User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Meta-analysis examines cancer risk concern for JAK inhibitors
MANCHESTER, ENGLAND – Janus kinase (JAK) inhibitors may be associated with a higher risk for cancer relative to tumor necrosis factor (TNF) inhibitors, according to a meta-analysis reported at the annual meeting of the British Society for Rheumatology.
Looking at all phase 2, 3, and 4 trials and long-term extension studies across the indications of rheumatoid arthritis, psoriatic arthritis, psoriasis, axial spondyloarthritis, inflammatory bowel disease, and atopic dermatitis, the risk ratio for any cancer developing was 1.63 when compared with anti-TNF therapy (95% confidence interval, 1.27-2.09).
By comparison, JAK inhibitor use was not significantly associated with any greater risk for cancer than methotrexate (RR, 1.06; 95% confidence interval, 0.58-1.94) or placebo (RR, 1.16; 95% CI, 0.75-1.80).
“Our data suggests that rather than JAK inhibitors necessarily being harmful, it could be more a case of TNF inhibitors being protective,” said Christopher Stovin, MBChB, a specialist registrar in rheumatology at the Princess Royal University Hospital, King’s College Hospital NHS Trust, London.
“We should stress that these are rare events in our study, roughly around 1 in every 100 patient-years of exposure,” Dr. Stovin said.
“Despite having over 80,000 years of patient exposure, the median follow-up duration for JAK inhibitors was still only 118 weeks, which for cancers [that] obviously have long latency periods is still a relatively small duration of time,” the researcher added.
“People worry about the drugs. But there is a possibility that [a] disturbed immune system plays a role per se in development of cancers,” consultant rheumatologist Anurag Bharadwaj, MD, DM, said in an interview.
“Although there are studies which attribute increased risk of cancer to different DMARDs [disease-modifying antirheumatic drugs] and biologics like TNF, but on other hand, it’s maybe that we are giving these drugs to patients who have got more serious immunological disease,” suggested Bharadwaj, who serves as the clinical lead for rheumatology at Basildon (England) Hospital, Mid & South Essex Foundation Trust.
“So, a possibility may be that the more severe or the more active the immunological inflammatory disease, the higher the chance of cancer, and these are the patients who go for the stronger medications,” Dr. Bharadwaj said.
There is an “immunological window of opportunity” when treating these inflammatory diseases, said Dr. Bharadwaj, noting that the first few months of treatment are vital. “For all immunological diseases, the more quickly you bring the immunological abnormality down, the chances of long-term complications go down, including [possibly that the] chances of cancer go down, chances of cardiovascular disease go down, and chances of lung disease go down. Hit it early, hit it hard.”
Concern over a possible higher risk for cancer with JAK inhibitors than with TNF inhibitors was raised following the release of data from the ORAL Surveillance trial, a postmarketing trial of tofacitinib (Xeljanz) that had been mandated by the Food and Drug Administration.
“This was a study looking at the coprimary endpoints of malignancy and major adverse cardiovascular events, and it was enriched with patients over the age of 50, with one additional cardiac risk factor, designed to amplify the detection of these rare events,” Dr. Stovin said.
“There was a signal of an increased risk of malignancy in the tofacitinib group, and this led to the FDA issuing a [boxed warning for all licensed JAK inhibitors] at that time,” he added.
Dr. Stovin and colleagues aimed to determine what, if any, cancer risk was associated with all available JAK inhibitors relative to placebo, TNF inhibitors, and methotrexate.
In all, data from 62 randomized controlled trials and 14 long-term extension studies were included in the meta-analysis, accounting for 82,366 patient years of follow-up. The JAK inhibitors analyzed included tofacitinib, baricitinib (Olumiant), upadacitinib (Rinvoq), filgotinib (Jyseleca), and peficitinib (Smyraf). (Filgotinib and peficitinib have not been approved by the FDA.)
The researchers performed sensitivity analyses that excluded cancers detected within the first 6 months of treatment, the use of higher than licensed JAK inhibitor doses, and patients with non-rheumatoid arthritis diagnoses, but the results remained largely unchanged, Dr. Stovin reported.
“Perhaps not surprisingly, when we removed ORAL Surveillance” from the analysis comparing JAK inhibitors and TNF inhibitors, “we lost statistical significance,” he said.
“Longitudinal observational data is needed but currently remains limited,” Dr. Stovin concluded.
Dr. Stovin and Dr. Bharadwaj reported no relevant financial relationships. The meta-analysis was independently supported. Dr. Bharadwaj was not involved in the study and provided comment ahead of the presentation.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – Janus kinase (JAK) inhibitors may be associated with a higher risk for cancer relative to tumor necrosis factor (TNF) inhibitors, according to a meta-analysis reported at the annual meeting of the British Society for Rheumatology.
Looking at all phase 2, 3, and 4 trials and long-term extension studies across the indications of rheumatoid arthritis, psoriatic arthritis, psoriasis, axial spondyloarthritis, inflammatory bowel disease, and atopic dermatitis, the risk ratio for any cancer developing was 1.63 when compared with anti-TNF therapy (95% confidence interval, 1.27-2.09).
By comparison, JAK inhibitor use was not significantly associated with any greater risk for cancer than methotrexate (RR, 1.06; 95% confidence interval, 0.58-1.94) or placebo (RR, 1.16; 95% CI, 0.75-1.80).
“Our data suggests that rather than JAK inhibitors necessarily being harmful, it could be more a case of TNF inhibitors being protective,” said Christopher Stovin, MBChB, a specialist registrar in rheumatology at the Princess Royal University Hospital, King’s College Hospital NHS Trust, London.
“We should stress that these are rare events in our study, roughly around 1 in every 100 patient-years of exposure,” Dr. Stovin said.
“Despite having over 80,000 years of patient exposure, the median follow-up duration for JAK inhibitors was still only 118 weeks, which for cancers [that] obviously have long latency periods is still a relatively small duration of time,” the researcher added.
“People worry about the drugs. But there is a possibility that [a] disturbed immune system plays a role per se in development of cancers,” consultant rheumatologist Anurag Bharadwaj, MD, DM, said in an interview.
“Although there are studies which attribute increased risk of cancer to different DMARDs [disease-modifying antirheumatic drugs] and biologics like TNF, but on other hand, it’s maybe that we are giving these drugs to patients who have got more serious immunological disease,” suggested Bharadwaj, who serves as the clinical lead for rheumatology at Basildon (England) Hospital, Mid & South Essex Foundation Trust.
“So, a possibility may be that the more severe or the more active the immunological inflammatory disease, the higher the chance of cancer, and these are the patients who go for the stronger medications,” Dr. Bharadwaj said.
There is an “immunological window of opportunity” when treating these inflammatory diseases, said Dr. Bharadwaj, noting that the first few months of treatment are vital. “For all immunological diseases, the more quickly you bring the immunological abnormality down, the chances of long-term complications go down, including [possibly that the] chances of cancer go down, chances of cardiovascular disease go down, and chances of lung disease go down. Hit it early, hit it hard.”
Concern over a possible higher risk for cancer with JAK inhibitors than with TNF inhibitors was raised following the release of data from the ORAL Surveillance trial, a postmarketing trial of tofacitinib (Xeljanz) that had been mandated by the Food and Drug Administration.
“This was a study looking at the coprimary endpoints of malignancy and major adverse cardiovascular events, and it was enriched with patients over the age of 50, with one additional cardiac risk factor, designed to amplify the detection of these rare events,” Dr. Stovin said.
“There was a signal of an increased risk of malignancy in the tofacitinib group, and this led to the FDA issuing a [boxed warning for all licensed JAK inhibitors] at that time,” he added.
Dr. Stovin and colleagues aimed to determine what, if any, cancer risk was associated with all available JAK inhibitors relative to placebo, TNF inhibitors, and methotrexate.
In all, data from 62 randomized controlled trials and 14 long-term extension studies were included in the meta-analysis, accounting for 82,366 patient years of follow-up. The JAK inhibitors analyzed included tofacitinib, baricitinib (Olumiant), upadacitinib (Rinvoq), filgotinib (Jyseleca), and peficitinib (Smyraf). (Filgotinib and peficitinib have not been approved by the FDA.)
The researchers performed sensitivity analyses that excluded cancers detected within the first 6 months of treatment, the use of higher than licensed JAK inhibitor doses, and patients with non-rheumatoid arthritis diagnoses, but the results remained largely unchanged, Dr. Stovin reported.
“Perhaps not surprisingly, when we removed ORAL Surveillance” from the analysis comparing JAK inhibitors and TNF inhibitors, “we lost statistical significance,” he said.
“Longitudinal observational data is needed but currently remains limited,” Dr. Stovin concluded.
Dr. Stovin and Dr. Bharadwaj reported no relevant financial relationships. The meta-analysis was independently supported. Dr. Bharadwaj was not involved in the study and provided comment ahead of the presentation.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – Janus kinase (JAK) inhibitors may be associated with a higher risk for cancer relative to tumor necrosis factor (TNF) inhibitors, according to a meta-analysis reported at the annual meeting of the British Society for Rheumatology.
Looking at all phase 2, 3, and 4 trials and long-term extension studies across the indications of rheumatoid arthritis, psoriatic arthritis, psoriasis, axial spondyloarthritis, inflammatory bowel disease, and atopic dermatitis, the risk ratio for any cancer developing was 1.63 when compared with anti-TNF therapy (95% confidence interval, 1.27-2.09).
By comparison, JAK inhibitor use was not significantly associated with any greater risk for cancer than methotrexate (RR, 1.06; 95% confidence interval, 0.58-1.94) or placebo (RR, 1.16; 95% CI, 0.75-1.80).
“Our data suggests that rather than JAK inhibitors necessarily being harmful, it could be more a case of TNF inhibitors being protective,” said Christopher Stovin, MBChB, a specialist registrar in rheumatology at the Princess Royal University Hospital, King’s College Hospital NHS Trust, London.
“We should stress that these are rare events in our study, roughly around 1 in every 100 patient-years of exposure,” Dr. Stovin said.
“Despite having over 80,000 years of patient exposure, the median follow-up duration for JAK inhibitors was still only 118 weeks, which for cancers [that] obviously have long latency periods is still a relatively small duration of time,” the researcher added.
“People worry about the drugs. But there is a possibility that [a] disturbed immune system plays a role per se in development of cancers,” consultant rheumatologist Anurag Bharadwaj, MD, DM, said in an interview.
“Although there are studies which attribute increased risk of cancer to different DMARDs [disease-modifying antirheumatic drugs] and biologics like TNF, but on other hand, it’s maybe that we are giving these drugs to patients who have got more serious immunological disease,” suggested Bharadwaj, who serves as the clinical lead for rheumatology at Basildon (England) Hospital, Mid & South Essex Foundation Trust.
“So, a possibility may be that the more severe or the more active the immunological inflammatory disease, the higher the chance of cancer, and these are the patients who go for the stronger medications,” Dr. Bharadwaj said.
There is an “immunological window of opportunity” when treating these inflammatory diseases, said Dr. Bharadwaj, noting that the first few months of treatment are vital. “For all immunological diseases, the more quickly you bring the immunological abnormality down, the chances of long-term complications go down, including [possibly that the] chances of cancer go down, chances of cardiovascular disease go down, and chances of lung disease go down. Hit it early, hit it hard.”
Concern over a possible higher risk for cancer with JAK inhibitors than with TNF inhibitors was raised following the release of data from the ORAL Surveillance trial, a postmarketing trial of tofacitinib (Xeljanz) that had been mandated by the Food and Drug Administration.
“This was a study looking at the coprimary endpoints of malignancy and major adverse cardiovascular events, and it was enriched with patients over the age of 50, with one additional cardiac risk factor, designed to amplify the detection of these rare events,” Dr. Stovin said.
“There was a signal of an increased risk of malignancy in the tofacitinib group, and this led to the FDA issuing a [boxed warning for all licensed JAK inhibitors] at that time,” he added.
Dr. Stovin and colleagues aimed to determine what, if any, cancer risk was associated with all available JAK inhibitors relative to placebo, TNF inhibitors, and methotrexate.
