User login
Low LDL cholesterol may increase women’s risk of hemorrhagic stroke
, according to research published in Neurology.
“Women with very low LDL cholesterol or low triglycerides should be monitored by their doctors for other stroke risk factors that can be modified, like high blood pressure and smoking, in order to reduce their risk of hemorrhagic stroke,” said Pamela M. Rist, ScD, instructor in epidemiology at Harvard Medical School, Boston. “Additional research is needed to determine how to lower the risk of hemorrhagic stroke in women with very low LDL and low triglycerides.”
Several meta-analyses have indicated that LDL cholesterol levels are inversely associated with the risk of hemorrhagic stroke. Because lipid-lowering treatments are used to prevent cardiovascular disease, this potential association has implications for clinical practice. Most of the studies included in these meta-analyses had low numbers of events among women, which prevented researchers from stratifying their results by sex. Because women are at greater risk of stroke than men, Dr. Rist and her colleagues sought to evaluate the association between lipid levels and risk of hemorrhagic stroke.
An analysis of the Women’s Health Study
The investigators examined data from the Women’s Health Study, a randomized, double-blind, placebo-controlled trial of low-dose aspirin and vitamin E for the primary prevention of cardiovascular disease and cancer among female American health professionals aged 45 years or older. The study ended in March 2004, but follow-up is ongoing. At regular intervals, the women complete a questionnaire about disease outcomes, including stroke. Some participants agreed to provide a fasting venous blood sample before randomization. With the subjects’ permission, a committee of physicians examined medical records for women who reported a stroke on a follow-up questionnaire.
Dr. Rist and her colleagues analyzed 27,937 samples for levels of LDL cholesterol, HDL cholesterol, total cholesterol, and triglycerides. They assigned each sample to one of five cholesterol level categories that were based on Adult Treatment Panel III guidelines. Cox proportional hazards models enabled the researchers to calculate the hazard ratio of incident hemorrhagic stroke events. They adjusted their results for covariates such as age, smoking status, menopausal status, body mass index, and alcohol consumption.
A U-shaped association
Women in the lowest category of LDL cholesterol level (less than 70 mg/dL) were younger, less likely to have a history of hypertension, and less likely to use cholesterol-lowering drugs than women in the reference group (100.0-129.9 mg/dL). Women with the lowest LDL cholesterol level were more likely to consume alcohol, have a normal weight, engage in physical activity, and be premenopausal than women in the reference group. The investigators confirmed 137 incident hemorrhagic stroke events during a mean 19.3 years of follow-up.
After data adjustment, the researchers found that women with the lowest level of LDL cholesterol had 2.17 times the risk of hemorrhagic stroke, compared with participants in the reference group. They found a trend toward increased risk among women with an LDL cholesterol level of 160 mg/dL or higher, but the result was not statistically significant. The highest risk for intracerebral hemorrhage (ICH) was among women with an LDL cholesterol level of less than 70 mg/dL (relative risk, 2.32), followed by women with a level of 160 mg/dL or higher (RR, 1.71).
In addition, after multivariable adjustment, women in the lowest quartile of triglycerides (less than or equal to 74 mg/dL for fasting and less than or equal to 85 mg/dL for nonfasting) had a significantly increased risk of hemorrhagic stroke, compared with women in the highest quartile (RR, 2.00). Low triglyceride levels were associated with an increased risk of subarachnoid hemorrhage, but not with an increased risk of ICH. Neither HDL cholesterol nor total cholesterol was associated with risk of hemorrhagic stroke, the researchers wrote.
Mechanism of increased risk unclear
The researchers do not yet know how low triglyceride and LDL cholesterol levels increase the risk of hemorrhagic stroke. One hypothesis is that low cholesterol promotes necrosis of the arterial medial layer’s smooth muscle cells. This impaired endothelium might be more susceptible to microaneurysms, which are common in patients with ICH, said the researchers.
The prospective design and the large sample size were two of the study’s strengths, but the study had important weaknesses as well, the researchers wrote. For example, few women were premenopausal at baseline, so the investigators could not evaluate whether menopausal status modifies the association between lipid levels and risk of hemorrhagic stroke. In addition, lipid levels were measured only at baseline, which prevented an analysis of whether change in lipid levels over time modifies the risk of hemorrhagic stroke.
Dr. Rist reported receiving a grant from the National Institutes of Health.
SOURCE: Rist PM et al. Neurology. 2019 April 10. doi: 10.1212/WNL.0000000000007454.
, according to research published in Neurology.
“Women with very low LDL cholesterol or low triglycerides should be monitored by their doctors for other stroke risk factors that can be modified, like high blood pressure and smoking, in order to reduce their risk of hemorrhagic stroke,” said Pamela M. Rist, ScD, instructor in epidemiology at Harvard Medical School, Boston. “Additional research is needed to determine how to lower the risk of hemorrhagic stroke in women with very low LDL and low triglycerides.”
Several meta-analyses have indicated that LDL cholesterol levels are inversely associated with the risk of hemorrhagic stroke. Because lipid-lowering treatments are used to prevent cardiovascular disease, this potential association has implications for clinical practice. Most of the studies included in these meta-analyses had low numbers of events among women, which prevented researchers from stratifying their results by sex. Because women are at greater risk of stroke than men, Dr. Rist and her colleagues sought to evaluate the association between lipid levels and risk of hemorrhagic stroke.
An analysis of the Women’s Health Study
The investigators examined data from the Women’s Health Study, a randomized, double-blind, placebo-controlled trial of low-dose aspirin and vitamin E for the primary prevention of cardiovascular disease and cancer among female American health professionals aged 45 years or older. The study ended in March 2004, but follow-up is ongoing. At regular intervals, the women complete a questionnaire about disease outcomes, including stroke. Some participants agreed to provide a fasting venous blood sample before randomization. With the subjects’ permission, a committee of physicians examined medical records for women who reported a stroke on a follow-up questionnaire.
Dr. Rist and her colleagues analyzed 27,937 samples for levels of LDL cholesterol, HDL cholesterol, total cholesterol, and triglycerides. They assigned each sample to one of five cholesterol level categories that were based on Adult Treatment Panel III guidelines. Cox proportional hazards models enabled the researchers to calculate the hazard ratio of incident hemorrhagic stroke events. They adjusted their results for covariates such as age, smoking status, menopausal status, body mass index, and alcohol consumption.
A U-shaped association
Women in the lowest category of LDL cholesterol level (less than 70 mg/dL) were younger, less likely to have a history of hypertension, and less likely to use cholesterol-lowering drugs than women in the reference group (100.0-129.9 mg/dL). Women with the lowest LDL cholesterol level were more likely to consume alcohol, have a normal weight, engage in physical activity, and be premenopausal than women in the reference group. The investigators confirmed 137 incident hemorrhagic stroke events during a mean 19.3 years of follow-up.
After data adjustment, the researchers found that women with the lowest level of LDL cholesterol had 2.17 times the risk of hemorrhagic stroke, compared with participants in the reference group. They found a trend toward increased risk among women with an LDL cholesterol level of 160 mg/dL or higher, but the result was not statistically significant. The highest risk for intracerebral hemorrhage (ICH) was among women with an LDL cholesterol level of less than 70 mg/dL (relative risk, 2.32), followed by women with a level of 160 mg/dL or higher (RR, 1.71).
In addition, after multivariable adjustment, women in the lowest quartile of triglycerides (less than or equal to 74 mg/dL for fasting and less than or equal to 85 mg/dL for nonfasting) had a significantly increased risk of hemorrhagic stroke, compared with women in the highest quartile (RR, 2.00). Low triglyceride levels were associated with an increased risk of subarachnoid hemorrhage, but not with an increased risk of ICH. Neither HDL cholesterol nor total cholesterol was associated with risk of hemorrhagic stroke, the researchers wrote.
Mechanism of increased risk unclear
The researchers do not yet know how low triglyceride and LDL cholesterol levels increase the risk of hemorrhagic stroke. One hypothesis is that low cholesterol promotes necrosis of the arterial medial layer’s smooth muscle cells. This impaired endothelium might be more susceptible to microaneurysms, which are common in patients with ICH, said the researchers.
The prospective design and the large sample size were two of the study’s strengths, but the study had important weaknesses as well, the researchers wrote. For example, few women were premenopausal at baseline, so the investigators could not evaluate whether menopausal status modifies the association between lipid levels and risk of hemorrhagic stroke. In addition, lipid levels were measured only at baseline, which prevented an analysis of whether change in lipid levels over time modifies the risk of hemorrhagic stroke.
Dr. Rist reported receiving a grant from the National Institutes of Health.
SOURCE: Rist PM et al. Neurology. 2019 April 10. doi: 10.1212/WNL.0000000000007454.
, according to research published in Neurology.
“Women with very low LDL cholesterol or low triglycerides should be monitored by their doctors for other stroke risk factors that can be modified, like high blood pressure and smoking, in order to reduce their risk of hemorrhagic stroke,” said Pamela M. Rist, ScD, instructor in epidemiology at Harvard Medical School, Boston. “Additional research is needed to determine how to lower the risk of hemorrhagic stroke in women with very low LDL and low triglycerides.”
Several meta-analyses have indicated that LDL cholesterol levels are inversely associated with the risk of hemorrhagic stroke. Because lipid-lowering treatments are used to prevent cardiovascular disease, this potential association has implications for clinical practice. Most of the studies included in these meta-analyses had low numbers of events among women, which prevented researchers from stratifying their results by sex. Because women are at greater risk of stroke than men, Dr. Rist and her colleagues sought to evaluate the association between lipid levels and risk of hemorrhagic stroke.
An analysis of the Women’s Health Study
The investigators examined data from the Women’s Health Study, a randomized, double-blind, placebo-controlled trial of low-dose aspirin and vitamin E for the primary prevention of cardiovascular disease and cancer among female American health professionals aged 45 years or older. The study ended in March 2004, but follow-up is ongoing. At regular intervals, the women complete a questionnaire about disease outcomes, including stroke. Some participants agreed to provide a fasting venous blood sample before randomization. With the subjects’ permission, a committee of physicians examined medical records for women who reported a stroke on a follow-up questionnaire.
Dr. Rist and her colleagues analyzed 27,937 samples for levels of LDL cholesterol, HDL cholesterol, total cholesterol, and triglycerides. They assigned each sample to one of five cholesterol level categories that were based on Adult Treatment Panel III guidelines. Cox proportional hazards models enabled the researchers to calculate the hazard ratio of incident hemorrhagic stroke events. They adjusted their results for covariates such as age, smoking status, menopausal status, body mass index, and alcohol consumption.
A U-shaped association
Women in the lowest category of LDL cholesterol level (less than 70 mg/dL) were younger, less likely to have a history of hypertension, and less likely to use cholesterol-lowering drugs than women in the reference group (100.0-129.9 mg/dL). Women with the lowest LDL cholesterol level were more likely to consume alcohol, have a normal weight, engage in physical activity, and be premenopausal than women in the reference group. The investigators confirmed 137 incident hemorrhagic stroke events during a mean 19.3 years of follow-up.
After data adjustment, the researchers found that women with the lowest level of LDL cholesterol had 2.17 times the risk of hemorrhagic stroke, compared with participants in the reference group. They found a trend toward increased risk among women with an LDL cholesterol level of 160 mg/dL or higher, but the result was not statistically significant. The highest risk for intracerebral hemorrhage (ICH) was among women with an LDL cholesterol level of less than 70 mg/dL (relative risk, 2.32), followed by women with a level of 160 mg/dL or higher (RR, 1.71).
In addition, after multivariable adjustment, women in the lowest quartile of triglycerides (less than or equal to 74 mg/dL for fasting and less than or equal to 85 mg/dL for nonfasting) had a significantly increased risk of hemorrhagic stroke, compared with women in the highest quartile (RR, 2.00). Low triglyceride levels were associated with an increased risk of subarachnoid hemorrhage, but not with an increased risk of ICH. Neither HDL cholesterol nor total cholesterol was associated with risk of hemorrhagic stroke, the researchers wrote.
Mechanism of increased risk unclear
The researchers do not yet know how low triglyceride and LDL cholesterol levels increase the risk of hemorrhagic stroke. One hypothesis is that low cholesterol promotes necrosis of the arterial medial layer’s smooth muscle cells. This impaired endothelium might be more susceptible to microaneurysms, which are common in patients with ICH, said the researchers.
The prospective design and the large sample size were two of the study’s strengths, but the study had important weaknesses as well, the researchers wrote. For example, few women were premenopausal at baseline, so the investigators could not evaluate whether menopausal status modifies the association between lipid levels and risk of hemorrhagic stroke. In addition, lipid levels were measured only at baseline, which prevented an analysis of whether change in lipid levels over time modifies the risk of hemorrhagic stroke.
Dr. Rist reported receiving a grant from the National Institutes of Health.
SOURCE: Rist PM et al. Neurology. 2019 April 10. doi: 10.1212/WNL.0000000000007454.
FROM NEUROLOGY
FDA modifies safety label for Addyi
The Food and Drug Administration has issued a safety labeling change for flibanserin (Addyi), a treatment for premenopausal women with acquired, generalized hypoactive sexual desire disorder, according to a press release issued April 11 by the agency. Previously, the warning said women should abstain from alcohol entirely.
According to the release, the manufacturer, Sprout, had hoped the FDA would remove the boxed warning and contraindication entirely. However, based on a review of two postmarket research studies, the agency chose to order these modifications to the warnings instead.
The first postmarket study was missing information related to participants’ blood pressure, which FDA officials thought was critical in determining risk; it appeared that this resulted from safety precautions built into the trial. The concern was that not only did this absent information provide further evidence of an interaction but that women at home would not have the benefit of these safety precautions and could suffer serious outcomes, including falls, accidents, and bodily harm. The other postmarketing trial showed that delaying administration of flibanserin until at least 2 hours after consuming alcohol reduced the risk of serious hypotension and syncope.
