User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Scattered Flesh-Colored Papules in a Linear Array in the Setting of Diffuse Skin Thickening
The Diagnosis: Scleromyxedema
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.
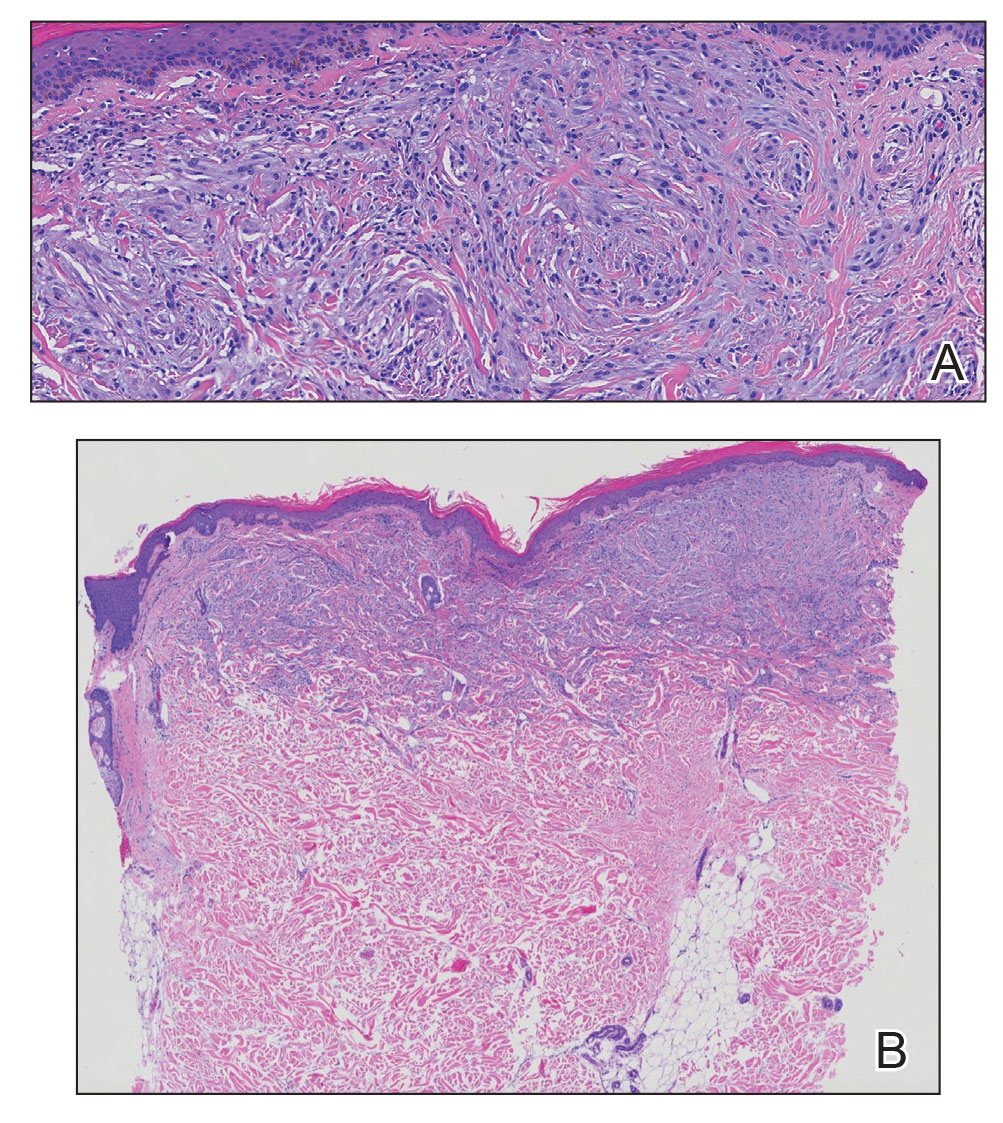
Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2
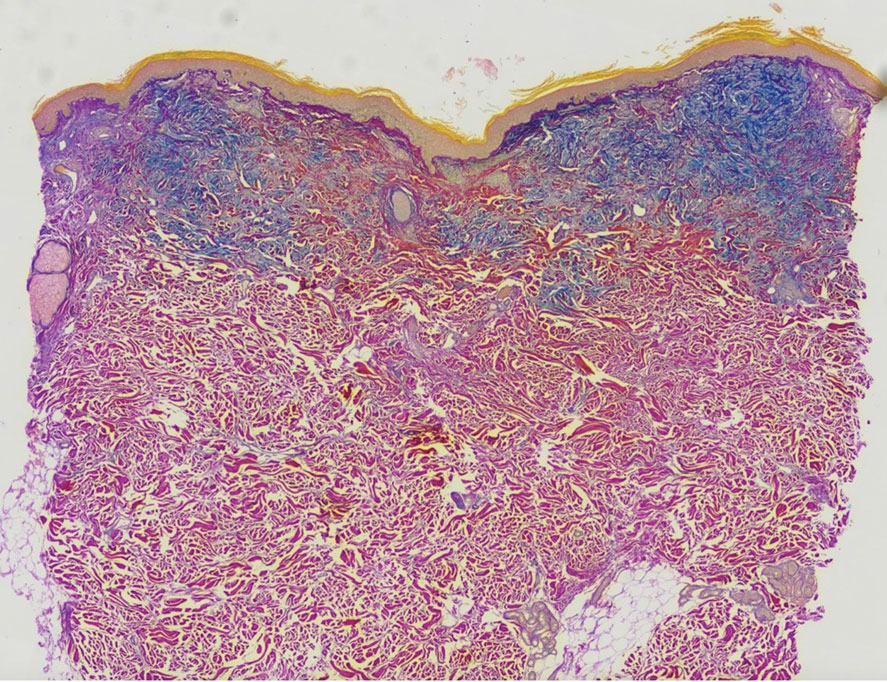
The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
The Diagnosis: Scleromyxedema
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.

Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2

The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
The Diagnosis: Scleromyxedema
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.

Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2

The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
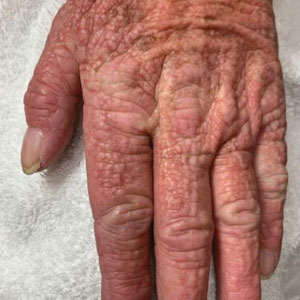
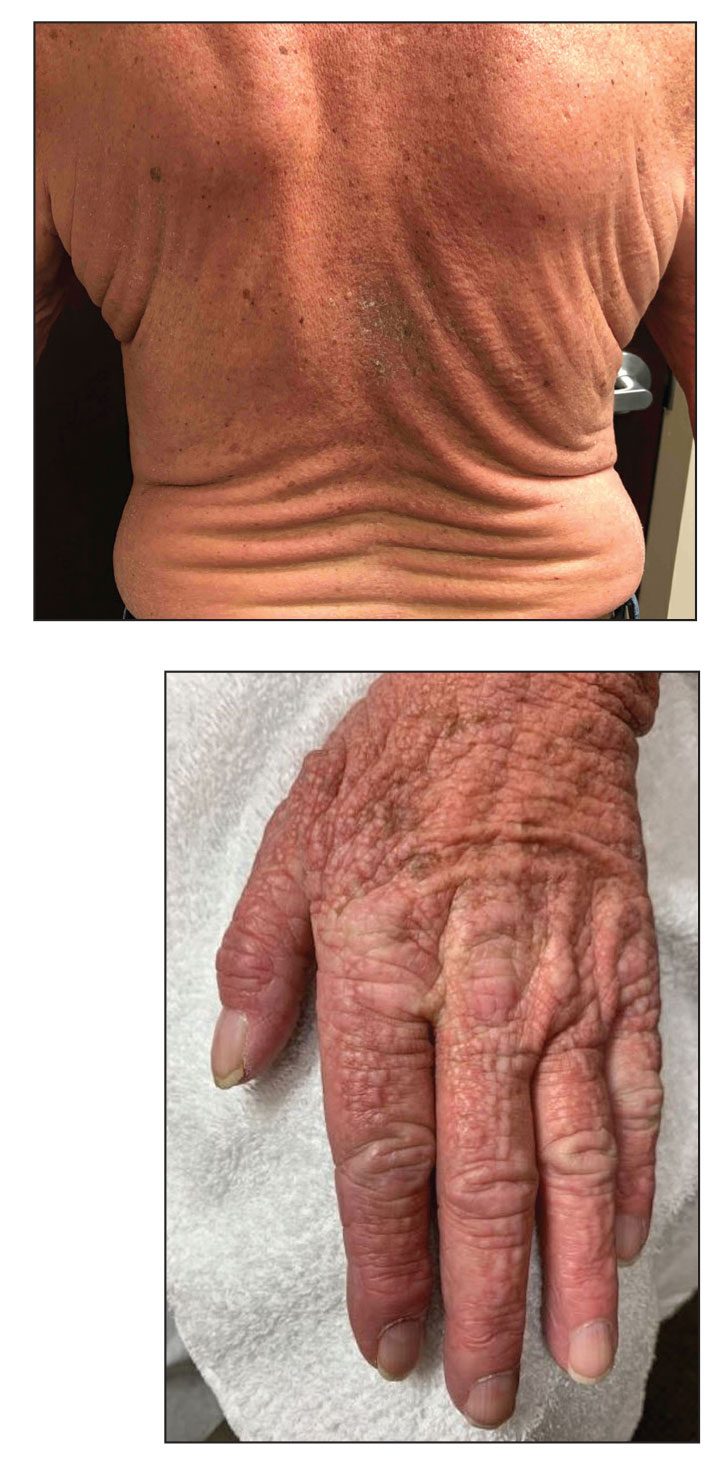
A 76-year-old man presented to our clinic with diffusely thickened and tightened skin that worsened over the course of 1 year, as well as numerous scattered small, firm, flesh-colored papules arranged in a linear pattern over the face, ears, neck, chest, abdomen, arms, hands, and knees. His symptoms progressed to include substantial skin thickening initially over the thighs followed by the arms, chest, back (top), and face. He developed confluent cobblestonelike plaques over the elbows and hands (bottom) and eventually developed decreased oral aperture limiting oral intake as well as decreased range of motion in the hands. The patient had a deep furrowed appearance of the brow accompanied by discrete, scattered, flesh-colored papules on the forehead and behind the ears. Deep furrows also were present on the back. When the proximal interphalangeal joints of the hands were extended, elevated rings with central depression were seen instead of horizontal folds.