In all, data from 62 randomized controlled trials and 14 long-term extension studies were included in the meta-analysis, accounting for 82,366 patient years of follow-up. The JAK inhibitors analyzed included tofacitinib, baricitinib (Olumiant), upadacitinib (Rinvoq), filgotinib (Jyseleca), and peficitinib (Smyraf). (Filgotinib and peficitinib have not been approved by the FDA.)
The researchers performed sensitivity analyses that excluded cancers detected within the first 6 months of treatment, the use of higher than licensed JAK inhibitor doses, and patients with non-rheumatoid arthritis diagnoses, but the results remained largely unchanged, Dr. Stovin reported.
“Perhaps not surprisingly, when we removed ORAL Surveillance” from the analysis comparing JAK inhibitors and TNF inhibitors, “we lost statistical significance,” he said.
“Longitudinal observational data is needed but currently remains limited,” Dr. Stovin concluded.
Dr. Stovin and Dr. Bharadwaj reported no relevant financial relationships. The meta-analysis was independently supported. Dr. Bharadwaj was not involved in the study and provided comment ahead of the presentation.
A version of this article first appeared on Medscape.com.
AT BSR 2023
Small study finds IPL-radiofrequency combination effective for dry eye disease
PHOENIX – and improved meibum quality in both upper and lower eyelids, results from an ongoing, novel study showed.
Dry eye disease affects a large proportion of people in the United States “and the factors that contribute to that are certainly not going away,” lead study author James G. Chelnis MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where he presented the results during an abstract session. “Prepandemic, we used to have meetings in person; now most are on a computer screen,” a common risk factor for worsening dry eyes, he said. Telltale dry eye symptoms include blurry vision, irritation, and corneal damage – mostly caused by meibomian gland dysfunction – which impacts the quality and quantity of meibum secreted. Common treatments include warm compresses, doxycycline, and artificial tears.
While some studies have shown IPL is helpful in treating dry eye disease caused by meibomian gland dysfunction, little information is available on its use alone or in combination with topical RF to preserve and improve the function of meibomian glands, said Dr. Chelnis, an ophthalmic plastic surgeon in New York City. “The theory here is that the radiofrequency would be able to vibrate the water molecules inside the meibomian glands, which would allow you to turn over the meibum faster, as well as improve the blink reflex response by building supporting collagen,” he said. “Our novel study explores the ability of this combined modality treatment to improve upon meibomian gland health.”
Study design, results
Dr. Chelnis and his colleagues enrolled 11 individuals with a previous diagnosis of dry eye disease and meibomian gland dysfunction with Ocular Surface Disease Index (OSDI) survey scores higher than 23, which indicate at least moderate dry eye symptoms. Inclusion criteria were being 22 years of age or older, signs of meibomian gland dysfunction as detected by biomicroscopy, a modified meibomian gland score over 12 in the lower eyelid of at least one eye, and type I-IV skin.
All patients received four treatments (each 2 weeks apart) of IPL to the lower eyelid, surrounding malar region, and nose, followed by 7 minutes of topical RF treatments at 1 MHz and 4 MHz extending to the inferior, lateral, and superior orbital rim. Evaluation of meibomian gland expression and quality of meibum upon expression was conducted following each treatment session, with a final evaluation 4 weeks after the final treatment session.
Meibum quality was evaluated on a scale of 0-3 representing clear (0), cloudy (1), inspissated (2), and blocked (3) meibum, respectively.
Following treatment, meibomian gland expression and meibum quality improved in all eyelids in all 11 patients. Specifically, in the right eye, the number of upper lid expressible glands increased from an average of 13 to 27.9 and the number of lower lid expressible glands increased from an average of 14.6 to 28.2; and in the left eye, the number of upper lid expressible glands increased from an average of 13.3 to 27.3 and the number of lower lid expressible glands increased from an average of 14.8 to 26.8 (P < .001 for all associations).
The overall percentage improvement in meibomian gland expression in the right eye was 82.7% for the upper lids and 136.6% for the lower lids, and in the left eye, 82.9% for the upper lids, and 112.2% for the lower lids.
When comparing upper against lower lids, meibomian gland expression increased 124.4% and 82.8%, respectively. Meibum quality improved in all four eyelids, although upper eyelids displayed a superior improvement compared with lower eyelids.
“We are finding that combining IPL plus RF produces a more complete and comprehensive improvement in the quality of their meibomian gland health, and as such, their dry eyes,” with “a large decrease in their symptom profile,” he concluded.
More patients to be studied
Dr. Chelnis acknowledged certain limitations of the study, including the small number of patients, but he and his colleagues have added an additional clinical site to expand the sample size. “Larger scale studies are needed to evaluate long-term effectiveness of IPL plus RF as well as a comparison with other treatment options.”
During a question-and-answer session Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, who served as one of the abstract session moderators, asked Dr. Chelnis to comment on what the mechanism of action of the IPL-RF combination in improving meibomian gland health.
“It’s not fully understood, but part of it is improved vascularity at the lid margin,” said Dr. Chelnis, who holds a faculty position in the department of ophthalmology at Icahn School of Medicine at Mount Sinai, New York. “Your ocular surface is sort of like your screen door; it catches everything that’s in the environment. An increase in vascularity and immunologic cytokines occurs in response to that. If you’re looking at the eye with a slit lamp, you can see a lot of vascularity that occurs at the lid margin and crowds the meibomian glands. When you decrease that crowding and immunogenic response, you move towards a normally functioning lid margin.”
Dr. Chelnis disclosed that he is a consultant to or an adviser for Lumenis, Horizon Therapeutics, and Soniquence.
PHOENIX – and improved meibum quality in both upper and lower eyelids, results from an ongoing, novel study showed.
Dry eye disease affects a large proportion of people in the United States “and the factors that contribute to that are certainly not going away,” lead study author James G. Chelnis MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where he presented the results during an abstract session. “Prepandemic, we used to have meetings in person; now most are on a computer screen,” a common risk factor for worsening dry eyes, he said. Telltale dry eye symptoms include blurry vision, irritation, and corneal damage – mostly caused by meibomian gland dysfunction – which impacts the quality and quantity of meibum secreted. Common treatments include warm compresses, doxycycline, and artificial tears.
While some studies have shown IPL is helpful in treating dry eye disease caused by meibomian gland dysfunction, little information is available on its use alone or in combination with topical RF to preserve and improve the function of meibomian glands, said Dr. Chelnis, an ophthalmic plastic surgeon in New York City. “The theory here is that the radiofrequency would be able to vibrate the water molecules inside the meibomian glands, which would allow you to turn over the meibum faster, as well as improve the blink reflex response by building supporting collagen,” he said. “Our novel study explores the ability of this combined modality treatment to improve upon meibomian gland health.”
Study design, results
Dr. Chelnis and his colleagues enrolled 11 individuals with a previous diagnosis of dry eye disease and meibomian gland dysfunction with Ocular Surface Disease Index (OSDI) survey scores higher than 23, which indicate at least moderate dry eye symptoms. Inclusion criteria were being 22 years of age or older, signs of meibomian gland dysfunction as detected by biomicroscopy, a modified meibomian gland score over 12 in the lower eyelid of at least one eye, and type I-IV skin.
All patients received four treatments (each 2 weeks apart) of IPL to the lower eyelid, surrounding malar region, and nose, followed by 7 minutes of topical RF treatments at 1 MHz and 4 MHz extending to the inferior, lateral, and superior orbital rim. Evaluation of meibomian gland expression and quality of meibum upon expression was conducted following each treatment session, with a final evaluation 4 weeks after the final treatment session.
Meibum quality was evaluated on a scale of 0-3 representing clear (0), cloudy (1), inspissated (2), and blocked (3) meibum, respectively.
Following treatment, meibomian gland expression and meibum quality improved in all eyelids in all 11 patients. Specifically, in the right eye, the number of upper lid expressible glands increased from an average of 13 to 27.9 and the number of lower lid expressible glands increased from an average of 14.6 to 28.2; and in the left eye, the number of upper lid expressible glands increased from an average of 13.3 to 27.3 and the number of lower lid expressible glands increased from an average of 14.8 to 26.8 (P < .001 for all associations).
The overall percentage improvement in meibomian gland expression in the right eye was 82.7% for the upper lids and 136.6% for the lower lids, and in the left eye, 82.9% for the upper lids, and 112.2% for the lower lids.
When comparing upper against lower lids, meibomian gland expression increased 124.4% and 82.8%, respectively. Meibum quality improved in all four eyelids, although upper eyelids displayed a superior improvement compared with lower eyelids.
“We are finding that combining IPL plus RF produces a more complete and comprehensive improvement in the quality of their meibomian gland health, and as such, their dry eyes,” with “a large decrease in their symptom profile,” he concluded.
More patients to be studied
Dr. Chelnis acknowledged certain limitations of the study, including the small number of patients, but he and his colleagues have added an additional clinical site to expand the sample size. “Larger scale studies are needed to evaluate long-term effectiveness of IPL plus RF as well as a comparison with other treatment options.”
During a question-and-answer session Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, who served as one of the abstract session moderators, asked Dr. Chelnis to comment on what the mechanism of action of the IPL-RF combination in improving meibomian gland health.
“It’s not fully understood, but part of it is improved vascularity at the lid margin,” said Dr. Chelnis, who holds a faculty position in the department of ophthalmology at Icahn School of Medicine at Mount Sinai, New York. “Your ocular surface is sort of like your screen door; it catches everything that’s in the environment. An increase in vascularity and immunologic cytokines occurs in response to that. If you’re looking at the eye with a slit lamp, you can see a lot of vascularity that occurs at the lid margin and crowds the meibomian glands. When you decrease that crowding and immunogenic response, you move towards a normally functioning lid margin.”
Dr. Chelnis disclosed that he is a consultant to or an adviser for Lumenis, Horizon Therapeutics, and Soniquence.
PHOENIX – and improved meibum quality in both upper and lower eyelids, results from an ongoing, novel study showed.
Dry eye disease affects a large proportion of people in the United States “and the factors that contribute to that are certainly not going away,” lead study author James G. Chelnis MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where he presented the results during an abstract session. “Prepandemic, we used to have meetings in person; now most are on a computer screen,” a common risk factor for worsening dry eyes, he said. Telltale dry eye symptoms include blurry vision, irritation, and corneal damage – mostly caused by meibomian gland dysfunction – which impacts the quality and quantity of meibum secreted. Common treatments include warm compresses, doxycycline, and artificial tears.
While some studies have shown IPL is helpful in treating dry eye disease caused by meibomian gland dysfunction, little information is available on its use alone or in combination with topical RF to preserve and improve the function of meibomian glands, said Dr. Chelnis, an ophthalmic plastic surgeon in New York City. “The theory here is that the radiofrequency would be able to vibrate the water molecules inside the meibomian glands, which would allow you to turn over the meibum faster, as well as improve the blink reflex response by building supporting collagen,” he said. “Our novel study explores the ability of this combined modality treatment to improve upon meibomian gland health.”
Study design, results
Dr. Chelnis and his colleagues enrolled 11 individuals with a previous diagnosis of dry eye disease and meibomian gland dysfunction with Ocular Surface Disease Index (OSDI) survey scores higher than 23, which indicate at least moderate dry eye symptoms. Inclusion criteria were being 22 years of age or older, signs of meibomian gland dysfunction as detected by biomicroscopy, a modified meibomian gland score over 12 in the lower eyelid of at least one eye, and type I-IV skin.
All patients received four treatments (each 2 weeks apart) of IPL to the lower eyelid, surrounding malar region, and nose, followed by 7 minutes of topical RF treatments at 1 MHz and 4 MHz extending to the inferior, lateral, and superior orbital rim. Evaluation of meibomian gland expression and quality of meibum upon expression was conducted following each treatment session, with a final evaluation 4 weeks after the final treatment session.
Meibum quality was evaluated on a scale of 0-3 representing clear (0), cloudy (1), inspissated (2), and blocked (3) meibum, respectively.
