User login
It’s time to start asking all patients about intimate partner violence
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.

What is intimate partner violence? How can you identify it?
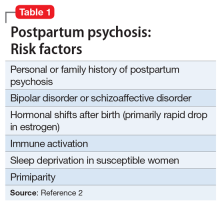
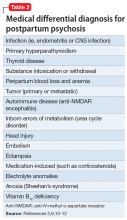
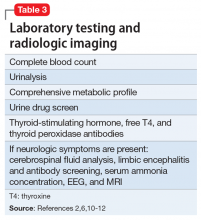
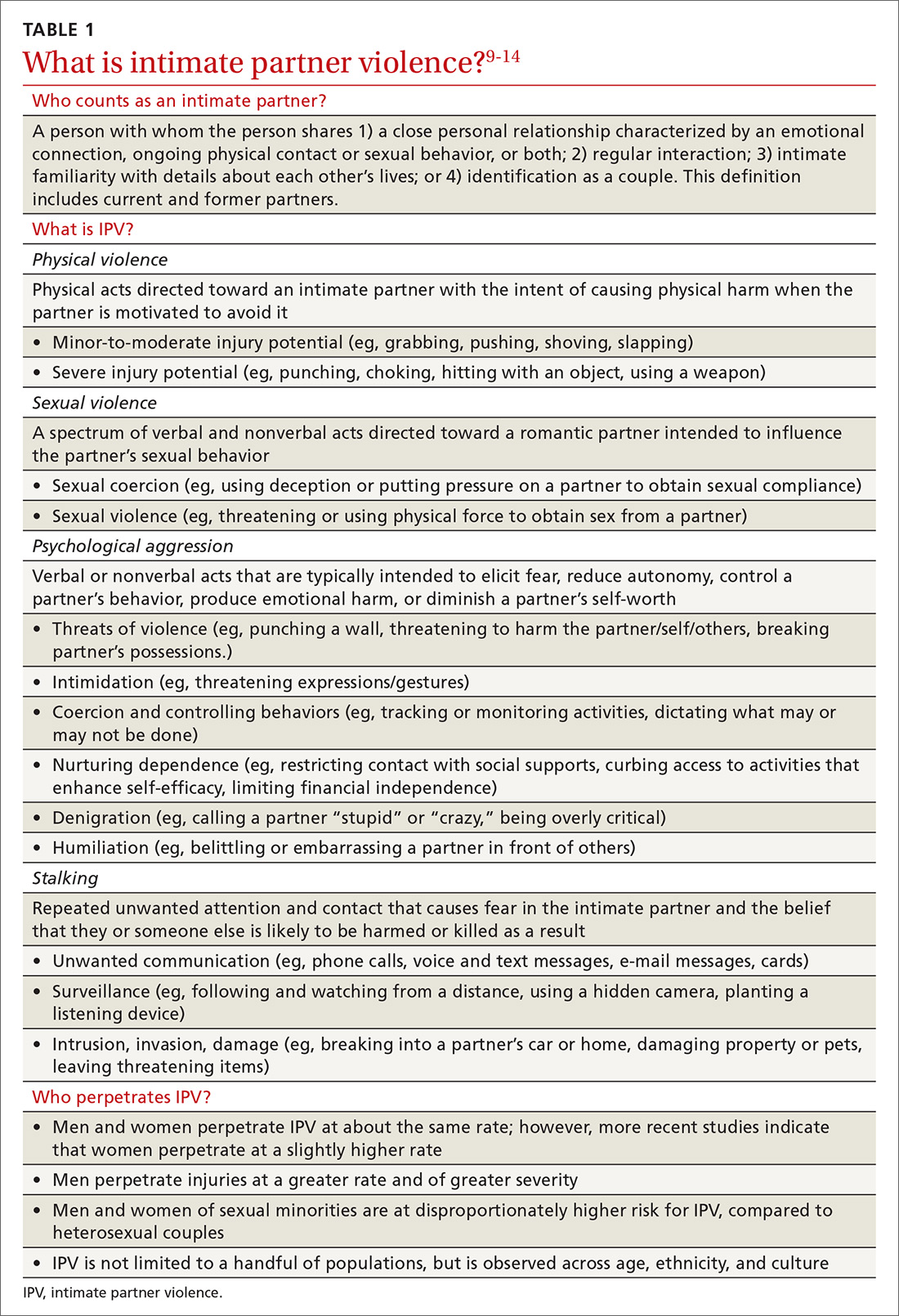
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16
Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30
What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.

Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).
Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; [email protected].
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.

What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16

Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
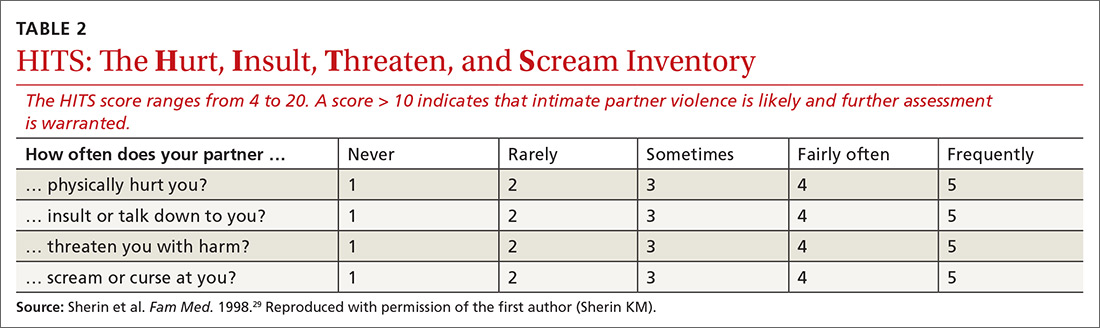
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30

What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.

Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
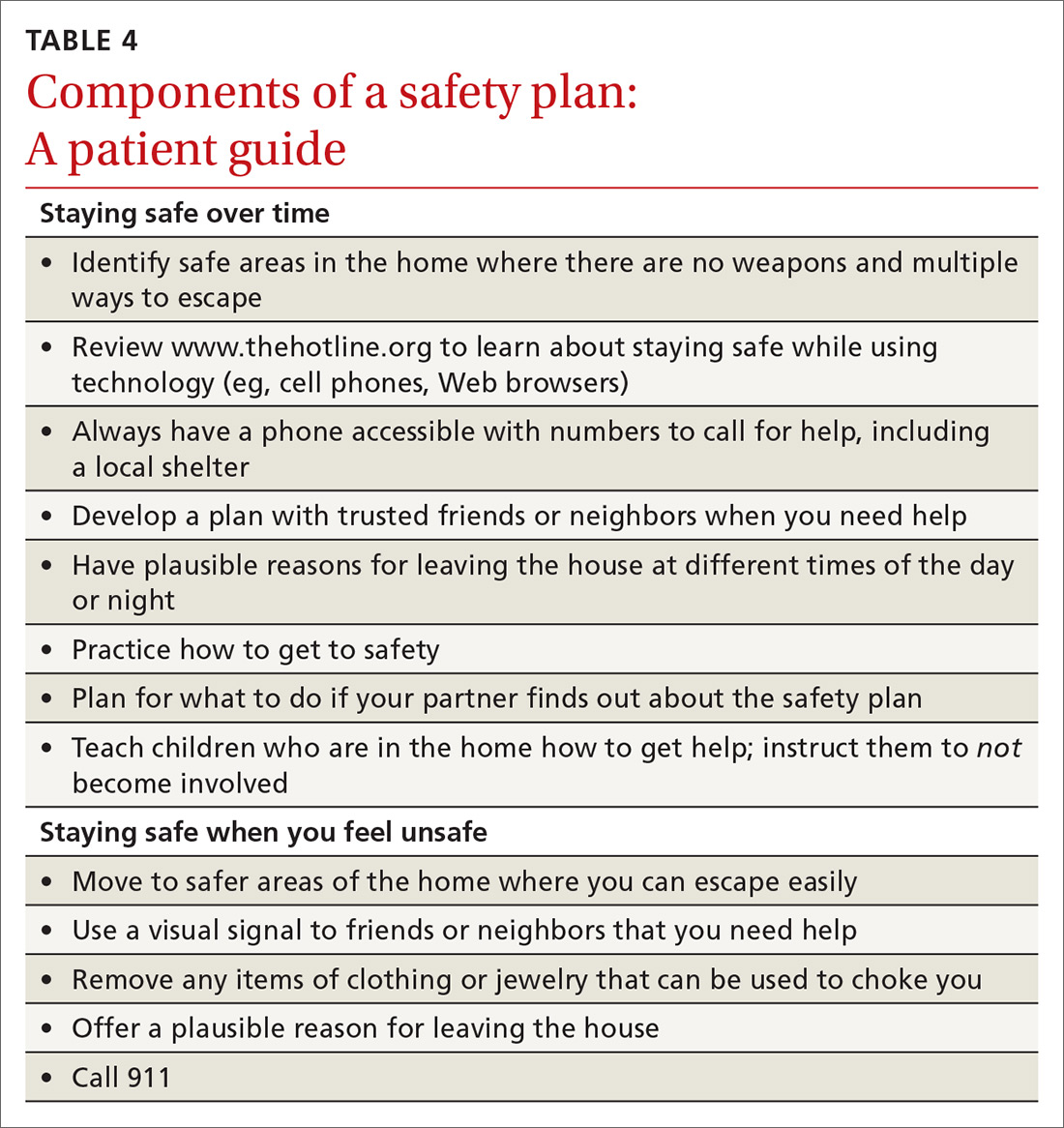
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).

Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; [email protected].
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.

What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16

Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30

What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.

Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).

Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; [email protected].
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
PRACTICE RECOMMENDATIONS
› Perform annual screening for intimate partner violence of all female patients of childbearing age; strongly consider a pilot program of universal screening (all male and female patients, across the lifespan). B
› Establish a protocol for intimate partner violence screening and referral—possibly the most effective means of identifying intimate partner violence at early and severe stages. B
› Collaborate with the patient in the safety planning and referral process; benefits include improved likelihood that the patient will adhere to a safety plan and follow through with the referral. B
› Utilize online resources to 1) ease the process of establishing relationships with local intimate partner violence referrals and 2) facilitate warm handoffs to increase the likelihood of patient engagement. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Screening and counseling interventions to prevent peripartum depression: A practical approach
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
Noninfected children of HIV-positive mothers have high rates of obesity
NEW ORLEANS – than are those with no such exposure, according to research that provides a compelling link between inflammatory activity in utero and subsequent risk of metabolic disorders.
Most supportive of that link was a near-linear inverse relationship between CD4 counts during the time of pregnancy and risk of both obesity and reactive respiratory disease more than a decade later, according to research presented by Lindsay Fourman, MD, an instructor in medicine at Massachusetts General Hospital, Boston, during the annual meeting of the Endocrine Society.
In this video interview, Dr. Fourman discusses the effort to understand the long-term health consequences of being exposed to HIV and antiretroviral therapies while in utero, a group known by the acronym HIV-exposed uninfected (HEU). With effective therapies now routinely preventing mother-to-child transmission, this population of children is growing quickly.
For this study, 50 HEU individuals were identified from a patient database. They were matched in a 3:1 ratio to a control group for a variety of demographic and socioeconomic variables. At a median age of 18 years, the HEU population was found to have a “strikingly” higher rate of obesity, compared with controls (42% vs. 25%, respectively; P = .04). The rate of reactive airway disease was similarly increased in the HEU group (40% vs. 24%; P = .04).
These data are important for considering health risks in an HEU population, but Dr. Fourman explained that it provides support for looking at metabolic risks from other in utero exposures linked to upregulated inflammation, such as gestational diabetes or obesity.
Dr Fourman and her colleagues reported no disclosures or financial conflicts of interest.
SOURCE: Fourman L et al. ENDO 2019, Session P10 (SAT-256).
NEW ORLEANS – than are those with no such exposure, according to research that provides a compelling link between inflammatory activity in utero and subsequent risk of metabolic disorders.
Most supportive of that link was a near-linear inverse relationship between CD4 counts during the time of pregnancy and risk of both obesity and reactive respiratory disease more than a decade later, according to research presented by Lindsay Fourman, MD, an instructor in medicine at Massachusetts General Hospital, Boston, during the annual meeting of the Endocrine Society.
In this video interview, Dr. Fourman discusses the effort to understand the long-term health consequences of being exposed to HIV and antiretroviral therapies while in utero, a group known by the acronym HIV-exposed uninfected (HEU). With effective therapies now routinely preventing mother-to-child transmission, this population of children is growing quickly.
For this study, 50 HEU individuals were identified from a patient database. They were matched in a 3:1 ratio to a control group for a variety of demographic and socioeconomic variables. At a median age of 18 years, the HEU population was found to have a “strikingly” higher rate of obesity, compared with controls (42% vs. 25%, respectively; P = .04). The rate of reactive airway disease was similarly increased in the HEU group (40% vs. 24%; P = .04).
These data are important for considering health risks in an HEU population, but Dr. Fourman explained that it provides support for looking at metabolic risks from other in utero exposures linked to upregulated inflammation, such as gestational diabetes or obesity.
Dr Fourman and her colleagues reported no disclosures or financial conflicts of interest.
SOURCE: Fourman L et al. ENDO 2019, Session P10 (SAT-256).
NEW ORLEANS – than are those with no such exposure, according to research that provides a compelling link between inflammatory activity in utero and subsequent risk of metabolic disorders.
Most supportive of that link was a near-linear inverse relationship between CD4 counts during the time of pregnancy and risk of both obesity and reactive respiratory disease more than a decade later, according to research presented by Lindsay Fourman, MD, an instructor in medicine at Massachusetts General Hospital, Boston, during the annual meeting of the Endocrine Society.
In this video interview, Dr. Fourman discusses the effort to understand the long-term health consequences of being exposed to HIV and antiretroviral therapies while in utero, a group known by the acronym HIV-exposed uninfected (HEU). With effective therapies now routinely preventing mother-to-child transmission, this population of children is growing quickly.
For this study, 50 HEU individuals were identified from a patient database. They were matched in a 3:1 ratio to a control group for a variety of demographic and socioeconomic variables. At a median age of 18 years, the HEU population was found to have a “strikingly” higher rate of obesity, compared with controls (42% vs. 25%, respectively; P = .04). The rate of reactive airway disease was similarly increased in the HEU group (40% vs. 24%; P = .04).
These data are important for considering health risks in an HEU population, but Dr. Fourman explained that it provides support for looking at metabolic risks from other in utero exposures linked to upregulated inflammation, such as gestational diabetes or obesity.
Dr Fourman and her colleagues reported no disclosures or financial conflicts of interest.
SOURCE: Fourman L et al. ENDO 2019, Session P10 (SAT-256).
REPORTING FROM ENDO 2019
Valproate, topiramate prescribed in young women despite known teratogenicity risks
results of a retrospective analysis suggest.
Topiramate, linked to increased risk of cleft palate and smaller-than-gestational-age newborns, was among the top three antiepileptic drugs (AEDs) prescribed to women 15-44 years of age in the population-based cohort study.
Valproate, linked to increases in both anatomic and behavioral teratogenicity, was less often prescribed, but nevertheless still prescribed in a considerable proportion of patients in the study, which looked at U.S. commercial, Medicare, and Medicaid claims data from 2009 to 2013.
Presence of comorbidities could be influencing whether or not a woman of childbearing age receives one of these AEDs, the investigators said. Specifically, they found valproate more often prescribed for women with epilepsy who also had mood or anxiety and dissociative disorder, while topiramate was more often prescribed in women with headaches or migraines.
Taken together, these findings suggest a lack of awareness of the teratogenic risks of valproate and topiramate, said the investigators, led by Hyunmi Kim, MD, PhD, MPH, of the department of neurology at Stanford (Calif.) University.
“To improve current practice, knowledge of the teratogenicity of certain AEDs should be disseminated to health care professionals and patients,” they wrote. The report is in JAMA Neurology.
The findings of Dr. Kim and her colleagues were based on data for 46,767 women of childbearing age: 8,003 incident (new) cases with a mean age of 27 years, and 38,764 prevalent cases with a mean age of 30 years.
Topiramate was the second- or third-most prescribed AED in the analyses, alongside levetiracetam and lamotrigine. In particular, topiramate prescriptions were found in incident cases receiving first-line monotherapy (15%), prevalent cases receiving first-line monotherapy (13%), and prevalent cases receiving polytherapy (29%).
Valproate was the fifth-most prescribed AED for incident and prevalent cases receiving first-line monotherapy (5% and 10%, respectively), and came in fourth place among prevalent cases receiving polytherapy (22%).
The somewhat lower rate of valproate prescriptions tracks with other recent analyses showing that valproate use decreased among women of childbearing age following recommendations against its use during pregnancy, according to Dr. Kim and her coauthors.
However, topiramate is another story: “Although the magnitude of risk and range of adverse reproductive outcomes associated with topiramate use appear substantially less than those associated with valproate, some reduction in the use of topiramate in this population might be expected after evidence emerged in 2008 of its association with cleft palate,” they said in their report.
UCB Pharma sponsored this study. Study authors reported disclosures related to UCB Pharma, Biogen, Eisai, SK Life Science, Brain Sentinel, UCB Pharma, and the University of Alabama at Birmingham.
SOURCE: Kim H et al. JAMA Neurol. 2019 Apr 1. doi: 10.1001/jamaneurol.2019.0447.
results of a retrospective analysis suggest.
Topiramate, linked to increased risk of cleft palate and smaller-than-gestational-age newborns, was among the top three antiepileptic drugs (AEDs) prescribed to women 15-44 years of age in the population-based cohort study.
Valproate, linked to increases in both anatomic and behavioral teratogenicity, was less often prescribed, but nevertheless still prescribed in a considerable proportion of patients in the study, which looked at U.S. commercial, Medicare, and Medicaid claims data from 2009 to 2013.
Presence of comorbidities could be influencing whether or not a woman of childbearing age receives one of these AEDs, the investigators said. Specifically, they found valproate more often prescribed for women with epilepsy who also had mood or anxiety and dissociative disorder, while topiramate was more often prescribed in women with headaches or migraines.
Taken together, these findings suggest a lack of awareness of the teratogenic risks of valproate and topiramate, said the investigators, led by Hyunmi Kim, MD, PhD, MPH, of the department of neurology at Stanford (Calif.) University.
“To improve current practice, knowledge of the teratogenicity of certain AEDs should be disseminated to health care professionals and patients,” they wrote. The report is in JAMA Neurology.
The findings of Dr. Kim and her colleagues were based on data for 46,767 women of childbearing age: 8,003 incident (new) cases with a mean age of 27 years, and 38,764 prevalent cases with a mean age of 30 years.
Topiramate was the second- or third-most prescribed AED in the analyses, alongside levetiracetam and lamotrigine. In particular, topiramate prescriptions were found in incident cases receiving first-line monotherapy (15%), prevalent cases receiving first-line monotherapy (13%), and prevalent cases receiving polytherapy (29%).
Valproate was the fifth-most prescribed AED for incident and prevalent cases receiving first-line monotherapy (5% and 10%, respectively), and came in fourth place among prevalent cases receiving polytherapy (22%).