Evolocumab benefits accrue with longer follow-up: FOURIER OLE
Long-term lipid lowering with evolocumab (Repatha) further reduces cardiovascular events, including CV death, without a safety signal, according to results from the FOURIER open-label extension (OLE) study.
In the parent FOURIER trial, treatment with the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor over a median of 2.2 years reduced the primary efficacy endpoint by 15% but showed no CV mortality signal, compared with placebo, in patients with atherosclerotic disease on background statin therapy.
Now with follow-up out to 8.4 years – the longest to date in any PCSK9 study – cardiovascular mortality was cut by 23% in patients who remained on evolocumab, compared with those originally assigned to placebo (3.32% vs. 4.45%; hazard ratio, 0.77; 95% confidence interval, 0.60-0.99).
The Kaplan-Meier curves during FOURIER were “essentially superimposed and it was not until the open-label extension period had begun with longer-term follow up that the benefit in terms of cardiovascular mortality reduction became apparent,” said principal investigator Michelle O’Donoghue, MD, MPH, of Brigham and Women’s Hospital, Boston.
The results were reported at the annual congress of the European Society of Cardiology and published simultaneously in Circulation.
Pivotal statin trials have median follow-up times of 4-5 years and demonstrated both a lag effect, meaning clinical benefit grew over time, and a legacy effect, where clinical benefit persisted in extended follow-up after the parent study, Dr. O’Donoghue observed.
With shorter follow-up in the parent FOURIER trial, there was evidence of a lag effect with the risk reduction in CV death, MI, and stroke increasing from 16% in the first year to 25% over time with evolocumab.
FOURIER-OLE enrolled 6,635 patients (3355 randomly assigned to evolocumab and 3280 to placebo), who completed the parent study and self-injected evolocumab subcutaneously with the choice of 140 mg every 2 weeks or 420 mg monthly. Study visits were at week 12 and then every 24 weeks. Median follow-up was 5 years.
Their mean age was 62 years, three-fourths were men, a third had diabetes. Three-fourths were on a high-intensity statin at the time of enrollment in FOURIER, and median LDL cholesterol at randomization was 91 mg/dL (2.4 mmol/L).
At week 12, the median LDL cholesterol was 30 mg/dL (0.78 mmol/L), and this was sustained throughout follow-up, Dr. O’Donoghue reported. Most patients achieved very low LDL cholesterol levels, with 63.2% achieving levels less than 40 mg/dL (1.04 mmol/L) and 26.6% less than 20 mg/dL (0.52 mmol/L).
Patients randomly assigned in the parent trial to evolocumab versus placebo had a 15% lower risk of the primary outcome of CV death, MI, stroke, hospitalization for unstable angina, or coronary revascularization (15.4% vs. 17.5%; HR, 0.85; 95% CI, 0.75-0.96).
Their risk of CV death, MI, or stroke was 20% lower (9.7% vs. 11.9%; HR, 0.80; 95% CI, 0.68-0.93), and, as noted previously, 23% lower for CV death.
When major adverse cardiovascular events data were parsed out by year, the largest LDL cholesterol reduction was in years 1 and 2 of the parent study (delta, 62 mg/dL between treatment arms), “highlighting that lag of benefit that continued to accrue with time,” Dr. O’Donoghue said.
“There was then carryover into the extension period, such that there was legacy effect from the LDL [cholesterol] delta that was seen during the parent study,” she said. “This benefit was most apparent early on during open-label extension and then, as one might expect when all patients were being treated with the same therapy, it began to attenuate somewhat with time.”
Although early studies raised concerns that very low LDL cholesterol may be associated with an increased risk of hemorrhagic stroke and neurocognitive effects, the frequency of adverse events did not increase over time with evolocumab exposure.
Annualized incidence rates for patients initially randomized to evolocumab did not exceed those for placebo-treated patients for any of the following events of interest: serious safety events (10% vs. 13%), hemorrhagic stroke (0.04% vs. 0.05%), new-onset diabetes (1.2% vs. 2.3%), muscle-related events (1.2% vs. 1.9%), injection-site reactions (0.4% vs. 0.7%), and drug-related allergic reactions (0.6% vs. 1.1%).
“Long-term use of evolocumab with a median follow-up of more than 7 years appears both safe and well tolerated,” Dr. O’Donoghue said.
Taken together with the continued accrual of cardiovascular benefit, including CV mortality, “these findings argue for early initiation of a marked and sustained LDL cholesterol reduction to maximize benefit,” she concluded.
Translating the benefits
Ulrich Laufs, MD, Leipzig (Germany) University Hospital, Germany, and invited commentator for the session, said the trial addresses two key issues: the long-term safety of low LDL cholesterol lowering and the long-term safety of inhibiting PCSK9, which is highly expressed not only in the liver but also in the brain, small intestine, and kidneys. Indeed, an LDL cholesterol level below 30 mg/dL is lower than the ESC treatment recommendation for very-high-risk patients and is, in fact, lower than most assays are reliable to interpret.
“So it is very important that we have these very clear data showing us that there were no adverse events, also including cataracts and hemorrhagic stroke, and these were on the level of placebo and did not increase over time,” he said.
The question of efficacy is triggered by observations of another PCSK9, the humanized monoclonal antibody bococizumab, which was associated in the SPIRE trial with an increase in LDL cholesterol over time because of neutralizing antibodies. Reassuringly, there was “completely sustained LDL [cholesterol] reduction” with no neutralizing antibodies with the fully human antibody evolocumab in FOURIER-OLE and in recent data from the OSLER-1 study, Dr. Laufs observed.
Acknowledging the potential for selection bias with an OLE program, Dr. Laufs said there are two important open questions: “Can the safety data observed for extracellular PCSK9 inhibition using an antibody be transferred to other mechanisms of PCSK9 inhibition? And obviously, from the perspective of patient care, how can we implement these important data into patient care and improve access to PCSK9 inhibitors?”
With regard to the latter point, he said physicians should be cautious in using the term “plaque regression,” opting instead for prevention and stabilization of atherosclerosis, and when using the term “legacy,” which may be misinterpreted by patients to imply there was cessation of therapy.
“From my perspective, [what] the open-label extension really shows is that earlier treatment is better,” Dr. Laufs said. “This should be our message.”
In a press conference prior to the presentation, ESC commentator Johann Bauersachs, MD, Hannover (Germany) Medical School, said “this is extremely important data because it confirms that it’s safe, and the criticism of the FOURIER study that mortality, cardiovascular mortality, was not reduced is now also reduced.”
Dr. Bauersachs said it would have been unethical to wait 7 years for a placebo-controlled trial and questioned whether data are available and suggestive of a legacy effect among patients who did not participate in the open-label extension.
Dr. O’Donoghue said unfortunately those data aren’t available but that Kaplan-Meier curves for the primary endpoint in the parent trial continued to diverge over time and that there was somewhat of a lag in terms of that divergence. “So, a median follow-up of 2 years may have been insufficient, especially for the emerging cardiovascular mortality that took longer to appear.”
The study was funded by Amgen. Dr. O’Donoghue reported receiving research grants from Amgen, AstraZeneca, Janssen, Intarcia, and Novartis, and consulting fees from Amgen, Novartis, AstraZeneca, and Janssen. Dr. Laufs reported receiving honoraria/reimbursement for lecture, study participation, and scientific cooperation with Saarland or Leipzig University, as well as relationships with multiple pharmaceutical and device makers.
A version of this article first appeared on Medscape.com.
Long-term lipid lowering with evolocumab (Repatha) further reduces cardiovascular events, including CV death, without a safety signal, according to results from the FOURIER open-label extension (OLE) study.
In the parent FOURIER trial, treatment with the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor over a median of 2.2 years reduced the primary efficacy endpoint by 15% but showed no CV mortality signal, compared with placebo, in patients with atherosclerotic disease on background statin therapy.
Now with follow-up out to 8.4 years – the longest to date in any PCSK9 study – cardiovascular mortality was cut by 23% in patients who remained on evolocumab, compared with those originally assigned to placebo (3.32% vs. 4.45%; hazard ratio, 0.77; 95% confidence interval, 0.60-0.99).
The Kaplan-Meier curves during FOURIER were “essentially superimposed and it was not until the open-label extension period had begun with longer-term follow up that the benefit in terms of cardiovascular mortality reduction became apparent,” said principal investigator Michelle O’Donoghue, MD, MPH, of Brigham and Women’s Hospital, Boston.
The results were reported at the annual congress of the European Society of Cardiology and published simultaneously in Circulation.
