should have micronutrient levels monitored on a yearly basis and should be taking at least a multivitamin with minerals (including zinc, copper, selenium and iron), a form of vitamin B12, and vitamin D with calcium supplementation. Additional supplements may be appropriate depending on the type of surgery the patient had or whether a deficiency is found.
The differential diagnosis for abdominal pain after bariatric surgery is unique
While the usual suspects such as diverticulitis or gastritis should be considered in postbariatric surgery patients just as in others, a few specific complications can arise after weight loss surgery.
Marginal ulcerations (ulcers at the surgical anastomotic sites) have been reported in up to a third of patients complaining of abdominal pain or dysphagia after RYGB, with tobacco, alcohol, or NSAID use conferring even greater risk.7 Early upper endoscopy may be warranted in symptomatic patients.
Small bowel obstruction (SBO) may occur due to surgical adhesions as in other patients, but catastrophic internal hernias with associated volvulus can occur due to specific anatomical defects that are created by the RYGB and BPD/DS procedures. CT imaging is insensitive and can miss up to 30% of these cases,8 and nasogastric tubes placed blindly for decompression of an SBO can lead to perforation of the end of the alimentary limb at the gastric pouch outlet, so post-RYGB or BPD/DS patients presenting with signs of small bowel obstruction should have an early surgical consult for expeditious surgical management rather than a trial of conservative medical management.9
Cholelithiasis is a very common postoperative complication, occurring in about 25% of SG patients and 32% of RYGB patients in the first year following surgery. The risk of gallstone formation can be significantly reduced with the postoperative use of ursodeoxycholic acid.10
Onset of abdominal cramping, nausea and diarrhea (sometimes accompanied by vasomotor symptoms) within 15-60 minutes of eating may be due to early dumping syndrome. Rapid delivery of food from the gastric pouch into the small intestine causes the release of gut peptides and an osmotic fluid shift into the intestinal lumen that can trigger these symptoms even in patients with a preserved pyloric sphincter, such as those who underwent SG. Simply eliminating sugars and simple carbohydrates from the diet usually resolves the problem, and eliminating lactose can often be helpful as well.
Postprandial hyperinsulinemic hypoglycemia (“late dumping syndrome”) can develop years after surgery
Vasomotor symptoms such as flushing/sweating, shaking, tachycardia/palpitations, lightheadedness, or difficulty concentrating occurring 1-3 hours after a meal should prompt blood glucose testing, as delayed hypoglycemia can occur after a large insulin surge.
Most commonly seen after RYGB, late dumping syndrome, like early dumping syndrome, can often be managed by eliminating sugars and simple carbohydrates from the diet. The onset of late dumping syndrome has been reported as late as 8 years after surgery,11 so the etiology of symptoms can be elusive. If the diagnosis is unclear, an oral glucose tolerance test may be helpful.
Alcohol use disorder is more prevalent after weight loss surgery
Changes to the gastrointestinal anatomy allow for more rapid absorption of ethanol into the bloodstream, making the drug more potent in postop patients. Simultaneously, many patients who undergo bariatric surgery have a history of using food to buffer negative emotions. Abruptly depriving them of that comfort in the context of the increased potency of alcohol could potentially leave bariatric surgery patients vulnerable to the development of alcohol use disorder, even when they did not misuse alcohol preoperatively.
Of note, alcohol misuse becomes more prevalent after the first postoperative year.12 Screening for alcohol misuse on admission to the hospital is wise in all cases, but perhaps even more so in the postbariatric surgery patient. If a patient does report excessive alcohol use, keep possible thiamine deficiency in mind.
The risk of suicide and self-harm increases after bariatric surgery
While all-cause mortality rates decrease after bariatric surgery compared with matched controls, the risk of suicide and nonfatal self-harm increases.
About half of bariatric surgery patients with nonfatal events have substance misuse.13 Notably, several studies have found reduced plasma levels of SSRIs in patients after RYGB,6 so pharmacotherapy for mood disorders could be less effective after bariatric surgery as well. The hospitalist could positively impact patients by screening for both substance misuse and depression and by having a low threshold for referral to a mental health professional.
As we see ever-increasing numbers of inpatients who have a history of bariatric surgery, being aware of these common and important complications can help today’s hospitalist provide the best care possible.
Dr. Kerns is a hospitalist and codirector of bariatric surgery at the Washington DC VA Medical Center.
References
1. Obesity and overweight. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published Feb 16, 2018.
2. Hales CM et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS data brief, no 288. Hyattsville, MD: National Center for Health Statistics. 2017.
3. Estimate of Bariatric Surgery Numbers, 2011-2018. ASMBS.org. Published June 2018.
4. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013 Mar;273(3):219-34. doi: 10.1111/joim.12012.
5. Neovius M et al. Health care use during 20 years following bariatric surgery. JAMA. 2012 Sep 19; 308(11):1132-41. doi: 10.1001/2012.jama.11792.
6. Azran C. et al. Oral drug therapy following bariatric surgery: An overview of fundamentals, literature and clinical recommendations. Obes Rev. 2016 Nov;17(11):1050-66. doi: 10.1111/obr.12434.
7. El-hayek KM et al. Marginal ulcer after Roux-en-Y gastric bypass: What have we really learned? Surg Endosc. 2012 Oct;26(10):2789-96. Epub 2012 Apr 28. (Abstract presented at Society of American Gastrointestinal and Endoscopic Surgeons 2012 annual meeting, San Diego.) 8. Iannelli A et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2006;16:1265-71. doi: 10.1381/096089206778663689.
9. Lim R et al. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018 Oct 9;3(1): e000219. doi: 10.1136/tsaco-2018-000219.
10. Coupaye M et al. Evaluation of incidence of cholelithiasis after bariatric surgery in subjects treated or not treated with ursodeoxycholic acid. Surg Obes Relat Dis. 2017;13(4):681-5. doi: 10.1016/j.soard.2016.11.022.
11. Eisenberg D et al. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2017 Mar;13(3):371-8. doi: 10.1016/j.soard.2016.12.005.
12. King WC et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012 Jun 20;307(23):2516-25. doi: 10.1001/jama.2012.6147.
13. Neovius M et al. Risk of suicide and non-fatal self-harm after bariatric surgery: Results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018 Mar;6(3):197-207. doi: 10.1016/S2213-8587(17)30437-0.
COVID-19 in China: Children have less severe disease, but are vulnerable
Clinical manifestations of COVID-19 infection among children in mainland China generally have been less severe than those among adults, but children of all ages – and infants in particular – are vulnerable to infection, according to a review of 2,143 cases.
Further, infection patterns in the nationwide series of all pediatric patients reported to the Chinese Center for Disease Control and Prevention from Jan. 16 to Feb. 8, 2020, provide strong evidence of human-to-human transmission, Yuanyuan Dong, MPH, a research assistant at Shanghai Children’s Medical Center, Shanghai Jiao Tong University, China, and colleagues reported in Pediatrics.
Of the 2,143 patients included in the review, 57% were boys and the median age was 7 years; 34% had laboratory-confirmed infection and 67% had suspected infection. More than 90% had asymptomatic, mild, or moderate disease (4%, 51%, and 39%, respectively), and 46% were from Hubei Province, where the first cases were reported, the investigators found.
The median time from illness onset to diagnosis was 2 days, and there was a trend of rapid increase of disease at the early stage of the epidemic – with rapid spread from Hubei Province to surrounding provinces – followed by a gradual and steady decrease, they noted.
“The total number of pediatric patients increased remarkably between mid-January and early February, peaked around February 1, and then declined since early February 2020,” they wrote. The proportion of severe and critical cases was 11% for infants under 1 year of age, compared with 7% for those aged 1-5 years; 4% for those aged 6-10 years; 4% for those 11-15 years; and 3% for those 16 years and older.