The somewhat lower rate of valproate prescriptions tracks with other recent analyses showing that valproate use decreased among women of childbearing age following recommendations against its use during pregnancy, according to Dr. Kim and her coauthors.
However, topiramate is another story: “Although the magnitude of risk and range of adverse reproductive outcomes associated with topiramate use appear substantially less than those associated with valproate, some reduction in the use of topiramate in this population might be expected after evidence emerged in 2008 of its association with cleft palate,” they said in their report.
UCB Pharma sponsored this study. Study authors reported disclosures related to UCB Pharma, Biogen, Eisai, SK Life Science, Brain Sentinel, UCB Pharma, and the University of Alabama at Birmingham.
SOURCE: Kim H et al. JAMA Neurol. 2019 Apr 1. doi: 10.1001/jamaneurol.2019.0447.
results of a retrospective analysis suggest.
Topiramate, linked to increased risk of cleft palate and smaller-than-gestational-age newborns, was among the top three antiepileptic drugs (AEDs) prescribed to women 15-44 years of age in the population-based cohort study.
Valproate, linked to increases in both anatomic and behavioral teratogenicity, was less often prescribed, but nevertheless still prescribed in a considerable proportion of patients in the study, which looked at U.S. commercial, Medicare, and Medicaid claims data from 2009 to 2013.
Presence of comorbidities could be influencing whether or not a woman of childbearing age receives one of these AEDs, the investigators said. Specifically, they found valproate more often prescribed for women with epilepsy who also had mood or anxiety and dissociative disorder, while topiramate was more often prescribed in women with headaches or migraines.
Taken together, these findings suggest a lack of awareness of the teratogenic risks of valproate and topiramate, said the investigators, led by Hyunmi Kim, MD, PhD, MPH, of the department of neurology at Stanford (Calif.) University.
“To improve current practice, knowledge of the teratogenicity of certain AEDs should be disseminated to health care professionals and patients,” they wrote. The report is in JAMA Neurology.
The findings of Dr. Kim and her colleagues were based on data for 46,767 women of childbearing age: 8,003 incident (new) cases with a mean age of 27 years, and 38,764 prevalent cases with a mean age of 30 years.
Topiramate was the second- or third-most prescribed AED in the analyses, alongside levetiracetam and lamotrigine. In particular, topiramate prescriptions were found in incident cases receiving first-line monotherapy (15%), prevalent cases receiving first-line monotherapy (13%), and prevalent cases receiving polytherapy (29%).
Valproate was the fifth-most prescribed AED for incident and prevalent cases receiving first-line monotherapy (5% and 10%, respectively), and came in fourth place among prevalent cases receiving polytherapy (22%).
The somewhat lower rate of valproate prescriptions tracks with other recent analyses showing that valproate use decreased among women of childbearing age following recommendations against its use during pregnancy, according to Dr. Kim and her coauthors.
However, topiramate is another story: “Although the magnitude of risk and range of adverse reproductive outcomes associated with topiramate use appear substantially less than those associated with valproate, some reduction in the use of topiramate in this population might be expected after evidence emerged in 2008 of its association with cleft palate,” they said in their report.
UCB Pharma sponsored this study. Study authors reported disclosures related to UCB Pharma, Biogen, Eisai, SK Life Science, Brain Sentinel, UCB Pharma, and the University of Alabama at Birmingham.
SOURCE: Kim H et al. JAMA Neurol. 2019 Apr 1. doi: 10.1001/jamaneurol.2019.0447.
FROM JAMA NEUROLOGY
Key clinical point: Both valproate and topiramate are prescribed relatively often in women of childbearing age despite known teratogenic risks.
Major finding: Topiramate was the second- or third-most prescribed AED in the analyses. Valproate was the fifth-most prescribed AED for incident and prevalent cases receiving first-line monotherapy.
Study details: Retrospective cohort study including nearly 47,000 women of childbearing age enrolled in claims databases between 2009 and 2013.
Disclosures: UCB Pharma sponsored the study. Study authors reported disclosures related to UCB Pharma, Biogen, Eisai, SK Life Science, Brain Sentinel, UCB Pharma, and the University of Alabama at Birmingham.
Source: Kim H et al. JAMA Neurol. 2019 Apr 1. doi: 10.1001/jamaneurol.2019.0447.
Spontaneous coronary artery dissection: An often unrecognized cause of acute coronary syndrome
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
KEY POINTS
- SCAD often presents with symptoms of acute coronary syndrome but can be asymptomatic or cause sudden death.
- Management is generally conservative, but a left main or severe proximal 2-vessel dissection, hemodynamic instability, or ongoing ischemic symptoms may warrant revascularization.
- All patients with SCAD should be screened for other vascular problems, especially fibromuscular dysplasia.
- Long-term aspirin therapy and 1 year of clopidogrel are recommended after an episode of SCAD.
How should I treat acute agitation in pregnancy?
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20 Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20 Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20 Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
A woman, age 35, with new-onset ascites
A 35-year-old woman is admitted to the hospital with a 5-day history of abdominal distention and jaundice. She reports no history of fever, chills, night sweats, abdominal pain, nausea, vomiting, diarrhea, changes in urine color, change in stool color, weight loss, weight gain, or loss of appetite.
She is petite, with a body mass index of 19.4 kg/m2. She has no known history of medical conditions or surgery and is not taking any medications. Her family history is unremarkable, and she denies current or past tobacco, alcohol, or illicit drug use.
RECENT TRAVEL
She says that during a trip to Central America several months ago, she had suffered a seizure and was taken to a local hospital, where laboratory testing revealed elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. She says that the rest of the workup at that time was normal.
About 1 week after that incident, she returned home and saw her primary care physician, who ordered further testing, which showed mild hyperbilirubinemia and mild elevation of AST and ALT levels. Her physician attributed the elevations to atovaquone, which she had been taking for malaria prophylaxis, as repeat testing 2 weeks later showed improvement in AST and ALT levels.
The patient says she returned to her normal state of health until about 5 days ago, when she noticed jaundice and abdominal distention, but without abdominal pain, dark urine, or clay-colored stools. She became concerned and went to her local hospital. Testing there noted mild elevation of AST and ALT, as well as an elevated international normalized ratio (INR) and hyperbilirubinemia. Computed tomography of the abdomen and pelvis showed hepatomegaly with possible fatty liver. Because of these results, the patient was transferred to our institution for further evaluation.
EVALUATION AT OUR INSTITUTION
On examination at our institution, she is afebrile, and vital signs are within normal ranges. She has bilateral scleral icterus and diffuse jaundice, but no other skin finding such as rash or spider angioma. She has no lymphadenopathy. Her abdomen is distended, with tense ascites, and her liver is tender to palpation. The tip of the spleen is not palpable.
The cardiovascular examination reveals no murmurs, rubs, or gallops, but she has jugular venous distention and +2 pitting edema of both lower extremities.
On respiratory examination, there is dullness to percussion, with slight crackles on auscultation at the right lung base. The neurologic examination is normal.
Table 1 shows the results of initial laboratory testing.
1. Which study would provide the most information on the cause of ascites?
- Abdominal ultrasonography
- Abdominal paracentesis with ascitic fluid analysis
- Chest radiography
- Echocardiography
- Urine protein-to-creatinine ratio
Abdominal paracentesis with ascitic fluid analysis is the essential study for any patient with clinically apparent new-onset ascites.1–3 It is the study that provides the most information on the cause of ascites.
In our patient, abdominal paracentesis yields 1,000 mL of straw-colored ascitic fluid, and analysis shows 86 nucleated cells, 28 of which are polymorphonuclear cells, and 0 red blood cells, with negative Gram stain and culture. The ascitic albumin level is 0.85 g/dL, with an ascitic protein of 1.1 g/dL.
Abdominal ultrasonography shows a diffusely echogenic liver, no focal lesions, moderate ascites, normal portal vein flow, no intrahepatic or extrahepatic biliary duct dilation, normal kidney sizes, no hydronephrosis, and no intra-abdominal mass. Chest radiography is clear with no sign of consolidation, edema, or effusion. Echocardiography shows a normal left ventricular ejection fraction with no valvular disease or pericardial effusion. A random urine protein-creatinine ratio is normal at 0.1 (reference range < 0.2).
2. What is the most likely cause of her ascites based on the workup to this point?
- Cirrhosis
- Heart failure
- Nephrotic syndrome
- Portal vein thrombus
- Abdominal malignancy
- Malaria
An initial approach to ascitic fluid analysis is to calculate the serum-ascites albumin gradient (SAAG). The SAAG is calculated as the serum albumin level minus the ascitic fluid albumin level.4,5 This is useful in determining the cause of the ascites (Figure 1).4,5 A gradient of 1.1 g/dL or higher indicates portal hypertension.4,5
Common causes of portal hypertension include cirrhosis, alcoholic hepatitis, heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome, portal vein thrombosis), idiopathic portal fibrosis, and metastatic liver disease.5,6
If portal hypertension is present based on the SAAG, the next step is to review the ascitic protein level to help distinguish between a hepatic and a cardiac etiology of the ascites. An ascitic protein level less than 2.5 g/dL indicates a primary liver pathology (eg, cirrhosis). An ascitic protein level of 2.5 g/dL or greater typically indicates a cardiac condition (eg, heart failure, pericardial disease) with secondary congestive hepatopathy.5,6
If the SAAG is less than 1.1 g/dL, the ascites is likely not from portal hypertension. Typical causes of a low SAAG include infection, malignancy, pancreatic ascites, and nephrotic syndrome.5,6
In our patient, the SAAG is 1.35 g/dL (2.2 g/dL minus 0.85 g/dL), ie, elevated and due to portal hypertension. With an SAAG of 1.1 g/dL or greater and an ascitic fluid protein level less than 2.5 g/dL, as in our patient, the most likely cause is cirrhosis.
Heart failure is unlikely based on her normal brain natriuretic peptide level, an ascitic fluid protein level below 2.5 g/dL, and normal results on echocardiography. Nephrotic syndrome is also very unlikely based on the patient’s normal random urine protein-creatinine ratio. Portal vein thrombus and abdominal malignancy are essentially ruled out by the negative results of Doppler abdominal ultrasonography, with normal venous flow and no intra-abdominal mass and coupled with an elevated SAAG.