It is recommended that flibanserin be taken at bedtime because of risks associated with hypotension and syncope, as well as risks associated with central nervous system depression (such as sleepiness). Furthermore, patients are encouraged to discontinue treatment with flibanserin if their hypoactive sexual desire disorder does not improve after 8 weeks. The most common adverse reactions include dizziness, sleepiness, nausea, fatigue, insomnia, and dry mouth.
Full prescribing information is available on the FDA website, as is the full release regarding these safety label modifications.
The Food and Drug Administration has issued a safety labeling change for flibanserin (Addyi), a treatment for premenopausal women with acquired, generalized hypoactive sexual desire disorder, according to a press release issued April 11 by the agency. Previously, the warning said women should abstain from alcohol entirely.
According to the release, the manufacturer, Sprout, had hoped the FDA would remove the boxed warning and contraindication entirely. However, based on a review of two postmarket research studies, the agency chose to order these modifications to the warnings instead.
The first postmarket study was missing information related to participants’ blood pressure, which FDA officials thought was critical in determining risk; it appeared that this resulted from safety precautions built into the trial. The concern was that not only did this absent information provide further evidence of an interaction but that women at home would not have the benefit of these safety precautions and could suffer serious outcomes, including falls, accidents, and bodily harm. The other postmarketing trial showed that delaying administration of flibanserin until at least 2 hours after consuming alcohol reduced the risk of serious hypotension and syncope.
It is recommended that flibanserin be taken at bedtime because of risks associated with hypotension and syncope, as well as risks associated with central nervous system depression (such as sleepiness). Furthermore, patients are encouraged to discontinue treatment with flibanserin if their hypoactive sexual desire disorder does not improve after 8 weeks. The most common adverse reactions include dizziness, sleepiness, nausea, fatigue, insomnia, and dry mouth.
Full prescribing information is available on the FDA website, as is the full release regarding these safety label modifications.
The Food and Drug Administration has issued a safety labeling change for flibanserin (Addyi), a treatment for premenopausal women with acquired, generalized hypoactive sexual desire disorder, according to a press release issued April 11 by the agency. Previously, the warning said women should abstain from alcohol entirely.
According to the release, the manufacturer, Sprout, had hoped the FDA would remove the boxed warning and contraindication entirely. However, based on a review of two postmarket research studies, the agency chose to order these modifications to the warnings instead.
The first postmarket study was missing information related to participants’ blood pressure, which FDA officials thought was critical in determining risk; it appeared that this resulted from safety precautions built into the trial. The concern was that not only did this absent information provide further evidence of an interaction but that women at home would not have the benefit of these safety precautions and could suffer serious outcomes, including falls, accidents, and bodily harm. The other postmarketing trial showed that delaying administration of flibanserin until at least 2 hours after consuming alcohol reduced the risk of serious hypotension and syncope.
It is recommended that flibanserin be taken at bedtime because of risks associated with hypotension and syncope, as well as risks associated with central nervous system depression (such as sleepiness). Furthermore, patients are encouraged to discontinue treatment with flibanserin if their hypoactive sexual desire disorder does not improve after 8 weeks. The most common adverse reactions include dizziness, sleepiness, nausea, fatigue, insomnia, and dry mouth.
Full prescribing information is available on the FDA website, as is the full release regarding these safety label modifications.
First-of-its-kind study looks at pregnancies in prison
according to a systematic study believed to be the first of its kind.
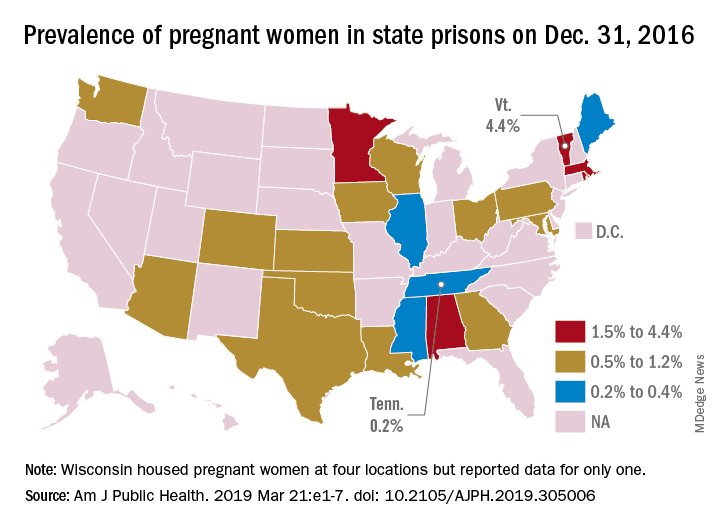
That works out to 0.6% of the 56,262 women housed in the 23 prison systems on Dec. 31, 2016, Carolyn Sufrin, MD, PhD, of Johns Hopkins University, Baltimore, and her associates wrote in the American Journal of Public Health.
Nearly 1,400 pregnant women were admitted to the 26 federal prisons that house women and 22 state prison systems over a 1-year period in 2016-2017. The prisons involved in the study represent 57% of all women incarcerated in the United States, they noted.
Among the pregnancies completed while women were in prison, there were 753 live births: 685 at state facilities and 68 at federal sites. About 6% of those births were preterm, compared with almost 10% nationally in 2016, and 32% were cesarean deliveries, Dr. Sufrin and her associates reported.
All but six births occurred in a hospital; three “were attributable to precipitous labor with prison nurses or paramedics in attendance, and details were not available for the others,” they wrote. Of the 8% of non–live birth pregnancies, 6% were miscarriages, 1% were abortions, and the remainder were stillbirths or ectopic pregnancies. There were three newborn deaths and no maternal deaths.
“That prison pregnancy data have previously not been systematically collected or reported signals a glaring disregard for the health and well-being of incarcerated pregnant women. The Bureau of Justice Statistics collects data on deaths during custody but not births during custody. Despite this marginalization, it is important to recognize that incarcerated women are still members of broader society, that most of them will be released, and that some will give birth while in custody; therefore, their pregnancies must be counted,” the investigators wrote.
The study was supported by the Society of Family Planning Research Fund and the Eunice Kennedy Shriver National Institute of Child Health and Development. The investigators had no conflicts of interest to report.
SOURCE: Sufrin C et al. Am J Public Health. 2019 Mar 21:e1-7. doi: 10.2105/AJPH.2019.305006.
according to a systematic study believed to be the first of its kind.

That works out to 0.6% of the 56,262 women housed in the 23 prison systems on Dec. 31, 2016, Carolyn Sufrin, MD, PhD, of Johns Hopkins University, Baltimore, and her associates wrote in the American Journal of Public Health.
Nearly 1,400 pregnant women were admitted to the 26 federal prisons that house women and 22 state prison systems over a 1-year period in 2016-2017. The prisons involved in the study represent 57% of all women incarcerated in the United States, they noted.
Among the pregnancies completed while women were in prison, there were 753 live births: 685 at state facilities and 68 at federal sites. About 6% of those births were preterm, compared with almost 10% nationally in 2016, and 32% were cesarean deliveries, Dr. Sufrin and her associates reported.
All but six births occurred in a hospital; three “were attributable to precipitous labor with prison nurses or paramedics in attendance, and details were not available for the others,” they wrote. Of the 8% of non–live birth pregnancies, 6% were miscarriages, 1% were abortions, and the remainder were stillbirths or ectopic pregnancies. There were three newborn deaths and no maternal deaths.
“That prison pregnancy data have previously not been systematically collected or reported signals a glaring disregard for the health and well-being of incarcerated pregnant women. The Bureau of Justice Statistics collects data on deaths during custody but not births during custody. Despite this marginalization, it is important to recognize that incarcerated women are still members of broader society, that most of them will be released, and that some will give birth while in custody; therefore, their pregnancies must be counted,” the investigators wrote.
The study was supported by the Society of Family Planning Research Fund and the Eunice Kennedy Shriver National Institute of Child Health and Development. The investigators had no conflicts of interest to report.
SOURCE: Sufrin C et al. Am J Public Health. 2019 Mar 21:e1-7. doi: 10.2105/AJPH.2019.305006.
according to a systematic study believed to be the first of its kind.

That works out to 0.6% of the 56,262 women housed in the 23 prison systems on Dec. 31, 2016, Carolyn Sufrin, MD, PhD, of Johns Hopkins University, Baltimore, and her associates wrote in the American Journal of Public Health.
Nearly 1,400 pregnant women were admitted to the 26 federal prisons that house women and 22 state prison systems over a 1-year period in 2016-2017. The prisons involved in the study represent 57% of all women incarcerated in the United States, they noted.
Among the pregnancies completed while women were in prison, there were 753 live births: 685 at state facilities and 68 at federal sites. About 6% of those births were preterm, compared with almost 10% nationally in 2016, and 32% were cesarean deliveries, Dr. Sufrin and her associates reported.
All but six births occurred in a hospital; three “were attributable to precipitous labor with prison nurses or paramedics in attendance, and details were not available for the others,” they wrote. Of the 8% of non–live birth pregnancies, 6% were miscarriages, 1% were abortions, and the remainder were stillbirths or ectopic pregnancies. There were three newborn deaths and no maternal deaths.
“That prison pregnancy data have previously not been systematically collected or reported signals a glaring disregard for the health and well-being of incarcerated pregnant women. The Bureau of Justice Statistics collects data on deaths during custody but not births during custody. Despite this marginalization, it is important to recognize that incarcerated women are still members of broader society, that most of them will be released, and that some will give birth while in custody; therefore, their pregnancies must be counted,” the investigators wrote.
The study was supported by the Society of Family Planning Research Fund and the Eunice Kennedy Shriver National Institute of Child Health and Development. The investigators had no conflicts of interest to report.
SOURCE: Sufrin C et al. Am J Public Health. 2019 Mar 21:e1-7. doi: 10.2105/AJPH.2019.305006.
FROM THE AMERICAN JOURNAL OF PUBLIC HEALTH
Stress incontinence surgery improves sexual dysfunction
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
REPORTING FROM SGS 2019
Romosozumab gets FDA approval for treating osteoporosis
“These are women who have a history of osteoporotic fracture or multiple risk factors or have failed other treatments for osteoporosis,” according to a news release from the agency.
The monthly treatment of two injections (given one after the other at one visit) mainly works by increasing new bone formation, but these effects wane after 12 doses. If patients still need osteoporosis therapy after that maximum of 12 doses, it’s recommended they are put on treatments that reduce bone breakdown. Romosozumab-aqqg is “a monoclonal antibody that blocks the effects of the protein sclerostin,” according to the news release.
The treatment’s efficacy and safety was evaluated in two clinical trials of more than 11,000 women with postmenopausal osteoporosis. In one trial, women received 12 months of either romosozumab-aqqg or placebo. The treatment arm had a 73% lower risk of vertebral fracture than did the placebo arm, and this benefit was maintained over a second year when both groups were switched to denosumab, another osteoporosis therapy. In the second trial, one group received romosozumab-aqqg for 1 year and then a year of alendronate, and the other group received 2 years of alendronate, another osteoporosis therapy, according to the news release. In this trial, the romosozumab-aqqg arm had 50% less risk of vertebral fractures than did the alendronate-only arm, as well as reduced risk of nonvertebral fractures.
Romosozumab-aqqg was associated with higher risks of cardiovascular death, heart attack, and stroke in the alendronate trial, so the treatment comes with a boxed warning regarding those risks and recommends that the drug not be used in patients who have had a heart attack or stroke within the previous year, according to the news release. Common side effects include joint pain and headache, as well as injection-site reactions.
“These are women who have a history of osteoporotic fracture or multiple risk factors or have failed other treatments for osteoporosis,” according to a news release from the agency.
The monthly treatment of two injections (given one after the other at one visit) mainly works by increasing new bone formation, but these effects wane after 12 doses. If patients still need osteoporosis therapy after that maximum of 12 doses, it’s recommended they are put on treatments that reduce bone breakdown. Romosozumab-aqqg is “a monoclonal antibody that blocks the effects of the protein sclerostin,” according to the news release.
The treatment’s efficacy and safety was evaluated in two clinical trials of more than 11,000 women with postmenopausal osteoporosis. In one trial, women received 12 months of either romosozumab-aqqg or placebo. The treatment arm had a 73% lower risk of vertebral fracture than did the placebo arm, and this benefit was maintained over a second year when both groups were switched to denosumab, another osteoporosis therapy. In the second trial, one group received romosozumab-aqqg for 1 year and then a year of alendronate, and the other group received 2 years of alendronate, another osteoporosis therapy, according to the news release. In this trial, the romosozumab-aqqg arm had 50% less risk of vertebral fractures than did the alendronate-only arm, as well as reduced risk of nonvertebral fractures.
Romosozumab-aqqg was associated with higher risks of cardiovascular death, heart attack, and stroke in the alendronate trial, so the treatment comes with a boxed warning regarding those risks and recommends that the drug not be used in patients who have had a heart attack or stroke within the previous year, according to the news release. Common side effects include joint pain and headache, as well as injection-site reactions.
“These are women who have a history of osteoporotic fracture or multiple risk factors or have failed other treatments for osteoporosis,” according to a news release from the agency.
The monthly treatment of two injections (given one after the other at one visit) mainly works by increasing new bone formation, but these effects wane after 12 doses. If patients still need osteoporosis therapy after that maximum of 12 doses, it’s recommended they are put on treatments that reduce bone breakdown. Romosozumab-aqqg is “a monoclonal antibody that blocks the effects of the protein sclerostin,” according to the news release.
The treatment’s efficacy and safety was evaluated in two clinical trials of more than 11,000 women with postmenopausal osteoporosis. In one trial, women received 12 months of either romosozumab-aqqg or placebo. The treatment arm had a 73% lower risk of vertebral fracture than did the placebo arm, and this benefit was maintained over a second year when both groups were switched to denosumab, another osteoporosis therapy. In the second trial, one group received romosozumab-aqqg for 1 year and then a year of alendronate, and the other group received 2 years of alendronate, another osteoporosis therapy, according to the news release. In this trial, the romosozumab-aqqg arm had 50% less risk of vertebral fractures than did the alendronate-only arm, as well as reduced risk of nonvertebral fractures.
