User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
National Practitioner Data Bank should go public, group says
arguing that extra public scrutiny could pressure state medical boards to be more aggressive watchdogs.
Public Citizen’s report includes an analysis of how frequently medical boards sanctioned physicians in 2019, 2020, and 2021. These sanctions include license revocations, suspensions, voluntary surrenders of licenses, and limitations on practice while under investigation.
The report used data from the National Practitioner Data Bank (NPDB), a federal repository of reports about state licensure, discipline, and certification actions as well as medical malpractice payments. The database is closed to the public, but hospitals, malpractice insurers, and investigators can query it.
According to Public Citizen’s calculations, states most likely to take serious disciplinary action against physicians were:
- Michigan: 1.74 serious disciplinary actions per 1,000 physicians per year
- Ohio: 1.61
- North Dakota: 1.60
- Colorado: 1.55
- Arizona: 1.53
- The states least likely to do so were:
- Nevada: 0.24 serious disciplinary actions per 1,000 physicians per year
- New Hampshire: 0.25
- Georgia: 0.27
- Indiana: 0.28
- Nebraska: 0.32
- California, the largest U.S. state by both population and number of physicians, landed near the middle, ranking 27th with a rate of 0.83 serious actions per 1,000 physicians, Public Citizen said.
“There is no evidence that physicians in any state are, overall, more or less likely to be incompetent or miscreant than the physicians in any other state,” said Robert Oshel, PhD, a former NPDB associate director for research and an author of the report.
The differences instead reflect variations in boards’ enforcement of medical practice laws, domination of licensing boards by physicians, and inadequate budgets, he noted.
Public Citizen said Congress should change federal law to let members of the public get information from the NPDB to do a background check on physicians whom they are considering seeing or are already seeing. This would not only help individuals but also would spur state licensing boards to do their own checks with the NPDB, the group said.
“If licensing boards routinely queried the NPDB, they would not be faulted by the public and state legislators for not knowing about malpractice payments or disciplinary actions affecting their licensees and therefore not taking reasonable actions concerning their licensees found to have poor records,” the report said.
Questioning NPDB access for consumers
Michelle Mello, JD, PhD, a professor of law and health policy at Stanford (Calif.) University, has studied the current applications of the NPDB. In 2019, she published an article in The New England Journal of Medicine examining changes in practice patterns for clinicians who faced multiple malpractice claims.
Dr. Mello questioned what benefit consumers would get from direct access to the NPDB’s information.
“It provides almost no context for the information it reports, making it even harder for patients to make sense of what they see there,” Dr. Mello said in an interview.
Hospitals are already required to routinely query the NPDB. This legal requirement should be expanded to include licensing boards, which the report called “the last line of defense for the public from incompetent and miscreant physicians,” Public Citizen said.
“Ideally, this amendment should include free continuous query access by medical boards for all their licensees,” the report said. “In the absence of any action by Congress, individual state legislatures should require their licensing boards to query all their licensees or enroll in continuous query, as a few states already do.”
The Federation of State Medical Boards agreed with some of the other suggestions Public Citizen offered in the report. The two concur on the need for increased funding to state medical boards to ensure that they have adequate resources and staffing to fulfill their duties, FSMB said in a statement.
But FSMB disagreed with Public Citizens’ approach to ranking boards, saying it could mislead. The report lacks context about how boards’ funding and authority vary, Humayun Chaudhry, DO, FSMB’s chief executive officer, said. He also questioned the decision to focus only on serious disciplinary actions.
“The Public Citizen report does not take into account the wide range of disciplinary steps boards can take such as letters of reprimand or fines, which are often enough to stop problem behaviors – preempting further problems in the future,” Dr. Chaudhry said.
D.C. gets worst rating
The District of Columbia earned the worst mark in the Public Citizen ranking, holding the 51st spot, the same place it held in the group’s similar ranking on actions taken in the 2017-2019 period. There were 0.19 serious disciplinary actions per 1,000 physicians a year in Washington, Public Citizen said.
In an email to this news organization, Dr. Oshel said that the Public Citizen analysis focused on the number of licensed physicians in each state and D.C. that can be obtained and compared reliably. It avoided using the term “practicing physicians” owing in part to doubts about the reliability of these counts, he said.
As many as 20% of physicians nationwide are focused primarily on work outside of clinical care, Dr. Oshel estimated. In D.C., perhaps 40% of physicians may fall into this category. Of the more than 13,700 physicians licensed in D.C., there may be only about 8,126 actively practicing, according to Dr. Oshel.
But even using that lower estimate of practicing physicians would only raise D.C.’s ranking to 46, signaling a need for stepped-up enforcement, Dr. Oshel said.
“[Whether it’s] 46th or 51st, both are bad,” Dr. Oshel said.
A version of this article first appeared on Medscape.com.
arguing that extra public scrutiny could pressure state medical boards to be more aggressive watchdogs.
Public Citizen’s report includes an analysis of how frequently medical boards sanctioned physicians in 2019, 2020, and 2021. These sanctions include license revocations, suspensions, voluntary surrenders of licenses, and limitations on practice while under investigation.
The report used data from the National Practitioner Data Bank (NPDB), a federal repository of reports about state licensure, discipline, and certification actions as well as medical malpractice payments. The database is closed to the public, but hospitals, malpractice insurers, and investigators can query it.
According to Public Citizen’s calculations, states most likely to take serious disciplinary action against physicians were:
- Michigan: 1.74 serious disciplinary actions per 1,000 physicians per year
- Ohio: 1.61
- North Dakota: 1.60
- Colorado: 1.55
- Arizona: 1.53
- The states least likely to do so were:
- Nevada: 0.24 serious disciplinary actions per 1,000 physicians per year
- New Hampshire: 0.25
- Georgia: 0.27
- Indiana: 0.28
- Nebraska: 0.32
- California, the largest U.S. state by both population and number of physicians, landed near the middle, ranking 27th with a rate of 0.83 serious actions per 1,000 physicians, Public Citizen said.
“There is no evidence that physicians in any state are, overall, more or less likely to be incompetent or miscreant than the physicians in any other state,” said Robert Oshel, PhD, a former NPDB associate director for research and an author of the report.
The differences instead reflect variations in boards’ enforcement of medical practice laws, domination of licensing boards by physicians, and inadequate budgets, he noted.
Public Citizen said Congress should change federal law to let members of the public get information from the NPDB to do a background check on physicians whom they are considering seeing or are already seeing. This would not only help individuals but also would spur state licensing boards to do their own checks with the NPDB, the group said.
“If licensing boards routinely queried the NPDB, they would not be faulted by the public and state legislators for not knowing about malpractice payments or disciplinary actions affecting their licensees and therefore not taking reasonable actions concerning their licensees found to have poor records,” the report said.
Questioning NPDB access for consumers
Michelle Mello, JD, PhD, a professor of law and health policy at Stanford (Calif.) University, has studied the current applications of the NPDB. In 2019, she published an article in The New England Journal of Medicine examining changes in practice patterns for clinicians who faced multiple malpractice claims.
Dr. Mello questioned what benefit consumers would get from direct access to the NPDB’s information.
“It provides almost no context for the information it reports, making it even harder for patients to make sense of what they see there,” Dr. Mello said in an interview.
Hospitals are already required to routinely query the NPDB. This legal requirement should be expanded to include licensing boards, which the report called “the last line of defense for the public from incompetent and miscreant physicians,” Public Citizen said.
“Ideally, this amendment should include free continuous query access by medical boards for all their licensees,” the report said. “In the absence of any action by Congress, individual state legislatures should require their licensing boards to query all their licensees or enroll in continuous query, as a few states already do.”
The Federation of State Medical Boards agreed with some of the other suggestions Public Citizen offered in the report. The two concur on the need for increased funding to state medical boards to ensure that they have adequate resources and staffing to fulfill their duties, FSMB said in a statement.
But FSMB disagreed with Public Citizens’ approach to ranking boards, saying it could mislead. The report lacks context about how boards’ funding and authority vary, Humayun Chaudhry, DO, FSMB’s chief executive officer, said. He also questioned the decision to focus only on serious disciplinary actions.
“The Public Citizen report does not take into account the wide range of disciplinary steps boards can take such as letters of reprimand or fines, which are often enough to stop problem behaviors – preempting further problems in the future,” Dr. Chaudhry said.
D.C. gets worst rating
The District of Columbia earned the worst mark in the Public Citizen ranking, holding the 51st spot, the same place it held in the group’s similar ranking on actions taken in the 2017-2019 period. There were 0.19 serious disciplinary actions per 1,000 physicians a year in Washington, Public Citizen said.
In an email to this news organization, Dr. Oshel said that the Public Citizen analysis focused on the number of licensed physicians in each state and D.C. that can be obtained and compared reliably. It avoided using the term “practicing physicians” owing in part to doubts about the reliability of these counts, he said.
As many as 20% of physicians nationwide are focused primarily on work outside of clinical care, Dr. Oshel estimated. In D.C., perhaps 40% of physicians may fall into this category. Of the more than 13,700 physicians licensed in D.C., there may be only about 8,126 actively practicing, according to Dr. Oshel.
But even using that lower estimate of practicing physicians would only raise D.C.’s ranking to 46, signaling a need for stepped-up enforcement, Dr. Oshel said.
“[Whether it’s] 46th or 51st, both are bad,” Dr. Oshel said.
A version of this article first appeared on Medscape.com.
arguing that extra public scrutiny could pressure state medical boards to be more aggressive watchdogs.
Public Citizen’s report includes an analysis of how frequently medical boards sanctioned physicians in 2019, 2020, and 2021. These sanctions include license revocations, suspensions, voluntary surrenders of licenses, and limitations on practice while under investigation.
The report used data from the National Practitioner Data Bank (NPDB), a federal repository of reports about state licensure, discipline, and certification actions as well as medical malpractice payments. The database is closed to the public, but hospitals, malpractice insurers, and investigators can query it.
According to Public Citizen’s calculations, states most likely to take serious disciplinary action against physicians were:
- Michigan: 1.74 serious disciplinary actions per 1,000 physicians per year
- Ohio: 1.61
- North Dakota: 1.60
- Colorado: 1.55
- Arizona: 1.53
- The states least likely to do so were:
- Nevada: 0.24 serious disciplinary actions per 1,000 physicians per year
- New Hampshire: 0.25
- Georgia: 0.27
- Indiana: 0.28
- Nebraska: 0.32
- California, the largest U.S. state by both population and number of physicians, landed near the middle, ranking 27th with a rate of 0.83 serious actions per 1,000 physicians, Public Citizen said.
“There is no evidence that physicians in any state are, overall, more or less likely to be incompetent or miscreant than the physicians in any other state,” said Robert Oshel, PhD, a former NPDB associate director for research and an author of the report.
The differences instead reflect variations in boards’ enforcement of medical practice laws, domination of licensing boards by physicians, and inadequate budgets, he noted.
Public Citizen said Congress should change federal law to let members of the public get information from the NPDB to do a background check on physicians whom they are considering seeing or are already seeing. This would not only help individuals but also would spur state licensing boards to do their own checks with the NPDB, the group said.
“If licensing boards routinely queried the NPDB, they would not be faulted by the public and state legislators for not knowing about malpractice payments or disciplinary actions affecting their licensees and therefore not taking reasonable actions concerning their licensees found to have poor records,” the report said.
Questioning NPDB access for consumers
Michelle Mello, JD, PhD, a professor of law and health policy at Stanford (Calif.) University, has studied the current applications of the NPDB. In 2019, she published an article in The New England Journal of Medicine examining changes in practice patterns for clinicians who faced multiple malpractice claims.
Dr. Mello questioned what benefit consumers would get from direct access to the NPDB’s information.
“It provides almost no context for the information it reports, making it even harder for patients to make sense of what they see there,” Dr. Mello said in an interview.
Hospitals are already required to routinely query the NPDB. This legal requirement should be expanded to include licensing boards, which the report called “the last line of defense for the public from incompetent and miscreant physicians,” Public Citizen said.
“Ideally, this amendment should include free continuous query access by medical boards for all their licensees,” the report said. “In the absence of any action by Congress, individual state legislatures should require their licensing boards to query all their licensees or enroll in continuous query, as a few states already do.”
The Federation of State Medical Boards agreed with some of the other suggestions Public Citizen offered in the report. The two concur on the need for increased funding to state medical boards to ensure that they have adequate resources and staffing to fulfill their duties, FSMB said in a statement.
But FSMB disagreed with Public Citizens’ approach to ranking boards, saying it could mislead. The report lacks context about how boards’ funding and authority vary, Humayun Chaudhry, DO, FSMB’s chief executive officer, said. He also questioned the decision to focus only on serious disciplinary actions.
“The Public Citizen report does not take into account the wide range of disciplinary steps boards can take such as letters of reprimand or fines, which are often enough to stop problem behaviors – preempting further problems in the future,” Dr. Chaudhry said.
D.C. gets worst rating
The District of Columbia earned the worst mark in the Public Citizen ranking, holding the 51st spot, the same place it held in the group’s similar ranking on actions taken in the 2017-2019 period. There were 0.19 serious disciplinary actions per 1,000 physicians a year in Washington, Public Citizen said.
In an email to this news organization, Dr. Oshel said that the Public Citizen analysis focused on the number of licensed physicians in each state and D.C. that can be obtained and compared reliably. It avoided using the term “practicing physicians” owing in part to doubts about the reliability of these counts, he said.
As many as 20% of physicians nationwide are focused primarily on work outside of clinical care, Dr. Oshel estimated. In D.C., perhaps 40% of physicians may fall into this category. Of the more than 13,700 physicians licensed in D.C., there may be only about 8,126 actively practicing, according to Dr. Oshel.
But even using that lower estimate of practicing physicians would only raise D.C.’s ranking to 46, signaling a need for stepped-up enforcement, Dr. Oshel said.
“[Whether it’s] 46th or 51st, both are bad,” Dr. Oshel said.
A version of this article first appeared on Medscape.com.
New COVID strain may evade vaccines, alarming health officials
The strain is called BA.2.86 and is of particular concern because of its more than 30 mutations, which means it may behave very differently than previous versions of the virus. That number of mutations is on par with the difference between variants so serious that they were formally named, such as between Delta and Omicron, the CDC explained in the risk assessment issued Aug. 23.
Worldwide, health agencies are issuing a flurry of updates on BA.2.86. The strain only recently landed on the World Health Organization’s radar when it was named a “variant under monitoring” on Aug. 17. The CDC announced the same day that it had been detected in the United States.
Among the characteristics the CDC monitors for are how contagious a strain is, how well it responds to treatment, and how severely it affects people.