Although the patient has a history of travel, the incubation period for malaria would not fit the time frame of presentation. Also, she did not have typical malarial symptoms, her rapid malaria test was negative, and a peripheral blood smear for blood parasites was negative. It should be noted, however, that Plasmodium malariae infection classically presents with flulike symptoms and can resemble nephrotic syndrome, including peripheral edema, ascites, heavy proteinuria, hypoalbuminemia, and hyperlipidemia.7
3. In which patients is antibiotic prophylaxis against spontaneous bacterial peritonitis (SBP) appropriate?
- Any patient with cirrhosis
- Any patient with cirrhosis who is hospitalized
- Any patient with cirrhosis and an ascitic fluid protein level below 2.0 g/dL
- Any patient with cirrhosis and a history of SBP
Any patient with cirrhosis and a history of SBP should receive prophylactic antibiotics,8 as should any patient deemed at high risk of SBP. It is indicated in the following patients:
- Patients with cirrhosis and gastrointestinal bleeding9,10
- Patients with cirrhosis and a previous episode of SBP8
- Patients with cirrhosis and an ascitic fluid protein level less than 1.5 g/dL with either impaired renal function (creatinine ≥ 1.2 mg/dL, blood urea nitrogen level ≥ 25 mg/dL, or serum sodium ≤ 130 mmol/L) or liver failure (Child-Pugh score ≥ 9 and a bilirubin ≥ 3 mg/dL)9
- Patients with cirrhosis who are hospitalized for other reasons and have an ascitic protein level < 1.0 g/dL.9
Our patient has no signs or symptoms of gastrointestinal bleeding and no history of SBP. Her ascitic fluid protein level is 1.1 g/dL, and she has normal renal function. However, her Child-Pugh score is 12 (3 points for total bilirubin > 3 mg/dL, 3 points for serum albumin < 2.8 g/dL, 2 points for an INR 1.7 to 2.2, 3 points for moderate ascites, and 1 point for no encephalopathy), with a bilirubin of 17.0 mg/dL. Based on this, she is placed on antibiotic prophylaxis for SBP.
Our patient then undergoes an extensive workup for liver disease. Results of tests for toxins, autoimmune diseases, and inheritable diseases are all within normal limits. At this point, despite the patient’s reported negative alcohol history, our leading diagnosis is alcoholic hepatitis.
To confirm this diagnosis, she subsequently undergoes transjugular liver biopsy, considered the gold standard for the diagnosis of alcoholic hepatitis. During the procedure, the hepatic venous pressure gradient is measured at 18 mm Hg (reference range 1–5 mm Hg), suggestive of portal hypertension. The pathology study shows severe fatty change, active steatohepatitis with ballooning degeneration, easily identifiable Mallory-Denk bodies, and prominent neutrophilic infiltration, as well as extensive bridging fibrosis (Figure 2). These findings point to alcoholic hepatitis.
After the biopsy results, we speak with the patient further about her alcohol habits. At this point, she informs us that she has consumed significant amounts of alcohol since the age of 18 (6 to 12 alcoholic beverages per day, including beer and hard liquor). Therefore, based on this new information, on her jaundice and ascites, and on results of laboratory testing and biopsy, we confirmed our diagnosis of alcoholic hepatitis.
4. When is drug treatment appropriate for alcoholic hepatitis?
- Model for End-stage Liver Disease (MELD) score greater than 12
- MELD score greater than 15
- Maddrey Discriminant Function score greater than 25
- Maddrey Discriminant Function score greater than 32
- Glasgow score greater than 5
- Glasgow score greater than 7
The best answer is a Maddrey Discriminant Function score greater than 32. A variety of scoring systems have been used to assess the severity of alcoholic hepatitis and to guide treatment, including the Maddrey Discriminant Function score, the MELD score, and the Glasgow score.11–16 They share similar laboratory values in their calculations, including prothrombin time (or INR) and total bilirubin.11–16 Typically, a Maddrey Discriminant Function score greater than 32, a Glasgow score of greater than 9, or a MELD score greater than 21 is used to determine whether pharmacologic treatment is indicated.11–16
The typical treatment is prednisolone or pentoxifylline.11,17–21 The Lille score is designed to help decide whether to stop corticosteroids after 1 week of administration due to lack of treatment response.22 It predicts mortality rates within 6 months; a score of 0.45 or less indicates a good prognosis, and corticosteroid therapy should continue for 28 days (Figure 3).22
Our patient’s discriminant function score is 50, her Glasgow score is 10, and her MELD score is 28; thus, she begins treatment with oral prednisolone. Her Lille score at 1 week is 0.119, indicating a good prognosis, and her corticosteroids are continued for a total of 28 days.
It should be highlighted that the most important treatment is abstinence from alcohol.11 Recent literature suggests that any benefit of prednisolone or pentoxifylline in terms of mortality rates is questionable,19–20 and there is evidence that giving both drugs simultaneously may improve mortality rates,11,21 but the evidence remains conflicting at this time.
ALCOHOLIC HEPATITIS
Alcoholic hepatitis is a clinical syndrome of jaundice and liver failure, often in the setting of heavy alcohol use for decades.11,12 The incidence is unknown, but the typical age of presentation is between 40 and 50.11,12 The chief sign is a rapid onset of jaundice (< 3 months); common signs and symptoms include fever, ascites, proximal muscle loss, and an enlarged, tender liver.12 Encephalopathy may be seen in severe alcoholic hepatitis.12
Our patient is 35 years old. She has jaundice with rapid onset, as well as ascites and a tender liver.
The diagnosis of alcoholic hepatitis must take into account the patient’s history, physical examination, and laboratory findings. Until proven otherwise, the diagnosis should be presumed in the following scenario: ascites and jaundice on examination (usually with a duration < 3 months); a history of heavy alcohol use; neutrophilic leukocytosis; an AST level that is elevated but below 300 U/L; an ALT level above the normal range but below 300 U/L; an AST-ALT ratio greater than 2; a total serum bilirubin level above 5 mg/dL; and an elevated INR.11,12 Liver biopsy is the gold standard for diagnosis. Though not routinely done because of risks associated with the procedure, it may help confirm the diagnosis if it is in question.
CASE CONCLUDED
We start our patient on oral prednisolone 40 mg daily for alcoholic hepatitis. Her symptoms and laboratory testing results including bilirubin improve. Her Lille score at 7 days indicates a good prognosis, prompting continuation of corticosteroid treatment for the full 28 days.
She is referred to an outpatient alcohol rehabilitation program and has remained sober as of the last outpatient note.
Alcoholic hepatitis is extremely difficult to diagnose, and no single blood test or imaging study confirms the diagnosis. The history, physical examination findings, and laboratory findings are crucial. If the diagnosis is still in doubt, liver biopsy may help confirm the diagnosis.
- Ruyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009; 49(6):2087–2107. doi:10.1002/hep.22853
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2(4):399–407. pmid:7095741
- Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med 2004; 350(16):1646–1654. doi:10.1056/NEJMra035021
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117(3):215–220. pmid:1616215
- Hernaez R, Hamilton JP. Unexplained ascites. Clin Liver Dis 2016; 7(3):53–56. https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/cld.537
- Huang LL, Xia HH, Zhu SL. Ascitic fluid analysis in the differential diagnosis of ascites: focus on cirrhotic ascites. J Clin Transl Hepatol 2014; 2(1):58–64. doi:10.14218/JCTH.2013.00010
- Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis 2012; 4(1):e2012026. doi:10.4084/MJHID.2012.026
- Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology 1988; 8(1):27–31. pmid:3257456
- Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131(4):1049–1056. doi:10.1053/j.gastro.2006.07.010
- Runyon B; The American Association for the Study of Liver Diseases (AASLD). Management of adult patients with ascites due to cirrhosis: update 2012. https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdf. Accessed September 4, 2018.
- Sidhu SS, Goyal O, Kishore H, Sidhu S. New paradigms in management of alcoholic hepatitis: a review. Hepatol Int 2017; 11(3):255–267. doi:10.1007/s12072-017-9790-5
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360(26):2758–2769. doi:10.1056/NEJMra0805786
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75(2):193–199. pmid:352788
- Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005; 54(8):1174–1179. doi:10.1136/gut.2004.050781
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41(2):353–358. doi:10.1002/hep.20503
- Sheth M, Riggs M, Patel T. Utility of the Mayo end-stage liver disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2. pmid:11835693
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119(6):1637–1648. pmid:11113085
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60(2):255–260. doi:10.1136/gut.2010.224097
- Thursz MR, Richardson P, Allison M, et al; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372(17):1619–1628. doi:10.1056/NEJMoa1412278
- Thursz M, Forrest E, Roderick P, et al. The clinical effectiveness and cost-effectiveness of steroids or pentoxifylline for alcoholic hepatitis (STOPAH): a 2 × 2 factorial randomised controlled trial. Health Technol Assess 2015; 19(102):1–104. doi:10.3310/hta191020
- Lee YS, Kim HJ, Kim JH, et al. Treatment of severe alcoholic hepatitis with corticosteroid, pentoxifylline, or dual therapy: a systematic review and meta-analysis. J Clin Gastroenterol 2017; 51(4):364–377. doi:10.1097/MCG.0000000000000674
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45(6):1348–1354. doi:10.1002/hep.21607
A 35-year-old woman is admitted to the hospital with a 5-day history of abdominal distention and jaundice. She reports no history of fever, chills, night sweats, abdominal pain, nausea, vomiting, diarrhea, changes in urine color, change in stool color, weight loss, weight gain, or loss of appetite.
She is petite, with a body mass index of 19.4 kg/m2. She has no known history of medical conditions or surgery and is not taking any medications. Her family history is unremarkable, and she denies current or past tobacco, alcohol, or illicit drug use.
RECENT TRAVEL
She says that during a trip to Central America several months ago, she had suffered a seizure and was taken to a local hospital, where laboratory testing revealed elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. She says that the rest of the workup at that time was normal.
About 1 week after that incident, she returned home and saw her primary care physician, who ordered further testing, which showed mild hyperbilirubinemia and mild elevation of AST and ALT levels. Her physician attributed the elevations to atovaquone, which she had been taking for malaria prophylaxis, as repeat testing 2 weeks later showed improvement in AST and ALT levels.
The patient says she returned to her normal state of health until about 5 days ago, when she noticed jaundice and abdominal distention, but without abdominal pain, dark urine, or clay-colored stools. She became concerned and went to her local hospital. Testing there noted mild elevation of AST and ALT, as well as an elevated international normalized ratio (INR) and hyperbilirubinemia. Computed tomography of the abdomen and pelvis showed hepatomegaly with possible fatty liver. Because of these results, the patient was transferred to our institution for further evaluation.
