User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Neuropsychiatric event etiology in lupus helps define predictors, outcomes
Different kinds of neuropsychiatric (NP) events in patients with systemic lupus erythematosus (SLE) have substantial variability in their occurrence, resolution, and recurrence over time, as well as in their predictors, according to new research from a large, prospective, international, inception cohort study.
Because “multiple NP events due to different causes may present concurrently in individual patients, the findings emphasize the importance of recognizing attribution of NP events as a determinant of clinical outcome,” John G. Hanly, MD, of Queen Elizabeth II Health Sciences Centre and Dalhousie University, Halifax, N.S., and colleagues wrote in Arthritis & Rheumatology.
In a previous study of the same group of 1,827 patients with SLE, NP events occurred in about half and approximately one-third of these events were deemed disease related. They also “occurred most frequently around the diagnosis of SLE and had a significant negative impact on health-related quality of life,” the researchers wrote.
Researchers involved with the Systemic Lupus International Collaborating Clinics recruited the 1,827 adults with SLE over an 11-year period during 1999-2011 from a total of 31 sites in Europe, Asia, and North America. The average age of the patients at study enrollment was 35 years, 89% were women, and 49% were White. The mean disease duration was 5.6 months, and 70% of patients were taking corticosteroids at enrollment.
Over an average follow-up period of 7.6 years, 955 patients (52.3%) experienced a single neuropsychiatric event, and 493 (27.0%) experienced two or more events; the total number of unique NP events was 1,910. Most of these unique events (92%) involved the central nervous system, and 8.4% involved the peripheral nervous system.
The researchers used multistate models to attribute NP events to SLE based on factors that included the temporal onset of NP events in relation to SLE diagnosis, concurrent non-SLE factors, and NP events that are common in healthy controls. The four states in the multistate models were no NP events, no current NP event but a history of at least one event, new or ongoing NP events, and death. The results included a multivariate analysis of a model involving 492 observed transitions into new or ongoing NP events.
In the multivariate analysis, factors positively associated with SLE-attributed NP events included male sex (hazard ratio, 1.35; P = .028), concurrent non-SLE NP events excluding headache (HR, 1.83; P < .001), active SLE based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (HR, 1.19; P = .012), and corticosteroid use (HR, 1.59; P = .008). The researchers also found that SLE-attributed NP events were negatively associated with Asian race/ethnicity, postsecondary education, and use of immunosuppressive drugs.
Another multivariate analysis found that non-SLE NP events were positively associated with only concurrent SLE-attributed NP events excluding headache (HR, 2.31; P < .001), but negative associations were seen with non-U.S. African race/ethnicity and Asian race/ethnicity.
The researchers found that SLE-attributed NP events had higher rates of resolution, compared with non-SLE NP events, with the exception of headache, which had similar resolution for both event groups.
“Resolution of SLE events was more likely in patients with Asian race/ethnicity and those with Central/Focal nervous system disease with no effect seen for age at diagnosis,” the researchers noted. “For non-SLE NP events, African race/ethnicity at non-U.S. sites and younger age at diagnosis was associated with a better outcome.”
The study findings were limited by several factors including the predominantly White patient population and the clustering of NP events into limited categories, which may have reduced the identification of more specific associations, the researchers noted. Also, the assessment of NP event outcomes did not include patient perceptions, and the relatively short follow-up period does not allow for assessment of later NP events such as cerebrovascular disease. However, “despite these limitations the current study provides valuable data on the presentation, outcome and predictors of NP disease in SLE patients enrolled in a long-term, international, disease inception cohort,” the researchers concluded.
The study received no outside funding. Dr. Hanly was supported by a grant from the Canadian Institutes of Health Research but had no financial conflicts to disclose. Several coauthors received grant support from various institutions, but not from industry, and had no financial conflicts to disclose.
Different kinds of neuropsychiatric (NP) events in patients with systemic lupus erythematosus (SLE) have substantial variability in their occurrence, resolution, and recurrence over time, as well as in their predictors, according to new research from a large, prospective, international, inception cohort study.
Because “multiple NP events due to different causes may present concurrently in individual patients, the findings emphasize the importance of recognizing attribution of NP events as a determinant of clinical outcome,” John G. Hanly, MD, of Queen Elizabeth II Health Sciences Centre and Dalhousie University, Halifax, N.S., and colleagues wrote in Arthritis & Rheumatology.
In a previous study of the same group of 1,827 patients with SLE, NP events occurred in about half and approximately one-third of these events were deemed disease related. They also “occurred most frequently around the diagnosis of SLE and had a significant negative impact on health-related quality of life,” the researchers wrote.
Researchers involved with the Systemic Lupus International Collaborating Clinics recruited the 1,827 adults with SLE over an 11-year period during 1999-2011 from a total of 31 sites in Europe, Asia, and North America. The average age of the patients at study enrollment was 35 years, 89% were women, and 49% were White. The mean disease duration was 5.6 months, and 70% of patients were taking corticosteroids at enrollment.
Over an average follow-up period of 7.6 years, 955 patients (52.3%) experienced a single neuropsychiatric event, and 493 (27.0%) experienced two or more events; the total number of unique NP events was 1,910. Most of these unique events (92%) involved the central nervous system, and 8.4% involved the peripheral nervous system.
The researchers used multistate models to attribute NP events to SLE based on factors that included the temporal onset of NP events in relation to SLE diagnosis, concurrent non-SLE factors, and NP events that are common in healthy controls. The four states in the multistate models were no NP events, no current NP event but a history of at least one event, new or ongoing NP events, and death. The results included a multivariate analysis of a model involving 492 observed transitions into new or ongoing NP events.
In the multivariate analysis, factors positively associated with SLE-attributed NP events included male sex (hazard ratio, 1.35; P = .028), concurrent non-SLE NP events excluding headache (HR, 1.83; P < .001), active SLE based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (HR, 1.19; P = .012), and corticosteroid use (HR, 1.59; P = .008). The researchers also found that SLE-attributed NP events were negatively associated with Asian race/ethnicity, postsecondary education, and use of immunosuppressive drugs.
Another multivariate analysis found that non-SLE NP events were positively associated with only concurrent SLE-attributed NP events excluding headache (HR, 2.31; P < .001), but negative associations were seen with non-U.S. African race/ethnicity and Asian race/ethnicity.
The researchers found that SLE-attributed NP events had higher rates of resolution, compared with non-SLE NP events, with the exception of headache, which had similar resolution for both event groups.
“Resolution of SLE events was more likely in patients with Asian race/ethnicity and those with Central/Focal nervous system disease with no effect seen for age at diagnosis,” the researchers noted. “For non-SLE NP events, African race/ethnicity at non-U.S. sites and younger age at diagnosis was associated with a better outcome.”
The study findings were limited by several factors including the predominantly White patient population and the clustering of NP events into limited categories, which may have reduced the identification of more specific associations, the researchers noted. Also, the assessment of NP event outcomes did not include patient perceptions, and the relatively short follow-up period does not allow for assessment of later NP events such as cerebrovascular disease. However, “despite these limitations the current study provides valuable data on the presentation, outcome and predictors of NP disease in SLE patients enrolled in a long-term, international, disease inception cohort,” the researchers concluded.
The study received no outside funding. Dr. Hanly was supported by a grant from the Canadian Institutes of Health Research but had no financial conflicts to disclose. Several coauthors received grant support from various institutions, but not from industry, and had no financial conflicts to disclose.
Different kinds of neuropsychiatric (NP) events in patients with systemic lupus erythematosus (SLE) have substantial variability in their occurrence, resolution, and recurrence over time, as well as in their predictors, according to new research from a large, prospective, international, inception cohort study.
Because “multiple NP events due to different causes may present concurrently in individual patients, the findings emphasize the importance of recognizing attribution of NP events as a determinant of clinical outcome,” John G. Hanly, MD, of Queen Elizabeth II Health Sciences Centre and Dalhousie University, Halifax, N.S., and colleagues wrote in Arthritis & Rheumatology.
In a previous study of the same group of 1,827 patients with SLE, NP events occurred in about half and approximately one-third of these events were deemed disease related. They also “occurred most frequently around the diagnosis of SLE and had a significant negative impact on health-related quality of life,” the researchers wrote.
Researchers involved with the Systemic Lupus International Collaborating Clinics recruited the 1,827 adults with SLE over an 11-year period during 1999-2011 from a total of 31 sites in Europe, Asia, and North America. The average age of the patients at study enrollment was 35 years, 89% were women, and 49% were White. The mean disease duration was 5.6 months, and 70% of patients were taking corticosteroids at enrollment.
Over an average follow-up period of 7.6 years, 955 patients (52.3%) experienced a single neuropsychiatric event, and 493 (27.0%) experienced two or more events; the total number of unique NP events was 1,910. Most of these unique events (92%) involved the central nervous system, and 8.4% involved the peripheral nervous system.
The researchers used multistate models to attribute NP events to SLE based on factors that included the temporal onset of NP events in relation to SLE diagnosis, concurrent non-SLE factors, and NP events that are common in healthy controls. The four states in the multistate models were no NP events, no current NP event but a history of at least one event, new or ongoing NP events, and death. The results included a multivariate analysis of a model involving 492 observed transitions into new or ongoing NP events.
In the multivariate analysis, factors positively associated with SLE-attributed NP events included male sex (hazard ratio, 1.35; P = .028), concurrent non-SLE NP events excluding headache (HR, 1.83; P < .001), active SLE based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (HR, 1.19; P = .012), and corticosteroid use (HR, 1.59; P = .008). The researchers also found that SLE-attributed NP events were negatively associated with Asian race/ethnicity, postsecondary education, and use of immunosuppressive drugs.
Another multivariate analysis found that non-SLE NP events were positively associated with only concurrent SLE-attributed NP events excluding headache (HR, 2.31; P < .001), but negative associations were seen with non-U.S. African race/ethnicity and Asian race/ethnicity.
The researchers found that SLE-attributed NP events had higher rates of resolution, compared with non-SLE NP events, with the exception of headache, which had similar resolution for both event groups.
“Resolution of SLE events was more likely in patients with Asian race/ethnicity and those with Central/Focal nervous system disease with no effect seen for age at diagnosis,” the researchers noted. “For non-SLE NP events, African race/ethnicity at non-U.S. sites and younger age at diagnosis was associated with a better outcome.”
The study findings were limited by several factors including the predominantly White patient population and the clustering of NP events into limited categories, which may have reduced the identification of more specific associations, the researchers noted. Also, the assessment of NP event outcomes did not include patient perceptions, and the relatively short follow-up period does not allow for assessment of later NP events such as cerebrovascular disease. However, “despite these limitations the current study provides valuable data on the presentation, outcome and predictors of NP disease in SLE patients enrolled in a long-term, international, disease inception cohort,” the researchers concluded.
The study received no outside funding. Dr. Hanly was supported by a grant from the Canadian Institutes of Health Research but had no financial conflicts to disclose. Several coauthors received grant support from various institutions, but not from industry, and had no financial conflicts to disclose.
FROM ARTHRITIS & RHEUMATOLOGY
Limited English proficiency linked with less health care in U.S.
Jessica Himmelstein, MD, a Harvard research fellow and primary care physician at Cambridge Health Alliance in Cambridge, Mass., led a study of more than 120,000 adults published July 6, 2021. The study population included 17,776 Hispanic adults with limited English proficiency, 14,936 Hispanic adults proficient in English and 87,834 non-Hispanic, English-proficient adults.
Researchers compared several measures of care usage from information in the Agency for Healthcare Research and Quality’s Medical Expenditure Panel Survey from 1998 to 2018.
They found that, in adjusted analyses, total use of care per capita from 2014-2018, measured by health care expenditures, was $1,463 lower (98% confidence interval, $1,030-$1,897), or 35% lower for primary-Spanish speakers than for Hispanic adults who were English proficient and $2,802 lower (98% CI, $2,356-$3,247), or 42% lower versus non-Hispanic adults who were English proficient.
Spanish speakers also had 36% fewer outpatient visits and 48% fewer prescription medications than non-Hispanic adults, and 35% fewer outpatient visits and 37% fewer prescription medications than English-proficient Hispanic adults.
Even when accounting for differences in health, age, sex, income and insurance, adults with language barriers fared worse.
Gaps span all types of care
The services that those with limited English skills are missing are “the types of care people need to lead a healthy life,” from routine visits and medications to urgent or emergency care, Dr. Himmelstein said in an interview.
She said the gaps were greater in outpatient care and in medication use, compared with emergency department visits and inpatient care, but the inequities were present in all the categories she and her coinvestigators studied.
Underlying causes for having less care may include that people who struggle with English may not feel comfortable accessing the health system or may feel unwelcome or discriminated against.
“An undercurrent of biases, including racism, could also be contributing,” she said.
The data show that, despite several federal policy changes aimed at promoting language services in hospitals and clinics, several language-based disparities have not improved over 2 decades.
Some of the changes have included an executive order in 2000 requiring interpreters to be available in federally funded health facilities. In 2010, the Affordable Care Act enhanced the definition of meaningful access to language services and setting standards for qualified interpreters.
Gap widened over 2 decades
The adjusted gap in annual health care expenditures per capita between adults with limited English skills and non-Hispanic, English-proficient adults widened by $1,596 (98% CI, $837-$2,356) between 1999-2000 and 2017-2018, after accounting for inflation.
Dr. Himmelstein said that though this study period predated COVID-19, its findings may help explain the disproportionate burden the pandemic placed on the Hispanic population.
“This is a community that traditionally wasn’t getting access to care and then suddenly something like COVID-19 comes and they were even more devastated,” she noted.
Telehealth, which proved an important way to access care during the pandemic, also added a degree of communication difficulty for those with fewer English skills, she said.
Many of the telehealth changes are here to stay, and it will be important to ask: “Are we ensuring equity in telehealth use for individuals who face language barriers?” Dr. Himmelstein said.
Olga Garcia-Bedoya, MD, an associate professor at University of Illinois at Chicago’s department of medicine and medical director of UIC’s Institute for Minority Health Research, said having access to interpreters with high accuracy is key to narrowing the gaps.
“The literature is very clear that access to professional medical interpreters is associated with decreased health disparities for patients with limited English proficiency,” she said.
More cultural training for clinicians is needed surrounding beliefs about illness and that some care may be declined not because of a person’s limited English proficiency, but because their beliefs may keep them from getting care, Dr. Garcia-Bedoya added. When it comes to getting a flu shot, for example, sometimes belief systems, rather than English proficiency, keep people from accessing care.
What can be done?
Addressing barriers caused by lack of English proficiency will likely take change in policies, including one related reimbursement for medical interpreters, Dr. Himmelstein said.
Currently, only 15 states’ Medicaid programs or Children’s Health Insurance Programs reimburse providers for language services, the paper notes, and neither Medicare nor private insurers routinely pay for those services.
Recruiting bilingual providers and staff at health care facilities and in medical and nursing schools will also be important to narrow the gaps, Dr. Himmelstein said.
Strengthening standards for interpreters also will help. “Currently such standards vary by state or by institution and are not necessarily enforced,” she explained.
It will also be important to make sure patients know that they are entitled by law to care, free of discriminatory practices and to have certain language services including qualified interpreters, Dr. Himmelstein said.
Dr. Garcia-Bedoya said changes need to come from health systems working in combination with clinicians, providing resources so that quality interpreters can be accessed and making sure that equipment supports clear communication in telehealth. Patients’ language preferences should also be noted as soon as they make the appointment.
The findings of the study may have large significance as one in seven people in the United States speak Spanish at home, and 25 million people in the United States have limited English proficiency, the authors noted.
Dr. Himmelstein receives funding support from an Institutional National Research Service Award. Dr. Garcia-Bedoya reports no relevant financial relationships.
Jessica Himmelstein, MD, a Harvard research fellow and primary care physician at Cambridge Health Alliance in Cambridge, Mass., led a study of more than 120,000 adults published July 6, 2021. The study population included 17,776 Hispanic adults with limited English proficiency, 14,936 Hispanic adults proficient in English and 87,834 non-Hispanic, English-proficient adults.
Researchers compared several measures of care usage from information in the Agency for Healthcare Research and Quality’s Medical Expenditure Panel Survey from 1998 to 2018.
They found that, in adjusted analyses, total use of care per capita from 2014-2018, measured by health care expenditures, was $1,463 lower (98% confidence interval, $1,030-$1,897), or 35% lower for primary-Spanish speakers than for Hispanic adults who were English proficient and $2,802 lower (98% CI, $2,356-$3,247), or 42% lower versus non-Hispanic adults who were English proficient.
Spanish speakers also had 36% fewer outpatient visits and 48% fewer prescription medications than non-Hispanic adults, and 35% fewer outpatient visits and 37% fewer prescription medications than English-proficient Hispanic adults.
Even when accounting for differences in health, age, sex, income and insurance, adults with language barriers fared worse.
Gaps span all types of care
The services that those with limited English skills are missing are “the types of care people need to lead a healthy life,” from routine visits and medications to urgent or emergency care, Dr. Himmelstein said in an interview.
She said the gaps were greater in outpatient care and in medication use, compared with emergency department visits and inpatient care, but the inequities were present in all the categories she and her coinvestigators studied.
Underlying causes for having less care may include that people who struggle with English may not feel comfortable accessing the health system or may feel unwelcome or discriminated against.
“An undercurrent of biases, including racism, could also be contributing,” she said.
The data show that, despite several federal policy changes aimed at promoting language services in hospitals and clinics, several language-based disparities have not improved over 2 decades.
Some of the changes have included an executive order in 2000 requiring interpreters to be available in federally funded health facilities. In 2010, the Affordable Care Act enhanced the definition of meaningful access to language services and setting standards for qualified interpreters.
Gap widened over 2 decades
The adjusted gap in annual health care expenditures per capita between adults with limited English skills and non-Hispanic, English-proficient adults widened by $1,596 (98% CI, $837-$2,356) between 1999-2000 and 2017-2018, after accounting for inflation.
Dr. Himmelstein said that though this study period predated COVID-19, its findings may help explain the disproportionate burden the pandemic placed on the Hispanic population.
“This is a community that traditionally wasn’t getting access to care and then suddenly something like COVID-19 comes and they were even more devastated,” she noted.
Telehealth, which proved an important way to access care during the pandemic, also added a degree of communication difficulty for those with fewer English skills, she said.
Many of the telehealth changes are here to stay, and it will be important to ask: “Are we ensuring equity in telehealth use for individuals who face language barriers?” Dr. Himmelstein said.
Olga Garcia-Bedoya, MD, an associate professor at University of Illinois at Chicago’s department of medicine and medical director of UIC’s Institute for Minority Health Research, said having access to interpreters with high accuracy is key to narrowing the gaps.
“The literature is very clear that access to professional medical interpreters is associated with decreased health disparities for patients with limited English proficiency,” she said.
More cultural training for clinicians is needed surrounding beliefs about illness and that some care may be declined not because of a person’s limited English proficiency, but because their beliefs may keep them from getting care, Dr. Garcia-Bedoya added. When it comes to getting a flu shot, for example, sometimes belief systems, rather than English proficiency, keep people from accessing care.
What can be done?
Addressing barriers caused by lack of English proficiency will likely take change in policies, including one related reimbursement for medical interpreters, Dr. Himmelstein said.
Currently, only 15 states’ Medicaid programs or Children’s Health Insurance Programs reimburse providers for language services, the paper notes, and neither Medicare nor private insurers routinely pay for those services.
Recruiting bilingual providers and staff at health care facilities and in medical and nursing schools will also be important to narrow the gaps, Dr. Himmelstein said.
Strengthening standards for interpreters also will help. “Currently such standards vary by state or by institution and are not necessarily enforced,” she explained.
It will also be important to make sure patients know that they are entitled by law to care, free of discriminatory practices and to have certain language services including qualified interpreters, Dr. Himmelstein said.
Dr. Garcia-Bedoya said changes need to come from health systems working in combination with clinicians, providing resources so that quality interpreters can be accessed and making sure that equipment supports clear communication in telehealth. Patients’ language preferences should also be noted as soon as they make the appointment.
The findings of the study may have large significance as one in seven people in the United States speak Spanish at home, and 25 million people in the United States have limited English proficiency, the authors noted.
Dr. Himmelstein receives funding support from an Institutional National Research Service Award. Dr. Garcia-Bedoya reports no relevant financial relationships.
Jessica Himmelstein, MD, a Harvard research fellow and primary care physician at Cambridge Health Alliance in Cambridge, Mass., led a study of more than 120,000 adults published July 6, 2021. The study population included 17,776 Hispanic adults with limited English proficiency, 14,936 Hispanic adults proficient in English and 87,834 non-Hispanic, English-proficient adults.
Researchers compared several measures of care usage from information in the Agency for Healthcare Research and Quality’s Medical Expenditure Panel Survey from 1998 to 2018.
They found that, in adjusted analyses, total use of care per capita from 2014-2018, measured by health care expenditures, was $1,463 lower (98% confidence interval, $1,030-$1,897), or 35% lower for primary-Spanish speakers than for Hispanic adults who were English proficient and $2,802 lower (98% CI, $2,356-$3,247), or 42% lower versus non-Hispanic adults who were English proficient.
Spanish speakers also had 36% fewer outpatient visits and 48% fewer prescription medications than non-Hispanic adults, and 35% fewer outpatient visits and 37% fewer prescription medications than English-proficient Hispanic adults.
Even when accounting for differences in health, age, sex, income and insurance, adults with language barriers fared worse.
Gaps span all types of care
The services that those with limited English skills are missing are “the types of care people need to lead a healthy life,” from routine visits and medications to urgent or emergency care, Dr. Himmelstein said in an interview.
She said the gaps were greater in outpatient care and in medication use, compared with emergency department visits and inpatient care, but the inequities were present in all the categories she and her coinvestigators studied.
Underlying causes for having less care may include that people who struggle with English may not feel comfortable accessing the health system or may feel unwelcome or discriminated against.
“An undercurrent of biases, including racism, could also be contributing,” she said.
The data show that, despite several federal policy changes aimed at promoting language services in hospitals and clinics, several language-based disparities have not improved over 2 decades.
Some of the changes have included an executive order in 2000 requiring interpreters to be available in federally funded health facilities. In 2010, the Affordable Care Act enhanced the definition of meaningful access to language services and setting standards for qualified interpreters.
Gap widened over 2 decades
The adjusted gap in annual health care expenditures per capita between adults with limited English skills and non-Hispanic, English-proficient adults widened by $1,596 (98% CI, $837-$2,356) between 1999-2000 and 2017-2018, after accounting for inflation.
Dr. Himmelstein said that though this study period predated COVID-19, its findings may help explain the disproportionate burden the pandemic placed on the Hispanic population.
“This is a community that traditionally wasn’t getting access to care and then suddenly something like COVID-19 comes and they were even more devastated,” she noted.
Telehealth, which proved an important way to access care during the pandemic, also added a degree of communication difficulty for those with fewer English skills, she said.
Many of the telehealth changes are here to stay, and it will be important to ask: “Are we ensuring equity in telehealth use for individuals who face language barriers?” Dr. Himmelstein said.
Olga Garcia-Bedoya, MD, an associate professor at University of Illinois at Chicago’s department of medicine and medical director of UIC’s Institute for Minority Health Research, said having access to interpreters with high accuracy is key to narrowing the gaps.
“The literature is very clear that access to professional medical interpreters is associated with decreased health disparities for patients with limited English proficiency,” she said.
More cultural training for clinicians is needed surrounding beliefs about illness and that some care may be declined not because of a person’s limited English proficiency, but because their beliefs may keep them from getting care, Dr. Garcia-Bedoya added. When it comes to getting a flu shot, for example, sometimes belief systems, rather than English proficiency, keep people from accessing care.
What can be done?
Addressing barriers caused by lack of English proficiency will likely take change in policies, including one related reimbursement for medical interpreters, Dr. Himmelstein said.
Currently, only 15 states’ Medicaid programs or Children’s Health Insurance Programs reimburse providers for language services, the paper notes, and neither Medicare nor private insurers routinely pay for those services.
Recruiting bilingual providers and staff at health care facilities and in medical and nursing schools will also be important to narrow the gaps, Dr. Himmelstein said.
Strengthening standards for interpreters also will help. “Currently such standards vary by state or by institution and are not necessarily enforced,” she explained.
It will also be important to make sure patients know that they are entitled by law to care, free of discriminatory practices and to have certain language services including qualified interpreters, Dr. Himmelstein said.
Dr. Garcia-Bedoya said changes need to come from health systems working in combination with clinicians, providing resources so that quality interpreters can be accessed and making sure that equipment supports clear communication in telehealth. Patients’ language preferences should also be noted as soon as they make the appointment.
The findings of the study may have large significance as one in seven people in the United States speak Spanish at home, and 25 million people in the United States have limited English proficiency, the authors noted.
Dr. Himmelstein receives funding support from an Institutional National Research Service Award. Dr. Garcia-Bedoya reports no relevant financial relationships.
FROM HEALTH AFFAIRS
From Buns to Braids and Ponytails: Entering a New Era of Female Military Hair-Grooming Standards
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
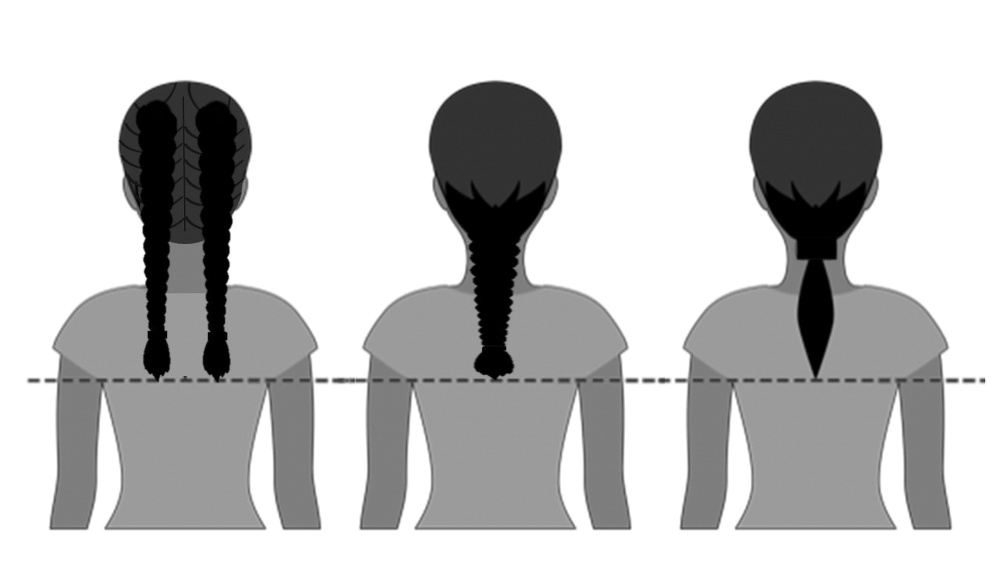
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.


Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
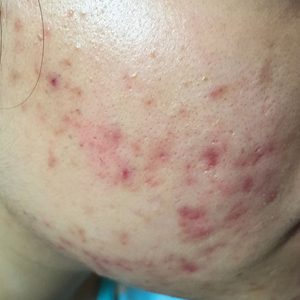
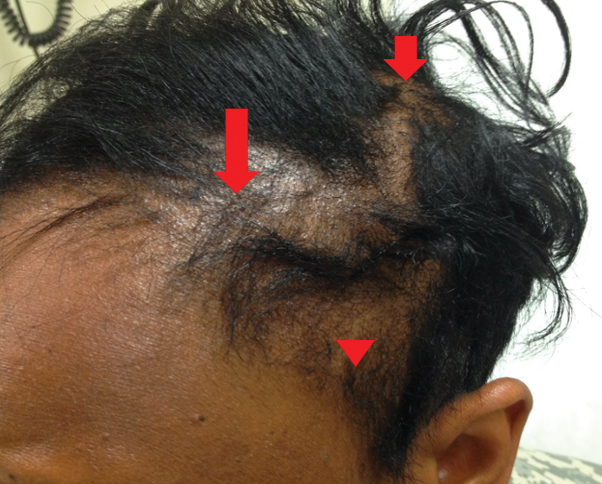
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18
Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.




Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18

Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.




Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18

Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
Practice Points
- Military hair-grooming standards have undergone considerable changes to foster inclusivity and acknowledge racial diversity in hair and skin types.
- The chronic wearing of tight hairstyles can lead to hair breakage, headaches, and traction alopecia.
- A deliberate focus on diversity and inclusivity has started to drive policy change that eliminates racial and gender bias.
Update on Contact Dermatitis and Patch Testing in Patients With Skin of Color
The world is an increasingly diverse place, which has particular relevance for the dermatologist. Skin color plays a significant role in diagnostic approach, as there are important differences in how cutaneous disease presents in patients with skin of color (SOC). Therefore, education about these differences is imperative. In this review, we focus on allergic contact dermatitis (ACD) and patch testing in patients with SOC. We discuss allergens common to this demographic and challenges encountered in patch testing patients with SOC. We also identify key health care disparities in the evaluation and management of ACD in this population.
Has contact allergy in SOC populations been studied in North America?
Over the last 2 decades, there have been only a handful of North American studies that address contact allergy in SOC populations. Patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic from 1988 to 1991 showed that overall allergy frequency was relatively similar (43.0% vs 43.6%). There were notable differences in allergen sensitization. Paraphenylenediamine (PPD), which is used in hair dye, had more positive patch test reactions in Black patients (10.6% vs 4.5%), and both PPD (21.2% vs 4.2%) and imidazolidinyl urea, a formaldehyde-releasing preservative (9.1% vs 2.6%), were more frequently allergenic in Black men compared to White men.1 Patch test results from the North American Contact Dermatitis Group from 1992 to 1998 described similar results, with minimal variation in the prevalence of ACD among 1014 Black and 8610 White patients (47%–49% vs 46%–49%).2 Positive patch test reactions to PPD were higher in Black patients for 2 of 3 test cycles (13.5% vs 5.8% [1994-1996] and 10.3% vs 5.3% [1996-1998]). Positive patch test reactions were higher in White patients for dimethylol dimethyl hydantoin, a formaldehyde-releasing preservative, also for 2 of 3 test cycles (1.8% vs 0% [1992-1994] and 2.8% vs 0.3% [1994-1996]). Finally, positive patch test reactions to thioureas (rubber accelerators) had a mixed picture: 2 test cycles were higher in Black patients (1.9% vs 1.0% [1992-1994] and 1.3% vs 0.7% [1994-1996]), but the third cycle (1996-1998) was lower (0.7% vs 1.4%). Positive patch test reactions to the metal cobalt chloride were higher in Black patients in just 1 test cycle (9.2% vs 6.6% [1992-1994]). The authors suggested that the use of darker hair dyes in the Black community may lead to more sensitization to PPD. They also theorized that this population’s more frequent use of ointment-based skin care products may make them less susceptible to sensitization to preservatives such as formaldehyde, which more commonly are found in water-based products such as creams. They concluded that differences in sensitization patterns likely were driven by cultural practices affecting exposures.2
In 2016, the North American Contact Dermatitis Group reported patch test results in 434 Black and 6634 White patients (1998-2006).3 Again, ACD prevalence was about the same in both groups (45.9% vs 43.6%). However, they reported several allergens with different reaction patterns. Black patients had higher risk ratios (RRs) for 3 rubber accelerators: mercaptobenzothiazole (RR, 2.10), mercapto mix (RR, 2.27), and thiuram mix (RR, 1.44). They also reacted to PPD (RR, 1.56) and the antibiotic bacitracin (RR, 1.34) at higher frequencies than White patients, who more frequently reacted to formaldehyde (RR, 0.58); the formaldehyde-releasing preservatives quaternium-15 (RR, 0.63) and diazolidinyl urea (in petrolatum: RR, 0.44; aqueous: RR, 0.47); the clothing finish ethylene urea melamine formalin resin (RR, 0.45); and the fragrances fragrance mix 1 (RR, 0.65) and balsam of Peru (RR, 0.55).3
Patch testing of 139 African American or Black patients at the Cleveland Clinic (2003-2012) revealed that this population most commonly had positive reactions to nickel (27.5%), fragrance mix (18.1%), bacitracin (13.0%), balsam of Peru (12.3%), and PPD (10.9%). The authors highlighted unique features of physical examination in patients with darker skin types, including lichenification and/or hyperpigmentation in those with ACD and the potential for lack of erythema and/or a papular reaction with patch test readings.4 Recently, data was presented at the American Contact Dermatitis Society Annual Meeting (March 2021) on patterns of ACD in Black and White patch tested patients in Philadelphia (2009-2019).5 Using the North American 80 comprehensive series, the researchers documented statistically significant differences in allergen sensitivity between the 2 groups. Black patients reacted to disperse blue dye (P=.019) and textile dye mix (P=.001) at higher frequencies. There was a nonsignificant trend of more frequent positive reactions to PPD in Black patients (11% vs 6%).5
Notably, all of these studies examined only 1 or 2 racial groups with a focus on Black patients. Some authors commented that this was due to low numbers of Hispanic, Asian and Pacific Islander, and Native American patients in tested populations.2,3,5 With approximately 13% of the US population self-identifying as Black,6 these patients and other minority races typically are underrepresented in large patch test studies. More data on patch test results for these groups is necessary for a complete understanding of patch testing in patients with SOC.
What are the challenges in patch testing SOC populations?
Patch testing in patients with SOC requires additional skills and experience. Darker skin does not reveal erythema as strikingly as lighter skin, making it more difficult to appreciate subtle color changes. Moreover, multiple studies have shown that ACD can have different presentations in Black patients.4,7,8 Lichenification and hyperpigmentation may be early signs of ACD in comparison to bright erythema and vesicles that can be seen in lighter skin types. It also has been reported that scalp ACD can be mistaken for seborrheic dermatitis due to lack of erythema.7 Without a high degree of clinical suspicion, a diagnosis of ACD can be missed in this patient population.
Patch test interpretation also can be challenging in patients with SOC. An early papular or follicular eruption with minimal erythema can signal a positive reaction.4,7 Because of these potentially subtle changes, patch testers should exercise care and attention when reading results for SOC populations. We recommend ample side lighting, palpation for adequate identification of positive reactions, and double-checking for positives that may have been overlooked on the initial review of findings.4,7
What health care disparities impact the evaluation and management of ACD?
There are many factors at play in this dialogue. The challenges we identified in diagnosing ACD in darker skin types are important to consider. Lack of familiarity with these unique features can lead to a delay in diagnosis and ultimately a delay in referral for patch testing. This is where dermatology training can help fill in the gap, but are the majority of programs equipped to do so? Inadequate education and exposure to patients with SOC is an issue for many dermatology residency programs. Surveys of residents and program directors in geographically less diverse regions may not receive adequate education or exposure to patients with SOC.9 Further, there is a lack of representation of SOC images for general dermatologic conditions in textbooks,10,11 which has a profound impact on the dermatologist’s ability to recognize common diseases in darker skin types. A 2019 survey of more than 5000 images from 2 dermatology textbooks showed SOC images comprised 22% to 32% of the total images.11 However, SOC images are overrepresented in textbooks for sexually transmitted infections, constituting 47% to 58% of the images; they made up 28% of images for nonvenereal infections.11 Why is that? In this article, we have shown the prevalence of ACD to be nearly equivalent in Black and White patients, yet a perusal of ACD images in dermatology textbooks will tell a different story. This trend deserves our attention; perhaps it is highlighting patterns of systemic racism seen in medicine. If our primary teaching materials are perpetuating stereotypes, we must consider the impact this can have on our personal implicit biases and the health care disparities that can ensue.
Additional factors impact time to diagnosis of ACD and referral for patch testing. A retrospective study examining distance to a North Carolina patch test referral clinic showed that patients living further from the clinic experienced a longer duration of dermatitis prior to patch test consultation and tended to live in areas with a higher county poverty rate.12 Specifically, a 17.9% increase (P<.001) in the median duration of dermatitis was observed for every 50-mile increase in distance to the patch test clinic. County poverty rate was measured by the percentage of residents living below the poverty threshold; for every 5% increase in county poverty rate, a 16.3% increase (P<.032) in duration of dermatitis was found.12
These data highlight a relationship with which many dermatologists are familiar and underscore a need for dermatologists to practice in areas that are more geographically accessible. The recently increased utilization of telehealth modalities can potentially help to bridge this gap by decreasing delays in diagnosis and providing more affordable options for evaluation by a dermatologist for patients with socioeconomic obstacles.
Final Interpretation
The prevalence of ACD among Black and White patients is similar; however, there are important differences in patch test reaction frequencies that may be related to the diverse exposure patterns for each group. Additionally, patients with SOC may have unique clinical presentations of ACD, such as lichenification and hyperpigmentation. Darker skin types also may require specialized techniques for accurate patch test readings. It is imperative that dermatologists are trained to recognize all of these features. Health care disparities come in many forms and, in this setting, can result in delayed referral for patch testing. Additional studies are needed to further examine these health care disparities and identify potential solutions.
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermat. 2001;12:77-82.
- Deleo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl understanding):S107-S112.
- Deleo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292.
- Yu SH, Khanna U, Taylor JS, et al. Patch testing in the African American population: a 10-year experience. Dermatitis. 2019;30:277-278.
- Garg VS, Zhan, T, Brod B, et al. Patterns of allergic contact dermatitis in African Americans and Caucasians in a major metropolitan area over a ten-year period. Presented at: 32nd American Contact Dermatitis Society Annual Meeting (virtual); March 17-18, 2021.
- United States Census Bureau. QuickFacts—United States. Accessed June 11, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Stallings A, Sood A. Hair-care practices in African American women: potential for allergic contact dermatitis. Semin Cutan Med Surg. 2016;35:207-210.
- Otrofanowei E, Ayanlowo OO, Akinkugbe A, et al. Clinico-etiologic profile of hand dermatitis and patch response of patients at a tertiary hospital in Lagos, Nigeria: results of a prospective observational study. Int J Dermatol. 2018;57:149-155.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264.
The world is an increasingly diverse place, which has particular relevance for the dermatologist. Skin color plays a significant role in diagnostic approach, as there are important differences in how cutaneous disease presents in patients with skin of color (SOC). Therefore, education about these differences is imperative. In this review, we focus on allergic contact dermatitis (ACD) and patch testing in patients with SOC. We discuss allergens common to this demographic and challenges encountered in patch testing patients with SOC. We also identify key health care disparities in the evaluation and management of ACD in this population.
Has contact allergy in SOC populations been studied in North America?
Over the last 2 decades, there have been only a handful of North American studies that address contact allergy in SOC populations. Patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic from 1988 to 1991 showed that overall allergy frequency was relatively similar (43.0% vs 43.6%). There were notable differences in allergen sensitization. Paraphenylenediamine (PPD), which is used in hair dye, had more positive patch test reactions in Black patients (10.6% vs 4.5%), and both PPD (21.2% vs 4.2%) and imidazolidinyl urea, a formaldehyde-releasing preservative (9.1% vs 2.6%), were more frequently allergenic in Black men compared to White men.1 Patch test results from the North American Contact Dermatitis Group from 1992 to 1998 described similar results, with minimal variation in the prevalence of ACD among 1014 Black and 8610 White patients (47%–49% vs 46%–49%).2 Positive patch test reactions to PPD were higher in Black patients for 2 of 3 test cycles (13.5% vs 5.8% [1994-1996] and 10.3% vs 5.3% [1996-1998]). Positive patch test reactions were higher in White patients for dimethylol dimethyl hydantoin, a formaldehyde-releasing preservative, also for 2 of 3 test cycles (1.8% vs 0% [1992-1994] and 2.8% vs 0.3% [1994-1996]). Finally, positive patch test reactions to thioureas (rubber accelerators) had a mixed picture: 2 test cycles were higher in Black patients (1.9% vs 1.0% [1992-1994] and 1.3% vs 0.7% [1994-1996]), but the third cycle (1996-1998) was lower (0.7% vs 1.4%). Positive patch test reactions to the metal cobalt chloride were higher in Black patients in just 1 test cycle (9.2% vs 6.6% [1992-1994]). The authors suggested that the use of darker hair dyes in the Black community may lead to more sensitization to PPD. They also theorized that this population’s more frequent use of ointment-based skin care products may make them less susceptible to sensitization to preservatives such as formaldehyde, which more commonly are found in water-based products such as creams. They concluded that differences in sensitization patterns likely were driven by cultural practices affecting exposures.2
In 2016, the North American Contact Dermatitis Group reported patch test results in 434 Black and 6634 White patients (1998-2006).3 Again, ACD prevalence was about the same in both groups (45.9% vs 43.6%). However, they reported several allergens with different reaction patterns. Black patients had higher risk ratios (RRs) for 3 rubber accelerators: mercaptobenzothiazole (RR, 2.10), mercapto mix (RR, 2.27), and thiuram mix (RR, 1.44). They also reacted to PPD (RR, 1.56) and the antibiotic bacitracin (RR, 1.34) at higher frequencies than White patients, who more frequently reacted to formaldehyde (RR, 0.58); the formaldehyde-releasing preservatives quaternium-15 (RR, 0.63) and diazolidinyl urea (in petrolatum: RR, 0.44; aqueous: RR, 0.47); the clothing finish ethylene urea melamine formalin resin (RR, 0.45); and the fragrances fragrance mix 1 (RR, 0.65) and balsam of Peru (RR, 0.55).3
Patch testing of 139 African American or Black patients at the Cleveland Clinic (2003-2012) revealed that this population most commonly had positive reactions to nickel (27.5%), fragrance mix (18.1%), bacitracin (13.0%), balsam of Peru (12.3%), and PPD (10.9%). The authors highlighted unique features of physical examination in patients with darker skin types, including lichenification and/or hyperpigmentation in those with ACD and the potential for lack of erythema and/or a papular reaction with patch test readings.4 Recently, data was presented at the American Contact Dermatitis Society Annual Meeting (March 2021) on patterns of ACD in Black and White patch tested patients in Philadelphia (2009-2019).5 Using the North American 80 comprehensive series, the researchers documented statistically significant differences in allergen sensitivity between the 2 groups. Black patients reacted to disperse blue dye (P=.019) and textile dye mix (P=.001) at higher frequencies. There was a nonsignificant trend of more frequent positive reactions to PPD in Black patients (11% vs 6%).5
Notably, all of these studies examined only 1 or 2 racial groups with a focus on Black patients. Some authors commented that this was due to low numbers of Hispanic, Asian and Pacific Islander, and Native American patients in tested populations.2,3,5 With approximately 13% of the US population self-identifying as Black,6 these patients and other minority races typically are underrepresented in large patch test studies. More data on patch test results for these groups is necessary for a complete understanding of patch testing in patients with SOC.
What are the challenges in patch testing SOC populations?
Patch testing in patients with SOC requires additional skills and experience. Darker skin does not reveal erythema as strikingly as lighter skin, making it more difficult to appreciate subtle color changes. Moreover, multiple studies have shown that ACD can have different presentations in Black patients.4,7,8 Lichenification and hyperpigmentation may be early signs of ACD in comparison to bright erythema and vesicles that can be seen in lighter skin types. It also has been reported that scalp ACD can be mistaken for seborrheic dermatitis due to lack of erythema.7 Without a high degree of clinical suspicion, a diagnosis of ACD can be missed in this patient population.
Patch test interpretation also can be challenging in patients with SOC. An early papular or follicular eruption with minimal erythema can signal a positive reaction.4,7 Because of these potentially subtle changes, patch testers should exercise care and attention when reading results for SOC populations. We recommend ample side lighting, palpation for adequate identification of positive reactions, and double-checking for positives that may have been overlooked on the initial review of findings.4,7
What health care disparities impact the evaluation and management of ACD?
There are many factors at play in this dialogue. The challenges we identified in diagnosing ACD in darker skin types are important to consider. Lack of familiarity with these unique features can lead to a delay in diagnosis and ultimately a delay in referral for patch testing. This is where dermatology training can help fill in the gap, but are the majority of programs equipped to do so? Inadequate education and exposure to patients with SOC is an issue for many dermatology residency programs. Surveys of residents and program directors in geographically less diverse regions may not receive adequate education or exposure to patients with SOC.9 Further, there is a lack of representation of SOC images for general dermatologic conditions in textbooks,10,11 which has a profound impact on the dermatologist’s ability to recognize common diseases in darker skin types. A 2019 survey of more than 5000 images from 2 dermatology textbooks showed SOC images comprised 22% to 32% of the total images.11 However, SOC images are overrepresented in textbooks for sexually transmitted infections, constituting 47% to 58% of the images; they made up 28% of images for nonvenereal infections.11 Why is that? In this article, we have shown the prevalence of ACD to be nearly equivalent in Black and White patients, yet a perusal of ACD images in dermatology textbooks will tell a different story. This trend deserves our attention; perhaps it is highlighting patterns of systemic racism seen in medicine. If our primary teaching materials are perpetuating stereotypes, we must consider the impact this can have on our personal implicit biases and the health care disparities that can ensue.
Additional factors impact time to diagnosis of ACD and referral for patch testing. A retrospective study examining distance to a North Carolina patch test referral clinic showed that patients living further from the clinic experienced a longer duration of dermatitis prior to patch test consultation and tended to live in areas with a higher county poverty rate.12 Specifically, a 17.9% increase (P<.001) in the median duration of dermatitis was observed for every 50-mile increase in distance to the patch test clinic. County poverty rate was measured by the percentage of residents living below the poverty threshold; for every 5% increase in county poverty rate, a 16.3% increase (P<.032) in duration of dermatitis was found.12
These data highlight a relationship with which many dermatologists are familiar and underscore a need for dermatologists to practice in areas that are more geographically accessible. The recently increased utilization of telehealth modalities can potentially help to bridge this gap by decreasing delays in diagnosis and providing more affordable options for evaluation by a dermatologist for patients with socioeconomic obstacles.
Final Interpretation
The prevalence of ACD among Black and White patients is similar; however, there are important differences in patch test reaction frequencies that may be related to the diverse exposure patterns for each group. Additionally, patients with SOC may have unique clinical presentations of ACD, such as lichenification and hyperpigmentation. Darker skin types also may require specialized techniques for accurate patch test readings. It is imperative that dermatologists are trained to recognize all of these features. Health care disparities come in many forms and, in this setting, can result in delayed referral for patch testing. Additional studies are needed to further examine these health care disparities and identify potential solutions.
The world is an increasingly diverse place, which has particular relevance for the dermatologist. Skin color plays a significant role in diagnostic approach, as there are important differences in how cutaneous disease presents in patients with skin of color (SOC). Therefore, education about these differences is imperative. In this review, we focus on allergic contact dermatitis (ACD) and patch testing in patients with SOC. We discuss allergens common to this demographic and challenges encountered in patch testing patients with SOC. We also identify key health care disparities in the evaluation and management of ACD in this population.
Has contact allergy in SOC populations been studied in North America?
Over the last 2 decades, there have been only a handful of North American studies that address contact allergy in SOC populations. Patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic from 1988 to 1991 showed that overall allergy frequency was relatively similar (43.0% vs 43.6%). There were notable differences in allergen sensitization. Paraphenylenediamine (PPD), which is used in hair dye, had more positive patch test reactions in Black patients (10.6% vs 4.5%), and both PPD (21.2% vs 4.2%) and imidazolidinyl urea, a formaldehyde-releasing preservative (9.1% vs 2.6%), were more frequently allergenic in Black men compared to White men.1 Patch test results from the North American Contact Dermatitis Group from 1992 to 1998 described similar results, with minimal variation in the prevalence of ACD among 1014 Black and 8610 White patients (47%–49% vs 46%–49%).2 Positive patch test reactions to PPD were higher in Black patients for 2 of 3 test cycles (13.5% vs 5.8% [1994-1996] and 10.3% vs 5.3% [1996-1998]). Positive patch test reactions were higher in White patients for dimethylol dimethyl hydantoin, a formaldehyde-releasing preservative, also for 2 of 3 test cycles (1.8% vs 0% [1992-1994] and 2.8% vs 0.3% [1994-1996]). Finally, positive patch test reactions to thioureas (rubber accelerators) had a mixed picture: 2 test cycles were higher in Black patients (1.9% vs 1.0% [1992-1994] and 1.3% vs 0.7% [1994-1996]), but the third cycle (1996-1998) was lower (0.7% vs 1.4%). Positive patch test reactions to the metal cobalt chloride were higher in Black patients in just 1 test cycle (9.2% vs 6.6% [1992-1994]). The authors suggested that the use of darker hair dyes in the Black community may lead to more sensitization to PPD. They also theorized that this population’s more frequent use of ointment-based skin care products may make them less susceptible to sensitization to preservatives such as formaldehyde, which more commonly are found in water-based products such as creams. They concluded that differences in sensitization patterns likely were driven by cultural practices affecting exposures.2
In 2016, the North American Contact Dermatitis Group reported patch test results in 434 Black and 6634 White patients (1998-2006).3 Again, ACD prevalence was about the same in both groups (45.9% vs 43.6%). However, they reported several allergens with different reaction patterns. Black patients had higher risk ratios (RRs) for 3 rubber accelerators: mercaptobenzothiazole (RR, 2.10), mercapto mix (RR, 2.27), and thiuram mix (RR, 1.44). They also reacted to PPD (RR, 1.56) and the antibiotic bacitracin (RR, 1.34) at higher frequencies than White patients, who more frequently reacted to formaldehyde (RR, 0.58); the formaldehyde-releasing preservatives quaternium-15 (RR, 0.63) and diazolidinyl urea (in petrolatum: RR, 0.44; aqueous: RR, 0.47); the clothing finish ethylene urea melamine formalin resin (RR, 0.45); and the fragrances fragrance mix 1 (RR, 0.65) and balsam of Peru (RR, 0.55).3
Patch testing of 139 African American or Black patients at the Cleveland Clinic (2003-2012) revealed that this population most commonly had positive reactions to nickel (27.5%), fragrance mix (18.1%), bacitracin (13.0%), balsam of Peru (12.3%), and PPD (10.9%). The authors highlighted unique features of physical examination in patients with darker skin types, including lichenification and/or hyperpigmentation in those with ACD and the potential for lack of erythema and/or a papular reaction with patch test readings.4 Recently, data was presented at the American Contact Dermatitis Society Annual Meeting (March 2021) on patterns of ACD in Black and White patch tested patients in Philadelphia (2009-2019).5 Using the North American 80 comprehensive series, the researchers documented statistically significant differences in allergen sensitivity between the 2 groups. Black patients reacted to disperse blue dye (P=.019) and textile dye mix (P=.001) at higher frequencies. There was a nonsignificant trend of more frequent positive reactions to PPD in Black patients (11% vs 6%).5
Notably, all of these studies examined only 1 or 2 racial groups with a focus on Black patients. Some authors commented that this was due to low numbers of Hispanic, Asian and Pacific Islander, and Native American patients in tested populations.2,3,5 With approximately 13% of the US population self-identifying as Black,6 these patients and other minority races typically are underrepresented in large patch test studies. More data on patch test results for these groups is necessary for a complete understanding of patch testing in patients with SOC.
What are the challenges in patch testing SOC populations?
Patch testing in patients with SOC requires additional skills and experience. Darker skin does not reveal erythema as strikingly as lighter skin, making it more difficult to appreciate subtle color changes. Moreover, multiple studies have shown that ACD can have different presentations in Black patients.4,7,8 Lichenification and hyperpigmentation may be early signs of ACD in comparison to bright erythema and vesicles that can be seen in lighter skin types. It also has been reported that scalp ACD can be mistaken for seborrheic dermatitis due to lack of erythema.7 Without a high degree of clinical suspicion, a diagnosis of ACD can be missed in this patient population.
Patch test interpretation also can be challenging in patients with SOC. An early papular or follicular eruption with minimal erythema can signal a positive reaction.4,7 Because of these potentially subtle changes, patch testers should exercise care and attention when reading results for SOC populations. We recommend ample side lighting, palpation for adequate identification of positive reactions, and double-checking for positives that may have been overlooked on the initial review of findings.4,7
What health care disparities impact the evaluation and management of ACD?
There are many factors at play in this dialogue. The challenges we identified in diagnosing ACD in darker skin types are important to consider. Lack of familiarity with these unique features can lead to a delay in diagnosis and ultimately a delay in referral for patch testing. This is where dermatology training can help fill in the gap, but are the majority of programs equipped to do so? Inadequate education and exposure to patients with SOC is an issue for many dermatology residency programs. Surveys of residents and program directors in geographically less diverse regions may not receive adequate education or exposure to patients with SOC.9 Further, there is a lack of representation of SOC images for general dermatologic conditions in textbooks,10,11 which has a profound impact on the dermatologist’s ability to recognize common diseases in darker skin types. A 2019 survey of more than 5000 images from 2 dermatology textbooks showed SOC images comprised 22% to 32% of the total images.11 However, SOC images are overrepresented in textbooks for sexually transmitted infections, constituting 47% to 58% of the images; they made up 28% of images for nonvenereal infections.11 Why is that? In this article, we have shown the prevalence of ACD to be nearly equivalent in Black and White patients, yet a perusal of ACD images in dermatology textbooks will tell a different story. This trend deserves our attention; perhaps it is highlighting patterns of systemic racism seen in medicine. If our primary teaching materials are perpetuating stereotypes, we must consider the impact this can have on our personal implicit biases and the health care disparities that can ensue.