“BA.2.86 may be more capable of causing infection in people who have previously had COVID-19 or who have received COVID-19 vaccines,” the CDC risk assessment stated.
The agency is evaluating how well the forthcoming updated vaccine, due out in September, performs against BA.2.86.
A new forecast also released this week by the CDC predicts hospitalizations due to the virus will continue their upward trend through at least mid-September. Currently, about 1,800 people are hospitalized daily with COVID-19. The new prediction shows that number has a small potential to drop as low as 1,100 daily, but it could also increase by as many as 7,500 per day. The most likely scenario lands somewhere in the middle of that range, with daily hospital admissions of between 2,000 and 4,000 people by Sept. 18.
The CDC said there is “no evidence” that BA.2.86 is causing more severe illness but said that could change as more information becomes available. Health experts typically gauge severity by the rate of COVID hospitalizations.
The journal Nature reported that many scientists see similarities between the emergence of BA.2.86 and that of Omicron, which rapidly spread around the world in late 2021.
“There’s a little bit of déjà vu all over again,” University of Michigan virologist Adam Lauring, MD, PhD, whose lab detected one of the first U.S. cases of BA.2.86, told Nature.
Dr. Lauring, as well as the CDC and the WHO, all caution that more data is needed to truly understand the threat posed by BA.2.86.
“There’s good reason to think it won’t be like the Omicron wave, but it’s early days,” Dr. Lauring said.
A version of this article first appeared on Medscape.com.
The strain is called BA.2.86 and is of particular concern because of its more than 30 mutations, which means it may behave very differently than previous versions of the virus. That number of mutations is on par with the difference between variants so serious that they were formally named, such as between Delta and Omicron, the CDC explained in the risk assessment issued Aug. 23.
Worldwide, health agencies are issuing a flurry of updates on BA.2.86. The strain only recently landed on the World Health Organization’s radar when it was named a “variant under monitoring” on Aug. 17. The CDC announced the same day that it had been detected in the United States.
Among the characteristics the CDC monitors for are how contagious a strain is, how well it responds to treatment, and how severely it affects people.
“BA.2.86 may be more capable of causing infection in people who have previously had COVID-19 or who have received COVID-19 vaccines,” the CDC risk assessment stated.
The agency is evaluating how well the forthcoming updated vaccine, due out in September, performs against BA.2.86.
A new forecast also released this week by the CDC predicts hospitalizations due to the virus will continue their upward trend through at least mid-September. Currently, about 1,800 people are hospitalized daily with COVID-19. The new prediction shows that number has a small potential to drop as low as 1,100 daily, but it could also increase by as many as 7,500 per day. The most likely scenario lands somewhere in the middle of that range, with daily hospital admissions of between 2,000 and 4,000 people by Sept. 18.
The CDC said there is “no evidence” that BA.2.86 is causing more severe illness but said that could change as more information becomes available. Health experts typically gauge severity by the rate of COVID hospitalizations.
The journal Nature reported that many scientists see similarities between the emergence of BA.2.86 and that of Omicron, which rapidly spread around the world in late 2021.
“There’s a little bit of déjà vu all over again,” University of Michigan virologist Adam Lauring, MD, PhD, whose lab detected one of the first U.S. cases of BA.2.86, told Nature.
Dr. Lauring, as well as the CDC and the WHO, all caution that more data is needed to truly understand the threat posed by BA.2.86.
“There’s good reason to think it won’t be like the Omicron wave, but it’s early days,” Dr. Lauring said.
A version of this article first appeared on Medscape.com.
The strain is called BA.2.86 and is of particular concern because of its more than 30 mutations, which means it may behave very differently than previous versions of the virus. That number of mutations is on par with the difference between variants so serious that they were formally named, such as between Delta and Omicron, the CDC explained in the risk assessment issued Aug. 23.
Worldwide, health agencies are issuing a flurry of updates on BA.2.86. The strain only recently landed on the World Health Organization’s radar when it was named a “variant under monitoring” on Aug. 17. The CDC announced the same day that it had been detected in the United States.
Among the characteristics the CDC monitors for are how contagious a strain is, how well it responds to treatment, and how severely it affects people.
“BA.2.86 may be more capable of causing infection in people who have previously had COVID-19 or who have received COVID-19 vaccines,” the CDC risk assessment stated.
The agency is evaluating how well the forthcoming updated vaccine, due out in September, performs against BA.2.86.
A new forecast also released this week by the CDC predicts hospitalizations due to the virus will continue their upward trend through at least mid-September. Currently, about 1,800 people are hospitalized daily with COVID-19. The new prediction shows that number has a small potential to drop as low as 1,100 daily, but it could also increase by as many as 7,500 per day. The most likely scenario lands somewhere in the middle of that range, with daily hospital admissions of between 2,000 and 4,000 people by Sept. 18.
The CDC said there is “no evidence” that BA.2.86 is causing more severe illness but said that could change as more information becomes available. Health experts typically gauge severity by the rate of COVID hospitalizations.
The journal Nature reported that many scientists see similarities between the emergence of BA.2.86 and that of Omicron, which rapidly spread around the world in late 2021.
“There’s a little bit of déjà vu all over again,” University of Michigan virologist Adam Lauring, MD, PhD, whose lab detected one of the first U.S. cases of BA.2.86, told Nature.
Dr. Lauring, as well as the CDC and the WHO, all caution that more data is needed to truly understand the threat posed by BA.2.86.
“There’s good reason to think it won’t be like the Omicron wave, but it’s early days,” Dr. Lauring said.
A version of this article first appeared on Medscape.com.
Atopic dermatitis may be a risk factor for GBS colonization in pregnancy
suggest.
“The rate of GBS colonization among pregnant females with a history of AD has not been previously reported, but AD could be a risk factor for maternal carriage of GBS,” corresponding author David J. Margolis, MD, PhD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and colleagues wrote in the study, which was published as a letter to the editor online in the Journal of Investigative Dermatology. “GBS reporting in a large administrative database represents a unique opportunity to conduct a population-based evaluation of GBS carriage with AD. Understanding this association could expand our understanding of microbial changes associated with AD,” they noted.
To determine if an association between GBS and AD in pregnant women exists, the researchers performed a cross-sectional study using a random sample from an Optum administrative database of pregnant women who had vaginal deliveries between May of 2007 and September 2021. The primary outcome of interest was the presence of GBS based on American College of Obstetricians and Gynecologists–recommended codes for GBS during 36 0/7 to 37 6/7 weeks of pregnancy. They used descriptive statistics to summarize categorical and continuous variables as proportions and means, and logistic regression to examine the association between AD and GBS status.
The cohort included 566,467 pregnant women with an average age of 38.8 years. Of these, 2.9% had a diagnosis of AD or a history of AD, and 24.9% had diagnoses of asthma, seasonal allergies, or both. Women with AD had an increased odds ratio of asthma (OR, 2.55), seasonal allergies (OR, 3.39), or both (OR, 5.35), compared with those without AD.
GBS was reported in 20.6% of the cohort. The median time of follow-up for those with and without GBS was 494 days and 468 days, respectively (P = .134). Among the women with AD, 24.1% had GBS, compared with 20.51% of the women without AD (P <.0001), which translated into an OR of 1.23 (95% confidence interval, 1.18-1.27).
Among the women with GBS, the OR of asthma was 1.08 (95% CI, 1.06-1.10) and was 1.07 (95% CI, 1.05-1.09) among those with seasonal allergies. When adjusted for potential confounders, these findings did not change substantively.
“It is not apparent why pregnant females with AD are more likely to specifically carry GBS,” the authors wrote. “However, several studies have shown that individuals with AD are more likely to carry [Staphylococcus] aureus and that individuals with AD might be deficient in host defenses against S. aureus and other pathogens,” they added.
“Individuals with AD frequently receive antibiotics as part of their AD treatment and this might alter their resident microbiome. Carriage rates may be enhanced by the inhibition of an important barrier protein called filaggrin (FLG) and FLG loss of function genetic variation is known to decrease barrier proteins thought to inhibit the colonization of S. aureus and other pathogens,” the researchers wrote.
They acknowledged certain limitations of their study, including its reliance on an administrative database that does not contain information on past disease.
Asked to comment on the results, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the study, characterized AD as “the poster child for cutaneous dysbiosis – an altered petri dish, so to speak, [that] facilitates survival of the few, leading to decreased microbial diversity that can both enable potential pathogen invasion and immune dysregulation.”
Though it’s not surprising that pregnant AD patients have dysbiosis, the focus on GBS, “which can be a bad actor in the perinatal period, is an interesting connection,” he said. “Will this change practices? Pregnant women should be screened for GBS regardless, but maybe more attention or counseling can be offered to AD patients about the importance of screening. Would decolonization regimens be employed early in pregnancy? This study can’t answer that but certainly raises good questions.”
Dr. Margolis disclosed that he is or recently has been a consultant for Pfizer, Leo, and Sanofi with respect to studies of atopic dermatitis and served on an advisory board for the National Eczema Association. Another author disclosed receiving grants from companies related to work with AD; other authors had no disclosures. Dr. Friedman reported having no relevant disclosures.
suggest.
“The rate of GBS colonization among pregnant females with a history of AD has not been previously reported, but AD could be a risk factor for maternal carriage of GBS,” corresponding author David J. Margolis, MD, PhD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and colleagues wrote in the study, which was published as a letter to the editor online in the Journal of Investigative Dermatology. “GBS reporting in a large administrative database represents a unique opportunity to conduct a population-based evaluation of GBS carriage with AD. Understanding this association could expand our understanding of microbial changes associated with AD,” they noted.
To determine if an association between GBS and AD in pregnant women exists, the researchers performed a cross-sectional study using a random sample from an Optum administrative database of pregnant women who had vaginal deliveries between May of 2007 and September 2021. The primary outcome of interest was the presence of GBS based on American College of Obstetricians and Gynecologists–recommended codes for GBS during 36 0/7 to 37 6/7 weeks of pregnancy. They used descriptive statistics to summarize categorical and continuous variables as proportions and means, and logistic regression to examine the association between AD and GBS status.
The cohort included 566,467 pregnant women with an average age of 38.8 years. Of these, 2.9% had a diagnosis of AD or a history of AD, and 24.9% had diagnoses of asthma, seasonal allergies, or both. Women with AD had an increased odds ratio of asthma (OR, 2.55), seasonal allergies (OR, 3.39), or both (OR, 5.35), compared with those without AD.
GBS was reported in 20.6% of the cohort. The median time of follow-up for those with and without GBS was 494 days and 468 days, respectively (P = .134). Among the women with AD, 24.1% had GBS, compared with 20.51% of the women without AD (P <.0001), which translated into an OR of 1.23 (95% confidence interval, 1.18-1.27).
Among the women with GBS, the OR of asthma was 1.08 (95% CI, 1.06-1.10) and was 1.07 (95% CI, 1.05-1.09) among those with seasonal allergies. When adjusted for potential confounders, these findings did not change substantively.
“It is not apparent why pregnant females with AD are more likely to specifically carry GBS,” the authors wrote. “However, several studies have shown that individuals with AD are more likely to carry [Staphylococcus] aureus and that individuals with AD might be deficient in host defenses against S. aureus and other pathogens,” they added.
“Individuals with AD frequently receive antibiotics as part of their AD treatment and this might alter their resident microbiome. Carriage rates may be enhanced by the inhibition of an important barrier protein called filaggrin (FLG) and FLG loss of function genetic variation is known to decrease barrier proteins thought to inhibit the colonization of S. aureus and other pathogens,” the researchers wrote.
They acknowledged certain limitations of their study, including its reliance on an administrative database that does not contain information on past disease.
Asked to comment on the results, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the study, characterized AD as “the poster child for cutaneous dysbiosis – an altered petri dish, so to speak, [that] facilitates survival of the few, leading to decreased microbial diversity that can both enable potential pathogen invasion and immune dysregulation.”
Though it’s not surprising that pregnant AD patients have dysbiosis, the focus on GBS, “which can be a bad actor in the perinatal period, is an interesting connection,” he said. “Will this change practices? Pregnant women should be screened for GBS regardless, but maybe more attention or counseling can be offered to AD patients about the importance of screening. Would decolonization regimens be employed early in pregnancy? This study can’t answer that but certainly raises good questions.”
Dr. Margolis disclosed that he is or recently has been a consultant for Pfizer, Leo, and Sanofi with respect to studies of atopic dermatitis and served on an advisory board for the National Eczema Association. Another author disclosed receiving grants from companies related to work with AD; other authors had no disclosures. Dr. Friedman reported having no relevant disclosures.
suggest.
“The rate of GBS colonization among pregnant females with a history of AD has not been previously reported, but AD could be a risk factor for maternal carriage of GBS,” corresponding author David J. Margolis, MD, PhD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and colleagues wrote in the study, which was published as a letter to the editor online in the Journal of Investigative Dermatology. “GBS reporting in a large administrative database represents a unique opportunity to conduct a population-based evaluation of GBS carriage with AD. Understanding this association could expand our understanding of microbial changes associated with AD,” they noted.
To determine if an association between GBS and AD in pregnant women exists, the researchers performed a cross-sectional study using a random sample from an Optum administrative database of pregnant women who had vaginal deliveries between May of 2007 and September 2021. The primary outcome of interest was the presence of GBS based on American College of Obstetricians and Gynecologists–recommended codes for GBS during 36 0/7 to 37 6/7 weeks of pregnancy. They used descriptive statistics to summarize categorical and continuous variables as proportions and means, and logistic regression to examine the association between AD and GBS status.
The cohort included 566,467 pregnant women with an average age of 38.8 years. Of these, 2.9% had a diagnosis of AD or a history of AD, and 24.9% had diagnoses of asthma, seasonal allergies, or both. Women with AD had an increased odds ratio of asthma (OR, 2.55), seasonal allergies (OR, 3.39), or both (OR, 5.35), compared with those without AD.
GBS was reported in 20.6% of the cohort. The median time of follow-up for those with and without GBS was 494 days and 468 days, respectively (P = .134). Among the women with AD, 24.1% had GBS, compared with 20.51% of the women without AD (P <.0001), which translated into an OR of 1.23 (95% confidence interval, 1.18-1.27).
Among the women with GBS, the OR of asthma was 1.08 (95% CI, 1.06-1.10) and was 1.07 (95% CI, 1.05-1.09) among those with seasonal allergies. When adjusted for potential confounders, these findings did not change substantively.