EVALUATION AT OUR INSTITUTION
On examination at our institution, she is afebrile, and vital signs are within normal ranges. She has bilateral scleral icterus and diffuse jaundice, but no other skin finding such as rash or spider angioma. She has no lymphadenopathy. Her abdomen is distended, with tense ascites, and her liver is tender to palpation. The tip of the spleen is not palpable.
The cardiovascular examination reveals no murmurs, rubs, or gallops, but she has jugular venous distention and +2 pitting edema of both lower extremities.
On respiratory examination, there is dullness to percussion, with slight crackles on auscultation at the right lung base. The neurologic examination is normal.
Table 1 shows the results of initial laboratory testing.
1. Which study would provide the most information on the cause of ascites?
- Abdominal ultrasonography
- Abdominal paracentesis with ascitic fluid analysis
- Chest radiography
- Echocardiography
- Urine protein-to-creatinine ratio
Abdominal paracentesis with ascitic fluid analysis is the essential study for any patient with clinically apparent new-onset ascites.1–3 It is the study that provides the most information on the cause of ascites.
In our patient, abdominal paracentesis yields 1,000 mL of straw-colored ascitic fluid, and analysis shows 86 nucleated cells, 28 of which are polymorphonuclear cells, and 0 red blood cells, with negative Gram stain and culture. The ascitic albumin level is 0.85 g/dL, with an ascitic protein of 1.1 g/dL.
Abdominal ultrasonography shows a diffusely echogenic liver, no focal lesions, moderate ascites, normal portal vein flow, no intrahepatic or extrahepatic biliary duct dilation, normal kidney sizes, no hydronephrosis, and no intra-abdominal mass. Chest radiography is clear with no sign of consolidation, edema, or effusion. Echocardiography shows a normal left ventricular ejection fraction with no valvular disease or pericardial effusion. A random urine protein-creatinine ratio is normal at 0.1 (reference range < 0.2).
2. What is the most likely cause of her ascites based on the workup to this point?
- Cirrhosis
- Heart failure
- Nephrotic syndrome
- Portal vein thrombus
- Abdominal malignancy
- Malaria
An initial approach to ascitic fluid analysis is to calculate the serum-ascites albumin gradient (SAAG). The SAAG is calculated as the serum albumin level minus the ascitic fluid albumin level.4,5 This is useful in determining the cause of the ascites (Figure 1).4,5 A gradient of 1.1 g/dL or higher indicates portal hypertension.4,5
Common causes of portal hypertension include cirrhosis, alcoholic hepatitis, heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome, portal vein thrombosis), idiopathic portal fibrosis, and metastatic liver disease.5,6
If portal hypertension is present based on the SAAG, the next step is to review the ascitic protein level to help distinguish between a hepatic and a cardiac etiology of the ascites. An ascitic protein level less than 2.5 g/dL indicates a primary liver pathology (eg, cirrhosis). An ascitic protein level of 2.5 g/dL or greater typically indicates a cardiac condition (eg, heart failure, pericardial disease) with secondary congestive hepatopathy.5,6
If the SAAG is less than 1.1 g/dL, the ascites is likely not from portal hypertension. Typical causes of a low SAAG include infection, malignancy, pancreatic ascites, and nephrotic syndrome.5,6
In our patient, the SAAG is 1.35 g/dL (2.2 g/dL minus 0.85 g/dL), ie, elevated and due to portal hypertension. With an SAAG of 1.1 g/dL or greater and an ascitic fluid protein level less than 2.5 g/dL, as in our patient, the most likely cause is cirrhosis.
Heart failure is unlikely based on her normal brain natriuretic peptide level, an ascitic fluid protein level below 2.5 g/dL, and normal results on echocardiography. Nephrotic syndrome is also very unlikely based on the patient’s normal random urine protein-creatinine ratio. Portal vein thrombus and abdominal malignancy are essentially ruled out by the negative results of Doppler abdominal ultrasonography, with normal venous flow and no intra-abdominal mass and coupled with an elevated SAAG.
Although the patient has a history of travel, the incubation period for malaria would not fit the time frame of presentation. Also, she did not have typical malarial symptoms, her rapid malaria test was negative, and a peripheral blood smear for blood parasites was negative. It should be noted, however, that Plasmodium malariae infection classically presents with flulike symptoms and can resemble nephrotic syndrome, including peripheral edema, ascites, heavy proteinuria, hypoalbuminemia, and hyperlipidemia.7
3. In which patients is antibiotic prophylaxis against spontaneous bacterial peritonitis (SBP) appropriate?
- Any patient with cirrhosis
- Any patient with cirrhosis who is hospitalized
- Any patient with cirrhosis and an ascitic fluid protein level below 2.0 g/dL
- Any patient with cirrhosis and a history of SBP
Any patient with cirrhosis and a history of SBP should receive prophylactic antibiotics,8 as should any patient deemed at high risk of SBP. It is indicated in the following patients:
- Patients with cirrhosis and gastrointestinal bleeding9,10
- Patients with cirrhosis and a previous episode of SBP8
- Patients with cirrhosis and an ascitic fluid protein level less than 1.5 g/dL with either impaired renal function (creatinine ≥ 1.2 mg/dL, blood urea nitrogen level ≥ 25 mg/dL, or serum sodium ≤ 130 mmol/L) or liver failure (Child-Pugh score ≥ 9 and a bilirubin ≥ 3 mg/dL)9
- Patients with cirrhosis who are hospitalized for other reasons and have an ascitic protein level < 1.0 g/dL.9
Our patient has no signs or symptoms of gastrointestinal bleeding and no history of SBP. Her ascitic fluid protein level is 1.1 g/dL, and she has normal renal function. However, her Child-Pugh score is 12 (3 points for total bilirubin > 3 mg/dL, 3 points for serum albumin < 2.8 g/dL, 2 points for an INR 1.7 to 2.2, 3 points for moderate ascites, and 1 point for no encephalopathy), with a bilirubin of 17.0 mg/dL. Based on this, she is placed on antibiotic prophylaxis for SBP.
Our patient then undergoes an extensive workup for liver disease. Results of tests for toxins, autoimmune diseases, and inheritable diseases are all within normal limits. At this point, despite the patient’s reported negative alcohol history, our leading diagnosis is alcoholic hepatitis.
To confirm this diagnosis, she subsequently undergoes transjugular liver biopsy, considered the gold standard for the diagnosis of alcoholic hepatitis. During the procedure, the hepatic venous pressure gradient is measured at 18 mm Hg (reference range 1–5 mm Hg), suggestive of portal hypertension. The pathology study shows severe fatty change, active steatohepatitis with ballooning degeneration, easily identifiable Mallory-Denk bodies, and prominent neutrophilic infiltration, as well as extensive bridging fibrosis (Figure 2). These findings point to alcoholic hepatitis.
After the biopsy results, we speak with the patient further about her alcohol habits. At this point, she informs us that she has consumed significant amounts of alcohol since the age of 18 (6 to 12 alcoholic beverages per day, including beer and hard liquor). Therefore, based on this new information, on her jaundice and ascites, and on results of laboratory testing and biopsy, we confirmed our diagnosis of alcoholic hepatitis.
4. When is drug treatment appropriate for alcoholic hepatitis?
- Model for End-stage Liver Disease (MELD) score greater than 12
- MELD score greater than 15
- Maddrey Discriminant Function score greater than 25
- Maddrey Discriminant Function score greater than 32
- Glasgow score greater than 5
- Glasgow score greater than 7
The best answer is a Maddrey Discriminant Function score greater than 32. A variety of scoring systems have been used to assess the severity of alcoholic hepatitis and to guide treatment, including the Maddrey Discriminant Function score, the MELD score, and the Glasgow score.11–16 They share similar laboratory values in their calculations, including prothrombin time (or INR) and total bilirubin.11–16 Typically, a Maddrey Discriminant Function score greater than 32, a Glasgow score of greater than 9, or a MELD score greater than 21 is used to determine whether pharmacologic treatment is indicated.11–16
The typical treatment is prednisolone or pentoxifylline.11,17–21 The Lille score is designed to help decide whether to stop corticosteroids after 1 week of administration due to lack of treatment response.22 It predicts mortality rates within 6 months; a score of 0.45 or less indicates a good prognosis, and corticosteroid therapy should continue for 28 days (Figure 3).22
Our patient’s discriminant function score is 50, her Glasgow score is 10, and her MELD score is 28; thus, she begins treatment with oral prednisolone. Her Lille score at 1 week is 0.119, indicating a good prognosis, and her corticosteroids are continued for a total of 28 days.
It should be highlighted that the most important treatment is abstinence from alcohol.11 Recent literature suggests that any benefit of prednisolone or pentoxifylline in terms of mortality rates is questionable,19–20 and there is evidence that giving both drugs simultaneously may improve mortality rates,11,21 but the evidence remains conflicting at this time.
ALCOHOLIC HEPATITIS
Alcoholic hepatitis is a clinical syndrome of jaundice and liver failure, often in the setting of heavy alcohol use for decades.11,12 The incidence is unknown, but the typical age of presentation is between 40 and 50.11,12 The chief sign is a rapid onset of jaundice (< 3 months); common signs and symptoms include fever, ascites, proximal muscle loss, and an enlarged, tender liver.12 Encephalopathy may be seen in severe alcoholic hepatitis.12
Our patient is 35 years old. She has jaundice with rapid onset, as well as ascites and a tender liver.
The diagnosis of alcoholic hepatitis must take into account the patient’s history, physical examination, and laboratory findings. Until proven otherwise, the diagnosis should be presumed in the following scenario: ascites and jaundice on examination (usually with a duration < 3 months); a history of heavy alcohol use; neutrophilic leukocytosis; an AST level that is elevated but below 300 U/L; an ALT level above the normal range but below 300 U/L; an AST-ALT ratio greater than 2; a total serum bilirubin level above 5 mg/dL; and an elevated INR.11,12 Liver biopsy is the gold standard for diagnosis. Though not routinely done because of risks associated with the procedure, it may help confirm the diagnosis if it is in question.
CASE CONCLUDED
We start our patient on oral prednisolone 40 mg daily for alcoholic hepatitis. Her symptoms and laboratory testing results including bilirubin improve. Her Lille score at 7 days indicates a good prognosis, prompting continuation of corticosteroid treatment for the full 28 days.
She is referred to an outpatient alcohol rehabilitation program and has remained sober as of the last outpatient note.
Alcoholic hepatitis is extremely difficult to diagnose, and no single blood test or imaging study confirms the diagnosis. The history, physical examination findings, and laboratory findings are crucial. If the diagnosis is still in doubt, liver biopsy may help confirm the diagnosis.