As of Feb. 8, 2020, only one child in this group of study patients died and most cases of COVID-19 symptoms were mild. There were many fewer severe and critical cases among the children (6%), compared with those reported in adult patients in other studies (19%). “It suggests that, compared with adult patients, clinical manifestations of children’s COVID-19 may be less severe,” the investigators suggested.
“As most of these children were likely to expose themselves to family members and/or other children with COVID-19, it clearly indicates person-to-person transmission ” of novel coronavirus 2019, they said, adding that similar evidence of such transmission also has been reported from studies of adult patients.
The reasons for reduced severity in children versus adults remain unclear, but may be related to both exposure and host factors, Ms. Dong and associates said. “Children were usually well cared for at home and might have relatively [fewer] opportunities to expose themselves to pathogens and/or sick patients.”
The findings demonstrate a pediatric distribution that varied across time and space, with most cases concentrated in the Hubei province and surrounding areas. No significant gender-related difference in infection rates was observed, and although the median patient age was 7 years, the range was 1 day to 18 years, suggesting that “all ages at childhood were susceptible” to the virus, they added.
The declining number of cases over time further suggests that disease control measures implemented by the government were effective, and that cases will “continue to decline, and finally stop in the near future unless sustained human-to-human transmissions occur,” Ms. Dong and associates concluded.
In an accompanying editorial, Andrea T. Cruz, MD, of Baylor College of Medicine, Houston, and Steven L. Zeichner, MD, PhD, of the University of Virginia, Charlottesville, said the findings regarding reduced severity among children versus adults with novel coronavirus 2019 infection are consistent with data on non-COVID-19 coronavirus.
They pointed out that Ms. Dong and associates did find that 13% of virologically-confirmed cases had asymptomatic infection, “a rate that almost certainly understates the true rate of asymptomatic infection, since many asymptomatic children are unlikely to be tested.”
Of the symptomatic children, “5% had dyspnea or hypoxemia (a substantially lower percentage than what has been reported for adults) and 0.6% progressed to acute respiratory distress syndrome (ARDS) or multiorgan system dysfunction”; this also is at a lower rate than seen in adults, they said.
Very young children –infants or children in preschool – were more likely to have severe clinical manifestations than children who were older.
Thus, it appears that certain subpopulations of children are at increased risk for more significant COVID-19 illness: “younger age, underlying pulmonary pathology, and immunocompromising conditions,” Dr. Cruz and Dr. Zeichner suggested.
The two editorialists said the findings suggest children “may play a major role in community-based viral transmission.” Evidence suggests that children may have more upper respiratory tract involvement and that fecal shedding may occur for several weeks after diagnosis; this raises concerns about fecal-oral transmission, particularly for infants and children, and about viral replication in the gastrointestinal tract, they said. This has substantial implications for community spread in day care centers, schools, and in the home.
A great deal has been learned about COVID-19 in a short time, but there still is much to learn about the effect of the virus on children, the impact of children on viral spread, and about possible vertical transmission, they said.
“Widespread availability of testing will allow for us to more accurately describe the spectrum of illness and may result in adjustment of the apparent morbidity and mortality rate as fewer ill individuals are diagnosed,” Dr. Cruz and Dr. Zeichner wrote, adding that “rigorously gauging the impact of COVID-19 on children will be important to accurately model the pandemic and to ensure that appropriate resources are allocated to children requiring care.”
They noted that understanding differences in children versus adults with COVID-19 “can yield important insights into disease pathogenesis, informing management and the development of therapeutics.”
This study was partially supported by the Science and Technology Commission of Shanghai Municipality. The authors reported having no disclosures. Dr. Cruz and Dr. Zeichner are associate editors for Pediatrics. Dr. Cruz reported having no disclosures. Dr. Zeichner is an inventor of new technologies for the rapid production of vaccines, for which the University of Virginia has filed patent applications.
SOURCE: Dong Y et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0702; Cruz A and Zeichner S. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0834.
Clinical manifestations of COVID-19 infection among children in mainland China generally have been less severe than those among adults, but children of all ages – and infants in particular – are vulnerable to infection, according to a review of 2,143 cases.
Further, infection patterns in the nationwide series of all pediatric patients reported to the Chinese Center for Disease Control and Prevention from Jan. 16 to Feb. 8, 2020, provide strong evidence of human-to-human transmission, Yuanyuan Dong, MPH, a research assistant at Shanghai Children’s Medical Center, Shanghai Jiao Tong University, China, and colleagues reported in Pediatrics.
Of the 2,143 patients included in the review, 57% were boys and the median age was 7 years; 34% had laboratory-confirmed infection and 67% had suspected infection. More than 90% had asymptomatic, mild, or moderate disease (4%, 51%, and 39%, respectively), and 46% were from Hubei Province, where the first cases were reported, the investigators found.
The median time from illness onset to diagnosis was 2 days, and there was a trend of rapid increase of disease at the early stage of the epidemic – with rapid spread from Hubei Province to surrounding provinces – followed by a gradual and steady decrease, they noted.
“The total number of pediatric patients increased remarkably between mid-January and early February, peaked around February 1, and then declined since early February 2020,” they wrote. The proportion of severe and critical cases was 11% for infants under 1 year of age, compared with 7% for those aged 1-5 years; 4% for those aged 6-10 years; 4% for those 11-15 years; and 3% for those 16 years and older.
As of Feb. 8, 2020, only one child in this group of study patients died and most cases of COVID-19 symptoms were mild. There were many fewer severe and critical cases among the children (6%), compared with those reported in adult patients in other studies (19%). “It suggests that, compared with adult patients, clinical manifestations of children’s COVID-19 may be less severe,” the investigators suggested.
“As most of these children were likely to expose themselves to family members and/or other children with COVID-19, it clearly indicates person-to-person transmission ” of novel coronavirus 2019, they said, adding that similar evidence of such transmission also has been reported from studies of adult patients.
The reasons for reduced severity in children versus adults remain unclear, but may be related to both exposure and host factors, Ms. Dong and associates said. “Children were usually well cared for at home and might have relatively [fewer] opportunities to expose themselves to pathogens and/or sick patients.”
The findings demonstrate a pediatric distribution that varied across time and space, with most cases concentrated in the Hubei province and surrounding areas. No significant gender-related difference in infection rates was observed, and although the median patient age was 7 years, the range was 1 day to 18 years, suggesting that “all ages at childhood were susceptible” to the virus, they added.
The declining number of cases over time further suggests that disease control measures implemented by the government were effective, and that cases will “continue to decline, and finally stop in the near future unless sustained human-to-human transmissions occur,” Ms. Dong and associates concluded.
In an accompanying editorial, Andrea T. Cruz, MD, of Baylor College of Medicine, Houston, and Steven L. Zeichner, MD, PhD, of the University of Virginia, Charlottesville, said the findings regarding reduced severity among children versus adults with novel coronavirus 2019 infection are consistent with data on non-COVID-19 coronavirus.
They pointed out that Ms. Dong and associates did find that 13% of virologically-confirmed cases had asymptomatic infection, “a rate that almost certainly understates the true rate of asymptomatic infection, since many asymptomatic children are unlikely to be tested.”
Of the symptomatic children, “5% had dyspnea or hypoxemia (a substantially lower percentage than what has been reported for adults) and 0.6% progressed to acute respiratory distress syndrome (ARDS) or multiorgan system dysfunction”; this also is at a lower rate than seen in adults, they said.
Very young children –infants or children in preschool – were more likely to have severe clinical manifestations than children who were older.
Thus, it appears that certain subpopulations of children are at increased risk for more significant COVID-19 illness: “younger age, underlying pulmonary pathology, and immunocompromising conditions,” Dr. Cruz and Dr. Zeichner suggested.