Following treatment, meibomian gland expression and meibum quality improved in all eyelids in all 11 patients. Specifically, in the right eye, the number of upper lid expressible glands increased from an average of 13 to 27.9 and the number of lower lid expressible glands increased from an average of 14.6 to 28.2; and in the left eye, the number of upper lid expressible glands increased from an average of 13.3 to 27.3 and the number of lower lid expressible glands increased from an average of 14.8 to 26.8 (P < .001 for all associations).
The overall percentage improvement in meibomian gland expression in the right eye was 82.7% for the upper lids and 136.6% for the lower lids, and in the left eye, 82.9% for the upper lids, and 112.2% for the lower lids.
When comparing upper against lower lids, meibomian gland expression increased 124.4% and 82.8%, respectively. Meibum quality improved in all four eyelids, although upper eyelids displayed a superior improvement compared with lower eyelids.
“We are finding that combining IPL plus RF produces a more complete and comprehensive improvement in the quality of their meibomian gland health, and as such, their dry eyes,” with “a large decrease in their symptom profile,” he concluded.
More patients to be studied
Dr. Chelnis acknowledged certain limitations of the study, including the small number of patients, but he and his colleagues have added an additional clinical site to expand the sample size. “Larger scale studies are needed to evaluate long-term effectiveness of IPL plus RF as well as a comparison with other treatment options.”
During a question-and-answer session Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, who served as one of the abstract session moderators, asked Dr. Chelnis to comment on what the mechanism of action of the IPL-RF combination in improving meibomian gland health.
“It’s not fully understood, but part of it is improved vascularity at the lid margin,” said Dr. Chelnis, who holds a faculty position in the department of ophthalmology at Icahn School of Medicine at Mount Sinai, New York. “Your ocular surface is sort of like your screen door; it catches everything that’s in the environment. An increase in vascularity and immunologic cytokines occurs in response to that. If you’re looking at the eye with a slit lamp, you can see a lot of vascularity that occurs at the lid margin and crowds the meibomian glands. When you decrease that crowding and immunogenic response, you move towards a normally functioning lid margin.”
Dr. Chelnis disclosed that he is a consultant to or an adviser for Lumenis, Horizon Therapeutics, and Soniquence.
AT ASLMS 2023
Painful Nodules With a Crawling Sensation
The Diagnosis: Cutaneous Furuncular Myiasis
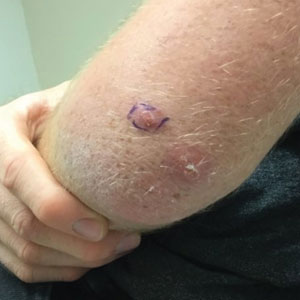
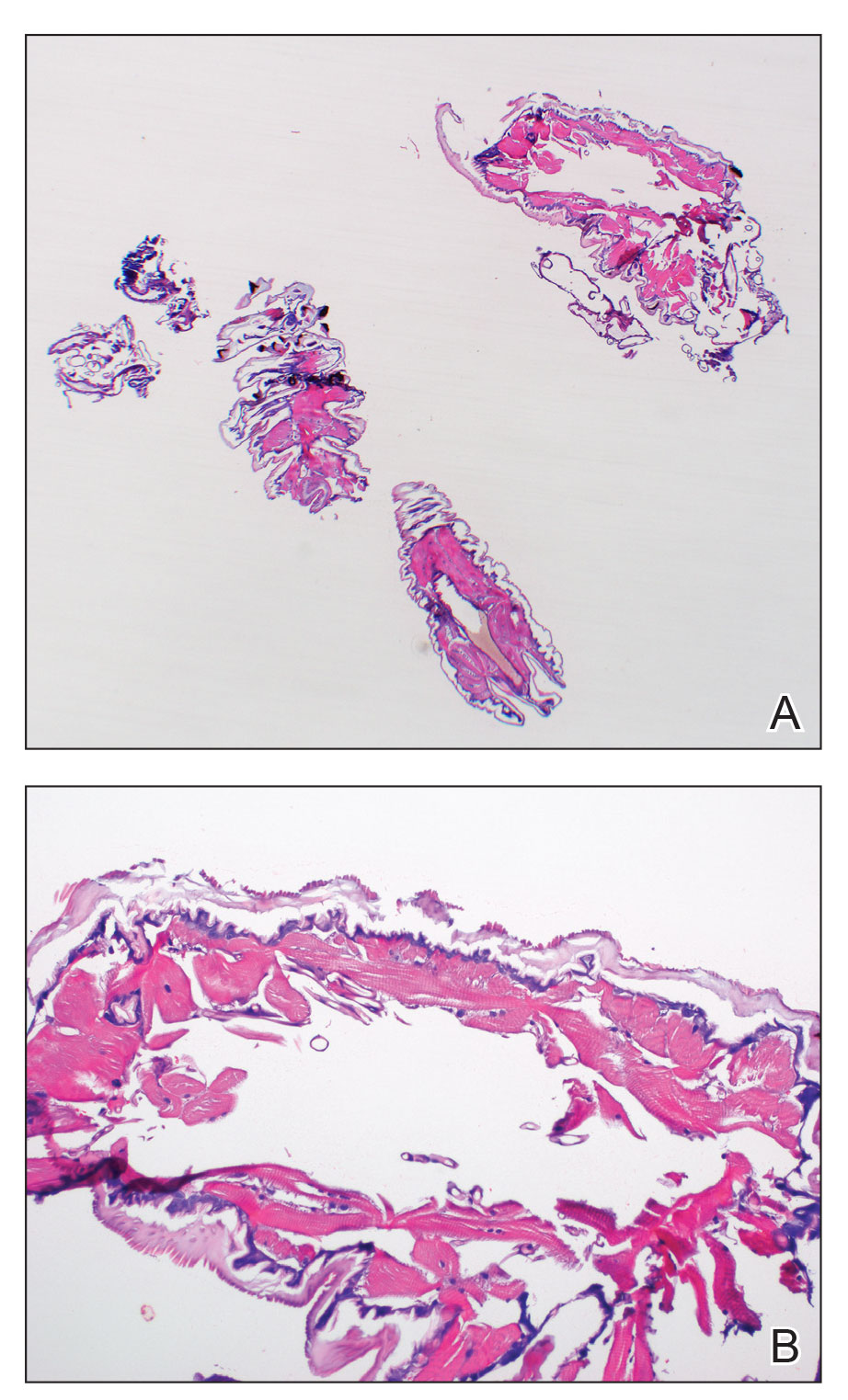
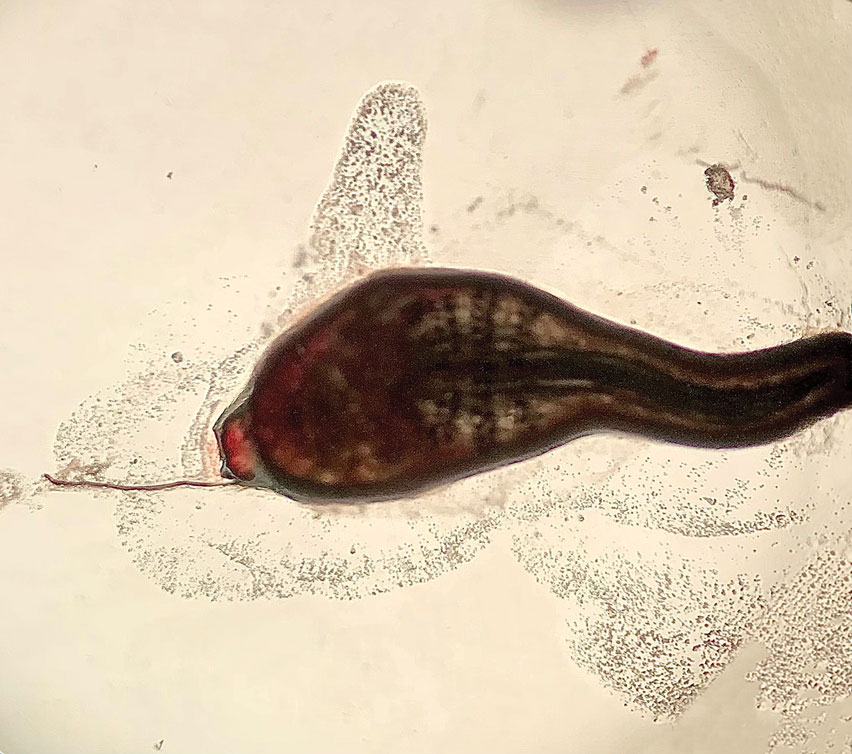
Histopathology of the punch biopsy showed an undulating chitinous exoskeleton and pigmented spines (setae) protruding from the exoskeleton with associated superficial perivascular lymphohistiocytic infiltrates on hematoxylin and eosin stain (Figure 1). Live insect larvae were observed and extracted, which immediately relieved the crawling sensation (Figure 2). Light microscopy of the larva showed a row of hooks surrounding a tapered body with a head attached anteriorly (Figure 3).
Myiasis is a parasitic infestation of the dipterous fly’s larvae in the host organ and tissue. There are 5 types of myiasis based on the location of the infestation: wound myiasis occurs with egg infestations on an open wound; furuncular myiasis results from egg placement by penetration of healthy skin by a mosquito vector; plaque myiasis comprises the placement of eggs on clothing through several maggots and flies; creeping myiasis involves the Gasterophilus fly delivering the larva intradermally; and body cavity myiasis may develop in the orbit, nasal cavity, urogenital system, and gastrointestinal tract.1-3

Furuncular myiasis infestation occurs via a complex life cycle in which mosquitoes act as a vector and transfer the eggs to the human or animal host.1-3 Botfly larvae then penetrate the skin and reside within the subdermis to mature. Adults then emerge after 1 month to repeat the cycle.1 Dermatobia hominis and Cordylobia anthropophaga are the most common causes of furuncular myiasis.2,3 Furuncular myiasis commonly presents in travelers that are returning from tropical countries. Initially, an itching erythematous papule develops. After the larvae mature, they can appear as boil-like lesions with a small central punctum.1-3 Dermoscopy can be utilized for visualization of different larvae anatomy such as a furuncularlike lesion, spines, and posterior breathing spiracle from the central punctum.4
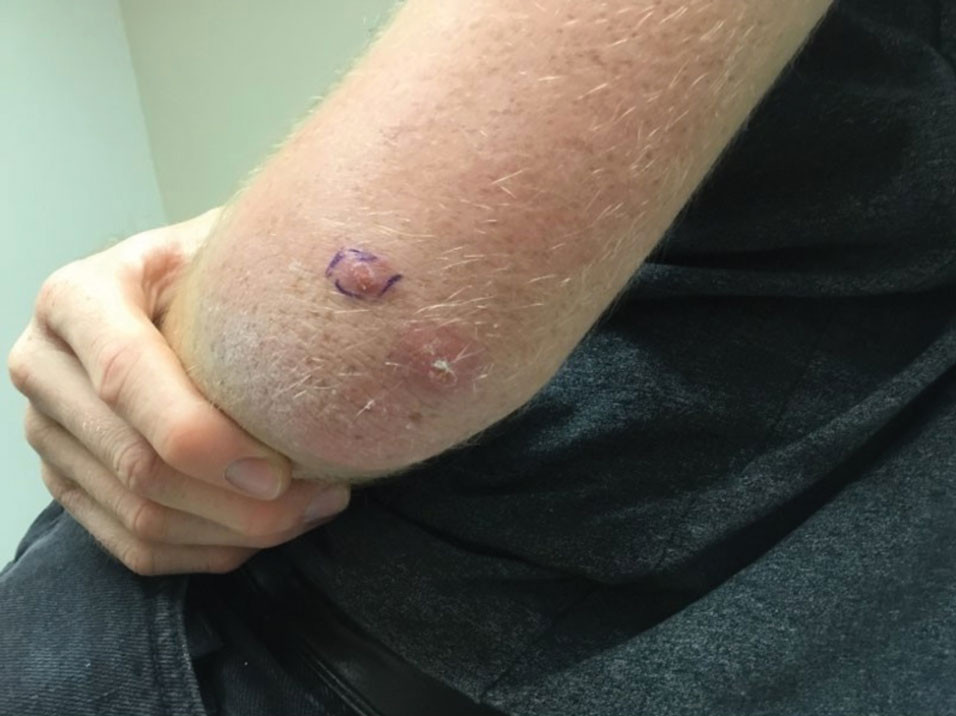
Our patient’s recent travel to the Amazon in Brazil, clinical history, and histopathologic findings ruled out other differential diagnoses such as cutaneous larva migrans, gnathostomiasis, loiasis, and tungiasis.
