User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Anatomic site influences ropivacaine duration during dermatologic surgery
DENVER – , results from a single-center study showed.
Ropivacaine is a long-acting anesthetic that may be used as a substitute for the more commonly local anesthetics such as lidocaine or bupivacaine in dermatologic surgery, lead study author Kira Minkis, MD, PhD, told this news organization following the annual meeting of the American Society for Dermatologic Surgery, where the study results were presented during an oral abstract session. By comparison, ropivacaine has been reported to have a faster onset, similar duration in the range of 6-14 hours, less pain upon injection, and inherent vasoconstrictive properties.
“With tumescent anesthesia, studies have previously shown that the rate and absorption of anesthetics is influenced by the site of administration,” said Dr. Minkis, director of Mohs and dermatologic surgery at Weill Cornell Medicine, New York. “In studies comparing absorption of local anesthetics in tumescent anesthesia by regions that differ in vascularity, peak serum concentrations are greater and rise more rapidly after use in the head and neck compared to the trunk and extremities. However, no studies to date have compared the duration of ropivacaine in highly vascularized tissue or compared duration between regions that differ in vascularity.” The aim of the study, she noted, was to characterize the difference in duration of ropivacaine’s effects between anatomic regions of rich and comparably poor vascularity, such as the face and extremities, respectively.
Dr. Minkis and her colleagues recruited 17 women and 12 men with a mean age of 72 years who underwent Mohs surgery on the nose or the shin at Weill Cornell Medicine. Patients were anesthetized at each site with a subcutaneous injection of 0.5 mL of ropivacaine, 0.2%. Sensation was determined by pinprick prior to injection, at baseline, and every 15 minutes until sensation returned or surgery concluded. The primary endpoint was time to return of pinprick sensation.
The researchers found that the duration of ropivacaine was significantly shorter on the nose (a median of 60 minutes) than on the shin (a median of 210 minutes). In fact, the upper limit of the range of duration at the shin was not determinable because 22 of the 29 (76%) of participants did not regain sensation on the shin prior to leaving the surgical suite and concluding the study. The proportion of study participants who regained sensation within 1 hour was 76% among those who were treated on the nose vs. 3% of those who were treated on the shin (P < .0001).
“With durations of up to 6-14 hours reported, our results indicate a strikingly shorter duration of local anesthesia in highly vascularized tissue,” Dr. Minkis said. “The brevity of local anesthesia is even more surprising given the intrinsic vasoconstrictive properties of ropivacaine. Often, we co-administer epinephrine to achieve vasoconstriction and reduce local blood flow, thus prolonging local concentrations of the anesthetic with the added benefit of reducing bleeding during surgery. The short duration we’ve observed in our study is emphasized in using a potent, long-acting local anesthetic with vasoconstrictive properties that otherwise should attenuate the effects of high local vascularity.”
In other findings, patients with history of hypertension were more likely to regain sensation on the nose by 60 minutes but this did not reach statistical significance (P = .079). Other comorbidities including underlying anxiety/depression, diabetes, and kidney disease did not significantly impact duration of ropivacaine action on the nose. The same held true for patients who were treated on the shin.
“We highlight an inconsistency between the reported duration of a long-lasting local anesthetic and the short-lived anesthesia experienced by our patients in a highly vascularized region,” Dr. Minkis said. “In practice, adjunctive use of a long-acting anesthetic to prolong anesthesia is common, which may provide relief from multiple injections of shorter-acting lidocaine. However, the duration of Mohs surgery can be unpredictable. Extended wait times between stages may exceed the duration we’ve observed in this study.”
In addition, she continued, “pain is frequently reported on postoperative days 0 to 3, leading some to recommend the use of long-acting local anesthetics to prevent overprescription or a gap in pain coverage. This emphasizes a gap in effective pain control, but also an opportunity to improve our patients’ surgical and recovery experiences.”
Impact on practice
Keith L. Duffy, MD, associate professor of dermatology at the University of Utah, Salt Lake City, who was asked to comment on the study, said that in light of current local anesthetic shortages and back orders, “we dermatologic surgeons have been experimenting with different anesthetics and concentrations that we can use in our patients. Ropivacaine may become the anesthetic of choice for many of our practices given its inherent properties.”
The duration of anesthetic effects by anatomic location in this study is “actually more impressive than I would have suspected as a practicing Mohs surgeon. The results of this study will immediately impact my Mohs surgery clinic,” he said, adding that he hoped that Dr. Minkis and others “will expand on this study to include more patients, different anesthetics, and more anatomic locations.”
Dr. Minkis acknowledged certain limitations of the study, including its single-center design and the fact that there were too few observations of medical and clinical characteristics for subgroup analysis.
She and Dr. Duffy reported having no financial disclosures.
DENVER – , results from a single-center study showed.
Ropivacaine is a long-acting anesthetic that may be used as a substitute for the more commonly local anesthetics such as lidocaine or bupivacaine in dermatologic surgery, lead study author Kira Minkis, MD, PhD, told this news organization following the annual meeting of the American Society for Dermatologic Surgery, where the study results were presented during an oral abstract session. By comparison, ropivacaine has been reported to have a faster onset, similar duration in the range of 6-14 hours, less pain upon injection, and inherent vasoconstrictive properties.
“With tumescent anesthesia, studies have previously shown that the rate and absorption of anesthetics is influenced by the site of administration,” said Dr. Minkis, director of Mohs and dermatologic surgery at Weill Cornell Medicine, New York. “In studies comparing absorption of local anesthetics in tumescent anesthesia by regions that differ in vascularity, peak serum concentrations are greater and rise more rapidly after use in the head and neck compared to the trunk and extremities. However, no studies to date have compared the duration of ropivacaine in highly vascularized tissue or compared duration between regions that differ in vascularity.” The aim of the study, she noted, was to characterize the difference in duration of ropivacaine’s effects between anatomic regions of rich and comparably poor vascularity, such as the face and extremities, respectively.
Dr. Minkis and her colleagues recruited 17 women and 12 men with a mean age of 72 years who underwent Mohs surgery on the nose or the shin at Weill Cornell Medicine. Patients were anesthetized at each site with a subcutaneous injection of 0.5 mL of ropivacaine, 0.2%. Sensation was determined by pinprick prior to injection, at baseline, and every 15 minutes until sensation returned or surgery concluded. The primary endpoint was time to return of pinprick sensation.
The researchers found that the duration of ropivacaine was significantly shorter on the nose (a median of 60 minutes) than on the shin (a median of 210 minutes). In fact, the upper limit of the range of duration at the shin was not determinable because 22 of the 29 (76%) of participants did not regain sensation on the shin prior to leaving the surgical suite and concluding the study. The proportion of study participants who regained sensation within 1 hour was 76% among those who were treated on the nose vs. 3% of those who were treated on the shin (P < .0001).
“With durations of up to 6-14 hours reported, our results indicate a strikingly shorter duration of local anesthesia in highly vascularized tissue,” Dr. Minkis said. “The brevity of local anesthesia is even more surprising given the intrinsic vasoconstrictive properties of ropivacaine. Often, we co-administer epinephrine to achieve vasoconstriction and reduce local blood flow, thus prolonging local concentrations of the anesthetic with the added benefit of reducing bleeding during surgery. The short duration we’ve observed in our study is emphasized in using a potent, long-acting local anesthetic with vasoconstrictive properties that otherwise should attenuate the effects of high local vascularity.”
In other findings, patients with history of hypertension were more likely to regain sensation on the nose by 60 minutes but this did not reach statistical significance (P = .079). Other comorbidities including underlying anxiety/depression, diabetes, and kidney disease did not significantly impact duration of ropivacaine action on the nose. The same held true for patients who were treated on the shin.