The two editorialists said the findings suggest children “may play a major role in community-based viral transmission.” Evidence suggests that children may have more upper respiratory tract involvement and that fecal shedding may occur for several weeks after diagnosis; this raises concerns about fecal-oral transmission, particularly for infants and children, and about viral replication in the gastrointestinal tract, they said. This has substantial implications for community spread in day care centers, schools, and in the home.
A great deal has been learned about COVID-19 in a short time, but there still is much to learn about the effect of the virus on children, the impact of children on viral spread, and about possible vertical transmission, they said.
“Widespread availability of testing will allow for us to more accurately describe the spectrum of illness and may result in adjustment of the apparent morbidity and mortality rate as fewer ill individuals are diagnosed,” Dr. Cruz and Dr. Zeichner wrote, adding that “rigorously gauging the impact of COVID-19 on children will be important to accurately model the pandemic and to ensure that appropriate resources are allocated to children requiring care.”
They noted that understanding differences in children versus adults with COVID-19 “can yield important insights into disease pathogenesis, informing management and the development of therapeutics.”
This study was partially supported by the Science and Technology Commission of Shanghai Municipality. The authors reported having no disclosures. Dr. Cruz and Dr. Zeichner are associate editors for Pediatrics. Dr. Cruz reported having no disclosures. Dr. Zeichner is an inventor of new technologies for the rapid production of vaccines, for which the University of Virginia has filed patent applications.
SOURCE: Dong Y et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0702; Cruz A and Zeichner S. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0834.
Clinical manifestations of COVID-19 infection among children in mainland China generally have been less severe than those among adults, but children of all ages – and infants in particular – are vulnerable to infection, according to a review of 2,143 cases.
Further, infection patterns in the nationwide series of all pediatric patients reported to the Chinese Center for Disease Control and Prevention from Jan. 16 to Feb. 8, 2020, provide strong evidence of human-to-human transmission, Yuanyuan Dong, MPH, a research assistant at Shanghai Children’s Medical Center, Shanghai Jiao Tong University, China, and colleagues reported in Pediatrics.
Of the 2,143 patients included in the review, 57% were boys and the median age was 7 years; 34% had laboratory-confirmed infection and 67% had suspected infection. More than 90% had asymptomatic, mild, or moderate disease (4%, 51%, and 39%, respectively), and 46% were from Hubei Province, where the first cases were reported, the investigators found.
The median time from illness onset to diagnosis was 2 days, and there was a trend of rapid increase of disease at the early stage of the epidemic – with rapid spread from Hubei Province to surrounding provinces – followed by a gradual and steady decrease, they noted.
“The total number of pediatric patients increased remarkably between mid-January and early February, peaked around February 1, and then declined since early February 2020,” they wrote. The proportion of severe and critical cases was 11% for infants under 1 year of age, compared with 7% for those aged 1-5 years; 4% for those aged 6-10 years; 4% for those 11-15 years; and 3% for those 16 years and older.
As of Feb. 8, 2020, only one child in this group of study patients died and most cases of COVID-19 symptoms were mild. There were many fewer severe and critical cases among the children (6%), compared with those reported in adult patients in other studies (19%). “It suggests that, compared with adult patients, clinical manifestations of children’s COVID-19 may be less severe,” the investigators suggested.
“As most of these children were likely to expose themselves to family members and/or other children with COVID-19, it clearly indicates person-to-person transmission ” of novel coronavirus 2019, they said, adding that similar evidence of such transmission also has been reported from studies of adult patients.
The reasons for reduced severity in children versus adults remain unclear, but may be related to both exposure and host factors, Ms. Dong and associates said. “Children were usually well cared for at home and might have relatively [fewer] opportunities to expose themselves to pathogens and/or sick patients.”
The findings demonstrate a pediatric distribution that varied across time and space, with most cases concentrated in the Hubei province and surrounding areas. No significant gender-related difference in infection rates was observed, and although the median patient age was 7 years, the range was 1 day to 18 years, suggesting that “all ages at childhood were susceptible” to the virus, they added.
The declining number of cases over time further suggests that disease control measures implemented by the government were effective, and that cases will “continue to decline, and finally stop in the near future unless sustained human-to-human transmissions occur,” Ms. Dong and associates concluded.
In an accompanying editorial, Andrea T. Cruz, MD, of Baylor College of Medicine, Houston, and Steven L. Zeichner, MD, PhD, of the University of Virginia, Charlottesville, said the findings regarding reduced severity among children versus adults with novel coronavirus 2019 infection are consistent with data on non-COVID-19 coronavirus.
They pointed out that Ms. Dong and associates did find that 13% of virologically-confirmed cases had asymptomatic infection, “a rate that almost certainly understates the true rate of asymptomatic infection, since many asymptomatic children are unlikely to be tested.”
Of the symptomatic children, “5% had dyspnea or hypoxemia (a substantially lower percentage than what has been reported for adults) and 0.6% progressed to acute respiratory distress syndrome (ARDS) or multiorgan system dysfunction”; this also is at a lower rate than seen in adults, they said.
Very young children –infants or children in preschool – were more likely to have severe clinical manifestations than children who were older.
Thus, it appears that certain subpopulations of children are at increased risk for more significant COVID-19 illness: “younger age, underlying pulmonary pathology, and immunocompromising conditions,” Dr. Cruz and Dr. Zeichner suggested.
The two editorialists said the findings suggest children “may play a major role in community-based viral transmission.” Evidence suggests that children may have more upper respiratory tract involvement and that fecal shedding may occur for several weeks after diagnosis; this raises concerns about fecal-oral transmission, particularly for infants and children, and about viral replication in the gastrointestinal tract, they said. This has substantial implications for community spread in day care centers, schools, and in the home.
A great deal has been learned about COVID-19 in a short time, but there still is much to learn about the effect of the virus on children, the impact of children on viral spread, and about possible vertical transmission, they said.
“Widespread availability of testing will allow for us to more accurately describe the spectrum of illness and may result in adjustment of the apparent morbidity and mortality rate as fewer ill individuals are diagnosed,” Dr. Cruz and Dr. Zeichner wrote, adding that “rigorously gauging the impact of COVID-19 on children will be important to accurately model the pandemic and to ensure that appropriate resources are allocated to children requiring care.”
They noted that understanding differences in children versus adults with COVID-19 “can yield important insights into disease pathogenesis, informing management and the development of therapeutics.”
This study was partially supported by the Science and Technology Commission of Shanghai Municipality. The authors reported having no disclosures. Dr. Cruz and Dr. Zeichner are associate editors for Pediatrics. Dr. Cruz reported having no disclosures. Dr. Zeichner is an inventor of new technologies for the rapid production of vaccines, for which the University of Virginia has filed patent applications.
SOURCE: Dong Y et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0702; Cruz A and Zeichner S. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0834.
FROM PEDIATRICS
COVID-19 guidance for children’s health care providers
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]
FDA advises stopping SGLT2 inhibitor treatment prior to surgery
The new changes affect canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, and were made because surgery may put patients being treated with SGLT2 inhibitors at a higher risk of ketoacidosis. Canagliflozin, dapagliflozin, and empagliflozin should be discontinued 3 days before scheduled surgery, and ertugliflozin should be stopped at least 4 days before, the agency noted in a press release. Blood glucose should be monitored after drug discontinuation and appropriately managed before surgery.
“The SGLT2 inhibitor may be restarted once the patient’s oral intake is back to baseline and any other risk factors for ketoacidosis are resolved,” the agency added.
SGLT2 inhibitors lower blood sugar by causing the kidney to remove sugar from the body through urine. Side effects for the drugs vary, but include urinary tract infections and genital mycotic infection. Patients with severe renal impairment or end-stage renal disease, who are on dialysis treatment, or who have a known hypersensitivity to the medication should not take SGLT2 inhibitors, the FDA said.