Romosozumab-aqqg was associated with higher risks of cardiovascular death, heart attack, and stroke in the alendronate trial, so the treatment comes with a boxed warning regarding those risks and recommends that the drug not be used in patients who have had a heart attack or stroke within the previous year, according to the news release. Common side effects include joint pain and headache, as well as injection-site reactions.
Expert gives tips on timing, managing lupus pregnancies
SAN FRANCISCO – Not that many years ago, women with systemic lupus erythematosus were told not to get pregnant. It was just one more lupus heartbreak.
Times have changed, according to Lisa Sammaritano, MD, a lupus specialist and associate professor of clinical medicine at Weill Cornell Medical College, New York.
While lupus certainly complicates pregnancy, it by no means rules it out these days. With careful management, the dream of motherhood can become a reality for many women. Dr. Sammaritano shared her insights about timing and treatment at an international congress on systemic lupus erythematosus.
It’s important that the disease is under control as much as possible; that means that timing – and contraception – are key. Antiphospholipid antibodies, common in lupus, complicate matters, but there are workarounds, she said.
SAN FRANCISCO – Not that many years ago, women with systemic lupus erythematosus were told not to get pregnant. It was just one more lupus heartbreak.
Times have changed, according to Lisa Sammaritano, MD, a lupus specialist and associate professor of clinical medicine at Weill Cornell Medical College, New York.
While lupus certainly complicates pregnancy, it by no means rules it out these days. With careful management, the dream of motherhood can become a reality for many women. Dr. Sammaritano shared her insights about timing and treatment at an international congress on systemic lupus erythematosus.
It’s important that the disease is under control as much as possible; that means that timing – and contraception – are key. Antiphospholipid antibodies, common in lupus, complicate matters, but there are workarounds, she said.
SAN FRANCISCO – Not that many years ago, women with systemic lupus erythematosus were told not to get pregnant. It was just one more lupus heartbreak.
Times have changed, according to Lisa Sammaritano, MD, a lupus specialist and associate professor of clinical medicine at Weill Cornell Medical College, New York.
While lupus certainly complicates pregnancy, it by no means rules it out these days. With careful management, the dream of motherhood can become a reality for many women. Dr. Sammaritano shared her insights about timing and treatment at an international congress on systemic lupus erythematosus.
It’s important that the disease is under control as much as possible; that means that timing – and contraception – are key. Antiphospholipid antibodies, common in lupus, complicate matters, but there are workarounds, she said.
AT LUPUS 2019
ACP: Average-risk women under 50 can postpone mammogram
(CBE) for screening in such women of any age, according to a new guideline from the American College of Physicians.
Further, clinicians should discuss whether to screen with mammography in average-risk women aged 40-49 years and consider potential harms and benefits, as well as patient preferences. Providers should discontinue screening average-risk women at age 75 years and women with a life expectancy of 10 years or less, Amir Qaseem, MD, PhD, of the ACP and colleagues wrote on behalf of the ACP Clinical Guidelines Committee.
The ACP guidance also addresses the varying recommendations from other organizations on the age at which to start and stop screening and on screening intervals, noting that “areas of disagreement include screening in women aged 40 to 49 years, screening in women aged 75 years or older, and recommended screening intervals,” and stresses the importance of patient input.
“Women should be informed participants in personalized decisions about breast cancer screening,” the authors wrote, adding that those under age 50 years without a clear preference for screening should not be screened.
However, the evidence shows that most average-risk women with no symptoms will benefit from mammography every other year beginning at age 50 years, they said.
The statement, published online April 8 in the Annals of Internal Medicine, was derived from a review of seven existing English-language breast cancer screening guidelines and the evidence cited in those guidelines. It’s intended to be a resource for all clinicians.
It differs from the 2017 American College of Obstetricians and Gynecologists (ACOG) guidelines in that ACOG recommends CBE and does not address screening in those with a life expectancy of less than 10 years. It also differs from the 2016 U.S. Preventive Services Task Force (USPSTF) guidelines, which make no recommendation on CBE and also do not address screening in those with a life expectancy of less than 10 years.
Other guidelines, such as those from the American College of Radiology, American Cancer Society (ACS), the Canadian Task Force on Preventive Health Care, and the National Comprehensive Cancer Network, recommend CBE, and the World Health Organization guidelines recommend CBE in low resource settings.
“Although CBE continues to be used as part of the examination of symptomatic women, data are sparse on screening asymptomatic women using CBE alone or combined with mammography,” the ACP guideline authors wrote. “The ACS recommends against CBE in average-risk women of any age because of the lack of demonstrated benefit and the potential for false-positive results.”
The guidance, which does not apply to patients with prior abnormal screening results or those at higher breast cancer risk, also includes an evidence-driven “talking points with patients” section based on frequently asked questions.
An important goal of the ACP Clinical Guidelines Committee in developing the guidance is to reduce overdiagnosis and overtreatment, which affects about 20% of women diagnosed over a 10-year period.
The committee reviewed all national guidelines published in English between January 1, 2013, and November 15, 2017, in the National Guideline Clearinghouse or Guidelines International Network library, and it also selected other guidelines commonly used in clinical practice. The committee evaluated the quality of each by using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument.
Alex Krist, MD, the USPSTF vice-chairperson, offered support for the “shift toward shared decision making that is emerging” and added it’s “part of a larger movement toward empowering people with information not only about the potential benefits but also the potential harms of screening tests.”
“In its 2016 recommendation, the Task Force found that the value of mammography increases with age, with women ages 50-74 benefiting most from screening. For women in their 40s, the Task Force also found that mammography screening every two years can be effective,” he told this publication. “We recommend that the decision to start screening should be an individual one, taking into account a woman’s health history, preferences, and how she values the different potential benefits and harms.”
Dr. Krist further noted that the USPSTF, ACP, and many others “have all affirmed that mammography is an important tool to reduce breast cancer mortality and that the benefits of mammography increase with age.”
Likewise, Robert Smith, PhD, vice president of cancer screening for the ACS, noted that the ACP guidance generally aligns with ACS and USPSTF guidelines because all “support informed decision making starting at age 40, and screening every two years starting at age 50 (USPSTF) or 55 (ACS).”
“The fact that all guidelines are not totally in sync is not unexpected. ... The most important thing to recognize is that all of these guidelines stress that regular mammography plays an important role in breast cancer early detection, and women should be aware of its benefits and limitations, and also remain vigilant and report any breast changes,” he said.
The guidance authors reported having no conflicts of interest.
SOURCE: Qaseem A et al., Ann Intern Med. 2019. doi: 10.7326/M18-2147.
The ACP guidance statements provide “clarity and simplicity amidst the chaos of diverging guidelines,” Joann G. Elmore, MD, and Christoph I. Lee, MD, wrote in an editorial that accompanied the guideline (Ann Intern Med. 2019. doi: 10.7326/M19-0726).
The four statements included in the guidance represent the convergence of differing recommendations, but they also highlight points for physicians to consider in shared decision making with patients, the editorial authors wrote.
Lacking, however, is advice on how clinicians should go about stopping screening in certain patients, they noted.
“We need reliable ways to determine life expectancy given comorbid conditions, as well as methods to appropriately manage the discussion about stopping screening. ... The cessation of routine screening is a highly uncomfortable situation for which we as clinicians currently have little guidance and few tools. At this crossroads of confusion, we need a clear path toward informed, tailored, risk-based screening for breast cancer,” they wrote adding that future guidance statements should “move beyond emphasizing variation across guidelines and instead provide more advice on how to implement high-value screening and deimplement low-value screening.”
Dr. Elmore is with the University of California, Los Angeles. Dr. Lee is with the University of Washington, Seattle.
The ACP guidance statements provide “clarity and simplicity amidst the chaos of diverging guidelines,” Joann G. Elmore, MD, and Christoph I. Lee, MD, wrote in an editorial that accompanied the guideline (Ann Intern Med. 2019. doi: 10.7326/M19-0726).
The four statements included in the guidance represent the convergence of differing recommendations, but they also highlight points for physicians to consider in shared decision making with patients, the editorial authors wrote.
Lacking, however, is advice on how clinicians should go about stopping screening in certain patients, they noted.
“We need reliable ways to determine life expectancy given comorbid conditions, as well as methods to appropriately manage the discussion about stopping screening. ... The cessation of routine screening is a highly uncomfortable situation for which we as clinicians currently have little guidance and few tools. At this crossroads of confusion, we need a clear path toward informed, tailored, risk-based screening for breast cancer,” they wrote adding that future guidance statements should “move beyond emphasizing variation across guidelines and instead provide more advice on how to implement high-value screening and deimplement low-value screening.”
Dr. Elmore is with the University of California, Los Angeles. Dr. Lee is with the University of Washington, Seattle.
The ACP guidance statements provide “clarity and simplicity amidst the chaos of diverging guidelines,” Joann G. Elmore, MD, and Christoph I. Lee, MD, wrote in an editorial that accompanied the guideline (Ann Intern Med. 2019. doi: 10.7326/M19-0726).
The four statements included in the guidance represent the convergence of differing recommendations, but they also highlight points for physicians to consider in shared decision making with patients, the editorial authors wrote.
Lacking, however, is advice on how clinicians should go about stopping screening in certain patients, they noted.
“We need reliable ways to determine life expectancy given comorbid conditions, as well as methods to appropriately manage the discussion about stopping screening. ... The cessation of routine screening is a highly uncomfortable situation for which we as clinicians currently have little guidance and few tools. At this crossroads of confusion, we need a clear path toward informed, tailored, risk-based screening for breast cancer,” they wrote adding that future guidance statements should “move beyond emphasizing variation across guidelines and instead provide more advice on how to implement high-value screening and deimplement low-value screening.”
Dr. Elmore is with the University of California, Los Angeles. Dr. Lee is with the University of Washington, Seattle.
(CBE) for screening in such women of any age, according to a new guideline from the American College of Physicians.
Further, clinicians should discuss whether to screen with mammography in average-risk women aged 40-49 years and consider potential harms and benefits, as well as patient preferences. Providers should discontinue screening average-risk women at age 75 years and women with a life expectancy of 10 years or less, Amir Qaseem, MD, PhD, of the ACP and colleagues wrote on behalf of the ACP Clinical Guidelines Committee.
The ACP guidance also addresses the varying recommendations from other organizations on the age at which to start and stop screening and on screening intervals, noting that “areas of disagreement include screening in women aged 40 to 49 years, screening in women aged 75 years or older, and recommended screening intervals,” and stresses the importance of patient input.
“Women should be informed participants in personalized decisions about breast cancer screening,” the authors wrote, adding that those under age 50 years without a clear preference for screening should not be screened.
However, the evidence shows that most average-risk women with no symptoms will benefit from mammography every other year beginning at age 50 years, they said.
The statement, published online April 8 in the Annals of Internal Medicine, was derived from a review of seven existing English-language breast cancer screening guidelines and the evidence cited in those guidelines. It’s intended to be a resource for all clinicians.
It differs from the 2017 American College of Obstetricians and Gynecologists (ACOG) guidelines in that ACOG recommends CBE and does not address screening in those with a life expectancy of less than 10 years. It also differs from the 2016 U.S. Preventive Services Task Force (USPSTF) guidelines, which make no recommendation on CBE and also do not address screening in those with a life expectancy of less than 10 years.
Other guidelines, such as those from the American College of Radiology, American Cancer Society (ACS), the Canadian Task Force on Preventive Health Care, and the National Comprehensive Cancer Network, recommend CBE, and the World Health Organization guidelines recommend CBE in low resource settings.
“Although CBE continues to be used as part of the examination of symptomatic women, data are sparse on screening asymptomatic women using CBE alone or combined with mammography,” the ACP guideline authors wrote. “The ACS recommends against CBE in average-risk women of any age because of the lack of demonstrated benefit and the potential for false-positive results.”
The guidance, which does not apply to patients with prior abnormal screening results or those at higher breast cancer risk, also includes an evidence-driven “talking points with patients” section based on frequently asked questions.
An important goal of the ACP Clinical Guidelines Committee in developing the guidance is to reduce overdiagnosis and overtreatment, which affects about 20% of women diagnosed over a 10-year period.
The committee reviewed all national guidelines published in English between January 1, 2013, and November 15, 2017, in the National Guideline Clearinghouse or Guidelines International Network library, and it also selected other guidelines commonly used in clinical practice. The committee evaluated the quality of each by using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument.
Alex Krist, MD, the USPSTF vice-chairperson, offered support for the “shift toward shared decision making that is emerging” and added it’s “part of a larger movement toward empowering people with information not only about the potential benefits but also the potential harms of screening tests.”
“In its 2016 recommendation, the Task Force found that the value of mammography increases with age, with women ages 50-74 benefiting most from screening. For women in their 40s, the Task Force also found that mammography screening every two years can be effective,” he told this publication. “We recommend that the decision to start screening should be an individual one, taking into account a woman’s health history, preferences, and how she values the different potential benefits and harms.”
Dr. Krist further noted that the USPSTF, ACP, and many others “have all affirmed that mammography is an important tool to reduce breast cancer mortality and that the benefits of mammography increase with age.”
Likewise, Robert Smith, PhD, vice president of cancer screening for the ACS, noted that the ACP guidance generally aligns with ACS and USPSTF guidelines because all “support informed decision making starting at age 40, and screening every two years starting at age 50 (USPSTF) or 55 (ACS).”
“The fact that all guidelines are not totally in sync is not unexpected. ... The most important thing to recognize is that all of these guidelines stress that regular mammography plays an important role in breast cancer early detection, and women should be aware of its benefits and limitations, and also remain vigilant and report any breast changes,” he said.
The guidance authors reported having no conflicts of interest.
SOURCE: Qaseem A et al., Ann Intern Med. 2019. doi: 10.7326/M18-2147.
(CBE) for screening in such women of any age, according to a new guideline from the American College of Physicians.