Treatment is curative with the extraction of the intact larva from the nodule. Localized skin anesthetic injection can be used to bulge the larva outward for easier extraction. A single dose of ivermectin 15 mg can treat the parasitic infestation of myiasis.1-3
- John DT, Petri WA, Markell EK, et al. Markell and Voge’s Medical Parasitology. 9th ed. Saunders Elsevier; 2006.
- Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
- Lachish T, Marhoom E, Mumcuoglu KY, et al. Myiasis in travelers. J Travel Med. 2015;22:232-236.
- Mello C, Magalhães R. Triangular black dots in dermoscopy of furuncular myiasis. JAAD Case Rep. 2021;12:49-50.
The Diagnosis: Cutaneous Furuncular Myiasis
Histopathology of the punch biopsy showed an undulating chitinous exoskeleton and pigmented spines (setae) protruding from the exoskeleton with associated superficial perivascular lymphohistiocytic infiltrates on hematoxylin and eosin stain (Figure 1). Live insect larvae were observed and extracted, which immediately relieved the crawling sensation (Figure 2). Light microscopy of the larva showed a row of hooks surrounding a tapered body with a head attached anteriorly (Figure 3).

Myiasis is a parasitic infestation of the dipterous fly’s larvae in the host organ and tissue. There are 5 types of myiasis based on the location of the infestation: wound myiasis occurs with egg infestations on an open wound; furuncular myiasis results from egg placement by penetration of healthy skin by a mosquito vector; plaque myiasis comprises the placement of eggs on clothing through several maggots and flies; creeping myiasis involves the Gasterophilus fly delivering the larva intradermally; and body cavity myiasis may develop in the orbit, nasal cavity, urogenital system, and gastrointestinal tract.1-3

Furuncular myiasis infestation occurs via a complex life cycle in which mosquitoes act as a vector and transfer the eggs to the human or animal host.1-3 Botfly larvae then penetrate the skin and reside within the subdermis to mature. Adults then emerge after 1 month to repeat the cycle.1 Dermatobia hominis and Cordylobia anthropophaga are the most common causes of furuncular myiasis.2,3 Furuncular myiasis commonly presents in travelers that are returning from tropical countries. Initially, an itching erythematous papule develops. After the larvae mature, they can appear as boil-like lesions with a small central punctum.1-3 Dermoscopy can be utilized for visualization of different larvae anatomy such as a furuncularlike lesion, spines, and posterior breathing spiracle from the central punctum.4

Our patient’s recent travel to the Amazon in Brazil, clinical history, and histopathologic findings ruled out other differential diagnoses such as cutaneous larva migrans, gnathostomiasis, loiasis, and tungiasis.
Treatment is curative with the extraction of the intact larva from the nodule. Localized skin anesthetic injection can be used to bulge the larva outward for easier extraction. A single dose of ivermectin 15 mg can treat the parasitic infestation of myiasis.1-3
The Diagnosis: Cutaneous Furuncular Myiasis
Histopathology of the punch biopsy showed an undulating chitinous exoskeleton and pigmented spines (setae) protruding from the exoskeleton with associated superficial perivascular lymphohistiocytic infiltrates on hematoxylin and eosin stain (Figure 1). Live insect larvae were observed and extracted, which immediately relieved the crawling sensation (Figure 2). Light microscopy of the larva showed a row of hooks surrounding a tapered body with a head attached anteriorly (Figure 3).

Myiasis is a parasitic infestation of the dipterous fly’s larvae in the host organ and tissue. There are 5 types of myiasis based on the location of the infestation: wound myiasis occurs with egg infestations on an open wound; furuncular myiasis results from egg placement by penetration of healthy skin by a mosquito vector; plaque myiasis comprises the placement of eggs on clothing through several maggots and flies; creeping myiasis involves the Gasterophilus fly delivering the larva intradermally; and body cavity myiasis may develop in the orbit, nasal cavity, urogenital system, and gastrointestinal tract.1-3

Furuncular myiasis infestation occurs via a complex life cycle in which mosquitoes act as a vector and transfer the eggs to the human or animal host.1-3 Botfly larvae then penetrate the skin and reside within the subdermis to mature. Adults then emerge after 1 month to repeat the cycle.1 Dermatobia hominis and Cordylobia anthropophaga are the most common causes of furuncular myiasis.2,3 Furuncular myiasis commonly presents in travelers that are returning from tropical countries. Initially, an itching erythematous papule develops. After the larvae mature, they can appear as boil-like lesions with a small central punctum.1-3 Dermoscopy can be utilized for visualization of different larvae anatomy such as a furuncularlike lesion, spines, and posterior breathing spiracle from the central punctum.4

Our patient’s recent travel to the Amazon in Brazil, clinical history, and histopathologic findings ruled out other differential diagnoses such as cutaneous larva migrans, gnathostomiasis, loiasis, and tungiasis.
Treatment is curative with the extraction of the intact larva from the nodule. Localized skin anesthetic injection can be used to bulge the larva outward for easier extraction. A single dose of ivermectin 15 mg can treat the parasitic infestation of myiasis.1-3
- John DT, Petri WA, Markell EK, et al. Markell and Voge’s Medical Parasitology. 9th ed. Saunders Elsevier; 2006.
- Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
- Lachish T, Marhoom E, Mumcuoglu KY, et al. Myiasis in travelers. J Travel Med. 2015;22:232-236.
- Mello C, Magalhães R. Triangular black dots in dermoscopy of furuncular myiasis. JAAD Case Rep. 2021;12:49-50.
- John DT, Petri WA, Markell EK, et al. Markell and Voge’s Medical Parasitology. 9th ed. Saunders Elsevier; 2006.
- Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
- Lachish T, Marhoom E, Mumcuoglu KY, et al. Myiasis in travelers. J Travel Med. 2015;22:232-236.
- Mello C, Magalhães R. Triangular black dots in dermoscopy of furuncular myiasis. JAAD Case Rep. 2021;12:49-50.
A 20-year-old man presented with progressively enlarging, painful lesions on the arm with a crawling sensation of 3 weeks’ duration. The lesions appeared after a recent trip to Brazil where he was hiking in the Amazon. He noted that the pain occurred suddenly and there was some serous drainage from the lesions. He denied any trauma to the area and reported no history of similar eruptions, treatments, or systemic symptoms. Physical examination revealed 2 tender erythematous nodules, each measuring 0.6 cm in diameter, with associated crust and a reported crawling sensation on the posterior aspect of the left arm. No drainage was seen. A punch biopsy was performed.
Guidelines for assessing cancer risk may need updating
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
FROM AACR 2023
Study focuses on adolescent data in upadacitinib AD trials
(AD), an analysis of three clinical trials reports.
Upadacitinib (Rinvoq) was approved by the Food and Drug Administration for treating adults and pediatric patients 12 years of age and older with refractory, moderate to severe AD, in January 2022. This study analyzed the adolescent data in three double-blind, placebo-controlled phase 3 randomized clinical trials, which included adults and 552 adolescents between 12 and 17 years of age with moderate to severe AD in more than 20 countries in Europe, North and South America, the Middle East, Oceania, and the Asia-Pacific region from July 2018 through December 2020.
In the studies, “treatment of moderate to severe AD in adolescents with upadacitinib was effective and generally well tolerated, with an overall efficacy and safety profile similar to that observed in adults, and patient-reported outcomes indicated an overall better health-related quality of life compared with placebo,” lead study author Amy S. Paller, MD, chair of the department of dermatology and professor of dermatology and pediatrics, at Northwestern University, Chicago, and her colleagues write in JAMA Dermatology.
Adolescents in the three studies – Measure Up 1, Measure Up 2, and AD Up – received once-daily oral upadacitinib 15 mg, 30 mg, or placebo. All participants in AD Up used topical corticosteroids.
At 16 weeks, in Measure Up 1, Measure Up 2, and AD Up, respectively, a greater proportion of adolescents improved by at least 75% in the Eczema Area and Severity Index (EASI 75) with upadacitinib 15 mg (73%, 69%, 63%); and with upadacitinib 30 mg (78%, 73%, 84%), compared with placebo (12%, 13%, 30%), (P < .001 for all comparisons vs. placebo).
Upadacitinib was generally well tolerated among the adolescents, with mild or moderate acne being the most common adverse event, reported in 10%-13% of those on 15 mg and 15%-16% of those on 30 mg vs. 2%-3% of those on placebo.
Asked to comment on the study, Peck Ong, MD, a pediatric allergist and immunologist at Children’s Hospital Los Angeles, said that he was not surprised by the drug’s effectiveness because JAK inhibitors are potent immunosuppressants. Strengths of the studies include the many pediatric participants, its international reach, and its use of standardized and validated measures, said Dr. Ong, who was not involved in the study.
“The effect of JAK inhibitors is more specific than traditional immunosuppressants such as cyclosporine and methotrexate but not as specific as biologics; therefore, long-term safety data are needed,” he advised. “16 weeks is a very short time to study a chronic disease like atopic dermatitis. We need safety data longer than 1 year.”
Given the disease’s potential impact on self-esteem, sleep, and other important areas of life, Sean Reynolds, MBBCH, a pediatric dermatologist at Children’s Mercy Kansas City (Mo.), welcomed the data on the newer pharmacologic agents.
“FDA-approved systemic treatment options for adolescents with AD are currently limited, which necessitates studies such as this that explore additional treatment options,” said Dr. Reynolds, who also was not involved in the study, told this news organization.
He added that oral upadacitinib may especially help patients who have not found relief with other topical or systemic treatments or who are needle phobic. While the overall efficacy and relatively mild side effects for most patients taking upadacitinib in the trials are encouraging, “the long-term efficacy and side effects in this population require further study, especially considering the limited systemic AD treatment options available in this age group,” he added.
“Given the reported use of other JAK inhibitors to treat myriad inflammatory skin conditions beyond atopic dermatitis, the potential use of upadacitinib and other JAK inhibitors to treat these skin diseases in children and adolescents represents an exciting area for future study in the field of pediatric dermatology,” Dr. Reynolds noted.
The study was funded by AbbVie, the developer and manufacturer of upadacitinib. Dr. Paller and almost all other authors reported relevant financial relationships with AbbVie and other pharmaceutical companies. Dr. Ong reported serving on an AbbVie advisory board, and Dr. Reynolds reported no conflict of interest with the study.
(AD), an analysis of three clinical trials reports.
Upadacitinib (Rinvoq) was approved by the Food and Drug Administration for treating adults and pediatric patients 12 years of age and older with refractory, moderate to severe AD, in January 2022. This study analyzed the adolescent data in three double-blind, placebo-controlled phase 3 randomized clinical trials, which included adults and 552 adolescents between 12 and 17 years of age with moderate to severe AD in more than 20 countries in Europe, North and South America, the Middle East, Oceania, and the Asia-Pacific region from July 2018 through December 2020.
In the studies, “treatment of moderate to severe AD in adolescents with upadacitinib was effective and generally well tolerated, with an overall efficacy and safety profile similar to that observed in adults, and patient-reported outcomes indicated an overall better health-related quality of life compared with placebo,” lead study author Amy S. Paller, MD, chair of the department of dermatology and professor of dermatology and pediatrics, at Northwestern University, Chicago, and her colleagues write in JAMA Dermatology.
Adolescents in the three studies – Measure Up 1, Measure Up 2, and AD Up – received once-daily oral upadacitinib 15 mg, 30 mg, or placebo. All participants in AD Up used topical corticosteroids.
At 16 weeks, in Measure Up 1, Measure Up 2, and AD Up, respectively, a greater proportion of adolescents improved by at least 75% in the Eczema Area and Severity Index (EASI 75) with upadacitinib 15 mg (73%, 69%, 63%); and with upadacitinib 30 mg (78%, 73%, 84%), compared with placebo (12%, 13%, 30%), (P < .001 for all comparisons vs. placebo).