Additional factors impact time to diagnosis of ACD and referral for patch testing. A retrospective study examining distance to a North Carolina patch test referral clinic showed that patients living further from the clinic experienced a longer duration of dermatitis prior to patch test consultation and tended to live in areas with a higher county poverty rate.12 Specifically, a 17.9% increase (P<.001) in the median duration of dermatitis was observed for every 50-mile increase in distance to the patch test clinic. County poverty rate was measured by the percentage of residents living below the poverty threshold; for every 5% increase in county poverty rate, a 16.3% increase (P<.032) in duration of dermatitis was found.12
These data highlight a relationship with which many dermatologists are familiar and underscore a need for dermatologists to practice in areas that are more geographically accessible. The recently increased utilization of telehealth modalities can potentially help to bridge this gap by decreasing delays in diagnosis and providing more affordable options for evaluation by a dermatologist for patients with socioeconomic obstacles.
Final Interpretation
The prevalence of ACD among Black and White patients is similar; however, there are important differences in patch test reaction frequencies that may be related to the diverse exposure patterns for each group. Additionally, patients with SOC may have unique clinical presentations of ACD, such as lichenification and hyperpigmentation. Darker skin types also may require specialized techniques for accurate patch test readings. It is imperative that dermatologists are trained to recognize all of these features. Health care disparities come in many forms and, in this setting, can result in delayed referral for patch testing. Additional studies are needed to further examine these health care disparities and identify potential solutions.
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermat. 2001;12:77-82.
- Deleo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl understanding):S107-S112.
- Deleo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292.
- Yu SH, Khanna U, Taylor JS, et al. Patch testing in the African American population: a 10-year experience. Dermatitis. 2019;30:277-278.
- Garg VS, Zhan, T, Brod B, et al. Patterns of allergic contact dermatitis in African Americans and Caucasians in a major metropolitan area over a ten-year period. Presented at: 32nd American Contact Dermatitis Society Annual Meeting (virtual); March 17-18, 2021.
- United States Census Bureau. QuickFacts—United States. Accessed June 11, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Stallings A, Sood A. Hair-care practices in African American women: potential for allergic contact dermatitis. Semin Cutan Med Surg. 2016;35:207-210.
- Otrofanowei E, Ayanlowo OO, Akinkugbe A, et al. Clinico-etiologic profile of hand dermatitis and patch response of patients at a tertiary hospital in Lagos, Nigeria: results of a prospective observational study. Int J Dermatol. 2018;57:149-155.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264.
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermat. 2001;12:77-82.
- Deleo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl understanding):S107-S112.
- Deleo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292.
- Yu SH, Khanna U, Taylor JS, et al. Patch testing in the African American population: a 10-year experience. Dermatitis. 2019;30:277-278.
- Garg VS, Zhan, T, Brod B, et al. Patterns of allergic contact dermatitis in African Americans and Caucasians in a major metropolitan area over a ten-year period. Presented at: 32nd American Contact Dermatitis Society Annual Meeting (virtual); March 17-18, 2021.
- United States Census Bureau. QuickFacts—United States. Accessed June 11, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Stallings A, Sood A. Hair-care practices in African American women: potential for allergic contact dermatitis. Semin Cutan Med Surg. 2016;35:207-210.
- Otrofanowei E, Ayanlowo OO, Akinkugbe A, et al. Clinico-etiologic profile of hand dermatitis and patch response of patients at a tertiary hospital in Lagos, Nigeria: results of a prospective observational study. Int J Dermatol. 2018;57:149-155.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264.
Practice Points
- Similar rates of allergic contact dermatitis (ACD) exist between Black and White patients, with some differences in allergen profiles.
- Patch testing in patients with skin of color (SOC) may require side lighting and palpation, as erythema may be absent or minimal.
- Dermatologic training in evaluation and management of patients with SOC and ACD is vital.
- Distance to clinic and county poverty rate may adversely affect timely referral to a contact dermatitis specialist.
Isotretinoin Meets COVID-19: Revisiting a Fragmented Paradigm
We cannot solve our problems with the same thinking we used when we created them.
Albert Einstein
Amidst the myriad of disruptions and corollary solutions budding from the ongoing global COVID-19 pandemic, management of acne with isotretinoin underwent a makeover. Firstly, as with any pharmaceutical prescribed in the last 1 to 2 years, patients asked the compelling question, “Will this prescription put me at higher risk for COVID-19?”, resulting in a complex set of answers from both clinical and basic science perspectives. Further, the practical use of telemedicine for clinical visits and pregnancy test reporting altered the shape of isotretinoin physician-patient communication and follow-up. Finally, the combination of these circumstances spurred us to revisit common quandaries in prescribing this drug: Can we trust what patients tell us when they are taking isotretinoin? Do we need to monitor laboratory values and follow patients on isotretinoin as closely and as frequently as we have in the past? Does the Risk Evaluation and Mitigation Strategy (REMS) program of iPLEDGE hold true utility?
Impact of COVID-19 on Isotretinoin Use
Isotretinoin may have varying influence on the ease of host entry and virulence of COVID-19. Because the majority of patients experience some degree of mucous membrane desiccation on isotretinoin, it originally was postulated that disruption of the nasal mucosa, thereby uncovering the basal epithelial layer where angiotensin-converting enzyme 2 (ACE2) receptors are expressed, could increase the risk for viral invasion, as ACE2 is the host receptor for COVID-19 entry.1,2 On the other hand, a study of 672 medications and their effect on regulation of ACE2 levels stratified isotretinoin in the highest category of ACE2 downregulators, therefore theoretically preventing cellular entry and replication of the virus.3 In conferring with many of my colleagues and reviewing available literature, I found that these data did not summarily deter providers from initiating or continuing isotretinoin during the pandemic, and research is ongoing to particularly earmark isotretinoin as a possible COVID-19 therapy option.4,5 Despite this, and despite the lower risk for COVID-19 in the customary isotretinoin adolescent and young adult age range, an Italian study reported that 14.7% of patients (5/34) prematurely interrupted isotretinoin therapy during lockdown because of fear of COVID-19 infection.6 Data also suggest that college towns (akin to where I practice, rife with isotretinoin-eligible patients) reflected higher COVID-19 infection and death rates, likely due to dense cohabitation and intermittent migration of students and staff to and from campuses and within their communities.7 Approximately 30% of my patients on isotretinoin in the last 18 months reported having COVID-19 at some point during the pandemic, though no data exist to guide us on whether isotretinoin should be discontinued in this scenario; my patients typically continued the drug unless their primary health care team discouraged it, and in those cases, all of them resumed it after COVID-19 symptomatology resolved.
Last spring, the US Department of Health and Human Services and the US Food and Drug Administration announced that health care professionals who prescribe and/or dispense drugs subject to REMS with laboratory testing or imaging requirements should consider whether there are compelling reasons not to complete the required testing/imaging during the current public health emergency and use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of these tests. It also was stressed that prescribers should effectively communicate with their patients regarding these benefits, risks, and altered protocols.8 Further, the iPLEDGE program concurred that telemedicine was an acceptable visit type for both initiating and maintaining isotretinoin, and home pregnancy tests were valid for females of childbearing potential if an accurate testing date and results were communicated by patients to the prescriber in the required reporting windows.9 This allowed dermatologists to foster what was one of our most important roles as outpatient clinicians during the pandemic: to maintain normalcy, continuity, and support for as many patients as possible.
Isotretinoin and Telemedicine
During the pandemic, continuation of isotretinoin therapy proved easier than initiating it, given that patients could access and maintain a clear connection to the online visit platform, display understanding of the REMS mandates (along with a guardian present for a minor), perform a home pregnancy test and report the result followed by the quiz (for females), and collect the prescription in the allotted window. For new patients, the absence of a detailed in-person examination and rapport with the patient (and guardians when applicable) as well as misalignment of the date of iPLEDGE registration with the timing of the pregnancy test results and prescribing window were more onerous using digital or mailed versions of consent forms and photodocumentation of urine pregnancy test results. This tangle of requirements perpetuated missed prescribing windows, increased patient portal and phone messages, resulted in more time on the phone with the iPLEDGE help desk, and intensified angst for clinical staff.
These telemedicine visits also required validation of the patient’s geographic location to verify the billability of the visit and whether the patient was in a secure location to have a US Health Insurance Portability and Accountability Act–compliant conversation as well as the abstract notion that the timing and result of the pregnancy tests for females reflected a true nonpregnant state.10,11 Verification of the pregnancy tests in these situations was approached by either the patient reporting the outcome verbally or displaying the pregnancy test kit result in a video or photograph form for the medical record, all of which leave room for error, doubt, and lower sensitivity than laboratory-based collection. That being said, the increased implementation of telemedicine visits during the pandemic sustained patient access, decreased cost with less laboratory testing and reduced time away from work or school, and resulted in high patient satisfaction with their care.12 Additionally, it allowed providers to continue to more comfortably inch away from frequent in-person serologic cholesterol and hepatic testing during therapy based on mounting data that it is not indicated.13
Accordingly, the complicated concepts of trust, practicality, and sustainability for the safe and effective management of isotretinoin patients re-emerged. For example, prior to COVID-19, we trusted patients who said they were regularly taking their oral contraceptives or were truly practicing abstinence as a form of contraception. During the pandemic, we then added a layer of trust with home pregnancy test reporting. If the patient or guardian signed the isotretinoin consent form and understood the risks of the medication, ideally the physician-patient relationship fostered the optimal goals of honest conversation, adherence to the drug, safety, and clear skin. However, there is yet another trust assay: iPLEDGE, in turn, trusts that we are reporting patient data accurately, provoking us to reiterate questions we asked ourselves before the pandemic. Is the extra provider and staff clerical work and validation necessary, compounded by prior data that iPLEDGE’s capacity to limit pregnancy-related morbidity with isotretinoin has been called into question in the last decade?14 Do males need to be followed every month? Is laboratory monitoring still necessary for all isotretinoin candidates? Will post–COVID-19 data show that during various versions of the lockdown, an increased number of isotretinoin patients developed unmonitored morbidity, including transaminitis, hypertriglyceridemia, and an increase in pregnancies? How long will telemedicine visits for isotretinoin be reimbursable beyond the pandemic? Are there other models to enhance and improve isotretinoin teledermatology and compliance?15
Final Thoughts
Dermatologists’ experience managing high volumes of isotretinoin patients paired with the creativity to maintain meaningful (and truthful) patient connections and decrease administrative burden lie front and center in 2021. Because the COVID-19 pandemic remains ambient with a dearth of data to guide us, I pose the questions above as points for commiseration and catapults for future study, discussion, collaboration, and innovation. Perhaps the neo–COVID-19 world provided us with the spark we needed to metaphorically clean up the dusty isotretinoin tenets that have frayed our time and patience so we can maintain excellent care for this worthy population.
- Hamming I, Timens W, Bulthuis MLC, et al. Tissue disruption of ACE2 protein, the functional receptor for SARS coronavirus. a first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637.
- British Association of Dermatologists. COVID-19—isotretinoin guidance. Published March 26, 2020. Accessed June 21, 2021. https://www.bad.org.uk/shared/get-file.ashx?itemtype=document&id=6661
- Sinha S, Cheng K, Schäffer AA, et al. In vitro and in vivo identification of clinically approved drugs that modify ACE2 expression. Mol Syst Biol. 2020;16:E9628.
- Öǧüt ND, Kutlu Ö, Erbaǧcı E. Oral isotretinoin treatment in patients with acne vulgaris during the COVID-19 pandemic: a retrospective cohort study in a tertiary care hospital [published online April 22, 2021]. J Cosmet Dermatol. doi:10.1111/jocd.14168
- Isotretinoin in treatment of COVID-19. National Library of Medicine website. ClinicalTrials.gov identifier: NCT04361422. Updated September 23, 2020. Accessed June 21, 2021. https://clinicaltrials.gov/ct2/show/NCT04361422
- Donnarumma M, Nocerino M, Lauro W, et al. Isotretinoin in acne treatment during the coronavirus disease 2019 (COVID-19): a retrospective analysis of adherence to therapy and side effects [published online December 22, 2020]. Dermatol Ther. 2021;34:E14677.
- Ivory D, Gebeloff R, Mervosh S. Young people have less COVID-19 risk, but in college towns, deaths rose fast. The New York Times. December 12, 2020. Accessed June 21, 2021. https://www.nytimes.com/2020/12/12/us/covid-colleges-nursing-homes.html
- US Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Published March 22, 2020. Accessed June 21, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
- Haelle T. iPledge allows at-home pregnancy tests during pandemic. Dermatology News. Published April 3, 2020. Accessed June 28, 2021. https://www.mdedge.com/dermatology/article/220186/acne/ipledge-allows-home-pregnancy-tests-during-pandemic
- Bressler MY, Siegel DM, Markowitz O. Virtual dermatology: a COVID-19 update. Cutis. 2020;105:163-164; E2.
- Telemedicine key issues and policy. Federation of State Medical Boards website. Accessed June 28, 2021. https://www.fsmb.org/advocacy/telemedicine
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663.
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Das S, et al. Asynchronous telemedicine for isotretinoin management: a direct care pilot [published online January 21, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.039
We cannot solve our problems with the same thinking we used when we created them.
Albert Einstein
Amidst the myriad of disruptions and corollary solutions budding from the ongoing global COVID-19 pandemic, management of acne with isotretinoin underwent a makeover. Firstly, as with any pharmaceutical prescribed in the last 1 to 2 years, patients asked the compelling question, “Will this prescription put me at higher risk for COVID-19?”, resulting in a complex set of answers from both clinical and basic science perspectives. Further, the practical use of telemedicine for clinical visits and pregnancy test reporting altered the shape of isotretinoin physician-patient communication and follow-up. Finally, the combination of these circumstances spurred us to revisit common quandaries in prescribing this drug: Can we trust what patients tell us when they are taking isotretinoin? Do we need to monitor laboratory values and follow patients on isotretinoin as closely and as frequently as we have in the past? Does the Risk Evaluation and Mitigation Strategy (REMS) program of iPLEDGE hold true utility?
Impact of COVID-19 on Isotretinoin Use
Isotretinoin may have varying influence on the ease of host entry and virulence of COVID-19. Because the majority of patients experience some degree of mucous membrane desiccation on isotretinoin, it originally was postulated that disruption of the nasal mucosa, thereby uncovering the basal epithelial layer where angiotensin-converting enzyme 2 (ACE2) receptors are expressed, could increase the risk for viral invasion, as ACE2 is the host receptor for COVID-19 entry.1,2 On the other hand, a study of 672 medications and their effect on regulation of ACE2 levels stratified isotretinoin in the highest category of ACE2 downregulators, therefore theoretically preventing cellular entry and replication of the virus.3 In conferring with many of my colleagues and reviewing available literature, I found that these data did not summarily deter providers from initiating or continuing isotretinoin during the pandemic, and research is ongoing to particularly earmark isotretinoin as a possible COVID-19 therapy option.4,5 Despite this, and despite the lower risk for COVID-19 in the customary isotretinoin adolescent and young adult age range, an Italian study reported that 14.7% of patients (5/34) prematurely interrupted isotretinoin therapy during lockdown because of fear of COVID-19 infection.6 Data also suggest that college towns (akin to where I practice, rife with isotretinoin-eligible patients) reflected higher COVID-19 infection and death rates, likely due to dense cohabitation and intermittent migration of students and staff to and from campuses and within their communities.7 Approximately 30% of my patients on isotretinoin in the last 18 months reported having COVID-19 at some point during the pandemic, though no data exist to guide us on whether isotretinoin should be discontinued in this scenario; my patients typically continued the drug unless their primary health care team discouraged it, and in those cases, all of them resumed it after COVID-19 symptomatology resolved.
Last spring, the US Department of Health and Human Services and the US Food and Drug Administration announced that health care professionals who prescribe and/or dispense drugs subject to REMS with laboratory testing or imaging requirements should consider whether there are compelling reasons not to complete the required testing/imaging during the current public health emergency and use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of these tests. It also was stressed that prescribers should effectively communicate with their patients regarding these benefits, risks, and altered protocols.8 Further, the iPLEDGE program concurred that telemedicine was an acceptable visit type for both initiating and maintaining isotretinoin, and home pregnancy tests were valid for females of childbearing potential if an accurate testing date and results were communicated by patients to the prescriber in the required reporting windows.9 This allowed dermatologists to foster what was one of our most important roles as outpatient clinicians during the pandemic: to maintain normalcy, continuity, and support for as many patients as possible.
Isotretinoin and Telemedicine
During the pandemic, continuation of isotretinoin therapy proved easier than initiating it, given that patients could access and maintain a clear connection to the online visit platform, display understanding of the REMS mandates (along with a guardian present for a minor), perform a home pregnancy test and report the result followed by the quiz (for females), and collect the prescription in the allotted window. For new patients, the absence of a detailed in-person examination and rapport with the patient (and guardians when applicable) as well as misalignment of the date of iPLEDGE registration with the timing of the pregnancy test results and prescribing window were more onerous using digital or mailed versions of consent forms and photodocumentation of urine pregnancy test results. This tangle of requirements perpetuated missed prescribing windows, increased patient portal and phone messages, resulted in more time on the phone with the iPLEDGE help desk, and intensified angst for clinical staff.
These telemedicine visits also required validation of the patient’s geographic location to verify the billability of the visit and whether the patient was in a secure location to have a US Health Insurance Portability and Accountability Act–compliant conversation as well as the abstract notion that the timing and result of the pregnancy tests for females reflected a true nonpregnant state.10,11 Verification of the pregnancy tests in these situations was approached by either the patient reporting the outcome verbally or displaying the pregnancy test kit result in a video or photograph form for the medical record, all of which leave room for error, doubt, and lower sensitivity than laboratory-based collection. That being said, the increased implementation of telemedicine visits during the pandemic sustained patient access, decreased cost with less laboratory testing and reduced time away from work or school, and resulted in high patient satisfaction with their care.12 Additionally, it allowed providers to continue to more comfortably inch away from frequent in-person serologic cholesterol and hepatic testing during therapy based on mounting data that it is not indicated.13
Accordingly, the complicated concepts of trust, practicality, and sustainability for the safe and effective management of isotretinoin patients re-emerged. For example, prior to COVID-19, we trusted patients who said they were regularly taking their oral contraceptives or were truly practicing abstinence as a form of contraception. During the pandemic, we then added a layer of trust with home pregnancy test reporting. If the patient or guardian signed the isotretinoin consent form and understood the risks of the medication, ideally the physician-patient relationship fostered the optimal goals of honest conversation, adherence to the drug, safety, and clear skin. However, there is yet another trust assay: iPLEDGE, in turn, trusts that we are reporting patient data accurately, provoking us to reiterate questions we asked ourselves before the pandemic. Is the extra provider and staff clerical work and validation necessary, compounded by prior data that iPLEDGE’s capacity to limit pregnancy-related morbidity with isotretinoin has been called into question in the last decade?14 Do males need to be followed every month? Is laboratory monitoring still necessary for all isotretinoin candidates? Will post–COVID-19 data show that during various versions of the lockdown, an increased number of isotretinoin patients developed unmonitored morbidity, including transaminitis, hypertriglyceridemia, and an increase in pregnancies? How long will telemedicine visits for isotretinoin be reimbursable beyond the pandemic? Are there other models to enhance and improve isotretinoin teledermatology and compliance?15
Final Thoughts
Dermatologists’ experience managing high volumes of isotretinoin patients paired with the creativity to maintain meaningful (and truthful) patient connections and decrease administrative burden lie front and center in 2021. Because the COVID-19 pandemic remains ambient with a dearth of data to guide us, I pose the questions above as points for commiseration and catapults for future study, discussion, collaboration, and innovation. Perhaps the neo–COVID-19 world provided us with the spark we needed to metaphorically clean up the dusty isotretinoin tenets that have frayed our time and patience so we can maintain excellent care for this worthy population.
We cannot solve our problems with the same thinking we used when we created them.
Albert Einstein
Amidst the myriad of disruptions and corollary solutions budding from the ongoing global COVID-19 pandemic, management of acne with isotretinoin underwent a makeover. Firstly, as with any pharmaceutical prescribed in the last 1 to 2 years, patients asked the compelling question, “Will this prescription put me at higher risk for COVID-19?”, resulting in a complex set of answers from both clinical and basic science perspectives. Further, the practical use of telemedicine for clinical visits and pregnancy test reporting altered the shape of isotretinoin physician-patient communication and follow-up. Finally, the combination of these circumstances spurred us to revisit common quandaries in prescribing this drug: Can we trust what patients tell us when they are taking isotretinoin? Do we need to monitor laboratory values and follow patients on isotretinoin as closely and as frequently as we have in the past? Does the Risk Evaluation and Mitigation Strategy (REMS) program of iPLEDGE hold true utility?
Impact of COVID-19 on Isotretinoin Use
Isotretinoin may have varying influence on the ease of host entry and virulence of COVID-19. Because the majority of patients experience some degree of mucous membrane desiccation on isotretinoin, it originally was postulated that disruption of the nasal mucosa, thereby uncovering the basal epithelial layer where angiotensin-converting enzyme 2 (ACE2) receptors are expressed, could increase the risk for viral invasion, as ACE2 is the host receptor for COVID-19 entry.1,2 On the other hand, a study of 672 medications and their effect on regulation of ACE2 levels stratified isotretinoin in the highest category of ACE2 downregulators, therefore theoretically preventing cellular entry and replication of the virus.3 In conferring with many of my colleagues and reviewing available literature, I found that these data did not summarily deter providers from initiating or continuing isotretinoin during the pandemic, and research is ongoing to particularly earmark isotretinoin as a possible COVID-19 therapy option.4,5 Despite this, and despite the lower risk for COVID-19 in the customary isotretinoin adolescent and young adult age range, an Italian study reported that 14.7% of patients (5/34) prematurely interrupted isotretinoin therapy during lockdown because of fear of COVID-19 infection.6 Data also suggest that college towns (akin to where I practice, rife with isotretinoin-eligible patients) reflected higher COVID-19 infection and death rates, likely due to dense cohabitation and intermittent migration of students and staff to and from campuses and within their communities.7 Approximately 30% of my patients on isotretinoin in the last 18 months reported having COVID-19 at some point during the pandemic, though no data exist to guide us on whether isotretinoin should be discontinued in this scenario; my patients typically continued the drug unless their primary health care team discouraged it, and in those cases, all of them resumed it after COVID-19 symptomatology resolved.
Last spring, the US Department of Health and Human Services and the US Food and Drug Administration announced that health care professionals who prescribe and/or dispense drugs subject to REMS with laboratory testing or imaging requirements should consider whether there are compelling reasons not to complete the required testing/imaging during the current public health emergency and use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of these tests. It also was stressed that prescribers should effectively communicate with their patients regarding these benefits, risks, and altered protocols.8 Further, the iPLEDGE program concurred that telemedicine was an acceptable visit type for both initiating and maintaining isotretinoin, and home pregnancy tests were valid for females of childbearing potential if an accurate testing date and results were communicated by patients to the prescriber in the required reporting windows.9 This allowed dermatologists to foster what was one of our most important roles as outpatient clinicians during the pandemic: to maintain normalcy, continuity, and support for as many patients as possible.
Isotretinoin and Telemedicine
During the pandemic, continuation of isotretinoin therapy proved easier than initiating it, given that patients could access and maintain a clear connection to the online visit platform, display understanding of the REMS mandates (along with a guardian present for a minor), perform a home pregnancy test and report the result followed by the quiz (for females), and collect the prescription in the allotted window. For new patients, the absence of a detailed in-person examination and rapport with the patient (and guardians when applicable) as well as misalignment of the date of iPLEDGE registration with the timing of the pregnancy test results and prescribing window were more onerous using digital or mailed versions of consent forms and photodocumentation of urine pregnancy test results. This tangle of requirements perpetuated missed prescribing windows, increased patient portal and phone messages, resulted in more time on the phone with the iPLEDGE help desk, and intensified angst for clinical staff.
These telemedicine visits also required validation of the patient’s geographic location to verify the billability of the visit and whether the patient was in a secure location to have a US Health Insurance Portability and Accountability Act–compliant conversation as well as the abstract notion that the timing and result of the pregnancy tests for females reflected a true nonpregnant state.10,11 Verification of the pregnancy tests in these situations was approached by either the patient reporting the outcome verbally or displaying the pregnancy test kit result in a video or photograph form for the medical record, all of which leave room for error, doubt, and lower sensitivity than laboratory-based collection. That being said, the increased implementation of telemedicine visits during the pandemic sustained patient access, decreased cost with less laboratory testing and reduced time away from work or school, and resulted in high patient satisfaction with their care.12 Additionally, it allowed providers to continue to more comfortably inch away from frequent in-person serologic cholesterol and hepatic testing during therapy based on mounting data that it is not indicated.13
Accordingly, the complicated concepts of trust, practicality, and sustainability for the safe and effective management of isotretinoin patients re-emerged. For example, prior to COVID-19, we trusted patients who said they were regularly taking their oral contraceptives or were truly practicing abstinence as a form of contraception. During the pandemic, we then added a layer of trust with home pregnancy test reporting. If the patient or guardian signed the isotretinoin consent form and understood the risks of the medication, ideally the physician-patient relationship fostered the optimal goals of honest conversation, adherence to the drug, safety, and clear skin. However, there is yet another trust assay: iPLEDGE, in turn, trusts that we are reporting patient data accurately, provoking us to reiterate questions we asked ourselves before the pandemic. Is the extra provider and staff clerical work and validation necessary, compounded by prior data that iPLEDGE’s capacity to limit pregnancy-related morbidity with isotretinoin has been called into question in the last decade?14 Do males need to be followed every month? Is laboratory monitoring still necessary for all isotretinoin candidates? Will post–COVID-19 data show that during various versions of the lockdown, an increased number of isotretinoin patients developed unmonitored morbidity, including transaminitis, hypertriglyceridemia, and an increase in pregnancies? How long will telemedicine visits for isotretinoin be reimbursable beyond the pandemic? Are there other models to enhance and improve isotretinoin teledermatology and compliance?15
Final Thoughts
Dermatologists’ experience managing high volumes of isotretinoin patients paired with the creativity to maintain meaningful (and truthful) patient connections and decrease administrative burden lie front and center in 2021. Because the COVID-19 pandemic remains ambient with a dearth of data to guide us, I pose the questions above as points for commiseration and catapults for future study, discussion, collaboration, and innovation. Perhaps the neo–COVID-19 world provided us with the spark we needed to metaphorically clean up the dusty isotretinoin tenets that have frayed our time and patience so we can maintain excellent care for this worthy population.
- Hamming I, Timens W, Bulthuis MLC, et al. Tissue disruption of ACE2 protein, the functional receptor for SARS coronavirus. a first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637.
- British Association of Dermatologists. COVID-19—isotretinoin guidance. Published March 26, 2020. Accessed June 21, 2021. https://www.bad.org.uk/shared/get-file.ashx?itemtype=document&id=6661
- Sinha S, Cheng K, Schäffer AA, et al. In vitro and in vivo identification of clinically approved drugs that modify ACE2 expression. Mol Syst Biol. 2020;16:E9628.