Further, clinicians should discuss whether to screen with mammography in average-risk women aged 40-49 years and consider potential harms and benefits, as well as patient preferences. Providers should discontinue screening average-risk women at age 75 years and women with a life expectancy of 10 years or less, Amir Qaseem, MD, PhD, of the ACP and colleagues wrote on behalf of the ACP Clinical Guidelines Committee.
The ACP guidance also addresses the varying recommendations from other organizations on the age at which to start and stop screening and on screening intervals, noting that “areas of disagreement include screening in women aged 40 to 49 years, screening in women aged 75 years or older, and recommended screening intervals,” and stresses the importance of patient input.
“Women should be informed participants in personalized decisions about breast cancer screening,” the authors wrote, adding that those under age 50 years without a clear preference for screening should not be screened.
However, the evidence shows that most average-risk women with no symptoms will benefit from mammography every other year beginning at age 50 years, they said.
The statement, published online April 8 in the Annals of Internal Medicine, was derived from a review of seven existing English-language breast cancer screening guidelines and the evidence cited in those guidelines. It’s intended to be a resource for all clinicians.
It differs from the 2017 American College of Obstetricians and Gynecologists (ACOG) guidelines in that ACOG recommends CBE and does not address screening in those with a life expectancy of less than 10 years. It also differs from the 2016 U.S. Preventive Services Task Force (USPSTF) guidelines, which make no recommendation on CBE and also do not address screening in those with a life expectancy of less than 10 years.
Other guidelines, such as those from the American College of Radiology, American Cancer Society (ACS), the Canadian Task Force on Preventive Health Care, and the National Comprehensive Cancer Network, recommend CBE, and the World Health Organization guidelines recommend CBE in low resource settings.
“Although CBE continues to be used as part of the examination of symptomatic women, data are sparse on screening asymptomatic women using CBE alone or combined with mammography,” the ACP guideline authors wrote. “The ACS recommends against CBE in average-risk women of any age because of the lack of demonstrated benefit and the potential for false-positive results.”
The guidance, which does not apply to patients with prior abnormal screening results or those at higher breast cancer risk, also includes an evidence-driven “talking points with patients” section based on frequently asked questions.
An important goal of the ACP Clinical Guidelines Committee in developing the guidance is to reduce overdiagnosis and overtreatment, which affects about 20% of women diagnosed over a 10-year period.
The committee reviewed all national guidelines published in English between January 1, 2013, and November 15, 2017, in the National Guideline Clearinghouse or Guidelines International Network library, and it also selected other guidelines commonly used in clinical practice. The committee evaluated the quality of each by using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument.
Alex Krist, MD, the USPSTF vice-chairperson, offered support for the “shift toward shared decision making that is emerging” and added it’s “part of a larger movement toward empowering people with information not only about the potential benefits but also the potential harms of screening tests.”
“In its 2016 recommendation, the Task Force found that the value of mammography increases with age, with women ages 50-74 benefiting most from screening. For women in their 40s, the Task Force also found that mammography screening every two years can be effective,” he told this publication. “We recommend that the decision to start screening should be an individual one, taking into account a woman’s health history, preferences, and how she values the different potential benefits and harms.”
Dr. Krist further noted that the USPSTF, ACP, and many others “have all affirmed that mammography is an important tool to reduce breast cancer mortality and that the benefits of mammography increase with age.”
Likewise, Robert Smith, PhD, vice president of cancer screening for the ACS, noted that the ACP guidance generally aligns with ACS and USPSTF guidelines because all “support informed decision making starting at age 40, and screening every two years starting at age 50 (USPSTF) or 55 (ACS).”
“The fact that all guidelines are not totally in sync is not unexpected. ... The most important thing to recognize is that all of these guidelines stress that regular mammography plays an important role in breast cancer early detection, and women should be aware of its benefits and limitations, and also remain vigilant and report any breast changes,” he said.
The guidance authors reported having no conflicts of interest.
SOURCE: Qaseem A et al., Ann Intern Med. 2019. doi: 10.7326/M18-2147.
REPORTING FROM THE ANNALS OF INTERNAL MEDICINE
Brexanolone approval ‘marks an important milestone’
In March 2019, the Food and Drug Administration approved a novel medication, Zulresso (brexanolone), for the treatment of postpartum depression. Brexanolone is the first FDA-approved medication for the treatment of postpartum depression, a serious illness that affects nearly one in nine women soon after giving birth.1
Mothers with postpartum depression experience feelings of sadness, irritability, and anxiety, as well as isolation from their loved ones (including their new baby) and exhaustion. The feelings of sadness and anxiety can be extreme, and can interfere with a woman’s ability to care for herself or her family. In some cases, these symptoms can be life threatening. Indeed, the most common cause of maternal death after childbirth in the developed world is suicide.2 Because of the severity of the symptoms and their impact on the family, postpartum depression usually requires treatment.
Until now, there have been no drugs specifically approved to treat postpartum depression. Commonly, postpartum depression is treated with medications that previously were approved for the treatment of major depressive disorder, despite limited evidence documenting their efficacy for postpartum depression. Other putative treatment alternatives include psychotherapy, estrogen therapy, and neuromodulation, such as electroconvulsive therapy and repetitive transcranial magnetic stimulation. Each of these treatments can take weeks or longer to take effect, time that is of elevated importance given the rapidly developing mother-infant relationship in the early postpartum period. Brexanolone addresses both the issue of efficacy and speed of onset, representing a major step forward in the care of women suffering from postpartum depression.
Importantly, the approval of brexanolone marks an important milestone for the psychiatric research community in general and the National Institute of Mental Health in particular, as it represents a compelling example of successful bench-to-bedside translation of basic neuroscience findings to benefit patients. As we have noted elsewhere,3 the research underlying the discovery of endogenous neurosteroids and their role in modulating GABA receptors laid the foundation for the development of brexanolone, an intravenous formulation of the neurosteroid allopregnanolone. The recognition that allopregnanolone was a protective factor induced by stress, that it derived from progesterone, and that its peripheral blood levels were dramatically reduced in the early postpartum period led to the hypothesis that it might be useful as a treatment for postpartum depression.
Sage Therapeutics took on the task of testing this hypothesis, designing a program, in consultation with the FDA, to test the efficacy of allopregnanolone in women with postpartum depression in a series of randomized, placebo-controlled studies assessing brexanolone. 4,5 It is a significant accomplishment of Sage Therapeutics to not only successfully complete the therapeutic program of studies (given past experience with difficulties recruiting these women for placebo-controlled treatment trials) but as well to demonstrate a robust therapeutic effect.
Although the FDA’s approval of a new and novel treatment is exciting for many women, there are still limitations to the broader use of brexanolone. It is delivered intravenously, requires an overnight stay in a certified medical center, and is likely to be considerably expensive, according to early reports – potentially limiting the access to the treatment. There also are potentially serious side effects, such as sedation, dizziness, or sudden loss of consciousness. Nonetheless, this is a promising first step and hopefully will spur further efforts to identify and optimize additional strategies to treat postpartum depression. In fact, other formulations of allopregnanolone and novel analogs to treat postpartum depression already are under study, including some that are orally bioavailable.6,7,8
Several important questions remain to be answered about both brexanolone and postpartum depression: What is the underlying mechanism through which allopregnanolone acts in the brain and reduces depressive symptoms? Is the mechanism unique to postpartum women, or might brexanolone also be effective in nonreproductive depressions in women and men? What causes postpartum depression, and what are the risk factors involved for women who develop this serious condition? Future work will focus on these and other important questions to the benefit of women who have suffered with this condition.
The FDA approval of brexanolone represents the second approval in a month of a new antidepressant treatment targeting different molecules in the brain. In early March 2019, the agency approved Spravato (esketamine) nasal spray as a therapy for treatment-resistant depression. Like brexanolone, esketamine is a fast-acting antidepressant that works through a novel mechanism, completely different from other antidepressants. These new treatment approvals are encouraging, as there has been a paucity for many years in approving new effective treatments for mood disorders.
However, treatment development in psychiatry still has a long way to go and the full underlying neurobiology of mood disorders, including postpartum depression, remains poorly understood. Many challenges are ahead of us in our efforts to develop new treatments and increase our understanding of mental illnesses. Nevertheless, the approval of brexanolone is an important milestone, giving hope to the many women who suffer from postpartum depression, and paving the way for the development of additional novel and effective medications to treat this serious and sometimes life-threatening condition.
Dr. Gordon is the director of the National Institute of Mental Health (NIMH), the lead federal agency for research on mental disorders. He oversees an extensive research portfolio of basic and clinical research that seeks to transform the understanding and treatment of mental illnesses, paving the way for prevention, recovery, and cure. Dr. Hillefors works at the NIMH and oversees the Translational Therapeutics Program in the division of translational research, focusing on the development of novel treatments and biomarkers and early phase clinical trials. She received her MD and PhD in neuroscience at the Karolinska Institute, Sweden. Dr. Schmidt joined the NIMH in 1986 after completing his psychiatric residency at the University of Toronto. He is the chief of the Section on Behavioral Endocrinology, within the Intramural Research Program at the NIMH, where his laboratory studies the relationship between hormones, stress, and mood – particularly in the areas of postpartum depression, severe premenstrual dysphoria, and perimenopausal depression.
References
1. J Psychiatric Res. 2018 Sep;104:235-48.
2. Br J Psychiatry. 2003 Oct;183:279-81.
3. NIMH Director’s Messages. 2019 Mar 20.
4. Lancet. 2017 Jul 29;390(10093):480-9.
5. Lancet. 2018 Sep 22; 392(10152):1058-70.
6. Sage Therapeutics News. 2019 Jan 7.
7. Marinus Pharmaceuticals. 2017 Jun 27.
8. ClinicalTrials.gov Identifier: NCT03460756. 2019 Mar.
In March 2019, the Food and Drug Administration approved a novel medication, Zulresso (brexanolone), for the treatment of postpartum depression. Brexanolone is the first FDA-approved medication for the treatment of postpartum depression, a serious illness that affects nearly one in nine women soon after giving birth.1
Mothers with postpartum depression experience feelings of sadness, irritability, and anxiety, as well as isolation from their loved ones (including their new baby) and exhaustion. The feelings of sadness and anxiety can be extreme, and can interfere with a woman’s ability to care for herself or her family. In some cases, these symptoms can be life threatening. Indeed, the most common cause of maternal death after childbirth in the developed world is suicide.2 Because of the severity of the symptoms and their impact on the family, postpartum depression usually requires treatment.
Until now, there have been no drugs specifically approved to treat postpartum depression. Commonly, postpartum depression is treated with medications that previously were approved for the treatment of major depressive disorder, despite limited evidence documenting their efficacy for postpartum depression. Other putative treatment alternatives include psychotherapy, estrogen therapy, and neuromodulation, such as electroconvulsive therapy and repetitive transcranial magnetic stimulation. Each of these treatments can take weeks or longer to take effect, time that is of elevated importance given the rapidly developing mother-infant relationship in the early postpartum period. Brexanolone addresses both the issue of efficacy and speed of onset, representing a major step forward in the care of women suffering from postpartum depression.
Importantly, the approval of brexanolone marks an important milestone for the psychiatric research community in general and the National Institute of Mental Health in particular, as it represents a compelling example of successful bench-to-bedside translation of basic neuroscience findings to benefit patients. As we have noted elsewhere,3 the research underlying the discovery of endogenous neurosteroids and their role in modulating GABA receptors laid the foundation for the development of brexanolone, an intravenous formulation of the neurosteroid allopregnanolone. The recognition that allopregnanolone was a protective factor induced by stress, that it derived from progesterone, and that its peripheral blood levels were dramatically reduced in the early postpartum period led to the hypothesis that it might be useful as a treatment for postpartum depression.
Sage Therapeutics took on the task of testing this hypothesis, designing a program, in consultation with the FDA, to test the efficacy of allopregnanolone in women with postpartum depression in a series of randomized, placebo-controlled studies assessing brexanolone. 4,5 It is a significant accomplishment of Sage Therapeutics to not only successfully complete the therapeutic program of studies (given past experience with difficulties recruiting these women for placebo-controlled treatment trials) but as well to demonstrate a robust therapeutic effect.
Although the FDA’s approval of a new and novel treatment is exciting for many women, there are still limitations to the broader use of brexanolone. It is delivered intravenously, requires an overnight stay in a certified medical center, and is likely to be considerably expensive, according to early reports – potentially limiting the access to the treatment. There also are potentially serious side effects, such as sedation, dizziness, or sudden loss of consciousness. Nonetheless, this is a promising first step and hopefully will spur further efforts to identify and optimize additional strategies to treat postpartum depression. In fact, other formulations of allopregnanolone and novel analogs to treat postpartum depression already are under study, including some that are orally bioavailable.6,7,8
Several important questions remain to be answered about both brexanolone and postpartum depression: What is the underlying mechanism through which allopregnanolone acts in the brain and reduces depressive symptoms? Is the mechanism unique to postpartum women, or might brexanolone also be effective in nonreproductive depressions in women and men? What causes postpartum depression, and what are the risk factors involved for women who develop this serious condition? Future work will focus on these and other important questions to the benefit of women who have suffered with this condition.
The FDA approval of brexanolone represents the second approval in a month of a new antidepressant treatment targeting different molecules in the brain. In early March 2019, the agency approved Spravato (esketamine) nasal spray as a therapy for treatment-resistant depression. Like brexanolone, esketamine is a fast-acting antidepressant that works through a novel mechanism, completely different from other antidepressants. These new treatment approvals are encouraging, as there has been a paucity for many years in approving new effective treatments for mood disorders.
However, treatment development in psychiatry still has a long way to go and the full underlying neurobiology of mood disorders, including postpartum depression, remains poorly understood. Many challenges are ahead of us in our efforts to develop new treatments and increase our understanding of mental illnesses. Nevertheless, the approval of brexanolone is an important milestone, giving hope to the many women who suffer from postpartum depression, and paving the way for the development of additional novel and effective medications to treat this serious and sometimes life-threatening condition.