“It is not apparent why pregnant females with AD are more likely to specifically carry GBS,” the authors wrote. “However, several studies have shown that individuals with AD are more likely to carry [Staphylococcus] aureus and that individuals with AD might be deficient in host defenses against S. aureus and other pathogens,” they added.
“Individuals with AD frequently receive antibiotics as part of their AD treatment and this might alter their resident microbiome. Carriage rates may be enhanced by the inhibition of an important barrier protein called filaggrin (FLG) and FLG loss of function genetic variation is known to decrease barrier proteins thought to inhibit the colonization of S. aureus and other pathogens,” the researchers wrote.
They acknowledged certain limitations of their study, including its reliance on an administrative database that does not contain information on past disease.
Asked to comment on the results, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the study, characterized AD as “the poster child for cutaneous dysbiosis – an altered petri dish, so to speak, [that] facilitates survival of the few, leading to decreased microbial diversity that can both enable potential pathogen invasion and immune dysregulation.”
Though it’s not surprising that pregnant AD patients have dysbiosis, the focus on GBS, “which can be a bad actor in the perinatal period, is an interesting connection,” he said. “Will this change practices? Pregnant women should be screened for GBS regardless, but maybe more attention or counseling can be offered to AD patients about the importance of screening. Would decolonization regimens be employed early in pregnancy? This study can’t answer that but certainly raises good questions.”
Dr. Margolis disclosed that he is or recently has been a consultant for Pfizer, Leo, and Sanofi with respect to studies of atopic dermatitis and served on an advisory board for the National Eczema Association. Another author disclosed receiving grants from companies related to work with AD; other authors had no disclosures. Dr. Friedman reported having no relevant disclosures.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Mohs found to confer survival benefit in localized Merkel cell carcinoma
results from a national retrospective cohort study suggest.
The study found that, in patients with pathologically confirmed, localized T1/T2 MCC, “treatment with MMS was associated with an approximately 40% reduction in hazard of death compared with WLE,” reported John A. Carucci, MD, PhD, and colleagues in the department of dermatology at NYU Langone Health, New York. The results provide “preliminary data suggesting that treatment of localized, early-stage MCC with MMS may result in the most optimal patient survival outcomes for this aggressive form of skin cancer,” they added. The study was published online in JAMA Dermatology.
“Although data for keratinocytic nonmelanoma skin cancers have been definitive in demonstrating the advantage of peripheral and deep en face margin assessment over conventional WLE or NME [narrow-margin excision], the data for MCC, likely because of the disease’s rarity and limitations of available data sets, have been mixed,” they wrote.
Results from national studies published in the Journal of the National Cancer Institute and the Journal of the American Academy of Dermatology found no difference in survival among patients with localized MCC treated with WLE versus MMS. “However, these studies did not have confirmed pathologic node status, a substantial limitation considering that clinically node-negative cases of localized MCC have sentinel lymph node positivity rates ranging from 25% to 40%,” the authors noted.
To evaluate the association of the surgical excision modality and patient survival for pathologically confirmed localized T1/T2 MCC, Dr. Carucci and coauthors examined a cohort of 2,313 patients from the National Cancer Database with T1/T2 MCC diagnosed between Jan. 1, 2004, and Dec. 31, 2018, with pathologically confirmed, negative regional lymph nodes and treated with surgery. Their mean age was 71 years and 57.9% were male. Of the 2,313 patients, 1,452 underwent WLE, 104 underwent MMS, and 757 underwent NME.
The unadjusted analysis revealed that, compared with WLE, excision with MMS had the best unadjusted mean survival rates: 87.4% versus 86.1%, respectively, at 3 years, 84.5% versus 76.9% at 5 years, and 81.8% versus 60.9% at 10 years. Patients treated with NME had similar mean survival rates as those treated with WLE: 84.8% at 3 years, 78.3% at 5 years, and 60.8% at 10 years.
Multivariable survival analysis demonstrated that treatment with MMS was associated with significantly improved survival, compared with WLE (hazard ratio, 0.59; 95% CI, 0.36-0.97; P = .04).
“These data suggest that MMS may provide a survival benefit in the treatment of localized MCC, although further prospective work studying this issue is required,” the authors concluded. “Future directions may also focus on elucidating the benefit of adjuvant radiotherapy in localized cases treated with MMS.”
They acknowledged certain limitations of the study, including the fewer numbers of patients receiving MMS surgery, lack of randomization, and potential for selection bias.
In an interview, Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the study, said that the field of MCC “has undergone rapid and robust transformation over the past 20 years. These changes encompass advancements in diagnosing the condition, identifying linked viruses, and developing systemic treatments.”
The study findings “imply that comprehensive assessment of histologic margins might offer advantages beyond minimizing scars, minimizing functional impact, and reducing the likelihood of local recurrence,” he said.
“It’s beyond doubt,” he added, that the study “furnishes us with yet another set of real-world insights that will undoubtedly influence patient outcomes. These insights serve to bring clarity to the ways in which we can deliver precisely targeted surgical treatment with durable outcomes for localized MCC.”
Patricia M. Richey, MD, director of Mohs surgery at Boston University, who was also asked to comment on the study, added that, because of the nature of the National Cancer Database, “the authors of this study were unfortunately unable to report disease-specific survival or immunosuppression status. That being said, the preliminary data presented are convincing and should result in us further exploring this topic, as well as readdressing and questioning related issues such as whether or not adjuvant radiotherapy is truly beneficial in cases with histologic clearance via Mohs.”
Dr. Carucci reported receiving grant funding from Regeneron for investigator-initiated basic research. No other author disclosures were reported. Neither Dr. Blalock nor Dr. Richey had relevant disclosures.
results from a national retrospective cohort study suggest.
The study found that, in patients with pathologically confirmed, localized T1/T2 MCC, “treatment with MMS was associated with an approximately 40% reduction in hazard of death compared with WLE,” reported John A. Carucci, MD, PhD, and colleagues in the department of dermatology at NYU Langone Health, New York. The results provide “preliminary data suggesting that treatment of localized, early-stage MCC with MMS may result in the most optimal patient survival outcomes for this aggressive form of skin cancer,” they added. The study was published online in JAMA Dermatology.
“Although data for keratinocytic nonmelanoma skin cancers have been definitive in demonstrating the advantage of peripheral and deep en face margin assessment over conventional WLE or NME [narrow-margin excision], the data for MCC, likely because of the disease’s rarity and limitations of available data sets, have been mixed,” they wrote.
Results from national studies published in the Journal of the National Cancer Institute and the Journal of the American Academy of Dermatology found no difference in survival among patients with localized MCC treated with WLE versus MMS. “However, these studies did not have confirmed pathologic node status, a substantial limitation considering that clinically node-negative cases of localized MCC have sentinel lymph node positivity rates ranging from 25% to 40%,” the authors noted.
To evaluate the association of the surgical excision modality and patient survival for pathologically confirmed localized T1/T2 MCC, Dr. Carucci and coauthors examined a cohort of 2,313 patients from the National Cancer Database with T1/T2 MCC diagnosed between Jan. 1, 2004, and Dec. 31, 2018, with pathologically confirmed, negative regional lymph nodes and treated with surgery. Their mean age was 71 years and 57.9% were male. Of the 2,313 patients, 1,452 underwent WLE, 104 underwent MMS, and 757 underwent NME.
The unadjusted analysis revealed that, compared with WLE, excision with MMS had the best unadjusted mean survival rates: 87.4% versus 86.1%, respectively, at 3 years, 84.5% versus 76.9% at 5 years, and 81.8% versus 60.9% at 10 years. Patients treated with NME had similar mean survival rates as those treated with WLE: 84.8% at 3 years, 78.3% at 5 years, and 60.8% at 10 years.
Multivariable survival analysis demonstrated that treatment with MMS was associated with significantly improved survival, compared with WLE (hazard ratio, 0.59; 95% CI, 0.36-0.97; P = .04).
“These data suggest that MMS may provide a survival benefit in the treatment of localized MCC, although further prospective work studying this issue is required,” the authors concluded. “Future directions may also focus on elucidating the benefit of adjuvant radiotherapy in localized cases treated with MMS.”
They acknowledged certain limitations of the study, including the fewer numbers of patients receiving MMS surgery, lack of randomization, and potential for selection bias.
In an interview, Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the study, said that the field of MCC “has undergone rapid and robust transformation over the past 20 years. These changes encompass advancements in diagnosing the condition, identifying linked viruses, and developing systemic treatments.”
The study findings “imply that comprehensive assessment of histologic margins might offer advantages beyond minimizing scars, minimizing functional impact, and reducing the likelihood of local recurrence,” he said.
“It’s beyond doubt,” he added, that the study “furnishes us with yet another set of real-world insights that will undoubtedly influence patient outcomes. These insights serve to bring clarity to the ways in which we can deliver precisely targeted surgical treatment with durable outcomes for localized MCC.”
Patricia M. Richey, MD, director of Mohs surgery at Boston University, who was also asked to comment on the study, added that, because of the nature of the National Cancer Database, “the authors of this study were unfortunately unable to report disease-specific survival or immunosuppression status. That being said, the preliminary data presented are convincing and should result in us further exploring this topic, as well as readdressing and questioning related issues such as whether or not adjuvant radiotherapy is truly beneficial in cases with histologic clearance via Mohs.”
Dr. Carucci reported receiving grant funding from Regeneron for investigator-initiated basic research. No other author disclosures were reported. Neither Dr. Blalock nor Dr. Richey had relevant disclosures.
results from a national retrospective cohort study suggest.
The study found that, in patients with pathologically confirmed, localized T1/T2 MCC, “treatment with MMS was associated with an approximately 40% reduction in hazard of death compared with WLE,” reported John A. Carucci, MD, PhD, and colleagues in the department of dermatology at NYU Langone Health, New York. The results provide “preliminary data suggesting that treatment of localized, early-stage MCC with MMS may result in the most optimal patient survival outcomes for this aggressive form of skin cancer,” they added. The study was published online in JAMA Dermatology.
“Although data for keratinocytic nonmelanoma skin cancers have been definitive in demonstrating the advantage of peripheral and deep en face margin assessment over conventional WLE or NME [narrow-margin excision], the data for MCC, likely because of the disease’s rarity and limitations of available data sets, have been mixed,” they wrote.
Results from national studies published in the Journal of the National Cancer Institute and the Journal of the American Academy of Dermatology found no difference in survival among patients with localized MCC treated with WLE versus MMS. “However, these studies did not have confirmed pathologic node status, a substantial limitation considering that clinically node-negative cases of localized MCC have sentinel lymph node positivity rates ranging from 25% to 40%,” the authors noted.
To evaluate the association of the surgical excision modality and patient survival for pathologically confirmed localized T1/T2 MCC, Dr. Carucci and coauthors examined a cohort of 2,313 patients from the National Cancer Database with T1/T2 MCC diagnosed between Jan. 1, 2004, and Dec. 31, 2018, with pathologically confirmed, negative regional lymph nodes and treated with surgery. Their mean age was 71 years and 57.9% were male. Of the 2,313 patients, 1,452 underwent WLE, 104 underwent MMS, and 757 underwent NME.
The unadjusted analysis revealed that, compared with WLE, excision with MMS had the best unadjusted mean survival rates: 87.4% versus 86.1%, respectively, at 3 years, 84.5% versus 76.9% at 5 years, and 81.8% versus 60.9% at 10 years. Patients treated with NME had similar mean survival rates as those treated with WLE: 84.8% at 3 years, 78.3% at 5 years, and 60.8% at 10 years.
Multivariable survival analysis demonstrated that treatment with MMS was associated with significantly improved survival, compared with WLE (hazard ratio, 0.59; 95% CI, 0.36-0.97; P = .04).
“These data suggest that MMS may provide a survival benefit in the treatment of localized MCC, although further prospective work studying this issue is required,” the authors concluded. “Future directions may also focus on elucidating the benefit of adjuvant radiotherapy in localized cases treated with MMS.”
They acknowledged certain limitations of the study, including the fewer numbers of patients receiving MMS surgery, lack of randomization, and potential for selection bias.
In an interview, Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the study, said that the field of MCC “has undergone rapid and robust transformation over the past 20 years. These changes encompass advancements in diagnosing the condition, identifying linked viruses, and developing systemic treatments.”
The study findings “imply that comprehensive assessment of histologic margins might offer advantages beyond minimizing scars, minimizing functional impact, and reducing the likelihood of local recurrence,” he said.
“It’s beyond doubt,” he added, that the study “furnishes us with yet another set of real-world insights that will undoubtedly influence patient outcomes. These insights serve to bring clarity to the ways in which we can deliver precisely targeted surgical treatment with durable outcomes for localized MCC.”
Patricia M. Richey, MD, director of Mohs surgery at Boston University, who was also asked to comment on the study, added that, because of the nature of the National Cancer Database, “the authors of this study were unfortunately unable to report disease-specific survival or immunosuppression status. That being said, the preliminary data presented are convincing and should result in us further exploring this topic, as well as readdressing and questioning related issues such as whether or not adjuvant radiotherapy is truly beneficial in cases with histologic clearance via Mohs.”
Dr. Carucci reported receiving grant funding from Regeneron for investigator-initiated basic research. No other author disclosures were reported. Neither Dr. Blalock nor Dr. Richey had relevant disclosures.
FROM JAMA DERMATOLOGY
Commentary: Newer Drugs for AD Plus Dupilumab and Other Issues, September 2023
Amlitelimab is a monoclonal antibody that targets the OX40 ligand (Weidinger et al). It is predicted to have broad potential therapeutic application for multiple immune diseases, including atopic dermatitis. I'm not looking for that. I've been spoiled by drugs that have narrow therapeutic application (like IL-23 blockade and IL-4/IL-13 blockade) that target a specific disease very effectively with very little in the way of side effects.
The OX40 ligand/receptor interaction may be too important. When I Google "OX40 deficiency," the first thing that pops up is a combined T- and B-cell immunodeficiency associated with possible aggressive, childhood-onset, disseminated, cutaneous, and systemic Kaposi sarcoma. That doesn't mean that such a horrible outcome will come with the level of pharmacologic OX40 blockade that we would try to achieve in our patients. Clinical trials don't show horrible adverse events — so far. I'm in no hurry to find out in my patients whether real-life efficacy in large numbers of people treated for long periods of time matches up with the short-term safety profiles seen in relatively small clinical trial populations.