Upadacitinib was generally well tolerated among the adolescents, with mild or moderate acne being the most common adverse event, reported in 10%-13% of those on 15 mg and 15%-16% of those on 30 mg vs. 2%-3% of those on placebo.
Asked to comment on the study, Peck Ong, MD, a pediatric allergist and immunologist at Children’s Hospital Los Angeles, said that he was not surprised by the drug’s effectiveness because JAK inhibitors are potent immunosuppressants. Strengths of the studies include the many pediatric participants, its international reach, and its use of standardized and validated measures, said Dr. Ong, who was not involved in the study.
“The effect of JAK inhibitors is more specific than traditional immunosuppressants such as cyclosporine and methotrexate but not as specific as biologics; therefore, long-term safety data are needed,” he advised. “16 weeks is a very short time to study a chronic disease like atopic dermatitis. We need safety data longer than 1 year.”
Given the disease’s potential impact on self-esteem, sleep, and other important areas of life, Sean Reynolds, MBBCH, a pediatric dermatologist at Children’s Mercy Kansas City (Mo.), welcomed the data on the newer pharmacologic agents.
“FDA-approved systemic treatment options for adolescents with AD are currently limited, which necessitates studies such as this that explore additional treatment options,” said Dr. Reynolds, who also was not involved in the study, told this news organization.
He added that oral upadacitinib may especially help patients who have not found relief with other topical or systemic treatments or who are needle phobic. While the overall efficacy and relatively mild side effects for most patients taking upadacitinib in the trials are encouraging, “the long-term efficacy and side effects in this population require further study, especially considering the limited systemic AD treatment options available in this age group,” he added.
“Given the reported use of other JAK inhibitors to treat myriad inflammatory skin conditions beyond atopic dermatitis, the potential use of upadacitinib and other JAK inhibitors to treat these skin diseases in children and adolescents represents an exciting area for future study in the field of pediatric dermatology,” Dr. Reynolds noted.
The study was funded by AbbVie, the developer and manufacturer of upadacitinib. Dr. Paller and almost all other authors reported relevant financial relationships with AbbVie and other pharmaceutical companies. Dr. Ong reported serving on an AbbVie advisory board, and Dr. Reynolds reported no conflict of interest with the study.
(AD), an analysis of three clinical trials reports.
Upadacitinib (Rinvoq) was approved by the Food and Drug Administration for treating adults and pediatric patients 12 years of age and older with refractory, moderate to severe AD, in January 2022. This study analyzed the adolescent data in three double-blind, placebo-controlled phase 3 randomized clinical trials, which included adults and 552 adolescents between 12 and 17 years of age with moderate to severe AD in more than 20 countries in Europe, North and South America, the Middle East, Oceania, and the Asia-Pacific region from July 2018 through December 2020.
In the studies, “treatment of moderate to severe AD in adolescents with upadacitinib was effective and generally well tolerated, with an overall efficacy and safety profile similar to that observed in adults, and patient-reported outcomes indicated an overall better health-related quality of life compared with placebo,” lead study author Amy S. Paller, MD, chair of the department of dermatology and professor of dermatology and pediatrics, at Northwestern University, Chicago, and her colleagues write in JAMA Dermatology.
Adolescents in the three studies – Measure Up 1, Measure Up 2, and AD Up – received once-daily oral upadacitinib 15 mg, 30 mg, or placebo. All participants in AD Up used topical corticosteroids.
At 16 weeks, in Measure Up 1, Measure Up 2, and AD Up, respectively, a greater proportion of adolescents improved by at least 75% in the Eczema Area and Severity Index (EASI 75) with upadacitinib 15 mg (73%, 69%, 63%); and with upadacitinib 30 mg (78%, 73%, 84%), compared with placebo (12%, 13%, 30%), (P < .001 for all comparisons vs. placebo).
Upadacitinib was generally well tolerated among the adolescents, with mild or moderate acne being the most common adverse event, reported in 10%-13% of those on 15 mg and 15%-16% of those on 30 mg vs. 2%-3% of those on placebo.
Asked to comment on the study, Peck Ong, MD, a pediatric allergist and immunologist at Children’s Hospital Los Angeles, said that he was not surprised by the drug’s effectiveness because JAK inhibitors are potent immunosuppressants. Strengths of the studies include the many pediatric participants, its international reach, and its use of standardized and validated measures, said Dr. Ong, who was not involved in the study.
“The effect of JAK inhibitors is more specific than traditional immunosuppressants such as cyclosporine and methotrexate but not as specific as biologics; therefore, long-term safety data are needed,” he advised. “16 weeks is a very short time to study a chronic disease like atopic dermatitis. We need safety data longer than 1 year.”
Given the disease’s potential impact on self-esteem, sleep, and other important areas of life, Sean Reynolds, MBBCH, a pediatric dermatologist at Children’s Mercy Kansas City (Mo.), welcomed the data on the newer pharmacologic agents.
“FDA-approved systemic treatment options for adolescents with AD are currently limited, which necessitates studies such as this that explore additional treatment options,” said Dr. Reynolds, who also was not involved in the study, told this news organization.
He added that oral upadacitinib may especially help patients who have not found relief with other topical or systemic treatments or who are needle phobic. While the overall efficacy and relatively mild side effects for most patients taking upadacitinib in the trials are encouraging, “the long-term efficacy and side effects in this population require further study, especially considering the limited systemic AD treatment options available in this age group,” he added.
“Given the reported use of other JAK inhibitors to treat myriad inflammatory skin conditions beyond atopic dermatitis, the potential use of upadacitinib and other JAK inhibitors to treat these skin diseases in children and adolescents represents an exciting area for future study in the field of pediatric dermatology,” Dr. Reynolds noted.
The study was funded by AbbVie, the developer and manufacturer of upadacitinib. Dr. Paller and almost all other authors reported relevant financial relationships with AbbVie and other pharmaceutical companies. Dr. Ong reported serving on an AbbVie advisory board, and Dr. Reynolds reported no conflict of interest with the study.
FROM JAMA DERMATOLOGY
What are the main reasons patients sue dermatologists?
PHOENIX – , and the defendants were more likely to be male.
Those are among key findings from a study that aimed to determine the reasons patients pursue litigation against dermatologists.
“The number of lawsuits against physicians continues to climb annually,” Young Lim, MD, PhD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the results were presented during an abstract session. “Depending on the study, anywhere between 75 to 99 percent of physicians will face a lawsuit by age 65. A clear understanding of prior litigations will help mitigate similar errors in future practice and promote safer, higher quality care.”
Dr. Lim, a dermatology resident at Massachusetts General Hospital and Harvard Medical School, Boston, along with Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at MGH, and H. Ray Jalian, MD, a cosmetic dermatologist who practices in Los Angeles, used two large national database repositories, WestlawNext and LexisNexis, to retrospectively analyze legal documents following a query using “dermatology” and “dermatologist” as search terms to capture all variety of litigations. They excluded cases in which litigation did not involve patient care as well as those in which the dermatologist was the plaintiff and those in which the dermatologist was involved as a third party.
The final analysis consisted of 54 claims, comprising 43 state and 11 federal cases. Of the 54 cases, 35 involved a male defendant, 12 involved a female defendant, and 7 cases either did not specify the gender of the defendant or involved multiple defendants. Of the 35 cases involving a male defendant, 23 (66%) were brought by female plaintiffs.
Most cases (49, or 91%) involved a defendant dermatologist in private practice while the remaining 5 involved a defendant dermatologist in an academic setting.
The most common reason for litigation was accidental injury (27 cases, or 50%), followed by incorrect or delayed diagnoses (22 cases, or 41%). Five cases resulted from the dermatologist failing to communicate important information, such as postop care instructions or obtaining informed consent.
Of all 54 cases 30 (56%) were dismissed prior to trial, while 24 (44%) resulted in a judgment for the plaintiff. According to Dr. Lim, payout information was available for only five cases, and ranged from $15,000 (injury from laser) to $1,950,000 (delayed diagnosis of malignant melanoma).
“While lawsuits from patients against dermatologists largely involve injury from elective procedures, clinicians should practice caution regarding missed or delayed diagnoses when practicing medical dermatology,” the authors concluded in their abstract. “Ensuring that critical information is shared with patients and obtaining proper written consent will also safeguard against easily-avoidable litigations.”
Christopher B. Zachary, MBBS, professor and chair emeritus of the department of dermatology at the University of California, Irvine, who was asked to comment on the study, said that the findings are a reminder that lack of attention to the most simply performed aspects of care can be the reasons patients will seek medical malpractice redress.
“Consent requires careful and thoughtful explanation of a planned procedure, which should then be recorded in the chart to avoid future confusion,” Dr. Zachary told this news organization. “A patient’s signature on a consent form obtained by a staff member is clearly inadequate if not accompanied by a clear and understandable preoperative discussion. Words, images, video are all elements that aid patients’ comprehension of a planned procedure. And postoperative instructions given to the patients while on the laser table are commonly forgotten by the patient and must be accompanied by written advice summary. Patients will frequently misremember instructions and can be overwhelmed by medical jargon.”
Neither the researchers nor Dr. Zachary reported having relevant financial disclosures.
PHOENIX – , and the defendants were more likely to be male.
Those are among key findings from a study that aimed to determine the reasons patients pursue litigation against dermatologists.
“The number of lawsuits against physicians continues to climb annually,” Young Lim, MD, PhD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the results were presented during an abstract session. “Depending on the study, anywhere between 75 to 99 percent of physicians will face a lawsuit by age 65. A clear understanding of prior litigations will help mitigate similar errors in future practice and promote safer, higher quality care.”
Dr. Lim, a dermatology resident at Massachusetts General Hospital and Harvard Medical School, Boston, along with Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at MGH, and H. Ray Jalian, MD, a cosmetic dermatologist who practices in Los Angeles, used two large national database repositories, WestlawNext and LexisNexis, to retrospectively analyze legal documents following a query using “dermatology” and “dermatologist” as search terms to capture all variety of litigations. They excluded cases in which litigation did not involve patient care as well as those in which the dermatologist was the plaintiff and those in which the dermatologist was involved as a third party.
The final analysis consisted of 54 claims, comprising 43 state and 11 federal cases. Of the 54 cases, 35 involved a male defendant, 12 involved a female defendant, and 7 cases either did not specify the gender of the defendant or involved multiple defendants. Of the 35 cases involving a male defendant, 23 (66%) were brought by female plaintiffs.
Most cases (49, or 91%) involved a defendant dermatologist in private practice while the remaining 5 involved a defendant dermatologist in an academic setting.
The most common reason for litigation was accidental injury (27 cases, or 50%), followed by incorrect or delayed diagnoses (22 cases, or 41%). Five cases resulted from the dermatologist failing to communicate important information, such as postop care instructions or obtaining informed consent.
Of all 54 cases 30 (56%) were dismissed prior to trial, while 24 (44%) resulted in a judgment for the plaintiff. According to Dr. Lim, payout information was available for only five cases, and ranged from $15,000 (injury from laser) to $1,950,000 (delayed diagnosis of malignant melanoma).
“While lawsuits from patients against dermatologists largely involve injury from elective procedures, clinicians should practice caution regarding missed or delayed diagnoses when practicing medical dermatology,” the authors concluded in their abstract. “Ensuring that critical information is shared with patients and obtaining proper written consent will also safeguard against easily-avoidable litigations.”
Christopher B. Zachary, MBBS, professor and chair emeritus of the department of dermatology at the University of California, Irvine, who was asked to comment on the study, said that the findings are a reminder that lack of attention to the most simply performed aspects of care can be the reasons patients will seek medical malpractice redress.
“Consent requires careful and thoughtful explanation of a planned procedure, which should then be recorded in the chart to avoid future confusion,” Dr. Zachary told this news organization. “A patient’s signature on a consent form obtained by a staff member is clearly inadequate if not accompanied by a clear and understandable preoperative discussion. Words, images, video are all elements that aid patients’ comprehension of a planned procedure. And postoperative instructions given to the patients while on the laser table are commonly forgotten by the patient and must be accompanied by written advice summary. Patients will frequently misremember instructions and can be overwhelmed by medical jargon.”