Pivotal statin trials have median follow-up times of 4-5 years and demonstrated both a lag effect, meaning clinical benefit grew over time, and a legacy effect, where clinical benefit persisted in extended follow-up after the parent study, Dr. O’Donoghue observed.
With shorter follow-up in the parent FOURIER trial, there was evidence of a lag effect with the risk reduction in CV death, MI, and stroke increasing from 16% in the first year to 25% over time with evolocumab.
FOURIER-OLE enrolled 6,635 patients (3355 randomly assigned to evolocumab and 3280 to placebo), who completed the parent study and self-injected evolocumab subcutaneously with the choice of 140 mg every 2 weeks or 420 mg monthly. Study visits were at week 12 and then every 24 weeks. Median follow-up was 5 years.
Their mean age was 62 years, three-fourths were men, a third had diabetes. Three-fourths were on a high-intensity statin at the time of enrollment in FOURIER, and median LDL cholesterol at randomization was 91 mg/dL (2.4 mmol/L).
At week 12, the median LDL cholesterol was 30 mg/dL (0.78 mmol/L), and this was sustained throughout follow-up, Dr. O’Donoghue reported. Most patients achieved very low LDL cholesterol levels, with 63.2% achieving levels less than 40 mg/dL (1.04 mmol/L) and 26.6% less than 20 mg/dL (0.52 mmol/L).
Patients randomly assigned in the parent trial to evolocumab versus placebo had a 15% lower risk of the primary outcome of CV death, MI, stroke, hospitalization for unstable angina, or coronary revascularization (15.4% vs. 17.5%; HR, 0.85; 95% CI, 0.75-0.96).
Their risk of CV death, MI, or stroke was 20% lower (9.7% vs. 11.9%; HR, 0.80; 95% CI, 0.68-0.93), and, as noted previously, 23% lower for CV death.
When major adverse cardiovascular events data were parsed out by year, the largest LDL cholesterol reduction was in years 1 and 2 of the parent study (delta, 62 mg/dL between treatment arms), “highlighting that lag of benefit that continued to accrue with time,” Dr. O’Donoghue said.
“There was then carryover into the extension period, such that there was legacy effect from the LDL [cholesterol] delta that was seen during the parent study,” she said. “This benefit was most apparent early on during open-label extension and then, as one might expect when all patients were being treated with the same therapy, it began to attenuate somewhat with time.”
Although early studies raised concerns that very low LDL cholesterol may be associated with an increased risk of hemorrhagic stroke and neurocognitive effects, the frequency of adverse events did not increase over time with evolocumab exposure.
Annualized incidence rates for patients initially randomized to evolocumab did not exceed those for placebo-treated patients for any of the following events of interest: serious safety events (10% vs. 13%), hemorrhagic stroke (0.04% vs. 0.05%), new-onset diabetes (1.2% vs. 2.3%), muscle-related events (1.2% vs. 1.9%), injection-site reactions (0.4% vs. 0.7%), and drug-related allergic reactions (0.6% vs. 1.1%).
“Long-term use of evolocumab with a median follow-up of more than 7 years appears both safe and well tolerated,” Dr. O’Donoghue said.
Taken together with the continued accrual of cardiovascular benefit, including CV mortality, “these findings argue for early initiation of a marked and sustained LDL cholesterol reduction to maximize benefit,” she concluded.
Translating the benefits
Ulrich Laufs, MD, Leipzig (Germany) University Hospital, Germany, and invited commentator for the session, said the trial addresses two key issues: the long-term safety of low LDL cholesterol lowering and the long-term safety of inhibiting PCSK9, which is highly expressed not only in the liver but also in the brain, small intestine, and kidneys. Indeed, an LDL cholesterol level below 30 mg/dL is lower than the ESC treatment recommendation for very-high-risk patients and is, in fact, lower than most assays are reliable to interpret.
“So it is very important that we have these very clear data showing us that there were no adverse events, also including cataracts and hemorrhagic stroke, and these were on the level of placebo and did not increase over time,” he said.
The question of efficacy is triggered by observations of another PCSK9, the humanized monoclonal antibody bococizumab, which was associated in the SPIRE trial with an increase in LDL cholesterol over time because of neutralizing antibodies. Reassuringly, there was “completely sustained LDL [cholesterol] reduction” with no neutralizing antibodies with the fully human antibody evolocumab in FOURIER-OLE and in recent data from the OSLER-1 study, Dr. Laufs observed.
Acknowledging the potential for selection bias with an OLE program, Dr. Laufs said there are two important open questions: “Can the safety data observed for extracellular PCSK9 inhibition using an antibody be transferred to other mechanisms of PCSK9 inhibition? And obviously, from the perspective of patient care, how can we implement these important data into patient care and improve access to PCSK9 inhibitors?”
With regard to the latter point, he said physicians should be cautious in using the term “plaque regression,” opting instead for prevention and stabilization of atherosclerosis, and when using the term “legacy,” which may be misinterpreted by patients to imply there was cessation of therapy.
“From my perspective, [what] the open-label extension really shows is that earlier treatment is better,” Dr. Laufs said. “This should be our message.”
In a press conference prior to the presentation, ESC commentator Johann Bauersachs, MD, Hannover (Germany) Medical School, said “this is extremely important data because it confirms that it’s safe, and the criticism of the FOURIER study that mortality, cardiovascular mortality, was not reduced is now also reduced.”
Dr. Bauersachs said it would have been unethical to wait 7 years for a placebo-controlled trial and questioned whether data are available and suggestive of a legacy effect among patients who did not participate in the open-label extension.
Dr. O’Donoghue said unfortunately those data aren’t available but that Kaplan-Meier curves for the primary endpoint in the parent trial continued to diverge over time and that there was somewhat of a lag in terms of that divergence. “So, a median follow-up of 2 years may have been insufficient, especially for the emerging cardiovascular mortality that took longer to appear.”
The study was funded by Amgen. Dr. O’Donoghue reported receiving research grants from Amgen, AstraZeneca, Janssen, Intarcia, and Novartis, and consulting fees from Amgen, Novartis, AstraZeneca, and Janssen. Dr. Laufs reported receiving honoraria/reimbursement for lecture, study participation, and scientific cooperation with Saarland or Leipzig University, as well as relationships with multiple pharmaceutical and device makers.
A version of this article first appeared on Medscape.com.
Long-term lipid lowering with evolocumab (Repatha) further reduces cardiovascular events, including CV death, without a safety signal, according to results from the FOURIER open-label extension (OLE) study.
In the parent FOURIER trial, treatment with the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor over a median of 2.2 years reduced the primary efficacy endpoint by 15% but showed no CV mortality signal, compared with placebo, in patients with atherosclerotic disease on background statin therapy.
Now with follow-up out to 8.4 years – the longest to date in any PCSK9 study – cardiovascular mortality was cut by 23% in patients who remained on evolocumab, compared with those originally assigned to placebo (3.32% vs. 4.45%; hazard ratio, 0.77; 95% confidence interval, 0.60-0.99).
The Kaplan-Meier curves during FOURIER were “essentially superimposed and it was not until the open-label extension period had begun with longer-term follow up that the benefit in terms of cardiovascular mortality reduction became apparent,” said principal investigator Michelle O’Donoghue, MD, MPH, of Brigham and Women’s Hospital, Boston.
The results were reported at the annual congress of the European Society of Cardiology and published simultaneously in Circulation.
Pivotal statin trials have median follow-up times of 4-5 years and demonstrated both a lag effect, meaning clinical benefit grew over time, and a legacy effect, where clinical benefit persisted in extended follow-up after the parent study, Dr. O’Donoghue observed.
With shorter follow-up in the parent FOURIER trial, there was evidence of a lag effect with the risk reduction in CV death, MI, and stroke increasing from 16% in the first year to 25% over time with evolocumab.
FOURIER-OLE enrolled 6,635 patients (3355 randomly assigned to evolocumab and 3280 to placebo), who completed the parent study and self-injected evolocumab subcutaneously with the choice of 140 mg every 2 weeks or 420 mg monthly. Study visits were at week 12 and then every 24 weeks. Median follow-up was 5 years.
Their mean age was 62 years, three-fourths were men, a third had diabetes. Three-fourths were on a high-intensity statin at the time of enrollment in FOURIER, and median LDL cholesterol at randomization was 91 mg/dL (2.4 mmol/L).
At week 12, the median LDL cholesterol was 30 mg/dL (0.78 mmol/L), and this was sustained throughout follow-up, Dr. O’Donoghue reported. Most patients achieved very low LDL cholesterol levels, with 63.2% achieving levels less than 40 mg/dL (1.04 mmol/L) and 26.6% less than 20 mg/dL (0.52 mmol/L).
Patients randomly assigned in the parent trial to evolocumab versus placebo had a 15% lower risk of the primary outcome of CV death, MI, stroke, hospitalization for unstable angina, or coronary revascularization (15.4% vs. 17.5%; HR, 0.85; 95% CI, 0.75-0.96).
Their risk of CV death, MI, or stroke was 20% lower (9.7% vs. 11.9%; HR, 0.80; 95% CI, 0.68-0.93), and, as noted previously, 23% lower for CV death.
When major adverse cardiovascular events data were parsed out by year, the largest LDL cholesterol reduction was in years 1 and 2 of the parent study (delta, 62 mg/dL between treatment arms), “highlighting that lag of benefit that continued to accrue with time,” Dr. O’Donoghue said.