It might be nice to give patients upadacitinib only as needed, for example for a flare of their atopic dermatitis, then cut down the dose or stop altogether until the next flare. The study by Guttman-Yassky and colleagues found that atopic dermatitis came back quickly when upadacitinib was stopped. However, their study looked at patients with chronically bad atopic dermatitis. If we have a patient who tends to flare only intermittently, it may be that we could use upadacitinib or other systemic treatments on an intermittent basis. I know when it came to my son's mild atopic dermatitis, intermittent use of a little triamcinolone ointment was all that was needed. Yes, I know that's a "reactive," roller-coaster approach. Yes, I know that a "proactive" keep-the-disease-away approach sounds better. But I'm realistic when it comes to patients' adherence behaviors. I think there's a lot to be said for minimizing drug exposure and just using treatments as needed. Guttman-Yassky's work makes me believe that a lot of patients will need continuous treatment to keep their severe disease under control. I'm not convinced that everyone will need continuous treatment to be happy with their treatment.
O'Connor and colleagues found that emollient bathing is associated with later development of atopic dermatitis. They defined emollient bathing as baths with oil or emulsifier-based additives. This study illustrates the importance of randomization in a controlled trial. Because their study was not randomized, we don't know whether the emollient bathing caused atopic dermatitis or whether families that had more dry skin or more family history of atopic dermatitis were more likely to use emollient bathing.
When dupilumab was first approved, I prescribed it to my patients to take every 2 weeks as recommended on the label. I'm not so sure how many patients actually used it that way. I suspect that a lot of them took the medicine less often than recommended, especially when they were doing well. This report by Sánchez-García and colleagues suggests that patients who are doing very well on dupilumab may be able to take the drug less often. That's probably not news to my patients who are already taking the drug less often than I told them to.
I think less frequent dosing may become even more common over time, particularly for drugs that may have more safety risks than dupilumab. Many patients with atopic dermatitis probably don't need to be taking drugs all the time. Patients who tend to have flare-ups but who do very well for a long period of time between flares may only need drugs intermittently. It will be interesting to see if our patients can use oral treatments for atopic dermatitis that way.
Siegfried and colleagues assessed how well dupilumab worked in children with atopic dermatitis in different areas of the body: head and neck, trunk, upper extremities, lower extremities. Dupilumab worked well in all these areas, as expected.
Xu and colleagues did a meta-analysis of studies of dupilumab for atopic dermatitis and concluded, not shockingly, that dupilumab is safe and effective for atopic dermatitis. Okay, I believe that. They further concluded: "More long-term, high-quality, controlled studies in different regions are needed for further verification." I don't think so. I think the evidence is clear already.
Studies that measure the levels of things are generally not particularly helpful. The study by García-Reyes and colleagues studied the levels of serum thymic stromal lymphopoietin (TSLP) in patients with atopic dermatitis. TSLP levels were higher in patients with atopic dermatitis compared with patients without atopic dermatitis. This basically tells us nothing about the role of TSLP in atopic dermatitis. The elevated levels could be causing atopic dermatitis or they could be the body's response to having atopic dermatitis.
To tell whether something is causal we have to look at either genetic studies or studies with specific inhibitors. A specific inhibitor study was done by atopic dermatitis expert Eric Simpson and colleagues.1 This was a randomized, placebo-controlled study in which an anti-TSLP antibody was given to patients with atopic dermatitis. Both the anti-TSLP antibody and placebo groups were permitted to use topical steroids. While the anti-TSLP antibody–treated patients did better than placebo-treated patients, the difference did not achieve statistical significance, probably, I believe, because the placebo-treated patients used more topical steroids. When you want to assess whether a drug for atopic dermatitis is better than placebo, you must be careful about how much topical steroid you let patients in the study use!
Additional Reference
- Simpson EL, Parnes JR, She D, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80(4):1013-1021. doi: 10.1016/j.jaad.2018.11.059
Amlitelimab is a monoclonal antibody that targets the OX40 ligand (Weidinger et al). It is predicted to have broad potential therapeutic application for multiple immune diseases, including atopic dermatitis. I'm not looking for that. I've been spoiled by drugs that have narrow therapeutic application (like IL-23 blockade and IL-4/IL-13 blockade) that target a specific disease very effectively with very little in the way of side effects.
The OX40 ligand/receptor interaction may be too important. When I Google "OX40 deficiency," the first thing that pops up is a combined T- and B-cell immunodeficiency associated with possible aggressive, childhood-onset, disseminated, cutaneous, and systemic Kaposi sarcoma. That doesn't mean that such a horrible outcome will come with the level of pharmacologic OX40 blockade that we would try to achieve in our patients. Clinical trials don't show horrible adverse events — so far. I'm in no hurry to find out in my patients whether real-life efficacy in large numbers of people treated for long periods of time matches up with the short-term safety profiles seen in relatively small clinical trial populations.
It might be nice to give patients upadacitinib only as needed, for example for a flare of their atopic dermatitis, then cut down the dose or stop altogether until the next flare. The study by Guttman-Yassky and colleagues found that atopic dermatitis came back quickly when upadacitinib was stopped. However, their study looked at patients with chronically bad atopic dermatitis. If we have a patient who tends to flare only intermittently, it may be that we could use upadacitinib or other systemic treatments on an intermittent basis. I know when it came to my son's mild atopic dermatitis, intermittent use of a little triamcinolone ointment was all that was needed. Yes, I know that's a "reactive," roller-coaster approach. Yes, I know that a "proactive" keep-the-disease-away approach sounds better. But I'm realistic when it comes to patients' adherence behaviors. I think there's a lot to be said for minimizing drug exposure and just using treatments as needed. Guttman-Yassky's work makes me believe that a lot of patients will need continuous treatment to keep their severe disease under control. I'm not convinced that everyone will need continuous treatment to be happy with their treatment.
O'Connor and colleagues found that emollient bathing is associated with later development of atopic dermatitis. They defined emollient bathing as baths with oil or emulsifier-based additives. This study illustrates the importance of randomization in a controlled trial. Because their study was not randomized, we don't know whether the emollient bathing caused atopic dermatitis or whether families that had more dry skin or more family history of atopic dermatitis were more likely to use emollient bathing.
When dupilumab was first approved, I prescribed it to my patients to take every 2 weeks as recommended on the label. I'm not so sure how many patients actually used it that way. I suspect that a lot of them took the medicine less often than recommended, especially when they were doing well. This report by Sánchez-García and colleagues suggests that patients who are doing very well on dupilumab may be able to take the drug less often. That's probably not news to my patients who are already taking the drug less often than I told them to.
I think less frequent dosing may become even more common over time, particularly for drugs that may have more safety risks than dupilumab. Many patients with atopic dermatitis probably don't need to be taking drugs all the time. Patients who tend to have flare-ups but who do very well for a long period of time between flares may only need drugs intermittently. It will be interesting to see if our patients can use oral treatments for atopic dermatitis that way.
Siegfried and colleagues assessed how well dupilumab worked in children with atopic dermatitis in different areas of the body: head and neck, trunk, upper extremities, lower extremities. Dupilumab worked well in all these areas, as expected.
Xu and colleagues did a meta-analysis of studies of dupilumab for atopic dermatitis and concluded, not shockingly, that dupilumab is safe and effective for atopic dermatitis. Okay, I believe that. They further concluded: "More long-term, high-quality, controlled studies in different regions are needed for further verification." I don't think so. I think the evidence is clear already.
Studies that measure the levels of things are generally not particularly helpful. The study by García-Reyes and colleagues studied the levels of serum thymic stromal lymphopoietin (TSLP) in patients with atopic dermatitis. TSLP levels were higher in patients with atopic dermatitis compared with patients without atopic dermatitis. This basically tells us nothing about the role of TSLP in atopic dermatitis. The elevated levels could be causing atopic dermatitis or they could be the body's response to having atopic dermatitis.
To tell whether something is causal we have to look at either genetic studies or studies with specific inhibitors. A specific inhibitor study was done by atopic dermatitis expert Eric Simpson and colleagues.1 This was a randomized, placebo-controlled study in which an anti-TSLP antibody was given to patients with atopic dermatitis. Both the anti-TSLP antibody and placebo groups were permitted to use topical steroids. While the anti-TSLP antibody–treated patients did better than placebo-treated patients, the difference did not achieve statistical significance, probably, I believe, because the placebo-treated patients used more topical steroids. When you want to assess whether a drug for atopic dermatitis is better than placebo, you must be careful about how much topical steroid you let patients in the study use!
Additional Reference
- Simpson EL, Parnes JR, She D, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80(4):1013-1021. doi: 10.1016/j.jaad.2018.11.059
Amlitelimab is a monoclonal antibody that targets the OX40 ligand (Weidinger et al). It is predicted to have broad potential therapeutic application for multiple immune diseases, including atopic dermatitis. I'm not looking for that. I've been spoiled by drugs that have narrow therapeutic application (like IL-23 blockade and IL-4/IL-13 blockade) that target a specific disease very effectively with very little in the way of side effects.
The OX40 ligand/receptor interaction may be too important. When I Google "OX40 deficiency," the first thing that pops up is a combined T- and B-cell immunodeficiency associated with possible aggressive, childhood-onset, disseminated, cutaneous, and systemic Kaposi sarcoma. That doesn't mean that such a horrible outcome will come with the level of pharmacologic OX40 blockade that we would try to achieve in our patients. Clinical trials don't show horrible adverse events — so far. I'm in no hurry to find out in my patients whether real-life efficacy in large numbers of people treated for long periods of time matches up with the short-term safety profiles seen in relatively small clinical trial populations.
It might be nice to give patients upadacitinib only as needed, for example for a flare of their atopic dermatitis, then cut down the dose or stop altogether until the next flare. The study by Guttman-Yassky and colleagues found that atopic dermatitis came back quickly when upadacitinib was stopped. However, their study looked at patients with chronically bad atopic dermatitis. If we have a patient who tends to flare only intermittently, it may be that we could use upadacitinib or other systemic treatments on an intermittent basis. I know when it came to my son's mild atopic dermatitis, intermittent use of a little triamcinolone ointment was all that was needed. Yes, I know that's a "reactive," roller-coaster approach. Yes, I know that a "proactive" keep-the-disease-away approach sounds better. But I'm realistic when it comes to patients' adherence behaviors. I think there's a lot to be said for minimizing drug exposure and just using treatments as needed. Guttman-Yassky's work makes me believe that a lot of patients will need continuous treatment to keep their severe disease under control. I'm not convinced that everyone will need continuous treatment to be happy with their treatment.
O'Connor and colleagues found that emollient bathing is associated with later development of atopic dermatitis. They defined emollient bathing as baths with oil or emulsifier-based additives. This study illustrates the importance of randomization in a controlled trial. Because their study was not randomized, we don't know whether the emollient bathing caused atopic dermatitis or whether families that had more dry skin or more family history of atopic dermatitis were more likely to use emollient bathing.
When dupilumab was first approved, I prescribed it to my patients to take every 2 weeks as recommended on the label. I'm not so sure how many patients actually used it that way. I suspect that a lot of them took the medicine less often than recommended, especially when they were doing well. This report by Sánchez-García and colleagues suggests that patients who are doing very well on dupilumab may be able to take the drug less often. That's probably not news to my patients who are already taking the drug less often than I told them to.
I think less frequent dosing may become even more common over time, particularly for drugs that may have more safety risks than dupilumab. Many patients with atopic dermatitis probably don't need to be taking drugs all the time. Patients who tend to have flare-ups but who do very well for a long period of time between flares may only need drugs intermittently. It will be interesting to see if our patients can use oral treatments for atopic dermatitis that way.
Siegfried and colleagues assessed how well dupilumab worked in children with atopic dermatitis in different areas of the body: head and neck, trunk, upper extremities, lower extremities. Dupilumab worked well in all these areas, as expected.
Xu and colleagues did a meta-analysis of studies of dupilumab for atopic dermatitis and concluded, not shockingly, that dupilumab is safe and effective for atopic dermatitis. Okay, I believe that. They further concluded: "More long-term, high-quality, controlled studies in different regions are needed for further verification." I don't think so. I think the evidence is clear already.
Studies that measure the levels of things are generally not particularly helpful. The study by García-Reyes and colleagues studied the levels of serum thymic stromal lymphopoietin (TSLP) in patients with atopic dermatitis. TSLP levels were higher in patients with atopic dermatitis compared with patients without atopic dermatitis. This basically tells us nothing about the role of TSLP in atopic dermatitis. The elevated levels could be causing atopic dermatitis or they could be the body's response to having atopic dermatitis.
To tell whether something is causal we have to look at either genetic studies or studies with specific inhibitors. A specific inhibitor study was done by atopic dermatitis expert Eric Simpson and colleagues.1 This was a randomized, placebo-controlled study in which an anti-TSLP antibody was given to patients with atopic dermatitis. Both the anti-TSLP antibody and placebo groups were permitted to use topical steroids. While the anti-TSLP antibody–treated patients did better than placebo-treated patients, the difference did not achieve statistical significance, probably, I believe, because the placebo-treated patients used more topical steroids. When you want to assess whether a drug for atopic dermatitis is better than placebo, you must be careful about how much topical steroid you let patients in the study use!
Additional Reference
- Simpson EL, Parnes JR, She D, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80(4):1013-1021. doi: 10.1016/j.jaad.2018.11.059
Financial Insecurity Among US Adults With Psoriasis
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.
