User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Poor representation of patients with darker skin phototypes in laser and light device studies
, according to a systematic review of the literature, the authors reported.
“While there broadly appears to be skin of color representation [in such studies], a more granular understanding of the data shows a large discrepancy in representation between ‘lighter’ and ‘darker’ skin of color patients,” Priya Manjaly and associates wrote in the Journal of Cosmetic Dermatology.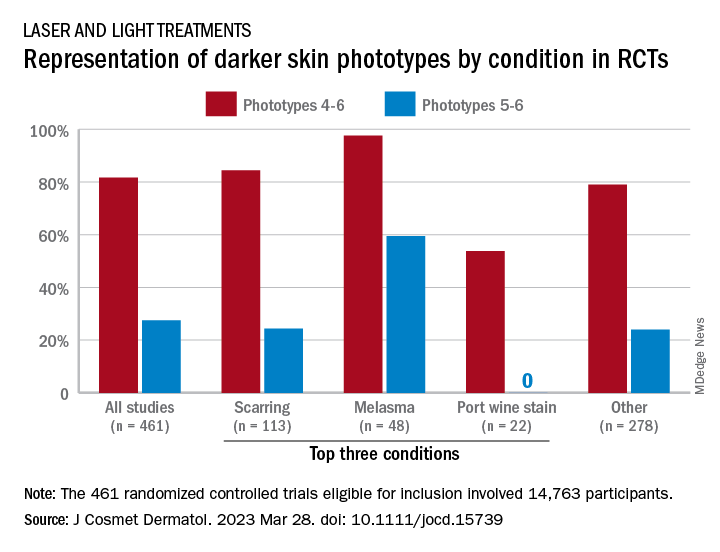
Among the 461 randomized controlled trials (RCTs) eligible for inclusion, most (81.7%) included participants with skin phototypes 4-6, which is considered skin of color. When only phototypes 5 and 6 were included, however, representation in studies involving laser and light devices was only 27.5%, said Ms. Manjaly, a research fellow in the department of dermatology at Boston University, and associates.
“This trend of excluding darker skin phototypes persisted when the results were stratified by condition, laser of study, study location, journal type, and funding source,” the investigators noted.
RCTs of laser/light devices for scarring, the most common dermatologic condition represented, included phototypes 5 and 6 in 24.4% of studies, compared with 84.4% for phototypes 4-6. The gap was smaller for melasma, but not for port wine stains. Among the devices examined, RCTs of diode lasers and intense pulsed light had the smallest gaps between inclusion of the two groups of phototypes, while pulsed-dye laser studies had the largest, they reported.
Stratification by journal showed the largest gap in studies published by Lasers in Medical Science and the smallest gap coming from Lasers in Surgery and Medicine. Funding was not specified for the majority of the eligible device RCTs, but those funded by industry had the smallest discrepancy between types 5-6 and types 4-6 and those supported by foundations/nonprofits the largest, Ms. Manjaly and associates said.
“With projections estimating that more than 50% of the U.S. population is set to identify as Hispanic or nonwhite by 2045 ... the lack of information has important consequences for clinical practice, as clinicians are unable to counsel patients on the efficacy and possible complications of various devices in patient with skin of color,” they wrote.
The investigators did not declare any conflicts of interest or funding sources.
, according to a systematic review of the literature, the authors reported.
“While there broadly appears to be skin of color representation [in such studies], a more granular understanding of the data shows a large discrepancy in representation between ‘lighter’ and ‘darker’ skin of color patients,” Priya Manjaly and associates wrote in the Journal of Cosmetic Dermatology.
Among the 461 randomized controlled trials (RCTs) eligible for inclusion, most (81.7%) included participants with skin phototypes 4-6, which is considered skin of color. When only phototypes 5 and 6 were included, however, representation in studies involving laser and light devices was only 27.5%, said Ms. Manjaly, a research fellow in the department of dermatology at Boston University, and associates.
“This trend of excluding darker skin phototypes persisted when the results were stratified by condition, laser of study, study location, journal type, and funding source,” the investigators noted.
RCTs of laser/light devices for scarring, the most common dermatologic condition represented, included phototypes 5 and 6 in 24.4% of studies, compared with 84.4% for phototypes 4-6. The gap was smaller for melasma, but not for port wine stains. Among the devices examined, RCTs of diode lasers and intense pulsed light had the smallest gaps between inclusion of the two groups of phototypes, while pulsed-dye laser studies had the largest, they reported.
Stratification by journal showed the largest gap in studies published by Lasers in Medical Science and the smallest gap coming from Lasers in Surgery and Medicine. Funding was not specified for the majority of the eligible device RCTs, but those funded by industry had the smallest discrepancy between types 5-6 and types 4-6 and those supported by foundations/nonprofits the largest, Ms. Manjaly and associates said.
“With projections estimating that more than 50% of the U.S. population is set to identify as Hispanic or nonwhite by 2045 ... the lack of information has important consequences for clinical practice, as clinicians are unable to counsel patients on the efficacy and possible complications of various devices in patient with skin of color,” they wrote.
The investigators did not declare any conflicts of interest or funding sources.
, according to a systematic review of the literature, the authors reported.
“While there broadly appears to be skin of color representation [in such studies], a more granular understanding of the data shows a large discrepancy in representation between ‘lighter’ and ‘darker’ skin of color patients,” Priya Manjaly and associates wrote in the Journal of Cosmetic Dermatology.
Among the 461 randomized controlled trials (RCTs) eligible for inclusion, most (81.7%) included participants with skin phototypes 4-6, which is considered skin of color. When only phototypes 5 and 6 were included, however, representation in studies involving laser and light devices was only 27.5%, said Ms. Manjaly, a research fellow in the department of dermatology at Boston University, and associates.
“This trend of excluding darker skin phototypes persisted when the results were stratified by condition, laser of study, study location, journal type, and funding source,” the investigators noted.
RCTs of laser/light devices for scarring, the most common dermatologic condition represented, included phototypes 5 and 6 in 24.4% of studies, compared with 84.4% for phototypes 4-6. The gap was smaller for melasma, but not for port wine stains. Among the devices examined, RCTs of diode lasers and intense pulsed light had the smallest gaps between inclusion of the two groups of phototypes, while pulsed-dye laser studies had the largest, they reported.
Stratification by journal showed the largest gap in studies published by Lasers in Medical Science and the smallest gap coming from Lasers in Surgery and Medicine. Funding was not specified for the majority of the eligible device RCTs, but those funded by industry had the smallest discrepancy between types 5-6 and types 4-6 and those supported by foundations/nonprofits the largest, Ms. Manjaly and associates said.
“With projections estimating that more than 50% of the U.S. population is set to identify as Hispanic or nonwhite by 2045 ... the lack of information has important consequences for clinical practice, as clinicians are unable to counsel patients on the efficacy and possible complications of various devices in patient with skin of color,” they wrote.
The investigators did not declare any conflicts of interest or funding sources.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
‘Exciting’ results for cancer vaccine plus pembro in melanoma
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
FROM AACR 2023
CMS inpatient payment rule for 2024: Key takeaways
The Centers for Medicare & Medicaid Services (CMS) released its annual update to the inpatient prospective payment system (IPPS) and long-term care hospital (LTCH) PPS on April 10, with many changes centered around improving health equity and quality as well as alleviating rural clinician shortages.
“This proposed rule reflects our person-centric approach to better measure health care quality and safety in hospitals to reduce preventable harm and our commitment to ensure that people with Medicare in rural and underserved areas have improved access to high-quality health care,” said CMS Administrator Chiquita Brooks-LaSure said in a statement.
Here are 14 things to know about the fiscal year (FY) 2024 proposal:
1. New payment rate: Acute-care hospitals that report inpatient quality data and participate in the EHR Meaningful Use program will receive a 2.8% net increase in payment rates. The rate adjustment will send approximately $3.3 billion more funding to hospitals compared with 2023.
2. LTCH payments: CMS projects that the LTCH standard payment rate will increase by 2.9%, whereas discharge payments will decrease by 2.5% or $59 million.
3. Disproportionate share hospital payments: Medicare disproportionate share hospital payments and Medicare uncompensated care payments will decrease by about $115 million for FY 2024.
4. Health equity categories: CMS proposes adding 15 new health equity hospital categorizations for IPPS payments to advance the goals of its Framework for Health Equity initiative.
5. Social determinants of health codes: To reflect increased resource utilization, the severity designation for the three International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes describing homelessness will change from noncomplication or comorbidity to complication or comorbidity.
6. Rural emergency hospitals: The proposed rule will allow designated rural emergency hospitals to serve as training sites and receive Medicare graduate medical education payments to address concerns over rural hospital closures.
7. COVID treatment add-on payments: If the public health emergency ends in May, add-on payments for discharges involving eligible products like convalescent plasma and nirmatrelvir-ritonavir will expire on Sept. 30.
8. Technology add-on payments: Requests for new technology add-on payments must include a complete, active Food and Drug Administration market authorization application. Beginning with FY 2025 applications, the FDA approval deadline will move from July 1 to May 1.
9. Physician-owned hospitals: To receive Medicare payment for services referred by a physician owner or investor, the hospital must satisfy all requirements of the whole hospital exception or the rural provider exception to the Stark Law. In either case, a hospital may not increase the aggregate number of operating rooms, procedure rooms, or beds above the level it was licensed for on March 23, 2010, unless CMS grants an exception.
10. Electronic clinical quality measures: The new rule will remove and modify several existing electronic clinical quality measures and add three new ones: hospital harm, pressure injury; hospital harm, acute kidney injury; and excessive radiation dose or inadequate image quality for diagnostic CT in adult inpatients.
11. HCAHPS survey: Beginning Jan. 1, 2025, modifications to the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey will extend the data collection period from 42 to 49 days, limit supplemental survey items to 12, and require an official Spanish translation for patients.
12. Safety-net hospitals request for information: CMS seeks public input about the unique challenges faced by safety-net hospitals and potential solutions to ensure that uninsured, underinsured, and other vulnerable populations have access to essential services.
13. LTCH quality reporting: CMS proposes several quality-measure updates, including a functional discharge score measure beginning in FY 2025 and reporting the percentage of patients current with Centers for Disease Control and Prevention–recommended COVID vaccinations starting in FY 2026.
14. Commenting period: CMS will accept comments on the proposed rule through June 9.
A version of this article originally appeared on Medscape.com.
The Centers for Medicare & Medicaid Services (CMS) released its annual update to the inpatient prospective payment system (IPPS) and long-term care hospital (LTCH) PPS on April 10, with many changes centered around improving health equity and quality as well as alleviating rural clinician shortages.
“This proposed rule reflects our person-centric approach to better measure health care quality and safety in hospitals to reduce preventable harm and our commitment to ensure that people with Medicare in rural and underserved areas have improved access to high-quality health care,” said CMS Administrator Chiquita Brooks-LaSure said in a statement.
Here are 14 things to know about the fiscal year (FY) 2024 proposal:
1. New payment rate: Acute-care hospitals that report inpatient quality data and participate in the EHR Meaningful Use program will receive a 2.8% net increase in payment rates. The rate adjustment will send approximately $3.3 billion more funding to hospitals compared with 2023.
2. LTCH payments: CMS projects that the LTCH standard payment rate will increase by 2.9%, whereas discharge payments will decrease by 2.5% or $59 million.
3. Disproportionate share hospital payments: Medicare disproportionate share hospital payments and Medicare uncompensated care payments will decrease by about $115 million for FY 2024.
4. Health equity categories: CMS proposes adding 15 new health equity hospital categorizations for IPPS payments to advance the goals of its Framework for Health Equity initiative.
5. Social determinants of health codes: To reflect increased resource utilization, the severity designation for the three International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes describing homelessness will change from noncomplication or comorbidity to complication or comorbidity.
6. Rural emergency hospitals: The proposed rule will allow designated rural emergency hospitals to serve as training sites and receive Medicare graduate medical education payments to address concerns over rural hospital closures.
7. COVID treatment add-on payments: If the public health emergency ends in May, add-on payments for discharges involving eligible products like convalescent plasma and nirmatrelvir-ritonavir will expire on Sept. 30.
8. Technology add-on payments: Requests for new technology add-on payments must include a complete, active Food and Drug Administration market authorization application. Beginning with FY 2025 applications, the FDA approval deadline will move from July 1 to May 1.
9. Physician-owned hospitals: To receive Medicare payment for services referred by a physician owner or investor, the hospital must satisfy all requirements of the whole hospital exception or the rural provider exception to the Stark Law. In either case, a hospital may not increase the aggregate number of operating rooms, procedure rooms, or beds above the level it was licensed for on March 23, 2010, unless CMS grants an exception.
10. Electronic clinical quality measures: The new rule will remove and modify several existing electronic clinical quality measures and add three new ones: hospital harm, pressure injury; hospital harm, acute kidney injury; and excessive radiation dose or inadequate image quality for diagnostic CT in adult inpatients.
11. HCAHPS survey: Beginning Jan. 1, 2025, modifications to the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey will extend the data collection period from 42 to 49 days, limit supplemental survey items to 12, and require an official Spanish translation for patients.
12. Safety-net hospitals request for information: CMS seeks public input about the unique challenges faced by safety-net hospitals and potential solutions to ensure that uninsured, underinsured, and other vulnerable populations have access to essential services.
13. LTCH quality reporting: CMS proposes several quality-measure updates, including a functional discharge score measure beginning in FY 2025 and reporting the percentage of patients current with Centers for Disease Control and Prevention–recommended COVID vaccinations starting in FY 2026.
14. Commenting period: CMS will accept comments on the proposed rule through June 9.
A version of this article originally appeared on Medscape.com.
The Centers for Medicare & Medicaid Services (CMS) released its annual update to the inpatient prospective payment system (IPPS) and long-term care hospital (LTCH) PPS on April 10, with many changes centered around improving health equity and quality as well as alleviating rural clinician shortages.
“This proposed rule reflects our person-centric approach to better measure health care quality and safety in hospitals to reduce preventable harm and our commitment to ensure that people with Medicare in rural and underserved areas have improved access to high-quality health care,” said CMS Administrator Chiquita Brooks-LaSure said in a statement.
Here are 14 things to know about the fiscal year (FY) 2024 proposal:
1. New payment rate: Acute-care hospitals that report inpatient quality data and participate in the EHR Meaningful Use program will receive a 2.8% net increase in payment rates. The rate adjustment will send approximately $3.3 billion more funding to hospitals compared with 2023.
2. LTCH payments: CMS projects that the LTCH standard payment rate will increase by 2.9%, whereas discharge payments will decrease by 2.5% or $59 million.