- Öǧüt ND, Kutlu Ö, Erbaǧcı E. Oral isotretinoin treatment in patients with acne vulgaris during the COVID-19 pandemic: a retrospective cohort study in a tertiary care hospital [published online April 22, 2021]. J Cosmet Dermatol. doi:10.1111/jocd.14168
- Isotretinoin in treatment of COVID-19. National Library of Medicine website. ClinicalTrials.gov identifier: NCT04361422. Updated September 23, 2020. Accessed June 21, 2021. https://clinicaltrials.gov/ct2/show/NCT04361422
- Donnarumma M, Nocerino M, Lauro W, et al. Isotretinoin in acne treatment during the coronavirus disease 2019 (COVID-19): a retrospective analysis of adherence to therapy and side effects [published online December 22, 2020]. Dermatol Ther. 2021;34:E14677.
- Ivory D, Gebeloff R, Mervosh S. Young people have less COVID-19 risk, but in college towns, deaths rose fast. The New York Times. December 12, 2020. Accessed June 21, 2021. https://www.nytimes.com/2020/12/12/us/covid-colleges-nursing-homes.html
- US Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Published March 22, 2020. Accessed June 21, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
- Haelle T. iPledge allows at-home pregnancy tests during pandemic. Dermatology News. Published April 3, 2020. Accessed June 28, 2021. https://www.mdedge.com/dermatology/article/220186/acne/ipledge-allows-home-pregnancy-tests-during-pandemic
- Bressler MY, Siegel DM, Markowitz O. Virtual dermatology: a COVID-19 update. Cutis. 2020;105:163-164; E2.
- Telemedicine key issues and policy. Federation of State Medical Boards website. Accessed June 28, 2021. https://www.fsmb.org/advocacy/telemedicine
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663.
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Das S, et al. Asynchronous telemedicine for isotretinoin management: a direct care pilot [published online January 21, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.039
- Hamming I, Timens W, Bulthuis MLC, et al. Tissue disruption of ACE2 protein, the functional receptor for SARS coronavirus. a first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637.
- British Association of Dermatologists. COVID-19—isotretinoin guidance. Published March 26, 2020. Accessed June 21, 2021. https://www.bad.org.uk/shared/get-file.ashx?itemtype=document&id=6661
- Sinha S, Cheng K, Schäffer AA, et al. In vitro and in vivo identification of clinically approved drugs that modify ACE2 expression. Mol Syst Biol. 2020;16:E9628.
- Öǧüt ND, Kutlu Ö, Erbaǧcı E. Oral isotretinoin treatment in patients with acne vulgaris during the COVID-19 pandemic: a retrospective cohort study in a tertiary care hospital [published online April 22, 2021]. J Cosmet Dermatol. doi:10.1111/jocd.14168
- Isotretinoin in treatment of COVID-19. National Library of Medicine website. ClinicalTrials.gov identifier: NCT04361422. Updated September 23, 2020. Accessed June 21, 2021. https://clinicaltrials.gov/ct2/show/NCT04361422
- Donnarumma M, Nocerino M, Lauro W, et al. Isotretinoin in acne treatment during the coronavirus disease 2019 (COVID-19): a retrospective analysis of adherence to therapy and side effects [published online December 22, 2020]. Dermatol Ther. 2021;34:E14677.
- Ivory D, Gebeloff R, Mervosh S. Young people have less COVID-19 risk, but in college towns, deaths rose fast. The New York Times. December 12, 2020. Accessed June 21, 2021. https://www.nytimes.com/2020/12/12/us/covid-colleges-nursing-homes.html
- US Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Published March 22, 2020. Accessed June 21, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
- Haelle T. iPledge allows at-home pregnancy tests during pandemic. Dermatology News. Published April 3, 2020. Accessed June 28, 2021. https://www.mdedge.com/dermatology/article/220186/acne/ipledge-allows-home-pregnancy-tests-during-pandemic
- Bressler MY, Siegel DM, Markowitz O. Virtual dermatology: a COVID-19 update. Cutis. 2020;105:163-164; E2.
- Telemedicine key issues and policy. Federation of State Medical Boards website. Accessed June 28, 2021. https://www.fsmb.org/advocacy/telemedicine
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663.
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Das S, et al. Asynchronous telemedicine for isotretinoin management: a direct care pilot [published online January 21, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.039
Ocular Manifestations of Patients With Cutaneous Rosacea With and Without Demodex Infection
Acne rosacea is a chronic inflammatory disease that may affect the facial skin, eyes, and eyelids.1 It is characterized by transient or persistent flushing, facial erythema, and telangiectases, generally located on the central portion of the face, and may progress to papules and pustules.2,3 At the late stage of the disease, dermal edema or fibroplasia and sebaceous gland hypertrophy may cause phymatous alterations in the skin. In 2004, the National Rosacea Society Expert Committee developed a classification system for rosacea to standardize subtypes and variants that has since been widely accepted and continues to aid in research and epidemiologic studies.4 The committee defined 4 subtypes based on clinical characteristics: erythematotelangiectatic (ETR), papulopustular (PPR), phymatous, and ocular rosacea.2,3
Ocular rosacea may accompany mild, moderate, and severe dermatologic disease or may occur in the absence of diagnostic skin disease.5 Ocular signs include eyelid margin telangiectasia, spade-shaped infiltrates in the cornea, scleritis, and sclerokeratitis. Common symptoms include burning, stinging, light sensitivity, and foreign-body sensation. Ocular signs commonly seen in rosacea are meibomian gland dysfunction characterized by inspissation and inflammation of the meibomian glands (chalazia), conjunctivitis, honey crust and cylindrical collarette accumulation at the base of the eyelashes, irregularity of the eyelid margin architecture, and evaporative tear dysfunction.5,6
The physiopathology of rosacea is still unknown. Potential factors include genetic predisposition, abnormal inflammation, vascular dysfunction, and involvement of several microbial agents, such as commensal Demodex mites. The number of Demodex mites on normal skin flora is less than 5/cm2; however, the increased vascular dilation and capillary permeability associated with rosacea that result from sunlight and heat exposure increase the density of Demodex folliculorum.7 Elevated Demodex mite density has been observed in the lumens of the sebaceous follicles in patients with rosacea. However, because the severity of the clinical manifestations of the disease is not directly associated with the density of D folliculorum, it generally is accepted that D folliculorum is not a pathogenetic but rather an exacerbating factor.8 It has been reported that this species of mite is mostly found on the face and around the eyelashes and scalp of patients and that it can cause ocular surface inflammation.8
Most studies have researched ocular manifestations of rosacea but not ocular involvement in rosacea patients with and without Demodex mite infestation. In our study, we sought to compare the ocular surface, meibomian gland characteristics, and tear film abnormalities among patients with cutaneous rosacea with and without Demodex infestation.
Materials and Methods
We conducted a retrospective study of 60 patients with cutaneous rosacea. This study was approved by the ethics committee of the local hospital (2018/002-003), and all patients provided verbal and written informed consent before participating in the study. The study was carried out according to the guidelines of the Declaration of Helsinki.
Patient Selection and Evaluation
Patients diagnosed with rosacea by a dermatologist within 6 months were included in the study. Diagnosis of the disease was made after a detailed anamnesis and dermatologic examination. Rosacea was diagnosed if patients had an itching sensation, erythema and/or erythema attacks, and papules and pustules, and fulfilled the diagnostic criteria according to the National Rosacea Society. The skin disease was classified according to the subtypes as ETR, PPR, phymatous rosacea, or ocular rosacea.
The standard skin surface biopsy method was used in 60 patients for detecting Demodex density. When more than 5 mites were detected per square centimeter, the result was recorded as positive. Thirty consecutive, newly diagnosed patients with cutaneous acne rosacea with Demodex infestation and 30 consecutive, newly diagnosed sex- and age-matched patients with acne rosacea without Demodex infestation admitted to the dermatology outpatient clinic were included to this study. The patients who did not have any known dermatologic, systemic, or ocular diseases were included in the study. Patients who met any of the following criteria were excluded from the study: prior anti-inflammatory topical and/or systemic treatment for rosacea during the last 3 months, contact lens wear, eyelid surgery, or autoimmune disease requiring treatment.
Microscopic Demodex Examination
Demodex count was determined using a standardized skin surface biopsy, which is a noninvasive method. Every patient gave samples from the cheeks. This biopsy was repeated from the same site. A drop of cyanoacrylate was placed on a clean slide, pressed against a skin lesion, held in place for 1 minute, and removed. The obtained samples were evaluated under a light microscope (Nikon E200) with oil immersion. When more than 5 mites were detected per square centimeter, the result was recorded as positive.
Ophthalmologic Examination
A complete ophthalmologic examination including visual acuity assessment, standardized slit lamp examination, and fundus examination was done for all patients. Ocular rosacea was diagnosed on detection of 1 or more of the following: watery or bloodshot appearance, foreign-body sensation, burning or stinging, dryness, itching, light sensitivity, blurred vision, telangiectases of the conjunctiva and eyelid margin, eyelid lid and periocular erythema, anterior blepharitis, meibomian gland dysfunction, or irregularity of eyelid margins. All patients were screened for the signs and symptoms of ocular rosacea and underwent other ophthalmologic examinations, including tear function tests. Tear functions were evaluated with Schirmer tests without anesthesia and fluorescein tear breakup time (TBUT). Tear film breakup time was assessed after instillation of 2% fluorescein staining under a cobalt blue filter. The time interval between the last complete blink and the appearance of the first dry spot was recorded. The mean of 3 consecutive measurements was obtained. The Schirmer test was performed without topical anesthesia using a standardized filter strip (Bio-Tech Vision Care). The amount of wetting was measured after 5 minutes. Meibomian gland expressibility was assessed by applying digital pressure to the eyelid margin.
Statistical Analysis
Statistical analysis of the study was performed with SPSS Statistics Version 22.0 (SPSS Inc). Continuous variables were reported as mean (SD), and categorical variables were reported as percentages and counts. Descriptive statistics for numerical variables were created. An independent sample t test was used for normally distributed continuous variables. The Kolmogorov-Smirnov test was used to determine normality. The Schirmer test without anesthesia and TBUT values among groups were compared using one-way analysis of variance. The differences were calculated using the multiple comparison Tukey test. P<.05 was considered statistically significant.
Results
Demographic Characteristics of Rosacea Patients
Sixty eyes of 30 newly diagnosed patients with acne rosacea with Demodex infestation and 60 eyes of 30 newly diagnosed patients with acne rosacea without Demodex infestation were enrolled in this study. The mean age (SD) of the 60 patients was 37.63 (10.01) years. The mean TBUT (SD) of the 120 eyes was 6.65 (3.44) seconds, and the mean Schirmer score (SD) was 12.59 (6.71) mm (Table 1).
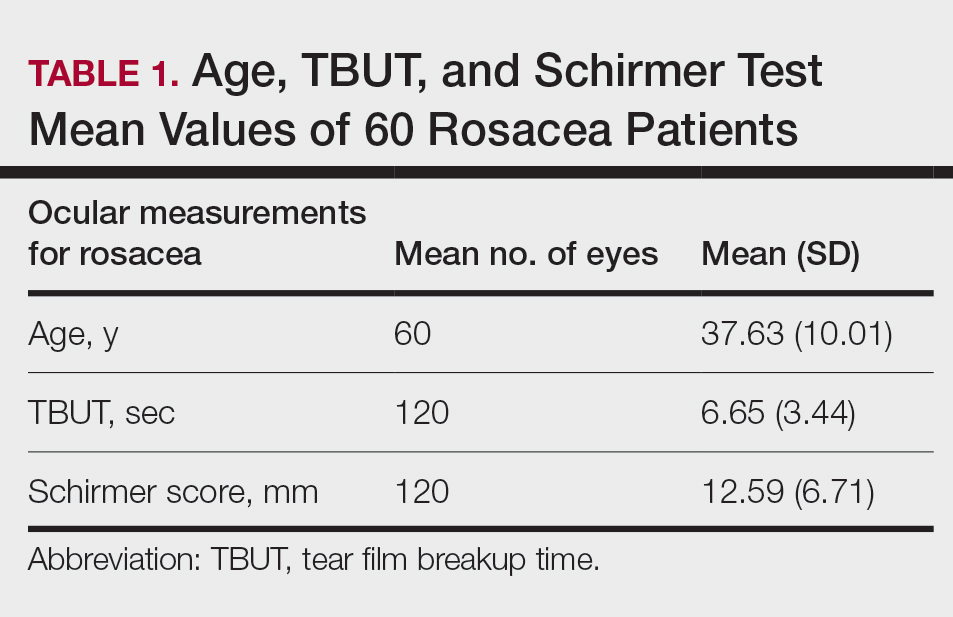
Meibomian Gland Dysfunction vs Subgroup of Rosacea Patients
Thirty-four (57%) patients had blepharitis, and 18 (30%) patients had meibomitis. Thirty-five (58.3%) patients had ETR, 5 (8.3%) patients had phymatous rosacea, and 20 (33.4%) patients had PPR (Table 2). Of the Demodex-negative patients, 73.3% (22/30) had ETR, 20% (6/30) had PPR, and 6.7% (2/30) had phymatous rosacea. Of the Demodex-positive patients, 43.3% (13/30) had ETR, 46.7% (14/30) had PPR, and 10% (3/30) had phymatous rosacea (Table 3). Papulopustular rosacea was found to be significantly associated with Demodex positivity (P=.003); neither ETR nor phymatous rosacea was found to be significantly associated with Demodex infestation (P=.66 and P=.13, respectively)(Table 3).
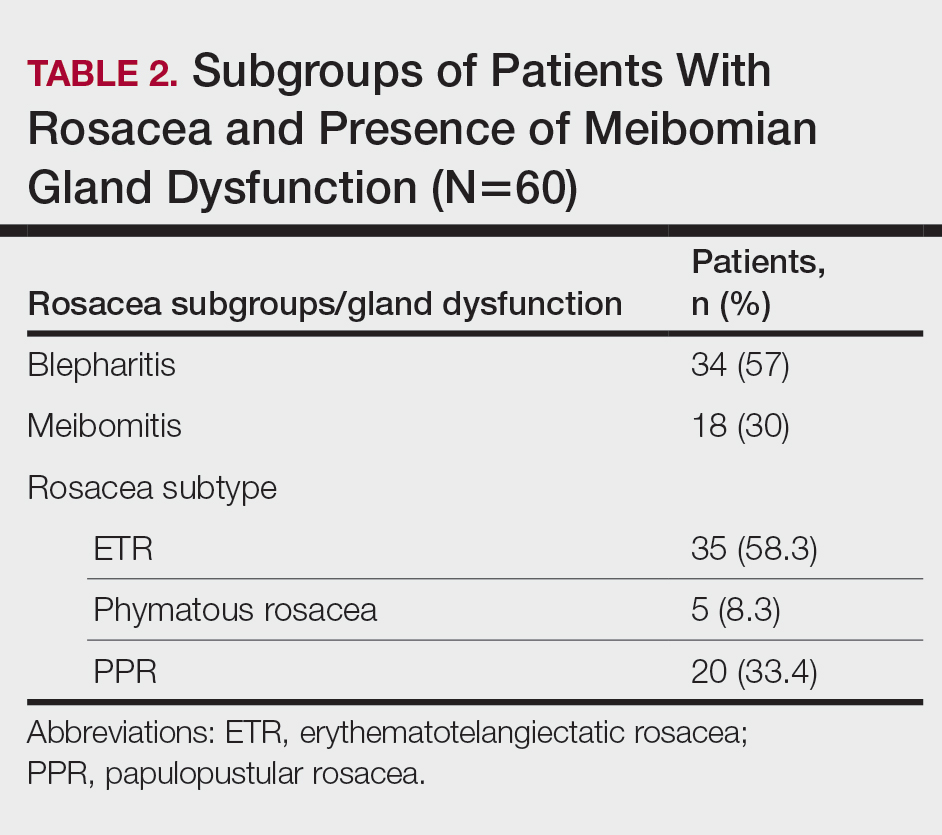
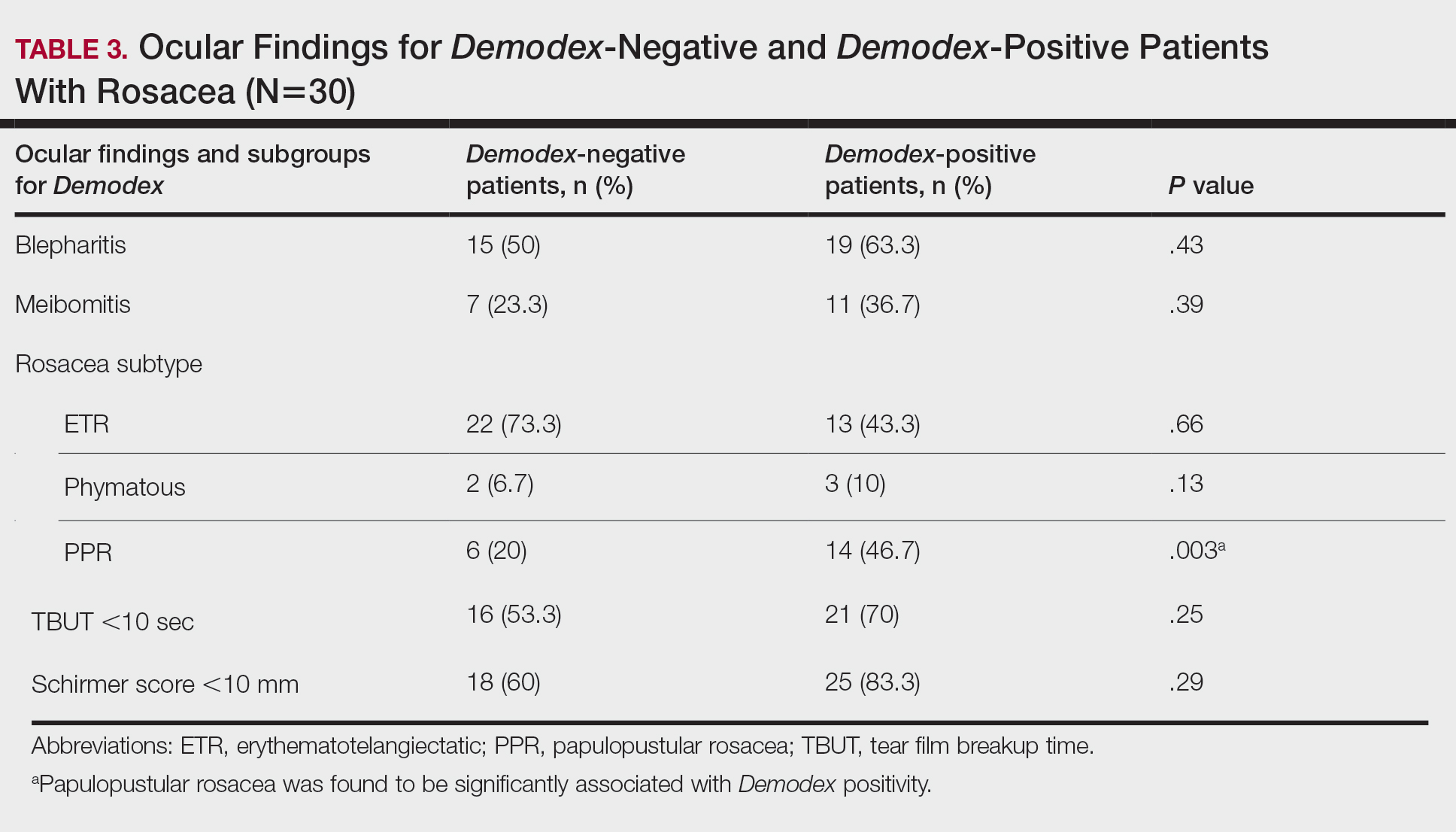
There was no statistically significant difference between the Demodex-negative and Demodex-positive groups for mean age (SD)(37.4 [11.54] years vs 37.87 [8.41] years; P=.85), mean TBUT (SD)(6.73 [3.62] seconds vs 6.57 [3.33] seconds; P=.85), and mean Schirmer score (SD)(13.68 [7.23] mm vs 11.5 [6.08] mm; P=.21)(Table 4).
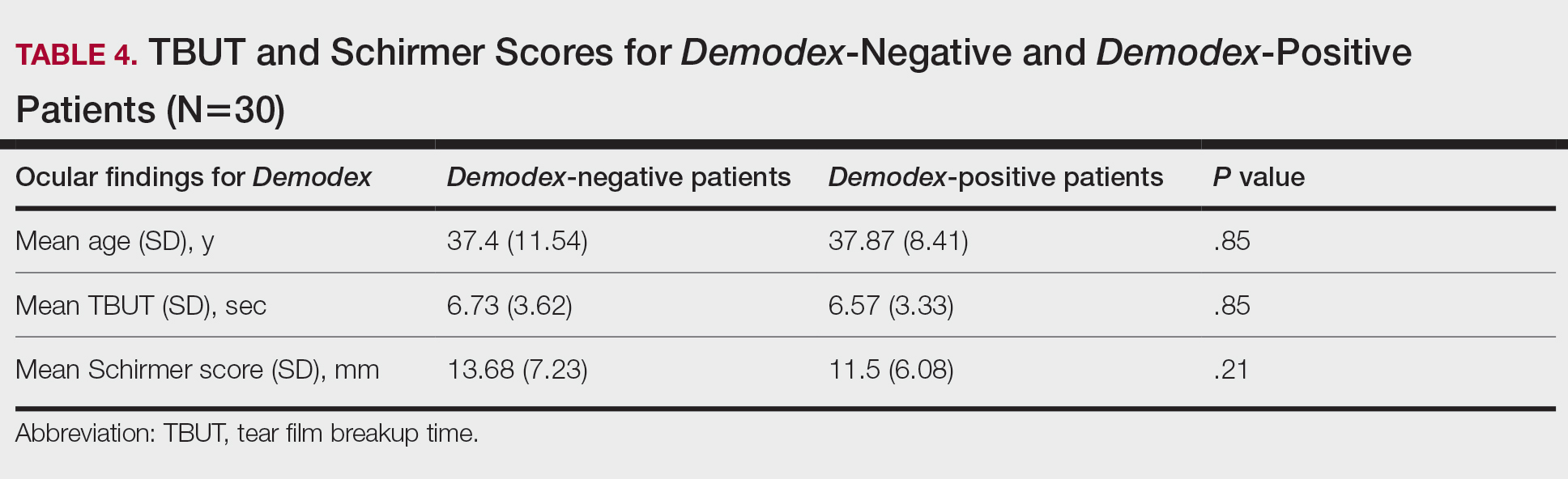
Fifteen (50%) patients (30 eyes) in the Demodex-negative group and 19 (63.3%) patients (38 eyes) in the Demodex-positive group had blepharitis, with no statistically significant difference between the groups (P=.43). Seven (23.3%) patients (14 eyes) in the Demodex-negative group and 11 (36.7%) patients (22 eyes) in the Demodex-positive group had meibomitis, with no statistically significant difference between the groups (P=.39)(Table 3).
Sixteen (53.3%) patients (32 eyes) in the Demodex-negative group and 21 (70%) patients (42 eyes) in the Demodex-positive group had TBUT values less than 10 seconds. Eighteen (60%) patients (36 eyes) in the Demodex-negative group and 25 (83.3%) patients (50 eyes) in the Demodex-positive group had Schirmer scores less than 10 mm (Table 3). The 2 groups were not significantly different in dry eye findings (P=.25 and P=.29, respectively).
Comment
Inflammation in Rosacea
It is known that the density of nonfloral bacteria as well as D folliculorum and Demodex brevis increases in skin affected by rosacea compared to normal skin. Vascular dilation associated with rosacea that results from sunlight and heat causes increased capillary permeability and creates the ideal environment for the proliferation of D folliculorum. Demodex is thought to act as a vector for the activity of certain other microorganisms, particularly Bacillus oleronius, and thus initiates the inflammatory response associated with rosacea.9
One study reported that the inflammation associated with rosacea that was caused by Demodex and other environmental stimuli occurred through toll-like receptor 2 and various cytokines.10 It has been reported that the abnormal function of toll-like receptor 2 in the epidermis leads to the increased production of cathelicidin. Cathelicidin is an antimicrobial peptide with both vasoactive and proinflammatory activity and has been used as a basis to explain the pathogenesis of facial erythema, flushing, and telangiectasia in the context of rosacea.11,12 In addition, it has been reported that the increased secretion of proinflammatory cytokines such as IL-1 and gelatinase B in ocular rosacea leads to tearing film abnormalities that result from increased bacterial flora in the eyelids, which subsequently leads to decreased tear drainage and dry eyes.13 In addition, B oleronius isolated from a D folliculorum mite from patients with PPR produced proteins that induced an inflammatory immune response in 73% (16/22) of patients with rosacea.14
Ocular Findings in Rosacea Patients
In our study, PPR was found to be significantly associated with Demodex positivity compared to ETR and phymatous rosacea (P=.003). However, ocular inflammation findings such as blepharitis and meibomitis were not significantly different between Demodex-positive and Demodex-negative patients. Although the mean Schirmer score of Demodex-positive patients was lower than Demodex-negative patients, this difference was not statistically significant. We evaluated a TBUT of less than 10 seconds and a Schirmer score less than 10 mm as dry eye. Accordingly, the number of patients with dry eye was higher in the Demodex-positive group, but this difference was not statistically significant.
Chronic blepharitis, conjunctival inflammation, and meibomian gland dysfunction are among the most common findings of ocular rosacea.15,16 Patients with ocular rosacea commonly have dry eye and abnormal TBUT and Schirmer scores.17 In our study, we found that the fluorescein TBUT and Schirmer scores were more likely to be abnormal in the Demodex-positive group, but the difference between the 2 groups was not statistically significant.
It has been reported that proinflammatory cytokines due to a weakened immune system in rosacea patients were increased. The weakened immune system was further supported by the increased concentrations of proinflammatory cytokines such as IL-1 and matrix metalloproteinase 9 in these patients’ tears and the improvement of symptoms after the inhibition of these cytokines.11 Luo et al18 reported that Demodex inflammation causes dry eye, particularly with D brevis. Ayyildiz and Sezgin19 reported that Schirmer scores were significantly lower and that the Ocular Surface Disease Index had significantly increased in the Demodex-positive group compared to the Demodex-negative group (P=.001 for both). A Korean study reported that Demodex density was correlated with age, sex, and TBUT results, but there was no significant relationship between Demodex density and Schirmer scores.16
Sobolewska et al20 administered ivermectin cream 1% to 10 patients with cutaneous and ocular rosacea, but only to the forehead, chin, nose, cheeks, and regions close to the eyelids, and observed a significant improvement in blepharitis (P=.004). They stated that ivermectin, as applied only to the face, suppressed the proinflammatory cytokines associated with rosacea and showed anti-inflammatory effects by reducing Demodex mites.20Li et al21 demonstrated a strong correlation between ocular Demodex inflammation and serum reactivity to these bacterial proteins in patients with ocular rosacea, and they found that eyelid margin inflammation and facial rosacea correlated with reactivity to these proteins. These studies suggest a possible role for Demodex infestation and bacterial proteins in the etiology of rosacea.