“We highlight an inconsistency between the reported duration of a long-lasting local anesthetic and the short-lived anesthesia experienced by our patients in a highly vascularized region,” Dr. Minkis said. “In practice, adjunctive use of a long-acting anesthetic to prolong anesthesia is common, which may provide relief from multiple injections of shorter-acting lidocaine. However, the duration of Mohs surgery can be unpredictable. Extended wait times between stages may exceed the duration we’ve observed in this study.”
In addition, she continued, “pain is frequently reported on postoperative days 0 to 3, leading some to recommend the use of long-acting local anesthetics to prevent overprescription or a gap in pain coverage. This emphasizes a gap in effective pain control, but also an opportunity to improve our patients’ surgical and recovery experiences.”
Impact on practice
Keith L. Duffy, MD, associate professor of dermatology at the University of Utah, Salt Lake City, who was asked to comment on the study, said that in light of current local anesthetic shortages and back orders, “we dermatologic surgeons have been experimenting with different anesthetics and concentrations that we can use in our patients. Ropivacaine may become the anesthetic of choice for many of our practices given its inherent properties.”
The duration of anesthetic effects by anatomic location in this study is “actually more impressive than I would have suspected as a practicing Mohs surgeon. The results of this study will immediately impact my Mohs surgery clinic,” he said, adding that he hoped that Dr. Minkis and others “will expand on this study to include more patients, different anesthetics, and more anatomic locations.”
Dr. Minkis acknowledged certain limitations of the study, including its single-center design and the fact that there were too few observations of medical and clinical characteristics for subgroup analysis.
She and Dr. Duffy reported having no financial disclosures.
DENVER – , results from a single-center study showed.
Ropivacaine is a long-acting anesthetic that may be used as a substitute for the more commonly local anesthetics such as lidocaine or bupivacaine in dermatologic surgery, lead study author Kira Minkis, MD, PhD, told this news organization following the annual meeting of the American Society for Dermatologic Surgery, where the study results were presented during an oral abstract session. By comparison, ropivacaine has been reported to have a faster onset, similar duration in the range of 6-14 hours, less pain upon injection, and inherent vasoconstrictive properties.
“With tumescent anesthesia, studies have previously shown that the rate and absorption of anesthetics is influenced by the site of administration,” said Dr. Minkis, director of Mohs and dermatologic surgery at Weill Cornell Medicine, New York. “In studies comparing absorption of local anesthetics in tumescent anesthesia by regions that differ in vascularity, peak serum concentrations are greater and rise more rapidly after use in the head and neck compared to the trunk and extremities. However, no studies to date have compared the duration of ropivacaine in highly vascularized tissue or compared duration between regions that differ in vascularity.” The aim of the study, she noted, was to characterize the difference in duration of ropivacaine’s effects between anatomic regions of rich and comparably poor vascularity, such as the face and extremities, respectively.
Dr. Minkis and her colleagues recruited 17 women and 12 men with a mean age of 72 years who underwent Mohs surgery on the nose or the shin at Weill Cornell Medicine. Patients were anesthetized at each site with a subcutaneous injection of 0.5 mL of ropivacaine, 0.2%. Sensation was determined by pinprick prior to injection, at baseline, and every 15 minutes until sensation returned or surgery concluded. The primary endpoint was time to return of pinprick sensation.
The researchers found that the duration of ropivacaine was significantly shorter on the nose (a median of 60 minutes) than on the shin (a median of 210 minutes). In fact, the upper limit of the range of duration at the shin was not determinable because 22 of the 29 (76%) of participants did not regain sensation on the shin prior to leaving the surgical suite and concluding the study. The proportion of study participants who regained sensation within 1 hour was 76% among those who were treated on the nose vs. 3% of those who were treated on the shin (P < .0001).
“With durations of up to 6-14 hours reported, our results indicate a strikingly shorter duration of local anesthesia in highly vascularized tissue,” Dr. Minkis said. “The brevity of local anesthesia is even more surprising given the intrinsic vasoconstrictive properties of ropivacaine. Often, we co-administer epinephrine to achieve vasoconstriction and reduce local blood flow, thus prolonging local concentrations of the anesthetic with the added benefit of reducing bleeding during surgery. The short duration we’ve observed in our study is emphasized in using a potent, long-acting local anesthetic with vasoconstrictive properties that otherwise should attenuate the effects of high local vascularity.”
In other findings, patients with history of hypertension were more likely to regain sensation on the nose by 60 minutes but this did not reach statistical significance (P = .079). Other comorbidities including underlying anxiety/depression, diabetes, and kidney disease did not significantly impact duration of ropivacaine action on the nose. The same held true for patients who were treated on the shin.
“We highlight an inconsistency between the reported duration of a long-lasting local anesthetic and the short-lived anesthesia experienced by our patients in a highly vascularized region,” Dr. Minkis said. “In practice, adjunctive use of a long-acting anesthetic to prolong anesthesia is common, which may provide relief from multiple injections of shorter-acting lidocaine. However, the duration of Mohs surgery can be unpredictable. Extended wait times between stages may exceed the duration we’ve observed in this study.”
In addition, she continued, “pain is frequently reported on postoperative days 0 to 3, leading some to recommend the use of long-acting local anesthetics to prevent overprescription or a gap in pain coverage. This emphasizes a gap in effective pain control, but also an opportunity to improve our patients’ surgical and recovery experiences.”
Impact on practice
Keith L. Duffy, MD, associate professor of dermatology at the University of Utah, Salt Lake City, who was asked to comment on the study, said that in light of current local anesthetic shortages and back orders, “we dermatologic surgeons have been experimenting with different anesthetics and concentrations that we can use in our patients. Ropivacaine may become the anesthetic of choice for many of our practices given its inherent properties.”
The duration of anesthetic effects by anatomic location in this study is “actually more impressive than I would have suspected as a practicing Mohs surgeon. The results of this study will immediately impact my Mohs surgery clinic,” he said, adding that he hoped that Dr. Minkis and others “will expand on this study to include more patients, different anesthetics, and more anatomic locations.”
Dr. Minkis acknowledged certain limitations of the study, including its single-center design and the fact that there were too few observations of medical and clinical characteristics for subgroup analysis.
She and Dr. Duffy reported having no financial disclosures.
AT ASDS 2022
First-in-class device for facial wrinkles, tightening hits the market
DENVER – .
“It’s early yet, but I have treated dozens of patients with this device, and they have been happy with the results,” Mathew M. Avram, MD, JD, said at the annual meeting of the American Society for Dermatologic Surgery. “This is a new technique that offers the ability to remove a significant amount of damaged, lax skin without concern for scarring,” he said.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the first-in-class device is cleared by the Food and Drug Administration for the treatment of moderate and severe wrinkles in the mid and lower face in adults aged 22 years or older with Fitzpatrick skin types I-IV. It features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“It doesn’t do anything equivalent to a facelift, but the concept is a facelift by thousands of micro-punch excisions,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “Rather than pulling up the skin and lifting it and cutting the excess skin like we do with a facelift, we are creating thousands of smaller-scale tissue removals with immediate closures to do the same thing. The micro-cores are about the size of a 22-gauge needle and there is no scarring due to the small size of these tissue extractions.”