3. Disproportionate share hospital payments: Medicare disproportionate share hospital payments and Medicare uncompensated care payments will decrease by about $115 million for FY 2024.
4. Health equity categories: CMS proposes adding 15 new health equity hospital categorizations for IPPS payments to advance the goals of its Framework for Health Equity initiative.
5. Social determinants of health codes: To reflect increased resource utilization, the severity designation for the three International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes describing homelessness will change from noncomplication or comorbidity to complication or comorbidity.
6. Rural emergency hospitals: The proposed rule will allow designated rural emergency hospitals to serve as training sites and receive Medicare graduate medical education payments to address concerns over rural hospital closures.
7. COVID treatment add-on payments: If the public health emergency ends in May, add-on payments for discharges involving eligible products like convalescent plasma and nirmatrelvir-ritonavir will expire on Sept. 30.
8. Technology add-on payments: Requests for new technology add-on payments must include a complete, active Food and Drug Administration market authorization application. Beginning with FY 2025 applications, the FDA approval deadline will move from July 1 to May 1.
9. Physician-owned hospitals: To receive Medicare payment for services referred by a physician owner or investor, the hospital must satisfy all requirements of the whole hospital exception or the rural provider exception to the Stark Law. In either case, a hospital may not increase the aggregate number of operating rooms, procedure rooms, or beds above the level it was licensed for on March 23, 2010, unless CMS grants an exception.
10. Electronic clinical quality measures: The new rule will remove and modify several existing electronic clinical quality measures and add three new ones: hospital harm, pressure injury; hospital harm, acute kidney injury; and excessive radiation dose or inadequate image quality for diagnostic CT in adult inpatients.
11. HCAHPS survey: Beginning Jan. 1, 2025, modifications to the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey will extend the data collection period from 42 to 49 days, limit supplemental survey items to 12, and require an official Spanish translation for patients.
12. Safety-net hospitals request for information: CMS seeks public input about the unique challenges faced by safety-net hospitals and potential solutions to ensure that uninsured, underinsured, and other vulnerable populations have access to essential services.
13. LTCH quality reporting: CMS proposes several quality-measure updates, including a functional discharge score measure beginning in FY 2025 and reporting the percentage of patients current with Centers for Disease Control and Prevention–recommended COVID vaccinations starting in FY 2026.
14. Commenting period: CMS will accept comments on the proposed rule through June 9.
A version of this article originally appeared on Medscape.com.
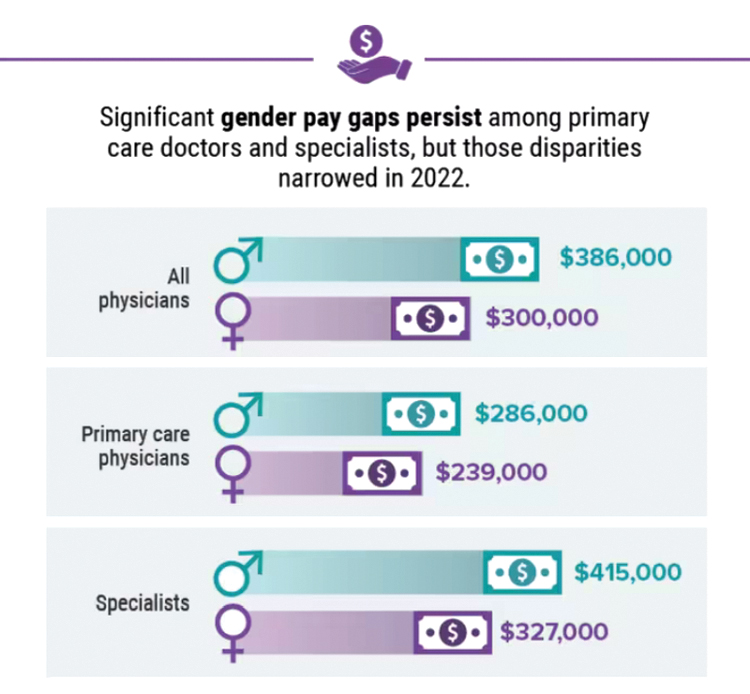
Infographic: Is your compensation rising as fast as your peers?
Did doctors’ salaries continue their zesty postpandemic rise in 2022? Are female physicians making pay gains versus their male counterparts that spark optimism for the future?
reveals which medical specialties pay better than others, and evaluates the current gender pay gap in medicine. If you’re interested in delving deeper into the data, check out Your Income vs. Your Peers’: Physician Compensation Report 2023.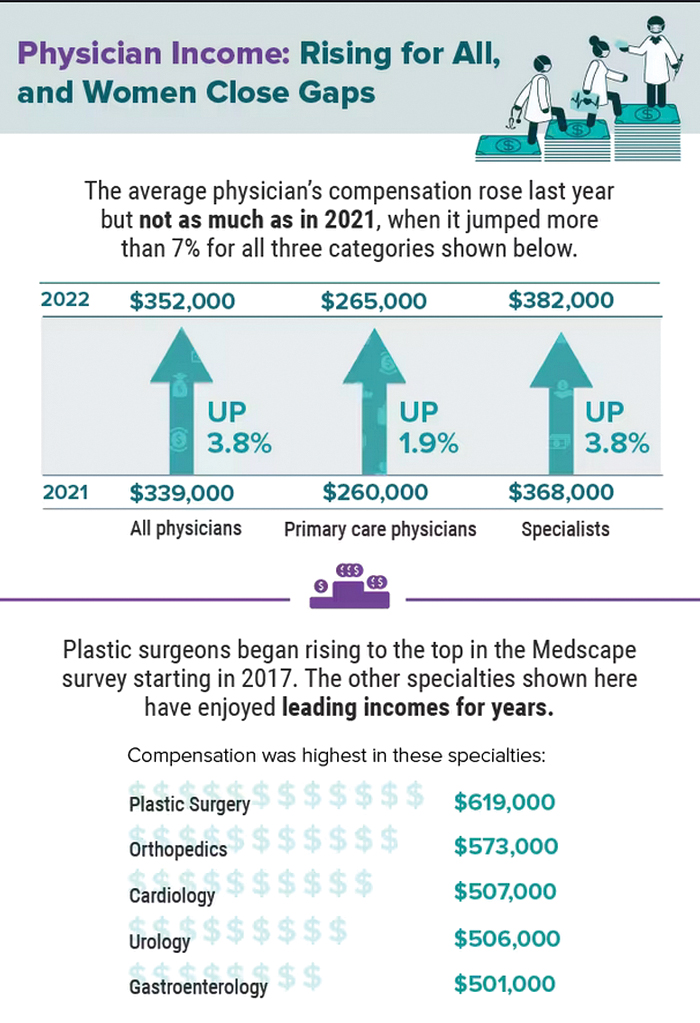
A version of this article first appeared on Medscape.com.
Did doctors’ salaries continue their zesty postpandemic rise in 2022? Are female physicians making pay gains versus their male counterparts that spark optimism for the future?
reveals which medical specialties pay better than others, and evaluates the current gender pay gap in medicine. If you’re interested in delving deeper into the data, check out Your Income vs. Your Peers’: Physician Compensation Report 2023.

A version of this article first appeared on Medscape.com.
Did doctors’ salaries continue their zesty postpandemic rise in 2022? Are female physicians making pay gains versus their male counterparts that spark optimism for the future?
reveals which medical specialties pay better than others, and evaluates the current gender pay gap in medicine. If you’re interested in delving deeper into the data, check out Your Income vs. Your Peers’: Physician Compensation Report 2023.

A version of this article first appeared on Medscape.com.
Five chronic mistakes that can sabotage your medical practice
A physician who in the past has led medical groups as both chief medical officer and president, Gerda Maissel, MD, president of My MD Advisor, a private patient advocacy group, has seen the good, bad, and ugly of practice administration. There’s a spectrum of infractions: Anything from doctors making inappropriate jokes with staff or patients, to failing to establish key relationships with other critical entities, says Dr. Maissel.
“Being a good physician who provides value is important in building a practice,” explained Dr. Maissel. “But it is not the be-all and end-all.”
While the number of physician-owned practices is declining, just under 50% are still in private practice, according to the American Medical Association’s 2020 survey. There’s also a continuing trend toward larger practices. Whatever the size, the physicians are responsible for strategy, marketing, building the practice, and maintaining profitability.
Catherine Lightfoot, CPA, CHBC, president of the National Society of Certified Healthcare Business Consultants (NSCHBC), has her finger on the pulse of what’s right and what’s wrong when it comes to running a medical practice. Although she says there are no hard and fast rules on how to run a thriving medical group, there are common mistakes that physicians often don’t recognize.
Here are the five key mistakes that commonly crop up, and the experts’ thoughts on how to prevent or fix them.
1. Failing to engage in outreach activities and community efforts to build your practice.
Yes, physicians earn good reputations through dedicated work, and that often precedes them when it comes to building a practice. But assuming that hanging a shingle backed by strong credentials is all it takes for success is akin to building a website and assuming people will find it organically. Maybe there was a time, in a small community, where this was good enough. But no longer.
It’s important to plan to get your practice and your name known to potential patients. “Most physicians think that means advertising, but that’s not the complete case,” Dr. Maissel said.
Much of the equation involves ensuring availability. This means setting office hours that work for your target audience of patients, and then ensuring you stick to those hours. This extends beyond scheduling your current patients and into referral patients, too. And it’s particularly true while in the building phase of a new practice.
“If one of your colleagues calls with a referral patient, and they consider the matter urgent, you need to heed that,” explained Dr. Maissel. “So have a breadth of availability for these referral cases.” Through word of mouth, you’ll get a good reputation for patient care and availability, and that will go a long way toward helping to grow your practice.
Establishing a culture that doesn’t involve canceling and rescheduling patients is part of the scheduling equation, too. “I’ve seen the full gamut of cancellation policies, ranging from a month’s notice on changes to 3 months’ notice,” said Dr. Maissel. “It all gets at the same issue, which is failing to set up a culture where doctors don’t change their schedules and leave patients hanging.”
In the end, wonky scheduling, cancellations, and a lack of respect for the urgency of referrals can cost a practice. Forge a reputation in reliability and word will get around, in all the right ways.
2. Not having enough oversight of your outsourced billing service
Billing is one of the biggest pieces of running a successful and profitable practice, yet too many practices ignore it once they’ve handed it off to a billing company. That can cost you in more ways than one, said Ms. Lightfoot. “Billing changes all the time, and if you’re not monitoring your billing partner, you don’t know what you’re getting,” she said.
Ms. Lightfoot said that a decade ago, billing was much more straightforward – essentially, you did the work and received payment. Today’s complex insurance, Medicare, and Medicaid environment have changed the landscape. “Now you have to fight for every dollar you’re billing,” said Ms. Lightfoot. “Rates get cut all the time, you might miss out on a claim, and the rules are constantly changing.”
The solution for many practices is to outsource billing, which Ms. Lightfoot supports. “They specialize in this, and that’s a great start,” she said. “But it’s not as simple as handing it off and forgetting it.”
Instead, ensure your internal staff is up to date on all things coding and billing so that they can catch what your outsourced billing partner doesn’t. Your internal staff should be prepared to carry out coding, check coding, and stay on top of the billing company if they aren’t processing claims quickly enough. For instance: If there’s a denial, how many times will the billing company go after that money?
Other questions to ask when entering a billing relationship: What does the billing company expect from your practice? Do they communicate what needs to be worked on or fixed? Are they providing you with monthly reports? “You want to make sure you’re getting those reports every month and reading them over carefully,” said Ms. Lightfoot.
This means that if you have a large practice, you should have a point person within your billing department to handle the relationship with your billing partner. If it’s a smaller practice, the task will likely fall to the office manager. The ‘who’ isn’t important, but having someone on the case is.
Another important aspect of this billing relationship is understanding what you’re receiving for your payment. “Sometimes going with the cheapest offer amounts to a billing partner who isn’t working on those claims and denials as much as they should,” said Ms. Lightfoot. “I’ve seen fees anywhere from 4% to 9%, and the lower end can mean you’ll need to chase down every penny.”
3. Neglecting to forge the right relationships in the community.
Another common mistake physicians make is failing to develop the professional relationships that will help you thrive. Successful practices need to establish relationships with the right people and organizations. While the occasional afternoon of golf used to serve this purpose, today outreach must go beyond that, said Dr. Maissel. “You need to create relationships with hospitals and hospital-based practices because you may have value to them,” she said. “You should also get into some sort of relationship with your local ACO (Accountable Care Organization) or PHO (Physician Hospital Organization). Identify the leaders there and let them know you exist.”
Establishing these relationships goes beyond that first step of introducing yourself, or you risk losing their benefits. You must also nurture and “fertilize” these relationships in an ongoing fashion. “For years, as the head of employee practice, I had a competitor who would go out of his way to invite me to lunch regularly,” said Dr. Maissel. “When there were opportunities for his group, I would connect him. I wouldn’t have done that had he not worked on our relationship over time.”
The adage of “it’s not what you know but who you know” holds up here. If you don’t do the reach out to the right people and organizations in your community, you will have a harder time succeeding as a practice.
4. Hiring the wrong person/a family member for the job.
When starting a new practice, or if you’re running a small practice, it can be tempting to look for affordable or reliable staffing from among family members or friends. That’s fine if your family member or friend is also qualified for the job. If they aren’t, however, you might be setting up for failure.
“When you hire someone without the right qualifications, you need to be willing to train them for the job,” said Ms. Lightfoot. “Doctors don’t have that kind of time.”
Too often, Ms. Lightfoot said, a doctor will have a position like officer manager open and fill it with an in-law, whether he or she is experienced or not. “Now you have someone in the role who is unqualified, and the rest of the office can’t speak up about that because it’s a relative to the lead physician,” she said. “That doesn’t create a good environment for anyone.”
Also, a setup for failure is hiring someone who might be qualified, but not possessing the right personality for the role. A front desk position, for instance, should be held by someone who’s a bit upbeat and able to multitask. “You can’t put a shy, quiet person in that job,” said Ms. Lightfoot. “So, if you see a person with 10 years’ experience in a medical practice, but they’re reserved, what will happen? You must think about this when hiring.”
One PA recalled a small family practice in which the lead physician’s wife was the office manager. To save money, the wife removed lights from the staff restroom and staff lunchroom and declined staff requests for earned vacation. The staff felt unable to speak up, and they – and all new office staff members – ultimately left the practice.