Dr. Gordon is the director of the National Institute of Mental Health (NIMH), the lead federal agency for research on mental disorders. He oversees an extensive research portfolio of basic and clinical research that seeks to transform the understanding and treatment of mental illnesses, paving the way for prevention, recovery, and cure. Dr. Hillefors works at the NIMH and oversees the Translational Therapeutics Program in the division of translational research, focusing on the development of novel treatments and biomarkers and early phase clinical trials. She received her MD and PhD in neuroscience at the Karolinska Institute, Sweden. Dr. Schmidt joined the NIMH in 1986 after completing his psychiatric residency at the University of Toronto. He is the chief of the Section on Behavioral Endocrinology, within the Intramural Research Program at the NIMH, where his laboratory studies the relationship between hormones, stress, and mood – particularly in the areas of postpartum depression, severe premenstrual dysphoria, and perimenopausal depression.
References
1. J Psychiatric Res. 2018 Sep;104:235-48.
2. Br J Psychiatry. 2003 Oct;183:279-81.
3. NIMH Director’s Messages. 2019 Mar 20.
4. Lancet. 2017 Jul 29;390(10093):480-9.
5. Lancet. 2018 Sep 22; 392(10152):1058-70.
6. Sage Therapeutics News. 2019 Jan 7.
7. Marinus Pharmaceuticals. 2017 Jun 27.
8. ClinicalTrials.gov Identifier: NCT03460756. 2019 Mar.
In March 2019, the Food and Drug Administration approved a novel medication, Zulresso (brexanolone), for the treatment of postpartum depression. Brexanolone is the first FDA-approved medication for the treatment of postpartum depression, a serious illness that affects nearly one in nine women soon after giving birth.1
Mothers with postpartum depression experience feelings of sadness, irritability, and anxiety, as well as isolation from their loved ones (including their new baby) and exhaustion. The feelings of sadness and anxiety can be extreme, and can interfere with a woman’s ability to care for herself or her family. In some cases, these symptoms can be life threatening. Indeed, the most common cause of maternal death after childbirth in the developed world is suicide.2 Because of the severity of the symptoms and their impact on the family, postpartum depression usually requires treatment.
Until now, there have been no drugs specifically approved to treat postpartum depression. Commonly, postpartum depression is treated with medications that previously were approved for the treatment of major depressive disorder, despite limited evidence documenting their efficacy for postpartum depression. Other putative treatment alternatives include psychotherapy, estrogen therapy, and neuromodulation, such as electroconvulsive therapy and repetitive transcranial magnetic stimulation. Each of these treatments can take weeks or longer to take effect, time that is of elevated importance given the rapidly developing mother-infant relationship in the early postpartum period. Brexanolone addresses both the issue of efficacy and speed of onset, representing a major step forward in the care of women suffering from postpartum depression.
Importantly, the approval of brexanolone marks an important milestone for the psychiatric research community in general and the National Institute of Mental Health in particular, as it represents a compelling example of successful bench-to-bedside translation of basic neuroscience findings to benefit patients. As we have noted elsewhere,3 the research underlying the discovery of endogenous neurosteroids and their role in modulating GABA receptors laid the foundation for the development of brexanolone, an intravenous formulation of the neurosteroid allopregnanolone. The recognition that allopregnanolone was a protective factor induced by stress, that it derived from progesterone, and that its peripheral blood levels were dramatically reduced in the early postpartum period led to the hypothesis that it might be useful as a treatment for postpartum depression.
Sage Therapeutics took on the task of testing this hypothesis, designing a program, in consultation with the FDA, to test the efficacy of allopregnanolone in women with postpartum depression in a series of randomized, placebo-controlled studies assessing brexanolone. 4,5 It is a significant accomplishment of Sage Therapeutics to not only successfully complete the therapeutic program of studies (given past experience with difficulties recruiting these women for placebo-controlled treatment trials) but as well to demonstrate a robust therapeutic effect.
Although the FDA’s approval of a new and novel treatment is exciting for many women, there are still limitations to the broader use of brexanolone. It is delivered intravenously, requires an overnight stay in a certified medical center, and is likely to be considerably expensive, according to early reports – potentially limiting the access to the treatment. There also are potentially serious side effects, such as sedation, dizziness, or sudden loss of consciousness. Nonetheless, this is a promising first step and hopefully will spur further efforts to identify and optimize additional strategies to treat postpartum depression. In fact, other formulations of allopregnanolone and novel analogs to treat postpartum depression already are under study, including some that are orally bioavailable.6,7,8
Several important questions remain to be answered about both brexanolone and postpartum depression: What is the underlying mechanism through which allopregnanolone acts in the brain and reduces depressive symptoms? Is the mechanism unique to postpartum women, or might brexanolone also be effective in nonreproductive depressions in women and men? What causes postpartum depression, and what are the risk factors involved for women who develop this serious condition? Future work will focus on these and other important questions to the benefit of women who have suffered with this condition.
The FDA approval of brexanolone represents the second approval in a month of a new antidepressant treatment targeting different molecules in the brain. In early March 2019, the agency approved Spravato (esketamine) nasal spray as a therapy for treatment-resistant depression. Like brexanolone, esketamine is a fast-acting antidepressant that works through a novel mechanism, completely different from other antidepressants. These new treatment approvals are encouraging, as there has been a paucity for many years in approving new effective treatments for mood disorders.
However, treatment development in psychiatry still has a long way to go and the full underlying neurobiology of mood disorders, including postpartum depression, remains poorly understood. Many challenges are ahead of us in our efforts to develop new treatments and increase our understanding of mental illnesses. Nevertheless, the approval of brexanolone is an important milestone, giving hope to the many women who suffer from postpartum depression, and paving the way for the development of additional novel and effective medications to treat this serious and sometimes life-threatening condition.
Dr. Gordon is the director of the National Institute of Mental Health (NIMH), the lead federal agency for research on mental disorders. He oversees an extensive research portfolio of basic and clinical research that seeks to transform the understanding and treatment of mental illnesses, paving the way for prevention, recovery, and cure. Dr. Hillefors works at the NIMH and oversees the Translational Therapeutics Program in the division of translational research, focusing on the development of novel treatments and biomarkers and early phase clinical trials. She received her MD and PhD in neuroscience at the Karolinska Institute, Sweden. Dr. Schmidt joined the NIMH in 1986 after completing his psychiatric residency at the University of Toronto. He is the chief of the Section on Behavioral Endocrinology, within the Intramural Research Program at the NIMH, where his laboratory studies the relationship between hormones, stress, and mood – particularly in the areas of postpartum depression, severe premenstrual dysphoria, and perimenopausal depression.
References
1. J Psychiatric Res. 2018 Sep;104:235-48.
2. Br J Psychiatry. 2003 Oct;183:279-81.
3. NIMH Director’s Messages. 2019 Mar 20.
4. Lancet. 2017 Jul 29;390(10093):480-9.
5. Lancet. 2018 Sep 22; 392(10152):1058-70.
6. Sage Therapeutics News. 2019 Jan 7.
7. Marinus Pharmaceuticals. 2017 Jun 27.
8. ClinicalTrials.gov Identifier: NCT03460756. 2019 Mar.
Powerful breast-implant testimony constrained by limited evidence
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
Postpartum anxiety: More common than you think
THE CASE
Julia* is a 31-year-old woman, gravida 3 para 3, who presents to your office for evaluation after a recent emergency department (ED) visit. Her husband and children are with her. She is 4 months postpartum after an uncomplicated normal spontaneous vaginal delivery. She is breastfeeding her healthy baby boy and is using an intrauterine device for birth control. She went to the ED last week after “choking on a chip” while having lunch with her children. It felt like she “couldn’t breathe.” She called 911 herself. The ED evaluation was unremarkable. Her discharge diagnosis was “panic attack,” and she was sent home with a prescription for lorazepam.
Since the incident, she has been unable to eat any solid foods and has lost 7 pounds. She also reports a globus sensation, extreme fear of swallowing, insomnia, and pervasive thoughts that she could die at any moment and leave her children motherless. She has not taken the lorazepam.
She has a history of self-reported anxiety dating back to high school but no history of panic attacks. She has never been diagnosed with an anxiety disorder and has never before been prescribed anti-anxiety medication. She doesn’t have a history of postpartum depression in prior pregnancies, and a depression screening at her postpartum visit 2 months ago was negative.
●
*The patient’s name has been changed to protect her identity.
During the perinatal period, women are particularly vulnerable to affective disorders, and primary care physicians are encouraged to routinely screen for and treat depression in pregnant and postpartum women.1 However, anxiety disorders have a higher incidence than mood disorders in the general population,2 and perinatal anxiety may be more widely underrecognized and undertreated than depression.3 In addition, higher depression scores early in pregnancy have been shown to predict higher anxiety later in pregnancy.4
As family physicians, we are well-trained to recognize and treat anxiety disorders in the general patient population; however, we may lack the awareness and tools to identify these conditions in the perinatal period. Given our frequent encounters with both mom and baby in a child’s first year of life, we are uniquely positioned to promptly recognize, diagnose, and treat postpartum anxiety and thereby improve health outcomes for families.
DEFINING PERINATAL ANXIETY
Anxiety disorders (including generalized anxiety disorder, panic, phobia, and social anxiety) are the most common mental health disorders evaluated and treated in the primary care setting, with a lifetime prevalence of close to 30%.2
Continue to: A recent report from...
A recent report from the Centers for Disease Control and Prevention (CDC) estimates that 1 in 9 women experience symptoms of postpartum depression.5 The prevalence of anxiety disorders during pregnancy and the early postpartum period is not as well-known, but studies suggest that perinatal anxiety is much more prevalent than depression. In one study, generalized anxiety disorder (GAD) in the pre- and postnatal periods was 15.8% and 17.1%, respectively; an incidence far exceeding that of perinatal depression (3.9% and 4.8%, for the same periods).6 Additional evidence suggests that even more women in the postnatal period experience clinically significant levels of anxiety but do not meet full diagnostic criteria for an anxiety disorder.7
In another study, 9.5% of women met criteria for GAD at some point during pregnancy, with highest anxiety levels in the first trimester.8 Women with a history of GAD, lower education, lack of social support, and personal history of child abuse have the highest risk for postpartum anxiety. Women with a history of posttraumatic stress disorder (PTSD) may be twice as likely to develop postpartum anxiety as healthy women.9
It has been well-documented that sleep disruption—which is very common in new mothers in the postnatal period—contributes to mood and anxiety disorders.10,11
Clarifying a diagnosis of postpartum anxiety
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)12 specifies no diagnosis of postpartum anxiety disorder. And no standardized diagnostic criteria exist. It is likely that in some cases, postpartum anxiety represents an exacerbation of underlying GAD, and in other cases it is a situational disorder brought about by specific circumstances of the peripartum period.
The DSM-5 does, however, provide a helpful diagnostic approach. It defines a diagnosis of postpartum depression as being a variant of major depressive disorder (MDD) in which a woman must 1) meet criteria for a major depressive episode; and 2) occur during pregnancy or within 4 weeks of delivery. In practice, many clinicians extend the second requirement to include the first year postpartum.13 There is a “with anxious distress” specifier for major depression in the DSM-5, but the 2 disorders are otherwise unlinked.
Continue to: To apply the...
To apply the DSM-5 principles for postpartum depression to postpartum anxiety, a patient would need to 1) meet the diagnostic criteria for an anxiety disorder that 2) have their onset within a specified perinatal period. Variant presentations of anxiety in the postpartum period might include panic disorder and phobias, which could also interfere with a woman’s ability to care for her child.
The DSM-5 offers the following criteria for GAD12:
- excessive worry about a variety of topics
- worry that is experienced as hard to control
- worry associated with at least 3 physical or cognitive symptoms: edginess/restlessness, tiring easily, impaired concentration, irritability
- anxiety, worry, or associated symptoms that make it hard to carry out day-to-day activities and responsibilities
- symptoms that are unrelated to any other medical conditions and cannot be explained by the effect of substances including a prescription medication, alcohol, or recreational drugs
- symptoms that are not better explained by a different mental disorder.
Debilitating effects of postpartum anxiety
Many women experience some level of anxiety during pregnancy and early postpartum—anxiety that may range from normal and adaptive to debilitating.14 While the challenges of caring for a newborn are likely to bring some level of anxiety, these symptoms should be transient and not interfere with a woman’s capacity to care for her infant, herself, or her family.
Postpartum anxiety has been associated with a prior fear of giving birth, fear of death (of both mother and baby), lack of control, lack of self-confidence, and lack of confidence in the medical system.9 The experience of such ongoing disturbing thoughts or feelings of worry and tension that affect a woman’s ability to manage from day to day should indicate an illness state that deserves medical attention.
Mothers with postpartum anxiety disorders report significantly less bonding with their infants than do mothers without anxiety.15 A recent narrative review describes numerous studies that illustrate the negative effects of postpartum anxiety on bonding, breastfeeding, infant temperament, early childhood development, and conduct disorders.16 Anxious women may be less likely to initiate breastfeeding, have more challenges with breastfeeding, and even have a different milk composition.17 Women with prenatal anxiety are also more likely to stop breastfeeding prematurely.18 Children of anxious mothers may be more likely to have a difficult temperament and to display more distress.19 There are small studies demonstrating deficits in early infant development and increases in conduct disorder in the male offspring of anxious women.20
Continue to: SCREENING FOR POSTPARTUM ANXIETY
SCREENING FOR POSTPARTUM ANXIETY
Screening for perinatal depression has become standard of care, and the Edinburgh Postnatal Depression Scale (EPDS) is a widely used instrument.1 The EPDS, a 10-question self-report scale, was created and validated to screen for perinatal depression, with a cutoff of > 10/30 usually considered a positive result.
Researchers have investigated the utility of the EPDS as a screening tool for perinatal anxiety as well.21-23 These studies show some promise, but there are questions as to whether a total score or a subscale score of the EPDS is most accurate in detecting anxiety. Women with perinatal anxiety may score low on the total EPDS, yet score higher on 3 anxiety-specific questions (TABLE 123). For this reason, several studies propose an EPDS anxiety subscore or subscale (referred to as EPDS-3A).