The device features needle cartridges capable of excising up to 24,000 cores per treatment. According to data from Cytrellis, the manufacturer, the equivalent of about 2 inches of skin can be removed during the procedure, which typically takes fewer than 30 minutes to perform. “There is no heat whatsoever,” Dr. Avram said. “In my experience, it especially helps with jawline definition, the lower medial cheek excess skin, and accordion lines in that area.”
In a pivotal trial of the device, 51 patients with mid to lower face wrinkles (moderately deep or deep wrinkles with well-defined edges) were treated 2-3 times with 7%-8% skin removal and up to a 5-mm needle coring depth). The investigators found that 40% of study participants achieved an improvement of 2 grades on the Lemperle Wrinkle Severity Scale and that the rate of overall satisfaction (slightly, somewhat, and extremely satisfied) was 86%.
In addition, 90% showed improvement of treated sites on the Global Aesthetic Improvement Scale, and 70% were comfortable enough to go out in public or return to work 3 days after treatment. Common side effects that can occur immediately post treatment include redness, swelling, and pinpoint bleeding, which typically clear in a few days.
Dr. Avram, immediate past president of the ASDS, has posted videos to his Instagram feed that show him treating patients with the Ellacor device and he admits that the procedure looks painful. “There are all these tear emojis and people cursing me out,” he said, referring to responses from his Instagram followers.
Proper local anesthesia prior to treatment is key. “I perform nerve blocks and infiltrate the skin,” he said. “You have to cover the whole treatment area. If you don’t, then it’s going to hurt. The average pain score is 1.9 out of 10. The highest pain score I’ve gotten from a patient is a 3 out of 10.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton, and has ownership and/or shareholder interest in Cytrellis.
DENVER – .
“It’s early yet, but I have treated dozens of patients with this device, and they have been happy with the results,” Mathew M. Avram, MD, JD, said at the annual meeting of the American Society for Dermatologic Surgery. “This is a new technique that offers the ability to remove a significant amount of damaged, lax skin without concern for scarring,” he said.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the first-in-class device is cleared by the Food and Drug Administration for the treatment of moderate and severe wrinkles in the mid and lower face in adults aged 22 years or older with Fitzpatrick skin types I-IV. It features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“It doesn’t do anything equivalent to a facelift, but the concept is a facelift by thousands of micro-punch excisions,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “Rather than pulling up the skin and lifting it and cutting the excess skin like we do with a facelift, we are creating thousands of smaller-scale tissue removals with immediate closures to do the same thing. The micro-cores are about the size of a 22-gauge needle and there is no scarring due to the small size of these tissue extractions.”
The device features needle cartridges capable of excising up to 24,000 cores per treatment. According to data from Cytrellis, the manufacturer, the equivalent of about 2 inches of skin can be removed during the procedure, which typically takes fewer than 30 minutes to perform. “There is no heat whatsoever,” Dr. Avram said. “In my experience, it especially helps with jawline definition, the lower medial cheek excess skin, and accordion lines in that area.”
In a pivotal trial of the device, 51 patients with mid to lower face wrinkles (moderately deep or deep wrinkles with well-defined edges) were treated 2-3 times with 7%-8% skin removal and up to a 5-mm needle coring depth). The investigators found that 40% of study participants achieved an improvement of 2 grades on the Lemperle Wrinkle Severity Scale and that the rate of overall satisfaction (slightly, somewhat, and extremely satisfied) was 86%.
In addition, 90% showed improvement of treated sites on the Global Aesthetic Improvement Scale, and 70% were comfortable enough to go out in public or return to work 3 days after treatment. Common side effects that can occur immediately post treatment include redness, swelling, and pinpoint bleeding, which typically clear in a few days.
Dr. Avram, immediate past president of the ASDS, has posted videos to his Instagram feed that show him treating patients with the Ellacor device and he admits that the procedure looks painful. “There are all these tear emojis and people cursing me out,” he said, referring to responses from his Instagram followers.
Proper local anesthesia prior to treatment is key. “I perform nerve blocks and infiltrate the skin,” he said. “You have to cover the whole treatment area. If you don’t, then it’s going to hurt. The average pain score is 1.9 out of 10. The highest pain score I’ve gotten from a patient is a 3 out of 10.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton, and has ownership and/or shareholder interest in Cytrellis.
DENVER – .
“It’s early yet, but I have treated dozens of patients with this device, and they have been happy with the results,” Mathew M. Avram, MD, JD, said at the annual meeting of the American Society for Dermatologic Surgery. “This is a new technique that offers the ability to remove a significant amount of damaged, lax skin without concern for scarring,” he said.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the first-in-class device is cleared by the Food and Drug Administration for the treatment of moderate and severe wrinkles in the mid and lower face in adults aged 22 years or older with Fitzpatrick skin types I-IV. It features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“It doesn’t do anything equivalent to a facelift, but the concept is a facelift by thousands of micro-punch excisions,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “Rather than pulling up the skin and lifting it and cutting the excess skin like we do with a facelift, we are creating thousands of smaller-scale tissue removals with immediate closures to do the same thing. The micro-cores are about the size of a 22-gauge needle and there is no scarring due to the small size of these tissue extractions.”
The device features needle cartridges capable of excising up to 24,000 cores per treatment. According to data from Cytrellis, the manufacturer, the equivalent of about 2 inches of skin can be removed during the procedure, which typically takes fewer than 30 minutes to perform. “There is no heat whatsoever,” Dr. Avram said. “In my experience, it especially helps with jawline definition, the lower medial cheek excess skin, and accordion lines in that area.”
In a pivotal trial of the device, 51 patients with mid to lower face wrinkles (moderately deep or deep wrinkles with well-defined edges) were treated 2-3 times with 7%-8% skin removal and up to a 5-mm needle coring depth). The investigators found that 40% of study participants achieved an improvement of 2 grades on the Lemperle Wrinkle Severity Scale and that the rate of overall satisfaction (slightly, somewhat, and extremely satisfied) was 86%.
In addition, 90% showed improvement of treated sites on the Global Aesthetic Improvement Scale, and 70% were comfortable enough to go out in public or return to work 3 days after treatment. Common side effects that can occur immediately post treatment include redness, swelling, and pinpoint bleeding, which typically clear in a few days.
Dr. Avram, immediate past president of the ASDS, has posted videos to his Instagram feed that show him treating patients with the Ellacor device and he admits that the procedure looks painful. “There are all these tear emojis and people cursing me out,” he said, referring to responses from his Instagram followers.
Proper local anesthesia prior to treatment is key. “I perform nerve blocks and infiltrate the skin,” he said. “You have to cover the whole treatment area. If you don’t, then it’s going to hurt. The average pain score is 1.9 out of 10. The highest pain score I’ve gotten from a patient is a 3 out of 10.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton, and has ownership and/or shareholder interest in Cytrellis.
AT ASDS 2022
Many specialists are on the wrong side of the patient-jargon relationship
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
IgA Vasculitis in the Setting of Biologic Therapy for Psoriasis and Recurrent Cutaneous Methicillin-Resistant Staphylococcus aureus Colonization
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
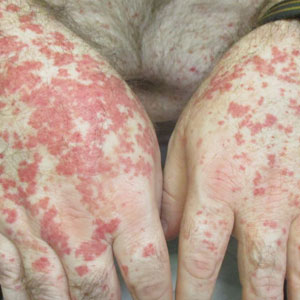
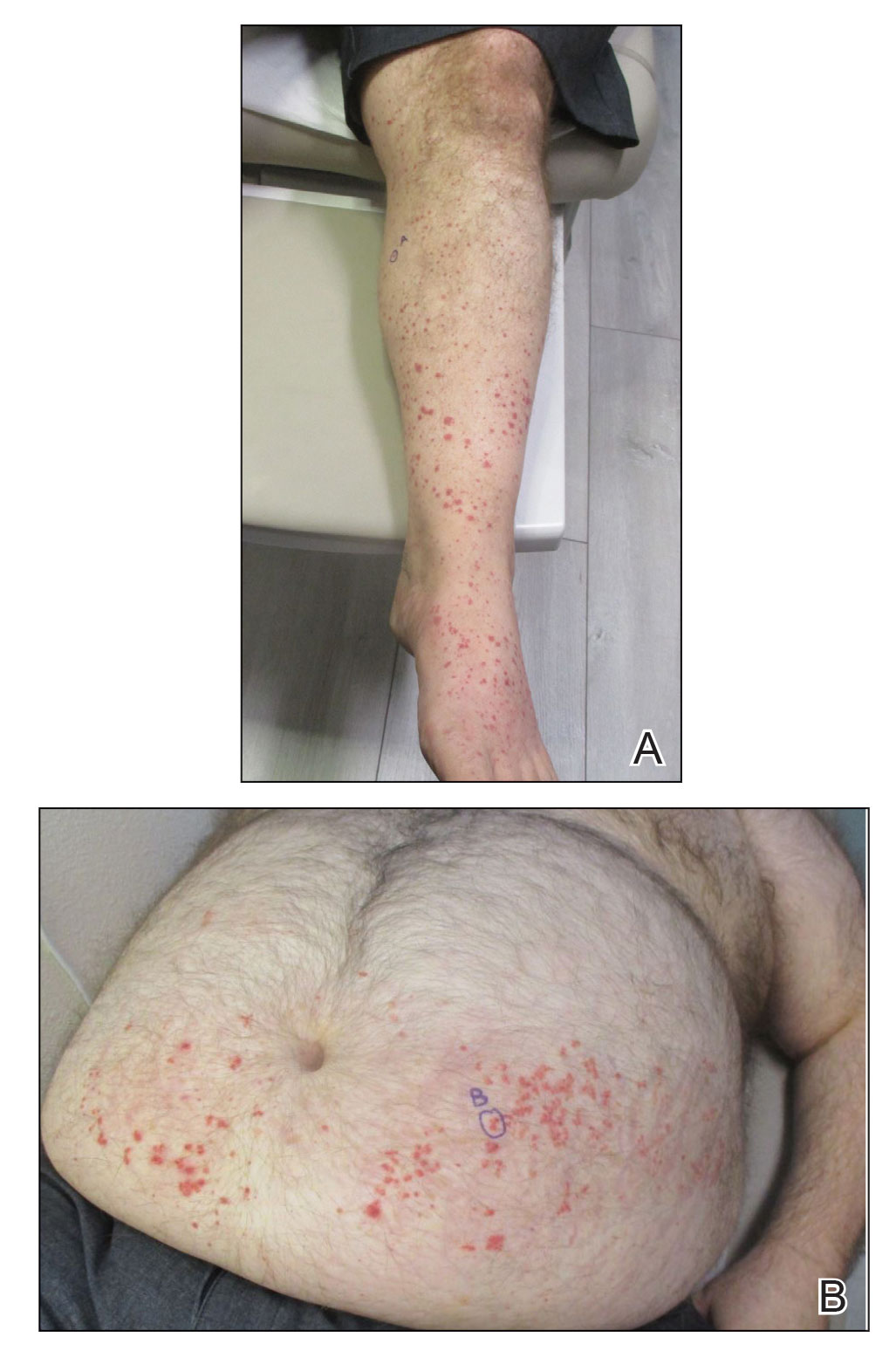
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.
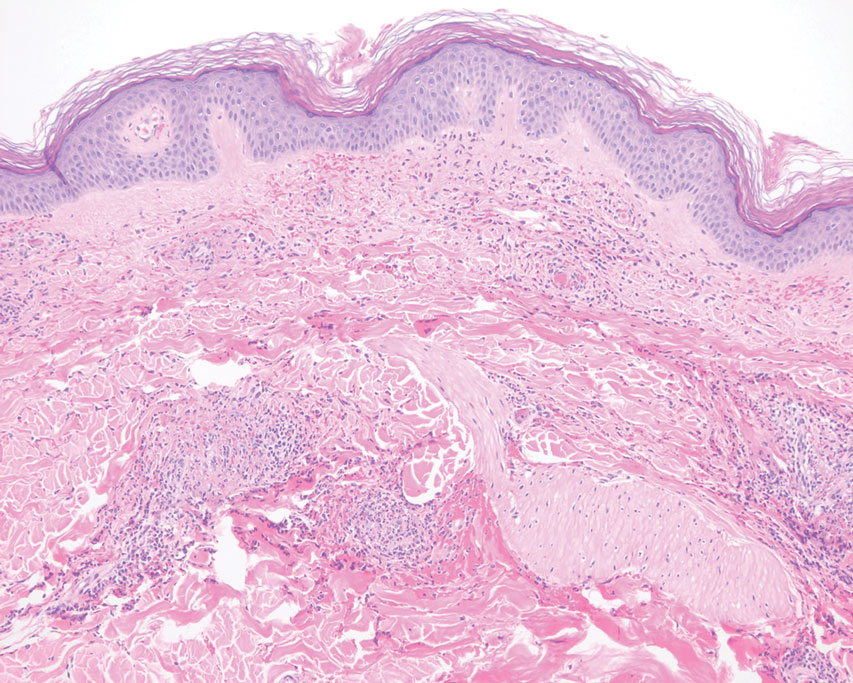
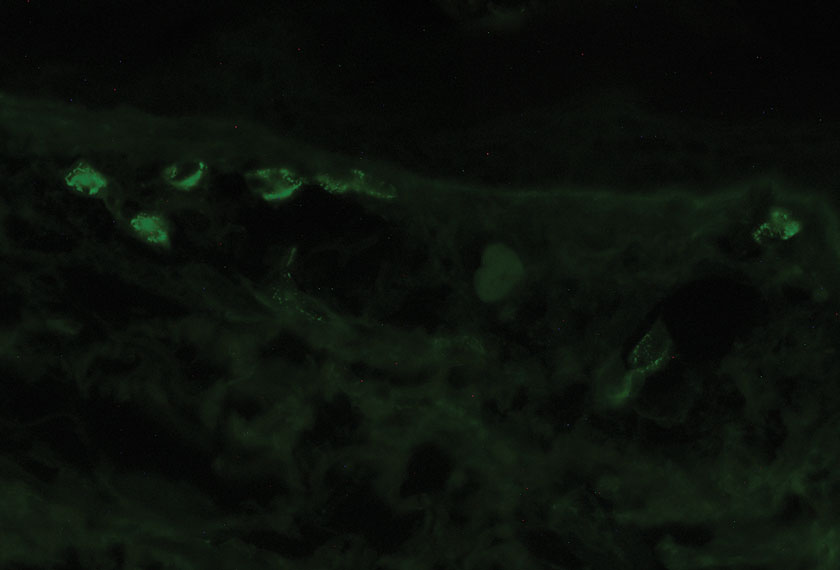
Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.
Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.
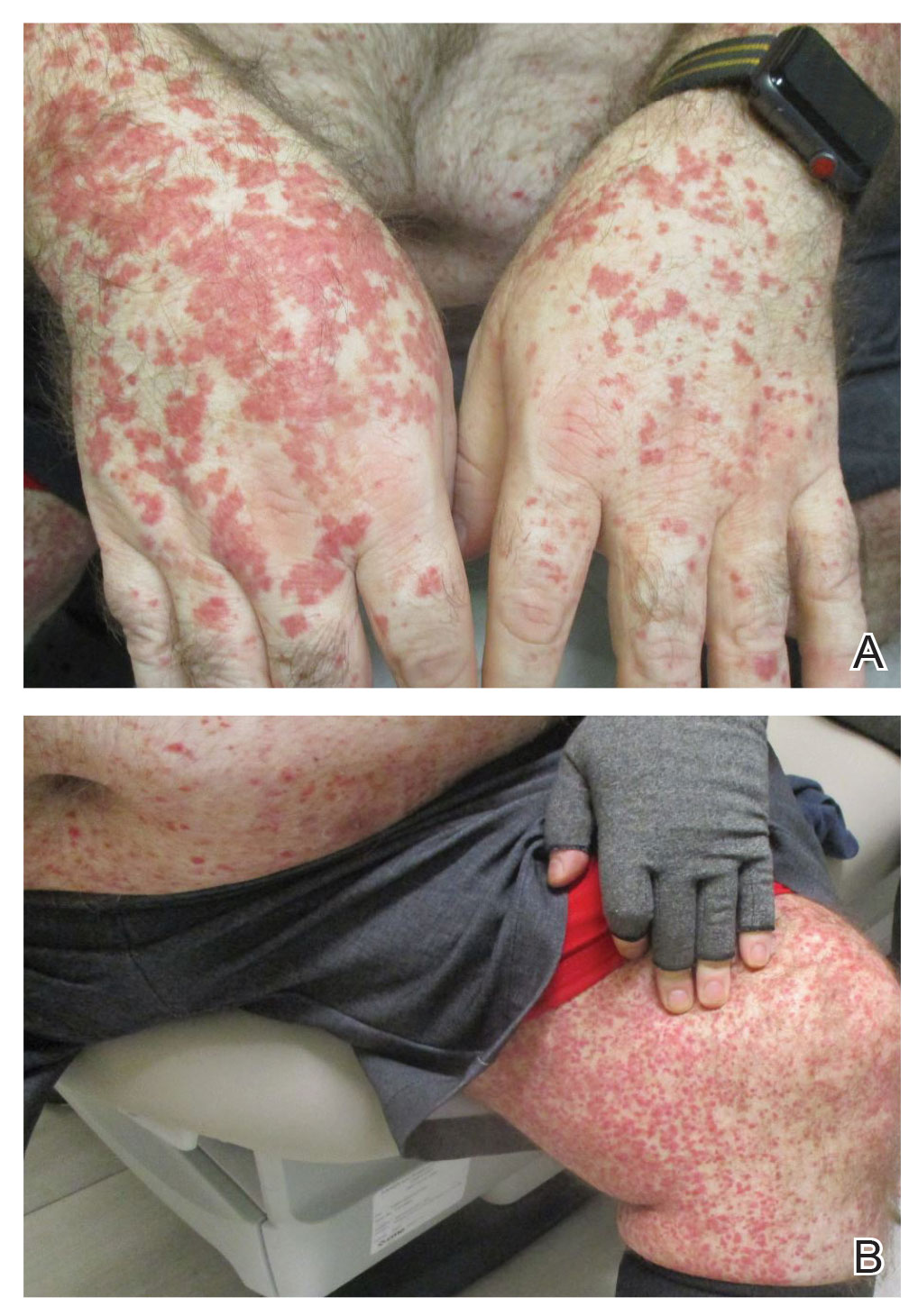
Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.
Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
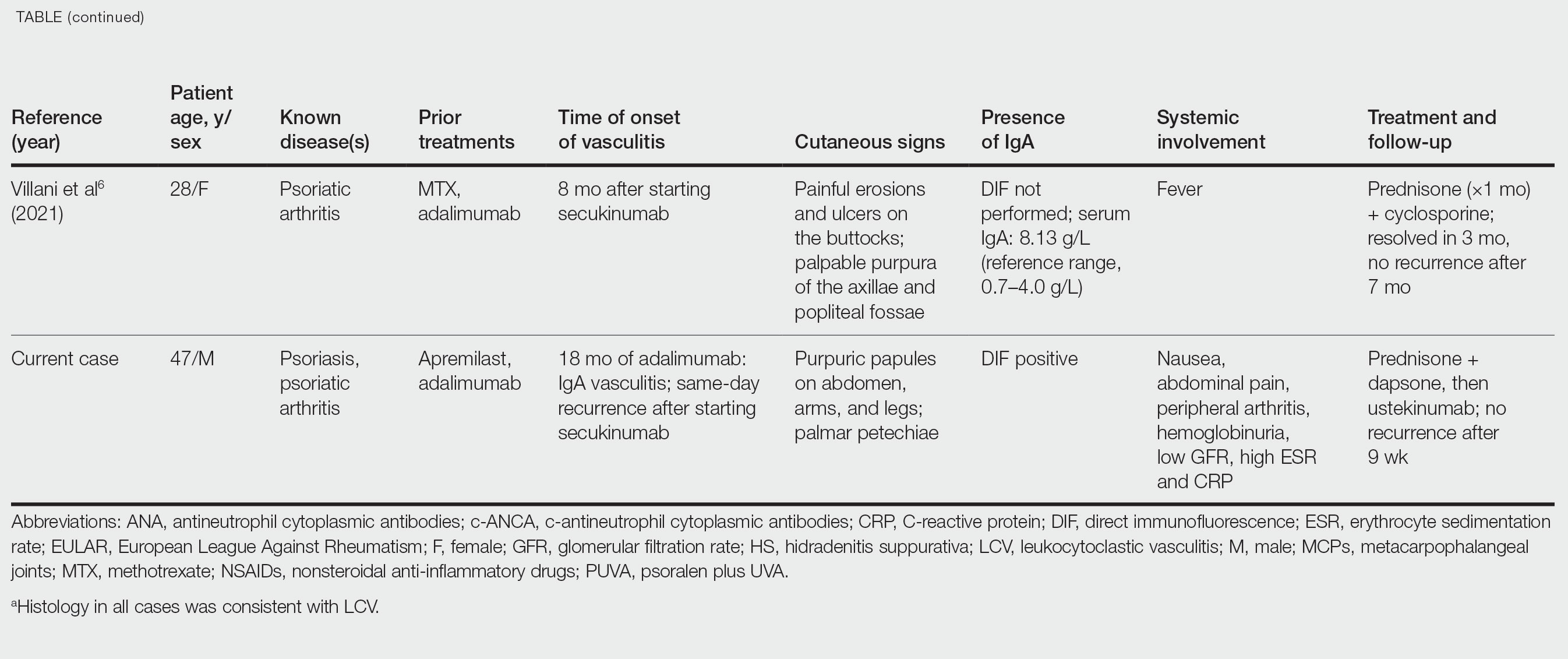
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7
The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10
There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.

Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.

Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.

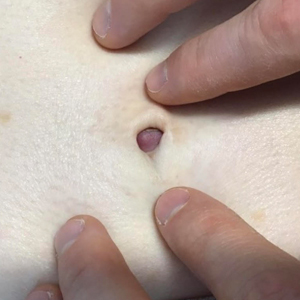
Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.

Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7

The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10

There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.

Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.

Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.

Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.

Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7

The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10

There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
Practice Points
- Biologic medications including adalimumab and more rarely secukinumab may be associated with leukocytoclastic vasculitis; a smaller subset of patients may experience IgA vasculitis.
- The IL-23 blocker ustekinumab may represent an ideal therapeutic agent when secukinumabassociated vasculitis is suspected. Because IL-23 is the main driver and sustainer of TH17 cell differentiation, it may cease the main causative cytokine cascades “upstream.”
Remote assessment of atopic dermatitis is feasible with patient-provided images: Study
MONTREAL – , as well as the possibility of conducting remote clinical trials that would be less expensive and less burdensome for participants, according to investigators, who presented the study at the annual meeting of the International Society of Atopic Dermatitis.
Still, practical barriers need to be addressed, particularly the problem of image quality, noted study investigator Aviël Ragamin, MD, from the department of dermatology, Erasmus MC, University Medical Center, Rotterdam, the Netherlands.
“Good-quality images are crucial, [and] in our study, patients didn’t have any incentive to provide images because they had already received their medical consultation,” he explained. He suggested that this problem could be overcome by providing technical support for patients and compensation for trial participants.
The study included 87 children (median age, 7 years), who were assessed for AD severity at an academic outpatient clinic. The in-person visit included assessment with the Eczema Area and Severity Index (EASI) score, as well as the collection of whole-body clinical images. Parents were then asked to return home and to provide their own clinical images and self-administered EASI assessments of their child for comparison. Four raters were asked to rate all images twice and to compare in-clinic and self-administered EASI scores based on the images.
At the in-clinic visit, the median EASI score of the group was 8.8. The majority of patients had moderate (46.6%) or severe (14.8%) AD. Roughly 40% of the patients had darker skin (Fitzpatrick skin types IV–VI).
Using Spearman rank correlation of 1,534 in-clinic and 425 patient-provided images, the study found good inter- and intra-rater reliability for clinical image assessment and strong agreement between images and the in-clinic EASI scores. The top outliers in the assessment were individuals with either darker skin or significant postinflammatory hyperpigmentation, which are “the most difficult cases to rate, based on images,” Dr. Ragamin noted.
There was only moderate correlation between the in-clinic and self-administered EASI scores, with a significant number of patients either underestimating or overestimating their AD severity, he added.
Overall, the main problem with remote assessment seems to be the feasibility of patients providing images, said Dr. Ragamin. Only 36.8% of parents provided any images at all, and of these, 1 of 5 were deemed too blurry, leaving just 13 for final assessment, he explained.
“Pragmatically, it’s tricky,” said Aaron Drucker, MD, a dermatologist at Women’s College Hospital and associate professor at the University of Toronto, who was asked to comment on the study. “It takes long enough to do an EASI score in person, let alone looking through blurry pictures that take too long to load into your electronic medical record. We know it works, but when our hospital went virtual [during the COVID pandemic] ... most of my patients with chronic eczema weren’t even sending me pictures.”
Regarding the utility of remote, full-body photography in clinical practice, he said, “There’s too many feasibility hoops to jump through at this point. The most promise I see is for clinical trials, where it’s hard to get people to come in.”
Dr. Ragamin and Dr. Drucker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – , as well as the possibility of conducting remote clinical trials that would be less expensive and less burdensome for participants, according to investigators, who presented the study at the annual meeting of the International Society of Atopic Dermatitis.
Still, practical barriers need to be addressed, particularly the problem of image quality, noted study investigator Aviël Ragamin, MD, from the department of dermatology, Erasmus MC, University Medical Center, Rotterdam, the Netherlands.
“Good-quality images are crucial, [and] in our study, patients didn’t have any incentive to provide images because they had already received their medical consultation,” he explained. He suggested that this problem could be overcome by providing technical support for patients and compensation for trial participants.
The study included 87 children (median age, 7 years), who were assessed for AD severity at an academic outpatient clinic. The in-person visit included assessment with the Eczema Area and Severity Index (EASI) score, as well as the collection of whole-body clinical images. Parents were then asked to return home and to provide their own clinical images and self-administered EASI assessments of their child for comparison. Four raters were asked to rate all images twice and to compare in-clinic and self-administered EASI scores based on the images.
At the in-clinic visit, the median EASI score of the group was 8.8. The majority of patients had moderate (46.6%) or severe (14.8%) AD. Roughly 40% of the patients had darker skin (Fitzpatrick skin types IV–VI).
Using Spearman rank correlation of 1,534 in-clinic and 425 patient-provided images, the study found good inter- and intra-rater reliability for clinical image assessment and strong agreement between images and the in-clinic EASI scores. The top outliers in the assessment were individuals with either darker skin or significant postinflammatory hyperpigmentation, which are “the most difficult cases to rate, based on images,” Dr. Ragamin noted.
There was only moderate correlation between the in-clinic and self-administered EASI scores, with a significant number of patients either underestimating or overestimating their AD severity, he added.
Overall, the main problem with remote assessment seems to be the feasibility of patients providing images, said Dr. Ragamin. Only 36.8% of parents provided any images at all, and of these, 1 of 5 were deemed too blurry, leaving just 13 for final assessment, he explained.
“Pragmatically, it’s tricky,” said Aaron Drucker, MD, a dermatologist at Women’s College Hospital and associate professor at the University of Toronto, who was asked to comment on the study. “It takes long enough to do an EASI score in person, let alone looking through blurry pictures that take too long to load into your electronic medical record. We know it works, but when our hospital went virtual [during the COVID pandemic] ... most of my patients with chronic eczema weren’t even sending me pictures.”
Regarding the utility of remote, full-body photography in clinical practice, he said, “There’s too many feasibility hoops to jump through at this point. The most promise I see is for clinical trials, where it’s hard to get people to come in.”
Dr. Ragamin and Dr. Drucker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – , as well as the possibility of conducting remote clinical trials that would be less expensive and less burdensome for participants, according to investigators, who presented the study at the annual meeting of the International Society of Atopic Dermatitis.
Still, practical barriers need to be addressed, particularly the problem of image quality, noted study investigator Aviël Ragamin, MD, from the department of dermatology, Erasmus MC, University Medical Center, Rotterdam, the Netherlands.
“Good-quality images are crucial, [and] in our study, patients didn’t have any incentive to provide images because they had already received their medical consultation,” he explained. He suggested that this problem could be overcome by providing technical support for patients and compensation for trial participants.
The study included 87 children (median age, 7 years), who were assessed for AD severity at an academic outpatient clinic. The in-person visit included assessment with the Eczema Area and Severity Index (EASI) score, as well as the collection of whole-body clinical images. Parents were then asked to return home and to provide their own clinical images and self-administered EASI assessments of their child for comparison. Four raters were asked to rate all images twice and to compare in-clinic and self-administered EASI scores based on the images.
At the in-clinic visit, the median EASI score of the group was 8.8. The majority of patients had moderate (46.6%) or severe (14.8%) AD. Roughly 40% of the patients had darker skin (Fitzpatrick skin types IV–VI).
Using Spearman rank correlation of 1,534 in-clinic and 425 patient-provided images, the study found good inter- and intra-rater reliability for clinical image assessment and strong agreement between images and the in-clinic EASI scores. The top outliers in the assessment were individuals with either darker skin or significant postinflammatory hyperpigmentation, which are “the most difficult cases to rate, based on images,” Dr. Ragamin noted.
There was only moderate correlation between the in-clinic and self-administered EASI scores, with a significant number of patients either underestimating or overestimating their AD severity, he added.
Overall, the main problem with remote assessment seems to be the feasibility of patients providing images, said Dr. Ragamin. Only 36.8% of parents provided any images at all, and of these, 1 of 5 were deemed too blurry, leaving just 13 for final assessment, he explained.
“Pragmatically, it’s tricky,” said Aaron Drucker, MD, a dermatologist at Women’s College Hospital and associate professor at the University of Toronto, who was asked to comment on the study. “It takes long enough to do an EASI score in person, let alone looking through blurry pictures that take too long to load into your electronic medical record. We know it works, but when our hospital went virtual [during the COVID pandemic] ... most of my patients with chronic eczema weren’t even sending me pictures.”
Regarding the utility of remote, full-body photography in clinical practice, he said, “There’s too many feasibility hoops to jump through at this point. The most promise I see is for clinical trials, where it’s hard to get people to come in.”
Dr. Ragamin and Dr. Drucker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Asymptomatic Umbilical Nodule
The Diagnosis: Sister Mary Joseph Nodule
Histopathologic analysis of the biopsy specimen revealed a dense infiltrate of large, hyperchromatic, mucin-producing cells exhibiting varying degrees of nuclear pleomorphism (Figure 1). Immunohistochemical (IHC) staining was negative for cytokeratin (CK) 20; however, CK7 was found positive (Figure 2), which confirmed the presence of a metastatic adenocarcinoma, consistent with the clinical diagnosis of a Sister Mary Joseph nodule (SMJN). Subsequent IHC workup to determine the site of origin revealed densely positive expression of both cancer antigen 125 and paired homeobox gene 8 (PAX-8)(Figure 3), consistent with primary ovarian disease. Furthermore, expression of estrogen receptor and p53 both were positive within the nuclei, illustrating an aberrant expression pattern. On the other hand, cancer antigen 19-9, caudal-type homeobox 2, gross cystic disease fluid protein 15, and mammaglobin were all determined negative, thus leading to the pathologic diagnosis of a metastatic ovarian adenocarcinoma. Additional workup with computed tomography of the abdomen and pelvis highlighted a large left ovarian mass with multiple omental nodules as well as enlarged retroperitoneal and pelvic lymph nodes.