5. Overlooking the importance of acting like a professional and respecting your staff.
This one might seem obvious, but many physicians get a bit too comfortable in the office environment, said Dr. Maissel. This can encompass a whole host of bad behaviors, from making inappropriate jokes to staff and patients, to trash-talking colleagues. None of this behavior is acceptable and can set you up for things to go wrong, especially when good labor is hard to come by. “Your staff is made up of people for whom 50 cents an hour is meaningful,” she said. “If they don’t have a warm, supportive office, they will look elsewhere.”
This is especially true of younger people now entering the workforce – they are less tolerant than generations past of egregious behavior. Try to establish a professional, yet nurturing environment for your staff. “Inquire about things that matter to them,” said Dr. Maissel. “Small talk can go a long way. See them as human beings, not cogs in the wheel.”
Inappropriate and uncaring behaviors will give physician leaders a reputation, one that sticks. “The medical community is pretty connected, and if you behave inappropriately enough times, it will circle back to you,” said Dr. Maissel.
Launching, and sustaining, a successful medical practice is never a given, but mistakes are. With the right approach, however, you can avoid these common – and impactful – errors and set your practice up for success.
A version of this article first appeared on Medscape.com.
A physician who in the past has led medical groups as both chief medical officer and president, Gerda Maissel, MD, president of My MD Advisor, a private patient advocacy group, has seen the good, bad, and ugly of practice administration. There’s a spectrum of infractions: Anything from doctors making inappropriate jokes with staff or patients, to failing to establish key relationships with other critical entities, says Dr. Maissel.
“Being a good physician who provides value is important in building a practice,” explained Dr. Maissel. “But it is not the be-all and end-all.”
While the number of physician-owned practices is declining, just under 50% are still in private practice, according to the American Medical Association’s 2020 survey. There’s also a continuing trend toward larger practices. Whatever the size, the physicians are responsible for strategy, marketing, building the practice, and maintaining profitability.
Catherine Lightfoot, CPA, CHBC, president of the National Society of Certified Healthcare Business Consultants (NSCHBC), has her finger on the pulse of what’s right and what’s wrong when it comes to running a medical practice. Although she says there are no hard and fast rules on how to run a thriving medical group, there are common mistakes that physicians often don’t recognize.
Here are the five key mistakes that commonly crop up, and the experts’ thoughts on how to prevent or fix them.
1. Failing to engage in outreach activities and community efforts to build your practice.
Yes, physicians earn good reputations through dedicated work, and that often precedes them when it comes to building a practice. But assuming that hanging a shingle backed by strong credentials is all it takes for success is akin to building a website and assuming people will find it organically. Maybe there was a time, in a small community, where this was good enough. But no longer.
It’s important to plan to get your practice and your name known to potential patients. “Most physicians think that means advertising, but that’s not the complete case,” Dr. Maissel said.
Much of the equation involves ensuring availability. This means setting office hours that work for your target audience of patients, and then ensuring you stick to those hours. This extends beyond scheduling your current patients and into referral patients, too. And it’s particularly true while in the building phase of a new practice.
“If one of your colleagues calls with a referral patient, and they consider the matter urgent, you need to heed that,” explained Dr. Maissel. “So have a breadth of availability for these referral cases.” Through word of mouth, you’ll get a good reputation for patient care and availability, and that will go a long way toward helping to grow your practice.
Establishing a culture that doesn’t involve canceling and rescheduling patients is part of the scheduling equation, too. “I’ve seen the full gamut of cancellation policies, ranging from a month’s notice on changes to 3 months’ notice,” said Dr. Maissel. “It all gets at the same issue, which is failing to set up a culture where doctors don’t change their schedules and leave patients hanging.”
In the end, wonky scheduling, cancellations, and a lack of respect for the urgency of referrals can cost a practice. Forge a reputation in reliability and word will get around, in all the right ways.
2. Not having enough oversight of your outsourced billing service
Billing is one of the biggest pieces of running a successful and profitable practice, yet too many practices ignore it once they’ve handed it off to a billing company. That can cost you in more ways than one, said Ms. Lightfoot. “Billing changes all the time, and if you’re not monitoring your billing partner, you don’t know what you’re getting,” she said.
Ms. Lightfoot said that a decade ago, billing was much more straightforward – essentially, you did the work and received payment. Today’s complex insurance, Medicare, and Medicaid environment have changed the landscape. “Now you have to fight for every dollar you’re billing,” said Ms. Lightfoot. “Rates get cut all the time, you might miss out on a claim, and the rules are constantly changing.”
The solution for many practices is to outsource billing, which Ms. Lightfoot supports. “They specialize in this, and that’s a great start,” she said. “But it’s not as simple as handing it off and forgetting it.”
Instead, ensure your internal staff is up to date on all things coding and billing so that they can catch what your outsourced billing partner doesn’t. Your internal staff should be prepared to carry out coding, check coding, and stay on top of the billing company if they aren’t processing claims quickly enough. For instance: If there’s a denial, how many times will the billing company go after that money?
Other questions to ask when entering a billing relationship: What does the billing company expect from your practice? Do they communicate what needs to be worked on or fixed? Are they providing you with monthly reports? “You want to make sure you’re getting those reports every month and reading them over carefully,” said Ms. Lightfoot.
This means that if you have a large practice, you should have a point person within your billing department to handle the relationship with your billing partner. If it’s a smaller practice, the task will likely fall to the office manager. The ‘who’ isn’t important, but having someone on the case is.
Another important aspect of this billing relationship is understanding what you’re receiving for your payment. “Sometimes going with the cheapest offer amounts to a billing partner who isn’t working on those claims and denials as much as they should,” said Ms. Lightfoot. “I’ve seen fees anywhere from 4% to 9%, and the lower end can mean you’ll need to chase down every penny.”
3. Neglecting to forge the right relationships in the community.
Another common mistake physicians make is failing to develop the professional relationships that will help you thrive. Successful practices need to establish relationships with the right people and organizations. While the occasional afternoon of golf used to serve this purpose, today outreach must go beyond that, said Dr. Maissel. “You need to create relationships with hospitals and hospital-based practices because you may have value to them,” she said. “You should also get into some sort of relationship with your local ACO (Accountable Care Organization) or PHO (Physician Hospital Organization). Identify the leaders there and let them know you exist.”
Establishing these relationships goes beyond that first step of introducing yourself, or you risk losing their benefits. You must also nurture and “fertilize” these relationships in an ongoing fashion. “For years, as the head of employee practice, I had a competitor who would go out of his way to invite me to lunch regularly,” said Dr. Maissel. “When there were opportunities for his group, I would connect him. I wouldn’t have done that had he not worked on our relationship over time.”
The adage of “it’s not what you know but who you know” holds up here. If you don’t do the reach out to the right people and organizations in your community, you will have a harder time succeeding as a practice.
4. Hiring the wrong person/a family member for the job.
When starting a new practice, or if you’re running a small practice, it can be tempting to look for affordable or reliable staffing from among family members or friends. That’s fine if your family member or friend is also qualified for the job. If they aren’t, however, you might be setting up for failure.
“When you hire someone without the right qualifications, you need to be willing to train them for the job,” said Ms. Lightfoot. “Doctors don’t have that kind of time.”
Too often, Ms. Lightfoot said, a doctor will have a position like officer manager open and fill it with an in-law, whether he or she is experienced or not. “Now you have someone in the role who is unqualified, and the rest of the office can’t speak up about that because it’s a relative to the lead physician,” she said. “That doesn’t create a good environment for anyone.”
Also, a setup for failure is hiring someone who might be qualified, but not possessing the right personality for the role. A front desk position, for instance, should be held by someone who’s a bit upbeat and able to multitask. “You can’t put a shy, quiet person in that job,” said Ms. Lightfoot. “So, if you see a person with 10 years’ experience in a medical practice, but they’re reserved, what will happen? You must think about this when hiring.”
One PA recalled a small family practice in which the lead physician’s wife was the office manager. To save money, the wife removed lights from the staff restroom and staff lunchroom and declined staff requests for earned vacation. The staff felt unable to speak up, and they – and all new office staff members – ultimately left the practice.
5. Overlooking the importance of acting like a professional and respecting your staff.
This one might seem obvious, but many physicians get a bit too comfortable in the office environment, said Dr. Maissel. This can encompass a whole host of bad behaviors, from making inappropriate jokes to staff and patients, to trash-talking colleagues. None of this behavior is acceptable and can set you up for things to go wrong, especially when good labor is hard to come by. “Your staff is made up of people for whom 50 cents an hour is meaningful,” she said. “If they don’t have a warm, supportive office, they will look elsewhere.”
This is especially true of younger people now entering the workforce – they are less tolerant than generations past of egregious behavior. Try to establish a professional, yet nurturing environment for your staff. “Inquire about things that matter to them,” said Dr. Maissel. “Small talk can go a long way. See them as human beings, not cogs in the wheel.”
Inappropriate and uncaring behaviors will give physician leaders a reputation, one that sticks. “The medical community is pretty connected, and if you behave inappropriately enough times, it will circle back to you,” said Dr. Maissel.
Launching, and sustaining, a successful medical practice is never a given, but mistakes are. With the right approach, however, you can avoid these common – and impactful – errors and set your practice up for success.
A version of this article first appeared on Medscape.com.
A physician who in the past has led medical groups as both chief medical officer and president, Gerda Maissel, MD, president of My MD Advisor, a private patient advocacy group, has seen the good, bad, and ugly of practice administration. There’s a spectrum of infractions: Anything from doctors making inappropriate jokes with staff or patients, to failing to establish key relationships with other critical entities, says Dr. Maissel.
“Being a good physician who provides value is important in building a practice,” explained Dr. Maissel. “But it is not the be-all and end-all.”
While the number of physician-owned practices is declining, just under 50% are still in private practice, according to the American Medical Association’s 2020 survey. There’s also a continuing trend toward larger practices. Whatever the size, the physicians are responsible for strategy, marketing, building the practice, and maintaining profitability.
Catherine Lightfoot, CPA, CHBC, president of the National Society of Certified Healthcare Business Consultants (NSCHBC), has her finger on the pulse of what’s right and what’s wrong when it comes to running a medical practice. Although she says there are no hard and fast rules on how to run a thriving medical group, there are common mistakes that physicians often don’t recognize.
Here are the five key mistakes that commonly crop up, and the experts’ thoughts on how to prevent or fix them.
1. Failing to engage in outreach activities and community efforts to build your practice.
Yes, physicians earn good reputations through dedicated work, and that often precedes them when it comes to building a practice. But assuming that hanging a shingle backed by strong credentials is all it takes for success is akin to building a website and assuming people will find it organically. Maybe there was a time, in a small community, where this was good enough. But no longer.
It’s important to plan to get your practice and your name known to potential patients. “Most physicians think that means advertising, but that’s not the complete case,” Dr. Maissel said.
Much of the equation involves ensuring availability. This means setting office hours that work for your target audience of patients, and then ensuring you stick to those hours. This extends beyond scheduling your current patients and into referral patients, too. And it’s particularly true while in the building phase of a new practice.
“If one of your colleagues calls with a referral patient, and they consider the matter urgent, you need to heed that,” explained Dr. Maissel. “So have a breadth of availability for these referral cases.” Through word of mouth, you’ll get a good reputation for patient care and availability, and that will go a long way toward helping to grow your practice.
Establishing a culture that doesn’t involve canceling and rescheduling patients is part of the scheduling equation, too. “I’ve seen the full gamut of cancellation policies, ranging from a month’s notice on changes to 3 months’ notice,” said Dr. Maissel. “It all gets at the same issue, which is failing to set up a culture where doctors don’t change their schedules and leave patients hanging.”
In the end, wonky scheduling, cancellations, and a lack of respect for the urgency of referrals can cost a practice. Forge a reputation in reliability and word will get around, in all the right ways.
2. Not having enough oversight of your outsourced billing service
Billing is one of the biggest pieces of running a successful and profitable practice, yet too many practices ignore it once they’ve handed it off to a billing company. That can cost you in more ways than one, said Ms. Lightfoot. “Billing changes all the time, and if you’re not monitoring your billing partner, you don’t know what you’re getting,” she said.
Ms. Lightfoot said that a decade ago, billing was much more straightforward – essentially, you did the work and received payment. Today’s complex insurance, Medicare, and Medicaid environment have changed the landscape. “Now you have to fight for every dollar you’re billing,” said Ms. Lightfoot. “Rates get cut all the time, you might miss out on a claim, and the rules are constantly changing.”
The solution for many practices is to outsource billing, which Ms. Lightfoot supports. “They specialize in this, and that’s a great start,” she said. “But it’s not as simple as handing it off and forgetting it.”
Instead, ensure your internal staff is up to date on all things coding and billing so that they can catch what your outsourced billing partner doesn’t. Your internal staff should be prepared to carry out coding, check coding, and stay on top of the billing company if they aren’t processing claims quickly enough. For instance: If there’s a denial, how many times will the billing company go after that money?
Other questions to ask when entering a billing relationship: What does the billing company expect from your practice? Do they communicate what needs to be worked on or fixed? Are they providing you with monthly reports? “You want to make sure you’re getting those reports every month and reading them over carefully,” said Ms. Lightfoot.
This means that if you have a large practice, you should have a point person within your billing department to handle the relationship with your billing partner. If it’s a smaller practice, the task will likely fall to the office manager. The ‘who’ isn’t important, but having someone on the case is.
Another important aspect of this billing relationship is understanding what you’re receiving for your payment. “Sometimes going with the cheapest offer amounts to a billing partner who isn’t working on those claims and denials as much as they should,” said Ms. Lightfoot. “I’ve seen fees anywhere from 4% to 9%, and the lower end can mean you’ll need to chase down every penny.”
3. Neglecting to forge the right relationships in the community.
Another common mistake physicians make is failing to develop the professional relationships that will help you thrive. Successful practices need to establish relationships with the right people and organizations. While the occasional afternoon of golf used to serve this purpose, today outreach must go beyond that, said Dr. Maissel. “You need to create relationships with hospitals and hospital-based practices because you may have value to them,” she said. “You should also get into some sort of relationship with your local ACO (Accountable Care Organization) or PHO (Physician Hospital Organization). Identify the leaders there and let them know you exist.”
Establishing these relationships goes beyond that first step of introducing yourself, or you risk losing their benefits. You must also nurture and “fertilize” these relationships in an ongoing fashion. “For years, as the head of employee practice, I had a competitor who would go out of his way to invite me to lunch regularly,” said Dr. Maissel. “When there were opportunities for his group, I would connect him. I wouldn’t have done that had he not worked on our relationship over time.”
The adage of “it’s not what you know but who you know” holds up here. If you don’t do the reach out to the right people and organizations in your community, you will have a harder time succeeding as a practice.