Neither the researchers nor Dr. Zachary reported having relevant financial disclosures.
PHOENIX – , and the defendants were more likely to be male.
Those are among key findings from a study that aimed to determine the reasons patients pursue litigation against dermatologists.
“The number of lawsuits against physicians continues to climb annually,” Young Lim, MD, PhD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the results were presented during an abstract session. “Depending on the study, anywhere between 75 to 99 percent of physicians will face a lawsuit by age 65. A clear understanding of prior litigations will help mitigate similar errors in future practice and promote safer, higher quality care.”
Dr. Lim, a dermatology resident at Massachusetts General Hospital and Harvard Medical School, Boston, along with Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at MGH, and H. Ray Jalian, MD, a cosmetic dermatologist who practices in Los Angeles, used two large national database repositories, WestlawNext and LexisNexis, to retrospectively analyze legal documents following a query using “dermatology” and “dermatologist” as search terms to capture all variety of litigations. They excluded cases in which litigation did not involve patient care as well as those in which the dermatologist was the plaintiff and those in which the dermatologist was involved as a third party.
The final analysis consisted of 54 claims, comprising 43 state and 11 federal cases. Of the 54 cases, 35 involved a male defendant, 12 involved a female defendant, and 7 cases either did not specify the gender of the defendant or involved multiple defendants. Of the 35 cases involving a male defendant, 23 (66%) were brought by female plaintiffs.
Most cases (49, or 91%) involved a defendant dermatologist in private practice while the remaining 5 involved a defendant dermatologist in an academic setting.
The most common reason for litigation was accidental injury (27 cases, or 50%), followed by incorrect or delayed diagnoses (22 cases, or 41%). Five cases resulted from the dermatologist failing to communicate important information, such as postop care instructions or obtaining informed consent.
Of all 54 cases 30 (56%) were dismissed prior to trial, while 24 (44%) resulted in a judgment for the plaintiff. According to Dr. Lim, payout information was available for only five cases, and ranged from $15,000 (injury from laser) to $1,950,000 (delayed diagnosis of malignant melanoma).
“While lawsuits from patients against dermatologists largely involve injury from elective procedures, clinicians should practice caution regarding missed or delayed diagnoses when practicing medical dermatology,” the authors concluded in their abstract. “Ensuring that critical information is shared with patients and obtaining proper written consent will also safeguard against easily-avoidable litigations.”
Christopher B. Zachary, MBBS, professor and chair emeritus of the department of dermatology at the University of California, Irvine, who was asked to comment on the study, said that the findings are a reminder that lack of attention to the most simply performed aspects of care can be the reasons patients will seek medical malpractice redress.
“Consent requires careful and thoughtful explanation of a planned procedure, which should then be recorded in the chart to avoid future confusion,” Dr. Zachary told this news organization. “A patient’s signature on a consent form obtained by a staff member is clearly inadequate if not accompanied by a clear and understandable preoperative discussion. Words, images, video are all elements that aid patients’ comprehension of a planned procedure. And postoperative instructions given to the patients while on the laser table are commonly forgotten by the patient and must be accompanied by written advice summary. Patients will frequently misremember instructions and can be overwhelmed by medical jargon.”
Neither the researchers nor Dr. Zachary reported having relevant financial disclosures.
AT ASLMS 2023
Proposed Medicare bill would raise docs’ pay with inflation
Introduced by four physician U.S. House representatives, HR 2474 would link Medicare fee schedule updates to the Medicare Economic Index, a measure of inflation related to physicians’ practice costs and wages.
That’s a long-sought goal of the American Medical Association, which is leading 120 state medical societies and medical specialty groups in championing the bill.
The legislation is essential to enabling physician practices to better absorb payment distributions triggered by budget neutrality rules, performance adjustments, and periods of high inflation, the groups wrote in a joint letter sent to the bill’s sponsors. The sponsors say they hope the legislation will improve access to care, as low reimbursements cause some physicians to limit their number of Medicare patients.
Physicians groups for years have urged federal lawmakers to scrap short-term fixes staving off Medicare pay cuts in favor of permanent reforms. Unlike nearly all other Medicare clinicians including hospitals, physicians’ Medicare payment updates aren’t currently tied to inflation.
Adjusted for inflation, Medicare payments to physicians have declined 26% between 2001 and 2023, including a 2% payment reduction in 2023, according to the AMA. Small and rural physician practices have been disproportionately affected by these reductions, as have doctors treating low-income or uninsured patients, the AMA said.
Last month, an influential federal advisory panel recommended permanently tying Medicare physician pay increases to inflation. Clinicians’ cost of providing services, measured by the Medicare Economic Index, rose by 2.6% in 2021 and are estimated to have risen 4.7% in 2022, significantly more than in recent years, the Medicare Payment Advisory Commission said.
A version of this article originally appeared on Medscape.com.
Introduced by four physician U.S. House representatives, HR 2474 would link Medicare fee schedule updates to the Medicare Economic Index, a measure of inflation related to physicians’ practice costs and wages.
That’s a long-sought goal of the American Medical Association, which is leading 120 state medical societies and medical specialty groups in championing the bill.
The legislation is essential to enabling physician practices to better absorb payment distributions triggered by budget neutrality rules, performance adjustments, and periods of high inflation, the groups wrote in a joint letter sent to the bill’s sponsors. The sponsors say they hope the legislation will improve access to care, as low reimbursements cause some physicians to limit their number of Medicare patients.
Physicians groups for years have urged federal lawmakers to scrap short-term fixes staving off Medicare pay cuts in favor of permanent reforms. Unlike nearly all other Medicare clinicians including hospitals, physicians’ Medicare payment updates aren’t currently tied to inflation.
Adjusted for inflation, Medicare payments to physicians have declined 26% between 2001 and 2023, including a 2% payment reduction in 2023, according to the AMA. Small and rural physician practices have been disproportionately affected by these reductions, as have doctors treating low-income or uninsured patients, the AMA said.
Last month, an influential federal advisory panel recommended permanently tying Medicare physician pay increases to inflation. Clinicians’ cost of providing services, measured by the Medicare Economic Index, rose by 2.6% in 2021 and are estimated to have risen 4.7% in 2022, significantly more than in recent years, the Medicare Payment Advisory Commission said.
A version of this article originally appeared on Medscape.com.
Introduced by four physician U.S. House representatives, HR 2474 would link Medicare fee schedule updates to the Medicare Economic Index, a measure of inflation related to physicians’ practice costs and wages.
That’s a long-sought goal of the American Medical Association, which is leading 120 state medical societies and medical specialty groups in championing the bill.
The legislation is essential to enabling physician practices to better absorb payment distributions triggered by budget neutrality rules, performance adjustments, and periods of high inflation, the groups wrote in a joint letter sent to the bill’s sponsors. The sponsors say they hope the legislation will improve access to care, as low reimbursements cause some physicians to limit their number of Medicare patients.
Physicians groups for years have urged federal lawmakers to scrap short-term fixes staving off Medicare pay cuts in favor of permanent reforms. Unlike nearly all other Medicare clinicians including hospitals, physicians’ Medicare payment updates aren’t currently tied to inflation.
Adjusted for inflation, Medicare payments to physicians have declined 26% between 2001 and 2023, including a 2% payment reduction in 2023, according to the AMA. Small and rural physician practices have been disproportionately affected by these reductions, as have doctors treating low-income or uninsured patients, the AMA said.
Last month, an influential federal advisory panel recommended permanently tying Medicare physician pay increases to inflation. Clinicians’ cost of providing services, measured by the Medicare Economic Index, rose by 2.6% in 2021 and are estimated to have risen 4.7% in 2022, significantly more than in recent years, the Medicare Payment Advisory Commission said.
A version of this article originally appeared on Medscape.com.
Surgeons, intensivists earn more than do colleagues from private insurance
General and orthopedic surgeons and intensivists earn the highest net reimbursements from private U.S. insurers, a new report estimates.
On average in 2021, they were paid $5.8 million, $4.9 million, and $3.3 million, respectively, according to figures compiled by AMN Healthcare, a Dallas-based health staffing company.
None of 15 other physician specialties topped $3 million in net reimbursement on average, and three – dermatology, pediatrics, and family medicine – didn’t reach $1 million.
The report doesn’t include data about reimbursement from Medicare and Medicaid, and its numbers assume that 50% of insurance claims are denied. Denial rates differ from practice to practice.
Still, the findings offer a “benchmark tool” to help clinicians understand how they rank against their peers, Linda Murphy, president of AMN Healthcare’s Revenue Cycle Solutions division, said in an interview.
This is the first year that the company has calculated physician reimbursement levels by using claim and clearinghouse data, Ms. Murphy said. Previously, a division of the firm compiled data by surveying chief financial officers from hospitals.
The report’s estimate that insurers deny 50% of claims is “conservative,” Ms. Murphy said. Miscoding is a significant factor behind that number.
The estimated 2021 net private insurance reimbursements by specialty for direct services, assuming a 50% denial rate:
- Anesthesiology: $1,665,510
- Cardiology: $1,703,013
- Critical Care (intensivist): $3,338,656
- Dermatology: $729,107
- Family medicine: $697,094
- Gastroenterology: $2,765,110
- Internal medicine: $1,297,200
- Neurology: $1,390,181
- Obstetrician/gynecology: $1,880,888
- Otolaryngology: $2,095,277
- Pediatrics: $661,552
- Psychiatry: $1,348,730
- Pulmonology: $1,561,617
- Radiology: $1,015,750
- Rheumatology: $1,705,140
- General surgery: $5,834,508
- Orthopedic surgery: $4,904,757
- Urology: $2,943,381
Among 18 physician specialties overall, the report estimated that the average net reimbursement in 2021 was $1.9 million.
The report also estimated that the net reimbursement amounts at $875,140 for certified registered nurse anesthetists and $388,696 for nurse practitioners.
Surprisingly, Ms. Murphy said, there’s “a really large swing” among reimbursement levels for individual specialties. The quartile of cardiologists with the lowest level of reimbursement, for example, submitted $2.1 million in claims in 2021, netting about $1 million at a 50% denial rate versus the $7.3 million made by those in the highest quartile, netting about $3.6 million.
The gap seems to be due to regional variations, she said, adding that a rural cardiologist will have different billing practices than does one practicing in New York City.
The quartile of general surgeons with the highest reimbursement levels billed for $21.1 million on average in 2021, making about $10.5 million at a 50% denial rate. The lowest quartile billed for $5.5 million, making about $2.7 million at a 50% denial rate.
The report noted that primary care physicians – that is, family medicine, internal medicine, and pediatrics specialists – have much lower levels of reimbursement, compared with most other specialties. But the work of primary care physicians “may lead to considerable ‘downstream revenue’ through the hospital admissions, tests and treatment they order.”
A previous analysis by a division of AMN Healthcare found that primary care physicians, on average, generate $2,113,273 a year in net annual revenue for their affiliated hospitals, nearing the $2,446,429 in net annual hospital revenue generated by specialists.
AMN Healthcare is preparing another report that will examine Medicare reimbursements, Ms. Murphy said. According to the new report, payments by nonprivate insurers amount to about one-third of the total amount of reimbursement by commercial insurers.
A version of this article originally appeared on Medscape.com.
General and orthopedic surgeons and intensivists earn the highest net reimbursements from private U.S. insurers, a new report estimates.
On average in 2021, they were paid $5.8 million, $4.9 million, and $3.3 million, respectively, according to figures compiled by AMN Healthcare, a Dallas-based health staffing company.
None of 15 other physician specialties topped $3 million in net reimbursement on average, and three – dermatology, pediatrics, and family medicine – didn’t reach $1 million.
The report doesn’t include data about reimbursement from Medicare and Medicaid, and its numbers assume that 50% of insurance claims are denied. Denial rates differ from practice to practice.
Still, the findings offer a “benchmark tool” to help clinicians understand how they rank against their peers, Linda Murphy, president of AMN Healthcare’s Revenue Cycle Solutions division, said in an interview.
This is the first year that the company has calculated physician reimbursement levels by using claim and clearinghouse data, Ms. Murphy said. Previously, a division of the firm compiled data by surveying chief financial officers from hospitals.