The SMJN is a rare presentation of internal malignancy that appears as a nodule that metastasizes to the umbilicus. It may be ulcerated or necrotic and is seen in up to 10% of patients with cutaneous metastases from internal malignancy.1 These nodules are named after Sister Mary Joseph, the surgical assistant of Dr. William Mayo who first described the relationship between umbilical nodules seen in patients with gastrointestinal and genitourinary cancer. The most common underlying malignancies include primary gastrointestinal and gynecologic adenocarcinomas. In a retrospective study of 34 patients by Chalya et al,2 the stomach was found to be the most common primary site (41.1%). The presence of an SMJN affords a poor prognosis, with a mean overall survival of 11 months from the time of diagnosis.3 The mechanism of disease dissemination remains unknown but is thought to occur through lymphovascular invasion of tumor cells and spread via the umbilical ligament.1,4
Merkel cell carcinoma is a cutaneous neuroendocrine tumor that most commonly presents in elderly patients as red-violet nodules or plaques. Although Merkel cell carcinoma most frequently is encountered on sun-exposed skin, they also can arise on the trunk and abdomen. Positive IHC staining for CK20 would be expected; however, it was negative in our case.5
Cutaneous endometriosis is a rare disease presentation and most commonly occurs as a secondary process due to surgical inoculation of the abdominal wall. Primary cutaneous endometriosis in which there is no history of abdominal surgery less frequently is encountered. Patients typically will report pain and cyclical bleeding with menses. Pathology demonstrates ectopic endometrial tissue with glands and uterine myxoid stroma.6
Amelanotic melanoma is an uncommon subtype of malignant melanoma that presents as nonpigmented nodules that have a propensity to ulcerate and bleed. Furthermore, the umbilicus is an exceedingly rare location for primary melanoma. However, one report does exist, and amelanotic melanoma should be considered in the differential for patients with umbilical nodules.7
Dermoid cysts are benign congenital lesions that typically present as a painless, slow-growing, and wellcircumscribed nodule, as similarly experienced by our patient. They most commonly are found on the testicles and ovaries but also are known to arise in embryologic fusion planes, and reports of umbilical lesions exist.8 Dermoid cysts are diagnosed based on histopathology, supporting the need for a biopsy to distinguish a malignant process from benign lesions.9
- Gabriele R, Conte M, Egidi F, et al. Umbilical metastases: current viewpoint. World J Surg Oncol. 2005;3:13.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph’s nodule at a university teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Leyrat B, Bernadach M, Ginzac A, et al. Sister Mary Joseph nodules: a case report about a rare location of skin metastasis. Case Rep Oncol. 2021;14:664-670.
- Yendluri V, Centeno B, Springett GM. Pancreatic cancer presenting as a Sister Mary Joseph’s nodule: case report and update of the literature. Pancreas. 2007;34:161-164.
- Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol. 2018;8:48.
- Bittar PG, Hryneewycz KT, Bryant EA. Primary cutaneous endometriosis presenting as an umbilical nodule. JAMA Dermatol. 2021;157:1227.
- Kovitwanichkanont T, Joseph S, Yip L. Hidden in plain sight: umbilical melanoma [published online January 28, 2020]. Med J Aust. 2020;212:154-155.e1.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Akinci O, Turker C, Erturk MS, et al. Umbilical dermoid cyst: a rare case. Cerrahpasa Med J. 2020;44:51-53.
The Diagnosis: Sister Mary Joseph Nodule
Histopathologic analysis of the biopsy specimen revealed a dense infiltrate of large, hyperchromatic, mucin-producing cells exhibiting varying degrees of nuclear pleomorphism (Figure 1). Immunohistochemical (IHC) staining was negative for cytokeratin (CK) 20; however, CK7 was found positive (Figure 2), which confirmed the presence of a metastatic adenocarcinoma, consistent with the clinical diagnosis of a Sister Mary Joseph nodule (SMJN). Subsequent IHC workup to determine the site of origin revealed densely positive expression of both cancer antigen 125 and paired homeobox gene 8 (PAX-8)(Figure 3), consistent with primary ovarian disease. Furthermore, expression of estrogen receptor and p53 both were positive within the nuclei, illustrating an aberrant expression pattern. On the other hand, cancer antigen 19-9, caudal-type homeobox 2, gross cystic disease fluid protein 15, and mammaglobin were all determined negative, thus leading to the pathologic diagnosis of a metastatic ovarian adenocarcinoma. Additional workup with computed tomography of the abdomen and pelvis highlighted a large left ovarian mass with multiple omental nodules as well as enlarged retroperitoneal and pelvic lymph nodes.

The SMJN is a rare presentation of internal malignancy that appears as a nodule that metastasizes to the umbilicus. It may be ulcerated or necrotic and is seen in up to 10% of patients with cutaneous metastases from internal malignancy.1 These nodules are named after Sister Mary Joseph, the surgical assistant of Dr. William Mayo who first described the relationship between umbilical nodules seen in patients with gastrointestinal and genitourinary cancer. The most common underlying malignancies include primary gastrointestinal and gynecologic adenocarcinomas. In a retrospective study of 34 patients by Chalya et al,2 the stomach was found to be the most common primary site (41.1%). The presence of an SMJN affords a poor prognosis, with a mean overall survival of 11 months from the time of diagnosis.3 The mechanism of disease dissemination remains unknown but is thought to occur through lymphovascular invasion of tumor cells and spread via the umbilical ligament.1,4

Merkel cell carcinoma is a cutaneous neuroendocrine tumor that most commonly presents in elderly patients as red-violet nodules or plaques. Although Merkel cell carcinoma most frequently is encountered on sun-exposed skin, they also can arise on the trunk and abdomen. Positive IHC staining for CK20 would be expected; however, it was negative in our case.5

Cutaneous endometriosis is a rare disease presentation and most commonly occurs as a secondary process due to surgical inoculation of the abdominal wall. Primary cutaneous endometriosis in which there is no history of abdominal surgery less frequently is encountered. Patients typically will report pain and cyclical bleeding with menses. Pathology demonstrates ectopic endometrial tissue with glands and uterine myxoid stroma.6
Amelanotic melanoma is an uncommon subtype of malignant melanoma that presents as nonpigmented nodules that have a propensity to ulcerate and bleed. Furthermore, the umbilicus is an exceedingly rare location for primary melanoma. However, one report does exist, and amelanotic melanoma should be considered in the differential for patients with umbilical nodules.7
Dermoid cysts are benign congenital lesions that typically present as a painless, slow-growing, and wellcircumscribed nodule, as similarly experienced by our patient. They most commonly are found on the testicles and ovaries but also are known to arise in embryologic fusion planes, and reports of umbilical lesions exist.8 Dermoid cysts are diagnosed based on histopathology, supporting the need for a biopsy to distinguish a malignant process from benign lesions.9
The Diagnosis: Sister Mary Joseph Nodule
Histopathologic analysis of the biopsy specimen revealed a dense infiltrate of large, hyperchromatic, mucin-producing cells exhibiting varying degrees of nuclear pleomorphism (Figure 1). Immunohistochemical (IHC) staining was negative for cytokeratin (CK) 20; however, CK7 was found positive (Figure 2), which confirmed the presence of a metastatic adenocarcinoma, consistent with the clinical diagnosis of a Sister Mary Joseph nodule (SMJN). Subsequent IHC workup to determine the site of origin revealed densely positive expression of both cancer antigen 125 and paired homeobox gene 8 (PAX-8)(Figure 3), consistent with primary ovarian disease. Furthermore, expression of estrogen receptor and p53 both were positive within the nuclei, illustrating an aberrant expression pattern. On the other hand, cancer antigen 19-9, caudal-type homeobox 2, gross cystic disease fluid protein 15, and mammaglobin were all determined negative, thus leading to the pathologic diagnosis of a metastatic ovarian adenocarcinoma. Additional workup with computed tomography of the abdomen and pelvis highlighted a large left ovarian mass with multiple omental nodules as well as enlarged retroperitoneal and pelvic lymph nodes.

The SMJN is a rare presentation of internal malignancy that appears as a nodule that metastasizes to the umbilicus. It may be ulcerated or necrotic and is seen in up to 10% of patients with cutaneous metastases from internal malignancy.1 These nodules are named after Sister Mary Joseph, the surgical assistant of Dr. William Mayo who first described the relationship between umbilical nodules seen in patients with gastrointestinal and genitourinary cancer. The most common underlying malignancies include primary gastrointestinal and gynecologic adenocarcinomas. In a retrospective study of 34 patients by Chalya et al,2 the stomach was found to be the most common primary site (41.1%). The presence of an SMJN affords a poor prognosis, with a mean overall survival of 11 months from the time of diagnosis.3 The mechanism of disease dissemination remains unknown but is thought to occur through lymphovascular invasion of tumor cells and spread via the umbilical ligament.1,4

Merkel cell carcinoma is a cutaneous neuroendocrine tumor that most commonly presents in elderly patients as red-violet nodules or plaques. Although Merkel cell carcinoma most frequently is encountered on sun-exposed skin, they also can arise on the trunk and abdomen. Positive IHC staining for CK20 would be expected; however, it was negative in our case.5