Of note, there are some women who will score high on the subscale who do not ultimately meet the criteria for an anxiety disorder diagnosis. Clinicians should not over-interpret these scores and should always use sound clinical judgment when making a diagnosis.
Research has also focused on using the GAD 7-item (GAD-7) scale (TABLE 224),25 and on the
Family physicians may consider using the EPDS subscale if they are already using the EPDS, or adding the GAD-7 as a separate screening instrument during a postpartum visit. To date there is no one standard recommendation or screening tool.
Continue to: NONPHARMACOLOGIC TREATMENT
NONPHARMACOLOGIC TREATMENT
As one would with any patient who has situational anxiety, help new mothers find ways to increase their coping skills, reduce stress, and mobilize social supports and family resources. Given the association between sleep disruption and perinatal anxiety, counsel new mothers, especially those at high risk for postpartum anxiety, to prioritize sleep during this vulnerable time. To that end, consider recommending that they ask partners, family members, or friends to help them take care of the infant at night (or during the day). Such nonmedical interventions may be sufficient for women with mild anxiety.
Very few studies have addressed nonpharmacologic management of postpartum anxiety, but cognitive behavioral therapy (CBT) has been shown to help in managing and treating anxiety disorders outside of pregnancy.28 A few small studies indicate promise for CBT and for mindfulness-based interventions (MBIs) during pregnancy.29
A 2016 systematic review of pharmacologic and nonpharmacologic treatment of anxiety in the perinatal period found support for the use of CBT for panic disorder and specific phobias both in pregnancy and postpartum.30 A very small study found that teaching mothers to massage their preterm infants decreased maternal anxiety.31
If the patient is amenable, it is reasonable to start with behavioral interventions like CBT or MBI before pharmacologic treatment—particularly when physicians have mental health professionals embedded in their primary care team.
PHARMACOLOGIC TREATMENT
Selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) are considered first-line treatment for moderate to severe anxiety disorders in the perinatal and postnatal period.
Continue to: SSRIs in pregnancy
SSRIs in pregnancy. Lacking support of randomized controlled trials, most recommendations regarding SSRIs in pregnancy come from expert consensus or cohort and case control studies. Studies have raised concerns for an increased rate of congenital heart defects among fetuses exposed to paroxetine32 and primary pulmonary hypertension with all SSRIs.33 But the absolute risks are quite small. There have also been concerns regarding low birth weight and preterm birth, but it is possible that these outcomes result from the depression itself rather than the medication.34
Unfortunately, there are very few studies evaluating the efficacy of SSRIs in treating postpartum depression35 and even fewer that specifically evaluate their effect on perinatal anxiety. Many experts believe that not treating anxiety/depression is actually more harmful than the fetal effects of SSRIs, and that SSRIs are largely safe in both pregnancy and while breastfeeding, with benefits outweighing the risks.
SSRIs while breastfeeding. SSRIs have been found to be present in varying levels in breastmilk but may or may not be present in the serum of nursing infants.36 A 2008 guideline from the American College of Obstetricians and Gynecologists lists paroxetine, sertraline, and fluvoxamine as slightly safer than fluoxetine, escitalopram, and citalopram.37 A 2015 systematic review similarly concluded that sertraline and paroxetine have the most safety data on lactation.38 Lowest effective dose is always recommended to minimize exposure.
Benzodiazepines. As in the general population, benzodiazepines should be reserved for short-term use in acute anxiety and panic because they are associated with such adverse effects as worsening of depression/anxiety and risk of dependence and overdose. Longer-acting benzodiazepines (eg, clonazepam) are generally not recommended in lactation because of reported effects on infants, including sedation. Shorter-acting benzodiazepines (eg, lorazepam) are considered safer in lactation.39
THE CASE
Julia saw her family physician 4 more times, was evaluated by an ear-nose-and-throat specialist for her throat complaints, saw a therapist for CBT and a psychiatrist for medication, had 3 more ED visits, and lost 23 pounds before she finally agreed to start an SSRI for postpartum anxiety. She screened high on the EPDS-3A (9/9) despite scoring low on the full EPDS for perinatal depression (total, 9/30).
Continue to: Because of her swallowing impediments...
Because of her swallowing impediments and because she was breastfeeding, sertraline solution was started at very small doses. It was titrated weekly to obtain therapeutic levels. By 4 weeks, her weight stabilized. By 8 weeks, she started gaining weight and sleeping better. She saw the therapist regularly to continue CBT techniques. Over the next several months she started eating a normal diet. She is currently maintained on her SSRI, is still breastfeeding, and has achieved insight into her perinatal anxiety disorder.
CORRESPONDENCE
Veronica Jordan, MD, 3569 Round Barn Cir #200, Santa Rosa, CA 95403; [email protected].
1. O’Connor E, Rossom RC, Henninger M, et al. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:388-406.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
3. Giardinelli L, Innocenti A, Benni L, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2012;15:21-30.
4. Rallis S, Skouteris H, McCabe M, et al. A prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. 2014;27:e36-e42.
5. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158.
6. Fairbrother N, Janssen P, Antony MM, et al. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148-155.
7. Phillips J, Sharpe L, Matthey S, et al. Maternally focused worry. Arch Womens Ment Health. 2009;12:409-418.
8. Buist A, Gotman N, Yonkers KA. Generalized anxiety disorder: course and risk factors in pregnancy. J Affect Disord. 2011;131:277-283.
9. Schlomi Polachek I, Huller Harari L, Baum M, et al. Postpartum anxiety in a cohort of women from the general population: risk factors and association with depression during last week of pregnancy, postpartum depression and postpartum PTSD. Isr J Psychiatry Relat Sci. 2014;51:128-134.
10. Bei B, Coo S, Trinder J. Sleep and mood during pregnancy and the postpartum period. Sleep Med Clin. 2015;10:25-33.
11. Lawson A, Murphy KE, Sloan E, et al. The relationship between sleep and postpartum mental disorders: a systematic review. J Affect Disord. 2015;176:65-77.
12. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013.
13. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
14. Ali E. Women’s experiences with postpartum anxiety disorders: a narrative literature review. Int J Womens Health. 2018;10:237-249.
15. Tietz A, Zietlow AL, Reck C. Maternal bonding in mothers with postpartum anxiety disorder: the crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Arch Womens Ment Health. 2014;17:433-442.
16. Field T. Postnatal anxiety prevalence, predictors and effects on development: a narrative review. Infant Behav Dev. 2018;51:24-32.
17. Serim Demirgoren B, Ozbek A, Ormen M, et al. Do mothers with high sodium levels in their breast milk have high depression and anxiety scores? J Int Med Res. 2017;45:843-848.
18. Ystrom E. Breastfeeding cessation and symptoms of anxiety and depression: a longitudinal cohort study. BMC Pregnancy Childbirth. 2012;12:36.
19. Britton JR. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health. 2011;51:55-71.
20. Glasheen C, Richardson GA, Kim KH, et al. Exposure to maternal pre- and postnatal depression and anxiety symptoms: risk for major depression, anxiety disorders, and conduct disorder in adolescent offspring. Dev Psychopathol. 2013;26:1045-1063.
21. Petrozzi A, Gagliardi L. Anxious and depressive components of Edinburgh Postnatal Depression Scale in maternal postpartum psychological problems. J Perinat Med. 2013;41:343-348.
22. Bina R, Harrington D. The Edinburgh Postnatal Depression Scale: screening tool for postpartum anxiety as well? Findings from a confirmatory factor analysis of the Hebrew version. Matern Child Health J. 2016;20:904-914.
23. Matthey S, Fisher J, Rowe H. Using the Edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations J Affect Disord. 2013;146:224-230.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. Simpson W, Glazer M, Michalski N, et al. Comparative efficacy of the Generalized Anxiety Disorder 7-Item Scale and the Edinburgh Postnatal Depression Scale as screening tools for generalized anxiety disorder in pregnancy and the postpartum period. Can J Psychiatry. 2014;59:434-440.
26. Moran TE, Polanin JR, Wenzel A. The Postpartum Worry Scale-Revised: an initial validation of a measure of postpartum worry. Arch Womens Ment Health. 2014;17:41-48.
27. Fallon V, Halford JCG, Bennett KM, et al. The Postpartum Specific Anxiety Scale: development and preliminary validation. Arch Womens Ment Health. 2016;19:1079-1090.
28. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
29. Goodman JH, Guarino A, Chenausky K, et al. CALM Pregnancy: results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Womens Ment Health. 2014;17:373-387.
30. Marchesi C, Ossola P, Amerio A, et al. Clinical management of perinatal anxiety disorders: a systematic review. J Affect Disord. 2016;190:543-550.
31. Feijó L, Hernandez-Reif M, Field T, et al. Mothers’ depressed mood and anxiety levels are reduced after massaging their preterm infants. Infant Behav Devel. 2006;29:476-480.
32. Bérard A, Iessa N, Chaabane S, et al. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:589-604.
33. Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313:2142-2151.
34. Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? A population-based investigation. PLoS One. 2016;11:e0168115.
35. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;11:CD002018.
36. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;70:e35.
37. ACOG Committee on Practice Bulletins—number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020.
38. Orsolini L, Bellantuono C. Serotonin reuptake inhibitors and breastfeeding: a systematic review. Hum Psychopharmacol. 2015;30:4-20.
39. NIH. Drugs and Lactation Database. https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 26, 2019.
THE CASE
Julia* is a 31-year-old woman, gravida 3 para 3, who presents to your office for evaluation after a recent emergency department (ED) visit. Her husband and children are with her. She is 4 months postpartum after an uncomplicated normal spontaneous vaginal delivery. She is breastfeeding her healthy baby boy and is using an intrauterine device for birth control. She went to the ED last week after “choking on a chip” while having lunch with her children. It felt like she “couldn’t breathe.” She called 911 herself. The ED evaluation was unremarkable. Her discharge diagnosis was “panic attack,” and she was sent home with a prescription for lorazepam.
Since the incident, she has been unable to eat any solid foods and has lost 7 pounds. She also reports a globus sensation, extreme fear of swallowing, insomnia, and pervasive thoughts that she could die at any moment and leave her children motherless. She has not taken the lorazepam.
She has a history of self-reported anxiety dating back to high school but no history of panic attacks. She has never been diagnosed with an anxiety disorder and has never before been prescribed anti-anxiety medication. She doesn’t have a history of postpartum depression in prior pregnancies, and a depression screening at her postpartum visit 2 months ago was negative.
●
*The patient’s name has been changed to protect her identity.
During the perinatal period, women are particularly vulnerable to affective disorders, and primary care physicians are encouraged to routinely screen for and treat depression in pregnant and postpartum women.1 However, anxiety disorders have a higher incidence than mood disorders in the general population,2 and perinatal anxiety may be more widely underrecognized and undertreated than depression.3 In addition, higher depression scores early in pregnancy have been shown to predict higher anxiety later in pregnancy.4
As family physicians, we are well-trained to recognize and treat anxiety disorders in the general patient population; however, we may lack the awareness and tools to identify these conditions in the perinatal period. Given our frequent encounters with both mom and baby in a child’s first year of life, we are uniquely positioned to promptly recognize, diagnose, and treat postpartum anxiety and thereby improve health outcomes for families.
DEFINING PERINATAL ANXIETY
Anxiety disorders (including generalized anxiety disorder, panic, phobia, and social anxiety) are the most common mental health disorders evaluated and treated in the primary care setting, with a lifetime prevalence of close to 30%.2
Continue to: A recent report from...
A recent report from the Centers for Disease Control and Prevention (CDC) estimates that 1 in 9 women experience symptoms of postpartum depression.5 The prevalence of anxiety disorders during pregnancy and the early postpartum period is not as well-known, but studies suggest that perinatal anxiety is much more prevalent than depression. In one study, generalized anxiety disorder (GAD) in the pre- and postnatal periods was 15.8% and 17.1%, respectively; an incidence far exceeding that of perinatal depression (3.9% and 4.8%, for the same periods).6 Additional evidence suggests that even more women in the postnatal period experience clinically significant levels of anxiety but do not meet full diagnostic criteria for an anxiety disorder.7
In another study, 9.5% of women met criteria for GAD at some point during pregnancy, with highest anxiety levels in the first trimester.8 Women with a history of GAD, lower education, lack of social support, and personal history of child abuse have the highest risk for postpartum anxiety. Women with a history of posttraumatic stress disorder (PTSD) may be twice as likely to develop postpartum anxiety as healthy women.9
It has been well-documented that sleep disruption—which is very common in new mothers in the postnatal period—contributes to mood and anxiety disorders.10,11
Clarifying a diagnosis of postpartum anxiety
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)12 specifies no diagnosis of postpartum anxiety disorder. And no standardized diagnostic criteria exist. It is likely that in some cases, postpartum anxiety represents an exacerbation of underlying GAD, and in other cases it is a situational disorder brought about by specific circumstances of the peripartum period.
The DSM-5 does, however, provide a helpful diagnostic approach. It defines a diagnosis of postpartum depression as being a variant of major depressive disorder (MDD) in which a woman must 1) meet criteria for a major depressive episode; and 2) occur during pregnancy or within 4 weeks of delivery. In practice, many clinicians extend the second requirement to include the first year postpartum.13 There is a “with anxious distress” specifier for major depression in the DSM-5, but the 2 disorders are otherwise unlinked.
Continue to: To apply the...
To apply the DSM-5 principles for postpartum depression to postpartum anxiety, a patient would need to 1) meet the diagnostic criteria for an anxiety disorder that 2) have their onset within a specified perinatal period. Variant presentations of anxiety in the postpartum period might include panic disorder and phobias, which could also interfere with a woman’s ability to care for her child.