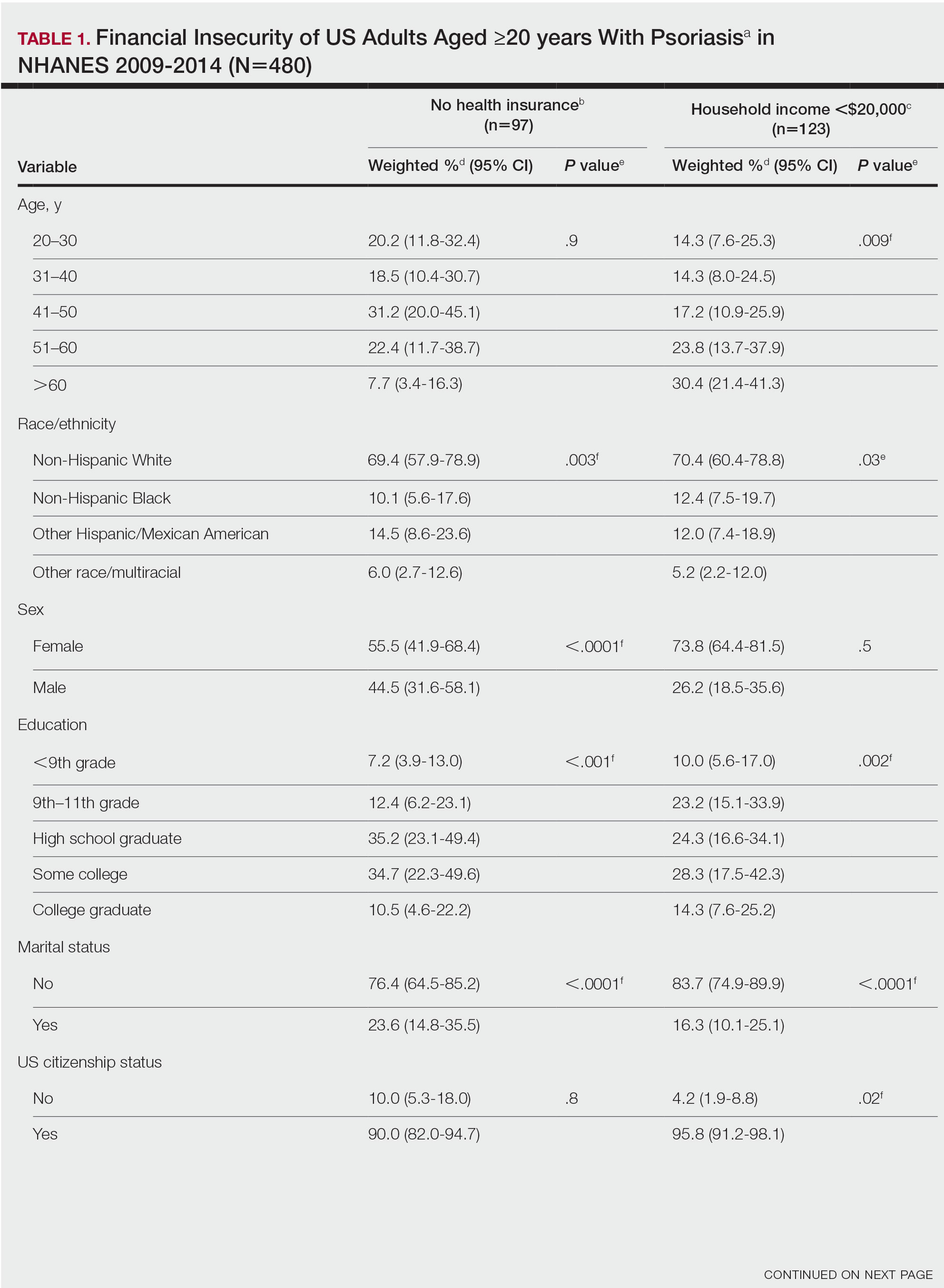

Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).
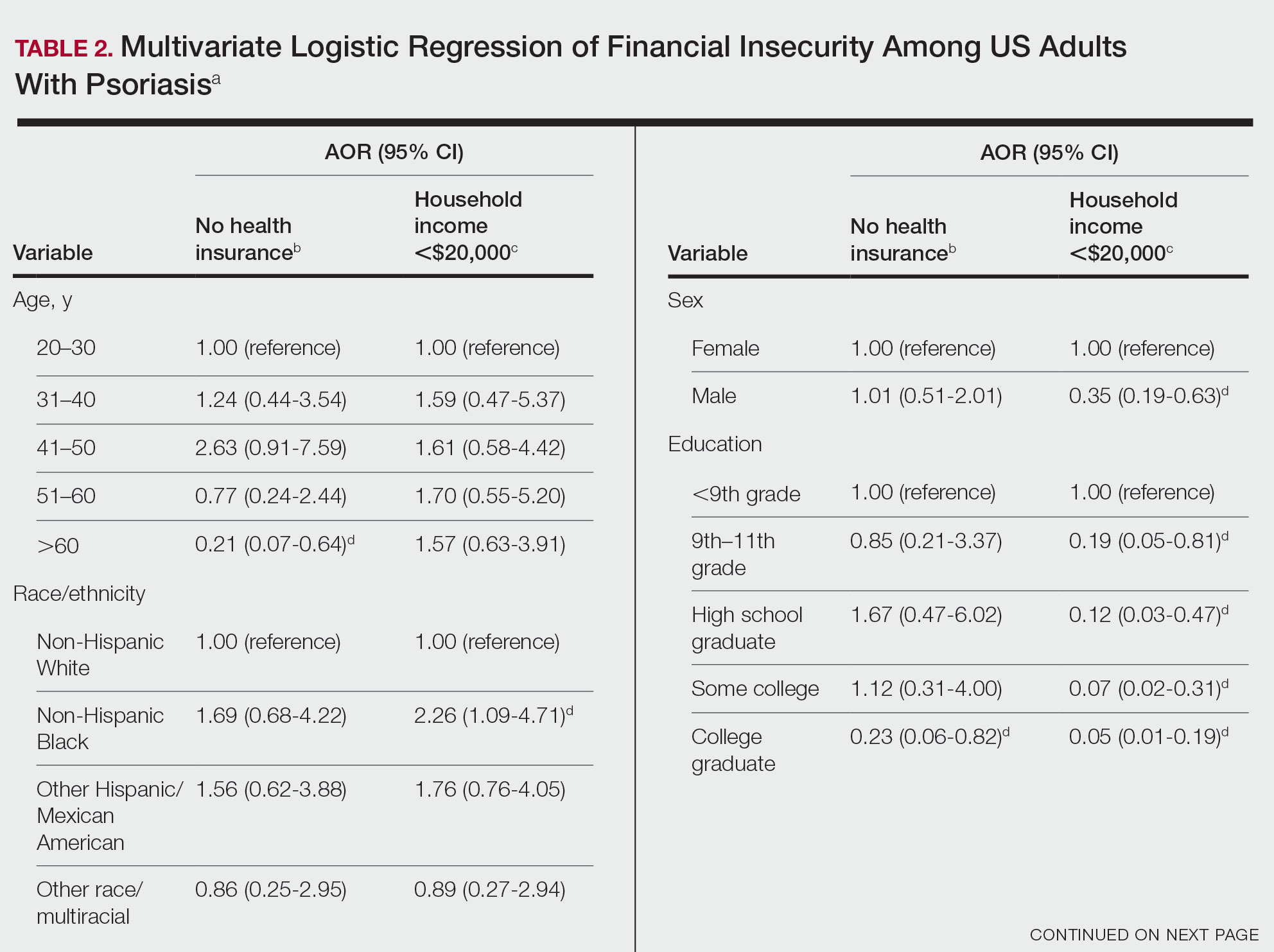
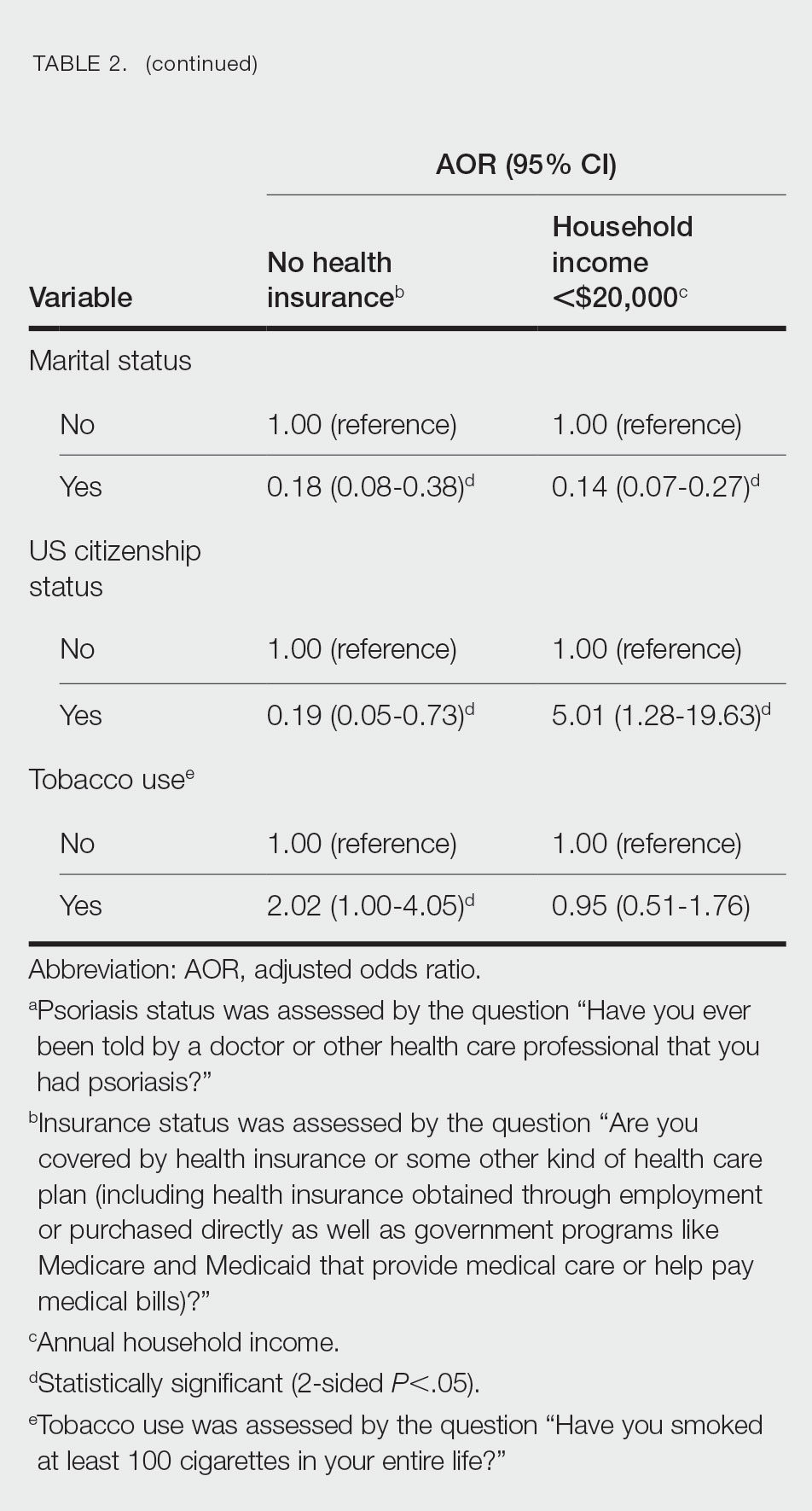
Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
Practice Points
- The economic burden on patients with psoriasis has been rising over time, as the disease impacts many aspects of patients’ lives.
- Various sociodemographic groups among patients with psoriasis are financially insecure. Knowing which groups are at higher risk for poor outcomes due to financial insecurity can assist with appropriate treatment regimens.
Consider housing insecurity, other issues when managing challenging skin diseases in children, expert says
ASHEVILLE, N.C. – , according to a pediatric dermatologist who addressed the annual meeting of the Society for Pediatric Dermatology.
As a general principle for treating chronic skin conditions in children who are not doing well, it is reasonable to draw out information about a patient’s access to adequate housing, nutrition, and other basic needs, George Hightower, MD, PhD, of the division of pediatric and adolescent dermatology, University of California, San Diego, said at the meeting.
“We need conversations about where patients play, learn, and rest their heads at night,” said Dr. Hightower, who conducts research in this area. Fundamental components of well-being, such as stable housing and secure access to nutrition “are inseparable” from a child’s health, he noted.
“What are the stakes?” he asked. For many children, these factors might mean the difference between effective and poor control of the diseases for which the patient is seeking care.
To illustrate the point, Dr. Hightower used hidradenitis suppurativa (HS), a disease that appears to be on the rise among adolescents, as an example of why patient circumstances matter and should be considered. A complex disorder that is more prevalent in resource-poor communities, HS is difficult to control, often requiring extended periods of treatment with medications that can involve complex dosing or regular infusions.
“There is a need for medical providers to help the patient plan for this chronic illness,” said Dr. Hightower, referring to the importance of close follow-up. In adolescents, HS can be sufficiently disruptive from both the physical and psychological perspective that poor control can “derail future aspirations” by complicating educational endeavors and social interactions.
Dr. Hightower acknowledged that simply documenting housing insecurity or other issues does not solve these problems, but he does believe that developing a sensitivity to these obstacles to health care is a first step. It is a process that should permeate into medical training, health care research, and strategies to improve outcomes.
“The connections between fair housing and clinical practice may appear tenuous and inconsequential to the care provided by medical specialists,” Dr. Hightower said, but he emphasized that there are clear consequences when these factors contribute to inadequate control of such diseases as HS. As a source of missed appointments and disjointed care, an unstable home life can be an important barrier to disease control – and because of scarring nodules, fistulae, pain, school absences, and social isolation, complications can be dire.
Solutions to insecure housing are not typically available to an individual clinician, but the awareness that this can be a factor can help both physicians and patients begin to think about the role this plays in impairing recovery and what solutions might be found to modify the impact. Awareness not just among individual clinicians but a broader consortium of those working to improve health care outcomes is needed to “challenge the way we are doing medicine,” he said.
While conversations about the social determinants of health, including access to resources within patients’ neighborhoods, schools, and environment, can demonstrate concern about how to address obstacles, it can also be part of a reorientation to think beyond treatment for the underlying pathology alone. Eliciting trust and emphasizing the importance of environmental barriers to adequate care can be positive steps on the path to solutions.
Participatory action research
Relevant to this orientation, Dr. Hightower spoke about participatory action research (PAR), which provides a framework for patients to participate in the planning of clinical studies to effect change, not just serve as subjects in these studies.
The assumption of PAR is that “all people have valuable knowledge about their lives and experiences,” Dr. Hightower said. From this assumption, individuals who have been historically marginalized by race, income, or other factors can help define the problems from the patient’s perspective and, from there, create studies to seek solutions.
PAR is consistent with a patient-centered approach to medical care, which Dr. Hightower called “the future of medicine.” It involves a big-picture approach to look beyond disease pathology and symptoms to factors that might be creating susceptibility to disease and undermining health care.
Organized medicine alone cannot solve the cause of social inequities leading to disparate risks for disease and risks of inadequate health care, but Dr. Hightower argued that these inequities should not be ignored. He believes medical trainees should learn how to elicit information about the barriers to adequate health care and be aware of solutions, such as fair housing policies.
While he believes that PAR is an example of a pathway to problem solving, he suggested that a comprehensive approach requires an effective method of communication between providers and patients that would lead to a collaborative and mutually reinforcing approach.
“How do we ensure that individuals from communities most impacted by health disparities are treated fairly and empowered to address these disparities?” Dr. Hightower asked. He said that this is the direction of his own research and the issues that inhibit adequate treatment of many dermatologic diseases, as well as other types of disease, in childhood.