Gonzalez-Hinojosa et al22 demonstrated that even though eyelash blepharitis was more common in PPR than ETR, there was no statistically significant association between rosacea and Demodex blepharitis. In our study, we found a significant correlation between PPR and Demodex positivity. Also, meibomian gland dysfunction was more common in the Demodex-positive group; however, this result was not statistically significant. One study compared patients with primary demodicosis and patients with rosacea with Demodex-induced blepharitis to healthy controls and found that patients with primary demodicosis and patients with rosacea did not have significantly different ocular findings.23 In contrast, Forton and De Maertelaer24 reported that patients with PPR had significantly more severe ocular manifestations compared with patients with demodicosis (P=.004).
Mizuno et al25 compared the normal (nonrosacea) population with and without Demodex-infested eyelashes and found that the 2 groups were not significantly different for meibomian gland dysfunction, fluorescein TBUT, or ocular surface discomfort.
Varying results have been reported regarding the association between Demodex and blepharitis or ocular surface discomfort with or without rosacea. In our study, we found that Demodex did not affect tear function tests or meibomian gland function in patients with rosacea. We believe this study is important because it demonstrates the effects of Demodex on ocular findings in patients with cutaneous rosacea.
Limitations
Our study has some limitations. The number of patients was relatively small, resulting in few significant differences between the comparison groups. A larger prospective research study is required to assess the prevalence of Demodex mites in the ocular rosacea population along with associated symptoms and findings.
Conclusion
Rosacea is a chronic disease associated with skin and ocular manifestations that range from mild to severe, that progresses in the form of attacks, and that requires long-term follow-up and treatment. Rosacea most often presents as a disease that causes ocular surface inflammation of varying degrees. Demodex infestation may increase cutaneous or ocular inflammation in rosacea. Therefore, every patient diagnosed with rosacea should be given a dermatologic examination to determine Demodex positivity and an ophthalmologic examination to determine ocular manifestations.
- O’Reilly N, Gallagher C, Reddy Katikireddy K, et al. Demodex-associated Bacillus proteins induce an aberrant wound healing response in a corneal epithelial cell line: possible implications for corneal ulcer formation in ocular rosacea. Invest Ophthalmol Vis Sci. 2012;53:3250-3259.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6 suppl 1):S42-S43.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089-3094.
- Fallen RS, Gooderham M. Rosacea: update on management and emerging therapies. Skin Therapy Lett. 2012;17:1-4.
- Erbagcı Z, Ozgoztası O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36:81‐86.
- Forton FMN, De Maertelaer V. Two consecutive standardized skin surface biopsies: an improved sampling method to evaluate Demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venereol. 2017;97:242‐248.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Gold LM, Draelos ZD. New and emerging treatments for rosacea. Am J Clin Dermatol. 2015;16:457-461.
- Two AM, Del Rosso JQ. Kallikrein 5-mediated inflammation in rosacea: clinically relevant correlations with acute and chronic manifestations in rosacea and how individual treatments may provide therapeutic benefit. J Clin Aesthet Dermatol. 2014;7:20-25.
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474-481.
- Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52:74-87.
- Lee SH, Chun YS, Kim JH, et al. The relationship between Demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51:2906-2911.
- Awais M, Anwar MI, Ilfikhar R, et al. Rosacea—the ophthalmic perspective. Cutan Ocul Toxicol. 2015;34:161-166.
- Luo X, Li J, Chen C, et al. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(suppl 1):S9-S14.
- Ayyildiz T, Sezgin FM. The effect of ocular Demodex colonization on Schirmer test and OSDI scores in newly diagnosed dry eye patients. Eye Contact Lens. 2020;46(suppl 1):S39-S41.
- Sobolewska B, Doycheva D, Deuter CM, et al. Efficacy of topical ivermectin for the treatment of cutaneous and ocular rosacea [published online April 7, 2020]. Ocul Immunol Inflamm. doi:10.1080/09273948.2020.1727531
- Li J, O‘Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. 2010;117:870-877.
- Gonzalez‐Hinojosa D, Jaime‐Villalonga A, Aguilar‐Montes G, et al. Demodex and rosacea: is there a relationship? Indian J Ophthalmol. 2018;66:36‐38.
- Sarac G, Cankaya C, Ozcan KN, et al. Increased frequency of Demodex blepharitis in rosacea and facial demodicosis patients. J Cosmet Dermatol. 2020;19:1260-1265.
- Forton FMN, De Maertelaer V. Rosacea and demodicosis: little-known diagnostic signs and symptoms. Acta Derm Venereol. 2019;99:47-52.
- Mizuno M, Kawashima M, Uchino M, et al. Demodex-mite infestation in cilia and its association with ocular surface parameters in Japanese volunteers. Eye Contact Lens. 2020;46:291-296.
Acne rosacea is a chronic inflammatory disease that may affect the facial skin, eyes, and eyelids.1 It is characterized by transient or persistent flushing, facial erythema, and telangiectases, generally located on the central portion of the face, and may progress to papules and pustules.2,3 At the late stage of the disease, dermal edema or fibroplasia and sebaceous gland hypertrophy may cause phymatous alterations in the skin. In 2004, the National Rosacea Society Expert Committee developed a classification system for rosacea to standardize subtypes and variants that has since been widely accepted and continues to aid in research and epidemiologic studies.4 The committee defined 4 subtypes based on clinical characteristics: erythematotelangiectatic (ETR), papulopustular (PPR), phymatous, and ocular rosacea.2,3
Ocular rosacea may accompany mild, moderate, and severe dermatologic disease or may occur in the absence of diagnostic skin disease.5 Ocular signs include eyelid margin telangiectasia, spade-shaped infiltrates in the cornea, scleritis, and sclerokeratitis. Common symptoms include burning, stinging, light sensitivity, and foreign-body sensation. Ocular signs commonly seen in rosacea are meibomian gland dysfunction characterized by inspissation and inflammation of the meibomian glands (chalazia), conjunctivitis, honey crust and cylindrical collarette accumulation at the base of the eyelashes, irregularity of the eyelid margin architecture, and evaporative tear dysfunction.5,6
The physiopathology of rosacea is still unknown. Potential factors include genetic predisposition, abnormal inflammation, vascular dysfunction, and involvement of several microbial agents, such as commensal Demodex mites. The number of Demodex mites on normal skin flora is less than 5/cm2; however, the increased vascular dilation and capillary permeability associated with rosacea that result from sunlight and heat exposure increase the density of Demodex folliculorum.7 Elevated Demodex mite density has been observed in the lumens of the sebaceous follicles in patients with rosacea. However, because the severity of the clinical manifestations of the disease is not directly associated with the density of D folliculorum, it generally is accepted that D folliculorum is not a pathogenetic but rather an exacerbating factor.8 It has been reported that this species of mite is mostly found on the face and around the eyelashes and scalp of patients and that it can cause ocular surface inflammation.8
Most studies have researched ocular manifestations of rosacea but not ocular involvement in rosacea patients with and without Demodex mite infestation. In our study, we sought to compare the ocular surface, meibomian gland characteristics, and tear film abnormalities among patients with cutaneous rosacea with and without Demodex infestation.
Materials and Methods
We conducted a retrospective study of 60 patients with cutaneous rosacea. This study was approved by the ethics committee of the local hospital (2018/002-003), and all patients provided verbal and written informed consent before participating in the study. The study was carried out according to the guidelines of the Declaration of Helsinki.
Patient Selection and Evaluation
Patients diagnosed with rosacea by a dermatologist within 6 months were included in the study. Diagnosis of the disease was made after a detailed anamnesis and dermatologic examination. Rosacea was diagnosed if patients had an itching sensation, erythema and/or erythema attacks, and papules and pustules, and fulfilled the diagnostic criteria according to the National Rosacea Society. The skin disease was classified according to the subtypes as ETR, PPR, phymatous rosacea, or ocular rosacea.
The standard skin surface biopsy method was used in 60 patients for detecting Demodex density. When more than 5 mites were detected per square centimeter, the result was recorded as positive. Thirty consecutive, newly diagnosed patients with cutaneous acne rosacea with Demodex infestation and 30 consecutive, newly diagnosed sex- and age-matched patients with acne rosacea without Demodex infestation admitted to the dermatology outpatient clinic were included to this study. The patients who did not have any known dermatologic, systemic, or ocular diseases were included in the study. Patients who met any of the following criteria were excluded from the study: prior anti-inflammatory topical and/or systemic treatment for rosacea during the last 3 months, contact lens wear, eyelid surgery, or autoimmune disease requiring treatment.
Microscopic Demodex Examination
Demodex count was determined using a standardized skin surface biopsy, which is a noninvasive method. Every patient gave samples from the cheeks. This biopsy was repeated from the same site. A drop of cyanoacrylate was placed on a clean slide, pressed against a skin lesion, held in place for 1 minute, and removed. The obtained samples were evaluated under a light microscope (Nikon E200) with oil immersion. When more than 5 mites were detected per square centimeter, the result was recorded as positive.
Ophthalmologic Examination
A complete ophthalmologic examination including visual acuity assessment, standardized slit lamp examination, and fundus examination was done for all patients. Ocular rosacea was diagnosed on detection of 1 or more of the following: watery or bloodshot appearance, foreign-body sensation, burning or stinging, dryness, itching, light sensitivity, blurred vision, telangiectases of the conjunctiva and eyelid margin, eyelid lid and periocular erythema, anterior blepharitis, meibomian gland dysfunction, or irregularity of eyelid margins. All patients were screened for the signs and symptoms of ocular rosacea and underwent other ophthalmologic examinations, including tear function tests. Tear functions were evaluated with Schirmer tests without anesthesia and fluorescein tear breakup time (TBUT). Tear film breakup time was assessed after instillation of 2% fluorescein staining under a cobalt blue filter. The time interval between the last complete blink and the appearance of the first dry spot was recorded. The mean of 3 consecutive measurements was obtained. The Schirmer test was performed without topical anesthesia using a standardized filter strip (Bio-Tech Vision Care). The amount of wetting was measured after 5 minutes. Meibomian gland expressibility was assessed by applying digital pressure to the eyelid margin.
Statistical Analysis
Statistical analysis of the study was performed with SPSS Statistics Version 22.0 (SPSS Inc). Continuous variables were reported as mean (SD), and categorical variables were reported as percentages and counts. Descriptive statistics for numerical variables were created. An independent sample t test was used for normally distributed continuous variables. The Kolmogorov-Smirnov test was used to determine normality. The Schirmer test without anesthesia and TBUT values among groups were compared using one-way analysis of variance. The differences were calculated using the multiple comparison Tukey test. P<.05 was considered statistically significant.
Results
Demographic Characteristics of Rosacea Patients
Sixty eyes of 30 newly diagnosed patients with acne rosacea with Demodex infestation and 60 eyes of 30 newly diagnosed patients with acne rosacea without Demodex infestation were enrolled in this study. The mean age (SD) of the 60 patients was 37.63 (10.01) years. The mean TBUT (SD) of the 120 eyes was 6.65 (3.44) seconds, and the mean Schirmer score (SD) was 12.59 (6.71) mm (Table 1).

Meibomian Gland Dysfunction vs Subgroup of Rosacea Patients
Thirty-four (57%) patients had blepharitis, and 18 (30%) patients had meibomitis. Thirty-five (58.3%) patients had ETR, 5 (8.3%) patients had phymatous rosacea, and 20 (33.4%) patients had PPR (Table 2). Of the Demodex-negative patients, 73.3% (22/30) had ETR, 20% (6/30) had PPR, and 6.7% (2/30) had phymatous rosacea. Of the Demodex-positive patients, 43.3% (13/30) had ETR, 46.7% (14/30) had PPR, and 10% (3/30) had phymatous rosacea (Table 3). Papulopustular rosacea was found to be significantly associated with Demodex positivity (P=.003); neither ETR nor phymatous rosacea was found to be significantly associated with Demodex infestation (P=.66 and P=.13, respectively)(Table 3).


There was no statistically significant difference between the Demodex-negative and Demodex-positive groups for mean age (SD)(37.4 [11.54] years vs 37.87 [8.41] years; P=.85), mean TBUT (SD)(6.73 [3.62] seconds vs 6.57 [3.33] seconds; P=.85), and mean Schirmer score (SD)(13.68 [7.23] mm vs 11.5 [6.08] mm; P=.21)(Table 4).

Fifteen (50%) patients (30 eyes) in the Demodex-negative group and 19 (63.3%) patients (38 eyes) in the Demodex-positive group had blepharitis, with no statistically significant difference between the groups (P=.43). Seven (23.3%) patients (14 eyes) in the Demodex-negative group and 11 (36.7%) patients (22 eyes) in the Demodex-positive group had meibomitis, with no statistically significant difference between the groups (P=.39)(Table 3).
Sixteen (53.3%) patients (32 eyes) in the Demodex-negative group and 21 (70%) patients (42 eyes) in the Demodex-positive group had TBUT values less than 10 seconds. Eighteen (60%) patients (36 eyes) in the Demodex-negative group and 25 (83.3%) patients (50 eyes) in the Demodex-positive group had Schirmer scores less than 10 mm (Table 3). The 2 groups were not significantly different in dry eye findings (P=.25 and P=.29, respectively).
Comment
Inflammation in Rosacea
It is known that the density of nonfloral bacteria as well as D folliculorum and Demodex brevis increases in skin affected by rosacea compared to normal skin. Vascular dilation associated with rosacea that results from sunlight and heat causes increased capillary permeability and creates the ideal environment for the proliferation of D folliculorum. Demodex is thought to act as a vector for the activity of certain other microorganisms, particularly Bacillus oleronius, and thus initiates the inflammatory response associated with rosacea.9
One study reported that the inflammation associated with rosacea that was caused by Demodex and other environmental stimuli occurred through toll-like receptor 2 and various cytokines.10 It has been reported that the abnormal function of toll-like receptor 2 in the epidermis leads to the increased production of cathelicidin. Cathelicidin is an antimicrobial peptide with both vasoactive and proinflammatory activity and has been used as a basis to explain the pathogenesis of facial erythema, flushing, and telangiectasia in the context of rosacea.11,12 In addition, it has been reported that the increased secretion of proinflammatory cytokines such as IL-1 and gelatinase B in ocular rosacea leads to tearing film abnormalities that result from increased bacterial flora in the eyelids, which subsequently leads to decreased tear drainage and dry eyes.13 In addition, B oleronius isolated from a D folliculorum mite from patients with PPR produced proteins that induced an inflammatory immune response in 73% (16/22) of patients with rosacea.14
Ocular Findings in Rosacea Patients
In our study, PPR was found to be significantly associated with Demodex positivity compared to ETR and phymatous rosacea (P=.003). However, ocular inflammation findings such as blepharitis and meibomitis were not significantly different between Demodex-positive and Demodex-negative patients. Although the mean Schirmer score of Demodex-positive patients was lower than Demodex-negative patients, this difference was not statistically significant. We evaluated a TBUT of less than 10 seconds and a Schirmer score less than 10 mm as dry eye. Accordingly, the number of patients with dry eye was higher in the Demodex-positive group, but this difference was not statistically significant.
Chronic blepharitis, conjunctival inflammation, and meibomian gland dysfunction are among the most common findings of ocular rosacea.15,16 Patients with ocular rosacea commonly have dry eye and abnormal TBUT and Schirmer scores.17 In our study, we found that the fluorescein TBUT and Schirmer scores were more likely to be abnormal in the Demodex-positive group, but the difference between the 2 groups was not statistically significant.
It has been reported that proinflammatory cytokines due to a weakened immune system in rosacea patients were increased. The weakened immune system was further supported by the increased concentrations of proinflammatory cytokines such as IL-1 and matrix metalloproteinase 9 in these patients’ tears and the improvement of symptoms after the inhibition of these cytokines.11 Luo et al18 reported that Demodex inflammation causes dry eye, particularly with D brevis. Ayyildiz and Sezgin19 reported that Schirmer scores were significantly lower and that the Ocular Surface Disease Index had significantly increased in the Demodex-positive group compared to the Demodex-negative group (P=.001 for both). A Korean study reported that Demodex density was correlated with age, sex, and TBUT results, but there was no significant relationship between Demodex density and Schirmer scores.16
Sobolewska et al20 administered ivermectin cream 1% to 10 patients with cutaneous and ocular rosacea, but only to the forehead, chin, nose, cheeks, and regions close to the eyelids, and observed a significant improvement in blepharitis (P=.004). They stated that ivermectin, as applied only to the face, suppressed the proinflammatory cytokines associated with rosacea and showed anti-inflammatory effects by reducing Demodex mites.20Li et al21 demonstrated a strong correlation between ocular Demodex inflammation and serum reactivity to these bacterial proteins in patients with ocular rosacea, and they found that eyelid margin inflammation and facial rosacea correlated with reactivity to these proteins. These studies suggest a possible role for Demodex infestation and bacterial proteins in the etiology of rosacea.
Gonzalez-Hinojosa et al22 demonstrated that even though eyelash blepharitis was more common in PPR than ETR, there was no statistically significant association between rosacea and Demodex blepharitis. In our study, we found a significant correlation between PPR and Demodex positivity. Also, meibomian gland dysfunction was more common in the Demodex-positive group; however, this result was not statistically significant. One study compared patients with primary demodicosis and patients with rosacea with Demodex-induced blepharitis to healthy controls and found that patients with primary demodicosis and patients with rosacea did not have significantly different ocular findings.23 In contrast, Forton and De Maertelaer24 reported that patients with PPR had significantly more severe ocular manifestations compared with patients with demodicosis (P=.004).
Mizuno et al25 compared the normal (nonrosacea) population with and without Demodex-infested eyelashes and found that the 2 groups were not significantly different for meibomian gland dysfunction, fluorescein TBUT, or ocular surface discomfort.
Varying results have been reported regarding the association between Demodex and blepharitis or ocular surface discomfort with or without rosacea. In our study, we found that Demodex did not affect tear function tests or meibomian gland function in patients with rosacea. We believe this study is important because it demonstrates the effects of Demodex on ocular findings in patients with cutaneous rosacea.
Limitations
Our study has some limitations. The number of patients was relatively small, resulting in few significant differences between the comparison groups. A larger prospective research study is required to assess the prevalence of Demodex mites in the ocular rosacea population along with associated symptoms and findings.
Conclusion
Rosacea is a chronic disease associated with skin and ocular manifestations that range from mild to severe, that progresses in the form of attacks, and that requires long-term follow-up and treatment. Rosacea most often presents as a disease that causes ocular surface inflammation of varying degrees. Demodex infestation may increase cutaneous or ocular inflammation in rosacea. Therefore, every patient diagnosed with rosacea should be given a dermatologic examination to determine Demodex positivity and an ophthalmologic examination to determine ocular manifestations.
Acne rosacea is a chronic inflammatory disease that may affect the facial skin, eyes, and eyelids.1 It is characterized by transient or persistent flushing, facial erythema, and telangiectases, generally located on the central portion of the face, and may progress to papules and pustules.2,3 At the late stage of the disease, dermal edema or fibroplasia and sebaceous gland hypertrophy may cause phymatous alterations in the skin. In 2004, the National Rosacea Society Expert Committee developed a classification system for rosacea to standardize subtypes and variants that has since been widely accepted and continues to aid in research and epidemiologic studies.4 The committee defined 4 subtypes based on clinical characteristics: erythematotelangiectatic (ETR), papulopustular (PPR), phymatous, and ocular rosacea.2,3
Ocular rosacea may accompany mild, moderate, and severe dermatologic disease or may occur in the absence of diagnostic skin disease.5 Ocular signs include eyelid margin telangiectasia, spade-shaped infiltrates in the cornea, scleritis, and sclerokeratitis. Common symptoms include burning, stinging, light sensitivity, and foreign-body sensation. Ocular signs commonly seen in rosacea are meibomian gland dysfunction characterized by inspissation and inflammation of the meibomian glands (chalazia), conjunctivitis, honey crust and cylindrical collarette accumulation at the base of the eyelashes, irregularity of the eyelid margin architecture, and evaporative tear dysfunction.5,6
The physiopathology of rosacea is still unknown. Potential factors include genetic predisposition, abnormal inflammation, vascular dysfunction, and involvement of several microbial agents, such as commensal Demodex mites. The number of Demodex mites on normal skin flora is less than 5/cm2; however, the increased vascular dilation and capillary permeability associated with rosacea that result from sunlight and heat exposure increase the density of Demodex folliculorum.7 Elevated Demodex mite density has been observed in the lumens of the sebaceous follicles in patients with rosacea. However, because the severity of the clinical manifestations of the disease is not directly associated with the density of D folliculorum, it generally is accepted that D folliculorum is not a pathogenetic but rather an exacerbating factor.8 It has been reported that this species of mite is mostly found on the face and around the eyelashes and scalp of patients and that it can cause ocular surface inflammation.8
Most studies have researched ocular manifestations of rosacea but not ocular involvement in rosacea patients with and without Demodex mite infestation. In our study, we sought to compare the ocular surface, meibomian gland characteristics, and tear film abnormalities among patients with cutaneous rosacea with and without Demodex infestation.
Materials and Methods
We conducted a retrospective study of 60 patients with cutaneous rosacea. This study was approved by the ethics committee of the local hospital (2018/002-003), and all patients provided verbal and written informed consent before participating in the study. The study was carried out according to the guidelines of the Declaration of Helsinki.
Patient Selection and Evaluation
Patients diagnosed with rosacea by a dermatologist within 6 months were included in the study. Diagnosis of the disease was made after a detailed anamnesis and dermatologic examination. Rosacea was diagnosed if patients had an itching sensation, erythema and/or erythema attacks, and papules and pustules, and fulfilled the diagnostic criteria according to the National Rosacea Society. The skin disease was classified according to the subtypes as ETR, PPR, phymatous rosacea, or ocular rosacea.
The standard skin surface biopsy method was used in 60 patients for detecting Demodex density. When more than 5 mites were detected per square centimeter, the result was recorded as positive. Thirty consecutive, newly diagnosed patients with cutaneous acne rosacea with Demodex infestation and 30 consecutive, newly diagnosed sex- and age-matched patients with acne rosacea without Demodex infestation admitted to the dermatology outpatient clinic were included to this study. The patients who did not have any known dermatologic, systemic, or ocular diseases were included in the study. Patients who met any of the following criteria were excluded from the study: prior anti-inflammatory topical and/or systemic treatment for rosacea during the last 3 months, contact lens wear, eyelid surgery, or autoimmune disease requiring treatment.
Microscopic Demodex Examination
Demodex count was determined using a standardized skin surface biopsy, which is a noninvasive method. Every patient gave samples from the cheeks. This biopsy was repeated from the same site. A drop of cyanoacrylate was placed on a clean slide, pressed against a skin lesion, held in place for 1 minute, and removed. The obtained samples were evaluated under a light microscope (Nikon E200) with oil immersion. When more than 5 mites were detected per square centimeter, the result was recorded as positive.
Ophthalmologic Examination
A complete ophthalmologic examination including visual acuity assessment, standardized slit lamp examination, and fundus examination was done for all patients. Ocular rosacea was diagnosed on detection of 1 or more of the following: watery or bloodshot appearance, foreign-body sensation, burning or stinging, dryness, itching, light sensitivity, blurred vision, telangiectases of the conjunctiva and eyelid margin, eyelid lid and periocular erythema, anterior blepharitis, meibomian gland dysfunction, or irregularity of eyelid margins. All patients were screened for the signs and symptoms of ocular rosacea and underwent other ophthalmologic examinations, including tear function tests. Tear functions were evaluated with Schirmer tests without anesthesia and fluorescein tear breakup time (TBUT). Tear film breakup time was assessed after instillation of 2% fluorescein staining under a cobalt blue filter. The time interval between the last complete blink and the appearance of the first dry spot was recorded. The mean of 3 consecutive measurements was obtained. The Schirmer test was performed without topical anesthesia using a standardized filter strip (Bio-Tech Vision Care). The amount of wetting was measured after 5 minutes. Meibomian gland expressibility was assessed by applying digital pressure to the eyelid margin.
Statistical Analysis
Statistical analysis of the study was performed with SPSS Statistics Version 22.0 (SPSS Inc). Continuous variables were reported as mean (SD), and categorical variables were reported as percentages and counts. Descriptive statistics for numerical variables were created. An independent sample t test was used for normally distributed continuous variables. The Kolmogorov-Smirnov test was used to determine normality. The Schirmer test without anesthesia and TBUT values among groups were compared using one-way analysis of variance. The differences were calculated using the multiple comparison Tukey test. P<.05 was considered statistically significant.
Results
Demographic Characteristics of Rosacea Patients
Sixty eyes of 30 newly diagnosed patients with acne rosacea with Demodex infestation and 60 eyes of 30 newly diagnosed patients with acne rosacea without Demodex infestation were enrolled in this study. The mean age (SD) of the 60 patients was 37.63 (10.01) years. The mean TBUT (SD) of the 120 eyes was 6.65 (3.44) seconds, and the mean Schirmer score (SD) was 12.59 (6.71) mm (Table 1).

Meibomian Gland Dysfunction vs Subgroup of Rosacea Patients
Thirty-four (57%) patients had blepharitis, and 18 (30%) patients had meibomitis. Thirty-five (58.3%) patients had ETR, 5 (8.3%) patients had phymatous rosacea, and 20 (33.4%) patients had PPR (Table 2). Of the Demodex-negative patients, 73.3% (22/30) had ETR, 20% (6/30) had PPR, and 6.7% (2/30) had phymatous rosacea. Of the Demodex-positive patients, 43.3% (13/30) had ETR, 46.7% (14/30) had PPR, and 10% (3/30) had phymatous rosacea (Table 3). Papulopustular rosacea was found to be significantly associated with Demodex positivity (P=.003); neither ETR nor phymatous rosacea was found to be significantly associated with Demodex infestation (P=.66 and P=.13, respectively)(Table 3).


There was no statistically significant difference between the Demodex-negative and Demodex-positive groups for mean age (SD)(37.4 [11.54] years vs 37.87 [8.41] years; P=.85), mean TBUT (SD)(6.73 [3.62] seconds vs 6.57 [3.33] seconds; P=.85), and mean Schirmer score (SD)(13.68 [7.23] mm vs 11.5 [6.08] mm; P=.21)(Table 4).

Fifteen (50%) patients (30 eyes) in the Demodex-negative group and 19 (63.3%) patients (38 eyes) in the Demodex-positive group had blepharitis, with no statistically significant difference between the groups (P=.43). Seven (23.3%) patients (14 eyes) in the Demodex-negative group and 11 (36.7%) patients (22 eyes) in the Demodex-positive group had meibomitis, with no statistically significant difference between the groups (P=.39)(Table 3).
Sixteen (53.3%) patients (32 eyes) in the Demodex-negative group and 21 (70%) patients (42 eyes) in the Demodex-positive group had TBUT values less than 10 seconds. Eighteen (60%) patients (36 eyes) in the Demodex-negative group and 25 (83.3%) patients (50 eyes) in the Demodex-positive group had Schirmer scores less than 10 mm (Table 3). The 2 groups were not significantly different in dry eye findings (P=.25 and P=.29, respectively).