Cutaneous endometriosis is a rare disease presentation and most commonly occurs as a secondary process due to surgical inoculation of the abdominal wall. Primary cutaneous endometriosis in which there is no history of abdominal surgery less frequently is encountered. Patients typically will report pain and cyclical bleeding with menses. Pathology demonstrates ectopic endometrial tissue with glands and uterine myxoid stroma.6
Amelanotic melanoma is an uncommon subtype of malignant melanoma that presents as nonpigmented nodules that have a propensity to ulcerate and bleed. Furthermore, the umbilicus is an exceedingly rare location for primary melanoma. However, one report does exist, and amelanotic melanoma should be considered in the differential for patients with umbilical nodules.7
Dermoid cysts are benign congenital lesions that typically present as a painless, slow-growing, and wellcircumscribed nodule, as similarly experienced by our patient. They most commonly are found on the testicles and ovaries but also are known to arise in embryologic fusion planes, and reports of umbilical lesions exist.8 Dermoid cysts are diagnosed based on histopathology, supporting the need for a biopsy to distinguish a malignant process from benign lesions.9
- Gabriele R, Conte M, Egidi F, et al. Umbilical metastases: current viewpoint. World J Surg Oncol. 2005;3:13.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph’s nodule at a university teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Leyrat B, Bernadach M, Ginzac A, et al. Sister Mary Joseph nodules: a case report about a rare location of skin metastasis. Case Rep Oncol. 2021;14:664-670.
- Yendluri V, Centeno B, Springett GM. Pancreatic cancer presenting as a Sister Mary Joseph’s nodule: case report and update of the literature. Pancreas. 2007;34:161-164.
- Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol. 2018;8:48.
- Bittar PG, Hryneewycz KT, Bryant EA. Primary cutaneous endometriosis presenting as an umbilical nodule. JAMA Dermatol. 2021;157:1227.
- Kovitwanichkanont T, Joseph S, Yip L. Hidden in plain sight: umbilical melanoma [published online January 28, 2020]. Med J Aust. 2020;212:154-155.e1.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Akinci O, Turker C, Erturk MS, et al. Umbilical dermoid cyst: a rare case. Cerrahpasa Med J. 2020;44:51-53.
- Gabriele R, Conte M, Egidi F, et al. Umbilical metastases: current viewpoint. World J Surg Oncol. 2005;3:13.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph’s nodule at a university teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Leyrat B, Bernadach M, Ginzac A, et al. Sister Mary Joseph nodules: a case report about a rare location of skin metastasis. Case Rep Oncol. 2021;14:664-670.
- Yendluri V, Centeno B, Springett GM. Pancreatic cancer presenting as a Sister Mary Joseph’s nodule: case report and update of the literature. Pancreas. 2007;34:161-164.
- Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol. 2018;8:48.
- Bittar PG, Hryneewycz KT, Bryant EA. Primary cutaneous endometriosis presenting as an umbilical nodule. JAMA Dermatol. 2021;157:1227.
- Kovitwanichkanont T, Joseph S, Yip L. Hidden in plain sight: umbilical melanoma [published online January 28, 2020]. Med J Aust. 2020;212:154-155.e1.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Akinci O, Turker C, Erturk MS, et al. Umbilical dermoid cyst: a rare case. Cerrahpasa Med J. 2020;44:51-53.
A 64-year-old woman with no notable medical history was referred to our dermatology clinic with an intermittent eczematous rash around the eyelids of 3 months’ duration. While performing a total-body skin examination, a firm pink nodule with a smooth surface incidentally was discovered on the umbilicus. The patient was uncertain when the lesion first appeared and denied any associated symptoms including pain and bleeding. Additionally, a lymph node examination revealed right inguinal lymphadenopathy. Upon further questioning, she reported worsening muscle weakness, fatigue, night sweats, and an unintentional weight loss of 10 pounds. A 6-mm punch biopsy of the umbilical lesion was obtained for routine histopathology.
Dupilumab-associated ocular surface disease in patients with AD: Unraveling the link
MONTREAL – , according to a study presented at the annual meeting of the International Society of Atopic Dermatitis.
In a prospective trial of 69 patients with AD starting dupilumab (Dupixent), baseline OSD was found in 91.3%, with about half of these patients reporting no symptoms, said investigator Roselie Achten, MD, from the National Expertise Center for Atopic Dermatitis at University Medical Center Utrecht, the Netherlands. Among these patients, ophthalmologic assessment revealed no OSD in 6 patients and mild OSD in 37 patients, but moderate and severe disease in 20 and 6 patients, respectively, she said, adding that 71% of the group also reported allergic conjunctivitis at baseline.
The patients enrolled in the study who started dupilumab were aged 36-38 years, with Eczema Area and Severity Index (EASI) scores of 14.7-16.5. Baseline ocular surface health was assessed with the Utrecht Ophthalmic Inflammatory and Allergic disease (UTOPIA) score. Tear fluid was collected to analyze biomarkers and dupilumab levels, and impression cytology was performed to collect conjunctival tissue cells for analysis of goblet cells. These measurements were repeated at 4 and 28 weeks after the start of therapy.
Over 28 weeks of treatment, 14.5% of patients experienced worsening of OSD, with worsening disease associated with a decline in the number of goblet cells. In addition, dupilumab treatment was associated with a significant decline in the production of Mucin5AC, suggesting a decline in function of the goblet cells. “Our hypothesis that the blocking effect of dupilumab on [interleukin-] IL-13 might lead to less goblet cells and less mucin production,” she explained.
In a subset of 48 patients, the researchers also detected significantly higher tear fluid dupilumab levels among those patients with more severe OSD, with comparable serum levels.
OSD has been reported in up to 34% of dupilumab-treated patients with AD and is the most frequently reported side-effect of this treatment, noted Dr. Achten. This side effect is not reported by other patients treated with dupilumab for other indications, she added, “suggesting that AD patients may have a predisposition to develop OSD during dupilumab treatment.”
Indeed, as recently noted by Vivian Shi, MD, from the department of dermatology, University of Arkansas for Medical Sciences, Little Rock, and colleagues, “for reasons not well understood, the incidence of conjunctivitis in dupilumab patients with asthma (0%-2.3%), chronic rhinosinusitis with nasal polyps (1.6%), or eosinophilic esophagitis (0%) is low to none; thus, patients with AD may be particularly susceptible.”
Dr. Achten said that dupilumab-treated patients with AD at her center are prescribed topical tacrolimus and ketotifen eye drops if they develop OSD.
Asked for comment, Melinda Gooderham, MD, who moderated the session, was impressed with the study. “I’d heard about the goblet cells, there were little bits of data here and there, but the tear analysis is something I hadn’t seen before. It was a nice series of experiments that pulled everything together,” she told this news organization. Dr. Gooderham, who is assistant professor at Queens University, in Kingston, Ontario, medical director at the SKiN Centre for Dermatology in Peterborough, Ontario, and consultant physician at Peterborough Regional Health Centre, said that she first began noticing dupilumab-related OSD as an early trial investigator for the drug. “When you put some patients on the drug it’s almost like tipping the balance – that little bit of mucin they’re dependent on is now reduced and it makes them more symptomatic,” she said.
Though she prescribes lubricating eye drops as prophylaxis for all her dupilumab-treated patients with AD, she recommends referring any patients who develop OSD to an ophthalmologist who is familiar with this specific side effect. “If they just see a random ophthalmologist who doesn’t know dupilumab, and doesn’t know the story around it, they could get any sort of diagnosis, or even be told to stop the medication altogether.”
The study was sponsored by Sanofi. Dr. Achten disclosed no other conflicts of interest. Dr. Gooderham is an investigator with Sanofi Genzyme for dupilumab.
A version of this article first appeared on Medscape.com.
MONTREAL – , according to a study presented at the annual meeting of the International Society of Atopic Dermatitis.
In a prospective trial of 69 patients with AD starting dupilumab (Dupixent), baseline OSD was found in 91.3%, with about half of these patients reporting no symptoms, said investigator Roselie Achten, MD, from the National Expertise Center for Atopic Dermatitis at University Medical Center Utrecht, the Netherlands. Among these patients, ophthalmologic assessment revealed no OSD in 6 patients and mild OSD in 37 patients, but moderate and severe disease in 20 and 6 patients, respectively, she said, adding that 71% of the group also reported allergic conjunctivitis at baseline.
The patients enrolled in the study who started dupilumab were aged 36-38 years, with Eczema Area and Severity Index (EASI) scores of 14.7-16.5. Baseline ocular surface health was assessed with the Utrecht Ophthalmic Inflammatory and Allergic disease (UTOPIA) score. Tear fluid was collected to analyze biomarkers and dupilumab levels, and impression cytology was performed to collect conjunctival tissue cells for analysis of goblet cells. These measurements were repeated at 4 and 28 weeks after the start of therapy.
Over 28 weeks of treatment, 14.5% of patients experienced worsening of OSD, with worsening disease associated with a decline in the number of goblet cells. In addition, dupilumab treatment was associated with a significant decline in the production of Mucin5AC, suggesting a decline in function of the goblet cells. “Our hypothesis that the blocking effect of dupilumab on [interleukin-] IL-13 might lead to less goblet cells and less mucin production,” she explained.
In a subset of 48 patients, the researchers also detected significantly higher tear fluid dupilumab levels among those patients with more severe OSD, with comparable serum levels.
OSD has been reported in up to 34% of dupilumab-treated patients with AD and is the most frequently reported side-effect of this treatment, noted Dr. Achten. This side effect is not reported by other patients treated with dupilumab for other indications, she added, “suggesting that AD patients may have a predisposition to develop OSD during dupilumab treatment.”
Indeed, as recently noted by Vivian Shi, MD, from the department of dermatology, University of Arkansas for Medical Sciences, Little Rock, and colleagues, “for reasons not well understood, the incidence of conjunctivitis in dupilumab patients with asthma (0%-2.3%), chronic rhinosinusitis with nasal polyps (1.6%), or eosinophilic esophagitis (0%) is low to none; thus, patients with AD may be particularly susceptible.”
Dr. Achten said that dupilumab-treated patients with AD at her center are prescribed topical tacrolimus and ketotifen eye drops if they develop OSD.
Asked for comment, Melinda Gooderham, MD, who moderated the session, was impressed with the study. “I’d heard about the goblet cells, there were little bits of data here and there, but the tear analysis is something I hadn’t seen before. It was a nice series of experiments that pulled everything together,” she told this news organization. Dr. Gooderham, who is assistant professor at Queens University, in Kingston, Ontario, medical director at the SKiN Centre for Dermatology in Peterborough, Ontario, and consultant physician at Peterborough Regional Health Centre, said that she first began noticing dupilumab-related OSD as an early trial investigator for the drug. “When you put some patients on the drug it’s almost like tipping the balance – that little bit of mucin they’re dependent on is now reduced and it makes them more symptomatic,” she said.
Though she prescribes lubricating eye drops as prophylaxis for all her dupilumab-treated patients with AD, she recommends referring any patients who develop OSD to an ophthalmologist who is familiar with this specific side effect. “If they just see a random ophthalmologist who doesn’t know dupilumab, and doesn’t know the story around it, they could get any sort of diagnosis, or even be told to stop the medication altogether.”
The study was sponsored by Sanofi. Dr. Achten disclosed no other conflicts of interest. Dr. Gooderham is an investigator with Sanofi Genzyme for dupilumab.
A version of this article first appeared on Medscape.com.
MONTREAL – , according to a study presented at the annual meeting of the International Society of Atopic Dermatitis.
In a prospective trial of 69 patients with AD starting dupilumab (Dupixent), baseline OSD was found in 91.3%, with about half of these patients reporting no symptoms, said investigator Roselie Achten, MD, from the National Expertise Center for Atopic Dermatitis at University Medical Center Utrecht, the Netherlands. Among these patients, ophthalmologic assessment revealed no OSD in 6 patients and mild OSD in 37 patients, but moderate and severe disease in 20 and 6 patients, respectively, she said, adding that 71% of the group also reported allergic conjunctivitis at baseline.
The patients enrolled in the study who started dupilumab were aged 36-38 years, with Eczema Area and Severity Index (EASI) scores of 14.7-16.5. Baseline ocular surface health was assessed with the Utrecht Ophthalmic Inflammatory and Allergic disease (UTOPIA) score. Tear fluid was collected to analyze biomarkers and dupilumab levels, and impression cytology was performed to collect conjunctival tissue cells for analysis of goblet cells. These measurements were repeated at 4 and 28 weeks after the start of therapy.
Over 28 weeks of treatment, 14.5% of patients experienced worsening of OSD, with worsening disease associated with a decline in the number of goblet cells. In addition, dupilumab treatment was associated with a significant decline in the production of Mucin5AC, suggesting a decline in function of the goblet cells. “Our hypothesis that the blocking effect of dupilumab on [interleukin-] IL-13 might lead to less goblet cells and less mucin production,” she explained.
In a subset of 48 patients, the researchers also detected significantly higher tear fluid dupilumab levels among those patients with more severe OSD, with comparable serum levels.
OSD has been reported in up to 34% of dupilumab-treated patients with AD and is the most frequently reported side-effect of this treatment, noted Dr. Achten. This side effect is not reported by other patients treated with dupilumab for other indications, she added, “suggesting that AD patients may have a predisposition to develop OSD during dupilumab treatment.”