4. Hiring the wrong person/a family member for the job.
When starting a new practice, or if you’re running a small practice, it can be tempting to look for affordable or reliable staffing from among family members or friends. That’s fine if your family member or friend is also qualified for the job. If they aren’t, however, you might be setting up for failure.
“When you hire someone without the right qualifications, you need to be willing to train them for the job,” said Ms. Lightfoot. “Doctors don’t have that kind of time.”
Too often, Ms. Lightfoot said, a doctor will have a position like officer manager open and fill it with an in-law, whether he or she is experienced or not. “Now you have someone in the role who is unqualified, and the rest of the office can’t speak up about that because it’s a relative to the lead physician,” she said. “That doesn’t create a good environment for anyone.”
Also, a setup for failure is hiring someone who might be qualified, but not possessing the right personality for the role. A front desk position, for instance, should be held by someone who’s a bit upbeat and able to multitask. “You can’t put a shy, quiet person in that job,” said Ms. Lightfoot. “So, if you see a person with 10 years’ experience in a medical practice, but they’re reserved, what will happen? You must think about this when hiring.”
One PA recalled a small family practice in which the lead physician’s wife was the office manager. To save money, the wife removed lights from the staff restroom and staff lunchroom and declined staff requests for earned vacation. The staff felt unable to speak up, and they – and all new office staff members – ultimately left the practice.
5. Overlooking the importance of acting like a professional and respecting your staff.
This one might seem obvious, but many physicians get a bit too comfortable in the office environment, said Dr. Maissel. This can encompass a whole host of bad behaviors, from making inappropriate jokes to staff and patients, to trash-talking colleagues. None of this behavior is acceptable and can set you up for things to go wrong, especially when good labor is hard to come by. “Your staff is made up of people for whom 50 cents an hour is meaningful,” she said. “If they don’t have a warm, supportive office, they will look elsewhere.”
This is especially true of younger people now entering the workforce – they are less tolerant than generations past of egregious behavior. Try to establish a professional, yet nurturing environment for your staff. “Inquire about things that matter to them,” said Dr. Maissel. “Small talk can go a long way. See them as human beings, not cogs in the wheel.”
Inappropriate and uncaring behaviors will give physician leaders a reputation, one that sticks. “The medical community is pretty connected, and if you behave inappropriately enough times, it will circle back to you,” said Dr. Maissel.
Launching, and sustaining, a successful medical practice is never a given, but mistakes are. With the right approach, however, you can avoid these common – and impactful – errors and set your practice up for success.
A version of this article first appeared on Medscape.com.
Living the introvert’s dream: Alone for 500 days, but never lonely
Beating the allegory of the cave
When Beatriz Flamini spoke with reporters on April 14, she knew nothing of the previous 18 months. The Russian invasion of Ukraine? Nope. The death of Queen Elizabeth? Also no. But before you make fun of her, she has an excuse. She’s been living under a rock.
As part of an experiment to test how social isolation and disorientation affect a person’s mind, sense of time, and sleeping patterns, Ms. Flamini lived in a 70-meter-deep cave in southern Spain for 500 days, starting in November 2021. Alone. No outside communication with the outside world in any way, though she was constantly monitored by a team of researchers. She also had multiple cameras filming her for an upcoming documentary.
This is a massive step up from the previous record for time spent underground for science: A team of 15 spent 50 days underground in 2021 to similar study of isolation and how it affected circadian rhythms. It’s also almost certainly a world record for time spent underground.
All that time alone certainly sounds like some sort of medieval torture, but Ms. Flamini had access to food, water, and a library of books. Which she made liberal use of, reading at least 60 books during her stay. She also had a panic button in case the isolation became too much or an emergency developed, but she never considered using it.
She lost track of time after 2 months, flies invaded the cave on occasion, and maintaining coherence was occasionally a struggle, but she kept things together very well. In fact, she didn’t even want to leave when her team came for her. She wasn’t even finished with her 61st book.
When she spoke with gathered reporters after the ordeal, words were obviously difficult to come by for her, having not spoken in nearly 18 months, but her mind was clearly still sharp and she had a very important question for everyone gathered around her.
Who’s buying the beer?
We approve of this request.
Staphylococcus and the speed of evolution
Bacteria, we know, are tough little buggers that are hard to see and even harder to get rid of. So hard, actually, that human bodies eventually gave up on the task and decided to just incorporate them into our organ systems. But why are bacteria so hard to eliminate?
Two words: rapid evolution. How rapid? For the first time, scientists have directly observed adaptive evolution by Staphylococcus aureus in a single person’s skin microbiome. That’s how rapid.
For their study, the researchers collected samples from the nostrils, backs of knees, insides of elbows, and forearms of 23 children with eczema. They eventually cultured almost 1,500 unique colonies of S. aureus cells from those samples and sequenced the cells’ genomes.
All that sampling and culturing and sequencing showed that it was rare for a new S. aureus strain to come in and replace the existing strain. “Despite the stability at the lineage level, we see a lot of dynamics at the whole genome level, where new mutations are constantly arising in these bacteria and then spreading throughout the entire body,” Tami D. Lieberman, PhD, of the Massachusetts Institute of Technology, Cambridge, said in a written statement from MIT.
One frequent mutation involved a gene called capD, which encodes an enzyme necessary for synthesizing the capsular polysaccharide – a coating that protects S. aureus from recognition by immune cells. In one patient, four different mutations of capD arose independently in different samples before one variant became dominant and spread over the entire microbiome, MIT reported.
The mutation, which actually results in the loss of the polysaccharide capsule, may allow cells to grow faster than those without the mutation because they have more fuel to power their own growth, the researchers suggested. It’s also possible that loss of the capsule allows S. aureus cells to stick to the skin better because proteins that allow them to adhere to the skin are more exposed.
Dr. Lieberman and her associates hope that these variant-containing cells could be a new target for eczema treatments, but we’re never optimistic when it comes to bacteria. That’s because some of us are old enough to remember evolutionary biologist Stephen Jay Gould, who wrote in his book “Full House”: “Our planet has always been in the ‘Age of Bacteria,’ ever since the first fossils – bacteria, of course – were entombed in rocks more than 3 billion years ago. On any possible, reasonable or fair criterion, bacteria are – and always have been – the dominant forms of life on Earth.”
In the distant future, long after humans have left the scene, the bacteria will be laughing at the last rats and cockroaches scurrying across the landscape. Wanna bet?
The height of genetic prediction
Genetics are practically a DNA Scrabble bag. Traits like eye color and hair texture are chosen in the same fashion, based on what gets pulled from our own genetic bag of letters, but what about height? Researchers may now have a way to predict adult height and make it more than just an educated guess.
How? By looking at the genes in our growth plates. The cartilage on the ends of our bones hardens as we age, eventually deciding an individual’s stature. In a recently published study, a research team looked at 600 million cartilage cells linked to maturation and cell growth in mice. Because everything starts with rodents.
After that search identified 145 genes linked to growth plate maturation and formation of the bones, they compared the mouse genes with data from genome-wide association studies (GWAS) of human height to look for hotspots where the height genes exist in human DNA.
The results showed which genes play a role in deciding height, and the GWAS data also suggested that genetic changes affecting cartilage cell maturation may strongly influence adult height, said the investigators, who hope that earlier interventions can improve outcomes in patients with conditions such as skeletal dysplasia.
So, yeah, you may want to be a little taller or shorter, but the outcome of that particular Scrabble game was determined when your parents, you know, dropped the letters in the bag.
Beating the allegory of the cave
When Beatriz Flamini spoke with reporters on April 14, she knew nothing of the previous 18 months. The Russian invasion of Ukraine? Nope. The death of Queen Elizabeth? Also no. But before you make fun of her, she has an excuse. She’s been living under a rock.
As part of an experiment to test how social isolation and disorientation affect a person’s mind, sense of time, and sleeping patterns, Ms. Flamini lived in a 70-meter-deep cave in southern Spain for 500 days, starting in November 2021. Alone. No outside communication with the outside world in any way, though she was constantly monitored by a team of researchers. She also had multiple cameras filming her for an upcoming documentary.
This is a massive step up from the previous record for time spent underground for science: A team of 15 spent 50 days underground in 2021 to similar study of isolation and how it affected circadian rhythms. It’s also almost certainly a world record for time spent underground.
All that time alone certainly sounds like some sort of medieval torture, but Ms. Flamini had access to food, water, and a library of books. Which she made liberal use of, reading at least 60 books during her stay. She also had a panic button in case the isolation became too much or an emergency developed, but she never considered using it.
She lost track of time after 2 months, flies invaded the cave on occasion, and maintaining coherence was occasionally a struggle, but she kept things together very well. In fact, she didn’t even want to leave when her team came for her. She wasn’t even finished with her 61st book.
When she spoke with gathered reporters after the ordeal, words were obviously difficult to come by for her, having not spoken in nearly 18 months, but her mind was clearly still sharp and she had a very important question for everyone gathered around her.
Who’s buying the beer?
We approve of this request.
Staphylococcus and the speed of evolution
Bacteria, we know, are tough little buggers that are hard to see and even harder to get rid of. So hard, actually, that human bodies eventually gave up on the task and decided to just incorporate them into our organ systems. But why are bacteria so hard to eliminate?
Two words: rapid evolution. How rapid? For the first time, scientists have directly observed adaptive evolution by Staphylococcus aureus in a single person’s skin microbiome. That’s how rapid.
For their study, the researchers collected samples from the nostrils, backs of knees, insides of elbows, and forearms of 23 children with eczema. They eventually cultured almost 1,500 unique colonies of S. aureus cells from those samples and sequenced the cells’ genomes.
All that sampling and culturing and sequencing showed that it was rare for a new S. aureus strain to come in and replace the existing strain. “Despite the stability at the lineage level, we see a lot of dynamics at the whole genome level, where new mutations are constantly arising in these bacteria and then spreading throughout the entire body,” Tami D. Lieberman, PhD, of the Massachusetts Institute of Technology, Cambridge, said in a written statement from MIT.
One frequent mutation involved a gene called capD, which encodes an enzyme necessary for synthesizing the capsular polysaccharide – a coating that protects S. aureus from recognition by immune cells. In one patient, four different mutations of capD arose independently in different samples before one variant became dominant and spread over the entire microbiome, MIT reported.
The mutation, which actually results in the loss of the polysaccharide capsule, may allow cells to grow faster than those without the mutation because they have more fuel to power their own growth, the researchers suggested. It’s also possible that loss of the capsule allows S. aureus cells to stick to the skin better because proteins that allow them to adhere to the skin are more exposed.
Dr. Lieberman and her associates hope that these variant-containing cells could be a new target for eczema treatments, but we’re never optimistic when it comes to bacteria. That’s because some of us are old enough to remember evolutionary biologist Stephen Jay Gould, who wrote in his book “Full House”: “Our planet has always been in the ‘Age of Bacteria,’ ever since the first fossils – bacteria, of course – were entombed in rocks more than 3 billion years ago. On any possible, reasonable or fair criterion, bacteria are – and always have been – the dominant forms of life on Earth.”
In the distant future, long after humans have left the scene, the bacteria will be laughing at the last rats and cockroaches scurrying across the landscape. Wanna bet?
The height of genetic prediction
Genetics are practically a DNA Scrabble bag. Traits like eye color and hair texture are chosen in the same fashion, based on what gets pulled from our own genetic bag of letters, but what about height? Researchers may now have a way to predict adult height and make it more than just an educated guess.
How? By looking at the genes in our growth plates. The cartilage on the ends of our bones hardens as we age, eventually deciding an individual’s stature. In a recently published study, a research team looked at 600 million cartilage cells linked to maturation and cell growth in mice. Because everything starts with rodents.
After that search identified 145 genes linked to growth plate maturation and formation of the bones, they compared the mouse genes with data from genome-wide association studies (GWAS) of human height to look for hotspots where the height genes exist in human DNA.
The results showed which genes play a role in deciding height, and the GWAS data also suggested that genetic changes affecting cartilage cell maturation may strongly influence adult height, said the investigators, who hope that earlier interventions can improve outcomes in patients with conditions such as skeletal dysplasia.
So, yeah, you may want to be a little taller or shorter, but the outcome of that particular Scrabble game was determined when your parents, you know, dropped the letters in the bag.
Beating the allegory of the cave
When Beatriz Flamini spoke with reporters on April 14, she knew nothing of the previous 18 months. The Russian invasion of Ukraine? Nope. The death of Queen Elizabeth? Also no. But before you make fun of her, she has an excuse. She’s been living under a rock.
As part of an experiment to test how social isolation and disorientation affect a person’s mind, sense of time, and sleeping patterns, Ms. Flamini lived in a 70-meter-deep cave in southern Spain for 500 days, starting in November 2021. Alone. No outside communication with the outside world in any way, though she was constantly monitored by a team of researchers. She also had multiple cameras filming her for an upcoming documentary.
This is a massive step up from the previous record for time spent underground for science: A team of 15 spent 50 days underground in 2021 to similar study of isolation and how it affected circadian rhythms. It’s also almost certainly a world record for time spent underground.
All that time alone certainly sounds like some sort of medieval torture, but Ms. Flamini had access to food, water, and a library of books. Which she made liberal use of, reading at least 60 books during her stay. She also had a panic button in case the isolation became too much or an emergency developed, but she never considered using it.
She lost track of time after 2 months, flies invaded the cave on occasion, and maintaining coherence was occasionally a struggle, but she kept things together very well. In fact, she didn’t even want to leave when her team came for her. She wasn’t even finished with her 61st book.
When she spoke with gathered reporters after the ordeal, words were obviously difficult to come by for her, having not spoken in nearly 18 months, but her mind was clearly still sharp and she had a very important question for everyone gathered around her.
Who’s buying the beer?
We approve of this request.
Staphylococcus and the speed of evolution
Bacteria, we know, are tough little buggers that are hard to see and even harder to get rid of. So hard, actually, that human bodies eventually gave up on the task and decided to just incorporate them into our organ systems. But why are bacteria so hard to eliminate?
Two words: rapid evolution. How rapid? For the first time, scientists have directly observed adaptive evolution by Staphylococcus aureus in a single person’s skin microbiome. That’s how rapid.
For their study, the researchers collected samples from the nostrils, backs of knees, insides of elbows, and forearms of 23 children with eczema. They eventually cultured almost 1,500 unique colonies of S. aureus cells from those samples and sequenced the cells’ genomes.
All that sampling and culturing and sequencing showed that it was rare for a new S. aureus strain to come in and replace the existing strain. “Despite the stability at the lineage level, we see a lot of dynamics at the whole genome level, where new mutations are constantly arising in these bacteria and then spreading throughout the entire body,” Tami D. Lieberman, PhD, of the Massachusetts Institute of Technology, Cambridge, said in a written statement from MIT.