The new changes affect canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, and were made because surgery may put patients being treated with SGLT2 inhibitors at a higher risk of ketoacidosis. Canagliflozin, dapagliflozin, and empagliflozin should be discontinued 3 days before scheduled surgery, and ertugliflozin should be stopped at least 4 days before, the agency noted in a press release. Blood glucose should be monitored after drug discontinuation and appropriately managed before surgery.
“The SGLT2 inhibitor may be restarted once the patient’s oral intake is back to baseline and any other risk factors for ketoacidosis are resolved,” the agency added.
SGLT2 inhibitors lower blood sugar by causing the kidney to remove sugar from the body through urine. Side effects for the drugs vary, but include urinary tract infections and genital mycotic infection. Patients with severe renal impairment or end-stage renal disease, who are on dialysis treatment, or who have a known hypersensitivity to the medication should not take SGLT2 inhibitors, the FDA said.
The new changes affect canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, and were made because surgery may put patients being treated with SGLT2 inhibitors at a higher risk of ketoacidosis. Canagliflozin, dapagliflozin, and empagliflozin should be discontinued 3 days before scheduled surgery, and ertugliflozin should be stopped at least 4 days before, the agency noted in a press release. Blood glucose should be monitored after drug discontinuation and appropriately managed before surgery.
“The SGLT2 inhibitor may be restarted once the patient’s oral intake is back to baseline and any other risk factors for ketoacidosis are resolved,” the agency added.
SGLT2 inhibitors lower blood sugar by causing the kidney to remove sugar from the body through urine. Side effects for the drugs vary, but include urinary tract infections and genital mycotic infection. Patients with severe renal impairment or end-stage renal disease, who are on dialysis treatment, or who have a known hypersensitivity to the medication should not take SGLT2 inhibitors, the FDA said.
ERAS for cesarean delivery: Postoperative care
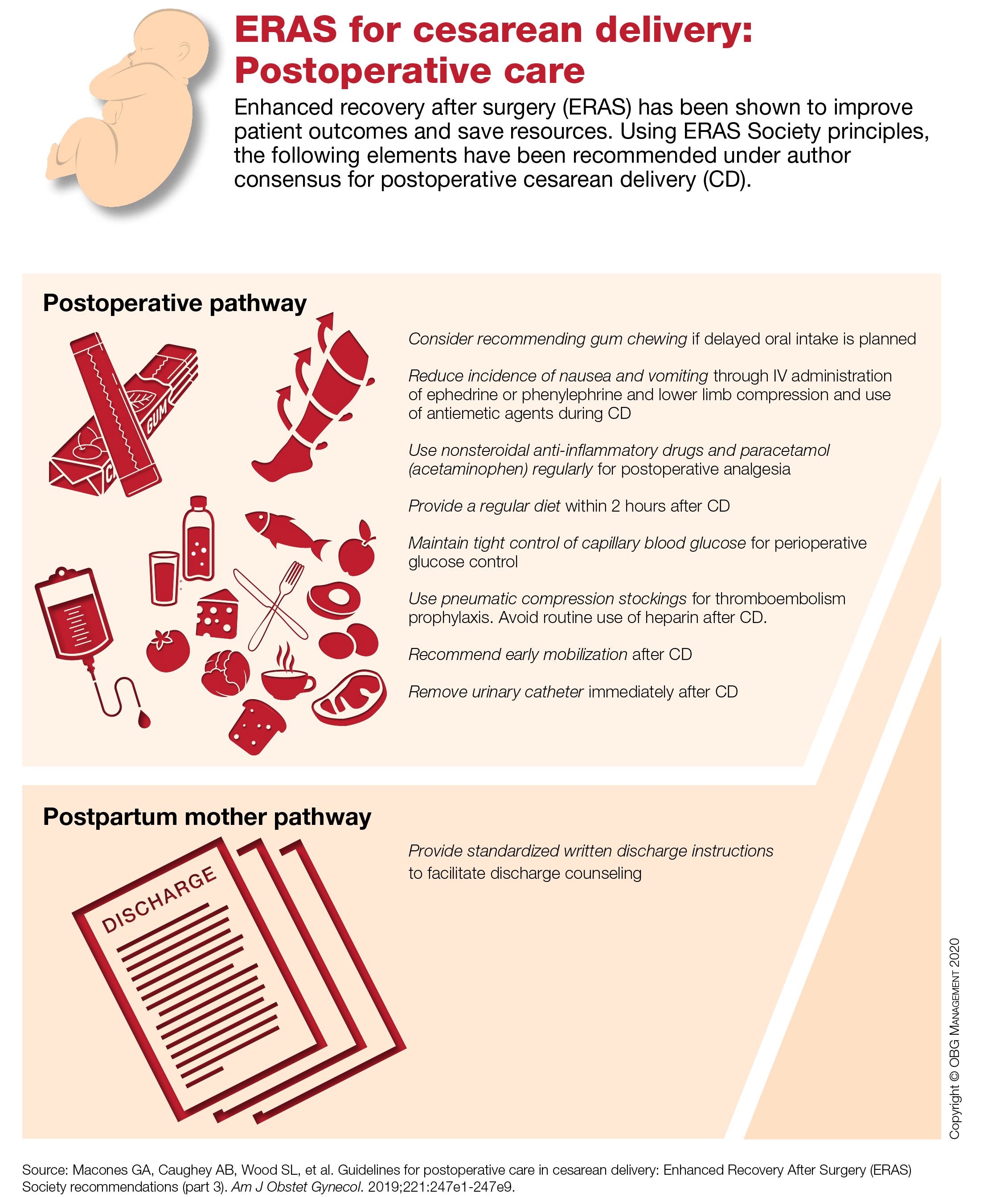


Wuhan case review: COVID-19 characteristics differ in children vs. adults
Pediatric cases of COVID-19 infection are typically mild, but underlying coinfection may be more common in children than in adults, according to an analysis of clinical, laboratory, and chest CT features of pediatric inpatients in Wuhan, China.

The findings point toward a need for early chest CT with corresponding pathogen detection in children with suspected COVID-19 infection, Wei Xia, MD, of Huazhong University of Science and Technology, Wuhan, China, and colleagues reported in Pediatric Pulmonology.
The most common symptoms in 20 pediatric patients hospitalized between Jan. 23 and Feb. 8, 2020, with COVID-19 infection confirmed by the pharyngeal swab COVID-19 nucleic acid test were fever and cough, which occurred in 60% and 65% of patients, respectively. Coinfection was detected in eight patients (40%), they noted.
Clinical manifestations were similar to those seen in adults, but overall symptoms were relatively mild and overall prognosis was good. Of particular note, 7 of the 20 (35%) patients had a previously diagnosed congenital or acquired diseases, suggesting that children with underlying conditions may be more susceptible, Dr. Xia and colleagues wrote.
Laboratory findings also were notable in that 80% of the children had procalcitonin (PCT) elevations not typically seen in adults with COVID-19. PCT is a marker for bacterial infection and “[this finding] may suggest that routine antibacterial treatment should be considered in pediatric patients,” the investigators wrote.
As for imaging results, chest CT findings in children were similar to those in adults.“The typical manifestations were unilateral or bilateral subpleural ground-glass opacities, and consolidations with surrounding halo signs,” Dr. Xia and associates wrote, adding that consolidations with surrounding halo sign accounted for about half the pediatric cases and should be considered as “typical signs in pediatric patients.”
Pediatric cases were “rather rare” in the early days of the COVID-19 outbreak in Wuhan, where the first cases of infection were reported.
“As a pediatric group is usually susceptible to upper respiratory tract infection, because of their developing immune system, the delayed presence of pediatric patients is confusing,” the investigators wrote, noting that a low detection rate of pharyngeal swab COVID-19 nucleic acid test, distinguishing the virus from other common respiratory tract infectious pathogens in pediatric patients, “is still a problem.”