A 35-year-old woman is admitted to the hospital with a 5-day history of abdominal distention and jaundice. She reports no history of fever, chills, night sweats, abdominal pain, nausea, vomiting, diarrhea, changes in urine color, change in stool color, weight loss, weight gain, or loss of appetite.
She is petite, with a body mass index of 19.4 kg/m2. She has no known history of medical conditions or surgery and is not taking any medications. Her family history is unremarkable, and she denies current or past tobacco, alcohol, or illicit drug use.
RECENT TRAVEL
She says that during a trip to Central America several months ago, she had suffered a seizure and was taken to a local hospital, where laboratory testing revealed elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. She says that the rest of the workup at that time was normal.
About 1 week after that incident, she returned home and saw her primary care physician, who ordered further testing, which showed mild hyperbilirubinemia and mild elevation of AST and ALT levels. Her physician attributed the elevations to atovaquone, which she had been taking for malaria prophylaxis, as repeat testing 2 weeks later showed improvement in AST and ALT levels.
The patient says she returned to her normal state of health until about 5 days ago, when she noticed jaundice and abdominal distention, but without abdominal pain, dark urine, or clay-colored stools. She became concerned and went to her local hospital. Testing there noted mild elevation of AST and ALT, as well as an elevated international normalized ratio (INR) and hyperbilirubinemia. Computed tomography of the abdomen and pelvis showed hepatomegaly with possible fatty liver. Because of these results, the patient was transferred to our institution for further evaluation.
EVALUATION AT OUR INSTITUTION
On examination at our institution, she is afebrile, and vital signs are within normal ranges. She has bilateral scleral icterus and diffuse jaundice, but no other skin finding such as rash or spider angioma. She has no lymphadenopathy. Her abdomen is distended, with tense ascites, and her liver is tender to palpation. The tip of the spleen is not palpable.
The cardiovascular examination reveals no murmurs, rubs, or gallops, but she has jugular venous distention and +2 pitting edema of both lower extremities.
On respiratory examination, there is dullness to percussion, with slight crackles on auscultation at the right lung base. The neurologic examination is normal.
Table 1 shows the results of initial laboratory testing.
1. Which study would provide the most information on the cause of ascites?
- Abdominal ultrasonography
- Abdominal paracentesis with ascitic fluid analysis
- Chest radiography
- Echocardiography
- Urine protein-to-creatinine ratio
Abdominal paracentesis with ascitic fluid analysis is the essential study for any patient with clinically apparent new-onset ascites.1–3 It is the study that provides the most information on the cause of ascites.
In our patient, abdominal paracentesis yields 1,000 mL of straw-colored ascitic fluid, and analysis shows 86 nucleated cells, 28 of which are polymorphonuclear cells, and 0 red blood cells, with negative Gram stain and culture. The ascitic albumin level is 0.85 g/dL, with an ascitic protein of 1.1 g/dL.
Abdominal ultrasonography shows a diffusely echogenic liver, no focal lesions, moderate ascites, normal portal vein flow, no intrahepatic or extrahepatic biliary duct dilation, normal kidney sizes, no hydronephrosis, and no intra-abdominal mass. Chest radiography is clear with no sign of consolidation, edema, or effusion. Echocardiography shows a normal left ventricular ejection fraction with no valvular disease or pericardial effusion. A random urine protein-creatinine ratio is normal at 0.1 (reference range < 0.2).
2. What is the most likely cause of her ascites based on the workup to this point?
- Cirrhosis
- Heart failure
- Nephrotic syndrome
- Portal vein thrombus
- Abdominal malignancy
- Malaria
An initial approach to ascitic fluid analysis is to calculate the serum-ascites albumin gradient (SAAG). The SAAG is calculated as the serum albumin level minus the ascitic fluid albumin level.4,5 This is useful in determining the cause of the ascites (Figure 1).4,5 A gradient of 1.1 g/dL or higher indicates portal hypertension.4,5
Common causes of portal hypertension include cirrhosis, alcoholic hepatitis, heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome, portal vein thrombosis), idiopathic portal fibrosis, and metastatic liver disease.5,6
If portal hypertension is present based on the SAAG, the next step is to review the ascitic protein level to help distinguish between a hepatic and a cardiac etiology of the ascites. An ascitic protein level less than 2.5 g/dL indicates a primary liver pathology (eg, cirrhosis). An ascitic protein level of 2.5 g/dL or greater typically indicates a cardiac condition (eg, heart failure, pericardial disease) with secondary congestive hepatopathy.5,6
If the SAAG is less than 1.1 g/dL, the ascites is likely not from portal hypertension. Typical causes of a low SAAG include infection, malignancy, pancreatic ascites, and nephrotic syndrome.5,6
In our patient, the SAAG is 1.35 g/dL (2.2 g/dL minus 0.85 g/dL), ie, elevated and due to portal hypertension. With an SAAG of 1.1 g/dL or greater and an ascitic fluid protein level less than 2.5 g/dL, as in our patient, the most likely cause is cirrhosis.
Heart failure is unlikely based on her normal brain natriuretic peptide level, an ascitic fluid protein level below 2.5 g/dL, and normal results on echocardiography. Nephrotic syndrome is also very unlikely based on the patient’s normal random urine protein-creatinine ratio. Portal vein thrombus and abdominal malignancy are essentially ruled out by the negative results of Doppler abdominal ultrasonography, with normal venous flow and no intra-abdominal mass and coupled with an elevated SAAG.
Although the patient has a history of travel, the incubation period for malaria would not fit the time frame of presentation. Also, she did not have typical malarial symptoms, her rapid malaria test was negative, and a peripheral blood smear for blood parasites was negative. It should be noted, however, that Plasmodium malariae infection classically presents with flulike symptoms and can resemble nephrotic syndrome, including peripheral edema, ascites, heavy proteinuria, hypoalbuminemia, and hyperlipidemia.7
3. In which patients is antibiotic prophylaxis against spontaneous bacterial peritonitis (SBP) appropriate?
- Any patient with cirrhosis
- Any patient with cirrhosis who is hospitalized
- Any patient with cirrhosis and an ascitic fluid protein level below 2.0 g/dL
- Any patient with cirrhosis and a history of SBP
Any patient with cirrhosis and a history of SBP should receive prophylactic antibiotics,8 as should any patient deemed at high risk of SBP. It is indicated in the following patients:
- Patients with cirrhosis and gastrointestinal bleeding9,10
- Patients with cirrhosis and a previous episode of SBP8
- Patients with cirrhosis and an ascitic fluid protein level less than 1.5 g/dL with either impaired renal function (creatinine ≥ 1.2 mg/dL, blood urea nitrogen level ≥ 25 mg/dL, or serum sodium ≤ 130 mmol/L) or liver failure (Child-Pugh score ≥ 9 and a bilirubin ≥ 3 mg/dL)9
- Patients with cirrhosis who are hospitalized for other reasons and have an ascitic protein level < 1.0 g/dL.9
Our patient has no signs or symptoms of gastrointestinal bleeding and no history of SBP. Her ascitic fluid protein level is 1.1 g/dL, and she has normal renal function. However, her Child-Pugh score is 12 (3 points for total bilirubin > 3 mg/dL, 3 points for serum albumin < 2.8 g/dL, 2 points for an INR 1.7 to 2.2, 3 points for moderate ascites, and 1 point for no encephalopathy), with a bilirubin of 17.0 mg/dL. Based on this, she is placed on antibiotic prophylaxis for SBP.
Our patient then undergoes an extensive workup for liver disease. Results of tests for toxins, autoimmune diseases, and inheritable diseases are all within normal limits. At this point, despite the patient’s reported negative alcohol history, our leading diagnosis is alcoholic hepatitis.
To confirm this diagnosis, she subsequently undergoes transjugular liver biopsy, considered the gold standard for the diagnosis of alcoholic hepatitis. During the procedure, the hepatic venous pressure gradient is measured at 18 mm Hg (reference range 1–5 mm Hg), suggestive of portal hypertension. The pathology study shows severe fatty change, active steatohepatitis with ballooning degeneration, easily identifiable Mallory-Denk bodies, and prominent neutrophilic infiltration, as well as extensive bridging fibrosis (Figure 2). These findings point to alcoholic hepatitis.
After the biopsy results, we speak with the patient further about her alcohol habits. At this point, she informs us that she has consumed significant amounts of alcohol since the age of 18 (6 to 12 alcoholic beverages per day, including beer and hard liquor). Therefore, based on this new information, on her jaundice and ascites, and on results of laboratory testing and biopsy, we confirmed our diagnosis of alcoholic hepatitis.
4. When is drug treatment appropriate for alcoholic hepatitis?
- Model for End-stage Liver Disease (MELD) score greater than 12
- MELD score greater than 15
- Maddrey Discriminant Function score greater than 25
- Maddrey Discriminant Function score greater than 32
- Glasgow score greater than 5
- Glasgow score greater than 7
The best answer is a Maddrey Discriminant Function score greater than 32. A variety of scoring systems have been used to assess the severity of alcoholic hepatitis and to guide treatment, including the Maddrey Discriminant Function score, the MELD score, and the Glasgow score.11–16 They share similar laboratory values in their calculations, including prothrombin time (or INR) and total bilirubin.11–16 Typically, a Maddrey Discriminant Function score greater than 32, a Glasgow score of greater than 9, or a MELD score greater than 21 is used to determine whether pharmacologic treatment is indicated.11–16
The typical treatment is prednisolone or pentoxifylline.11,17–21 The Lille score is designed to help decide whether to stop corticosteroids after 1 week of administration due to lack of treatment response.22 It predicts mortality rates within 6 months; a score of 0.45 or less indicates a good prognosis, and corticosteroid therapy should continue for 28 days (Figure 3).22
Our patient’s discriminant function score is 50, her Glasgow score is 10, and her MELD score is 28; thus, she begins treatment with oral prednisolone. Her Lille score at 1 week is 0.119, indicating a good prognosis, and her corticosteroids are continued for a total of 28 days.
It should be highlighted that the most important treatment is abstinence from alcohol.11 Recent literature suggests that any benefit of prednisolone or pentoxifylline in terms of mortality rates is questionable,19–20 and there is evidence that giving both drugs simultaneously may improve mortality rates,11,21 but the evidence remains conflicting at this time.
ALCOHOLIC HEPATITIS
Alcoholic hepatitis is a clinical syndrome of jaundice and liver failure, often in the setting of heavy alcohol use for decades.11,12 The incidence is unknown, but the typical age of presentation is between 40 and 50.11,12 The chief sign is a rapid onset of jaundice (< 3 months); common signs and symptoms include fever, ascites, proximal muscle loss, and an enlarged, tender liver.12 Encephalopathy may be seen in severe alcoholic hepatitis.12