Comment
Inflammation in Rosacea
It is known that the density of nonfloral bacteria as well as D folliculorum and Demodex brevis increases in skin affected by rosacea compared to normal skin. Vascular dilation associated with rosacea that results from sunlight and heat causes increased capillary permeability and creates the ideal environment for the proliferation of D folliculorum. Demodex is thought to act as a vector for the activity of certain other microorganisms, particularly Bacillus oleronius, and thus initiates the inflammatory response associated with rosacea.9
One study reported that the inflammation associated with rosacea that was caused by Demodex and other environmental stimuli occurred through toll-like receptor 2 and various cytokines.10 It has been reported that the abnormal function of toll-like receptor 2 in the epidermis leads to the increased production of cathelicidin. Cathelicidin is an antimicrobial peptide with both vasoactive and proinflammatory activity and has been used as a basis to explain the pathogenesis of facial erythema, flushing, and telangiectasia in the context of rosacea.11,12 In addition, it has been reported that the increased secretion of proinflammatory cytokines such as IL-1 and gelatinase B in ocular rosacea leads to tearing film abnormalities that result from increased bacterial flora in the eyelids, which subsequently leads to decreased tear drainage and dry eyes.13 In addition, B oleronius isolated from a D folliculorum mite from patients with PPR produced proteins that induced an inflammatory immune response in 73% (16/22) of patients with rosacea.14
Ocular Findings in Rosacea Patients
In our study, PPR was found to be significantly associated with Demodex positivity compared to ETR and phymatous rosacea (P=.003). However, ocular inflammation findings such as blepharitis and meibomitis were not significantly different between Demodex-positive and Demodex-negative patients. Although the mean Schirmer score of Demodex-positive patients was lower than Demodex-negative patients, this difference was not statistically significant. We evaluated a TBUT of less than 10 seconds and a Schirmer score less than 10 mm as dry eye. Accordingly, the number of patients with dry eye was higher in the Demodex-positive group, but this difference was not statistically significant.
Chronic blepharitis, conjunctival inflammation, and meibomian gland dysfunction are among the most common findings of ocular rosacea.15,16 Patients with ocular rosacea commonly have dry eye and abnormal TBUT and Schirmer scores.17 In our study, we found that the fluorescein TBUT and Schirmer scores were more likely to be abnormal in the Demodex-positive group, but the difference between the 2 groups was not statistically significant.
It has been reported that proinflammatory cytokines due to a weakened immune system in rosacea patients were increased. The weakened immune system was further supported by the increased concentrations of proinflammatory cytokines such as IL-1 and matrix metalloproteinase 9 in these patients’ tears and the improvement of symptoms after the inhibition of these cytokines.11 Luo et al18 reported that Demodex inflammation causes dry eye, particularly with D brevis. Ayyildiz and Sezgin19 reported that Schirmer scores were significantly lower and that the Ocular Surface Disease Index had significantly increased in the Demodex-positive group compared to the Demodex-negative group (P=.001 for both). A Korean study reported that Demodex density was correlated with age, sex, and TBUT results, but there was no significant relationship between Demodex density and Schirmer scores.16
Sobolewska et al20 administered ivermectin cream 1% to 10 patients with cutaneous and ocular rosacea, but only to the forehead, chin, nose, cheeks, and regions close to the eyelids, and observed a significant improvement in blepharitis (P=.004). They stated that ivermectin, as applied only to the face, suppressed the proinflammatory cytokines associated with rosacea and showed anti-inflammatory effects by reducing Demodex mites.20Li et al21 demonstrated a strong correlation between ocular Demodex inflammation and serum reactivity to these bacterial proteins in patients with ocular rosacea, and they found that eyelid margin inflammation and facial rosacea correlated with reactivity to these proteins. These studies suggest a possible role for Demodex infestation and bacterial proteins in the etiology of rosacea.
Gonzalez-Hinojosa et al22 demonstrated that even though eyelash blepharitis was more common in PPR than ETR, there was no statistically significant association between rosacea and Demodex blepharitis. In our study, we found a significant correlation between PPR and Demodex positivity. Also, meibomian gland dysfunction was more common in the Demodex-positive group; however, this result was not statistically significant. One study compared patients with primary demodicosis and patients with rosacea with Demodex-induced blepharitis to healthy controls and found that patients with primary demodicosis and patients with rosacea did not have significantly different ocular findings.23 In contrast, Forton and De Maertelaer24 reported that patients with PPR had significantly more severe ocular manifestations compared with patients with demodicosis (P=.004).
Mizuno et al25 compared the normal (nonrosacea) population with and without Demodex-infested eyelashes and found that the 2 groups were not significantly different for meibomian gland dysfunction, fluorescein TBUT, or ocular surface discomfort.
Varying results have been reported regarding the association between Demodex and blepharitis or ocular surface discomfort with or without rosacea. In our study, we found that Demodex did not affect tear function tests or meibomian gland function in patients with rosacea. We believe this study is important because it demonstrates the effects of Demodex on ocular findings in patients with cutaneous rosacea.
Limitations
Our study has some limitations. The number of patients was relatively small, resulting in few significant differences between the comparison groups. A larger prospective research study is required to assess the prevalence of Demodex mites in the ocular rosacea population along with associated symptoms and findings.
Conclusion
Rosacea is a chronic disease associated with skin and ocular manifestations that range from mild to severe, that progresses in the form of attacks, and that requires long-term follow-up and treatment. Rosacea most often presents as a disease that causes ocular surface inflammation of varying degrees. Demodex infestation may increase cutaneous or ocular inflammation in rosacea. Therefore, every patient diagnosed with rosacea should be given a dermatologic examination to determine Demodex positivity and an ophthalmologic examination to determine ocular manifestations.
- O’Reilly N, Gallagher C, Reddy Katikireddy K, et al. Demodex-associated Bacillus proteins induce an aberrant wound healing response in a corneal epithelial cell line: possible implications for corneal ulcer formation in ocular rosacea. Invest Ophthalmol Vis Sci. 2012;53:3250-3259.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6 suppl 1):S42-S43.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089-3094.
- Fallen RS, Gooderham M. Rosacea: update on management and emerging therapies. Skin Therapy Lett. 2012;17:1-4.
- Erbagcı Z, Ozgoztası O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36:81‐86.
- Forton FMN, De Maertelaer V. Two consecutive standardized skin surface biopsies: an improved sampling method to evaluate Demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venereol. 2017;97:242‐248.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Gold LM, Draelos ZD. New and emerging treatments for rosacea. Am J Clin Dermatol. 2015;16:457-461.
- Two AM, Del Rosso JQ. Kallikrein 5-mediated inflammation in rosacea: clinically relevant correlations with acute and chronic manifestations in rosacea and how individual treatments may provide therapeutic benefit. J Clin Aesthet Dermatol. 2014;7:20-25.
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474-481.
- Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52:74-87.
- Lee SH, Chun YS, Kim JH, et al. The relationship between Demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51:2906-2911.
- Awais M, Anwar MI, Ilfikhar R, et al. Rosacea—the ophthalmic perspective. Cutan Ocul Toxicol. 2015;34:161-166.
- Luo X, Li J, Chen C, et al. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(suppl 1):S9-S14.
- Ayyildiz T, Sezgin FM. The effect of ocular Demodex colonization on Schirmer test and OSDI scores in newly diagnosed dry eye patients. Eye Contact Lens. 2020;46(suppl 1):S39-S41.
- Sobolewska B, Doycheva D, Deuter CM, et al. Efficacy of topical ivermectin for the treatment of cutaneous and ocular rosacea [published online April 7, 2020]. Ocul Immunol Inflamm. doi:10.1080/09273948.2020.1727531
- Li J, O‘Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. 2010;117:870-877.
- Gonzalez‐Hinojosa D, Jaime‐Villalonga A, Aguilar‐Montes G, et al. Demodex and rosacea: is there a relationship? Indian J Ophthalmol. 2018;66:36‐38.
- Sarac G, Cankaya C, Ozcan KN, et al. Increased frequency of Demodex blepharitis in rosacea and facial demodicosis patients. J Cosmet Dermatol. 2020;19:1260-1265.
- Forton FMN, De Maertelaer V. Rosacea and demodicosis: little-known diagnostic signs and symptoms. Acta Derm Venereol. 2019;99:47-52.
- Mizuno M, Kawashima M, Uchino M, et al. Demodex-mite infestation in cilia and its association with ocular surface parameters in Japanese volunteers. Eye Contact Lens. 2020;46:291-296.
- O’Reilly N, Gallagher C, Reddy Katikireddy K, et al. Demodex-associated Bacillus proteins induce an aberrant wound healing response in a corneal epithelial cell line: possible implications for corneal ulcer formation in ocular rosacea. Invest Ophthalmol Vis Sci. 2012;53:3250-3259.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6 suppl 1):S42-S43.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089-3094.
- Fallen RS, Gooderham M. Rosacea: update on management and emerging therapies. Skin Therapy Lett. 2012;17:1-4.
- Erbagcı Z, Ozgoztası O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36:81‐86.
- Forton FMN, De Maertelaer V. Two consecutive standardized skin surface biopsies: an improved sampling method to evaluate Demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venereol. 2017;97:242‐248.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Gold LM, Draelos ZD. New and emerging treatments for rosacea. Am J Clin Dermatol. 2015;16:457-461.
- Two AM, Del Rosso JQ. Kallikrein 5-mediated inflammation in rosacea: clinically relevant correlations with acute and chronic manifestations in rosacea and how individual treatments may provide therapeutic benefit. J Clin Aesthet Dermatol. 2014;7:20-25.
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474-481.
- Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52:74-87.
- Lee SH, Chun YS, Kim JH, et al. The relationship between Demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51:2906-2911.
- Awais M, Anwar MI, Ilfikhar R, et al. Rosacea—the ophthalmic perspective. Cutan Ocul Toxicol. 2015;34:161-166.
- Luo X, Li J, Chen C, et al. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(suppl 1):S9-S14.
- Ayyildiz T, Sezgin FM. The effect of ocular Demodex colonization on Schirmer test and OSDI scores in newly diagnosed dry eye patients. Eye Contact Lens. 2020;46(suppl 1):S39-S41.
- Sobolewska B, Doycheva D, Deuter CM, et al. Efficacy of topical ivermectin for the treatment of cutaneous and ocular rosacea [published online April 7, 2020]. Ocul Immunol Inflamm. doi:10.1080/09273948.2020.1727531
- Li J, O‘Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. 2010;117:870-877.
- Gonzalez‐Hinojosa D, Jaime‐Villalonga A, Aguilar‐Montes G, et al. Demodex and rosacea: is there a relationship? Indian J Ophthalmol. 2018;66:36‐38.
- Sarac G, Cankaya C, Ozcan KN, et al. Increased frequency of Demodex blepharitis in rosacea and facial demodicosis patients. J Cosmet Dermatol. 2020;19:1260-1265.
- Forton FMN, De Maertelaer V. Rosacea and demodicosis: little-known diagnostic signs and symptoms. Acta Derm Venereol. 2019;99:47-52.
- Mizuno M, Kawashima M, Uchino M, et al. Demodex-mite infestation in cilia and its association with ocular surface parameters in Japanese volunteers. Eye Contact Lens. 2020;46:291-296.
Practice Points
- Rosacea is a common chronic inflammatory skin disease of the central facial skin and is of unknown origin. Patients with ocular rosacea may report dryness, itching, and photophobia.
- Demodex infestation may increase cutaneous or ocular inflammation in rosacea.
Results of Laboratory Monitoring in Patients Taking Isotretinoin for Acne
Introduced in 1982, isotretinoin is a retinoid derivative that has been widely used to treat various dermatologic conditions such as acne vulgaris, rosacea, hidradenitis suppurativa, and hair folliculitis. 1 It remains one of the most effective drugs for the treatment of all forms of acne vulgaris, especially the nodulocystic type, and exerts its effects via different mechanisms that affect the major domains involved in the pathogenesis of acne. 2 One month after treatment initiation, isotretinoin suppresses sebum production by decreasing the size and activity of sebaceous glands. In addition, it notably stabilizes keratinization of the skin and decreases the number of Propionibacterium acnes, which will minimize the inflammation associated with acne. 3,4 Despite its beneficial effects, isotretinoin therapy has been associated with several complications. The most commonly reported adverse effects include fissured lips, dry skin, eczema, epistaxis, dry eyes, gastrointestinal tract upset, angular stomatitis, and back pain. Less frequent systemic adverse effects have been reported and relate mainly to teratogenicity, pancreatitis, drug-induced hepatotoxicity, leukopenia, and thrombocytopenia. 5
Isotretinoin use has been associated with alterations in hepatic and lipid profiles; elevations of serum liver enzymes and triglycerides (TGs) following isotretinoin treatment have been reported.4 Consequently, different protocols for laboratory monitoring during isotretinoin therapy have been established and utilized by various health care institutes.6 Despite the time and economic investment involved, certain protocols recommend repetition of liver function tests and several other laboratory parameters following a baseline test.7 The aim of this study was to determine the prevalence of laboratory changes in alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholesterol, and TGs among patients with acne receiving isotretinoin therapy, as well as to link the initial and second laboratory readings of the aforementioned parameters following initiation of isotretinoin treatment.
Materials and Methods
This retrospective cohort design study obtained patient data, including laboratory test results, from the Electronic System for Integrated Health Information at King Khalid University Hospital (KKUH)(Riyadh, Saudi Arabia). All patients older than 16 years who presented with acne vulgaris to the dermatology department at KKUH; who received a course of isotretinoin for at least 4 weeks between 2011 and 2016; and who had available baseline readings of ALT, AST, cholesterol, and TGs, as well as 2 concurrent follow-up readings after isotretinoin treatment initiation, were included in this study. Patients with only 1 reading following treatment initiation and those receiving isotretinoin treatment for reasons other than acne were excluded. This study was approved by the institutional review board of the College of Medicine at King Saud University (Riyadh, Saudi Arabia)(E-18-3310).
Statistical Analysis
Data were entered into a Microsoft Excel document, and statistical analysis was performed using SPSS (version 22.0). Data were represented as numbers and percentages. Repeated measures analysis was performed using the Cochran Q test to compare proportions of abnormal laboratory values among 3 groups: baseline, first reading, and second reading. When test results were significant, a post hoc test was used to compare proportions between any 2 groups. Moreover, a Spearman rank correlation was performed to investigate the association between the daily isotretinoin dose and the laboratory parameters. Results with P<.05 were considered statistically significant.
Results
During the study period, treatment with oral isotretinoin was undertaken by 386 patients at KKUH. Several of these patients were excluded due to incomplete medical records. The age of the studied patients ranged from 17 to 60 years, with a median age of 24 years (interquartile range, 20−28 years). The daily administered dose ranged from 10 to 80 mg, with a median dose of 30 mg (interquartile range, 20−40 mg), as illustrated in the Table. Repeated-measures analysis of liver enzymes (AST and ALT), total cholesterol, and TGs is detailed in eTable 1. Eight (2.2%) of 371 patients showed abnormal baseline AST levels. The first follow-up measurements of AST revealed high levels in 7 (1.9%) patients. This figure doubled (14 [3.8%] patients) at the second follow-up, with no statistically significant differences (P>.05). Likewise, ALT showed abnormally high levels at baseline and at both the first and second follow-ups (47/371 [12.7%], 49/371 [13.2%], and 37/371 [10.0%], respectively) with no significant differences (P>.05). Furthermore, the proportions of high cholesterol levels at baseline and at both the first and second follow-ups (40/331 [12.1%], 72/331 [21.8%], and 62/331 [18.7%], respectively) showed a statistically significant difference (P=.001). The proportions of high cholesterol levels in both the first and second follow-ups were significantly higher than the baseline proportions (P=.001 and P=.002, respectively). However, the percentages of high cholesterol were reduced at the second reading relative to the first but with no significant differences. Regarding TGs, there was a statistically significant difference in the proportions of high levels over time (5/320 [1.6%], 12/320 [3.8%], and 14/320 [4.4%] at baseline and at the first and second readings, respectively). Moreover, pairwise comparison among the 3 readings revealed a significant difference between the second follow-up and the baseline levels (P=.048). eTable 2 demonstrates statistically significant positive weak associations between the daily administered isotretinoin dose and each of the cholesterol and TG levels, both at the first and second follow-up readings (P<.05).
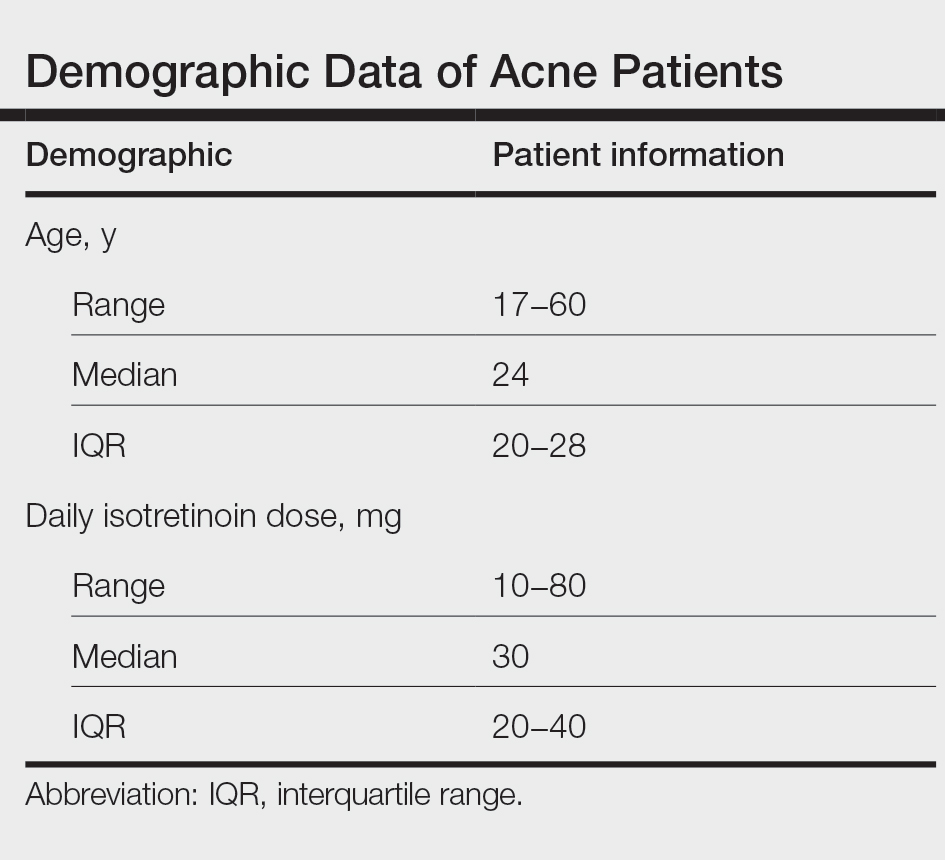
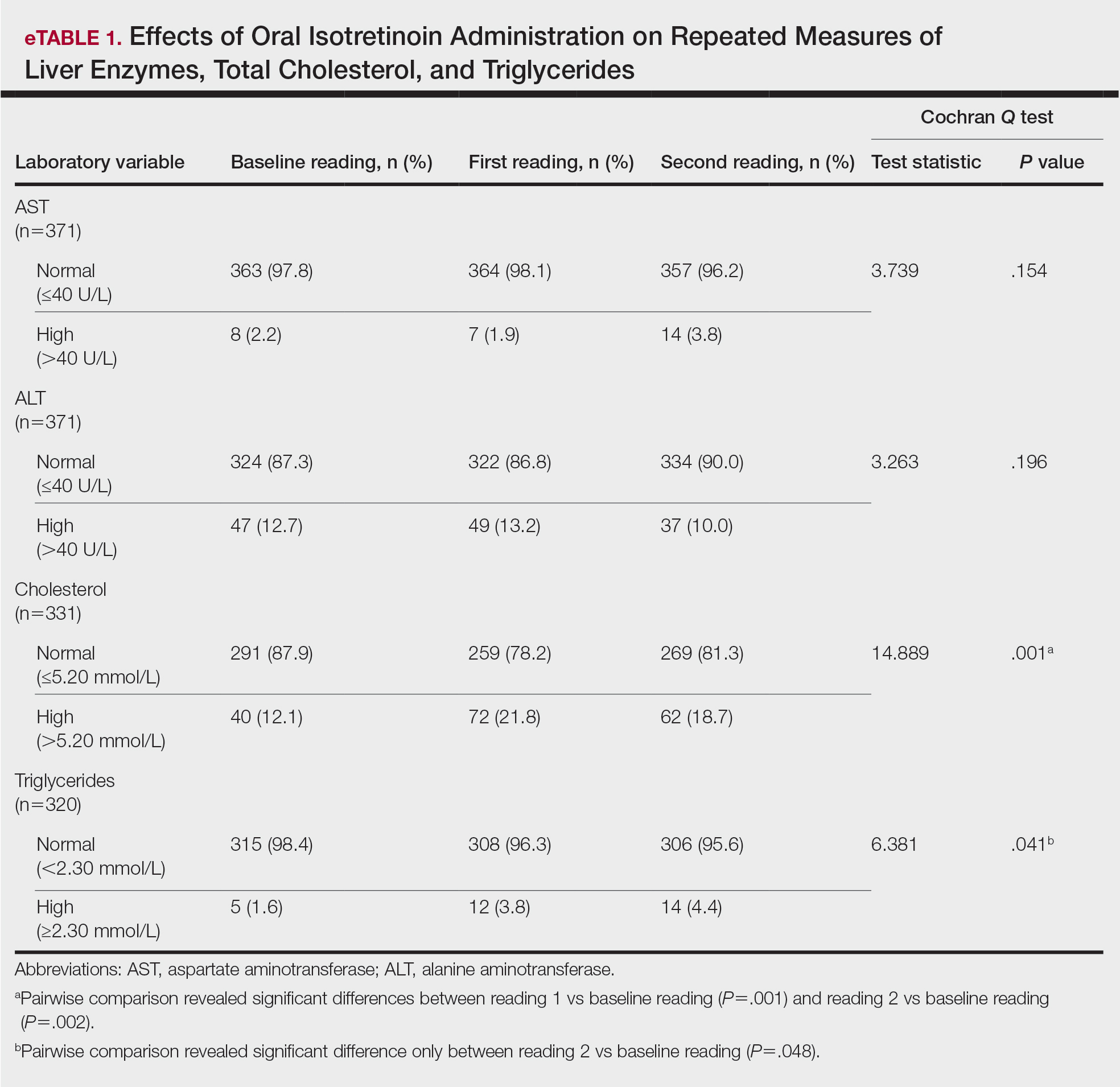
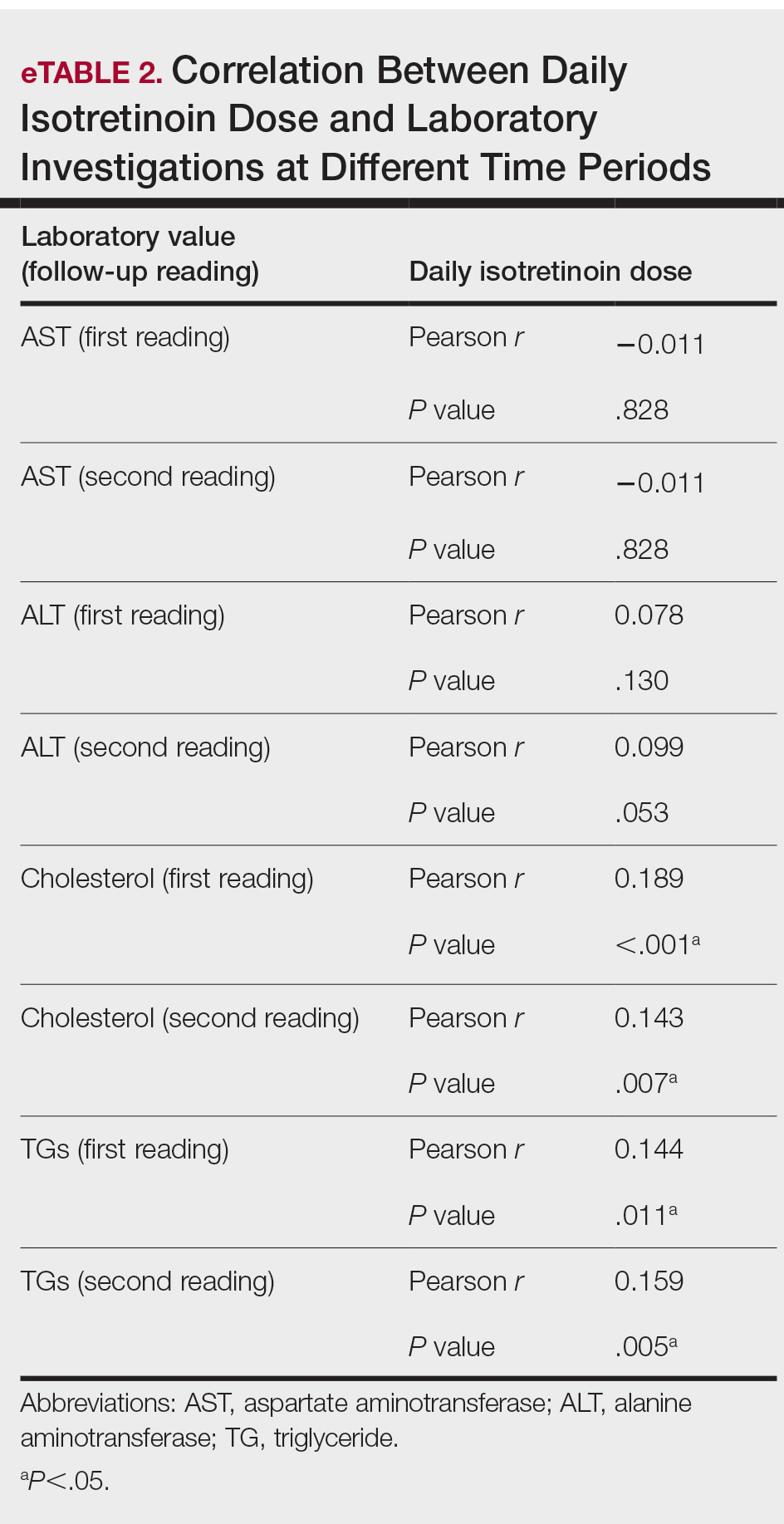
Comment
Evaluation of the effects of isotretinoin on liver enzymes and lipids has suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT) and lipid profiles to various degrees.8 Furthermore, there are controversies regarding the routine laboratory monitoring of these patients. Some studies have reported severe alterations in serum liver transaminase and lipid levels, and they support the need for careful monitoring when treating patients with isotretinoin. However, other studies have reported that adverse effects are minimal, with no need for costly laboratory monitoring.9
Our study explored the profile of changes in liver aminotransferases (AST and ALT), cholesterol, and TGs in patients with acne who had been treated with oral isotretinoin. The cholesterol levels showed a nonprogressive increase, with a prevalence rate of 21.8% and 18.7% at the first and second follow-ups, respectively. Likewise, the frequency of high TG levels was 3.8% and 4.4%, respectively, with significant differences from the baseline levels (P=.041). However, liver enzymes were less affected by isotretinoin therapy than lipid profiles. Both AST and ALT showed nonsignificant minimal elevations during follow-up of the patients.