Craig Burkhart, MD, director of a private pediatric and adolescent dermatology practice in Cary, N.C., said that Dr. Hightower’s message is relevant. The value of considering and addressing the psychological well-being of patients of any age is not a new concept, but he acknowledged that he, for one, has not routinely inquired about obstacles to follow-up care if there is a signal that this might be an issue.
“As dermatologists, we focus on the acute complaints. We want to make the patient better,” said Dr. Burkhart, who moderated the session in which Dr. Hightower spoke. He agreed with Dr. Hightower that environmental factors make a difference on the road to recovery for a patient, and his presentation was a good reminder, he said, to consider the patient’s circumstances when response to treatment is inadequate, particularly in chronic diseases like HS, for which comprehensive care and close follow-up are needed.
Dr. Hightower and Dr. Burkhart report no potential conflicts of interest.
ASHEVILLE, N.C. – , according to a pediatric dermatologist who addressed the annual meeting of the Society for Pediatric Dermatology.
As a general principle for treating chronic skin conditions in children who are not doing well, it is reasonable to draw out information about a patient’s access to adequate housing, nutrition, and other basic needs, George Hightower, MD, PhD, of the division of pediatric and adolescent dermatology, University of California, San Diego, said at the meeting.
“We need conversations about where patients play, learn, and rest their heads at night,” said Dr. Hightower, who conducts research in this area. Fundamental components of well-being, such as stable housing and secure access to nutrition “are inseparable” from a child’s health, he noted.
“What are the stakes?” he asked. For many children, these factors might mean the difference between effective and poor control of the diseases for which the patient is seeking care.
To illustrate the point, Dr. Hightower used hidradenitis suppurativa (HS), a disease that appears to be on the rise among adolescents, as an example of why patient circumstances matter and should be considered. A complex disorder that is more prevalent in resource-poor communities, HS is difficult to control, often requiring extended periods of treatment with medications that can involve complex dosing or regular infusions.
“There is a need for medical providers to help the patient plan for this chronic illness,” said Dr. Hightower, referring to the importance of close follow-up. In adolescents, HS can be sufficiently disruptive from both the physical and psychological perspective that poor control can “derail future aspirations” by complicating educational endeavors and social interactions.
Dr. Hightower acknowledged that simply documenting housing insecurity or other issues does not solve these problems, but he does believe that developing a sensitivity to these obstacles to health care is a first step. It is a process that should permeate into medical training, health care research, and strategies to improve outcomes.
“The connections between fair housing and clinical practice may appear tenuous and inconsequential to the care provided by medical specialists,” Dr. Hightower said, but he emphasized that there are clear consequences when these factors contribute to inadequate control of such diseases as HS. As a source of missed appointments and disjointed care, an unstable home life can be an important barrier to disease control – and because of scarring nodules, fistulae, pain, school absences, and social isolation, complications can be dire.
Solutions to insecure housing are not typically available to an individual clinician, but the awareness that this can be a factor can help both physicians and patients begin to think about the role this plays in impairing recovery and what solutions might be found to modify the impact. Awareness not just among individual clinicians but a broader consortium of those working to improve health care outcomes is needed to “challenge the way we are doing medicine,” he said.
While conversations about the social determinants of health, including access to resources within patients’ neighborhoods, schools, and environment, can demonstrate concern about how to address obstacles, it can also be part of a reorientation to think beyond treatment for the underlying pathology alone. Eliciting trust and emphasizing the importance of environmental barriers to adequate care can be positive steps on the path to solutions.
Participatory action research
Relevant to this orientation, Dr. Hightower spoke about participatory action research (PAR), which provides a framework for patients to participate in the planning of clinical studies to effect change, not just serve as subjects in these studies.
The assumption of PAR is that “all people have valuable knowledge about their lives and experiences,” Dr. Hightower said. From this assumption, individuals who have been historically marginalized by race, income, or other factors can help define the problems from the patient’s perspective and, from there, create studies to seek solutions.
PAR is consistent with a patient-centered approach to medical care, which Dr. Hightower called “the future of medicine.” It involves a big-picture approach to look beyond disease pathology and symptoms to factors that might be creating susceptibility to disease and undermining health care.
Organized medicine alone cannot solve the cause of social inequities leading to disparate risks for disease and risks of inadequate health care, but Dr. Hightower argued that these inequities should not be ignored. He believes medical trainees should learn how to elicit information about the barriers to adequate health care and be aware of solutions, such as fair housing policies.
While he believes that PAR is an example of a pathway to problem solving, he suggested that a comprehensive approach requires an effective method of communication between providers and patients that would lead to a collaborative and mutually reinforcing approach.
“How do we ensure that individuals from communities most impacted by health disparities are treated fairly and empowered to address these disparities?” Dr. Hightower asked. He said that this is the direction of his own research and the issues that inhibit adequate treatment of many dermatologic diseases, as well as other types of disease, in childhood.
Craig Burkhart, MD, director of a private pediatric and adolescent dermatology practice in Cary, N.C., said that Dr. Hightower’s message is relevant. The value of considering and addressing the psychological well-being of patients of any age is not a new concept, but he acknowledged that he, for one, has not routinely inquired about obstacles to follow-up care if there is a signal that this might be an issue.
“As dermatologists, we focus on the acute complaints. We want to make the patient better,” said Dr. Burkhart, who moderated the session in which Dr. Hightower spoke. He agreed with Dr. Hightower that environmental factors make a difference on the road to recovery for a patient, and his presentation was a good reminder, he said, to consider the patient’s circumstances when response to treatment is inadequate, particularly in chronic diseases like HS, for which comprehensive care and close follow-up are needed.
Dr. Hightower and Dr. Burkhart report no potential conflicts of interest.
ASHEVILLE, N.C. – , according to a pediatric dermatologist who addressed the annual meeting of the Society for Pediatric Dermatology.
As a general principle for treating chronic skin conditions in children who are not doing well, it is reasonable to draw out information about a patient’s access to adequate housing, nutrition, and other basic needs, George Hightower, MD, PhD, of the division of pediatric and adolescent dermatology, University of California, San Diego, said at the meeting.
“We need conversations about where patients play, learn, and rest their heads at night,” said Dr. Hightower, who conducts research in this area. Fundamental components of well-being, such as stable housing and secure access to nutrition “are inseparable” from a child’s health, he noted.
“What are the stakes?” he asked. For many children, these factors might mean the difference between effective and poor control of the diseases for which the patient is seeking care.
To illustrate the point, Dr. Hightower used hidradenitis suppurativa (HS), a disease that appears to be on the rise among adolescents, as an example of why patient circumstances matter and should be considered. A complex disorder that is more prevalent in resource-poor communities, HS is difficult to control, often requiring extended periods of treatment with medications that can involve complex dosing or regular infusions.
“There is a need for medical providers to help the patient plan for this chronic illness,” said Dr. Hightower, referring to the importance of close follow-up. In adolescents, HS can be sufficiently disruptive from both the physical and psychological perspective that poor control can “derail future aspirations” by complicating educational endeavors and social interactions.
Dr. Hightower acknowledged that simply documenting housing insecurity or other issues does not solve these problems, but he does believe that developing a sensitivity to these obstacles to health care is a first step. It is a process that should permeate into medical training, health care research, and strategies to improve outcomes.
“The connections between fair housing and clinical practice may appear tenuous and inconsequential to the care provided by medical specialists,” Dr. Hightower said, but he emphasized that there are clear consequences when these factors contribute to inadequate control of such diseases as HS. As a source of missed appointments and disjointed care, an unstable home life can be an important barrier to disease control – and because of scarring nodules, fistulae, pain, school absences, and social isolation, complications can be dire.
Solutions to insecure housing are not typically available to an individual clinician, but the awareness that this can be a factor can help both physicians and patients begin to think about the role this plays in impairing recovery and what solutions might be found to modify the impact. Awareness not just among individual clinicians but a broader consortium of those working to improve health care outcomes is needed to “challenge the way we are doing medicine,” he said.
While conversations about the social determinants of health, including access to resources within patients’ neighborhoods, schools, and environment, can demonstrate concern about how to address obstacles, it can also be part of a reorientation to think beyond treatment for the underlying pathology alone. Eliciting trust and emphasizing the importance of environmental barriers to adequate care can be positive steps on the path to solutions.
Participatory action research
Relevant to this orientation, Dr. Hightower spoke about participatory action research (PAR), which provides a framework for patients to participate in the planning of clinical studies to effect change, not just serve as subjects in these studies.
The assumption of PAR is that “all people have valuable knowledge about their lives and experiences,” Dr. Hightower said. From this assumption, individuals who have been historically marginalized by race, income, or other factors can help define the problems from the patient’s perspective and, from there, create studies to seek solutions.
PAR is consistent with a patient-centered approach to medical care, which Dr. Hightower called “the future of medicine.” It involves a big-picture approach to look beyond disease pathology and symptoms to factors that might be creating susceptibility to disease and undermining health care.
Organized medicine alone cannot solve the cause of social inequities leading to disparate risks for disease and risks of inadequate health care, but Dr. Hightower argued that these inequities should not be ignored. He believes medical trainees should learn how to elicit information about the barriers to adequate health care and be aware of solutions, such as fair housing policies.
While he believes that PAR is an example of a pathway to problem solving, he suggested that a comprehensive approach requires an effective method of communication between providers and patients that would lead to a collaborative and mutually reinforcing approach.
“How do we ensure that individuals from communities most impacted by health disparities are treated fairly and empowered to address these disparities?” Dr. Hightower asked. He said that this is the direction of his own research and the issues that inhibit adequate treatment of many dermatologic diseases, as well as other types of disease, in childhood.
Craig Burkhart, MD, director of a private pediatric and adolescent dermatology practice in Cary, N.C., said that Dr. Hightower’s message is relevant. The value of considering and addressing the psychological well-being of patients of any age is not a new concept, but he acknowledged that he, for one, has not routinely inquired about obstacles to follow-up care if there is a signal that this might be an issue.
“As dermatologists, we focus on the acute complaints. We want to make the patient better,” said Dr. Burkhart, who moderated the session in which Dr. Hightower spoke. He agreed with Dr. Hightower that environmental factors make a difference on the road to recovery for a patient, and his presentation was a good reminder, he said, to consider the patient’s circumstances when response to treatment is inadequate, particularly in chronic diseases like HS, for which comprehensive care and close follow-up are needed.
Dr. Hightower and Dr. Burkhart report no potential conflicts of interest.
AT SPD 2023
Cystic Presentation of High-Grade Ductal Carcinoma In Situ in an Inframammary Accessory Nipple
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.
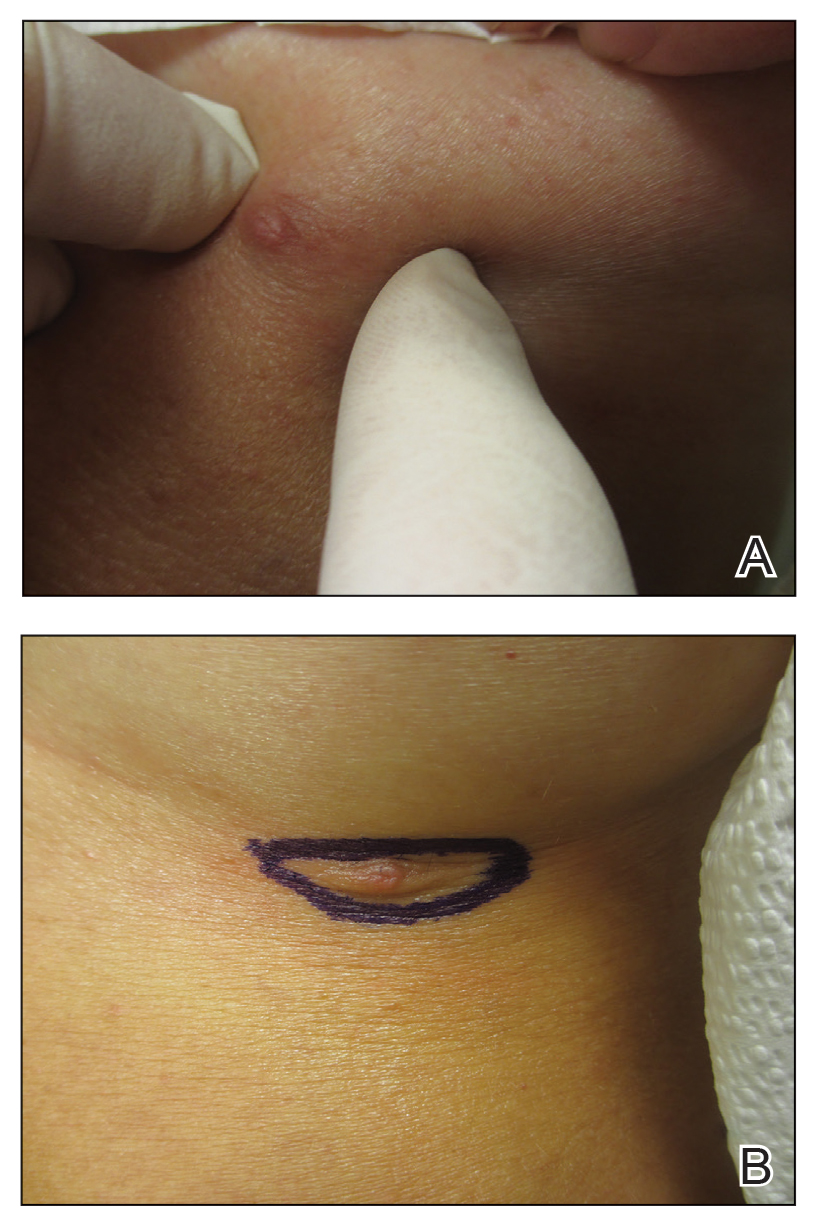
A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).