One frequent mutation involved a gene called capD, which encodes an enzyme necessary for synthesizing the capsular polysaccharide – a coating that protects S. aureus from recognition by immune cells. In one patient, four different mutations of capD arose independently in different samples before one variant became dominant and spread over the entire microbiome, MIT reported.
The mutation, which actually results in the loss of the polysaccharide capsule, may allow cells to grow faster than those without the mutation because they have more fuel to power their own growth, the researchers suggested. It’s also possible that loss of the capsule allows S. aureus cells to stick to the skin better because proteins that allow them to adhere to the skin are more exposed.
Dr. Lieberman and her associates hope that these variant-containing cells could be a new target for eczema treatments, but we’re never optimistic when it comes to bacteria. That’s because some of us are old enough to remember evolutionary biologist Stephen Jay Gould, who wrote in his book “Full House”: “Our planet has always been in the ‘Age of Bacteria,’ ever since the first fossils – bacteria, of course – were entombed in rocks more than 3 billion years ago. On any possible, reasonable or fair criterion, bacteria are – and always have been – the dominant forms of life on Earth.”
In the distant future, long after humans have left the scene, the bacteria will be laughing at the last rats and cockroaches scurrying across the landscape. Wanna bet?
The height of genetic prediction
Genetics are practically a DNA Scrabble bag. Traits like eye color and hair texture are chosen in the same fashion, based on what gets pulled from our own genetic bag of letters, but what about height? Researchers may now have a way to predict adult height and make it more than just an educated guess.
How? By looking at the genes in our growth plates. The cartilage on the ends of our bones hardens as we age, eventually deciding an individual’s stature. In a recently published study, a research team looked at 600 million cartilage cells linked to maturation and cell growth in mice. Because everything starts with rodents.
After that search identified 145 genes linked to growth plate maturation and formation of the bones, they compared the mouse genes with data from genome-wide association studies (GWAS) of human height to look for hotspots where the height genes exist in human DNA.
The results showed which genes play a role in deciding height, and the GWAS data also suggested that genetic changes affecting cartilage cell maturation may strongly influence adult height, said the investigators, who hope that earlier interventions can improve outcomes in patients with conditions such as skeletal dysplasia.
So, yeah, you may want to be a little taller or shorter, but the outcome of that particular Scrabble game was determined when your parents, you know, dropped the letters in the bag.
Physicians may retire en masse soon. What does that mean for medicine?
The double whammy of pandemic burnout and the aging of baby boomer physicians has, indeed, the makings of some scary headlines. A recent survey by Elsevier Health predicts that up to 75% of health care workers will leave the profession by 2025. And a 2020 study conducted by the Association of American Medical Colleges (AAMC) projected a shortfall of up to 139,000 physicians by 2033.
“We’ve paid a lot of attention to physician retirement,” says Michael Dill, AAMC’s director of workforce studies. “It’s a significant concern in terms of whether we have an adequate supply of physicians in the U.S. to meet our nation’s medical care needs. Anyone who thinks otherwise is incorrect.”
To Mr. Dill,
“The physician workforce as a whole is aging,” he said. “Close to a quarter of the physicians in the U.S. are 65 and over. So, you don’t need any extraordinary events driving retirement in order for retirement to be a real phenomenon of which we should all be concerned.”
And, although Mr. Dill said there aren’t any data to suggest that doctors in rural or urban areas are retiring faster than in the suburbs, that doesn’t mean retirement will have the same impact depending on where patients live.
“If you live in a rural area with one small practice in town and that physician retires, there goes the entirety of the physician supply,” he said. “In a major metro area, that’s not as big a deal.”
Why younger doctors are fast-tracking retirement
Fernando Mendoza, MD, 54, a pediatric emergency department physician in Miami, worries that physicians are getting so bogged down by paperwork that this may lead to even more doctors, at younger ages, leaving the profession.
“I love taking care of kids, but there’s going to be a cost to doing your work when you’re spending as much time as we need to spend on charts, pharmacy requests, and making sure all of the Medicare and Medicaid compliance issues are worked out.”
These stressors may compel some younger doctors to consider carving out a second career or fast-track younger physicians toward retirement.
“A medical degree carries a lot of weight, which helps when pivoting,” said Dr. Mendoza, who launched Scrivas, a Miami-based medical scribe agency, to help reduce the paperwork workload for physicians. “It might be that a doctor wants to get involved in the acquisition of medical equipment, or maybe they can focus on their investments. Either way, by leaving medicine, they’re not dealing with the hassle and churn-and-burn of seeing patients.”
What this means for patients
The time is now to stem the upcoming tide of retirement, said Mr. Dill. But the challenges remain daunting. For starters, the country needs more physicians trained now – but it will take years to replace those baby boomer doctors ready to hang up their white coats.
The medical profession also needs to find ways to support physicians who spend their days juggling an endless array of responsibilities, he said.
The AAMC study found that patients already feel the physician shortfall. Their public opinion research in 2019 said 35% of patients had trouble finding a physician over the past 2 or 3 years, up 10 percentage points since they asked the question in 2015.
Moreover, according to the report, the over-65 population is expected to grow by 45.1%, leaving a specialty care gap because older people generally have more complicated health cases that require specialists. In addition, physician burnout may lead more physicians under 65 to retire much earlier than expected.
Changes in how medicine is practiced, telemedicine care, and medical education – such as disruption of classes or clinical rotations, regulatory changes, and a lack of interest in certain specialties – could also be affected by a mass physician retirement.
What can we do about mass retirement?
The AAMC reports in “The Complexities of Physician Supply and Demand: Projections From 2019 to 2034” that federally funded GME support is in the works to train 15,000 physicians per year, with 3,000 new residency slots added per year over 5 years. The proposed model will add 3,750 new physicians each year beginning in 2026.
Other efforts include increasing use of APRNs and PAs, whose population is estimated to more than double by 2034, improve population health through preventive care, increase equity in health outcomes, and improve access and affordable care.
Removing licensing barriers for immigrant doctors can also help alleviate the shortage.
“We need to find better ways to leverage the entirety of the health care team so that not as much falls on physicians,” Mr. Dill said. “It’s also imperative that we focus on ways to support physician wellness and allow physicians to remain active in the field, but at a reduced rate.”
That’s precisely what Marie Brown, MD, director of practice redesign at the American Medical Association, is seeing nationwide. Cutting back their hours is not only trending, but it’s also helping doctors cope with burnout.
“We’re seeing physicians take a 20% or more cut in salary in order to decrease their burden,” she said. “They’ll spend 4 days on clinical time with patients so that on that fifth ‘day off,’ they’re doing the paperwork and documentation they need to do so they don’t compromise care on the other 4 days of the week.”
And this may only be a Band-Aid solution, she fears.
“If a physician is spending 3 hours a day doing unnecessary work that could be done by another team member, that’s contributing to burnout,” Dr. Brown said. “It’s no surprise that they’ll want to escape and retire if they’re in a financial situation to do so.”
“I advocate negotiating within your organization so you’re doing more of what you like, such as mentoring or running a residency, and less of what you don’t, while cutting back from full-time to something less than full-time while maintaining benefits,” said Joel Greenwald, MD, a certified financial planner in Minneapolis, who specializes in helping physicians manage their financial affairs.
“Falling into the ‘like less’ bucket are usually things like working weekends and taking calls,” he said.
“This benefits everyone on a large scale because those doctors who find things they enjoy are generally working to a later age but working less hard,” he said. “Remaining comfortably and happily gainfully employed for a longer period, even if you’re not working full-time, has a very powerful effect on your financial planning, and you’ll avoid the risk of running out of money.”
A version of this article first appeared on Medscape.com.
The double whammy of pandemic burnout and the aging of baby boomer physicians has, indeed, the makings of some scary headlines. A recent survey by Elsevier Health predicts that up to 75% of health care workers will leave the profession by 2025. And a 2020 study conducted by the Association of American Medical Colleges (AAMC) projected a shortfall of up to 139,000 physicians by 2033.
“We’ve paid a lot of attention to physician retirement,” says Michael Dill, AAMC’s director of workforce studies. “It’s a significant concern in terms of whether we have an adequate supply of physicians in the U.S. to meet our nation’s medical care needs. Anyone who thinks otherwise is incorrect.”
To Mr. Dill,
“The physician workforce as a whole is aging,” he said. “Close to a quarter of the physicians in the U.S. are 65 and over. So, you don’t need any extraordinary events driving retirement in order for retirement to be a real phenomenon of which we should all be concerned.”
And, although Mr. Dill said there aren’t any data to suggest that doctors in rural or urban areas are retiring faster than in the suburbs, that doesn’t mean retirement will have the same impact depending on where patients live.
“If you live in a rural area with one small practice in town and that physician retires, there goes the entirety of the physician supply,” he said. “In a major metro area, that’s not as big a deal.”
Why younger doctors are fast-tracking retirement
Fernando Mendoza, MD, 54, a pediatric emergency department physician in Miami, worries that physicians are getting so bogged down by paperwork that this may lead to even more doctors, at younger ages, leaving the profession.
“I love taking care of kids, but there’s going to be a cost to doing your work when you’re spending as much time as we need to spend on charts, pharmacy requests, and making sure all of the Medicare and Medicaid compliance issues are worked out.”
These stressors may compel some younger doctors to consider carving out a second career or fast-track younger physicians toward retirement.
“A medical degree carries a lot of weight, which helps when pivoting,” said Dr. Mendoza, who launched Scrivas, a Miami-based medical scribe agency, to help reduce the paperwork workload for physicians. “It might be that a doctor wants to get involved in the acquisition of medical equipment, or maybe they can focus on their investments. Either way, by leaving medicine, they’re not dealing with the hassle and churn-and-burn of seeing patients.”
What this means for patients
The time is now to stem the upcoming tide of retirement, said Mr. Dill. But the challenges remain daunting. For starters, the country needs more physicians trained now – but it will take years to replace those baby boomer doctors ready to hang up their white coats.
The medical profession also needs to find ways to support physicians who spend their days juggling an endless array of responsibilities, he said.
The AAMC study found that patients already feel the physician shortfall. Their public opinion research in 2019 said 35% of patients had trouble finding a physician over the past 2 or 3 years, up 10 percentage points since they asked the question in 2015.
Moreover, according to the report, the over-65 population is expected to grow by 45.1%, leaving a specialty care gap because older people generally have more complicated health cases that require specialists. In addition, physician burnout may lead more physicians under 65 to retire much earlier than expected.
Changes in how medicine is practiced, telemedicine care, and medical education – such as disruption of classes or clinical rotations, regulatory changes, and a lack of interest in certain specialties – could also be affected by a mass physician retirement.
What can we do about mass retirement?
The AAMC reports in “The Complexities of Physician Supply and Demand: Projections From 2019 to 2034” that federally funded GME support is in the works to train 15,000 physicians per year, with 3,000 new residency slots added per year over 5 years. The proposed model will add 3,750 new physicians each year beginning in 2026.
Other efforts include increasing use of APRNs and PAs, whose population is estimated to more than double by 2034, improve population health through preventive care, increase equity in health outcomes, and improve access and affordable care.
Removing licensing barriers for immigrant doctors can also help alleviate the shortage.
“We need to find better ways to leverage the entirety of the health care team so that not as much falls on physicians,” Mr. Dill said. “It’s also imperative that we focus on ways to support physician wellness and allow physicians to remain active in the field, but at a reduced rate.”
That’s precisely what Marie Brown, MD, director of practice redesign at the American Medical Association, is seeing nationwide. Cutting back their hours is not only trending, but it’s also helping doctors cope with burnout.
“We’re seeing physicians take a 20% or more cut in salary in order to decrease their burden,” she said. “They’ll spend 4 days on clinical time with patients so that on that fifth ‘day off,’ they’re doing the paperwork and documentation they need to do so they don’t compromise care on the other 4 days of the week.”
And this may only be a Band-Aid solution, she fears.
“If a physician is spending 3 hours a day doing unnecessary work that could be done by another team member, that’s contributing to burnout,” Dr. Brown said. “It’s no surprise that they’ll want to escape and retire if they’re in a financial situation to do so.”
“I advocate negotiating within your organization so you’re doing more of what you like, such as mentoring or running a residency, and less of what you don’t, while cutting back from full-time to something less than full-time while maintaining benefits,” said Joel Greenwald, MD, a certified financial planner in Minneapolis, who specializes in helping physicians manage their financial affairs.
“Falling into the ‘like less’ bucket are usually things like working weekends and taking calls,” he said.
“This benefits everyone on a large scale because those doctors who find things they enjoy are generally working to a later age but working less hard,” he said. “Remaining comfortably and happily gainfully employed for a longer period, even if you’re not working full-time, has a very powerful effect on your financial planning, and you’ll avoid the risk of running out of money.”
A version of this article first appeared on Medscape.com.
The double whammy of pandemic burnout and the aging of baby boomer physicians has, indeed, the makings of some scary headlines. A recent survey by Elsevier Health predicts that up to 75% of health care workers will leave the profession by 2025. And a 2020 study conducted by the Association of American Medical Colleges (AAMC) projected a shortfall of up to 139,000 physicians by 2033.
“We’ve paid a lot of attention to physician retirement,” says Michael Dill, AAMC’s director of workforce studies. “It’s a significant concern in terms of whether we have an adequate supply of physicians in the U.S. to meet our nation’s medical care needs. Anyone who thinks otherwise is incorrect.”
To Mr. Dill,
“The physician workforce as a whole is aging,” he said. “Close to a quarter of the physicians in the U.S. are 65 and over. So, you don’t need any extraordinary events driving retirement in order for retirement to be a real phenomenon of which we should all be concerned.”
And, although Mr. Dill said there aren’t any data to suggest that doctors in rural or urban areas are retiring faster than in the suburbs, that doesn’t mean retirement will have the same impact depending on where patients live.
“If you live in a rural area with one small practice in town and that physician retires, there goes the entirety of the physician supply,” he said. “In a major metro area, that’s not as big a deal.”
Why younger doctors are fast-tracking retirement
Fernando Mendoza, MD, 54, a pediatric emergency department physician in Miami, worries that physicians are getting so bogged down by paperwork that this may lead to even more doctors, at younger ages, leaving the profession.
“I love taking care of kids, but there’s going to be a cost to doing your work when you’re spending as much time as we need to spend on charts, pharmacy requests, and making sure all of the Medicare and Medicaid compliance issues are worked out.”
These stressors may compel some younger doctors to consider carving out a second career or fast-track younger physicians toward retirement.