Indeed, as recently noted by Vivian Shi, MD, from the department of dermatology, University of Arkansas for Medical Sciences, Little Rock, and colleagues, “for reasons not well understood, the incidence of conjunctivitis in dupilumab patients with asthma (0%-2.3%), chronic rhinosinusitis with nasal polyps (1.6%), or eosinophilic esophagitis (0%) is low to none; thus, patients with AD may be particularly susceptible.”
Dr. Achten said that dupilumab-treated patients with AD at her center are prescribed topical tacrolimus and ketotifen eye drops if they develop OSD.
Asked for comment, Melinda Gooderham, MD, who moderated the session, was impressed with the study. “I’d heard about the goblet cells, there were little bits of data here and there, but the tear analysis is something I hadn’t seen before. It was a nice series of experiments that pulled everything together,” she told this news organization. Dr. Gooderham, who is assistant professor at Queens University, in Kingston, Ontario, medical director at the SKiN Centre for Dermatology in Peterborough, Ontario, and consultant physician at Peterborough Regional Health Centre, said that she first began noticing dupilumab-related OSD as an early trial investigator for the drug. “When you put some patients on the drug it’s almost like tipping the balance – that little bit of mucin they’re dependent on is now reduced and it makes them more symptomatic,” she said.
Though she prescribes lubricating eye drops as prophylaxis for all her dupilumab-treated patients with AD, she recommends referring any patients who develop OSD to an ophthalmologist who is familiar with this specific side effect. “If they just see a random ophthalmologist who doesn’t know dupilumab, and doesn’t know the story around it, they could get any sort of diagnosis, or even be told to stop the medication altogether.”
The study was sponsored by Sanofi. Dr. Achten disclosed no other conflicts of interest. Dr. Gooderham is an investigator with Sanofi Genzyme for dupilumab.
A version of this article first appeared on Medscape.com.
FROM ISAD 2022
Achieving diversity, equity and inclusion: Invite everyone and build a team
What you really don’t want to do, if you want to improve diversity, equity, and inclusion (DEI) at your academic institution, is to recruit diverse people to your program and then have them come and feel not included, said Vivian Asare, MD. “That can work against your efforts,” she stated in an oral presentation at the annual meeting of the American College of Chest Physicians (CHEST). Dr. Asare is assistant professor and vice chief of DEI for Yale Pulmonary, Critical Care, and Sleep Medicine, and associate medical director of Yale Centers for Sleep Medicine, New Haven, Conn.
In offering a path to successful DEI, Dr. Asare said: “The first step is to build a team and discuss your mission. Invite everyone to participate and include your leadership because they’re the ones who set the stage, ensure sustainability, and can be a liaison with faculty.” Then a DEI leader should be elected, she added.
The next and very important step is to survey the current institutional climate. That entails speaking directly with the stakeholders (faculty, staff, trainees) and identifying their specific concerns and what they think is lacking. Retreats, serious group discussions, and self-reflecting (asking “what initiatives would be good for us?”), and meeting one-on-one with individuals for a truly personalized approach are among potentially productive strategies for identifying the priorities and DEI-related topics specific to a particular academic sleep program.
Dr. Asare offered up a sample DEI survey (Am J Obstet Gynecol. 2020 Nov;223[5]:715.e1-715.e7), that made direct statements inviting the respondent to check off one of the following responses: Yes, No, Somewhat, Do not know, and Not applicable. Among sample statements:
- Our department is actively committed to issues of diversity, equity, and inclusion.
- Faculty searches in the department regularly attract a diverse pool of highly qualified candidates and/or attract a pool that represents the availability of MDs in this field.
- Our outreach and recruitment processes employ targeted practices for attracting diverse populations.
Dr. Asare said that a survey can be a simple approach for garnering information that can be useful for prioritizing DEI topics of concern and igniting interest in them. Engagement requires regular DEI committee meetings with minutes or a newsletter and with updates and topics brought to faculty meetings.
Key DEI areas of focus
Dr. Asare listed several key DEI areas: Recruitment/retention, mentorship, scholarship, and inclusion and community engagement. Under scholarship, for example, she cited topics for potential inclusion in a DEI curriculum: Unconscious bias and anti-racism training, racism, discrimination and microaggression education (bystander/deescalation training), cultural competency and awareness, workplace civility, and health disparities. “We all know that implicit bias in providers is a reality, unfortunately,” Dr. Asare said. Being aware of these implicit biases is a start, but instruction on how to actively overcome them has to be provided. Tools may include perspective-taking, exploring common identity, and self-reflection.
To create an inclusive environment for all faculty, trainees, and staff may involve establishing a “welcome committee” for new faculty, perhaps with designating a “peer buddy,” creating social events and other opportunities for all opinions and ideas to be heard and valued. Particularly for underserved and disadvantaged patient populations, patient advocacy and community service need to be fostered through support groups and provision of resources.
Summarizing, Dr. Asare reiterated several key elements for a successful DEI program: Build a team and discuss the mission, survey the current climate allowing open communication and dialogue, plan and engage, organize, and form areas of DEI focus. Find out where you are and where you want to be with respect to DEI, she concluded.
Dr. Asare declared that she had no conflicts of interest.
What you really don’t want to do, if you want to improve diversity, equity, and inclusion (DEI) at your academic institution, is to recruit diverse people to your program and then have them come and feel not included, said Vivian Asare, MD. “That can work against your efforts,” she stated in an oral presentation at the annual meeting of the American College of Chest Physicians (CHEST). Dr. Asare is assistant professor and vice chief of DEI for Yale Pulmonary, Critical Care, and Sleep Medicine, and associate medical director of Yale Centers for Sleep Medicine, New Haven, Conn.
In offering a path to successful DEI, Dr. Asare said: “The first step is to build a team and discuss your mission. Invite everyone to participate and include your leadership because they’re the ones who set the stage, ensure sustainability, and can be a liaison with faculty.” Then a DEI leader should be elected, she added.
The next and very important step is to survey the current institutional climate. That entails speaking directly with the stakeholders (faculty, staff, trainees) and identifying their specific concerns and what they think is lacking. Retreats, serious group discussions, and self-reflecting (asking “what initiatives would be good for us?”), and meeting one-on-one with individuals for a truly personalized approach are among potentially productive strategies for identifying the priorities and DEI-related topics specific to a particular academic sleep program.
Dr. Asare offered up a sample DEI survey (Am J Obstet Gynecol. 2020 Nov;223[5]:715.e1-715.e7), that made direct statements inviting the respondent to check off one of the following responses: Yes, No, Somewhat, Do not know, and Not applicable. Among sample statements:
- Our department is actively committed to issues of diversity, equity, and inclusion.
- Faculty searches in the department regularly attract a diverse pool of highly qualified candidates and/or attract a pool that represents the availability of MDs in this field.
- Our outreach and recruitment processes employ targeted practices for attracting diverse populations.
Dr. Asare said that a survey can be a simple approach for garnering information that can be useful for prioritizing DEI topics of concern and igniting interest in them. Engagement requires regular DEI committee meetings with minutes or a newsletter and with updates and topics brought to faculty meetings.
Key DEI areas of focus
Dr. Asare listed several key DEI areas: Recruitment/retention, mentorship, scholarship, and inclusion and community engagement. Under scholarship, for example, she cited topics for potential inclusion in a DEI curriculum: Unconscious bias and anti-racism training, racism, discrimination and microaggression education (bystander/deescalation training), cultural competency and awareness, workplace civility, and health disparities. “We all know that implicit bias in providers is a reality, unfortunately,” Dr. Asare said. Being aware of these implicit biases is a start, but instruction on how to actively overcome them has to be provided. Tools may include perspective-taking, exploring common identity, and self-reflection.
To create an inclusive environment for all faculty, trainees, and staff may involve establishing a “welcome committee” for new faculty, perhaps with designating a “peer buddy,” creating social events and other opportunities for all opinions and ideas to be heard and valued. Particularly for underserved and disadvantaged patient populations, patient advocacy and community service need to be fostered through support groups and provision of resources.
Summarizing, Dr. Asare reiterated several key elements for a successful DEI program: Build a team and discuss the mission, survey the current climate allowing open communication and dialogue, plan and engage, organize, and form areas of DEI focus. Find out where you are and where you want to be with respect to DEI, she concluded.
Dr. Asare declared that she had no conflicts of interest.
What you really don’t want to do, if you want to improve diversity, equity, and inclusion (DEI) at your academic institution, is to recruit diverse people to your program and then have them come and feel not included, said Vivian Asare, MD. “That can work against your efforts,” she stated in an oral presentation at the annual meeting of the American College of Chest Physicians (CHEST). Dr. Asare is assistant professor and vice chief of DEI for Yale Pulmonary, Critical Care, and Sleep Medicine, and associate medical director of Yale Centers for Sleep Medicine, New Haven, Conn.
In offering a path to successful DEI, Dr. Asare said: “The first step is to build a team and discuss your mission. Invite everyone to participate and include your leadership because they’re the ones who set the stage, ensure sustainability, and can be a liaison with faculty.” Then a DEI leader should be elected, she added.
The next and very important step is to survey the current institutional climate. That entails speaking directly with the stakeholders (faculty, staff, trainees) and identifying their specific concerns and what they think is lacking. Retreats, serious group discussions, and self-reflecting (asking “what initiatives would be good for us?”), and meeting one-on-one with individuals for a truly personalized approach are among potentially productive strategies for identifying the priorities and DEI-related topics specific to a particular academic sleep program.
Dr. Asare offered up a sample DEI survey (Am J Obstet Gynecol. 2020 Nov;223[5]:715.e1-715.e7), that made direct statements inviting the respondent to check off one of the following responses: Yes, No, Somewhat, Do not know, and Not applicable. Among sample statements:
- Our department is actively committed to issues of diversity, equity, and inclusion.
- Faculty searches in the department regularly attract a diverse pool of highly qualified candidates and/or attract a pool that represents the availability of MDs in this field.
- Our outreach and recruitment processes employ targeted practices for attracting diverse populations.
Dr. Asare said that a survey can be a simple approach for garnering information that can be useful for prioritizing DEI topics of concern and igniting interest in them. Engagement requires regular DEI committee meetings with minutes or a newsletter and with updates and topics brought to faculty meetings.
Key DEI areas of focus
Dr. Asare listed several key DEI areas: Recruitment/retention, mentorship, scholarship, and inclusion and community engagement. Under scholarship, for example, she cited topics for potential inclusion in a DEI curriculum: Unconscious bias and anti-racism training, racism, discrimination and microaggression education (bystander/deescalation training), cultural competency and awareness, workplace civility, and health disparities. “We all know that implicit bias in providers is a reality, unfortunately,” Dr. Asare said. Being aware of these implicit biases is a start, but instruction on how to actively overcome them has to be provided. Tools may include perspective-taking, exploring common identity, and self-reflection.
To create an inclusive environment for all faculty, trainees, and staff may involve establishing a “welcome committee” for new faculty, perhaps with designating a “peer buddy,” creating social events and other opportunities for all opinions and ideas to be heard and valued. Particularly for underserved and disadvantaged patient populations, patient advocacy and community service need to be fostered through support groups and provision of resources.
Summarizing, Dr. Asare reiterated several key elements for a successful DEI program: Build a team and discuss the mission, survey the current climate allowing open communication and dialogue, plan and engage, organize, and form areas of DEI focus. Find out where you are and where you want to be with respect to DEI, she concluded.
Dr. Asare declared that she had no conflicts of interest.
FROM CHEST 2022
Ready or not, hands-free devices are coming
Denver – When Anne Chapas, MD, was asked to help conduct a clinical trial of a wearable, hands-free device for remodeling of the face and submental area, she responded with a healthy dose of skepticism.
“My first thought was, ‘this is crazy. It looks like a Storm Trooper helmet,’ ” Dr. Chapas, founder and medical director of UnionDerm, New York, said at the annual meeting of the American Society for Dermatologic Surgery. “But it’s the first FDA-cleared device that uses bipolar radiofrequency to target the lower third of the face and the submental area of the face. We wanted to see how it works.”
Its bipolar radiofrequency (RF) component reaches 4 mm in depth and travels from central to outer electrodes. The device features real-time temperature monitoring and the ability to delivery energy at lower temps for longer periods of time compared with hands-on approaches. No cooling is required.