To better characterize the clinical and imaging features in children versus adults with COVID-19, Dr. Xia and associates reviewed these 20 pediatric cases, including 13 boys and 7 girls with ages ranging from less than 1 month to 14 years, 7 months (median 2 years, 1.5 months). Thirteen had an identified close contact with a COVID-19–diagnosed family member, and all were treated in an isolation ward. A total of 18 children were cured and discharged after an average stay of 13 days, and 2 neonates remained under observation because of positive swab results with negative CT findings. The investigators speculated that the different findings in neonates were perhaps caused by the influence of delivery on sampling or the specific CT manifestations for neonates, adding that more samples are needed for further clarification.
Based on these findings, “the CT imaging of COVID-19 infection should be differentiated with other virus pneumonias such as influenza virus, parainfluenza virus, respiratory syncytial virus, and adenovirus,” they concluded. It also should “be differentiated from bacterial pneumonia, mycoplasma pneumonia, and chlamydia pneumonia ... the density of pneumonia lesions caused by the latter pathogens is relatively higher.”
However, Dr. Xia and colleagues noted that chest CT manifestations of pneumonia caused by different pathogens overlap, and COVID-19 pneumonia “can be superimposed with serious and complex imaging manifestations, so epidemiological and etiological examinations should be combined.”
The investigators concluded that COVID-19 virus pneumonia in children is generally mild, and that the characteristic changes of subpleural ground-glass opacities and consolidations with surrounding halo on chest CT provide an “effective means for follow-up and evaluating the changes of lung lesions.”
“In the case that the positive rate of COVID-19 nucleic acid test from pharyngeal swab samples is not high, the early detection of lesions by CT is conducive to reasonable management and early treatment for pediatric patients. However, the diagnosis of COVID-19 pneumonia by CT imaging alone is not sufficient enough, especially in the case of coinfection with other pathogens,” Dr. Xia and associates wrote. “Therefore, early chest CT screening and timely follow-up, combined with corresponding pathogen detection, is a feasible clinical protocol in children.”
An early study
In a separate retrospective analysis described in a letter to the editor of the New England Journal of Medicine, Weiyong Liu, PhD, of Tongji Hospital of Huazhong University of Science and Technology and colleagues found that the most frequently detected pathogens in 366 children under the age of 16 years hospitalized with respiratory infections in Wuhan during Jan. 7-15, 2020, were influenza A virus (6.3% of cases) and influenza B virus (5.5% of cases), whereas COVID-19 was detected in 1.6% of cases.
The median age of the COVID-19 patients in that series was 3 years (range 1-7 years), and in contrast to the findings of Xia et al., all previously had been “completely healthy.” Common characteristics were high fever and cough in all six patients, and vomiting in four patients. Five had pneumonia as assessed by X-ray, and CTs showed typical viral pneumonia patterns.
One patient was admitted to a pediatric ICU. All patients received antiviral agents, antibiotic agents, and supportive therapies; all recovered after a median hospital stay of 7.5 days (median range, 5-13 days).
In contrast with the findings of Xia et al., the findings of Liu et al. showed COVID-19 caused moderate to severe respiratory illness in children, and that infections in children were occurring early in the epidemic.
Some perspective
In an interview regarding the findings by Xia et al., Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University, and director of pediatric infectious diseases at Boston Medical Center, noted the absence of fever in 40% of cases.
“This is important, as the criteria for testing by public health departments has been high fever, cough, and shortness of breath,” he said. “The absence of fever is not inconsistent with COVID-19 disease.”
Another important point regarding the findings by Xia et al. is that the highest attack rates appear to be in children under 1 year of age, he said, further noting that the finding of concurrent influenza A, influenza B, or respiratory syncytial virus underscores that “concurrent infection can occur, and the presence of another virus in diagnostic tests does not mean that COVID-19 is not causal.”
As for whether the finding of elevated procalcitonin levels in 80% of cases reflects COVID-19 disease or coinfection with bacteria, the answer is unclear. But none of the children in the study were proven to have bacterial disease, he said, adding that “this marker will need to be interpreted with caution in the setting of COVID-19 disease.”
Dr. Xia and colleagues reported having no disclosures. Dr. Liu and associates also reported having no disclosures. The study by Liu et al. was supported by the Ministry of Science and Technology of China, the National Mega Project on Major Infectious Disease Prevention, and the National Key Research and Development Program of China.
SOURCES: Xia W et al. Ped Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718; Liu W et al. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Pediatric cases of COVID-19 infection are typically mild, but underlying coinfection may be more common in children than in adults, according to an analysis of clinical, laboratory, and chest CT features of pediatric inpatients in Wuhan, China.

The findings point toward a need for early chest CT with corresponding pathogen detection in children with suspected COVID-19 infection, Wei Xia, MD, of Huazhong University of Science and Technology, Wuhan, China, and colleagues reported in Pediatric Pulmonology.
The most common symptoms in 20 pediatric patients hospitalized between Jan. 23 and Feb. 8, 2020, with COVID-19 infection confirmed by the pharyngeal swab COVID-19 nucleic acid test were fever and cough, which occurred in 60% and 65% of patients, respectively. Coinfection was detected in eight patients (40%), they noted.
Clinical manifestations were similar to those seen in adults, but overall symptoms were relatively mild and overall prognosis was good. Of particular note, 7 of the 20 (35%) patients had a previously diagnosed congenital or acquired diseases, suggesting that children with underlying conditions may be more susceptible, Dr. Xia and colleagues wrote.
Laboratory findings also were notable in that 80% of the children had procalcitonin (PCT) elevations not typically seen in adults with COVID-19. PCT is a marker for bacterial infection and “[this finding] may suggest that routine antibacterial treatment should be considered in pediatric patients,” the investigators wrote.
As for imaging results, chest CT findings in children were similar to those in adults.“The typical manifestations were unilateral or bilateral subpleural ground-glass opacities, and consolidations with surrounding halo signs,” Dr. Xia and associates wrote, adding that consolidations with surrounding halo sign accounted for about half the pediatric cases and should be considered as “typical signs in pediatric patients.”
Pediatric cases were “rather rare” in the early days of the COVID-19 outbreak in Wuhan, where the first cases of infection were reported.
“As a pediatric group is usually susceptible to upper respiratory tract infection, because of their developing immune system, the delayed presence of pediatric patients is confusing,” the investigators wrote, noting that a low detection rate of pharyngeal swab COVID-19 nucleic acid test, distinguishing the virus from other common respiratory tract infectious pathogens in pediatric patients, “is still a problem.”
To better characterize the clinical and imaging features in children versus adults with COVID-19, Dr. Xia and associates reviewed these 20 pediatric cases, including 13 boys and 7 girls with ages ranging from less than 1 month to 14 years, 7 months (median 2 years, 1.5 months). Thirteen had an identified close contact with a COVID-19–diagnosed family member, and all were treated in an isolation ward. A total of 18 children were cured and discharged after an average stay of 13 days, and 2 neonates remained under observation because of positive swab results with negative CT findings. The investigators speculated that the different findings in neonates were perhaps caused by the influence of delivery on sampling or the specific CT manifestations for neonates, adding that more samples are needed for further clarification.
Based on these findings, “the CT imaging of COVID-19 infection should be differentiated with other virus pneumonias such as influenza virus, parainfluenza virus, respiratory syncytial virus, and adenovirus,” they concluded. It also should “be differentiated from bacterial pneumonia, mycoplasma pneumonia, and chlamydia pneumonia ... the density of pneumonia lesions caused by the latter pathogens is relatively higher.”
However, Dr. Xia and colleagues noted that chest CT manifestations of pneumonia caused by different pathogens overlap, and COVID-19 pneumonia “can be superimposed with serious and complex imaging manifestations, so epidemiological and etiological examinations should be combined.”