“A medical degree carries a lot of weight, which helps when pivoting,” said Dr. Mendoza, who launched Scrivas, a Miami-based medical scribe agency, to help reduce the paperwork workload for physicians. “It might be that a doctor wants to get involved in the acquisition of medical equipment, or maybe they can focus on their investments. Either way, by leaving medicine, they’re not dealing with the hassle and churn-and-burn of seeing patients.”
What this means for patients
The time is now to stem the upcoming tide of retirement, said Mr. Dill. But the challenges remain daunting. For starters, the country needs more physicians trained now – but it will take years to replace those baby boomer doctors ready to hang up their white coats.
The medical profession also needs to find ways to support physicians who spend their days juggling an endless array of responsibilities, he said.
The AAMC study found that patients already feel the physician shortfall. Their public opinion research in 2019 said 35% of patients had trouble finding a physician over the past 2 or 3 years, up 10 percentage points since they asked the question in 2015.
Moreover, according to the report, the over-65 population is expected to grow by 45.1%, leaving a specialty care gap because older people generally have more complicated health cases that require specialists. In addition, physician burnout may lead more physicians under 65 to retire much earlier than expected.
Changes in how medicine is practiced, telemedicine care, and medical education – such as disruption of classes or clinical rotations, regulatory changes, and a lack of interest in certain specialties – could also be affected by a mass physician retirement.
What can we do about mass retirement?
The AAMC reports in “The Complexities of Physician Supply and Demand: Projections From 2019 to 2034” that federally funded GME support is in the works to train 15,000 physicians per year, with 3,000 new residency slots added per year over 5 years. The proposed model will add 3,750 new physicians each year beginning in 2026.
Other efforts include increasing use of APRNs and PAs, whose population is estimated to more than double by 2034, improve population health through preventive care, increase equity in health outcomes, and improve access and affordable care.
Removing licensing barriers for immigrant doctors can also help alleviate the shortage.
“We need to find better ways to leverage the entirety of the health care team so that not as much falls on physicians,” Mr. Dill said. “It’s also imperative that we focus on ways to support physician wellness and allow physicians to remain active in the field, but at a reduced rate.”
That’s precisely what Marie Brown, MD, director of practice redesign at the American Medical Association, is seeing nationwide. Cutting back their hours is not only trending, but it’s also helping doctors cope with burnout.
“We’re seeing physicians take a 20% or more cut in salary in order to decrease their burden,” she said. “They’ll spend 4 days on clinical time with patients so that on that fifth ‘day off,’ they’re doing the paperwork and documentation they need to do so they don’t compromise care on the other 4 days of the week.”
And this may only be a Band-Aid solution, she fears.
“If a physician is spending 3 hours a day doing unnecessary work that could be done by another team member, that’s contributing to burnout,” Dr. Brown said. “It’s no surprise that they’ll want to escape and retire if they’re in a financial situation to do so.”
“I advocate negotiating within your organization so you’re doing more of what you like, such as mentoring or running a residency, and less of what you don’t, while cutting back from full-time to something less than full-time while maintaining benefits,” said Joel Greenwald, MD, a certified financial planner in Minneapolis, who specializes in helping physicians manage their financial affairs.
“Falling into the ‘like less’ bucket are usually things like working weekends and taking calls,” he said.
“This benefits everyone on a large scale because those doctors who find things they enjoy are generally working to a later age but working less hard,” he said. “Remaining comfortably and happily gainfully employed for a longer period, even if you’re not working full-time, has a very powerful effect on your financial planning, and you’ll avoid the risk of running out of money.”
A version of this article first appeared on Medscape.com.
Acute Onset of Vitiligolike Depigmentation After Nivolumab Therapy for Systemic Melanoma
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.
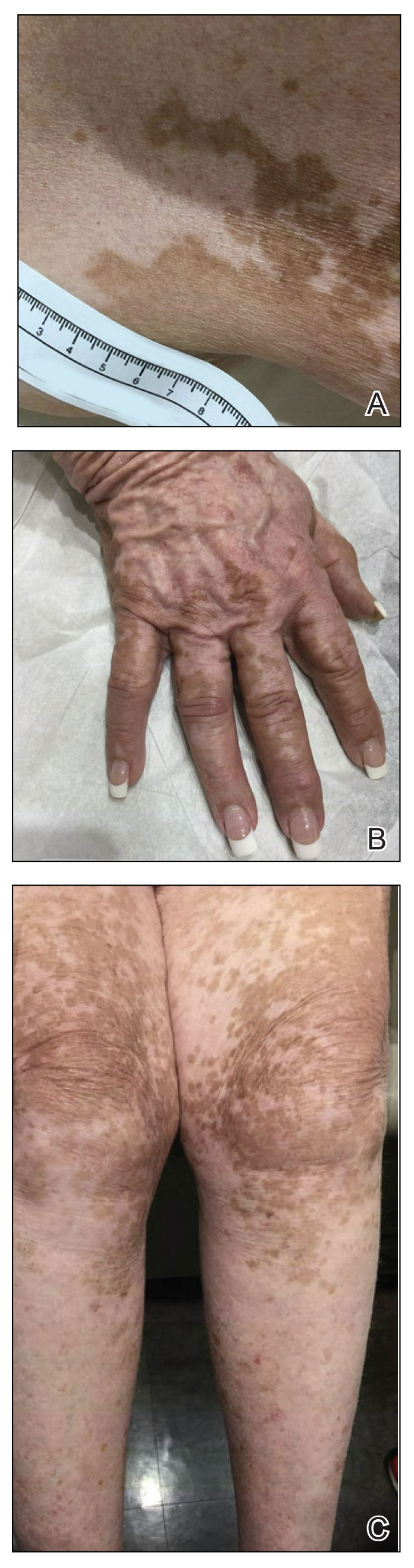
At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.

At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.

At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
Practice Points
- New-onset vitiligo coinciding with malignant melanoma should be considered a good prognostic indicator.
- Daily use of hydroquinone cream 4% in conjunction with diligent photoprotection was shown to even overall skin tone in a patient experiencing leukoderma from nivolumab therapy.
Collision Course of a Basal Cell Carcinoma and Apocrine Hidrocystoma on the Scalp
To the Editor:
A collision tumor is the coexistence of 2 discrete tumors in the same neoplasm, possibly comprising a malignant tumor and a benign tumor, and thereby complicating appropriate diagnosis and treatment. We present a case of a basal cell carcinoma (BCC) of the scalp that was later found to be in collision with an apocrine hidrocystoma that might have arisen from a nevus sebaceus. Although rare, BCC can coexist with apocrine hidrocystoma. Jayaprakasam and Rene1 reported a case of a collision tumor containing BCC and hidrocystoma on the eyelid.1 We present a case of a BCC on the scalp that was later found to be in collision with an apocrine hidrocystoma that possibly arose from a nevus sebaceus.
A 92-year-old Black woman with a biopsy-confirmed primary BCC of the left parietal scalp presented for Mohs micrographic surgery. The pathology report from an outside facility was reviewed. The initial diagnosis had been made with 2 punch biopsies from separate areas of the large nodule—one consistent with nodular and pigmented BCC (Figure 1), and the other revealed nodular ulcerated BCC. Physical examination prior to Mohs surgery revealed a mobile, flesh-colored, 6.2×6.0-cm nodule with minimal overlying hair on the left parietal scalp (Figure 2). During stage-I processing by the histopathology laboratory, large cystic structures were encountered; en face frozen sections showed a cystic tumor. Excised tissue was submitted for permanent processing to aid in diagnosis; the initial diagnostic biopsy slides were requested from the outside facility for review.
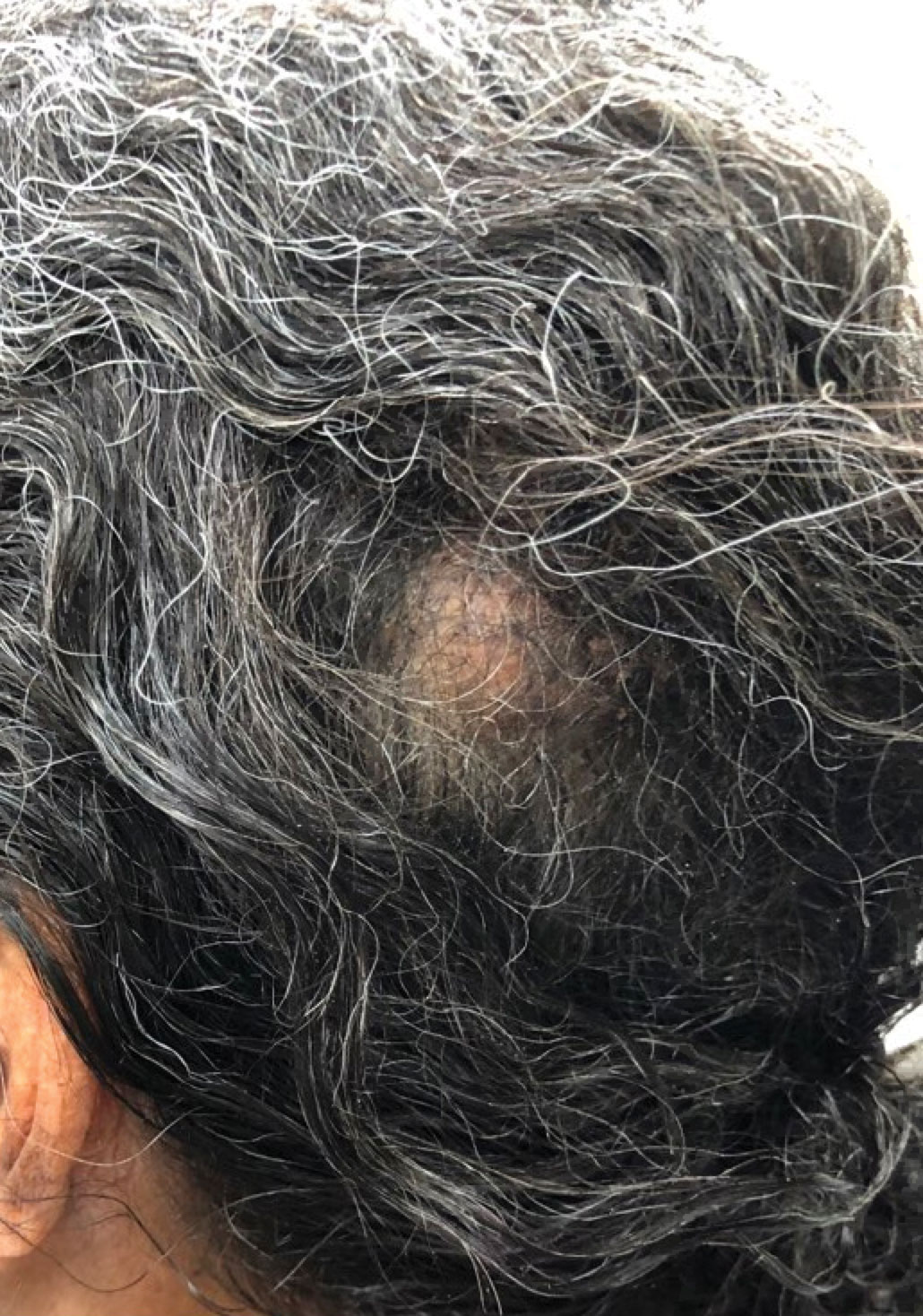
The initial diagnostic biopsy slides were reviewed and found to be consistent with nodular and pigmented BCC, as previously reported. Findings from hematoxylin and eosin staining of tissue obtained from Mohs sections were consistent with a combined neoplasm comprising BCC (Figure 3A) and apocrine hidrocystoma (Figure 3B). In addition, one section was characterized by acanthosis, papillomatosis, and sebaceous glands—similar to findings that are seen in a nevus sebaceus (Figure 3C).
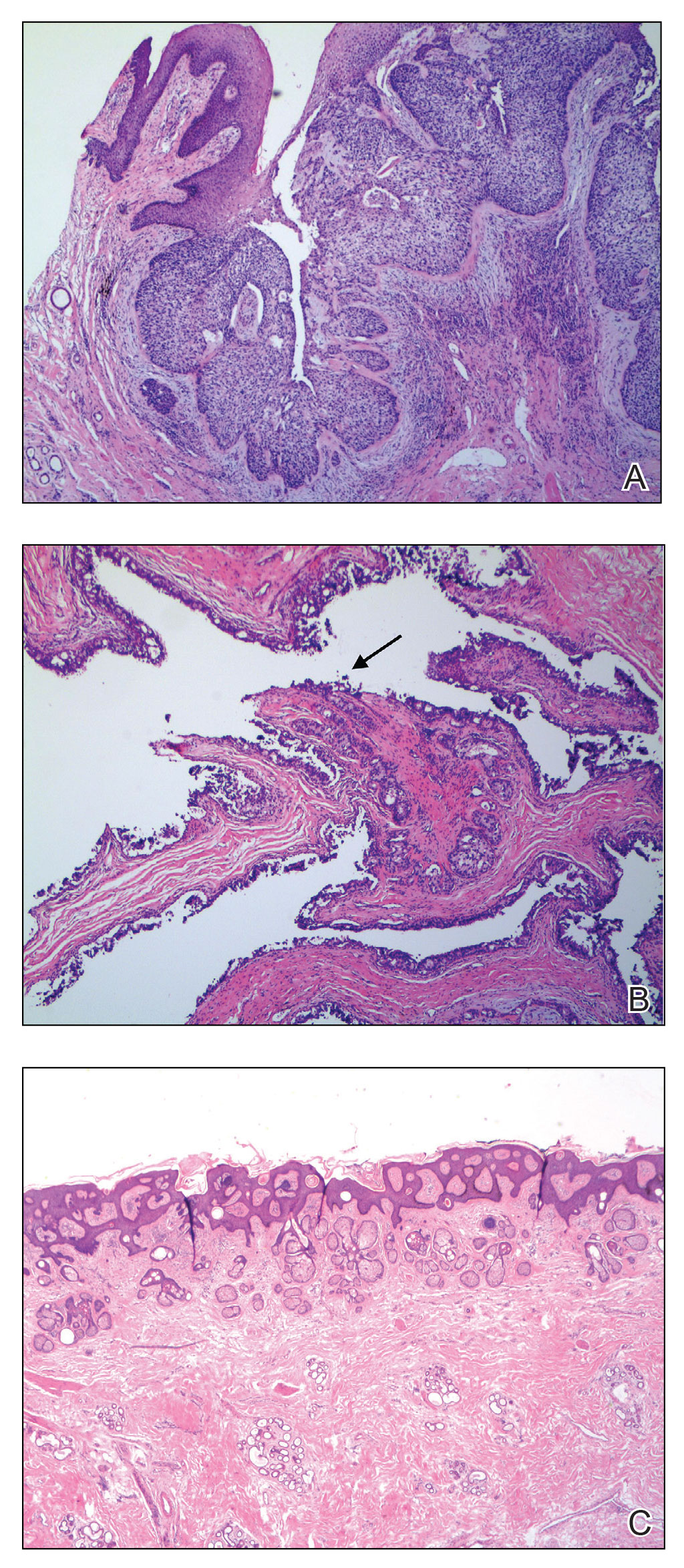
The BCC was cleared after stage I; the final wound size was 7×6.6 cm. Although benign apocrine hidrocystoma was still evident at the margin, further excision was not performed at the request of the patient and her family. Partial primary wound closure was performed with pulley sutures. A xenograft was placed over the unclosed central portion. The wound was permitted to heal by second intention.