“It is able to treat a large surface area simultaneously to achieve maximal tissue contraction,” Dr. Chapas said. “What we’ve learned in decades of RF technology is that it’s not just about heat. It has to be the right amount of heat for the right amount of time. That’s what’s difficult when we’re doing our own individual treatments. How many pulses do we need? How is that heat dissipating? Are we getting the amount of heat we need? Is the patient in pain? We need to take that data from the individual provider and come up with an automated system. That’s what this device is trying to accomplish.”
In a prospective trial, she and her colleagues enrolled 40 patients between the ages of 36 and 75 years with visible signs of facial aging who were seeking skin tightening treatments at one of three centers in the United States. They underwent three biweekly treatments with the Evoke device to the lower face and submental area where a target temperature of 42°-43° C was maintained for 41 minutes, or about 20 minutes for each site.
For the primary safety endpoint, investigators and blinded evaluators used a 4-point Likert scale before treatment, and 1, 3, and 6 months post-treatment. Follow-up visit satisfaction metrics were the patient’s skin appearance evaluation and overall satisfaction, and the investigator improvement rating based on an analysis of volumetric data from 3D imaging software. Chin and cheek discomfort metrics were assessed at all treatments. The subject satisfaction metrics were measured on an 11-point scale where 0 is most comfortable and 10 is most uncomfortable.
In terms of safety, patients tolerated the treatments well and rated their average discomfort from 0.643 to 1.45 on the 11-point Likert scale. “The subject satisfaction rate was about 80%, which is in line with other devices, such as microfocused ultrasound,” said Dr. Chapas, who is also a clinical instructor of dermatology at the Mount Sinai Medical Center, New York.
“The physicians were a little tougher on their assessments. We felt there was about a 65%-70% success rate after the three treatment timepoints.” One possible reason for the disparity between the patient and physician assessments is that patients “may be more accepting of meager results from a hands-free treatment.”
Expect to see more hands-free devices hit the dermatology market in the coming months and years ahead, Dr. Chapas said. Before clinicians incorporate such systems into their practices, she advises them to review existing evidence for the technology, including published data and asking for demonstrations. “If it’s not efficacious, you’ve just wasted everybody’s time,” she said. “Also, is it practical for your office? Do you have the space for it? What staff training is involved? Is it truly automated?”
She added, “If you have a device that’s hands-free but someone must stay in the room with the patient for an hour, does that really help the flow of your practice? And finally, what do your patients want? Do they want to come back multiple times, or do they prefer one-and-done treatments?”
Other questions to consider, she said, include, who benefits from these treatments. Does it fill an unmet need for patients, and for clinicians? Does it help with operator fatigue? How are more consistent treatments achieved? Can the technology be applied to broad body areas?
“The hands-free revolution has been building,” Dr. Chapas commented. “The next generation of lasers and energy devices are going to be coming into our offices, so we should think carefully about how to incorporate them.”
Dr. Chapas disclosed that she is an investigator for InMode (the manufacturer of Evoke), Cutera, and Galderma, and a speaker for Allergan.
Denver – When Anne Chapas, MD, was asked to help conduct a clinical trial of a wearable, hands-free device for remodeling of the face and submental area, she responded with a healthy dose of skepticism.
“My first thought was, ‘this is crazy. It looks like a Storm Trooper helmet,’ ” Dr. Chapas, founder and medical director of UnionDerm, New York, said at the annual meeting of the American Society for Dermatologic Surgery. “But it’s the first FDA-cleared device that uses bipolar radiofrequency to target the lower third of the face and the submental area of the face. We wanted to see how it works.”
Its bipolar radiofrequency (RF) component reaches 4 mm in depth and travels from central to outer electrodes. The device features real-time temperature monitoring and the ability to delivery energy at lower temps for longer periods of time compared with hands-on approaches. No cooling is required.
“It is able to treat a large surface area simultaneously to achieve maximal tissue contraction,” Dr. Chapas said. “What we’ve learned in decades of RF technology is that it’s not just about heat. It has to be the right amount of heat for the right amount of time. That’s what’s difficult when we’re doing our own individual treatments. How many pulses do we need? How is that heat dissipating? Are we getting the amount of heat we need? Is the patient in pain? We need to take that data from the individual provider and come up with an automated system. That’s what this device is trying to accomplish.”
In a prospective trial, she and her colleagues enrolled 40 patients between the ages of 36 and 75 years with visible signs of facial aging who were seeking skin tightening treatments at one of three centers in the United States. They underwent three biweekly treatments with the Evoke device to the lower face and submental area where a target temperature of 42°-43° C was maintained for 41 minutes, or about 20 minutes for each site.
For the primary safety endpoint, investigators and blinded evaluators used a 4-point Likert scale before treatment, and 1, 3, and 6 months post-treatment. Follow-up visit satisfaction metrics were the patient’s skin appearance evaluation and overall satisfaction, and the investigator improvement rating based on an analysis of volumetric data from 3D imaging software. Chin and cheek discomfort metrics were assessed at all treatments. The subject satisfaction metrics were measured on an 11-point scale where 0 is most comfortable and 10 is most uncomfortable.
In terms of safety, patients tolerated the treatments well and rated their average discomfort from 0.643 to 1.45 on the 11-point Likert scale. “The subject satisfaction rate was about 80%, which is in line with other devices, such as microfocused ultrasound,” said Dr. Chapas, who is also a clinical instructor of dermatology at the Mount Sinai Medical Center, New York.
“The physicians were a little tougher on their assessments. We felt there was about a 65%-70% success rate after the three treatment timepoints.” One possible reason for the disparity between the patient and physician assessments is that patients “may be more accepting of meager results from a hands-free treatment.”
Expect to see more hands-free devices hit the dermatology market in the coming months and years ahead, Dr. Chapas said. Before clinicians incorporate such systems into their practices, she advises them to review existing evidence for the technology, including published data and asking for demonstrations. “If it’s not efficacious, you’ve just wasted everybody’s time,” she said. “Also, is it practical for your office? Do you have the space for it? What staff training is involved? Is it truly automated?”
She added, “If you have a device that’s hands-free but someone must stay in the room with the patient for an hour, does that really help the flow of your practice? And finally, what do your patients want? Do they want to come back multiple times, or do they prefer one-and-done treatments?”
Other questions to consider, she said, include, who benefits from these treatments. Does it fill an unmet need for patients, and for clinicians? Does it help with operator fatigue? How are more consistent treatments achieved? Can the technology be applied to broad body areas?
“The hands-free revolution has been building,” Dr. Chapas commented. “The next generation of lasers and energy devices are going to be coming into our offices, so we should think carefully about how to incorporate them.”
Dr. Chapas disclosed that she is an investigator for InMode (the manufacturer of Evoke), Cutera, and Galderma, and a speaker for Allergan.
Denver – When Anne Chapas, MD, was asked to help conduct a clinical trial of a wearable, hands-free device for remodeling of the face and submental area, she responded with a healthy dose of skepticism.
“My first thought was, ‘this is crazy. It looks like a Storm Trooper helmet,’ ” Dr. Chapas, founder and medical director of UnionDerm, New York, said at the annual meeting of the American Society for Dermatologic Surgery. “But it’s the first FDA-cleared device that uses bipolar radiofrequency to target the lower third of the face and the submental area of the face. We wanted to see how it works.”
Its bipolar radiofrequency (RF) component reaches 4 mm in depth and travels from central to outer electrodes. The device features real-time temperature monitoring and the ability to delivery energy at lower temps for longer periods of time compared with hands-on approaches. No cooling is required.
“It is able to treat a large surface area simultaneously to achieve maximal tissue contraction,” Dr. Chapas said. “What we’ve learned in decades of RF technology is that it’s not just about heat. It has to be the right amount of heat for the right amount of time. That’s what’s difficult when we’re doing our own individual treatments. How many pulses do we need? How is that heat dissipating? Are we getting the amount of heat we need? Is the patient in pain? We need to take that data from the individual provider and come up with an automated system. That’s what this device is trying to accomplish.”
In a prospective trial, she and her colleagues enrolled 40 patients between the ages of 36 and 75 years with visible signs of facial aging who were seeking skin tightening treatments at one of three centers in the United States. They underwent three biweekly treatments with the Evoke device to the lower face and submental area where a target temperature of 42°-43° C was maintained for 41 minutes, or about 20 minutes for each site.
For the primary safety endpoint, investigators and blinded evaluators used a 4-point Likert scale before treatment, and 1, 3, and 6 months post-treatment. Follow-up visit satisfaction metrics were the patient’s skin appearance evaluation and overall satisfaction, and the investigator improvement rating based on an analysis of volumetric data from 3D imaging software. Chin and cheek discomfort metrics were assessed at all treatments. The subject satisfaction metrics were measured on an 11-point scale where 0 is most comfortable and 10 is most uncomfortable.
In terms of safety, patients tolerated the treatments well and rated their average discomfort from 0.643 to 1.45 on the 11-point Likert scale. “The subject satisfaction rate was about 80%, which is in line with other devices, such as microfocused ultrasound,” said Dr. Chapas, who is also a clinical instructor of dermatology at the Mount Sinai Medical Center, New York.
“The physicians were a little tougher on their assessments. We felt there was about a 65%-70% success rate after the three treatment timepoints.” One possible reason for the disparity between the patient and physician assessments is that patients “may be more accepting of meager results from a hands-free treatment.”
Expect to see more hands-free devices hit the dermatology market in the coming months and years ahead, Dr. Chapas said. Before clinicians incorporate such systems into their practices, she advises them to review existing evidence for the technology, including published data and asking for demonstrations. “If it’s not efficacious, you’ve just wasted everybody’s time,” she said. “Also, is it practical for your office? Do you have the space for it? What staff training is involved? Is it truly automated?”
She added, “If you have a device that’s hands-free but someone must stay in the room with the patient for an hour, does that really help the flow of your practice? And finally, what do your patients want? Do they want to come back multiple times, or do they prefer one-and-done treatments?”
Other questions to consider, she said, include, who benefits from these treatments. Does it fill an unmet need for patients, and for clinicians? Does it help with operator fatigue? How are more consistent treatments achieved? Can the technology be applied to broad body areas?
“The hands-free revolution has been building,” Dr. Chapas commented. “The next generation of lasers and energy devices are going to be coming into our offices, so we should think carefully about how to incorporate them.”
Dr. Chapas disclosed that she is an investigator for InMode (the manufacturer of Evoke), Cutera, and Galderma, and a speaker for Allergan.
AT ASDS 2022
Alcohol use disorder and atopic dermatitis: Is there a link?
Key clinical point: Patients with atopic dermatitis (AD) have a 50% higher chance of developing alcohol use disorder (AUD) than people without AD and should be screened when appropriate.
Major finding: Patients with AD had a significantly higher chance of developing AUD (odds ratio 1.50; P < .001) than controls without AD.
Study details: Findings are from the All of Us cohort including 11,752 patients with AD and 47,008 matched controls.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Fan R et al. Alcohol use disorder among adults with atopic dermatitis: A case-control study in the All of Us research program. J Am Acad Dermatol. 2022 (Sep 20). Doi: 10.1016/j.jaad.2022.09.015
Key clinical point: Patients with atopic dermatitis (AD) have a 50% higher chance of developing alcohol use disorder (AUD) than people without AD and should be screened when appropriate.
Major finding: Patients with AD had a significantly higher chance of developing AUD (odds ratio 1.50; P < .001) than controls without AD.
Study details: Findings are from the All of Us cohort including 11,752 patients with AD and 47,008 matched controls.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Fan R et al. Alcohol use disorder among adults with atopic dermatitis: A case-control study in the All of Us research program. J Am Acad Dermatol. 2022 (Sep 20). Doi: 10.1016/j.jaad.2022.09.015
Key clinical point: Patients with atopic dermatitis (AD) have a 50% higher chance of developing alcohol use disorder (AUD) than people without AD and should be screened when appropriate.
Major finding: Patients with AD had a significantly higher chance of developing AUD (odds ratio 1.50; P < .001) than controls without AD.
Study details: Findings are from the All of Us cohort including 11,752 patients with AD and 47,008 matched controls.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Fan R et al. Alcohol use disorder among adults with atopic dermatitis: A case-control study in the All of Us research program. J Am Acad Dermatol. 2022 (Sep 20). Doi: 10.1016/j.jaad.2022.09.015