“There was then carryover into the extension period, such that there was legacy effect from the LDL [cholesterol] delta that was seen during the parent study,” she said. “This benefit was most apparent early on during open-label extension and then, as one might expect when all patients were being treated with the same therapy, it began to attenuate somewhat with time.”
Although early studies raised concerns that very low LDL cholesterol may be associated with an increased risk of hemorrhagic stroke and neurocognitive effects, the frequency of adverse events did not increase over time with evolocumab exposure.
Annualized incidence rates for patients initially randomized to evolocumab did not exceed those for placebo-treated patients for any of the following events of interest: serious safety events (10% vs. 13%), hemorrhagic stroke (0.04% vs. 0.05%), new-onset diabetes (1.2% vs. 2.3%), muscle-related events (1.2% vs. 1.9%), injection-site reactions (0.4% vs. 0.7%), and drug-related allergic reactions (0.6% vs. 1.1%).
“Long-term use of evolocumab with a median follow-up of more than 7 years appears both safe and well tolerated,” Dr. O’Donoghue said.
Taken together with the continued accrual of cardiovascular benefit, including CV mortality, “these findings argue for early initiation of a marked and sustained LDL cholesterol reduction to maximize benefit,” she concluded.
Translating the benefits
Ulrich Laufs, MD, Leipzig (Germany) University Hospital, Germany, and invited commentator for the session, said the trial addresses two key issues: the long-term safety of low LDL cholesterol lowering and the long-term safety of inhibiting PCSK9, which is highly expressed not only in the liver but also in the brain, small intestine, and kidneys. Indeed, an LDL cholesterol level below 30 mg/dL is lower than the ESC treatment recommendation for very-high-risk patients and is, in fact, lower than most assays are reliable to interpret.
“So it is very important that we have these very clear data showing us that there were no adverse events, also including cataracts and hemorrhagic stroke, and these were on the level of placebo and did not increase over time,” he said.
The question of efficacy is triggered by observations of another PCSK9, the humanized monoclonal antibody bococizumab, which was associated in the SPIRE trial with an increase in LDL cholesterol over time because of neutralizing antibodies. Reassuringly, there was “completely sustained LDL [cholesterol] reduction” with no neutralizing antibodies with the fully human antibody evolocumab in FOURIER-OLE and in recent data from the OSLER-1 study, Dr. Laufs observed.
Acknowledging the potential for selection bias with an OLE program, Dr. Laufs said there are two important open questions: “Can the safety data observed for extracellular PCSK9 inhibition using an antibody be transferred to other mechanisms of PCSK9 inhibition? And obviously, from the perspective of patient care, how can we implement these important data into patient care and improve access to PCSK9 inhibitors?”
With regard to the latter point, he said physicians should be cautious in using the term “plaque regression,” opting instead for prevention and stabilization of atherosclerosis, and when using the term “legacy,” which may be misinterpreted by patients to imply there was cessation of therapy.
“From my perspective, [what] the open-label extension really shows is that earlier treatment is better,” Dr. Laufs said. “This should be our message.”
In a press conference prior to the presentation, ESC commentator Johann Bauersachs, MD, Hannover (Germany) Medical School, said “this is extremely important data because it confirms that it’s safe, and the criticism of the FOURIER study that mortality, cardiovascular mortality, was not reduced is now also reduced.”
Dr. Bauersachs said it would have been unethical to wait 7 years for a placebo-controlled trial and questioned whether data are available and suggestive of a legacy effect among patients who did not participate in the open-label extension.
Dr. O’Donoghue said unfortunately those data aren’t available but that Kaplan-Meier curves for the primary endpoint in the parent trial continued to diverge over time and that there was somewhat of a lag in terms of that divergence. “So, a median follow-up of 2 years may have been insufficient, especially for the emerging cardiovascular mortality that took longer to appear.”
The study was funded by Amgen. Dr. O’Donoghue reported receiving research grants from Amgen, AstraZeneca, Janssen, Intarcia, and Novartis, and consulting fees from Amgen, Novartis, AstraZeneca, and Janssen. Dr. Laufs reported receiving honoraria/reimbursement for lecture, study participation, and scientific cooperation with Saarland or Leipzig University, as well as relationships with multiple pharmaceutical and device makers.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
New ovulatory disorder classifications from FIGO replace 50-year-old system
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
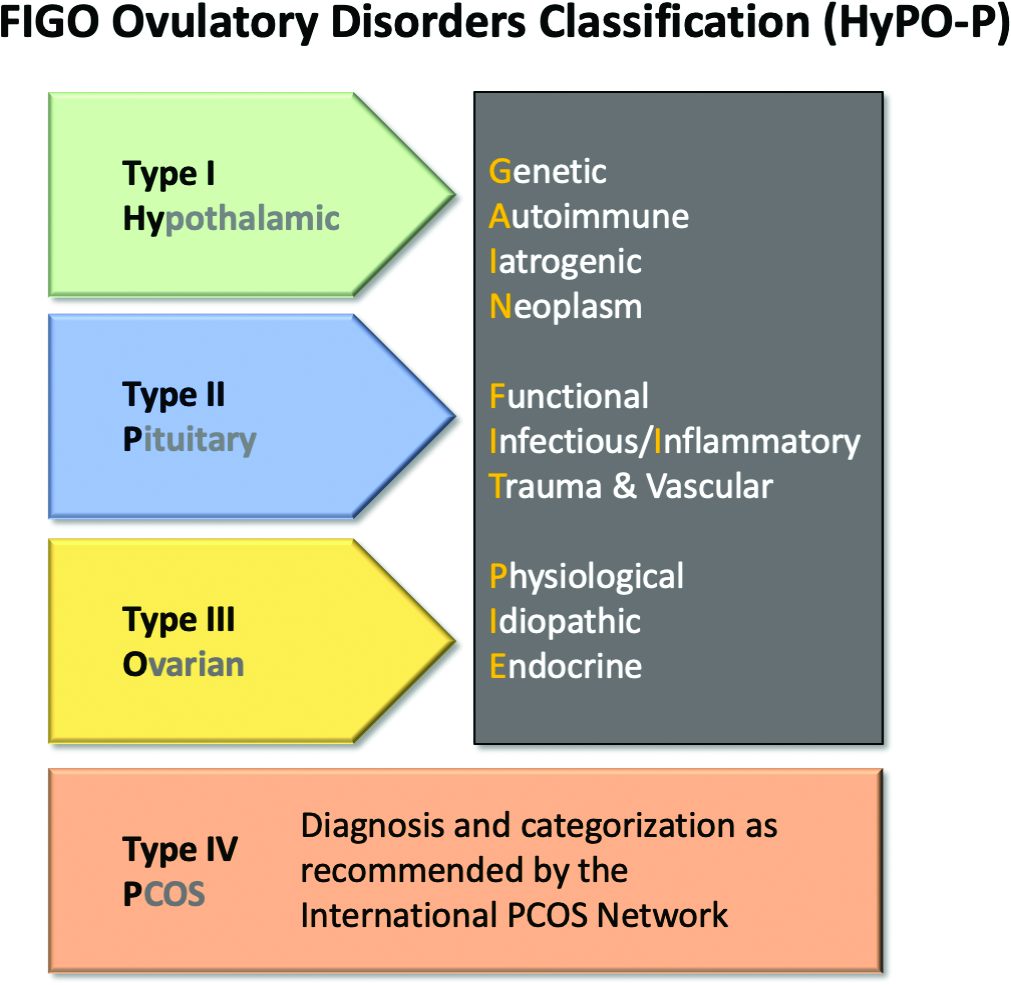
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
FROM INTERNATIONAL JOURNAL OF GYNECOLOGY AND OBSTETRICS
Children and COVID: New cases increase; hospital admissions could follow
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.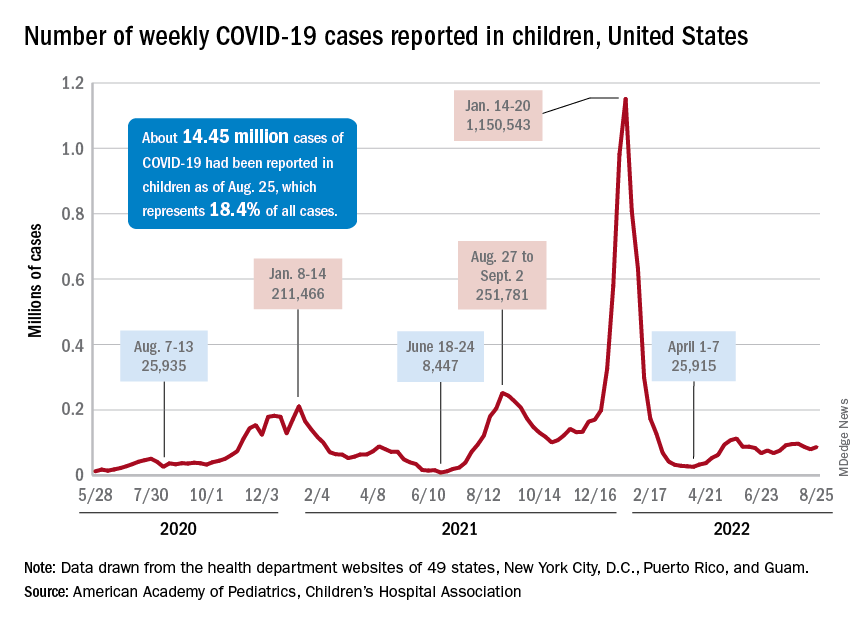
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.