The clinical differential diagnosis of a scalp nodule includes a pilar cyst, BCC, squamous cell carcinoma, melanoma, cutaneous metastasis, adnexal tumor, atypical fibroxanthoma, and collision tumor. A collision tumor—the association of 2 or more benign or malignant neoplasms—represents a well-known pitfall in making a correct clinical and pathologic diagnosis.2 Many theories have been proposed to explain the pathophysiology of collision tumors. Some authors have speculated that they arise from involvement of related cell types.1 Other theories include induction by cytokines and growth factors secreted from one tumor that provides an ideal environment for proliferation of other cell types, a field cancerization effect of sun-damaged skin, or a coincidence.2
In our case, it is possible that the 2 tumors arose from a nevus sebaceus. One retrospective study of 706 cases of nevus sebaceus (707 specimens) found that 22.5% of cases developed secondary proliferation; of those cases, 18.9% were benign.3 Additionally, in 4.2% of cases of nevus sebaceus, proliferation of 2 or more tumors developed. The most common malignant neoplasm to develop from nevus sebaceus was BCC, followed by squamous cell carcinoma and sebaceous carcinoma. The most common benign neoplasm to develop from nevus sebaceus was trichoblastoma, followed by syringocystadenoma papilliferum.3
Our case highlights the possibility of a sampling error when performing a biopsy of any large neoplasm. Additionally, Mohs surgeons should maintain high clinical suspicion for collision tumors when encountering a large tumor with pathology inconsistent with the original biopsy. Apocrine hidrocystoma should be considered in the differential diagnosis of a large cystic mass of the scalp. Also, it is important to recognize that malignant lesions, such as BCC, can coexist with another benign tumor. Basal cell carcinoma is rare in Black patients, supporting our belief that our patient’s tumors arose from a nevus sebaceus.
It also is important for Mohs surgeons to consider any potential discrepancy between the initial pathology report and Mohs intraoperative pathology that can impact diagnosis, the aggressiveness of the tumors identified, and how such aggressiveness may affect management options.4,5 Some dermatology practices request biopsy slides from patients who are referred for Mohs micrographic surgery for internal review by a dermatopathologist before surgery is performed; however, this protocol requires additional time and adds costs for the overall health care system.4 One study found that internal review of outside biopsy slides resulted in a change in diagnosis in 2.2% of patients (N=3345)—affecting management in 61% of cases in which the diagnosis was changed.4 Another study (N=163) found that the reported aggressiveness of 50.5% of nonmelanoma cases in an initial biopsy report was changed during Mohs micrographic surgery.5 Mohs surgeons should be aware that discrepancies can occur, and if a discrepancy is discovered, the procedure may be paused until the initial biopsy slide is reviewed and further information is collected.
- Jayaprakasam A, Rene C. A benign or malignant eyelid lump—can you tell? an unusual collision tumour highlighting the difficulty differentiating a hidrocystoma from a basal cell carcinoma. BMJ Case Reports. 2012;2012:bcr1220115307. doi:10.1136/bcr.12.2011.5307
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603. doi:10.1097/DAD.0b013e3181a88116
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Butler ST, Youker SR, Mandrell J, et al. The importance of reviewing pathology specimens before Mohs surgery. Dermatol Surg. 2009;35:407-412. doi:10.1111/j.1524-4725.2008.01056.x
- Stiegel E, Lam C, Schowalter M, et al. Correlation between original biopsy pathology and Mohs intraoperative pathology. Dermatol Surg. 2018;44:193-197. doi:10.1097/DSS.0000000000001276
To the Editor:
A collision tumor is the coexistence of 2 discrete tumors in the same neoplasm, possibly comprising a malignant tumor and a benign tumor, and thereby complicating appropriate diagnosis and treatment. We present a case of a basal cell carcinoma (BCC) of the scalp that was later found to be in collision with an apocrine hidrocystoma that might have arisen from a nevus sebaceus. Although rare, BCC can coexist with apocrine hidrocystoma. Jayaprakasam and Rene1 reported a case of a collision tumor containing BCC and hidrocystoma on the eyelid.1 We present a case of a BCC on the scalp that was later found to be in collision with an apocrine hidrocystoma that possibly arose from a nevus sebaceus.

A 92-year-old Black woman with a biopsy-confirmed primary BCC of the left parietal scalp presented for Mohs micrographic surgery. The pathology report from an outside facility was reviewed. The initial diagnosis had been made with 2 punch biopsies from separate areas of the large nodule—one consistent with nodular and pigmented BCC (Figure 1), and the other revealed nodular ulcerated BCC. Physical examination prior to Mohs surgery revealed a mobile, flesh-colored, 6.2×6.0-cm nodule with minimal overlying hair on the left parietal scalp (Figure 2). During stage-I processing by the histopathology laboratory, large cystic structures were encountered; en face frozen sections showed a cystic tumor. Excised tissue was submitted for permanent processing to aid in diagnosis; the initial diagnostic biopsy slides were requested from the outside facility for review.

The initial diagnostic biopsy slides were reviewed and found to be consistent with nodular and pigmented BCC, as previously reported. Findings from hematoxylin and eosin staining of tissue obtained from Mohs sections were consistent with a combined neoplasm comprising BCC (Figure 3A) and apocrine hidrocystoma (Figure 3B). In addition, one section was characterized by acanthosis, papillomatosis, and sebaceous glands—similar to findings that are seen in a nevus sebaceus (Figure 3C).

The BCC was cleared after stage I; the final wound size was 7×6.6 cm. Although benign apocrine hidrocystoma was still evident at the margin, further excision was not performed at the request of the patient and her family. Partial primary wound closure was performed with pulley sutures. A xenograft was placed over the unclosed central portion. The wound was permitted to heal by second intention.
The clinical differential diagnosis of a scalp nodule includes a pilar cyst, BCC, squamous cell carcinoma, melanoma, cutaneous metastasis, adnexal tumor, atypical fibroxanthoma, and collision tumor. A collision tumor—the association of 2 or more benign or malignant neoplasms—represents a well-known pitfall in making a correct clinical and pathologic diagnosis.2 Many theories have been proposed to explain the pathophysiology of collision tumors. Some authors have speculated that they arise from involvement of related cell types.1 Other theories include induction by cytokines and growth factors secreted from one tumor that provides an ideal environment for proliferation of other cell types, a field cancerization effect of sun-damaged skin, or a coincidence.2
In our case, it is possible that the 2 tumors arose from a nevus sebaceus. One retrospective study of 706 cases of nevus sebaceus (707 specimens) found that 22.5% of cases developed secondary proliferation; of those cases, 18.9% were benign.3 Additionally, in 4.2% of cases of nevus sebaceus, proliferation of 2 or more tumors developed. The most common malignant neoplasm to develop from nevus sebaceus was BCC, followed by squamous cell carcinoma and sebaceous carcinoma. The most common benign neoplasm to develop from nevus sebaceus was trichoblastoma, followed by syringocystadenoma papilliferum.3
Our case highlights the possibility of a sampling error when performing a biopsy of any large neoplasm. Additionally, Mohs surgeons should maintain high clinical suspicion for collision tumors when encountering a large tumor with pathology inconsistent with the original biopsy. Apocrine hidrocystoma should be considered in the differential diagnosis of a large cystic mass of the scalp. Also, it is important to recognize that malignant lesions, such as BCC, can coexist with another benign tumor. Basal cell carcinoma is rare in Black patients, supporting our belief that our patient’s tumors arose from a nevus sebaceus.
It also is important for Mohs surgeons to consider any potential discrepancy between the initial pathology report and Mohs intraoperative pathology that can impact diagnosis, the aggressiveness of the tumors identified, and how such aggressiveness may affect management options.4,5 Some dermatology practices request biopsy slides from patients who are referred for Mohs micrographic surgery for internal review by a dermatopathologist before surgery is performed; however, this protocol requires additional time and adds costs for the overall health care system.4 One study found that internal review of outside biopsy slides resulted in a change in diagnosis in 2.2% of patients (N=3345)—affecting management in 61% of cases in which the diagnosis was changed.4 Another study (N=163) found that the reported aggressiveness of 50.5% of nonmelanoma cases in an initial biopsy report was changed during Mohs micrographic surgery.5 Mohs surgeons should be aware that discrepancies can occur, and if a discrepancy is discovered, the procedure may be paused until the initial biopsy slide is reviewed and further information is collected.
To the Editor:
A collision tumor is the coexistence of 2 discrete tumors in the same neoplasm, possibly comprising a malignant tumor and a benign tumor, and thereby complicating appropriate diagnosis and treatment. We present a case of a basal cell carcinoma (BCC) of the scalp that was later found to be in collision with an apocrine hidrocystoma that might have arisen from a nevus sebaceus. Although rare, BCC can coexist with apocrine hidrocystoma. Jayaprakasam and Rene1 reported a case of a collision tumor containing BCC and hidrocystoma on the eyelid.1 We present a case of a BCC on the scalp that was later found to be in collision with an apocrine hidrocystoma that possibly arose from a nevus sebaceus.

A 92-year-old Black woman with a biopsy-confirmed primary BCC of the left parietal scalp presented for Mohs micrographic surgery. The pathology report from an outside facility was reviewed. The initial diagnosis had been made with 2 punch biopsies from separate areas of the large nodule—one consistent with nodular and pigmented BCC (Figure 1), and the other revealed nodular ulcerated BCC. Physical examination prior to Mohs surgery revealed a mobile, flesh-colored, 6.2×6.0-cm nodule with minimal overlying hair on the left parietal scalp (Figure 2). During stage-I processing by the histopathology laboratory, large cystic structures were encountered; en face frozen sections showed a cystic tumor. Excised tissue was submitted for permanent processing to aid in diagnosis; the initial diagnostic biopsy slides were requested from the outside facility for review.

The initial diagnostic biopsy slides were reviewed and found to be consistent with nodular and pigmented BCC, as previously reported. Findings from hematoxylin and eosin staining of tissue obtained from Mohs sections were consistent with a combined neoplasm comprising BCC (Figure 3A) and apocrine hidrocystoma (Figure 3B). In addition, one section was characterized by acanthosis, papillomatosis, and sebaceous glands—similar to findings that are seen in a nevus sebaceus (Figure 3C).

The BCC was cleared after stage I; the final wound size was 7×6.6 cm. Although benign apocrine hidrocystoma was still evident at the margin, further excision was not performed at the request of the patient and her family. Partial primary wound closure was performed with pulley sutures. A xenograft was placed over the unclosed central portion. The wound was permitted to heal by second intention.
The clinical differential diagnosis of a scalp nodule includes a pilar cyst, BCC, squamous cell carcinoma, melanoma, cutaneous metastasis, adnexal tumor, atypical fibroxanthoma, and collision tumor. A collision tumor—the association of 2 or more benign or malignant neoplasms—represents a well-known pitfall in making a correct clinical and pathologic diagnosis.2 Many theories have been proposed to explain the pathophysiology of collision tumors. Some authors have speculated that they arise from involvement of related cell types.1 Other theories include induction by cytokines and growth factors secreted from one tumor that provides an ideal environment for proliferation of other cell types, a field cancerization effect of sun-damaged skin, or a coincidence.2
In our case, it is possible that the 2 tumors arose from a nevus sebaceus. One retrospective study of 706 cases of nevus sebaceus (707 specimens) found that 22.5% of cases developed secondary proliferation; of those cases, 18.9% were benign.3 Additionally, in 4.2% of cases of nevus sebaceus, proliferation of 2 or more tumors developed. The most common malignant neoplasm to develop from nevus sebaceus was BCC, followed by squamous cell carcinoma and sebaceous carcinoma. The most common benign neoplasm to develop from nevus sebaceus was trichoblastoma, followed by syringocystadenoma papilliferum.3
Our case highlights the possibility of a sampling error when performing a biopsy of any large neoplasm. Additionally, Mohs surgeons should maintain high clinical suspicion for collision tumors when encountering a large tumor with pathology inconsistent with the original biopsy. Apocrine hidrocystoma should be considered in the differential diagnosis of a large cystic mass of the scalp. Also, it is important to recognize that malignant lesions, such as BCC, can coexist with another benign tumor. Basal cell carcinoma is rare in Black patients, supporting our belief that our patient’s tumors arose from a nevus sebaceus.
It also is important for Mohs surgeons to consider any potential discrepancy between the initial pathology report and Mohs intraoperative pathology that can impact diagnosis, the aggressiveness of the tumors identified, and how such aggressiveness may affect management options.4,5 Some dermatology practices request biopsy slides from patients who are referred for Mohs micrographic surgery for internal review by a dermatopathologist before surgery is performed; however, this protocol requires additional time and adds costs for the overall health care system.4 One study found that internal review of outside biopsy slides resulted in a change in diagnosis in 2.2% of patients (N=3345)—affecting management in 61% of cases in which the diagnosis was changed.4 Another study (N=163) found that the reported aggressiveness of 50.5% of nonmelanoma cases in an initial biopsy report was changed during Mohs micrographic surgery.5 Mohs surgeons should be aware that discrepancies can occur, and if a discrepancy is discovered, the procedure may be paused until the initial biopsy slide is reviewed and further information is collected.