Our patient is 35 years old. She has jaundice with rapid onset, as well as ascites and a tender liver.
The diagnosis of alcoholic hepatitis must take into account the patient’s history, physical examination, and laboratory findings. Until proven otherwise, the diagnosis should be presumed in the following scenario: ascites and jaundice on examination (usually with a duration < 3 months); a history of heavy alcohol use; neutrophilic leukocytosis; an AST level that is elevated but below 300 U/L; an ALT level above the normal range but below 300 U/L; an AST-ALT ratio greater than 2; a total serum bilirubin level above 5 mg/dL; and an elevated INR.11,12 Liver biopsy is the gold standard for diagnosis. Though not routinely done because of risks associated with the procedure, it may help confirm the diagnosis if it is in question.
CASE CONCLUDED
We start our patient on oral prednisolone 40 mg daily for alcoholic hepatitis. Her symptoms and laboratory testing results including bilirubin improve. Her Lille score at 7 days indicates a good prognosis, prompting continuation of corticosteroid treatment for the full 28 days.
She is referred to an outpatient alcohol rehabilitation program and has remained sober as of the last outpatient note.
Alcoholic hepatitis is extremely difficult to diagnose, and no single blood test or imaging study confirms the diagnosis. The history, physical examination findings, and laboratory findings are crucial. If the diagnosis is still in doubt, liver biopsy may help confirm the diagnosis.
- Ruyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009; 49(6):2087–2107. doi:10.1002/hep.22853
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2(4):399–407. pmid:7095741
- Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med 2004; 350(16):1646–1654. doi:10.1056/NEJMra035021
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117(3):215–220. pmid:1616215
- Hernaez R, Hamilton JP. Unexplained ascites. Clin Liver Dis 2016; 7(3):53–56. https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/cld.537
- Huang LL, Xia HH, Zhu SL. Ascitic fluid analysis in the differential diagnosis of ascites: focus on cirrhotic ascites. J Clin Transl Hepatol 2014; 2(1):58–64. doi:10.14218/JCTH.2013.00010
- Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis 2012; 4(1):e2012026. doi:10.4084/MJHID.2012.026
- Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology 1988; 8(1):27–31. pmid:3257456
- Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131(4):1049–1056. doi:10.1053/j.gastro.2006.07.010
- Runyon B; The American Association for the Study of Liver Diseases (AASLD). Management of adult patients with ascites due to cirrhosis: update 2012. https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdf. Accessed September 4, 2018.
- Sidhu SS, Goyal O, Kishore H, Sidhu S. New paradigms in management of alcoholic hepatitis: a review. Hepatol Int 2017; 11(3):255–267. doi:10.1007/s12072-017-9790-5
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360(26):2758–2769. doi:10.1056/NEJMra0805786
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75(2):193–199. pmid:352788
- Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005; 54(8):1174–1179. doi:10.1136/gut.2004.050781
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41(2):353–358. doi:10.1002/hep.20503
- Sheth M, Riggs M, Patel T. Utility of the Mayo end-stage liver disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2. pmid:11835693
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119(6):1637–1648. pmid:11113085
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60(2):255–260. doi:10.1136/gut.2010.224097
- Thursz MR, Richardson P, Allison M, et al; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372(17):1619–1628. doi:10.1056/NEJMoa1412278
- Thursz M, Forrest E, Roderick P, et al. The clinical effectiveness and cost-effectiveness of steroids or pentoxifylline for alcoholic hepatitis (STOPAH): a 2 × 2 factorial randomised controlled trial. Health Technol Assess 2015; 19(102):1–104. doi:10.3310/hta191020
- Lee YS, Kim HJ, Kim JH, et al. Treatment of severe alcoholic hepatitis with corticosteroid, pentoxifylline, or dual therapy: a systematic review and meta-analysis. J Clin Gastroenterol 2017; 51(4):364–377. doi:10.1097/MCG.0000000000000674
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45(6):1348–1354. doi:10.1002/hep.21607
- Ruyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009; 49(6):2087–2107. doi:10.1002/hep.22853
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2(4):399–407. pmid:7095741
- Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med 2004; 350(16):1646–1654. doi:10.1056/NEJMra035021
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117(3):215–220. pmid:1616215
- Hernaez R, Hamilton JP. Unexplained ascites. Clin Liver Dis 2016; 7(3):53–56. https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/cld.537
- Huang LL, Xia HH, Zhu SL. Ascitic fluid analysis in the differential diagnosis of ascites: focus on cirrhotic ascites. J Clin Transl Hepatol 2014; 2(1):58–64. doi:10.14218/JCTH.2013.00010
- Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis 2012; 4(1):e2012026. doi:10.4084/MJHID.2012.026
- Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology 1988; 8(1):27–31. pmid:3257456
- Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131(4):1049–1056. doi:10.1053/j.gastro.2006.07.010
- Runyon B; The American Association for the Study of Liver Diseases (AASLD). Management of adult patients with ascites due to cirrhosis: update 2012. https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdf. Accessed September 4, 2018.
- Sidhu SS, Goyal O, Kishore H, Sidhu S. New paradigms in management of alcoholic hepatitis: a review. Hepatol Int 2017; 11(3):255–267. doi:10.1007/s12072-017-9790-5
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360(26):2758–2769. doi:10.1056/NEJMra0805786
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75(2):193–199. pmid:352788
- Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005; 54(8):1174–1179. doi:10.1136/gut.2004.050781
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41(2):353–358. doi:10.1002/hep.20503
- Sheth M, Riggs M, Patel T. Utility of the Mayo end-stage liver disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2. pmid:11835693
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119(6):1637–1648. pmid:11113085
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60(2):255–260. doi:10.1136/gut.2010.224097
- Thursz MR, Richardson P, Allison M, et al; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372(17):1619–1628. doi:10.1056/NEJMoa1412278
- Thursz M, Forrest E, Roderick P, et al. The clinical effectiveness and cost-effectiveness of steroids or pentoxifylline for alcoholic hepatitis (STOPAH): a 2 × 2 factorial randomised controlled trial. Health Technol Assess 2015; 19(102):1–104. doi:10.3310/hta191020
- Lee YS, Kim HJ, Kim JH, et al. Treatment of severe alcoholic hepatitis with corticosteroid, pentoxifylline, or dual therapy: a systematic review and meta-analysis. J Clin Gastroenterol 2017; 51(4):364–377. doi:10.1097/MCG.0000000000000674
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45(6):1348–1354. doi:10.1002/hep.21607
Personalizing guideline-driven cancer screening
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
AGA publishes care pathway for IBD in pregnancy
Ideally, pregnant women with inflammatory bowel disease (IBD) should receive coordinated care from gastroenterologists and maternal-fetal medicine specialists, plus additional input from nutritionists, lactation counselors, and colorectal surgeons as needed, states a new report from the American Gastroenterological Association.
But in reality, these women often receive scant and conflicting advice from health care providers, writes Uma Mahadevan, MD, of the University of California, San Francisco, with her associates in Gastroenterology.
An “explosion” of new treatments in the past 15 years has given hope to many women with IBD who wish to be healthy enough to conceive, the experts noted. But in a recent AGA survey, more than 40% of obstetrician/gynecologist (OB/GYN) providers felt that women with IBD received inadequate information about pregnancy, compared with patients with other immune-mediated diseases. Strikingly, 94% of surveyed clinicians said they had patients stop taking their IBD medications during pregnancy because they feared harm to the fetus. In doing so, these patients actually risked greater disease activity, perinatal flares, and adverse pregnancy outcomes.
Therefore, the AGA, in partnership with the Crohn’s & Colitis Foundation, the Society for Maternal-Fetal Medicine, and Girls With Guts, crafted a standardized, evidence-based care pathway for health care providers from diverse disciplines who treat women with IBD in all stages of family planning. Its authors recommended that a maternal-fetal medicine specialist oversee obstetric care whenever possible. A gastroenterologist should continue IBD care by seeing the patient once during the first or second trimester and thereafter depending on IBD severity. The patient should receive a “clear and easily understandable consensus plan” for managing complex care during and after pregnancy, according to the pathway.
Aminosalicylates, biologics, and immunomodulators can be continued during pregnancy and delivery. Biologics have not shown teratogenicity in large studies, but monotherapy is preferred to reduce infection risk in infants. Clinicians should calculate weight-based doses according to prepregnancy weight. Doses can be tweaked to achieve minimal trough levels near delivery.
During pregnancy, patients should stop antidiarrheal therapy with loperamide and diphenoxylate when possible. Proinflammatory mediators are known to damage hippocampal neurogenesis and neuronal cytoarchitecture during brain development, so patients should understand the need for good inflammatory control during pregnancy. However, biologic therapy is preferred, and patients should only use corticosteroids adjunctively if needed for flares.
The usual indications guide the choice between a vaginal or cesarean delivery, the pathway states. Vaginal delivery often is possible for patients without active perineal disease, while cesarean is recommended for women with prior perineal surgery or active perineal disease or rectovaginal fistulas. The perineal area can be examined for active disease during the routine visit for group B streptococcus screening culture at 35-37 weeks’ gestation. For women who have had ileal-pouch anal anastomosis surgery, mode of delivery does not seem to affect pouch function, but cesarean delivery is thought to prevent anal sphincter injury and the accompanying risk of incontinence.
For ostomy patients, stretching of the abdominal wall during pregnancy can lead to stomal problems, such as displacement, enlargement, retraction, stenosis, and prolapse. A nutritionist can help ostomy patients avoid excess weight gain, and a colorectal surgeon and ostomy/wound nurse can help coordinate postpartum care. If cesarean delivery is needed, simply covering the ostomy with gauze sufficiently protects the operative field.
Since IBD increases the risk of venous thromboembolism, clinicians should consider prophylactic anticoagulation after cesarean delivery and during a hospitalization for IBD flares, according to the care pathway. Breastfeeding women can receive unfractionated heparin, low-molecular-weight heparin, or warfarin up to 3-6 weeks post partum, but they should not receive oral direct thrombin or factor Xa inhibitors.