The report’s estimate that insurers deny 50% of claims is “conservative,” Ms. Murphy said. Miscoding is a significant factor behind that number.
The estimated 2021 net private insurance reimbursements by specialty for direct services, assuming a 50% denial rate:
- Anesthesiology: $1,665,510
- Cardiology: $1,703,013
- Critical Care (intensivist): $3,338,656
- Dermatology: $729,107
- Family medicine: $697,094
- Gastroenterology: $2,765,110
- Internal medicine: $1,297,200
- Neurology: $1,390,181
- Obstetrician/gynecology: $1,880,888
- Otolaryngology: $2,095,277
- Pediatrics: $661,552
- Psychiatry: $1,348,730
- Pulmonology: $1,561,617
- Radiology: $1,015,750
- Rheumatology: $1,705,140
- General surgery: $5,834,508
- Orthopedic surgery: $4,904,757
- Urology: $2,943,381
Among 18 physician specialties overall, the report estimated that the average net reimbursement in 2021 was $1.9 million.
The report also estimated that the net reimbursement amounts at $875,140 for certified registered nurse anesthetists and $388,696 for nurse practitioners.
Surprisingly, Ms. Murphy said, there’s “a really large swing” among reimbursement levels for individual specialties. The quartile of cardiologists with the lowest level of reimbursement, for example, submitted $2.1 million in claims in 2021, netting about $1 million at a 50% denial rate versus the $7.3 million made by those in the highest quartile, netting about $3.6 million.
The gap seems to be due to regional variations, she said, adding that a rural cardiologist will have different billing practices than does one practicing in New York City.
The quartile of general surgeons with the highest reimbursement levels billed for $21.1 million on average in 2021, making about $10.5 million at a 50% denial rate. The lowest quartile billed for $5.5 million, making about $2.7 million at a 50% denial rate.
The report noted that primary care physicians – that is, family medicine, internal medicine, and pediatrics specialists – have much lower levels of reimbursement, compared with most other specialties. But the work of primary care physicians “may lead to considerable ‘downstream revenue’ through the hospital admissions, tests and treatment they order.”
A previous analysis by a division of AMN Healthcare found that primary care physicians, on average, generate $2,113,273 a year in net annual revenue for their affiliated hospitals, nearing the $2,446,429 in net annual hospital revenue generated by specialists.
AMN Healthcare is preparing another report that will examine Medicare reimbursements, Ms. Murphy said. According to the new report, payments by nonprivate insurers amount to about one-third of the total amount of reimbursement by commercial insurers.
A version of this article originally appeared on Medscape.com.
General and orthopedic surgeons and intensivists earn the highest net reimbursements from private U.S. insurers, a new report estimates.
On average in 2021, they were paid $5.8 million, $4.9 million, and $3.3 million, respectively, according to figures compiled by AMN Healthcare, a Dallas-based health staffing company.
None of 15 other physician specialties topped $3 million in net reimbursement on average, and three – dermatology, pediatrics, and family medicine – didn’t reach $1 million.
The report doesn’t include data about reimbursement from Medicare and Medicaid, and its numbers assume that 50% of insurance claims are denied. Denial rates differ from practice to practice.
Still, the findings offer a “benchmark tool” to help clinicians understand how they rank against their peers, Linda Murphy, president of AMN Healthcare’s Revenue Cycle Solutions division, said in an interview.
This is the first year that the company has calculated physician reimbursement levels by using claim and clearinghouse data, Ms. Murphy said. Previously, a division of the firm compiled data by surveying chief financial officers from hospitals.
The report’s estimate that insurers deny 50% of claims is “conservative,” Ms. Murphy said. Miscoding is a significant factor behind that number.
The estimated 2021 net private insurance reimbursements by specialty for direct services, assuming a 50% denial rate:
- Anesthesiology: $1,665,510
- Cardiology: $1,703,013
- Critical Care (intensivist): $3,338,656
- Dermatology: $729,107
- Family medicine: $697,094
- Gastroenterology: $2,765,110
- Internal medicine: $1,297,200
- Neurology: $1,390,181
- Obstetrician/gynecology: $1,880,888
- Otolaryngology: $2,095,277
- Pediatrics: $661,552
- Psychiatry: $1,348,730
- Pulmonology: $1,561,617
- Radiology: $1,015,750
- Rheumatology: $1,705,140
- General surgery: $5,834,508
- Orthopedic surgery: $4,904,757
- Urology: $2,943,381
Among 18 physician specialties overall, the report estimated that the average net reimbursement in 2021 was $1.9 million.
The report also estimated that the net reimbursement amounts at $875,140 for certified registered nurse anesthetists and $388,696 for nurse practitioners.
Surprisingly, Ms. Murphy said, there’s “a really large swing” among reimbursement levels for individual specialties. The quartile of cardiologists with the lowest level of reimbursement, for example, submitted $2.1 million in claims in 2021, netting about $1 million at a 50% denial rate versus the $7.3 million made by those in the highest quartile, netting about $3.6 million.
The gap seems to be due to regional variations, she said, adding that a rural cardiologist will have different billing practices than does one practicing in New York City.
The quartile of general surgeons with the highest reimbursement levels billed for $21.1 million on average in 2021, making about $10.5 million at a 50% denial rate. The lowest quartile billed for $5.5 million, making about $2.7 million at a 50% denial rate.
The report noted that primary care physicians – that is, family medicine, internal medicine, and pediatrics specialists – have much lower levels of reimbursement, compared with most other specialties. But the work of primary care physicians “may lead to considerable ‘downstream revenue’ through the hospital admissions, tests and treatment they order.”
A previous analysis by a division of AMN Healthcare found that primary care physicians, on average, generate $2,113,273 a year in net annual revenue for their affiliated hospitals, nearing the $2,446,429 in net annual hospital revenue generated by specialists.
AMN Healthcare is preparing another report that will examine Medicare reimbursements, Ms. Murphy said. According to the new report, payments by nonprivate insurers amount to about one-third of the total amount of reimbursement by commercial insurers.
A version of this article originally appeared on Medscape.com.
NPF provides guidance for virtual psoriasis visits
.
The success of telemedicine in managing chronic inflammatory skin conditions including psoriasis during the COVID-19 pandemic “highlighted that teledermatology can be used beyond the context of a global health crisis to provide continuity of care and improve access to health care more broadly,” the task force wrote in a paper published online in JAAD International.
Co–senior author George Han, MD, PhD, said in an interview that the impetus for the guidelines came from NPF patient advocates, who realized that the organization needed something to take to payers and governmental agencies to advocate for better access to dermatologic care. He is associate professor of dermatology and director of teledermatology at the Hofstra/Northwell department of dermatology, Hyde Park, New York.
“We realized that, in many places around the country, people don’t have access to dermatology.” In upstate New York, said Dr. Han, his anecdotal research has revealed wait times of 6 months or more.
As a guiding principle, the authors pronounce teledermatology “a reasonable alternative for providing long-term management of patients with psoriasis.” Research shows that nearly all dermatologists used teledermatology during the pandemic, the authors noted, and that well-run programs improve Psoriasis Area and Severity Index (PASI) scores and other measures on par with in-person care. Telemedicine may be especially useful for initial visits, they added, particularly when distance, patient incapacity, and circumstances prevent face-to-face evaluation.
Additional position statements emphasize that teledermatology should support rather than supplant in-person visits, and that this balance may be particularly important in cases involving psoriatic arthritis (PsA). “Even though we can’t do a physical exam and palpate some of those joints in person,” said Dr. Han, “tools have been developed that, through a series of questions the patient can answer, can guide you towards whether there is a high index of suspicion for psoriatic arthritis.” Such patients require in-person evaluation with urgency, he said, because delays in PsA diagnosis and treatment can lead to irreversible joint damage and significant functional impairment.
Another motivation for producing the guidelines, said Dr. Han, was that, even when underserved patients get a dermatology appointment, some providers may not have all the latest tools or medicines available for treating psoriasis. In such cases, telemedicine may allow dermatologists specializing in psoriasis care to extend their reach in comanaging patients with primary care physicians and community dermatologists.
Before the appointment, guidelines suggest determining what form of teledermatology will best suit each patient. Authors recommended gauging patients’ savviness with computers and cameras, and counseling patients regarding available virtual evaluation tools – such as live video visits, store-and-forward photo strategies, and assessment-tool training videos.
A subsequent guideline underscores the importance of continuously improving technology to support expeditious image capture and workflows that emulate in-person practice. Dr. Han explained, “we wanted to make sure that on the back end there’s adequate support such that – if through teledermatology, we determine that the patient should get, say, a systemic treatment – the patient is able to get the appropriate lab tests, get the medicine, and know how to inject it.”
Regarding reimbursement, Dr. Han said that policies varied prepandemic, but many commercial insurers covered telemedicine at a rate 20% lower than the in-person rate. During the pandemic, he said, insurers shifted to provide the higher rate for telemedicine, consistent with policies adopted by the Centers for Medicare & Medicaid Services.
“There are differences in coverage and reimbursement from plan to plan,” Dr. Han added. “And even within the same plan, there are carve-outs so that some plans don’t allow certain services. The big picture is that for the most part these services are covered at a level comparable to an in-person visit at present.”
With the Department of Health & Human Services’ public health emergency declaration expiring in May, he said, physicians have worried that some of the allowances made by CMS – such as lifting requirements that Medicare patients in rural areas be seen at care sites – will expire. “It seems that some of those limitations have been addressed, and those allowances are going to be extended until Congress is able to pass something that gives us durable access to telemedicine care. We think that based on the current environment telemedicine is here to stay.”
The study was funded by the NPF. Dr. Han has been an investigator, adviser, speaker, or researcher for AbbVie, Amgen, Apogee Therapeutics, Arcutis, Athenex, Bausch Health, Beiersdorf, Boehringer Ingelheim, Bond Avillion, Bristol Myers Squibb, Celgene, CeraVe, Dermavant, DermTech, Eli Lilly, EPI Health, Janssen Pharmaceuticals, LEO Pharma, L’Oreal, MC2 Therapeutics, Novartis, Ortho Dermatologics, PellePharm, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, SUN Pharmaceuticals, and UCB.
.
The success of telemedicine in managing chronic inflammatory skin conditions including psoriasis during the COVID-19 pandemic “highlighted that teledermatology can be used beyond the context of a global health crisis to provide continuity of care and improve access to health care more broadly,” the task force wrote in a paper published online in JAAD International.
Co–senior author George Han, MD, PhD, said in an interview that the impetus for the guidelines came from NPF patient advocates, who realized that the organization needed something to take to payers and governmental agencies to advocate for better access to dermatologic care. He is associate professor of dermatology and director of teledermatology at the Hofstra/Northwell department of dermatology, Hyde Park, New York.
“We realized that, in many places around the country, people don’t have access to dermatology.” In upstate New York, said Dr. Han, his anecdotal research has revealed wait times of 6 months or more.
As a guiding principle, the authors pronounce teledermatology “a reasonable alternative for providing long-term management of patients with psoriasis.” Research shows that nearly all dermatologists used teledermatology during the pandemic, the authors noted, and that well-run programs improve Psoriasis Area and Severity Index (PASI) scores and other measures on par with in-person care. Telemedicine may be especially useful for initial visits, they added, particularly when distance, patient incapacity, and circumstances prevent face-to-face evaluation.
Additional position statements emphasize that teledermatology should support rather than supplant in-person visits, and that this balance may be particularly important in cases involving psoriatic arthritis (PsA). “Even though we can’t do a physical exam and palpate some of those joints in person,” said Dr. Han, “tools have been developed that, through a series of questions the patient can answer, can guide you towards whether there is a high index of suspicion for psoriatic arthritis.” Such patients require in-person evaluation with urgency, he said, because delays in PsA diagnosis and treatment can lead to irreversible joint damage and significant functional impairment.
Another motivation for producing the guidelines, said Dr. Han, was that, even when underserved patients get a dermatology appointment, some providers may not have all the latest tools or medicines available for treating psoriasis. In such cases, telemedicine may allow dermatologists specializing in psoriasis care to extend their reach in comanaging patients with primary care physicians and community dermatologists.