The DSM-5 offers the following criteria for GAD12:
- excessive worry about a variety of topics
- worry that is experienced as hard to control
- worry associated with at least 3 physical or cognitive symptoms: edginess/restlessness, tiring easily, impaired concentration, irritability
- anxiety, worry, or associated symptoms that make it hard to carry out day-to-day activities and responsibilities
- symptoms that are unrelated to any other medical conditions and cannot be explained by the effect of substances including a prescription medication, alcohol, or recreational drugs
- symptoms that are not better explained by a different mental disorder.
Debilitating effects of postpartum anxiety
Many women experience some level of anxiety during pregnancy and early postpartum—anxiety that may range from normal and adaptive to debilitating.14 While the challenges of caring for a newborn are likely to bring some level of anxiety, these symptoms should be transient and not interfere with a woman’s capacity to care for her infant, herself, or her family.
Postpartum anxiety has been associated with a prior fear of giving birth, fear of death (of both mother and baby), lack of control, lack of self-confidence, and lack of confidence in the medical system.9 The experience of such ongoing disturbing thoughts or feelings of worry and tension that affect a woman’s ability to manage from day to day should indicate an illness state that deserves medical attention.
Mothers with postpartum anxiety disorders report significantly less bonding with their infants than do mothers without anxiety.15 A recent narrative review describes numerous studies that illustrate the negative effects of postpartum anxiety on bonding, breastfeeding, infant temperament, early childhood development, and conduct disorders.16 Anxious women may be less likely to initiate breastfeeding, have more challenges with breastfeeding, and even have a different milk composition.17 Women with prenatal anxiety are also more likely to stop breastfeeding prematurely.18 Children of anxious mothers may be more likely to have a difficult temperament and to display more distress.19 There are small studies demonstrating deficits in early infant development and increases in conduct disorder in the male offspring of anxious women.20
Continue to: SCREENING FOR POSTPARTUM ANXIETY
SCREENING FOR POSTPARTUM ANXIETY
Screening for perinatal depression has become standard of care, and the Edinburgh Postnatal Depression Scale (EPDS) is a widely used instrument.1 The EPDS, a 10-question self-report scale, was created and validated to screen for perinatal depression, with a cutoff of > 10/30 usually considered a positive result.
Researchers have investigated the utility of the EPDS as a screening tool for perinatal anxiety as well.21-23 These studies show some promise, but there are questions as to whether a total score or a subscale score of the EPDS is most accurate in detecting anxiety. Women with perinatal anxiety may score low on the total EPDS, yet score higher on 3 anxiety-specific questions (TABLE 123). For this reason, several studies propose an EPDS anxiety subscore or subscale (referred to as EPDS-3A).

Of note, there are some women who will score high on the subscale who do not ultimately meet the criteria for an anxiety disorder diagnosis. Clinicians should not over-interpret these scores and should always use sound clinical judgment when making a diagnosis.
Research has also focused on using the GAD 7-item (GAD-7) scale (TABLE 224),25 and on the

Family physicians may consider using the EPDS subscale if they are already using the EPDS, or adding the GAD-7 as a separate screening instrument during a postpartum visit. To date there is no one standard recommendation or screening tool.
Continue to: NONPHARMACOLOGIC TREATMENT
NONPHARMACOLOGIC TREATMENT
As one would with any patient who has situational anxiety, help new mothers find ways to increase their coping skills, reduce stress, and mobilize social supports and family resources. Given the association between sleep disruption and perinatal anxiety, counsel new mothers, especially those at high risk for postpartum anxiety, to prioritize sleep during this vulnerable time. To that end, consider recommending that they ask partners, family members, or friends to help them take care of the infant at night (or during the day). Such nonmedical interventions may be sufficient for women with mild anxiety.
Very few studies have addressed nonpharmacologic management of postpartum anxiety, but cognitive behavioral therapy (CBT) has been shown to help in managing and treating anxiety disorders outside of pregnancy.28 A few small studies indicate promise for CBT and for mindfulness-based interventions (MBIs) during pregnancy.29
A 2016 systematic review of pharmacologic and nonpharmacologic treatment of anxiety in the perinatal period found support for the use of CBT for panic disorder and specific phobias both in pregnancy and postpartum.30 A very small study found that teaching mothers to massage their preterm infants decreased maternal anxiety.31
If the patient is amenable, it is reasonable to start with behavioral interventions like CBT or MBI before pharmacologic treatment—particularly when physicians have mental health professionals embedded in their primary care team.
PHARMACOLOGIC TREATMENT
Selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) are considered first-line treatment for moderate to severe anxiety disorders in the perinatal and postnatal period.
Continue to: SSRIs in pregnancy
SSRIs in pregnancy. Lacking support of randomized controlled trials, most recommendations regarding SSRIs in pregnancy come from expert consensus or cohort and case control studies. Studies have raised concerns for an increased rate of congenital heart defects among fetuses exposed to paroxetine32 and primary pulmonary hypertension with all SSRIs.33 But the absolute risks are quite small. There have also been concerns regarding low birth weight and preterm birth, but it is possible that these outcomes result from the depression itself rather than the medication.34
Unfortunately, there are very few studies evaluating the efficacy of SSRIs in treating postpartum depression35 and even fewer that specifically evaluate their effect on perinatal anxiety. Many experts believe that not treating anxiety/depression is actually more harmful than the fetal effects of SSRIs, and that SSRIs are largely safe in both pregnancy and while breastfeeding, with benefits outweighing the risks.
SSRIs while breastfeeding. SSRIs have been found to be present in varying levels in breastmilk but may or may not be present in the serum of nursing infants.36 A 2008 guideline from the American College of Obstetricians and Gynecologists lists paroxetine, sertraline, and fluvoxamine as slightly safer than fluoxetine, escitalopram, and citalopram.37 A 2015 systematic review similarly concluded that sertraline and paroxetine have the most safety data on lactation.38 Lowest effective dose is always recommended to minimize exposure.
Benzodiazepines. As in the general population, benzodiazepines should be reserved for short-term use in acute anxiety and panic because they are associated with such adverse effects as worsening of depression/anxiety and risk of dependence and overdose. Longer-acting benzodiazepines (eg, clonazepam) are generally not recommended in lactation because of reported effects on infants, including sedation. Shorter-acting benzodiazepines (eg, lorazepam) are considered safer in lactation.39
THE CASE
Julia saw her family physician 4 more times, was evaluated by an ear-nose-and-throat specialist for her throat complaints, saw a therapist for CBT and a psychiatrist for medication, had 3 more ED visits, and lost 23 pounds before she finally agreed to start an SSRI for postpartum anxiety. She screened high on the EPDS-3A (9/9) despite scoring low on the full EPDS for perinatal depression (total, 9/30).
Continue to: Because of her swallowing impediments...
Because of her swallowing impediments and because she was breastfeeding, sertraline solution was started at very small doses. It was titrated weekly to obtain therapeutic levels. By 4 weeks, her weight stabilized. By 8 weeks, she started gaining weight and sleeping better. She saw the therapist regularly to continue CBT techniques. Over the next several months she started eating a normal diet. She is currently maintained on her SSRI, is still breastfeeding, and has achieved insight into her perinatal anxiety disorder.
CORRESPONDENCE
Veronica Jordan, MD, 3569 Round Barn Cir #200, Santa Rosa, CA 95403; [email protected].
THE CASE
Julia* is a 31-year-old woman, gravida 3 para 3, who presents to your office for evaluation after a recent emergency department (ED) visit. Her husband and children are with her. She is 4 months postpartum after an uncomplicated normal spontaneous vaginal delivery. She is breastfeeding her healthy baby boy and is using an intrauterine device for birth control. She went to the ED last week after “choking on a chip” while having lunch with her children. It felt like she “couldn’t breathe.” She called 911 herself. The ED evaluation was unremarkable. Her discharge diagnosis was “panic attack,” and she was sent home with a prescription for lorazepam.
Since the incident, she has been unable to eat any solid foods and has lost 7 pounds. She also reports a globus sensation, extreme fear of swallowing, insomnia, and pervasive thoughts that she could die at any moment and leave her children motherless. She has not taken the lorazepam.
She has a history of self-reported anxiety dating back to high school but no history of panic attacks. She has never been diagnosed with an anxiety disorder and has never before been prescribed anti-anxiety medication. She doesn’t have a history of postpartum depression in prior pregnancies, and a depression screening at her postpartum visit 2 months ago was negative.
●
*The patient’s name has been changed to protect her identity.
During the perinatal period, women are particularly vulnerable to affective disorders, and primary care physicians are encouraged to routinely screen for and treat depression in pregnant and postpartum women.1 However, anxiety disorders have a higher incidence than mood disorders in the general population,2 and perinatal anxiety may be more widely underrecognized and undertreated than depression.3 In addition, higher depression scores early in pregnancy have been shown to predict higher anxiety later in pregnancy.4
As family physicians, we are well-trained to recognize and treat anxiety disorders in the general patient population; however, we may lack the awareness and tools to identify these conditions in the perinatal period. Given our frequent encounters with both mom and baby in a child’s first year of life, we are uniquely positioned to promptly recognize, diagnose, and treat postpartum anxiety and thereby improve health outcomes for families.
DEFINING PERINATAL ANXIETY
Anxiety disorders (including generalized anxiety disorder, panic, phobia, and social anxiety) are the most common mental health disorders evaluated and treated in the primary care setting, with a lifetime prevalence of close to 30%.2
Continue to: A recent report from...
A recent report from the Centers for Disease Control and Prevention (CDC) estimates that 1 in 9 women experience symptoms of postpartum depression.5 The prevalence of anxiety disorders during pregnancy and the early postpartum period is not as well-known, but studies suggest that perinatal anxiety is much more prevalent than depression. In one study, generalized anxiety disorder (GAD) in the pre- and postnatal periods was 15.8% and 17.1%, respectively; an incidence far exceeding that of perinatal depression (3.9% and 4.8%, for the same periods).6 Additional evidence suggests that even more women in the postnatal period experience clinically significant levels of anxiety but do not meet full diagnostic criteria for an anxiety disorder.7
In another study, 9.5% of women met criteria for GAD at some point during pregnancy, with highest anxiety levels in the first trimester.8 Women with a history of GAD, lower education, lack of social support, and personal history of child abuse have the highest risk for postpartum anxiety. Women with a history of posttraumatic stress disorder (PTSD) may be twice as likely to develop postpartum anxiety as healthy women.9
It has been well-documented that sleep disruption—which is very common in new mothers in the postnatal period—contributes to mood and anxiety disorders.10,11
Clarifying a diagnosis of postpartum anxiety
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)12 specifies no diagnosis of postpartum anxiety disorder. And no standardized diagnostic criteria exist. It is likely that in some cases, postpartum anxiety represents an exacerbation of underlying GAD, and in other cases it is a situational disorder brought about by specific circumstances of the peripartum period.
The DSM-5 does, however, provide a helpful diagnostic approach. It defines a diagnosis of postpartum depression as being a variant of major depressive disorder (MDD) in which a woman must 1) meet criteria for a major depressive episode; and 2) occur during pregnancy or within 4 weeks of delivery. In practice, many clinicians extend the second requirement to include the first year postpartum.13 There is a “with anxious distress” specifier for major depression in the DSM-5, but the 2 disorders are otherwise unlinked.
Continue to: To apply the...
To apply the DSM-5 principles for postpartum depression to postpartum anxiety, a patient would need to 1) meet the diagnostic criteria for an anxiety disorder that 2) have their onset within a specified perinatal period. Variant presentations of anxiety in the postpartum period might include panic disorder and phobias, which could also interfere with a woman’s ability to care for her child.
The DSM-5 offers the following criteria for GAD12:
- excessive worry about a variety of topics
- worry that is experienced as hard to control
- worry associated with at least 3 physical or cognitive symptoms: edginess/restlessness, tiring easily, impaired concentration, irritability
- anxiety, worry, or associated symptoms that make it hard to carry out day-to-day activities and responsibilities
- symptoms that are unrelated to any other medical conditions and cannot be explained by the effect of substances including a prescription medication, alcohol, or recreational drugs
- symptoms that are not better explained by a different mental disorder.
Debilitating effects of postpartum anxiety
Many women experience some level of anxiety during pregnancy and early postpartum—anxiety that may range from normal and adaptive to debilitating.14 While the challenges of caring for a newborn are likely to bring some level of anxiety, these symptoms should be transient and not interfere with a woman’s capacity to care for her infant, herself, or her family.
Postpartum anxiety has been associated with a prior fear of giving birth, fear of death (of both mother and baby), lack of control, lack of self-confidence, and lack of confidence in the medical system.9 The experience of such ongoing disturbing thoughts or feelings of worry and tension that affect a woman’s ability to manage from day to day should indicate an illness state that deserves medical attention.
Mothers with postpartum anxiety disorders report significantly less bonding with their infants than do mothers without anxiety.15 A recent narrative review describes numerous studies that illustrate the negative effects of postpartum anxiety on bonding, breastfeeding, infant temperament, early childhood development, and conduct disorders.16 Anxious women may be less likely to initiate breastfeeding, have more challenges with breastfeeding, and even have a different milk composition.17 Women with prenatal anxiety are also more likely to stop breastfeeding prematurely.18 Children of anxious mothers may be more likely to have a difficult temperament and to display more distress.19 There are small studies demonstrating deficits in early infant development and increases in conduct disorder in the male offspring of anxious women.20
Continue to: SCREENING FOR POSTPARTUM ANXIETY
SCREENING FOR POSTPARTUM ANXIETY
Screening for perinatal depression has become standard of care, and the Edinburgh Postnatal Depression Scale (EPDS) is a widely used instrument.1 The EPDS, a 10-question self-report scale, was created and validated to screen for perinatal depression, with a cutoff of > 10/30 usually considered a positive result.
Researchers have investigated the utility of the EPDS as a screening tool for perinatal anxiety as well.21-23 These studies show some promise, but there are questions as to whether a total score or a subscale score of the EPDS is most accurate in detecting anxiety. Women with perinatal anxiety may score low on the total EPDS, yet score higher on 3 anxiety-specific questions (TABLE 123). For this reason, several studies propose an EPDS anxiety subscore or subscale (referred to as EPDS-3A).