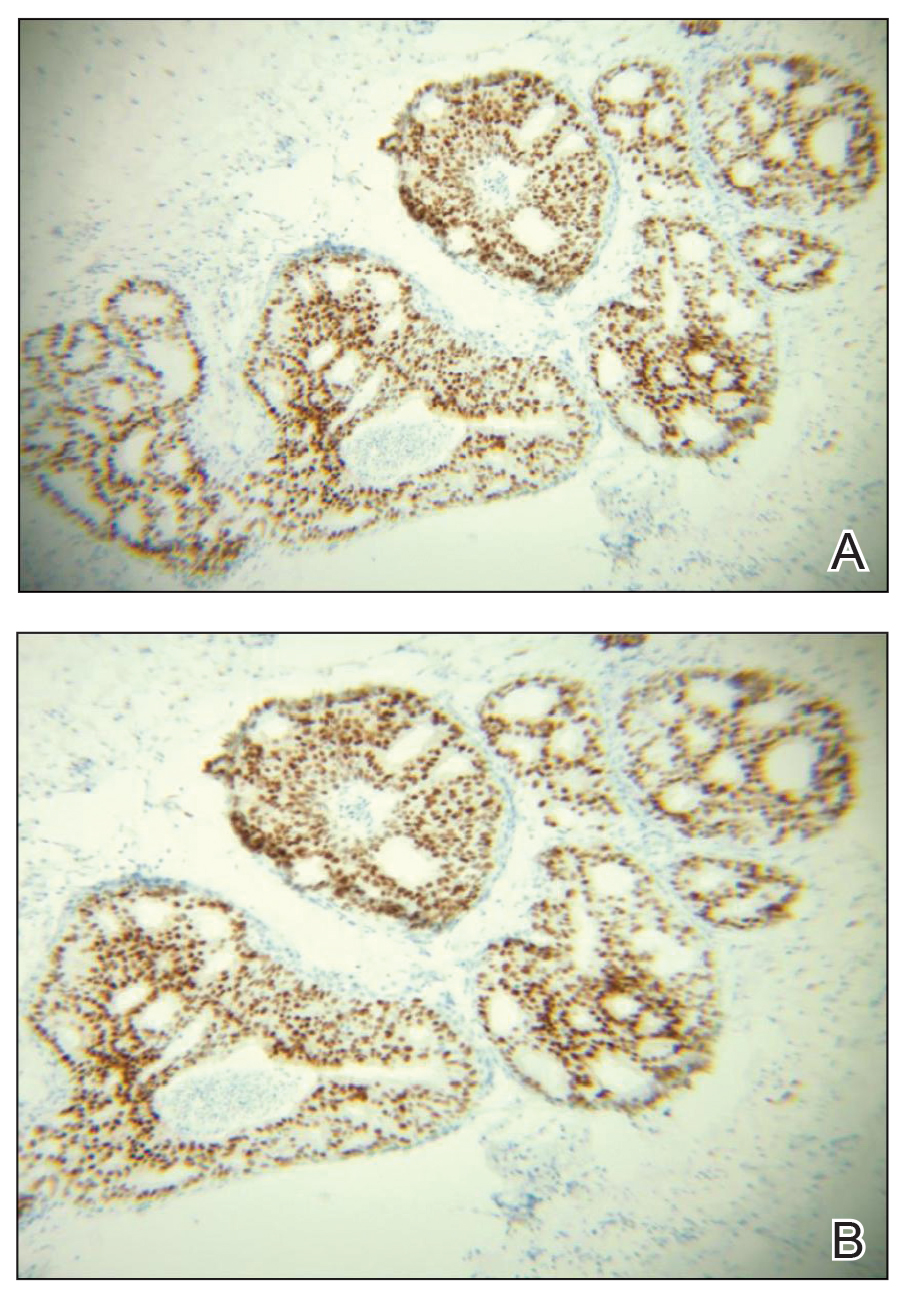
Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11
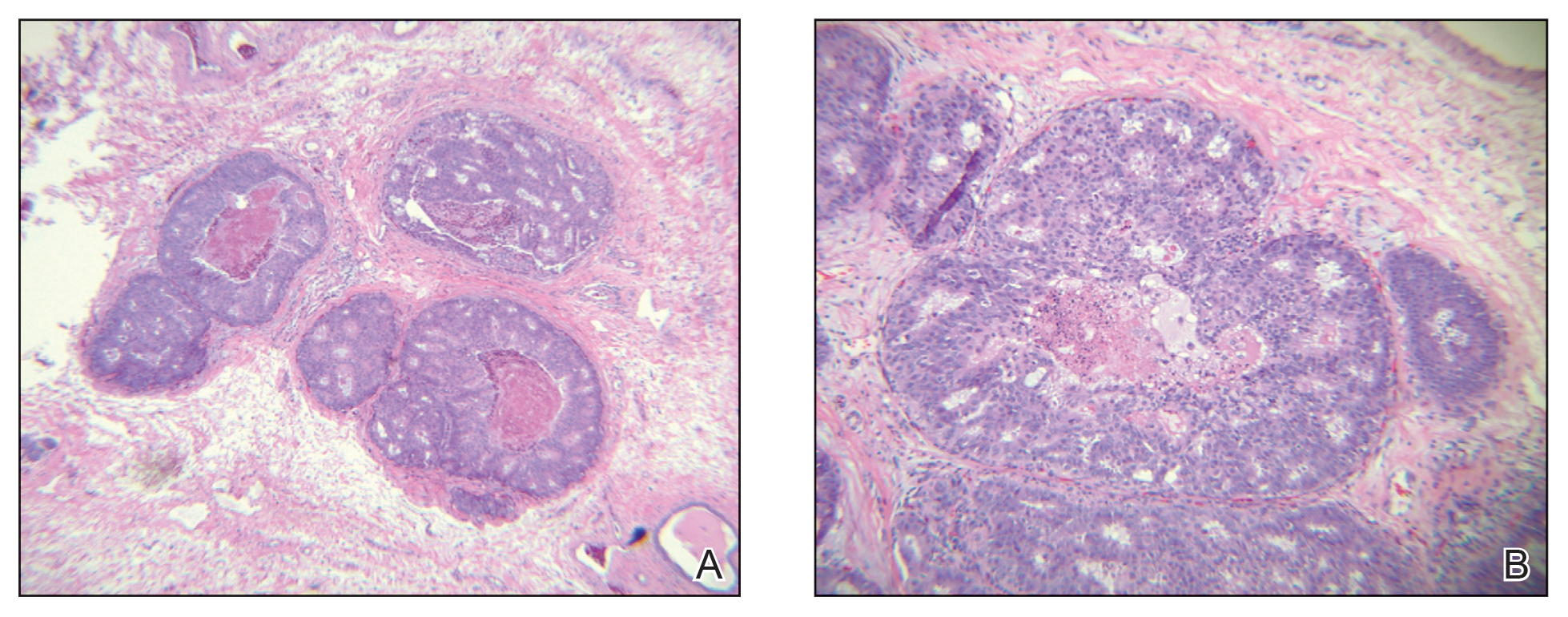
There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
Practice Points
- Accessory breasts (also referred to as ectopic breast tissue) develop when breast tissue is retained along the mammary ridge outside of the usual pectoral regions.
- Because accessory breasts may contain the same structures as anatomically correct breasts, they can be subject to the same benign or malignant changes.
- Clinical and pathologic correlation is prudent when interpreting ectopic mammary tissue, as various benign or malignant neoplasms may arise in this setting, especially if there are underlying genetic aberrancies or genodermatoses.
ChatGPT in Dermatology Clinical Practice: Potential Uses and Pitfalls
Artificial intelligence (AI) technology has increasingly been incorporated in medicine. In dermatology, AI has been used to detect and diagnose skin lesions, including skin cancer.1 ChatGPT (OpenAI) is a novel, highly popular development in generative AI technology. A large language model released in 2022, ChatGPT is a chatbot designed to mimic human conversation and generate specific detailed information when prompted. Free and publicly available, it has been used by millions of people. ChatGPT’s application in the medical field currently is being evaluated across several specialties, including plastic surgery, radiology, and urology.2-4 ChatGPT has the potential to assist health care professionals, including dermatologists, though its use raises important ethical considerations. Herein, we focus on the potential benefits as well as the pitfalls of using ChatGPT in dermatology clinical practice.
Potential Uses of ChatGPT in Practice
A major benefit of ChatGPT is its ability to improve clinical efficiency. First, ChatGPT can provide quick access to general medical information, similar to a search engine but with more natural language processing and contextual understanding to synthesize information.5 This function is useful for rapid concise answers to specific and directed questions. ChatGPT also can interact with its user by asking follow-up questions to produce more precise and relevant responses; this feature may help dermatologists form more accurate differential diagnoses. Additionally, ChatGPT can increase efficiency in clinical practice by drafting generic medical documents,2 including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. Another useful feature of ChatGPT is its ability to output information modeling human conversation. Because of this feature, ChatGPT also could be employed in clinical practice to serve as an interpreter for patients during clinic visits. Currently, the use of virtual translators can be cumbersome and subject to technical constraints. ChatGPT can provide accurate and conversational translations for patients and dermatologists, improving the patient-provider relationship.
ChatGPT also can contribute to major advancements in the field of dermatology beyond the clinical setting. Because of its ability to draw from extensive data that have already been uploaded, there are some uses of ChatGPT in a research context: to assist in finding resources for research and reviews, formulating hypotheses, drafting study protocols, and collecting large amounts of data within seconds.6
ChatGPT also has potential in advancing medical education. It could be used by medical schools to model interactive patient encounters to help students practice taking a patient’s history and creating differential diagnoses.6 This application of ChatGPT may help medical students hone their clinical skills in a low-stress environment without the restrictions that can come with hiring and training standardized patients, especially when mimicking dermatologic clinical encounters.
Other possibilities for ChatGPT in dermatologic practice include survey administration, clinical trial recruitment, and even automatic high-risk medication monitoring. Despite the many potential applications of ChatGPT in clinical practice, the question raised in each scenario is the quality, accuracy, and safety of what it produces.
Potential Pitfalls of ChatGPT in Practice and Possible Mitigation Strategies
A main concern in using ChatGPT in clinical practice is its potential to produce inaccurate or biased information. When prompted to create a research abstract based on previously published research, ChatGPT drafted abstracts that were clear and digestible but supplemented with incorrect data.7 A group of medical researchers who reviewed these ChatGPT-generated abstracts mistook 32% of the abstracts as having been written by human researchers. The implications of this finding are worrisome. If inaccurate or false information is used by ChatGPT in documents sent to insurance companies or patients, the patient’s safety as well as the dermatologist’s license and credibility are at stake. Thus, dermatologists looking to use ChatGPT to draft generic medical documents should actively review the output to ensure that the information is accurate. Importantly, ChatGPT also is only currently programmed with information up to 2021, limiting its access to recently published research articles and updated International Classification of Diseases, Tenth Revision codes.5 The continued development of ChatGPT will lead to regular updates by OpenAI that resolve this shortcoming in the future. Further, AI models may encode and perpetuate harmful stereotypes and social biases that are present in training data.8
When considering its potential in clinical practice, ChatGPT itself states that it can aid in clinical decision-making by processing patient information, including history, current symptoms, and biopsy and test results. This is uncharted territory, and providers who use ChatGPT at this stage to aid in decision-making should regard it similarly to information retrieved from a search engine. The information produced by ChatGPT should be used to substantiate but not definitively confirm a diagnosis or dictate management. Clinical suspicion by the dermatologist should always trump ChatGPT output. If physicians recommend ChatGPT output over their own advice, it can become a liability, as the technology is not regulated to any degree. Patients also should be cautious when seeking and following medical advice from ChatGPT, as it could be misleading or incorrect and could undermine the patient-physician relationship.6
Additionally, the specific use of ChatGPT to evaluate patient health records raises concerns related to patient privacy and confidentiality. Providers should never enter patient information protected by the Health Insurance Portability and Accountability Act into ChatGPT or anywhere else on the internet. Mitigation of this risk is not currently possible; ChatGPT requires more development and further understanding to even consider its integration into current electronic health records without compromising patient confidentiality.
The Bottom Line
Overall, ChatGPT has the potential to improve efficiency in clinical practice by assisting with informational searches and document drafting. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. However, dermatologists should carefully weigh the ethical issues of nonmaleficence and justice in evaluating ChatGPT for accuracy, biases, and the protection of patient confidentiality.
Finally, we asked ChatGPT on February 28, 2023, what it thinks:
User: Should dermatologists use ChatGPT in clinical practice?
ChatGPT: As an AI language model, ChatGPT can be a useful tool in dermatology clinical practice, but it should be used with caution in conjunction with other sources of information and clinical judgement.
Dermatologists should be aware of the potential benefits and risks of using ChatGPT and make informed decisions about how to integrate it into their clinical practice. It’s important to remember that ChatGPT is not a substitute for clinical expertise or human interaction with patients, and it should only be used as a supplement to enhance the quality of patient care.
Dermatologists who choose to use ChatGPT should also be aware of its limitations and potential risks, such as limited accuracy, lack of context, and liability issues if it provides incorrect information or recommendations that lead to patient harm. Dermatologists should use their professional judgement and consult with other health care professionals when making important decisions regarding patient care.
- Thomsen K, Iversen L, Titlestad TL, et al. Systematic review of machine learning for diagnosis and prognosis in dermatology. J Dermatol Treat. 2020;31:496-510. doi:10.1080/09546634.2019.1682500
- Shen Y, Heacock L, Elias J, et al. ChatGPT and other large language models are double-edged swords. Radiology. 2023;307:E230163. doi:10.1148/radiol.230163
- Gupta R, Pande P, Herzog I, et al. Application of ChatGPT in cosmetic plastic surgery: ally or antagonist? Aesthet Surg J. 2023;43:NP587-NP590. doi: 10.1093/asj/sjad042
- Gabrielson AT, Odisho AY, Canes D. Harnessing generative artificial intelligence to improve efficiency among urologists: welcome ChatGPT. J Urol. 2023;209:827-829. doi:10.1097/JU.0000000000003383
- What is ChatGPT? OpenAI. Accessed August 10, 2023. https://help.openai.com/en/articles/6783457-chatgpt-general-faq
- Haupt CE, Marks M. AI-generated medical advice—GPT and beyond. JAMA. 2023;329:1349-1350. doi:10.1001/jama.2023.5321
- Gao CA, Howard FM, Markov NS, et al. Comparing Scientific Abstracts Generated by ChatGPT to Original Abstracts Using an Artificial Intelligence Output Detector, Plagiarism Detector, and Blinded Human Reviewers. Scientific Communication and Education; 2022. doi:10.1101/2022.12.23.521610
- Weidinger L, Mellor J, Rauh M, et al. Ethical and social risks of harm from language models. arXiv. Preprint posted online December 8, 2021. https://doi.org/10.48550/arXiv.2112.04359
Artificial intelligence (AI) technology has increasingly been incorporated in medicine. In dermatology, AI has been used to detect and diagnose skin lesions, including skin cancer.1 ChatGPT (OpenAI) is a novel, highly popular development in generative AI technology. A large language model released in 2022, ChatGPT is a chatbot designed to mimic human conversation and generate specific detailed information when prompted. Free and publicly available, it has been used by millions of people. ChatGPT’s application in the medical field currently is being evaluated across several specialties, including plastic surgery, radiology, and urology.2-4 ChatGPT has the potential to assist health care professionals, including dermatologists, though its use raises important ethical considerations. Herein, we focus on the potential benefits as well as the pitfalls of using ChatGPT in dermatology clinical practice.