Before the appointment, guidelines suggest determining what form of teledermatology will best suit each patient. Authors recommended gauging patients’ savviness with computers and cameras, and counseling patients regarding available virtual evaluation tools – such as live video visits, store-and-forward photo strategies, and assessment-tool training videos.
A subsequent guideline underscores the importance of continuously improving technology to support expeditious image capture and workflows that emulate in-person practice. Dr. Han explained, “we wanted to make sure that on the back end there’s adequate support such that – if through teledermatology, we determine that the patient should get, say, a systemic treatment – the patient is able to get the appropriate lab tests, get the medicine, and know how to inject it.”
Regarding reimbursement, Dr. Han said that policies varied prepandemic, but many commercial insurers covered telemedicine at a rate 20% lower than the in-person rate. During the pandemic, he said, insurers shifted to provide the higher rate for telemedicine, consistent with policies adopted by the Centers for Medicare & Medicaid Services.
“There are differences in coverage and reimbursement from plan to plan,” Dr. Han added. “And even within the same plan, there are carve-outs so that some plans don’t allow certain services. The big picture is that for the most part these services are covered at a level comparable to an in-person visit at present.”
With the Department of Health & Human Services’ public health emergency declaration expiring in May, he said, physicians have worried that some of the allowances made by CMS – such as lifting requirements that Medicare patients in rural areas be seen at care sites – will expire. “It seems that some of those limitations have been addressed, and those allowances are going to be extended until Congress is able to pass something that gives us durable access to telemedicine care. We think that based on the current environment telemedicine is here to stay.”
The study was funded by the NPF. Dr. Han has been an investigator, adviser, speaker, or researcher for AbbVie, Amgen, Apogee Therapeutics, Arcutis, Athenex, Bausch Health, Beiersdorf, Boehringer Ingelheim, Bond Avillion, Bristol Myers Squibb, Celgene, CeraVe, Dermavant, DermTech, Eli Lilly, EPI Health, Janssen Pharmaceuticals, LEO Pharma, L’Oreal, MC2 Therapeutics, Novartis, Ortho Dermatologics, PellePharm, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, SUN Pharmaceuticals, and UCB.
.
The success of telemedicine in managing chronic inflammatory skin conditions including psoriasis during the COVID-19 pandemic “highlighted that teledermatology can be used beyond the context of a global health crisis to provide continuity of care and improve access to health care more broadly,” the task force wrote in a paper published online in JAAD International.
Co–senior author George Han, MD, PhD, said in an interview that the impetus for the guidelines came from NPF patient advocates, who realized that the organization needed something to take to payers and governmental agencies to advocate for better access to dermatologic care. He is associate professor of dermatology and director of teledermatology at the Hofstra/Northwell department of dermatology, Hyde Park, New York.
“We realized that, in many places around the country, people don’t have access to dermatology.” In upstate New York, said Dr. Han, his anecdotal research has revealed wait times of 6 months or more.
As a guiding principle, the authors pronounce teledermatology “a reasonable alternative for providing long-term management of patients with psoriasis.” Research shows that nearly all dermatologists used teledermatology during the pandemic, the authors noted, and that well-run programs improve Psoriasis Area and Severity Index (PASI) scores and other measures on par with in-person care. Telemedicine may be especially useful for initial visits, they added, particularly when distance, patient incapacity, and circumstances prevent face-to-face evaluation.
Additional position statements emphasize that teledermatology should support rather than supplant in-person visits, and that this balance may be particularly important in cases involving psoriatic arthritis (PsA). “Even though we can’t do a physical exam and palpate some of those joints in person,” said Dr. Han, “tools have been developed that, through a series of questions the patient can answer, can guide you towards whether there is a high index of suspicion for psoriatic arthritis.” Such patients require in-person evaluation with urgency, he said, because delays in PsA diagnosis and treatment can lead to irreversible joint damage and significant functional impairment.
Another motivation for producing the guidelines, said Dr. Han, was that, even when underserved patients get a dermatology appointment, some providers may not have all the latest tools or medicines available for treating psoriasis. In such cases, telemedicine may allow dermatologists specializing in psoriasis care to extend their reach in comanaging patients with primary care physicians and community dermatologists.
Before the appointment, guidelines suggest determining what form of teledermatology will best suit each patient. Authors recommended gauging patients’ savviness with computers and cameras, and counseling patients regarding available virtual evaluation tools – such as live video visits, store-and-forward photo strategies, and assessment-tool training videos.
A subsequent guideline underscores the importance of continuously improving technology to support expeditious image capture and workflows that emulate in-person practice. Dr. Han explained, “we wanted to make sure that on the back end there’s adequate support such that – if through teledermatology, we determine that the patient should get, say, a systemic treatment – the patient is able to get the appropriate lab tests, get the medicine, and know how to inject it.”
Regarding reimbursement, Dr. Han said that policies varied prepandemic, but many commercial insurers covered telemedicine at a rate 20% lower than the in-person rate. During the pandemic, he said, insurers shifted to provide the higher rate for telemedicine, consistent with policies adopted by the Centers for Medicare & Medicaid Services.
“There are differences in coverage and reimbursement from plan to plan,” Dr. Han added. “And even within the same plan, there are carve-outs so that some plans don’t allow certain services. The big picture is that for the most part these services are covered at a level comparable to an in-person visit at present.”
With the Department of Health & Human Services’ public health emergency declaration expiring in May, he said, physicians have worried that some of the allowances made by CMS – such as lifting requirements that Medicare patients in rural areas be seen at care sites – will expire. “It seems that some of those limitations have been addressed, and those allowances are going to be extended until Congress is able to pass something that gives us durable access to telemedicine care. We think that based on the current environment telemedicine is here to stay.”
The study was funded by the NPF. Dr. Han has been an investigator, adviser, speaker, or researcher for AbbVie, Amgen, Apogee Therapeutics, Arcutis, Athenex, Bausch Health, Beiersdorf, Boehringer Ingelheim, Bond Avillion, Bristol Myers Squibb, Celgene, CeraVe, Dermavant, DermTech, Eli Lilly, EPI Health, Janssen Pharmaceuticals, LEO Pharma, L’Oreal, MC2 Therapeutics, Novartis, Ortho Dermatologics, PellePharm, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, SUN Pharmaceuticals, and UCB.
FROM JAAD INTERNATIONAL
Ten-year analysis finds relatively low complication rate from fractional resurfacing lasers
PHOENIX – over a decade showed.
To investigate, Dr. Hashemi, a third-year dermatology resident at Harvard University and Massachusetts General Hospital, Boston, and Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at MGH, drew from the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database, which compiles medical device reports for suspected injuries from device use or malfunction and represents the largest repository of device adverse effects. Medical device reports are submitted by manufacturers, clinicians, patients, and others.
The researchers limited their query to MDRs related to ablative and nonablative fractional resurfacing lasers over the 10-year period from 2013 to 2022. The query was performed in January 2023 using a comprehensive list of product names and manufacturers.
The initial search yielded 240 MDRs, which were individually reviewed for duplicate records or insufficient data, and the final analysis included 165 MDRs. The 10 most reported adverse events were burns (30%), followed by dyspigmentation (14%), scarring (12%), other (11%), postoperative infection (8%), blisters (6%), pain (5%), hypertrophic scar (4%), post-treatment inflammation (4%), and textural changes (3%). Within the 10-year period analyzed, 56% of MDRs occurred between 2016 and 2019, with a disproportionately low percentage of MDRs occurring in 2022 (5%).
“Adverse events due to ablative and nonablative fractional resurfacing lasers are rare but potentially serious,” Dr. Hashemi concluded. “Care must be taken with counseling, patient selection, and treatment settings to optimize safety, informed consent, and patient satisfaction. Given the relatively low number of adverse events seen with fractional resurfacing lasers, factors driving their safety should be further explored.”
He added that he was surprised by the relatively low number of reported issues, referring to the total of 165 cases over 10 years. By comparison, he said, body contouring had 660 cases reported over a 7-year period in one recent study.
According to the MAUDE website, submitting MDRs to MAUDE is mandatory for manufacturers, importers, and device user facilities, and are voluntary for other groups, such as health care professionals, patients, and consumers.
Dr. Hashemi disclosed that he is a consultant for Castle Biosciences. He is also an entrepreneur in residence for Gore Range Capital.
PHOENIX – over a decade showed.
To investigate, Dr. Hashemi, a third-year dermatology resident at Harvard University and Massachusetts General Hospital, Boston, and Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at MGH, drew from the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database, which compiles medical device reports for suspected injuries from device use or malfunction and represents the largest repository of device adverse effects. Medical device reports are submitted by manufacturers, clinicians, patients, and others.
The researchers limited their query to MDRs related to ablative and nonablative fractional resurfacing lasers over the 10-year period from 2013 to 2022. The query was performed in January 2023 using a comprehensive list of product names and manufacturers.
The initial search yielded 240 MDRs, which were individually reviewed for duplicate records or insufficient data, and the final analysis included 165 MDRs. The 10 most reported adverse events were burns (30%), followed by dyspigmentation (14%), scarring (12%), other (11%), postoperative infection (8%), blisters (6%), pain (5%), hypertrophic scar (4%), post-treatment inflammation (4%), and textural changes (3%). Within the 10-year period analyzed, 56% of MDRs occurred between 2016 and 2019, with a disproportionately low percentage of MDRs occurring in 2022 (5%).
“Adverse events due to ablative and nonablative fractional resurfacing lasers are rare but potentially serious,” Dr. Hashemi concluded. “Care must be taken with counseling, patient selection, and treatment settings to optimize safety, informed consent, and patient satisfaction. Given the relatively low number of adverse events seen with fractional resurfacing lasers, factors driving their safety should be further explored.”
He added that he was surprised by the relatively low number of reported issues, referring to the total of 165 cases over 10 years. By comparison, he said, body contouring had 660 cases reported over a 7-year period in one recent study.
According to the MAUDE website, submitting MDRs to MAUDE is mandatory for manufacturers, importers, and device user facilities, and are voluntary for other groups, such as health care professionals, patients, and consumers.
Dr. Hashemi disclosed that he is a consultant for Castle Biosciences. He is also an entrepreneur in residence for Gore Range Capital.
PHOENIX – over a decade showed.
To investigate, Dr. Hashemi, a third-year dermatology resident at Harvard University and Massachusetts General Hospital, Boston, and Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at MGH, drew from the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database, which compiles medical device reports for suspected injuries from device use or malfunction and represents the largest repository of device adverse effects. Medical device reports are submitted by manufacturers, clinicians, patients, and others.
The researchers limited their query to MDRs related to ablative and nonablative fractional resurfacing lasers over the 10-year period from 2013 to 2022. The query was performed in January 2023 using a comprehensive list of product names and manufacturers.
The initial search yielded 240 MDRs, which were individually reviewed for duplicate records or insufficient data, and the final analysis included 165 MDRs. The 10 most reported adverse events were burns (30%), followed by dyspigmentation (14%), scarring (12%), other (11%), postoperative infection (8%), blisters (6%), pain (5%), hypertrophic scar (4%), post-treatment inflammation (4%), and textural changes (3%). Within the 10-year period analyzed, 56% of MDRs occurred between 2016 and 2019, with a disproportionately low percentage of MDRs occurring in 2022 (5%).
“Adverse events due to ablative and nonablative fractional resurfacing lasers are rare but potentially serious,” Dr. Hashemi concluded. “Care must be taken with counseling, patient selection, and treatment settings to optimize safety, informed consent, and patient satisfaction. Given the relatively low number of adverse events seen with fractional resurfacing lasers, factors driving their safety should be further explored.”
He added that he was surprised by the relatively low number of reported issues, referring to the total of 165 cases over 10 years. By comparison, he said, body contouring had 660 cases reported over a 7-year period in one recent study.
According to the MAUDE website, submitting MDRs to MAUDE is mandatory for manufacturers, importers, and device user facilities, and are voluntary for other groups, such as health care professionals, patients, and consumers.
Dr. Hashemi disclosed that he is a consultant for Castle Biosciences. He is also an entrepreneur in residence for Gore Range Capital.
AT ASLMS 2023