- Jayaprakasam A, Rene C. A benign or malignant eyelid lump—can you tell? an unusual collision tumour highlighting the difficulty differentiating a hidrocystoma from a basal cell carcinoma. BMJ Case Reports. 2012;2012:bcr1220115307. doi:10.1136/bcr.12.2011.5307
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603. doi:10.1097/DAD.0b013e3181a88116
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Butler ST, Youker SR, Mandrell J, et al. The importance of reviewing pathology specimens before Mohs surgery. Dermatol Surg. 2009;35:407-412. doi:10.1111/j.1524-4725.2008.01056.x
- Stiegel E, Lam C, Schowalter M, et al. Correlation between original biopsy pathology and Mohs intraoperative pathology. Dermatol Surg. 2018;44:193-197. doi:10.1097/DSS.0000000000001276
- Jayaprakasam A, Rene C. A benign or malignant eyelid lump—can you tell? an unusual collision tumour highlighting the difficulty differentiating a hidrocystoma from a basal cell carcinoma. BMJ Case Reports. 2012;2012:bcr1220115307. doi:10.1136/bcr.12.2011.5307
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603. doi:10.1097/DAD.0b013e3181a88116
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Butler ST, Youker SR, Mandrell J, et al. The importance of reviewing pathology specimens before Mohs surgery. Dermatol Surg. 2009;35:407-412. doi:10.1111/j.1524-4725.2008.01056.x
- Stiegel E, Lam C, Schowalter M, et al. Correlation between original biopsy pathology and Mohs intraoperative pathology. Dermatol Surg. 2018;44:193-197. doi:10.1097/DSS.0000000000001276
PRACTICE POINTS
- When collision tumors are encountered during Mohs micrographic surgery, review of the initial diagnostic material is recommended.
- Permanent processing of Mohs excisions may be helpful in determining the diagnosis of the occult second tumor diagnosis.
Physician compensation continues to climb amid postpandemic change
In addition, gender-based pay disparity among primary care physicians shrank, and the number of physicians who declined to take new Medicare patients rose.
The annual report is based on a survey of more than 10,000 physicians in over 29 specialties who answered questions about their income, workload, challenges, and level of satisfaction.
Average compensation across specialties rose to $352,000 – up nearly 17% from the 2018 average of $299,000. Fallout from the COVID-19 public health emergency continued to affect both physician compensation and job satisfaction, including Medicare reimbursements and staffing shortages due to burnout or retirement.
“Many physicians reevaluated what drove them to be a physician,” says Marc Adam, a recruiter at MASC Medical, a Florida physician recruiting firm.
Adam cites telehealth as an example. “An overwhelming majority of physicians prefer telehealth because of the convenience, but some really did not want to do it long term. They miss the patient interaction.”
The report also revealed that the gender-based pay gap in primary physicians fell, with men earning 19% more – down from 25% more in recent years. Among specialists, the gender gap was 27% on average, down from 31% last year. One reason may be an increase in compensation transparency, which Mr. Adam says should be the norm.
Income increases will likely continue, owing in large part to the growing disparity between physician supply and demand.
The projected physician shortage is expected to grow to 124,000 by 2034, according to the American Association of Medical Colleges. Federal lawmakers are considering passing the Resident Physician Shortage Reduction Act of 2023, which would add 14,000 Medicare-funded residency positions to help alleviate shortages.
Patient needs, Medicare rules continue to shift
Specialties with the biggest increases in compensation include oncology, anesthesiology, gastroenterology, radiology, critical care, and urology. Many procedure-related specialties saw more volume post pandemic.
Some respondents identified Medicare cuts and low reimbursement rates as a factor in tamping down compensation hikes. The number of physicians who expect to continue to take new Medicare patients is 65%, down from 71% 5 years ago.
For example, Medicare reimbursements for telehealth are expected to scale down in May, when the COVID-19 Public Health Emergency, which expanded telehealth services for Medicare patients, winds down.
“Telehealth will still exist,” says Mr. Adam, “but certain requirements will shape it going forward.”
Medicare isn’t viewed negatively across the board, however. Florida is among the top-earning states for physicians – along with Indiana, Connecticut, and Missouri. One reason is Florida’s unique health care environment, explains Mr. Adam, whose Florida-based firm places physicians nationwide.
“Florida is very progressive in terms of health care. For one thing, we have a large aging population and a large Medicare population.” Several growing organizations that focus on quality-based care are based in Florida, including ChenMed and Cano Health. Add to that the fact that owners of Florida’s health care organizations don’t have to be physicians, he explains, and the stage is set for experimentation.
“Being able to segment tasks frees up physicians to be more focused on medicine and provide better care while other people focus on the business and innovation.”
If Florida’s high compensation ranking continues, it may help employers there fulfill a growing need. The state is among those expected to experience the largest physician shortages in 2030, along with California, Texas, Arizona, and Georgia.
Side gigs up, satisfaction (slightly) down
In general, physicians aren’t fazed by these challenges. Many reported taking side gigs, some for additional income. Even so, 73% say they would still choose medicine, and more than 90% of physicians in 10 specialties would choose their specialty again. Still, burnout and stressors have led some to stop practicing altogether.
More and more organizations are hiring “travel physicians,” Mr. Adam says, and more physicians are choosing to take contract work (“locum tenens”) and practice in many different regions. Contract physicians typically help meet patient demand or provide coverage during the hiring process as well as while staff are on vacation or maternity leave.
Says Mr. Adam, “There’s no security, but there’s higher income and more flexibility.”
According to CHG Healthcare, locum tenens staffing is rising – approximately 7% of U.S. physicians (around 50,000) filled assignments in 2022, up 88% from 2015. In 2022, 56% of locum tenens employers reported a reduction in staff burnout, up from 30% in 2020.
The report indicates that more than half of physicians are satisfied with their income, down slightly from 55% 5 years ago (prepandemic). Physicians in some of the lower-paying specialties are among those most satisfied with their income. It’s not very surprising to Mr. Adam: “Higher earners generally suffer the most from burnout.
“They’re overworked, they have the largest number of patients, and they’re performing in high-stress situations doing challenging procedures on a daily basis – and they probably have worse work-life balance.” These physicians know going in that they need to be paid more to deal with such burdens. “That’s the feedback I get when I speak to high earners,” says Mr. Adam.
“The experienced ones are very clear about their [compensation] expectations.”
A version of this article first appeared on Medscape.com.
In addition, gender-based pay disparity among primary care physicians shrank, and the number of physicians who declined to take new Medicare patients rose.
The annual report is based on a survey of more than 10,000 physicians in over 29 specialties who answered questions about their income, workload, challenges, and level of satisfaction.
Average compensation across specialties rose to $352,000 – up nearly 17% from the 2018 average of $299,000. Fallout from the COVID-19 public health emergency continued to affect both physician compensation and job satisfaction, including Medicare reimbursements and staffing shortages due to burnout or retirement.
“Many physicians reevaluated what drove them to be a physician,” says Marc Adam, a recruiter at MASC Medical, a Florida physician recruiting firm.
Adam cites telehealth as an example. “An overwhelming majority of physicians prefer telehealth because of the convenience, but some really did not want to do it long term. They miss the patient interaction.”
The report also revealed that the gender-based pay gap in primary physicians fell, with men earning 19% more – down from 25% more in recent years. Among specialists, the gender gap was 27% on average, down from 31% last year. One reason may be an increase in compensation transparency, which Mr. Adam says should be the norm.
Income increases will likely continue, owing in large part to the growing disparity between physician supply and demand.
The projected physician shortage is expected to grow to 124,000 by 2034, according to the American Association of Medical Colleges. Federal lawmakers are considering passing the Resident Physician Shortage Reduction Act of 2023, which would add 14,000 Medicare-funded residency positions to help alleviate shortages.
Patient needs, Medicare rules continue to shift
Specialties with the biggest increases in compensation include oncology, anesthesiology, gastroenterology, radiology, critical care, and urology. Many procedure-related specialties saw more volume post pandemic.
Some respondents identified Medicare cuts and low reimbursement rates as a factor in tamping down compensation hikes. The number of physicians who expect to continue to take new Medicare patients is 65%, down from 71% 5 years ago.
For example, Medicare reimbursements for telehealth are expected to scale down in May, when the COVID-19 Public Health Emergency, which expanded telehealth services for Medicare patients, winds down.
“Telehealth will still exist,” says Mr. Adam, “but certain requirements will shape it going forward.”
Medicare isn’t viewed negatively across the board, however. Florida is among the top-earning states for physicians – along with Indiana, Connecticut, and Missouri. One reason is Florida’s unique health care environment, explains Mr. Adam, whose Florida-based firm places physicians nationwide.
“Florida is very progressive in terms of health care. For one thing, we have a large aging population and a large Medicare population.” Several growing organizations that focus on quality-based care are based in Florida, including ChenMed and Cano Health. Add to that the fact that owners of Florida’s health care organizations don’t have to be physicians, he explains, and the stage is set for experimentation.
“Being able to segment tasks frees up physicians to be more focused on medicine and provide better care while other people focus on the business and innovation.”
If Florida’s high compensation ranking continues, it may help employers there fulfill a growing need. The state is among those expected to experience the largest physician shortages in 2030, along with California, Texas, Arizona, and Georgia.
Side gigs up, satisfaction (slightly) down
In general, physicians aren’t fazed by these challenges. Many reported taking side gigs, some for additional income. Even so, 73% say they would still choose medicine, and more than 90% of physicians in 10 specialties would choose their specialty again. Still, burnout and stressors have led some to stop practicing altogether.
More and more organizations are hiring “travel physicians,” Mr. Adam says, and more physicians are choosing to take contract work (“locum tenens”) and practice in many different regions. Contract physicians typically help meet patient demand or provide coverage during the hiring process as well as while staff are on vacation or maternity leave.
Says Mr. Adam, “There’s no security, but there’s higher income and more flexibility.”
According to CHG Healthcare, locum tenens staffing is rising – approximately 7% of U.S. physicians (around 50,000) filled assignments in 2022, up 88% from 2015. In 2022, 56% of locum tenens employers reported a reduction in staff burnout, up from 30% in 2020.
The report indicates that more than half of physicians are satisfied with their income, down slightly from 55% 5 years ago (prepandemic). Physicians in some of the lower-paying specialties are among those most satisfied with their income. It’s not very surprising to Mr. Adam: “Higher earners generally suffer the most from burnout.
“They’re overworked, they have the largest number of patients, and they’re performing in high-stress situations doing challenging procedures on a daily basis – and they probably have worse work-life balance.” These physicians know going in that they need to be paid more to deal with such burdens. “That’s the feedback I get when I speak to high earners,” says Mr. Adam.
“The experienced ones are very clear about their [compensation] expectations.”
A version of this article first appeared on Medscape.com.
In addition, gender-based pay disparity among primary care physicians shrank, and the number of physicians who declined to take new Medicare patients rose.
The annual report is based on a survey of more than 10,000 physicians in over 29 specialties who answered questions about their income, workload, challenges, and level of satisfaction.
Average compensation across specialties rose to $352,000 – up nearly 17% from the 2018 average of $299,000. Fallout from the COVID-19 public health emergency continued to affect both physician compensation and job satisfaction, including Medicare reimbursements and staffing shortages due to burnout or retirement.
“Many physicians reevaluated what drove them to be a physician,” says Marc Adam, a recruiter at MASC Medical, a Florida physician recruiting firm.
Adam cites telehealth as an example. “An overwhelming majority of physicians prefer telehealth because of the convenience, but some really did not want to do it long term. They miss the patient interaction.”
The report also revealed that the gender-based pay gap in primary physicians fell, with men earning 19% more – down from 25% more in recent years. Among specialists, the gender gap was 27% on average, down from 31% last year. One reason may be an increase in compensation transparency, which Mr. Adam says should be the norm.
Income increases will likely continue, owing in large part to the growing disparity between physician supply and demand.
The projected physician shortage is expected to grow to 124,000 by 2034, according to the American Association of Medical Colleges. Federal lawmakers are considering passing the Resident Physician Shortage Reduction Act of 2023, which would add 14,000 Medicare-funded residency positions to help alleviate shortages.
Patient needs, Medicare rules continue to shift
Specialties with the biggest increases in compensation include oncology, anesthesiology, gastroenterology, radiology, critical care, and urology. Many procedure-related specialties saw more volume post pandemic.
Some respondents identified Medicare cuts and low reimbursement rates as a factor in tamping down compensation hikes. The number of physicians who expect to continue to take new Medicare patients is 65%, down from 71% 5 years ago.
For example, Medicare reimbursements for telehealth are expected to scale down in May, when the COVID-19 Public Health Emergency, which expanded telehealth services for Medicare patients, winds down.
“Telehealth will still exist,” says Mr. Adam, “but certain requirements will shape it going forward.”
Medicare isn’t viewed negatively across the board, however. Florida is among the top-earning states for physicians – along with Indiana, Connecticut, and Missouri. One reason is Florida’s unique health care environment, explains Mr. Adam, whose Florida-based firm places physicians nationwide.
“Florida is very progressive in terms of health care. For one thing, we have a large aging population and a large Medicare population.” Several growing organizations that focus on quality-based care are based in Florida, including ChenMed and Cano Health. Add to that the fact that owners of Florida’s health care organizations don’t have to be physicians, he explains, and the stage is set for experimentation.
“Being able to segment tasks frees up physicians to be more focused on medicine and provide better care while other people focus on the business and innovation.”
If Florida’s high compensation ranking continues, it may help employers there fulfill a growing need. The state is among those expected to experience the largest physician shortages in 2030, along with California, Texas, Arizona, and Georgia.
Side gigs up, satisfaction (slightly) down
In general, physicians aren’t fazed by these challenges. Many reported taking side gigs, some for additional income. Even so, 73% say they would still choose medicine, and more than 90% of physicians in 10 specialties would choose their specialty again. Still, burnout and stressors have led some to stop practicing altogether.
More and more organizations are hiring “travel physicians,” Mr. Adam says, and more physicians are choosing to take contract work (“locum tenens”) and practice in many different regions. Contract physicians typically help meet patient demand or provide coverage during the hiring process as well as while staff are on vacation or maternity leave.
Says Mr. Adam, “There’s no security, but there’s higher income and more flexibility.”
According to CHG Healthcare, locum tenens staffing is rising – approximately 7% of U.S. physicians (around 50,000) filled assignments in 2022, up 88% from 2015. In 2022, 56% of locum tenens employers reported a reduction in staff burnout, up from 30% in 2020.
The report indicates that more than half of physicians are satisfied with their income, down slightly from 55% 5 years ago (prepandemic). Physicians in some of the lower-paying specialties are among those most satisfied with their income. It’s not very surprising to Mr. Adam: “Higher earners generally suffer the most from burnout.
“They’re overworked, they have the largest number of patients, and they’re performing in high-stress situations doing challenging procedures on a daily basis – and they probably have worse work-life balance.” These physicians know going in that they need to be paid more to deal with such burdens. “That’s the feedback I get when I speak to high earners,” says Mr. Adam.
“The experienced ones are very clear about their [compensation] expectations.”
A version of this article first appeared on Medscape.com.