Potential Uses of ChatGPT in Practice
A major benefit of ChatGPT is its ability to improve clinical efficiency. First, ChatGPT can provide quick access to general medical information, similar to a search engine but with more natural language processing and contextual understanding to synthesize information.5 This function is useful for rapid concise answers to specific and directed questions. ChatGPT also can interact with its user by asking follow-up questions to produce more precise and relevant responses; this feature may help dermatologists form more accurate differential diagnoses. Additionally, ChatGPT can increase efficiency in clinical practice by drafting generic medical documents,2 including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. Another useful feature of ChatGPT is its ability to output information modeling human conversation. Because of this feature, ChatGPT also could be employed in clinical practice to serve as an interpreter for patients during clinic visits. Currently, the use of virtual translators can be cumbersome and subject to technical constraints. ChatGPT can provide accurate and conversational translations for patients and dermatologists, improving the patient-provider relationship.
ChatGPT also can contribute to major advancements in the field of dermatology beyond the clinical setting. Because of its ability to draw from extensive data that have already been uploaded, there are some uses of ChatGPT in a research context: to assist in finding resources for research and reviews, formulating hypotheses, drafting study protocols, and collecting large amounts of data within seconds.6
ChatGPT also has potential in advancing medical education. It could be used by medical schools to model interactive patient encounters to help students practice taking a patient’s history and creating differential diagnoses.6 This application of ChatGPT may help medical students hone their clinical skills in a low-stress environment without the restrictions that can come with hiring and training standardized patients, especially when mimicking dermatologic clinical encounters.
Other possibilities for ChatGPT in dermatologic practice include survey administration, clinical trial recruitment, and even automatic high-risk medication monitoring. Despite the many potential applications of ChatGPT in clinical practice, the question raised in each scenario is the quality, accuracy, and safety of what it produces.
Potential Pitfalls of ChatGPT in Practice and Possible Mitigation Strategies
A main concern in using ChatGPT in clinical practice is its potential to produce inaccurate or biased information. When prompted to create a research abstract based on previously published research, ChatGPT drafted abstracts that were clear and digestible but supplemented with incorrect data.7 A group of medical researchers who reviewed these ChatGPT-generated abstracts mistook 32% of the abstracts as having been written by human researchers. The implications of this finding are worrisome. If inaccurate or false information is used by ChatGPT in documents sent to insurance companies or patients, the patient’s safety as well as the dermatologist’s license and credibility are at stake. Thus, dermatologists looking to use ChatGPT to draft generic medical documents should actively review the output to ensure that the information is accurate. Importantly, ChatGPT also is only currently programmed with information up to 2021, limiting its access to recently published research articles and updated International Classification of Diseases, Tenth Revision codes.5 The continued development of ChatGPT will lead to regular updates by OpenAI that resolve this shortcoming in the future. Further, AI models may encode and perpetuate harmful stereotypes and social biases that are present in training data.8
When considering its potential in clinical practice, ChatGPT itself states that it can aid in clinical decision-making by processing patient information, including history, current symptoms, and biopsy and test results. This is uncharted territory, and providers who use ChatGPT at this stage to aid in decision-making should regard it similarly to information retrieved from a search engine. The information produced by ChatGPT should be used to substantiate but not definitively confirm a diagnosis or dictate management. Clinical suspicion by the dermatologist should always trump ChatGPT output. If physicians recommend ChatGPT output over their own advice, it can become a liability, as the technology is not regulated to any degree. Patients also should be cautious when seeking and following medical advice from ChatGPT, as it could be misleading or incorrect and could undermine the patient-physician relationship.6
Additionally, the specific use of ChatGPT to evaluate patient health records raises concerns related to patient privacy and confidentiality. Providers should never enter patient information protected by the Health Insurance Portability and Accountability Act into ChatGPT or anywhere else on the internet. Mitigation of this risk is not currently possible; ChatGPT requires more development and further understanding to even consider its integration into current electronic health records without compromising patient confidentiality.
The Bottom Line
Overall, ChatGPT has the potential to improve efficiency in clinical practice by assisting with informational searches and document drafting. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. However, dermatologists should carefully weigh the ethical issues of nonmaleficence and justice in evaluating ChatGPT for accuracy, biases, and the protection of patient confidentiality.
Finally, we asked ChatGPT on February 28, 2023, what it thinks:
User: Should dermatologists use ChatGPT in clinical practice?
ChatGPT: As an AI language model, ChatGPT can be a useful tool in dermatology clinical practice, but it should be used with caution in conjunction with other sources of information and clinical judgement.
Dermatologists should be aware of the potential benefits and risks of using ChatGPT and make informed decisions about how to integrate it into their clinical practice. It’s important to remember that ChatGPT is not a substitute for clinical expertise or human interaction with patients, and it should only be used as a supplement to enhance the quality of patient care.
Dermatologists who choose to use ChatGPT should also be aware of its limitations and potential risks, such as limited accuracy, lack of context, and liability issues if it provides incorrect information or recommendations that lead to patient harm. Dermatologists should use their professional judgement and consult with other health care professionals when making important decisions regarding patient care.
Artificial intelligence (AI) technology has increasingly been incorporated in medicine. In dermatology, AI has been used to detect and diagnose skin lesions, including skin cancer.1 ChatGPT (OpenAI) is a novel, highly popular development in generative AI technology. A large language model released in 2022, ChatGPT is a chatbot designed to mimic human conversation and generate specific detailed information when prompted. Free and publicly available, it has been used by millions of people. ChatGPT’s application in the medical field currently is being evaluated across several specialties, including plastic surgery, radiology, and urology.2-4 ChatGPT has the potential to assist health care professionals, including dermatologists, though its use raises important ethical considerations. Herein, we focus on the potential benefits as well as the pitfalls of using ChatGPT in dermatology clinical practice.
Potential Uses of ChatGPT in Practice
A major benefit of ChatGPT is its ability to improve clinical efficiency. First, ChatGPT can provide quick access to general medical information, similar to a search engine but with more natural language processing and contextual understanding to synthesize information.5 This function is useful for rapid concise answers to specific and directed questions. ChatGPT also can interact with its user by asking follow-up questions to produce more precise and relevant responses; this feature may help dermatologists form more accurate differential diagnoses. Additionally, ChatGPT can increase efficiency in clinical practice by drafting generic medical documents,2 including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. Another useful feature of ChatGPT is its ability to output information modeling human conversation. Because of this feature, ChatGPT also could be employed in clinical practice to serve as an interpreter for patients during clinic visits. Currently, the use of virtual translators can be cumbersome and subject to technical constraints. ChatGPT can provide accurate and conversational translations for patients and dermatologists, improving the patient-provider relationship.
ChatGPT also can contribute to major advancements in the field of dermatology beyond the clinical setting. Because of its ability to draw from extensive data that have already been uploaded, there are some uses of ChatGPT in a research context: to assist in finding resources for research and reviews, formulating hypotheses, drafting study protocols, and collecting large amounts of data within seconds.6
ChatGPT also has potential in advancing medical education. It could be used by medical schools to model interactive patient encounters to help students practice taking a patient’s history and creating differential diagnoses.6 This application of ChatGPT may help medical students hone their clinical skills in a low-stress environment without the restrictions that can come with hiring and training standardized patients, especially when mimicking dermatologic clinical encounters.
Other possibilities for ChatGPT in dermatologic practice include survey administration, clinical trial recruitment, and even automatic high-risk medication monitoring. Despite the many potential applications of ChatGPT in clinical practice, the question raised in each scenario is the quality, accuracy, and safety of what it produces.
Potential Pitfalls of ChatGPT in Practice and Possible Mitigation Strategies
A main concern in using ChatGPT in clinical practice is its potential to produce inaccurate or biased information. When prompted to create a research abstract based on previously published research, ChatGPT drafted abstracts that were clear and digestible but supplemented with incorrect data.7 A group of medical researchers who reviewed these ChatGPT-generated abstracts mistook 32% of the abstracts as having been written by human researchers. The implications of this finding are worrisome. If inaccurate or false information is used by ChatGPT in documents sent to insurance companies or patients, the patient’s safety as well as the dermatologist’s license and credibility are at stake. Thus, dermatologists looking to use ChatGPT to draft generic medical documents should actively review the output to ensure that the information is accurate. Importantly, ChatGPT also is only currently programmed with information up to 2021, limiting its access to recently published research articles and updated International Classification of Diseases, Tenth Revision codes.5 The continued development of ChatGPT will lead to regular updates by OpenAI that resolve this shortcoming in the future. Further, AI models may encode and perpetuate harmful stereotypes and social biases that are present in training data.8
When considering its potential in clinical practice, ChatGPT itself states that it can aid in clinical decision-making by processing patient information, including history, current symptoms, and biopsy and test results. This is uncharted territory, and providers who use ChatGPT at this stage to aid in decision-making should regard it similarly to information retrieved from a search engine. The information produced by ChatGPT should be used to substantiate but not definitively confirm a diagnosis or dictate management. Clinical suspicion by the dermatologist should always trump ChatGPT output. If physicians recommend ChatGPT output over their own advice, it can become a liability, as the technology is not regulated to any degree. Patients also should be cautious when seeking and following medical advice from ChatGPT, as it could be misleading or incorrect and could undermine the patient-physician relationship.6
Additionally, the specific use of ChatGPT to evaluate patient health records raises concerns related to patient privacy and confidentiality. Providers should never enter patient information protected by the Health Insurance Portability and Accountability Act into ChatGPT or anywhere else on the internet. Mitigation of this risk is not currently possible; ChatGPT requires more development and further understanding to even consider its integration into current electronic health records without compromising patient confidentiality.
The Bottom Line
Overall, ChatGPT has the potential to improve efficiency in clinical practice by assisting with informational searches and document drafting. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. However, dermatologists should carefully weigh the ethical issues of nonmaleficence and justice in evaluating ChatGPT for accuracy, biases, and the protection of patient confidentiality.
Finally, we asked ChatGPT on February 28, 2023, what it thinks:
User: Should dermatologists use ChatGPT in clinical practice?
ChatGPT: As an AI language model, ChatGPT can be a useful tool in dermatology clinical practice, but it should be used with caution in conjunction with other sources of information and clinical judgement.
Dermatologists should be aware of the potential benefits and risks of using ChatGPT and make informed decisions about how to integrate it into their clinical practice. It’s important to remember that ChatGPT is not a substitute for clinical expertise or human interaction with patients, and it should only be used as a supplement to enhance the quality of patient care.
Dermatologists who choose to use ChatGPT should also be aware of its limitations and potential risks, such as limited accuracy, lack of context, and liability issues if it provides incorrect information or recommendations that lead to patient harm. Dermatologists should use their professional judgement and consult with other health care professionals when making important decisions regarding patient care.
- Thomsen K, Iversen L, Titlestad TL, et al. Systematic review of machine learning for diagnosis and prognosis in dermatology. J Dermatol Treat. 2020;31:496-510. doi:10.1080/09546634.2019.1682500
- Shen Y, Heacock L, Elias J, et al. ChatGPT and other large language models are double-edged swords. Radiology. 2023;307:E230163. doi:10.1148/radiol.230163
- Gupta R, Pande P, Herzog I, et al. Application of ChatGPT in cosmetic plastic surgery: ally or antagonist? Aesthet Surg J. 2023;43:NP587-NP590. doi: 10.1093/asj/sjad042
- Gabrielson AT, Odisho AY, Canes D. Harnessing generative artificial intelligence to improve efficiency among urologists: welcome ChatGPT. J Urol. 2023;209:827-829. doi:10.1097/JU.0000000000003383
- What is ChatGPT? OpenAI. Accessed August 10, 2023. https://help.openai.com/en/articles/6783457-chatgpt-general-faq
- Haupt CE, Marks M. AI-generated medical advice—GPT and beyond. JAMA. 2023;329:1349-1350. doi:10.1001/jama.2023.5321
- Gao CA, Howard FM, Markov NS, et al. Comparing Scientific Abstracts Generated by ChatGPT to Original Abstracts Using an Artificial Intelligence Output Detector, Plagiarism Detector, and Blinded Human Reviewers. Scientific Communication and Education; 2022. doi:10.1101/2022.12.23.521610
- Weidinger L, Mellor J, Rauh M, et al. Ethical and social risks of harm from language models. arXiv. Preprint posted online December 8, 2021. https://doi.org/10.48550/arXiv.2112.04359
- Thomsen K, Iversen L, Titlestad TL, et al. Systematic review of machine learning for diagnosis and prognosis in dermatology. J Dermatol Treat. 2020;31:496-510. doi:10.1080/09546634.2019.1682500
- Shen Y, Heacock L, Elias J, et al. ChatGPT and other large language models are double-edged swords. Radiology. 2023;307:E230163. doi:10.1148/radiol.230163
- Gupta R, Pande P, Herzog I, et al. Application of ChatGPT in cosmetic plastic surgery: ally or antagonist? Aesthet Surg J. 2023;43:NP587-NP590. doi: 10.1093/asj/sjad042
- Gabrielson AT, Odisho AY, Canes D. Harnessing generative artificial intelligence to improve efficiency among urologists: welcome ChatGPT. J Urol. 2023;209:827-829. doi:10.1097/JU.0000000000003383
- What is ChatGPT? OpenAI. Accessed August 10, 2023. https://help.openai.com/en/articles/6783457-chatgpt-general-faq
- Haupt CE, Marks M. AI-generated medical advice—GPT and beyond. JAMA. 2023;329:1349-1350. doi:10.1001/jama.2023.5321
- Gao CA, Howard FM, Markov NS, et al. Comparing Scientific Abstracts Generated by ChatGPT to Original Abstracts Using an Artificial Intelligence Output Detector, Plagiarism Detector, and Blinded Human Reviewers. Scientific Communication and Education; 2022. doi:10.1101/2022.12.23.521610
- Weidinger L, Mellor J, Rauh M, et al. Ethical and social risks of harm from language models. arXiv. Preprint posted online December 8, 2021. https://doi.org/10.48550/arXiv.2112.04359
Practice Points
- ChatGPT potentially can play a beneficial role in dermatologic practice by quickly accessing and synthesizing information, drafting generic medical documents, interpreting visits, advancing medical education, and more.
- Dermatologists using ChatGPT should be extremely cautious, as it can produce false or biased information, perpetuate harmful stereotypes, and present information that is not up-to-date.
Dupilumab gains off-label uses as clinicians turn to drug for more indications
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.