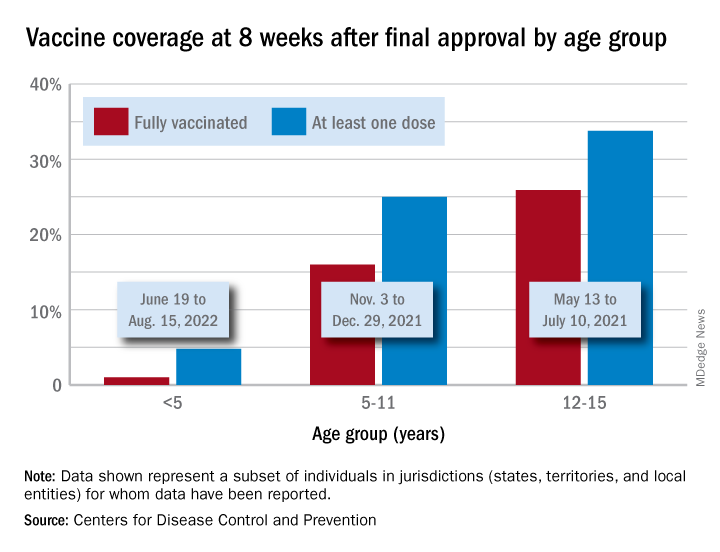
Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
Distorted time perception during the pandemic tied to stress, poor mental health
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
FROM PSYCHOLOGICAL TRAUMA: THEORY, RESEARCH, PRACTICE, AND POLICY
Nearly 30% of U.S. cancer deaths linked to smoking
Nearly 123,000 cancer deaths – or
That corresponds to more than 2 million person-years of lost life and nearly $21 billion in annual lost earnings.
“During the past few decades, smoking has substantially declined in the U.S., followed by great declines in mortality from lung cancer and some other smoking-related cancers,” said lead author Farhad Islami, MD, senior scientific director of cancer disparity research at the American Cancer Society.
Despite this “remarkable progress, our results indicate that smoking is still associated with about 30% of all cancer deaths and substantial lost earnings in the U.S., and that more work should be done to further reduce smoking in the country,” he said.
The study was published online in the International Journal of Cancer.
Dr. Islami and colleagues had found that lost earnings from cancer deaths in 2015 came to nearly $95 billion. Other research showed that a substantial portion of lost earnings from cancer deaths could be traced to cigarette smoking, but estimates were more than a decade old.
To provide more recent estimates and help guide tobacco control policies, Dr. Islami and colleagues estimated person-years of life lost (PYLL) and lost earnings from cigarette smoking-related cancer deaths in 2019.
Of the 418,563 cancer deaths in adults ages 25-79 years, an estimated 122,951 could be linked to cigarette smoking. That corresponds to 29.4% of all cancer deaths and roughly 2.2 million PYLL. Translated to lost earnings, the authors estimated $20.9 billion total, with average lost earnings of $170,000 per cancer death linked to smoking.
By cancer type, lung cancer accounted for about 62%, or $12.9 billion, of the total lost earnings linked to smoking, followed by esophageal cancer (7%, or $1.5 billion), colorectal cancer (6%, or $1.2 billion), and liver cancer (5%, or $1.1 billion).
Smoking-related death rates were highest in the 13 “tobacco nation” states with weaker tobacco control policies and a higher rate of cigarette smoking. These states are Alabama, Arkansas, Indiana, Kentucky, Louisiana, Michigan, Mississippi, Missouri, Ohio, Oklahoma, South Carolina, Tennessee, and West Virginia.
The lost earnings rate in all 13 tobacco nation states combined was about 44% higher, compared with other states and the District of Columbia combined, and the annual PYLL rate was 47% higher in tobacco nation states.
The researchers estimated that if PYLL and lost earnings rates in all states matched those in Utah, which has the lowest rates, more than half of the total PYLL and lost earnings nationally would have been avoided. In other words, that would mean 1.27 million PYLL and $10.5 billion saved in 2019.
Ending the ‘scourge of tobacco’
To kick the smoking habit, health providers should “screen patients for tobacco use, document tobacco use status, advise people who smoke to quit, and assist in attempts to quit,” Dr. Islami said.
Getting more people to screen for lung cancer in the United States is also important, given that only 6.6% of eligible people in 2019 received screening.
In a statement, Lisa Lacasse, president of the American Cancer Society Cancer Action Network, said this report “further demonstrates just how critical reducing tobacco use is to ending suffering and death from cancer.”
To end the “scourge of tobacco,” local, state, and federal lawmakers need to pass proven tobacco control policies, she said.
These include regular and significant tobacco tax increases, thorough statewide smoke-free laws, and enough funding for state programs to prevent and stop smoking. It also means ensuring all Medicaid enrollees have access to all services that can help smokers quit as well as access to all FDA-approved medications that help users stop smoking.
“We have the tools to get this done, we just need lawmakers to act,” Ms. Lacasse said.
A version of this article first appeared on WebMD.com.
Nearly 123,000 cancer deaths – or
That corresponds to more than 2 million person-years of lost life and nearly $21 billion in annual lost earnings.
“During the past few decades, smoking has substantially declined in the U.S., followed by great declines in mortality from lung cancer and some other smoking-related cancers,” said lead author Farhad Islami, MD, senior scientific director of cancer disparity research at the American Cancer Society.
Despite this “remarkable progress, our results indicate that smoking is still associated with about 30% of all cancer deaths and substantial lost earnings in the U.S., and that more work should be done to further reduce smoking in the country,” he said.
The study was published online in the International Journal of Cancer.
Dr. Islami and colleagues had found that lost earnings from cancer deaths in 2015 came to nearly $95 billion. Other research showed that a substantial portion of lost earnings from cancer deaths could be traced to cigarette smoking, but estimates were more than a decade old.
To provide more recent estimates and help guide tobacco control policies, Dr. Islami and colleagues estimated person-years of life lost (PYLL) and lost earnings from cigarette smoking-related cancer deaths in 2019.
Of the 418,563 cancer deaths in adults ages 25-79 years, an estimated 122,951 could be linked to cigarette smoking. That corresponds to 29.4% of all cancer deaths and roughly 2.2 million PYLL. Translated to lost earnings, the authors estimated $20.9 billion total, with average lost earnings of $170,000 per cancer death linked to smoking.
By cancer type, lung cancer accounted for about 62%, or $12.9 billion, of the total lost earnings linked to smoking, followed by esophageal cancer (7%, or $1.5 billion), colorectal cancer (6%, or $1.2 billion), and liver cancer (5%, or $1.1 billion).
Smoking-related death rates were highest in the 13 “tobacco nation” states with weaker tobacco control policies and a higher rate of cigarette smoking. These states are Alabama, Arkansas, Indiana, Kentucky, Louisiana, Michigan, Mississippi, Missouri, Ohio, Oklahoma, South Carolina, Tennessee, and West Virginia.
The lost earnings rate in all 13 tobacco nation states combined was about 44% higher, compared with other states and the District of Columbia combined, and the annual PYLL rate was 47% higher in tobacco nation states.
The researchers estimated that if PYLL and lost earnings rates in all states matched those in Utah, which has the lowest rates, more than half of the total PYLL and lost earnings nationally would have been avoided. In other words, that would mean 1.27 million PYLL and $10.5 billion saved in 2019.
Ending the ‘scourge of tobacco’
To kick the smoking habit, health providers should “screen patients for tobacco use, document tobacco use status, advise people who smoke to quit, and assist in attempts to quit,” Dr. Islami said.
Getting more people to screen for lung cancer in the United States is also important, given that only 6.6% of eligible people in 2019 received screening.
In a statement, Lisa Lacasse, president of the American Cancer Society Cancer Action Network, said this report “further demonstrates just how critical reducing tobacco use is to ending suffering and death from cancer.”
To end the “scourge of tobacco,” local, state, and federal lawmakers need to pass proven tobacco control policies, she said.
These include regular and significant tobacco tax increases, thorough statewide smoke-free laws, and enough funding for state programs to prevent and stop smoking. It also means ensuring all Medicaid enrollees have access to all services that can help smokers quit as well as access to all FDA-approved medications that help users stop smoking.
“We have the tools to get this done, we just need lawmakers to act,” Ms. Lacasse said.
A version of this article first appeared on WebMD.com.
Nearly 123,000 cancer deaths – or
That corresponds to more than 2 million person-years of lost life and nearly $21 billion in annual lost earnings.
“During the past few decades, smoking has substantially declined in the U.S., followed by great declines in mortality from lung cancer and some other smoking-related cancers,” said lead author Farhad Islami, MD, senior scientific director of cancer disparity research at the American Cancer Society.
Despite this “remarkable progress, our results indicate that smoking is still associated with about 30% of all cancer deaths and substantial lost earnings in the U.S., and that more work should be done to further reduce smoking in the country,” he said.
The study was published online in the International Journal of Cancer.
Dr. Islami and colleagues had found that lost earnings from cancer deaths in 2015 came to nearly $95 billion. Other research showed that a substantial portion of lost earnings from cancer deaths could be traced to cigarette smoking, but estimates were more than a decade old.
To provide more recent estimates and help guide tobacco control policies, Dr. Islami and colleagues estimated person-years of life lost (PYLL) and lost earnings from cigarette smoking-related cancer deaths in 2019.
Of the 418,563 cancer deaths in adults ages 25-79 years, an estimated 122,951 could be linked to cigarette smoking. That corresponds to 29.4% of all cancer deaths and roughly 2.2 million PYLL. Translated to lost earnings, the authors estimated $20.9 billion total, with average lost earnings of $170,000 per cancer death linked to smoking.