Similar to our findings, Zane et al6 at the University of California, San Francisco, studied 13,772 patients with acne who underwent oral isotretinoin therapy between 1995 and 2002. They reported a cumulative incidence of new abnormalities in patients with normal values at baseline at a frequency of 44% for TG levels, 31% for total cholesterol levels, and 11% for transaminase levels. Moreover, they suggested that these abnormalities generally were transient and reversible.6 Another retrospective study in Brazil included 130 patients who were treated with isotretinoin for 3 months and reported that TG levels had increased beyond the normal range in 11% of patients, whereas 8.6% had elevated AST levels and 7.3% had elevated ALT levels.8 Comparable to our findings, Kizilyel et al10 concluded that isotretinoin appeared to have a greater effect on lipids than on liver enzymes, and they recommended its use with careful monitoring.
The transient effects of isotretinoin therapy on lipid profiles were highlighted in an earlier study. It has been reported that the changes in low-density lipoprotein and TGs returned to baseline levels 2 months following termination of treatment.11 Although many studies have reported alterations in serum transaminase and lipid levels, other studies fail to report any such effects. Alcalay et al7 investigated 907 patients who completed a treatment course lasting 5 to 9 months. They reported that only 1.5% of patients had serum TG levels above 400 mg. Additionally, serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of treatment. They concluded that isotretinoin is a safe therapeutic drug and suggested that there is no need for routine laboratory follow-up in young healthy patients apart from a pregnancy test for females.7 In addition, Brito et al12 conducted a prospective clinical and laboratory evaluation of 150 patients being treated with oral isotretinoin prior to the start of therapy, 1 month after therapy initiation, and every 3 months thereafter until the completion of treatment. They found no statistically significant changes in liver transaminase, TG, or cholesterol levels.12 In another study of 30 participants, Baxter et al13 also reported no significant changes in TG or cholesterol levels measured at baseline or during treatment with isotretinoin. Furthermore, a systematic review and meta-analysis has estimated the laboratory changes that occur during isotretinoin therapy of acne vulgaris.14 The evidence revealed in this study does not support monthly laboratory testing for use of standard doses of oral isotretinoin for the typical patient with acne.
Conclusion
In our study, liver enzymes were less affected than lipids in patients who were treated with isotretinoin. Additionally, laboratory alterations in lipid profiles were nonprogressive and nonsevere. Consequently, isotretinoin may be administered with minimal concern for changes in serum transaminase and lipid profile. However, physicians should exercise caution when administering isotretinoin in patients with a history of abnormal findings.
- Kaymak Y, Ilter N. The results and side effects of systemic isotretinoin treatment in 100 patients with acne vulgaris. Dermatol Nurs. 2006;18:576-580.
- Al-Mutairi N, Manchanda Y, Nour-Eldin O, et al. Isotretinoin in acne vulgaris: a prospective analysis of 160 cases from Kuwait. J Drugs Dermatol. 2005;4:369-373.
- Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: a randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77:688-694.
- Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
- Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
- Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglycerides and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
- Bauer LB, Ornelas JN, Elston DM, et al. Isotretinoin: controversies, facts, and recommendations. Expert Rev Clin Pharmacol. 2016;9:1435-1442.
- Kizilyel O, Metin MS, Elmas ÖF, et al. Effects of oral isotretinoin on lipids and liver enzymes in acne patients. Cutis. 2014;94:234-238.
- Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
- Brito MDFDM, Sant’Anna IP, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin. An Bras Dermatol. 2010;85:331-337.
- Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2004;14:216-218.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
Introduced in 1982, isotretinoin is a retinoid derivative that has been widely used to treat various dermatologic conditions such as acne vulgaris, rosacea, hidradenitis suppurativa, and hair folliculitis. 1 It remains one of the most effective drugs for the treatment of all forms of acne vulgaris, especially the nodulocystic type, and exerts its effects via different mechanisms that affect the major domains involved in the pathogenesis of acne. 2 One month after treatment initiation, isotretinoin suppresses sebum production by decreasing the size and activity of sebaceous glands. In addition, it notably stabilizes keratinization of the skin and decreases the number of Propionibacterium acnes, which will minimize the inflammation associated with acne. 3,4 Despite its beneficial effects, isotretinoin therapy has been associated with several complications. The most commonly reported adverse effects include fissured lips, dry skin, eczema, epistaxis, dry eyes, gastrointestinal tract upset, angular stomatitis, and back pain. Less frequent systemic adverse effects have been reported and relate mainly to teratogenicity, pancreatitis, drug-induced hepatotoxicity, leukopenia, and thrombocytopenia. 5
Isotretinoin use has been associated with alterations in hepatic and lipid profiles; elevations of serum liver enzymes and triglycerides (TGs) following isotretinoin treatment have been reported.4 Consequently, different protocols for laboratory monitoring during isotretinoin therapy have been established and utilized by various health care institutes.6 Despite the time and economic investment involved, certain protocols recommend repetition of liver function tests and several other laboratory parameters following a baseline test.7 The aim of this study was to determine the prevalence of laboratory changes in alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholesterol, and TGs among patients with acne receiving isotretinoin therapy, as well as to link the initial and second laboratory readings of the aforementioned parameters following initiation of isotretinoin treatment.
Materials and Methods
This retrospective cohort design study obtained patient data, including laboratory test results, from the Electronic System for Integrated Health Information at King Khalid University Hospital (KKUH)(Riyadh, Saudi Arabia). All patients older than 16 years who presented with acne vulgaris to the dermatology department at KKUH; who received a course of isotretinoin for at least 4 weeks between 2011 and 2016; and who had available baseline readings of ALT, AST, cholesterol, and TGs, as well as 2 concurrent follow-up readings after isotretinoin treatment initiation, were included in this study. Patients with only 1 reading following treatment initiation and those receiving isotretinoin treatment for reasons other than acne were excluded. This study was approved by the institutional review board of the College of Medicine at King Saud University (Riyadh, Saudi Arabia)(E-18-3310).
Statistical Analysis
Data were entered into a Microsoft Excel document, and statistical analysis was performed using SPSS (version 22.0). Data were represented as numbers and percentages. Repeated measures analysis was performed using the Cochran Q test to compare proportions of abnormal laboratory values among 3 groups: baseline, first reading, and second reading. When test results were significant, a post hoc test was used to compare proportions between any 2 groups. Moreover, a Spearman rank correlation was performed to investigate the association between the daily isotretinoin dose and the laboratory parameters. Results with P<.05 were considered statistically significant.
Results
During the study period, treatment with oral isotretinoin was undertaken by 386 patients at KKUH. Several of these patients were excluded due to incomplete medical records. The age of the studied patients ranged from 17 to 60 years, with a median age of 24 years (interquartile range, 20−28 years). The daily administered dose ranged from 10 to 80 mg, with a median dose of 30 mg (interquartile range, 20−40 mg), as illustrated in the Table. Repeated-measures analysis of liver enzymes (AST and ALT), total cholesterol, and TGs is detailed in eTable 1. Eight (2.2%) of 371 patients showed abnormal baseline AST levels. The first follow-up measurements of AST revealed high levels in 7 (1.9%) patients. This figure doubled (14 [3.8%] patients) at the second follow-up, with no statistically significant differences (P>.05). Likewise, ALT showed abnormally high levels at baseline and at both the first and second follow-ups (47/371 [12.7%], 49/371 [13.2%], and 37/371 [10.0%], respectively) with no significant differences (P>.05). Furthermore, the proportions of high cholesterol levels at baseline and at both the first and second follow-ups (40/331 [12.1%], 72/331 [21.8%], and 62/331 [18.7%], respectively) showed a statistically significant difference (P=.001). The proportions of high cholesterol levels in both the first and second follow-ups were significantly higher than the baseline proportions (P=.001 and P=.002, respectively). However, the percentages of high cholesterol were reduced at the second reading relative to the first but with no significant differences. Regarding TGs, there was a statistically significant difference in the proportions of high levels over time (5/320 [1.6%], 12/320 [3.8%], and 14/320 [4.4%] at baseline and at the first and second readings, respectively). Moreover, pairwise comparison among the 3 readings revealed a significant difference between the second follow-up and the baseline levels (P=.048). eTable 2 demonstrates statistically significant positive weak associations between the daily administered isotretinoin dose and each of the cholesterol and TG levels, both at the first and second follow-up readings (P<.05).



Comment
Evaluation of the effects of isotretinoin on liver enzymes and lipids has suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT) and lipid profiles to various degrees.8 Furthermore, there are controversies regarding the routine laboratory monitoring of these patients. Some studies have reported severe alterations in serum liver transaminase and lipid levels, and they support the need for careful monitoring when treating patients with isotretinoin. However, other studies have reported that adverse effects are minimal, with no need for costly laboratory monitoring.9
Our study explored the profile of changes in liver aminotransferases (AST and ALT), cholesterol, and TGs in patients with acne who had been treated with oral isotretinoin. The cholesterol levels showed a nonprogressive increase, with a prevalence rate of 21.8% and 18.7% at the first and second follow-ups, respectively. Likewise, the frequency of high TG levels was 3.8% and 4.4%, respectively, with significant differences from the baseline levels (P=.041). However, liver enzymes were less affected by isotretinoin therapy than lipid profiles. Both AST and ALT showed nonsignificant minimal elevations during follow-up of the patients.
Similar to our findings, Zane et al6 at the University of California, San Francisco, studied 13,772 patients with acne who underwent oral isotretinoin therapy between 1995 and 2002. They reported a cumulative incidence of new abnormalities in patients with normal values at baseline at a frequency of 44% for TG levels, 31% for total cholesterol levels, and 11% for transaminase levels. Moreover, they suggested that these abnormalities generally were transient and reversible.6 Another retrospective study in Brazil included 130 patients who were treated with isotretinoin for 3 months and reported that TG levels had increased beyond the normal range in 11% of patients, whereas 8.6% had elevated AST levels and 7.3% had elevated ALT levels.8 Comparable to our findings, Kizilyel et al10 concluded that isotretinoin appeared to have a greater effect on lipids than on liver enzymes, and they recommended its use with careful monitoring.
The transient effects of isotretinoin therapy on lipid profiles were highlighted in an earlier study. It has been reported that the changes in low-density lipoprotein and TGs returned to baseline levels 2 months following termination of treatment.11 Although many studies have reported alterations in serum transaminase and lipid levels, other studies fail to report any such effects. Alcalay et al7 investigated 907 patients who completed a treatment course lasting 5 to 9 months. They reported that only 1.5% of patients had serum TG levels above 400 mg. Additionally, serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of treatment. They concluded that isotretinoin is a safe therapeutic drug and suggested that there is no need for routine laboratory follow-up in young healthy patients apart from a pregnancy test for females.7 In addition, Brito et al12 conducted a prospective clinical and laboratory evaluation of 150 patients being treated with oral isotretinoin prior to the start of therapy, 1 month after therapy initiation, and every 3 months thereafter until the completion of treatment. They found no statistically significant changes in liver transaminase, TG, or cholesterol levels.12 In another study of 30 participants, Baxter et al13 also reported no significant changes in TG or cholesterol levels measured at baseline or during treatment with isotretinoin. Furthermore, a systematic review and meta-analysis has estimated the laboratory changes that occur during isotretinoin therapy of acne vulgaris.14 The evidence revealed in this study does not support monthly laboratory testing for use of standard doses of oral isotretinoin for the typical patient with acne.
Conclusion
In our study, liver enzymes were less affected than lipids in patients who were treated with isotretinoin. Additionally, laboratory alterations in lipid profiles were nonprogressive and nonsevere. Consequently, isotretinoin may be administered with minimal concern for changes in serum transaminase and lipid profile. However, physicians should exercise caution when administering isotretinoin in patients with a history of abnormal findings.
Introduced in 1982, isotretinoin is a retinoid derivative that has been widely used to treat various dermatologic conditions such as acne vulgaris, rosacea, hidradenitis suppurativa, and hair folliculitis. 1 It remains one of the most effective drugs for the treatment of all forms of acne vulgaris, especially the nodulocystic type, and exerts its effects via different mechanisms that affect the major domains involved in the pathogenesis of acne. 2 One month after treatment initiation, isotretinoin suppresses sebum production by decreasing the size and activity of sebaceous glands. In addition, it notably stabilizes keratinization of the skin and decreases the number of Propionibacterium acnes, which will minimize the inflammation associated with acne. 3,4 Despite its beneficial effects, isotretinoin therapy has been associated with several complications. The most commonly reported adverse effects include fissured lips, dry skin, eczema, epistaxis, dry eyes, gastrointestinal tract upset, angular stomatitis, and back pain. Less frequent systemic adverse effects have been reported and relate mainly to teratogenicity, pancreatitis, drug-induced hepatotoxicity, leukopenia, and thrombocytopenia. 5
Isotretinoin use has been associated with alterations in hepatic and lipid profiles; elevations of serum liver enzymes and triglycerides (TGs) following isotretinoin treatment have been reported.4 Consequently, different protocols for laboratory monitoring during isotretinoin therapy have been established and utilized by various health care institutes.6 Despite the time and economic investment involved, certain protocols recommend repetition of liver function tests and several other laboratory parameters following a baseline test.7 The aim of this study was to determine the prevalence of laboratory changes in alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholesterol, and TGs among patients with acne receiving isotretinoin therapy, as well as to link the initial and second laboratory readings of the aforementioned parameters following initiation of isotretinoin treatment.
Materials and Methods
This retrospective cohort design study obtained patient data, including laboratory test results, from the Electronic System for Integrated Health Information at King Khalid University Hospital (KKUH)(Riyadh, Saudi Arabia). All patients older than 16 years who presented with acne vulgaris to the dermatology department at KKUH; who received a course of isotretinoin for at least 4 weeks between 2011 and 2016; and who had available baseline readings of ALT, AST, cholesterol, and TGs, as well as 2 concurrent follow-up readings after isotretinoin treatment initiation, were included in this study. Patients with only 1 reading following treatment initiation and those receiving isotretinoin treatment for reasons other than acne were excluded. This study was approved by the institutional review board of the College of Medicine at King Saud University (Riyadh, Saudi Arabia)(E-18-3310).
Statistical Analysis
Data were entered into a Microsoft Excel document, and statistical analysis was performed using SPSS (version 22.0). Data were represented as numbers and percentages. Repeated measures analysis was performed using the Cochran Q test to compare proportions of abnormal laboratory values among 3 groups: baseline, first reading, and second reading. When test results were significant, a post hoc test was used to compare proportions between any 2 groups. Moreover, a Spearman rank correlation was performed to investigate the association between the daily isotretinoin dose and the laboratory parameters. Results with P<.05 were considered statistically significant.
Results
During the study period, treatment with oral isotretinoin was undertaken by 386 patients at KKUH. Several of these patients were excluded due to incomplete medical records. The age of the studied patients ranged from 17 to 60 years, with a median age of 24 years (interquartile range, 20−28 years). The daily administered dose ranged from 10 to 80 mg, with a median dose of 30 mg (interquartile range, 20−40 mg), as illustrated in the Table. Repeated-measures analysis of liver enzymes (AST and ALT), total cholesterol, and TGs is detailed in eTable 1. Eight (2.2%) of 371 patients showed abnormal baseline AST levels. The first follow-up measurements of AST revealed high levels in 7 (1.9%) patients. This figure doubled (14 [3.8%] patients) at the second follow-up, with no statistically significant differences (P>.05). Likewise, ALT showed abnormally high levels at baseline and at both the first and second follow-ups (47/371 [12.7%], 49/371 [13.2%], and 37/371 [10.0%], respectively) with no significant differences (P>.05). Furthermore, the proportions of high cholesterol levels at baseline and at both the first and second follow-ups (40/331 [12.1%], 72/331 [21.8%], and 62/331 [18.7%], respectively) showed a statistically significant difference (P=.001). The proportions of high cholesterol levels in both the first and second follow-ups were significantly higher than the baseline proportions (P=.001 and P=.002, respectively). However, the percentages of high cholesterol were reduced at the second reading relative to the first but with no significant differences. Regarding TGs, there was a statistically significant difference in the proportions of high levels over time (5/320 [1.6%], 12/320 [3.8%], and 14/320 [4.4%] at baseline and at the first and second readings, respectively). Moreover, pairwise comparison among the 3 readings revealed a significant difference between the second follow-up and the baseline levels (P=.048). eTable 2 demonstrates statistically significant positive weak associations between the daily administered isotretinoin dose and each of the cholesterol and TG levels, both at the first and second follow-up readings (P<.05).



Comment
Evaluation of the effects of isotretinoin on liver enzymes and lipids has suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT) and lipid profiles to various degrees.8 Furthermore, there are controversies regarding the routine laboratory monitoring of these patients. Some studies have reported severe alterations in serum liver transaminase and lipid levels, and they support the need for careful monitoring when treating patients with isotretinoin. However, other studies have reported that adverse effects are minimal, with no need for costly laboratory monitoring.9
Our study explored the profile of changes in liver aminotransferases (AST and ALT), cholesterol, and TGs in patients with acne who had been treated with oral isotretinoin. The cholesterol levels showed a nonprogressive increase, with a prevalence rate of 21.8% and 18.7% at the first and second follow-ups, respectively. Likewise, the frequency of high TG levels was 3.8% and 4.4%, respectively, with significant differences from the baseline levels (P=.041). However, liver enzymes were less affected by isotretinoin therapy than lipid profiles. Both AST and ALT showed nonsignificant minimal elevations during follow-up of the patients.
Similar to our findings, Zane et al6 at the University of California, San Francisco, studied 13,772 patients with acne who underwent oral isotretinoin therapy between 1995 and 2002. They reported a cumulative incidence of new abnormalities in patients with normal values at baseline at a frequency of 44% for TG levels, 31% for total cholesterol levels, and 11% for transaminase levels. Moreover, they suggested that these abnormalities generally were transient and reversible.6 Another retrospective study in Brazil included 130 patients who were treated with isotretinoin for 3 months and reported that TG levels had increased beyond the normal range in 11% of patients, whereas 8.6% had elevated AST levels and 7.3% had elevated ALT levels.8 Comparable to our findings, Kizilyel et al10 concluded that isotretinoin appeared to have a greater effect on lipids than on liver enzymes, and they recommended its use with careful monitoring.
The transient effects of isotretinoin therapy on lipid profiles were highlighted in an earlier study. It has been reported that the changes in low-density lipoprotein and TGs returned to baseline levels 2 months following termination of treatment.11 Although many studies have reported alterations in serum transaminase and lipid levels, other studies fail to report any such effects. Alcalay et al7 investigated 907 patients who completed a treatment course lasting 5 to 9 months. They reported that only 1.5% of patients had serum TG levels above 400 mg. Additionally, serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of treatment. They concluded that isotretinoin is a safe therapeutic drug and suggested that there is no need for routine laboratory follow-up in young healthy patients apart from a pregnancy test for females.7 In addition, Brito et al12 conducted a prospective clinical and laboratory evaluation of 150 patients being treated with oral isotretinoin prior to the start of therapy, 1 month after therapy initiation, and every 3 months thereafter until the completion of treatment. They found no statistically significant changes in liver transaminase, TG, or cholesterol levels.12 In another study of 30 participants, Baxter et al13 also reported no significant changes in TG or cholesterol levels measured at baseline or during treatment with isotretinoin. Furthermore, a systematic review and meta-analysis has estimated the laboratory changes that occur during isotretinoin therapy of acne vulgaris.14 The evidence revealed in this study does not support monthly laboratory testing for use of standard doses of oral isotretinoin for the typical patient with acne.
Conclusion
In our study, liver enzymes were less affected than lipids in patients who were treated with isotretinoin. Additionally, laboratory alterations in lipid profiles were nonprogressive and nonsevere. Consequently, isotretinoin may be administered with minimal concern for changes in serum transaminase and lipid profile. However, physicians should exercise caution when administering isotretinoin in patients with a history of abnormal findings.
- Kaymak Y, Ilter N. The results and side effects of systemic isotretinoin treatment in 100 patients with acne vulgaris. Dermatol Nurs. 2006;18:576-580.
- Al-Mutairi N, Manchanda Y, Nour-Eldin O, et al. Isotretinoin in acne vulgaris: a prospective analysis of 160 cases from Kuwait. J Drugs Dermatol. 2005;4:369-373.
- Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: a randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77:688-694.
- Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
- Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
- Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglycerides and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
- Bauer LB, Ornelas JN, Elston DM, et al. Isotretinoin: controversies, facts, and recommendations. Expert Rev Clin Pharmacol. 2016;9:1435-1442.
- Kizilyel O, Metin MS, Elmas ÖF, et al. Effects of oral isotretinoin on lipids and liver enzymes in acne patients. Cutis. 2014;94:234-238.
- Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
- Brito MDFDM, Sant’Anna IP, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin. An Bras Dermatol. 2010;85:331-337.
- Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2004;14:216-218.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
- Kaymak Y, Ilter N. The results and side effects of systemic isotretinoin treatment in 100 patients with acne vulgaris. Dermatol Nurs. 2006;18:576-580.
- Al-Mutairi N, Manchanda Y, Nour-Eldin O, et al. Isotretinoin in acne vulgaris: a prospective analysis of 160 cases from Kuwait. J Drugs Dermatol. 2005;4:369-373.
- Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: a randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77:688-694.
- Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
- Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
- Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglycerides and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
- Bauer LB, Ornelas JN, Elston DM, et al. Isotretinoin: controversies, facts, and recommendations. Expert Rev Clin Pharmacol. 2016;9:1435-1442.
- Kizilyel O, Metin MS, Elmas ÖF, et al. Effects of oral isotretinoin on lipids and liver enzymes in acne patients. Cutis. 2014;94:234-238.
- Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
- Brito MDFDM, Sant’Anna IP, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin. An Bras Dermatol. 2010;85:331-337.
- Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2004;14:216-218.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
Practice Points
- Isotretinoin is the mainstay treatment for severe acne.
- Cost and convenience to patients should always be considered.
- Frequent monitoring for laboratory changes during isotretinoin treatment is not warranted.
Study eyes impact of isotretinoin on triglycerides, other lab measures
.
“Isotretinoin is a very effective treatment for severe acne,” Varsha Parthasarathy said at the annual meeting of the Society for Pediatric Dermatology. “However, initiating this medication requires a complex process of laboratory testing,” which includes human chorionic gonadotropin pregnancy testing, because isotretinoin is a teratogen, as well as lipid labs and liver function tests, she noted. “Importantly, triglycerides are measured due to an association in adults between isotretinoin and hypertriglyceridemia-associated pancreatitis. However, these findings in children are limited to case reports, as are findings of retinoid-induced hepatotoxicity.”
To identify the role of isotretinoin on changes in lipids, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and to determine the impact on treatment course, Ms. Parthasarathy, a 4-year medical student at George Washington University, Washington, and colleagues retrospectively reviewed the charts of 130 patients aged 12-21 years who were cared for at Children’s National Hospital between January 2012 and October 2020. Nearly two-thirds (65%) were male, their average age was 16 years, and the mean time to obtain follow-up labs after starting isotretinoin was 3.25 months.
Between baseline and follow-up, the researchers observed increases in total cholesterol, triglycerides, and LDL (P less than .001 for all associations) and a decrease in HDL (P = .001), but there were no significant changes in AST or ALT levels. These findings were consistent with prior studies in adults examining the utility of these laboratory tests, most notably a 2016 study by Timothy J. Hansen, MD, and colleagues.
Among the 13 patients with elevated triglycerides at baseline, 9 (69%) were overweight or obese. Of the 20 patients with elevated triglycerides at follow-up, 11 patients (55%) were obese. At follow-up, 11 patients had levels of 200-500 mg/dL (grade I elevation), and 2 patients had levels of 501-1,000 mg/dL (grade II elevation). Isotretinoin was stopped in the latter two patients, who also had obesity as a risk factor for their hypertriglyceridemia.
“None of these patients had clinical sequelae from their hypertriglyceridemia, such as pancreatitis at baseline or follow-up,” Ms. Parthasarathy said. “However, since pancreatitis would be expected to be exceedingly rare, the sample size may be limited in identifying this adverse effect.”
She noted that while isotretinoin might cause a significant increase in lipid levels, the mean levels remained within normal limits at both baseline and follow-up. “Of the patients with elevated triglycerides at baseline and follow-up, obesity may have been a potential risk factor,” she said. “This could suggest a possible strategy for reduced testing in nonobese isotretinoin patients, which can be further explored in larger study populations.”
In addition, “there was a lack of significant change in AST and ALT in this study and adult studies, as well as minimal evidence for pediatric retinoid-induced hepatotoxicity, which raises the question of the necessity of baseline and follow-up comprehensive metabolic panel testing,” Ms. Parthasarathy added. “Clinicians must weigh the laboratory values with the costs of laboratory testing, including opportunity costs such as time, monetary costs, and the discomfort of testing for pediatric patients.”
The study’s senior author was A. Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital, Washington. The researchers reported having no relevant financial disclosures.
.
“Isotretinoin is a very effective treatment for severe acne,” Varsha Parthasarathy said at the annual meeting of the Society for Pediatric Dermatology. “However, initiating this medication requires a complex process of laboratory testing,” which includes human chorionic gonadotropin pregnancy testing, because isotretinoin is a teratogen, as well as lipid labs and liver function tests, she noted. “Importantly, triglycerides are measured due to an association in adults between isotretinoin and hypertriglyceridemia-associated pancreatitis. However, these findings in children are limited to case reports, as are findings of retinoid-induced hepatotoxicity.”
To identify the role of isotretinoin on changes in lipids, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and to determine the impact on treatment course, Ms. Parthasarathy, a 4-year medical student at George Washington University, Washington, and colleagues retrospectively reviewed the charts of 130 patients aged 12-21 years who were cared for at Children’s National Hospital between January 2012 and October 2020. Nearly two-thirds (65%) were male, their average age was 16 years, and the mean time to obtain follow-up labs after starting isotretinoin was 3.25 months.
Between baseline and follow-up, the researchers observed increases in total cholesterol, triglycerides, and LDL (P less than .001 for all associations) and a decrease in HDL (P = .001), but there were no significant changes in AST or ALT levels. These findings were consistent with prior studies in adults examining the utility of these laboratory tests, most notably a 2016 study by Timothy J. Hansen, MD, and colleagues.