Of note, there are some women who will score high on the subscale who do not ultimately meet the criteria for an anxiety disorder diagnosis. Clinicians should not over-interpret these scores and should always use sound clinical judgment when making a diagnosis.
Research has also focused on using the GAD 7-item (GAD-7) scale (TABLE 224),25 and on the

Family physicians may consider using the EPDS subscale if they are already using the EPDS, or adding the GAD-7 as a separate screening instrument during a postpartum visit. To date there is no one standard recommendation or screening tool.
Continue to: NONPHARMACOLOGIC TREATMENT
NONPHARMACOLOGIC TREATMENT
As one would with any patient who has situational anxiety, help new mothers find ways to increase their coping skills, reduce stress, and mobilize social supports and family resources. Given the association between sleep disruption and perinatal anxiety, counsel new mothers, especially those at high risk for postpartum anxiety, to prioritize sleep during this vulnerable time. To that end, consider recommending that they ask partners, family members, or friends to help them take care of the infant at night (or during the day). Such nonmedical interventions may be sufficient for women with mild anxiety.
Very few studies have addressed nonpharmacologic management of postpartum anxiety, but cognitive behavioral therapy (CBT) has been shown to help in managing and treating anxiety disorders outside of pregnancy.28 A few small studies indicate promise for CBT and for mindfulness-based interventions (MBIs) during pregnancy.29
A 2016 systematic review of pharmacologic and nonpharmacologic treatment of anxiety in the perinatal period found support for the use of CBT for panic disorder and specific phobias both in pregnancy and postpartum.30 A very small study found that teaching mothers to massage their preterm infants decreased maternal anxiety.31
If the patient is amenable, it is reasonable to start with behavioral interventions like CBT or MBI before pharmacologic treatment—particularly when physicians have mental health professionals embedded in their primary care team.
PHARMACOLOGIC TREATMENT
Selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) are considered first-line treatment for moderate to severe anxiety disorders in the perinatal and postnatal period.
Continue to: SSRIs in pregnancy
SSRIs in pregnancy. Lacking support of randomized controlled trials, most recommendations regarding SSRIs in pregnancy come from expert consensus or cohort and case control studies. Studies have raised concerns for an increased rate of congenital heart defects among fetuses exposed to paroxetine32 and primary pulmonary hypertension with all SSRIs.33 But the absolute risks are quite small. There have also been concerns regarding low birth weight and preterm birth, but it is possible that these outcomes result from the depression itself rather than the medication.34
Unfortunately, there are very few studies evaluating the efficacy of SSRIs in treating postpartum depression35 and even fewer that specifically evaluate their effect on perinatal anxiety. Many experts believe that not treating anxiety/depression is actually more harmful than the fetal effects of SSRIs, and that SSRIs are largely safe in both pregnancy and while breastfeeding, with benefits outweighing the risks.
SSRIs while breastfeeding. SSRIs have been found to be present in varying levels in breastmilk but may or may not be present in the serum of nursing infants.36 A 2008 guideline from the American College of Obstetricians and Gynecologists lists paroxetine, sertraline, and fluvoxamine as slightly safer than fluoxetine, escitalopram, and citalopram.37 A 2015 systematic review similarly concluded that sertraline and paroxetine have the most safety data on lactation.38 Lowest effective dose is always recommended to minimize exposure.
Benzodiazepines. As in the general population, benzodiazepines should be reserved for short-term use in acute anxiety and panic because they are associated with such adverse effects as worsening of depression/anxiety and risk of dependence and overdose. Longer-acting benzodiazepines (eg, clonazepam) are generally not recommended in lactation because of reported effects on infants, including sedation. Shorter-acting benzodiazepines (eg, lorazepam) are considered safer in lactation.39
THE CASE
Julia saw her family physician 4 more times, was evaluated by an ear-nose-and-throat specialist for her throat complaints, saw a therapist for CBT and a psychiatrist for medication, had 3 more ED visits, and lost 23 pounds before she finally agreed to start an SSRI for postpartum anxiety. She screened high on the EPDS-3A (9/9) despite scoring low on the full EPDS for perinatal depression (total, 9/30).
Continue to: Because of her swallowing impediments...
Because of her swallowing impediments and because she was breastfeeding, sertraline solution was started at very small doses. It was titrated weekly to obtain therapeutic levels. By 4 weeks, her weight stabilized. By 8 weeks, she started gaining weight and sleeping better. She saw the therapist regularly to continue CBT techniques. Over the next several months she started eating a normal diet. She is currently maintained on her SSRI, is still breastfeeding, and has achieved insight into her perinatal anxiety disorder.
CORRESPONDENCE
Veronica Jordan, MD, 3569 Round Barn Cir #200, Santa Rosa, CA 95403; [email protected].
1. O’Connor E, Rossom RC, Henninger M, et al. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:388-406.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
3. Giardinelli L, Innocenti A, Benni L, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2012;15:21-30.
4. Rallis S, Skouteris H, McCabe M, et al. A prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. 2014;27:e36-e42.
5. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158.
6. Fairbrother N, Janssen P, Antony MM, et al. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148-155.
7. Phillips J, Sharpe L, Matthey S, et al. Maternally focused worry. Arch Womens Ment Health. 2009;12:409-418.
8. Buist A, Gotman N, Yonkers KA. Generalized anxiety disorder: course and risk factors in pregnancy. J Affect Disord. 2011;131:277-283.
9. Schlomi Polachek I, Huller Harari L, Baum M, et al. Postpartum anxiety in a cohort of women from the general population: risk factors and association with depression during last week of pregnancy, postpartum depression and postpartum PTSD. Isr J Psychiatry Relat Sci. 2014;51:128-134.
10. Bei B, Coo S, Trinder J. Sleep and mood during pregnancy and the postpartum period. Sleep Med Clin. 2015;10:25-33.
11. Lawson A, Murphy KE, Sloan E, et al. The relationship between sleep and postpartum mental disorders: a systematic review. J Affect Disord. 2015;176:65-77.
12. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013.
13. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
14. Ali E. Women’s experiences with postpartum anxiety disorders: a narrative literature review. Int J Womens Health. 2018;10:237-249.
15. Tietz A, Zietlow AL, Reck C. Maternal bonding in mothers with postpartum anxiety disorder: the crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Arch Womens Ment Health. 2014;17:433-442.
16. Field T. Postnatal anxiety prevalence, predictors and effects on development: a narrative review. Infant Behav Dev. 2018;51:24-32.
17. Serim Demirgoren B, Ozbek A, Ormen M, et al. Do mothers with high sodium levels in their breast milk have high depression and anxiety scores? J Int Med Res. 2017;45:843-848.
18. Ystrom E. Breastfeeding cessation and symptoms of anxiety and depression: a longitudinal cohort study. BMC Pregnancy Childbirth. 2012;12:36.
19. Britton JR. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health. 2011;51:55-71.
20. Glasheen C, Richardson GA, Kim KH, et al. Exposure to maternal pre- and postnatal depression and anxiety symptoms: risk for major depression, anxiety disorders, and conduct disorder in adolescent offspring. Dev Psychopathol. 2013;26:1045-1063.
21. Petrozzi A, Gagliardi L. Anxious and depressive components of Edinburgh Postnatal Depression Scale in maternal postpartum psychological problems. J Perinat Med. 2013;41:343-348.
22. Bina R, Harrington D. The Edinburgh Postnatal Depression Scale: screening tool for postpartum anxiety as well? Findings from a confirmatory factor analysis of the Hebrew version. Matern Child Health J. 2016;20:904-914.
23. Matthey S, Fisher J, Rowe H. Using the Edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations J Affect Disord. 2013;146:224-230.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. Simpson W, Glazer M, Michalski N, et al. Comparative efficacy of the Generalized Anxiety Disorder 7-Item Scale and the Edinburgh Postnatal Depression Scale as screening tools for generalized anxiety disorder in pregnancy and the postpartum period. Can J Psychiatry. 2014;59:434-440.
26. Moran TE, Polanin JR, Wenzel A. The Postpartum Worry Scale-Revised: an initial validation of a measure of postpartum worry. Arch Womens Ment Health. 2014;17:41-48.
27. Fallon V, Halford JCG, Bennett KM, et al. The Postpartum Specific Anxiety Scale: development and preliminary validation. Arch Womens Ment Health. 2016;19:1079-1090.
28. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
29. Goodman JH, Guarino A, Chenausky K, et al. CALM Pregnancy: results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Womens Ment Health. 2014;17:373-387.
30. Marchesi C, Ossola P, Amerio A, et al. Clinical management of perinatal anxiety disorders: a systematic review. J Affect Disord. 2016;190:543-550.
31. Feijó L, Hernandez-Reif M, Field T, et al. Mothers’ depressed mood and anxiety levels are reduced after massaging their preterm infants. Infant Behav Devel. 2006;29:476-480.
32. Bérard A, Iessa N, Chaabane S, et al. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:589-604.
33. Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313:2142-2151.
34. Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? A population-based investigation. PLoS One. 2016;11:e0168115.
35. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;11:CD002018.
36. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;70:e35.
37. ACOG Committee on Practice Bulletins—number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020.
38. Orsolini L, Bellantuono C. Serotonin reuptake inhibitors and breastfeeding: a systematic review. Hum Psychopharmacol. 2015;30:4-20.
39. NIH. Drugs and Lactation Database. https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 26, 2019.
1. O’Connor E, Rossom RC, Henninger M, et al. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:388-406.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
3. Giardinelli L, Innocenti A, Benni L, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2012;15:21-30.
4. Rallis S, Skouteris H, McCabe M, et al. A prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. 2014;27:e36-e42.
5. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158.
6. Fairbrother N, Janssen P, Antony MM, et al. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148-155.
7. Phillips J, Sharpe L, Matthey S, et al. Maternally focused worry. Arch Womens Ment Health. 2009;12:409-418.
8. Buist A, Gotman N, Yonkers KA. Generalized anxiety disorder: course and risk factors in pregnancy. J Affect Disord. 2011;131:277-283.
9. Schlomi Polachek I, Huller Harari L, Baum M, et al. Postpartum anxiety in a cohort of women from the general population: risk factors and association with depression during last week of pregnancy, postpartum depression and postpartum PTSD. Isr J Psychiatry Relat Sci. 2014;51:128-134.
10. Bei B, Coo S, Trinder J. Sleep and mood during pregnancy and the postpartum period. Sleep Med Clin. 2015;10:25-33.
11. Lawson A, Murphy KE, Sloan E, et al. The relationship between sleep and postpartum mental disorders: a systematic review. J Affect Disord. 2015;176:65-77.
12. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013.
13. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
14. Ali E. Women’s experiences with postpartum anxiety disorders: a narrative literature review. Int J Womens Health. 2018;10:237-249.
15. Tietz A, Zietlow AL, Reck C. Maternal bonding in mothers with postpartum anxiety disorder: the crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Arch Womens Ment Health. 2014;17:433-442.
16. Field T. Postnatal anxiety prevalence, predictors and effects on development: a narrative review. Infant Behav Dev. 2018;51:24-32.
17. Serim Demirgoren B, Ozbek A, Ormen M, et al. Do mothers with high sodium levels in their breast milk have high depression and anxiety scores? J Int Med Res. 2017;45:843-848.
18. Ystrom E. Breastfeeding cessation and symptoms of anxiety and depression: a longitudinal cohort study. BMC Pregnancy Childbirth. 2012;12:36.
19. Britton JR. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health. 2011;51:55-71.
20. Glasheen C, Richardson GA, Kim KH, et al. Exposure to maternal pre- and postnatal depression and anxiety symptoms: risk for major depression, anxiety disorders, and conduct disorder in adolescent offspring. Dev Psychopathol. 2013;26:1045-1063.
21. Petrozzi A, Gagliardi L. Anxious and depressive components of Edinburgh Postnatal Depression Scale in maternal postpartum psychological problems. J Perinat Med. 2013;41:343-348.
22. Bina R, Harrington D. The Edinburgh Postnatal Depression Scale: screening tool for postpartum anxiety as well? Findings from a confirmatory factor analysis of the Hebrew version. Matern Child Health J. 2016;20:904-914.
23. Matthey S, Fisher J, Rowe H. Using the Edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations J Affect Disord. 2013;146:224-230.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. Simpson W, Glazer M, Michalski N, et al. Comparative efficacy of the Generalized Anxiety Disorder 7-Item Scale and the Edinburgh Postnatal Depression Scale as screening tools for generalized anxiety disorder in pregnancy and the postpartum period. Can J Psychiatry. 2014;59:434-440.
26. Moran TE, Polanin JR, Wenzel A. The Postpartum Worry Scale-Revised: an initial validation of a measure of postpartum worry. Arch Womens Ment Health. 2014;17:41-48.
27. Fallon V, Halford JCG, Bennett KM, et al. The Postpartum Specific Anxiety Scale: development and preliminary validation. Arch Womens Ment Health. 2016;19:1079-1090.
28. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
29. Goodman JH, Guarino A, Chenausky K, et al. CALM Pregnancy: results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Womens Ment Health. 2014;17:373-387.
30. Marchesi C, Ossola P, Amerio A, et al. Clinical management of perinatal anxiety disorders: a systematic review. J Affect Disord. 2016;190:543-550.
31. Feijó L, Hernandez-Reif M, Field T, et al. Mothers’ depressed mood and anxiety levels are reduced after massaging their preterm infants. Infant Behav Devel. 2006;29:476-480.
32. Bérard A, Iessa N, Chaabane S, et al. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:589-604.
33. Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313:2142-2151.
34. Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? A population-based investigation. PLoS One. 2016;11:e0168115.
35. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;11:CD002018.
36. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;70:e35.
37. ACOG Committee on Practice Bulletins—number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020.
38. Orsolini L, Bellantuono C. Serotonin reuptake inhibitors and breastfeeding: a systematic review. Hum Psychopharmacol. 2015;30:4-20.
39. NIH. Drugs and Lactation Database. https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 26, 2019.