The investigators concluded that COVID-19 virus pneumonia in children is generally mild, and that the characteristic changes of subpleural ground-glass opacities and consolidations with surrounding halo on chest CT provide an “effective means for follow-up and evaluating the changes of lung lesions.”
“In the case that the positive rate of COVID-19 nucleic acid test from pharyngeal swab samples is not high, the early detection of lesions by CT is conducive to reasonable management and early treatment for pediatric patients. However, the diagnosis of COVID-19 pneumonia by CT imaging alone is not sufficient enough, especially in the case of coinfection with other pathogens,” Dr. Xia and associates wrote. “Therefore, early chest CT screening and timely follow-up, combined with corresponding pathogen detection, is a feasible clinical protocol in children.”
An early study
In a separate retrospective analysis described in a letter to the editor of the New England Journal of Medicine, Weiyong Liu, PhD, of Tongji Hospital of Huazhong University of Science and Technology and colleagues found that the most frequently detected pathogens in 366 children under the age of 16 years hospitalized with respiratory infections in Wuhan during Jan. 7-15, 2020, were influenza A virus (6.3% of cases) and influenza B virus (5.5% of cases), whereas COVID-19 was detected in 1.6% of cases.
The median age of the COVID-19 patients in that series was 3 years (range 1-7 years), and in contrast to the findings of Xia et al., all previously had been “completely healthy.” Common characteristics were high fever and cough in all six patients, and vomiting in four patients. Five had pneumonia as assessed by X-ray, and CTs showed typical viral pneumonia patterns.
One patient was admitted to a pediatric ICU. All patients received antiviral agents, antibiotic agents, and supportive therapies; all recovered after a median hospital stay of 7.5 days (median range, 5-13 days).
In contrast with the findings of Xia et al., the findings of Liu et al. showed COVID-19 caused moderate to severe respiratory illness in children, and that infections in children were occurring early in the epidemic.
Some perspective
In an interview regarding the findings by Xia et al., Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University, and director of pediatric infectious diseases at Boston Medical Center, noted the absence of fever in 40% of cases.
“This is important, as the criteria for testing by public health departments has been high fever, cough, and shortness of breath,” he said. “The absence of fever is not inconsistent with COVID-19 disease.”
Another important point regarding the findings by Xia et al. is that the highest attack rates appear to be in children under 1 year of age, he said, further noting that the finding of concurrent influenza A, influenza B, or respiratory syncytial virus underscores that “concurrent infection can occur, and the presence of another virus in diagnostic tests does not mean that COVID-19 is not causal.”
As for whether the finding of elevated procalcitonin levels in 80% of cases reflects COVID-19 disease or coinfection with bacteria, the answer is unclear. But none of the children in the study were proven to have bacterial disease, he said, adding that “this marker will need to be interpreted with caution in the setting of COVID-19 disease.”
Dr. Xia and colleagues reported having no disclosures. Dr. Liu and associates also reported having no disclosures. The study by Liu et al. was supported by the Ministry of Science and Technology of China, the National Mega Project on Major Infectious Disease Prevention, and the National Key Research and Development Program of China.
SOURCES: Xia W et al. Ped Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718; Liu W et al. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Pediatric cases of COVID-19 infection are typically mild, but underlying coinfection may be more common in children than in adults, according to an analysis of clinical, laboratory, and chest CT features of pediatric inpatients in Wuhan, China.

The findings point toward a need for early chest CT with corresponding pathogen detection in children with suspected COVID-19 infection, Wei Xia, MD, of Huazhong University of Science and Technology, Wuhan, China, and colleagues reported in Pediatric Pulmonology.
The most common symptoms in 20 pediatric patients hospitalized between Jan. 23 and Feb. 8, 2020, with COVID-19 infection confirmed by the pharyngeal swab COVID-19 nucleic acid test were fever and cough, which occurred in 60% and 65% of patients, respectively. Coinfection was detected in eight patients (40%), they noted.
Clinical manifestations were similar to those seen in adults, but overall symptoms were relatively mild and overall prognosis was good. Of particular note, 7 of the 20 (35%) patients had a previously diagnosed congenital or acquired diseases, suggesting that children with underlying conditions may be more susceptible, Dr. Xia and colleagues wrote.
Laboratory findings also were notable in that 80% of the children had procalcitonin (PCT) elevations not typically seen in adults with COVID-19. PCT is a marker for bacterial infection and “[this finding] may suggest that routine antibacterial treatment should be considered in pediatric patients,” the investigators wrote.
As for imaging results, chest CT findings in children were similar to those in adults.“The typical manifestations were unilateral or bilateral subpleural ground-glass opacities, and consolidations with surrounding halo signs,” Dr. Xia and associates wrote, adding that consolidations with surrounding halo sign accounted for about half the pediatric cases and should be considered as “typical signs in pediatric patients.”
Pediatric cases were “rather rare” in the early days of the COVID-19 outbreak in Wuhan, where the first cases of infection were reported.
“As a pediatric group is usually susceptible to upper respiratory tract infection, because of their developing immune system, the delayed presence of pediatric patients is confusing,” the investigators wrote, noting that a low detection rate of pharyngeal swab COVID-19 nucleic acid test, distinguishing the virus from other common respiratory tract infectious pathogens in pediatric patients, “is still a problem.”
To better characterize the clinical and imaging features in children versus adults with COVID-19, Dr. Xia and associates reviewed these 20 pediatric cases, including 13 boys and 7 girls with ages ranging from less than 1 month to 14 years, 7 months (median 2 years, 1.5 months). Thirteen had an identified close contact with a COVID-19–diagnosed family member, and all were treated in an isolation ward. A total of 18 children were cured and discharged after an average stay of 13 days, and 2 neonates remained under observation because of positive swab results with negative CT findings. The investigators speculated that the different findings in neonates were perhaps caused by the influence of delivery on sampling or the specific CT manifestations for neonates, adding that more samples are needed for further clarification.
Based on these findings, “the CT imaging of COVID-19 infection should be differentiated with other virus pneumonias such as influenza virus, parainfluenza virus, respiratory syncytial virus, and adenovirus,” they concluded. It also should “be differentiated from bacterial pneumonia, mycoplasma pneumonia, and chlamydia pneumonia ... the density of pneumonia lesions caused by the latter pathogens is relatively higher.”
However, Dr. Xia and colleagues noted that chest CT manifestations of pneumonia caused by different pathogens overlap, and COVID-19 pneumonia “can be superimposed with serious and complex imaging manifestations, so epidemiological and etiological examinations should be combined.”
The investigators concluded that COVID-19 virus pneumonia in children is generally mild, and that the characteristic changes of subpleural ground-glass opacities and consolidations with surrounding halo on chest CT provide an “effective means for follow-up and evaluating the changes of lung lesions.”
“In the case that the positive rate of COVID-19 nucleic acid test from pharyngeal swab samples is not high, the early detection of lesions by CT is conducive to reasonable management and early treatment for pediatric patients. However, the diagnosis of COVID-19 pneumonia by CT imaging alone is not sufficient enough, especially in the case of coinfection with other pathogens,” Dr. Xia and associates wrote. “Therefore, early chest CT screening and timely follow-up, combined with corresponding pathogen detection, is a feasible clinical protocol in children.”
An early study
In a separate retrospective analysis described in a letter to the editor of the New England Journal of Medicine, Weiyong Liu, PhD, of Tongji Hospital of Huazhong University of Science and Technology and colleagues found that the most frequently detected pathogens in 366 children under the age of 16 years hospitalized with respiratory infections in Wuhan during Jan. 7-15, 2020, were influenza A virus (6.3% of cases) and influenza B virus (5.5% of cases), whereas COVID-19 was detected in 1.6% of cases.