By cancer type, lung cancer accounted for about 62%, or $12.9 billion, of the total lost earnings linked to smoking, followed by esophageal cancer (7%, or $1.5 billion), colorectal cancer (6%, or $1.2 billion), and liver cancer (5%, or $1.1 billion).
Smoking-related death rates were highest in the 13 “tobacco nation” states with weaker tobacco control policies and a higher rate of cigarette smoking. These states are Alabama, Arkansas, Indiana, Kentucky, Louisiana, Michigan, Mississippi, Missouri, Ohio, Oklahoma, South Carolina, Tennessee, and West Virginia.
The lost earnings rate in all 13 tobacco nation states combined was about 44% higher, compared with other states and the District of Columbia combined, and the annual PYLL rate was 47% higher in tobacco nation states.
The researchers estimated that if PYLL and lost earnings rates in all states matched those in Utah, which has the lowest rates, more than half of the total PYLL and lost earnings nationally would have been avoided. In other words, that would mean 1.27 million PYLL and $10.5 billion saved in 2019.
Ending the ‘scourge of tobacco’
To kick the smoking habit, health providers should “screen patients for tobacco use, document tobacco use status, advise people who smoke to quit, and assist in attempts to quit,” Dr. Islami said.
Getting more people to screen for lung cancer in the United States is also important, given that only 6.6% of eligible people in 2019 received screening.
In a statement, Lisa Lacasse, president of the American Cancer Society Cancer Action Network, said this report “further demonstrates just how critical reducing tobacco use is to ending suffering and death from cancer.”
To end the “scourge of tobacco,” local, state, and federal lawmakers need to pass proven tobacco control policies, she said.
These include regular and significant tobacco tax increases, thorough statewide smoke-free laws, and enough funding for state programs to prevent and stop smoking. It also means ensuring all Medicaid enrollees have access to all services that can help smokers quit as well as access to all FDA-approved medications that help users stop smoking.
“We have the tools to get this done, we just need lawmakers to act,” Ms. Lacasse said.
A version of this article first appeared on WebMD.com.
FROM THE INTERNATIONAL JOURNAL OF CANCER
Drinking black tea linked to lower risk of dying from cardiovascular disease
Drinking tea has several reported health benefits, but most studies have been conducted in regions where green tea predominates.
The findings come from a prospective study of nearly 500,000 participants in the UK Biobank cohort, among whom drinking black tea was common. They suggest that drinking black tea may be associated with a moderately lower all-cause mortality risk, and the risk was lowest among those drinking two or more cups of tea per day.
The study was published online in Annals of Internal Medicine.
During a median follow-up of 11.2 years, those who drank at least two cups of tea each day had a lower all-cause mortality risk, reported Maki Inoue-Choi, PhD, and colleagues from the National Cancer Institute in Bethesda, Md.
After multivariate adjustment, the hazard ratios for death among tea drinkers, compared with no tea intake, were similar across intake levels: 0.95 for daily intake of up to 1 cup, 0.87 for 2-3 cups, 0.88 for 4-5 cups, 0.88 for 6-7 cups, 0.91 for 8-9 cups, and 0.89 for 10 or more cups.
Drinking tea also showed an inverse association with mortality from cardiovascular disease (adjusted HRs ranging from 0.98 to 0.76), ischemic heart disease (aHRs ranging from 1.03 to 0.74), and stroke (aHRs ranging from 0.92 to 0.48 ), However, the researchers added that “no clear trend was seen for cancer or respiratory disease mortality, with associations among higher intake categories tending toward the null.”
There is “no clear answer” as to why no association was observed between tea consumption and cancer mortality in the current study, Dr. Inoue-Choi said at a press briefing. Notably, the effects were apparent regardless of whether milk or sugar was added to tea, tea temperature, or genetic variations in caffeine metabolism among participants.
She and her colleagues controlled for these factors, as well as numerous others that could confound the results, including coffee consumption and baseline health and demographic characteristics..
The study subjects were 498,043 adults with a mean baseline age of 56.5 years. About 85% reported drinking tea, 90% reported drinking black tea, and most drank two to three cups (29%), four to five cups (26%), or six to seven cups (12%) per day.
A limitation of the study is the lack of information on certain aspects of tea intake, such as portion size and tea strength, the authors noted.
Tea is among the most frequently consumed beverages worldwide, and studies from places where green tea is popular, like China and Japan, have demonstrated health benefits. Data from places where black tea is more commonly consumed have been lacking and have provided conflicting results, Dr. Inoue-Choi said.
A presumed mechanism of action related to tea consumption is reduced oxidative stress and inflammation thanks to "polyphenols and flavonoids, namely catechins and their oxidated products," the authors explained. Oxidative stress and inflammation may promote carcinogenesis; therefore, reducing oxidative stress and inflammation may improve endothelial function, they added.
“While these findings may offer reassurance to tea drinkers, they do not indicate that people should start drinking tea or change their tea consumption for health benefits,” Dr. Inoue-Choi said, explaining that “the results need to be replicated in future studies and extended in other diverse populations.”
This study was funded by the National Cancer Institute Intramural Research Program and the NCI division of cancer epidemiology & genetics. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This article was updated 8/31/22.
Drinking tea has several reported health benefits, but most studies have been conducted in regions where green tea predominates.
The findings come from a prospective study of nearly 500,000 participants in the UK Biobank cohort, among whom drinking black tea was common. They suggest that drinking black tea may be associated with a moderately lower all-cause mortality risk, and the risk was lowest among those drinking two or more cups of tea per day.
The study was published online in Annals of Internal Medicine.
During a median follow-up of 11.2 years, those who drank at least two cups of tea each day had a lower all-cause mortality risk, reported Maki Inoue-Choi, PhD, and colleagues from the National Cancer Institute in Bethesda, Md.
After multivariate adjustment, the hazard ratios for death among tea drinkers, compared with no tea intake, were similar across intake levels: 0.95 for daily intake of up to 1 cup, 0.87 for 2-3 cups, 0.88 for 4-5 cups, 0.88 for 6-7 cups, 0.91 for 8-9 cups, and 0.89 for 10 or more cups.
Drinking tea also showed an inverse association with mortality from cardiovascular disease (adjusted HRs ranging from 0.98 to 0.76), ischemic heart disease (aHRs ranging from 1.03 to 0.74), and stroke (aHRs ranging from 0.92 to 0.48 ), However, the researchers added that “no clear trend was seen for cancer or respiratory disease mortality, with associations among higher intake categories tending toward the null.”
There is “no clear answer” as to why no association was observed between tea consumption and cancer mortality in the current study, Dr. Inoue-Choi said at a press briefing. Notably, the effects were apparent regardless of whether milk or sugar was added to tea, tea temperature, or genetic variations in caffeine metabolism among participants.
She and her colleagues controlled for these factors, as well as numerous others that could confound the results, including coffee consumption and baseline health and demographic characteristics..
The study subjects were 498,043 adults with a mean baseline age of 56.5 years. About 85% reported drinking tea, 90% reported drinking black tea, and most drank two to three cups (29%), four to five cups (26%), or six to seven cups (12%) per day.
A limitation of the study is the lack of information on certain aspects of tea intake, such as portion size and tea strength, the authors noted.
Tea is among the most frequently consumed beverages worldwide, and studies from places where green tea is popular, like China and Japan, have demonstrated health benefits. Data from places where black tea is more commonly consumed have been lacking and have provided conflicting results, Dr. Inoue-Choi said.
A presumed mechanism of action related to tea consumption is reduced oxidative stress and inflammation thanks to "polyphenols and flavonoids, namely catechins and their oxidated products," the authors explained. Oxidative stress and inflammation may promote carcinogenesis; therefore, reducing oxidative stress and inflammation may improve endothelial function, they added.
“While these findings may offer reassurance to tea drinkers, they do not indicate that people should start drinking tea or change their tea consumption for health benefits,” Dr. Inoue-Choi said, explaining that “the results need to be replicated in future studies and extended in other diverse populations.”
This study was funded by the National Cancer Institute Intramural Research Program and the NCI division of cancer epidemiology & genetics. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This article was updated 8/31/22.
Drinking tea has several reported health benefits, but most studies have been conducted in regions where green tea predominates.
The findings come from a prospective study of nearly 500,000 participants in the UK Biobank cohort, among whom drinking black tea was common. They suggest that drinking black tea may be associated with a moderately lower all-cause mortality risk, and the risk was lowest among those drinking two or more cups of tea per day.
The study was published online in Annals of Internal Medicine.
During a median follow-up of 11.2 years, those who drank at least two cups of tea each day had a lower all-cause mortality risk, reported Maki Inoue-Choi, PhD, and colleagues from the National Cancer Institute in Bethesda, Md.
After multivariate adjustment, the hazard ratios for death among tea drinkers, compared with no tea intake, were similar across intake levels: 0.95 for daily intake of up to 1 cup, 0.87 for 2-3 cups, 0.88 for 4-5 cups, 0.88 for 6-7 cups, 0.91 for 8-9 cups, and 0.89 for 10 or more cups.
Drinking tea also showed an inverse association with mortality from cardiovascular disease (adjusted HRs ranging from 0.98 to 0.76), ischemic heart disease (aHRs ranging from 1.03 to 0.74), and stroke (aHRs ranging from 0.92 to 0.48 ), However, the researchers added that “no clear trend was seen for cancer or respiratory disease mortality, with associations among higher intake categories tending toward the null.”