Among the 13 patients with elevated triglycerides at baseline, 9 (69%) were overweight or obese. Of the 20 patients with elevated triglycerides at follow-up, 11 patients (55%) were obese. At follow-up, 11 patients had levels of 200-500 mg/dL (grade I elevation), and 2 patients had levels of 501-1,000 mg/dL (grade II elevation). Isotretinoin was stopped in the latter two patients, who also had obesity as a risk factor for their hypertriglyceridemia.
“None of these patients had clinical sequelae from their hypertriglyceridemia, such as pancreatitis at baseline or follow-up,” Ms. Parthasarathy said. “However, since pancreatitis would be expected to be exceedingly rare, the sample size may be limited in identifying this adverse effect.”
She noted that while isotretinoin might cause a significant increase in lipid levels, the mean levels remained within normal limits at both baseline and follow-up. “Of the patients with elevated triglycerides at baseline and follow-up, obesity may have been a potential risk factor,” she said. “This could suggest a possible strategy for reduced testing in nonobese isotretinoin patients, which can be further explored in larger study populations.”
In addition, “there was a lack of significant change in AST and ALT in this study and adult studies, as well as minimal evidence for pediatric retinoid-induced hepatotoxicity, which raises the question of the necessity of baseline and follow-up comprehensive metabolic panel testing,” Ms. Parthasarathy added. “Clinicians must weigh the laboratory values with the costs of laboratory testing, including opportunity costs such as time, monetary costs, and the discomfort of testing for pediatric patients.”
The study’s senior author was A. Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital, Washington. The researchers reported having no relevant financial disclosures.
.
“Isotretinoin is a very effective treatment for severe acne,” Varsha Parthasarathy said at the annual meeting of the Society for Pediatric Dermatology. “However, initiating this medication requires a complex process of laboratory testing,” which includes human chorionic gonadotropin pregnancy testing, because isotretinoin is a teratogen, as well as lipid labs and liver function tests, she noted. “Importantly, triglycerides are measured due to an association in adults between isotretinoin and hypertriglyceridemia-associated pancreatitis. However, these findings in children are limited to case reports, as are findings of retinoid-induced hepatotoxicity.”
To identify the role of isotretinoin on changes in lipids, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and to determine the impact on treatment course, Ms. Parthasarathy, a 4-year medical student at George Washington University, Washington, and colleagues retrospectively reviewed the charts of 130 patients aged 12-21 years who were cared for at Children’s National Hospital between January 2012 and October 2020. Nearly two-thirds (65%) were male, their average age was 16 years, and the mean time to obtain follow-up labs after starting isotretinoin was 3.25 months.
Between baseline and follow-up, the researchers observed increases in total cholesterol, triglycerides, and LDL (P less than .001 for all associations) and a decrease in HDL (P = .001), but there were no significant changes in AST or ALT levels. These findings were consistent with prior studies in adults examining the utility of these laboratory tests, most notably a 2016 study by Timothy J. Hansen, MD, and colleagues.
Among the 13 patients with elevated triglycerides at baseline, 9 (69%) were overweight or obese. Of the 20 patients with elevated triglycerides at follow-up, 11 patients (55%) were obese. At follow-up, 11 patients had levels of 200-500 mg/dL (grade I elevation), and 2 patients had levels of 501-1,000 mg/dL (grade II elevation). Isotretinoin was stopped in the latter two patients, who also had obesity as a risk factor for their hypertriglyceridemia.
“None of these patients had clinical sequelae from their hypertriglyceridemia, such as pancreatitis at baseline or follow-up,” Ms. Parthasarathy said. “However, since pancreatitis would be expected to be exceedingly rare, the sample size may be limited in identifying this adverse effect.”
She noted that while isotretinoin might cause a significant increase in lipid levels, the mean levels remained within normal limits at both baseline and follow-up. “Of the patients with elevated triglycerides at baseline and follow-up, obesity may have been a potential risk factor,” she said. “This could suggest a possible strategy for reduced testing in nonobese isotretinoin patients, which can be further explored in larger study populations.”
In addition, “there was a lack of significant change in AST and ALT in this study and adult studies, as well as minimal evidence for pediatric retinoid-induced hepatotoxicity, which raises the question of the necessity of baseline and follow-up comprehensive metabolic panel testing,” Ms. Parthasarathy added. “Clinicians must weigh the laboratory values with the costs of laboratory testing, including opportunity costs such as time, monetary costs, and the discomfort of testing for pediatric patients.”
The study’s senior author was A. Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital, Washington. The researchers reported having no relevant financial disclosures.
FROM SPD 2021
Delta variant key to breakthrough infections in vaccinated Israelis
Israeli officials are reporting a 30% decrease in the effectiveness of the Pfizer/BioNTech vaccine to prevent SARS-CoV-2 infection and mild to moderate cases of COVID-19. At the same time, protection against hospitalization and severe illness remains robust.
The country’s Ministry of Health data cited high levels of circulating Delta variant and a relaxation of public health measures in early June for the drop in the vaccine’s prevention of “breakthrough” cases from 94% to 64% in recent weeks.
However, it is important to consider the findings in context, experts cautioned.
“My overall take on this that the vaccine is highly protective against the endpoints that matter – hospitalization and severe disease,” Anna Durbin, MD, told this news organization.
“I was very pleasantly surprised with the very high efficacy against hospitalization and severe disease – even against the Delta variant,” added Dr. Durbin, professor of medicine at Johns Hopkins University, Baltimore.
Ali Mokdad, PhD, of the Institute for Health Metrics at the University of Washington, Seattle, agreed that the high degree of protection against severe outcomes should be the focus.
“That’s the whole idea. You want to defend against COVID-19. So even if someone is infected, they don’t end up in the hospital or in the morgue,” he said in an interview.
Compared with an earlier report, the efficacy of the Pfizer vaccine against hospitalization fell slightly from 98% to 93%.
“For me, the fact that there is increased infection from the Delta variant after the vaccines such as Pfizer is of course a concern. But the positive news is that there is 93% prevention against severe disease or mortality,” added Dr. Mokdad, who is also professor of global health at University of Washington.
In addition, the absolute numbers remain relatively small. The Ministry of Health data show that, of the 63 Israelis hospitalized with COVID-19 nationwide on July 3, 34 were in critical condition.
Unrealistic expectations?
People may have unrealistic expectations regarding breakthrough infections, Dr. Durbin said. “It seems that people are almost expecting ‘sterilizing immunity’ from these vaccines,” she said, explaining that would mean complete protection from infection.
Expectations may be high “because these vaccines have been so effective,” added Dr. Durbin, who is also affiliated with the Johns Hopkins Center for Global Health.
The higher the number of vaccinated residents, the more breakthrough cases will be reported, epidemiologist Katelyn Jetelina, PhD, MPH, assistant professor of epidemiology, human genetics, and environmental sciences at the University of Texas Science Center at Houston, wrote in her “Your Local Epidemiologist” blog.
This could apply to Israel, with an estimated 60% of adults in Israel fully vaccinated and 65% receiving at least one dose as of July 5, Our World in Data figures show.
How the updated figures were reported could be confusing, Dr. Jetelina said. Israel’s Health Minister Chezy Levy noted that “55% of the newly infected had been vaccinated” in a radio interview announcing the results.
“This language is important because it’s very different than ‘half of vaccinated people were infected,’ ” Dr. Jetelina noted.
Israel had a 7-day rolling average of 324 new confirmed COVID-19 cases as of July 5. Assuming 55% of these cases were among vaccinated people, that would mean 178 people experienced breakthrough infections.
In contrast, almost 6 million people in Israel are fully vaccinated. If 55% of them experienced breakthrough infections, the number would be much higher – more than 3 million.
Dr. Jetelina added that more details about the new Israel figures would be helpful, including the severity of COVID-19 among the vaccinated cases and breakdown of infections between adults and children.
Next steps
Israeli health officials are weighing the necessity of a third or booster dose of the vaccine. Whether they will reinstate public health measures to prevent spread of COVID-19 also remains unknown.
Going forward, Israel intends to study whether factors such as age, comorbidities, or time since immunization affect risk for breakthrough infections among people vaccinated against COVID-19.
“We want to prevent people from getting hospitalized, seriously ill, and of course, dying. It’s encouraging these vaccines will be able to have a high impact on those outcomes,” Dr. Durbin said. “We just need to get people vaccinated.”
A call for better global surveillance
A global surveillance system is a potential solution to track and respond to the growing threat of the Delta variant and other variants of concern, Scott P. Layne, MD, and Jeffery K. Taubenberger, MD, PhD, wrote in a July 7, 2021, editorial in Science Translational Medicine.
One goal, Dr. Layne said in an interview, is to highlight “the compelling need for a new global COVID-19 program of surveillance and offer a blueprint for building it.” A second aim is to promote global cooperation among key advisers and leaders in the G7, G20, and Asia-Pacific Economic Cooperation nations.
“It’s an uphill struggle with superpower discords, global warming, cybersecurity, and pandemics all competing for finite attention,” Dr. Layne said. “However, what other options do we have for taming the so-called forever virus?”
Dr. Mokdad and Dr. Jetelina had no relevant disclosures. Dr. Durban disclosed she was the site primary investigator for the phase 3 AstraZeneca vaccine trial and an investigator on the Pfizer COVID-19 vaccine trial.
A version of this article first appeared on Medscape.com.
Israeli officials are reporting a 30% decrease in the effectiveness of the Pfizer/BioNTech vaccine to prevent SARS-CoV-2 infection and mild to moderate cases of COVID-19. At the same time, protection against hospitalization and severe illness remains robust.
The country’s Ministry of Health data cited high levels of circulating Delta variant and a relaxation of public health measures in early June for the drop in the vaccine’s prevention of “breakthrough” cases from 94% to 64% in recent weeks.
However, it is important to consider the findings in context, experts cautioned.
“My overall take on this that the vaccine is highly protective against the endpoints that matter – hospitalization and severe disease,” Anna Durbin, MD, told this news organization.
“I was very pleasantly surprised with the very high efficacy against hospitalization and severe disease – even against the Delta variant,” added Dr. Durbin, professor of medicine at Johns Hopkins University, Baltimore.
Ali Mokdad, PhD, of the Institute for Health Metrics at the University of Washington, Seattle, agreed that the high degree of protection against severe outcomes should be the focus.
“That’s the whole idea. You want to defend against COVID-19. So even if someone is infected, they don’t end up in the hospital or in the morgue,” he said in an interview.
Compared with an earlier report, the efficacy of the Pfizer vaccine against hospitalization fell slightly from 98% to 93%.
“For me, the fact that there is increased infection from the Delta variant after the vaccines such as Pfizer is of course a concern. But the positive news is that there is 93% prevention against severe disease or mortality,” added Dr. Mokdad, who is also professor of global health at University of Washington.
In addition, the absolute numbers remain relatively small. The Ministry of Health data show that, of the 63 Israelis hospitalized with COVID-19 nationwide on July 3, 34 were in critical condition.
Unrealistic expectations?
People may have unrealistic expectations regarding breakthrough infections, Dr. Durbin said. “It seems that people are almost expecting ‘sterilizing immunity’ from these vaccines,” she said, explaining that would mean complete protection from infection.
Expectations may be high “because these vaccines have been so effective,” added Dr. Durbin, who is also affiliated with the Johns Hopkins Center for Global Health.
The higher the number of vaccinated residents, the more breakthrough cases will be reported, epidemiologist Katelyn Jetelina, PhD, MPH, assistant professor of epidemiology, human genetics, and environmental sciences at the University of Texas Science Center at Houston, wrote in her “Your Local Epidemiologist” blog.
This could apply to Israel, with an estimated 60% of adults in Israel fully vaccinated and 65% receiving at least one dose as of July 5, Our World in Data figures show.
How the updated figures were reported could be confusing, Dr. Jetelina said. Israel’s Health Minister Chezy Levy noted that “55% of the newly infected had been vaccinated” in a radio interview announcing the results.
“This language is important because it’s very different than ‘half of vaccinated people were infected,’ ” Dr. Jetelina noted.
Israel had a 7-day rolling average of 324 new confirmed COVID-19 cases as of July 5. Assuming 55% of these cases were among vaccinated people, that would mean 178 people experienced breakthrough infections.
In contrast, almost 6 million people in Israel are fully vaccinated. If 55% of them experienced breakthrough infections, the number would be much higher – more than 3 million.
Dr. Jetelina added that more details about the new Israel figures would be helpful, including the severity of COVID-19 among the vaccinated cases and breakdown of infections between adults and children.
Next steps
Israeli health officials are weighing the necessity of a third or booster dose of the vaccine. Whether they will reinstate public health measures to prevent spread of COVID-19 also remains unknown.
Going forward, Israel intends to study whether factors such as age, comorbidities, or time since immunization affect risk for breakthrough infections among people vaccinated against COVID-19.
“We want to prevent people from getting hospitalized, seriously ill, and of course, dying. It’s encouraging these vaccines will be able to have a high impact on those outcomes,” Dr. Durbin said. “We just need to get people vaccinated.”
A call for better global surveillance
A global surveillance system is a potential solution to track and respond to the growing threat of the Delta variant and other variants of concern, Scott P. Layne, MD, and Jeffery K. Taubenberger, MD, PhD, wrote in a July 7, 2021, editorial in Science Translational Medicine.
One goal, Dr. Layne said in an interview, is to highlight “the compelling need for a new global COVID-19 program of surveillance and offer a blueprint for building it.” A second aim is to promote global cooperation among key advisers and leaders in the G7, G20, and Asia-Pacific Economic Cooperation nations.
“It’s an uphill struggle with superpower discords, global warming, cybersecurity, and pandemics all competing for finite attention,” Dr. Layne said. “However, what other options do we have for taming the so-called forever virus?”
Dr. Mokdad and Dr. Jetelina had no relevant disclosures. Dr. Durban disclosed she was the site primary investigator for the phase 3 AstraZeneca vaccine trial and an investigator on the Pfizer COVID-19 vaccine trial.
A version of this article first appeared on Medscape.com.
Israeli officials are reporting a 30% decrease in the effectiveness of the Pfizer/BioNTech vaccine to prevent SARS-CoV-2 infection and mild to moderate cases of COVID-19. At the same time, protection against hospitalization and severe illness remains robust.
The country’s Ministry of Health data cited high levels of circulating Delta variant and a relaxation of public health measures in early June for the drop in the vaccine’s prevention of “breakthrough” cases from 94% to 64% in recent weeks.
However, it is important to consider the findings in context, experts cautioned.
“My overall take on this that the vaccine is highly protective against the endpoints that matter – hospitalization and severe disease,” Anna Durbin, MD, told this news organization.
“I was very pleasantly surprised with the very high efficacy against hospitalization and severe disease – even against the Delta variant,” added Dr. Durbin, professor of medicine at Johns Hopkins University, Baltimore.
Ali Mokdad, PhD, of the Institute for Health Metrics at the University of Washington, Seattle, agreed that the high degree of protection against severe outcomes should be the focus.
“That’s the whole idea. You want to defend against COVID-19. So even if someone is infected, they don’t end up in the hospital or in the morgue,” he said in an interview.
Compared with an earlier report, the efficacy of the Pfizer vaccine against hospitalization fell slightly from 98% to 93%.
“For me, the fact that there is increased infection from the Delta variant after the vaccines such as Pfizer is of course a concern. But the positive news is that there is 93% prevention against severe disease or mortality,” added Dr. Mokdad, who is also professor of global health at University of Washington.
In addition, the absolute numbers remain relatively small. The Ministry of Health data show that, of the 63 Israelis hospitalized with COVID-19 nationwide on July 3, 34 were in critical condition.
Unrealistic expectations?
People may have unrealistic expectations regarding breakthrough infections, Dr. Durbin said. “It seems that people are almost expecting ‘sterilizing immunity’ from these vaccines,” she said, explaining that would mean complete protection from infection.
Expectations may be high “because these vaccines have been so effective,” added Dr. Durbin, who is also affiliated with the Johns Hopkins Center for Global Health.
The higher the number of vaccinated residents, the more breakthrough cases will be reported, epidemiologist Katelyn Jetelina, PhD, MPH, assistant professor of epidemiology, human genetics, and environmental sciences at the University of Texas Science Center at Houston, wrote in her “Your Local Epidemiologist” blog.
This could apply to Israel, with an estimated 60% of adults in Israel fully vaccinated and 65% receiving at least one dose as of July 5, Our World in Data figures show.
How the updated figures were reported could be confusing, Dr. Jetelina said. Israel’s Health Minister Chezy Levy noted that “55% of the newly infected had been vaccinated” in a radio interview announcing the results.
“This language is important because it’s very different than ‘half of vaccinated people were infected,’ ” Dr. Jetelina noted.
Israel had a 7-day rolling average of 324 new confirmed COVID-19 cases as of July 5. Assuming 55% of these cases were among vaccinated people, that would mean 178 people experienced breakthrough infections.
In contrast, almost 6 million people in Israel are fully vaccinated. If 55% of them experienced breakthrough infections, the number would be much higher – more than 3 million.
Dr. Jetelina added that more details about the new Israel figures would be helpful, including the severity of COVID-19 among the vaccinated cases and breakdown of infections between adults and children.
Next steps
Israeli health officials are weighing the necessity of a third or booster dose of the vaccine. Whether they will reinstate public health measures to prevent spread of COVID-19 also remains unknown.
Going forward, Israel intends to study whether factors such as age, comorbidities, or time since immunization affect risk for breakthrough infections among people vaccinated against COVID-19.
“We want to prevent people from getting hospitalized, seriously ill, and of course, dying. It’s encouraging these vaccines will be able to have a high impact on those outcomes,” Dr. Durbin said. “We just need to get people vaccinated.”
A call for better global surveillance
A global surveillance system is a potential solution to track and respond to the growing threat of the Delta variant and other variants of concern, Scott P. Layne, MD, and Jeffery K. Taubenberger, MD, PhD, wrote in a July 7, 2021, editorial in Science Translational Medicine.
One goal, Dr. Layne said in an interview, is to highlight “the compelling need for a new global COVID-19 program of surveillance and offer a blueprint for building it.” A second aim is to promote global cooperation among key advisers and leaders in the G7, G20, and Asia-Pacific Economic Cooperation nations.
“It’s an uphill struggle with superpower discords, global warming, cybersecurity, and pandemics all competing for finite attention,” Dr. Layne said. “However, what other options do we have for taming the so-called forever virus?”
Dr. Mokdad and Dr. Jetelina had no relevant disclosures. Dr. Durban disclosed she was the site primary investigator for the phase 3 AstraZeneca vaccine trial and an investigator on the Pfizer COVID-19 vaccine trial.
A version of this article first appeared on Medscape.com.
Isotretinoin benefits similar in overweight, obese adolescents, and those in normal weight range
a retrospective cohort study found.
“Oral isotretinoin is among the most effective treatments for acne and is indicated for the treatment of severe acne or when first-line regimens have failed,” Maggie Tallmadge said at the annual meeting of the Society for Pediatric Dermatology. In adolescents with acne, isotretinoin is prescribed at a dose of 0.5-1 mg/kg per day “with the goal of reaching a cumulative dose of 120-150 mg/kg and clinical clearance with durable remission,” she said. “Most providers do not prescribe a daily dose over 80 mg due to perceived increased risk of side effects, including xerosis, cheilitis, liver dysfunction, and acne flare. However, many adolescents weigh over 80 kg and are therefore effectively underdosed, prolonging treatment time and possibly increasing the risk of side effects due to prolonged therapy.”
To evaluate differences in treatment courses among normal-weight, overweight, and obese adolescents, and the efficacy and safety of treatment, Ms. Tallmadge, a third-year medical student at the Medical College of Wisconsin, Milwaukee, and colleagues completed a retrospective chart review of 550 dermatology patients at Children’s Wisconsin, also in Milwaukee, who completed at least 2 months of isotretinoin treatment for acne when they were between the ages of 10 and 24, from November 2012 to January 2020. They collected data on age, weight, height, daily dose, cumulative dose, time to acne clearance, side effects, and acne recurrence after treatment, and classified patients as normal weight, overweight, or obese based on their body mass index for age percentile.
Of the 550 patients, 367 (67%) were normal weight, 101 (18%) were overweight, and 82 (15%) were obese. The median age of those in the normal-weight and overweight groups was 16, and was 15 in the obese group.
There was were significant differences in the median cumulative dose in each weight group: 143.7 mg/kg for normal-weight patients, 138.2 mg/kg for overweight patients, and 140.6 mg/kg for obese patients (P < .001).
“Despite achieving different cumulative doses, there was no difference in acne clearance, relapse, and most side effects among the three [body mass index] cohorts,” Ms. Tallmadge said. “Thus, it appears that current treatment strategies may be appropriate for overweight and obese adolescents.”
The proportion of patients with acne clearance did not differ significantly among the three groups of patients: 62% who were in the normal weight range, 60% who were overweight, and 59% who were obese had clearance of facial acne with treatment (P = .84).
Of patients whose treatment course was completed by the time of data collection, the proportion with acne recurrences was similar between the three groups: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients (P > .05). Of patients whose treatment course was completed by the time of data collection, there was no significant differences in acne recurrence: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients.
However, the proportion of patients reporting headaches differed significantly between the groups: 29% of normal-weight patients, compared with 40% of both overweight and obese patients (P = .035). The researchers also observed a significant positive correlation between increased BMI and increased triglyceride and ALT levels during treatment (P < .001 for both associations), yet no elevations required clinical action.
Funding for the study was provided by the MCW Medical Student Summer Research Program and the American Acne & Rosacea Society.
a retrospective cohort study found.
“Oral isotretinoin is among the most effective treatments for acne and is indicated for the treatment of severe acne or when first-line regimens have failed,” Maggie Tallmadge said at the annual meeting of the Society for Pediatric Dermatology. In adolescents with acne, isotretinoin is prescribed at a dose of 0.5-1 mg/kg per day “with the goal of reaching a cumulative dose of 120-150 mg/kg and clinical clearance with durable remission,” she said. “Most providers do not prescribe a daily dose over 80 mg due to perceived increased risk of side effects, including xerosis, cheilitis, liver dysfunction, and acne flare. However, many adolescents weigh over 80 kg and are therefore effectively underdosed, prolonging treatment time and possibly increasing the risk of side effects due to prolonged therapy.”
To evaluate differences in treatment courses among normal-weight, overweight, and obese adolescents, and the efficacy and safety of treatment, Ms. Tallmadge, a third-year medical student at the Medical College of Wisconsin, Milwaukee, and colleagues completed a retrospective chart review of 550 dermatology patients at Children’s Wisconsin, also in Milwaukee, who completed at least 2 months of isotretinoin treatment for acne when they were between the ages of 10 and 24, from November 2012 to January 2020. They collected data on age, weight, height, daily dose, cumulative dose, time to acne clearance, side effects, and acne recurrence after treatment, and classified patients as normal weight, overweight, or obese based on their body mass index for age percentile.
Of the 550 patients, 367 (67%) were normal weight, 101 (18%) were overweight, and 82 (15%) were obese. The median age of those in the normal-weight and overweight groups was 16, and was 15 in the obese group.
There was were significant differences in the median cumulative dose in each weight group: 143.7 mg/kg for normal-weight patients, 138.2 mg/kg for overweight patients, and 140.6 mg/kg for obese patients (P < .001).
“Despite achieving different cumulative doses, there was no difference in acne clearance, relapse, and most side effects among the three [body mass index] cohorts,” Ms. Tallmadge said. “Thus, it appears that current treatment strategies may be appropriate for overweight and obese adolescents.”
The proportion of patients with acne clearance did not differ significantly among the three groups of patients: 62% who were in the normal weight range, 60% who were overweight, and 59% who were obese had clearance of facial acne with treatment (P = .84).
Of patients whose treatment course was completed by the time of data collection, the proportion with acne recurrences was similar between the three groups: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients (P > .05). Of patients whose treatment course was completed by the time of data collection, there was no significant differences in acne recurrence: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients.
However, the proportion of patients reporting headaches differed significantly between the groups: 29% of normal-weight patients, compared with 40% of both overweight and obese patients (P = .035). The researchers also observed a significant positive correlation between increased BMI and increased triglyceride and ALT levels during treatment (P < .001 for both associations), yet no elevations required clinical action.
Funding for the study was provided by the MCW Medical Student Summer Research Program and the American Acne & Rosacea Society.
a retrospective cohort study found.
“Oral isotretinoin is among the most effective treatments for acne and is indicated for the treatment of severe acne or when first-line regimens have failed,” Maggie Tallmadge said at the annual meeting of the Society for Pediatric Dermatology. In adolescents with acne, isotretinoin is prescribed at a dose of 0.5-1 mg/kg per day “with the goal of reaching a cumulative dose of 120-150 mg/kg and clinical clearance with durable remission,” she said. “Most providers do not prescribe a daily dose over 80 mg due to perceived increased risk of side effects, including xerosis, cheilitis, liver dysfunction, and acne flare. However, many adolescents weigh over 80 kg and are therefore effectively underdosed, prolonging treatment time and possibly increasing the risk of side effects due to prolonged therapy.”
To evaluate differences in treatment courses among normal-weight, overweight, and obese adolescents, and the efficacy and safety of treatment, Ms. Tallmadge, a third-year medical student at the Medical College of Wisconsin, Milwaukee, and colleagues completed a retrospective chart review of 550 dermatology patients at Children’s Wisconsin, also in Milwaukee, who completed at least 2 months of isotretinoin treatment for acne when they were between the ages of 10 and 24, from November 2012 to January 2020. They collected data on age, weight, height, daily dose, cumulative dose, time to acne clearance, side effects, and acne recurrence after treatment, and classified patients as normal weight, overweight, or obese based on their body mass index for age percentile.
Of the 550 patients, 367 (67%) were normal weight, 101 (18%) were overweight, and 82 (15%) were obese. The median age of those in the normal-weight and overweight groups was 16, and was 15 in the obese group.
There was were significant differences in the median cumulative dose in each weight group: 143.7 mg/kg for normal-weight patients, 138.2 mg/kg for overweight patients, and 140.6 mg/kg for obese patients (P < .001).
“Despite achieving different cumulative doses, there was no difference in acne clearance, relapse, and most side effects among the three [body mass index] cohorts,” Ms. Tallmadge said. “Thus, it appears that current treatment strategies may be appropriate for overweight and obese adolescents.”
The proportion of patients with acne clearance did not differ significantly among the three groups of patients: 62% who were in the normal weight range, 60% who were overweight, and 59% who were obese had clearance of facial acne with treatment (P = .84).
Of patients whose treatment course was completed by the time of data collection, the proportion with acne recurrences was similar between the three groups: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients (P > .05). Of patients whose treatment course was completed by the time of data collection, there was no significant differences in acne recurrence: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients.
However, the proportion of patients reporting headaches differed significantly between the groups: 29% of normal-weight patients, compared with 40% of both overweight and obese patients (P = .035). The researchers also observed a significant positive correlation between increased BMI and increased triglyceride and ALT levels during treatment (P < .001 for both associations), yet no elevations required clinical action.
Funding for the study was provided by the MCW Medical Student Summer Research Program and the American Acne & Rosacea Society.
FROM SPD 2021