The median age of the COVID-19 patients in that series was 3 years (range 1-7 years), and in contrast to the findings of Xia et al., all previously had been “completely healthy.” Common characteristics were high fever and cough in all six patients, and vomiting in four patients. Five had pneumonia as assessed by X-ray, and CTs showed typical viral pneumonia patterns.
One patient was admitted to a pediatric ICU. All patients received antiviral agents, antibiotic agents, and supportive therapies; all recovered after a median hospital stay of 7.5 days (median range, 5-13 days).
In contrast with the findings of Xia et al., the findings of Liu et al. showed COVID-19 caused moderate to severe respiratory illness in children, and that infections in children were occurring early in the epidemic.
Some perspective
In an interview regarding the findings by Xia et al., Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University, and director of pediatric infectious diseases at Boston Medical Center, noted the absence of fever in 40% of cases.
“This is important, as the criteria for testing by public health departments has been high fever, cough, and shortness of breath,” he said. “The absence of fever is not inconsistent with COVID-19 disease.”
Another important point regarding the findings by Xia et al. is that the highest attack rates appear to be in children under 1 year of age, he said, further noting that the finding of concurrent influenza A, influenza B, or respiratory syncytial virus underscores that “concurrent infection can occur, and the presence of another virus in diagnostic tests does not mean that COVID-19 is not causal.”
As for whether the finding of elevated procalcitonin levels in 80% of cases reflects COVID-19 disease or coinfection with bacteria, the answer is unclear. But none of the children in the study were proven to have bacterial disease, he said, adding that “this marker will need to be interpreted with caution in the setting of COVID-19 disease.”
Dr. Xia and colleagues reported having no disclosures. Dr. Liu and associates also reported having no disclosures. The study by Liu et al. was supported by the Ministry of Science and Technology of China, the National Mega Project on Major Infectious Disease Prevention, and the National Key Research and Development Program of China.
SOURCES: Xia W et al. Ped Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718; Liu W et al. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
FROM PEDIATRIC PULMONOLOGY
Managing children’s fear, anxiety in the age of COVID-19
With coronavirus disease (COVID-19) reaching epidemic proportions, many US children are growing increasingly anxious about what this means for their own health and safety and that of their friends and family.
The constantly changing numbers of people affected by the virus and the evolving situation mean daily life for many children is affected in some way, with school trips, sports tournaments, and family vacations being postponed or canceled.
All children may have a heightened level of worry, and some who are normally anxious might be obsessing more about handwashing or getting sick.
Experts say there are ways to manage this fear to help children feel safe and appropriately informed.
Clinicians and other adults should provide children with honest and accurate information geared to their age and developmental level, said David Fassler, MD, clinical professor of psychiatry, University of Vermont Larner College of Medicine, Burlington, and member of the Consumer Issues Committee of the American Academy of Child and Adolescent Psychiatry.
That said, it’s also acceptable to let children know that some questions can’t be answered, said Fassler.
Be truthful, calm
“This is partly because the information keeps changing as we learn more about how the virus spreads, how to best protect communities, and how to treat people who get sick,” he added.
Clinicians and parents should remind children “that there are a lot of adults who are working very hard to keep them safe,” said Eli R. Lebowitz, PhD, associate professor in the Child Study Center, Yale School of Medicine, New Haven, Connecticut, who directs a program for anxiety.
It’s important for adults to pay attention not only to what they say to children but also how they say it, said Lebowitz. He highlighted the importance of talking about the virus “in a calm and matter-of-fact way” rather than in an anxious way.
“If you look scared or tense or your voice is conveying that you’re really scared, the child is going to absorb that and feel anxious as well,” he noted.
This advice also applies when adults are discussing the issue among themselves. They should be aware that “children are listening” and are picking up any anxiety or panic adults are expressing.
Children are soaking up information about this virus from the Internet, the media, friends, teachers, and elsewhere. Lebowitz suggests asking children what they have already heard, which provides an opportunity to correct rumors and inaccurate information.
“A child might have a very inflated sense of what the actual risk is. For example, they may think that anyone who gets the virus dies,” he said.
Myth busting
Adults should let children know that not everything they hear from friends or on the Internet “is necessarily correct,” he added.
Some children who have experienced serious illness or losses may be particularly vulnerable to experiencing intense reactions to graphic news reports or images of illness or death and may need extra support, said Fassler.
Adults could use the “framework of knowledge” that children already have, said Lebowitz. He noted that all children are aware of sickness.
“They know people get sick, and they themselves have probably been sick, so you can tell them that this is a sickness like a bad flu,” he said.
Children should be encouraged to approach adults they trust, such as their pediatrician, a parent, or a teacher, with their questions, said Lebowitz. “Those are the people who are able to give them the most accurate information.”
Fassler noted that accurate, up-to-date information is available via fact sheets developed by the Centers for Disease Control and Prevention and the World Health Organization.
Although it’s helpful and appropriate to be reassuring, Fassler advises not to make unrealistic promises.
“It’s fine to tell kids that you’ll deal with whatever happens, even if it means altering travel plans or work schedules, but you can’t promise that no one in your state or community will get sick,” he said.
Maintain healthy habits
Physicians and other adults can tell children “in an age-appropriate way” how the virus is transmitted and what the symptoms are, but it’s important to emphasize that most people who are sick don’t have COVID-19, said Lebowitz.
“I would emphasize that the people who are the sickest are the elderly who are already sick, rather than healthy younger people,” he said.
Lebowitz recommends continuing to follow guidelines on staying healthy, including coughing into a sleeve instead of your hand and regular handwashing.
It’s also important at this time for children to maintain healthy habits – getting enough physical activity and sleep, eating well, and being outside – because this regime will go a long way toward reducing anxiety, said Lebowitz. Deep breathing and muscle-relaxing exercises can also help, he said.
Lebowitz also suggests maintaining a supportive attitude and showing “some acceptance and validation of what children are feeling, as well as some confidence that they can cope and tolerate feeling uncomfortable sometimes, that they can handle some anxiety.”
While accepting that the child could be anxious, it’s important not to encourage excessive avoidance or unhealthy coping strategies. Fassler and Lebowitz agree that children who are overly anxious or preoccupied with concerns about the coronavirus should be evaluated by a trained, qualified mental health professional.
Signs that a child may need additional help include ongoing sleep difficulties, intrusive thoughts or worries, obsessive-compulsive behaviors, or reluctance or refusal to go to school, said Fassler.
The good news is that most children are resilient, said Fassler. “They’ll adjust, adapt, and go on with their lives.”
This article first appeared on Medscape.com.
With coronavirus disease (COVID-19) reaching epidemic proportions, many US children are growing increasingly anxious about what this means for their own health and safety and that of their friends and family.
The constantly changing numbers of people affected by the virus and the evolving situation mean daily life for many children is affected in some way, with school trips, sports tournaments, and family vacations being postponed or canceled.
All children may have a heightened level of worry, and some who are normally anxious might be obsessing more about handwashing or getting sick.
Experts say there are ways to manage this fear to help children feel safe and appropriately informed.
Clinicians and other adults should provide children with honest and accurate information geared to their age and developmental level, said David Fassler, MD, clinical professor of psychiatry, University of Vermont Larner College of Medicine, Burlington, and member of the Consumer Issues Committee of the American Academy of Child and Adolescent Psychiatry.
That said, it’s also acceptable to let children know that some questions can’t be answered, said Fassler.
Be truthful, calm
“This is partly because the information keeps changing as we learn more about how the virus spreads, how to best protect communities, and how to treat people who get sick,” he added.
Clinicians and parents should remind children “that there are a lot of adults who are working very hard to keep them safe,” said Eli R. Lebowitz, PhD, associate professor in the Child Study Center, Yale School of Medicine, New Haven, Connecticut, who directs a program for anxiety.