There is “no clear answer” as to why no association was observed between tea consumption and cancer mortality in the current study, Dr. Inoue-Choi said at a press briefing. Notably, the effects were apparent regardless of whether milk or sugar was added to tea, tea temperature, or genetic variations in caffeine metabolism among participants.
She and her colleagues controlled for these factors, as well as numerous others that could confound the results, including coffee consumption and baseline health and demographic characteristics..
The study subjects were 498,043 adults with a mean baseline age of 56.5 years. About 85% reported drinking tea, 90% reported drinking black tea, and most drank two to three cups (29%), four to five cups (26%), or six to seven cups (12%) per day.
A limitation of the study is the lack of information on certain aspects of tea intake, such as portion size and tea strength, the authors noted.
Tea is among the most frequently consumed beverages worldwide, and studies from places where green tea is popular, like China and Japan, have demonstrated health benefits. Data from places where black tea is more commonly consumed have been lacking and have provided conflicting results, Dr. Inoue-Choi said.
A presumed mechanism of action related to tea consumption is reduced oxidative stress and inflammation thanks to "polyphenols and flavonoids, namely catechins and their oxidated products," the authors explained. Oxidative stress and inflammation may promote carcinogenesis; therefore, reducing oxidative stress and inflammation may improve endothelial function, they added.
“While these findings may offer reassurance to tea drinkers, they do not indicate that people should start drinking tea or change their tea consumption for health benefits,” Dr. Inoue-Choi said, explaining that “the results need to be replicated in future studies and extended in other diverse populations.”
This study was funded by the National Cancer Institute Intramural Research Program and the NCI division of cancer epidemiology & genetics. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This article was updated 8/31/22.
FROM ANNALS OF INTERNAL MEDICINE
At-home test for oral/throat cancer launched in U.S.
Recently, a home test for oral and throat cancer was launched in the United States, and it is being marketed directly to the general public, aimed at former or current tobacco users and anyone 50 years or older.
Individuals can order the test – CancerDetect Test for Oral & Throat Cancer – directly from its maker, Viome Life Sciences, for $399.
The test is being marketed under the agency’s “laboratory developed test” rubric.
People who qualify and buy the test are mailed a saliva collection tube, which they fill and mail back. The company then analyzes the RNA for changes in human cells and the oropharyngeal microbiome that are associated with cancer. During a 15-minute telemedicine conference – included in the $399 cost – those who test positive are told to follow up with a secondary care center for a definitive diagnosis.
For people who test positive but have no visible lesion to biopsy, doctors will likely opt for surveillance, computer scientist Guruduth Banavar, PhD, Viome’s chief technology officer, told this news organization.
Dr. Banavar said people have been buying the test every day since it was launched in early August, but he declined to give specific sales figures.
CancerDetect’s tagline is “test at home for peace of mind.” The test “brings unprecedented accuracy to early cancer detection and prevention,” the company said in a press release announcing the launch.
The test showed an overall specificity of 94% and sensitivity of 84.2%-90% for cancer in Viome’s latest study, which is posted on medRxiv.org as a preprint. Banavar said it has been submitted to a top-tier medical journal.
Viome plans to market CancerDetect “in every possible way” to consumers, including social media, Dr. Banavar said. CancerDetect is not sold on Amazon at the moment, but the company sells another at-home test for gut microbiome plus cellular health on the website.
As for outreach to the medical community, “we will start doing that with dentists first” and then eventually oncologists and other doctors, but “our primary target is to get out to the consumers themselves,” Dr. Banavar said.
Viome’s main goal is to help consumers be proactive regarding their health, he said.
An expert opinion
The marketing push means that sooner or later, oncologists will likely have to deal with a patient who tests positive on CancerDetect, so this news organization turned to numerous experts for their thoughts. None had heard about the test, but one responded with comments.
“I am happy to see industry working on strategies for the early detection of oral and throat cancers,” and CancerDetect has “potential,” said surgical oncologist Saral Mehra, MD, MBA, chief of head and neck surgery at Yale University, New Haven, Conn.
However, after reviewing the study posted on medRxiv, Dr. Mehra advised caution. He said he was concerned about false negative results leading to missed cancers and false positives leading to unnecessary anxiety and testing.
According to the medRxiv preprint, the test was developed and validated using saliva samples from 1,175 people 50 years or older as well as adults with a history of tobacco use.
In the 230-sample validation cohort, CancerDetect correctly classified 18 out of 20 people with oral squamous cell carcinoma (OSCC) and 64/76 with oropharyngeal squamous cell carcinoma (OPSCC), yielding sensitivities of 90% and 84.2%, respectively.
The test also correctly identified 126/134 people as cancer free, for a specificity of 94%.
Results were similar between early and late-stage disease, but mixed in subgroups. Among people younger than age 50, for instance, 4/4 (100%) with OSCC and 2/3 (66.7%) with OPSCC were correctly classified as positive. Among older people, 15/17 (88.2%) with OSCC and 62/73 (84.9%) with OPSCC were correctly classified
Commenting on the results, Dr. Mehra noted that “the power of the study, especially for subgroup analysis, was low,” and investigators “used both advanced-stage and early-stage cancer patients in the model, while the target population for this test is early stage.
“The research needs to be tightened significantly on specific target populations, the models adjusted to really limit false negatives, and a plan [put in place] to act upon positive results,” he said.
Also, the ability of CancerDetect to pick up premalignant lesions – “the greatest value in a screening test” – is not clear, he added.
Viome’s Dr. Banavar said that CancerDetect is in its first iteration, and the test uses machine learning, so its diagnostic performance will improve with the ongoing addition of real-world data.
The company is organizing a pivotal trial to gain formal FDA approval, with results expected in a year and a half or so, he said.
Viome is pushing ahead with its RNA diagnosis technology for the entire range of alimentary canal cancers and disorders, including inflammatory bowel disease. The company has partnered with pharmaceutical companies, including GSK, for vaccines, Dr. Banavar said.
Dr. Mehra reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recently, a home test for oral and throat cancer was launched in the United States, and it is being marketed directly to the general public, aimed at former or current tobacco users and anyone 50 years or older.
Individuals can order the test – CancerDetect Test for Oral & Throat Cancer – directly from its maker, Viome Life Sciences, for $399.
The test is being marketed under the agency’s “laboratory developed test” rubric.
People who qualify and buy the test are mailed a saliva collection tube, which they fill and mail back. The company then analyzes the RNA for changes in human cells and the oropharyngeal microbiome that are associated with cancer. During a 15-minute telemedicine conference – included in the $399 cost – those who test positive are told to follow up with a secondary care center for a definitive diagnosis.
For people who test positive but have no visible lesion to biopsy, doctors will likely opt for surveillance, computer scientist Guruduth Banavar, PhD, Viome’s chief technology officer, told this news organization.
Dr. Banavar said people have been buying the test every day since it was launched in early August, but he declined to give specific sales figures.
CancerDetect’s tagline is “test at home for peace of mind.” The test “brings unprecedented accuracy to early cancer detection and prevention,” the company said in a press release announcing the launch.
The test showed an overall specificity of 94% and sensitivity of 84.2%-90% for cancer in Viome’s latest study, which is posted on medRxiv.org as a preprint. Banavar said it has been submitted to a top-tier medical journal.
Viome plans to market CancerDetect “in every possible way” to consumers, including social media, Dr. Banavar said. CancerDetect is not sold on Amazon at the moment, but the company sells another at-home test for gut microbiome plus cellular health on the website.
As for outreach to the medical community, “we will start doing that with dentists first” and then eventually oncologists and other doctors, but “our primary target is to get out to the consumers themselves,” Dr. Banavar said.
Viome’s main goal is to help consumers be proactive regarding their health, he said.
An expert opinion
The marketing push means that sooner or later, oncologists will likely have to deal with a patient who tests positive on CancerDetect, so this news organization turned to numerous experts for their thoughts. None had heard about the test, but one responded with comments.
“I am happy to see industry working on strategies for the early detection of oral and throat cancers,” and CancerDetect has “potential,” said surgical oncologist Saral Mehra, MD, MBA, chief of head and neck surgery at Yale University, New Haven, Conn.
However, after reviewing the study posted on medRxiv, Dr. Mehra advised caution. He said he was concerned about false negative results leading to missed cancers and false positives leading to unnecessary anxiety and testing.
According to the medRxiv preprint, the test was developed and validated using saliva samples from 1,175 people 50 years or older as well as adults with a history of tobacco use.
In the 230-sample validation cohort, CancerDetect correctly classified 18 out of 20 people with oral squamous cell carcinoma (OSCC) and 64/76 with oropharyngeal squamous cell carcinoma (OPSCC), yielding sensitivities of 90% and 84.2%, respectively.
The test also correctly identified 126/134 people as cancer free, for a specificity of 94%.
Results were similar between early and late-stage disease, but mixed in subgroups. Among people younger than age 50, for instance, 4/4 (100%) with OSCC and 2/3 (66.7%) with OPSCC were correctly classified as positive. Among older people, 15/17 (88.2%) with OSCC and 62/73 (84.9%) with OPSCC were correctly classified
Commenting on the results, Dr. Mehra noted that “the power of the study, especially for subgroup analysis, was low,” and investigators “used both advanced-stage and early-stage cancer patients in the model, while the target population for this test is early stage.
“The research needs to be tightened significantly on specific target populations, the models adjusted to really limit false negatives, and a plan [put in place] to act upon positive results,” he said.
Also, the ability of CancerDetect to pick up premalignant lesions – “the greatest value in a screening test” – is not clear, he added.