In addition, most IBD medications are either undetectable in breast milk or are secreted at such low concentrations that they pose no known risk to infants. Therefore, patients can continue IBD medications after delivery – except methotrexate, which has not been sufficiently studied to assess its safety. Breastfeeding women with IBD should avoid using fenugreek to increase milk production, since it can cause diarrhea and bleeding.
Finally, infants should not receive live vaccines during the first 6 months after birth if their mothers received biologics besides certolizumab during the third trimester, the pathway notes. In the United States, this applies only to the oral rotavirus vaccine.
For more information about the care pathway and resources for your patients, visit IBDParenthoodProject.org.
SOURCE: Mahadevan U et al. Gastroenterology. 2019 Jan 15. doi: 10.1053/j.gastro.2018.12.022.
Ideally, pregnant women with inflammatory bowel disease (IBD) should receive coordinated care from gastroenterologists and maternal-fetal medicine specialists, plus additional input from nutritionists, lactation counselors, and colorectal surgeons as needed, states a new report from the American Gastroenterological Association.
But in reality, these women often receive scant and conflicting advice from health care providers, writes Uma Mahadevan, MD, of the University of California, San Francisco, with her associates in Gastroenterology.
An “explosion” of new treatments in the past 15 years has given hope to many women with IBD who wish to be healthy enough to conceive, the experts noted. But in a recent AGA survey, more than 40% of obstetrician/gynecologist (OB/GYN) providers felt that women with IBD received inadequate information about pregnancy, compared with patients with other immune-mediated diseases. Strikingly, 94% of surveyed clinicians said they had patients stop taking their IBD medications during pregnancy because they feared harm to the fetus. In doing so, these patients actually risked greater disease activity, perinatal flares, and adverse pregnancy outcomes.
Therefore, the AGA, in partnership with the Crohn’s & Colitis Foundation, the Society for Maternal-Fetal Medicine, and Girls With Guts, crafted a standardized, evidence-based care pathway for health care providers from diverse disciplines who treat women with IBD in all stages of family planning. Its authors recommended that a maternal-fetal medicine specialist oversee obstetric care whenever possible. A gastroenterologist should continue IBD care by seeing the patient once during the first or second trimester and thereafter depending on IBD severity. The patient should receive a “clear and easily understandable consensus plan” for managing complex care during and after pregnancy, according to the pathway.
Aminosalicylates, biologics, and immunomodulators can be continued during pregnancy and delivery. Biologics have not shown teratogenicity in large studies, but monotherapy is preferred to reduce infection risk in infants. Clinicians should calculate weight-based doses according to prepregnancy weight. Doses can be tweaked to achieve minimal trough levels near delivery.
During pregnancy, patients should stop antidiarrheal therapy with loperamide and diphenoxylate when possible. Proinflammatory mediators are known to damage hippocampal neurogenesis and neuronal cytoarchitecture during brain development, so patients should understand the need for good inflammatory control during pregnancy. However, biologic therapy is preferred, and patients should only use corticosteroids adjunctively if needed for flares.
The usual indications guide the choice between a vaginal or cesarean delivery, the pathway states. Vaginal delivery often is possible for patients without active perineal disease, while cesarean is recommended for women with prior perineal surgery or active perineal disease or rectovaginal fistulas. The perineal area can be examined for active disease during the routine visit for group B streptococcus screening culture at 35-37 weeks’ gestation. For women who have had ileal-pouch anal anastomosis surgery, mode of delivery does not seem to affect pouch function, but cesarean delivery is thought to prevent anal sphincter injury and the accompanying risk of incontinence.
For ostomy patients, stretching of the abdominal wall during pregnancy can lead to stomal problems, such as displacement, enlargement, retraction, stenosis, and prolapse. A nutritionist can help ostomy patients avoid excess weight gain, and a colorectal surgeon and ostomy/wound nurse can help coordinate postpartum care. If cesarean delivery is needed, simply covering the ostomy with gauze sufficiently protects the operative field.
Since IBD increases the risk of venous thromboembolism, clinicians should consider prophylactic anticoagulation after cesarean delivery and during a hospitalization for IBD flares, according to the care pathway. Breastfeeding women can receive unfractionated heparin, low-molecular-weight heparin, or warfarin up to 3-6 weeks post partum, but they should not receive oral direct thrombin or factor Xa inhibitors.
In addition, most IBD medications are either undetectable in breast milk or are secreted at such low concentrations that they pose no known risk to infants. Therefore, patients can continue IBD medications after delivery – except methotrexate, which has not been sufficiently studied to assess its safety. Breastfeeding women with IBD should avoid using fenugreek to increase milk production, since it can cause diarrhea and bleeding.
Finally, infants should not receive live vaccines during the first 6 months after birth if their mothers received biologics besides certolizumab during the third trimester, the pathway notes. In the United States, this applies only to the oral rotavirus vaccine.
For more information about the care pathway and resources for your patients, visit IBDParenthoodProject.org.
SOURCE: Mahadevan U et al. Gastroenterology. 2019 Jan 15. doi: 10.1053/j.gastro.2018.12.022.
Ideally, pregnant women with inflammatory bowel disease (IBD) should receive coordinated care from gastroenterologists and maternal-fetal medicine specialists, plus additional input from nutritionists, lactation counselors, and colorectal surgeons as needed, states a new report from the American Gastroenterological Association.
But in reality, these women often receive scant and conflicting advice from health care providers, writes Uma Mahadevan, MD, of the University of California, San Francisco, with her associates in Gastroenterology.
An “explosion” of new treatments in the past 15 years has given hope to many women with IBD who wish to be healthy enough to conceive, the experts noted. But in a recent AGA survey, more than 40% of obstetrician/gynecologist (OB/GYN) providers felt that women with IBD received inadequate information about pregnancy, compared with patients with other immune-mediated diseases. Strikingly, 94% of surveyed clinicians said they had patients stop taking their IBD medications during pregnancy because they feared harm to the fetus. In doing so, these patients actually risked greater disease activity, perinatal flares, and adverse pregnancy outcomes.
Therefore, the AGA, in partnership with the Crohn’s & Colitis Foundation, the Society for Maternal-Fetal Medicine, and Girls With Guts, crafted a standardized, evidence-based care pathway for health care providers from diverse disciplines who treat women with IBD in all stages of family planning. Its authors recommended that a maternal-fetal medicine specialist oversee obstetric care whenever possible. A gastroenterologist should continue IBD care by seeing the patient once during the first or second trimester and thereafter depending on IBD severity. The patient should receive a “clear and easily understandable consensus plan” for managing complex care during and after pregnancy, according to the pathway.
Aminosalicylates, biologics, and immunomodulators can be continued during pregnancy and delivery. Biologics have not shown teratogenicity in large studies, but monotherapy is preferred to reduce infection risk in infants. Clinicians should calculate weight-based doses according to prepregnancy weight. Doses can be tweaked to achieve minimal trough levels near delivery.
During pregnancy, patients should stop antidiarrheal therapy with loperamide and diphenoxylate when possible. Proinflammatory mediators are known to damage hippocampal neurogenesis and neuronal cytoarchitecture during brain development, so patients should understand the need for good inflammatory control during pregnancy. However, biologic therapy is preferred, and patients should only use corticosteroids adjunctively if needed for flares.
The usual indications guide the choice between a vaginal or cesarean delivery, the pathway states. Vaginal delivery often is possible for patients without active perineal disease, while cesarean is recommended for women with prior perineal surgery or active perineal disease or rectovaginal fistulas. The perineal area can be examined for active disease during the routine visit for group B streptococcus screening culture at 35-37 weeks’ gestation. For women who have had ileal-pouch anal anastomosis surgery, mode of delivery does not seem to affect pouch function, but cesarean delivery is thought to prevent anal sphincter injury and the accompanying risk of incontinence.
For ostomy patients, stretching of the abdominal wall during pregnancy can lead to stomal problems, such as displacement, enlargement, retraction, stenosis, and prolapse. A nutritionist can help ostomy patients avoid excess weight gain, and a colorectal surgeon and ostomy/wound nurse can help coordinate postpartum care. If cesarean delivery is needed, simply covering the ostomy with gauze sufficiently protects the operative field.
Since IBD increases the risk of venous thromboembolism, clinicians should consider prophylactic anticoagulation after cesarean delivery and during a hospitalization for IBD flares, according to the care pathway. Breastfeeding women can receive unfractionated heparin, low-molecular-weight heparin, or warfarin up to 3-6 weeks post partum, but they should not receive oral direct thrombin or factor Xa inhibitors.
In addition, most IBD medications are either undetectable in breast milk or are secreted at such low concentrations that they pose no known risk to infants. Therefore, patients can continue IBD medications after delivery – except methotrexate, which has not been sufficiently studied to assess its safety. Breastfeeding women with IBD should avoid using fenugreek to increase milk production, since it can cause diarrhea and bleeding.
Finally, infants should not receive live vaccines during the first 6 months after birth if their mothers received biologics besides certolizumab during the third trimester, the pathway notes. In the United States, this applies only to the oral rotavirus vaccine.
For more information about the care pathway and resources for your patients, visit IBDParenthoodProject.org.
SOURCE: Mahadevan U et al. Gastroenterology. 2019 Jan 15. doi: 10.1053/j.gastro.2018.12.022.
FROM THE AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Postpartum psychosis: Protecting mother and infant
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).

Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36

Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31

Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).

Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36

Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31

Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).

Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36

Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.