It’s important for adults to pay attention not only to what they say to children but also how they say it, said Lebowitz. He highlighted the importance of talking about the virus “in a calm and matter-of-fact way” rather than in an anxious way.
“If you look scared or tense or your voice is conveying that you’re really scared, the child is going to absorb that and feel anxious as well,” he noted.
This advice also applies when adults are discussing the issue among themselves. They should be aware that “children are listening” and are picking up any anxiety or panic adults are expressing.
Children are soaking up information about this virus from the Internet, the media, friends, teachers, and elsewhere. Lebowitz suggests asking children what they have already heard, which provides an opportunity to correct rumors and inaccurate information.
“A child might have a very inflated sense of what the actual risk is. For example, they may think that anyone who gets the virus dies,” he said.
Myth busting
Adults should let children know that not everything they hear from friends or on the Internet “is necessarily correct,” he added.
Some children who have experienced serious illness or losses may be particularly vulnerable to experiencing intense reactions to graphic news reports or images of illness or death and may need extra support, said Fassler.
Adults could use the “framework of knowledge” that children already have, said Lebowitz. He noted that all children are aware of sickness.
“They know people get sick, and they themselves have probably been sick, so you can tell them that this is a sickness like a bad flu,” he said.
Children should be encouraged to approach adults they trust, such as their pediatrician, a parent, or a teacher, with their questions, said Lebowitz. “Those are the people who are able to give them the most accurate information.”
Fassler noted that accurate, up-to-date information is available via fact sheets developed by the Centers for Disease Control and Prevention and the World Health Organization.
Although it’s helpful and appropriate to be reassuring, Fassler advises not to make unrealistic promises.
“It’s fine to tell kids that you’ll deal with whatever happens, even if it means altering travel plans or work schedules, but you can’t promise that no one in your state or community will get sick,” he said.
Maintain healthy habits
Physicians and other adults can tell children “in an age-appropriate way” how the virus is transmitted and what the symptoms are, but it’s important to emphasize that most people who are sick don’t have COVID-19, said Lebowitz.
“I would emphasize that the people who are the sickest are the elderly who are already sick, rather than healthy younger people,” he said.
Lebowitz recommends continuing to follow guidelines on staying healthy, including coughing into a sleeve instead of your hand and regular handwashing.
It’s also important at this time for children to maintain healthy habits – getting enough physical activity and sleep, eating well, and being outside – because this regime will go a long way toward reducing anxiety, said Lebowitz. Deep breathing and muscle-relaxing exercises can also help, he said.
Lebowitz also suggests maintaining a supportive attitude and showing “some acceptance and validation of what children are feeling, as well as some confidence that they can cope and tolerate feeling uncomfortable sometimes, that they can handle some anxiety.”
While accepting that the child could be anxious, it’s important not to encourage excessive avoidance or unhealthy coping strategies. Fassler and Lebowitz agree that children who are overly anxious or preoccupied with concerns about the coronavirus should be evaluated by a trained, qualified mental health professional.
Signs that a child may need additional help include ongoing sleep difficulties, intrusive thoughts or worries, obsessive-compulsive behaviors, or reluctance or refusal to go to school, said Fassler.
The good news is that most children are resilient, said Fassler. “They’ll adjust, adapt, and go on with their lives.”
This article first appeared on Medscape.com.
With coronavirus disease (COVID-19) reaching epidemic proportions, many US children are growing increasingly anxious about what this means for their own health and safety and that of their friends and family.
The constantly changing numbers of people affected by the virus and the evolving situation mean daily life for many children is affected in some way, with school trips, sports tournaments, and family vacations being postponed or canceled.
All children may have a heightened level of worry, and some who are normally anxious might be obsessing more about handwashing or getting sick.
Experts say there are ways to manage this fear to help children feel safe and appropriately informed.
Clinicians and other adults should provide children with honest and accurate information geared to their age and developmental level, said David Fassler, MD, clinical professor of psychiatry, University of Vermont Larner College of Medicine, Burlington, and member of the Consumer Issues Committee of the American Academy of Child and Adolescent Psychiatry.
That said, it’s also acceptable to let children know that some questions can’t be answered, said Fassler.
Be truthful, calm
“This is partly because the information keeps changing as we learn more about how the virus spreads, how to best protect communities, and how to treat people who get sick,” he added.
Clinicians and parents should remind children “that there are a lot of adults who are working very hard to keep them safe,” said Eli R. Lebowitz, PhD, associate professor in the Child Study Center, Yale School of Medicine, New Haven, Connecticut, who directs a program for anxiety.
It’s important for adults to pay attention not only to what they say to children but also how they say it, said Lebowitz. He highlighted the importance of talking about the virus “in a calm and matter-of-fact way” rather than in an anxious way.
“If you look scared or tense or your voice is conveying that you’re really scared, the child is going to absorb that and feel anxious as well,” he noted.
This advice also applies when adults are discussing the issue among themselves. They should be aware that “children are listening” and are picking up any anxiety or panic adults are expressing.
Children are soaking up information about this virus from the Internet, the media, friends, teachers, and elsewhere. Lebowitz suggests asking children what they have already heard, which provides an opportunity to correct rumors and inaccurate information.
“A child might have a very inflated sense of what the actual risk is. For example, they may think that anyone who gets the virus dies,” he said.
Myth busting
Adults should let children know that not everything they hear from friends or on the Internet “is necessarily correct,” he added.
Some children who have experienced serious illness or losses may be particularly vulnerable to experiencing intense reactions to graphic news reports or images of illness or death and may need extra support, said Fassler.
Adults could use the “framework of knowledge” that children already have, said Lebowitz. He noted that all children are aware of sickness.
“They know people get sick, and they themselves have probably been sick, so you can tell them that this is a sickness like a bad flu,” he said.
Children should be encouraged to approach adults they trust, such as their pediatrician, a parent, or a teacher, with their questions, said Lebowitz. “Those are the people who are able to give them the most accurate information.”
Fassler noted that accurate, up-to-date information is available via fact sheets developed by the Centers for Disease Control and Prevention and the World Health Organization.
Although it’s helpful and appropriate to be reassuring, Fassler advises not to make unrealistic promises.
“It’s fine to tell kids that you’ll deal with whatever happens, even if it means altering travel plans or work schedules, but you can’t promise that no one in your state or community will get sick,” he said.
Maintain healthy habits
Physicians and other adults can tell children “in an age-appropriate way” how the virus is transmitted and what the symptoms are, but it’s important to emphasize that most people who are sick don’t have COVID-19, said Lebowitz.
“I would emphasize that the people who are the sickest are the elderly who are already sick, rather than healthy younger people,” he said.
Lebowitz recommends continuing to follow guidelines on staying healthy, including coughing into a sleeve instead of your hand and regular handwashing.
It’s also important at this time for children to maintain healthy habits – getting enough physical activity and sleep, eating well, and being outside – because this regime will go a long way toward reducing anxiety, said Lebowitz. Deep breathing and muscle-relaxing exercises can also help, he said.
Lebowitz also suggests maintaining a supportive attitude and showing “some acceptance and validation of what children are feeling, as well as some confidence that they can cope and tolerate feeling uncomfortable sometimes, that they can handle some anxiety.”
While accepting that the child could be anxious, it’s important not to encourage excessive avoidance or unhealthy coping strategies. Fassler and Lebowitz agree that children who are overly anxious or preoccupied with concerns about the coronavirus should be evaluated by a trained, qualified mental health professional.
Signs that a child may need additional help include ongoing sleep difficulties, intrusive thoughts or worries, obsessive-compulsive behaviors, or reluctance or refusal to go to school, said Fassler.
The good news is that most children are resilient, said Fassler. “They’ll adjust, adapt, and go on with their lives.”
This article first appeared on Medscape.com.
In the management of cesarean scar defects, is there a superior surgical method for treatment?
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.