Viome’s Dr. Banavar said that CancerDetect is in its first iteration, and the test uses machine learning, so its diagnostic performance will improve with the ongoing addition of real-world data.
The company is organizing a pivotal trial to gain formal FDA approval, with results expected in a year and a half or so, he said.
Viome is pushing ahead with its RNA diagnosis technology for the entire range of alimentary canal cancers and disorders, including inflammatory bowel disease. The company has partnered with pharmaceutical companies, including GSK, for vaccines, Dr. Banavar said.
Dr. Mehra reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recently, a home test for oral and throat cancer was launched in the United States, and it is being marketed directly to the general public, aimed at former or current tobacco users and anyone 50 years or older.
Individuals can order the test – CancerDetect Test for Oral & Throat Cancer – directly from its maker, Viome Life Sciences, for $399.
The test is being marketed under the agency’s “laboratory developed test” rubric.
People who qualify and buy the test are mailed a saliva collection tube, which they fill and mail back. The company then analyzes the RNA for changes in human cells and the oropharyngeal microbiome that are associated with cancer. During a 15-minute telemedicine conference – included in the $399 cost – those who test positive are told to follow up with a secondary care center for a definitive diagnosis.
For people who test positive but have no visible lesion to biopsy, doctors will likely opt for surveillance, computer scientist Guruduth Banavar, PhD, Viome’s chief technology officer, told this news organization.
Dr. Banavar said people have been buying the test every day since it was launched in early August, but he declined to give specific sales figures.
CancerDetect’s tagline is “test at home for peace of mind.” The test “brings unprecedented accuracy to early cancer detection and prevention,” the company said in a press release announcing the launch.
The test showed an overall specificity of 94% and sensitivity of 84.2%-90% for cancer in Viome’s latest study, which is posted on medRxiv.org as a preprint. Banavar said it has been submitted to a top-tier medical journal.
Viome plans to market CancerDetect “in every possible way” to consumers, including social media, Dr. Banavar said. CancerDetect is not sold on Amazon at the moment, but the company sells another at-home test for gut microbiome plus cellular health on the website.
As for outreach to the medical community, “we will start doing that with dentists first” and then eventually oncologists and other doctors, but “our primary target is to get out to the consumers themselves,” Dr. Banavar said.
Viome’s main goal is to help consumers be proactive regarding their health, he said.
An expert opinion
The marketing push means that sooner or later, oncologists will likely have to deal with a patient who tests positive on CancerDetect, so this news organization turned to numerous experts for their thoughts. None had heard about the test, but one responded with comments.
“I am happy to see industry working on strategies for the early detection of oral and throat cancers,” and CancerDetect has “potential,” said surgical oncologist Saral Mehra, MD, MBA, chief of head and neck surgery at Yale University, New Haven, Conn.
However, after reviewing the study posted on medRxiv, Dr. Mehra advised caution. He said he was concerned about false negative results leading to missed cancers and false positives leading to unnecessary anxiety and testing.
According to the medRxiv preprint, the test was developed and validated using saliva samples from 1,175 people 50 years or older as well as adults with a history of tobacco use.
In the 230-sample validation cohort, CancerDetect correctly classified 18 out of 20 people with oral squamous cell carcinoma (OSCC) and 64/76 with oropharyngeal squamous cell carcinoma (OPSCC), yielding sensitivities of 90% and 84.2%, respectively.
The test also correctly identified 126/134 people as cancer free, for a specificity of 94%.
Results were similar between early and late-stage disease, but mixed in subgroups. Among people younger than age 50, for instance, 4/4 (100%) with OSCC and 2/3 (66.7%) with OPSCC were correctly classified as positive. Among older people, 15/17 (88.2%) with OSCC and 62/73 (84.9%) with OPSCC were correctly classified
Commenting on the results, Dr. Mehra noted that “the power of the study, especially for subgroup analysis, was low,” and investigators “used both advanced-stage and early-stage cancer patients in the model, while the target population for this test is early stage.
“The research needs to be tightened significantly on specific target populations, the models adjusted to really limit false negatives, and a plan [put in place] to act upon positive results,” he said.
Also, the ability of CancerDetect to pick up premalignant lesions – “the greatest value in a screening test” – is not clear, he added.
Viome’s Dr. Banavar said that CancerDetect is in its first iteration, and the test uses machine learning, so its diagnostic performance will improve with the ongoing addition of real-world data.
The company is organizing a pivotal trial to gain formal FDA approval, with results expected in a year and a half or so, he said.
Viome is pushing ahead with its RNA diagnosis technology for the entire range of alimentary canal cancers and disorders, including inflammatory bowel disease. The company has partnered with pharmaceutical companies, including GSK, for vaccines, Dr. Banavar said.
Dr. Mehra reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
How do you live with COVID? One doctor’s personal experience
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Leg rash
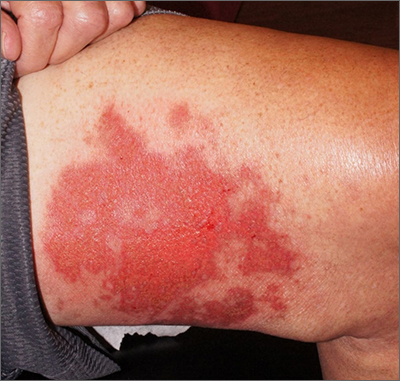
Punch biopsies for standard pathology and direct immunofluorescence were performed and ruled out vesiculobullous disease. Further conversation with the patient revealed that this was a phototoxic drug eruption that resulted from a medication mix-up. The patient had intended to treat an eczema flare with a topical steroid but had inadvertently applied 5-fluorouracil (5-FU), which he had left over from a previous bout of actinic keratosis. While selective to precancerous cells with rapid DNA replication, 5-FU can trigger a significant photodermatitis when applied to heavily sun-exposed skin.
Phototoxic skin reactions can be an adverse result of multiple systemic and topical therapies. Common systemic examples include amiodarone, chlorpromazine, doxycycline, hydrochlorothiazide, isotretinoin, nalidixic acid, naproxen, piroxicam, tetracycline, thioridazine, vemurafenib, and voriconazole.1 Topical examples include retinoids, levulinic acid, and 5-FU. Treatment requires that the patient stop the offending medication and use photoprotection. The patient followed this protocol and his erosions resolved over the course of a few weeks.
This case demonstrates that topical therapies, like systemic medications, can have chemical names that are confusing to patients. Further complicating matters can be the practice of folding metal tubes of cream over their life of use, thus obscuring the label.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Blakely KM, Drucker AM, Rosen CF. Drug-induced photosensitivity-an update: culprit drugs, prevention, and management. Drug Saf. 2019;42:827-847. doi: 10.1007/s40264-019-00806-5

Punch biopsies for standard pathology and direct immunofluorescence were performed and ruled out vesiculobullous disease. Further conversation with the patient revealed that this was a phototoxic drug eruption that resulted from a medication mix-up. The patient had intended to treat an eczema flare with a topical steroid but had inadvertently applied 5-fluorouracil (5-FU), which he had left over from a previous bout of actinic keratosis. While selective to precancerous cells with rapid DNA replication, 5-FU can trigger a significant photodermatitis when applied to heavily sun-exposed skin.
Phototoxic skin reactions can be an adverse result of multiple systemic and topical therapies. Common systemic examples include amiodarone, chlorpromazine, doxycycline, hydrochlorothiazide, isotretinoin, nalidixic acid, naproxen, piroxicam, tetracycline, thioridazine, vemurafenib, and voriconazole.1 Topical examples include retinoids, levulinic acid, and 5-FU. Treatment requires that the patient stop the offending medication and use photoprotection. The patient followed this protocol and his erosions resolved over the course of a few weeks.
This case demonstrates that topical therapies, like systemic medications, can have chemical names that are confusing to patients. Further complicating matters can be the practice of folding metal tubes of cream over their life of use, thus obscuring the label.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Punch biopsies for standard pathology and direct immunofluorescence were performed and ruled out vesiculobullous disease. Further conversation with the patient revealed that this was a phototoxic drug eruption that resulted from a medication mix-up. The patient had intended to treat an eczema flare with a topical steroid but had inadvertently applied 5-fluorouracil (5-FU), which he had left over from a previous bout of actinic keratosis. While selective to precancerous cells with rapid DNA replication, 5-FU can trigger a significant photodermatitis when applied to heavily sun-exposed skin.
Phototoxic skin reactions can be an adverse result of multiple systemic and topical therapies. Common systemic examples include amiodarone, chlorpromazine, doxycycline, hydrochlorothiazide, isotretinoin, nalidixic acid, naproxen, piroxicam, tetracycline, thioridazine, vemurafenib, and voriconazole.1 Topical examples include retinoids, levulinic acid, and 5-FU. Treatment requires that the patient stop the offending medication and use photoprotection. The patient followed this protocol and his erosions resolved over the course of a few weeks.
This case demonstrates that topical therapies, like systemic medications, can have chemical names that are confusing to patients. Further complicating matters can be the practice of folding metal tubes of cream over their life of use, thus obscuring the label.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Blakely KM, Drucker AM, Rosen CF. Drug-induced photosensitivity-an update: culprit drugs, prevention, and management. Drug Saf. 2019;42:827-847. doi: 10.1007/s40264-019-00806-5
1. Blakely KM, Drucker AM, Rosen CF. Drug-induced photosensitivity-an update: culprit drugs, prevention, and management. Drug Saf. 2019;42:827-847. doi: 10.1007/s40264-019-00806-5